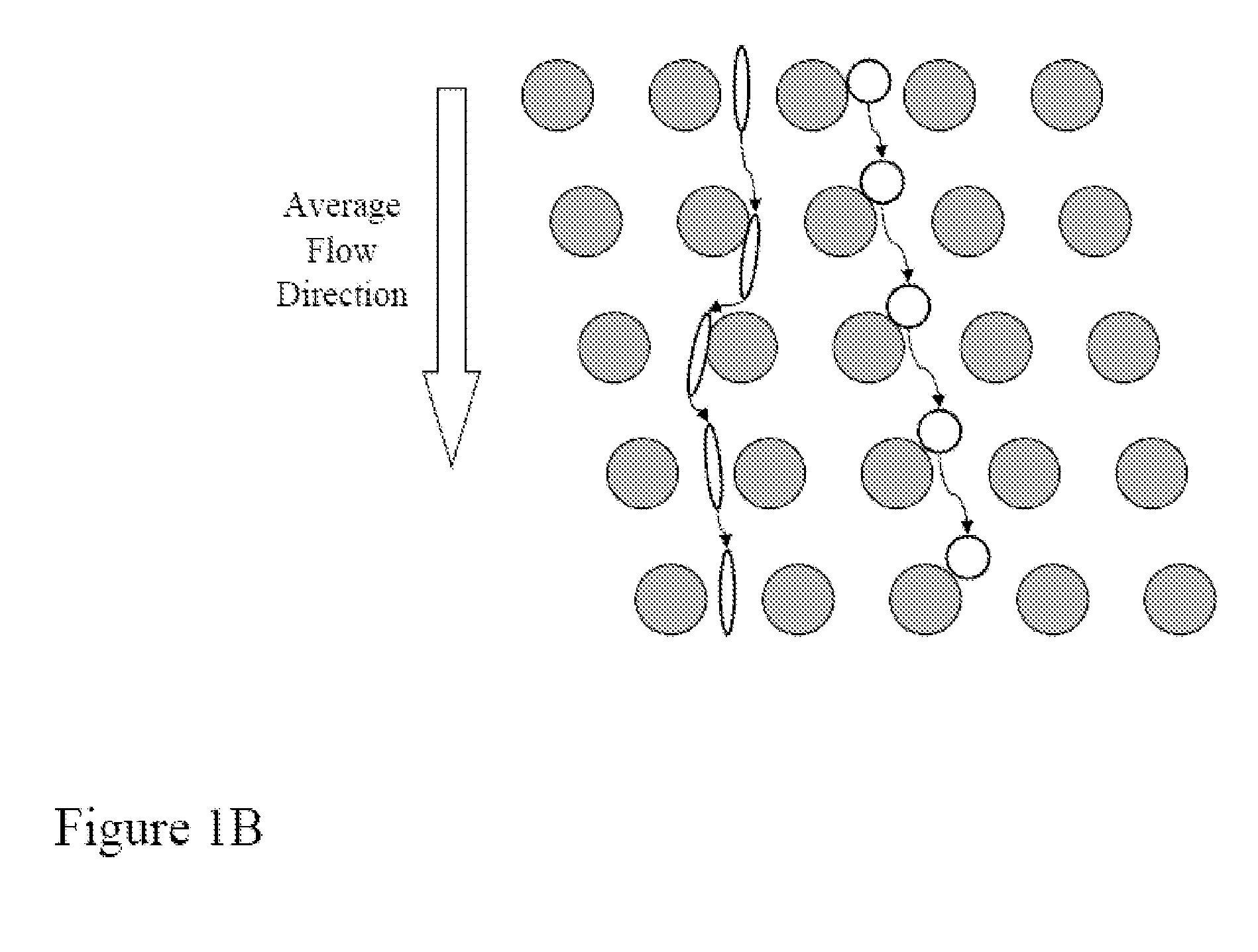Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
21232results about "Disease diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
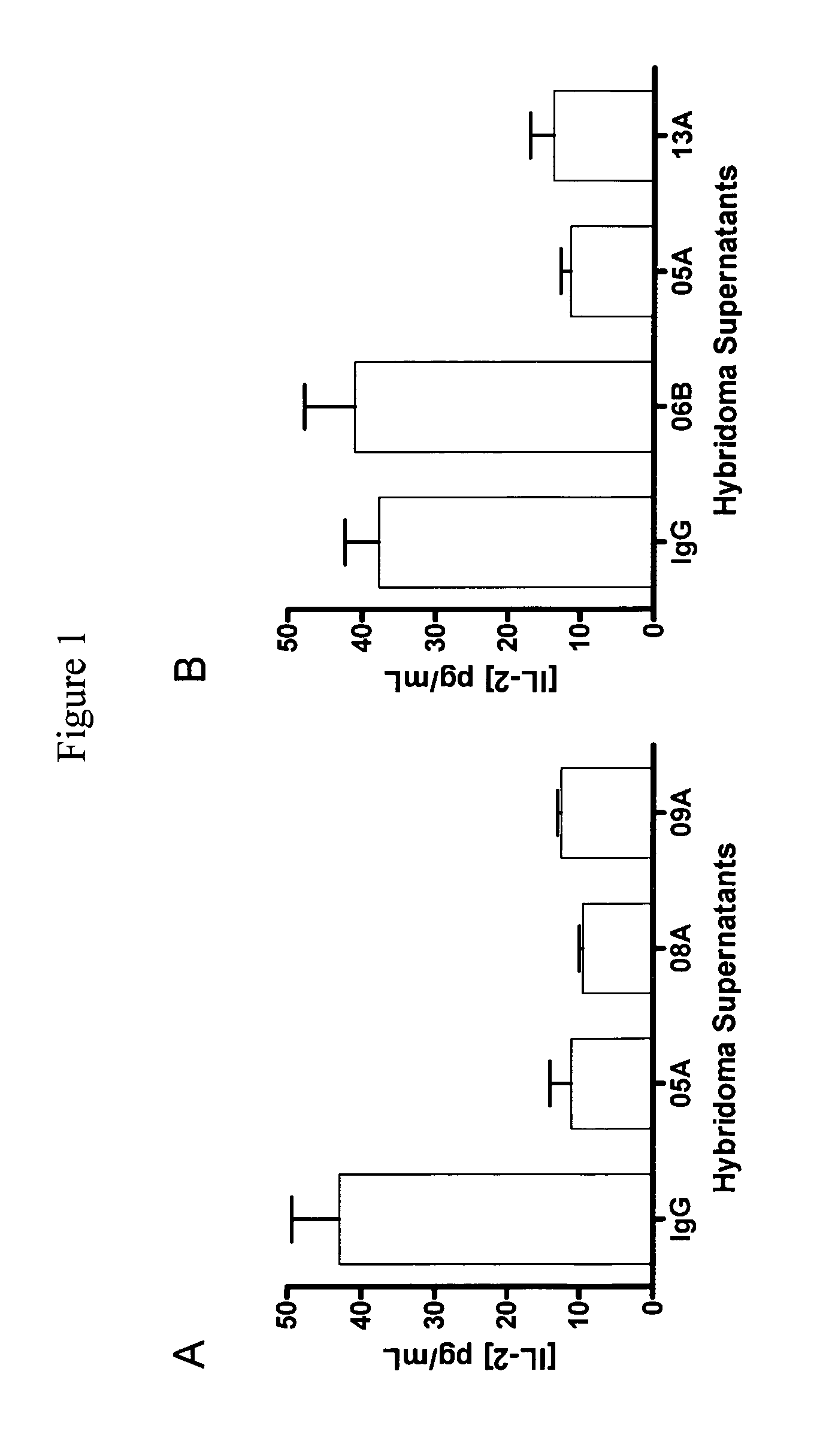
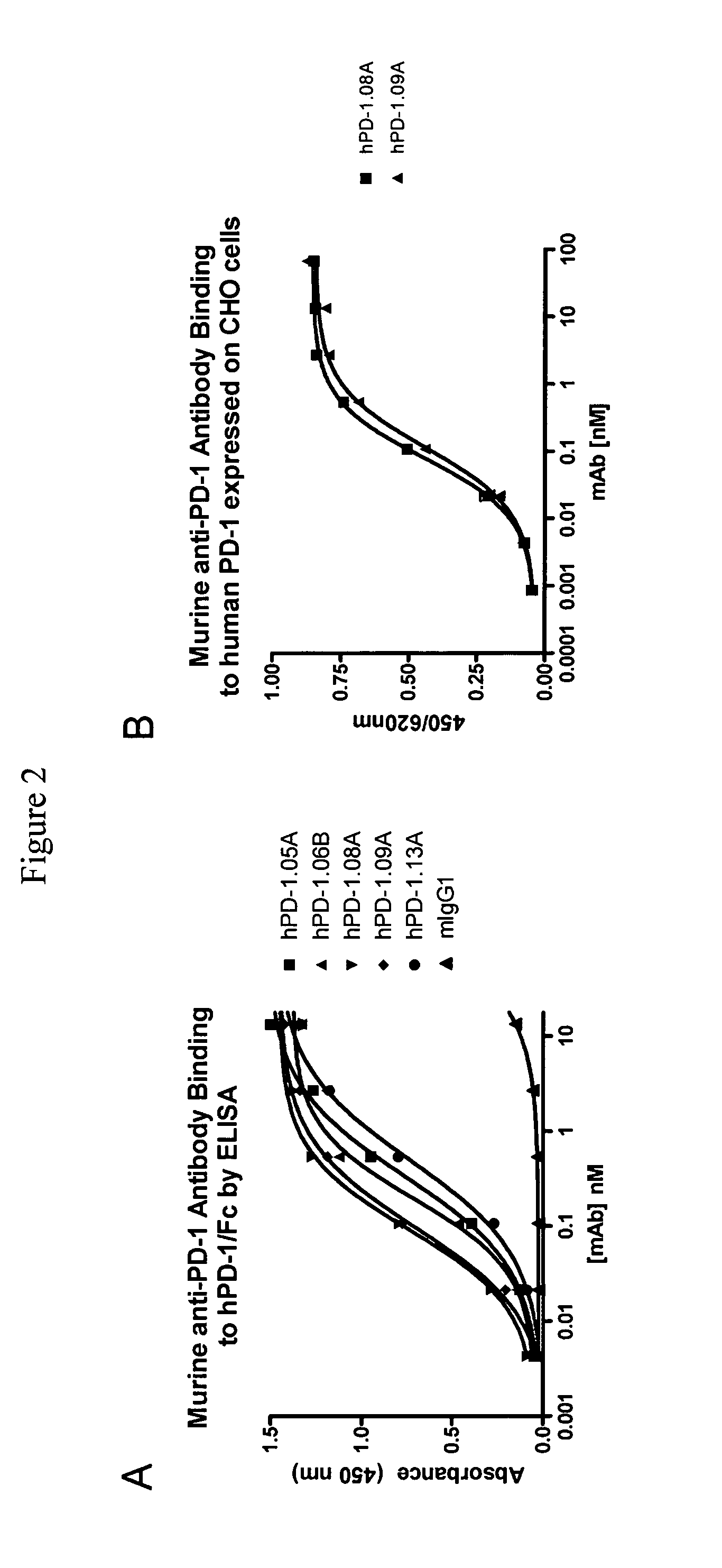
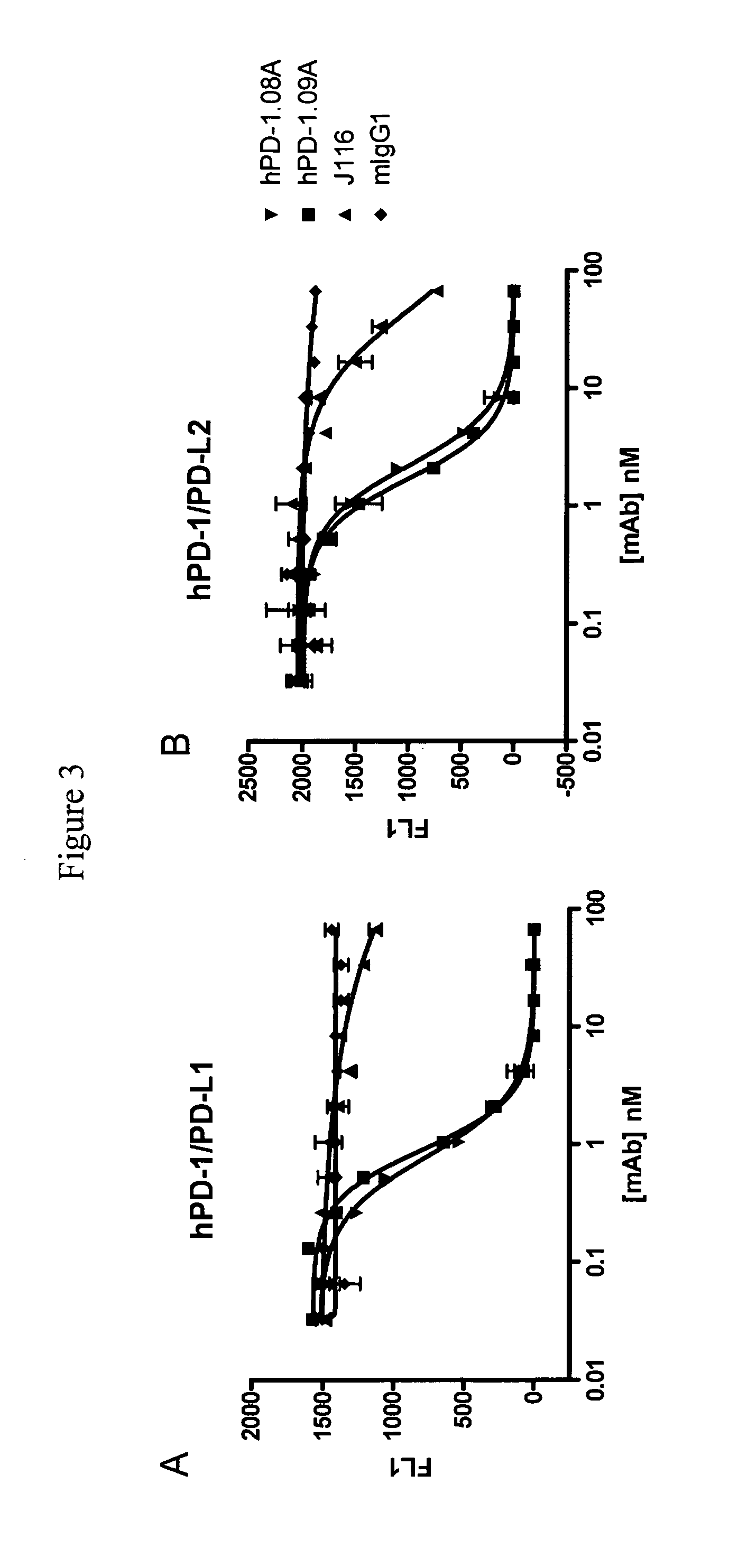
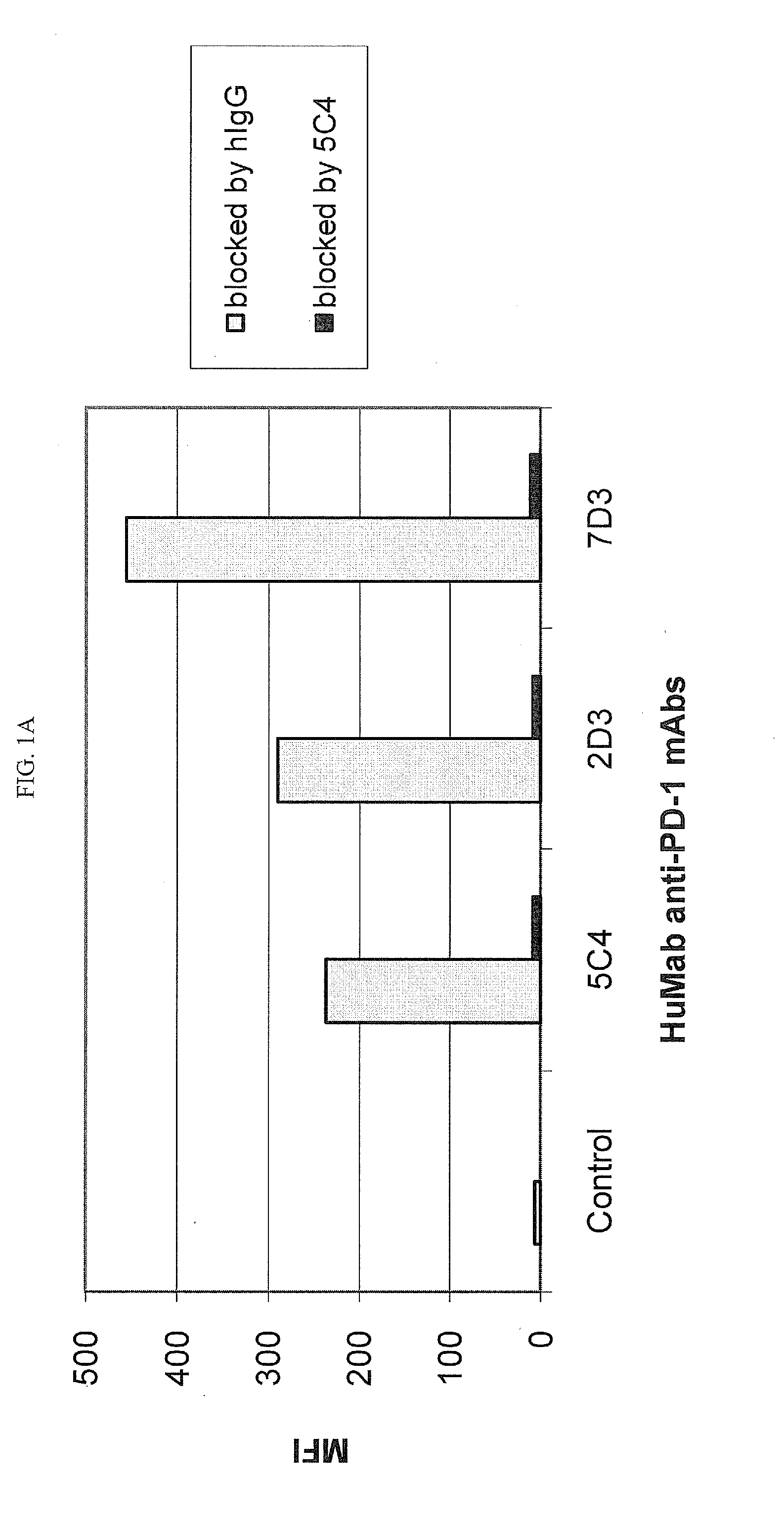
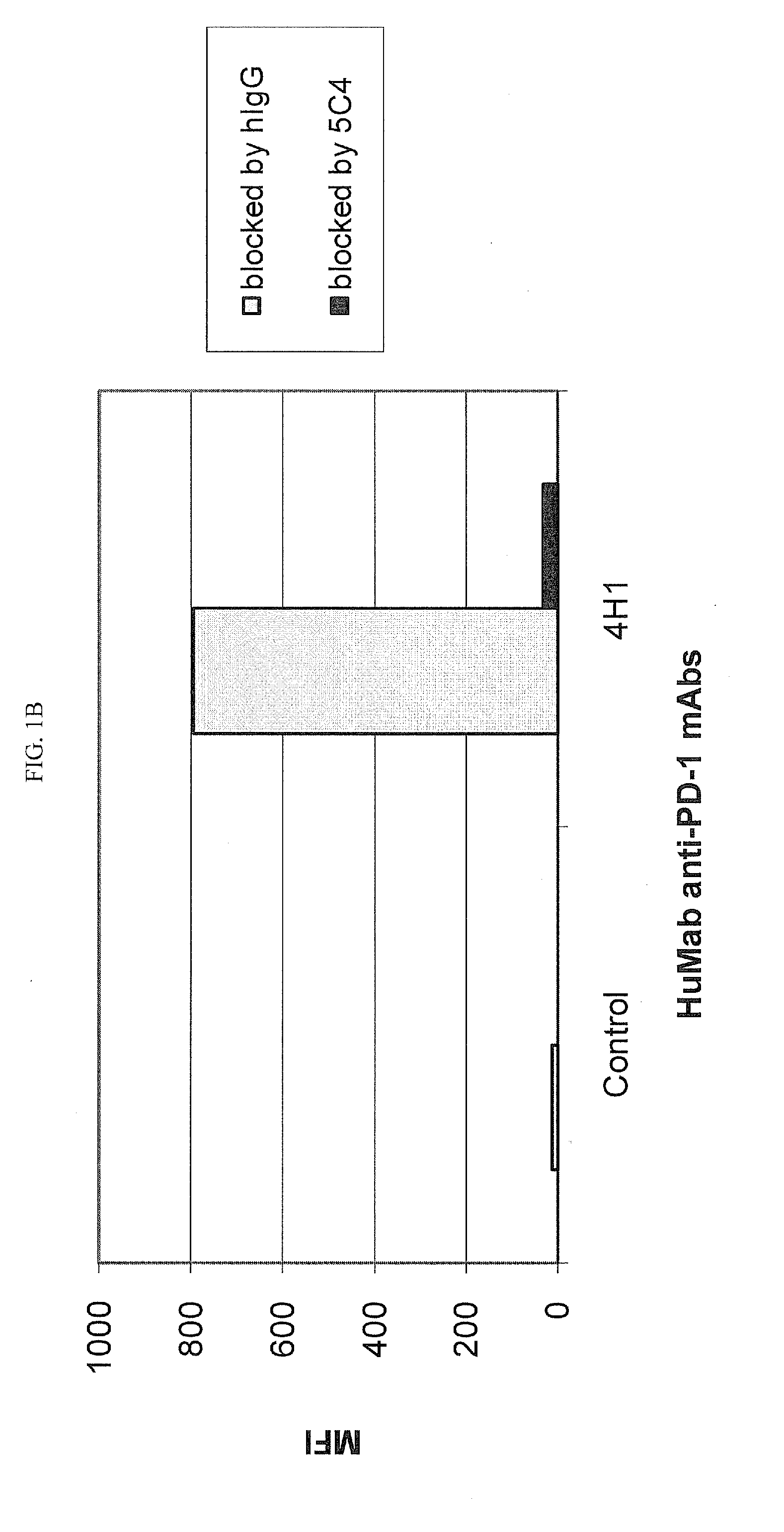
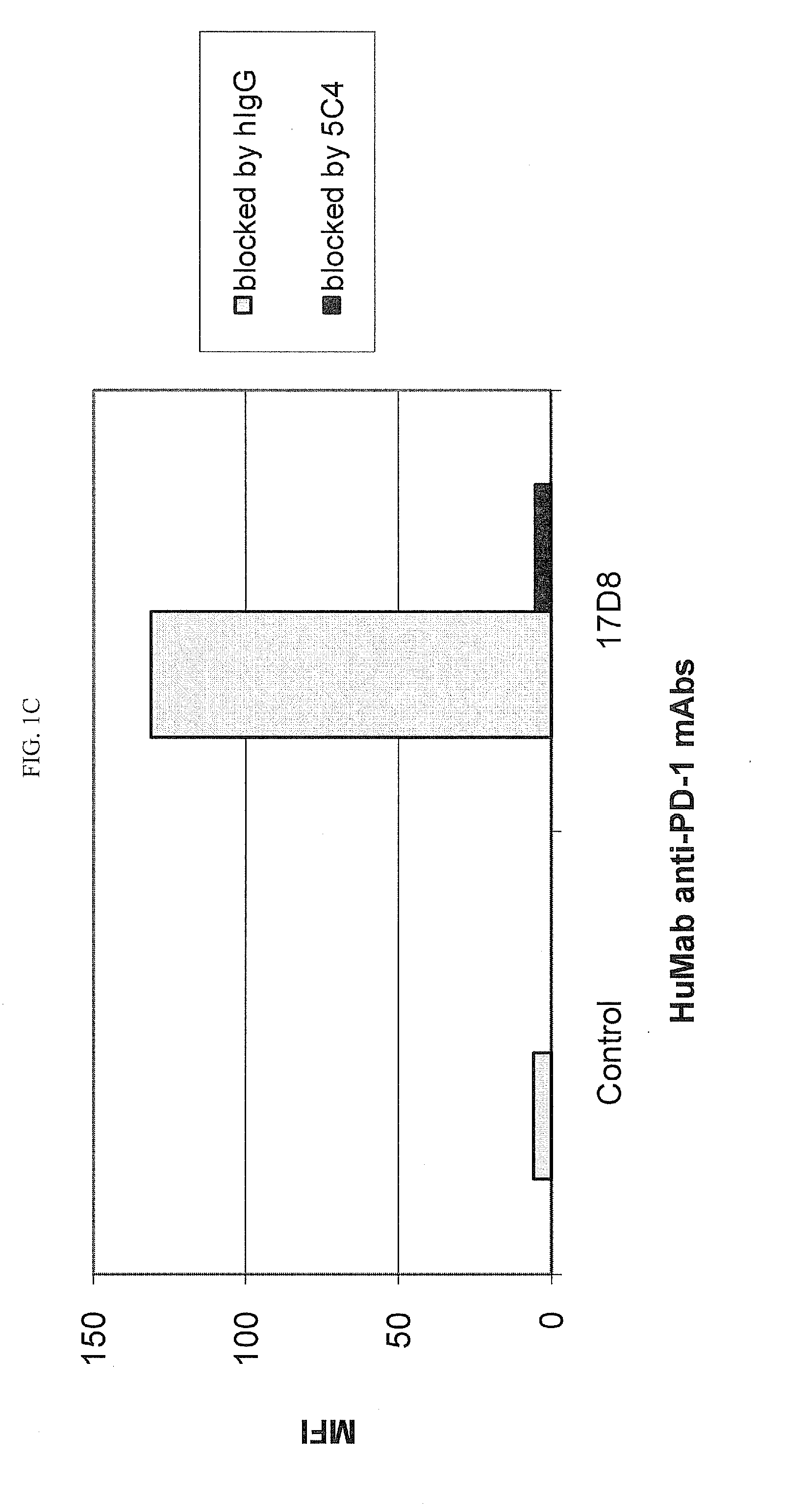
Antibodies to human programmed death receptor PD-1
ActiveUS8354509B2Increased activationIncreased proliferationSugar derivativesAntibody ingredientsProgrammed deathReceptor for activated C kinase 1
Antibodies which block the binding of human Programmed Death Receptor 1 (hPD-1) to its ligands (hPD-L1 or hPD-L2) and their variable region sequences are disclosed. A method of increasing the activity (or reducing downmodulation) of an immune response through the PD-I pathway is also disclosed.
