Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Lipoprotein particle" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A spherical particle containing non-covalently associated proteins and lipids. Examples are plasma lipoprotein particles which transport lipids in the blood or lymph. [GOC:vesicles]
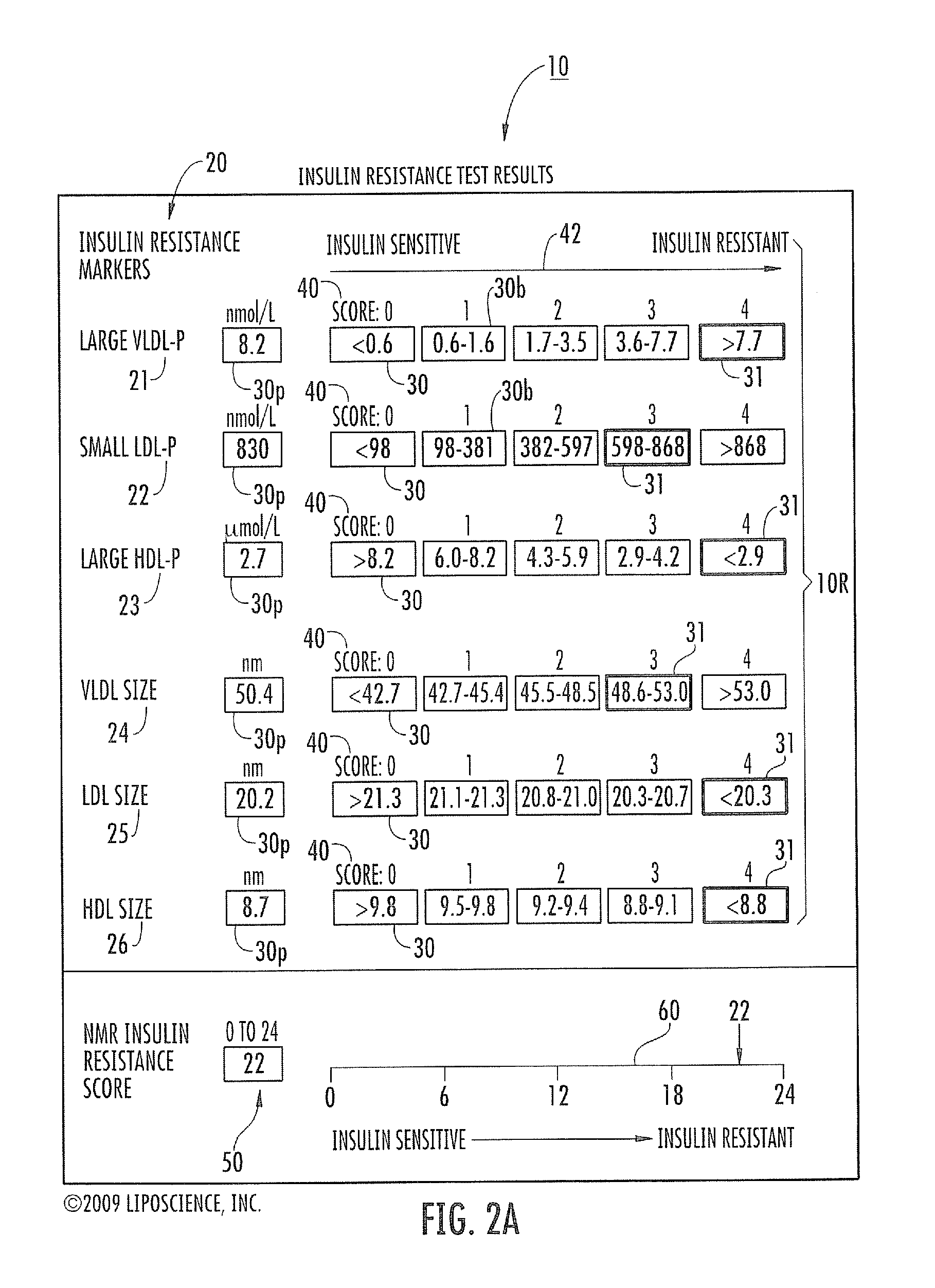
Lipoprotein insulin resistance indexes and related methods, systems and computer programs for generating same
ActiveUS20100100334A1Effective preventionMagnetic measurementsPeptide/protein ingredientsLipoprotein particleInsulin sensitivity
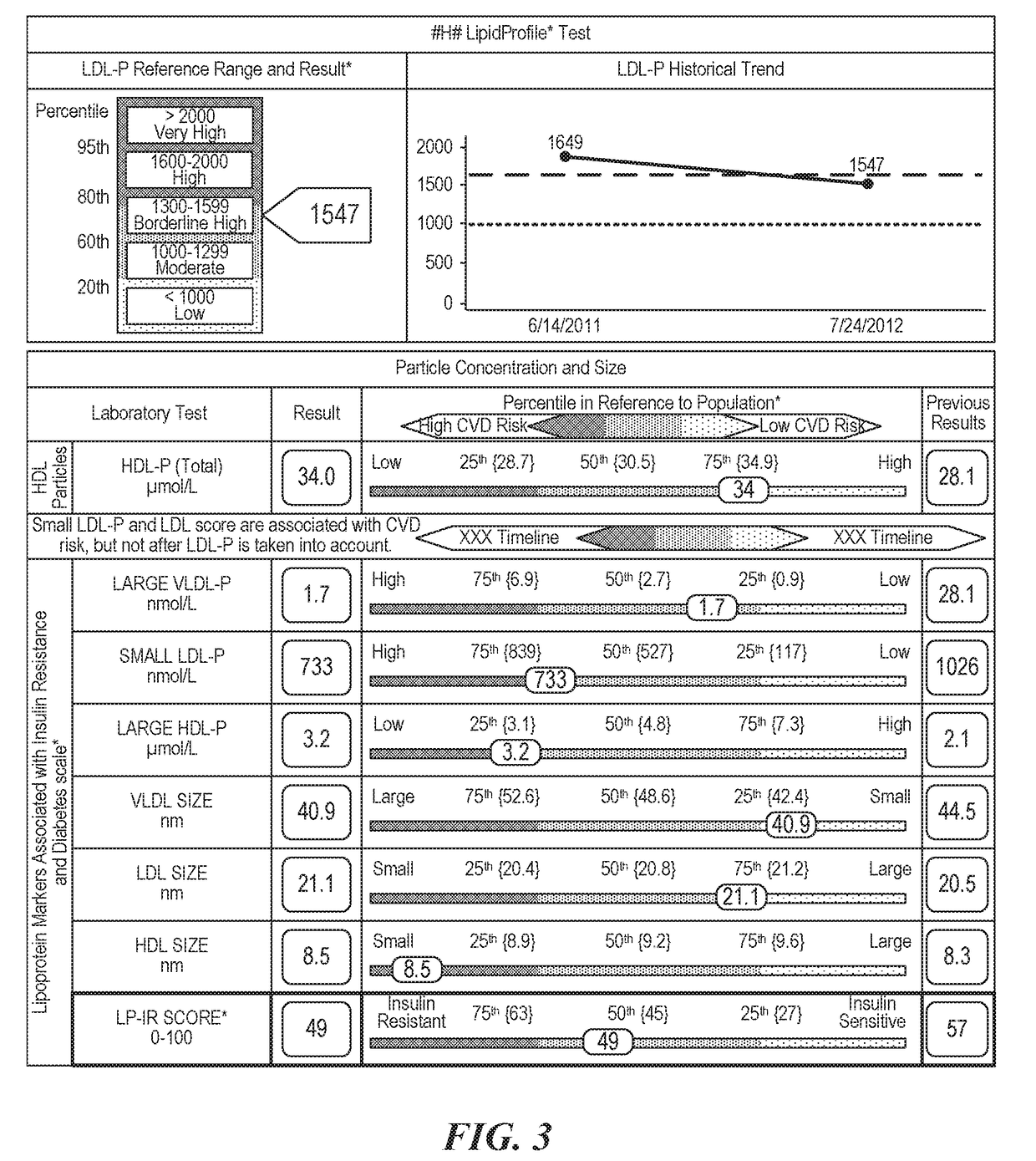
Methods, reports and systems for generating insulin resistance indexes for assessing decreased insulin sensitivity and / or levels of insulin resistance using a plurality of different measured lipoprotein particle parameters.
Owner:LIPOSCI
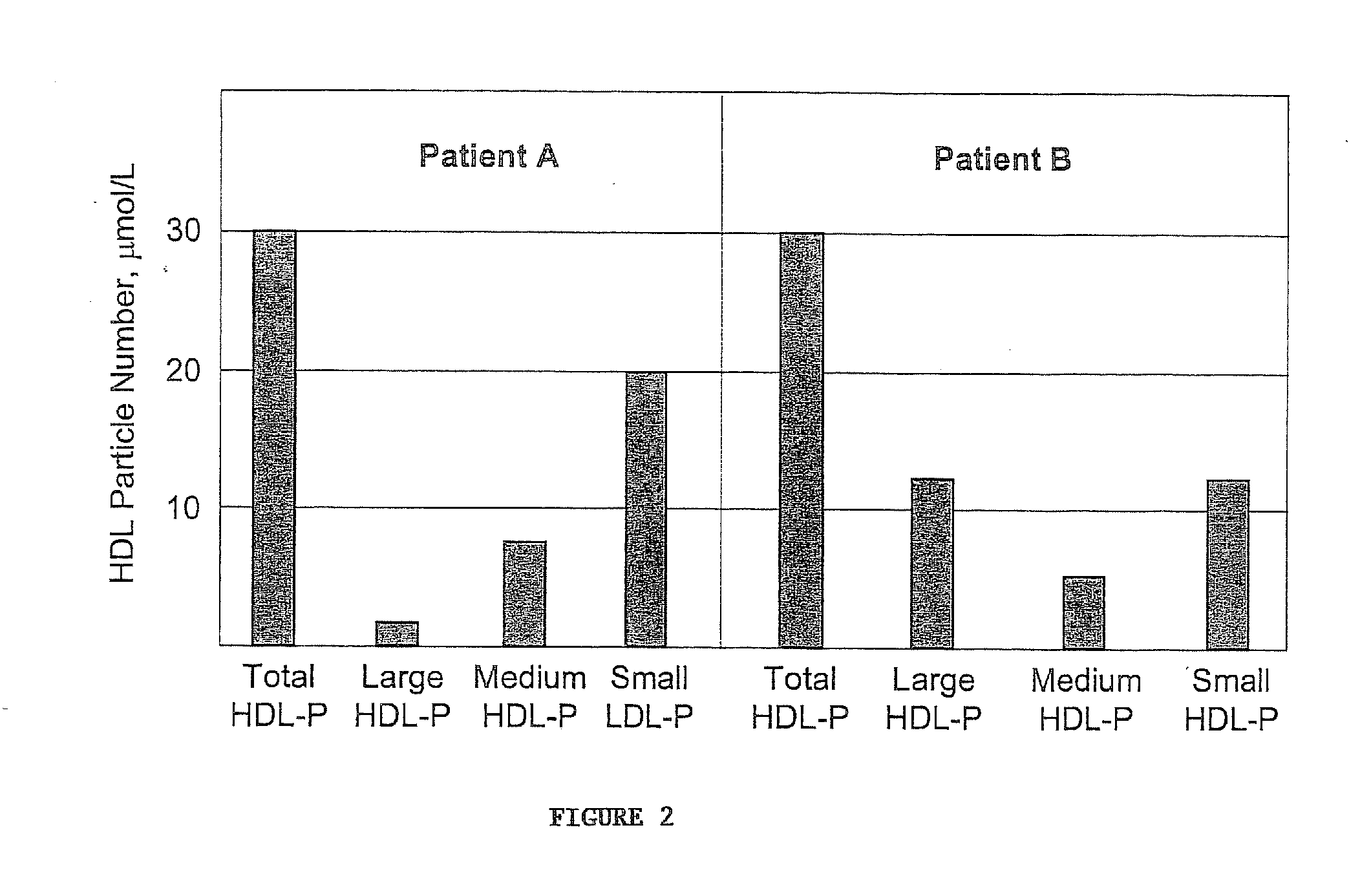
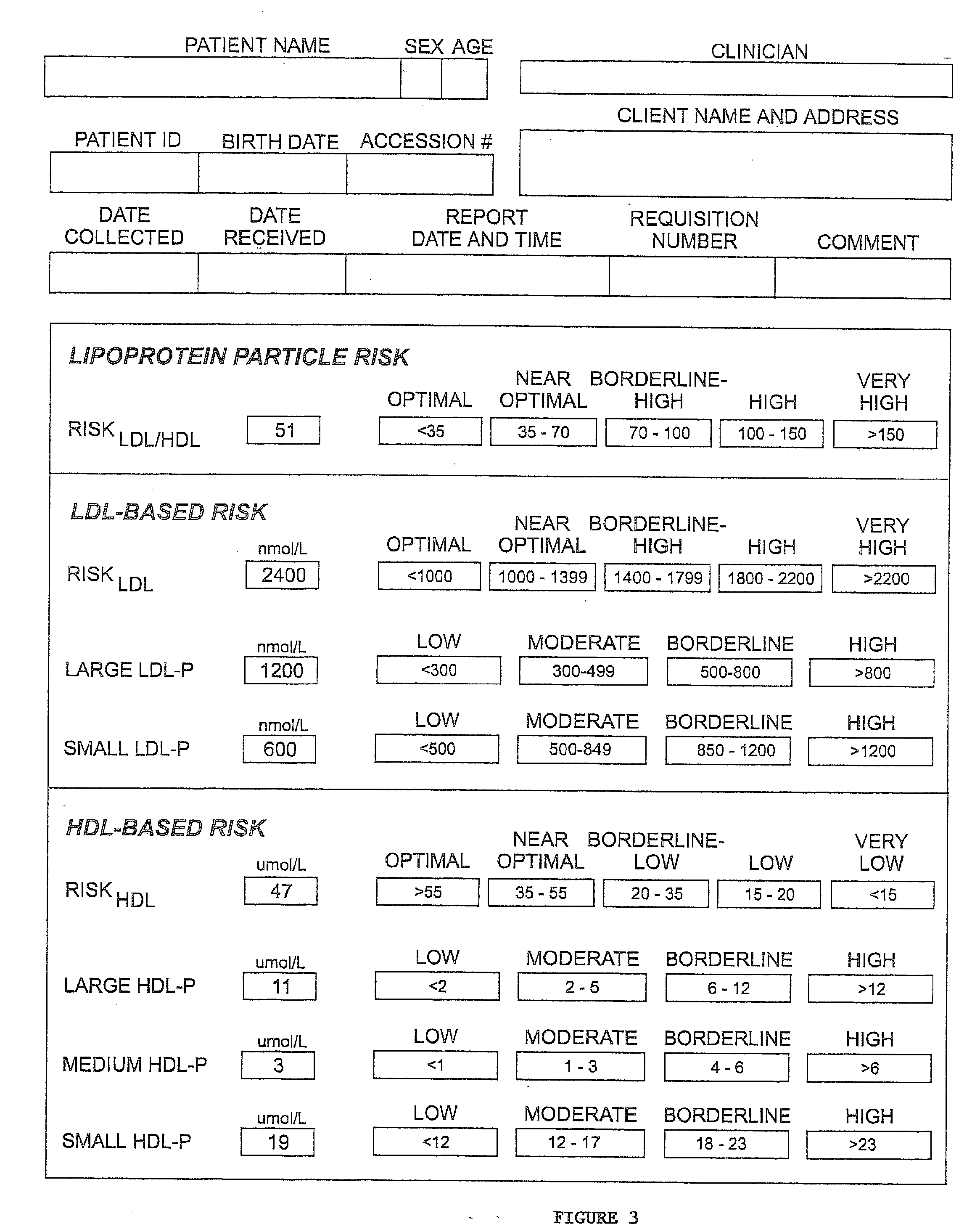
Methods, systems and computer programs for assessing chd risk using adjusted hdl particle number measurements
