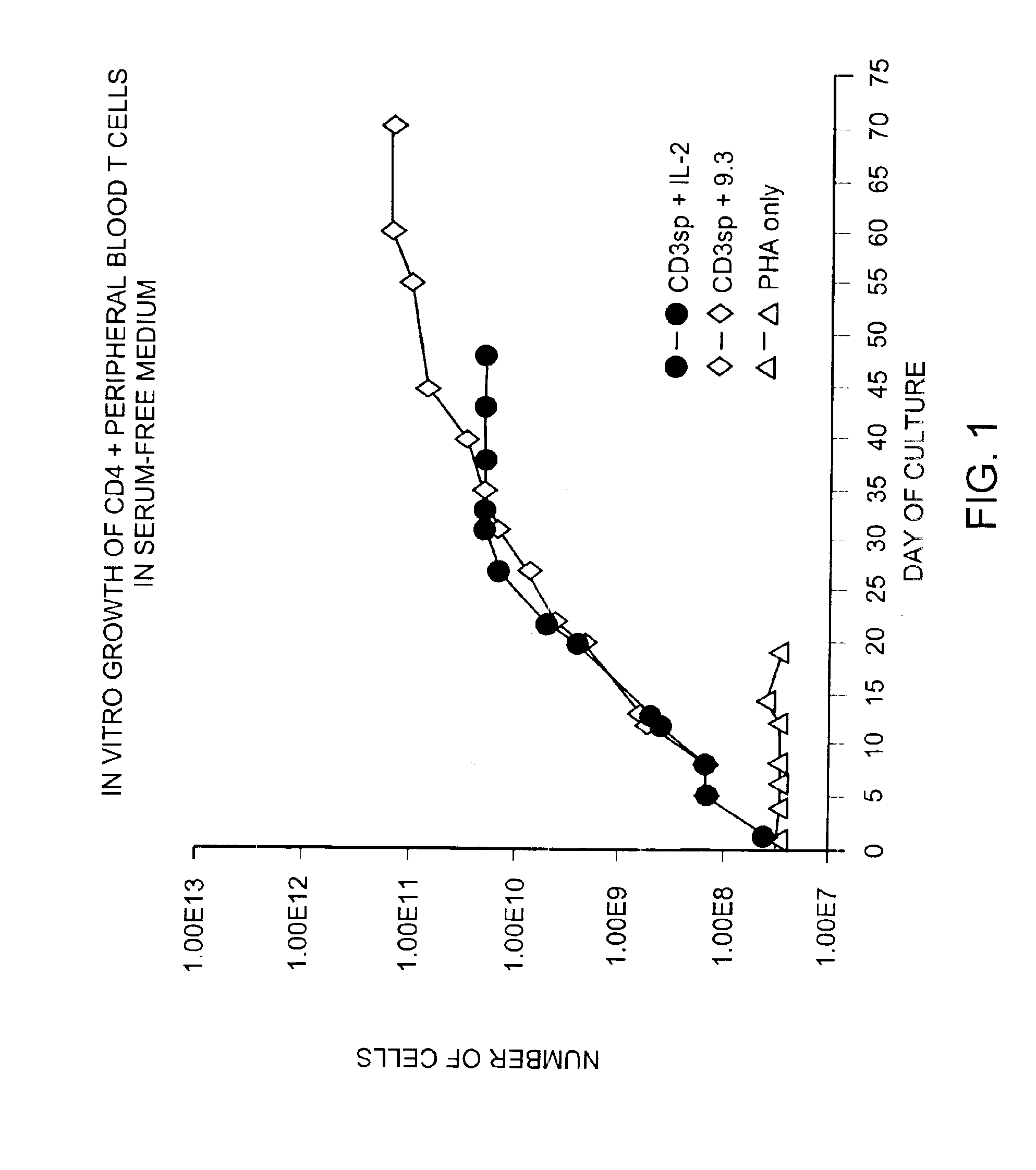
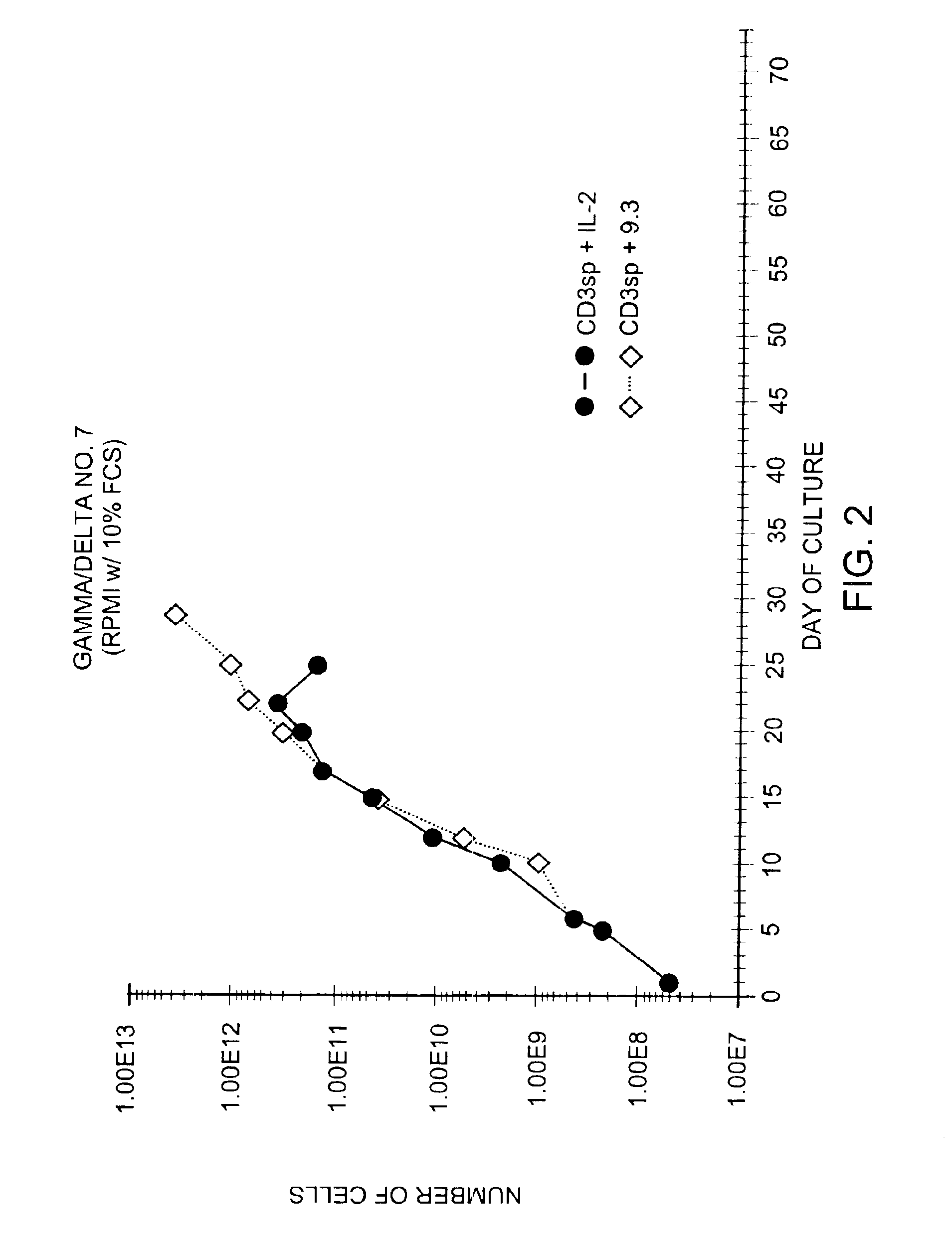
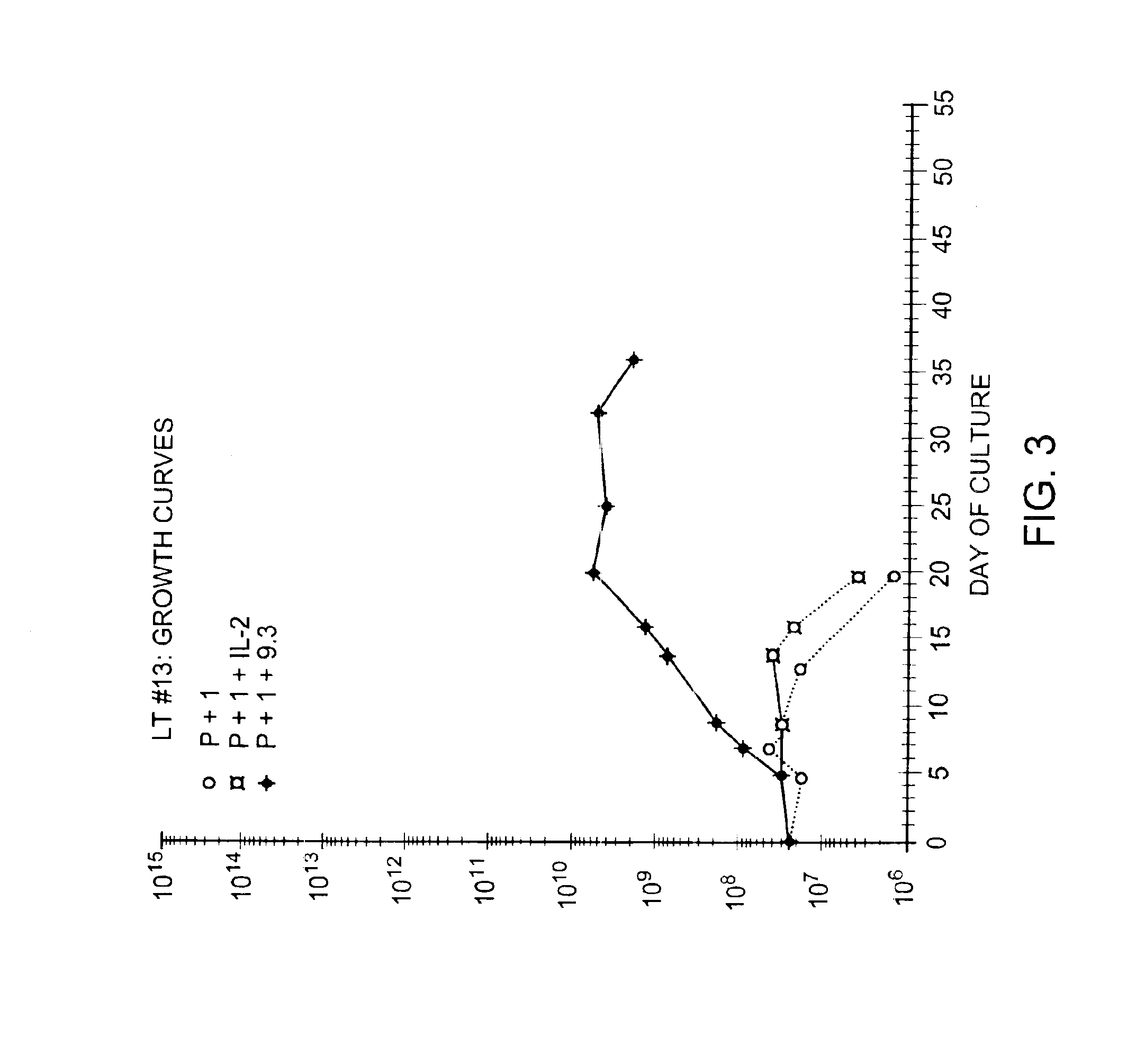
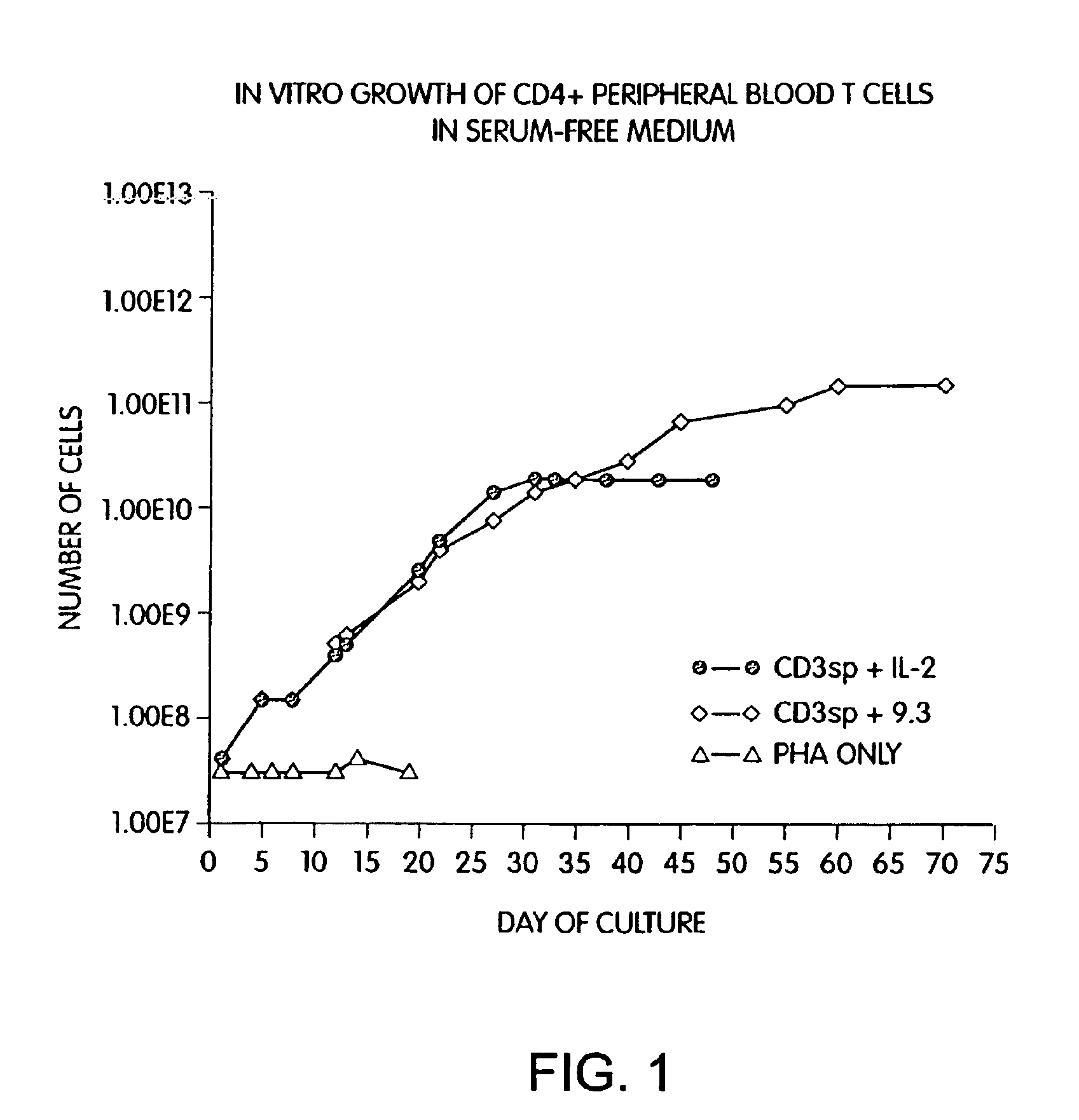
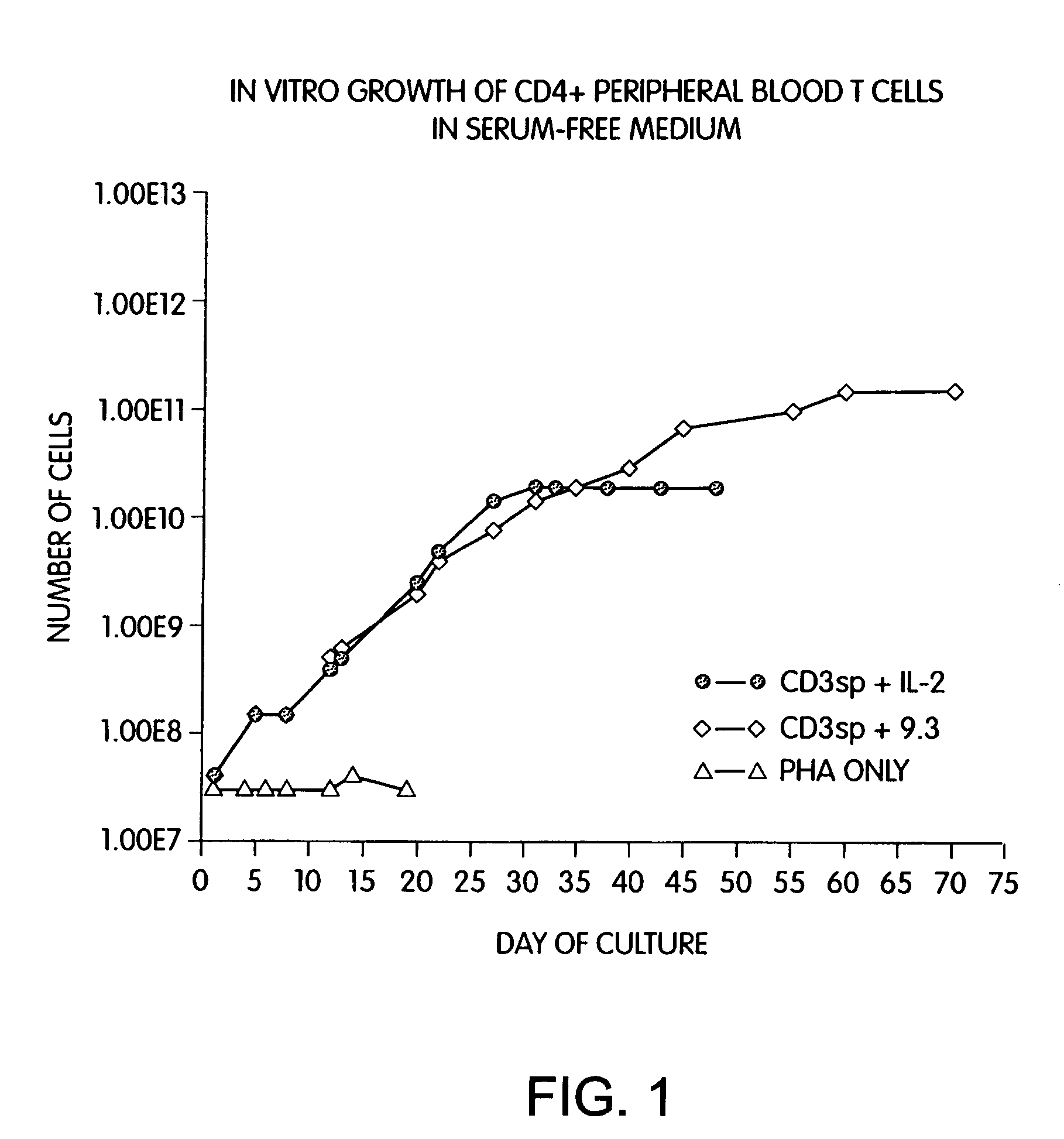
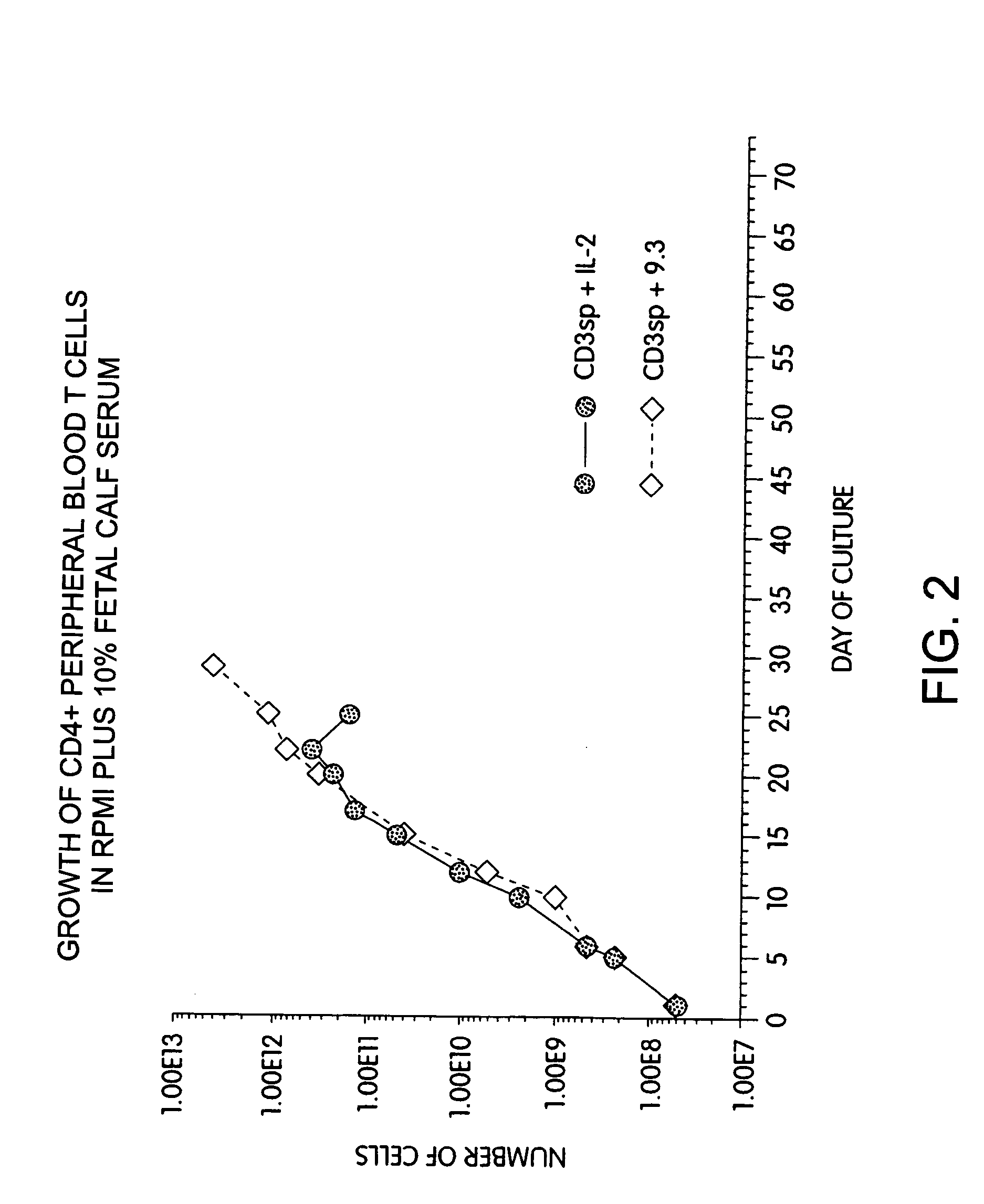
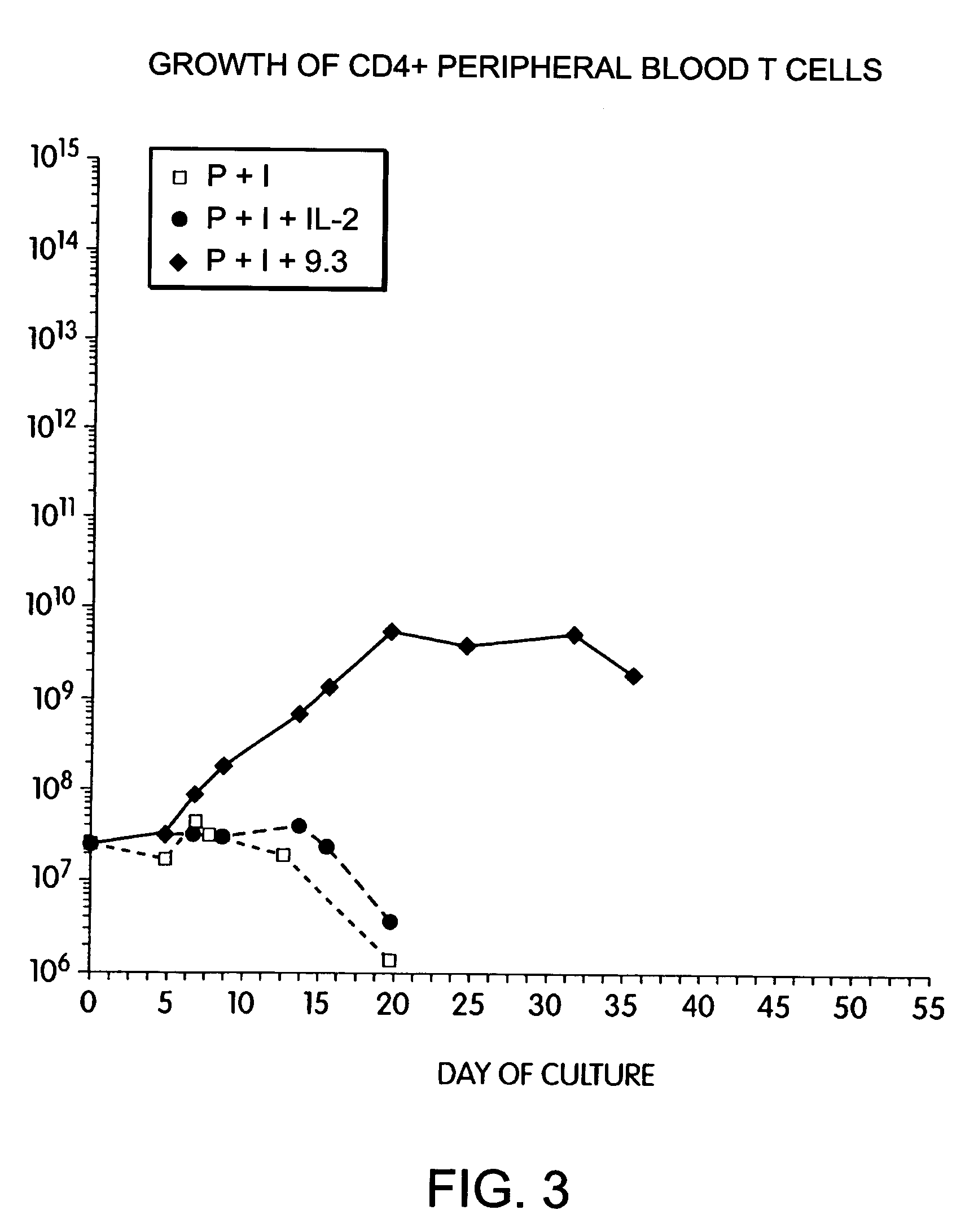
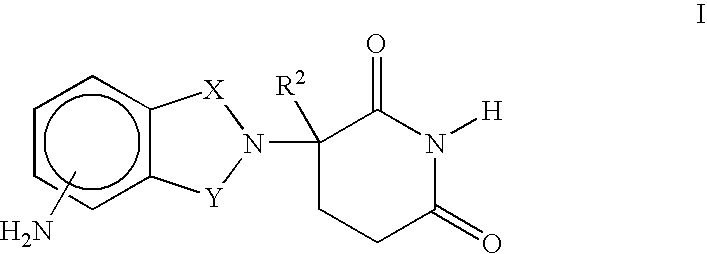
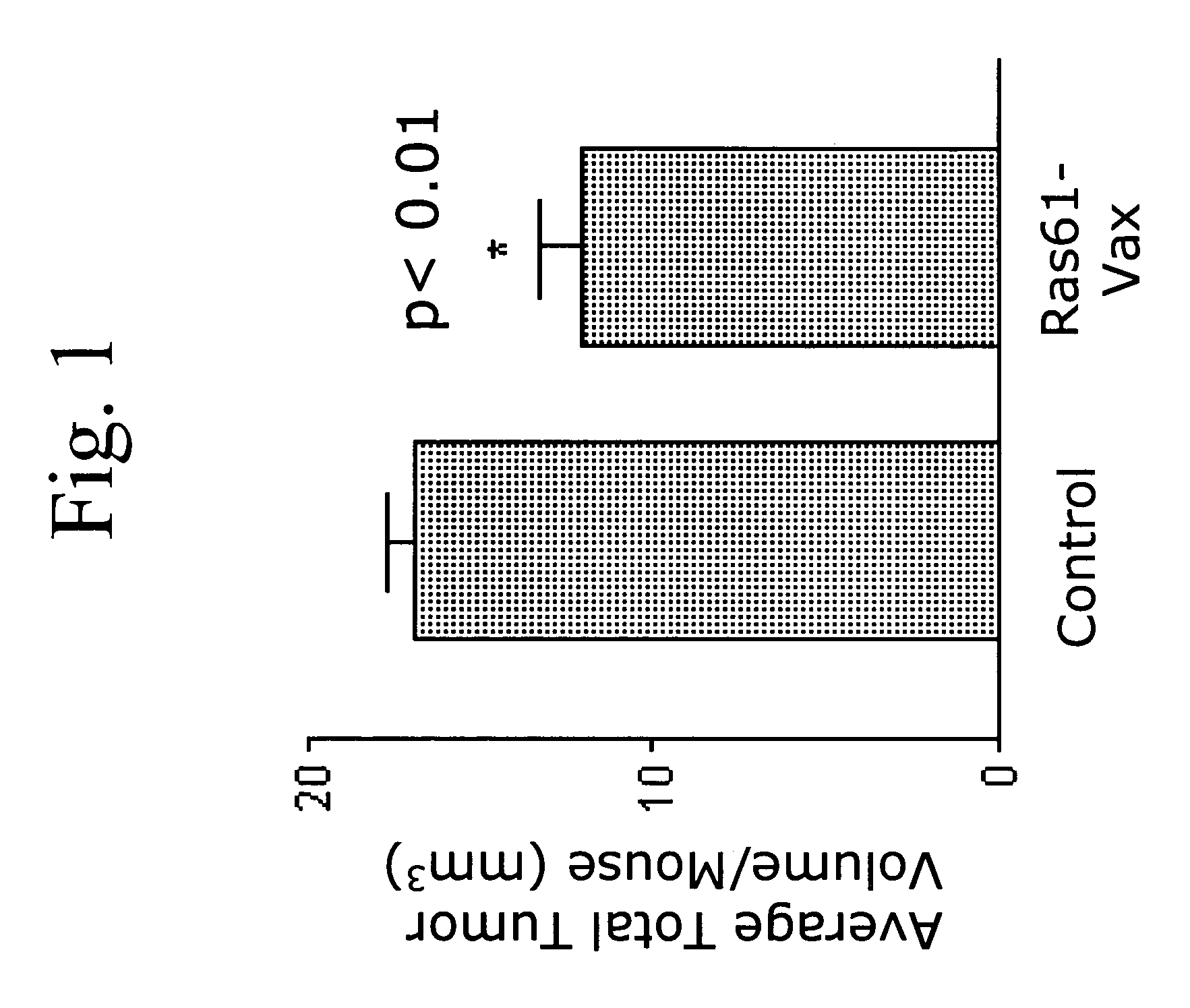
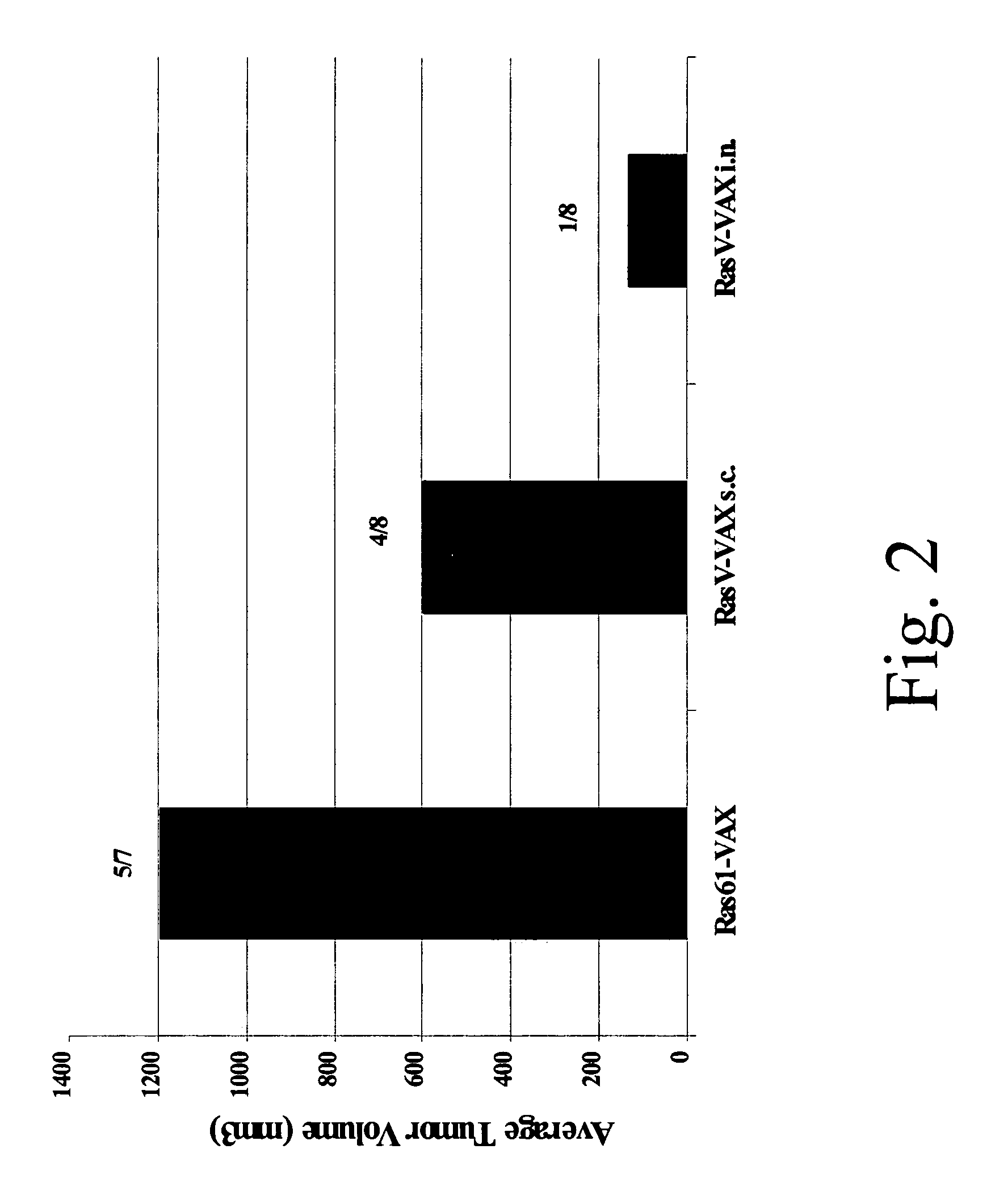
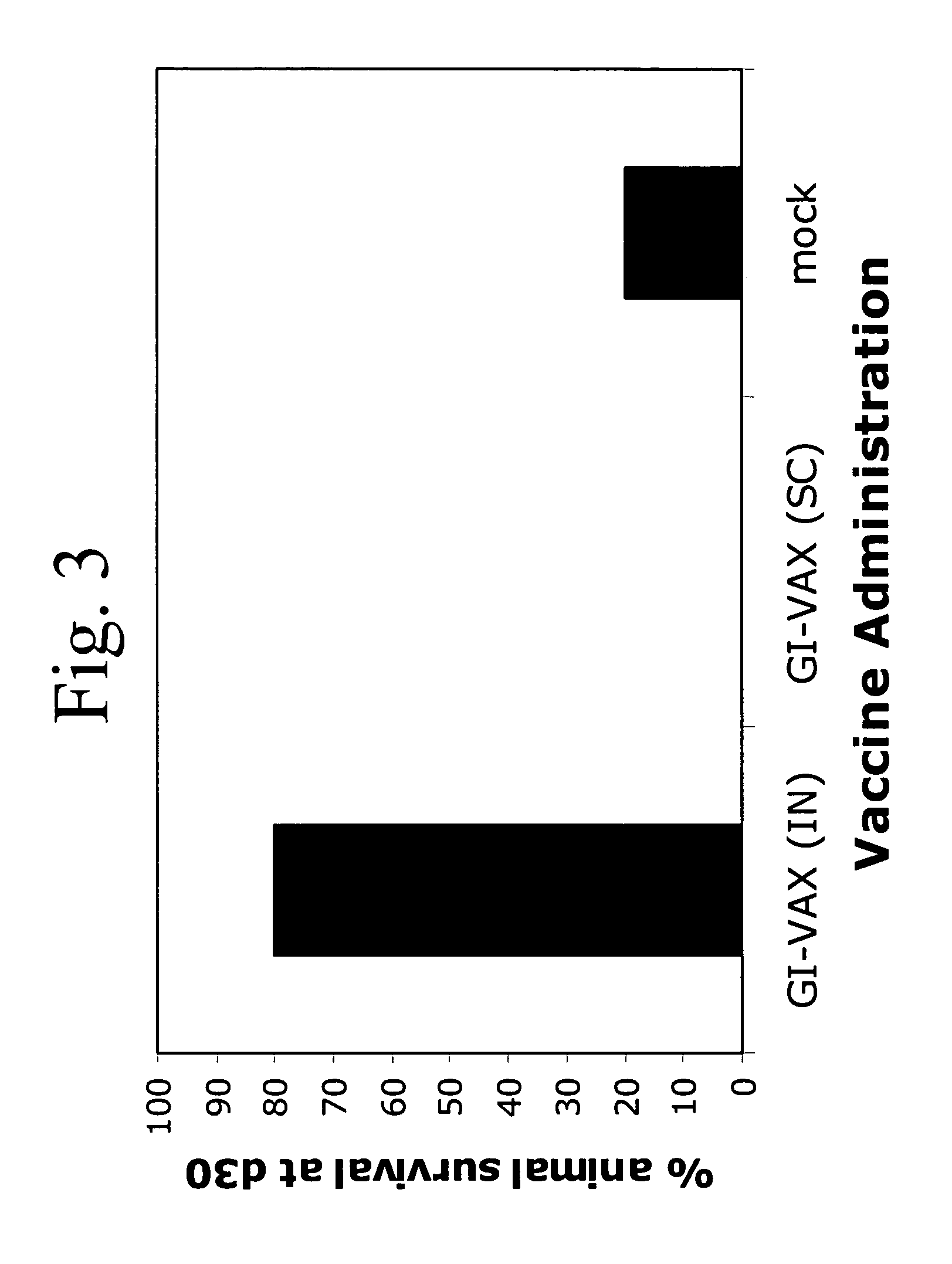
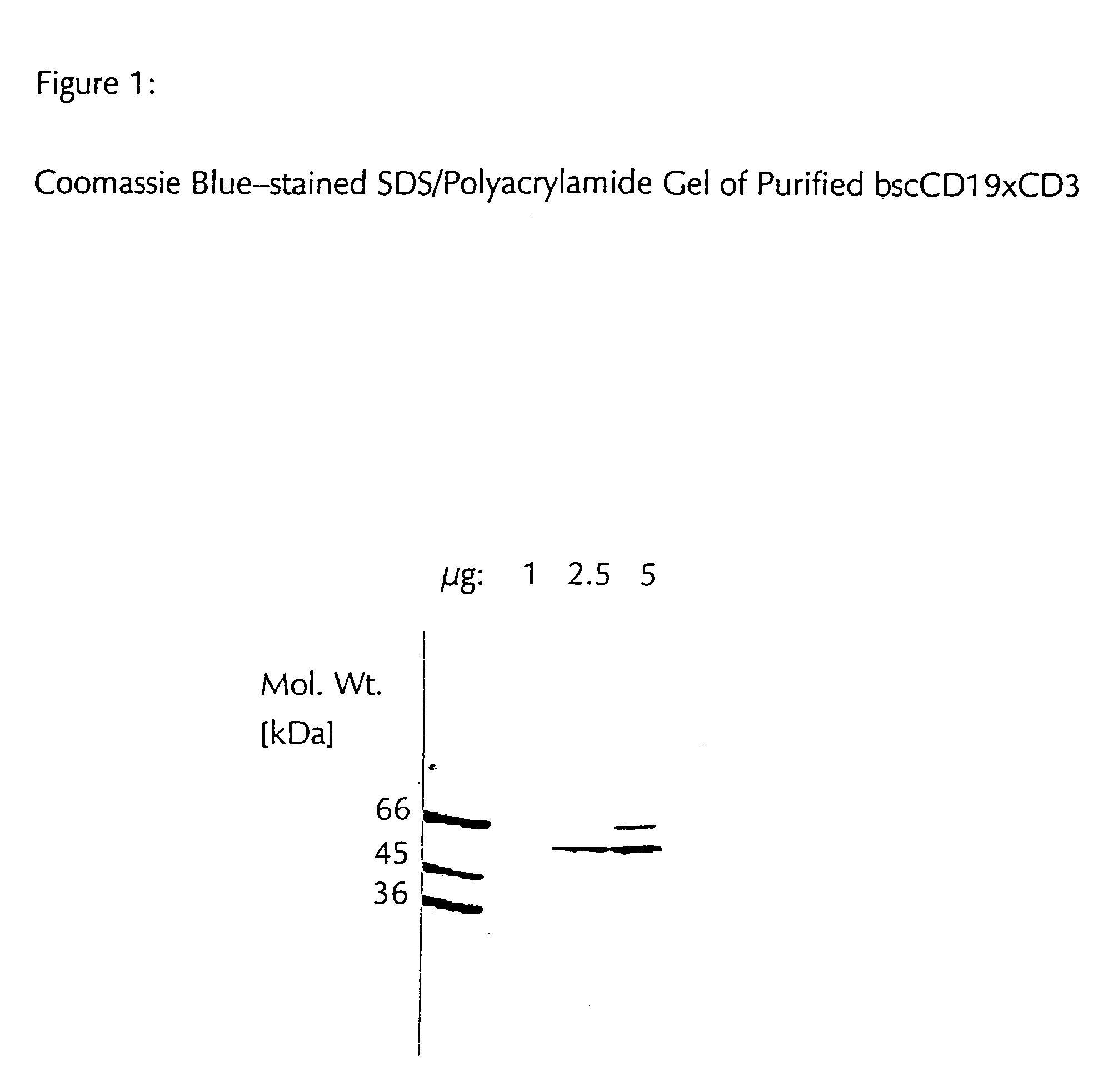
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2435 results about "Immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunotherapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies.
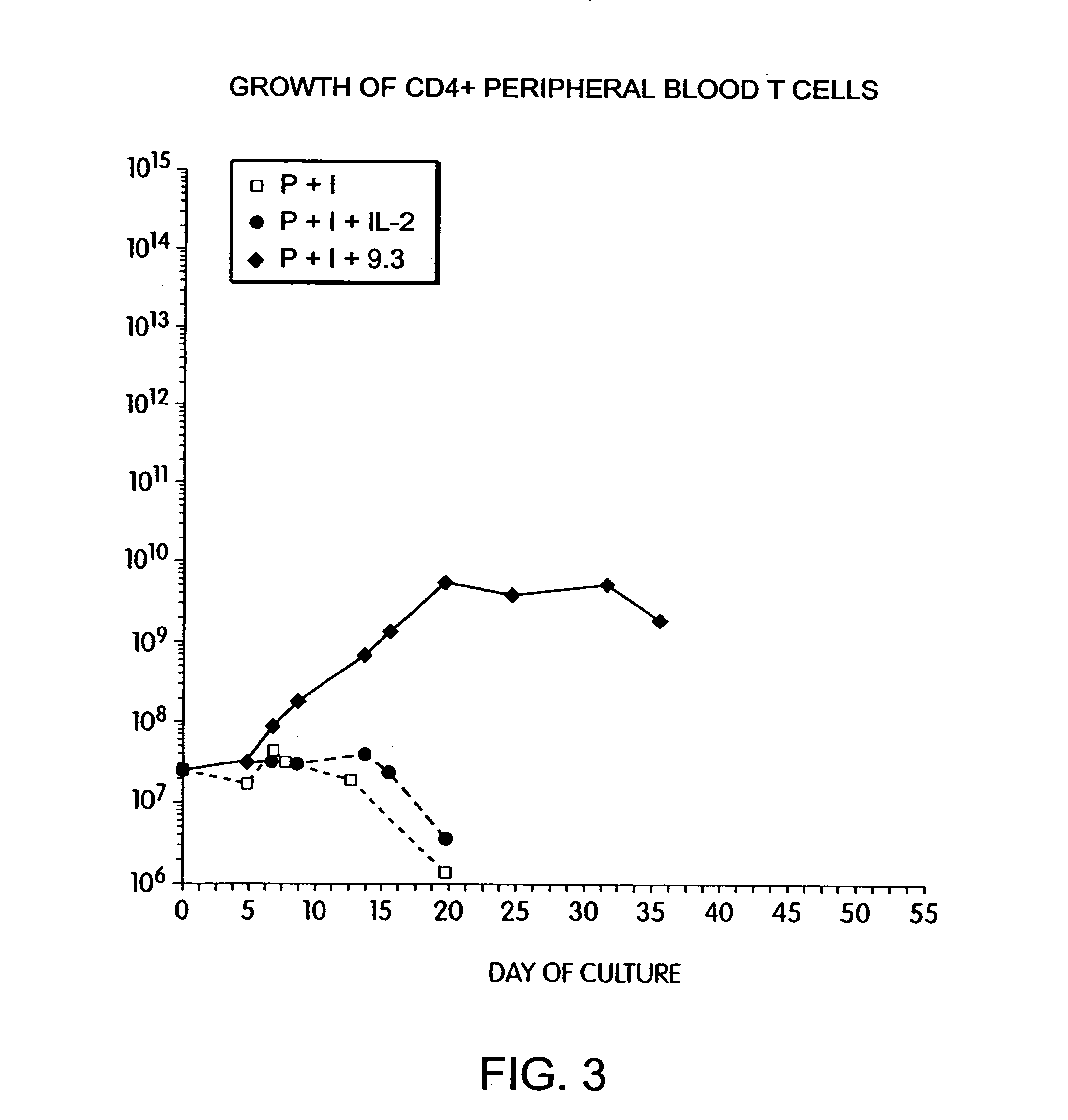
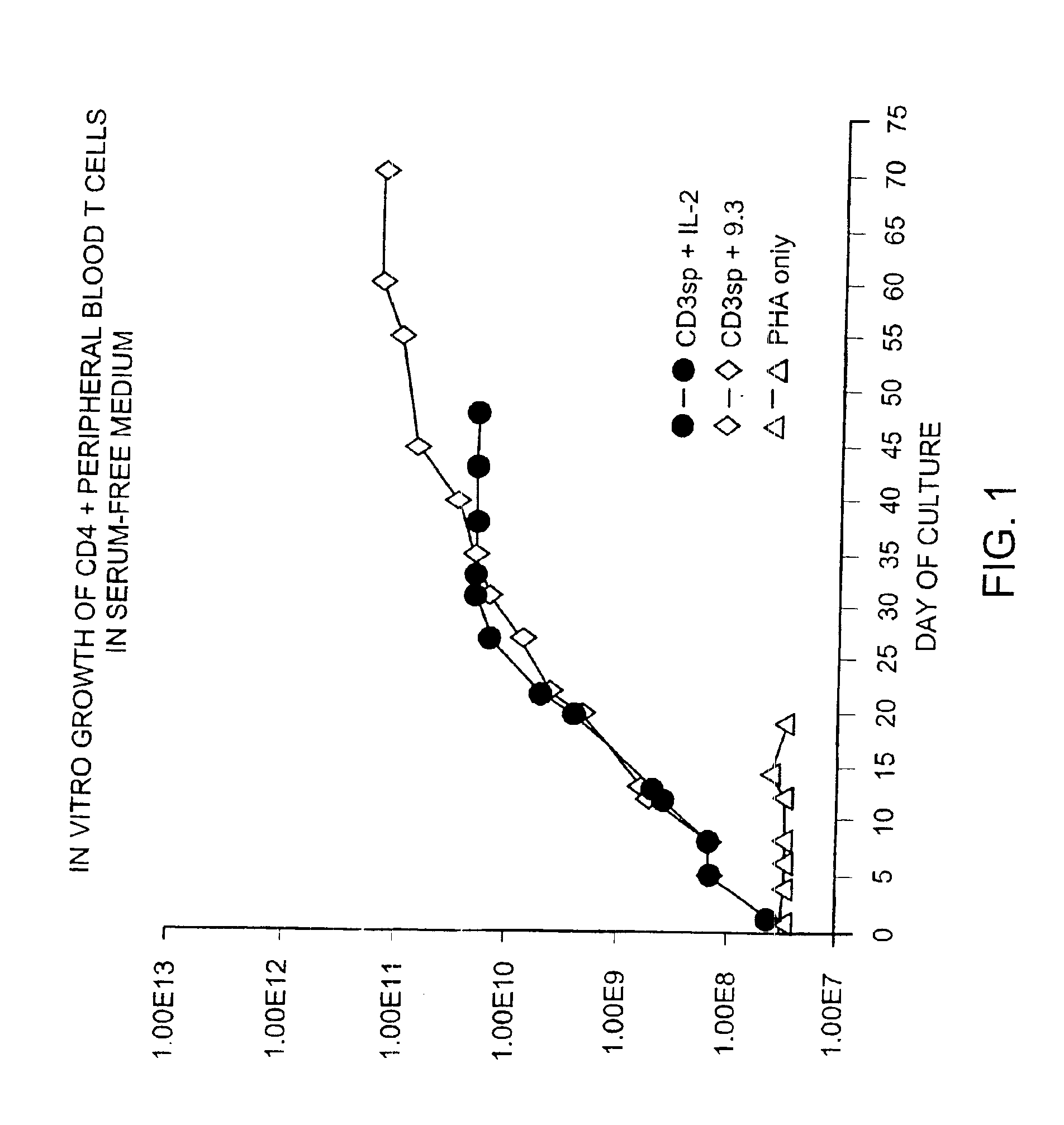
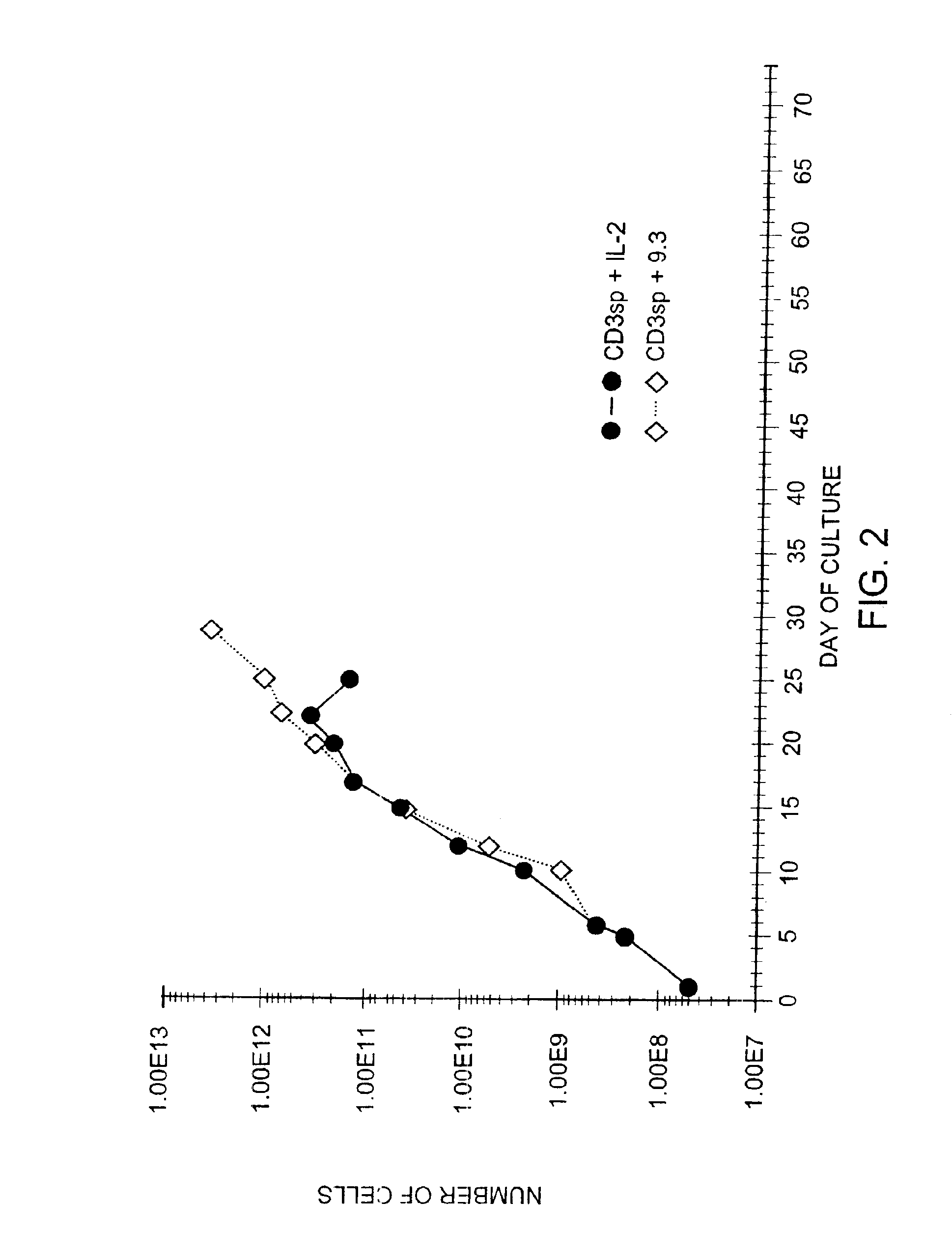
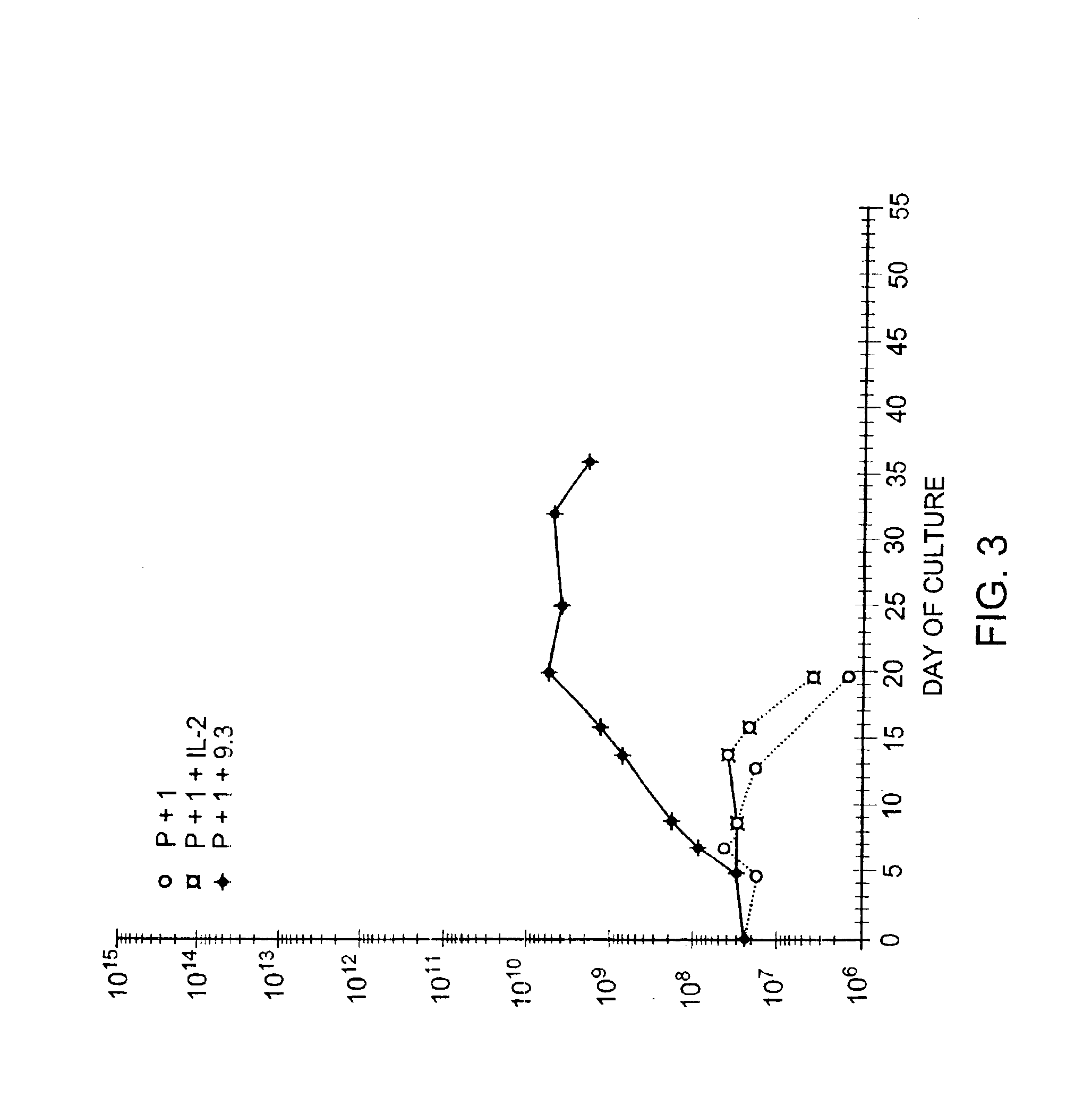
Methods for selectively stimulating proliferation of T cells
InactiveUS6905681B1Increase the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS6887466B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
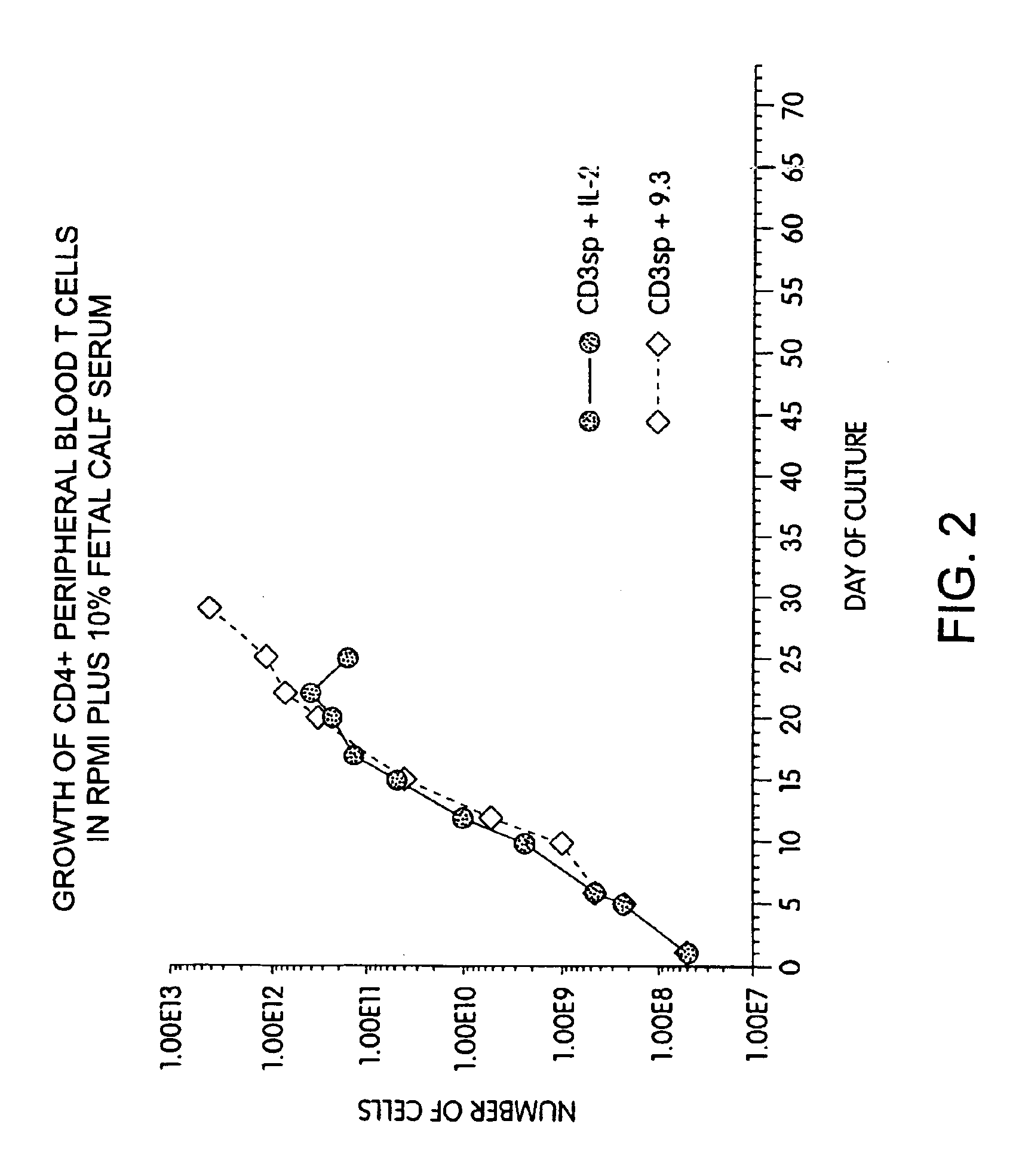
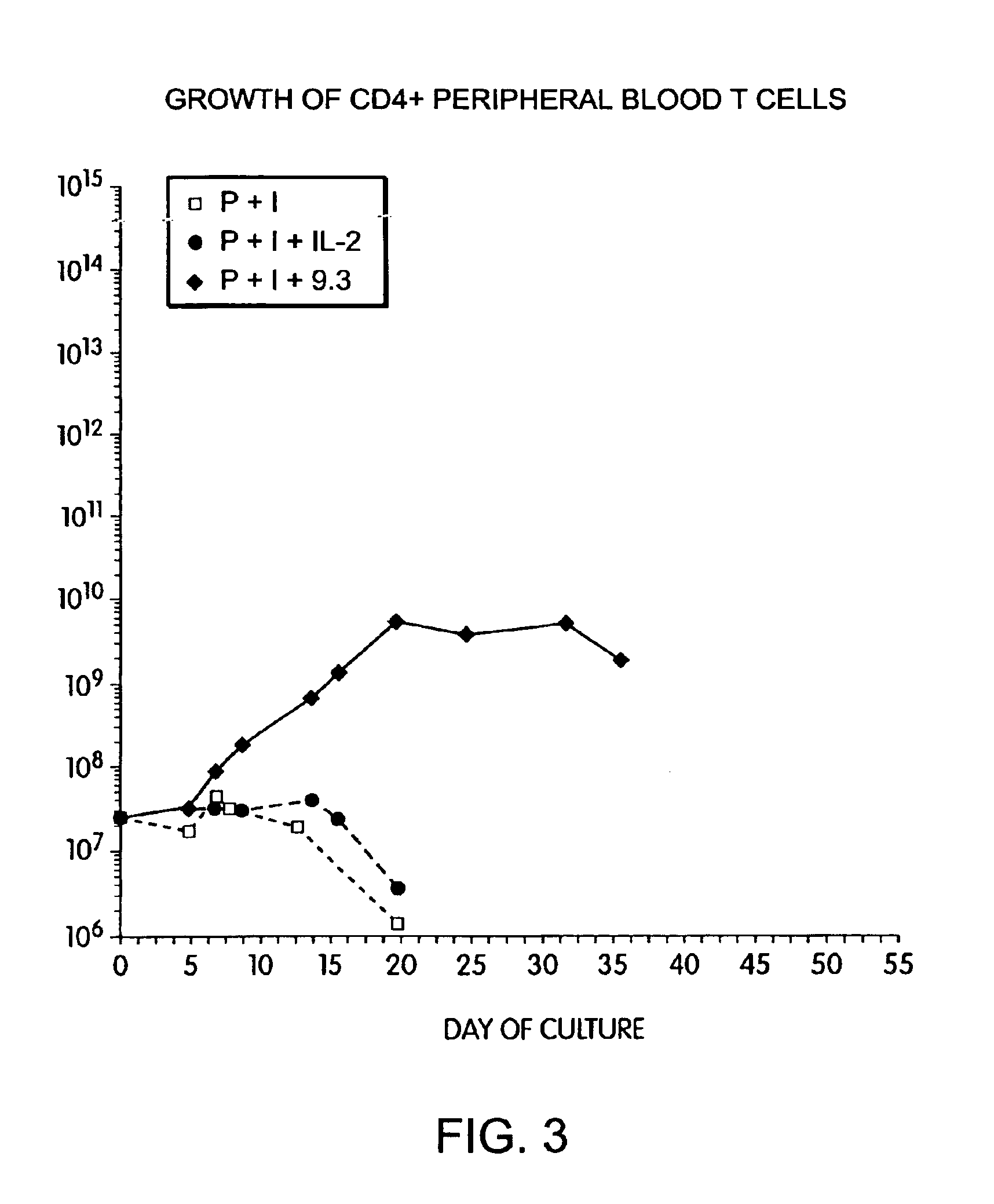
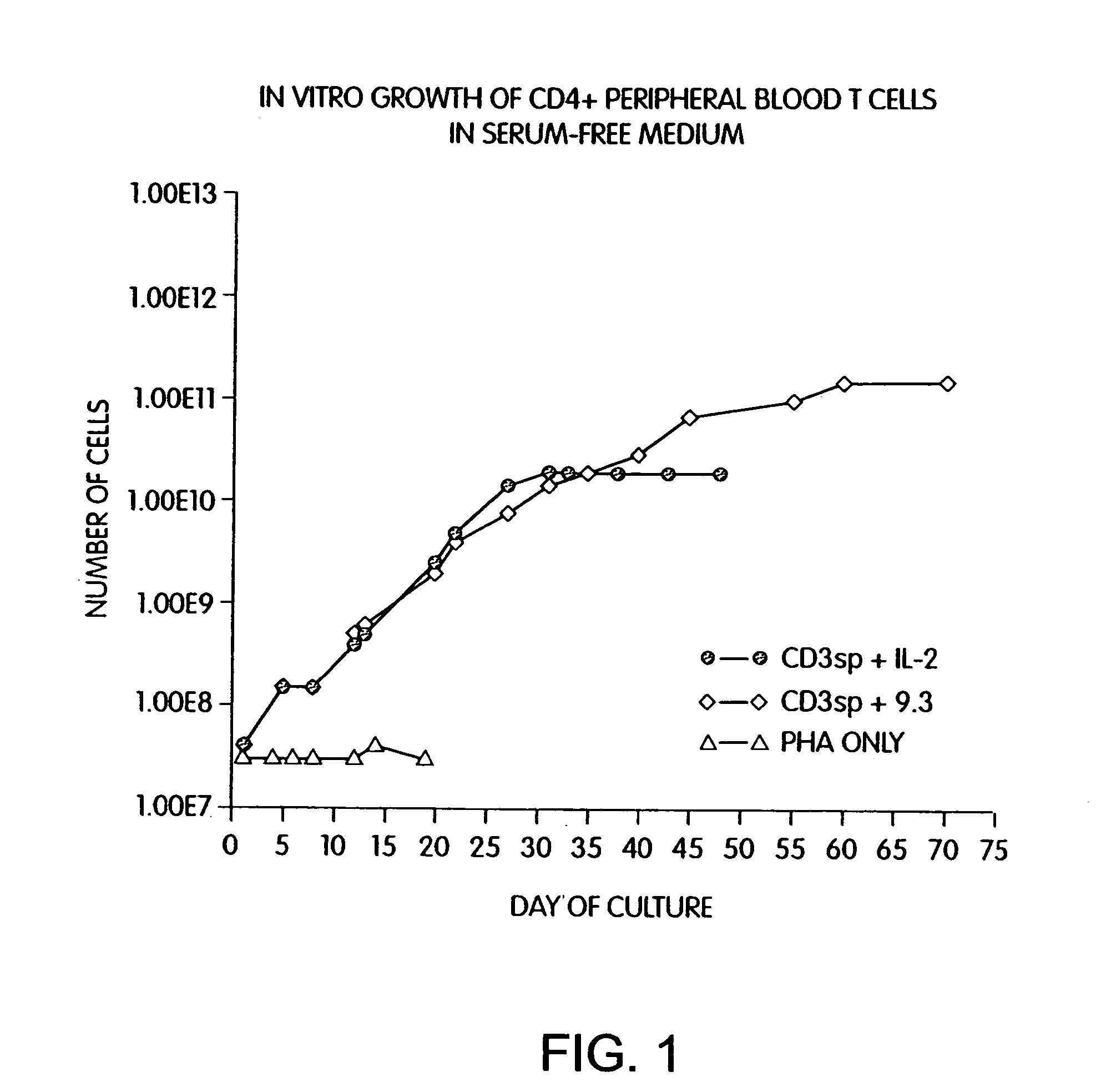
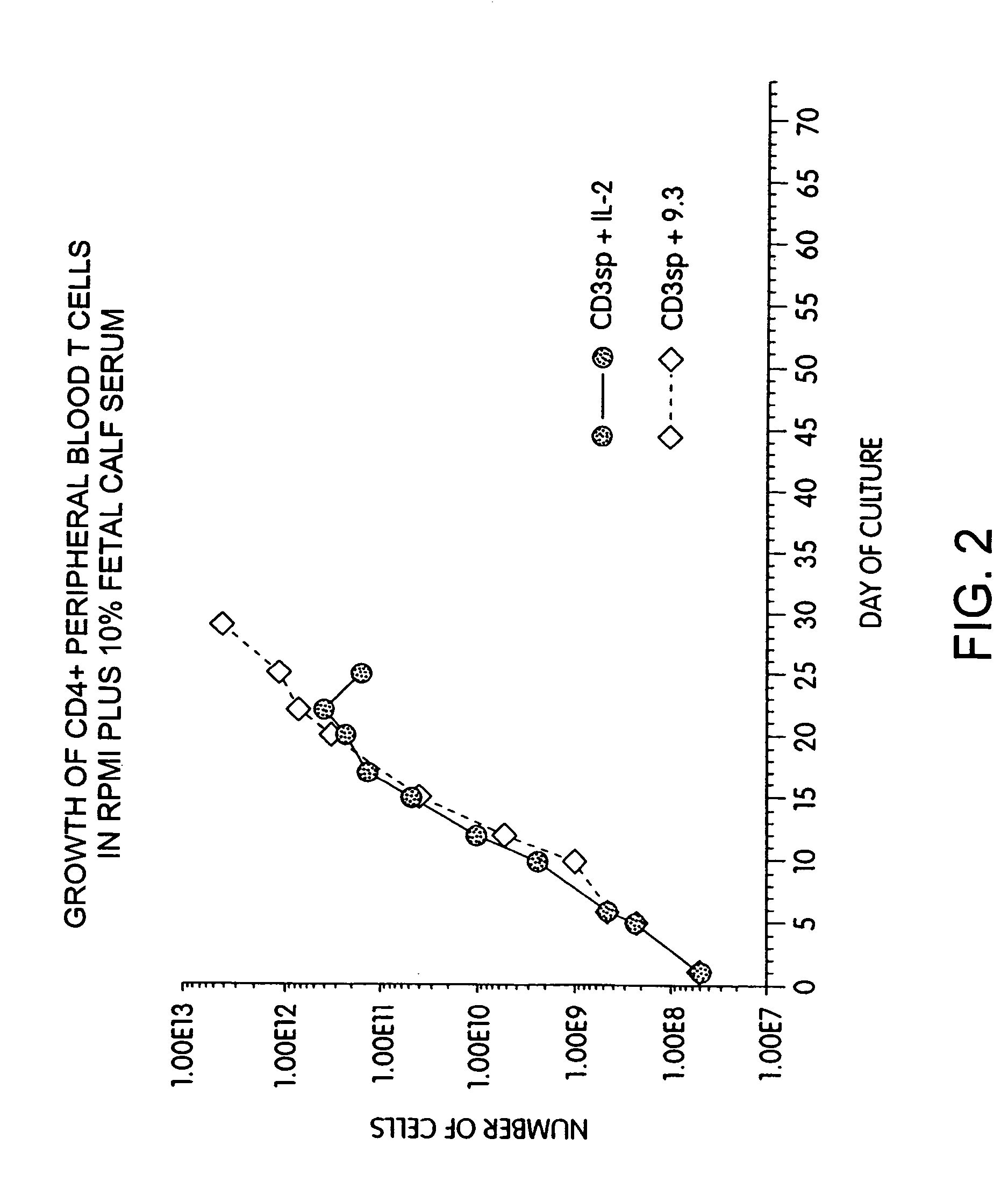
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS7175843B2Increase the number ofBiocideCell receptors/surface-antigens/surface-determinantsAccessory moleculeExogenous growth
