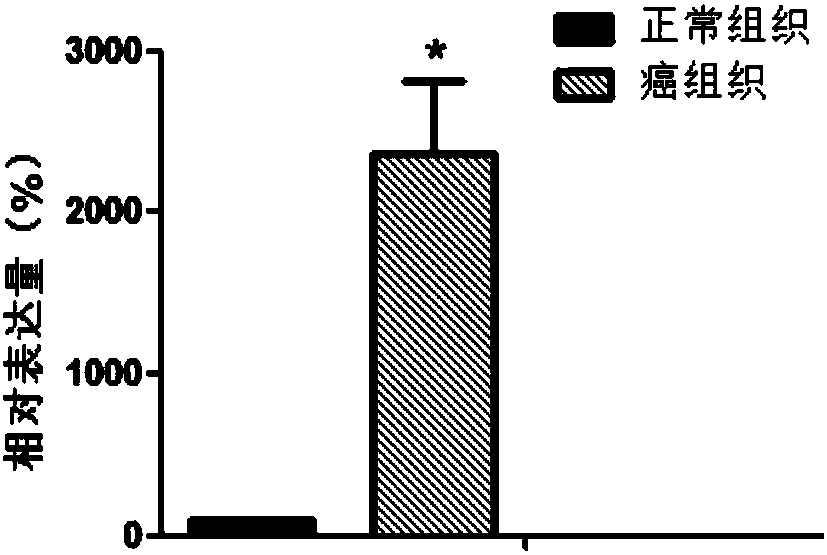
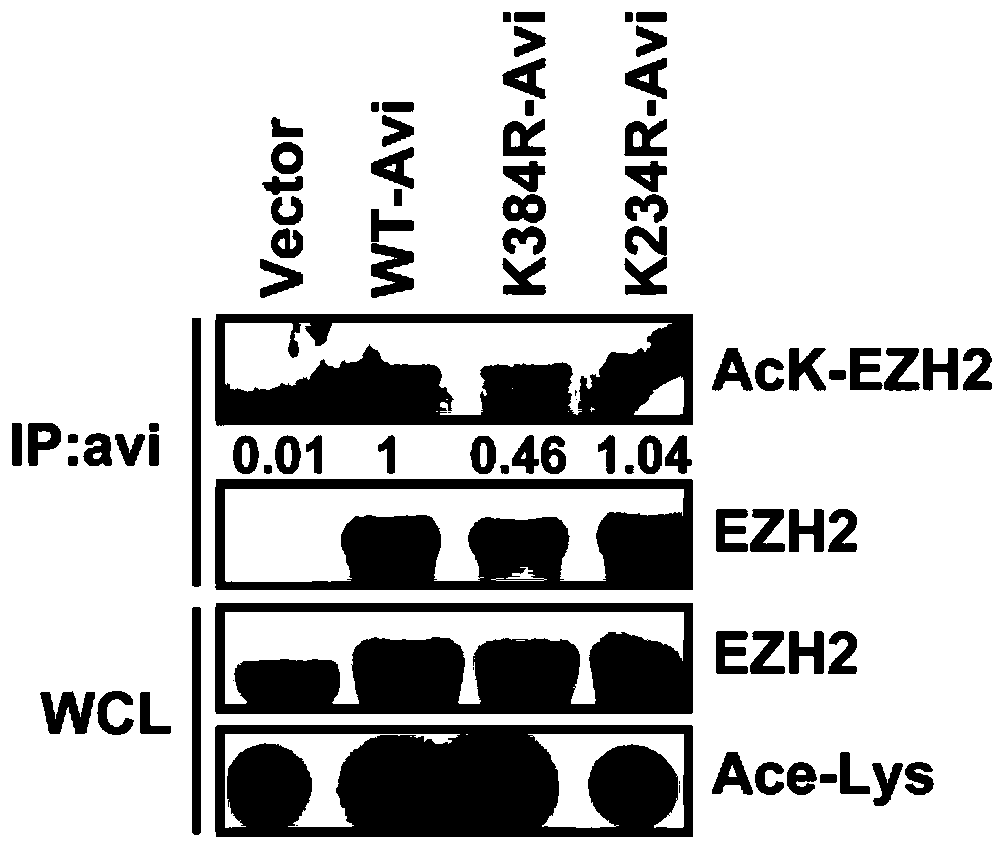
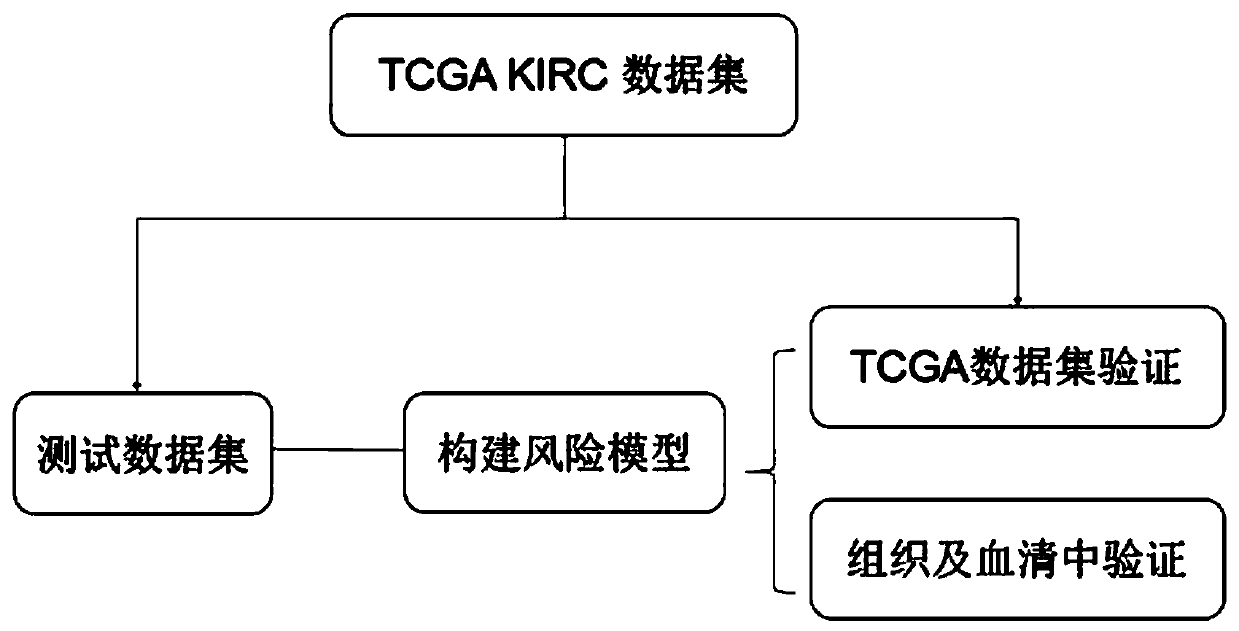
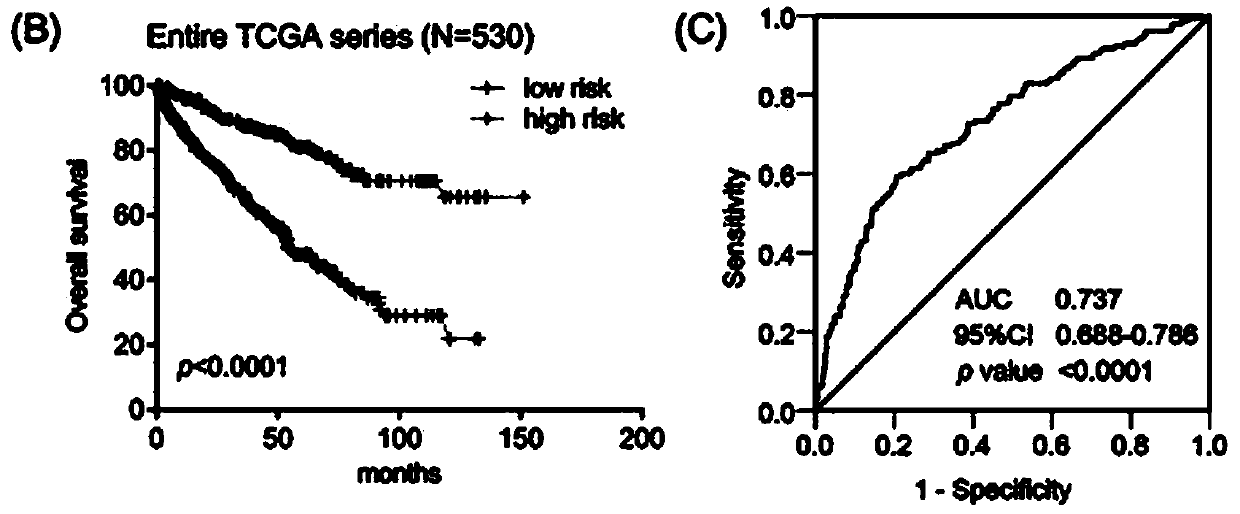
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "Renal clear cell carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
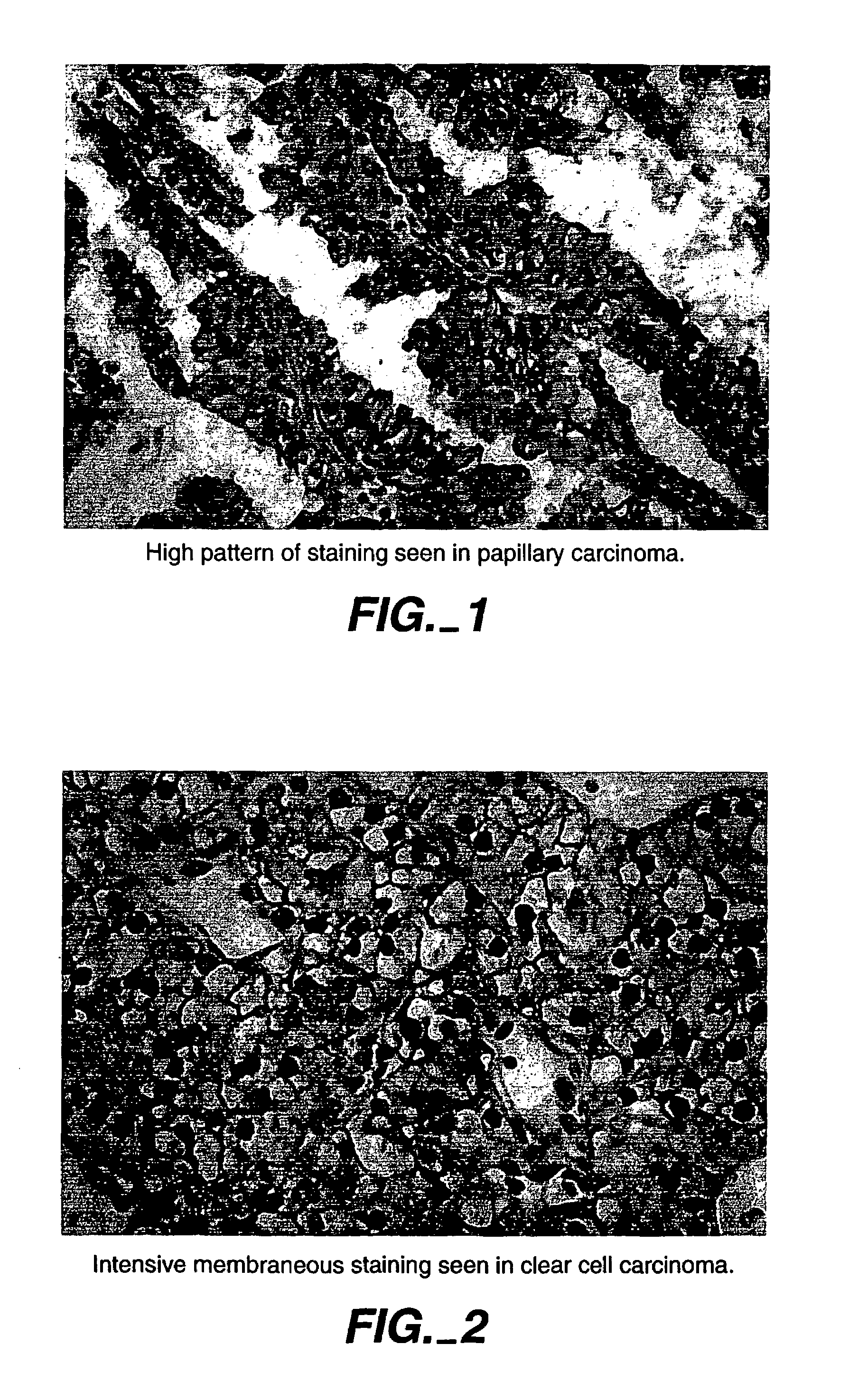
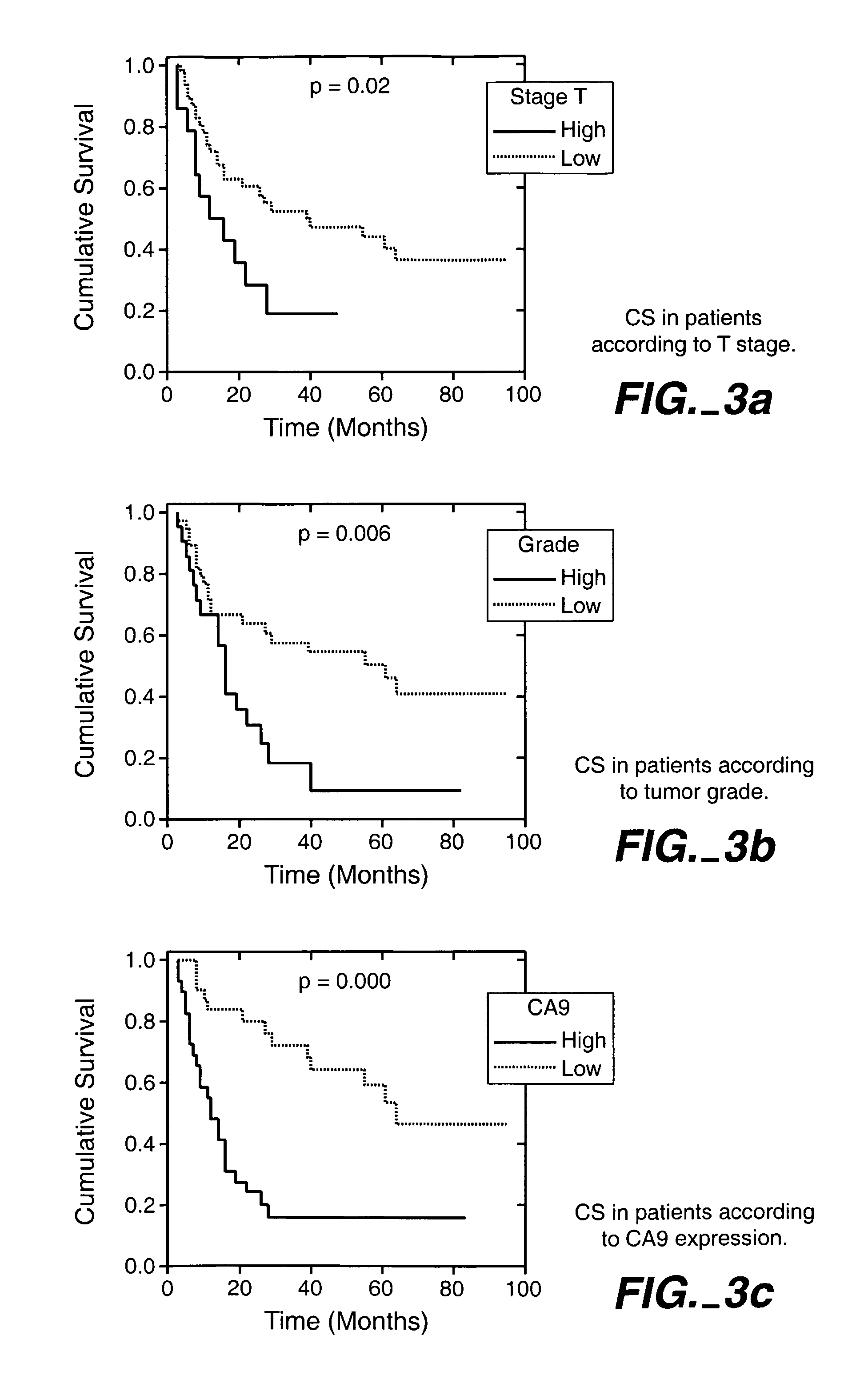
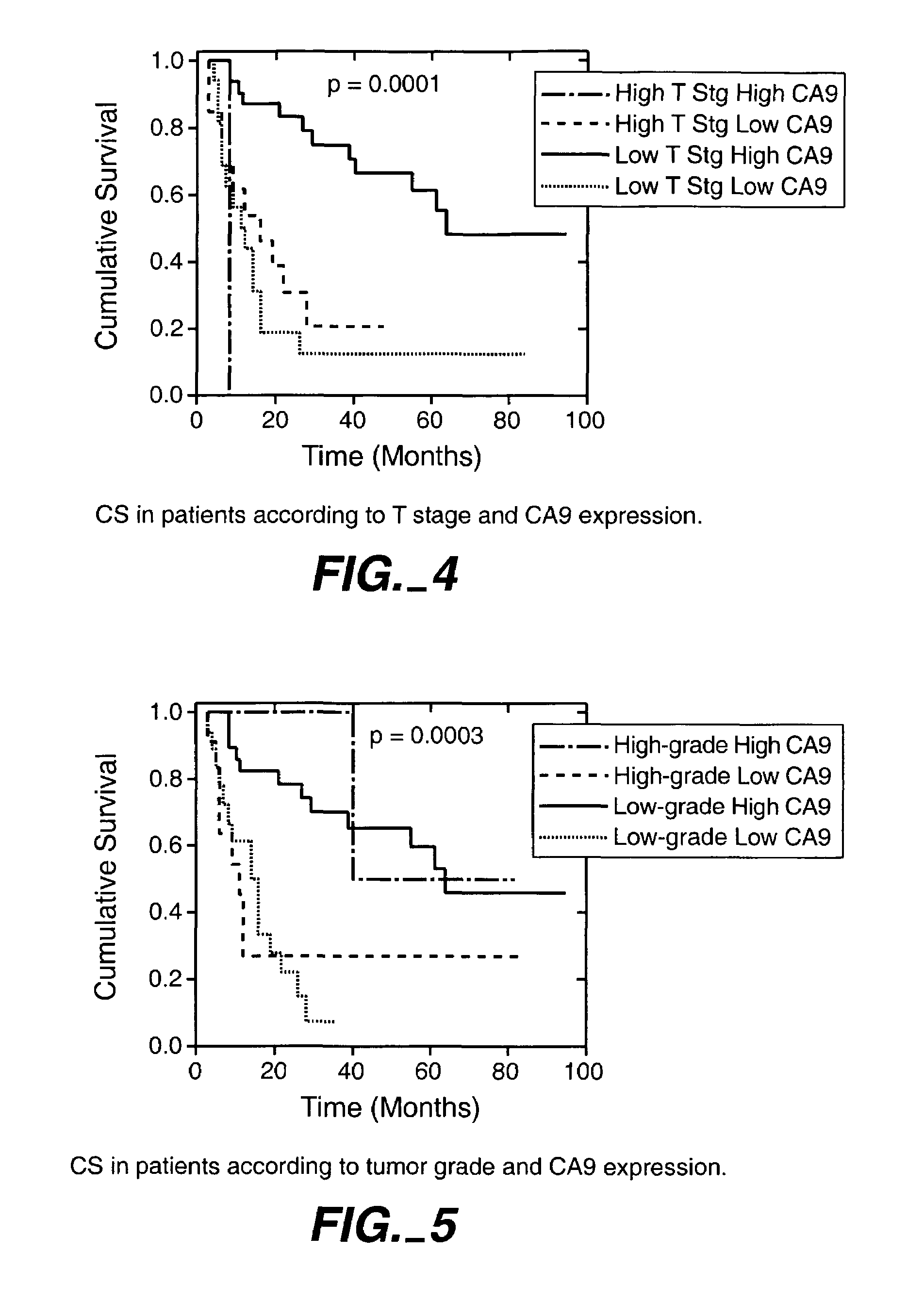
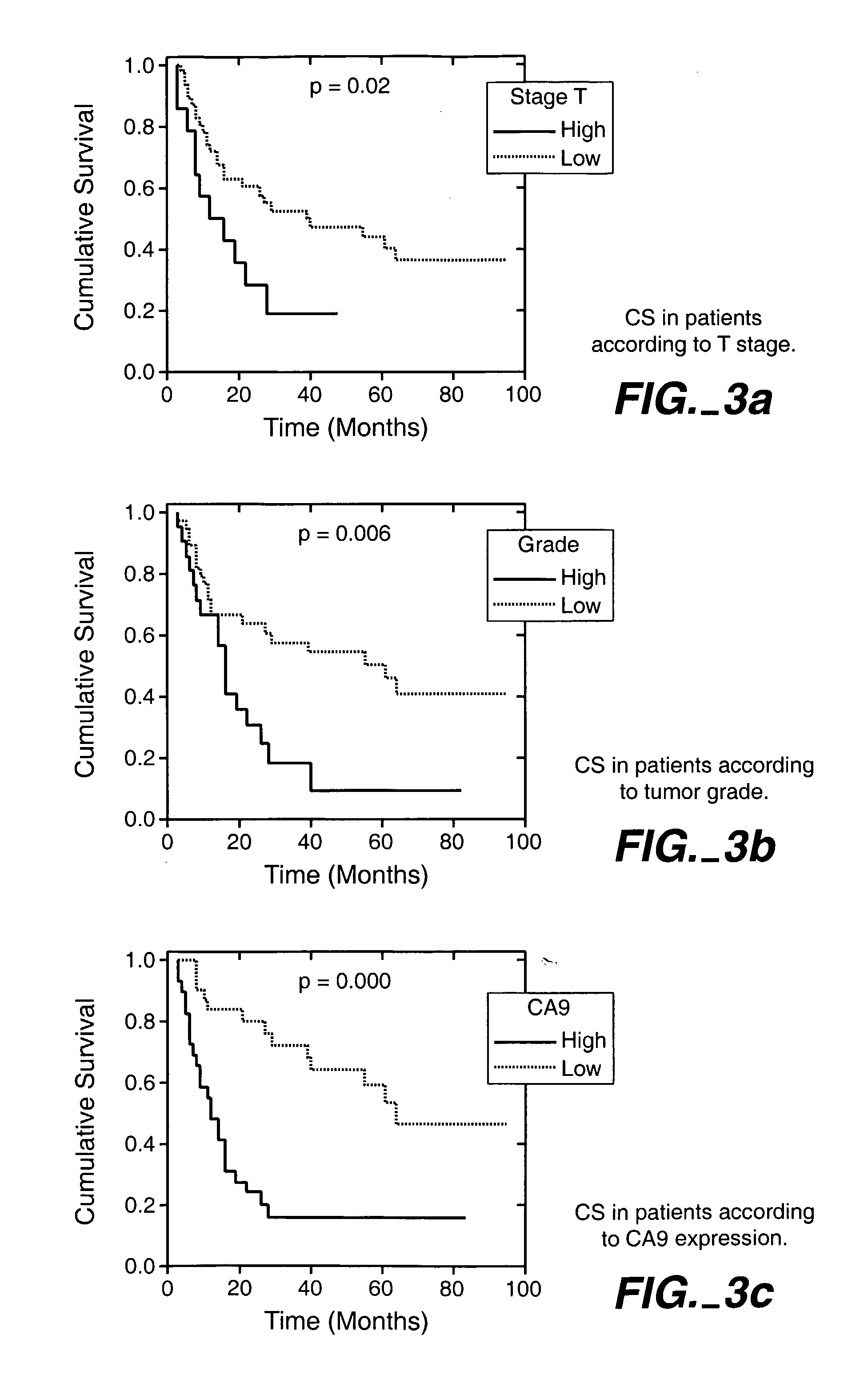
MN/CA IX/CA9 and Renal Cancer Prognosis
InactiveUS7482129B2Microbiological testing/measurementBiological testingRegimenRenal clear cell carcinoma
Herein disclosed are methods that are prognostic for renal cell carcinoma, particularly renal clear cell carcinoma, afflicting a vertebrate. An exemplary prognostic method comprises detecting the presence of, and quantitating the level and / or extent of a MN / CA9 gene expression product in a sample from the affected subject, wherein if 50% or fewer cells are found to express the MN / CA9 gene, then the subject is considered to have a poorer prognosis. MN / CA9 gene expression products useful in the prognostic methods include MN protein, MN polypeptide and / or MN nucleic acids. The methods are useful as an aid in the selection of treatment for a patient with renal cell carcinoma, alone or in combination with conventional tumor stage and / or grade information. The methods of the invention can be used, for example, to identify those patients requiring more aggressive therapy regimens, or those patients most likely to respond to adjuvant immunotherapies, particularly MN / CA IX / CA9-targeted therapies.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI +1
Adjuvant theraphy of G250-expressing tumors
ActiveUS7691375B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsRenal clear cell carcinomaOncology
The invention relates to a method for the treatment of G250-antigen-expressing tumors, in particular renal clear cell carcinoma comprising the administration of G250-antigen-specific antibodies to high-risk patients diagnosed with non-metastasizing disease.
