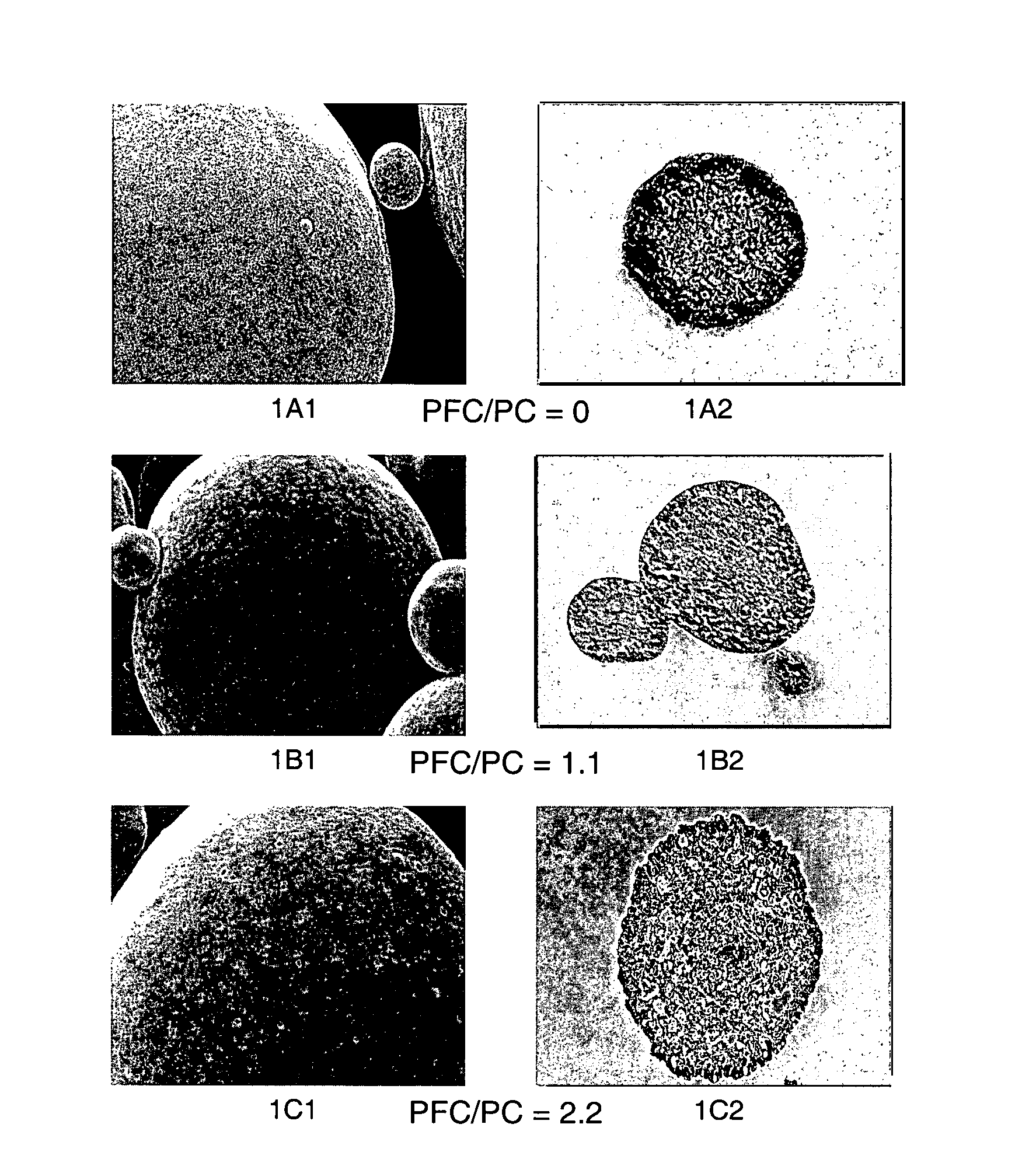
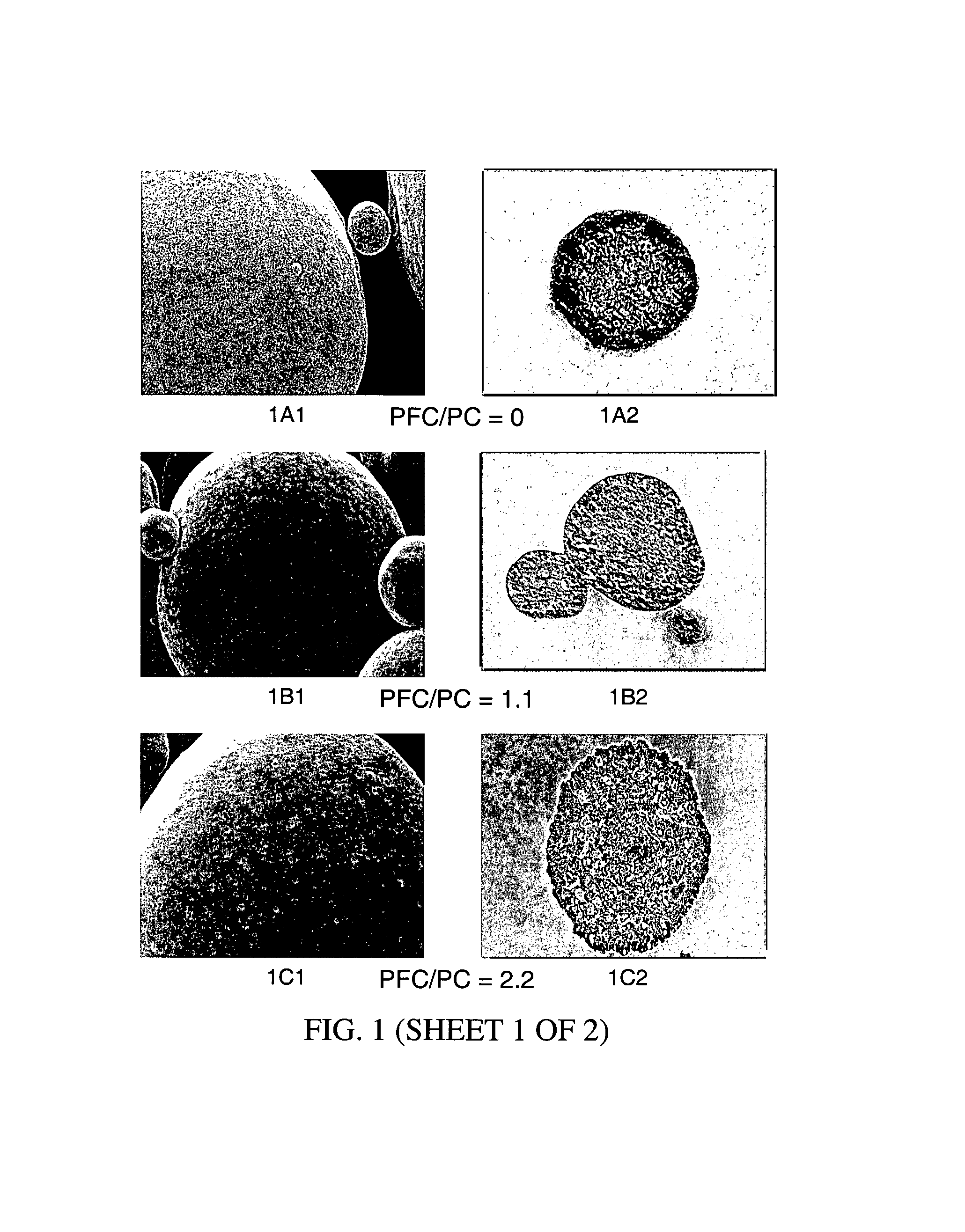
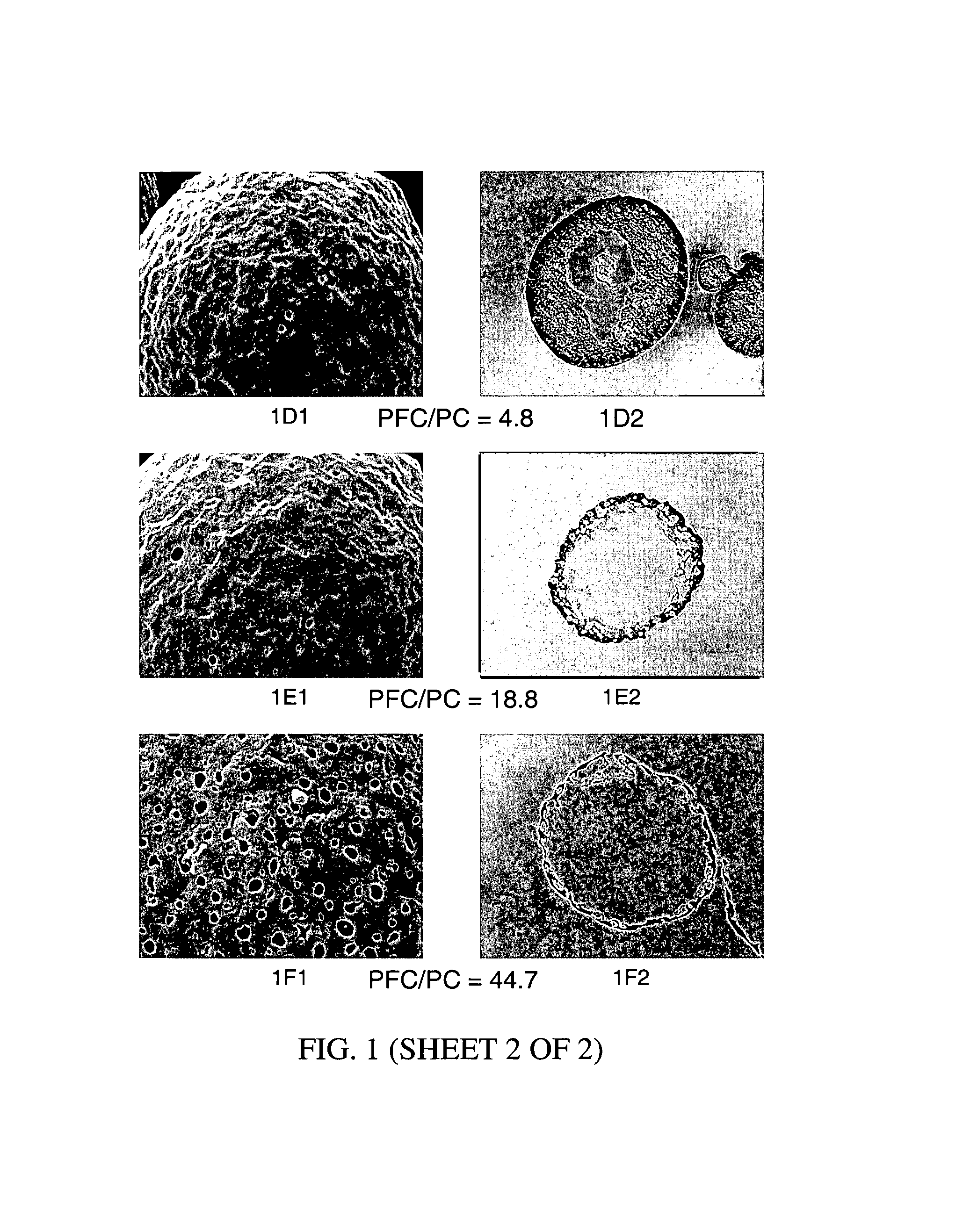
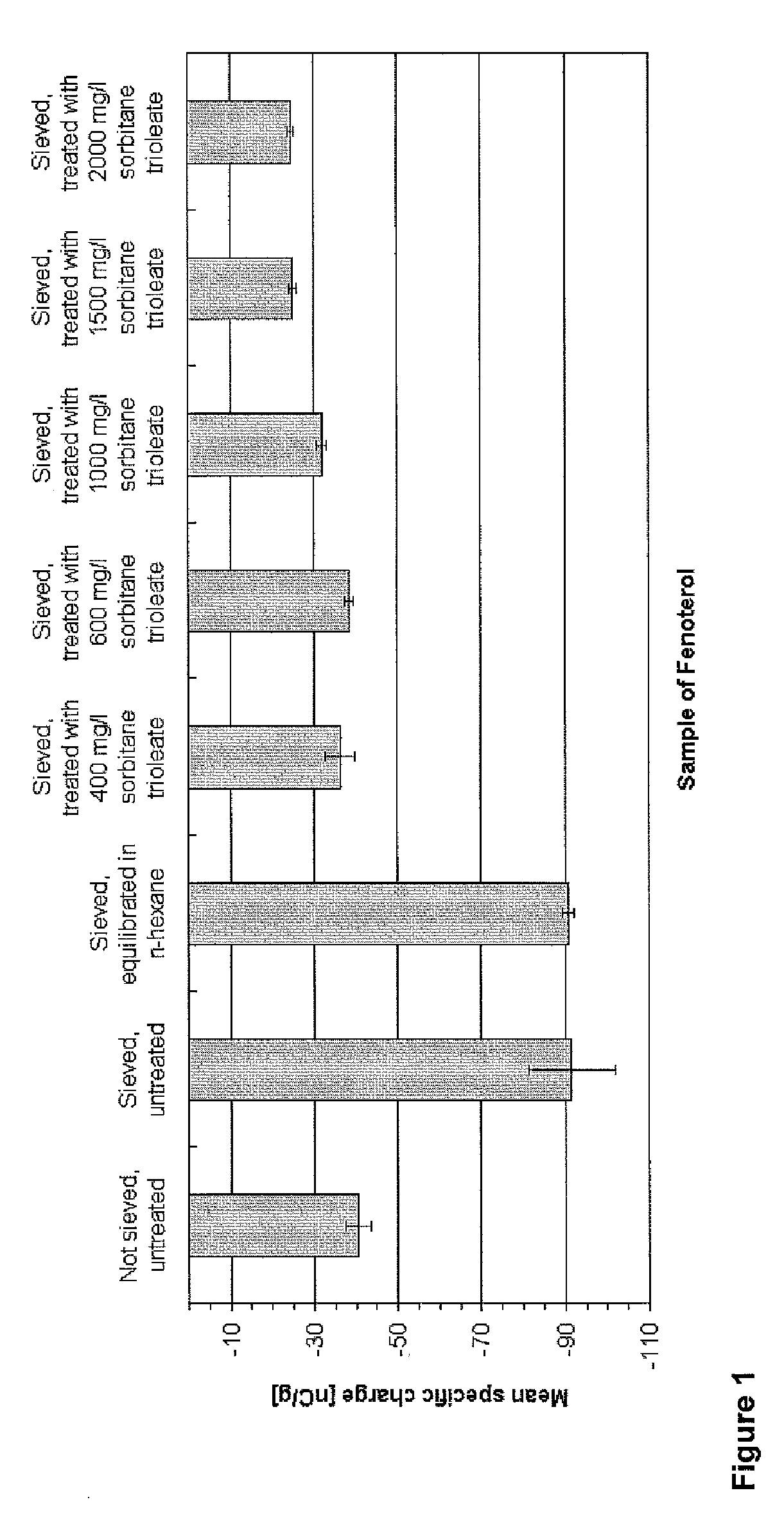
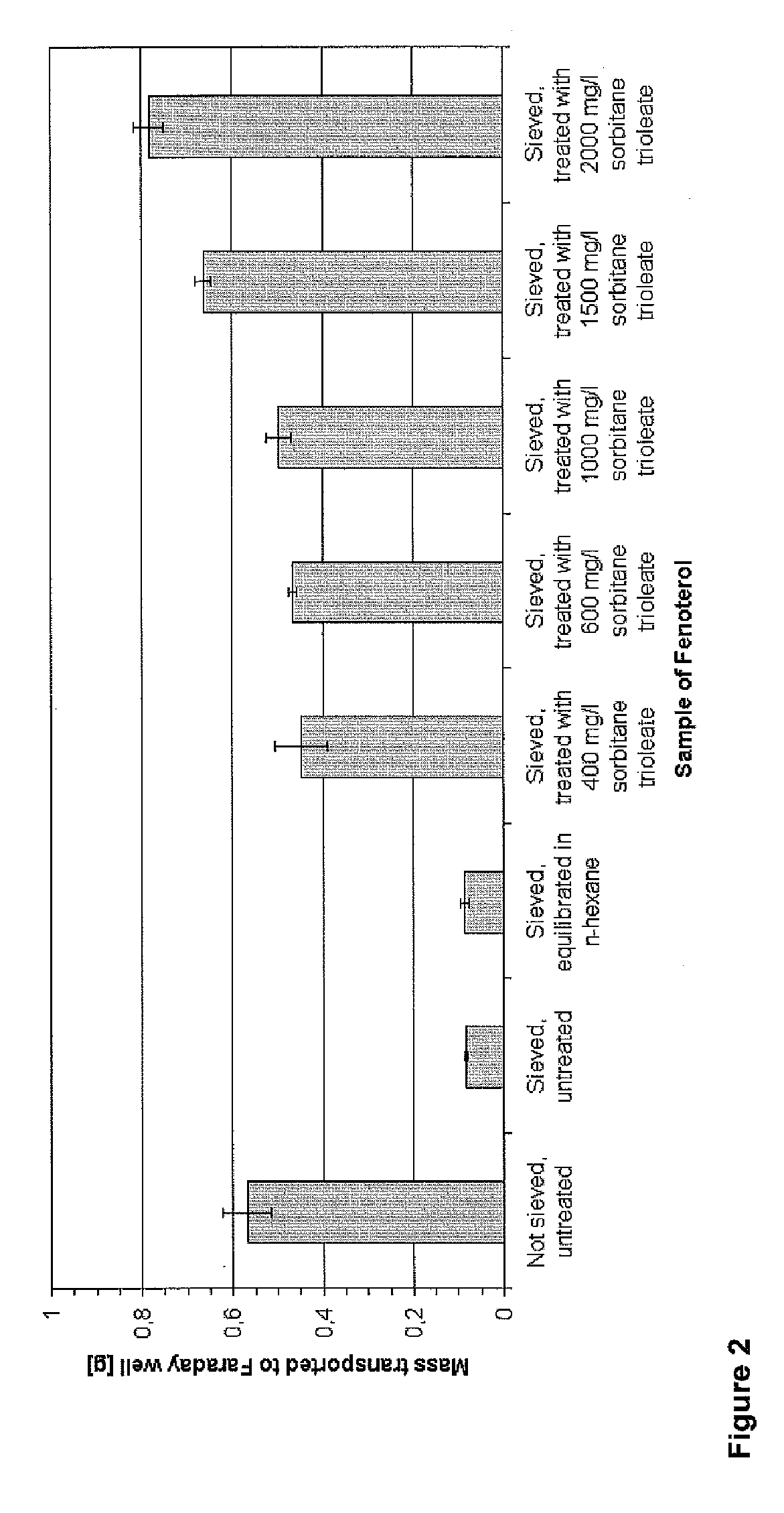
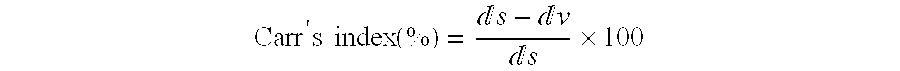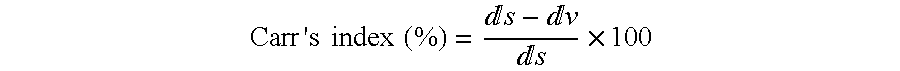Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
142 results about "Powder Inhaler" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
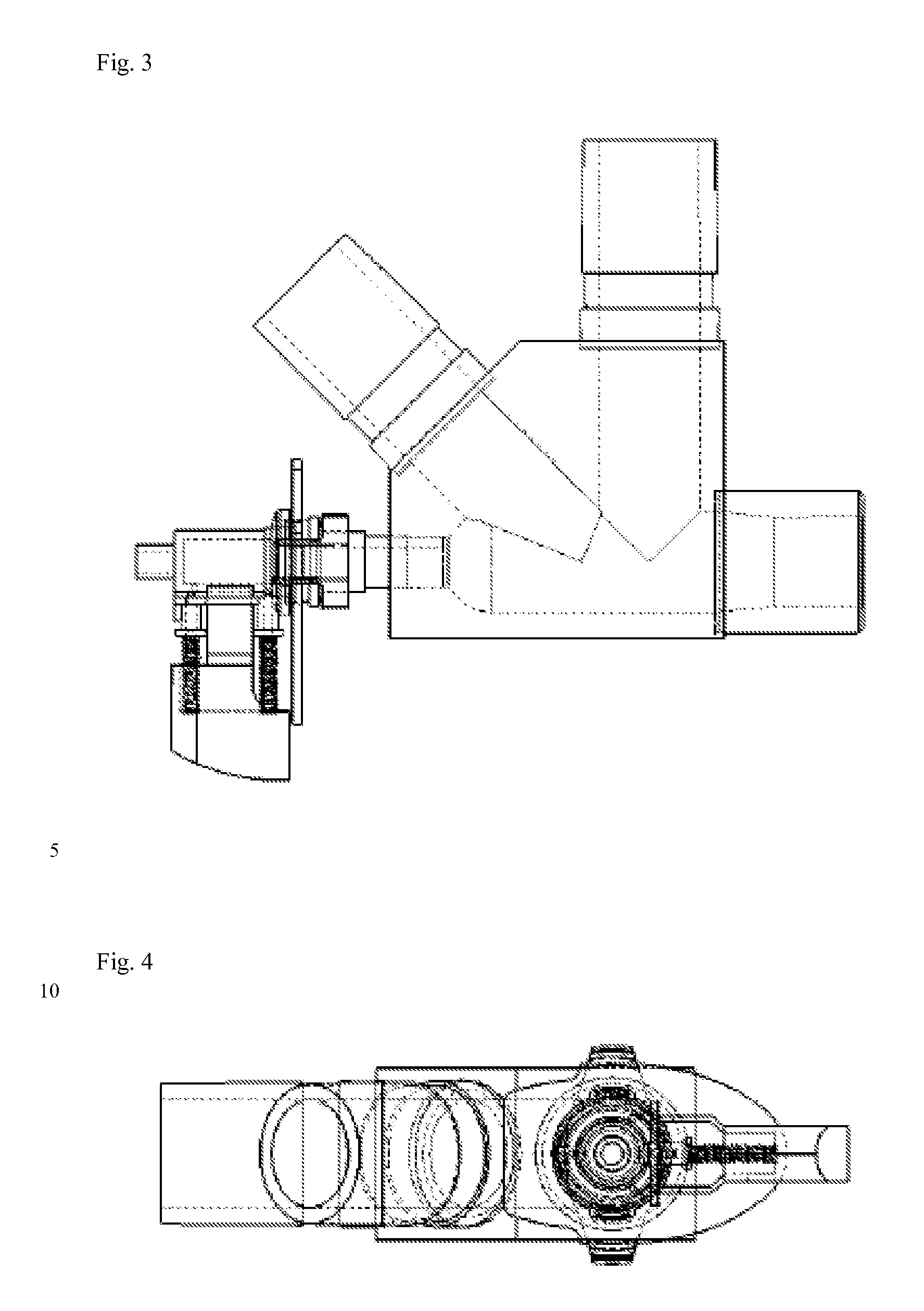
A dry-powder inhaler (DPI) is a device that delivers medication to the lungs in the form of a dry powder. DPIs are commonly used to treat respiratory diseases such as asthma, bronchitis, emphysema and COPD although DPIs (such as inhalable insulin Afrezza) have also been used in the treatment of diabetes mellitus.