Owner:MERCK SHARP & DOHME BV
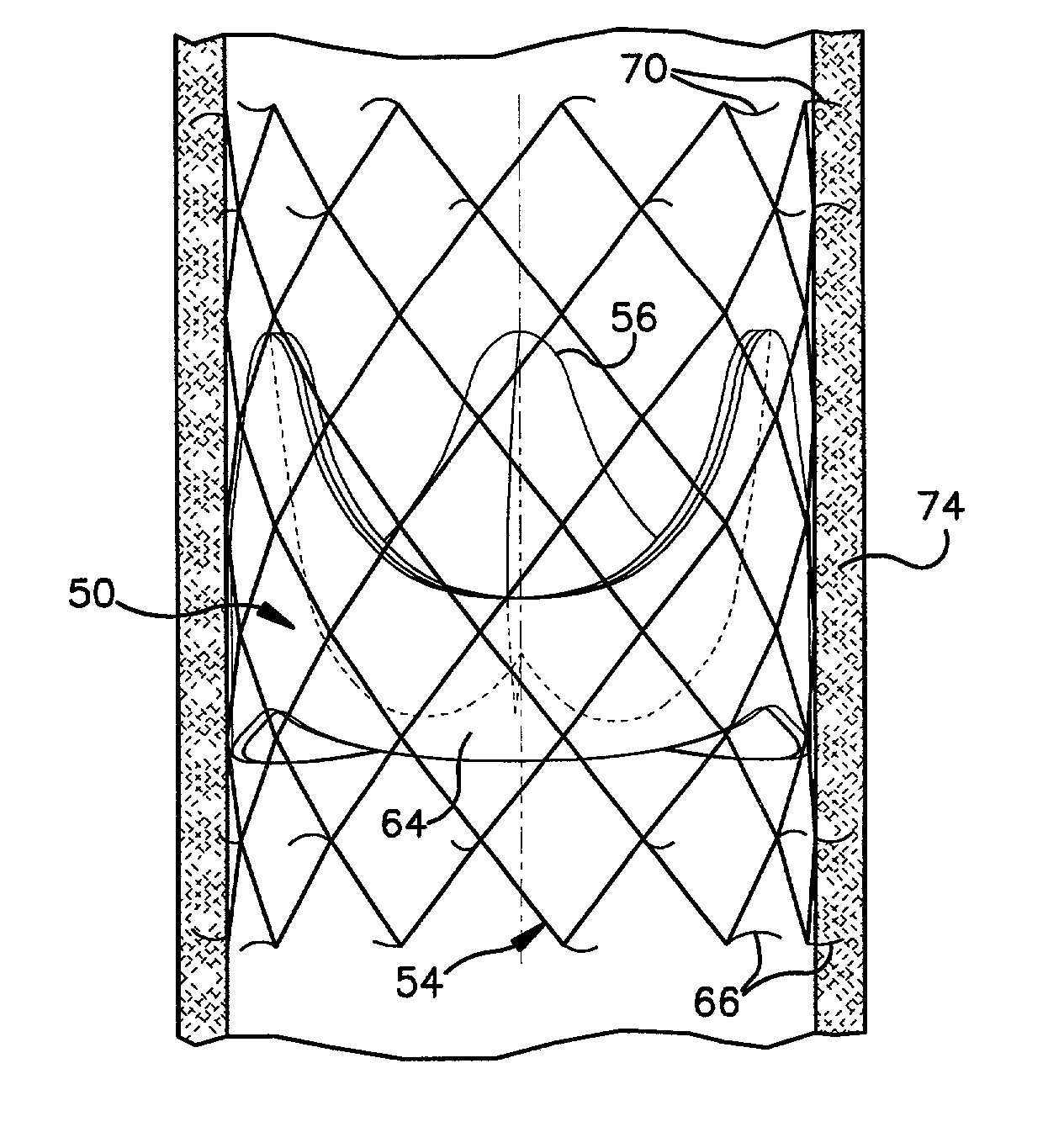
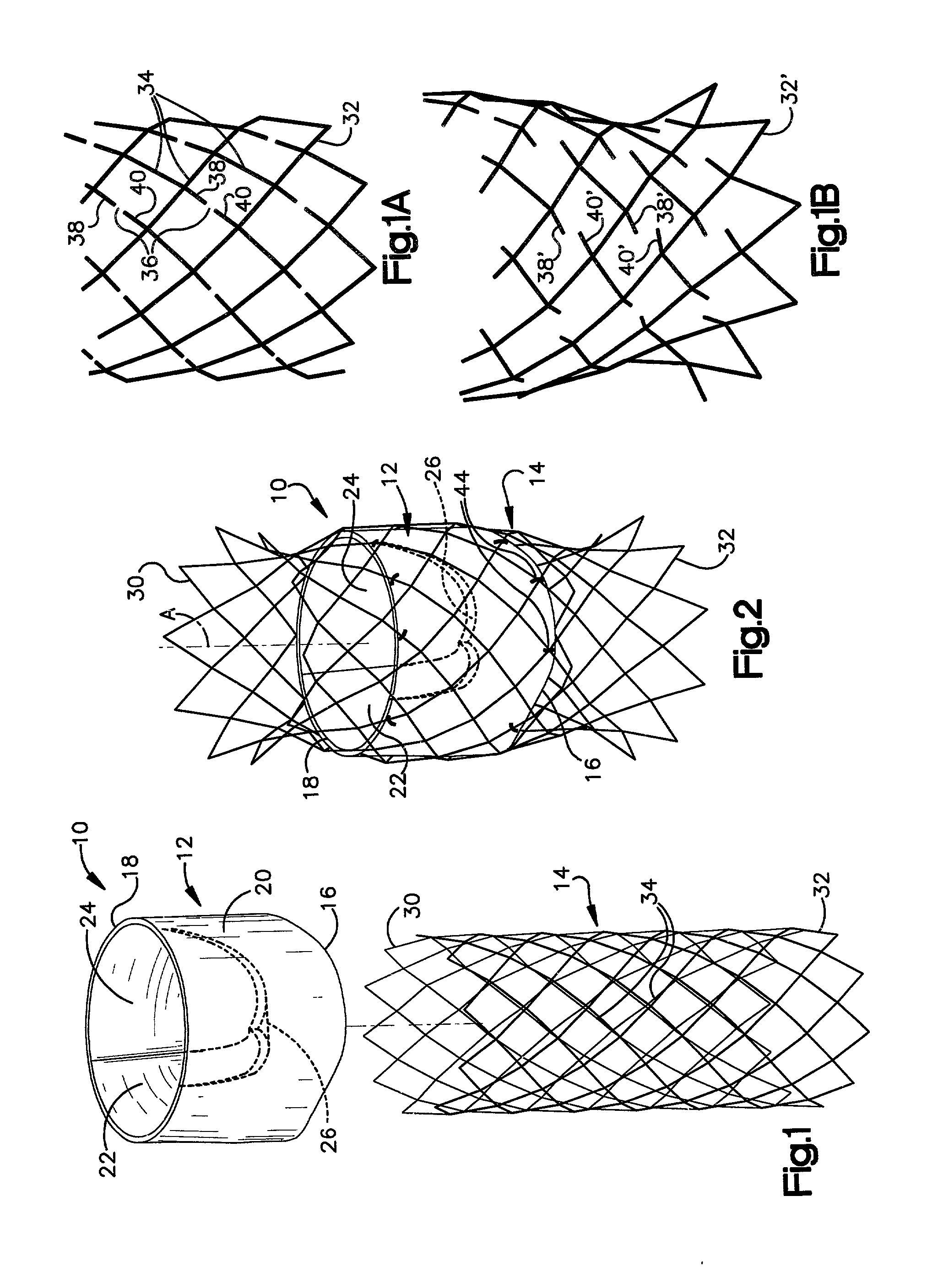
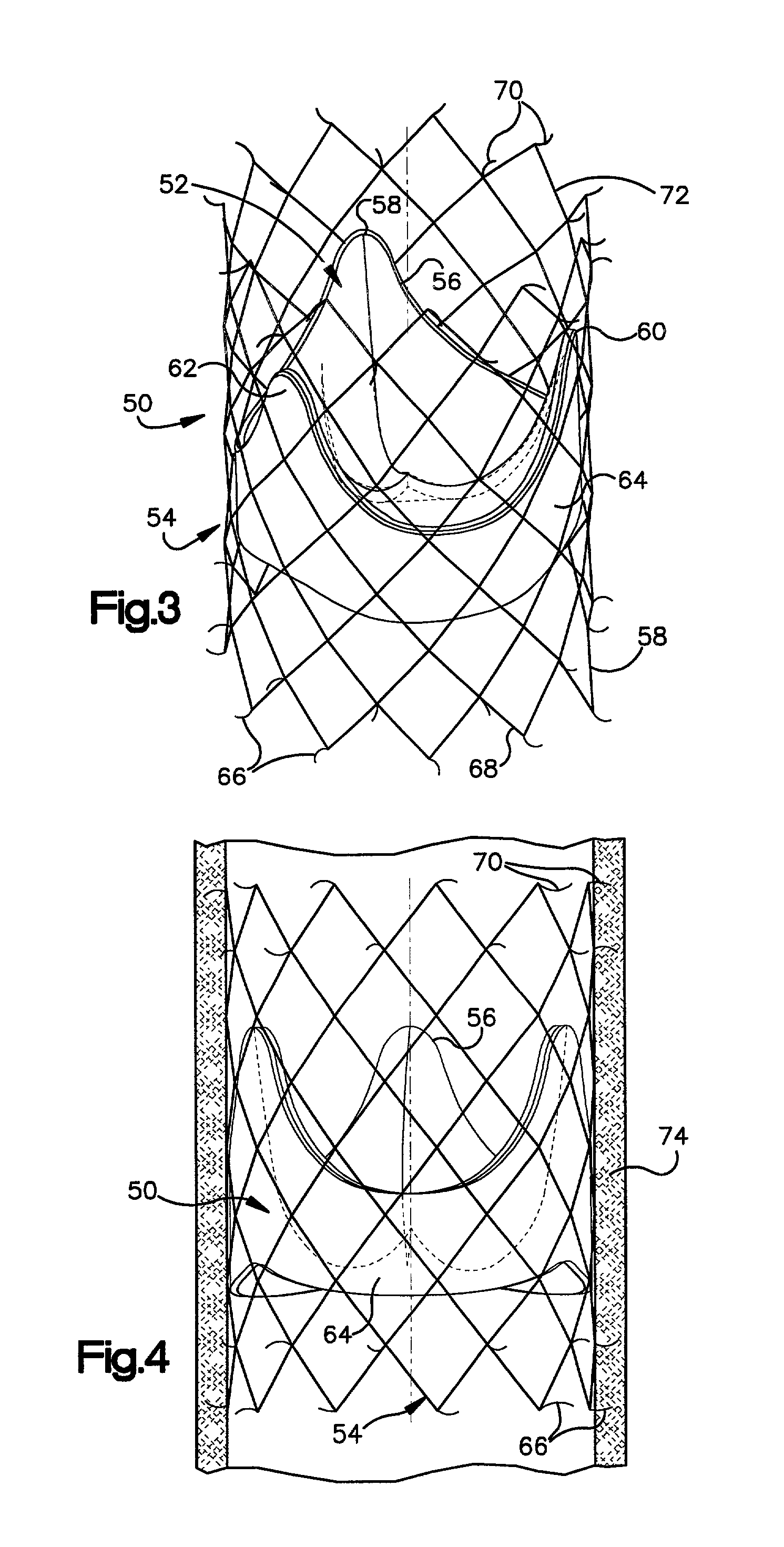
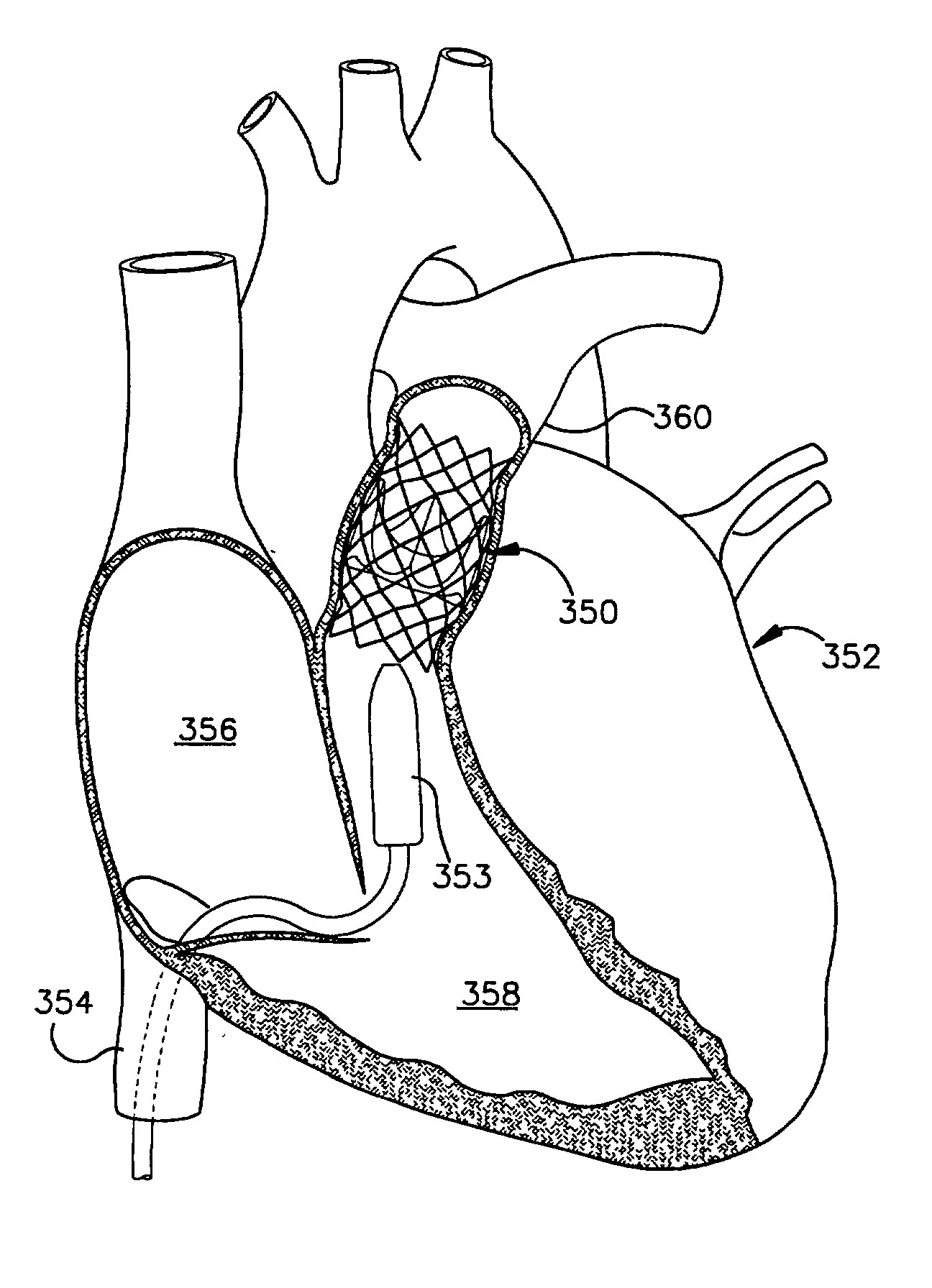
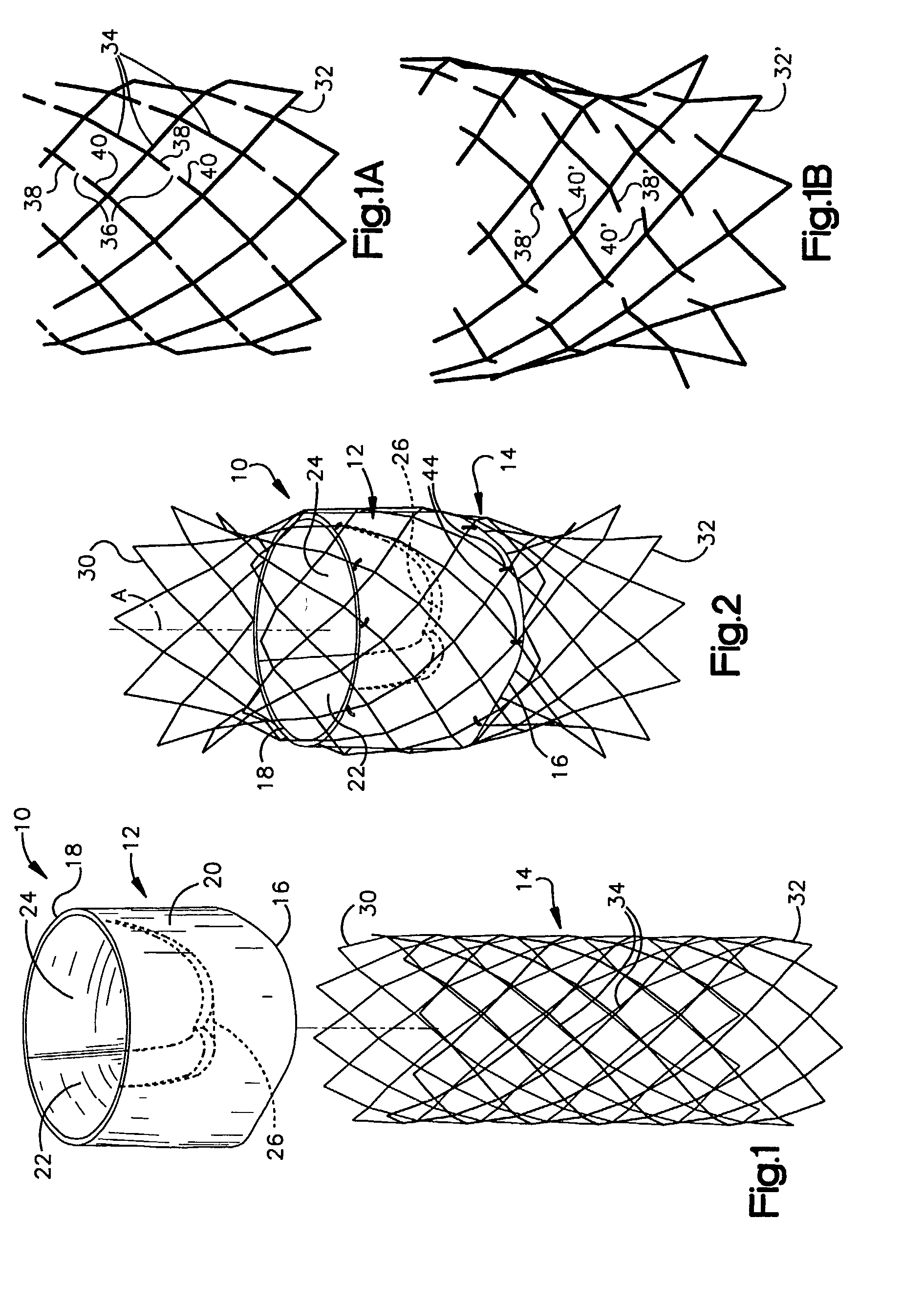
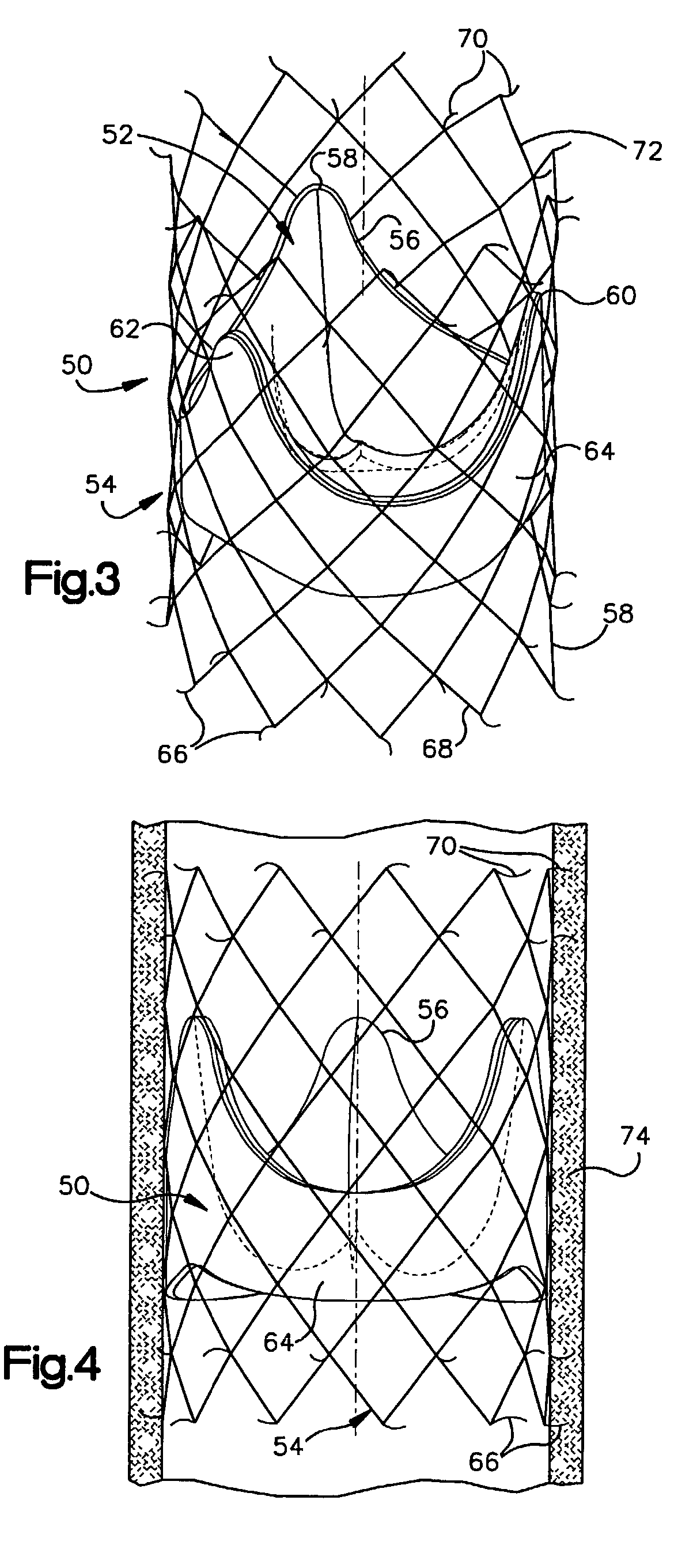
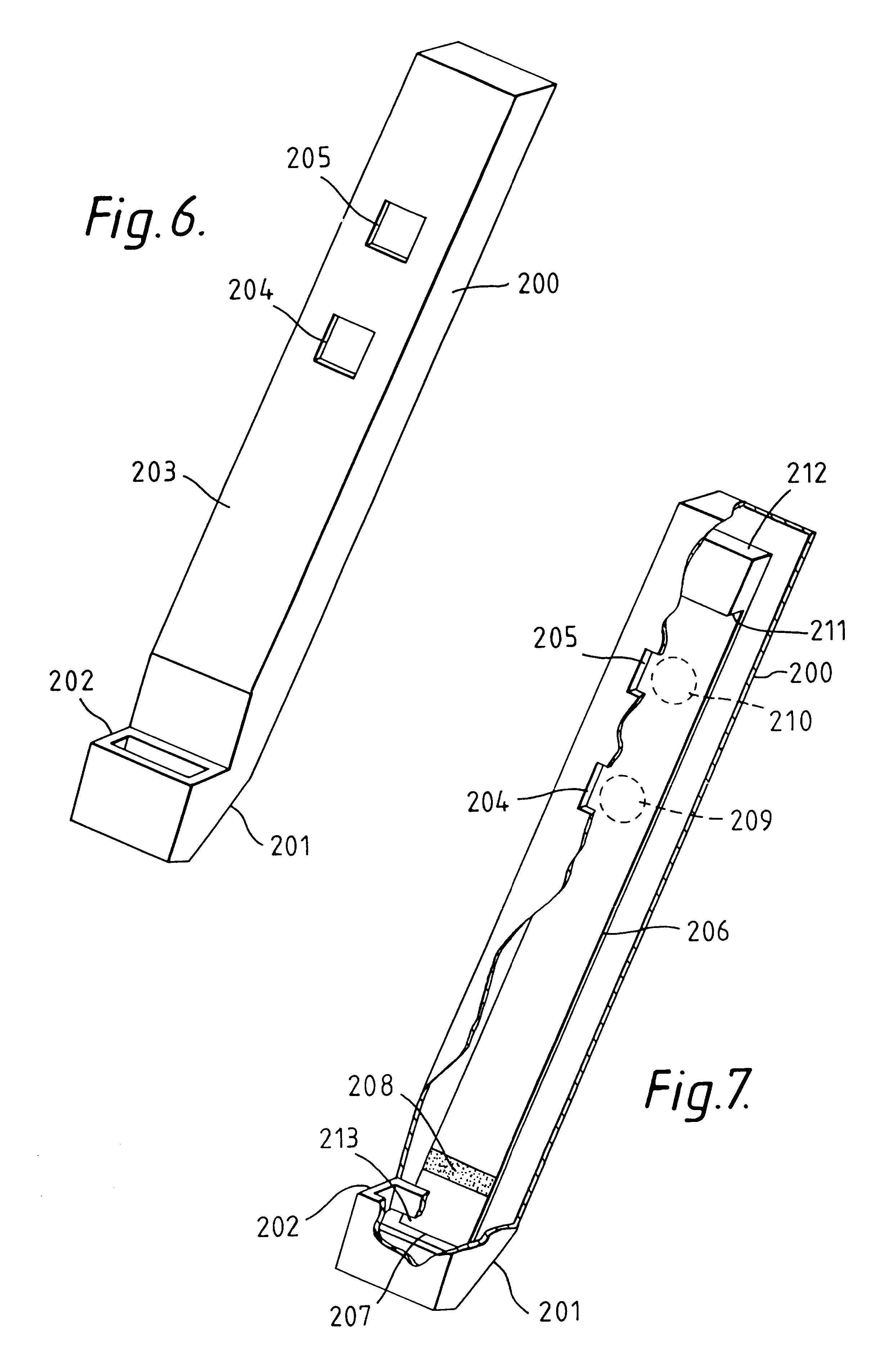
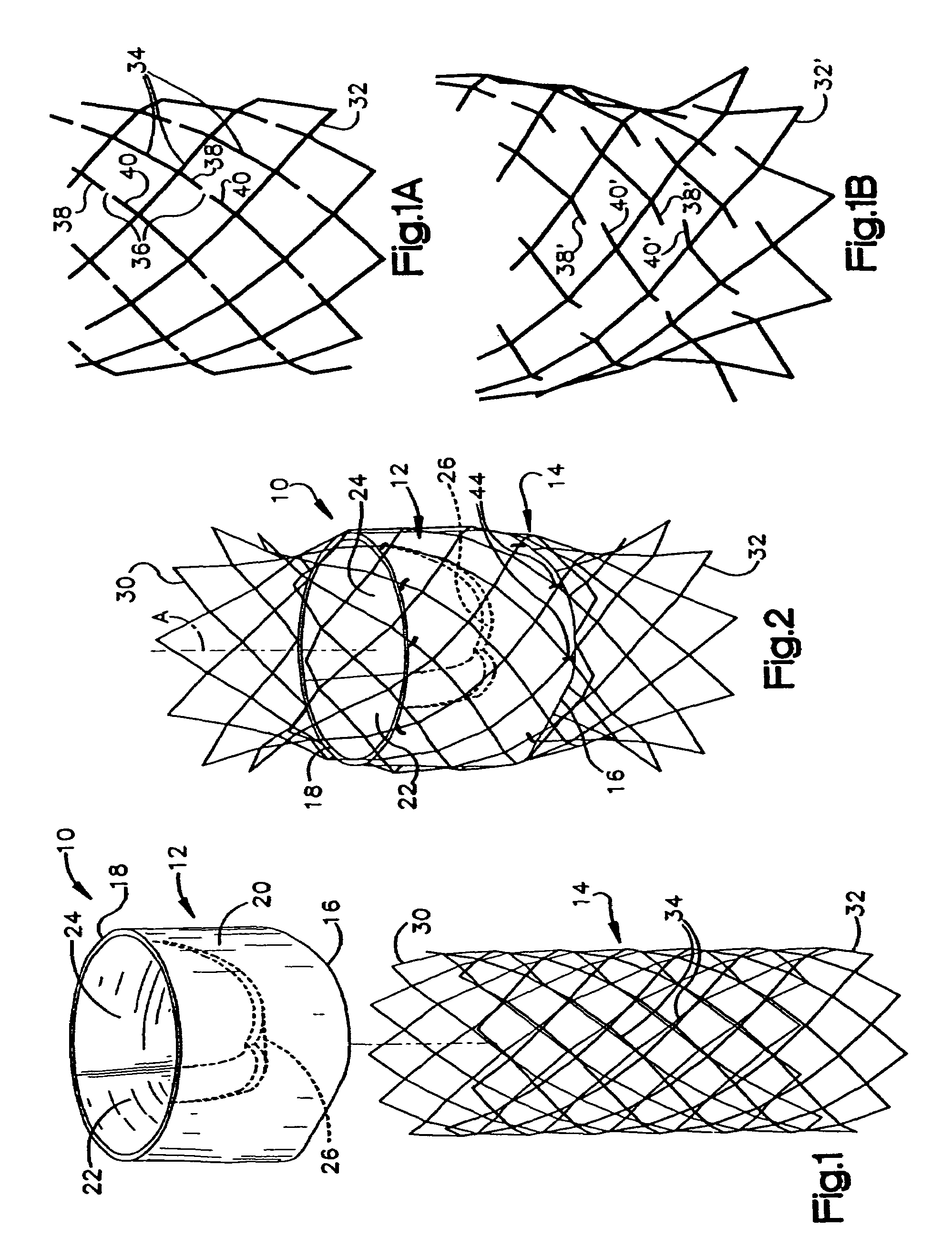
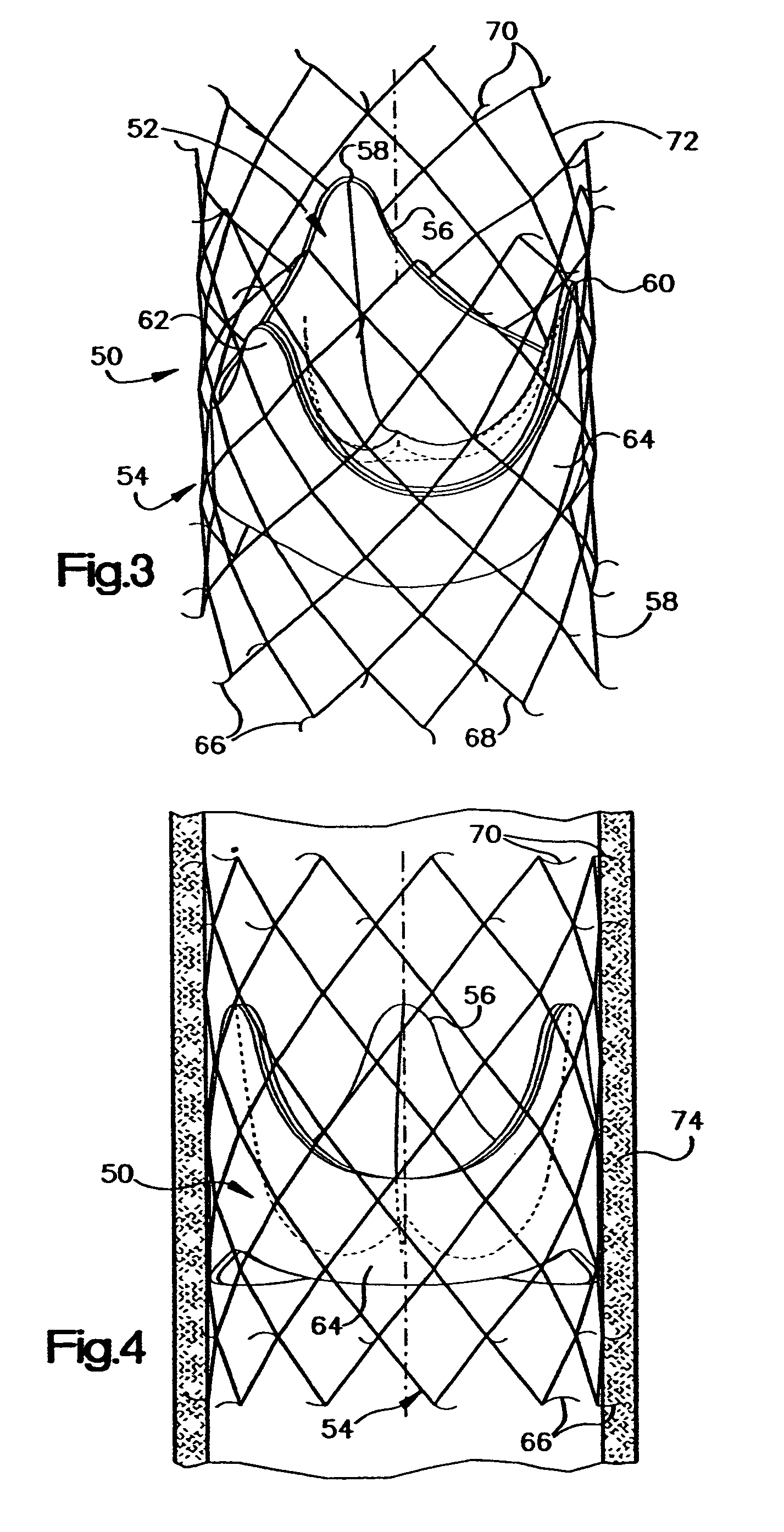
Heart valve prosthesis and sutureless implantation of a heart valve prosthesis
A heart valve prosthesis and method of implanting the prosthesis are disclosed. A valve is mounted within a support apparatus that is deformable between a first condition and a second condition. The prosthesis has a cross-sectional dimension in the second condition that is less than a cross-sectional dimension of the supported valve when in first condition. The prosthesis can be implanted into a patient's heart, such as during a direct vision procedure through a tubular implantation apparatus that maintains the prosthesis in its second condition until discharged from the tubular apparatus.
