Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
340 results about "Device implant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
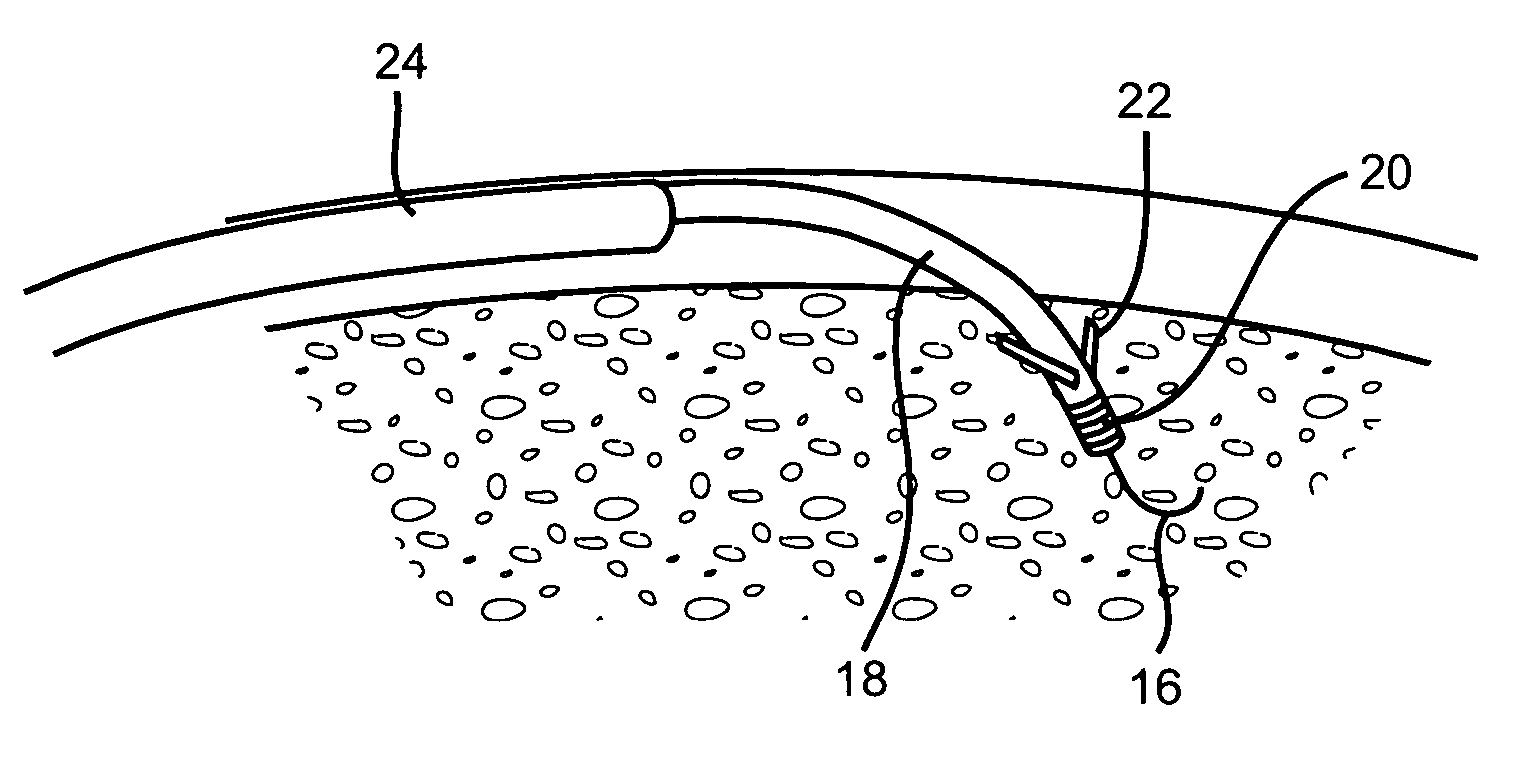
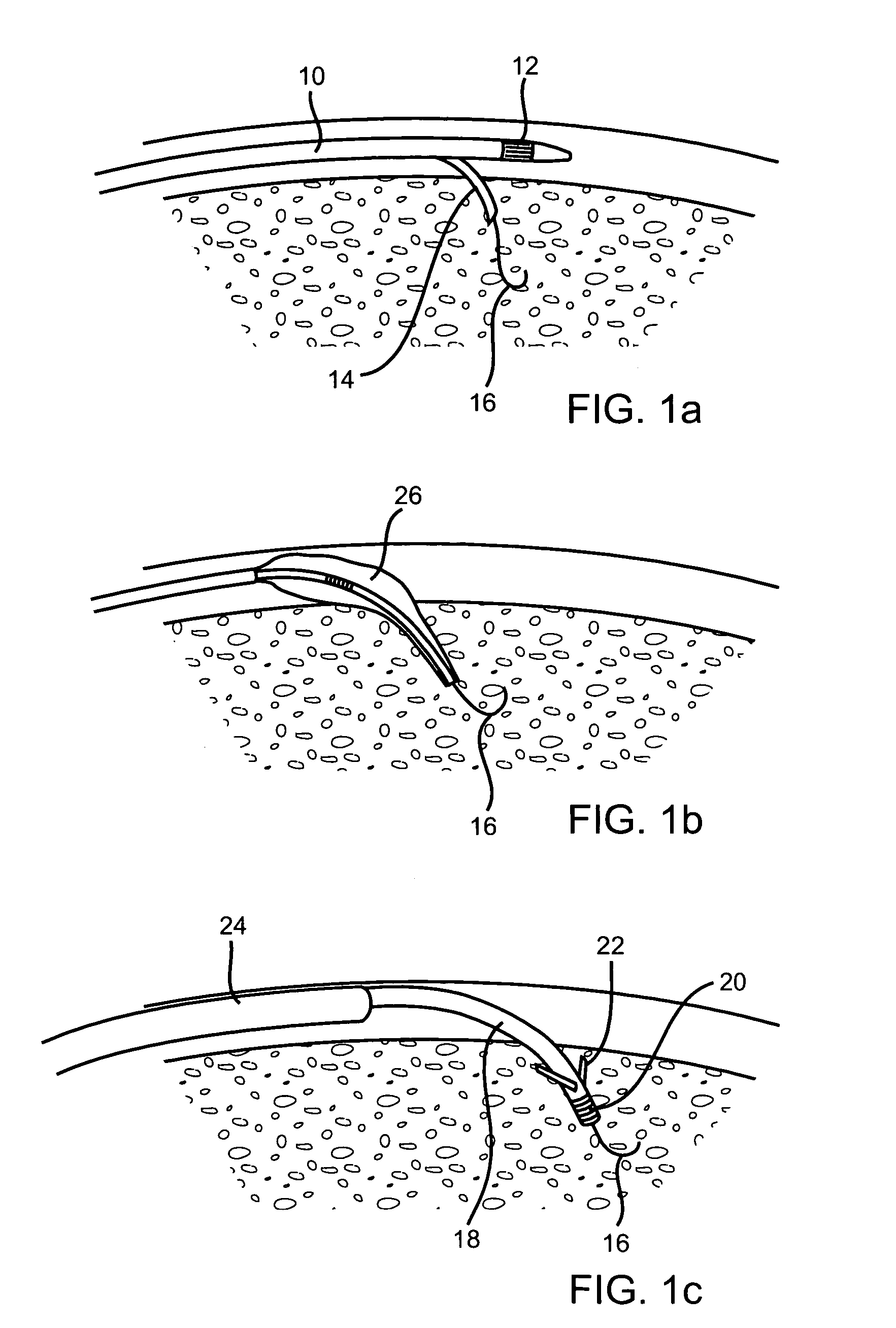
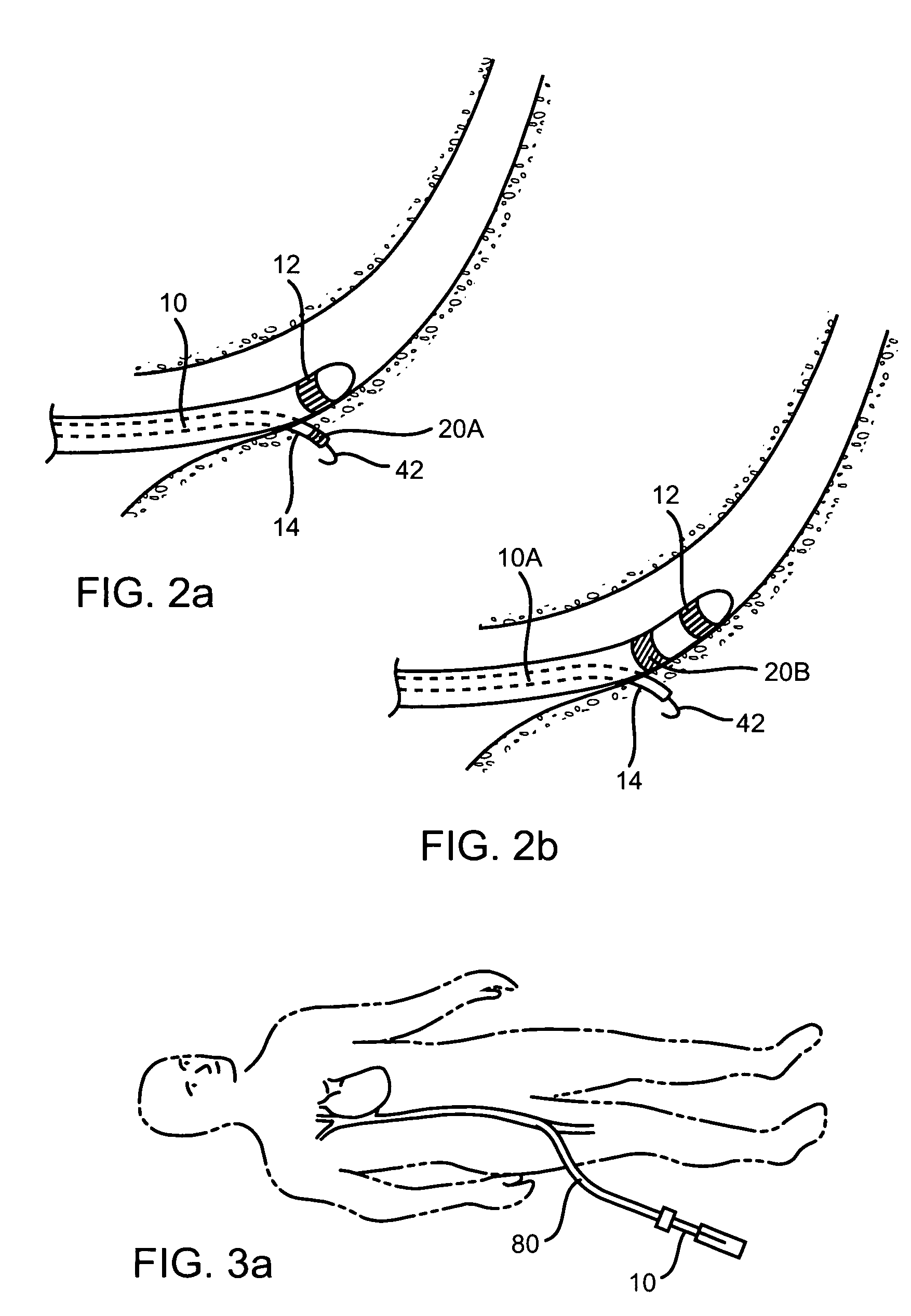
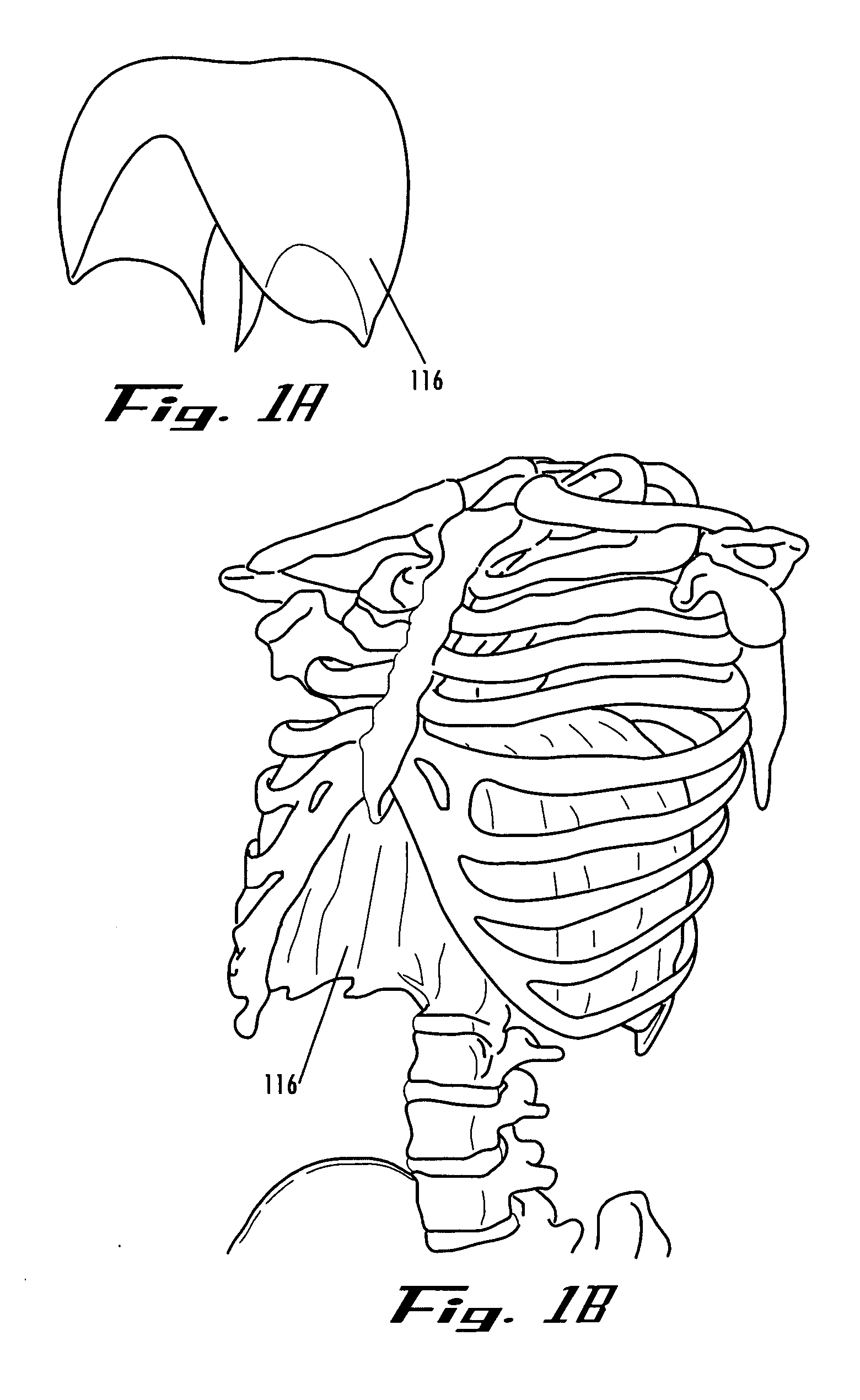
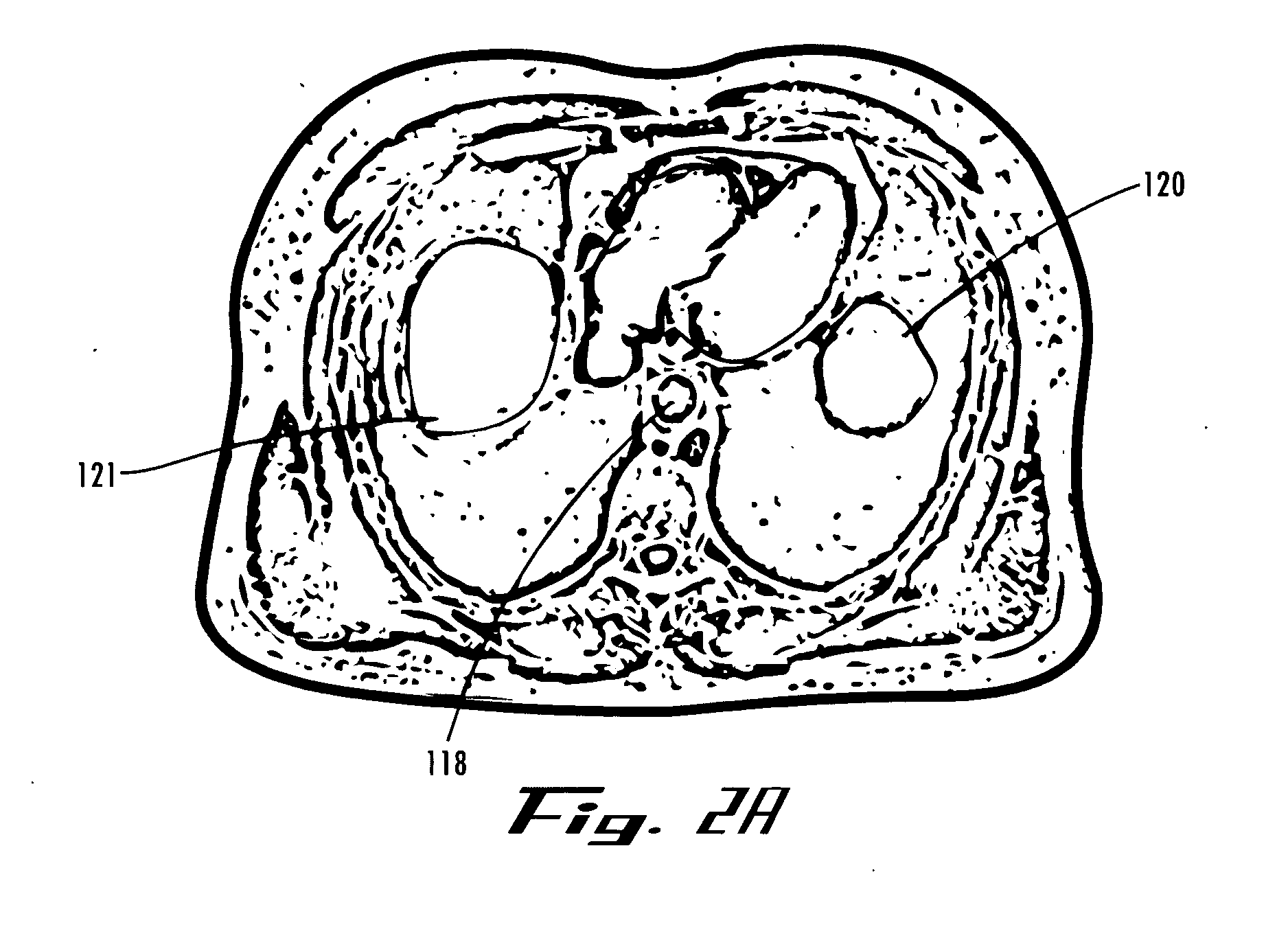
An implant is a medical device manufactured to replace a missing biological structure, support a damaged biological structure, or enhance an existing biological structure. Medical implants are man-made devices, in contrast to a transplant, which is a transplanted biomedical tissue.