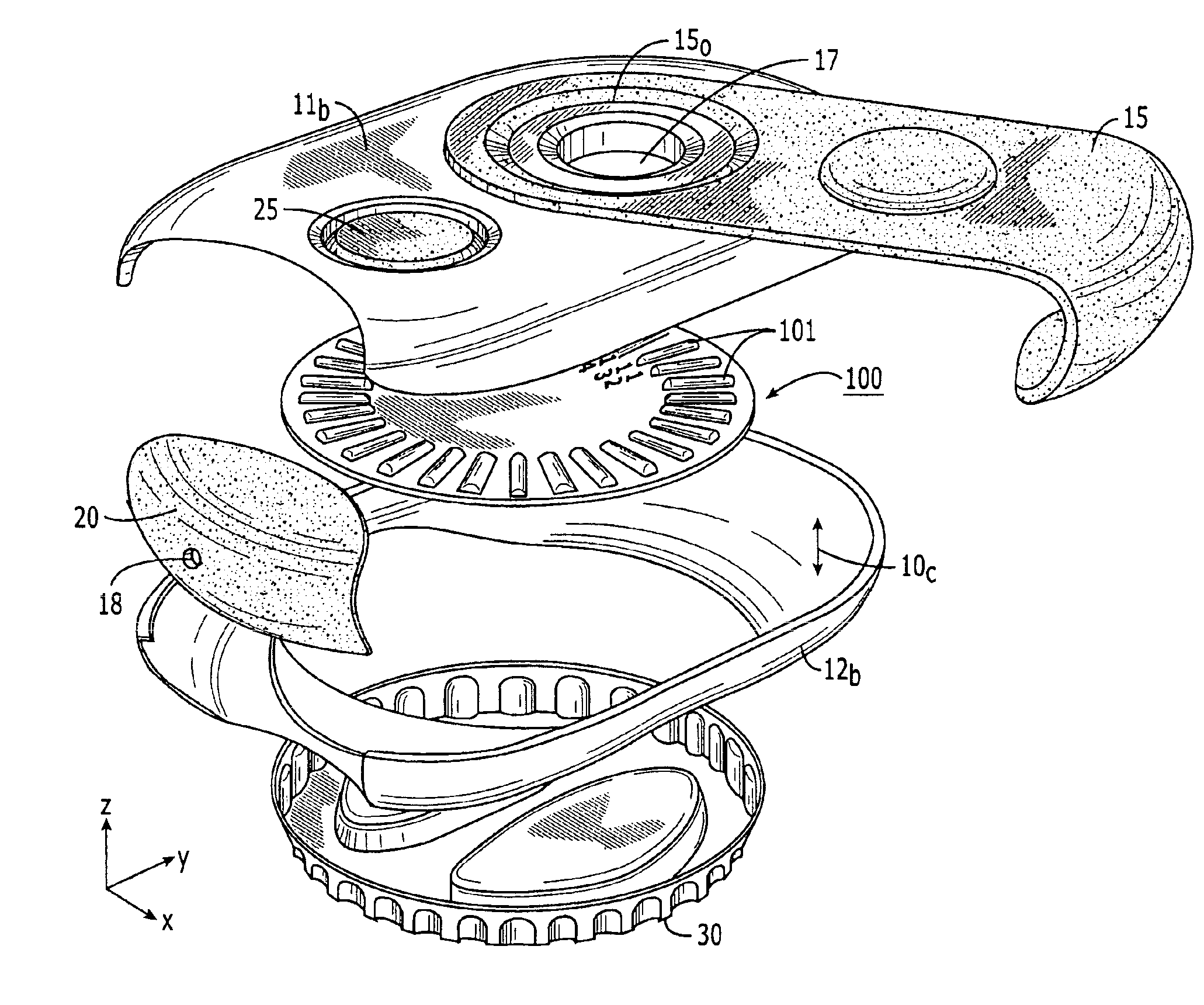
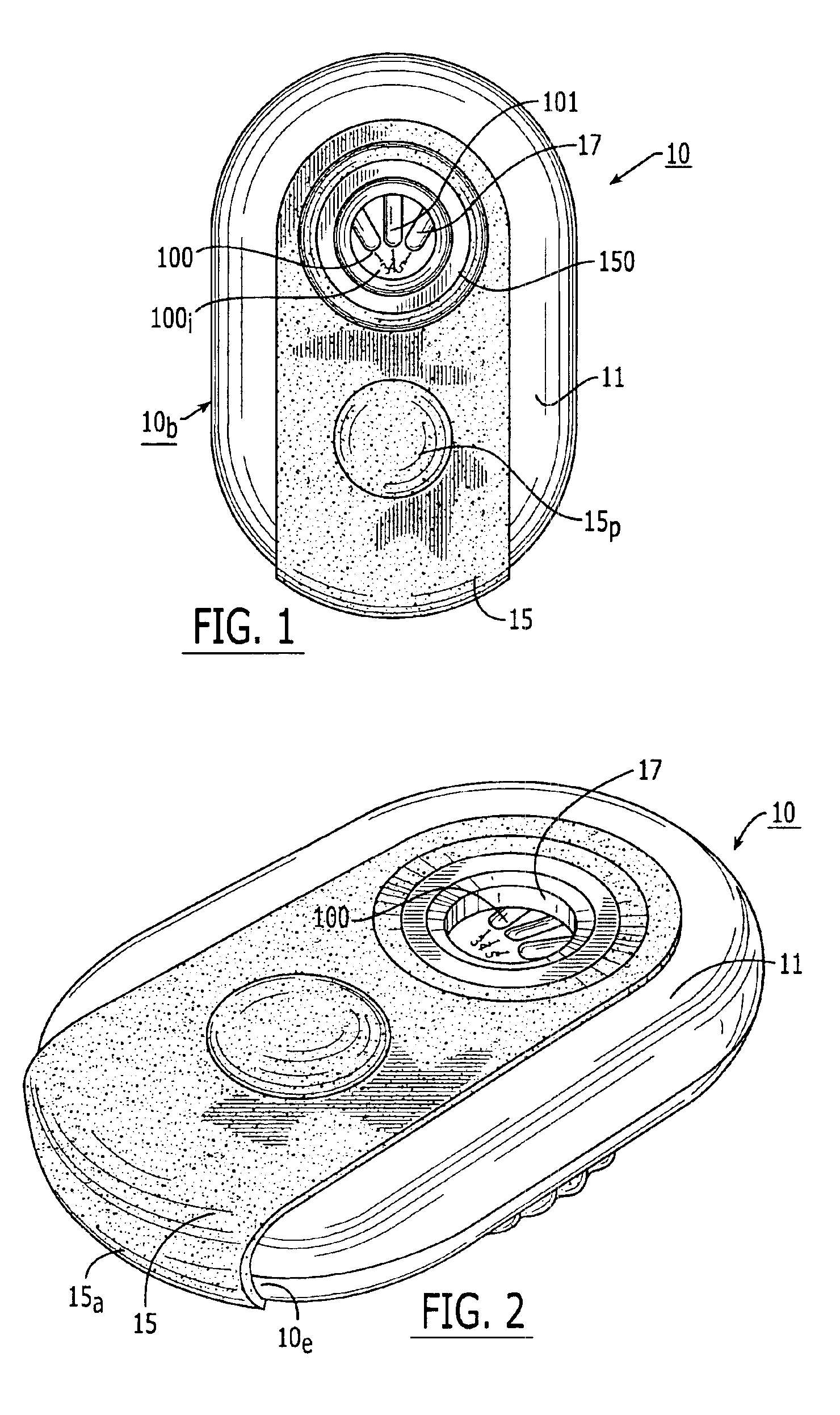
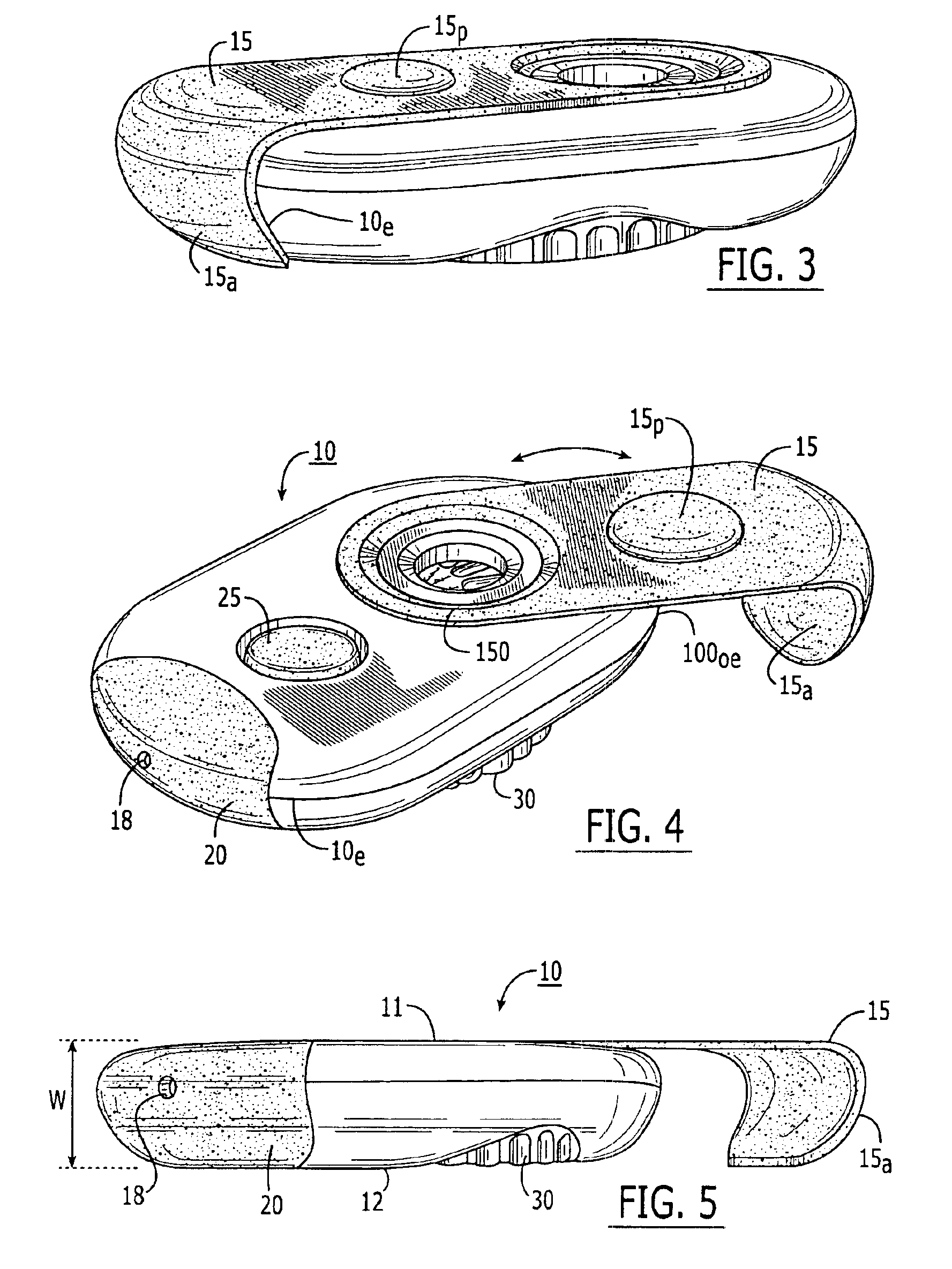
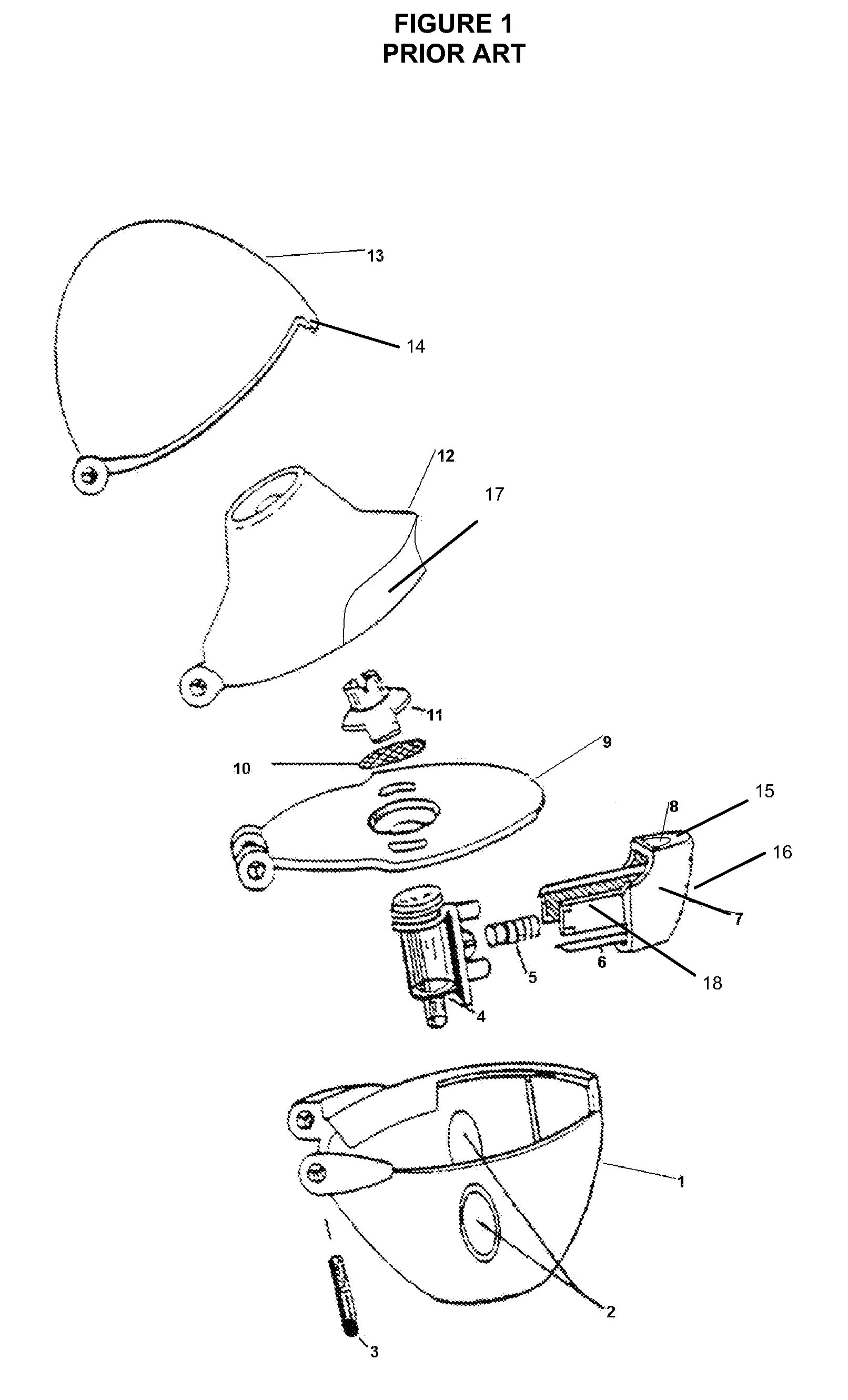
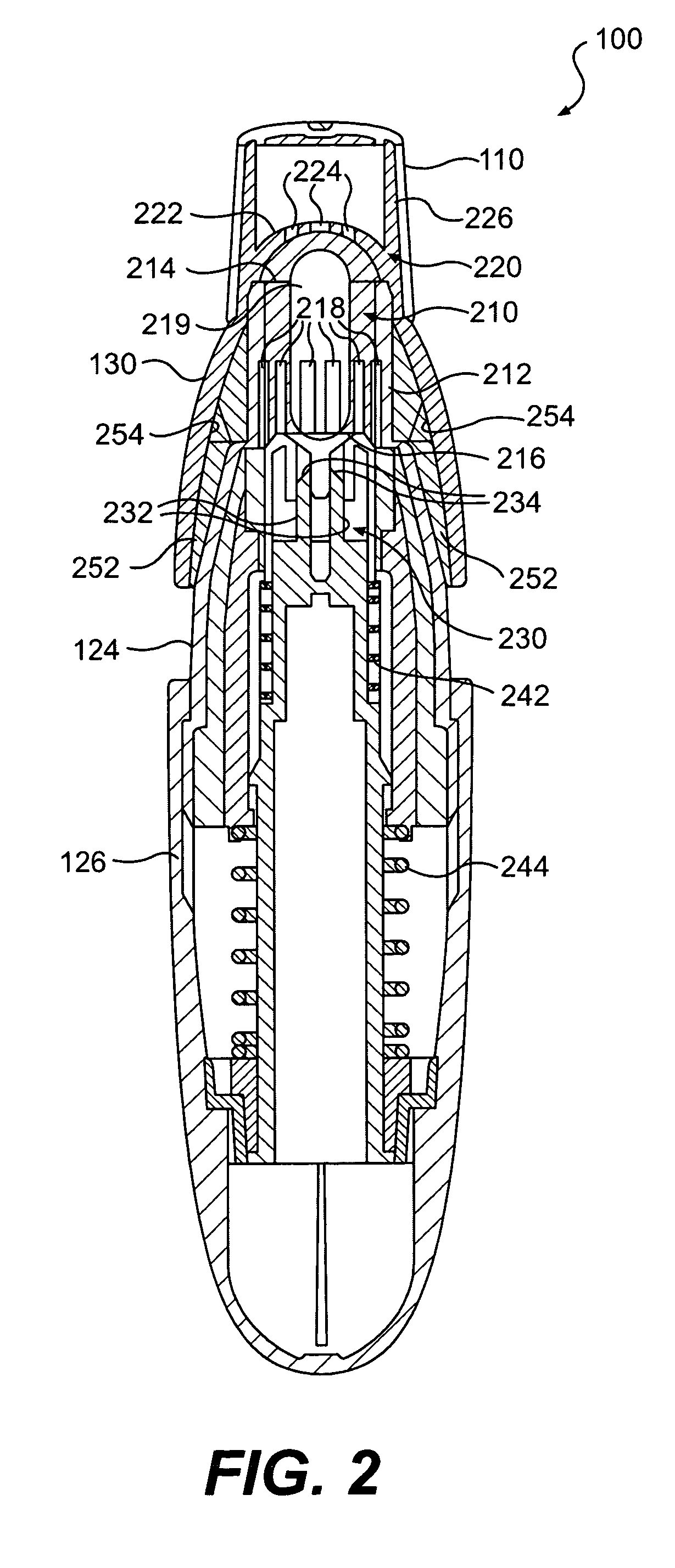
Dry powder inhalers, related blister devices, and associated methods of dispensing dry powder substances and fabricating blister packages
InactiveUS6889690B2Easy to optimizeLimit amount of resistanceSmall article dispensingLiquid surface applicatorsPowder InhalerInhalation
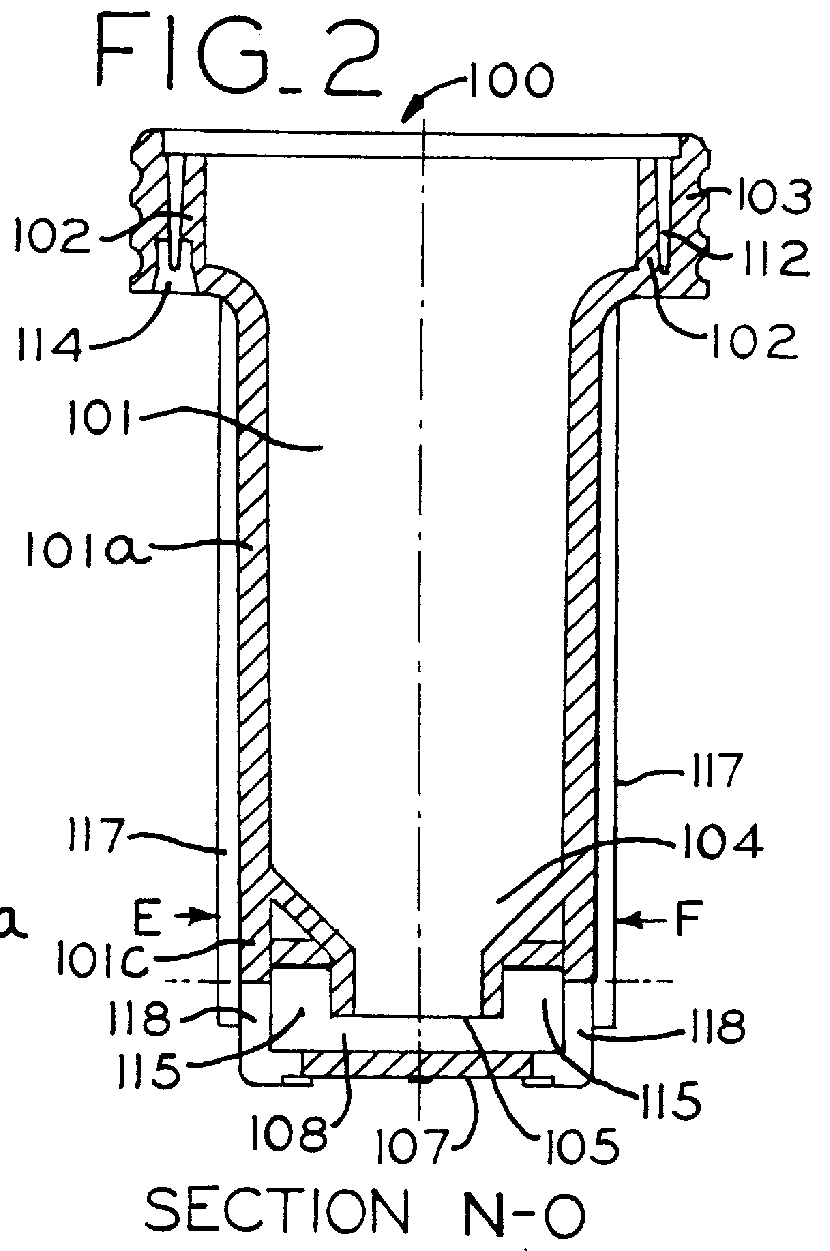
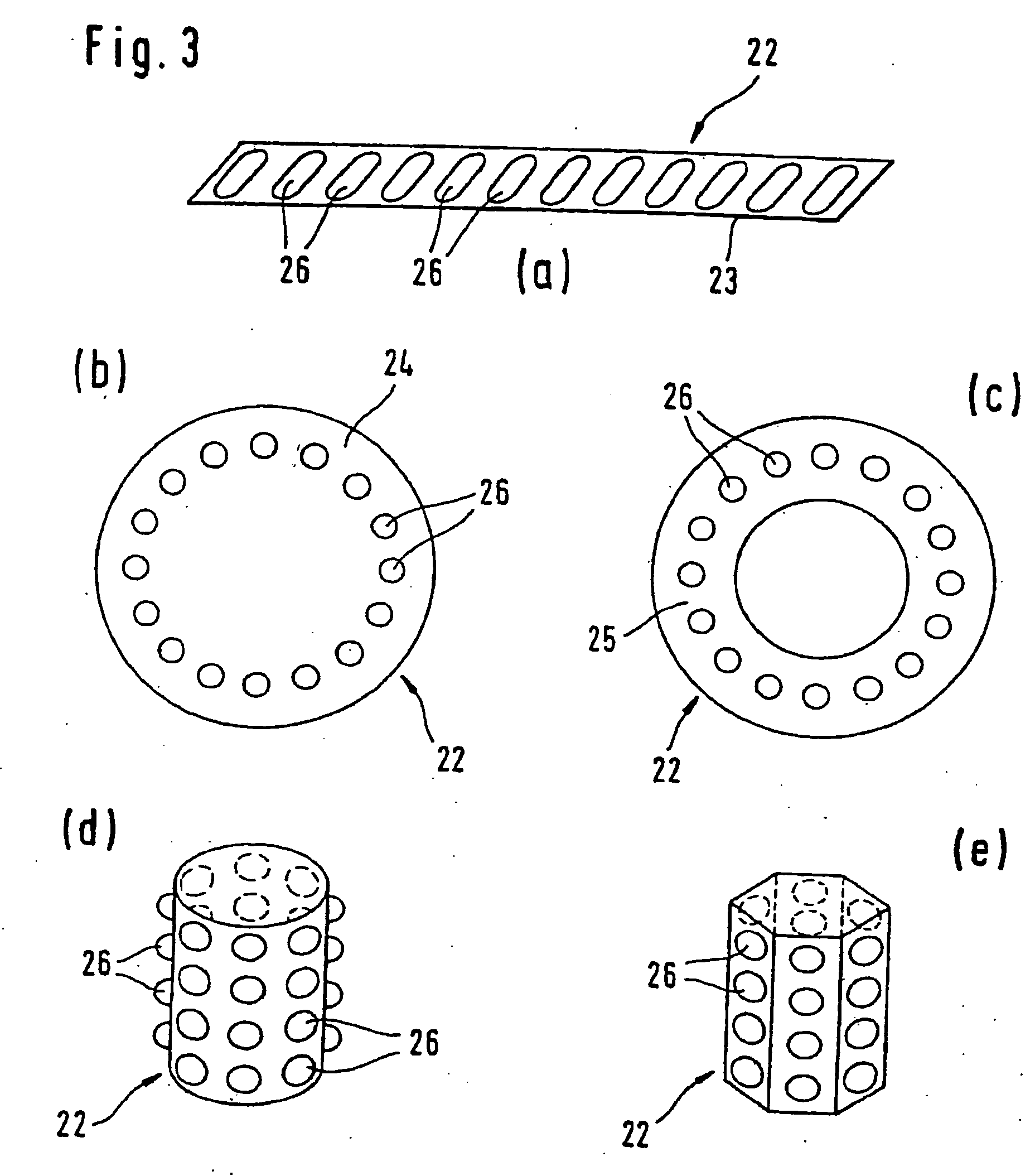
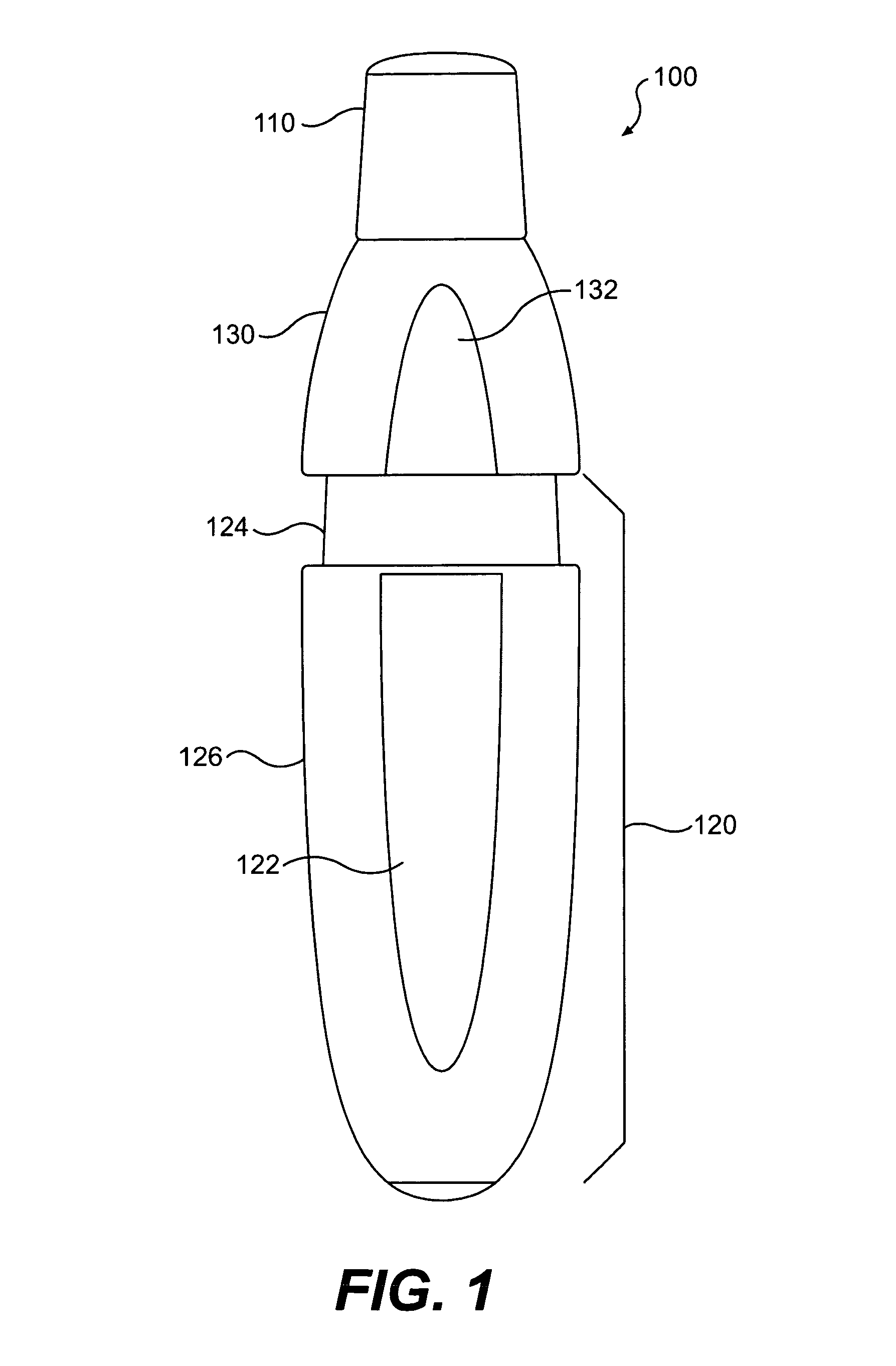
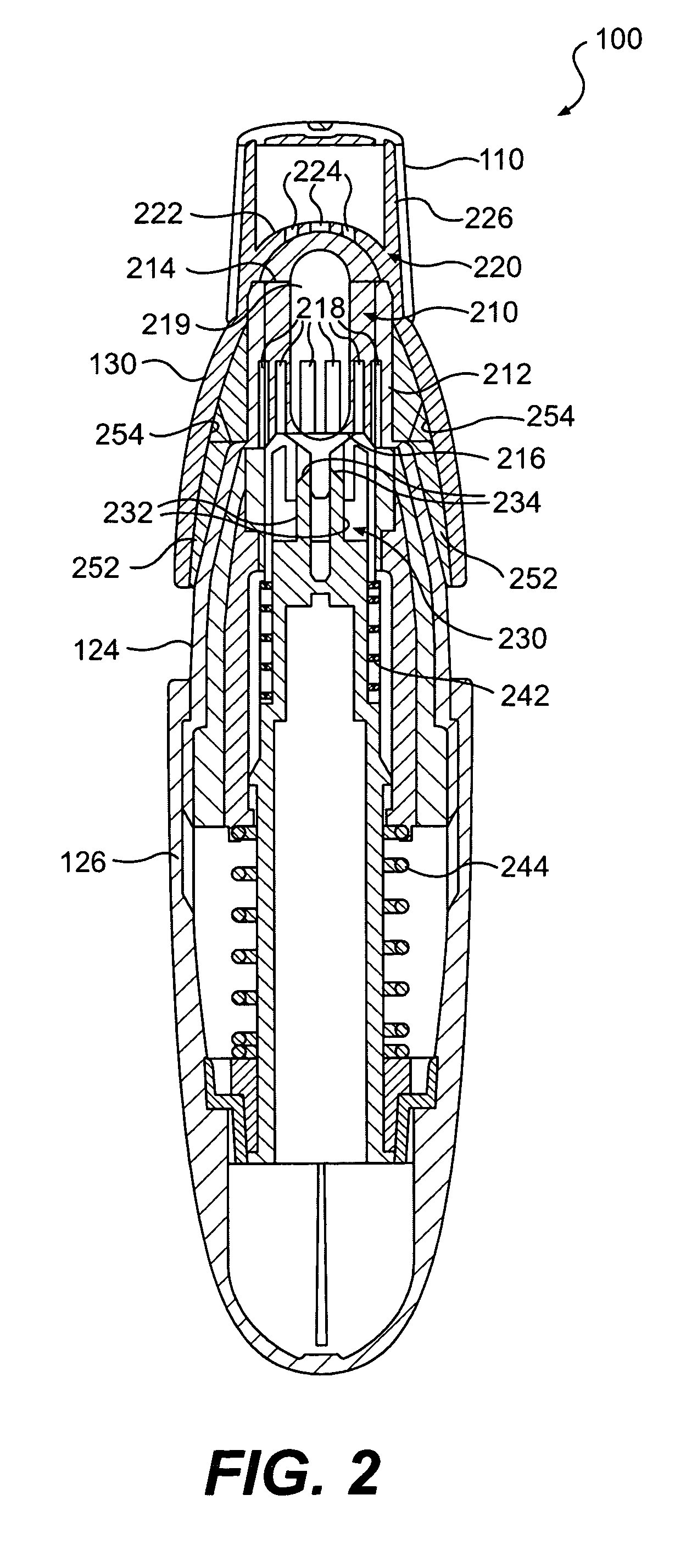
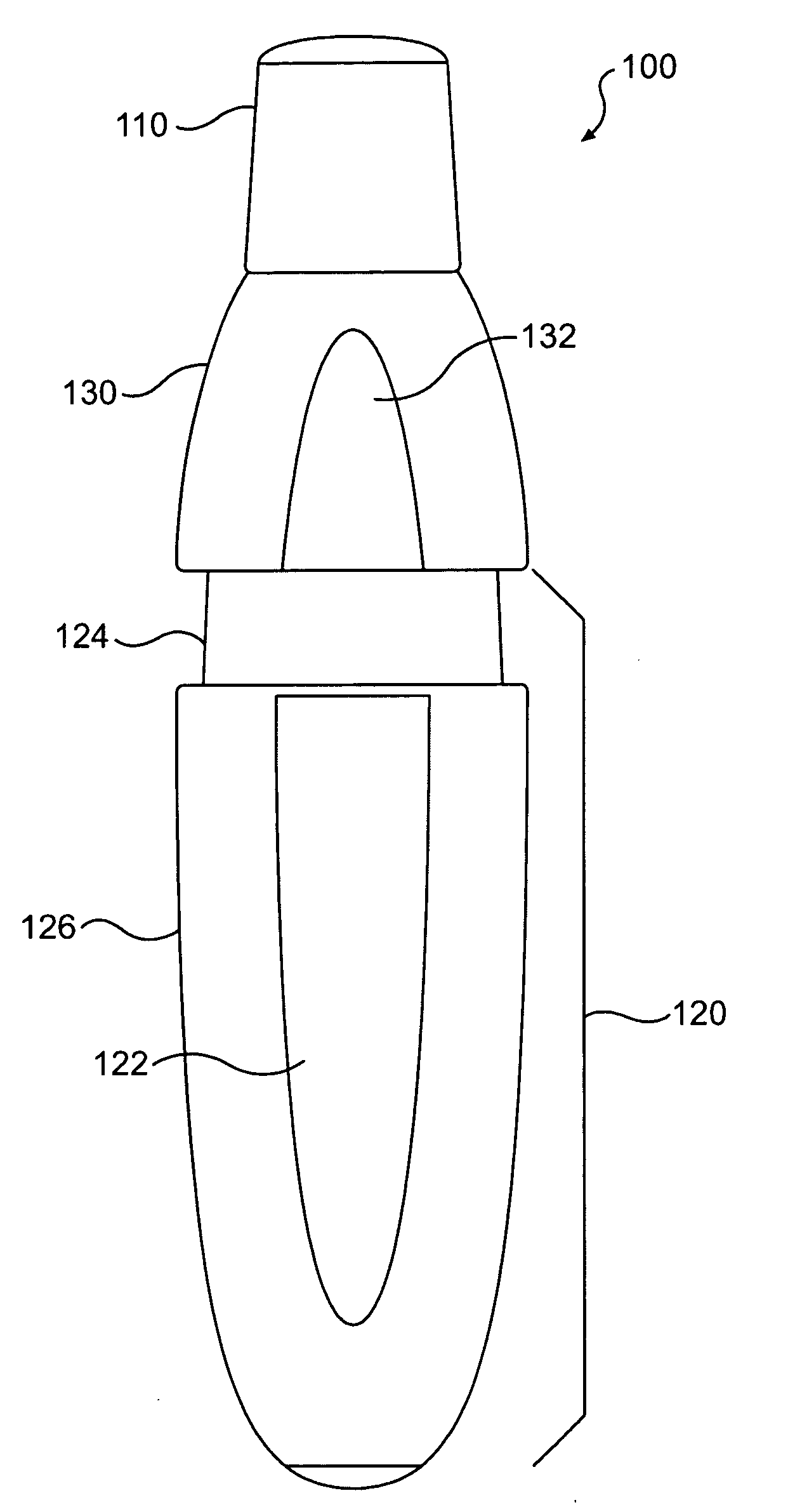
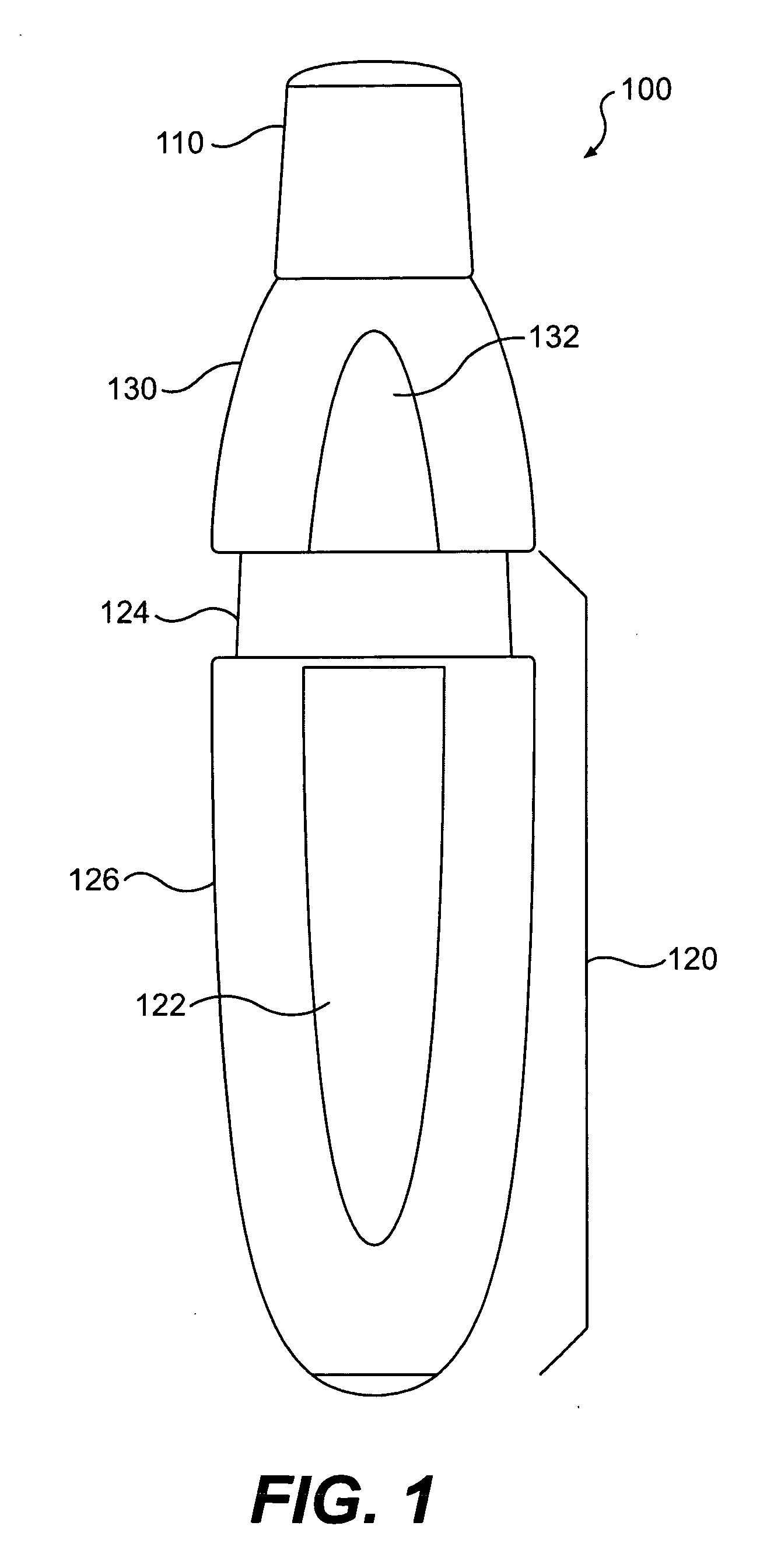
The present invention includes dry powder inhalers and associated multi-dose dry powder packages for holding inhalant formulated dry powder substances and associated fabrication and dispensing methods. The multi-dose package can include a platform body comprising at least one thin piezoelectric polymer material layer defining at least a portion of a plurality of spatially separated discrete elongate dry powder channels having an associated length, width and height; and a metallic material attached to selected portions of the piezoelectric polymer material including each of the regions corresponding to the elongate dry powder channels to, in operation, define active energy releasing vibratory channels. In operation, the elongate channels can be selectively individually activated to vibrate upon exposure to an electrical input.The dry powder inhaler includes an elongate body having opposing first and second outer primary surfaces with a cavity therebetween and having opposing top and bottom end portions and a multi-dose sealed blister package holding a plurality of discrete meted doses of a dry powder inhalable product