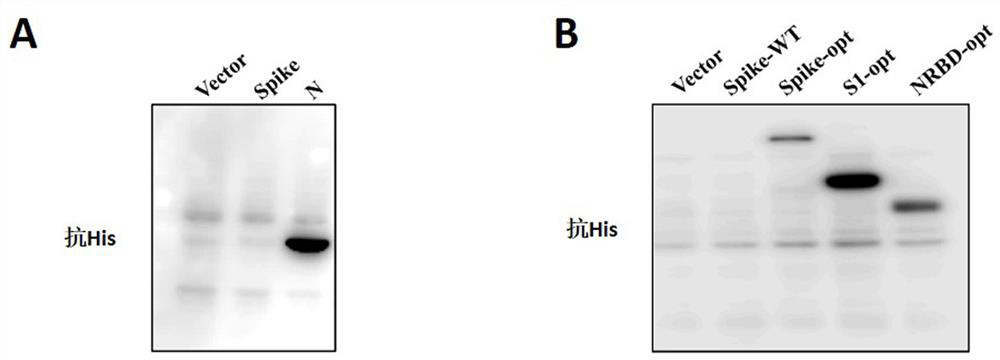
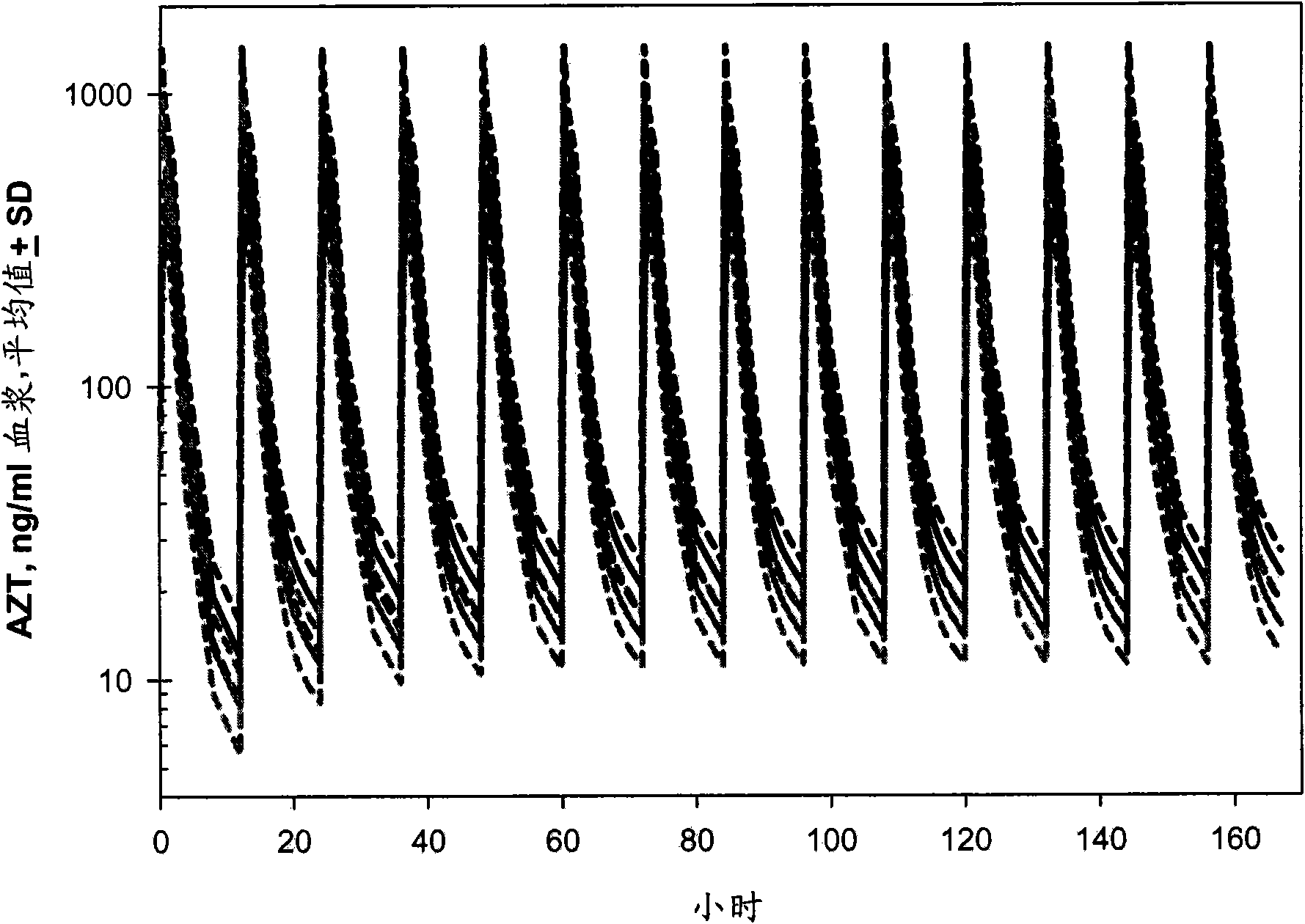
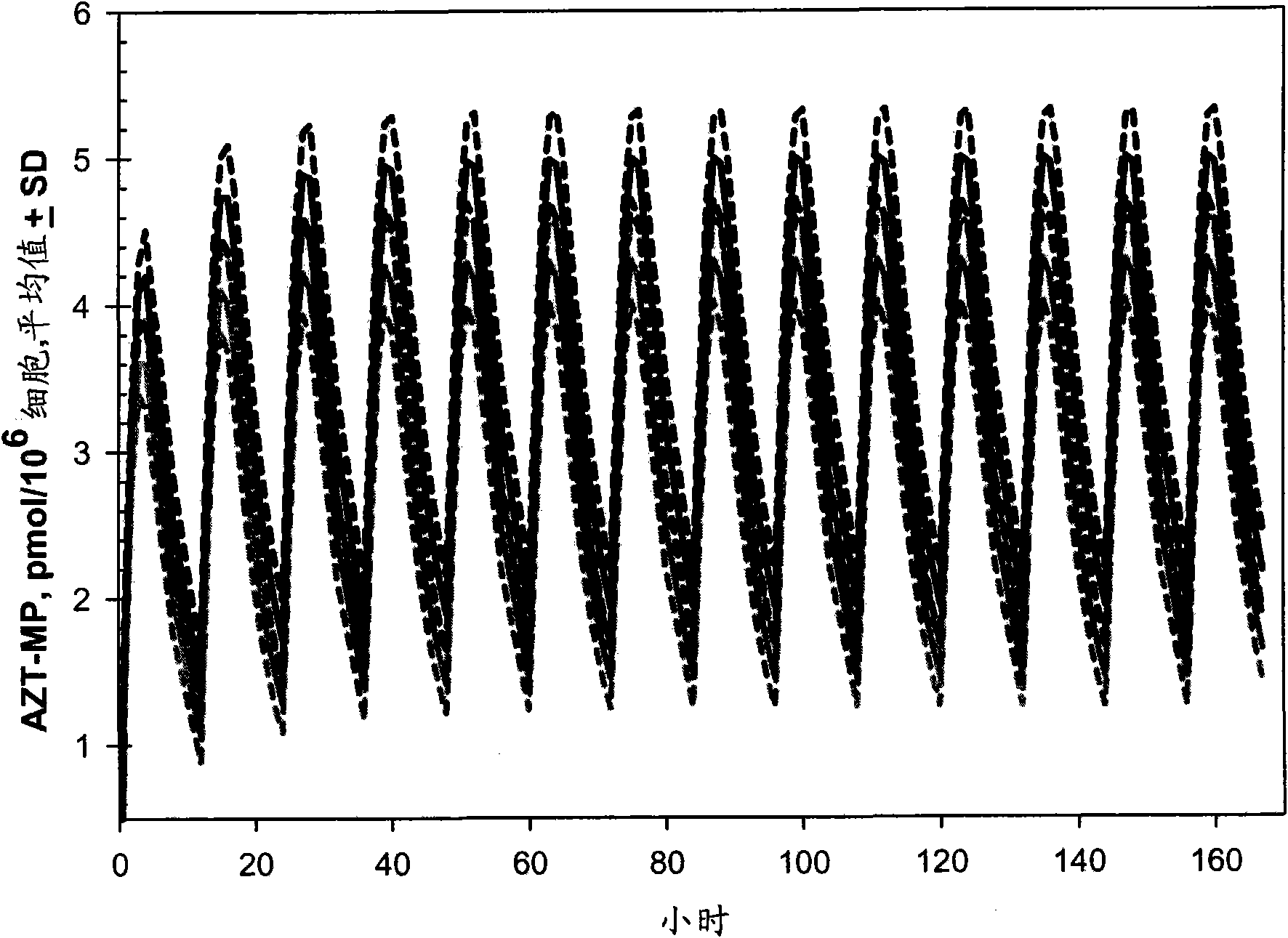
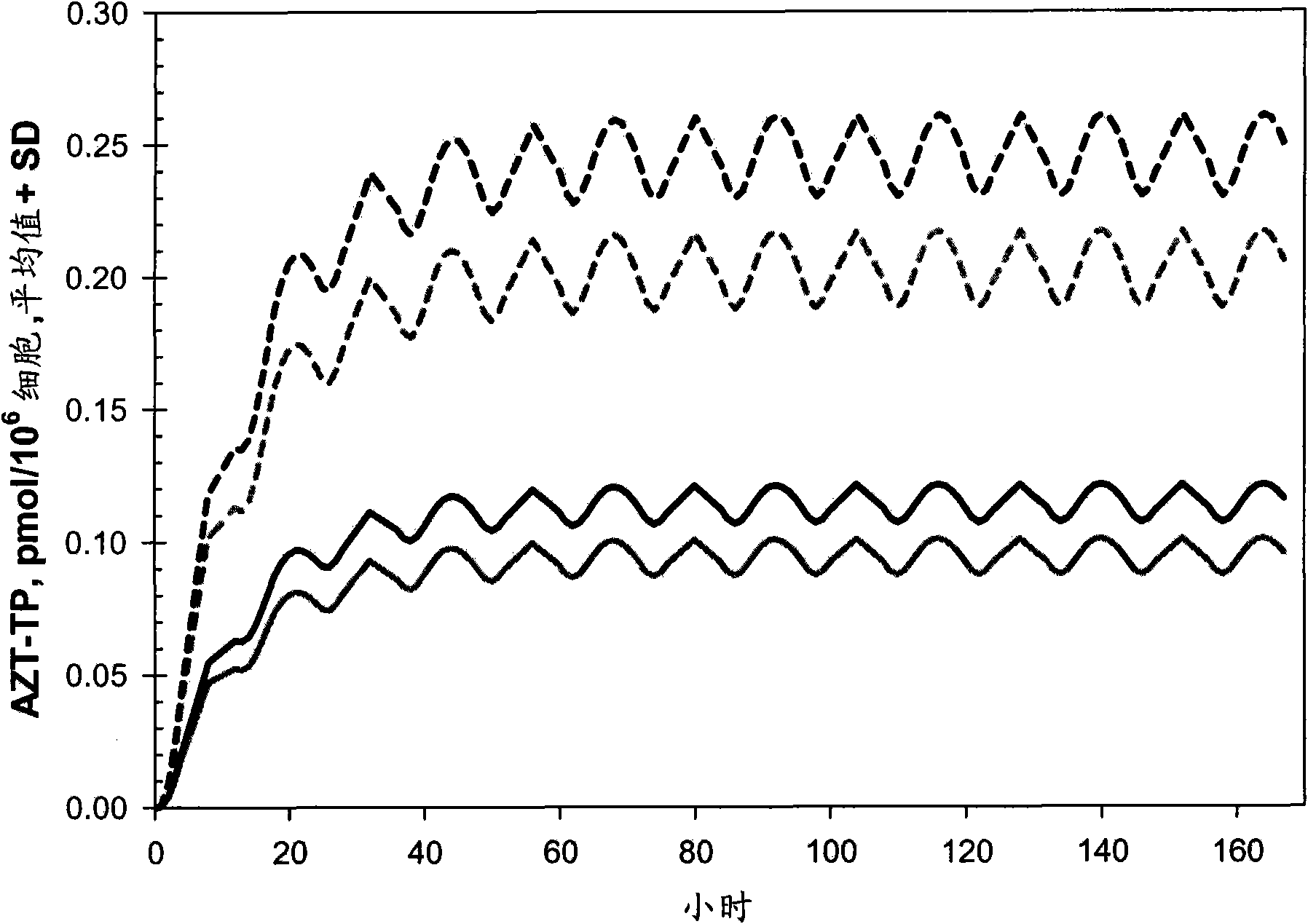
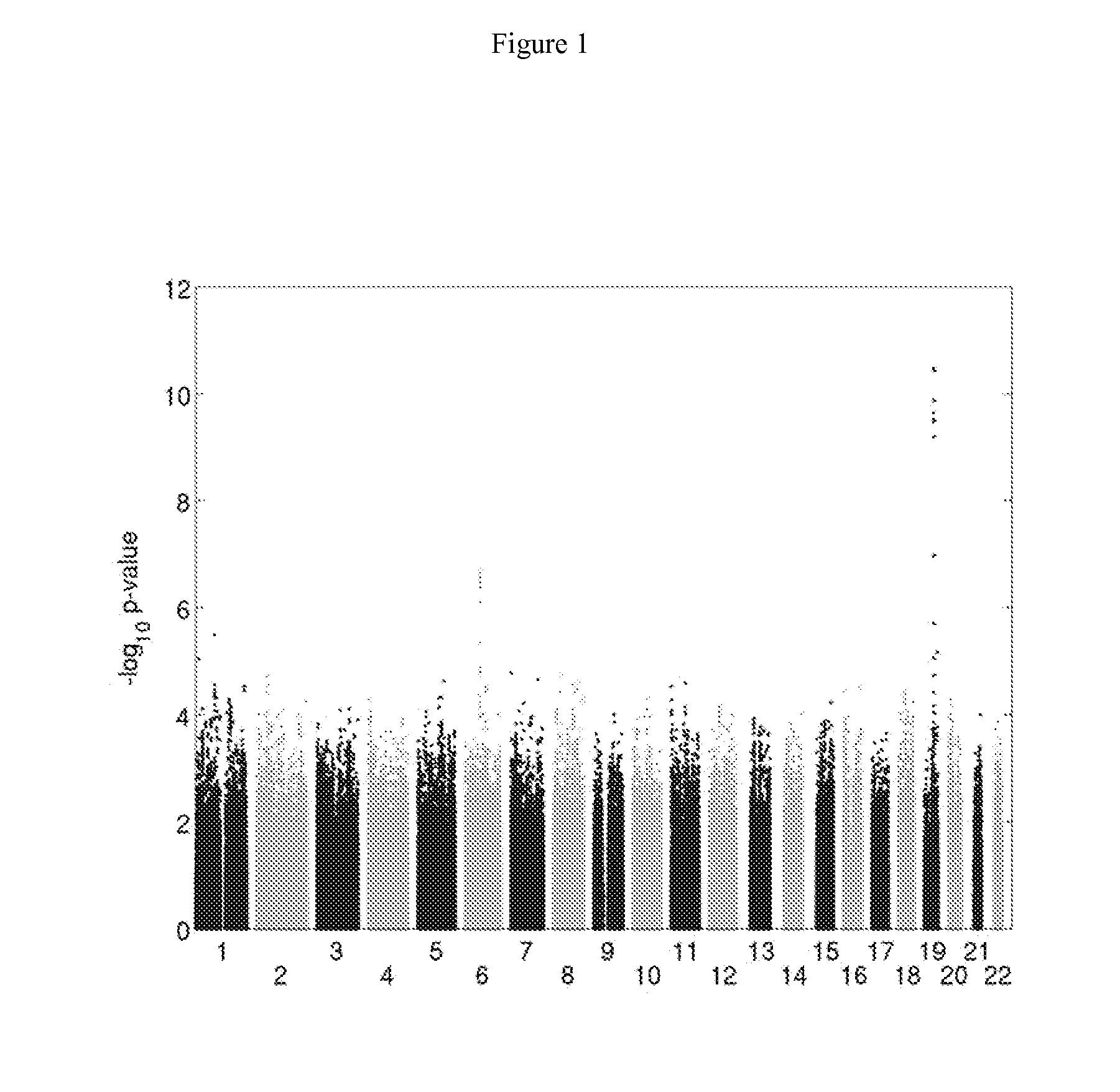
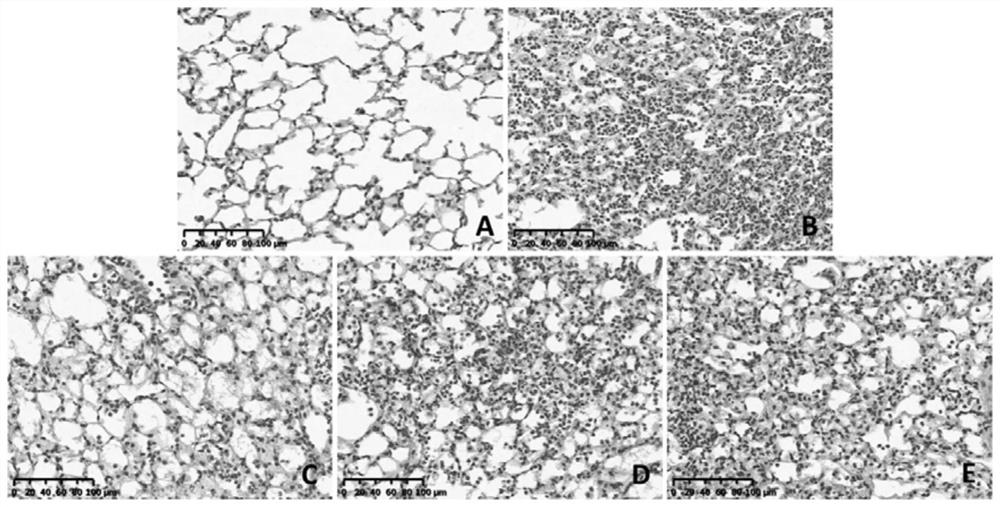
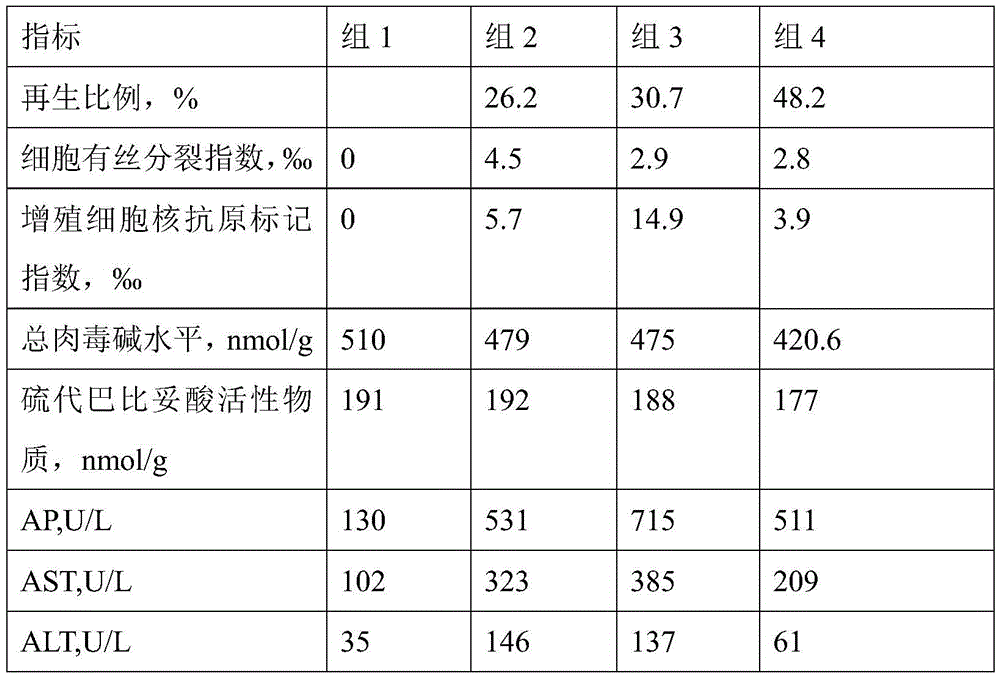
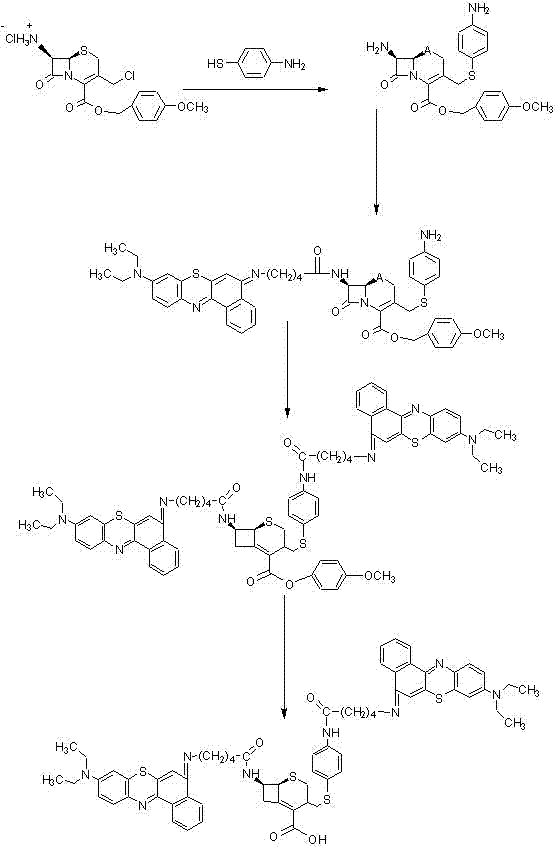
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
141 results about "Infected patient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
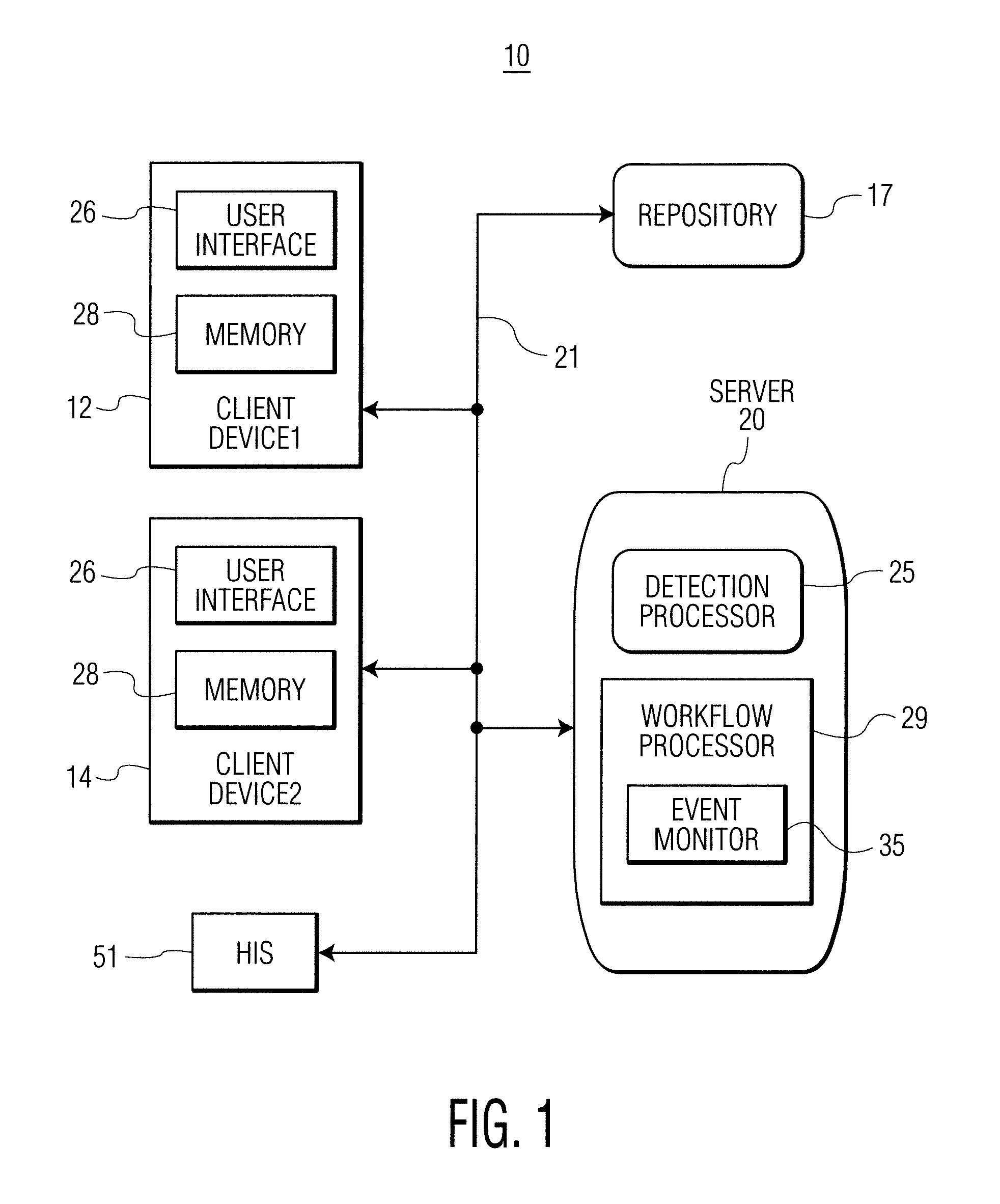
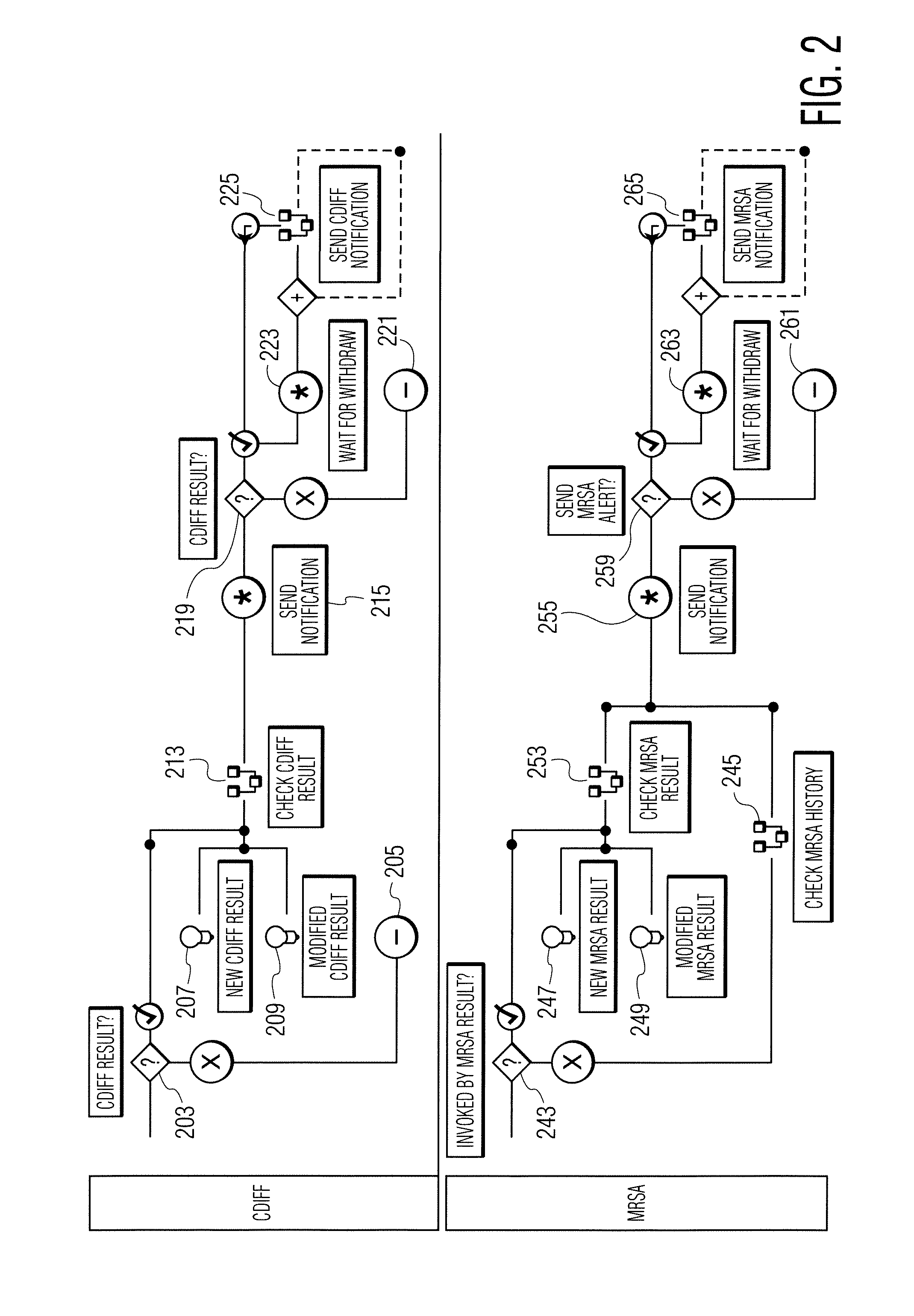
Infection control management and workflow system
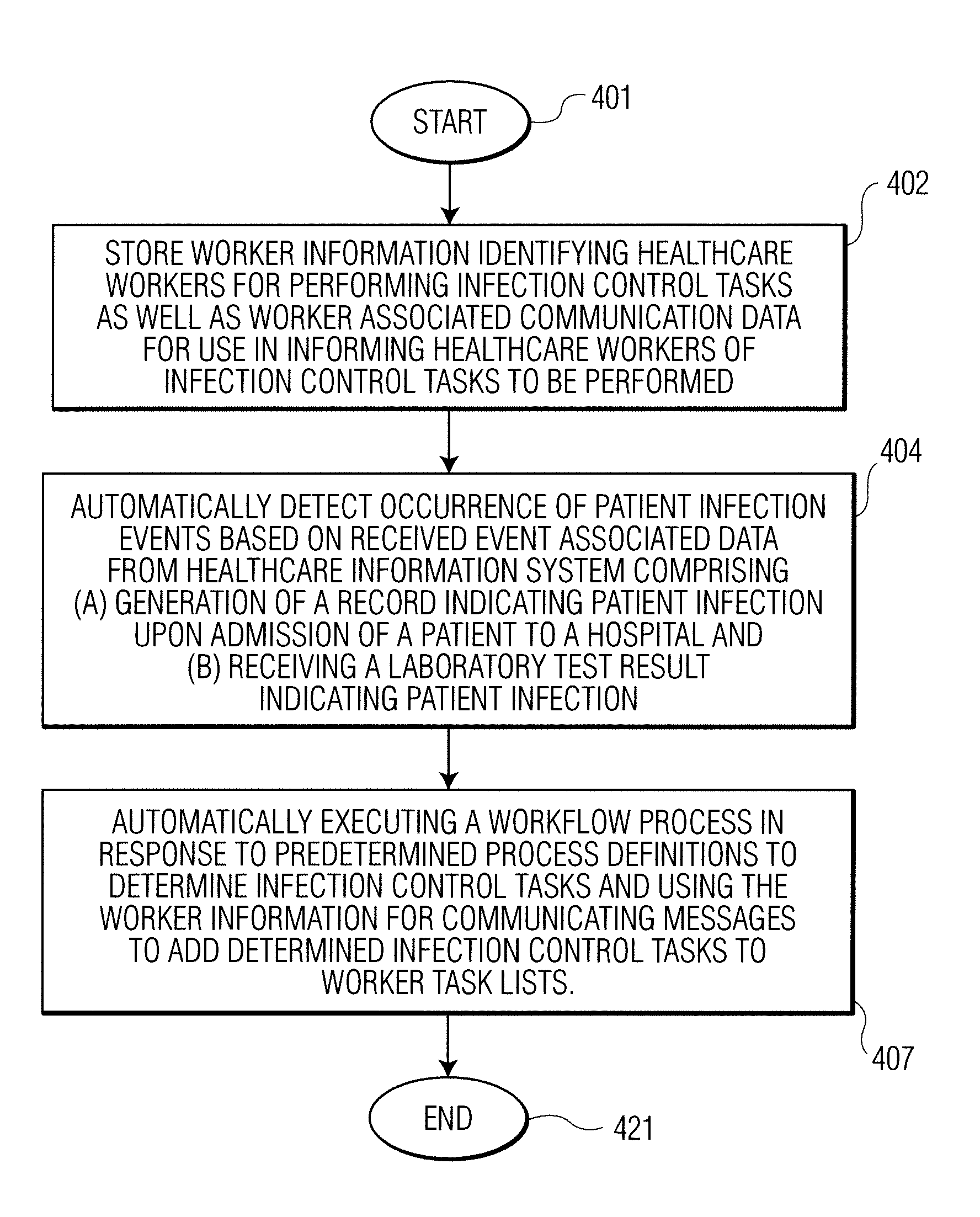
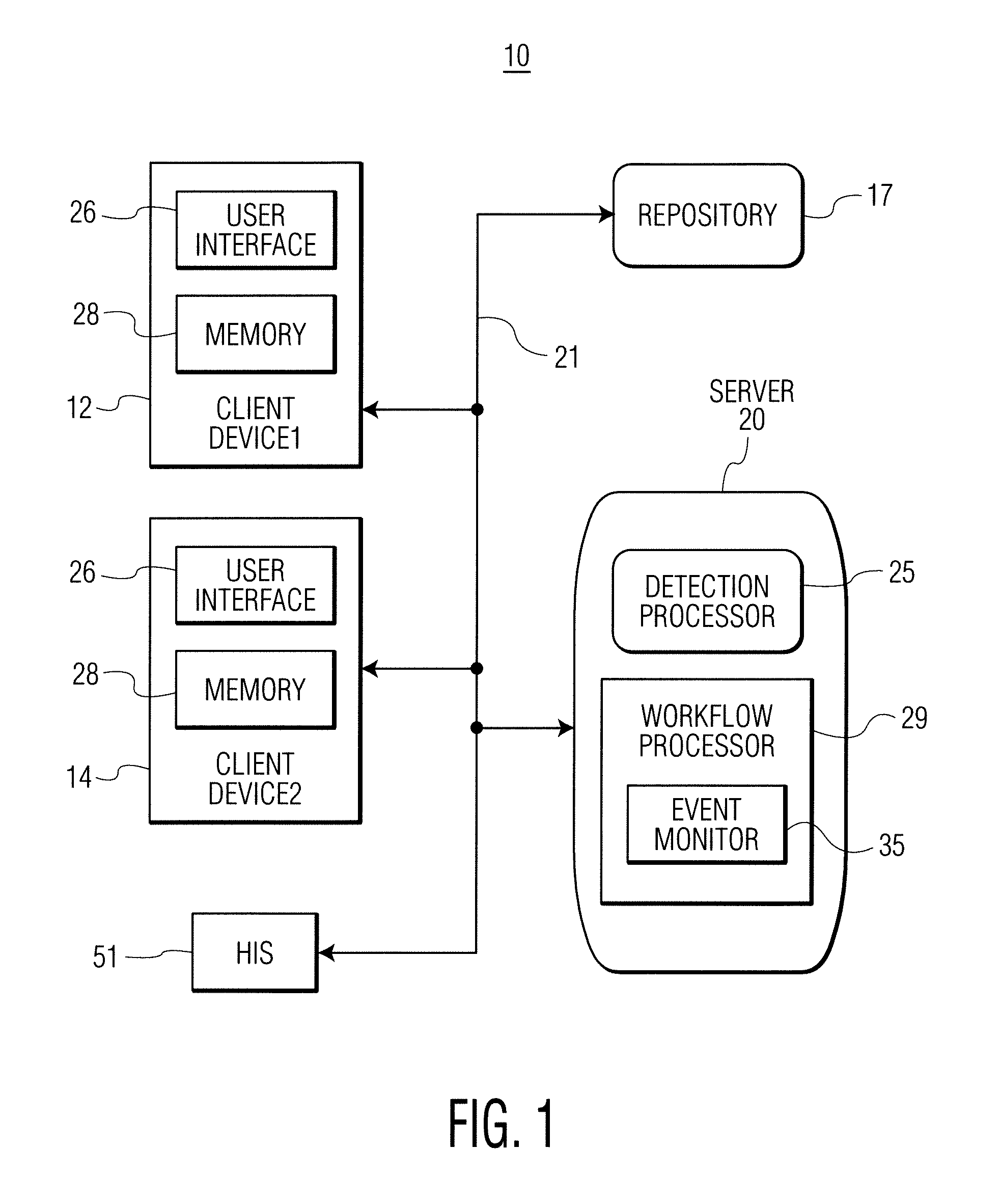
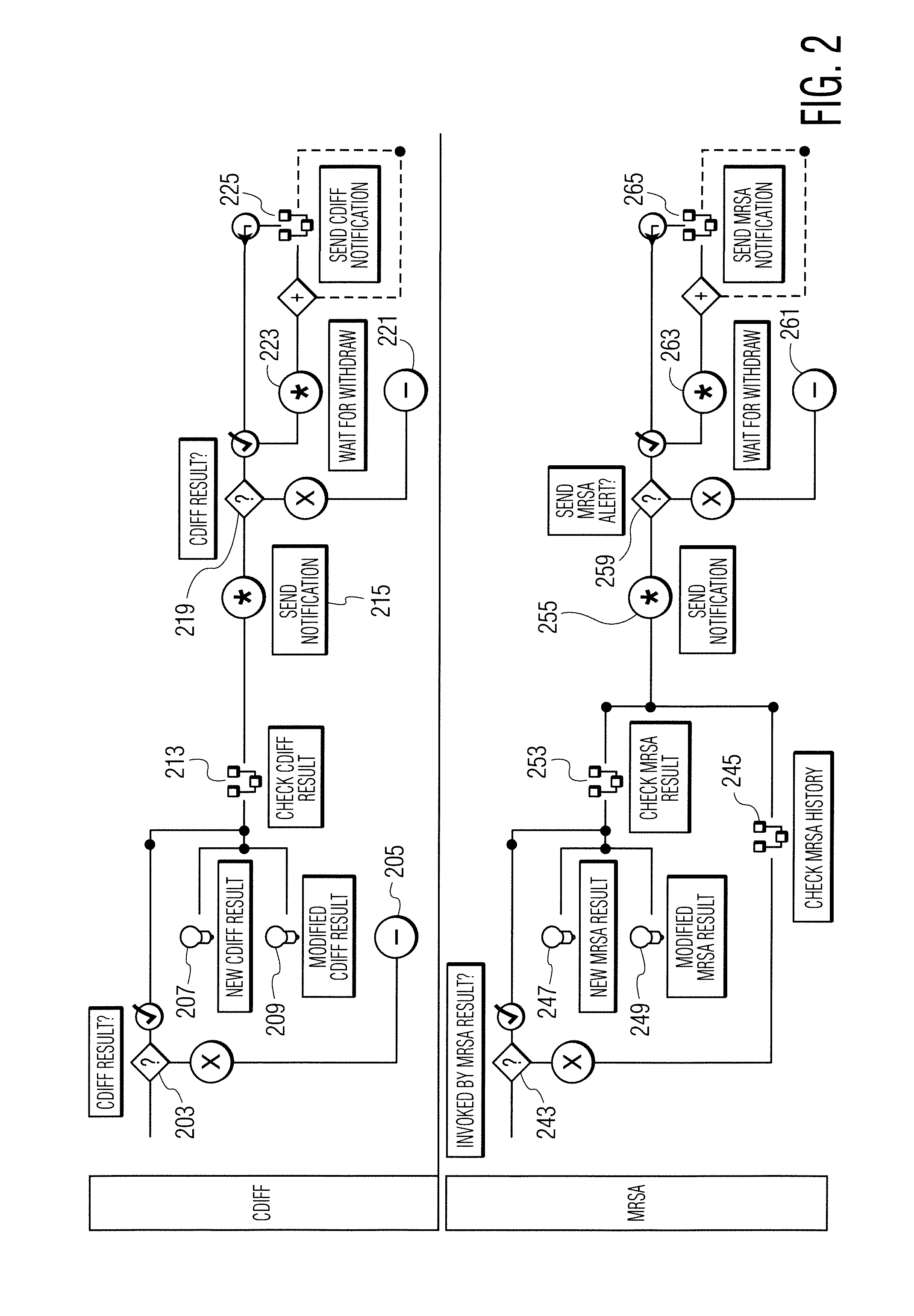
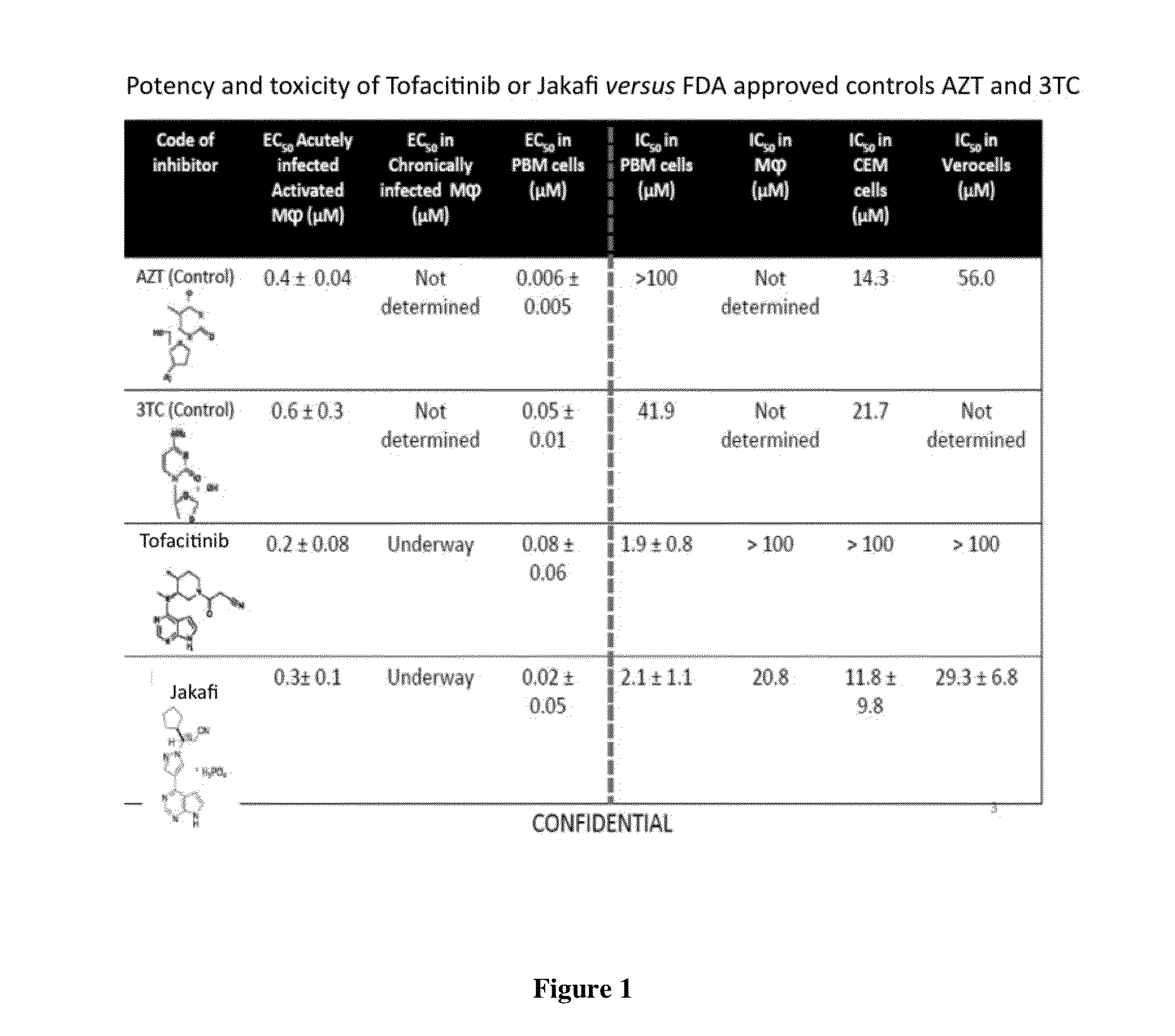
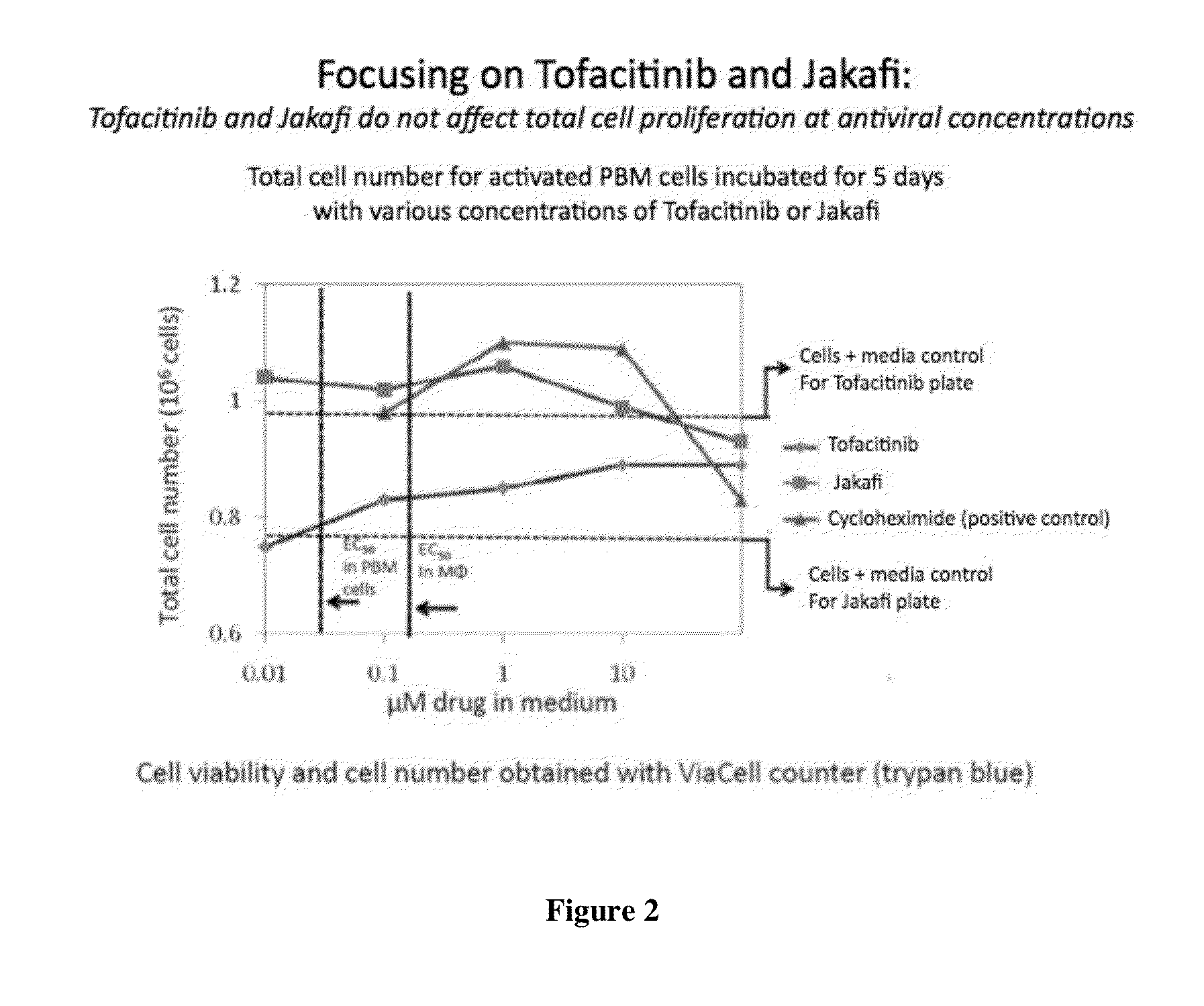
