Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
38 results about "Tissue expander" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
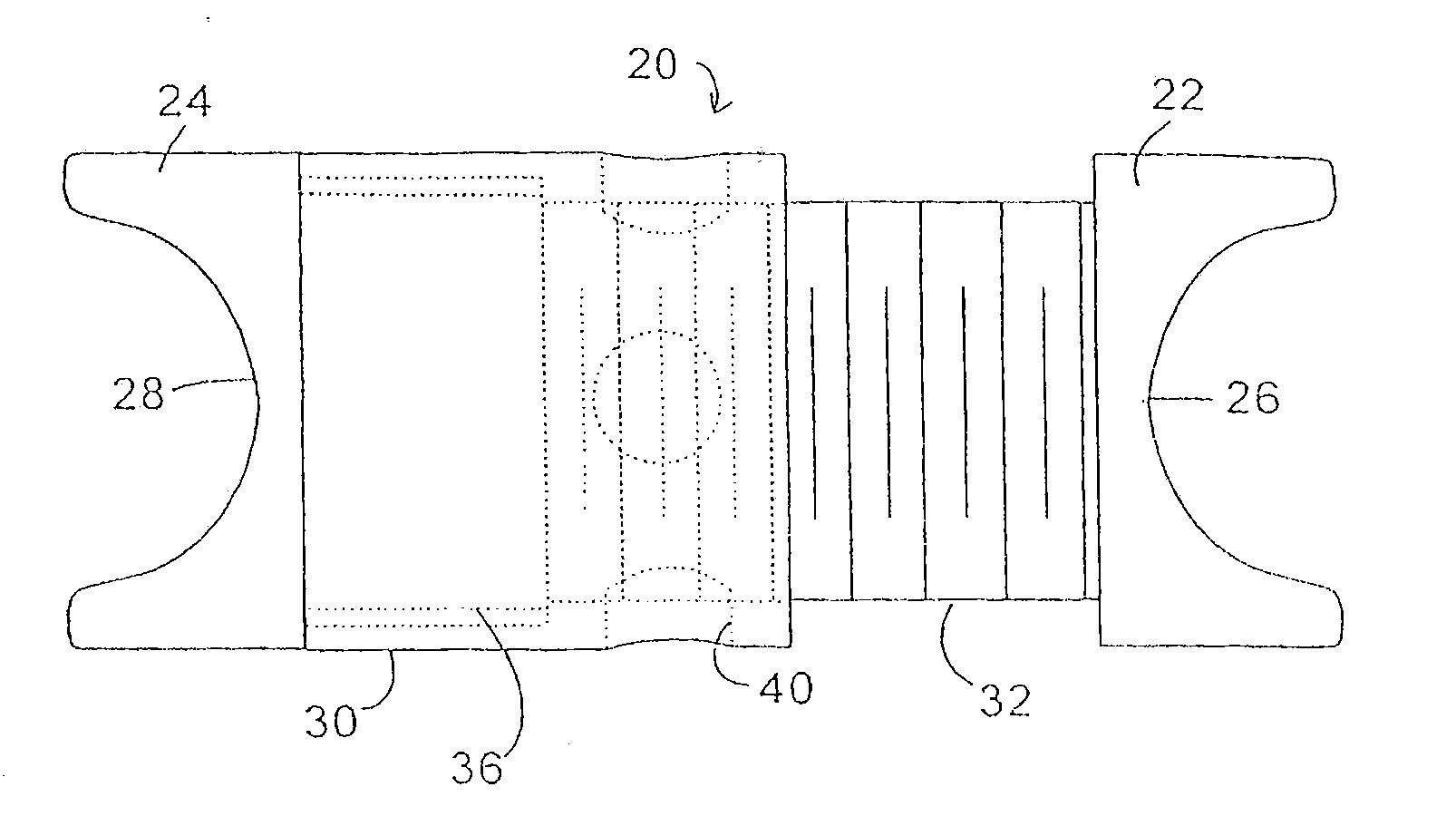
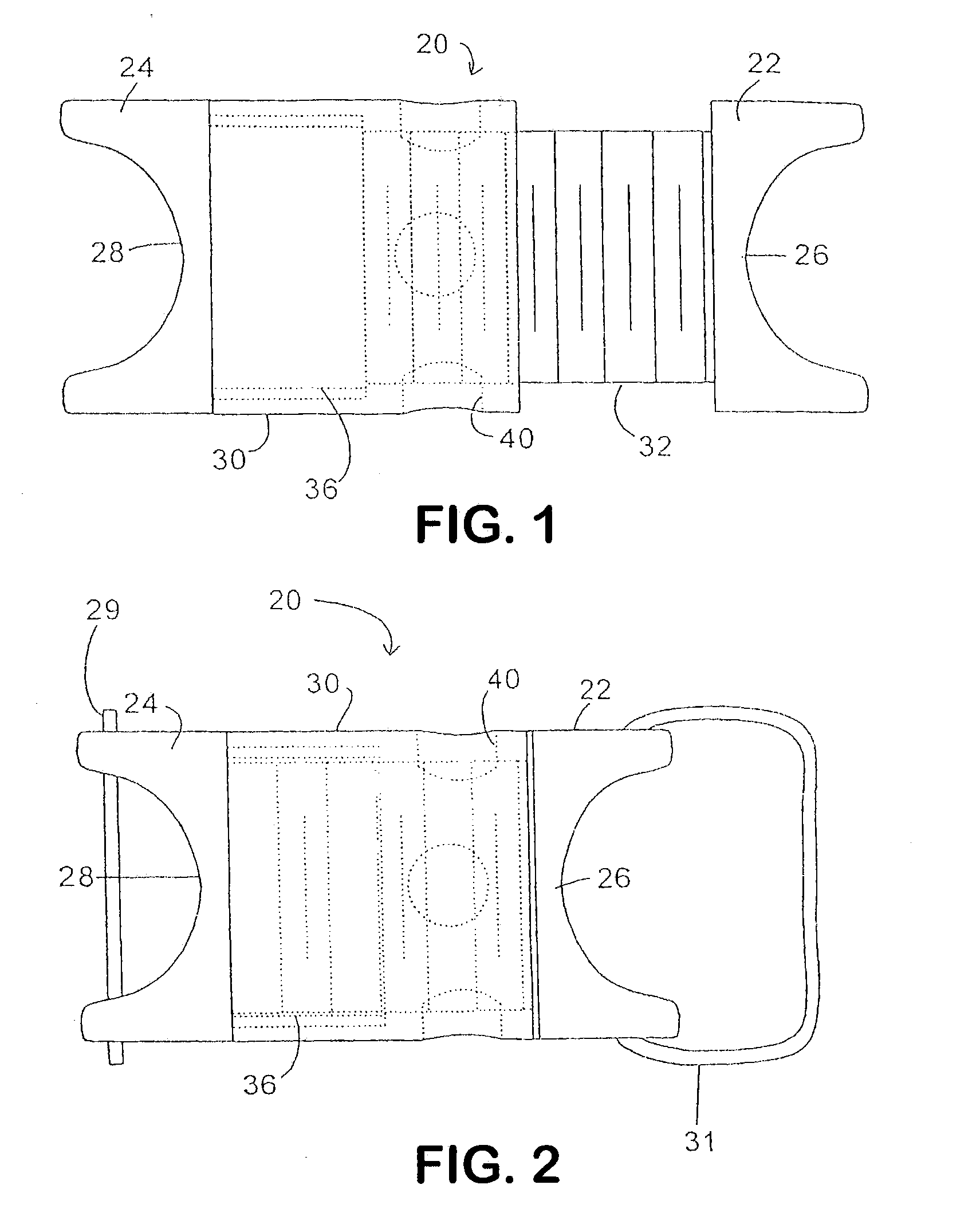
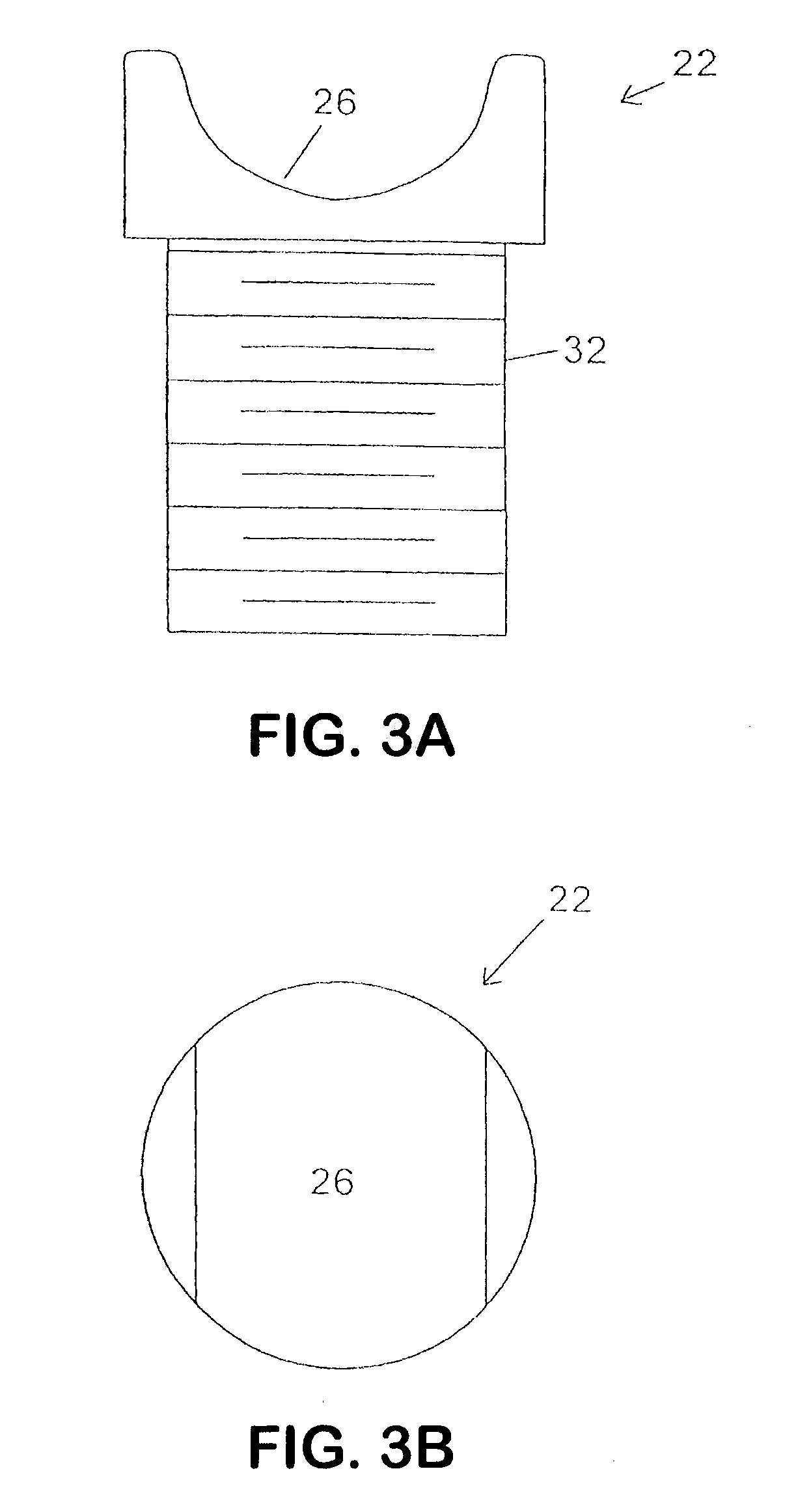
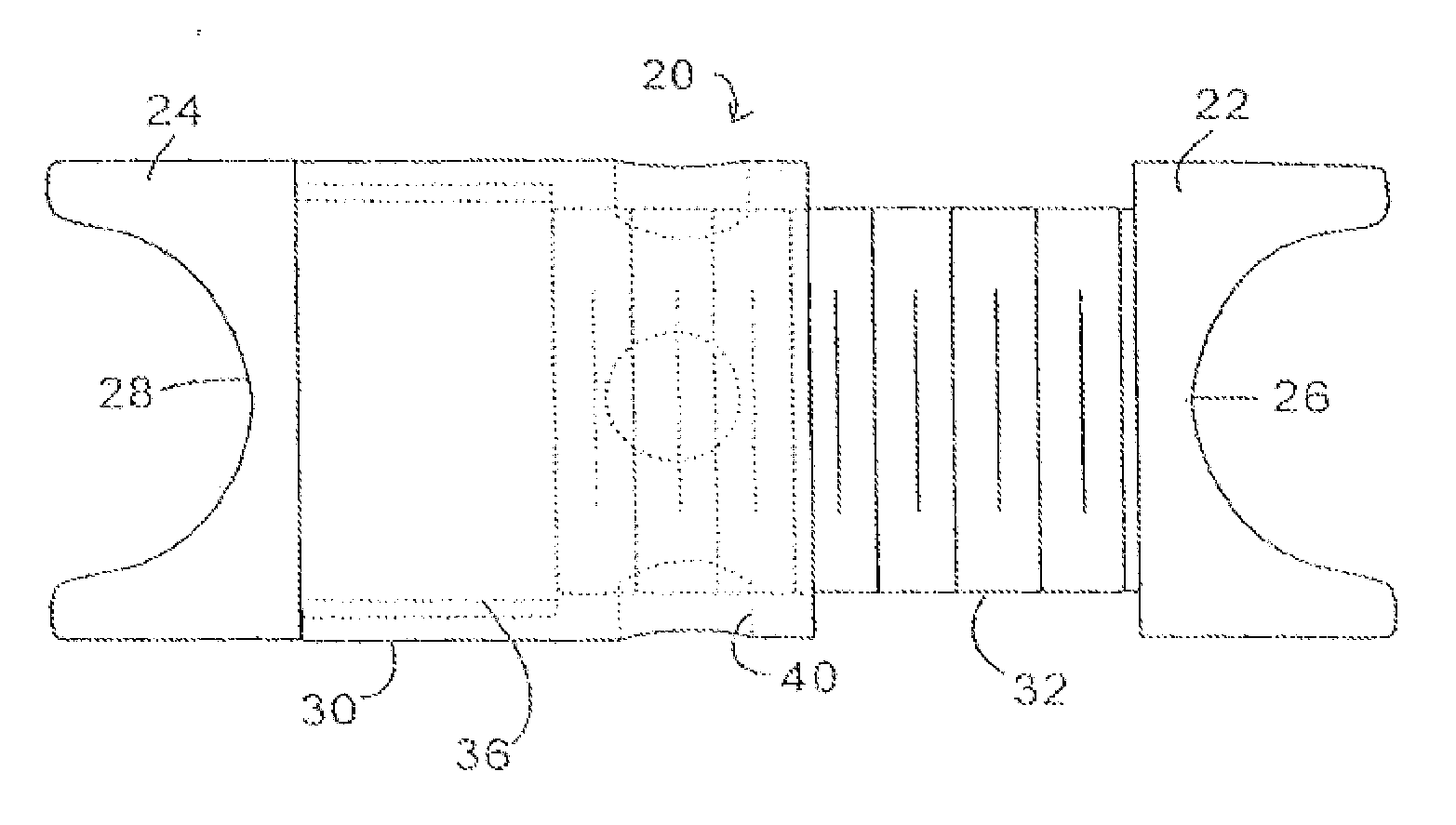
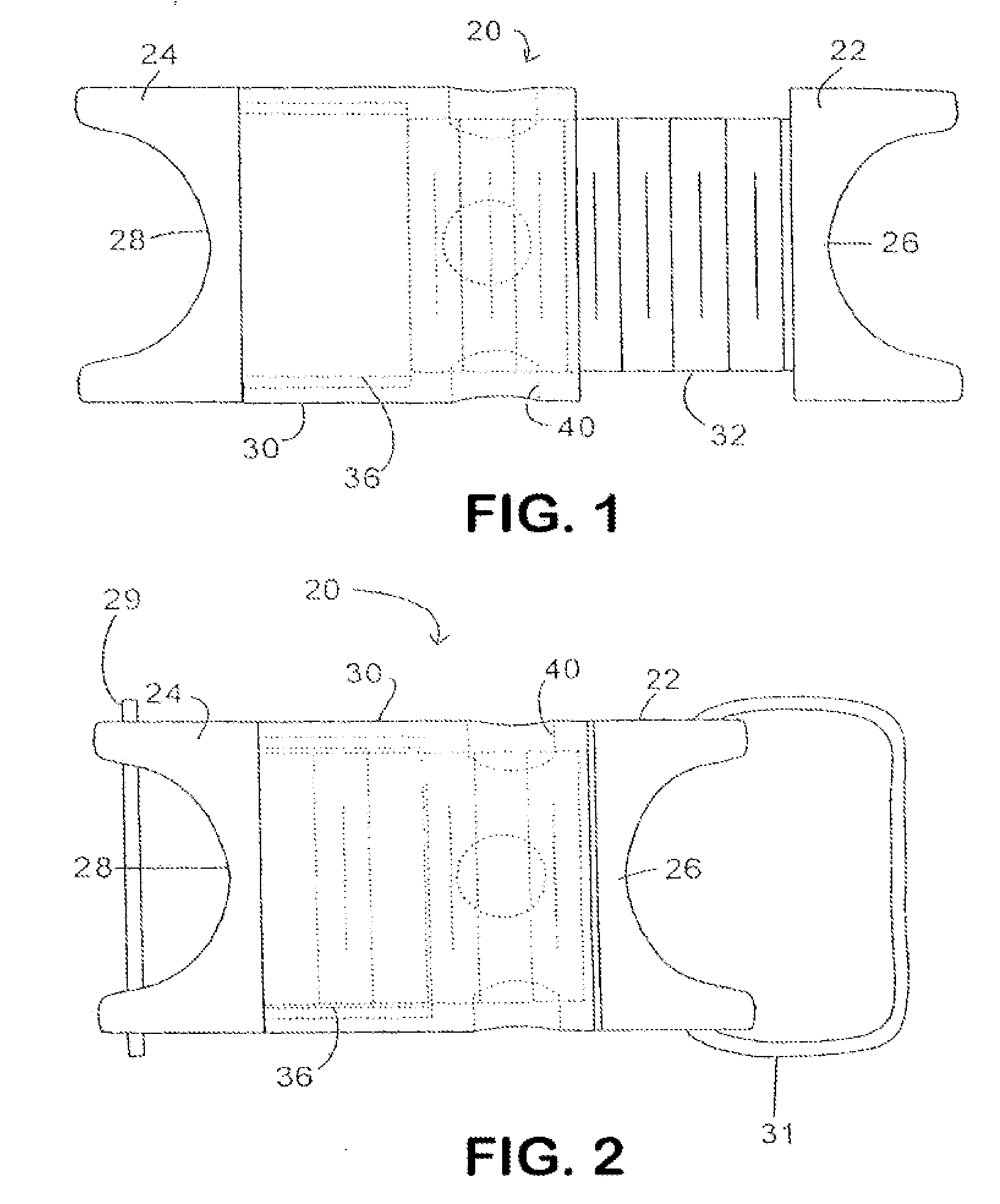
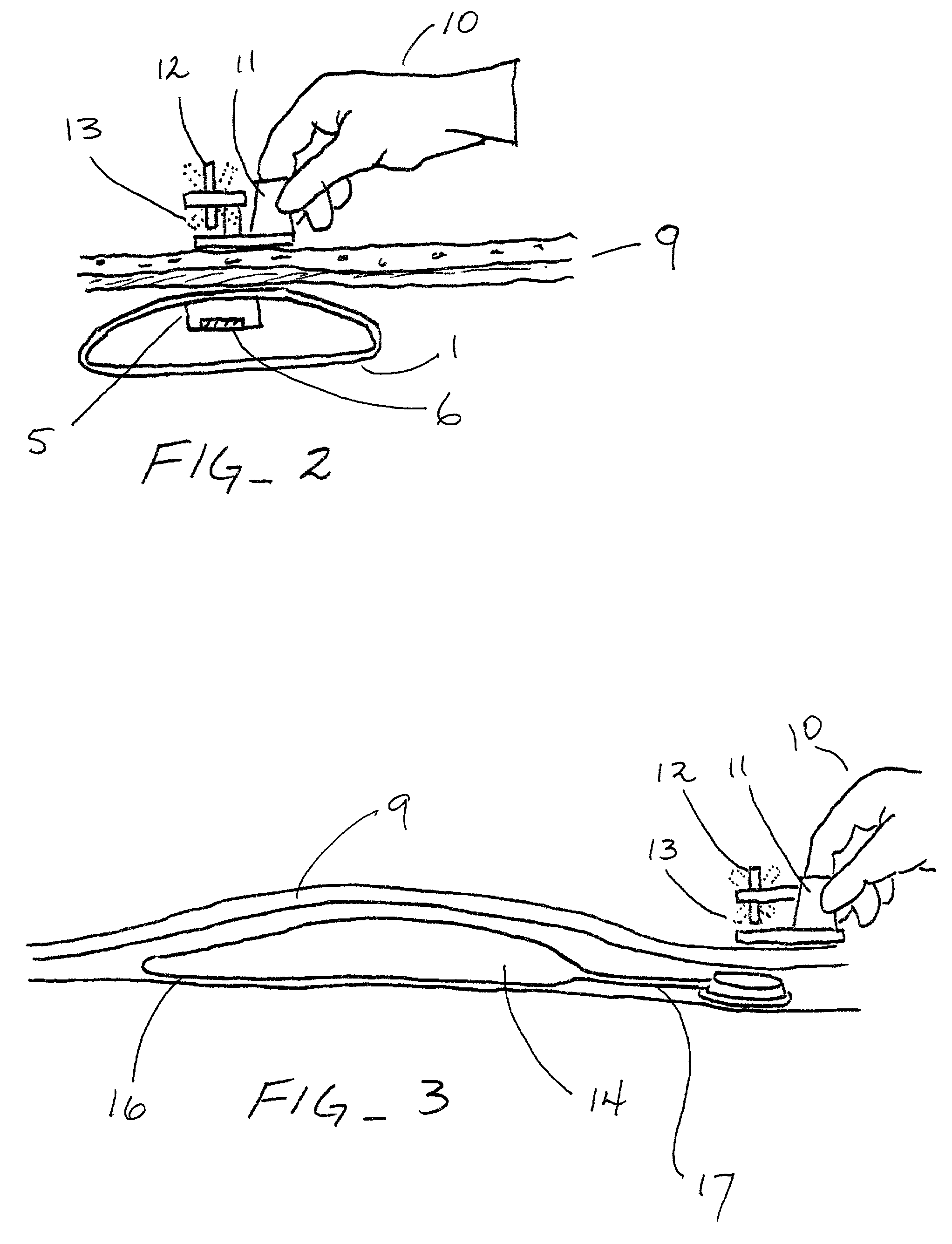
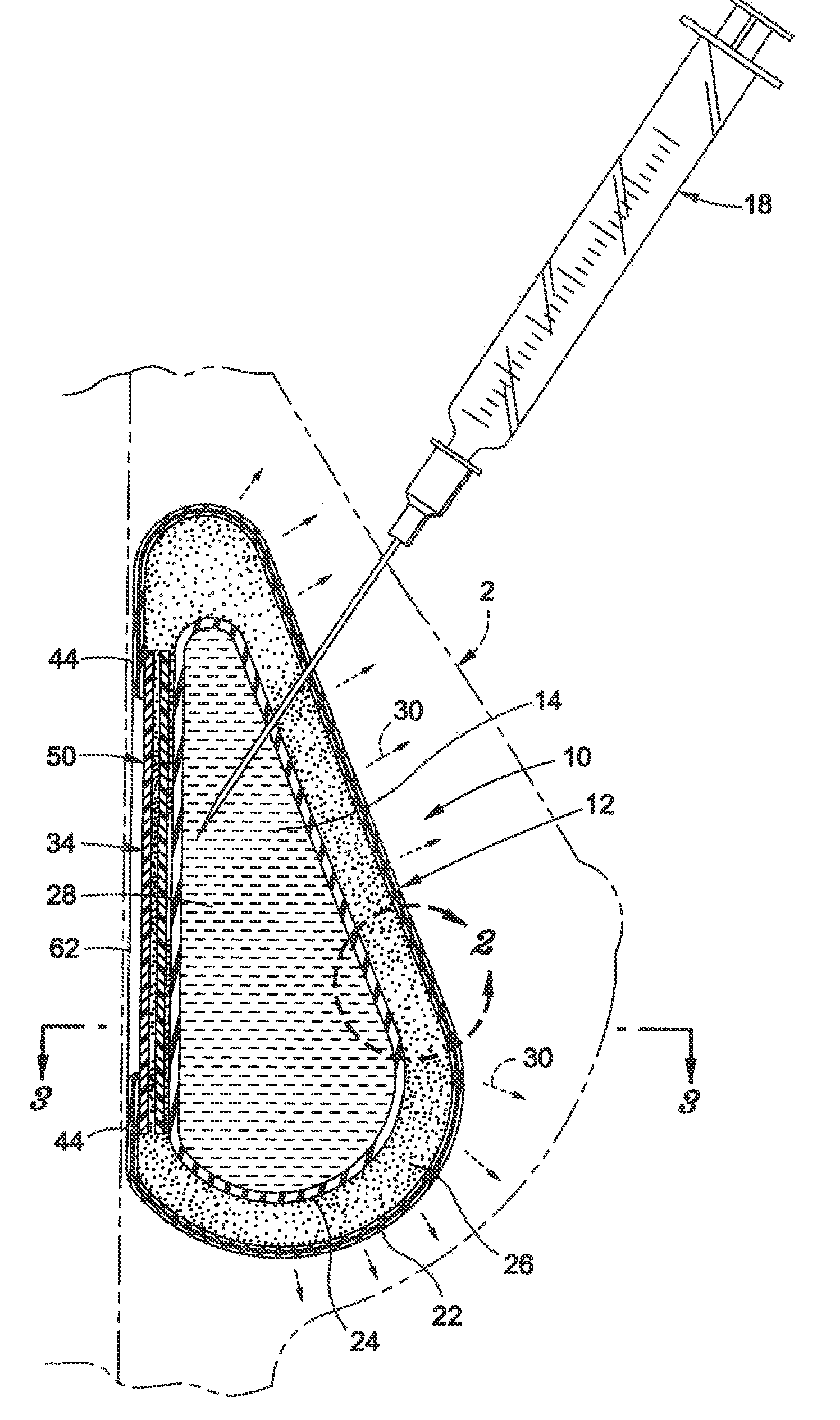
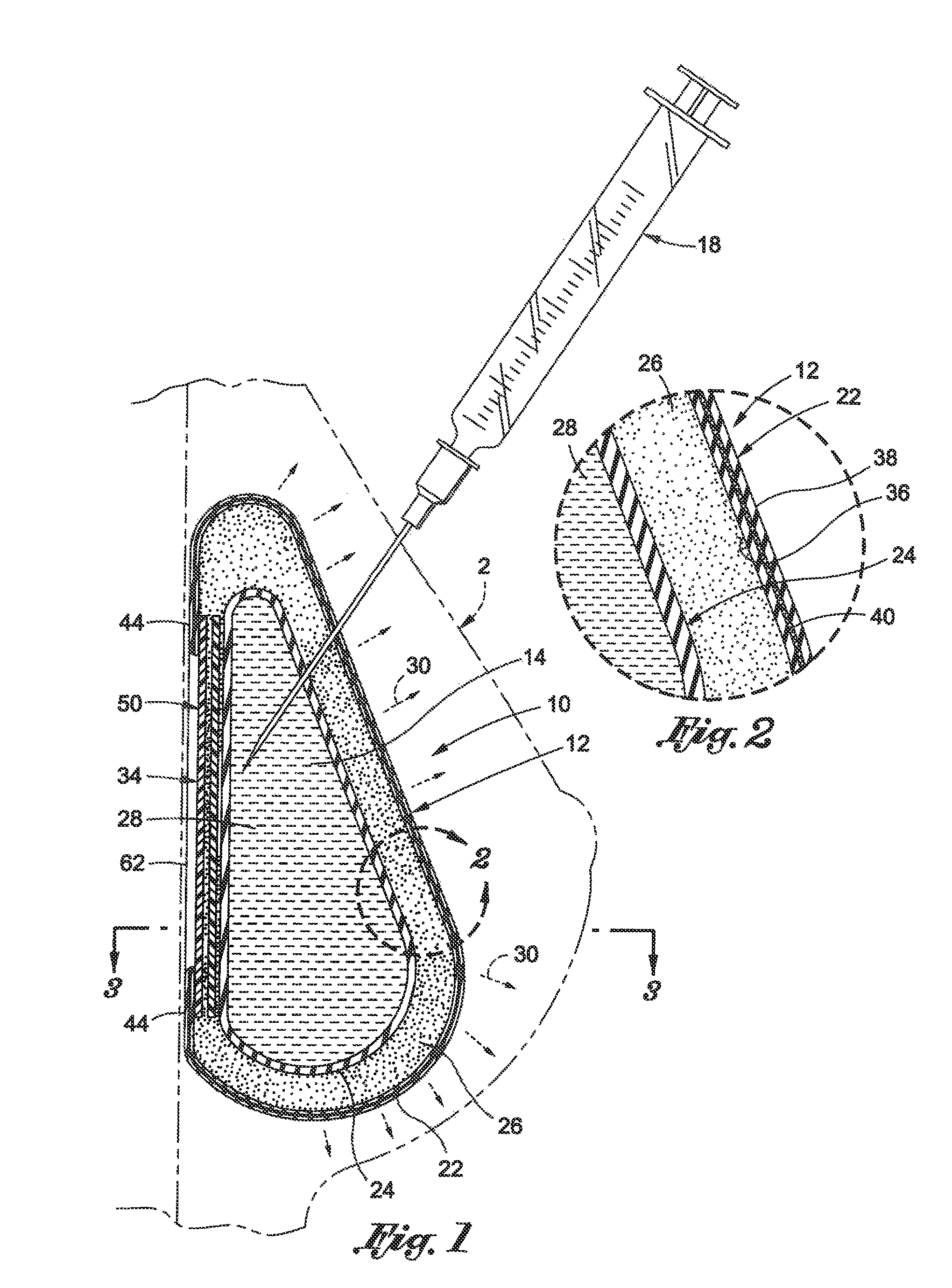
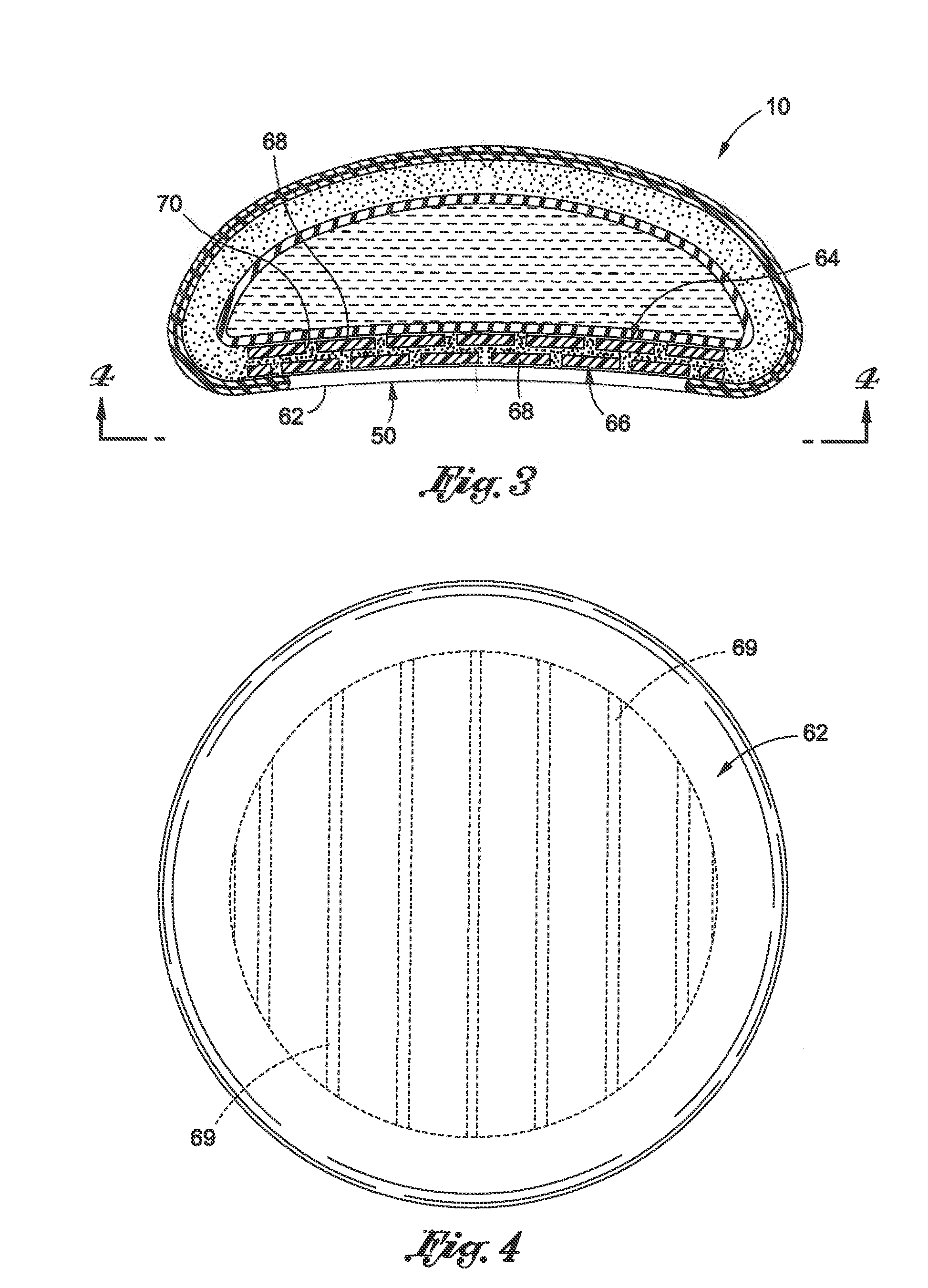
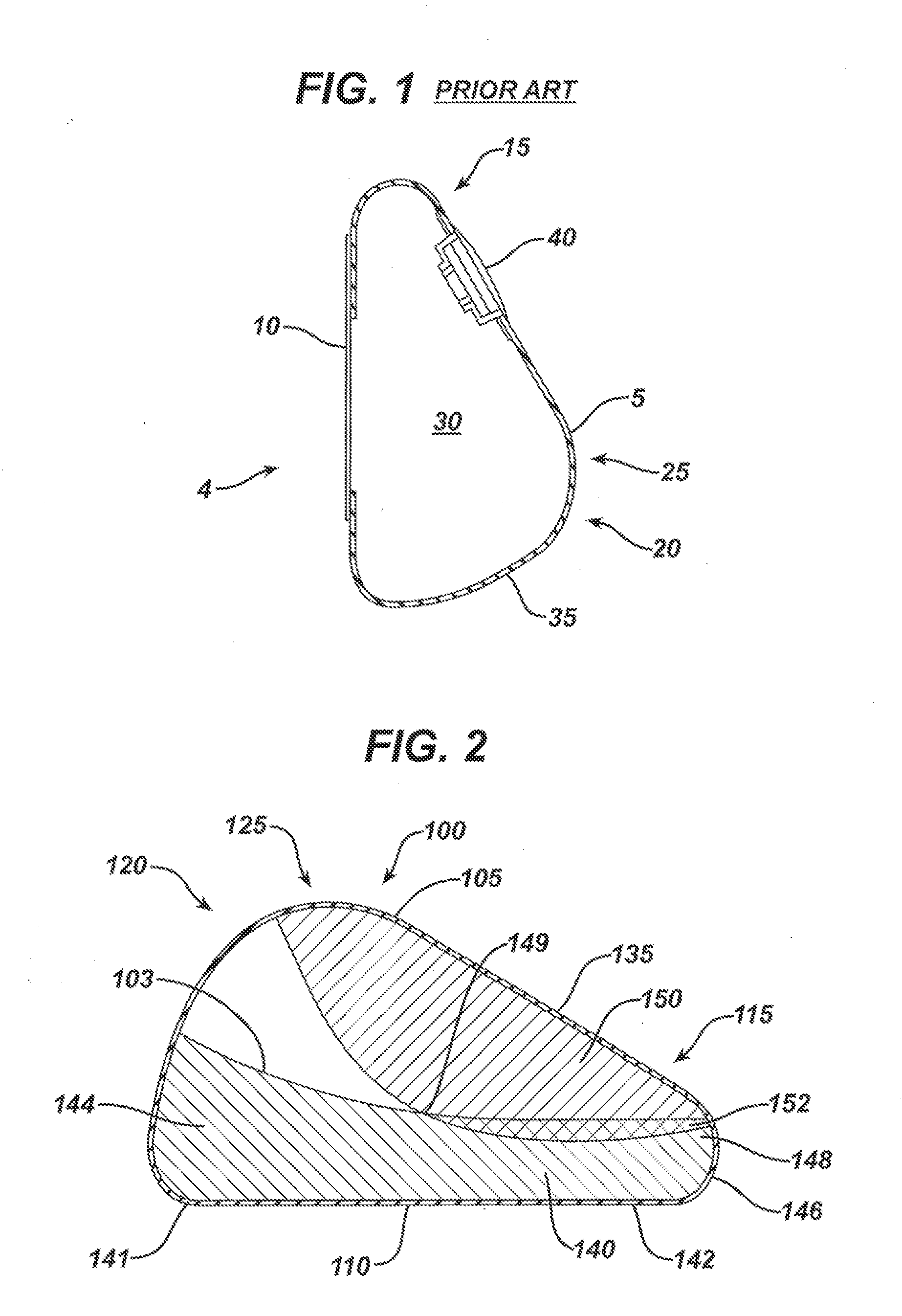
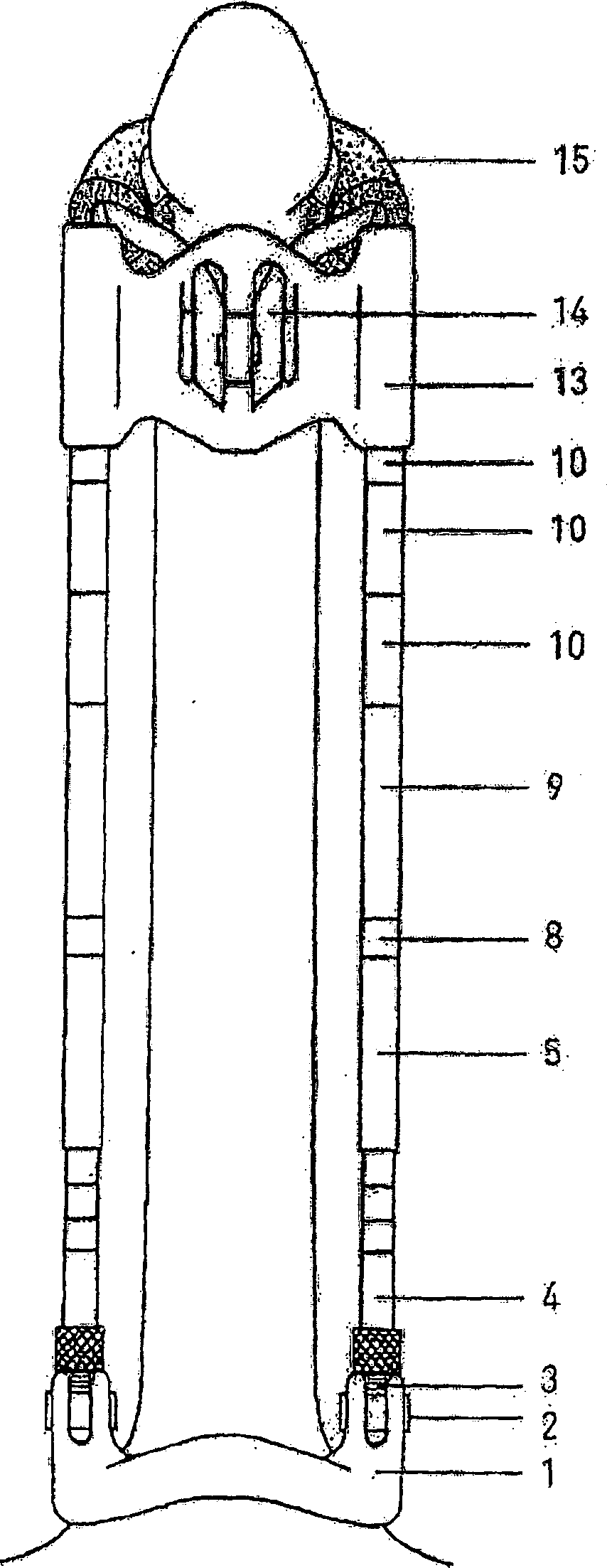
Interspinous process implant with radiolucent spacer and lead-in tissue expander