ActiveUS20070264677A1Facilitate patient risk stratificationEfficiently decideMagnetic measurementsTherapiesMathematical modelA lipoprotein
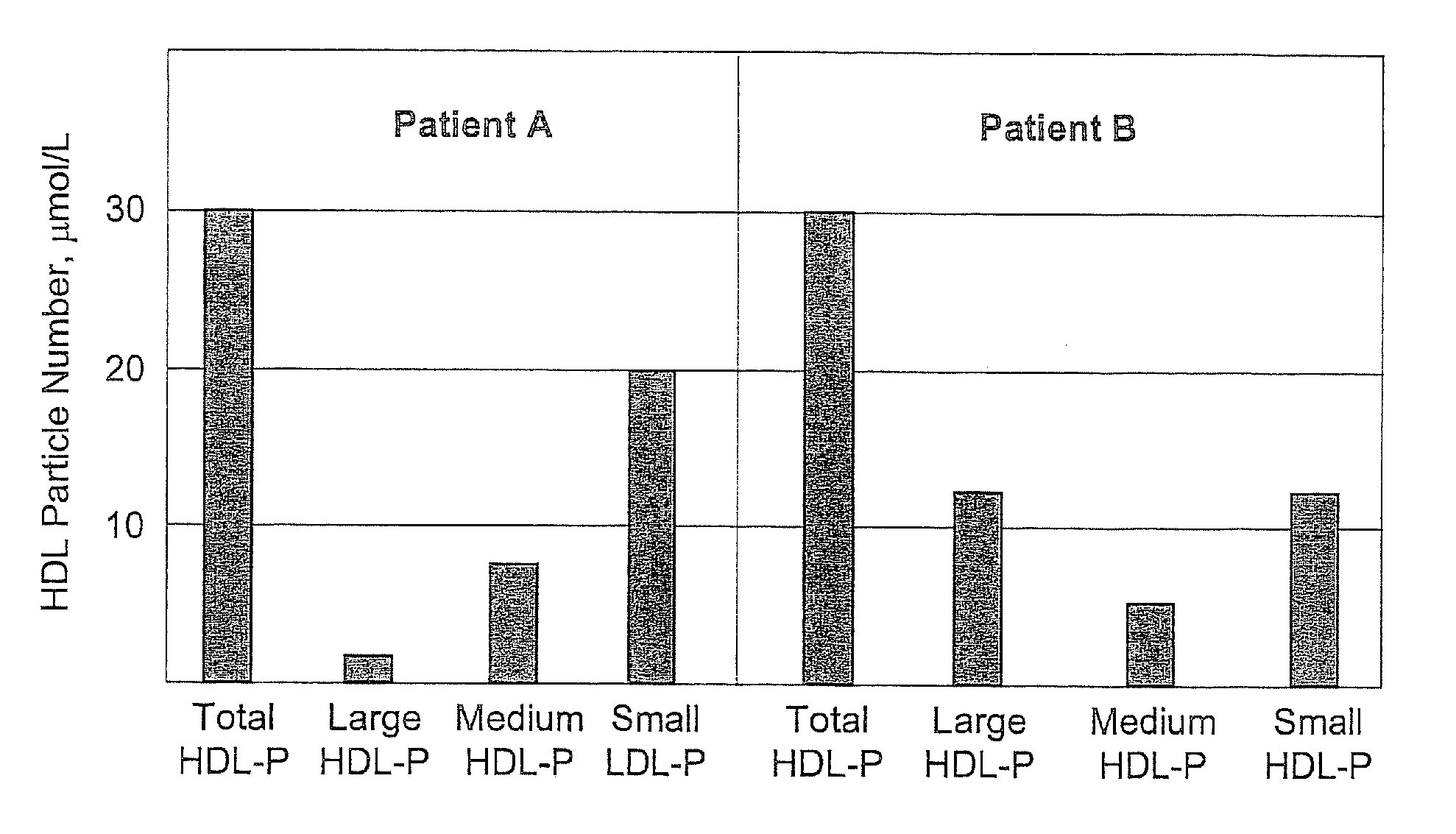
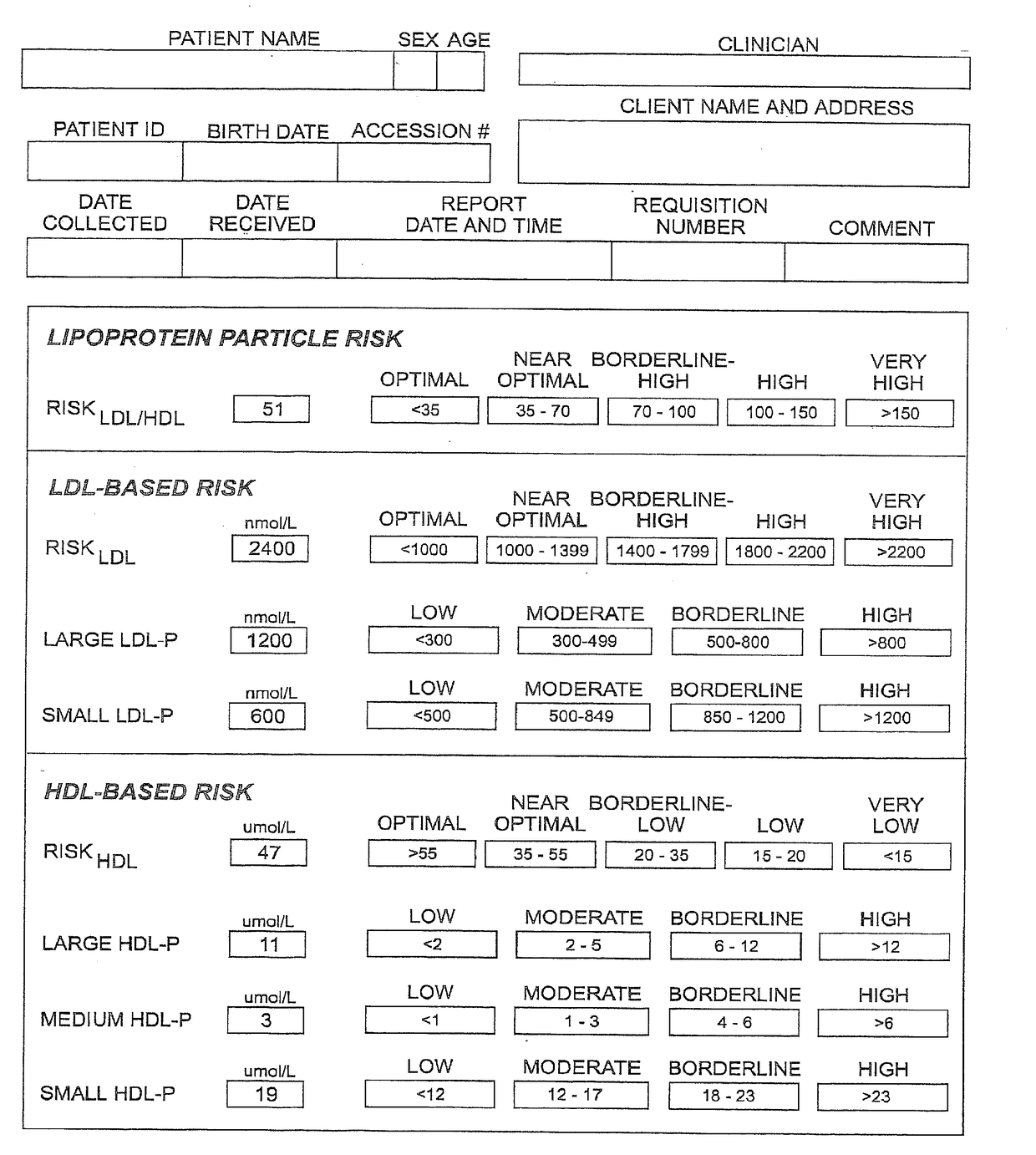
Methods, computer program products and apparatus determine a subject's risk of having or developing CHD using a calculated HDL particle risk number and / or a mathematical model of risk associated with HDL particles that adjusts concentrations of at least one of the subclasses of small, medium and large HDL particle measurements to reflect predicted CHD risk. A calculated LDL particle risk number may also be generated as well as a lipoprotein particle index derived from the ratio of RLDL / RHDL.
Owner:LIPOSCI
Lipoprotein insulin resistance indexes and related methods, systems and computer programs for generating same
ActiveUS8386187B2Magnetic measurementsPeptide/protein ingredientsLipoprotein particleInsulin sensitivity
Methods, reports and systems for generating insulin resistance indexes for assessing decreased insulin sensitivity and / or levels of insulin resistance using a plurality of different measured lipoprotein particle parameters.