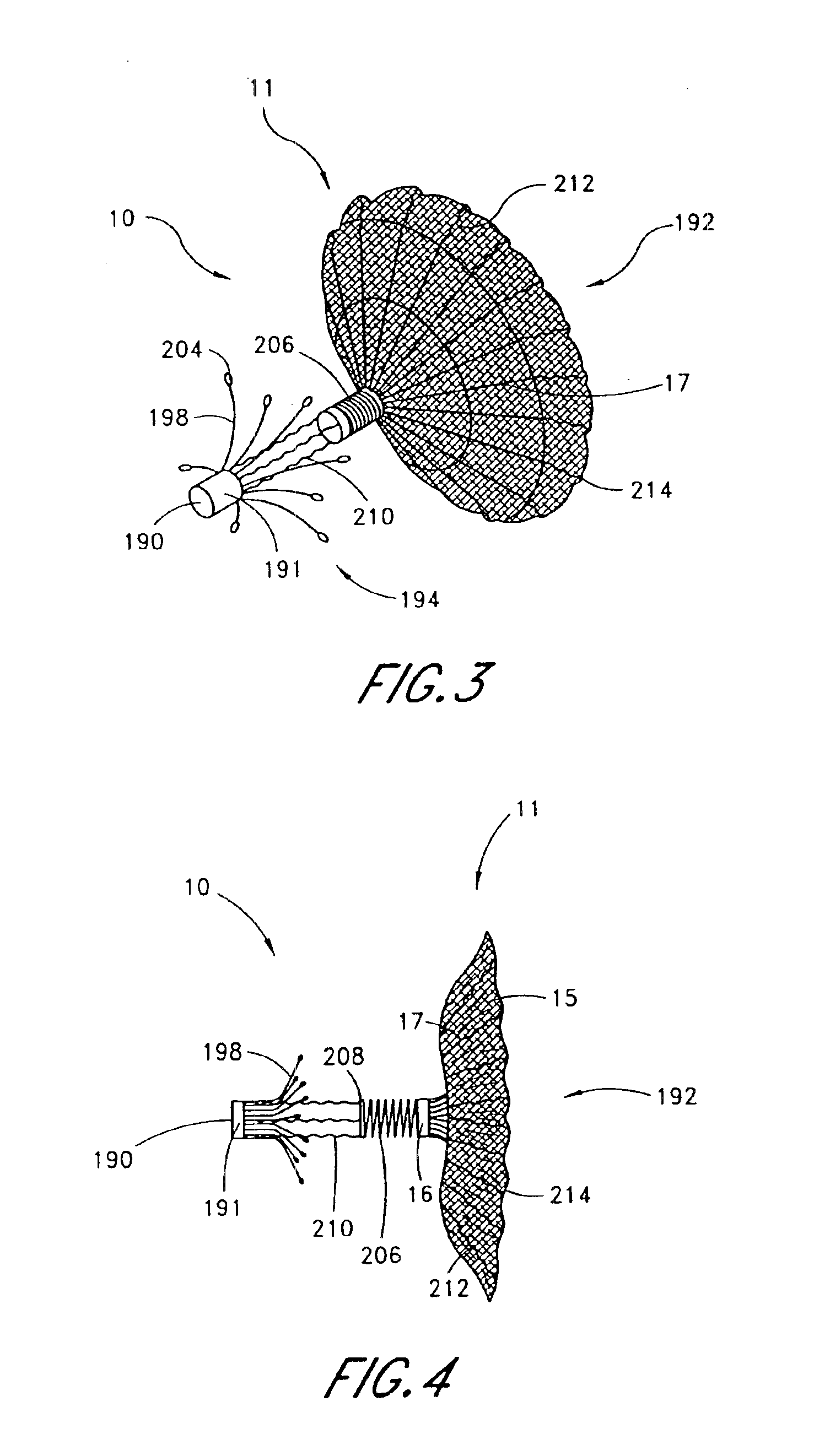
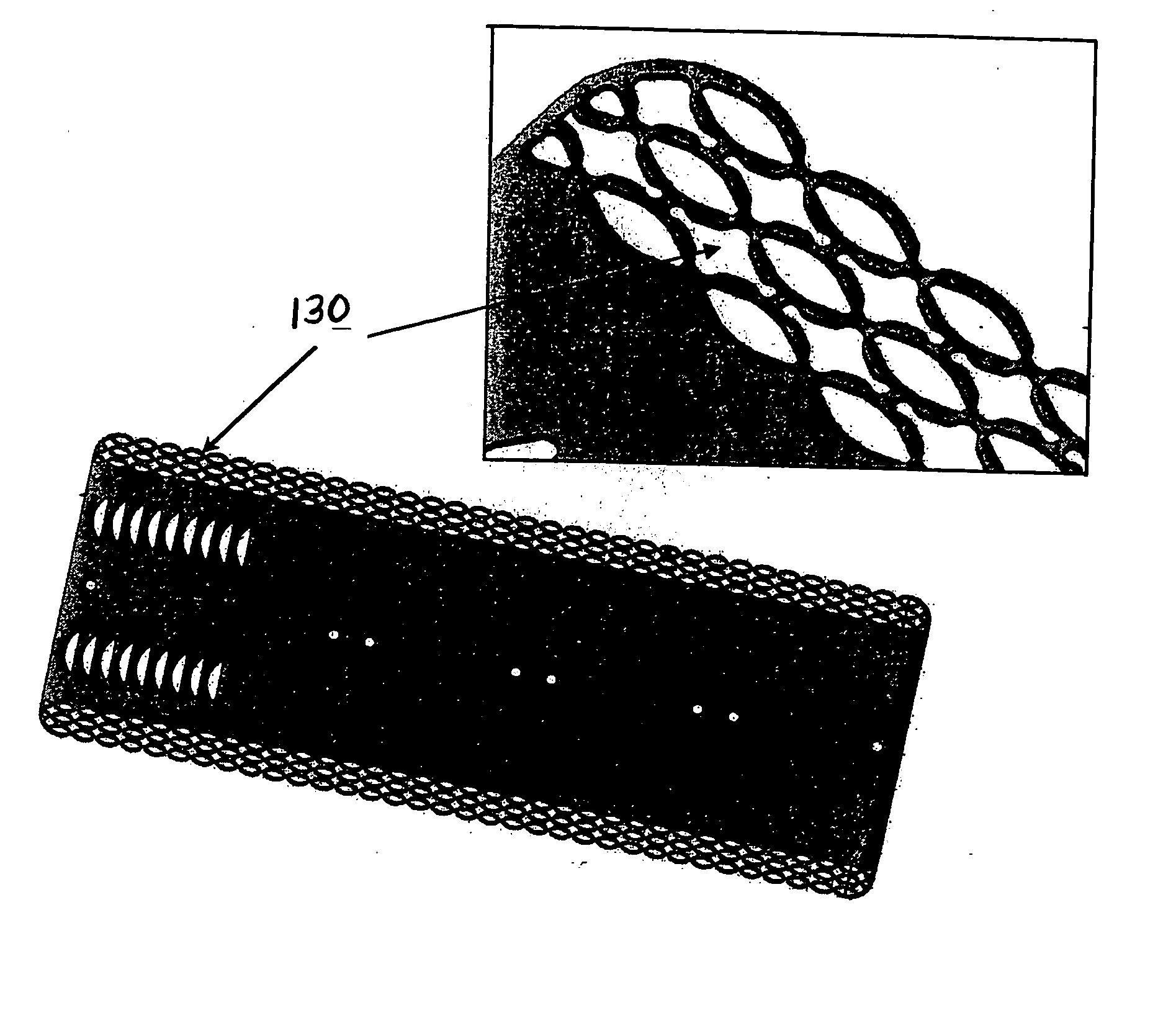
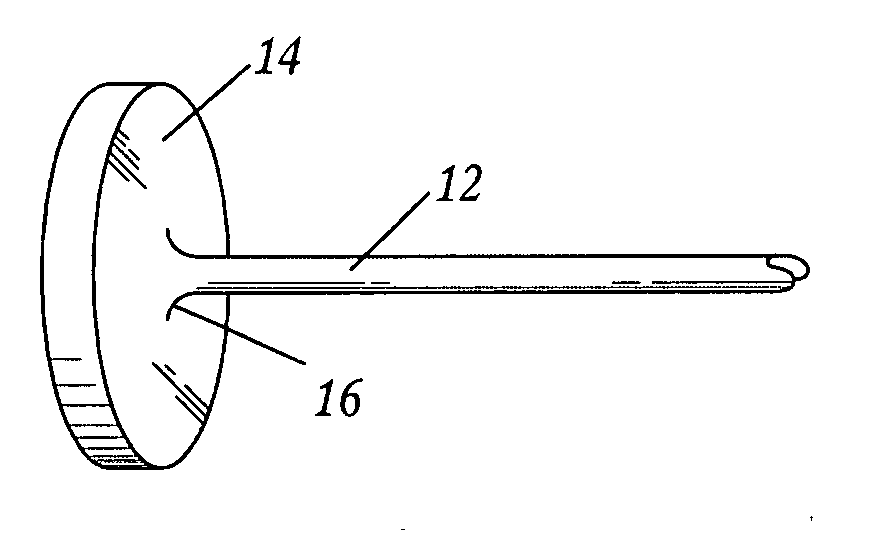
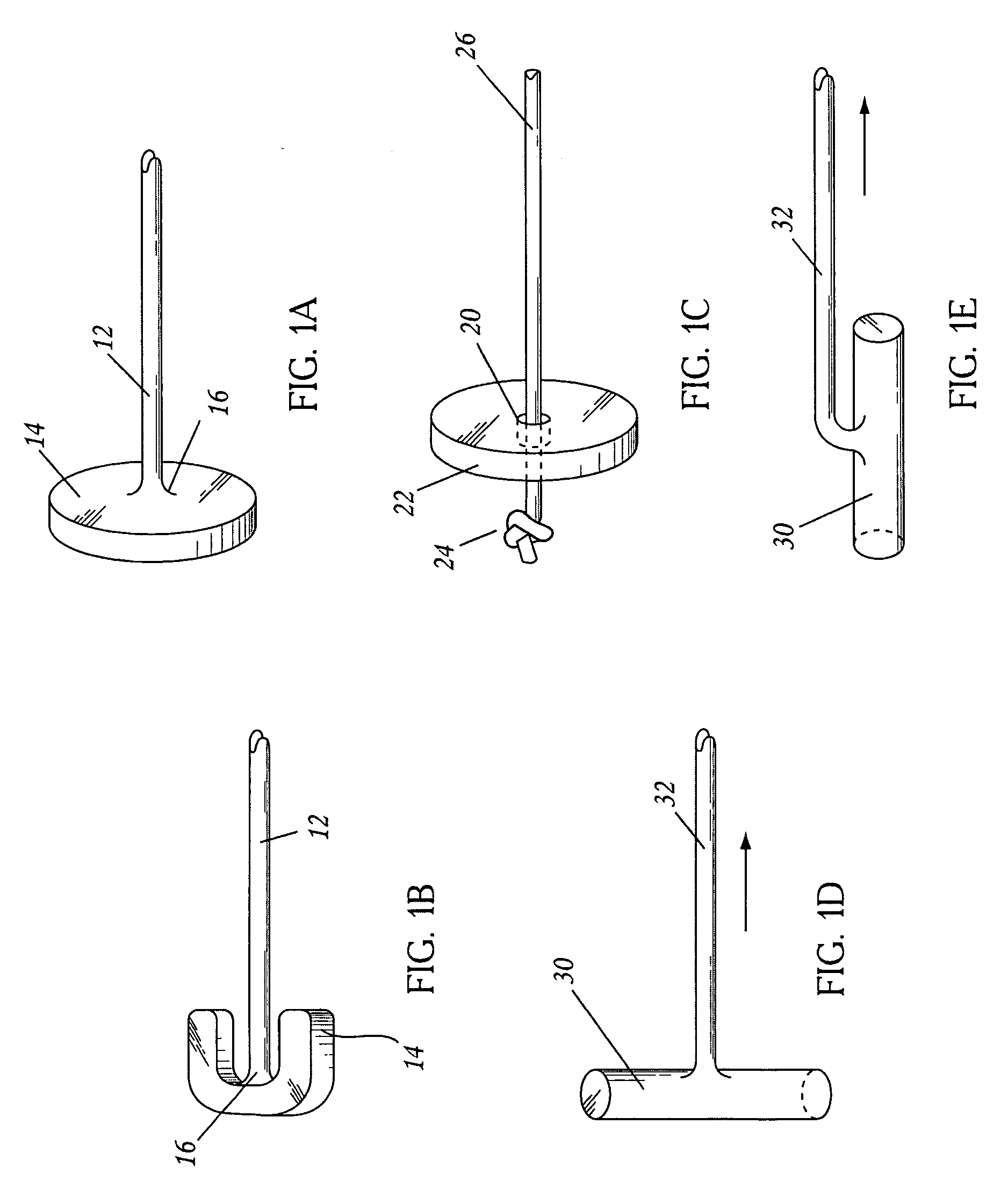
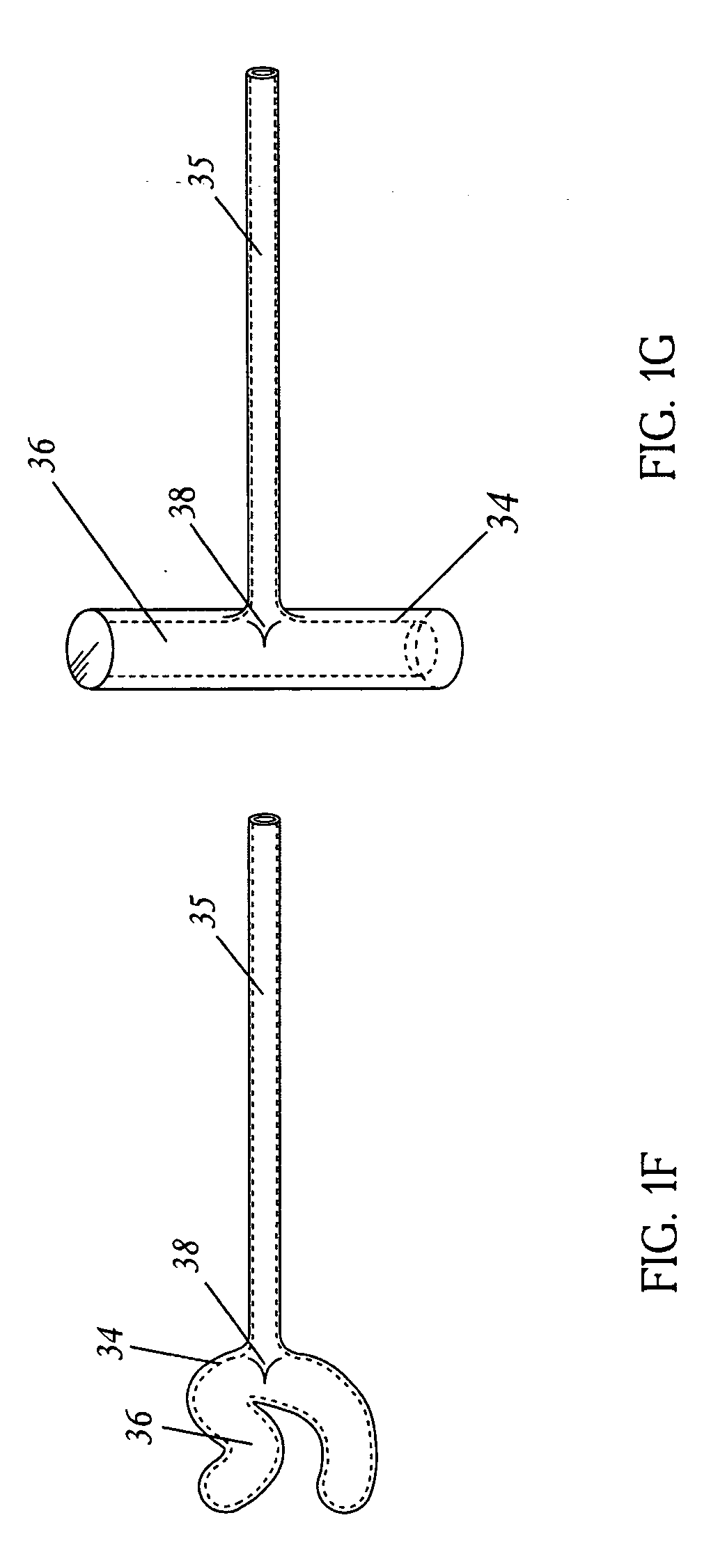
Surgical fasteners and related implant devices having bioabsorbable components
InactiveUS20060142772A1High strengthSufficient flexibilityInternal osteosythesisJoint implantsDevice implantPlastic materials
Surgical implants including fasteners and related devices which are partially bioabsorbable. The implants are constructed of a non-bioabsorbable base comprising metals and or high strength plastic materials. The base is partially or completely coated with a bioabsorbable material which can have its own mechanical features, such as the threads on a screw. Attachment elements are provided on the base to enhance the mechanical attachment of the bioabsorbable material to the base. The implants can withstand the torques and stresses encountered during surgery and, following implantation, in the body.
Owner:BIODYNAMICS
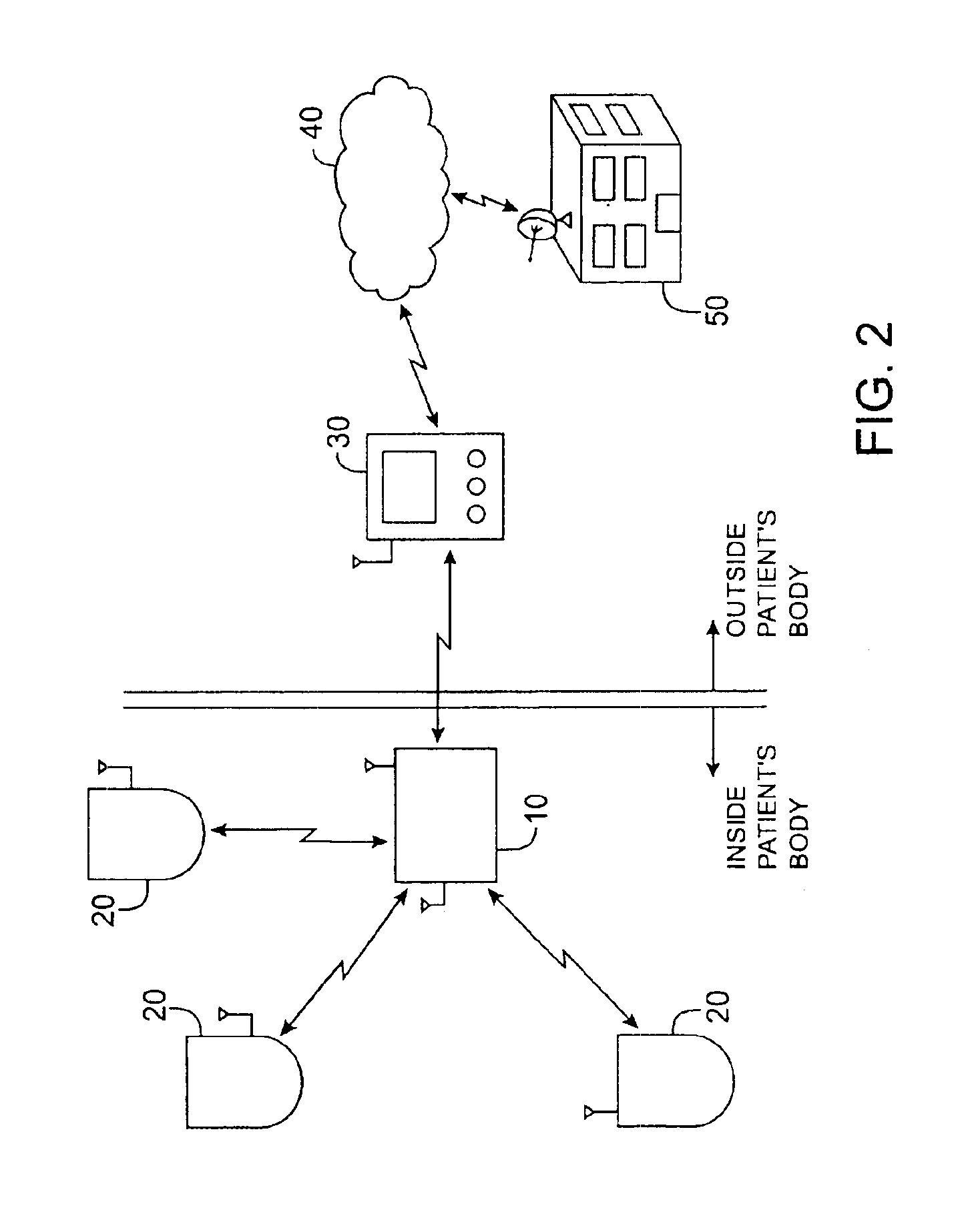
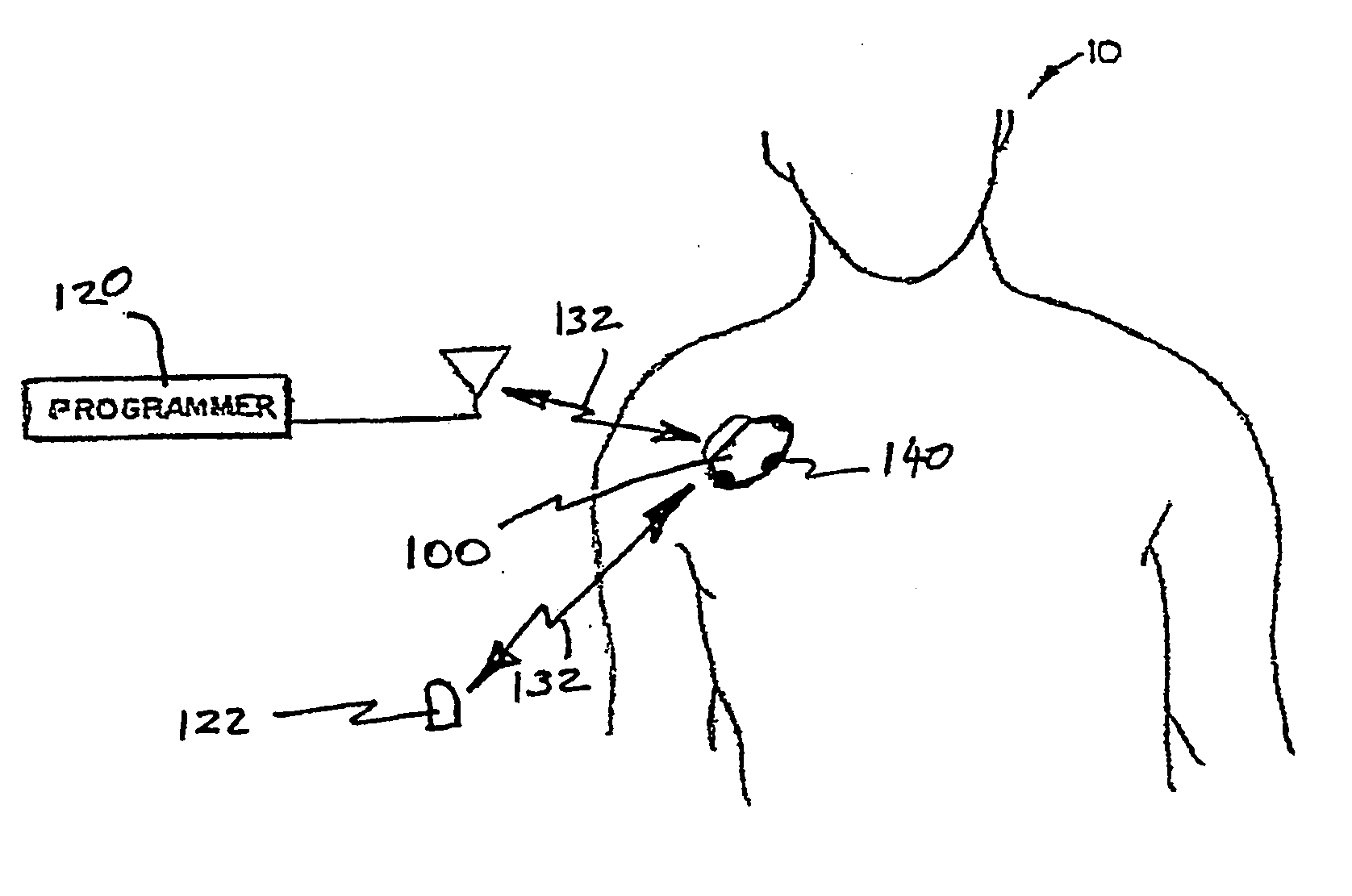
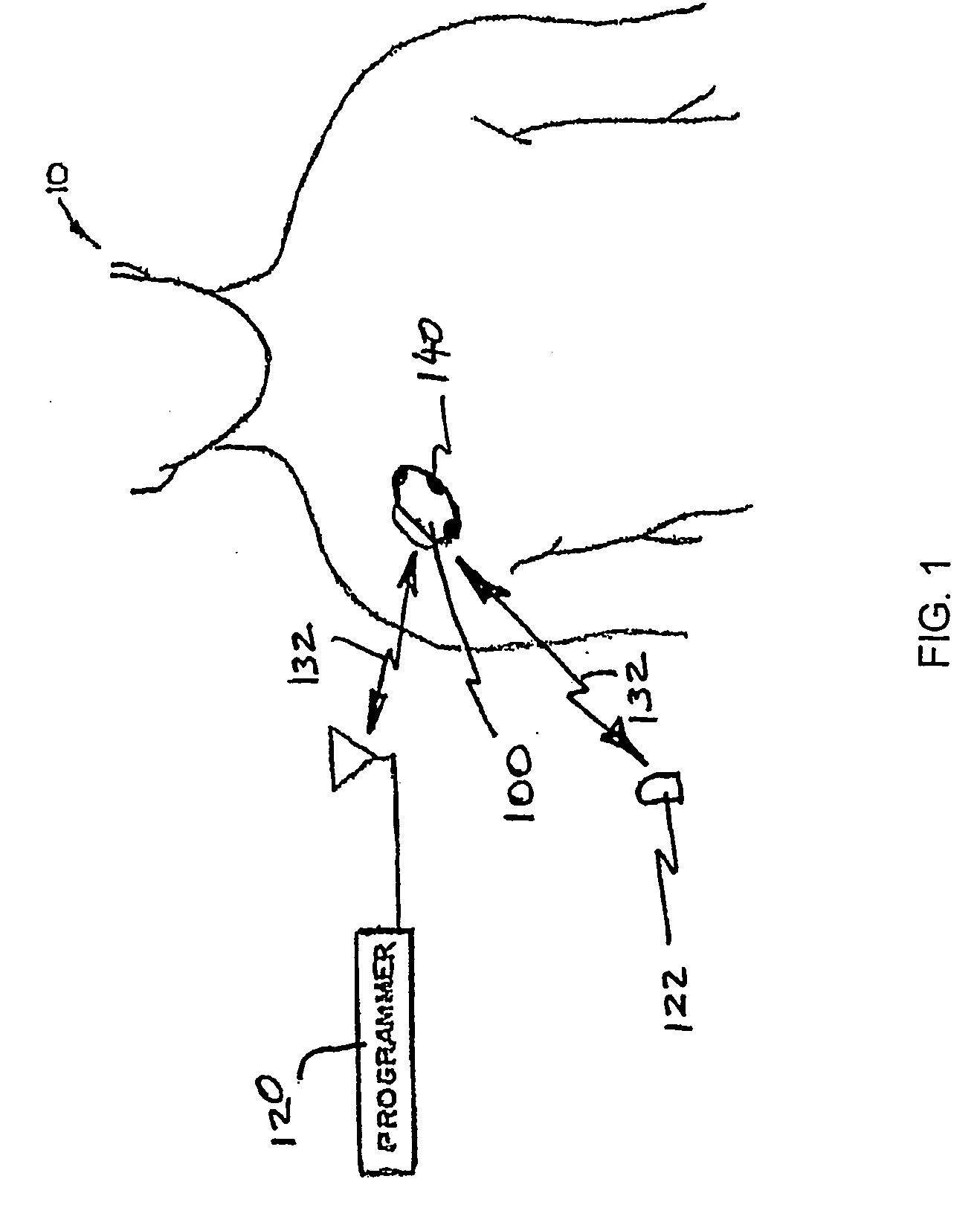
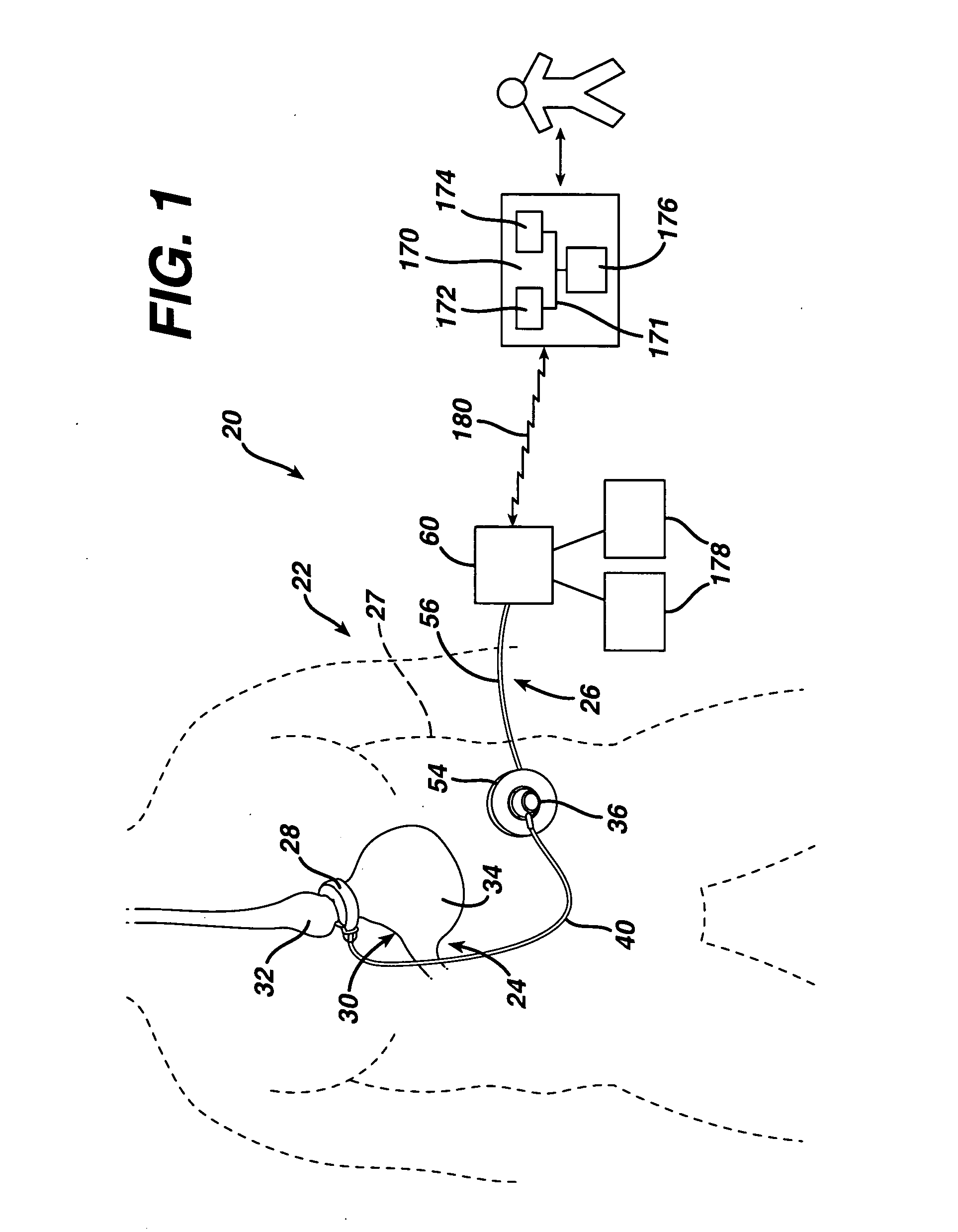
Initiating medical system communications
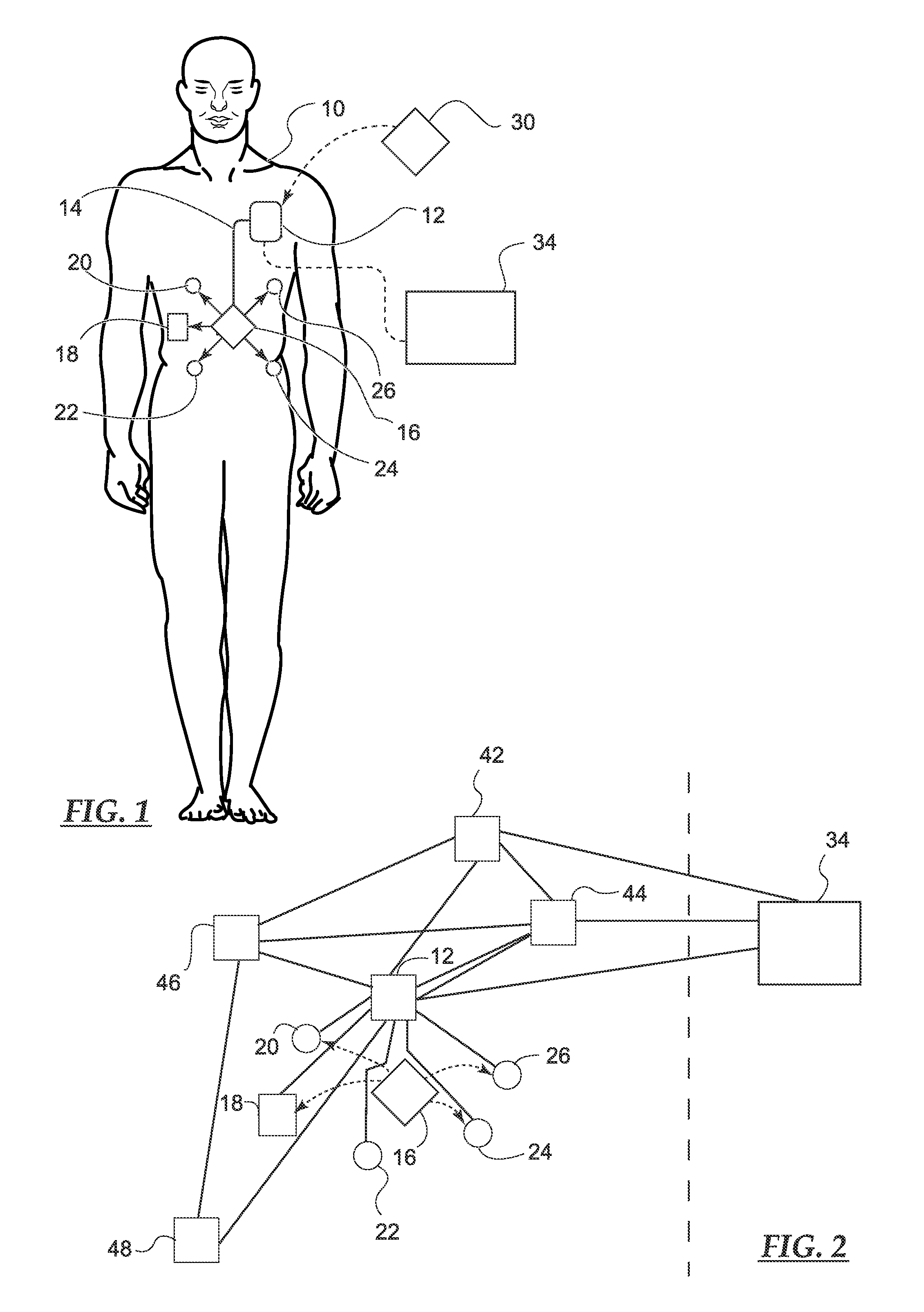
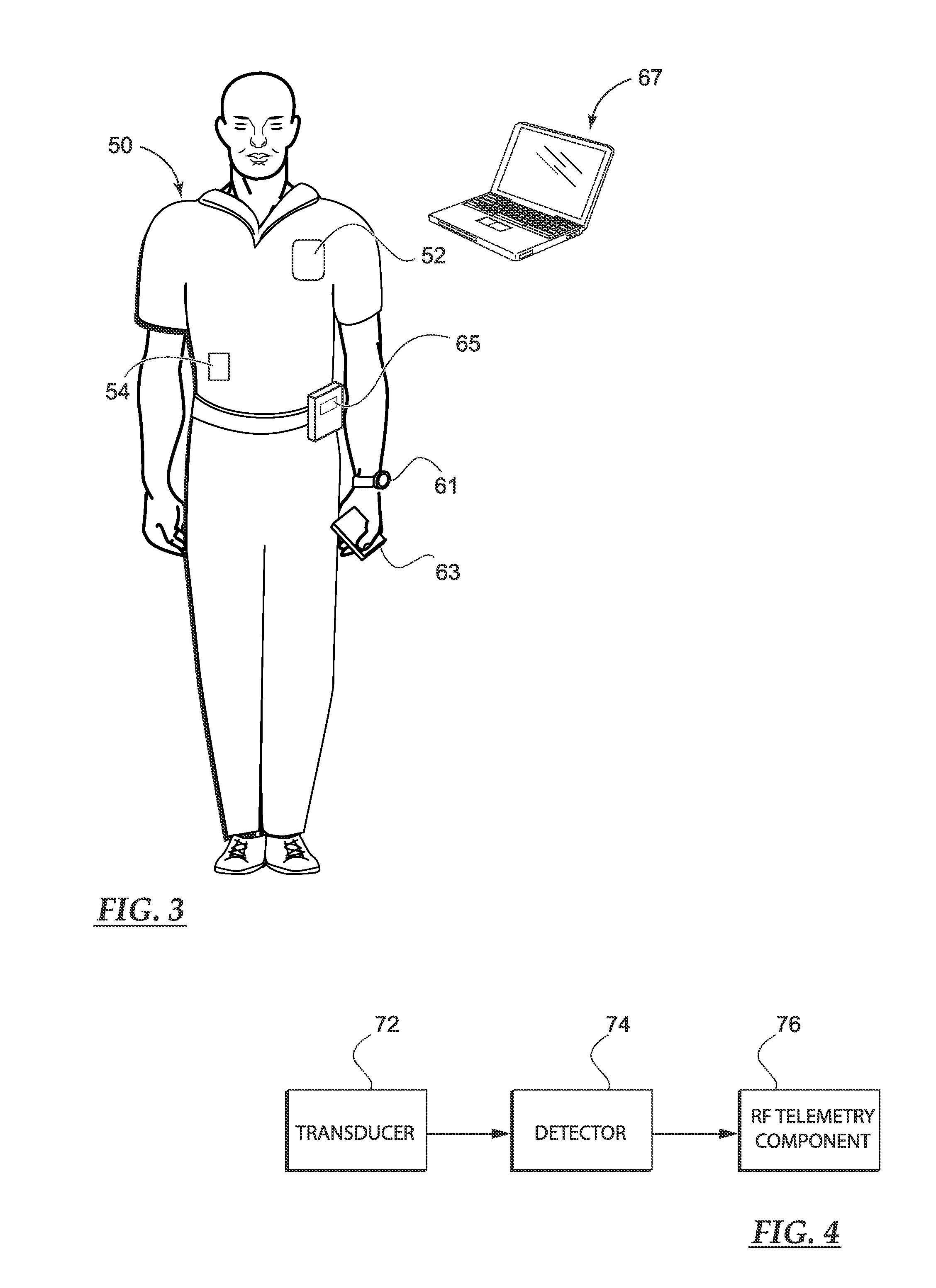
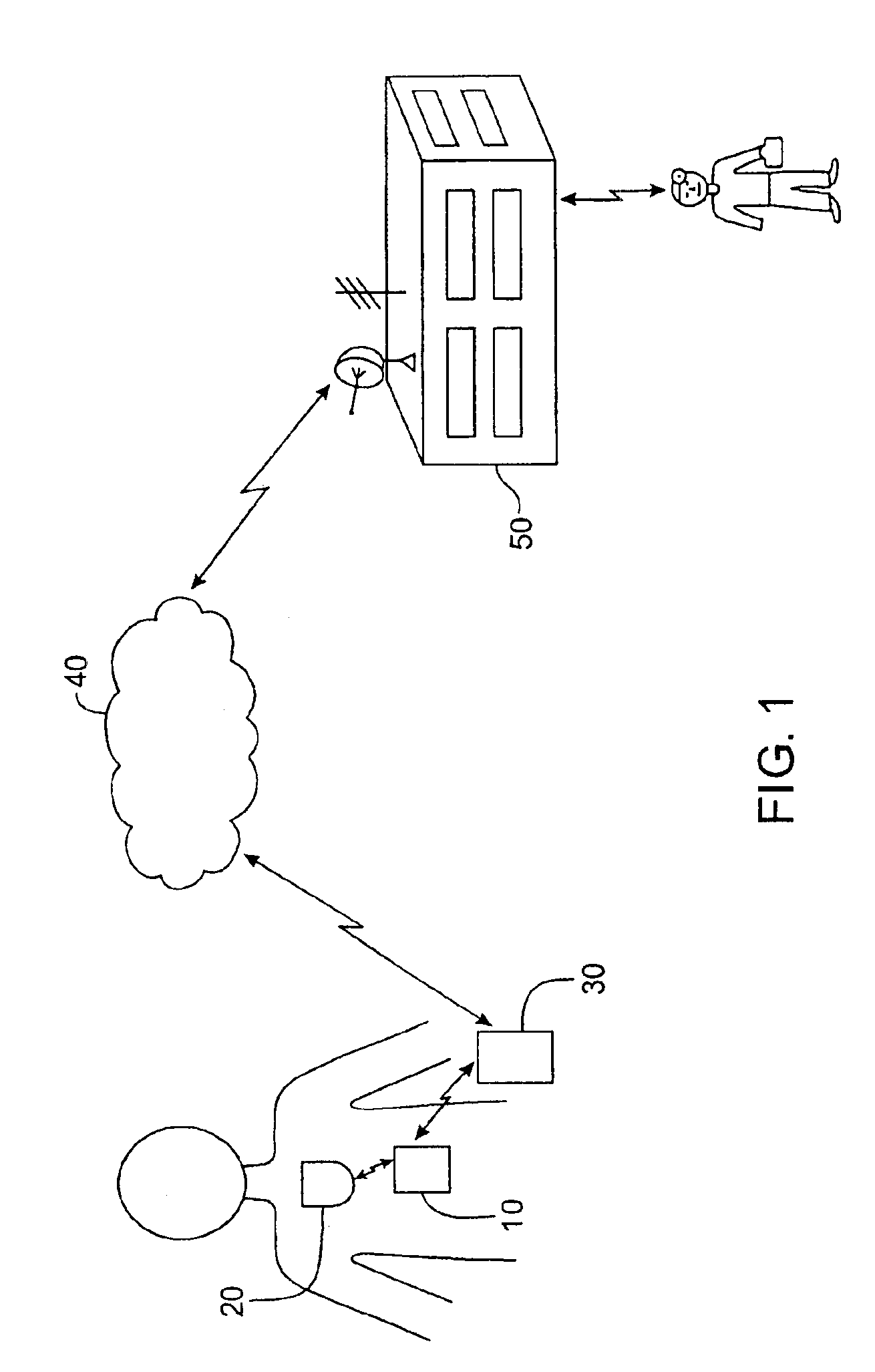
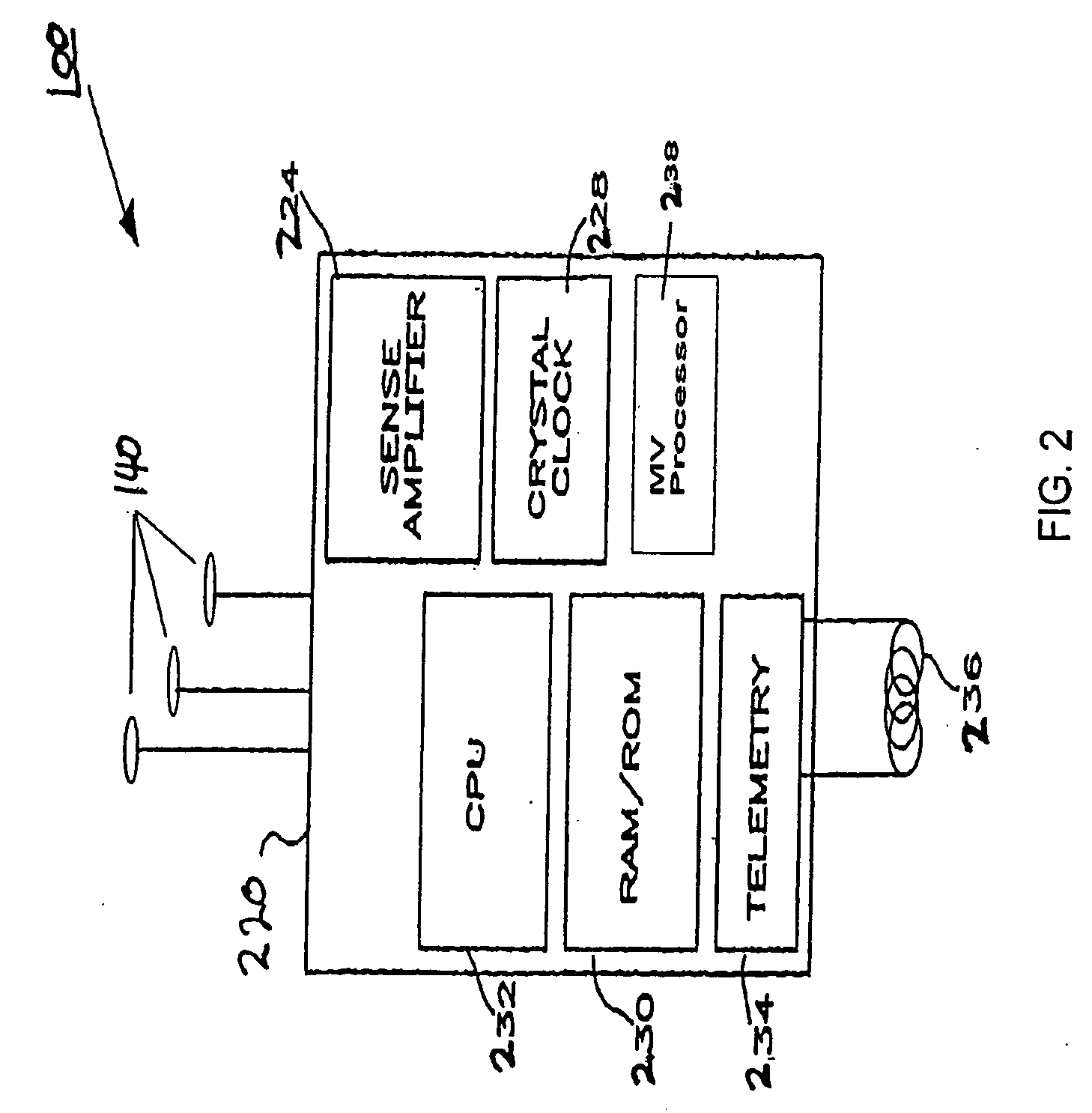
A medical system includes a body-contacting signal source adapted to transmit an oscillatory signal though a body to a transducer of a device implanted therein. A detector that is coupled to the transducer, upon detection of a response of the transducer to the signal, activates a radio-frequency (RF) telemetry component of the device.
Owner:MEDTRONIC INC
Method and apparatus for monitoring and communicating with an implanted medical device
InactiveUS6957107B2Simplify the surgical processMinimize impactElectrotherapySurgeryCommunication interfaceDevice implant
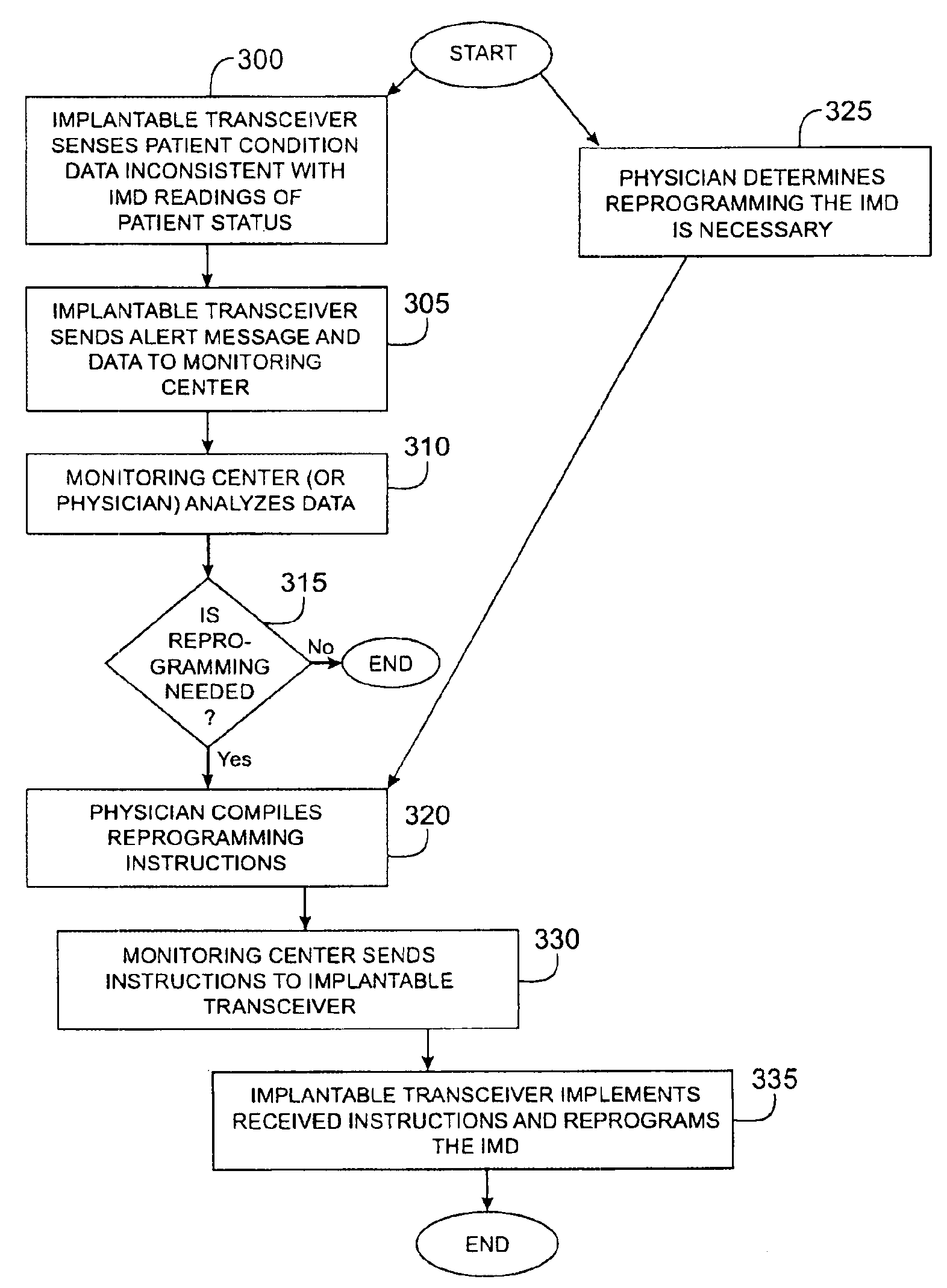
