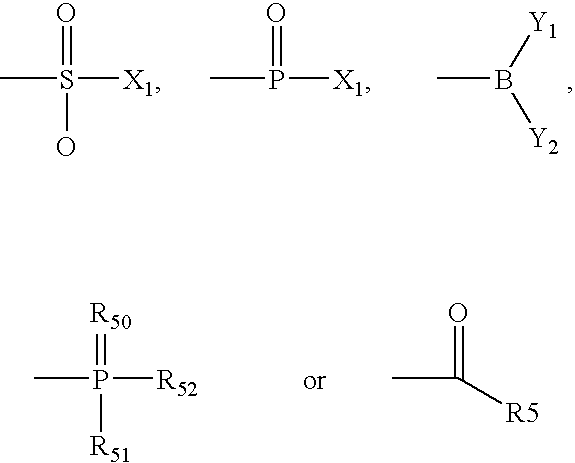
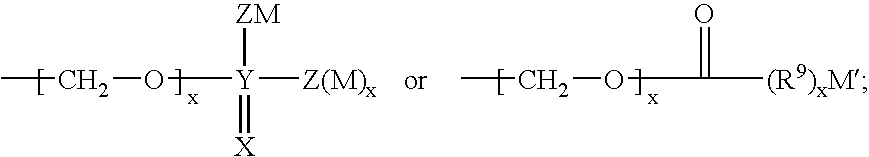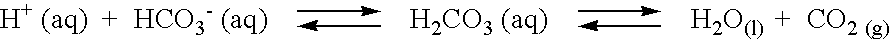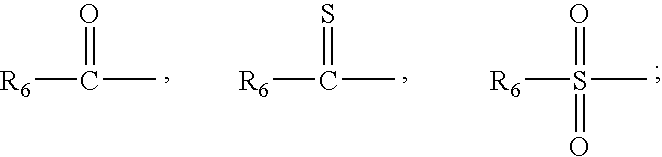Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2806results about "Phosphorous compound active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
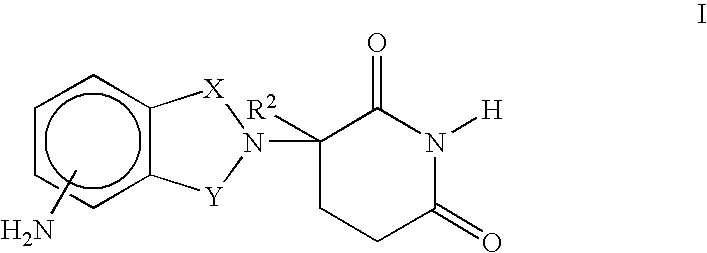
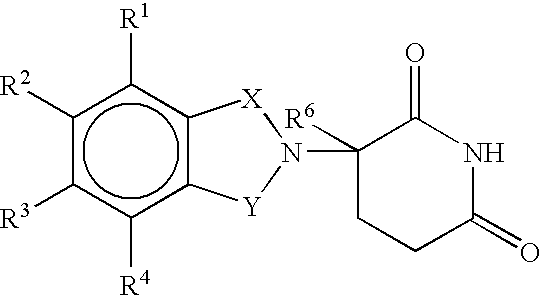
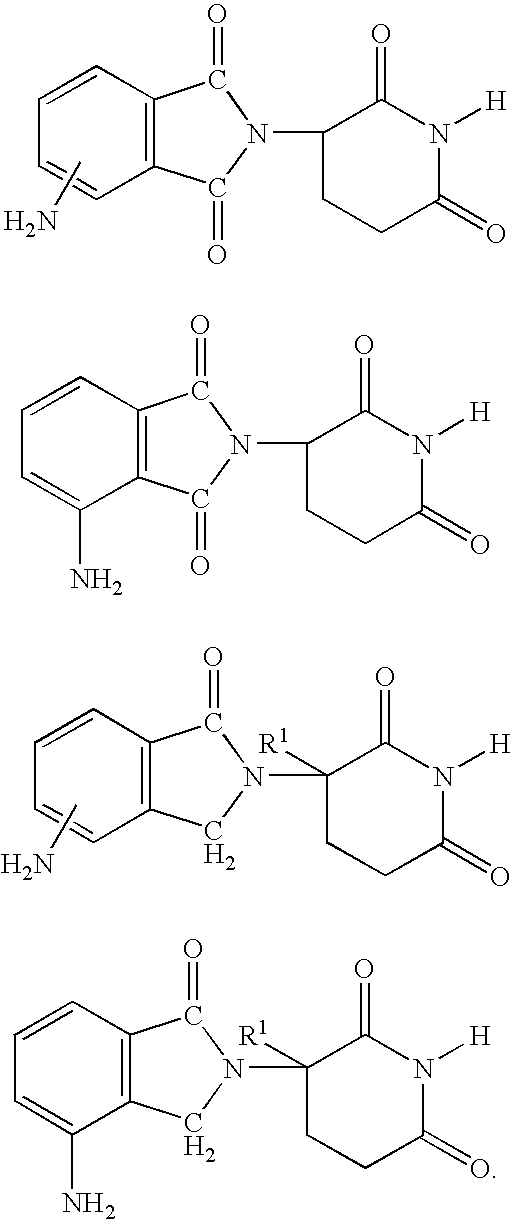
Glucocorticoid blocking agents for increasing blood-brain barrier permeability stan-261con
InactiveUS20050124533A1Improve breathabilityIncrease volumeAntibacterial agentsBiocideBlood brain barrier permeabilityDisease cause
Glucocorticoid blockers, including glucocorticoid receptor antagonists, are effective to prevent glucocorticoid-induced decrease in permeability of the blood-brain barrier and to increase the permeability of the blood-brain barrier. Administration of glucocorticoid blockers, including glucocorticoid receptor antagonists, concomitant with administration of drugs for treating diseases of the central nervous system increases delivery of such drugs into the central nervous system.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Therapeutic Use of a TLR Agonist and Combination Therapy
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Ophthalmic formulations including selective alpha 1 antagonists
InactiveUS20050080056A1Easy to manageImprove eyesightBiocideSenses disorderActive agentPupil light reflex
Ophthalmic formulations are provided. The ophthalmic formulations include one or more active agents that act to optimize pupil light reflex while minimizing, or effectively eliminating, any undesired eye redness in response to application thereof. The active agents include, for example, alpha 1 antagonists, such as alpha la selective antagonists.
Owner:OCUPHIRE PHARM INC
Combination therapies for treating neurological disorders
InactiveUS20130116215A1Effective treatmentBiocidePhosphorous compound active ingredientsDiseaseMedicine
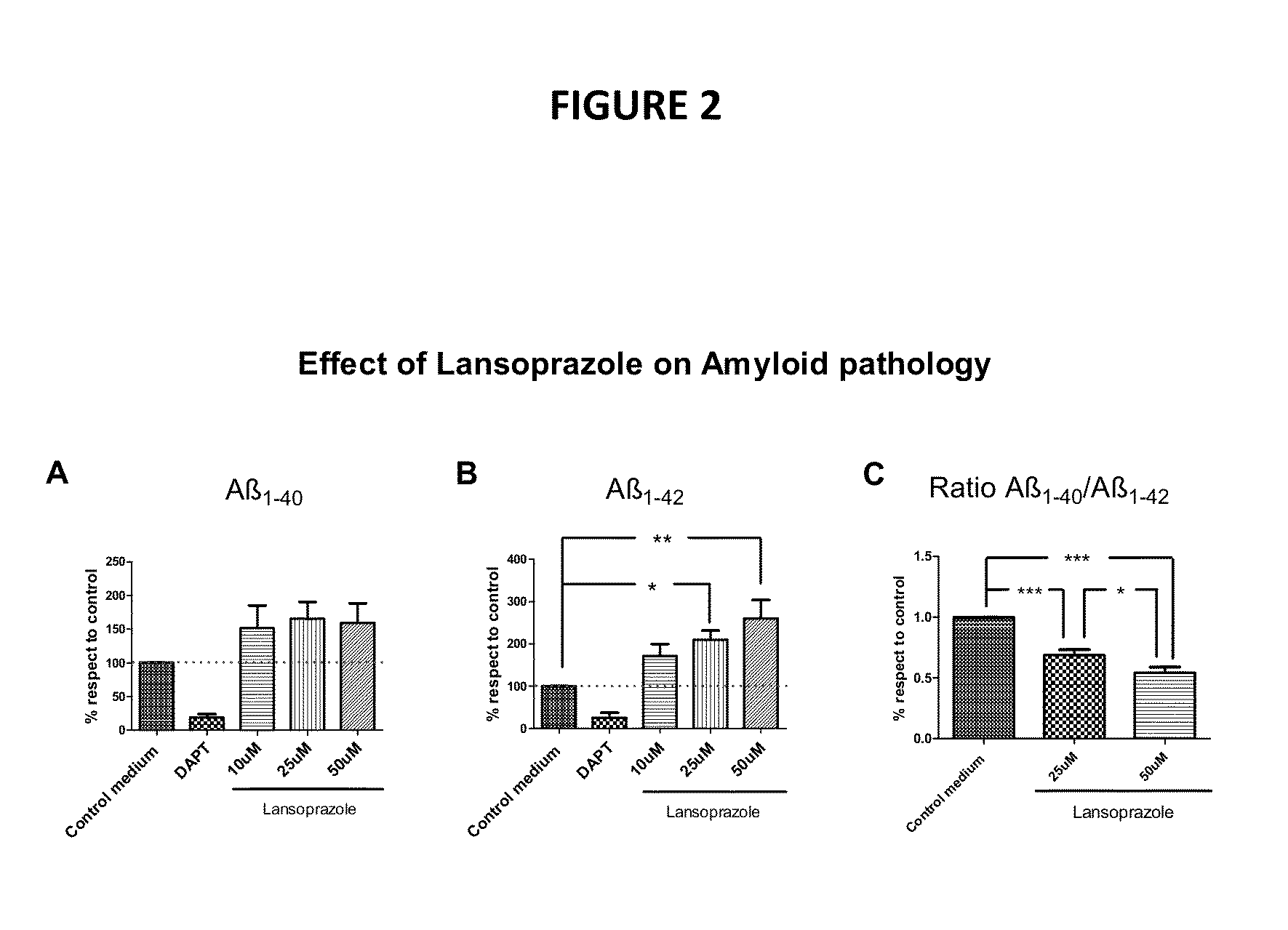
The invention features novel pharmaceutical combinations useful for the treatment of neurological diseases, specifically neurodegenerative diseases. The novel pharmaceutical combinations of the invention demonstrate additive or synergistic effect in silico and in vivo. The invention also relates to methods of treatment of neurological and neurodegenerative diseases including the pharmaceutical combinations of the invention.
Owner:ANAXOMICS BIOTECH SL
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of asbestos-related diseases and disorders
Methods of treating, preventing and managing an asbestos-related disease or disorder are disclosed. Specific embodiments encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent and / or chemotherapy, surgery, or radiation therapy. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
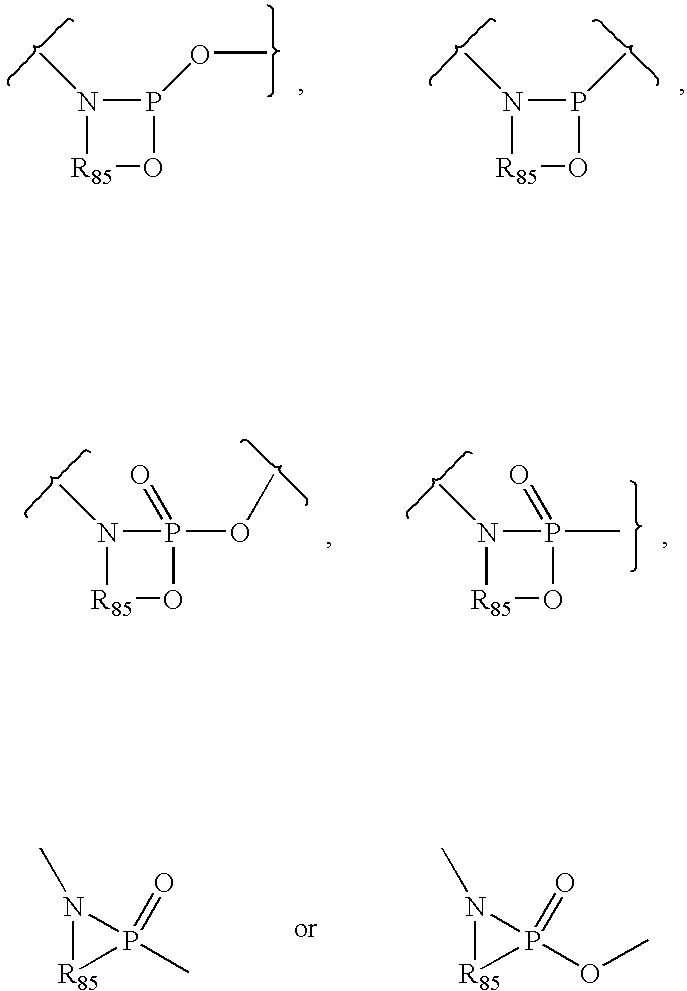
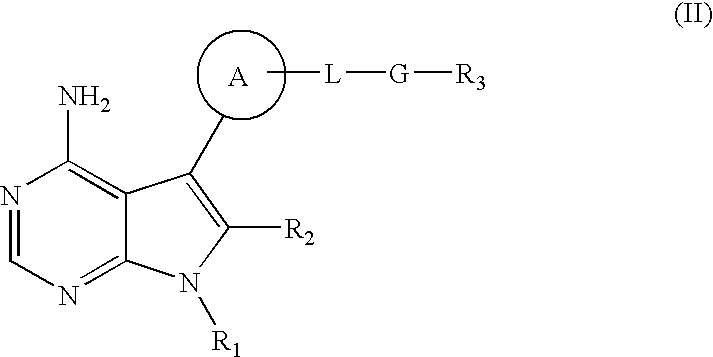
Nucleoside phosphoramidates
Disclosed herein are nucleoside phosphoramidates and their use as agents for treating viral diseases. These compounds are inhibitors of RNA-dependent 5 RNA viral replication and are useful as inhibitors of HCV NS5B polymerase, as inhibitors of HCV replication and for treatment of hepatitis C infection in mammals.