Owner:LIPOSCI
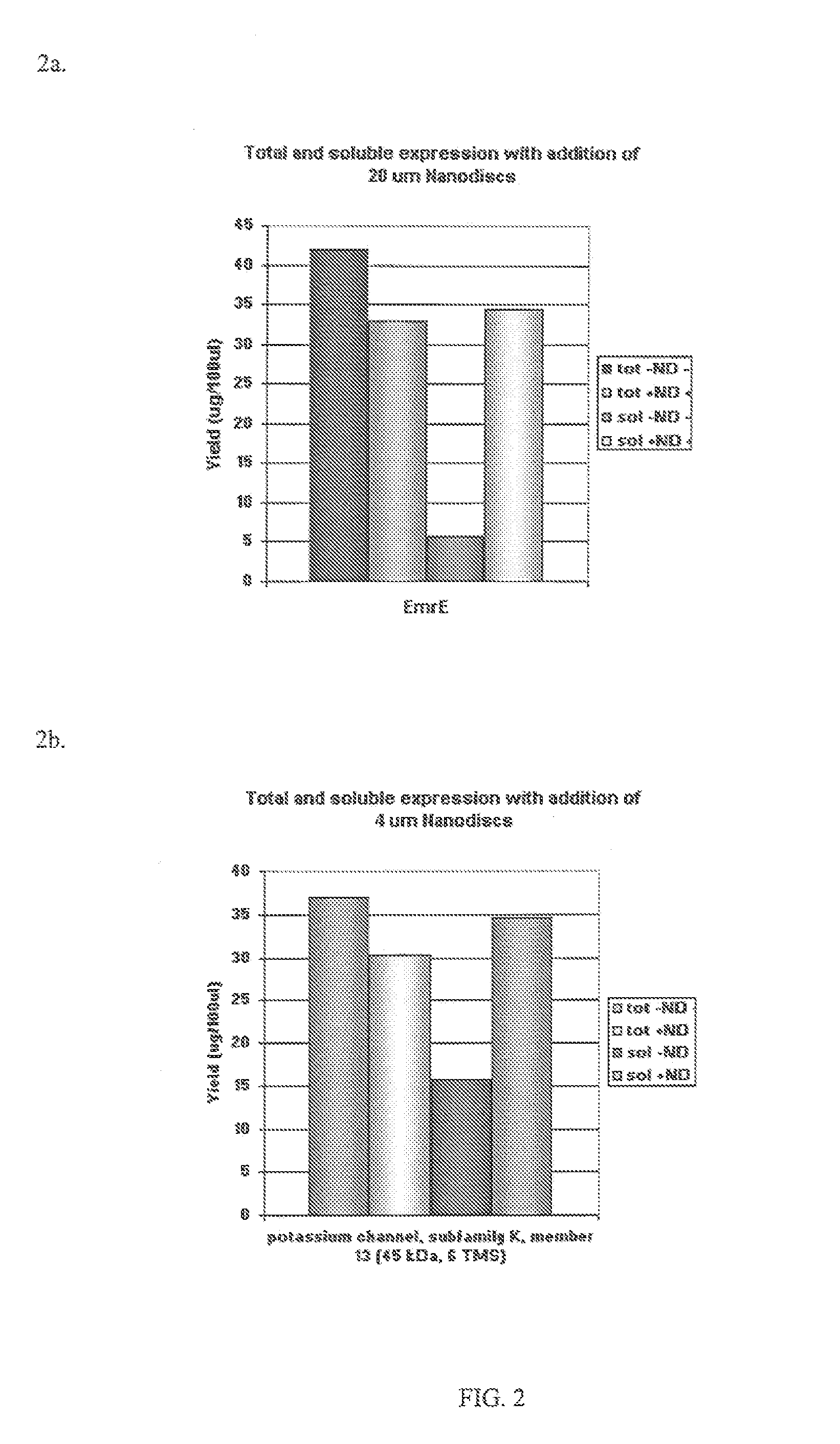
In Vitro Protein Synthesis Systems for Membrane Proteins that Include Adolipoproteins and Phospholipid Adolipoprotein Particles
In vitro protein synthesis systems and methods are provided that produce membrane proteins in soluble form. In some aspects, the invention provides methods of synthesizing proteins using in vitro protein synthesis systems that include an apolipoprotein, in which higher yields of soluble protein are produced than in the absence of the apolipoprotein. Apolipoproteins useful in the present invention include naturally occurring apolipoproteins, as well as sequence variants of wild-type apolipoproteins, and engineered apolipoproteins. The apolipoproteins can be provided in an in vitro protein synthesis system associated with lipid or not associated with lipid. The invention also provides compositions and kits for synthesis of proteins in soluble form, in which the compositions and kits include cell extracts for protein translation and at least one apolipoprotein biomolecule.
Owner:LIFE TECH CORP
Method for Measuring Lipoprotein-Specific Apolipoproteins
The present invention is directed to methods of measuring the concentration of lipoprotein particles and / or lipoprotein-specific apolipoproteins in a biological fluid using an immunoassay, without the need of preliminary physical separation of the various types of lipoprotein particles present in the biological fluid.
Owner:MAINE STANDARDS
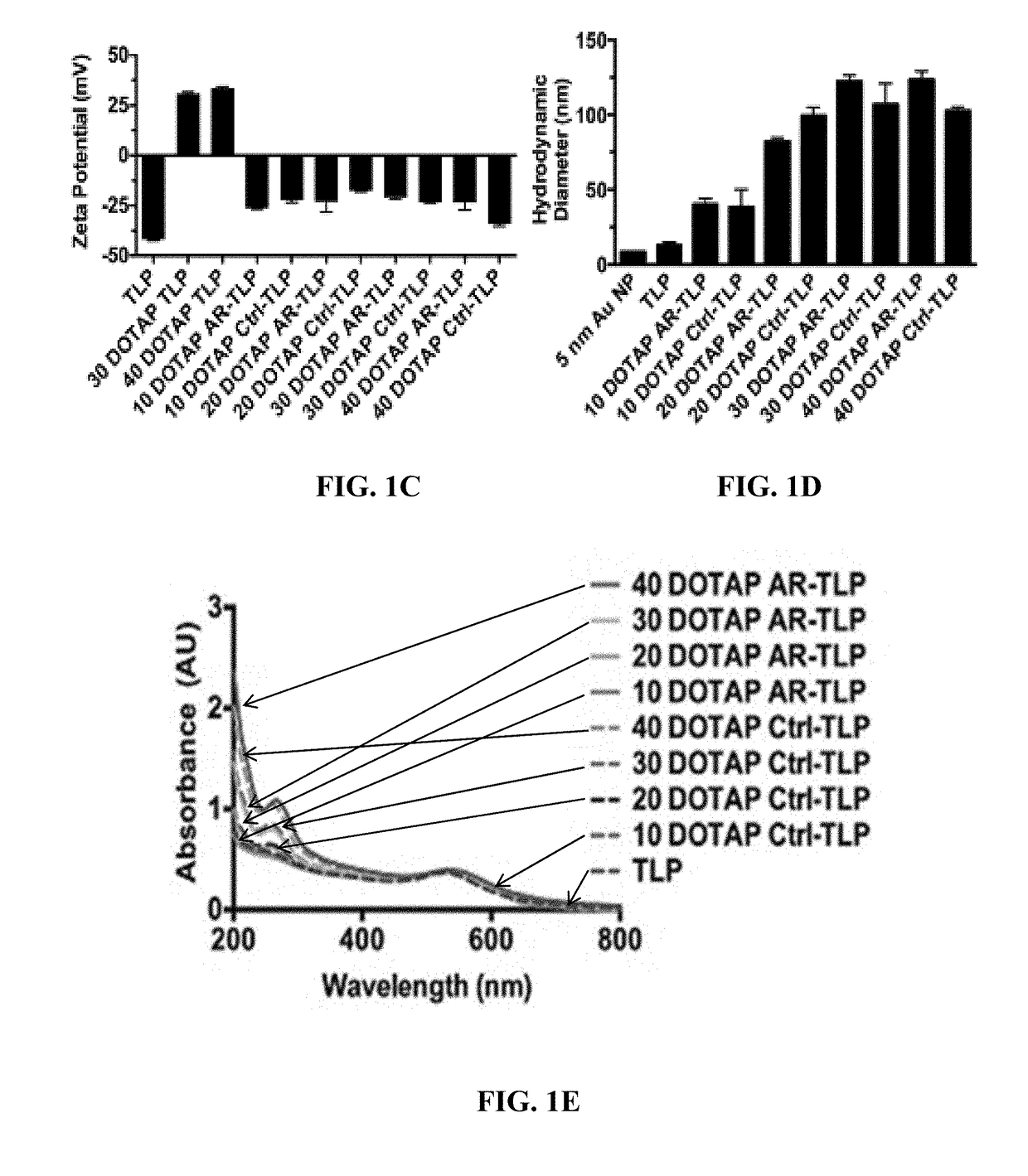
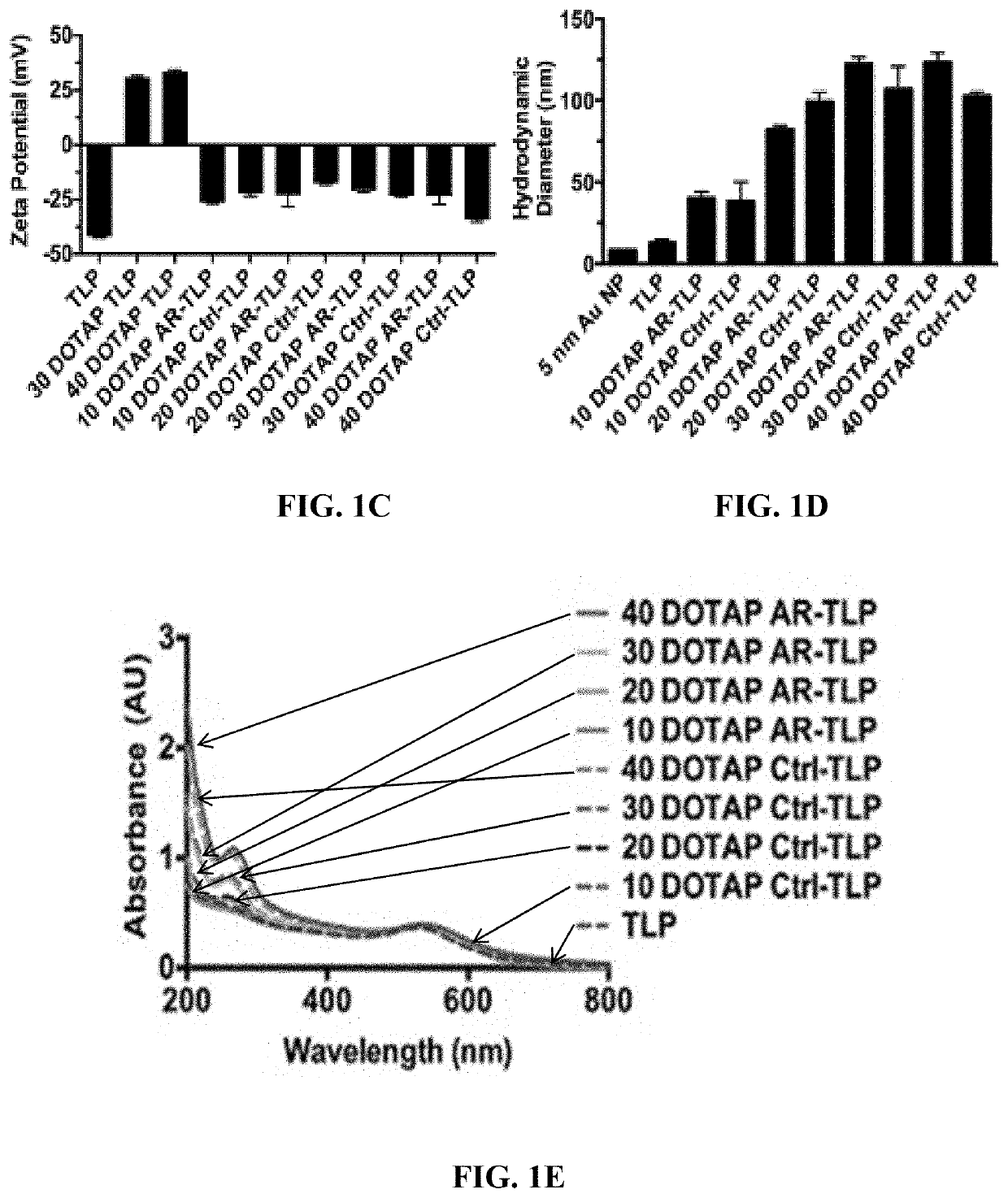
Short interfering RNA templated lipoprotein particles (sirna-tlp)
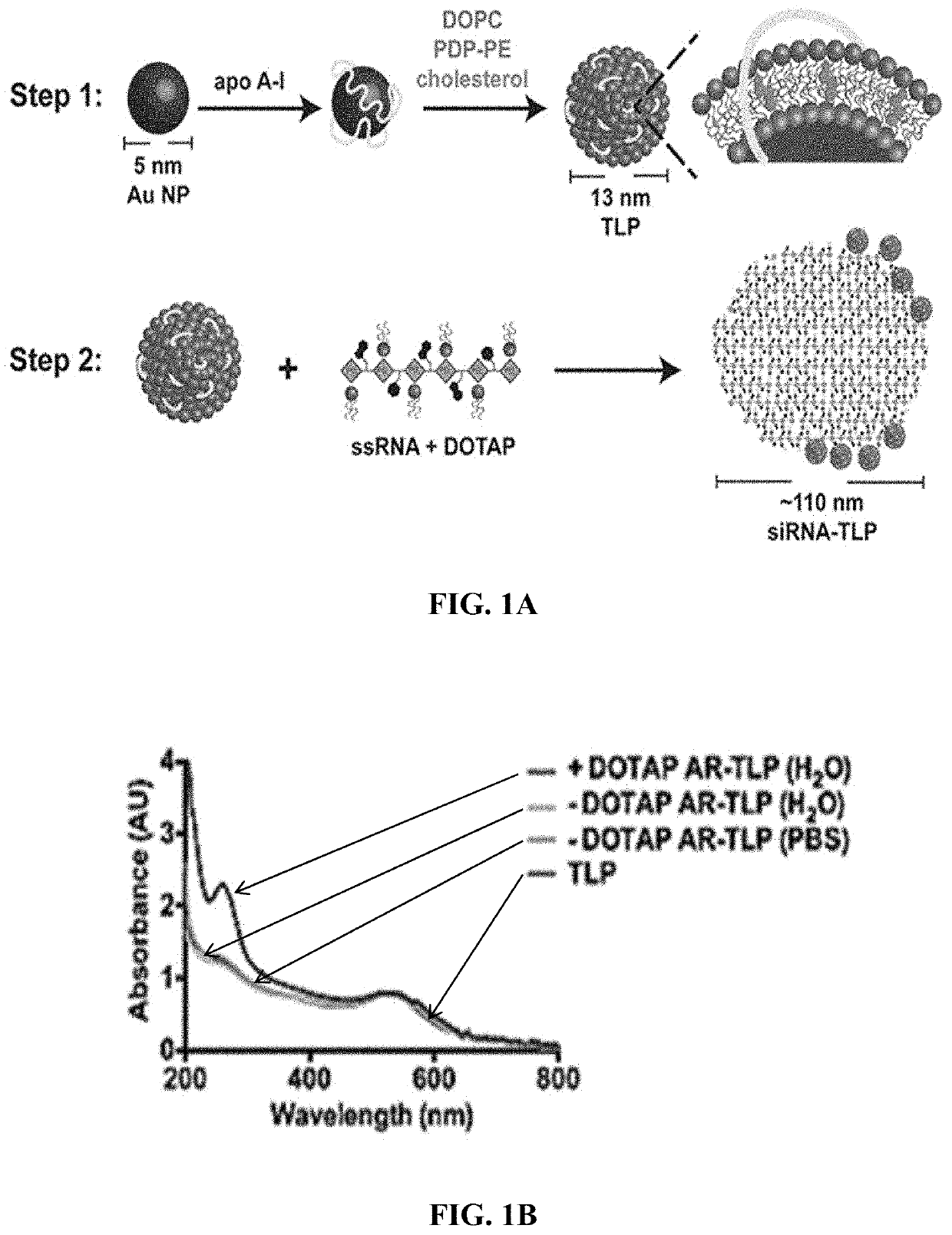
Nanostructures for the systemic delivery of nucleic acids, such as RNA, are provided herein. The nanostructures include templated lipoprotein nanoparticles (TLPs) composed of a core decorated with proteins, a lipid bilayer and hydrophobic molecules that self-assemble with nucleic acids, such as RNA. The nanostructures are useful for research, therapeutic and diagnostic applications.