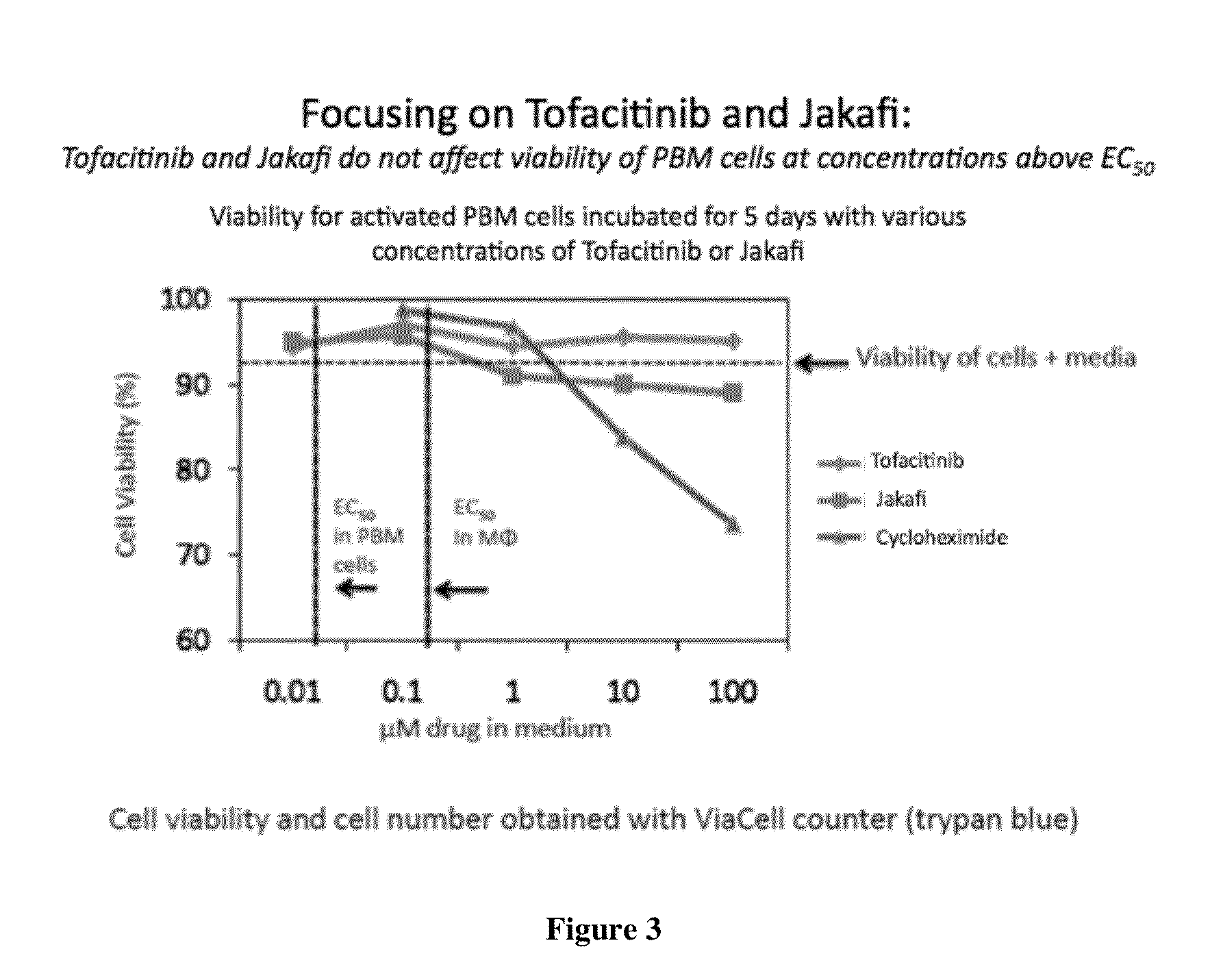
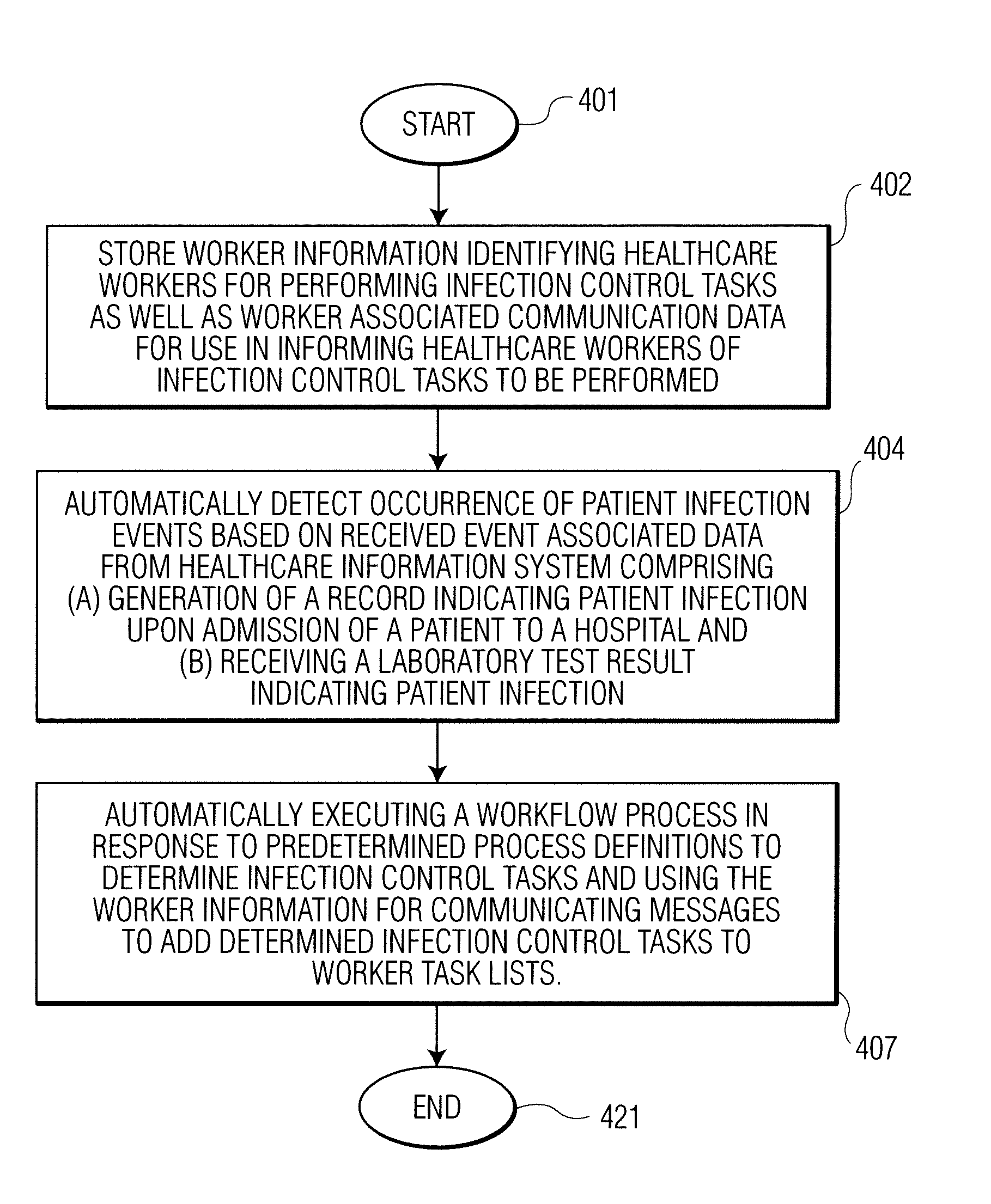
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
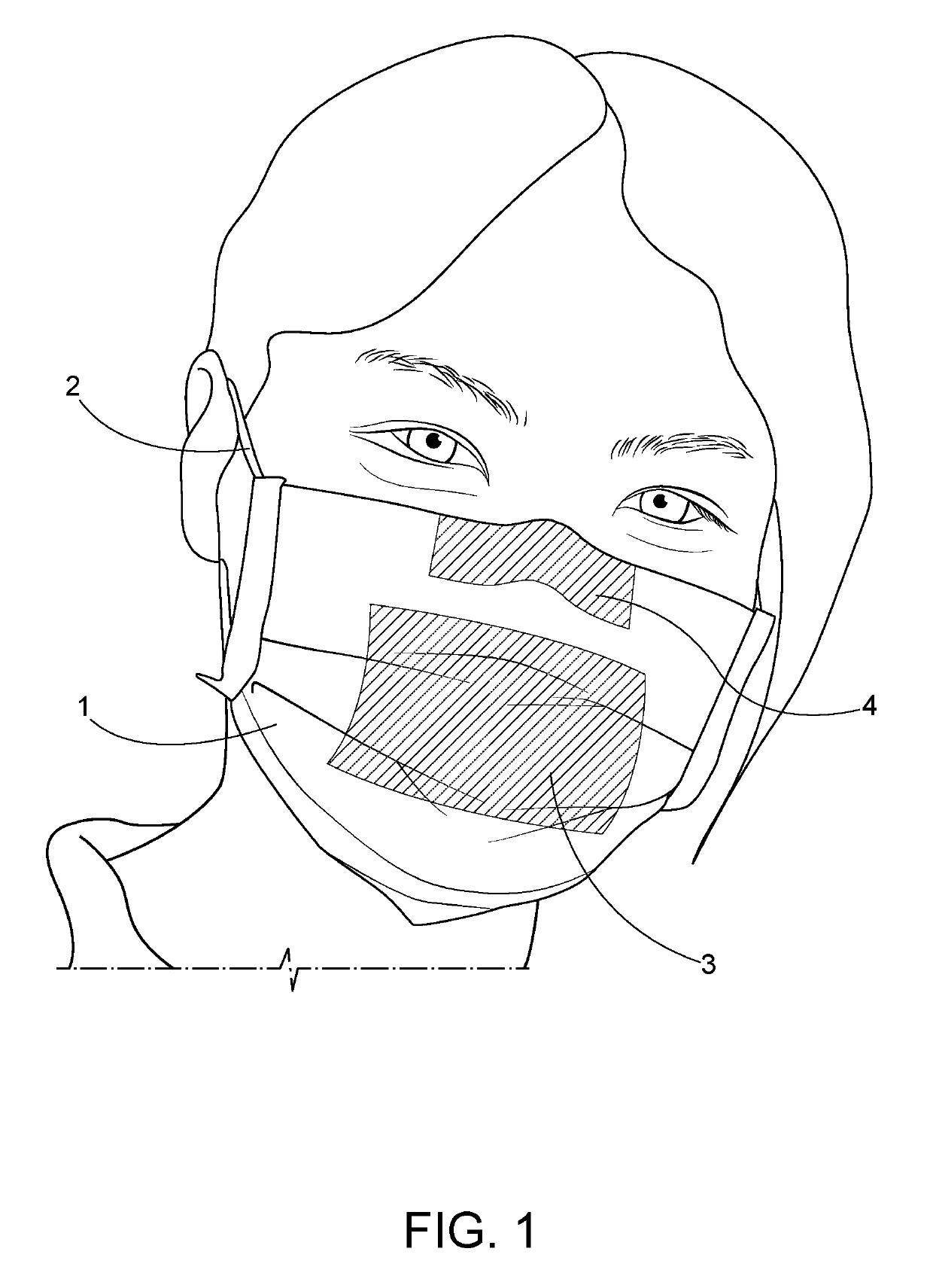
Temperature sensitive surgical face mask for identifying at risk patients and reducing viral infection
InactiveUS20190125011A1Increase temperatureReduce riskGarment special featuresBreathing filtersInfected patientChange color
Owner:THOMAS JEFFERSON UNIV
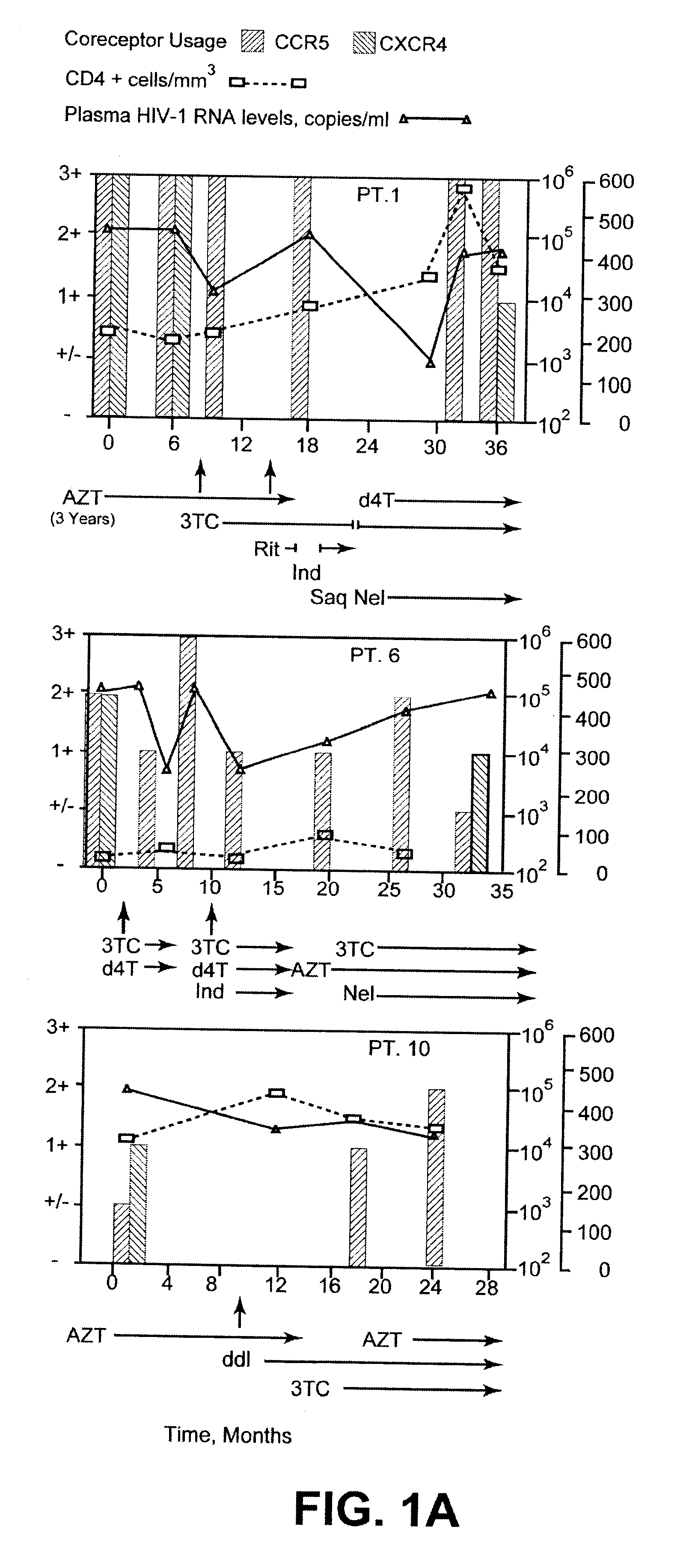
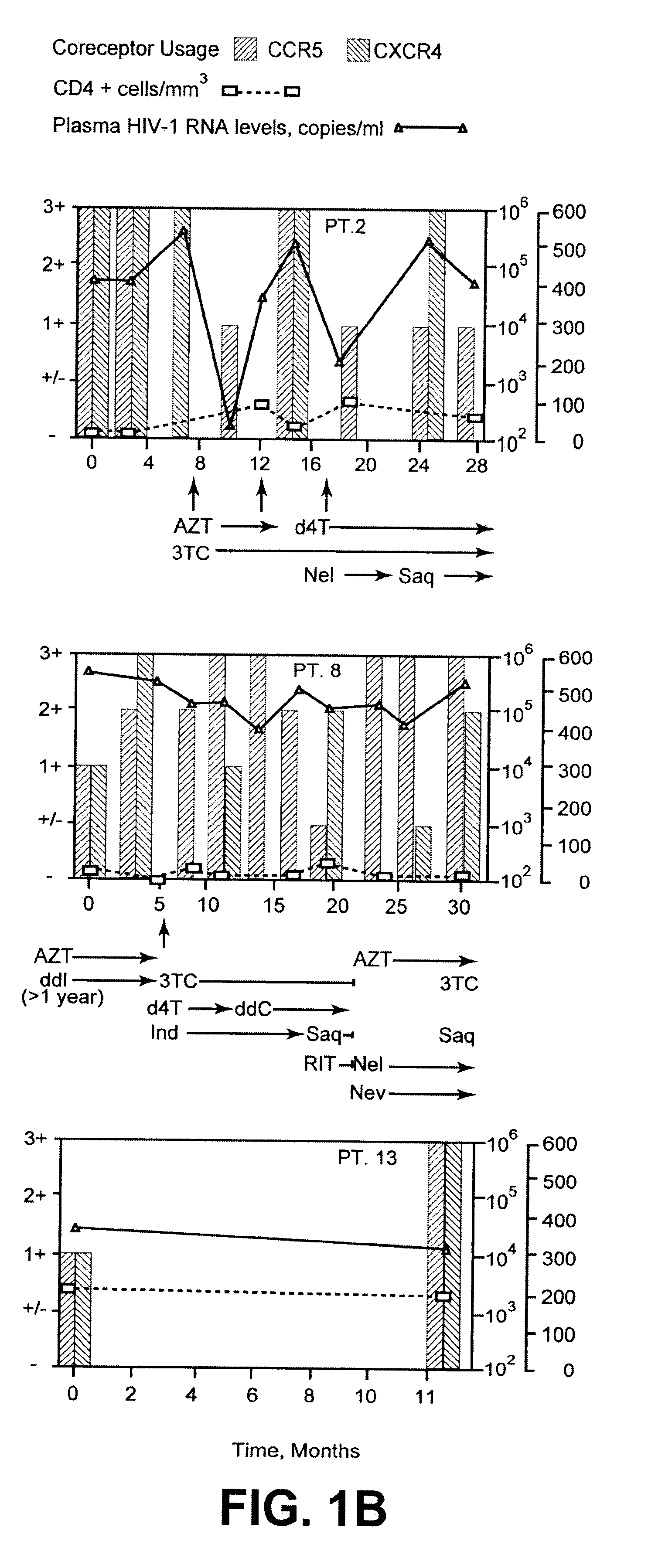
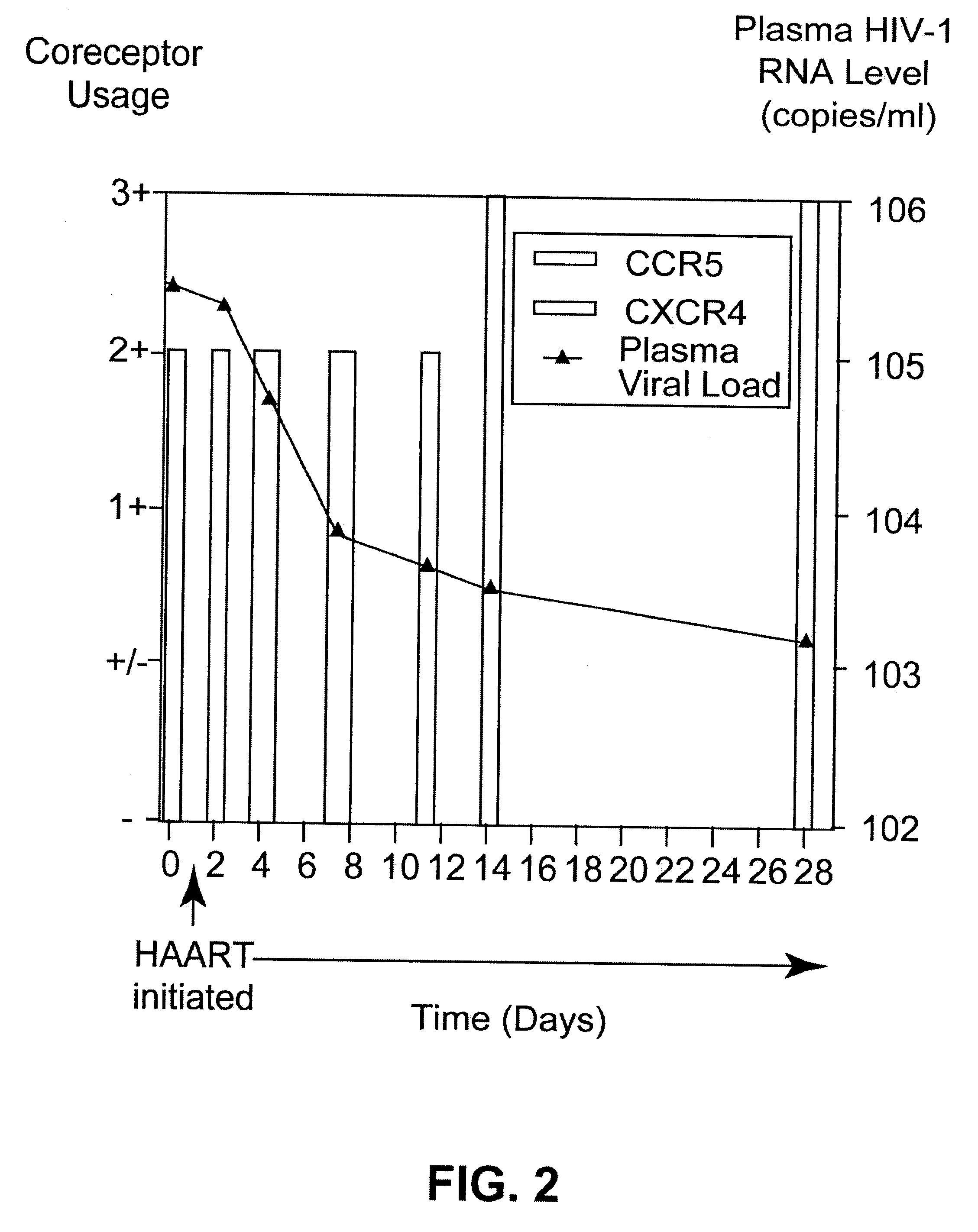
Analysis of HIV-1 coreceptor use in the clinical care of HIV-1-infected patients
InactiveUS6727060B2Accurate predictionMore effectivenessMicrobiological testing/measurementArtificial cell constructsImmunodeficiency virusHIV positives
A change in viral tropism occurs in many HIV positive individuals over time and can be indicated by a shift in coreceptor use from CCR5 to CXCR4. The shift in coreceptor use to CXCR4 has been shown to correlate with increased disease progression. In patients undergoing HAART, the predominant populations of virus can be shifted back to CCR5-mediated entry after the CXCR4-specific strains have emerged. The present invention relates to a diagnostic method to monitor coreceptor use in the treatment of human immunodeficiency virus (HIV) infection. The present invention further relates to a diagnostic method applied to HIV-positive individuals undergoing HAART to monitor the suppression of CXCR4 specific strains. The diagnostic methods can be used to assist in selecting antiretroviral therapy and to improve predictions of disease prognosis over time.