Owner:EDWARDS LIFESCI CARDIAQ
Heart valve prosthesis and sutureless implantation of a heart valve prosthesis
InactiveUS20030040792A1Reduced cross-sectional dimensionReduce exerciseStentsBalloon catheterDirect visionProsthesis
Owner:EDWARDS LIFESCI CARDIAQ
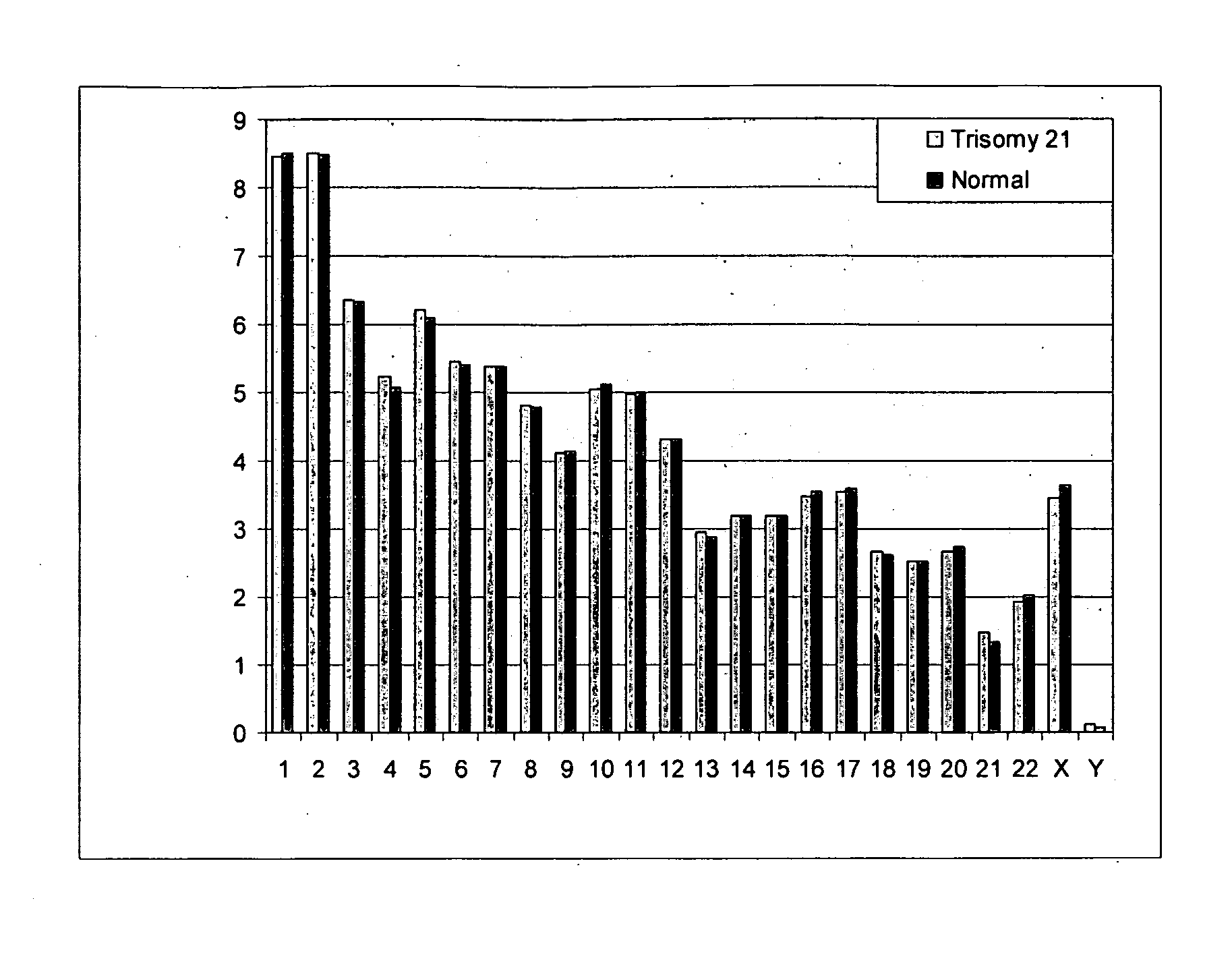
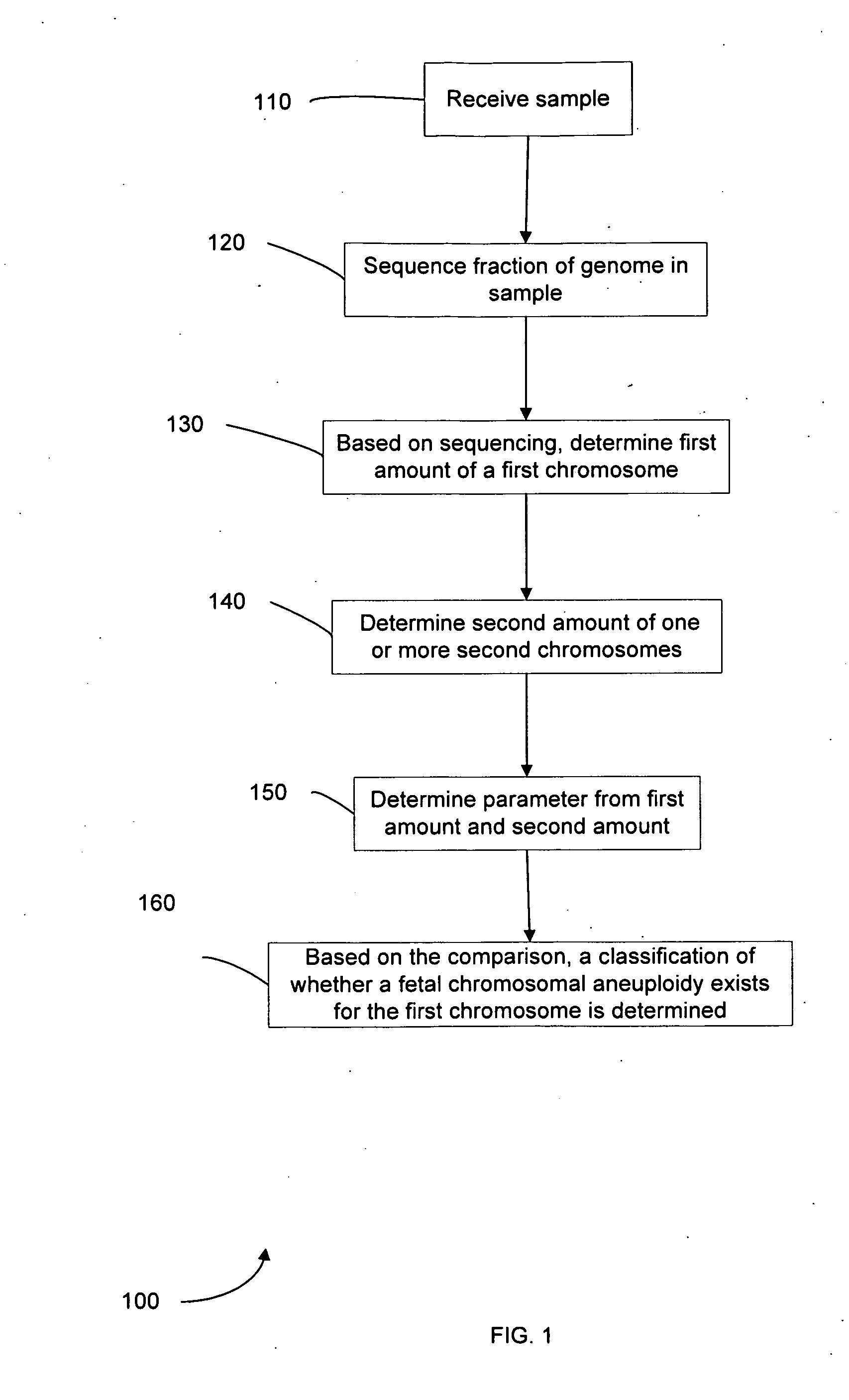
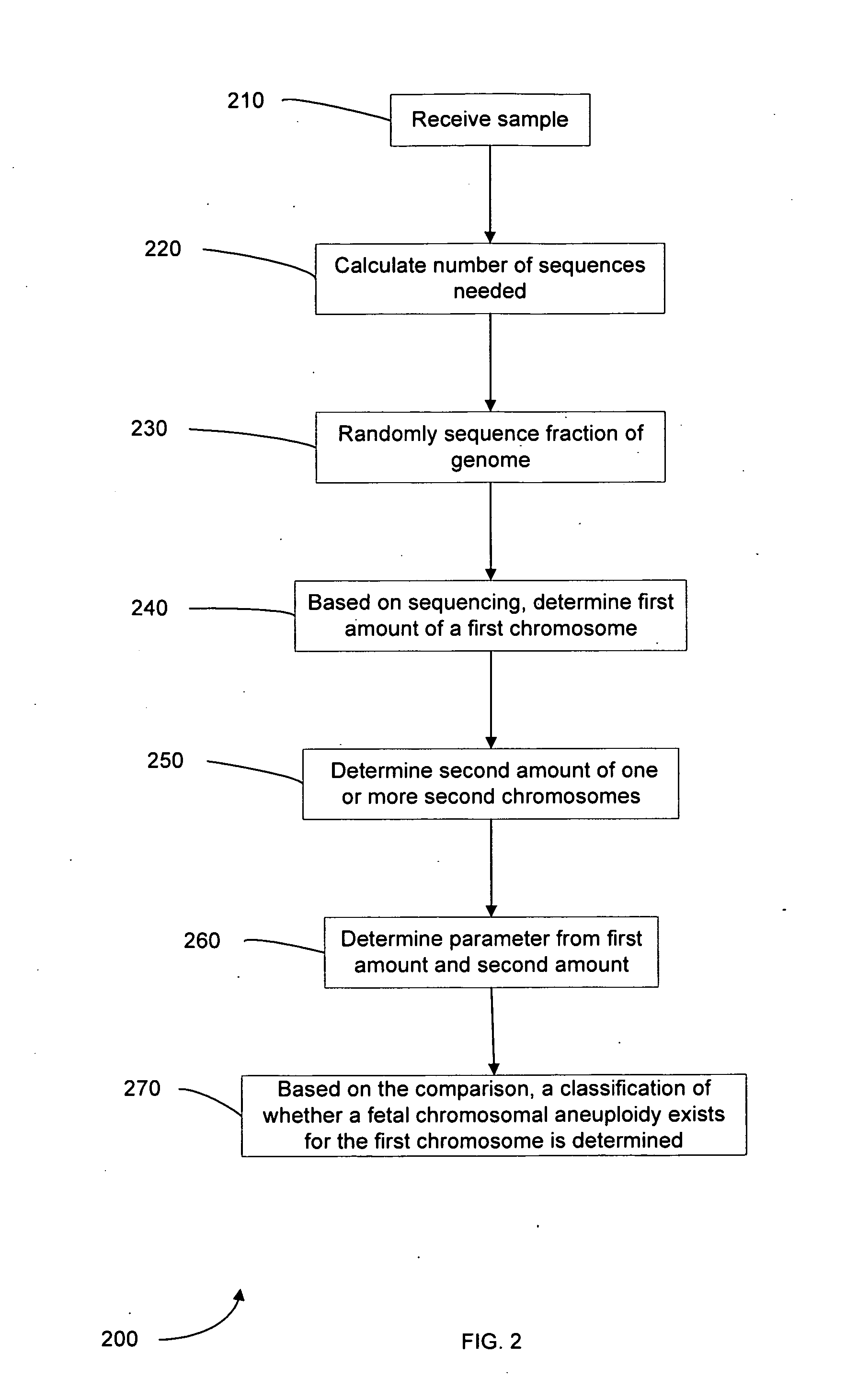
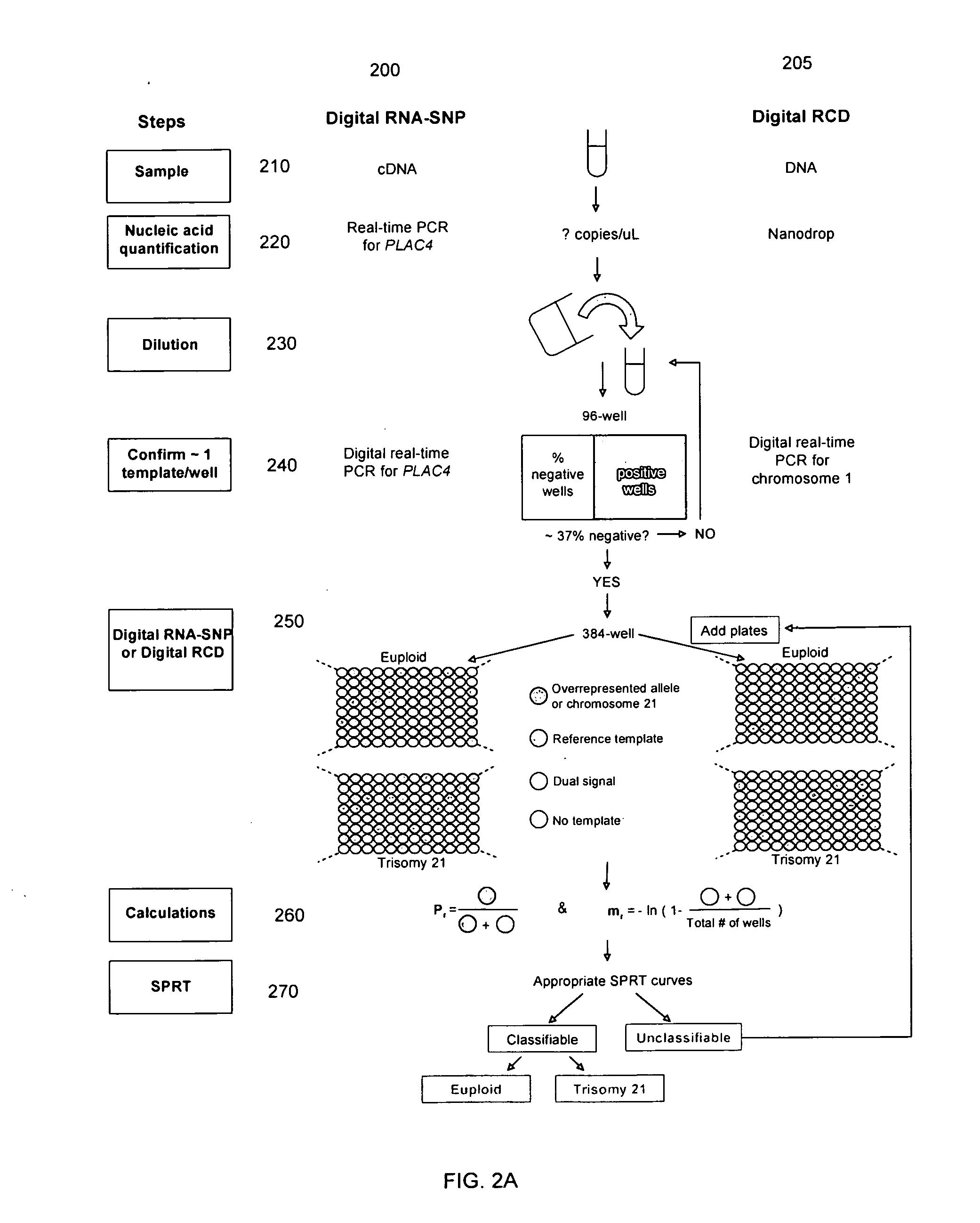
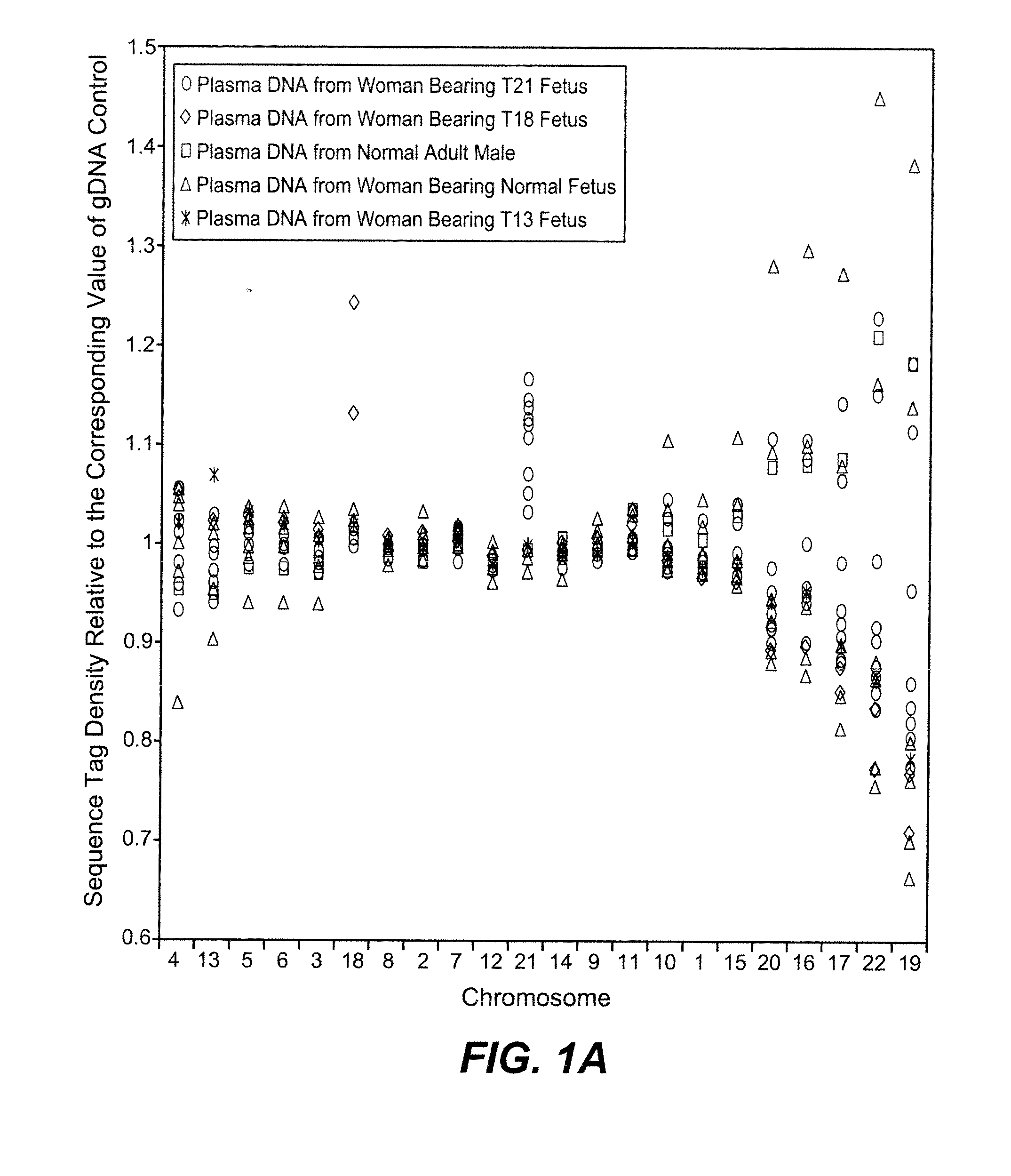
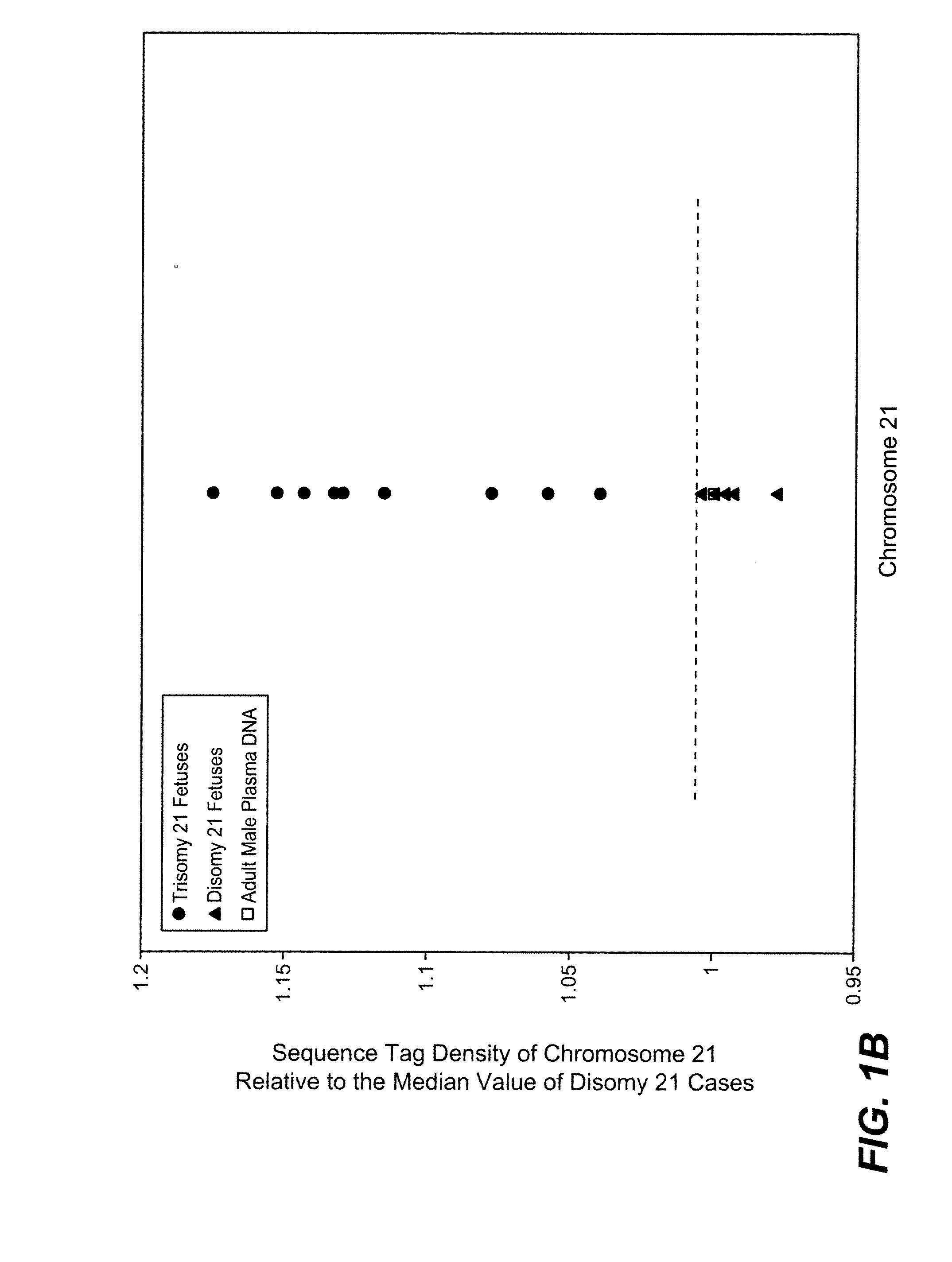
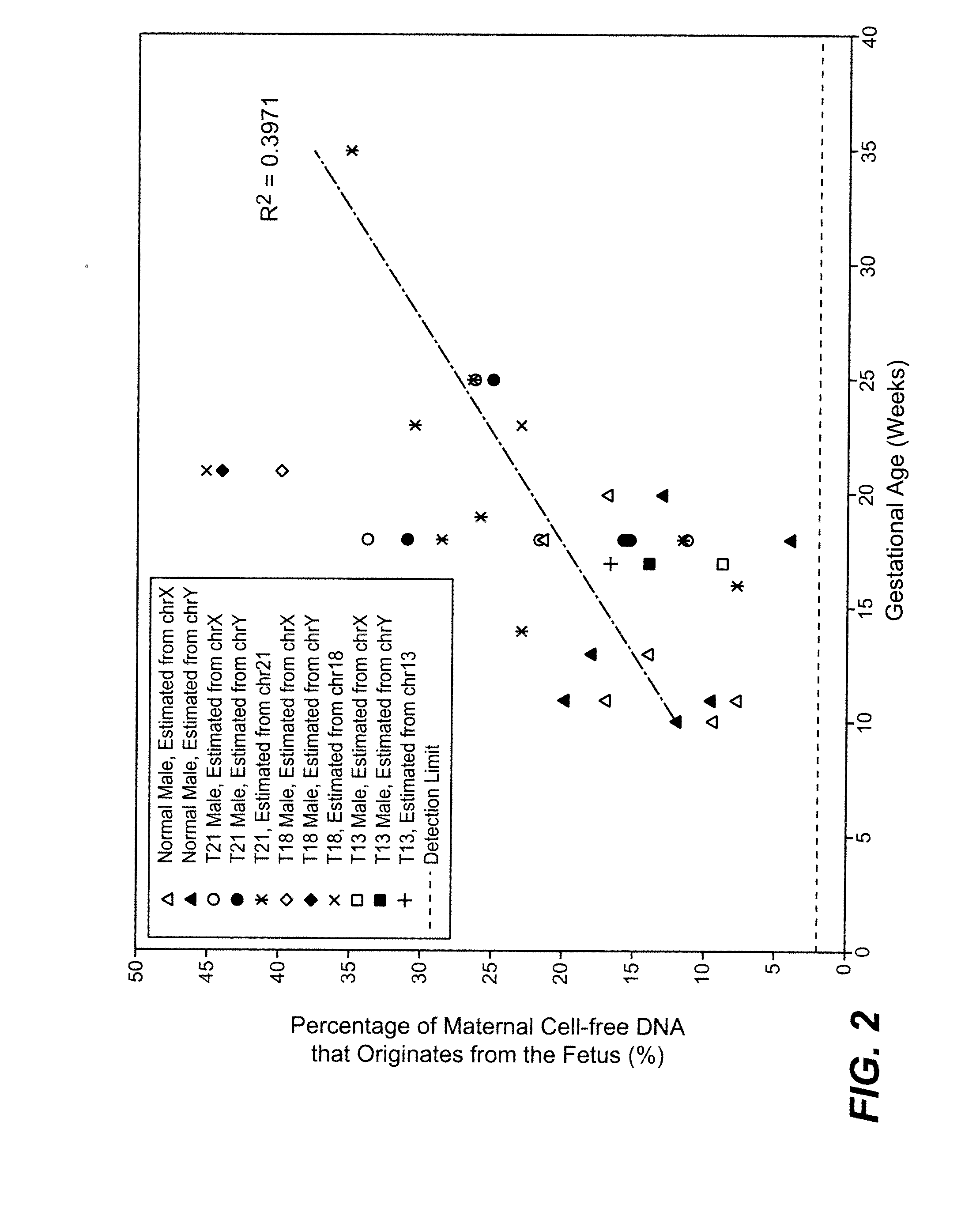
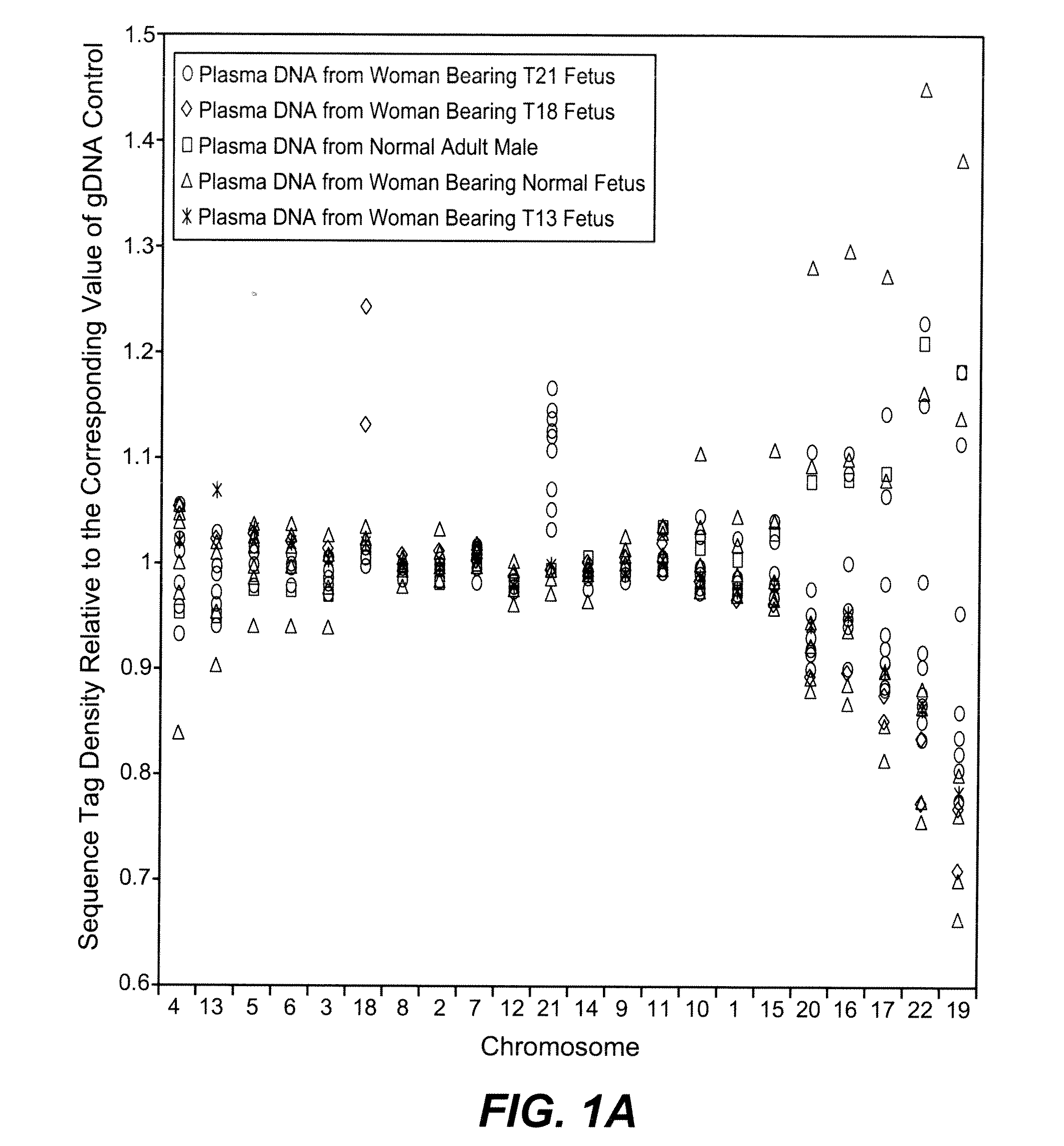
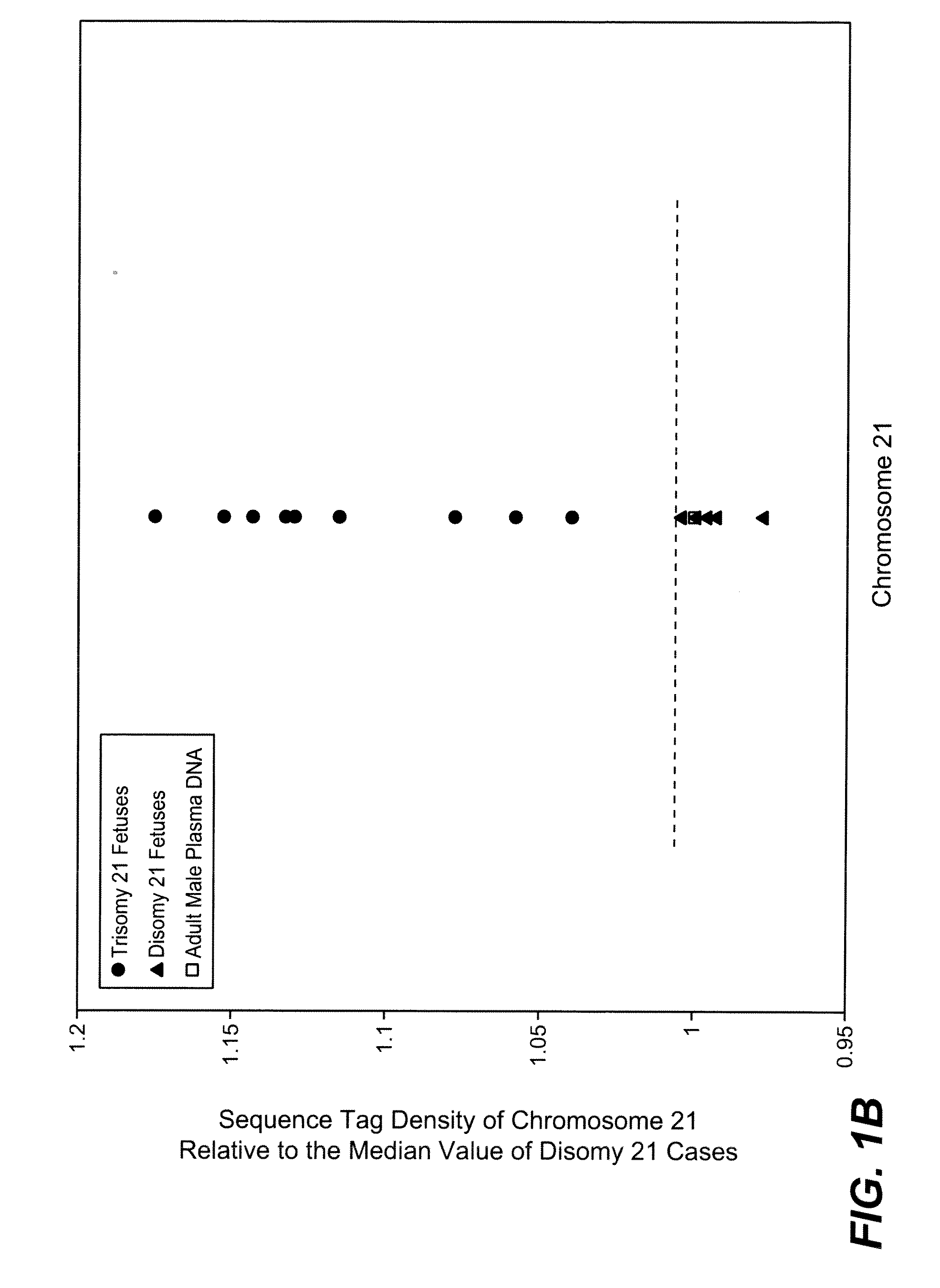
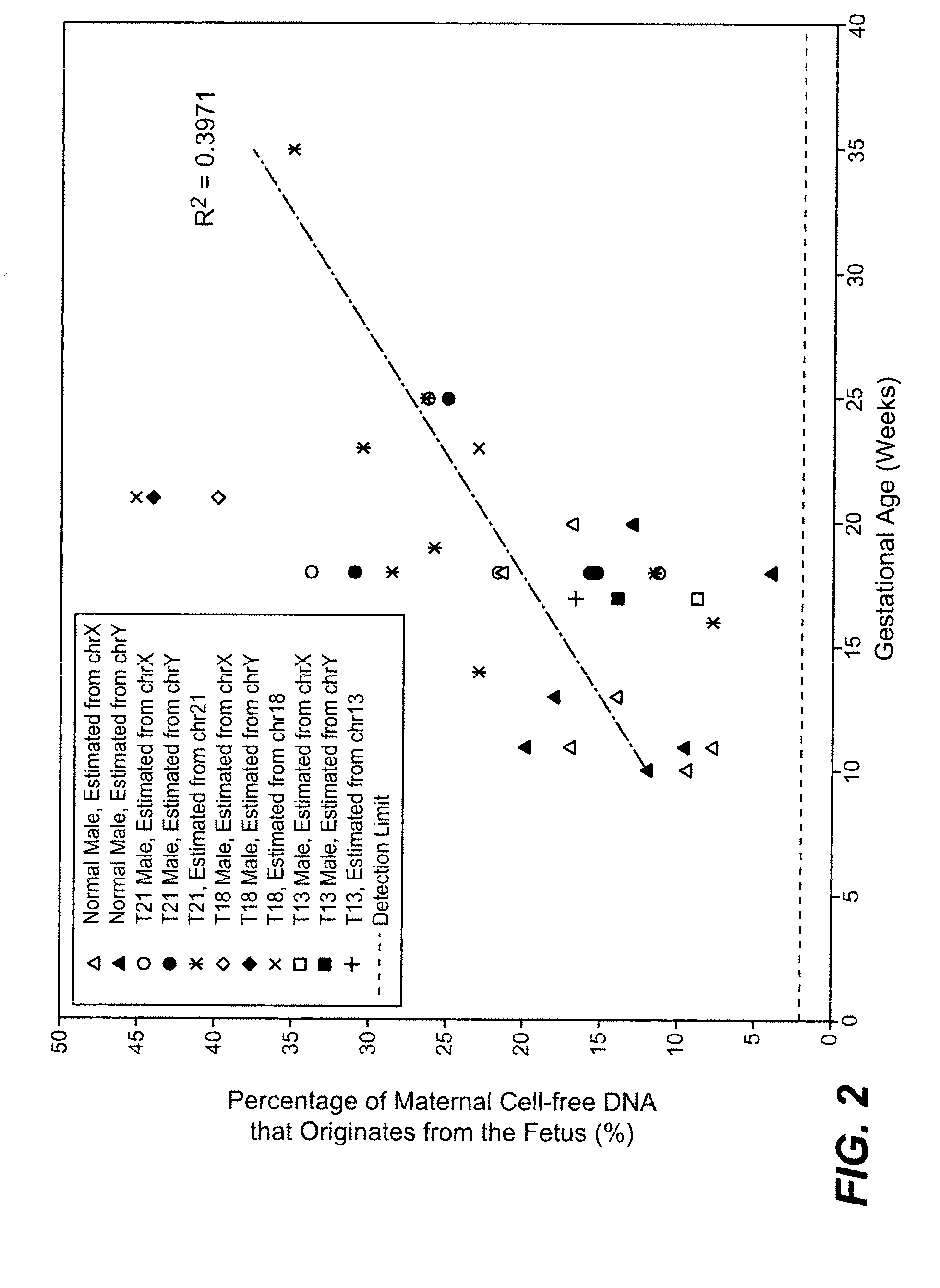
Diagnosing fetal chromosomal aneuploidy using massively parallel genomic sequencing
PendingUS20090029377A1Quantity maximizationSufficient amountMicrobiological testing/measurementDisease diagnosisGenomic sequencingGenome
Embodiments of this invention provide methods, systems, and apparatus for determining whether a fetal chromosomal aneuploidy exists from a biological sample obtained from a pregnant female. Nucleic acid molecules of the biological sample are sequenced, such that a fraction of the genome is sequenced. Respective amounts of a clinically-relevant chromosome and of background chromosomes are determined from results of the sequencing. A parameter derived from these amounts (e.g. a ratio) is compared to one or more cutoff values, thereby determining a classification of whether a fetal chromosomal aneuploidy exists.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
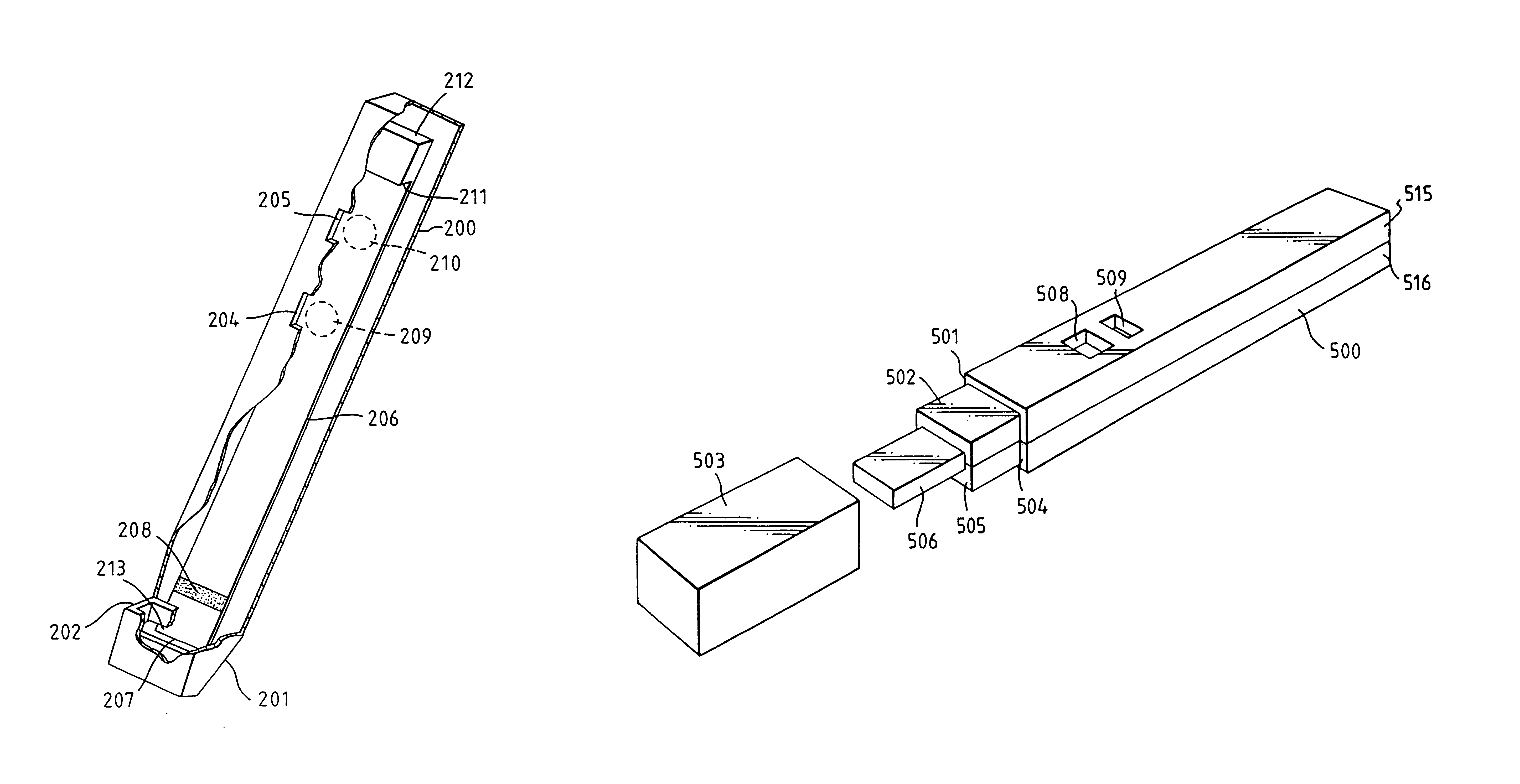
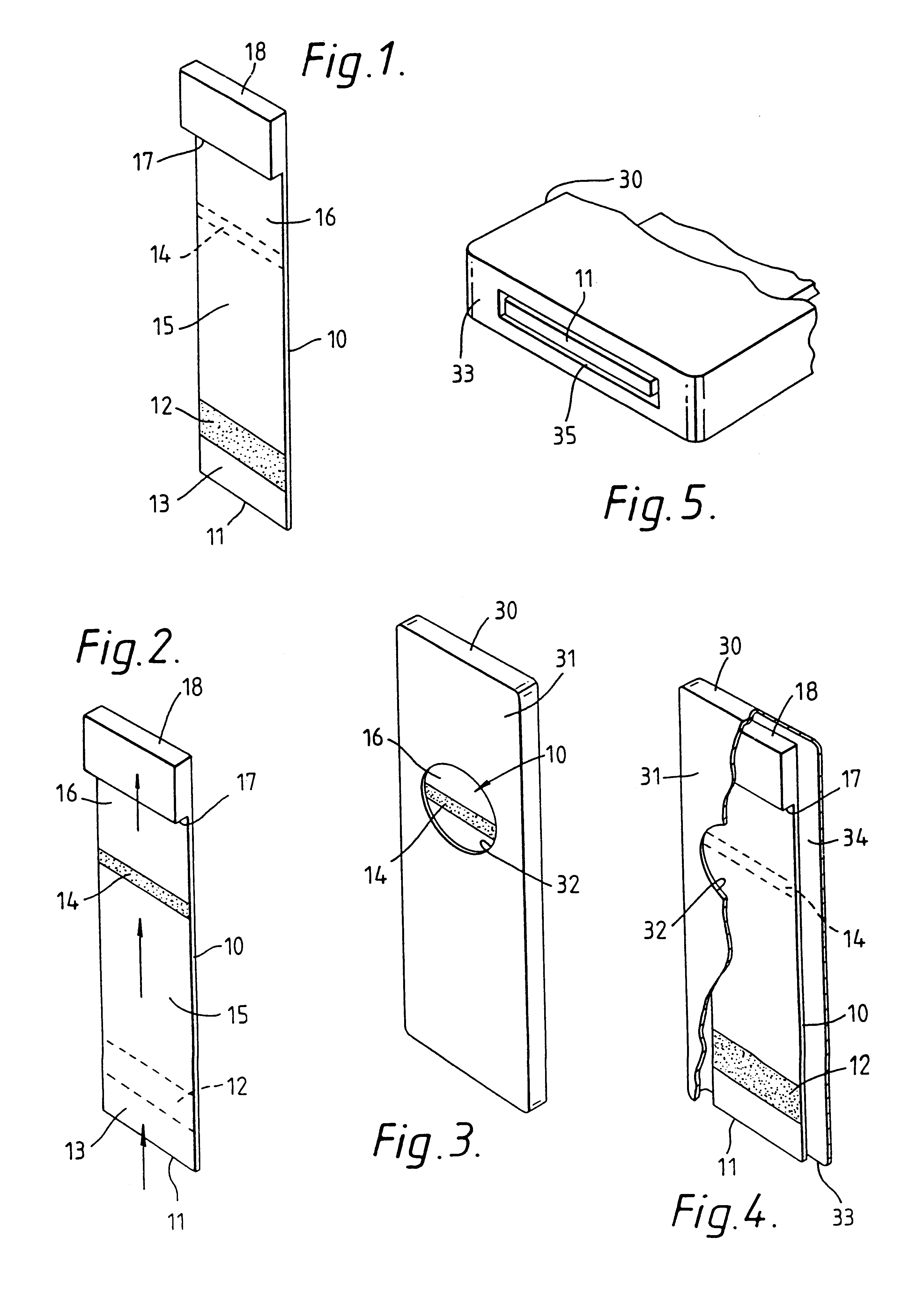
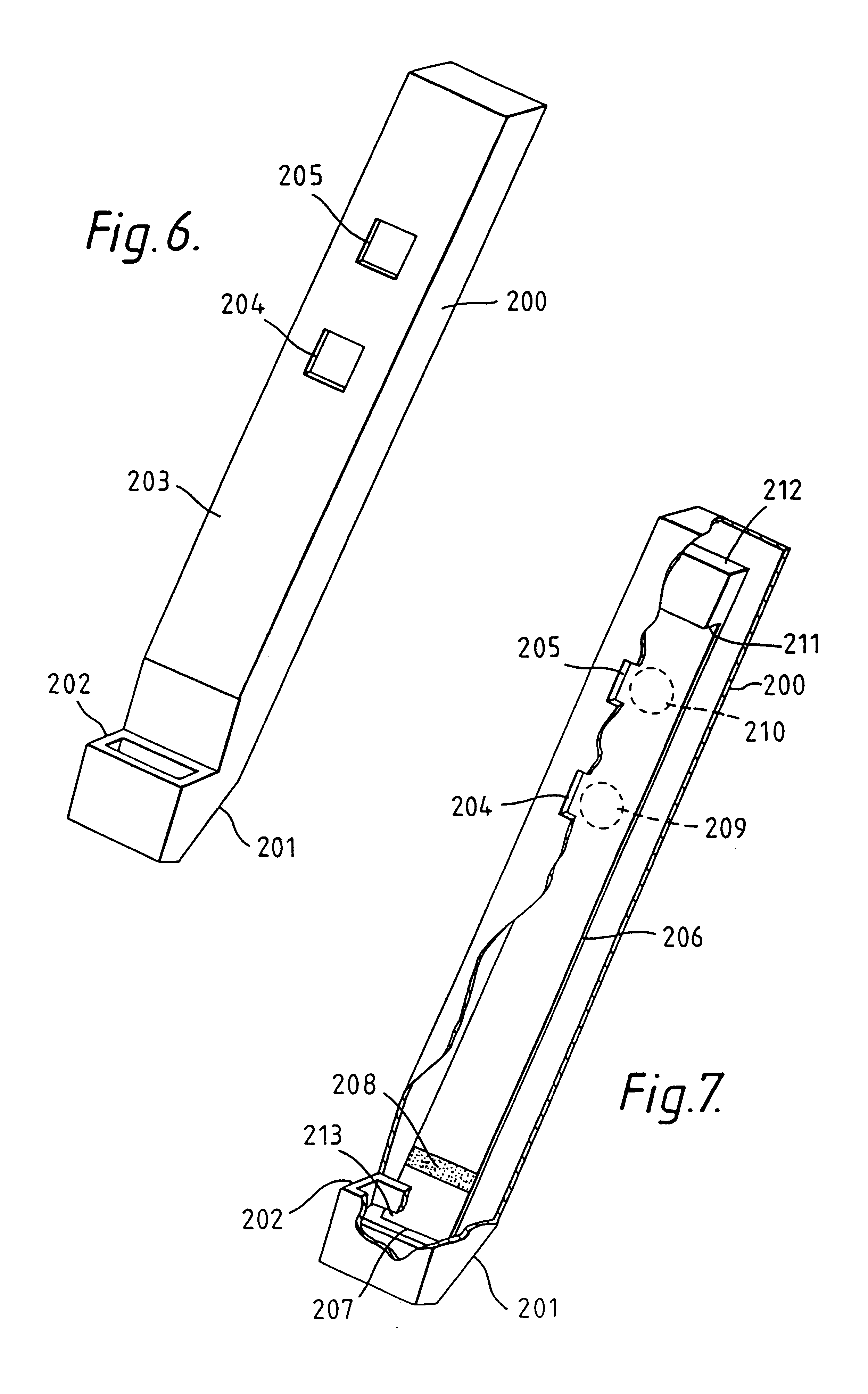
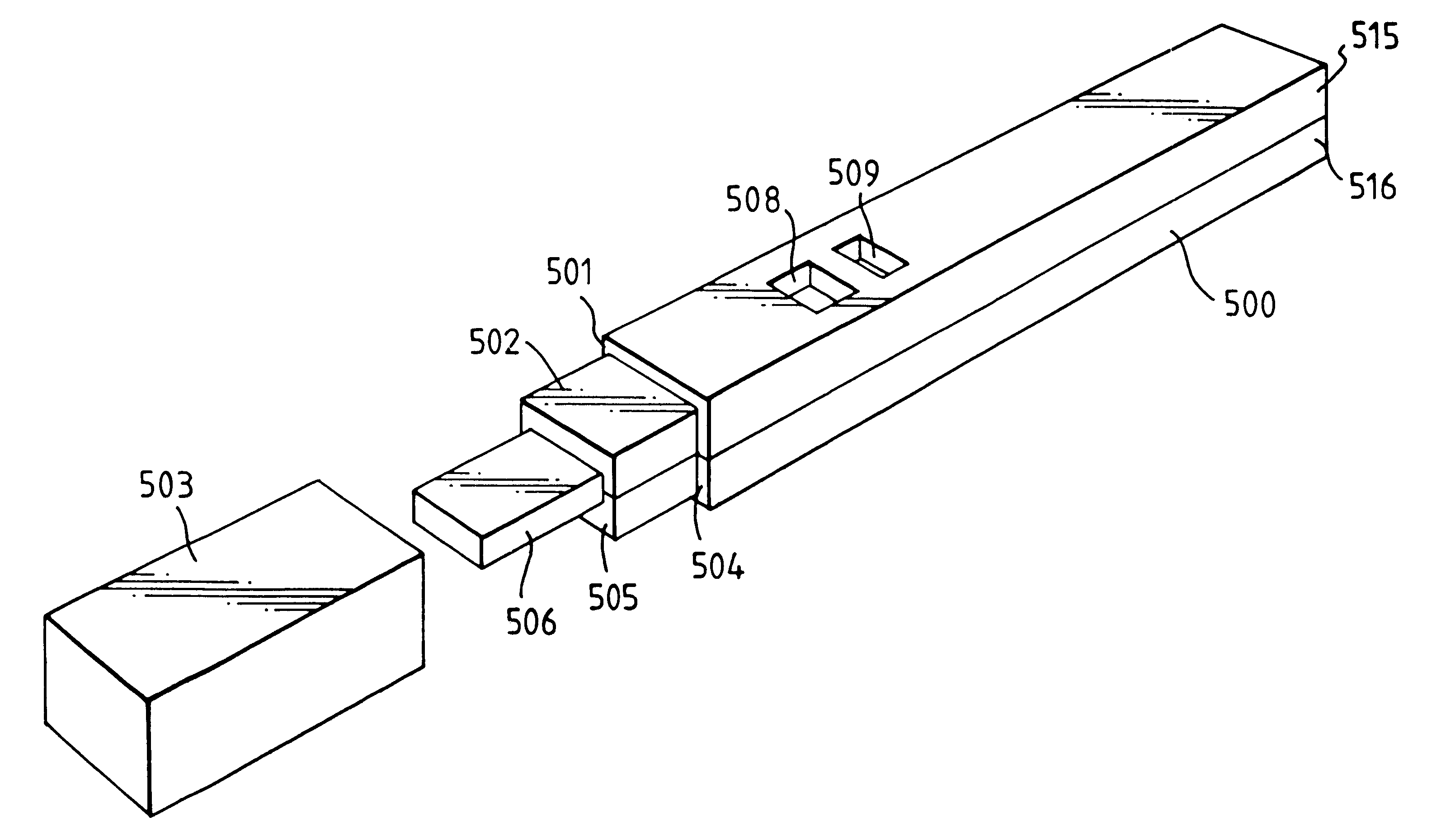
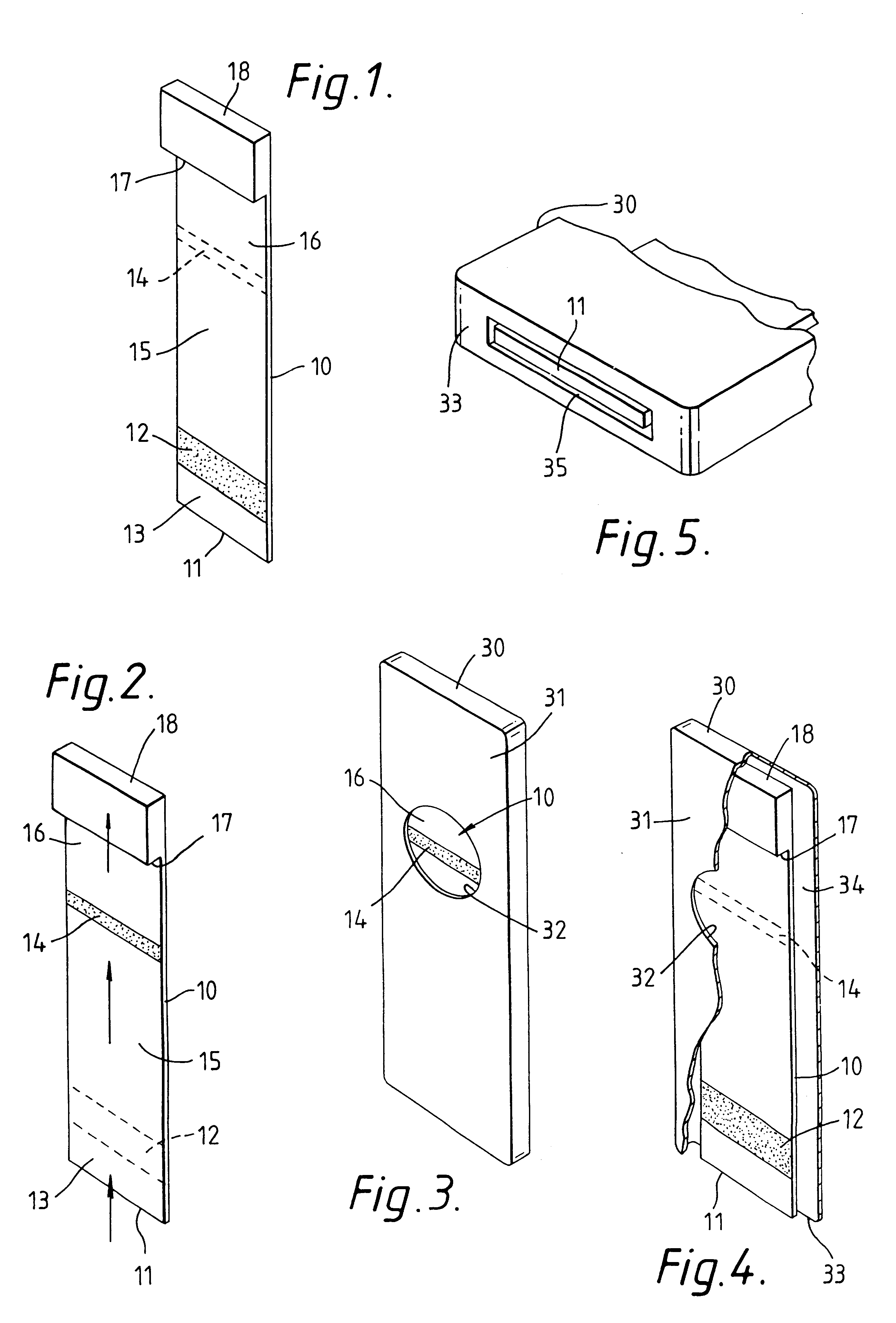
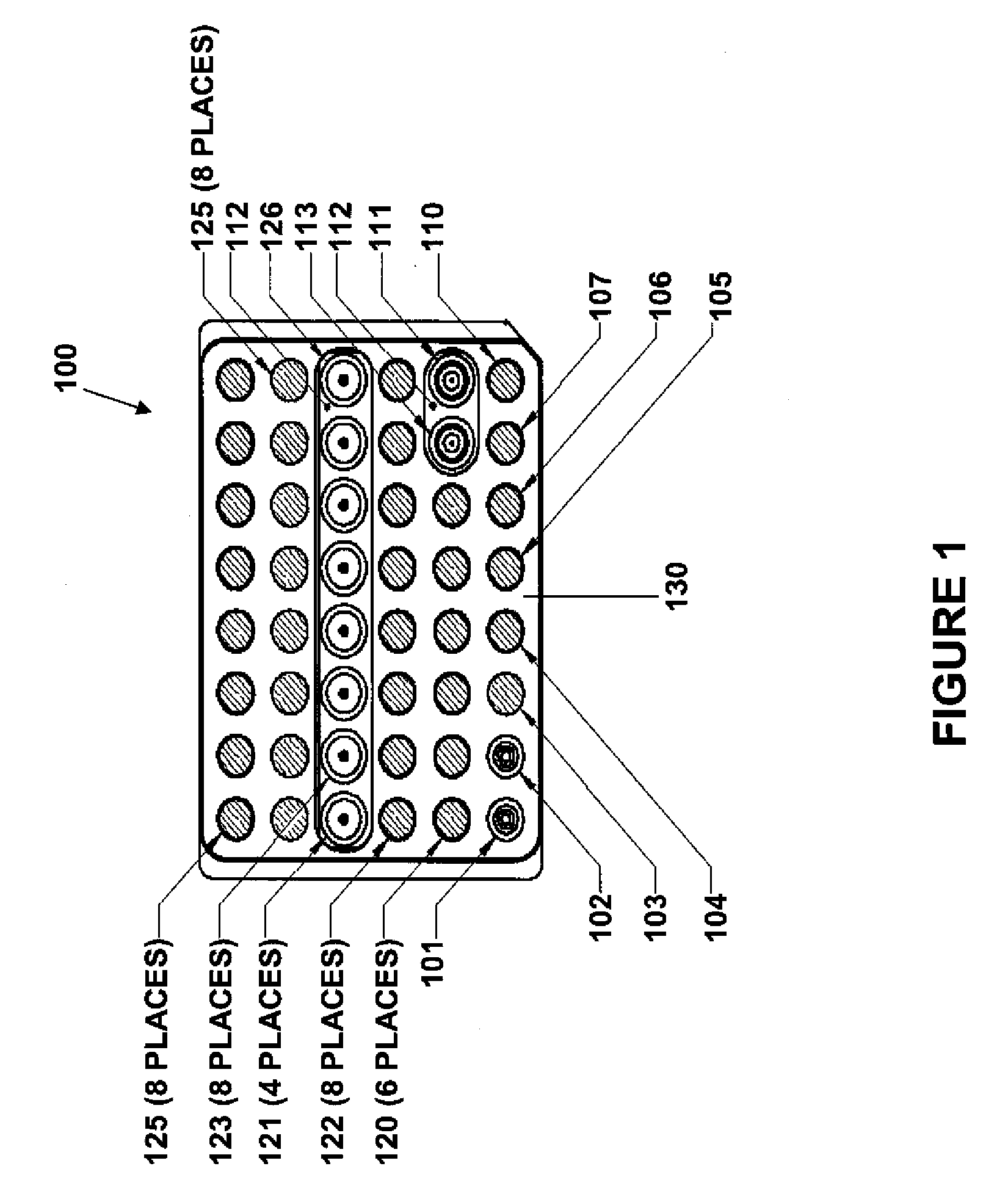
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6228660B1Improve completenessAnalysis using chemical indicatorsComponent separationParticulatesAnalyte
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilized on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:CONOPCO INC D B A UNILEVER
Nanoparticles for manipulation of biopolymers and methods of thereof
InactiveUS20060177855A1Material nanotechnologyMicrobiological testing/measurementCrystallographyNanoparticle
Matrices for manipulation of biopolymers, including the separation, purification, bilization and archival storage of biopolymers is disclosed.
Owner:ARGYLLA TECH
Capillary immunoassay and device therefor comprising mobilizable particulate labelled reagents
InactiveUS6187598B1Improve completenessBioreactor/fermenter combinationsBiological substance pretreatmentsPlastic materialsCapillary Tubing
