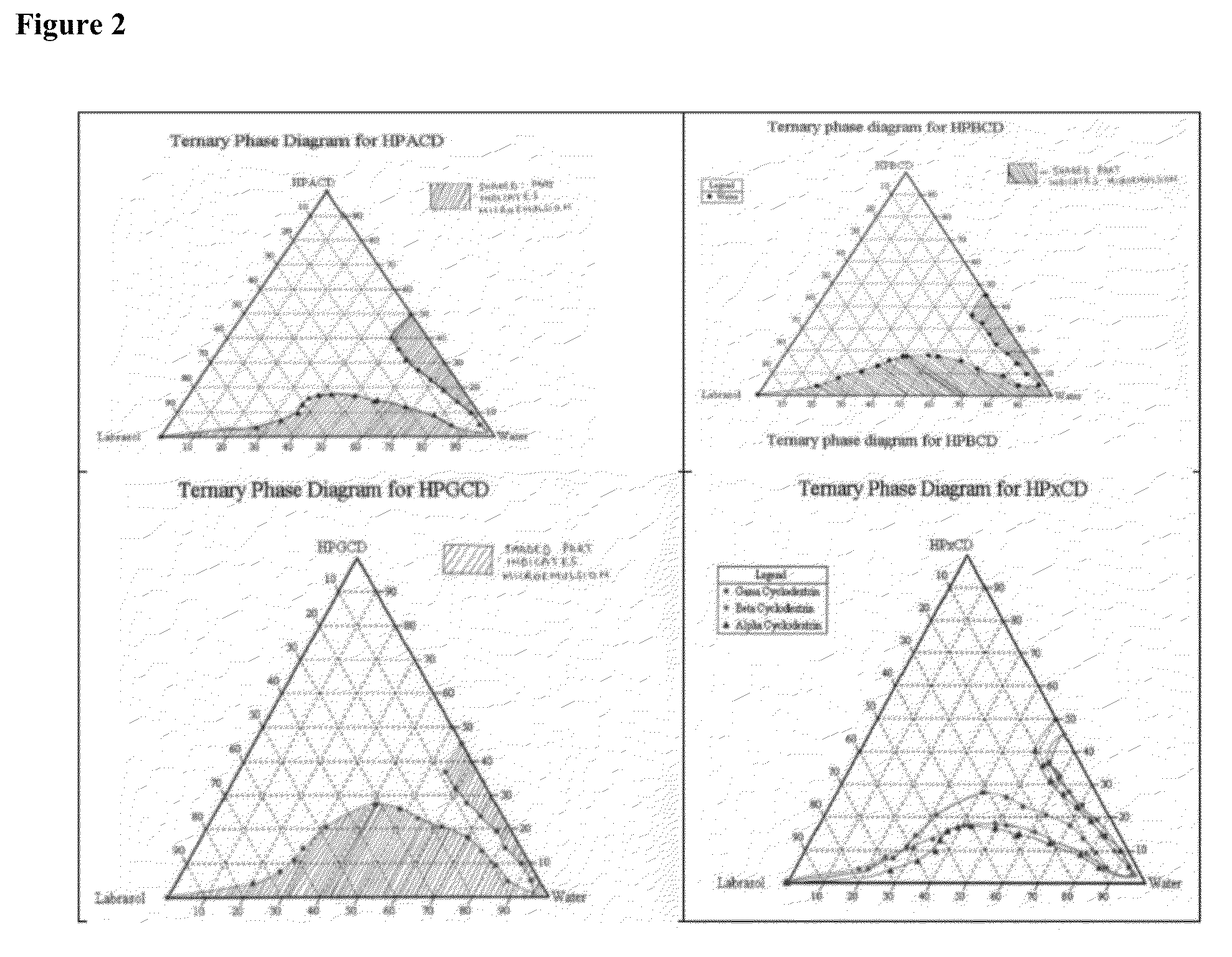
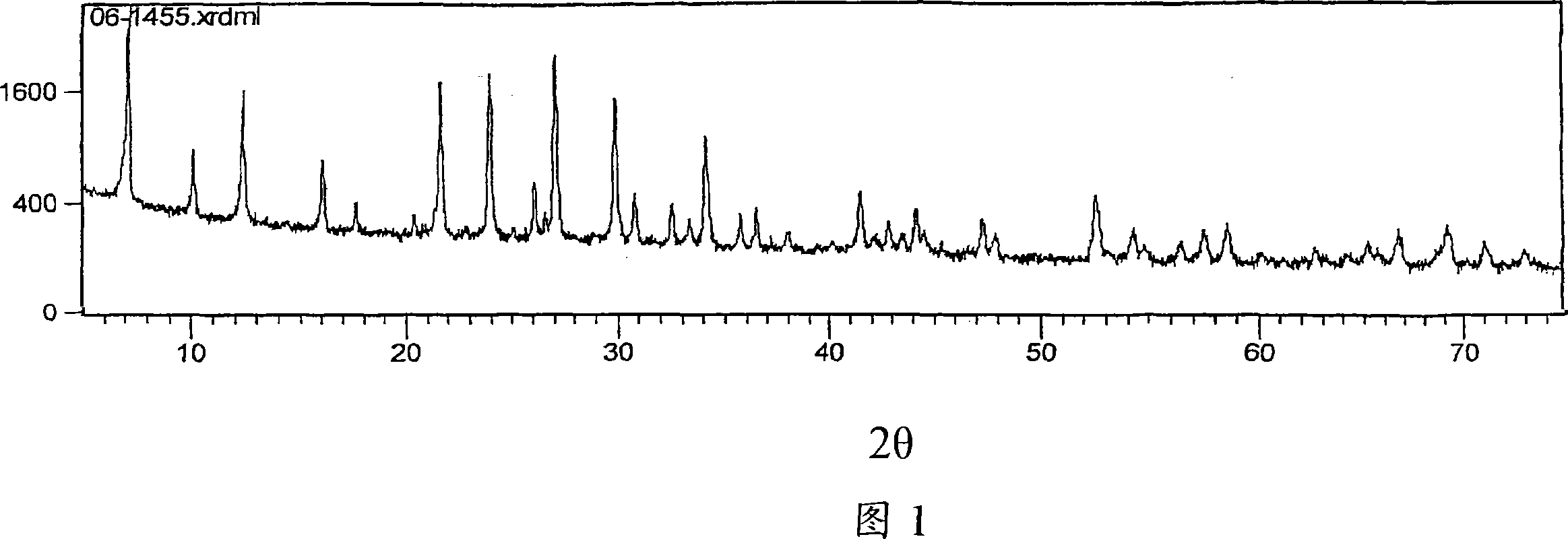
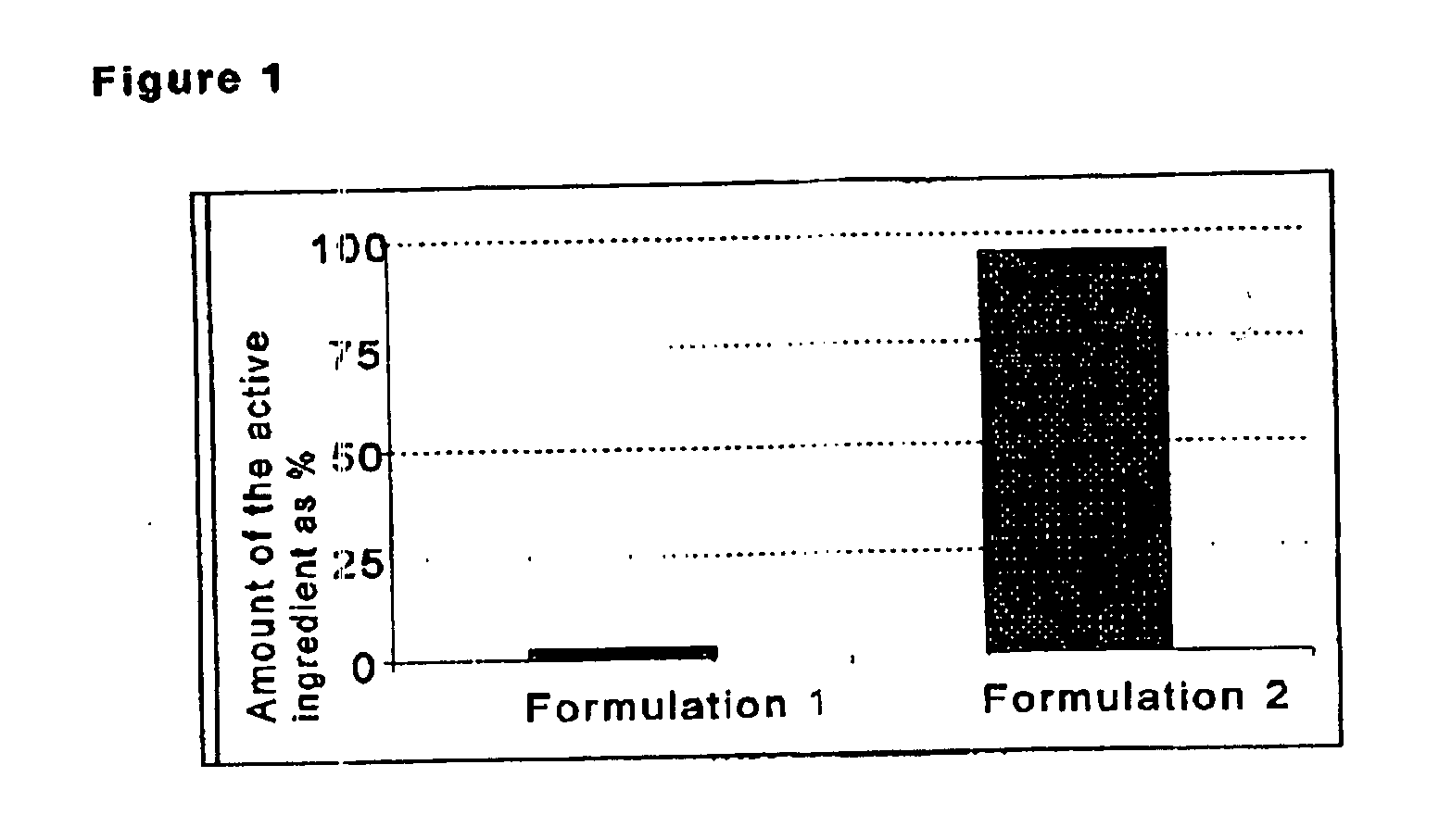
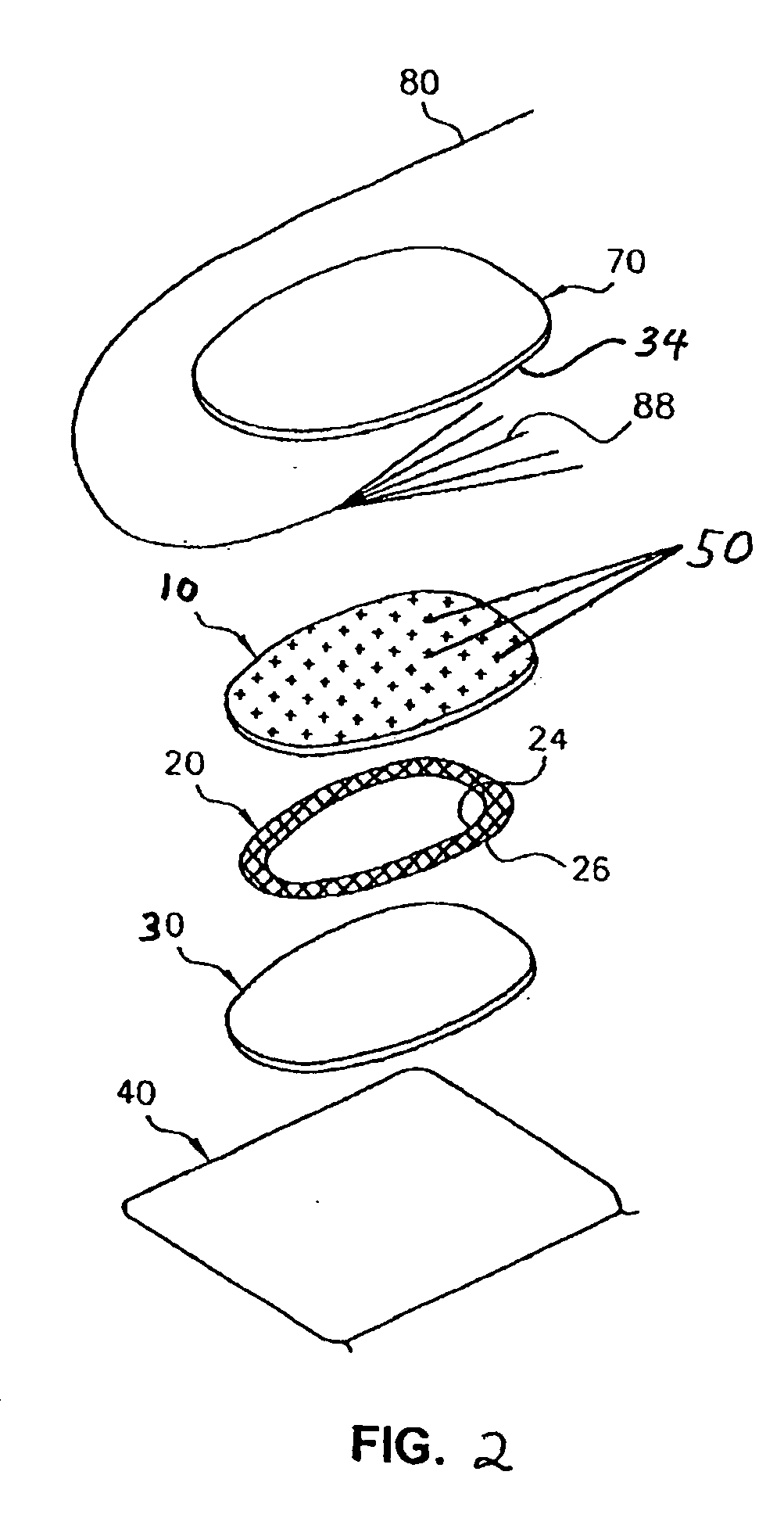
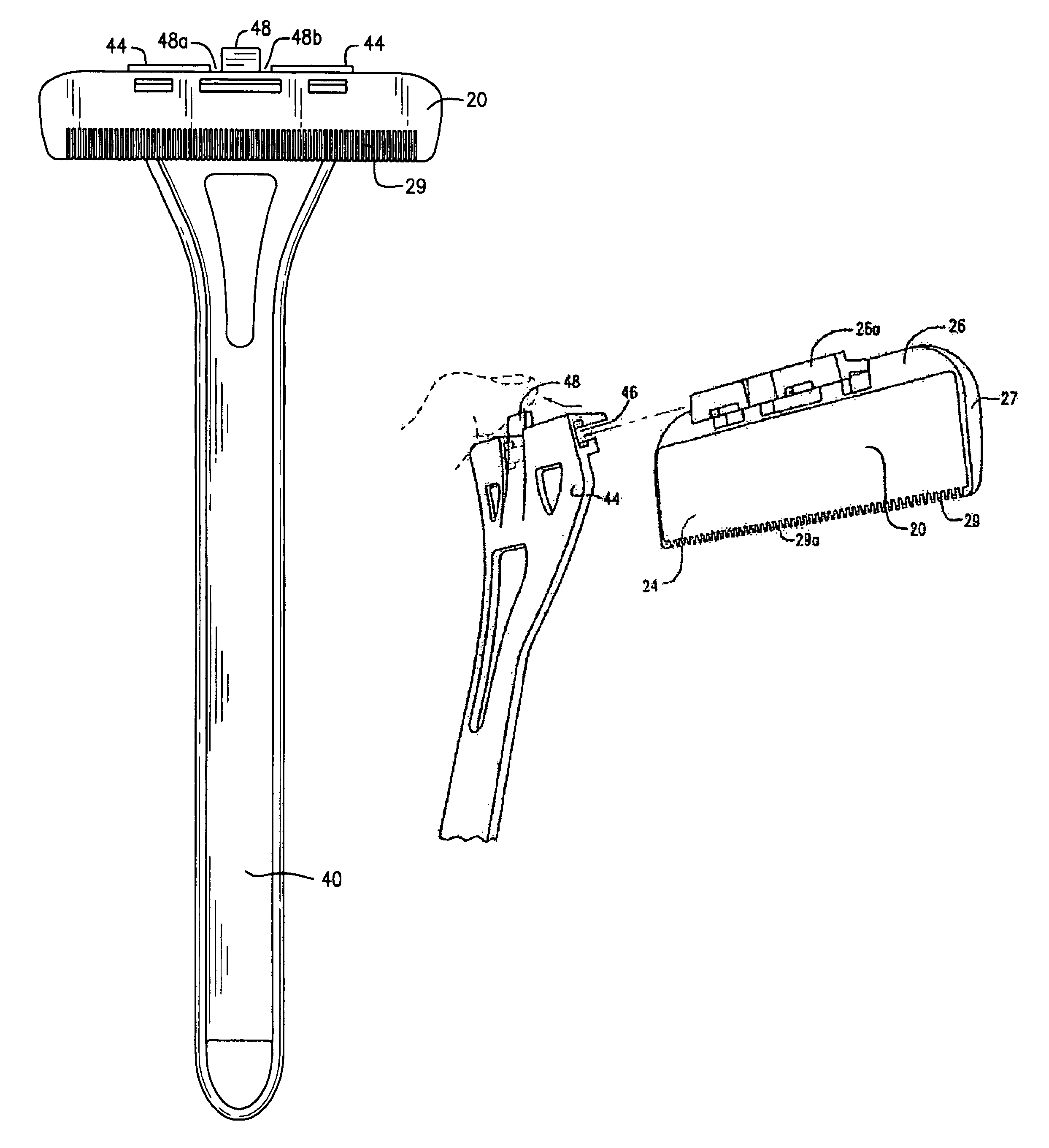
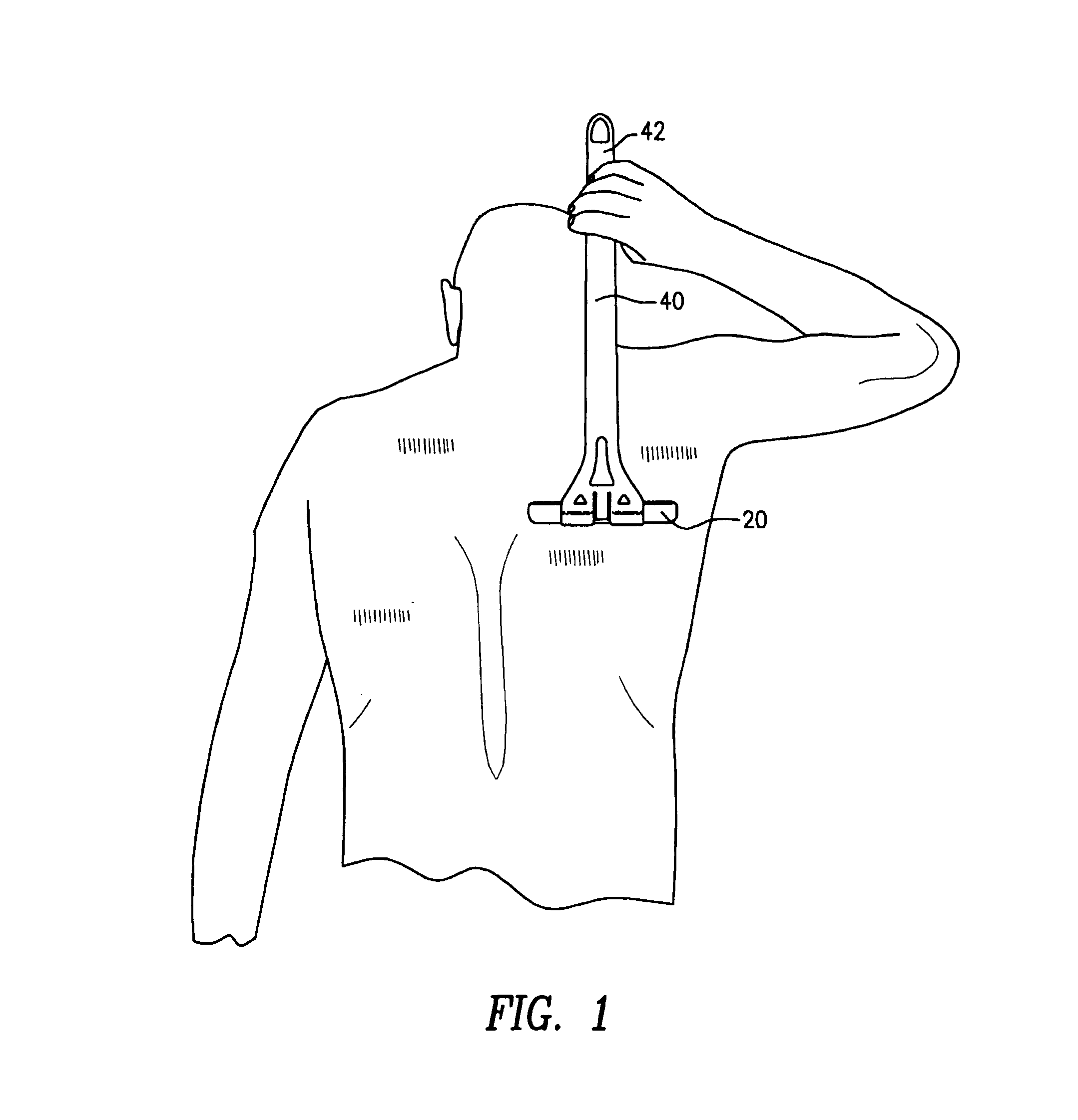
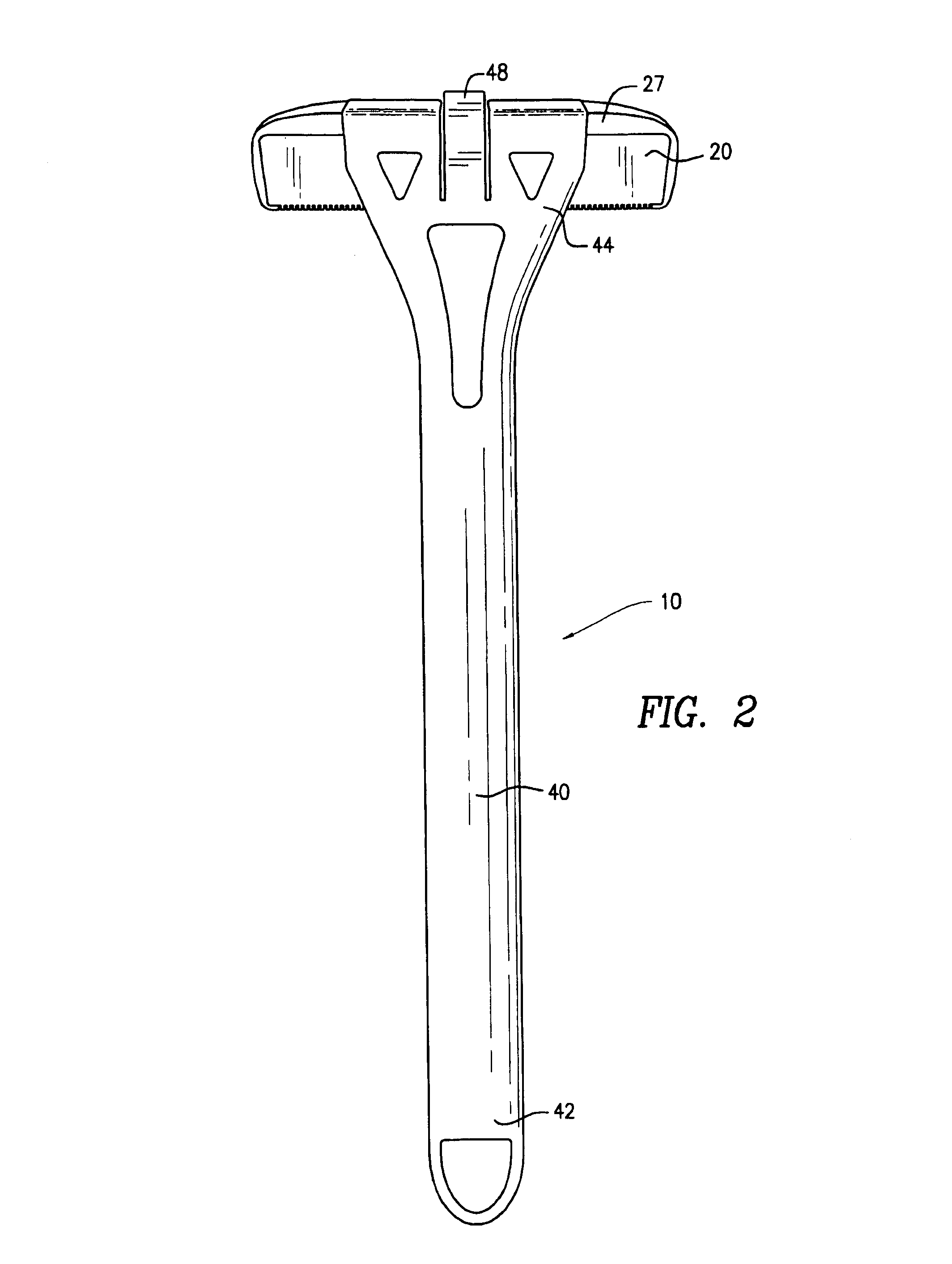
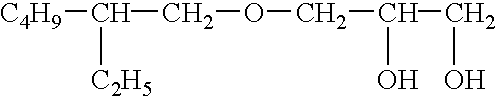Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
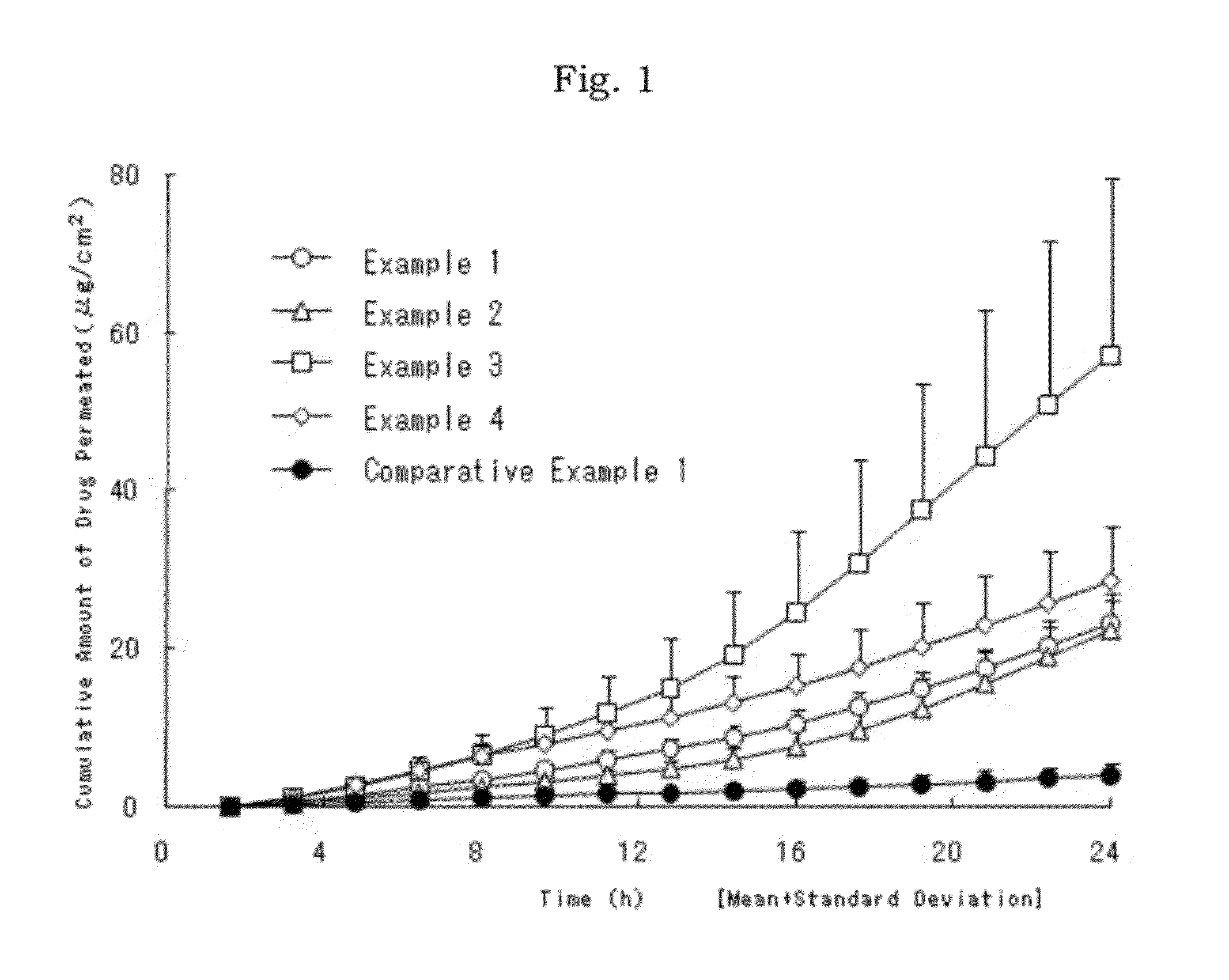
2414 results about "Skin irritation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Gentle-acting skin disinfectants
InactiveUS6846846B2Minimize skin irritationUnexpected antimicrobial effectivenessCosmetic preparationsBiocideOctoxyglycerinMedicine
Antimicrobial compositions having synergistic combinations of octoxyglycerin and at least one other antimicrobial agent in formulations which are more effective than prior art compositions without causing increased irritation to the skin of the average user. In certain embodiments, skin irritation may be minimized by low concentrations of antimicrobials and / or the presence of soothing compounds such as zinc. Preferred embodiments include combinations of octoxyglycerin, a quaternary compound, and at least one other antimicrobial agent. Without being bound to any particular theory, it is hypothesized that the unexpected antimicrobial effectiveness of combinations of octoxyglycerin may result from an enhancement of the permeability of microbes to antimicrobials caused by octoxyglycerin.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Skin abrader for biomedical electrode
InactiveUS6136008AEfficiently and effectively removesEfficiently and effectively removedElectrocardiographySurgical furnitureStratum corneumBiomedical engineering
A skin abrader for a biomedical electrode is disclosed wherein the abrader has a geometrically structured surface abrasive selected to provide sufficient abrasive effect to easily remove a portion of the stratum corneum of mammalian skin with minimal skin irritation, in order to reduce skin impedance encountered during diagnosis or monitoring of a mammalian patient.