Owner:GENETICS INST LLC +2
Methods of treating HIV infected subjects
InactiveUS6905680B2Expanding population of cellIncrease the number ofVirusesPeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:GENETICS INST INC +2
Methods for selectively stimulating proliferation of T cells
InactiveUS7144575B2Increase the number ofBiocidePeptide/protein ingredientsAccessory moleculeExogenous growth
Methods for inducing a population of T cells to proliferate by activating the population of T cells and stimulating an accessory molecule on the surface of the T cells with a ligand which binds the accessory molecule are described. T cell proliferation occurs in the absence of exogenous growth factors or accessory cells. T cell activation is accomplished by stimulating the T cell receptor (TCR) / CD3 complex or the CD2 surface protein. To induce proliferation of an activated population T cells, an accessory molecule on the surface of the T cells, such as CD28, is stimulated with a ligand which binds the accessory molecule. The T cell population expanded by the method of the invention can be genetically transduced and used for immunotherapy or can be used in methods of diagnosis.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY +2
Methods and compositions using immunomodulatory compounds for treatment and management of cancers and other diseases
ActiveUS20040029832A1Prevent proliferationAntibacterial agentsBiocideSide effectBiologically-Based Therapy
Methods of treating, preventing and / or managing cancer as well as and diseases and disorders associated with, or characterized by, undesired angiogenesis are disclosed. Specific methods encompass the administration of an immunomodulatory compound alone or in combination with a second active ingredient. The invention further relates to methods of reducing or avoiding adverse side effects associated with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy which comprise the administration of an immunomodulatory compound. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Owner:GLAXO SMITHKLINE LLC
Radioimmunotherapy of lymphoma using anti-CD20 antibodies
Methods for the treatment of lymphoma by administration of a B cell-specific antibody are described. The invention encompasses providing to a patient both unlabeled antibodies and antibodies labeled with a radioisotope. A principal advantage of the method is that tumor responses can be obtained in a radiometric dose range that does not require hematopoietic stem cell replacement as an adjunct therapy also described is a composition useful in the treatment of lymphoma.