InactiveUS20050075634A1Less discomfortHighly structuredInternal osteosythesisJoint implantsDistractionRange of motion
The present invention is directed to an interspinous process device with a deflectable spacer which can be placed between adjacent spinous processes to limit the movement of the vertebrae. The device limits the range of motion of the spinous processes. The spacer and a lead-in distraction guide or tissue expander can be radiolucent.
Owner:KYPHON
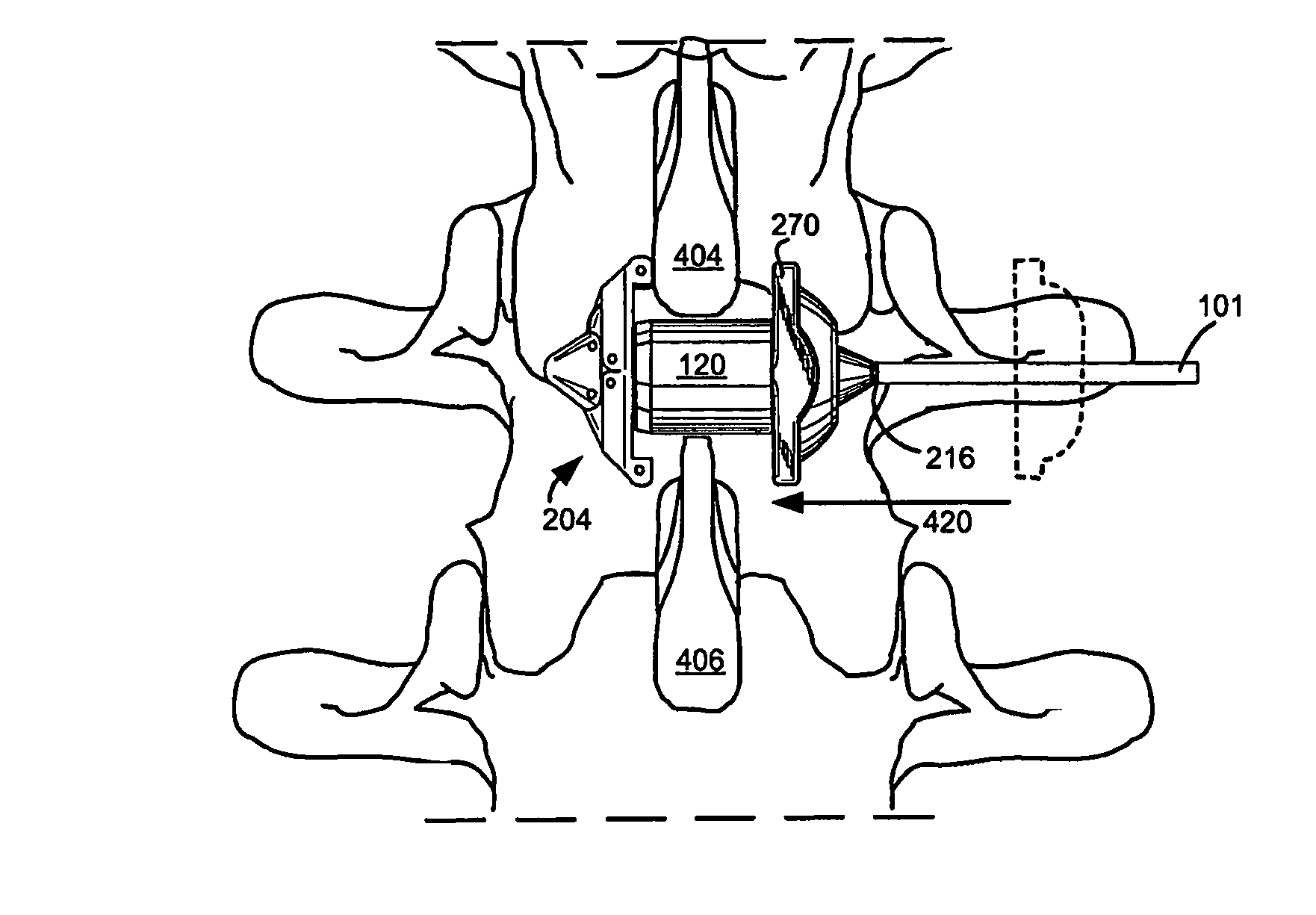
Systems and Methods for In Situ Assembly of an Interspinous Process Distraction Implant
