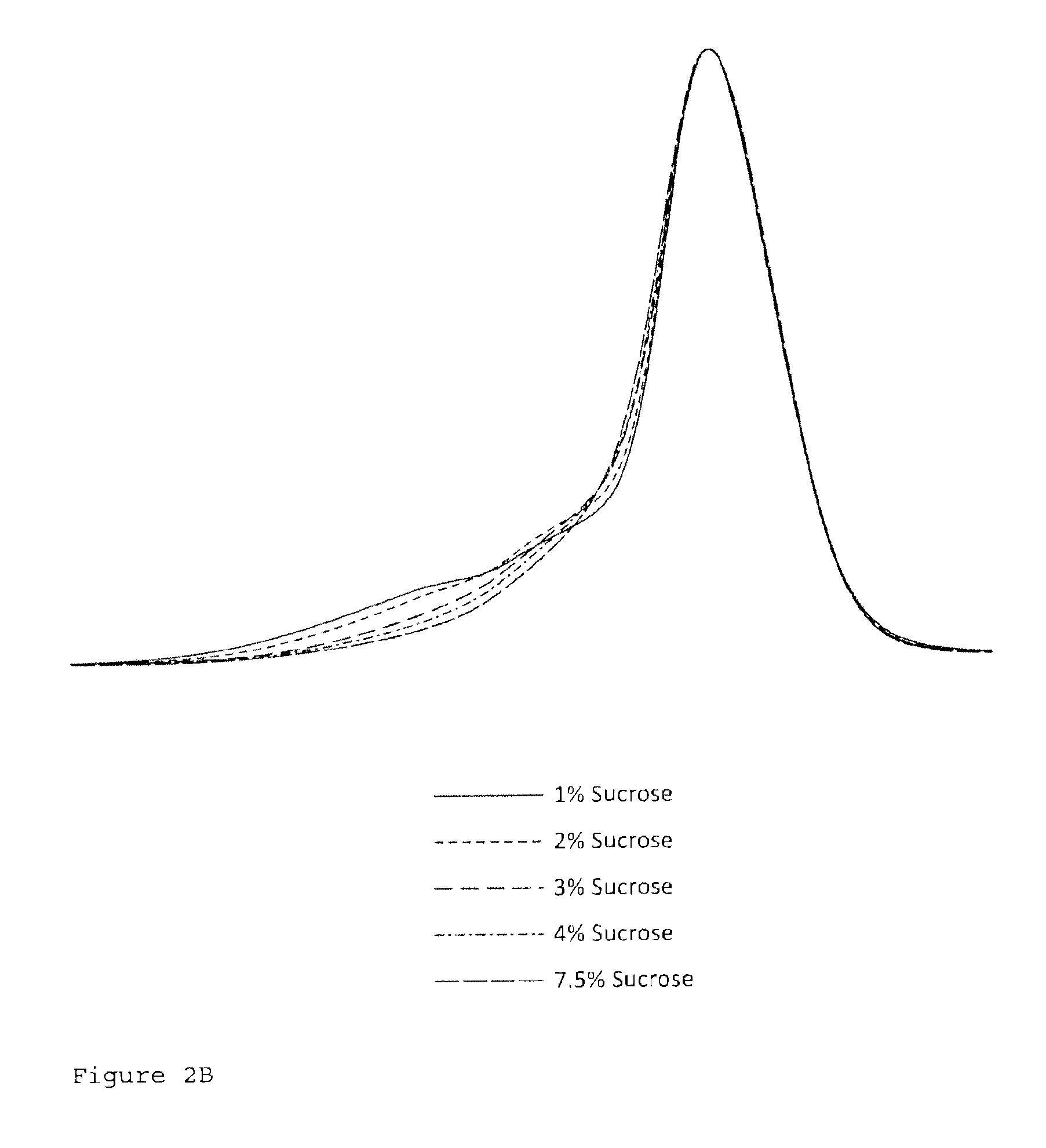
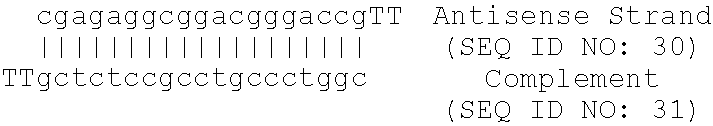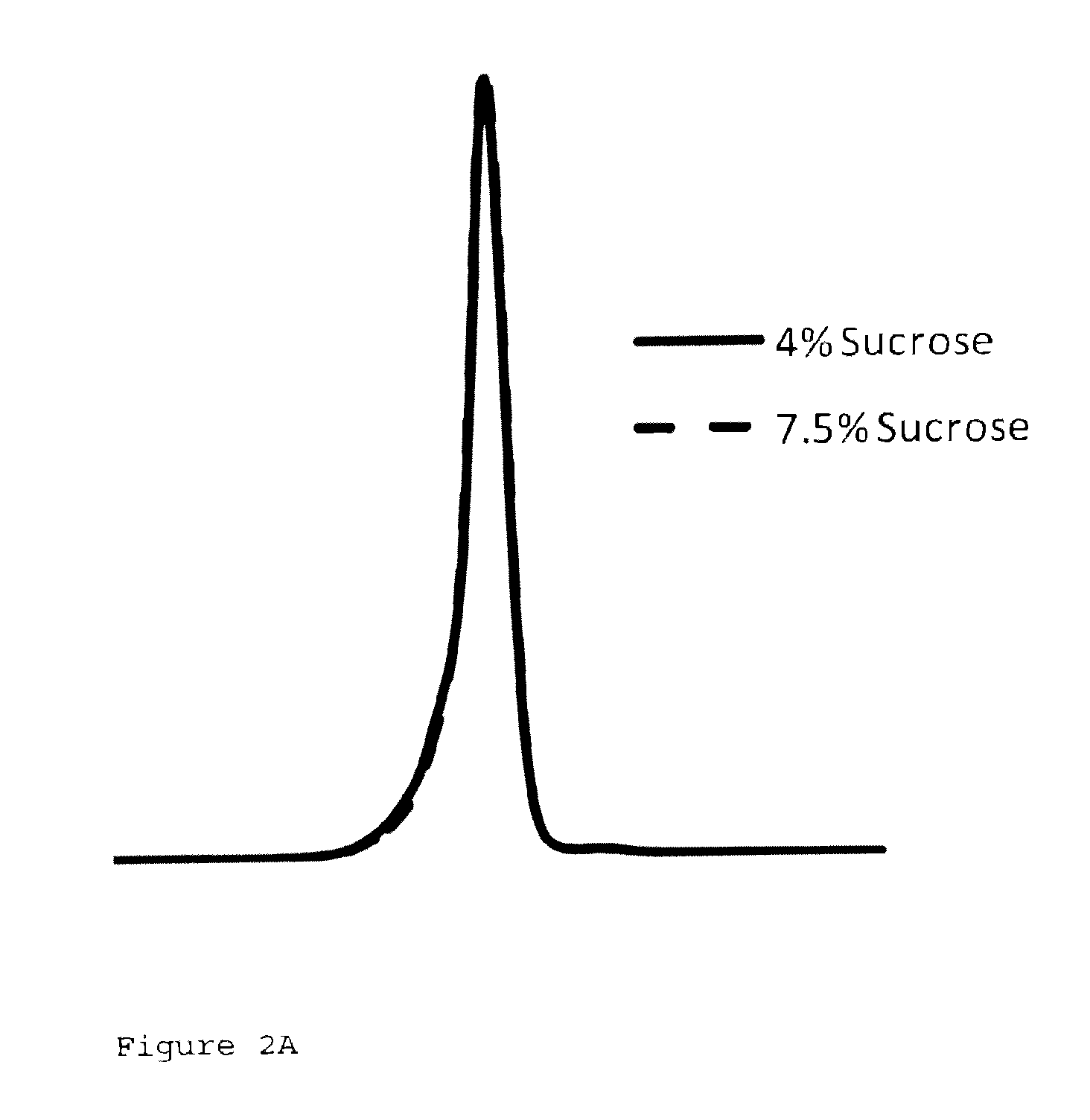Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
127 results about "Apolipoproteins E" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
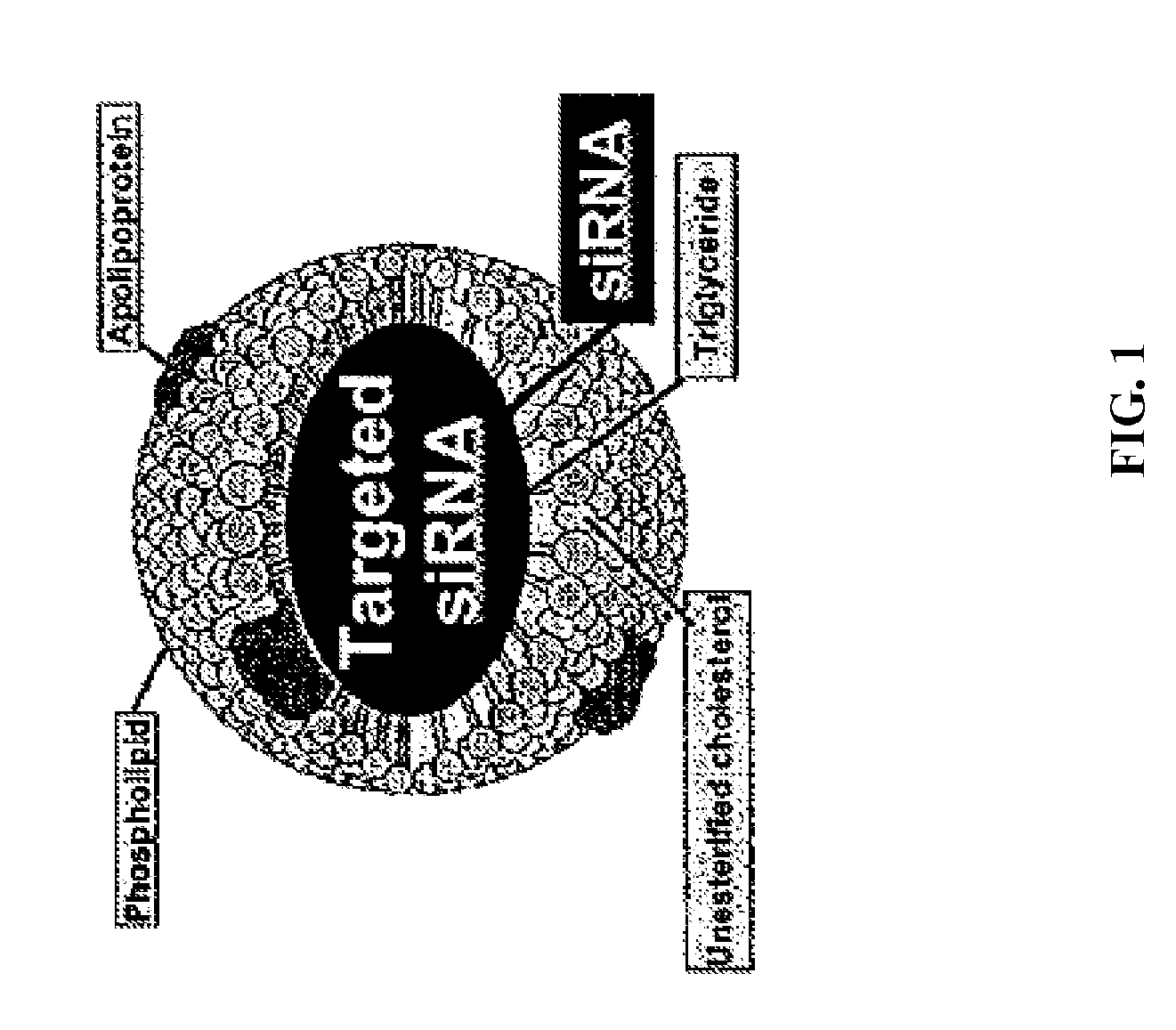
A class of protein components which can be found in several lipoproteins including HIGH-DENSITY LIPOPROTEINS; VERY-LOW-DENSITY LIPOPROTEINS; and CHYLOMICRONS. Synthesized in most organs, Apo E is important in the global transport of lipids and cholesterol throughout the body. Apo E is also a ligand for LDL receptors (RECEPTORS, LDL) that mediates the binding, internalization, and catabolism of lipoprotein particles in cells. There are several allelic isoforms (such as E2, E3, and E4). Deficiency or defects in Apo E are causes of HYPERLIPOPROTEINEMIA TYPE III.