An analytical test device useful for example in pregnancy testing, comprises a hollow casing (500) constructed of moisture-impervious solid material, such as plastics materials, containing a dry porous carrier (510) which communicates indirectly with the exterior of the casing via a bibulous sample receiving member (506) which protrudes from the casing such that a liquid test sample can be applied to the receiving member and permeate therefrom to the porous carrier, the carrier containing in a first zone a labelled specific binding reagent is freely mobile within the porous carrier when in the moist state, and in a second zone spatially distinct from the first zone unlabelled specific binding reagent for the same analyte which unlabelled reagent is permanently immobilised on the carrier material and is therefore not mobile in the moist state, the two zones being arranged such that liquid sample applied to the porous carrier can permeate via the first zone into the second zone, and the device incorporating means, such as an aperture (508) in the casing, enabling the extent (if any) to which the labelled reagent becomes bound in the second zone to be observed. Preferably the device includes a removable cap for the protruding bibulous member.
Owner:INVERNESS SWITZERLAND GMBH
Implantation system for delivery of a heart valve prosthesis
A heart valve prosthesis and method of implanting the prosthesis are disclosed. A valve is mounted within a support apparatus that is deformable between a first condition and a second condition. The prosthesis has a cross-sectional dimension in the second condition that is less than a cross-sectional dimension of the supported valve when in first condition. The prosthesis can be implanted into a patient's heart, such as during a direct vision procedure through a tubular implantation apparatus that maintains the prosthesis in its second condition until discharged from the tubular apparatus.
Owner:EDWARDS LIFESCI CARDIAQ
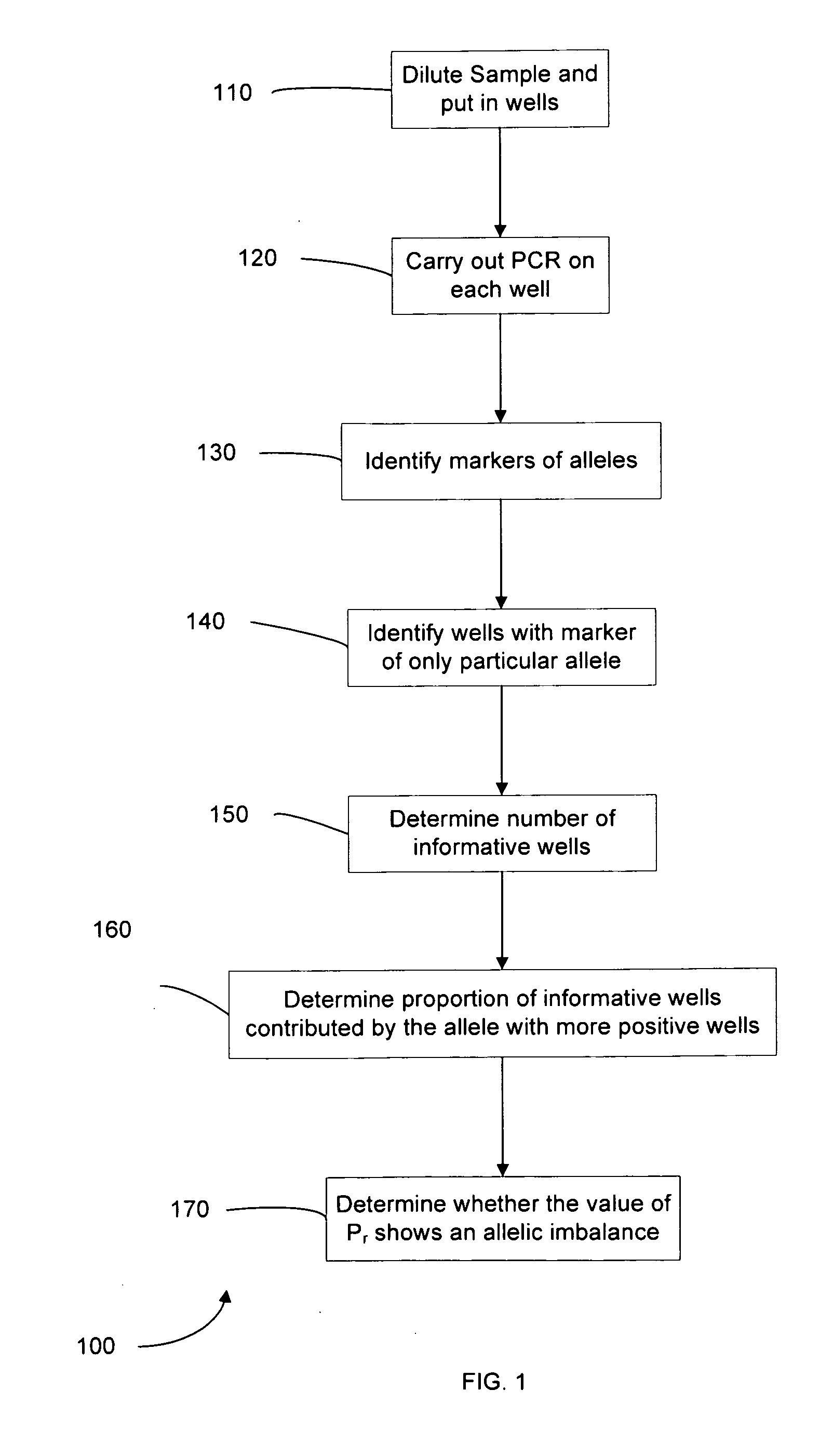
Determining a nucleic acid sequence imbalance
ActiveUS20090087847A1Quantity minimizationMicrobiological testing/measurementDisease diagnosisNucleic acid sequencingNucleic acid sequence
Methods, systems, and apparatus are provided for determining whether a nucleic acid sequence imbalance exists within a biological sample. One or more cutoff values for determining an imbalance of, for example, the ratio of the two sequences (or sets of sequences) are chosen. The cutoff value may be determined based at least in part on the percentage of fetal DNA in a sample, such as maternal plasma, containing a background of maternal nucleic acid sequences. The cutoff value may also be determined based on an average concentration of a sequence per reaction. In one aspect, the cutoff value is determined from a proportion of informative wells that are estimated to contain a particular nucleic acid sequence, where the proportion is determined based on the above-mentioned percentage and / or average concentration. The cutoff value may be determined using many different types of methods, such as sequential probability ratio testing (SPRT).
