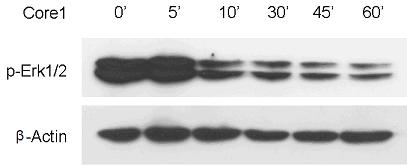
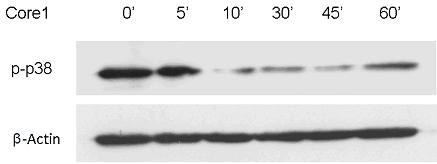
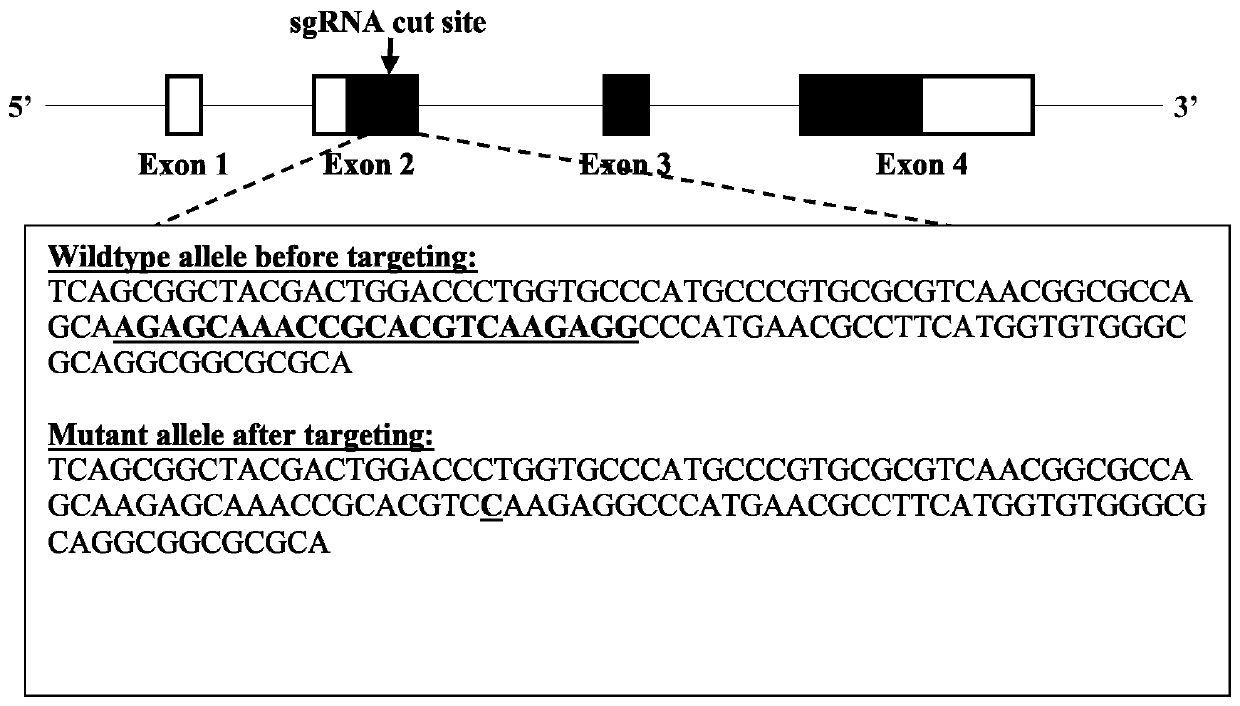
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Hypoplasia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hypoplasia (from Ancient Greek ὑπo- hypo-, "under" + πλάσις plasis, "formation"; adjective form hypoplastic) is underdevelopment or incomplete development of a tissue or organ. Although the term is not always used precisely, it properly refers to an inadequate or below-normal number of cells. Hypoplasia is similar to aplasia, but less severe. It is technically not the opposite of hyperplasia (too many cells). Hypoplasia is a congenital condition, while hyperplasia generally refers to excessive cell growth later in life. (Atrophy, the wasting away of already existing cells, is technically the direct opposite of both hyperplasia and hypertrophy.)
Method and kit for imaging and treating organs and tissues
InactiveUS6331175B1High resolutionStrong specificityElectrotherapyNanomedicineMammalHypoplastic genitalia
Provided are methods and compositions for detecting and treating normal, hypoplastic, ectopic or remnant tissue, organ or cells in a mammal. The method comprises parenterally injecting a mammalian subject, at a locus and by a route providing access to above-mentioned tissue or organ, with an composition comprising antibody / fragment which specifically binds to targeted organ, tissue or cell. The antibody / fragment may be administered alone, or labeled or conjugated with an imaging, therapeutic, cytoprotective or activating agent.
Owner:IMMUNOMEDICS INC
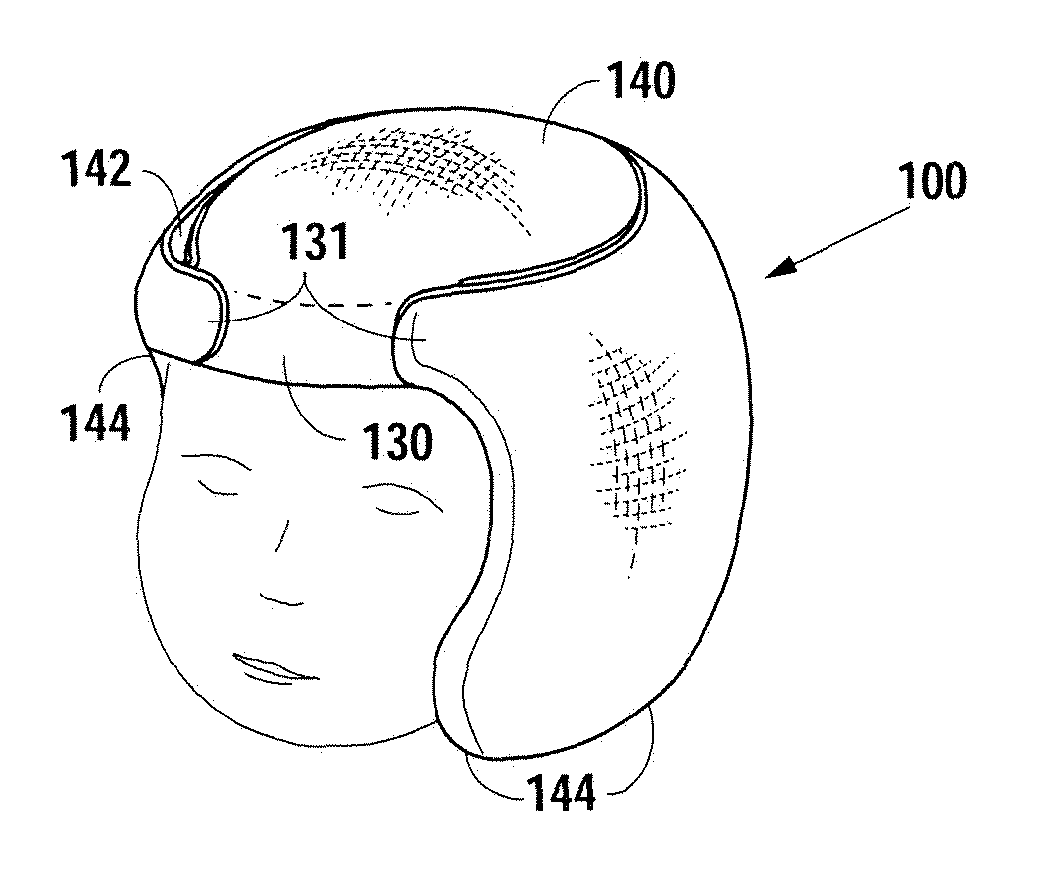
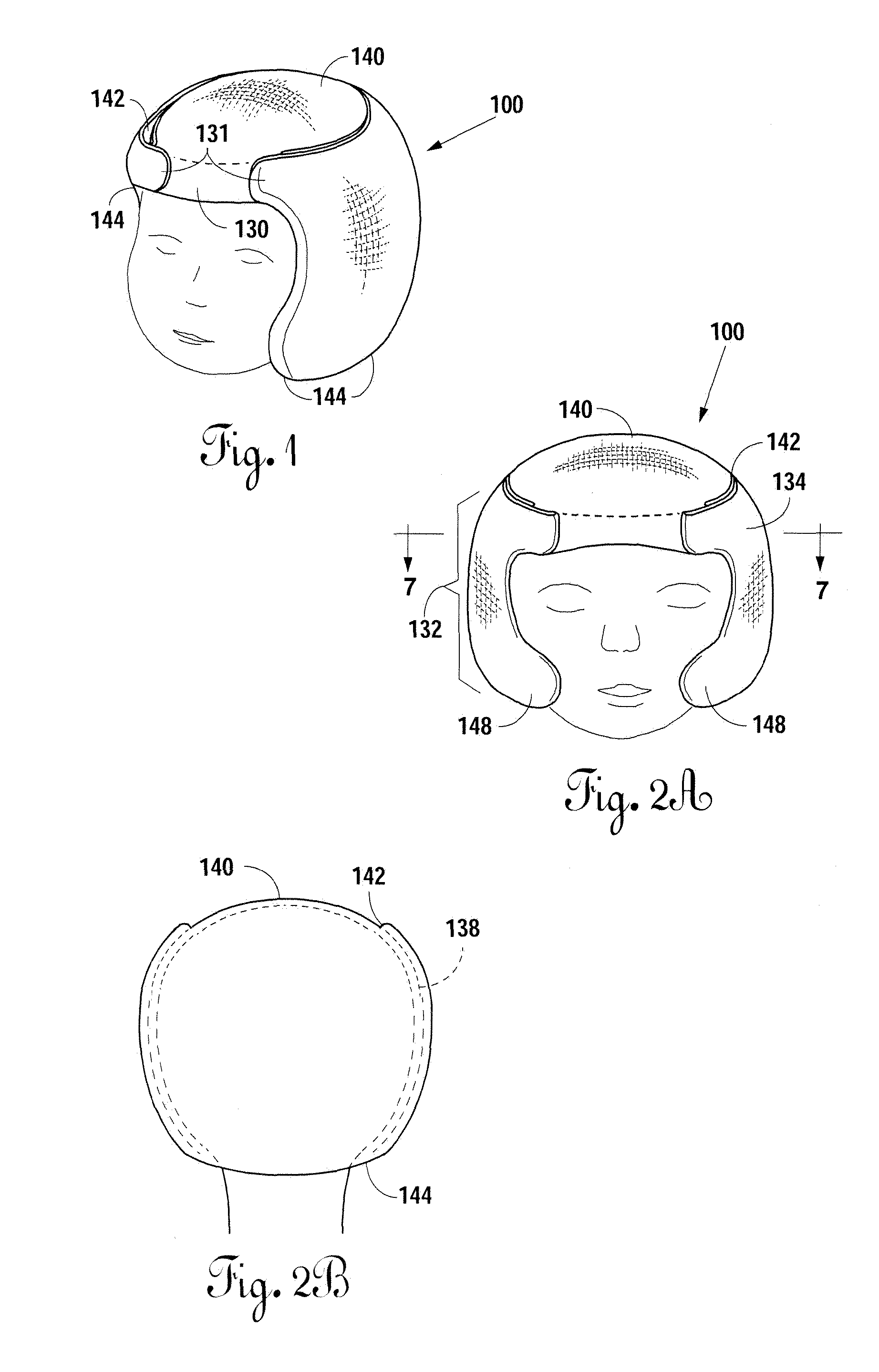
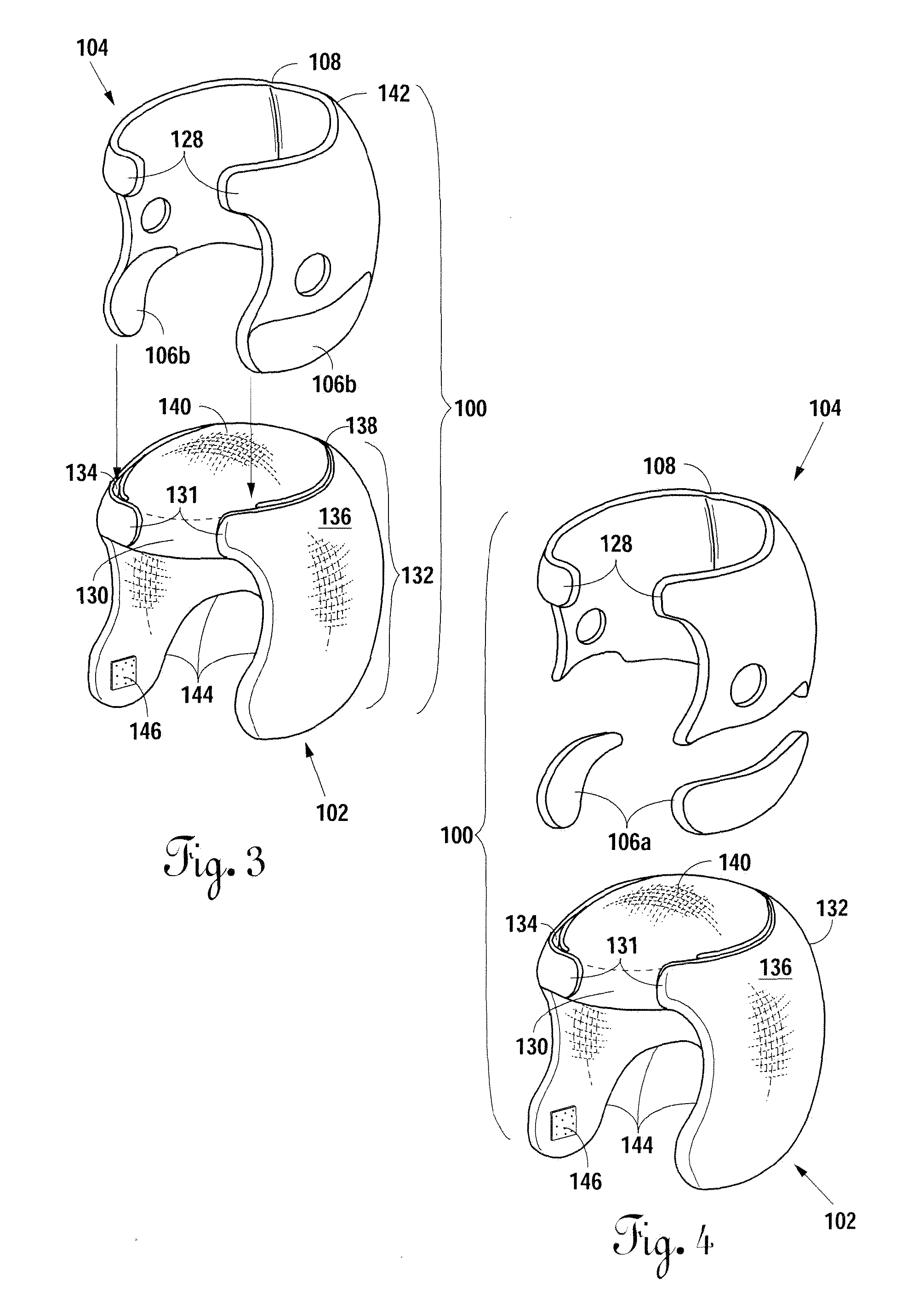
Neonatal cranial support bonnet
InactiveUS20130046219A1Maintain proper shapeEliminate pointHead bandagesNeck bandagesSIDS - Sudden infant death syndromeGuideline
A neonatal cranial support bonnet is configured to prevent a premature child's head from deforming under the force of its own weight, because of underdeveloped cranial plates. The bonnet includes a thin cotton shell with contoured gel packs inside. The gel packs are configured to distribute weight around the skull and eliminate or reduce high-pressure points so as to maintain proper shape of the cranium. The bonnet is also configured to not interfere with development, and to not obstruct airways for breathing, so as to meet current Sudden Infant Death Syndrome prevention guidelines.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
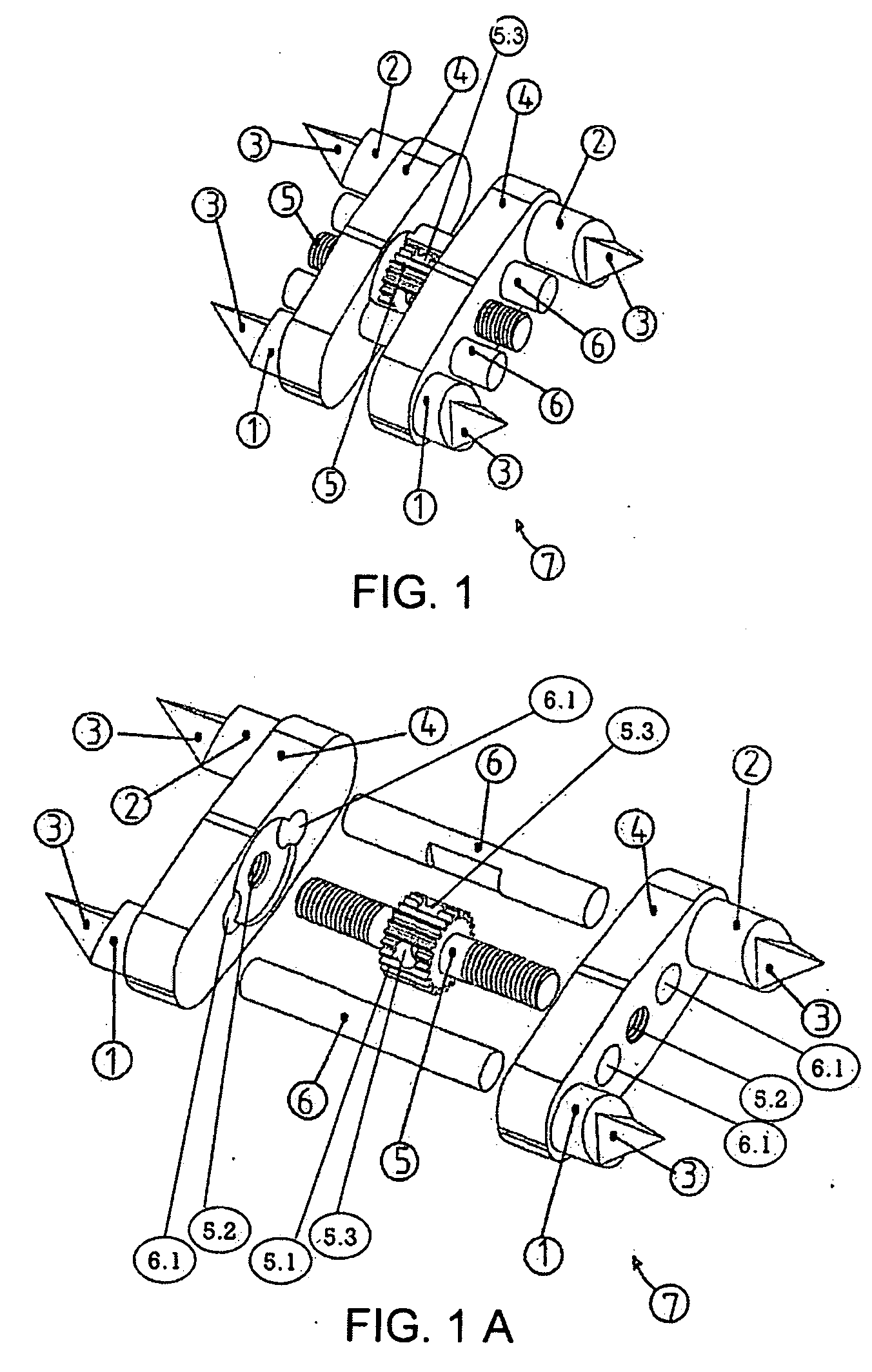
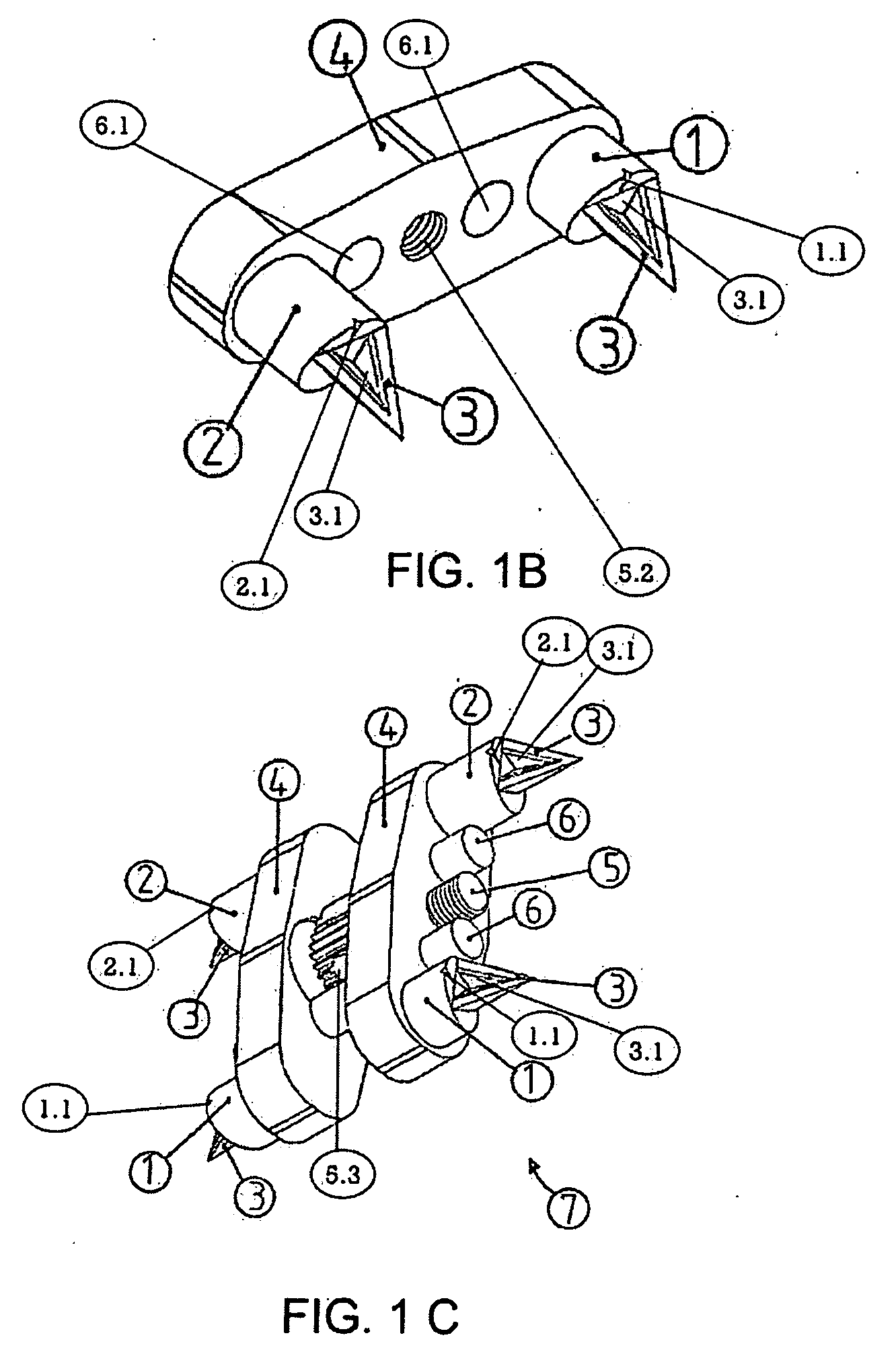
Automatic maxillary expander and transfering apparatus
InactiveUS20090081602A1Improve stabilityImprove dental crowdingOthrodonticsDental toolsSurgical operationHypoplasia
