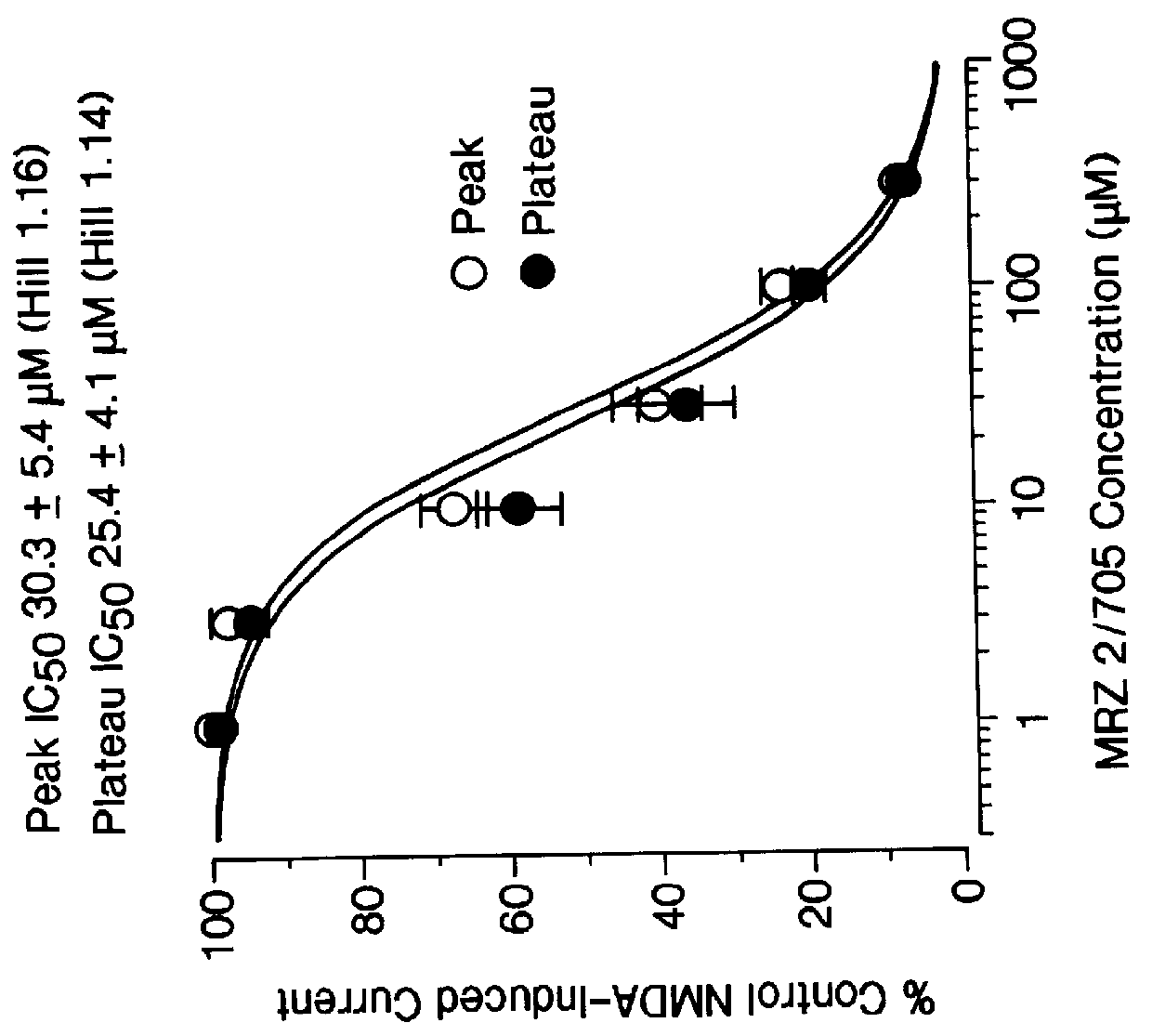
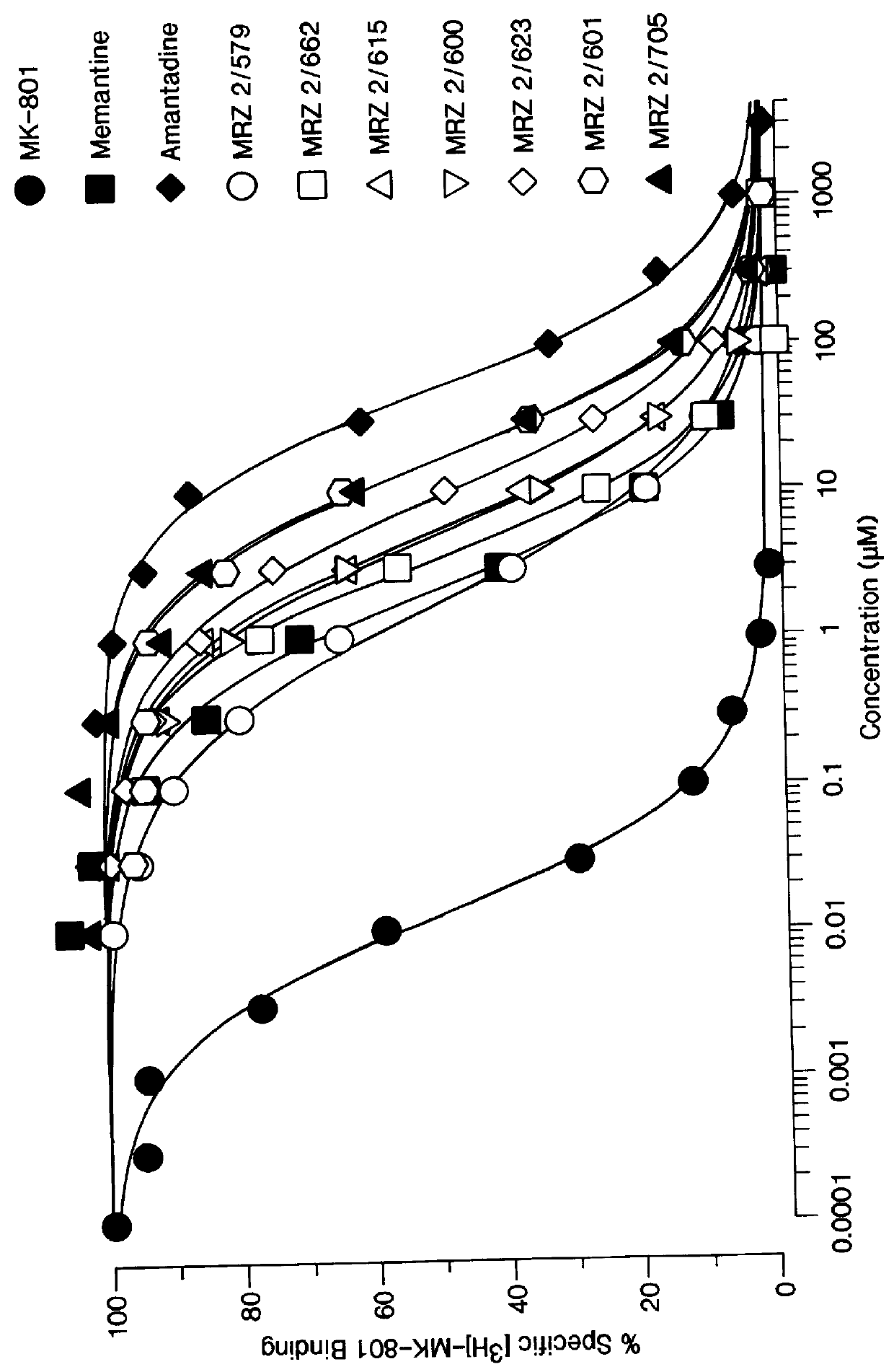
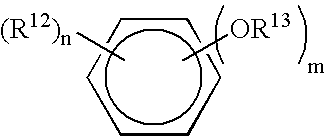
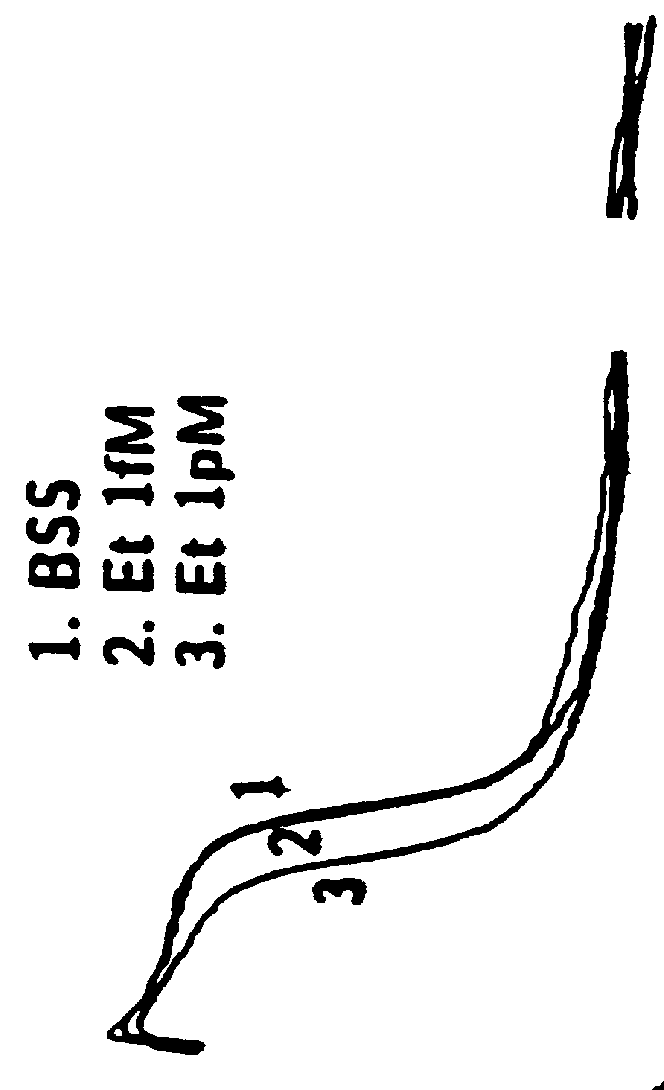
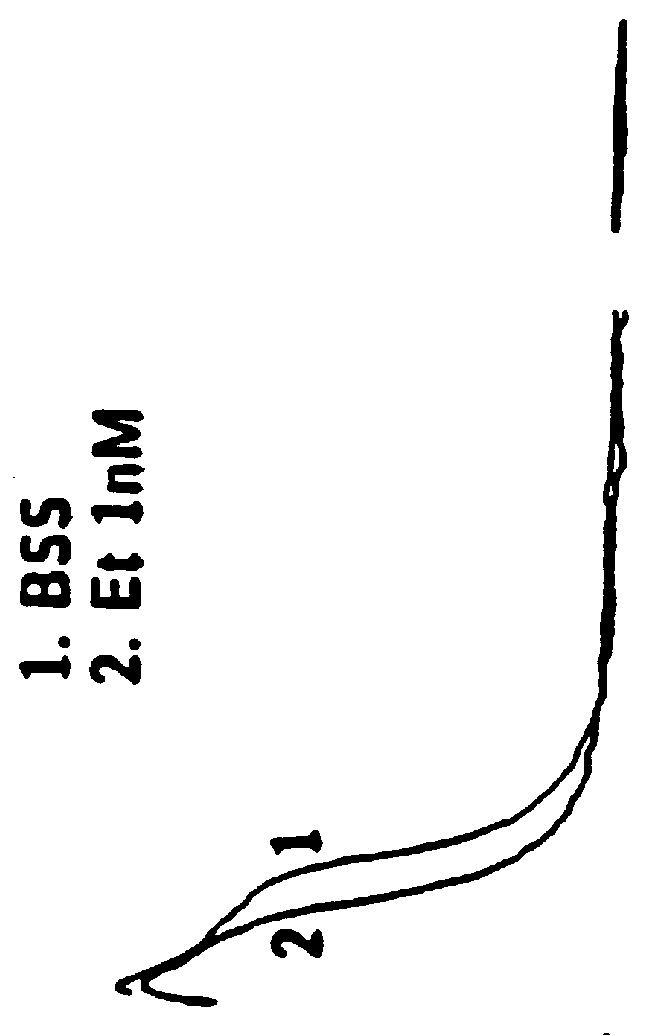
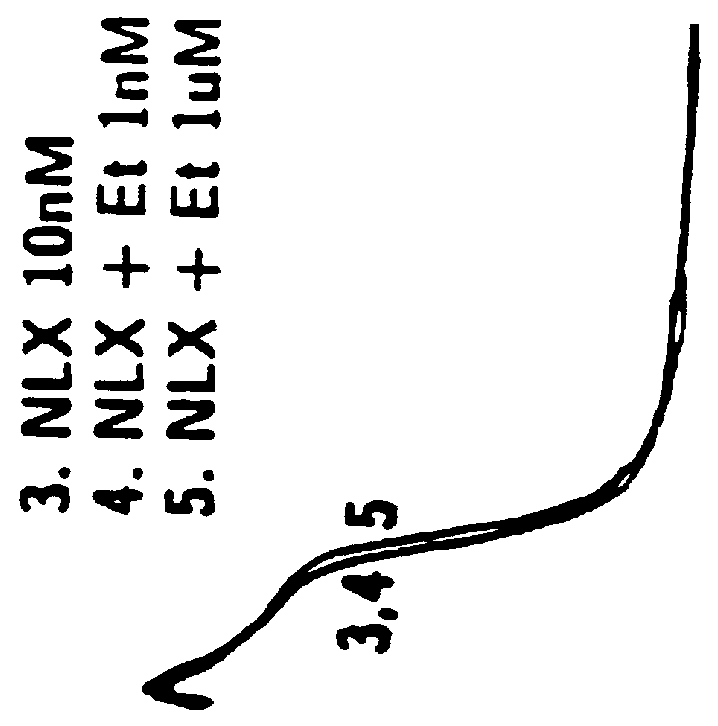
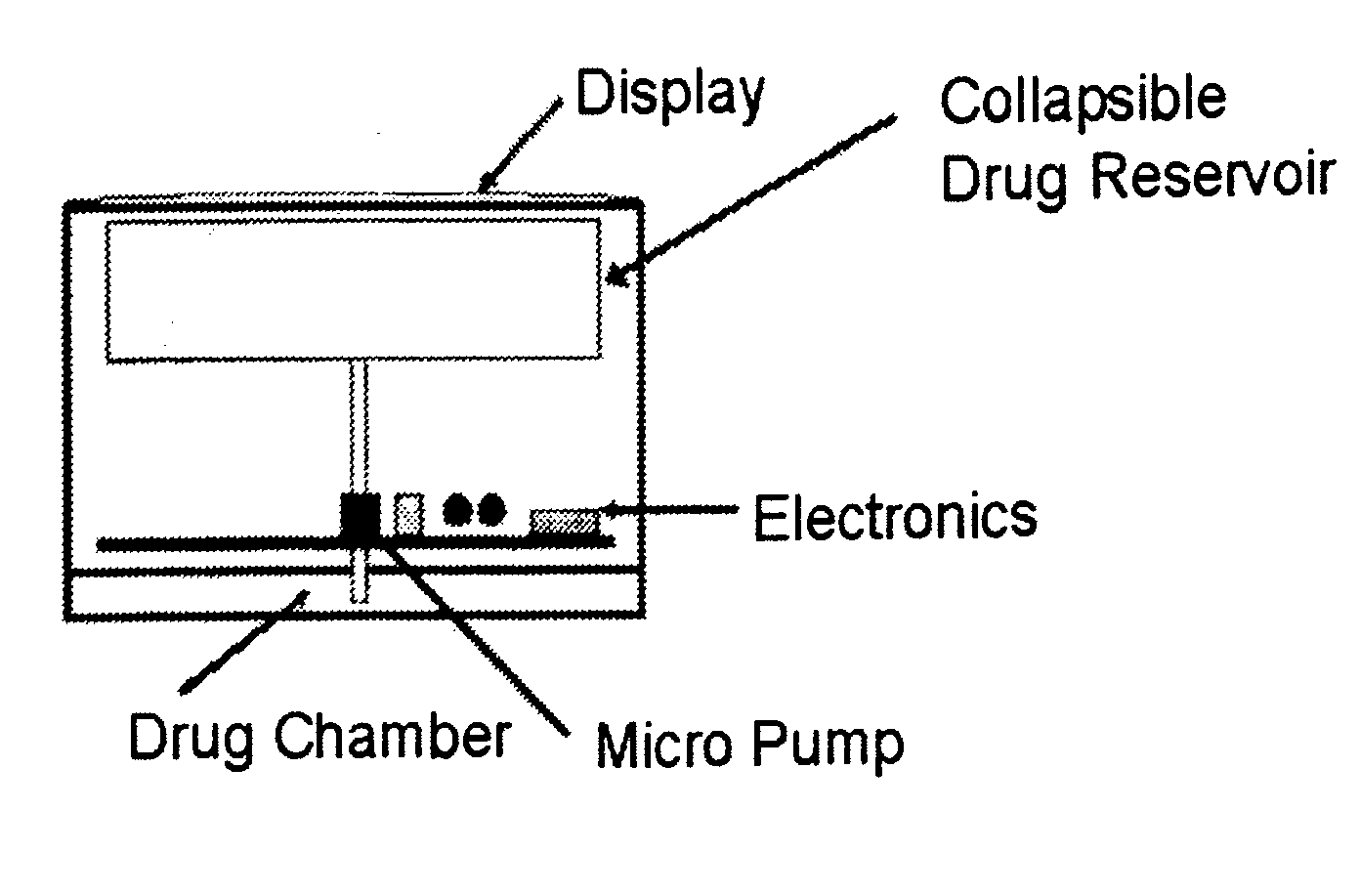
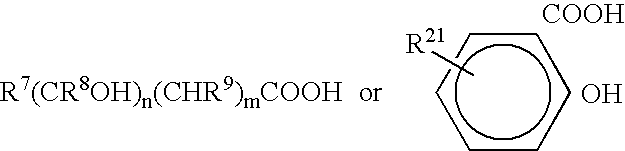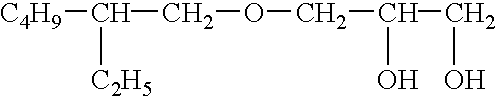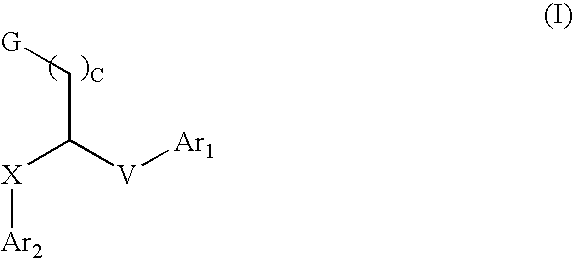Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
4636results about "Amine active ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
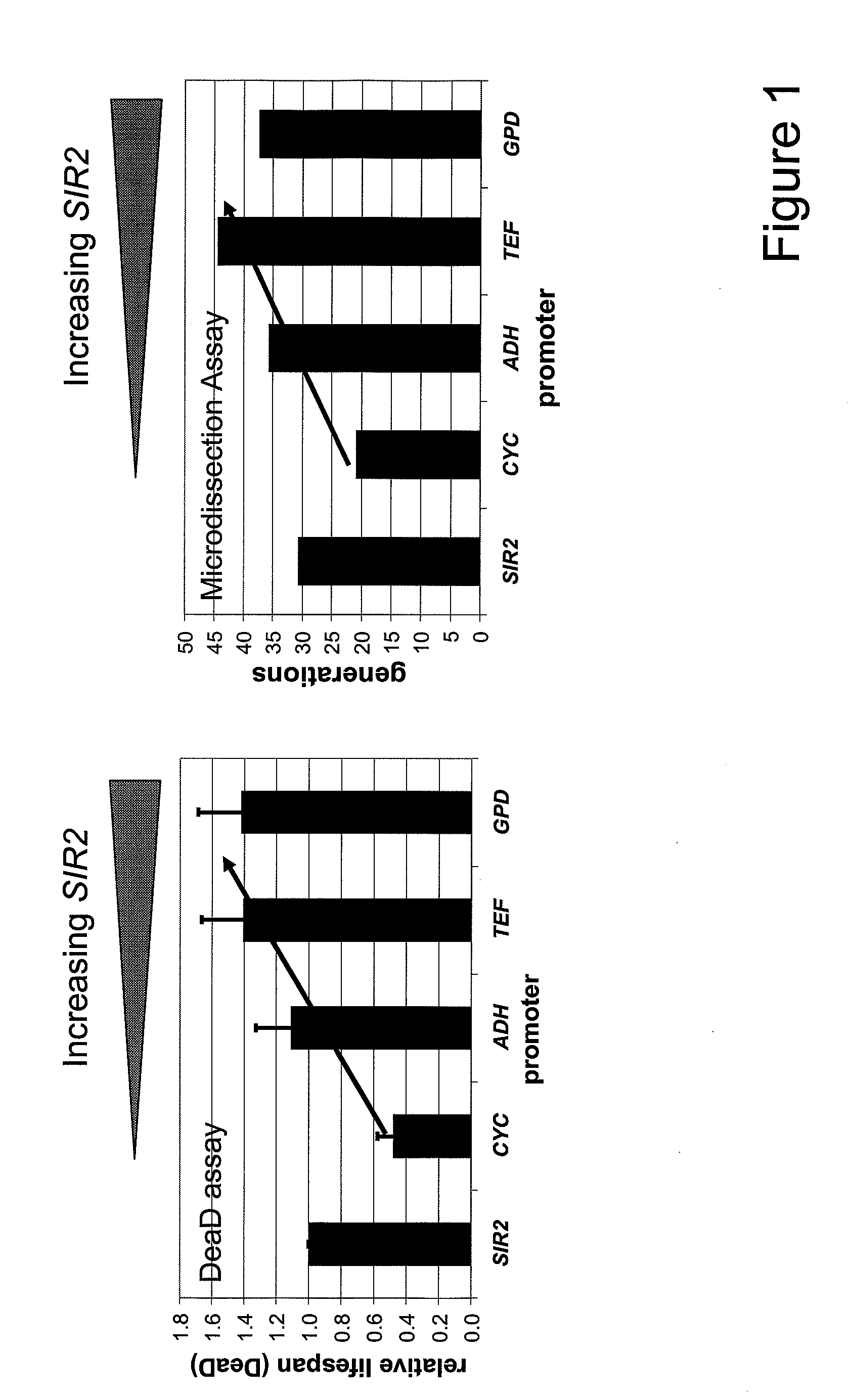
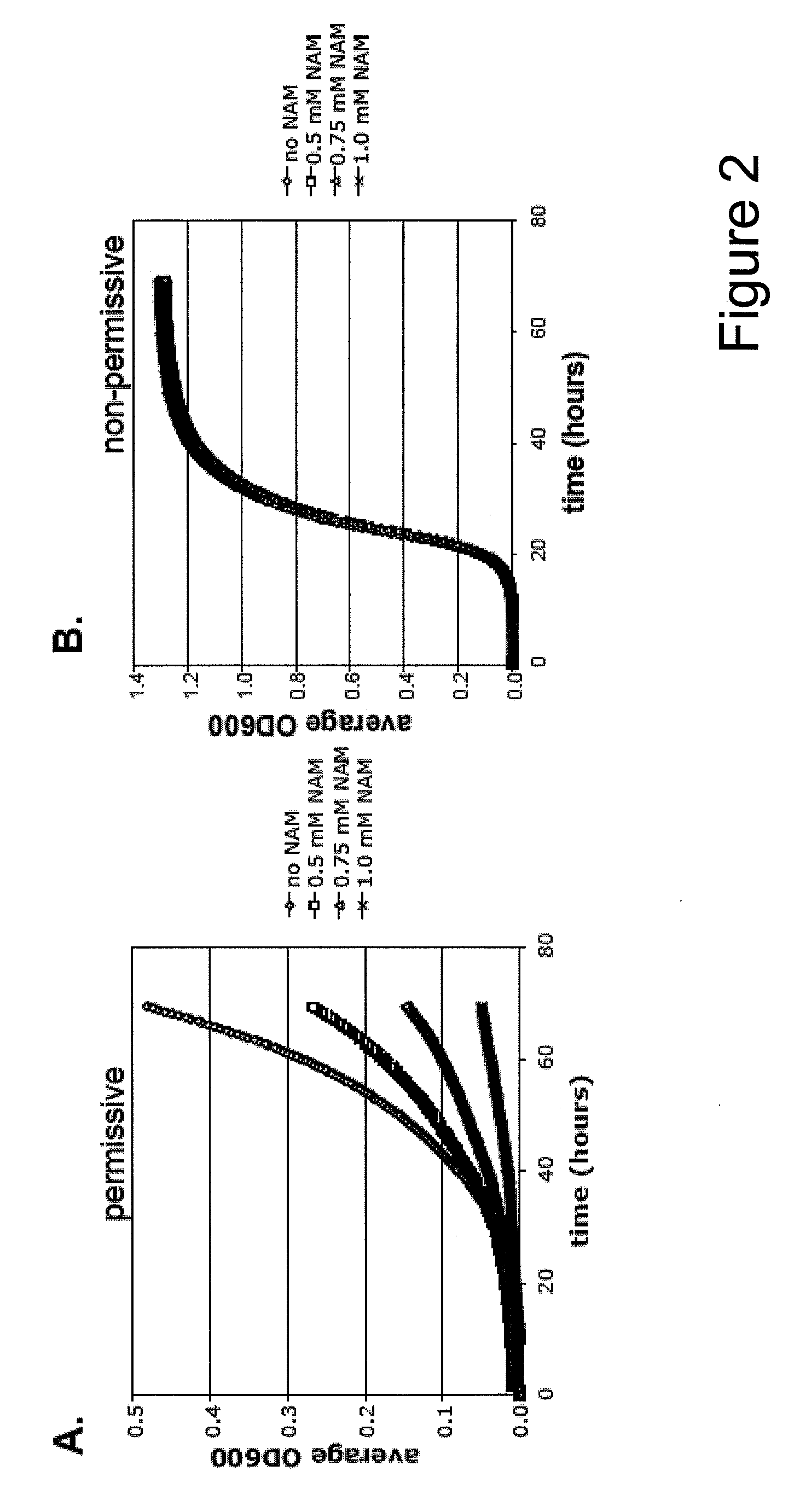
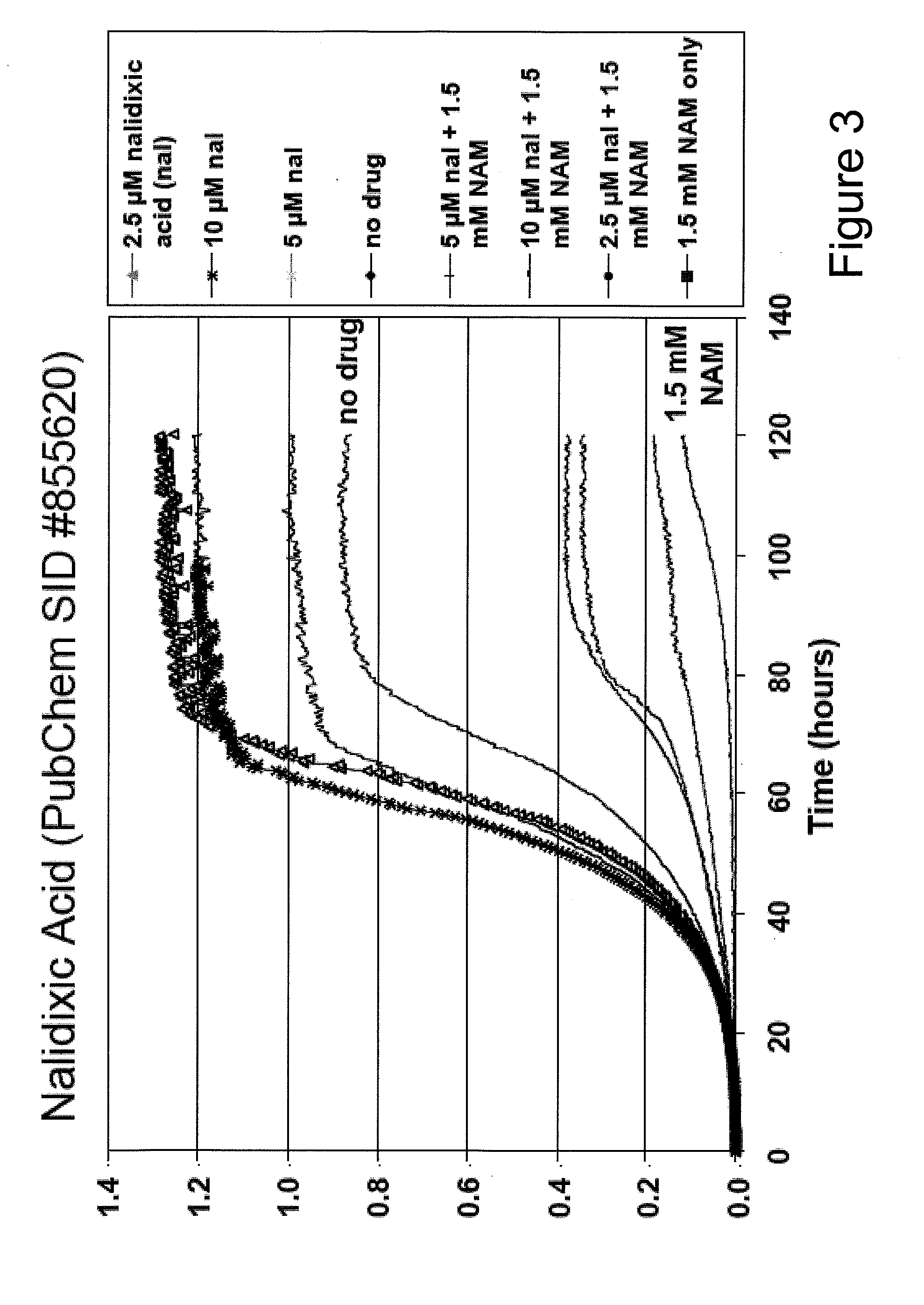
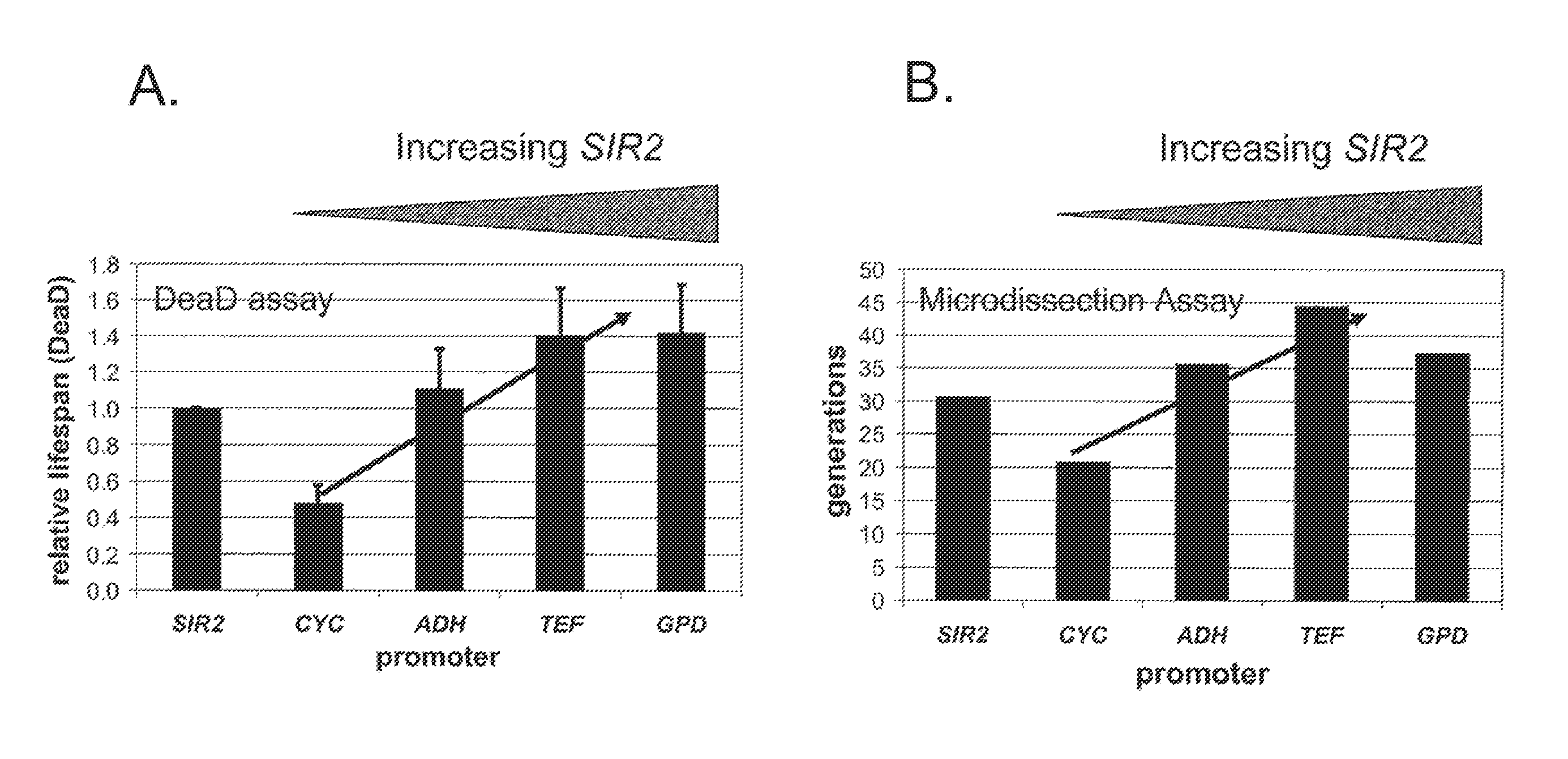
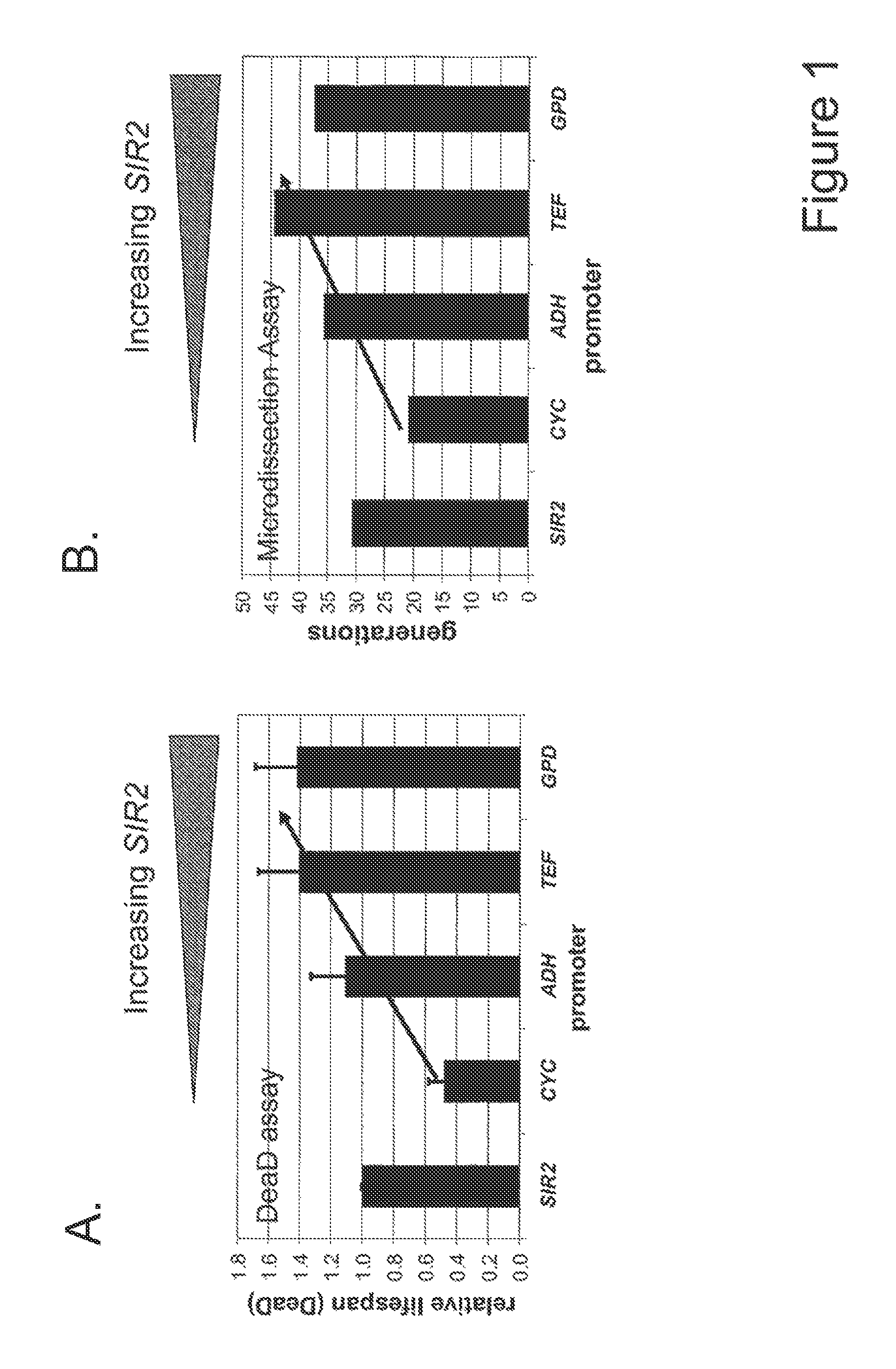
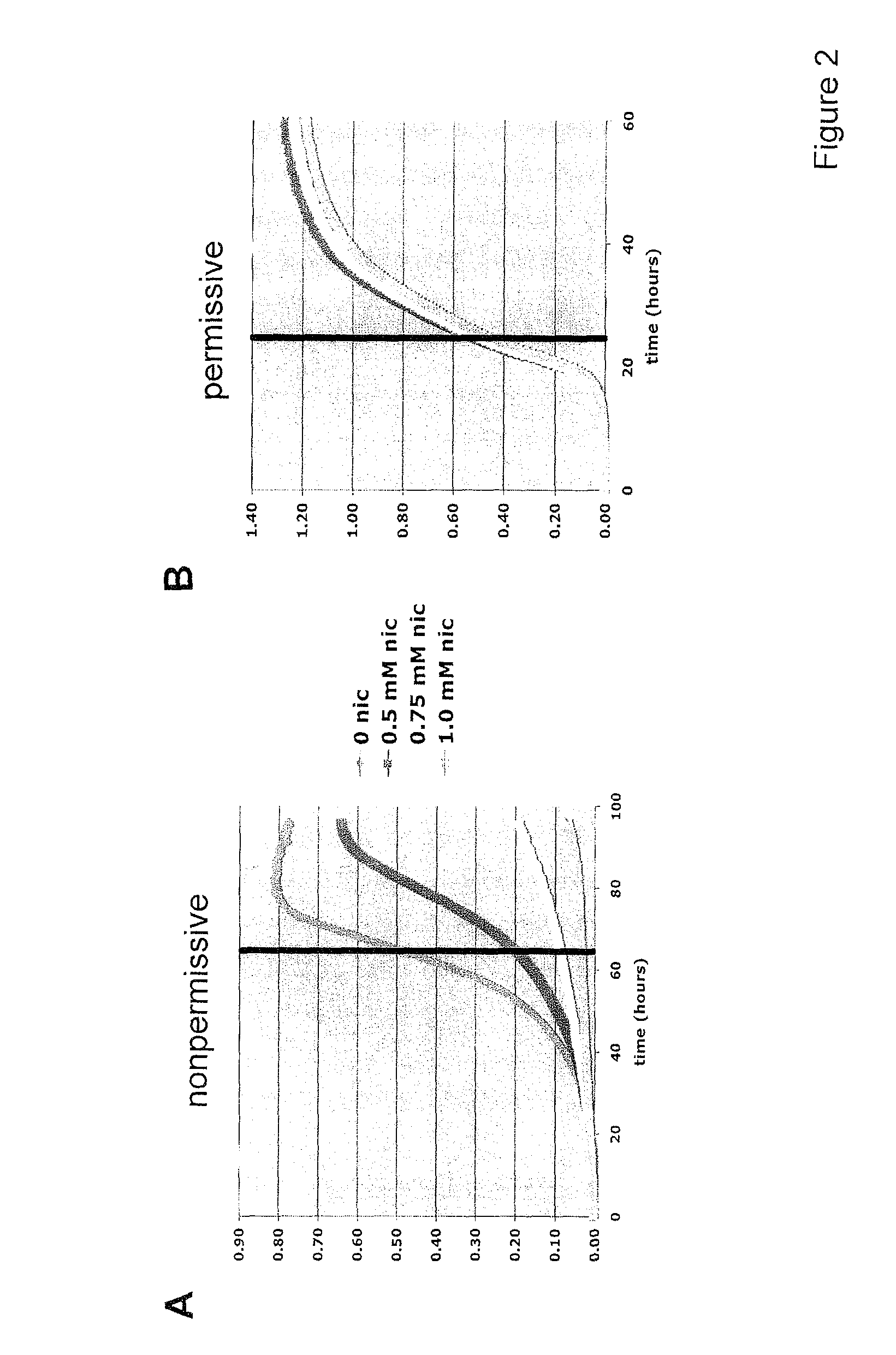
Method For Altering The Lifespan Of Eukaryotic Organisms
A method for altering the lifespan of a eukaryotic organism. The method comprises the steps of providing a lifespan altering compound, and administering an effective amount of the compound to a eukaryotic organism, such that the lifespan of the organism is altered. In one embodiment, the compound is identified using the DeaD assay.
Owner:UNIVERSITY OF ROCHESTER
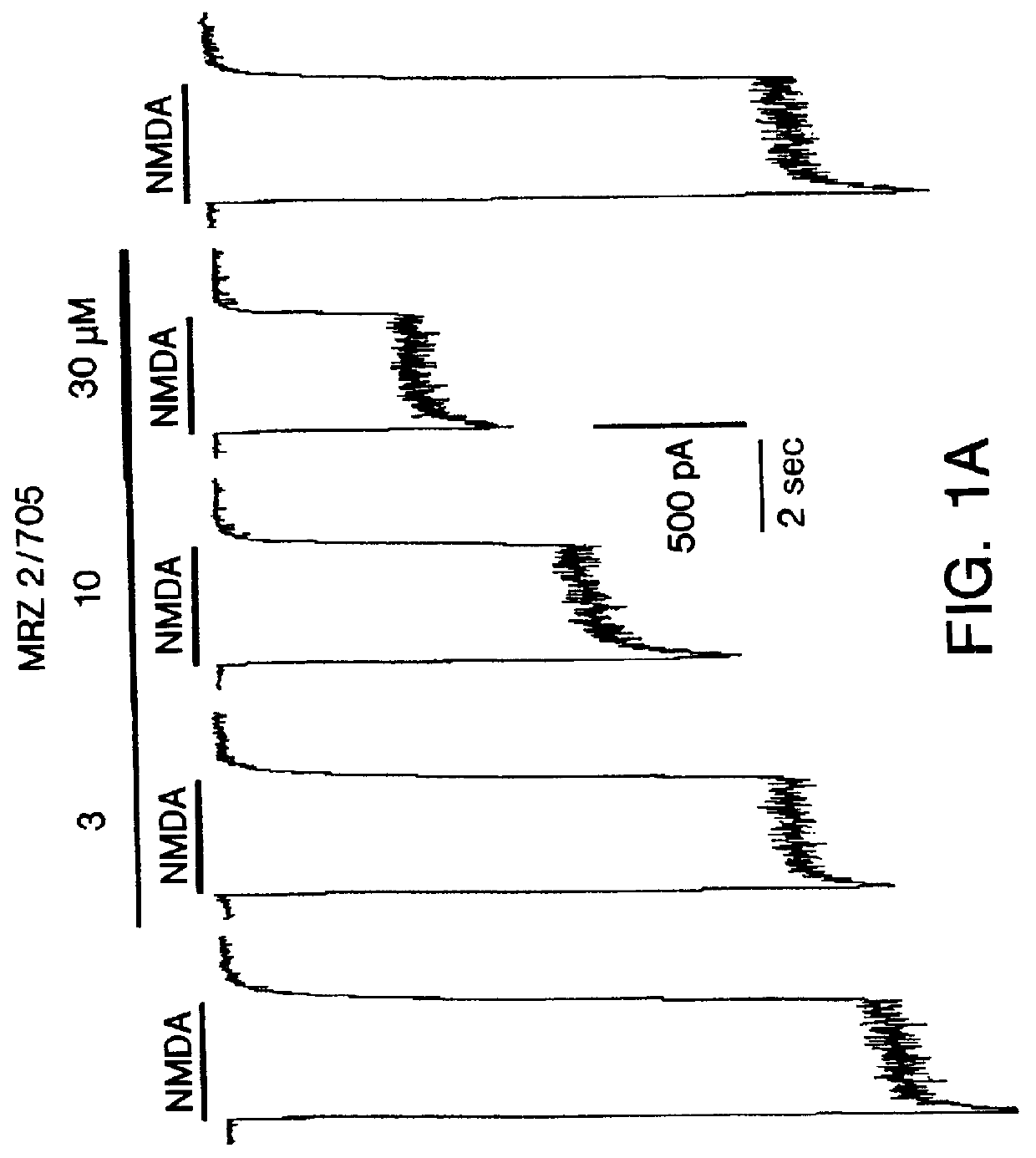
1-Amino-alkylcyclohexane NMDA receptor antagonists
Certain 1-aminoalkylcyclohexanes are systemically-active uncompetitive NMDA receptor antagonists having rapid blocking / unblocking kinetics and strong voltage-dependency and are therefore useful in the alleviation of conditions resulting from disturbances of glutamatergic transmission giving them a wide range of utility in the treatment of CNS disorders involving the same, as well as in non-NMDA