Owner:RGT UNIV OF MICHIGAN +2
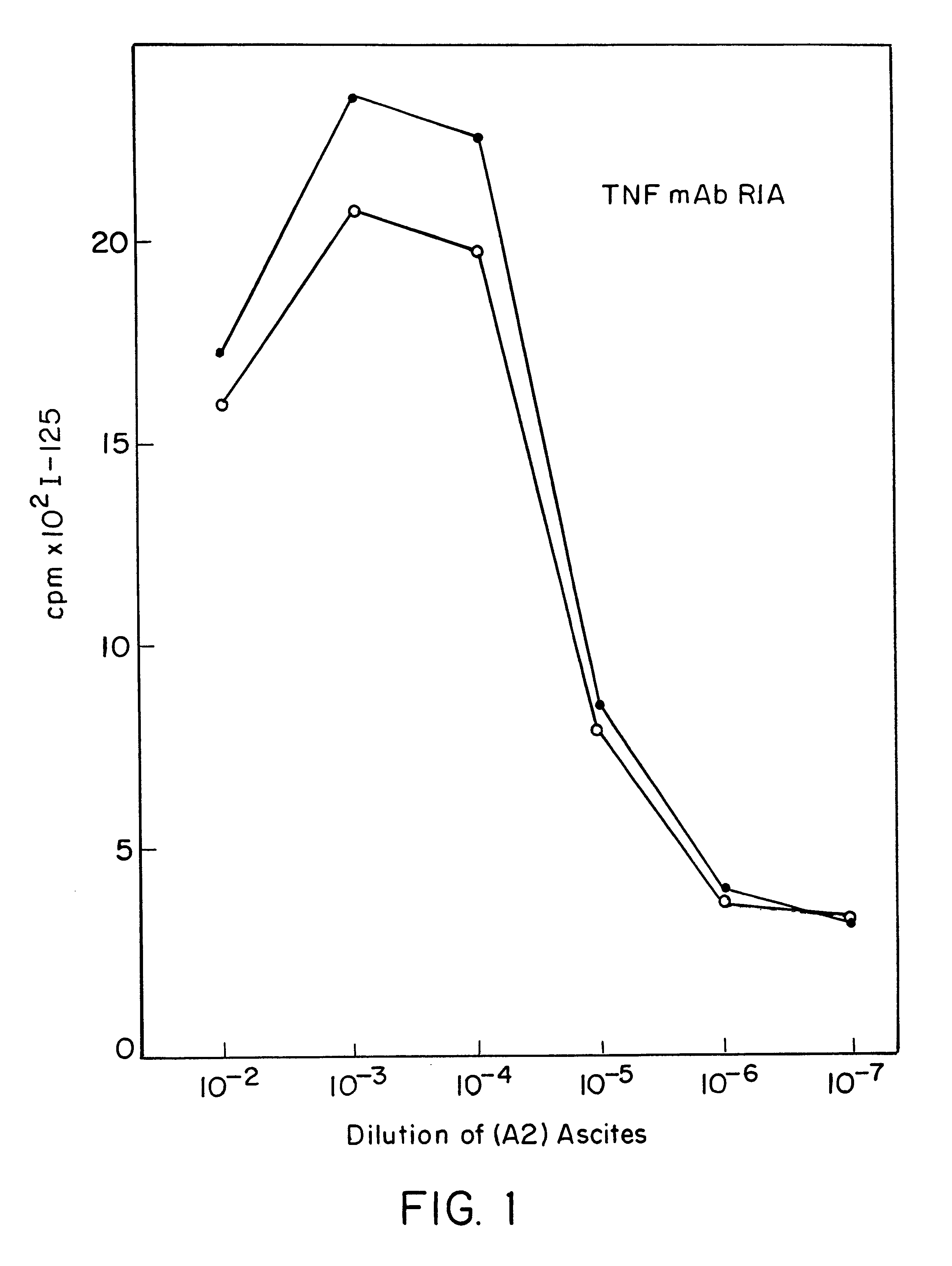
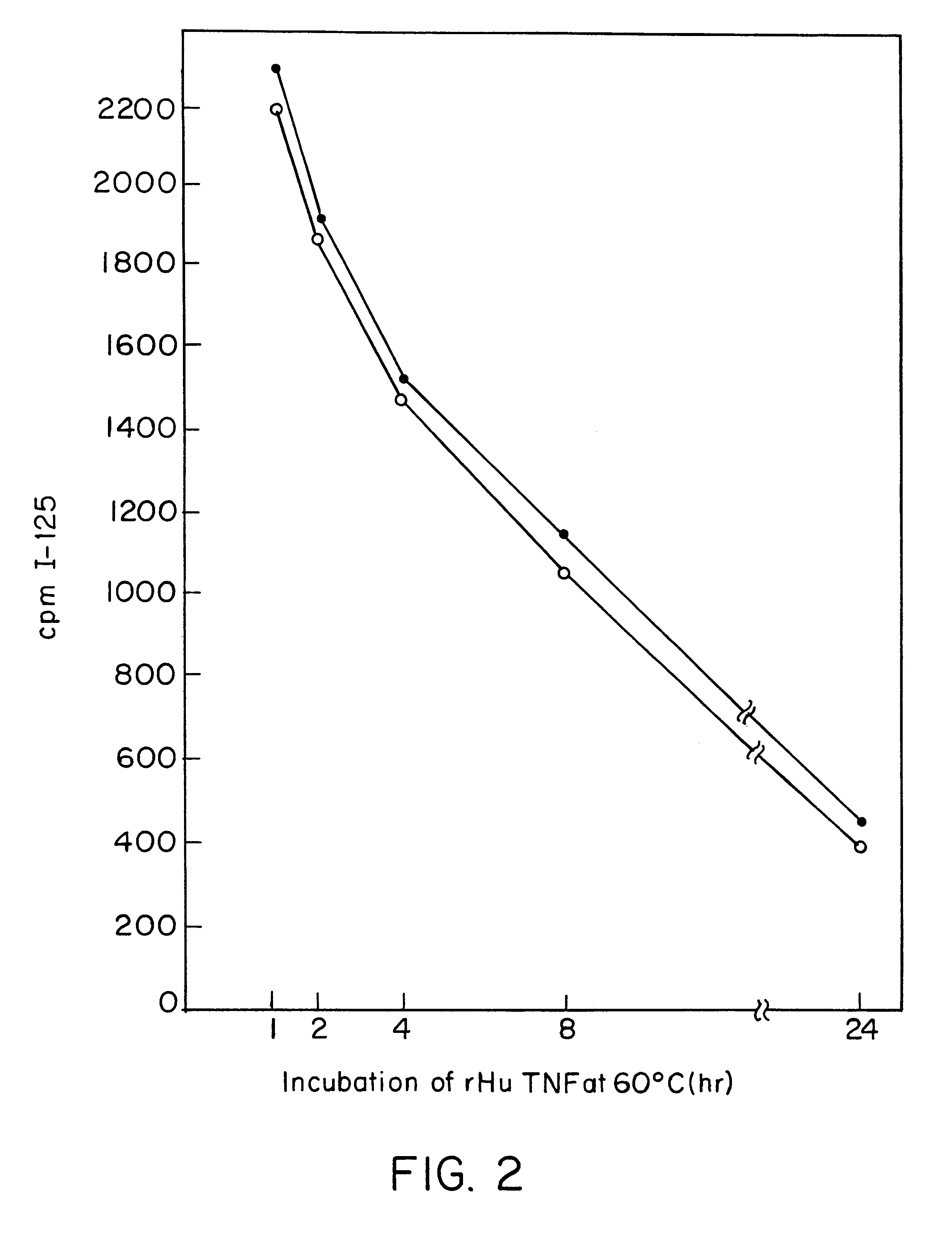
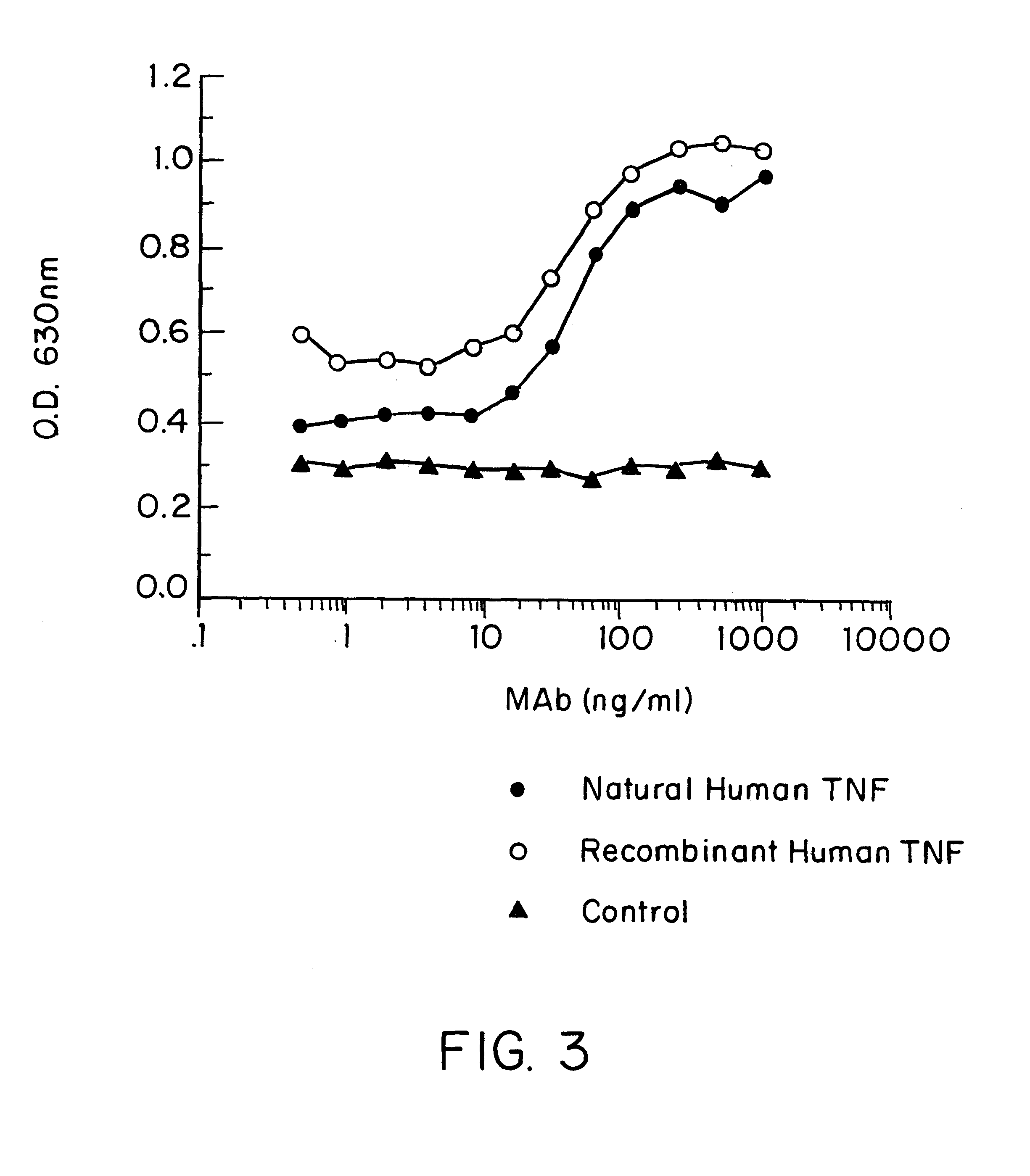
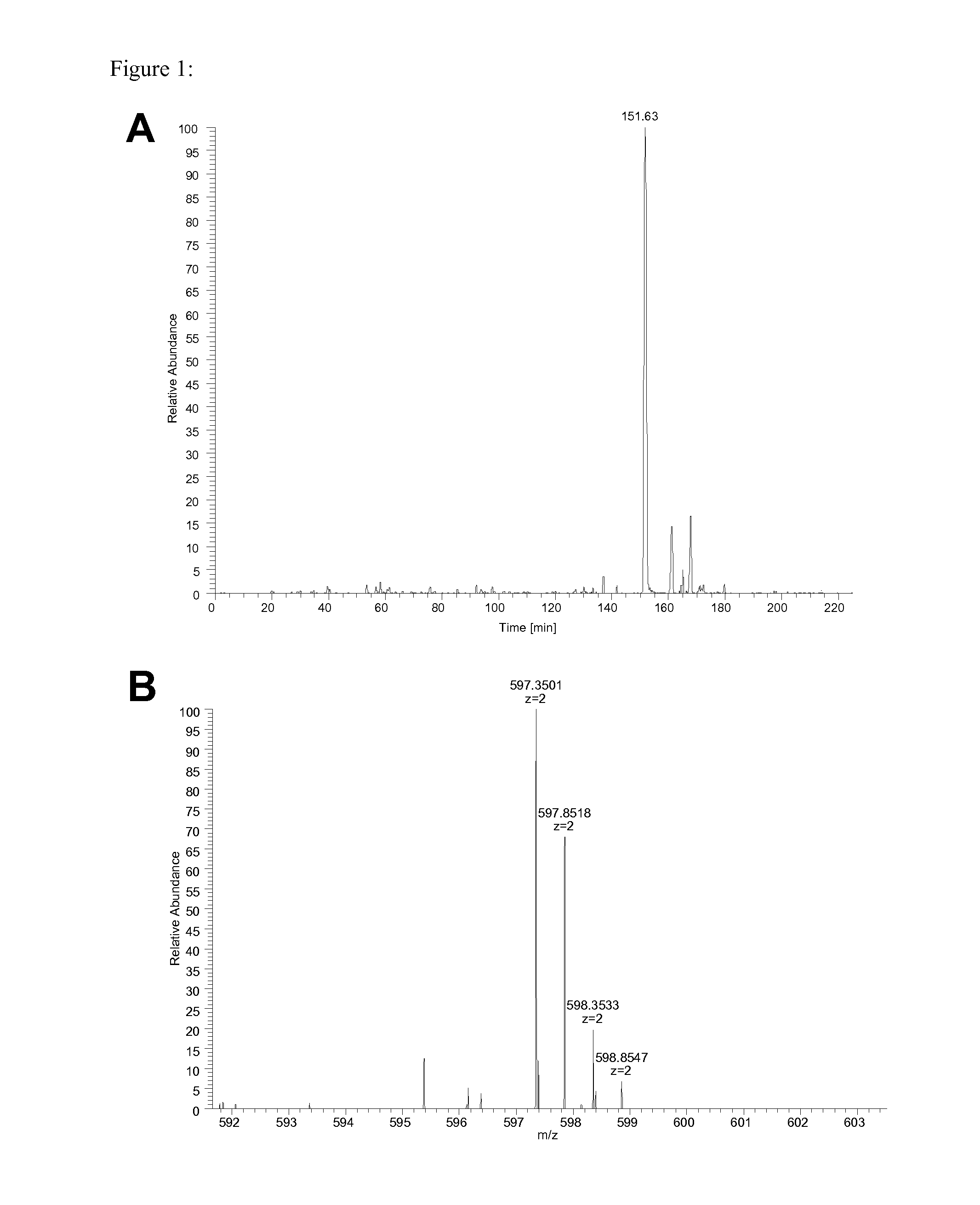
Anti-TNF antibodies and peptides of human tumor necrosis factor
Anti-TNF antibodies, fragments and regions thereof which are specific for human tumor necrosis factor-alpha (TNFalpha) and are useful in vivo diagnosis and therapy of a number of TNFalpha-mediated pathologies and conditions, as well as polynucleotides coding for murine and chimeric antibodies, methods of producing the antibody, methods of use of the anti-TNF antibody, or fragment, region or derivative thereof, in immunoassays and immunotherapeutic approaches are provided.
Owner:NEW YORK UNIV +1
Method for differentially quantifying naturally processed hla-restricted peptides for cancer, autoimmune and infectious diseases immunotherapy development
ActiveUS20130096016A1Efficient use ofBiological material analysisLibrary member identificationDiseaseAntigen
The invention relates to a method for quantitatively identifying relevant HLA-bound peptide antigens from primary tissue specimens on a large scale without labeling approaches. This method can not only be used for the development of peptide vaccines, but is also highly valuable for a molecularly defined immunomonitoring and the identification of new antigens for any immunotherapeutic strategy in which HLA-restricted antigenic determinants function as targets, such as a variety of subunit vaccines or adoptive T-cell transfer approaches in cancer, or infectious and autoimmune diseases.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
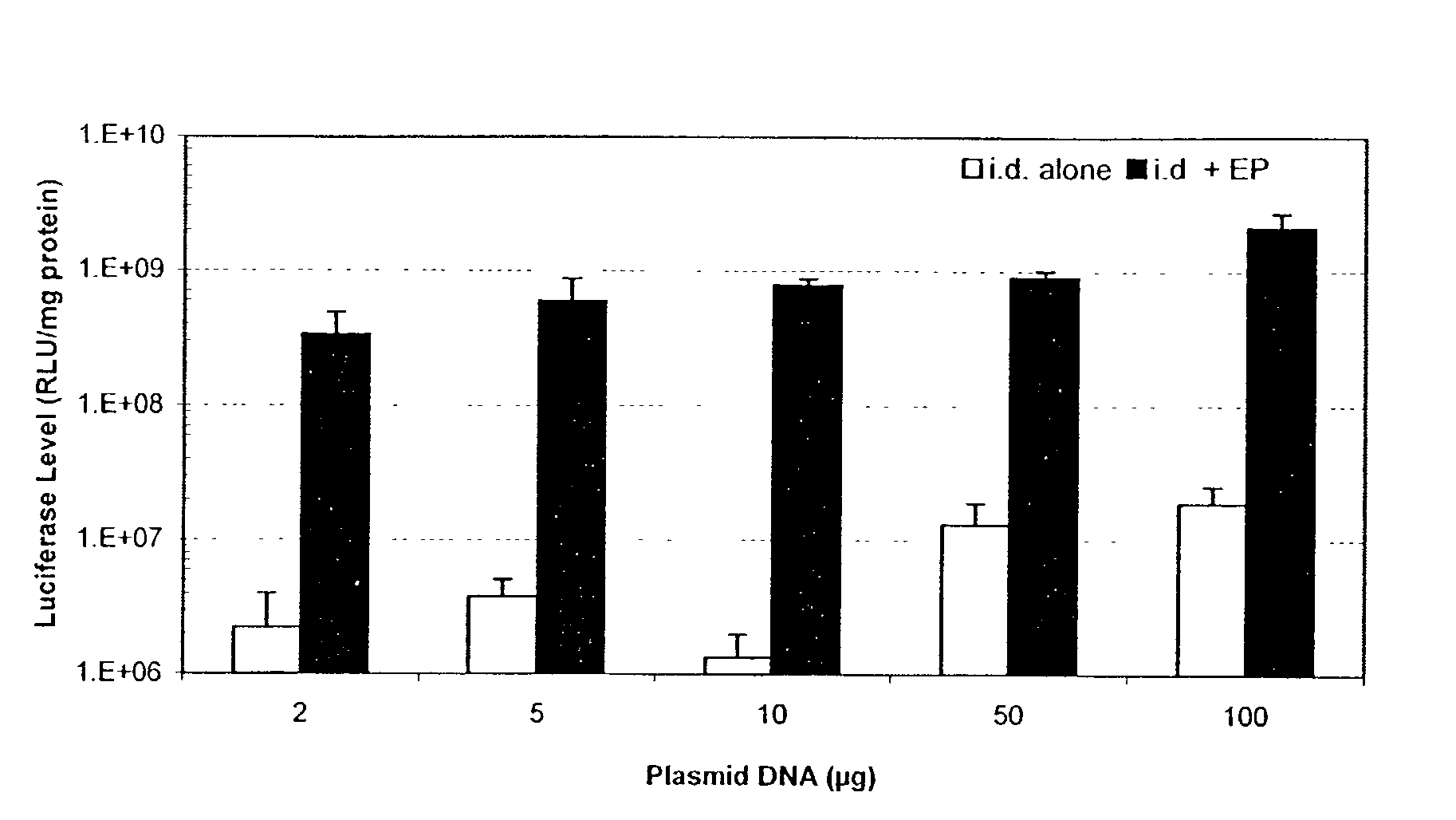
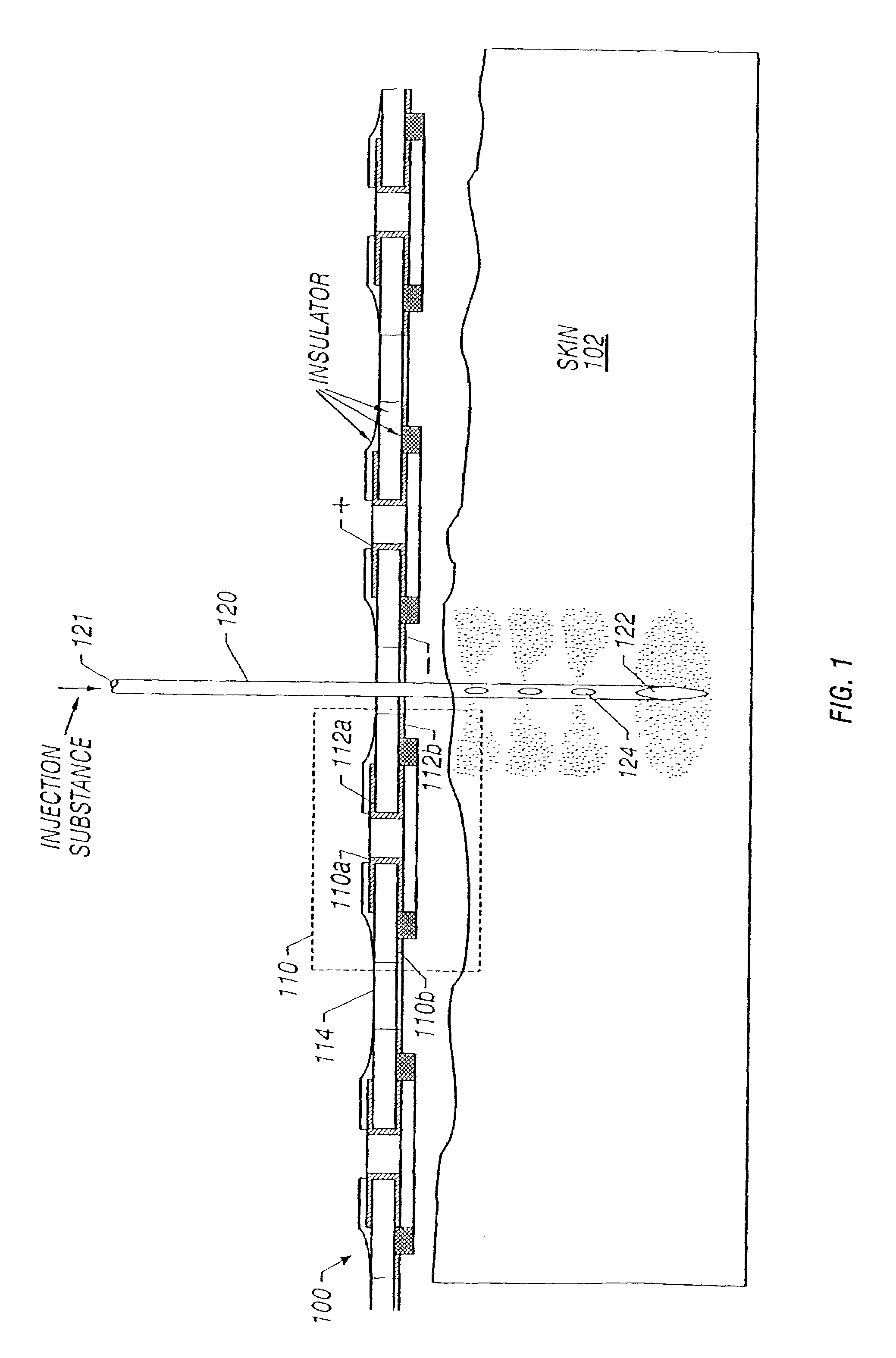
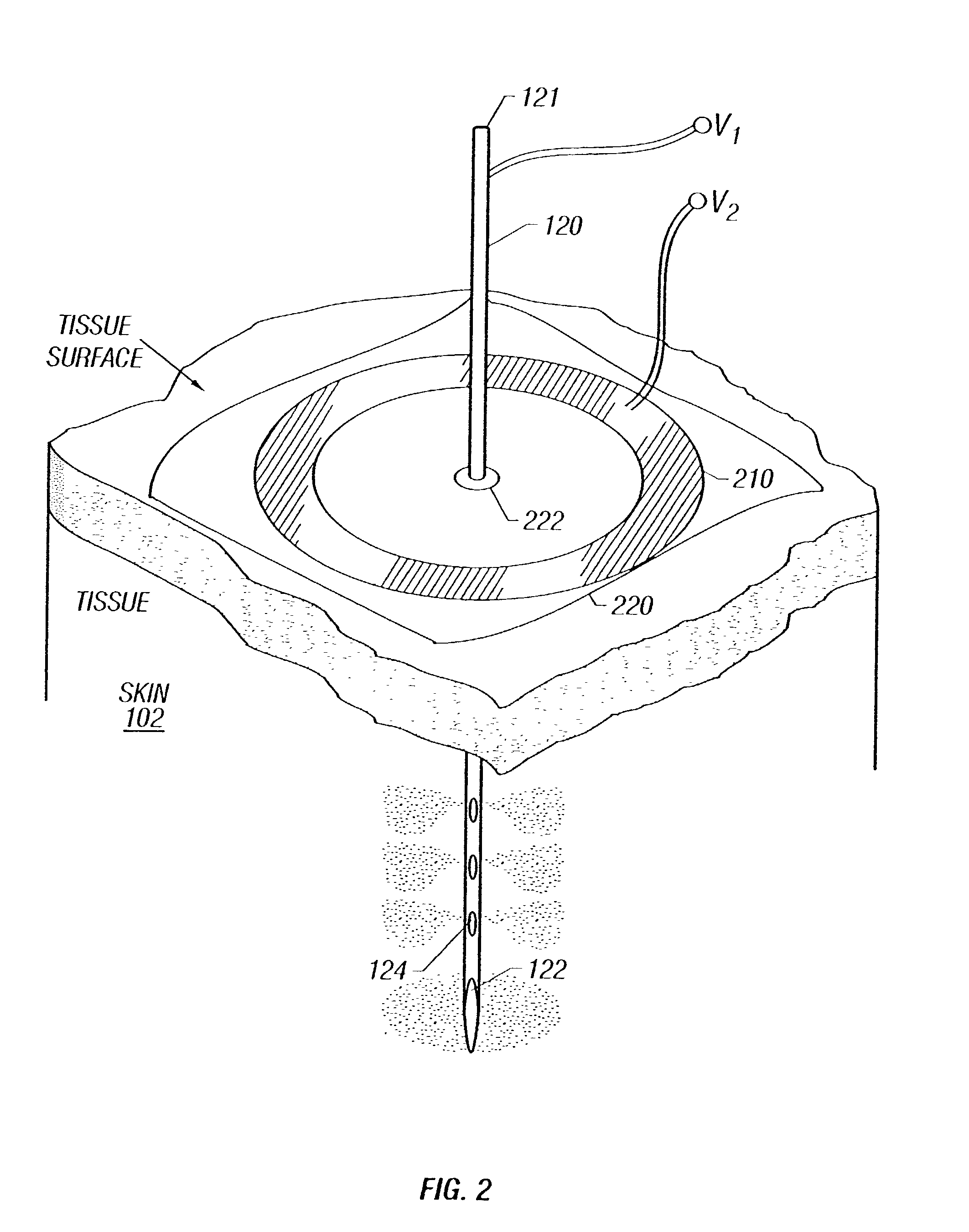
Enhanced delivery of naked DNA to skin by non-invasive in vivo electroporation
InactiveUS6972013B1Formidable physical barrier to gene transferHigh expressionElectrotherapyMedical devicesWhole bodyIn vivo
In vivo methods are provided for using an electric field to delivery therapeutic or immunizing treatment to a subject by applying non-invasive, user-friendly electrodes to the surface of the skin. Thus, therapeutic or immunizing agents can be delivered into cells of skin for local and systemic treatments or for immunization with optimal gene expression and minimal tissue damage. In particular, therapeutic agents include naked or formulated nucleic acid, polypeptides and chemotherapeutic agents.
Owner:INOVIO PHARMA
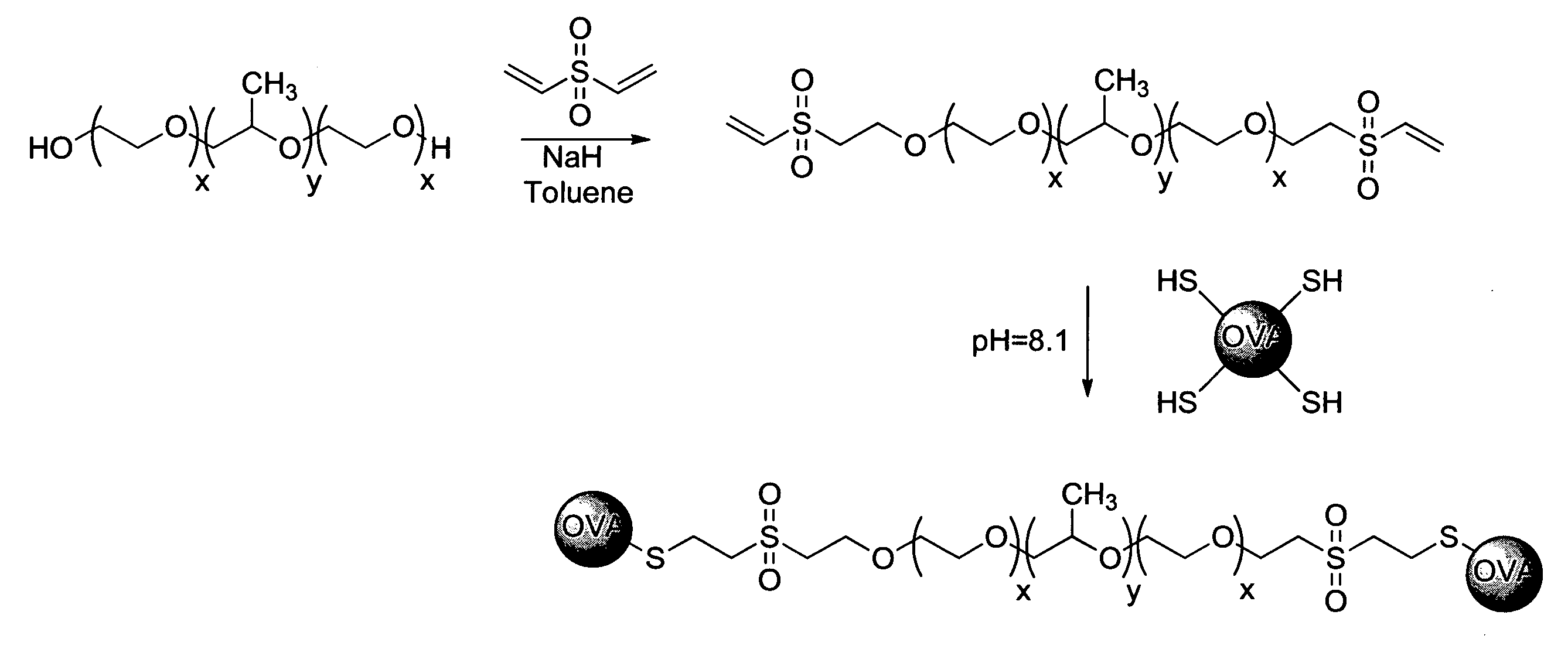
Nanoparticles for immunotherapy
ActiveUS20080031899A1High expressionPowder deliveryCarrier-bound antigen/hapten ingredientsNanoparticleImmunotherapy
Nanoparticles that activate complement in the absence of biological molecules are described. The nanoparticles are shown to specifically target antigen presenting cells in specifically in lymph nodes, without the use of a biological molecule for targeting. These particles are useful vehicles for delivering immunotherapeutics.