An automatic Maxillary Expander, which is a bone-borne distractor for expanding the maxillary bone in adult and adolescents having transversal maxillary hypoplasia. It fixes itself to the palatal vault in a way without any need for screwing, by the asymmetrical triangular prism-shaped spikes on the anterior, posterior columns. Both being hygienic and not wasting a bulky space in the mouth, it provides a high patient comfort. The maxillary expanding process does not interrupt orthodontic treatment of patients and minimizes damage to the texture of the mouth. An automatic Maxillary Expander Transferring Apparatus enables the practitioner to place the Automatic Maxillary Expander into the palatal surface with ease and precision into the palate under local anesthesia quickly, without any surgical operation. In addition, this apparatus is composed of a very simple mechanism. It has rounded ends in order not to hurt the practitioner or patient.
Owner:AYAN MUSTAFA
Method and kit for imaging and treating organs and tissues
InactiveUS20020052594A1High resolutionStrong specificityElectrotherapyMedical devicesAntibody fragmentsImaging agent
Provided are methods and compositions for detecting and treating normal, hypoplastic, ectopic or remnant tissue, organ or cells in a mammal. The method comprises parenterally injecting a mammalian subject, at a locus and by a route providing access to said tissue or organ, with an composition comprising antibody / fragment which specifically binds to targeted organ, tissue or cell. The antibody / fragment may be administered alone, or labeled or conjugated with an imaging, therapeutic, cytoprotective or activating agent.
Owner:IMMUNOMEDICS INC
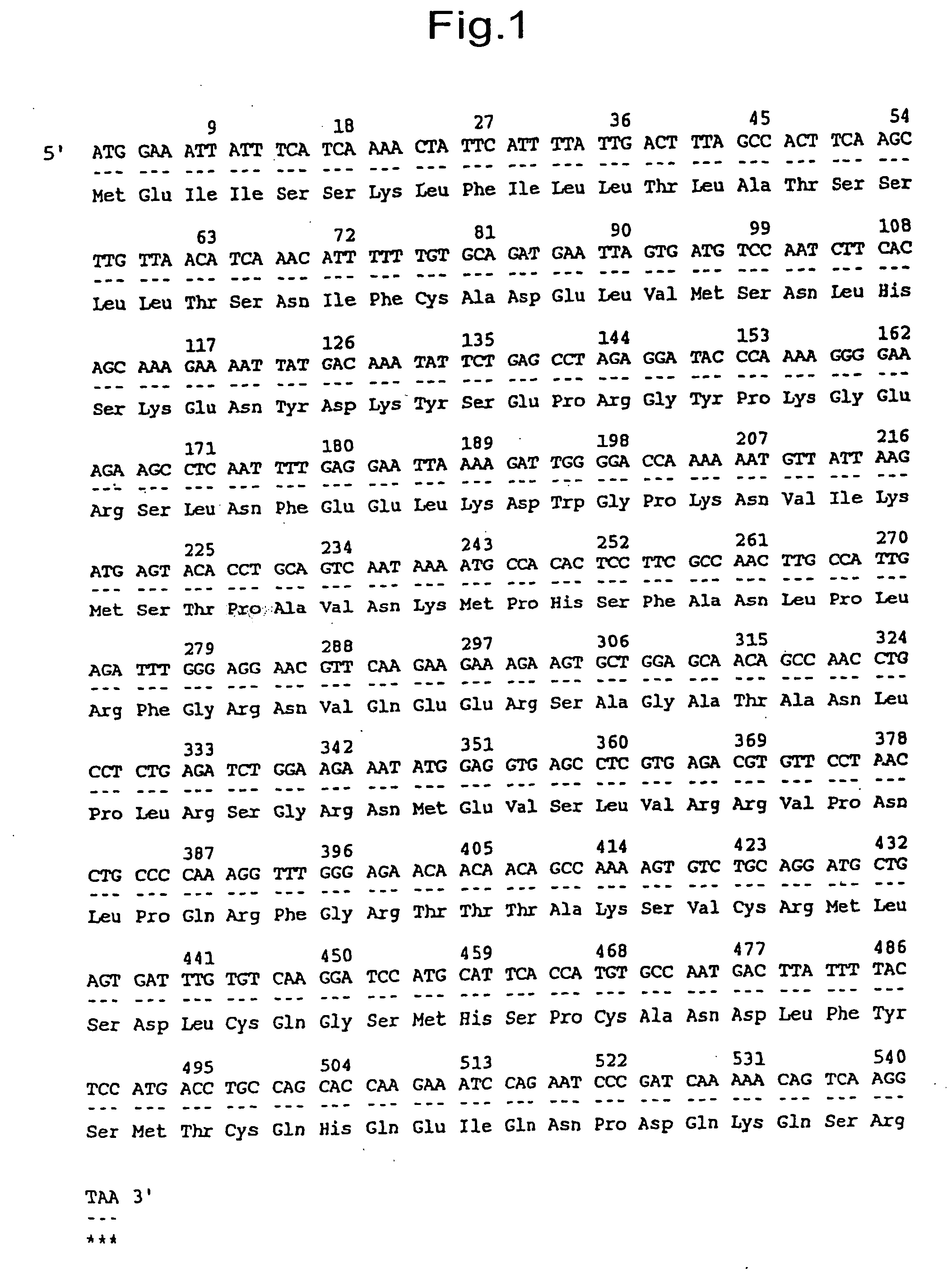
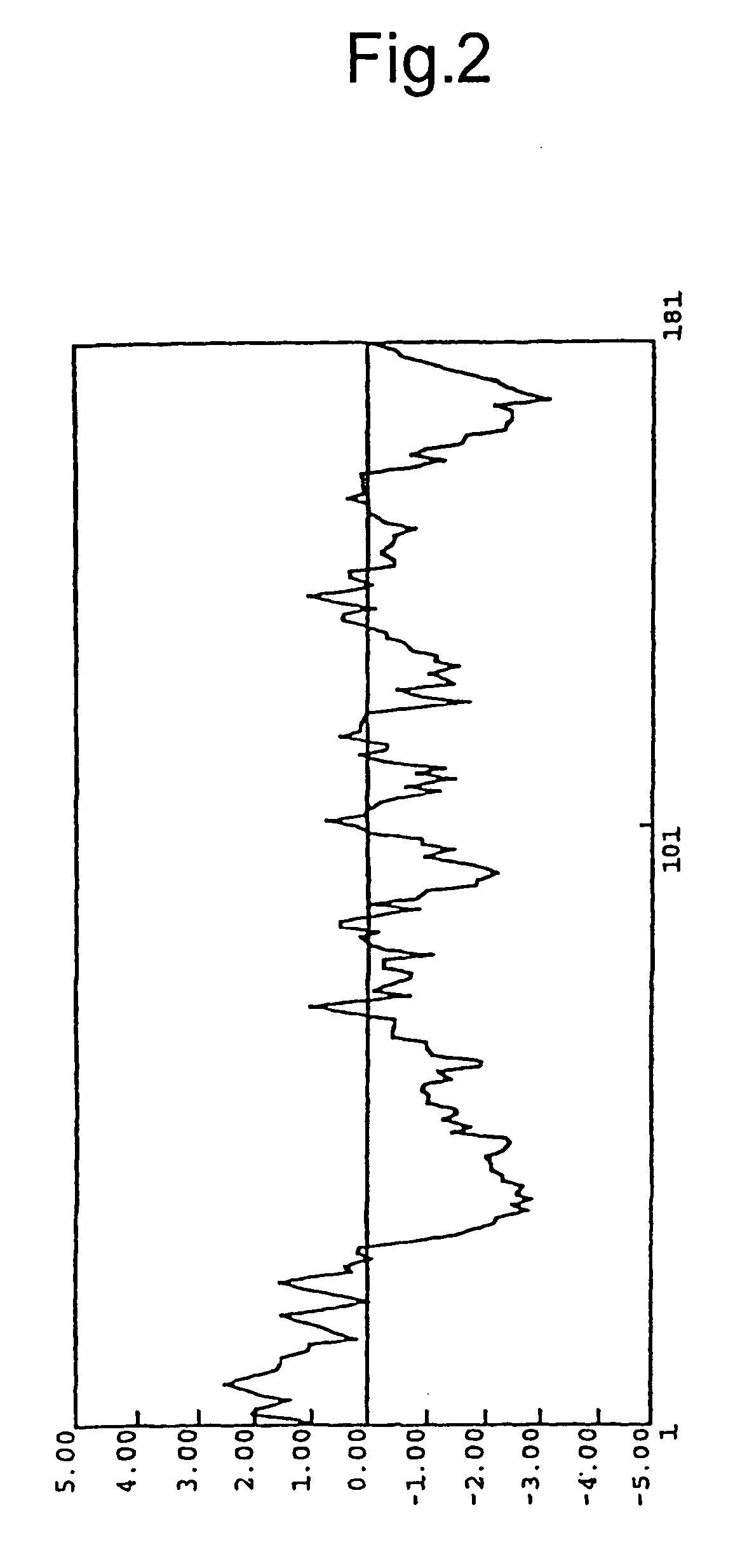
G protein-coupled receptor protein, its DNA and ligand thereof
The polypeptides in the present invention possess the effects of promoting and inhibiting the secretion of prolactin, and are thus useful as drugs for the prevention and treatment of various diseases, in terms of prolactin secretion stimulants, which are associated with the secretion of prolactin, such as hypoovarianism, spermatic underdevelopment, menopausal symptoms, hypothyroidism, etc. The polypeptides are useful as drugs for the prevention and treatment of various diseases, in terms of prolactin secretion inhibitors, which are associated with the secretion of prolactin, such as pituitary tumor, diencephalon tumor, menstrual disorder, autoimmune diseases, prolactinoma, sterility, impotence, amenorrhea, lactorrhea, acromegaly, Chiari-Frommel syndrome, Argonz-del Castilo syndrome, Forbes-Albright syndrome, lymphoma, Sheehan's syndrome, spermatogenesis disorder, etc.