indications, due to their immunomodulatory, antimalarial, anti-Borna virus, and anti-Hepatitis C activities and utilities. Pharmaceutical compositions thereof and a method-of-treating conditions which are alleviated by the employment of an NMDA receptor antagonist, as well as the aforementioned non-NMDA indications, and a method for the preparation of the active 1-aminoalkylcyclohexane compounds involved.
Owner:MERZ PHARMA GMBH & CO KGAA
Cationic antiseptic compositions and methods of use
ActiveUS20060051385A1Reduce eliminateReduce and eliminate clinical signAntibacterial agentsBiocideAmmonium compoundsCetylpyridinium
Antimicrobial compositions, especially those useful when applied topically, particularly to mucosal tissues (i.e., mucous membranes), including a cationic antiseptic such as biguanides and bisbiguanides such as chlorhexidine and its various salts including but not limited to the digluconate, diacetate, dimethosulfate, and dilactate salts; polymeric quaternary ammonium compounds such as polyhexamethylenebiguanide; silver and various silver complexes; small molecule quaternary ammonium compounds such as benzalkoium chloride and alkyl substituted derivatives; di-long chain alkyl (C8-C18) quaternary ammonium compounds; cetylpyridinium halides and their derivatives; benzethonium chloride and its alkyl substituted derivatives; and octenidine. The compositions can also include an enhancer component, a surfactant, a hydrophobic component, and / or a hydrophilic component. Such compositions provide effective topical antimicrobial activity and are accordingly useful in the treatment and / or prevention of conditions that are caused, or aggravated by, microorganisms (including viruses).
