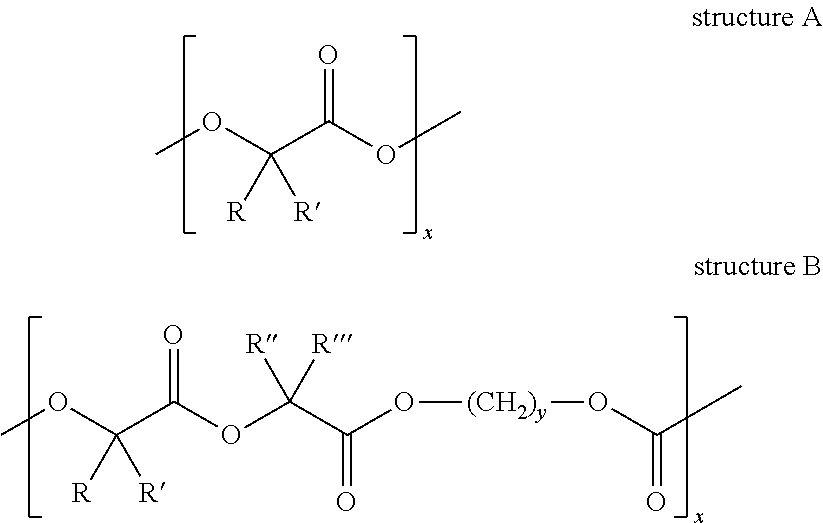
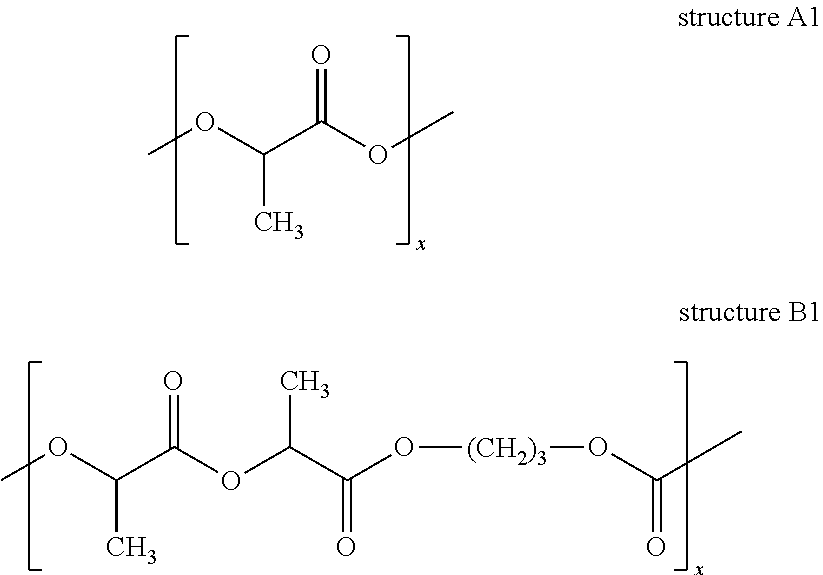
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1113 results about "Medical product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical product. A generic term for any product used to diagnose or manage patients. Examples. Exam gloves, swabs, mattresses, lab products, protective garments, drains, dressings, etc.
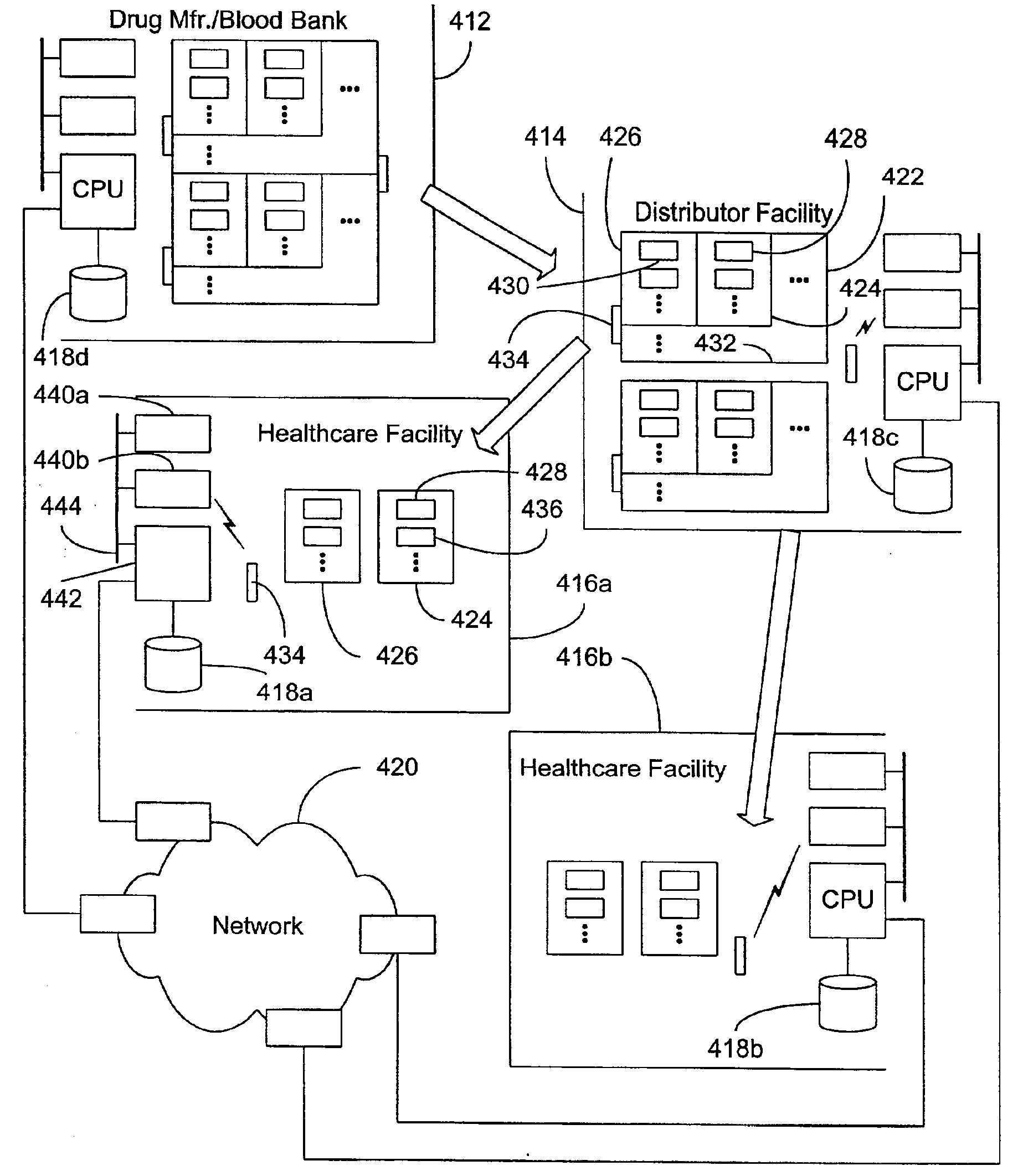
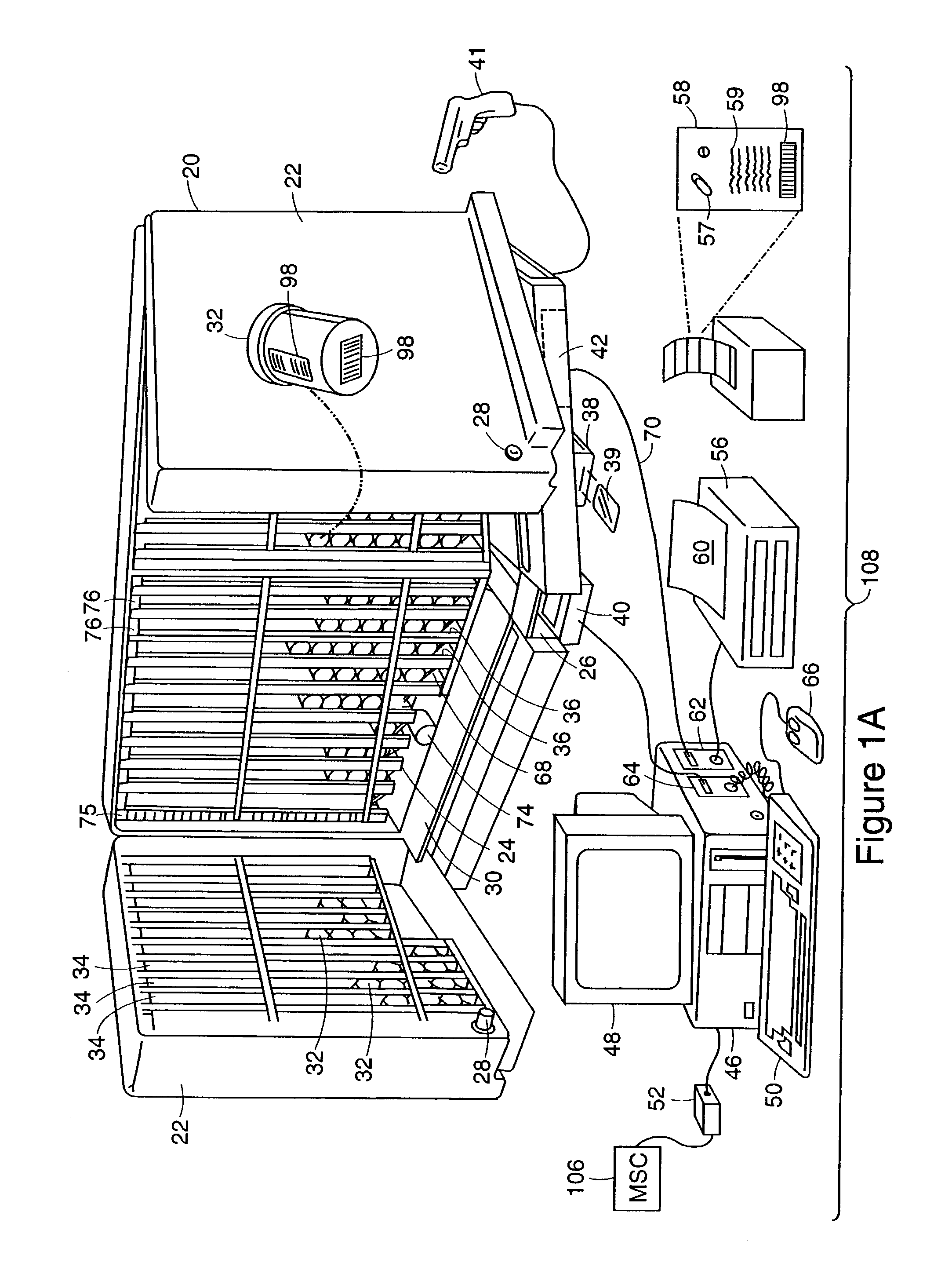
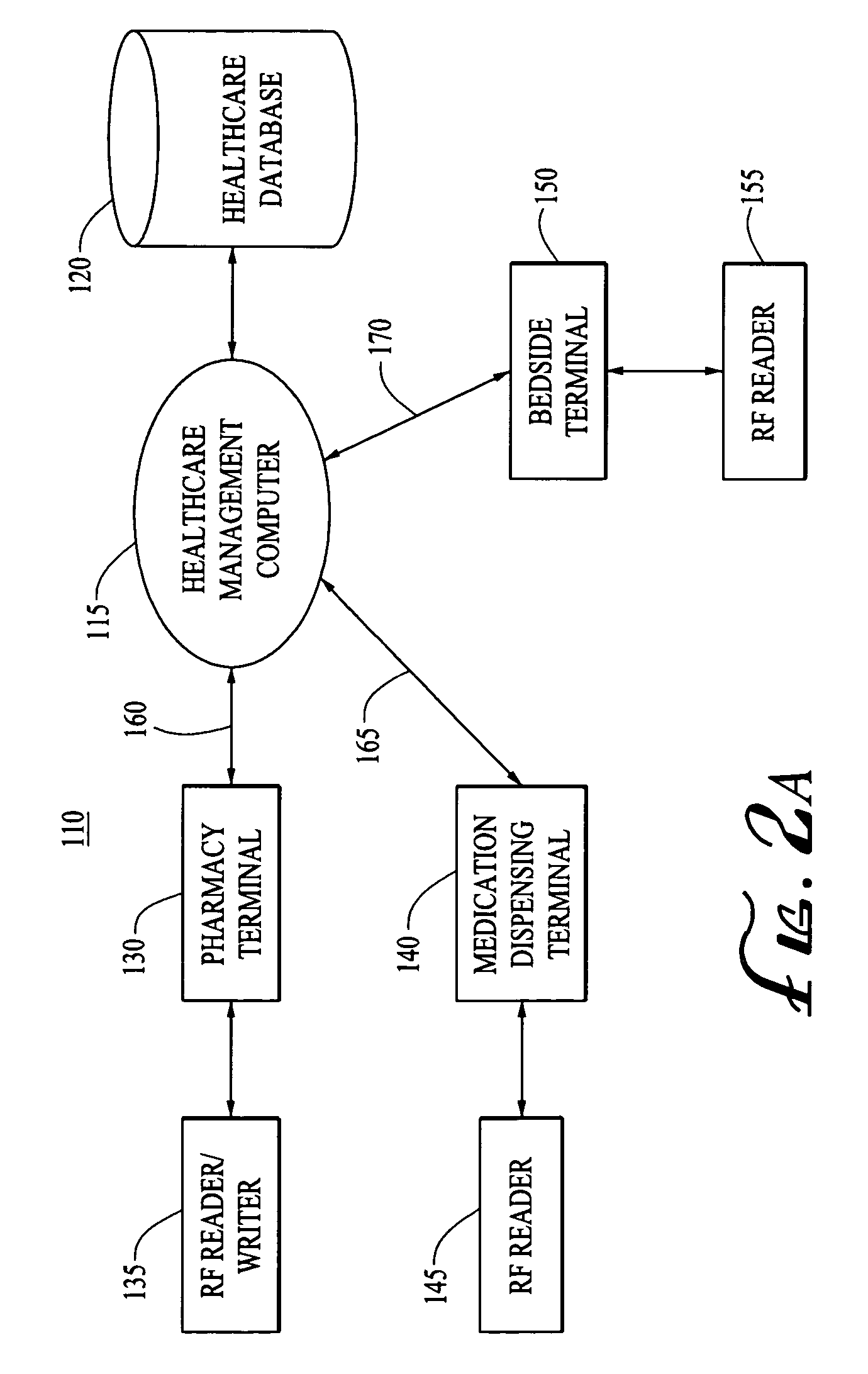
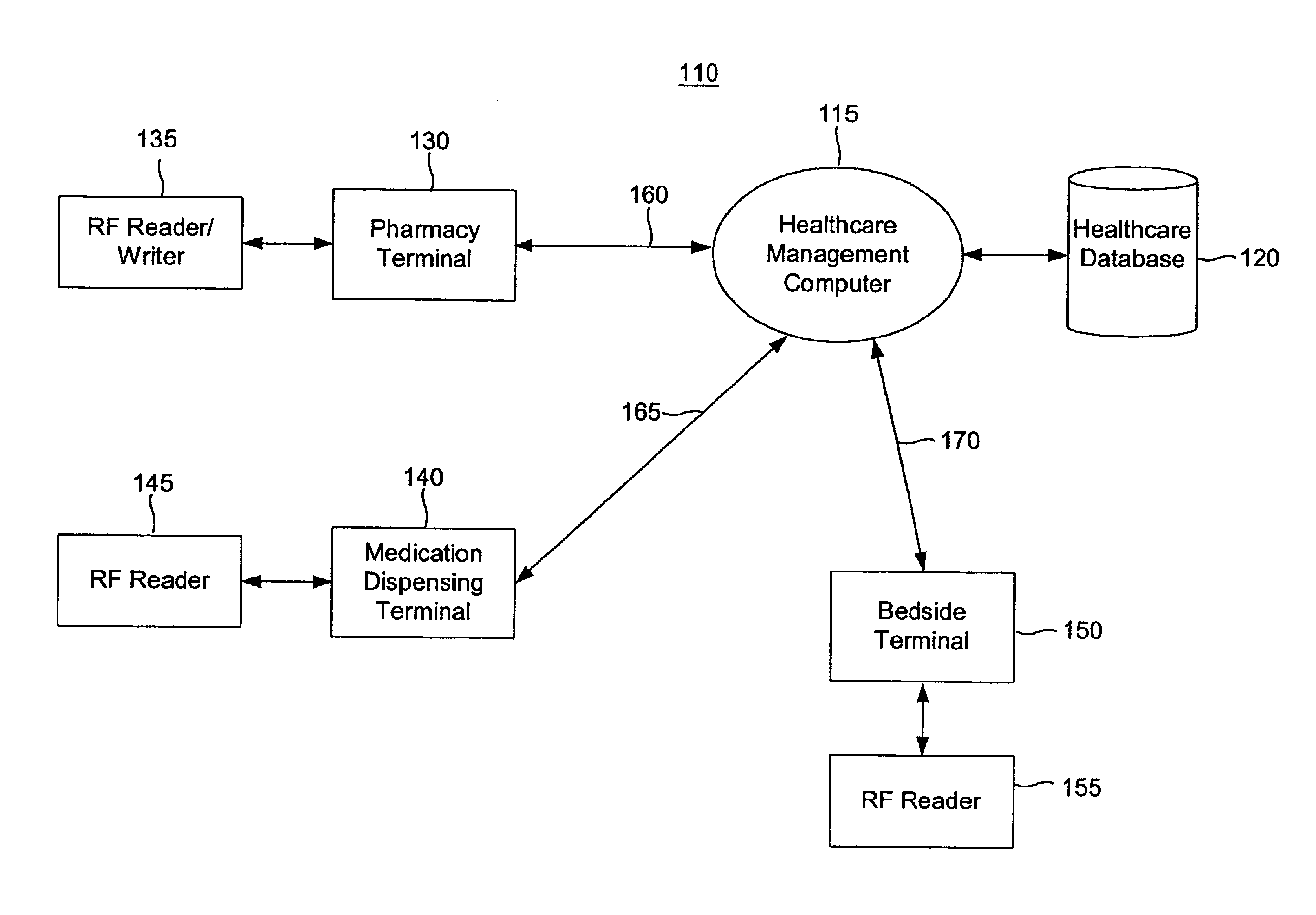
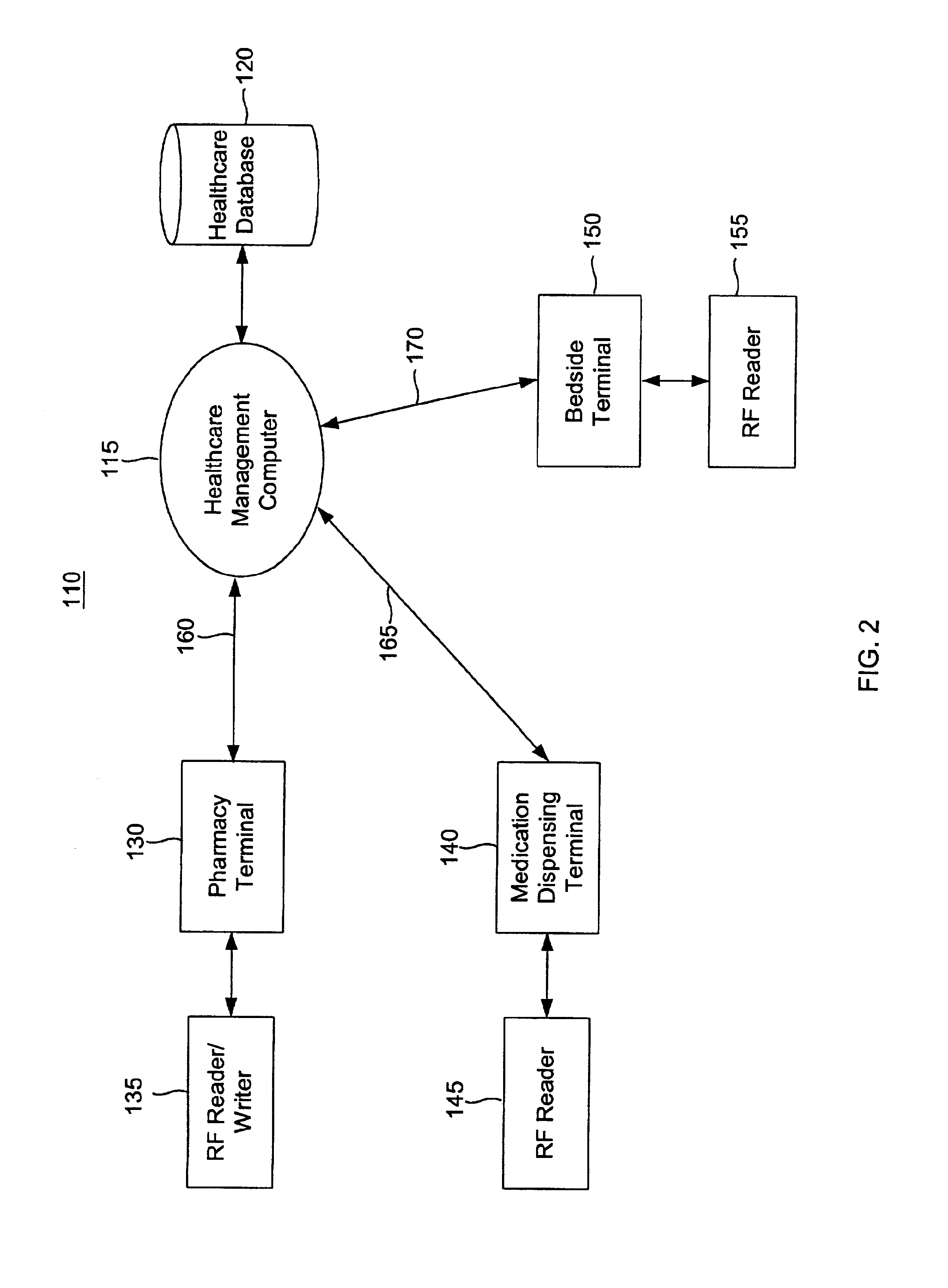
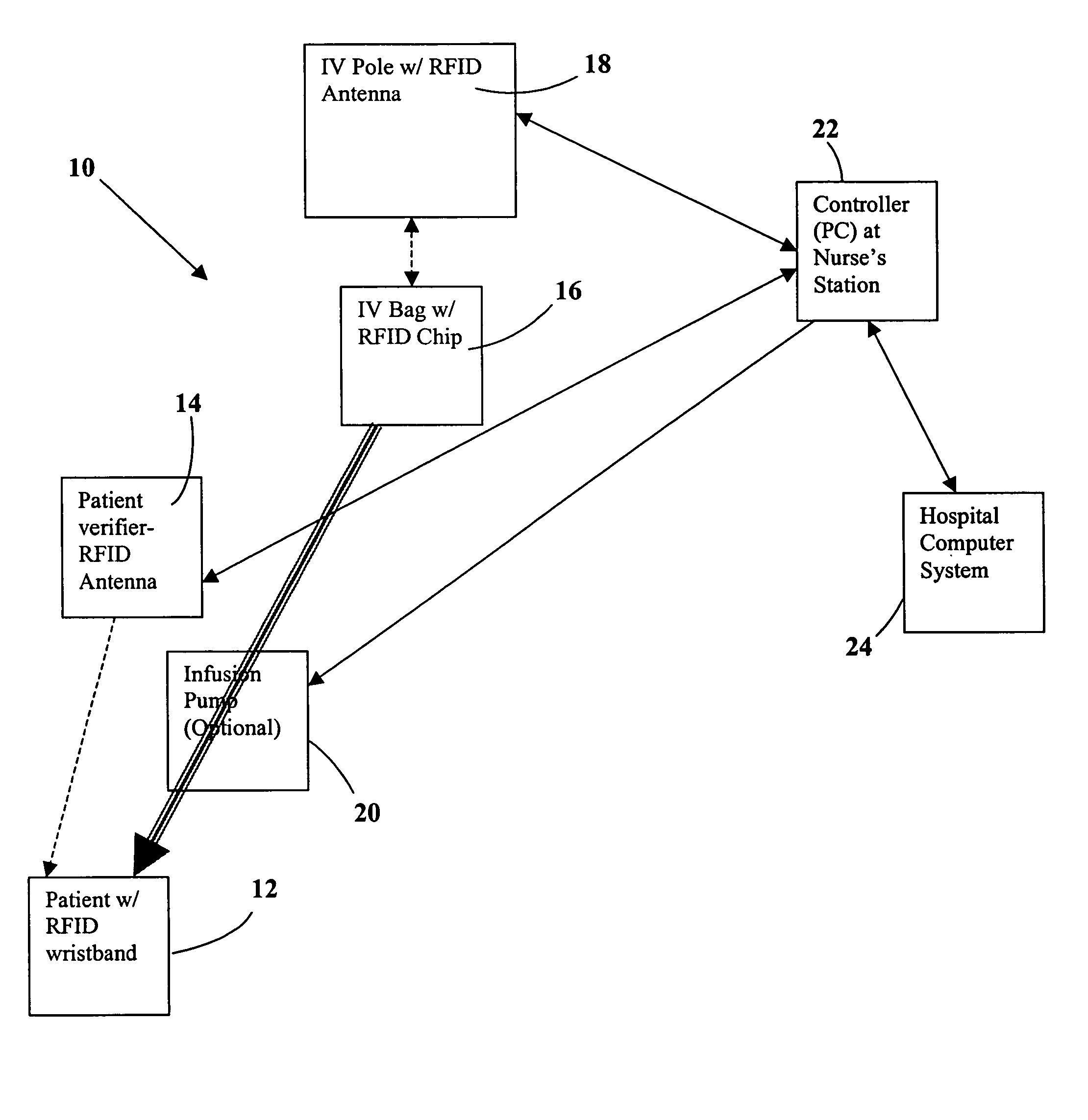
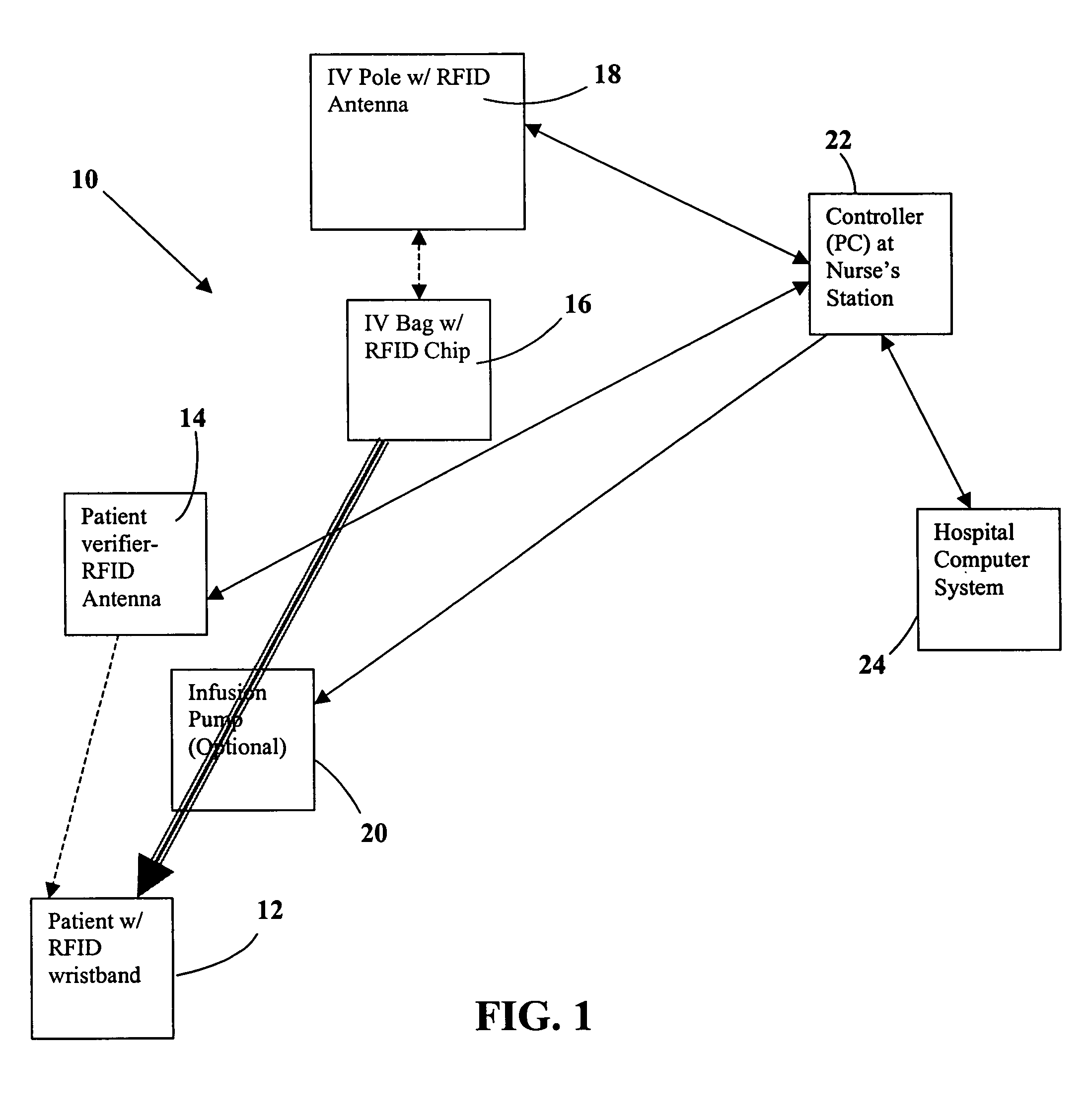
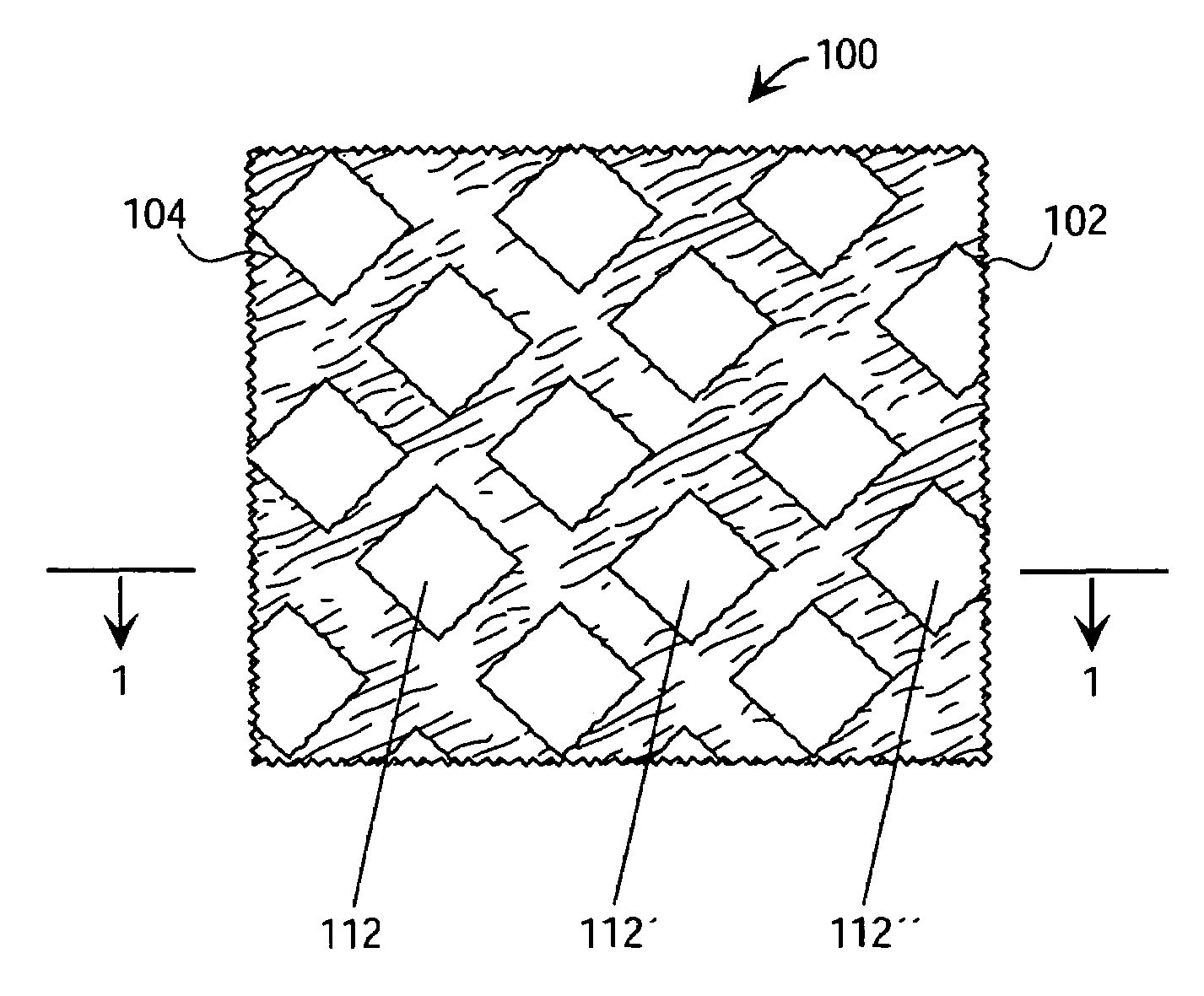
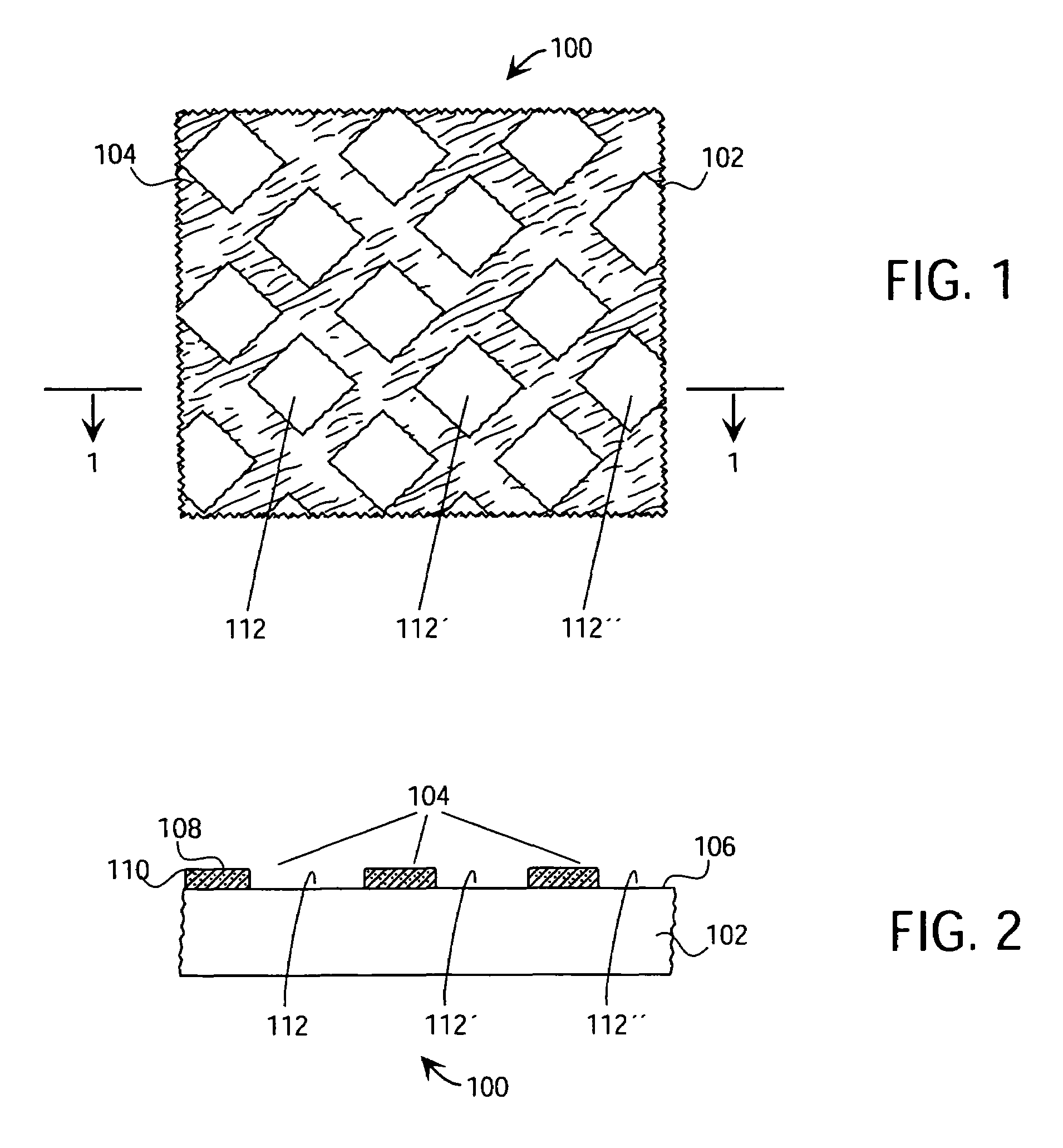
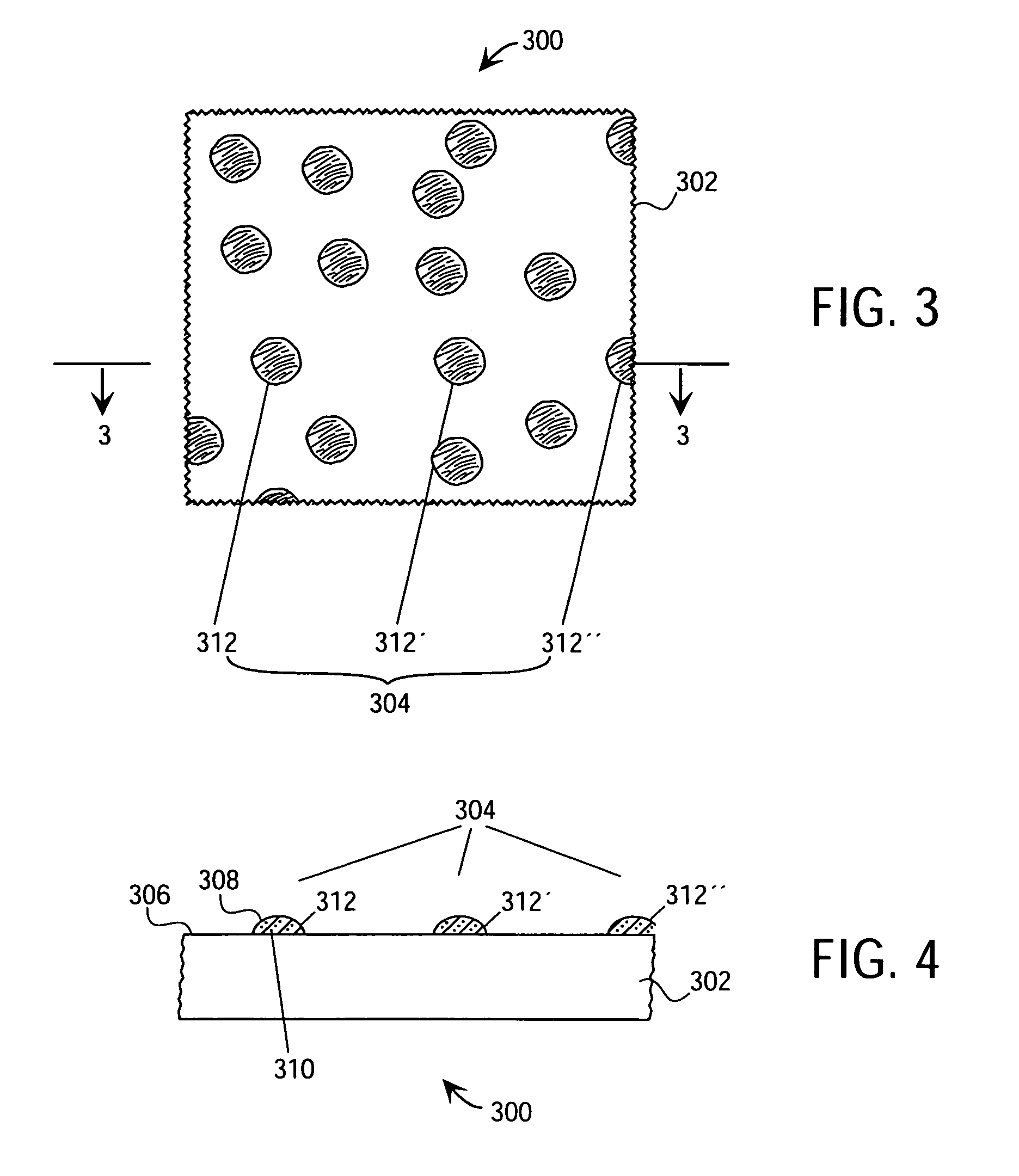
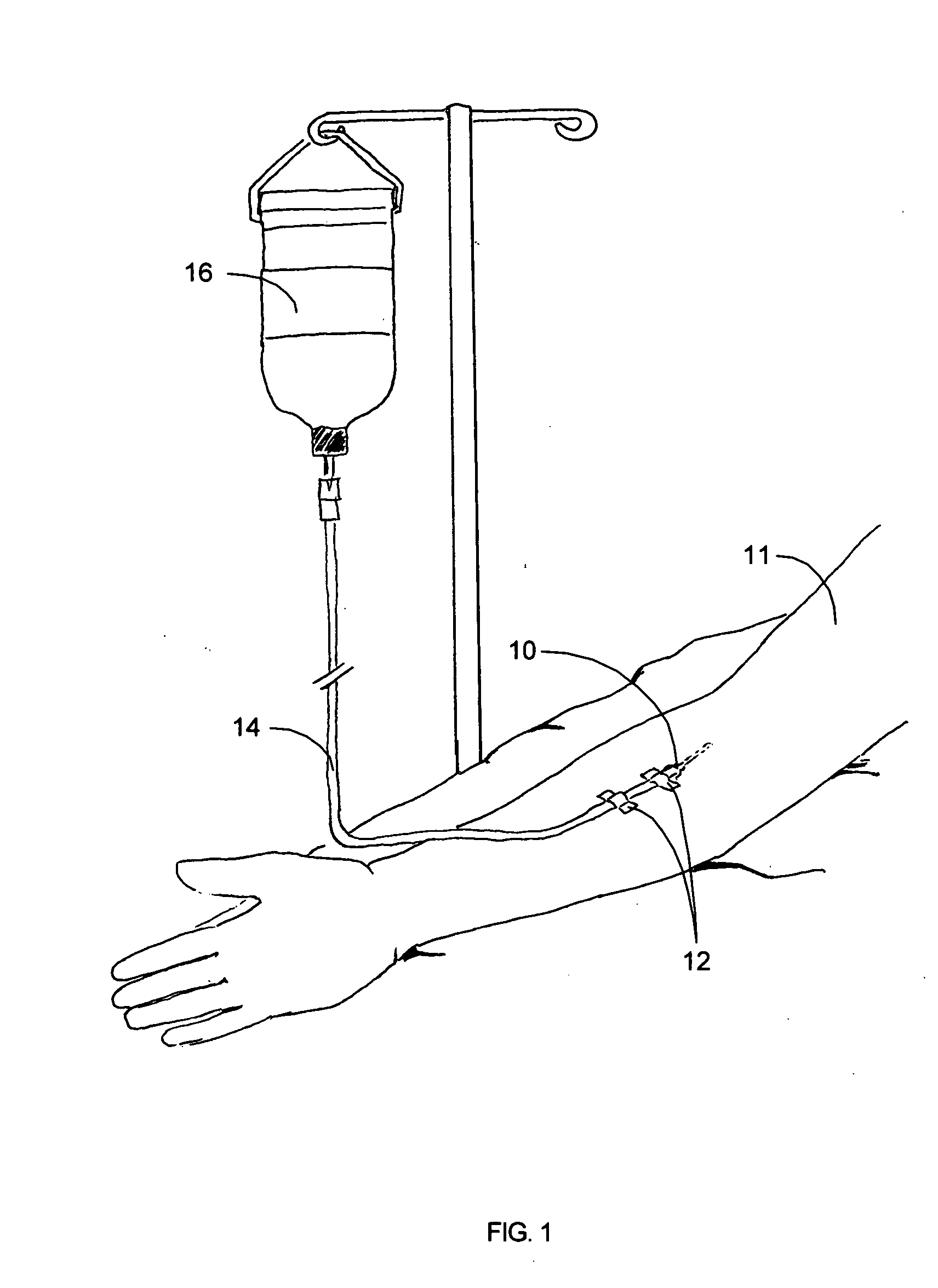
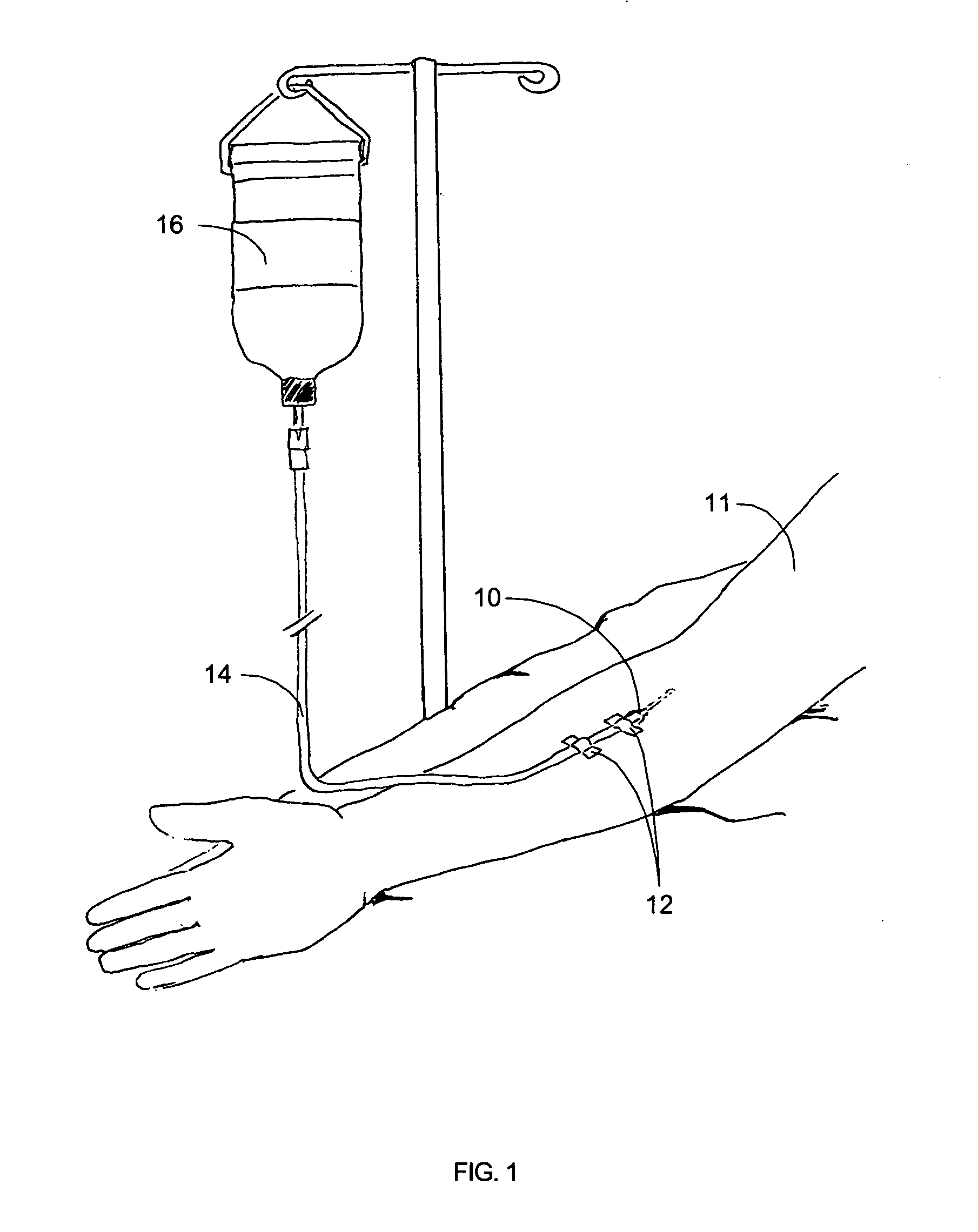
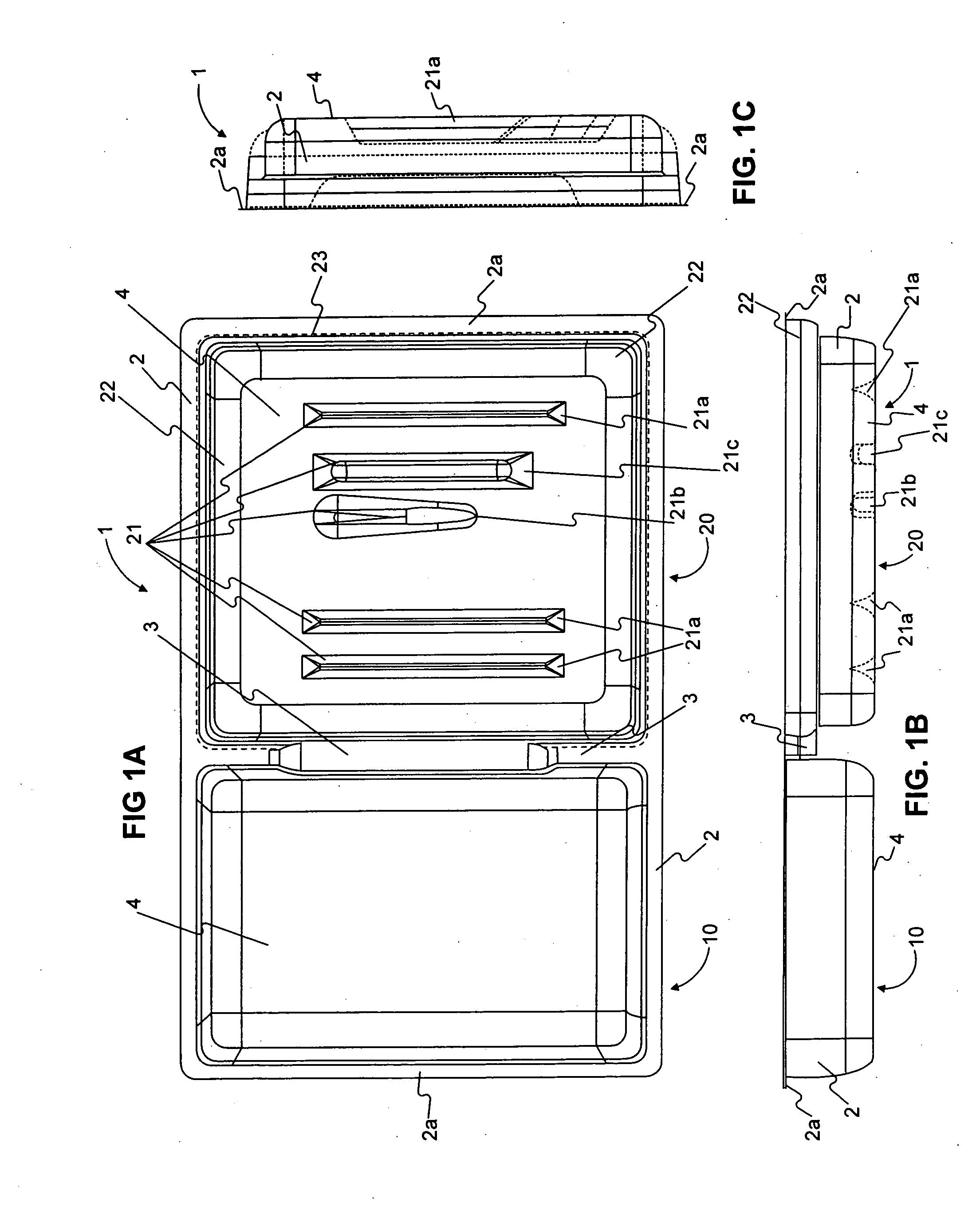
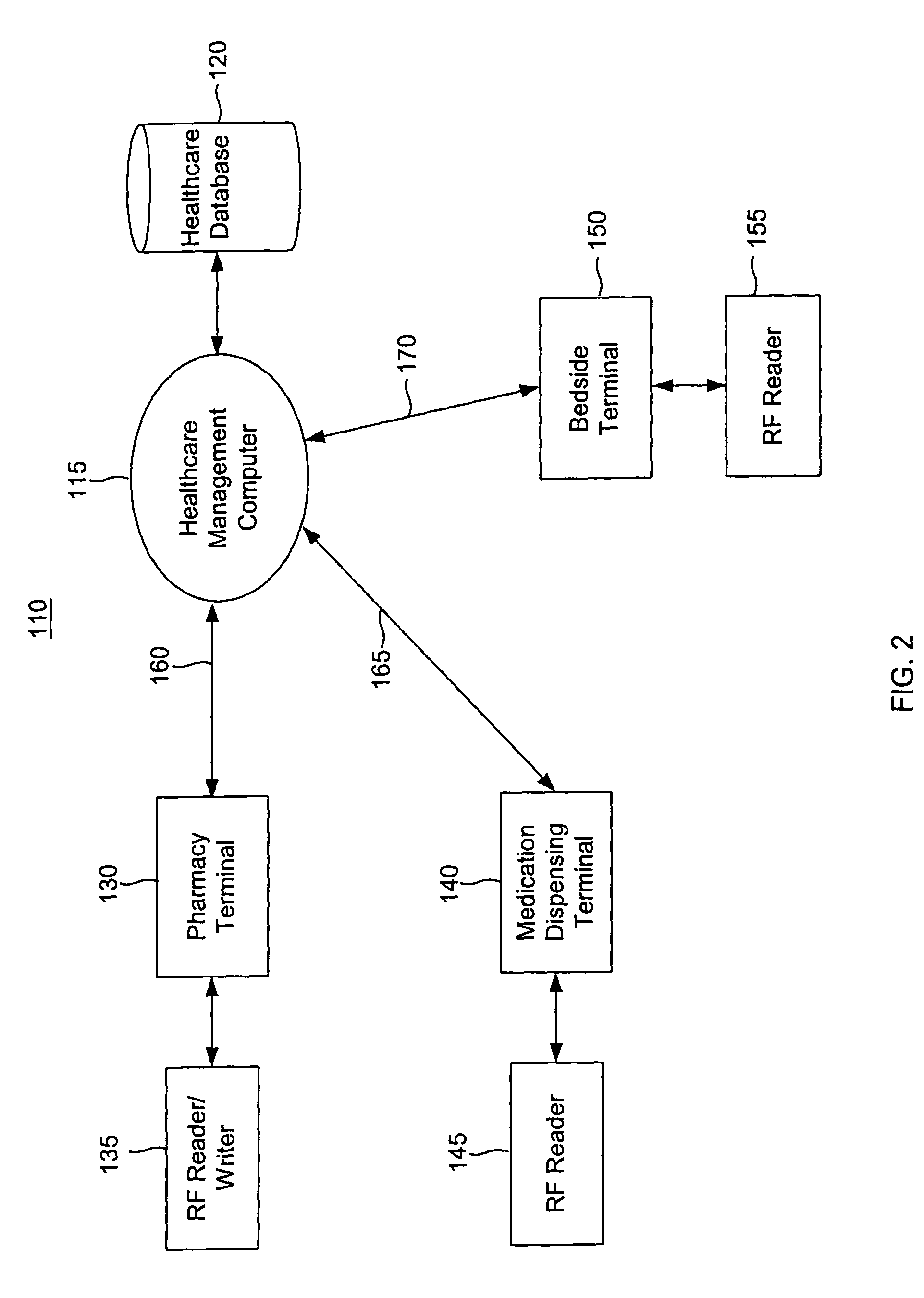
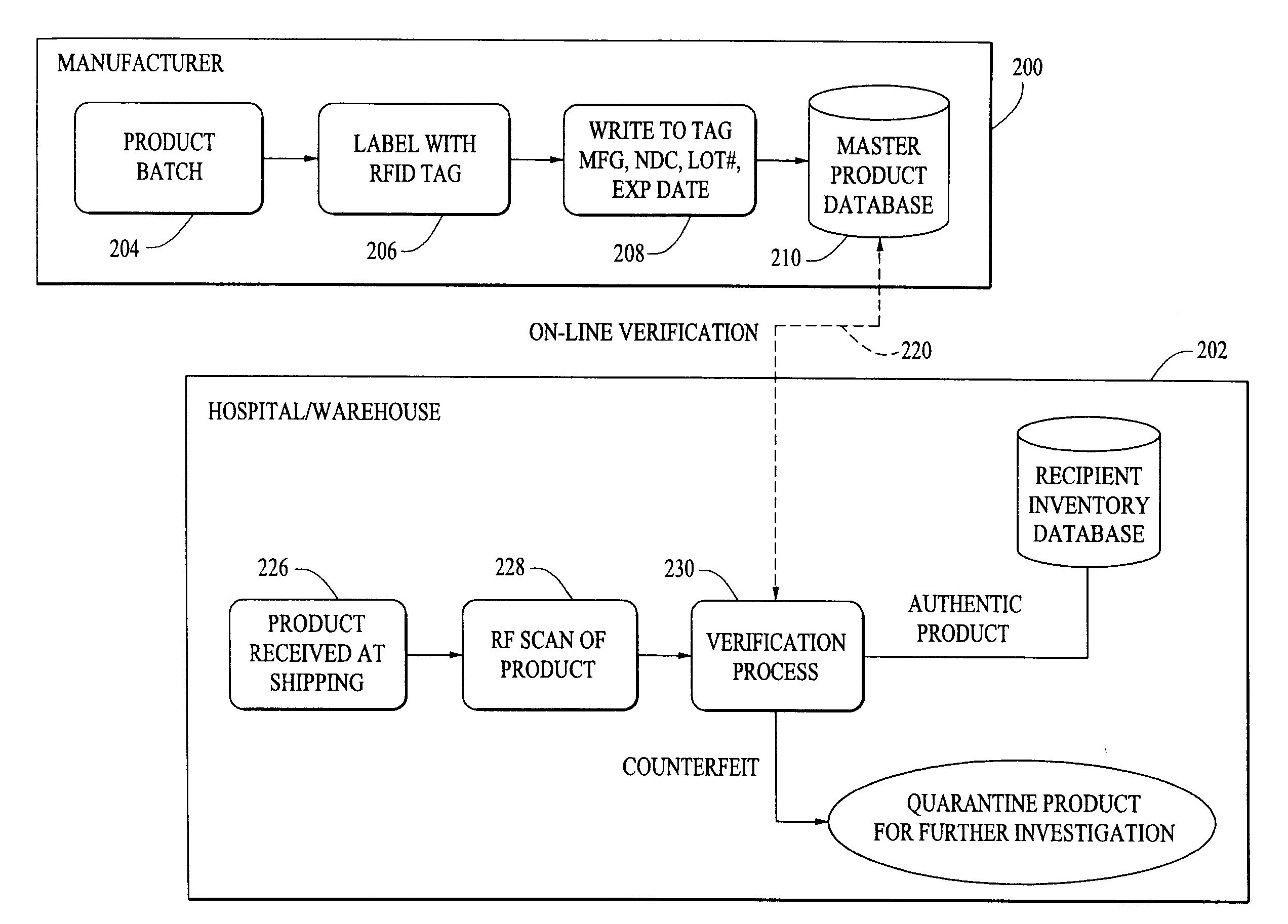
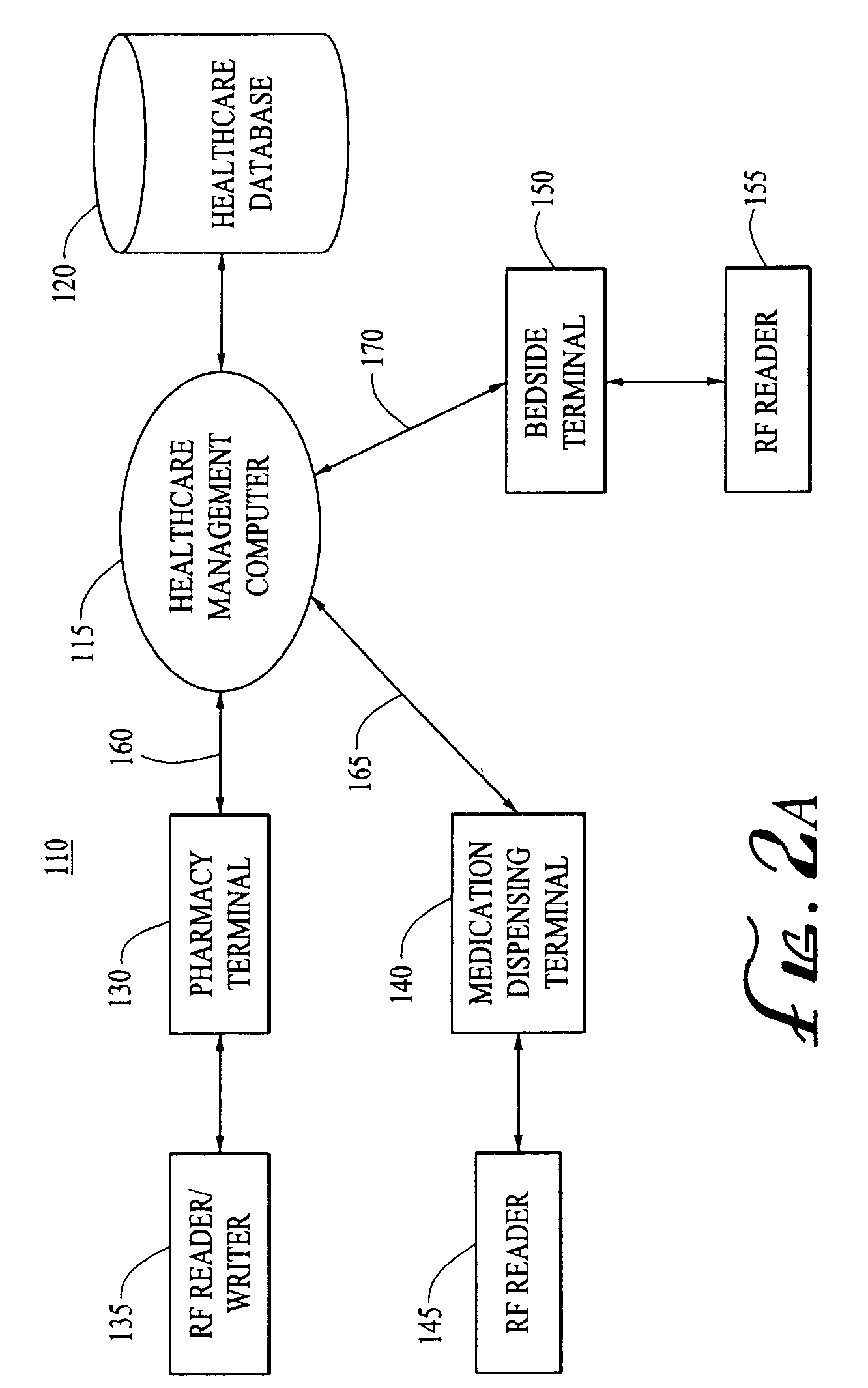
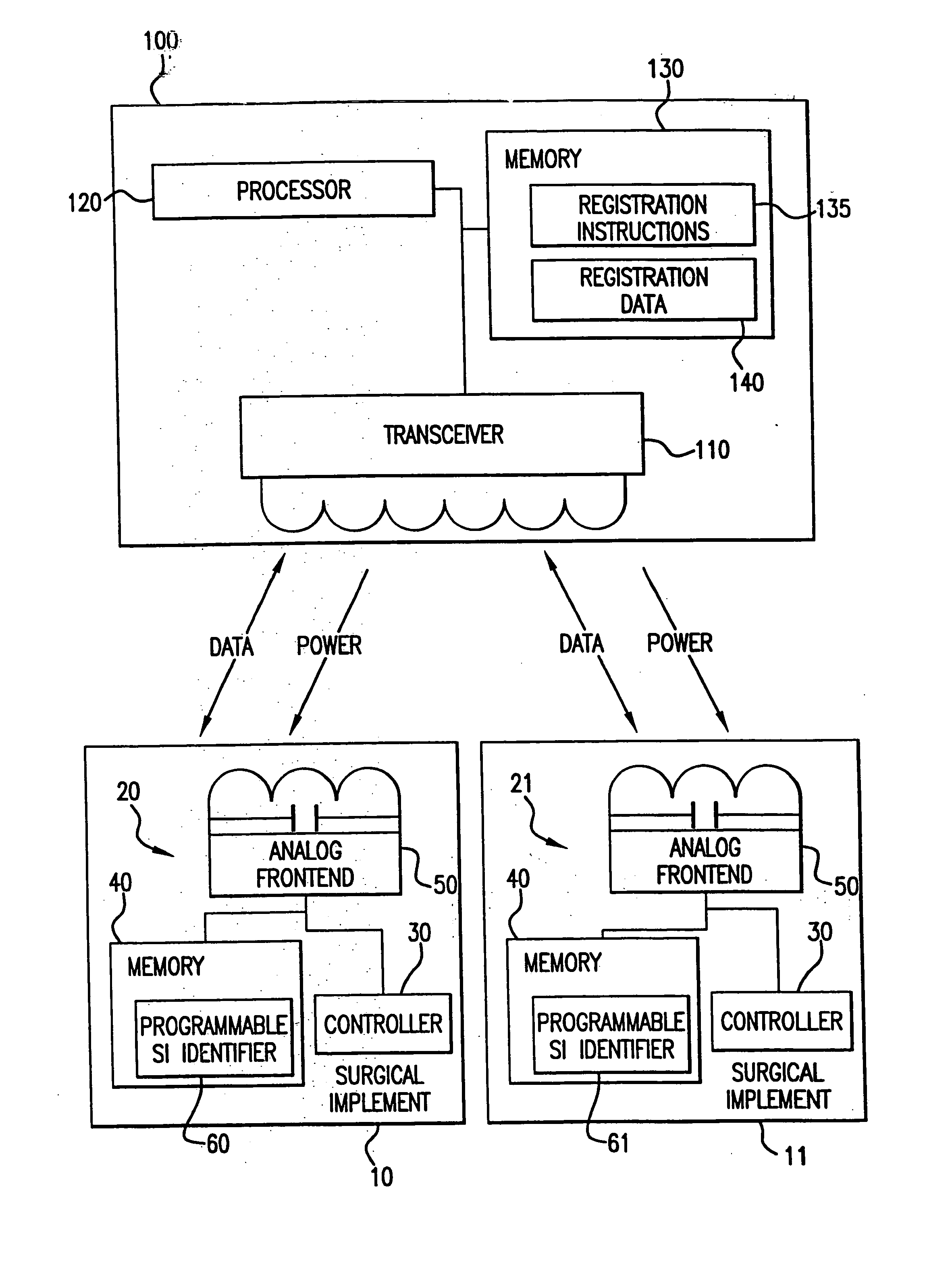
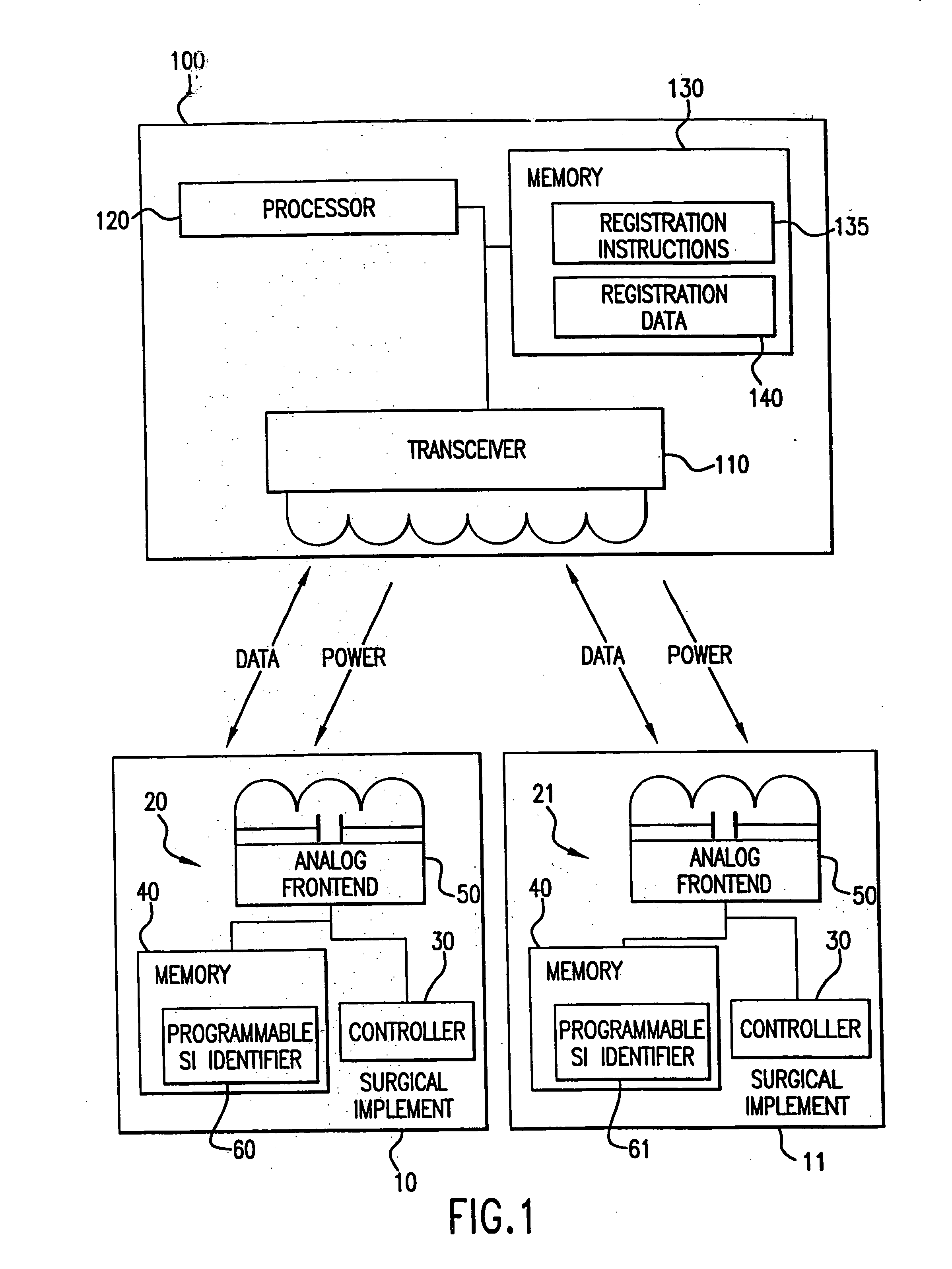
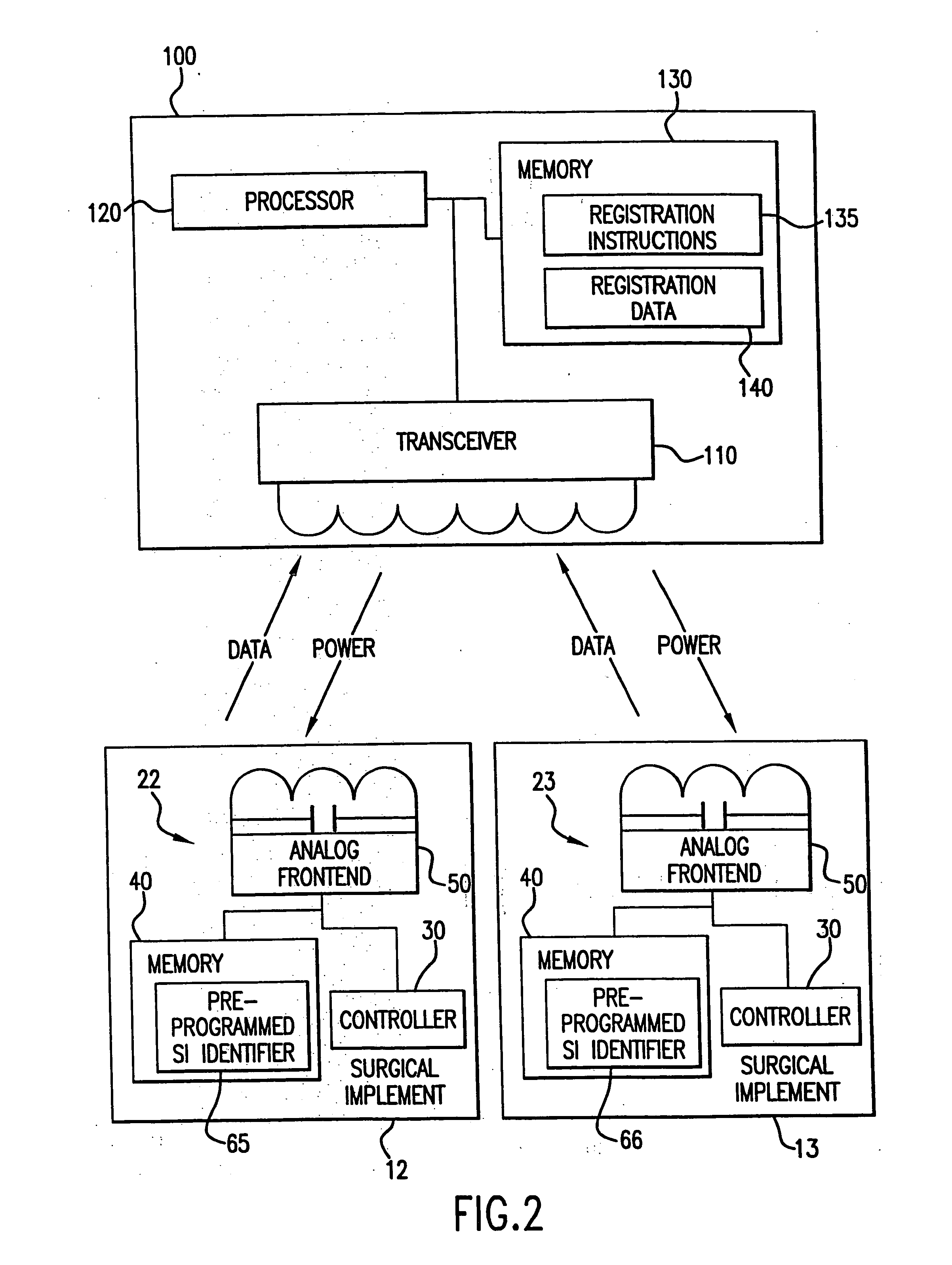
Tracking medical products with integrated circuits
A system and method of tracking medical products provides for associating a group of medical products with a group location based on a group radio frequency identification (RF ID) device signal, where the group includes a first unit and a second unit. The first unit is associated with a first remote location based on a first unit RF ID device signal. The method further provides for associating the second unit with a second remote location based on a second remote location based on a second unit RF ID device signal. The signals uniquely identify the units and the group.