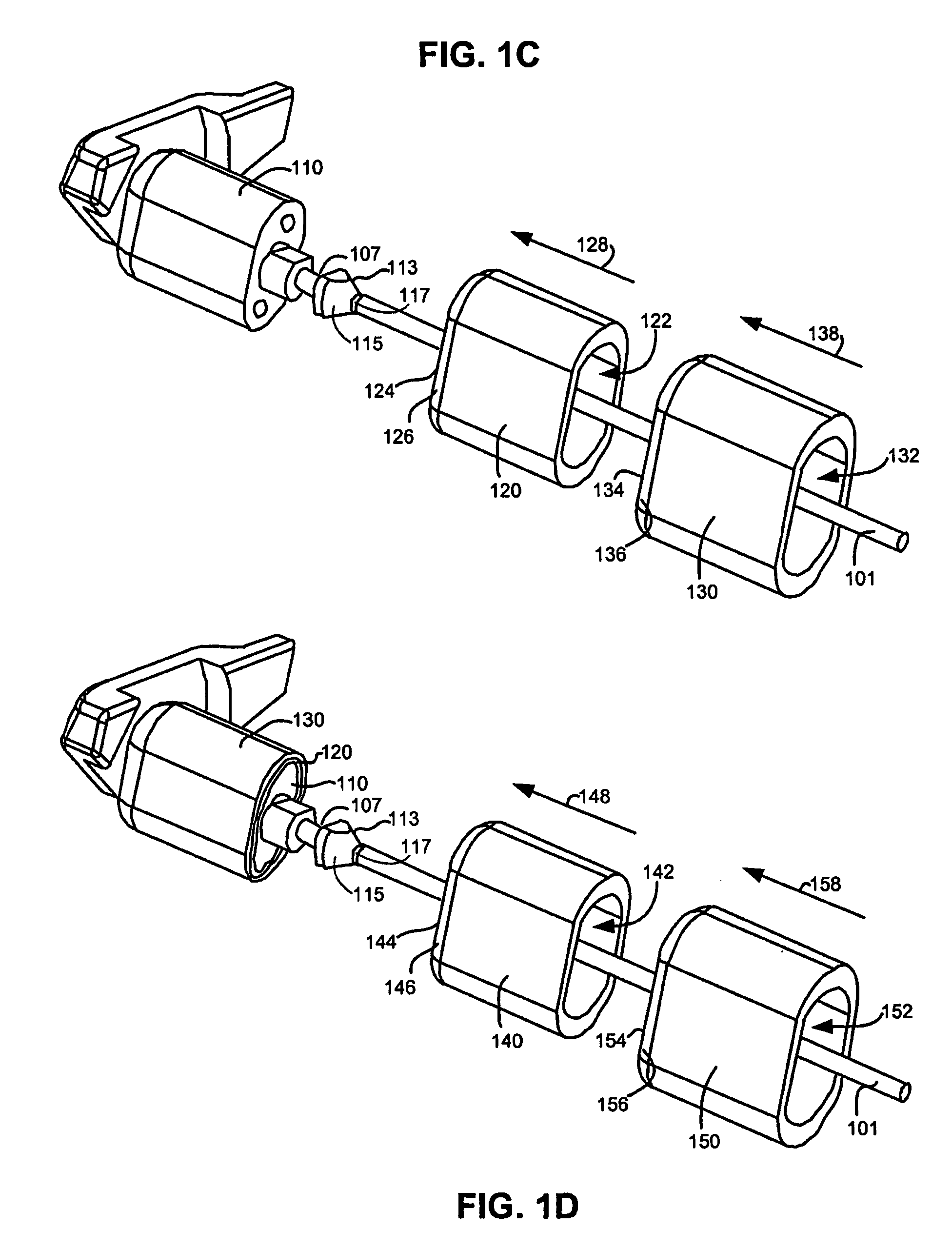
An implant system for implantation between adjacent spinous processes for the relief of pain associated with the spine. The implant has a series of spacers which may be inserted over a shaft located between adjacent spinous processes thus allowing the implant to be assembled in situ. The spacers may rotate on the shaft relative to the wings. To minimize trauma to the patient, each spacer has a tapered tissue expander to distract the opening between the spinous processes during assembly. The shaft is connected to a wing, and a second wing or deployable wing may be inserted over the shaft and locked into place.