Owner:GILEAD SCI INC
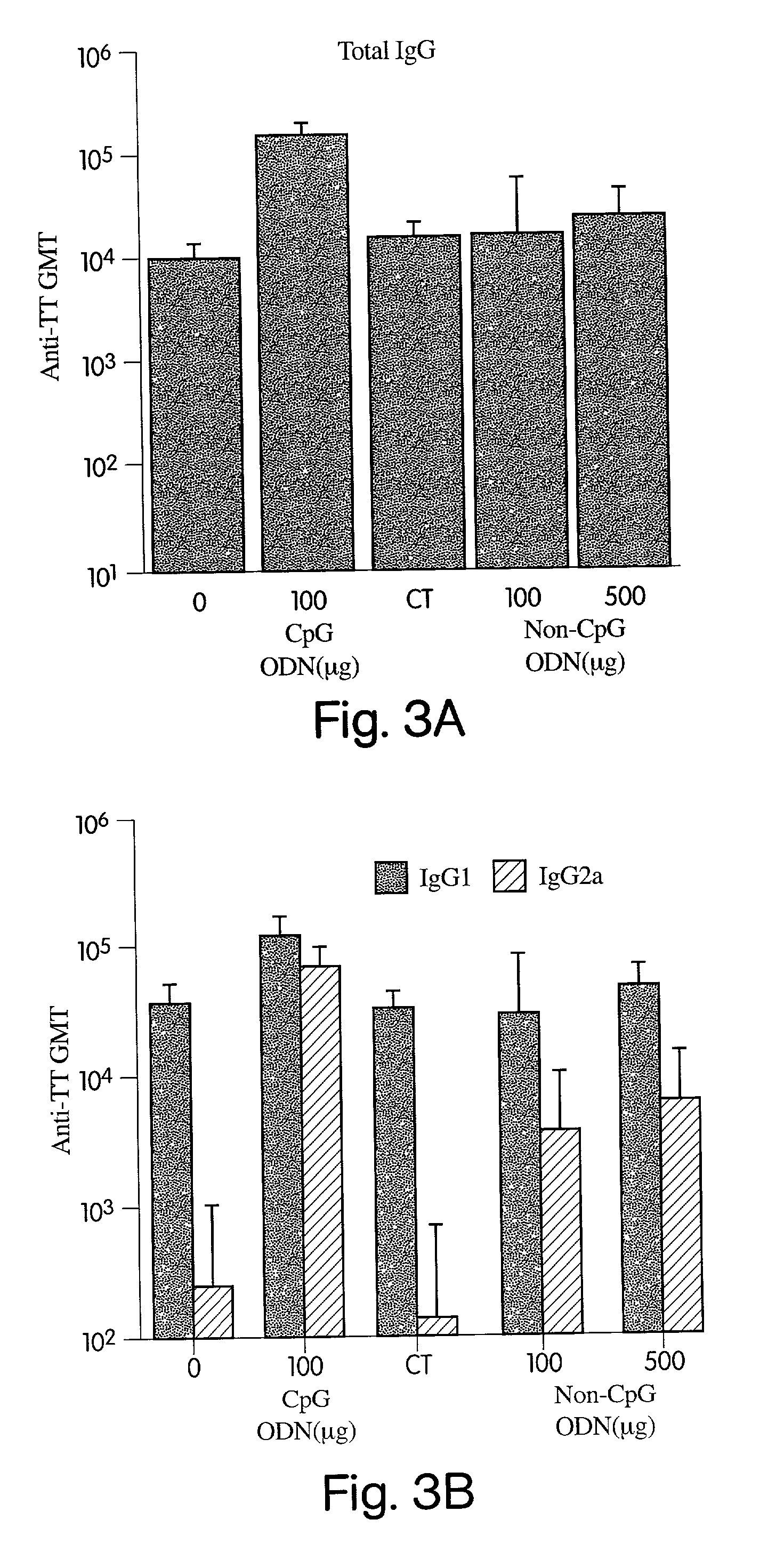
Immunostimulatory nucleic acids for inducing a Th2 immune response
Owner:OTTAVA HEALTH RES INST (CA)
Method of prevention and treatment of aging, age-related disorders and/or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimers disease and cancer
InactiveUS20060275294A1Halogenated hydrocarbon active ingredientsBiocideAbnormal tissue growthSTAT Transcription Factors
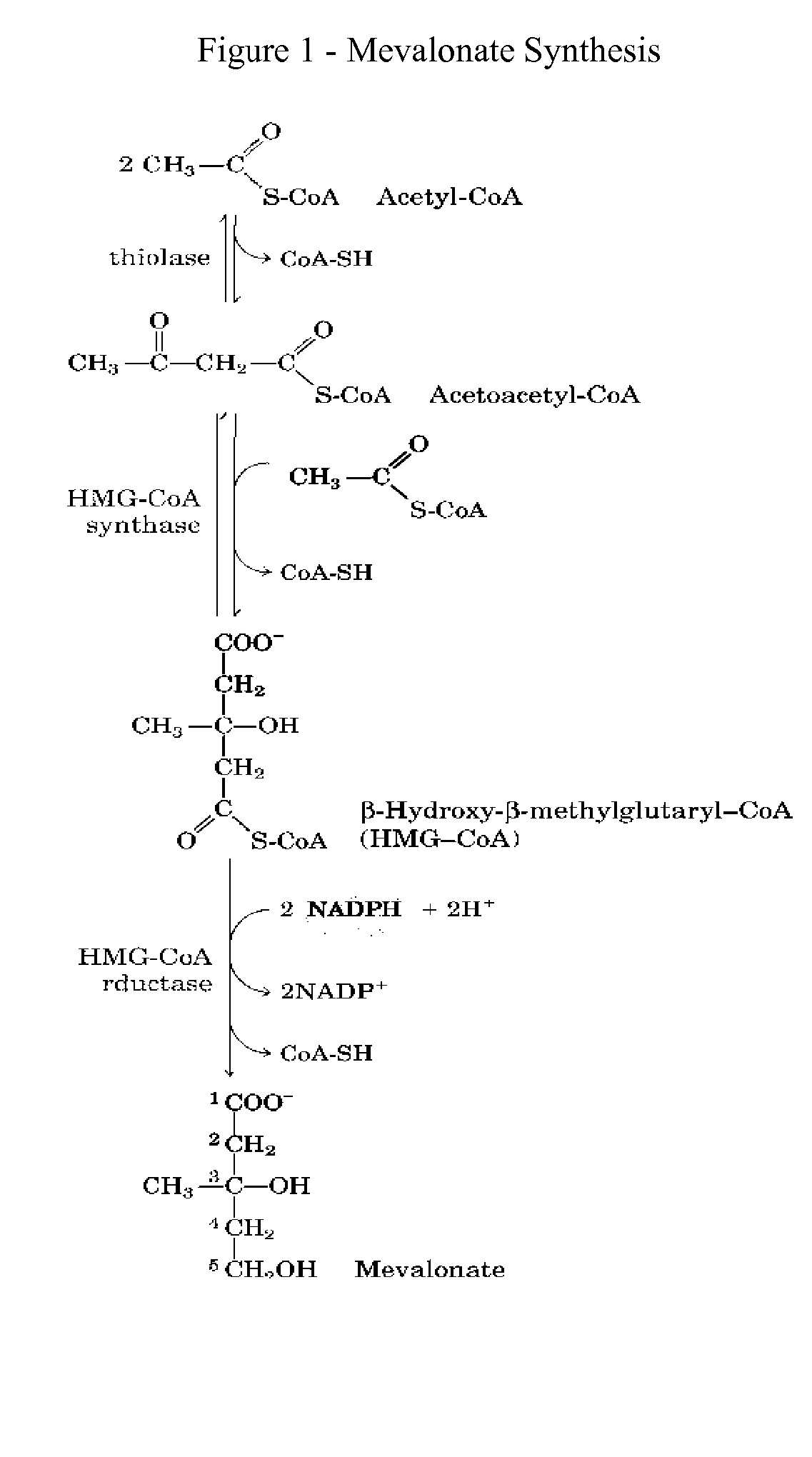
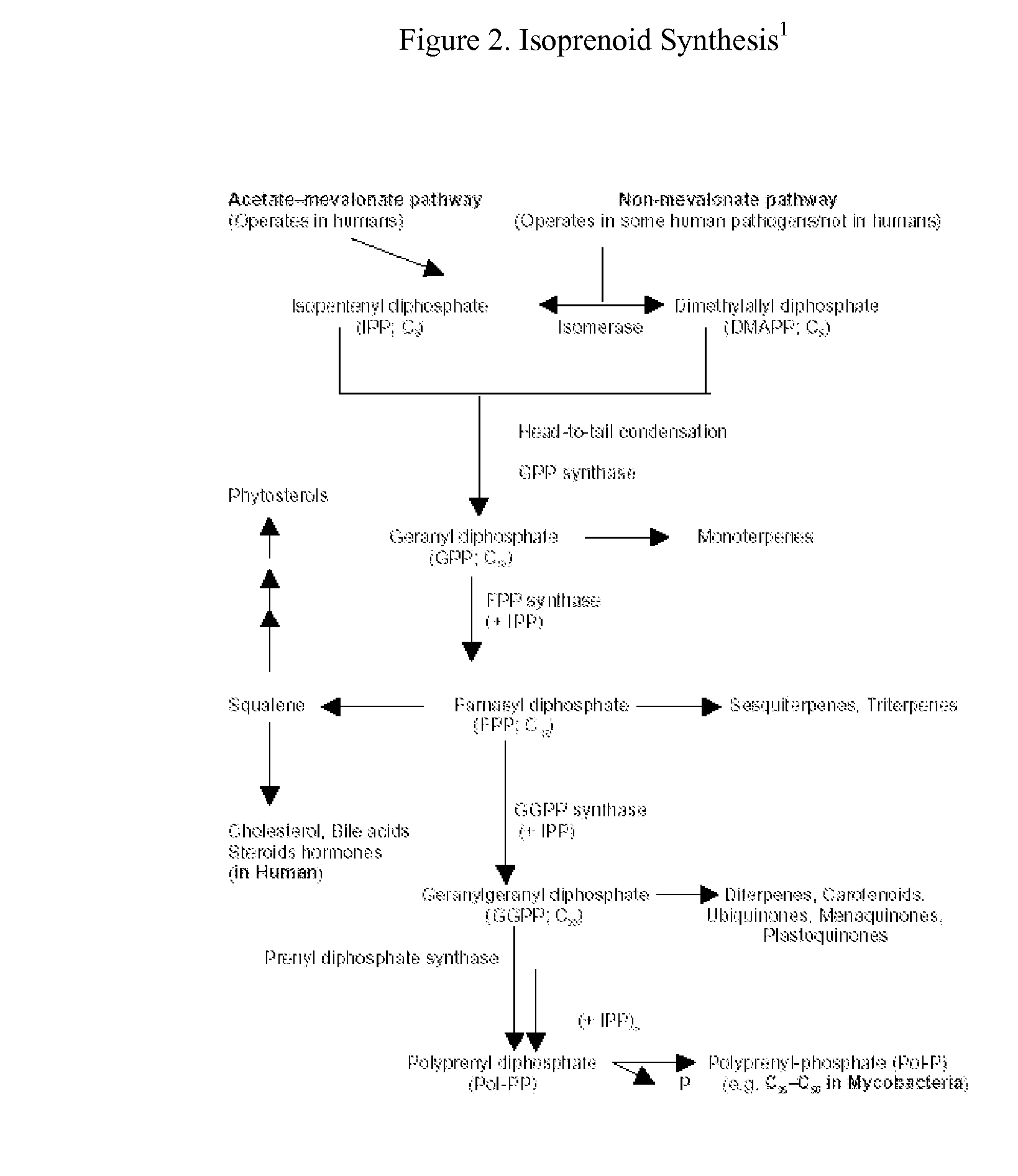
This invention relates to a method for prevention and treatment of aging, age-related disorders and / or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, type 2 diabetes, dementia and some forms of arthritis and cancer in a subject comprising administering to said subject, separately, sequentially or simultaneously a therapeutically effective dosage of each component or combination of statins, bisphosphonates, cholesterol lowering agents or techniques, interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof, administered separately, in sequence or simultaneously. Inhibition of the signal transduction pathway for Interleukin 6 mediated inflammation is key to the prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, aging, age-related disorders and / or age-related manifestations including osteoporosis, type 2 diabetes, dementia and some forms of arthritis and tumors. Inhibition of Interleukin 6 mediated inflammation may be achieved indirectly through regulation of endogenous cholesterol synthesis and isoprenoid depletion or by direct inhibition of the signal transduction pathway utilizing interleukin-6 inhibitor / antibody, interleukin-6 receptor inhibitor / antibody, interleukin-6 antisense oligonucleotide (ASON), gp130 protein inhibitor / antibody, tyrosine kinases inhibitors / antibodies, serine / threonine kinases inhibitors / antibodies, mitogen-activated protein (MAP) kinase inhibitors / antibodies, phosphatidylinositol 3-kinase (PI3K) inhibitors / antibodies, Nuclear factor κB (NF-κB) inhibitors / antibodies, IκB kinase (IKK) inhibitors / antibodies, activator protein-1 (AP-1) inhibitors / antibodies, STAT transcription factors inhibitors / antibodies, altered IL-6, partial peptides of IL-6 or IL-6 receptor, or SOCS (suppressors of cytokine signaling) protein, or a functional fragment thereof. Said method for prevention and treatment of said disorders is based on inhibition of Interleukin-6 inflammation through regulation of cholesterol metabolism, isoprenoid depletion and / or inhibition of the signal transduction pathway
Owner:OMOIGUI OSEMWOTA SOTA
Method of treating transplant rejection
This invention relates to a method of treating transplant rejection comprising administering to a patient a pharmaceutical composition comprising an lck inhibitor and a calcineurin inhibitor or an immunosuppressant.
Owner:ABBOTT LAB INC
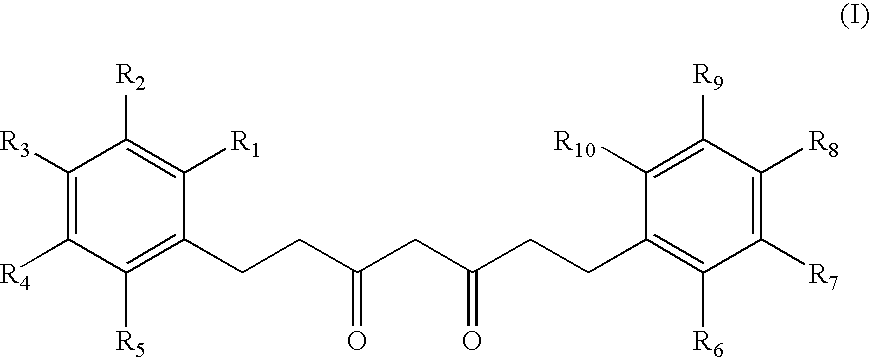
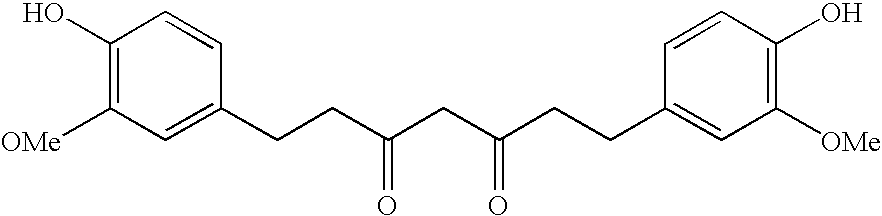
Cosmetic/dermatological compositions comprising a tetrahydrocurcuminoid and an amide oil
The invention relates to a cosmetic or dermatological composition containing a carrier comprising at least one fatty phase characterized in that it contains at least one derivative or a mixture of derivatives of 1,7-diphenyl-3,5-heptanedione having a particular structure and at least one oil having, in its structure, at least one amide unit.The invention also relates to its uses in cosmetics and dermatology, in particular for preventing or combating the harmful effects of UV radiation and pollution on human keratinous materials, and more particularly for preventing and / or treating photoaging of the skin.The invention also relates to a method for solubilizing a derivative or a mixture of derivatives of 1,7-diphenyl-3,5-heptanedione having a particular structure with at least one oil having, in its structure, at least one amide unit.
