Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
159 results about "Infection control" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Infection control is the discipline concerned with preventing nosocomial or healthcare-associated infection, a practical (rather than academic) sub-discipline of epidemiology. It is an essential, though often underrecognized and undersupported, part of the infrastructure of health care. Infection control and hospital epidemiology are akin to public health practice, practiced within the confines of a particular health-care delivery system rather than directed at society as a whole. Anti-infective agents include antibiotics, antibacterials, antifungals, antivirals and antiprotozoals.
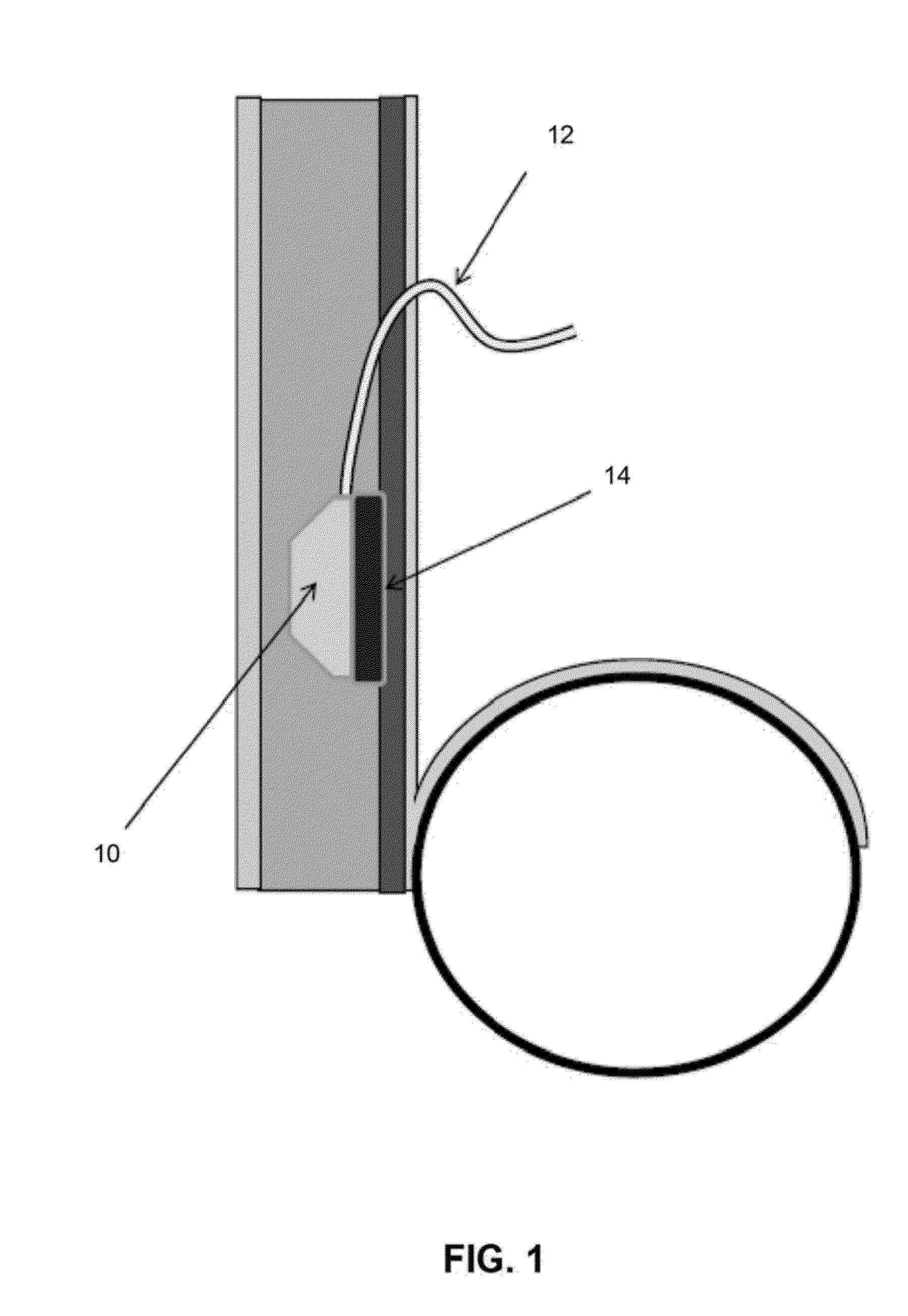
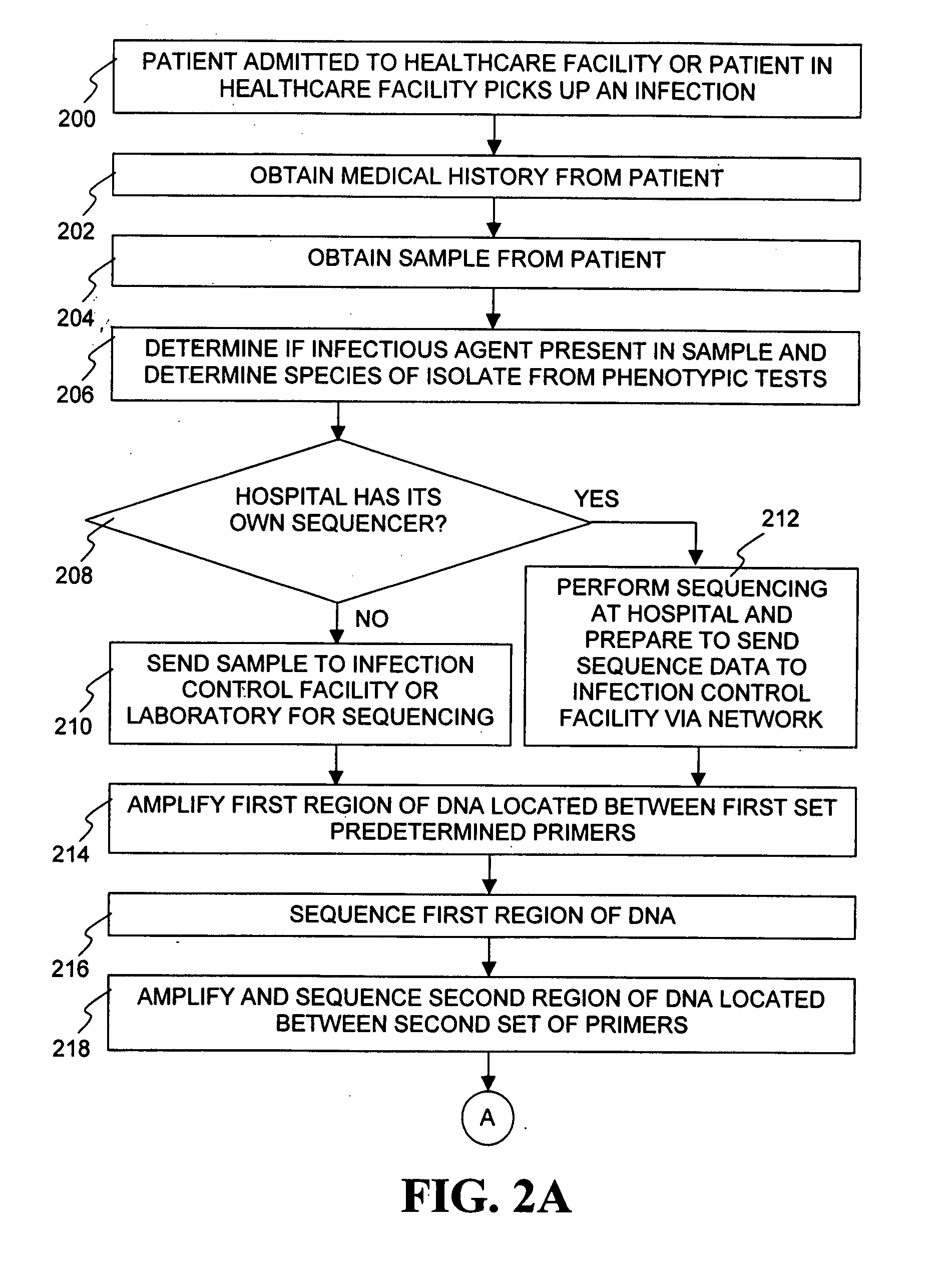
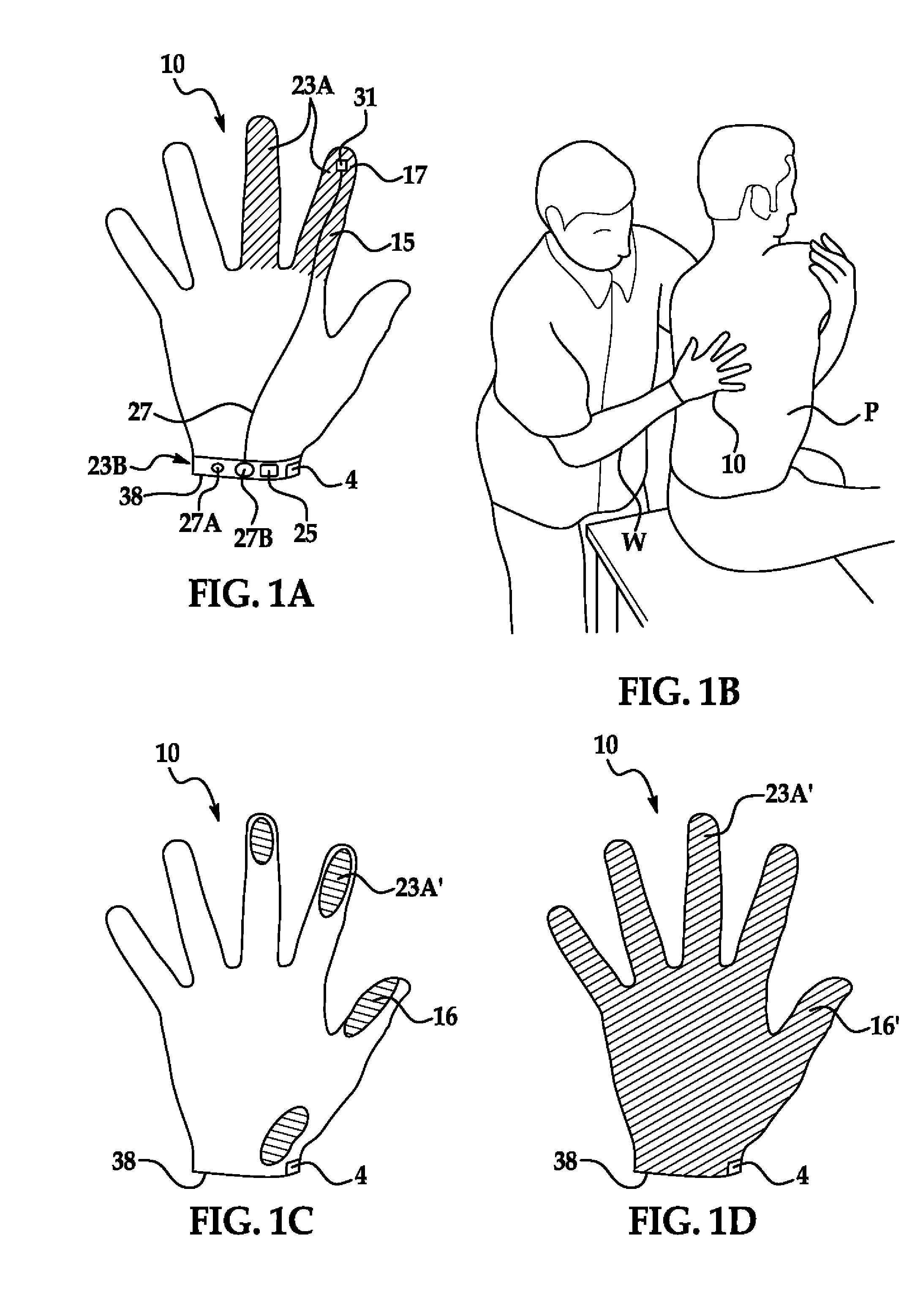
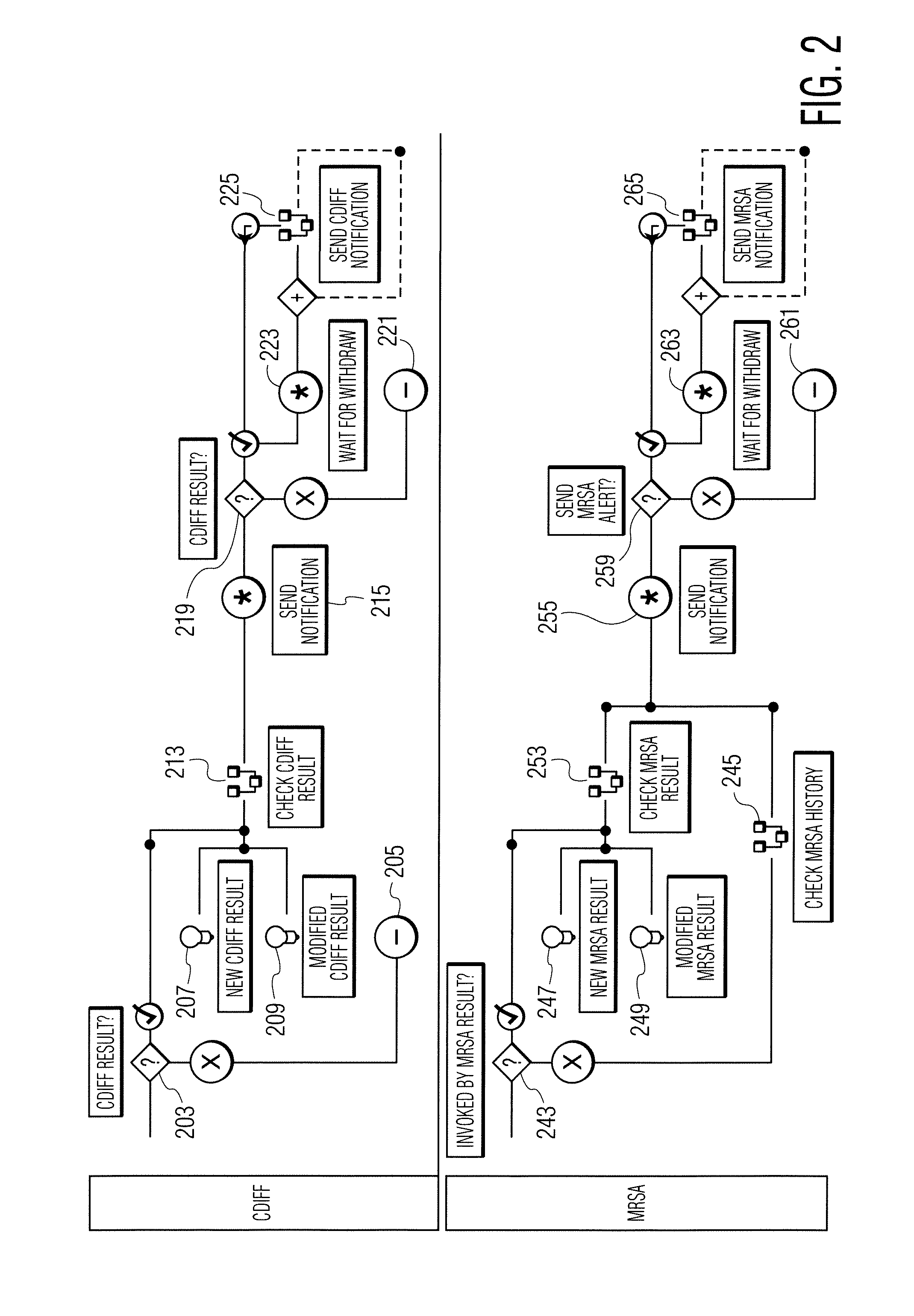
Method for tracking and controlling infections
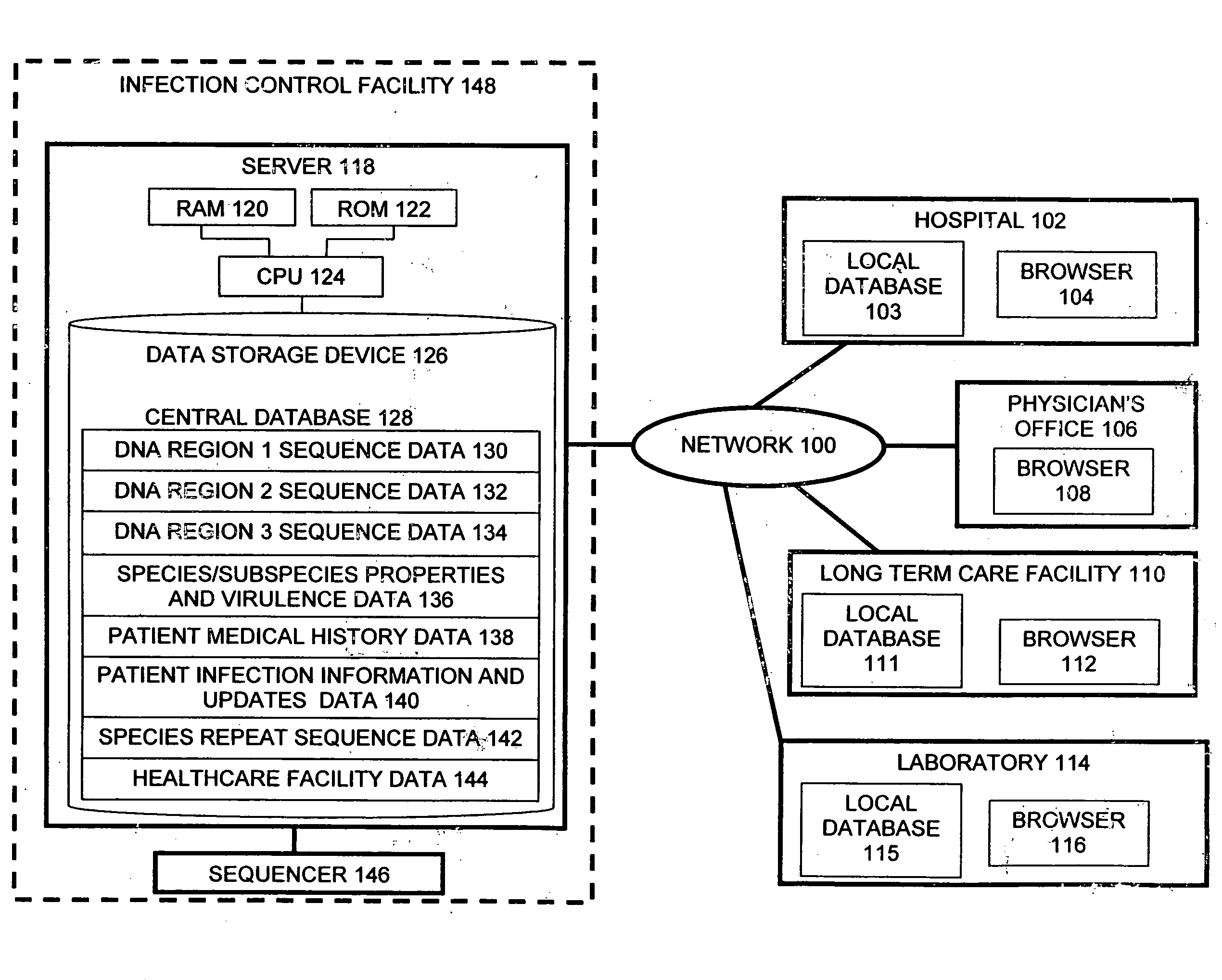
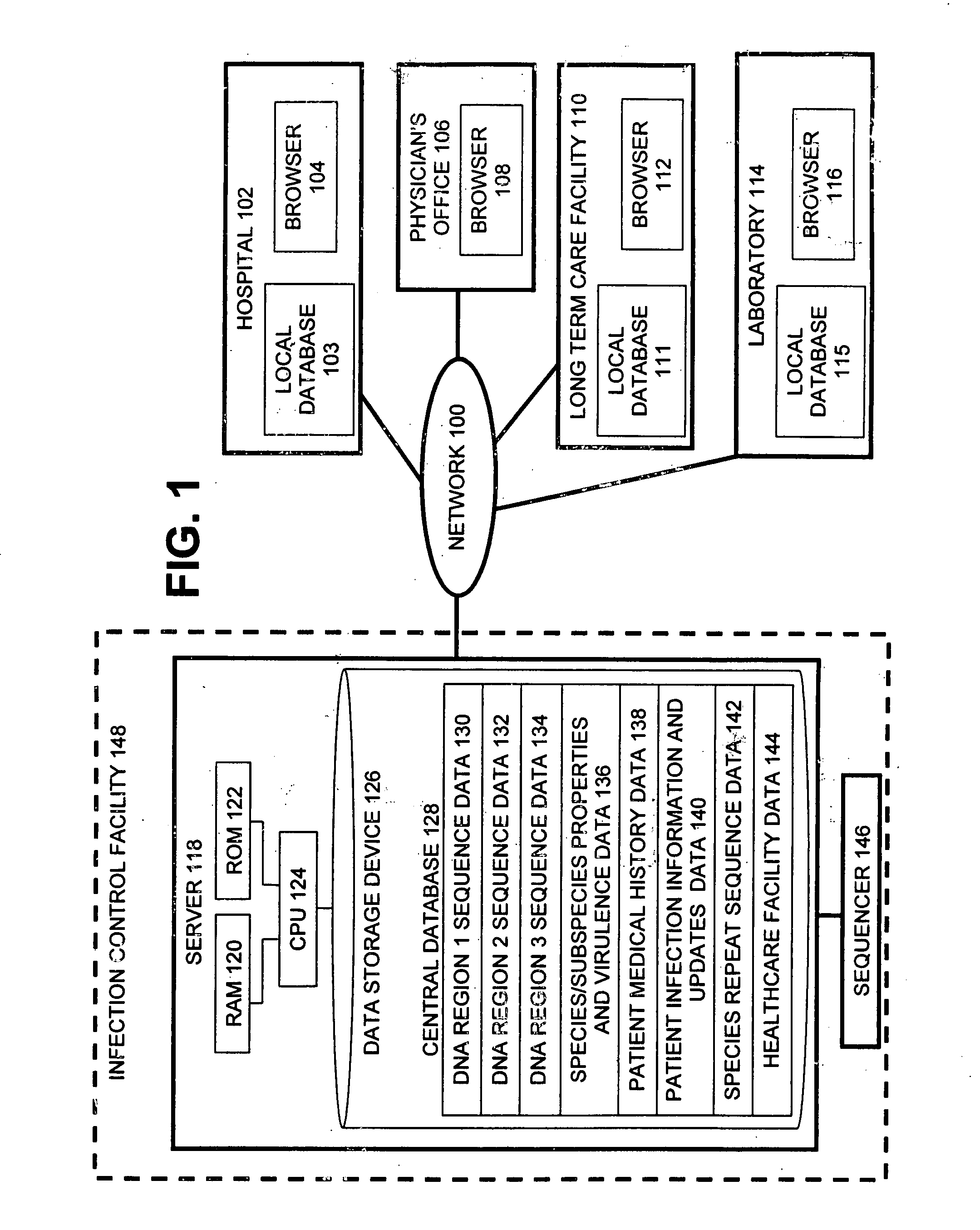
InactiveUS20060020391A1Computer-assisted medical data acquisitionMedical imagesPhylogenetic relatednessMicroorganism
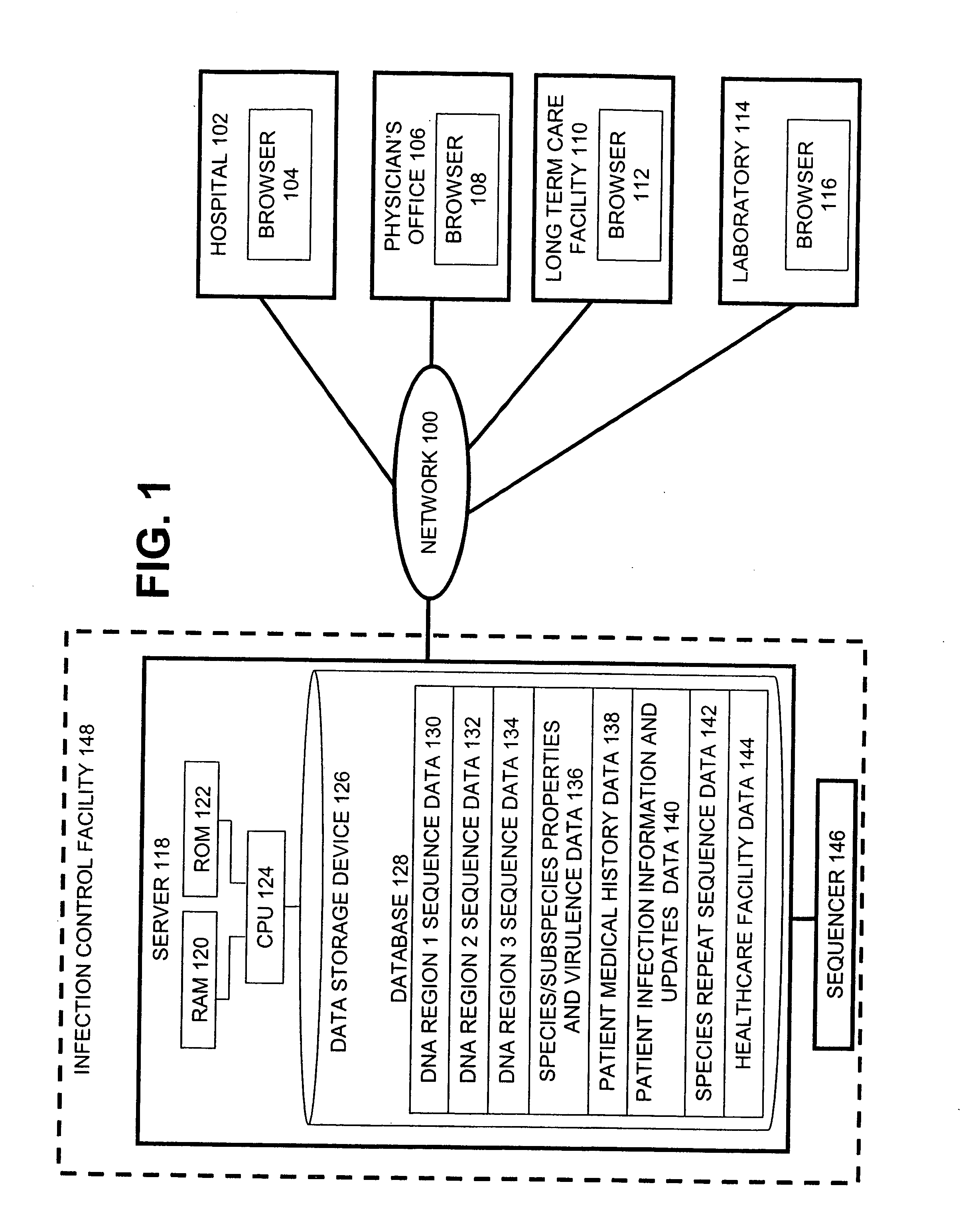
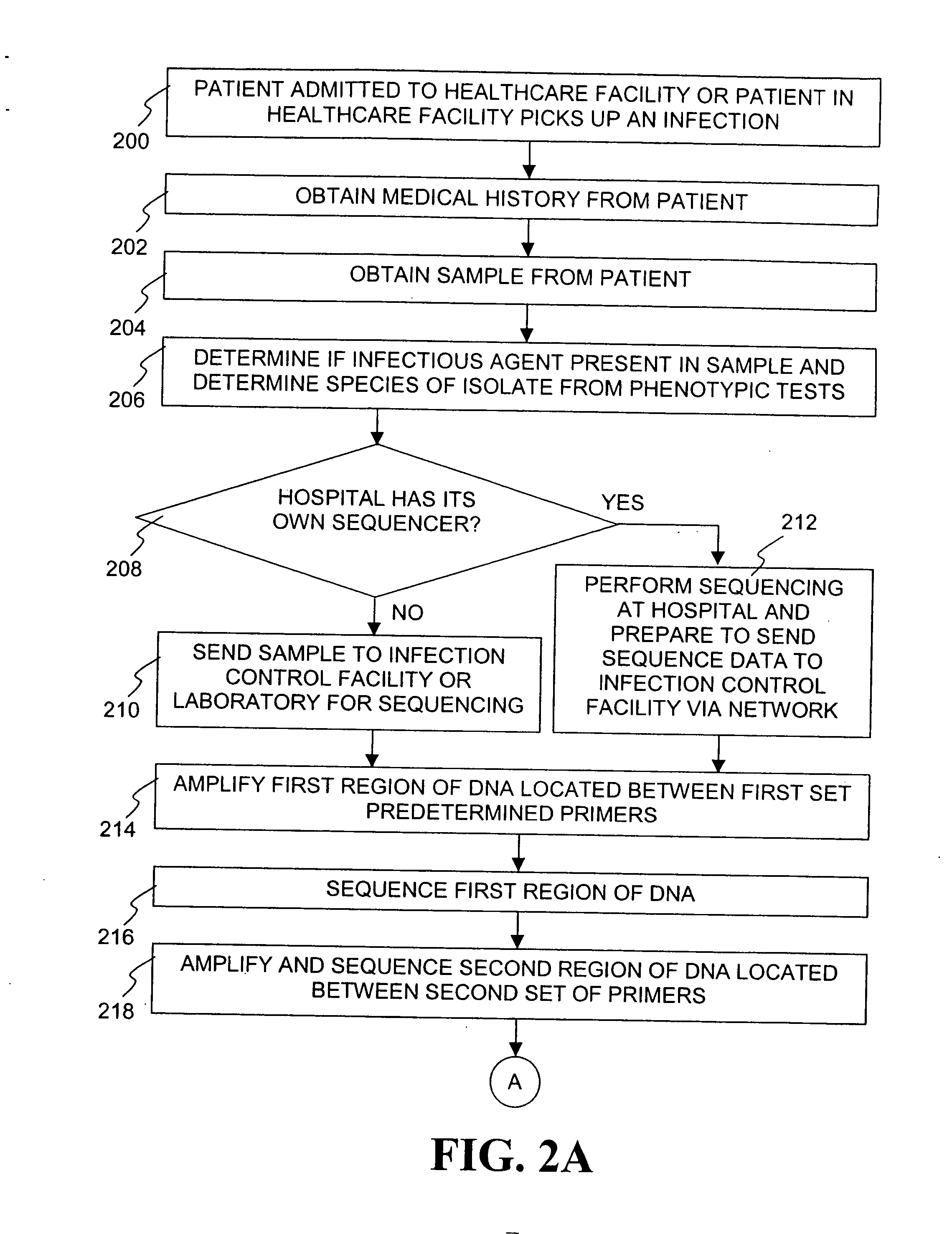
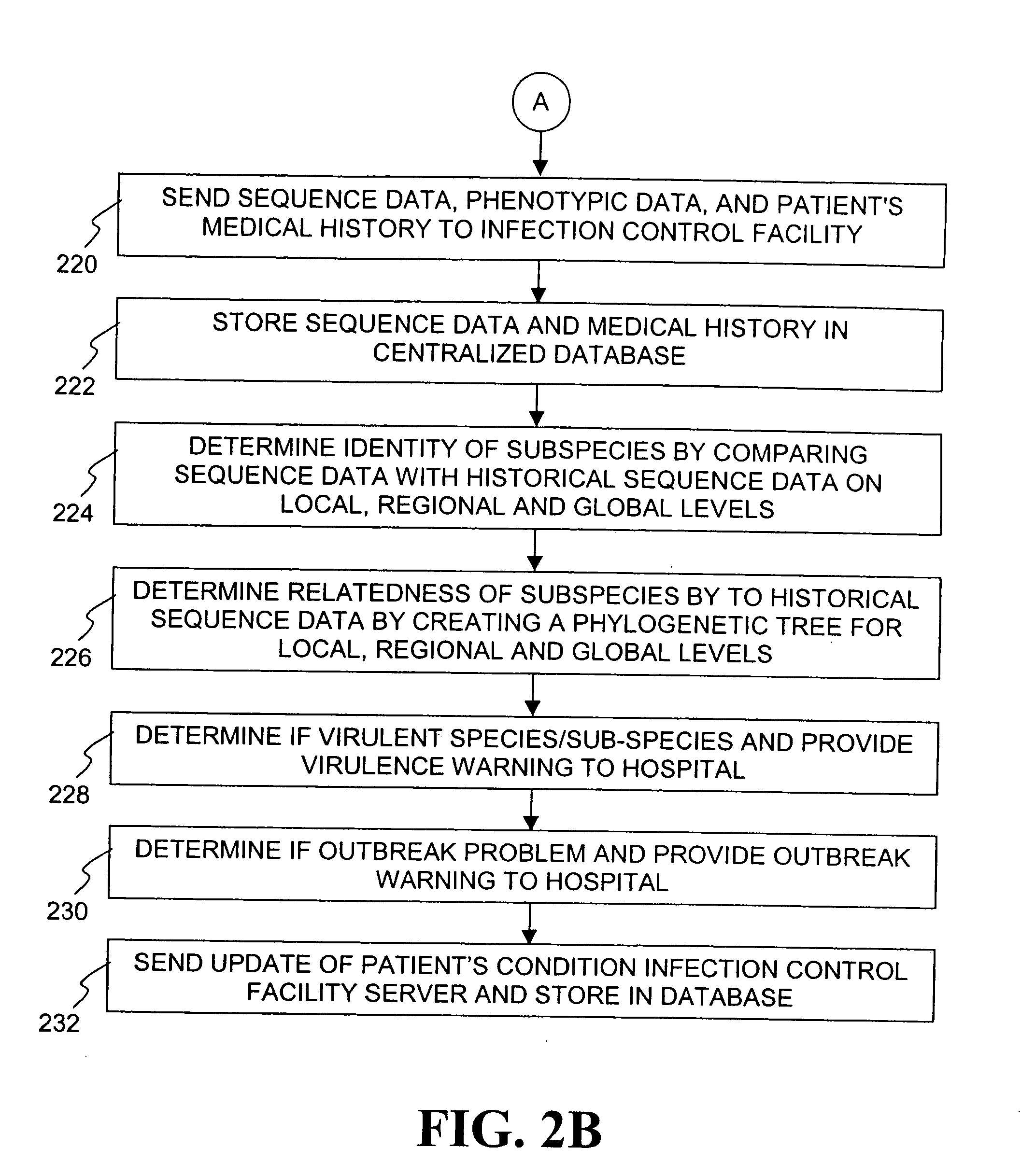
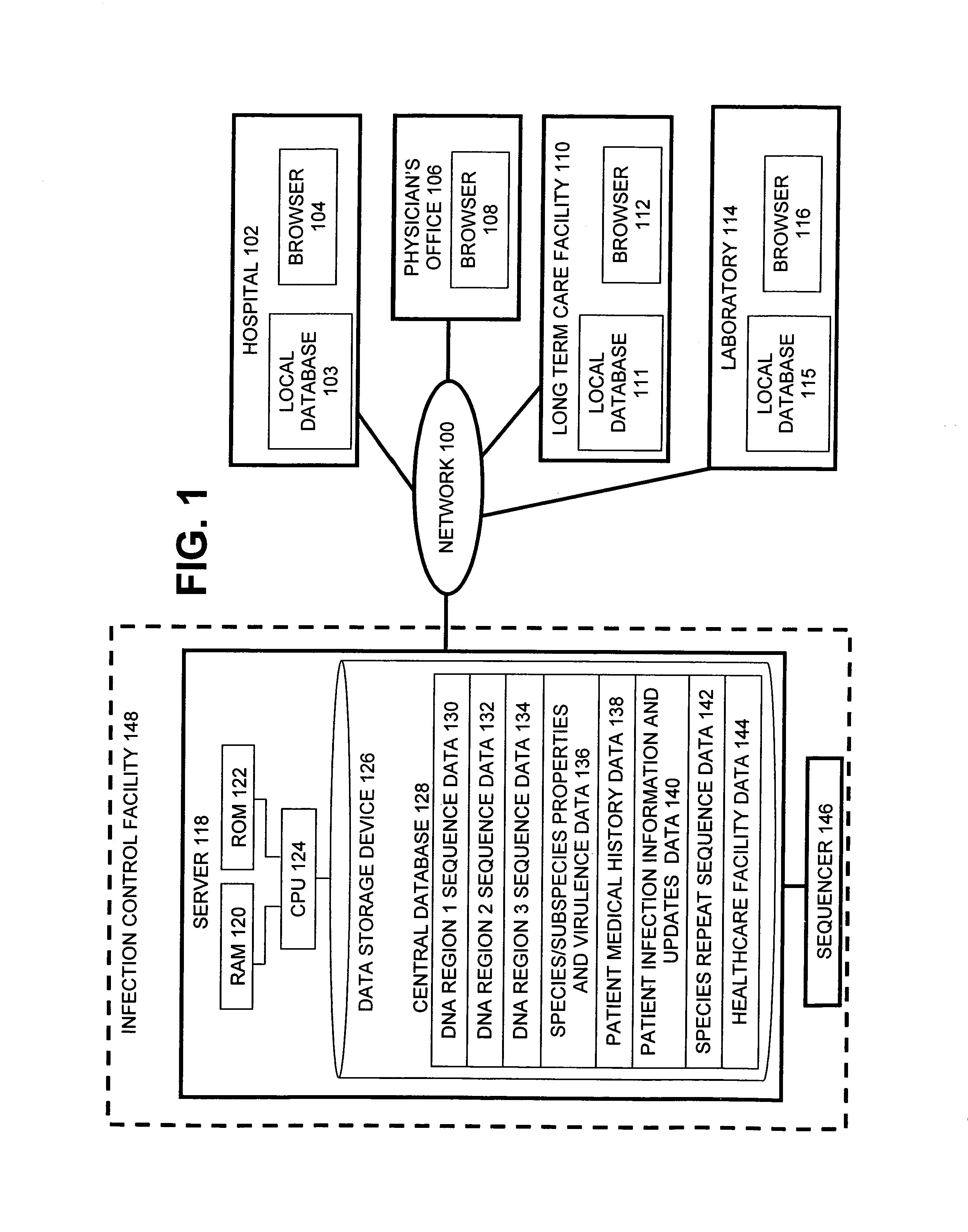
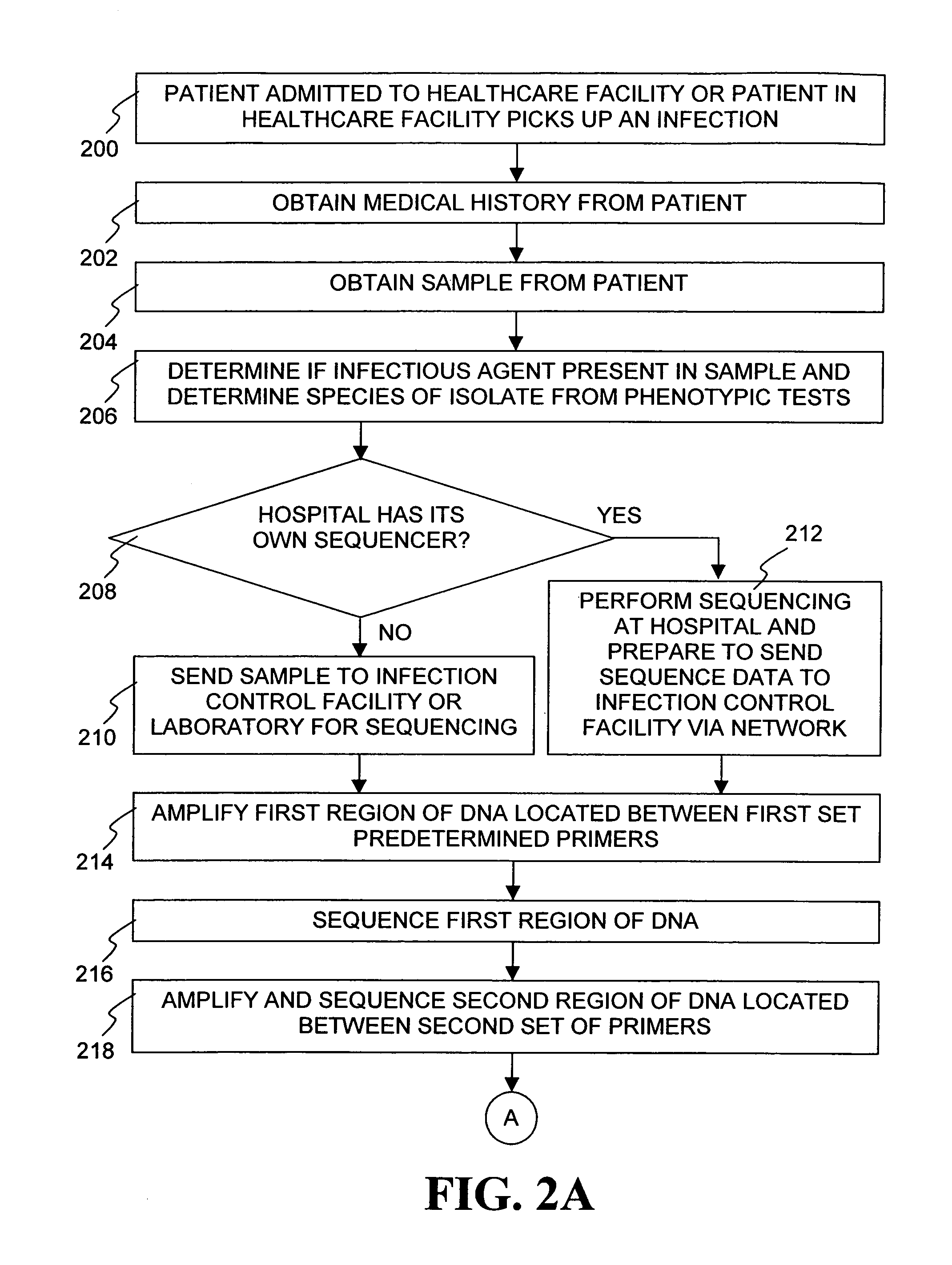
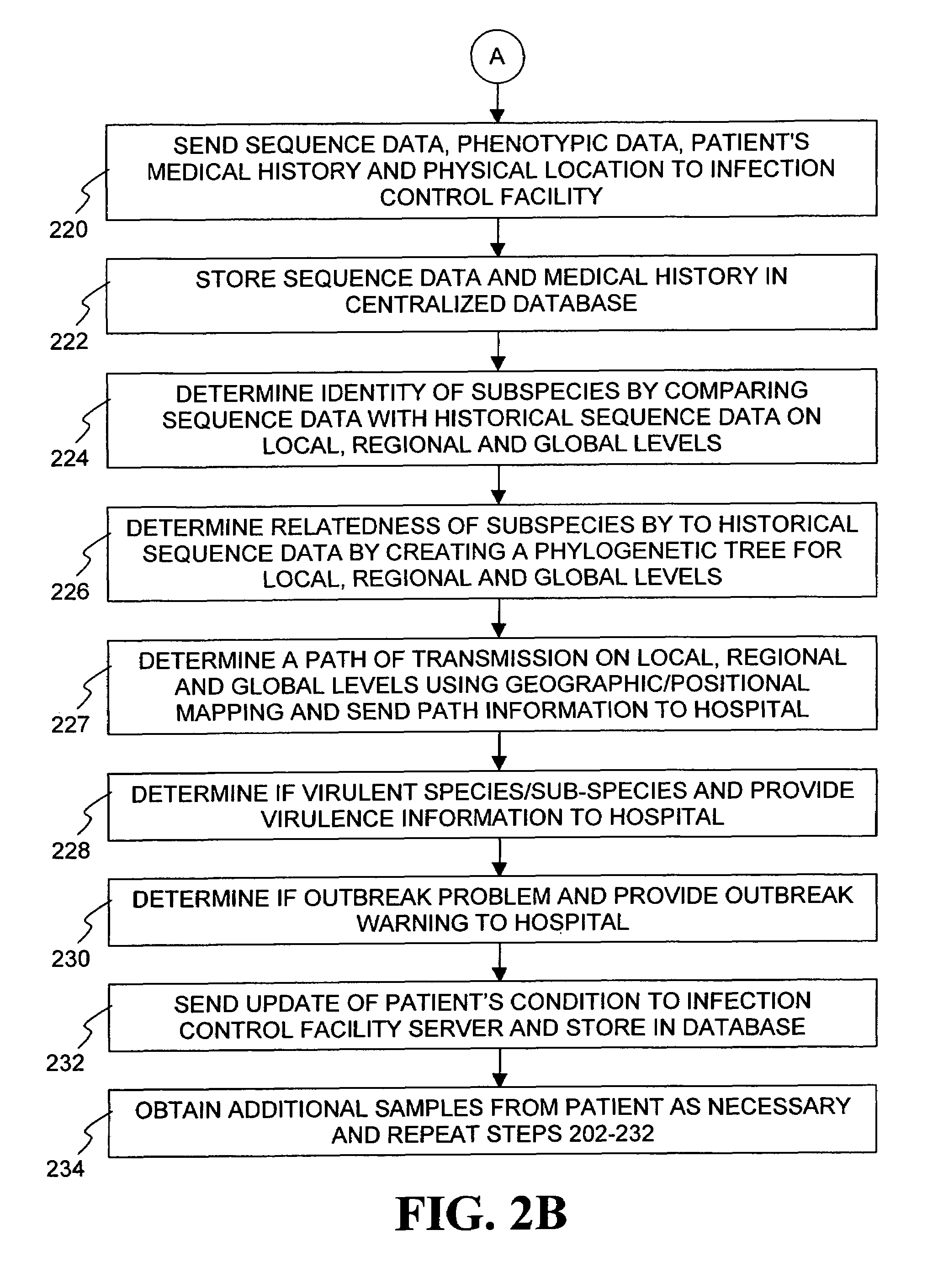
The present invention is a system and method for performing real-time infection control over a computer network. The method comprises obtaining a sample of a microorganism at a health care facility, sequencing a first region of a nucleic acid from the microorganism sample, comparing the first sequenced region with historical sequence data stored in a database, determining a measure of phylogenetic relatedness between the microorganism sample and historical samples stored in the database, and providing infection control information based on the phylogenetic relatedness determination to the health care facility, thereby allowing the health care facility to use the infection control information to control or prevent the spread of an infection.
Owner:EGENOMICS INC
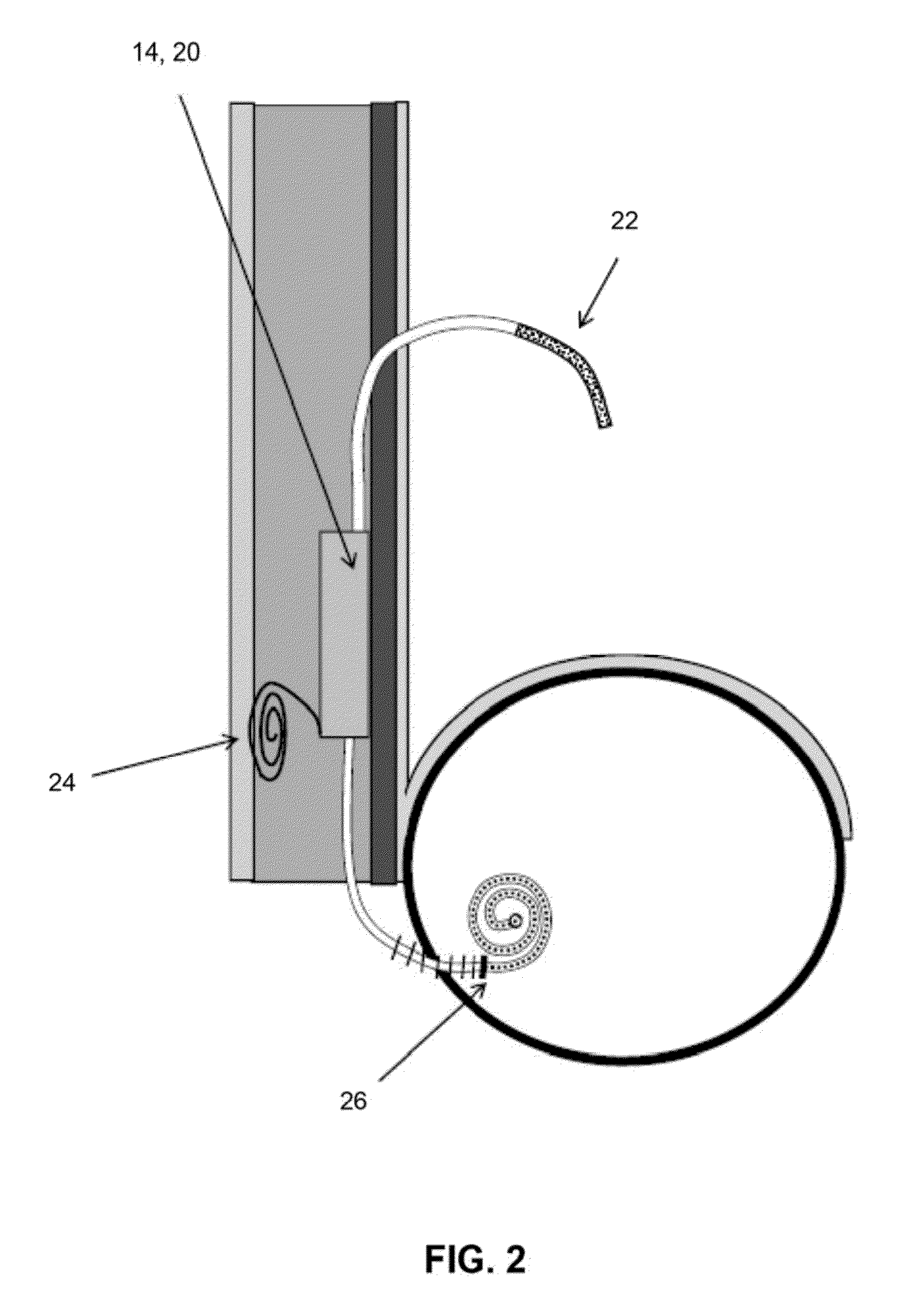
System and method for tracking and controlling infections
InactiveUS7349808B1Digital data processing detailsAnalogue computers for chemical processesMicroorganismPhylogenetic relatedness