Owner:NORTHWESTERN UNIV
Optical method and system to determine distribution of lipid particles in a sample
InactiveUS7700360B2Bioreactor/fermenter combinationsBiological substance pretreatmentsLipid formationLipid particle
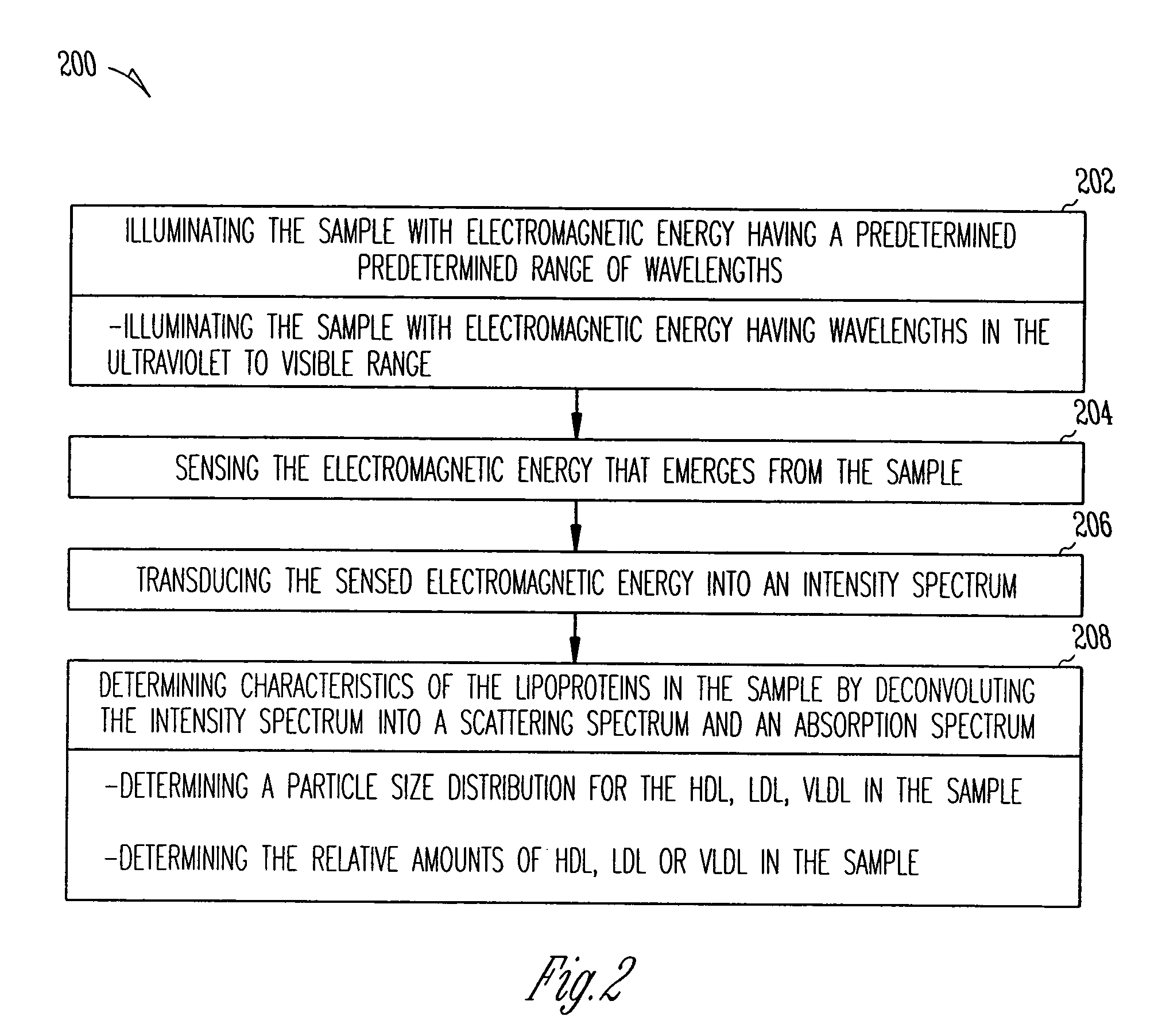
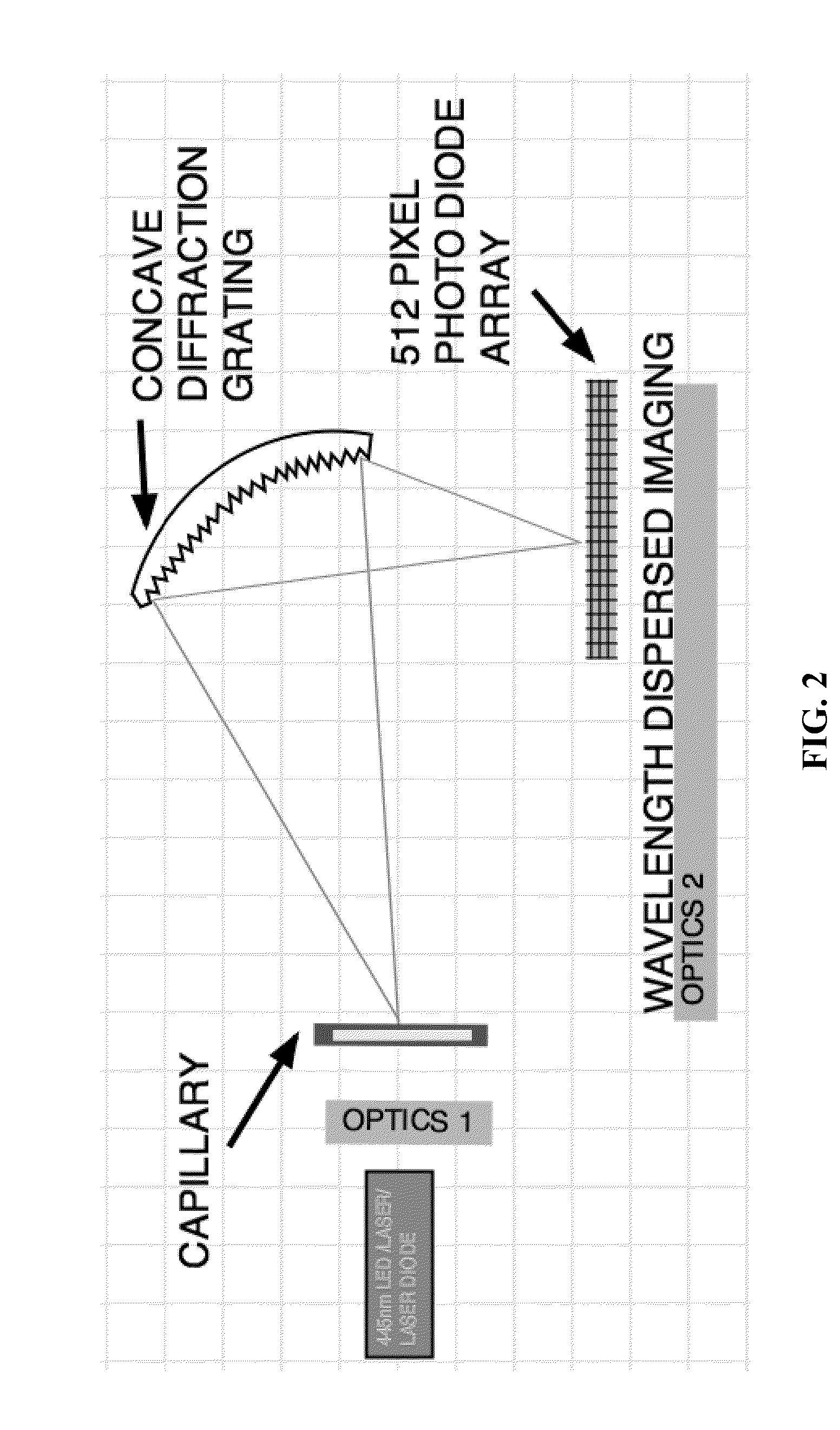
The system and method of the present invention relate to characterizing lipoproteins in a sample. The system includes a light source that delivers electromagnetic energy in a predetermined range of wavelengths to the sample and a sensor that senses an intensity spectrum which emerges from the sample when the sample is illuminated by the light source. A processor determines a chemical composition of the sample to determine the presence of lipoprotein particles. The processor then characterizes lipoproteins that are within in the sample by deconvoluting the intensity spectrum into a scattering spectrum and absorption spectrum. The method includes illuminating the sample with electromagnetic energy having a predetermined range of wavelengths and sensing the electromagnetic energy that emerges from the sample. The method further includes transducing the sensed electromagnetic energy which emerges from the sample into an intensity spectrum that determines the types of lipoproteins that are within the sample.
Owner:KIMBERLY-CLARK WORLDWIDE INC +1
Process of producing active protein and oil with maggot and earth worm
InactiveCN101019603APrevent or reduce oxidationPrevent or reduce decompositionAnimal feeding stuffWorking-up animal fodderMaggotManufacturing technology
The process of producing active protein and oil with maggot and earthworm as material belongs to the field of feed producing technology. The automatic production process can produce full protein powder, granular full protein product, defatted protein powder, defatted granular protein product and separated maggot or earthworm oil. The production process can maintain the activity of the protein, amino acids and other active matter unchanged, raise the biological potency and digesting and absorbing rate of the products. The present invention also provides relevant large scale production process.
Owner:李金穗 +1
System and method for assessing quantities or sizes of lipoprotein particles from lipoprotein particle compositions
InactiveUS20170343464A1Improved clinical decisionLow costIndividual particle analysisBiological testingFree cholesterolVascular disease
This application discloses methods for assessing quantities of spherical or substantially spherical lipoprotein particles or portions thereof present in a biological sample based on the measurement of free cholesterol and / or phospholipid content in the lipoprotein particles. Methods of treating a subject at increased risk for cardiovascular disease and / or cardiodiabetes are also disclosed.