Owner:BANK ONE N A +1
Penetration Enhancer Combinations for Transdermal Delivery
InactiveUS20070269379A1Easy to transportLess irritatingOrganic active ingredientsBiocideHigh-Throughput Screening MethodsIrritation
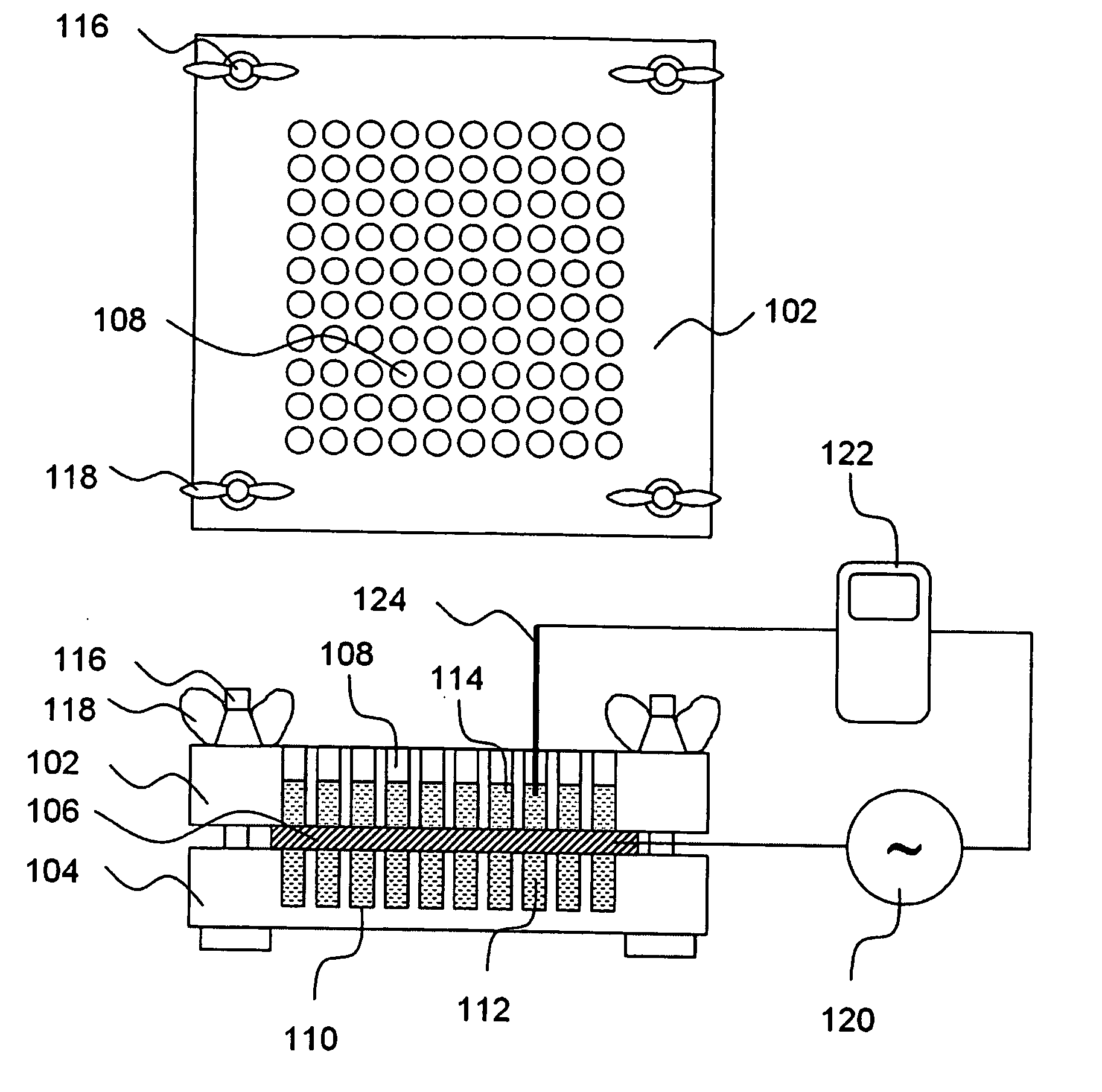
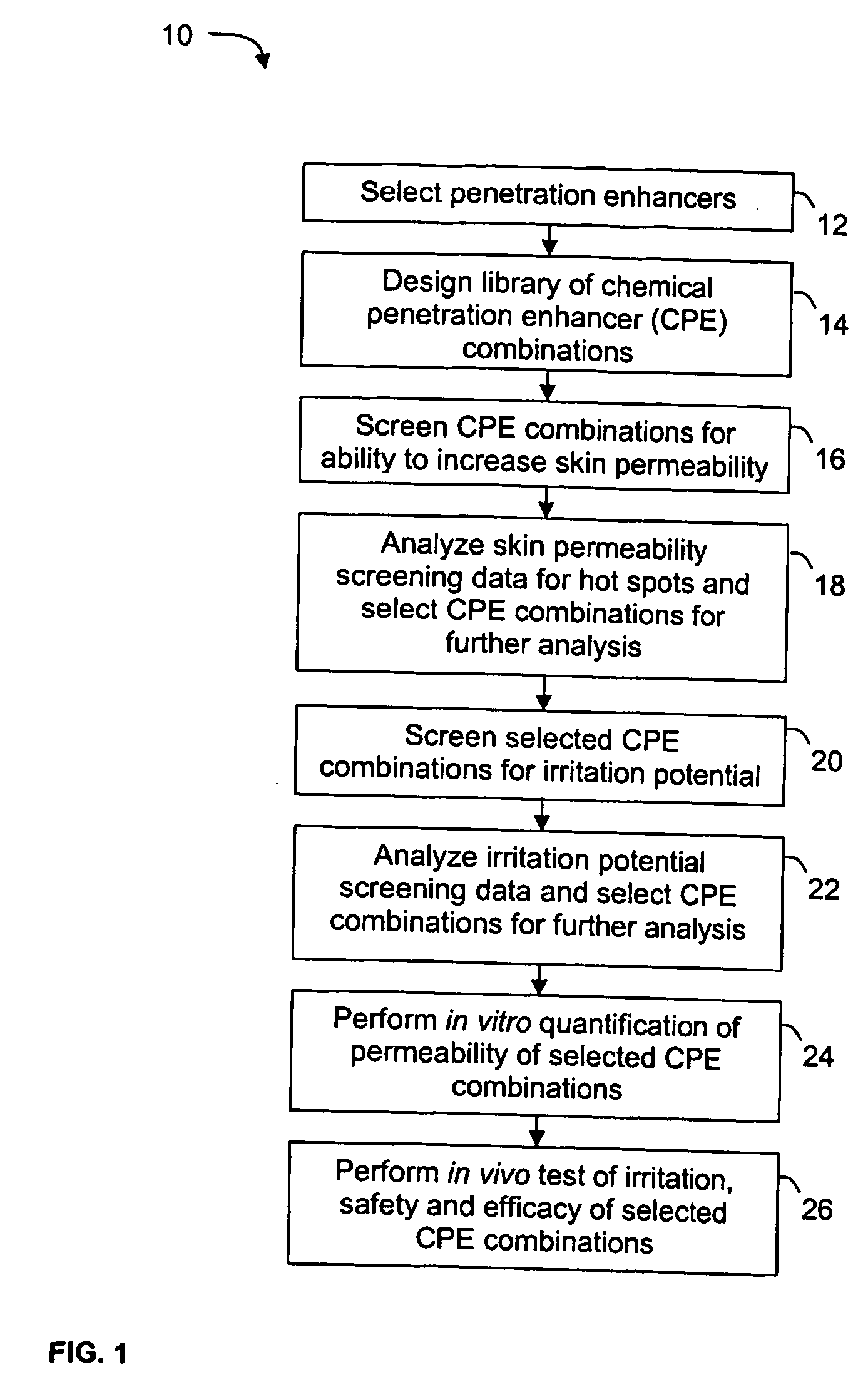
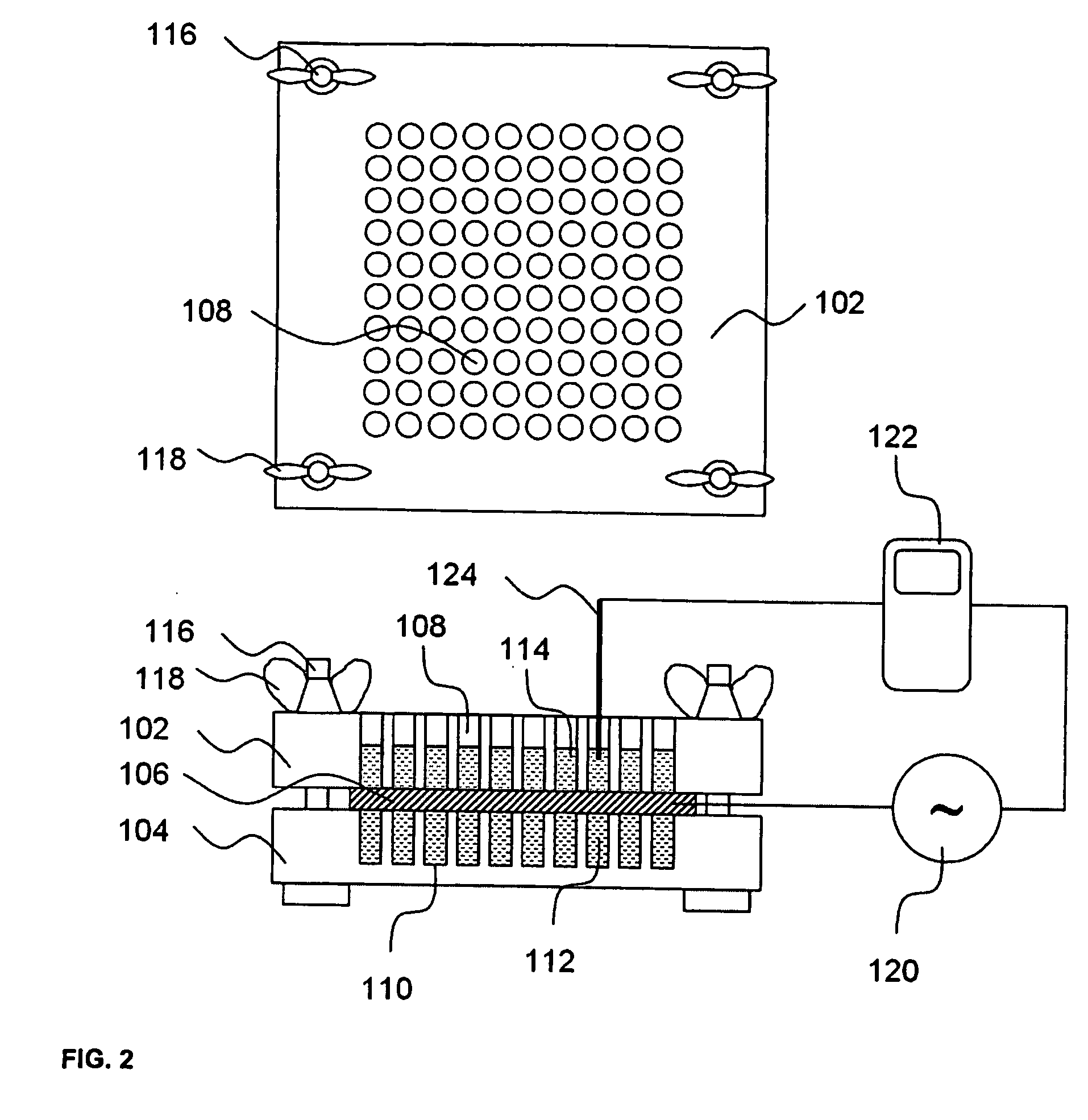
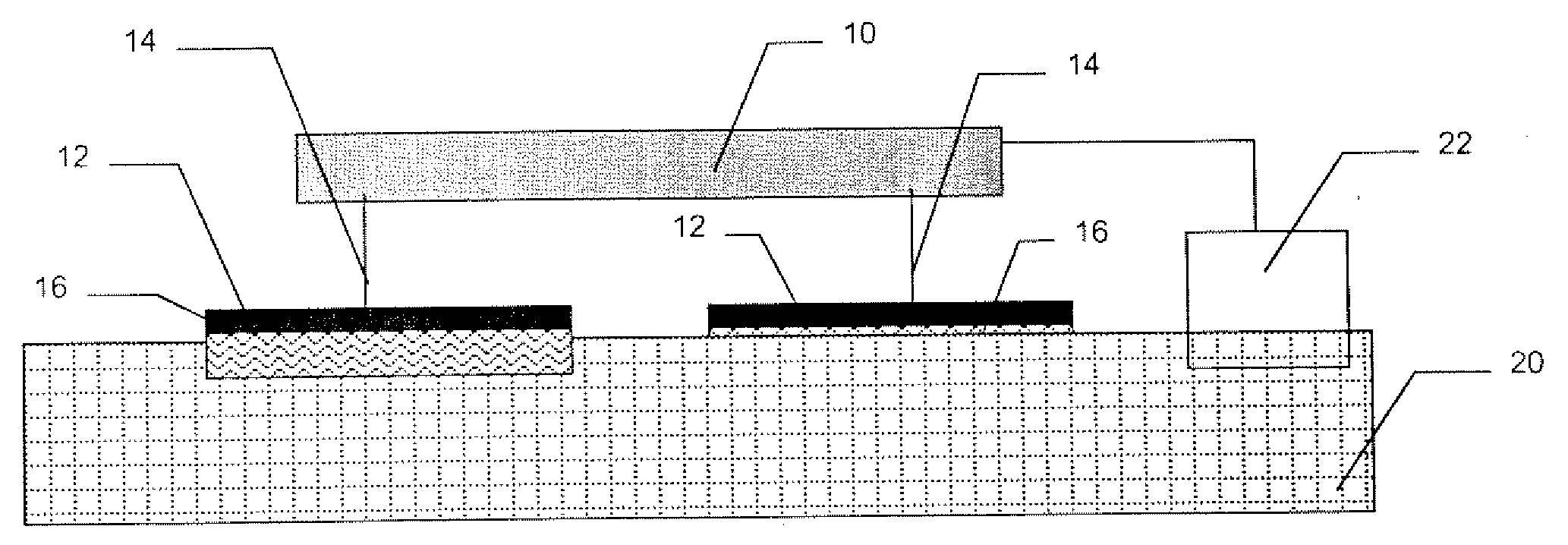
A high throughput screening and isolation system identifies rare enhancer mixtures from a candidate pool of penetration enhancer combinations. The combinations are screened for high penetration but low irritation potential using a unique data mining method to find new potent and safe chemical penetration enhancer combinations. The members of a library of chemical penetration enhancer combinations are screened with a high throughput device to identify “hot spots”, particular combinations that show higher chemical penetration enhancement compared to neighboring compositions. The irritation potentials of the hot spot combinations are measured to identify combinations that also show low irritation potential. A active component, such as a drug, is then combined with the combination in a formulation which is tested for the ability of the drug to penetrate into or through skin. It is then assessed whether the formulation can deliver the quantity of drug required, and animal tests are conducted to confirm in vivo the ability of the chemical penetration enhancer combinations to facilitate transport of sufficient active molecules across the skin to achieve therapeutic levels of the active molecule in the animal's blood. The invention provides specific unique and rare mixtures of chemical penetration enhancers that enhance skin permeability to hydrophilic macromolecules by more than 50-fold without inducing skin irritation, such as combinations of sodium laurel ether sulfate and 1-phenyl piperazine, and combinations of N-lauryl sarcosine and Span 20 / sorbitan monolaurate.