Owner:OLIVE SHADE LLC
Systems for dispensing medical products
InactiveUS7006893B2Safe and convenientPreserving confidentialityControlling coin-freed apparatusDrug and medicationsCommunications systemMedical product
Owner:ARXIUM INC
Pharmaceutical tracking
InactiveUS7175081B2Easy to trackDrug and medicationsDigital data processing detailsMedical productRadio frequency
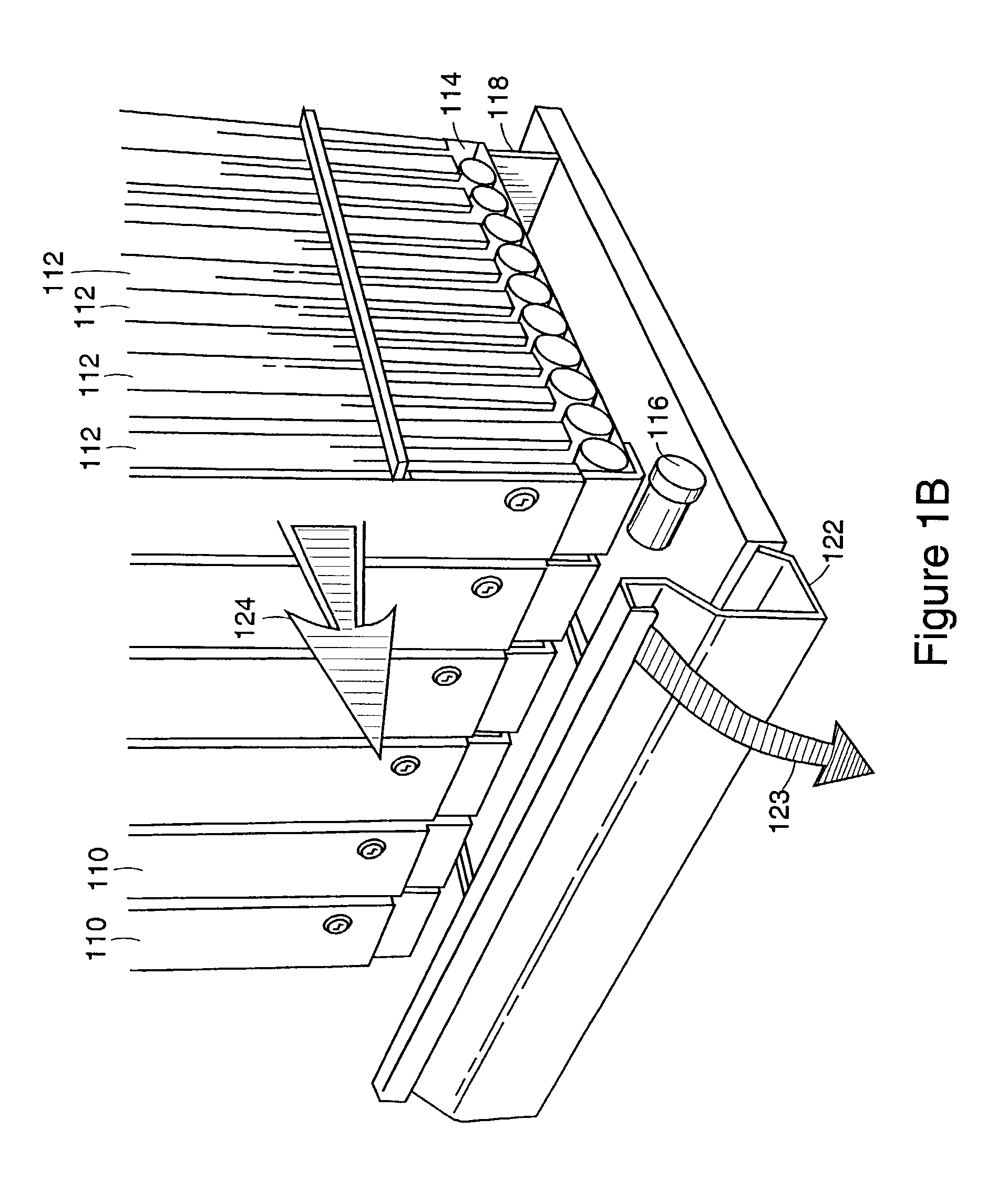
A medication dispensing unit is provided for tracking medical products having a Radio Frequency Identification (RFID) tag uniquely associated therewith. The dispensing unit includes compartments for receiving medical products therein, and readers for reading the RFID tags associated with the medical products in the compartments. A processor is coupled to the readers for receiving and processing readings of the RFID tags in the compartment to identify the medical products in the compartments. The processor may identify a medical product removed from a compartment by determining a difference between readings of the RFID tags in the compartment taken before and after the medical product is removed from the compartment. The processor may verify that the medical product removed from the compartment is authorized to be removed or confirmed that an identified patient is intended to receive the medical product being removed from the compartment. A system and method for counterfeit prevention is disclose, as is specimen, blood, organ and the like, tracking.
Owner:MEPS REAL TIME
Systems and methods for tracking pharmaceuticals within a facility
InactiveUS6935560B2Digital data processing detailsDrug and medicationsMedical productRadio frequency
A medication-dispensing unit is provided for tracking medical products having a Radio Frequency Identification (RFID) tag uniquely associated therewith. The dispensing unit includes compartments for receiving medical products therein, and readers for reading the RFID tags associated with the medical products in the compartments. A processor is coupled to the readers for receiving and processing readings of the RFID tags in the compartment to identify the medical products in the compartments. The processor may identify a medical product removed from a compartment by determining a difference between readings of the RFID tags in the compartment taken before and after the medical product is removed from the compartment. The processor may verify that the medical product removed from the compartment is authorized to be removed or confirm that an identified patient is intended to receive the medical product being removed from the compartment.
Owner:MEPS REAL TIME
System and method for monitored administration of medical products to patients
A system and method for monitored administration of medical products to patients. In one implementation, the system includes: an RFID tag article disposable on or in proximity to a patient, including a first RFID tag containing first information relevant to administration of medical product to the patient; a medical product labeled with a second RFID tag containing second information relevant to administration of the medical product to the patient; a reader arranged to receive signals from at least one of the first and second RFIDs; and a controller operatively coupleable in communication with the reader to process information received by the reader and to responsively generate an output relating to treatment of the patient.