Owner:LOREAL SA
Method for reducing adenosine levels with a dehydroepiandrosterone and optionally a ubiquinone
A method and composition for reducing adenosine levels comprises administering a dehydroepiandrosterone, and optionally a ubiquinone.
Owner:EAST CAROLINA UNIVERISTY
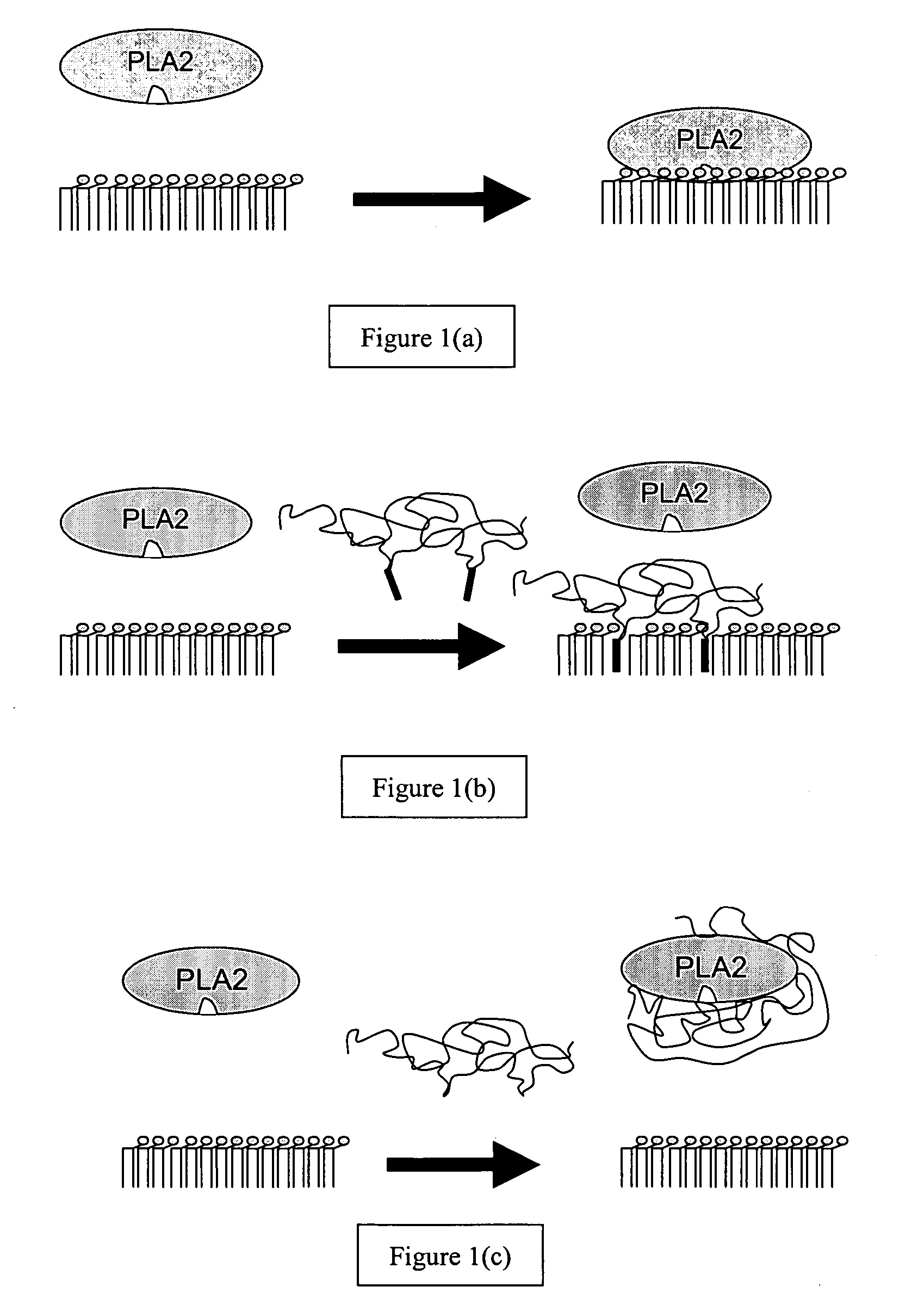
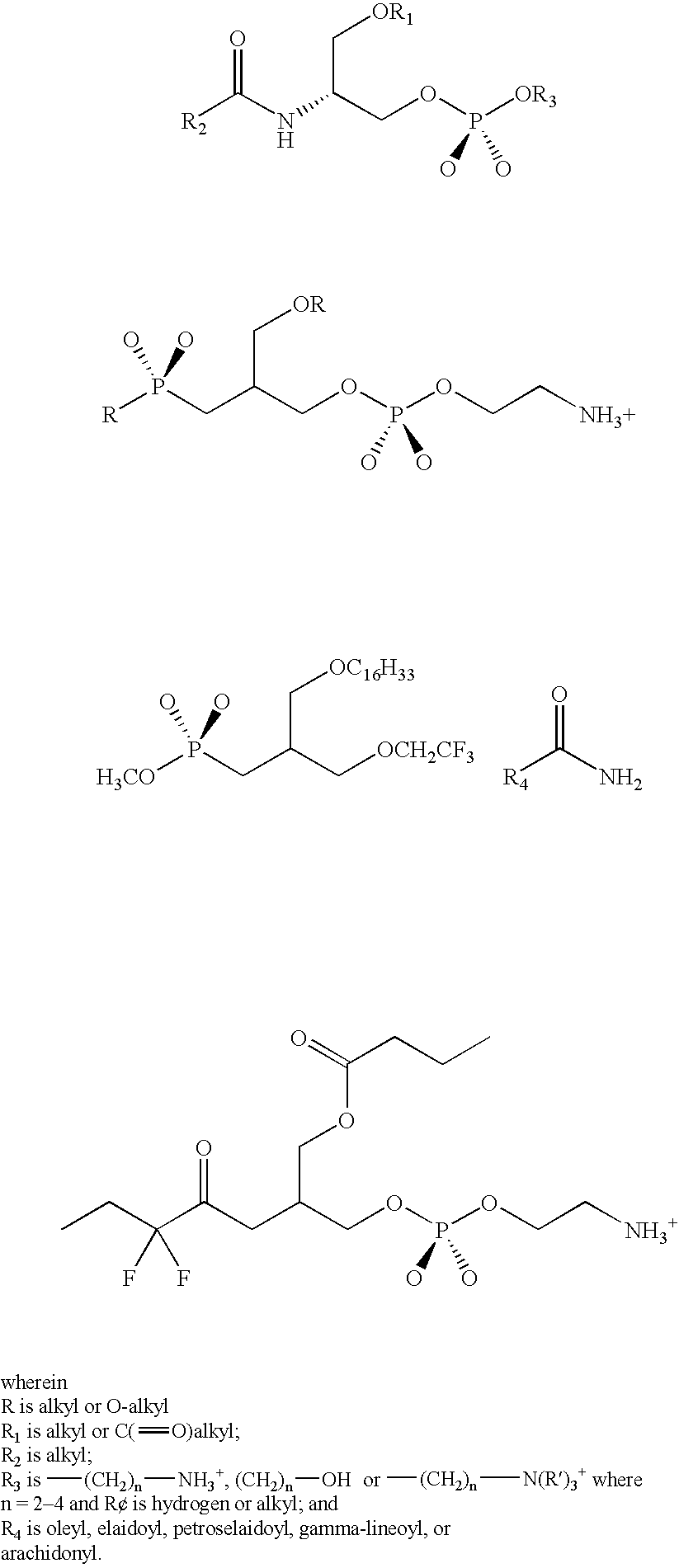
Phospholipase inhibitors localized in the gastrointestinal lumen
The present invention provides methods and compositions for the treatment of phospholipase-related conditions. In particular, the invention provides a method of treating insulin-related, weight-related conditions and / or cholesterol-related conditions in an animal subject. The method generally involves the administration of a non-absorbed and / or effluxed phospholipase A2 inhibitor that is localized in a gastrointestinal lumen.
Owner:ILYPSA
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Dosage forms of bisphosphonates
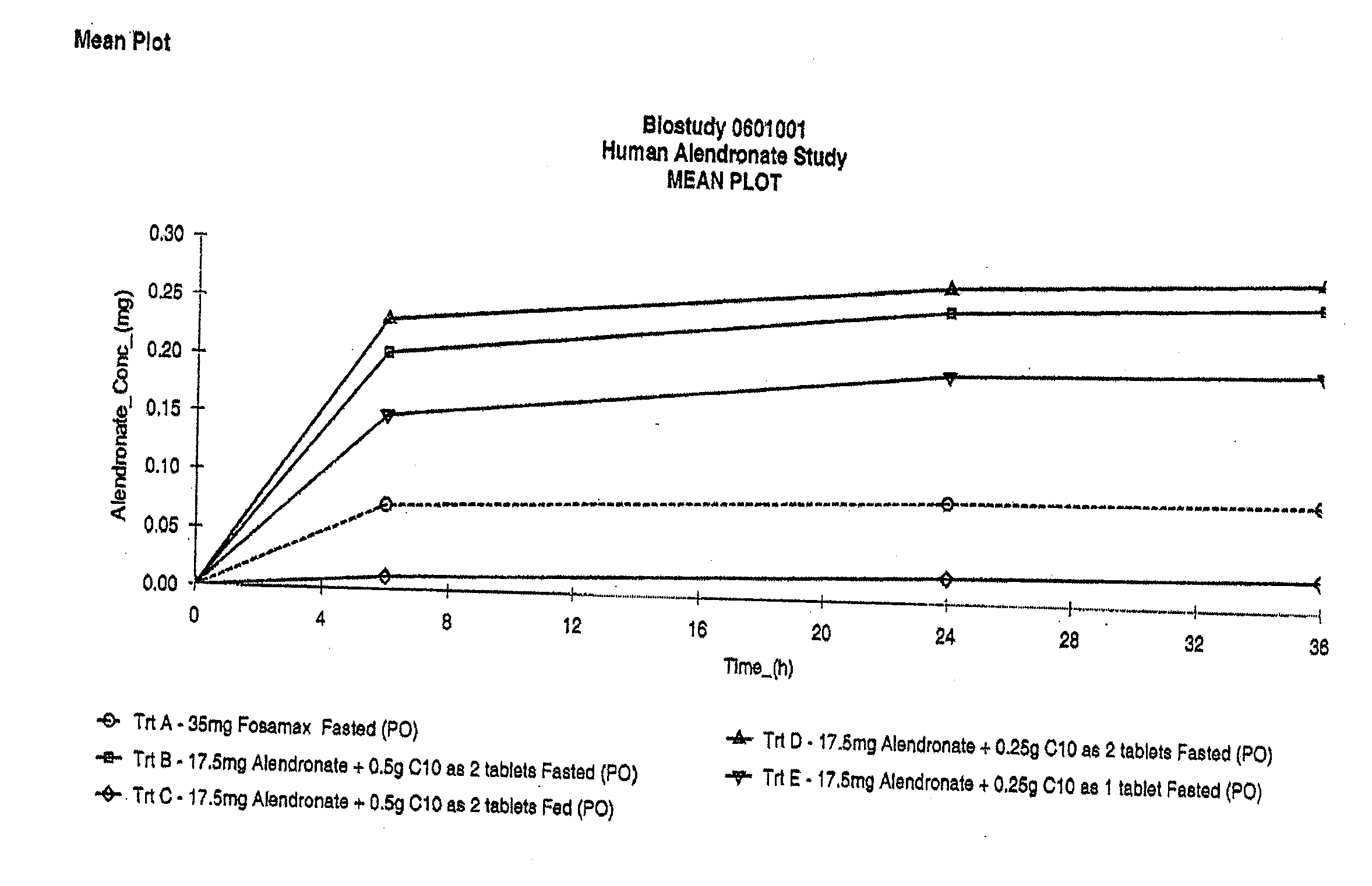
ActiveUS20050260262A1Effective absorptionReduce interactionBiocideMetabolism disorderBisphosphonate therapyUpper gastrointestinal
Oral dosage forms of a bisphosphonate comprised of a safe and effective amount of a pharmaceutical composition comprising a bisphosphonate, a chelating agent, and, means for effecting delayed release of the bisphosphonate and the chelating agent in the lower gastrointestinal tract provide delivery of the pharmaceutical composition to the lower gastrointestinal tract of the mammal subject and pharmaceutically effective absorption of the bisphosphonate with or without food or beverages. The present invention substantially alleviates the interaction between bisphosphonates and food or beverages, which interaction results in the bisphosphonate active ingredient not being available for absorption. The resulting oral dosage form may thus be taken with or without food. Further, the present invention effects delivery of the bisphosphonate and the chelating agent to the lower GI tract, substantially alleviating the upper GI irritation associated with bisphosphonate therapies. These benefits simplify previously complex treatment regimens and can lead to increased patient compliance with bisphosphonate therapies.