Owner:ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL)
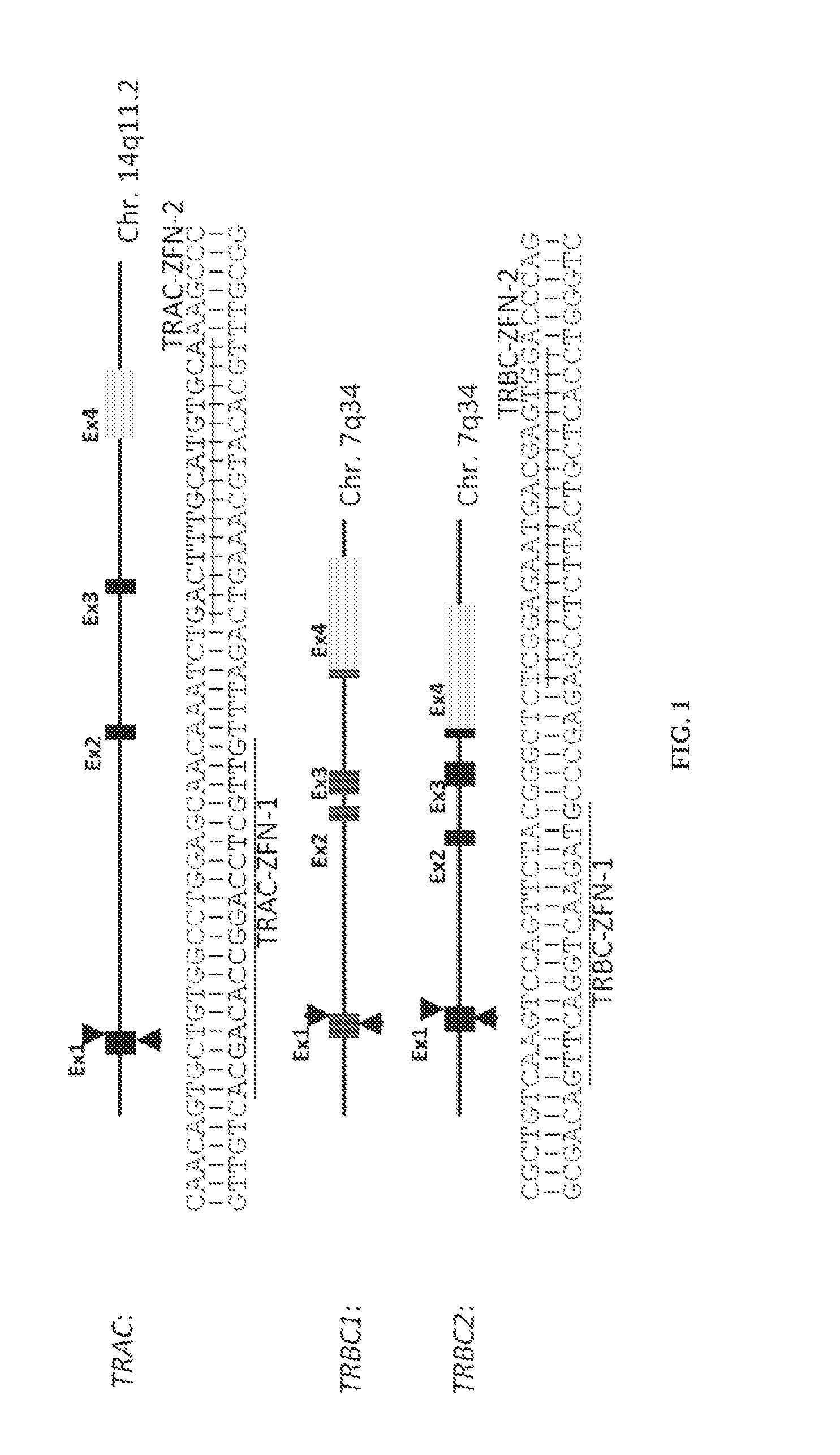
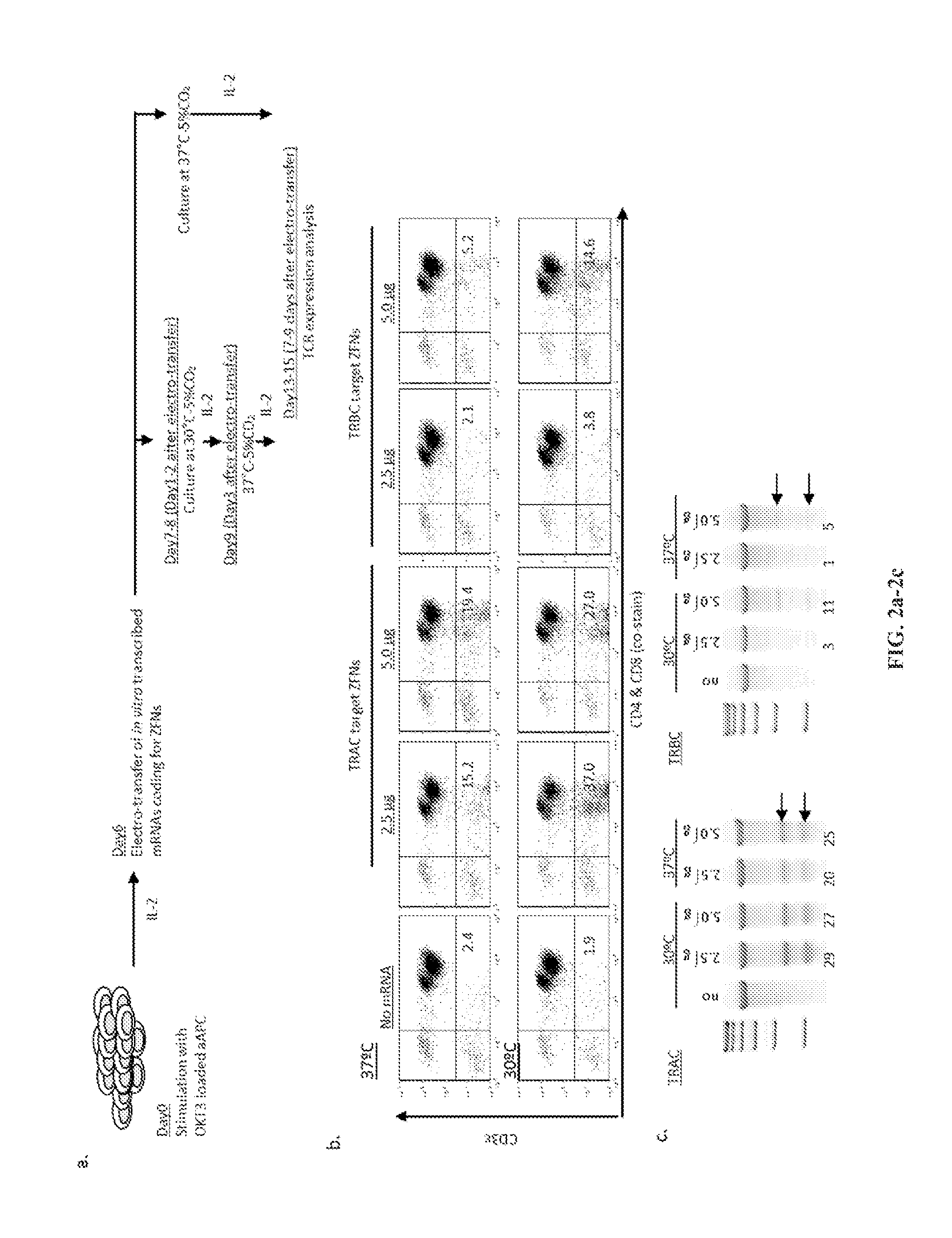
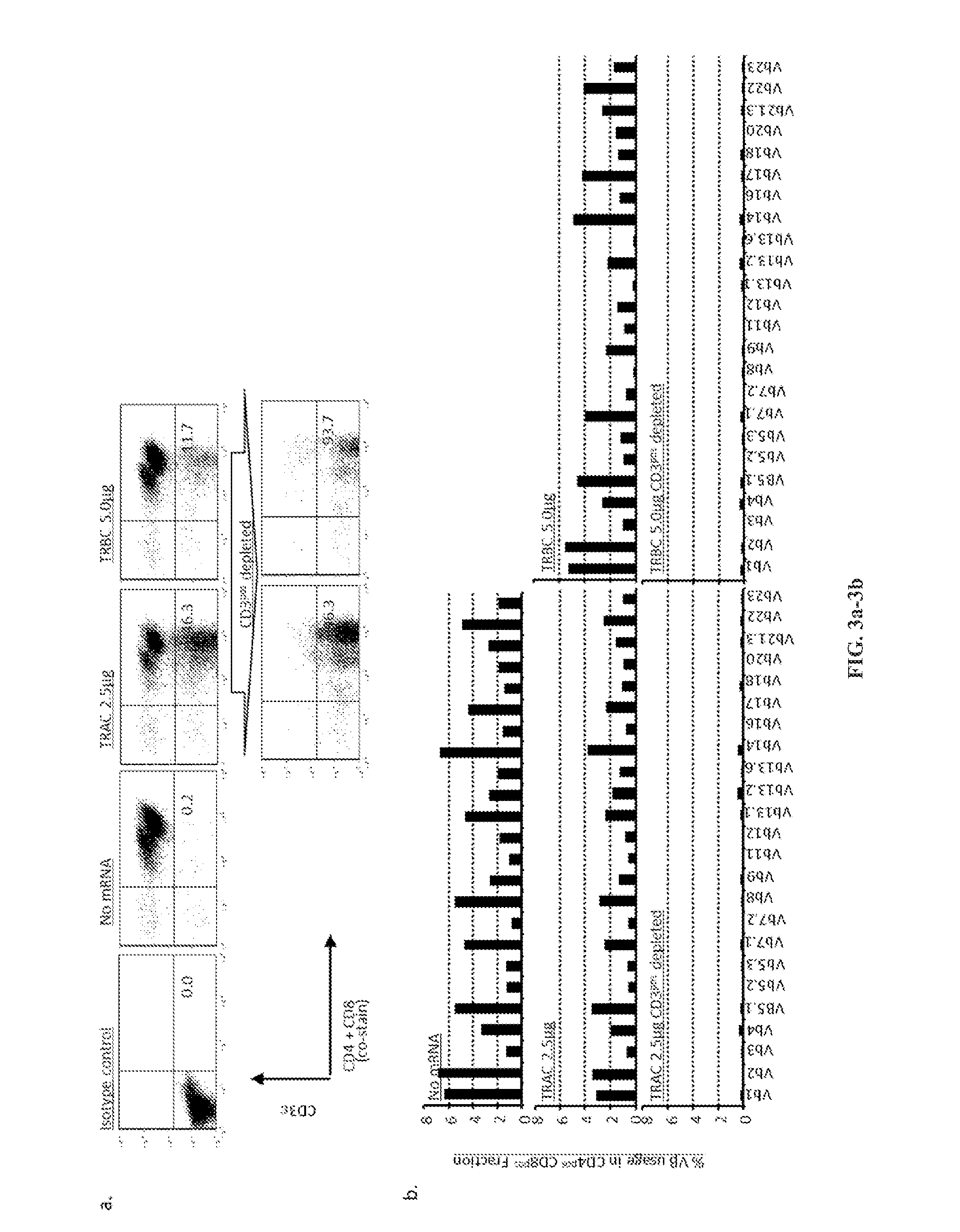
Car+ t cells genetically modified to eliminate expression of t-cell receptor and/or HLA
ActiveUS20140349402A1Suitable for storageReduce/eliminate T cellsGenetically modified cellsMammal material medical ingredientsAntigen receptorsAutoimmunity
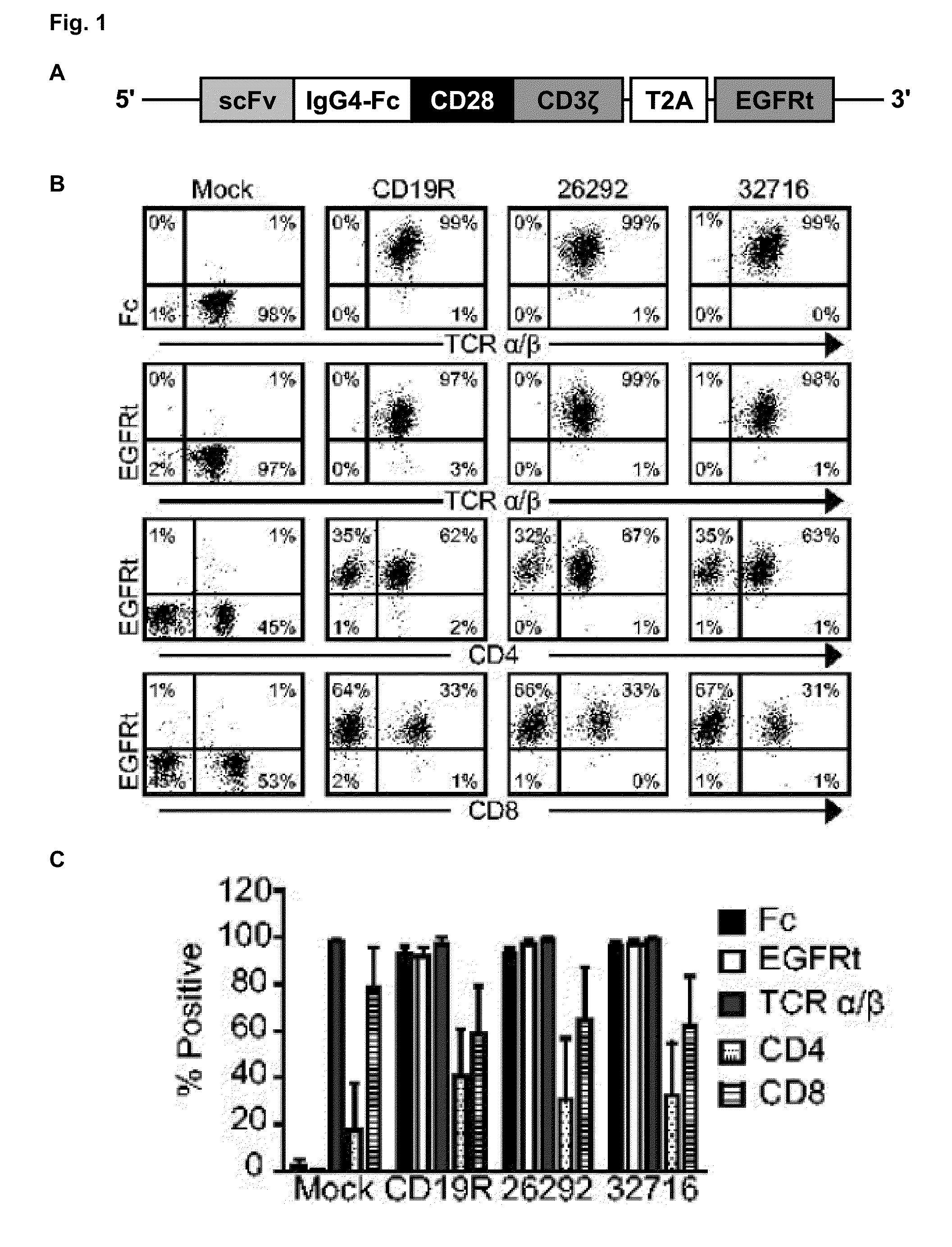
The present invention concerns methods and compositions for immunotherapy employing a modified T cell comprising disrupted T cell receptor and / or HLA and comprising a chimeric antigen receptor. In certain embodiments, the compositions are employed allogeneically as universal reagents for “off-the-shelf treatment of medical conditions such as cancer, autoimmunity, and infection. In particular embodiments, the T cell receptor-negative and / or HLA-negative T cells are generated using zinc finger nucleases, for example.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
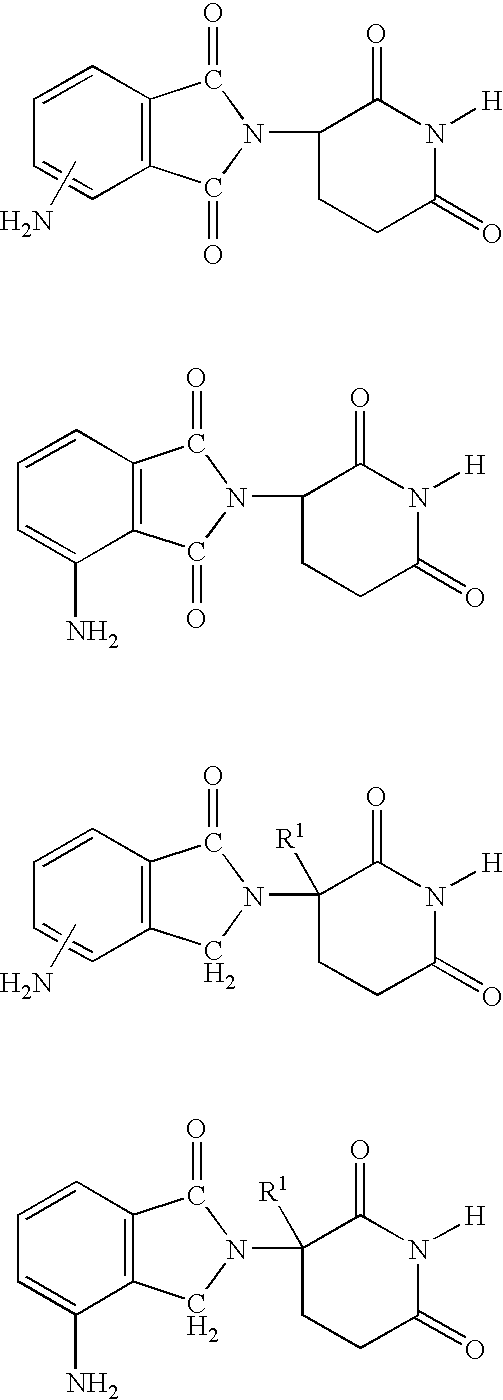
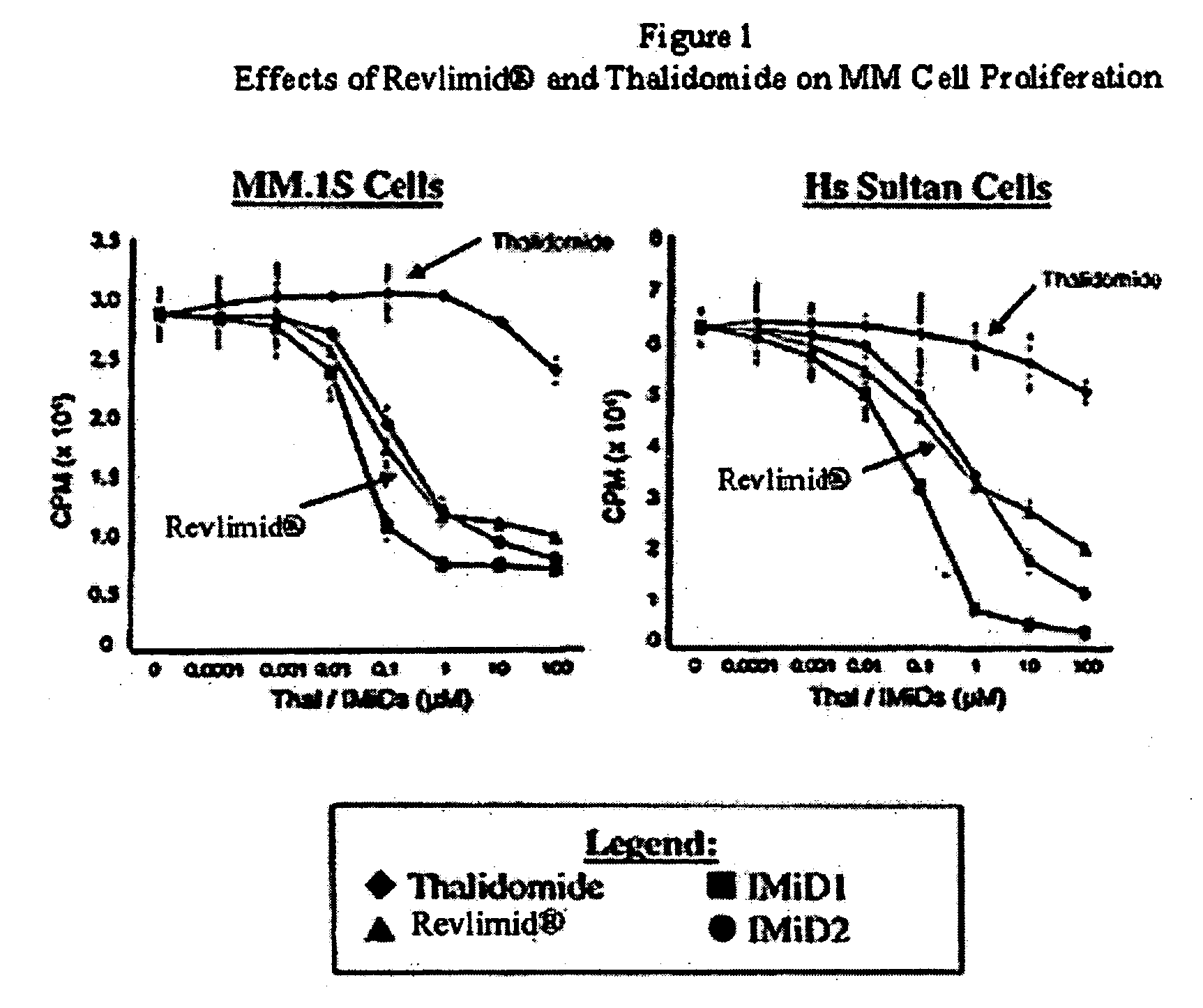
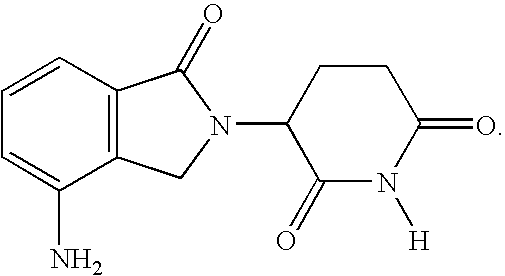
Method using 3-(4-amino-1-oxo-1,3-dihydro-isoindol-2-yl)-piperidine-2,6-dione for treatment of certain leukemias
InactiveUS20060030594A1Prevent proliferationBiocidePeptide/protein ingredientsCompound (substance)Radiation therapy
Methods of treating, preventing or managing leukemias are disclosed. The methods encompass the administration of an immunomodulatory compound of the invention known as Revlimid® or lenalidomide. The invention further relates to methods of treatment using this compound with chemotherapy, radiation therapy, hormonal therapy, biological therapy or immunotherapy. Pharmaceutical compositions and single unit dosage forms suitable for use in the methods of the invention are also disclosed.
Owner:CELGENE CORP
Combination therapy for treating, preventing or managing proliferative disorders and cancers
InactiveUS20040067953A1Improve efficiencyImprove toleranceBiocideHeavy metal active ingredientsCancer preventionBiologically-Based Therapy
The present invention relates to methods and compositions designed for the treatment, management or prevention of cancer. The methods of the invention comprise the administration of an effective amount of one or more inhibitors of JNK in combination with the administration of an effective amount of one or more other agents useful for cancer therapy. The invention also provides pharmaceutical compositions comprising one or more inhibitors of JNK in combination with one or more other agents useful for cancer therapy. In particular, the invention is directed to methods of treatment and prevention of cancer by the administration of an effective amount of one or more inhibitors of JNK in combination with standard and experimental chemotherapies, hormonal therapies, bone marrow transplants, stem cell replacement therapies, biological therapies / immunotherapies and / or radiation therapies for treatment or prevention of cancer. Also included are methods of treatment of cancer by the administration of one or more inhibitors of JNK in combination with surgery, alone or in further combination with standard and experimental chemotherapies, hormonal therapies, bone marrow transplants, stem cell replacement therapies, biological therapies / immunotherapies and / or radiation therapies.
Owner:SIGNAL PHARMA LLC
Immunotherapy Regimes Dependent On APOE Status
InactiveUS20090155256A1Improve securityReducing brain volume declineNervous disorderData processing applicationsDiseaseGenotype
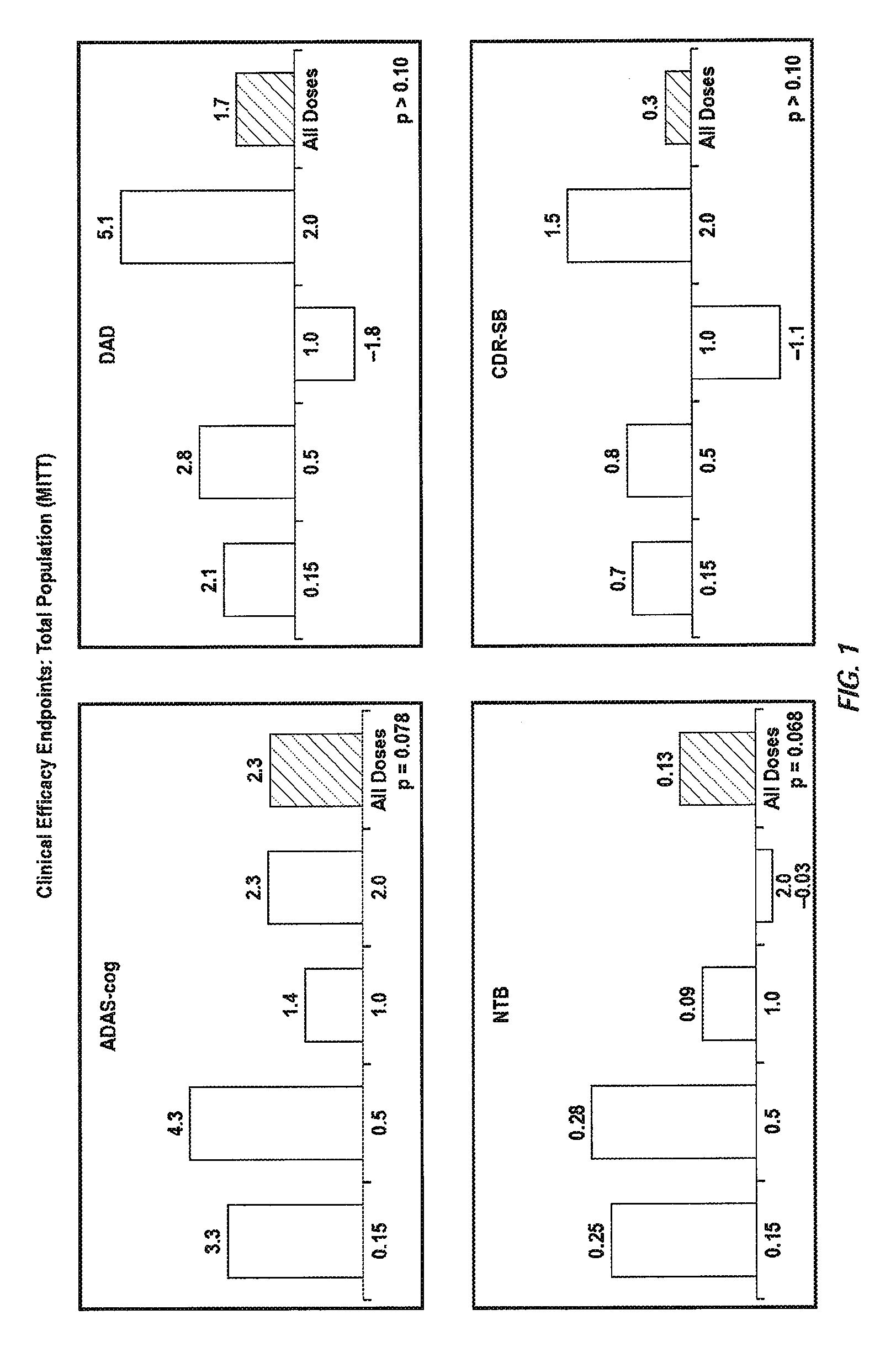
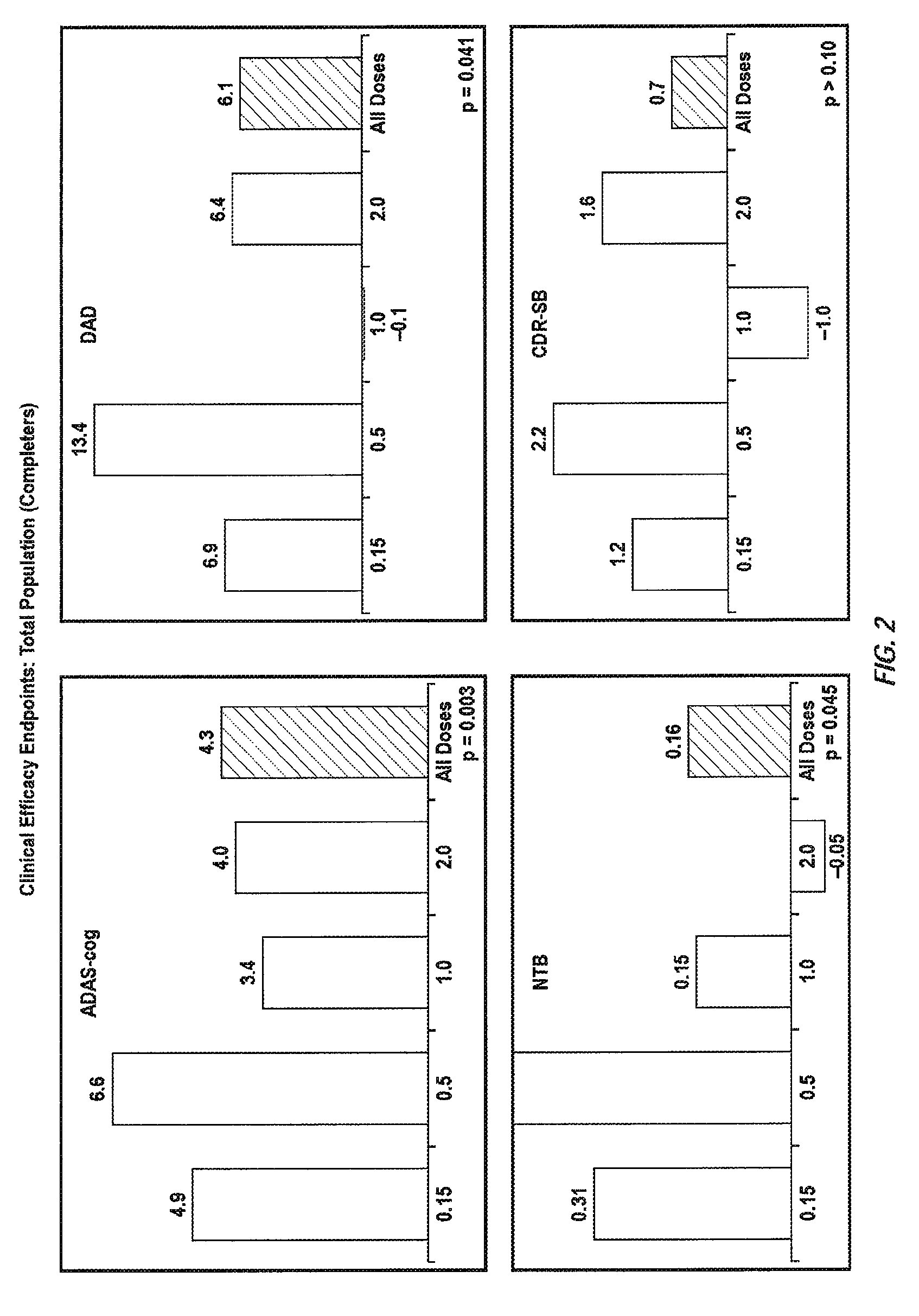
The invention provides methods of immunotherapy of Alzheimer's and similar diseases in which the regime administered to a patient depends on the ApoE genotype of the patient.