Owner:3M INNOVATIVE PROPERTIES CO
Microbicidal composition
Owner:DDP SPECIALTY ELECTRONICS MATERIALS US 8 LLC
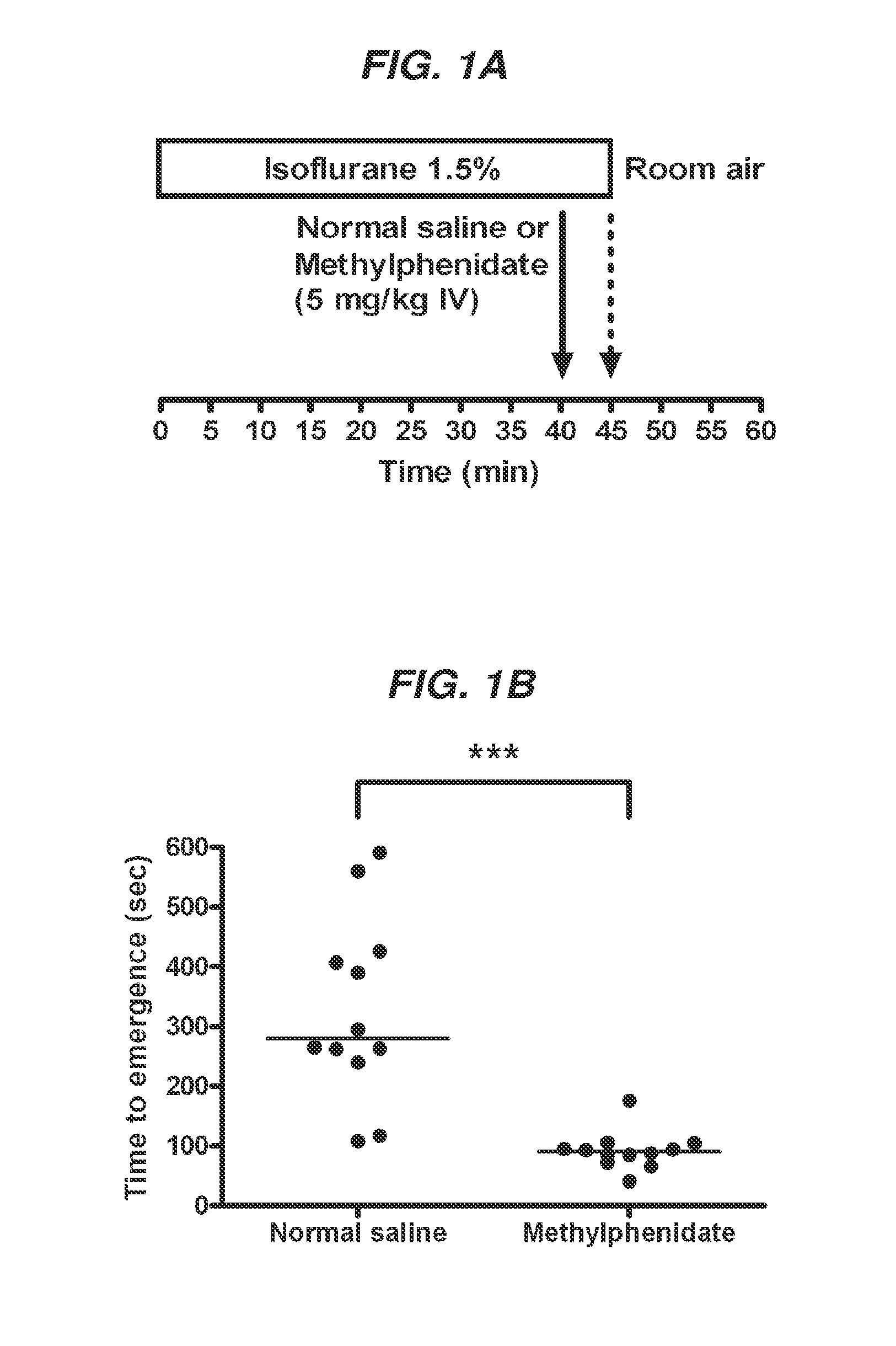
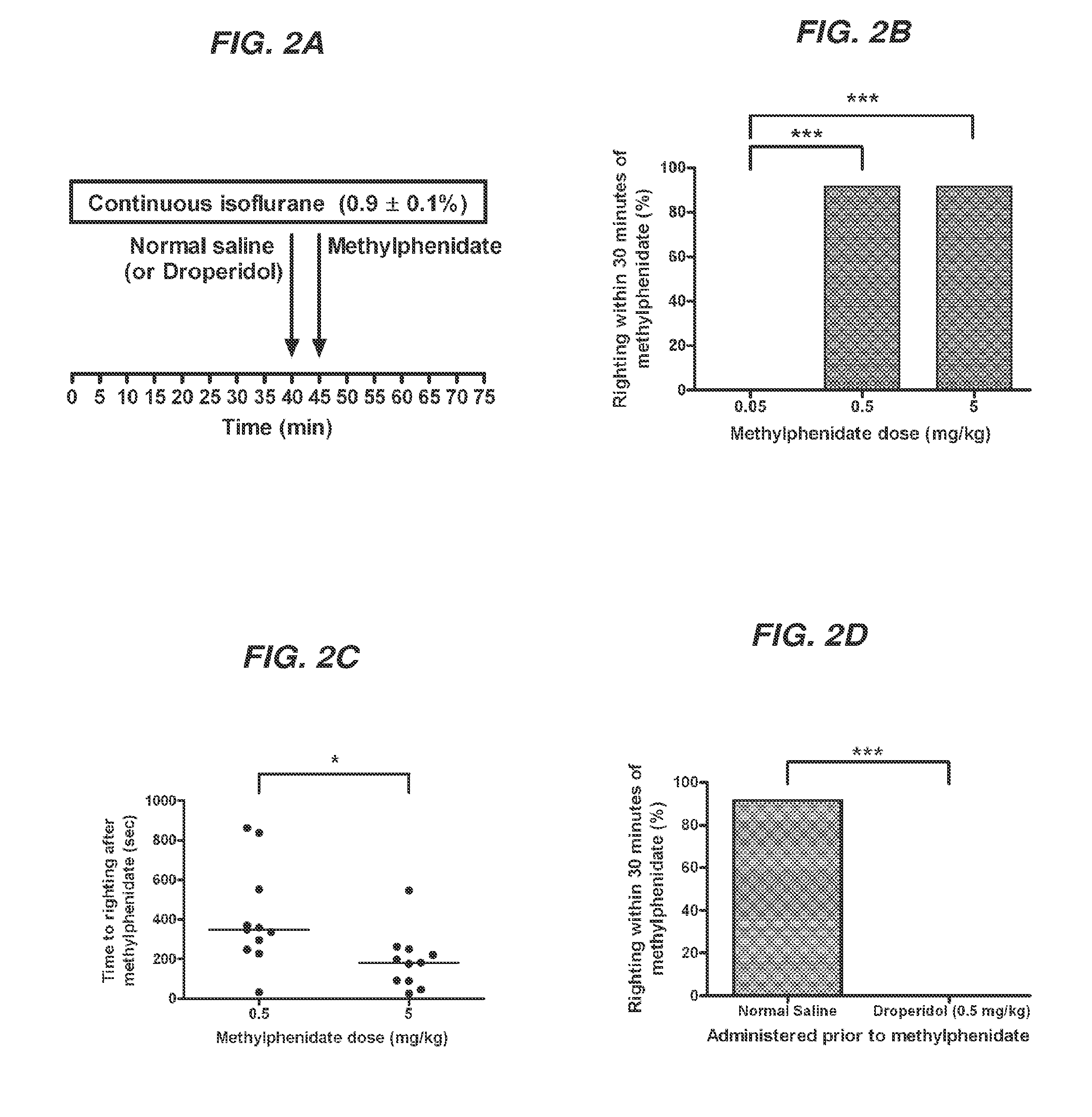
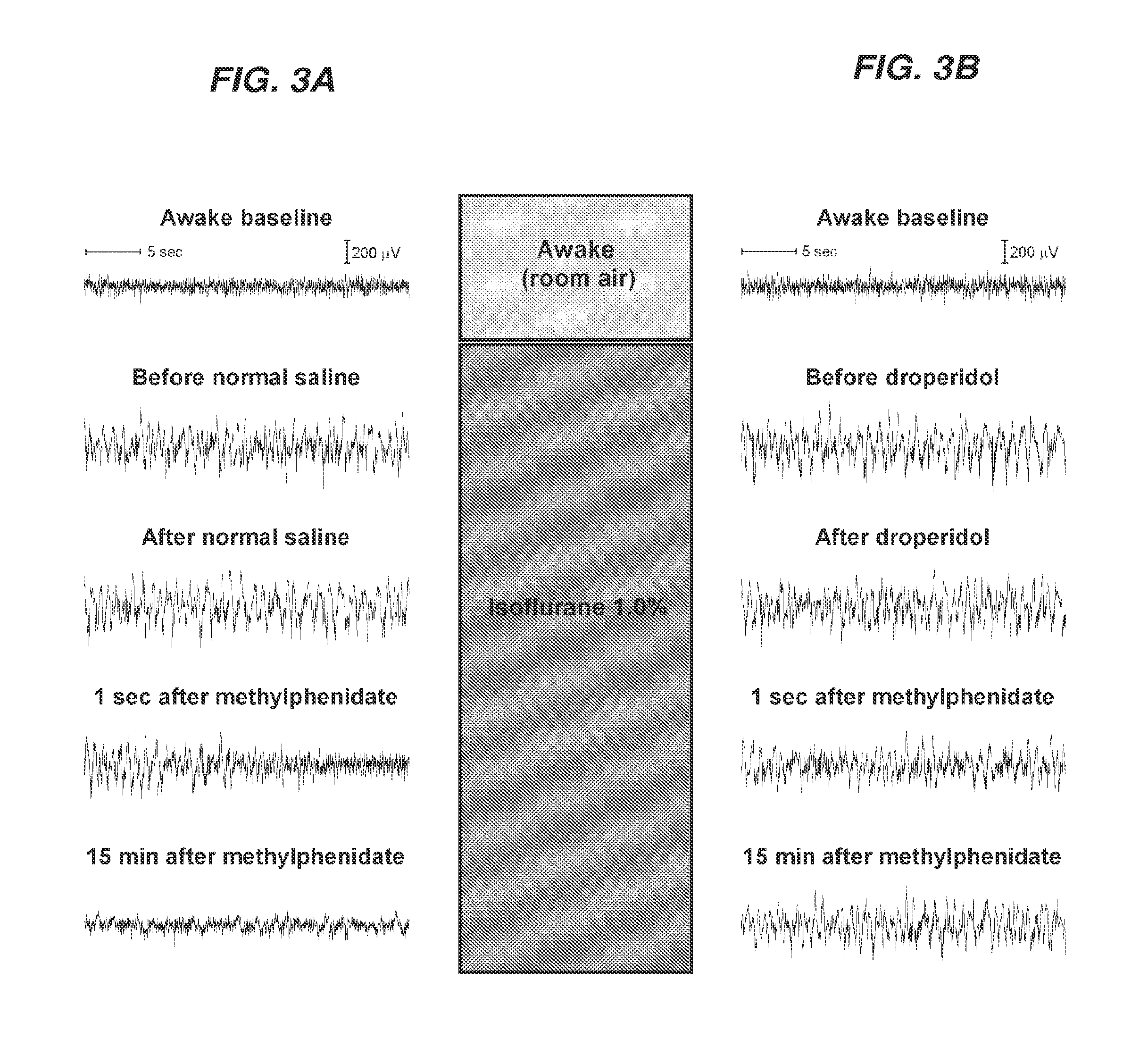
Reversal of General Anesthesia by Administration of Methylphenidate, Amphetamine, Modafinil, Amantadine, and/or Caffeine
ActiveUS20150196249A1High speedReduces and eliminates effectElectroencephalographyPharmaceutical delivery mechanismUnconsciousnessWhole body
Owner:THE GENERAL HOSPITAL CORP
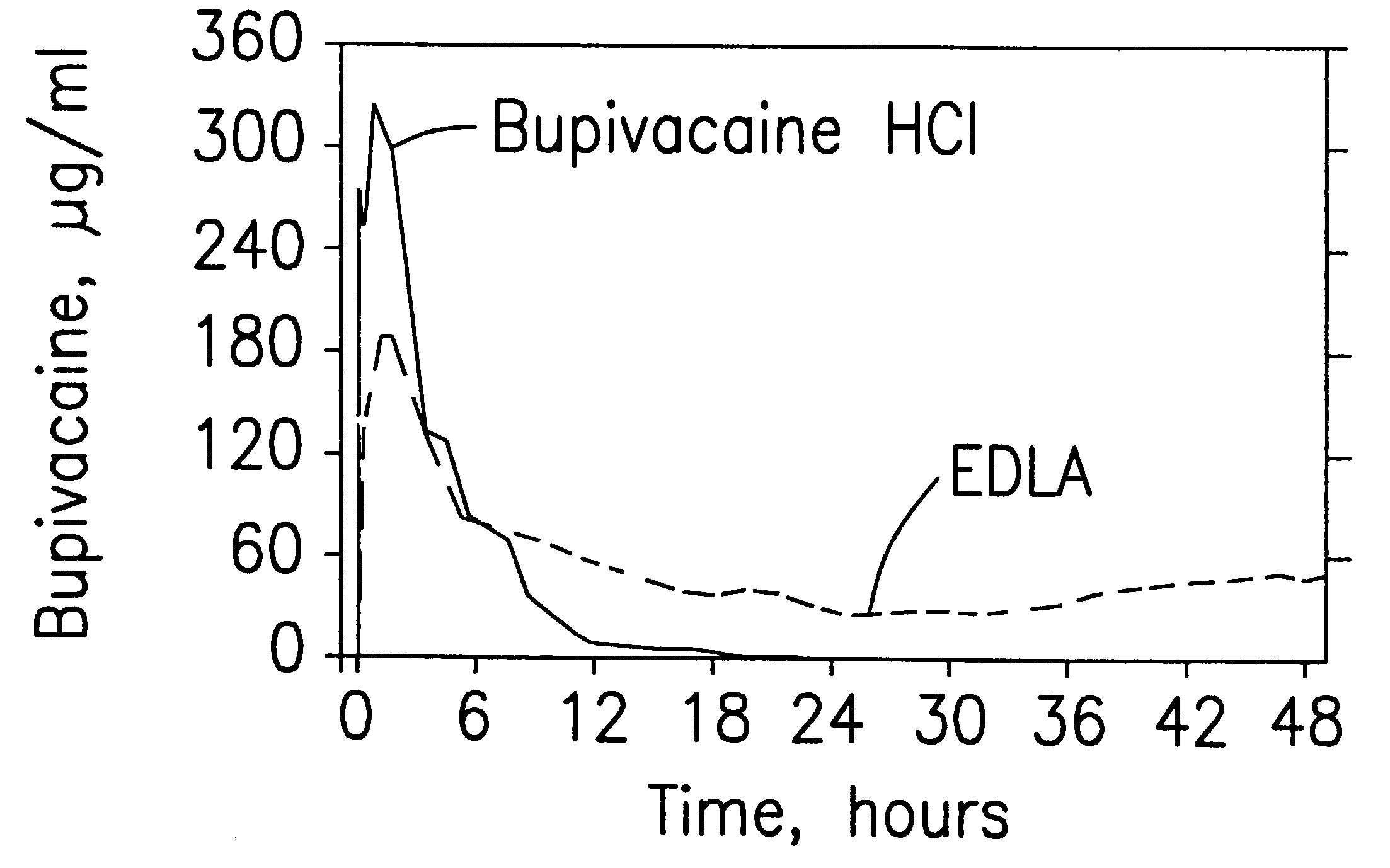
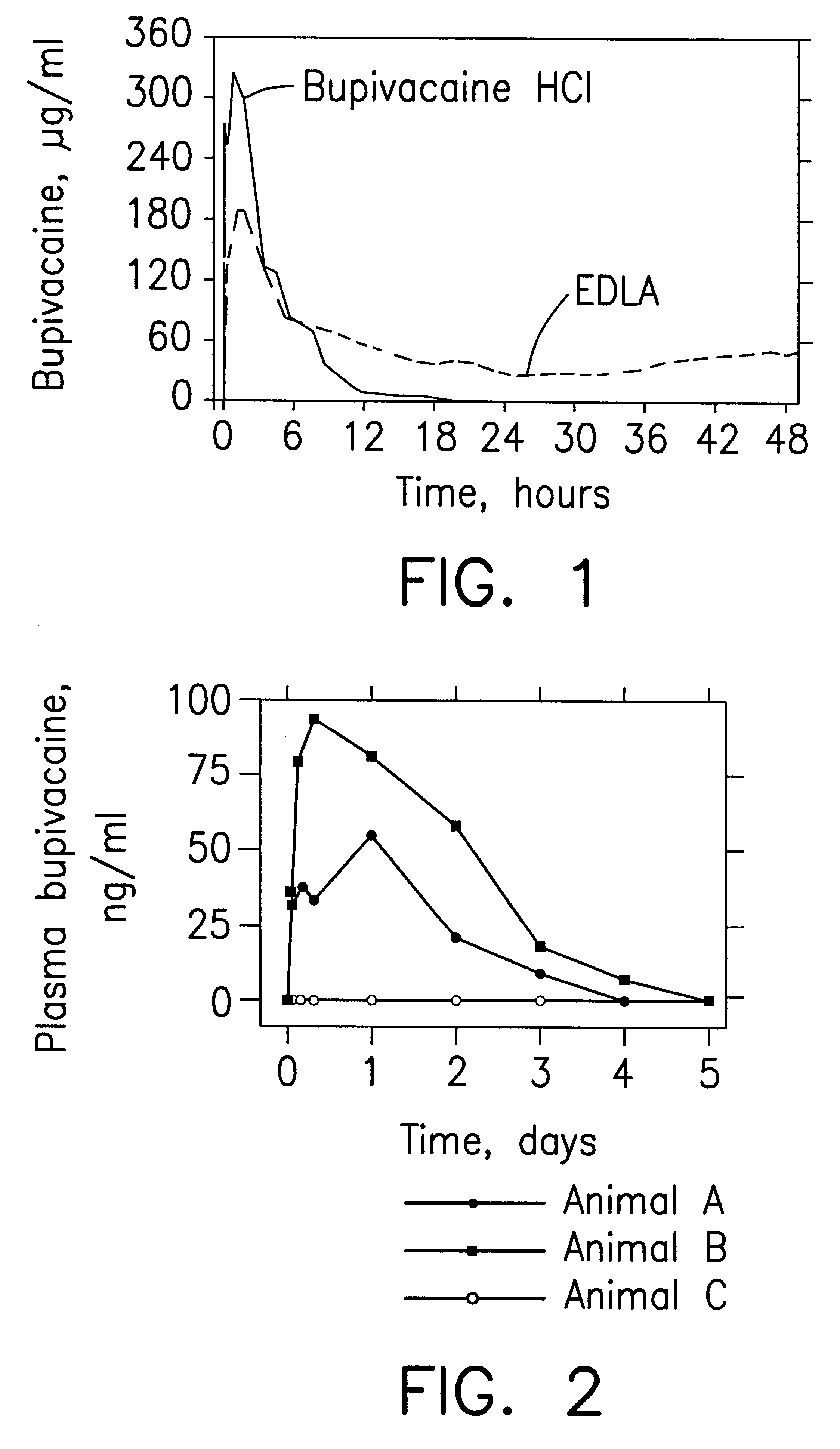
Prolonged anesthesia in joints and body spaces
InactiveUS6248345B1Enhance and prolong local anesthesiaImprovement in administrationInorganic non-active ingredientsAnaestheticsAnesthetic AgentPharmaceutical medicine
Sustained release local anesthetic formulations are administered intra articularly and / or into body spaces / cavities. The formulation is preferably a plurality of injectable microparticles including a local anesthetic and an effective amount of a biocompatible, biodegradable, sustained release material prolonging the release of the local anesthetic and optionally and a pharmaceutically acceptable, i.e., non-toxic, augmenting agent effective to prolong the duration of the local anesthesia for a time period longer than that obtainable without the augmenting agent.
Owner:PURDUE PHARMA LP
Method for altering the lifespan of eukaryotic organisms
A method for altering the lifespan of a eukaryotic organism. The method comprises the steps of providing a lifespan altering compound, and administering an effective amount of the compound to a eukaryotic organism, such that the lifespan of the organism is altered. In one embodiment, the compound is identified using the DeaD assay.