Owner:RGT UNIV OF CALIFORNIA
Microcurrent device with a sensory cue
The present invention is directed to an apparatus that includes a microcurrent delivery device portion and at least one independent sensory cue delivery means. The independent sensory cue delivery means can provide an independent sensory cue selected from the group consisting of vibration, heat, cool, skin irritation, tingling, fragrance or auditory.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Hydroxy acids based delivery systems for skin resurfacing and anti-aging compositions
This invention relates to in-situ preparation of the derivatives of various hydroxy acids (HA), such as alpha-(Alpha) Hydroxy Acids (AHA), beta-(Beta) Hydroxy Acids (BHA), and Poly-Hydroxy Acids (PHA) with certain skin beneficial organic hetero-atom bases and their application in skin resurfacing (exfoliation), and in the synergistic treatment and regulation of topical disorders of skin such as skin aging, wrinkles, acne, rosacea, age-spots, canker sores, striae distensae (stretch marks), pimples, skin redness, and dry skin conditions of cracking, flaking, and scaling. Most HA derivatives produced by the in-situ method do not cause skin irritation and skin redness effects that are commonly experienced with AHA and BHA, yet there is no loss of their skin beneficial effects. These compositions can be traditional water and oil emulsions, liposomes, suspensions, colloids, solutions, masks, muds, serums, sprays, gels, lotions, creams, cleansers, and anhydrous systems, thus offering a wide choice of formulations to meet their consumer appeal and acceptance requirements.
Owner:GUPTA SHYAM K
Direct emulsion for bleaching hair
ActiveUS20060242773A1Prevent degradationEasy accessCosmetic preparationsHair cosmeticsSolubilityFiber
A direct emulsion for bleaching keratin fibers, preferably human keratin fibers such as hair, having an inert phase containing of a nonoxygenated and nonperfluorinated liquid compound having a water-solubility at 25° C. of less than 1% and an aqueous hydrogen peroxide solution, a bleaching method using this direct emulsion, as it is or in the form of a ready-to-use composition, and the use of this direct emulsion for bleaching keratin fibers. The direct emulsion in accordance with the invention makes it possible to rapidly obtain substantial lightening of keratin fibers while limiting the degradation of the keratin fibers and skin irritation.
Owner:LOREAL SA
Fabric or garment containing fecal enzyme inhibitor
Skin irritation, such as diaper rash, appearing when the skin is allowed to remain in contact with proteolytic enzymes found in feces is prevented by inactivating the fecal proteolytic enzymes by contact with organophilic clays. The organophilic clays are applied to the skin in areas likely to come into contact with feces or to garments such as diapers. A composition suitable for practicing the method of the invention comprises an amount of an organophilic clay effective to inactivate irritating fecal proteolytic enzymes dispersed in a pharmaceutically acceptable non-toxic dermatological vehicle. A fabric incorporating organophilic clay, preferably dispersed in a matrix of a superabsorbent polymer is useful for preparing diapers for infants that can help to prevent skin irritation by fecal enzymes.
Owner:SUD CHEM INC
Microstructures and method for treating and conditioning skin which cause less irritation during exfoliation
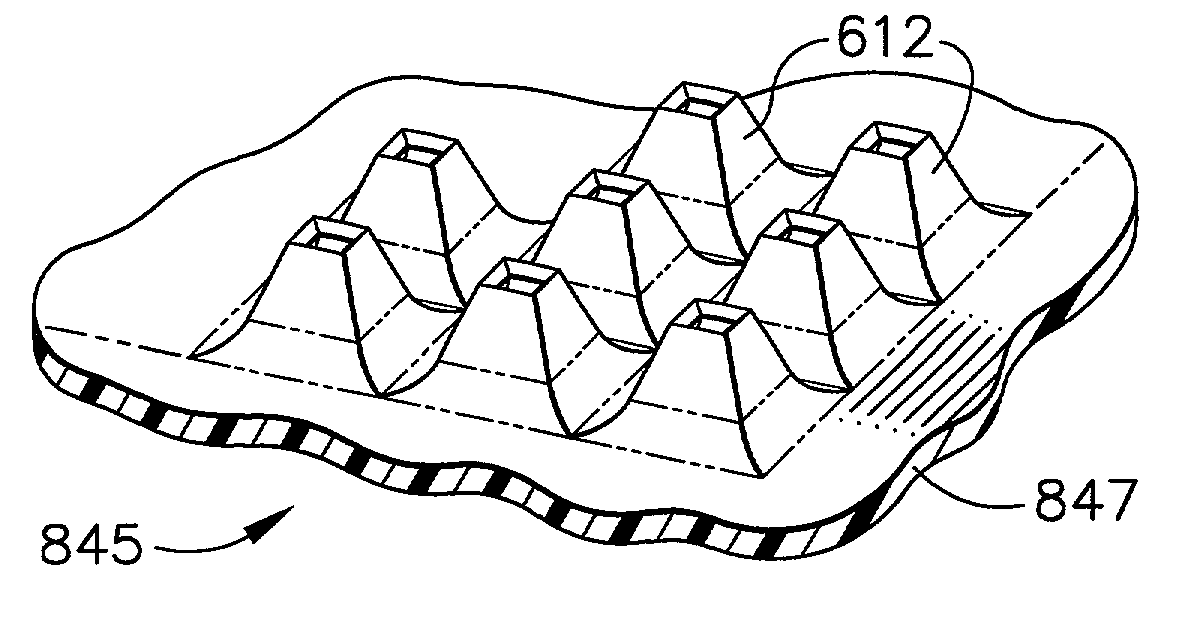
An improved method is provided to enhance skin appearance and health, in which skin is cleaned (or exfoliated) and conditioned by use of microelements affixed to a base element or hand-held patch. For the microstructure used in the improved method, the dimensions of the microelements are controlled so as to remove a certain number of layers of skin cells and to accumulate and retain those skin cells, along with other foreign substances, into areas between the microelements. In another embodiment of the improved method, a conditioning compound or therapeutic active can be applied to the exfoliated skin to enhance the skin. Moreover, the amount of skin cells accumulated using the improved method represents a self-limiting maximum quantity that cannot be substantially exceeded regardless of the number of attempts by a user to re-use the microstructure apparatus associated with the improved method. Some of the microelement shapes are purposefully designed with distal ends that exhibit sharp edges rather than sharp tips to reduce skin irritation resulting from use of the improved method.
Owner:CORIUM PHARMA SOLUTIONS INC
Wound or surgical dressing
InactiveUS20070141130A1Improve efficiencyImprove bindingBiocideAdhesive dressingsOligomerSurgical incision
A wound or surgical dressing is disclosed. The wound or surgical dressing is configured to cover or surround a wound, a surgical incision, or any type of skin irritation. In accordance with the present disclosure, the wound or surgical dressing is treated with a bacteriostatic composition that is capable of binding and trapping negatively charged matter, such as bacteria, pathogens, and the like. The bacteriostatic composition comprises a cationic polymer, a cationic oligomer, or particles coated with a cationic material. The bacteriostatic composition is bonded to the wound or surgical dressing in a manner such that the bacteriostatic composition is not substantially transferred to a patient being treated.
Owner:AVENT INC
Moisturizing sunless tanning composition
InactiveUS20070160548A1Mitigates and eliminates skin irritationMitigates and eliminates and tanning active occlusionCosmetic preparationsHair removalMedicineActive systems
There is provided a sunless tanning composition having a tanning active system and a moisturizing system. The moisturizing system is oil-free, which mitigates and / or eliminates skin irritation and / or tanning active occlusion.