Owner:TAKEDA PHARMA CO LTD
Chemiluminescence quantitative detection kit for estradiol
InactiveCN102095880AQuick checkEasy to detectChemiluminescene/bioluminescenceBiological testingDiseaseHypoplasia
The invention discloses a chemiluminescence quantitative detection kit for estradiol. The kit comprises an estradiol detection reaction plate, an enzyme conjugate, a luminescent substrate, a calibrator, a quality control material and washing concentrate, wherein the reaction plate is coated with an estradiol antibody. The kit can specifically and quantitatively detect the content of the estradiol in patient serum, and is used for the auxiliary diagnosis of diseases such as ovarian hypoplasia, primary ovarian failure, pituitary amenorrhea or infertility, Cushing's syndrome, Addison's disease, malignant tumors, focal lesion of brain and hypophysis, and the like. Compared with the conventional enzyme linked immunosorbent assay (ELISA) technology, the chemiluminescence immunoassay keeps high specificity of the ELISA technology, stability and reliability of a detection result, and convenience of operation, and can improve detection sensitivity simultaneously.
Owner:上海裕隆生物科技有限公司
Chinese herba preparation for recuperating and curing ovarian hypoplasia of young girls or adult women
InactiveCN102614446AStrong targetingStrong orientationSexual disorderMolluscs material medical ingredientsDiseaseHypoplasia
A Chinese herba preparation for recuperating and curing ovarian hypoplasia of young girls or adult women is hot drink particles or a tea bag or a granular preparation consisting of kudzu roots, hawthorn, soya beans, red beans, black beans, fructus ligustri lucidi, eclipta alba, honeysuckle, Chinese rose, raw liquorice, dried tangerine or orange peels, fructus amomi, folium ginseng, dipsacus roots, eucommia bark, mistletoe, Japanese raspberry roots, winged euonymus twig, radix trichosanthis, pearl powder, yam flour, medicine beans and a packaging material. The Chinese herba preparation is used for preventing, recuperating and curing diseases including ovarian dysplasia or retardation of the young girls, ovarian hypofunction or hyperfunction, too little or too much estrogen secretion and ovarian and uterus dysgenesis of young married women, premature ovarian failure of older women, and barrenness of fertile women due to hypoplasia of ovum of ovarian, is used for moistening skin, retaining youthful looks and eliminating macula, and has the advantages that total effective rate of clinical recuperating and curing reaches 100% and the like.
Owner:李运乃
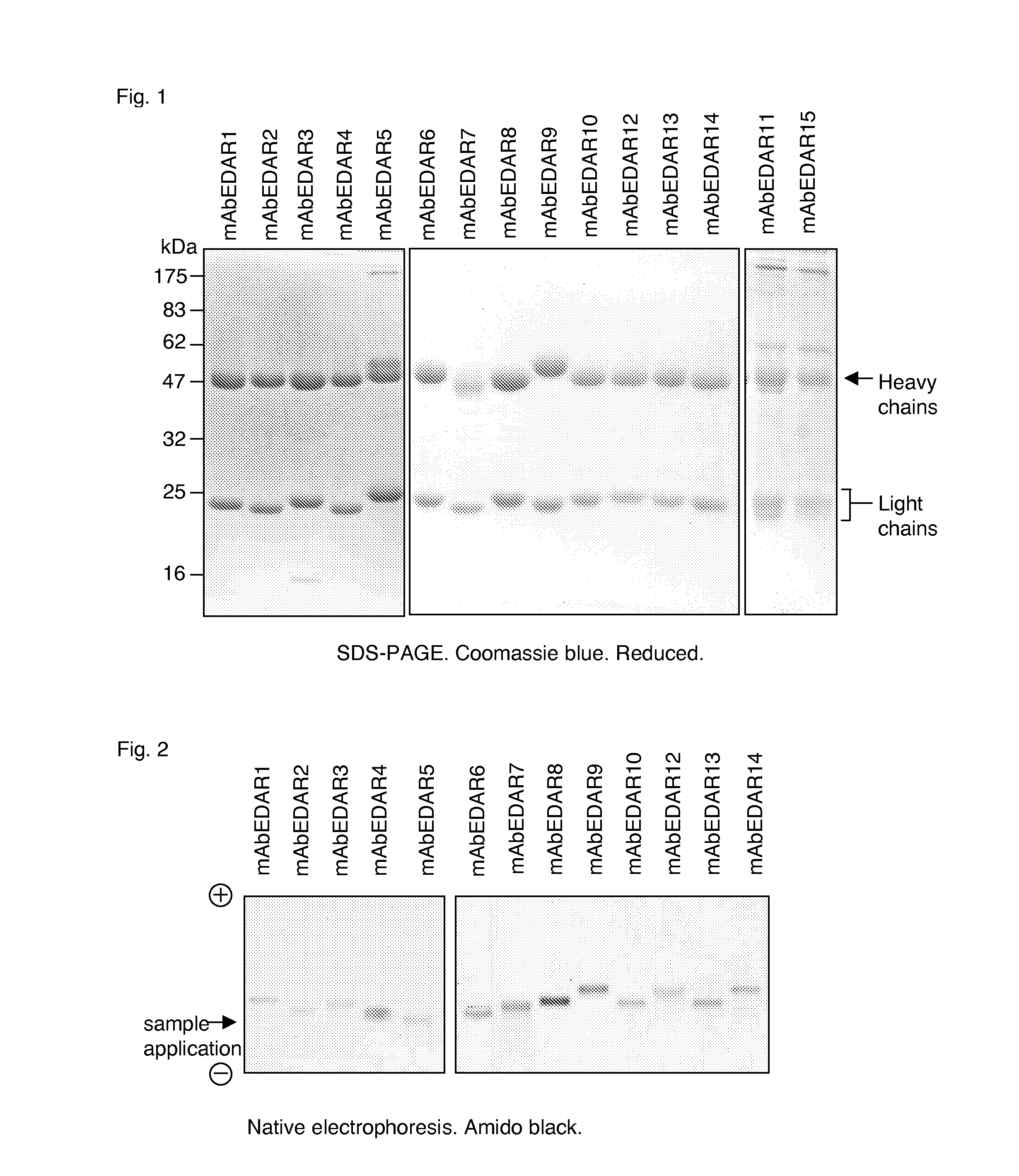
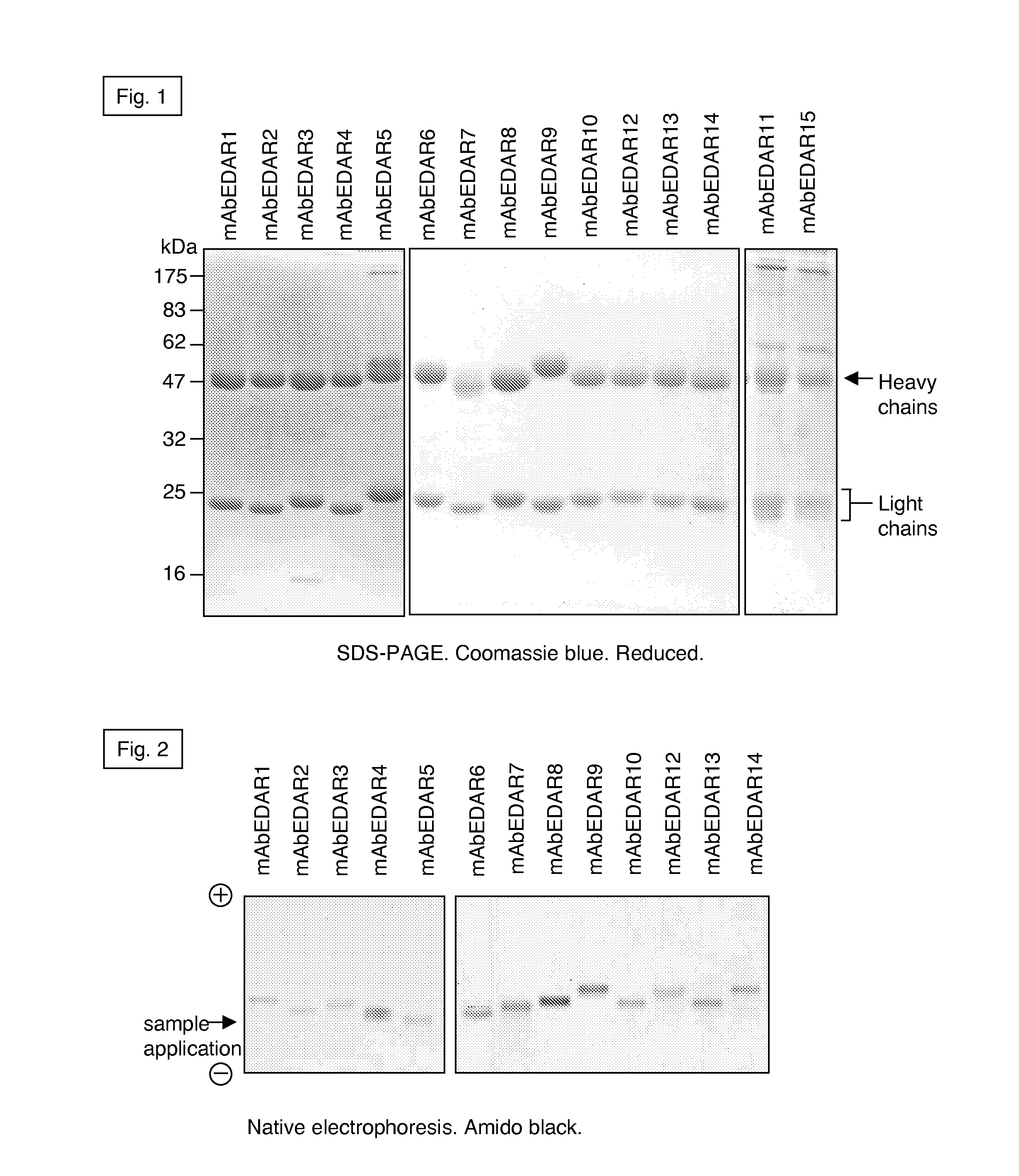
Preparation of isolated agonist anti-EDAR monoclonal antibodies
The present invention concerns the preparation of substantially purified agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof. The invention further relates to isolated agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof as well as their use in the treatment of X-linked hypohidrotic ectodermal dysplasia and tooth agenesis. The invention also relates to a pharmaceutical composition comprising said isolated agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof and to a method of treating X-linked hypohidrotic ectodermal dysplasia and tooth agenesis. Finally, the present invention concerns a pharmaceutical kit comprising said isolated agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof.
Owner:ESPOIRXLHED SARL +1
Preparation of isolated agonist Anti-edar monoclonal antibodies
The present invention concerns the preparation of substantially purified agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof. The invention further relates to isolated agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof as well as their use in the treatment of X-linked hypohidrotic ectodermal dysplasia and tooth agenesis. The invention also relates to a pharmaceutical composition comprising said isolated agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof and to a method of treating X-linked hypohidrotic ectodermal dysplasia and tooth agenesis. Finally, the present invention concerns a pharmaceutical kit comprising said isolated agonist anti-EDAR monoclonal antibodies or isolated monoclonal antibody fragments or antigen binding portions or fragments thereof.