Owner:UNIVERSITY OF ROCHESTER
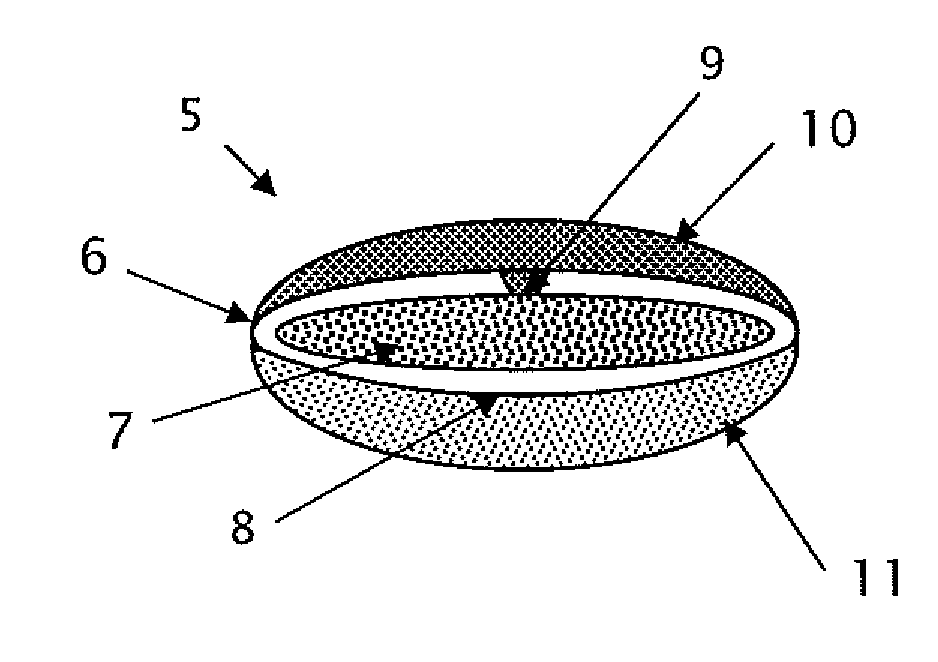
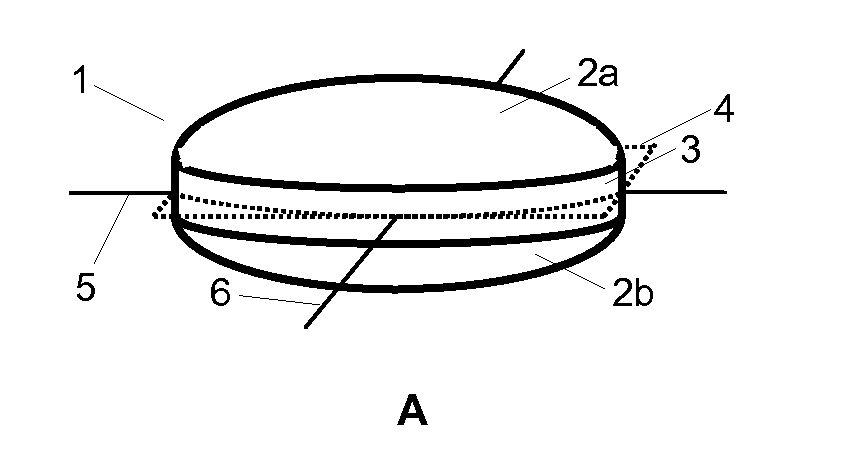
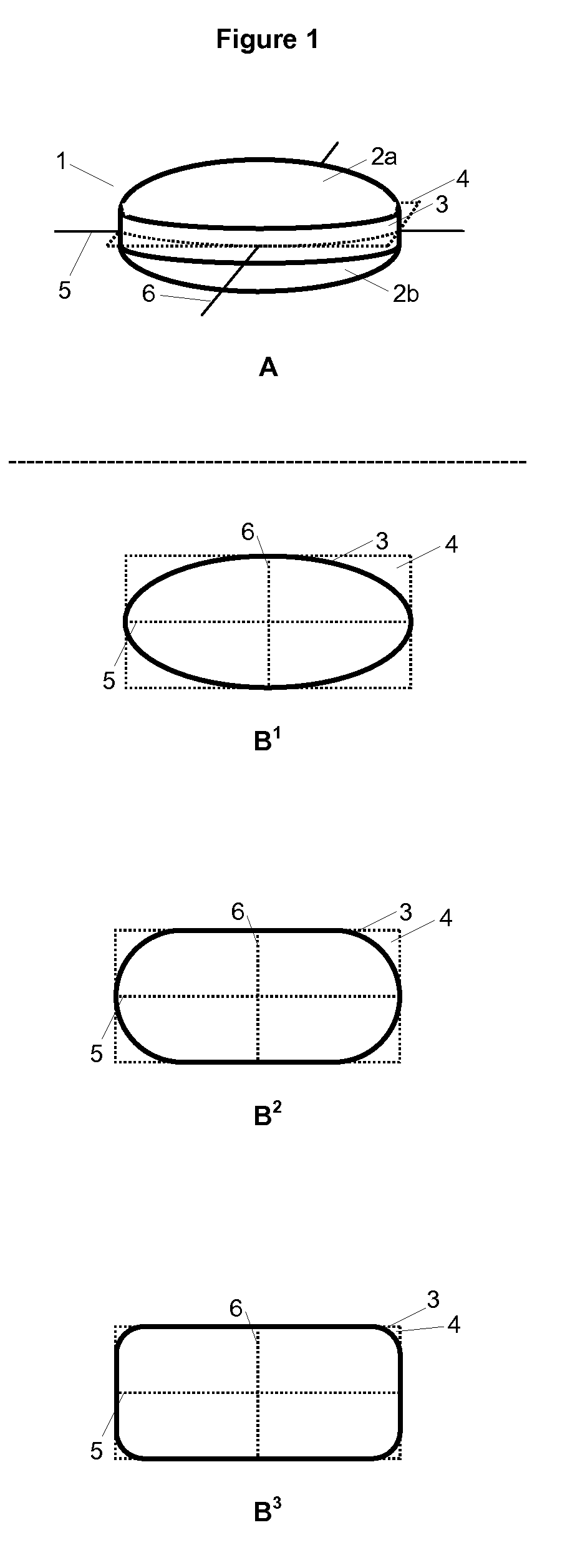
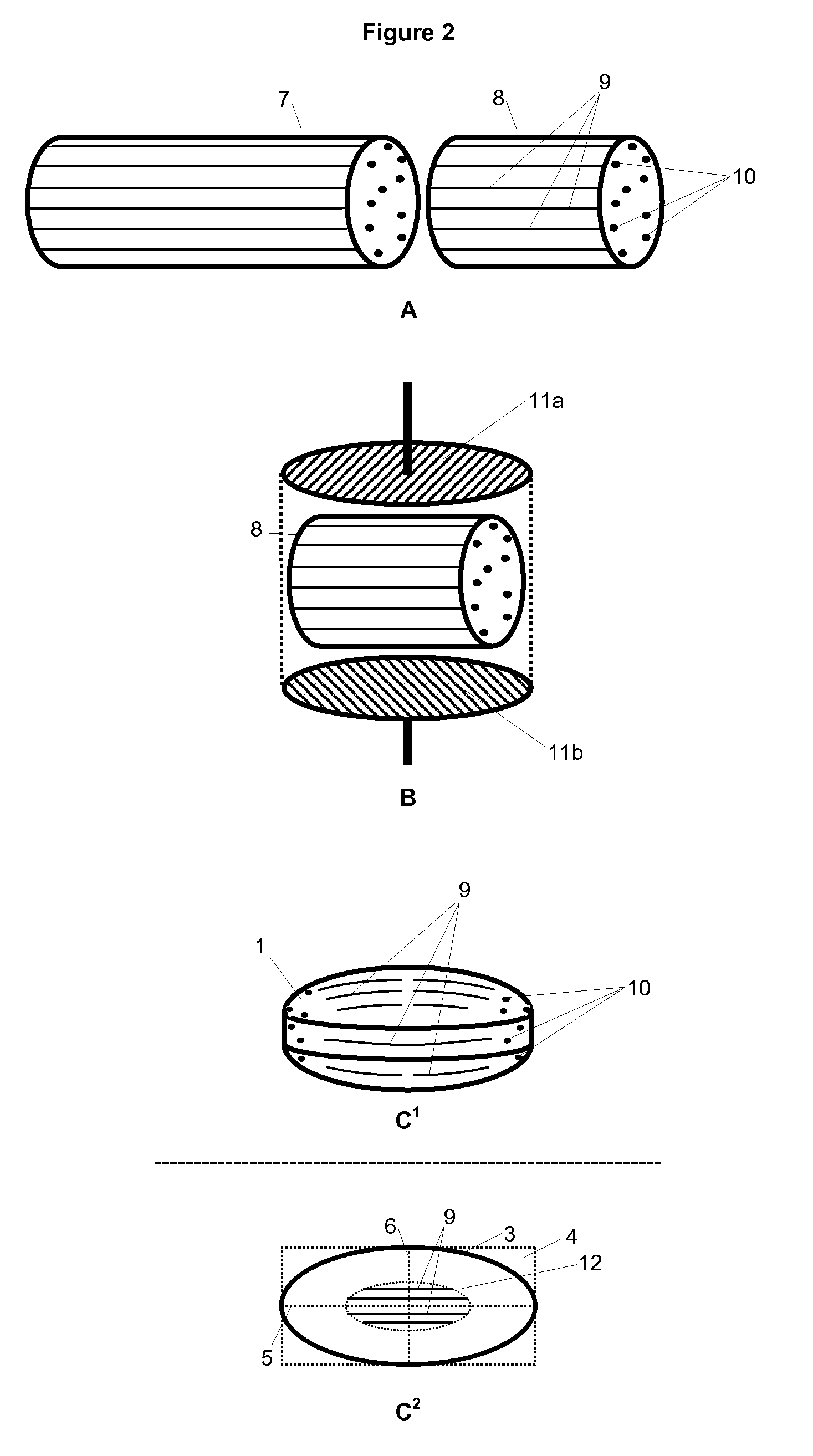
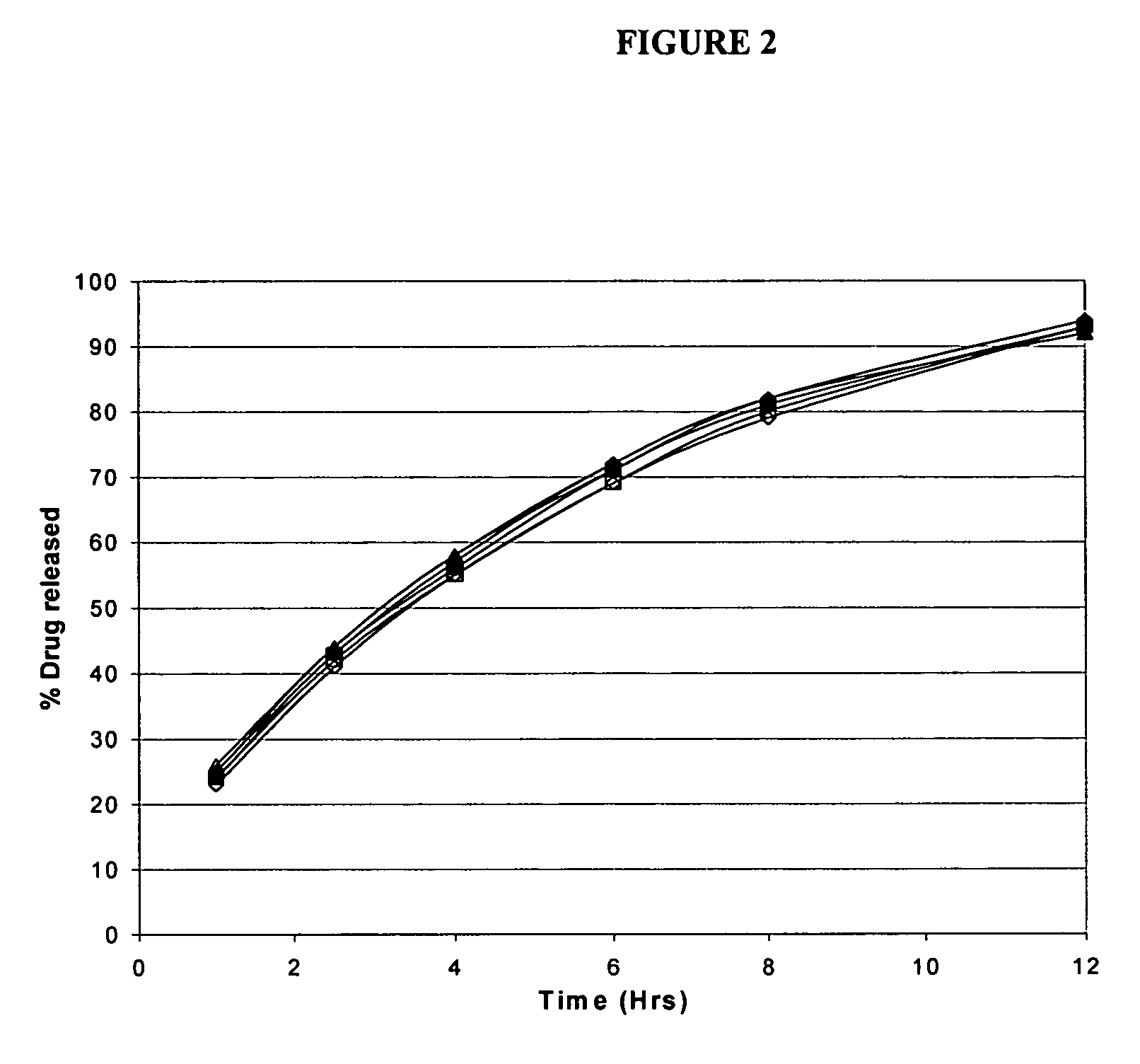
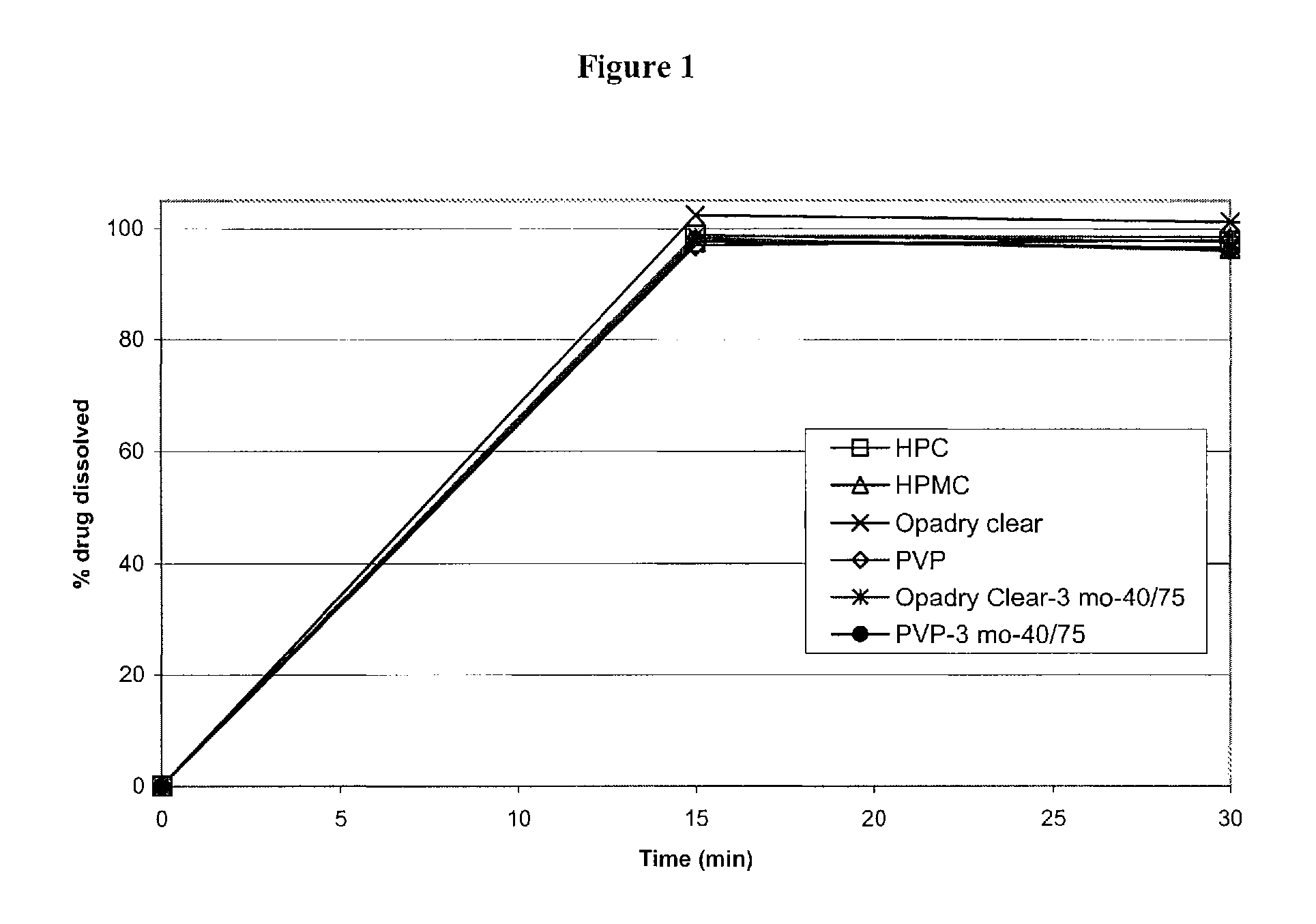
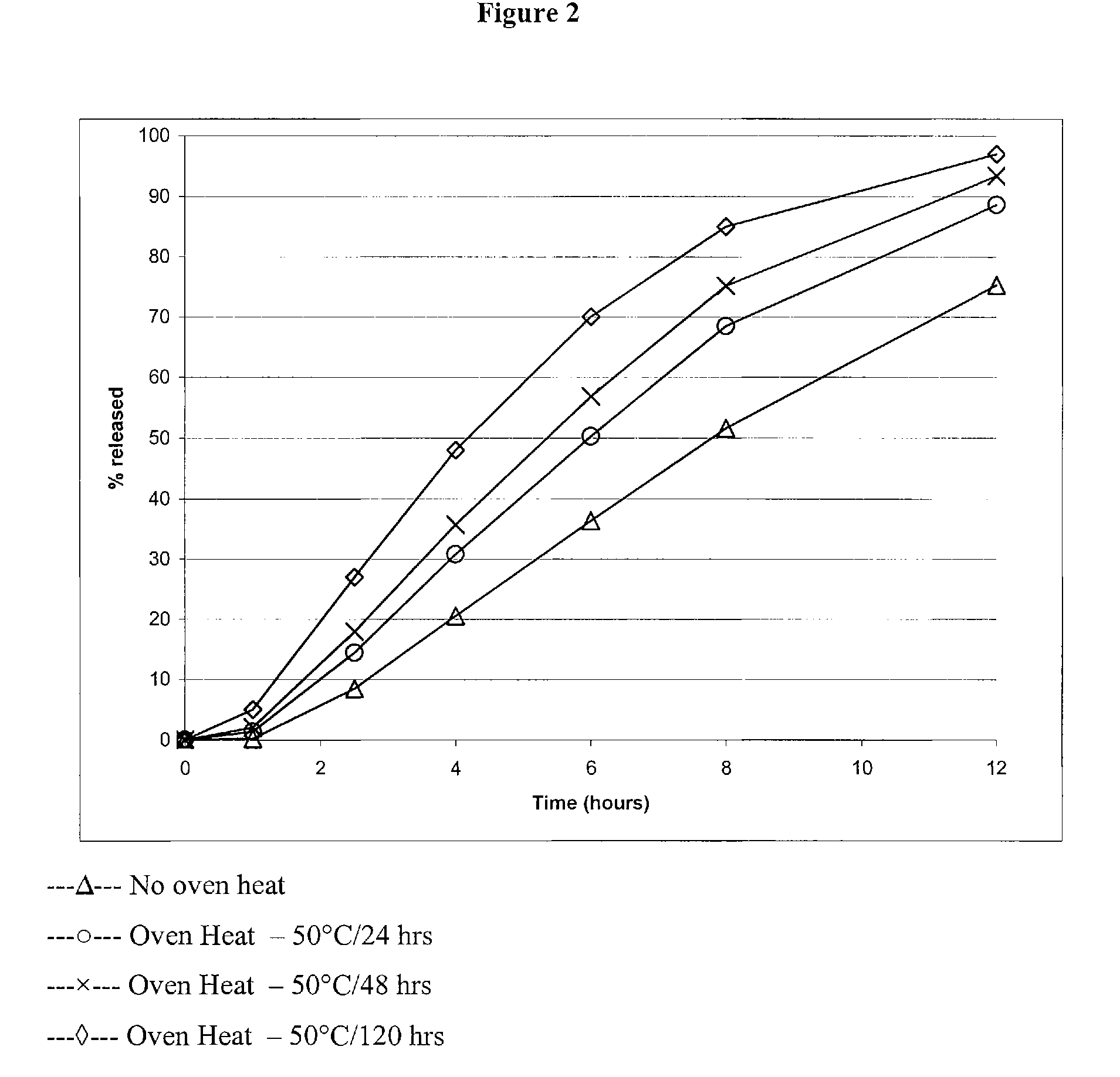
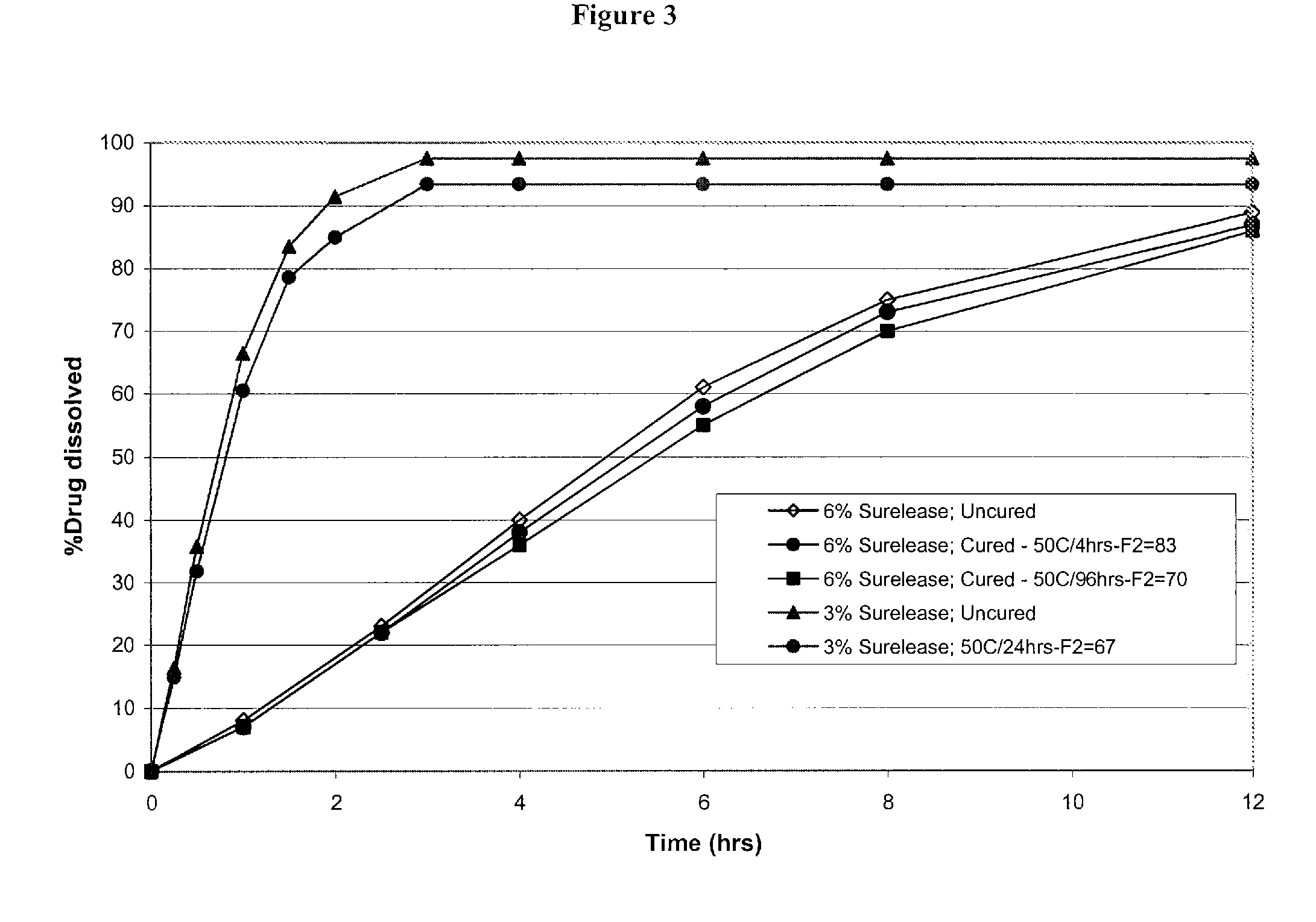
Triple Combination Release Multi-Layered Tablet
This invention pertains to a multi-layered tablet for a triple combination release of active agents to an environment of use. More particularly, the invention pertains to a multi-layered tablet (1) comprising two external drug-containing layers (2 and 3) in stacked arrangement with respect to and on opposite sides of an oral dosage form (4) that provides a triple combination release of at least one active agent. In one embodiment of the invention the dosage form is an osmotic device. In another embodiment of the invention the dosage form is a gastro-resistant coated core. In yet another embodiment of the invention the dosage form is a matrix tablet. In a different embodiment the dosage form is a hard capsule.