Owner:PLAYTEX PROD INC
Gentle-acting skin-disinfectants
InactiveUS20030152644A1Minimize skin irritationUnexpected antimicrobial effectivenessBiocideCosmetic preparationsOctoxyglycerinDisinfectant
Antimicrobial compositions having synergistic combinations of octoxyglycerin and at least one other antimicrobial agent in formulations which are more effective than prior art compositions without causing increased irritation to the skin of the average user. In certain embodiments, skin irritation may be minimized by low concentrations of antimicrobials and / or the presence of soothing compounds such as zinc. Preferred embodiments include combinations of octoxyglycerin, a quaternary compound, and at least one other antimicrobial agent. Without being bound to any particular theory, it is hypothesized that the unexpected antimicrobial effectiveness of combinations of octoxyglycerin may result from an enhancement of the permeability of microbes to antimicrobials caused by octoxyglycerin.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Atraumatic high-retention headpiece
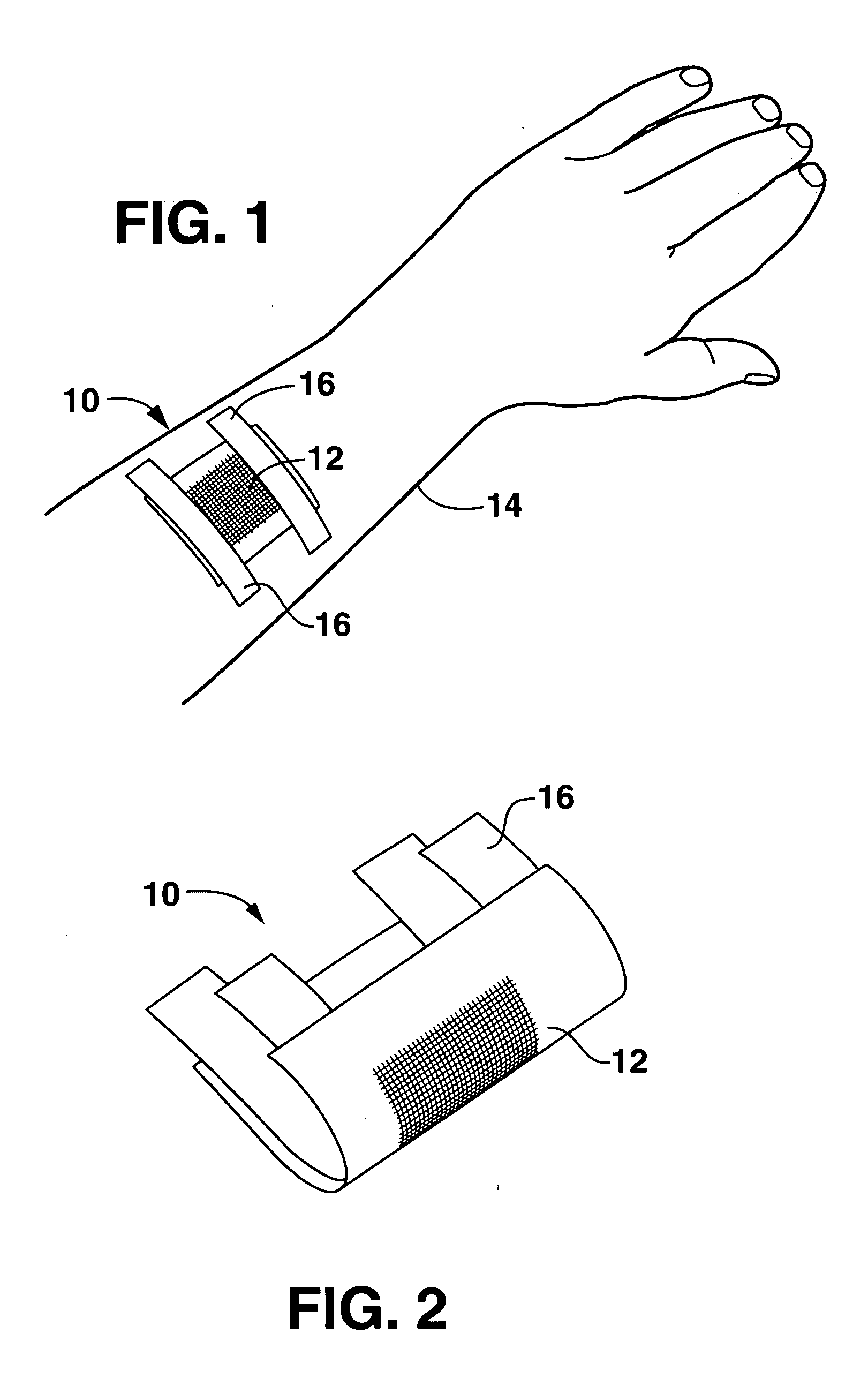
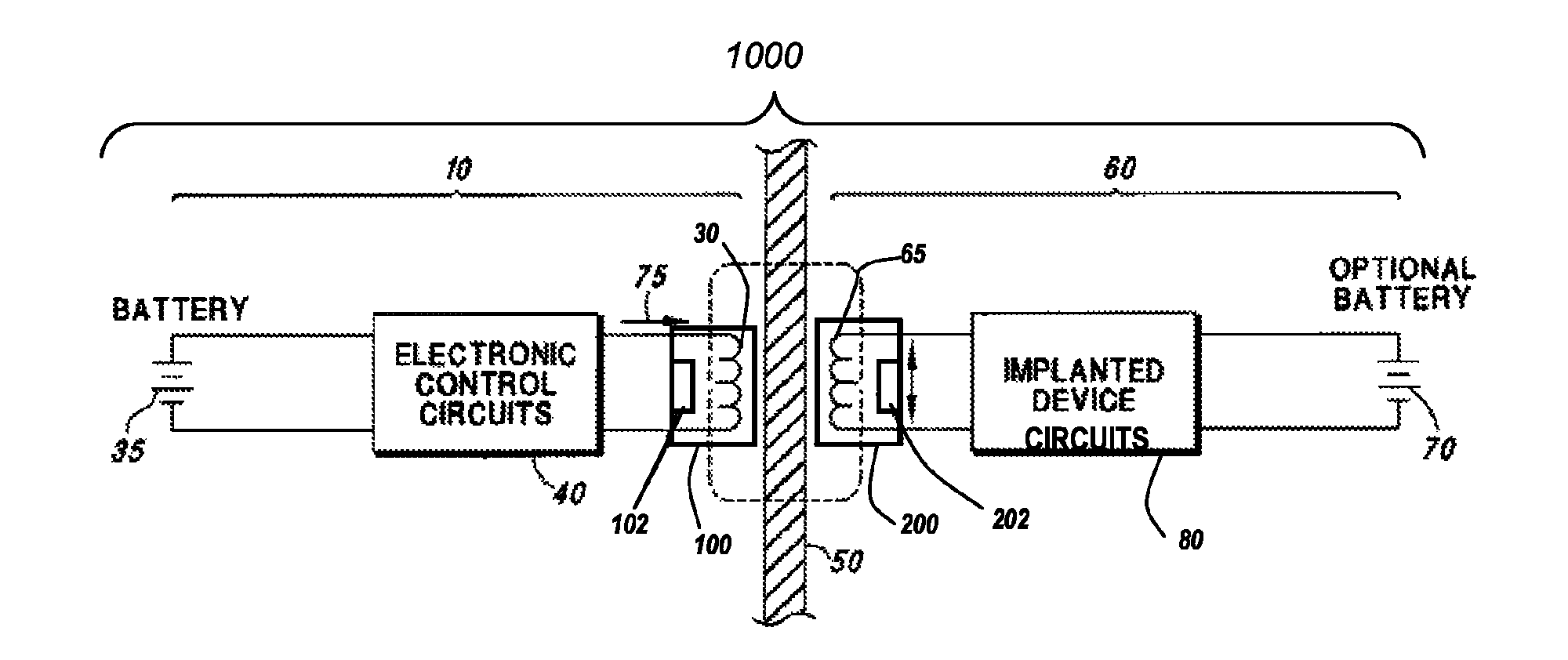
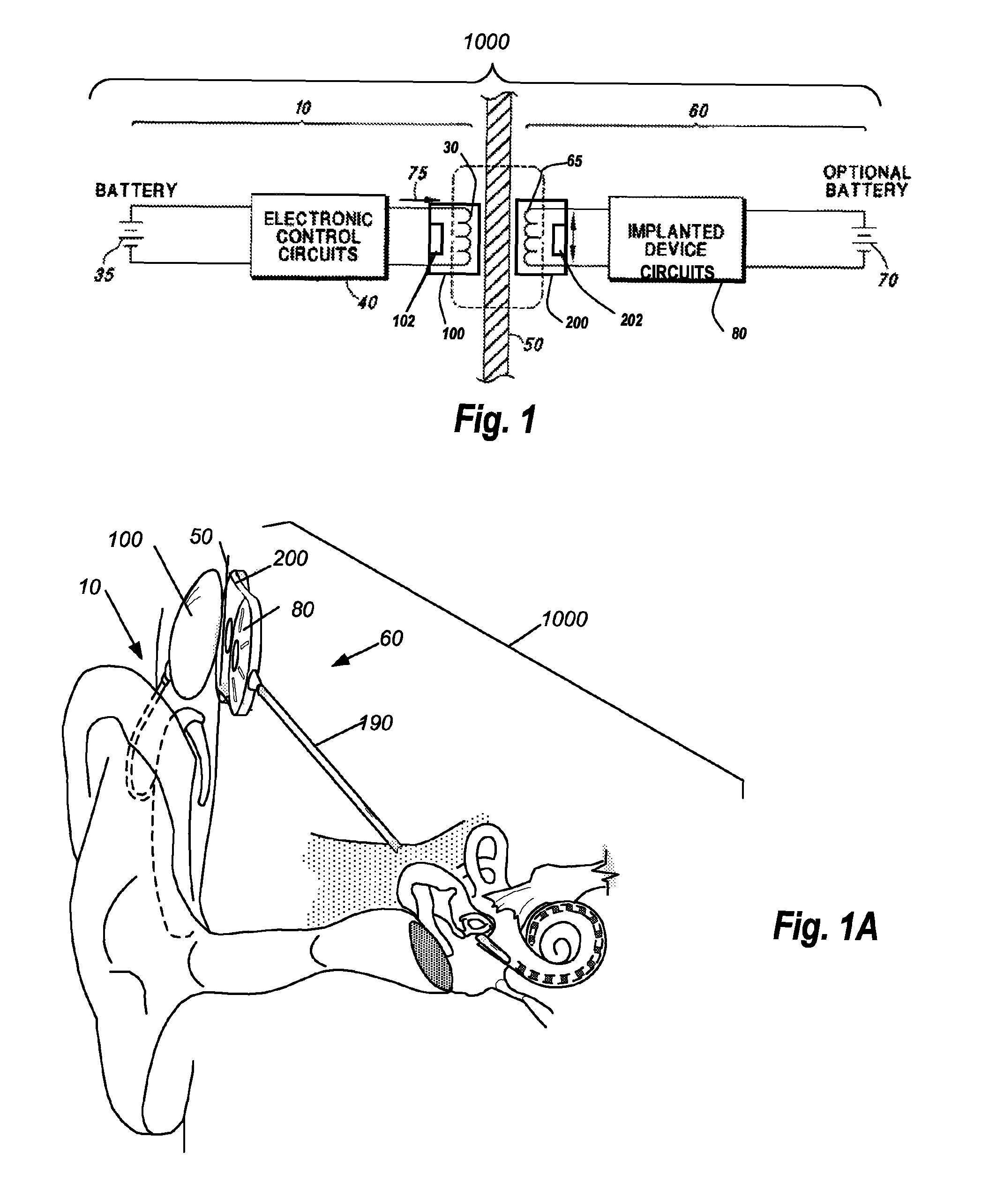
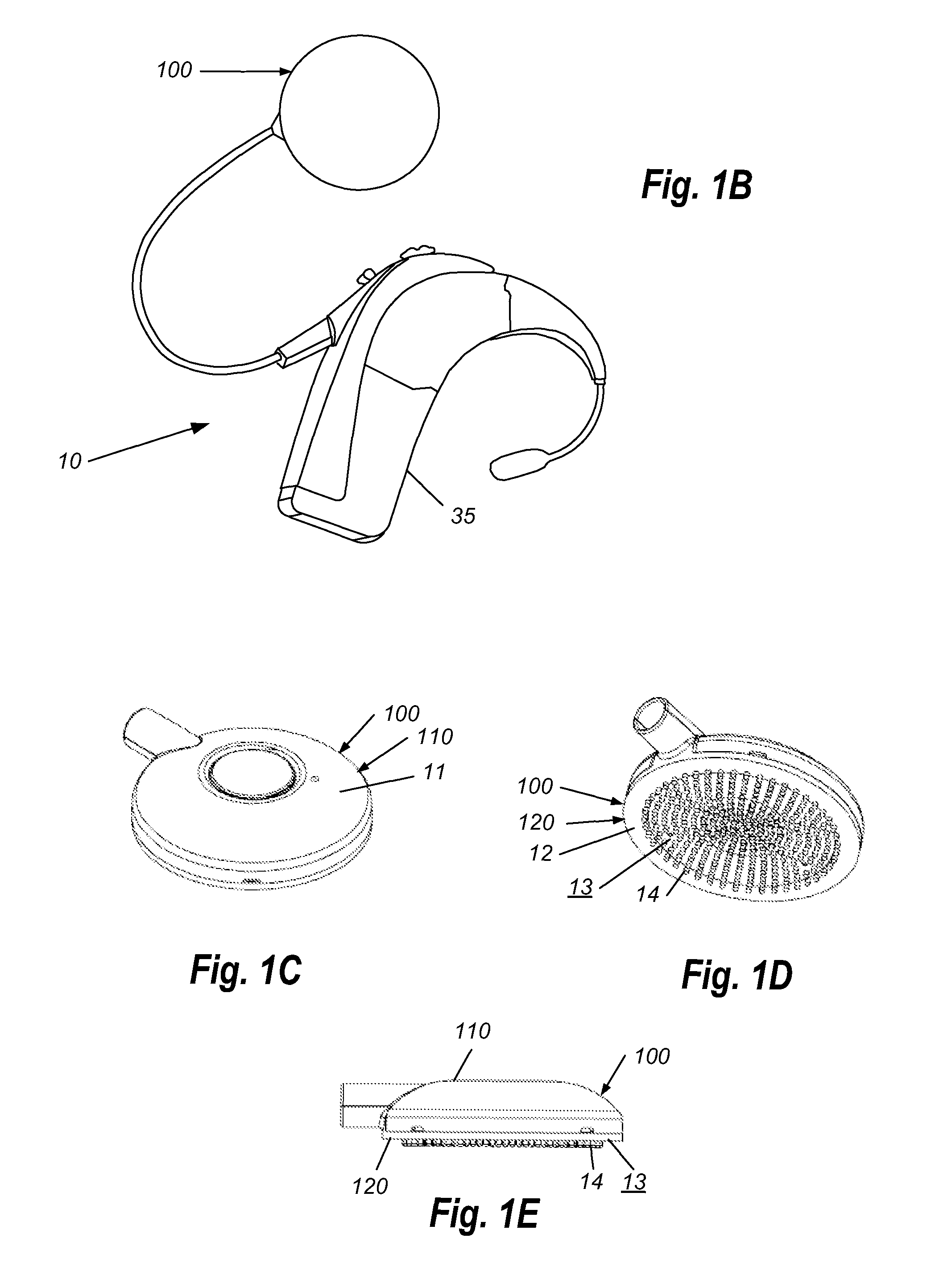
A headpiece for a cochlear implant system includes a transcutaneous transmission coil that transfers power and / or data to an implantable device implanted under a user's skin. The headpiece includes a magnet for holding the transmission coil in close proximity to the receiver coil in the implanted device, which also contains a magnet, and provides the desired alignment between the coils so that inductive coupling may efficiently occur. The headpiece has a bottom surface for skin contact that includes a plurality of flexible bumps configured to distribute pressure over a large surface area while allowing blood flow throughout the area. This also provides friction contact with the skin to help secure the headpiece, reducing movement due to lateral loading, while reducing skin irritation and erosion.
Owner:ADVANCED BIONICS AG
Transdermal drug delivery system for liquid active ingredient
InactiveUS20100087768A1Reduce lossesModerate shearPowder deliveryBiocideAdditive ingredientCross linker
A monolithic device for transdermal administration of an active pharmaceutical ingredient which is selected from propargylamines and rivastigmine and is liquid at 25° C., has an adhesive matrix layer which includes the active ingredient in an acrylic polymer pressure sensitive adhesive without cross-linker agent containing a metal atom, the adhesive having a shear value of between 1.5 and 15 hours, and further includes a non-volatile coadjuvant selected from squalene and triethylcitrate present in the layer in an amount of 1 to 15 wt %. The combination provides good release of the drug in use, reduces loss of the drug during a drying step in manufacture, reduces chemical interaction of the layer with the drug and achieves low level of skin irritation.