Owner:ADVANCED TECH MATERIALS INC
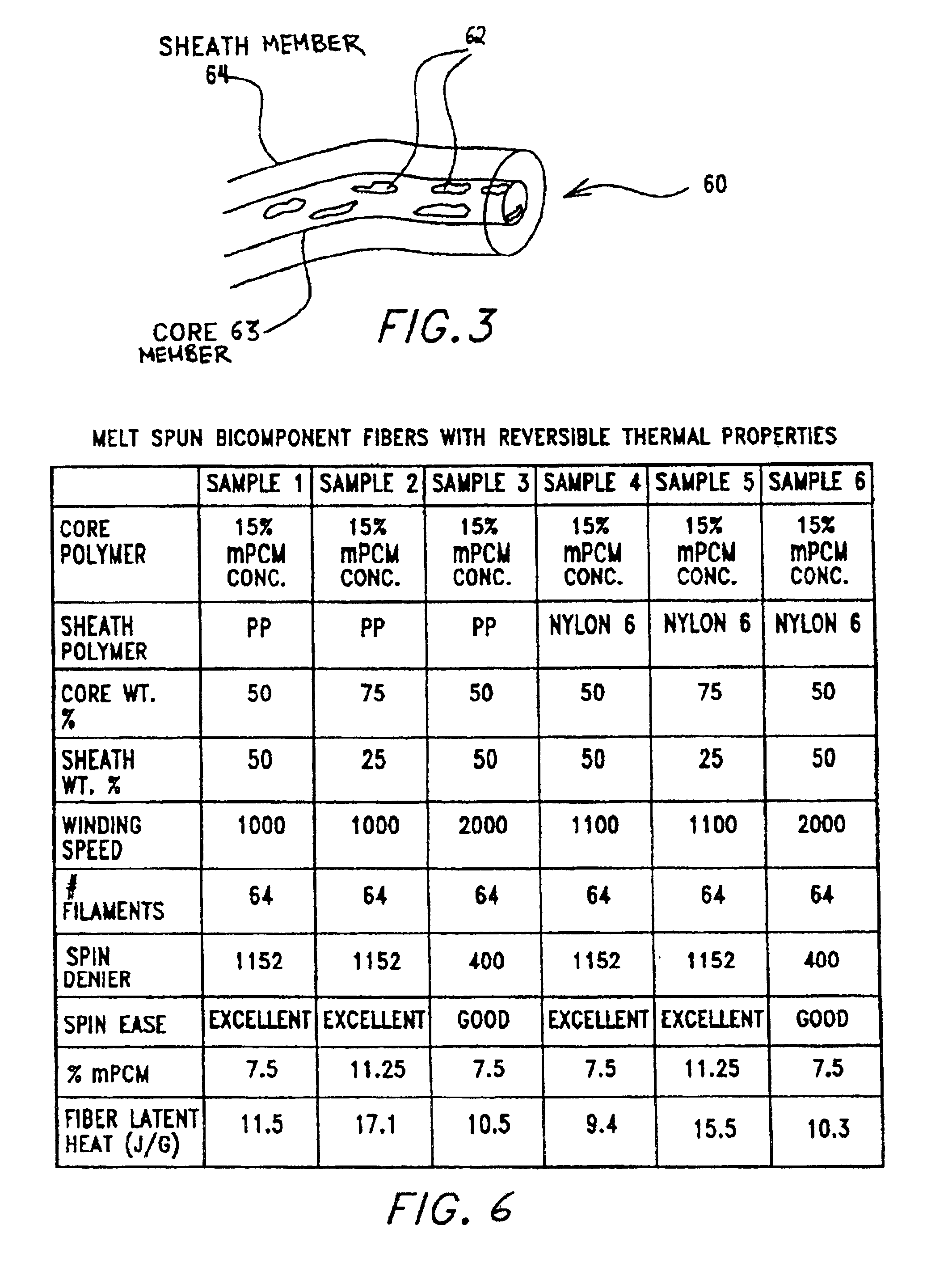
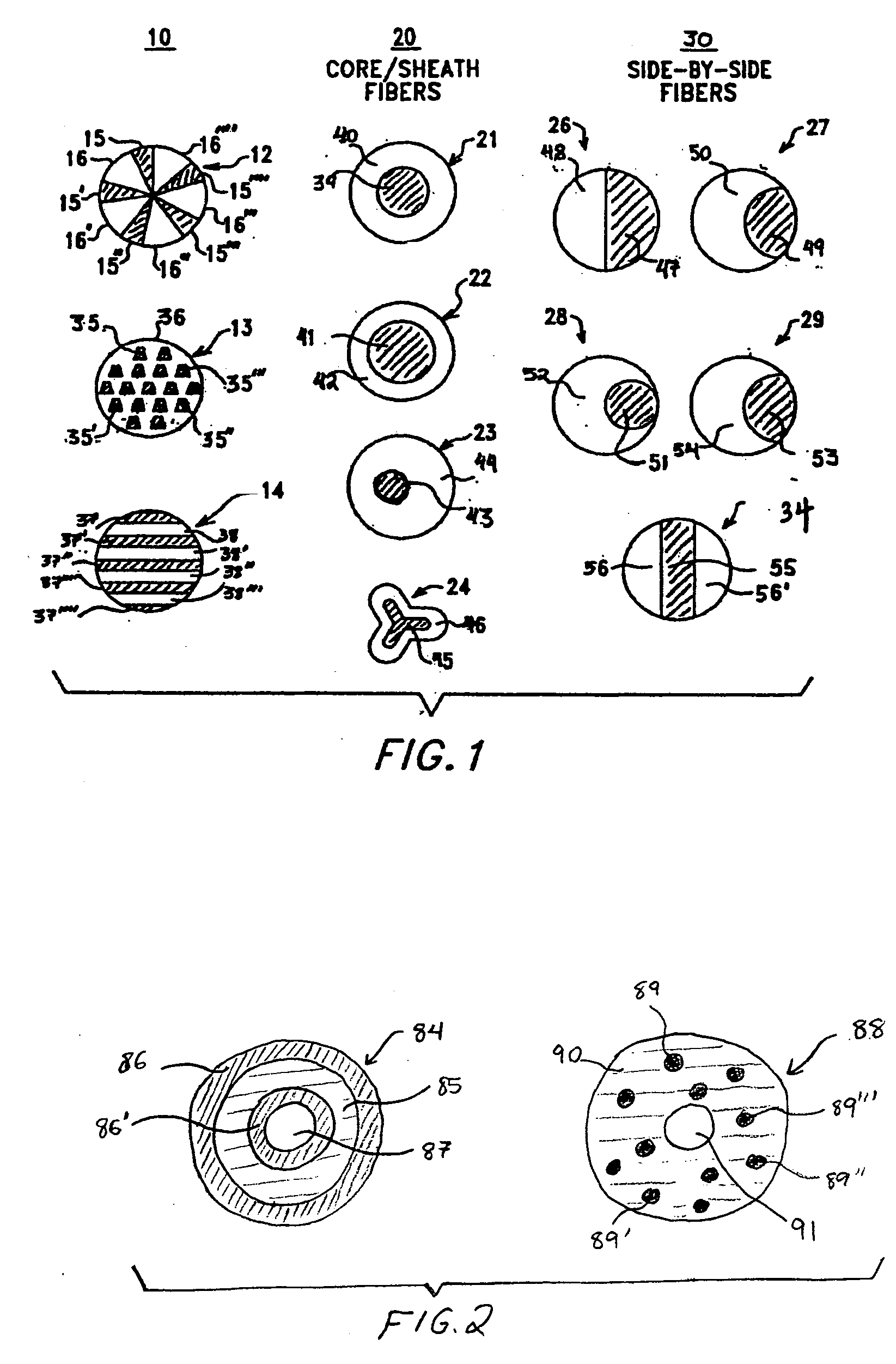
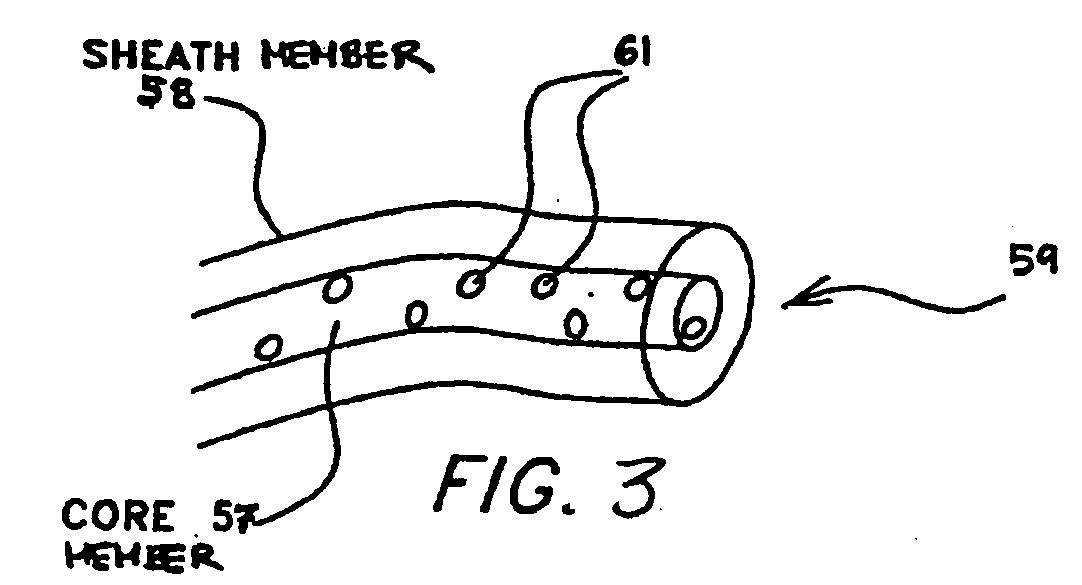
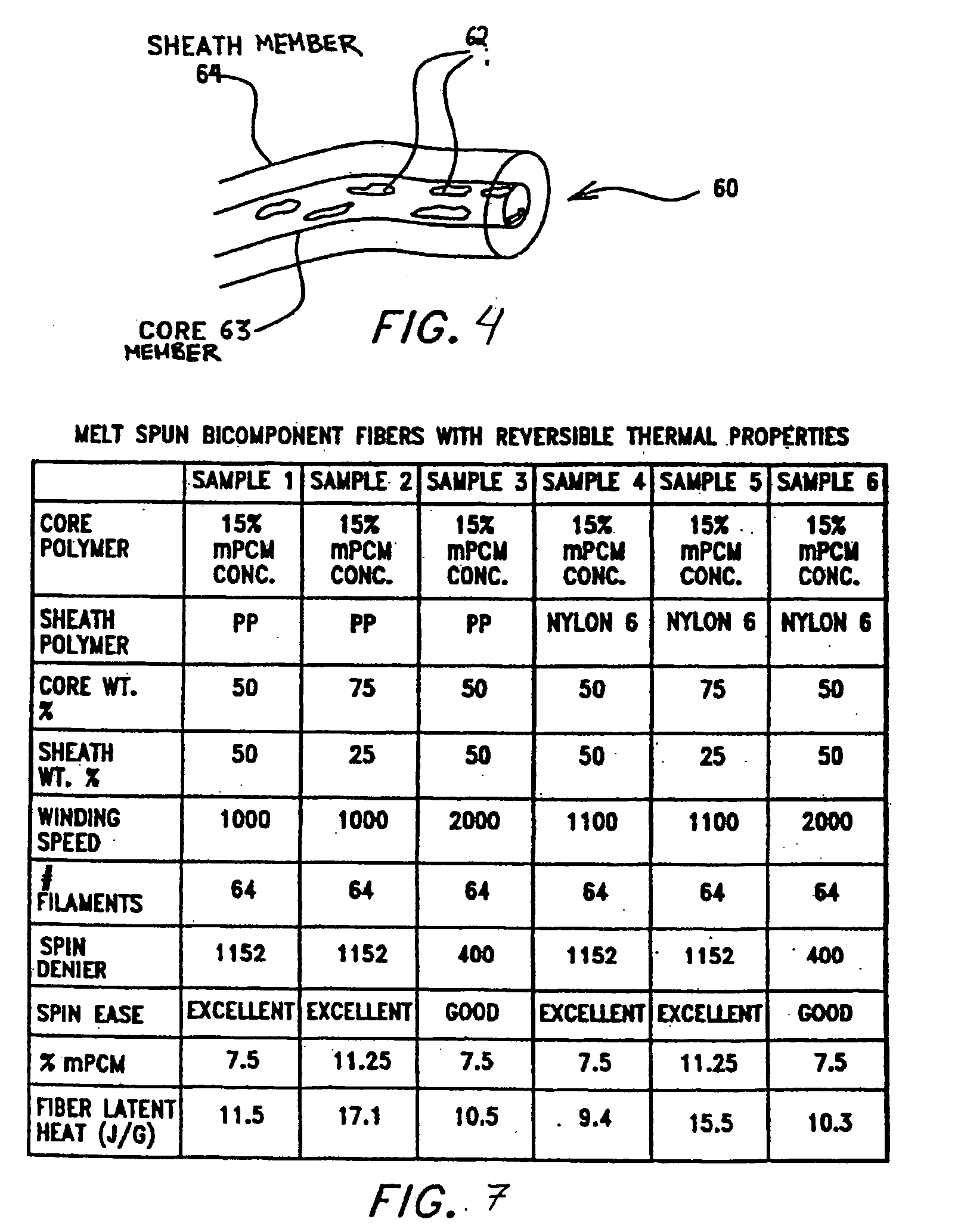
Multi-component fibers having enhanced reversible thermal properties and methods of manufacturing thereof
InactiveUS6855422B2Easy to processImprove propertiesHeat storage plantsWarp knittingMedical productPhase-change material
The invention relates to a multi-component fiber having enhanced reversible thermal properties and methods of manufacturing thereof. The multi-component fiber comprises a fiber body formed from a plurality of elongated members, at least one of the elongated members comprising a temperature regulating material dispersed therein. The temperature regulating material comprises a phase change material. The multi-component fiber may be formed via a melt spinning process or a solution spinning process and may be used or incorporated in various products where a thermal regulating property is desired. For example, the multi-component fiber may be used in textiles, apparel, footwear, medical products, containers and packagings, buildings, appliances, and other products.
Owner:HILLS CO
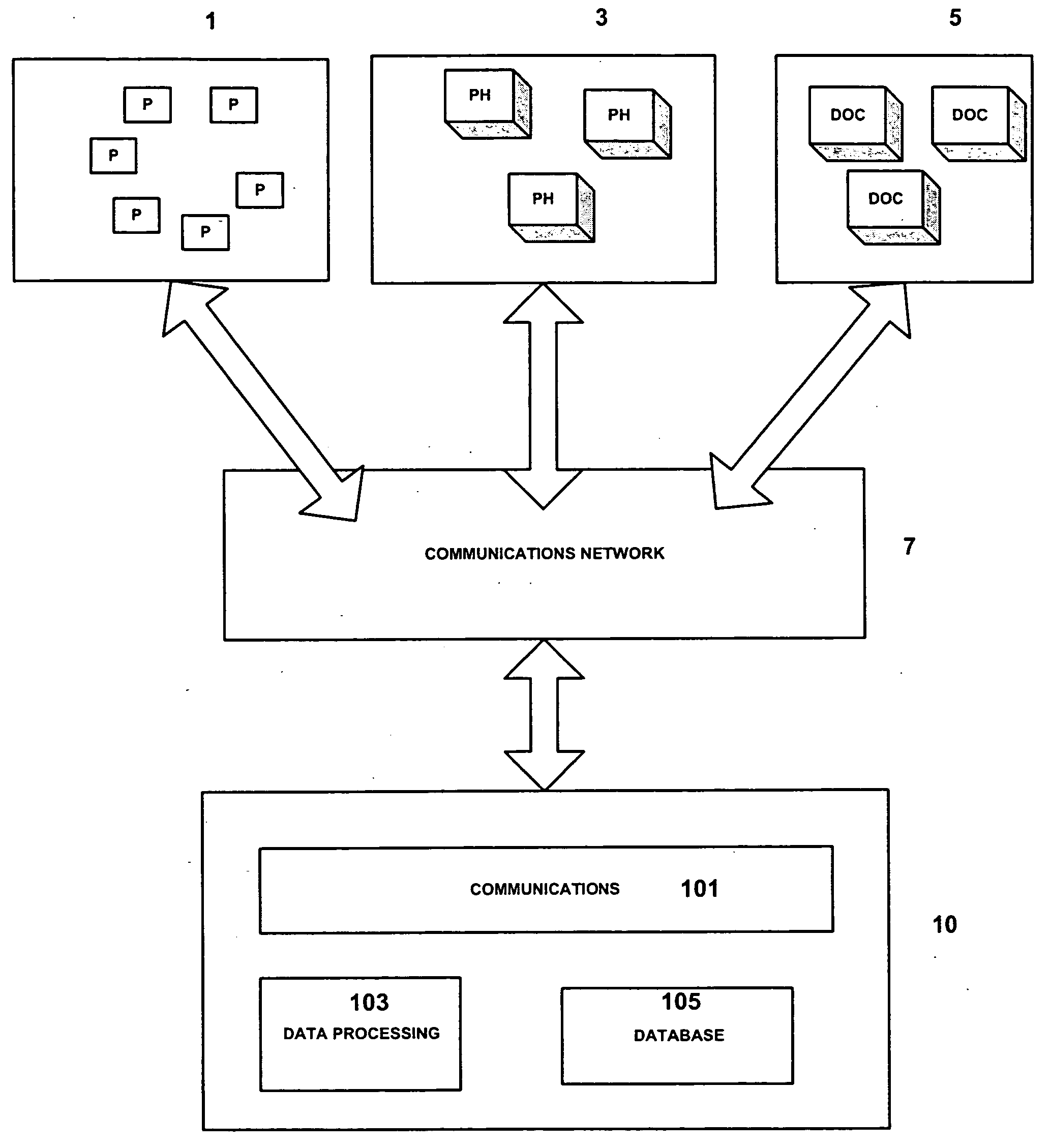
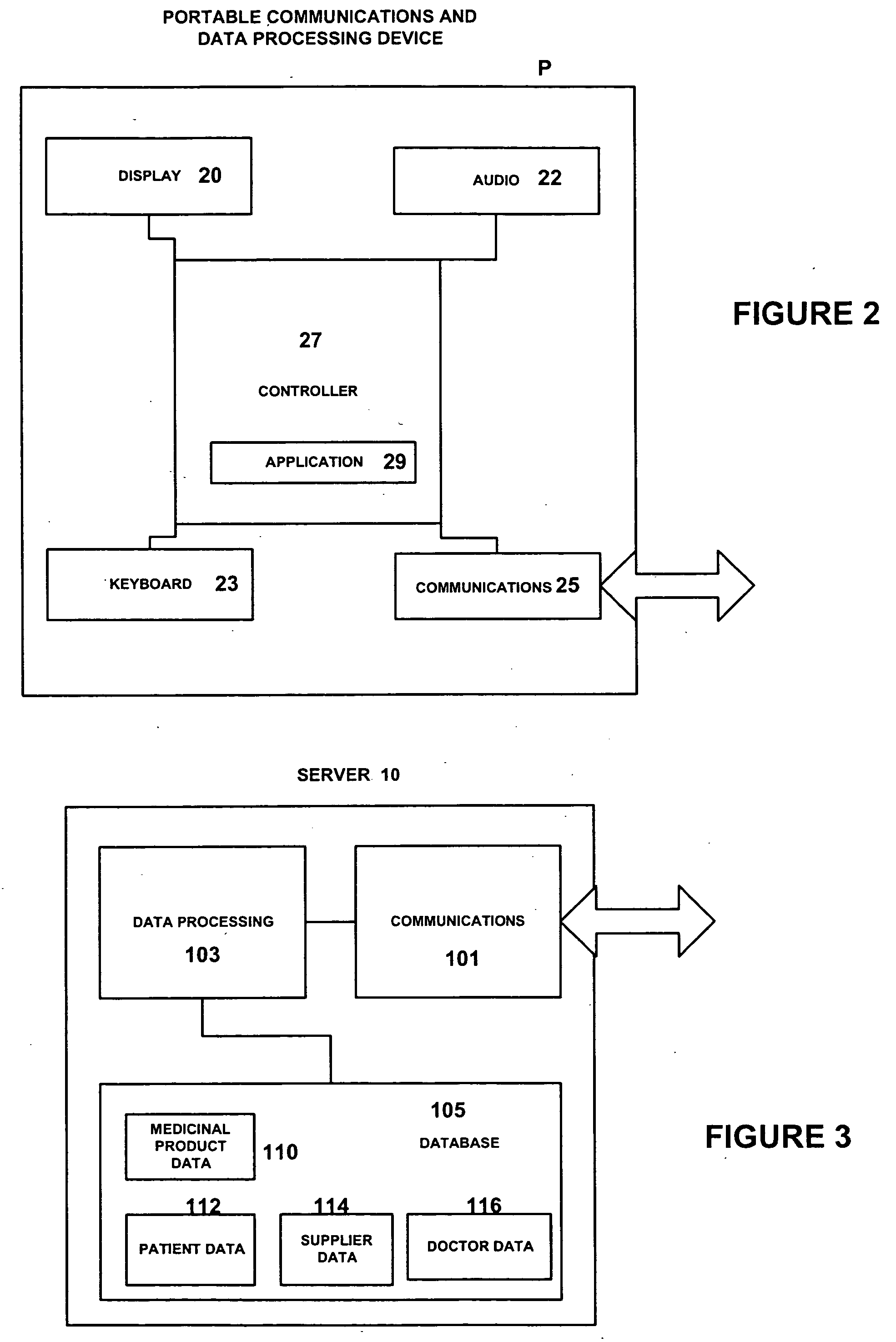
Medicinal product order processing system
InactiveUS20060136266A1Improve monitoring qualityProcess is repeatedDrug and medicationsPatient personal data managementRepeated prescriptionRegimen
A repeat prescription ordering system for allowing patients requiring resupply of medication or medical products to access a server using a portable communications and data processing device such as a smart phone or personal digital assistant. The server supplies to the patient a list of medication and medical products which they are authorized to order. The patient can select the products required, and the order is logged by the server and allocated to a supplier for completion of the order. The server maintains an estimate of the amount of medication or medical product held by the patient, this being based on the prescribed dosage regimen and information entered by the patient on their usage and, optionally, on checks on their own health. The patient may be alerted when the estimate indicates that their supplies are running low. The estimate is allowed to go below zero, this implying a possible departure from the prescribed medication regimen.
Owner:E SAN LTD
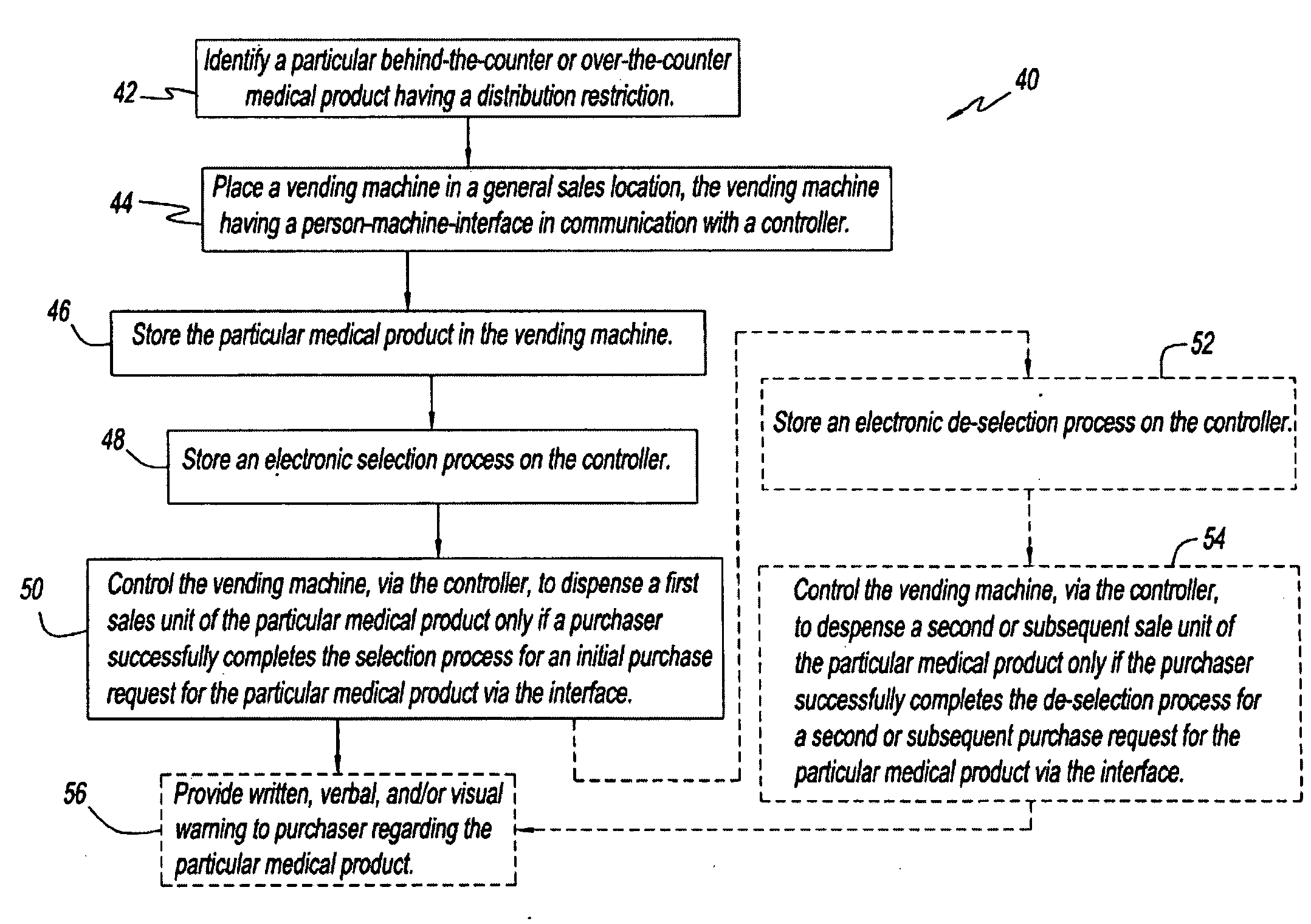
Coated articles having enhanced reversible thermal properties and exhibiting improved flexibility, softness, air permeability, or water vapor transport properties
InactiveUS7135424B2Enhanced reversible thermal propertyImprove breathabilityHeat storage plantsDecorative surface effectsWater vaporMedical product
The invention relates to a coated article having enhanced reversible thermal properties. The coated article comprises a substrate having a surface and a coating covering a portion of the surface and comprising a polymeric material and a temperature regulating material dispersed in the polymeric material. The coating is formed with a plurality of regions of discontinuity that are separated from one another and expose a remaining portion of the surface to provide improved flexibility, softness, air permeability, or water vapor transport properties. The coated article may be used in apparel, footwear, medical products, containers and packagings, building materials, appliances, and other products.
Owner:OUTLAST TECH LLC
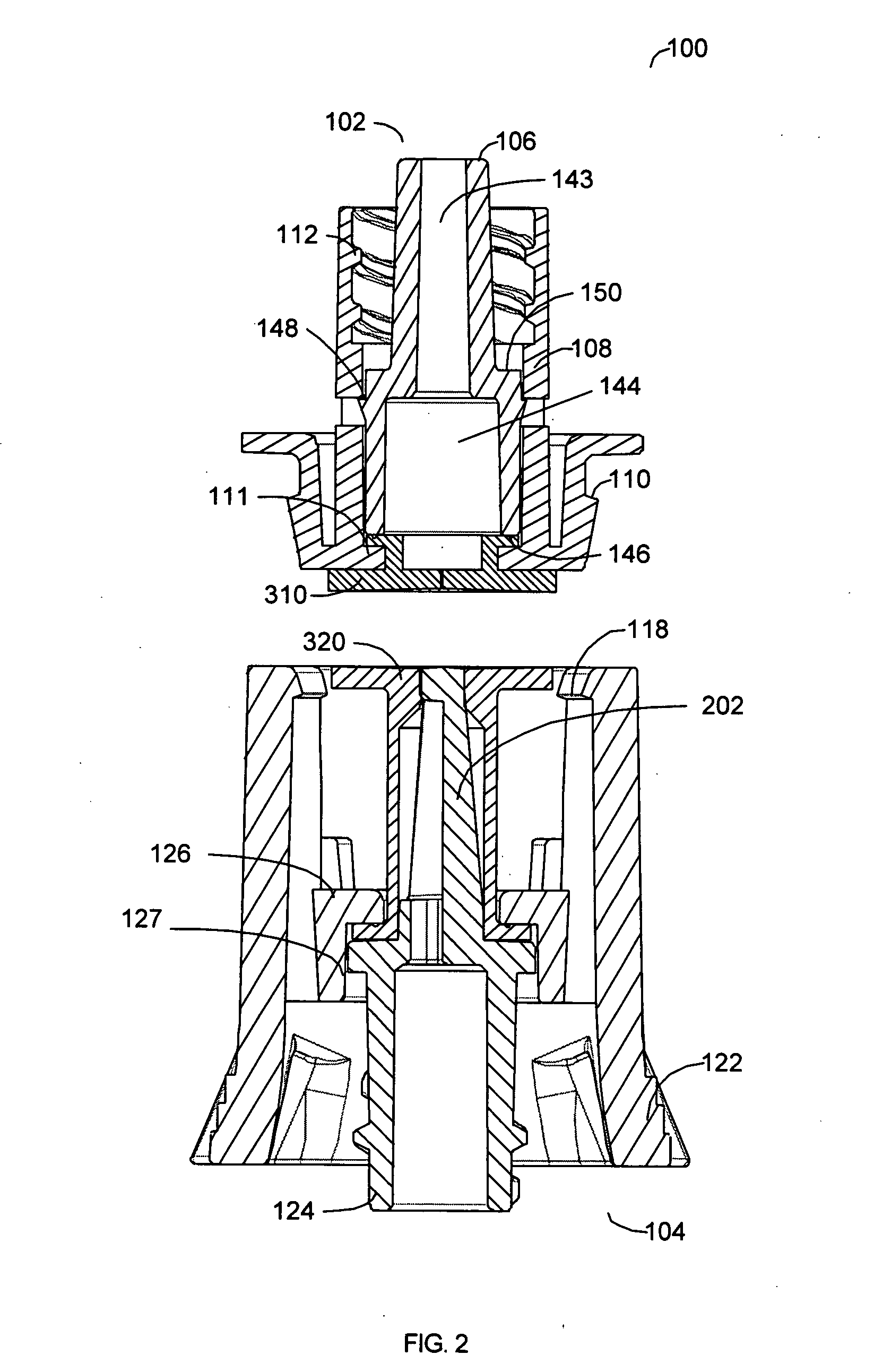
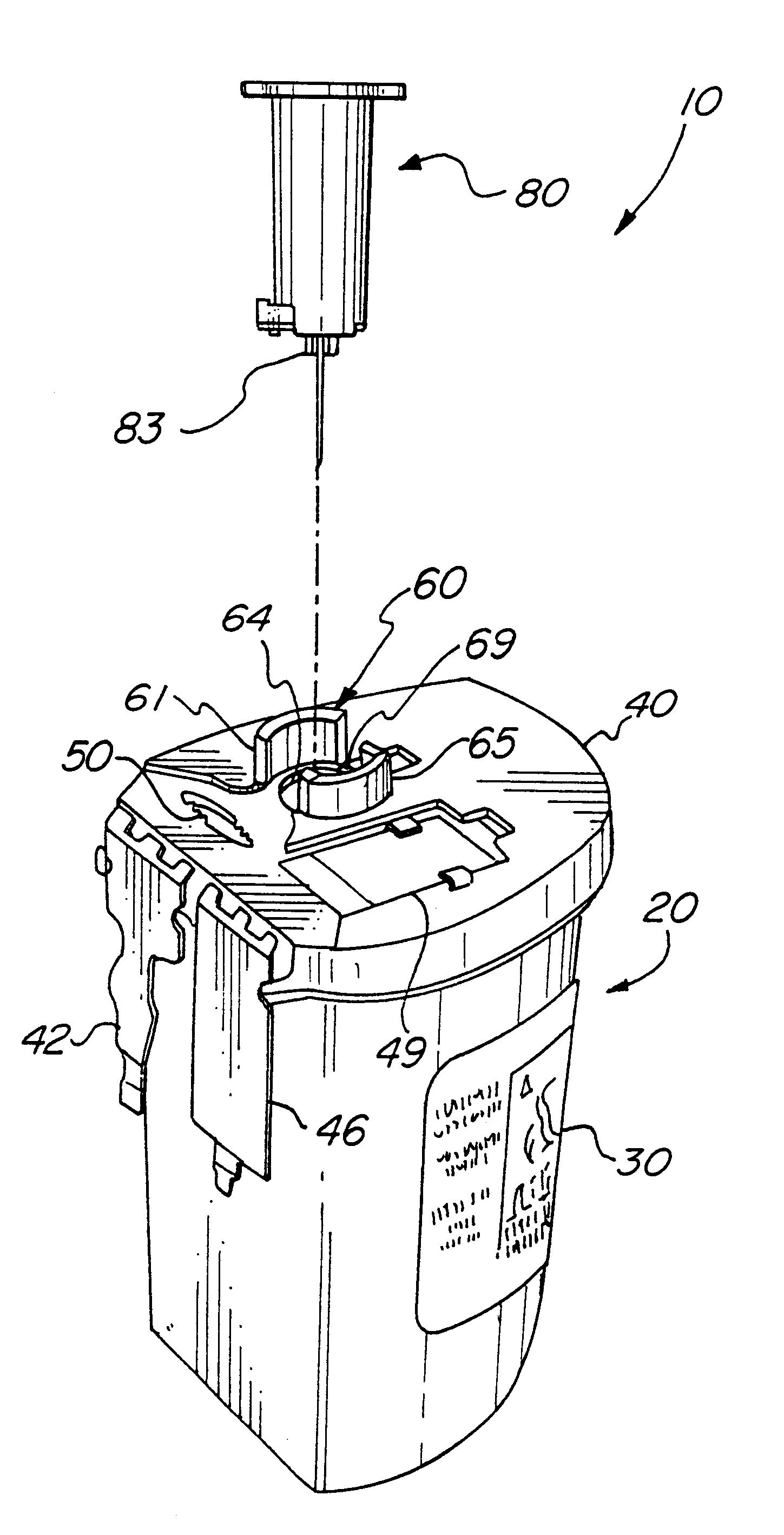
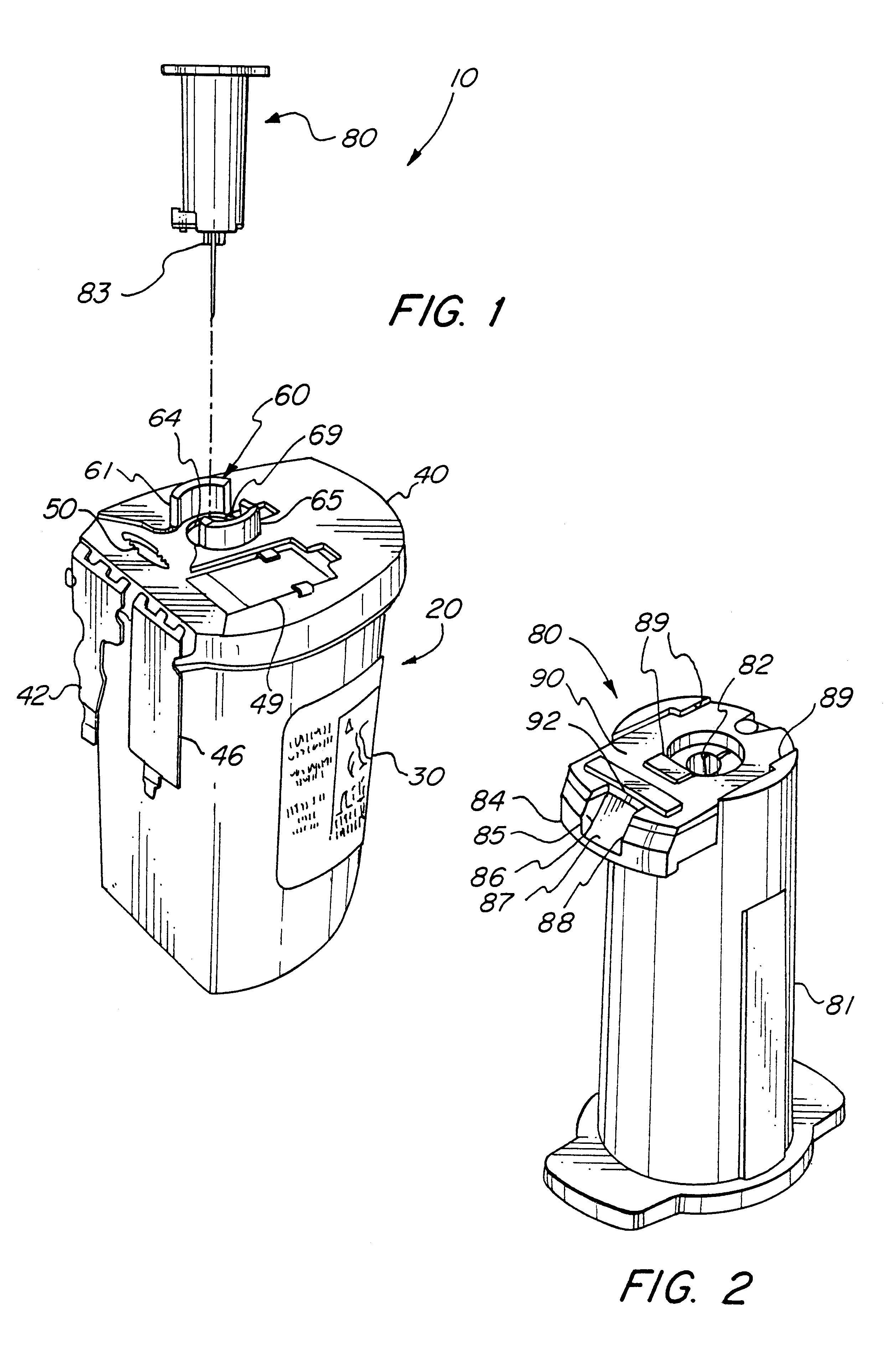
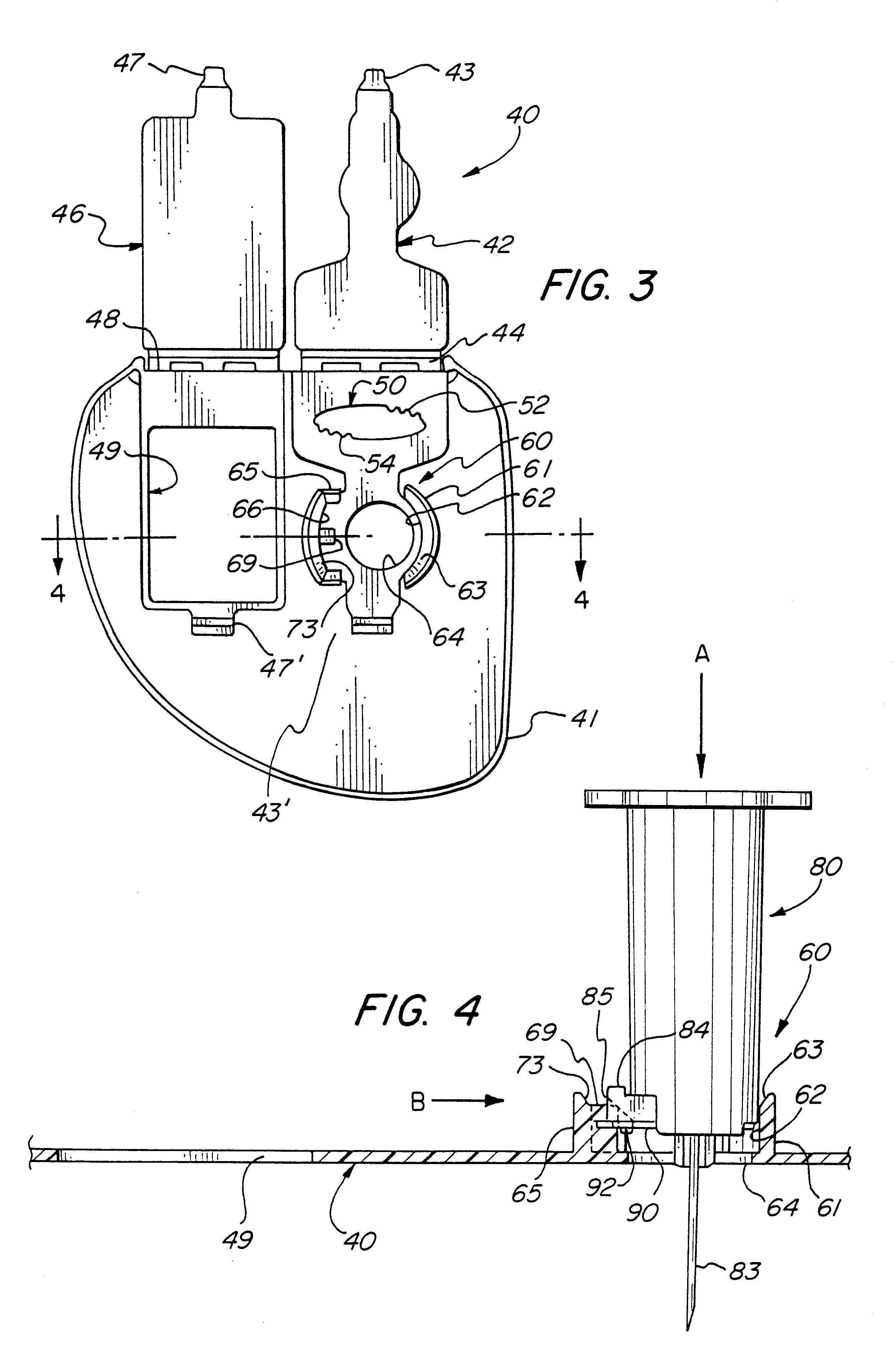
Reconnectable disconnect device for fluid transfer line
InactiveUS20060129109A1Avoid injuryEasy to disinfectIntravenous devicesValvesMedical productAxial force
An improved disconnect device suitable for use with an IV tube or other medical tubing device which can be either manually disconnected or automatically disconnected by the application of an axial force sufficiently low to prevent patient injury; which can be sterilely reattached after disconnection; which allows fluid flow in either direction; which shuts off fluid flow from both directions when disconnected; and which can be simply and inexpensively manufactured and assembled with techniques common to the injection molding, and medical products manufacturing industry.