Owner:KYPHON
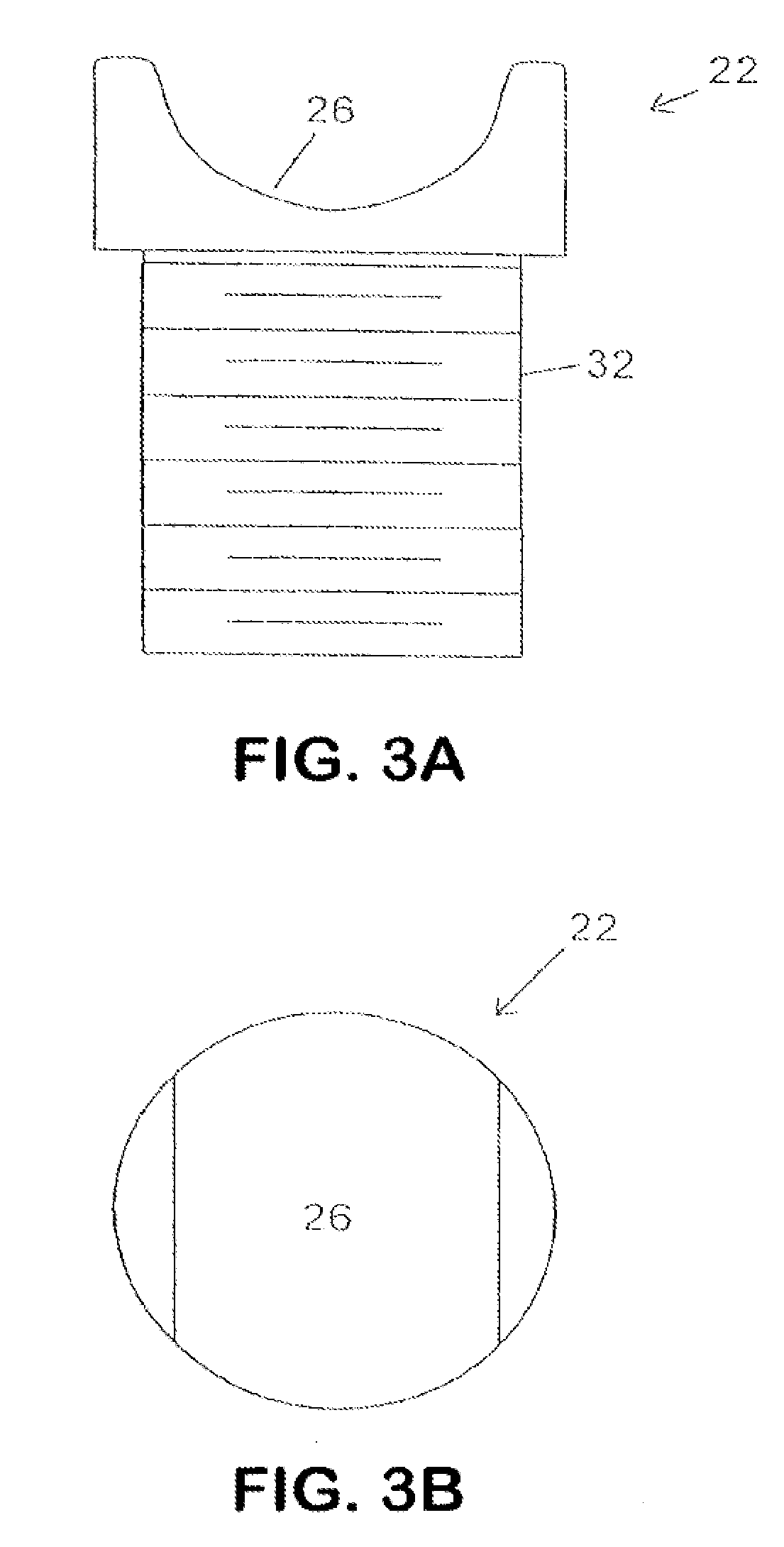
Spine distraction implant
InactiveUS20080086212A1Increase volumeReduce restrictionsInternal osteosythesisSpinal implantsDistractionDevice implant
A spine distraction implant alleviates pain associated with spinal stenosis and facet arthropathy by expanding the volume in the spine canal and / or neural foramen. The implant provides a spinal extension stop while allowing freedom of spinal flexion. An interspinous process implant with a selectably expandable spacer can be placed between adjacent spinous processes. a device implanted between the spinous processes of adjacent vertebrae of the spine can be used for relieving pain associated with the vertebrae and surrounding tissues and structures by maintaining and / or adding distraction between adjacent vertebrae. A tissue expander can be adapted to move from a first insertion position, for ease of implantation between spinous processes, to a second retention position that prevents displacement of the implant. An embodiment of a system can include an implant having a spacer with a thickness and a wing, wherein a first configuration of the wing has a first height substantially similar to the thickness and wherein the wing is adapted to be selectably arranged in a second configuration such that the wing has a second height greater than the first height. A periphery of the implant has a shape generally conformal with a shape of an inner surface of a cannula and a cross-sectional diameter smaller than an inner diameter of the cannula. The cannula is inserted such that a proximal end of the cannula is arranged between the adjacent spinous processes. The implant is then urged into position between the adjacent spinous processes by way of the cannula, and subsequently arranged in a second configuration to fix the implant in position.
Owner:KYPHON
Unitized penile erection system and tissue expander
InactiveUS7922649B2Small volumeNatural and full feelNon-surgical orthopedic devicesPenis implantsControl releaseTransfer system
A surgically implantable device is a single unit comprised of a hydraulically activated extendable cavernosal component and a manually controlled fluid transfer system composed of a pump. Flow from the pump is directed by a one way valve interposed between a bypass from an included elastic reservoir, the pump and a second one way valve interposed between the pump and the cavernosal unit. A manually controlled release valve, interposed between the outlet of the cavernosal unit and the reservoir, permits de-activation of the cavernosal unit and returns fluid to the reservoir. These components are housed by a molded tailpiece which stabilizes the device against the symphysis pubis, positions the cavernosal implant properly within the shaft of the penis and presents the controls for easy access. An access port permits adjustment of the fluid volume. The unit has utility as a tissue expander and penile re-construction in event of congenital or traumatic deformity.
Owner:WALCH JOHN R
Spine distraction implant
InactiveUS20100262243A1Increase volumeReduce restrictionsInternal osteosythesisJoint implantsDistractionDilator
Owner:MEDTRONIC EURO SARL
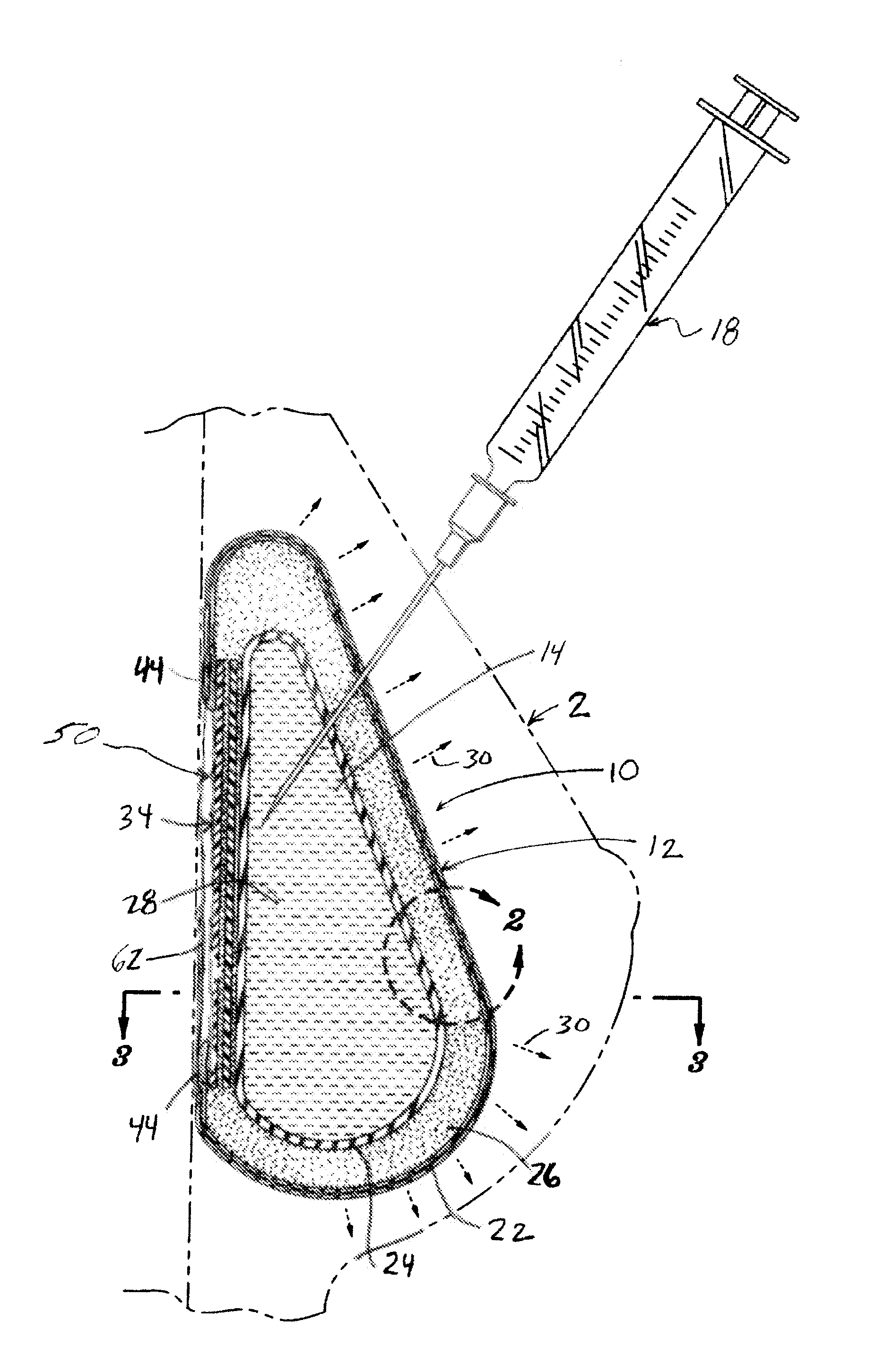
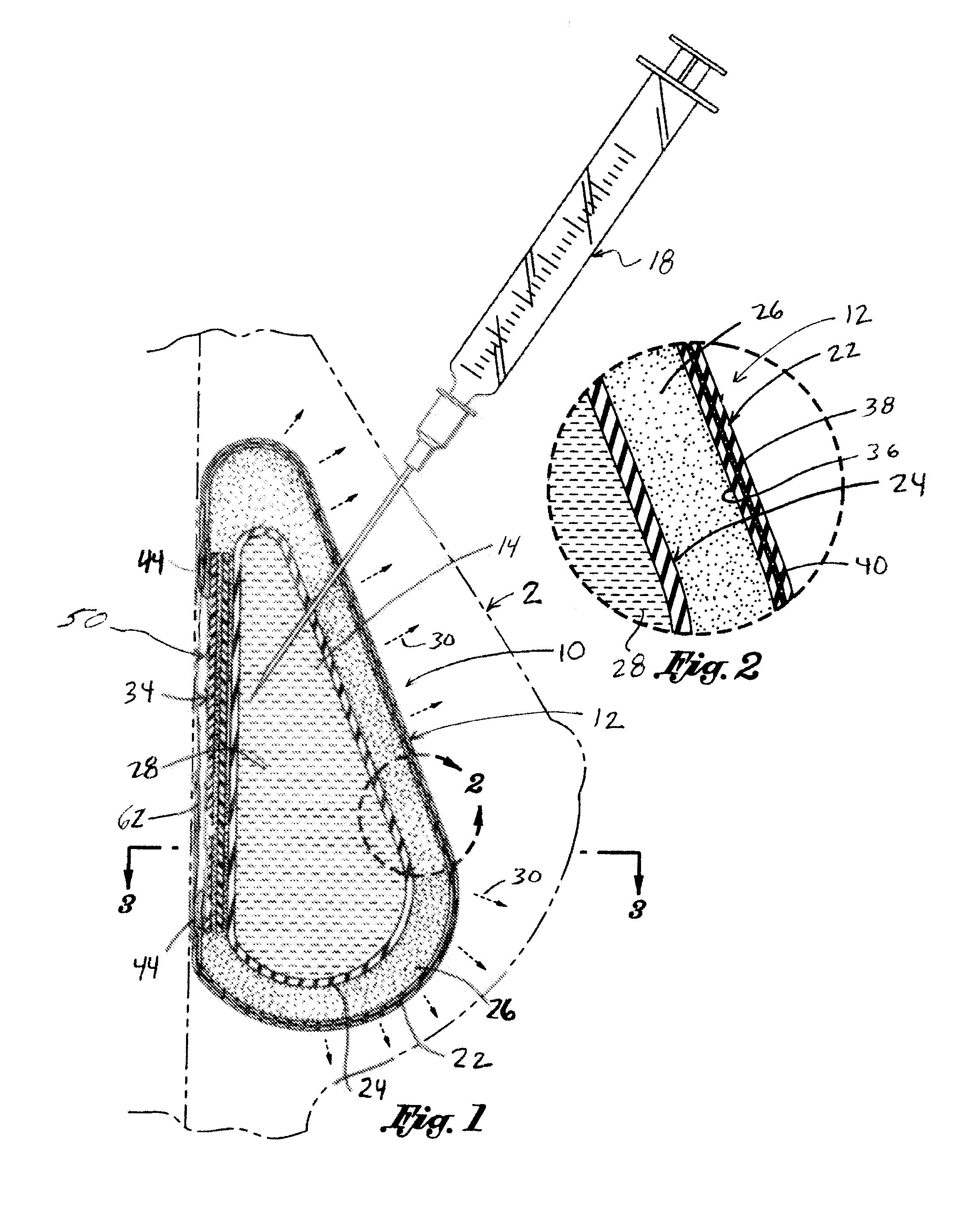
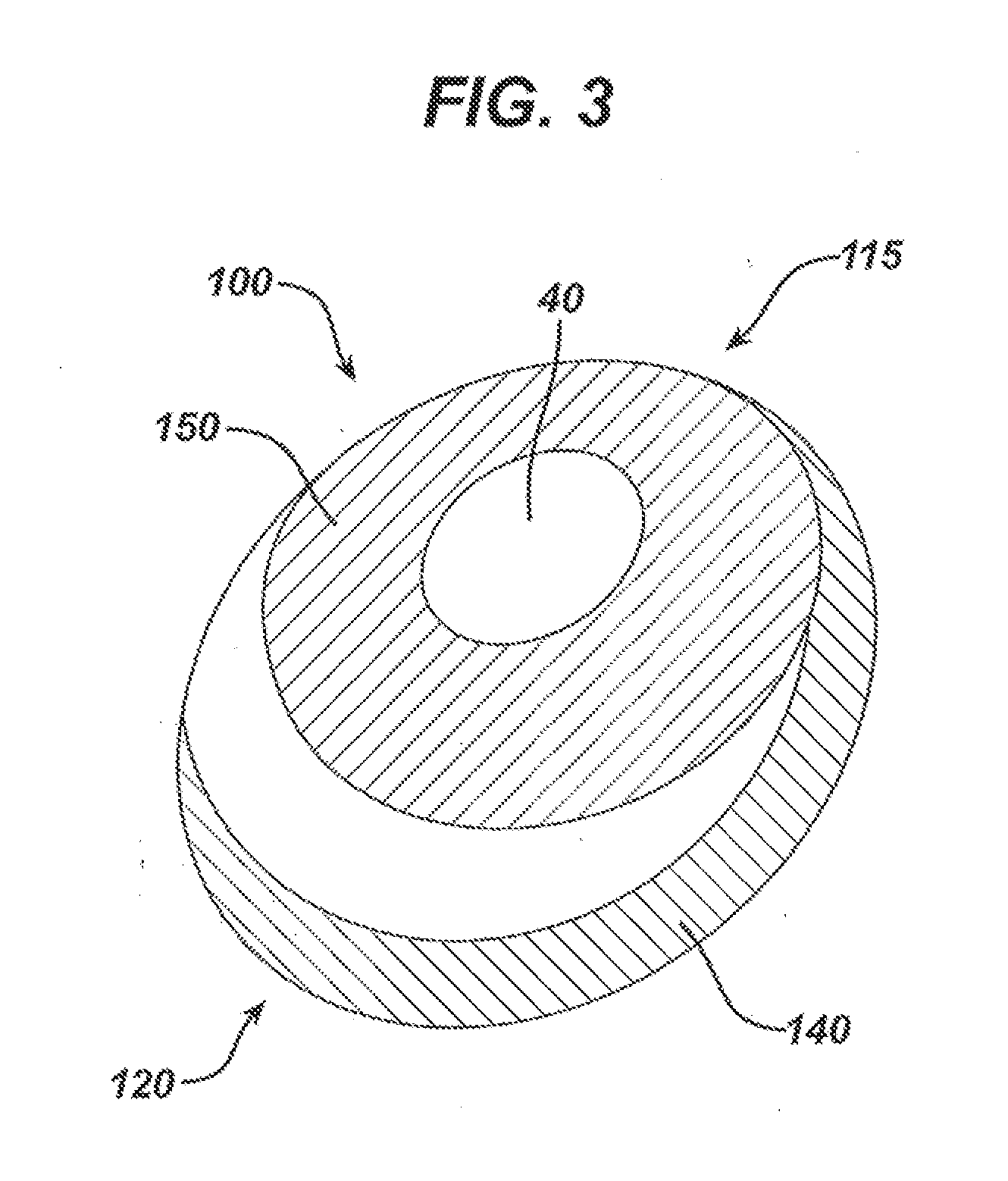
Inflatable prostheses and methods of making same
ActiveUS20110270391A1Sacrificing flexibility of assemblyMammary implantsDiagnosticsElastomerPermanent prosthesis
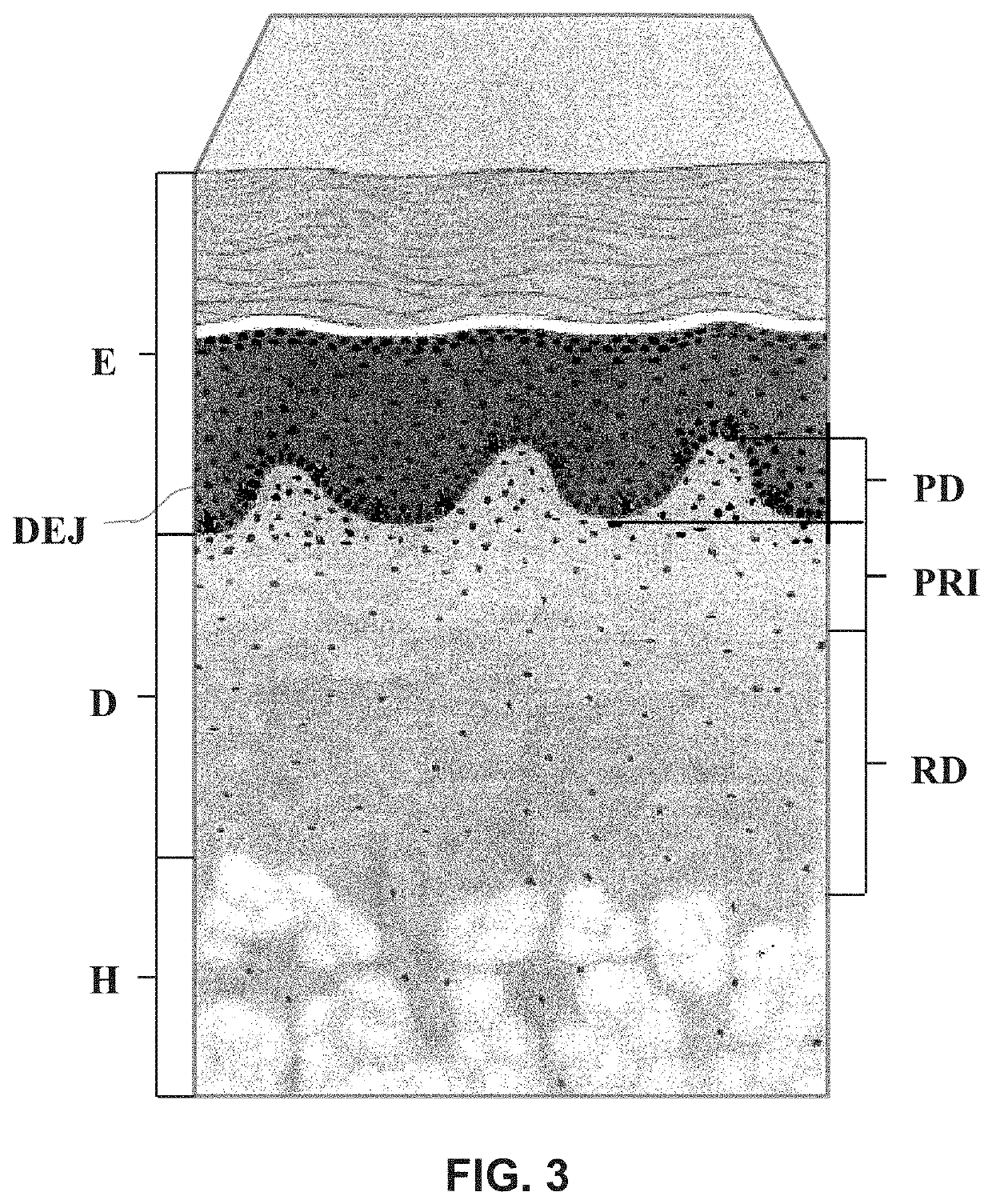
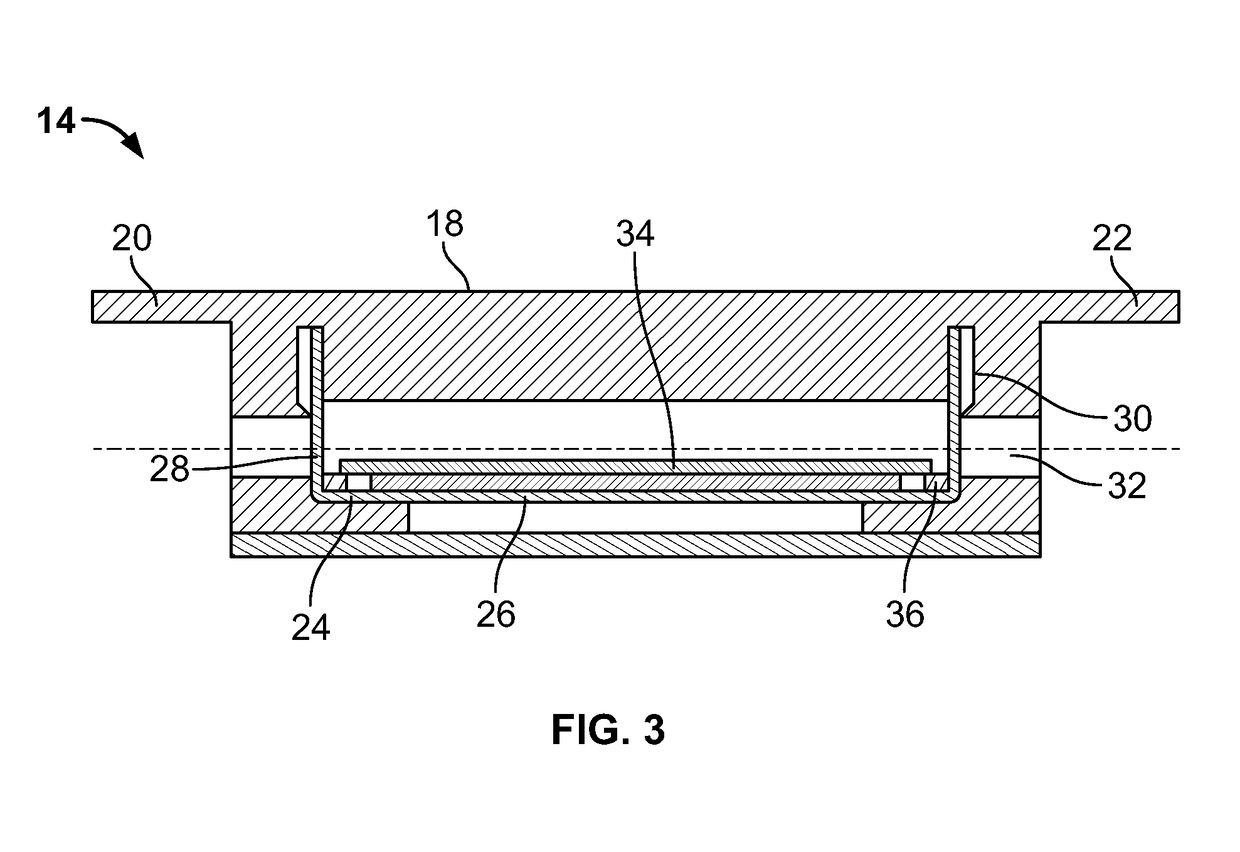
An inflatable tissue expander or more permanent prosthesis, suitable for implantation in a breast, is provided. The tissue expander includes a puncturable, self-sealing anterior portion forming a fillable cavity, and posterior portion that is puncture resistant. The anterior portion includes a silicone-based elastomer material having a mesh embedded therein. The posterior portion includes a first composite guard and a second composite guard, each composite guard including an arrangement of puncture resistant members and a flexible substrate having a first side on which the puncture resistant members are disposed in a spaced apart fashion.
Owner:ALLERGAN INC
Systems and methods for tissue expansion with fluid delivery and drainage system
ActiveUS20110153017A1Eliminate biofilm formationMammary implantsDiagnosticsTissue expansionBiomedical engineering
The invention provides systems and methods for tissue expansion. A tissue expander may have an implant portion which may be used to expand the tissue. A delivery / drainage system may be also be provided, which may be in fluid communication with a pocket surrounding the tissue expander. Various port configurations may be provided that may provide access to the implant portion and / or the delivery / drainage system. The tissue expander may advantageously help prevent or treat infection, or check the state of the pocket surrounding the tissue expander.