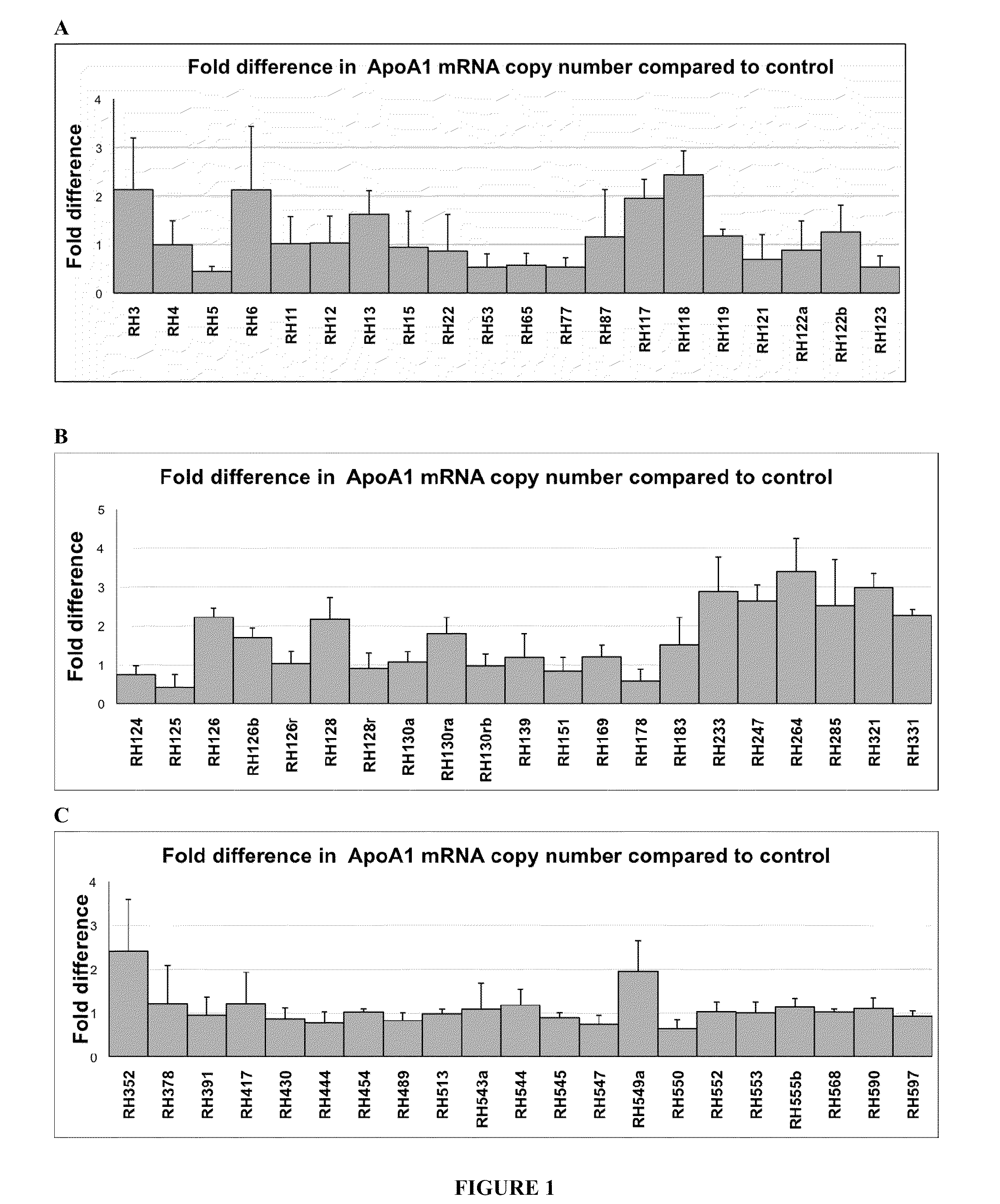
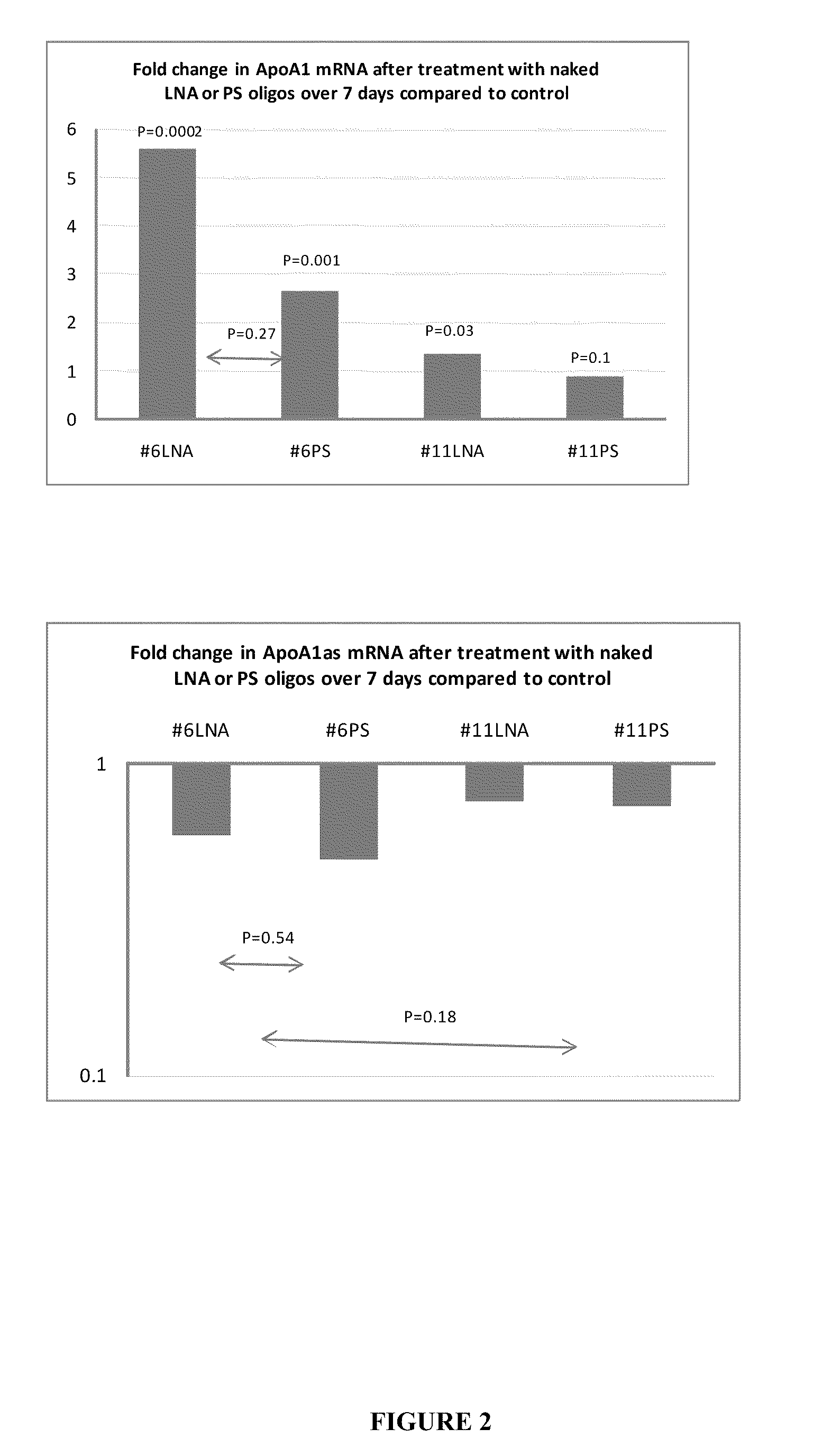
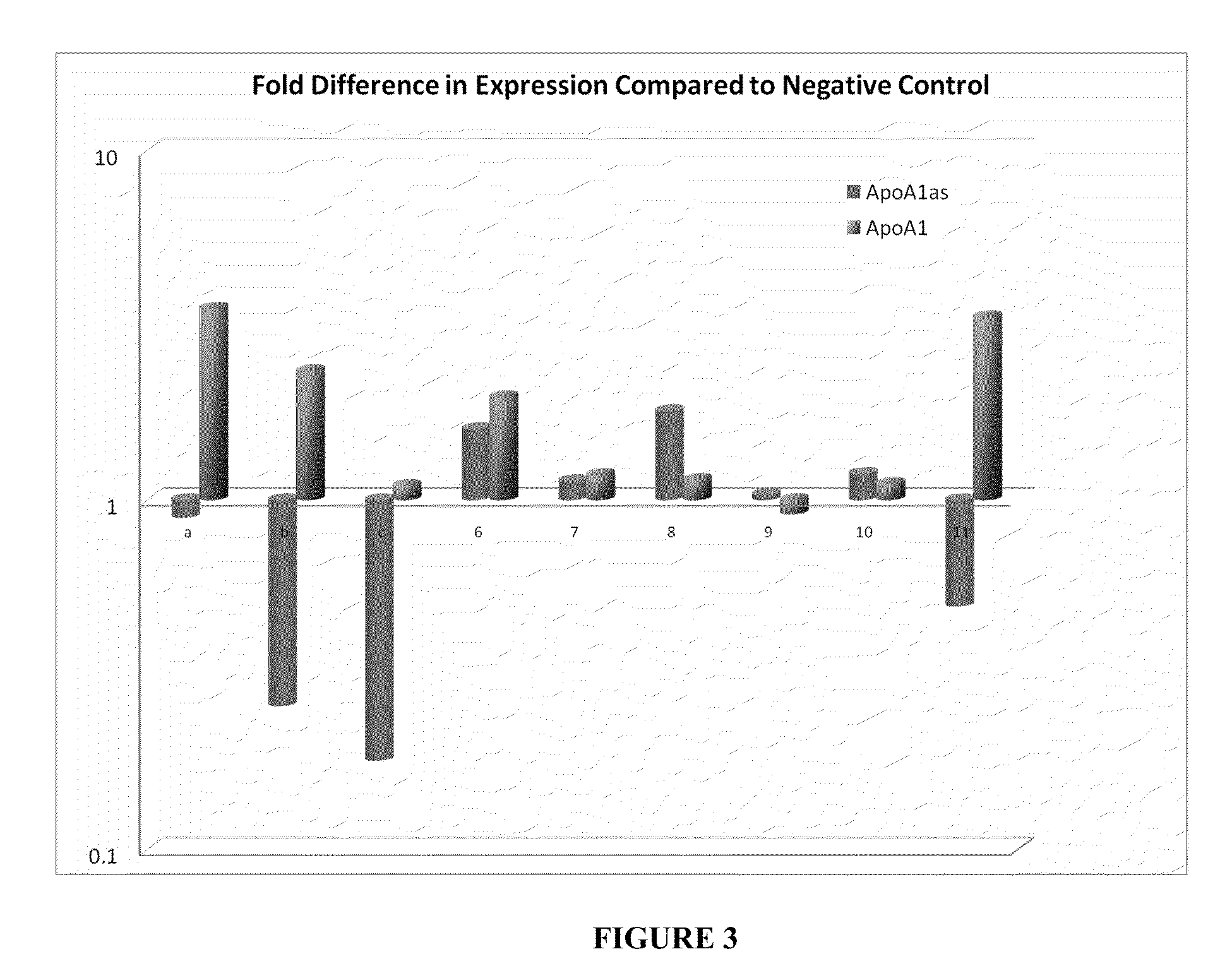
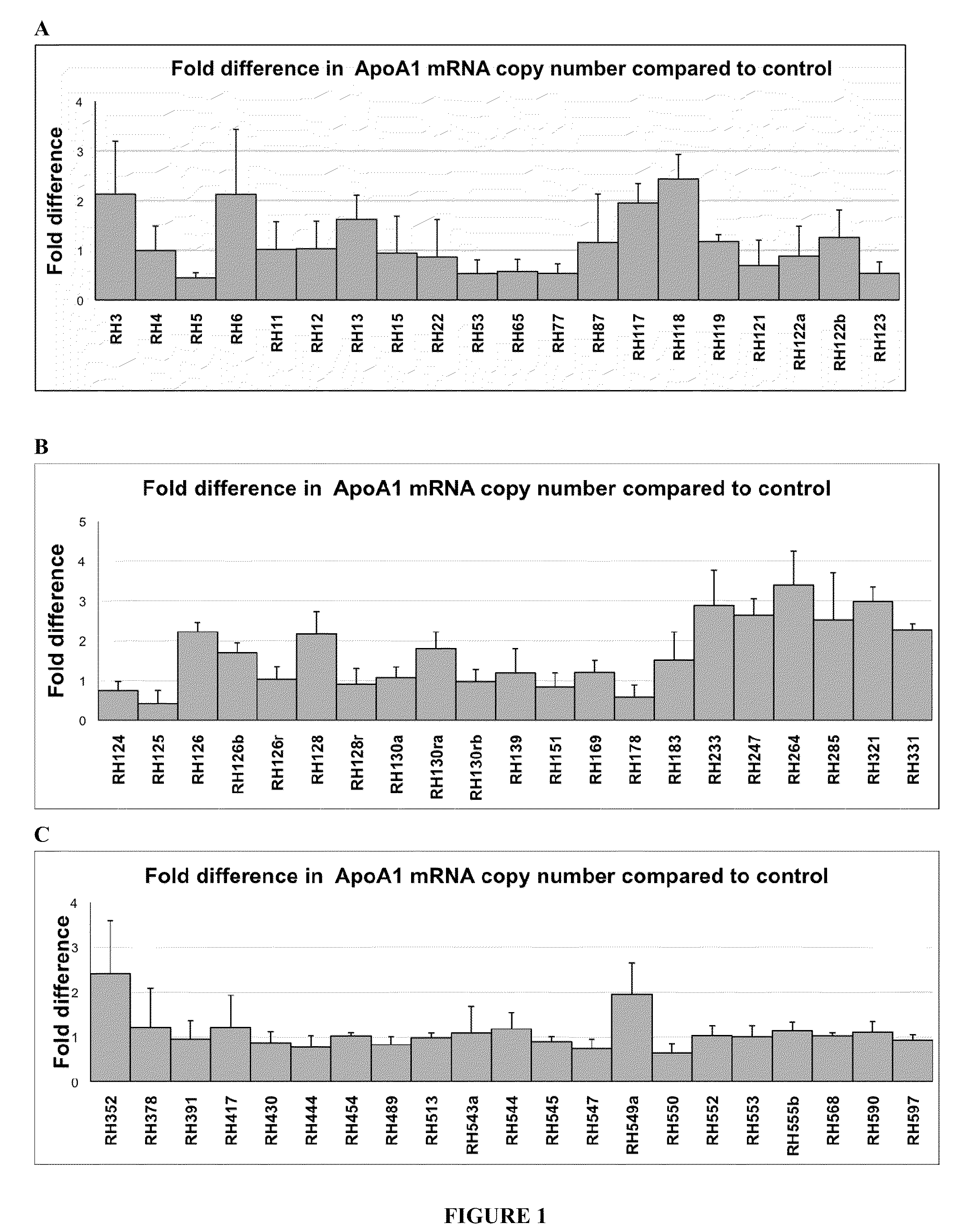
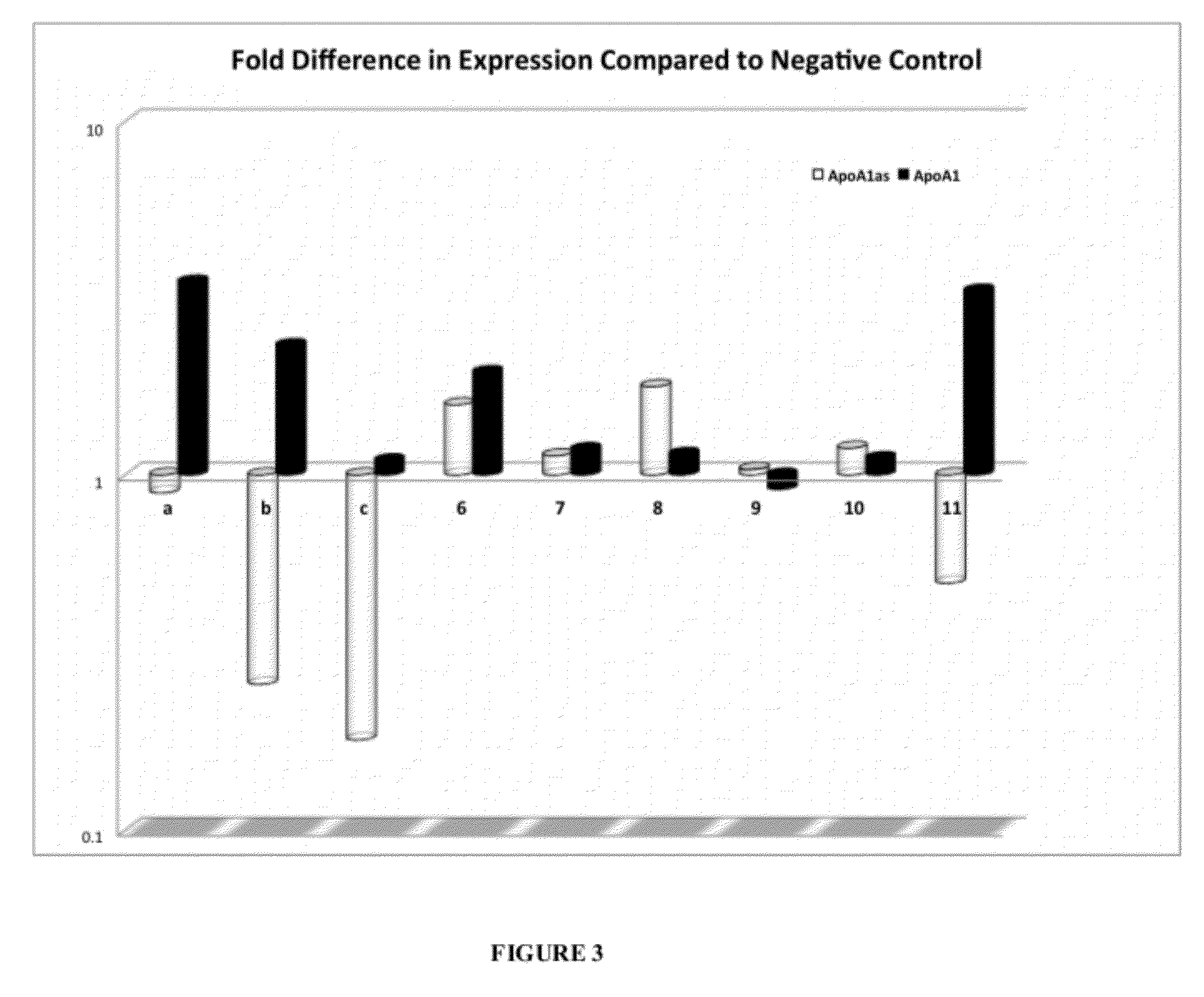
Treatment of Apolipoprotein-A1 Related Diseases by Inhibition of Natural Antisense Transcript to Apolipoprotein-A1
ActiveUS20100105760A1Modulate its functionNervous disorderAntipyreticApolipoproteins EPolynucleotide
Oligonucleotide compounds modulate expression and / or function of an apolipoprotein (ApoA1) polynucleotides and encoded products thereof. Methods for treating diseases associated with apolipoprotein-A1 (ApoA1) comprise administering one or more Oligonucleotide compounds designed to inhibit the Apo-A1 natural antisense transcript to patients.
Owner:CURNA INC
Statin and omega-3 fatty acids for reduction of apo-b levels
InactiveUS20080085911A1Reduce additionalAvoid loweringBiocideMetabolism disorderDyslipidemiaApolipoproteins E
Methods of utilizing a combined administration or a unit dosage of a combination of an HMG-CoA inhibitor and omega-3 fatty acids for the reduction of apolipoprotein-B levels. The methods are especially useful in the treatment of patients with hypertriglyceridemia or hypercholesterolemia or mixed dyslipidemia, coronary heart disease (CHD), vascular disease, atherosclerotic disease and related conditions, and for the prevention or reduction of cardiovascular, cardiac, and vascular events.
Owner:RELIANT PHARMACEUTICALS INC
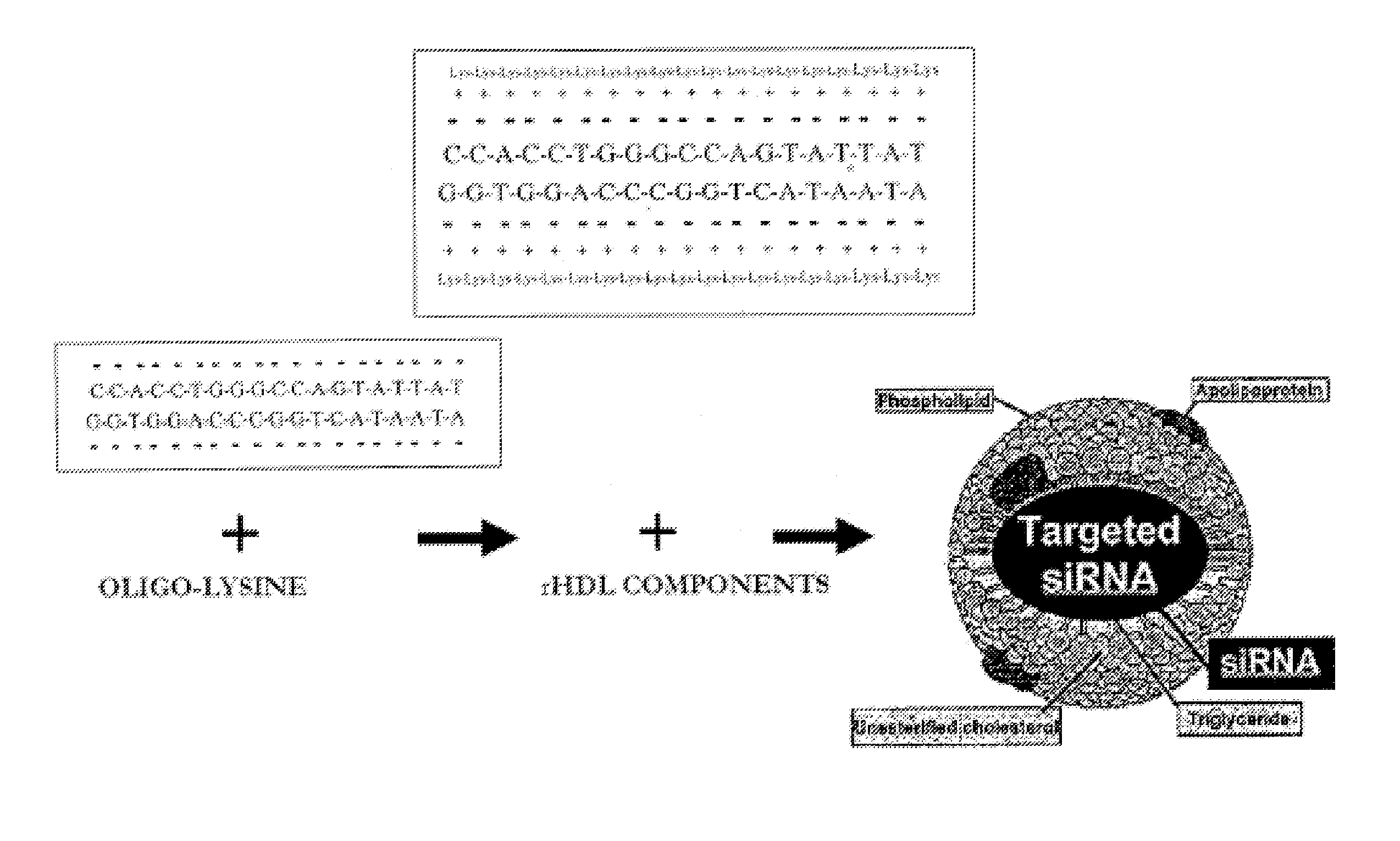
HDL particles for delivery of nucleic acids
InactiveUS8734853B2Efficient deliveryHigh densityPowder deliveryOrganic active ingredientsApolipoproteins EHDL particle
Disclosed are high density lipoprotein-nucleic acid particles, wherein the particles include (a) an apolipoprotein; (b) a nucleic acid component comprising a therapeutic nucleic acid segment; and (c) a polypeptide comprising a positively charged region, wherein the positively-charged region of the polypeptide associates with the nucleic acid component. Also disclosed are pharmaceutical compositions that include a) an apolipoprotein; (b) a nucleic acid component comprising a therapeutic nucleic acid segment; and (c) a polypeptide comprising a positively charged region. Methods that concern the particles and pharmaceutical compositions of the present invention are also set forth, as well as kits.