Owner:ESPOIRXLHED SARL +1
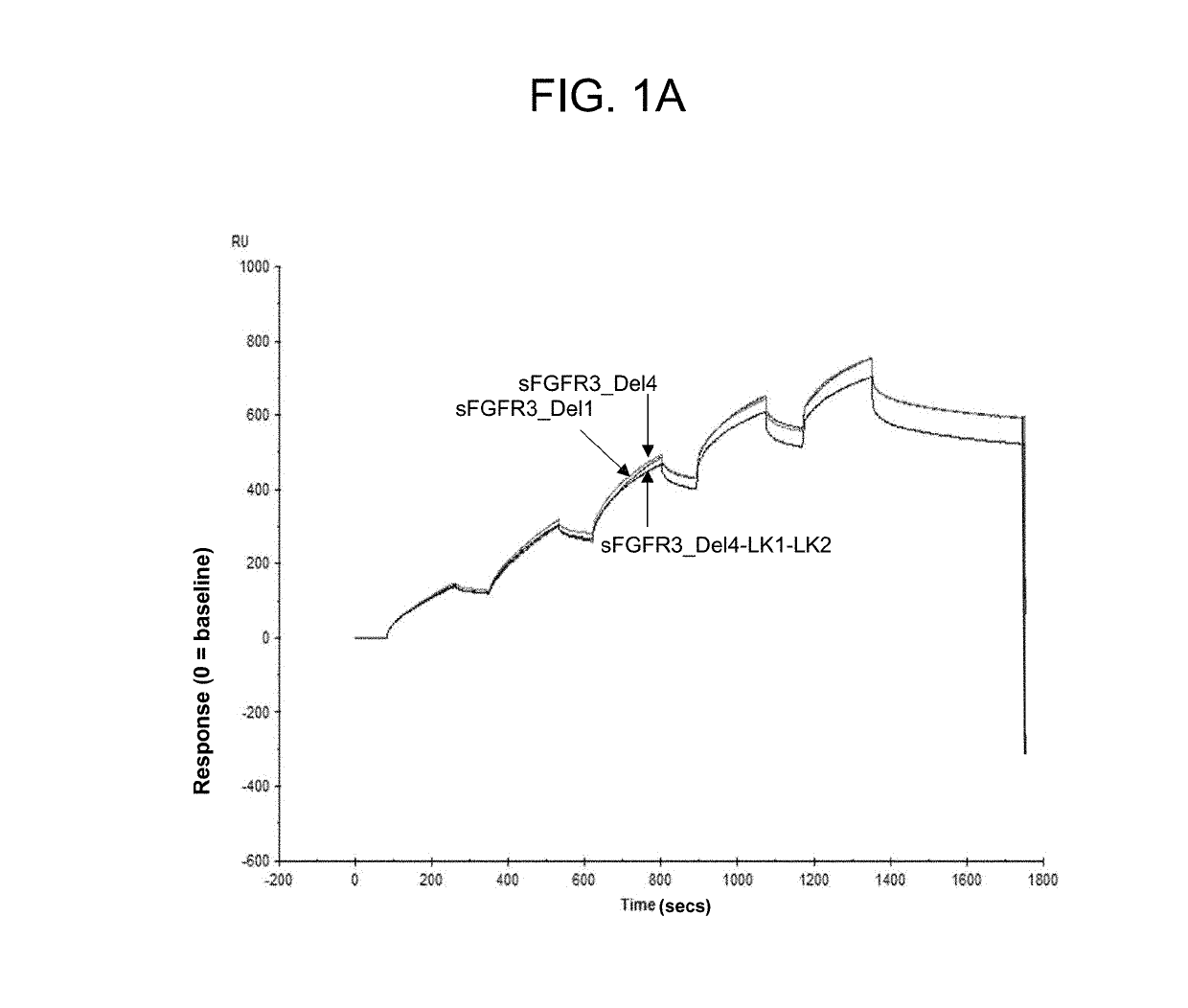
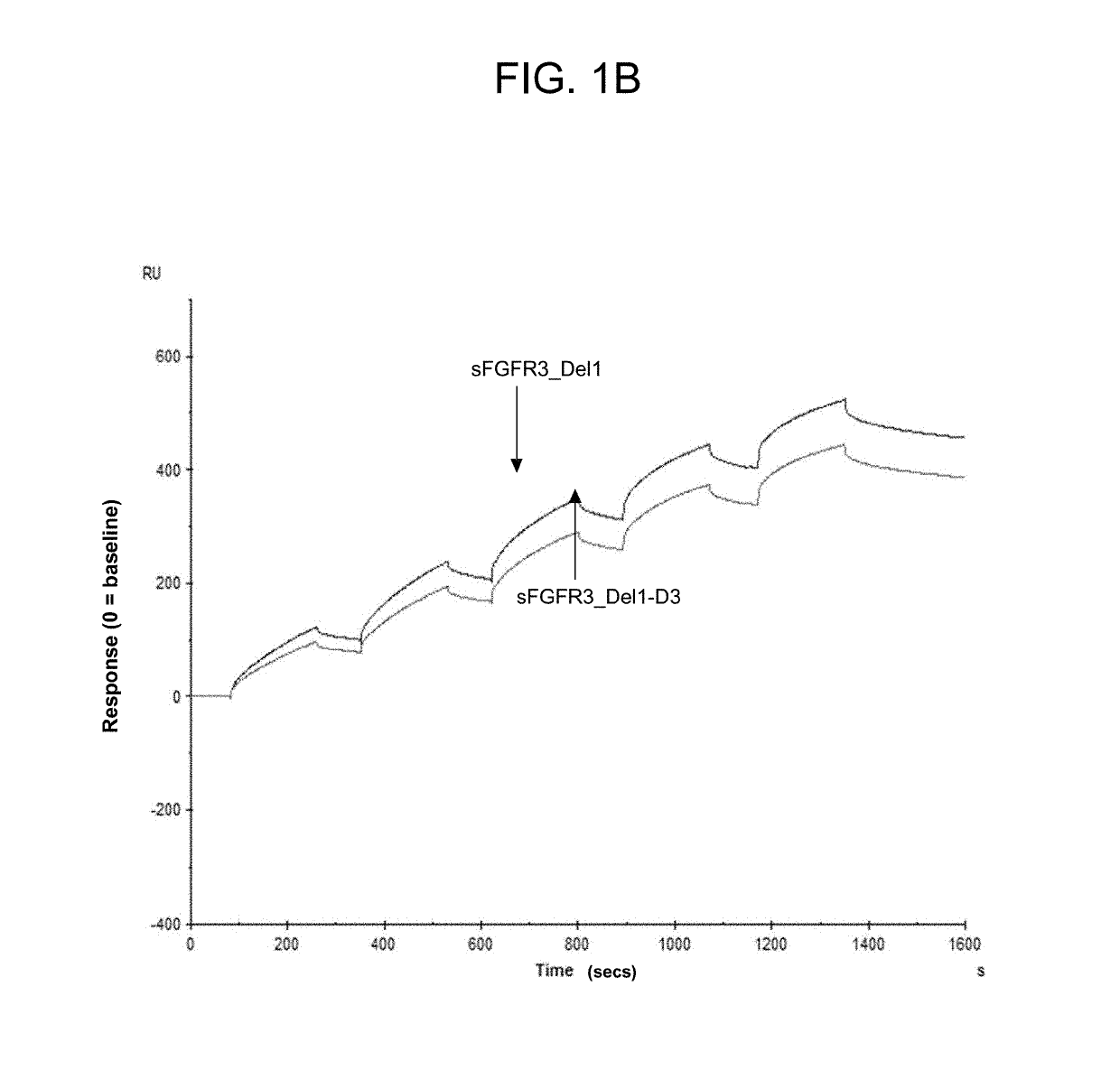
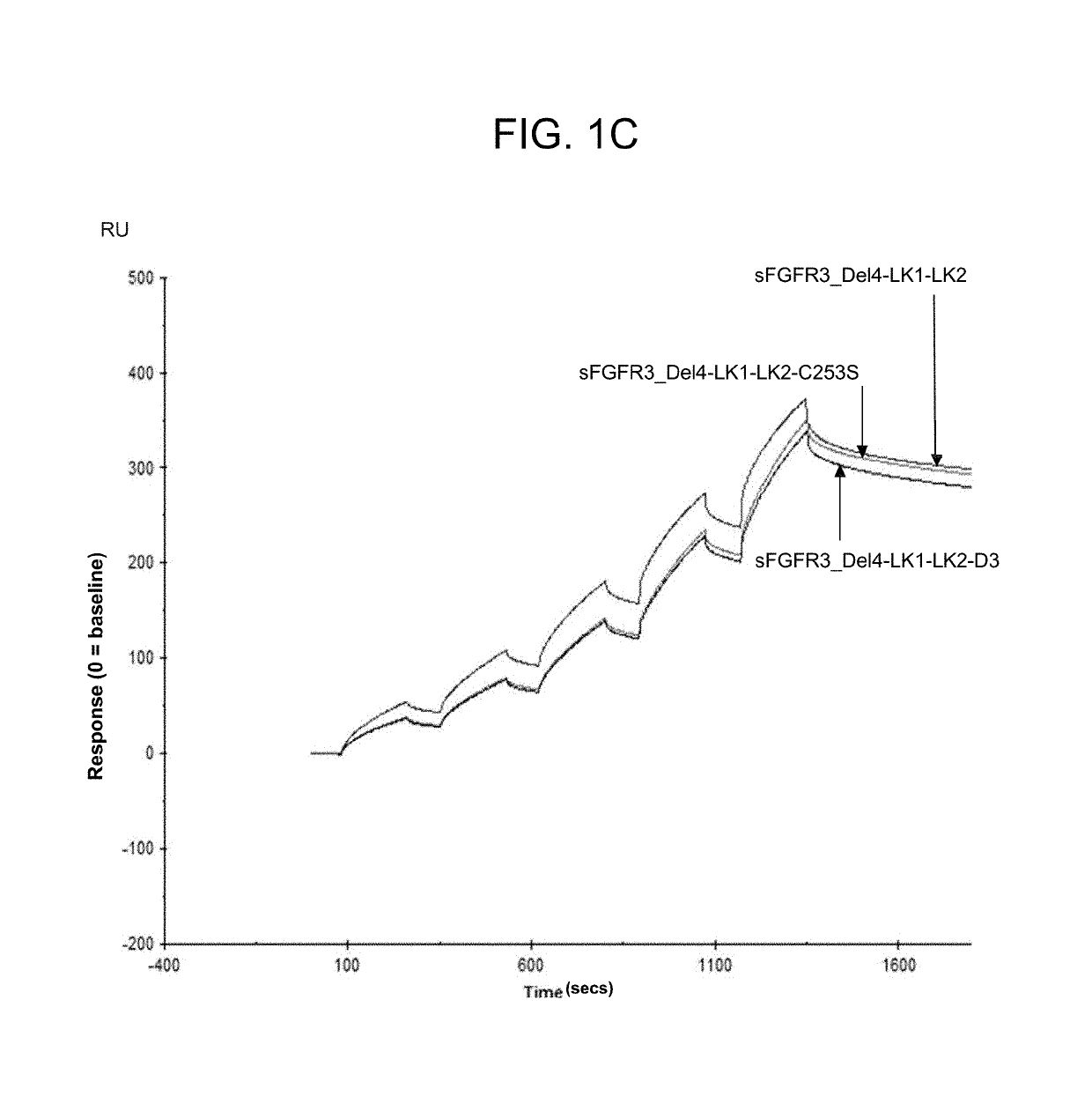
Soluble fibroblast growth factor receptor 3 (SFGFR3) polypeptides and uses thereof
ActiveUS10294289B2Improve survivalPromote sportsPeptide/protein ingredientsSkeletal disorderHypoplasiaBiochemistry
The invention features soluble fibroblast growth factor receptor 3 (sFGFR3) polypeptides. The invention also features methods of using sFGFR3 polypeptides to treat skeletal growth retardation disorders, such as achondroplasia.
Owner:PFIZER INC +2
Soluble fgfr3 decoys for treating skeletal growth disorders
InactiveUS20180148494A1Easy genetic manipulationReduce contentPeptide/protein ingredientsAntibody mimetics/scaffoldsChemistryAggrecan
The invention features soluble FGF decoy polypeptides and fusion polypeptides comprising an FGF decoy polypeptide linked to a heterologous polypeptide, such as an aggrecan binding protein. Both soluble FGF decoy polypeptides and fusion polypeptides can be used to prevent or treat skeletal disorders, such as achondroplasia.
Owner:UNIV COTE DAZUR +3
Application of CTRP3 protein (C1qTNF related protein 3)
ActiveCN106177912AIncreased sensitivityEnhancement and effectPeptide/protein ingredientsSexual disorderDiseaseOvarian tissue
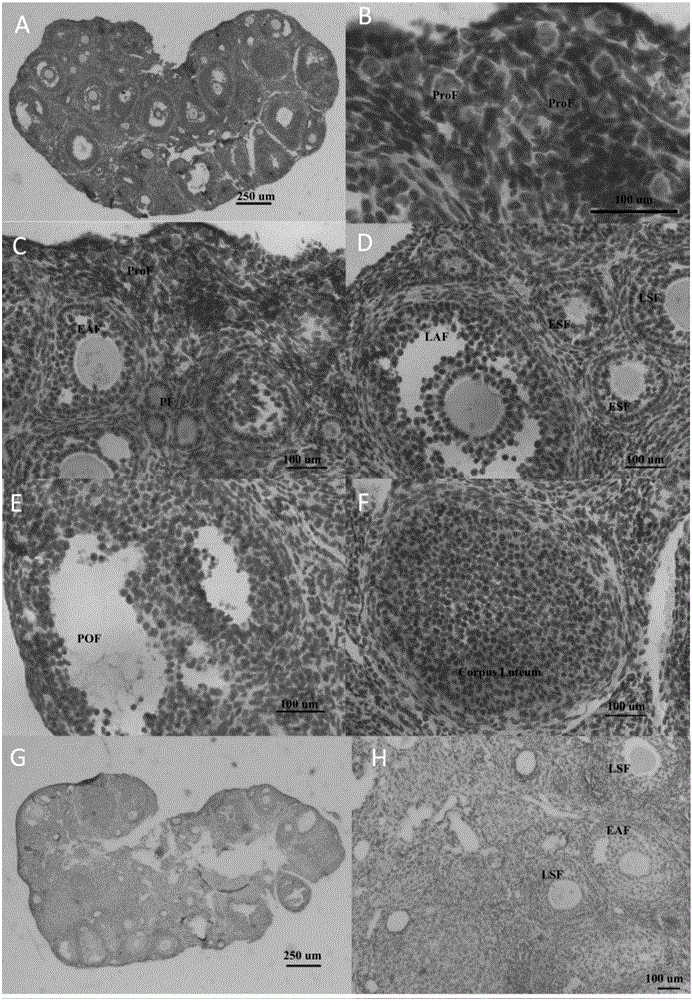
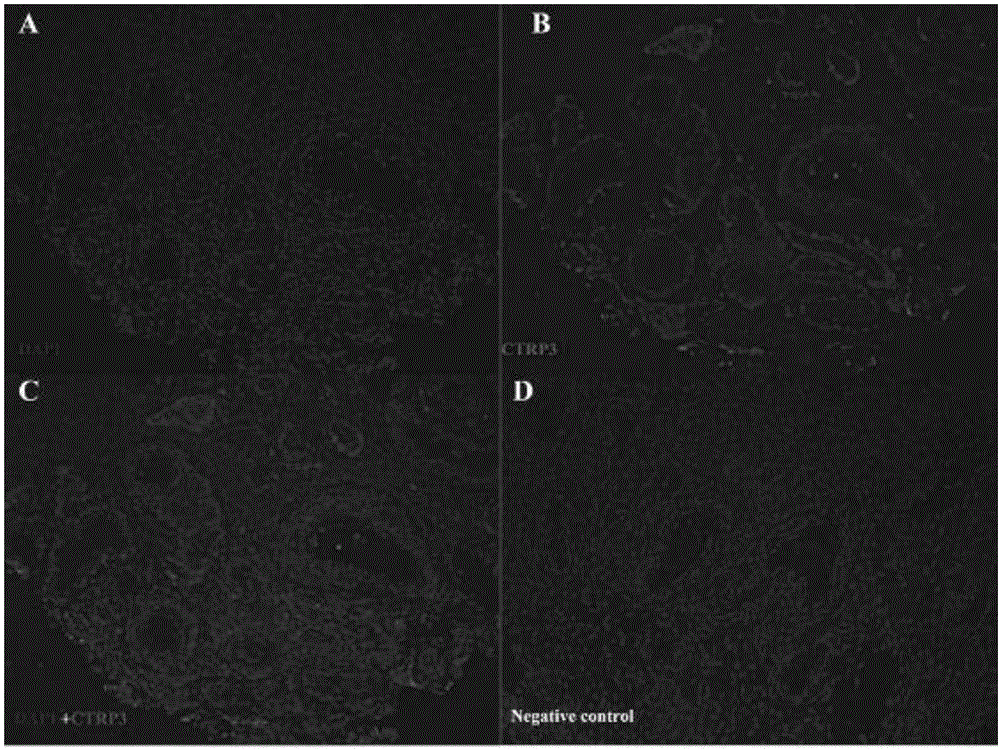
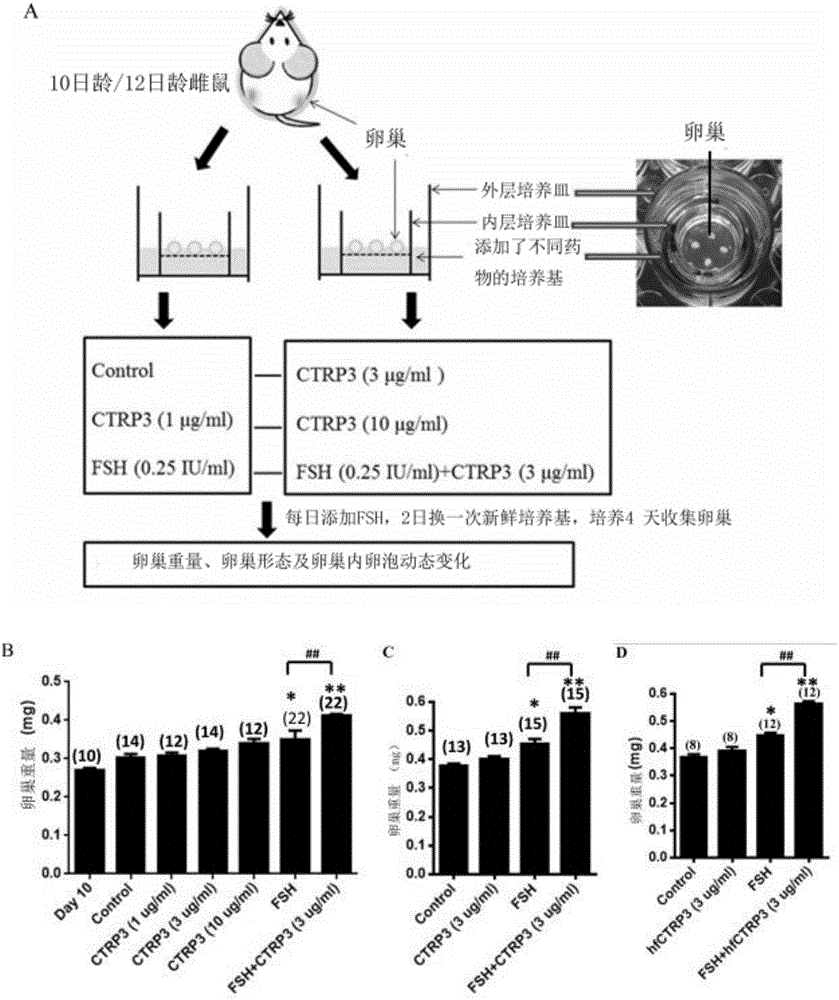
The invention discloses an application of CTRP3 protein (C1qTNF related protein 3), and belongs to the fields of biotechnology and medicines. The CTRP3 protein provided by the invention can enhance the sensitivity of follicle to follicle stimulating hormone; and by identifying the expression of the CTRP3 protein in human and mouse ovaries, it indicates that the CTRP3 protein is autologous protein of the ovaries; therefore, the the CTRP3 protein can be safely developed and utilized as a drug. The effect of the CTRP3, as a follicle stimulating hormone sensitizer, on promoting follicle development is verified from three different levels, namely isolated ovarian tissue culture, isolated preantral follicle culture and living mesovarium injection, indicating that the CTRP3 protein, as the follicle stimulating hormone sensitizer, is effective on promoting preantral follicle and antral follicle development. The invention provides the effect and the mechanism of the CTRP3 participating in follicle development regulation and indicates that the CTRP3 protein, as the follicle stimulating hormone sensitizer, is applicable to the treatment of female ovarian hypoplasia diseases, so that the effect of the follicle stimulating hormone on the development of the follicle is enhanced.