The present invention is a system and method for performing real-time infection control over a computer network. The method comprises obtaining a sample of a microorganism at a health care facility, sequencing a first region of a nucleic acid from the microorganism sample, comparing the first sequenced region with historical sequence data stored in a database, determining a measure of phylogenetic relatedness between the microorganism sample and historical samples stored in the database, and providing infection control information based on the phylogenetic relatedness determination to the health care facility, thereby allowing the health care facility to use the infection control information to control or prevent the spread of an infection.
Owner:EGENOMICS INC +1
Antimicrobial treatment of nonwoven materials for infection control
A material substrate having at least part of a surface treated with an antimicrobial composition is described. The antimicrobial composition exhibits at least a 3 log10 CFU reduction within a period of about 30 minutes after contact with various species of a broad spectrum of microorganisms. The substrate can be a nonwoven material that has good fluid barrier properties, which can be used in protective garments and sheets. Methods for manufacturing and imparting the antimicrobial treatment to the substrate are also provided.
Owner:KIMBERLY-CLARK WORLDWIDE INC
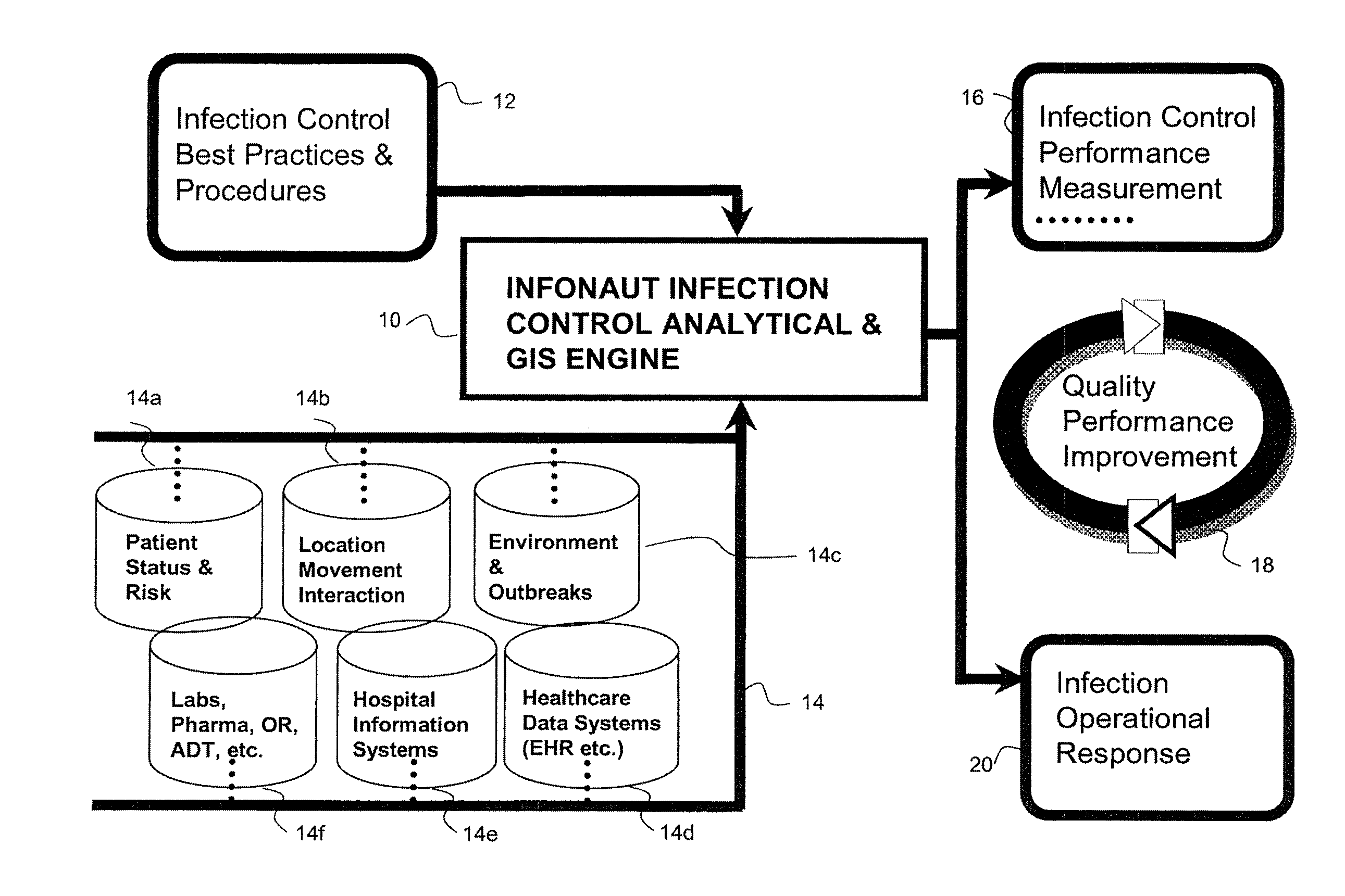
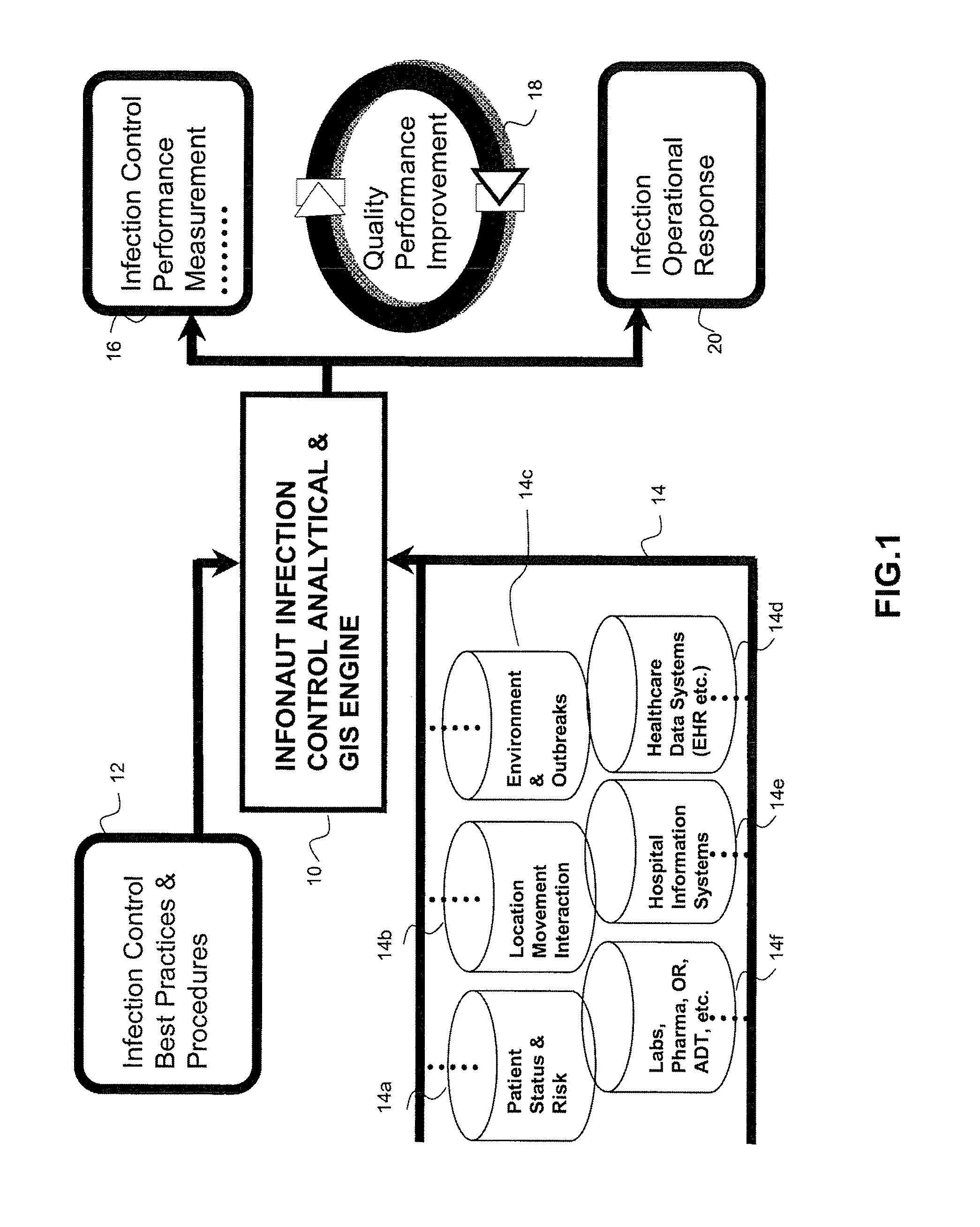
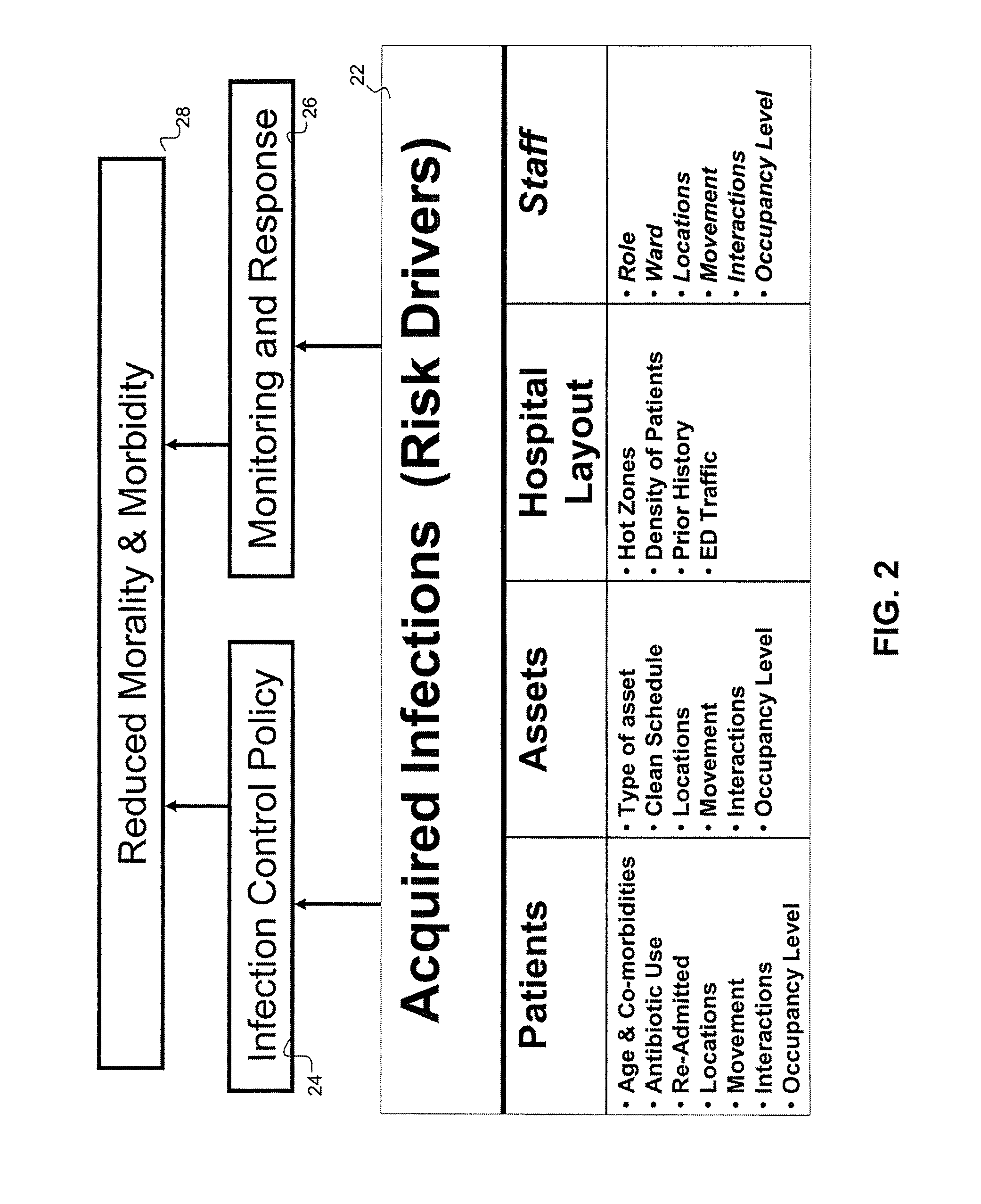
Disease Mapping and Infection Control System and Method
Owner:INFONAUT
Semisolid system and combination semisolid, multiparticulate system for sealing tissues and/or controlling biological fluids
InactiveUS20060127437A1Alter viscosityAlter consistencyAbsorbent padsSynthetic polymeric active ingredientsSolid massMedical product
A semisolid system and combination semisolid, multiparticulate system for controlling biological fluids is provided which includes a body fluid control composition. The body fluid control composition is adapted for utilization both as an independent mixture for direct topical application to body tissue for human or veterinary application and for incorporation with a standard medical products in order to facilitate absorption, moisture balance, hemostasis, infection control, and wound healing.