Owner:WILEX AG
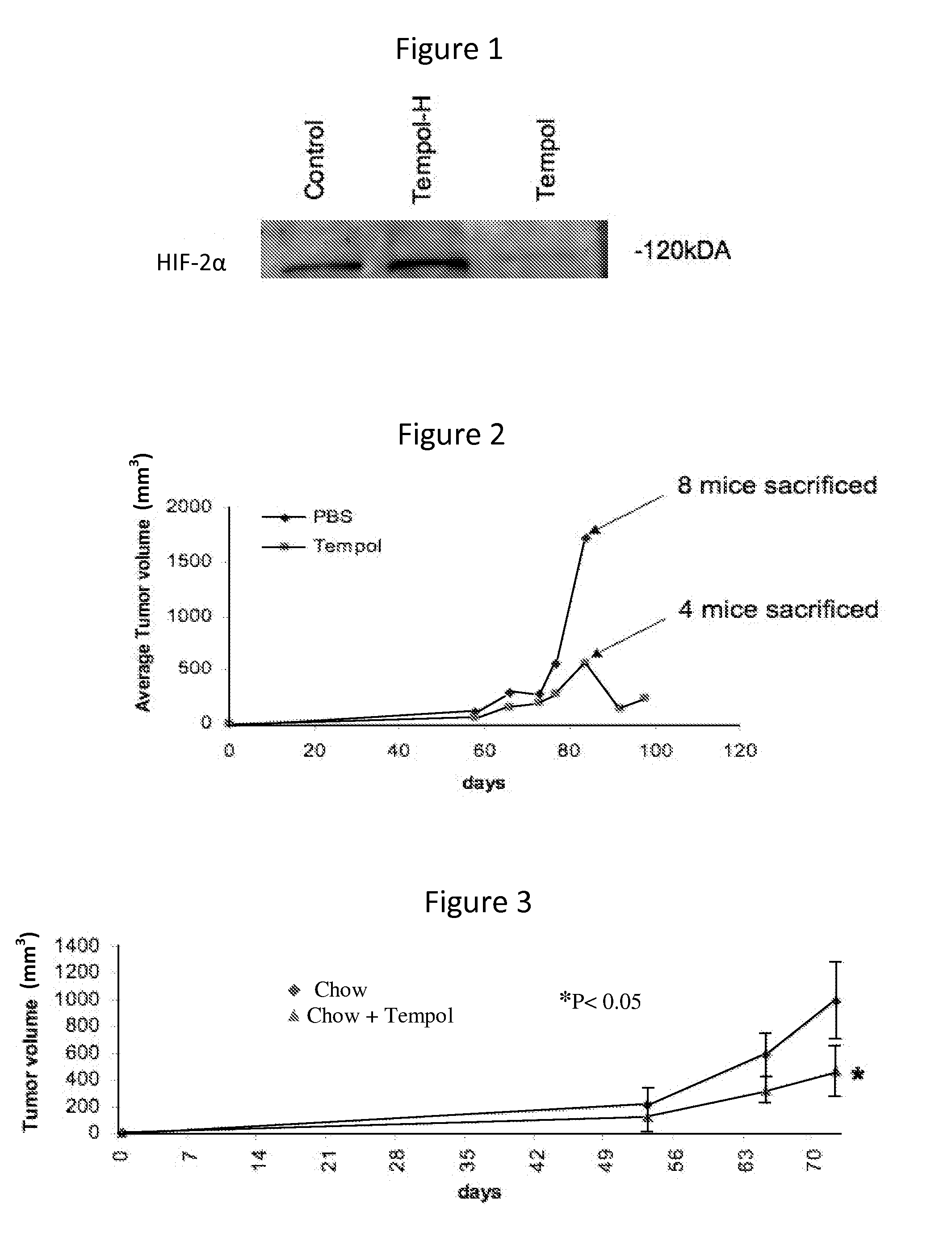
Nitroxide therapy for the treatment of von hippel - lindau disease (VHL) and renal clear cell carcinoma (RCC)
ActiveUS20120295937A1Reduce transcriptionLower Level RequirementsBiocideAnimal repellantsRenal clear cell carcinomaLindau' disease
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Renal cancer sunitinib drug-resistant cell system and establishing method thereof
InactiveCN104988122AHigh scientific researchIncrease production capacityMicrobiological testing/measurementMicroorganism based processesCancer cellKidney cancer
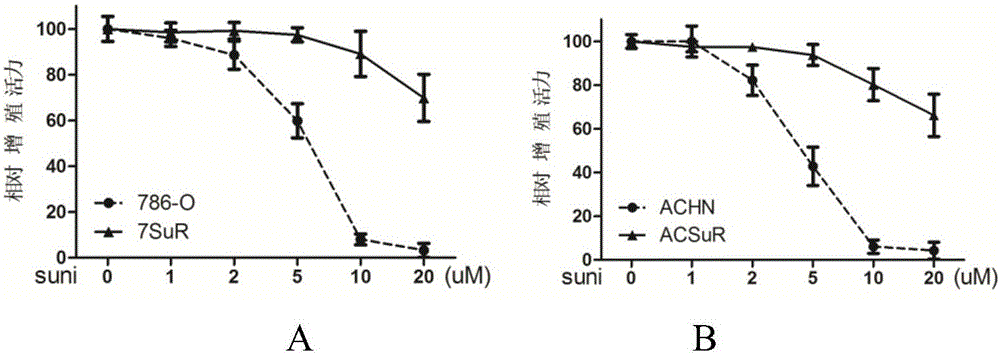
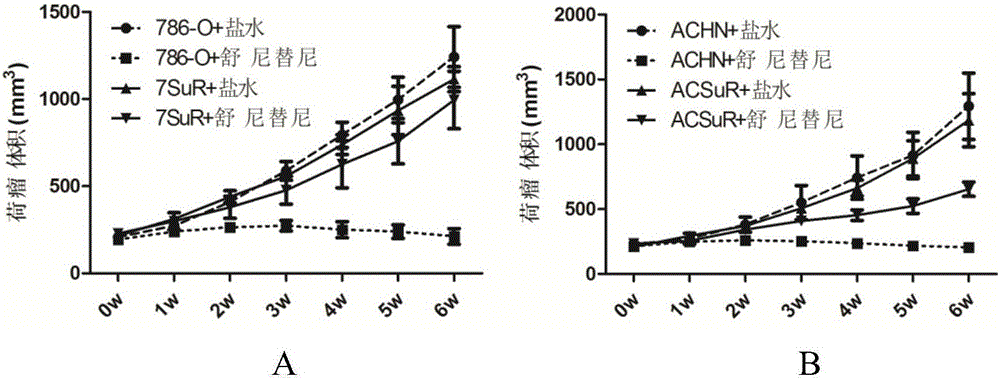
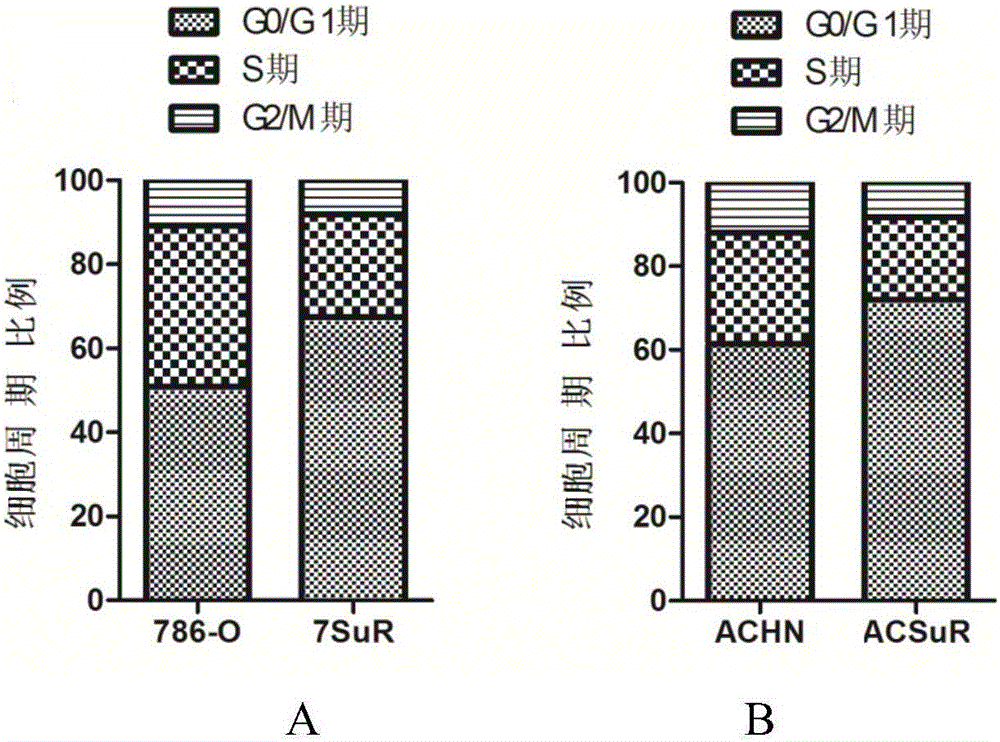
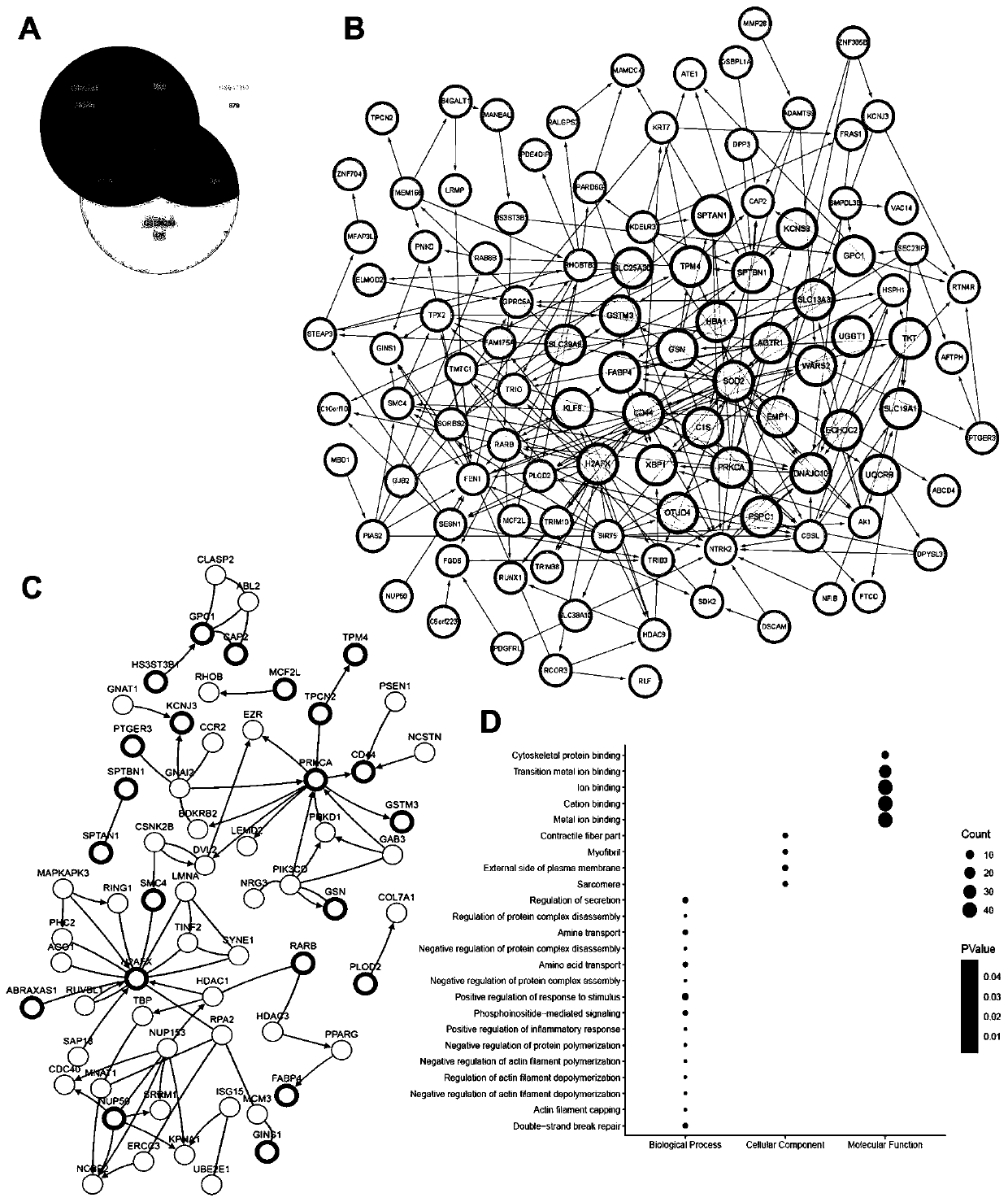
The invention relates to the technical field of biomedicine, and in particular to a renal cancer sunitinib drug-resistant cell system and an establishing method and application of the renal cancer sunitinib drug-resistant cell system. 786-O cells and ACHN cells of a human renal clear cell carcinoma system are adopted as objects for establishing a subcutaneous tumor-bearing nude mouse model; the clinical conventional dose of the sunitinib is used for treating a tumor-bearing nude mouse; and through multiple times of in-vivo continuous passage and drug pressure screening, the renal cancer drug-resistant cell system free of restraints of the sunitinib in vitro and vivo is finally obtained through separate culture and named as 7SuR and ACSuR. The established renal cancer sunitinib drug-resistant cell system can better simulate clinical drug resistance and provides an important platform for studying renal cancer targeted drug resistance mechanism, reversing renal cancer cell drug resistance and developing and evaluating new anti-cancer drugs.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Combined genome for evaluating prognosis of clear cell renal cell carcinoma (ccRCC) and application of combined genome
PendingCN111575376AImprove survival rateMicrobiological testing/measurementDNA/RNA fragmentationRenal clear cell carcinomaClear cell renal cell carcinoma
The invention discloses a combined genome for evaluating prognosis of clear cell renal cell carcinoma (ccRCC) and application of the combined genome, relates to the technical field of medical biological detection, and provides novel application of the combined genome of ADAMTS9, C1S, DPYSL3, H2AFX, MINA, PLOD2, RUNX1, SLC19A1, TPX2 and TRIB3, particularly application in preparation of a ccRCC prognosis evaluation reagent or kit. The genome is derived from a molecular marker significantly correlated with a ccRCC metastasis way, and the discovery of the genome model provides a new strategy for predicting the ccRCC recurrent risk and the long-term survival condition of a patient after operation, plays important roles in judging the prognosis of the ccRCC patient, can evaluate the risk level of tumor progression or death of the patient after ccRCC operation, contributes to guiding a clinician to perform an individualized precise therapeutic strategy, can increase the postoperative survivalrate of the patient, and has important guide significance postoperative follow-up monitoring and sequential therapy management of the ccRCC patient.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