Owner:JINAN UNIVERSITY
Soluble fgfr3 decoys for treating skeletal growth disorders
PendingUS20210009657A1Easy genetic manipulationReduce contentPeptide/protein ingredientsAntibody mimetics/scaffoldsHeterologousDisease
Owner:THERACHON HLDG GMBH +3
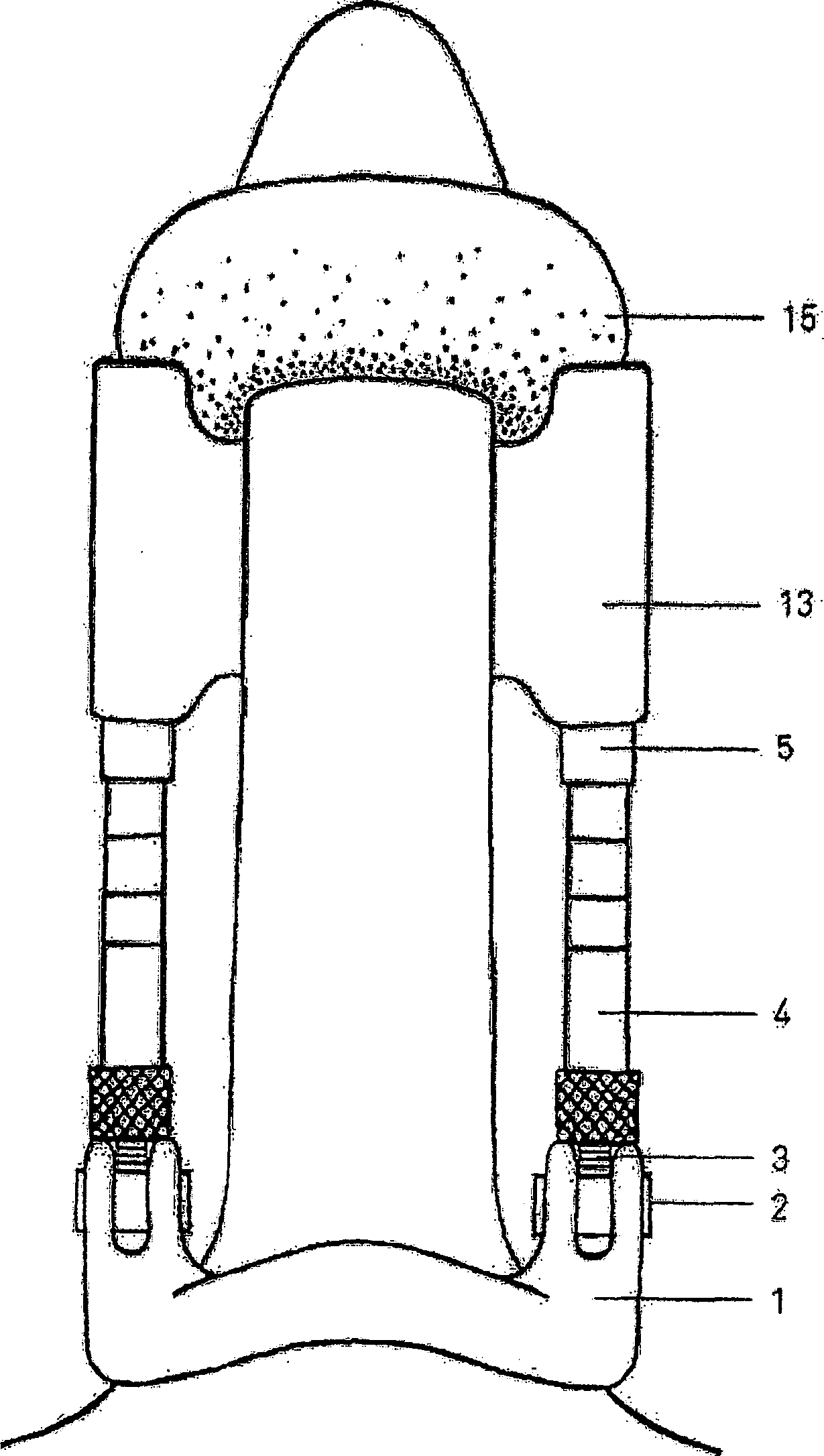
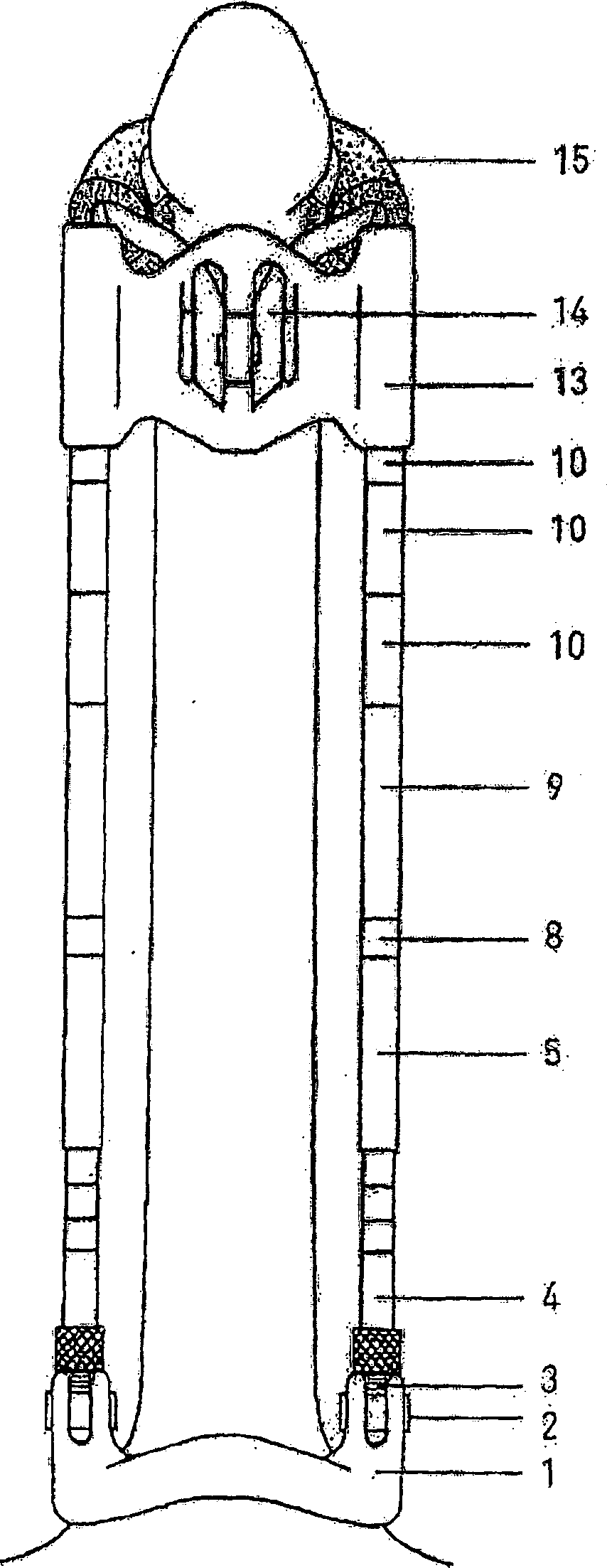
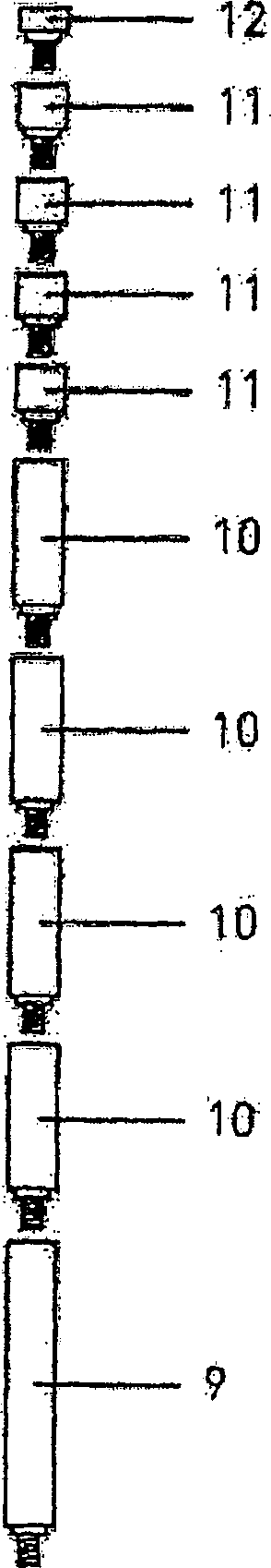
Genitalia external prothesis for treatment of male external genitalia hypoplasia
InactiveCN1284517CAvoid woundsNo discomfortPenis support devicesRestraining devicesUser deviceMale gender
The present invention relates to a device for treating male genital hypoplasia, growing its tissue and correcting existing curvature. The device of the present invention is unique in that it employs an external genital fixation system (1; 13) to which a diameter device (5) and a gland protection system (15) are connected. The external genital fixation system (1) and the upper element (13) create a traction force on the male external genitalia. The diameter device (5) creates a tissue expansion on the male genitalia. The gland protection system (15) avoids possible wounds, which makes it possible to use it for a long time without discomfort. Users of the device can wear it for months, several hours a day. Thus, for the treatment of male genital hypoplasia, timely tissue expansion and the opportunity to prolong the expansion are fundamental.
Owner:爱德华多.A.戈麦斯.德迭戈
Fgfr3 antagonists
The invention pertains to novel FG-FR3antagonists of general formula (I), The compounds are useful for the treatments and prevention of achondroplasia and cancer.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
Polypeptide with effect of inhibiting activity of fibroblast growth factor receptor 3 and application thereof
InactiveCN102627686AEnhanced inhibitory effectSmall molecular weightPeptide/protein ingredientsSkeletal disorderDiseaseHypoplasia
The invention discloses polypeptide with an effect of inhibiting activity of fibroblast growth factor receptor 3 (FGFR3). The polypeptide consists of an amino acid sequence shown by Thr-Leu-Gly-Gln-Leu-Leu, can be combined with specificity of an extracellular fragment of the FGFR3, has an obvious inhibition effect on the activity of the extracellular fragment of the FGFR3, has the advantages of low molecular weight, simplicity in preparation, easily controlled quality, low immunogenicity and the like, can be prepared into a medicament for preventing and controlling FGFR3 function enhanced diseases, and has potential good application prospect in prevention and treatment of the FGFR3 function enhanced diseases such as thanatophoric dysplasia and achondroplasia.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
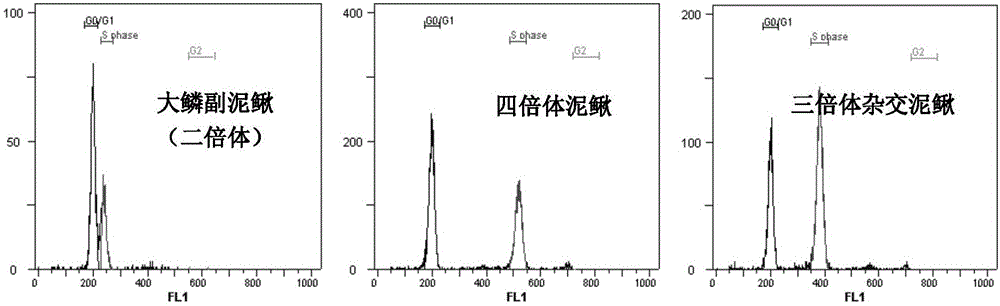
Method for obtaining high-proportion male loaches through interspecies cross
InactiveCN103875569ADysplasiaBreeding generations are fewClimate change adaptationPisciculture and aquariaAbdominal cavityRed mullet
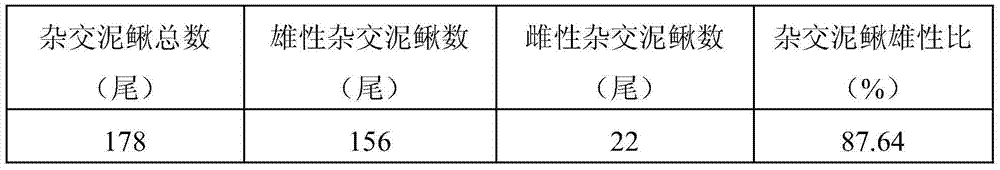
The invention discloses a method for obtaining high-proportion male loaches through interspecies cross. The method includes the steps that female paramisgurnus dabryanus and male tetraploid loaches serve as parents for cross; oxytocic medicine is injected into abdominal cavities of the female paramisgurnus dabryanus and abdominal cavities of the male tetraploid loaches, after hasten parturition is carried out for 12-16 hours, testes of the male tetraploid loaches and egg grains of the female paramisgurnus dabryanus are collected, the testes and preserving liquid are grinded and mixed evenly and then mixed with the egg grains evenly, fertilized eggs are obtained and then incubated, and the high-proportion male loaches are obtained from the hybrid loach fries obtained through breeding and incubation. In triploid hybrid loach groups obtained through the method, the proportion of the male hybrid loaches reaches up to 87.64% to 98.53%, and the ovaries of the female loaches are in a hypoplasia state.