Owner:MCCLELLAN WILLIAM T
Inflatable prostheses and methods of making same
InactiveUS20130245758A1Sacrificing flexibility of assemblyMammary implantsProtective equipmentPermanent prosthesisDevice prosthetic
An inflatable tissue expander or more permanent prosthesis, suitable for implantation in a breast, is provided.
Owner:ALLERGAN INC
Tissue expander apparatus with magnetically locatable injection site and methods for use
InactiveUS20010004709A1Precise positioningMinimize the potential for the thinnerMammary implantsDiagnosticsMedicineTissue expander
Owner:DUBRUL WILLIAM R
Inflatable prostheses and methods of making same
An inflatable tissue expander, suitable for implantation in a breast, is provided. The tissue expander includes a puncturable, self-sealing anterior portion forming a fillable cavity, and posterior portion that is puncture resistant. The anterior portion includes a silicone-based elastomer material having a mesh embedded therein. The posterior portion includes a first composite guard and a second composite guard, each composite guard including an arrangement of puncture resistant members and a flexible substrate having a first side on which the puncture resistant members are disposed in a spaced apart fashion.
Owner:ALLERGAN INC
Method and apparatus for expanding tissue
An implantable tissue expander for supporting a body tissue including a support member positioned with respect to the body tissue and adapted to expand to a support configuration, in the support configuration, the support member shaping the body tissue.
Owner:VARDI GIL
Inflatable prostheses and methods of making same
ActiveUS8636797B2Sacrificing flexibility of assemblyMammary implantsDiagnosticsElastomerPermanent prosthesis
An inflatable tissue expander or more permanent prosthesis, suitable for implantation in a breast, is provided. The tissue expander includes a puncturable, self-sealing anterior portion forming a fillable cavity, and posterior portion that is puncture resistant. The anterior portion includes a silicone-based elastomer material having a mesh embedded therein. The posterior portion includes a first composite guard and a second composite guard, each composite guard including an arrangement of puncture resistant members and a flexible substrate having a first side on which the puncture resistant members are disposed in a spaced apart fashion.
Owner:ALLERGAN INC
Systems and methods for in situ assembly of an interspinous process distraction implant
An implant system for implantation between adjacent spinous processes for the relief of pain associated with the spine. The implant has a series of spacers which may be inserted over a shaft located between adjacent spinous processes thus allowing the implant to be assembled in situ. The spacers may rotate on the shaft relative to the wings. To minimize trauma to the patient, each spacer has a tapered tissue expander to distract the opening between the spinous processes during assembly. The shaft is connected to a wing, and a second wing or deployable wing may be inserted over the shaft and locked into place.
Owner:KYPHON
Systems and methods for tissue expansion with fluid delivery and drainage system
The invention provides systems and methods for tissue expansion. A tissue expander may have an implant portion which may be used to expand the tissue. A delivery / drainage system may be also be provided, which may be in fluid communication with a pocket surrounding the tissue expander. Various port configurations may be provided that may provide access to the implant portion and / or the delivery / drainage system. The tissue expander may advantageously help prevent or treat infection, or check the state of the pocket surrounding the tissue expander.
Owner:MCCLELLAN WILLIAM T
Tissue Expander with Means to Deliver Antibiotics or Medication Uniformly on its Surface Using Multiple Channels Comprising Pores
InactiveUS20170035999A1The process is simple and effectiveAvoid pollutionDiagnosticsSurgeryEffusionAntibiotic Y
A tissue expander distributes antibiotics or other drugs and treats infections to the surrounding tissue. The tissue expander is provided with a manifold that can be accessed from outside via an injection needle which is connected to a series of channels containing drug effusion ports. When the tissue is infected antibiotics are injected into the manifold which in turn runs through the channels, effuse out the strategically placed ports to treat the infection. The procedure can be repeated until the infection is resolved. Any fluids effusing from the tissue are drained using the drainage channels that connect to a central drainage cavity and is later drained out of the body using a needle or cannula placed into a special drainage collection cavity.