Owner:UNIV OF NORTH TEXAS HEALTH SCI CENT +1
Lipoproteins as nucleic acid vectors
InactiveUS6635623B1Restores growth suppression controlReduce the numberBiocideApolipeptidesApolipoproteins ELow-density lipoprotein
The present invention relates to materials and methods for the in vivo transport and deliver of nucleic acids. More particularly, it concerns the use of lipoproteins, including but not limited to, low density lipoproteins ("LDL"), and / or apolipoproteins for the binding and in vivo transport of nucleic acids. In addition, the present invention relates to the use of lipoproteins in the early detection of cancer and / or metastatic cancer and / or arteriosclerosis.
Owner:BAYLOR COLLEGE OF MEDICINE
Apolipoprotein analogues
InactiveUS20020156007A1Antibody mimetics/scaffoldsGenetic material ingredientsCubilinLipid formation
The invention relates to a pharmaceutical composition comprising an apolipoprotein construct, to an apolipoprotein construct, a nucleic acid sequence encoding the apolipoprotein construct, a vector comprising the nucleic acid sequence, a method for producing the apolipoprotein construct, and a method of treatment comprising administering the apolipoprotein construct. The presented data document that the constructs according to the invention are capable of binding lipids, are capable of binding cubilin, which is a strong Apo Al receptor, stronger than native Apo A-I and that the plasma half life of the constructs is at least tripled compared to native Apo A-I. Together these data document that the constructs according to the invention are strong candidates for treatment of cardiovascular diseases.
Owner:F HOFFMANN LA ROCHE & CO AG
Modulation of apolipoprotein (a) expression
Compounds, compositions and methods are provided for modulating the expression of apolipoprotein(a). The compositions comprise oligonucleotides, targeted to nucleic acid encoding apolipoprotein(a). Methods of using these compounds for modulation of apolipoprotein(a) expression and for diagnosis and treatment of disease associated with expression of apolipoprotein(a) are provided.
Owner:IONIS PHARMA INC
Antisense modulation of apolipoprotein b expression
InactiveUS20080242629A1Organic active ingredientsSugar derivativesApolipoproteins bApolipoproteins E
Antisense compounds, compositions and methods are provided for modulating the expression of apolipoprotein B. The compositions comprise antisense compounds, particularly antisense oligonucleotides, targeted to nucleic acids encoding apolipoprotein B. Methods of using these compounds for modulation of apolipoprotein B expression and for treatment of diseases associated with expression of apolipoprotein B are provided.
Owner:KASTLE THERAPEUTICS LLC
Method of treating dyslipidemic disorders
InactiveUS20040067873A1Faster and easy administrationImproved patient comfortOrganic active ingredientsPeptide/protein ingredientsDyslipidemiaMedicine
The invention provides methods of treating or preventing a condition or disorder associated with dyslipidemia with compositions comprising apolipoprotein-sphingomyelin complexes. The methods of the invention permit reduction, by 4- to 20-fold, of the amount of apolipoprotein required for therapeutic administration to bring about an ameliorative effect.
Owner:PFIZER INC
Effects of apolipoprotein b inhibition on gene expression profiles in animals
Methods are provided for modulating the expression of genes involved in lipid metabolism, useful in the treatment of conditions associated with cardiovascular risk. Antisense oligonucleotides targeted to apolipoprotein B reduce the level of apolipoprotein B mRNA, lower serum cholesterol and shift liver gene expression profiles from those of an obese animal towards those of a lean animal. Further provided are methods for improving the cardiovascular risk of a subject through antisense inhibition of apolipoprotein B. Also provided are methods for employing antisense oligonucleotides targeted to apolipoprotein B to modulate a cellular pathway or metabolic process.
Owner:KASTLE THERAPEUTICS LLC
Topical delivery of therapeutic agents using cell-penetrating peptides for the treatment of age-related macular degeneration and other eye diseases
ActiveUS20190015521A1Hydroxy compound active ingredientsPeptide/protein ingredientsAntioxidantApolipoproteins E
The present disclosure provides therapeutic agents for the treatment of age-related macular degeneration (AMD) and other eye disorders. One or more therapeutic agents can be used to treat any stages (including the early, intermediate and advance stages) of AMD, and any phenotypes of AMD, including geographic atrophy (including non-central GA and central GA) and neovascularization (including types 1, 2 and 3 NV). In some embodiments, the one or more therapeutic agents are or include an anti-dyslipidemic agent, an antioxidant, an anti-inflammatory agent, a complement inhibitor, a neuroprotector or an anti-angiogenic agent, or any combination thereof. In certain embodiments, the one or more therapeutic agents are or include an anti-dyslipidemic agent (e.g., an apolipoprotein mimetic or / and a statin). In some embodiments, the one or more therapeutic agents are mixed with, non-covalently associated with or covalently bonded to a cell-penetrating peptide (CPP), encapsulated in CPP-conjugated nanoparticles, micelles or liposomes, or modified (e.g., stapled, prenylated, lipidated or coupled to a small-molecule α-helix mimic) to acquire membrane-translocating ability. In certain embodiments, the one or more therapeutic agents are administered by eye drop.
Owner:MACREGEN INC
Antisense modulation of apolipoprotein b expression
InactiveUS20090326040A1Organic active ingredientsMetabolism disorderApolipoproteins EApolipoproteins b
Methods for the rapid and long-term lowering of lipid levels in human subjects and for the treatment of conditions associated with elevated LDL-cholesterol and elevated apolipoprotein B are provided.
Owner:KASTLE THERAPEUTICS LLC
Pharmaceutical composition comprising apolipoproteins for the treatment of human diseases
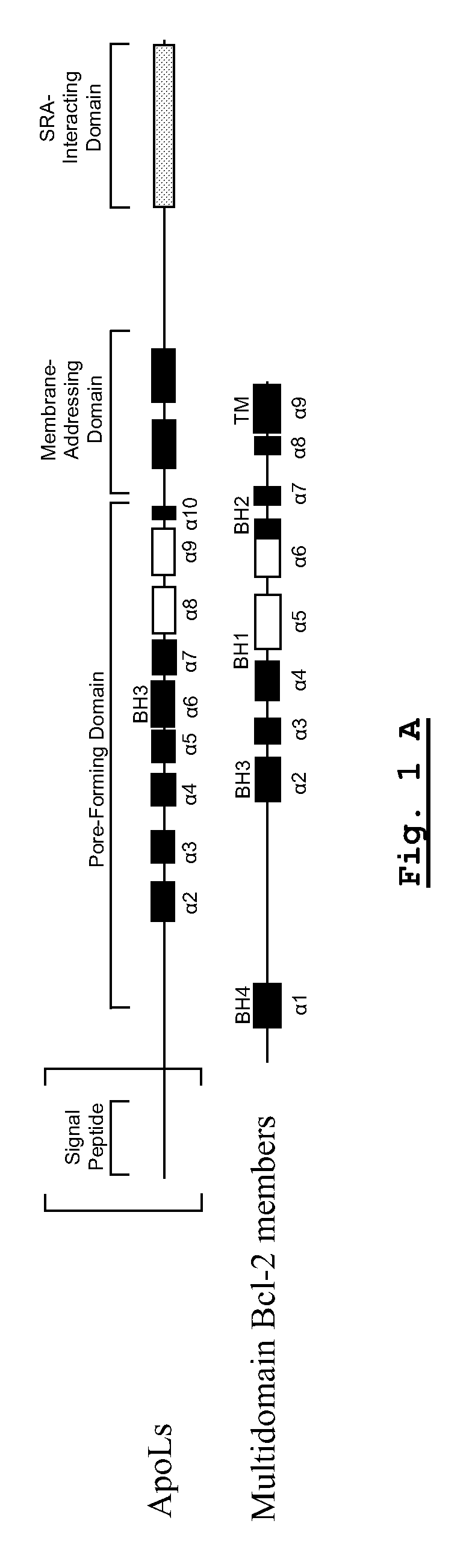
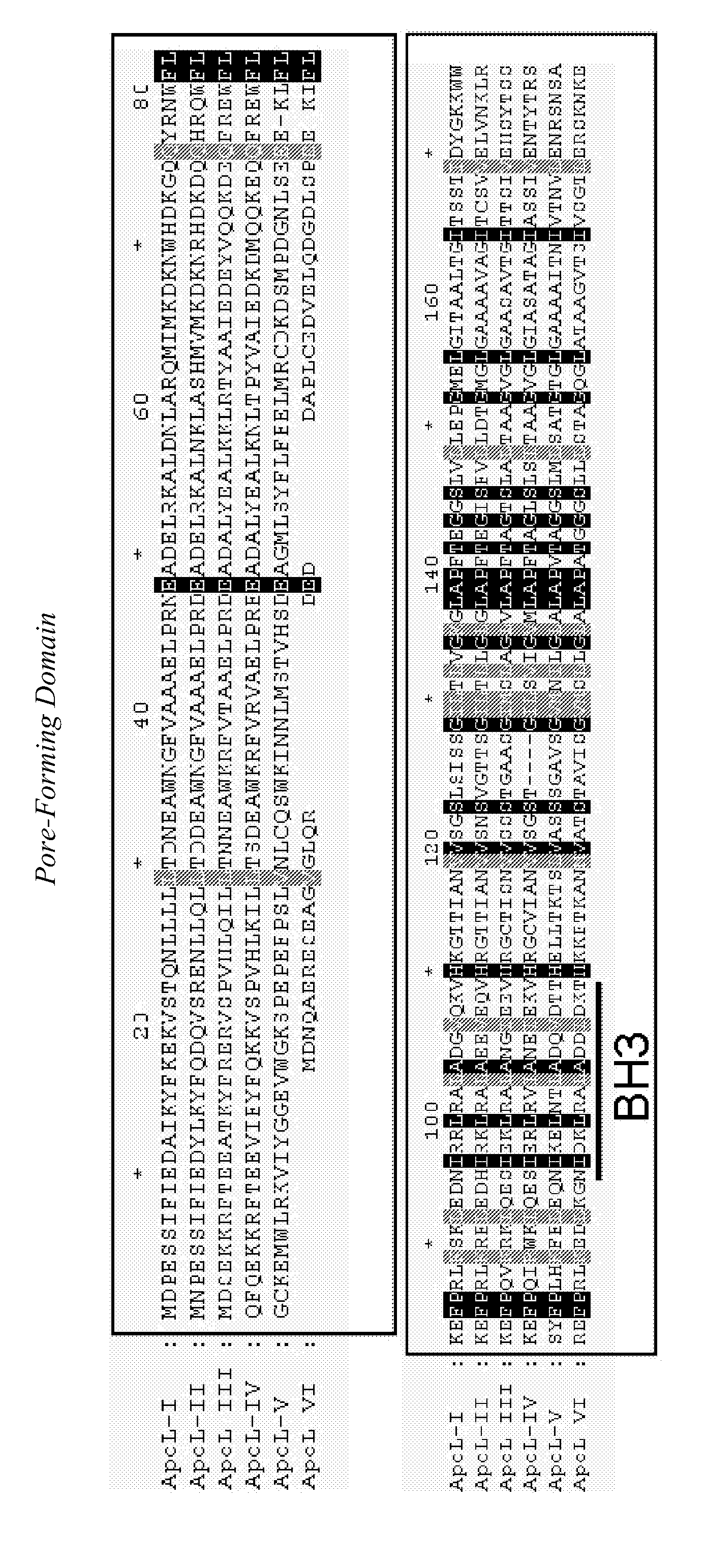
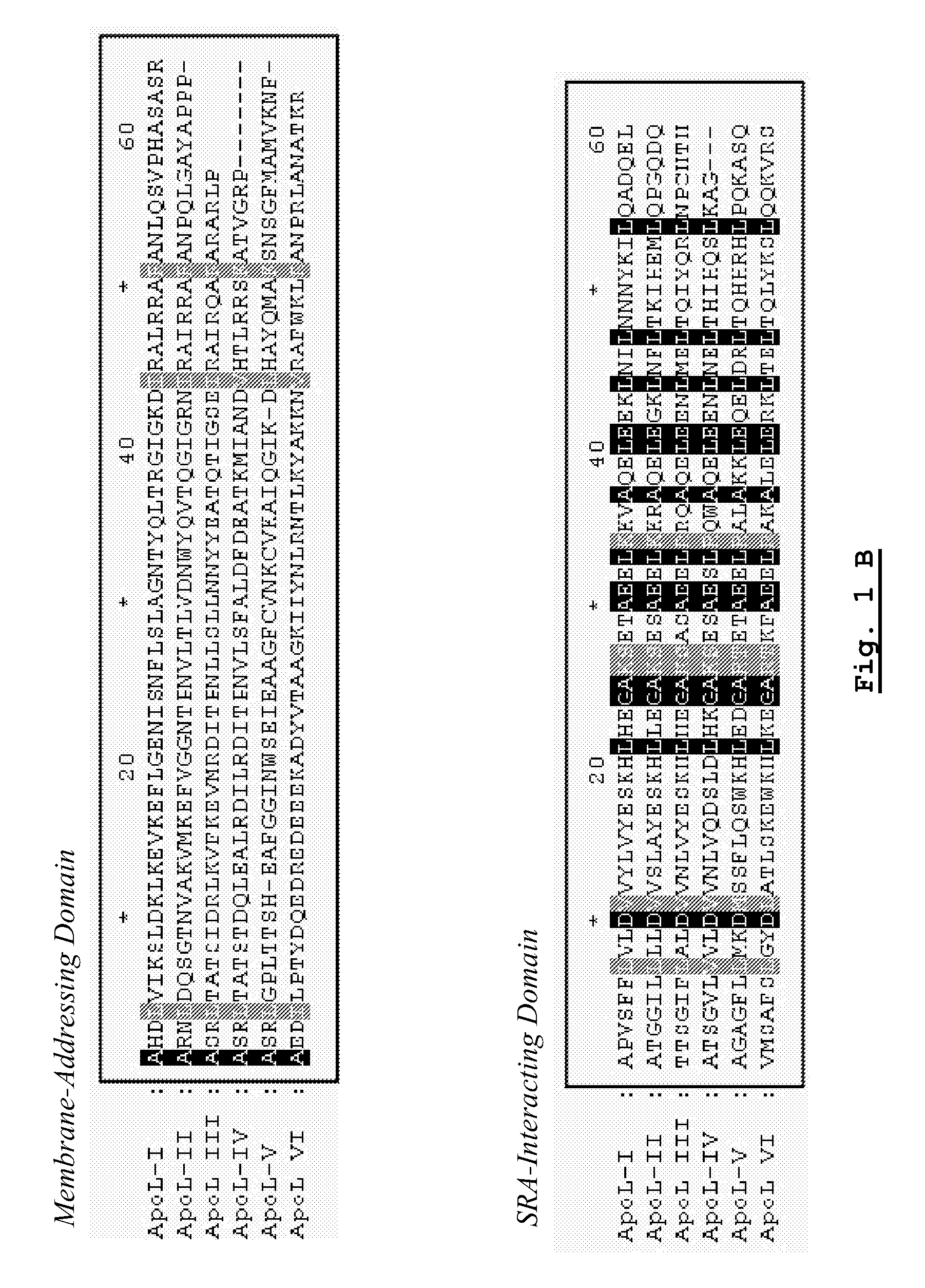
A pharmaceutical composition has an adequate pharmaceutical carrier or diluent and an element selected from a pharmaceutical composition having an adequate pharmaceutical carrier or diluent and an element selected from: —apolipoprotein apoL-III; —a pharmaceutically active fragment of this apolipoprotein apoL-III; —a nucleotide sequence encoding this apolipoprotein apoL-III or the fragment; —a vector having the nucleotide sequence; —a cell transformed by the nucleotide sequence or the vector; —an inhibitor or activator directed against the apolipoprotein apoL-III, its fragment or its nucleotide sequence, —an anti-inhibitor or anti-activator directed against the inhibitor or activator or its encoding nucleotide sequence.