Owner:HUAZHONG AGRI UNIV
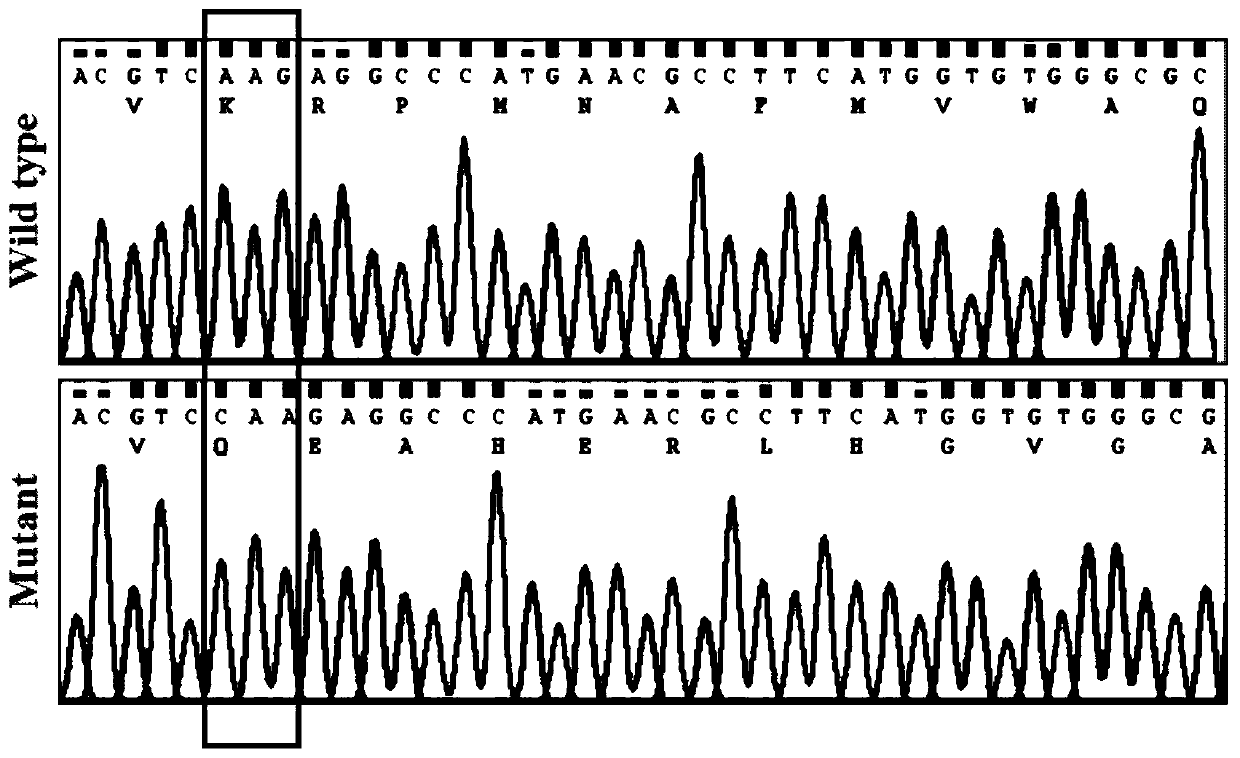
Pig SOX10 mutant gene causing inner ear hypoplasia and application of pig SOX10 mutant gene
The invention relates to a pig SOX10 mutant gene causing inner ear hypoplasia and an application of the pig SOX10 mutant gene. The pig SOX10 mutant gene is characterized in that the 321st basic groupcytosine C of an SOX10 gene coding region is subjected to replication (SOX10 c.321 dupC), the mutation can cause infertility of the inner ear and is autosomal dominant inheritance, and can be used forpathogenesis, prevention, diagnosis and treatment research of infertility of the inner ear of a human body. The pig SOX10 mutant gene can be used for preparation of a disease animal model with incomplete development of the inner ear. The model can be used for research on pathogenesis, prevention, diagnosis and treatment on human inner ear hypoplasia, and is of great significance to research on inner ear hypoplasia.
Owner:ARMY MEDICAL UNIV
One-plate five-combined TORCH-IgM antibody colloidal gold rapid detection test strip and preparation method thereof
The invention discloses a one-plate five-combined TORCH-IgM antibody colloidal gold rapid detection test strip. One end of a chromatographic membrane is connected with a gold-labeled pad, the other end of the gold-labeled pad is connected with a sample pad, and the other end of the chromatographic membrane is connected with an absorption pad; the absorption pad, the chromatographic membrane, the gold-labeled pad and the sample pad are arranged on a base plate; goat-anti-human IgM-labeled colloidal gold conjugate particles are adsorbed on the gold-labeled pad; detection lines of five TORCH antigens and a control line of a rabbit-anti-goat IgG antibody are coated on the chromatographic membrane; and the absorption pad is provided with labeling regions corresponding to the detection lines on the chromatographic membrane. The invention also discloses a preparation method of the one-plate five-combined TORCH-IgM antibody colloidal gold rapid detection test strip. The test strip can detect whether a pregnant woman suffers from the acute infection of the five TORCH pathogens through one-step blood serum sampling; and the result can be observed with the naked eye and can be easily accepted by the user. Thus, the test strip is especially suitable for basic health centers, and can effectively prevent congenital or perinatal newborn infection caused by the TORCH infection of the pregnant woman, thereby avoiding abortion, teratism, stillbirth, premature delivery, hypoplasia or the like.
Owner:广州精达医学科技有限公司
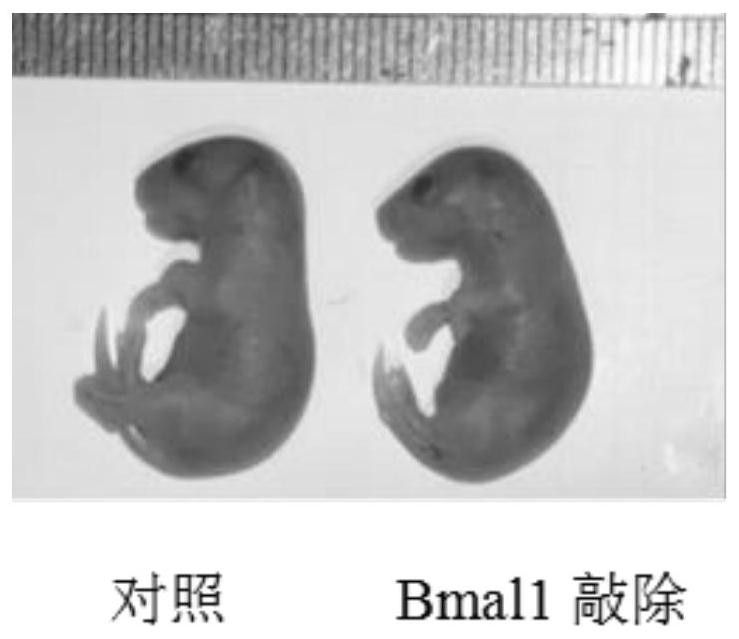
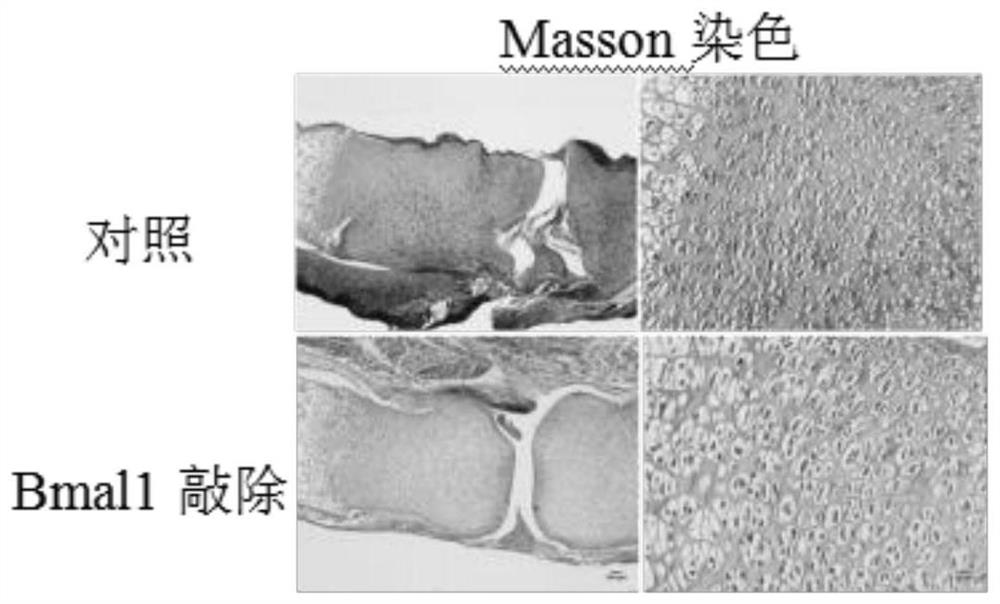
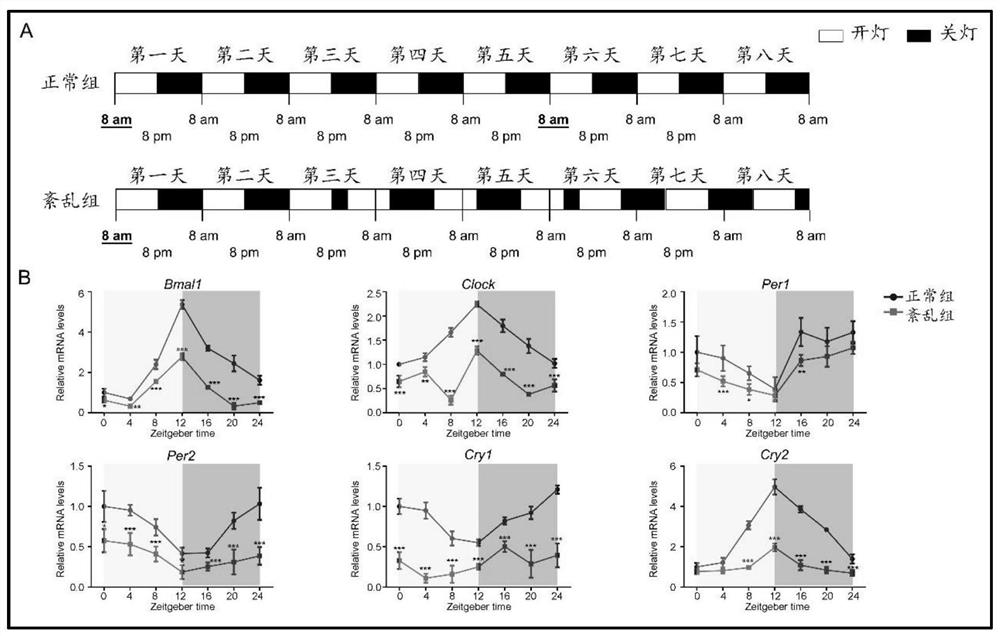
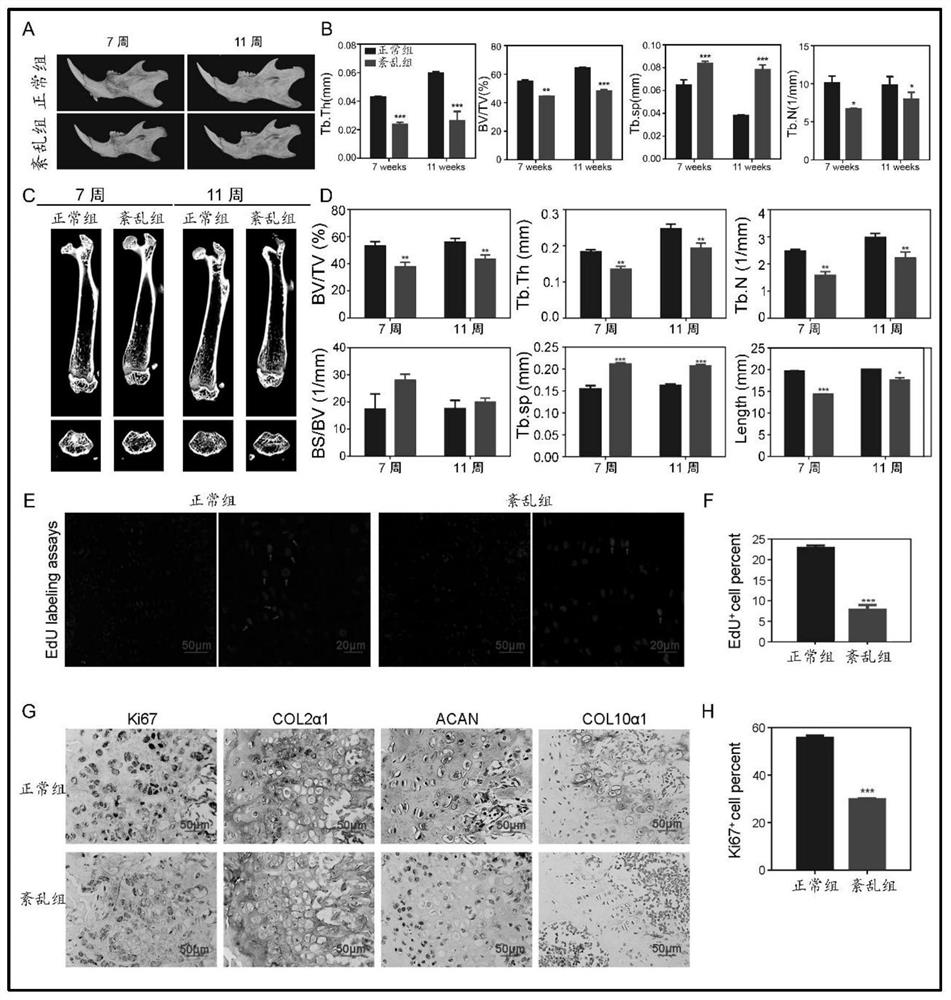
Application of Bmal1 gene in preparation of product for detecting and treating cartilage hypoplasia disease
The invention discloses application of a Bmal1 gene in preparation of a product for detecting and treating cartilage hypoplasia diseases, and belongs to the technical field of biological medicines. The nucleotide sequence of the Bmal1 gene in the invention is as shown in SEQ ID NO. 1, and the amino acid sequence of the Bmal1 gene is as shown in SEQ ID NO. 2. According to the invention, a molecular mechanism of the Bmal1 gene for regulating and controlling the biological process of cartilage growth and development is clarified, and a foundation is laid for research and development of drugs taking the P4ha1 gene as a target based on the biological clock gene Bmal1.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Method and system for treatment of maxillary deficiency using Hyrax
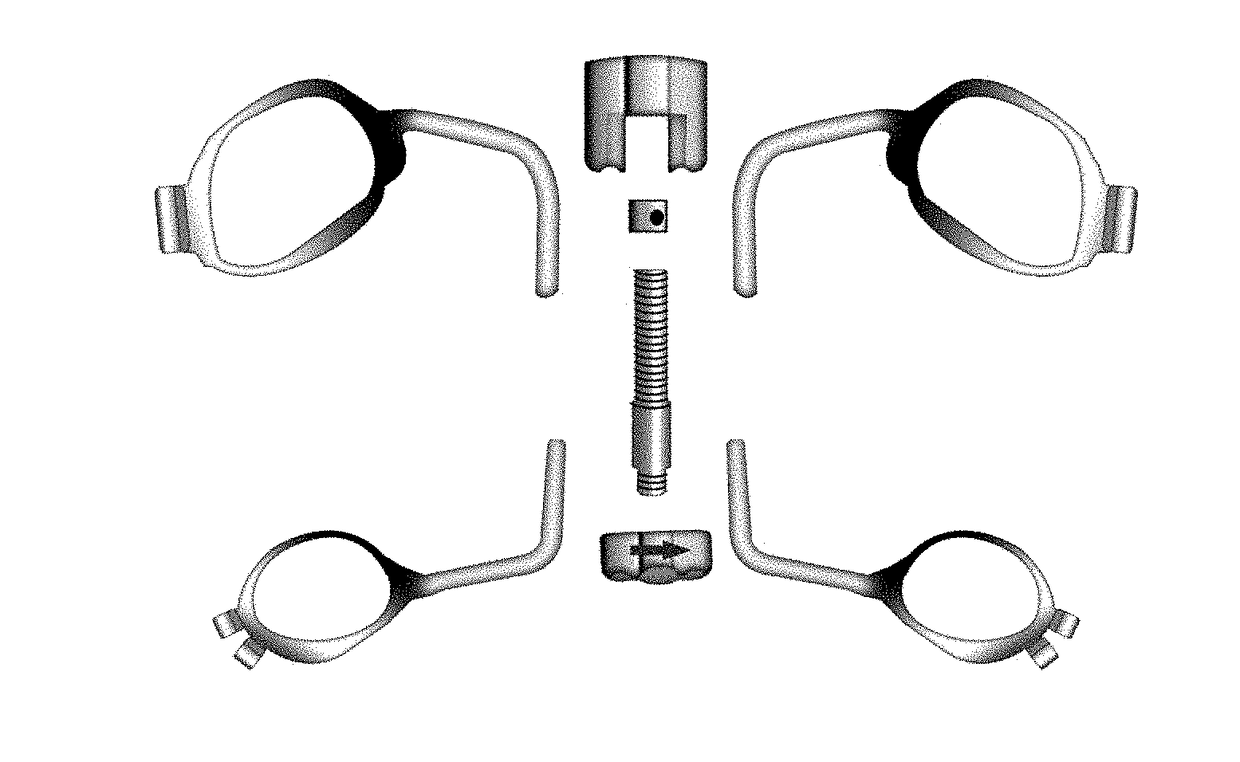
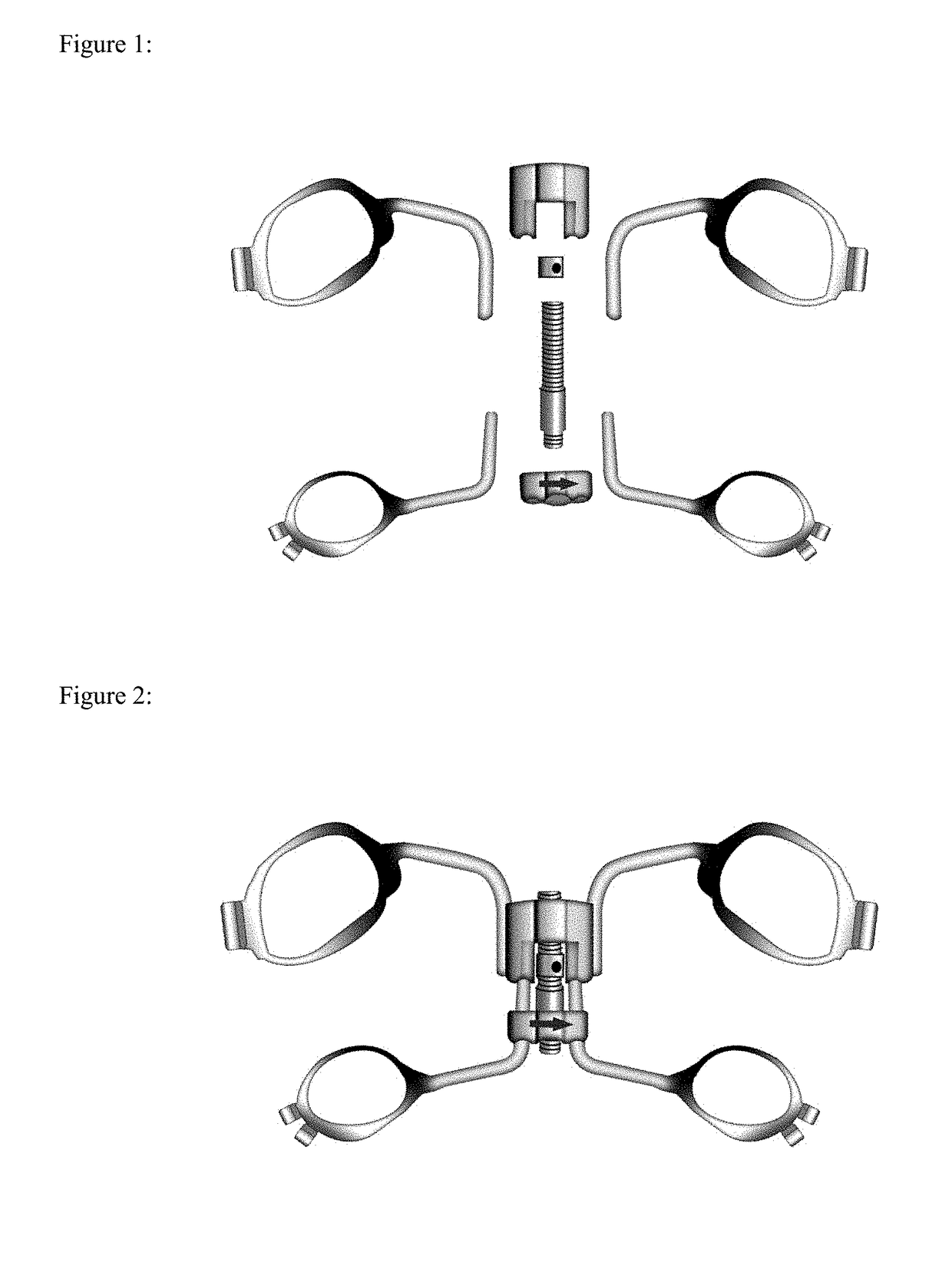
InactiveUS20170209239A1Eliminate the problemOthrodonticsMaxillary premolar toothMaxillary deficiency
A method and system for treating maxillary transverse discrepancies in young orthodontic patients and in skeletally mature patients. The maxillary protraction device comprises a Hyrax provided with an adjustable screw and four bands connected to the Hyrax through connecting wires.Placement of Hyrax on first maxillary molars and second maxillary premolars would create enough space for effective treatment of maxillary hypoplasia.