Marine bacillus polypeptide and preparation and application thereof
InactiveCN104610432ASolve key problemsStrong cytotoxicityPeptide/protein ingredientsMicroorganism based processesMetaboliteCytotoxicity
The invention discloses a marine bacillus polypeptide and a preparation and an application thereof and belongs to the technical field of marine microorganism medicines. The marine bacillus polypeptide is from metabolites of antarctic marine bacillus N11-8 subjected to fermentation cultivation; 15 amino acid sequences at N-ends of the active polypeptide are ASTGSQKVTVYAVAD; the active polypeptide has relatively high cytotoxicity to a plurality of tumor cells such as human hepatoma carcinoma cell BEL-7402, human ovarian carcinoma cells NIH:OVCAR-3, human renal clear cell carcinoma cells 786-0 and human large cell lung cancer cells NCI-H460, and has relatively good research and application value. The polypeptide can be applied to medicines for preventing and / or treating the cancers.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Diagnosis marker and treatment target of renal clear cell carcinoma
InactiveCN107385083ATreatment reachedCompound screeningApoptosis detectionLymphatic SpreadCancer cell
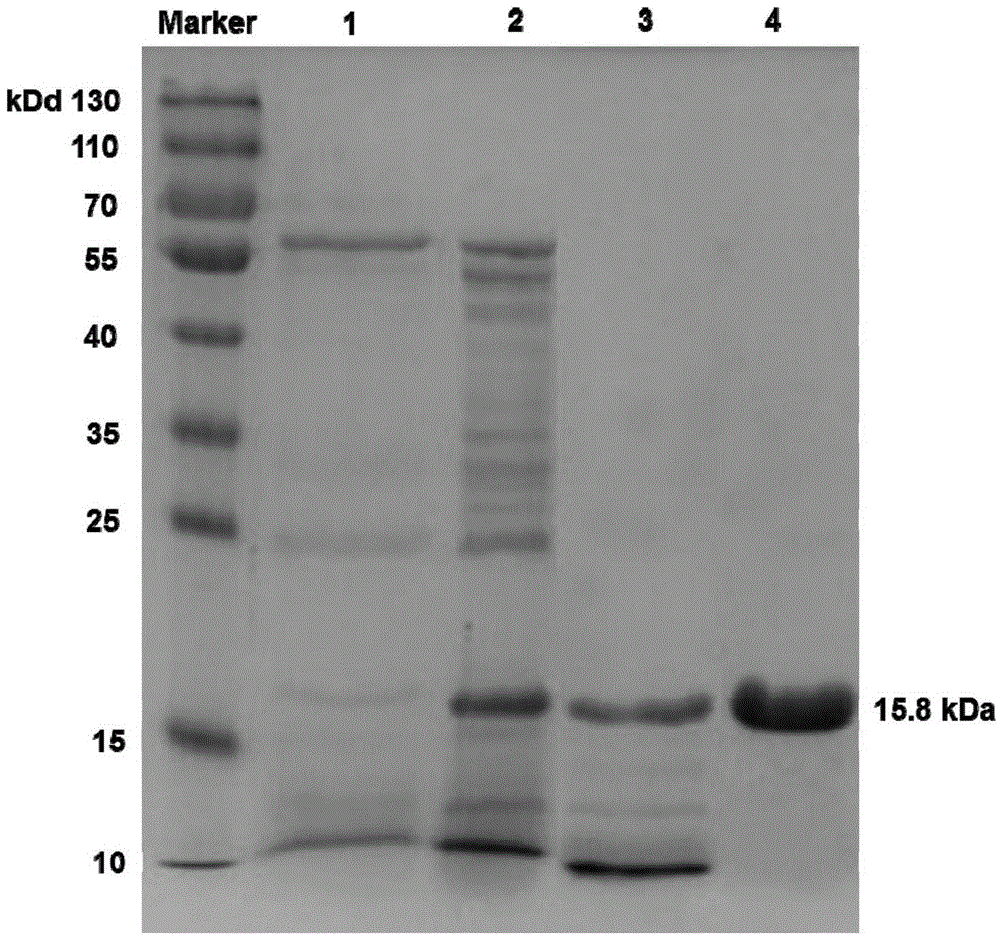
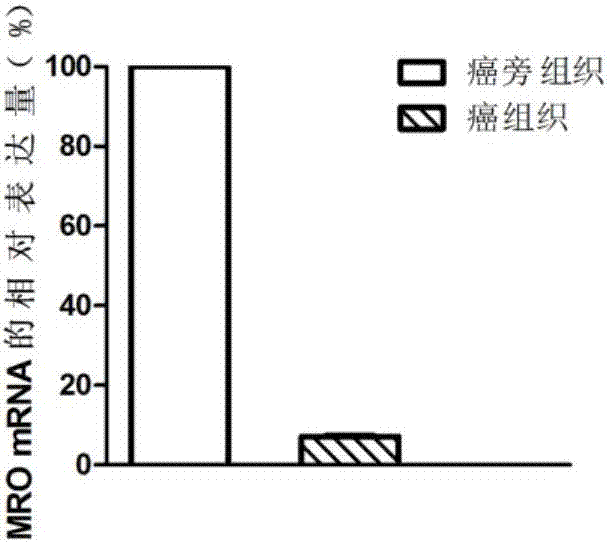
The invention discloses a diagnosis marker and treatment target of renal clear cell carcinoma and belongs to the technical field of gene new application. The diagnosis marker and treatment target is MRO. The diagnosis marker and treatment target has the advantages that the fact that the expression of the marker MRO in a renal clear cell carcinoma is reduced is discovered for the first time, the proliferation, migration and invasion of cancer cells can be lowered by increasing the expression of the MRO, and the MRO serving as the target can be used for treating the renal clear cell carcinoma and renal carcinoma metastasis.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Mn/Ca Ix/Ca9 and Renal Cancer Prognosis
InactiveUS20070224606A1Microbiological testing/measurementBiological testingRegimenRenal clear cell carcinoma
Herein disclosed are methods that are prognostic for renal cell carcinoma, particularly renal clear cell carcinoma, afflicting a vertebrate. An exemplary prognostic method comprises detecting the presence of, and quantitating the level and / or extent of a MN / CA9 gene expression product in a sample from the affected subject, wherein if 50% or fewer cells are found to express the MN / CA9 gene, then the subject is considered to have a poorer prognosis. MN / CA9 gene expression products useful in the prognostic methods include MN protein, MN polypeptide and / or MN nucleic acids. The methods are useful as an aid in the selection of treatment for a patient with renal cell carcinoma, alone or in combination with conventional tumor stage and / or grade information. The methods of the invention can be used, for example, to identify those patients requiring more aggressive therapy regimens, or those patients most likely to respond to adjuvant immunotherapies, particularly MN / CA IX / CA9-targeted therapies.