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Cancer immunotherapy by disrupting pd-1/pd-l1 signaling
ActiveUS20130309250A1Reduces and suppresses signalingReliable responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
The disclosure provides a method for immunotherapy of a subject afflicted with cancer, comprises administering to the subject a composition comprising a therapeutically effective amount of an antibody that inhibits signaling from the PD-1 / PD-L1 signaling pathway. This disclosure also provides a method for immunotherapy of a subject afflicted with cancer comprising selecting a subject that is a suitable candidate for immunotherapy based on an assessment that the proportion of cells in a test tissue sample from the subject that express PD-L1 on the cell surface exceeds a predetermined threshold level, and administering a therapeutically effective amount of an anti-PD-1 antibody to the selected subject. The invention additionally provides rabbit mAbs that bind specifically to a cell surface-expressed PD-L1 antigen in a FFPE tissue sample, and an automated IHC method for assessing cell surface expression in FFPE tissues using the provided anti-PD-L1 Abs.
Owner:BRISTOL MYERS SQUIBB CO
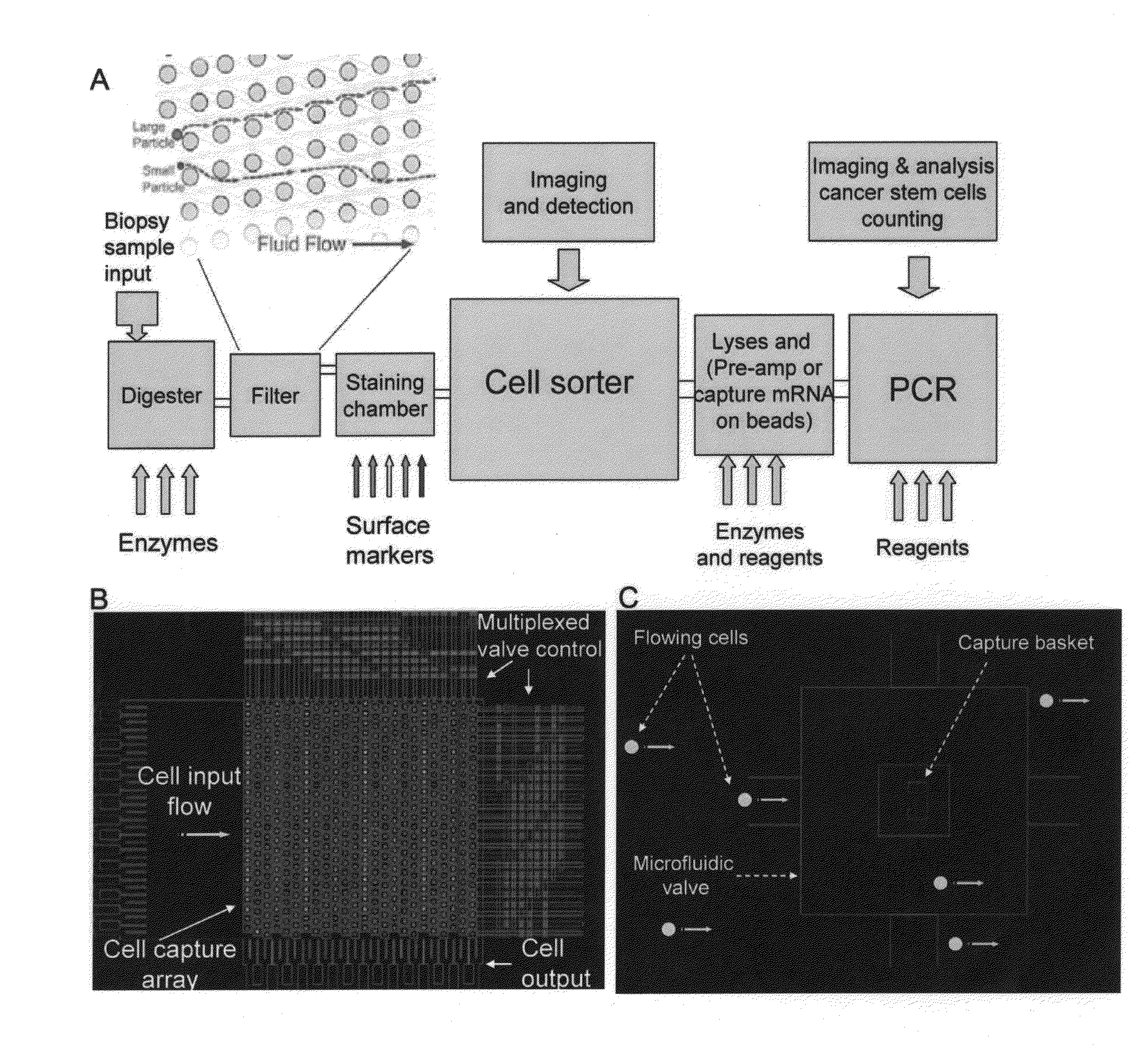
Methods for the Diagnosis of Fetal Abnormalities
ActiveUS20080138809A1Reliable and accurate clinical diagnosisAccurate and reliable diagnosisMicrobiological testing/measurementPreparing sample for investigationFetal abnormalityRare cell
The present invention relates to methods for detecting, enriching, and analyzing rare cells that are present in the blood, e.g. fetal cells. The invention further features methods of analyzing rare cell(s) to determine the presence of an abnormality, disease or condition in a subject, e.g. a fetus by analyzing a cellular sample from the subject.
Owner:VERINATA HEALTH INC +2
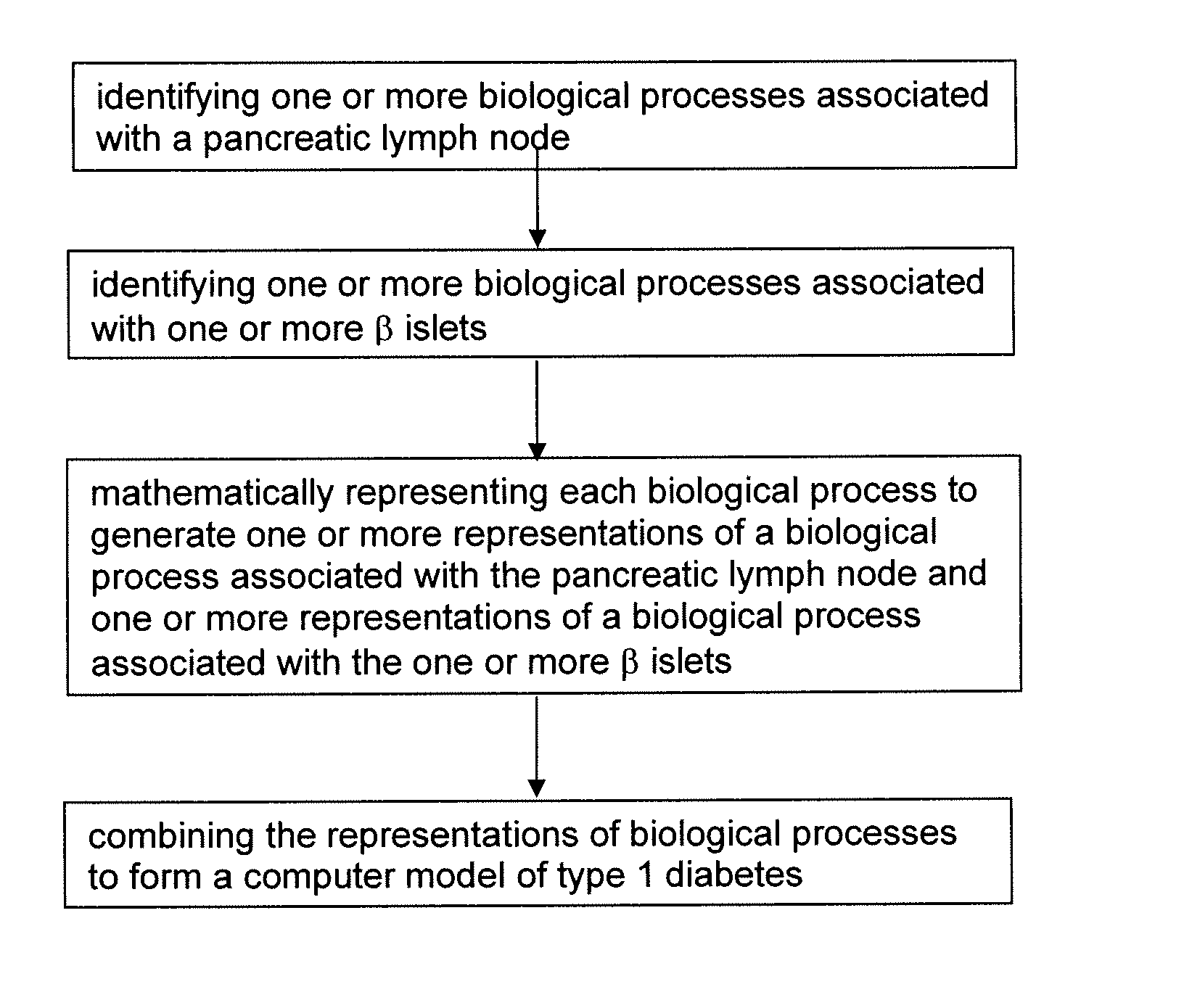
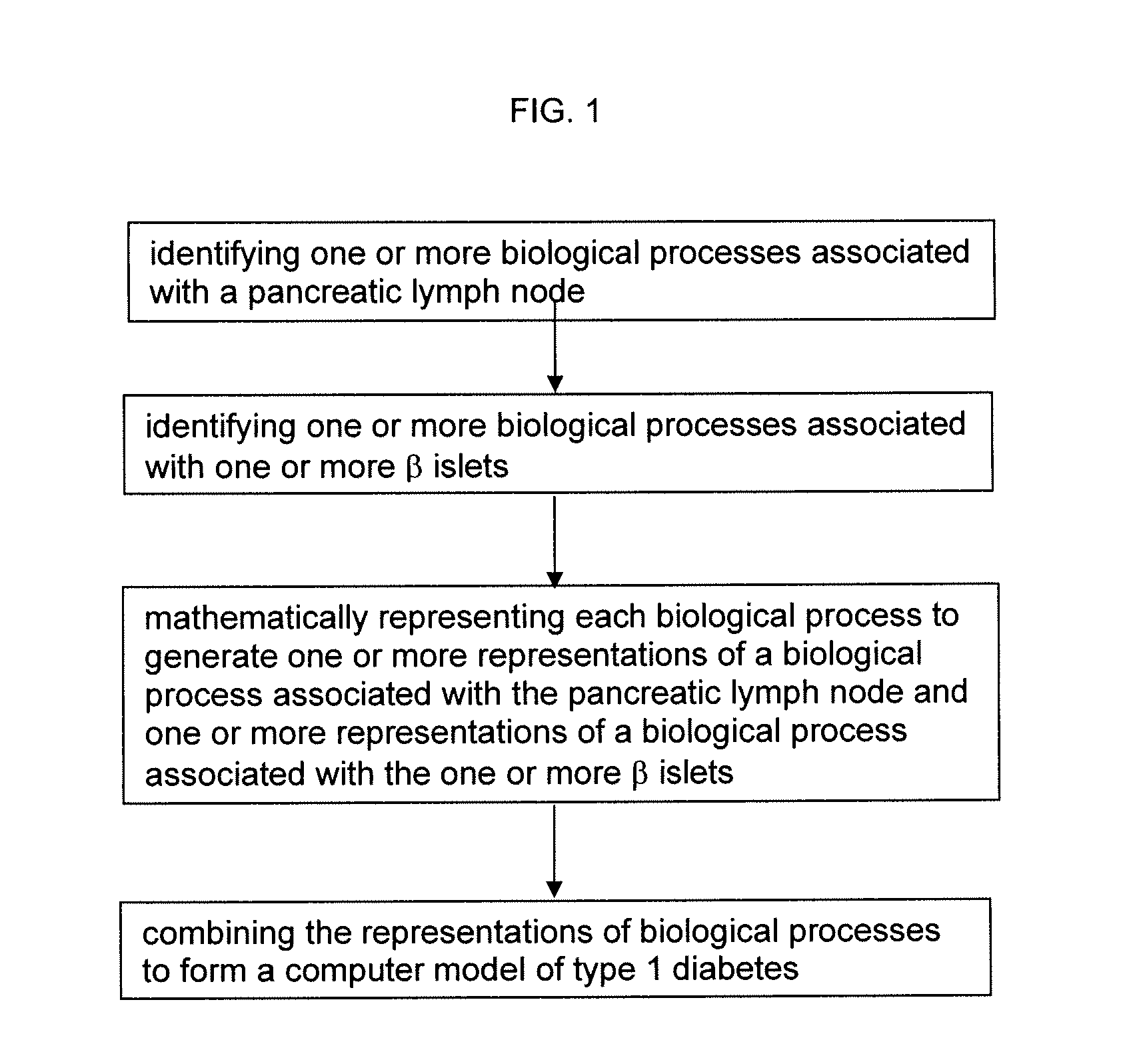
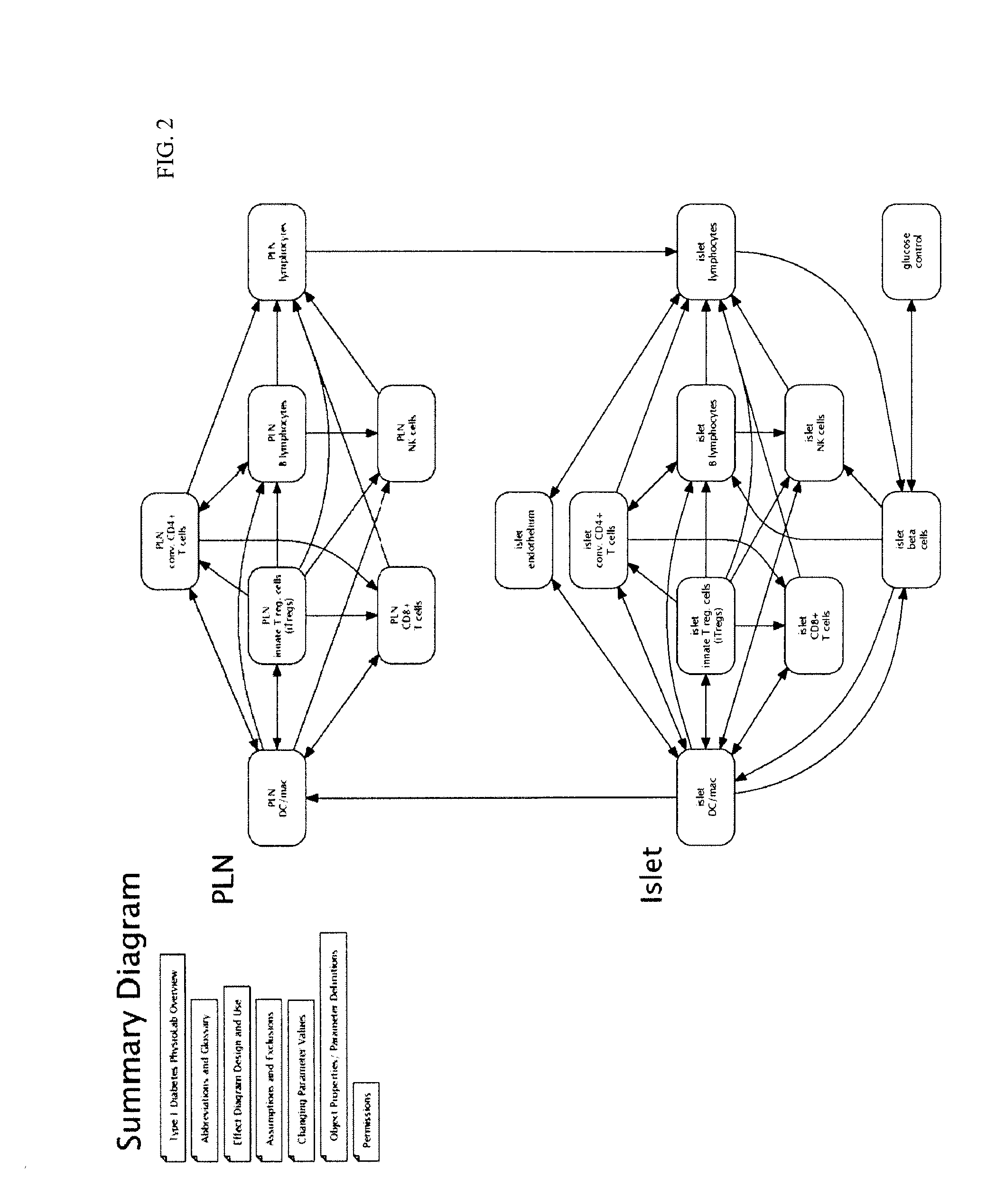
Apparatus and method for computer modeling type 1 diabetes
The invention encompasses novel methods for developing a computer model of type 1 diabetes in a mammal. In particular, the models can include representations of biological processes associated with a pancreatic lymph node and one or more pancreatic islets. Alternatively, the models can include representations of biological processes associated with at least two conditions selected from the group consisting of autoreactive T cell production, autoreactive T cell priming, insulitis and hyperglycemia. The invention also provides methods for developing a computer model of a non-insulin replacement treatment of type 1 diabetes. The invention also encompasses computer models of type 1 diabetes, methods of simulating type 1 diabetes and computer systems for simulating type 1 diabetes and the uses thereof.
Owner:ENTELOS INC
Noninvasive Diagnosis of Fetal Aneuploidy by Sequencing
InactiveUS20100112575A1Maximum resultMicrobiological testing/measurementDisease diagnosisMassive parallel sequencingFetal aneuploidy
Disclosed is a method to achieve digital quantification of DNA (i.e., counting differences between identical sequences) using direct shotgun sequencing followed by mapping to the chromosome of origin and enumeration of fragments per chromosome. The preferred method uses massively parallel sequencing, which can produce tens of millions of short sequence tags in a single run and enabling a sampling that can be statistically evaluated. By counting the number of sequence tags mapped to a predefined window in each chromosome, the over- or under-representation of any chromosome in maternal plasma DNA contributed by an aneuploid fetus can be detected. This method does not require the differentiation of fetal versus maternal DNA. The median count of autosomal values is used as a normalization constant to account for differences in total number of sequence tags is used for comparison between samples and between chromosomes.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
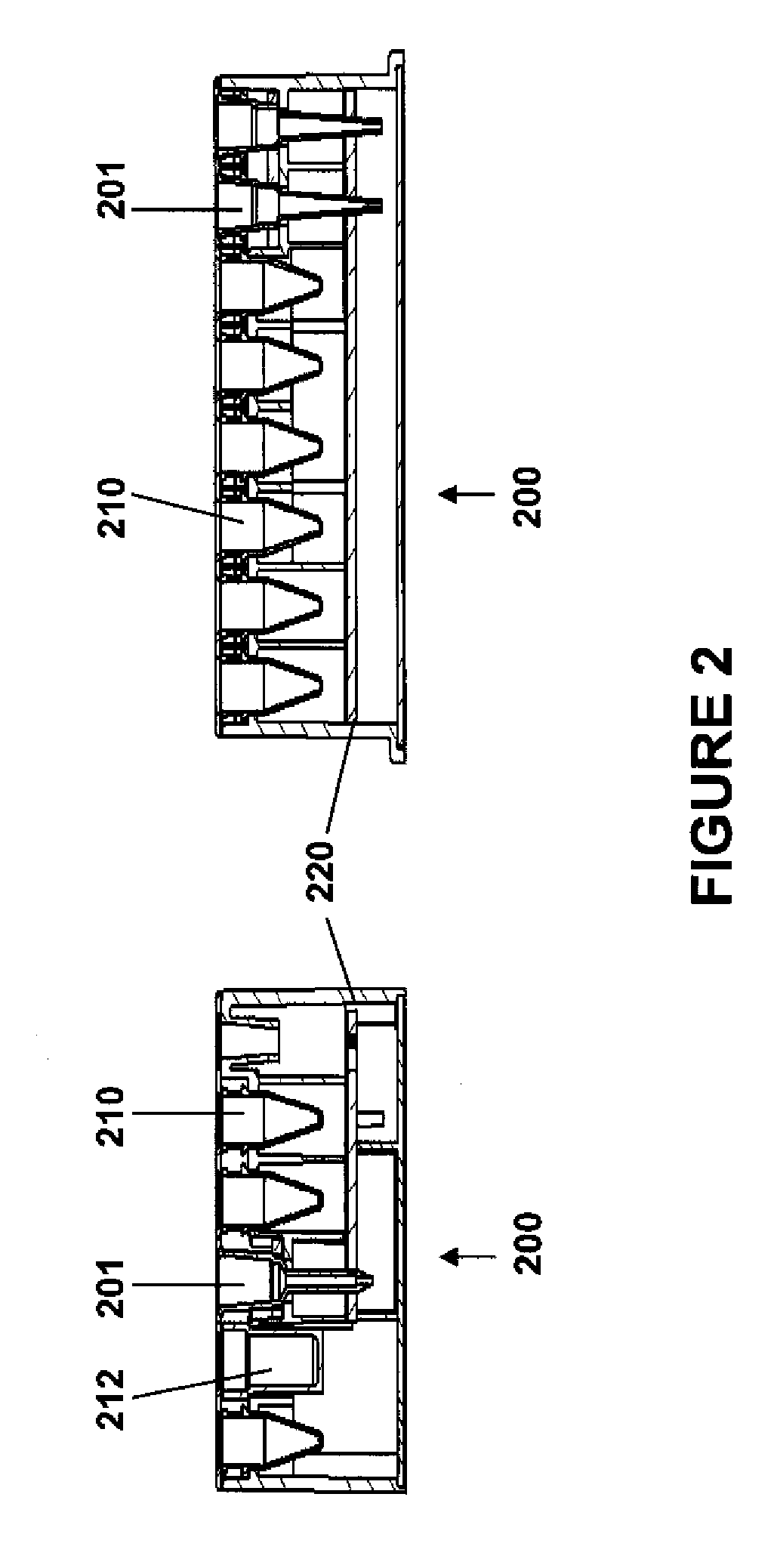
Modular point-of-care devices, systems, and uses thereof
ActiveUS20090088336A1Sequential/parallel process reactionsHeating or cooling apparatusAnalytePoint of care device
The present invention provides devices and systems for use at the point of care. The methods devices of the invention are directed toward automatic detection of analytes in a bodily fluid. The components of the device are modular to allow for flexibility and robustness of use with the disclosed methods for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
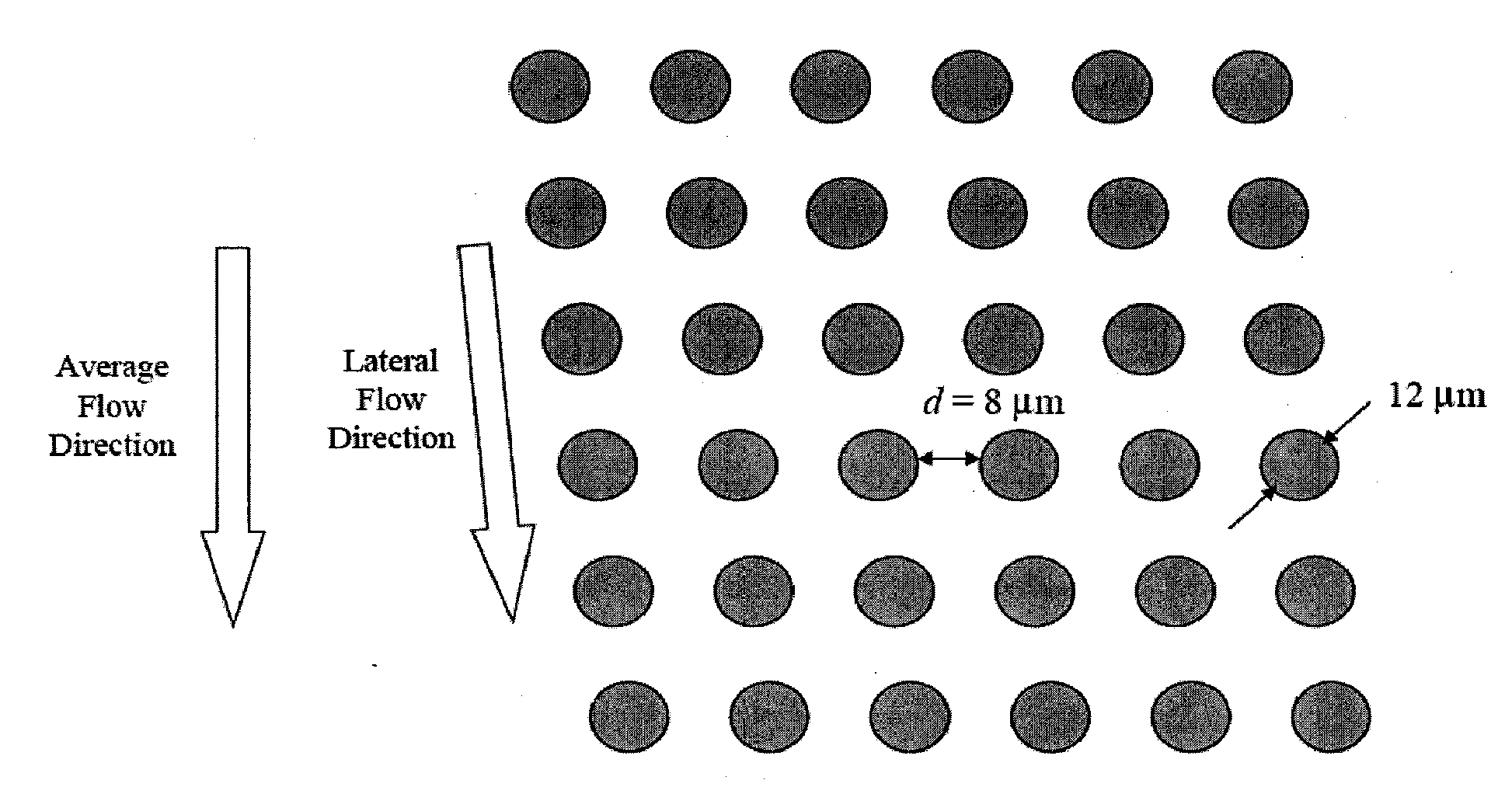
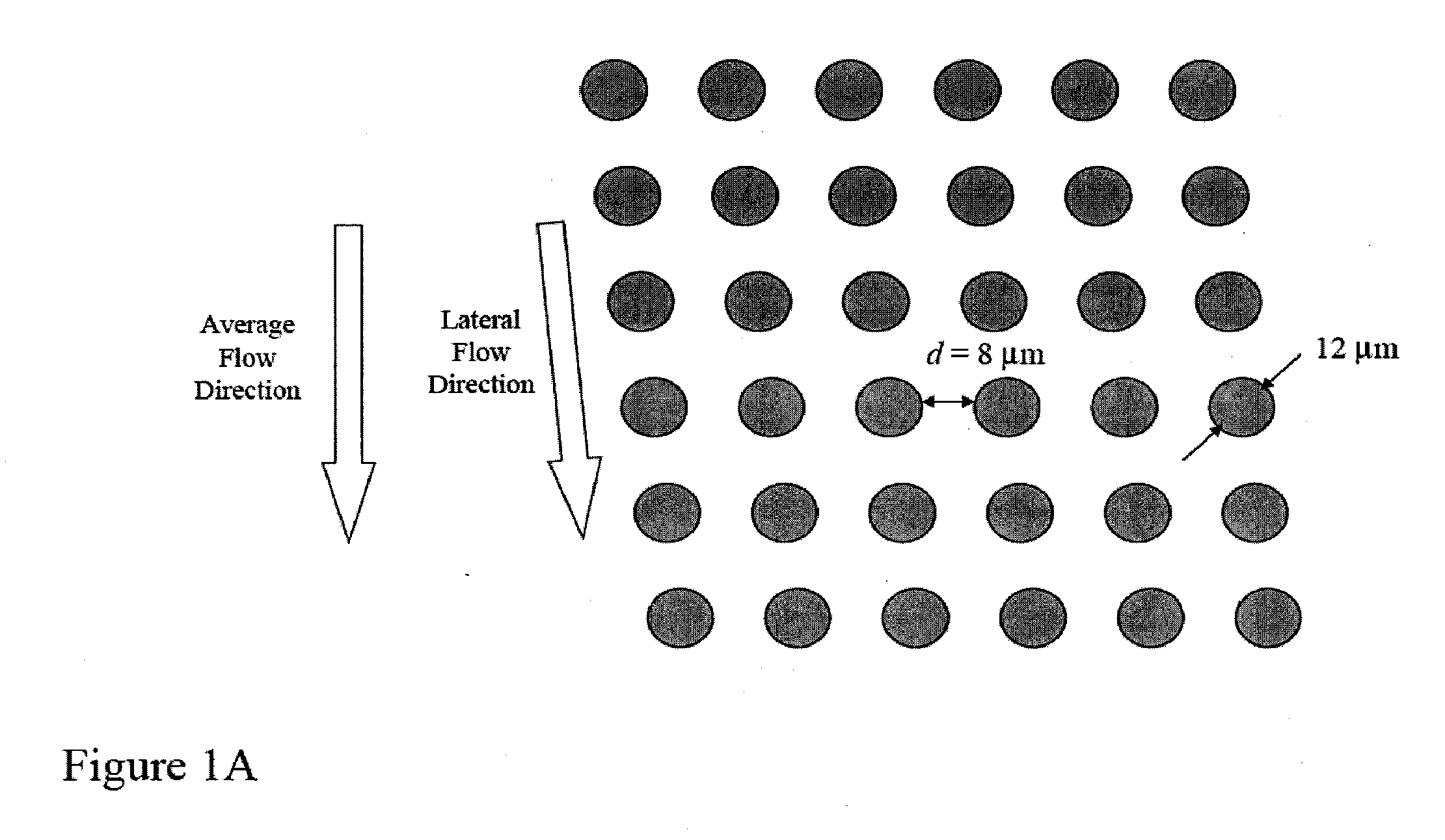
Rare cell analysis using sample splitting and DNA tags
Described herein are methods to diagnose or prognose cancer in a subject by enriching, detecting, and analyzing individual rare cells, e.g., epithelial cells, in a sample from the subject. Also described are methods for labeling regions of genomic DNA in individual cells in said mixed sample with different labels wherein each label is specific to each cell and quantifying the labeled regions of genomic DNA from each cell in the mixed sample. More particularly the method includes detecting the presence of gene mutations in individual rare cells in a subsample.
Owner:GPB SCI +2
Device for the testing of fluid samples and process for making the device
InactiveUS6372515B1Simple processBioreactor/fermenter combinationsBiological substance pretreatmentsBusiness cardAnalyte
A test card for drugs of abuse has a thin flat member having the size and shape of a business card. A plurality of immunoassay test strips extend longitudinally from top to bottom at the card and are fastened side by side in parallel on one or both sides of the test card within the outline of the card. Each test strip is reactive to provide a visual indication in response to a particular drug of abuse. The test card thus provides for the simultaneous detection of multiple analytes. Processes are also disclosed for making the drug test card with test strips on one and both sides of the card.