Owner:SHOWKATBAKHSH ABDOLRAHMAN +1
Traditional Chinese medicine composition for treating infertility and preparation method of traditional Chinese medicine composition
InactiveCN104474468AReduce tensionSimple processing methodSexual disorderDrageesHabitual AbortionsWolfiporia extensa
The invention discloses a traditional Chinese medicine composition for treating infertility and a preparation method of the traditional Chinese medicine composition. The traditional Chinese medicine composition is prepared from the following raw materials: codonopsis pilosula, semen cuscutae, rhizoma atractylodis macrocephalae stir-fried with bran, poria cocos, Chinese yam, mistletoe, curcuma aromatic, radix dipsaci, parched white peony root, salted eucommia ulmoides, liquorice, radix rehmanniae praeparata, herba epimedii and prepared lumbricus in combination. The traditional Chinese medicine composition has accurate curative effects on threatened abortion of female patients, metrorrhagia and metrostaxis, morbid leukorrhoea, qi deficiency and blood stasis, adverse main and collateral channels, deficiency of vital energy and blood, unsmooth fallopian tubes, meridian disharmony, amenorrhea, dysmenorrhea, abnormal leukorrhea, endometriosis, hypoplasia of uterus, habitual abortion, pregnancy blood leakage, threatened abortion, threatened abortion, metrorrhagia and metrostaxis, irregular menstruation, metrorrhagia embolism in blood and the like.
Owner:高春娟
Method for verifying effect of SAG in preventing environmental anomaly related bone dysplasia
PendingCN112790155AAvoid stunted growthMicrobiological testing/measurementDisease diagnosisCartilage cellsBone morphology
The invention provides a method for verifying the effect of SAG in preventing environmental anomaly related bone dysplasia. The method comprises the following steps of changing the illumination condition in the environment, and inducing the establishment of a bone dysplasia model of an environmental rhythm disorder mouse; analyzing the bone mass and bone morphology of mandible and femur tissues of the rhythm disorder mouse, the multiplication capacity and collagen secretion capacity of corresponding cartilage cells and Hedgehog pathway related gene expression; and finally analyzing the treatment effect of the mandible and femur tissues of the mouse subjected to SAG treatment. According to the method for verifying the effect of the SAG in preventing the environmental anomaly related bone dysplasia provided by the invention, the influence of an illumination factor and time difference in the natural environment on bone growth and development can be simulated by constructing the illumination factor induced mouse bone dysplasia model, and by comparing the development conditions of the mandible and femur tissues before and after injection of the SAG, the effect of the SAG in preventing mandibular deficiency, short and deformed femur and the like can be effectively verified.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Methods of use of soluble cd24 for treating immune related adverse events in cancer therapies
The present invention relates to a CD24 protein for treating immune-related adverse events (irAEs) associated with cancer immunotherapy. Provided herein is a method of treating, mitigating, minimizing, or preventing immunerelated adverse events (irAEs) associated with a cancer immunotherapy by administering a CD24 protein to a subject in need thereof. The irAE may be diarrhea or another gastrointestinal disorder, pure red cell aplasia, microcytic anemia, lupus, autoimmune nephritism, autoimmune hepatitis, pneumonitis, myocarditis, pericarditis, endocrinopathy, Addison's disease, hypogonadism,Sjogren's syndrome, or type I diabetes. The CD24 protein may comprise a mature human CD24 or a variant thereof.
Owner:ONCOIMMUNE INC +1
Novel G protein-coupled receptor protein, its DNA and ligand thereof
InactiveUS20070202550A1Receptors for hormonesImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune conditionPhysiology
Owner:TAKEDA PHARMA CO LTD
Biphasic lipid-vesicle compositions and methods for treating cervical dysplasia by intravaginal delivery
ActiveUS10159646B2Improved shelfPrevent oxidationPeptide/protein ingredientsAerosol deliveryVaginaDysplasia