Owner:HEALTH RES INC
Blood transcriptional signature of mycobacterium tuberculosis infection
InactiveCN102150043AMicrobiological testing/measurementDisease diagnosisInfected patientMycobacterium Infections
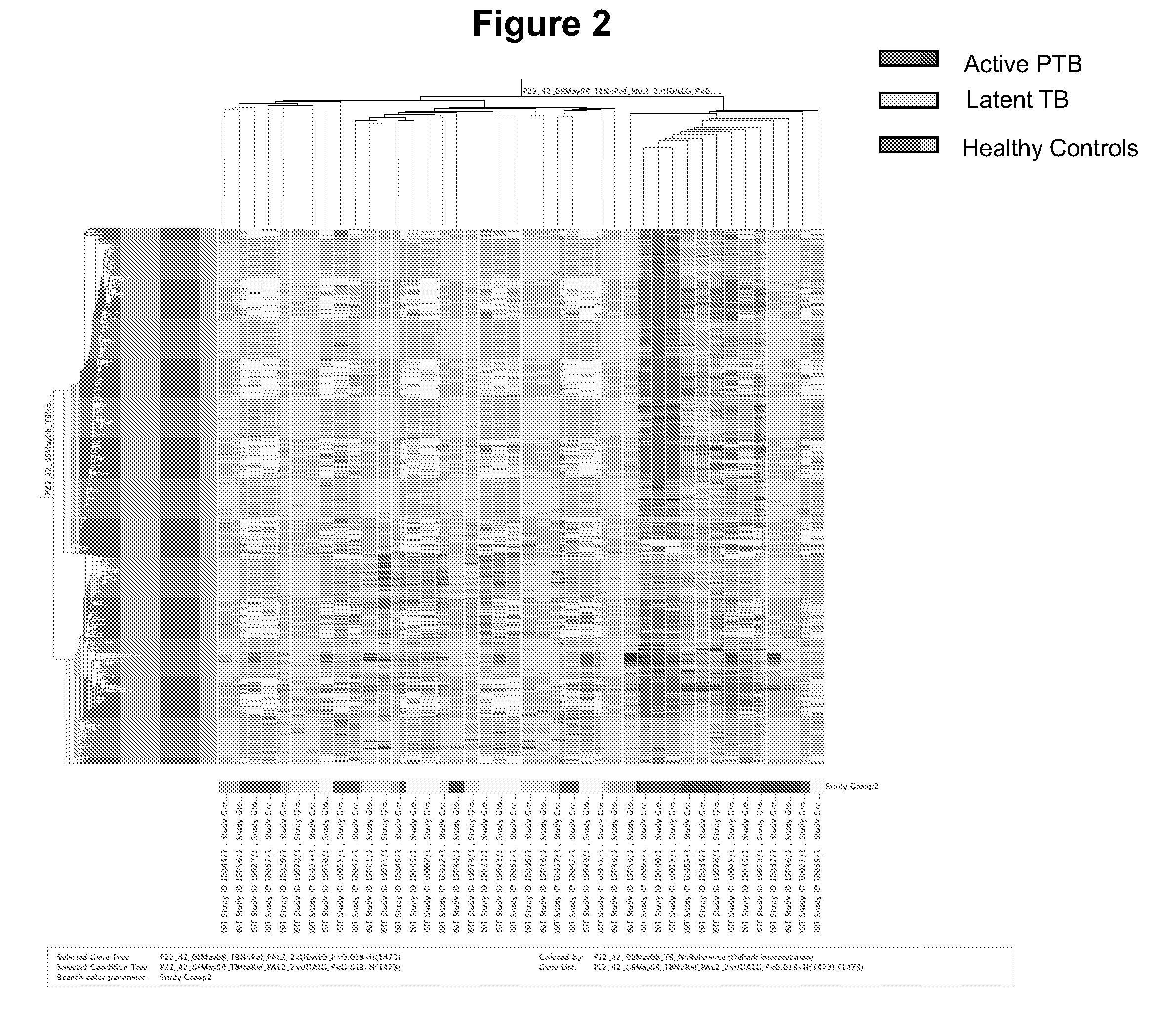
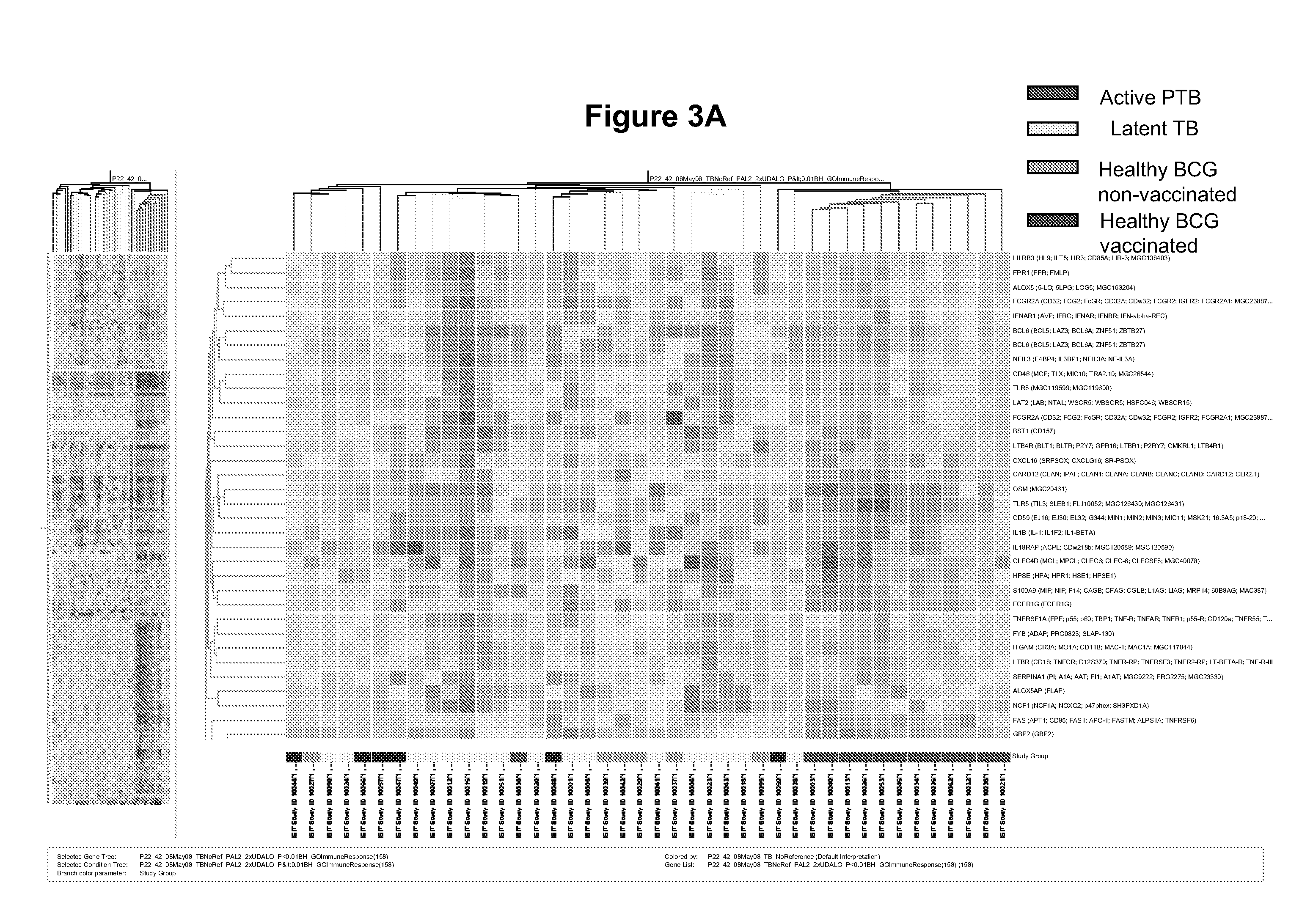
The present invention includes methods, systems and kits for distinguishing between active and latent mycobacterium tuberculosis infection in a patient suspected of being infected with mycobacterium tuberculosis, and distinguishing such patients from uninfected individuals, the method including the steps of obtaining a gene expression dataset from a whole blood obtained sample from the patient and determining the differential expression of one or more transcriptional gene expression modules that distinguish between infected and non-infected patients, wherein the dataset demonstrates an aggregate change in the levels of polynucleotides in the one or more transcriptional gene expression modules as compared to matched non- infected patients, thereby distinguishing between active and latent mycobacterium tuberculosis infection.
Owner:BAYLOR RES INST +2
Three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as kit thereof
InactiveCN101886138AEasy to detectHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCoxsackievirus a16Reverse transcription polymerase chain reaction
The invention provides a three-color fluorescent RT-PCR (Reverse Transcription-Polymerase Chain Reaction) combined detection method of enterovirus 71, Coxsackie virus A16 and other subtypes of enterovirus as well as a kit thereof. The method can rapidly and accurately detect the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of enterovirus in a sample. The method comprises the following steps of: (1) acquiring and conveying a sample of an infected patient or a suspected patient; (2) preprocessing the sample and extracting RNA; (3) detecting the sample by adopting a one-step PCR-three-color fluorescent probe in-vitro amplification method; and (4) analyzing the corresponding sample according to the fluorescence intensity of each amplification reaction after the amplification reaction is finished, thereby judging the existence of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus in the acquired sample and being capable of carrying out accurate quantitation (a figure 3) on the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus. The invention realizes the aim of carrying out rapid and accurate combined detection of the enterovirus 71, the Coxsackie virus A16 and the other subtypes of nucleic acids of the enterovirus.
Owner:BEIJING SUOAO BIOTECH
Blood transcriptional signature of mycobacterium tuberculosis infection
InactiveUS20110196614A1Microbiological testing/measurementDisease diagnosisInfected patientMycobacterium Infections
The present invention includes methods, systems and kits for distinguishing between active and latent mycobacterium tuberculosis infection in a patient suspected of being infected with mycobacterium tuberculosis, and distinguishing such patients from uninfected individuals, the method including the steps of obtaining a gene expression dataset from a whole blood obtained sample from the patient and determining the differential expression of one or more transcriptional gene expression modules that distinguish between infected and non-infected patients, wherein the dataset demonstrates an aggregate change in the levels of polynucleotides in the one or more transcriptional gene expression modules as compared to matched non-infected patients, thereby distinguishing between active and latent mycobacterium tuberculosis infection.
Owner:BAYLOR RES INST +2
Novel vaccine for preventing COVID-19 and preparation method thereof
ActiveCN111939250AHighly conservativeAntibody induction ability is weakSsRNA viruses positive-senseAntibody mimetics/scaffoldsNucleotideReceptor
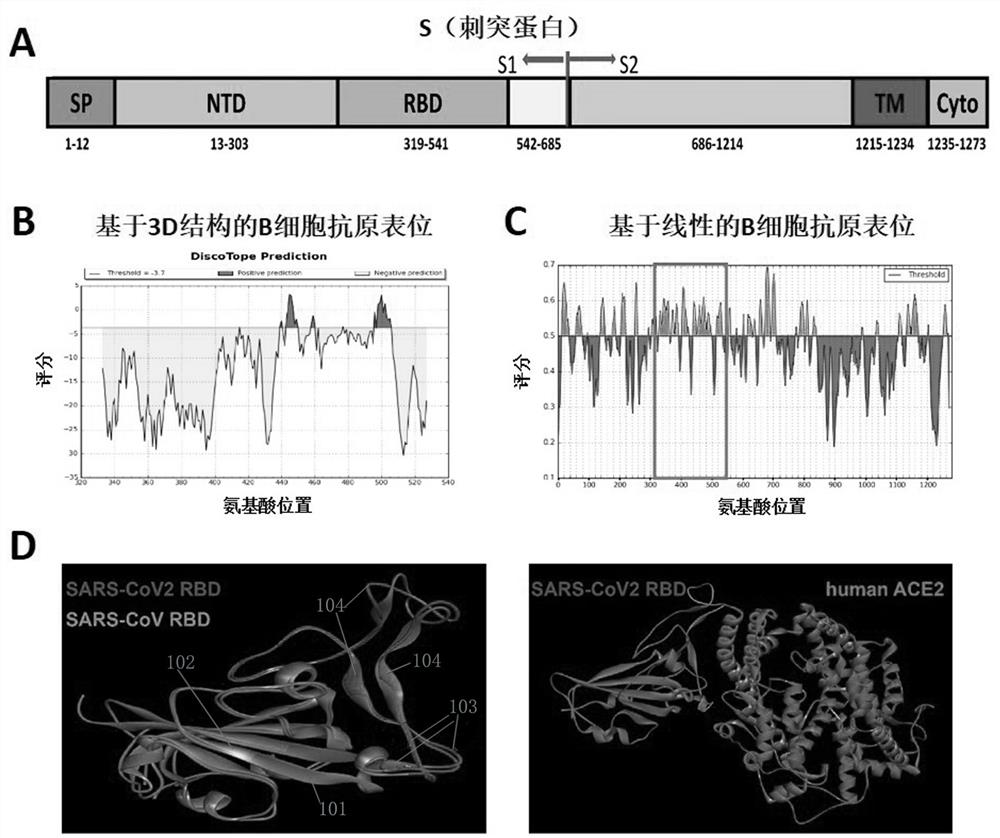
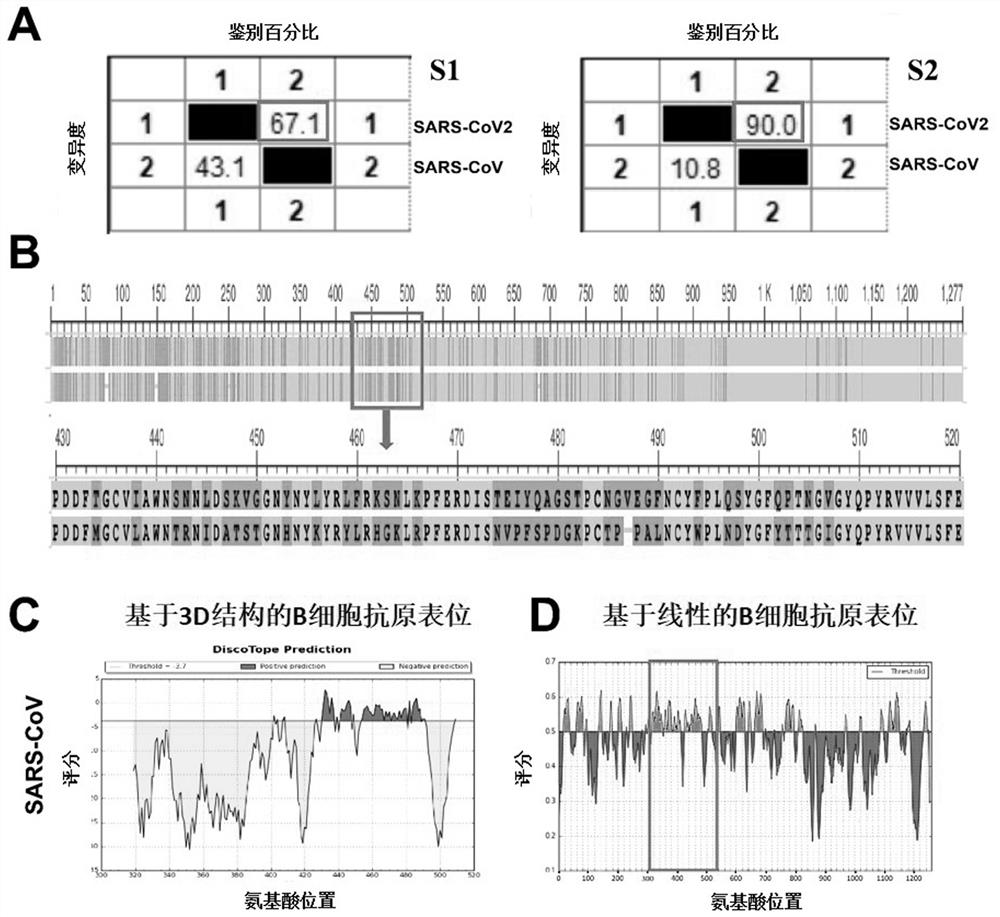
Provided is a novel vaccine for preventing COVID-19, the nucleotide sequence of an antigen of the novel vaccine is SEQ NO: 1, the amino acid sequence of the antigen of the novel vaccine is SEQ NO: 2,and the antigen of the vaccine comprises two functional parts: an S protein receptor binding structural domain capable of inducing a specific neutralizing antibody and a T cell related N protein truncated peptide fragment capable of inducing and activating effector T cells; The vaccine disclosed by the invention has the characteristics that the T cell related N protein truncated peptide fragment has weak capability of inducing the generation of the N protein antibody, so that a vaccine inoculator and a COVID-19 infected patient can be identified by using the N protein antibody, and the vaccineantigen does not induce the generation of the N protein antibody, so that lung injuries can be reduced, and the vaccine is safer. The cell vaccine disclosed by the invention is low in manufacturing cost, and can induce generation of virus-specific neutralizing antibodies and T cell immune response.