Owner:HEALGEN SCI LLC
Non-immunostimulatory antibody and compositions containing the same
ActiveUS20070148167A1Immunoglobulins against animals/humansEnzymologyTherapeutic antibodyFc-Gamma Receptor
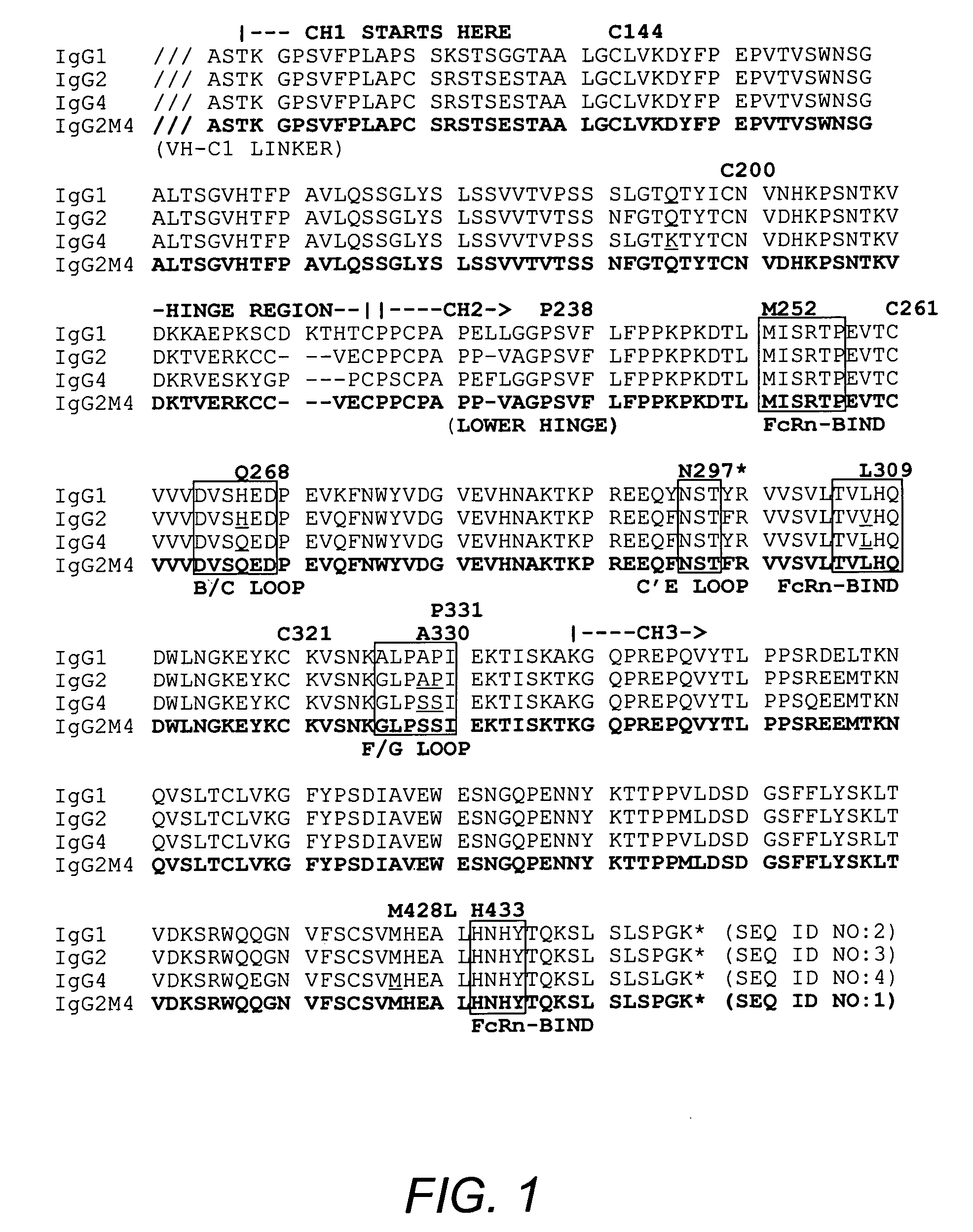
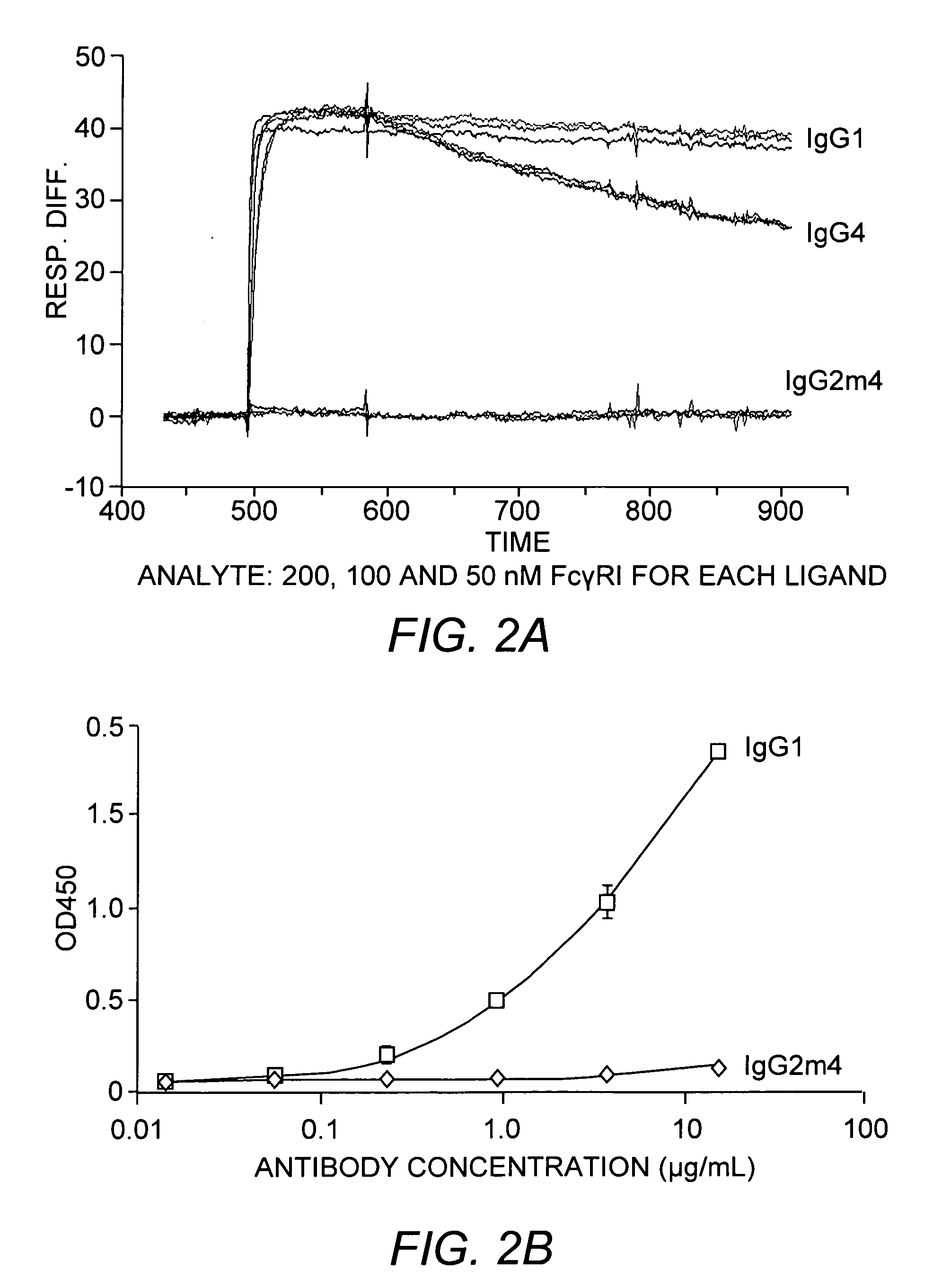
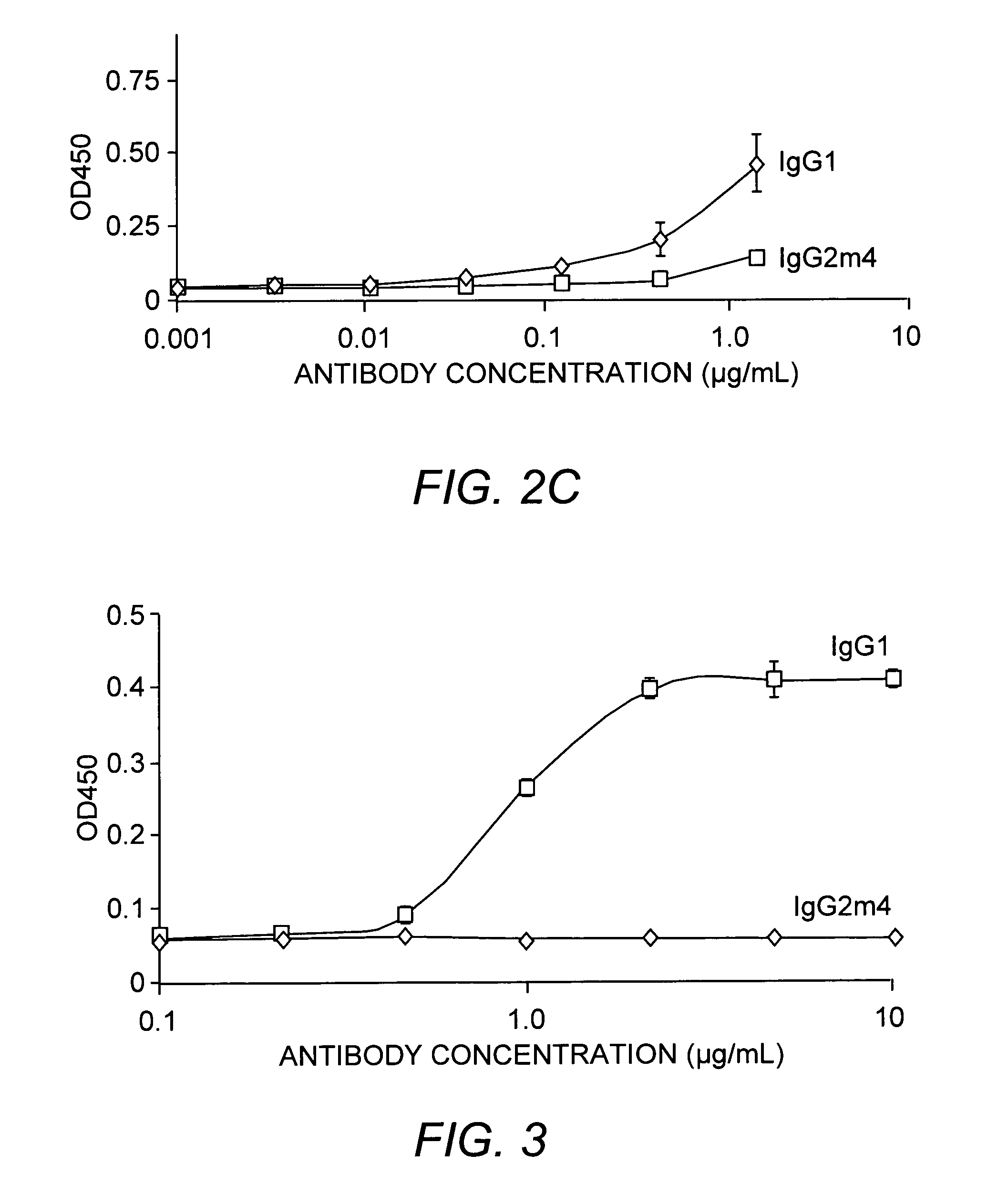
The present invention relates to a non-immunostimulatory antibody which lacks antibody-dependent cell-mediated cytotoxicity, Fc gamma receptor binding and complement-mediated cytotoxicity. In some embodiments, the antibody contains a modified immunoglobulin G2 (IgG2) Fc region with at least one substitution in the B / C loop, FcRn binding domain, and the F / G loop. The antibody of the invention is useful in the preparation of therapeutic antibodies and pharmaceutical compositions and kits containing the same.
Owner:MERCK SHARP & DOHME CORP
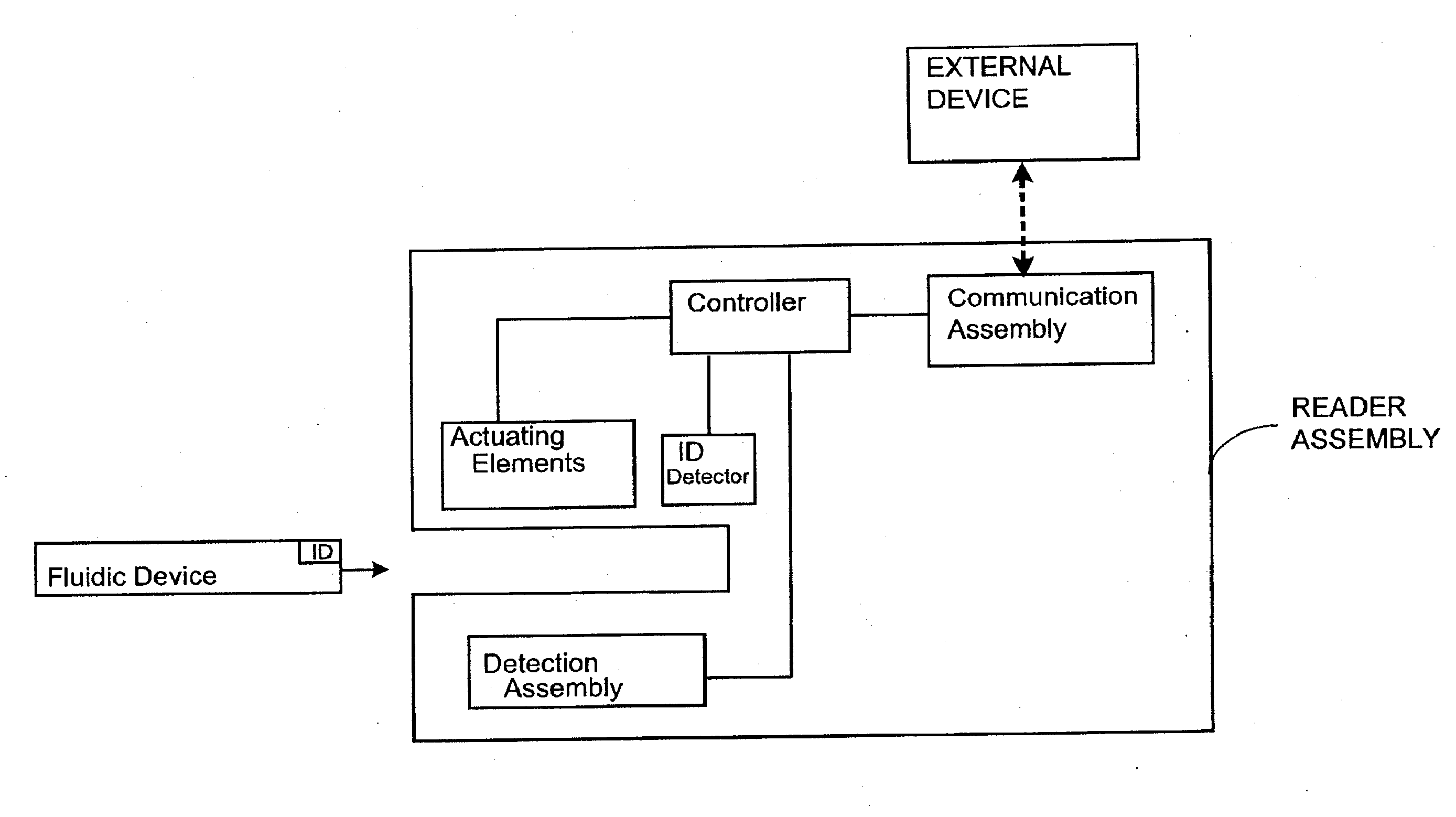
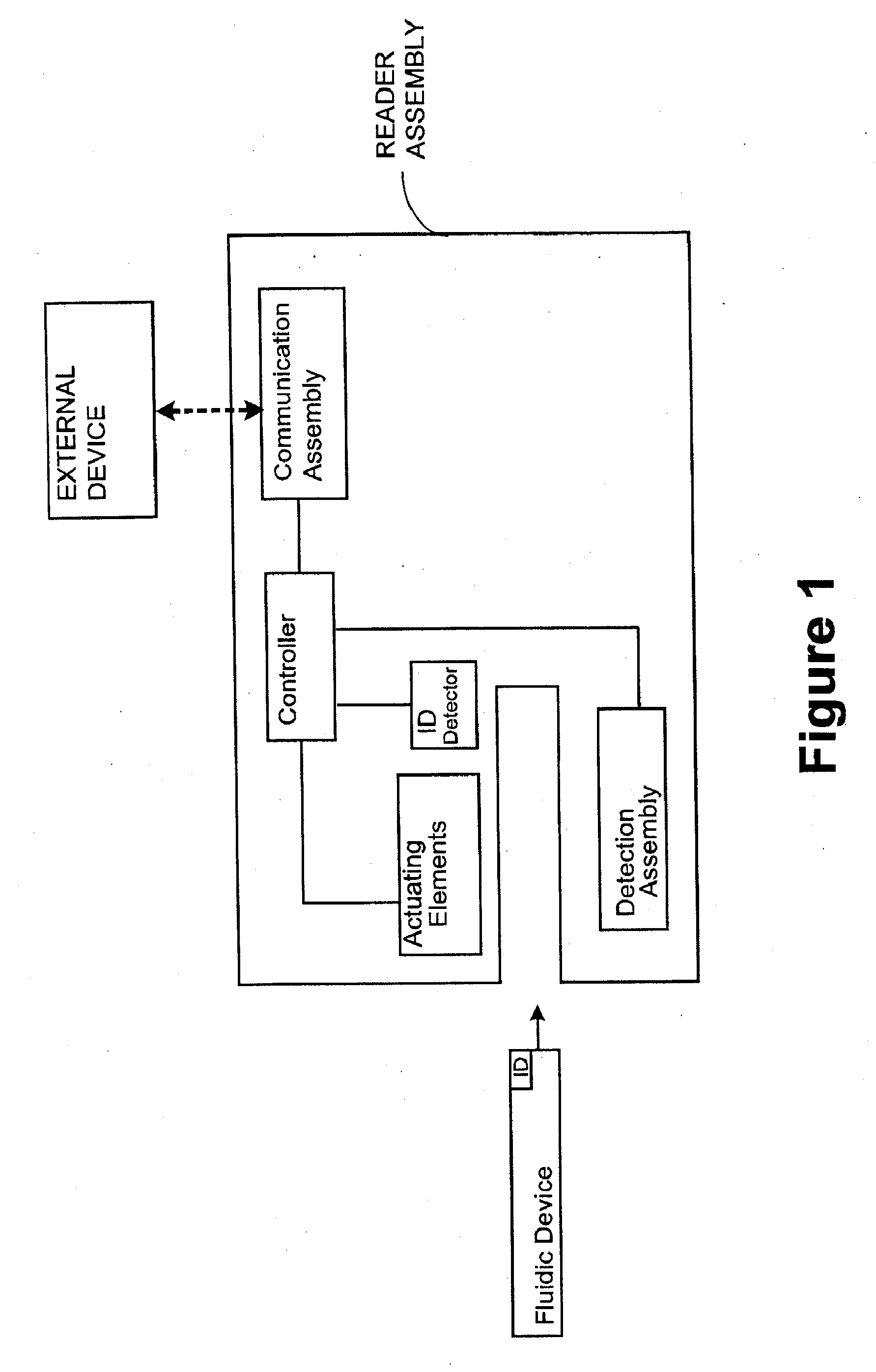
Systems and Methods of Sample Processing and Fluid Control in a Fluidic System
ActiveUS20070224084A1Reduce Interfering SignalsMicrobiological testing/measurementCatheterAnalyteFluid control
This invention is in the field of medical devices. Specifically, the present invention provides portable medical devices that allow real-time detection of analytes from a biological fluid. The methods and devices are particularly useful for providing point-of-care testing for a variety of medical applications.
Owner:GOLDEN DIAGNOSTICS CORP
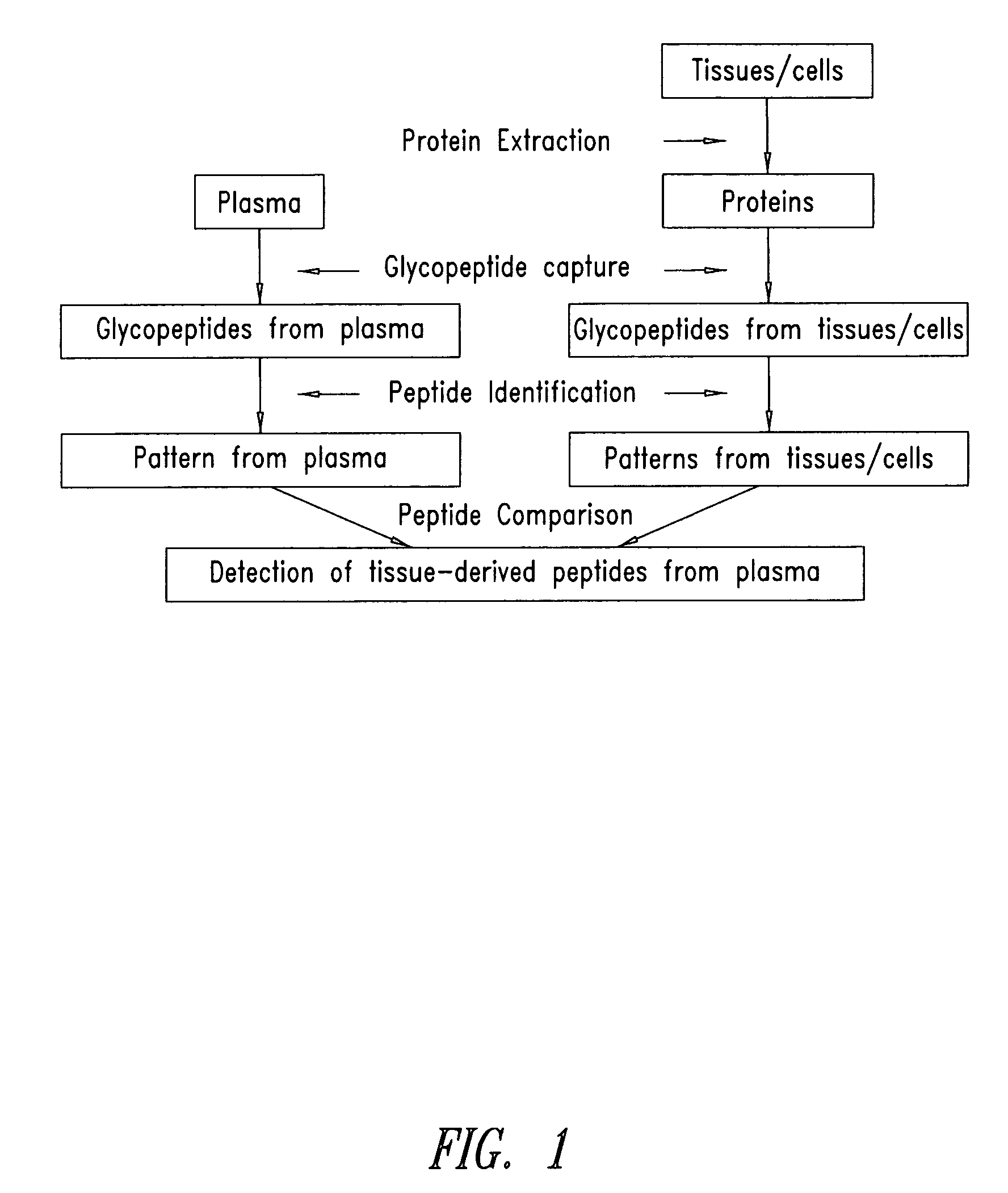
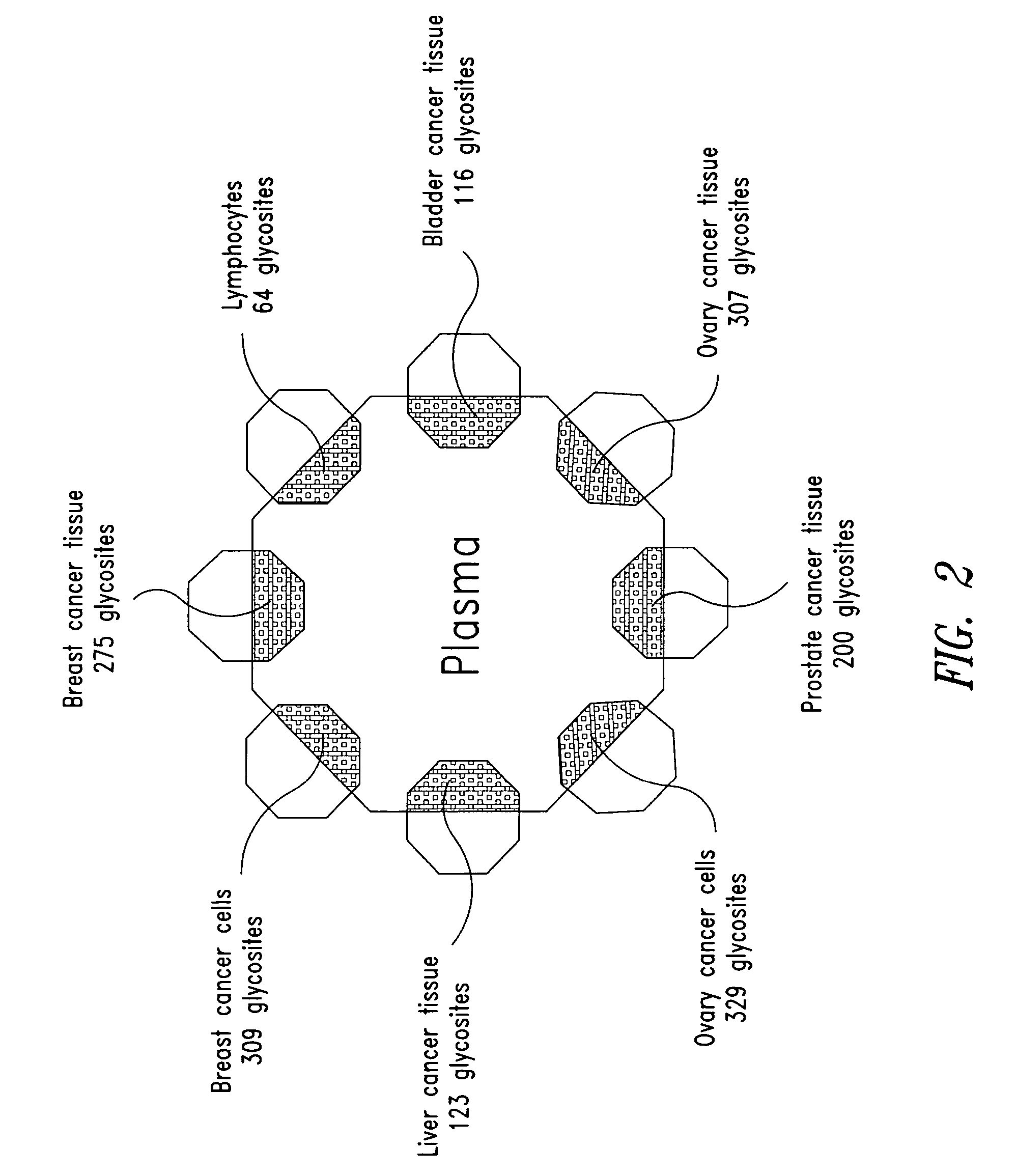
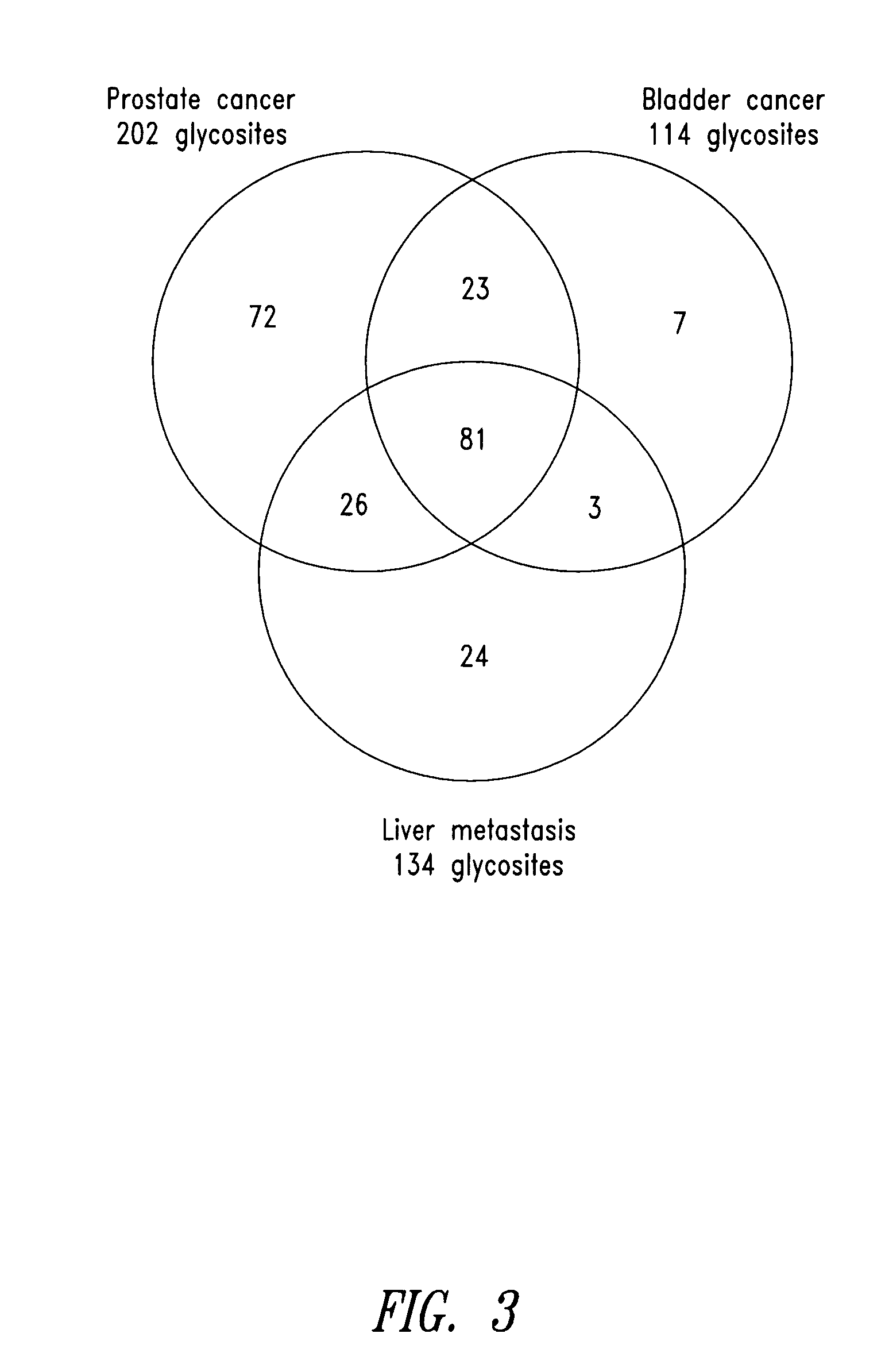
Tissue-and serum-derived glycoproteins and methods of their use
InactiveUS20070099251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBlood plasmaBiology
The present invention is directed generally to tissue-derived glycoproteins and glycosites detectable in plasma via mass spectrometric analysis of glycoproteins from both tissues and blood. The invention also provides methods for identifying tissue-derived glycoproteins and glycosites in plasma, panels of detection reagents for detecting same, as well methods for detecting disease using such panels. The invention further provides a database of tissue-derived glycoproteins and glycosites detectable in plasma.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Modular point-of-care devices, systems, and uses thereof
ActiveUS8088593B2Sequential/parallel process reactionsHeating or cooling apparatusAnalytePoint of care device
The present invention provides devices and systems for use at the point of care. The methods devices of the invention are directed toward automatic detection of analytes in a bodily fluid. The components of the device are modular to allow for flexibility and robustness of use with the disclosed methods for a variety of medical applications.