This invention relates to biphasic lipid-vesicle compositions and methods for treating cervical displasia by intravaginal delivery.
Owner:ALTUM AVRO PHARMA PARTNERSHIP
A method to obtain a high proportion of male loach through interspecific hybridization
InactiveCN103875569BDysplasiaBreeding generations are fewClimate change adaptationPisciculture and aquariaWeatherfishAbdominal cavity
The invention discloses a method for obtaining high-proportion male loaches through interspecies cross. The method includes the steps that female paramisgurnus dabryanus and male tetraploid loaches serve as parents for cross; oxytocic medicine is injected into abdominal cavities of the female paramisgurnus dabryanus and abdominal cavities of the male tetraploid loaches, after hasten parturition is carried out for 12-16 hours, testes of the male tetraploid loaches and egg grains of the female paramisgurnus dabryanus are collected, the testes and preserving liquid are grinded and mixed evenly and then mixed with the egg grains evenly, fertilized eggs are obtained and then incubated, and the high-proportion male loaches are obtained from the hybrid loach fries obtained through breeding and incubation. In triploid hybrid loach groups obtained through the method, the proportion of the male hybrid loaches reaches up to 87.64% to 98.53%, and the ovaries of the female loaches are in a hypoplasia state.
Owner:HUAZHONG AGRI UNIV
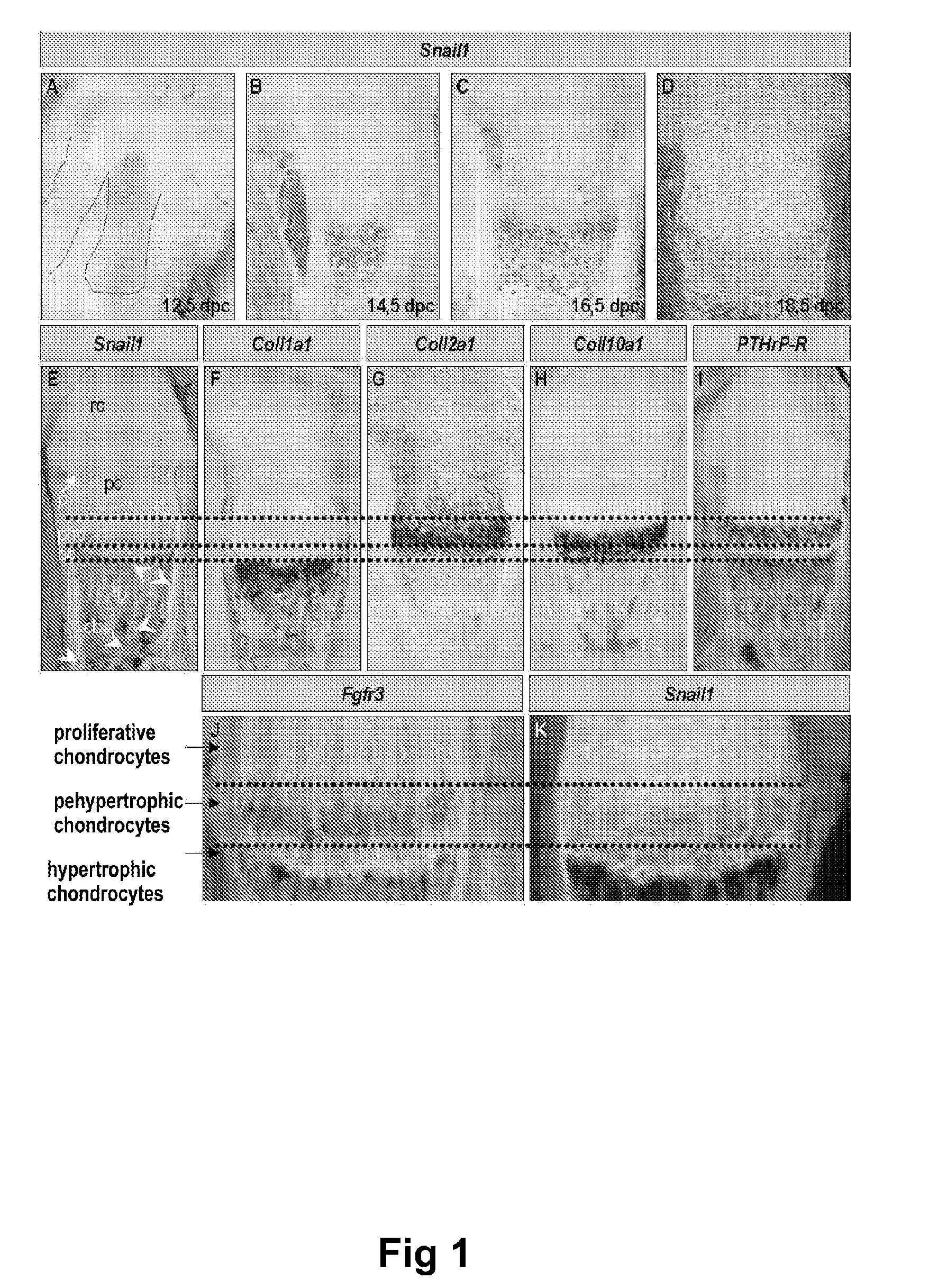
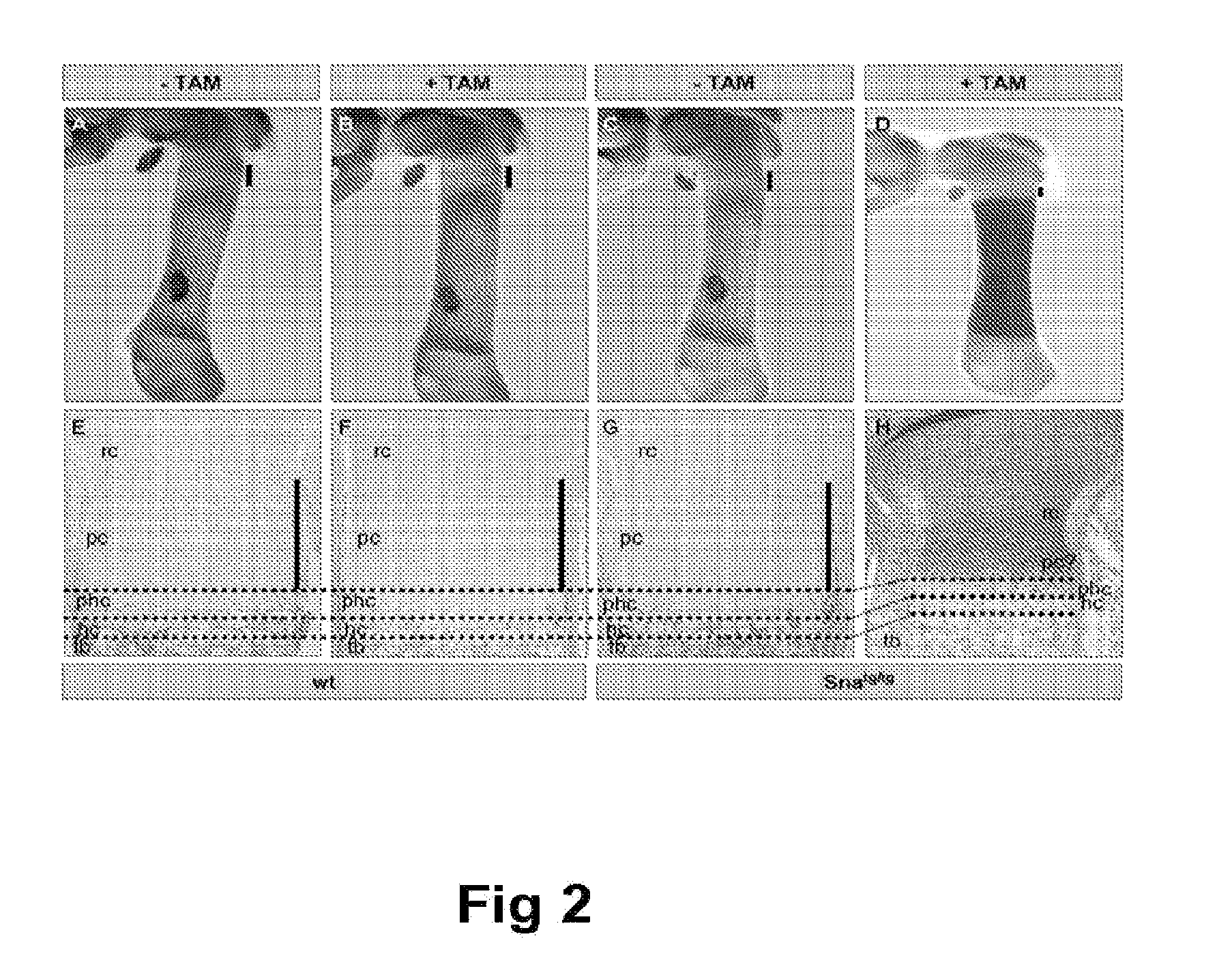
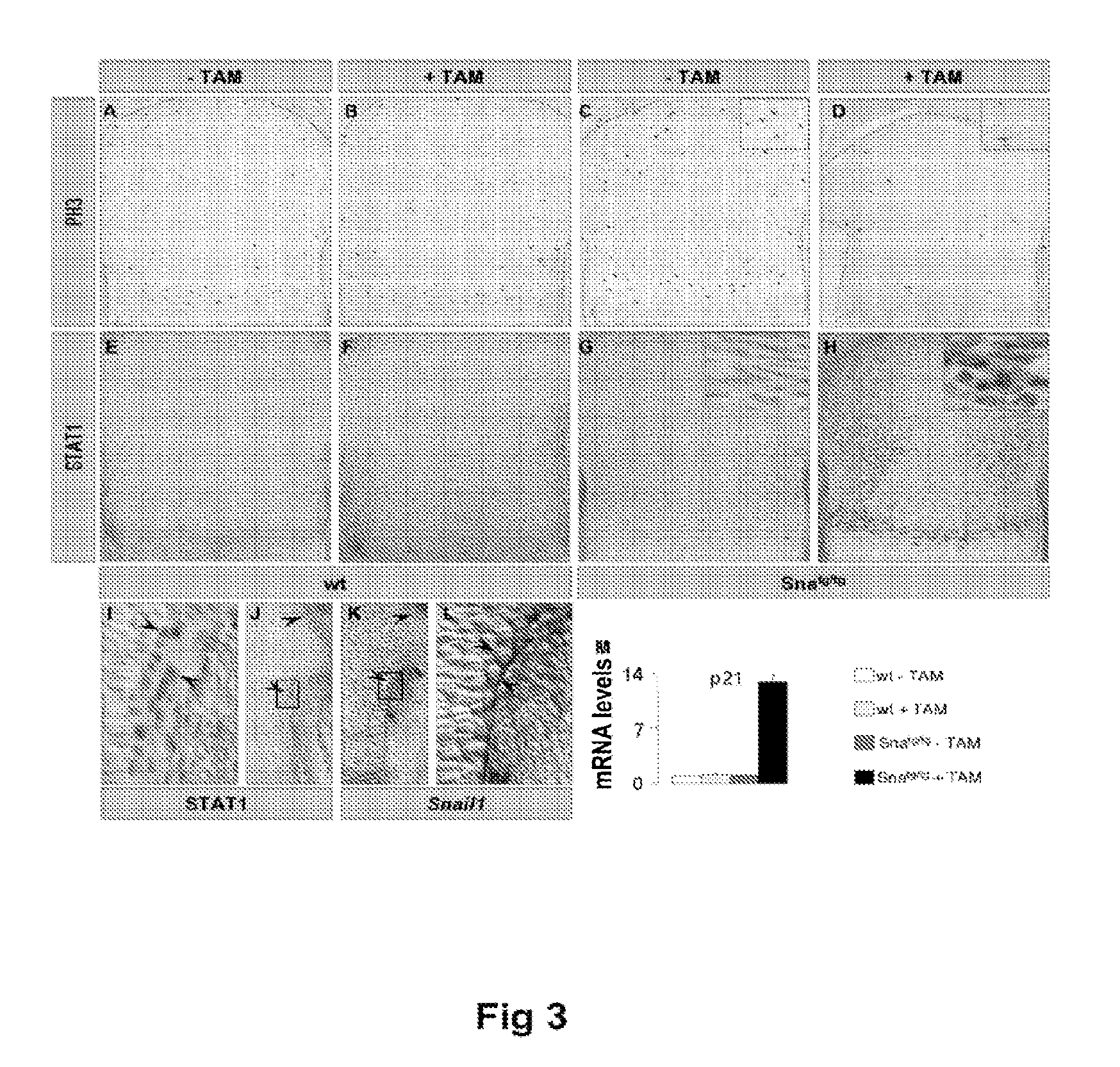
Methods of identifying and using snail1 inhibitory compounds in chondrodysplasia treatment and preparation of pharmaceutical compositions
InactiveUS20100218266A1Inhibit chondrodysplasia processOrganic active ingredientsSugar derivativesThanatophoric dysplasiaHypochondroplasia
Exemplary embodiments disclosed herein demonstrate that the Snail1 gene contributes to FGFR3 receptor signal transduction, which contributes to chondrodysplasias (achondroplasia (ACH), thanatophoric dysplasia (TD) and hypochondroplasia (HCH)). The exemplary embodiments identify Snail1 as a therapeutic and diagnostic target for chondrodysplasia, as well as the use of inhibitors thereof as drugs for the treatment of these diseases.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS (CSIC) +1
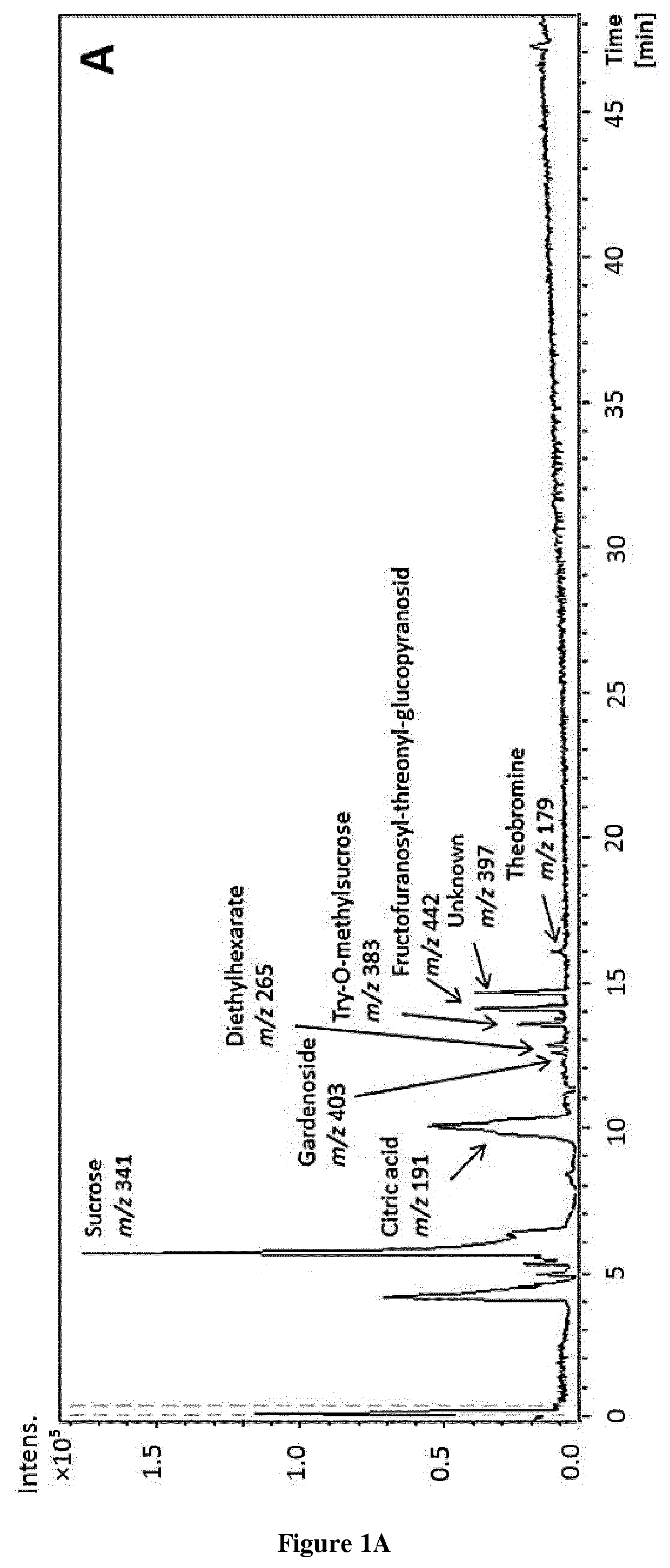
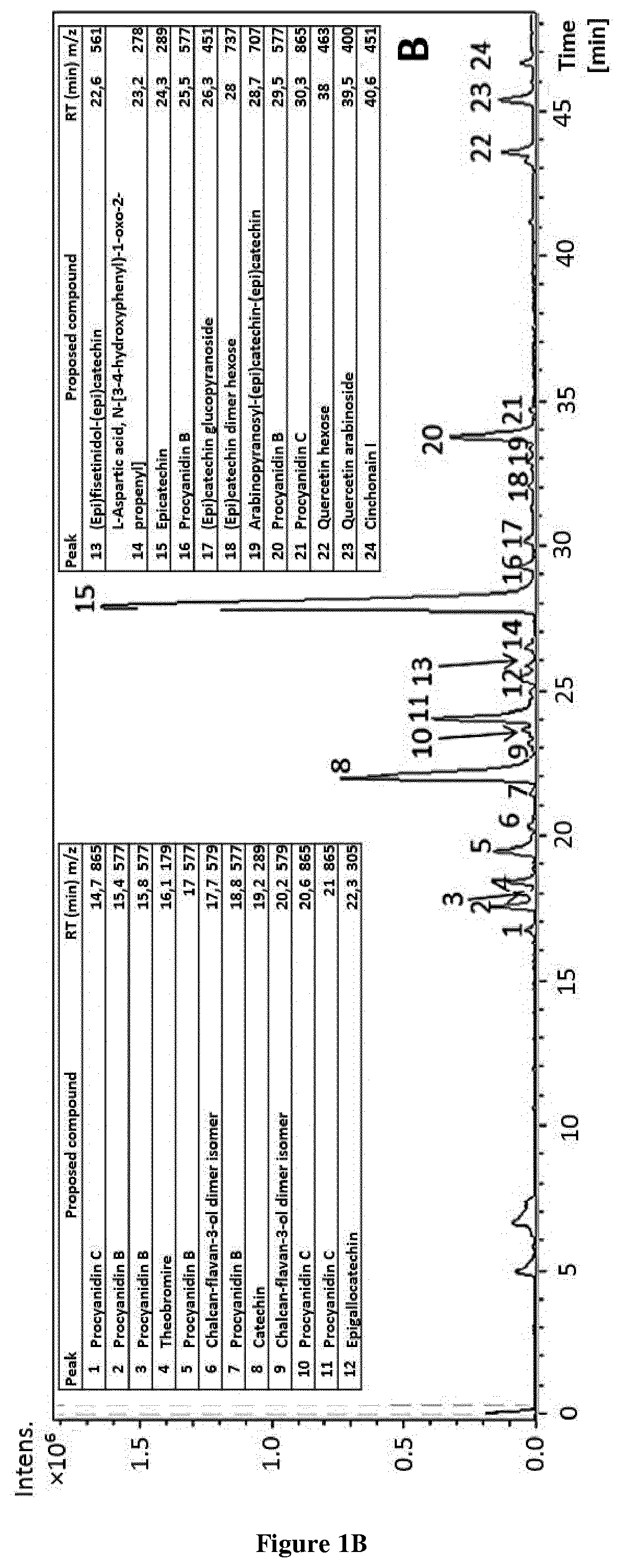
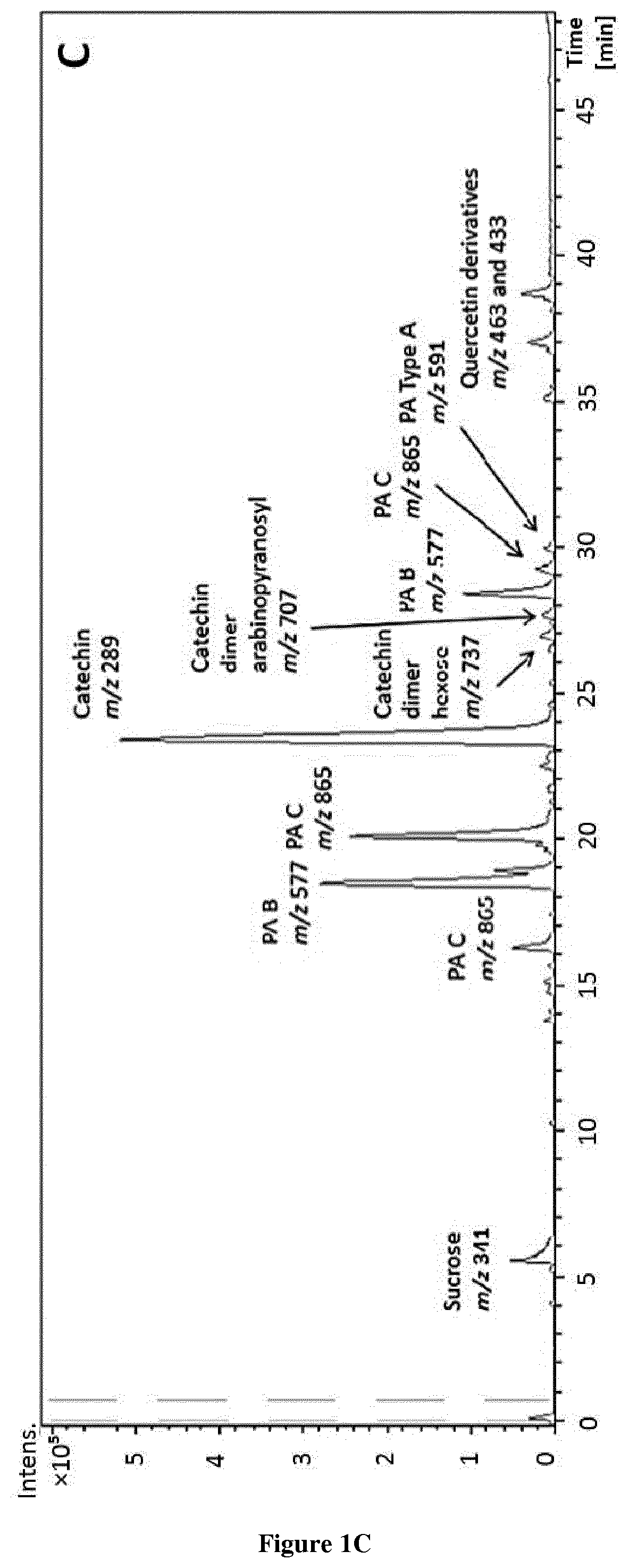
Methods and pharmaceutical compositions for the treatment of fgfr3-related chondrodysplasias
FGFR3-related chondrodysplasias represent a group of rare diseases. Among them, achondroplasia, a nonlethal form of chondrodysplasia, is the most common type of dwarfism. The mutation, which produce an increase of FGFR3 function, affects many tissues, most strikingly the cartilaginous growth plate and bone in the growing skeleton, leading to a variety of manifestations and complications. In attempt to find a new therapeutic approach for FGFR3-related chondrodysplasia, the inventors purified (−)-epicatechin from T. cacao and showed that (−)-epicatechin treatment significantly increases the length of the Fgfr3Y367C / + femurs comparing to Fgfr3+ / + femurs and improves the whole growth plate cartilage. The present invention thus relates to the use of (−)-epicatechin for the treatment of FGFR3-related chondrodysplasias.
Owner:UNIVERSITÉ PARIS CITÉ +4
Combined use of selective serotonin reuptake inhibitors and hematopoietic growth factors for treating hematopoietic diseases
PendingUS20220008514A1Convenient treatmentShorten the lengthPeptide/protein ingredientsAmine active ingredientsSerotonin reuptakePharmaceutical drug
The invention relates to the combined use of selective serotonin reuptake inhibitors (SSRIs) and hematopoietic growth factors as a drug and particularly for treating cytopenia related to hematopoietic diseases or chemotherapy, and also to a pharmaceutical kit comprising both SSRIs and hematopoietic growth factors. This combination is more particularly used for treating patients presenting cytopenia, and patients in need of chemotherapy and more particularly to reduce length of chemotherapy-induced aplasia.
Owner:UNIVERSITÉ PARIS CITÉ +5
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com