Owner:BIOMEDICAL RES CENT OF THE SLOVAK ACADEMY OF SCI +1
Biomarker relating to renal clear cell carcinoma
ActiveCN107496923APrecision therapyAchieve early detectionOrganic active ingredientsGenetic material ingredientsCell cancerCancer cell
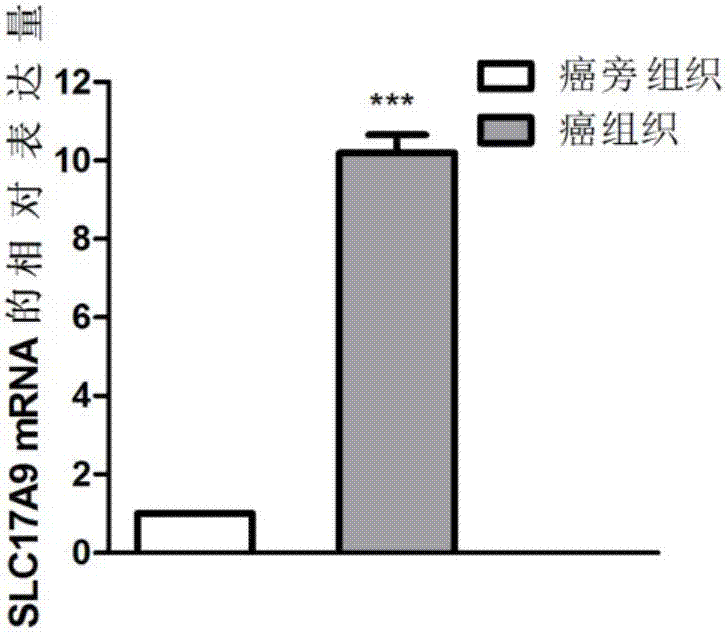
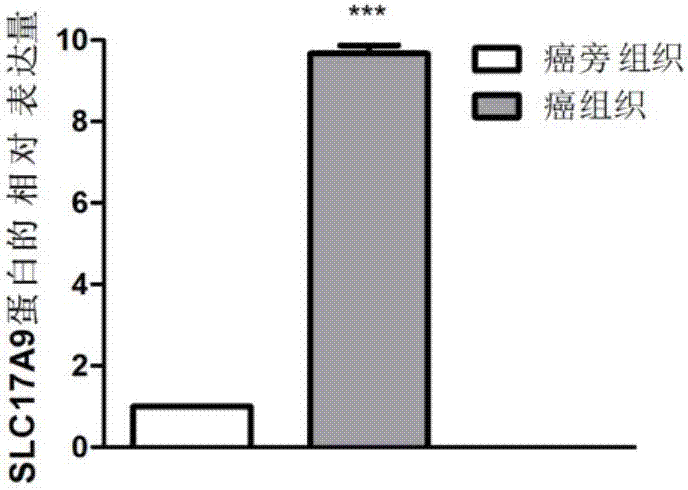
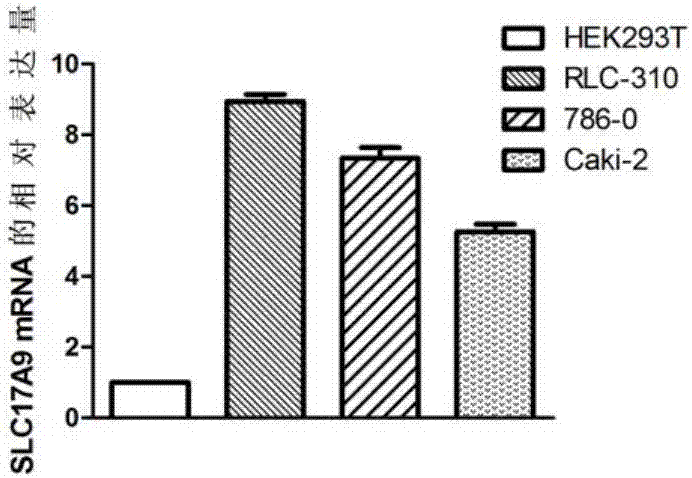
The invention discloses a biomarker relating to renal clear cell carcinoma. The biomarker is SLC17A9. Experiments prove that the expression of the SLC17A9 is up-regulated in patients suffering from the renal clear cell carcinoma, proliferation and invasion of the renal clear cell carcinoma cells can be inhibited, and the SLC17A9 can serve as a diagnosis and / or treatment target to be applied to clinical renal clear cell carcinoma diagnosis and treatment.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Application of lncRNA combination to preparation of product for predicting renal clear cell carcinoma prognosis and molecular targeted medicine treatment sensitivity
InactiveCN108277283ASensitivity PreciseSensitivity Rapid JudgmentMicrobiological testing/measurementMolecular targeted drugRenal clear cell carcinoma
The invention discloses application of lncRNA combination to preparation of a product for predicting renal clear cell carcinoma prognosis and molecular targeted medicine treatment sensitivity. The lncRNA or the lncRNA combination serving as a detection target point is applied to preparation of the product for predicting renal clear cell carcinoma prognosis and molecular targeted medicine treatmentsensitivity, and the lncRNA or the lncRNA combination is selected from any one or more of SEQ ID NO.1 to SEQ ID NO.4. A new clinical method is provided for evaluating prognosis of patients sufferingfrom renal clear cell carcinoma, new reference advice is provided for auxiliary treatment after operation of the renal clear cell carcinoma, and important clinical significance and popularization andapplication prospect in the aspects of timely taking effective clinical measures, formulating an individualized diagnosis and treatment scheme and finally increasing the survival rate of the patientssuffering from the renal clear cell carcinoma are achieved.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
MiR-125b-2-3p as molecular marker for differential diagnosis of renal cancer subtypes and use thereof in tumor metastasis
The present invention relates to a MiR-125b-2-3p as a molecular marker for differential diagnosis of renal cancer subtypes and a use thereof in tumor metastasis. The biomolecular marker MiR-125b-2-3prelated to genesis and development of renal clear cell carcinoma is firstly discovered. By detecting an expression of the MiR-125b-2-3p in patient tissues and blood, early diagnosis of the renal clearcell carcinoma is experimented. Besides, a molecular target for treatment of the renal clear cell carcinoma is firstly provided. The treatment by the targeting and molecular marker in the metastaticrenal clear cell carcinoma has sensitivity and specificity.