located in the cavity of the elongate body. The inhaler also includes an inhalation port formed in the bottom end portion of the elongate body, the inhalation port configured to be in fluid communication with at least one of the discrete meted doses during use and a cover member that is pivotably attached to the elongate body so that it remains attached to the body during normal operational periods of use and moves to a first closed position to overlie the inhalation port at the bottom end portion of the body during periods of non-use and moves to a second open position away from the inhalation port during periods of use to allow a user to access the inhalation port.
Owner:ORIEL THERAPEUTICS INC
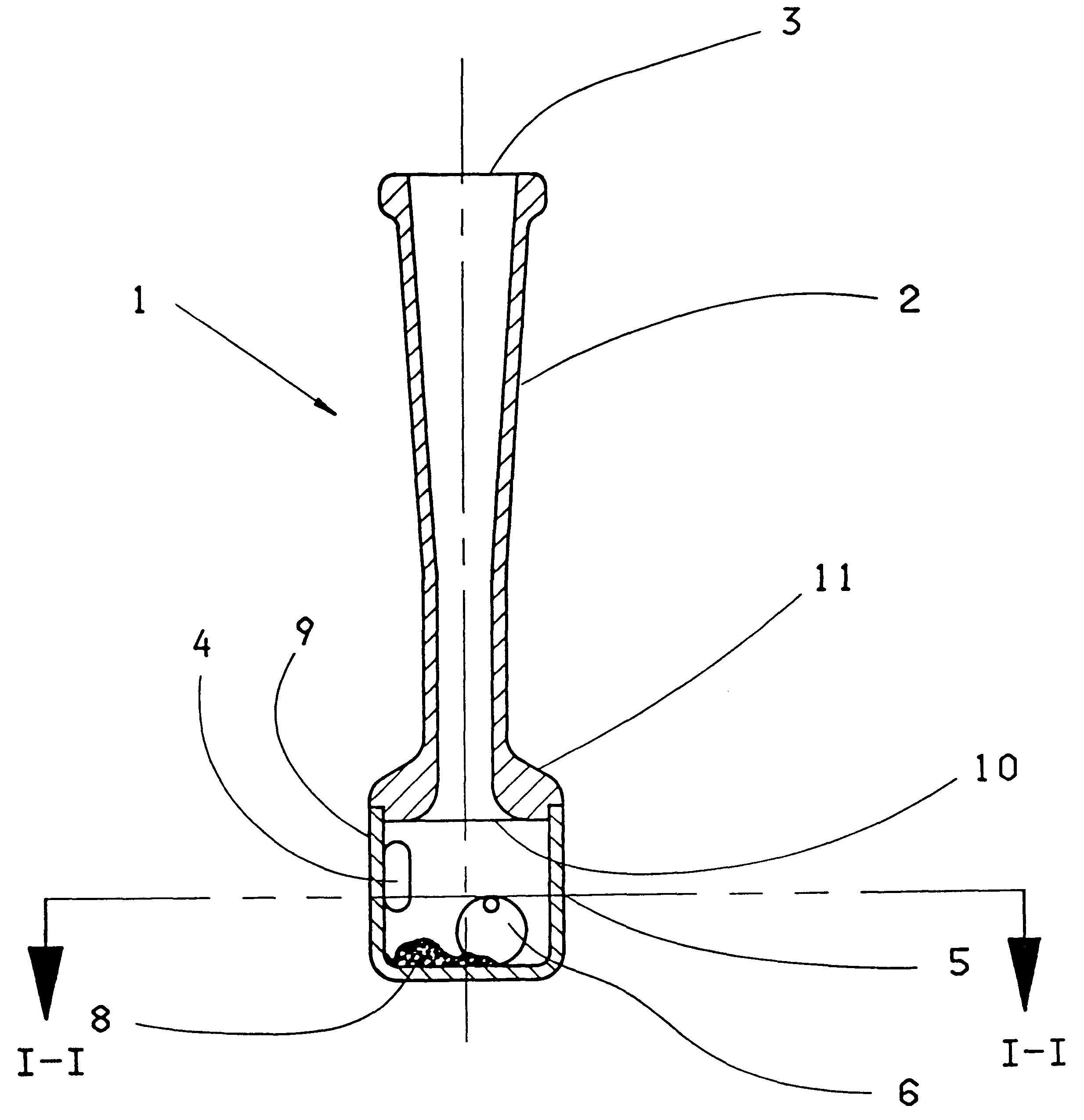
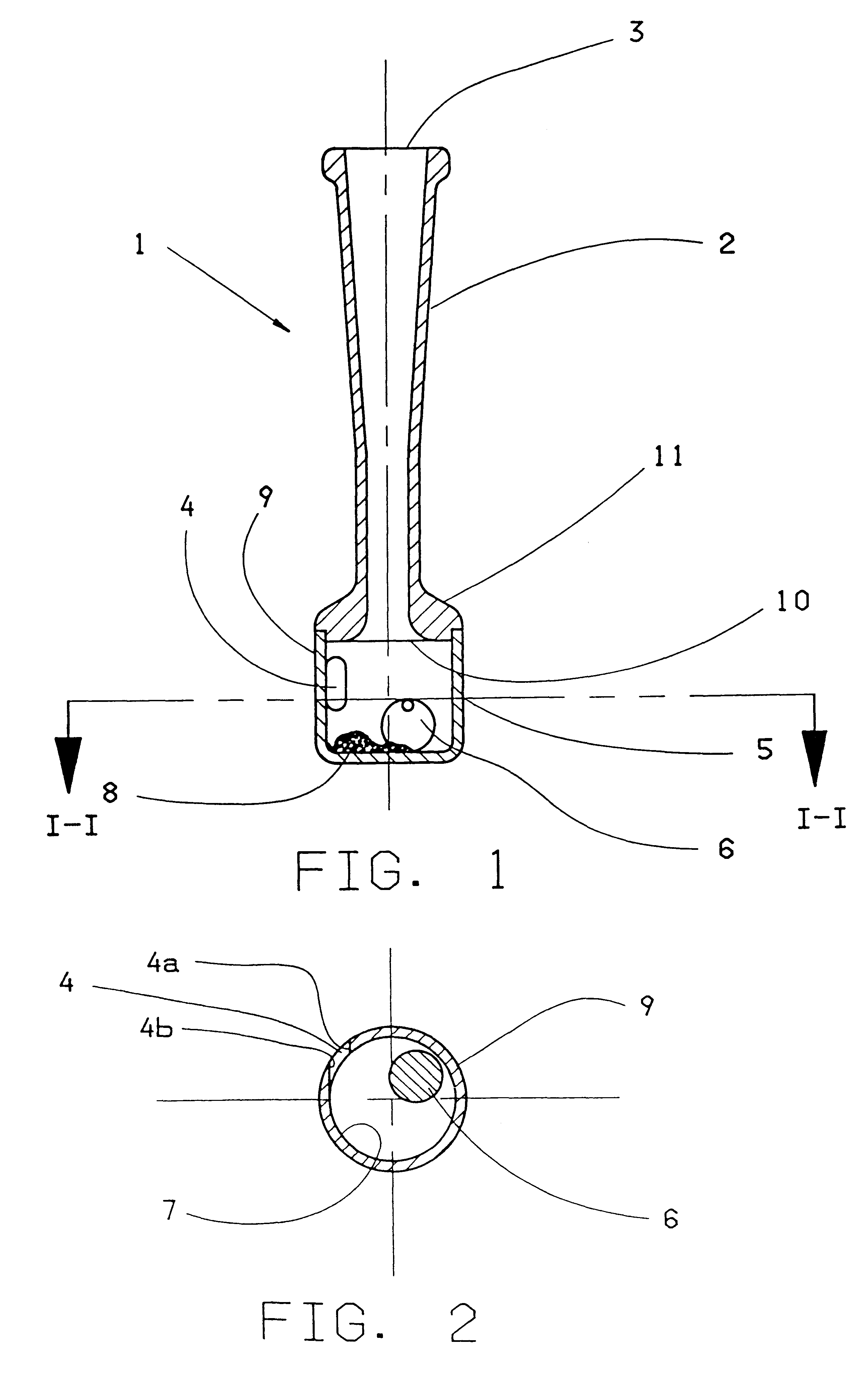
Powder inhaler
InactiveUS6230707B1Good dispersion propertiesEasily reaches lungRespiratorsLiquid surface applicatorsPowder InhalerEngineering
The invention concerns an inhaler device (1) having a hollow tubular member (2, 102, 402) connected to a chamber (5, 105, 405). The tubular member has a first opening (3, 103, 203) at one end through which air can be sucked and the chamber (5, 105, 405) has a hole (4, 104, 204) therein for entry of air. When air is sucked through the first opening, air enters the chamber through the hole swirls and moves towards the first opening (3, 103, 203). To maintain or increase the swirling effect of the air a single restriction (10, 110) is arranged between the opening and the hole (4, 104, 204). A powdered substance (8) within the chamber is picked up by the swirling air within the chamber and is uniformly and finely divided by the swirling effect of the air. The effect can be enhanced by adding a movable element (6) such as a ball inside the chamber (5) and / or by providing a central core element (112, 412) inside the chamber.