Owner:ACELLA HLDG LLC +1
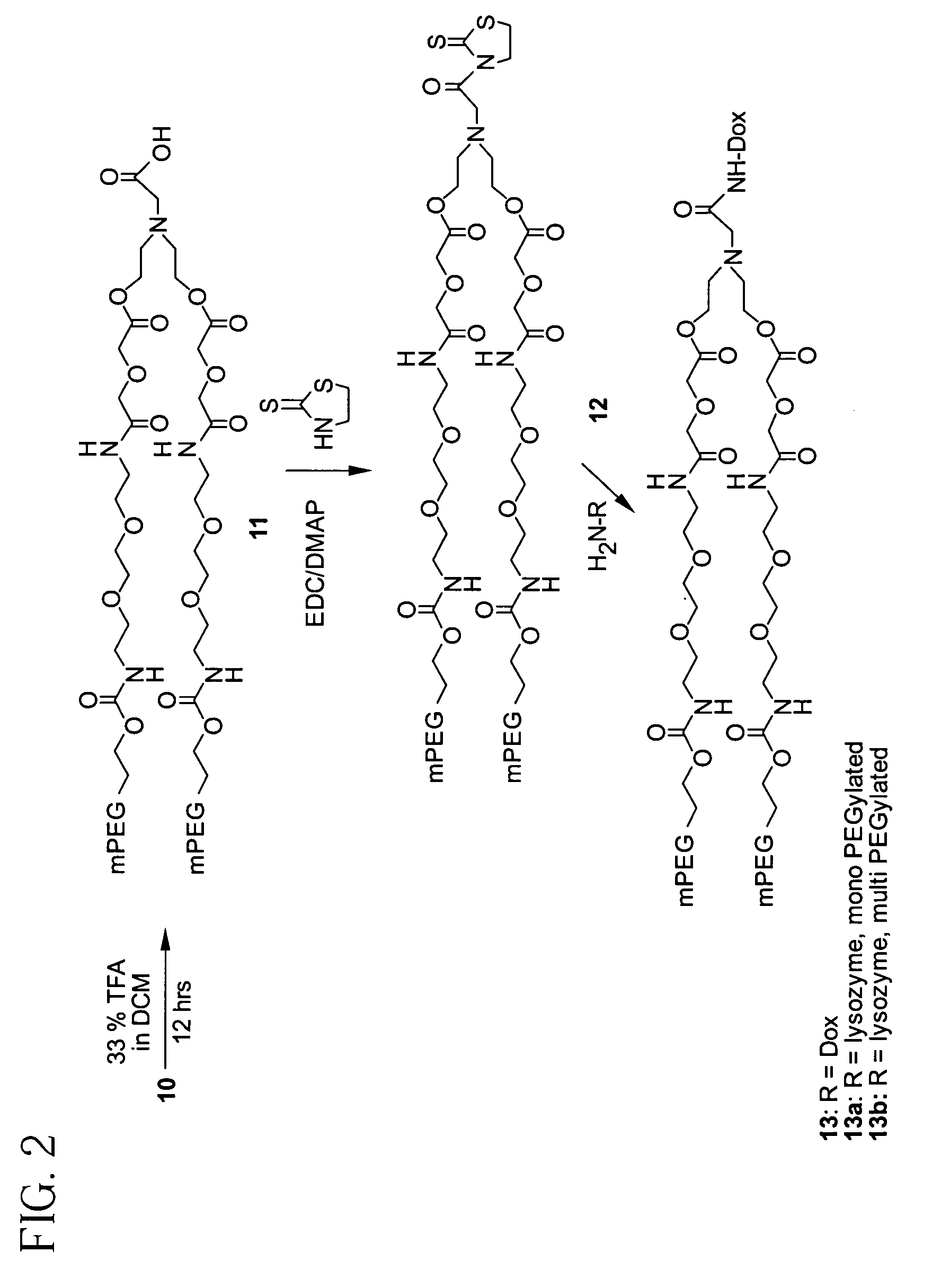
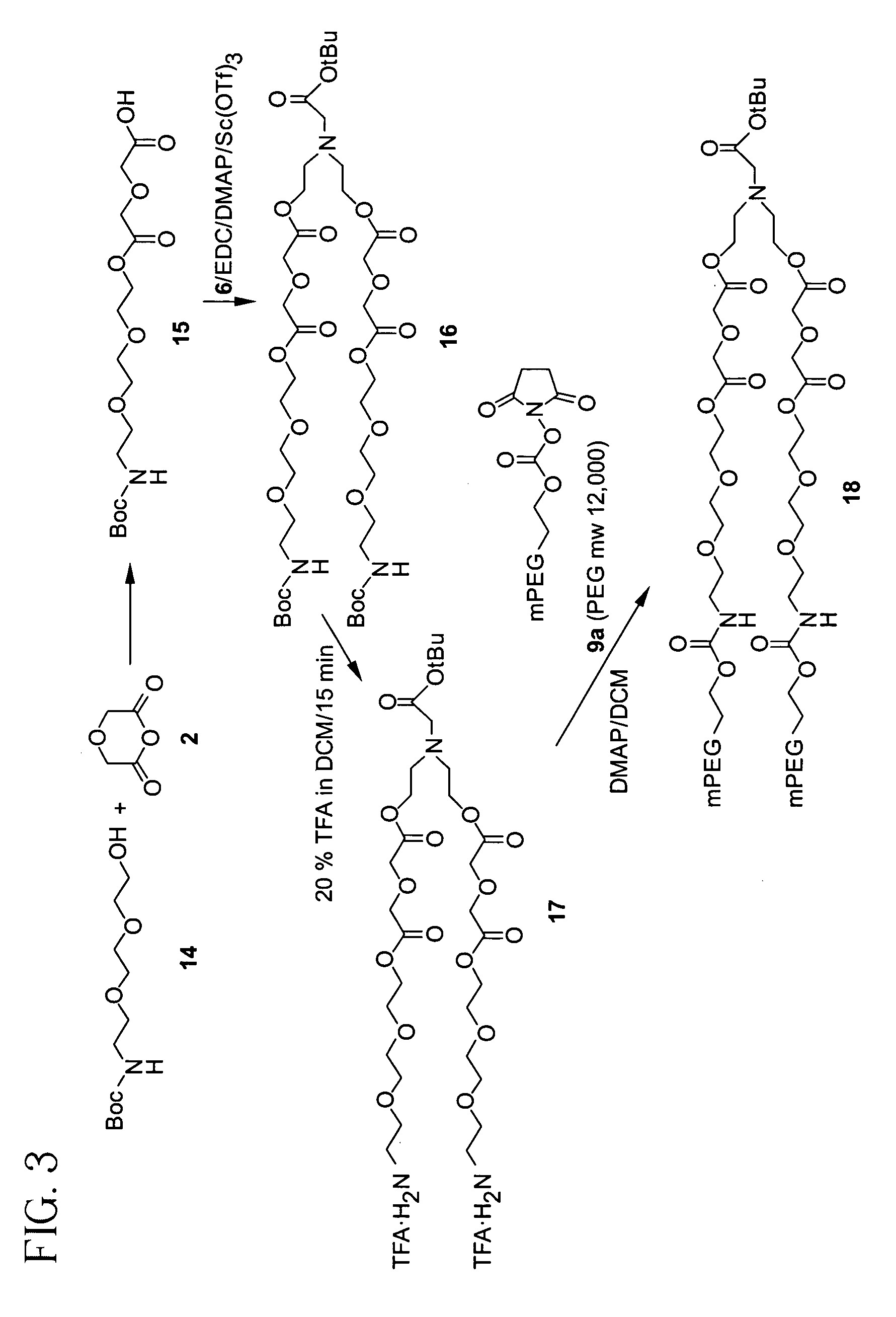
Releasable polymeric conjugates based on aliphatic biodegradable linkers
InactiveUS7087229B2Modulate the rate of hydrolysis of the prodrug and/or cellular uptakeGood water solubilityBiocideEther/acetal active ingredientsPolymerStereochemistry
Owner:BELROSE PHARMA
Gentle-acting skin disinfectants
InactiveUS6846846B2Minimize skin irritationUnexpected antimicrobial effectivenessCosmetic preparationsBiocideOctoxyglycerinMedicine
Antimicrobial compositions having synergistic combinations of octoxyglycerin and at least one other antimicrobial agent in formulations which are more effective than prior art compositions without causing increased irritation to the skin of the average user. In certain embodiments, skin irritation may be minimized by low concentrations of antimicrobials and / or the presence of soothing compounds such as zinc. Preferred embodiments include combinations of octoxyglycerin, a quaternary compound, and at least one other antimicrobial agent. Without being bound to any particular theory, it is hypothesized that the unexpected antimicrobial effectiveness of combinations of octoxyglycerin may result from an enhancement of the permeability of microbes to antimicrobials caused by octoxyglycerin.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
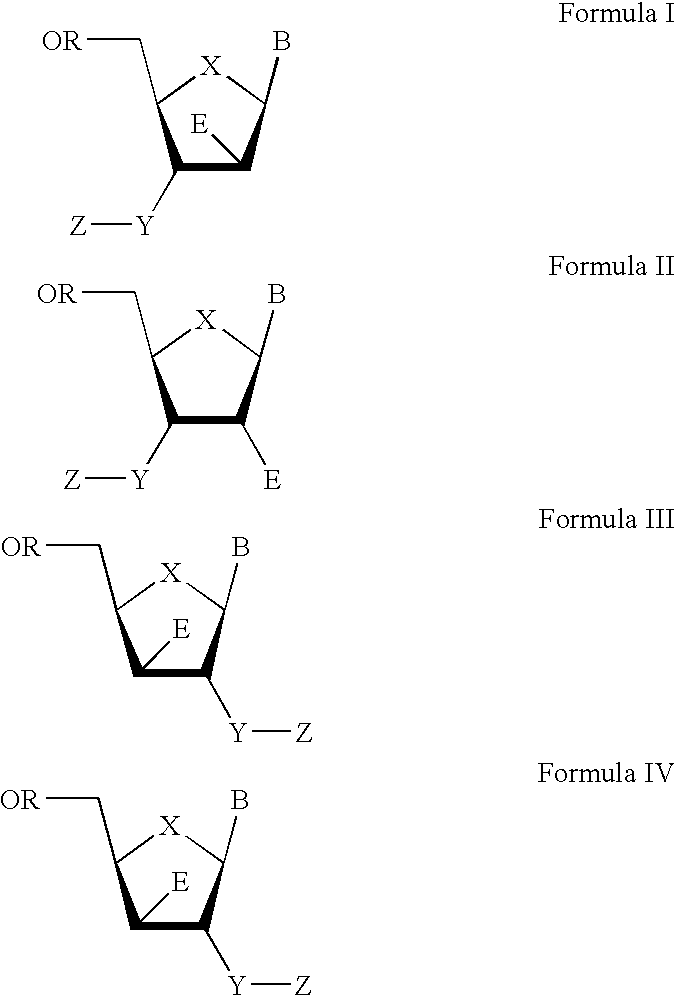
3′-or 2′-hydroxymethyl substituted nucleoside derivatives for treatment of hepatitis virus infections
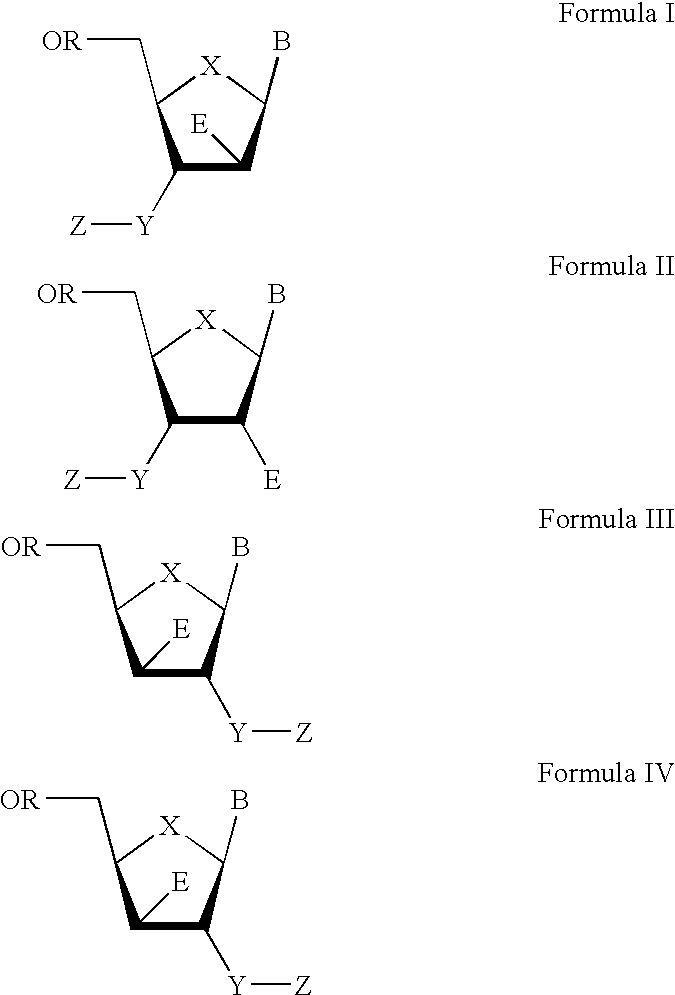
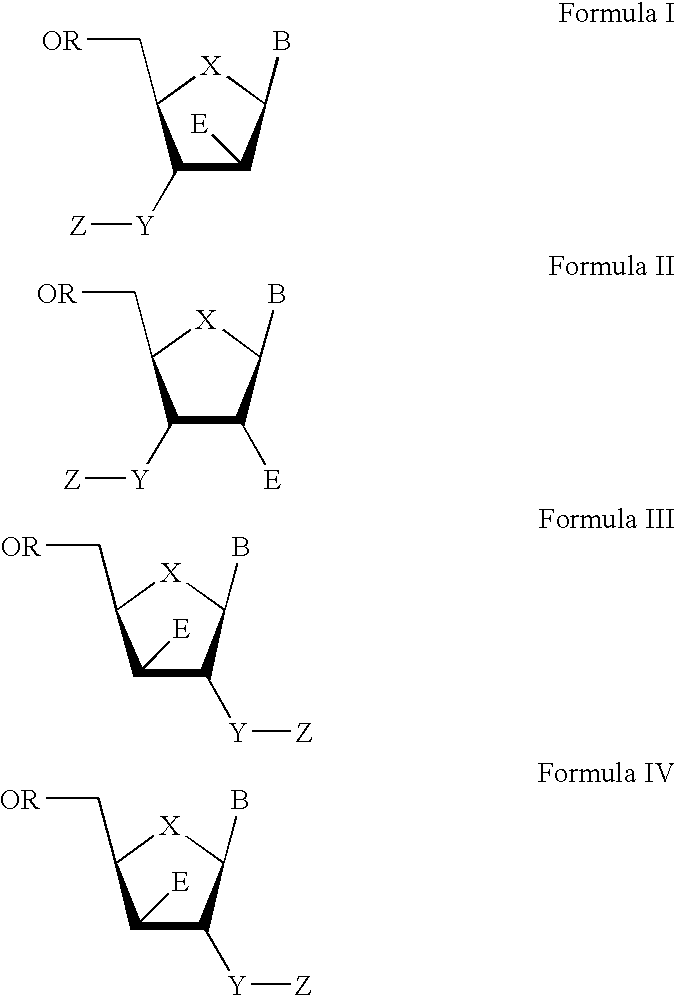
The present invention relates to a composition for and a method of treating hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, hepatitis D virus (HDV) infection or a proliferative disorder in a patient using an effective amount of a compound selected from the group consisting of formulas [I]–[IV] below and mixtures of two or more thereof:wherein the substituents are as defined herein. Pharmaceutical compositions comprising these compounds in combination with other HBV, HCV, or HDV agents is also disclosed.
Owner:PHARMASSET
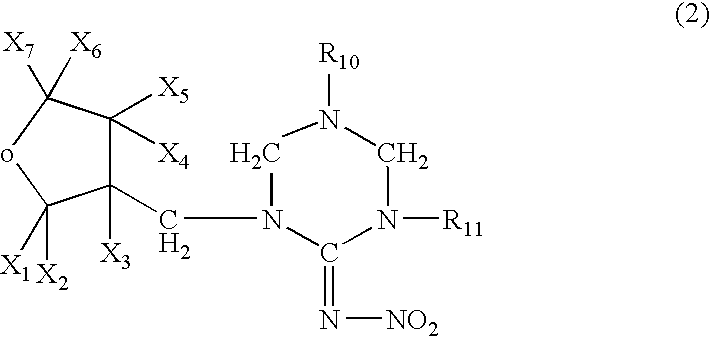
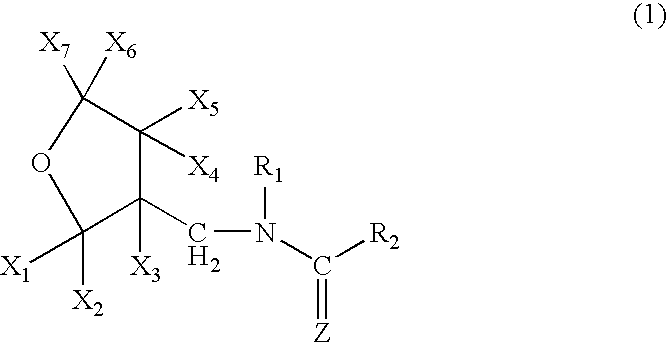
Amine Compounds
InactiveUS20080200535A1Potent immunosuppressive actionBiocideSenses disorderUveitisAutoimmune disease
There is provided a compound exhibiting an activity of suppressing immune response with reduced adverse drug reactions, which compound is useful in the chemotherapy for preventing or treating, for example, a wide range of various autoimmune diseases including systemic erythematodes, chronic rheumatoid arthritis, Type I diabetes, inflammatory bowel disease, biliary cirrhosis, uveitis, multiple sclerosis or other disorders, or chronic inflammatory diseases, or cancers, lymphoma or leukemia, or resistance to organ or tissue transplantation or rejection against transplantation.Novel amine compounds having an S1P1 / Edg1 receptor agonist effect, possible stereoisomers or racemic bodies of the compounds, or pharmacologically acceptable salts, hydrates or solvates of the compound, the stereoisomers or the racemic bodies, or prodrugs of the compounds, the stereoisomers, the racemic bodies, the salts, the hydrates or the solvates, are provided.
Owner:ASAHI KASEI PHARMA
Topical nitric oxide donor compositions
InactiveUS6287601B1Reduced and failing organ functionPoor appetitePowder deliveryBiocideLipid formationEquine Species
Compositions and methods of the topical treatment of equine laminitis are disclosed. In particular, combinations of a fast acting nitric oxide (NO) donor, a sustained acting NO donor and an NSAID mixed in a lipid-based carrier are described. The application of such combinations to the affected areas, e.g., the hoofs and surrounding tissues, of an equine afflicted with laminitis provides relief from the debilitating effects of this painful, often life-threatening condition.