Owner:ZHENGZHOU UNIV
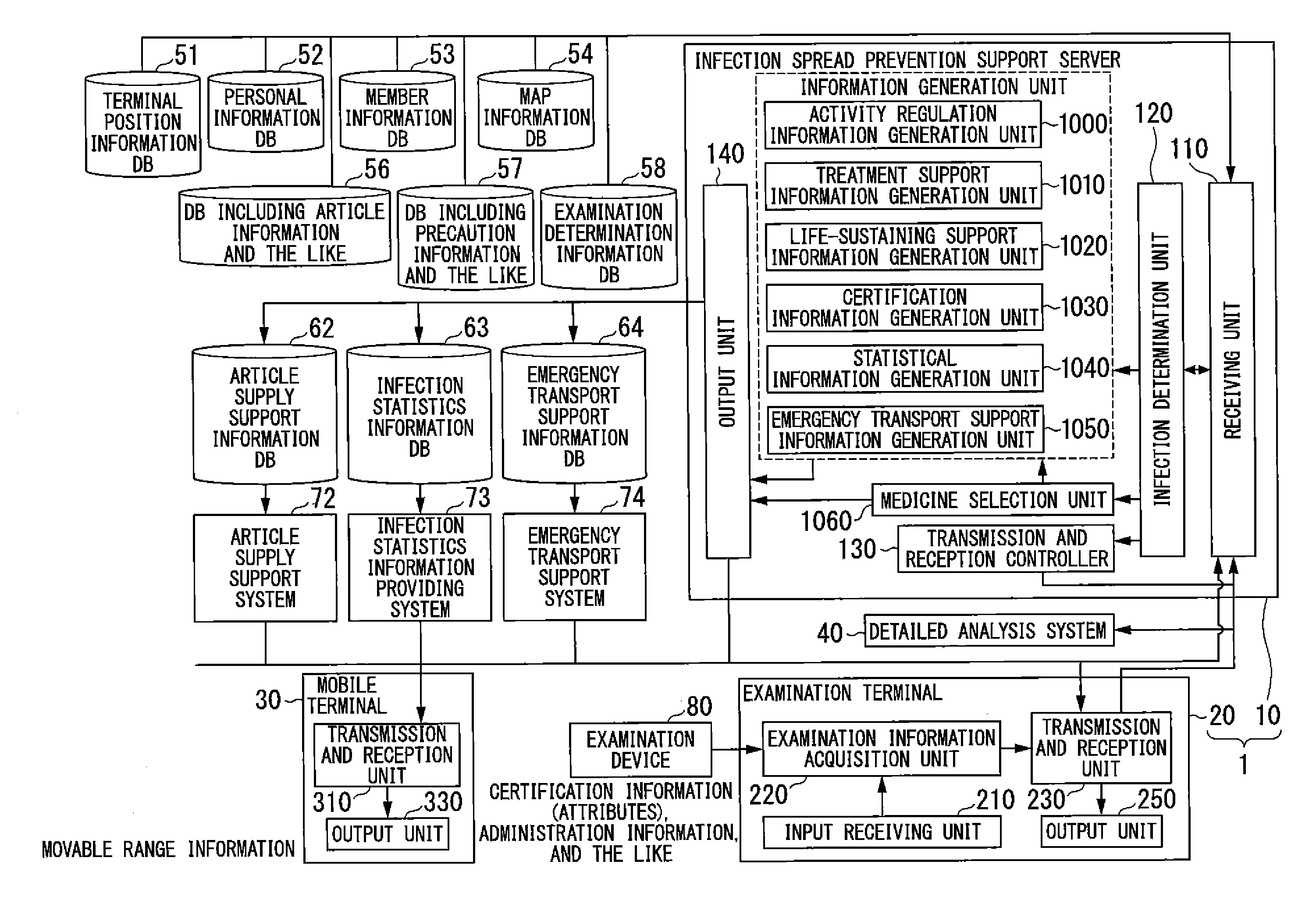
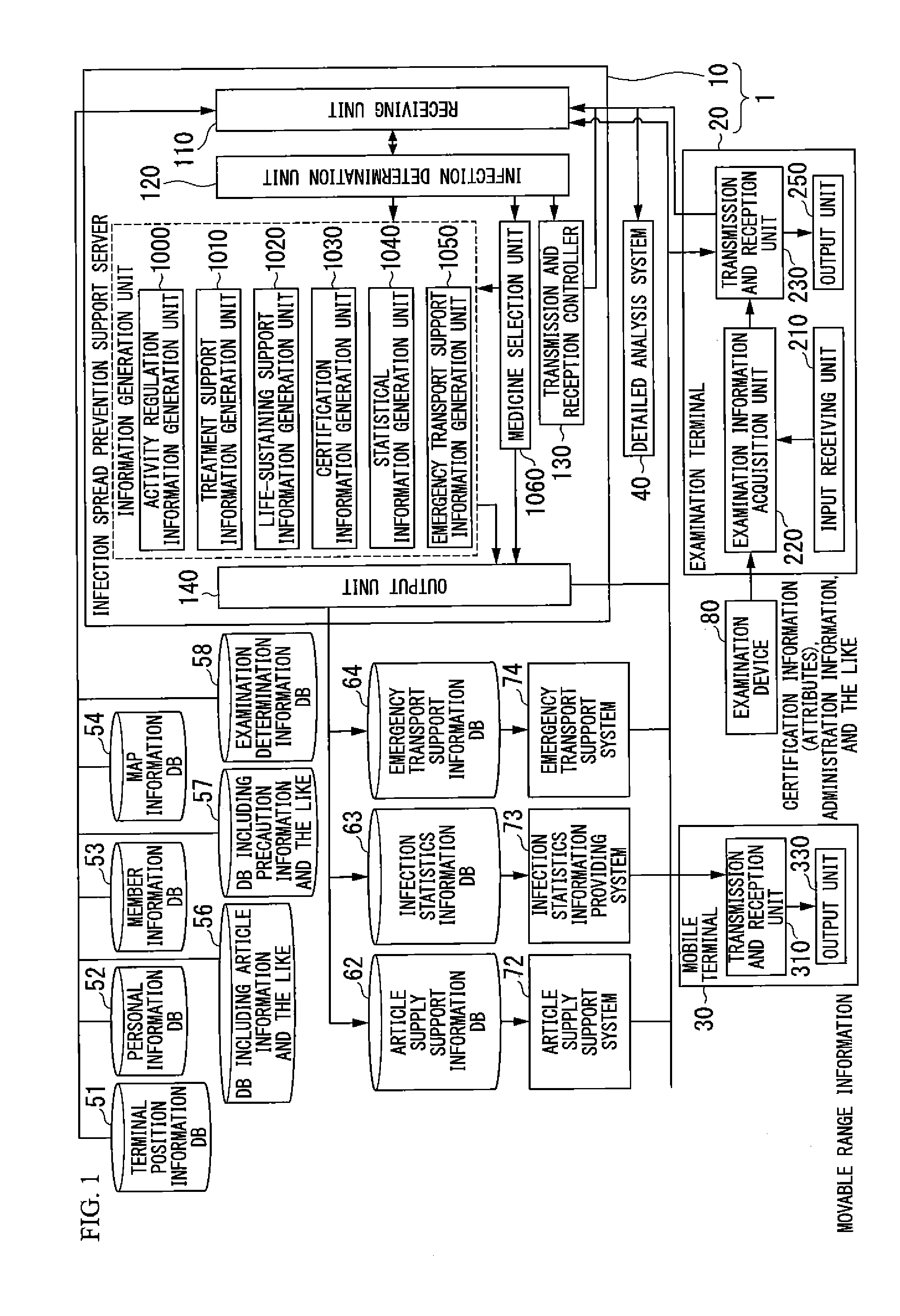
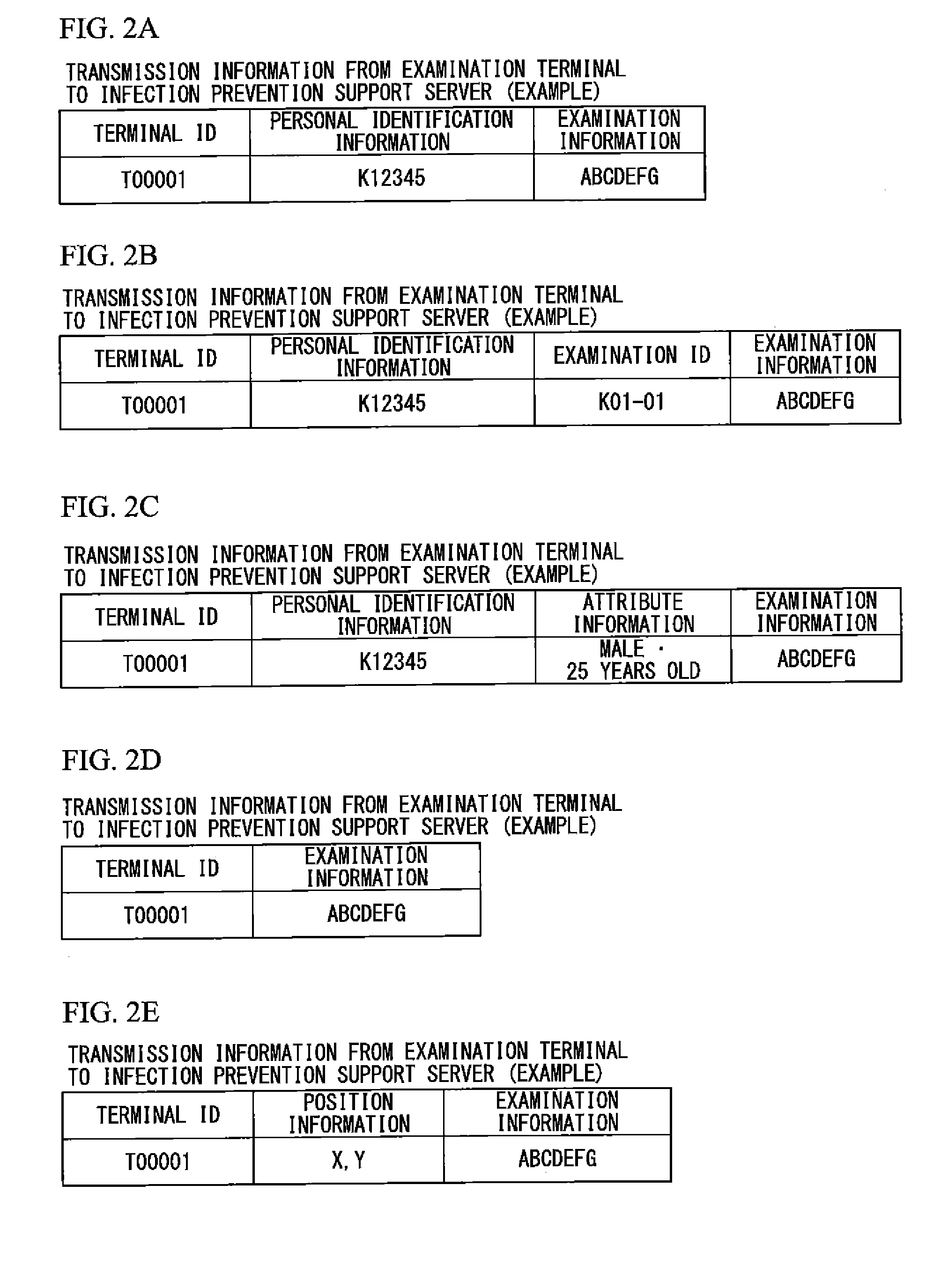
Infection spread prevention support system, infection spread prevention support server, examination terminal, mobile terminal and program
InactiveUS20130138451A1Avoid spreadingEpidemiological alert systemsOffice automationSupporting systemInfected patient
An infection spread prevention support system includes an examination terminal and an infection spread prevention support server. The examination terminal includes an examination information acquisition unit that acquires examination information to examine the infection of an infectious disease in subjects and a transmission and reception unit that transmits the examination information to the infection spread prevention support server. The infection spread prevention support server includes a receiving unit that receives examination information, an infection determination unit that determines the presence or degree of infection of the infectious disease using the examination information, an activity regulation information generation unit that generates activity regulation information to regulate the an activity of the infected patient based on infection determination information, which is a determination result of the infection determination unit, and information regarding the subjects, and an output unit that outputs the activity regulation information.
Owner:NIKON CORP
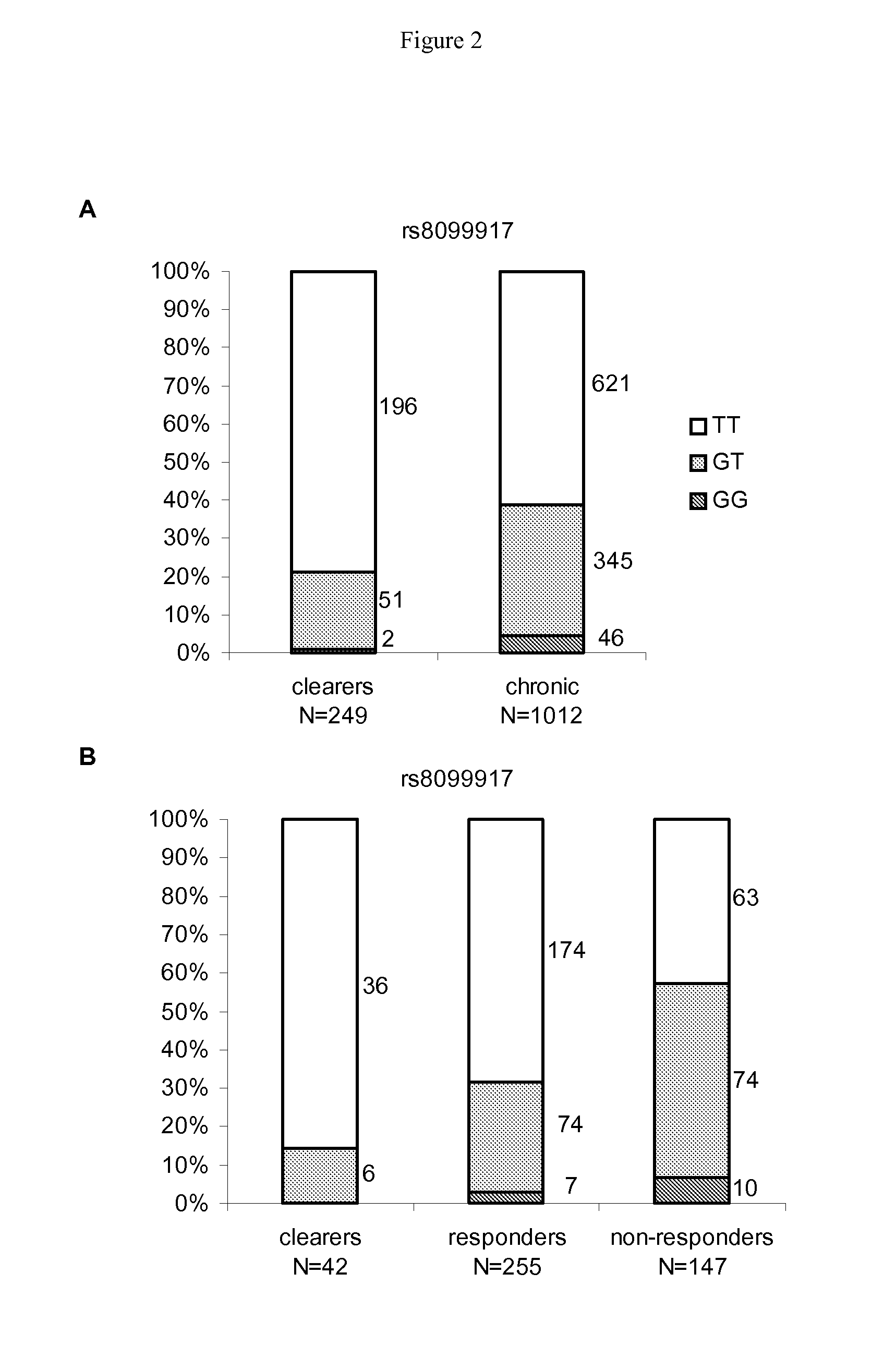
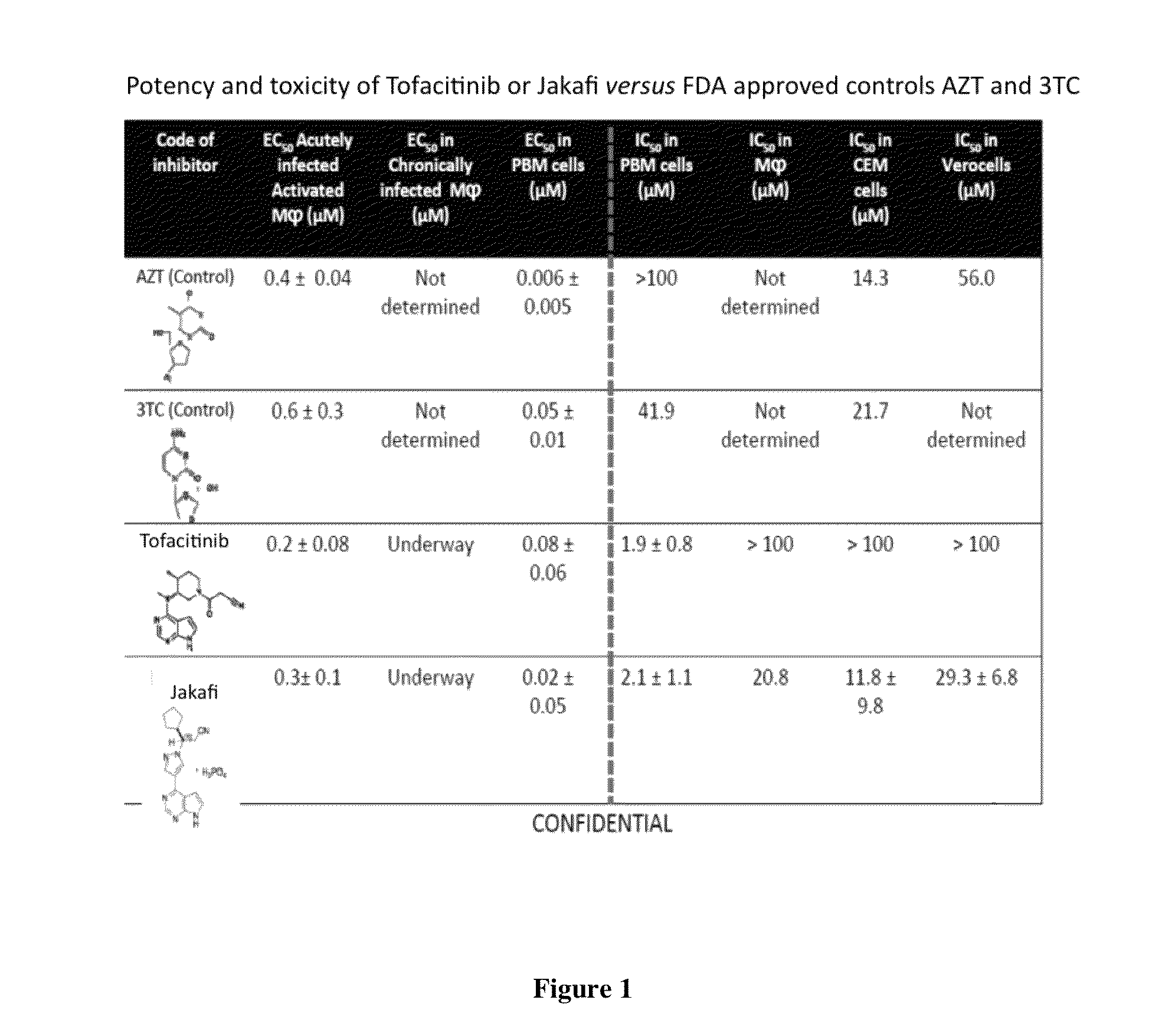
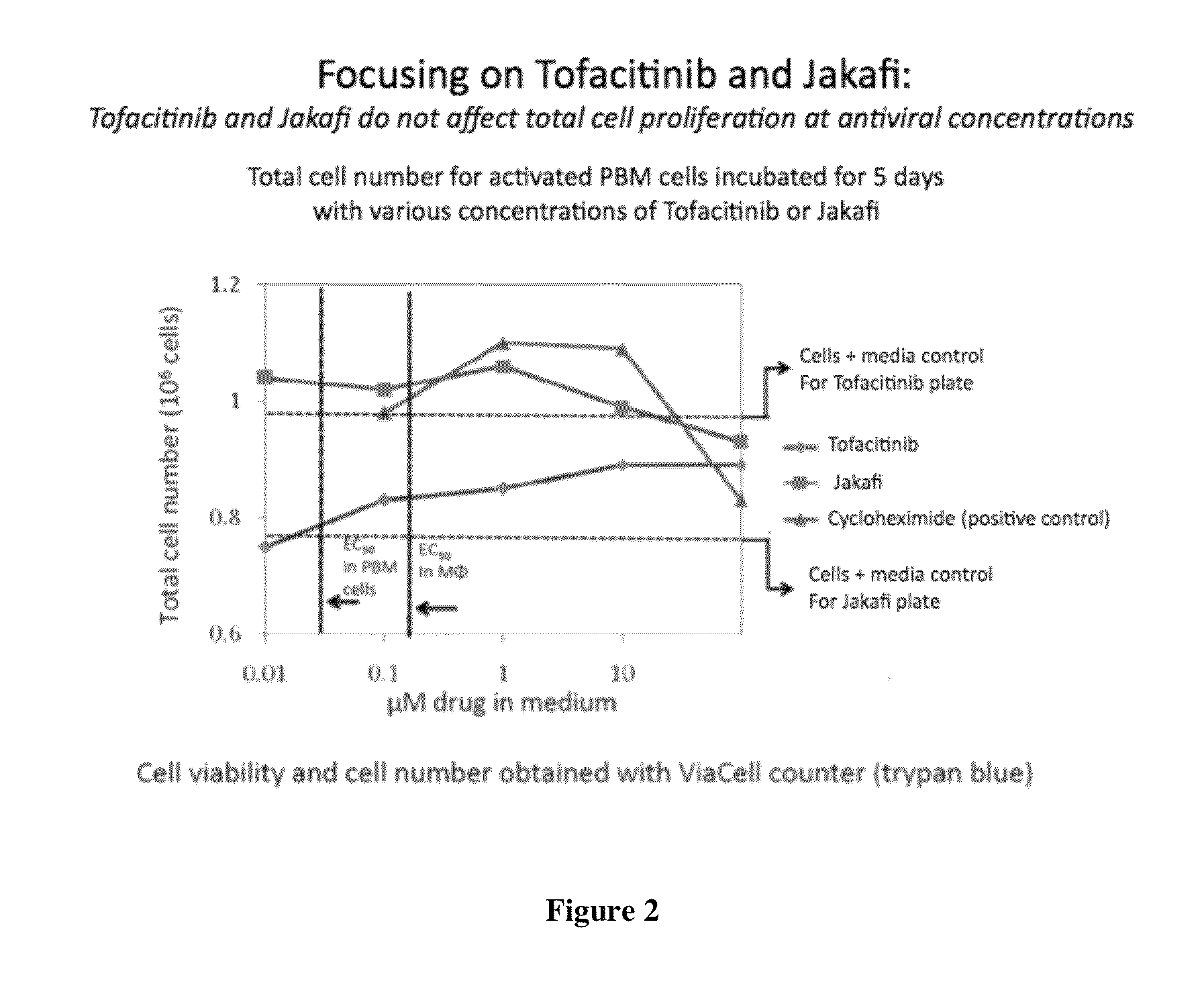
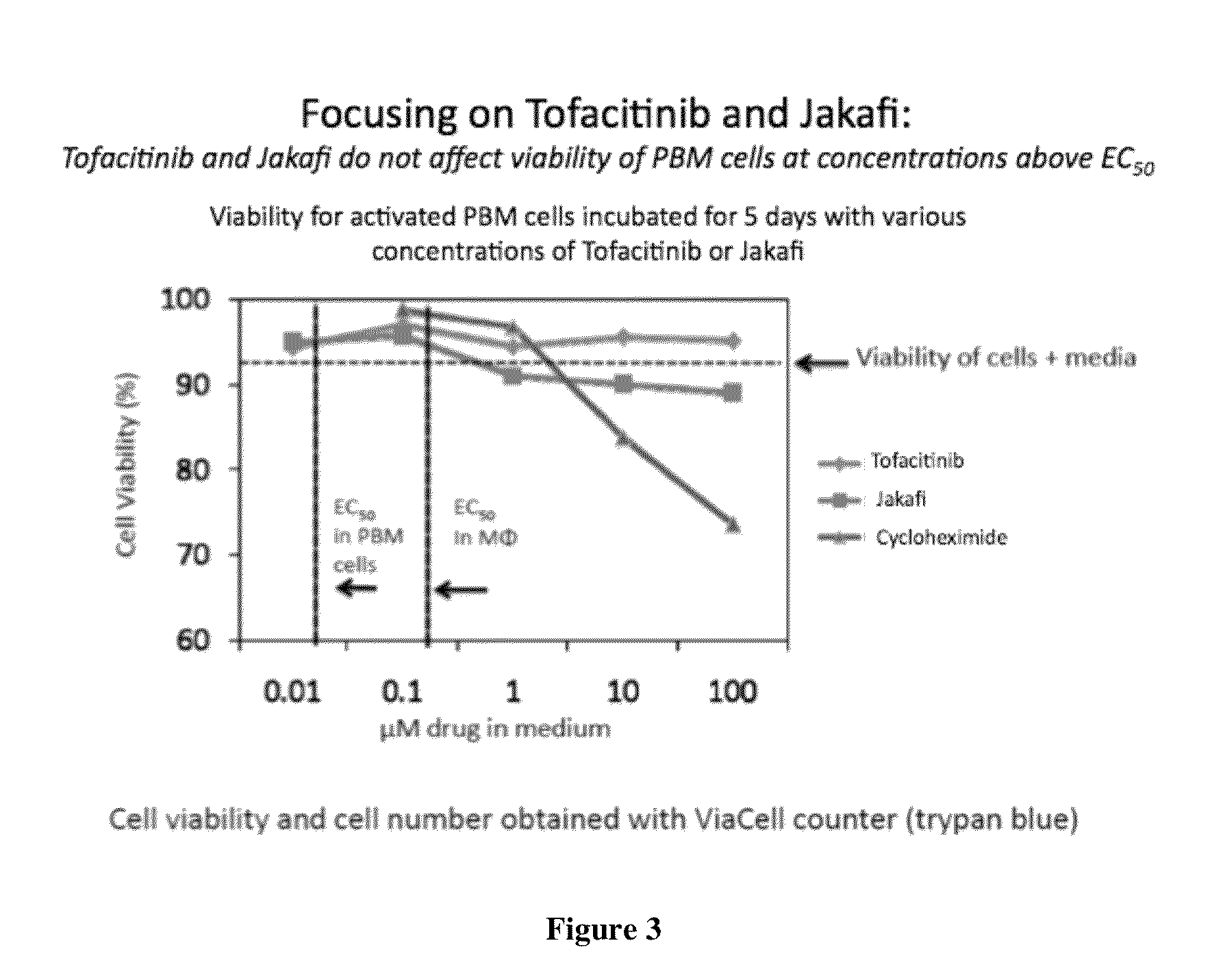
Antiviral jak inhibitors useful in treating or preventing retroviral and other viral infections
ActiveUS20140328793A1Improve their absolute antiviral effectLow toxicityBiocidePeptide/protein ingredientsProteinase inhibitorThymidine
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Infection Control Management and Workflow System
A system identifies multiple medical conditions, observations, and laboratory test results using active sensors and predetermined rules to identify infected patients. An infection control and workflow management system includes a repository of worker information identifying healthcare workers for performing infection control tasks as well as worker associated communication data for use in informing healthcare workers of infection control tasks to be performed. A detection processor automatically detects infection in patients from multiple different sources including from at least one of, (a) a medical record evaluated upon admission of a patient to a hospital and (b) a laboratory test result. A workflow processor uses the worker information for automatically communicating a message to inform a healthcare worker of a task to be performed to initiate infection control tasks using communication data in response to detection of an infected patient.