Owner:UNIV LIBRE DE BRUXELIES
Methods for purification of alpha-1-antitrypsin andapolipoprotein a-1
ActiveUS20110087008A1Minimize deamidationMinimize denaturationApolipeptidesPeptide/protein ingredientsPurification methodsApolipoproteins E
This invention relates to protein separation and purification methods for both alpha-1-antitrypsin (AAT, also known as alpha-1 proteinase inhibitor, API, and A1-PI) and Apolipoprotein A-I (ApoA-1) from, for example, a fraction of human blood plasma. In certain embodiments, the invention provides methods for separating AAT from ApoA-1 at the initial stage of purification, so that the same starting material can be used as a source for both proteins. The methods further pertain to providing compositions of AAT and of ApoA-1 suitable for pharmaceutical use and are suitable for large-scale purification.
Owner:CSL BEHRING GMBH
Telodendrimer nanodiscs without apolipoprotein
The present invention provides a nanodisc without a membrane scaffold protein. The nanodisc includes a telodendrimer and a lipid; the nanodisc does not include a membrane scaffold protein. The telodendrimer has the general formula PEG-L-D-(R)n, wherein D is a dendritic polymer; L is a bond or a linker linked to the focal point group of the dendritic polymer; each PEG is a poly(ethylene glycol) polymer; each R is and end group of the dendritic polymer, or and end group with a covalently bound hydrophobic group, hydrophilic group, amphiphilic compound, or drug; and subscript n is an integer from 2 to 20. Methods of making the nanodiscs are also provided.
Owner:RGT UNIV OF CALIFORNIA
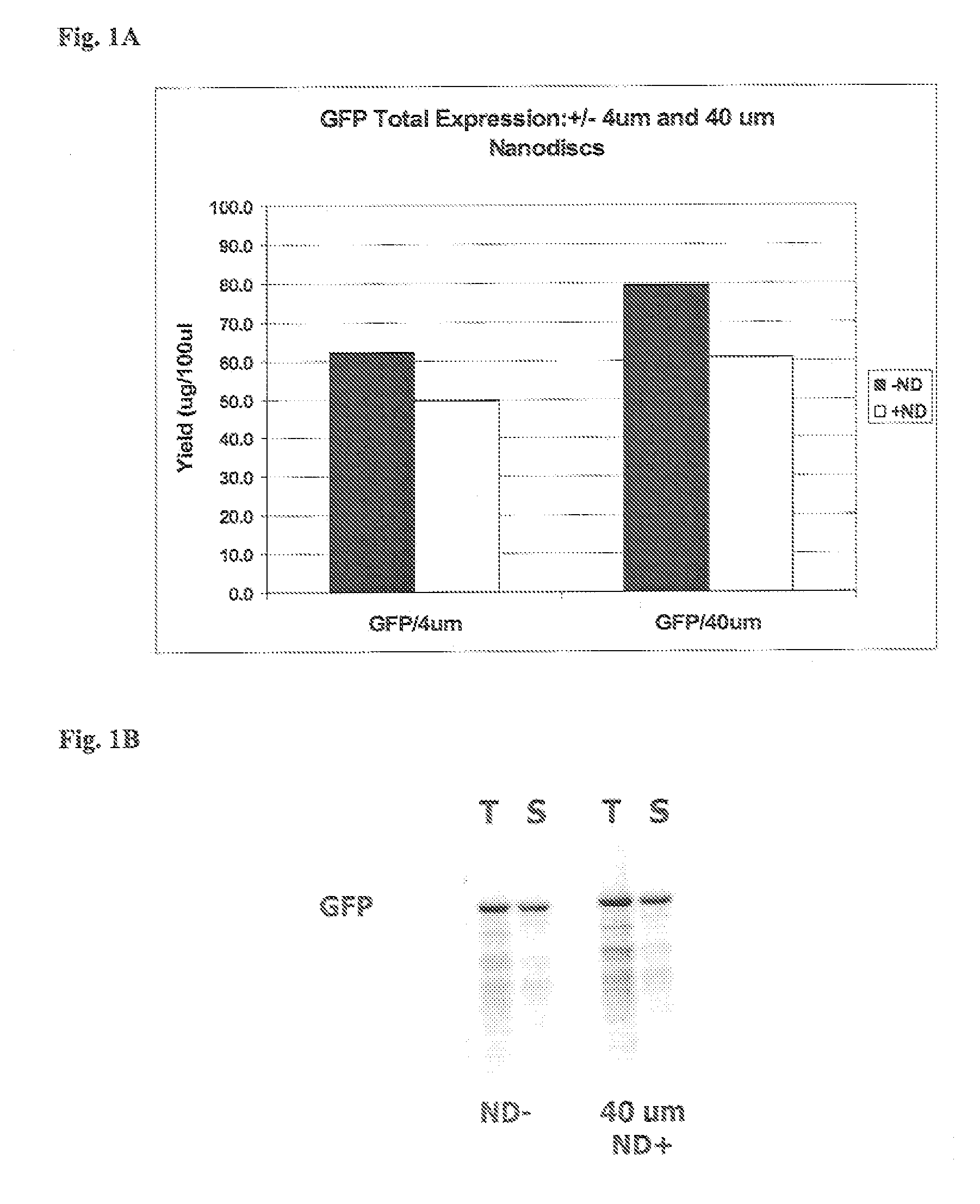
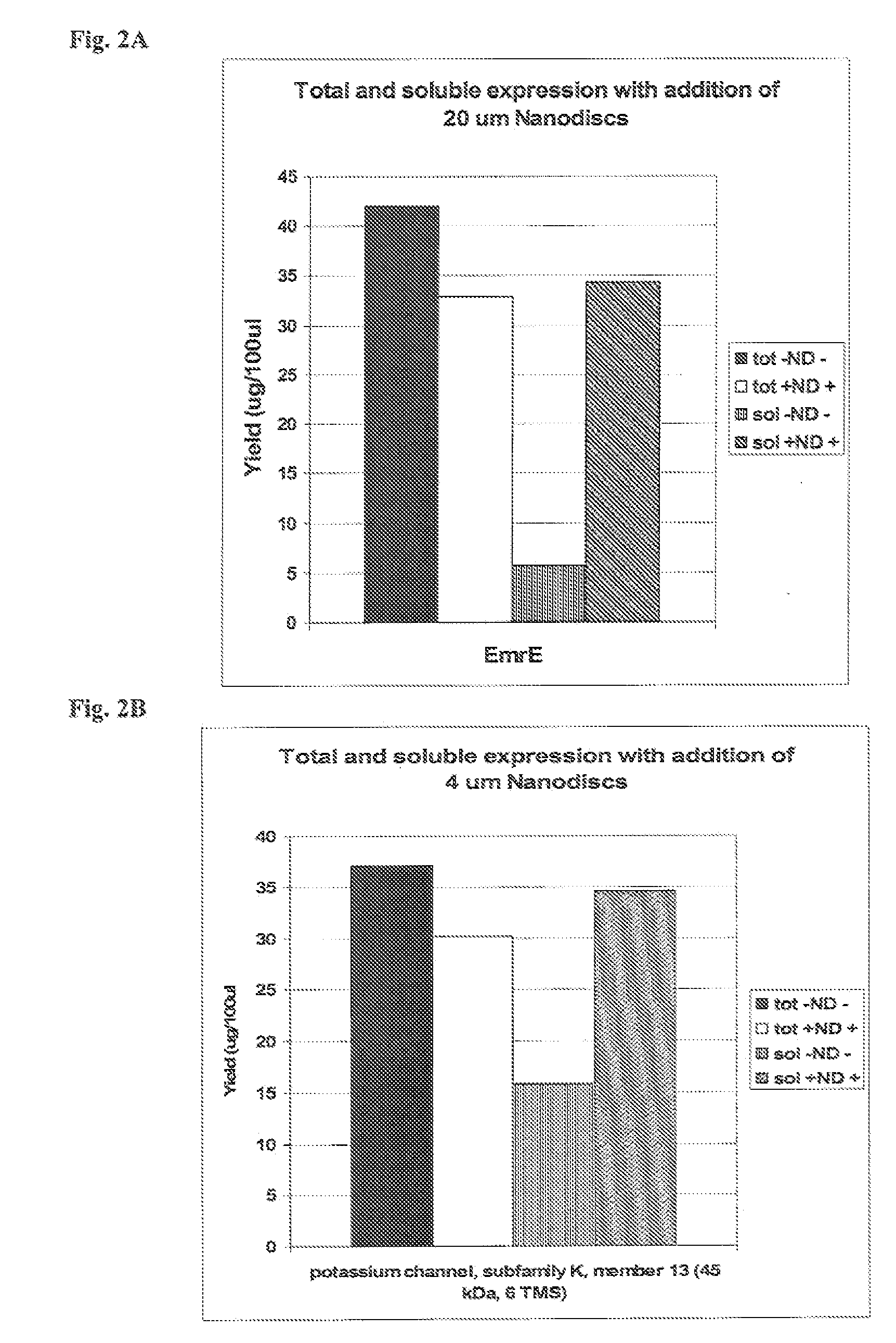
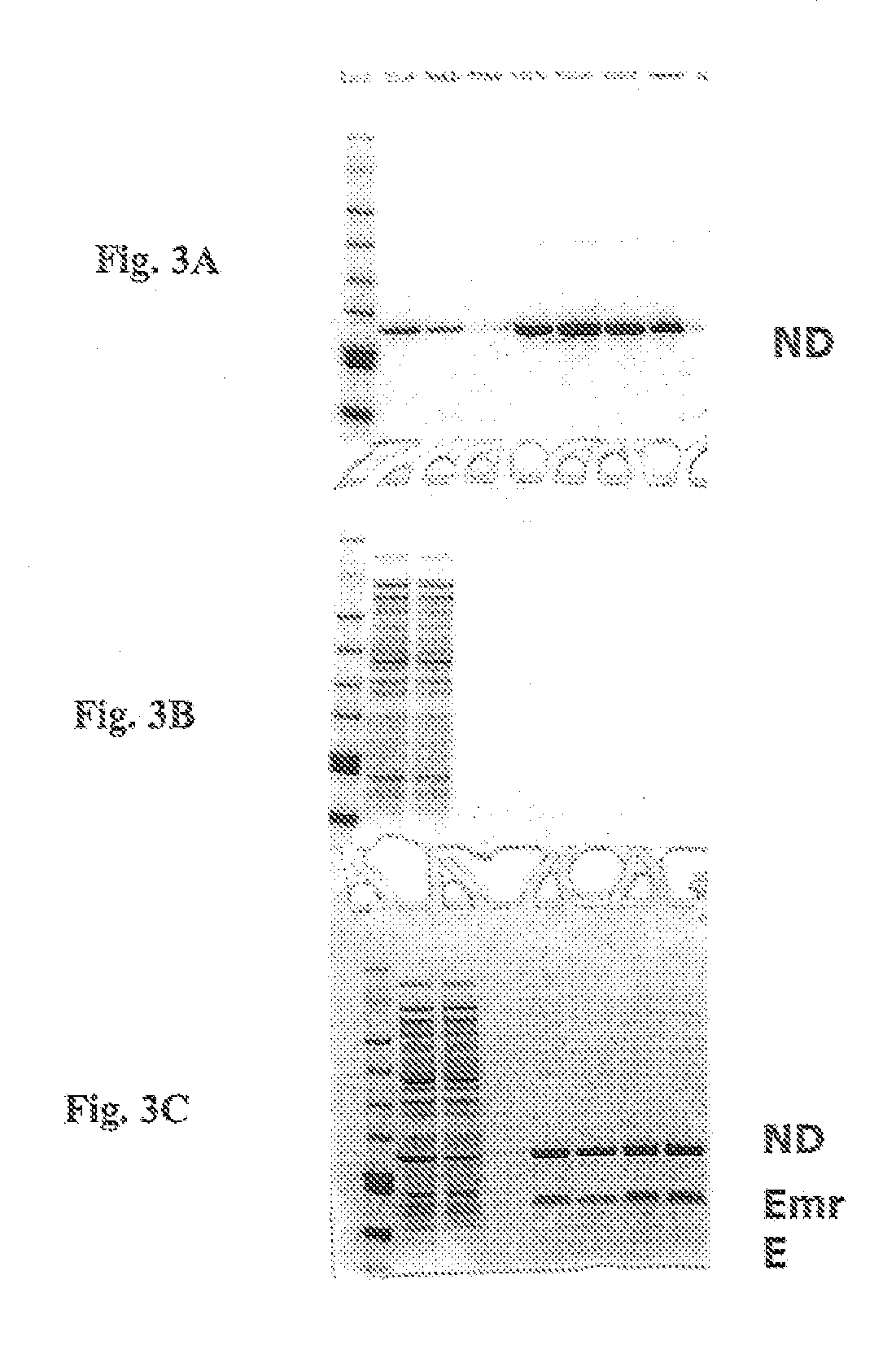
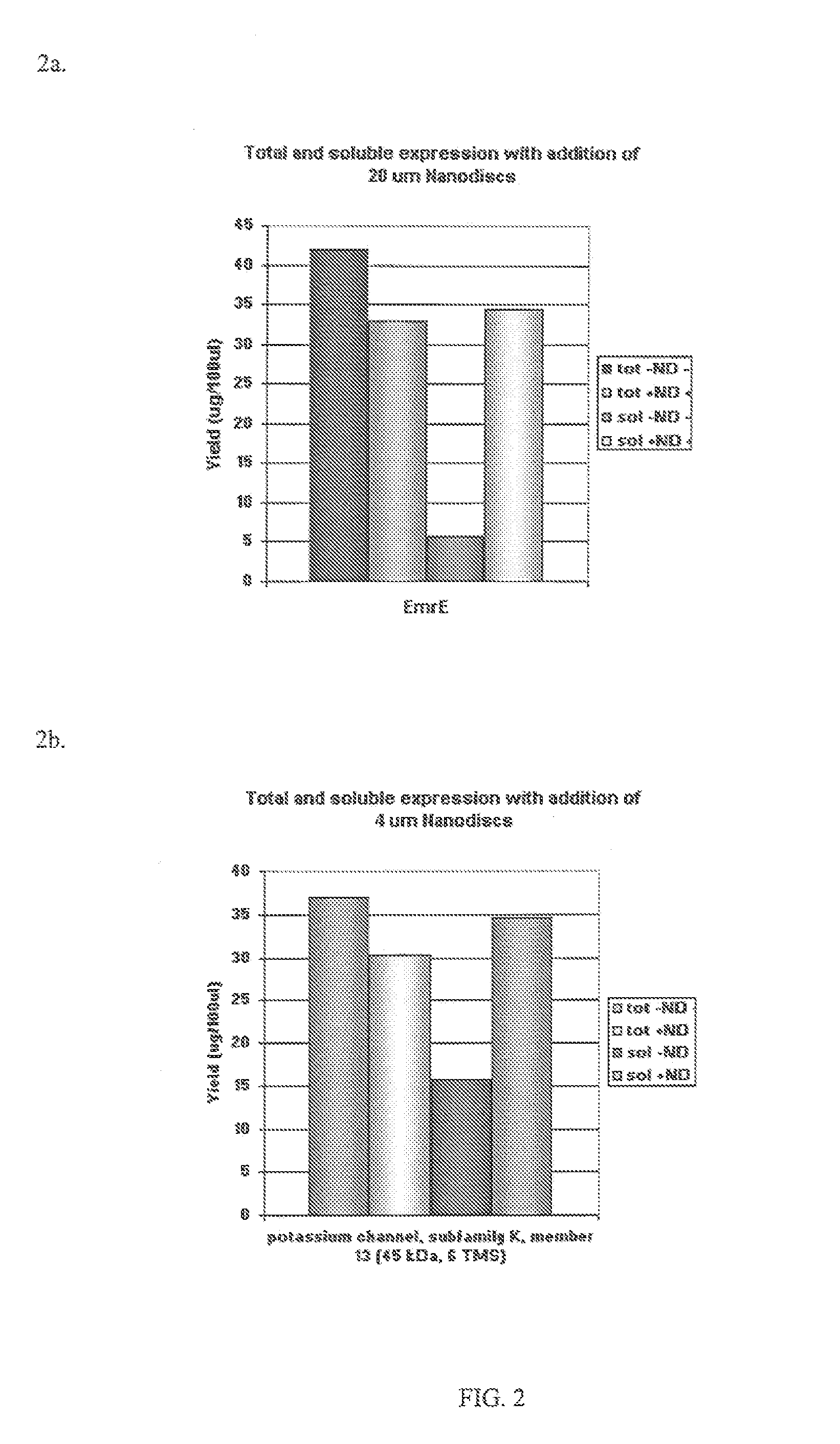
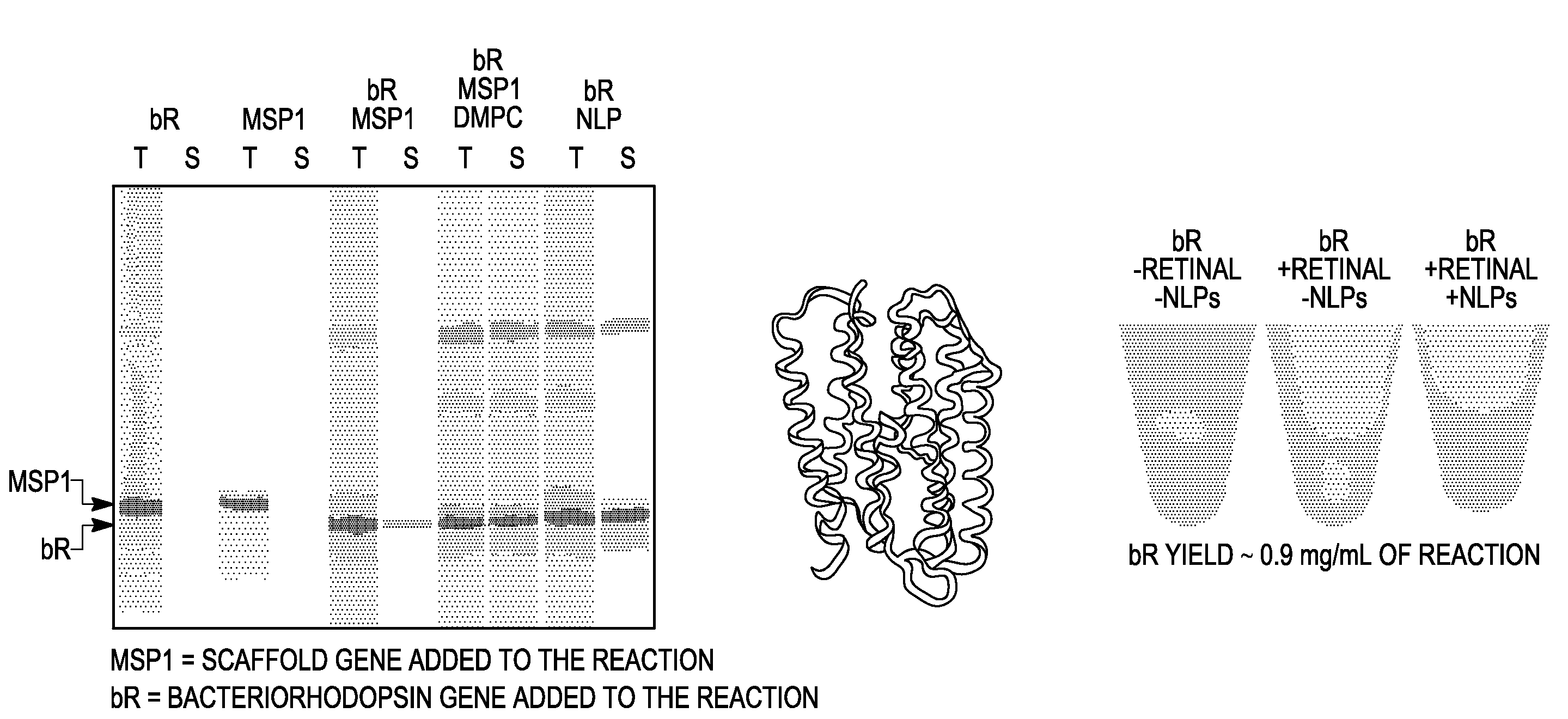
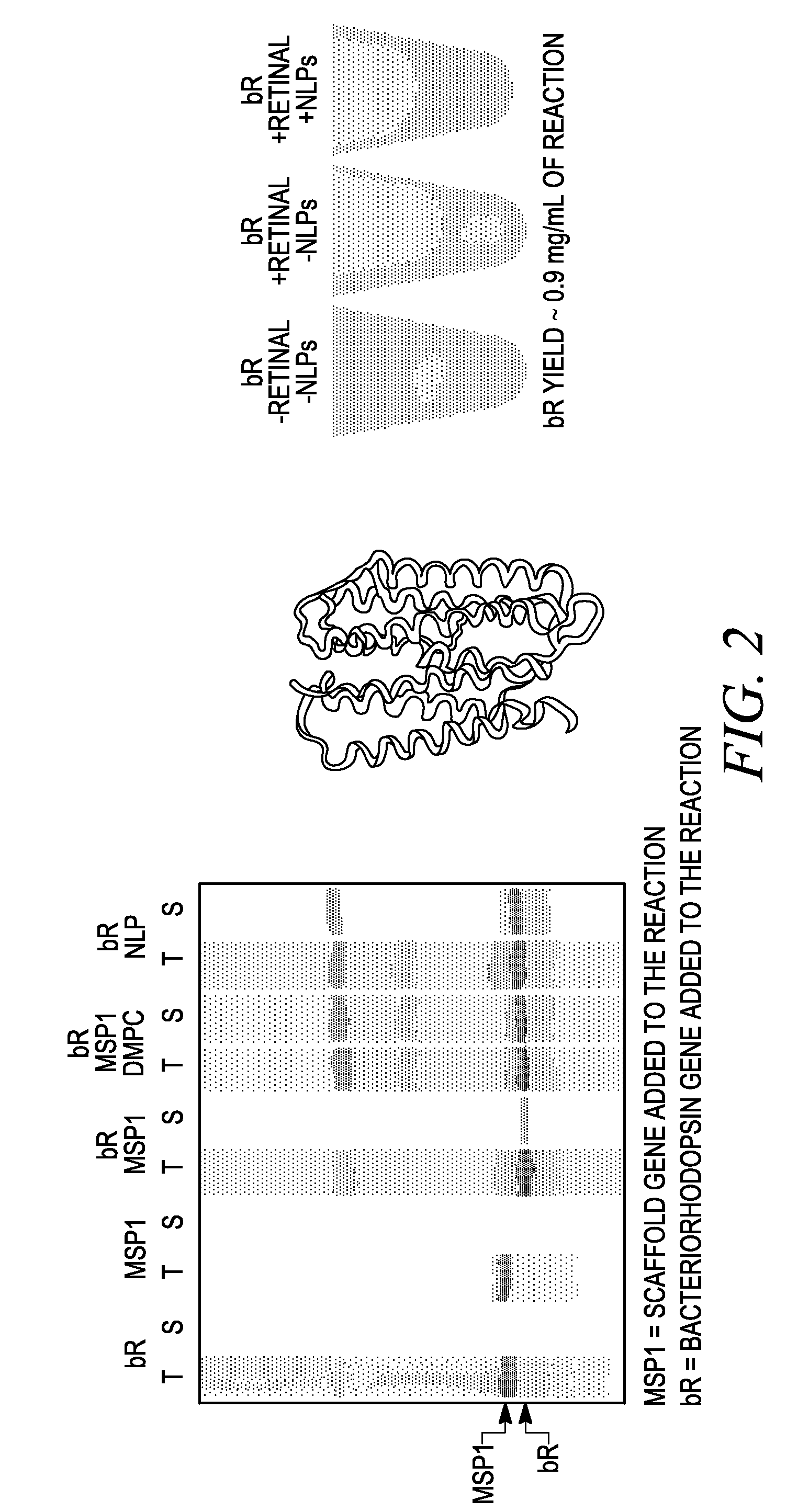
In vitro protein synthesis systems for membrane proteins that include adolipoproteins and phospholipid-adolipoprotein particles
In vitro protein synthesis systems and methods are provided that produce membrane proteins in soluble form. In some aspects, the invention provides methods of synthesizing proteins using in vitro protein synthesis systems that include an apolipoprotein, in which higher yields of soluble protein are produced than in the absence of the apolipoprotein. Apolipoproteins useful in the present invention include naturally occurring apolipoproteins, as well as sequence variants of wild-type