Owner:HELENA LAB
Methods, systems and computer programs for assessing CHD risk using adjusted HDL particle number measurements
ActiveUS9435870B2Easy to understandPromote stratificationMagnetic measurementsTherapiesMathematical modelMedicine
Owner:LIPOSCI
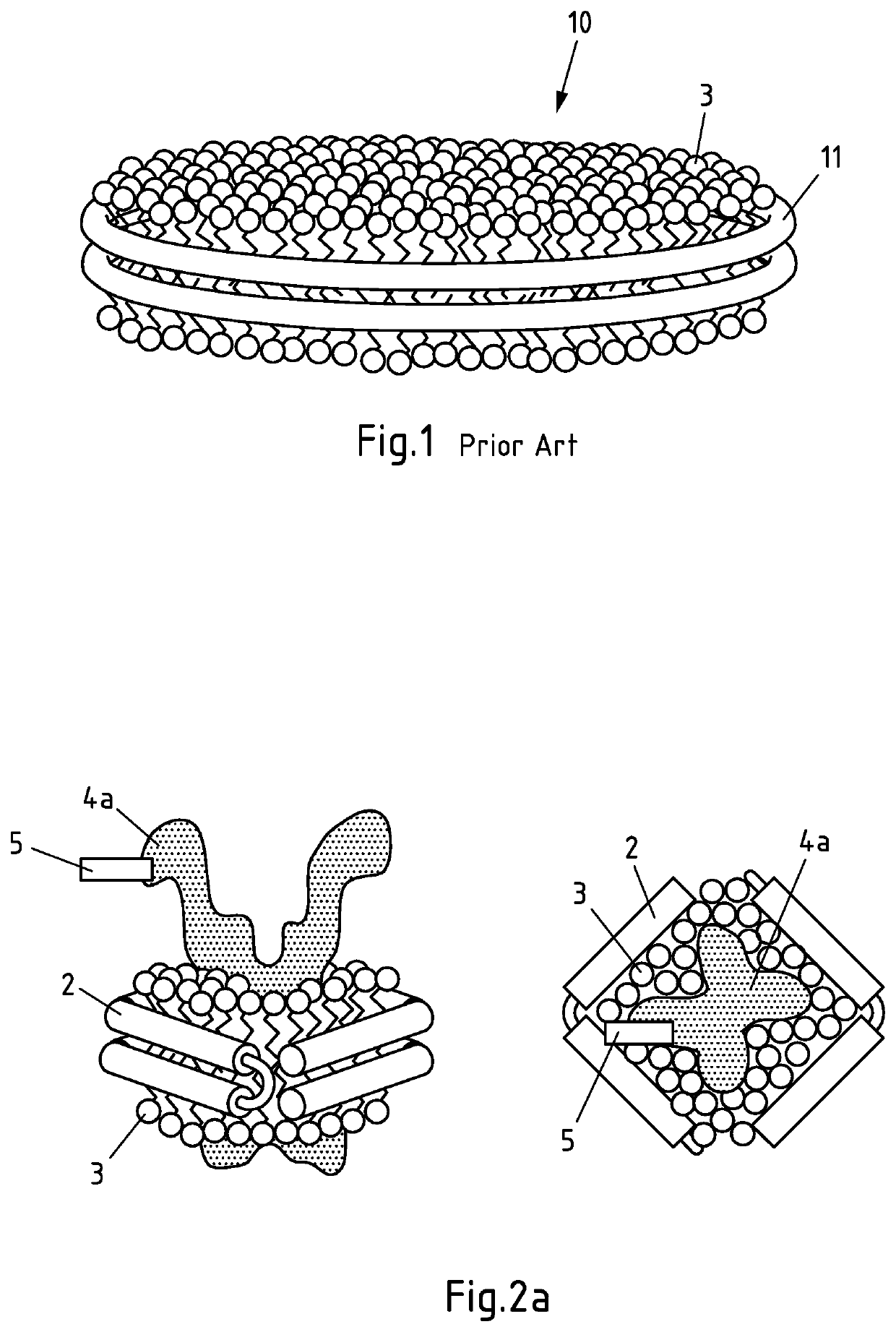
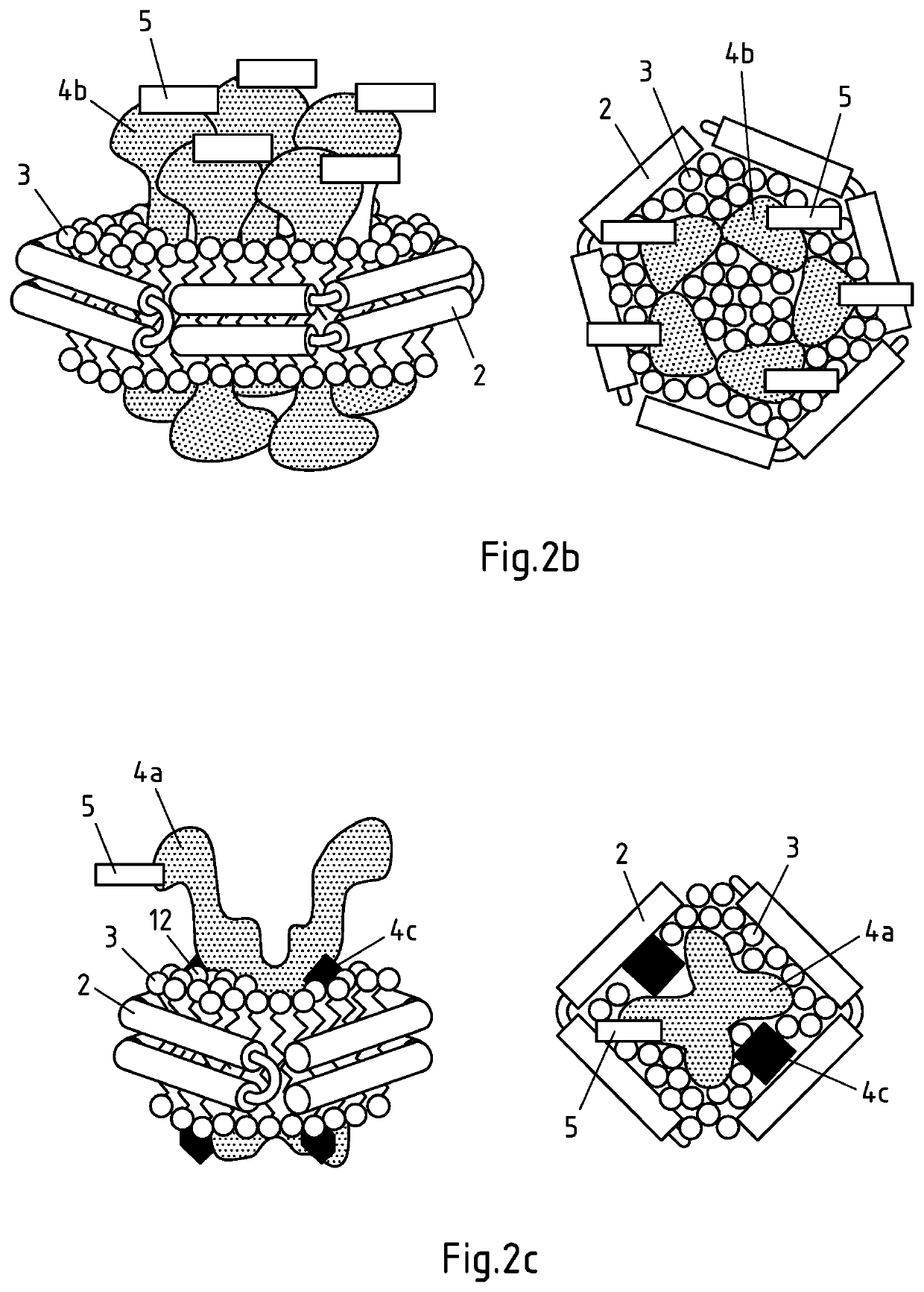
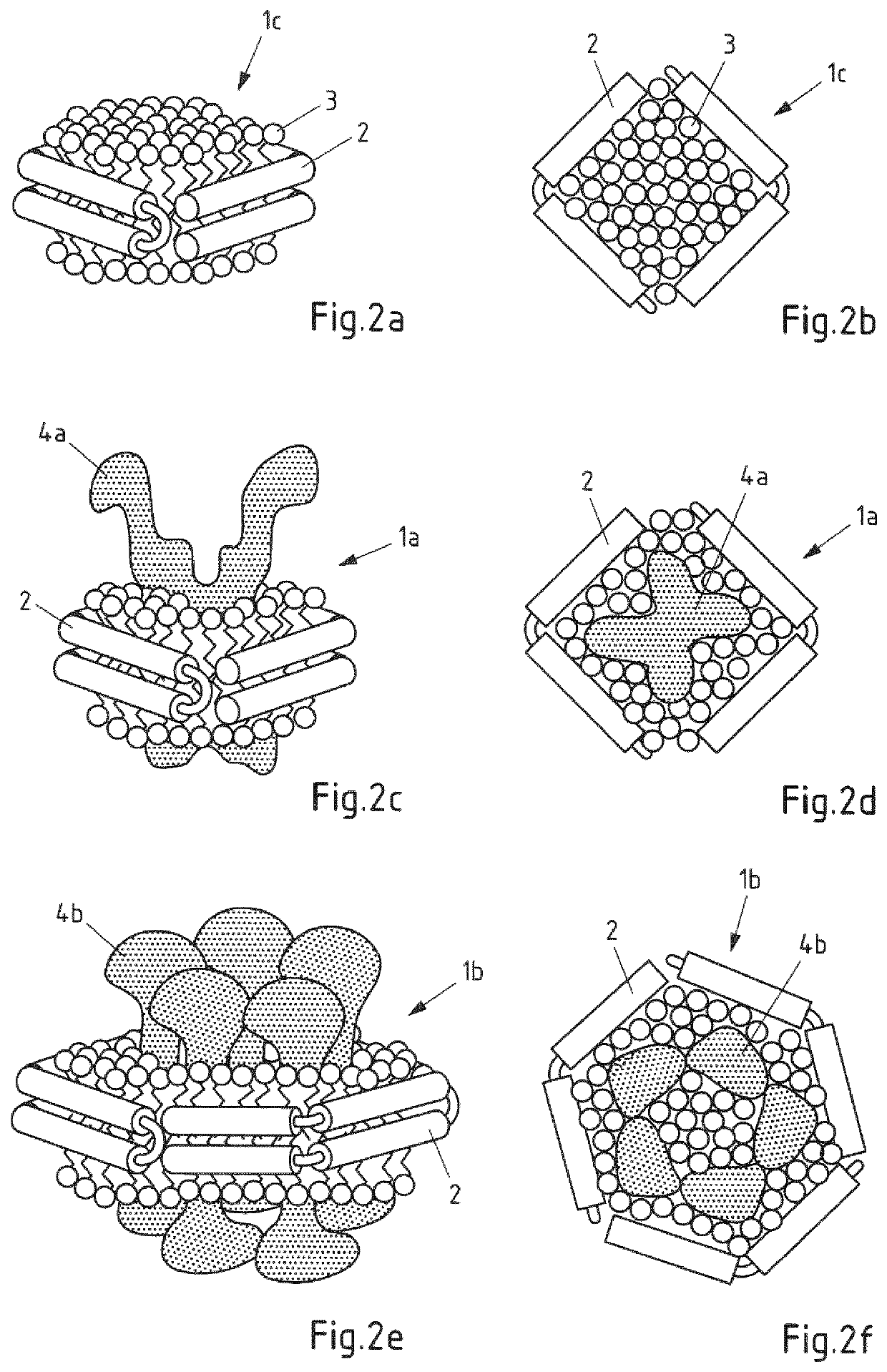
Non-naturally occurring lipoprotein particle
InactiveUS6670452B2Increased proliferationIncrease concentrationPeptide/protein ingredientsTissue cultureBinding siteLipoprotein particle
Non-naturally occurring receptor competent LDL particle comprising at least one peptide component wherein the said peptide component comprises at least a binding site for an Apo B protein receptor and at least one lipophilic substituent.