Owner:WYETH LLC +1
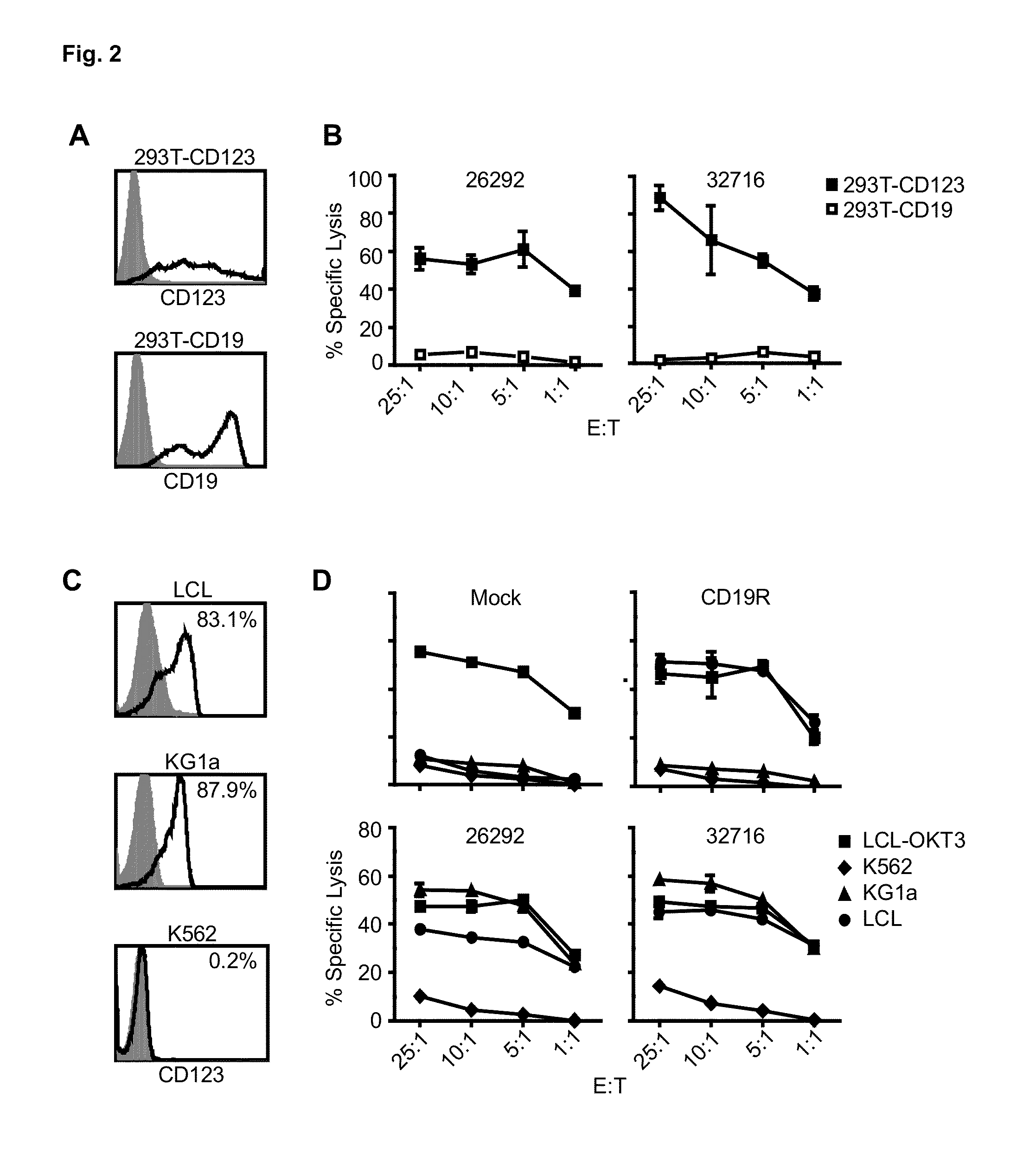
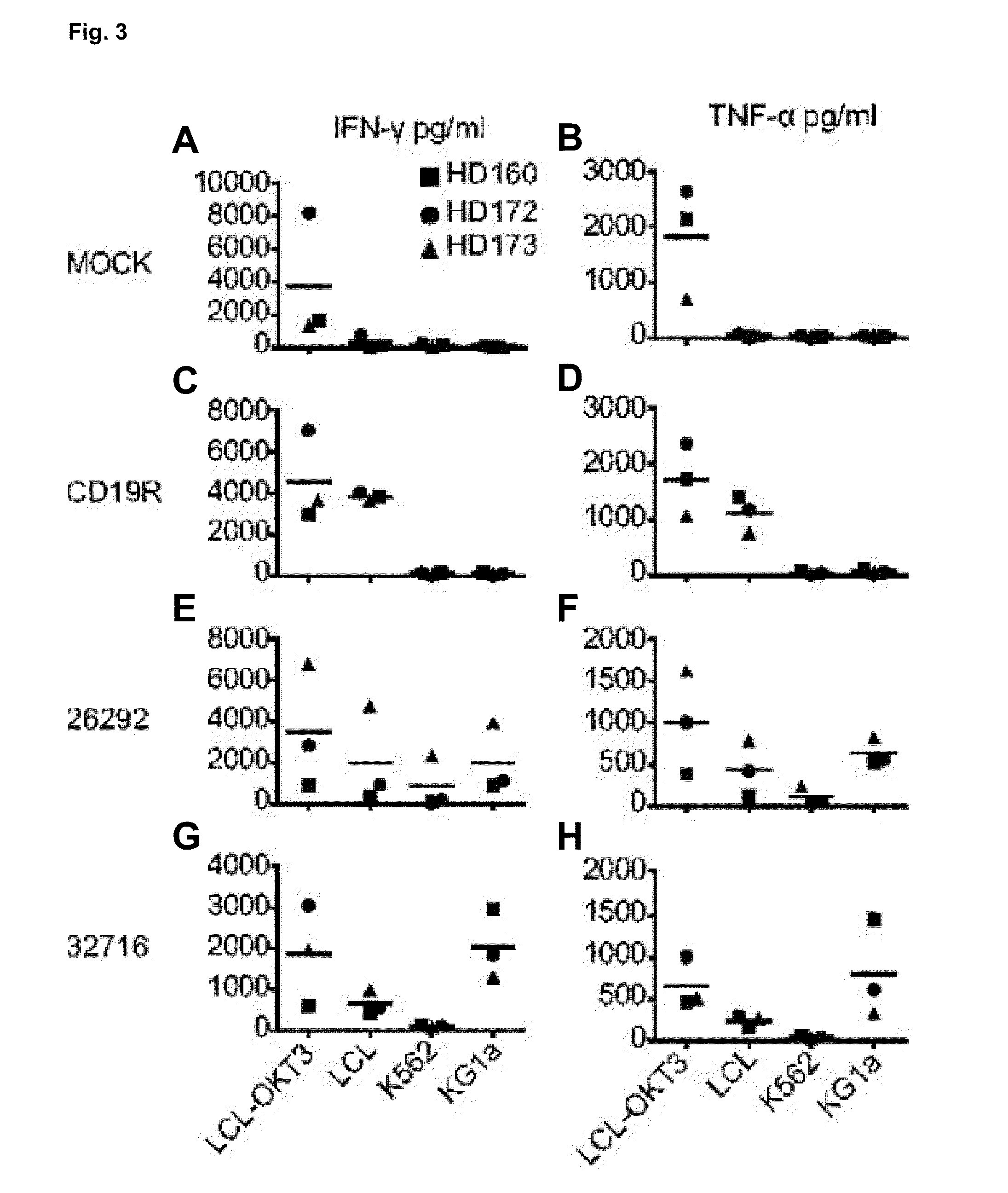
Cd123-specific chimeric antigen receptor redirected t cells and methods of their use
ActiveUS20140271582A1BiocideAntibody mimetics/scaffoldsCD19-specific chimeric antigen receptorT-Cell Specificity
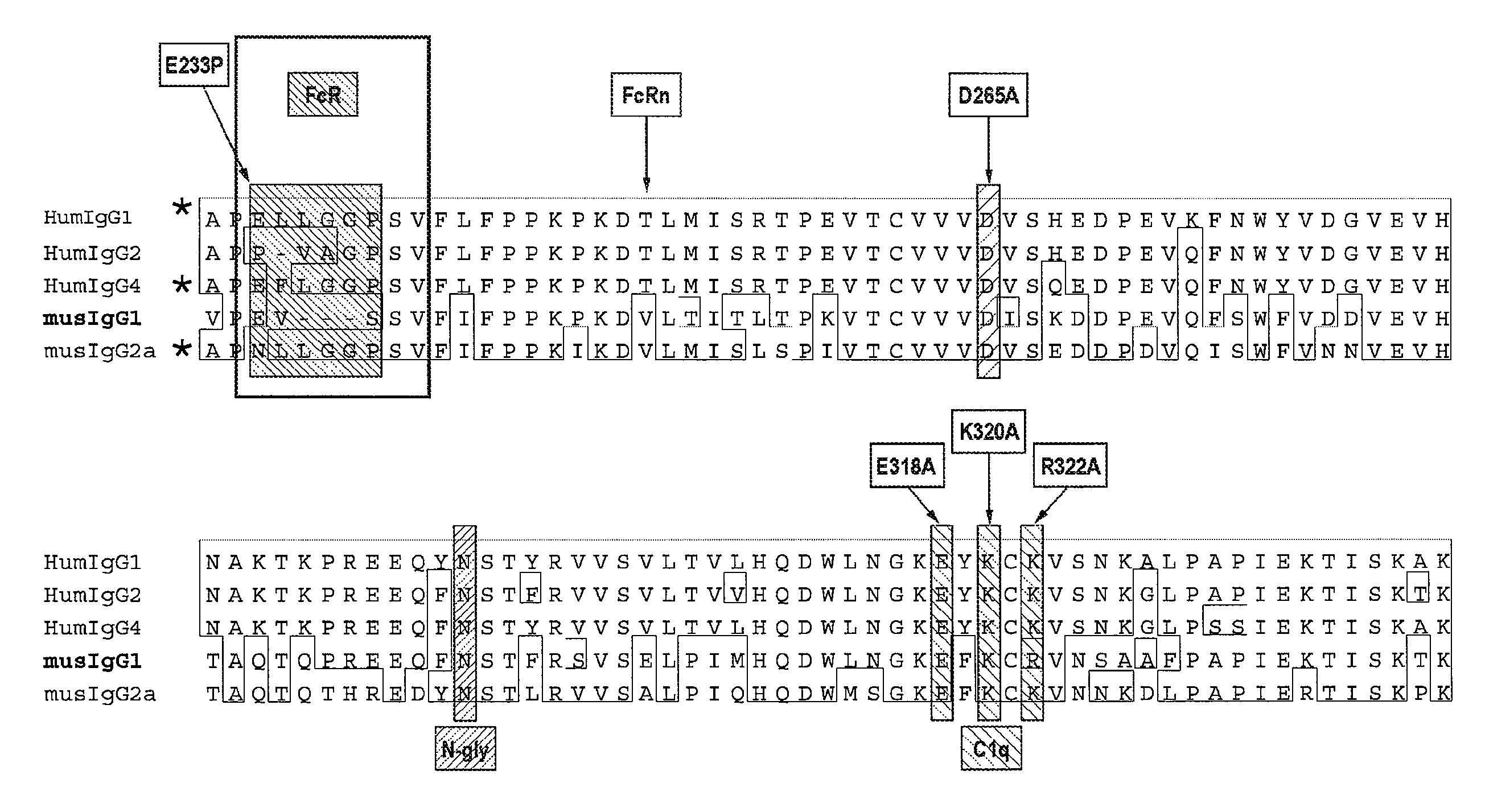
A family of chimeric antigen receptors (CARs) containing a CD123 specific scFv was developed to target different epitopes on CD123. In some embodiments, such a CD123 chimeric antigen receptor (CD123CAR) gene includes an anti-CD123 scFv region fused in frame to a modified IgG4 hinge region comprising an S228P substitution, an L235E substitution, and optionally an N297Q substitution; a costimulatory signaling domain; and a T cell receptor (TCR) zeta chain signaling domain. When expressed in healthy donor T cells (CD4 / CD8), the CD123CARs redirect T cell specificity and mediated potent effector activity against CD123+ cell lines as well as primary AML patient samples. Further, T cells obtained from patients with active AML can be modified to express CD123CAR genes and are able to lyse autologous AML blasts in vitro. Finally, a single dose of 5.0×106 CAR123 T cells results in significantly delayed leukemic progression in mice. These results suggest that CD123CAR-transduced T cells may be used as an immunotherapy for the treatment of high risk AML.
Owner:CITY OF HOPE
Compounds and methods for diagnosis and immunotherapy of tuberculosis
InactiveUS7087713B2Preventing and diagnosing tuberculosisAntibacterial agentsBacteriaProtective immunityImmunotherapy
Compounds and methods for diagnosing tuberculosis or for inducing protective immunity against tuberculosis are disclosed. The compounds provided include polypeptides that contain at least one immunogenic portion of one or more Mycobacterium proteins and DNA molecules encoding such polypeptides. Diagnostic kits containing such polypeptides or DNA sequences and a suitable detection reagent may be used for the detection of Mycobacterium infection in patients and biological samples. Antibodies directed against such polypeptides are also provided. In addition, such compounds may be formulated into vaccines and / or pharmaceutical compositions for immunization against Mycobacterium infection.
Owner:CORIXA CORP
Cd37 immunotherapeutic and combination with bifunctional chemotherapeutic thereof
The present disclosure provides a humanized anti-CD37 small modular immunopharmaceutical (SMIP) molecule, as well as synergistic combination therapies of CD37-specific binding molecules (such as anti-CD37 SMIP proteins or antibodies) with bifunctional chemotherapeutics (such as bendamustine) that can be administered concurrently or sequentially, for use in treating or preventing B-cell related autoimmune, inflammatory, or hyperproliferative diseases.
Owner:APTEVO RES & DEV LLC
Yeast-based vaccines as immunotherapy
InactiveUS7465454B2Enhance immune responseExtended half-lifeBiocideAntibody mimetics/scaffoldsYeastDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
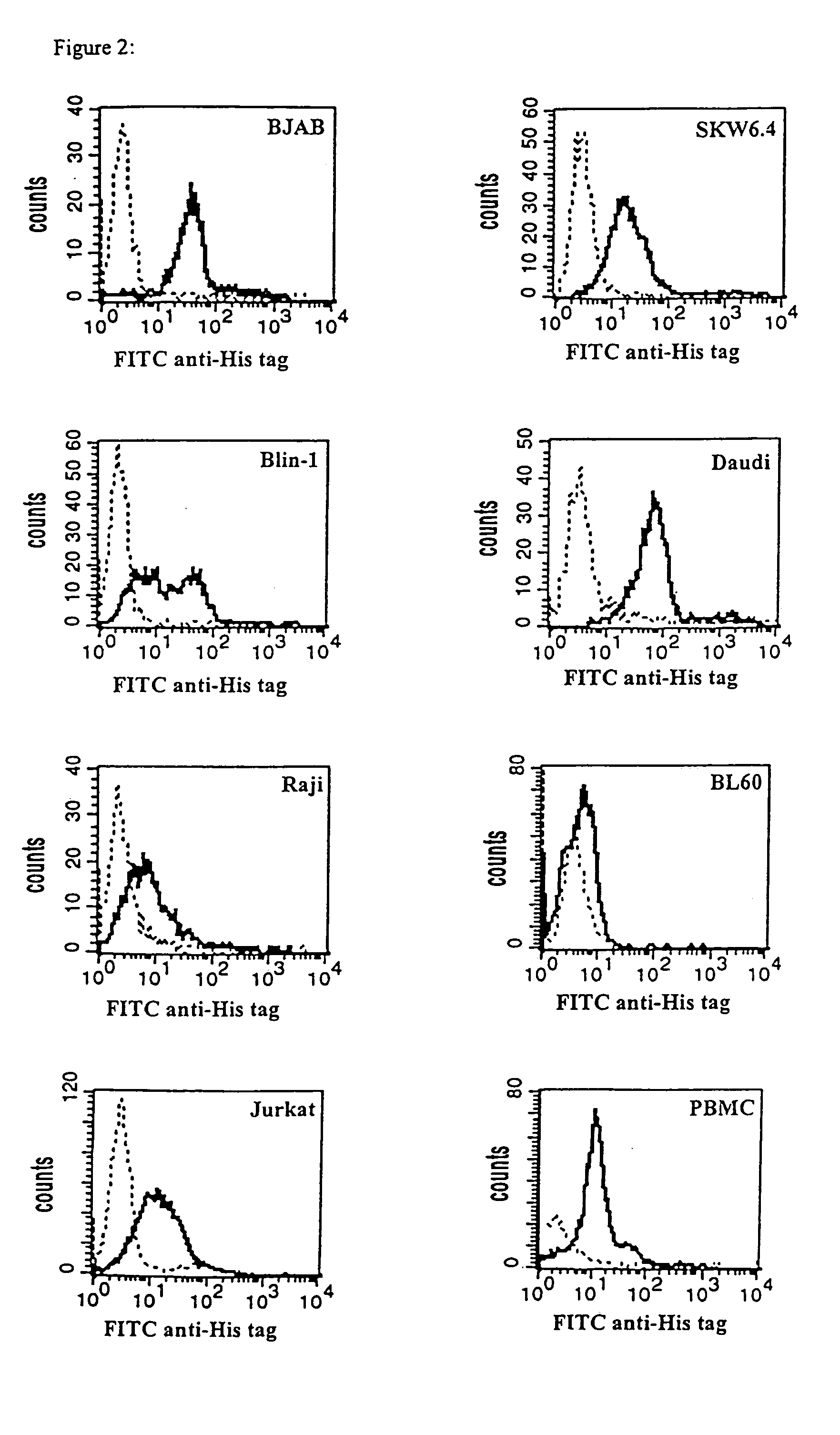
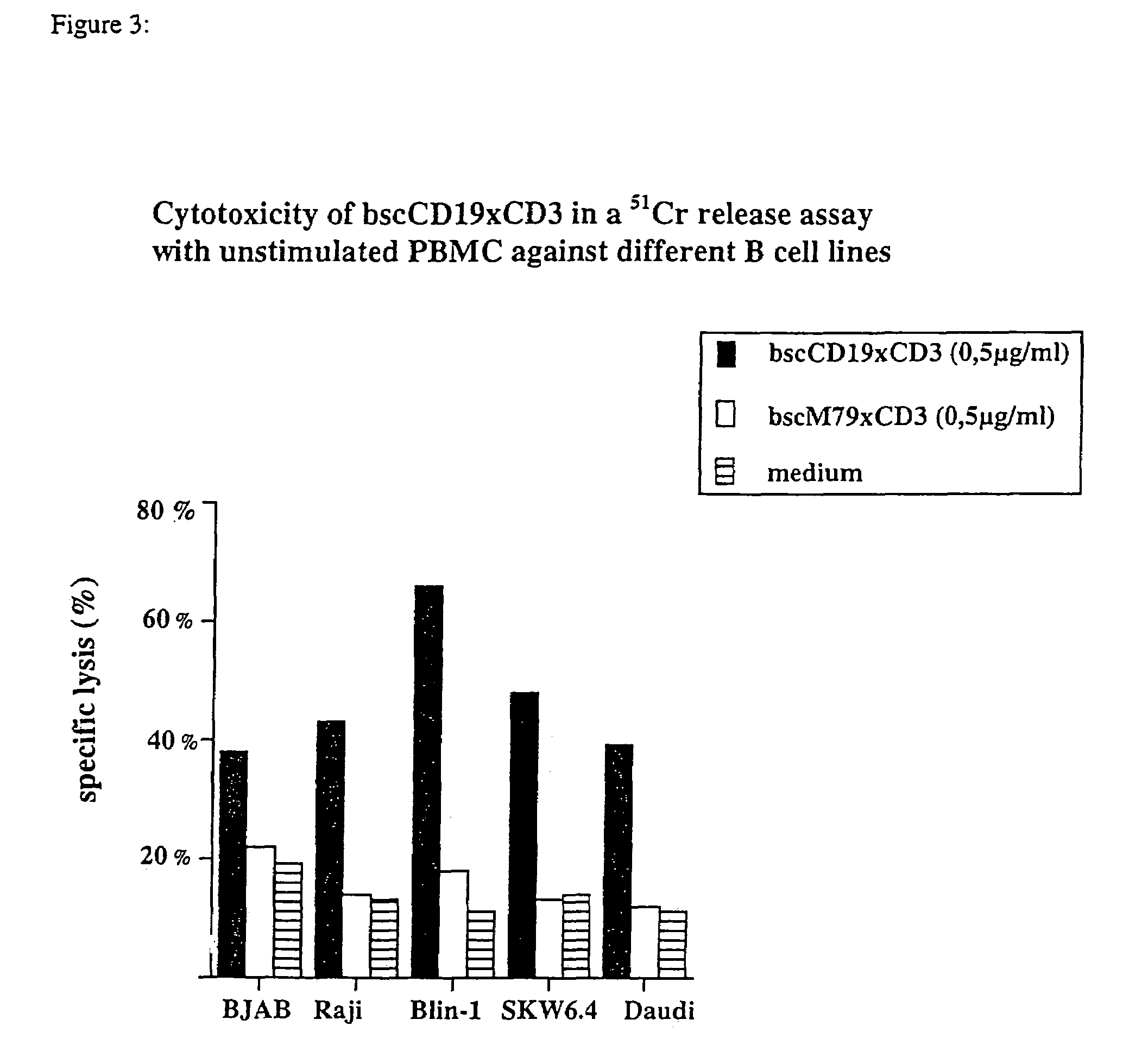
CD19xCD3 specific polypeptides and uses thereof
Described are novel single-chain multifunctional polypeptides comprising at least two binding sites specific for the CD19 and CD3 antigen, respectively. Further provided are polypeptides, wherein the above-described polypeptide comprises at least one further domain, preferably of pre-determined function. Furthermore, polynucleotides encoding said polypeptides as well as to vectors comprising said polynucleotides and host cells transformed therewith and their use in the production of said polypeptides are described. In addition, compositions, preferably pharmaceutical and diagnostic compositions are provided comprising any of the afore-described polypeptides, polynucleotides or vectors. Described is also the use of the afore-mentioned polypeptides, polynucleotides and vectors for the preparation of pharmaceutical compositions for immunotherapy, preferably against B-cell malignancies such as non-Hodgkin lymphoma.