Owner:SHAW SCOTT RANDALL +1
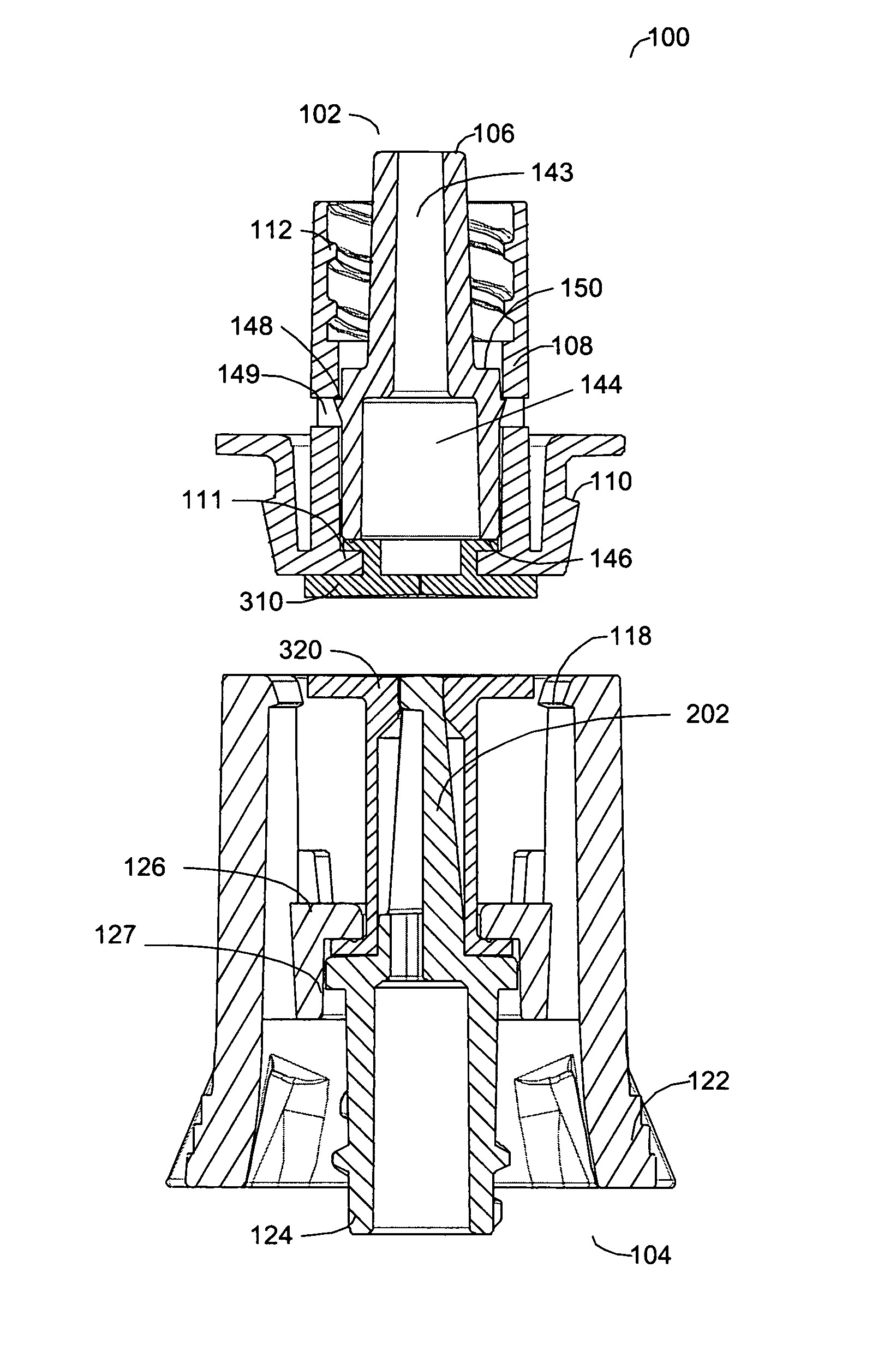
Reconnectable disconnect device for fluid transfer line
InactiveUS20050090805A1Simply and inexpensively manufacturedSimply and inexpensively and assembledCatheterTube connectorsMedical productAxial force
An improved disconnect device suitable for use with an IV tube or other medical tubing device which can be either manually disconnected or automatically disconnected by the application of an axial force sufficiently low to prevent patient injury; which can be sterilely reattached after disconnection; which allows fluid flow in either direction; which shuts off fluid flow from both directions when disconnected; and which can be simply and inexpensively manufactured and assembled with techniques common to the injection molding, and medical products manufacturing industry.
Owner:SHAW SCOTT RANDALL +1
Multi-component fibers having enhanced reversible thermal properties and methods of manufacturing thereof
Multi-component fibers having enhanced reversible thermal properties and methods of manufacturing thereof are described. In one embodiment, a multi-component fiber includes a fiber body formed from a set of elongated members, and at least one of the set of elongated members includes a temperature regulating material having a latent heat of at least 40 J / g and a transition temperature in the range of 22° C. to 40° C. The temperature regulating material provides thermal regulation based on at least one of absorption and release of the latent heat at the transition temperature. The multi-component fiber can be formed via a melt spinning process or a solution spinning process and can be used or incorporated in various products where a thermal regulating property is desired. For example, the multi-component fiber can be used in textiles, apparel, footwear, medical products, containers and packagings, buildings, appliances, and other products.
Owner:HILLS CO
Low-density, open-cell, soft, flexible, thermoplastic, absorbent foam and method of making foam
A soft, flexible, low-density, open-cell, thermoplastic, absorbent foam formed from a foam polymer formula including a balanced amount of a plasticizing agent and a surfactant in combination with a base resin. Thermoplastic elastomers can be added to the foam polymer formula to improve softness, flexibility, elasticity, and resiliency of the resulting foam. The surfactant may be either a single surfactant or a multi-surfactant system. The foam possesses a number of qualities, such as softness and strength, which render the foam particularly suitable for use in a variety of personal care products, medical products, and the like.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Packaging for a kit, and related methods of use
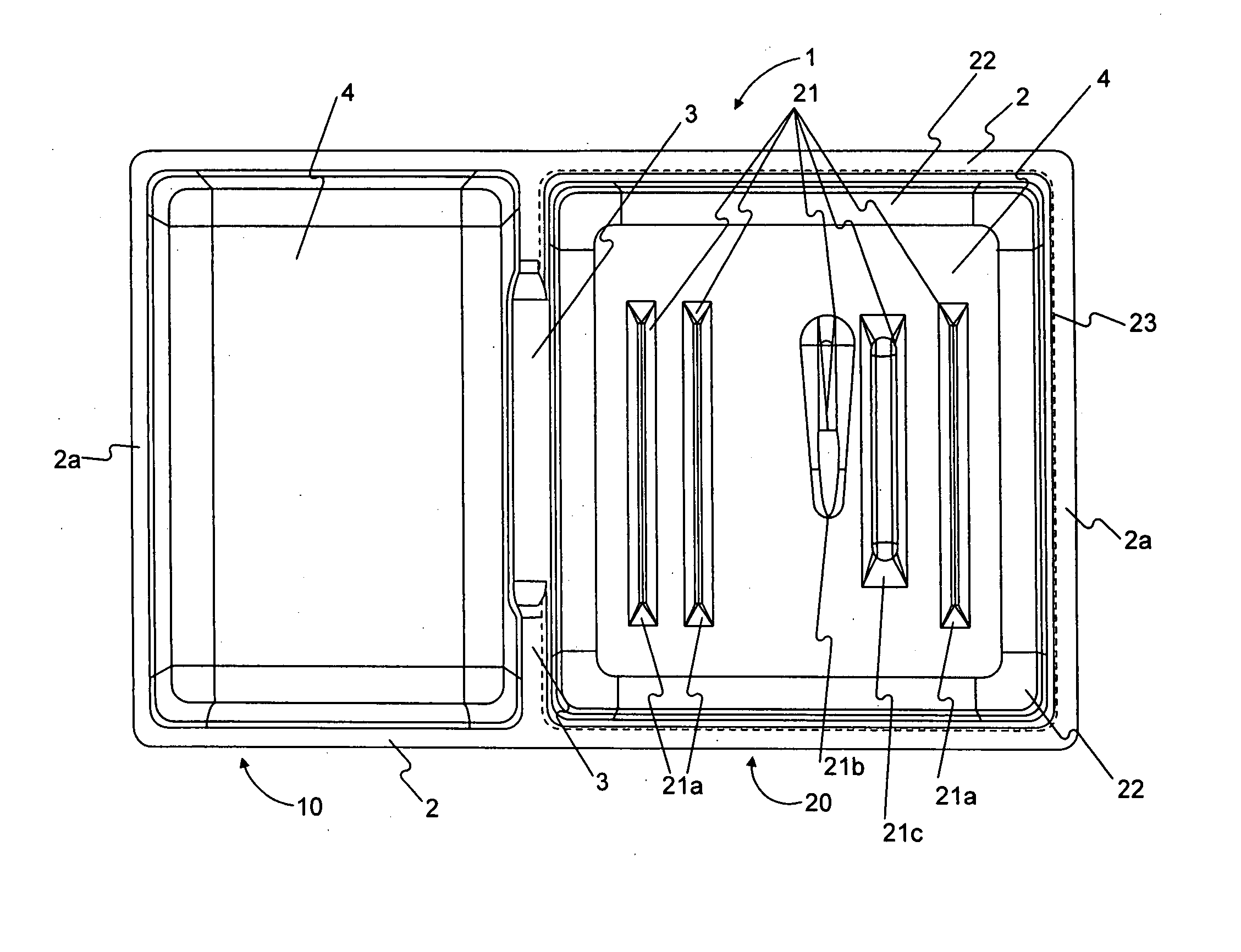
InactiveUS20060011506A1Restrict movementSuture equipmentsSurgical furnitureMedical productBiomedical engineering
Embodiments of the invention include a packaging for a medical kit. The packaging includes a bottom configured to receive a plurality of medical products and a top having at least one protrusion extending therefrom. The bottom and the top are shaped so that the bottom receives the top with the at least one protrusion extending towards the bottom. The at least one protrusion is configured to deform about the plurality of medical products to restrict movement of the plurality of medical products.
Owner:BOSTON SCI SCIMED INC
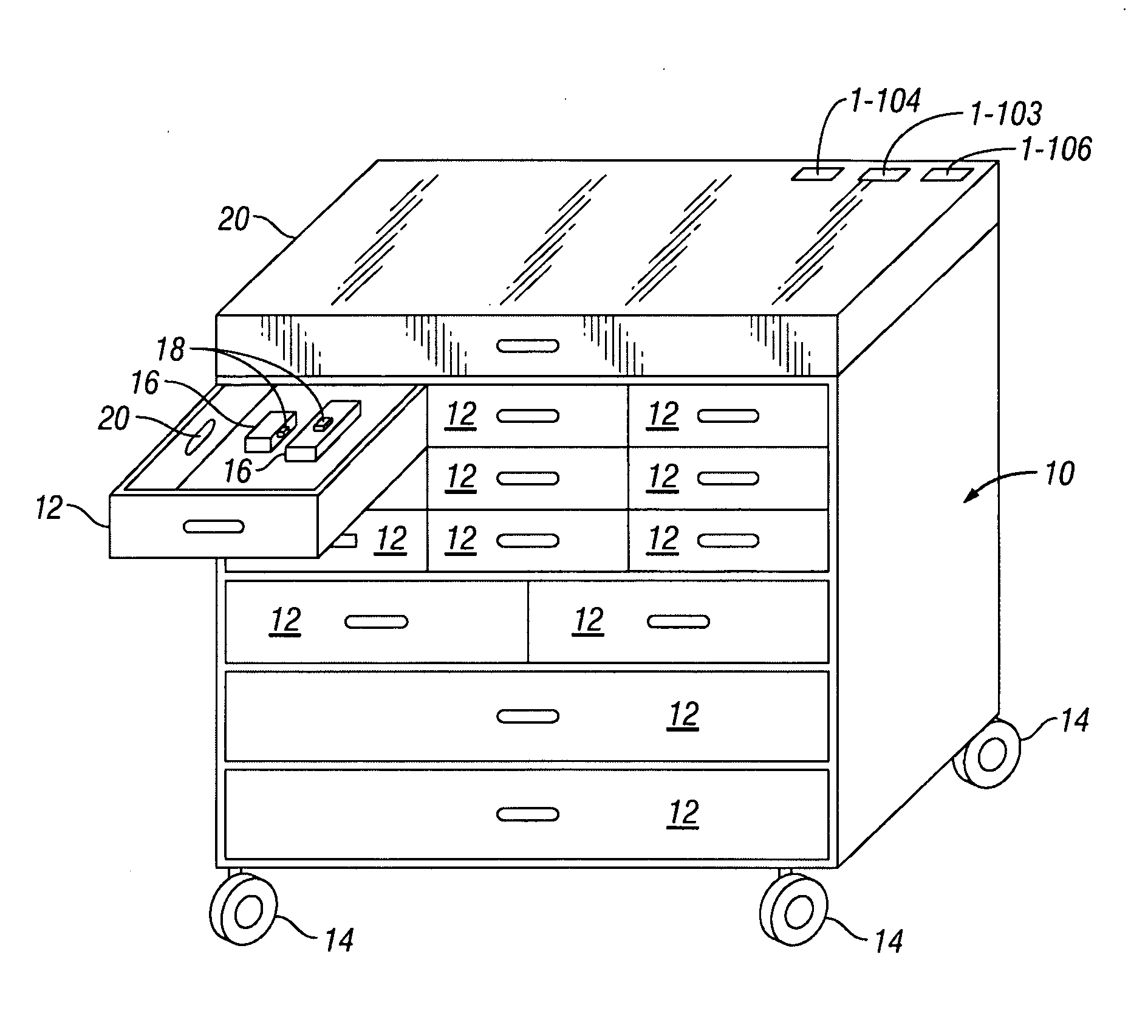
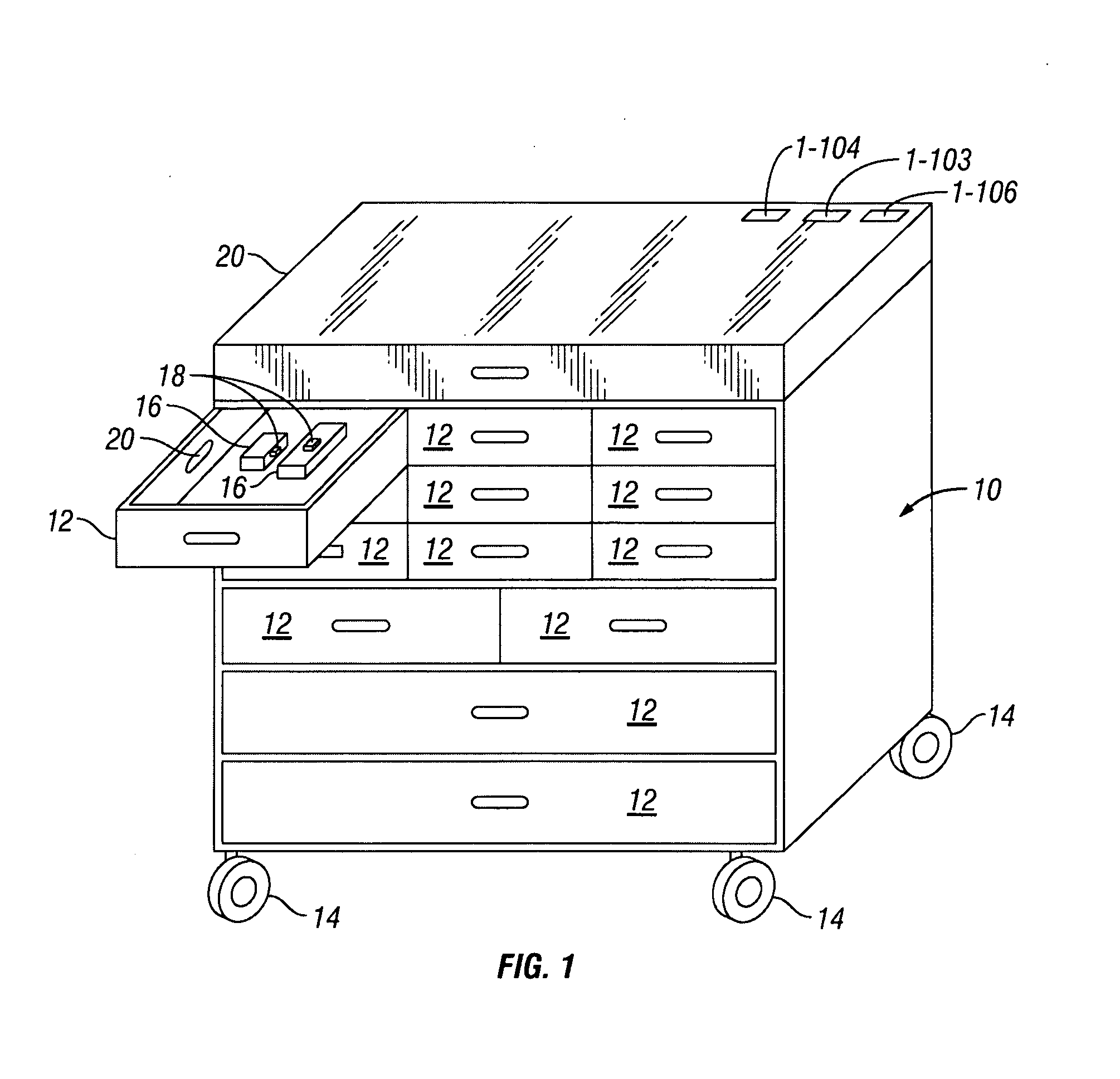
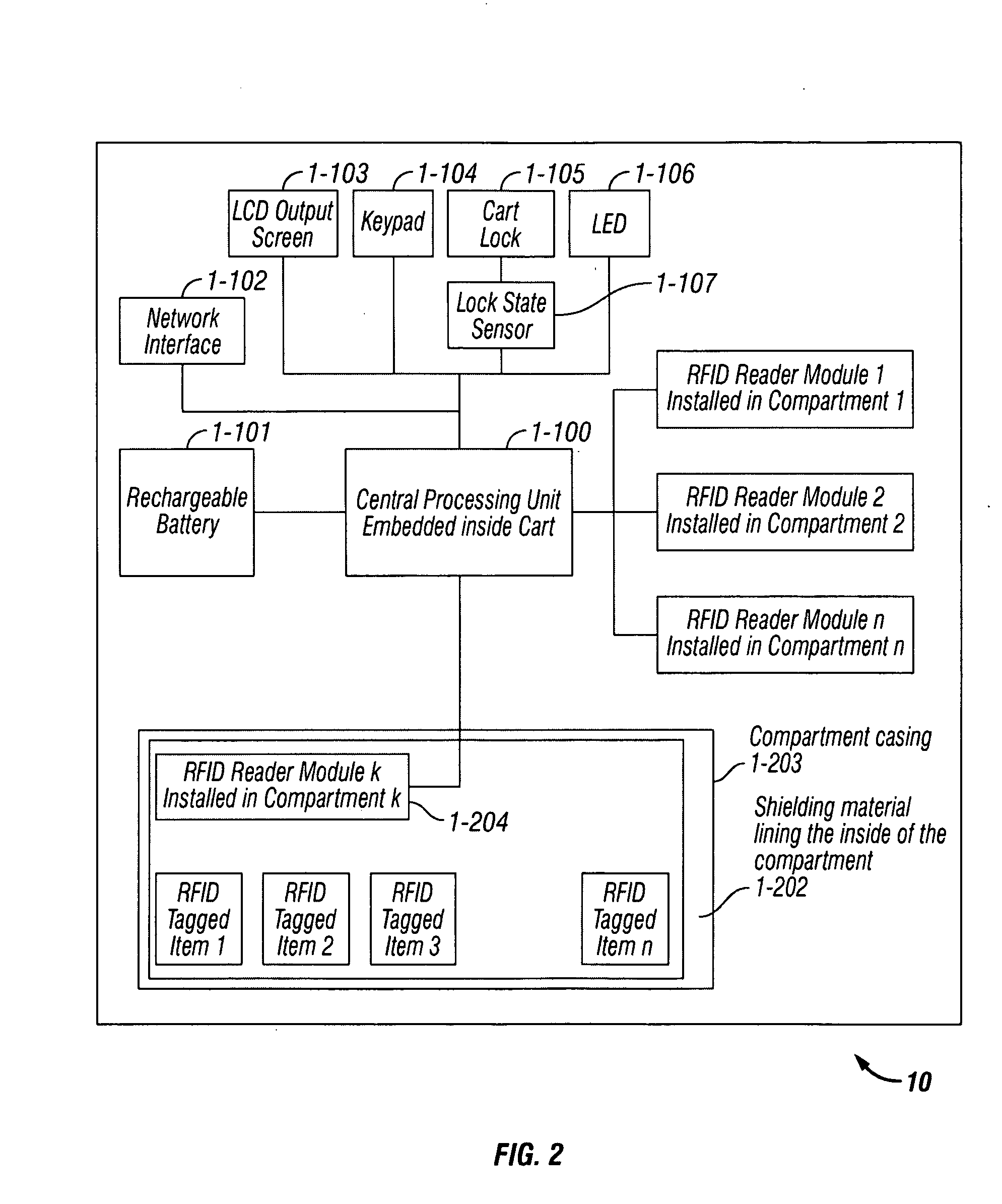
Payload Aware Medical Cart, System and Method
InactiveUS20090267772A1Improve efficiencyReduce errorsDrug and medicationsCoin-freed apparatus detailsMedical productComputerized system
A payload aware medical cart, system and method utilizes a computer system to take inventory of the medical products stored in the medical cart. Each medical product carries an ID tag that provides a unique identifier when queried by an ID sensor, and the medical cart detects the contents of each compartment by reading the ID tags of the products placed in that compartment thereby producing an inventory enumerating all products and the quantity of each product per compartment.