Owner:STREHKEHN INT LTD
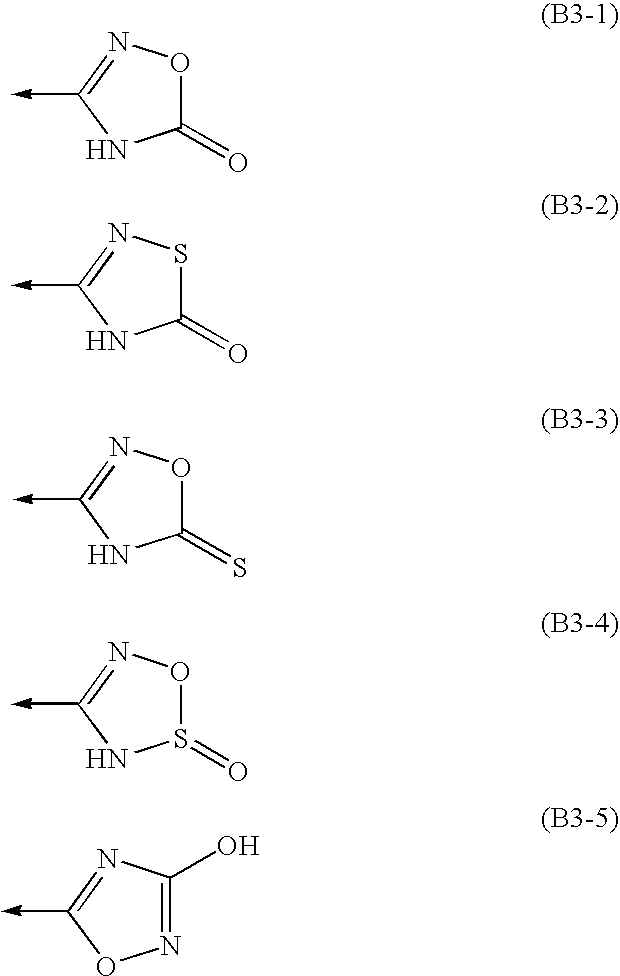
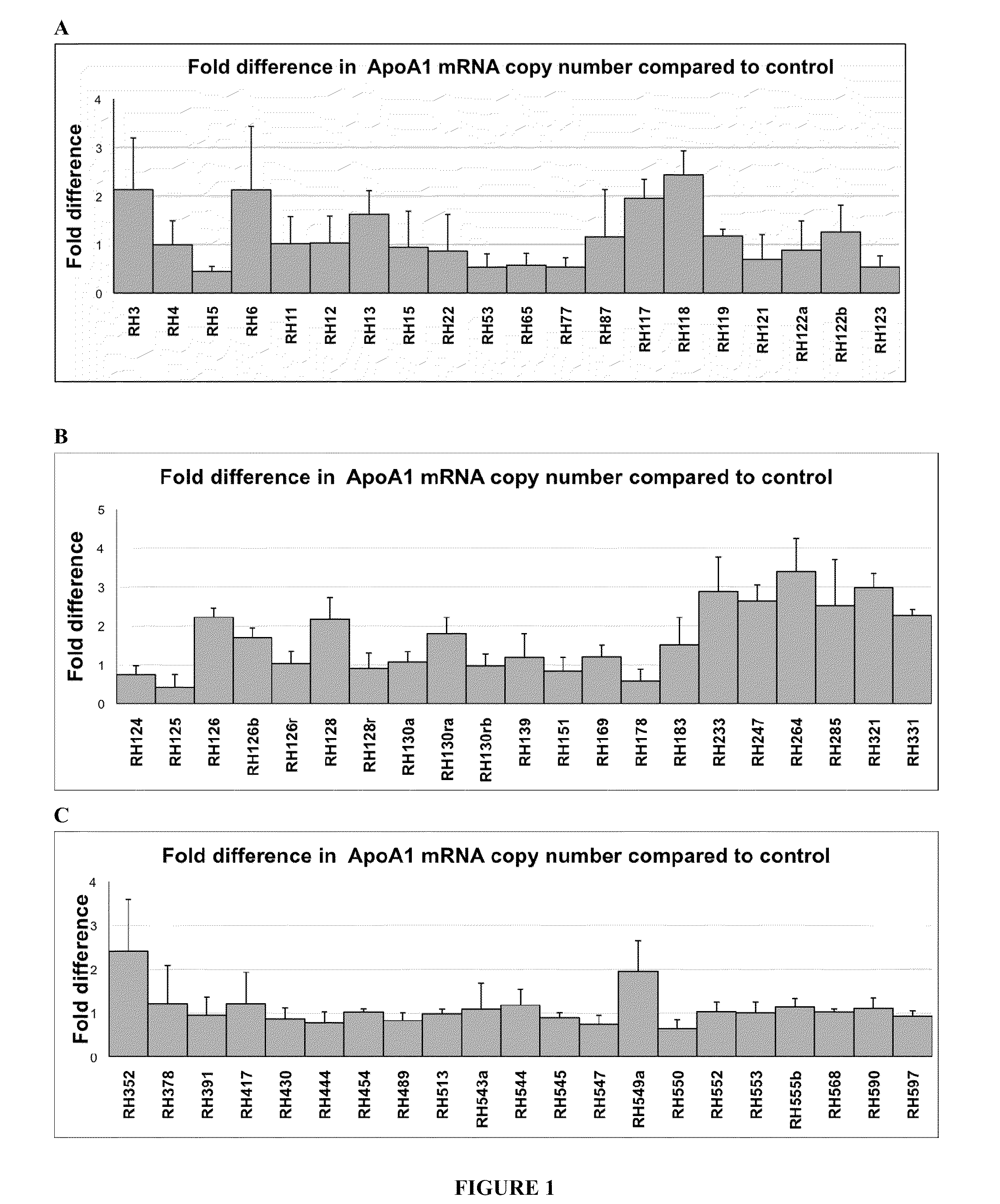
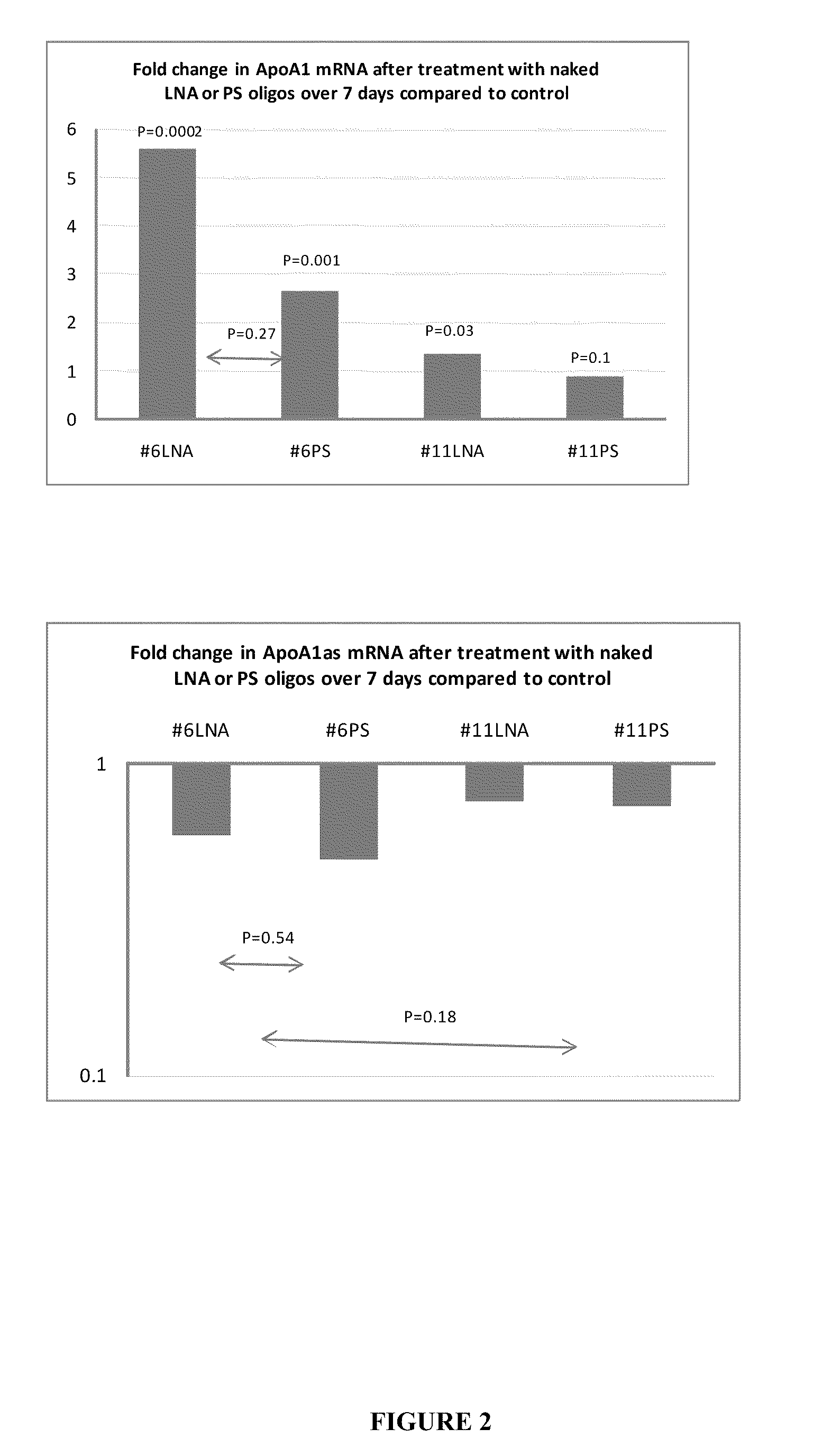
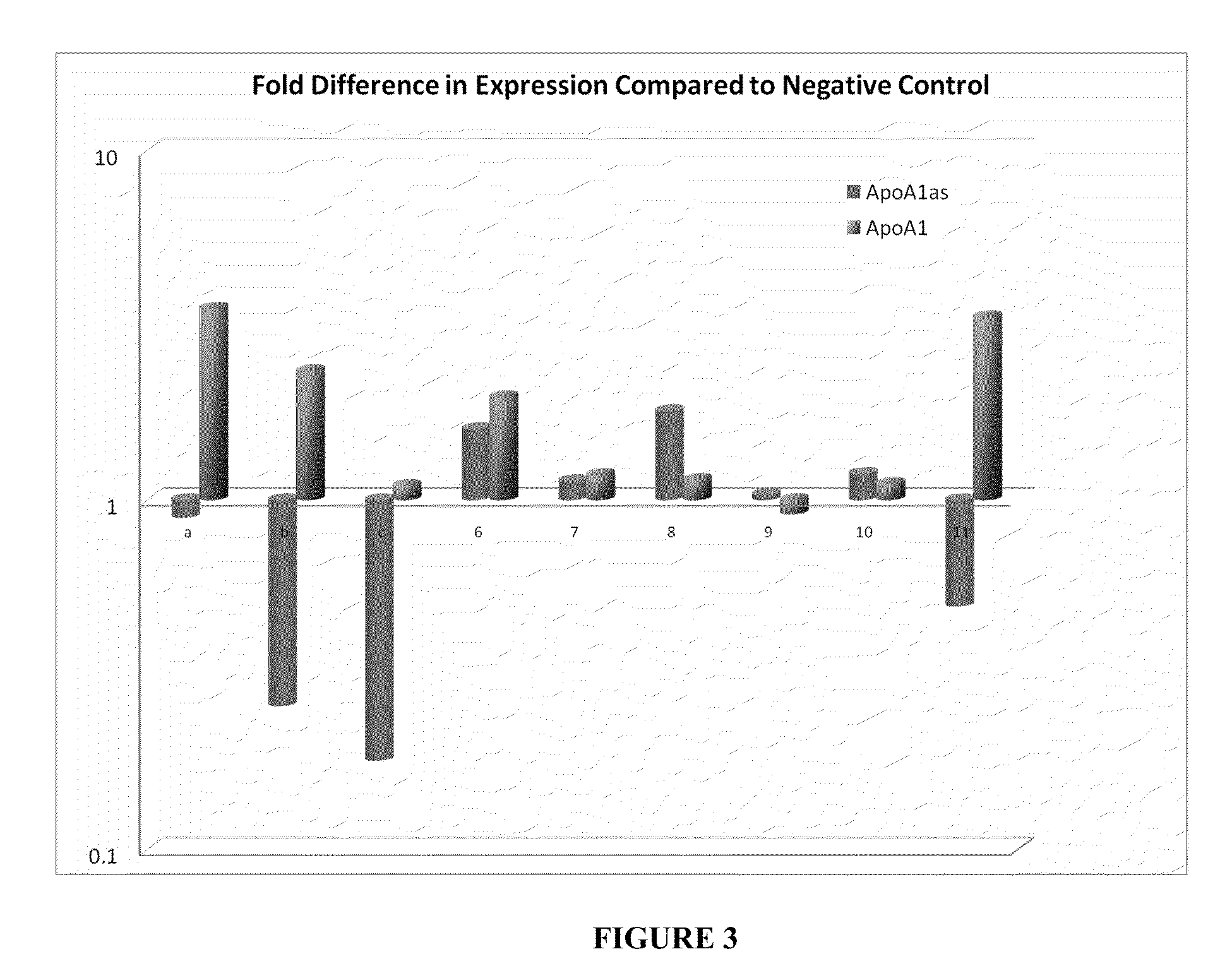
Treatment of Apolipoprotein-A1 Related Diseases by Inhibition of Natural Antisense Transcript to Apolipoprotein-A1
ActiveUS20100105760A1Modulate its functionNervous disorderAntipyreticApolipoproteins EPolynucleotide
Oligonucleotide compounds modulate expression and / or function of an apolipoprotein (ApoA1) polynucleotides and encoded products thereof. Methods for treating diseases associated with apolipoprotein-A1 (ApoA1) comprise administering one or more Oligonucleotide compounds designed to inhibit the Apo-A1 natural antisense transcript to patients.
Owner:CURNA INC
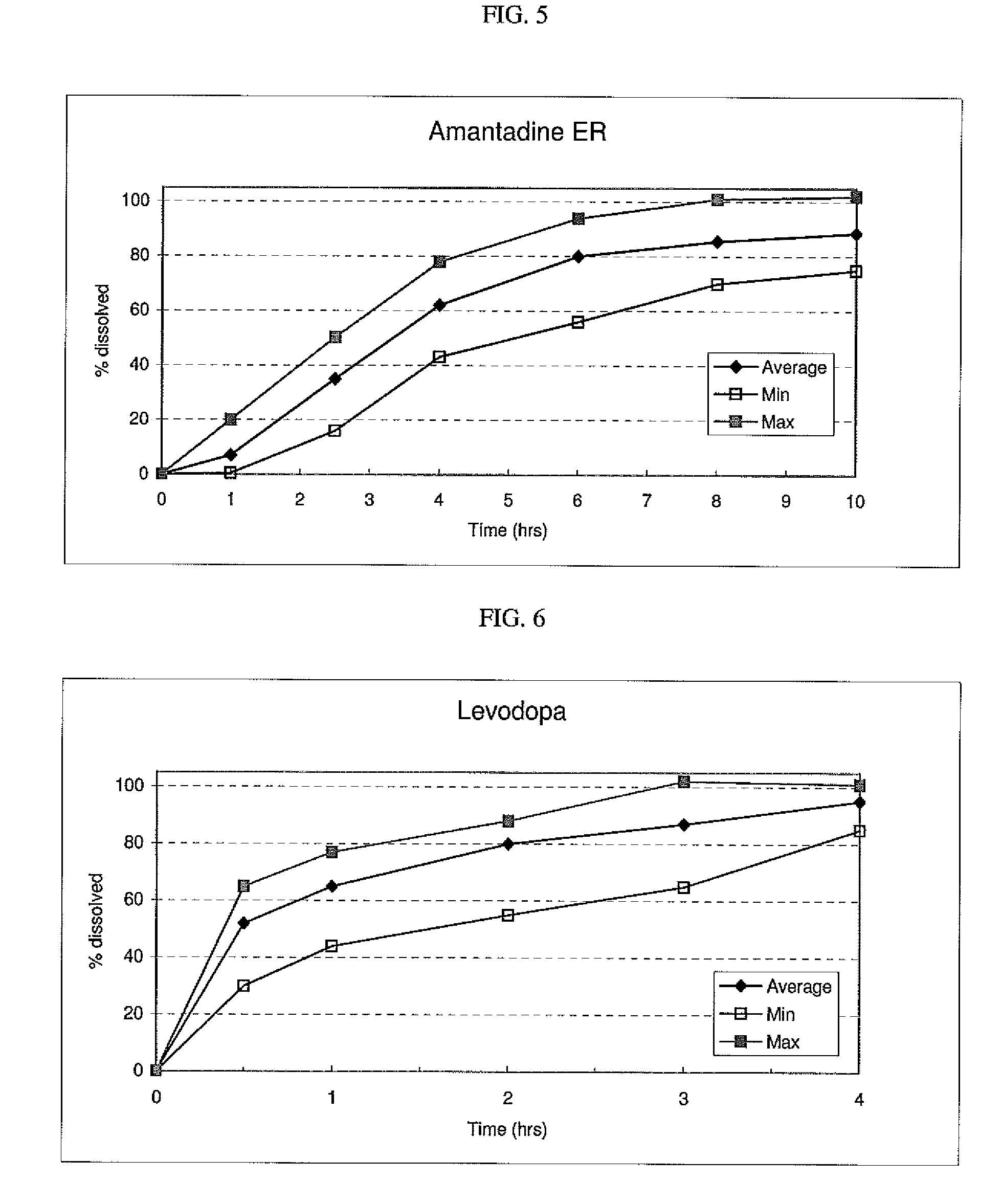
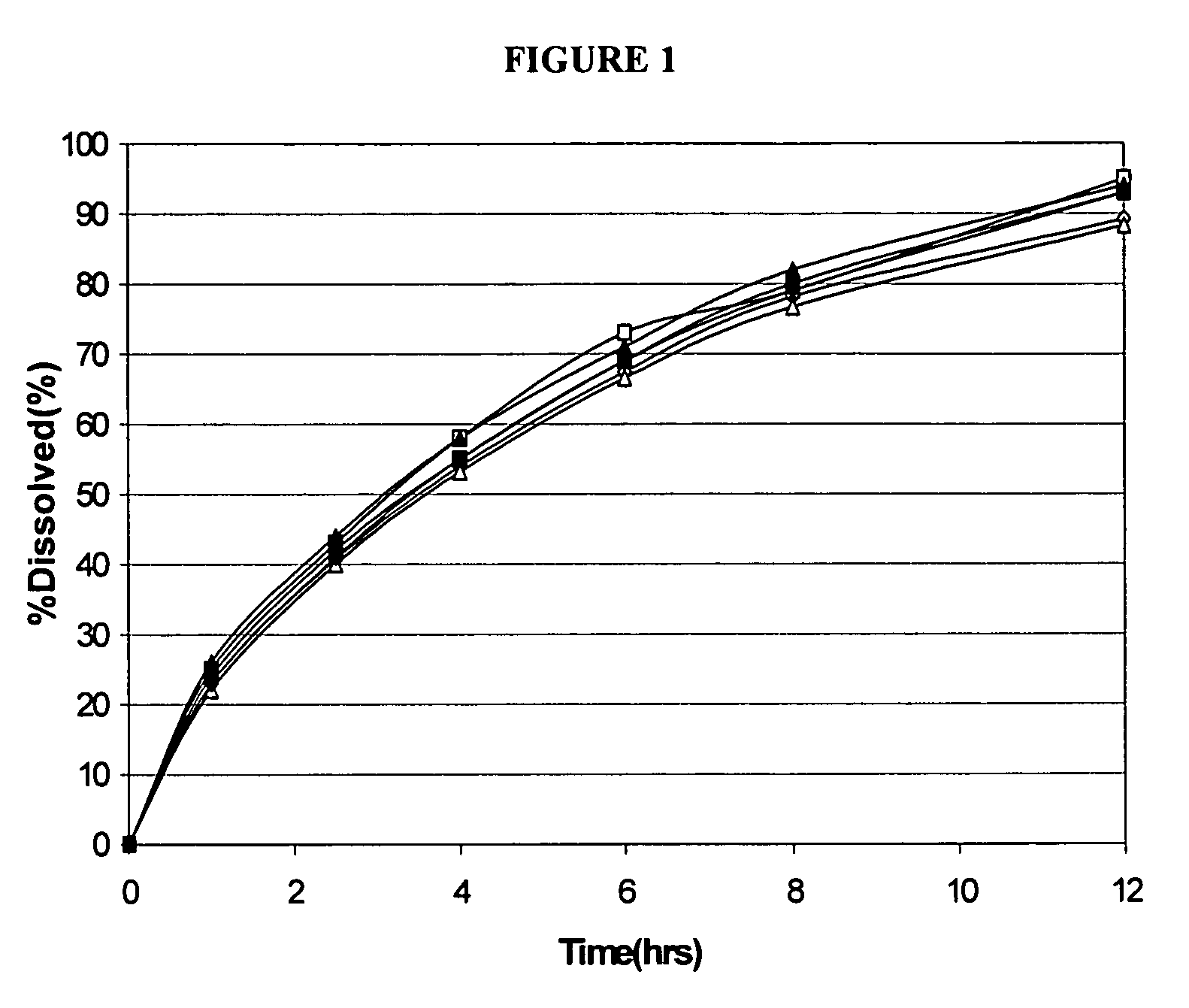
Hot-melt extruded pharmaceutical dosage form
ActiveUS20110038930A1Quick releaseRate of releaseBiocideNervous disorderBreaking strengthControlled release
The invention relates to a hot-melt extruded pharmaceutical dosage form with controlled release of a pharmacologically active ingredient (A) embedded in a matrix comprising a polymer (C), the dosage form exhibiting a breaking strength of at least 300 N and having an oblong shape comprising a longitudinal direction of extension, a transversal direction of extension orthogonal to the longitudinal direction of extension, a front side, an opposite back side and a circumferential rim between said front and back side;whereinthe core of the pharmaceutical dosage form has a morphological orientation caused by hot-melt extrusion that is substantially orthogonal to the longitudinal direction of extension of the dosage form; and / orthe release per area of the pharmacologically active ingredient (A) through the front side and the opposite back side is faster than the release through the circumferential rim.
Owner:GRUNENTHAL GMBH
Methods of decreasing sebum production in the skin
ActiveUS20120156163A1Reduce sebum productionEliminate and prevent in skinBiocideCosmetic preparationsSmall moleculeSebum production
Provided herein are methods of using gaseous nitric oxide and / or at least one nitric oxide source to or decrease sebum production in the skin of a subject. In some embodiments, the nitric oxide source includes small molecule and / or macromolecular NO-releasing compounds.