apolipoproteins, and engineered apolipoproteins. The apolipoproteins can be provided in an in vitro protein synthesis system associated with lipid or not associated with lipid. The invention also provides compositions and kits for synthesis of proteins in soluble form, in which the compositions and kits include cell extracts for protein translation and at least one apolipoprotein biomolecule.
Owner:LIFE TECH CORP
Treatment of apolipoprotein-A1 related diseases by inhibition of natural antisense transcript to apolipoprotein-A1
Oligonucleotide compounds modulate expression and / or function of an apolipoprotein (ApoA1) polynucleotides and encoded products thereof. Methods for treating diseases associated with apolipoprotein-A1 (ApoA1) comprise administering one or more Oligonucleotide compounds designed to inhibit the Apo-A1 natural antisense transcript to patients.
Owner:CURNA INC
In Vitro Protein Synthesis Systems for Membrane Proteins that Include Adolipoproteins and Phospholipid Adolipoprotein Particles
In vitro protein synthesis systems and methods are provided that produce membrane proteins in soluble form. In some aspects, the invention provides methods of synthesizing proteins using in vitro protein synthesis systems that include an apolipoprotein, in which higher yields of soluble protein are produced than in the absence of the apolipoprotein. Apolipoproteins useful in the present invention include naturally occurring apolipoproteins, as well as sequence variants of wild-type apolipoproteins, and engineered apolipoproteins. The apolipoproteins can be provided in an in vitro protein synthesis system associated with lipid or not associated with lipid. The invention also provides compositions and kits for synthesis of proteins in soluble form, in which the compositions and kits include cell extracts for protein translation and at least one apolipoprotein biomolecule.
Owner:LIFE TECH CORP
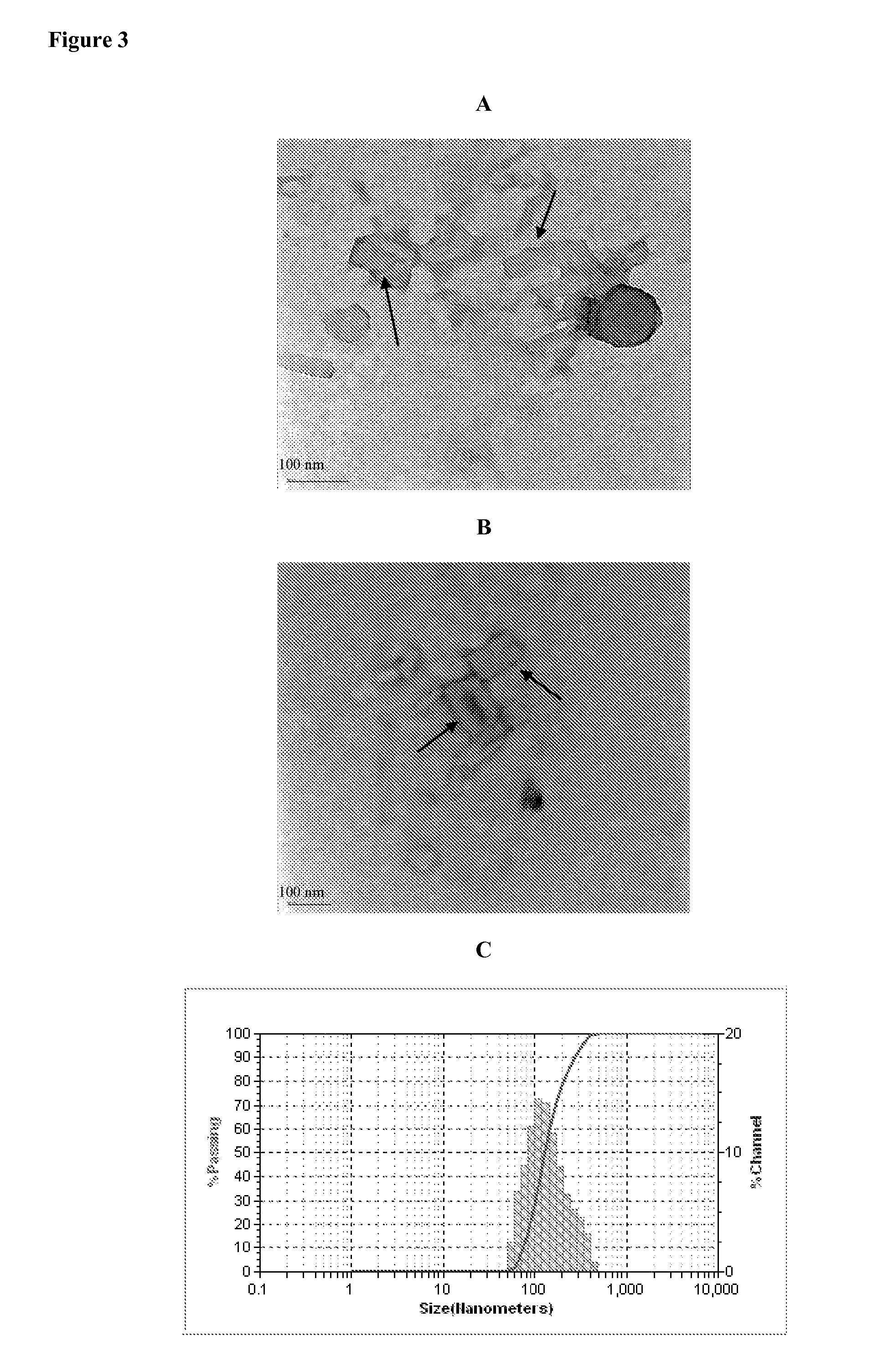
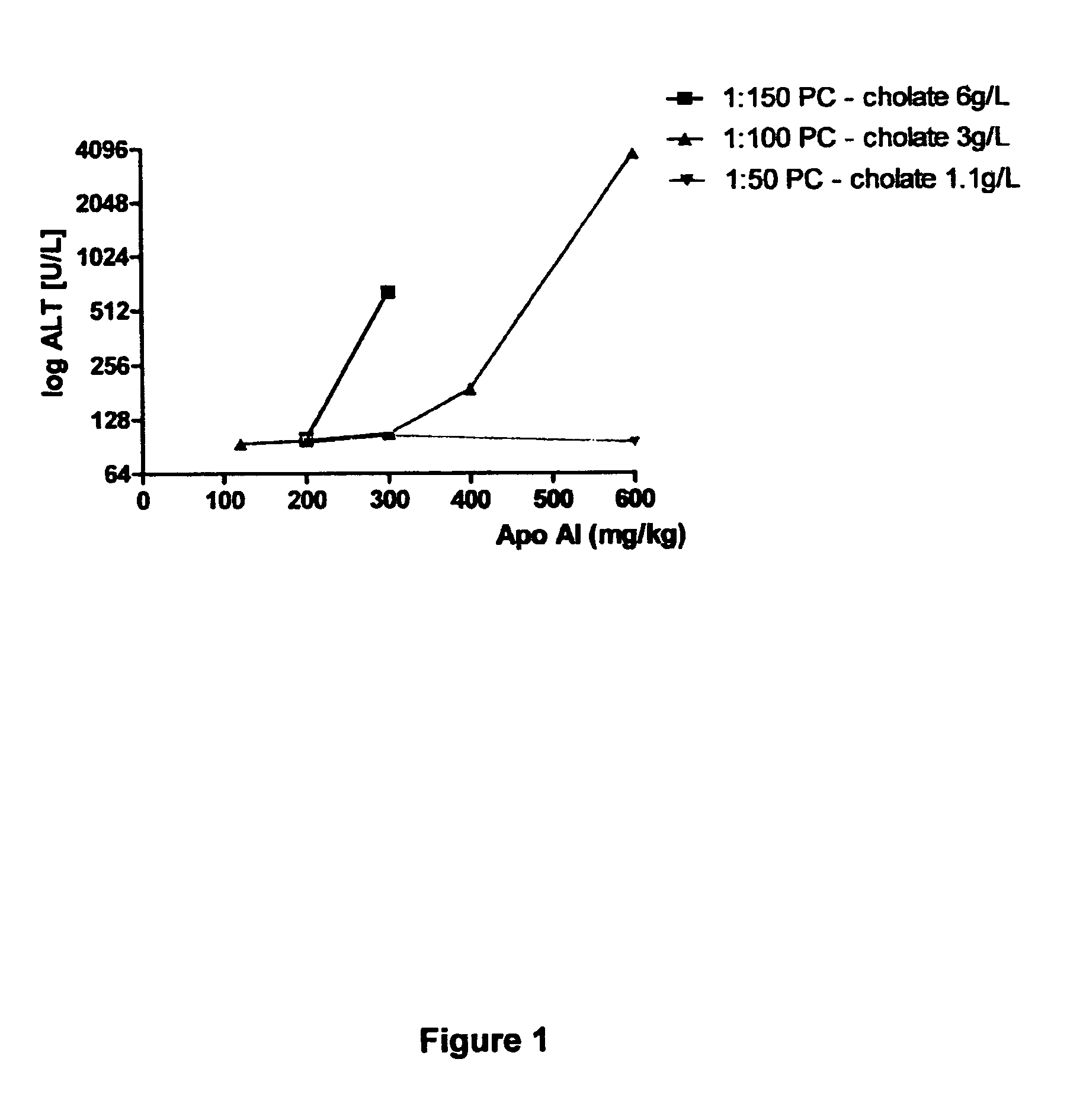
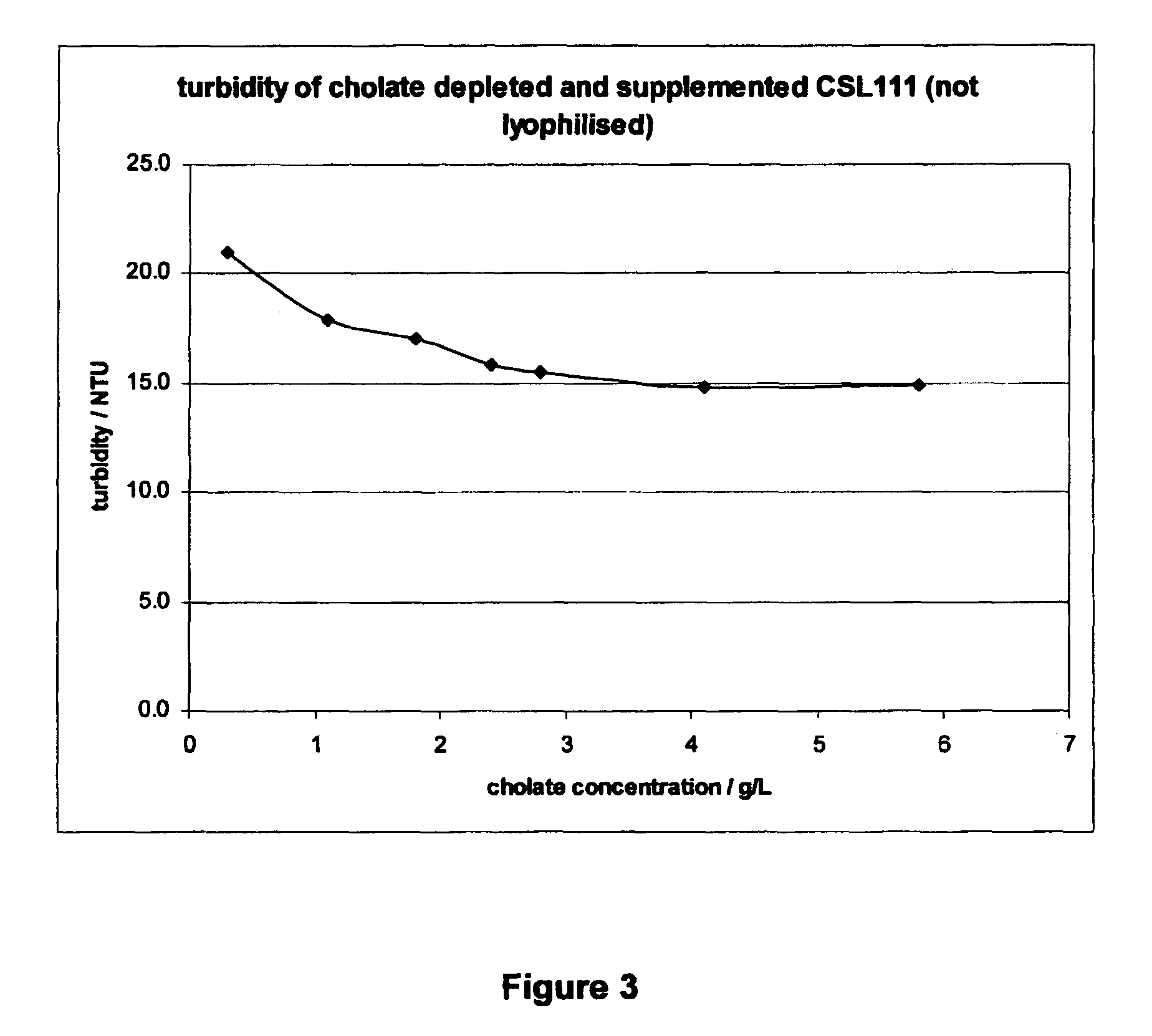
Reconstituted HDL formulation
ActiveUS9125943B2Reduce riskImprove stabilityApolipeptidesPeptide/protein ingredientsApolipoproteins EHigh-density lipoprotein
The present invention relates to reconstituted high density lipoprotein (rHDL) formulations comprising an apolipoprotein, a lipid and a lyophilization stabilizer. Said formulations have reduced renal toxicity and good long-term stability, especially in lyophilized form.