Owner:SOUTHEASTERN MEDICAL TECH
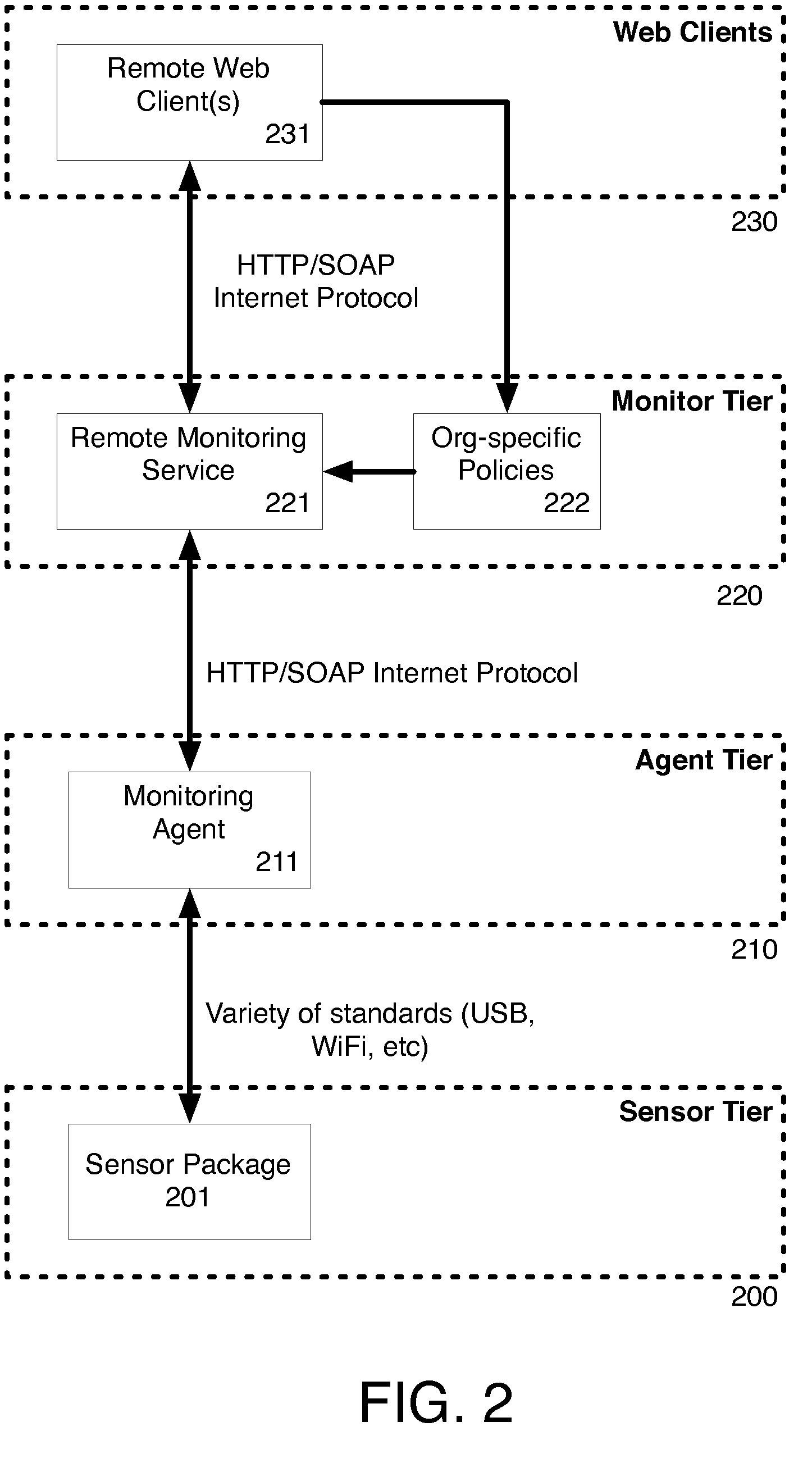
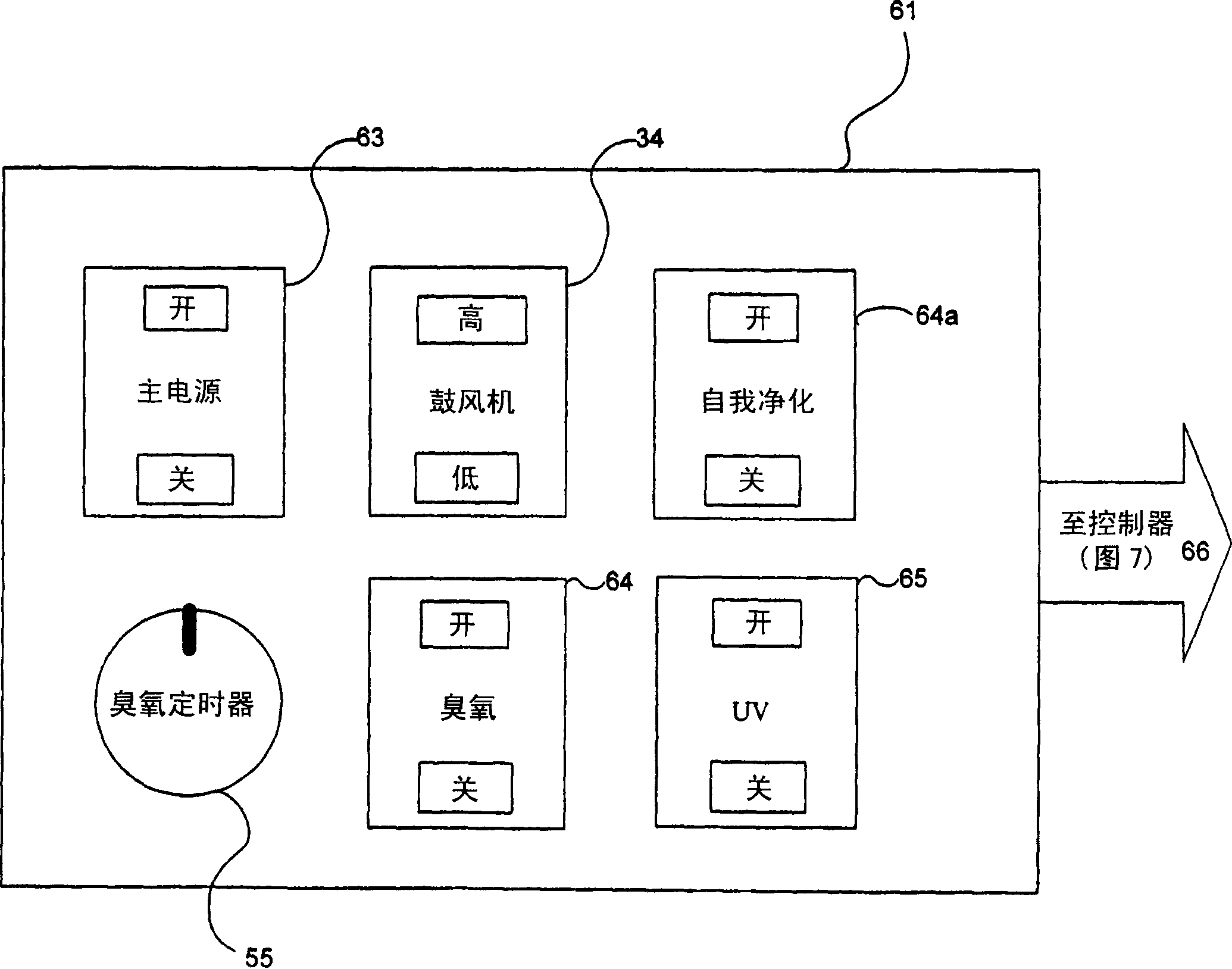
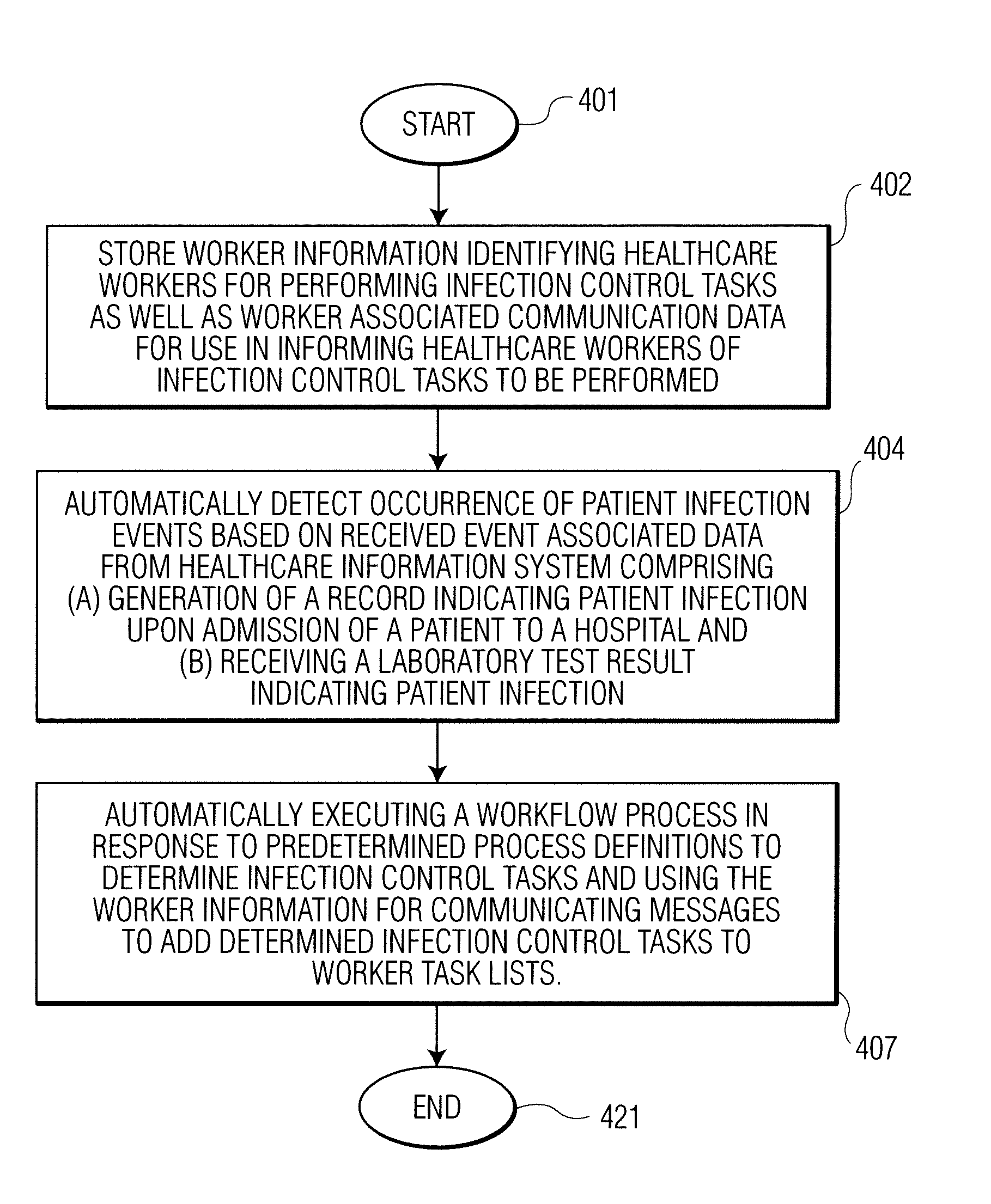
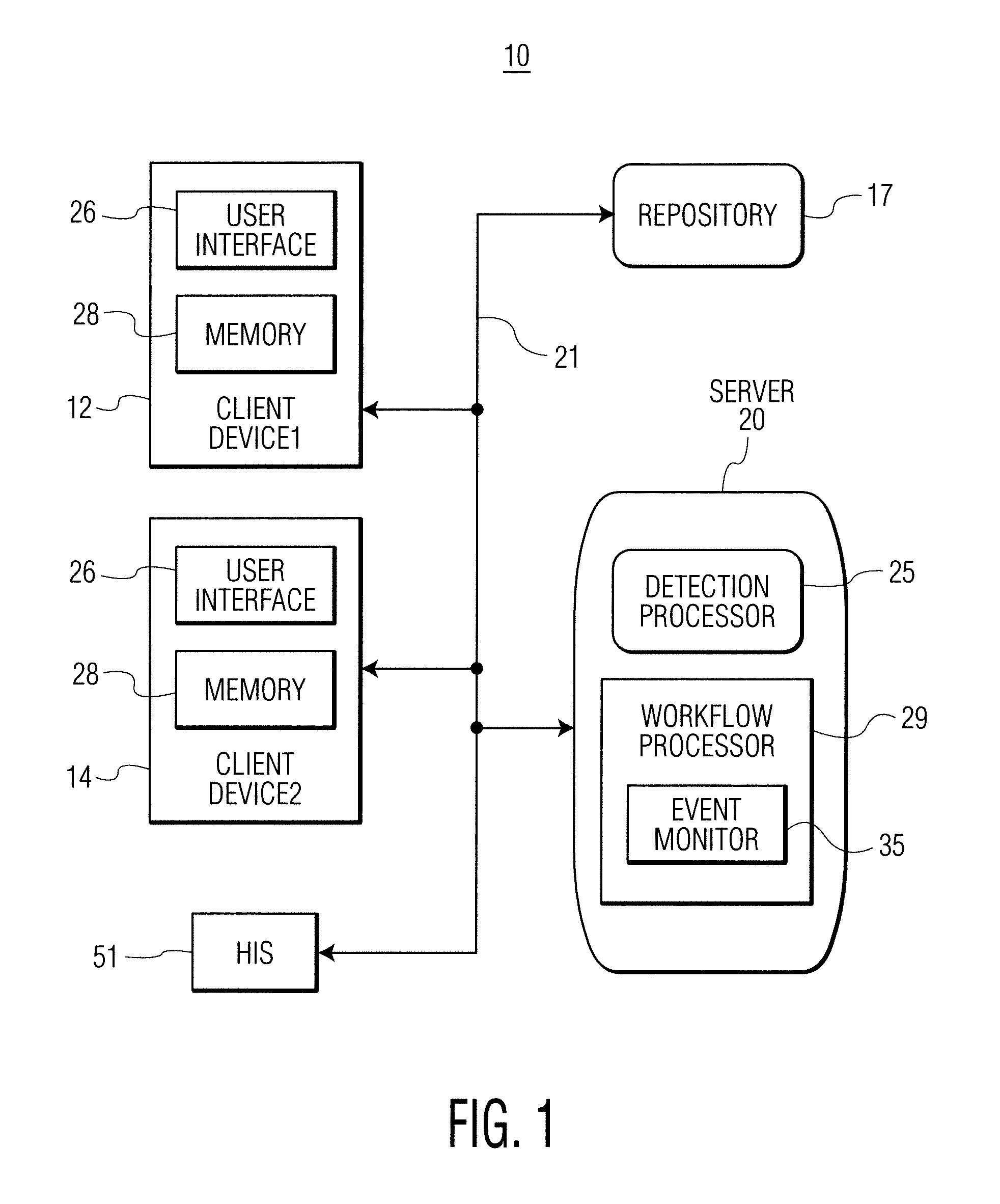
Infection control management and workflow system
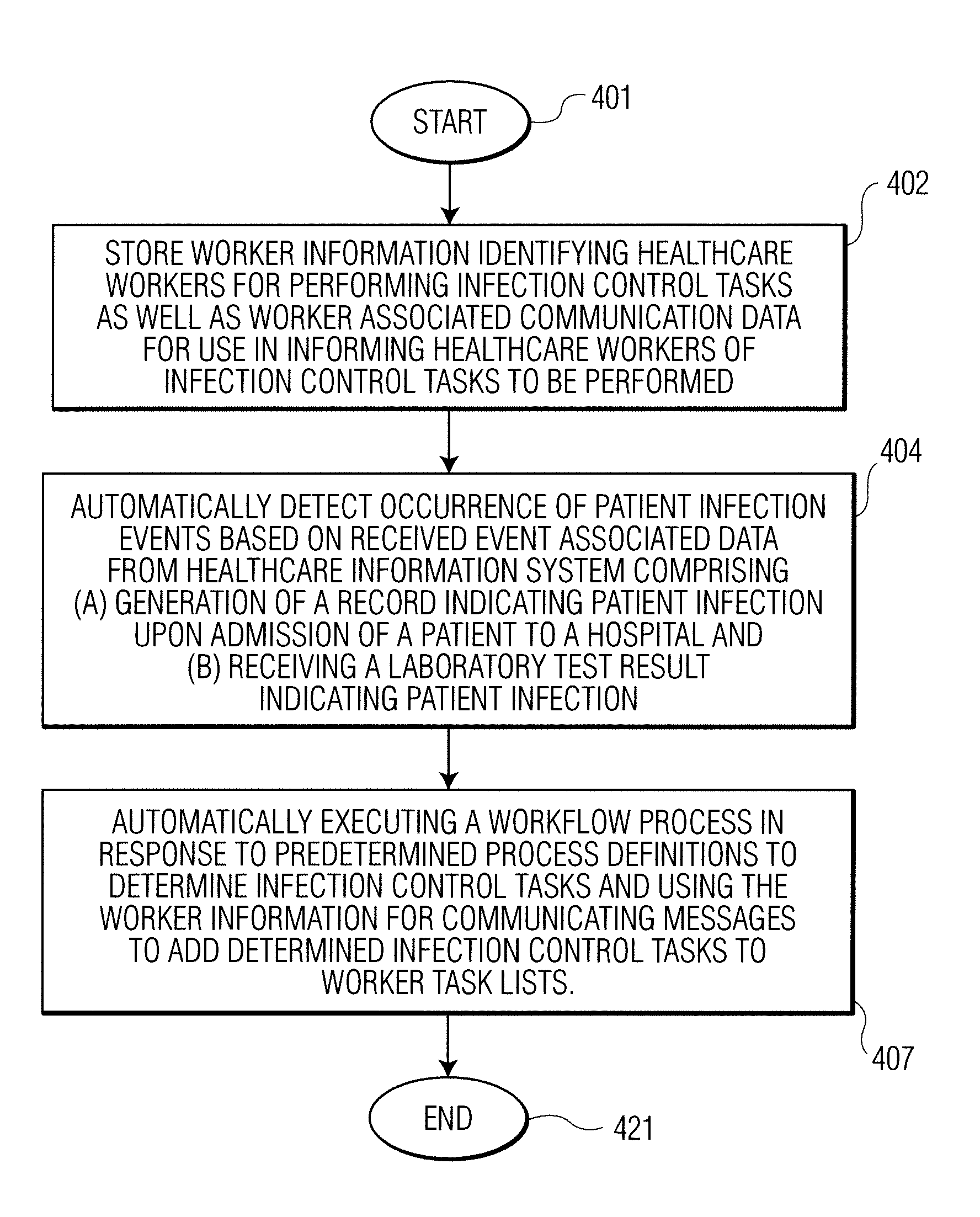
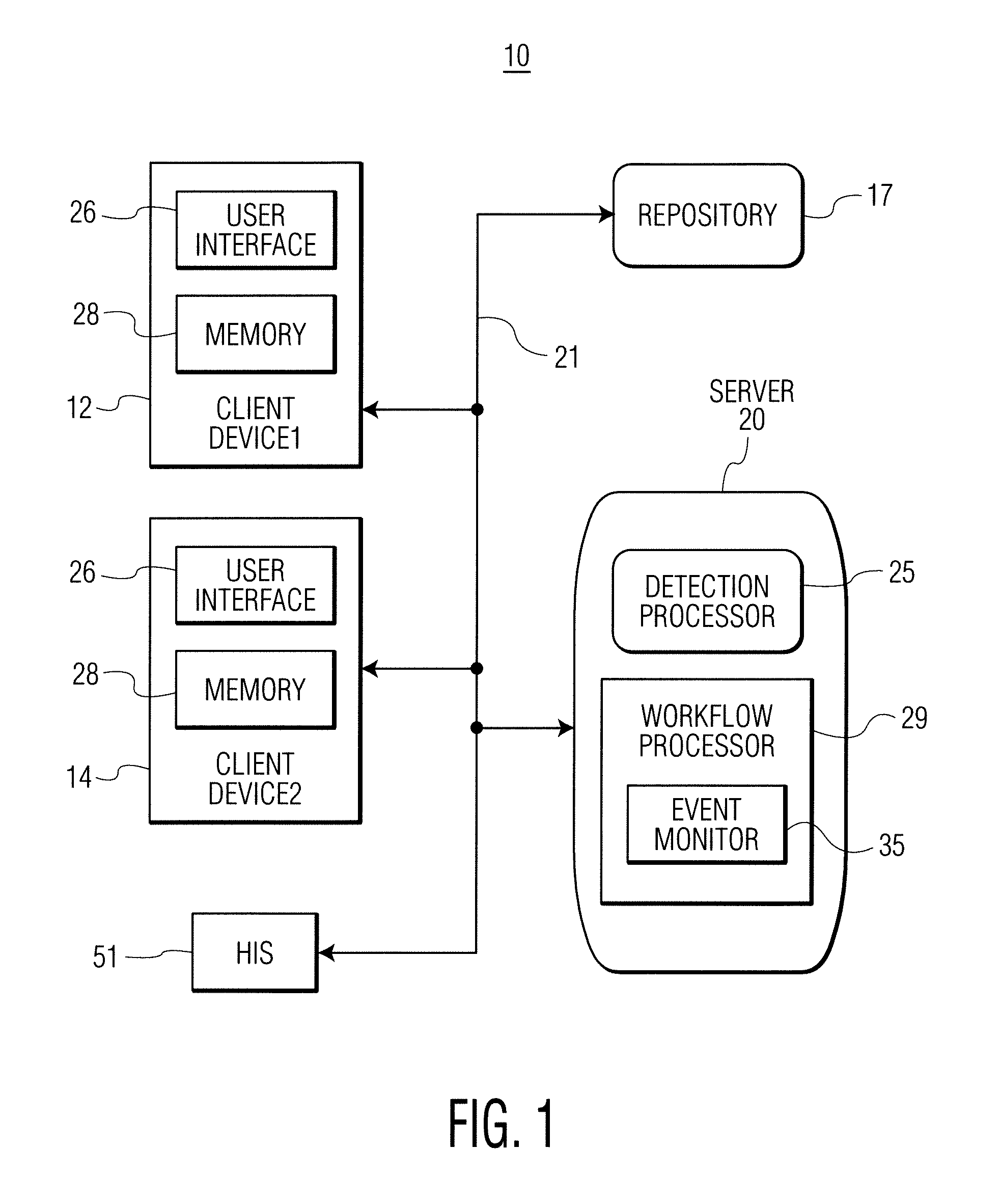
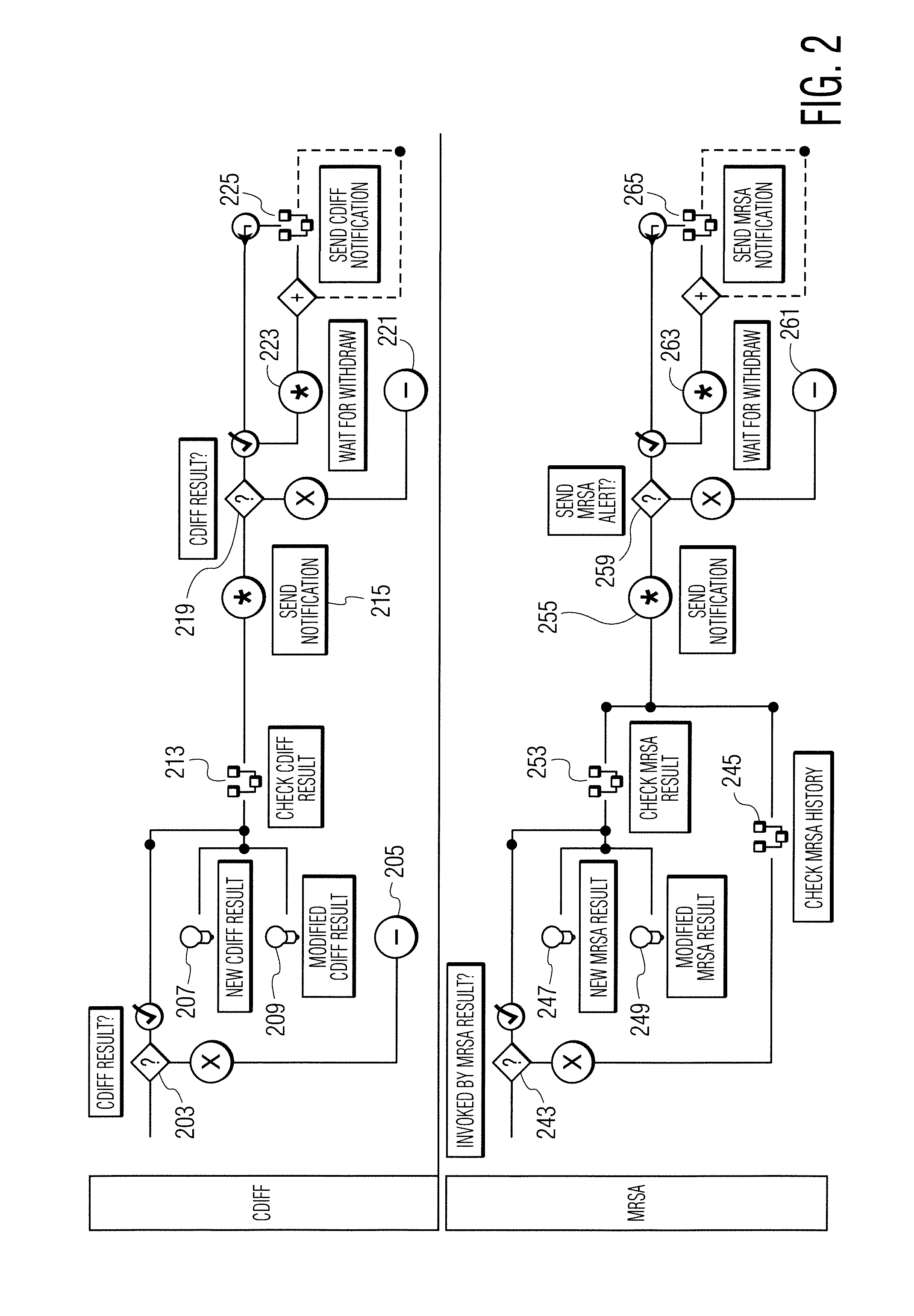
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
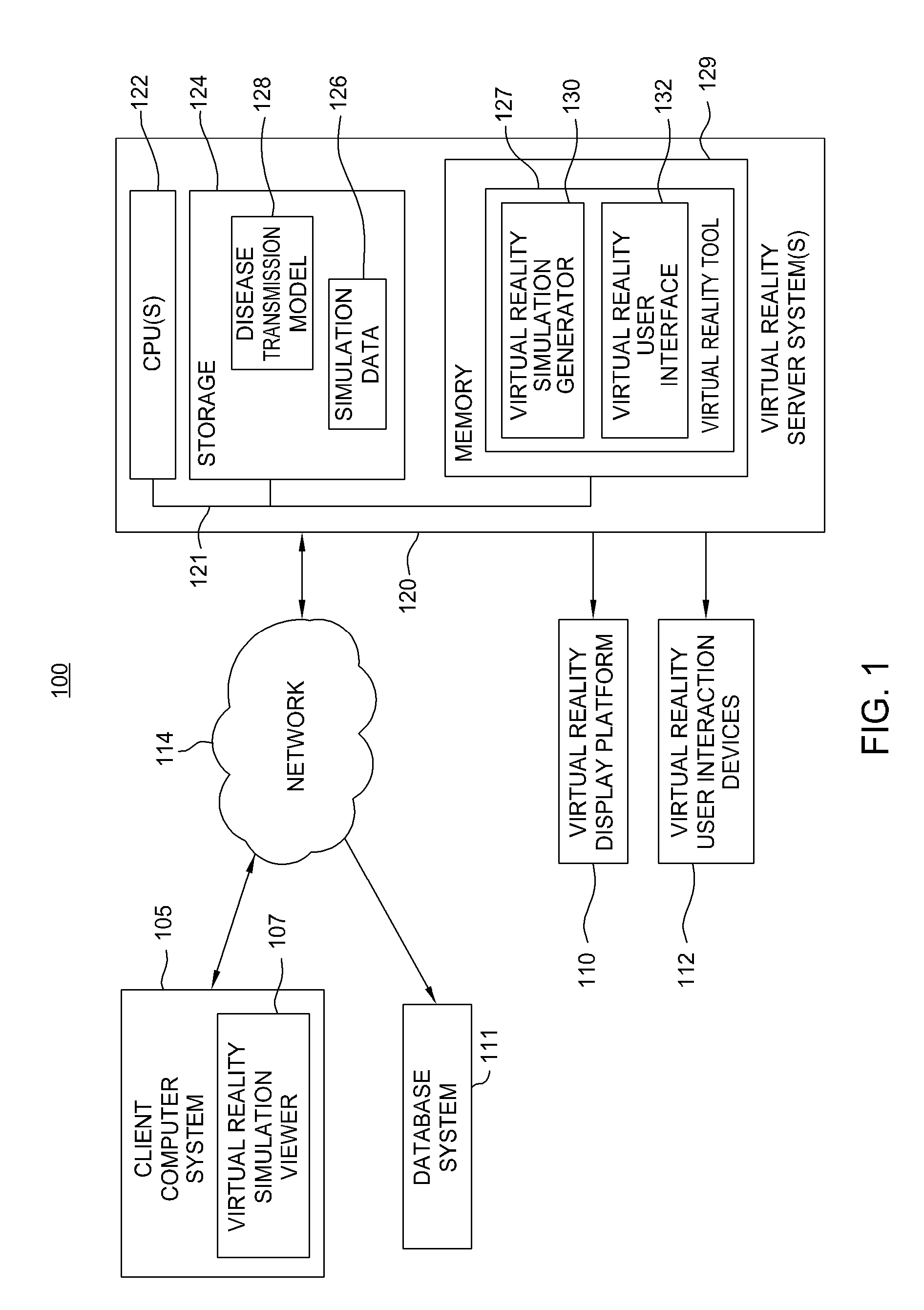
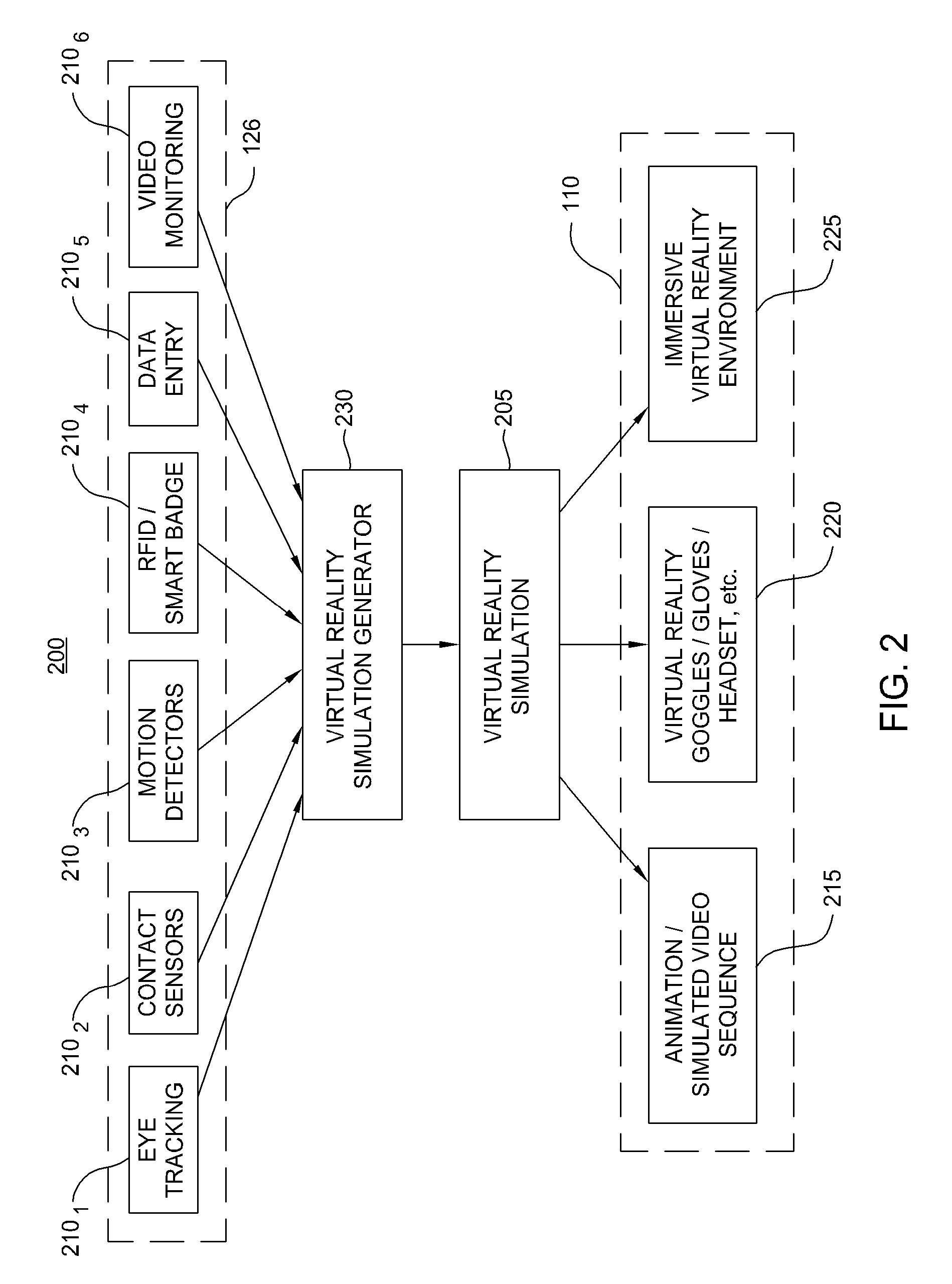
Virtual reality tools for development of infection control solutions
InactiveUS20090112541A1Analogue computers for chemical processesTeaching apparatusHuman behaviorComputer science
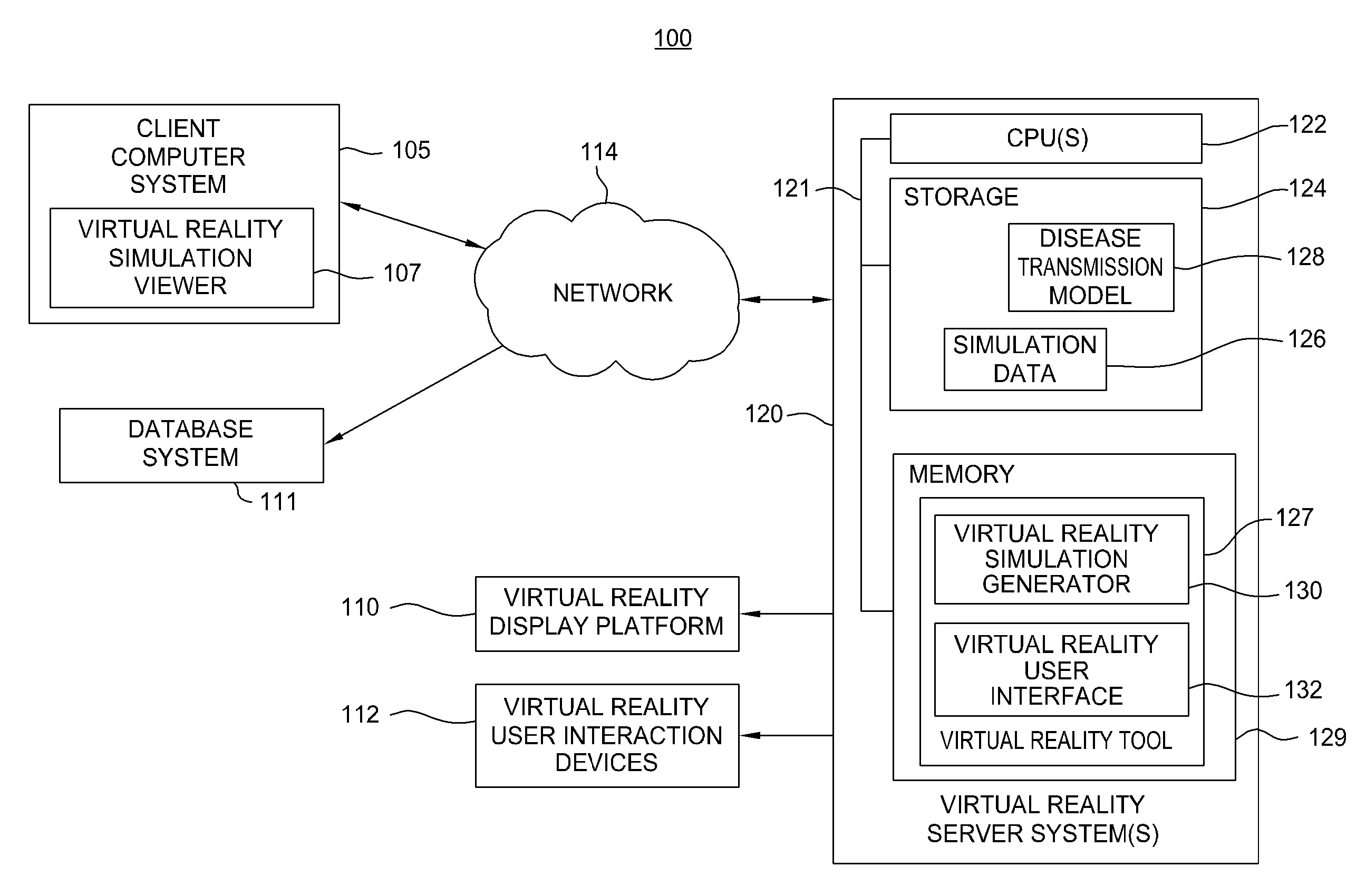
Embodiments of the invention provide virtual reality tools used to develop improved infection control solutions. The virtual reality tools may be applied to a variety of health care environments to develop improved infection control solutions, including improved training systems for modifying human behavior regarding hand washing (or other infection control behaviors), improved systems for