A method and apparatus for communicating with and monitoring the operation of a device implanted within a patient. A transceiver capable of being implanted within a patient provides a communication interface between an implanted medical device and a monitor external to the patient's body. The external monitor can communicate with a remote monitoring center over a communication network. The external monitor also provides control signals to the implanted device via the transceiver unit. The transceiver apparatus is capable of two-way communication between the implanted device and the external monitor. The transceiver apparatus is also capable of detecting actions performed by the implanted device and physiological signals directly from the patient's body. Thus, the transceiver apparatus provides circuitry for determining whether an implanted medical device is operating properly. The transceiver apparatus provides a way to remotely reprogram one or more implanted medical devices.
Owner:BRAEMAR MFG
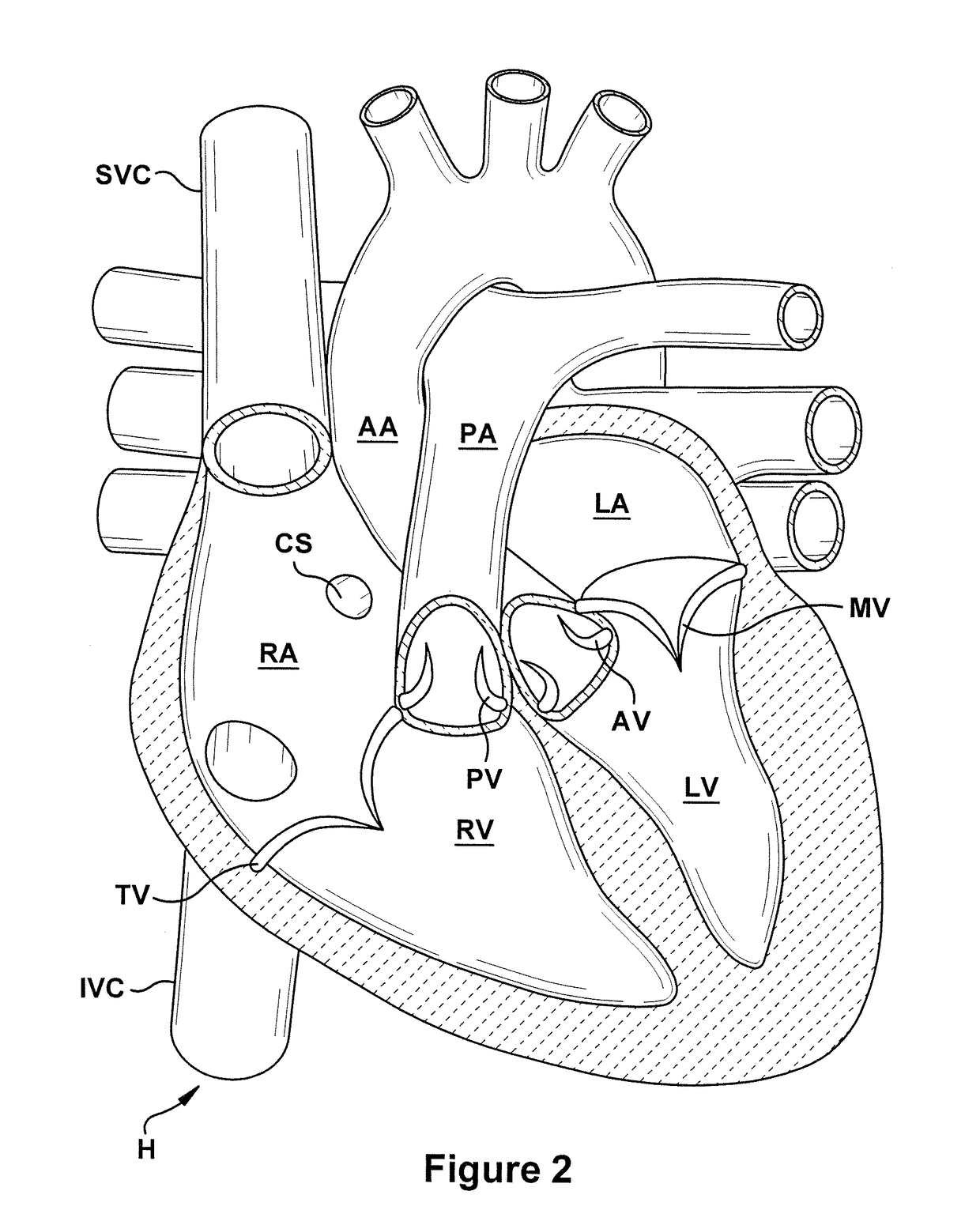
Devices and methods for transluminal or transthoracic interstitial electrode placement
ActiveUS7191015B2Epicardial electrodesTransvascular endocardial electrodesElectrode placementDevice implant
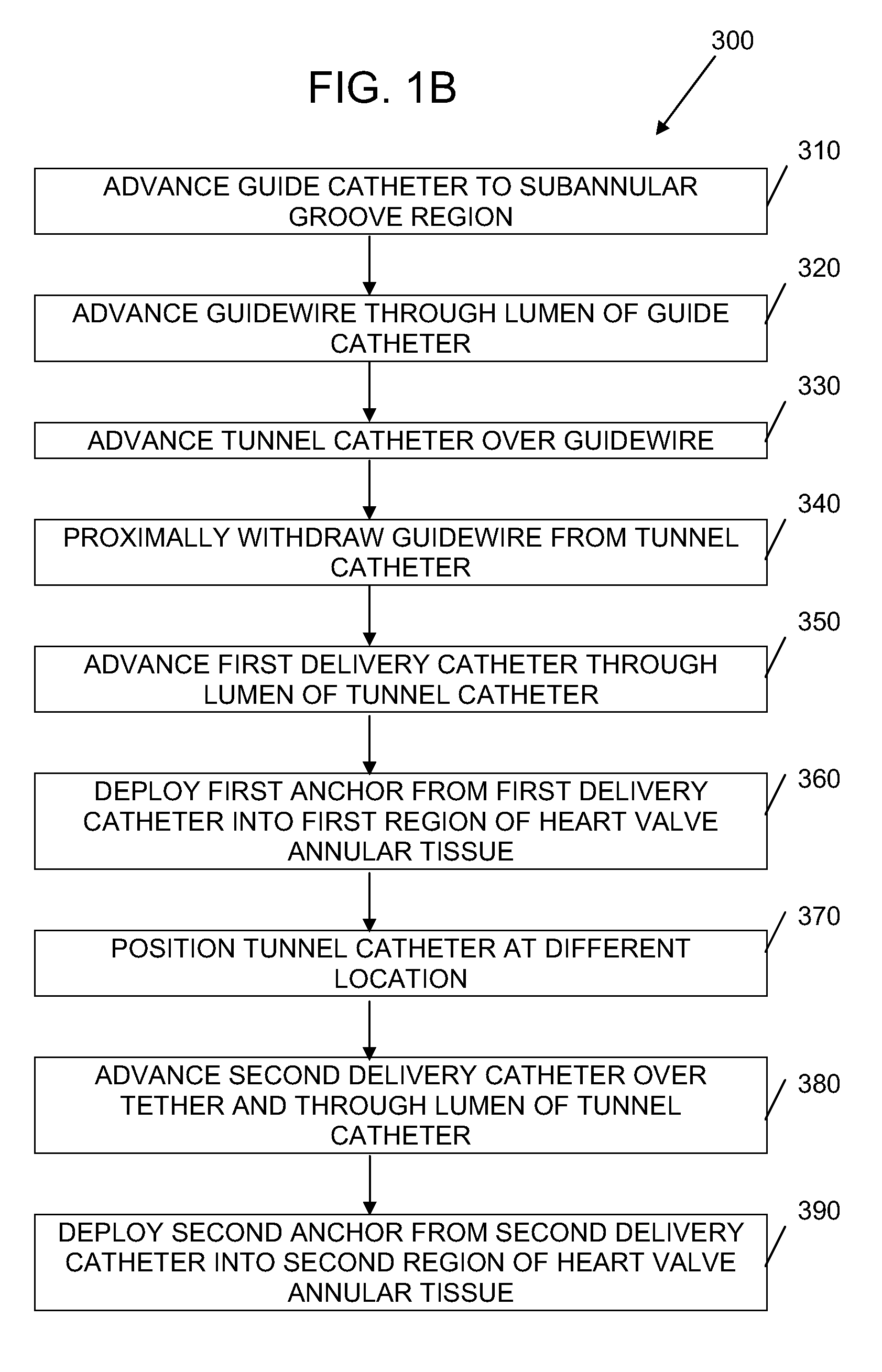
Methods and devices for implanting pacing electrodes or other apparatus, or for delivering substances, to the heart of other tissues within the body. A guided tissue penetrating catheter is inserted into a body lumen (e.g., blood vessel) or into a body cavity or space (e.g., the pericardial space) and a penetrator is advanced from the catheter to a target location. In some embodiments, a substance or an apparatus (such as an electrode) may be delivered through a lumen in the penetrator. In other embodiments, a guidewire may be advanced through the penetrator, the penetrating catheter may then be removed and an apparatus (e.g., electrode) may then be advanced over that guidewire. Also disclosed are various implantable electrodes and electrode anchoring apparatus.
Owner:MEDTRONIC VASCULAR INC
Methods and devices for the surgical creation of satiety and biofeedback pathways
InactiveUS20050267533A1Increase heightLonger-term implantationSuture equipmentsSurgical needlesDevice implantPatient feedback
Disclosed are devices and systems which to sense parameters related to the mechanical forces imposed on devices implanted in the stomach. The parameters of the system are further translated into patient feedback systems to create satiety inducing pathways in the stomach.