Owner:APTALIS PHARMA
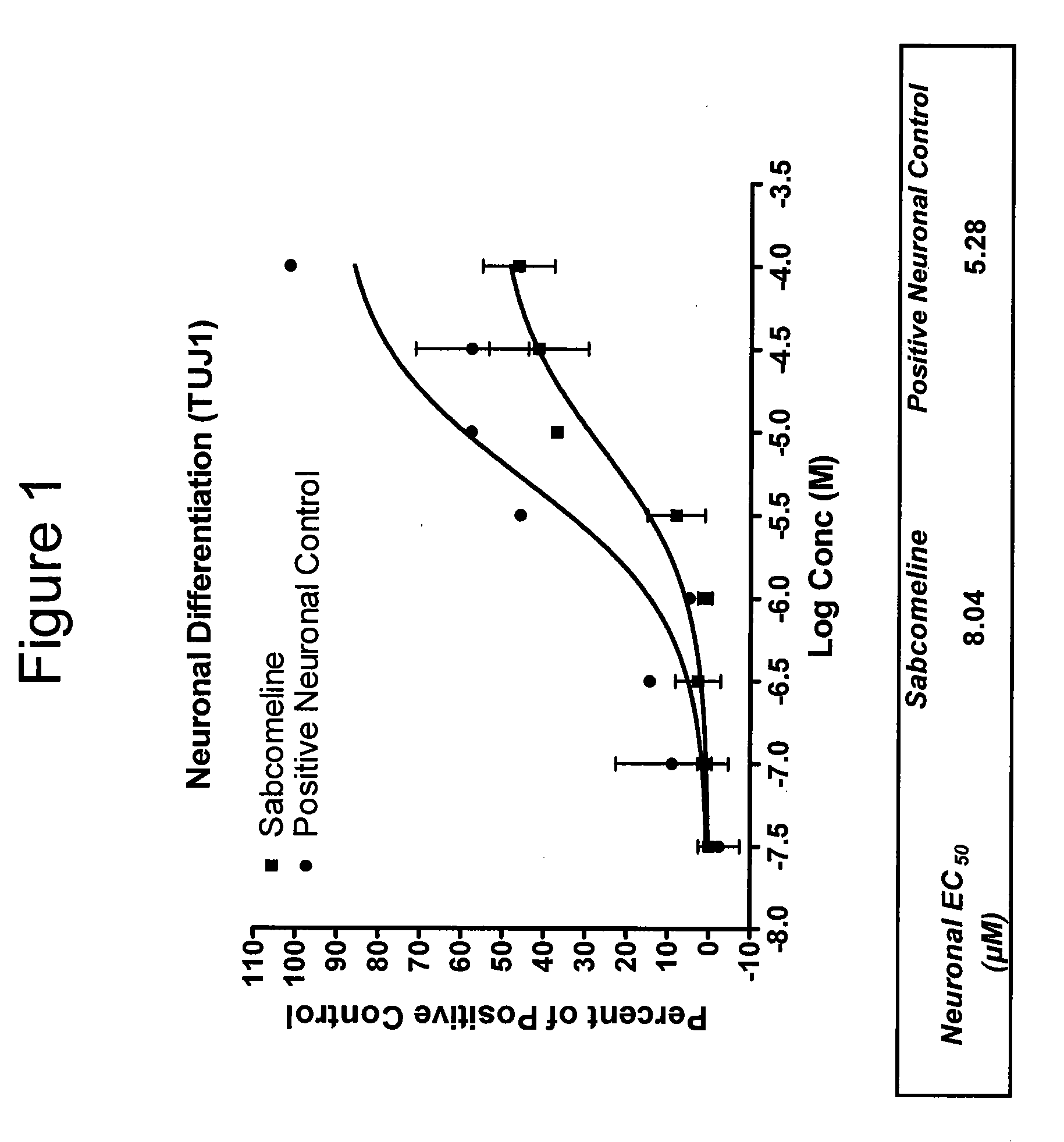
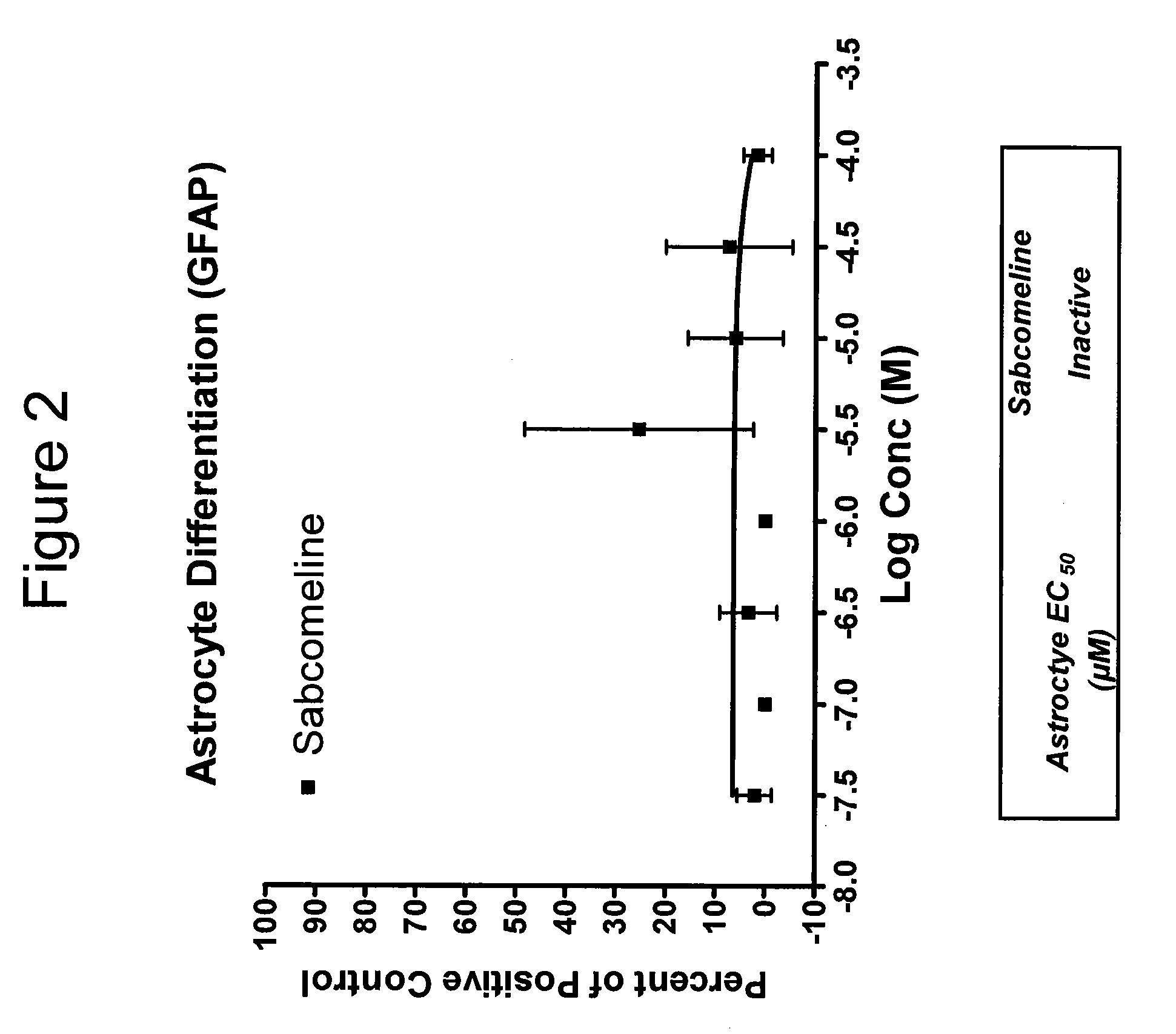
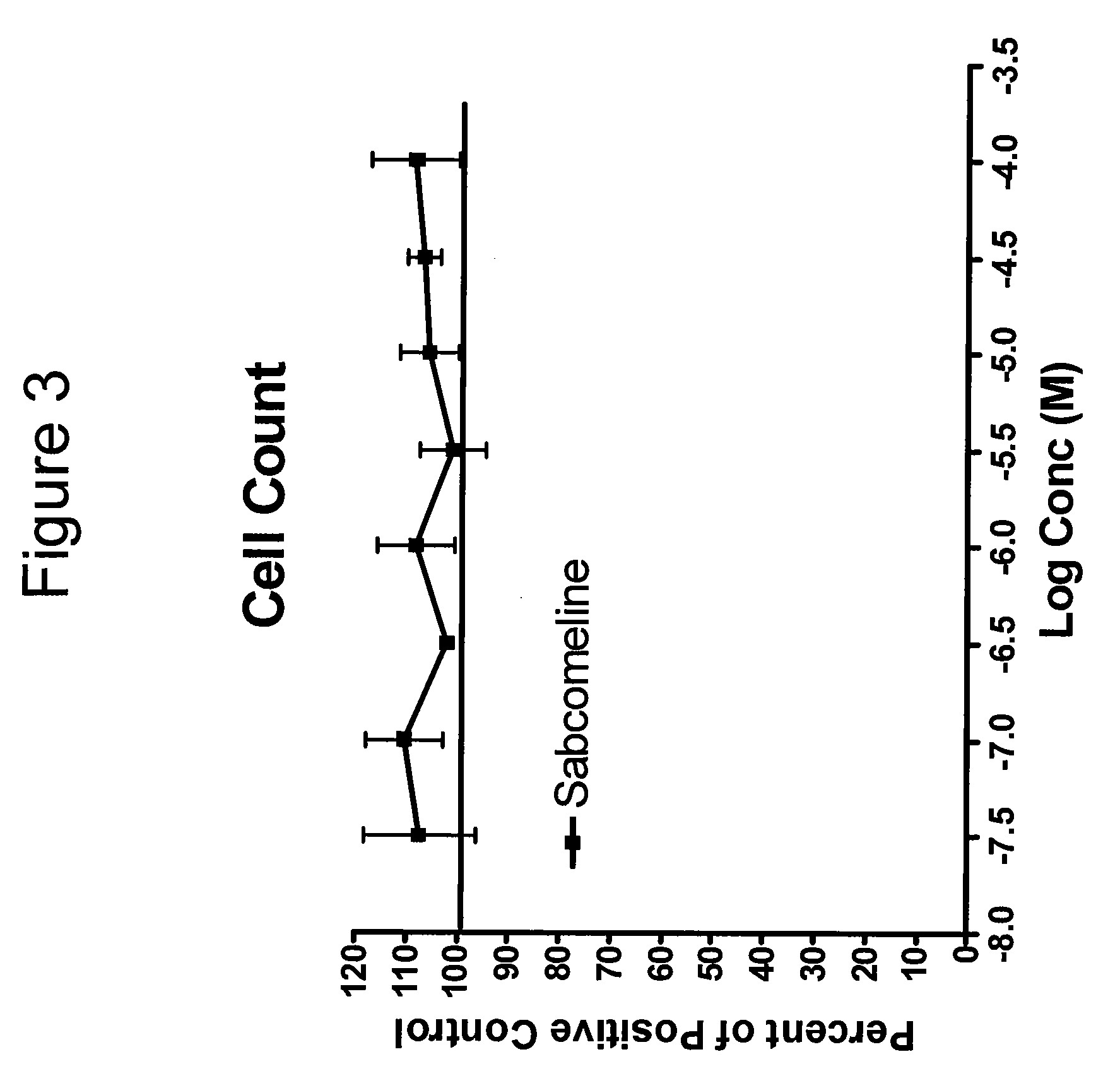
Neurogenesis by muscarinic receptor modulation
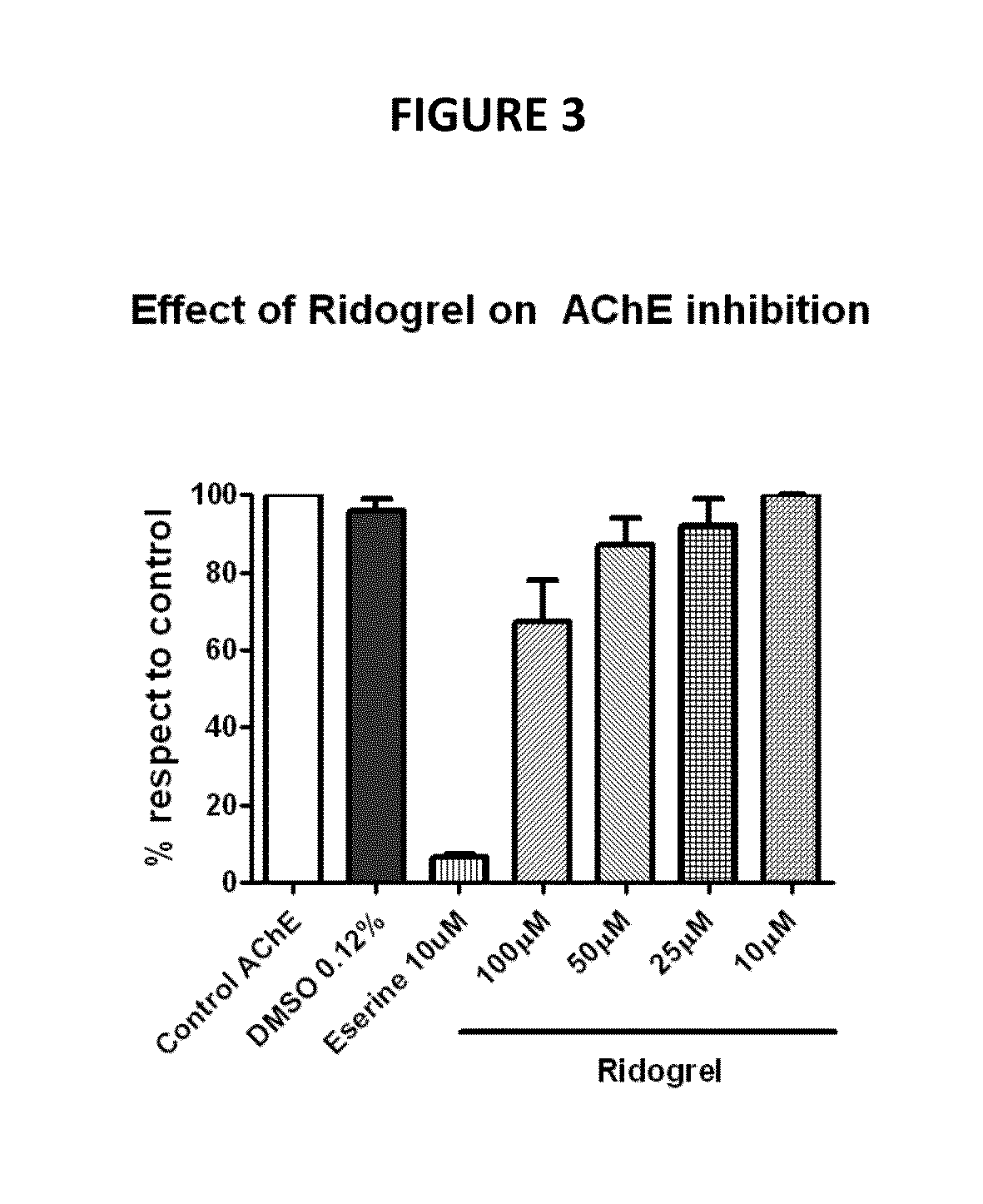
The instant disclosure describes methods for treating diseases and conditions of the central and peripheral nervous system by stimulating or increasing neurogenesis. The disclosure includes compositions and methods based on muscarinic receptor modulation, such as via inhibition of acetylcholine esterase (AChE) activity, alone or in combination with another neurogenic agent to stimulate or activate the formation of new nerve cells.