evaluating the effectiveness of a proposed infection control solution, and for a producer / seller of anti-microbial products to demonstrate the superiority of one product over another or the superiority of one proposed solution over another.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Temperature probe adapter
InactiveUS20050249263A1Improve accuracyImprove performanceThermometer detailsThermometers using electric/magnetic elementsElectrical resistance and conductanceThermistor
An electronic thermometer that reduces patient exposure to all sources of cross-contamination, aids in infection control, and provides a clean, uncontaminated, readily accessible source of probe covers. A probe assembly for an electronic thermometer, which does not require expensive calibration procedures during manufacturing and allows the use of inexpensive thermisters. A memory component such as an EEPROM integrated circuit stores calibration information and identifying information in each particular probe assembly which improves performance and reduces manufacturing costs.
Owner:YERLIKAYA Y DENNIS +1
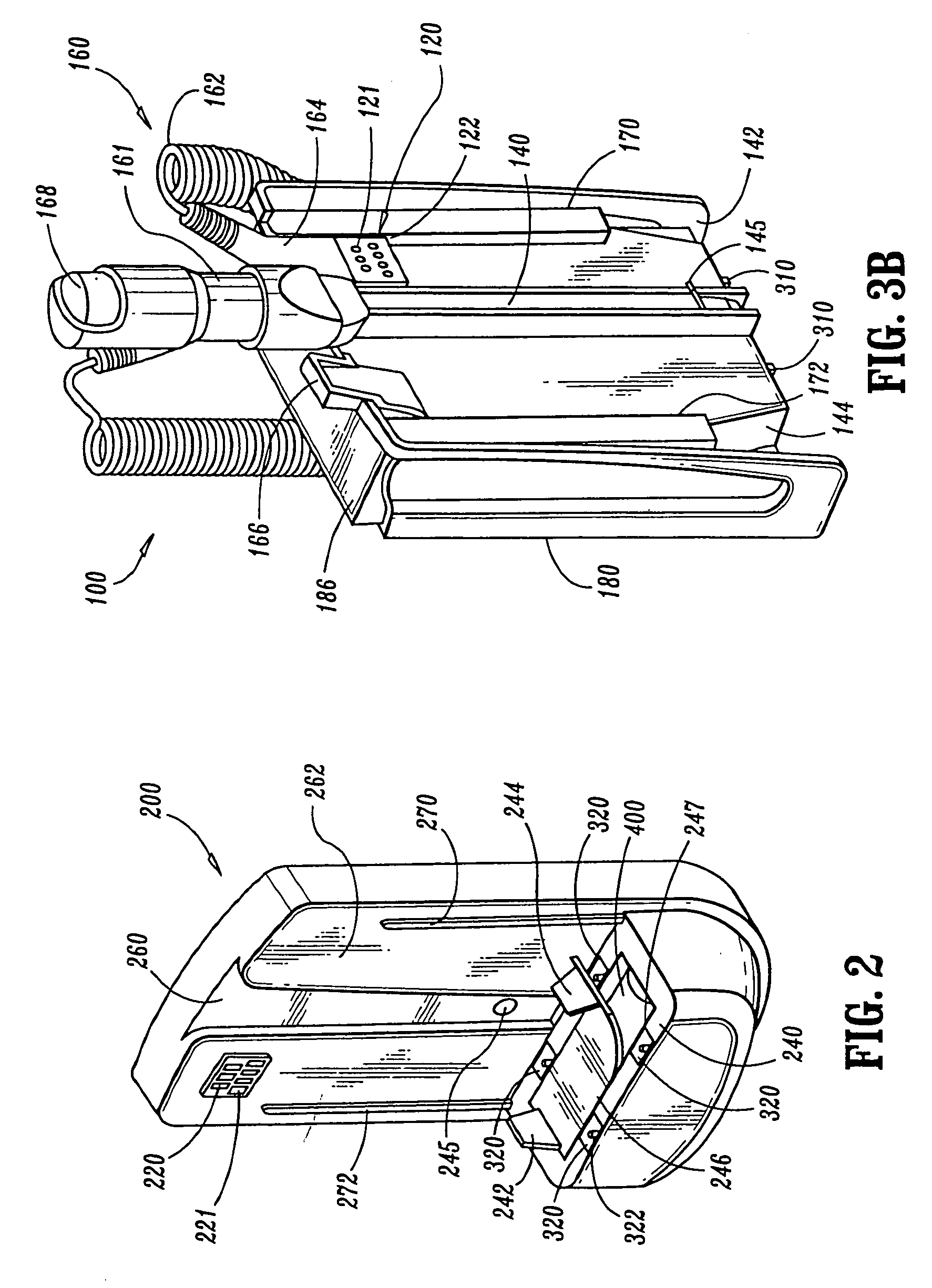