Owner:UNIV OF STRATHCLYDE
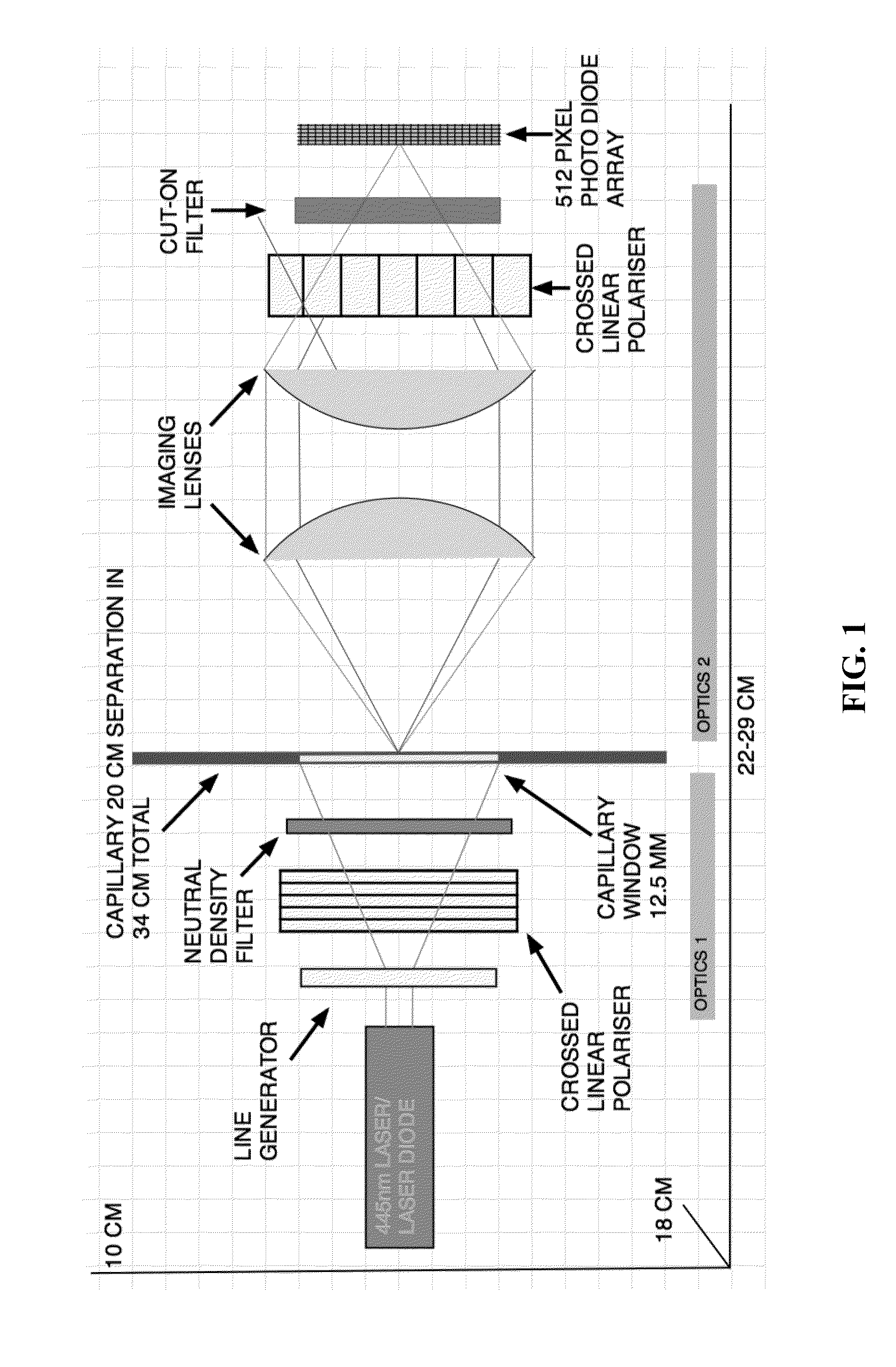
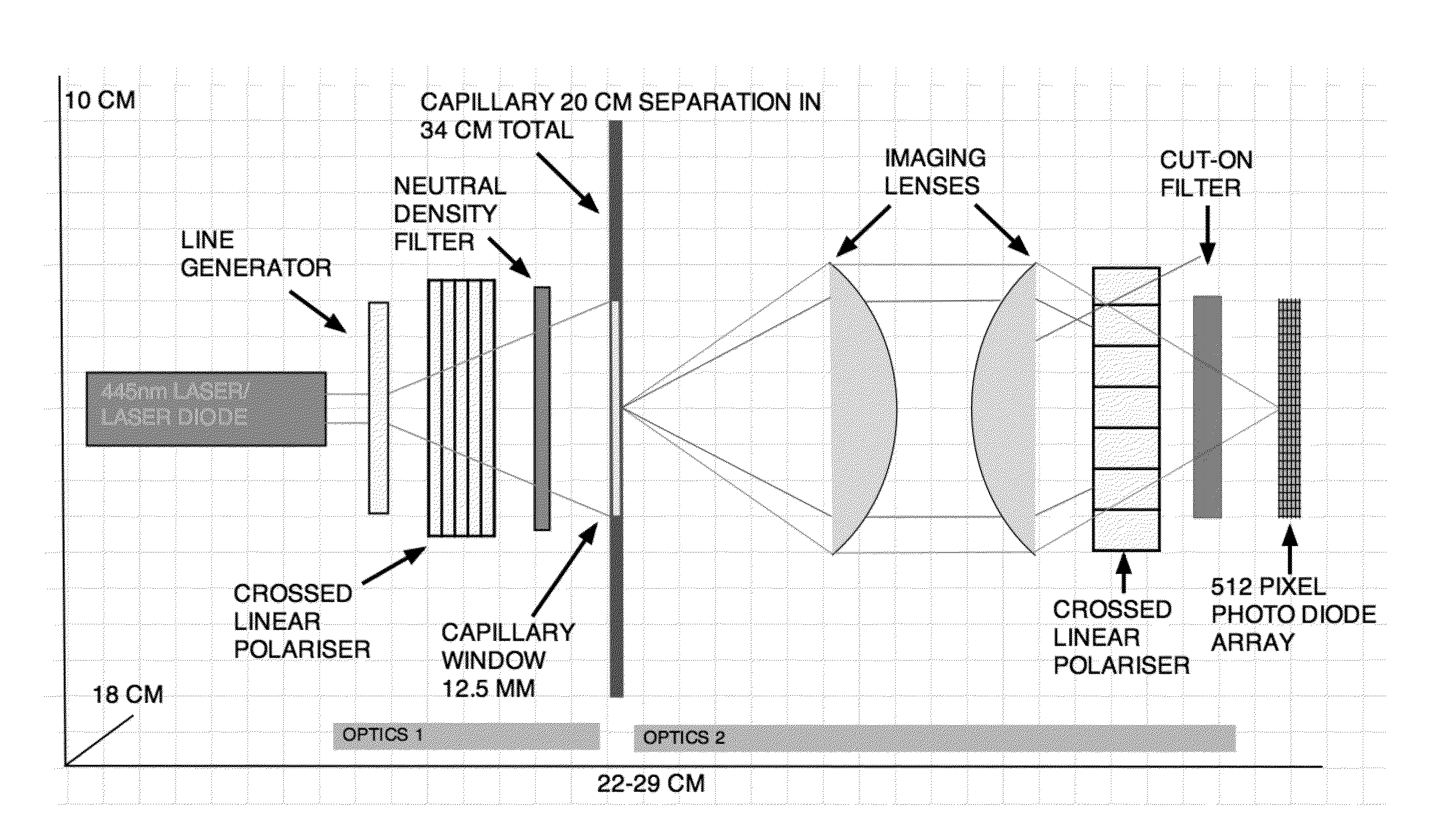
Quantitative molar concentration detection of specific apolipoprotein-containing particles present in bodily fluids by using capillary electrophoresis
InactiveUS20160109472A1Fast and accurate and detailed resolutionBiocideComponent separationDiseaseLipid particle
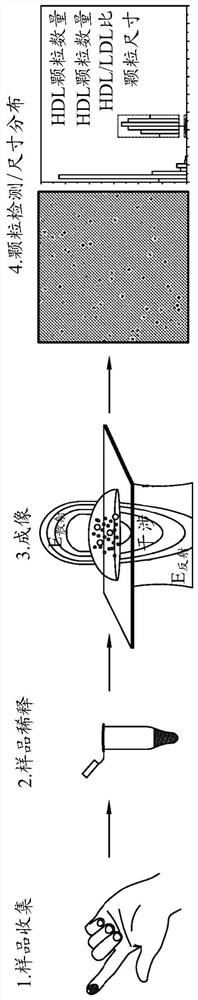
A method for determining the molar concentration of specific lipoprotein particles present in a bodily fluid is presented. Multipixel Capillary Isotachophoresis Laser Induced Fluorescence is applied to fluorescently-labeled lipoproteins or immunologically-labeled apolipoproteins, facilitating quantification of lipoproteins and / or lipid particles and / or their associated apolipoproteins in a sample. The measurements are used to predict the risks of developing, progressing in severity of diseases related to lipoprotein particles, including cardiovascular and metabolic disorders.
Owner:HELENA LAB
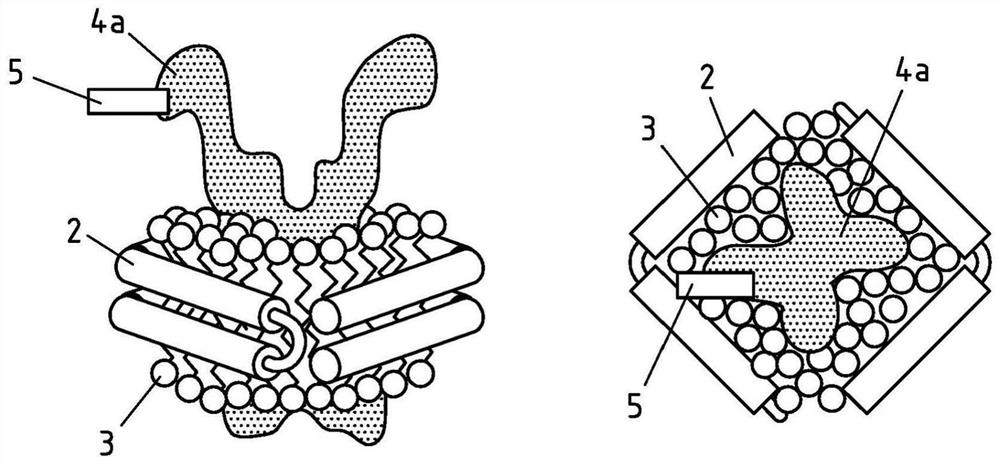
Production of salipro particles
PendingCN113873999AKeep dryBiological Functional EffectsMacromolecular non-active ingredientsLiposomal deliveryA lipoproteinLipoprotein particle
The invention relates to a process for preparing saposin lipoprotein particles, comprising a saposin-like protein, lipids and optionally a hydrophobic agent wherein the saposin-like protein or the hydrophobic agent is selectively bound to a support to allow the self-assembly of the saposin lipoprotein particles. The process of the invention comprises the step of a.) providing the hydrophobic agent and lipids, b. 1) / b.2) contacting the hydrophobic agent or the saposin-like protein with a support that is capable of selectively binding either of the two molecules to the support, c.1) / c.2) contacting the support-bound particle components with the remaining particle components, either the saposin-like protein or the hydrophobic agent, to allow fpr the self-assembly of the saposin lipoprotein particle on the support and d.) optionally eluting the support-bound saposin lipoprotein particles.
Owner:塞利普洛生物技术有限责任公司
Detection system used for simultaneously detecting lipoprotein sub-fraction cholesterol and lipoprotein particle concentration
InactiveCN110108673AScattering properties measurementsColor/spectral properties measurementsLipoprotein particleLipoprotein cholesterol
The invention relates to a detection system used for simultaneously detecting lipoprotein sub-fraction cholesterol and lipoprotein particle concentration. The detection system is characterized in thatthe system comprises a sample container, a reagent container, a cleaning fluid container, a reaction module, a first detection module and a second detection module; the sample container is connectedto the inlet pipeline of the first detection module through a pipeline; the cleaning liquid container is connected to a pipeline which is connected with the sample container and the first detection module through a pipeline; the outlet of the first detection module is connected with the inlet of the reaction module through a pipeline; the reagent container is connected to a pipeline which is connected with the first detection module and the reaction module through a pipeline; and the outlet end of the reaction module is connected with the second detection module. With the detection system adopted, lipoprotein cholesterol sub-fractions can be detected, and lipoprotein particle concentration can be also detected; and since the detection system does not need to perform detection twice, and time and cost are saved.
Owner:江西美康盛德生物科技有限公司
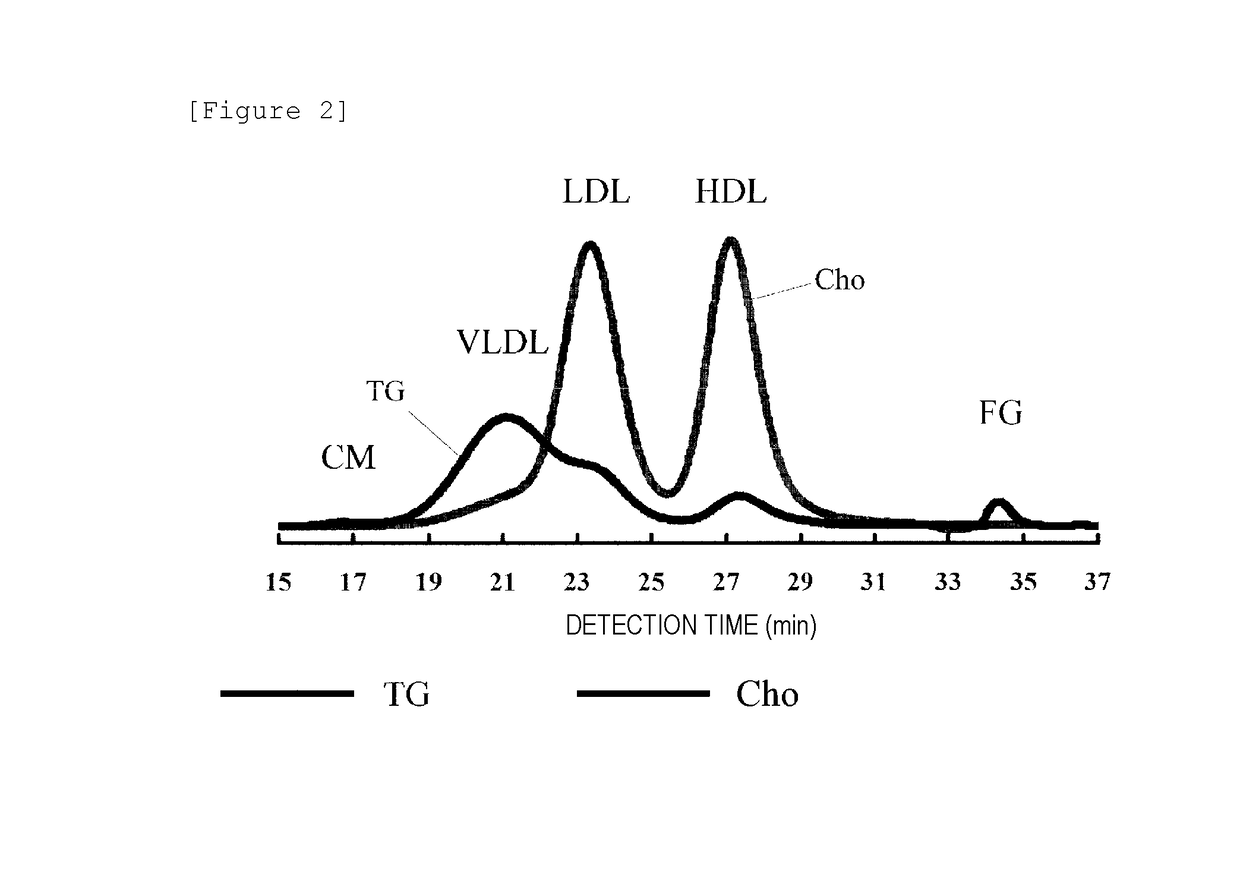
Method for analyzing lipoproteins
A concentration of cholesterol ester or free cholesterol can be calculated using the concentrations of total cholesterol and triglyceride in lipoproteins contained in a sample. Further, a concentration of lipoprotein particles in a fraction can be calculated using the lipoprotein particle size in the fraction. The concentrations of total cholesterol and triglyceride contained in a subject sample are detected. Using the concentrations of total cholesterol and triglyceride thus detected, the concentration of cholesterol ester or free cholesterol is calculated. Further, using the concentration of cholesterol ester or free cholesterol thus calculated, the concentration of lipoprotein particles in a fraction can be calculated.