Owner:CERNER INNOVATION
Polypeptide chip and application thereof in virus detection
The invention provides a polypeptide chip, which comprises a substrate and n polypeptides distributed on the substrate in an array, each polypeptide sequence has 10-20 amino acids, a group consistingof sequences of first to nth polypeptides covers at least 95% of a viral protein sequence, the adjacent polypeptides have an overlap of 3-8 amino acids, and n is 810-1370. According to the method, proteomics and system biology strategies are adopted, all coded protein sequences of COVID-19 are extracted from NCBI data, an SARS-CoV-2 virus proteome polypeptide chip is designed and prepared, and panoramic scanning of all SARS-CoV-2 virus antibodies in blood of a novel pneumonia virus infected patient is achieved.
Owner:ACADEMY OF MILITARY MEDICAL SCI
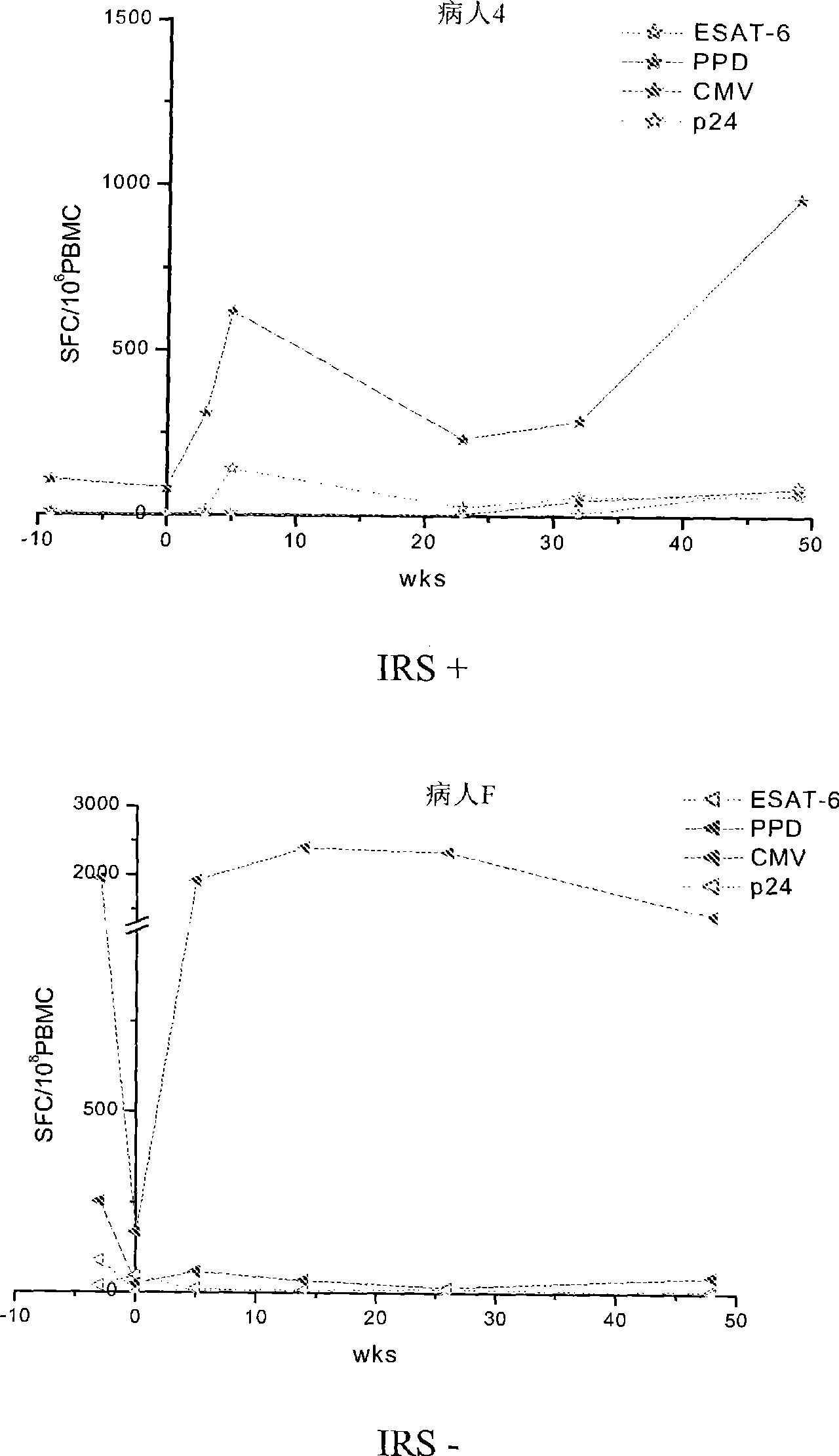
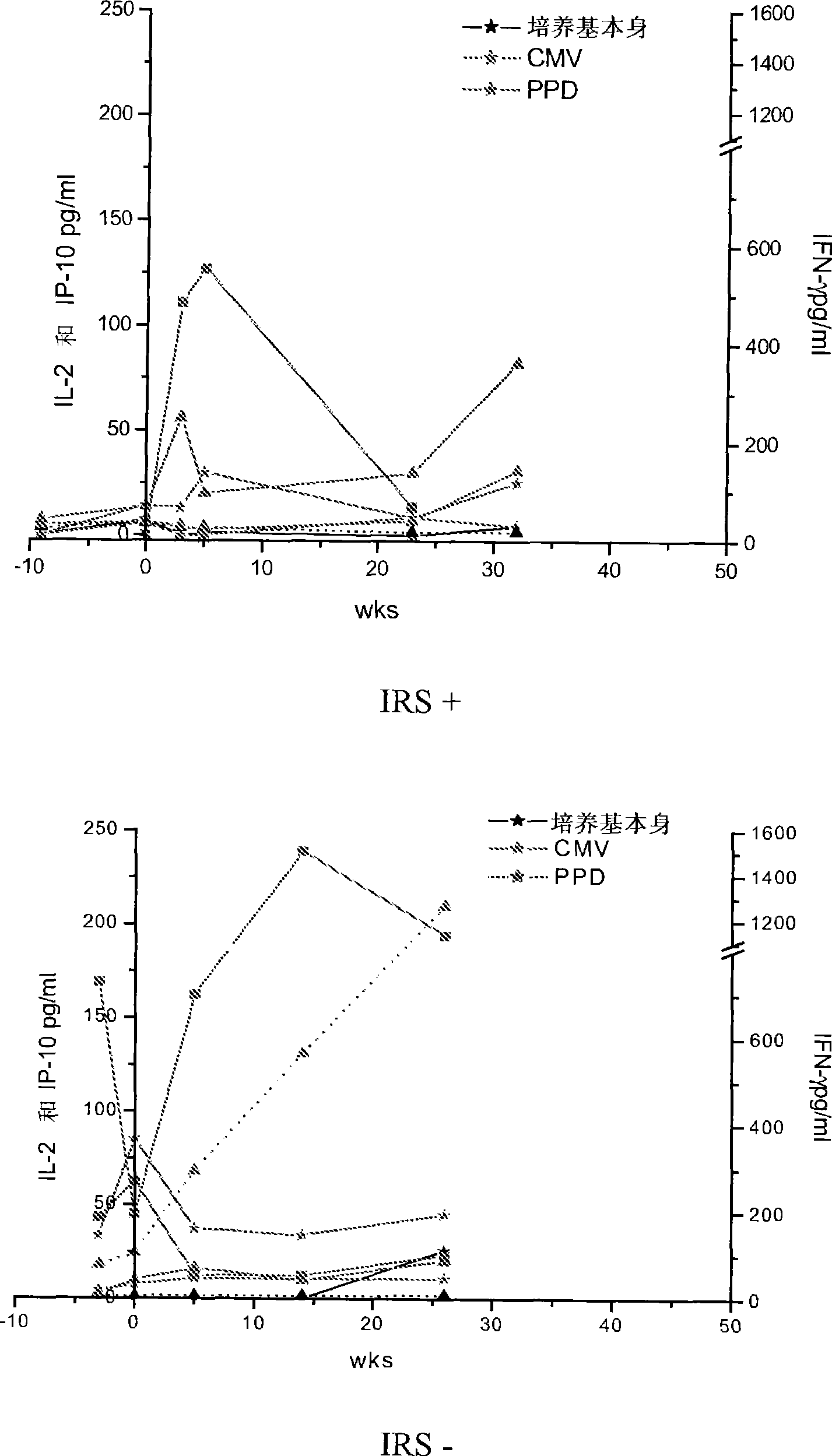
Method of diagnosis of tuberculosis related immune restoration syndrome (IRS)
The present invention relates to a method and kit of diagnosis of Immune Restoration Syndrome associated with tuberculosis (TB-IRS) in patients infected with tuberculosis (TB) as well as in HIV co-infected patients comprising detecting an acute increase in Th1 response following exposure to mycobacterial extract, referred as tuberculin or PPD (Purified Protein Derivative) as well as to the 16kDa protein, but not to ESAT-6 or CFP-10, two antigens from mycobacterium tuberculosis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
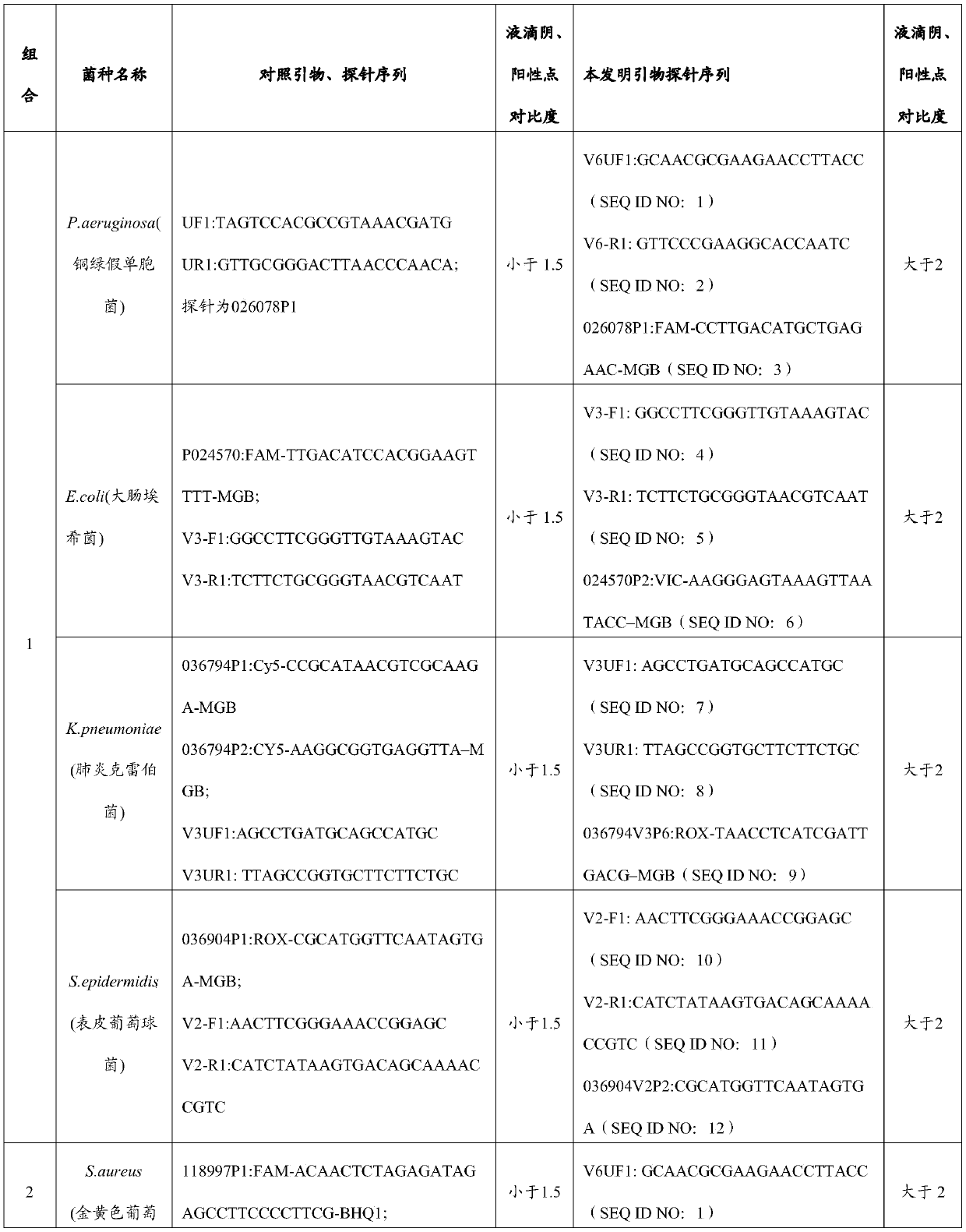
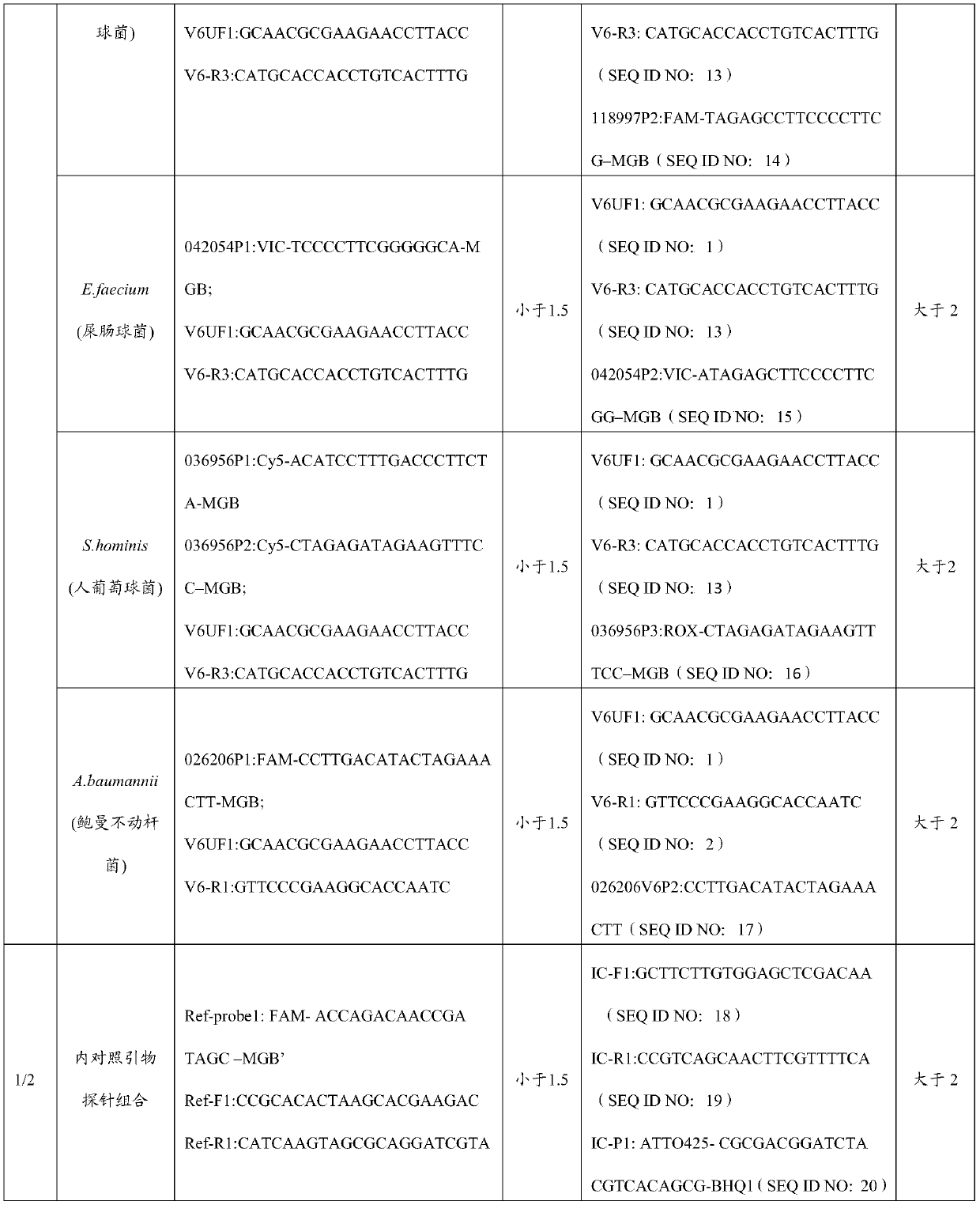
Primer and probe combination and kit for detecting human pathogenic bacteria
PendingCN110564824AShorten identification timeImprove positive detectionMicrobiological testing/measurementDNA/RNA fragmentationInfected patientTreatment effect
The invention relates to the technical field of medical detection, and in particular, relates to a primer and probe combination and a kit for detecting human pathogenic bacteria. The kit comprises theprimer and probe combination for detecting pseudomonas aeruginosa, escherichia coli, klebsiella pneumoniae, staphylococcus epidermidis, staphylococcus aureus, enterococcus faecium, human staphylococcus and acinetobacter baumannii. According to the kit, the primer and probe combination is adopted, multiple digital PCR is combined, the pathogenic bacteria types and the target nucleic acid copy number in the detection range can be rapidly and accurately identified, the detection method has high sensitivity, and positive detection of low-copy nucleic acid fragments of pathogenic bacteria is guaranteed; meanwhile, the variety of the pathogenic bacteria or the change of the copy number of the target nucleic acid fragment in the body of a pathogenic bacteria infected patient can be dynamically monitored, the treatment effect is assisted and evaluated in time, and reference is provided for optimization of a doctor clinical scheme.
Owner:PILOT GENE TECH HANGZHOU CO LTD
Potent combinations of zidovudine and drugs that select for the K65R mutation in the HIV polymerase
InactiveCN101878032ALow toxicityImprove absolute antiviral effectAntiviralsCarbohydrate active ingredientsNucleoside Reverse Transcriptase InhibitorRetroviral infection
Owner:EMORY UNIVERSITY
Methods for diagnosing or predicting hepatitis c outcome in hcv infected patients
InactiveUS20110165124A1Increased susceptibilityPeptide/protein ingredientsMicrobiological testing/measurementInfected patientHepatitis X
Owner:CENT HOSPITALIER UNIV VAUDOIS C H U V +1
Antiviral JAK inhibitors useful in treating or preventing retroviral and other viral infections
Compounds, compositions, and methods of treatment and prevention of HIV infection are disclosed. The compounds are pyrrolo[2,3-b]pyridines and pyrrolo[2,3-b]pyrimidine JAK inhibitors. Combinations of these JAK inhibitors and additional antiretroviral compounds, such as NRTI, NNRTI, integrase inhibitors, entry inhibitors, protease inhibitors, and the like, are also disclosed. In one embodiment, the combinations include a combination of adenine, cytosine, thymidine, and guanine nucleoside antiviral agents, optionally in further combination with at least one additional antiviral agent that works via a different mechanism than a nucleoside analog. This combination has the potential to eliminate the presence of HIV in an infected patient.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Method and system for detecting drug resistance of beta-lactam antibiotics and application of beta-lactam antibiotics
ActiveCN103645273AHigh sensitivityImprove accuracyComponent separationMicroorganismBeta lactam antibiotic
The invention relates to the filed of drug detection, and particularly relates to a method and system for detecting drug resistance of beta-lactam antibiotics and application of beta-lactam antibiotics. The method comprises steps of mixing turbid liquid of microorganism to be tested with standard liquid of beta-lactam antibiotics, then removing the microorganism to be tested so as to obtain the liquid to be tested; obtaining liquid chromatogram of the liquid to be tested; judging the tolerance of the microorganism to be tested to the beta-lactam antibiotic according to the liquid chromatogram. The detection is not affected by concentration and purity of the microorganism and operation technique of an operator, so that the method has the advantages of high sensitivity, high accuracy, high detection speed and short detection time, and can be implemented within two hours. The detection method uses fewer microorganisms, does not need subculture for many times, greatly shortens the time of the whole experiment, provides prompt basis for rapid and early clinical drug use, especially for seriously infected patients; and since the detection time is greatly shortened, the consumable cost is low.
Owner:宁波诺威科技有限公司
Plasmodium vivax PvMSP1 recombinant antigenic protein as well as preparation method and application thereof
ActiveCN103570817AIncreased sensitivityImprove featuresBiological material analysisMicroorganism based processesEpidemiologic surveyGlycosylphosphatidylinositol
The invention discloses a plasmodium vivax PvMSP1 recombinant antigenic protein, which is protein of which the amino acid sequence is shown in SEQ ID NO:1 which has glycophosphatidylinositol (GPI) anchor and epidermal growth factor-like (EGF-like) structure domain. Furthermore, the invention also discloses a preparation method of the recombinant antigenic protein, which comprises the steps of amplifying a plasmodium vivax PvMSP1 gene sequence, constructing and identifying recombinant plasmid, inducibly expressing and purifying recombinant protein and the like. Experiments prove that the PvMSP1 recombinant antigenic protein has the advantages of high sensitivity, strong specificity and the like to assay of the serum antibody of a plasmodium vivax infected patient, and has a wide application prospect in the aspect of plasmodium vivax epidemiological investigation.
Owner:中国疾病预防控制中心寄生虫病预防控制所国家热带病研究中心
Serratia marcescens
InactiveCN102181382ARaw materials are simpleRaw materials are easy to getBacteriaMicroorganism based processesSide effectInstability
The invention discloses serratia marcescens ZJH20098. The officinal bacterium prodigiosum lipopolysaccharide produced at present has serious side effects, such as pyrogen and allergic phenomena. Besides the unspecified process, the main reasons are as follows: the strain is not explicitly stipulated, the mutation is generated by instability, and the functional component change is caused by extraction. The strain provided by the invention is preserved in the China General Microbiological Culture Collection Center (CGMCC) with the preservation number of CGMCC No.4010; and as the strain is collected from the bodies of clinical infected patients, and is a high-activity strain obtained through separation, purification, physical mutagism and optimization, the serratia marcescens ZJH20098 provided by the invention is used as a raw material for producing 'bacterium prodigiosum lipopolysaccharide' for human or livestock use.