Owner:CSL LTD
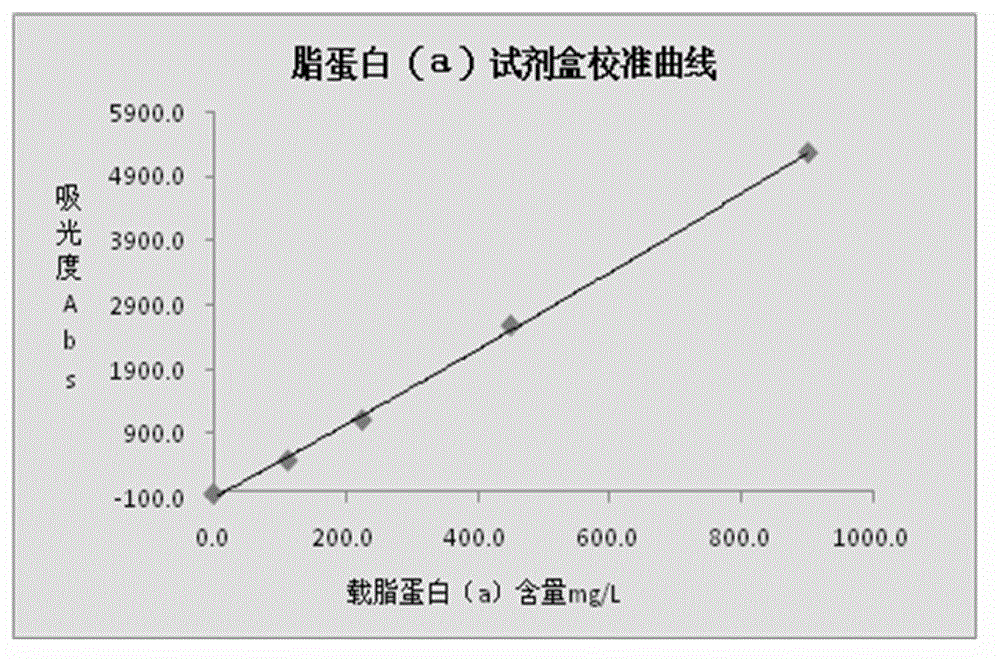
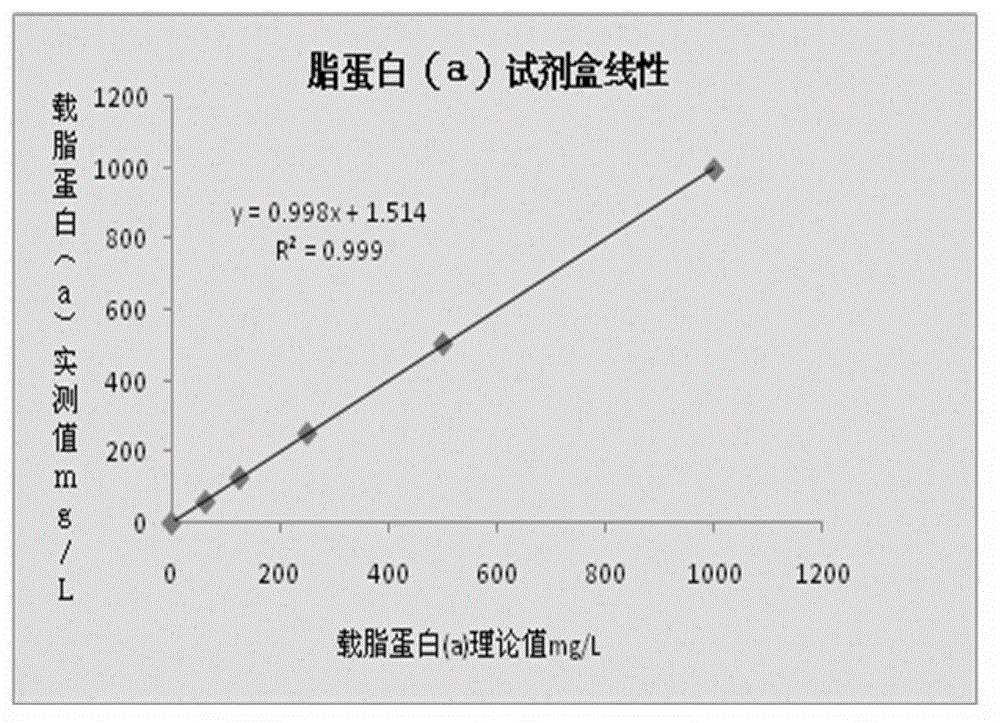
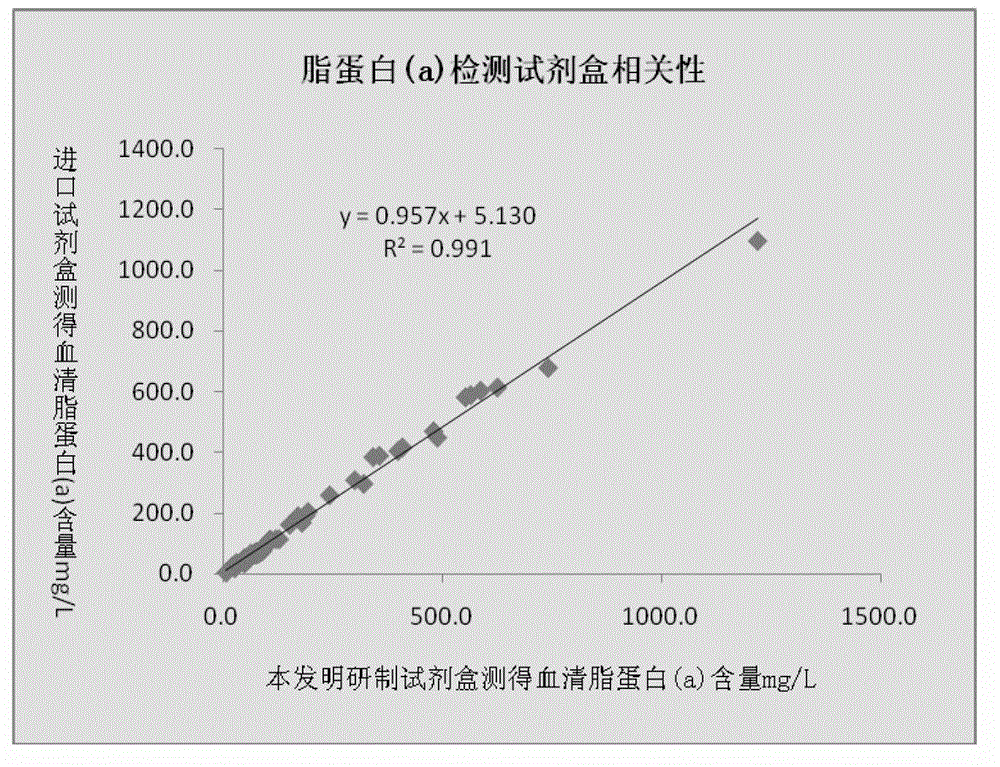
Lipoprotein (a) detection kit
ActiveCN103149370AImprove stabilityImprove bindingBiological testingHuman apolipoproteinA lipoprotein
The invention relates to a lipoprotein (a) detection kit which comprises a reagent R1, a reagent R2 and a reference substance, wherein the reagent R1 is an HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer system which comprises 0.5-10 g / L HEPES, 2-20 g / L sodium chloride, 0.05-1.0 ml / L Tween-20, 0.1-2 g / L bovine serum albumin, 5-25 g / L polyethyleneglycol-6000, 1-5 g / L EDTA (ethylene diamine tetraacetic acid) and 0.1-2 g / L Proclin 300; the reagent R2 comprises a polystyrene latex particle mixture which is prepared by a chemical crosslinking method, coated with an anti-human-apolipoprotein (a) polyclonal antibody and provided with carboxylic groups on the surface, a 0.5-10 g / L HEPES buffer solution and aspartame, wherein the weight-to-volume ratio of the aspartame to the reagent R2 is (0.01-0.5):100 (g / L); and the reference substance is a human serum or serum-matrix-like liquid with human recombinant apolipoprotein (a). The kit provided by the invention has the advantages of high detection sensitivity, high accuracy, favorable precision, favorable linearity within detection range, high stability, low production cost and low blank absorbance, and has anti-interference performance.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
A beta targeted recombinant lipoprotein nano drug carrier and preparation method and application thereof
InactiveCN104138600AAddressing Scarcity of SourcesSimple preparationNervous disorderGenetic material ingredientsDiseaseLipid formation
The invention discloses an A beta targeted recombinant lipoprotein nano drug carrier and a preparation method and application thereof. The A beta targeted recombinant lipoprotein nano drug carrier comprises lipid, apolipoprotein and a drug, the drug is an alzheimer disease targeting therapeutic or diagnostic drug, and comprises one or a plurality of small molecule chemical therapeutic drugs and large molecule polypeptide, protein, gene therapeutic and diagnostic drugs. By construction of the drug carrier, the defects of scarce sources, cumbersome preparation, low quality controllability and the like of natural lipoprotein can be solved, and the drug carrier provides a new and more effective and safe solution for delivery of the alzheimer disease targeting therapeutic or diagnostic drug, and has the important research value and clinical application prospects.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Artificial low-density lipoprotein carriers for transport of substances across the blood-brain barrier
InactiveUS7803400B2Convenience to mergeFacilitate and improve treatmentBiocidePowder deliveryDiseaseLipid formation
This invention relates to a highly efficient artificial low-density lipoprotein (LDL) carrier system for the targeted delivery therapeutic agents across the blood-brain barrier (BBB). In particular, this invention relates to artificial LDL particles comprised of three lipid elements: phosphatidyl choline, fatty-acyl-cholesterol esters, and at least one apolipoprotein. The present invention further relates to compositions, methods and kits comprising artificial LDL particles for targeting drugs to and across the BBB for the prevention and treatment of brain diseases.