Owner:AMGEN RES (MUNICH) GMBH
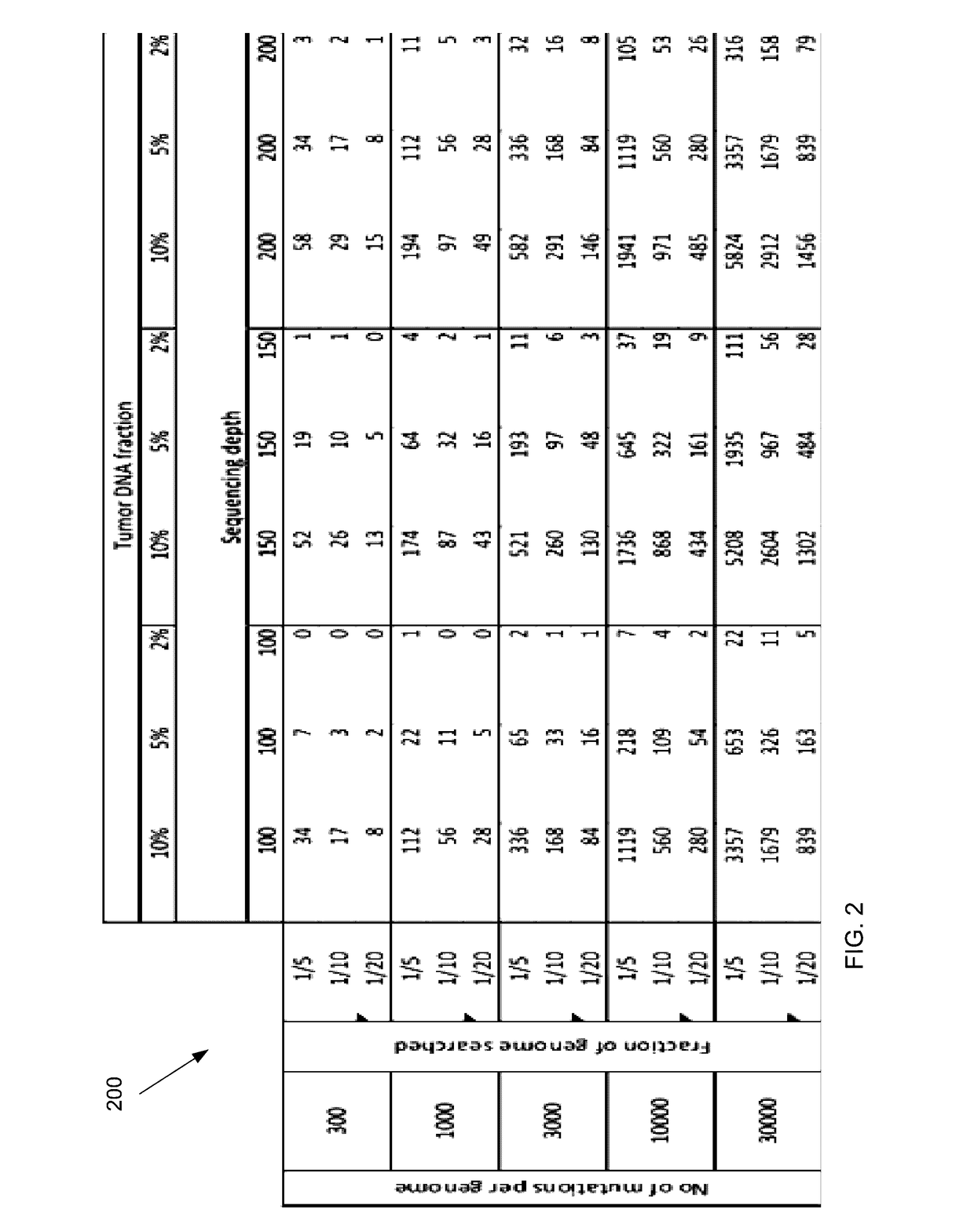
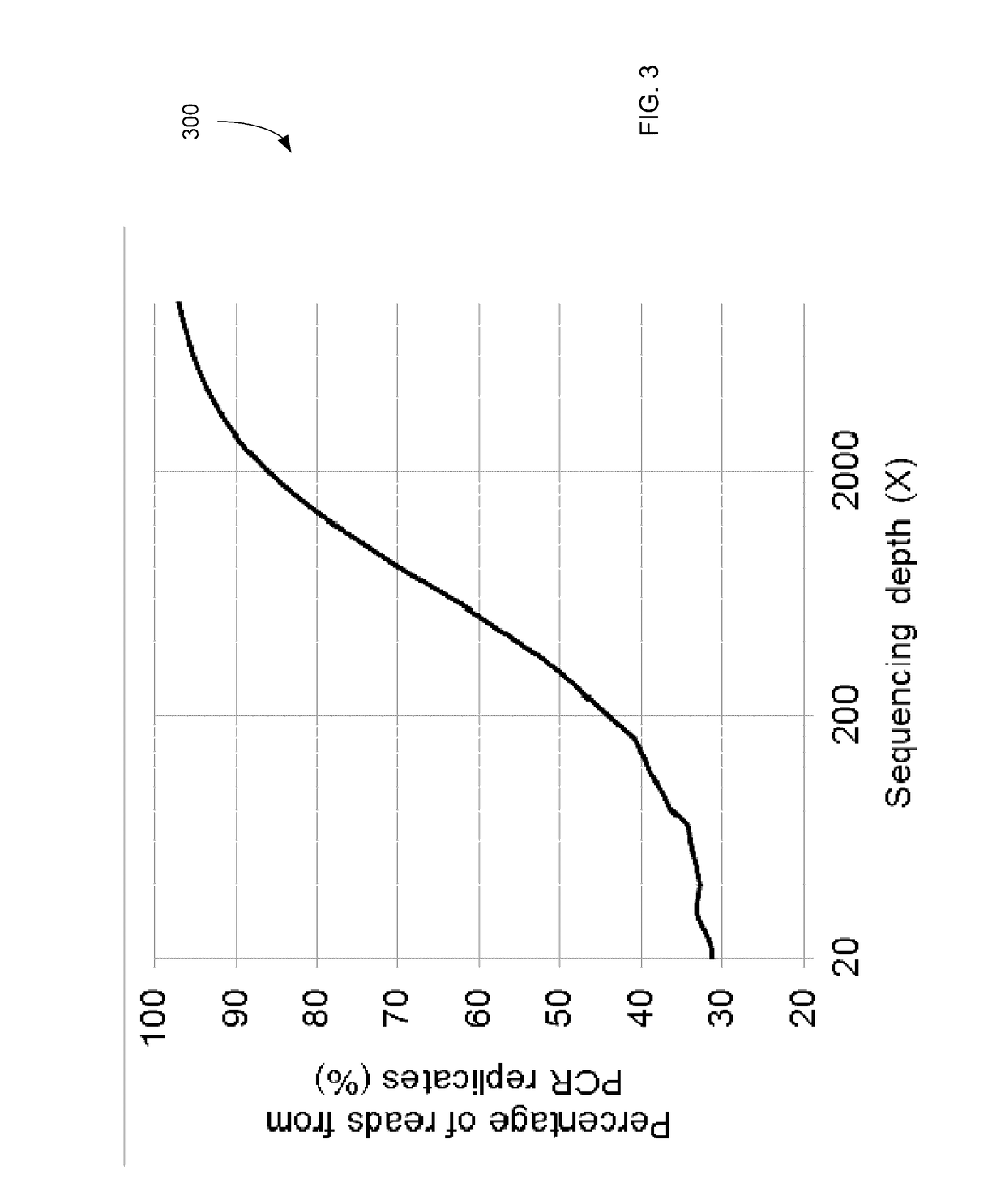
Detecting mutations for cancer screening and fetal analysis
ActiveUS20170073774A1Accurate detectionMicrobiological testing/measurementProteomicsCell freeEmbolization Therapy
Embodiments are related to the accurate detection of somatic mutations in the plasma (or other samples containing cell-free DNA) of cancer patients and for subjects being screened for cancer. The detection of these molecular markers would be useful for the screening, detection, monitoring, management, and prognostication of cancer patients. For example, a mutational load can be determined from the identified somatic mutations, and the mutational load can be used to screen for any or various types of cancers, where no prior knowledge about a tumor or possible cancer of the subject may be required. Embodiments can be useful for guiding the use of therapies (e.g. targeted therapy, immunotherapy, genome editing, surgery, chemotherapy, embolization therapy, anti-angiogenesis therapy) for cancers. Embodiments are also directed to identifying de novo mutations in a fetus by analyzing a maternal sample having cell-free DNA from the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Immunotherapy of B cell malignancies and autoimmune diseases using unconjugated antibodies and conjugated antibodies and antibody combinations and fusion proteins
ActiveUS7534427B2Easy to manageSimilar response ratePeptide/protein ingredientsAntipyreticDiseaseMammal
The invention is directed to a method for treating a treating and diagnosing a B cell-related disease, T cell-related disease or an autoimmune disease in a mammal by concurrently or sequentially administering to the mammal a therapeutic composition that comprises a pharmaceutically acceptable vehicle and at least one conjugated antibody, wherein predosing with a non-radiolabeled antibody is not performed.
Owner:IMMUNOMEDICS INC
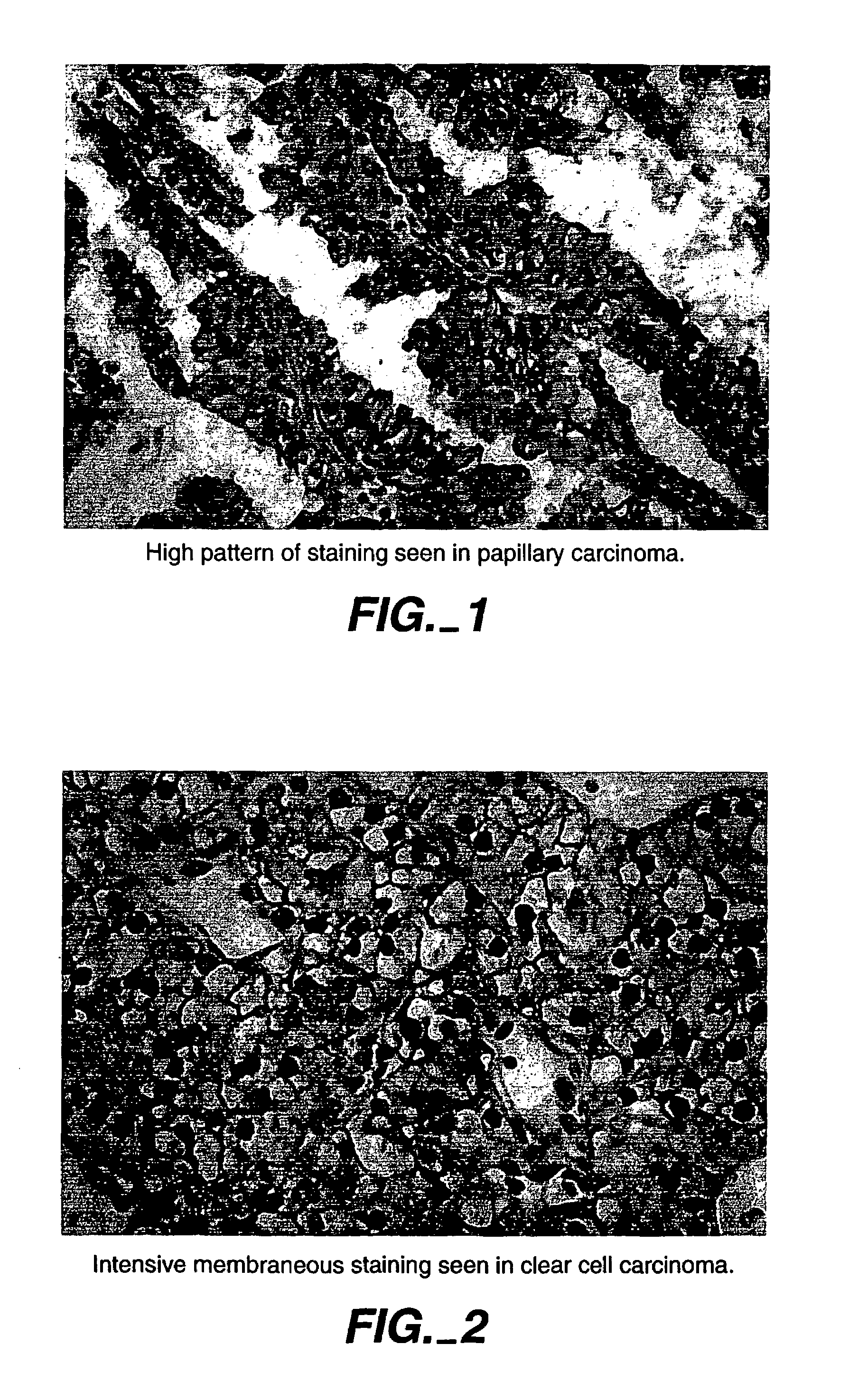
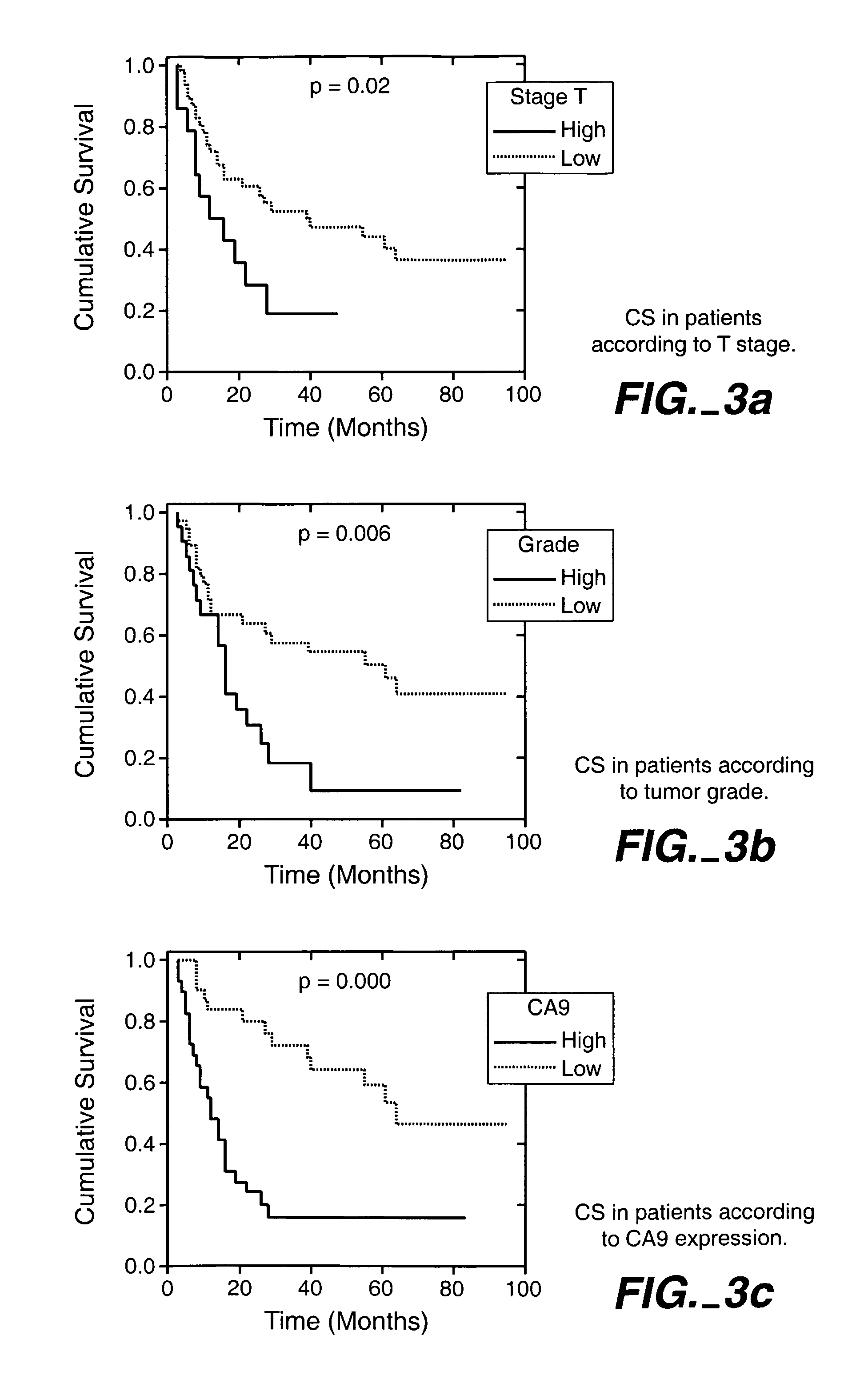
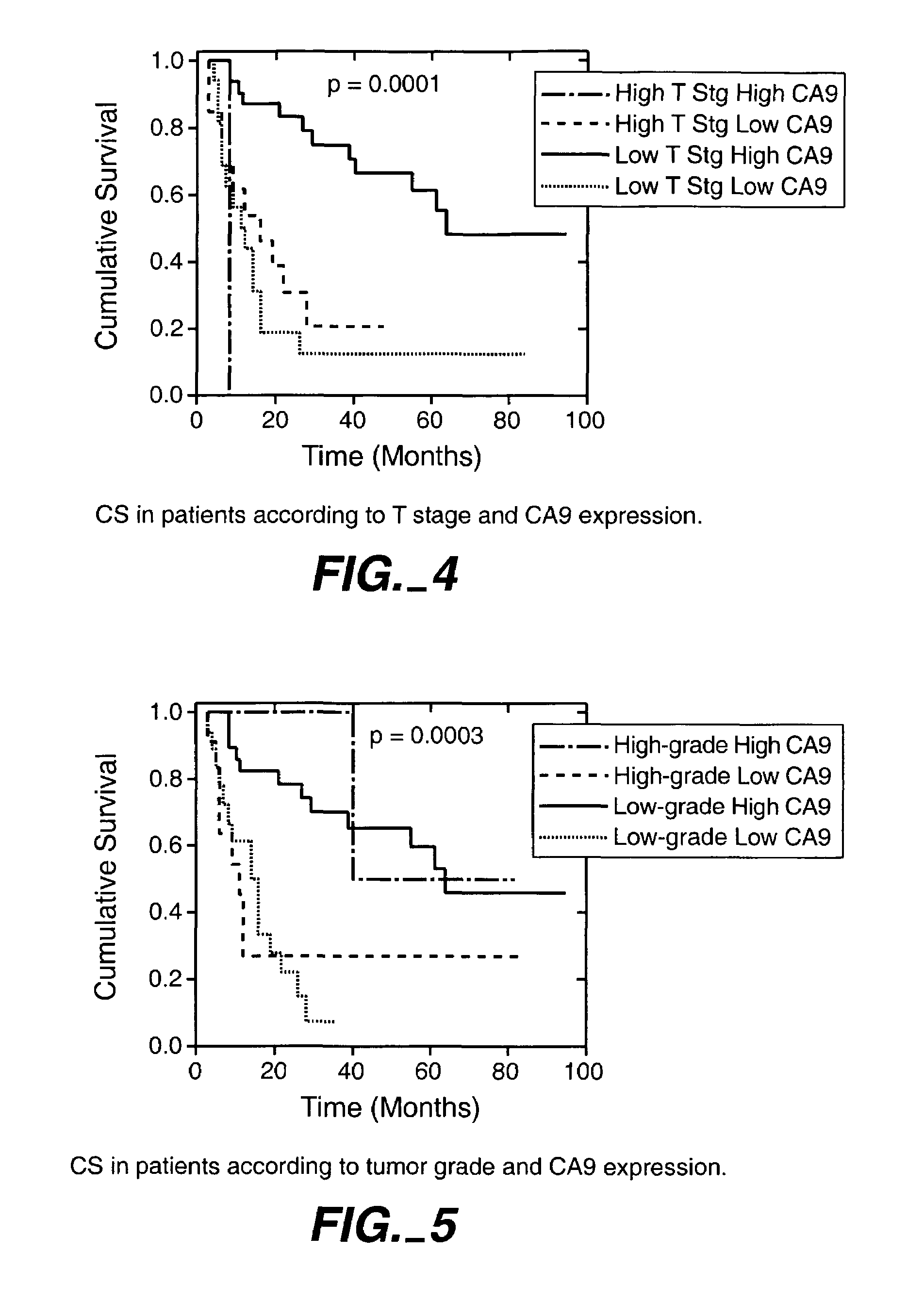
MN/CA IX/CA9 and Renal Cancer Prognosis
InactiveUS7482129B2Microbiological testing/measurementBiological testingRegimenRenal clear cell carcinoma
Herein disclosed are methods that are prognostic for renal cell carcinoma, particularly renal clear cell carcinoma, afflicting a vertebrate. An exemplary prognostic method comprises detecting the presence of, and quantitating the level and / or extent of a MN / CA9 gene expression product in a sample from the affected subject, wherein if 50% or fewer cells are found to express the MN / CA9 gene, then the subject is considered to have a poorer prognosis. MN / CA9 gene expression products useful in the prognostic methods include MN protein, MN polypeptide and / or MN nucleic acids. The methods are useful as an aid in the selection of treatment for a patient with renal cell carcinoma, alone or in combination with conventional tumor stage and / or grade information. The methods of the invention can be used, for example, to identify those patients requiring more aggressive therapy regimens, or those patients most likely to respond to adjuvant immunotherapies, particularly MN / CA IX / CA9-targeted therapies.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI +1
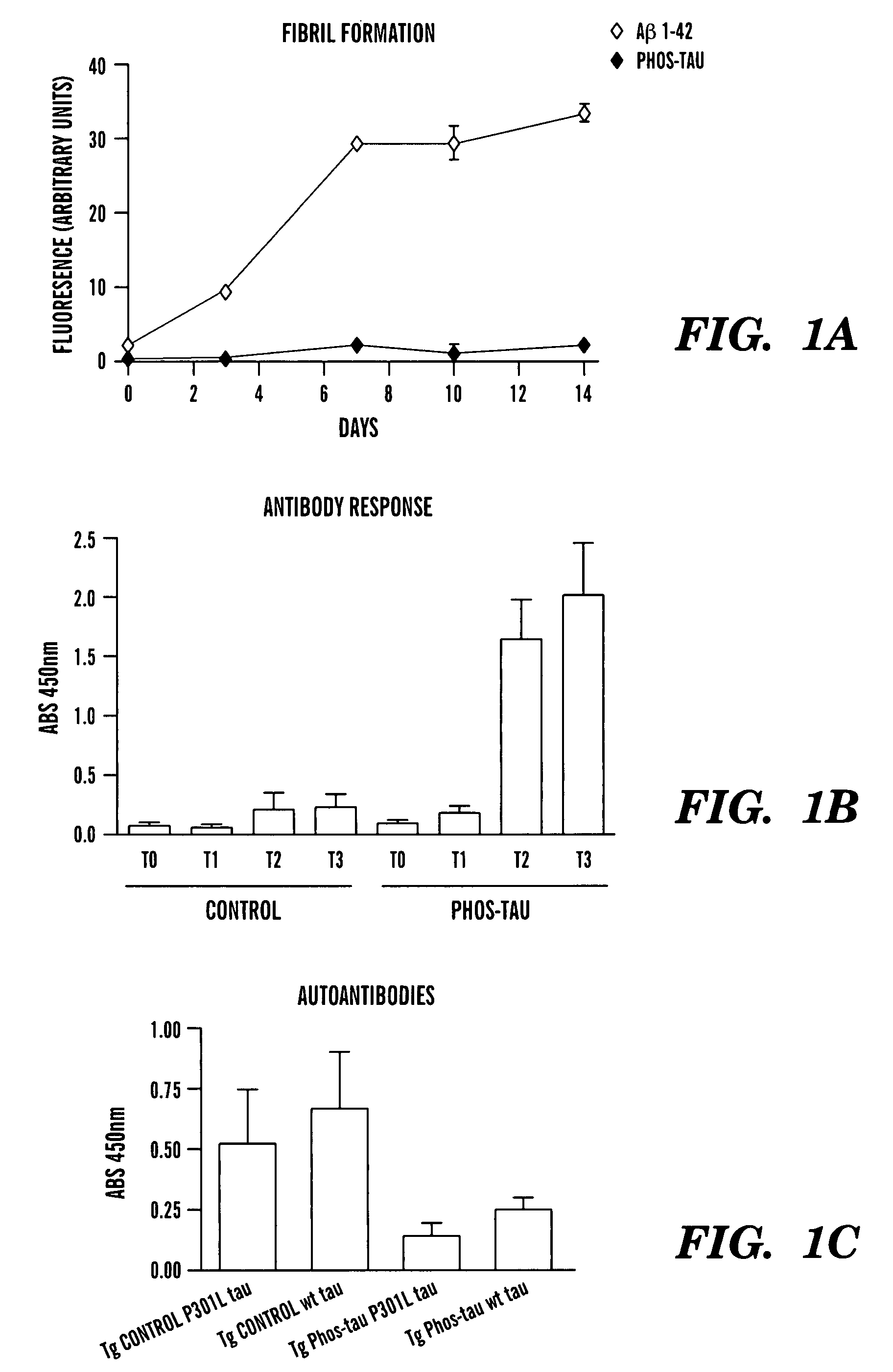
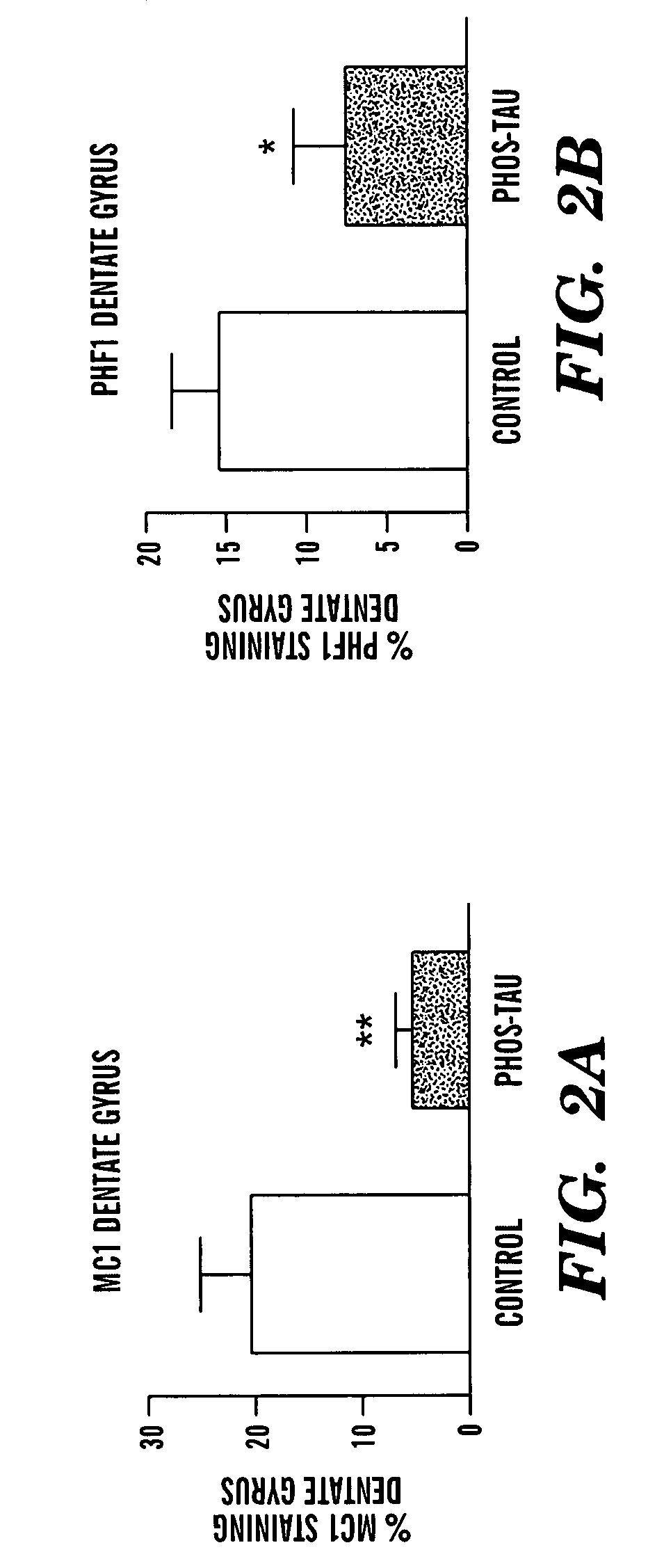
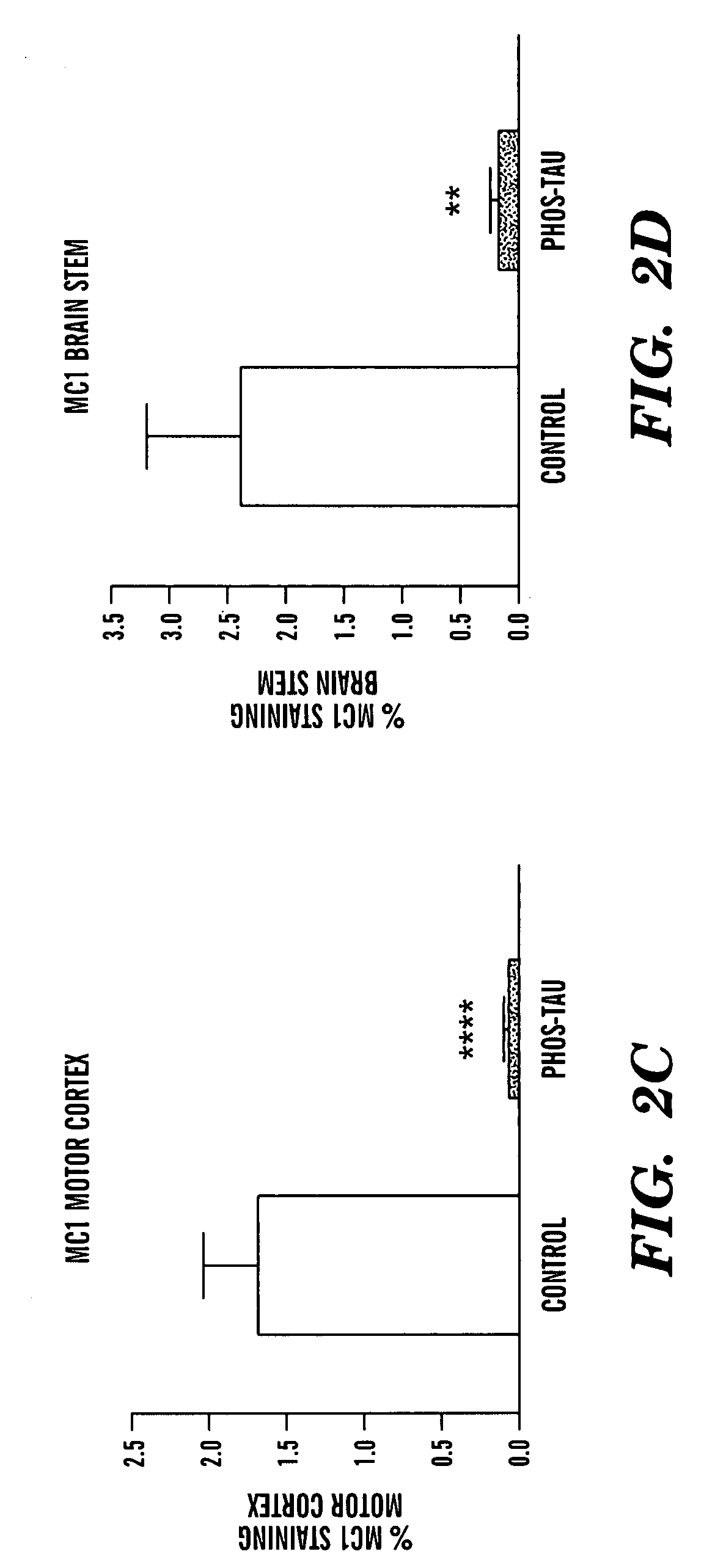
Tau fragments for immunotherapy
The present invention relates to methods of treating and preventing Alzheimer's Disease or other tauopathies in a subject by administering a tau protein, its immunogenic epitopes, or antibodies recognizing the tau protein or its immunogenic epitopes under conditions effective to treat or prevent Alzheimer's Disease of other tauopathies. Also disclosed are methods of promoting clearance of aggregates from the brain of the subject and of slowing progression of tangle-related behavioral phenotype in a subject.