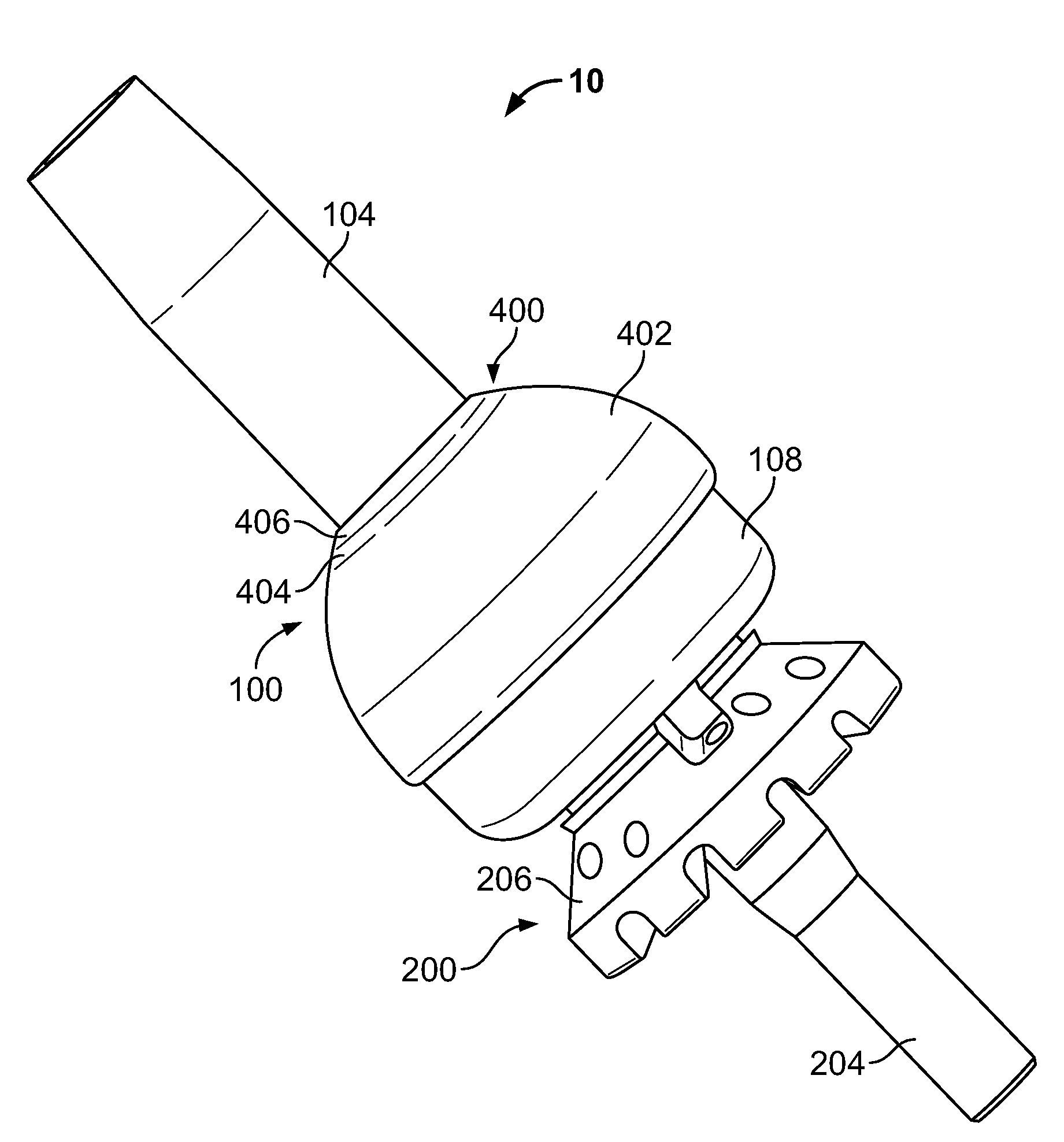
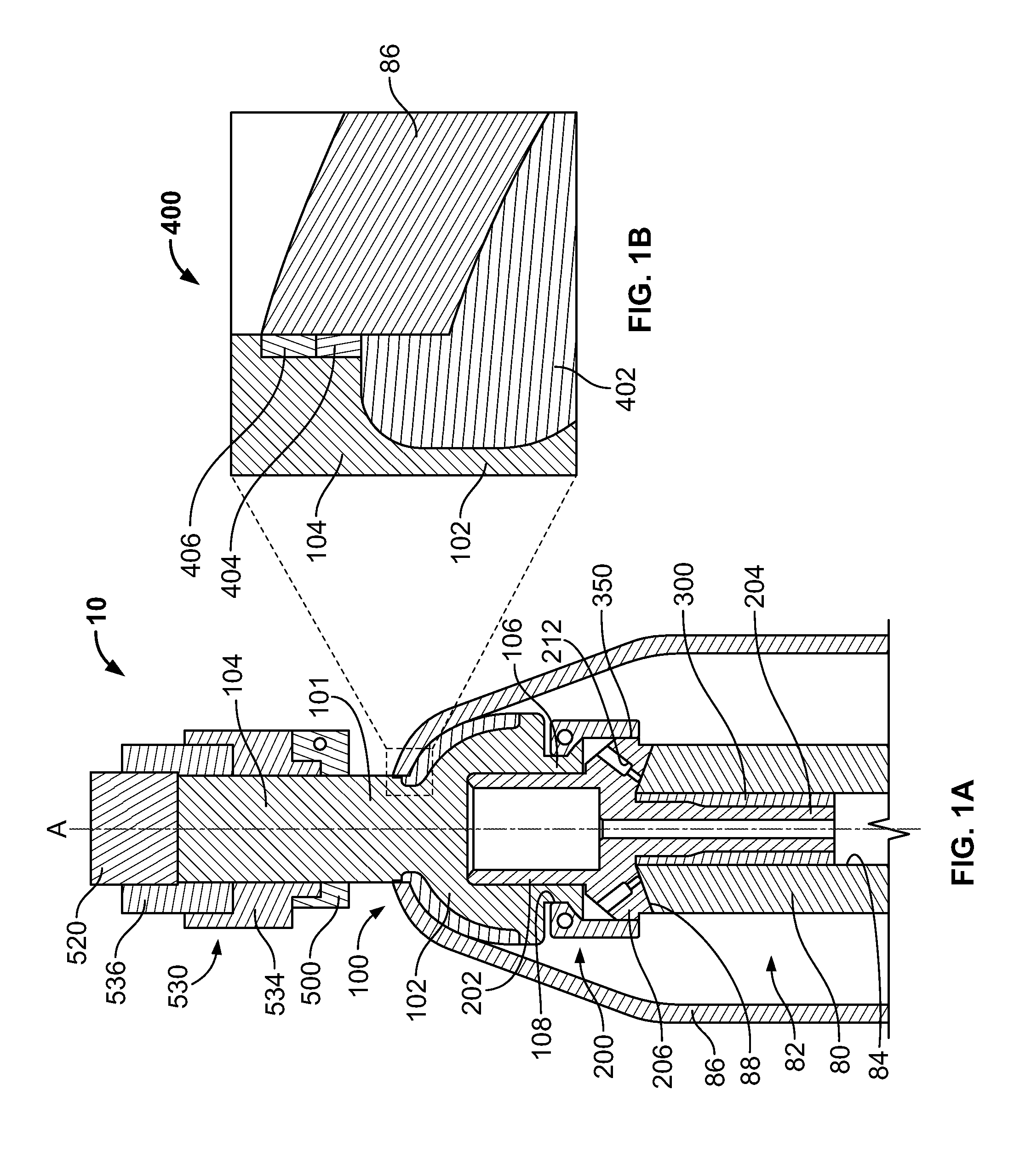
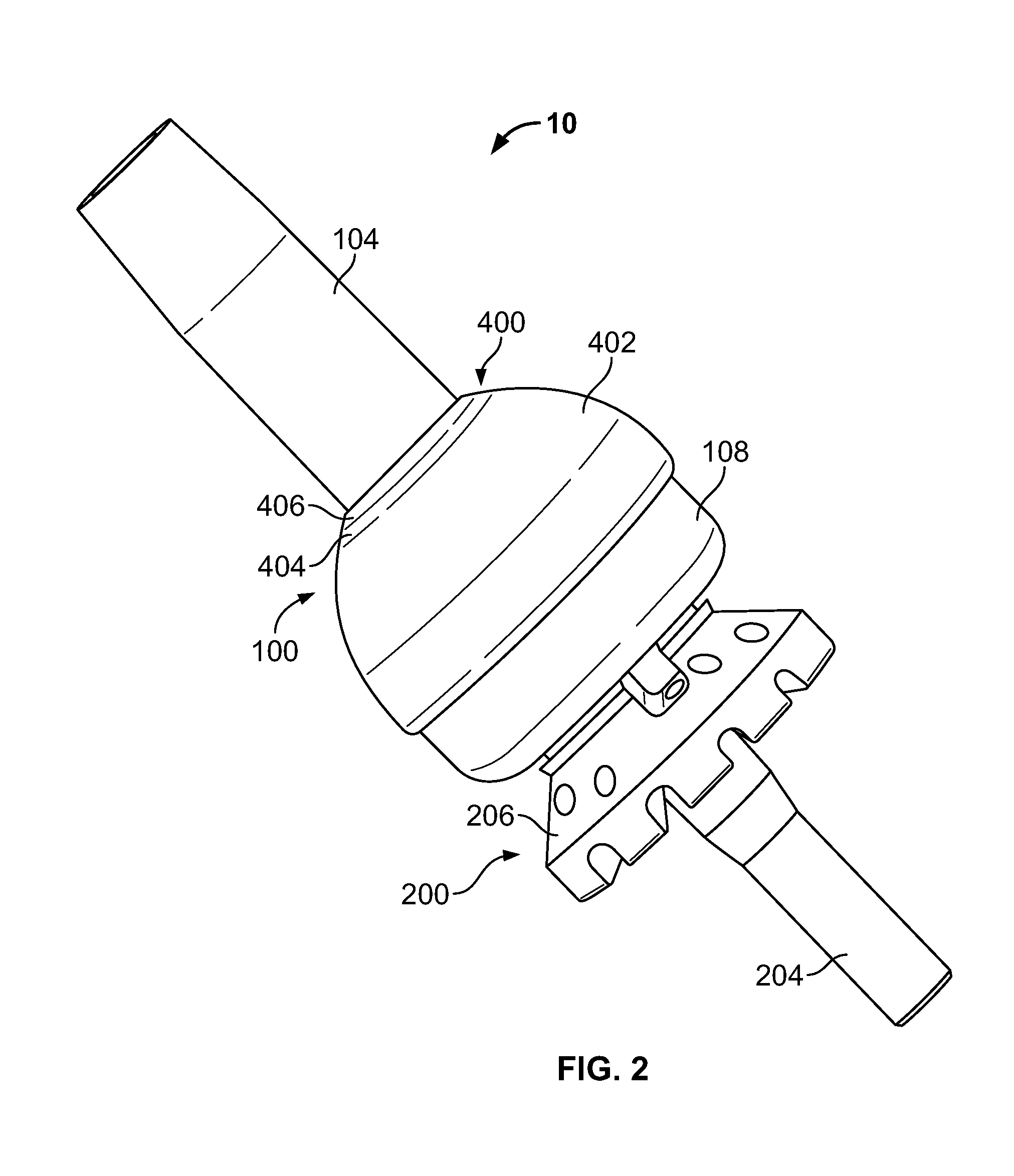
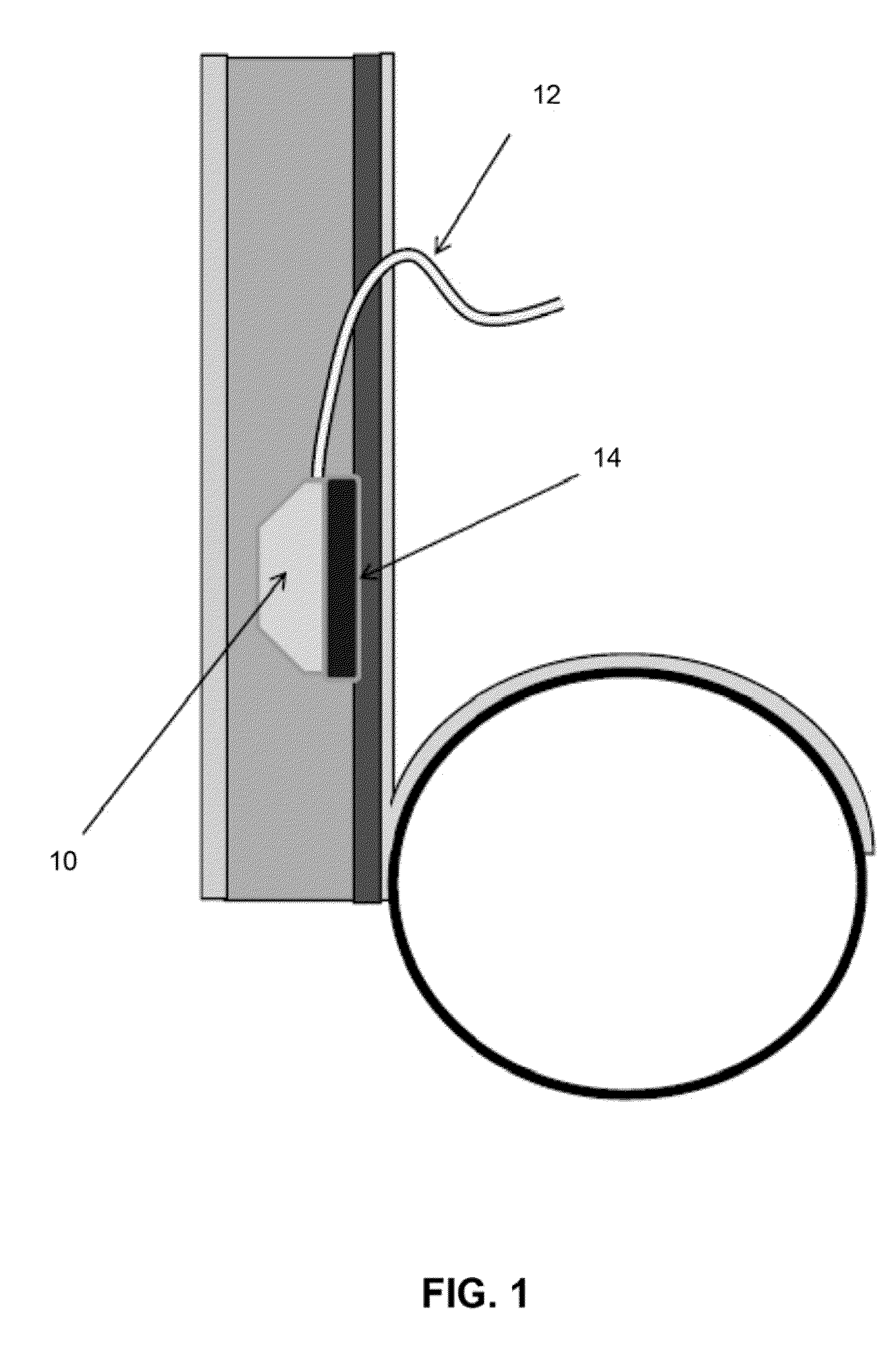
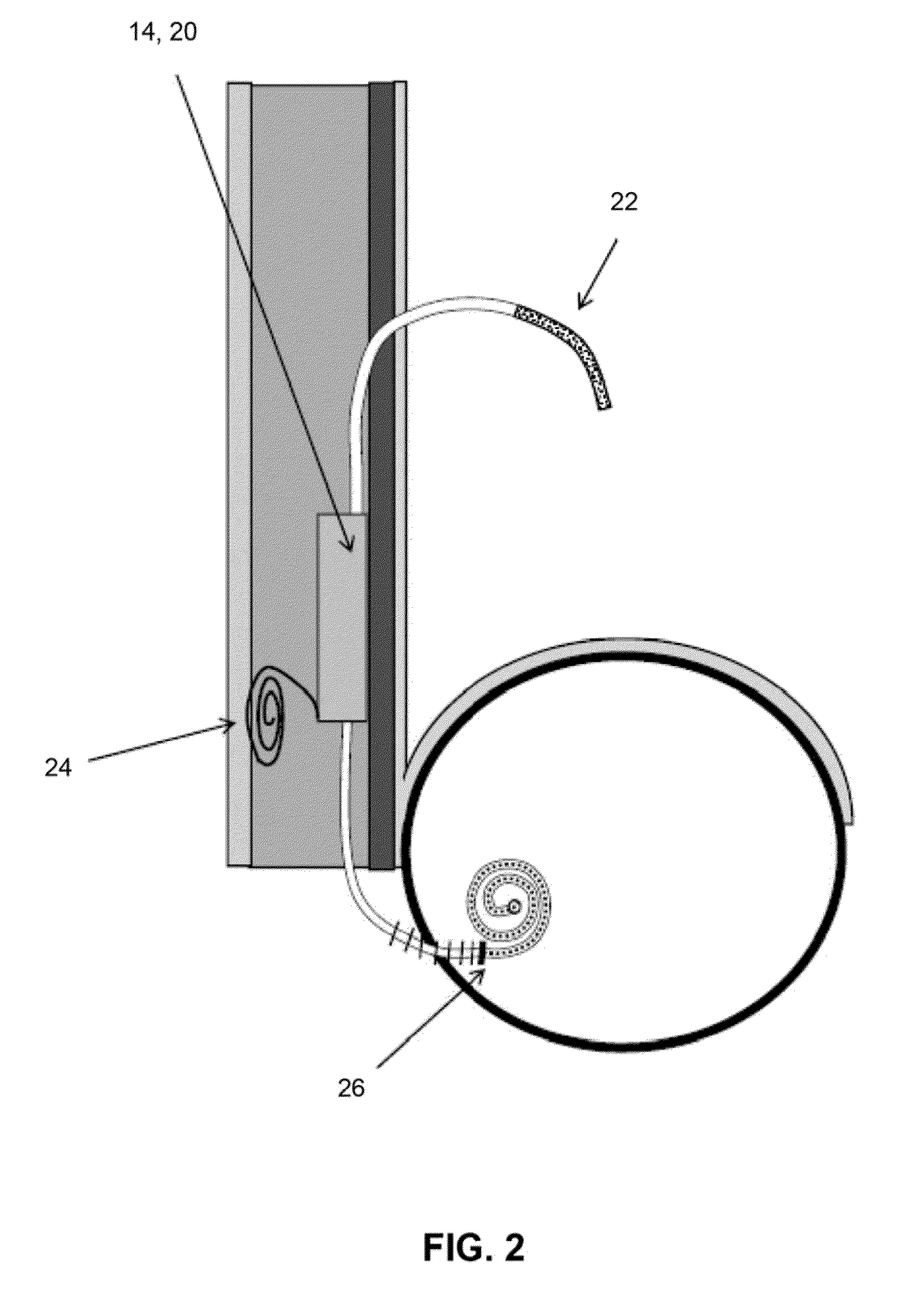
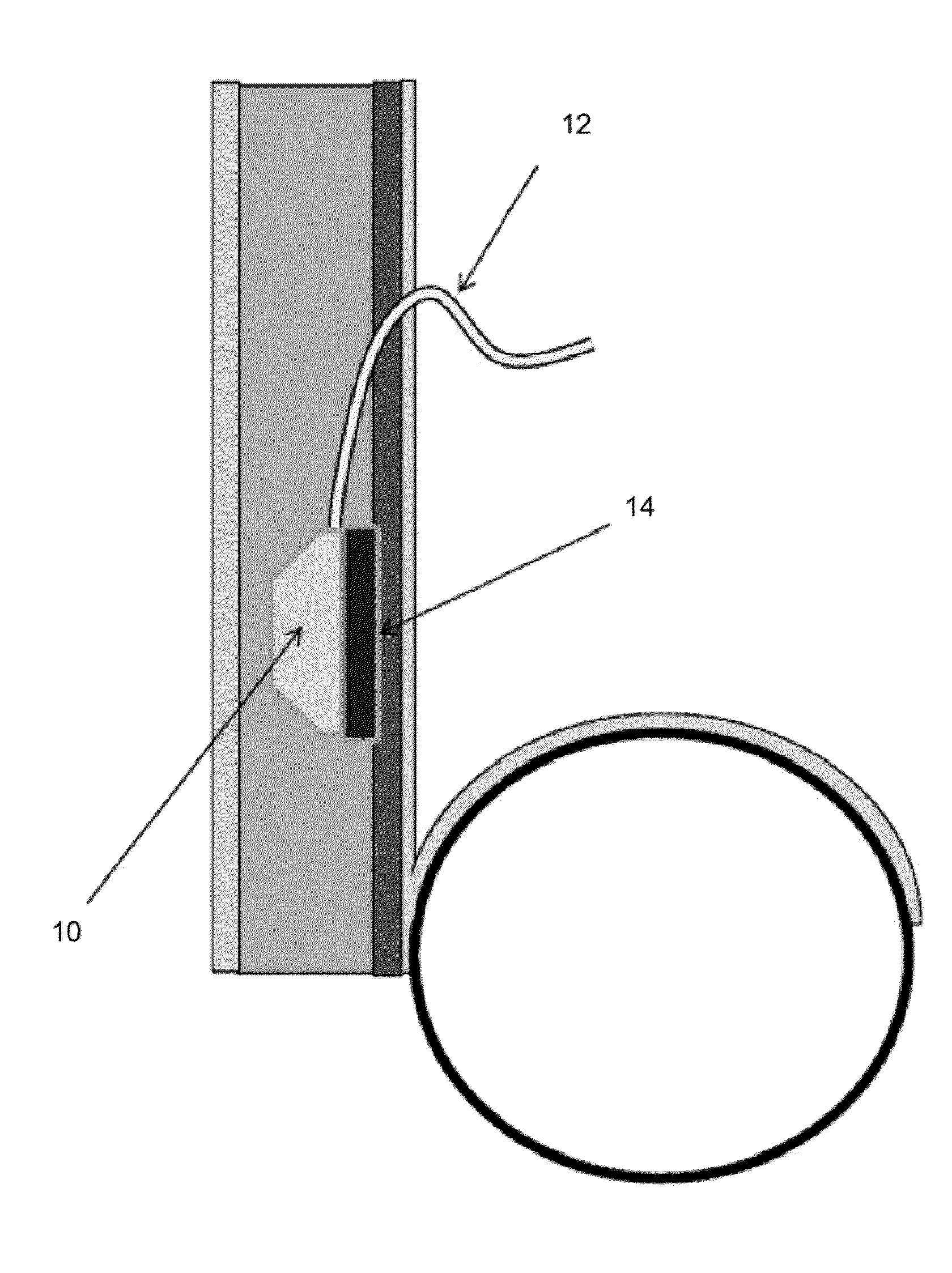
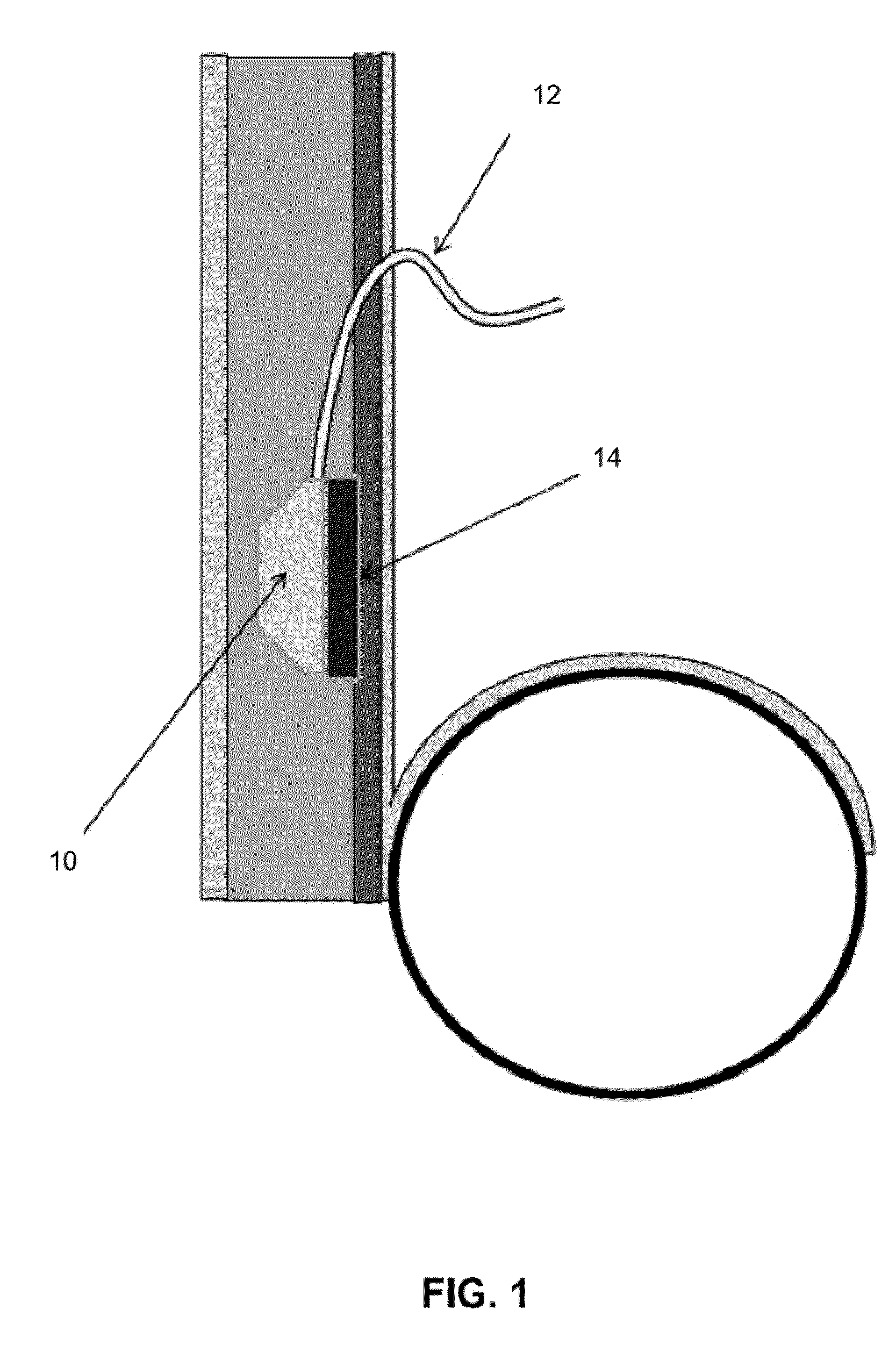
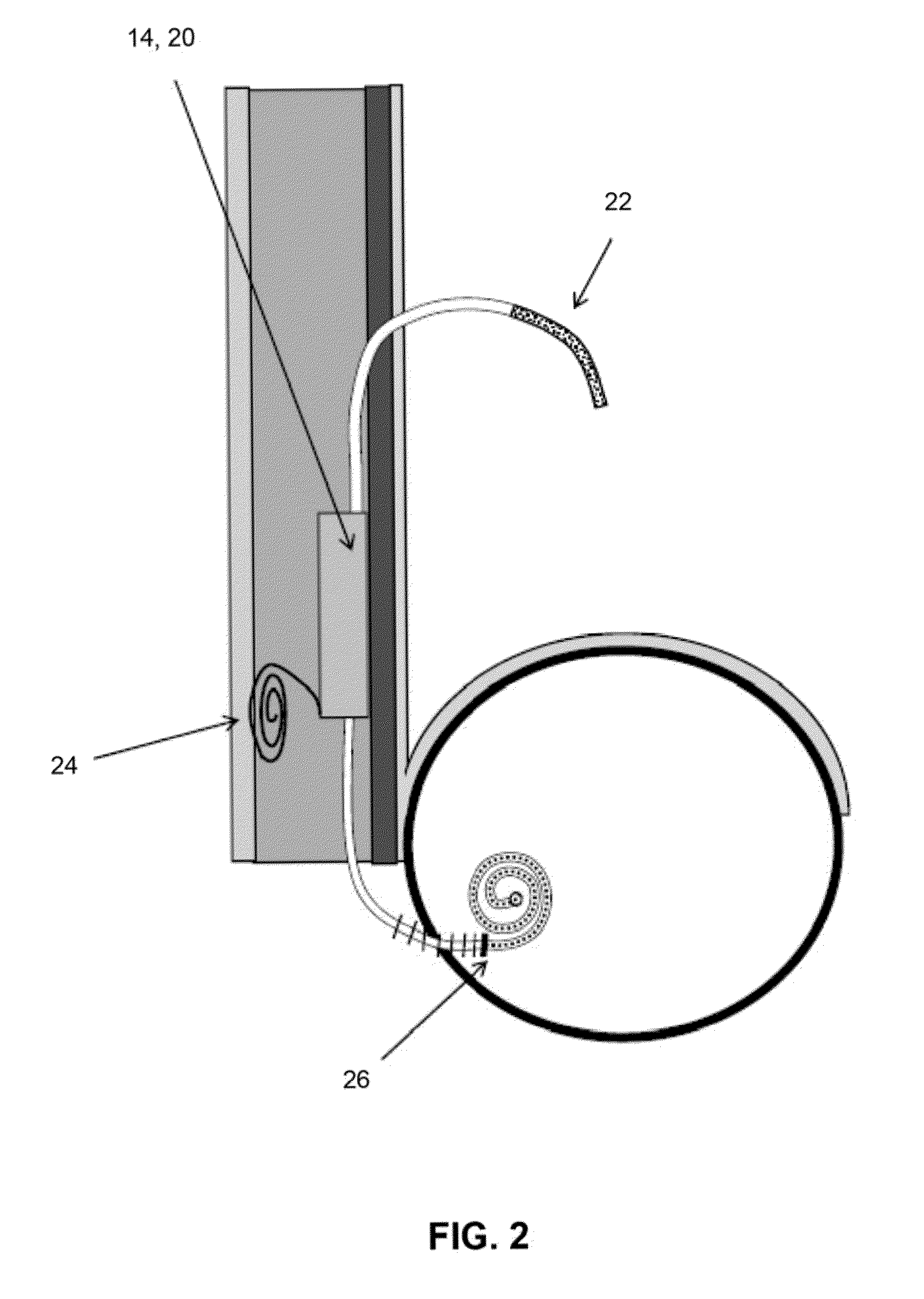
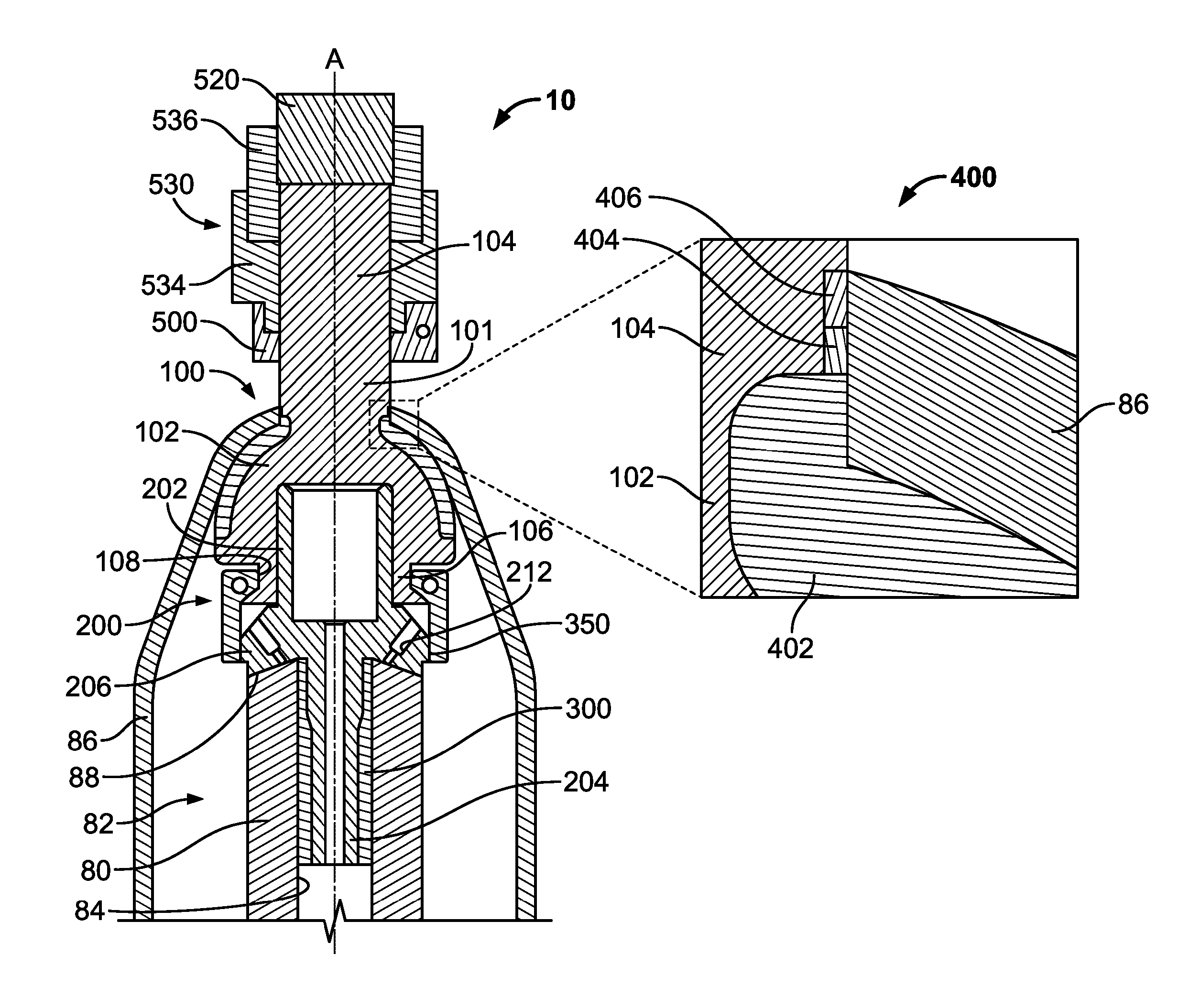
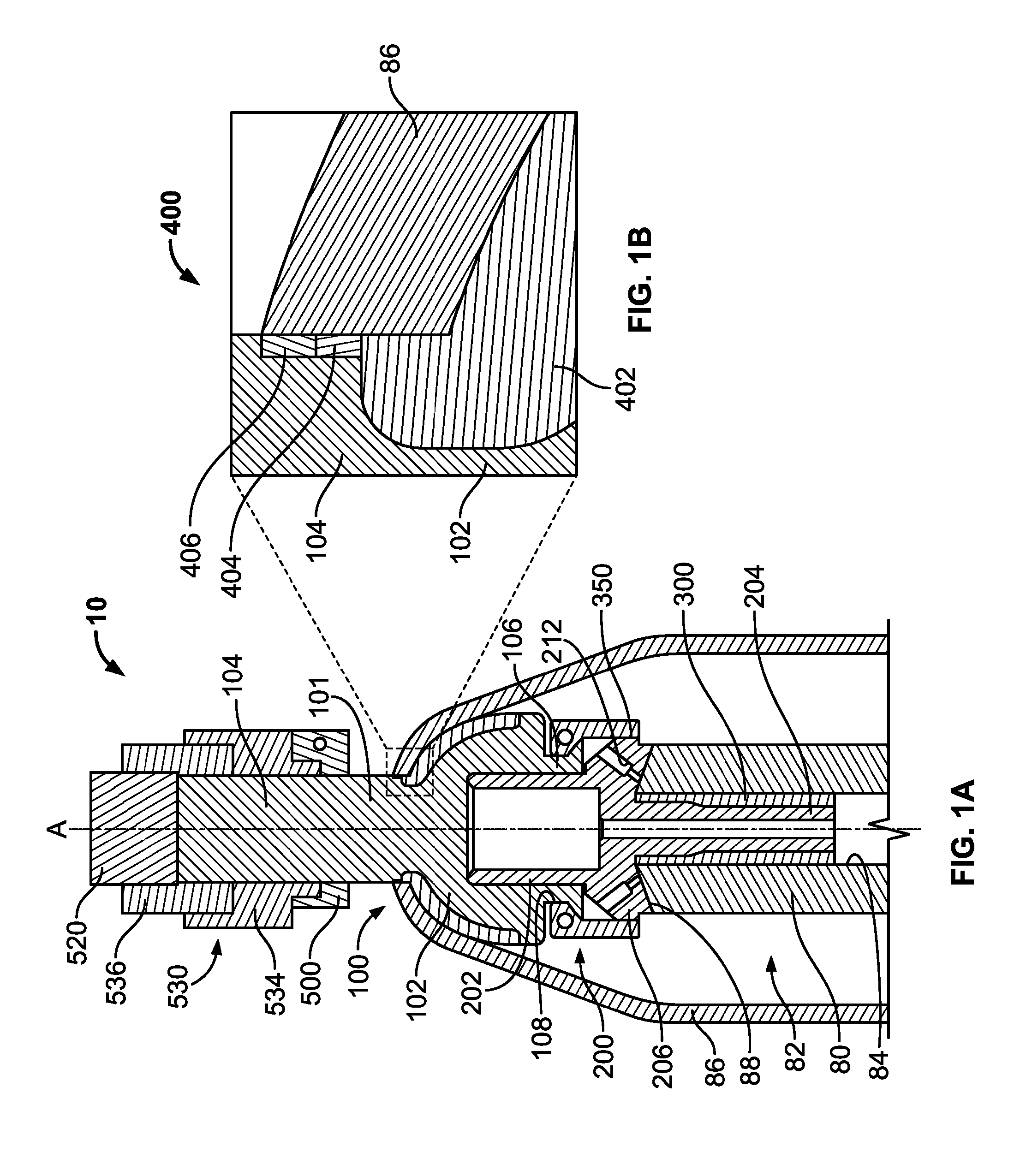
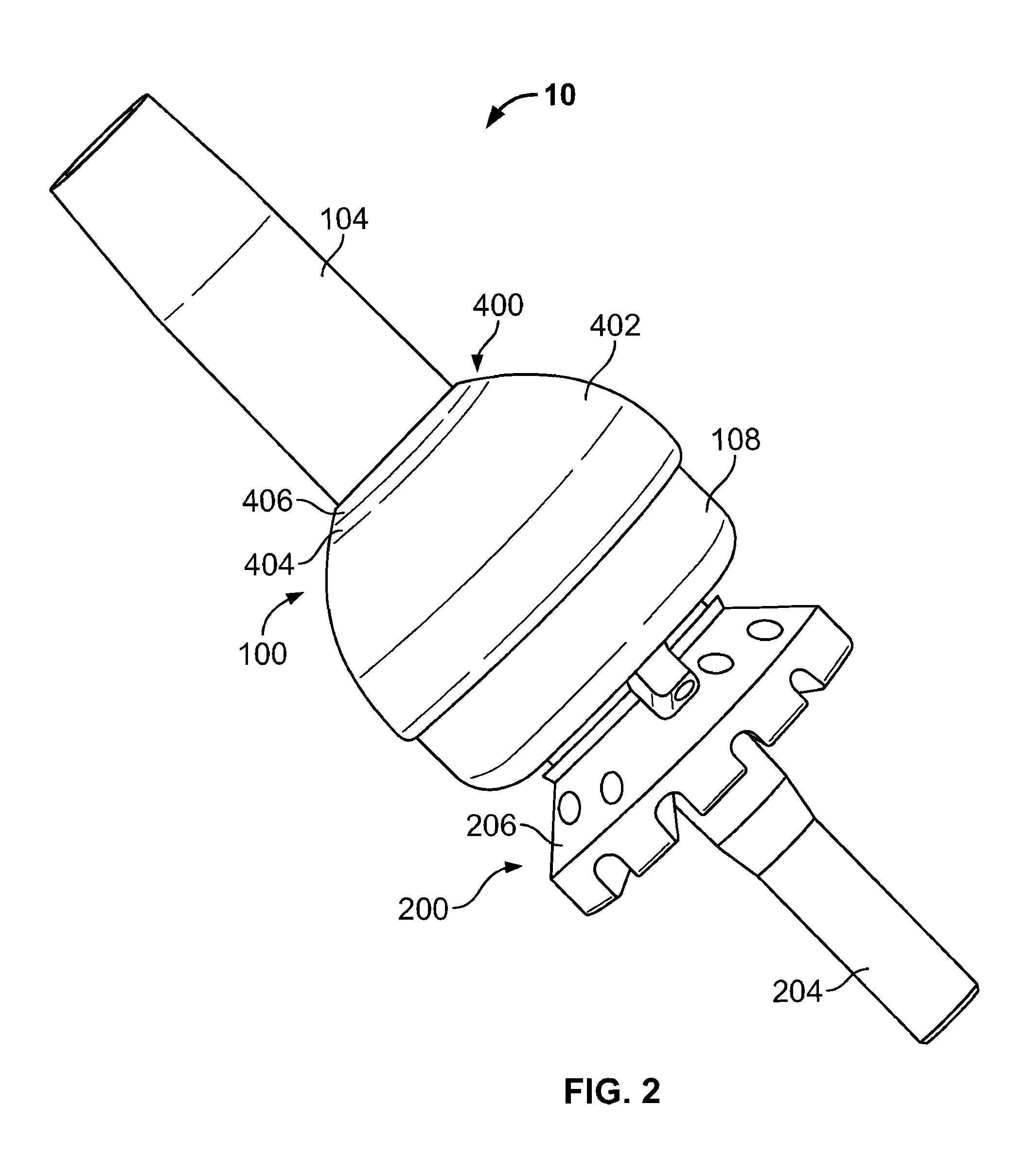
Transdermal Intraosseous Device
A transdermal intraosseous device includes a transdermal adapter for an external prosthetic device for a bone of a patient and a bone fixator including a distal portion coupled to the transdermal adapter and a proximal portion for anchoring into the bone. The transdermal adapter includes a dome-shaped portion for transcutaneous implantation and an external shaft extending from the dome-shaped portion. A dermal transition structure is configured to include a controlled roughness gradient from the external shaft to the dome-shaped portion and configured for use in infection control at a dermis layer of the patient.