Owner:GERTNER MICHAEL
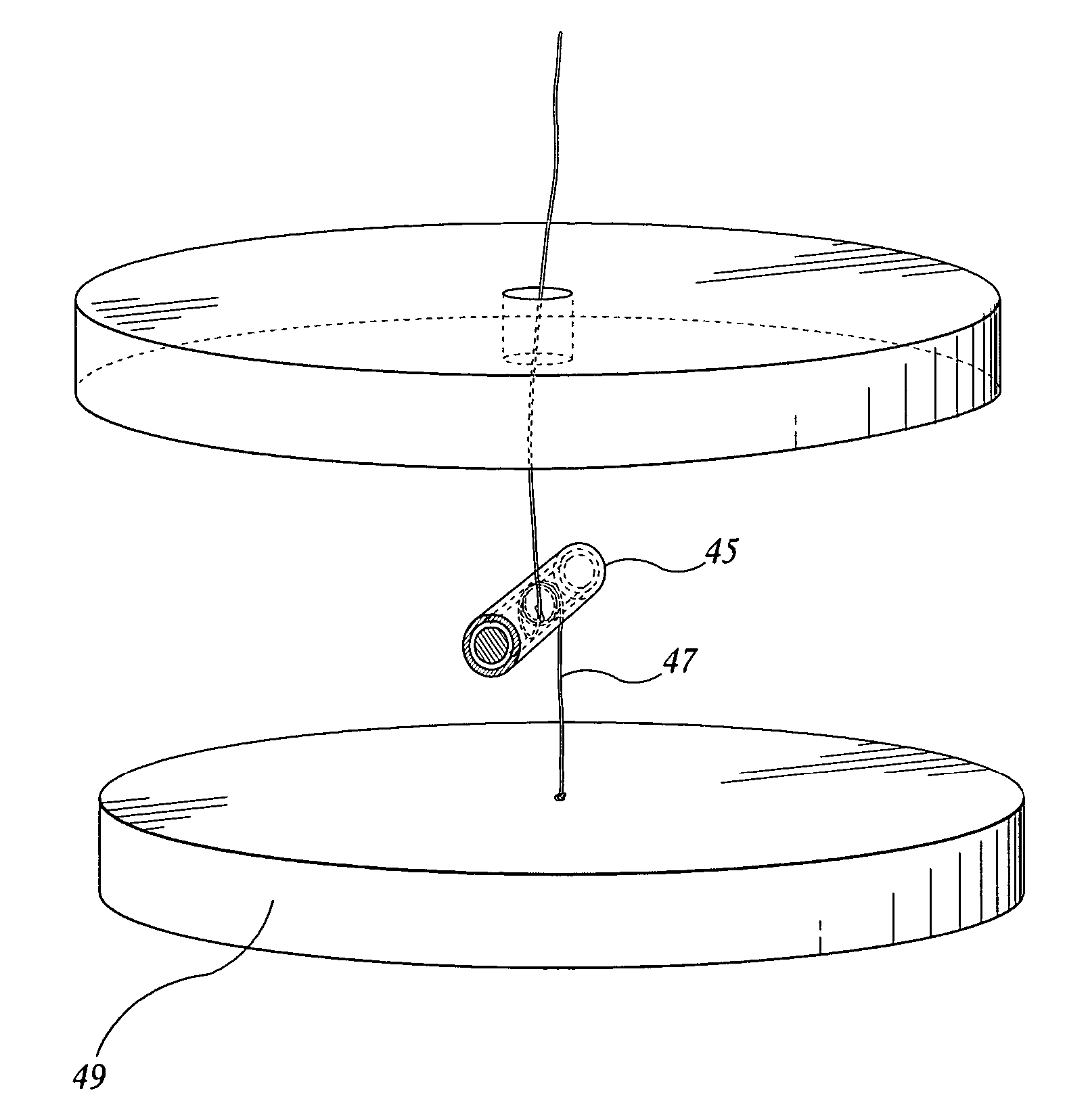
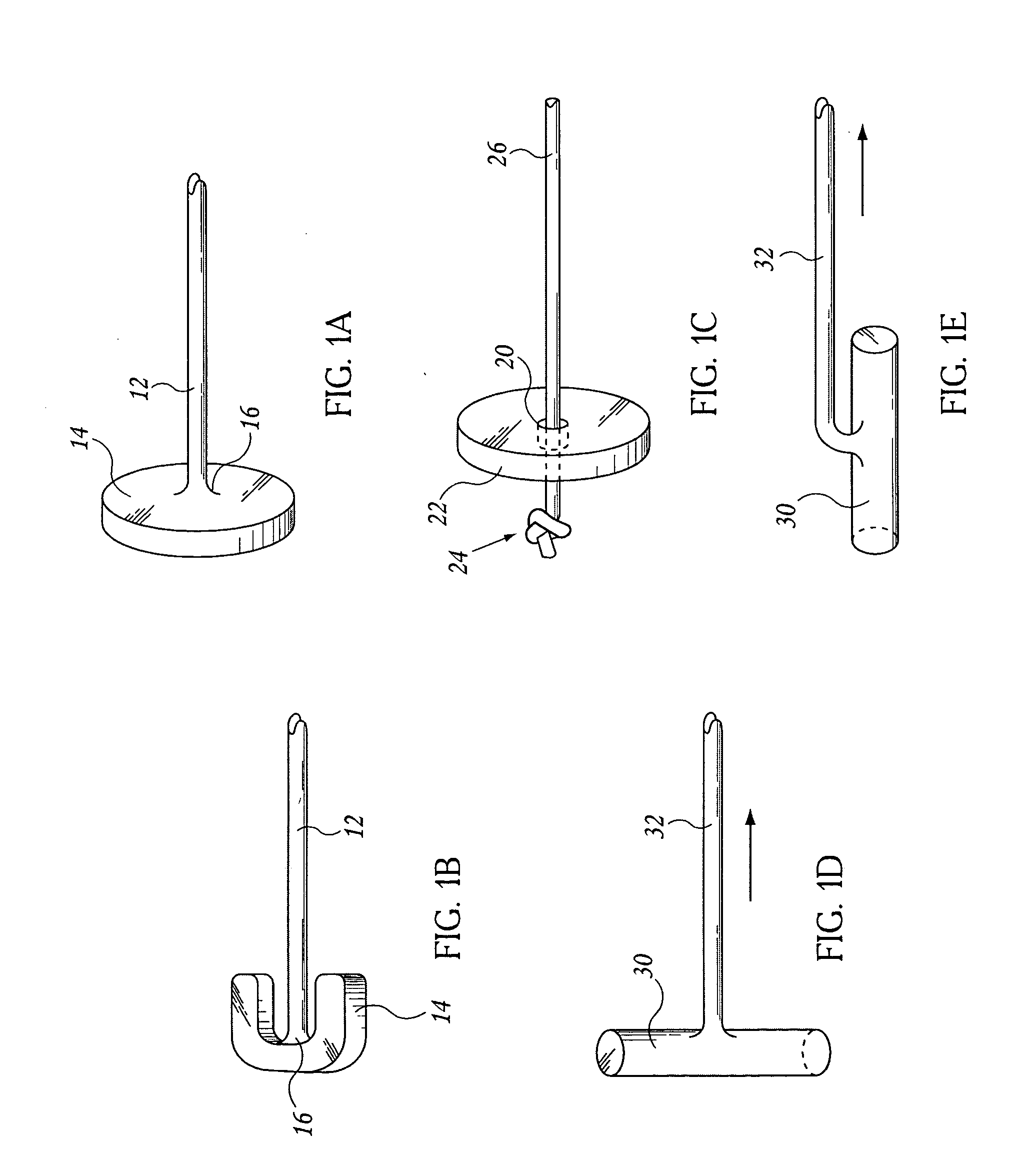
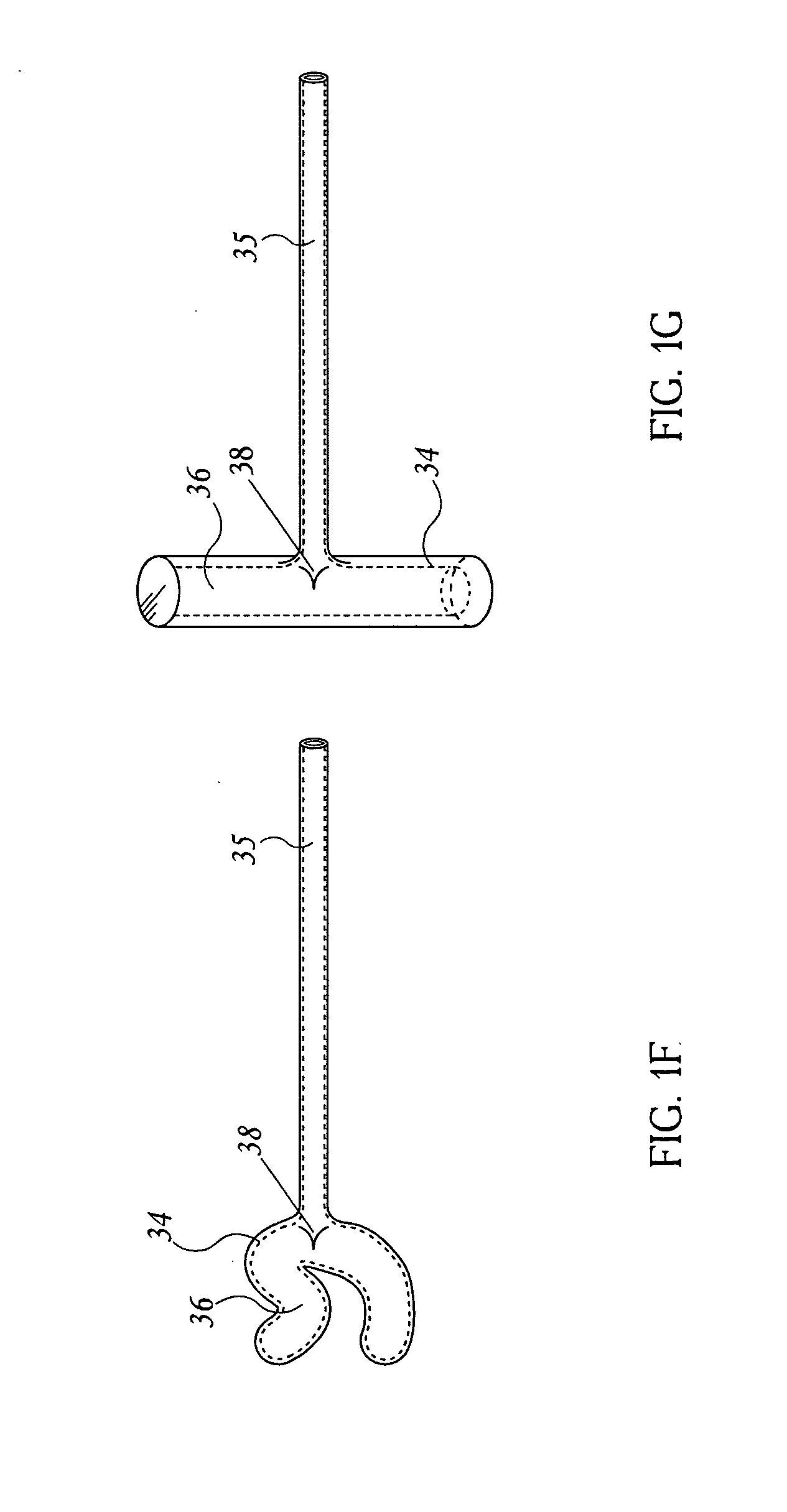
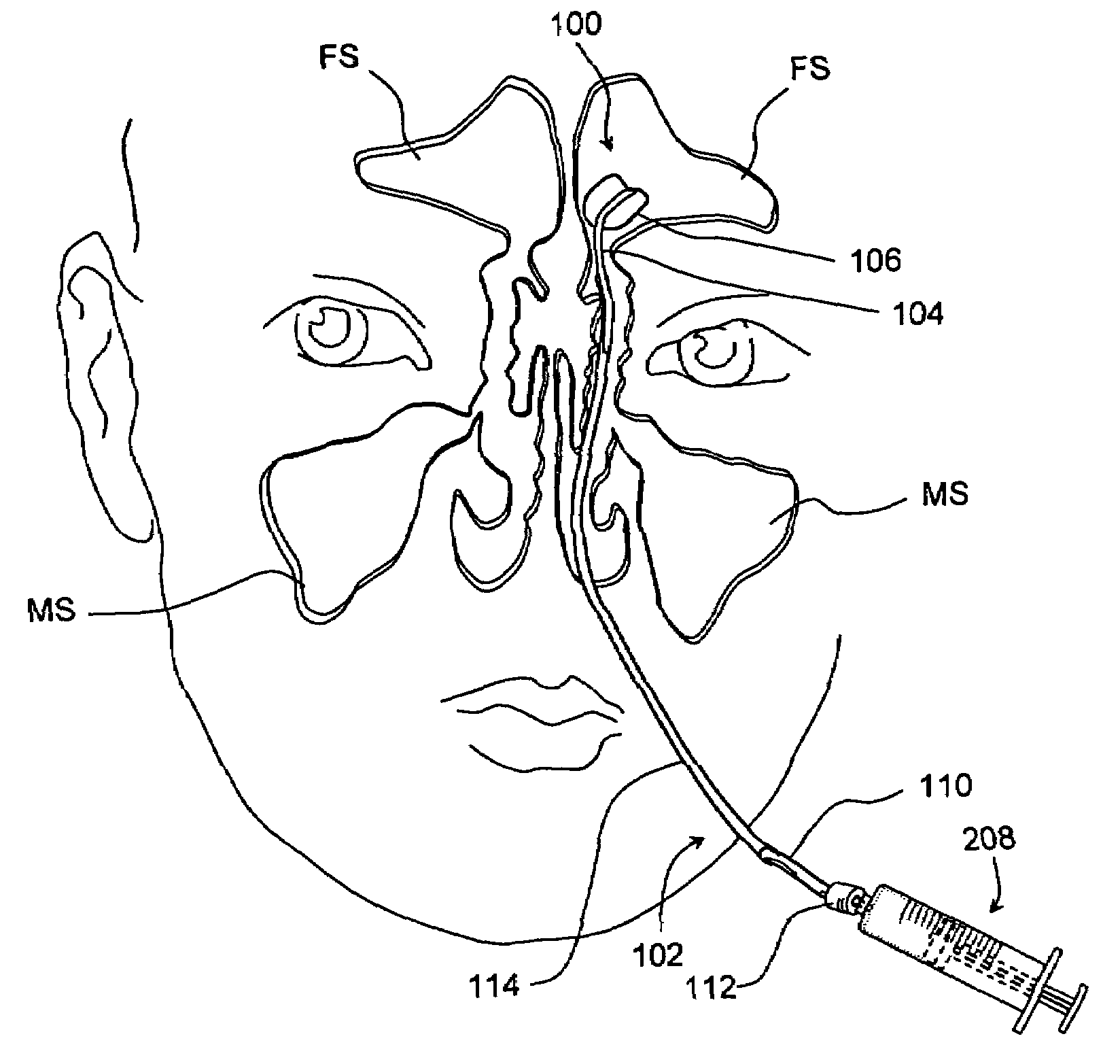
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
Method of implanting a device in the left atrial appendage
InactiveUS7044134B2Reduce decreaseReducing the implantable deviceStentsBalloon catheterDevice implantImplanted device
An adjustable occlusion device and a method of implanting the device in a body lumen such as the left atrial appendage. The occlusion device is removably carried by a deployment catheter. The device may be enlarged or reduced to facilitate optimal placement or removal. Methods are also disclosed.
Owner:BOSTON SCI SCIMED INC
Devices and methods to treat a patient
InactiveUS20050216040A1Reduce internal volumeReduction procedureSuture equipmentsElectrotherapyDevice implantSurgical device
Disclosed are methods and apparatus for implantation into the walls of an organ such as the stomach. Deformable or inflatable anchors with a connector between are used to pull the walls of the organ together, or to implant devices in the wall of the organ. Also disclosed are surgical instruments useful in practicing the disclosed methods.
Owner:GERTNER MICHAEL +2
Systems and methods for timing-based communication between implantable medical devices
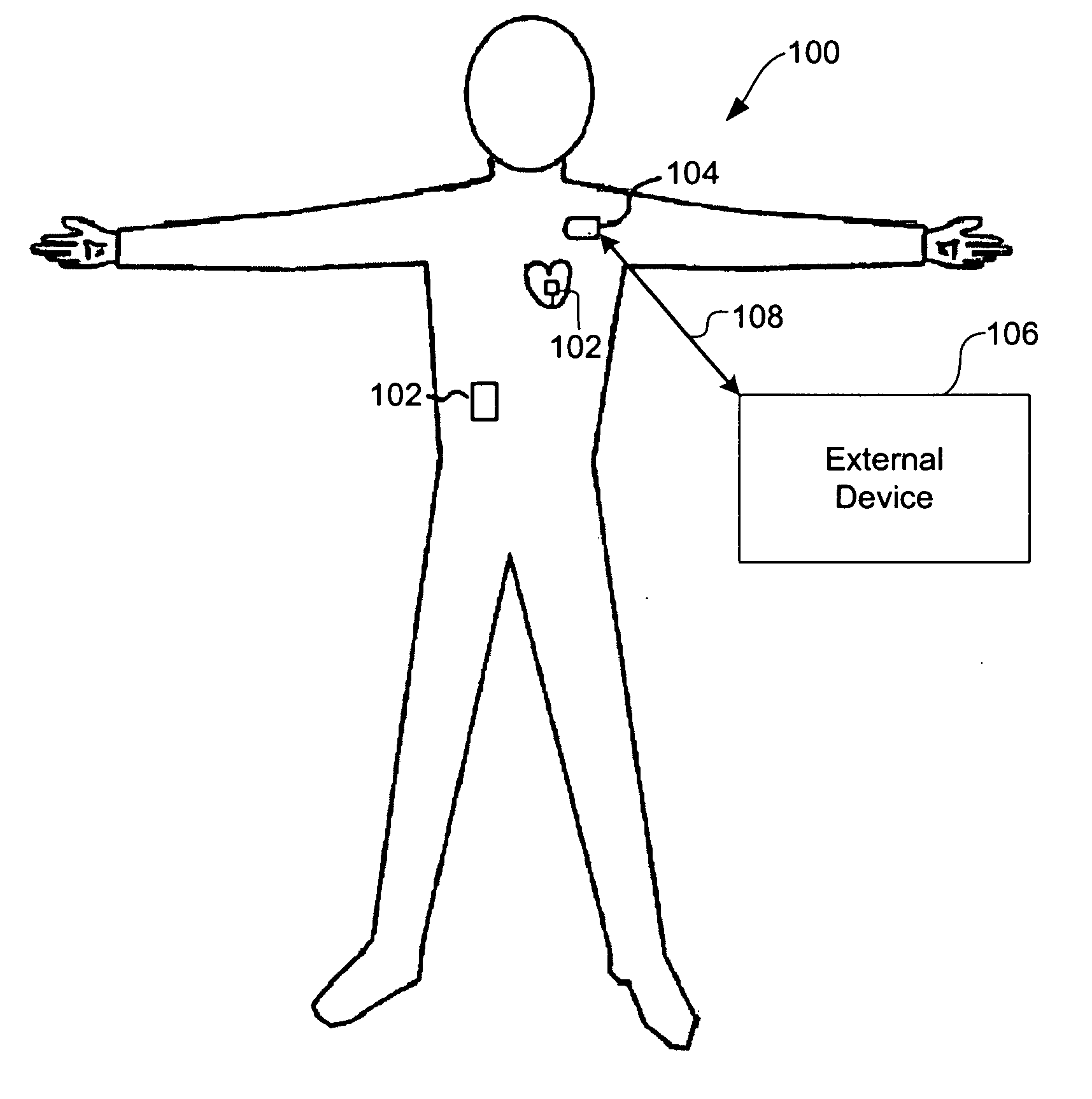
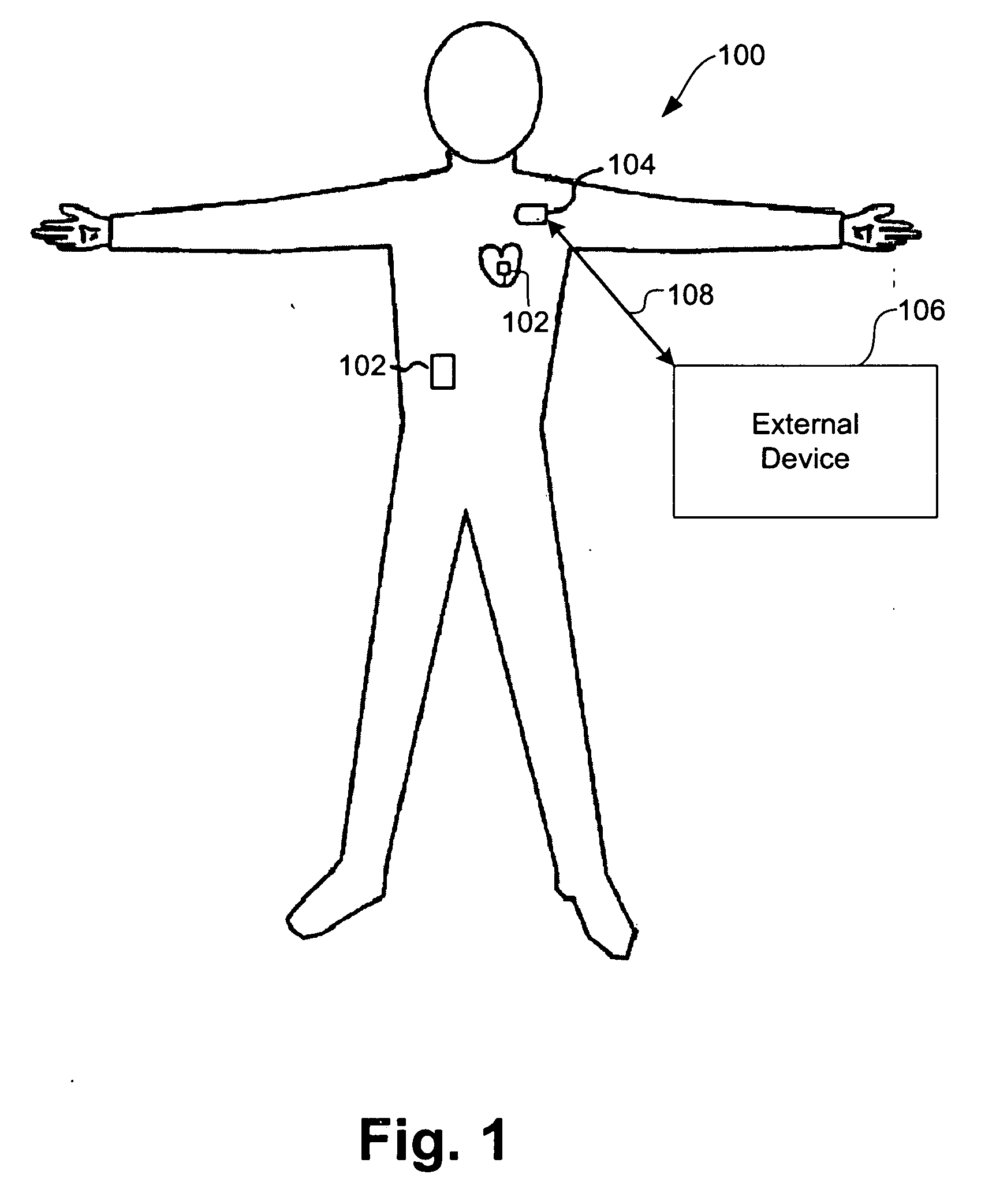
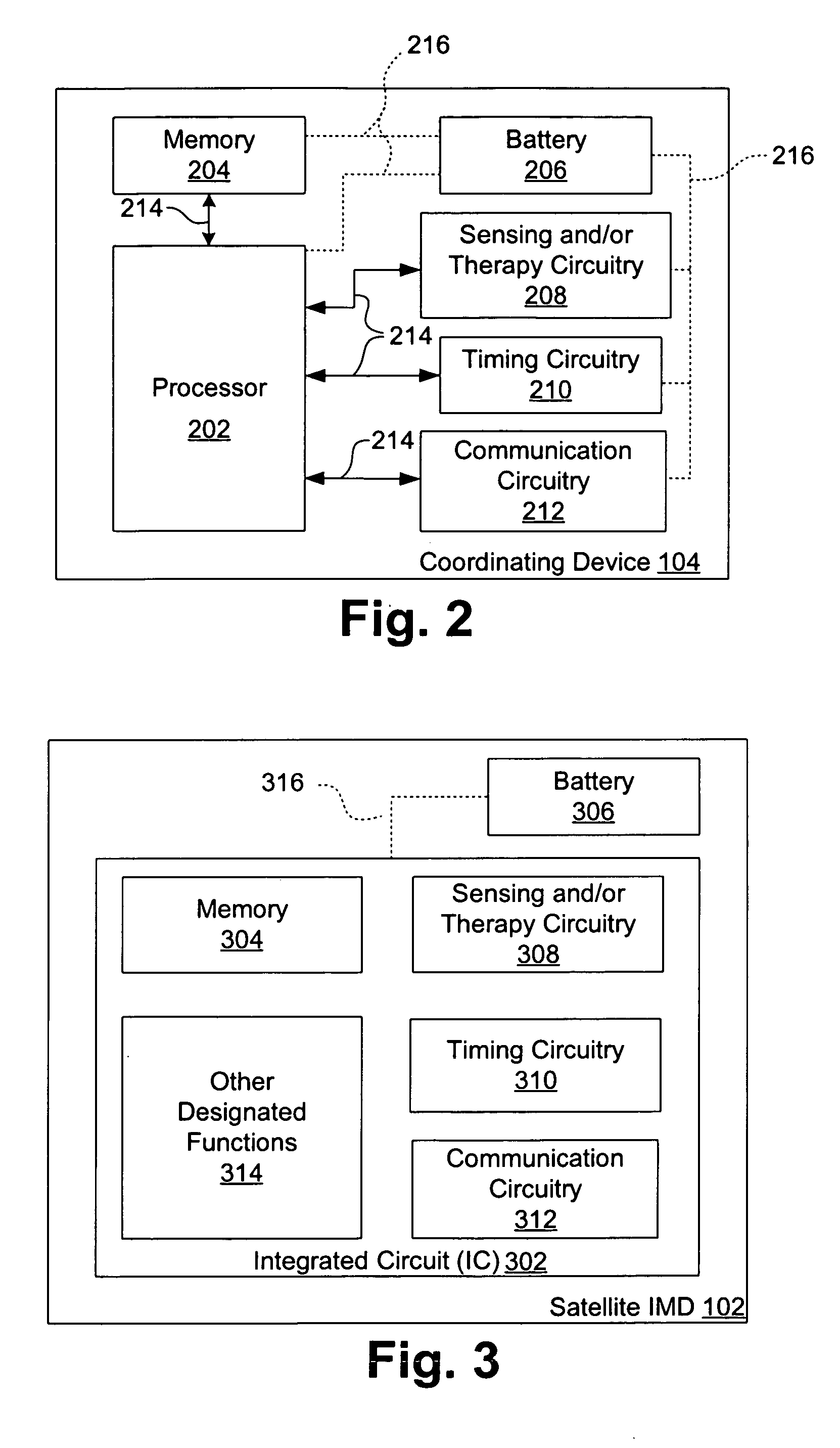
A method carried out by an implantable medical device (IMD) for coordinating performance of one or more designated functions includes waiting in a low-power state for a predetermined event, detecting the predetermined event, and, responsive to detecting the predetermined event, searching for a wake-up command from a coordinating device implanted in the human body. The method further includes, receiving the wake-up command, and responsive to receiving the wake-up command, performing the one or more designated functions, and returning to the low-power state. A system includes a network of one or more implantable medical devices (IMDs) implanted in a human body. The system includes a satellite IMD operable to change between a plurality of power states, search for a wake-up command, and transmit an identification signal. The system may include a primary unit operable to receive the signal and coordinate a wake-up time based on the signal.