Owner:DEHNADI POURYA M
System for disposal of contaminated medical products
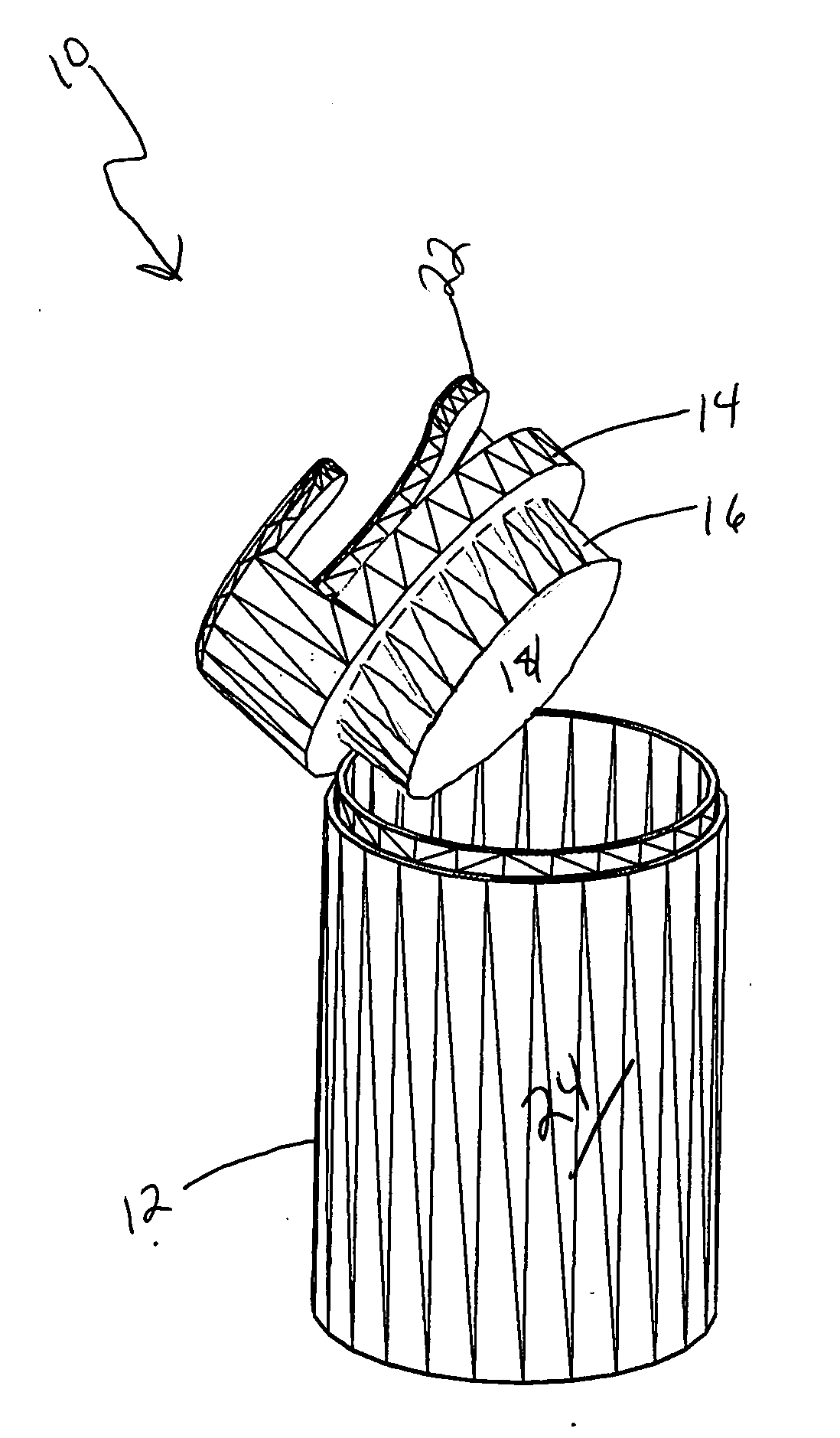
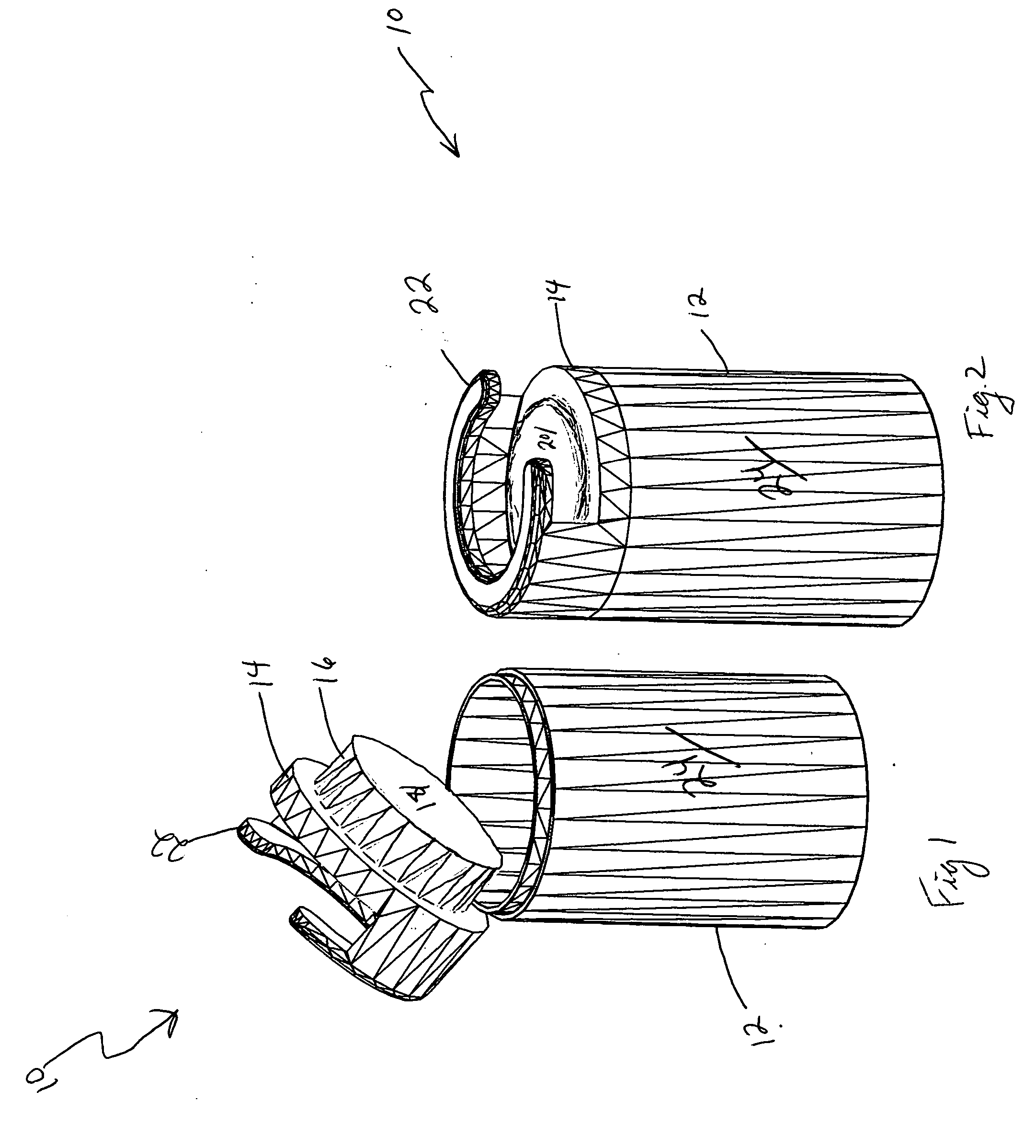
InactiveUS6247592B1Precise and reliable single-handedPrecise and reliable detachmentDispensing apparatusRefuse receptaclesMedical wasteMedical product
A disposed system for medical waste, and particularly sharp ended waste, includes a sharps receptacle and a companion needle holder. The needle holder has an actuation mechanism capable of detachably mounting an externally threaded hub of a needle assembly to the holder. The sharps receptacle has a top with an automatic release structure disposed thereon for engagement with the actuation mechanism of the needle holder. The automatic release structure cooperates with the actuation mechanism of the needle holder to automatically detach a mounted needle assembly from the holder into the sharps receptacle in response to placement of the holder into the automatic release structure.
Owner:ICU MEDICAL SALES +1
Stethoscope cleansing unit and business method for providing advertising through the use of stethoscope cleansing unit
InactiveUS20050214185A1The process is convenient and fastHighly practicalOther printing matterSurgeryMedical productPatient assessment
The invention is of a stethoscope cleansing unit and of the use of the stethoscope cleansing unit as a novel business method for promoting a marketer's logo and advertisement to medical personnel and patients in the examining room or hospital. The system attaches such advertisements to a useful and conveniently used stethoscope cleansing unit that will be used by medical personnel to sterilize stethoscope diaphragms between patient assessments, thereby preventing the spread of infectious diseases. This apparatus can be utilized in a novel business method as a way for marketers to distinguish their product from the myriad of others by 1) attracting the attention of busy medical personnel during patient examinations, which is the exact time that medical product marketers would benefit most from such attention; and by 2) attracting the attention of the patient through its presence in the examining room at a time when the patient is typically waiting for attention and has time to notice such advertisements.
Owner:CASTANEDA C ROBERT
Systems and methods for tracking pharmaceuticals within a facility
A pad is provided for monitoring administration of medical products to a patient, each of the medical products including a Radio Frequency Identification (RFID) tag for storing data related to the respective medical product. The pad includes an RF antenna for reading RFID tags associated with medical products placed in close proximity to the pad to obtain data stored in the RFID tags. A processor coupled to the antenna compares the data with data associated with a patient to verify that the patient is intended to receive the medical products. The pad may include an output device that is activated when the processor detects a mismatch between the data from the RFID tags and the patient data. Optionally, the patient data may be accessed by a remote computer device communicating with the processor to verify that the patient is intended to receive the medical products.
Owner:MEPS REAL TIME
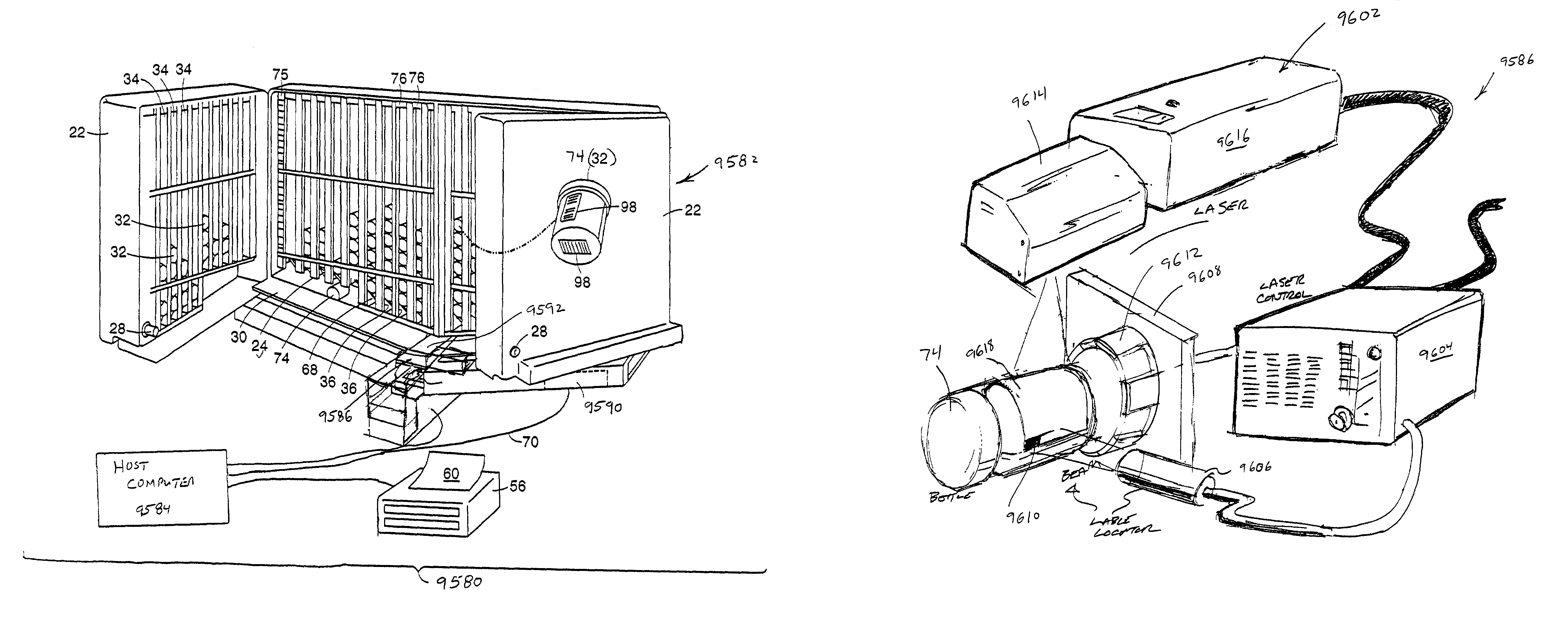
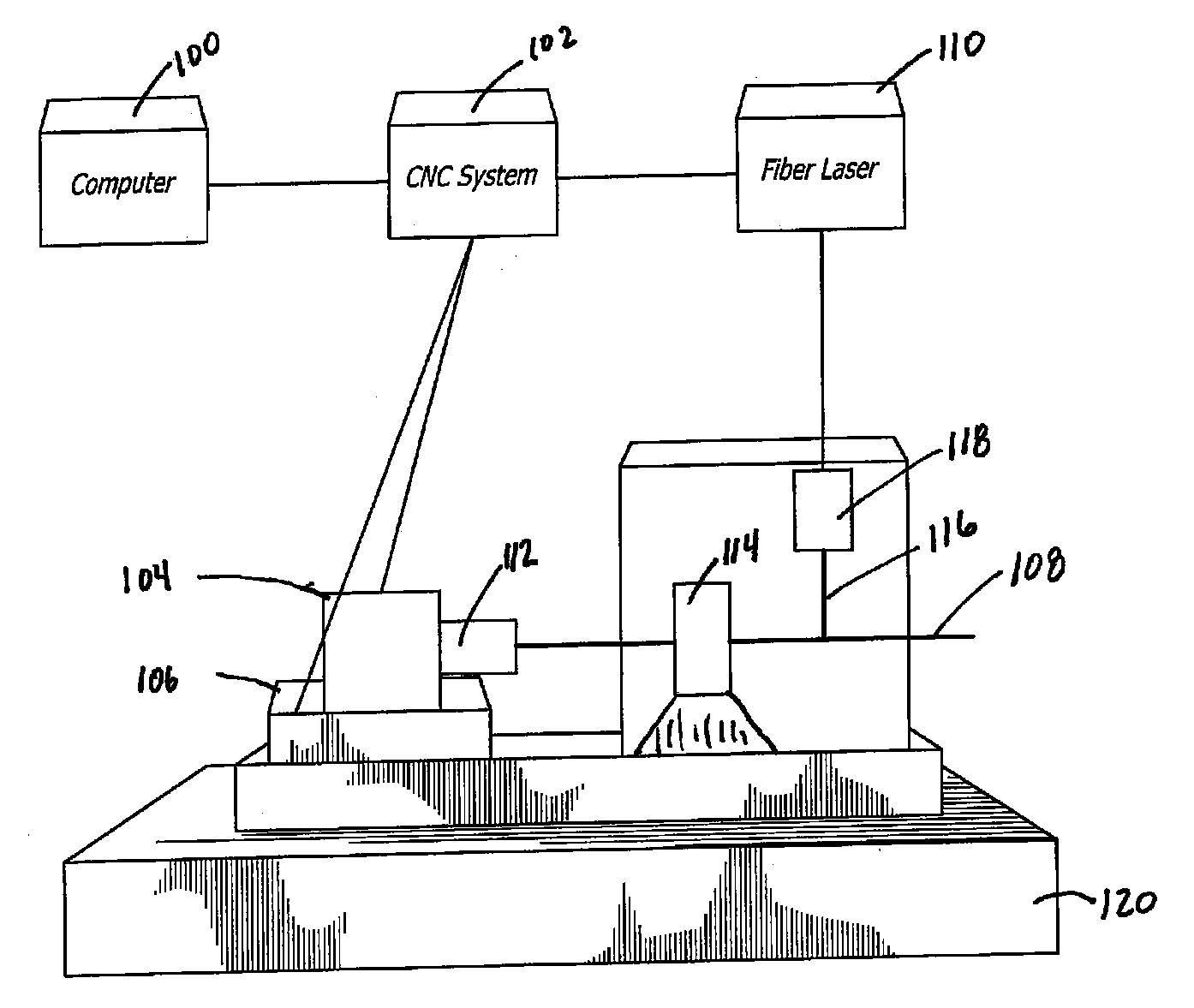
Pulsed Synchronized Laser Cutting of Stents
InactiveUS20070228023A1High resolutionMinimal heat build-upStentsSpecial data processing applicationsPulse controlInsertion stent
A system for pulsed synchronized laser cutting of stents and / or other medical products includes a numerical controller and a machine for moving a tube of material during cutting. A pulsed fiber laser is configured to cut the tube into, for example, a stent, the numerical controller being in communication with the machine and configured to send movement control information to the machine. The numerical controller may also receive movement speed information from the machine. The numerical controller is also in communication with the pulsed fiber laser and is configured to send pulse control information to the pulsed fiber laser. The numerical controller is configured to cause average laser power to decrease by decreasing frequency of laser pulses as stent cutting speed decreases, and to cause average laser power to increase by increasing frequency of laser pulses as stent cutting speed increases.
Owner:ABBOTT CARDIOVASCULAR
Implantable products comprising nanoparticles
The present disclosure relates to nanoparticle-containing implantable and preferably biodegradable medical products and their use for the thermotherapeutic after-treatment after surgical removal of tumors and cancerous ulcers.
Owner:MAGFORCE AG
Pharmaceutical tracking
InactiveUS20070023513A1Easy to trackMix-up can be avoidedDrug and medicationsCash registersMedical productEngineering
A medication-dispensing unit is provided for tracking medical products having a Radio Frequency Identification (RFID) tag uniquely associated therewith. The dispensing unit includes compartments for receiving medical products therein, and readers for reading the RFID tags associated with the medical products in the compartments. A processor is coupled to the readers for receiving and processing readings of the RFID tags in the compartment to identify the medical products in the compartments. The processor may identify a medical product removed from a compartment by determining a difference between readings of the RFID tags in the compartment taken before and after the medical product is removed from the compartment. The processor may verify that the medical product removed from the compartment is authorized to be removed or confirmed that an identified patient is intended to receive the medical product being removed from the compartment. A system and method for counterfeit prevention is disclose, as is specimen, blood, organ and the like, tracking.
Owner:MEPS REAL TIME
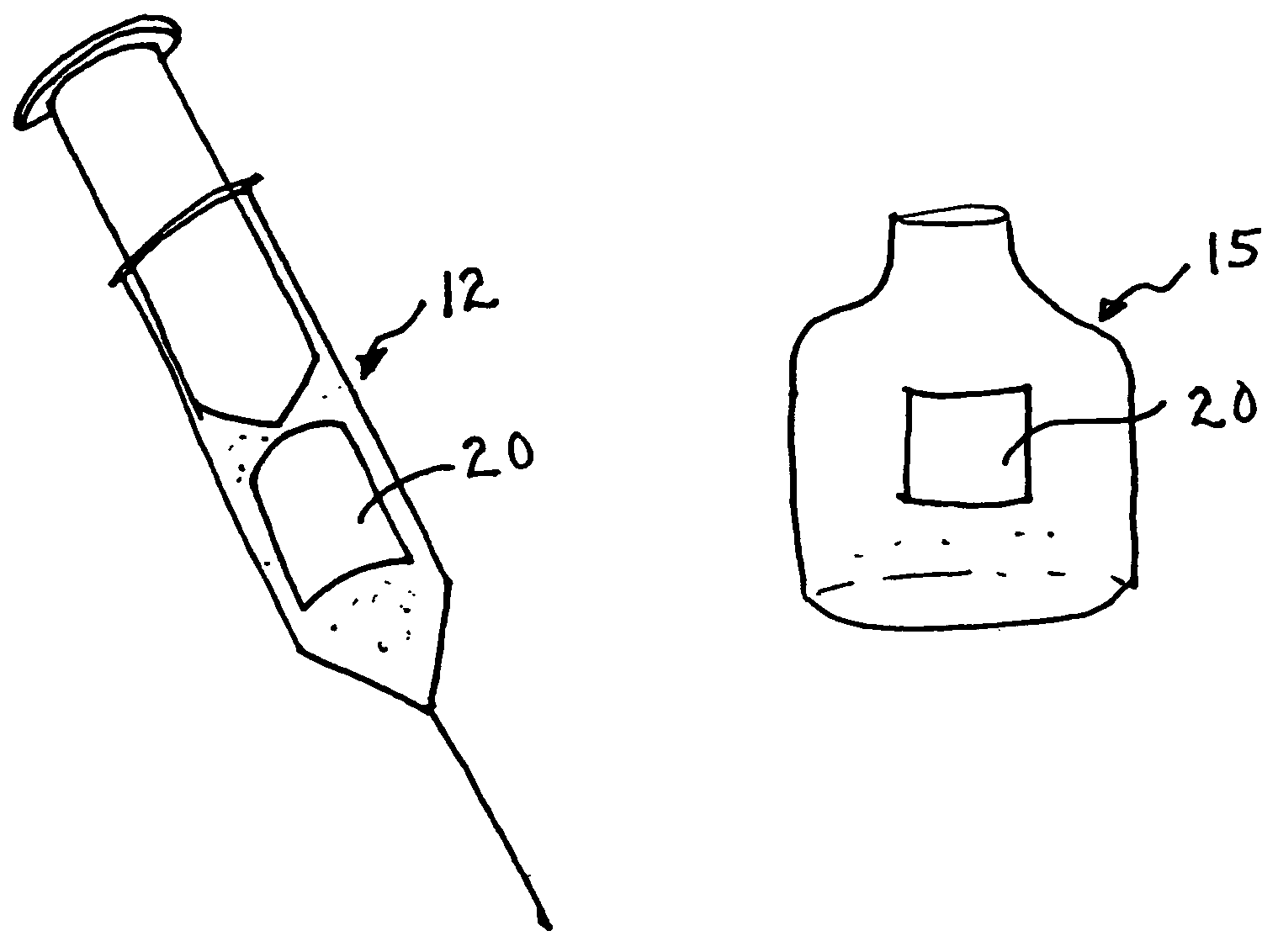
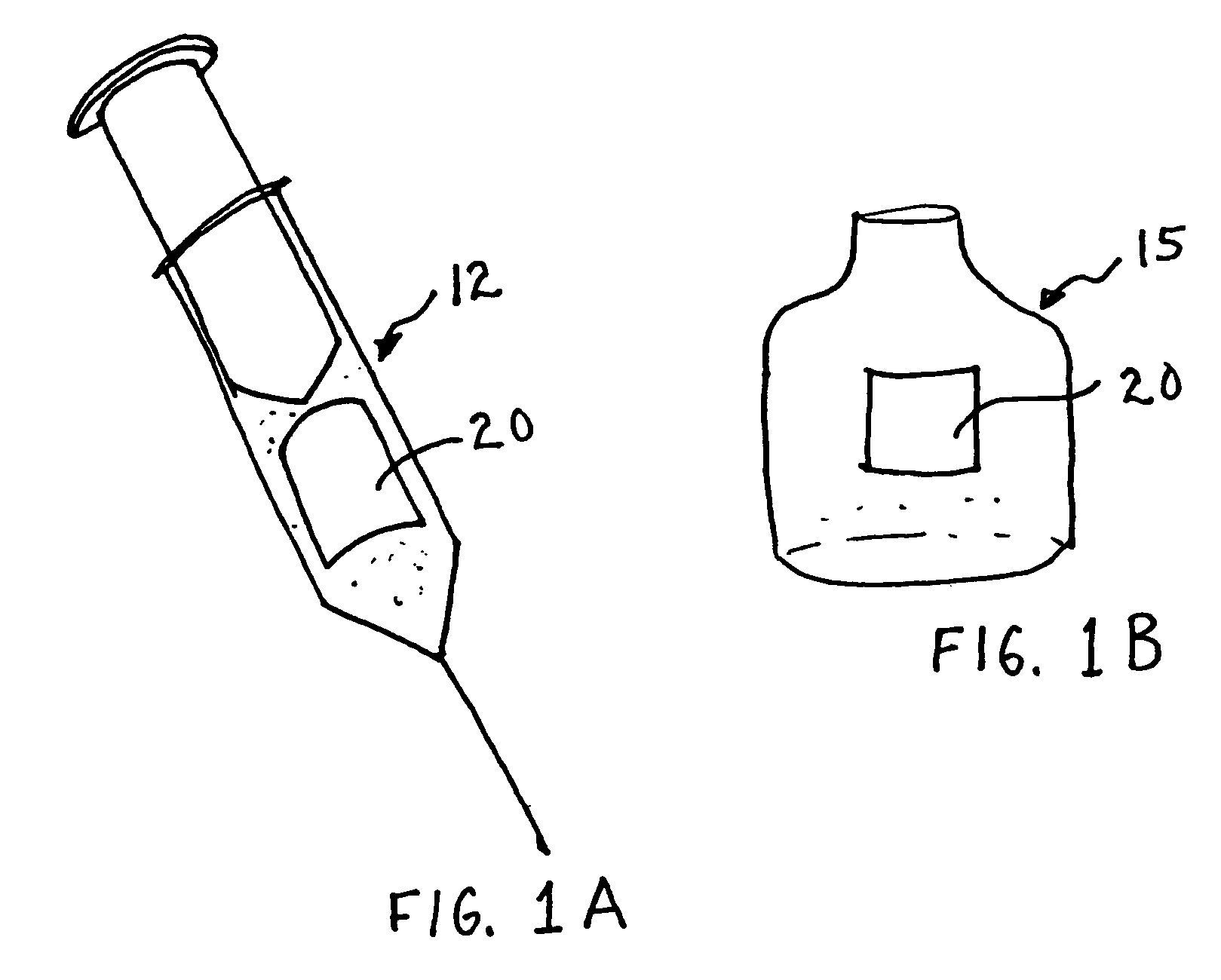
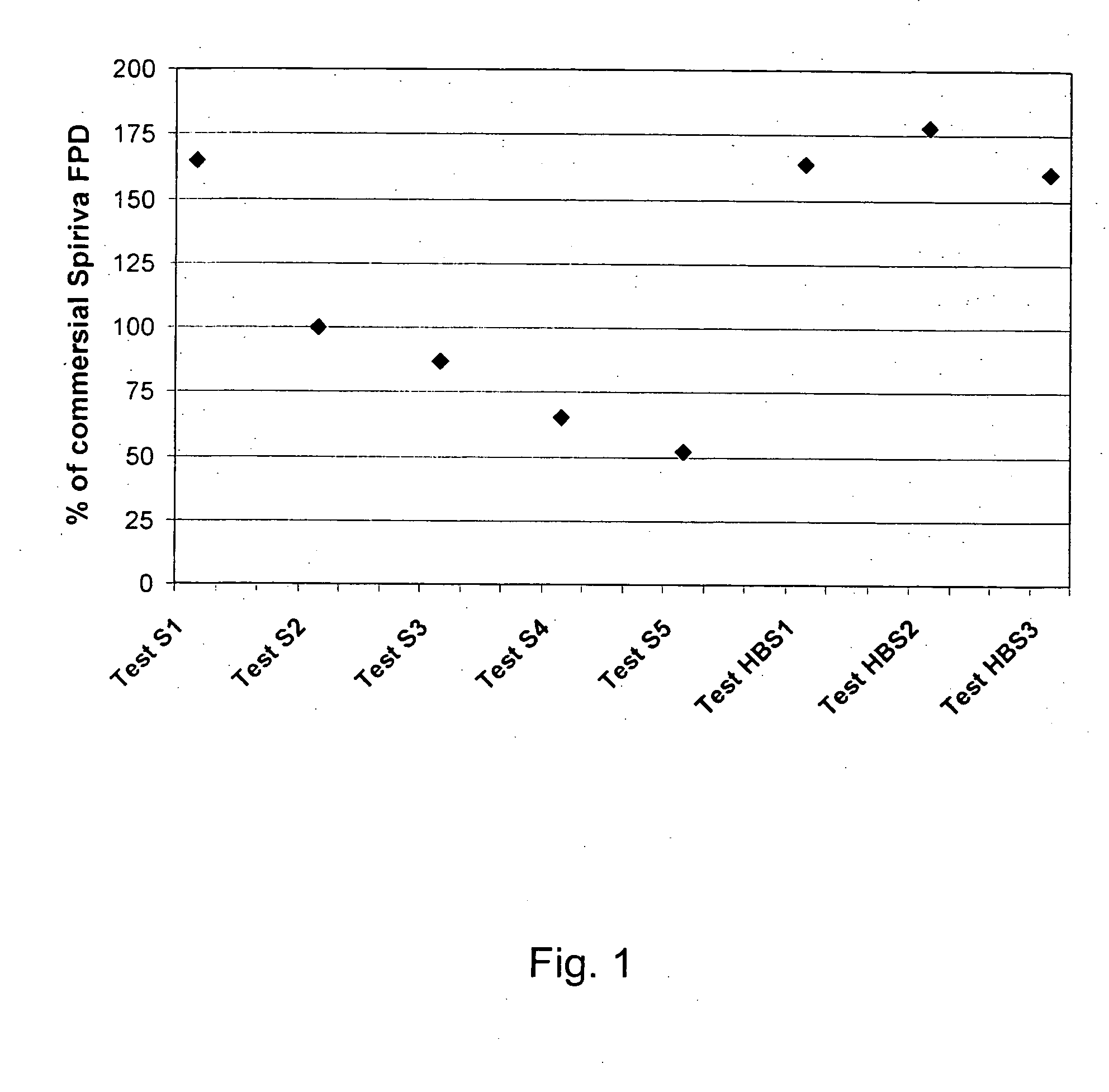
Inhalable tiotropium and container therefor
A medical product suitable for storing and delivering a pre-metered dose of tiotropium, devices containing the same, and methods of using the same.