Owner:BIOMET MFG CORP
Hand health and hygiene system for hand health and infection control
ActiveUS20090191248A1Prevent and overcome skin damageGood for healthCosmetic preparationsBiocideIrritationHand sanitizer
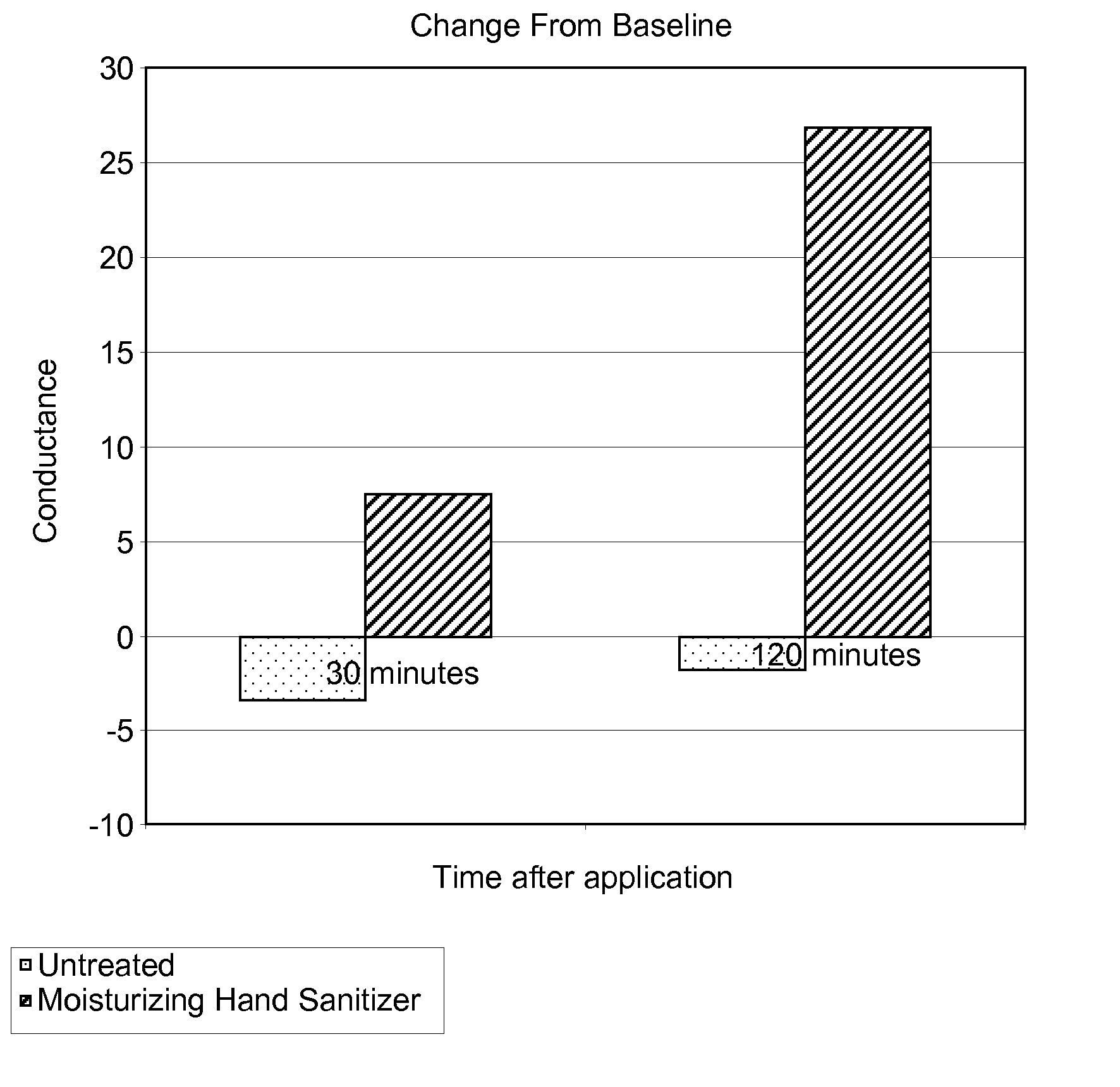
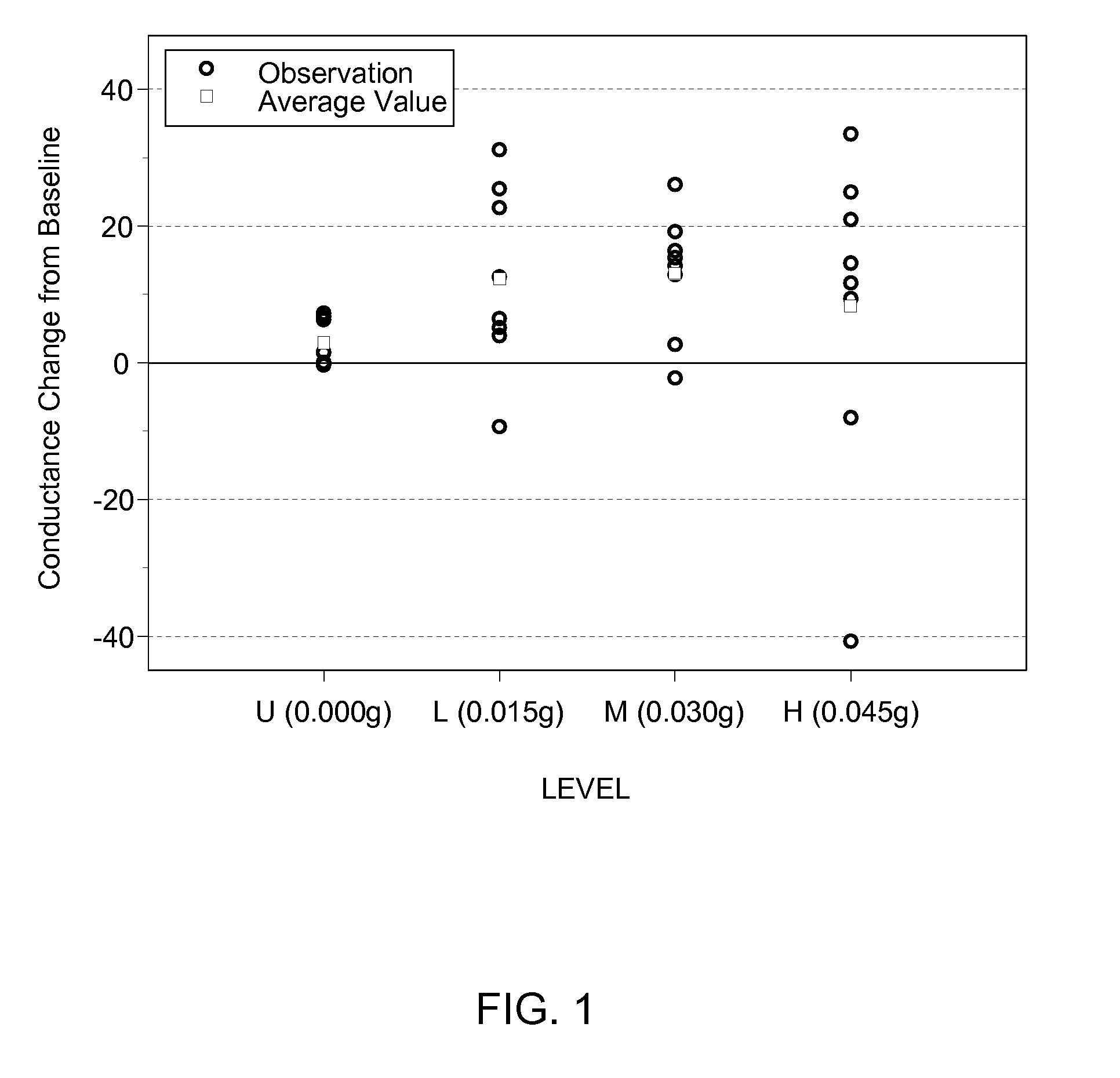
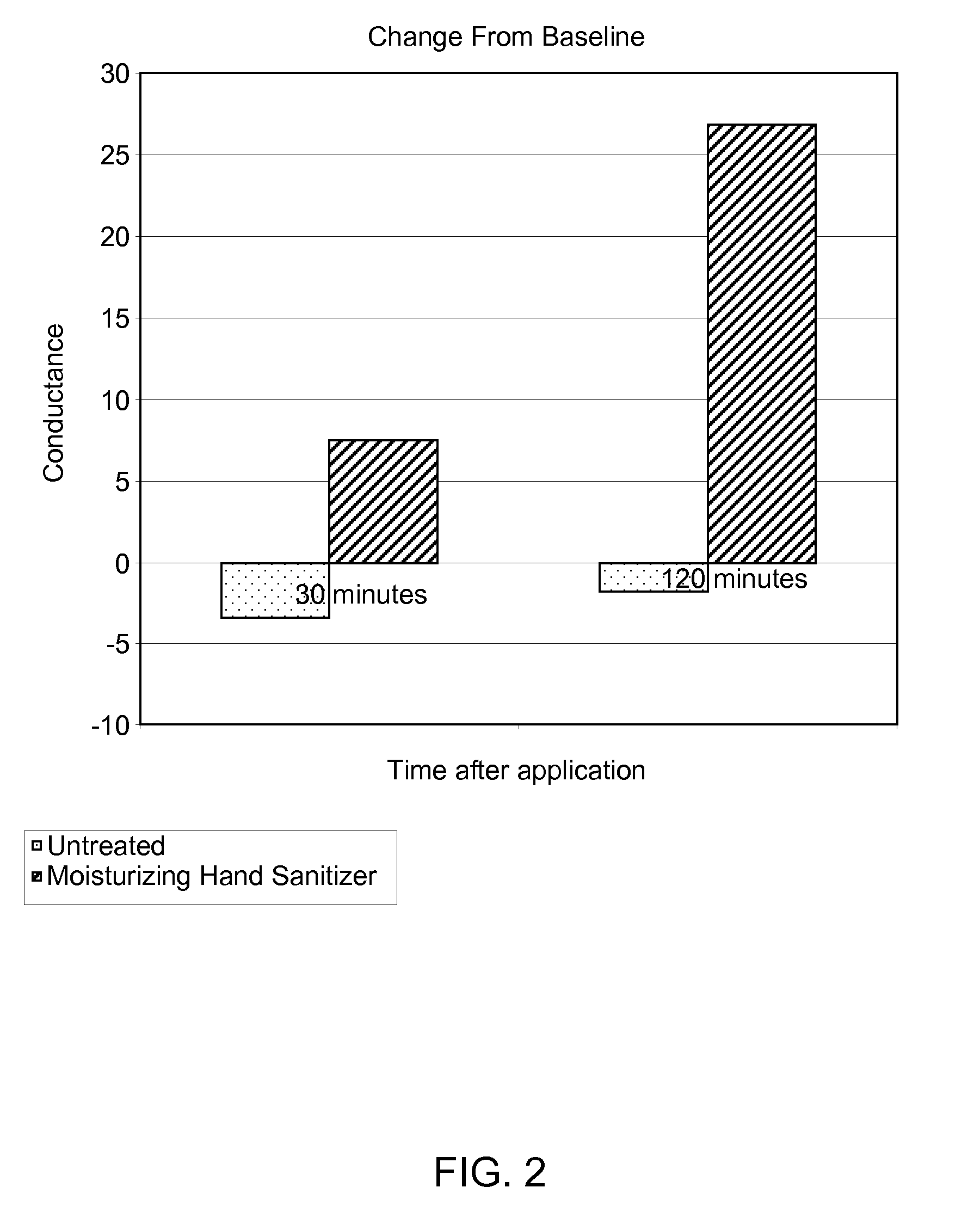
The present disclosure is directed to a system and methods for maintaining or improving hand health and hygiene. More particularly, the system and methods prevent or overcome skin damage caused by frequent hand washing and use of hand sanitizers and / or cleansers. The hand care system comprises an article, such as an elastomeric glove, that has a moisturizing liquid liner composition on the skin-contacting surface of the article that delivers a skin health benefit to the skin, a non-irritating hand cleanser capable of moisturizing the skin, and a moisturizing hand sanitizer.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Method and apparatus for automated active sterilization of fully implanted devices
InactiveUS20150011928A1Effective sterilizationPromote resultsUrinary bladderStentsUltravioletProsthetic knee
The current invention provides this advance in infection control via its unique application of active sterilization to a catheter or implant. While most catheters, and many implants, are passive devices, the current invention will provide an active component as a integral part of the implanted catheter or device to continuously or intermittently sterilize the exposed surfaces / areas of the device. This active sterilization may be accomplished by a variety of mechanisms, including, application of heat, RF, microwave, ultrasound, ultraviolet radiation or other energy capable of sterilizing the device or dislodging any problematic Biofilm that may form. The active sterilization may also employ the pumping of a sterilizing chemical from all attached drug reservoir, the use of electricity or freezing temperatures or any other mechanism for either inhibiting, killing or dislodging any infectious material in contact with the implant. One major advantage of this design is that through the use of small, battery powered or inductively powered sterilization element, the implanted catheter or device can be effectively sterilized without requiring the standard removal surgery, waiting period, then replacement of the infected device. This is expected to translate into greatly improved outcomes (particularly for devices where infection may be catastrophic, ie a prosthetic knee or hip), greatly improved costs, and greatly improved longevity of susceptible devices (ie IV ports, etc.).
Owner:THERANOVA LLC
System for monitoring infection control and prevention processes
The present invention is a centralized system for the automated monitoring and reporting of compliance to infection prevention policies. It is focused on the proactive reduction (elimination) of pathogenic organisms causing infection within the facility by enforcing best practices for infection prevention.
Owner:APPLE INC
Method and apparatus for automated active sterilization of fully implanted devices
InactiveUS20100256607A1Avoid spreadingEffective sterilizationUrinary bladderStentsProsthetic kneeCatheter device
The current invention provides this advance in infection control via its unique application of active sterilization to a catheter or implant. While most catheters, and many implants, are passive devices, the current invention will provide an active component as a integral part of the implanted catheter or device to continuously or intermittently sterilize the exposed surfaces / areas of the device. This active sterilization may be accomplished by a variety of mechanisms, including, application of heat, RF, microwave, ultrasound, ultraviolet radiation or other energy capable of sterilizing the device or dislodging any problematic Biofilm that may form. The active sterilization may also employ the pumping of a sterilizing chemical from an attached drug reservoir, the use of electricity or freezing temperatures or any other mechanism for either inhibiting, killing or dislodging any infectious material in contact with the implant. One major advantage of this design is that through the use of a small, battery powered or inductively powered sterilization element, the implanted catheter or device can be effectively sterilized without requiring the standard removal surgery, waiting period, then replacement of the infected device. This is expected to translate into greatly improved outcomes (particularly for devices where infection may be catastrophic, ie a prosthetic knee or hip), greatly improved costs, and greatly improved longevity of susceptible devices (ie IV ports, etc.).
Owner:THERANOVA LLC
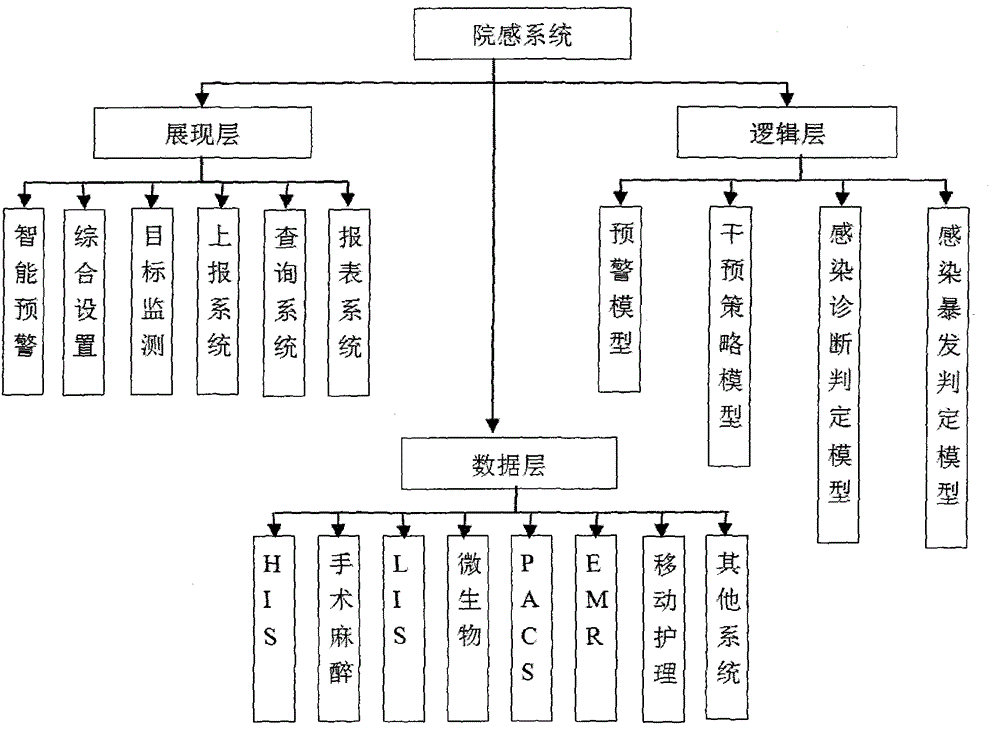
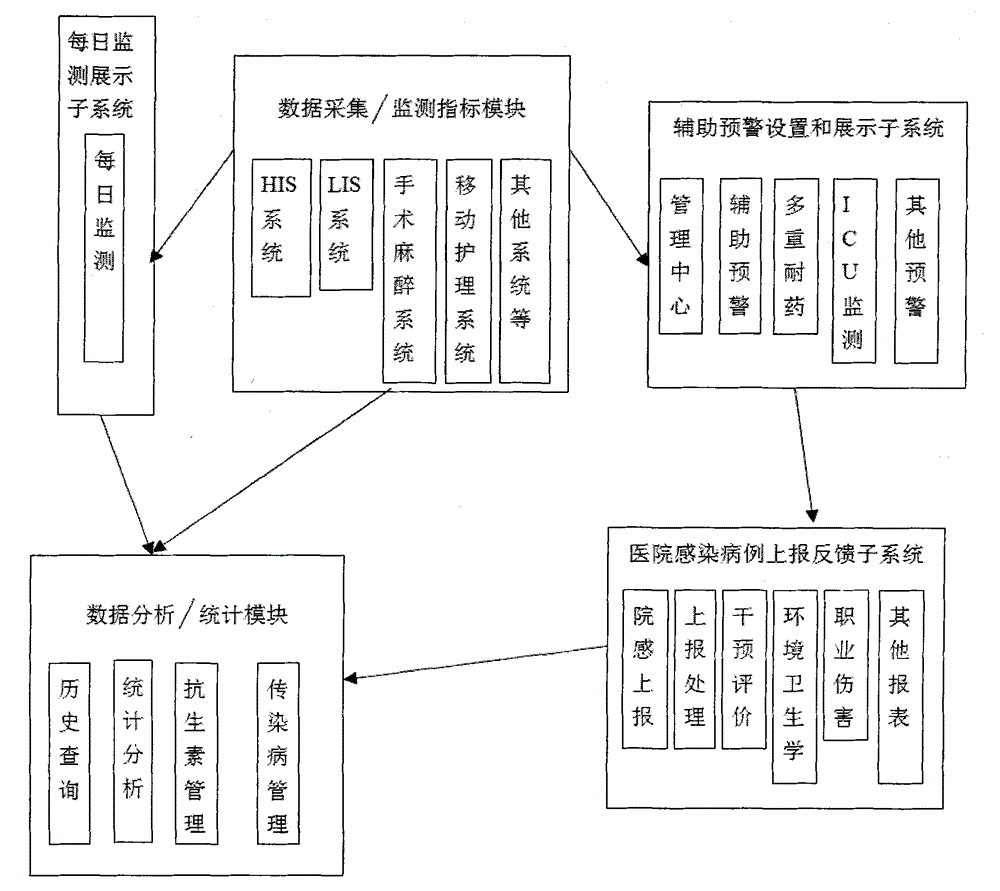
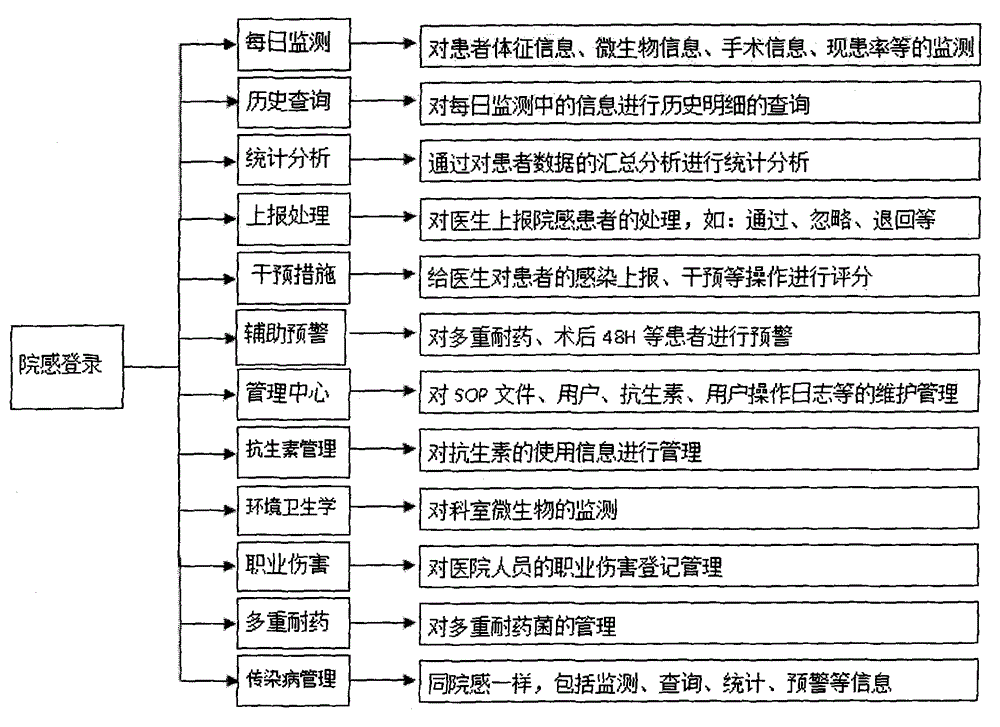
Management system for an entire process of hospital infection prevention and control, and method thereof
ActiveCN105893725AEasy to find out in timeData processing applicationsSpecial data processing applicationsThird partyData acquisition
The present invention discloses a management system for an entire process of hospital infection prevention and control, and a method thereof. The system comprises a data collection / monitoring indicator module, and an interface module with a third-party system. The data collection / monitoring indicator module is connected with a daily monitoring and displaying subsystem, a data analysis / statistics module, and an auxiliary alarm setting and displaying subsystem; the daily monitoring and displaying subsystem is connected with the data analysis / statistics module; the auxiliary alarm setting and displaying subsystem is connected with a hospital infections reporting and feedback subsystem; the hospital infections reporting and feedback subsystem is connected with the data analysis / statistics module. The interface module with the third-party system is used for interfacing the various systems. The beneficial effects of the system and method disclosed by the present invention are that comprehensive, fast and accurate retrieval is carried out through the set hospital infection alarm determination condition information, a case range required to be consulted by the infection control staff is reduced, and infection control efficiency is improved.