Owner:BRAINCELLS INC
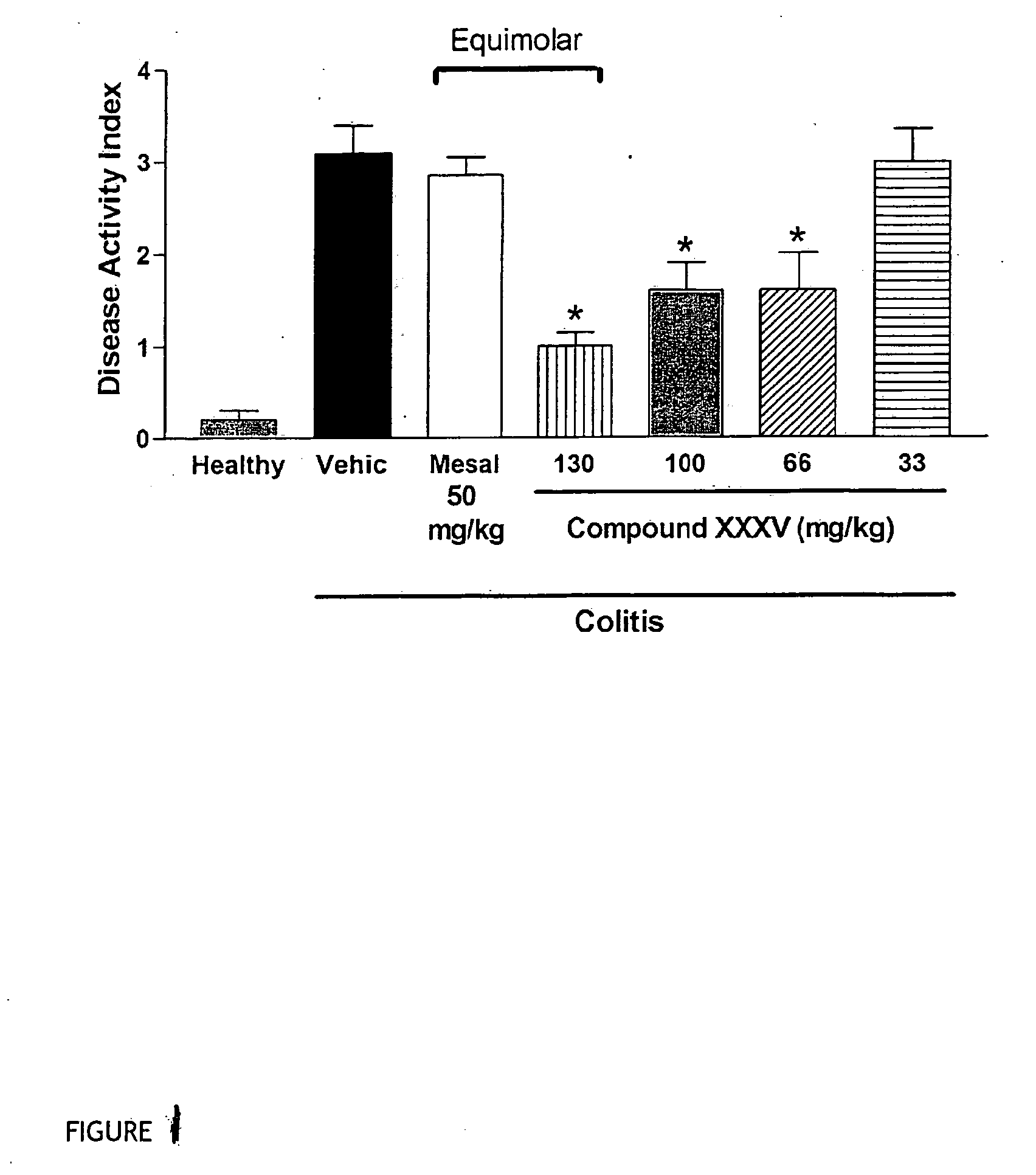
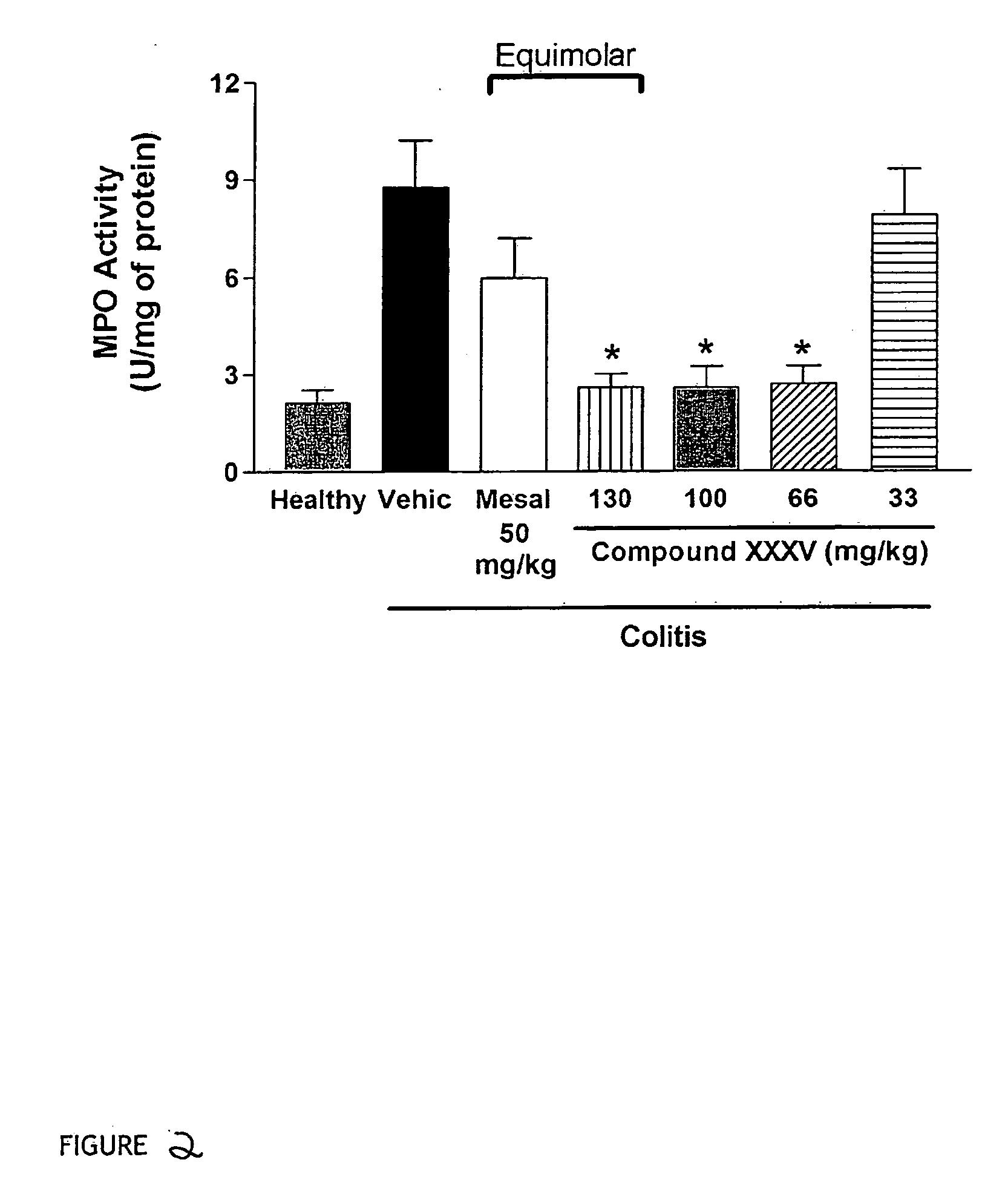
Derivatives of 4- or 5-aminosalicylic acid
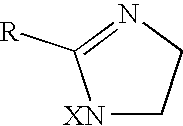
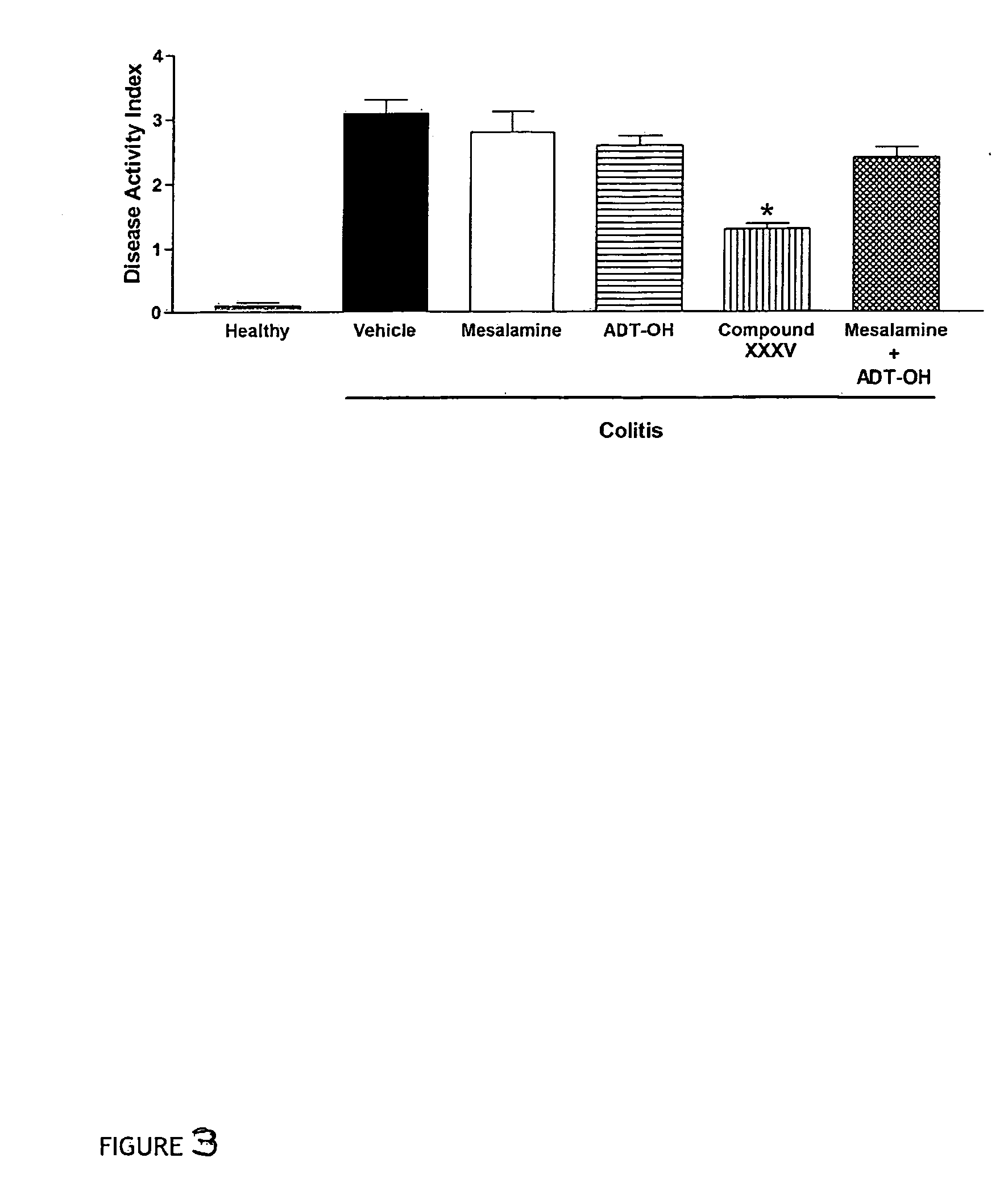
InactiveUS20060270635A1Less readily absorbedEasily reach colonBiocidePhosphorous compound active ingredientsThioester synthesisSalicylic acid
The present invention provides new derivatives of 4- or 5-aminosalicylic acid, and a pharmaceutical composition containing these derivatives of 4- or 5-aminosalicylic acid as active ingredients, useful for the treatment of intestinal diseases such as inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) and for the prevention / treatment of colon cancer. More particularly, these derivatives comprise a hydrogen sulfide releasing moiety linked via an azo, an ester, an anhydride, a thioester or an amide linkage to a molecule of 4- or 5-aminosalicylic acid. Furthermore, the present invention provides a process for preparing these compounds and their use for treating IBD and IBS and the prevention / treatment of colon cancer.
Owner:ANTIBE THERAPEUTICS INC
Method of biochemical treatment of persistent pain
InactiveUS20050152905A1Reduce releaseAvoid exposureBiocidePeptide/protein ingredientsInterleukin 6Interleukin-1beta
This invention relates to a method for the biochemical treatment of persistent pain disorders by inhibiting the biochemical mediators of inflammation in a subject comprising administering to said subject any one of several combinations of components that are inhibitors of biochemical mediators of inflammation. Said process for biochemical treatment of persistent pain disorders is based on Sota Omoigui's Law, which states: ‘The origin of all pain is inflammation and the inflammatory response’. Sota Omoigui's Law of Pain unifies all pain syndromes as sharing a common origin of inflammation and the inflammatory response. The various biochemical mediators of inflammation are present in differing amounts in all pain syndromes and are responsible for the pain experience. Classification and treatment of pain syndromes should depend on the complex inflammatory profile. A variety of mediators are generated by tissue injury and inflammation. These include substances produced by damaged tissue, substances of vascular origin as well as substances released by nerve fibers themselves, sympathetic fibers and various immune cells. Biochemical mediators of inflammation that are targeted for inhibition include but are not limited to: prostaglandin, nitric oxide, tumor necrosis factor alpha, interleukin 1-alpha, interleukin 1-beta, interleukin-4, Interleukin-6 and interleukin-8, histamine and serotonin, substance P, Matrix Metallo-Proteinase, calcitonin gene-related peptide, vasoactive intestinal peptide as well as the potent inflammatory mediator peptide proteins neurokinin A, bradykinin, kallidin and T-kinin.
Owner:OMOIGUI OSEMWOTA SOTA
Hepatitis c virus inhibitors
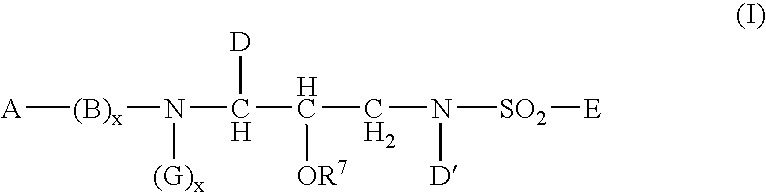
The present invention discloses compounds of Formula (I), or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Sulphonamide derivatives as prodrugs of aspartyl protease inhibitors
InactiveUS20050148548A1Good water solubilityImprove bioavailabilityBiocideSugar derivativesSulfur drugPatient compliance
The present invention relates to prodrugs of a class of sulfonamides which are aspartyl protease inhibitors. In one embodiment, this invention relates to a novel class of prodrugs of HIV aspartyl protease inhibitors characterized by favorable aqueous solubility, high oral bioavailability and facile in vivo generation of the active ingredient. This invention also relates to pharmaceutical compositions comprising these prodrugs. The prodrugs and pharmaceutical compositions of this invention are particularly well suited for decreasing the pill burden and increasing patient compliance. This invention also relates to methods of treating mammals with these prodrugs and pharmaceutical compositions.