Owner:NINGBO UNIV
Application of miR-577 for preparing nephrosis diagnosis marker
InactiveCN108624693AMicrobiological testing/measurementAntineoplastic agentsNephrosisTarget analysis
The invention relates to application of miR-577 for preparing a nephrosis diagnosis marker, in particular to application of miR-577 and a target gene thereof in preparing a renal clear cell carcinomadiagnosis marker. Database renal clear cell carcinoma mRNA (Ribonucleic Acid) and miRNA data are retrieved, and miR-577 and a target gene regulated and controlled by the miR-577 are selected to carryout molecular verification through data integration, differential expression analysis and targeted analysis. A result indicates that the miR-577 and the target genes CD1D, TMEM133 and SLFN11 regulatedand controlled by the miR-577 can serve as the diagnosis marker of the renal clear cell carcinoma, and therefore good clinic application value is performed.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
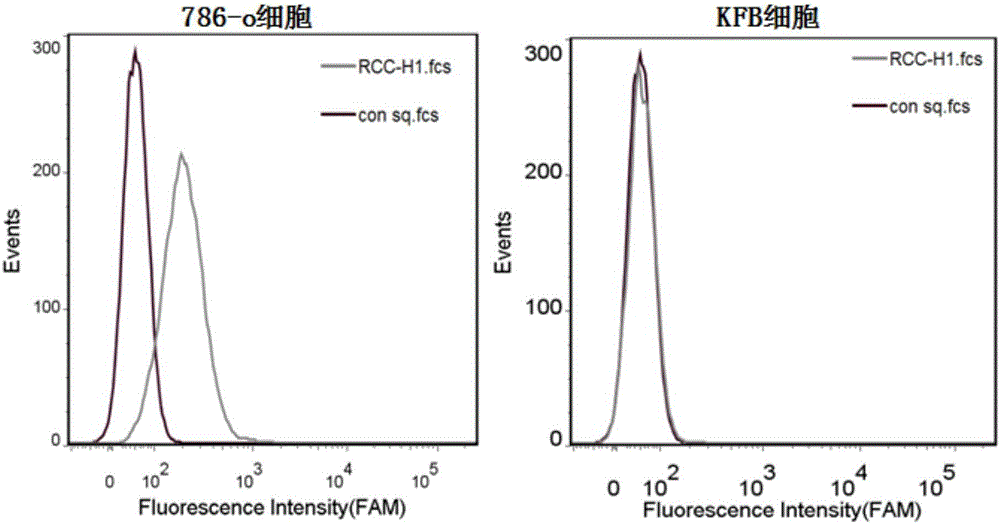
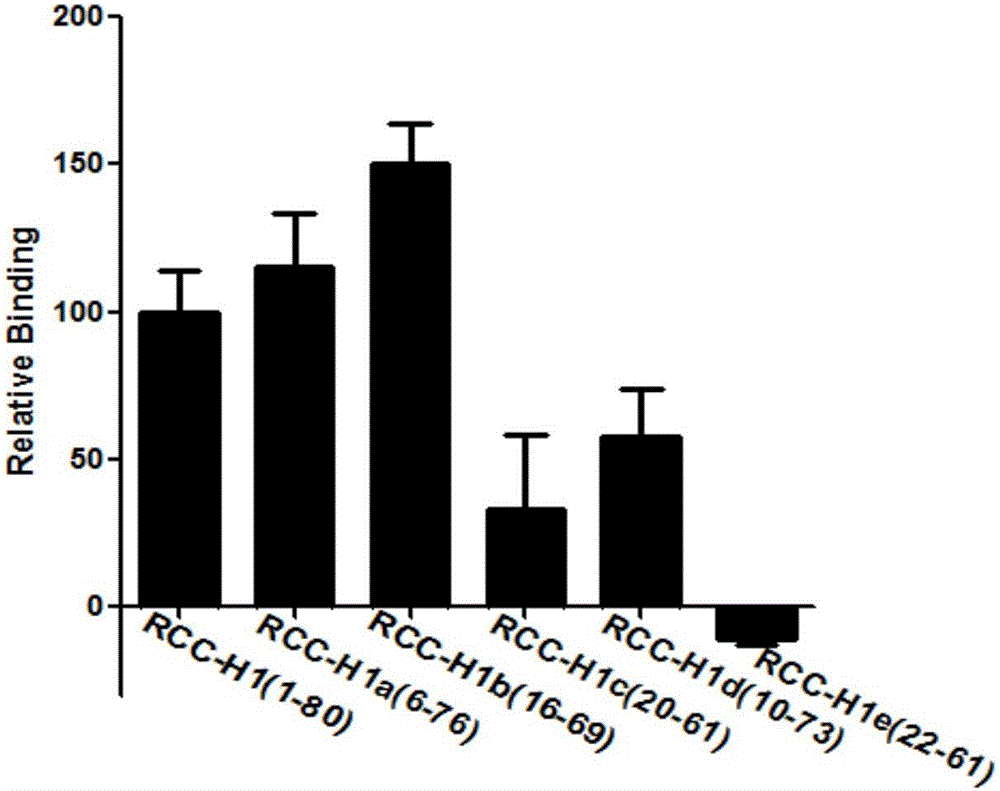
Aptamer for detecting human renal clear cell carcinoma cells and application of aptamer in preparing detection preparation
ActiveCN106754938AHigh affinityImprove featuresMaterial analysisDNA/RNA fragmentationAptamerCancer cell
The invention discloses an aptamer for detecting human renal clear cell carcinoma cells (786-O) and application of the aptamer in a preparing detection preparation. Compared with the prior art, the aptamer has affinity and specificity higher than those of an associated protein antibody, is free of immunogenicity, can be chemically synthesized in vitro and is small in molecular weight. Furthermore, different parts of the aptamer can be modified and replaced, so that sequences are stable, storage is convenient, and labeling is convenient. When the aptamer disclosed by the invention is adopted to detect the human renal clear cell carcinoma cells, operations are simpler and quicker; and moreover, synthesis cost of the aptamer is lower than preparation cost of the antibody, the period is short and repeatability is good.
Owner:HUNAN UNIV
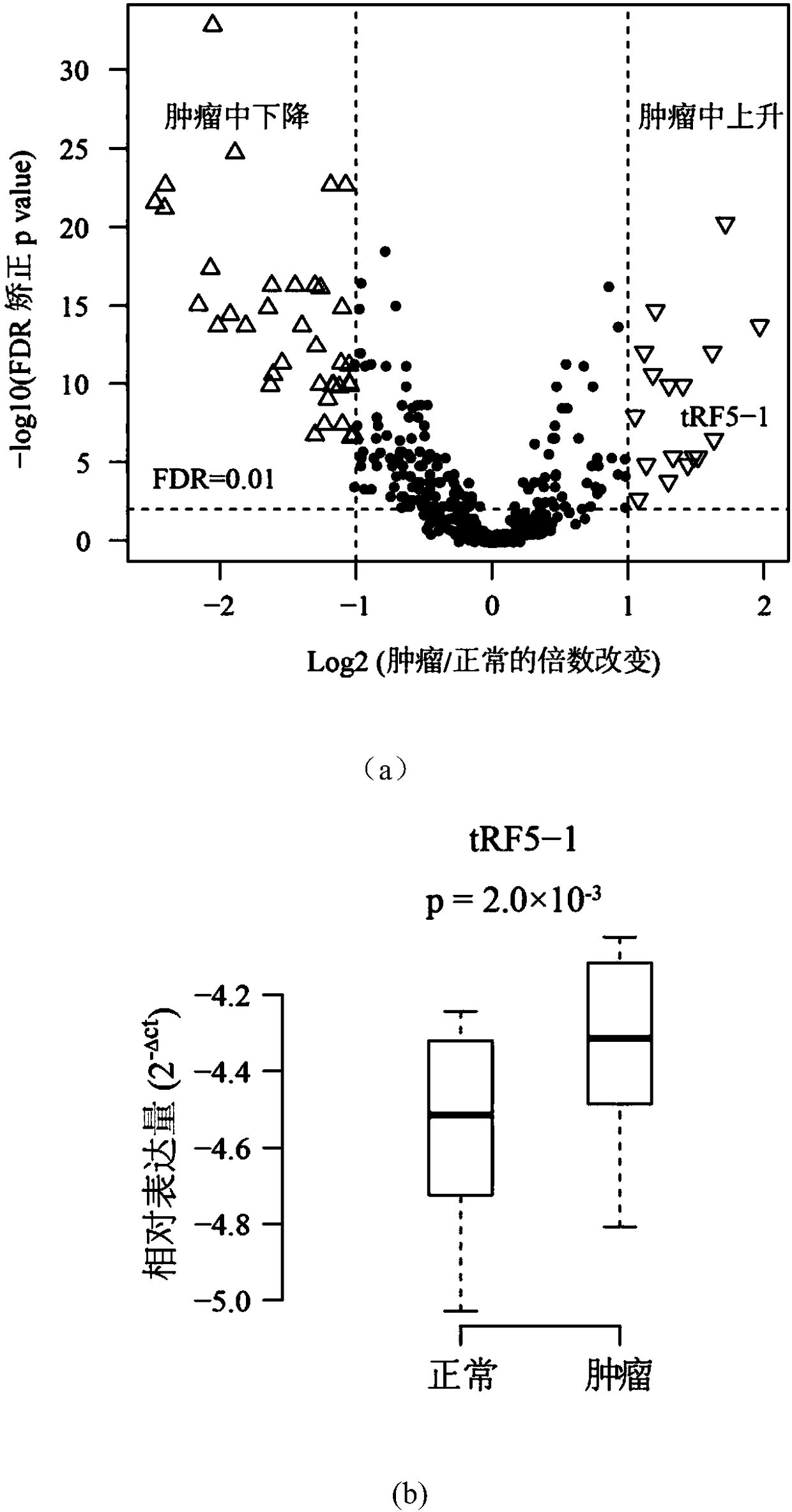
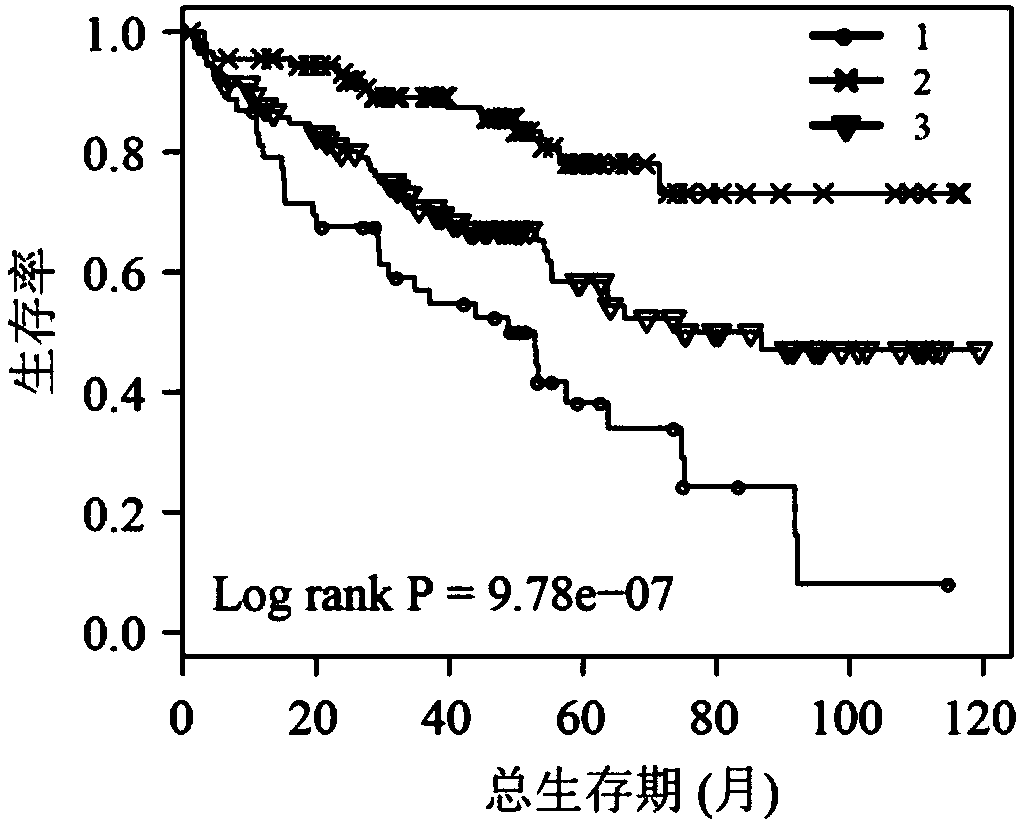
Application of novel molecular marker to preparation of kit for diagnosis and prognosis of renal clear cell carcinoma
ActiveCN108559777AHigh sensitivityStrong specificityMicrobiological testing/measurementProteomicsMathematical modelTumor Sample
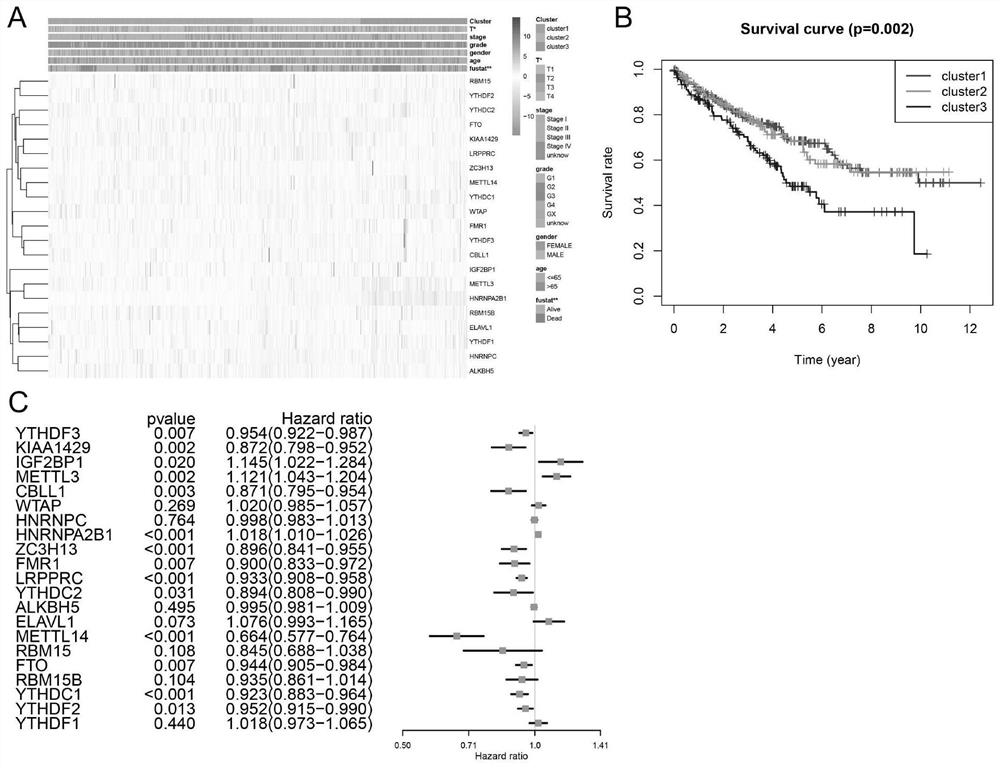
The invention discloses application of a novel molecular marker to the preparation of a kit for diagnosis and prognosis of renal clear cell carcinoma. The RNA sequence of the novel molecular marker isshown in SEQ ID NO: 1 to SEQ ID NO: 57. A mathematical model for diagnosis of renal clear cell carcinoma is constructed by taking the marker as a base; the model is high in sensitivity and good in specificity, AUC can be as high as 0.997, and the diagnosis effect is good. In addition, 57 tRF fragments can serve molecular markers for classifying renal clear cell carcinoma and predicating the survival periods of patients; in test data, a renal clear cell carcinoma tumor sample is clustered into 3 subtypes according to rRFs expression, and survivorship curve analysis shows that the survival periods of the subtypes have obvious difference. The novel tRF molecular marker has excellent diagnosis index characteristic, can be effectively applied to the diagnosis, classification and prognosis of renal clear cell carcinoma, and has high clinical application and promotion values.