Owner:BEIJING ZHONGZHI HUIYI SCI & TECH CO LTD
Method and apparatus for automated active sterilization of fully implanted devices
Owner:THERANOVA LLC
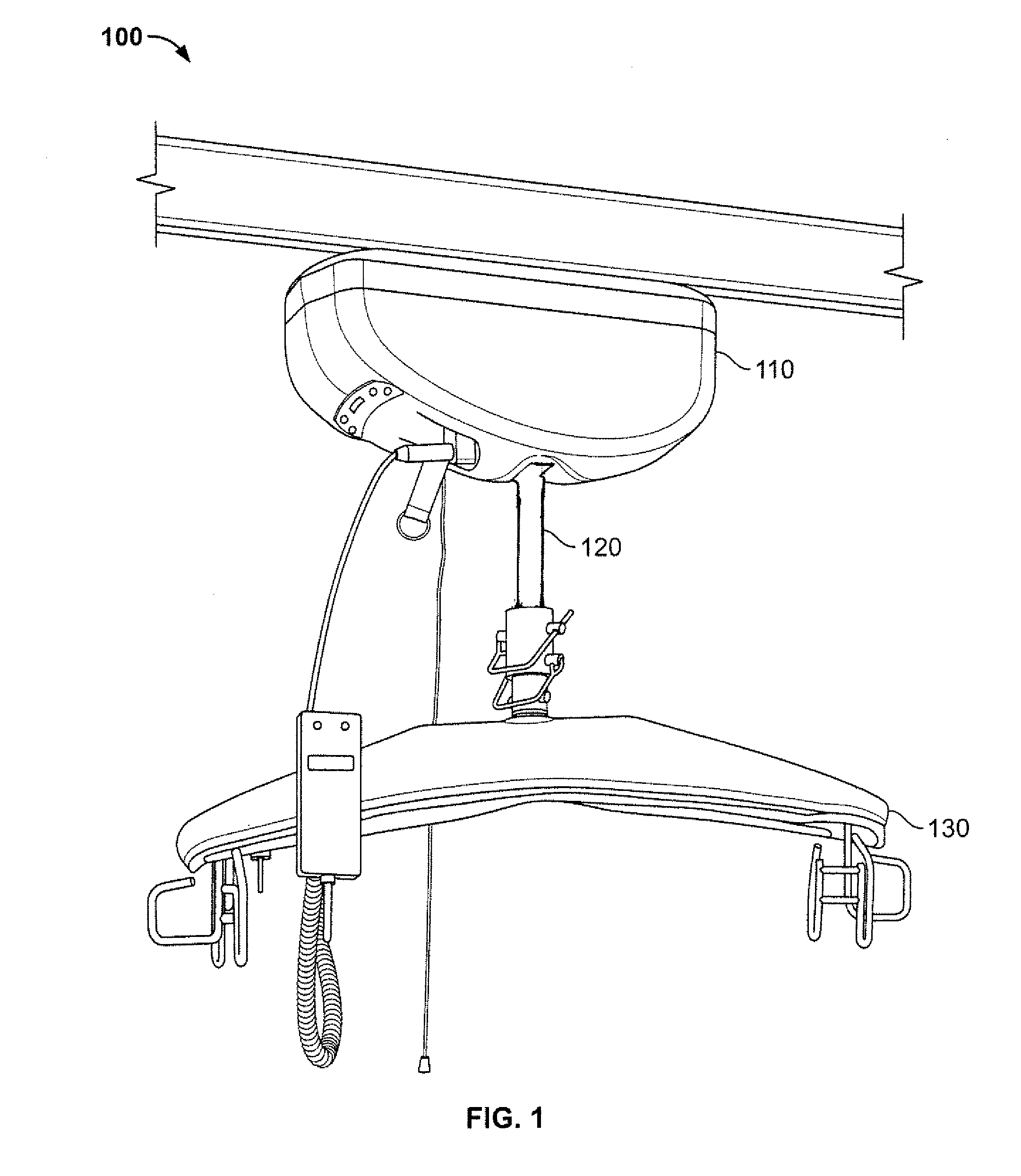
Infection control lifting strap
InactiveUS20100064432A1Risk minimizationEasily and quickly wiped downNursing bedsElevatorsWinchLeather belt
A support member for use in a patient lifting device is described. The support member includes an improved lift strap having an inner core and an outer plastic layer that can be easily and effectively cleaned with standard disinfectant. The lift strap is secured to a patient lifting device with a spool assembly that guides the lift strap and a belt clamp assembly that compresses the lift strap and holds it into place. In the preferred embodiment, the support member is used in an electric ceiling- or floor-mounted patient lifting device. The preferred lifting device is composed of a track component attached to a winch assembly. The winch assembly has an electric motor that raises and lowers the lift strap by means of a spool assembly and belt clamp assembly. The belt clamp assembly attaches to a sling that supports the patient while he or she is displaced.
Owner:T H E MEDICAL
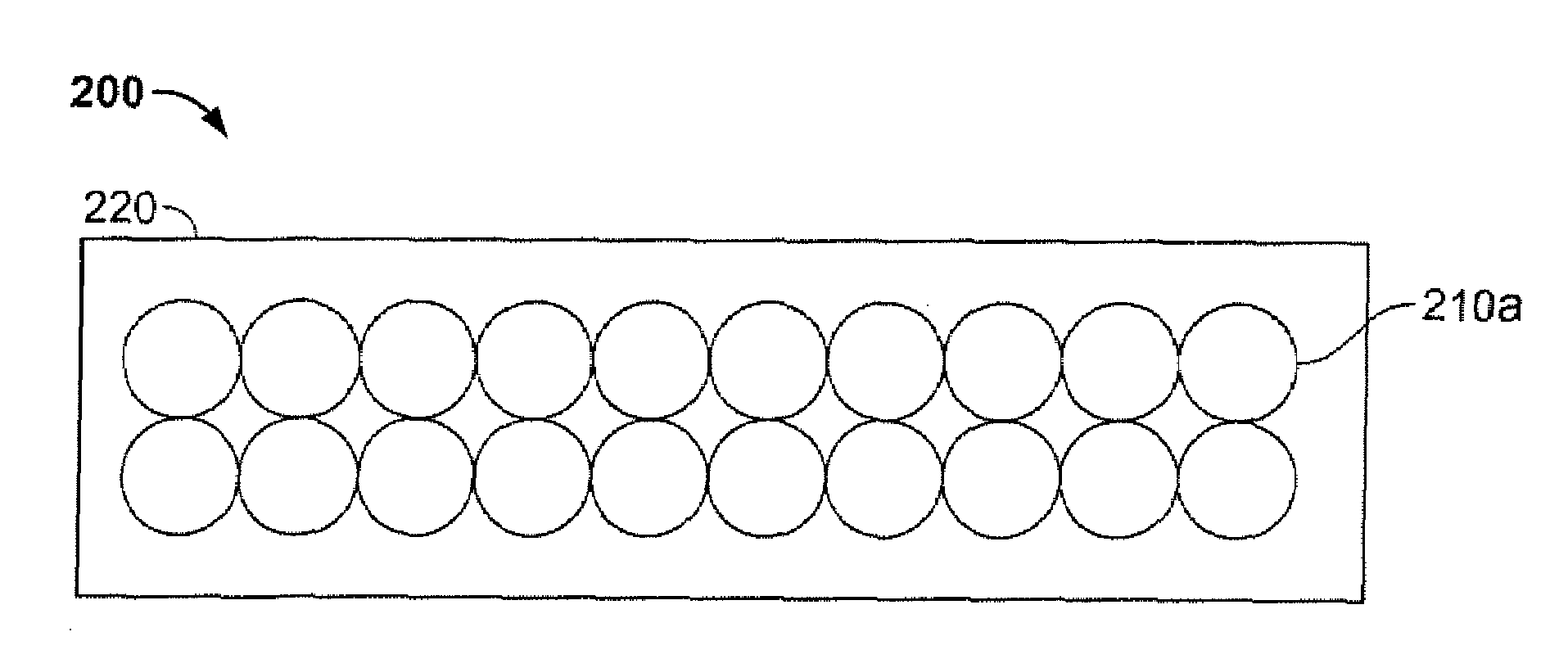
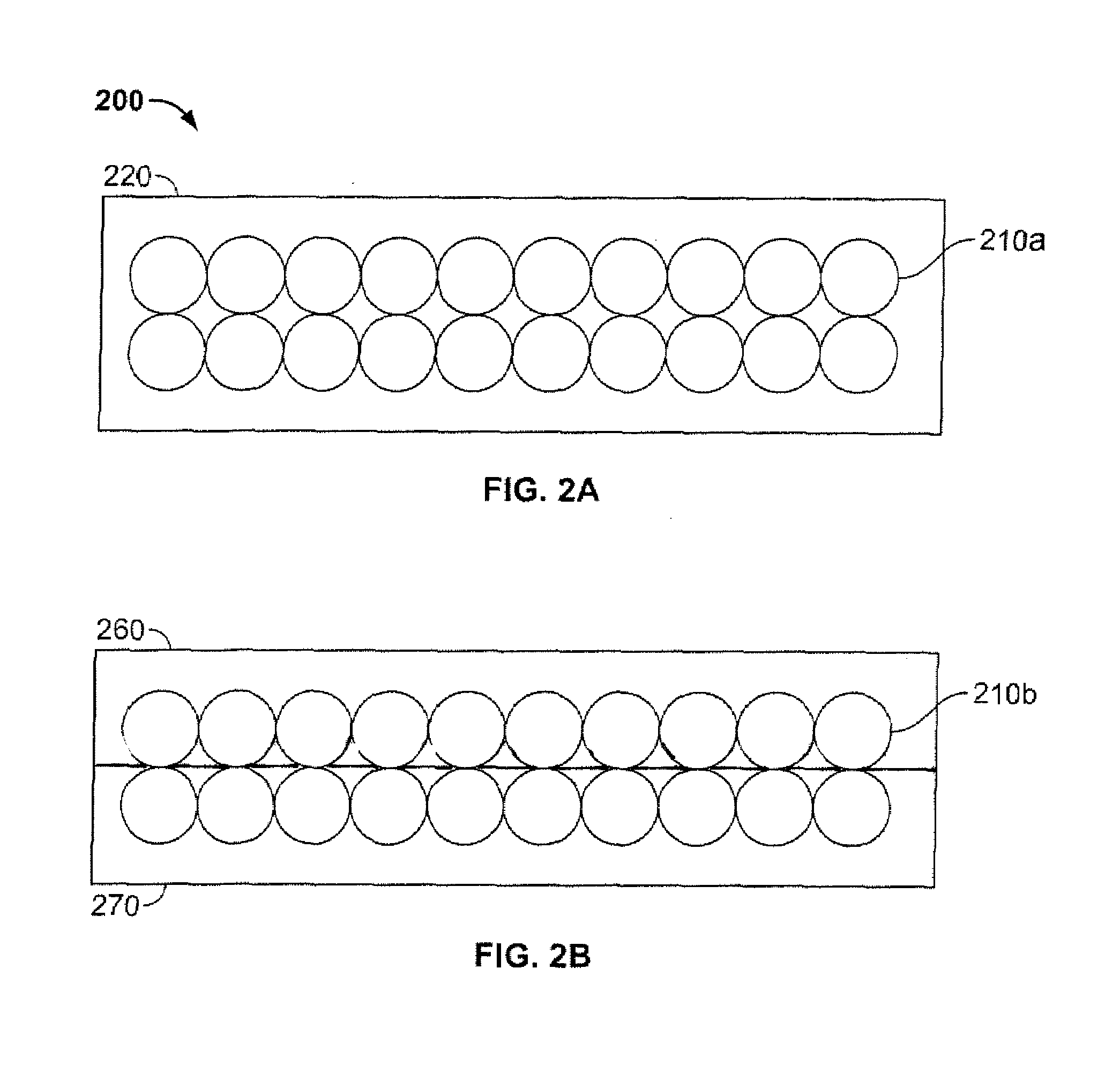
Antimicrobial, infection-control and odor-control film and film composite
InactiveUS20080171068A1Small amountEliminate disadvantagesBiocideDead animal preservationOdor controlProduct gas
A novel method of producing an odor-controlling textile is disclosed. More specifically, a textile structure is disclosed that contains thin breathable film layer having an activatable antimicrobial odor controlling material. Most specifically, the present invention relates to a textile composite that is composed of a flexible breathable film layer with a measurable gas or vapor transmission rate, that contains metallic silver, zinc and / or copper metallic ions, and is combined with fabric and / or foam layers to form an antimicrobial, odor and infection control laminated structure.
Owner:ETCETERA
Cold Plasma Treatment Devices and Associated Methods
ActiveUS20130072859A1Utility in controlIncrease blood flowElectrotherapyElectric discharge tubesClosed head injuryEngineering
A cold plasma helmet application device for delivery of cold plasma benefits to the head of a patient. An appropriate gas is introduced into a helmet receptacle within a containment dome of the helmet. The gas is energized by one or more dielectric barrier devices that receive energy from a pulsed source. The dielectric barrier devices can be configured to match the treatment area. Such a device and method can be used to treat large surface areas treatment sites associated with the head, head trauma, brain cancer, the control of brain swelling with closed head injury or infection, as well as treating male pattern baldness.
Owner:COLD PLASMA MEDICAL TECH
Automated infection control surfaces
InactiveUS20110286882A1Avoid contactAvoid damageOperating means/releasing devices for valvesLavatory sanitoryUv disinfectionElectronic switch
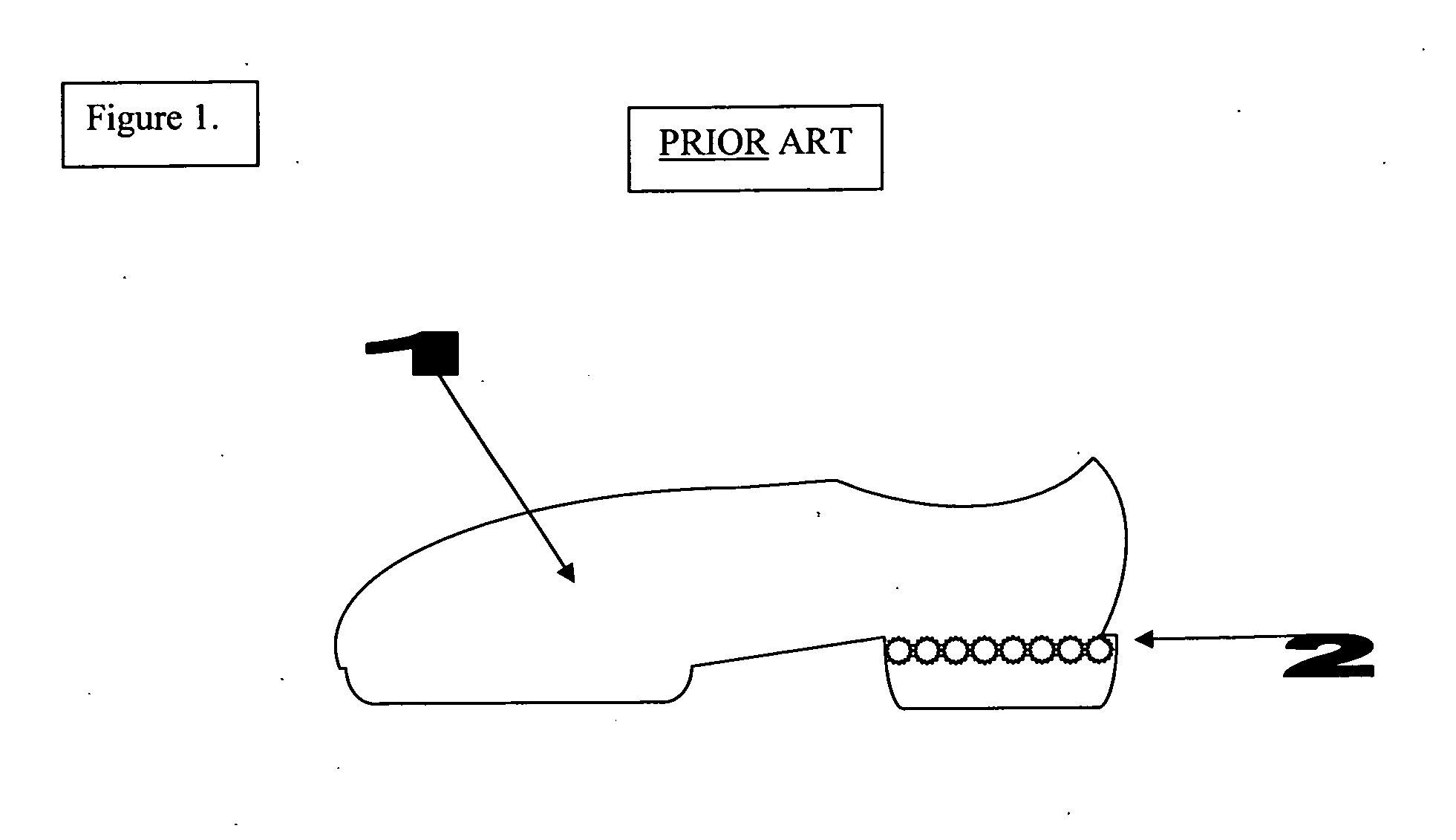
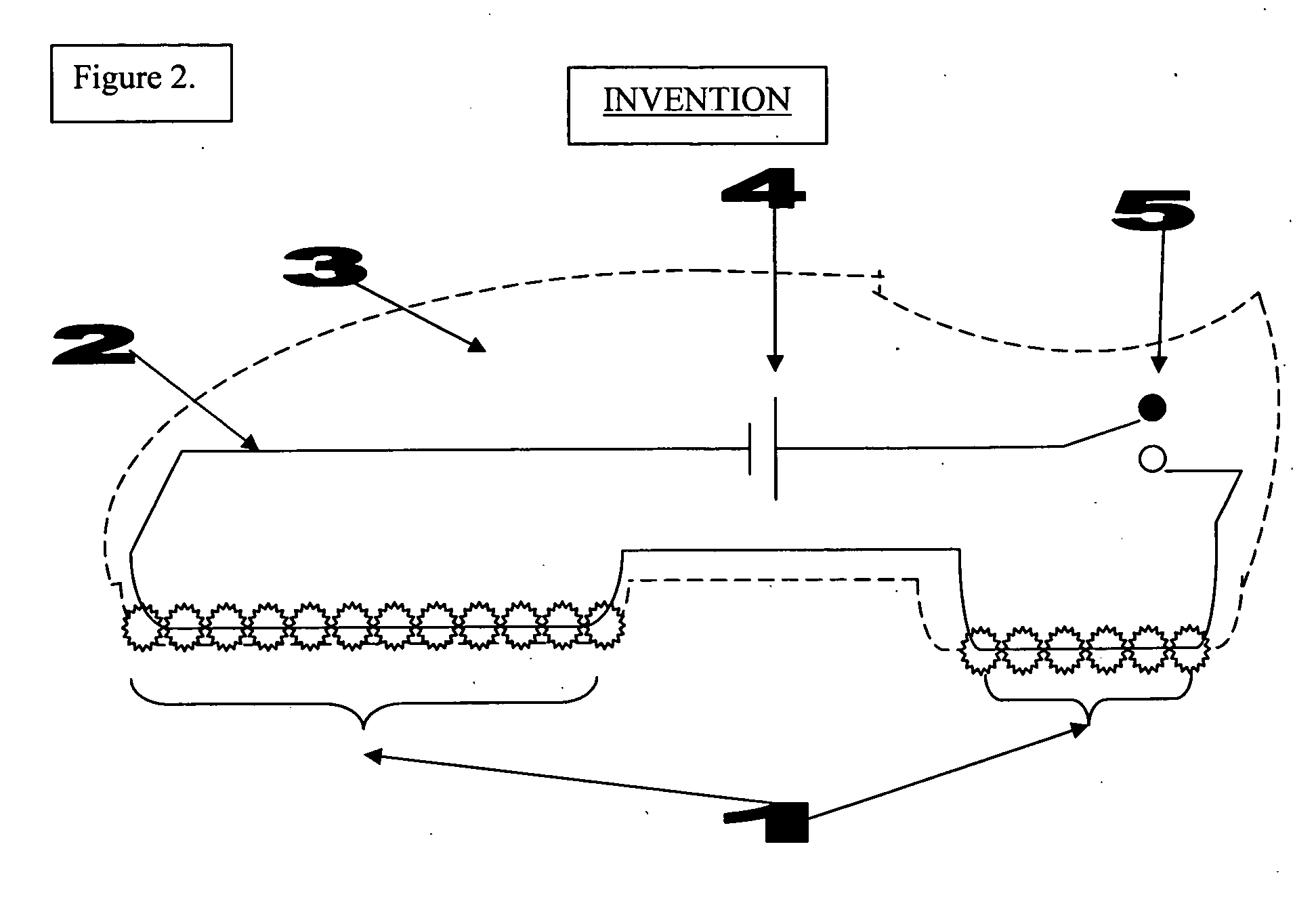
The invention is an automated infection control surface, which may be placed on the sole of a shoe or on the ground in the form of a floor mat / tile. The invention is composed of a series of individual light sources or LEDs (light emitting diodes) capable of emitting ultraviolet sanitizing light. When in the format of a shoe, there is an electronic switch in the form of a pressure sensor which is triggered when the operator's foot is planted within the shoe and standing firmly on the ground. When the pressure sensor is triggered the electrical current passes from the power source to the light sources or LEDs and illumination of sanitizing light occurs. When the shoe is lifted off the ground and when there is no weight or pressure within the shoe, the pressure sensor is turned off and the LEDs turn off. In this way the shoe is only capable of triggering UV microbicidal activity when the sole is flush to the ground, thus preventing inadvertent UV light scatter and potential skin and eye damage. The invention may also be composed in an array like fashion in the form of a floor mat or tile, such that the LEDs are oriented upwardly to shine towards the sole of the operator's shoe, but always concealed underneath the shoe. In either embodiment the LEDs are turned off when there is no weight on the sole of the shoe or floor mat / tile. Both embodiments of the invention could also be used simultaneously to provide maximum sterilization activity.
Owner:ALLAN YANG WU
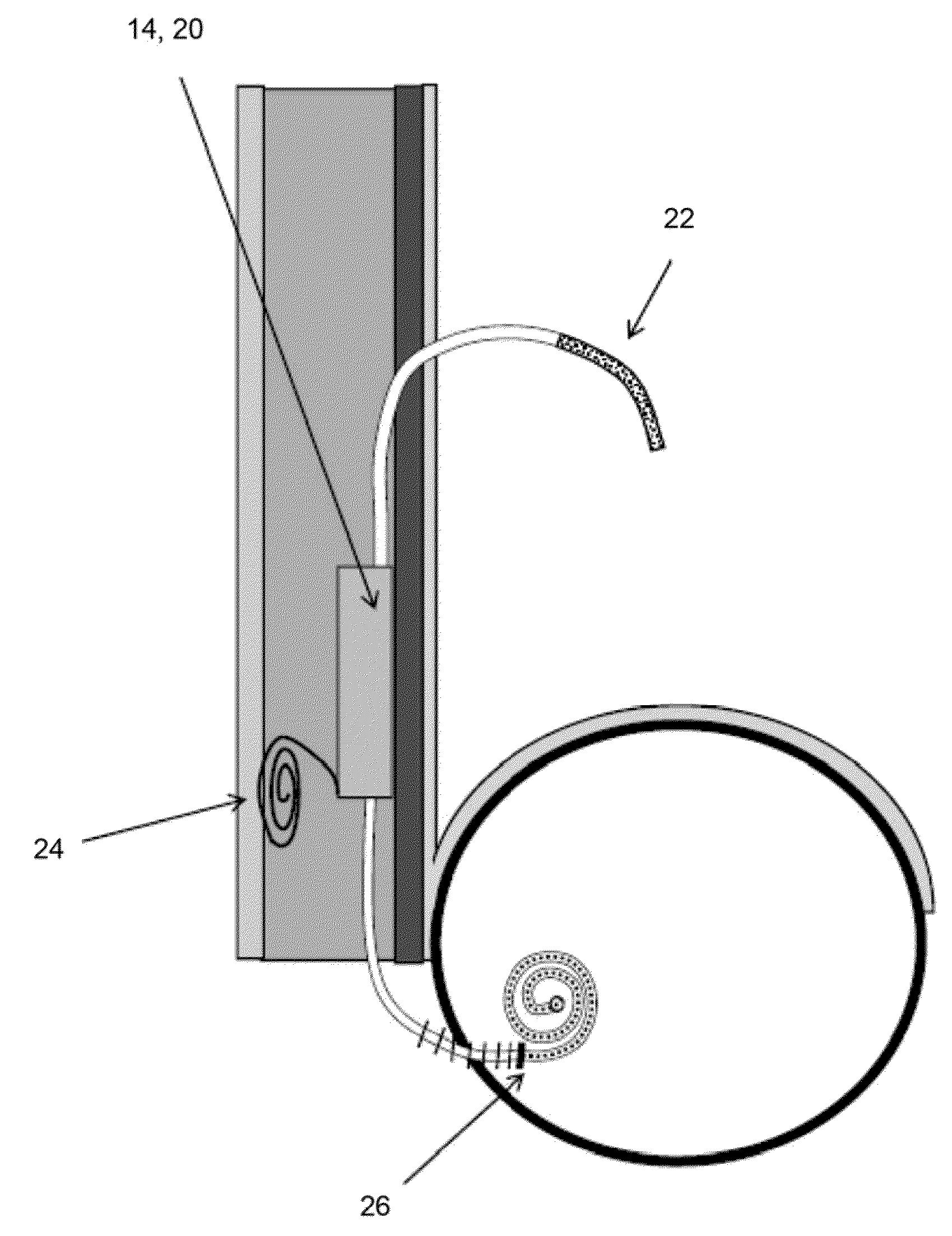
System and method for tracking and controlling infections
InactiveUS20080206767A1Health-index calculationMicrobiological testing/measurementMicroorganismPhylogenetic relatedness
The present invention is a system and method for performing real-time infection control over a computer network. The method comprises obtaining a sample of a microorganism at a health care facility, sequencing a first region of a nucleic acid from the microorganism sample, comparing the first sequenced region with historical sequence data stored in a database, determining a measure of phylogenetic relatedness between the microorganism sample and historical samples stored in the database, and providing infection control information based on the phylogenetic relatedness determination to the health care facility, thereby allowing the health care facility to use the infection control information to control or prevent the spread of an infection.
Owner:EGENOMICS INC
Easy sticking type device for sucking and lavaging skin and soft tissue wound surface
InactiveCN101474432AEfficient removalPromote growthWound drainsSuction devicesDressing changeWound surface
The invention discloses an easy-to-stick skin and soft tissue wound attracting and lavaging device which comprises a medical sponge block, build-in tubes and a sticking membrane. The sticking membrane enwraps the whole medical sponge block from the back. The lower surface of the sponge block is a region which is in contact with the wound. The sticking membrane forms a circle of an enclosed blending surface on the periphery of the lower surface to be blended with skin. The build-in tubes which are provided with a plurality of lateral holes are inserted in the sponge block, eduction ends of which are provided with atomseal ports at sticking membrane piercing out parts for maintaining the subpressure in the sponge during the attracting process. The product can carry out the vacuum sealing drainage on the wound and the enclosed lavage on infective wounds, has the advantages of lightness and using convenience and can relieve trivial works of repeated wound cleaning and change dressing of medical staff by one-time operation and achieve cleaning and draining effects better than repeated dressing changes, thereby promoting the infection control on the infective wounds and the rapid growth of granulation tissues.