Owner:HEILONGJIANG UNIV
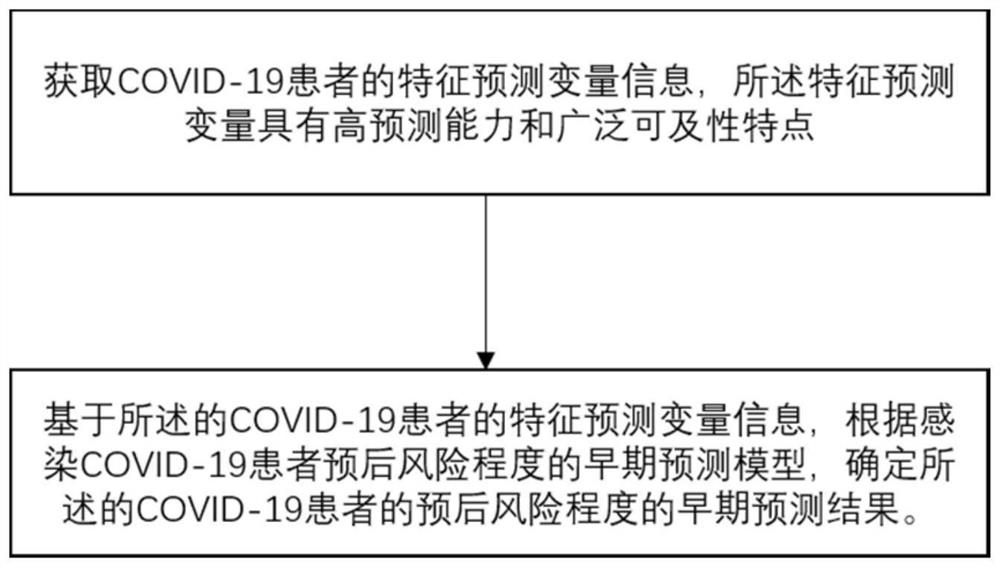
Early prediction method and system for prognosis risk degree of patient infected with COVID-19
PendingCN112185560AAccurate identificationQuick identificationHealth-index calculationEpidemiological alert systemsInfected patientEarly prediction
The invention discloses an early prediction method and system for prognosis risk degree of a patient infected with COVID-19. According to the method and the system, an early prediction model of the prognosis risk degree of the patient infected with COVID-19 is established based on the admission information and the dynamic change of the COVID-19 infected patient, and the prediction model can provide important risk indexes for early identification of important critical patients, so that in the prevalence period of report case increase and medical resource shortage, severe COVID-19 cases can be recognized more accurately and quickly, and the treatment effect of the patient is improved.
Owner:JIANGSU PROVINCE HOSPITAL THE FIRST AFFILIATED HOSPITAL WITH NANJING MEDICAL UNIV
Cefoperazone sodium and sulbactam sodium composition
InactiveCN101143146AReduce contentReduce manufacturing costAntibacterial agentsPharmaceutical delivery mechanismInfected patientCurative effect
The invention provides a combination of cefoperazone sodium and sulbactam sodium. The combination consists of the cefoperazone sodium and the sulbactam sodium, and the weight ratio of which is 3 to 1. The combination of cefoperazone sodium and sulbactam sodium of the invention has the comparative curative effect at the aspect of antibacterial effect with a compound preparation of 1 to 1 or 2 to 1 of the cefoperazone sodium / the sulbactam sodium in markets. The invention reduces the corresponding content of the sulbactam sodium in the combination, so the invention has wider clinical application range and can reduce the production cost of the drug. Compared with the prior compound preparation of the cefoperazone sodium / the sulbactam sodium, the invention is characterized by being fit for the anti-infection remedy of the patient with the renal dysfunction and the remedy of the seriously infected patient.
Owner:CHENGDU BOAOTONG TECH
Pharmaceutical compositions of anti-viral compounds and process for preparation thereof
InactiveUS20150141376A1Improve efficacyImprove securityBiocideOrganic active ingredientsInfected patientEmtricitabine
Pharmaceutical compositions of anti-viral compounds, process for preparation and method of using the same are provided. Particularly, the present invention relates to chemically stable pharmaceutical compositions of efavirenz, emtricitabine and tenofovir disoproxil fumarate with optionally one or more pharmaceutically acceptable excipients, process for preparation and method for the treatment or prevention of the symptoms or effects of an HIV infection in an infected patient.
Owner:AUROBINDO PHARMA LTD
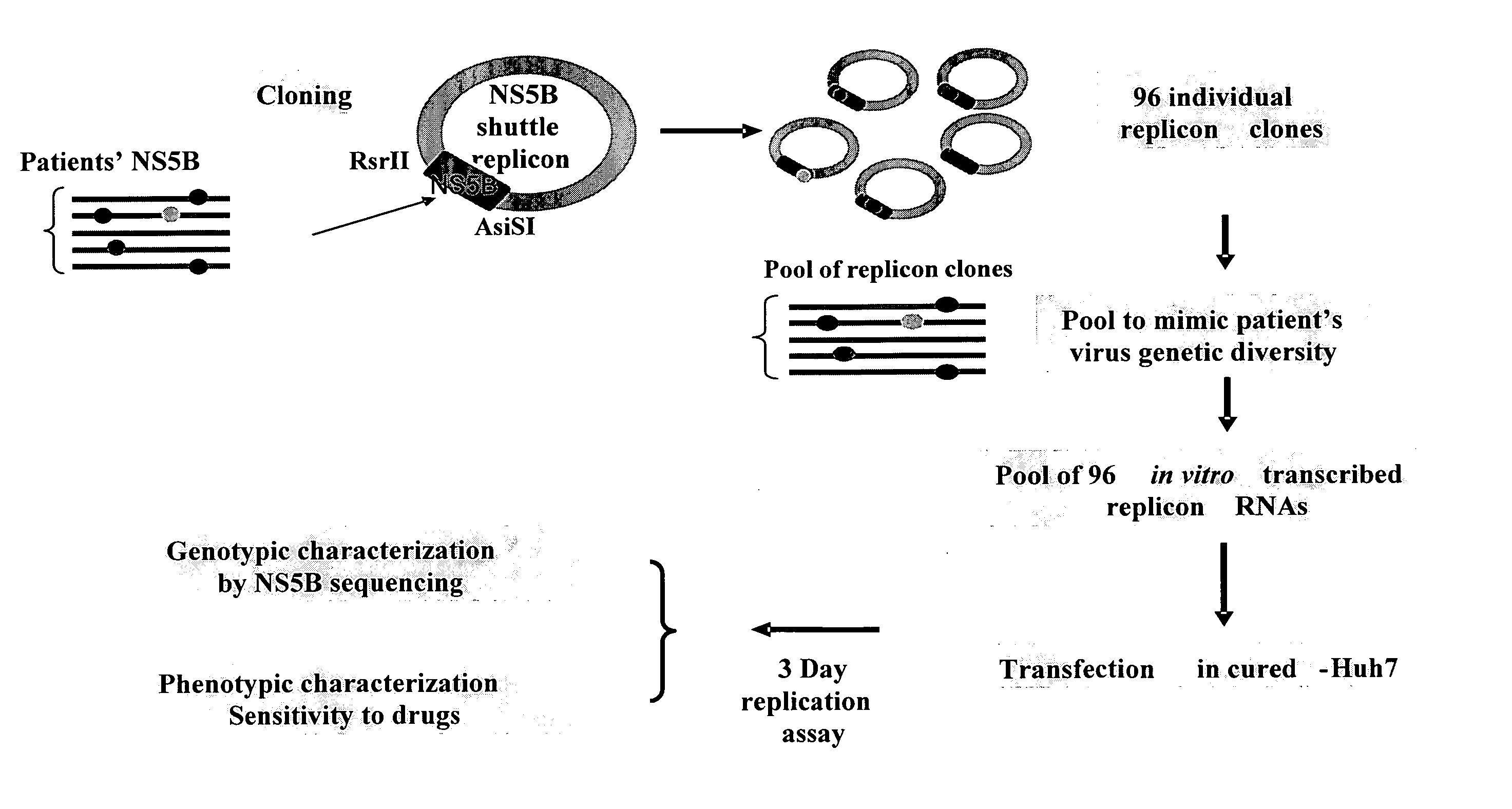
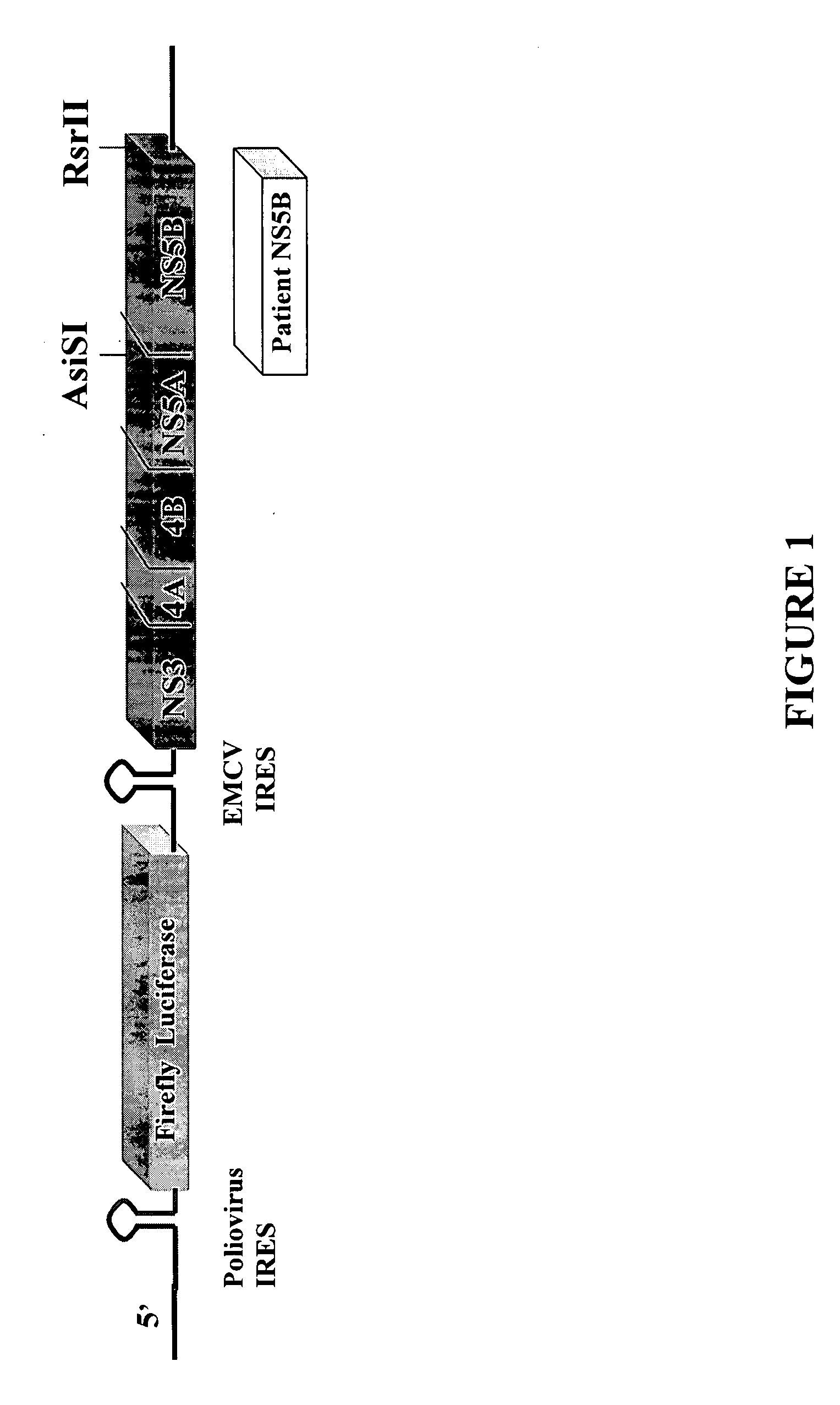
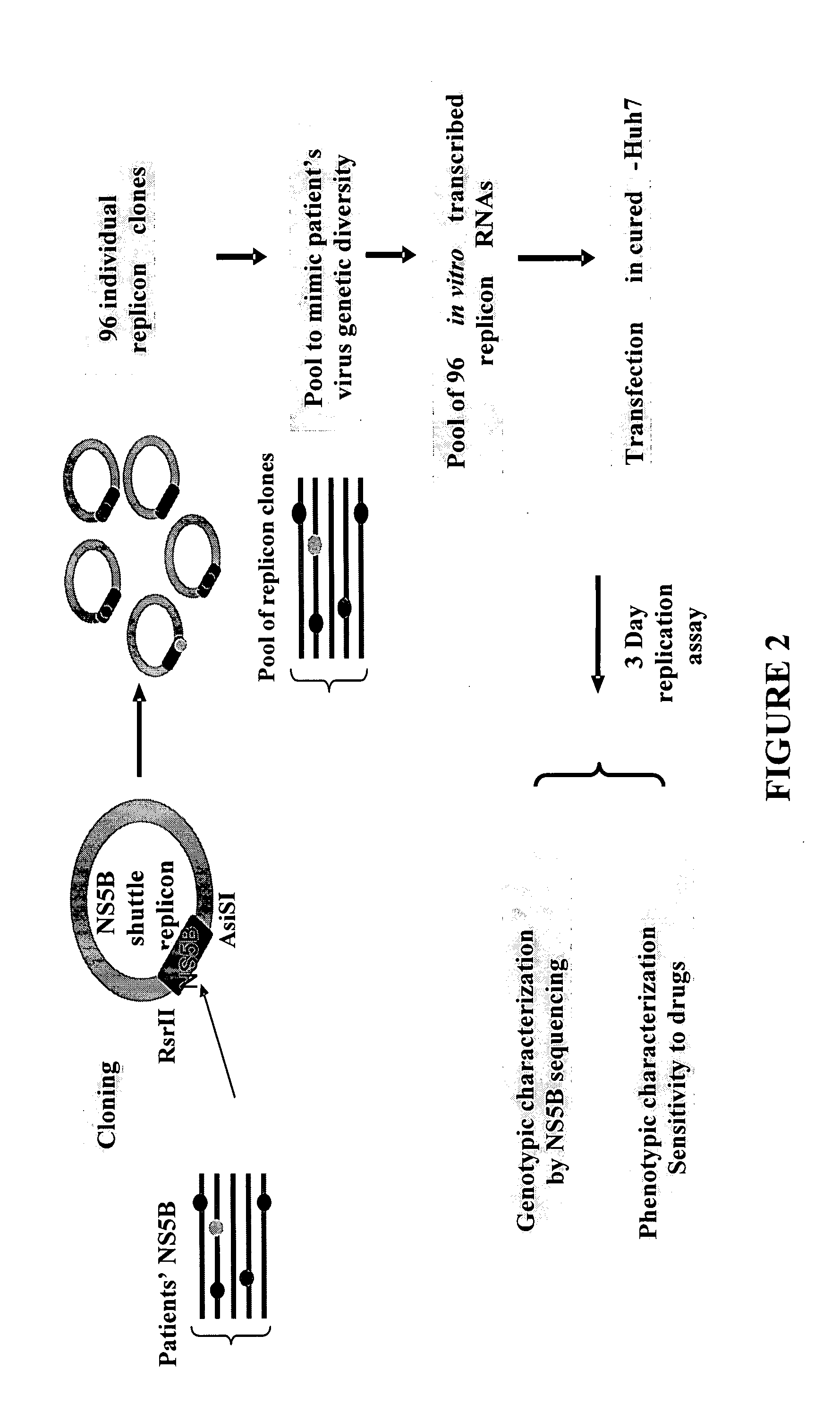
HCV replicon shuttle vectors
InactiveUS20080026952A1High error rateSensitivity and resistanceSsRNA viruses positive-senseVirus peptidesInfected patientShuttle vector
The present invention provides for novel HCV replicon shuttle vectors useful for cloning in HCV polynucleotide sequences from samples of HCV-infected patients and testing the resulting replicons for drug susceptibility.
Owner:ROCHE PALO ALTO LLC
II-type parainfluenza virus (PIV) fluorescence quantitative polymerase chain reaction (PCR) kit and detection method thereof
InactiveCN102061340AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceRNA extractionFluorescence
The invention discloses a II-type parainfluenza virus (PIV) detection kit and application thereof. The kit comprises ribonucleic acid (RNA) extract, reverse transcriptase, an RNA enzyme inhibitor, a standard positive template, Taq deoxyribonucleic acid (DNA) polymerase, fluorescence quantitative polymerase chain reaction (PCR) reaction liquid and a standard negative quality control product. A detection method of the kit comprises the following steps of: extracting pathogen RNA of a sample to be tested; performing fluorescence quantitativeRT-PCR on the pathogen RNA, a standard positive quality control product, an inner control template and the negative quality control product; and calculating the starting concentration of the II-type PIV of the sample to be tested according to software of a fluorescence quantitative PCR instrument. The kit is high in detection speed, convenient and safe to operate, time-saving and efficient, and can effectively detect virus RNA of II-type PIV infected patient so as to realize early diagnosis and effectively prevent II-type PIV infection.
Owner:SHANGHAI XINGYAO MED TECH DEV CO LTD +1
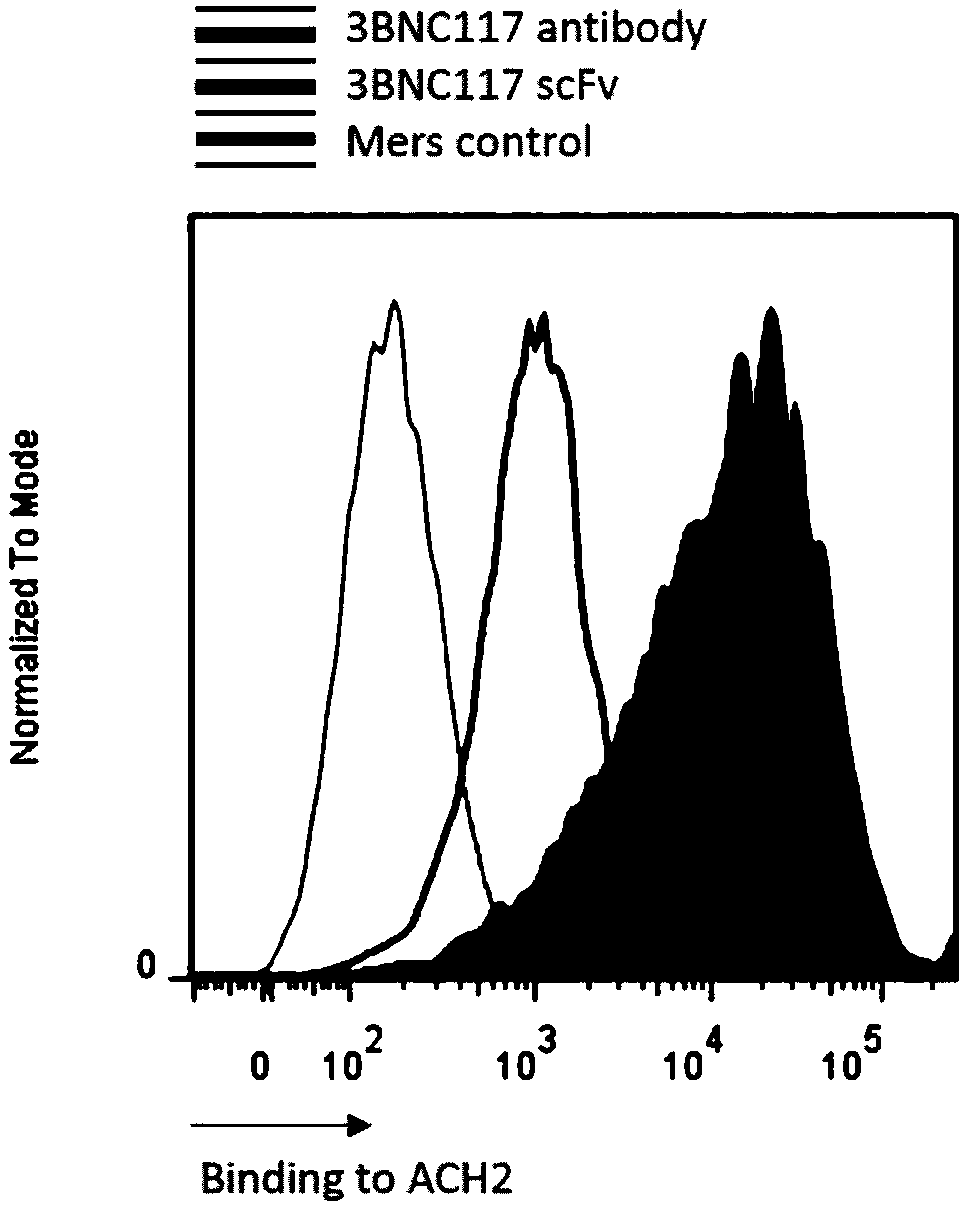
Use of T cell comprising chimeric antigen receptor (CAR) modification for preparing cell drug
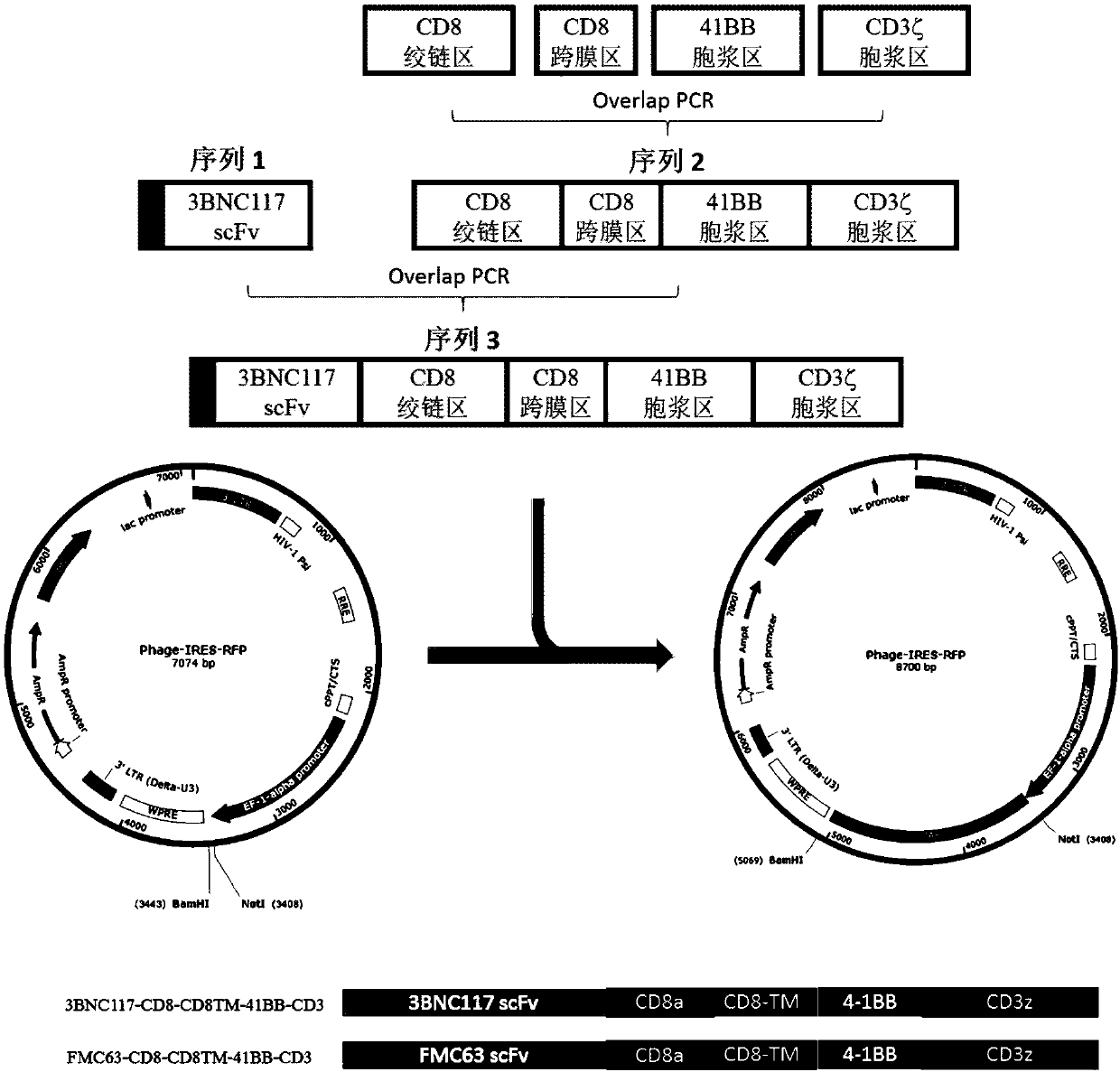
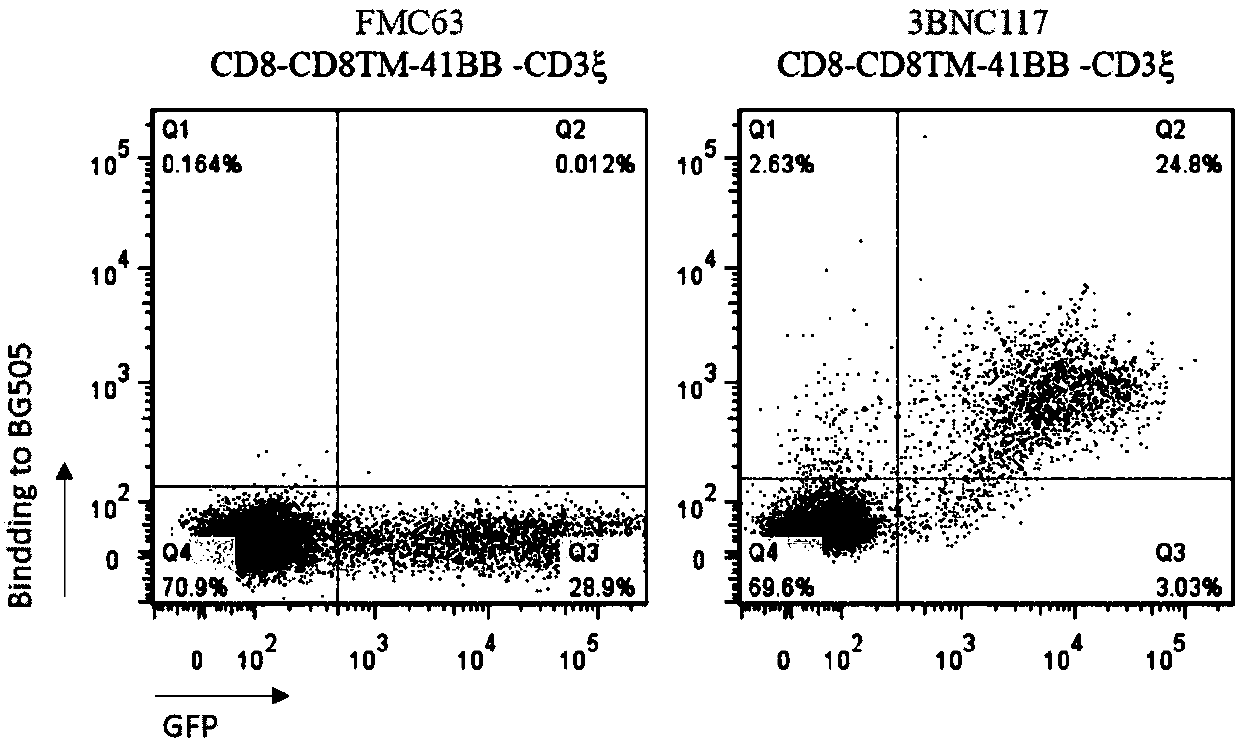
The invention discloses a T cell comprising a chimeric antigen receptor (CAR) modification, a preparation or a medicine prepared from the T cells, the use of the T cells for preparing a cell drug, andthe preparation or the drug for treating and removing a cell storage bank of human immunodeficiency virus (HIV) in a HIV infected patient. The invention further discloses a cell immunotherapy using gp120 antibody CART, and specifically using 3BNC117 scFv-CART, which achieves MHC independent recognition and killing specific for HIV latent cells, so that the goal of eradicating AIDS is achieved.