Owner:VERTEX PHARMA INC
Solid oral dosage form containing an enhancer
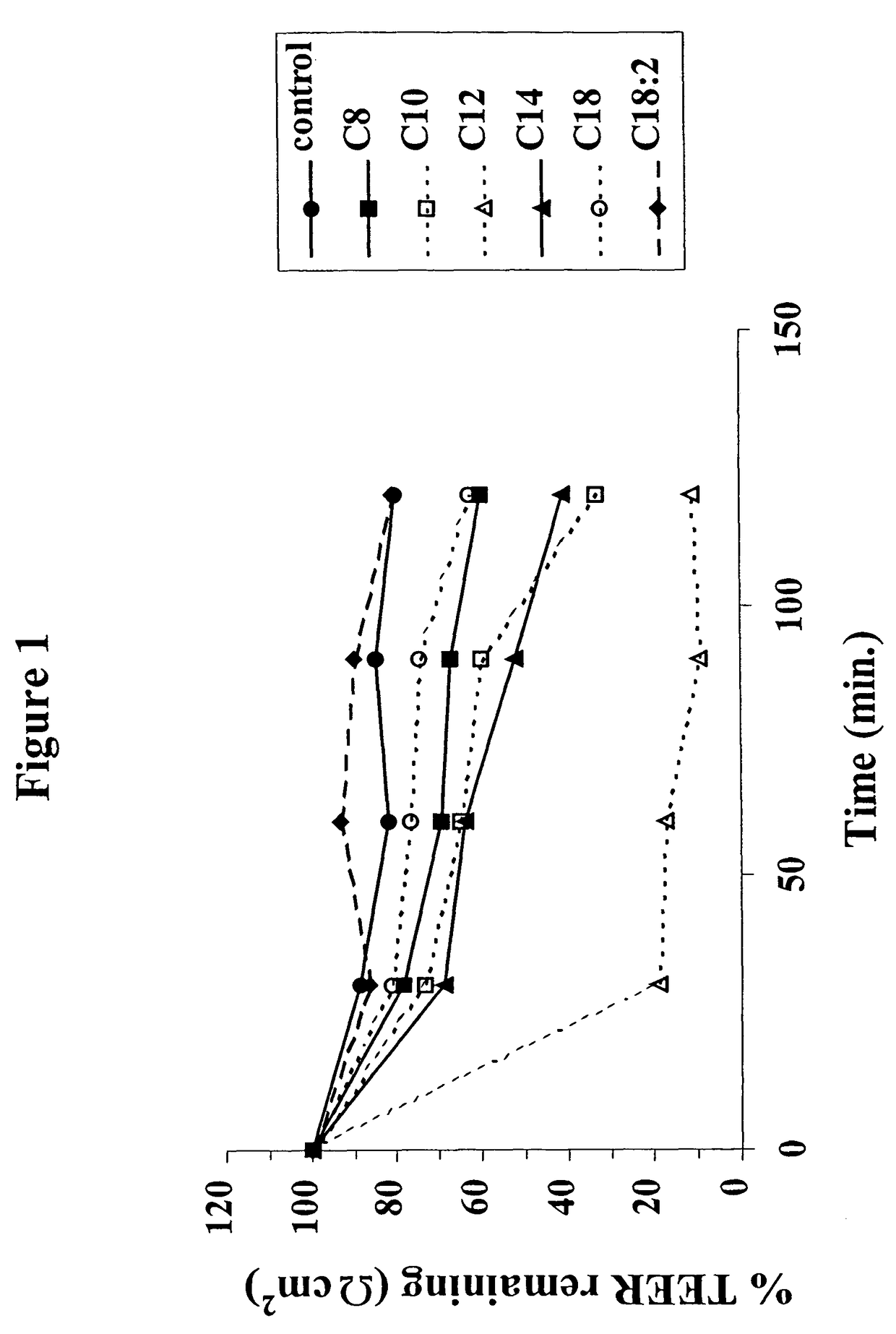
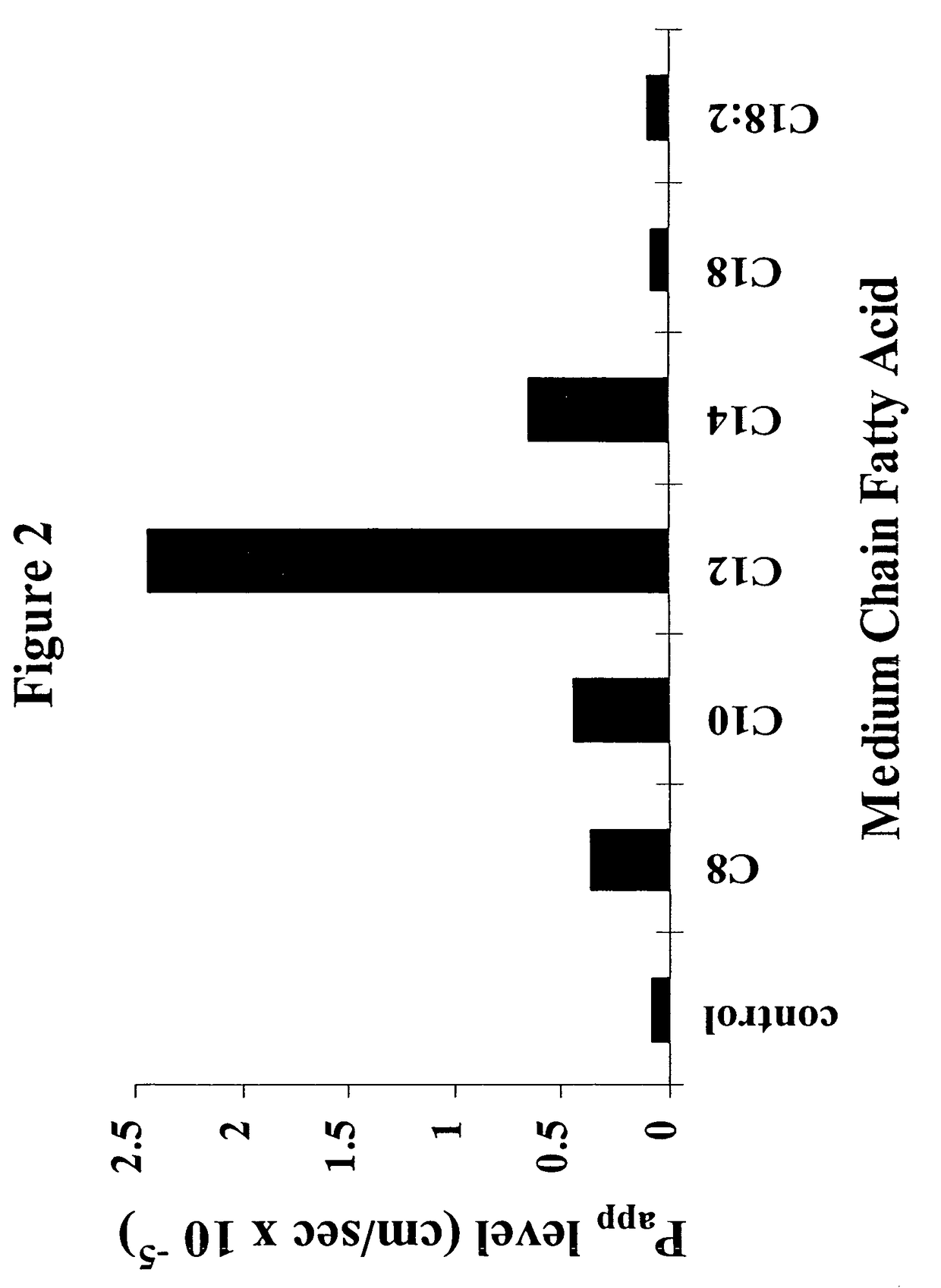
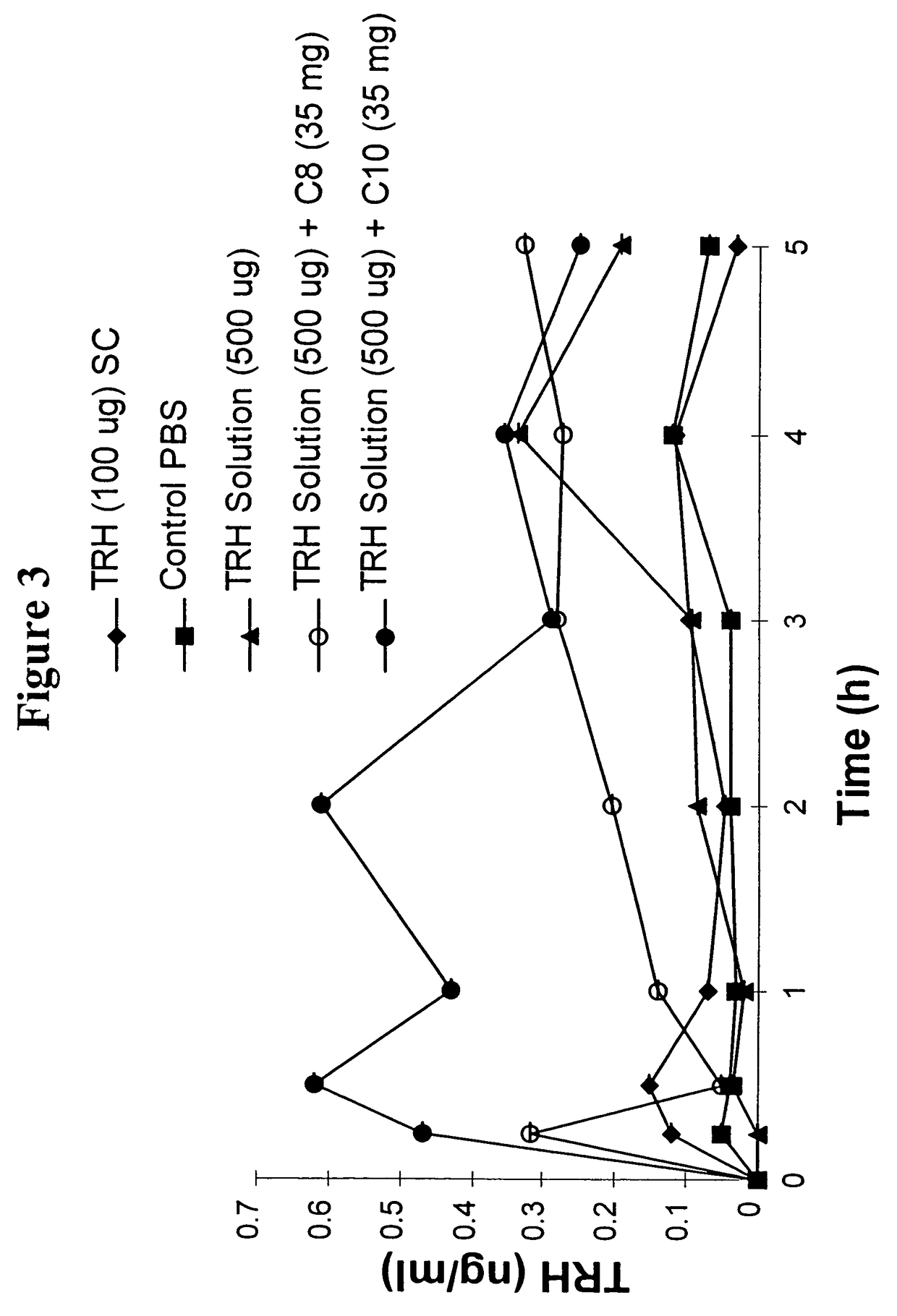
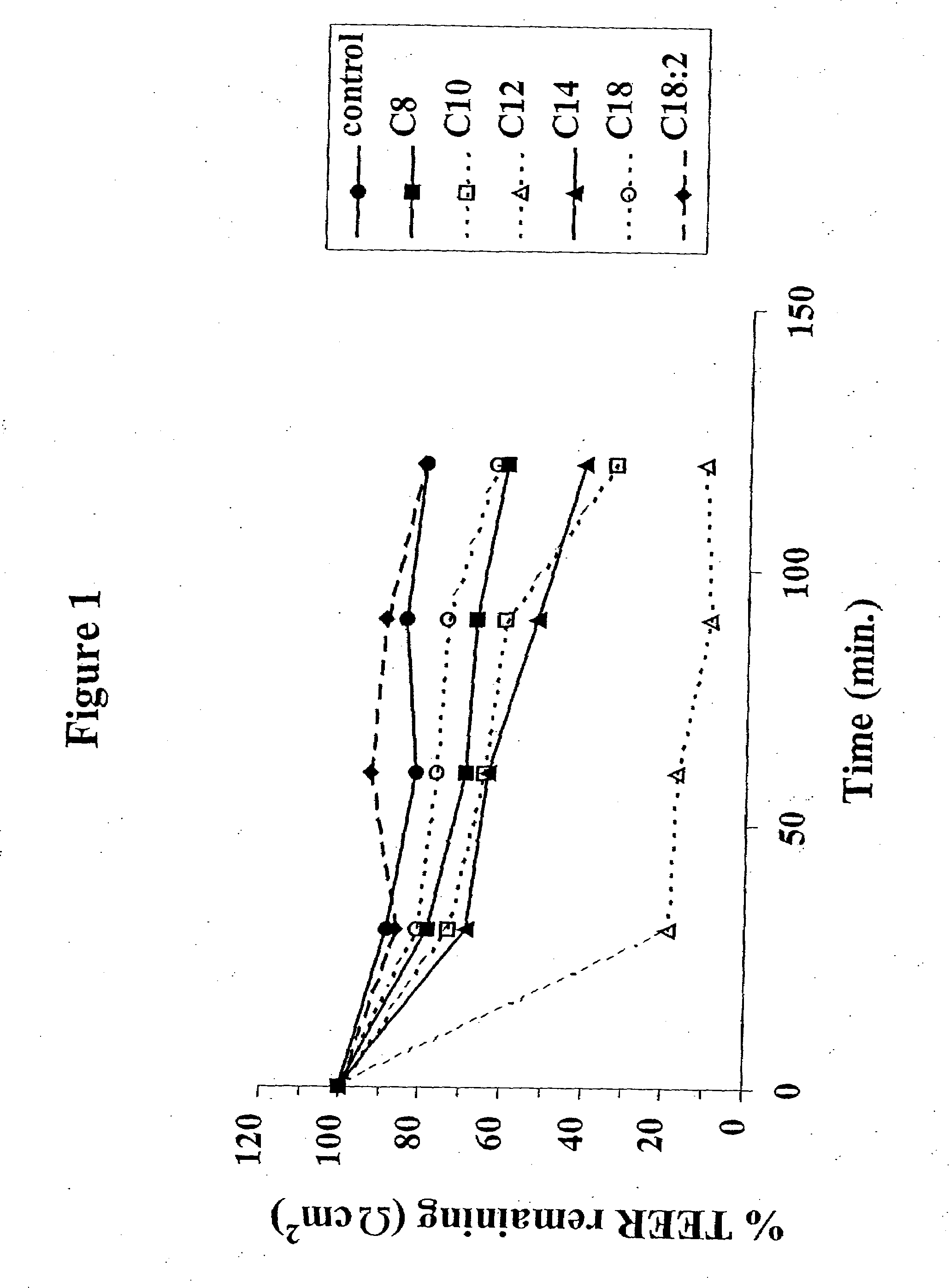
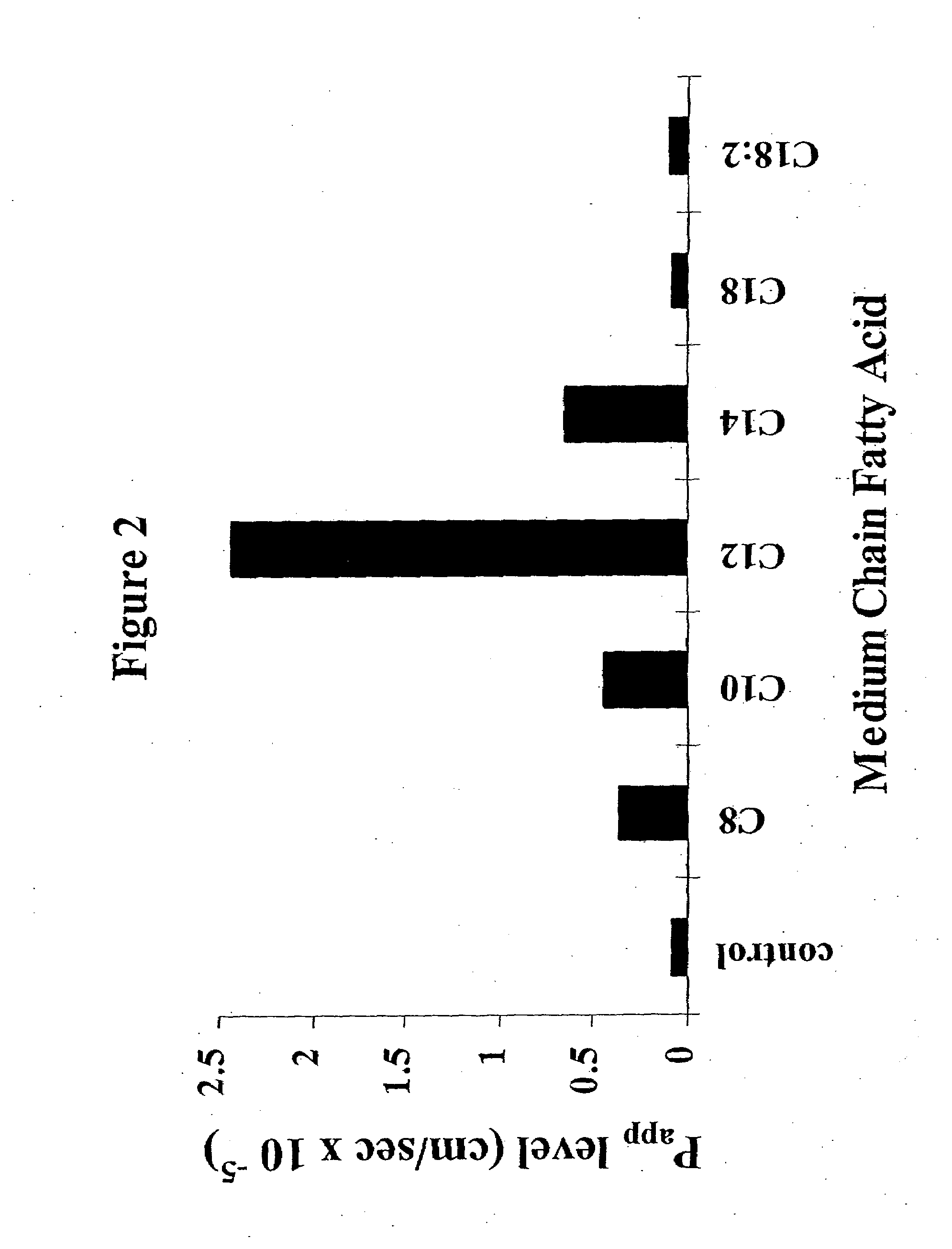
InactiveUS8119159B2Minimizes risk of local irritationImprove oral bioavailabilityBiocidePhosphorous compound active ingredientsDelayed Release Dosage FormCarbon chain
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to promote absorption of the bisphosphonate at the GIT cell lining. The enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms. Preferably, the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Bioeffective krill oil compositions
InactiveUS20080274203A1Increasing flesh colorationPromote growthBiocideMetabolism disorderInsulin resistanceAnti oxidant
This invention discloses new krill oil compositions characterized by having high amounts of phospholipids, astaxanthin esters and / or omega-3 contents. The krill oils are obtained from krill meal using supercritical fluid extraction in a two stage process. Stage 1 removes the neutral lipid by extracting with neat supercritical CO2 or CO2 plus approximately 5% of a co-solvent. Stage 2 extracts the actual krill oils by using supercritical CO2 in combination with approximately 20% ethanol. The krill oil materials obtained are compared with commercially available krill oil and found to be more bioeffective in a number of areas such as anti-inflammation, anti-oxidant effects, improving insulin resistances and improving blood lipid profile.