Owner:CARDIAC PACEMAKERS INC
Percutaneous gastroplasty
InactiveUS20050216042A1Lower the volumeEfficient use ofSuture equipmentsSurgical needlesDevice implantSurgical device
Disclosed are methods and apparatus for implantation into the walls of an organ such as the stomach. Deformable or inflatable anchors with a connector between are used to pull the walls of the organ together, or to implant devices in the wall of the organ. Also disclosed are surgical instruments useful in practicing the disclosed methods.
Owner:GERTNER MICHAEL
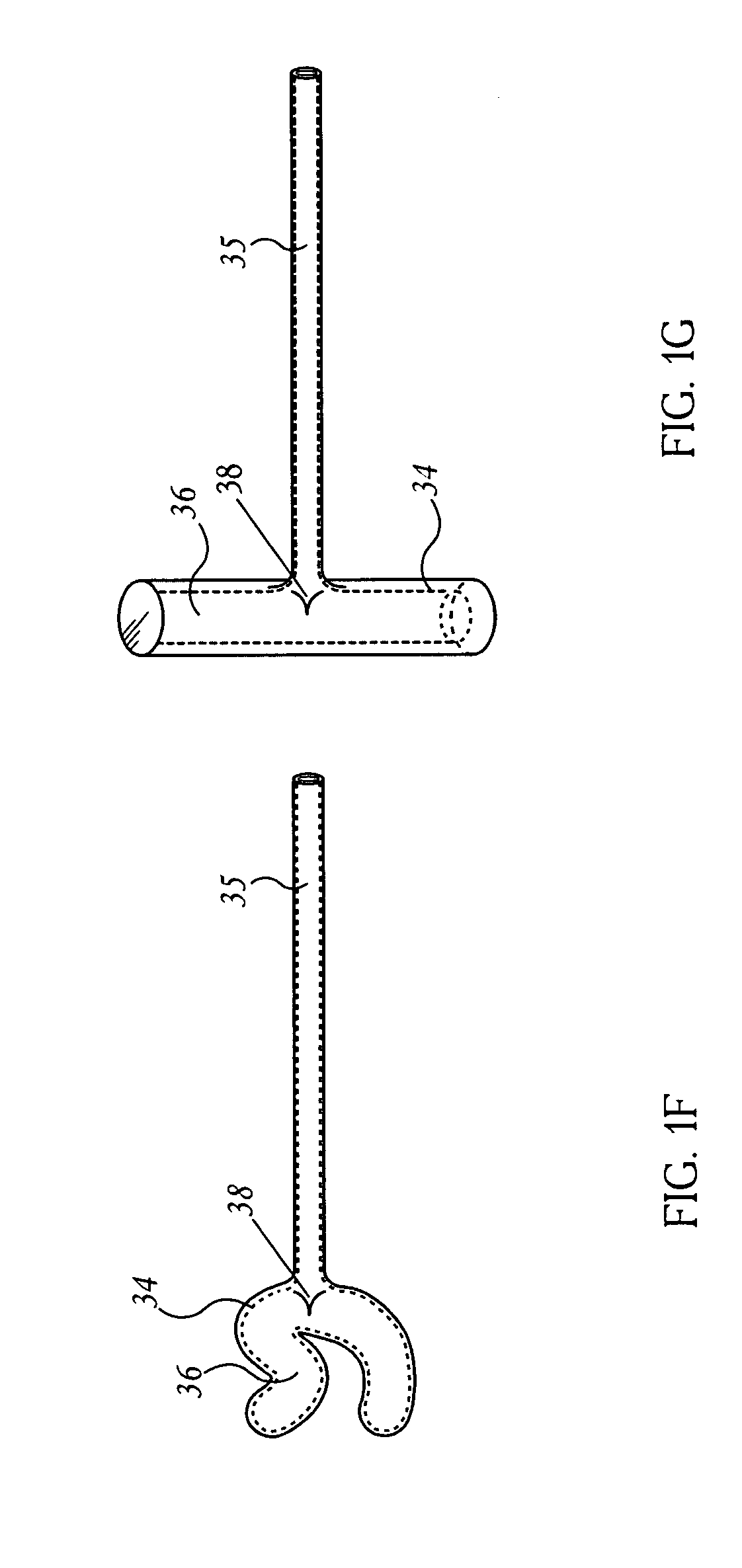
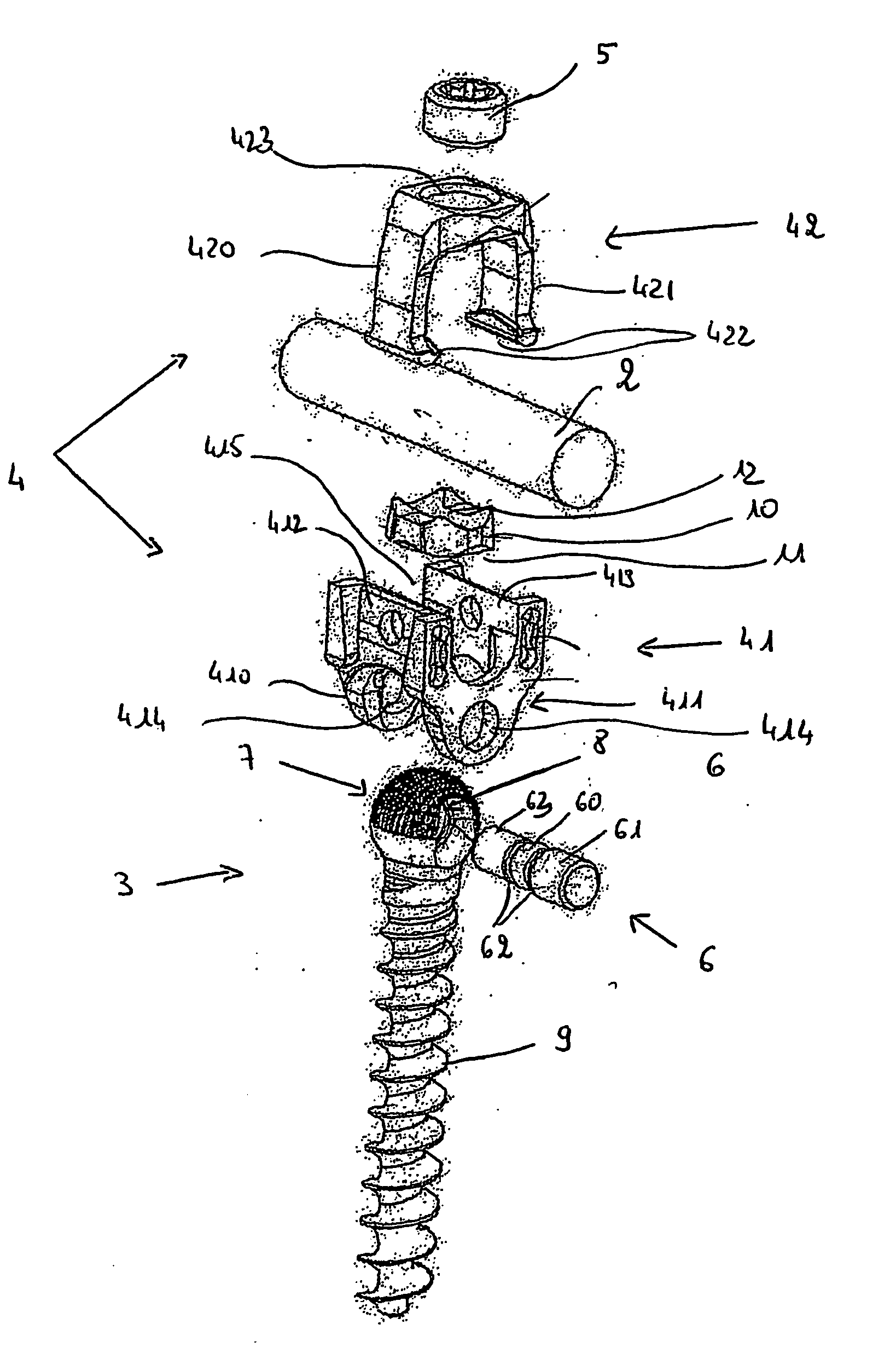
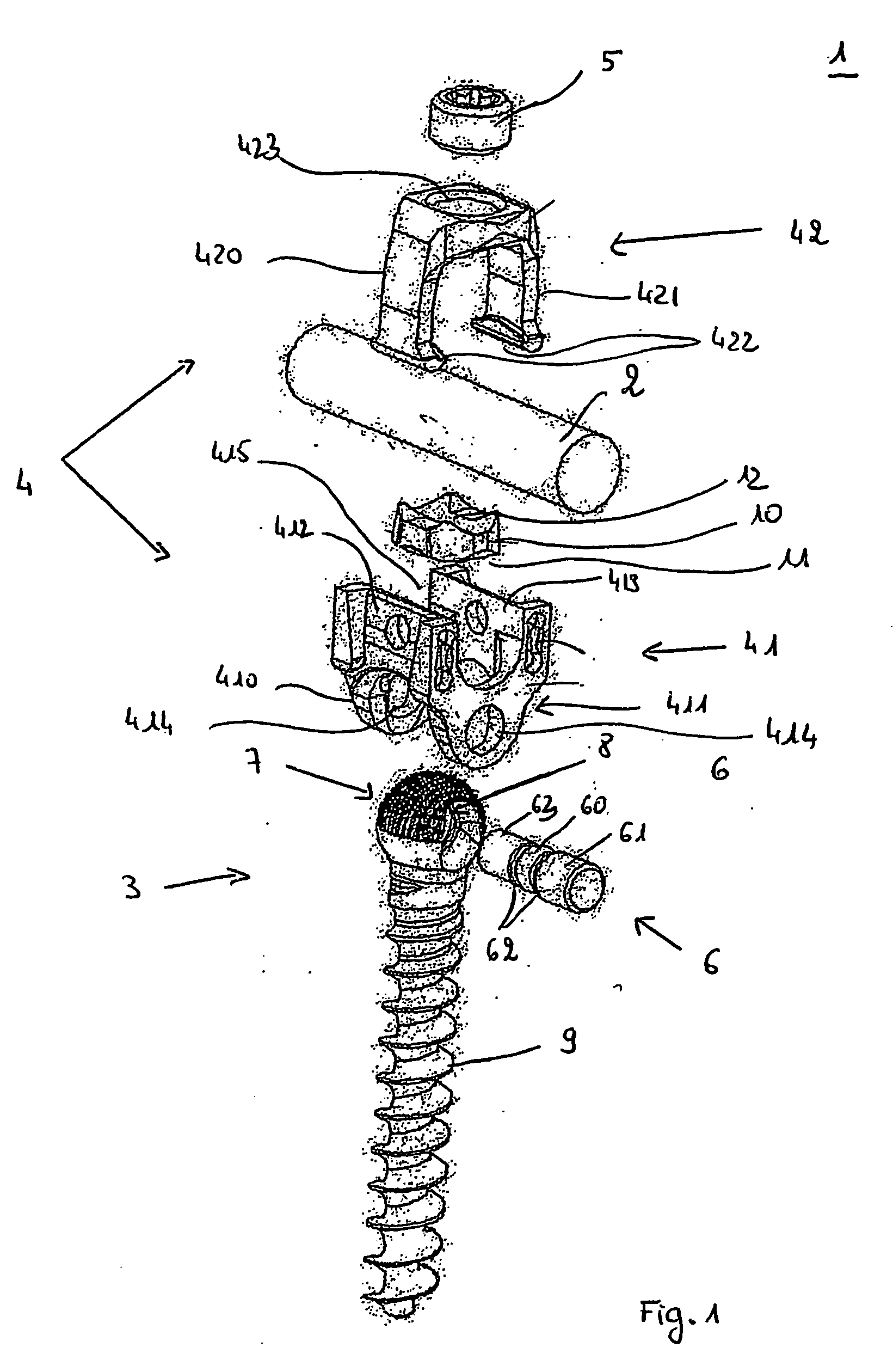
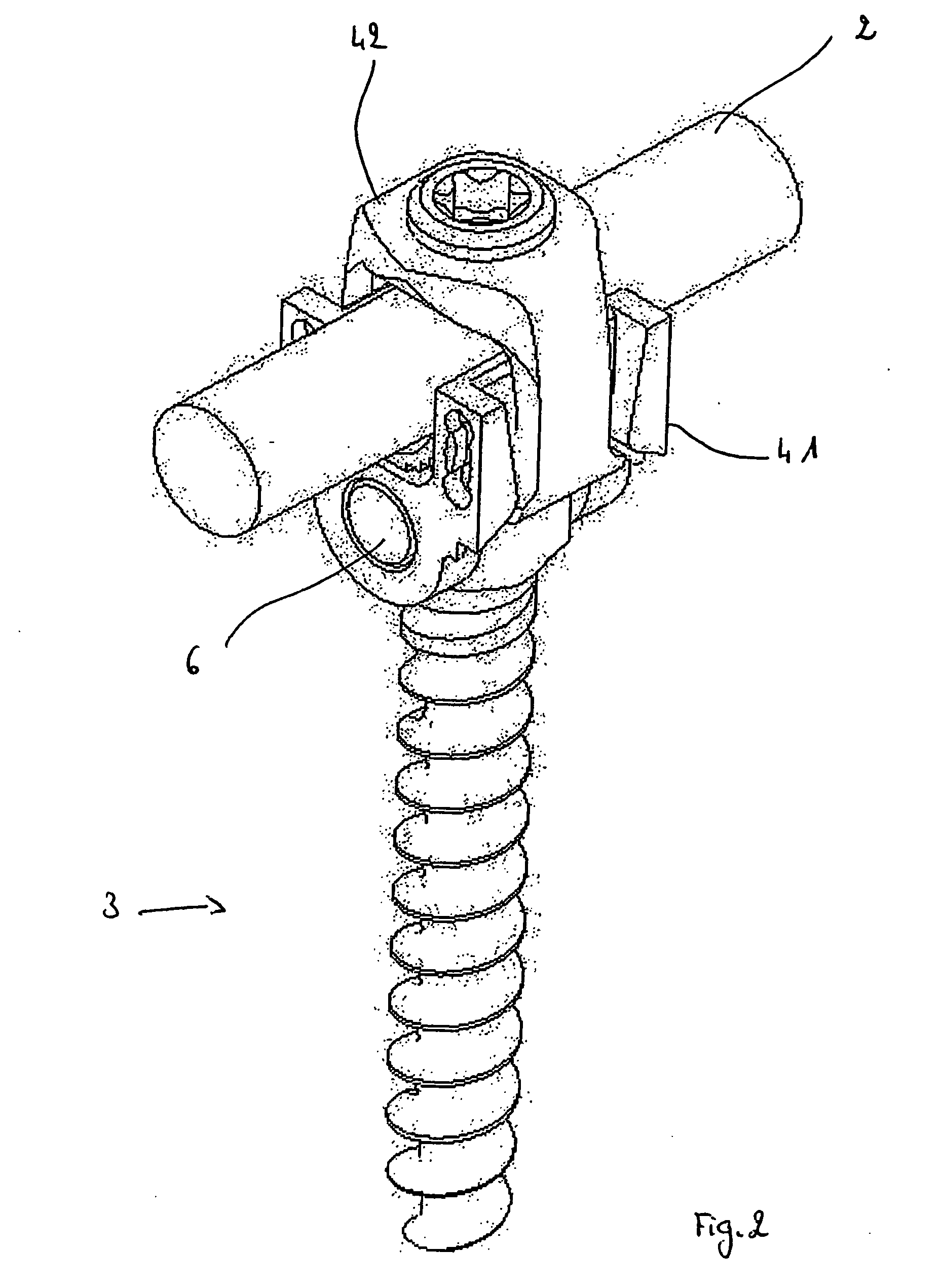
Connecting device for spinal osteosynthesis
ActiveUS20070043355A1Improve completenessSimple processInternal osteosythesisJoint implantsDevice implantPivot joint
A connecting device for spinal osteosynthesis has an osseous anchoring device, a connector that accommodates a joining shaft and is fixed to one end of the anchoring device, and a tightening device for immobilizing the joining shaft. The connecting device is characterized in that the connector is fixed to the anchoring device with the aid of a pin that penetrates the anchoring device and embodies a pivot joint. Furthermore, the device comprises an arrangement for stabilizing the connector relative to the anchoring device when the device is implanted in the patient, the stabilizing arrangement being located on the plane encompassing the shaft and the anchoring device.
Owner:SPINEVISION
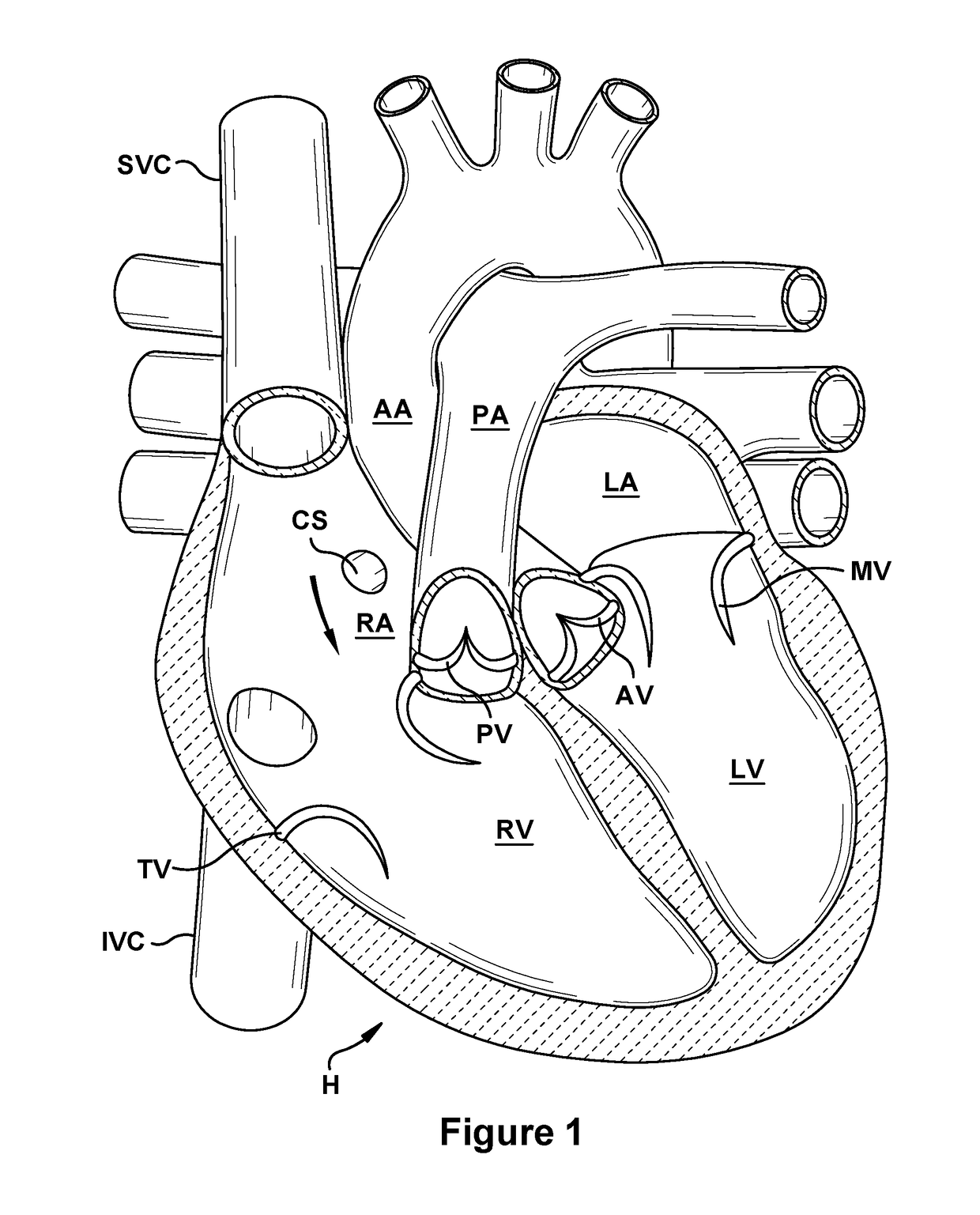
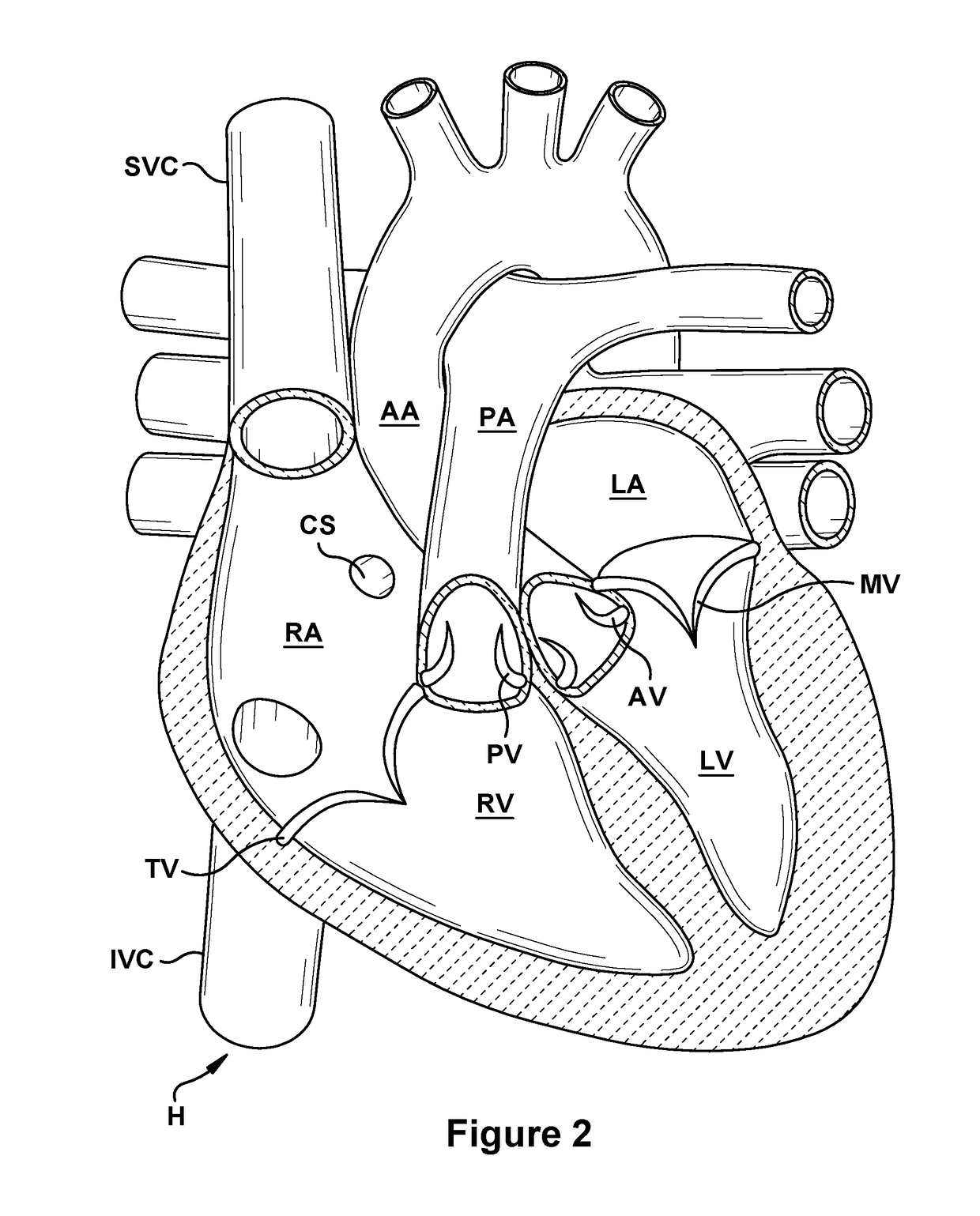
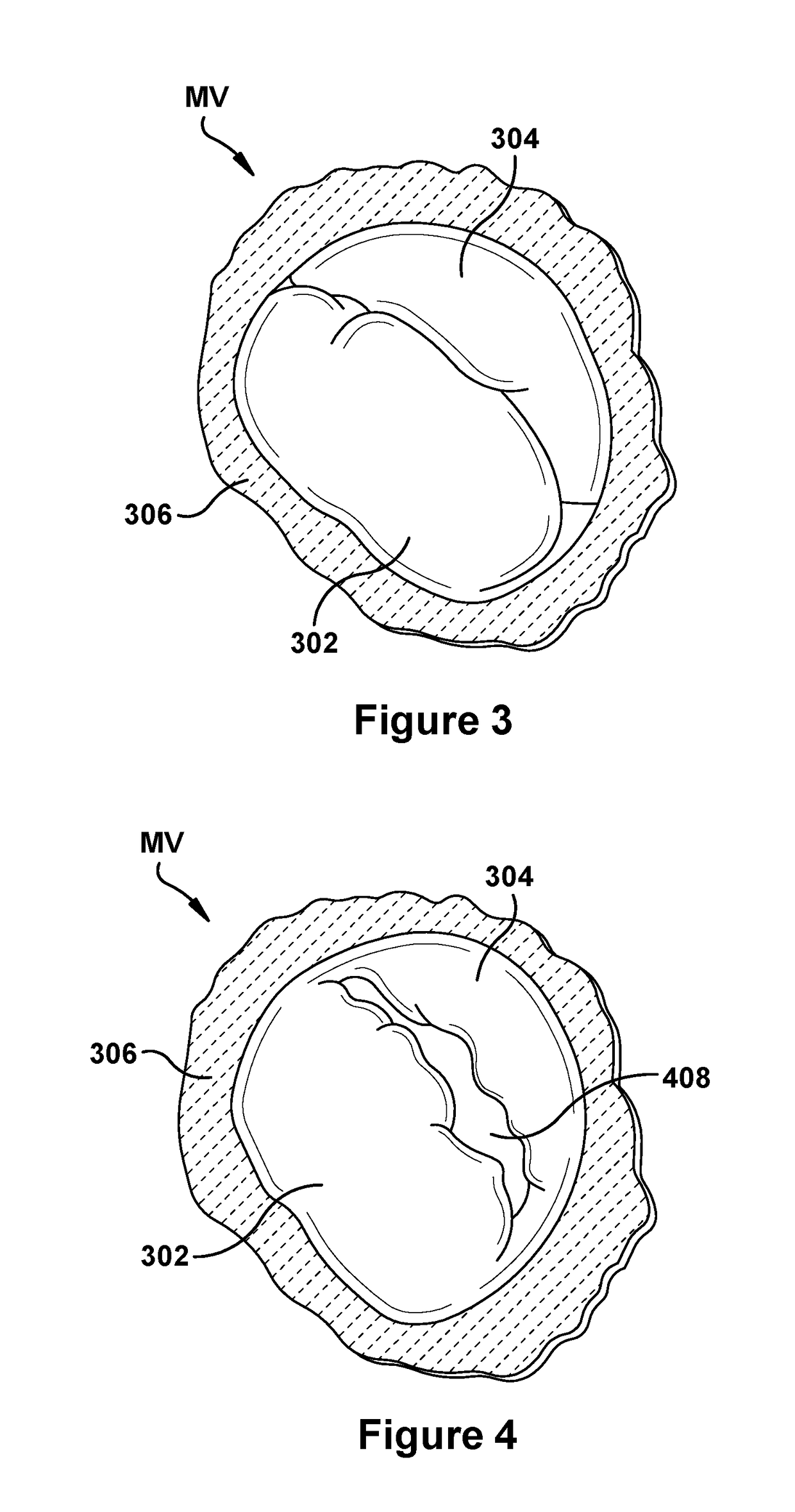
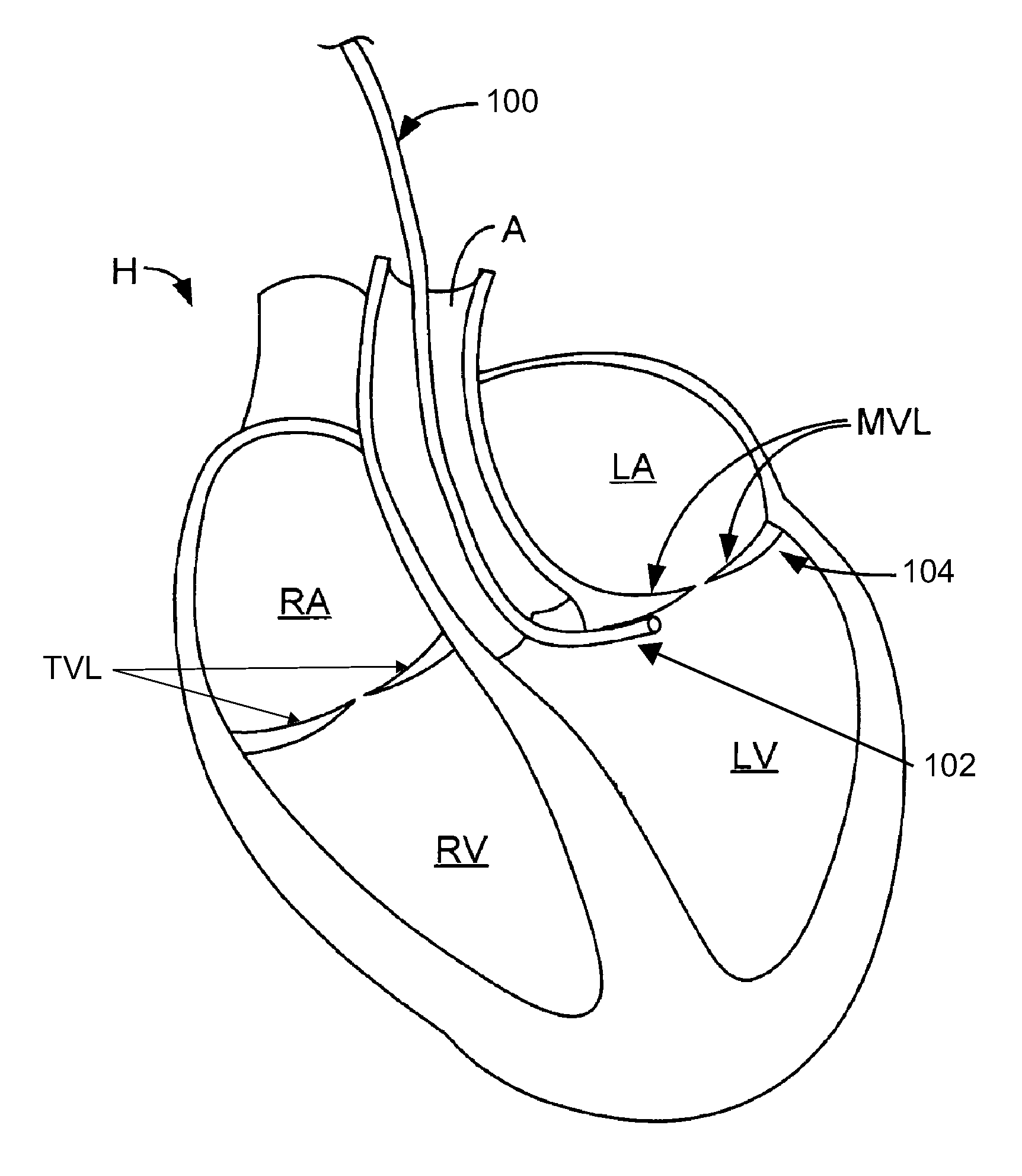
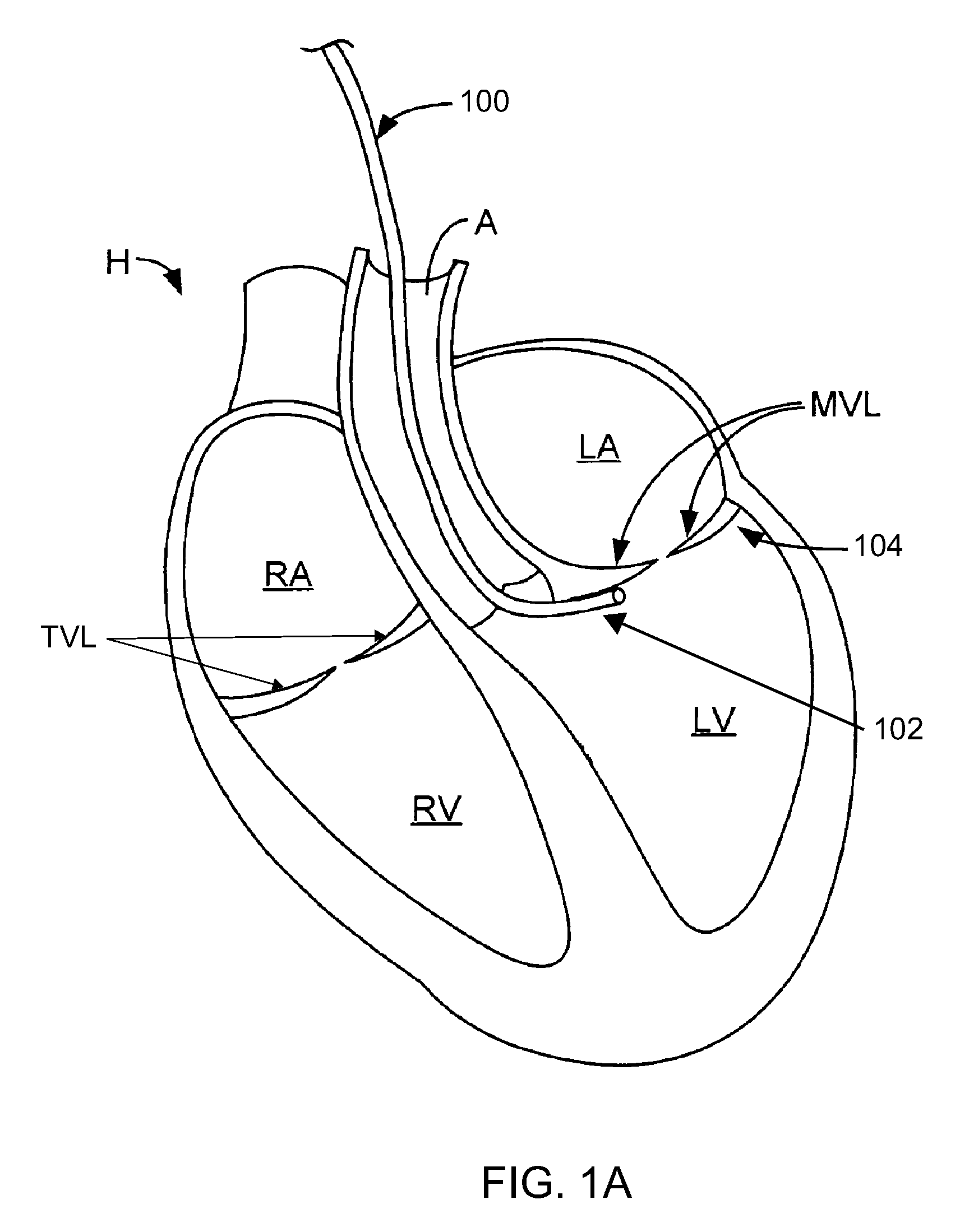
Native valve repair devices and procedures
A system for implanting a repair device onto a native valve of a natural heart to repair the native valve of a patient during a non-open-heart procedure. The system includes a base assembly having a plurality of pivoting links, a pair of paddles attached to the base assembly, a pair of gripping members attached to the base assembly, and at least one coaptation or spacer element disposed between the pair of gripping members. Movement of the pivoting links of the base assembly causes the paddles to move between an open position and a closed position. The paddles and the gripping members are configured to attach to valve leaflets of the patient. The coaptation or spacer element is configured to fill at least a portion of a gap between the valve leaflets of the patient.
Owner:EDWARDS LIFESCIENCES CORP
Devices and methods for treatment of obesity
ActiveUS20070250020A1Avoid expansionAvoid insufficient lengthSuture equipmentsDilatorsDevice implantPERITONEOSCOPE
Implantable devices, instruments, kits and methods for treatment of obesity. One or more devices can be implanted adjacent to or in contact with the stomach to occupy a space to prevent the stomach from expanding into that space as food is taken into the stomach. Alternatively, one or more devices may be implanted and expanded to displace at least a portion of the wall of the stomach to decrease the internal volume of the stomach that is available to receive food. Devices may be anchored to one or more internal structures at one or more locations without piercing through the wall of the stomach. Devices can be implanted using minimally invasive methods, such as percutaneous or laparoscopic methods. Delivery instruments are also provided. An intra-gastric sizing device is provided to facilitate implantation of an extra-gastric device in some method embodiments.
Owner:VIBRYNT
Apparatus for data retention in an implantable medical device
ActiveUS20060095091A1Physical therapies and activitiesHeart stimulatorsDevice implantImplanted device
An apparatus for storing data records associated with a medical monitoring event in a data structure. An implanted device obtains data and stores the data in the data record in a first data structure that is age-based. Before an oldest data record is lost, the oldest data record may be stored in a second data structure that is priority index-based. The priority index may be determined by a severity level and may be further determined by associated factors. The implanted device may organize, off-load, report, and / or display a plurality of data records based on an associated priority index. Additionally, the implanted device may select a subset or composite of physiologic channels from the available physiologic channels based on a selection criterion.
Owner:MEDTRONIC INC
Native valve repair devices and procedures
A system for implanting a repair device onto a native valve of a natural heart to repairing the native valve of a patient during a non-open-heart procedure. The system includes a surgical delivery instrument, a valve repair device, and a gripper control mechanism. The surgical delivery instrument has at least one lumen. The valve repair device is configured to be delivered through the lumen of the surgical delivery instrument and to be attached to a native valve of a patient. The valve repair device has a pair of paddles, and first and second gripping members. The paddles are movable between an open position and a closed position. The paddles and the first and second gripping members are configured to attach to the native valve of the patient. The gripper control mechanisms each include a suture and a wire having a loop at a distal end. The suture extends from the surgical delivery instrument and through the loop of the wire. The suture is removably attached to the gripping member. The loop of the wire is configured to engage the gripping member to move the gripping member between one or more positions.
Owner:EDWARDS LIFESCIENCES CORP
Expandable implant for repairing a defect in a nucleus of an intervertebral disc