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions containing lipid micro- or nanoparticles for the enhancement of the dermal action of solid particles
The invention is related to compositions which can be used as dermal formulations for supporting the skin to restore normal conditions in case of e.g. irritated skin, or to support medical therapy of skin with atopic dermatitis symptoms, atopic dermatitis, psoriasis or related diseases (e.g. accompanied by distorted barrier function of the skin and microbial load). The compositions of the invention can be used for dermo-cosmetic products but also for pharmaceutical / -medical products, depending on the composition and the additional actives incorporated (cosmetic actives or drugs). The invention is based on the synergistic effect of metallic particles, in particular silver particles (such as microsilver, nanosilver) and lipid particles (lipid nanoparticles or lipid microparticles). As alternatives to silver particles, other metallic particles (e.g. zinc, copper) or nanocrystalline actives can be incorporated (e.g. replacing the anti-oxidative silver by anti-oxidative nanocrystals of plant molecules such as hesperitin). This leads to combinations of lipid particles with nanocrystals for dermal use.
Owner:PHARMASOL +1
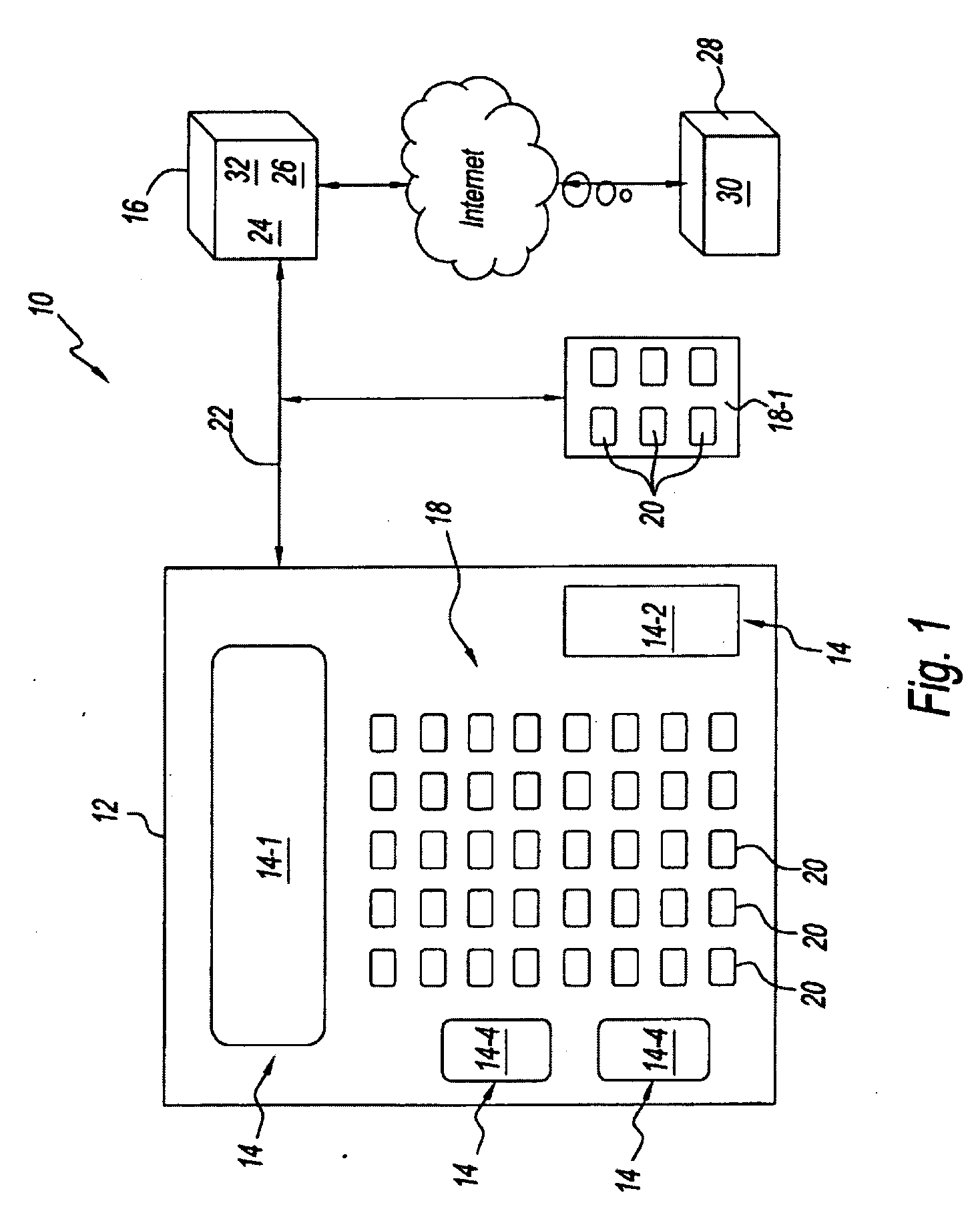
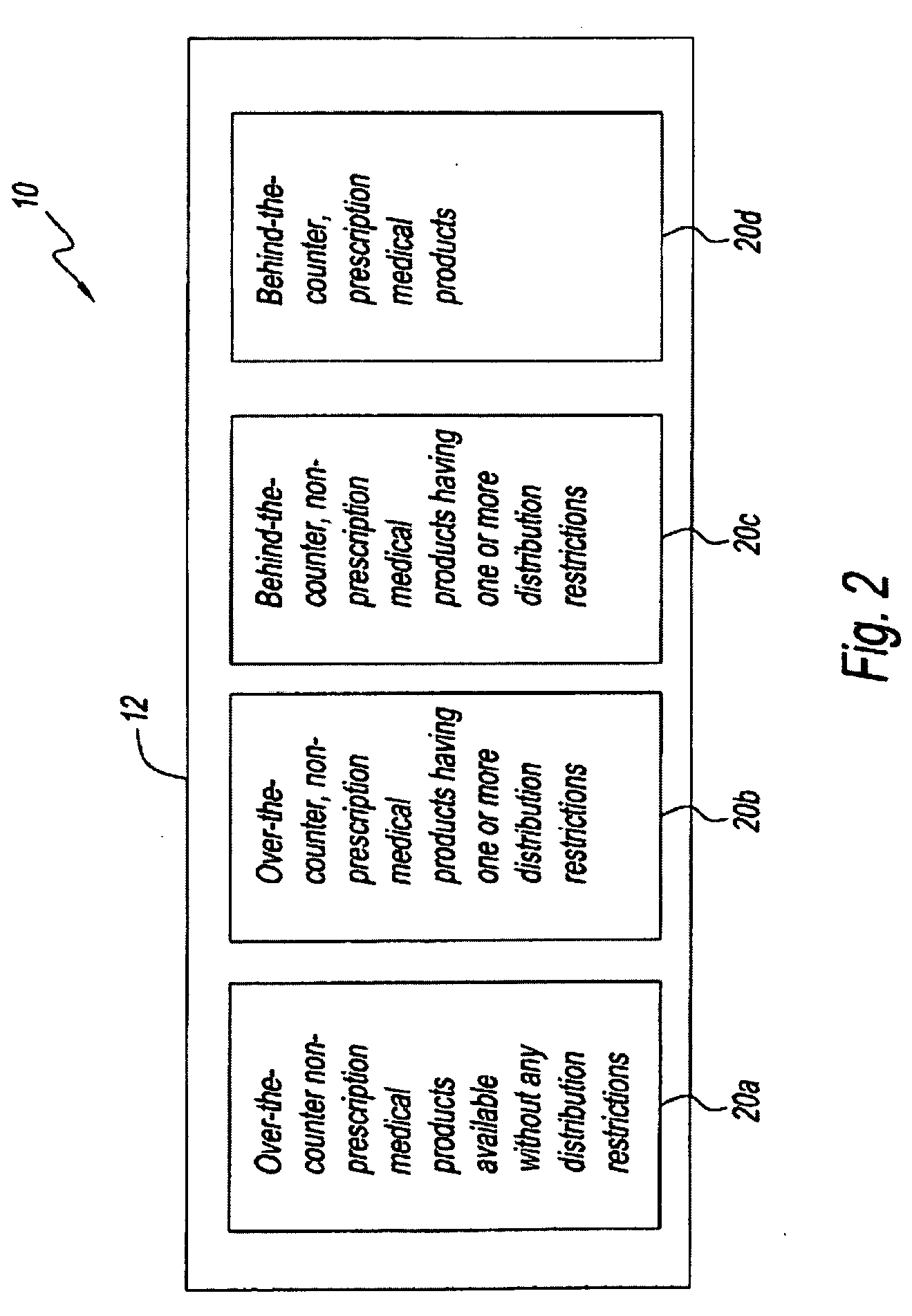
Medical product dispensing systems and methods
Methods and systems for transferring restricted distribution medical products to an over-the-counter general sales environment are provided. Methods and systems of dispensing non-prescription, behind-the-counter medical products from a vending machine in a general sales location are also provided. In some embodiments, methods and systems are provided for dispensing a medical product from a vending machine in a general sales location based, at least in part, on biometric data collected from the purchaser and, in some instances, based on self-selection and / or de-selection criteria, is provided. Further, methods and systems of switching prescription medical products to non-prescription, over-the-counter medical products are provided.
Owner:GLAXOSMITHKLINE CONSUMER HEALTHCARE (UK) IP LTD
Multi-component fibers having enhanced reversible thermal properties and methods of manufacturing thereof
Multi-component fibers having enhanced reversible thermal properties and methods of manufacturing thereof are described. In one embodiment, a multi-component fiber includes a fiber body formed from a set of elongated members, and at least one of the set of elongated members includes a temperature regulating material having a latent heat of at least 40 J / g and a transition temperature in the range of 22° C. to 40° C. The temperature regulating material provides thermal regulation based on at least one of absorption and release of the latent heat at the transition temperature. The multi-component fiber can be formed via a melt spinning process or a solution spinning process and can be used or incorporated in various products where a thermal regulating property is desired. For example, the multi-component fiber can be used in textiles, apparel, footwear, medical products, containers and packagings, buildings, appliances, and other products.
Owner:HILLS CO
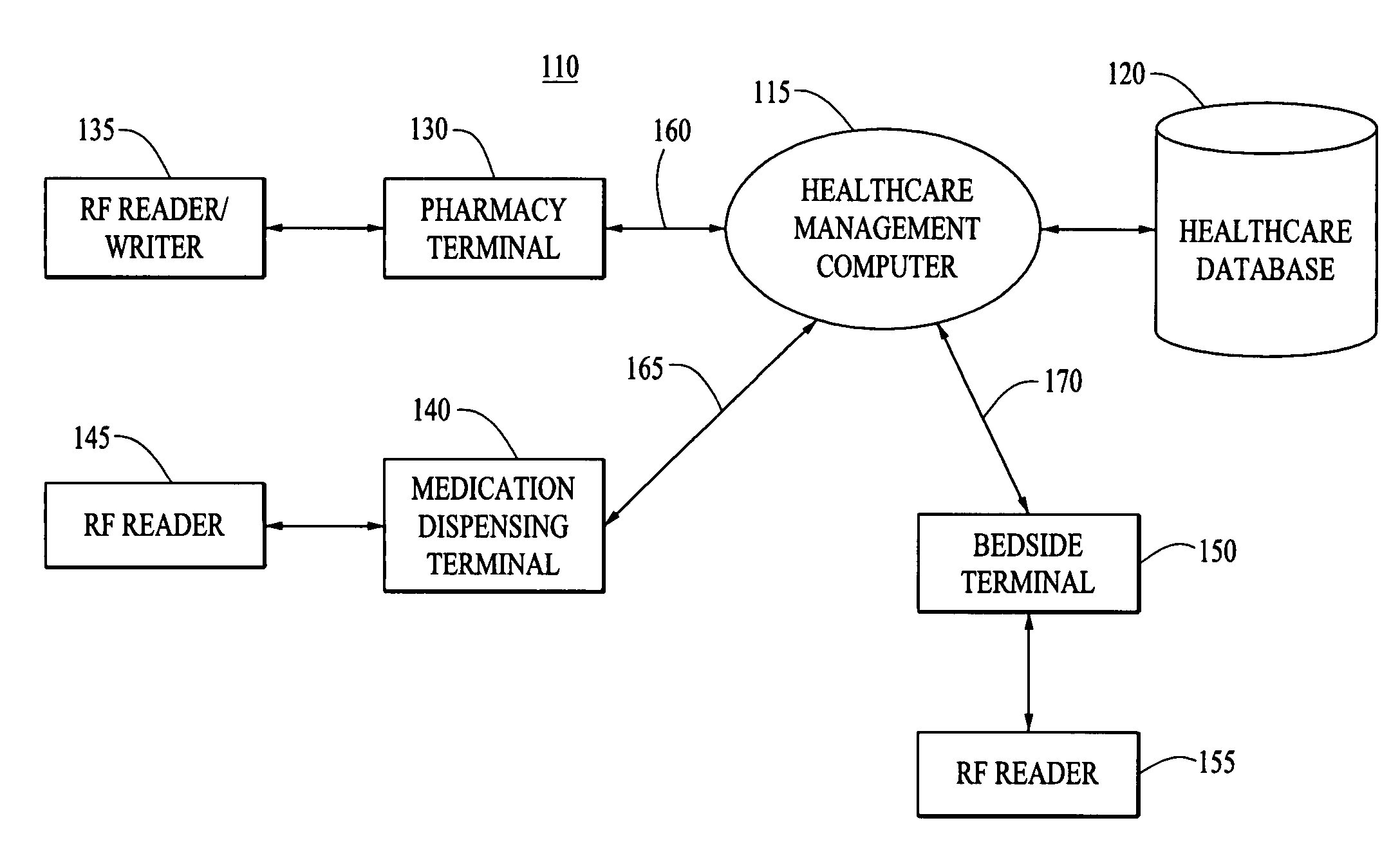
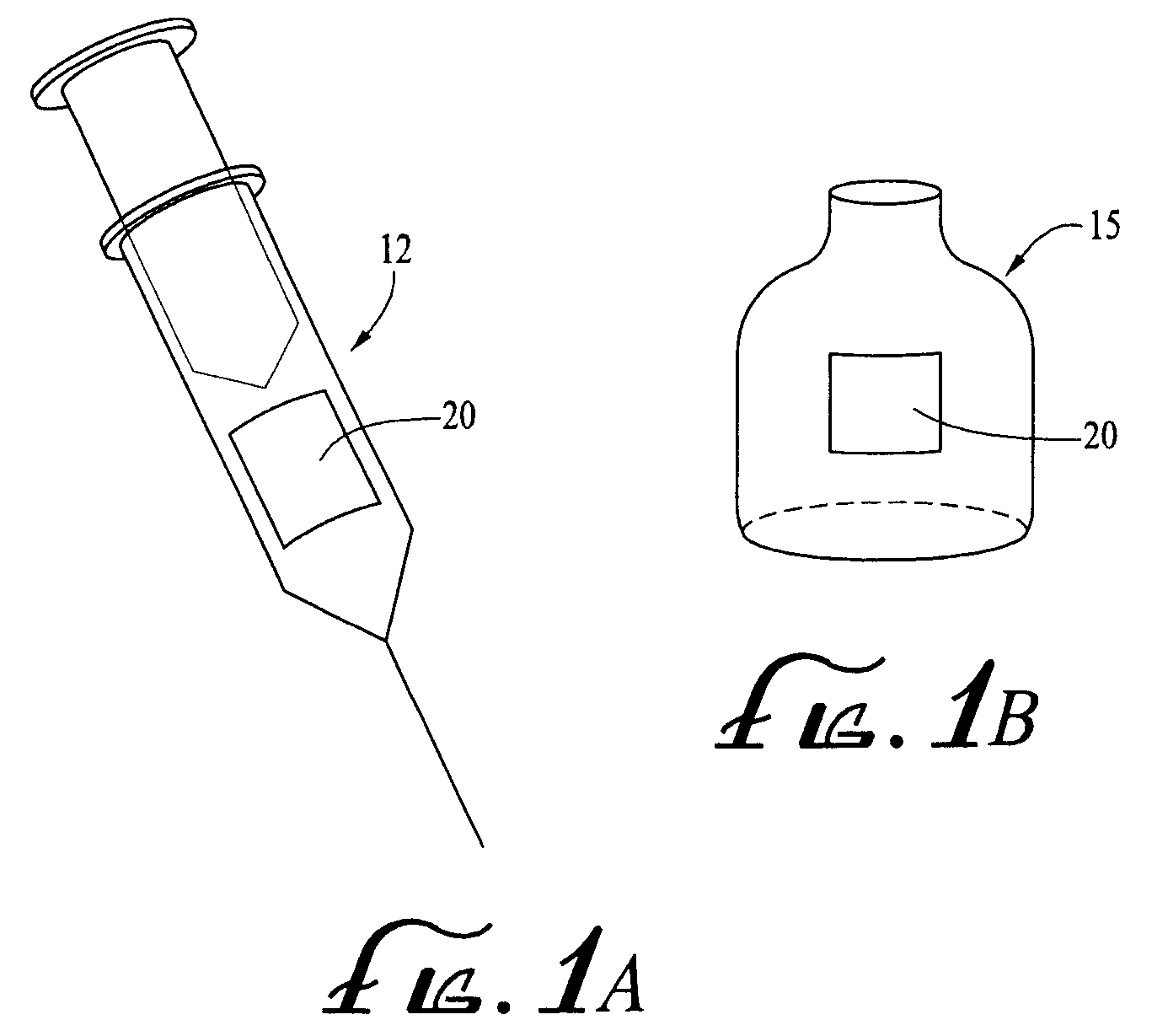
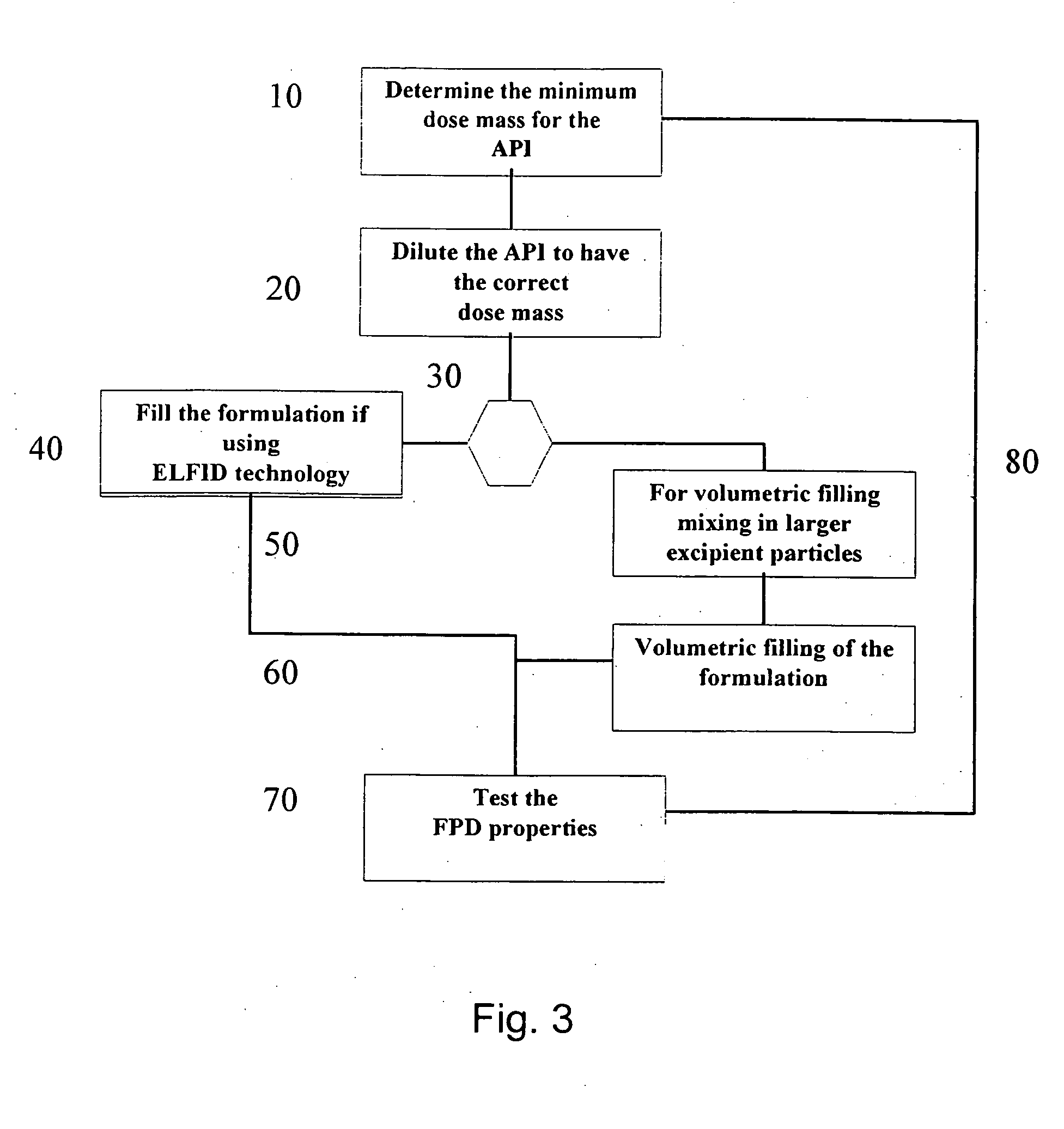
Methods and apparatuses for assuring quality and safety of drug administration and medical products and kits
InactiveUS20070213684A1Prevent unsafe reuseNervous disorderDrug and medicationsQuality assuranceMedical product
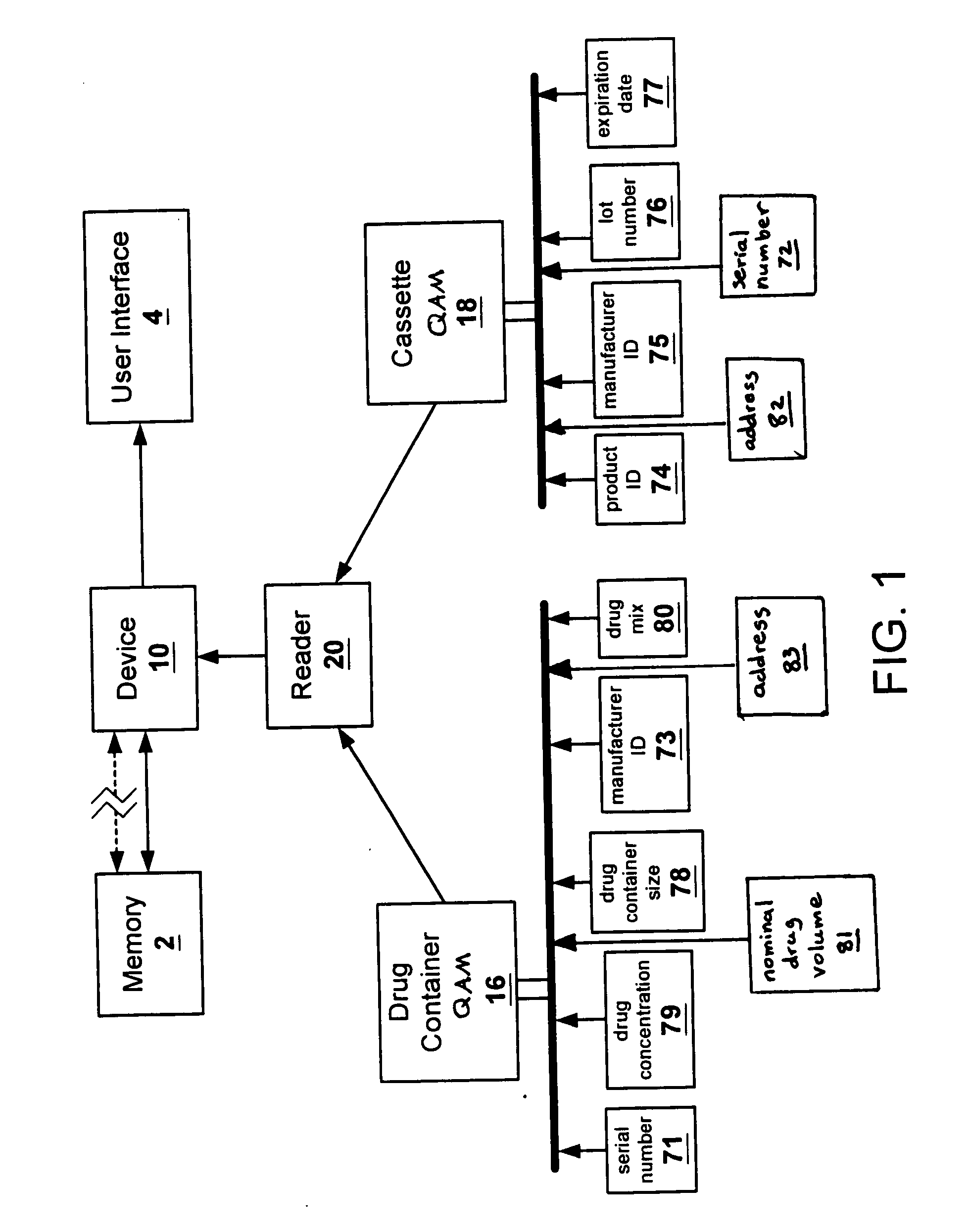
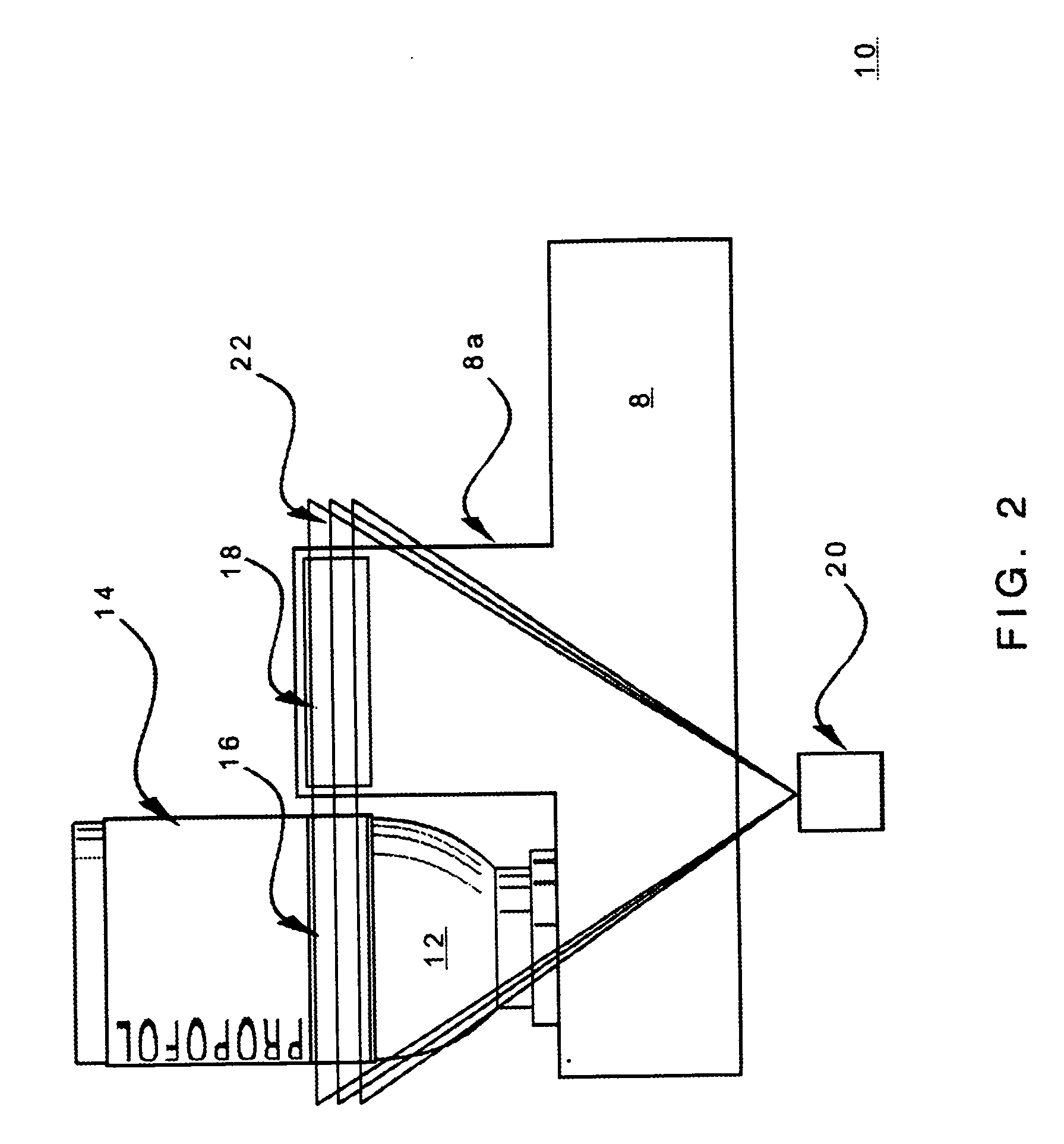
The present invention provides apparatuses and methods for marking components, supplies and kits of drug administration devices and other medical systems with quality assurance information. The invention also provides apparatuses and methods for tracking time of use of such components, supplies and kits and various apparatuses and methods for preventing use or reuse of tainted, recalled or unrecognized components, supplies and kits. Quality assurance markers (QAMs) are described which store information regarding the identity and manufacturer of disposable components, supplies and kits. The invention utilizes several QAM modalities, such as, among others, 1-D and 2-D bar codes, 1-D and 2-D symbologies, holograms, written text, radio frequency identification devices (RFIDs), integrated chip smart cards, and EEPROMs.