Owner:AVENTIS PHARMA LTD
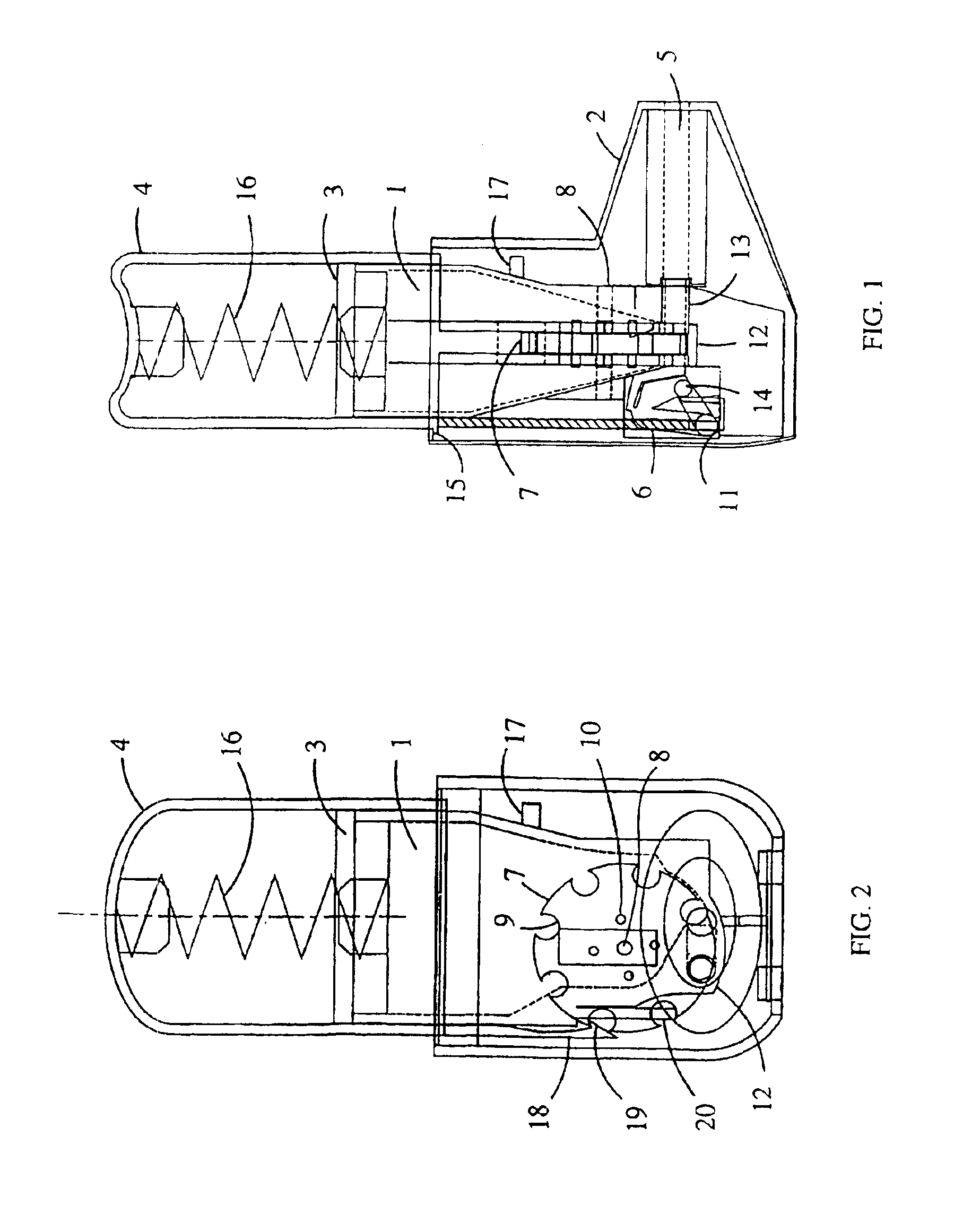
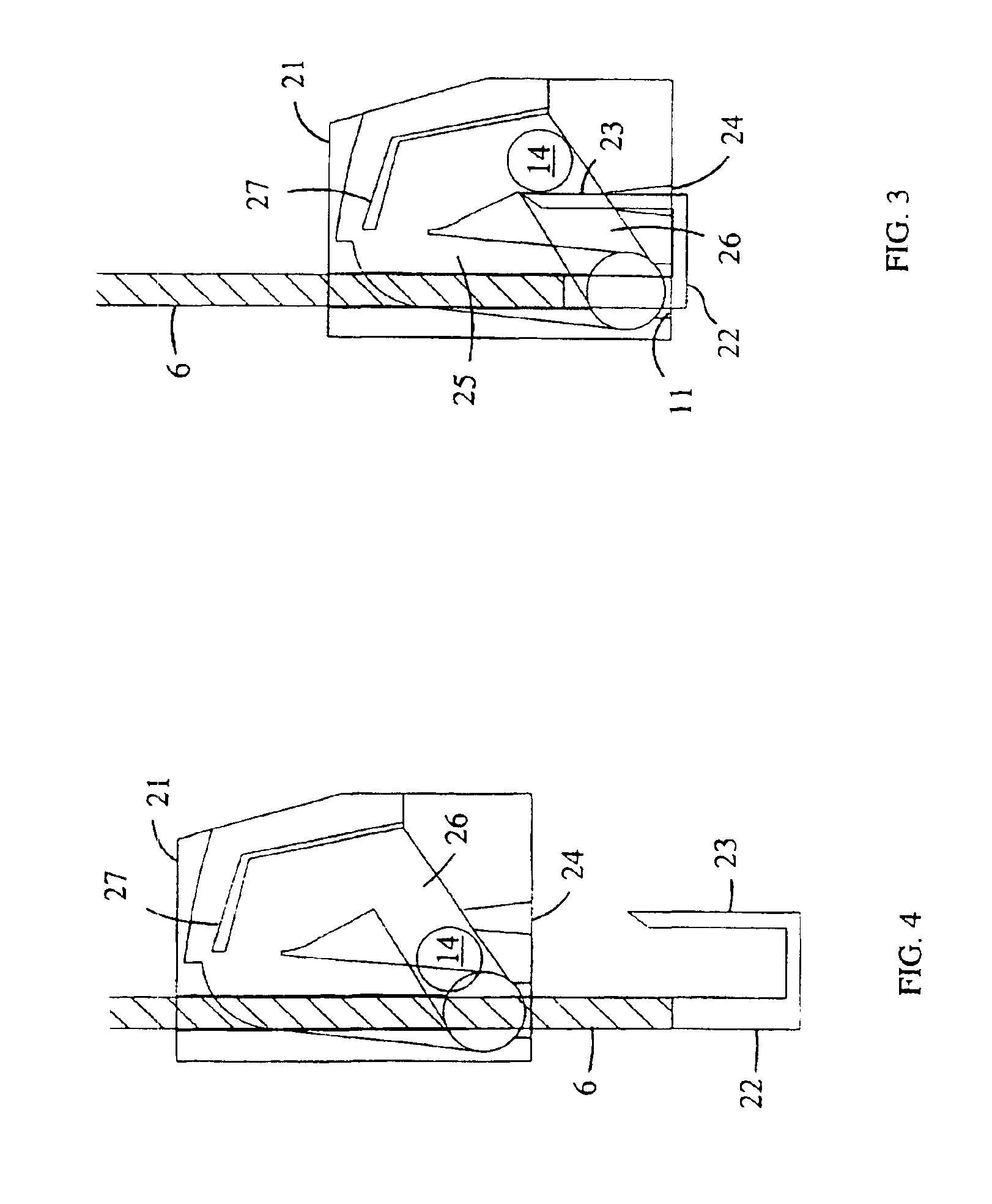
Dry powder inhaler devices, multi-dose dry powder drug packages, control systems, and associated methods
InactiveUS6971383B2Evenly dispersedFacilitate dispersion and releaseRespiratorsLiquid surface applicatorsPowder InhalerPhysical medicine and rehabilitation
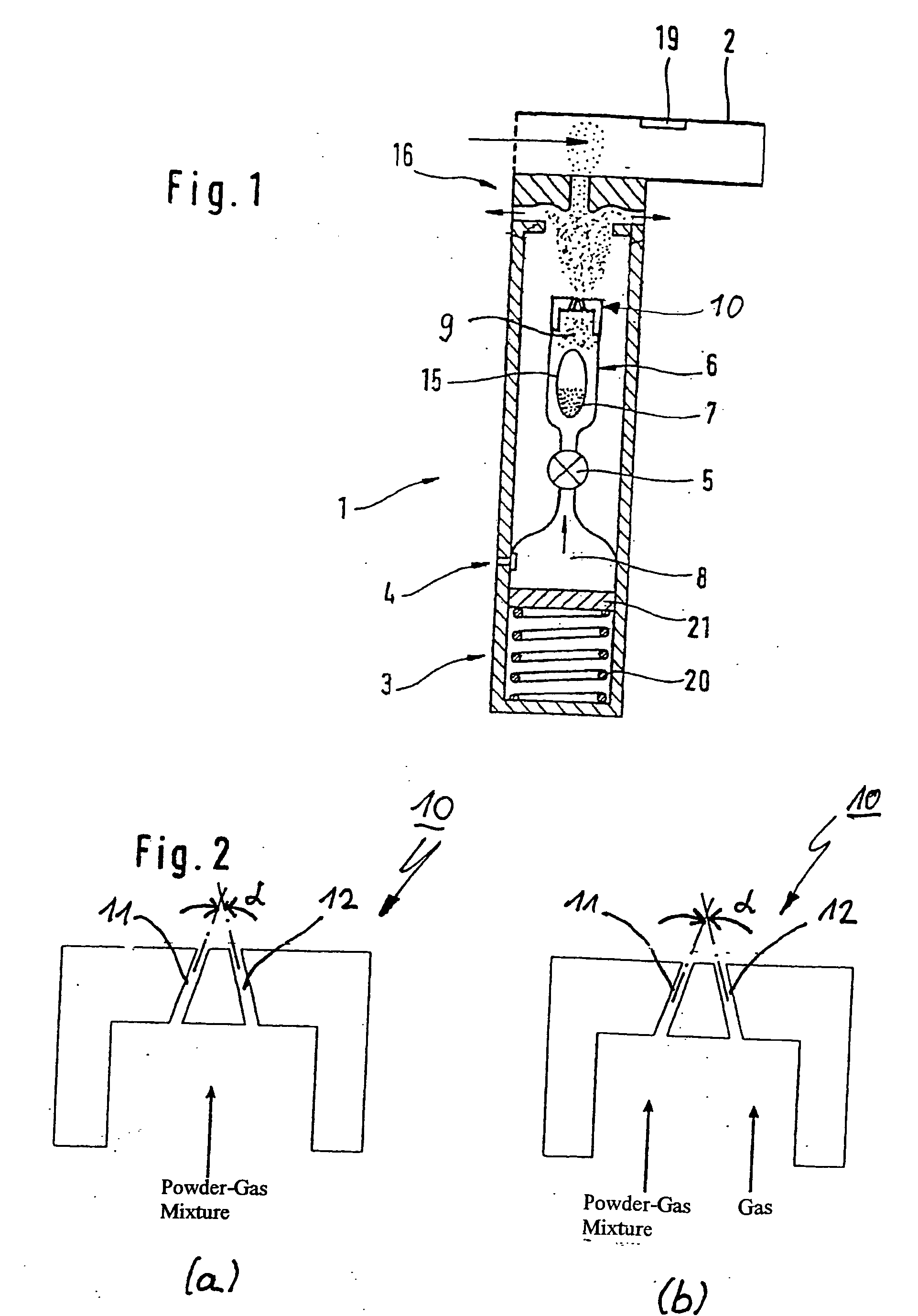
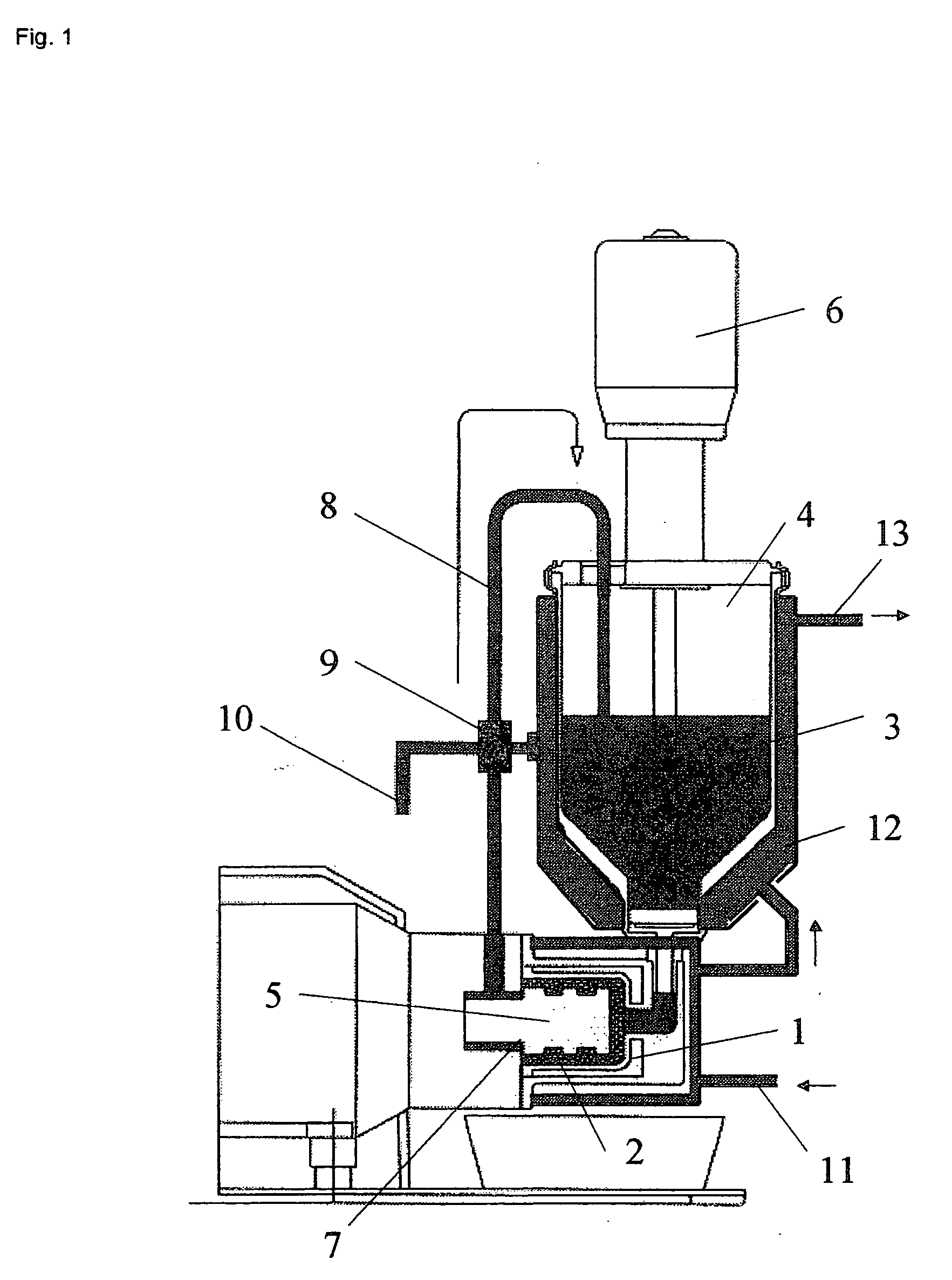
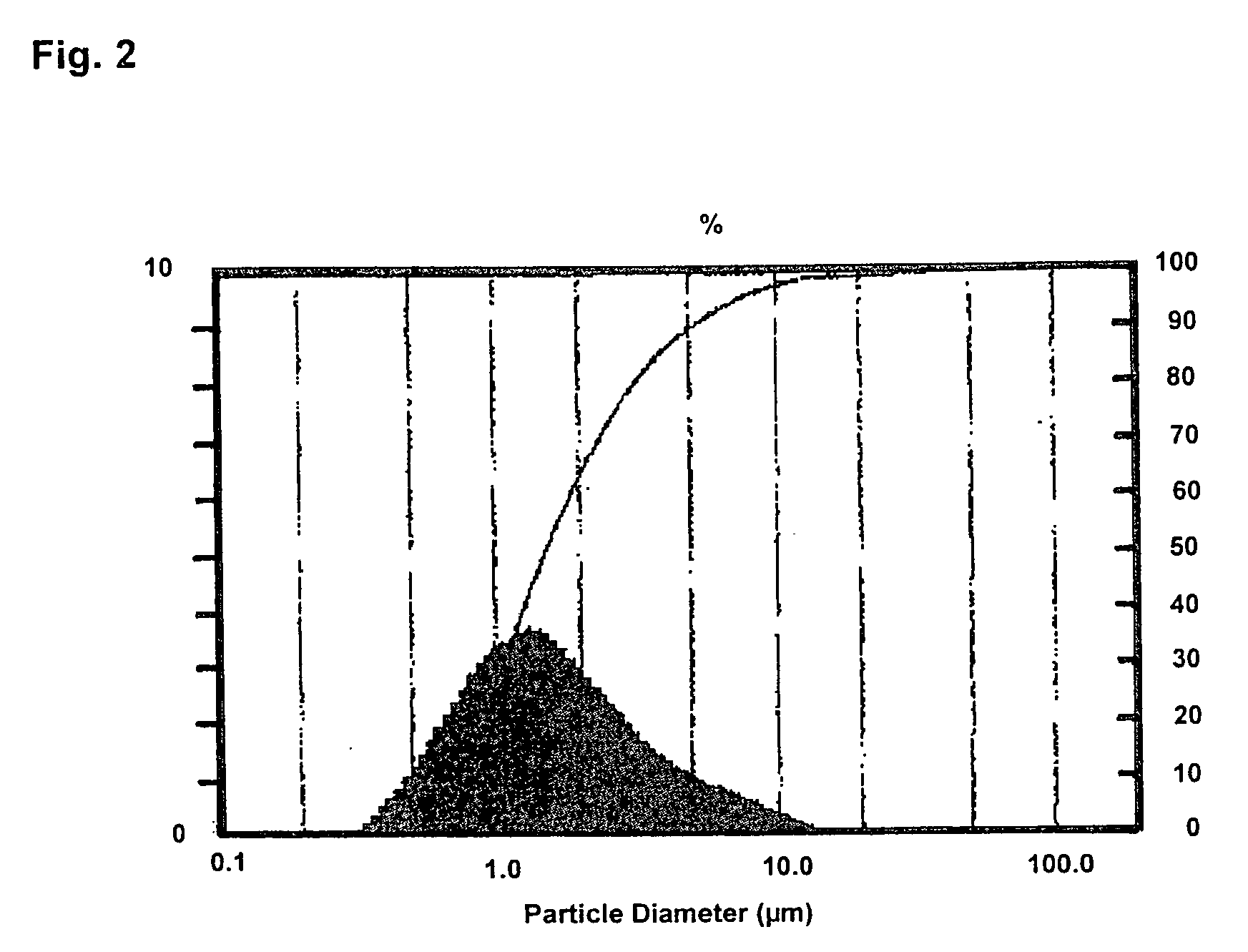
Dry powder inhalers (FIG. 1) with integrated active energy patient assist dispersal systems are configured with control systems which provide adjustable energy output responsive to the user's inspiratory capabilities and / or the flowability of the dry powder being administered. The multi-dose dry drug package (FIG. 2) a piezoelectric polymer substrate which flexes to deform and provide mechanical oscillation in a selected region of the package corresponding to the dry powder drug which is dispersed during inhalation by a user. Control system (FIG. 12) employs fuzzy logic to relate in response to a user's inspiratory effort.
Owner:ORIEL THERAPEUTICS INC
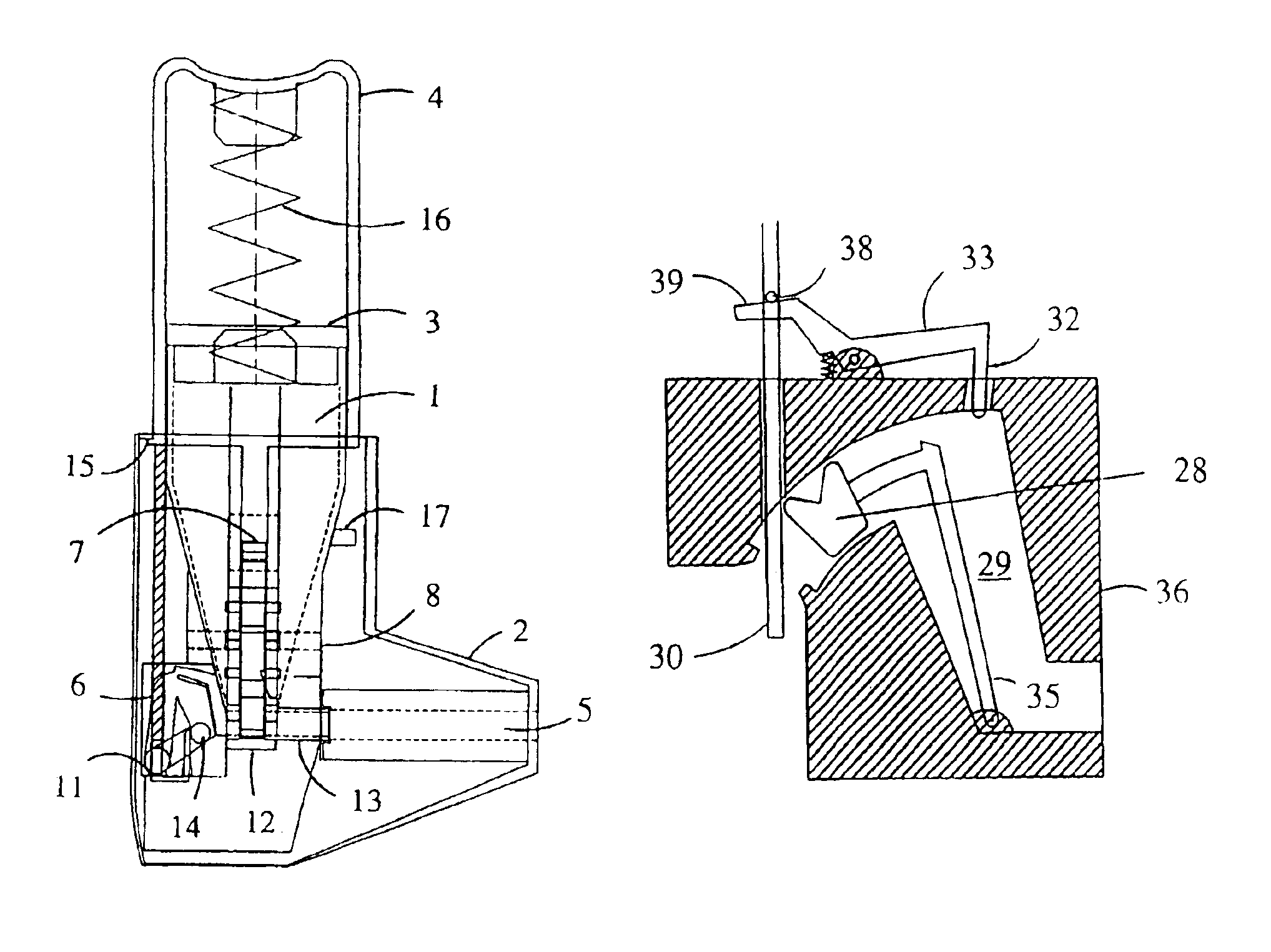
Inhaler for powdered medicaments
InactiveUS6071498ASimple and safe operationSimply and economically manufacturedPowder deliveryAerosol deliveryPowder InhalerInhaled drug
Pharmaceutical powder cartridge for powder inhalers for receiving a medicament depot for a large number of pharmaceutical powder doses, having an integrated metering device which comprises at least one metering cavity for receiving a predetermined quantity of a pharmaceutical powder, the integrated metering device being capable of being moved at least out of a filling position into an emptying position approximately transversely with respect to the flow direction of the pharmaceutical powder, and an inhaler for powdered medicaments, in which inhaler the medicament can be received by a patient by means of an air stream and which has a receptacle for such a pharmaceutical powder cartridge.