Expandable implants for repairing a nuclear defect of an intervertebral disc, and methods and apparatuses for delivering the same into the disc. The implants generally have a compressed form having a size adapted for insertion into the intervertebral disc, and a composition that allows the implant to expand from the compressed form into an expanded form after the implant is inserted into the nucleus. The expanded form of the implant has a configuration that fills the nuclear defect. The composition used to make the implant can include a shape memory alloy (SMA) or any other suitable material. Various devices can be used to insert the implants into the area being treated.
Owner:THOMAS JR JAMES C
Low profile bioactive agent delivery device
InactiveUS20080057106A1Low external profileMinimize damageEye implantsPharmaceutical delivery mechanismDevice implantDistal portion
Disclosed are implantable devices that are configured for implantation through tissue or membrane and into an implantation site comprising viscoelastic fluid or non-osseous tissue. In embodiments of the invention, the implantable devices comprise: (a) a nonlinear body member having a direction of extension, a longitudinal axis along the direction of extension, and a proximal portion and a distal portion, wherein at least a portion of the body member deviates from the direction of extension, (b) a retention element at the proximal portion of the body member, the retention element configured to retain the implantable device at the implantation site, the retention element presenting an external profile of no greater than 0.5 mm when the device is implanted in a patient; and (c) a bioactive agent delivery system at the distal portion of the body member, the bioactive agent delivery system comprising one or more bioactive agents. Also disclosed are methods of delivering a bioactive agent to a patient using the devices.
Owner:SURMODICS INC
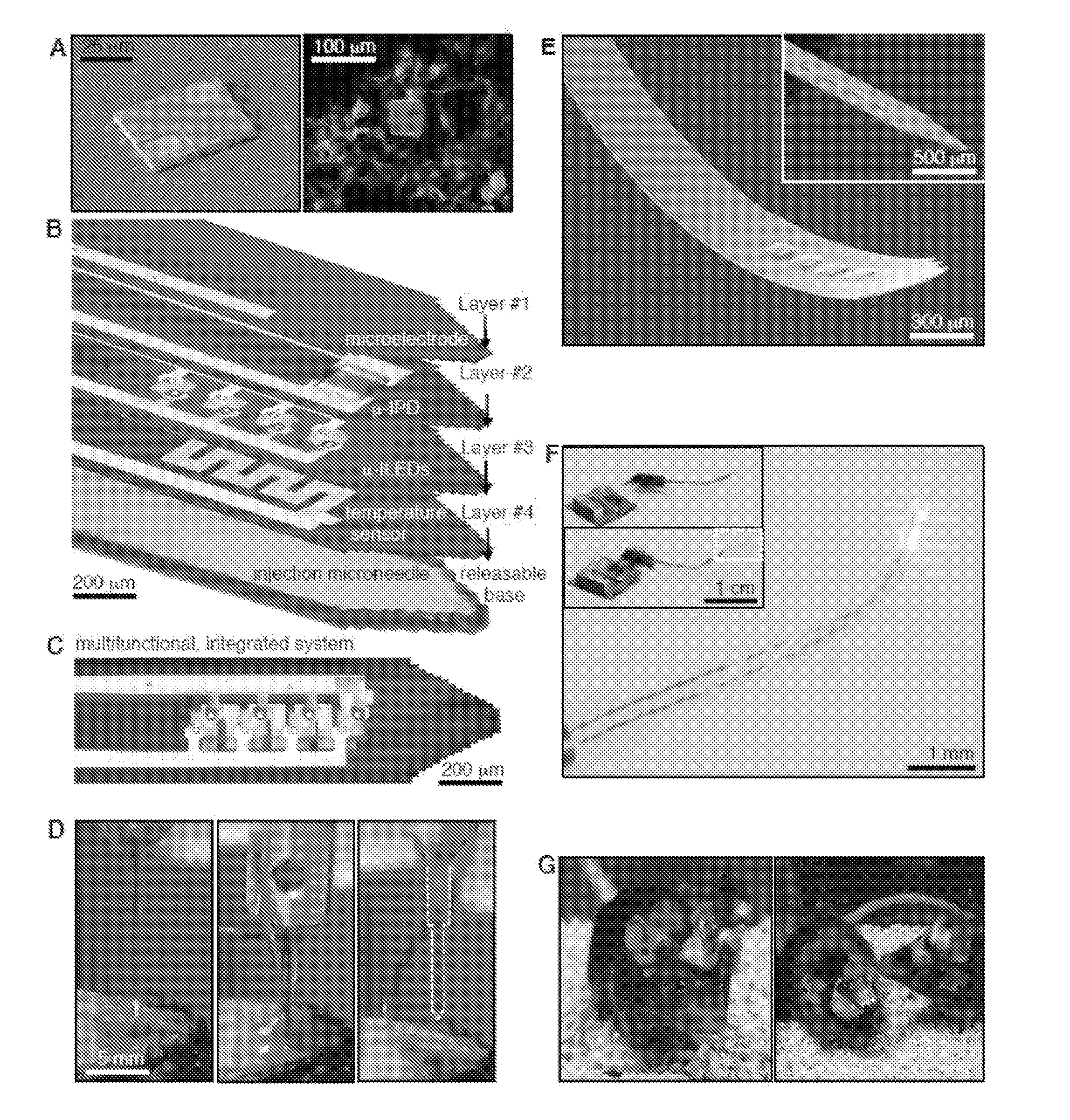
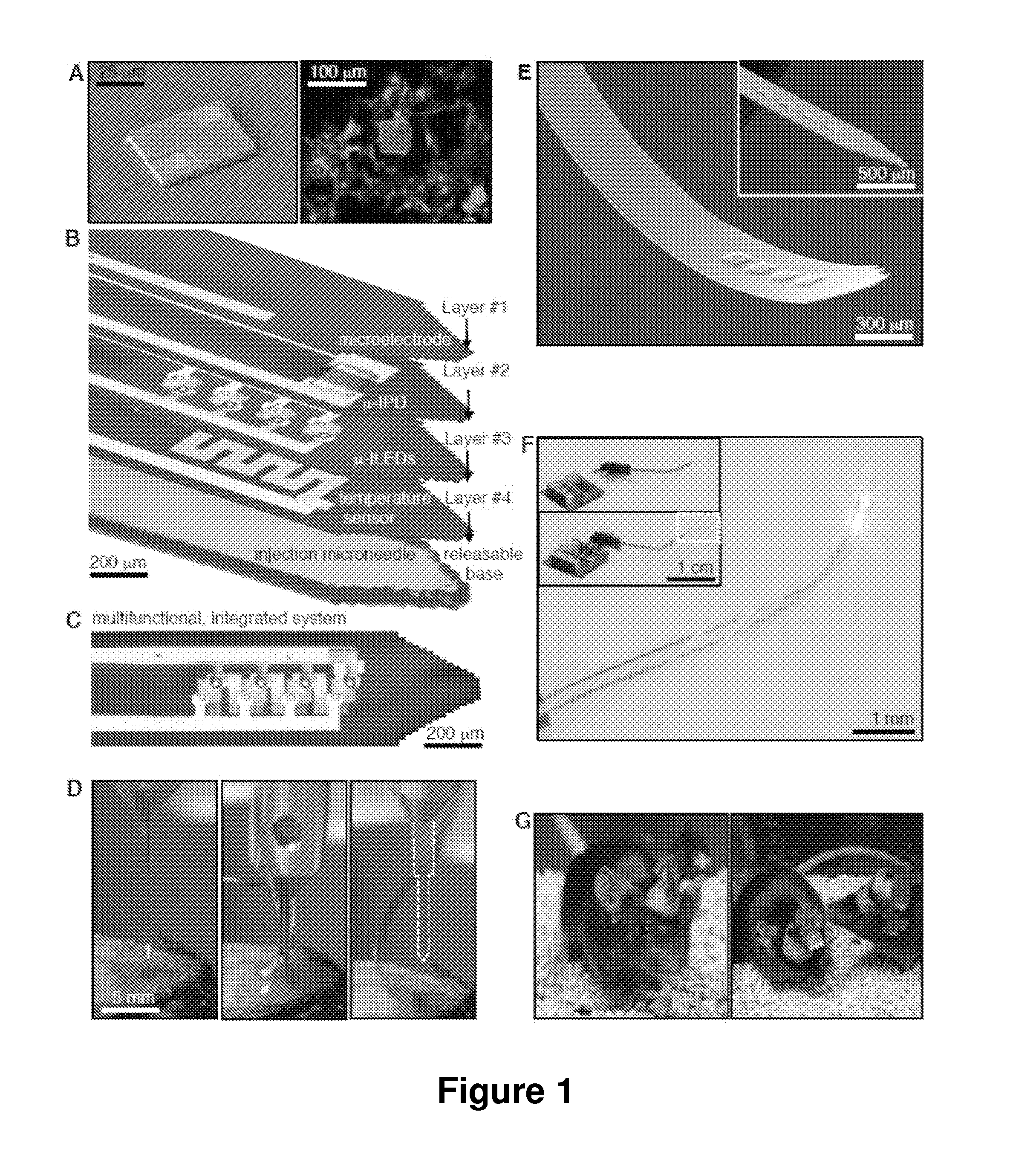
Injectable and implantable cellular-scale electronic devices
ActiveUS20160066789A1Accurate monitoringEasy to controlInternal electrodesSurgerySurface mountingTarget tissue
Provided are implantable or surface mounted biomedical devices and related methods for interfacing with a target tissue. The devices have a substrate and device component supported by the substrate. The components of the device are specially configured and packaged to be ultra-thin and mechanically compliant. In particular, device thicknesses are less than 1 mm and have lateral dimensions between about 1 μm and 10 mm, depending on the application. Delivery substrates may be incorporated to assist with device implantation and handling. The devices can be shaped to provide injection in a minimally invasive manner, thereby avoiding unnecessary tissue damage and providing a platform for long-term implantation for interfacing with biological tissue.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
Use of magnetic implants to treat body tissue structures
Plural (at least two) magnetic devices are implanted in a patient so that magnetic interaction between those devices modifies the patient's body in one or more respects (e.g., by modifying the shape and / or performance of some part of the body) Prior to implantation, a location in the body may be marked for later reference during the implantation. The magnetism of one or more of the magnetic devices may be changed in vivo after implantation. One or more of the implanted devices may be subsequently removed from the patient if desired. Use of the magnetic devices inside tissue conduits (e.g., as a treatment for GERD) is especially considered by way of illustration.
Owner:TORAX MEDICAL
Non-invasive measurement of fluid pressure in an adjustable gastric band
A food intake restriction device for forming a restriction in a patient's gastro-intestinal tract and non-invasively communicating pressure data regarding the restriction to an external monitor. The device includes a food intake restriction device implanted substantially about a patient's gastro-intestinal tract to form a restricted opening in the tract. A port is connected to the restriction device. The port contains a working fluid for affecting the size of the restricted opening. A pressure sensing system communicates with the working fluid to measure the pressure of the working fluid. A transmitter communicates the measured fluid pressure to the external monitor.
Owner:ETHICON ENDO SURGERY INC
Remote monitoring and adjustment of a food intake restriction device
InactiveUS20080009680A1Diagnostic recording/measuringTourniquetsDevice implantTelecommunications link
A bi-directional communication system for use with a restrictive opening device implanted within a patient. The system includes a sensor for measuring an operational parameter within the restrictive opening device. The system further includes a means for communicating a measured parameter data from the sensor means to a local unit external to the patient. The system further includes a base unit at a remote location from the patient, the base unit including user interface means for evaluating the measured parameter data. And, a communication link between the local and base units for transmitting data between the units, the transmitted data including the measured parameter data.
Owner:ETHICON ENDO SURGERY INC
Passive wear-indicating sensor for implantable prosthetic device
InactiveUS20070088442A1Easy to detectUltrasonic/sonic/infrasonic diagnosticsJoint implantsDevice implantMechanical wear
A method is provided for non-invasively detecting mechanical wear of a prosthetic device implanted in a patient, the method comprises using a non-invasive imaging technique to image the prosthetic device that includes a wear indicating composition; and detecting whether the wear indicating composition has been released from the prosthetic device, and, if so, the location, type, and / or amount thereof. The implant device includes a prosthetic device body having at least one outer surface area; at least one reservoir (e.g., a plurality of discretely spaced micro-reservoirs) in the device body; a wear indicator composition disposed in said at least one reservoir, wherein mechanical wear of the at least one outer surface area of the device body in vivo causes release of at least part of the wear indicator composition. The prosthetic device body may be one for replacement of a hip, a knee, a shoulder, an elbow, or a vertebra.