Owner:WEST VIRGINIA UNIVERSITY
Methods of modulating lipid metabolism and storage
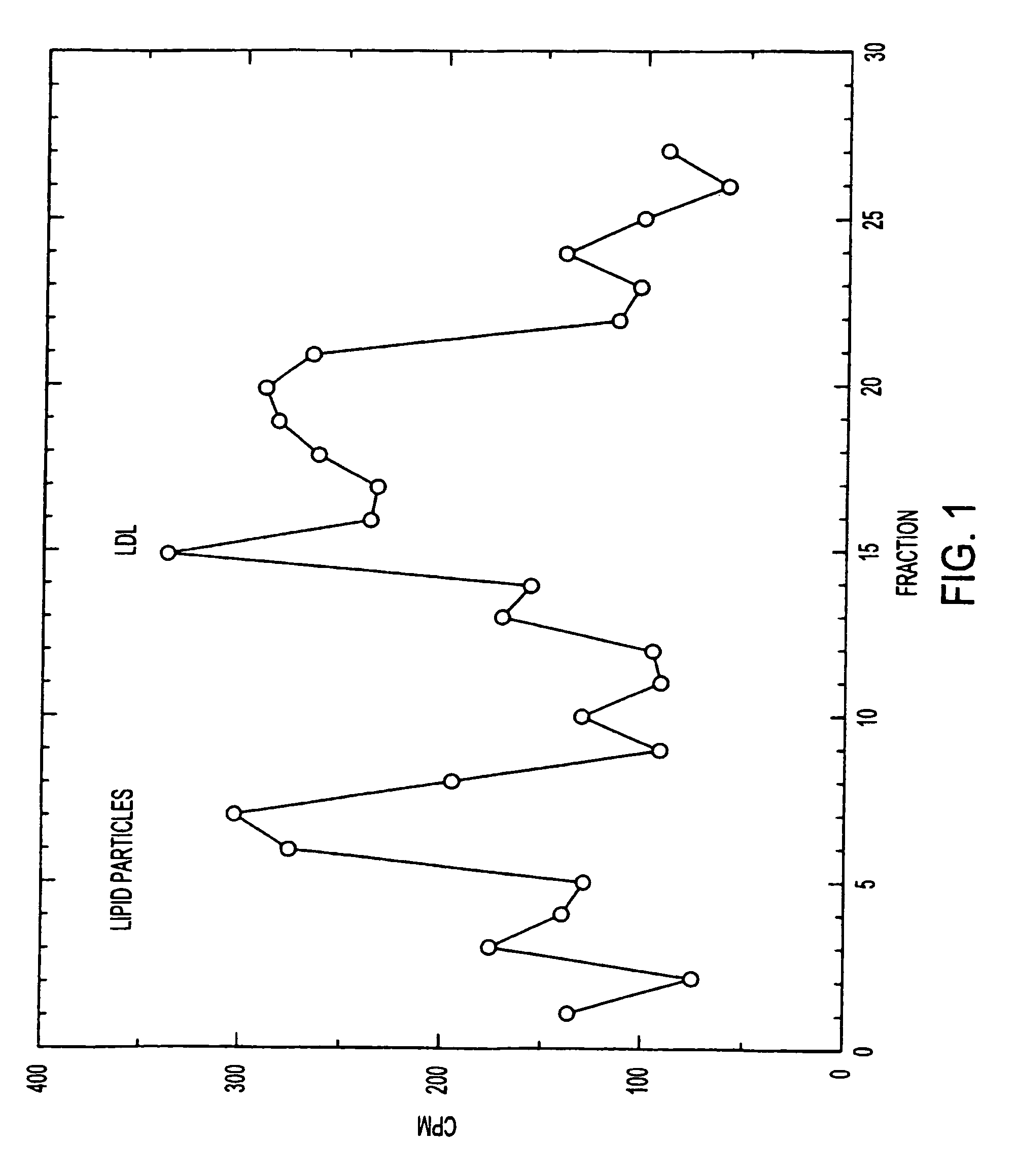
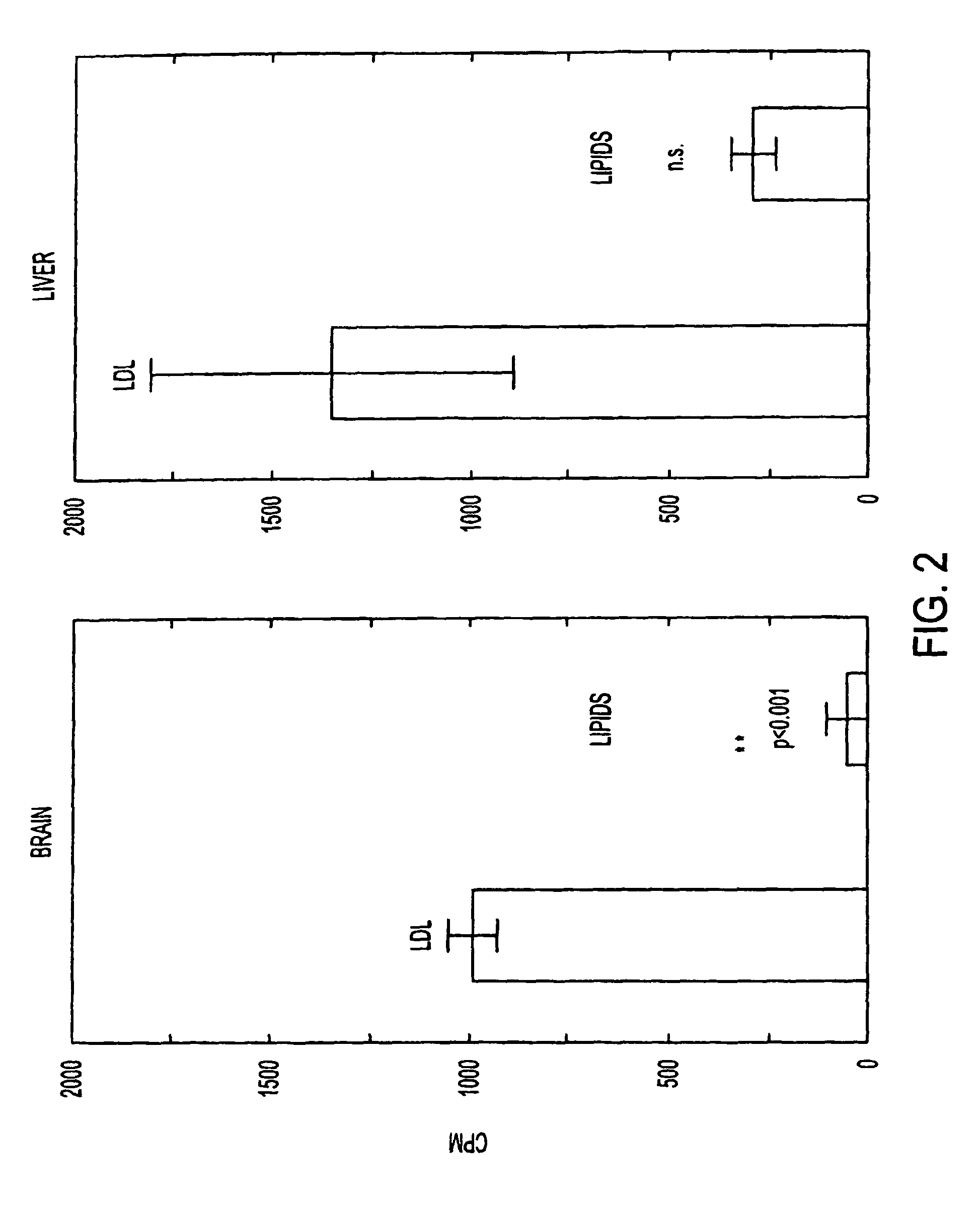
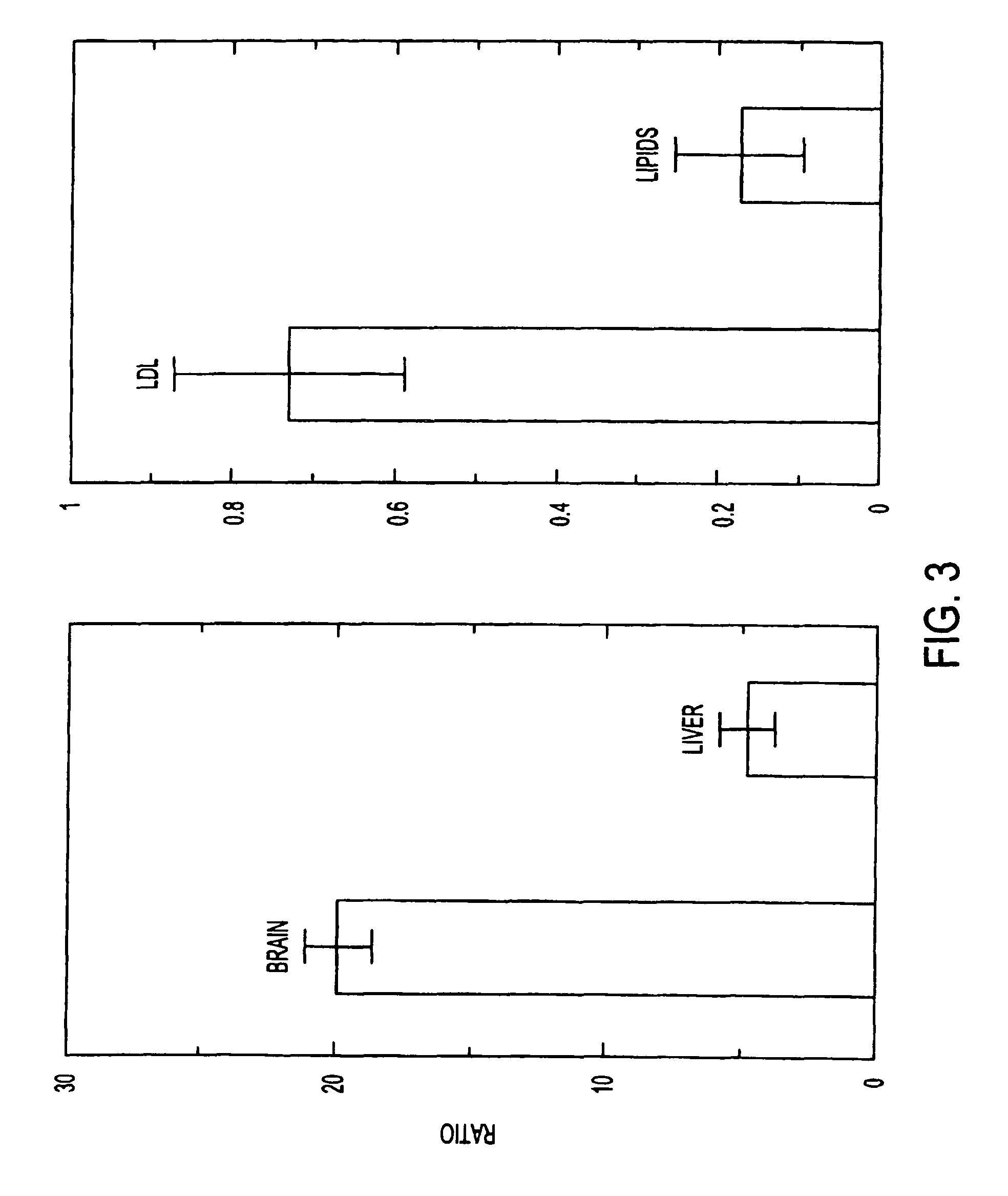
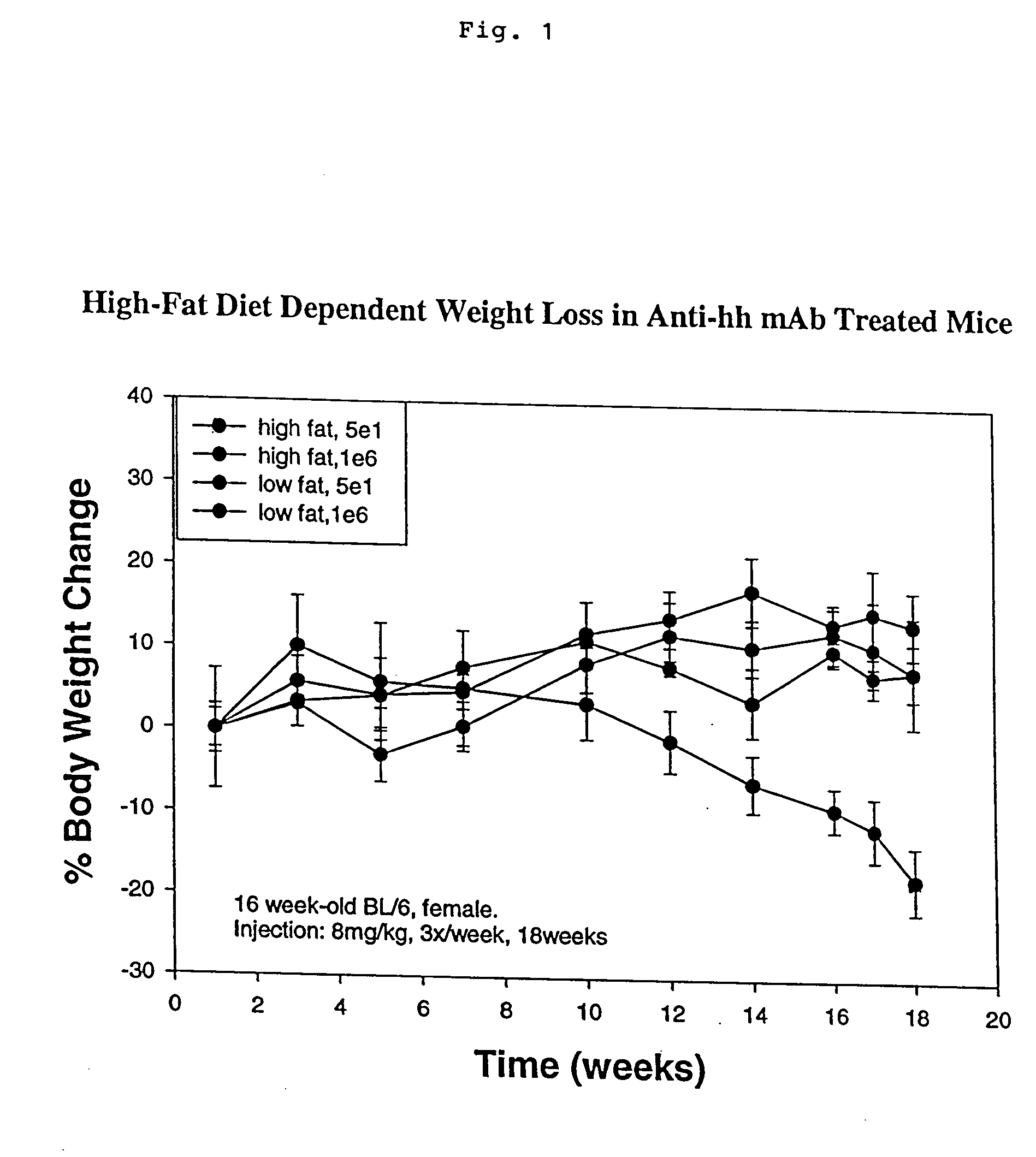
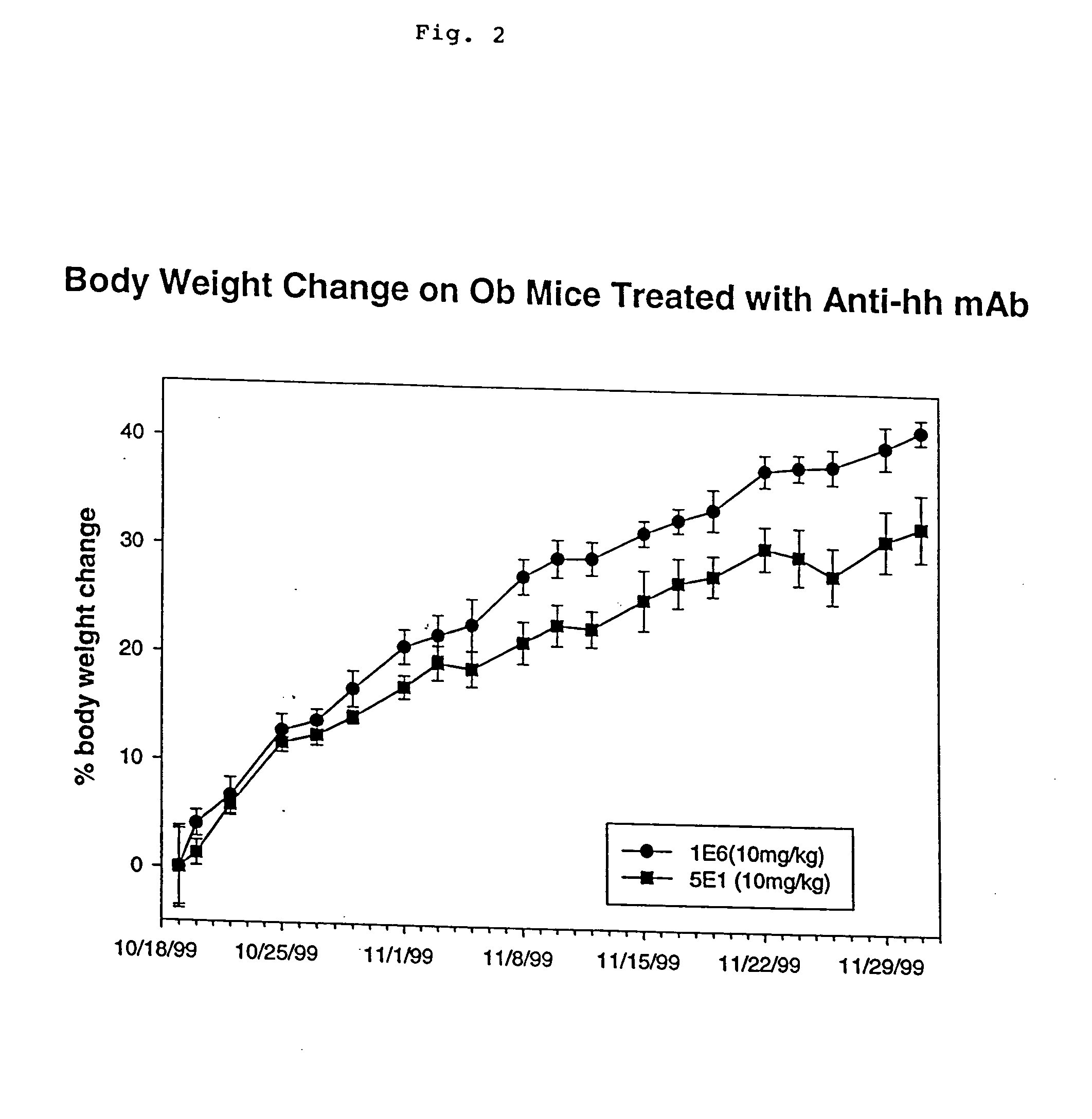
Anti-hedgehog antibodies directed at blocking the binding of hedgehog to its receptor patched-1, will impair lipid metabolism and storage. This invention presents methods for the treatment of a variety of lipid metabolism and lipid storage disorders, aberrant apolipoprotein expression, atherosclerosis and other lipid associated disorders using lipid modulators such as hedgehog antagonists or hedgehog agonists.