Owner:ASTRAZENECA AB
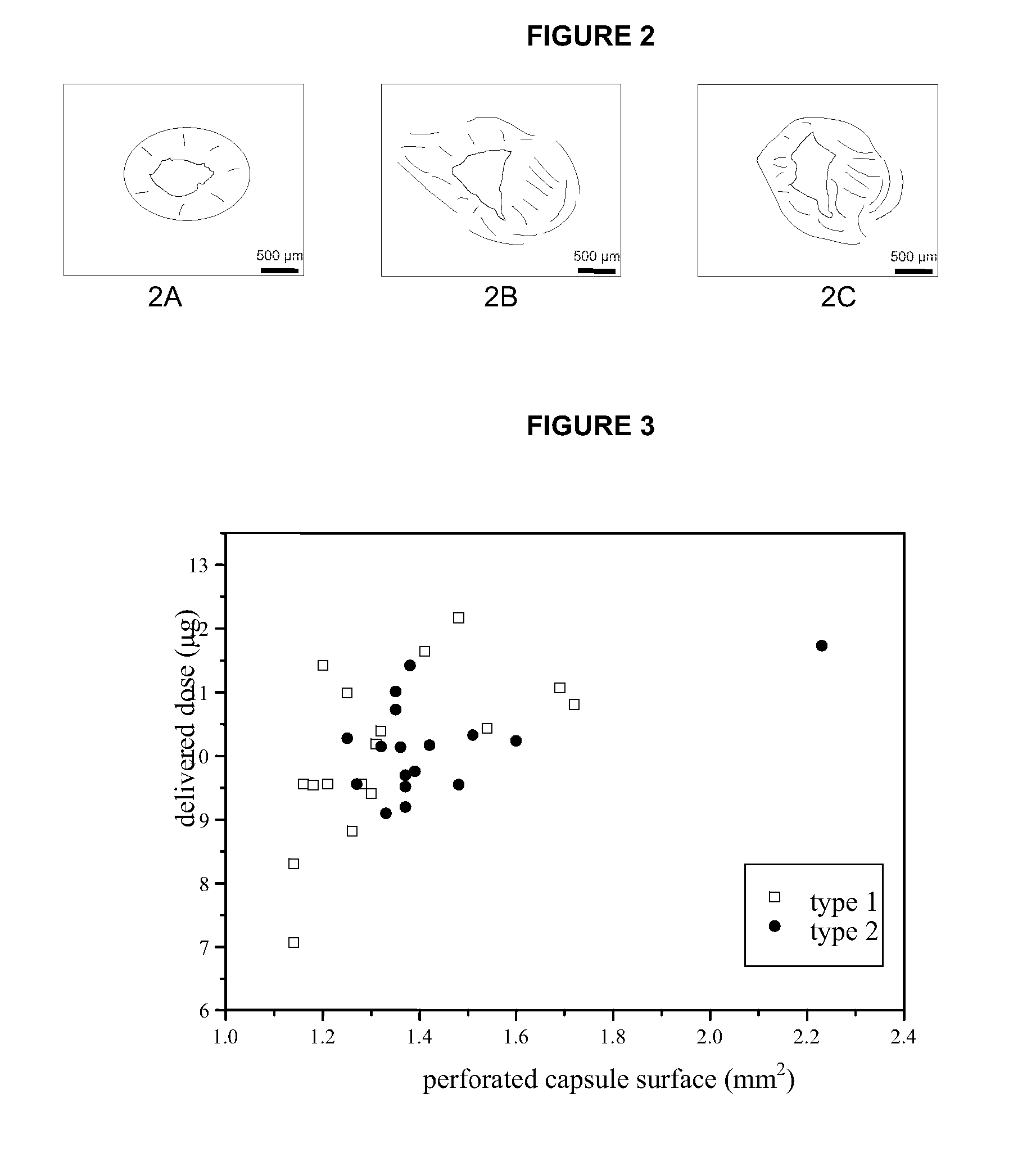
Pharmaceutical formulations for dry powder inhalers in the form of hard-pellets
InactiveUS20030180227A1Reduce intensityEfficient deliveryPowder deliveryDispersion deliveryPrillInhalation
The invention provides a formulation to be administered as dry powder for inhalation suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering of pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation freely flowable, which can be produced in a simple way, physically and chemically stable and able of delivering either accurate doses and high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:CHIESI FARM SPA
Dry powder inhaler devices, multi-dose dry powder drug packages, control systems, and associated methods
InactiveUS20040123864A1Evenly dispersedFacilitate dispersion and releaseRespiratorsLiquid surface applicatorsElectricityPowder Inhaler
Dry powder inhalers (FIG. 1) with integrated active energy patient assist dispersal systems are configured with control systems which provide adjustable energy output responsive to the user's inspiratory capabilities and / or the flowability of the dry powder being administered. The multi-dose dry drug package (FIG. 2) a piezoelectric polymer substrate which flexes to deform and provide mechanical oscillation in a selected region of the package corresponding to the dry powder drug which is dispersed during inhalation by a user. Control system (FIG. 12) employs fuzzy logic to relate in response to a user's inspiratory effort.
Owner:ORIEL THERAPEUTICS INC
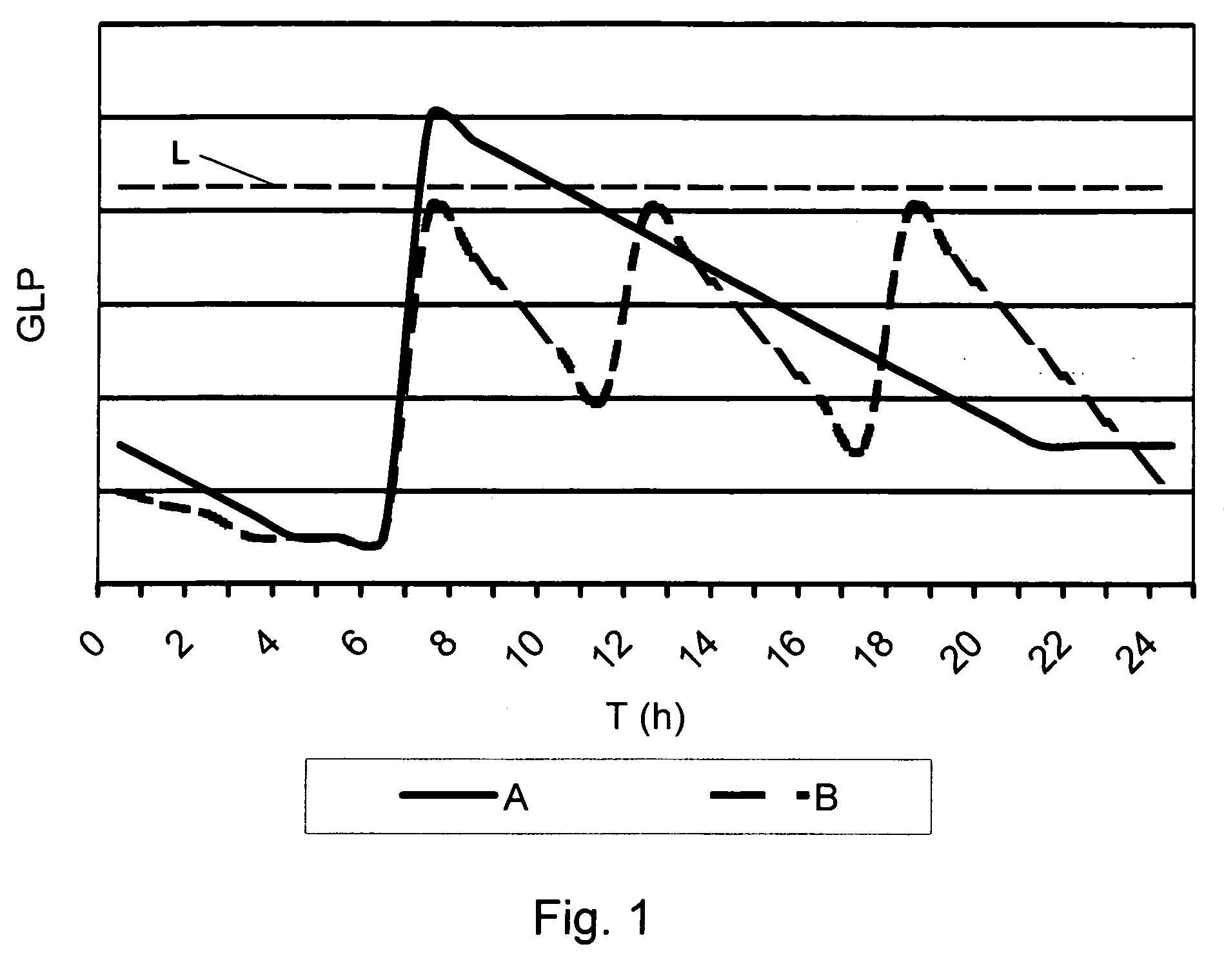
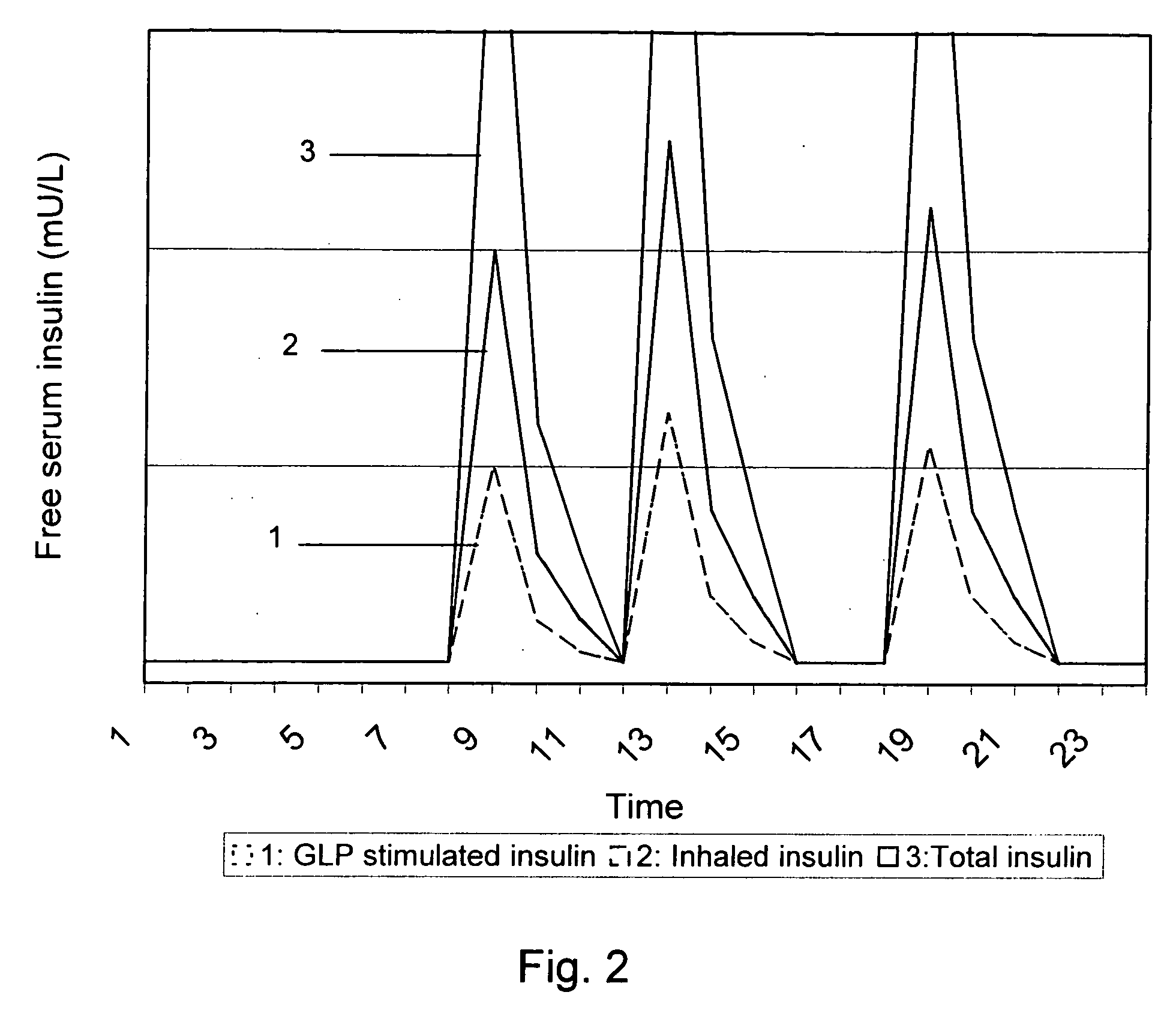
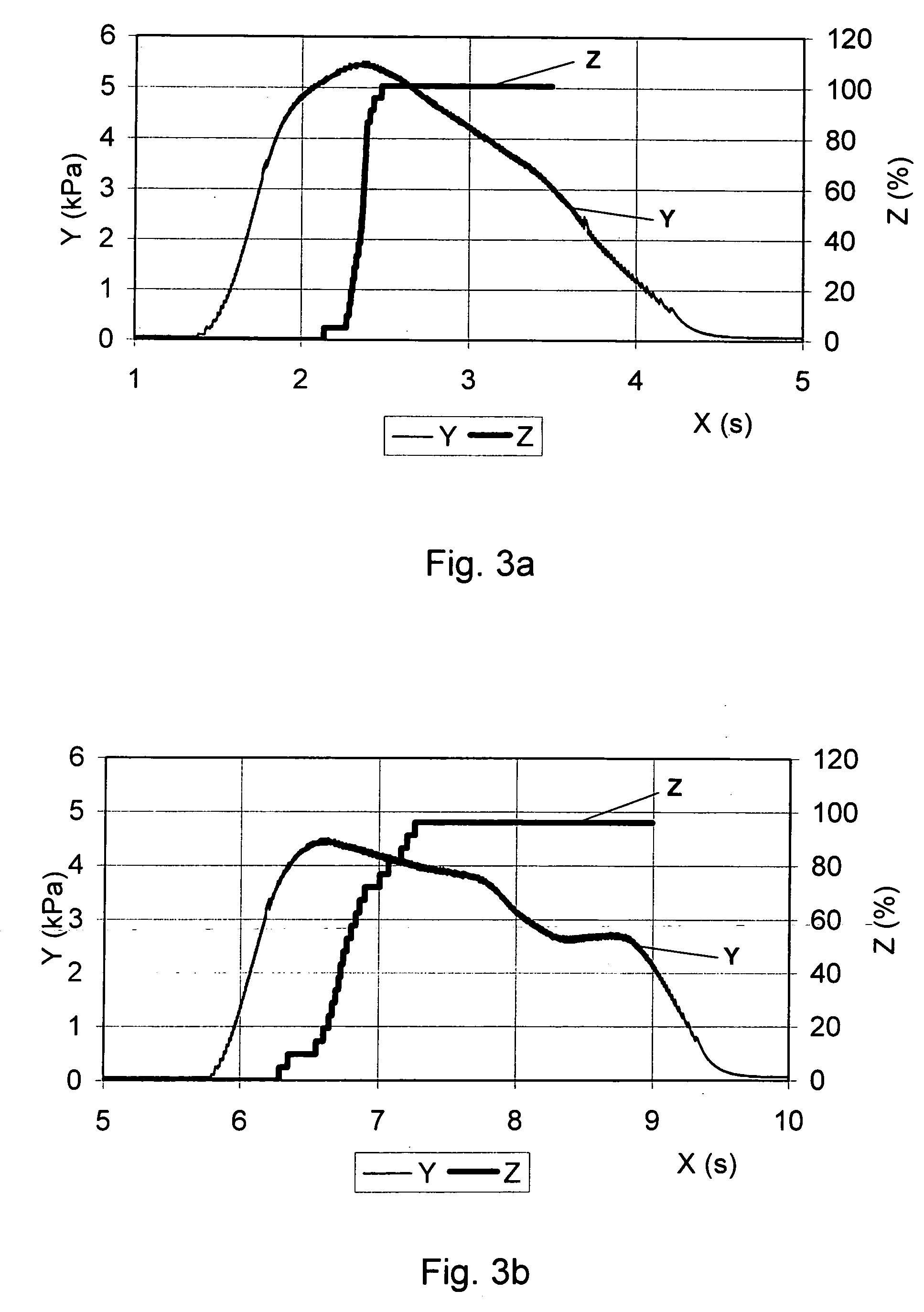
Medical product for inhalation containing glucagon-like peptide-1 (GLP-1)
InactiveUS20060120969A1Reduce deliveryAccurate dosePowder deliverySpray deliveryPharmaceutical preservativesPulmonary inhalation
A medical product containing an accurately metered dose of a GLP-1 medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is preferably adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Two-part capsule to accept pharmaceutical preparations for powder inhalers
InactiveUS20010008637A1Releasing their contentPowder deliveryMedical devicesPowder InhalerWater insoluble
The present invention relates to capsules for holding pharmaceutical preparations for powder inhalers with increased drug safety and capsules for pharmaceutical preparations for powder inhalers with improved adaptation to their use in powder inhalers. The capsules consist of water-insoluble hydrophobic synthetic materials which do not significantly affect the pharmaceutical quality of the contents themselves, but which improve the usability of the filled capsules with regard to their function, their longevity and / or the geographic location of their use, and are advantageous at various stages from manufacture up to utilisation.
Owner:HOCHRAINER DIETER +1
Two-part capsule to accept pharmaceutical preparations for powder inhalers
InactiveUS20040131668A1Releasing their contentPowder deliveryMedical devicesPowder InhalerWater insoluble
The present invention relates to capsules for holding pharmaceutical preparations for powder inhalers with increased drug safety and capsules for pharmaceutical preparations for powder inhalers with improved adaptation to their use in powder inhalers. The capsules consist of water-insoluble hydrophobic synthetic materials which do not significantly affect the pharmaceutical quality of the contents themselves, but which improve the usability of the filled capsules with regard to their function, their longevity and / or the geographic location of their use, and are advantageous at various stages from manufacture up to utilisation.