Owner:LABRADOR DIAGNOSTICS LLC
Methods of predicting and monitoring tyrosine kinase inhibitor therapy
InactiveUS20070254295A1Eliminate side effectsCompound screeningApoptosis detectionAbnormal tissue growthSide effect
Owner:SOC DES PROD NESTLE SA
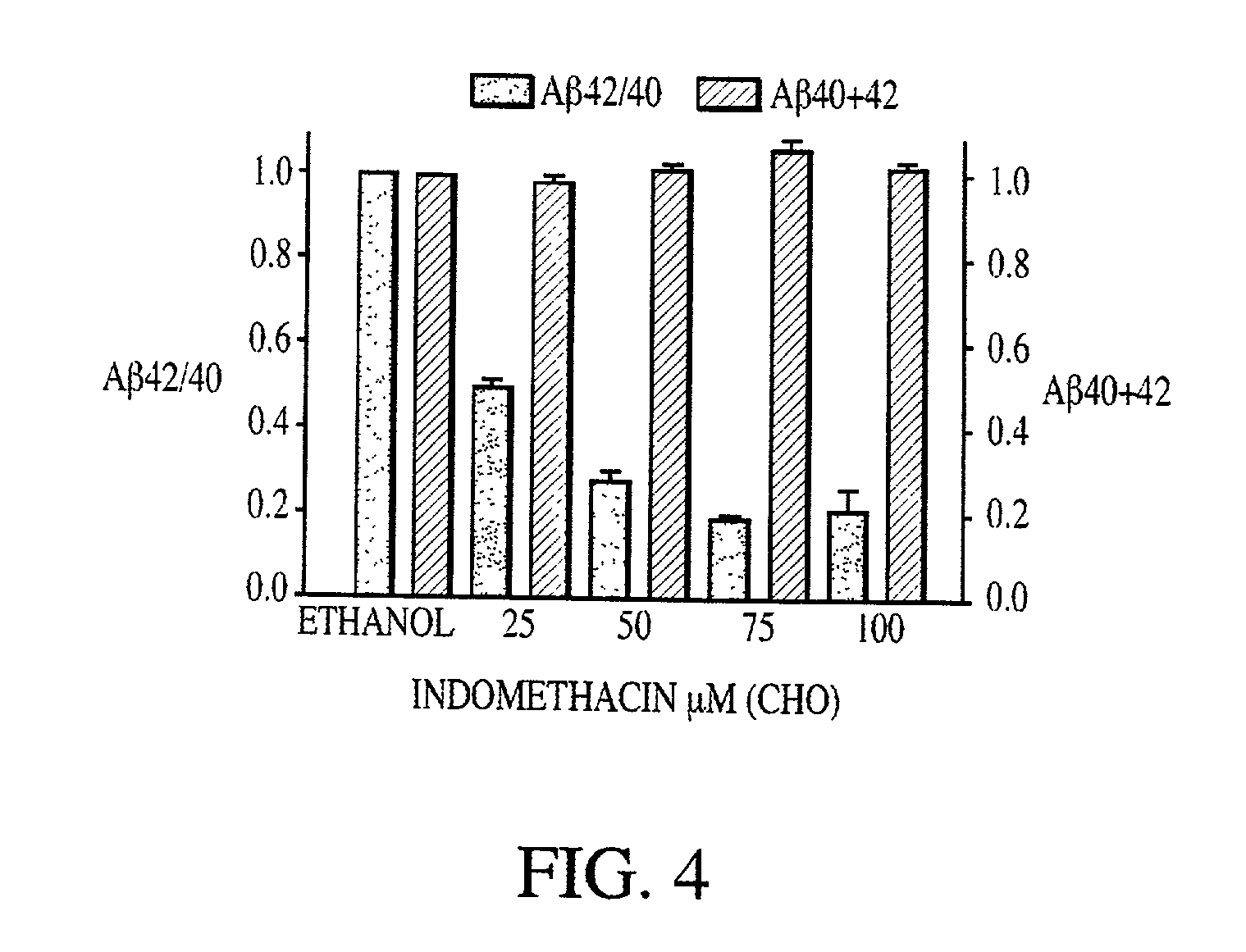
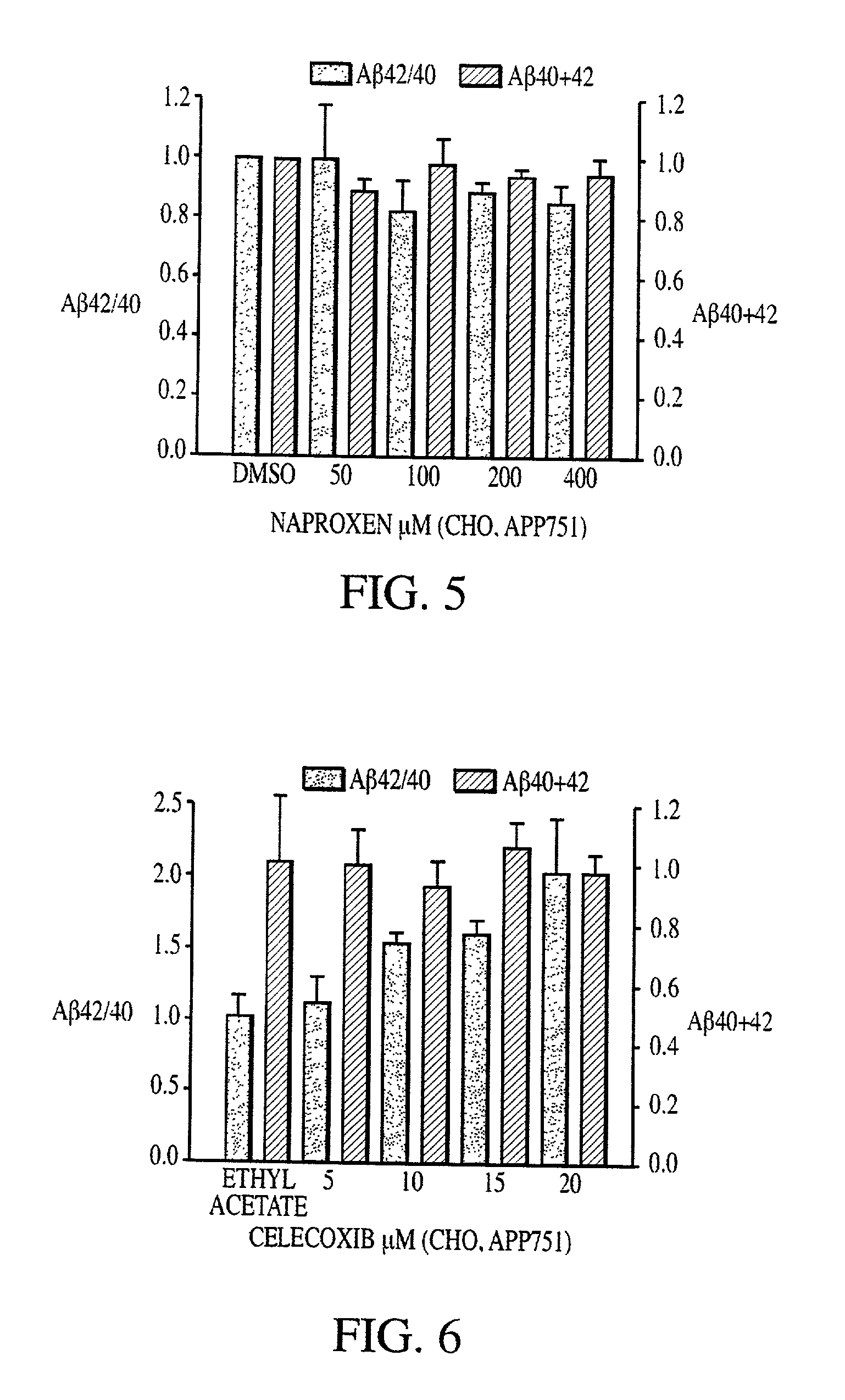
Abeta 42 lowering agents
InactiveUS20020128319A1Prevent and delay and reverse progressionLower Level RequirementsCompounds screening/testingCompound screeningRegimenCholinesterase inhibition
The invention provides a method of preventing, delaying, or reversing the progression of Alzheimer's disease by administering an A.beta..sub.42 lowering agent to a mammal under conditions in which levels of A.beta..sub.42 are selectively reduced, levels of A.beta..sub.38 are increased, and levels of A.beta..sub.40 are unchanged. The invention provides methods and materials for developing and identifying A.beta..sub.42 lowering agents. In addition, the invention provides methods for identifying agents that increase the risk of developing, or hasten progression of, Alzheimer's disease. The invention also provides compositions of A.beta..sub.42 lowering agents and antioxidants, A.beta..sub.42 lowering agents and non-selective secretase inhibitors, as well as A.beta..sub.42 lowering agents and acetylcholinesterase inhibitors. The invention also provides kits containing A.beta..sub.42 lowering agents, antioxidants, non-selective secretase inhibitors, and / or acetylcholinesterase inhibitors as well as instructions related to dose regimens for A.beta..sub.42 lowering agents, antioxidants, non-selective secretase inhibitors, and acetylcholinesterase inhibitors.
Owner:RGT UNIV OF CALIFORNIA
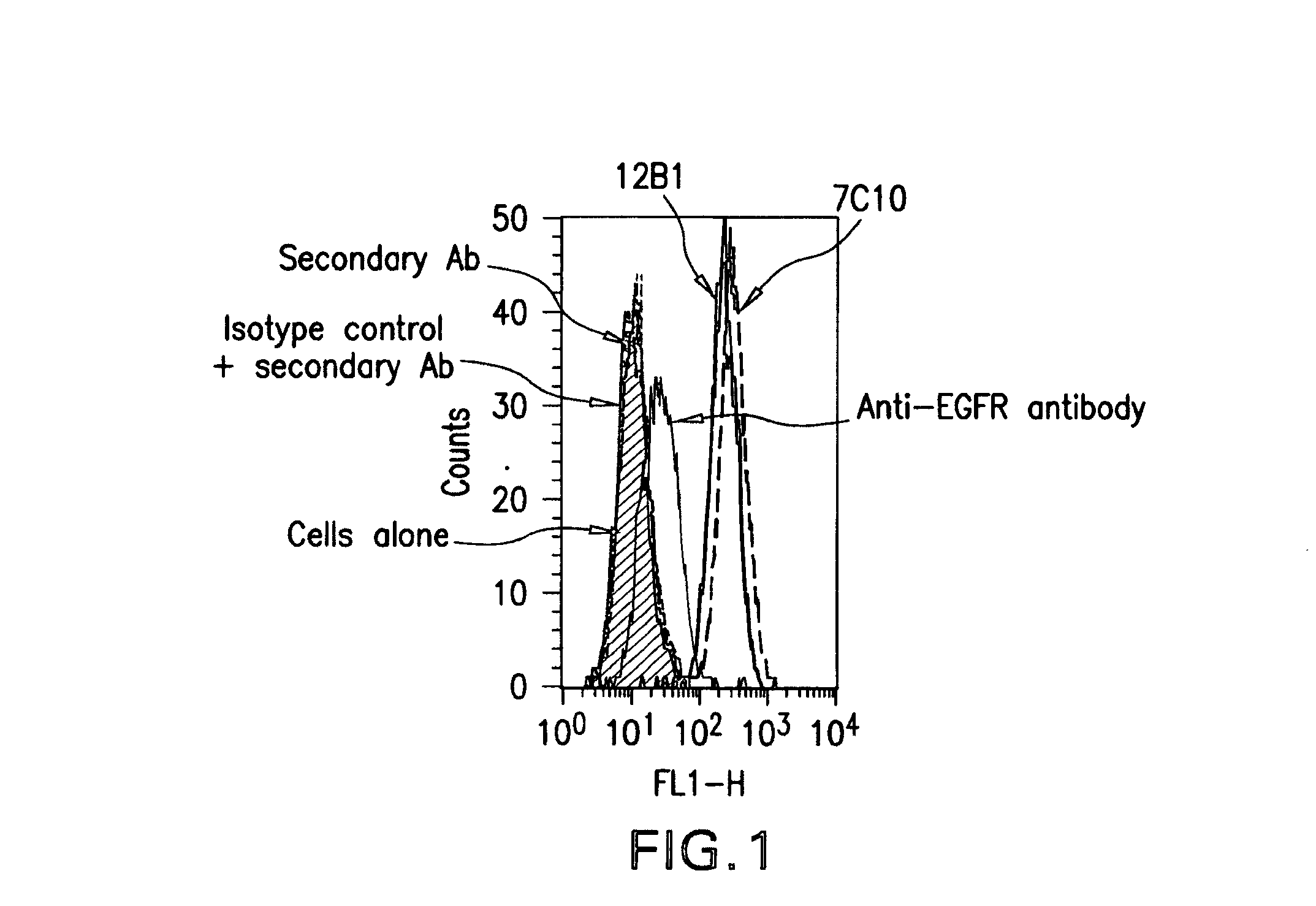
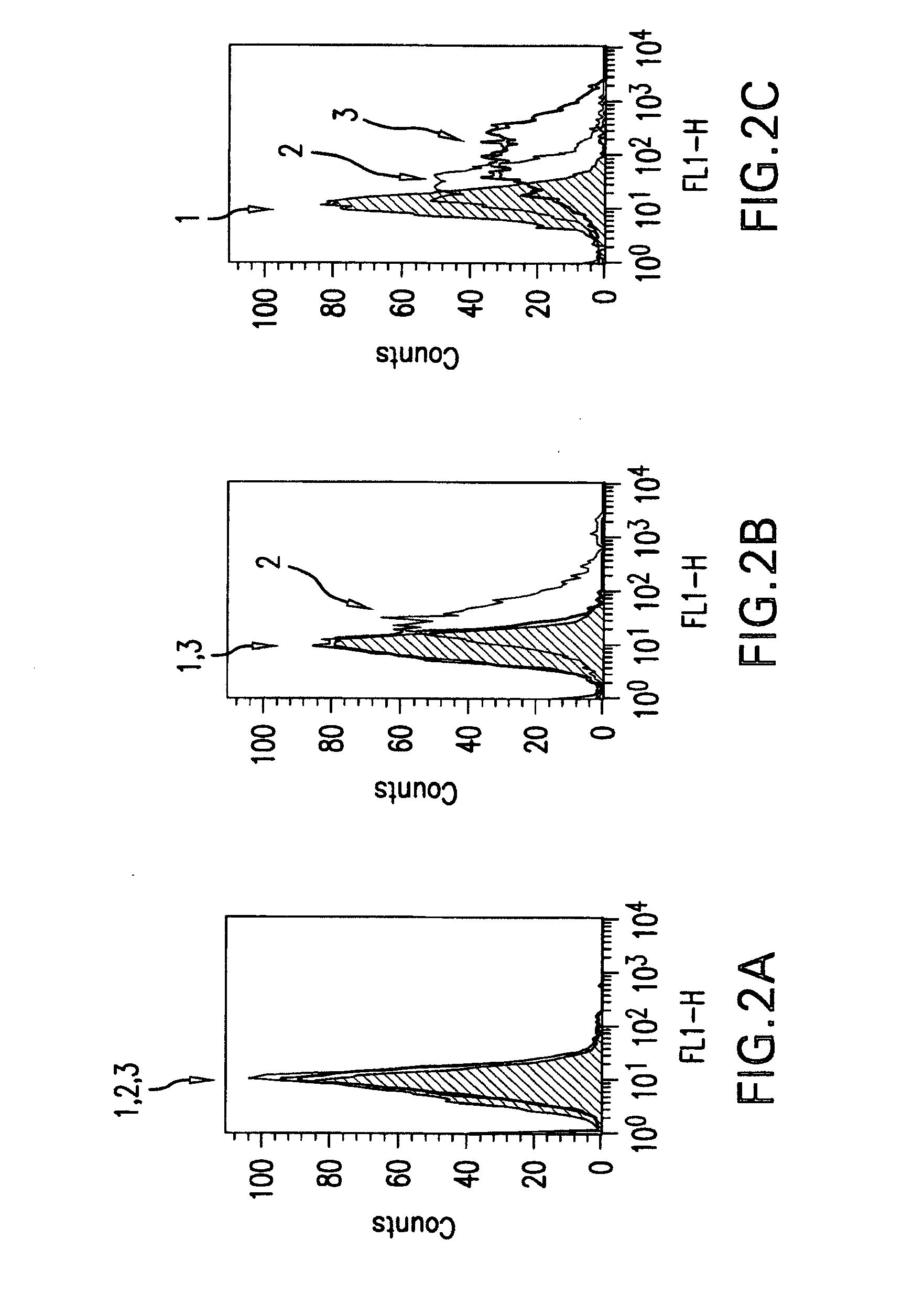
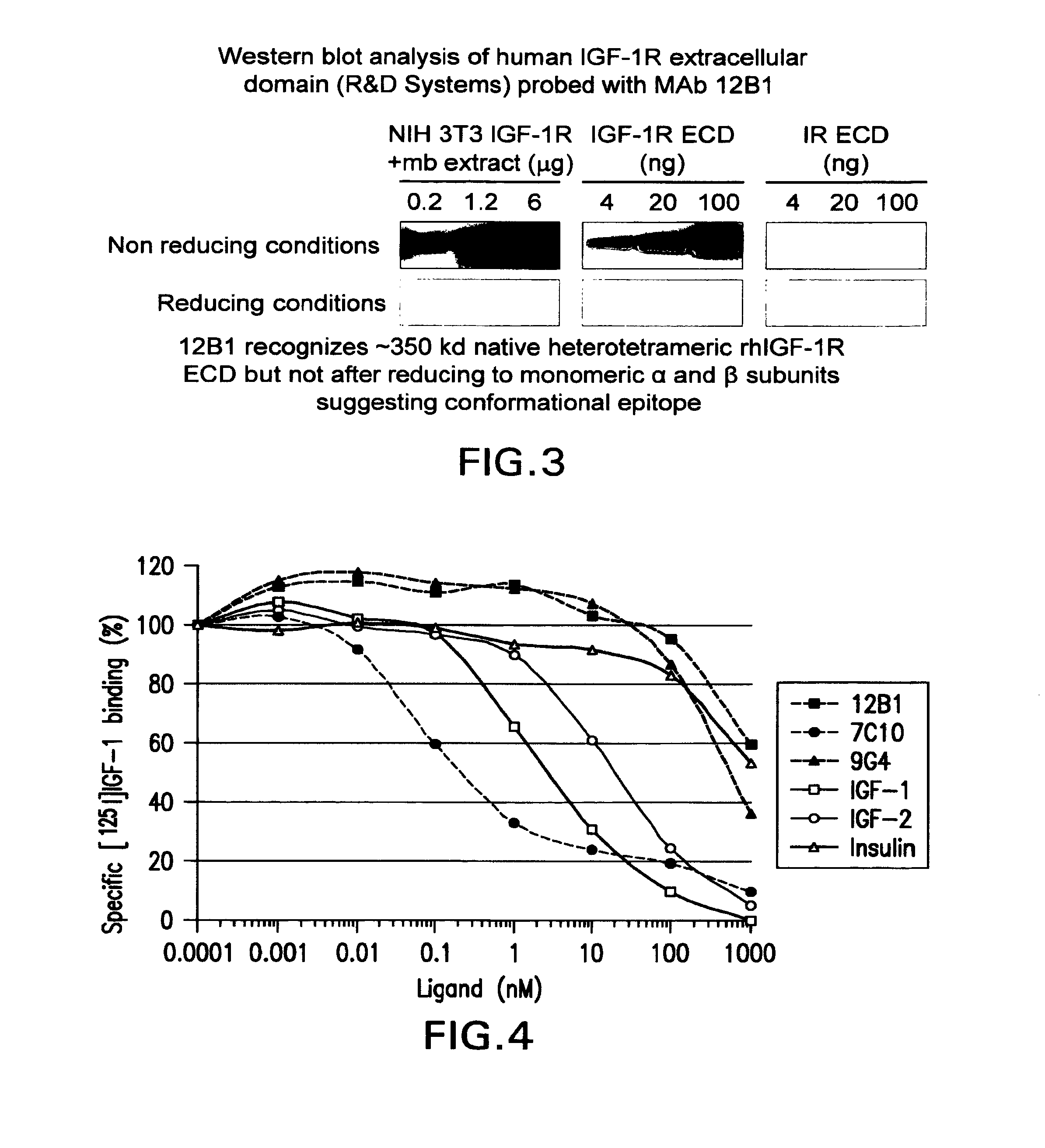
Igf-1r specific antibodies useful in the detection and diagnosis of cellular proliferative disorders
InactiveUS20130084243A1High affinityUseful in detectionAnimal cellsIn-vivo radioactive preparationsDiseaseSingle-Chain Antibodies
The present invention relates to mammalian antibodies, designated 12B1 and antigen-binding portions thereof that specifically bind to insulin-like growth factor I receptor (IGF-IR), preferably human IGF-IR. Also included are chimeric, bispecific, derivatized, single chain antibodies derived from the antibodies disclosed herein. Nucleic acid molecules encoding the mammalian antibodies as well as methods of use thereof are also disclosed. Also included are pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for treatment and diagnosis of pathological hyperproliferative oncogenic disorders associated with expression of IGf-1R.
Owner:GOETSCH LILIANE +4
Methods of activating a melanocortin-4 receptor pathway in obese subjects
ActiveUS8476227B2Induce weight lossOrganic active ingredientsPeptide/protein ingredientsDefined ProcedureMC4 Receptor
Methods and therapeutics are provided for treating metabolic disorders by activation of melanocortin signaling pathways. Generally, the methods and therapeutics can induce activation of melanocortin receptor signaling to increase energy expenditure and induce weight loss. In one embodiment, a method for performing a diagnostic procedure can be chosen, energy expenditure then assess in light of the diagnostic procedure and a definitive procedure(s) can be selected dependent on the outcome of the energy assessment. In another embodiment, a diagnostic procedure can be chosen to activate melanocortin receptor pathways, energy expenditure can be assessed and a definitive procedure(s) can be chosen that selectively and optimally activate melanocortin receptor pathways.
Owner:ETHICON ENDO SURGERY INC +1
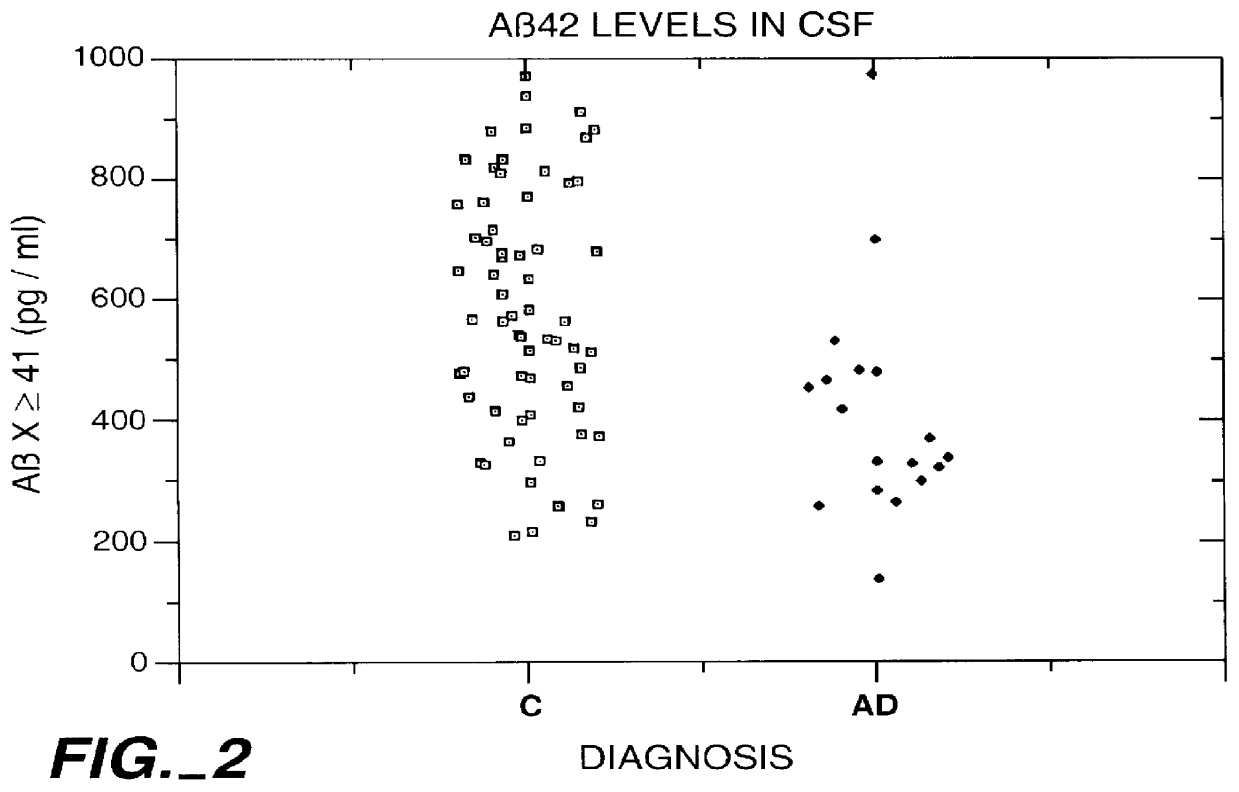
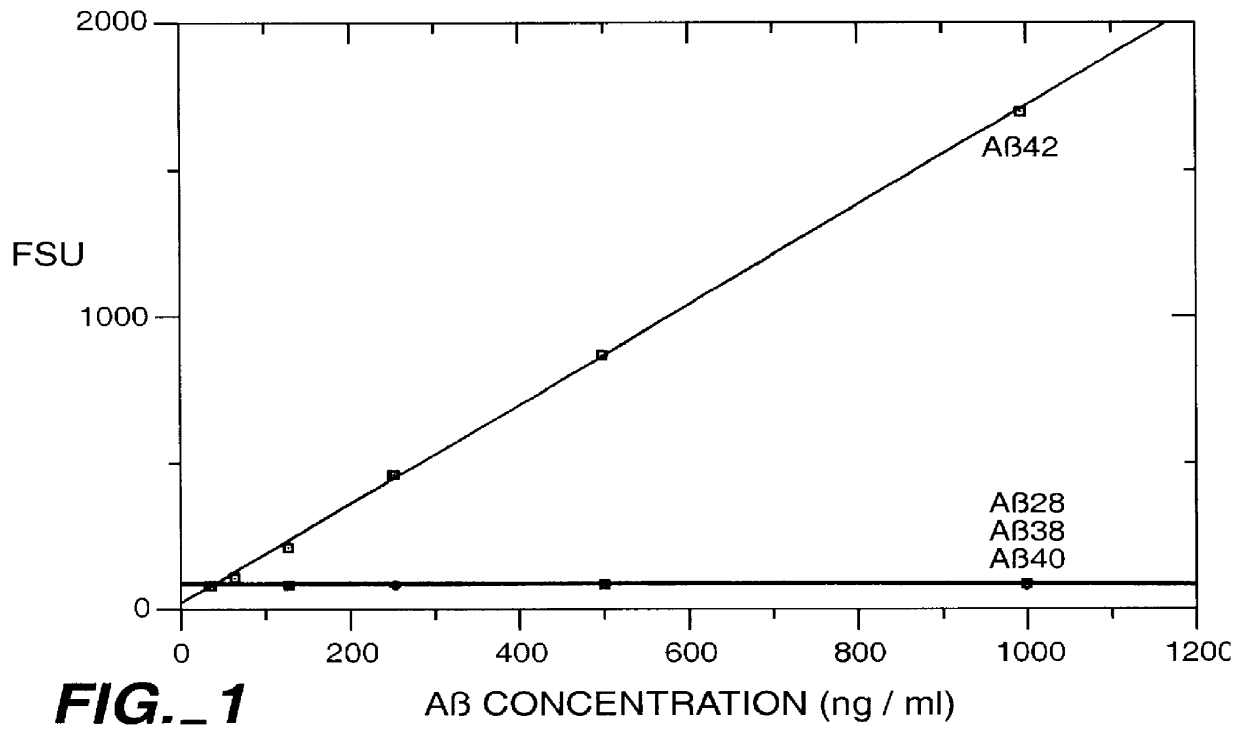
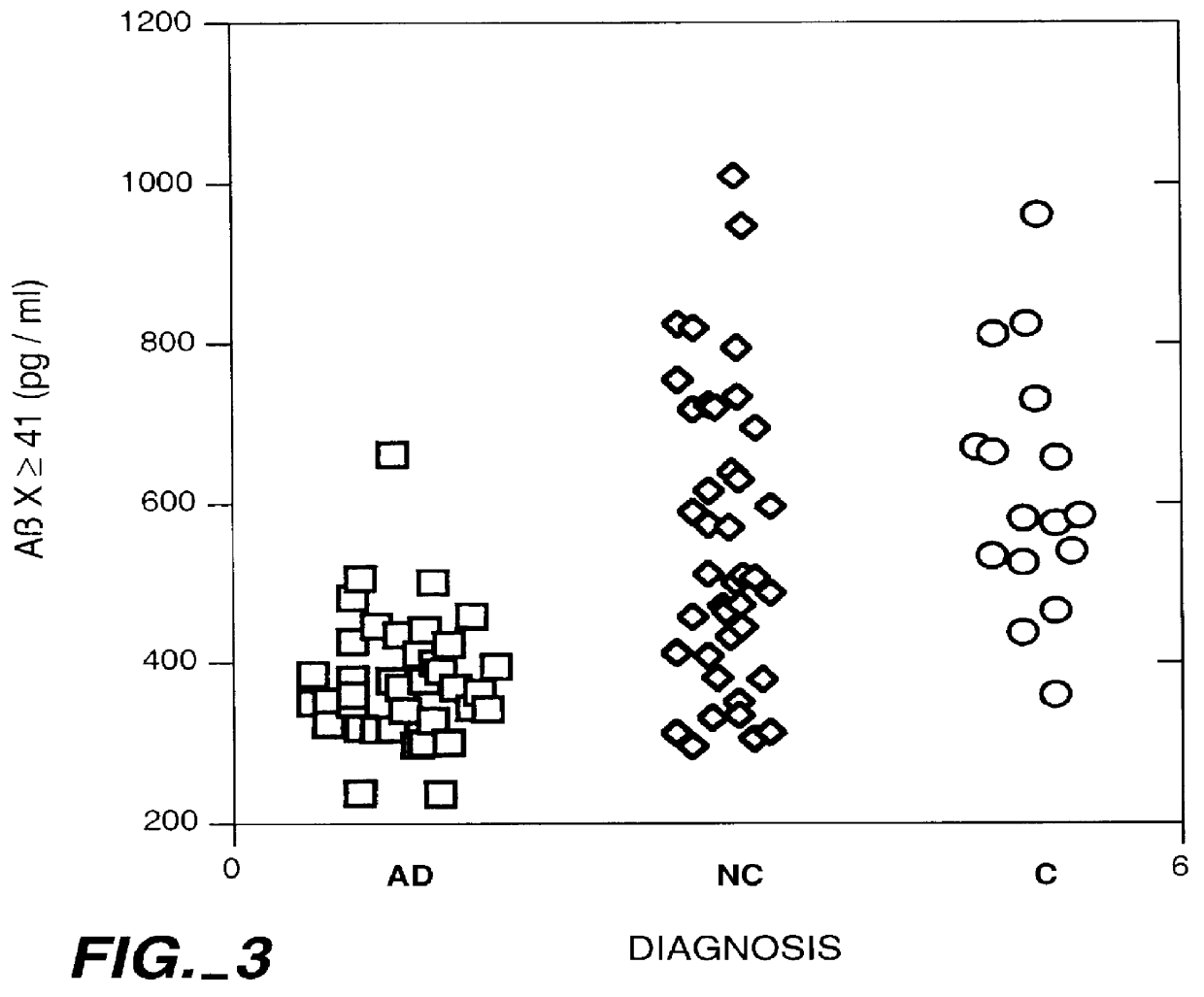
Methods for aiding in the diagnosis of Alzheimer's disease by measuring amyloid- beta peptide (x->/=41)
InactiveUS6114133AImmunoglobulins against animals/humansDisease diagnosisAlzheimer SyndromeDisease cause
This invention provides methods useful in aiding in the diagnosis of Alzheimer's disease. The methods involve measuring the amount of amyloid- beta peptide (x-> / =41) in the cerebrospinal fluid of a patient. High levels of the peptide generally are inconsistent with a diagnosis of Alzheimer's. Low levels of the peptide are consistent with the disease and, with other tests, can provide a positive diagnosis.
Owner:ELAN PHARM INC
UsiRNA Complexes
ActiveUS20110313020A1Reduce off-target effectsImprove propertiesOrganic active ingredientsNervous disorderNucleotideSense strand
This disclosure provides double-stranded RNA complexes having one or more hydroxymethyl substituted nucleomonomer(s) in the passenger strand (or sense strand) of an RNA complex. RNA complexes of the disclosure may be useful for therapeutic applications, diagnostic applications or research applications. RNA complexes include short interfering RNA complexes (siRNA) capable of modulating gene expression comprising an antisense strand and a continuous or a discontinuous passenger strand (“sense strand”). Further, one or more hydroxymethyl substituted nucleomonomer(s) of this disclosure may be positioned at the 3′-end, at the 5′-end, at both the 3′-end and 5′end.