Owner:WIJAY BANDULA
Soft tissue repair grafts and processes for preparing and using same
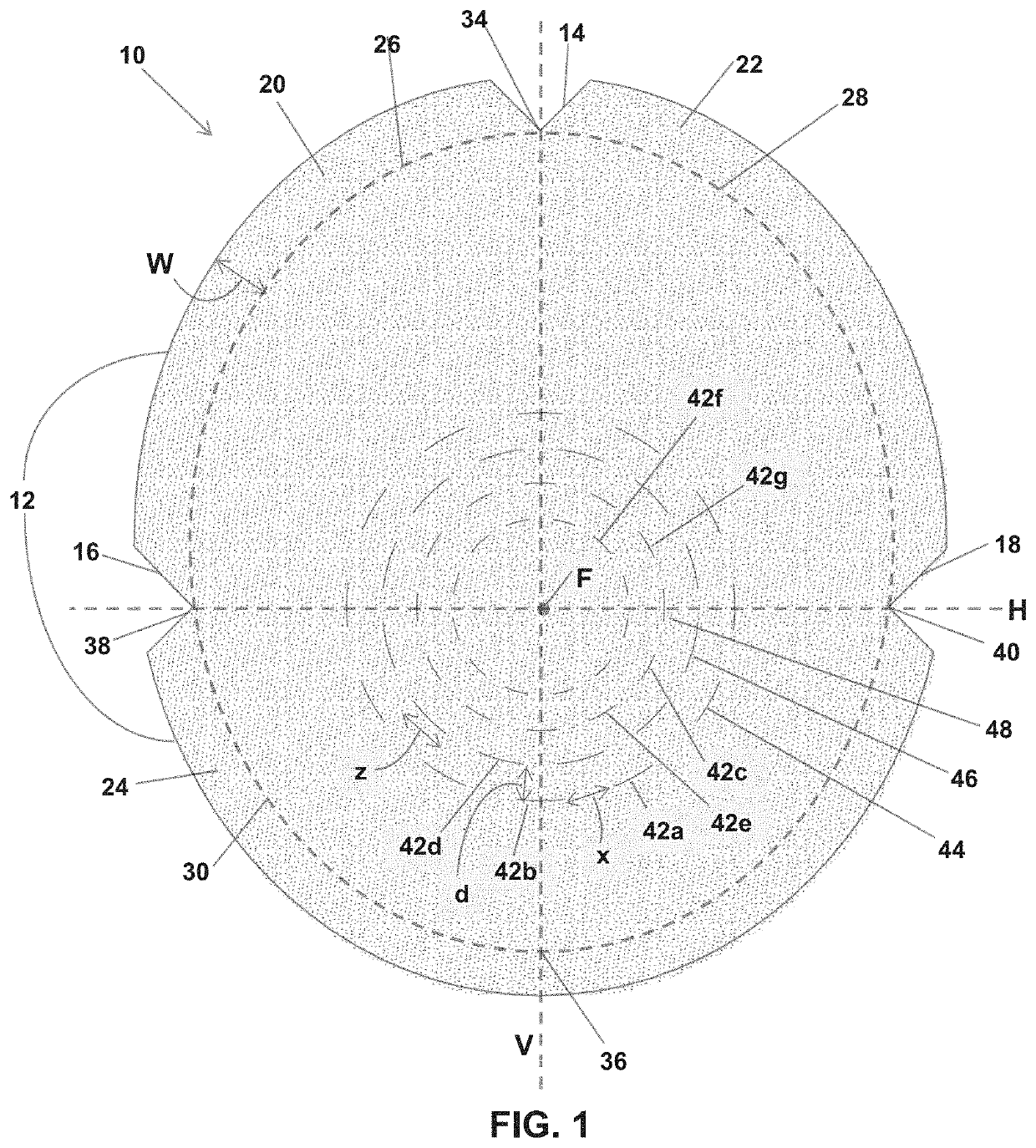
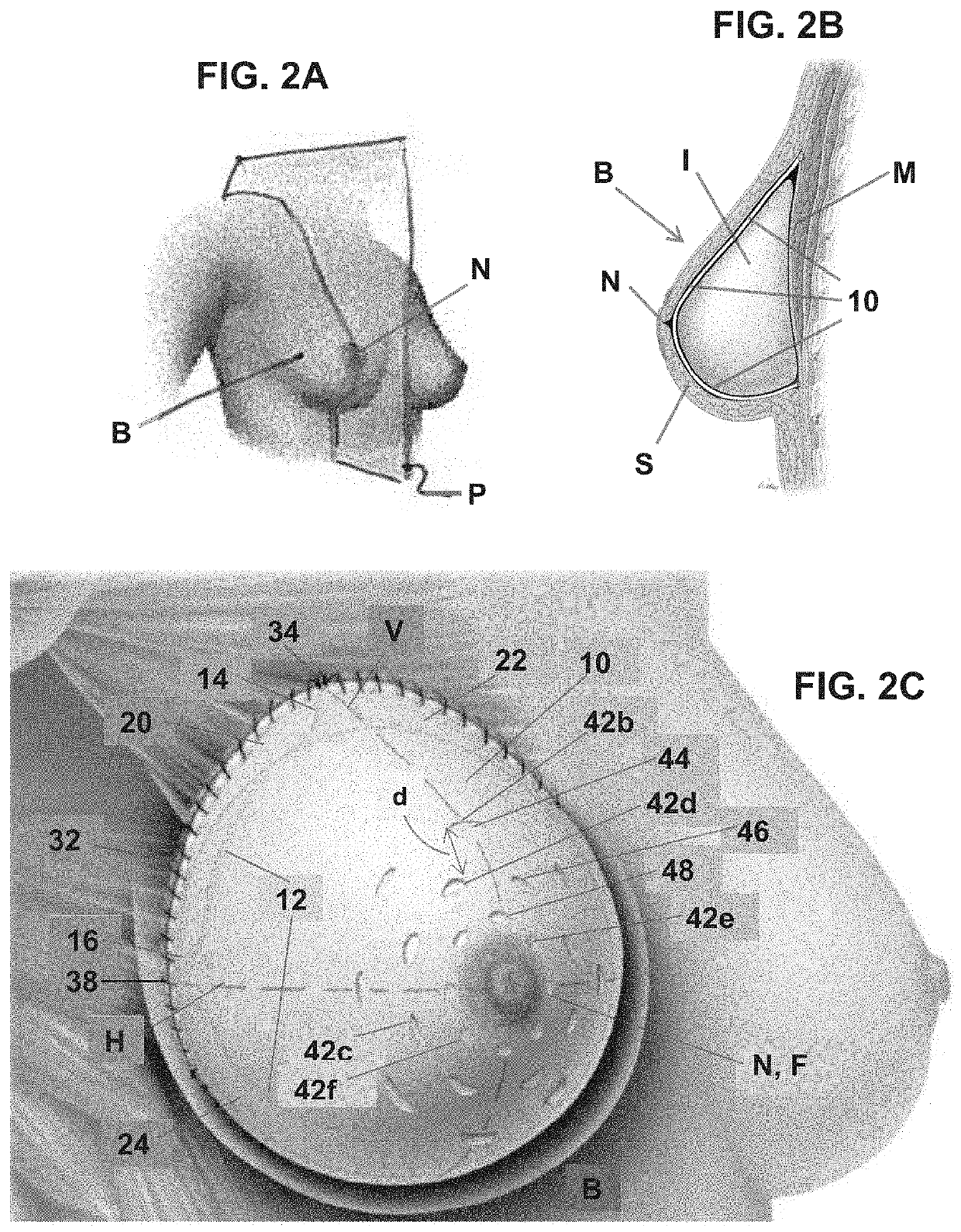
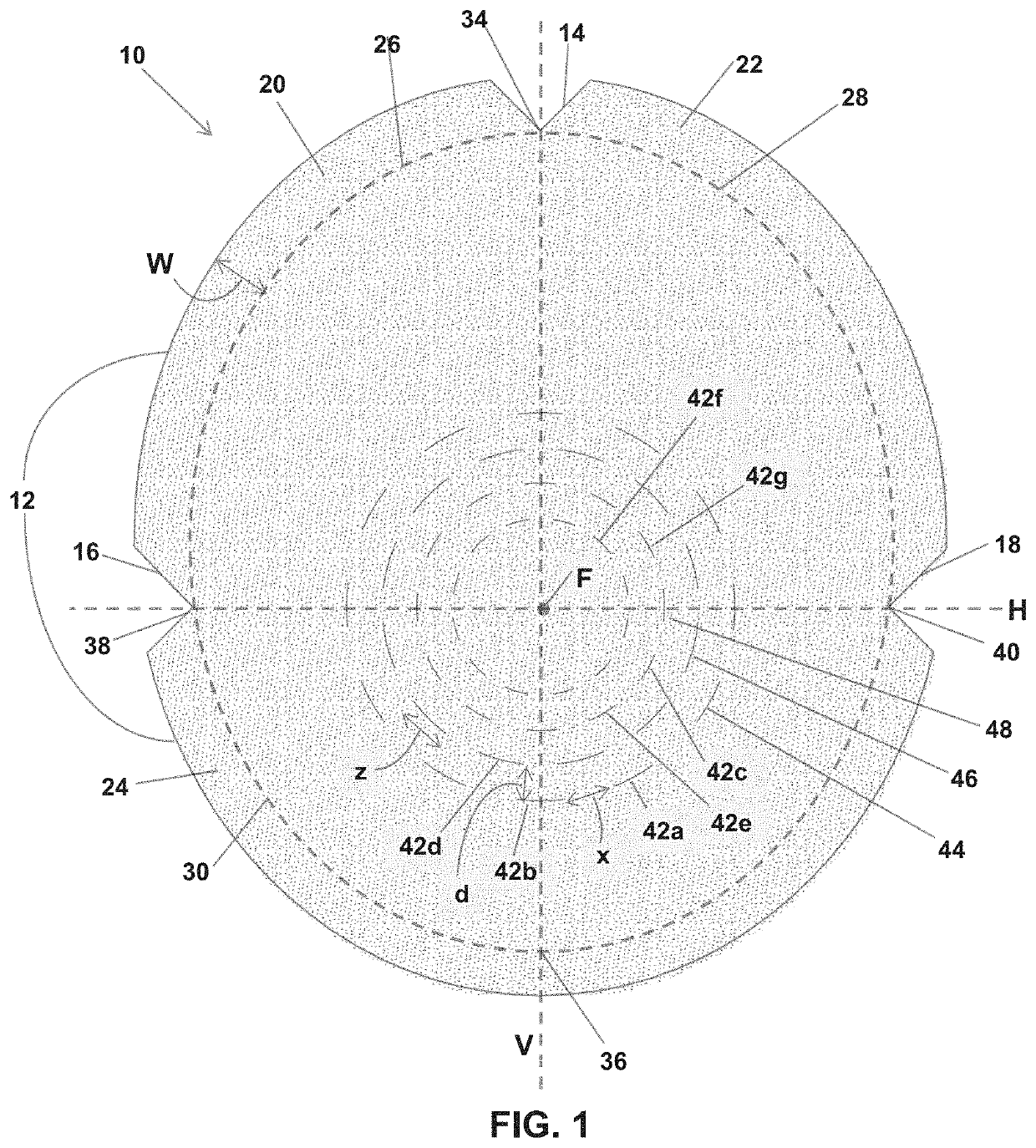
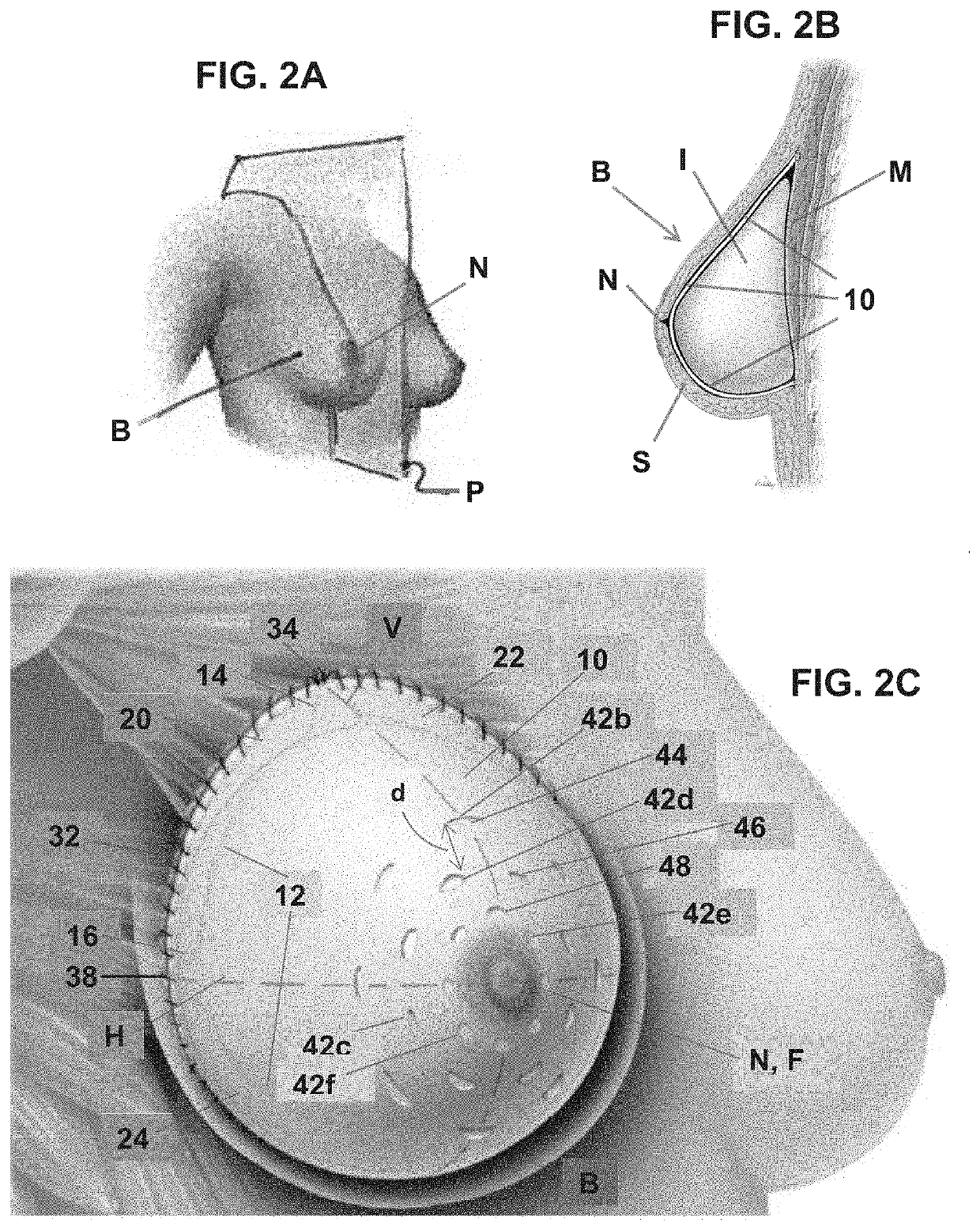
Soft tissue repair grafts are provided for supporting, covering, and / or retaining an implant positioned in the body of a subject. The grafts are particularly suitable for use for pre-pectoral breast reconstruction with a breast implant or tissue expander. The grafts include positional notches for more accurate positioning in a subject. The grafts also include at least one cuff element which is folded to form a reinforced folded edge for suturing the graft more securely to adjacent tissues than previously known grafts. The grafts also include a plurality of arcuate slots which form a plurality of circular patterns arranged concentrically about a focal point, thereby enabling the grafts to expand without tearing and to conform more closely to the implant and / or adjacent body tissues such as the breast pocket, than previously known grafts. Acellular dermal matrices are particularly suitable for making the soft tissue repair grafts.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
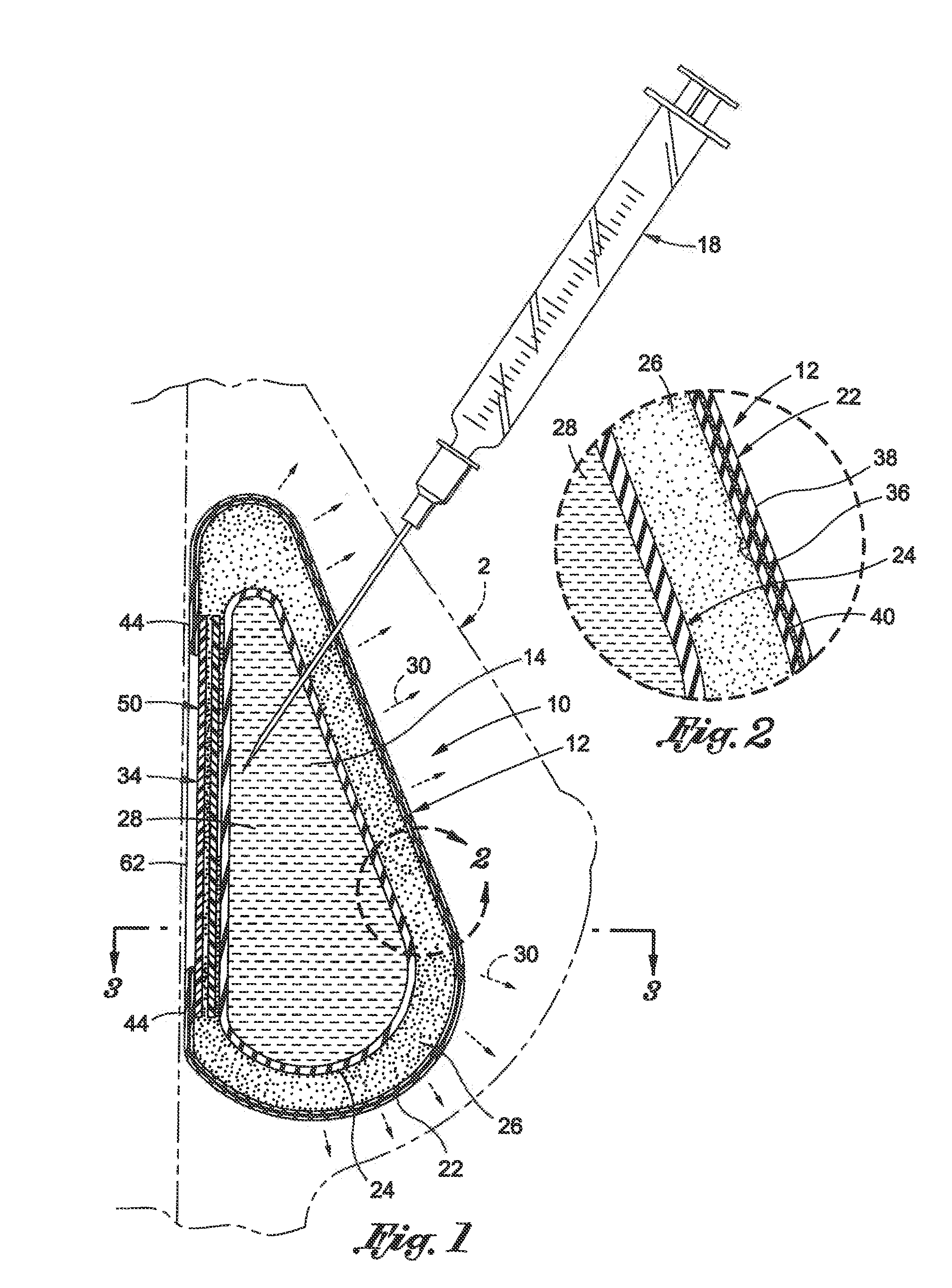
Tissue expander implant with self-sealing safety patch
ActiveUS9700404B2Add featureAccurate locationMammary implantsDiagnosticsHypodermic needleHypodermoclysis
Disclosed is a self-sealing patch for use with a tissue expander implant. The self-sealing patch serves as protection for the tissue expander against a hypodermic needle inadvertently missing a fluid injection port thereof by sealing a puncture through the patch. The patch includes a first sheet having a first sheet perimeter, a second sheet having a second sheet perimeter attached to the first sheet perimeter to form a pocket between the first sheet and the second sheet. Material is disposed within the pocket, where the material is hydrophobic material having a viscosity high enough that the material is prevented from flowing outside the pocket when either the first or second sheet is punctured with a hypodermic needle but low enough that the material flows to close a track made by a hypodermic needle puncturing the first or second sheet.