Owner:AKER BIOMARINE ANTARCTIC
Hydroxy acids based delivery systems for skin resurfacing and anti-aging compositions
This invention relates to in-situ preparation of the derivatives of various hydroxy acids (HA), such as alpha-(Alpha) Hydroxy Acids (AHA), beta-(Beta) Hydroxy Acids (BHA), and Poly-Hydroxy Acids (PHA) with certain skin beneficial organic hetero-atom bases and their application in skin resurfacing (exfoliation), and in the synergistic treatment and regulation of topical disorders of skin such as skin aging, wrinkles, acne, rosacea, age-spots, canker sores, striae distensae (stretch marks), pimples, skin redness, and dry skin conditions of cracking, flaking, and scaling. Most HA derivatives produced by the in-situ method do not cause skin irritation and skin redness effects that are commonly experienced with AHA and BHA, yet there is no loss of their skin beneficial effects. These compositions can be traditional water and oil emulsions, liposomes, suspensions, colloids, solutions, masks, muds, serums, sprays, gels, lotions, creams, cleansers, and anhydrous systems, thus offering a wide choice of formulations to meet their consumer appeal and acceptance requirements.
Owner:GUPTA SHYAM K
Combination methods of treating cancer
InactiveUS20070190022A1Dosage of each agent in a combination therapy can be reducedAntitumor effectBiocidePeptide/protein ingredientsAnticarcinogenOncology
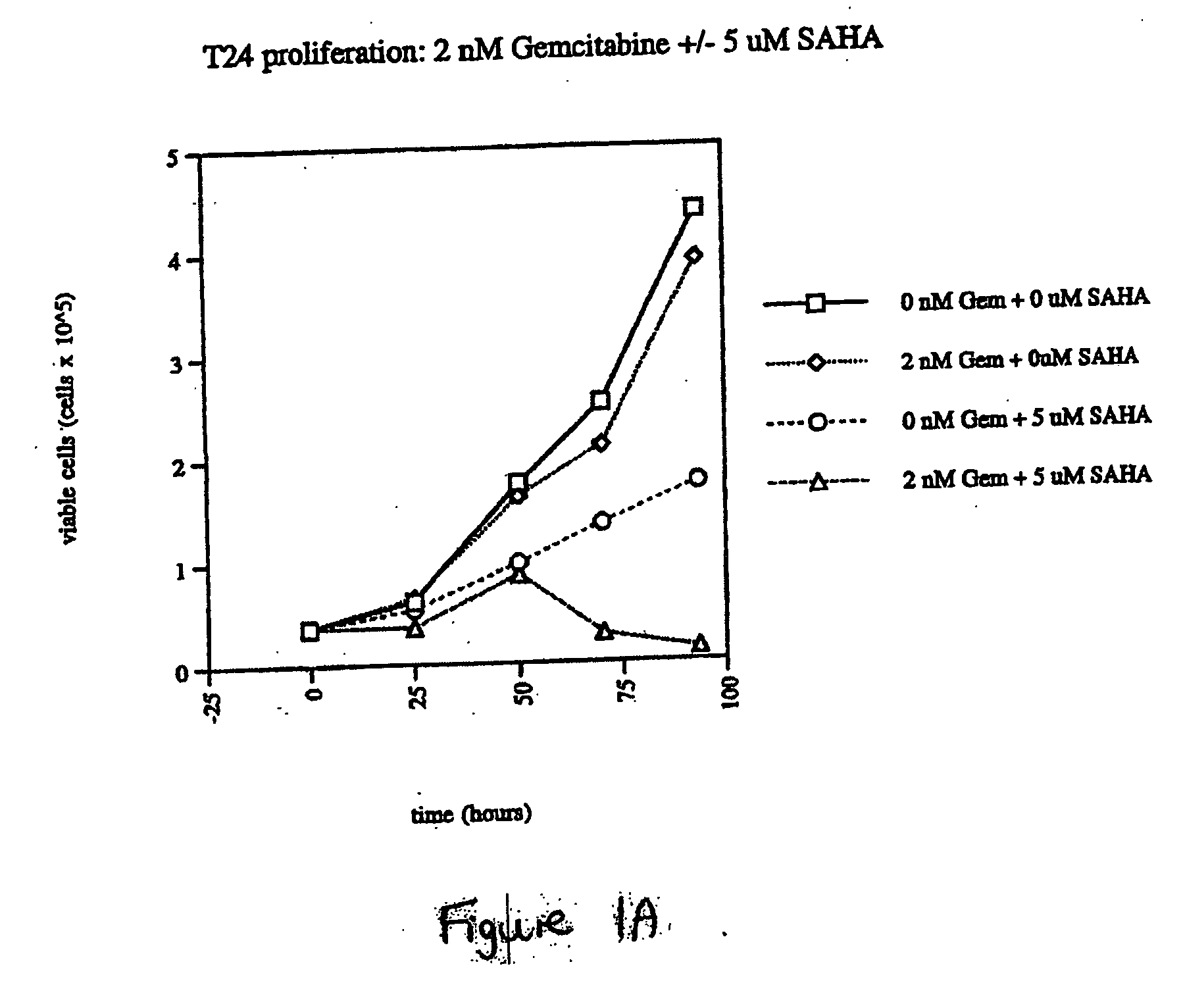
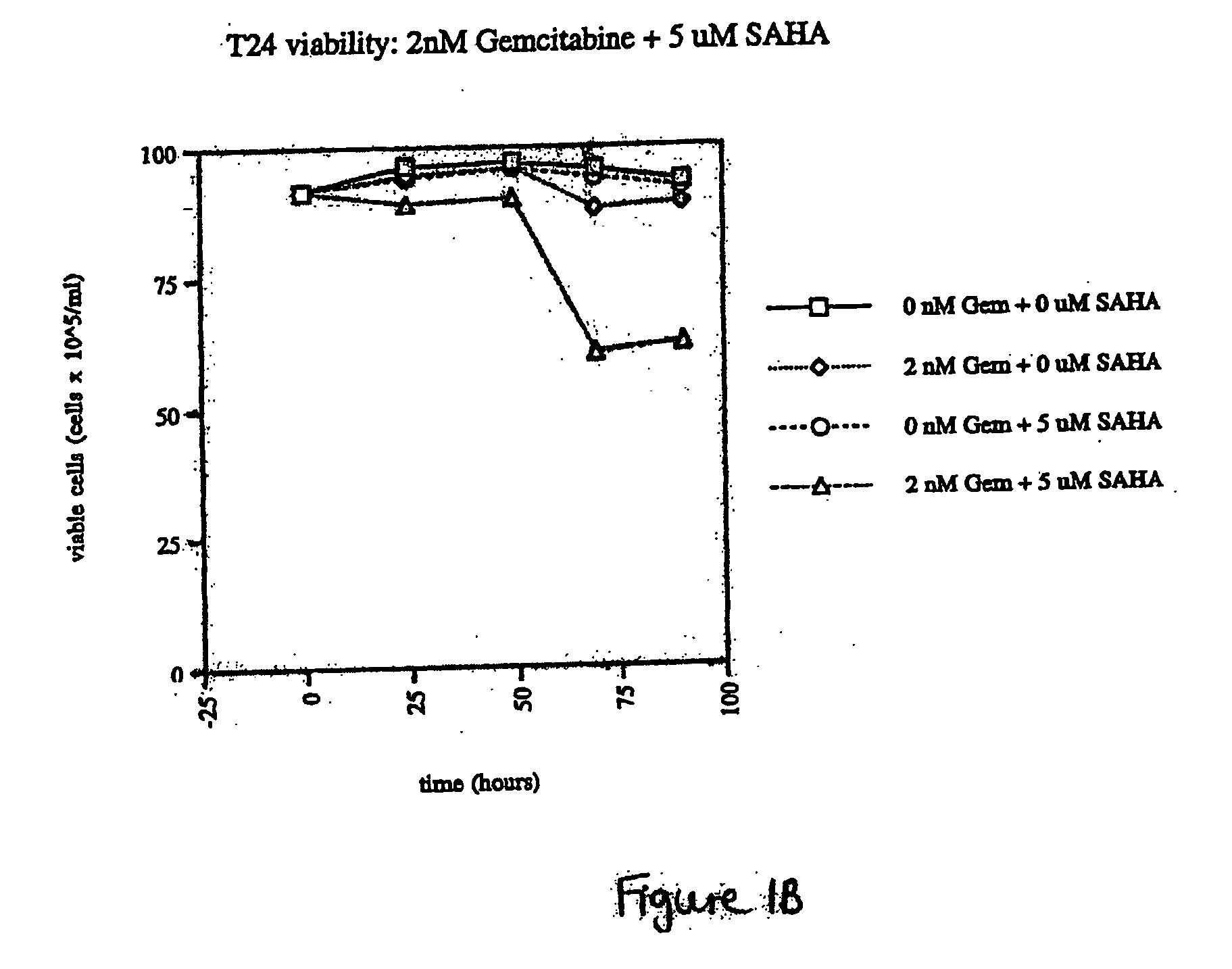
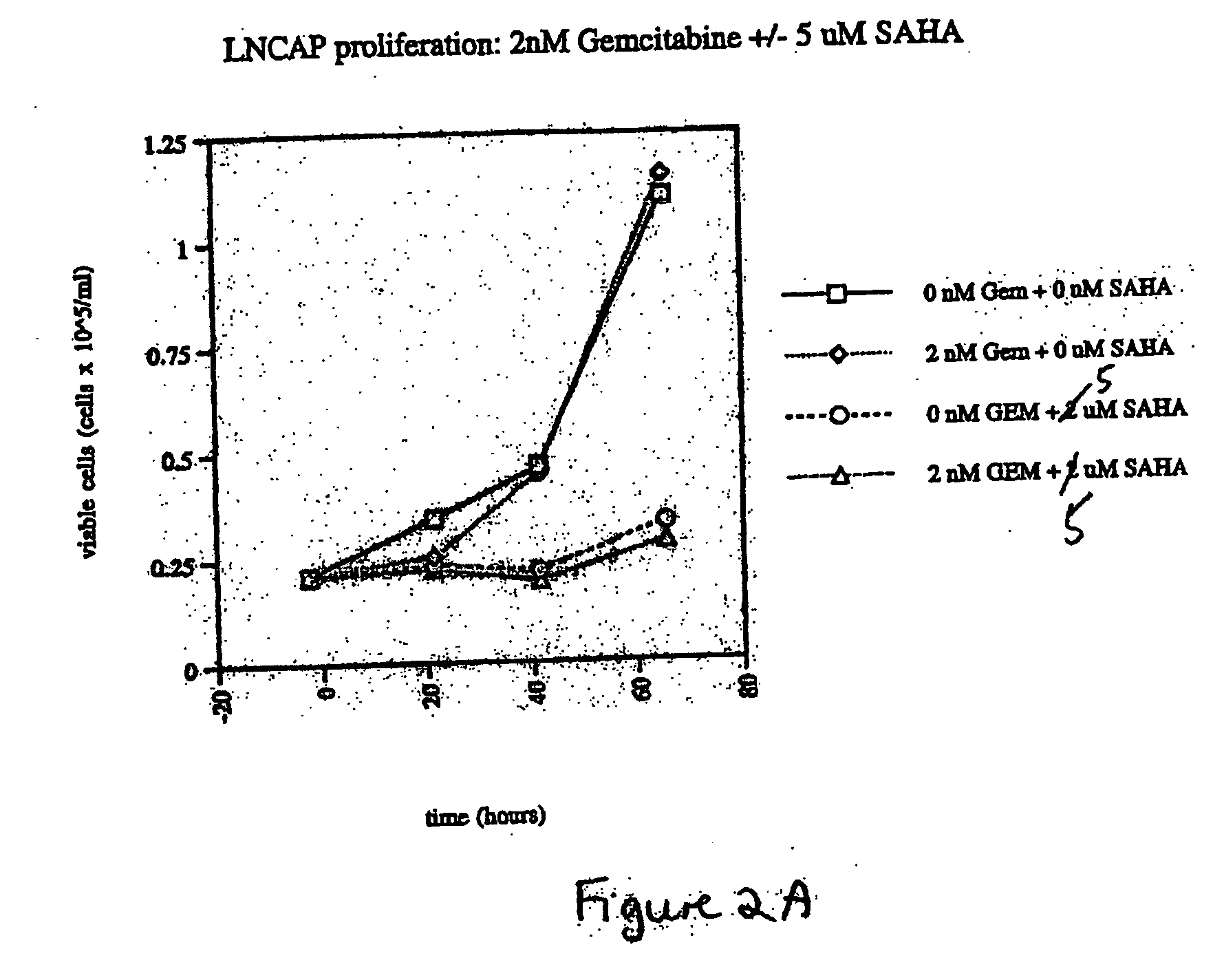
The present invention relates to a method of treating cancer in a subject in need thereof, by administering to a subject in need thereof a first amount of a histone deacetylase (HDAC) inhibitor or a pharmaceutically acceptable salt or hydrate thereof, in a first treatment procedure, and a second amount of an anti-cancer agent in a second treatment procedure. The first and second amounts together comprise a therapeutically effective amount. The effect of the HDAC inhibitor and the anti-cancer agent may be additive or synergistic.