Owner:NAVAN INC
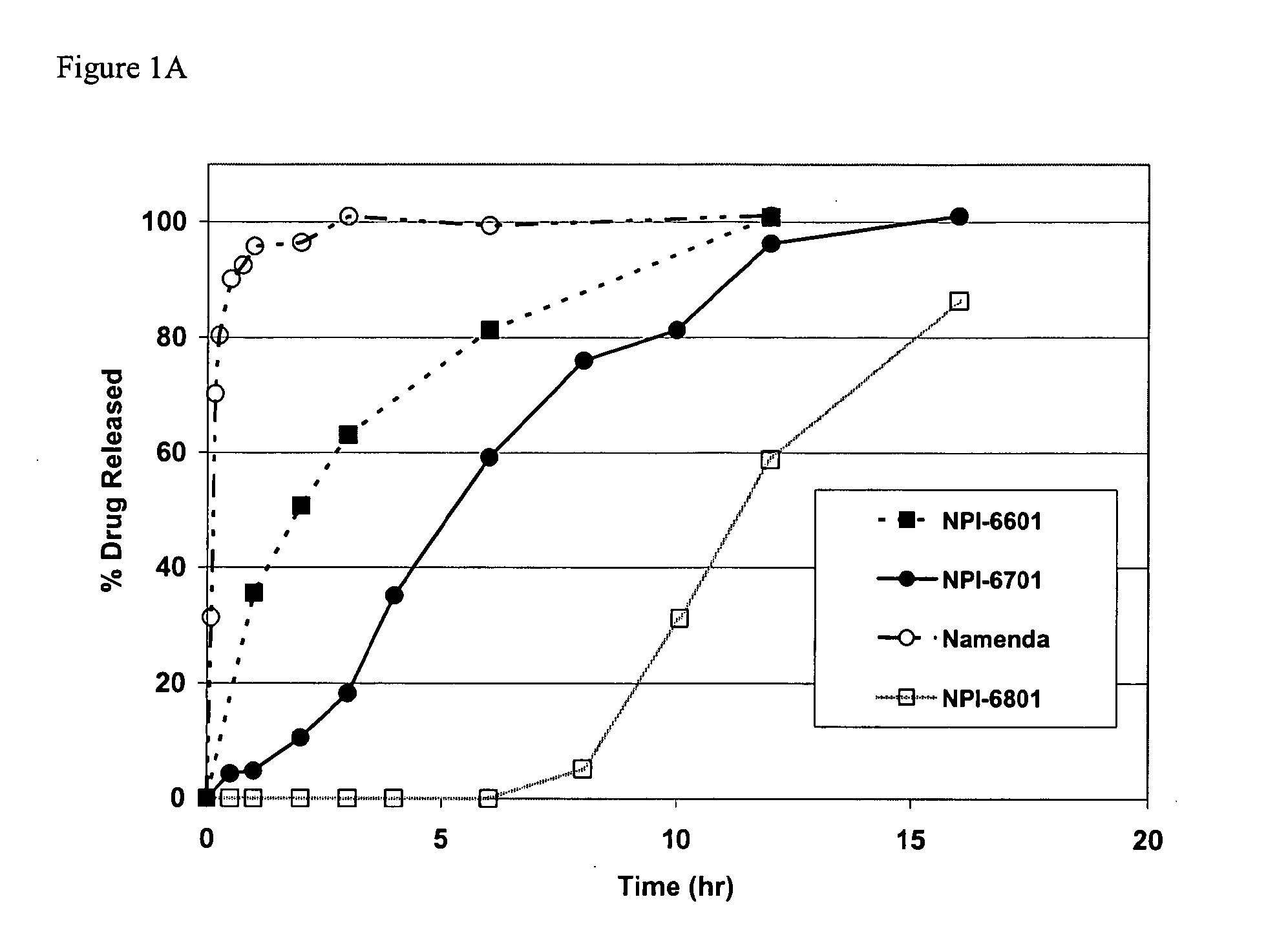
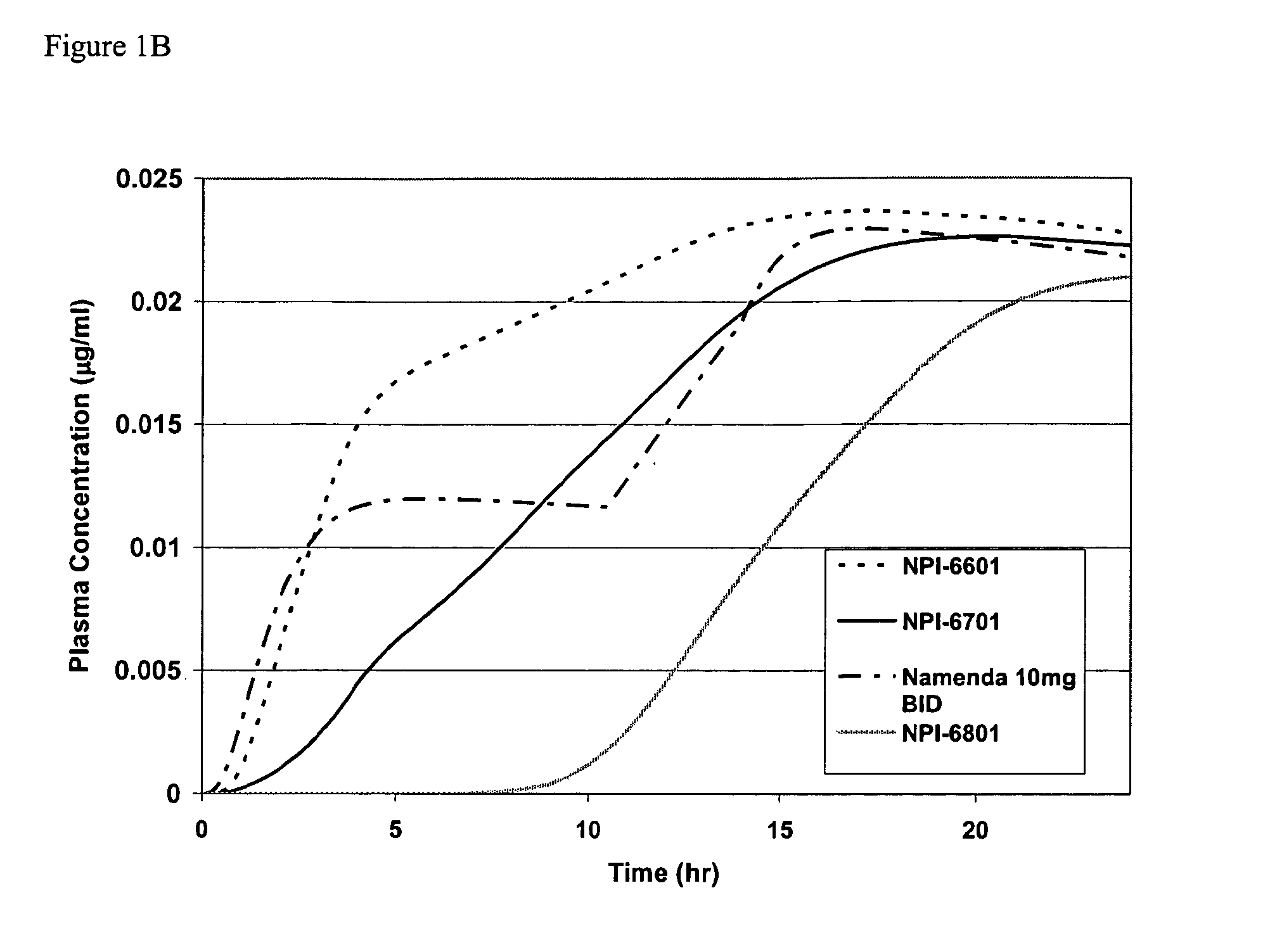
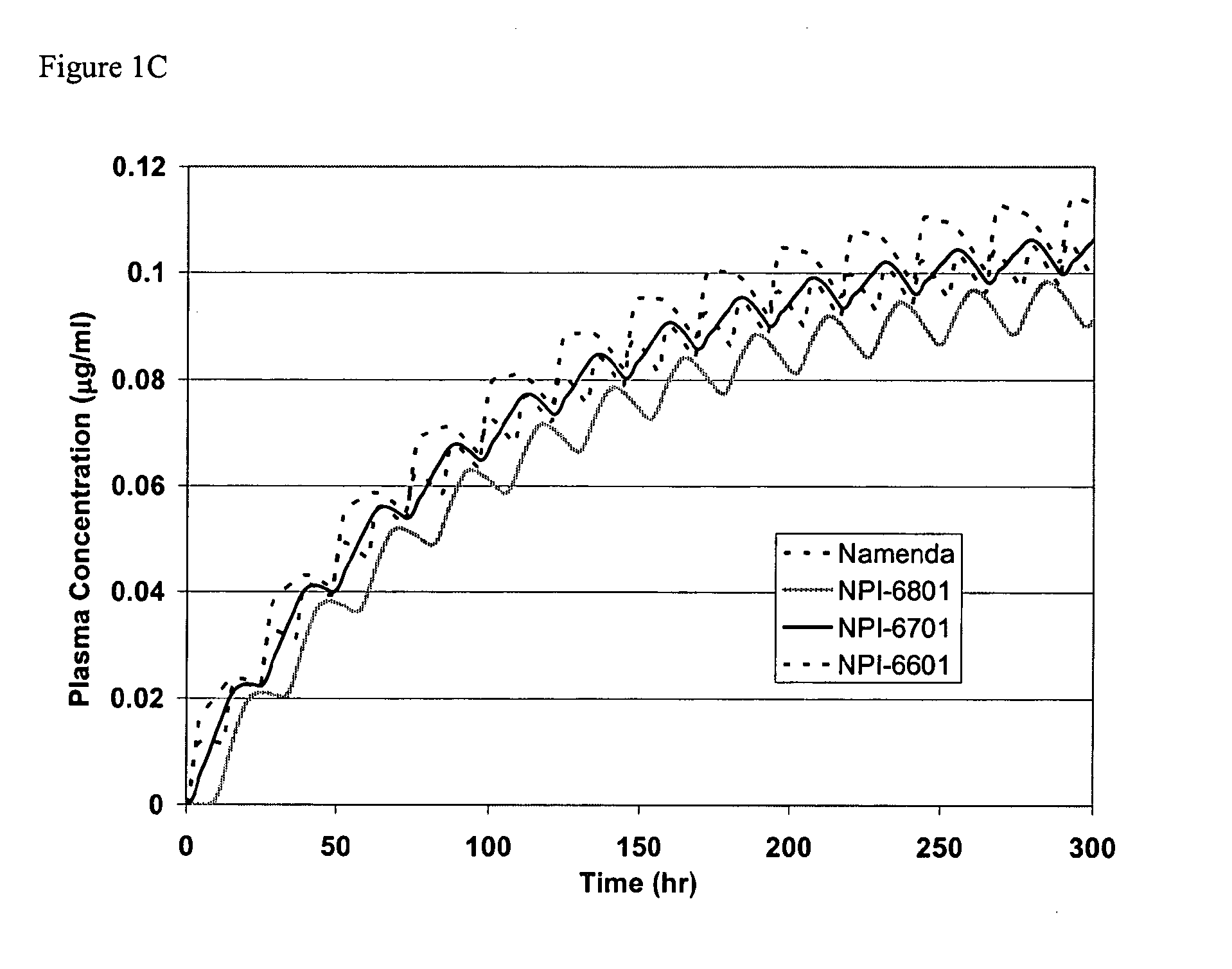
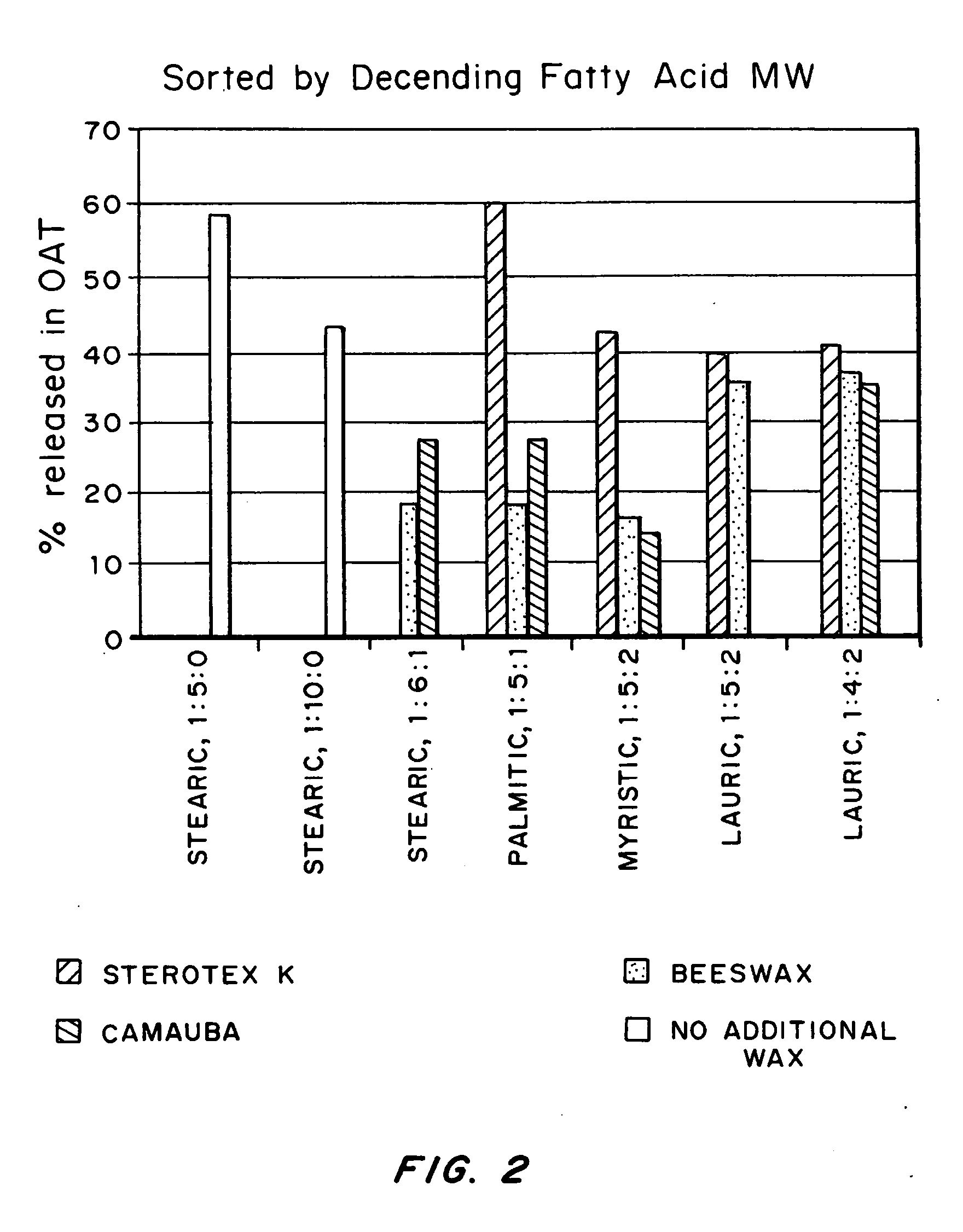
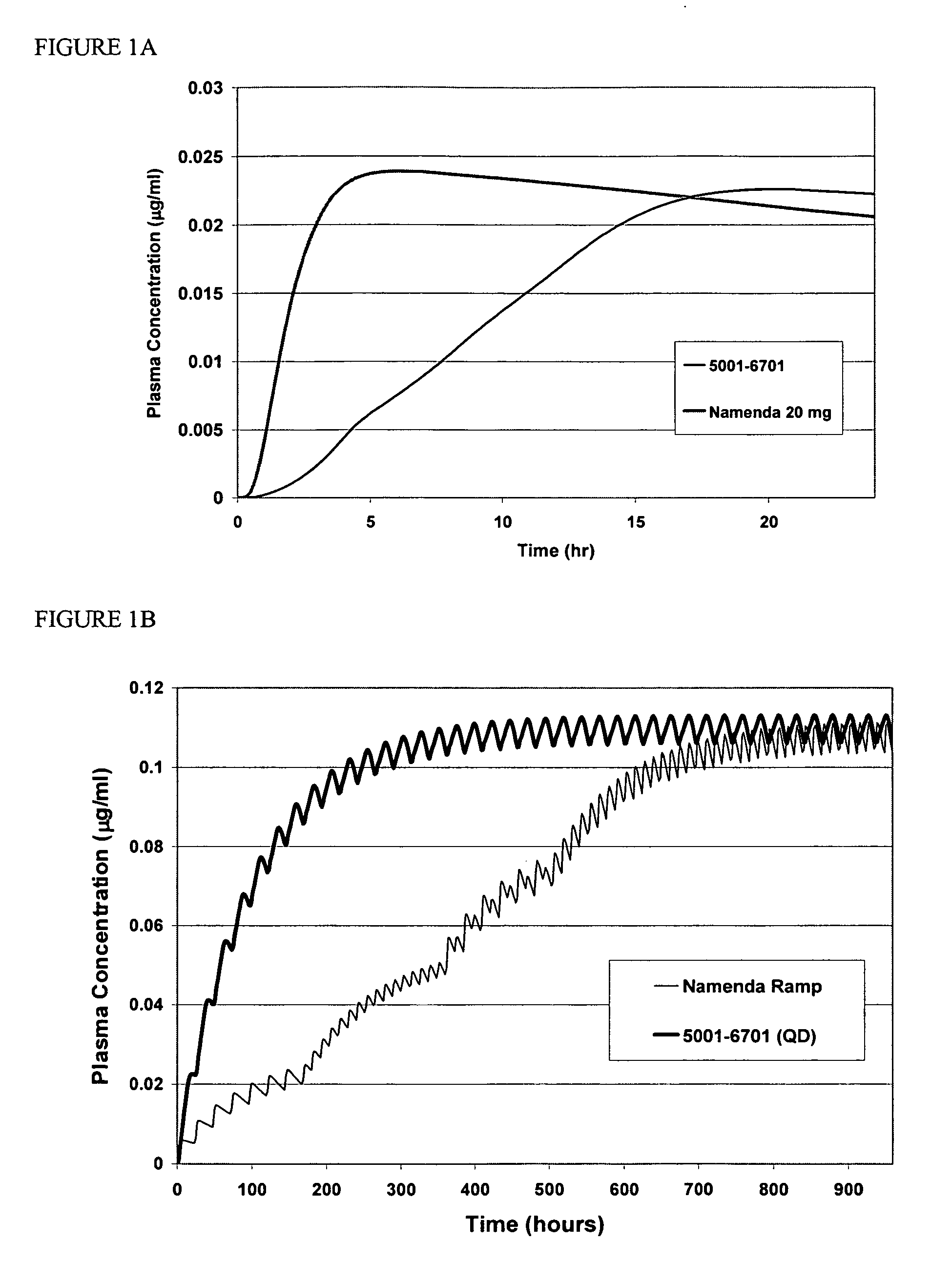
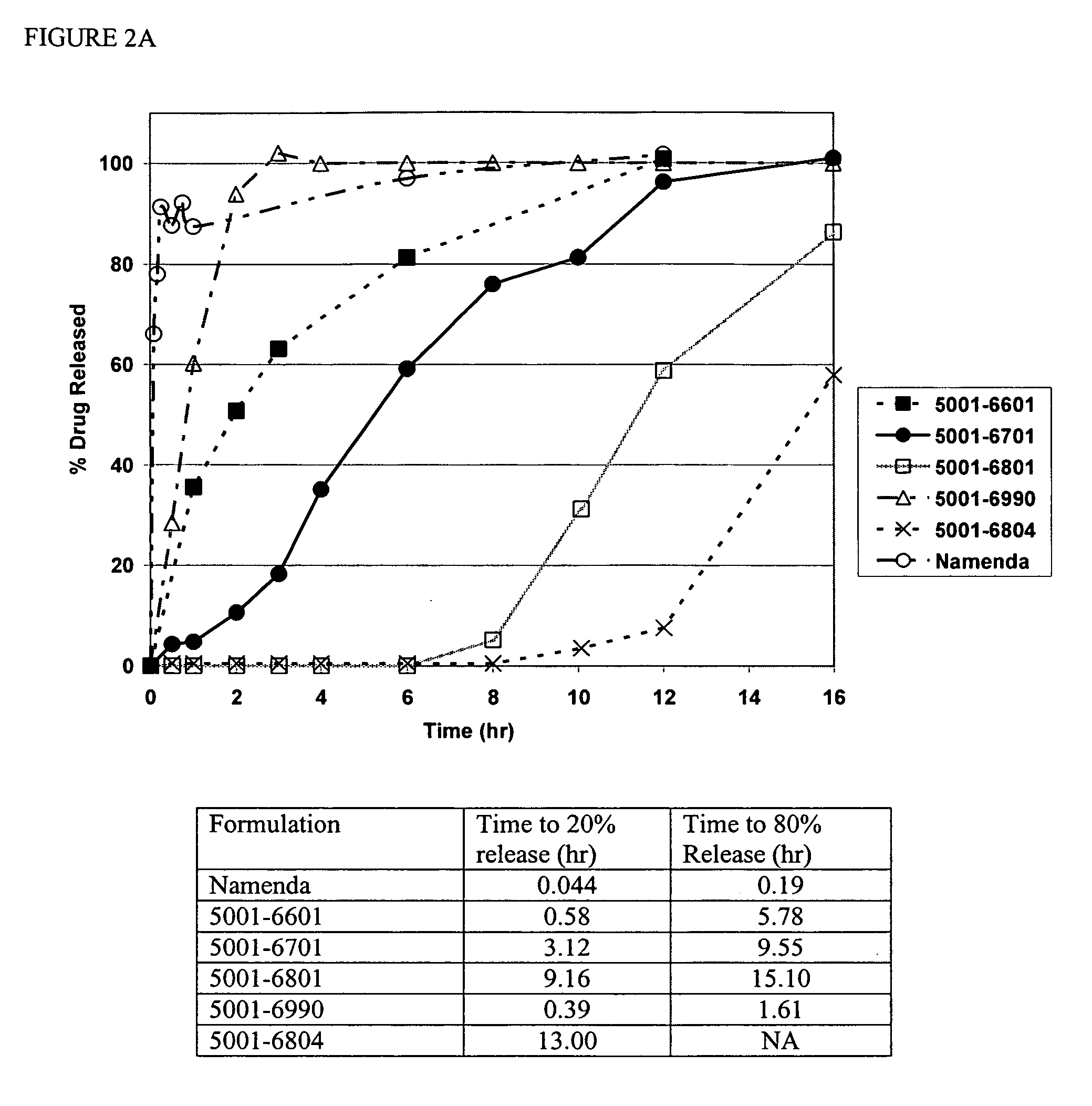
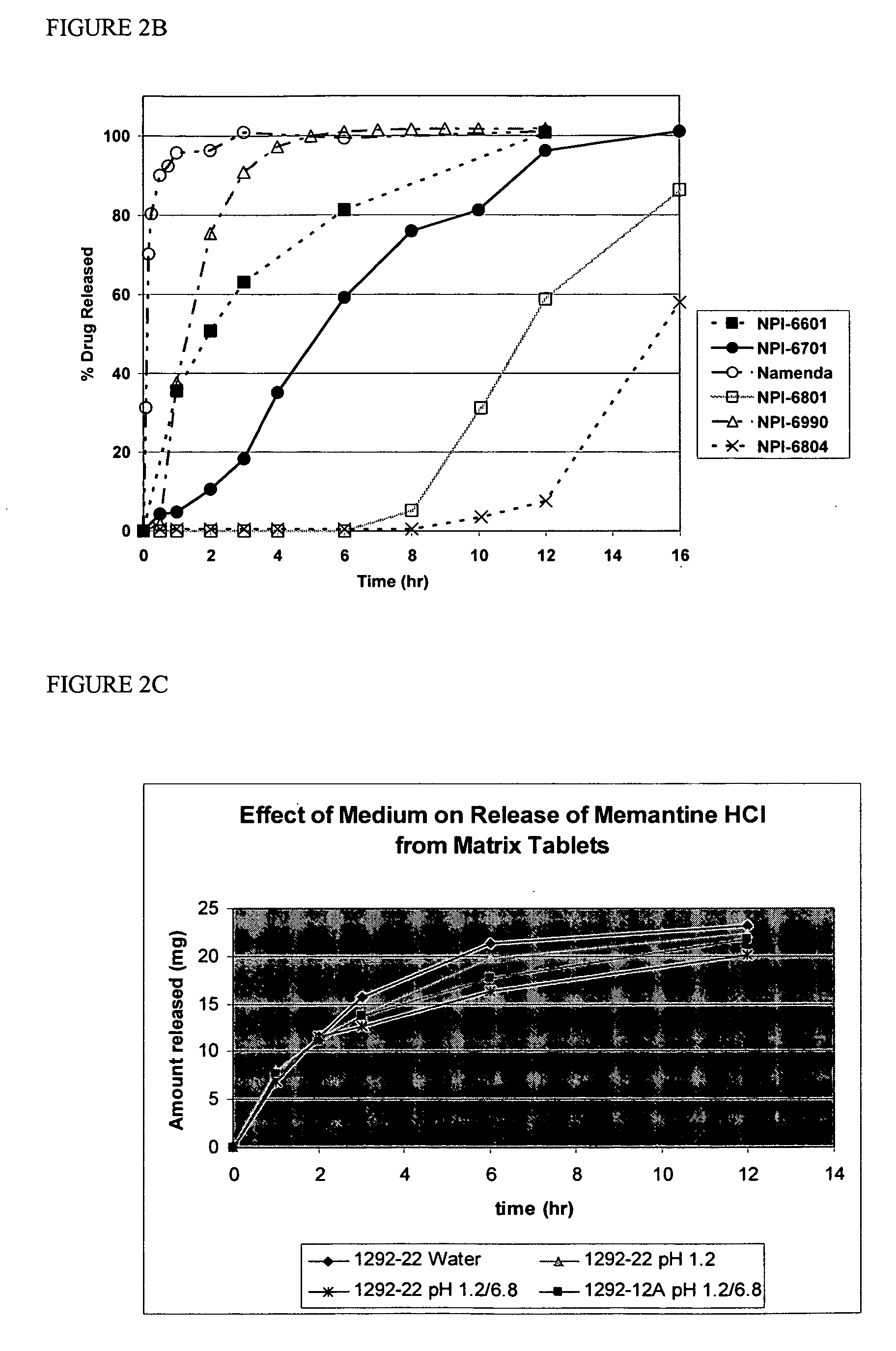
Modified release formulations of memantine oral dosage forms
The present invention provides pharmaceutical compositions given once daily containing at least one therapeutically active ingredient selected from the group consisting of memantine and a pharmaceutically acceptable salt of memantine, and a pharmaceutically acceptable polymeric matrix carrier. The dosage forms of the invention sustain the release of the therapeutically active agent from about 4 to about 24 hours when said dosage form is exposed to aqueous solutions. following entry of said form into a use environment, wherein said dosage form has a dissolution rate of more than about 80% after passage of about 6 hours to about 12 hours following said entry into said use environment.
Owner:FOREST LAB HLDG LTD
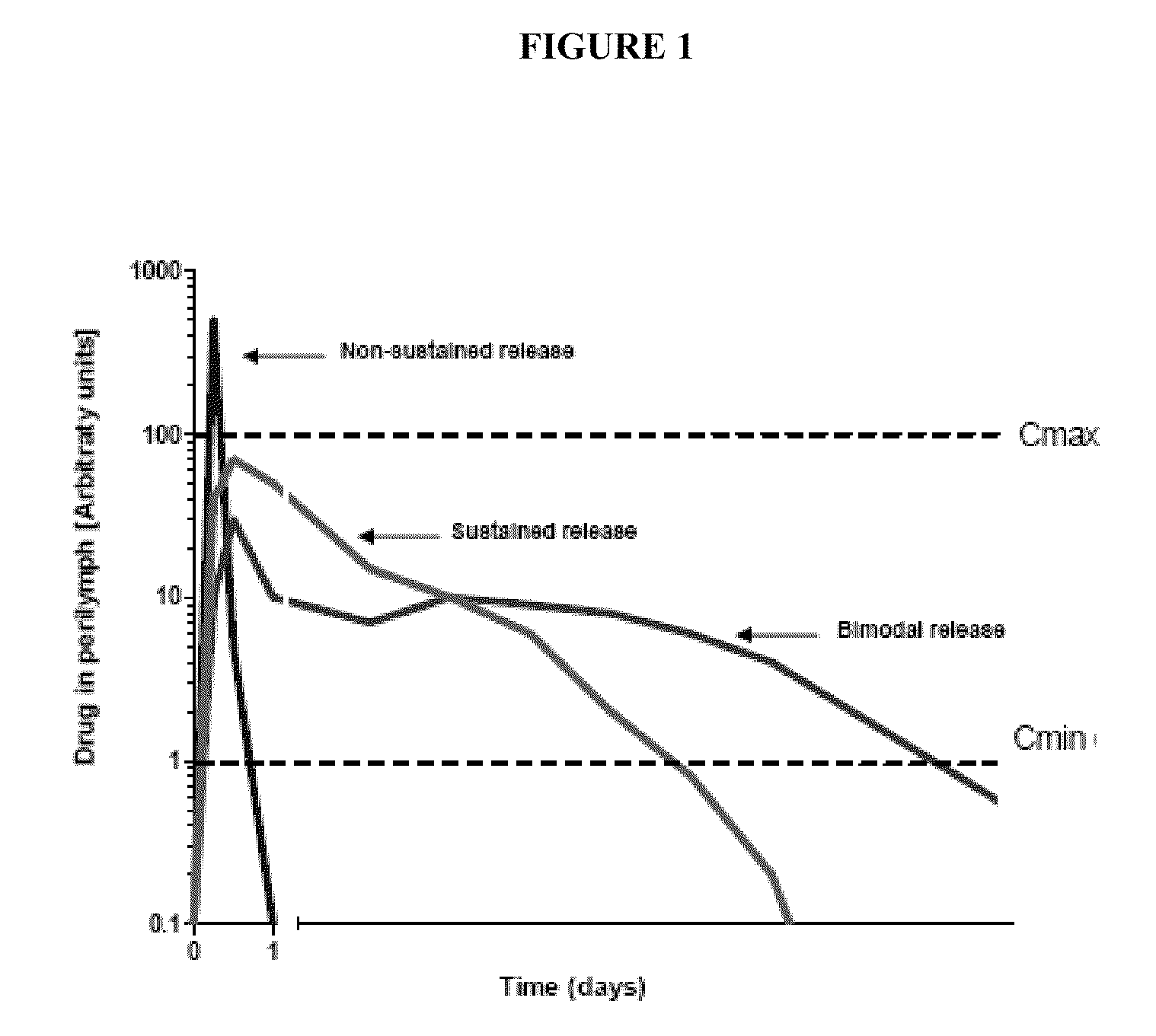
Auris formulations for treating otic diseases and conditions
Disclosed herein are compositions and methods for the treatment of otic disorders with immunomodulating agents and auris pressure modulators. In these methods, the auris compositions and formulations are administered locally to an individual afflicted with an otic disorder, through direct application of the immunomodulating and / or auris pressure modulating compositions and formulations onto the auris media and / or auris interna target areas, or via perfusion into the auris media and / or auris interna structures.