Owner:MENTOR WORLDWIDE
Tissue expander with pectoral attachment
A mammary tissue expander device including an inflatable shell having an anterior side and a posterior side, the anterior side having an upper pole portion and a lower pole portion meeting at an apex, and an injection port for receiving fluid therethrough to inflate the expander, and a substantially flat attachment flap coupled to the posterior side of the shell, and extending in an inferior direction beyond an inferior edge of the shell by a distance D such that the attachment flap may be extended around the inferior edge and upwards to overlay at least a portion of the anterior side of the shell.
Owner:MENTOR WORLDWIDE
Inflatable prostheses and methods of making same
InactiveUS20140121771A1Sacrificing flexibility of assemblyMammary implantsDiagnosticsElastomerPermanent prosthesis
An inflatable tissue expander or more permanent prosthesis, suitable for implantation in a breast, is provided. The tissue expander includes a puncturable, self-sealing anterior portion forming a fillable cavity, and posterior portion that is puncture resistant. The anterior portion includes a silicone-based elastomer material having a mesh embedded therein. The posterior portion includes a first composite guard and a second composite guard, each composite guard including an arrangement of puncture resistant members and a flexible substrate having a first side on which the puncture resistant members are disposed in a spaced apart fashion.
Owner:ALLERGAN INC
Directional tissue expander
An expandable mammary implant including a shell having an anterior face and a posterior face. The anterior has an upper pole portion and a lower pole portion meeting at an apex, and an injection zone for receiving fluid therethrough to inflate the implant. A reinforcement material is coupled to the shell in a reinforcement zone. The reinforcement material is coupled to the shell so as to at least coincide with the upper pole portion and a peripheral rim portion that extends from the posterior face upwardly into said anterior face by a predetermined distance.
Owner:MENTOR WORLDWIDE
Hybrid breast implant and tissue expander, methods of making and use of same
An implant includes a first container and a plurality of members disposed in the first container. The implant can be made by attaching a member to a second container disposed in the first container and inserting the second container in the first container. The implant can be used by disposing the implant into a subject and adjusting a volume of a fluid in the implant. Additionally, a tissue expander includes an outer container; and an inner container disposed in the outer container and comprising: a reticulated frame comprising a plurality of struts which interconnect; a void disposed between the struts in the reticulated frame; and a projection connected to the struts to cover the void.
Owner:TECHNO INVESTMENTS
Soft tissue repair grafts and processes for preparing and using same
Soft tissue repair grafts are provided for supporting, covering, and / or retaining an implant positioned in the body of a subject. The grafts are particularly suitable for use for pre-pectoral breast reconstruction with a breast implant or tissue expander. The grafts include positional notches for more accurate positioning in a subject. The grafts also include at least one cuff element which is folded to form a reinforced folded edge for suturing the graft more securely to adjacent tissues than previously known grafts. The grafts also include a plurality of arcuate slots which form a plurality of circular patterns arranged concentrically about a focal point, thereby enabling the grafts to expand without tearing and to conform more closely to the implant and / or adjacent body tissues such as the breast pocket, than previously known grafts. Acellular dermal matrices are particularly suitable for making the soft tissue repair grafts.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC +1
Directional Tissue Expander
An expandable mammary tissue implant including a shell having an anterior face and a posterior face, the anterior face having an upper pole portion and a lower pole portion meeting at an apex, and an injection zone for receiving fluid therethrough to inflate the implant. The implant further includes a vertical tether member having first and second ends and a central region therebetween having an aperture therethrough. The central region is coupled to the anterior face of the implant at a location such that the injection zone is positioned within the aperture in the central region. The first and second ends of the vertical tether member are coupled to the posterior face of the implant.
Owner:MENTOR WORLDWIDE
An automatic constant pressure skin dilator
InactiveCN102274054AAccurate control of pressureAvoid displacementSurgeryDilatorsMedical equipmentDilator
The invention belongs to medical equipment, and relates to an automatic constant-pressure skin dilator, which includes a silicon capsule and a water injection device connected by a water injection pipe. hole. The beneficial effect of the present invention is that it can detect the pressure, accurately grasp the internal pressure of the silicon capsule, facilitate the operation, and is convenient to use and store, and the hanging hole can be hung on the wall or shelf, and the sensor and the water pump can be used constantly. Manual operation is required, just hang it up to prevent it from being accidentally touched. After use, it can be hung on the utensil rack for easy storage. The thermometer can accurately measure the temperature of the liquid injected into the silicon capsule, and injecting a liquid with a suitable temperature is beneficial to reduce the pain of the patient.
Owner:赵东红
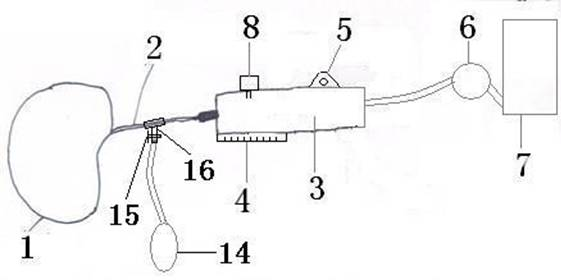
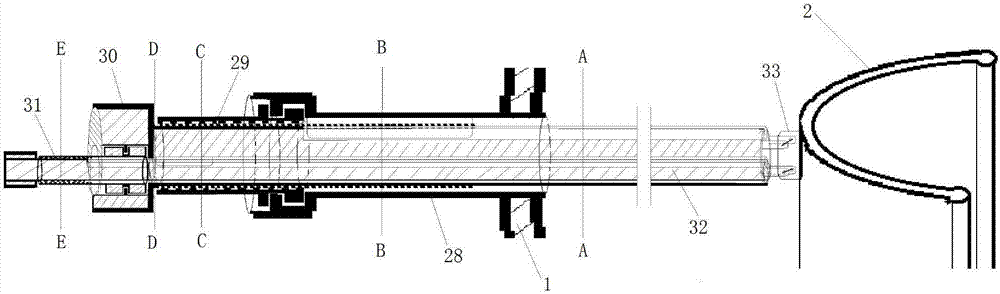
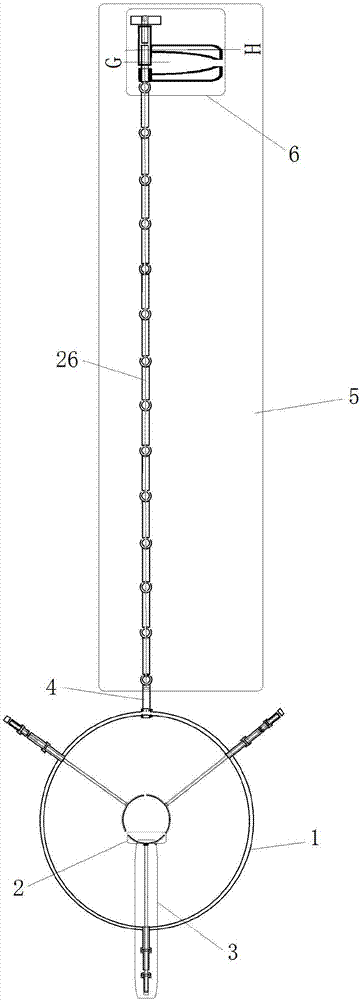
Genitalia external prothesis for treatment of male external genitalia hypoplasia
InactiveCN1284517CAvoid woundsNo discomfortPenis support devicesRestraining devicesUser deviceMale gender
The present invention relates to a device for treating male genital hypoplasia, growing its tissue and correcting existing curvature. The device of the present invention is unique in that it employs an external genital fixation system (1; 13) to which a diameter device (5) and a gland protection system (15) are connected. The external genital fixation system (1) and the upper element (13) create a traction force on the male external genitalia. The diameter device (5) creates a tissue expansion on the male genitalia. The gland protection system (15) avoids possible wounds, which makes it possible to use it for a long time without discomfort. Users of the device can wear it for months, several hours a day. Thus, for the treatment of male genital hypoplasia, timely tissue expansion and the opportunity to prolong the expansion are fundamental.
Owner:爱德华多.A.戈麦斯.德迭戈
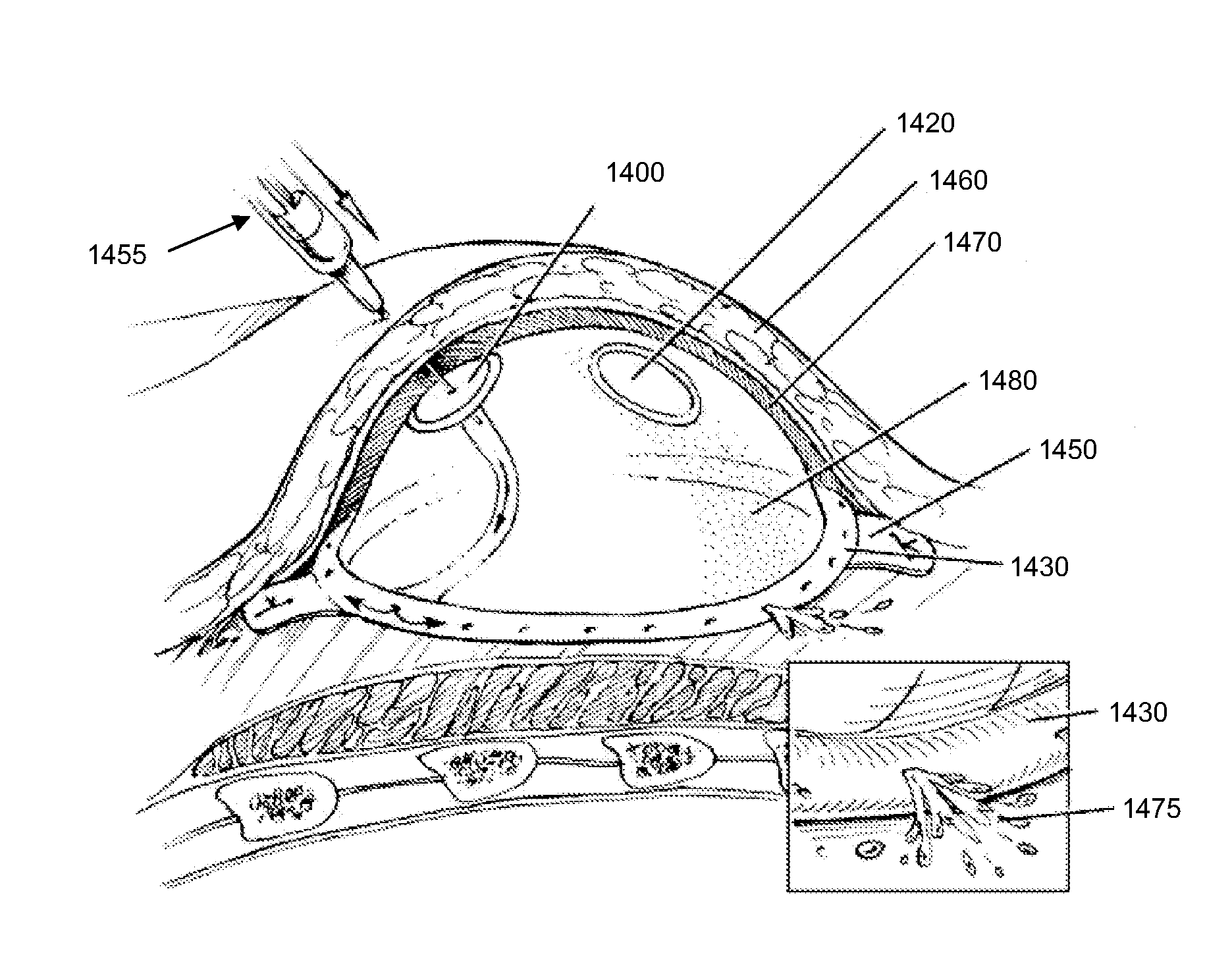
Minimally invasive tissue expander
The invention relates to a drag hook, and especially relates to a minimally invasive tissue expander, and belongs to the technical field of medical instruments. According to the provided technical scheme, the minimally invasive tissue expander comprises a drag hook base and a plurality of drag hook sheets arranged in the drag hook base. The drag hook sheet is arranged in the drag hook base througha drag hook sheet positioning control mechanism. The drag hook sheets and the drag hook sheet positioning control mechanisms are connected with each other in a one-to-one manner. When the drag hook sheets in the drag hook base are close to each other, a cylindrical structure can be formed. Through the drag hook sheet positioning control mechanisms, required adjustment is performed on traction depth and traction orientations of the connected drag hook sheets. The adjustable drag hook sheets can be adjusted at will according to requirement of an operation, so that traction depth and angles of the adjustable drag hook sheets meet operation requirement, to pull a brain tissue by proper external forces and fully expose view of an operative site. The minimally invasive tissue expander is safe and reliable.
Owner:WUXI SHENGNUOYA TECH CO LTD
Oval dilator and retractor set and method
Two groups of orderly placed tissue expanders which are oriented to become axial lines are positioned in a plane parallel to a vertebral axis, the last non-circular expander encloses the two groups of expanders, then a non-circular tubular retractor provides a working channel extended along the longitudinal direction of the vertebra, therefore an operation can enter into the larger area of a surgical site from the smallest cut.
Owner:WARSAW ORTHOPEDIC INC
Prosthesis for repairing upper jaw bone defect
The invention discloses a prosthesis for repairing a maxillary bone defect and a preparation method thereof, a silicone rubber outer layer adapted to the maxillary bone defect area, and a columnar skin soft tissue expansion is arranged inside the silicone rubber outer layer The inner layer of the cylindrical soft tissue expander is connected with an inflation device extending outside the outer layer of silicone rubber, and the outer layer of silicone rubber is connected to the inner layer of the cylindrical soft tissue expander. Or the inflatable device is provided with a corresponding magnetic retainer. The invention is a prosthetic body for repairing maxillary defects with better repair and retention and convenient removal and wear, and establishes a preparation method for the prosthetic body.