Owner:BOEHRINGER INGELHEIM PHARM KG
Pre-metered dry powder inhaler for moisture-sensitive medicaments
Owner:BOEHRINGER INGELHEIM INT GMBH
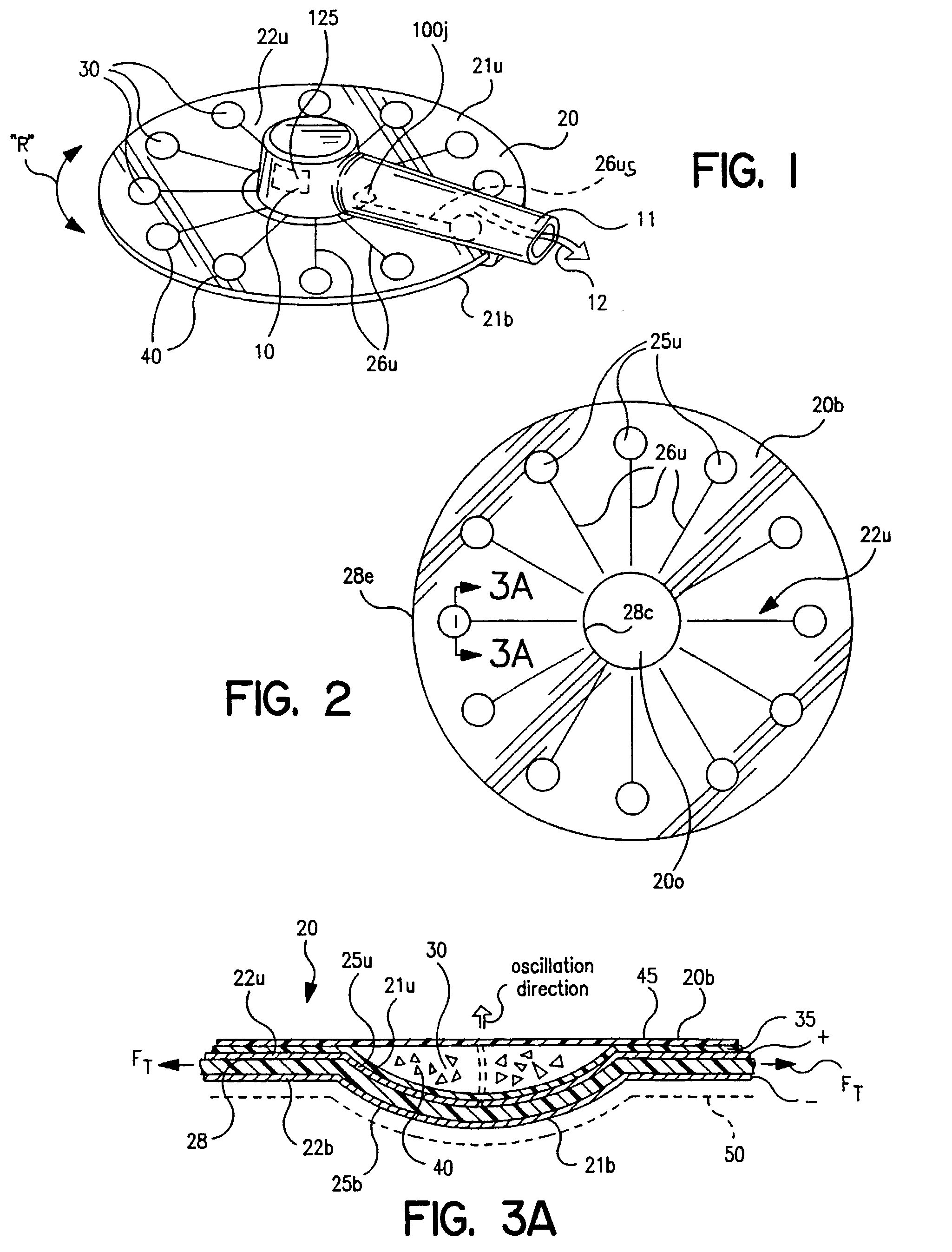
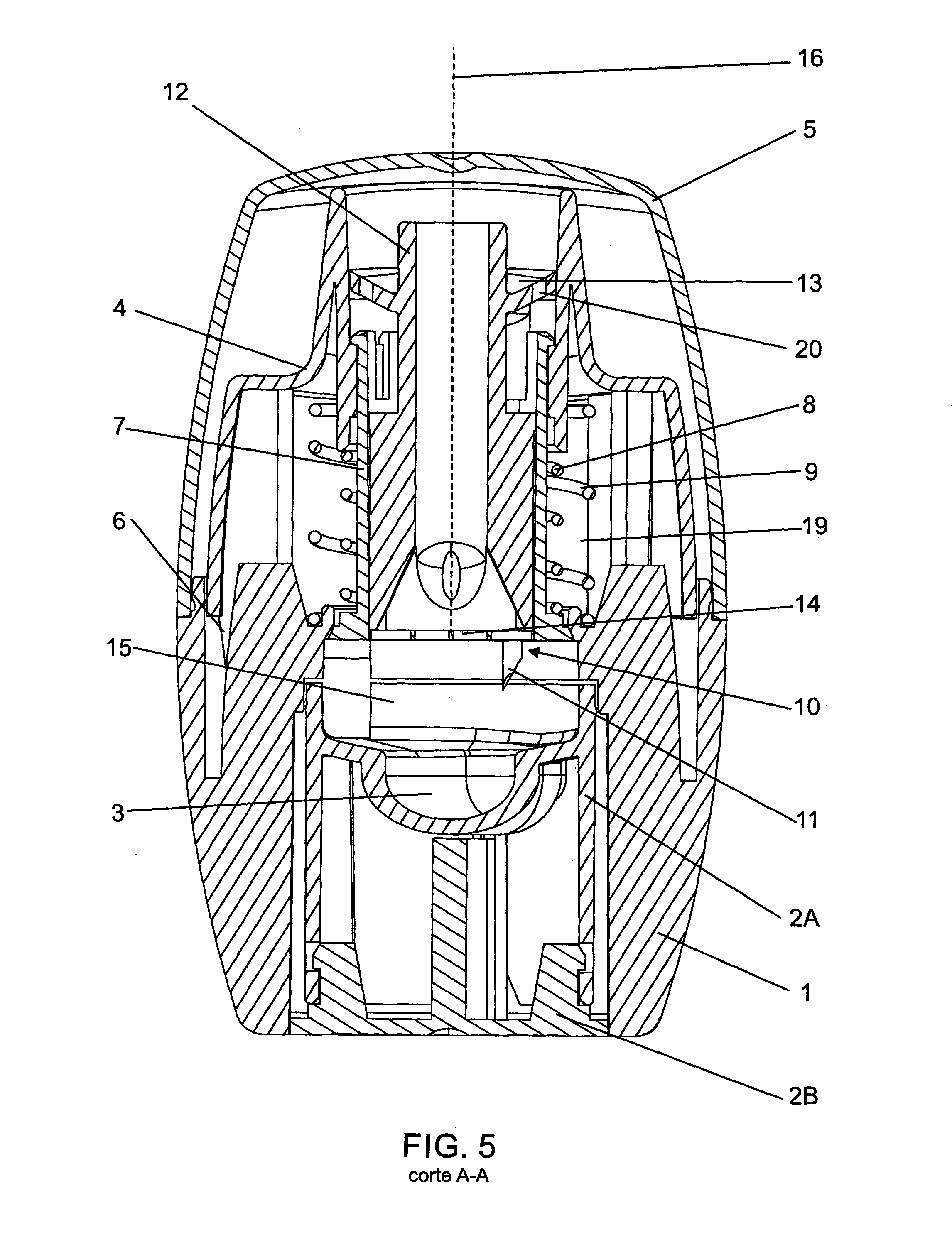
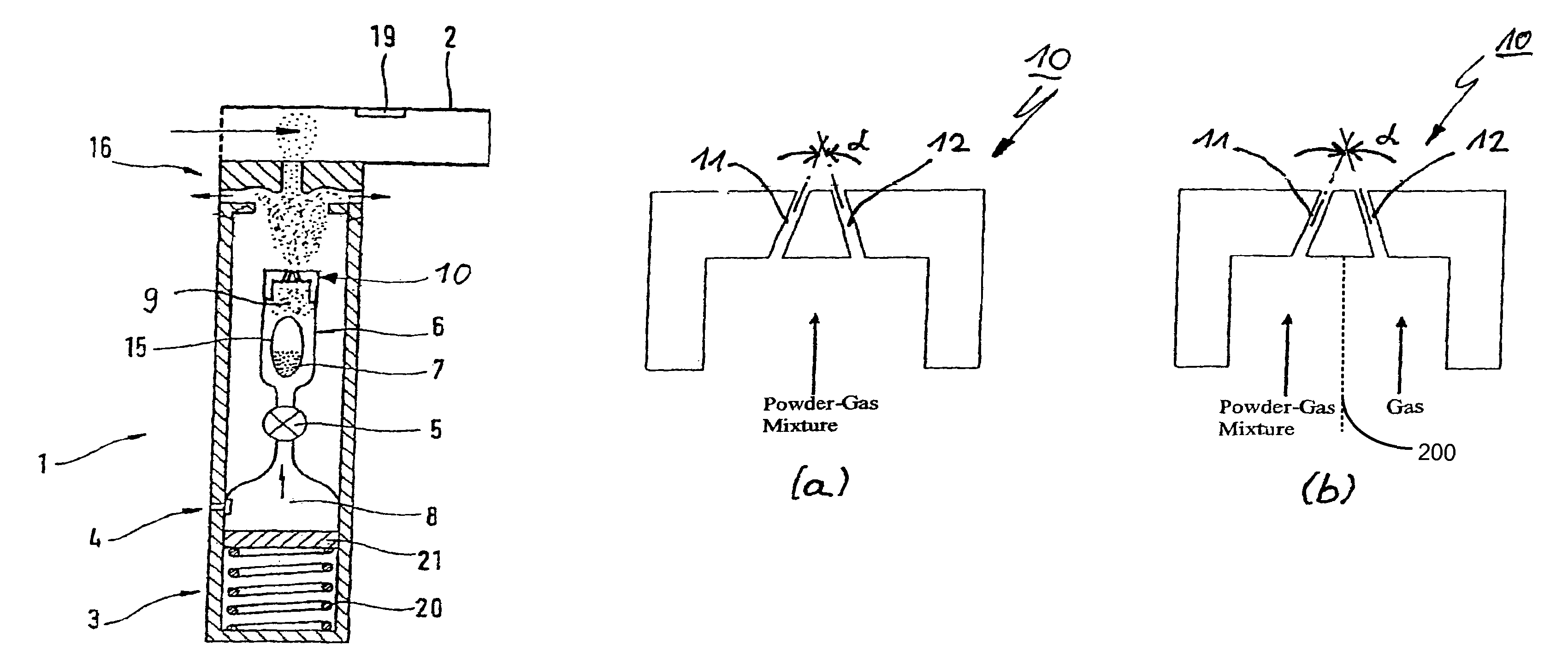
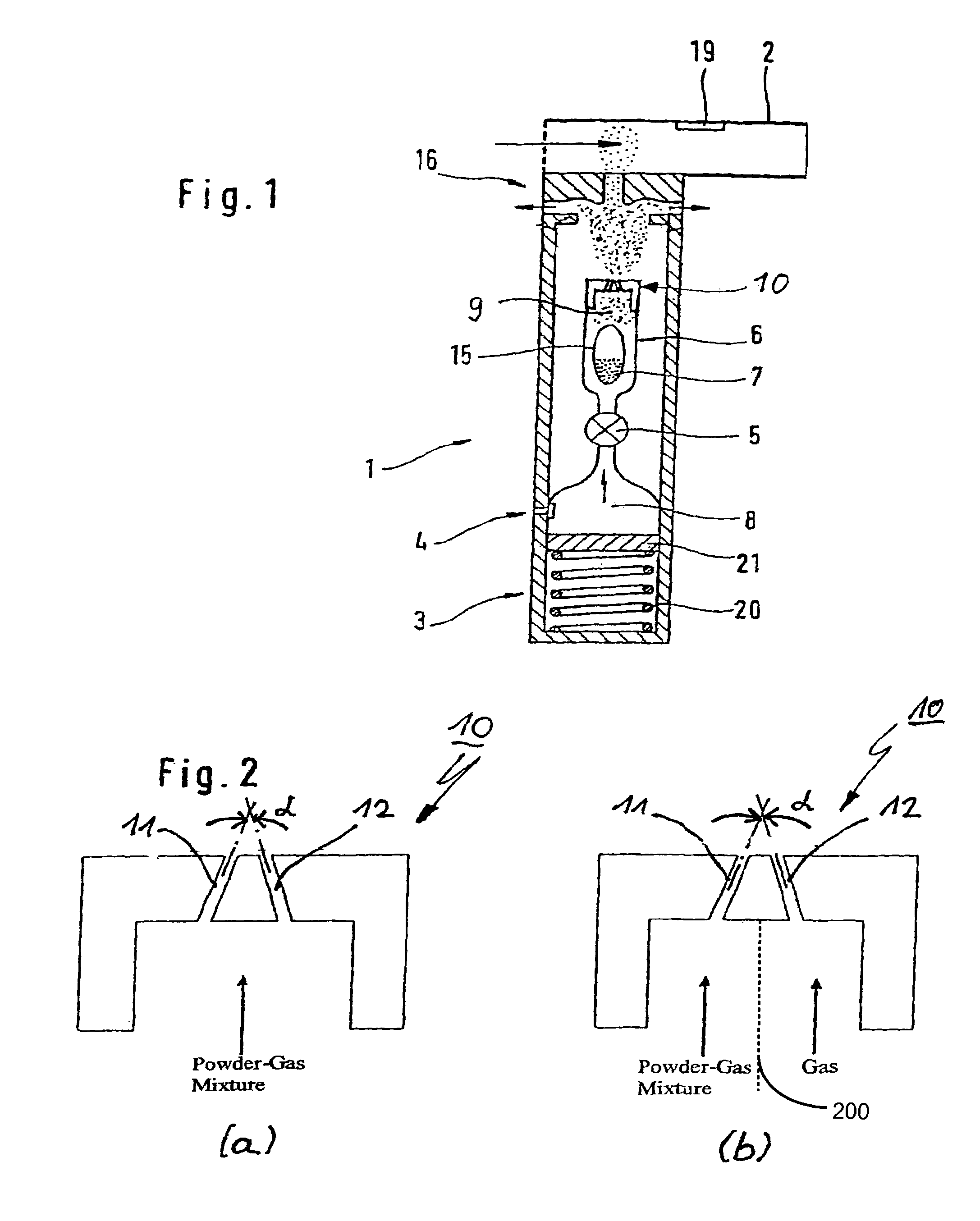
Powder inhaler having a nozzle with a plurality of channels
ActiveUS20050263151A1Small and convenientRespiratorsLiquid surface applicatorsPowder InhalerJet flow
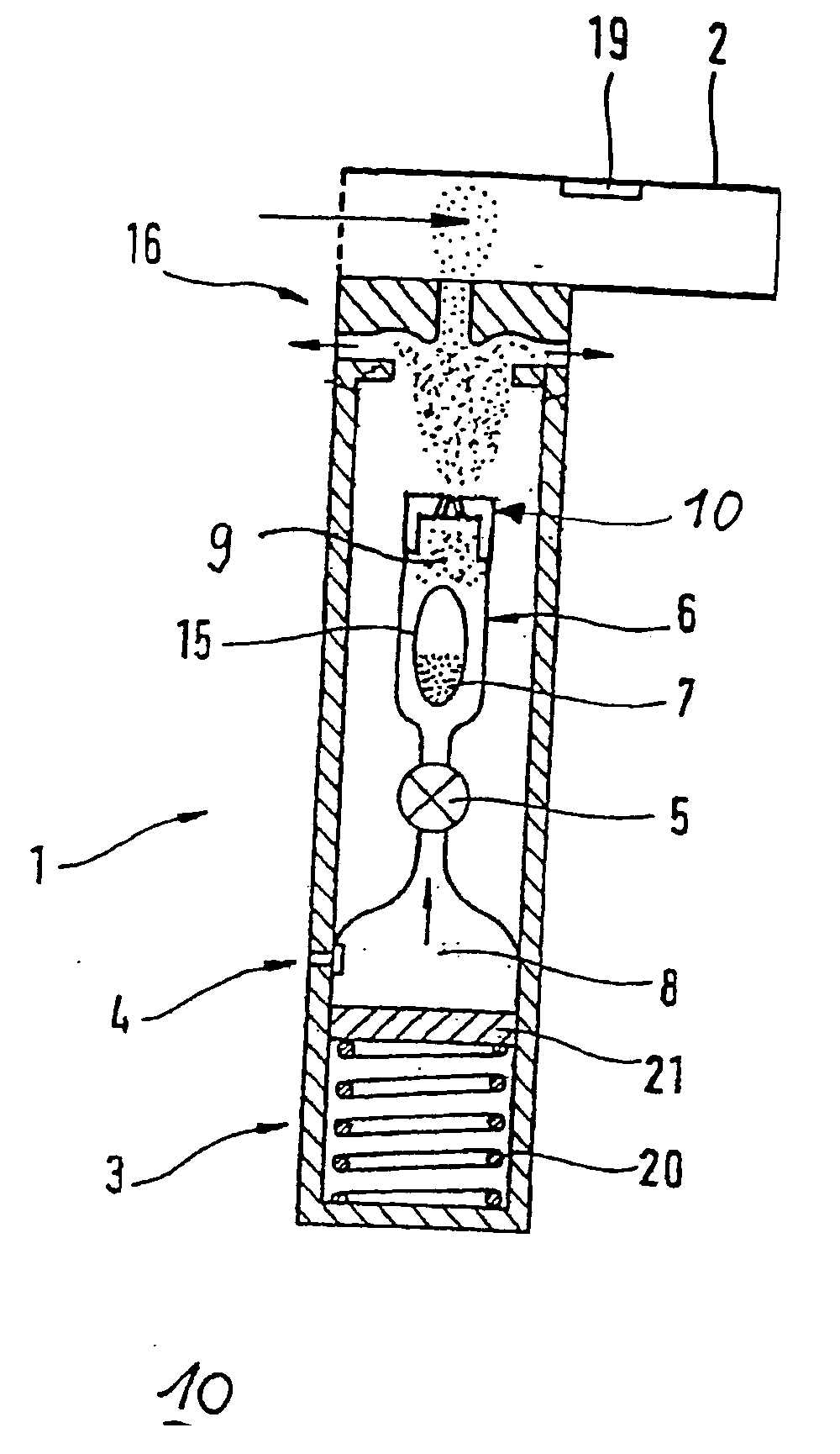
The invention relates to a powder inhaler (1) with mouthpiece (2) for dispersing pharmaceutical medicament formulations, which comprises an auxiliary energy source in the form of a pressure medium system (3), having a device for supplying (6) a powder formulation (7), while on activation of the pressure medium system a gaseous pressure medium (8) released by the pressure medium system (3) forms an aerosol (9) with the powder formulation (7) such that the powder particles are present in the gaseous pressure medium (8) in dispersed form. This is achieved by means of a powder inhaler (1) comprising a multi-channel nozzle, the channels of which are inclined to one another at an angle such that the aerosol jets flowing through them meet one another downstream behind the nozzle (10).
Owner:BOEHRINGER INGELHEIM INT GMBH
Pharmaceutical formulations for dry powder inhalers in the form of hard-pellets
InactiveUS6884794B2High fine particle fractionReduce intensityBiocidePowder deliveryDiseasePowder Inhaler
The invention provides a formulation to be administered as dry powder for inhalation suitable for efficacious delivery of active ingredients into the low respiratory tract of patients suffering of pulmonary diseases such as asthma. In particular, the invention provides a formulation to be administered as dry powder for inhalation freely flowable, which can be produced in a simple way, physically and chemically stable and able of delivering either accurate doses and high fine particle fraction of low strength active ingredients by using a high- or medium resistance device.
Owner:CHIESI FARM SPA
Powder inhalers
InactiveUS20090235929A1RespiratorsLiquid surface applicatorsPowder InhalerPharmaceutical formulation
Owner:EGEN MARC +2
Method and apparatus for sealing medicinal capsules
InactiveUS6949154B2Reduce penetrationQuality improvementLamination ancillary operationsLaminationPowder InhalerNebulizer
A method of sealing parts of a plastic capsule by forming a weld seam in an overlapping region of the parts of the capsule, wherein the capsule comprises a capsule cap having an open end and a capsule body having an open end, the method comprising:(a) holding the capsule cap and the capsule body in a capsule holder comprising a first holding part and a second holding part which can be guided synchronously with one another, wherein the first holding part interlockingly surrounds the capsule cap and the second holding part interlockingly surrounds the capsule body;(b) closing the capsule holder holding the capsule cap and the capsule body so that the open end of the capsule cap and the open end of the capsule body form a sealed cavity therebetween and form an overlapping region, wherein the overlapping region is not covered by the capsule holder, to obtain a closed capsule; and(c) welding the closed capsule using an energy beam of hot gas or laser light on the overlapping region by forming the weld seam thereon. The capsules produced by the process according to the invention are disposable capsules and preferably contain a single dose of a pharmaceutical formulation in the form of a powder or liquid suitable for inhalation and are suitable, by their form and function, for use in powder inhalers or liquid nebulizers for producing aerosols. Aerosols thus produced can be inhaled, for example, in order to administer a pharmaceutical formulation to the lungs.
Owner:BOEHRINGER INGELHEIM PHARM KG
Needle for piercing a powder capsule for inhalation
ActiveUS20070107722A1Enhances expulsionReduce effortRespiratorsLiquid surface applicatorsPowder InhalerInhalation
The invention relates to a powder inhaler with at least one specially sharpened needle, to ensure the precise piercing or cutting open of capsules and hence the optimum delivery of powdered medicament compositions, medicament formulations or medicament mixtures, as well as ensuring that little effort is needed to perforate or cut open the capsule.
Owner:BOEHRINGER INGELHEIM PHARM KG
Powder particles with smooth surface for use in inhalation therapy
InactiveUS6780508B1Change surface propertiesChange propertiesPowder deliveryBiocidePowder InhalerInhalation powder
Carriers for use in the preparation of mixtures for inhalation powders intended for pulmonary administration of micronized drugs by means of a dry powder inhaler and the method for their preparation are described.
Owner:CHIESI FARM SPA
Powder Inhalers
InactiveUS20110203586A1RespiratorsLiquid surface applicatorsPowder InhalerPharmaceutical formulation
Owner:BOEHRINGER INGELHEIM INT GMBH
Engineered particles and methods of use
Engineered particles are provided may be used for the delivery of a bioactive agent to the respiratory tract of a patient. The particles may be used in the form of dry powders or in the form of stabilized dispersions comprising a nonaqueous continuous phase. In particularly preferred embodiments the particles may be used in conjunction with an inhalation device such as a dry powder inhaler, metered dose inhaler or a nebulizer.
Owner:NOVARTIS AG
Powder inhaler devices
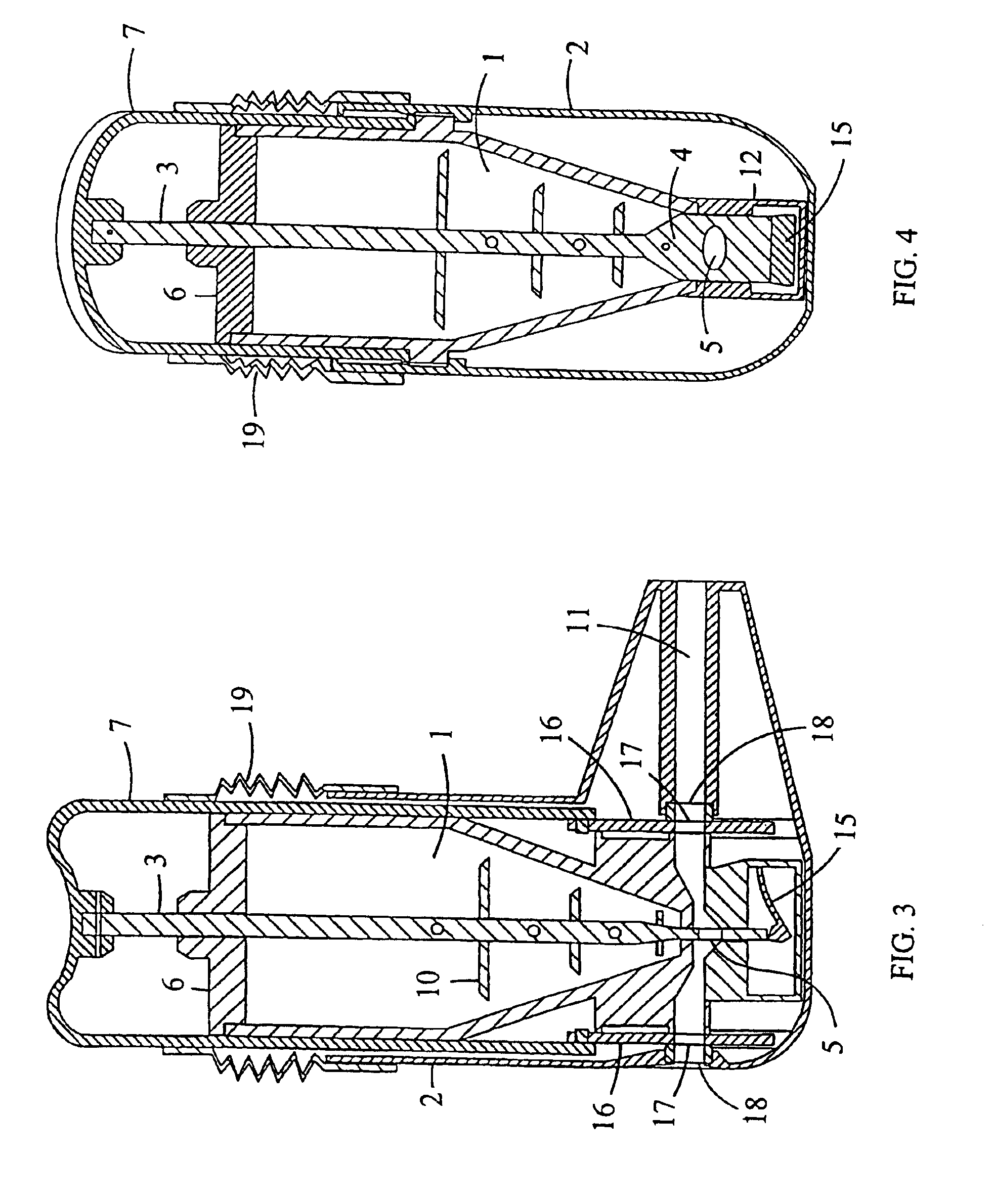
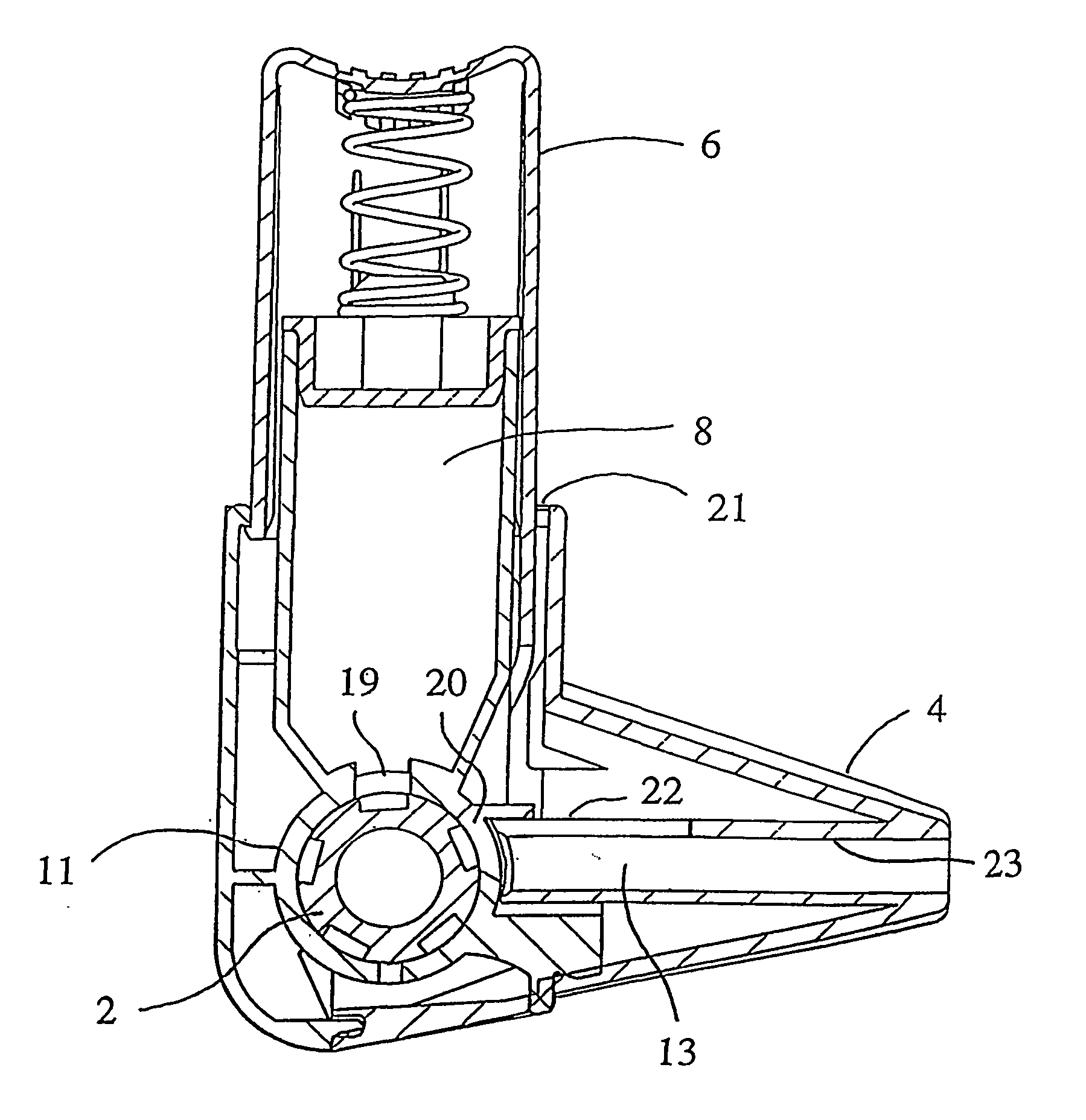
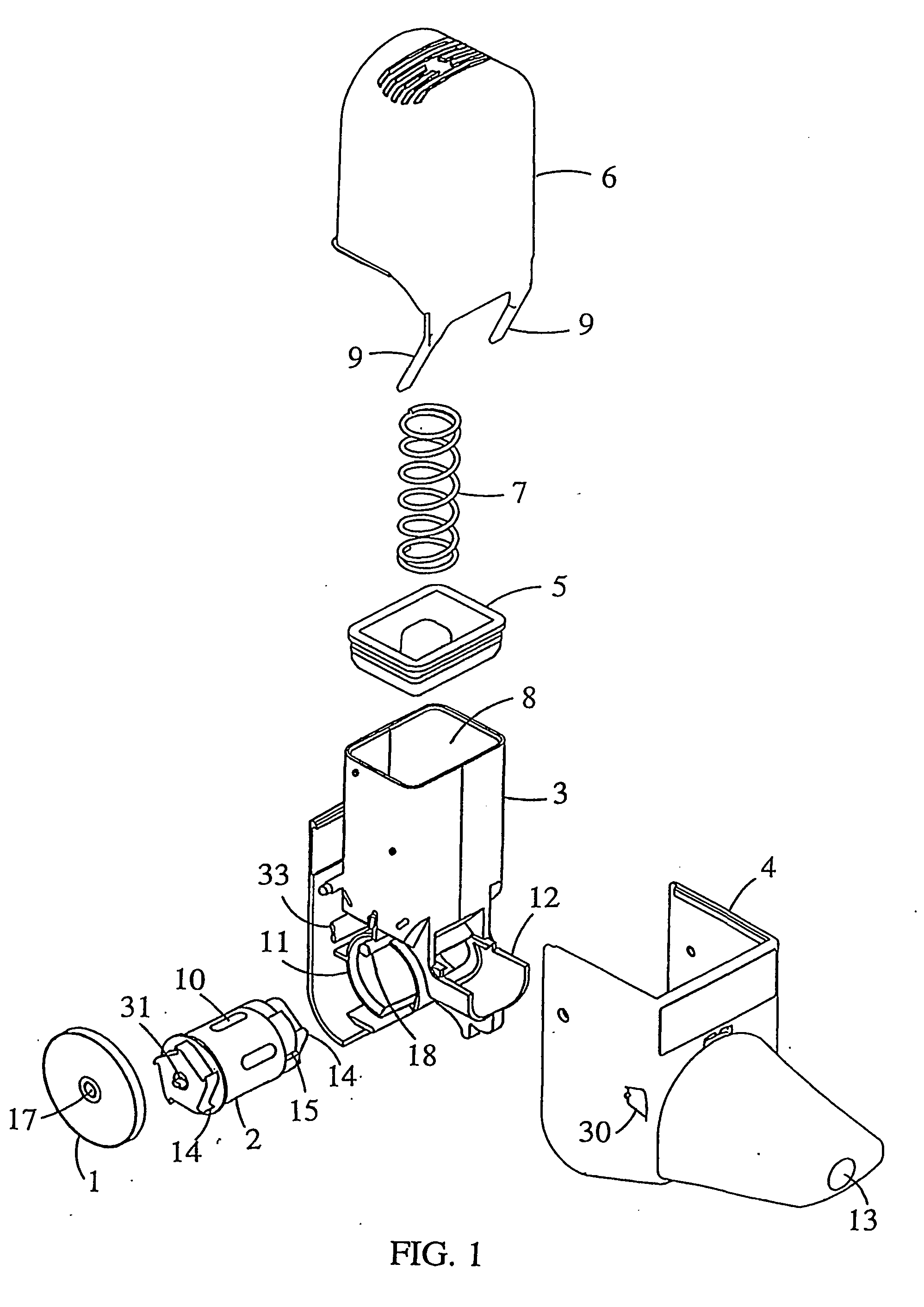
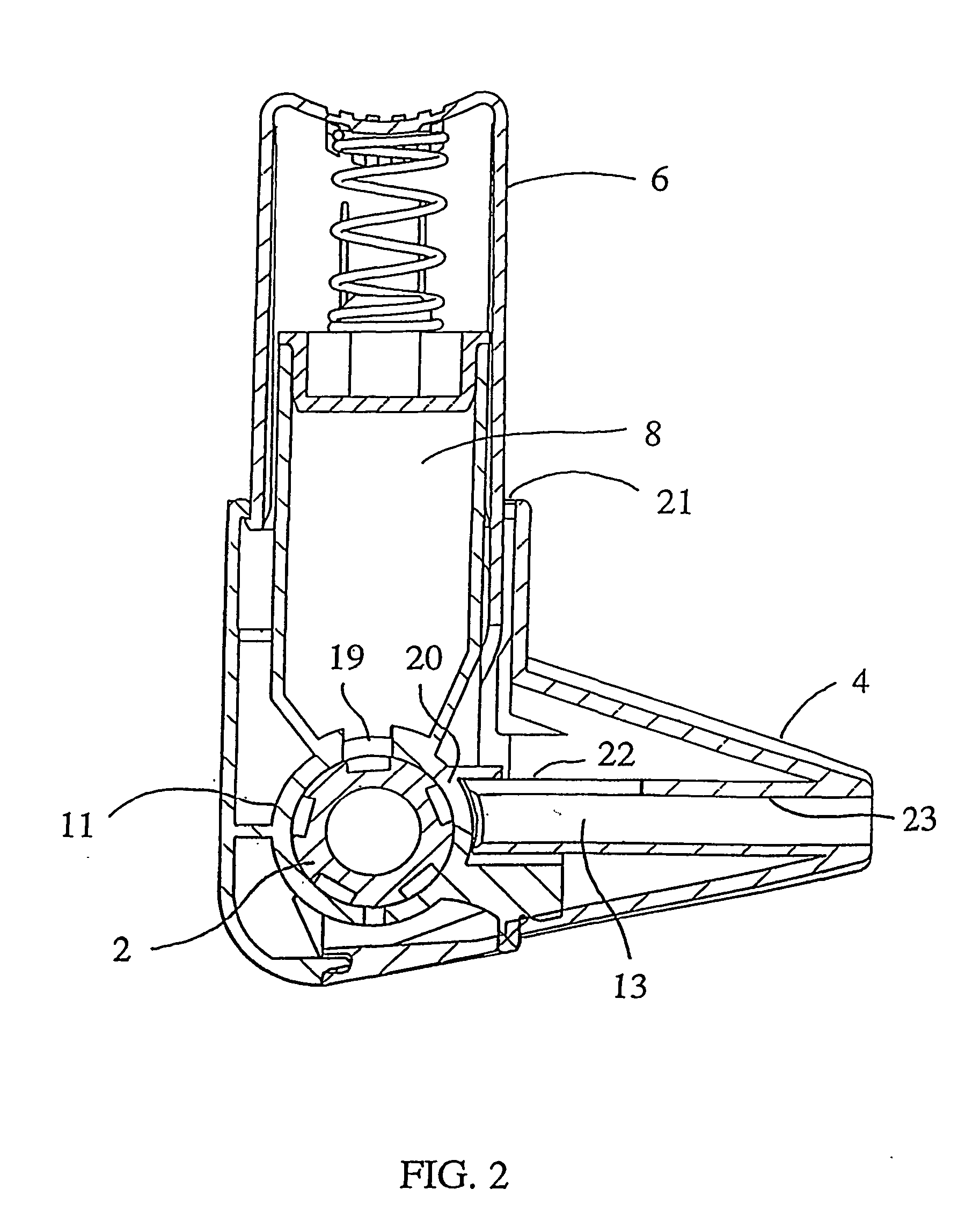
An improved inhalation device is provided for facilitating inhalation by a patient of powder medicaments contained in a receptacle. The inhalation device includes a staple assembly comprising a plunger and staple that are securely and robustly coupled to one another. Embodiments of the inhalation device have a cap that prevents or reduces the amount of dust and grime entering into the device. The cap is additionally configured to prevent or reduce inadvertent and unintentional operation of the device. The body portion and first casing portion of certain embodiments of the inhalation device are sealably coupled, restricting or reducing undesirable flow pathways that have an adverse effect on the operation of the device.