Owner:OKAZAKI MITSUYO
Lipoprotein particle number from measurements of lipoprotein particle phospholipid concentration in lipoprotein particle membrane bilayer
InactiveUS20160109471A1Fast and reliable and accurateBiological testingFluorescence/phosphorescencePhospholipidCvd risk
This application describes a method for measuring the molar concentrations of lipoprotein particles and lipoprotein subclass particles in bodily fluid by Multipixel Capillary Isotachophoresis Laser Induced Fluorescence (MPCE-ITP-LIF) and compositional analysis of spherical lipoprotein particles. The ability to measure several kinds of lipoproteins and particles in one unified system provides a useful diagnostic tool for predicting the risk of developing metabolic diseases such as cardiovascular disease and cardiodiabetes.
Owner:HELENA LAB
Vaccines for malaria
InactiveUS9364525B2Antibody mimetics/scaffoldsAntiinfectivesPolyclonal antibodiesLipoprotein particle
The present invention relates to a novel lipoprotein particle, methods for preparing and purifying the same, its use in medicine, particularly in the prevention of malarial infections, compositions / vaccines containing the particle or antibodies against the protein particle such as monoclonal or polyclonal antibodies and use of the same, particularly in therapy. In particular it relates to an immunogenic protein particle comprising the following monomers:a. a fusion protein comprising sequences derived from a CS protein of P. vivax and the S antigen of Hepatitis B (CSV-S), andb. a fusion protein comprising sequences derived from CS protein of P. falciparum and S antigen of Hepatitis B (RTS), andc. optionally the S antigen derived from Hepatitis B.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA +1
Methods, Systems and Computer Programs for Assessing CHD Risk Using Adjusted HDL Particle Number Measurements
ActiveUS20170083663A1Easy to understandPromote stratificationMagnetic measurementsTherapiesMathematical modelA lipoprotein
Methods, computer program products and apparatus determine a subject's risk of having or developing CHD using a calculated HDL particle risk number and / or a mathematical model of risk associated with HDL particles that adjusts concentrations of at least one of the subclasses of small, medium and large HDL particle measurements to reflect predicted CHD risk. A calculated LDL particle risk number may also be generated as well as a lipoprotein particle index derived from the ratio of RLDL / RHDL.
Owner:LIPOSCI
Method of determining lipoprotein concentration in solution using light scattering
PendingCN111684285AScattering properties measurementsDisease diagnosisA lipoproteinLipoprotein particle
The invention relates the use of single particle light scattering, preferably interferometric scattering microscopy (also referred to herein as iSCAT), to measure the concentration of a particle in asolution. The invention furthermore relates to the use of light scattering to detect lipoprotein particles in a sample, and to related diagnostic and treatment methods.
Owner:OXFORD UNIV INNOVATION LTD
Short interfering RNA templated lipoprotein particles (siRNA-TLP)
ActiveUS10967072B2Powder deliveryPharmaceutical non-active ingredientsMolecular compositionA lipoprotein
Nanostructures for the systemic delivery of nucleic acids, such as RNA, are provided herein. The nanostructures include templated lipoprotein nanoparticles (TLPs) composed of a core decorated with proteins, a lipid bilayer and hydrophobic molecules that self-assemble with nucleic acids, such as RNA. The nanostructures are useful for research, therapeutic and diagnostic applications.
Owner:NORTHWESTERN UNIV
System and method for assessing quanitites or sizes of lipoprotein particles from lipoprotein particle compositions
InactiveUS20150260631A1Shorten the timeLow costElectrolysis componentsVolume/mass flow measurementFree cholesterolLipoprotein particle
This invention relates to methods for assessing quantities of spherical or substantially spherical lipoprotein particles or portions thereof present in a biological sample based on the measurement of free cholesterol and / or phospholipid content in the lipoprotein particles. The invention also relates to the use of the assessed quantities of the lipoprotein particles or portions thereof to determine whether a subject is at increased risk for cardiovascular diseases and cardiodiabetes.
Owner:HELENA LAB
Fluorescence assay for measuring the rate of cholesterol ester transfer
An improved method of obtaining fluorescence measurements which may be used as a measure of the CETP-catalyzed rate of transfer of a lipophilic non-polar fluorescent cholesteryl ester between lipoprotein particles is disclosed. The method may be used in assays for screening CETP inhibitors.
Owner:MERCK SHARP & DOHME CORP
Non-naturally occurring lipoprotein particle
Non-naturally occurring lipoprotein particles, process for preparing such particles and uses thereof.
Owner:UNIV OF STRATHCLYDE
Method for analyzing lipoproteins
A concentration of cholesterol ester or free cholesterol can be calculated using the concentrations of total cholesterol and triglyceride in lipoproteins contained in a sample. Further, a concentration of lipoprotein particles in a fraction can be calculated using the lipoprotein particle size in the fraction. The concentrations of total cholesterol and triglyceride contained in a subject sample are detected. Using the concentrations of total cholesterol and triglyceride thus detected, the concentration of cholesterol ester or free cholesterol is calculated. Further, using the concentration of cholesterol ester or free cholesterol thus calculated, the concentration of lipoprotein particles in a fraction can be calculated.
Owner:OKAZAKI MITSUYO
Non-naturally occurring lipoprotein particle
Non-naturally occurring lipoprotein particles, process for preparing such particles and uses thereof.
Owner:UNIV OF STRATHCLYDE
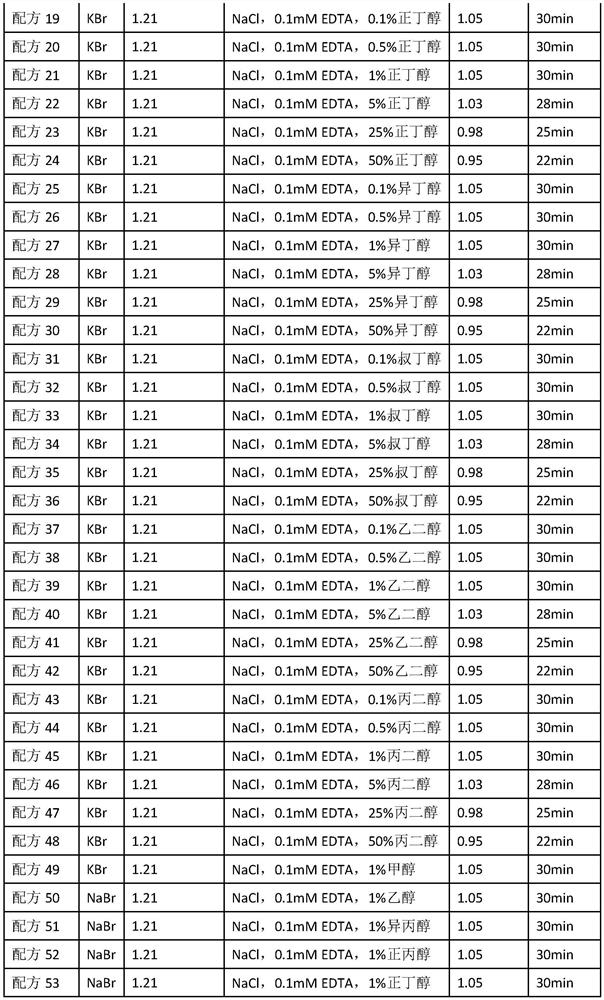
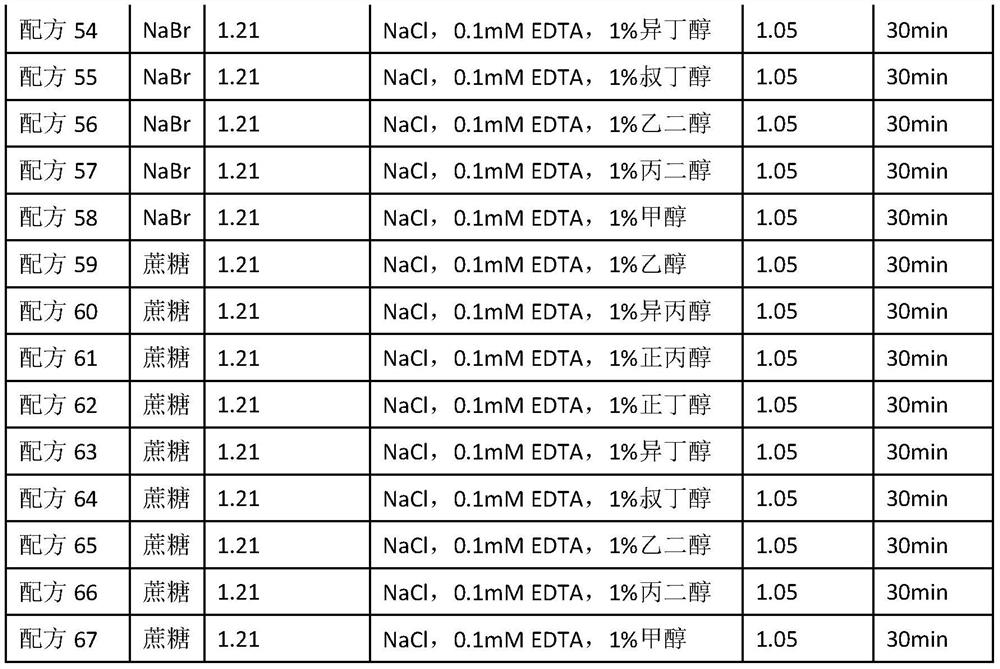
A reagent for detecting the concentration of lipoprotein particles and its application method
ActiveCN112798481BHigh sensitivityBiological testingParticle suspension analysisAlcoholAlcohol substance
The invention discloses a reagent for detecting the concentration of lipoprotein particles. The reagent is composed of the following components: a diluent, a density liquid, and a buffer, and the density liquid contains alcohols, and the alcohols The concentration of the substance is 0.1%‑50%. The invention belongs to the field of in vitro diagnosis. The invention provides a reagent for detecting the concentration of lipoprotein particles. The reagent can detect the concentration of protein particles alone, and can be used in combination with other instrument reagents to simultaneously detect lipoprotein cholesterol subcomponents without having to detect twice, saving It saves time and cost, has high commercial value, and can be widely used in clinical tests and scientific research experiments.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Production of salipro particles
PendingUS20220192982A1Macromolecular non-active ingredientsLiposomal deliveryA lipoproteinLipoprotein particle
The invention relates to a process for preparing saposin lipoprotein particles, comprising a saposin-like protein, lipids and optionally a hydrophobic agent wherein the saposin-like protein or the hydrophobic agent is selectively bound to a support to allow the self-assembly of the saposin lipoprotein particles. The process of the invention comprises the step of a.) providing the hydrophobic agent and lipids, b. 1) / b.2 contacting the hydrophobic agent or the saposin-like protein with a support that is capable of selectively binding either of the two molecules to the support, c.1) / c.2) contacting the support-bound particle components with the remaining particle components, either the saposin-like protein or the hydrophobic agent, to allow for the self-assembly of the saposin lipoprotein particle on the support and d.) optionally eluting the support-bound saposin lipoprotein particles.