Owner:NEW YORK UNIV
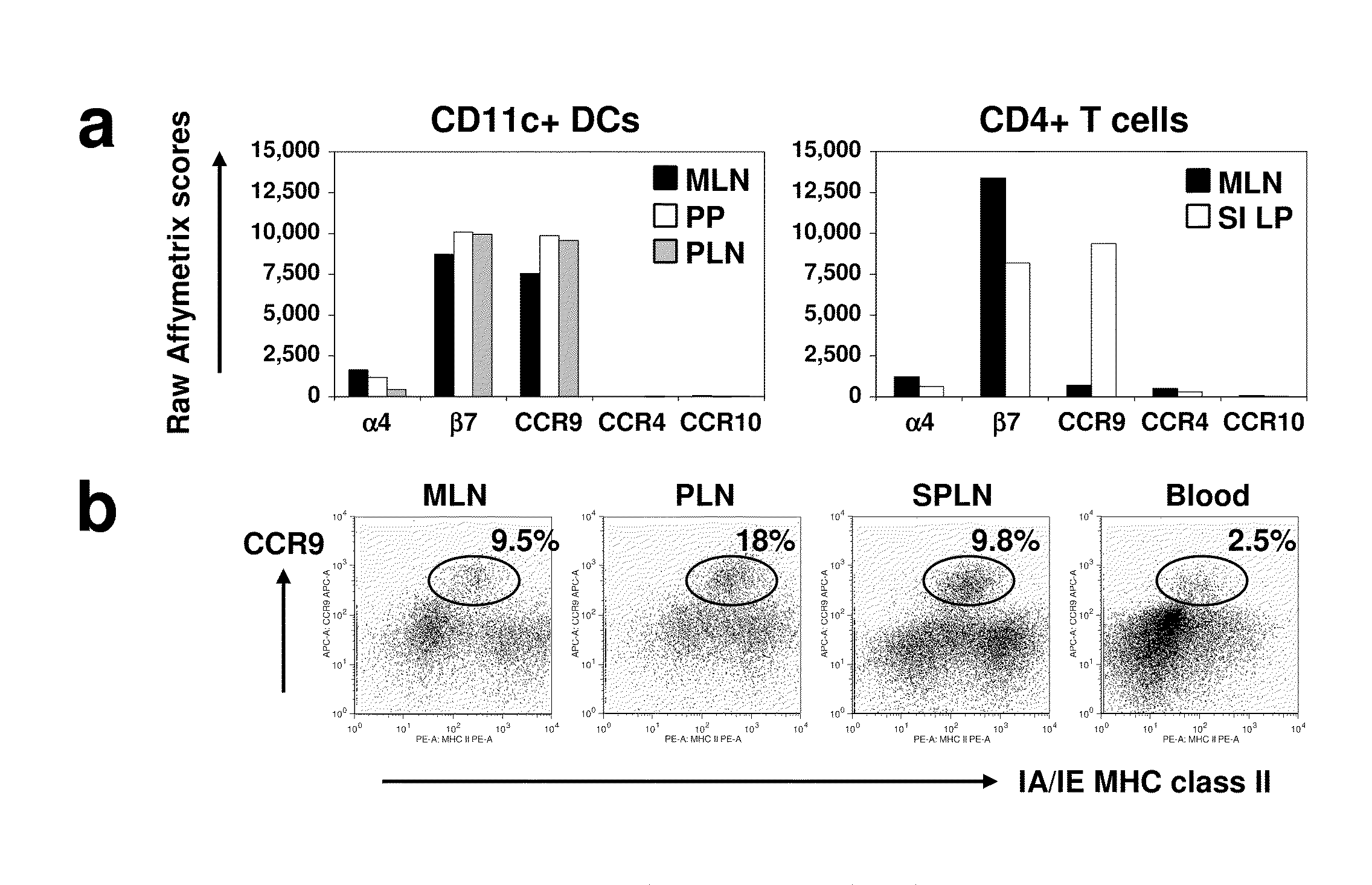
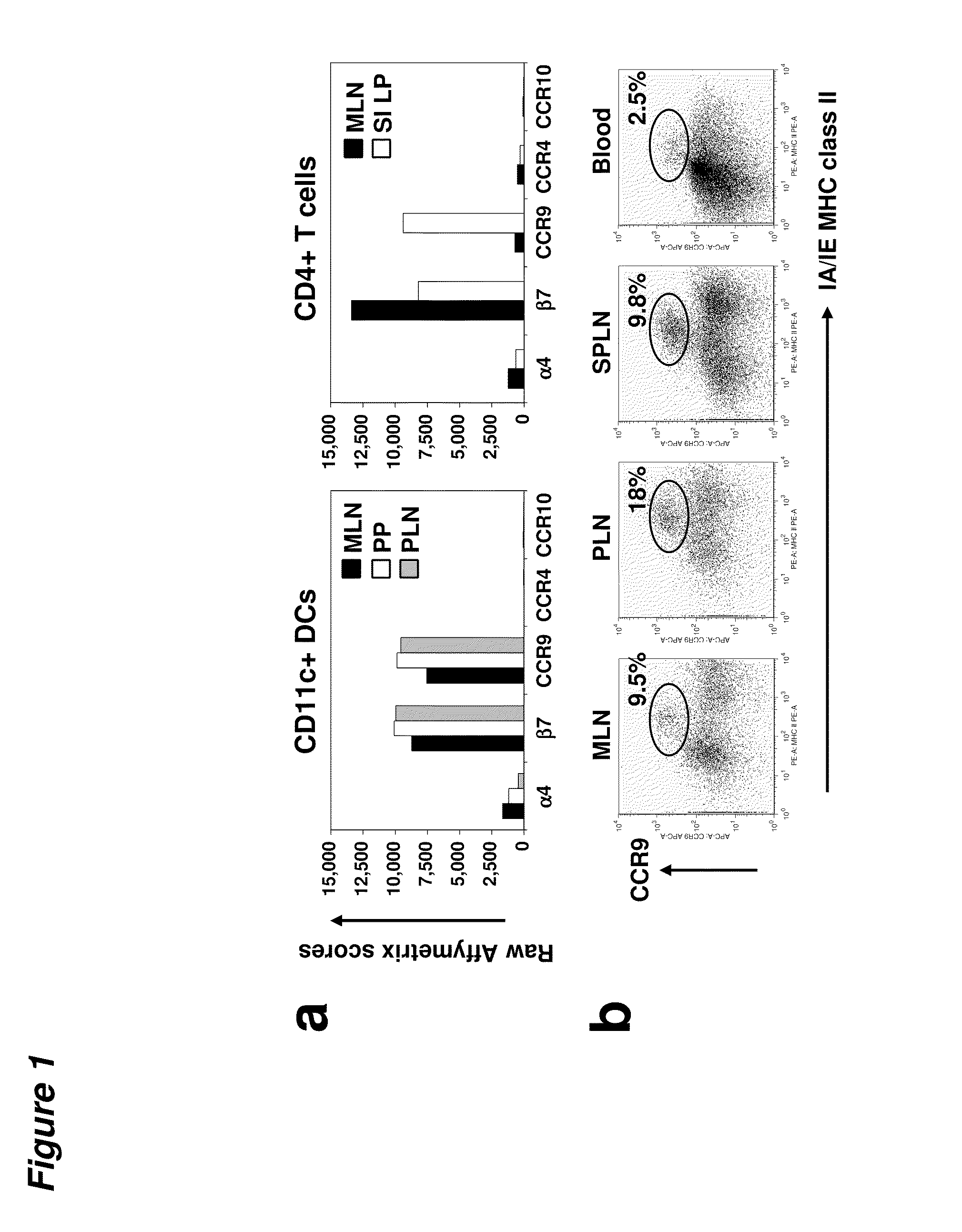
Tolerogenic populations of dendritic cells
InactiveUS20100080816A1Snake antigen ingredientsArtificial cell constructsAutoimmune responsesCMKLR1
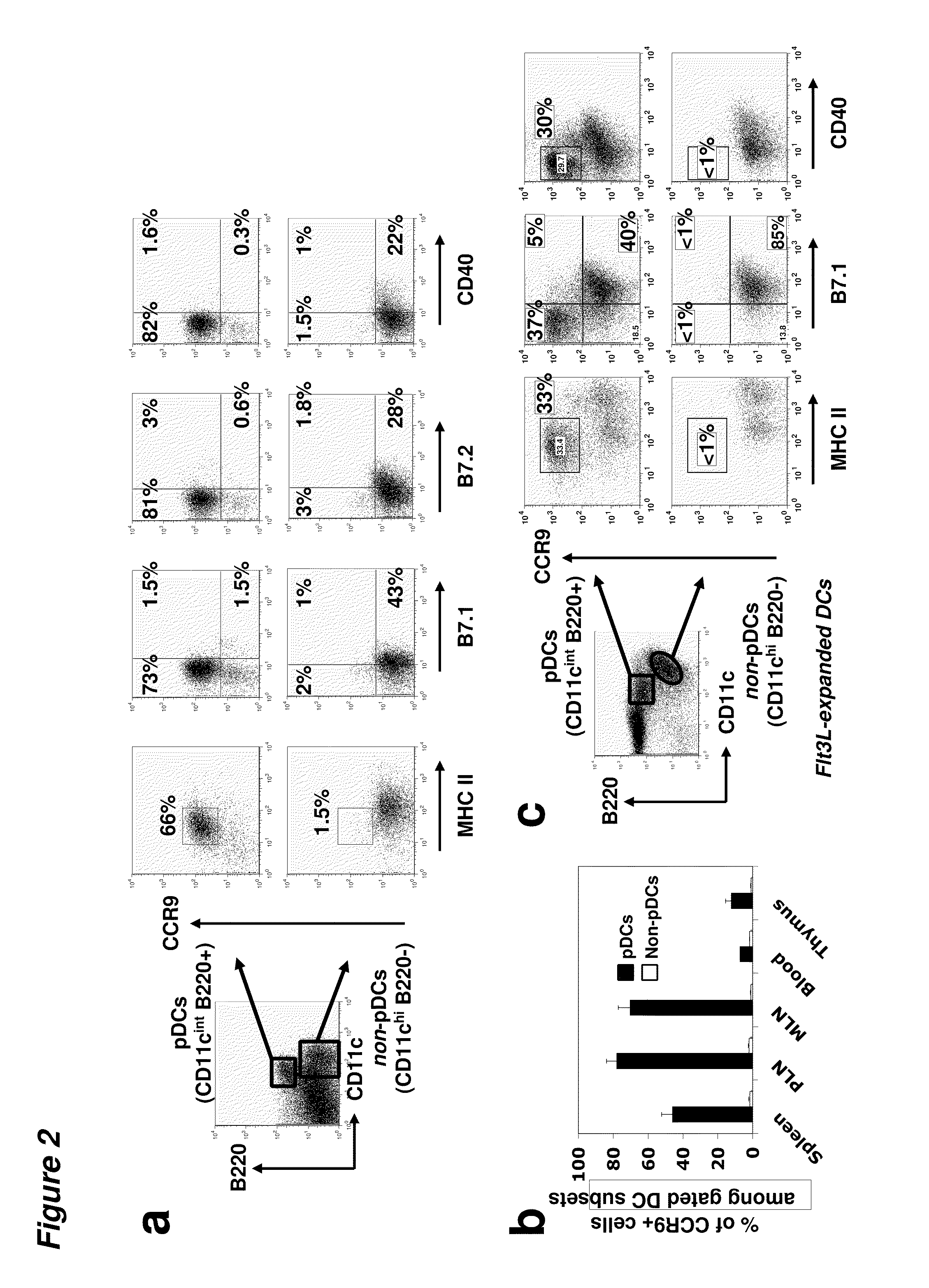
Tolerogenic populations of dendritic cells are provided, where the dendritic cells are characterized by expression of select tissue-specific homing receptors including the chemokine receptors CCR9; or CMKLR1; or the integrin CD103. The dendritic cells may be conventional / myeloid or plasmacytoid dendritic cells. The cells may be isolated from lymphoid tissue, from blood, or from in vitro culture, e.g. bone marrow culture, etc. Methods are provided for their identification, isolation and targeting in immunotherapeutic interventions in suppressing inflammatory disorders including autoimmunity, transplantation responses and allergic diseases. In some embodiments dendritic cell populations are fixed to render them immunosuppressive, thus allowing the cells to be typed and banked for future use.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Cd37 immunotherapeutic and combination with bifunctional chemotherapeutic thereof
The present disclosure provides a humanized anti-CD37 small modular immunopharmaceutical (SMIP) molecule, as well as synergistic combination therapies of CD37-specific binding molecules (such as anti-CD37 SMIP proteins or antibodies) with bifunctional chemotherapeutics (such as bendamustine) that can be administered concurrently or sequentially, for use in treating or preventing B cell related autoimmune, inflammatory, or hyperproliferative diseases.
Owner:APTEVO RES & DEV LLC
Peptides and combination of peptides for use in immunotherapy against small cell lung cancer and other cancers
ActiveUS10253077B2Increase in motilityAid in diagnosisPeptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthAdditive ingredient
The present invention relates to peptides, proteins, nucleic acids and cells for use in immunotherapeutic methods. In particular, the present invention relates to the immunotherapy of cancer. The present invention furthermore relates to tumor-associated T-cell peptide epitopes, alone or in combination with other tumor-associated peptides that can for example serve as active pharmaceutical ingredients of vaccine compositions that stimulate anti-tumor immune responses, or to stimulate T cells ex vivo and transfer into patients. Peptides bound to molecules of the major histocompatibility complex (MHC), or peptides as such, can also be targets of antibodies, soluble T-cell receptors, and other binding molecules.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Method for treating cancer harboring EGFR mutations
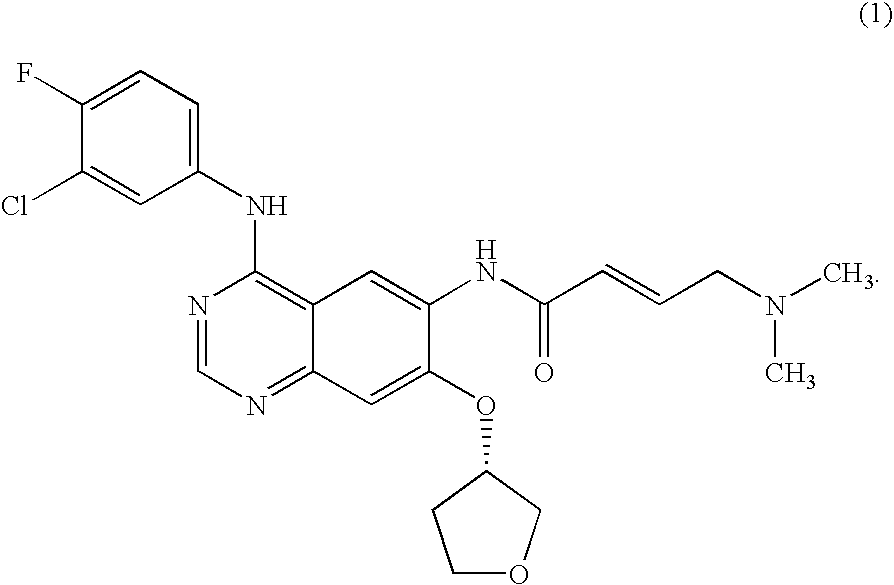
ActiveUS20090318480A1Advantageously effective in treatmentReduced responseBiocideOrganic active ingredientsAcquired resistanceActivating mutation
The present invention relates to a method of treatment of patients suffering from cancer and harbouring mutations of EGFR in the tumour, for instance an activating mutation of the EGFR or a mutation responsible for resistance or the emergence of acquired resistance to treatment with reversible EGFR and / or HER2 inhibitors or irreversible inhibitors such as CI-1033, EKB-569, HKI-272 or HKI-357, comprising administering an effective amount of the irreversible EGFR inhibitor BIBW2992 (1) 4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino}-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, to a person in need of such treatment, optionally in combination with the administration of a further chemotherapeutic agent, in combination with radiotherapy, radio-immunotherapy and / or tumour resection by surgery, and to the use of a BIBW 2992 (1) for preparing a pharmaceutical composition for the treatment of patients suffering from cancer and harbouring mutations of EGFR in the tumour.
Owner:BOEHRINGER INGELHEIM INT GMBH
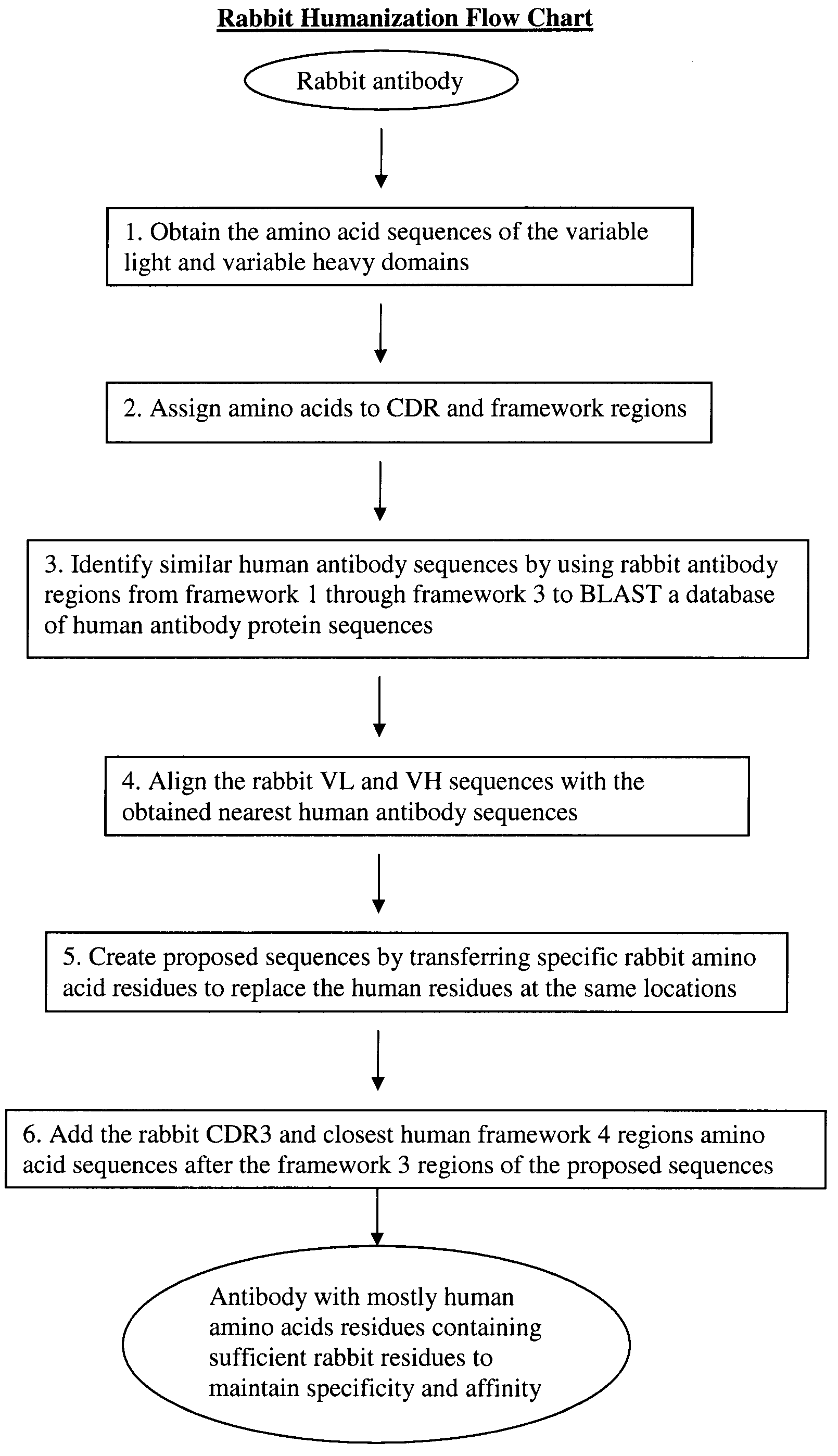
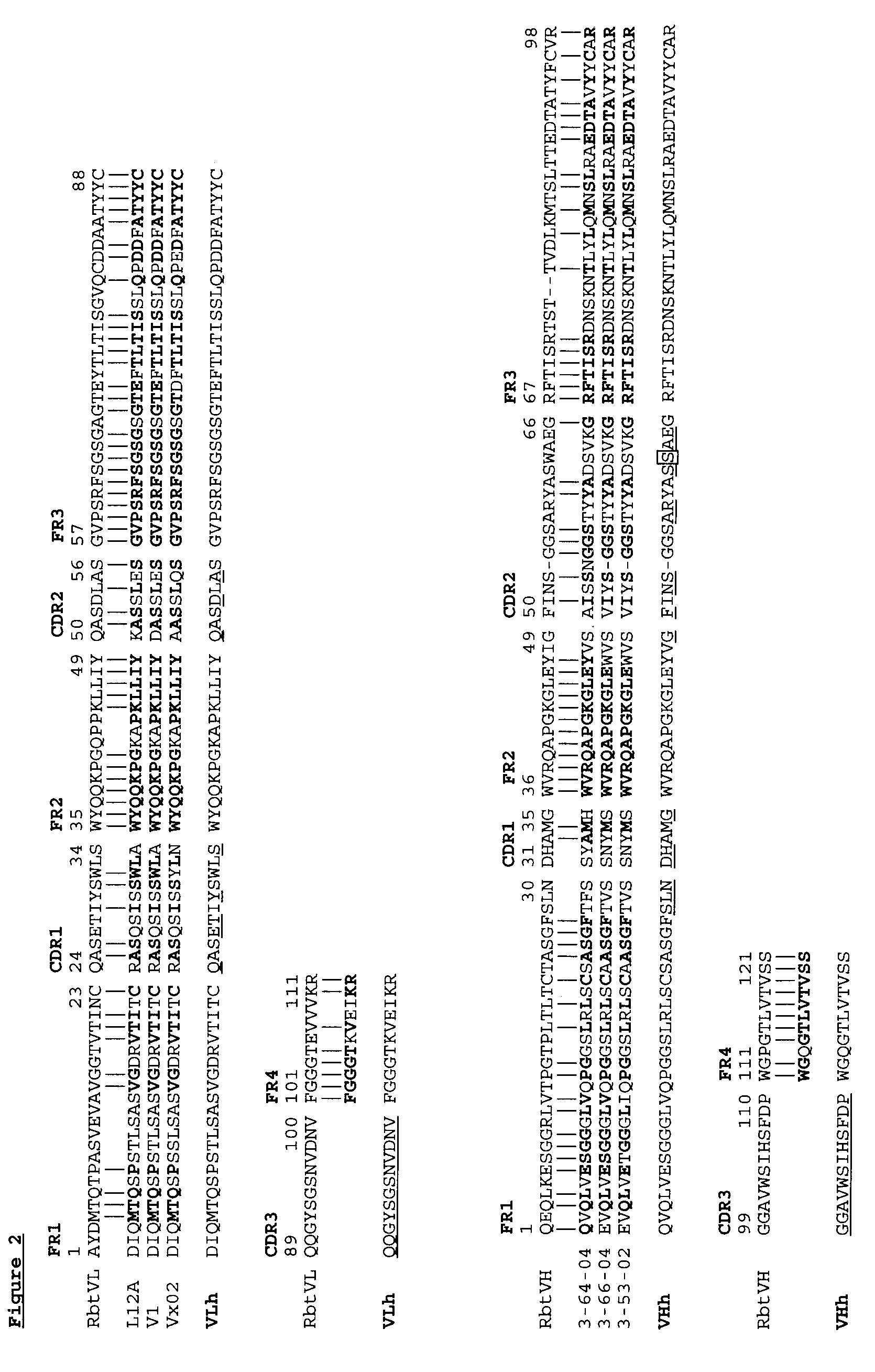
Novel Rabbit Antibody Humanization Methods and Humanized Rabbit Antibodies
The present invention is directed to novel and improved methods for humanizing rabbit heavy and light variable regions. The resulting humanized rabbit heavy and light chains and antibodies and antibody fragments containing are well suited for use in immunotherapy and immunodiagnosis as they retain the antigen binding affinity of the parent antibody and based on their very high level of sequence identity to human antibody sequences should be essentially non-immunogenic in humans. The invention exemplifies the protocol for the manufacture of therapeutic humanized anti-human TNF-alpha and anti-human IL-6 antibodies.
Owner:ALDERBIO HLDG LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com