Owner:AMARIN TECH
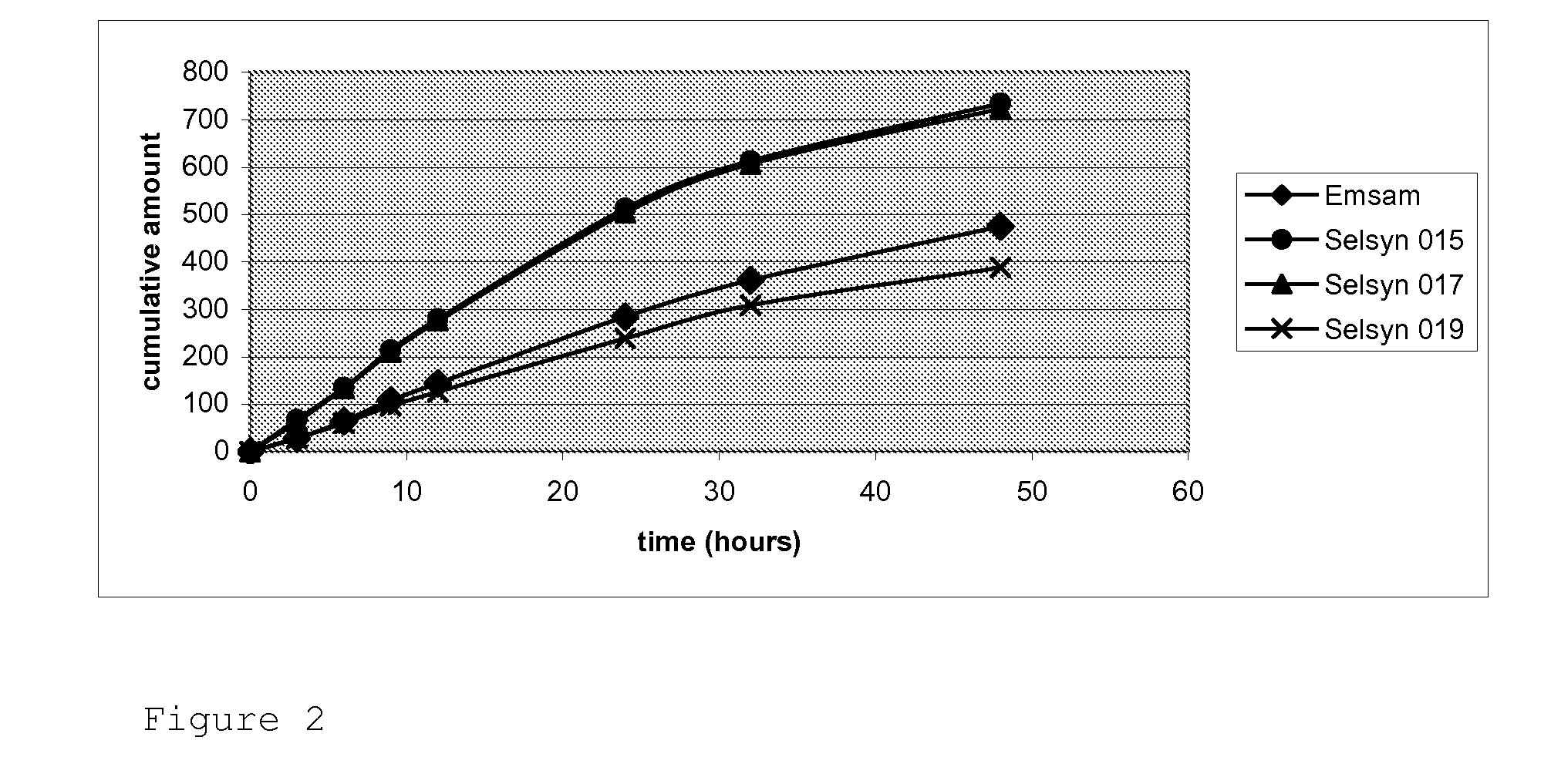
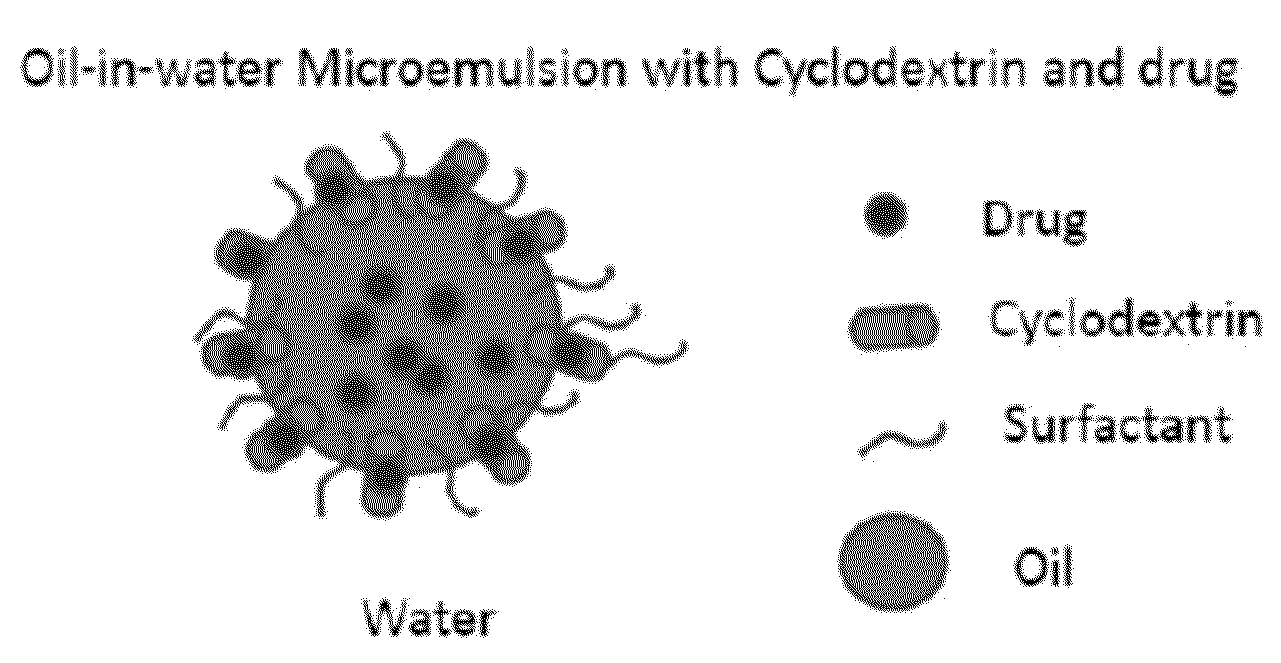
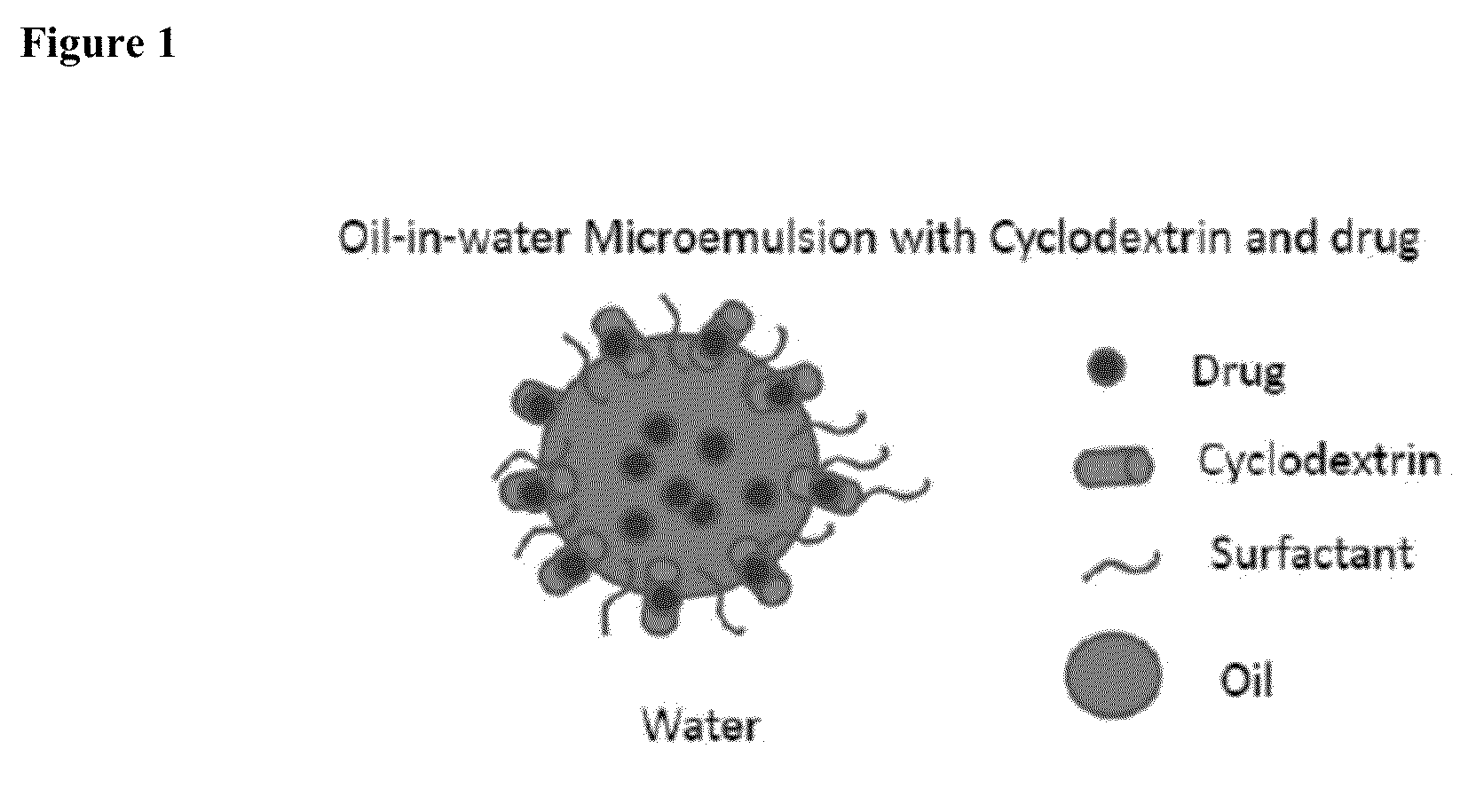
Cyclodextrin-Based Microemulsions, and Dermatological Uses Thereof
InactiveUS20130251644A1Large average pore sizeUneven skinBiocideCosmetic preparationsSolubilityActive agent
Described herein are cyclodextrin-stabilized microemulsion systems useful for increasing the solubility, stability, bioavailability, or safety of an active agent for delivery to the skin. The microemulsions may reduce the occurrence of skin irritation or odor upon application. In certain embodiments, the active agent is substantially insoluble in water. The microemulsions may be formulated as semi-solids, for example creams, or as aerosol or non-aerosol foams. Also described are methods of treating skin disorders, comprising the step of applying to an affected area of a subject in need thereof a therapeutically-effective amount of an inventive microemulsion.
Owner:PRECISION DERMATOLOGY
Direct emulsion for bleaching hair
A direct emulsion for bleaching keratin fibers, preferably human keratin fibers such as hair, having an inert phase containing of a nonoxygenated and nonperfluorinated liquid compound having a water-solubility at 25° C. of less than 1% and an aqueous hydrogen peroxide solution, a bleaching method using this direct emulsion, as it is or in the form of a ready-to-use composition, and the use of this direct emulsion for bleaching keratin fibers. The direct emulsion in accordance with the invention makes it possible to rapidly obtain substantial lightening of keratin fibers while limiting the degradation of the keratin fibers and skin irritation.
Owner:LOREAL SA
Zeolite hemostatic dressings and preparation method and application thereof
The invention relates to the high degree of exchange Ca-A type zeolite hemostasis dressing and the preparation method and the purpose. The zeolite hemostasis dressing of the invention containing the zeolite has fast hemostasis speed, and has no bacterium, no pyrogen, no cell toxicity, no hypersensitive reaction and no skin irritation, the using is convenient and the cost is low.
Owner:深圳泰明嘉业药业有限公司
Antioxidant composition for topical/transdermal prevention and treatment of wrinkles
InactiveUS6180133B1Significant free radical protectionHead bandagesCosmetic preparationsWrinkle skinVitamin C
An anti-wrinkle skin treating composition comprises a pressure sensitive matrix patch having dissolved in the adhesive a mixture of antioxidants in the form of a Vitamins C ester and Vitamin E. Also preferably dissolved in the adhesive are glycerine and a polydiorganosiloxane adhesion-adjusting agent. Optionally dissolved in the adhesive is also one or more members selected from the group consisting of moisturizing agents, skin collagen synthesis promoting agents and exfoliating agents. When applied to a wrinkled skin area the composition acts to diminish fine wrinkles and improves the overall thickness, elasticity, firmness and smoothness of the skin. The modified adhesive properties of the patch are sufficient to maintain the patch in place on the skin for the recommended treatment period while allowing the patch to be readily removed without causing skin irritation or leaving adhesive residue on the skin.