Owner:SLOAN KETTERING INST FOR CANCER RES +1
Compounds, methods, and treatments for abnormal signaling pathways for prenatal and postnatal development
The present invention relates to prevention of congenital deformations. The invention further relates to cancer inhibition and prevention. The invention further relates to methods and compositions to modulate, antagonize, or agonize disparate signaling pathways that may converge to regulate patterning events and gene expression during prenatal development, post-natal development, and during development in the adult organism.
Owner:JENNINGS BARBARA BROOKE
Method of regulating glucose metabolism, and reagents related thereto
InactiveUS20030153509A1Reduce insulin resistanceExcellent hostBiocideDipeptide ingredientsAcute hyperglycaemiaChylomicron
The present invention provides methods and compositions for modification and regulation of glucose and lipid metabolism, generally to reduce insulin resistance, hyperglycemia, hyperinsulinemia, obesity, hyperlipidemia, hyperlipoprotein-emia (such as chylomicrons, VLDL and LDL), and to regulate body fat and more generally lipid stores, and, more generally, for the improvement of metabolism disorders, especially those associated with diabetes, obesity and / or atherosclerosis.
Owner:1149336 ONTARIO +2
Antimicrobial composition
An antimicrobial composition that involves a synergistic mixture in terms of active agents, of a primary antimicrobial agent, such as polyhexamethylene biguanide (PHMB), a secondary antimicrobial agent, and optionally an organic acid against various kinds of microbes is described. Various additional processing aids, such as alcohols and surfactants, may also be incorporated within the mixture. The composition allows one to use a significantly less concentration of individual constituent antimicrobial agents to achieve the same or a better degree of antimicrobial efficacy. The antimicrobial composition can be applied to the surface of almost any kind of substrate material, and can achieve a killing-efficacy of about 3 Log10 reduction in microbes within 30 minutes under ambient conditions.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Solid Oral Dosage Form Containing an Enhancer
InactiveUS20070238707A1Improve oral bioavailabilityMinimizes risk of local irritationBiocideAntipyreticDelayed Release Dosage FormDiphosphonates
The invention relates to a pharmaceutical composition and oral dosage forms comprising a bisphosphonate in combination with an enhancer to enhance intestinal delivery of the bisphosphonate to the underlying circulation. Preferably, the enhancer is a medium chain fatty acid or a medium chain fatty acid derivative having a carbon chain length of from 6 to 20 carbon atoms, and the solid oral dosage form is a controlled release dosage form such as a delayed release dosage form.
Owner:NOVO NORDISK AS
Treatment of Spinal Mechanical Pain
The invention is directed to a method of treating chronic spinal mechanical pain by intravenous administration to a subject in need of chronic spinal mechanical pain relief of an effective amount of bisphosphonate.
Owner:NEW YORK UNIV
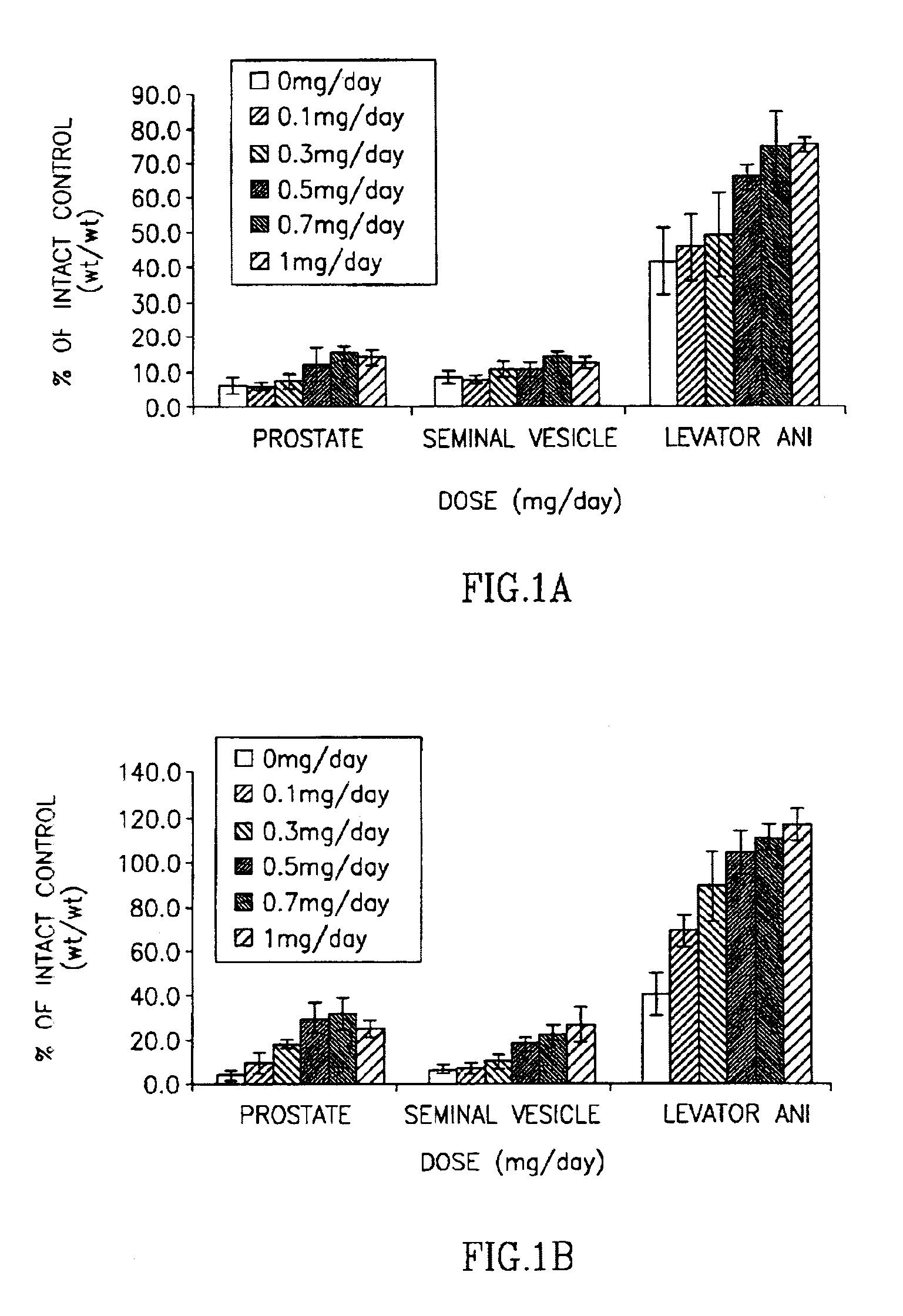
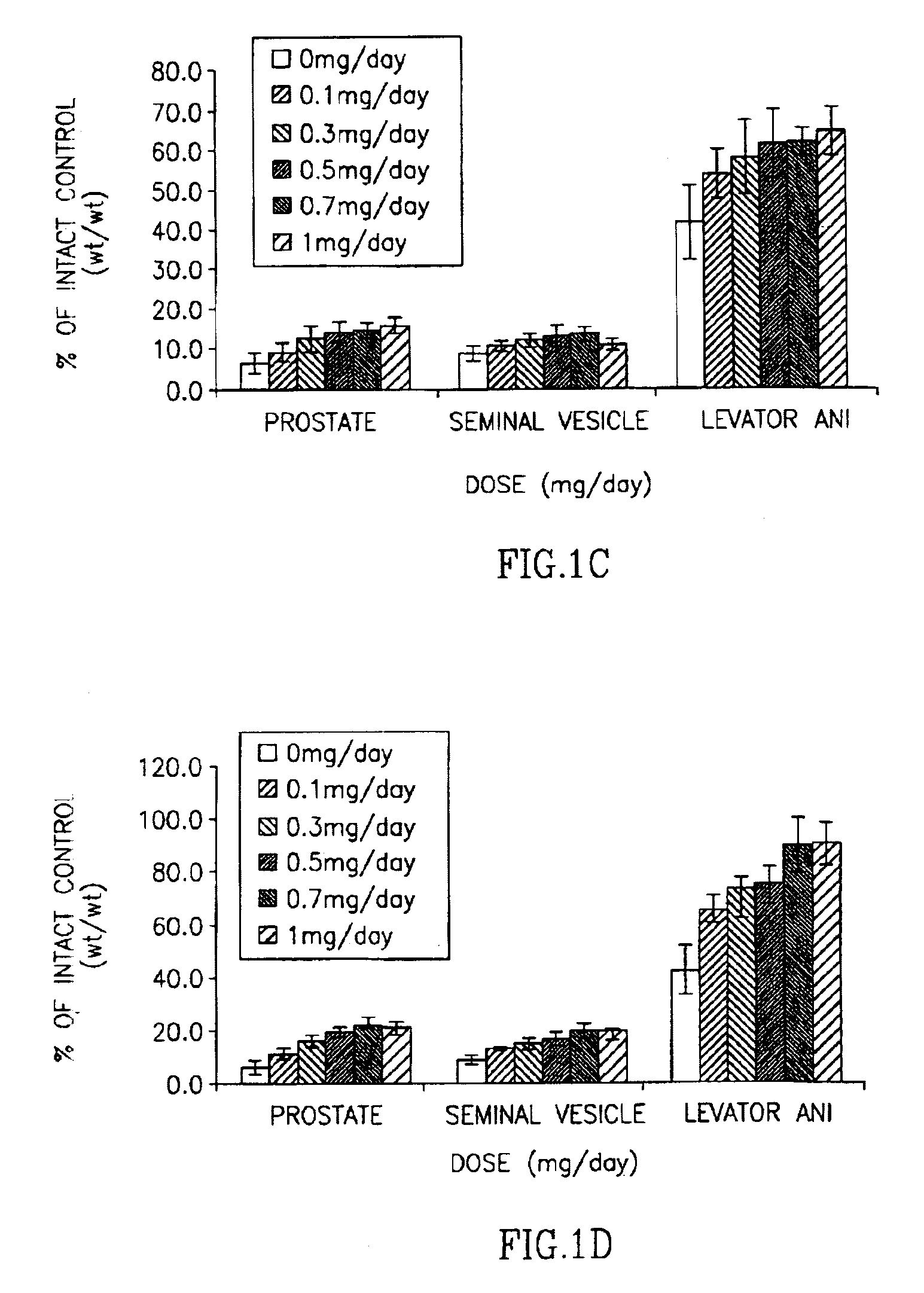
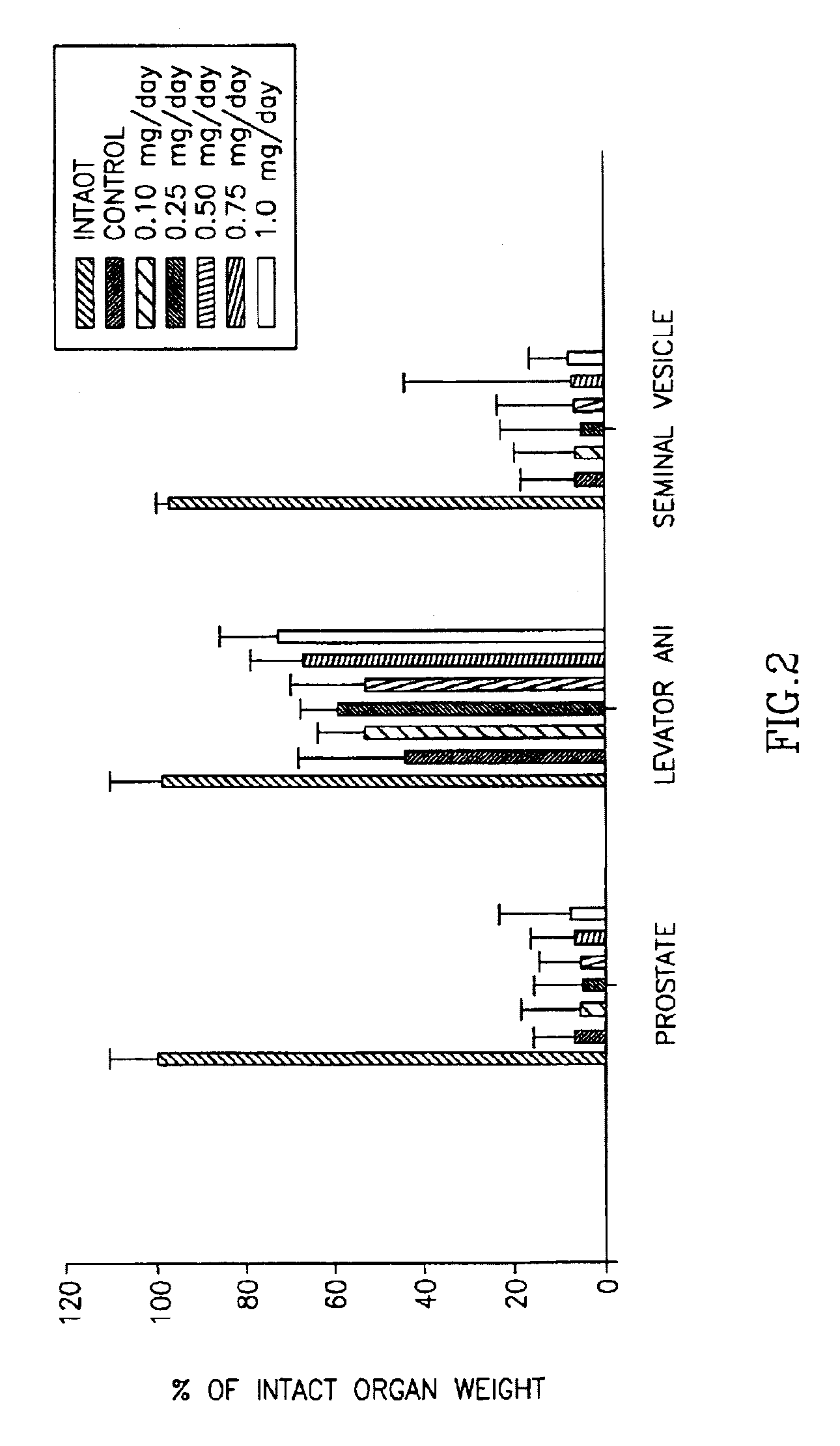
Halogenated selective androgen receptor modulators and methods of use thereof
InactiveUS7026500B2Unexpected anabolicUnexpected androgenicBiocideUrea derivatives preparationDiseaseAging male
This invention provides a class of androgen receptor targeting agents (ARTA). The agents define a new subclass of compounds, which are selective androgen receptor modulators (SARM). Several of the SARM compounds have been found to have an unexpected androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. Other SARM compounds have been found to have an unexpected antiandrogenic activity of a nonsteroidal ligand for the androgen receptor. The SARM compounds, either alone or as a composition, are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of acute and / or chronic muscular wasting conditions; e) preventing and / or treating dry eye conditions; f) oral androgen replacement therapy; and / or g) decreasing the incidence of, halting or causing a regression of prostate cancer.
Owner:UNIV OF TENNESSEE RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com