Owner:ARCTURUS THERAPEUTICS
Noninvasive Diagnosis of Fetal Aneuploidy by Sequencing
ActiveUS20100138165A1Maximum resultMicrobiological testing/measurementDisease diagnosisMassive parallel sequencingFetal aneuploidy
Disclosed is a method to achieve digital quantification of DNA (i.e., counting differences between identical sequences) using direct shotgun sequencing followed by mapping to the chromosome of origin and enumeration of fragments per chromosome. The preferred method uses massively parallel sequencing, which can produce tens of millions of short sequence tags in a single run and enabling a sampling that can be statistically evaluated. By counting the number of sequence tags mapped to a predefined window in each chromosome, the over- or under-representation of any chromosome in maternal plasma DNA contributed by an aneuploid fetus can be detected. This method does not require the differentiation of fetal versus maternal DNA. The median count of autosomal values is used as a normalization constant to account for differences in total number of sequence tags is used for comparison between samples and between chromosomes.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Single cell gene expression for diagnosis, prognosis and identification of drug targets
InactiveUS20100255471A1Microbiological testing/measurementDisease diagnosisDrug targetDisease status
Methods are provided for diagnosis and prognosis of disease by analyzing expression of a set of genes obtained from single cell analysis. Classification allows optimization of treatment, and determination of whether on whether to proceed with a specific therapy, and how to optimize dose, choice of treatment, and the like. Single cell analysis also provides for the identification and development of therapies which target mutations and / or pathways in disease-state cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
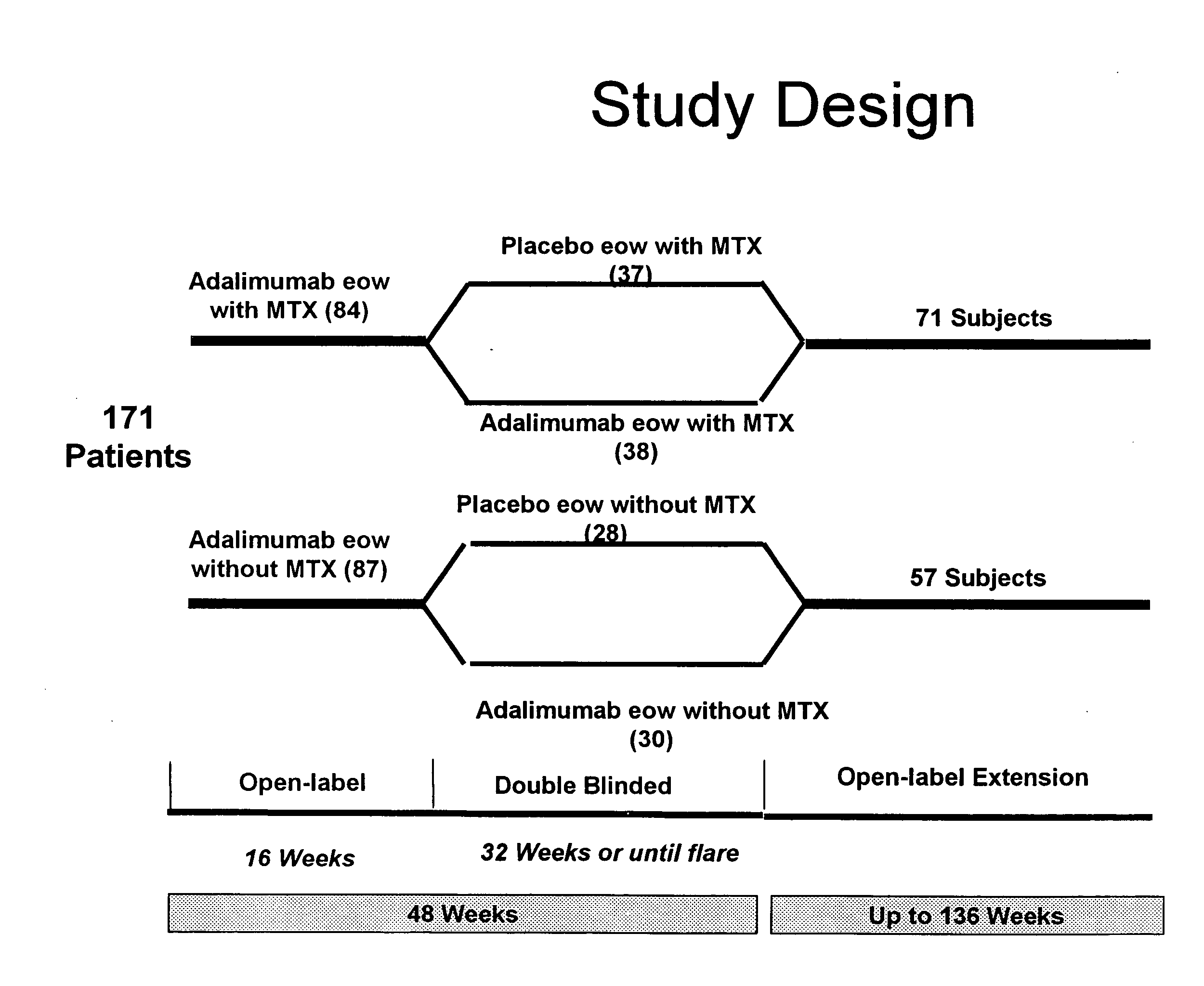
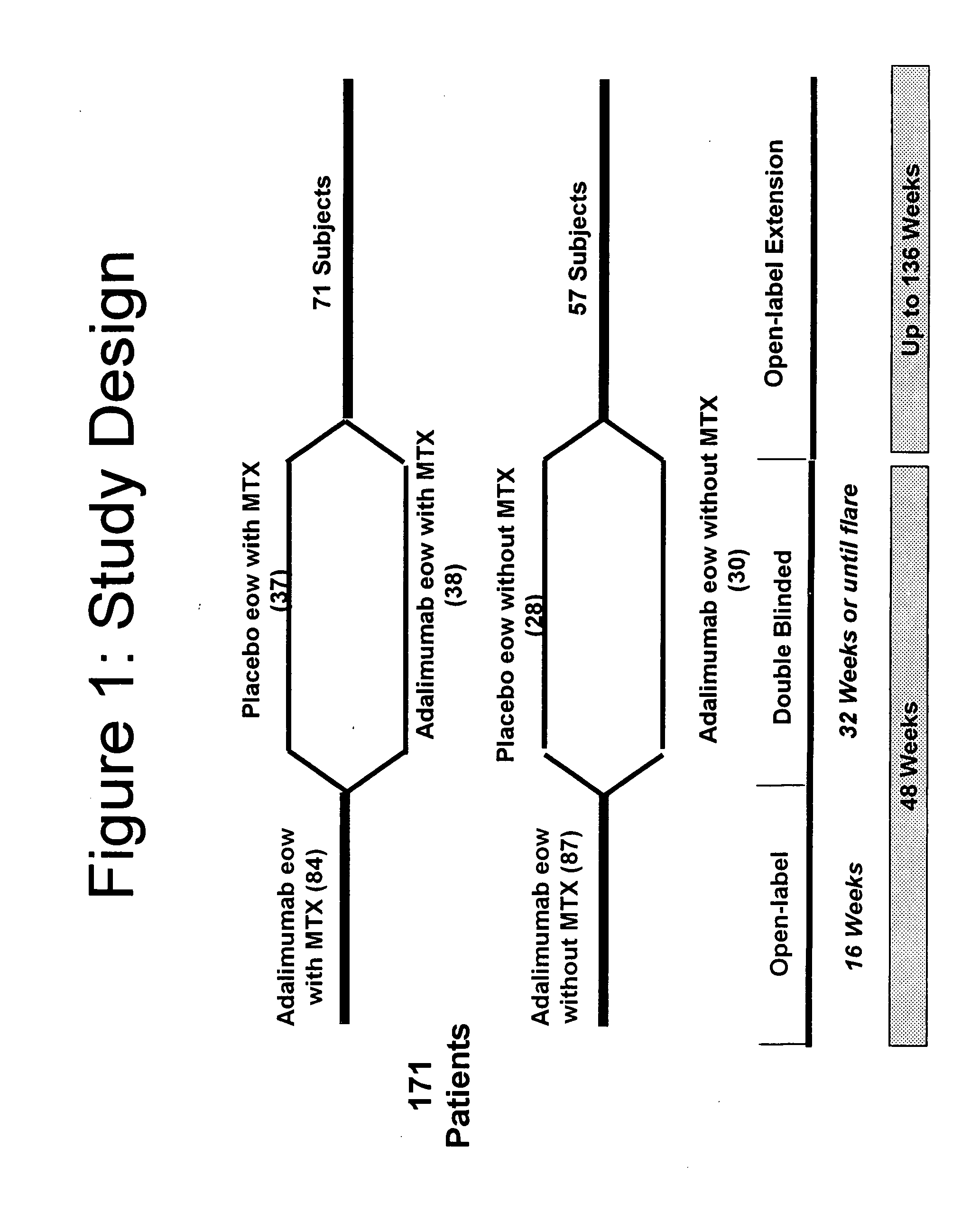
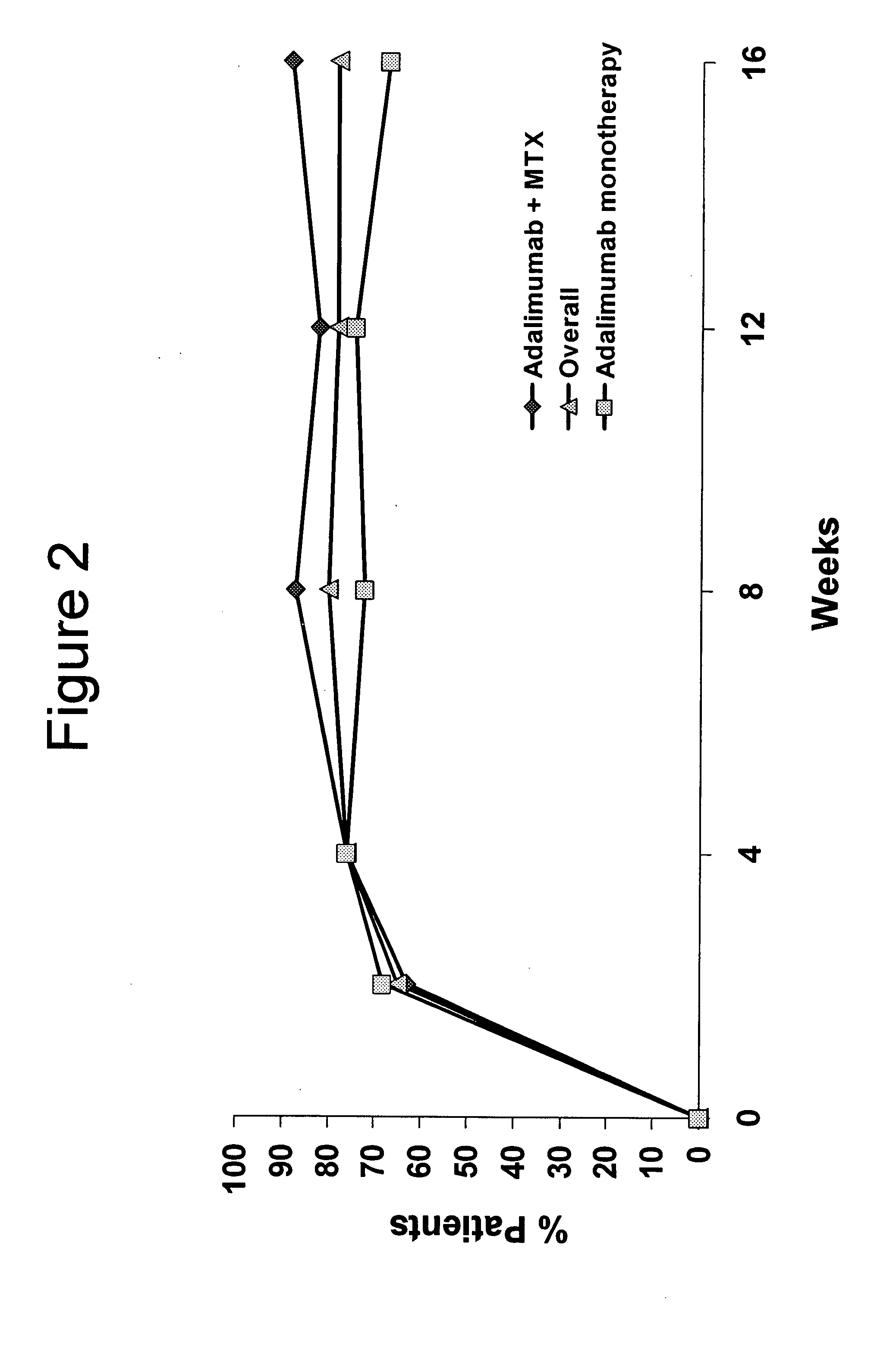
Uses and compositions for treatment of juvenile rheumatoid arthritis
InactiveUS20080118496A1Prevent outbreakTreatment safetyLarge containersAntibody ingredientsMedicineAntigen binding
The invention provides methods, uses and compositions for the treatment of juvenile rheumatoid arthritis (JRA). The invention describes methods and uses for treating JRA, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to prevent flare-ups associated with JRA. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of JRA in a subject.
Owner:MEDICH JOHN R +4
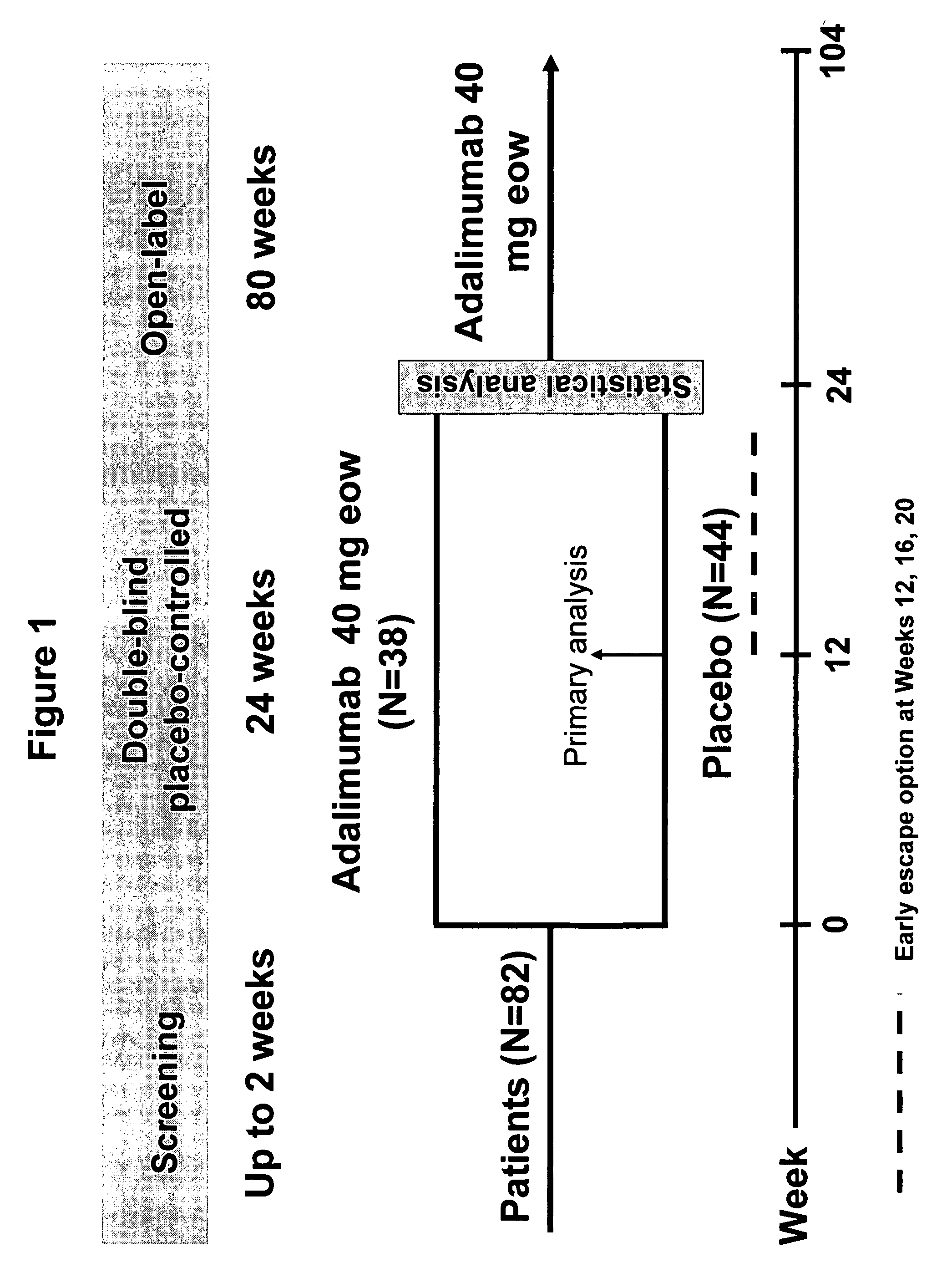
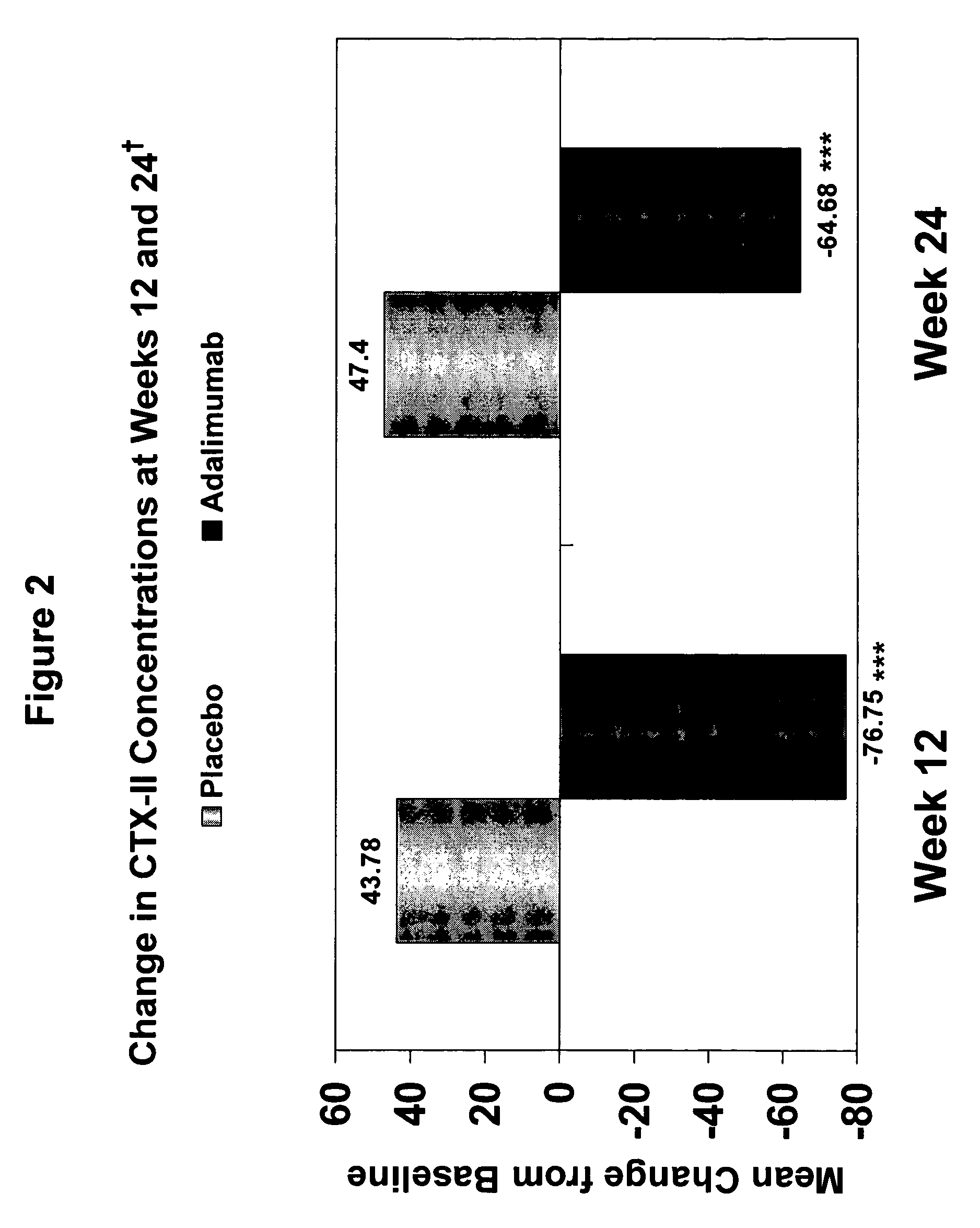
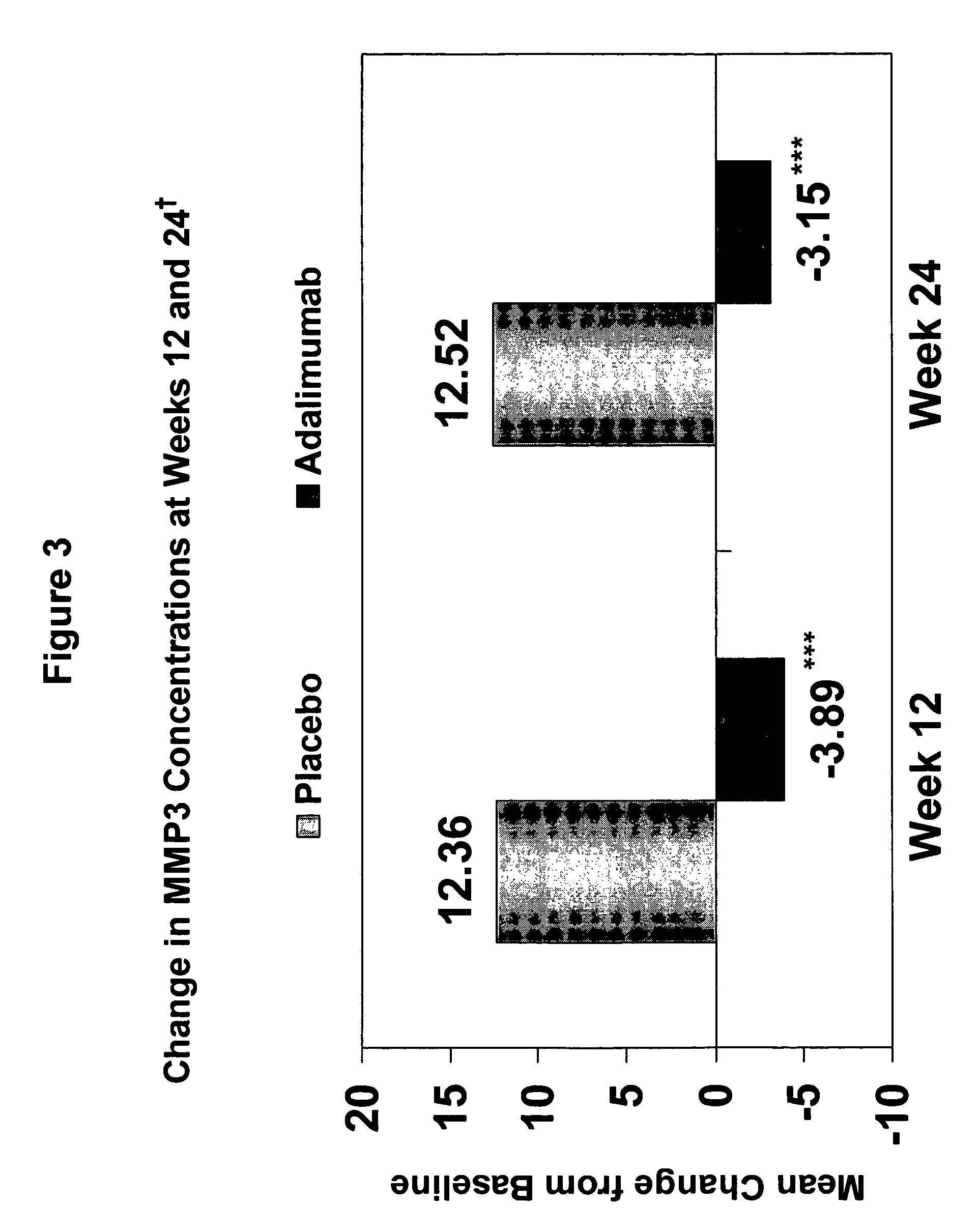
Methods and compositions for determining the efficacy of a treatment for ankylosing spondylitis using biomarkers
ActiveUS7919264B2Better assess improvements in the patient's disease statusUseful in treatingBioreactor/fermenter combinationsBiological substance pretreatmentsAnkylosing spondylitisAntigen binding
The invention provides a method for determining the efficacy of a TNFα inhibitor, such as a TNFα antibody, or an antigen-binding portion thereof, for treating ankylosing spondylitis (AS), using a collagen degradation biomarker and / or a synovitis biomarker.
Owner:AMERICAN TELEPHONE & TELEGRAPH CO +1
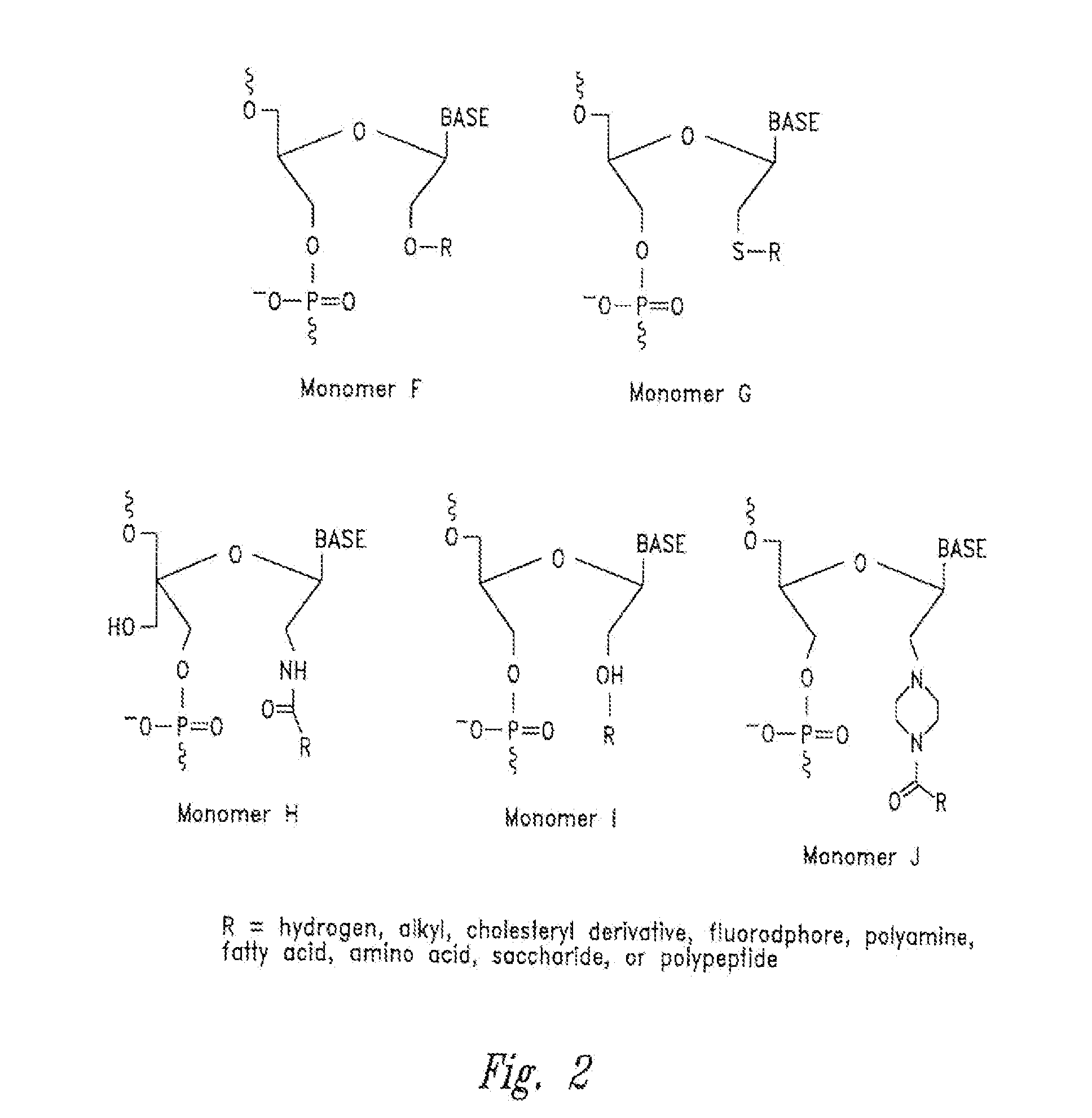
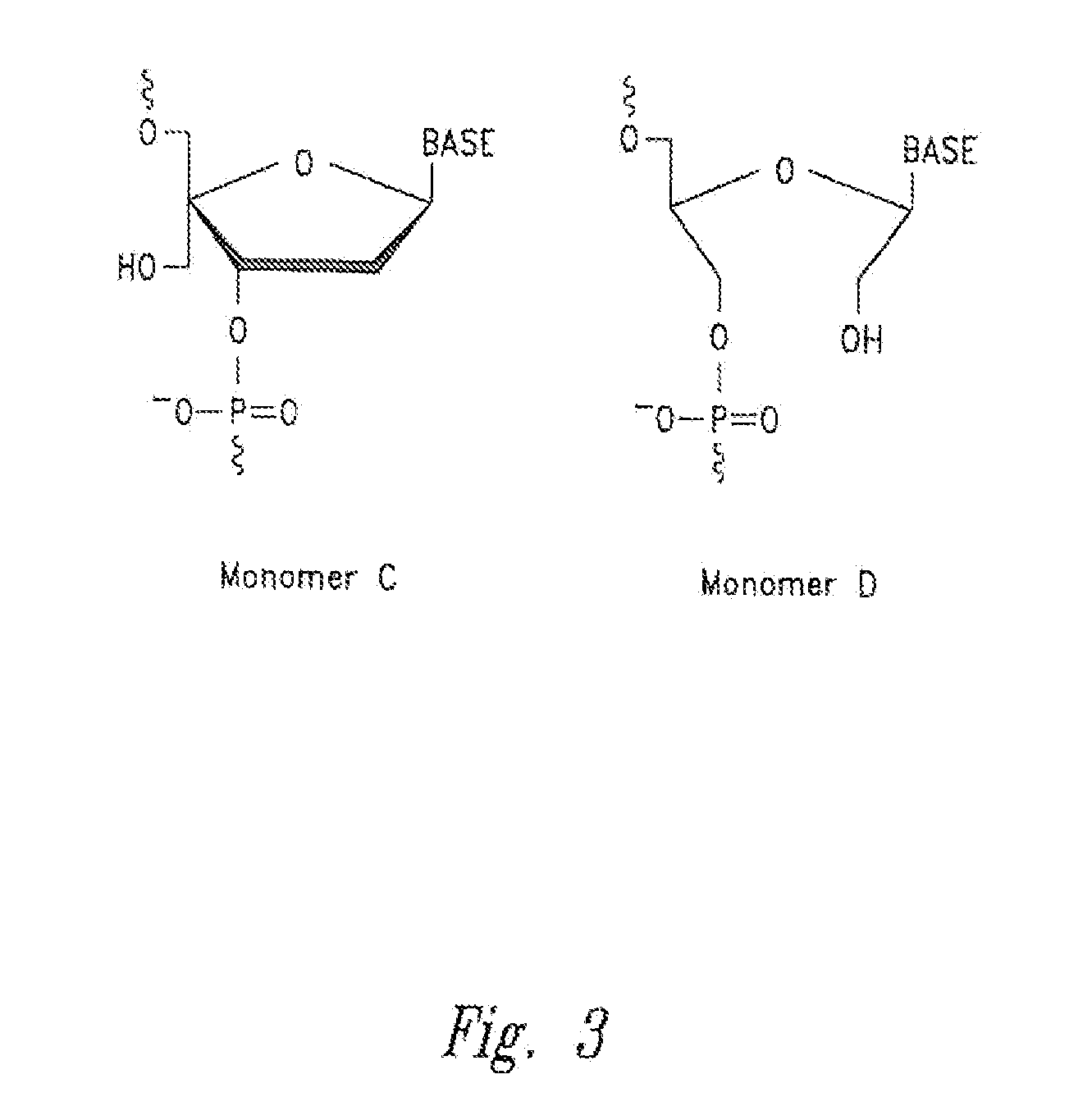
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com