Owner:CURIS INC
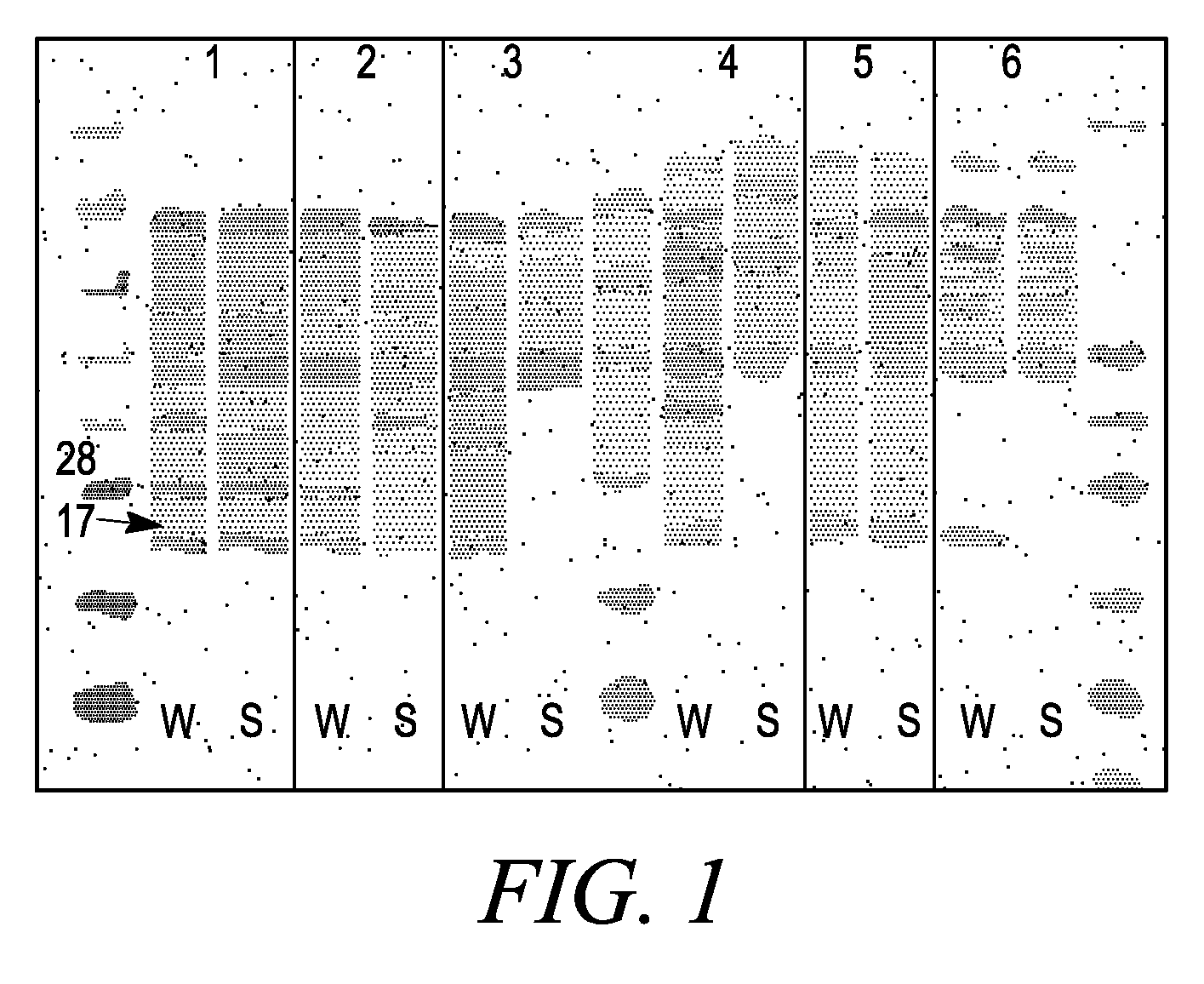
Isolated phospholipid-protein particles
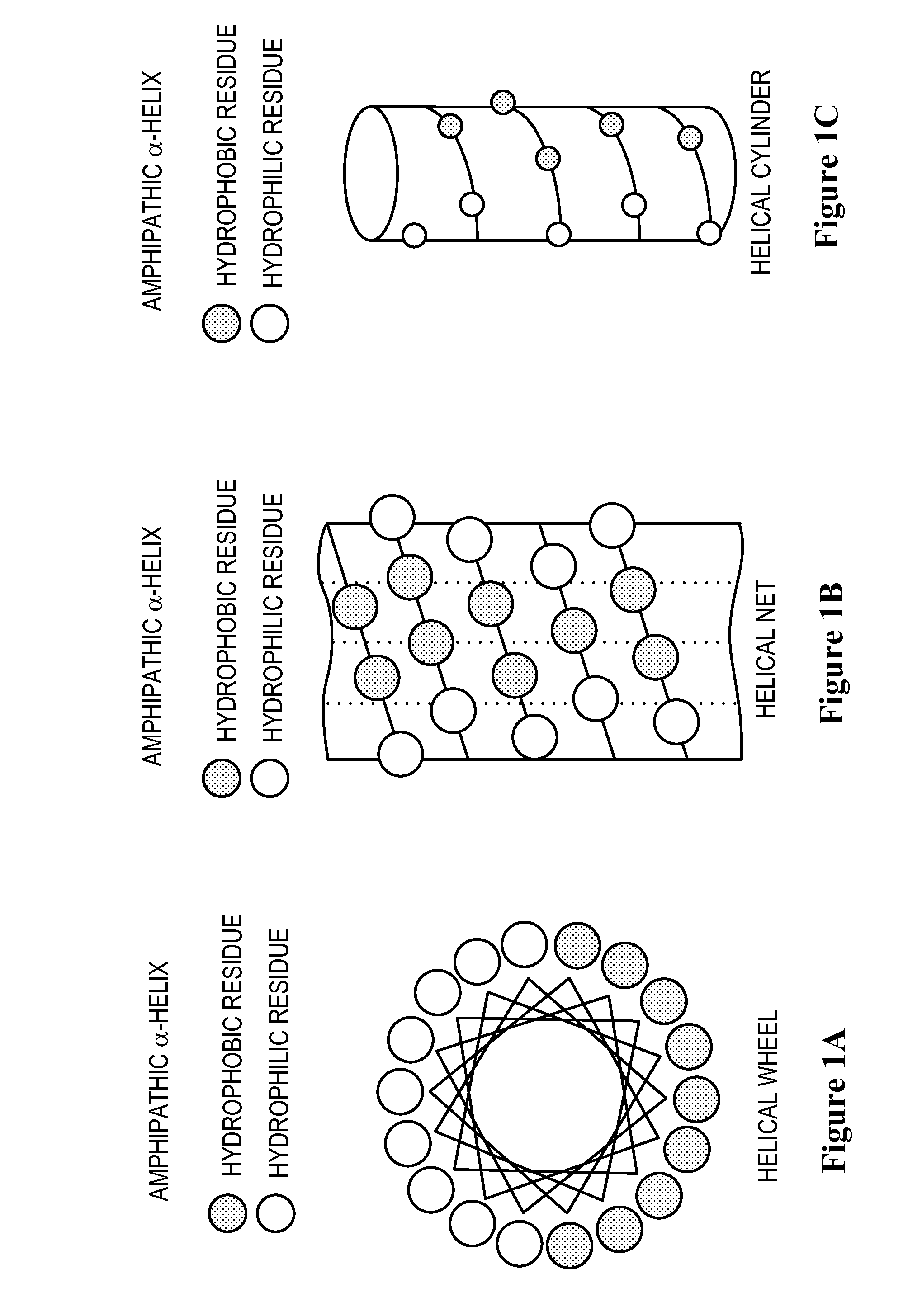
InactiveUS20080248565A1Speed up the processImprove solubilityPeptide preparation methodsDepsipeptidesADAMTS ProteinsApolipoproteins E
Systems and methods are provided for producing a protein of interest that is typically not amenable to expression in soluble form in in vitro expression systems. In some aspects, the invention provides methods of synthesizing proteins using in vitro protein synthesis systems that include a scaffold protein such as apolipoprotein or an amphipathic alpha helix containing (“AAHC”) protein, in which higher yields of soluble protein are produced than in the absence of the scaffold protein. The scaffold proteins may be provided in an in vitro protein synthesis system associated with lipid or not associated with lipid. The scaffold protein may be provided as a protein per se or may be encoded by a nucleic acid template and co-expressed with the protein of interest. The invention also provides compositions and kits for synthesis of proteins in soluble form, in which the compositions and kits include cell extracts for protein expression and isolation.
Owner:LIFE TECH CORP
Reconstituted high density lipoprotein formulation and production method thereof
ActiveUS8999920B2Reduced and minimal toxicityOrganic active ingredientsPeptide/protein ingredientsLipid formationApolipoproteins E
A reconstituted high density lipoprotein formulation having relatively low toxicity comprises an apolipoprotein such as ApoAI or fragment thereof, a lipid and a detergent at a level which is about 5-50% of that which would normally cause liver toxicity upon administration to a human. The lipid is optimally phosphatidylcholine at about 30-50 g / L and the molar ratio of apolipoprotein:lipid is optimally in the range 1:40 to 1:75. The formulation is useful for treating diseases or conditions such as cardiovascular disease, hypercholesterolaemia and hypocholesterolaemia inclusive of acute coronary syndrome (ACS), atherosclerosis and myocardial infarction.
Owner:CSL LTD
Method for Measuring Lipoprotein-Specific Apolipoproteins
The present invention is directed to methods of measuring the concentration of lipoprotein particles and / or lipoprotein-specific apolipoproteins in a biological fluid using an immunoassay, without the need of preliminary physical separation of the various types of lipoprotein particles present in the biological fluid.
Owner:MAINE STANDARDS
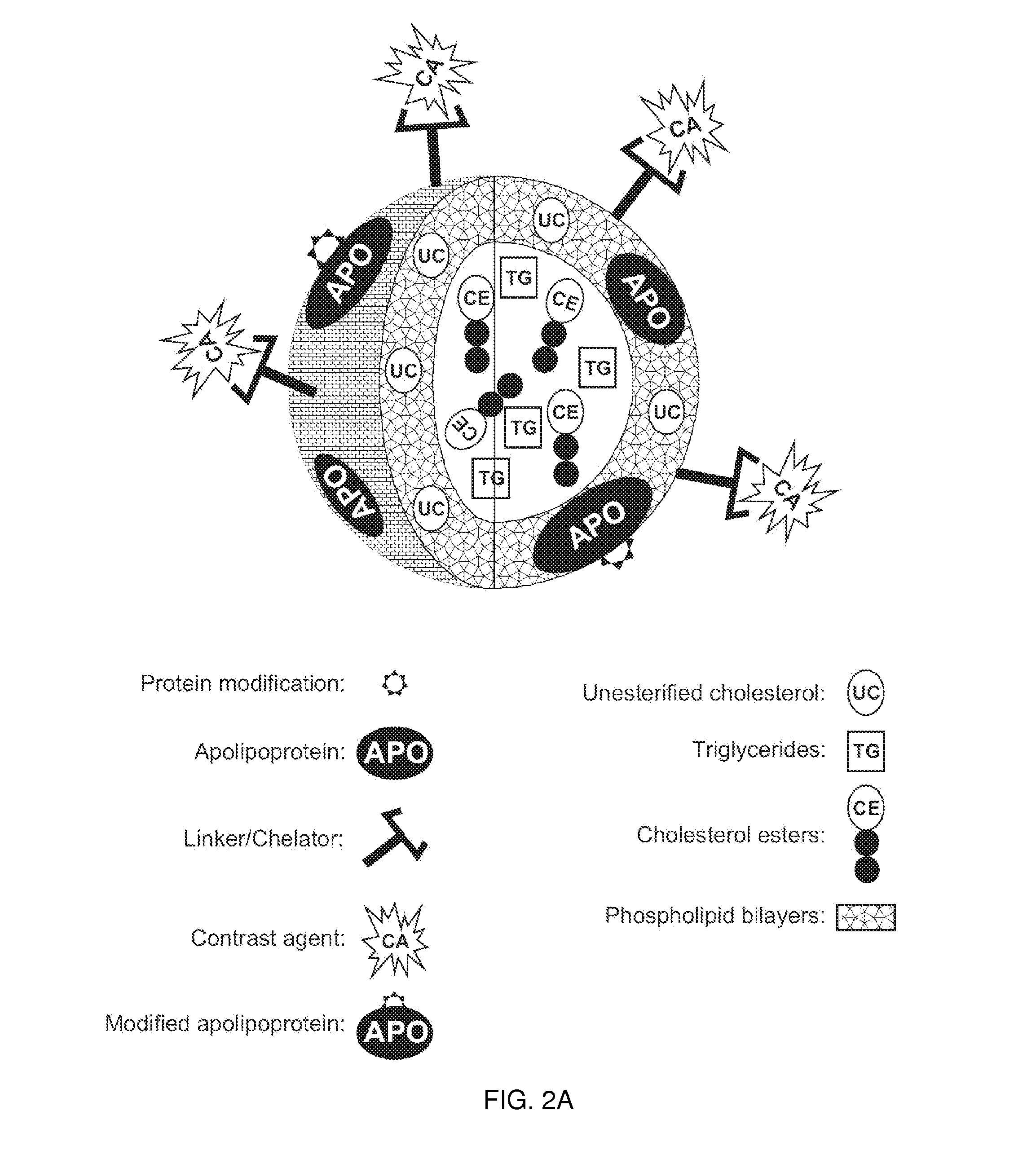
Methods and compositions for targeted imaging
A new approach to targeting imaging agents to macrophage-rich sites of interest is disclosed. Compositions of the invention are rHDL and HDL-like liposomal compositions, protein constituents of which, apolipoproteins A-I and / or A-II or fragments thereof are used not only as structural but also as targeting agents. This is achieved by certain controlled chemical or enzymatic modification of apolipoproteins A-I or A-II or fragments thereof. Such modification converts these apolipoproteins to substrates for macrophage scavenger receptors and results in the improvement of contrast agent-(HDL / modified apolipoprotein)-particle association with macrophages and / or absorption (uptake) by macrophages when compared to that of the contrast agent-(HDL / apolipoprotein)-particle constructed with non-modified naturally occurring apo A-I. The compositions can be used for noninvasive specific in vivo molecular detection and localization of macrophage-rich sites of interest using imaging techniques such as computed tomography (CT), gamma-scintigraphy, positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI).
Owner:SIGNABLOK
Method of treating dyslipidemic disorder
InactiveUS20080269111A1Low costReduce adverse effectsOrganic active ingredientsPeptide/protein ingredientsDyslipidemiaMedicine
The invention provides methods of treating or preventing a condition or disorder associated with dyslipidemia with compositions comprising apolipoprotein-sphingomyelin complexes. The methods of the invention permit reduction, by 4- to 20-fold, of the amount of apolipoprotein required for therapeutic administration to bring about an ameliorative effect.
Owner:PFIZER INC
Apolipoprotein a-i mimics
Provided are peptides, compositions thereof, and methods for treating or preventing dyslipidemia, a cardiovascular disease, endothelial dysfunction, a macrovascular disorder, or a microvascular disorder.
Owner:CERENIS THERAPEUTICS HOLDINGS SA
Statin and omega-3 fatty acids for reduction of apo-b levels
Methods of utilizing a combined administration or a unit dosage of a combination of an HMG-CoA inhibitor and omega-3 fatty acids for the reduction of apolipoprotein-B levels. The methods are especially useful in the treatment of patients with hypertriglyceridemia or hypercholesterolemia or mixed dyslipidemia, coronary heart disease (CHD), vascular disease, atherosclerotic disease and related conditions, and for the prevention or reduction of cardiovascular, cardiac, and vascular events.
Owner:GLAXO SMITHKLINE LLC
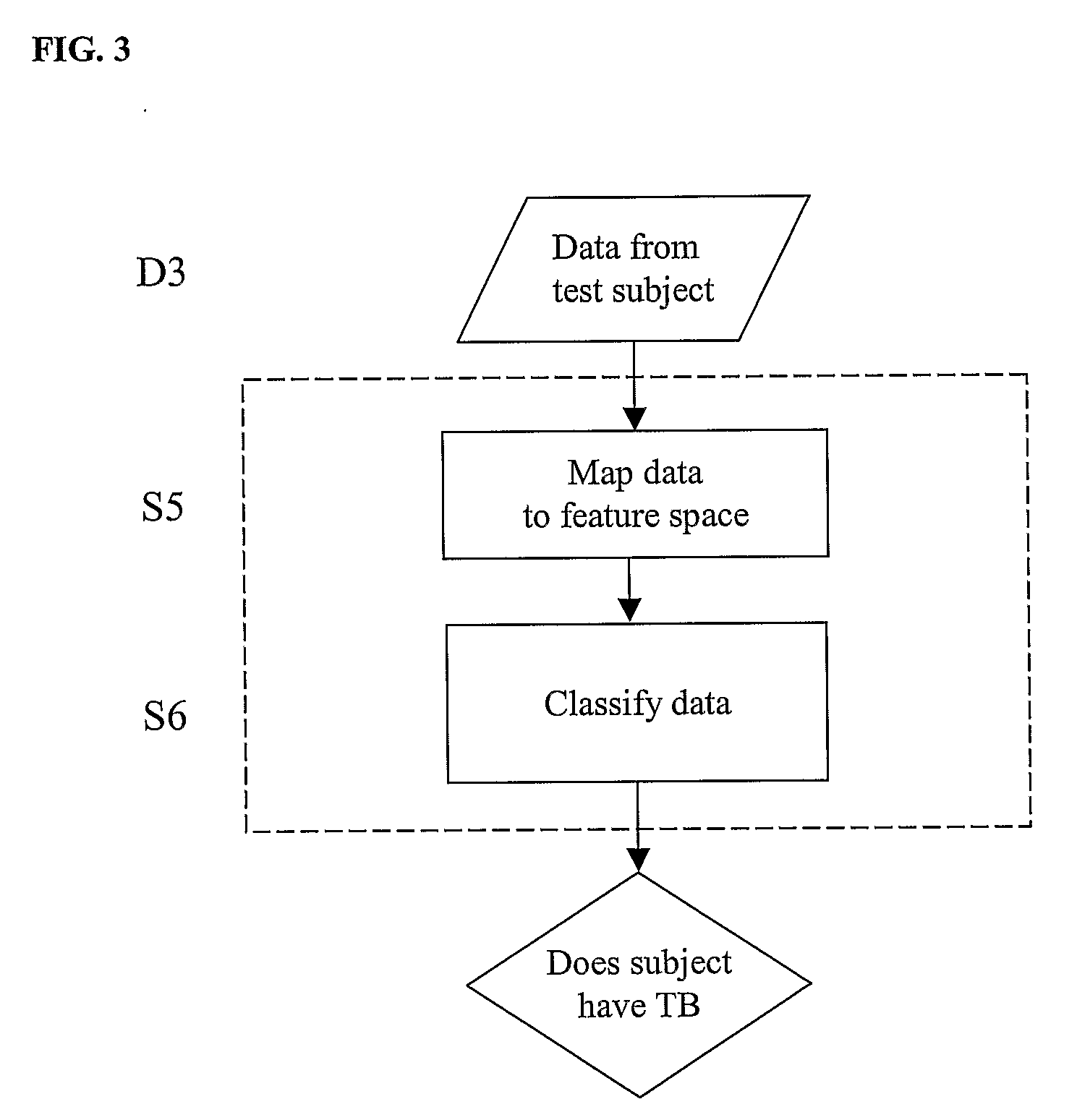
Diagnosis of Tuberculosis
InactiveUS20090104602A1Microbiological testing/measurementMaterial analysisSerum protein albuminApolipoproteins E
The invention provides a method of diagnosing tuberculosis (TB) in a test subject, said method comprising: (i) providing expression data of two or more markers in a subject, wherein at least two of said markers are selected from transthyretin, neopterin, C-reactive protein (CRP), serum amyloid A (SAA), serum albumin, apoliopoprotein-A1 (Apo-A1), apolipoprotein-A2 (Apo-A2), hemoglobin beta, haptoglobin protein, DEP domain protein, leucine-rich alpha-2-glycoprotein (A2GL) and hypothetical protein DFKZp667I032; and (ii) comparing said expression data to expression data of said marker from a group of control subjects, wherein said control subjects comprise patients suffering from inflammatory conditions other than TB, thereby determining whether or not said test subject has TB.
Owner:FERNANDEZ REYES DELMIRO +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com