Owner:ACTAVIS HOLDCO US INC
Solid transdermal therapeutic system with UV absorber
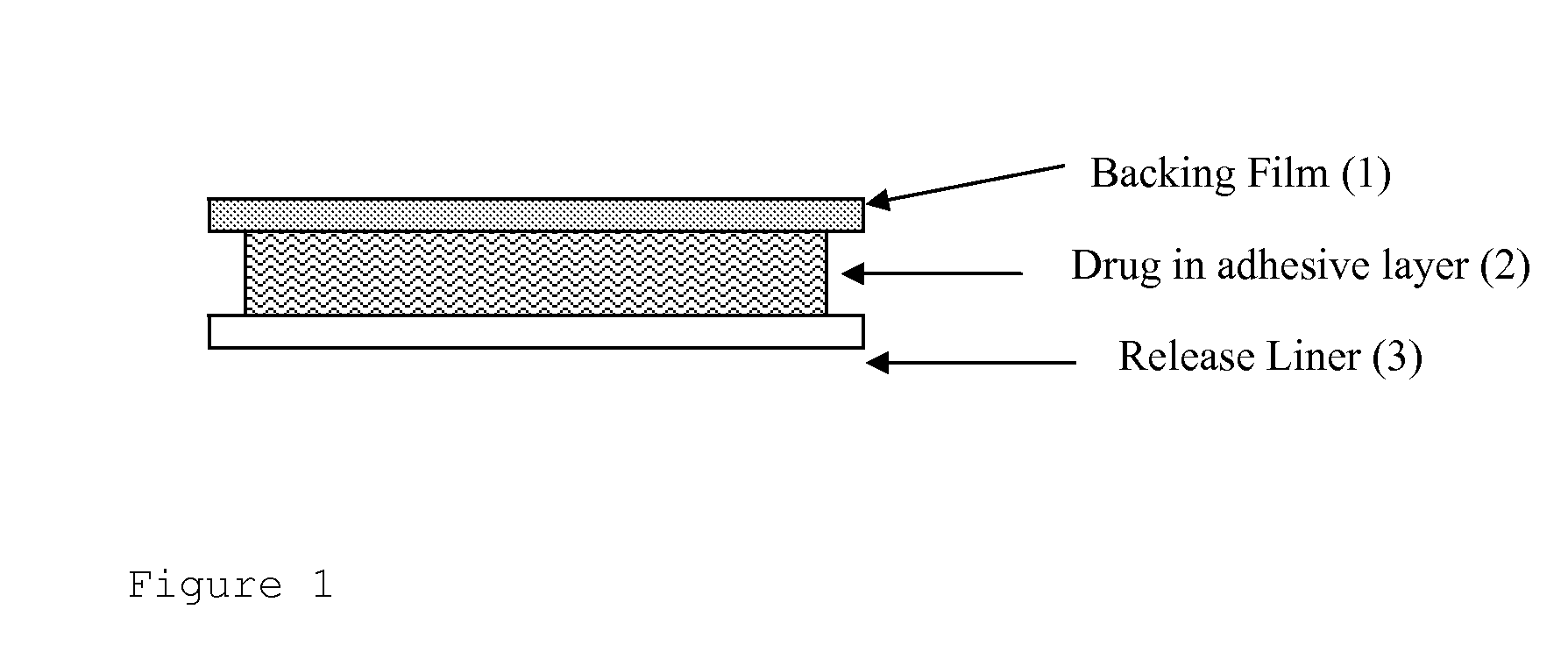
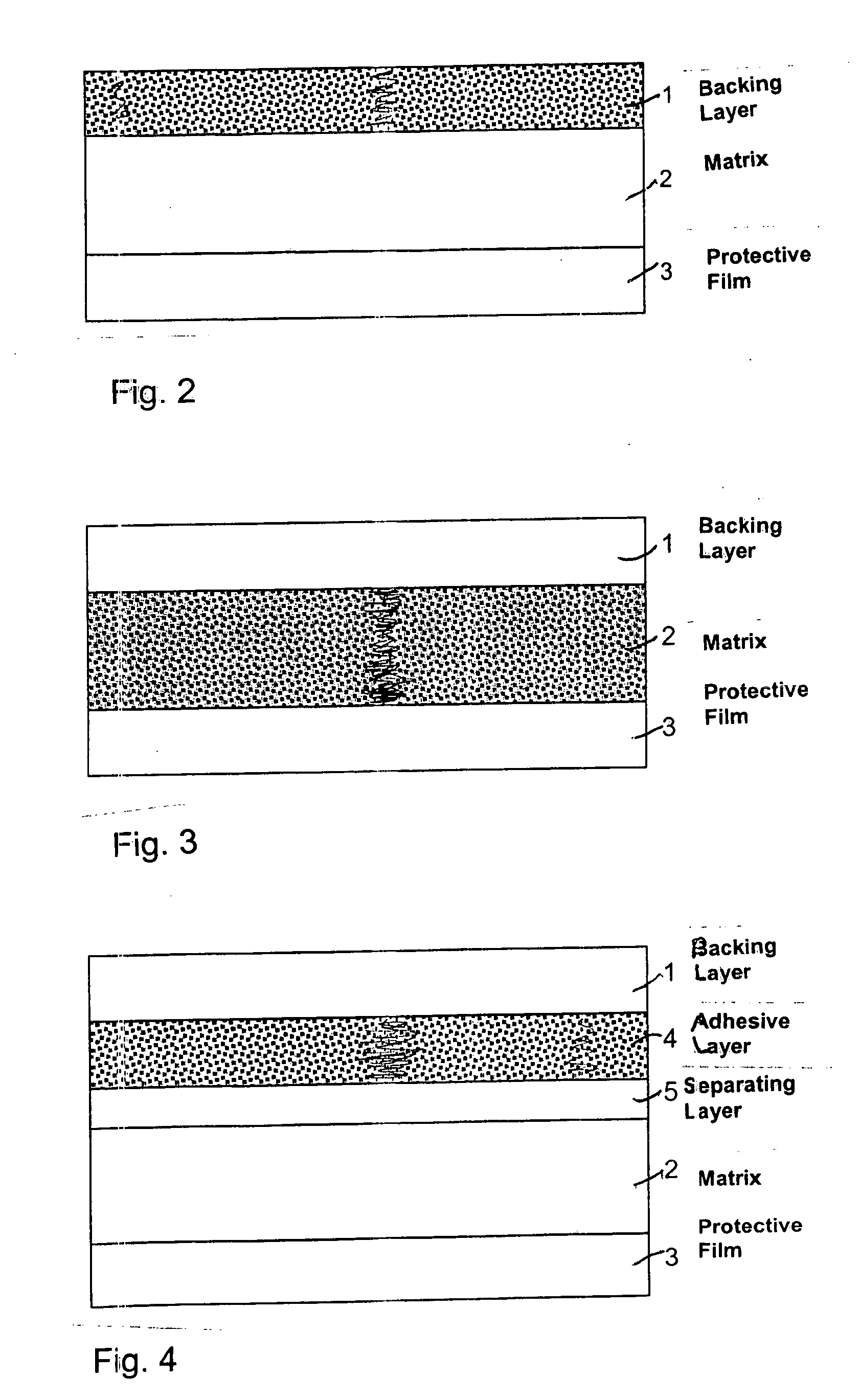
ActiveUS20060246122A1Improve the protective effectBiocidePharmaceutical non-active ingredientsTectorial membraneMedicine
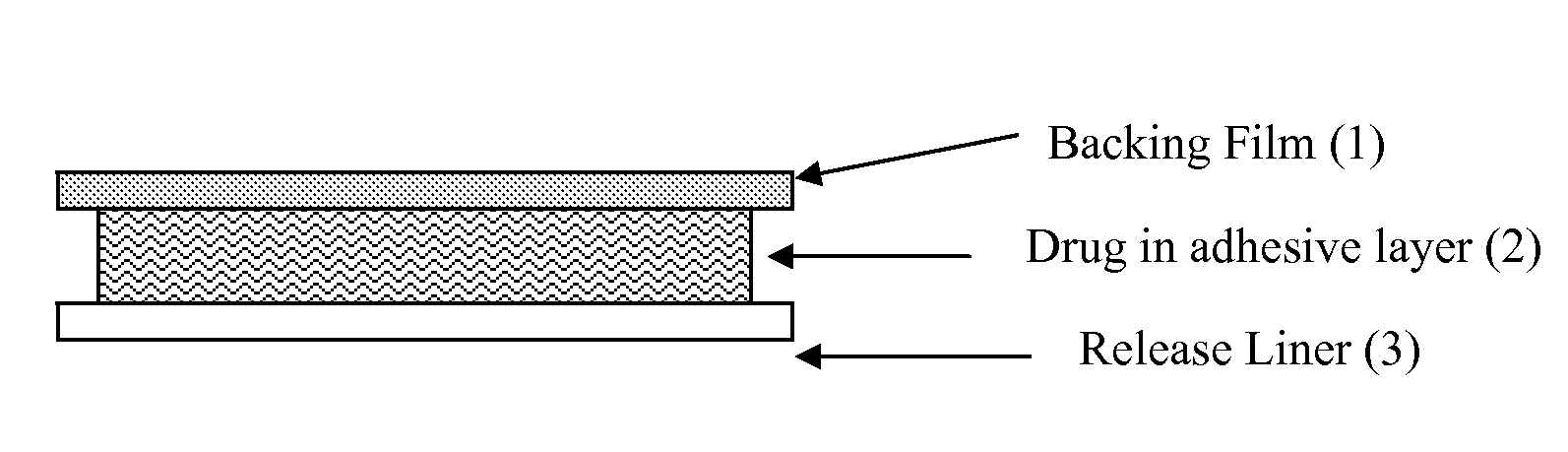
The UV-stable solid transdermal therapeutic system (TTS) with UV absorber for photosensitive active pharmaceutical ingredients has a backing layer (1), at least one active ingredient-containing matrix (2), and a detachable protective film (3). Optionally an adhesive layer (4) and a separating layer (5) are introduced between the backing layer (1) and the at least one active ingredient-containing matrix (2). At least one hydroxyphenyltriazine compound acting as UV absorber is embedded in the backing layer (1), in the active ingredient-containing matrix (2), or in the adhesive layer (4). The TTS according to the invention achieves high stability at low concentrations of UV absorber, preferably 0.5 to 3% (m / m), so as to reduce or avoid skin irritation.
Owner:LUYE PHARMA SWITZERLAND AG
Anti-inflammatory analgesic adhesive patch for external use
ActiveUS20120283671A1Promote absorptionLess irritatingAntipyreticAnalgesicsAdditive ingredientTackifier
An external patch containing diclofenac hydroxyethylpyrrolidine prepared by laminating an adhesive layer on a backing, wherein said adhesive layer is characterized by comprising 5-50% by weight of styrene•isoprene•styrene block copolymer, 20-50% by weight of a tackifier resin, 5-70% by weight of a softening agent, and 0.5-20% by weight of one or more solubilizers selected from N-methyl-2-pyrrolidone, propylene glycol and dimethyl sulfoxide as essential ingredients, and 0.5-20% by weight of diclofenac hydroxyethylpyrrolidine as an active ingredient. The patch has excellent transdermal absorption, less skin-irritation and excellent stability of the drug.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD +1
Medical electrode
A medical electrode, and a method of making a medical electrode. The electrode comprises an electrode member having a top face and a bottom face; disconnected regions of electrically conductive material in electrical contact with the top face of the electrode member, patient contacting layer and an electrical connector in electrical contact with the disconnected regions. The disconnected regions reduce patient skin irritation and burning while optimizing electrical impedance of the electrode.
Owner:KPR U S LLC
Electrically conductive adhesive hydrogels with two initiators
A composition providing electrically conductive adhesive hydrogels suitable for use as skin contact adhesives and, particularly, suitable for use as an electrical interface for disposable medical devices. The present hydrogels provide for reduced skin irritation and / or malodor properties, hydrate a subject's skin, readily wet around a subject's skin surface hair, and protect against burning of a subject upon or due to electrical stimulation through the hydrogel. These hydrogels generally include a monomer, a first initiator, a second initiator, and a cross-linking agent. The present hydrogels also desirably include a solubilizer. The present hydrogels also desirably may include a buffer system to help prevent discoloration of the hydrogels and / or hydrolysis of the hydrogels as well as to improve shelf-life. Other additives such as conductivity enhancers, pharmaceuticals, humectants, plasticizers, skin health agents, etc. may be added to the present hydrogels either before or after curing.
Owner:CONMED CORP
Method for treating erectile dysfunction and increasing libido in men
InactiveUS20050049233A1Little and no skin irritationImprove efficiencySenses disorderNervous disorderIncreasing libidoLibido
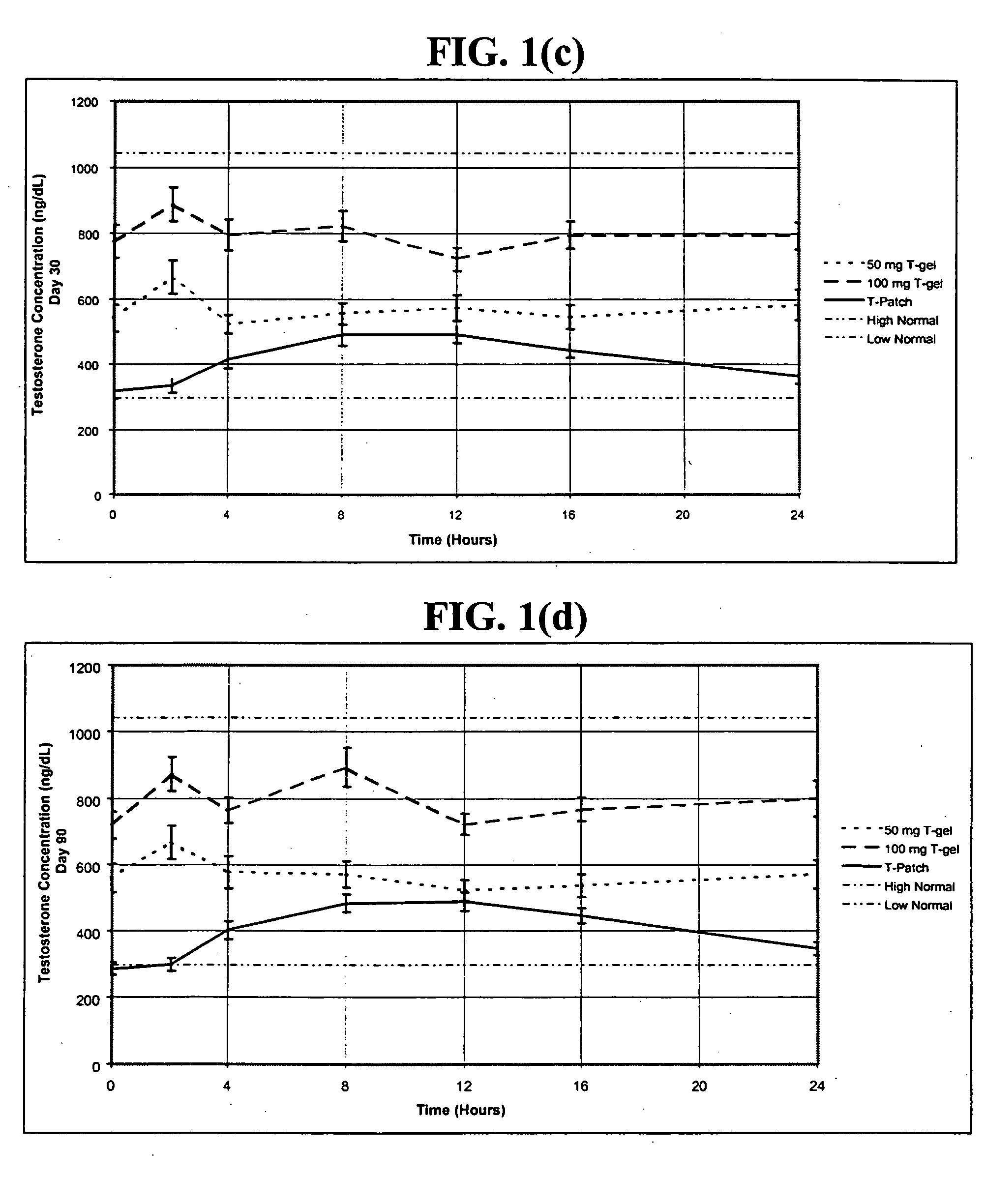
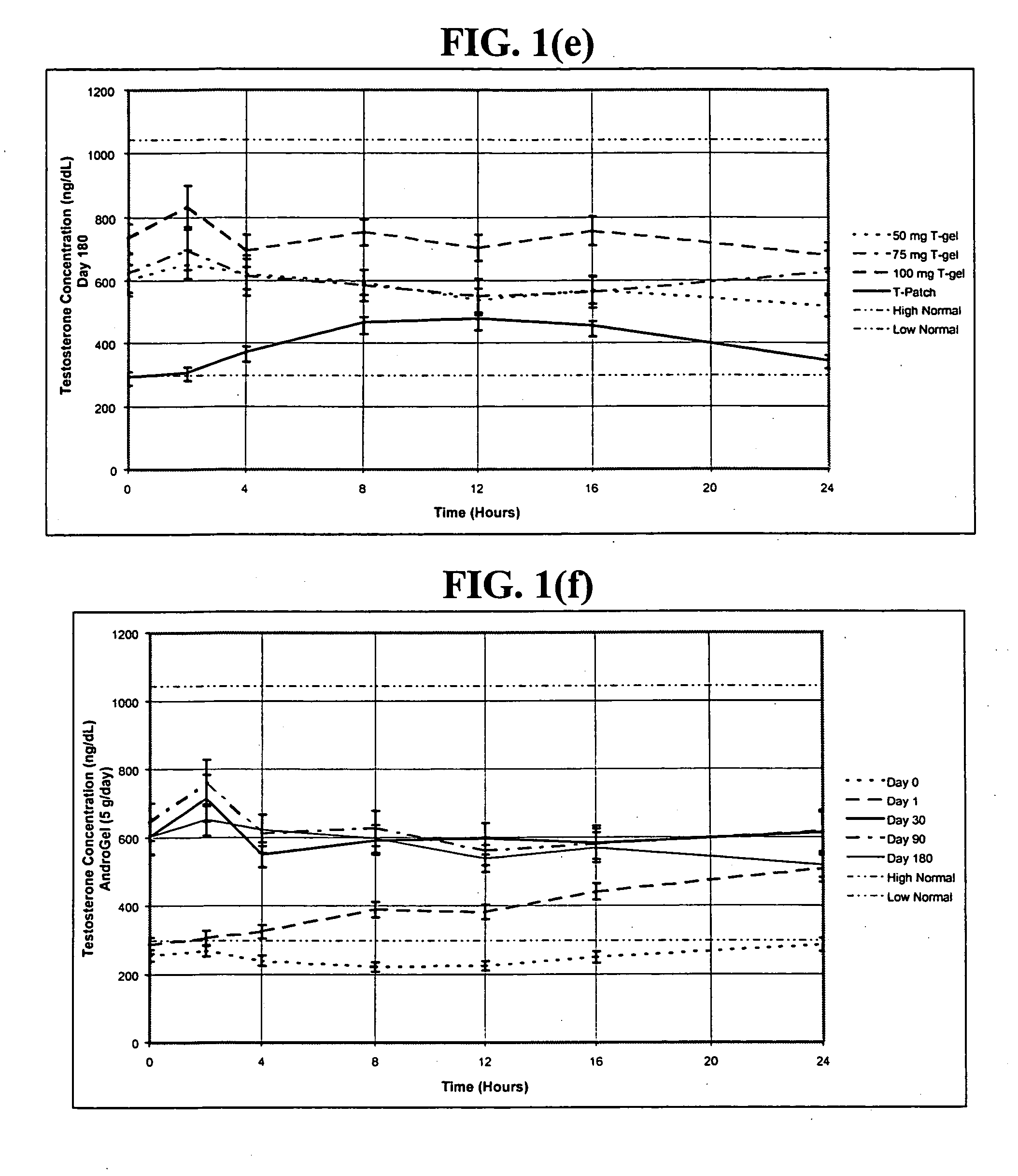
The present invention relates to a transdermal hydroalcoholic testosterone gel formulation that overcomes the problems associated with other testosterone delivery mechanisms by providing, among other things, a desirable pharmacokinetic hormone profile with little or no skin irritation. In addition, the gel is used in conjunction with pharmaceuticals aimed at treating erectile dysfunction, such as VIAGRA®, to enhance their effectiveness.