Owner:MICROCHIPS INC
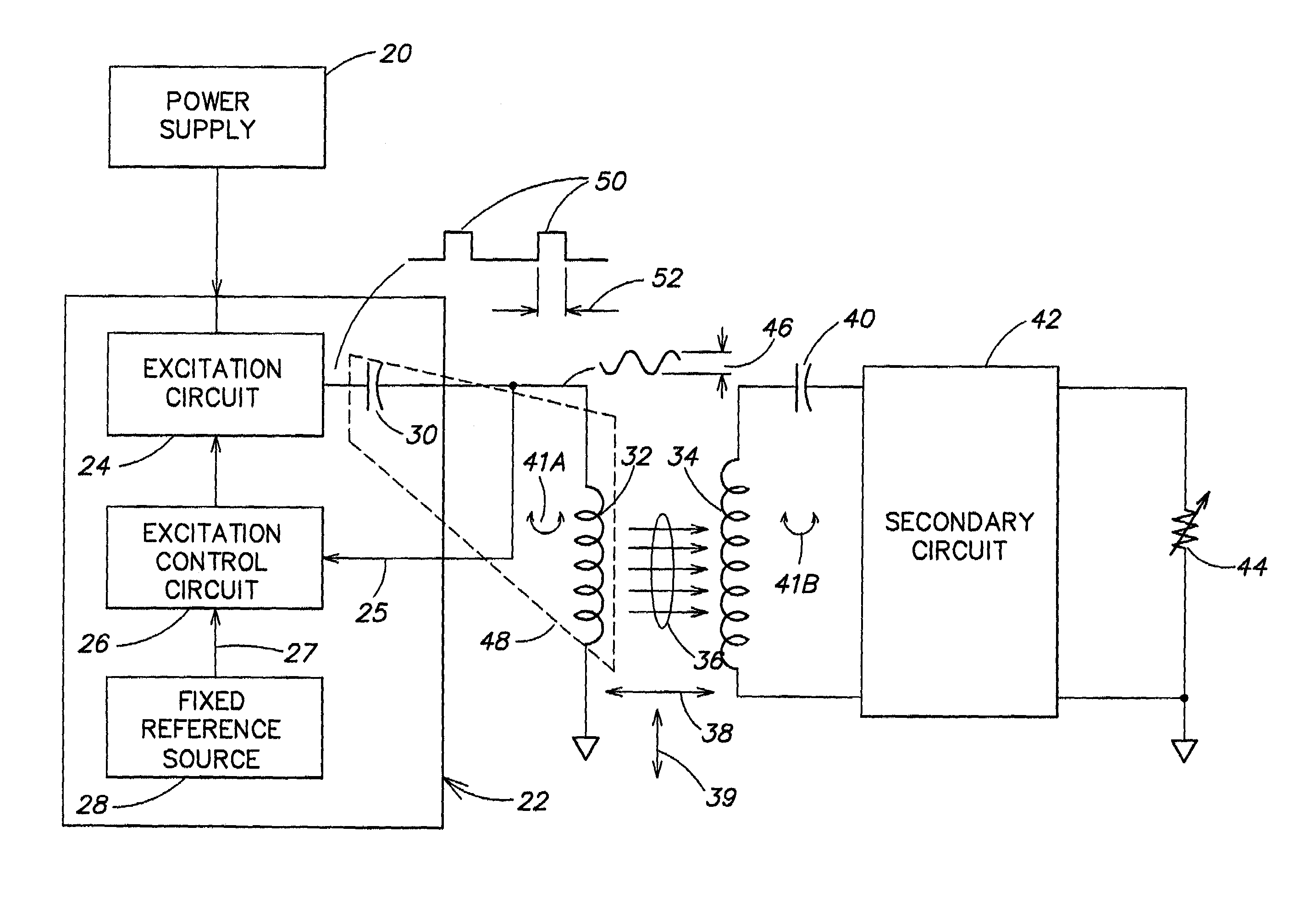
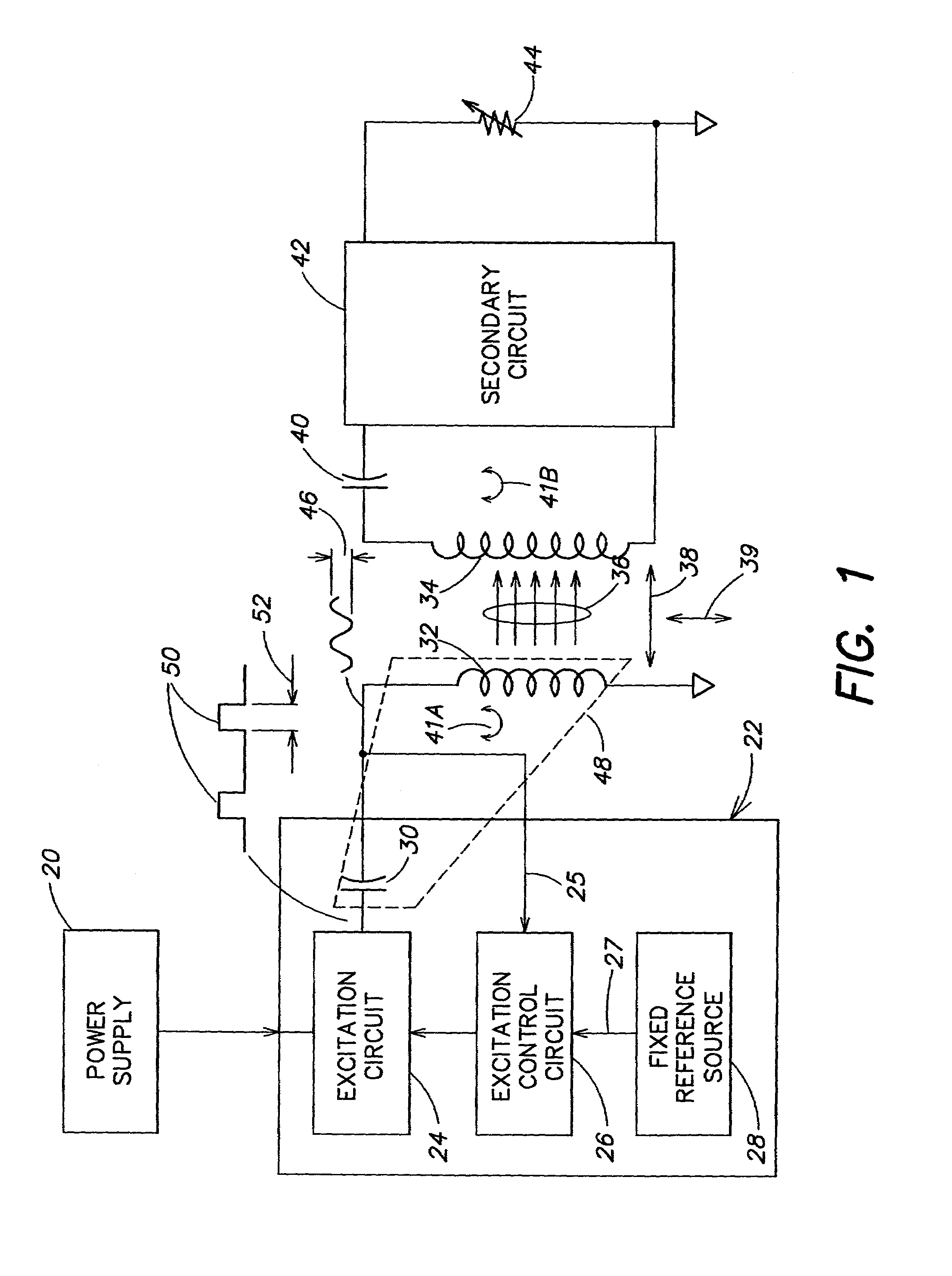
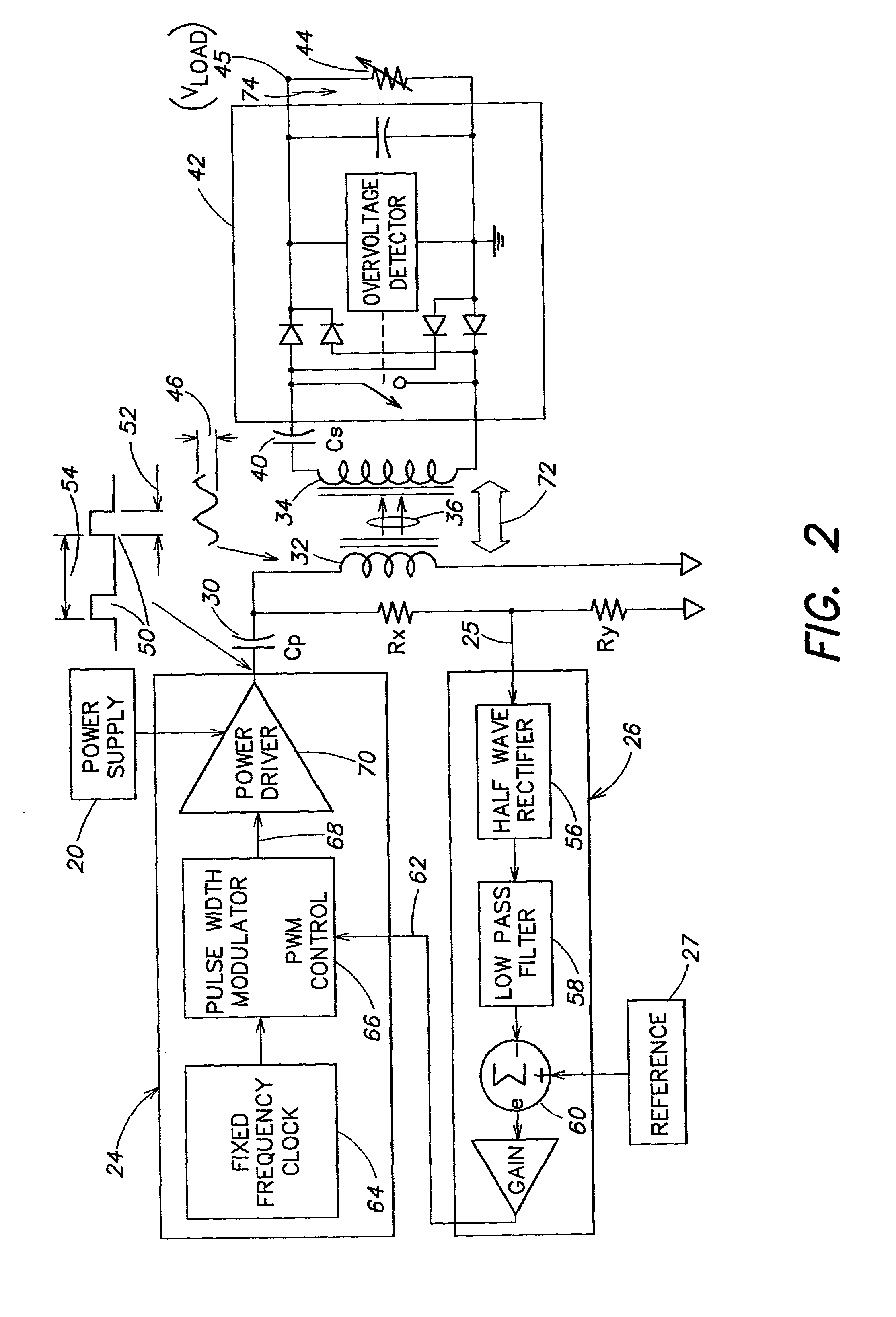
Methods and apparatus for providing a sufficiently stable power to a load in an energy transfer system
Owner:ABIOMED
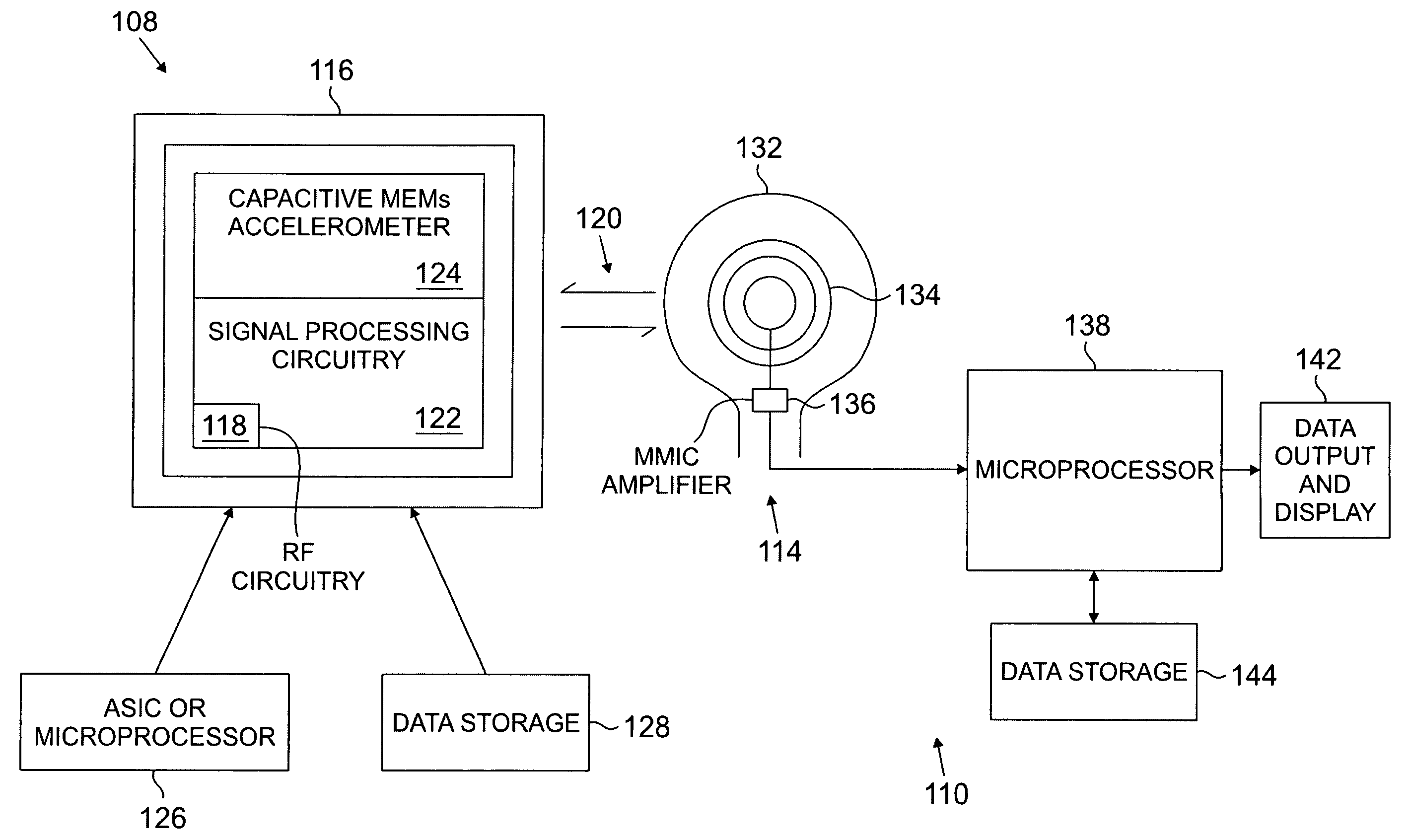
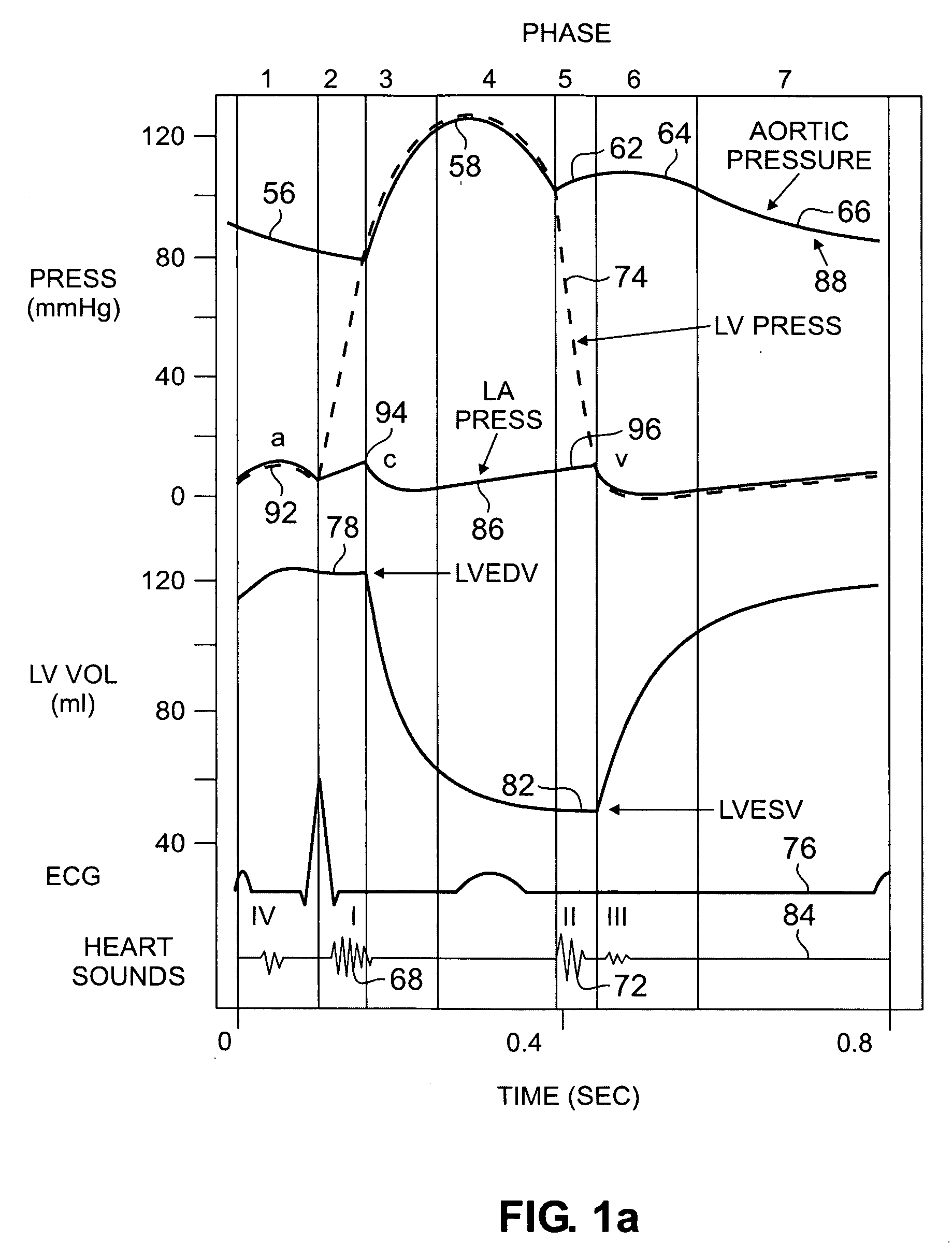
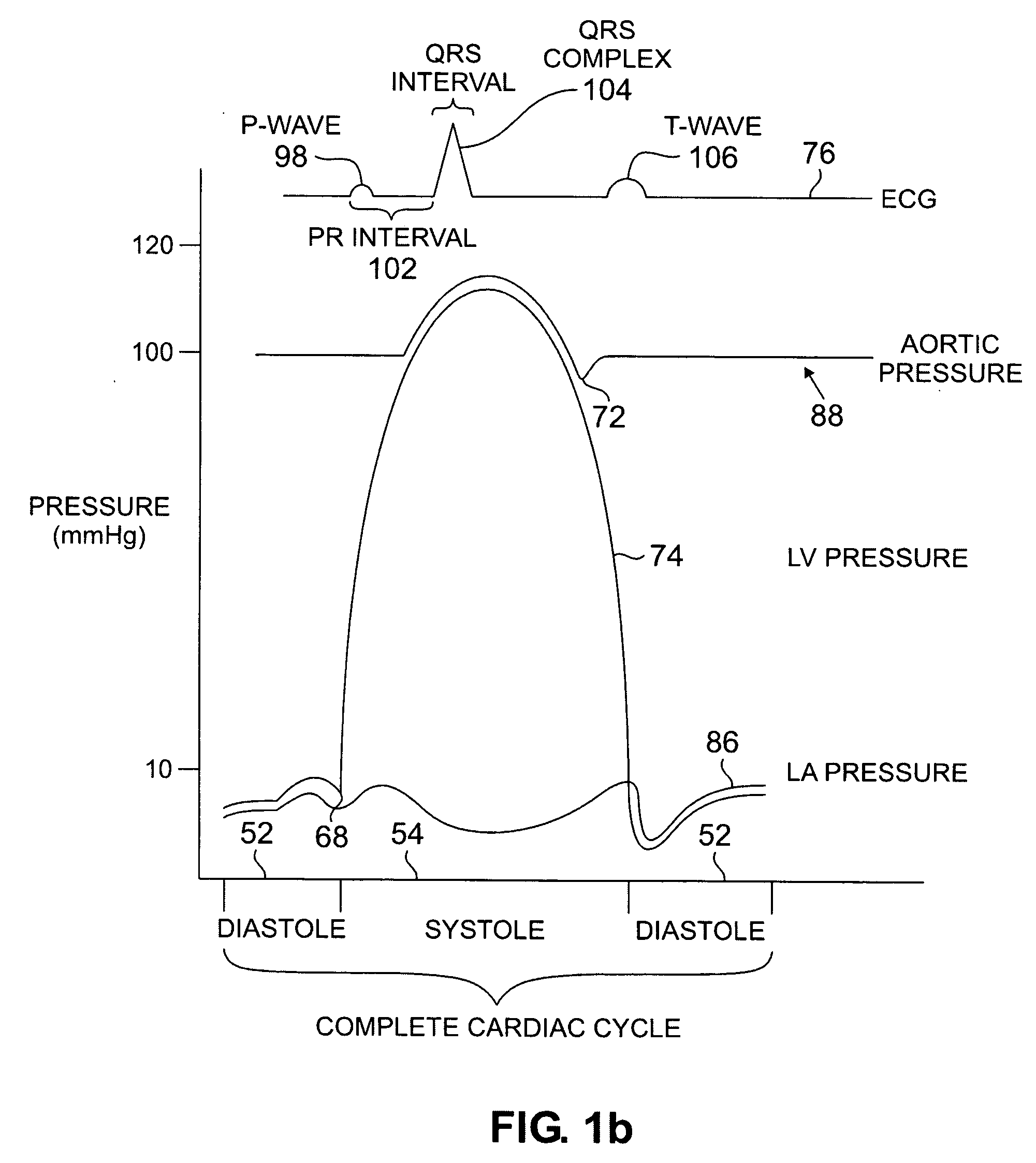
Devices and methods for accelerometer-based characterization of cardiac function and identification of LV target pacing zones
InactiveUS20060178586A1Optimize therapyEasy to identifyElectrocardiographyTransvascular endocardial electrodesCardiac featureZone System
Systems according to the invention employ an acceleration sensor to characterize displacement and vibrational LV motion, and uses this motion data to characterize the different phases of the LV cycle for analyzing LV function. Systems may identify a target pacing region or regions in the LV or RV using the acceleration sensor by localizing regions of late onset of motion relative to the QRS, or isovolumic contraction, or mitral valve closure, or by pacing of target regions and measuring LV function in response to pacing. Systems further provide an implantable or non-implantable acceleration sensor device for measuring LV motion and characterizing LV function. An implantable myocardial acceleration sensing system (“IAD”) includes at least one acceleration sensor, a data acquisition and processing device, and an electromagnetic, e.g., RF, communication device. The IAD may be integrated into the pacing lead of a CRT device and can operate independently of the CRT IPG.
Owner:CARDIOSYNC
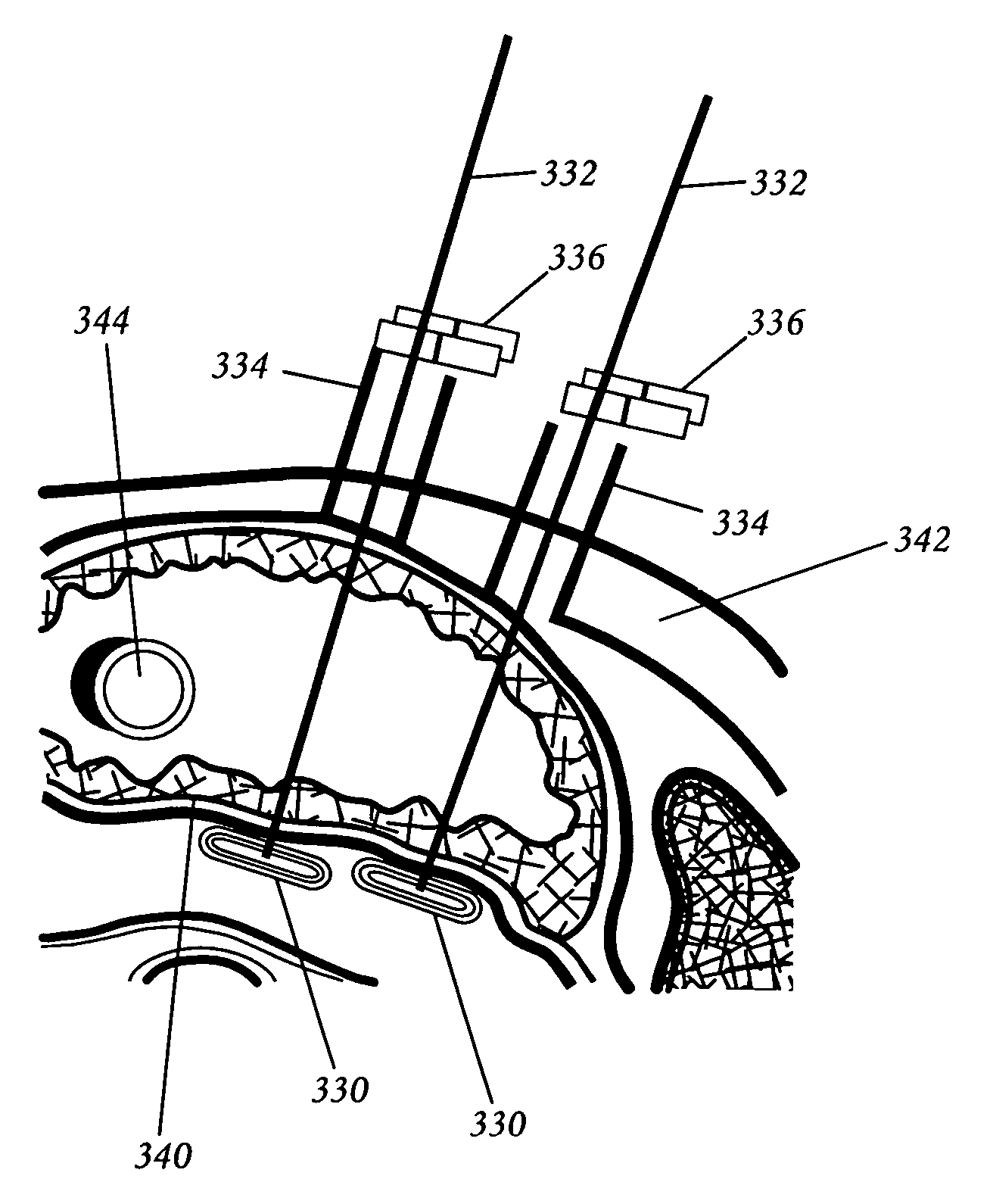
Spine distraction implant
InactiveUS20080086212A1Increase volumeReduce restrictionsInternal osteosythesisSpinal implantsDistractionDevice implant
A spine distraction implant alleviates pain associated with spinal stenosis and facet arthropathy by expanding the volume in the spine canal and / or neural foramen. The implant provides a spinal extension stop while allowing freedom of spinal flexion. An interspinous process implant with a selectably expandable spacer can be placed between adjacent spinous processes. a device implanted between the spinous processes of adjacent vertebrae of the spine can be used for relieving pain associated with the vertebrae and surrounding tissues and structures by maintaining and / or adding distraction between adjacent vertebrae. A tissue expander can be adapted to move from a first insertion position, for ease of implantation between spinous processes, to a second retention position that prevents displacement of the implant. An embodiment of a system can include an implant having a spacer with a thickness and a wing, wherein a first configuration of the wing has a first height substantially similar to the thickness and wherein the wing is adapted to be selectably arranged in a second configuration such that the wing has a second height greater than the first height. A periphery of the implant has a shape generally conformal with a shape of an inner surface of a cannula and a cross-sectional diameter smaller than an inner diameter of the cannula. The cannula is inserted such that a proximal end of the cannula is arranged between the adjacent spinous processes. The implant is then urged into position between the adjacent spinous processes by way of the cannula, and subsequently arranged in a second configuration to fix the implant in position.
Owner:KYPHON
Methods and devices for the surgical creation of satiety and biofeedback pathways
Disclosed are devices and systems which to sense parameters related to the mechanical forces imposed on devices implanted in the stomach. The parameters of the system are further translated into patient feedback systems to create satiety inducing pathways in the stomach.