Owner:CIVITAS THERAPEUTICS
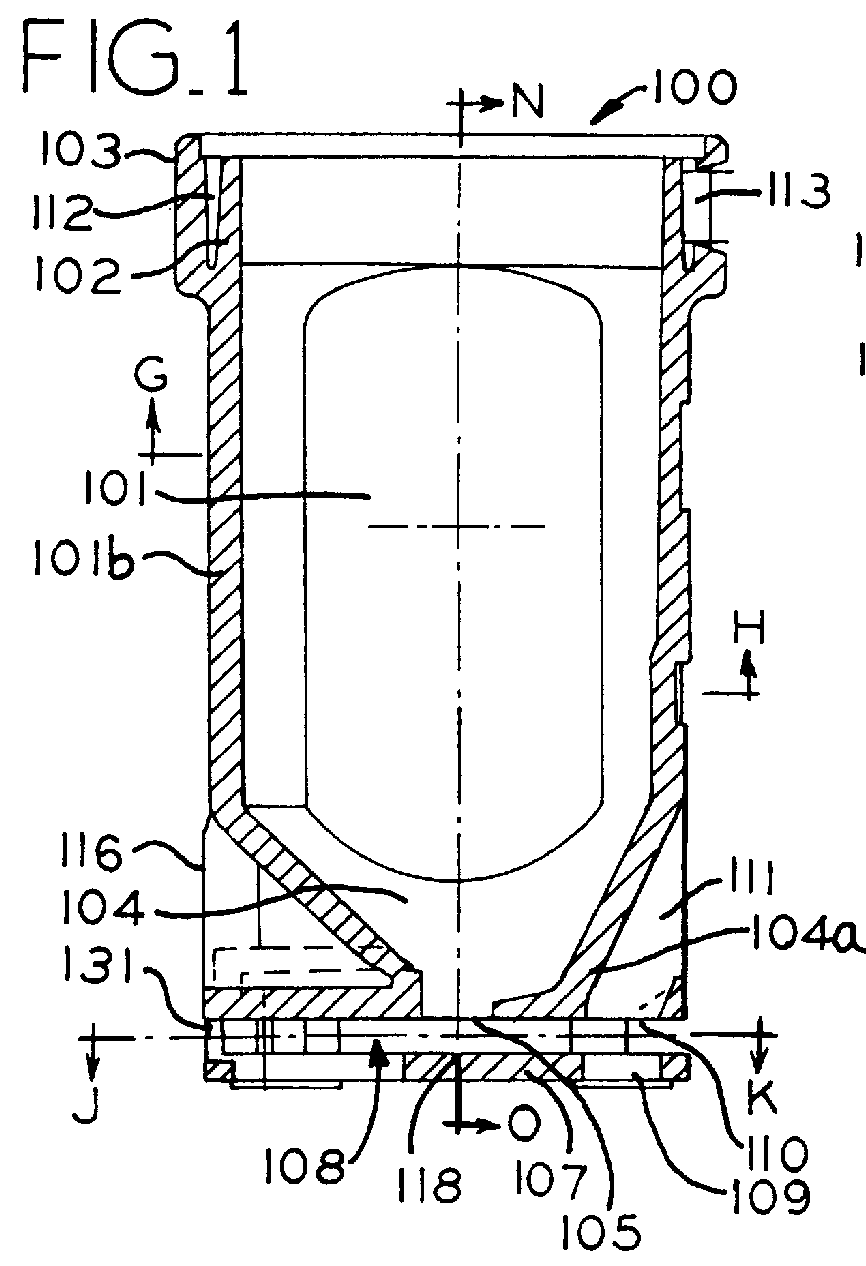
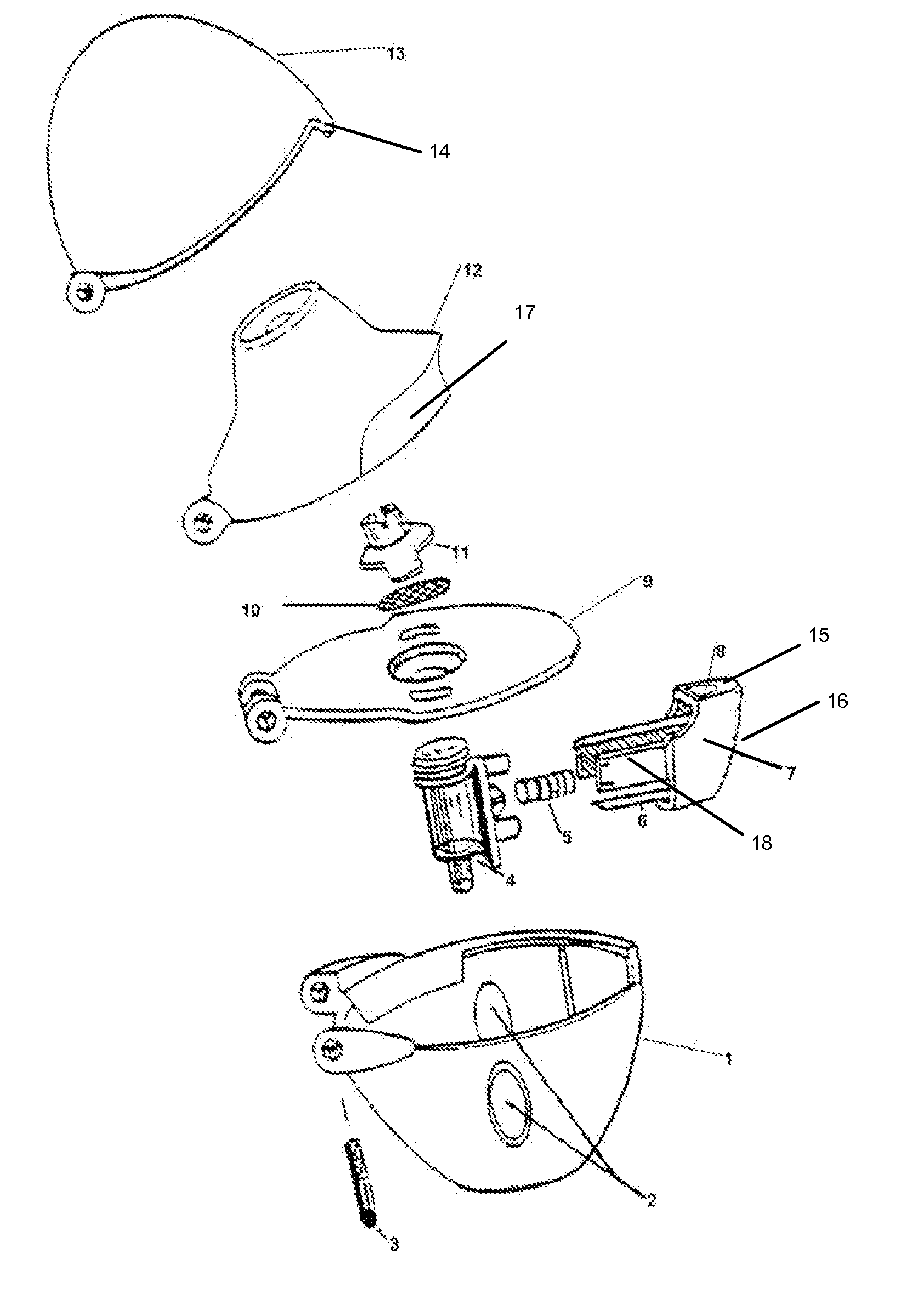
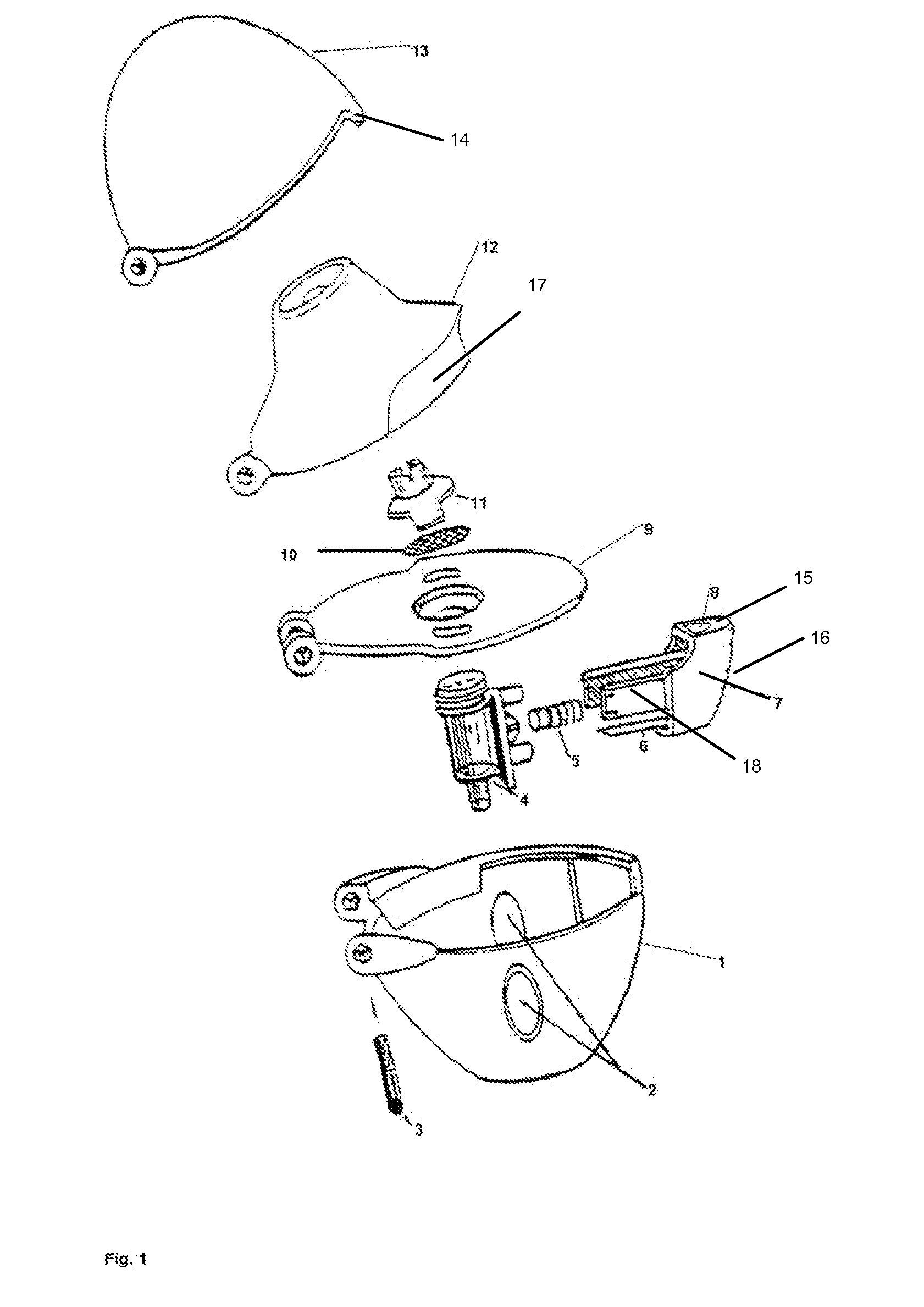
Powder inhaler
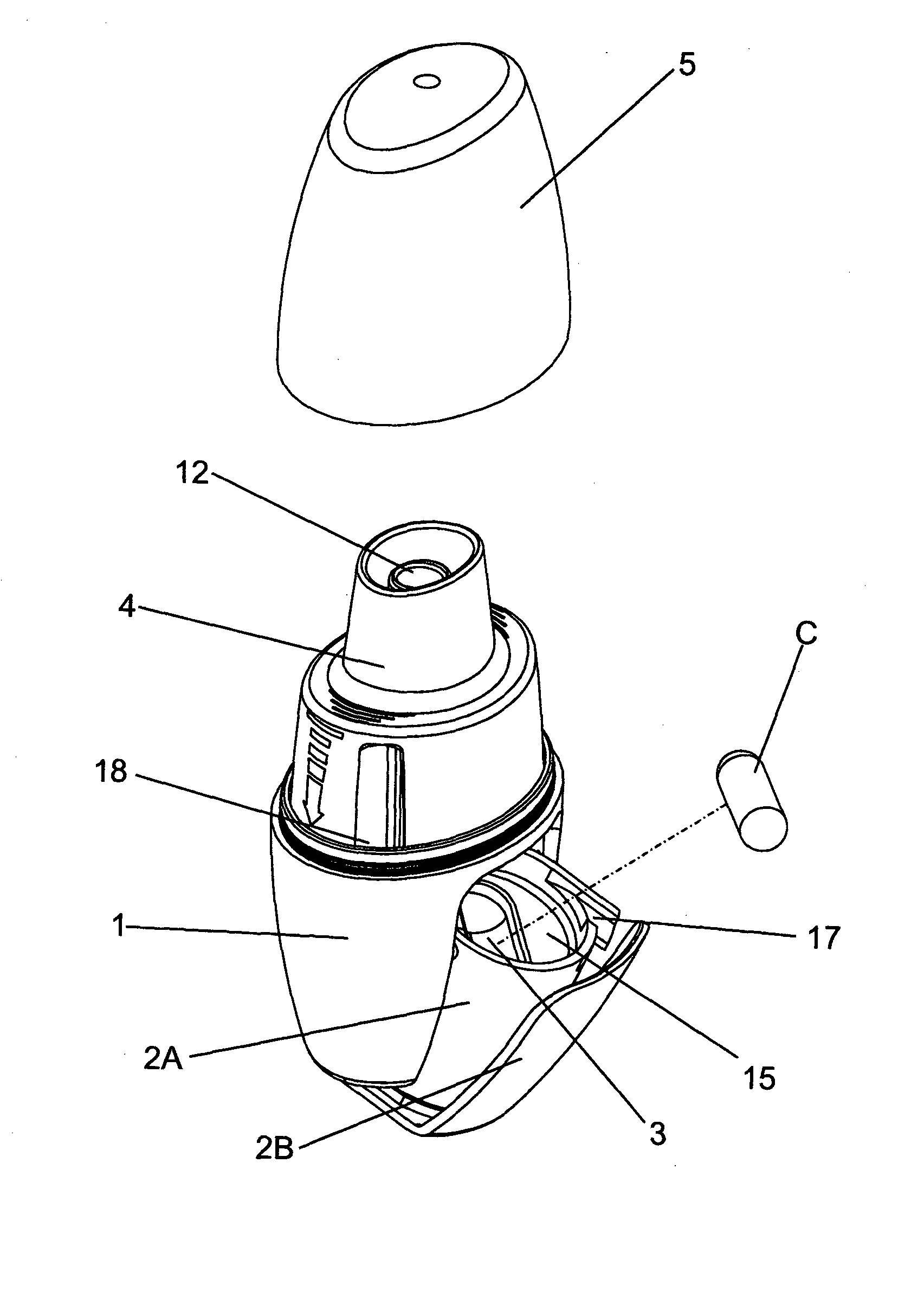
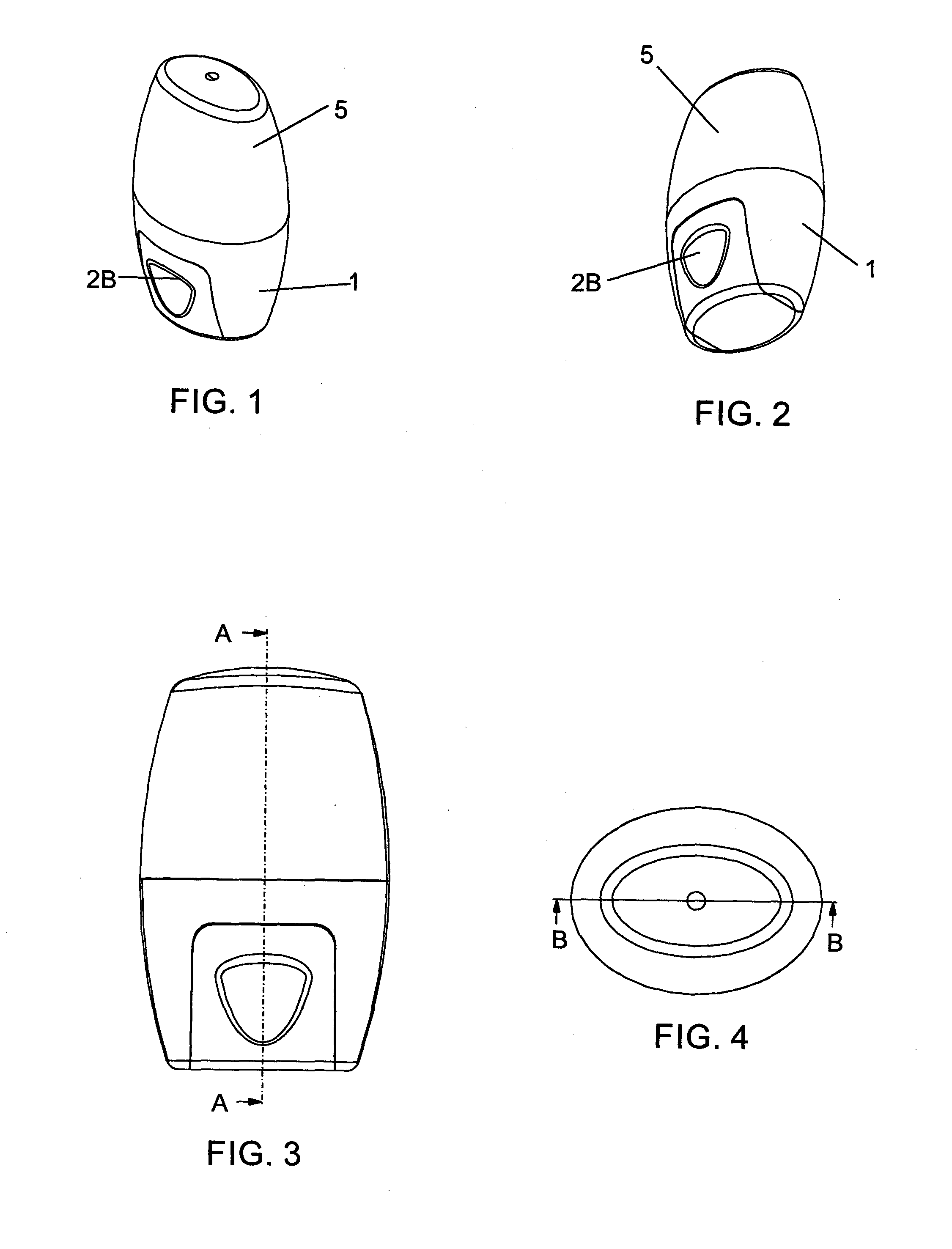
Powder inhaler, consisting of a base housing (1), snap-in capsule receptacle (2a) mounted together with a lid (2b); a moveable mouthpiece (4) with cap (5) and guided by lateral stems (6) and vertical guide (7); a perforation device (10) for opening the capsule; a flow guide tube, centralized and housed in the mouthpiece and on the guide; a de-agglomeration chamber formed above the housing of the capsule; a vertical passage formed between said de-agglomeration chamber and the upper edge of the mouthpiece; a secondary air intake point positioned between the walls of the capsule receptacle and the base housing which in turn has one or two air intake points with a pocket and include one or more secondary air flow passages.