Owner:CENT SOUTH UNIV
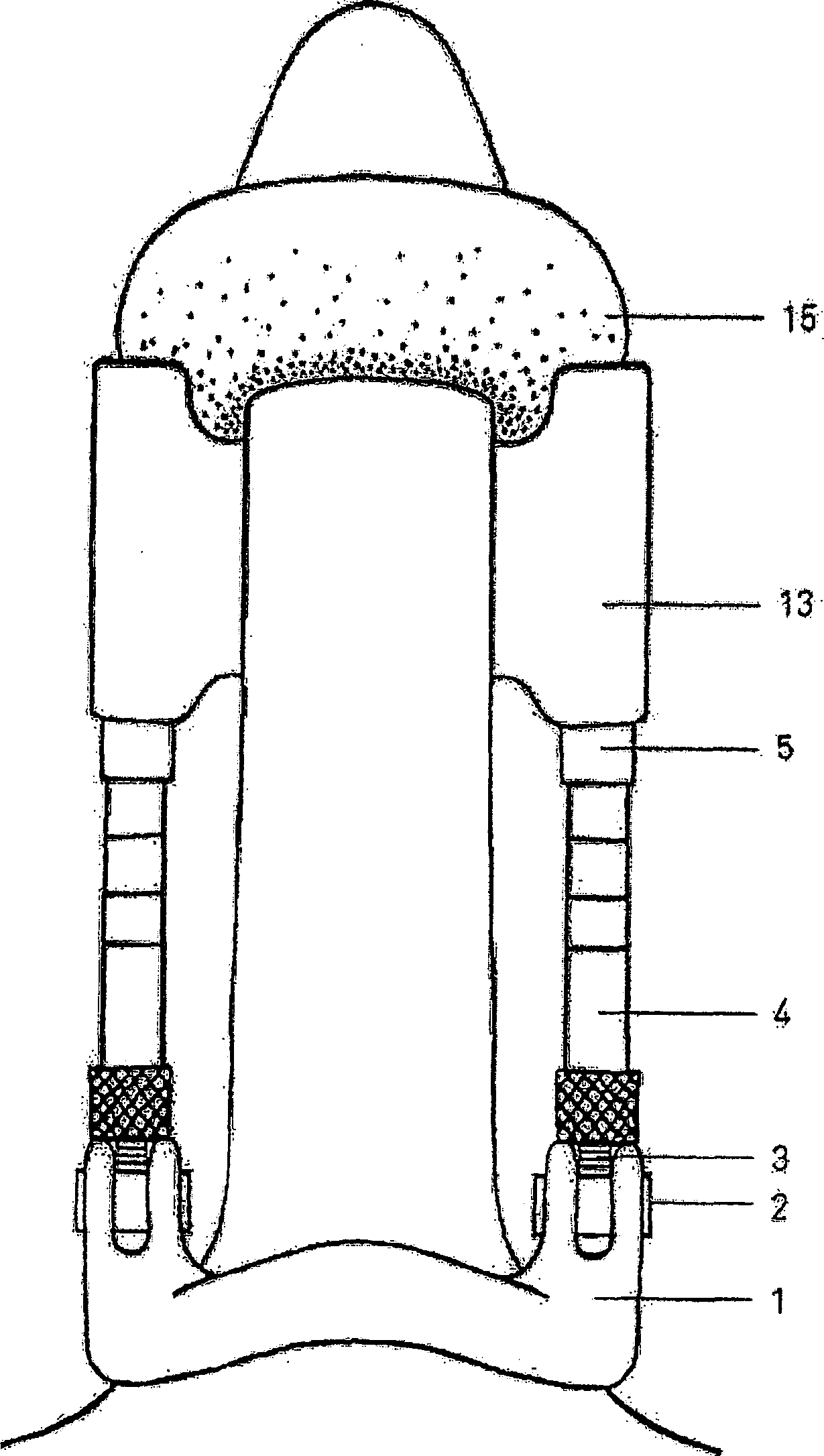
Tissue expander with separable coating
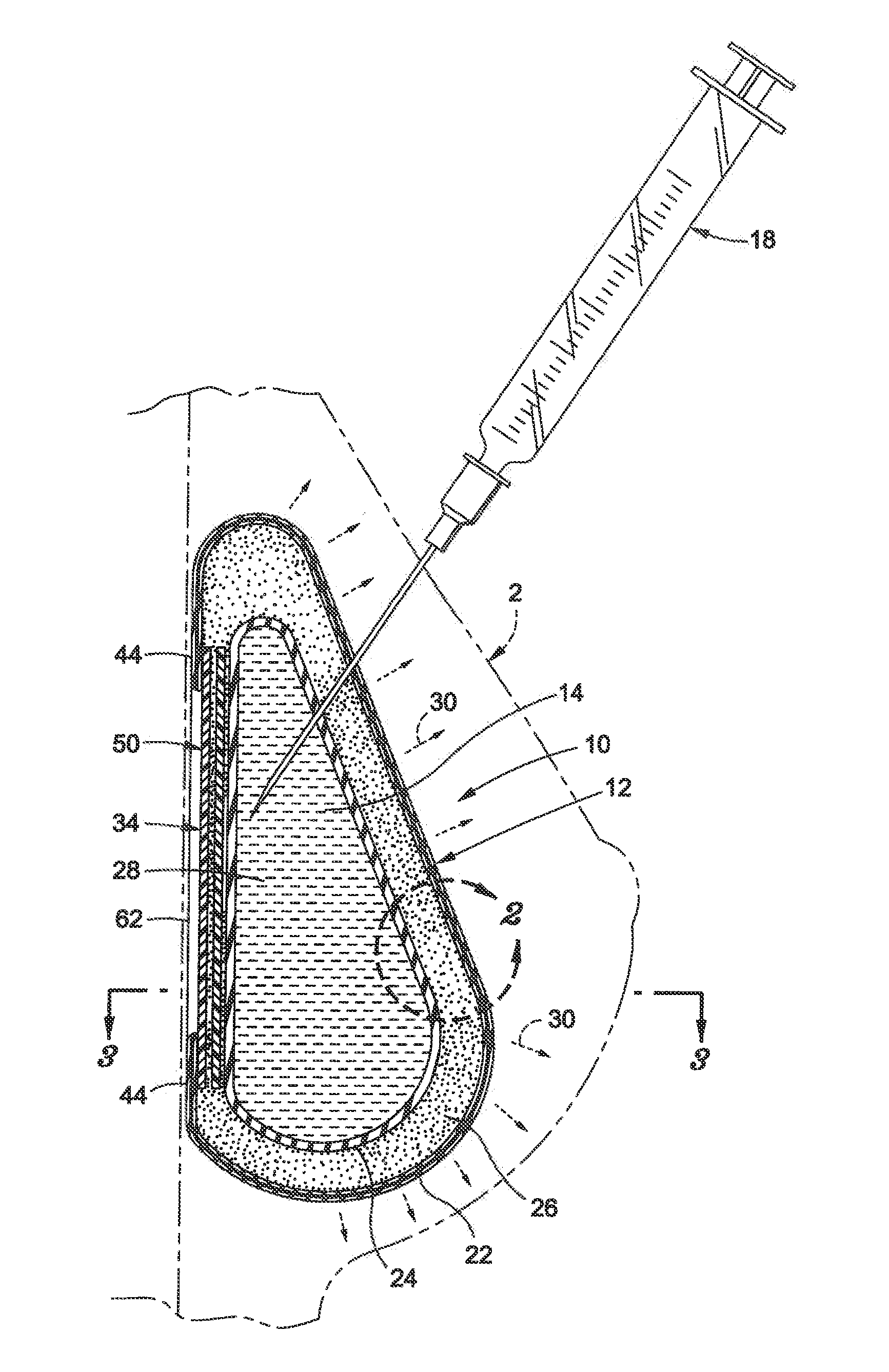
The present invention provides a tissue expander (10) comprising a self-inflating core (12or 2e) having a non-inflated state and an inflated state and a coating (14) surrounding said core (12or 12e), in which said core (12or 12e) comprises a compacted self-inflating material and said coating comprises a fluid impermeable material having a plurality of apertures therethrough for allowing the passage of fluid to the core (12or12e) and in which the coating (14) is separable or separated from the core (12or 12e) such as to allow the coating to provide a support function during use. The invention also provides a method of manufacturing the product.
Owner:OXTOX LIMITED
Skin dilator
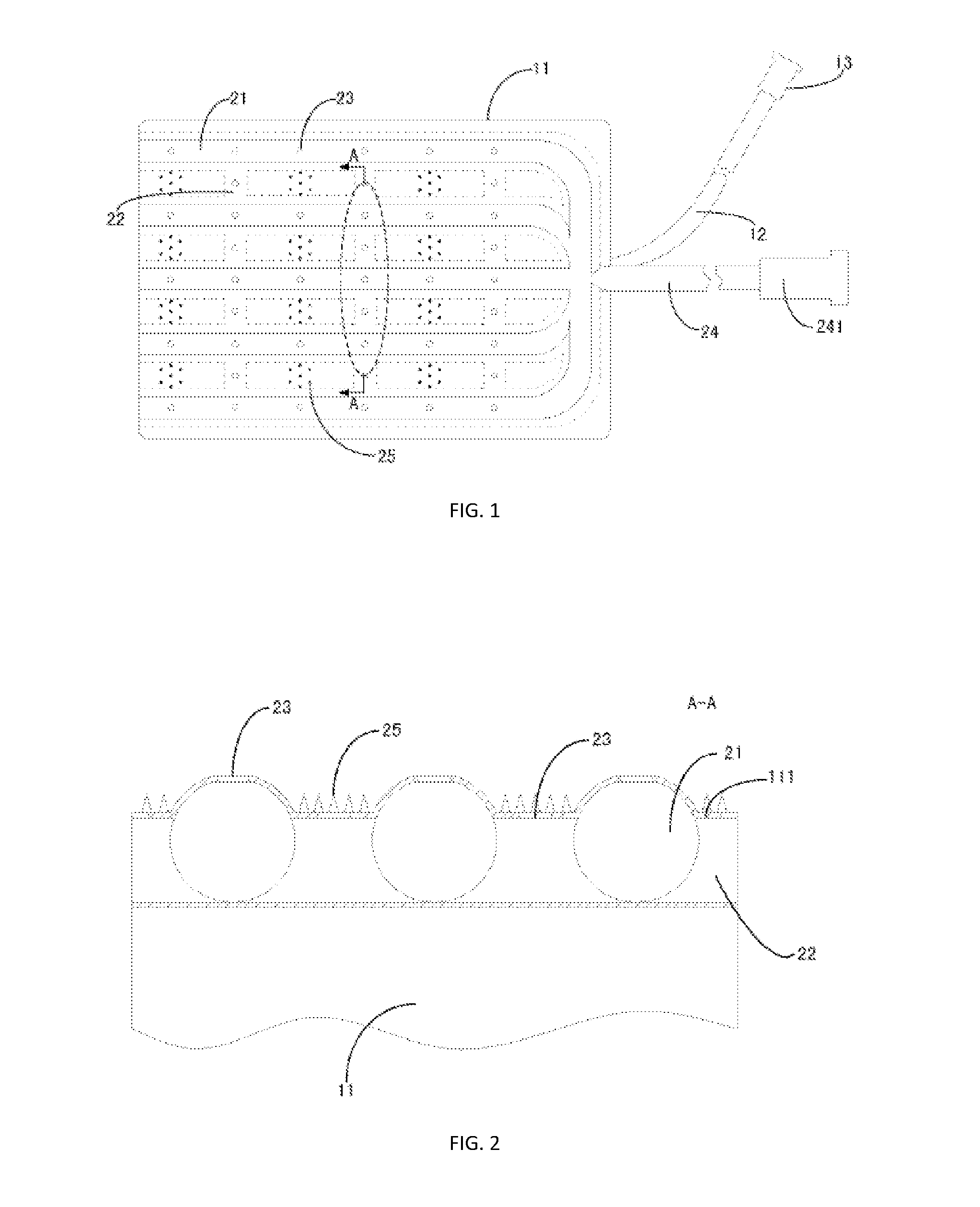
ActiveUS20160242864A1Improve biological effectGood treatment effectDiagnosticsSurgeryDilatorReticular formation
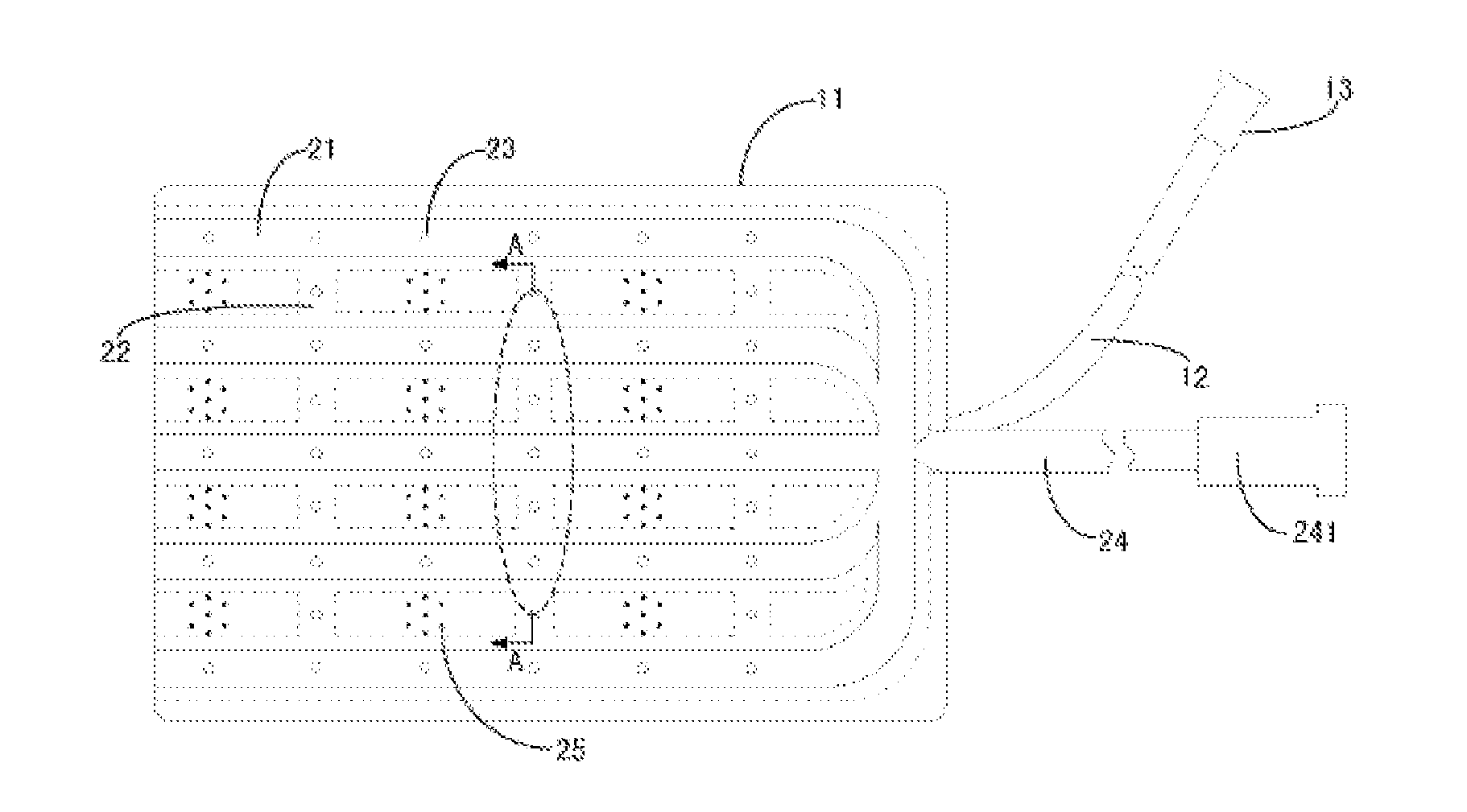
Disclosed is a skin dilator, comprising an expander (11), a pipe network provided on the skin-side surface wall (111) of the expander (11) composed of longitudinal tube (22) and horizontal tube (21) having a reticular structure and communicating with each other, and a guide tube (24) communicating with the pipe network. The skin dilator further comprises several burrs (25) distributed on the skin-side surface wall (111) of the expander (11), and several small apertures (23) communicating the inner cavity of the tube with the outside are provided on both of the longitudinal tube (22) and horizontal tube(21). The tissue expander can be infused biological agents by the filling port to the skin expanding site through the small apertures (23), which may accelerate the expansion growth of skin, prevent skin complications, such as necrosis.
Owner:SHANGHAI NINTH PEOPLES HOSPITAL SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com