Owner:SALIPRO BIOTECH AB
Methods and kits for reducing the susceptibility of lipoprotein particles to atherogenic aggregation induced by arterial-wall enzymes
InactiveCN109789217APromoted activationCosmetic preparationsPeptide/protein ingredientsCholesterol crystalsA lipoprotein
Owner:凯文乔恩威廉姆斯
Saposin lipoprotein particles and libraries from crude membranes
The invention is directed to a process for preparing a library of saposin lipoprotein particles, wherein the particles comprise membrane components from a cell or an organelle membrane and a lipid binding polypeptide that is a saposin-like protein belonging to the SAPLIP family of lipid interacting proteins or a derivative form thereof, wherein the process comprises the steps of a) providing a mixture of crude membrane vesicles obtained from a cell or an organelle membrane; b) contacting the mixture of step a) with the lipid binding polypeptide in a liquid environment; and c) allowing for self-assembly of the particles. The invention also provides a process for preparing a purified saposin lipoprotein particle comprising the steps of preparing a library according to the process described above and the additional step of f) purifying the saposin lipoprotein particle from the library. In addition, the invention provides a library of saposin lipoprotein particles and saposin lipoprotein particles obtainable according to the processes of the invention. These can be used in medicine, in particular in preventing, treating or lessening the severity of a disease or for use in a diagnostic method, a cosmetic treatment or for use as vaccination formulation or as a tool for drug development, drug screening, drug discovery, antibody development, development of therapeutic biologies, for membrane or membrane protein purification, for membrane protein expression, for membrane and / or membrane protein research, in particular lipidomics and proteomics, preferably for the isolation, identification and / or study of membranes and / or membrane proteins or creation of a lipidome or proteome database.
Owner:SALIPRO BIOTECH AB
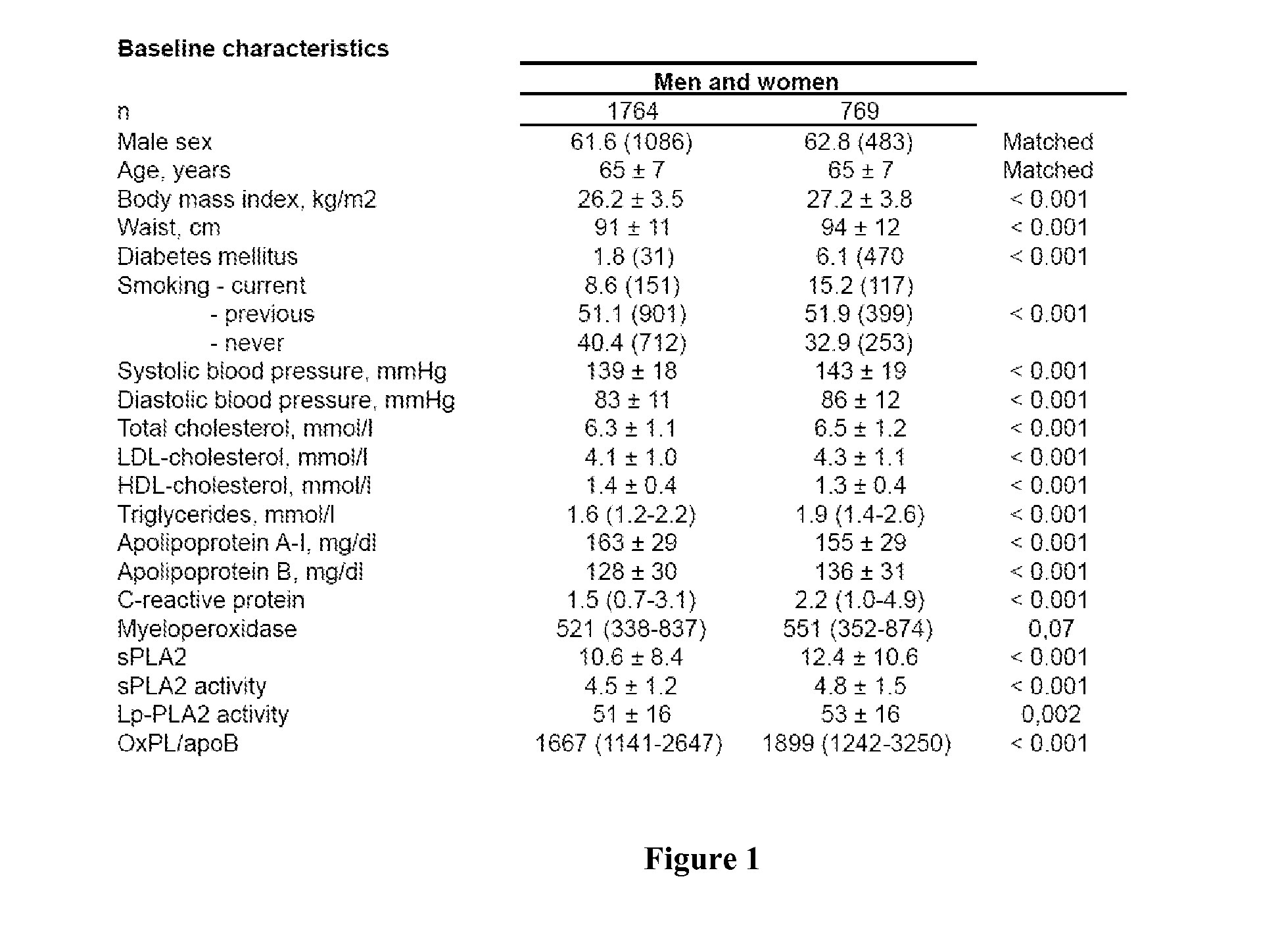
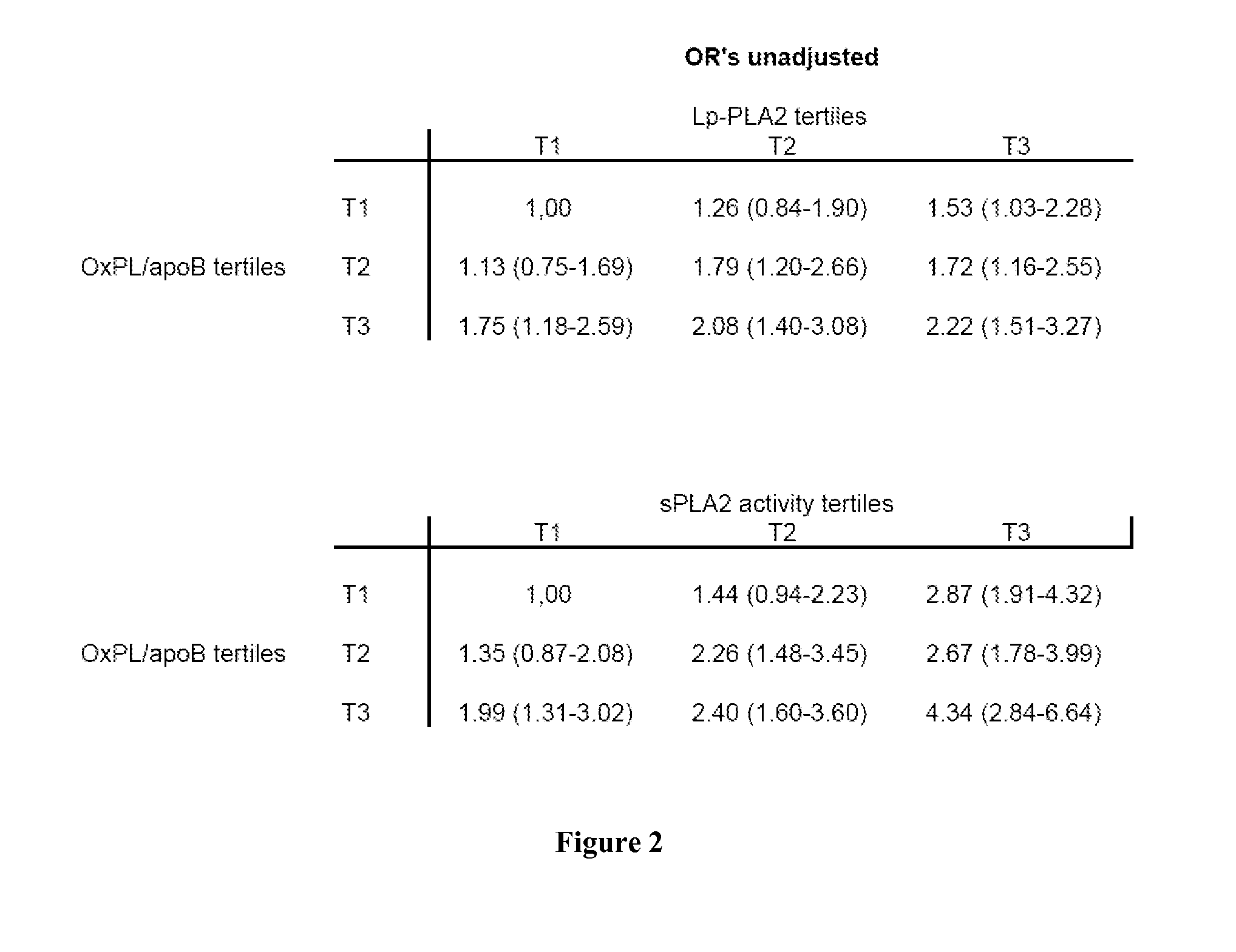
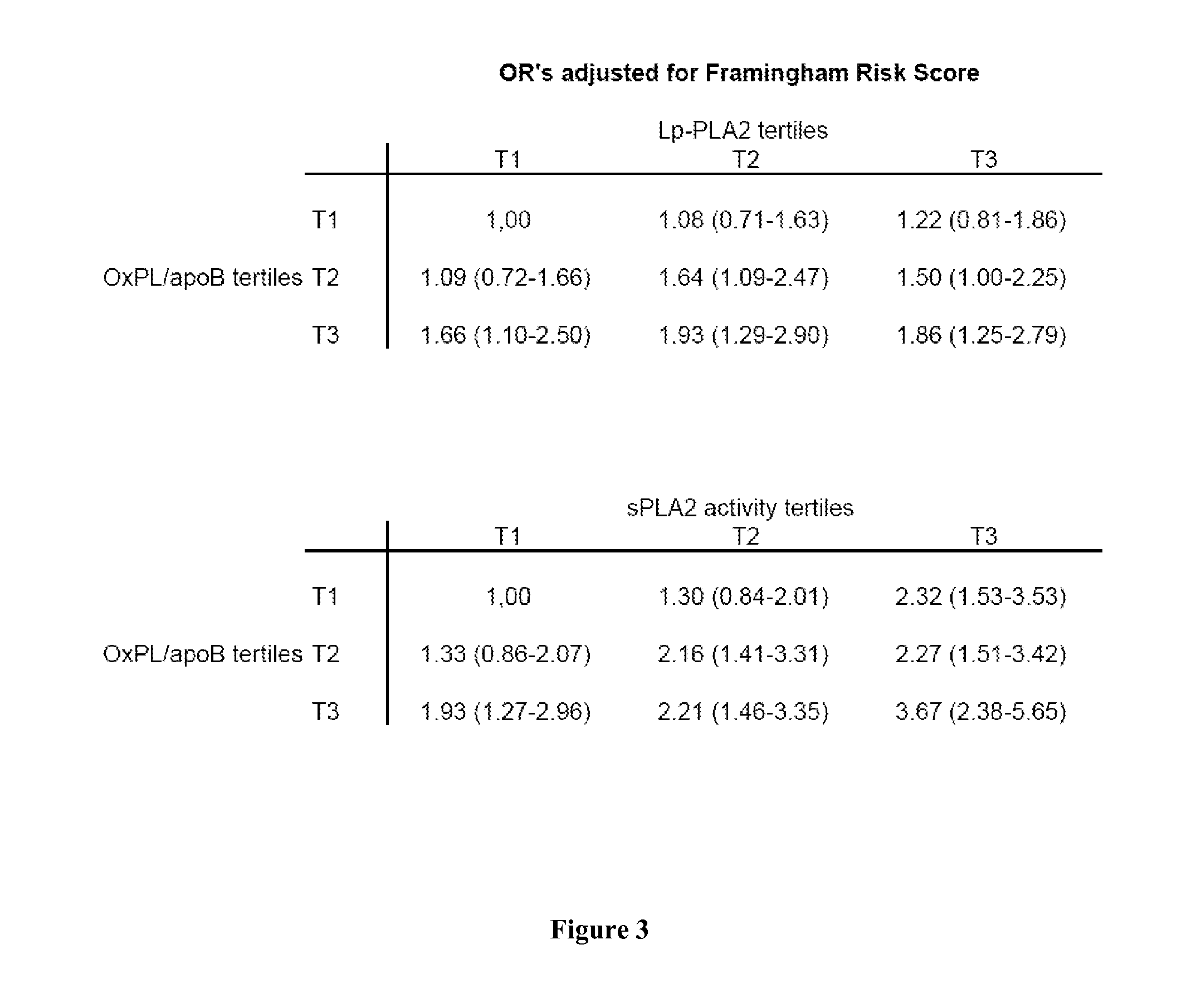
Combination of spla2 activity and oxpl/apob cardiovascular risk factors for the diagnosis/prognosis of a cardiovascular disease/event.
InactiveUS20110229919A1Small sample volumeHigh precisionMicrobiological testing/measurementDisease diagnosisApolipoproteins bPhospholipid
A method of identifying a subject having or at risk of having or developing a cardiovascular disease and / or a cardiovascular event, includes: measuring, in a sample obtained from the subject, at least two cardiovascular risk factors: a) sPLA2 activity and b) oxidized phospholipids on apolipoprotein B-100 particles (OxPL / apoB), combining the measurements, the combined value of sPLA2 activity and OxPL / apoB being indicative of having or a risk of having or developing a cardiovascular disease and / or cardiovascular event.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com