Owner:TSINGHUA UNIV +1
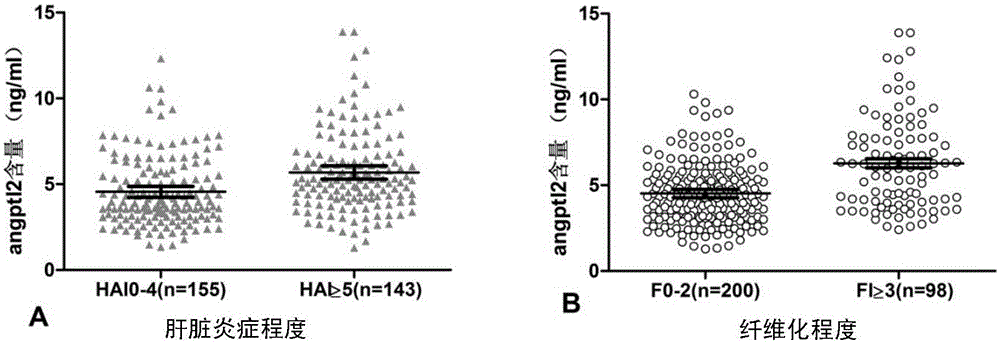
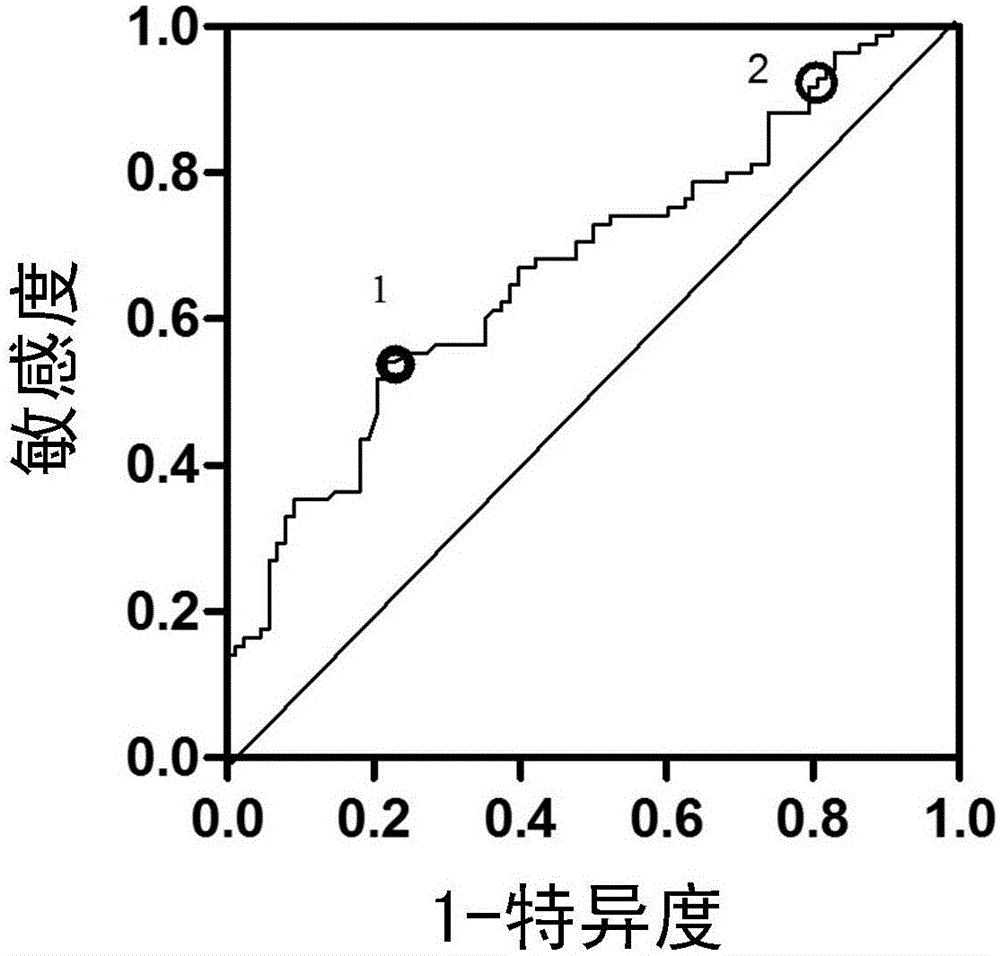
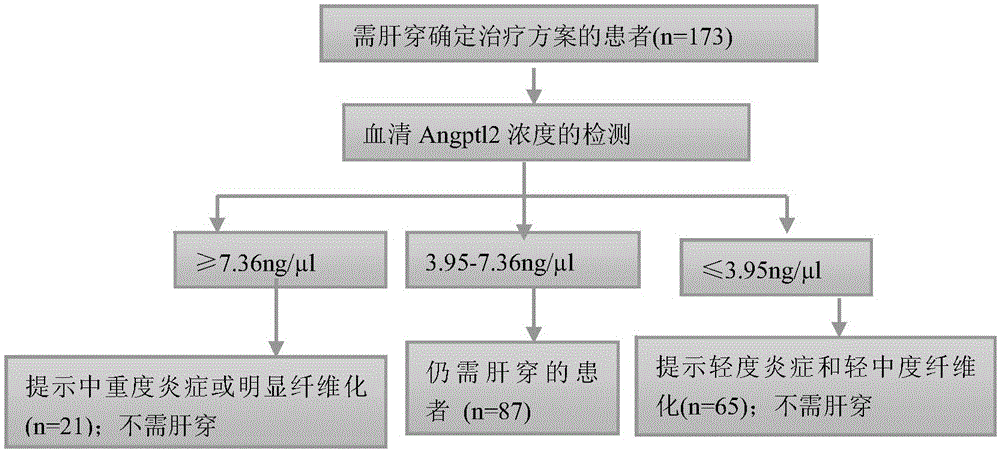
Application of substance for detecting content of serum angiopoetin-like protein 2 in preparation of product for detecting hepatitis and fibrosis degree
The invention discloses an application of a substance for detecting the content of a serum angiopoetin-like protein 2 (angptl2) in preparation of a product for detecting hepatitis and fibrosis degree. Experiments find out that the content of the angptl2 in serum has correlation with the hepatitis degree and fibrosis degree of a chronic hepatitis B virus infected patient, the content of the angptl2 can be used as a noninvasive single indicator for judging the hepatitis degree and fibrosis degree, the substance for detecting the content of the serum angptl2 or a substance for detecting other common clinical indicators of hepatopath can be combined together to prepare the product for detecting the human hepatitis degree and / or fibrosis degree, a product for diagnosing or auxiliarily diagnosing whether patients with hepatopathy need liver biopsy detection or a product for screening or auxiliarily screening the patients with hepatopathy who need or do not need liver biopsy.
Owner:PEKING UNIV FIRST HOSPITAL
Method for detection of infection with human cytomegalovirus
InactiveUS20130109010A1High detection sensitivitySurely detectedPeptide/protein ingredientsMicrobiological testing/measurementEscherichia coliAntigen
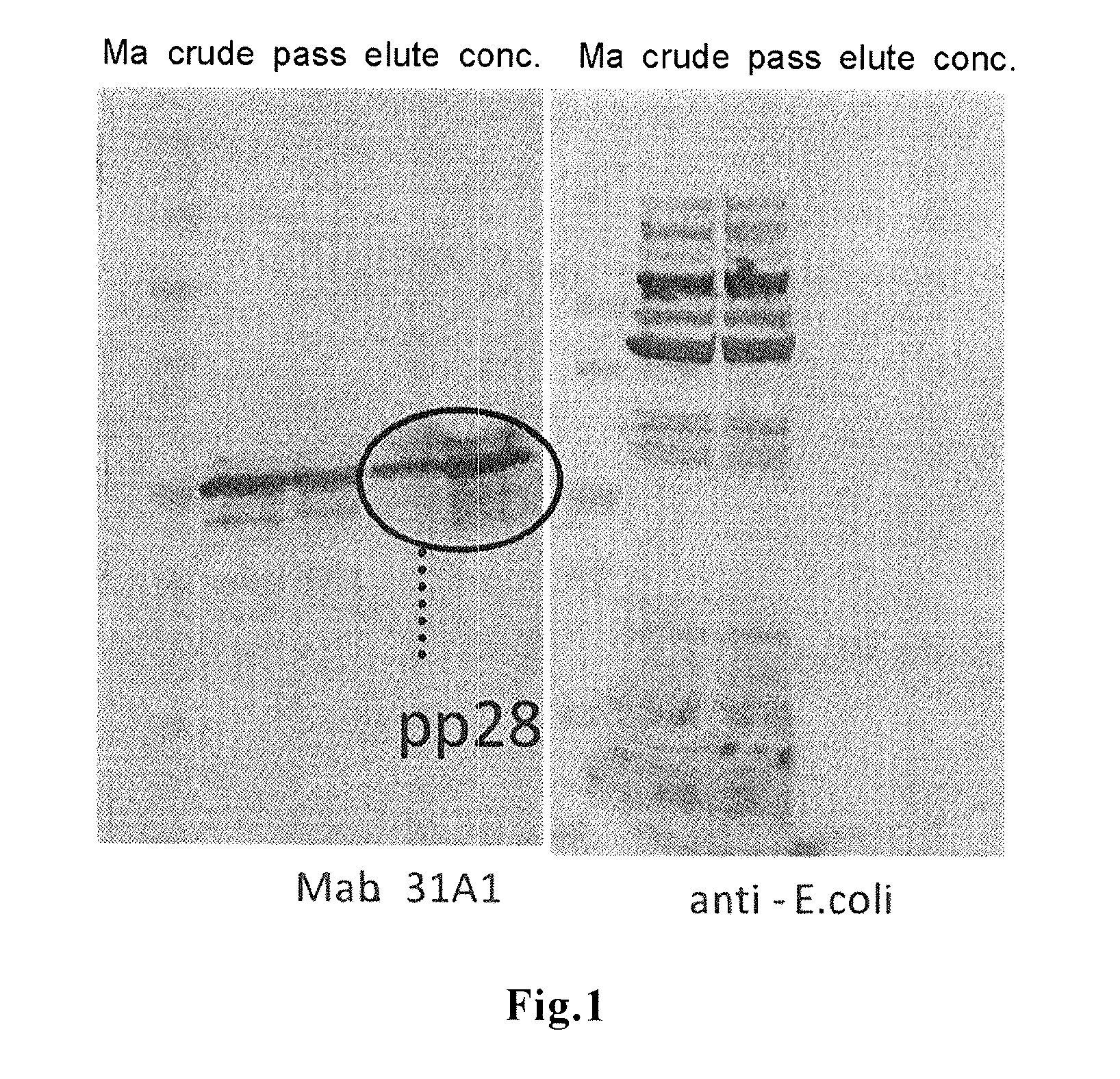
A novel means by which HCMV infection can be detected with a high sensitivity and whose practicality is high is disclosed. The present inventors synthesized as many as 15 kinds of HCMV proteins in their full length forms, and intensively studied their reactivities with sera from HCMV-infected patients to find that all the infected patients can be detected without fail when using the pp28 full length protein as an antigen. The pp28 full length protein can be synthesized and purified as a recombinant protein in a large scale by using Escherichia coli, and can be commercially used as an antigen for HCMV tests.
Owner:FUJIREBIO CO LTD
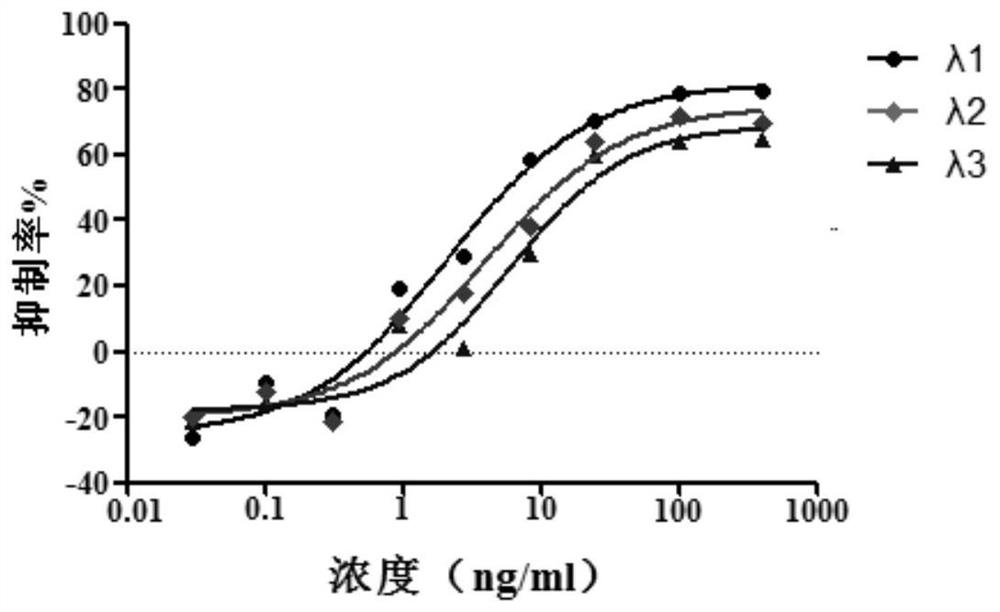
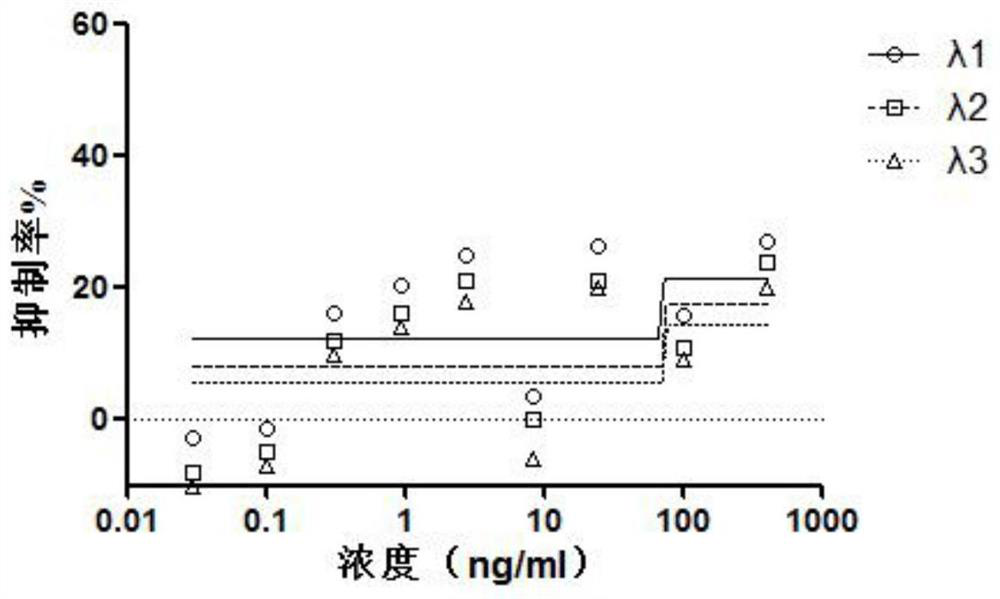
Application of interferon lambda in treatment of novel coronavirus (2019-nCoV) infection
The invention relates to application of IFN-lambda in preparation of a drug for treating a patient infected with a novel coronavirus. The level of the novel coronavirus in the infected patient can be effectively reduced, and the IFN-lambda shows more excellent performance in the aspects of controlling cytokine storm caused in the treatment process and relieving respiratory distress syndrome caused by the cytokine storm.
Owner:HANGZHOU SCIWIND BIOSCI CO LTD
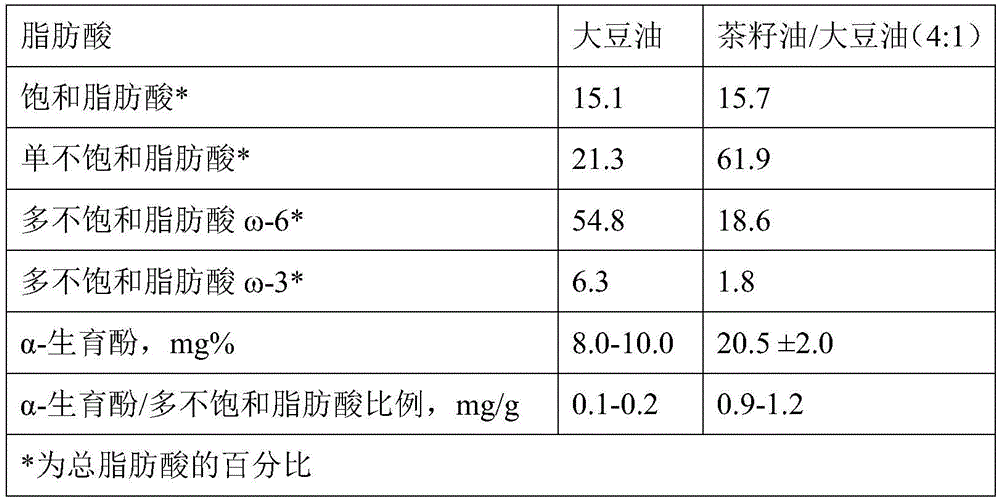
Tea-seed oil fat emulsion injection and preparing method and application thereof
InactiveCN105616710AReduce adverse reactionsRaise the ratioMetabolism disorderEmulsion deliveryInfected patientOMEGA-3 POLYUNSATURATED FATTY ACIDS
The invention discloses tea-seed oil fat emulsion injection and a preparing method and application thereof. Each liter of injection water of the tea-seed oil fat emulsion injection is prepared from following components: 0.01-30kg of tea-seed oil, 0.01-20kg of injection long chain oil, 0.5-10kg of emulsifier, 0.1-15kg of isoosmotic adjusting agent and 0-0.50kg of antioxygen and an adjusted pH is 5.5-9.50. By reducing the proportion of omega-6 polyunsaturated fatty acids while increasing the proportion of omega-3 polyunsaturated fatty acids, compatibility of various fats is optimized, a lipid metabolism level is accelerated, and organism material metabolism and synthesis of protein are helped. The injection can be widely used for various patients of parenteral nutrition treatment and particularly suitable for the low-immunity and various infected patients.
Owner:GUANGZHOU HANFANG PHARMA
Compound and medicament for treating MRSA (Methicillin-resistant Staphylococcus Aureus) infection
InactiveCN103360385AStrong specificityLittle side effectsAntibacterial agentsOrganic active ingredientsInfected patientPhotosens
The invention provides a compound and a preparation method thereof. The compound has a structure shown in a structural formula (I), wherein MRAS-expressed beta-lactamase can activate photosensitive groups in the compound provided by the invention, so as to generate active oxygen for exterminating MRAS without influencing a microbe and normal tissue which have unexpressed beta-lactamase, and therefore, the compound provided by the invention can achieve the beneficial effects of high efficiency, high specificity and less side effect, can be used as a photosensitizer for PACT treatment of MRSA infection and can provide a powerful tool for treating clinical patients with MRSA injection.
Owner:上海交通大学医学院附属第三人民医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com