Owner:SCOTT LAB
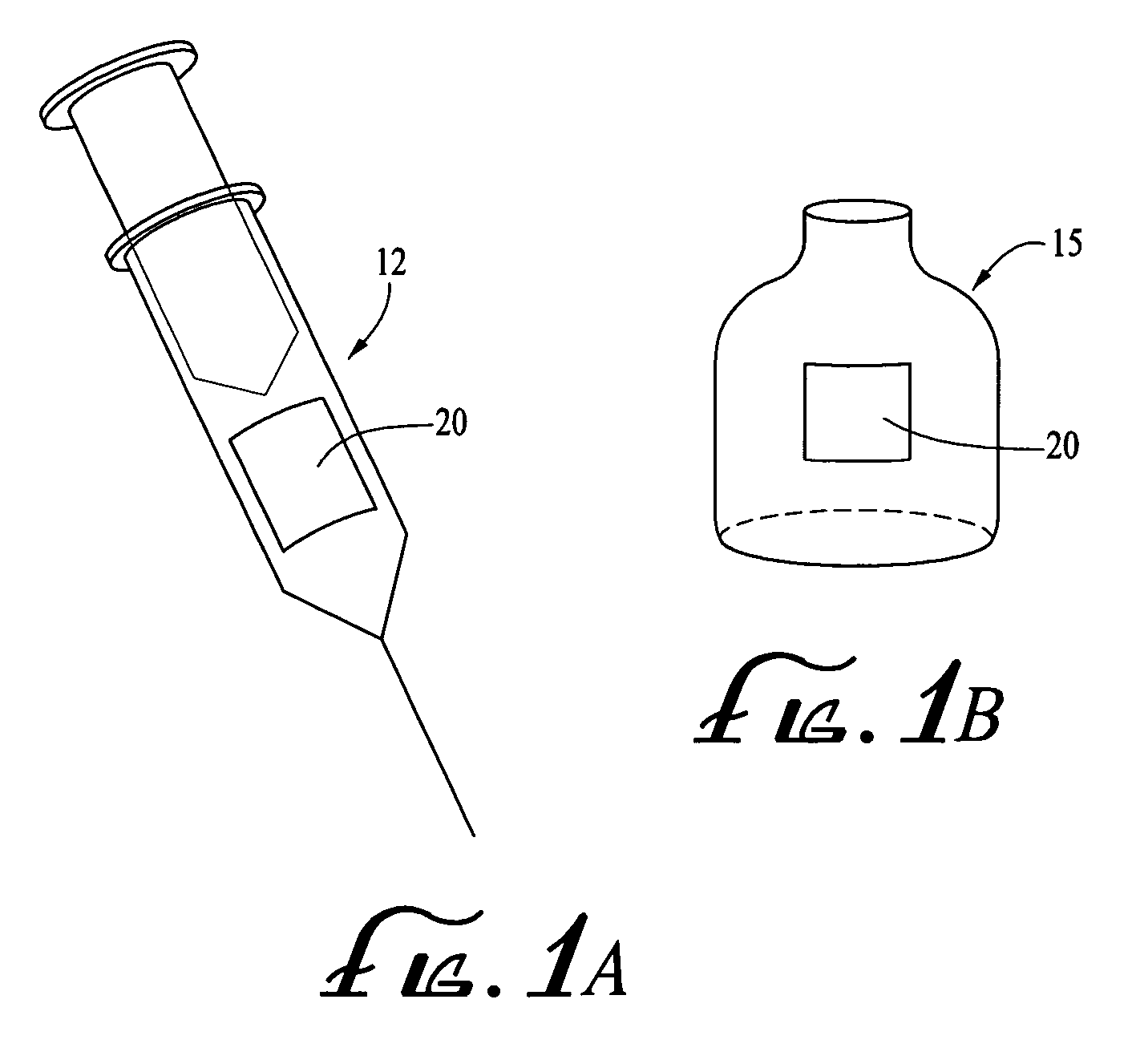
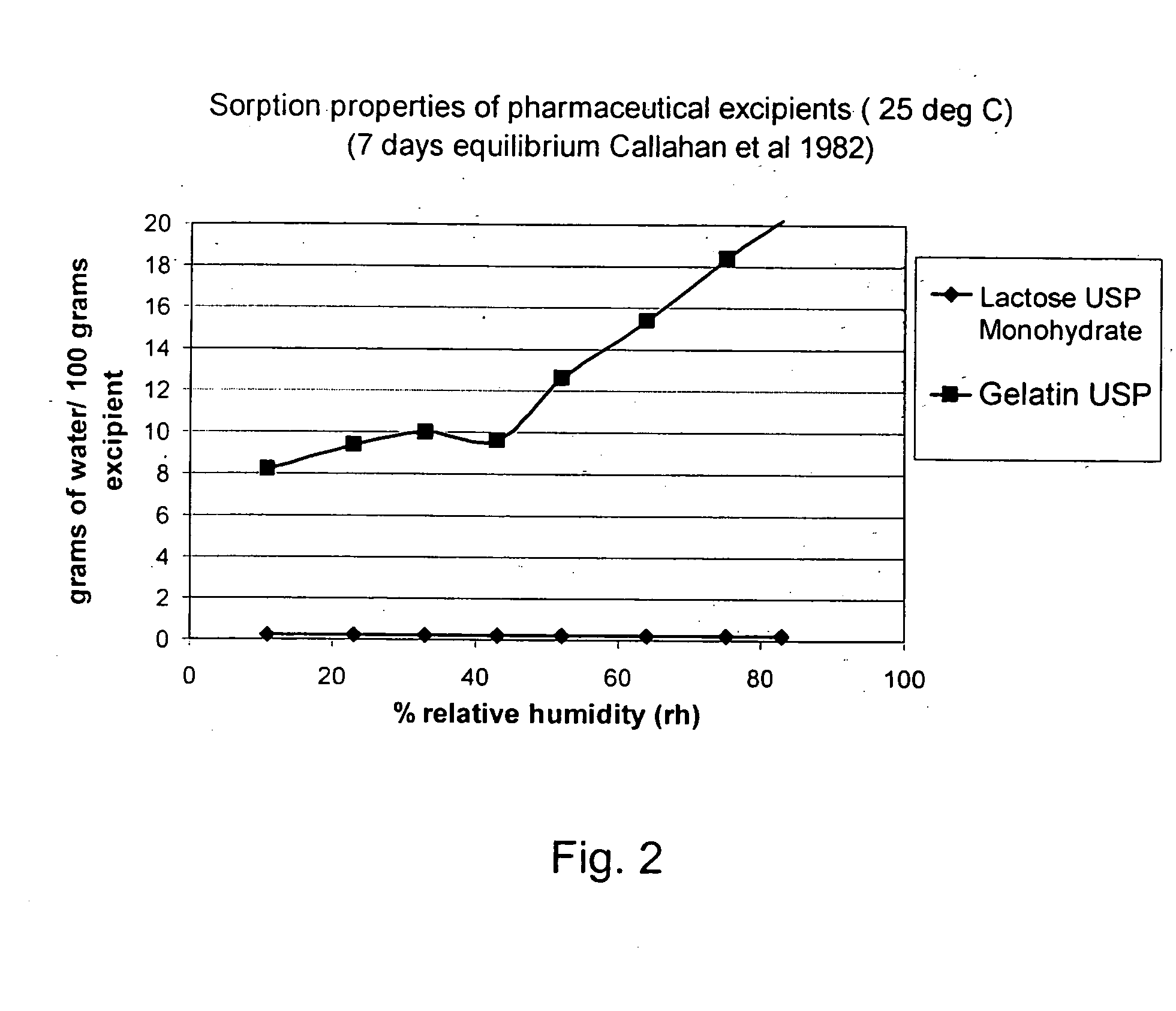
Medical product
InactiveUS20060239933A1Powder deliveryPeptide/protein ingredientsPulmonary inhalationMedical product
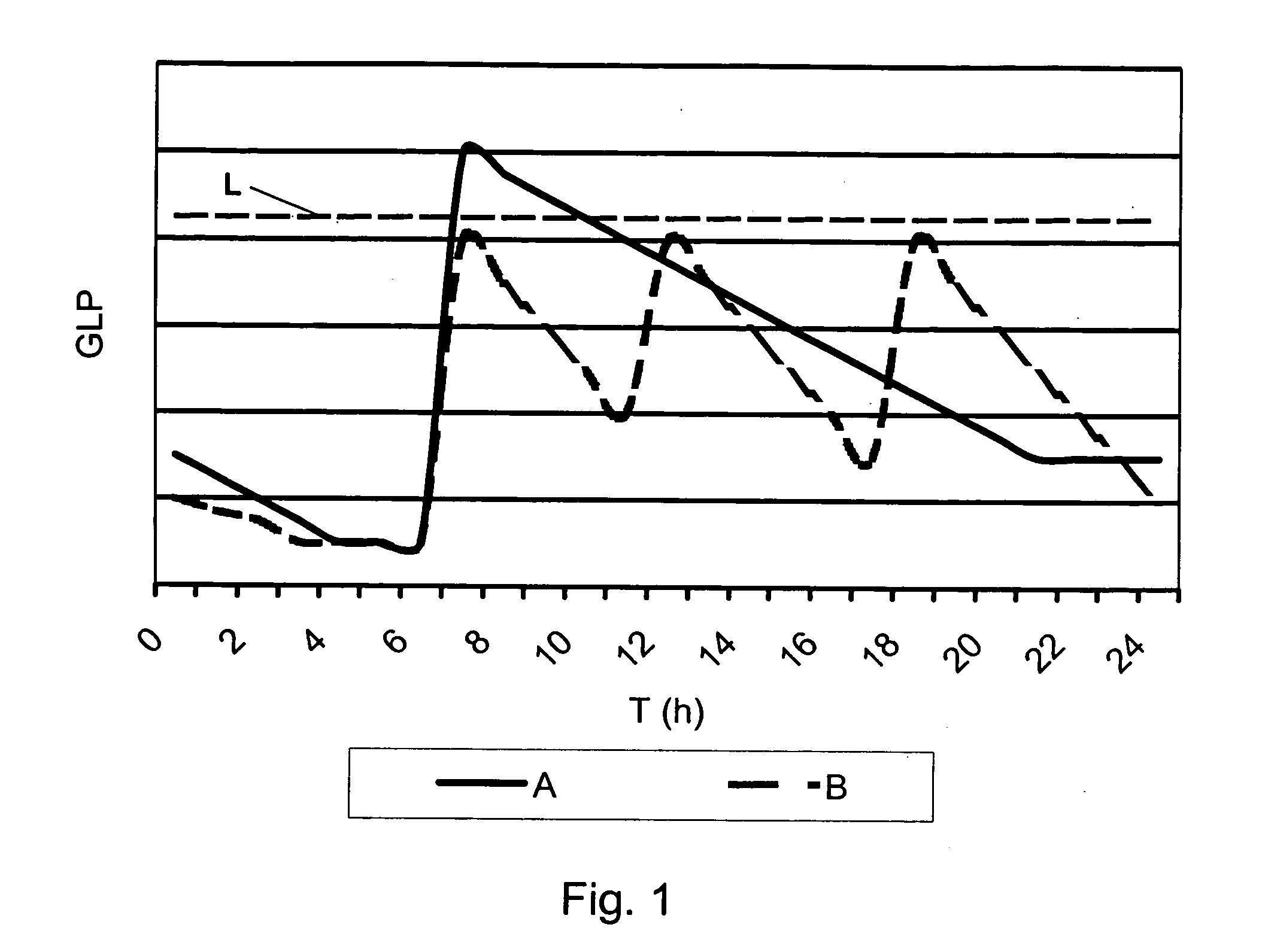
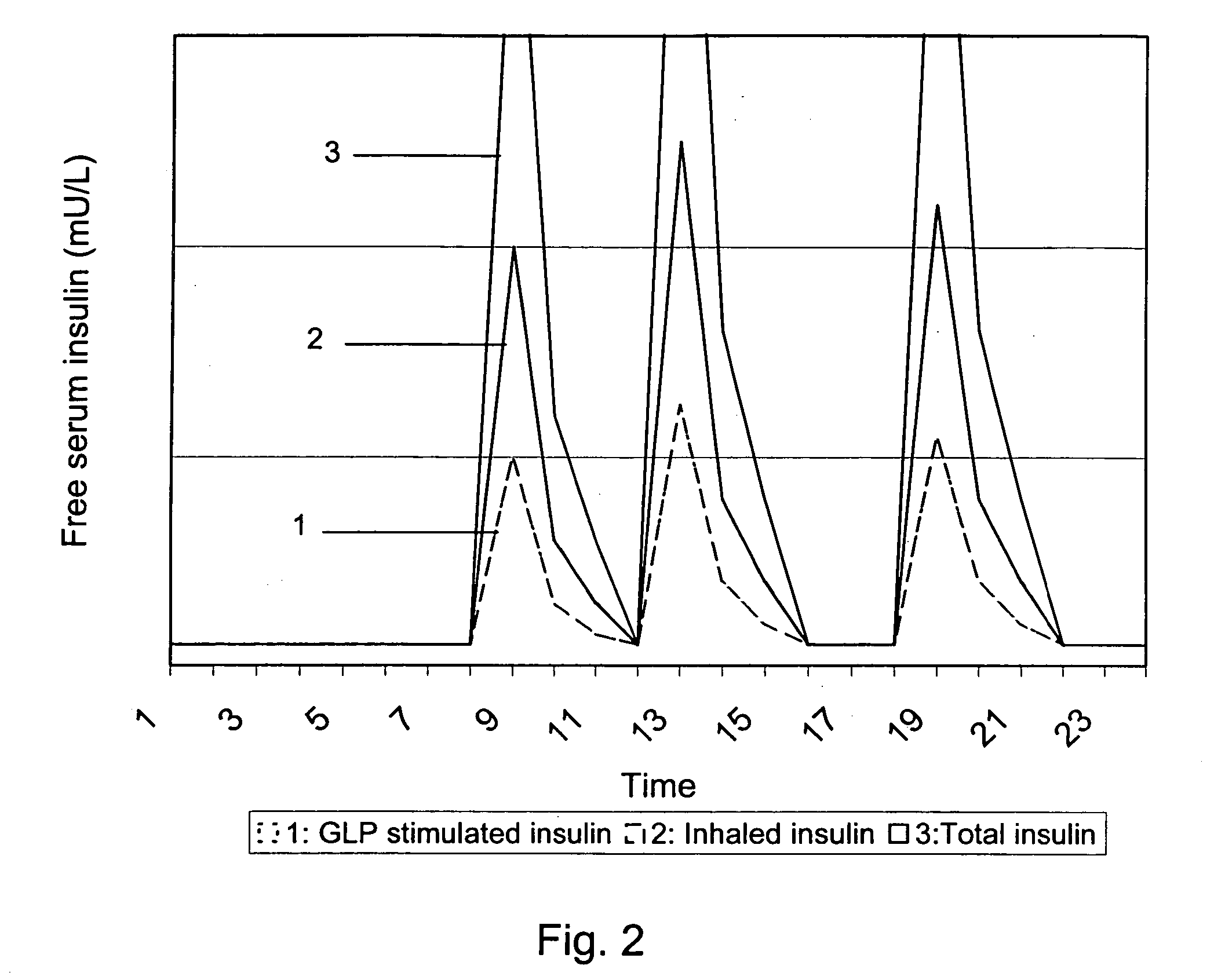
A medical product is disclosed. The medical product contains an accurately metered dose of at least one GLP medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Cellulosic fibers having enhanced reversible thermal properties and methods of forming thereof
Cellulosic fibers having enhanced reversible thermal properties and methods of forming such cellulosic fibers are described. In one embodiment, a cellulosic fiber includes a fiber body formed of an elongated member. The elongated member includes a cellulosic material and a temperature regulating material dispersed within the cellulosic material. The temperature regulating material includes a phase change material having a transition temperature in the range of −5° C. to 125° C. The cellulosic fiber can be formed via a solution spinning process and can be used in various products where thermal regulating properties are desired. For example, the cellulosic fiber can be used in textiles, apparel, footwear, medical products, containers and packagings, buildings, appliances, and other products.
Owner:OUTLAST TECH GMBH
Semisolid system and combination semisolid, multiparticulate system for sealing tissues and/or controlling biological fluids
InactiveUS20060127437A1Alter viscosityAlter consistencyAbsorbent padsSynthetic polymeric active ingredientsSolid massMedical product
A semisolid system and combination semisolid, multiparticulate system for controlling biological fluids is provided which includes a body fluid control composition. The body fluid control composition is adapted for utilization both as an independent mixture for direct topical application to body tissue for human or veterinary application and for incorporation with a standard medical products in order to facilitate absorption, moisture balance, hemostasis, infection control, and wound healing.
Owner:SOUTHEASTERN MEDICAL TECH
Low-density, open-cell, soft, flexible, thermoplastic, absorbent foam and method of making foam
A soft, flexible, low-density, open-cell, thermoplastic, absorbent foam formed from a foam polymer formula including a balanced amount of a plasticizing agent and a surfactant in combination with a base resin. Thermoplastic elastomers can be added to the foam polymer formula to improve softness, flexibility, elasticity, and resiliency of the resulting foam. The surfactant may be either a single surfactant or a multi-surfactant system. The foam possesses a number of qualities, such as softness and strength, which render the foam particularly suitable for use in a variety of personal care products, medical products, and the like.
Owner:KIMBERLY-CLARK WORLDWIDE INC
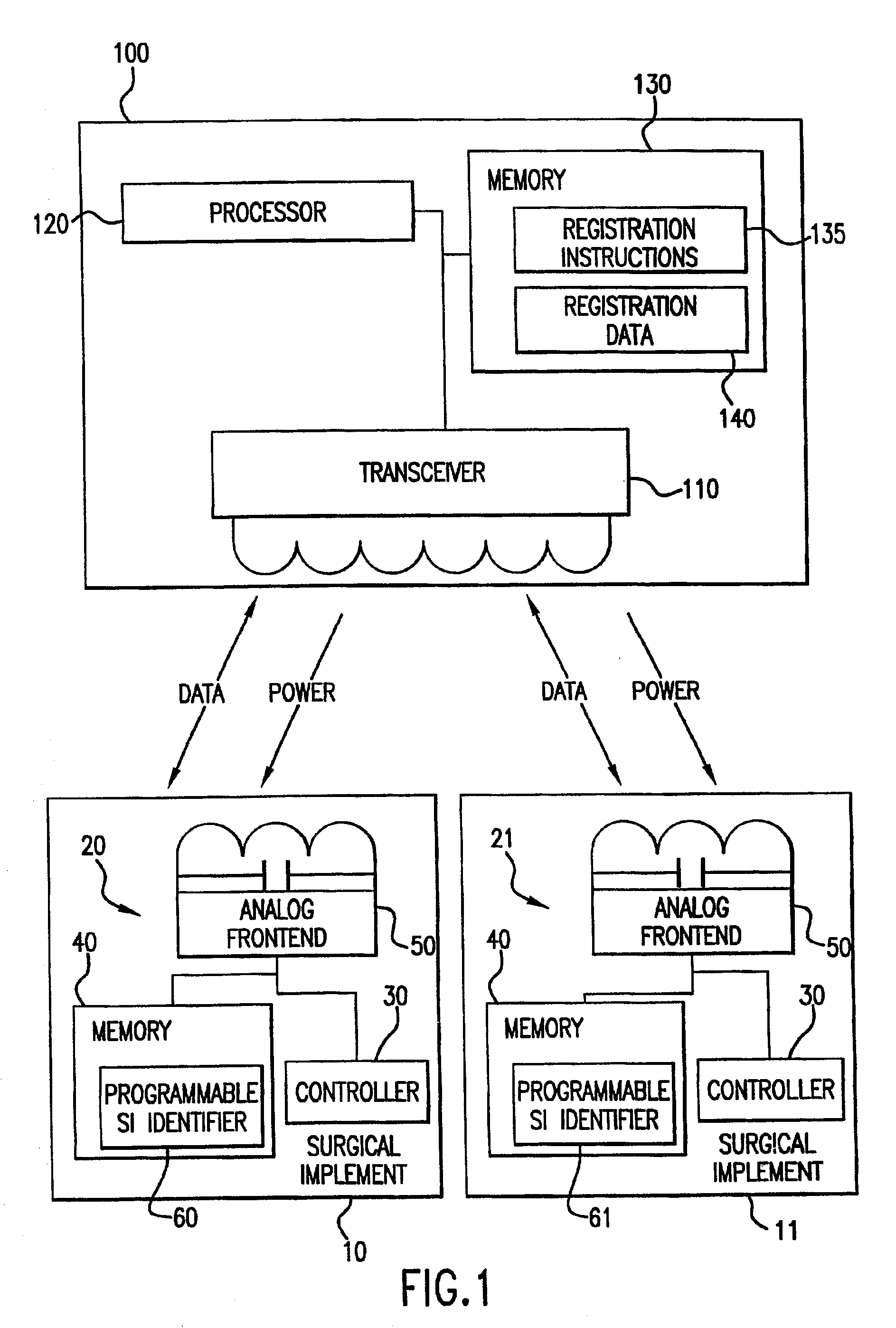
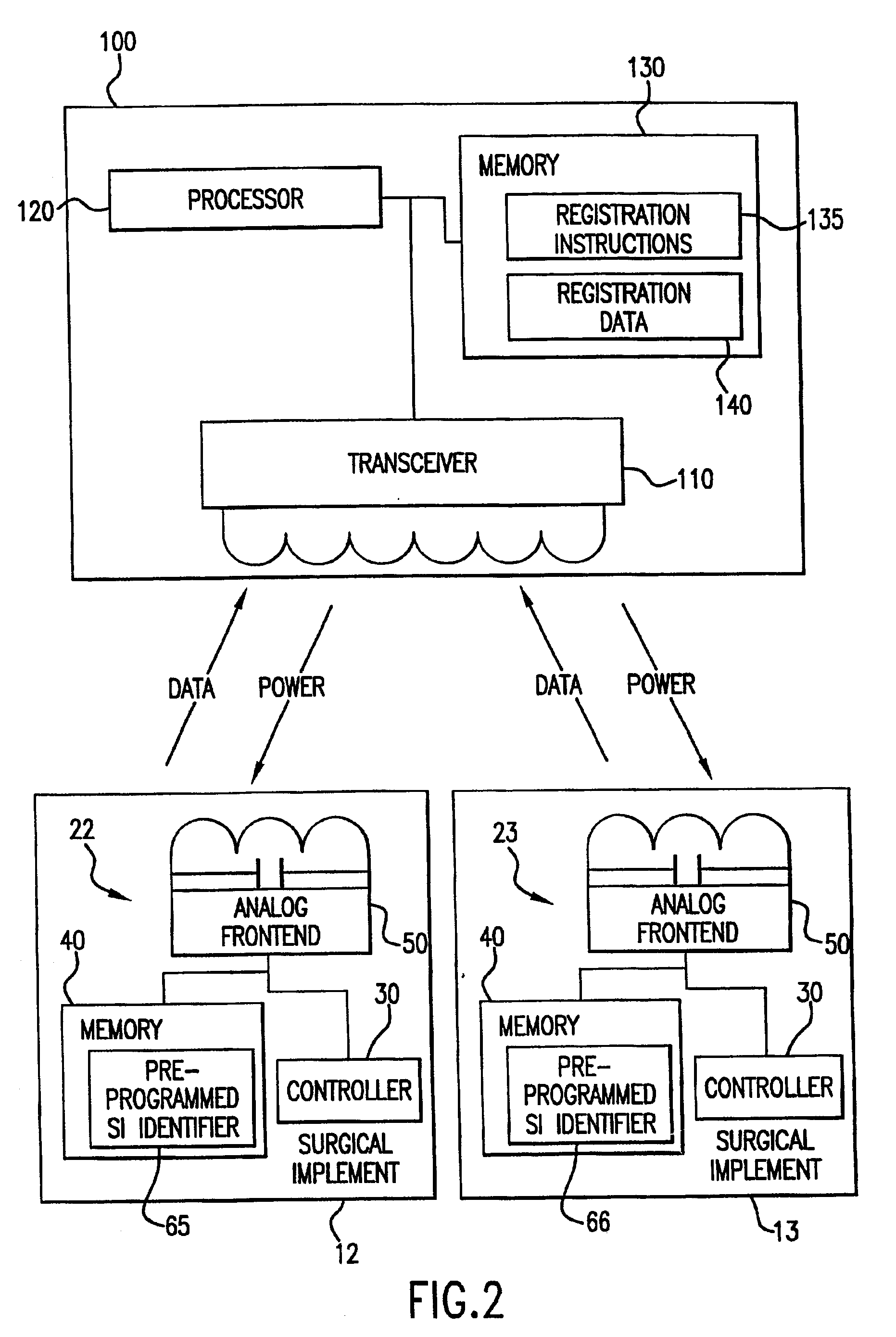
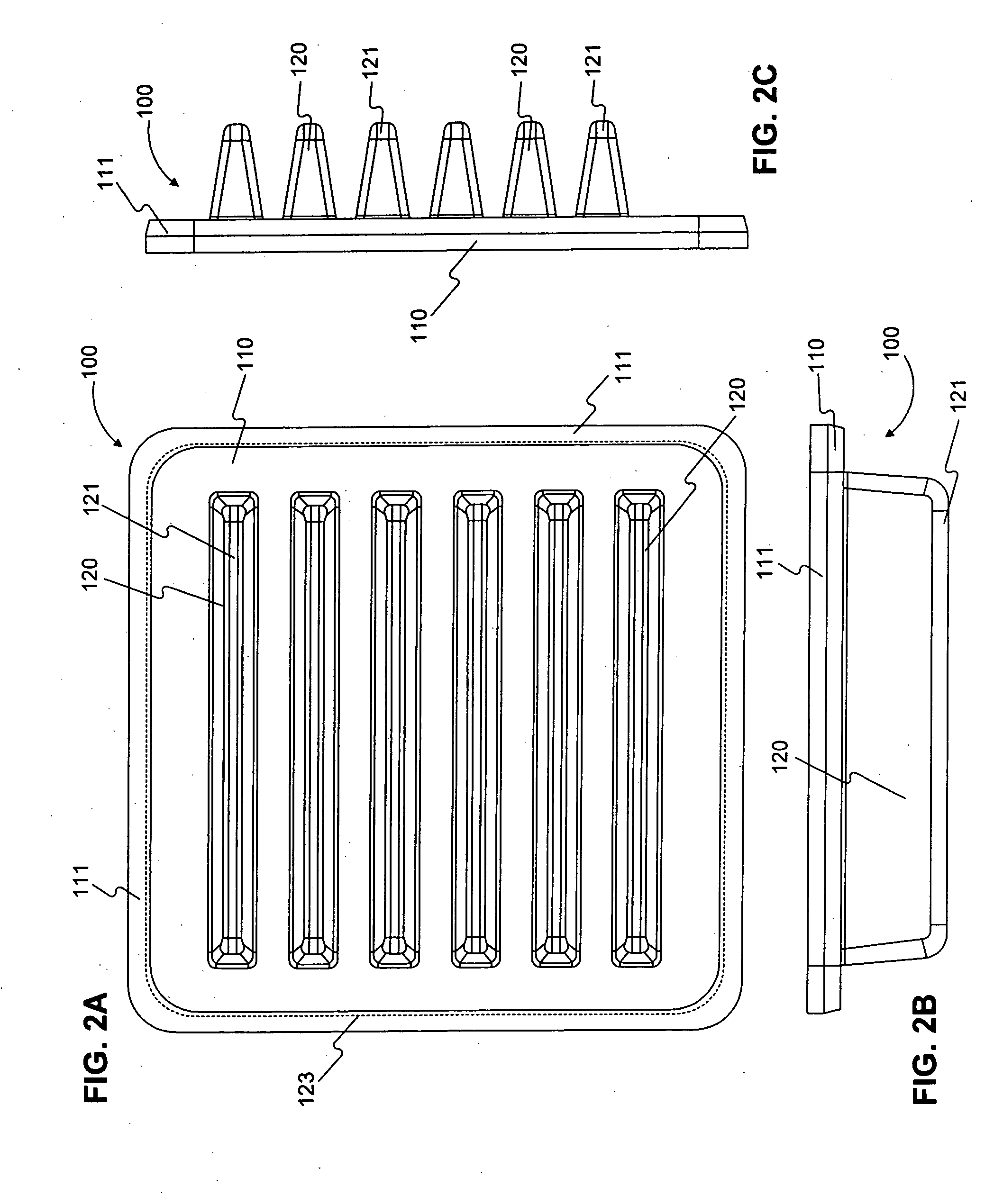
Tracking surgical implements with integrated circuits
A system and method of tracking medical products provides for associating a group of medical products with a group location based on a group radio frequency identification (RF ID) device signal, where the group includes a first unit and a second unit. The first unit is associated with a first remote location based on a first unit RF ID device signal. The method further provides for associating the second unit with a second remote location based on a second remote location based on a second unit RF ID device signal. The signals uniquely identify the units and the group.
Owner:OLIVE SHADE LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com