Owner:LABORATORIES BESINS INTERNATIONAL SAS
Back hair removal using comb and integrated blade
A method and apparatus of removing hair from the back, shoulders and arms of a man utilizes a device comprising a comb having teeth on a lower end and a blade embedded in the teeth so that a lower active edge of the blade does not reach a lower edge of the teeth and a s result there is no exposed blade and no skin irritation or safety concerns. A rigid or semi-rigid one-piece elongated handle, a proximal end of the handle having a channel shaped to slidably receive the upper end of the comb, the proximal end also having a lever pressing against the comb to hold the comb securely to the proximal end of the handle, the lever capable of being bent to release the comb. The device has a center of gravity approximately one-third of the way down the handle from the proximal end of the handle.
Owner:BAKBLADE LTD
Absorbent articles with non-aqueous compositions containing anionic polymers
InactiveUS20020128615A1Soften, smooth, plasticize, lubricate, moisturizeGood lookingCosmetic preparationsInfusion syringesPolymer scienceSkin contact
The present invention relates to absorbent articles including non-aqueous compositions for protecting the barrier function of the skin. The compositions can be applied to the bodyfacing surfaces of absorbent articles so that the compositions come into contact with the skin. The compositions of the invention have improved stability on the bodyfacing surfaces after processing. The compositions of the invention provide several benefits including prevention and alleviation of skin irritations associated with the use of absorbent articles. The compositions can include emollients, viscosity enhancers and anionic polymers.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Disposable medical article with multiple adhesives for skin attachment
InactiveUS20050013957A1Reduce riskImproved painless removalLayered productsPlastersWound dressingUltimate tensile strength
A disposable medical article for skin attachment is provided with two or more stacked layers of pressure-sensitive adhesives with progressively higher skin peel strength separated by protective covers therebetween to accommodate a wide range of skin conditions such as wet or oily skin while reducing the risk of skin irritation. A lower strength adhesive is preferably exposed first by removing its protective cover to attach the article to the skin. If the article separates from the skin, the next protective cover is removed revealing an additional more aggressive adhesive layer so the article may be reattached. Alternatively, the more aggressive adhesive can be exposed right away by removing another adhesive layer and its protective cover as a unit. The article may be used advantageously for wound dressings, EKG electrodes, hemostasis patches, ostomy bag attachments and alike to provide a more secure attachment over a wide range of patients.
Owner:DATASCOPE INVESTMENT
Electrically conductive adhesive hydrogels with solubilizer
A composition providing electrically conductive adhesive hydrogels suitable for use as skin contact adhesives and, particularly, suitable for use as an electrical interface for disposable medical devices. The present hydrogels provide for reduced skin irritation and / or malodor properties, hydrate a subject's skin, readily wet around a subject's skin surface hair, and protect against burning of a subject upon or due to electrical stimulation through the hydrogel. These hydrogels generally include a monomer, a first initiator, a solubilizer, and a cross-linking agent. The present hydrogels also desirably include a buffer system to help prevent discoloration of the hydrogels and / or hydrolysis of the hydrogels as well as to improve shelf-life. Other additives such as conductivity enhancers, pharmaceuticals, humectants, plasticizers, skin health agents, etc. may be added to the present hydrogels either before or after curing.
Owner:CONMED CORP
Method for treating erectile dysfunction and increasing libido in men
InactiveUS20050054623A1Little and no skin irritationImprove sexual performanceSenses disorderNervous disorderSexual impotenceCombined use
The present invention relates to a transdermal hydroalcoholic testosterone gel formulation that overcomes the problems associated with other testosterone delivery mechanisms by providing, among other things, a desirable pharmacokinetic hormone profile with little or no skin irritation. The gel may be used as a method of improving sexual performance, including treating erectile dysfunction, and increasing libido by increasing testosterone levels in men. In addition, the gel may be used in conjunction with pharmaceuticals aimed at treating erectile dysfunction, such as VIAGRA®, to enhance their effectiveness.
Owner:UNIMED PHARMA LLC
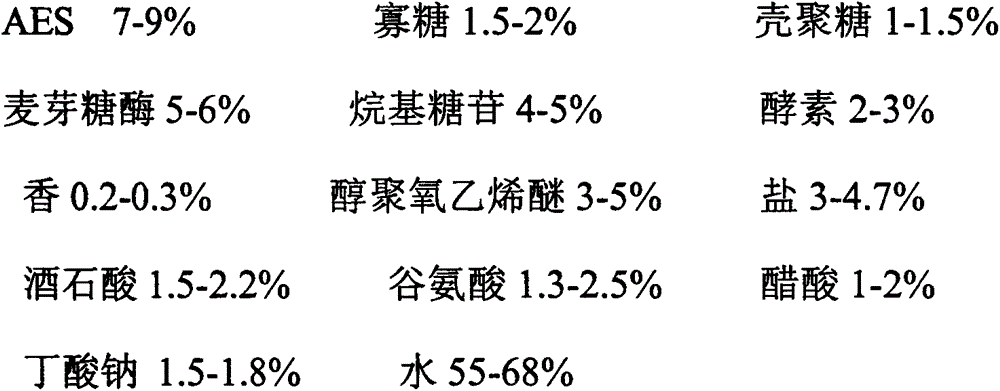
Preparation method for enzyme laundry detergent
InactiveCN105542996ANot allergicWill not turn yellow and hardenInorganic/elemental detergent compounding agentsNon-ionic surface-active compoundsCleansing AgentsBULK ACTIVE INGREDIENT
The invention relates to a preparation method for enzyme laundry detergent. At present, most detergent in the market is prepared from chemical raw materials, skin irritation, irritability and pruritus are caused, the detergent has certain toxicity, environment is polluted severely, and health of people is harmed. The enzyme laundry detergent is prepared by organically combining natural active ingredients AES, oligose, chitosan, maltase, alkyl glycoside, enzyme, perfume, alcohol ethoxylates, salt, tartaric acid, glutamic acid, acetic acid, sodium butyrate and water. The enzyme laundry detergent has a biodegradation property, decomposes dirty marks automatically, removes various stubborn dirty marks, has the quick-acting and whitening effects, and can carry out sterilization and resist static electricity, harm to clothes and skin is avoided, and the clothes are kept shining and bright.
Owner:长沙满旺生物工程有限公司
Method for treating erectile dysfunction and increasing libido in men
InactiveUS20060211664A1Little and skin irritationImprove efficiencyBiocideSenses disorderIncreasing libidoLibido
Owner:LABORATORIES BESINS INTERNATIONAL SAS
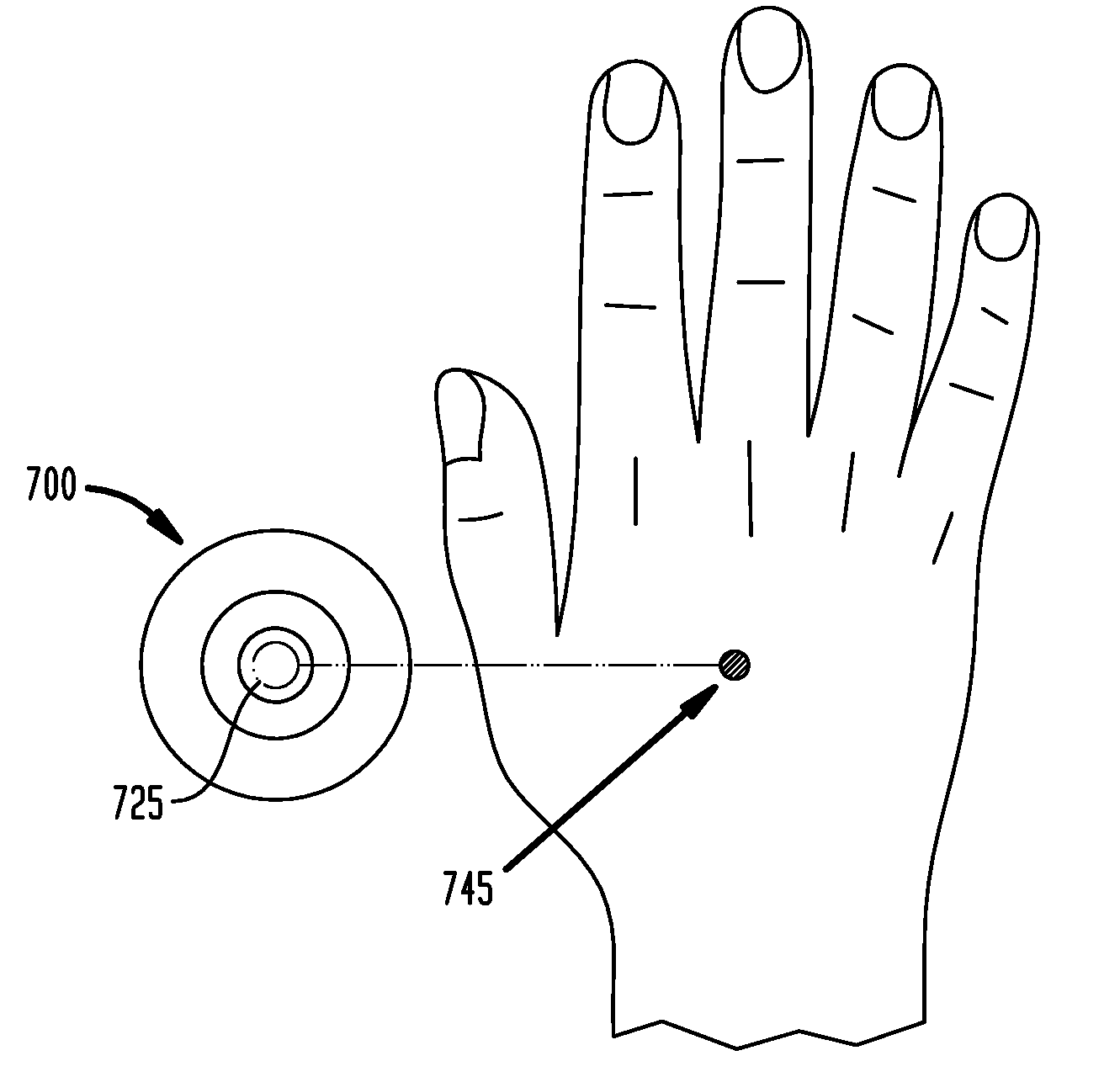
Self-locating, multiple application, and multiple location medical patch systems and methods therefor
ActiveUS20100239648A1Minimizing localized skin irritationMinimize skin irritationPlastersAdhesive dressingsSurgeryMultiple applications
A medical patch system has a first medical patch including an outer locating ring secureable to a surface and an inner patch, separable from the outer locating ring, disposed within the outer locating ring. The medical patch system includes a second medical patch adapted for insertion into the central area bounded by the outer locating ring after the inner patch is separated from the outer locating ring. The second medical patch includes a second outer locating ring secureable to the surface, and a second inner patch section, separable from the second outer locating ring, disposed within a second central area bounded by the second outer locating ring. The first and second outer locating rings have adhesive layers for securing the outer locating rings to a surface. The adhesive layers on the respective outer locating rings have different sizes, shapes or patterns to minimize skin irritation.
Owner:ETHICON INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com