Owner:GERTNER MICHAEL
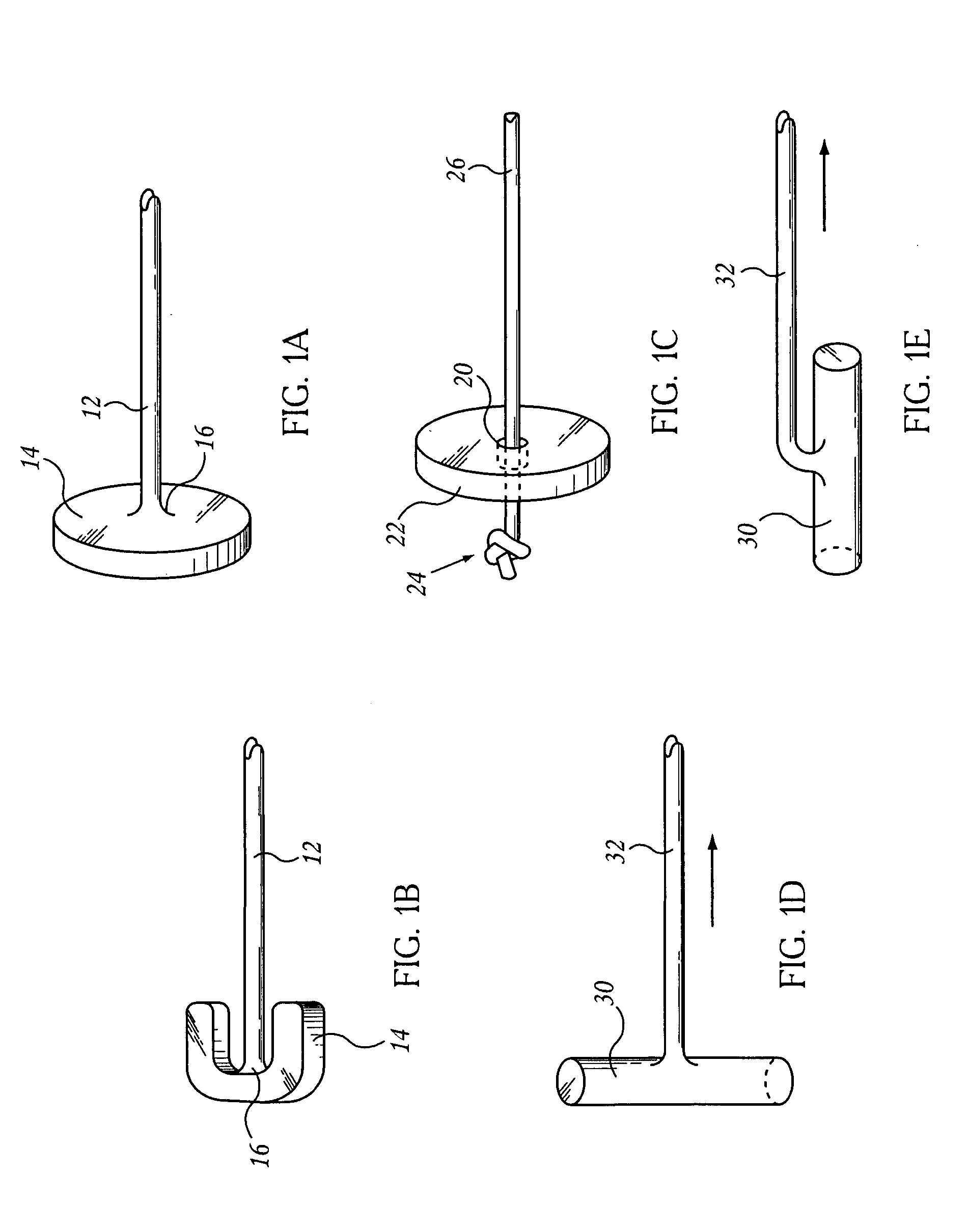
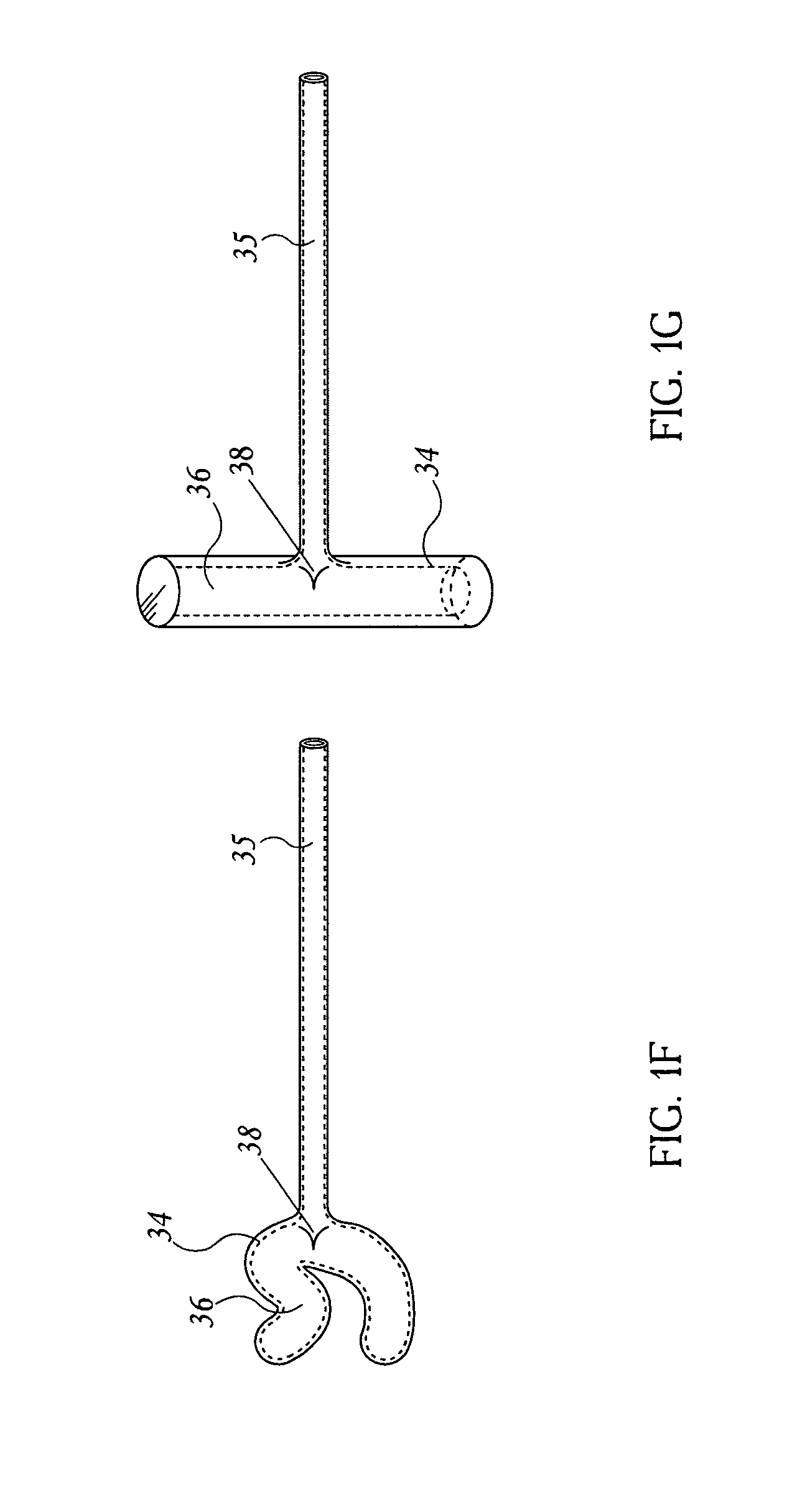
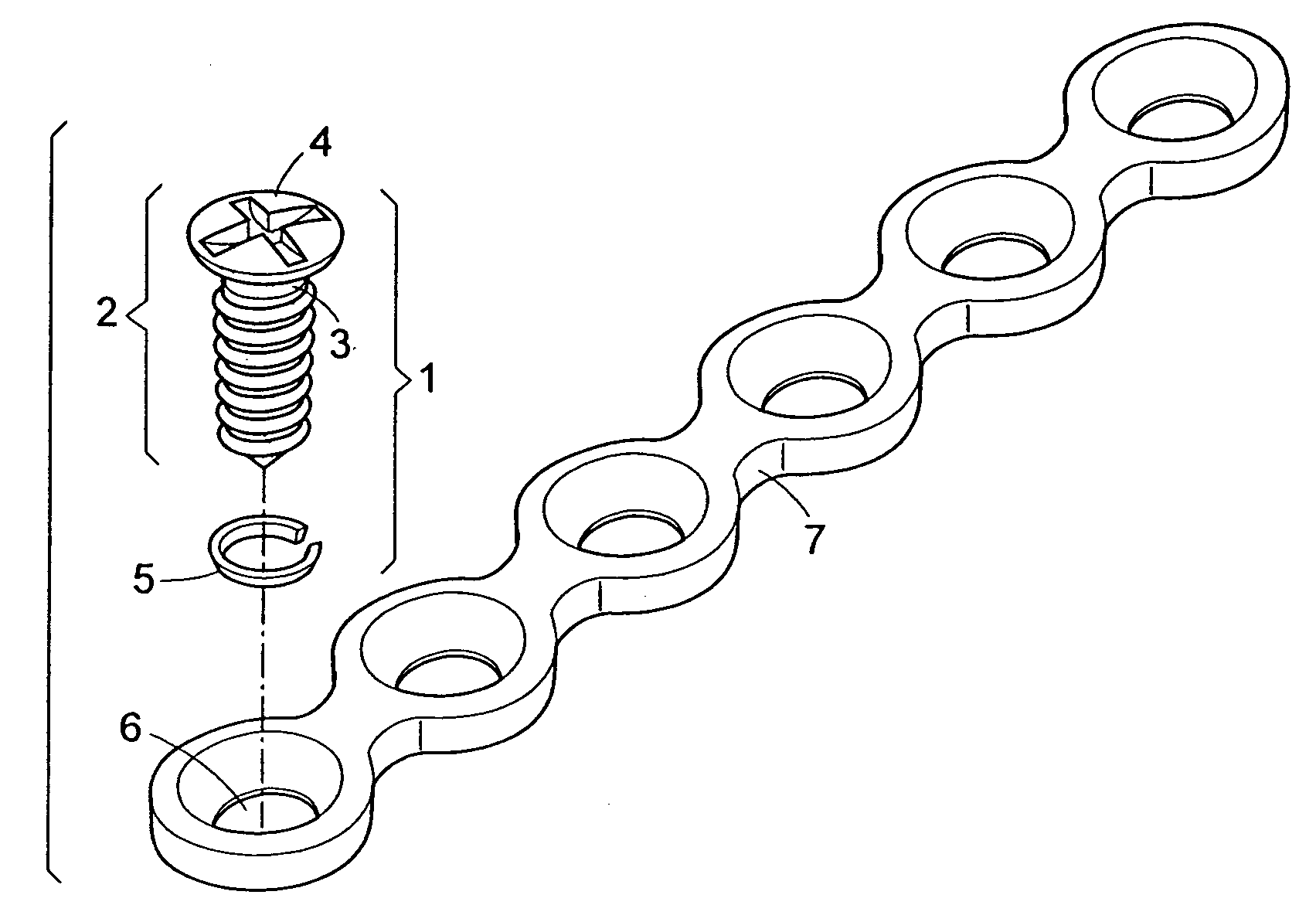
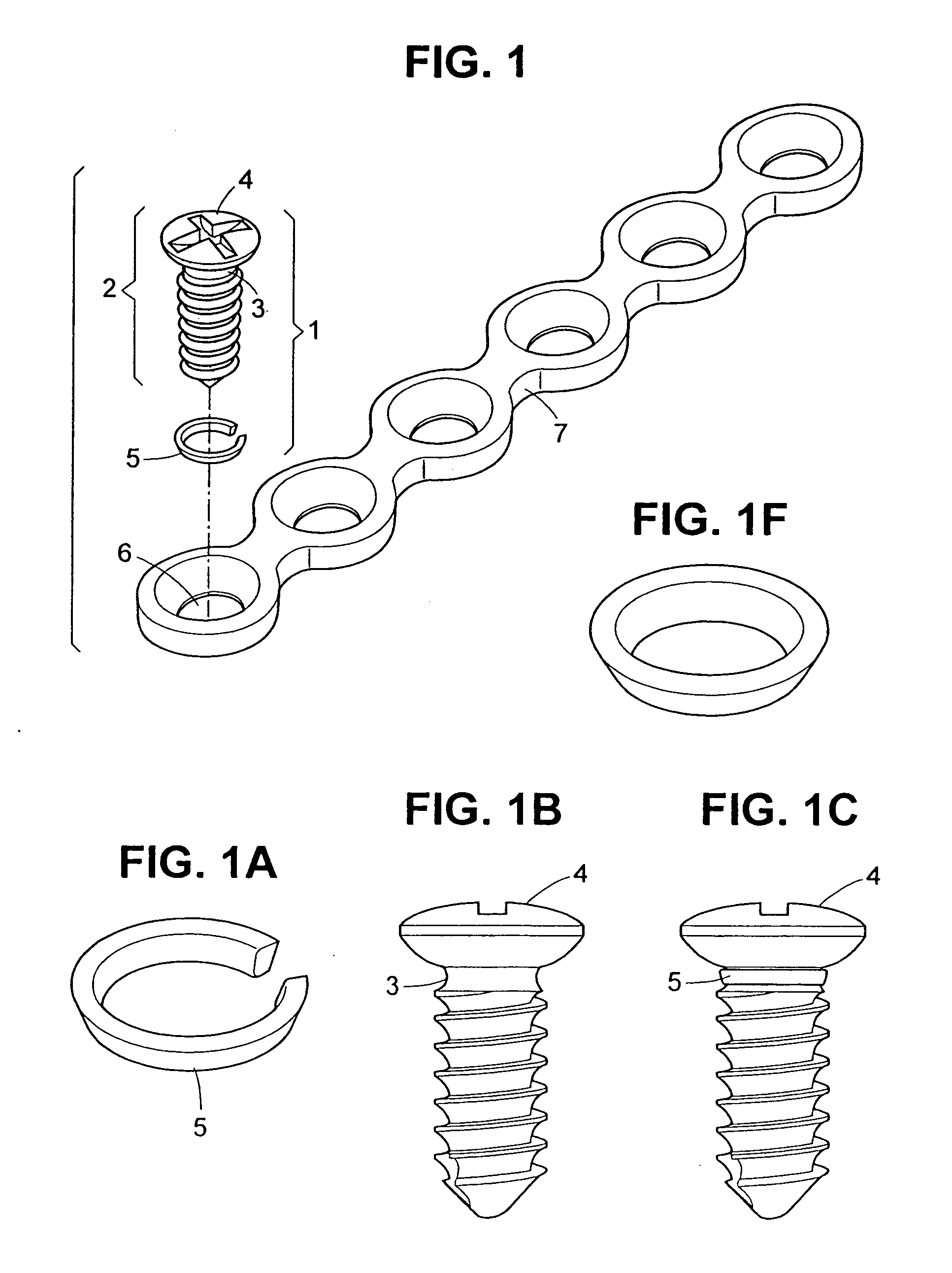
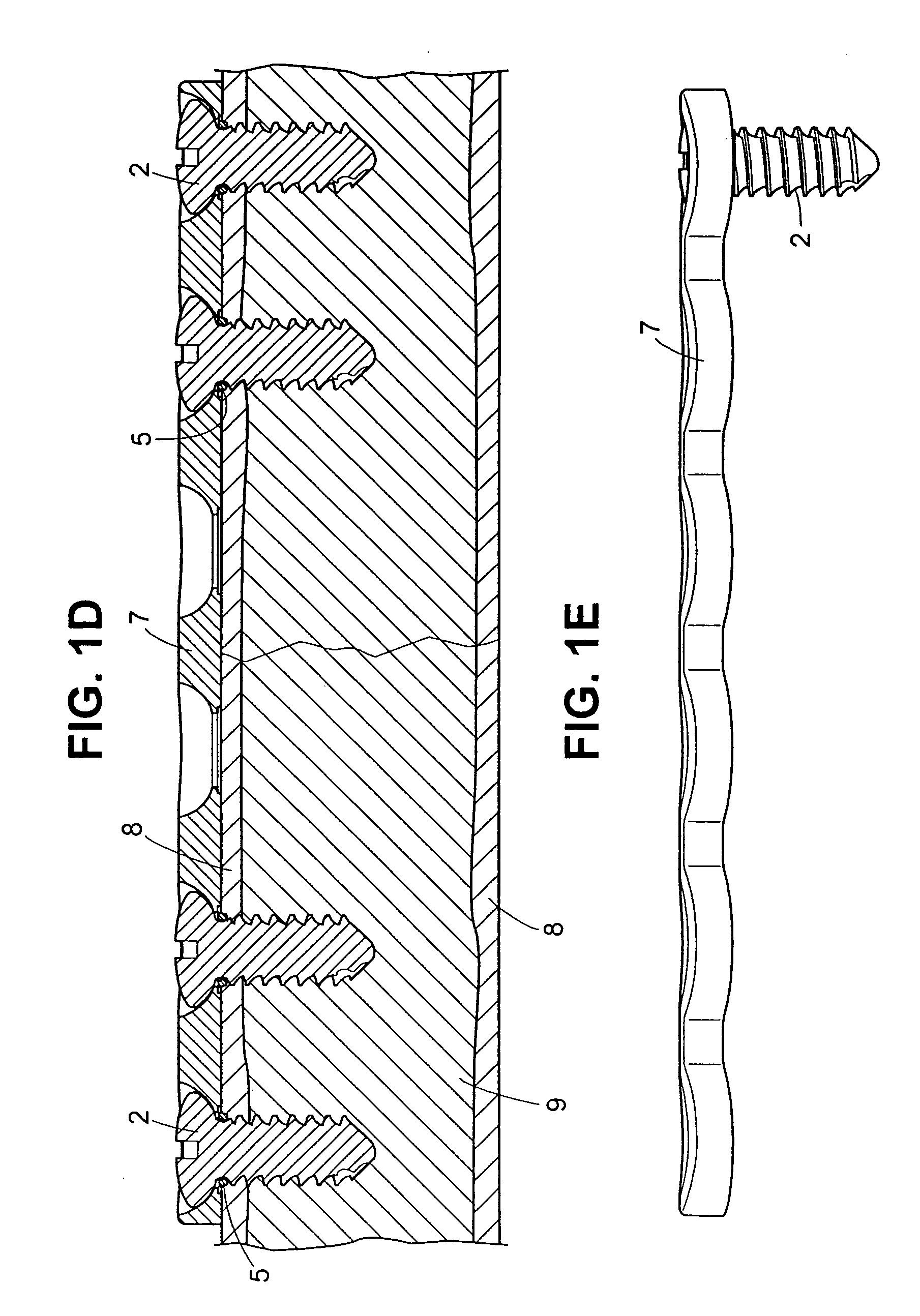
Self-locking screws for medical implants
Self-locking surgical screw assemblies are described for fastening a medical implant to a bone. The invention also includes combinations of self-locking screws and implants, devices implanted with self-locking screws and related methods which insure the stable attachment of the implant to the bone. Each self-locking surgical screw assembly is comprised of a screw having a non-threaded head affixed to a shank, a threaded portion on the shank, a neck on the shank and a locking device externally affixed to or disposed on the head and / or neck. The locking device lockingly engages the head and / or neck of the screw when the screw is in fastening engagement with the medical implant and the bone.
Owner:FIRST COMMERCE BANK
Methods and devices for combined gastric restriction and electrical stimulation
InactiveUS20060074473A1Reduce internal volumeReduction procedureSuture equipmentsSurgical needlesDevice implantGastric restriction
Disclosed are methods and apparatus for implantation into the walls of an organ such as the stomach. Deformable or inflatable anchors with a connector between are used to pull the walls of the organ together, or to implant devices in the wall of the organ. Also disclosed are surgical instruments useful in practicing the disclosed methods.
Owner:GERTNER MICHAEL
Systems and methods for cardiac remodeling
ActiveUS9125632B2Reduced dimensionReduce distanceSuture equipmentsAnnuloplasty ringsDevice implantHemodynamics
Owner:ANCORA HEART INC
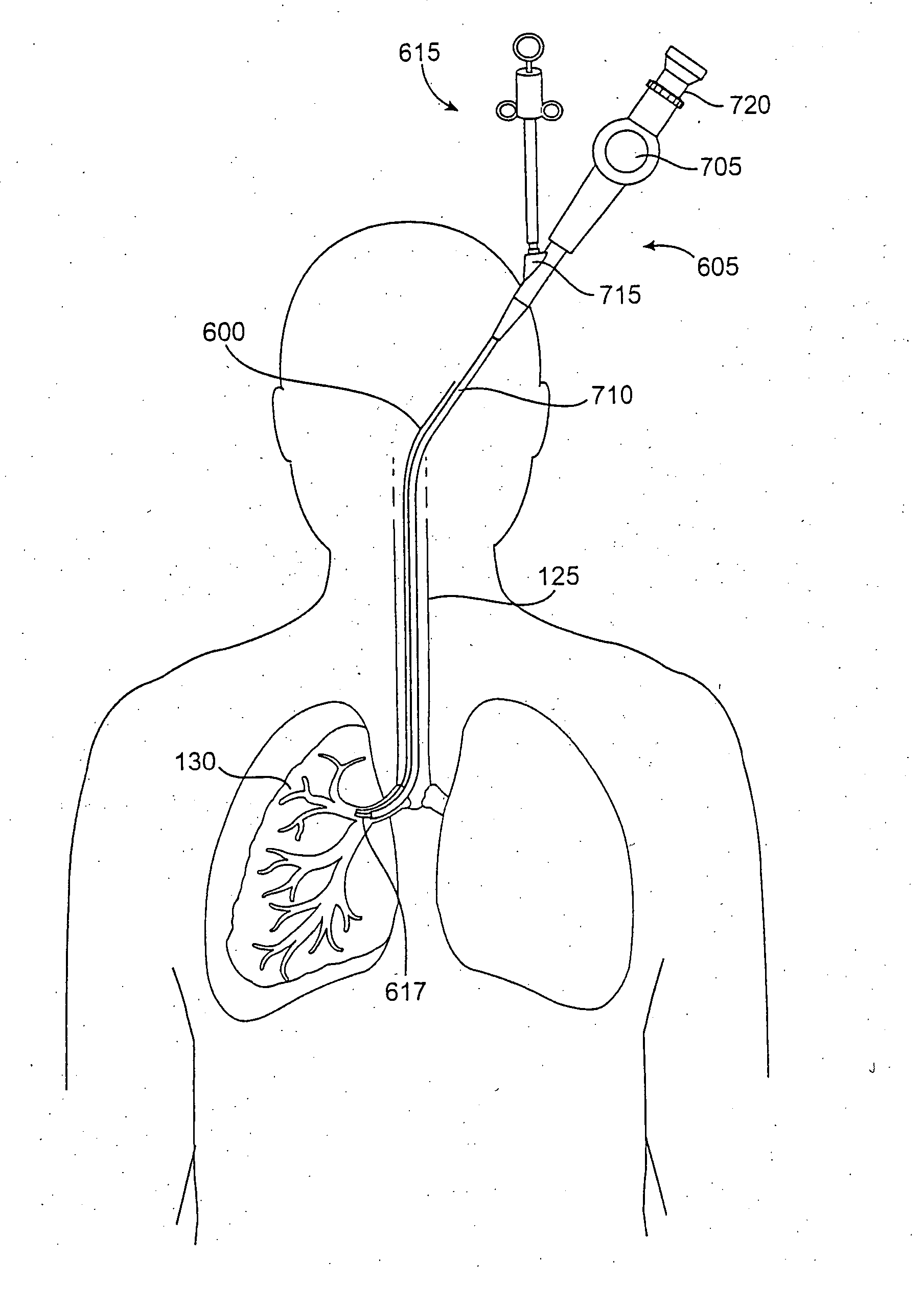
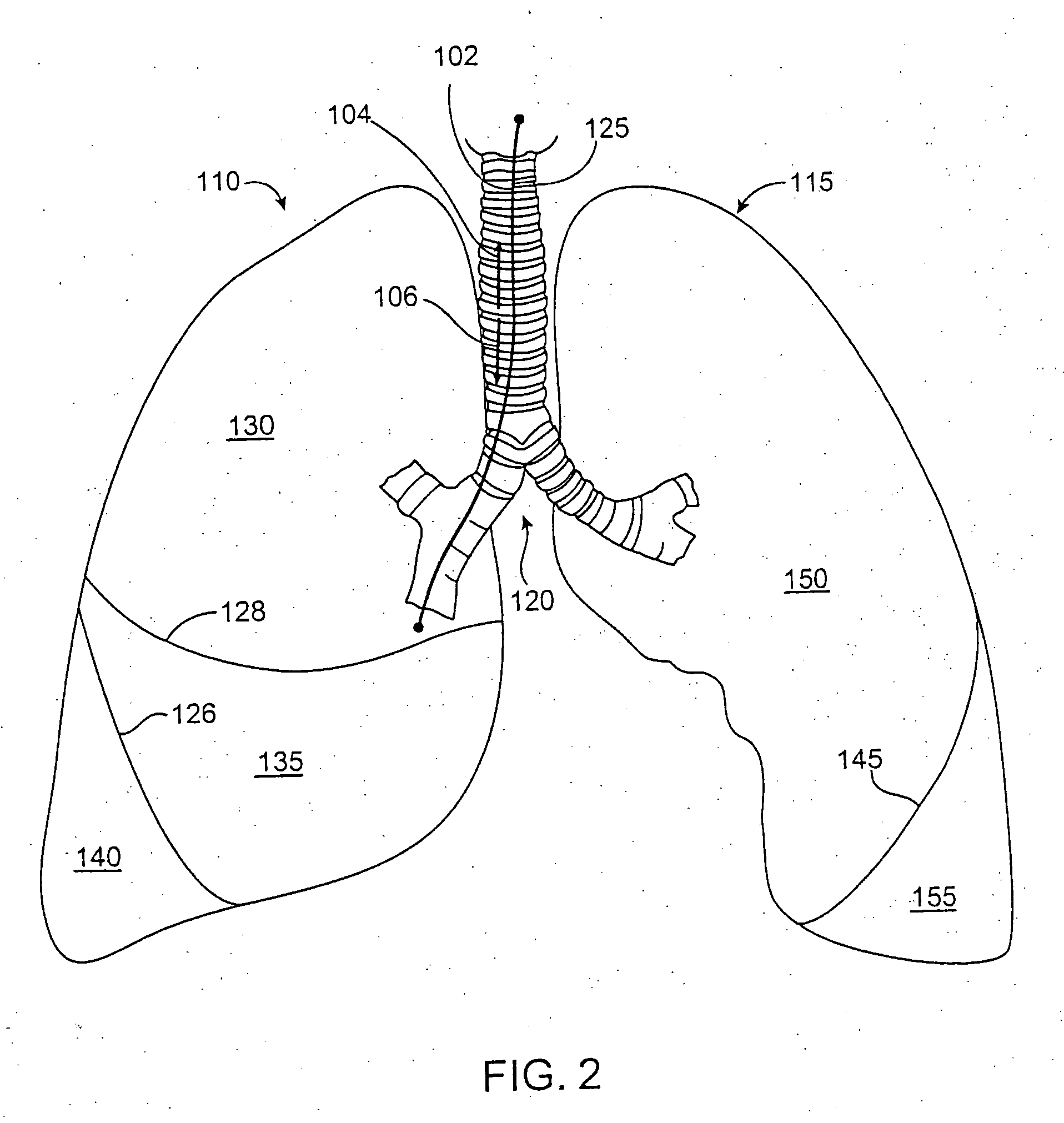
Modification of lung region flow dynamics using flow control devices implanted in bronchial wall channels
A desired fluid flow dynamic to a lung region can be achieved by deploying various combinations of flow control devices and bronchial isolation devices in one or more bronchial passageways that communicate with the lung region. The flow control devices are implanted in channels that are formed in the walls of bronchial passageways of the lung. The flow control devices regulate fluid flow through the channels. The bronchial isolation devices are implanted in lumens of bronchial passageways that communicate with the lung region in order to regulate fluid flow to and from the lung region through the bronchial passageways.
Owner:PULMONX
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com