Owner:ESTEVE VICTOR +1
Powder inhaler having a nozzle with a plurality of channels
The invention relates to a powder inhaler (1) with mouthpiece (2) for dispersing pharmaceutical medicament formulations, which comprises an auxiliary energy source in the form of a pressure medium system (3), having a device for supplying (6) a powder formulation (7), while on activation of the pressure medium system a gaseous pressure medium (8) released by the pressure medium system (3) forms an aerosol (9) with the powder formulation (7) such that the powder particles are present in the gaseous pressure medium (8) in dispersed form. This is achieved by means of a powder inhaler (1) comprising a multi-channel nozzle, the channels of which are inclined to one another at an angle such that the aerosol jets flowing through them meet one another downstream behind the nozzle (10).
Owner:BOEHRINGER INGELHEIM INT GMBH
Powder inhaler devices
An improved inhalation device is provided for facilitating inhalation by a patient of powder medicaments contained in a receptacle. The inhalation device includes a staple assembly comprising a plunger and staple that are securely and robustly coupled to one another. Embodiments of the inhalation device have a cap that prevents or reduces the amount of dust and grime entering into the device. The cap is additionally configured to prevent or reduce inadvertent and unintentional operation of the device. The body portion and first casing portion of certain embodiments of the inhalation device are sealably coupled, restricting or reducing undesirable flow pathways that have an adverse effect on the operation of the device.
Owner:CIVITAS THERAPEUTICS
Powder inhaler formulations
The present invention relates to new methods for the surface modification of powders. Furthermore the present invention relates to new, improved pharmaceutical dosage forms obtainable by the new methods for surface modification of drugs according to the invention and to the use of these pharmaceutical dosage forms within dry powder inhalation devices (DPI).
Owner:BECHTOLD PETERS KAROLINE +2
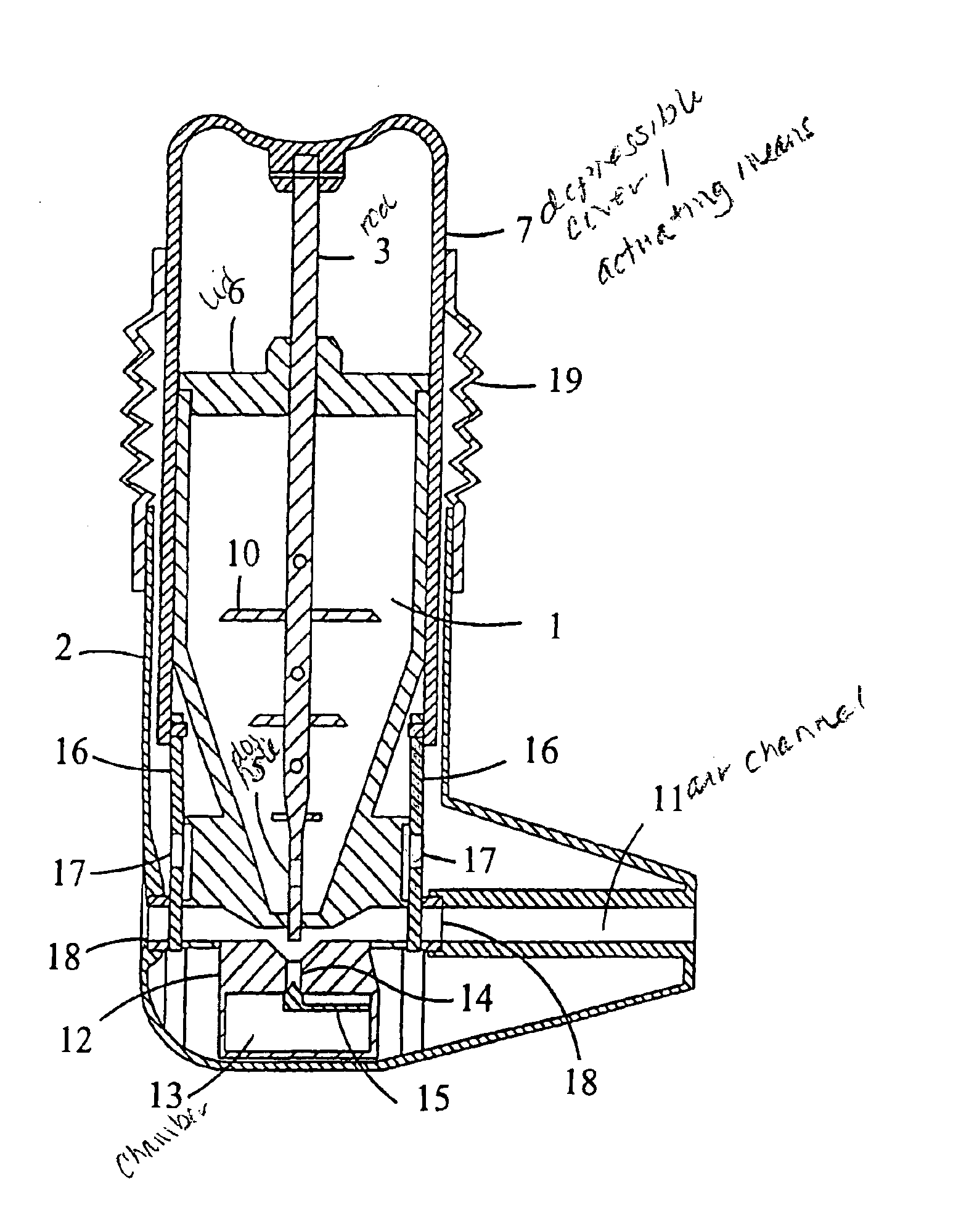
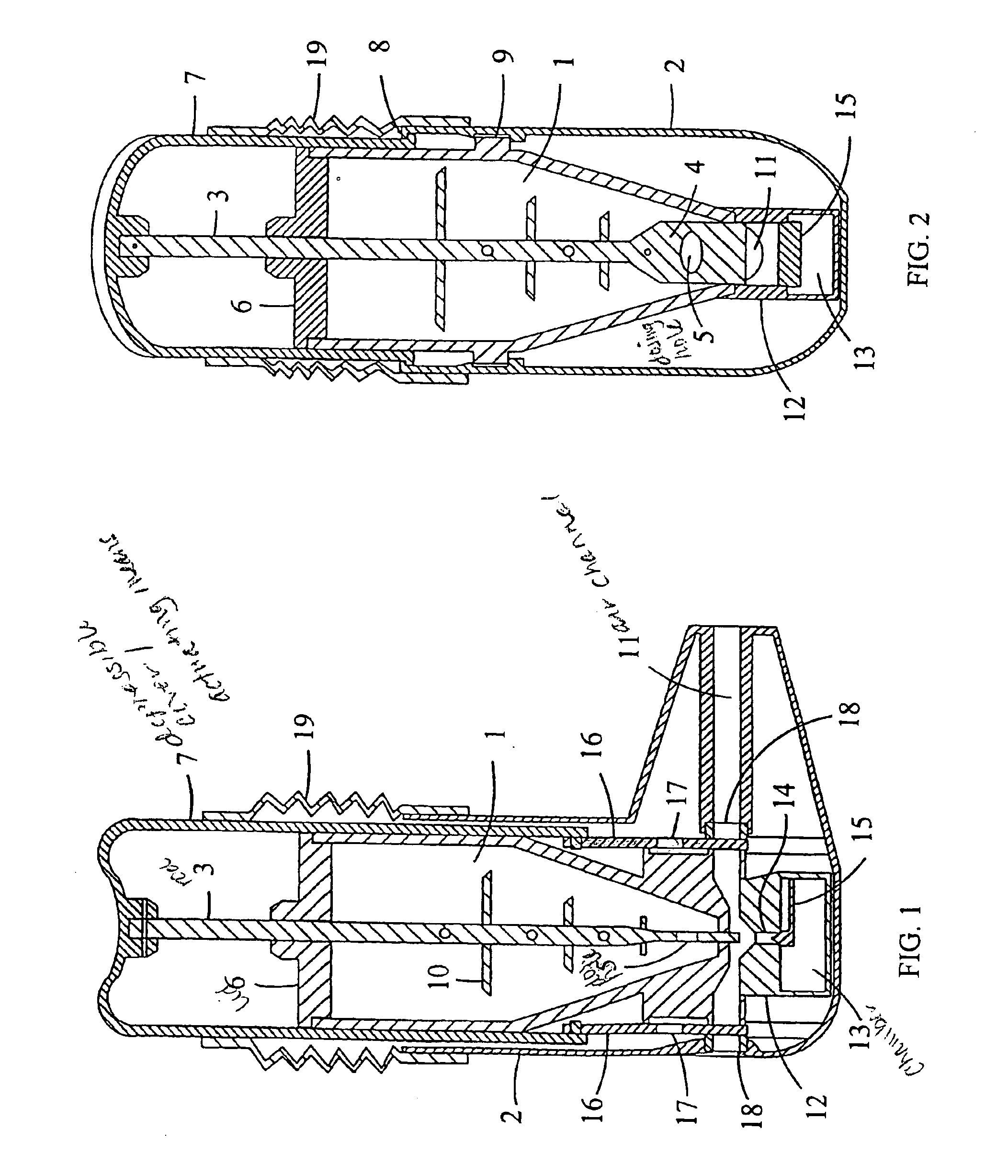
Moisture protected powder inhaler
InactiveUS6886560B1Improve accuracyAvoid disadvantagesRespiratorsLiquid surface applicatorsPowder InhalerInhalation
A powder inhaler comprising a powder container, an air channel, a metering member equipped with a dosing recess, an actuating means for the displacement of the metering member between the filling and the inhalation position, and a closure element for plugging the air channel in a substantially water-proof manner when the metering member is in the filling position and opening the air channel when the metering member is in the inhalation position. When the inhaler is not in use, the closure element prevents moisture and dirt from entering the sensitive parts of the device.
Owner:ORION CORPORATION
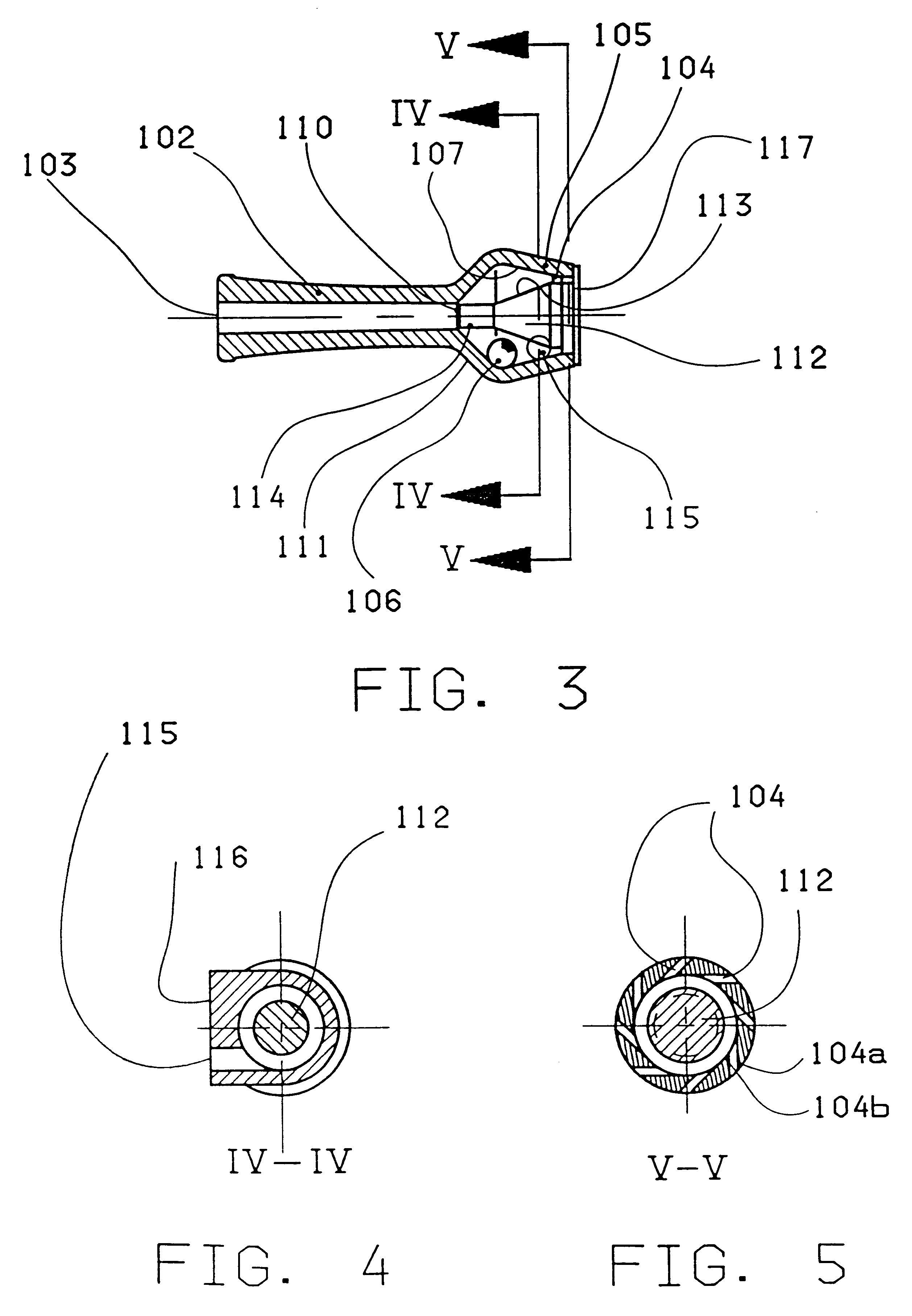
Powder inhaler
ActiveUS20050005933A1Efficient deagglomerationIncrease volumeRespiratorsLiquid surface applicatorsPowder InhalerCatheter
An inhaler device includes an air conduit including a mouthpiece and a dosing means adapted to provide a dose of powder to the air conduit for entrainment in the stream of air. In the area downstream from the dosing means the wall of the air conduit is provided with a secondary air inlet extending to the direction of the mouthpiece such that the entry of secondary air occurs over an extended length of the air conduit downstream from the dosing means.
Owner:ORION CORPORATION
Solid peptide preparations for inhalation and their preparation
InactiveUS20050014677A1Broaden applicationGood dispersionPowder deliveryDispersion deliveryPowder InhalerInhalation
Owner:VIATRIS GMBH & CO KG
Solid peptide preparations for inhalation and their preparation
InactiveUS20090142407A1Good dispersionReduce the risk of contaminationBiocidePowder deliveryPowder InhalerInhalation
Owner:VIATRIS GMBH & CO KG
Adapter for inhalation appliances for treatment of artificially ventilated patients
The invention relates to a device for delivery of respiratory gases in conjunction with a powder inhaler for inhaled administration of pharmaceuticals, pharmaceutical mixtures or pharmaceutical formulations to patients who are attached to respirators. The pharmaceuticals, pharmaceutical mixtures or pharmaceutical formulations are preferably administered to patients who are being artificially ventilated through a mask or via a tracheal tube under anesthesia.
Owner:BOEHRINGER INGELHEIM PHARM KG
Powder inhaler
InactiveUS6948495B2Simple and reliable structureReduce stepsRespiratorsLiquid surface applicatorsDiseasePowder Inhaler
An inhaler device comprising a container for powdered medicament, an inhalation channel, a movable metering member, a depressible actuator and a multiple dosage preventing means for locking the depressible actuator after use such that the actuator is unlocked only upon inhalation. The inhaler device is useful in the treatment of respiratory diseases such as asthma. The multiple dosage preventing means of the device eliminates the risk of unintentional overdosing.
Owner:ORION CORPORATION
Improved dry powder inhaler
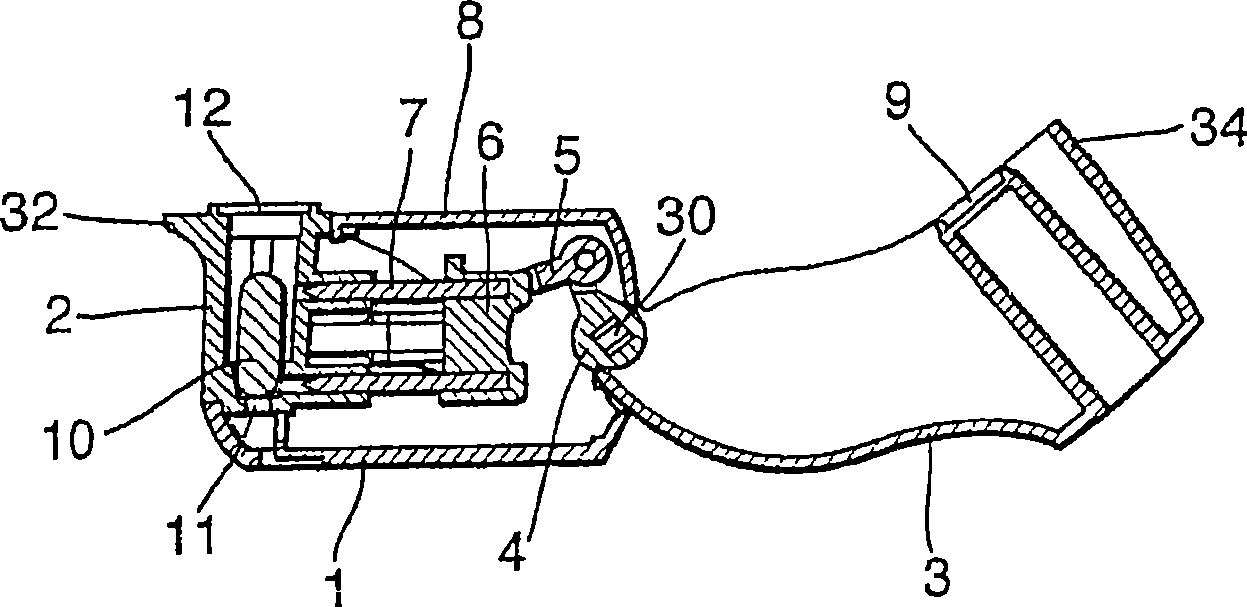
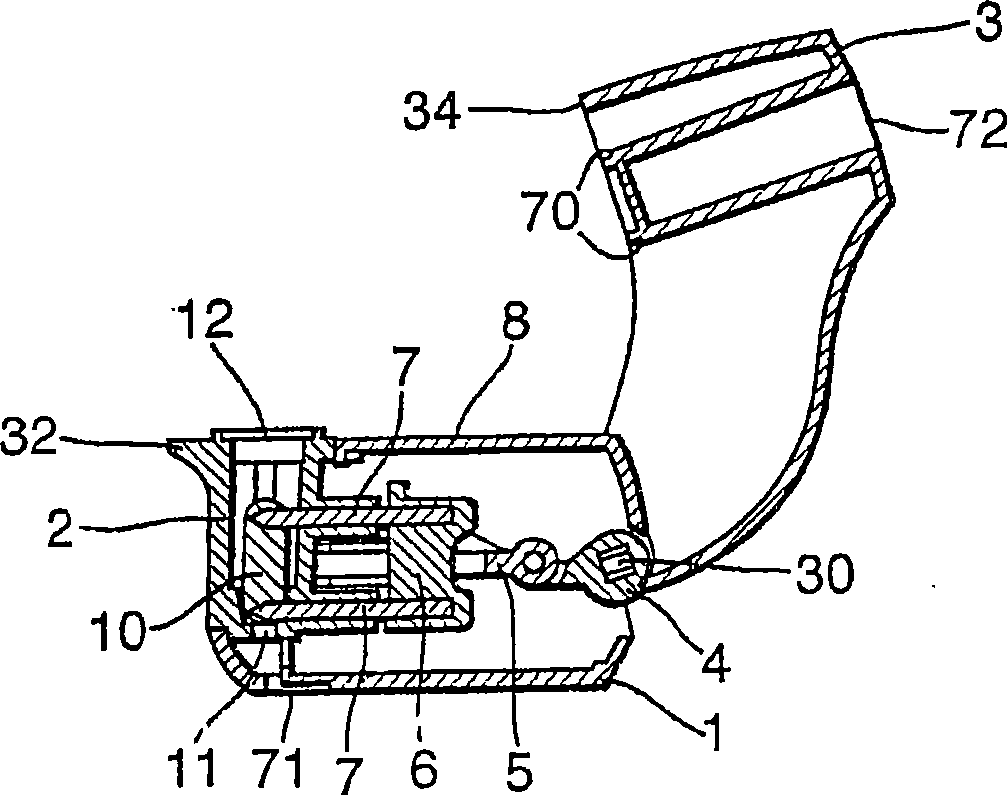
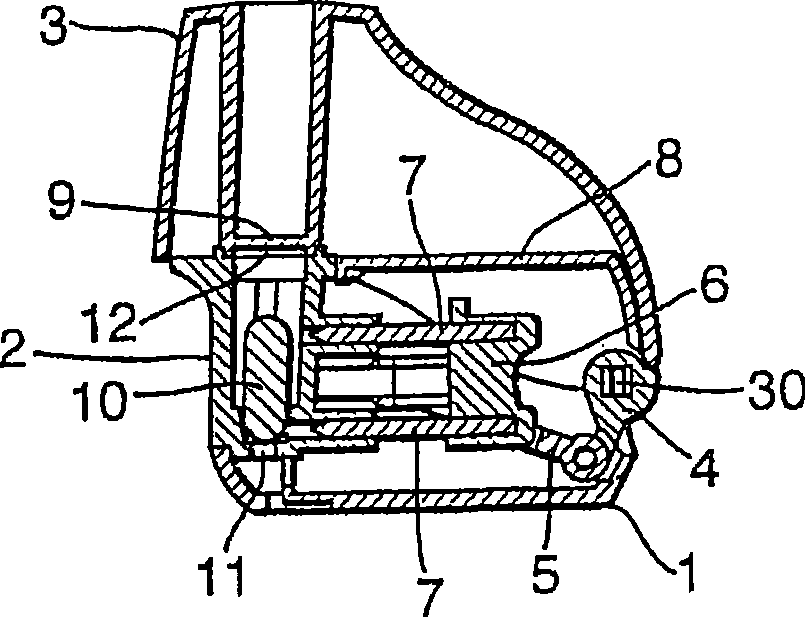
An inhaler (1) device for inhalation of a medicament from a pierceable capsule (10) comprises a housing (2) for receiving a medicament, capsule, closure means (3) for closing the housing, said closure means being moveable relative to the housing; piercing means (7) suitable for piercing a medicament capsule; wherein movement of the closure means relative to the housing causes movement of the piercing means, and wherein the device comprises an air inlet (71) and an air outlet (72) defining an inhalation passage therebetween, the passage comprising one or more vents (70).
Owner:CIPLA LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com