Owner:RGT UNIV OF CALIFORNIA +1
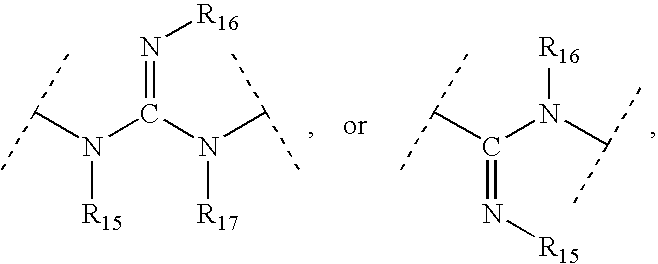
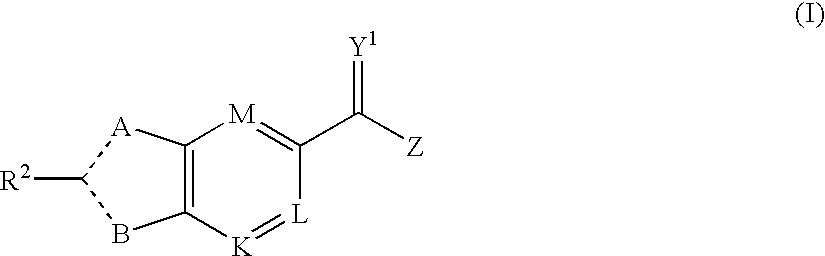
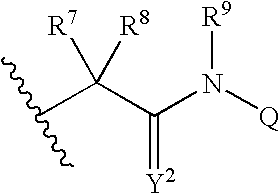
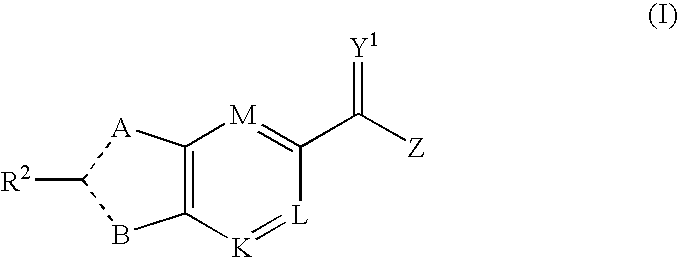
Viral Polymerase Inhibitors
An isomer, enantiomer, diastereoisomer, or tautomer of a compound, represented by formula I: wherein: A is O, S, NR1, or CR1, wherein R1 is defined herein; — represents either a single or a double bond; R2 is selected from: H, halogen, R21, OR21, SR21, COOR21, SO2N(R22)2, N(R22)2, CON(R22)2, NR22C(O)R22 or NR22C(O)NR22 wherein R21 and each R22 is defined herein; B is NR3 or CR3, with the proviso that one of A or B is either CR1 or CR3, wherein R3 is defined herein; K is N or CR4, wherein R4 is defined herein; L is N or CR5 wherein R5 has the same definition as R4 defined above; M is N or CR7, wherein R7 has the same definition as R4 defined above; Y1 is O or S; Z is N(R6a)R6 or OR6, wherein R6a is H or alkyl or NR61R62 wherein R61 and R62 are defined herein; a salt or a derivative thereof, as an inhibitor of HCV NS5B polymerase.
Owner:BOEHRINGER INGELHEIM INT GMBH
Compositions and methods
Non-aerosol spray-on skin patch compositions as described comprising at least one substantially water insoluble film forming agent, at least one film plasticizer agent, at least one water soluble compound, and at least one organic solvent, the composition forming a flexible, porous and physiologically compatible skin patch when sprayed on to skin and allowed to dry. Also described are methods of improving wound healing by administering a physiologically active ingredient to a patient in need of such treatment.
Owner:KO THOMAS SAI YING
Methods and Compositions for Treating Migraine Pain
InactiveUS20100029665A1Benefit maximizationEliminate side effectsBiocideNervous disorderHeadache severeVascular headache
The present invention provides novel methods and compositions for the treatment and prevention of headaches, vascular headaches, migraine headaches, cluster headaches, and migraine. One of the headaches, vascular headaches, migraine headaches, cluster headaches, and migraine treated by the methods and compositions of the invention is migraine.
Owner:MEYERSON LAURENCE R +3
High concentration topical insecticide containing insect growth regulator
InactiveUS20050009880A1Easy to useOvercomes drawbackBiocideDead animal preservationHigh concentrationSide effect
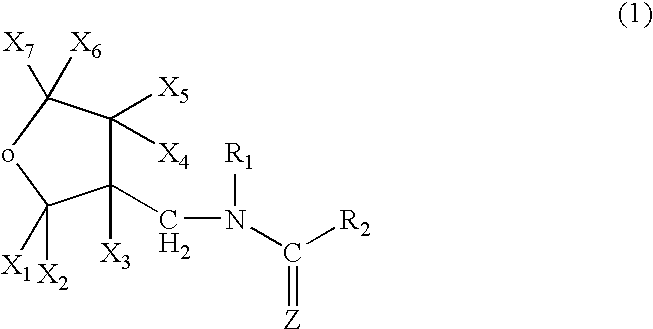
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. The insecticide contains an insecticide and an insect growth regulator effective for killing fleas, flea larvae and flea eggs. The insecticide is formulated by dissolving an insecticidal (tetrahydro-3-furanyl) methylamine derivative or a chloronicotinyl insecticide and an insect growth regulator (IGR) in a solvent containing a quaternary ammonium salt to increase the solvency of the IGR component, thereby providing an insecticide having high insecticidal activity.
Owner:CEVA ANIMAL HEALTH
High concentration topical insecticide containing insect growth regulator
ActiveUS20050096386A1Easy to useOvercomes drawbackBiocideDead animal preservationHigh concentrationSide effect
A topical insecticide is provided which can be safe to use and avoids many common deleterious side effects of conventional topical insecticides. The insecticide contains an insecticide and an insect growth regulator effective for killing fleas, flea larvae and flea eggs. The insecticide is formulated by dissolving an insecticidal (tetrahydro-3-furanyl) methylamine derivative and an insect growth regulator (IGR) in a solvent containing a quaternary ammonium salt to increase the solvency of the IGR component, thereby providing an insecticide having high insecticidal activity.
Owner:CEVA ANIMAL HEALTH
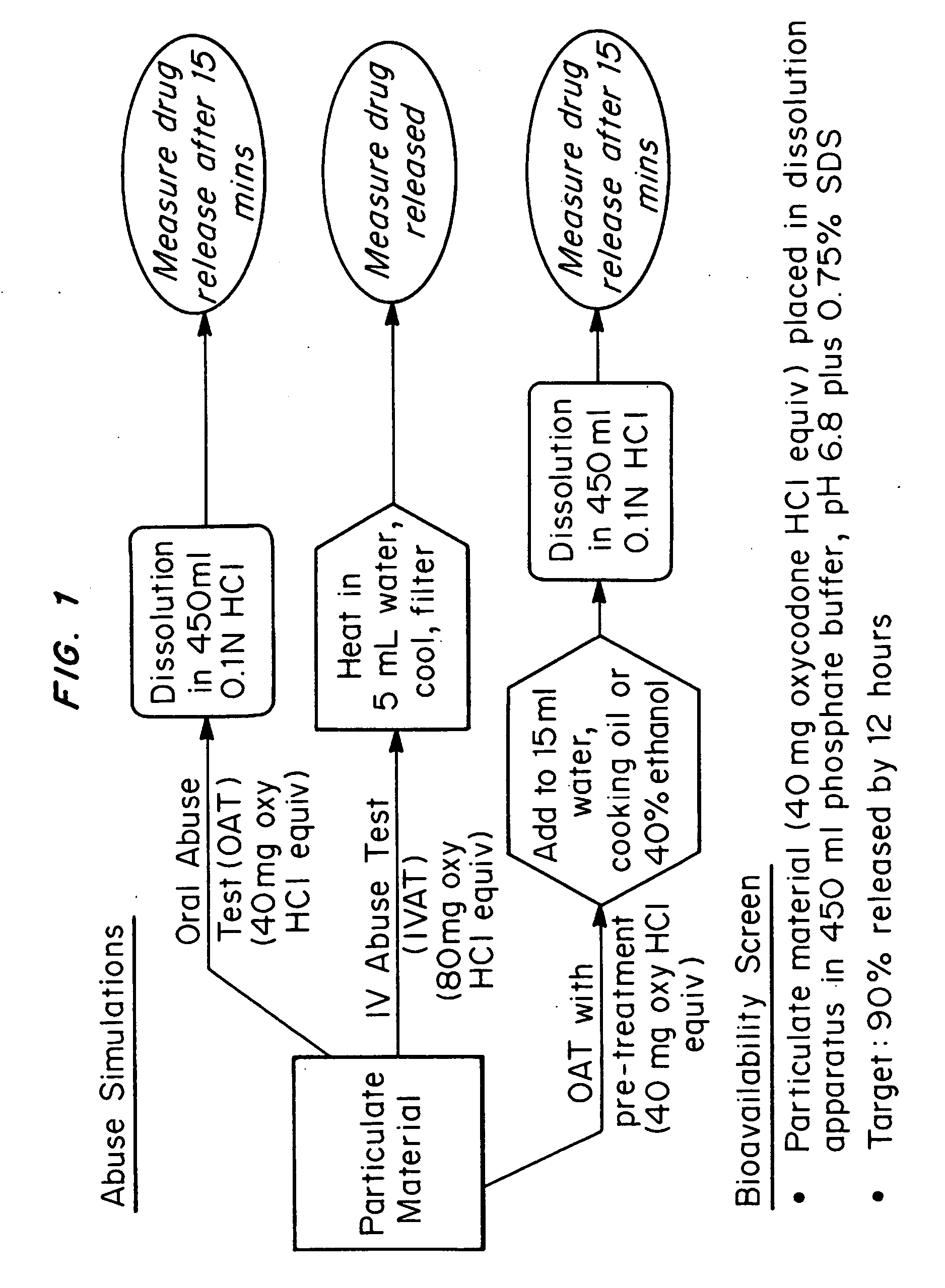
Abuse-deterrent drug formulations
ActiveUS20050281748A1Reduce the possibilityImprove lipophilicityTelevision system detailsPowder deliveryImmediate releaseActive agent
An abuse-deterrent pharmaceutical composition has been developed to reduce the likelihood of improper administration of drugs, especially drugs such as opiods. In the preferred embodiment, the drug is modified to increase its lipophilicity by forming a salt between the drug and one or more fatty acids wherein the concentration of the one or more fatty acids is one to 15 times the molar amount of the active agent, preferably two to ten times the molar amount of the active agent. In one embodiment the modified drug is homogeneously dispersed within microparticles composed of a material that is either slowly soluble or not soluble in water. In some embodiments the drug containing microparticles or drug particles are coated with one or more coating layers, where at least one coating is water insoluble and preferably organic solvent insoluble. The abuse-deterrent composition prevents the immediate release of a substantial portion of drug, even if the physical integrity of the formulation is compromised (for example, by chopping with a blade or crushing) and the resulting material is placed in water, snorted, or swallowed. However, when administered as directed, the drug is slowly released from the composition as the composition is broken down or dissolved gradually within the GI tract by a combination of enzymatic degradation, surfactant action of bile acids, and mechanical erosion.
Owner:COLLEGIUM PHARMA INC
Method and composition for adminstering an NMDA receptor antagonist to a subject
ActiveUS20060142398A1Prevent adverse side effectsPatient compliance is goodBiocideNervous disorderNR1 NMDA receptorPharmacology
The invention provides methods and compositions for administering an NMDA receptor antagonist (e.g., memantine) to a subject.
Owner:ADAMAS PHARMA LLC
Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by exogenous and endogenous opioid agonists
InactiveUSRE36547E1Enhance analgesic potencyDecrease dependence liabilityCompound screeningBiocideEndogenous OpiatesNervous system
This invention relates to a method of selectively enhancing the analgesic potency of morphine and other clinically used bimodally-acting opioid agonists and simultaneously attenuating development of physical dependence, tolerance and other undesirable side effects caused by the chronic administration of said bimodally-acting opioid agonists comprising the co-administration of a bimodally-acting opioid agonist which activates both inhibitory and excitatory opioid receptor-mediated functions of neurons in the nociceptive (pain) pathways of the nervous system and an opioid receptor antagonist which selectively inactivates excitatory opioid receptor-mediated side effects. This invention also relates to a method of using excitatory opioid receptor antagonists alone to block the undesirable excitatory side effects of endogenous bimodally-acting opioid agonists which may be markedly elevated during chronic pain. This invention further relates to a method of long-term treatment of previously detoxified opiate, cocaine and alcohol addicts utilizing said excitatory opioid receptor antagonists, either alone or in combination with low-dose methadone, to prevent protracted physical dependence, and to compositions comprising an excitatory opioid receptor antagonist of the invention and a bimodally-acting opioid agonist.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
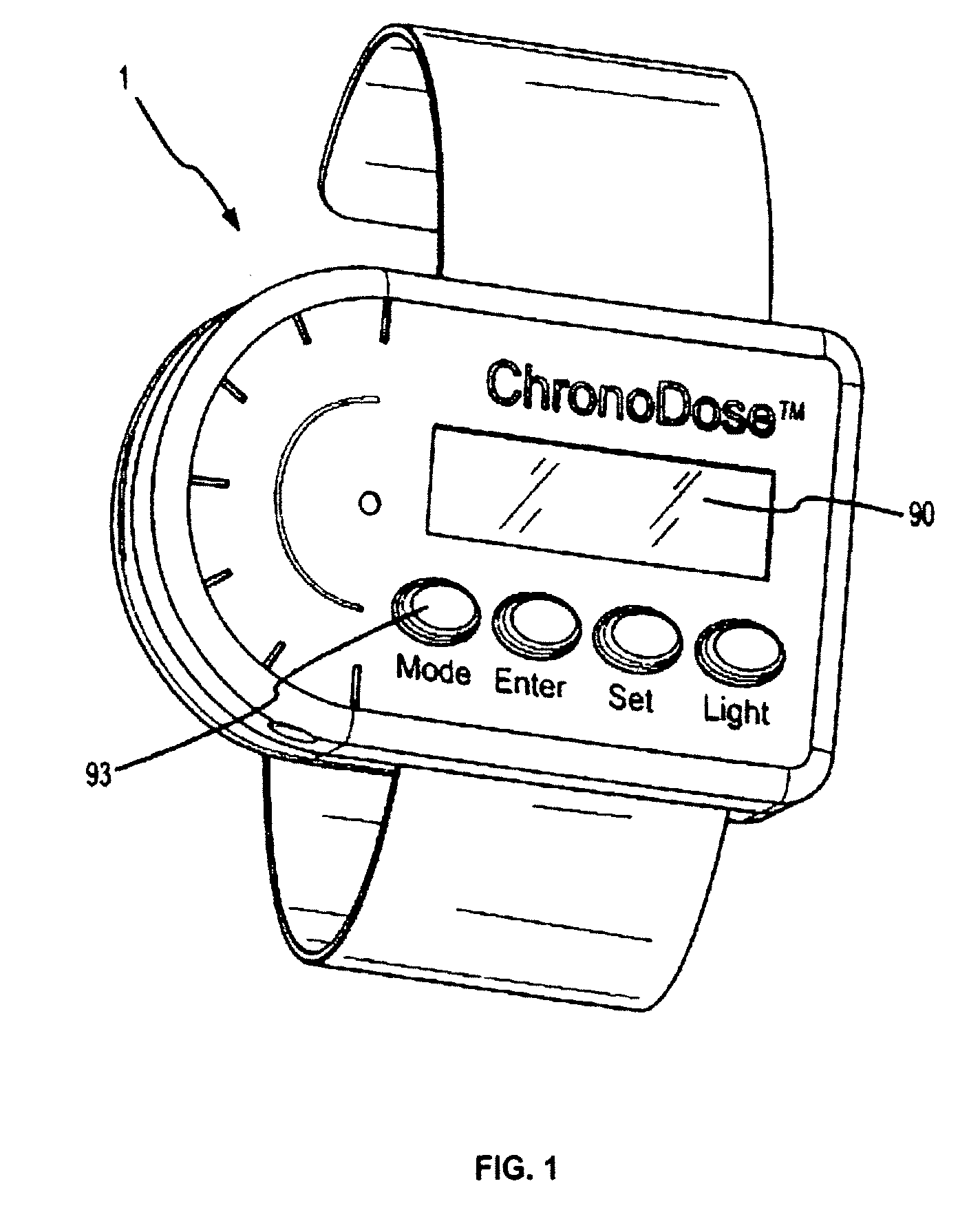
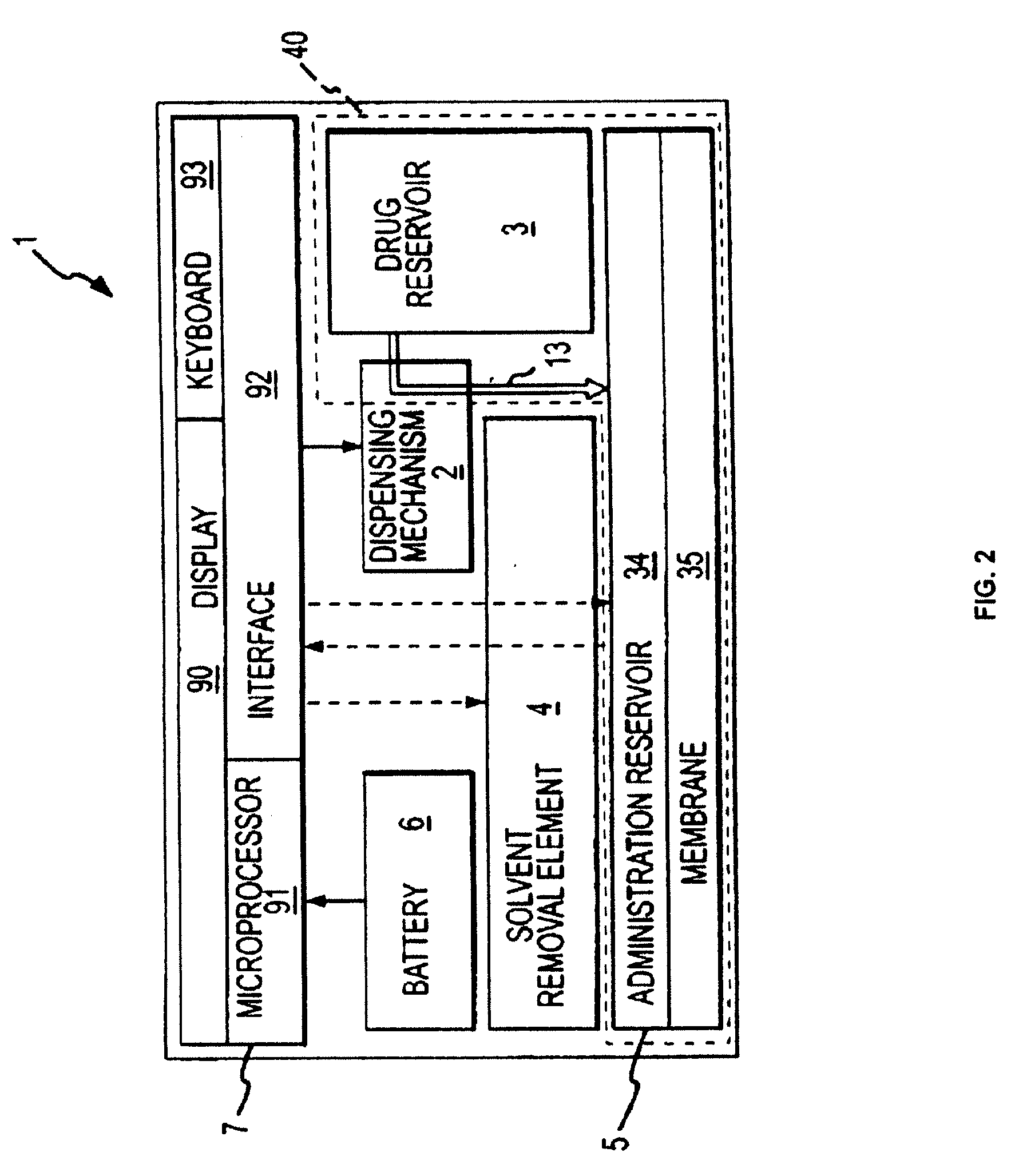
Biosynchronous transdermal drug delivery for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and the treatment of hyperglycemia, alzheimer's disease, sleep disorders, parkinson's disease, aids, epilepsy, attention deficit disorder, nicotine addiction, cancer, headache and pain control, asthma, angina, hypertension, depression, cold, flu and the like
ActiveUS20080220092A1Improve performanceReduce the amount requiredHeavy metal active ingredientsBiocidePhytochemicalAntioxidant
Systems and methods for longevity, anti-aging, fatigue management, obesity, weight loss, weight management, delivery of nutraceuticals, and treating hyperglycemia, Alzheimer's disease, sleep disorders, Parkinson's disease, Attention Deficit Disorder and nicotine addiction involve synchronizing and tailoring the administration of nutraceuticals, medications and other substances (for example, stimulants) in accordance with the body's natural circadian rhythms, meal times and other factors. Improved control of blood glucose levels, extended alertness, and weight control, and counteracting of disease symptoms when they are at their worst are possible. An automated, pre-programmable transdermal administration system is used to provide pulsed doses of medications, pharmaceuticals, hormones, neuropeptides, anorexigens, pro-drugs, stimulants, plant extracts, botanicals, nutraceuticals, cosmeceuticals, phytochemicals, phytonutrients, enzymes, antioxidants, essential oils, fatty acids, minerals, vitamins, amino acids, coenzymes, or other physiological active ingredient or precursor. The system can utilize a pump, pressurized reservoir, a system for removing depleted carrier solution, or other modulated dispensing actuator, in conjunction with porous membranes or micro-fabricated structures.
Owner:MORNINGSIDE VENTURE INVESTMENTS
Aryl and heteroaryl compounds, compositions and methods of use
InactiveUS20050049310A1Reduce hypoxiaInduce red blood cell productionBiocideOrganic chemistryArylErythropoietin receptor
This invention provides aryl and heteroaryl compounds of Formula (I) as described herein, and methods of their preparation. Also provided are pharmaceutical compositions made with the compounds of Formula (I) and methods for making such compositions. The compounds of Formula (I) may activate an erythropoietin receptor and thus, may be useful to induce red blood cell production. The compounds of Formula (I) and compositions including compounds of Formula (I) may be useful in a variety of applications including the management, treatment and / or control of diseases caused at least in part by deficient (or inefficient) EPO production relative to hemoglobin level.
Owner:VTV THERAPEUTICS LLC
Viral polymerase inhibitors
Owner:BOEHRINGER INGELHEIM CANADA LTD
Modified and immediate release formulations of memantine
InactiveUS20070065512A1Reliable absorptionImprove toleranceBiocideNervous disorderImmediate releaseNeuropathic pain
The present invention provides immediate release and modified release oral dosage forms. Specifically, the invention provides modified and immediate release pharmaceutical dosage forms containing memantine that exhibit an enhanced release profile and provide reliable absorption. The dosage forms may be used to treat mild, moderate or severe Alzheimer's disease or neuropathic pain.
Owner:FOREST LAB HLDG LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com