Owner:ARMY MEDICAL UNIV
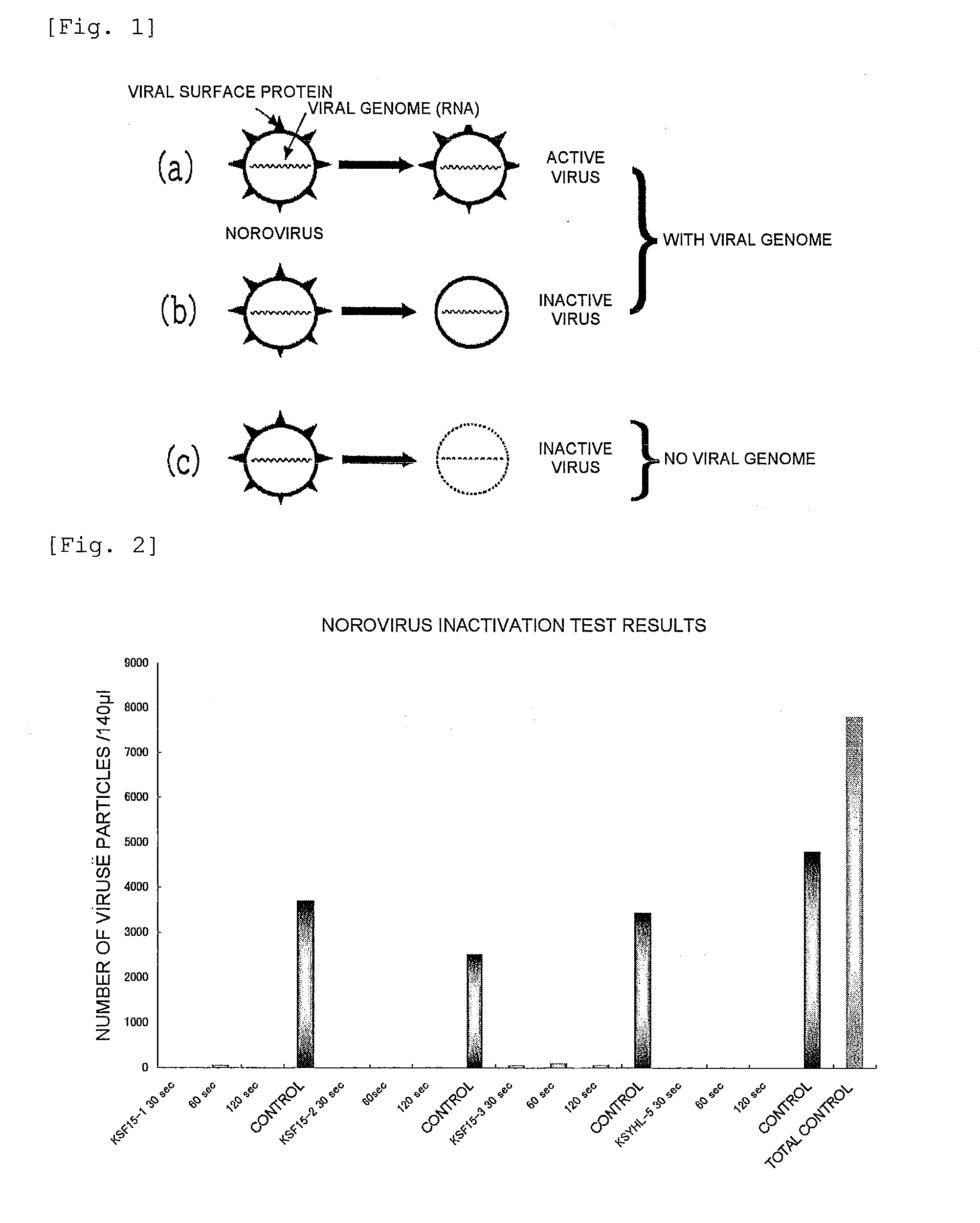
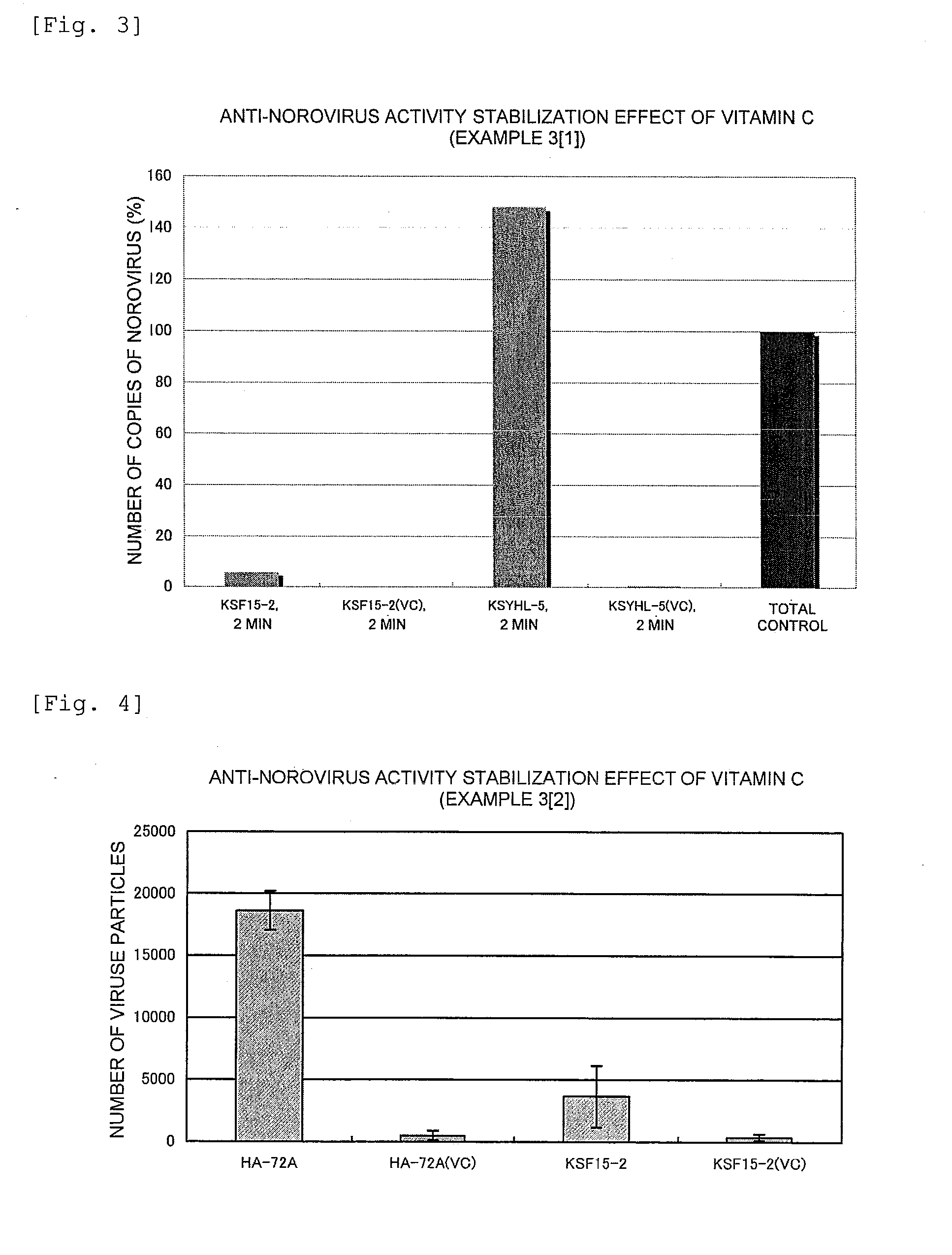
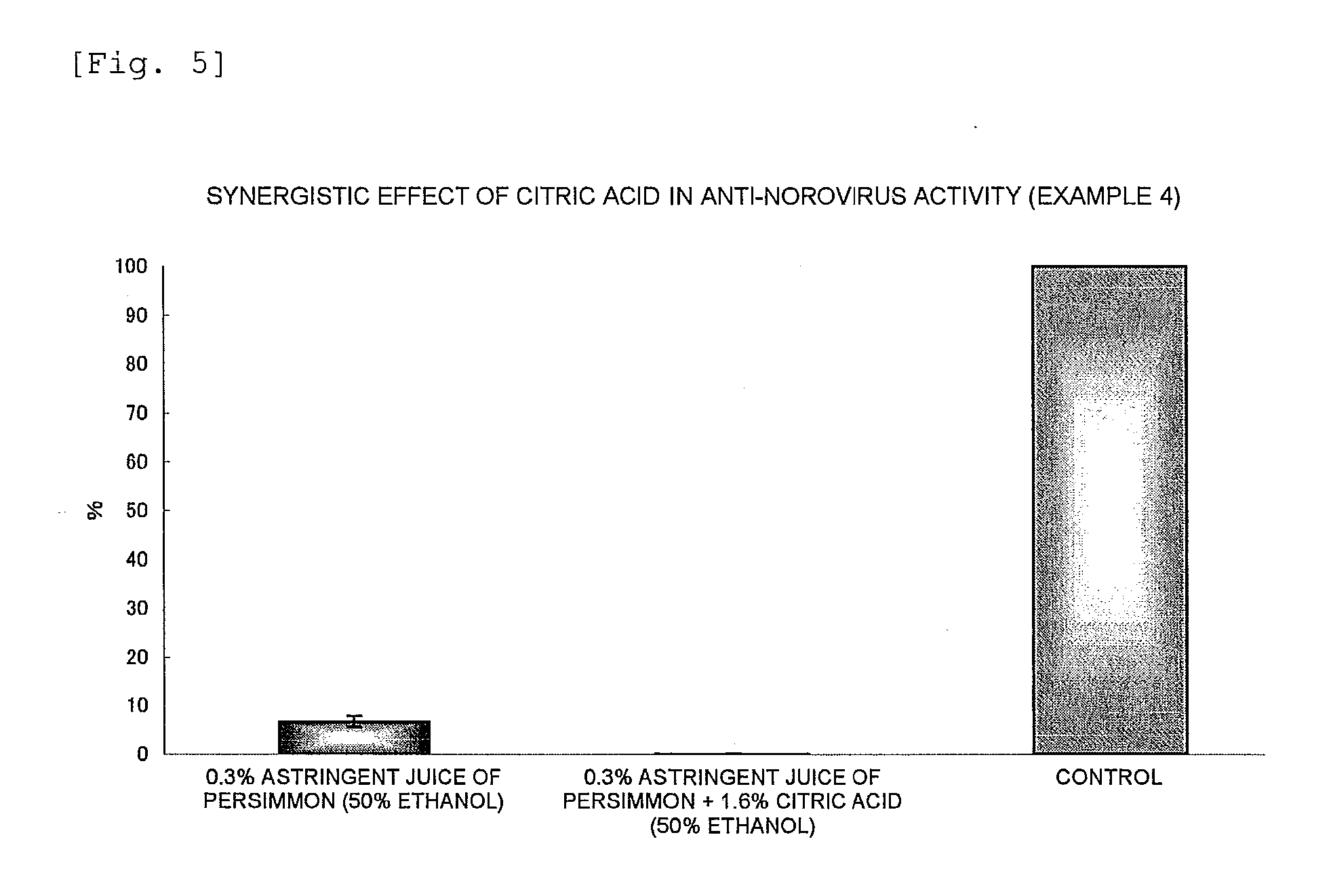
Anti-Norovirus Agent and Composition Containing the Same
ActiveUS20100240600A1Excellent anti-norovirus characteristicEffective disinfectionAntibacterial agentsBiocideFruit juiceVitamin C
Provided is an anti-norovirus agent that has high norovirus-inactivating activity and is safe for the human body, and an anti-norovirus composition that contains the anti-norovirus agent and is useful for disinfection and infection control against the norovirus. The anti-norovirus agent includes, as an active ingredient, an extract from a plant of the genus Diospyros containing tannin (hereinafter referred to as a “persimmon extract”), preferably a persimmon extract produced by heating squeezed juice or an extract from the fruit of a plant of the genus Diospyros or treating the squeezed juice or the extract with an alcohol. The anti-norovirus composition contains the anti-norovirus agent and at least one selected from the group consisting of alcohols, surfactants, antimicrobial agents, humectants, and cosmetic fats and oils, and preferably further containing an organic acid, such as citric acid, and / or a salt thereof or vitamin C.
Owner:HIROSHIMA UNIVERSITY +1
Infection Control Station
A infection control station includes: a mounting unit; a cover rotatably attached to the mounting unit, adapted to transition between an open position and a closed position; a pump adapted to dispense the hand sanitizer; a site window to view the hand sanitizer when the cover is in the closed position; an aperture adapted to dispense the towel; and a site window to view the towel when the cover is in the closed position. The station may also include a hinge that pivotally attaches a first side of the cover to the mounting unit; a lock that releasably secures a second side of the cover against the mounting unit; and a shelf that is accessible when the cover is in the open position and is not accessible when the cover is in the closed position.
Owner:SIMMONS ROBERT
Infection control bedding product
ActiveUS20100306923A1Promote degradationHinders viabilityPillowsBlanketFilling materialsFilter media
A hermetically sealed infection control bedding product such as a pillow, duvet, mattress, cushion or such like having a sealed cover and having a resiliently deformable filling material, comprising a vent means in the cover, characterised in that the vent means comprises a filter medium including a filter membrane for the removal of particles of microbial size and wherein the filter medium also comprises a strengthening layer of material for providing mechanical strength to the filter medium whereby the filter medium allows air flow therethrough but is substantially impermeable to liquid while maintaining a barrier to particles of microbial size.
Owner:PNEUMA PURE I P
Air decontamination devices
Air decontamination method and device designed for bioterrorism, nerve gas, toxic mold, small pox, Ebola, anthrax and other agents require built in air sampling, rapid filter changes and the ability to use a mobile, transportable and connectable system in positive mode to push contaminates away or in negative mode to contain a toxin from spreading. This application combines features in respirators, industrial and hospital grade air filtration with the ability to provide air testing to guide the connection of the device with other treatment modules or existing HVAC and other equipment. With this new flexibility, ozone, UV, absorption, Thermal destruction, filters and liquid chemical neutralization can be manually or automatically adapted for emergency response to both daily airborne contamination and military grade terrorist threats of airborne contamination. The air decontamination units may be used to decontaminate the air after industrial and medical contaminations and terrorist biological, chemical and radiological attacks, for example. Mobile isolation units, and methods of decontaminating rooms, are disclosed, as well as Well as infection control and emergency response usage as an emergency clean air supply when connected to escape hoods, decon tents, or containment barriers to protect structures from homes to business from outside toxic agents. The unit can be powered by normal AC, 120 volts or 240 or be adapted to battery or field power supply units.
Owner:西奥多・A・M・阿尔茨
Transdermal intraosseous device
A transdermal intraosseous device includes a transdermal adapter for an external prosthetic device for a bone of a patient and a bone fixator including a distal portion coupled to the transdermal adapter and a proximal portion for anchoring into the bone. The transdermal adapter includes a dome-shaped portion for transcutaneous implantation and an external shaft extending from the dome-shaped portion. A dermal transition structure is configured to include a controlled roughness gradient from the external shaft to the dome-shaped portion and configured for use in infection control at a dermis layer of the patient.
Owner:BIOMET MFG CORP
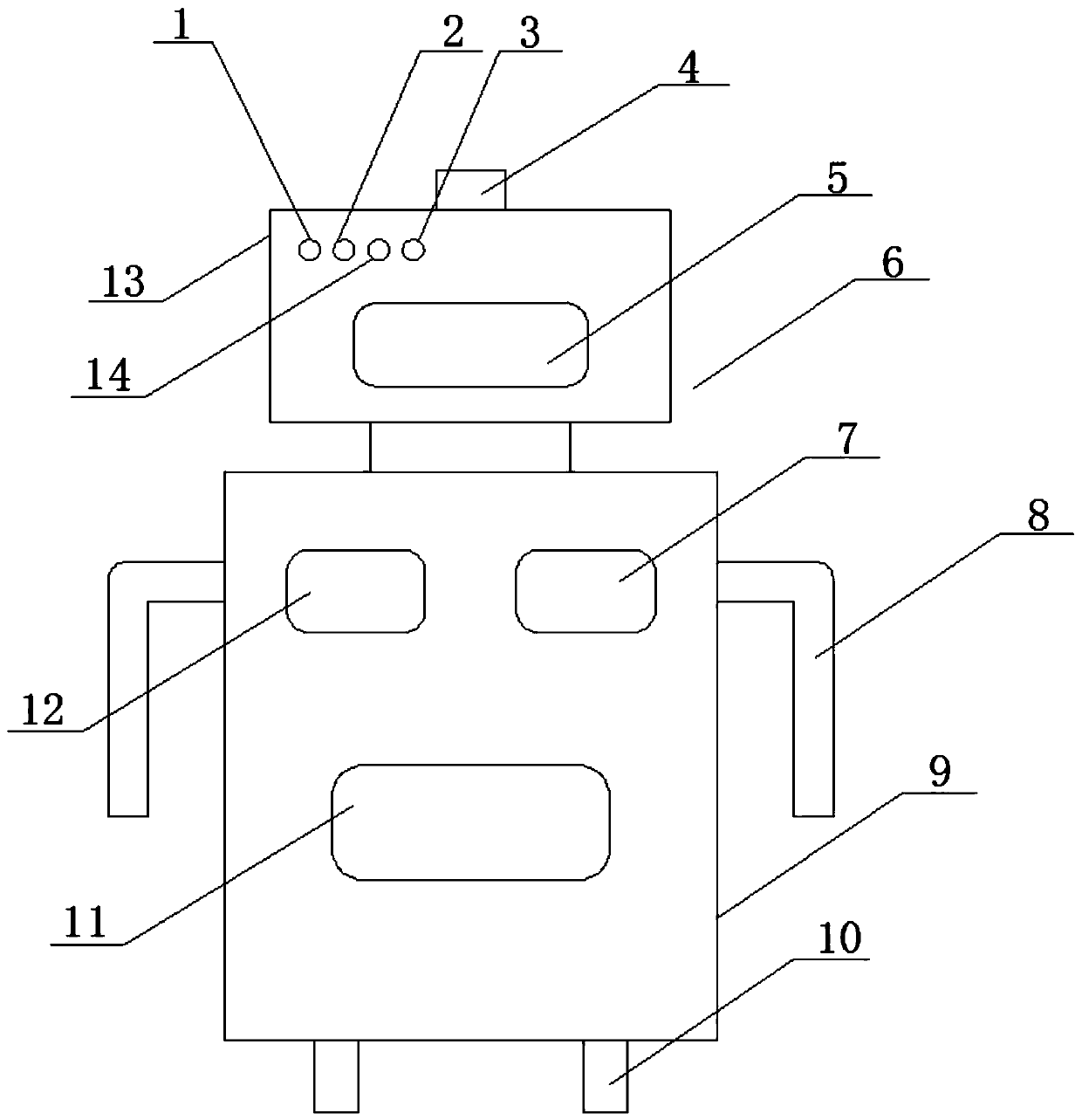
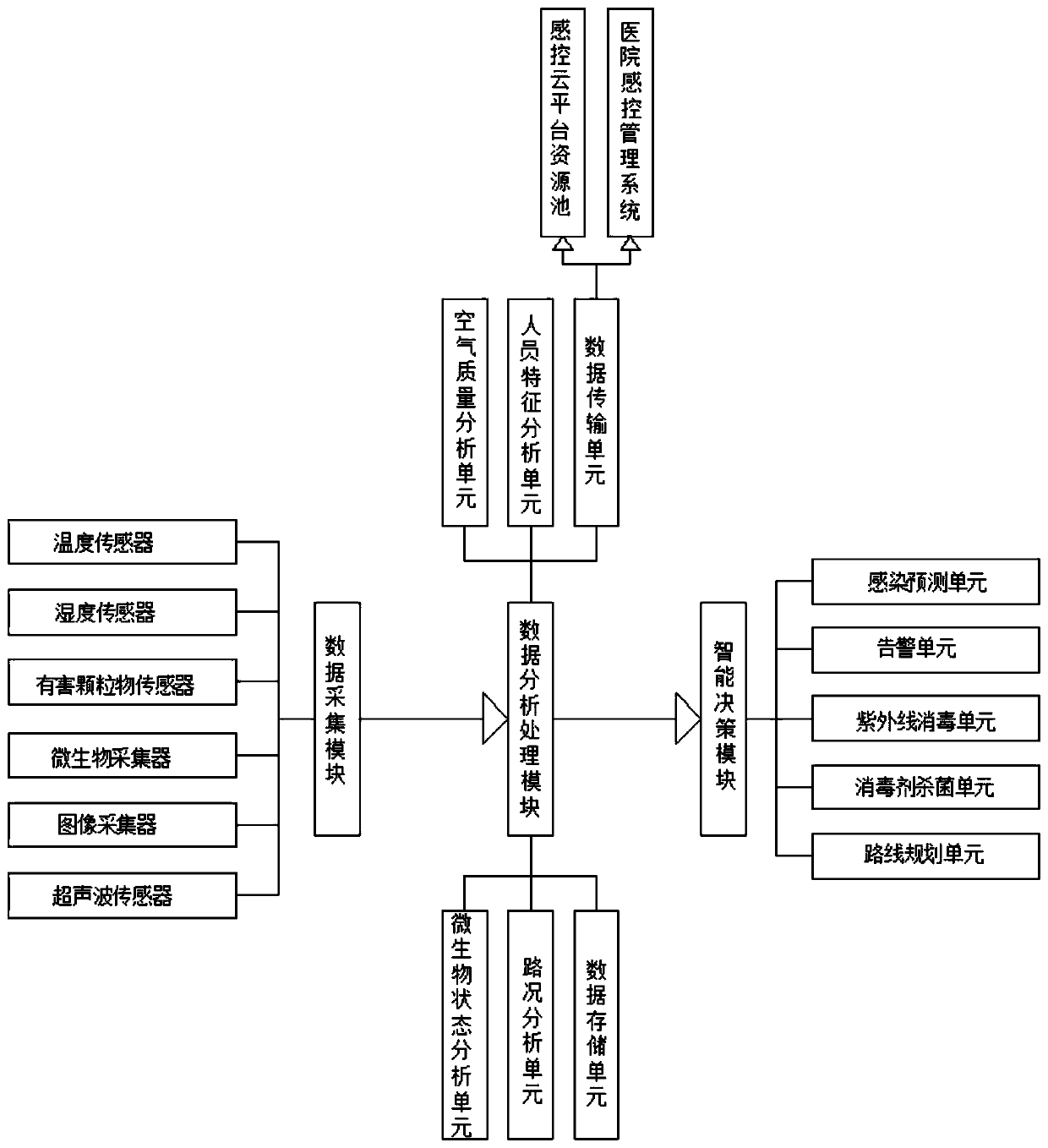
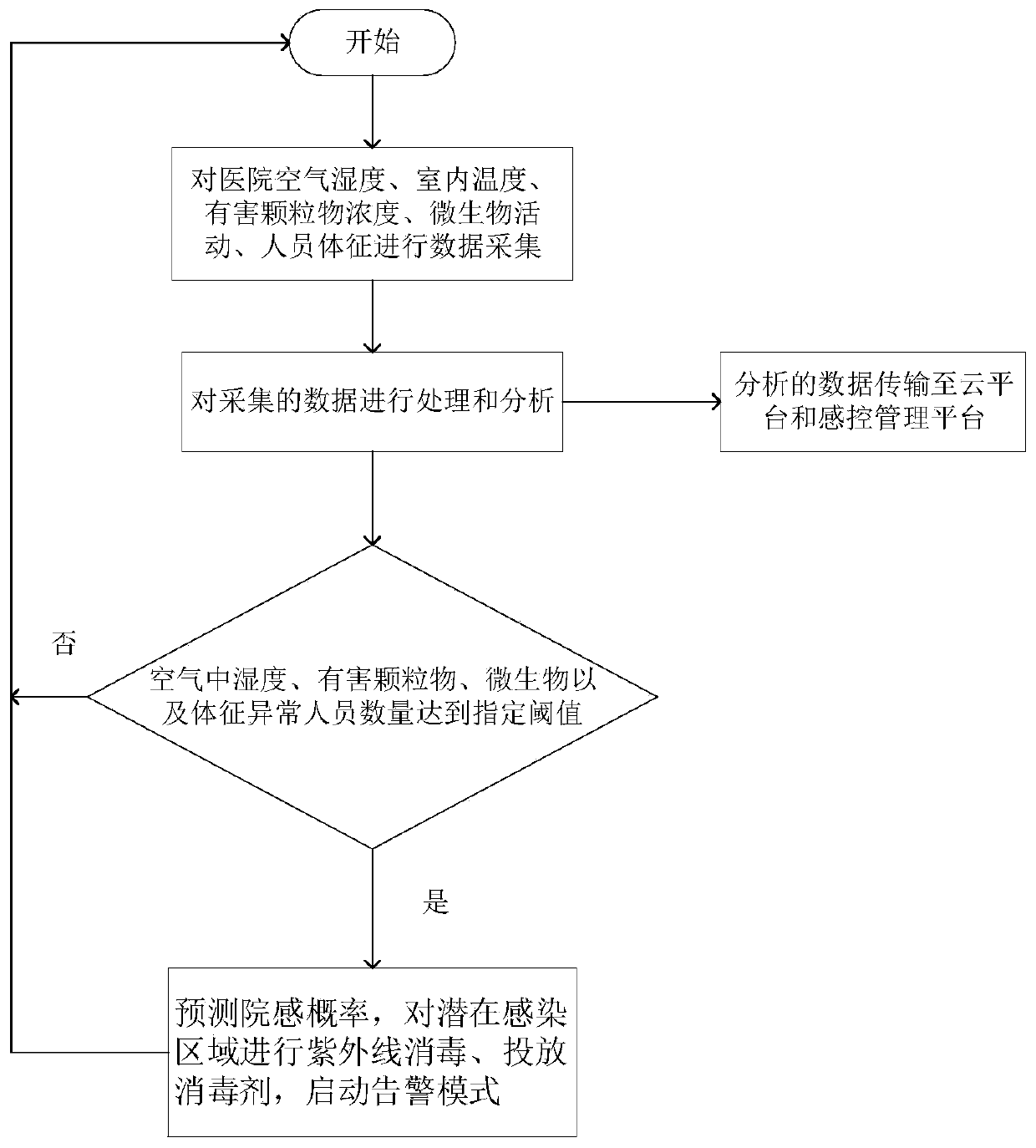
Infection control robot based on artificial intelligence
InactiveCN110497420AEffective monitoringReduce complexityMeasurement devicesAlarmsData acquisitionArtificial Intelligence System
The invention discloses an infection control robot based on artificial intelligence. The robot comprises a robot body, a data acquisition module, a data analysis and processing module and an intelligent decision-making module; when the infection probability reaches a certain threshold value, the robot can automatically send an alarm through an audible and visual alarm, furthermore, the intelligentdecision-making module can be used for automatically planning a route for the robot according to the analysis of air quality and infection probability in different areas, so that the robot can intelligently move toward areas with poor air quality and high infection probability, and infection control management is carried out on the areas; and meanwhile, distributed storage and calculation can becarried out on infection control monitoring data, so that medical staff can master the environment and infection control conditions in a hospital anytime and anywhere, and the real-time performance, effectiveness and accuracy of the infection controlling work can be realized; and according to the infection control robot based on the artificial intelligence, a data analysis result can be intelligently decided, potential infectious diseases in the hospital can be effectively controlled and warned in time, so that huge medical loss and casualties caused by epidemic situation outbreak are avoided.
Owner:EDGE INTELLIGENCE RES INST NANJING CO LTD +1
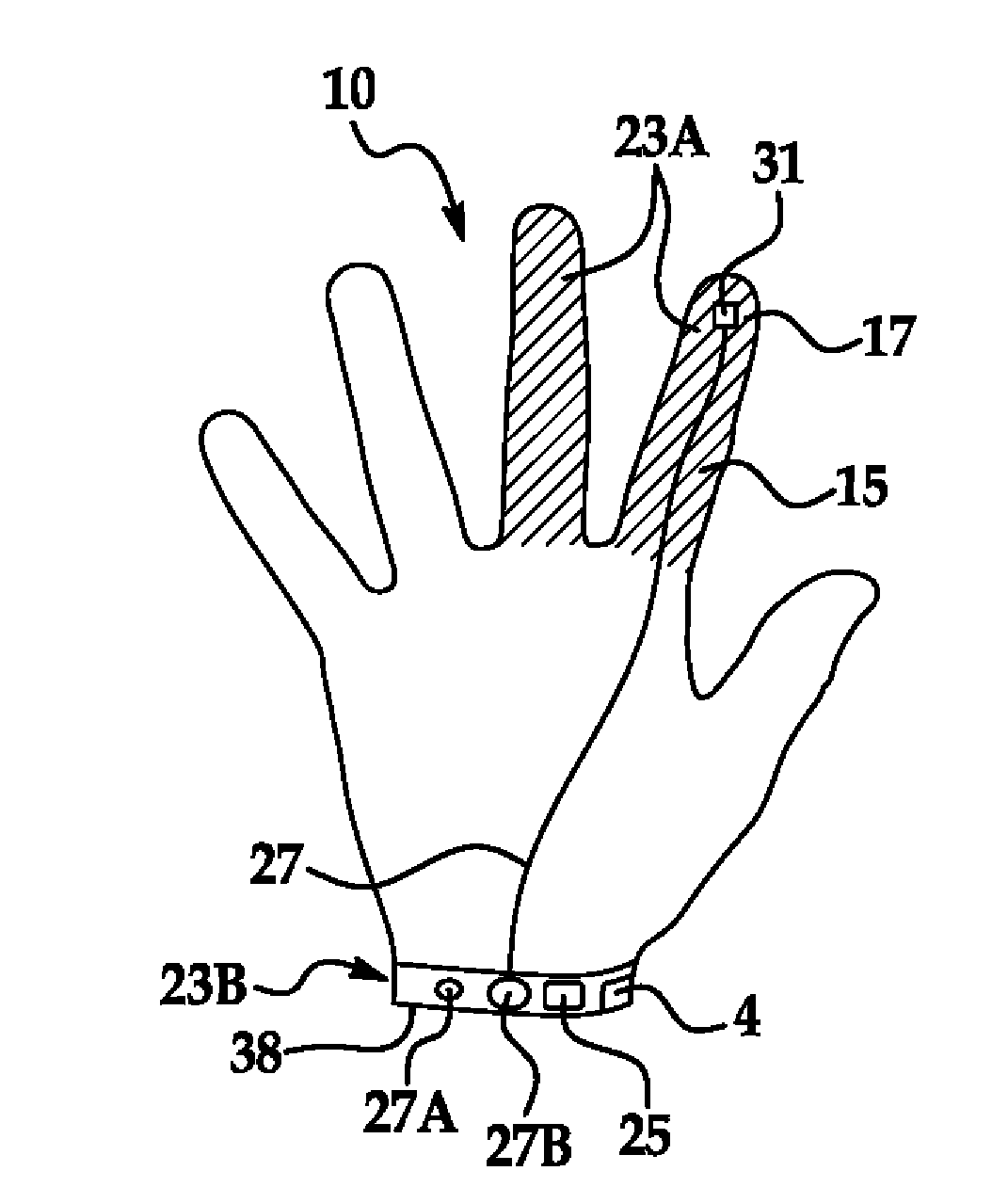
Infection control glove with sensory contamination indicator
InactiveUS20130104284A1Avoid spreadingImprove abilitiesGarment special featuresDiagnosticsInfectious agentBiomedical engineering
A barrier protection device and dispensing system are provided in the form of a medical service or examination glove with sensory indicators that respond to contact with infectious agents or substances. The glove and dispensing system prevent transmission of infectious agents from a healthcare worker to a patient or vice versa.
Owner:KANTROWITZ ALLEN B +1
Infection Control Management and Workflow System
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com