Owner:ZHEJIANG UNIV
Diagnostic marker-C16orf74 gene of renal clear cell carcinoma
ActiveCN108531607AProvide survival rateMicrobiological testing/measurementBiological material analysisRenal clear cell carcinomaBiomarker (petroleum)
The invention discloses a C16orf74 gene which can be taken as a biomarker for the renal clear cell carcinoma. The experiment proves that compared with normal nephridial tissues, the C16orf74 gene expression in renal clear cell carcinoma tissues is significantly improved. According to the research result, the C16orf74 gene can be applied to research and development of a kit used for diagnosing therenal clear cell carcinoma and also can be used for researching and developing medicines capable of inhibiting the C16orf74 gene expression, thereby achieving clinical prevention and treatment for therenal clear cell carcinoma.
Owner:THE FIRST AFFILIATED HOSPITAL OF ARMY MEDICAL UNIV
Medicine combination for overcoming drug resistance of liver cancer and kidney cancer tumors and application of medicine combination
ActiveCN111420059AIncreased sensitivityImprove efficacyOrganic active ingredientsAntineoplastic agentsHepatocellular carcinomaRenal clear cell carcinoma
The invention discloses a medicine combination for overcoming drug resistance of liver cancer and kidney cancer tumors and an application of the medicine combination. The research of the invention shows that through combined use of an acetylase inhibitor anacardic acid (AA) and an EZH2 inhibitor GSK-126, tumor sensitivity of the EZH2 inhibitor is enhanced from two aspects of reducing stability ofthe EZH2 and inhibiting activity of the EZH2, the effect is obvious, and a new method and a new idea are provided for improving the drug effect and the application range of the EZH2 inhibitor. Experiments show that combined use of the acetylase inhibitor and the EZH2 inhibitor has strong killing effect on hepatocellular carcinoma and renal clear cell carcinoma cells, and the effect of combined useis obviously superior to the effect of single use of the EZH2 inhibitor, so that combined use of the acetylase inhibitor and the EZH2 inhibitor can really exert a synergistic effect, and a new methodis provided for improving the drug effect and application range of the EZH2 inhibitor.
Owner:SUN YAT SEN UNIV
Cyclic snoRNA biomarker used for diagnosing renal clear cell carcinoma, kit and applications
ActiveCN110229912AAvoid damageReduce medical costsMicrobiological testing/measurementDNA/RNA fragmentationDiseaseRenal clear cell carcinoma
The invention belongs to the field of biological detection, and specifically relates to a cyclic snoRNA biomarker used for diagnosing renal clear cell carcinoma, a kit and applications. The biomarkerconsists of the following cyclic snoRNA: SNORA2, SNORD12B, SNORA59B, SNORA70B, SNORD93 and SNORD116-2. The snoRNA that can obviously influence the lifetime of renal clear cell carcinoma patients can be screened, and differential expression and diagnosis capabilities can be verified in tissues and serum; and the biomarker can be effectively used for the diagnosis and prognosis of renal clear cell carcinoma, and the development and utilization of the biomarker can provide a novel direction for the diagnosis of tumor and other diseases and further treatment.
Owner:中国医科大学
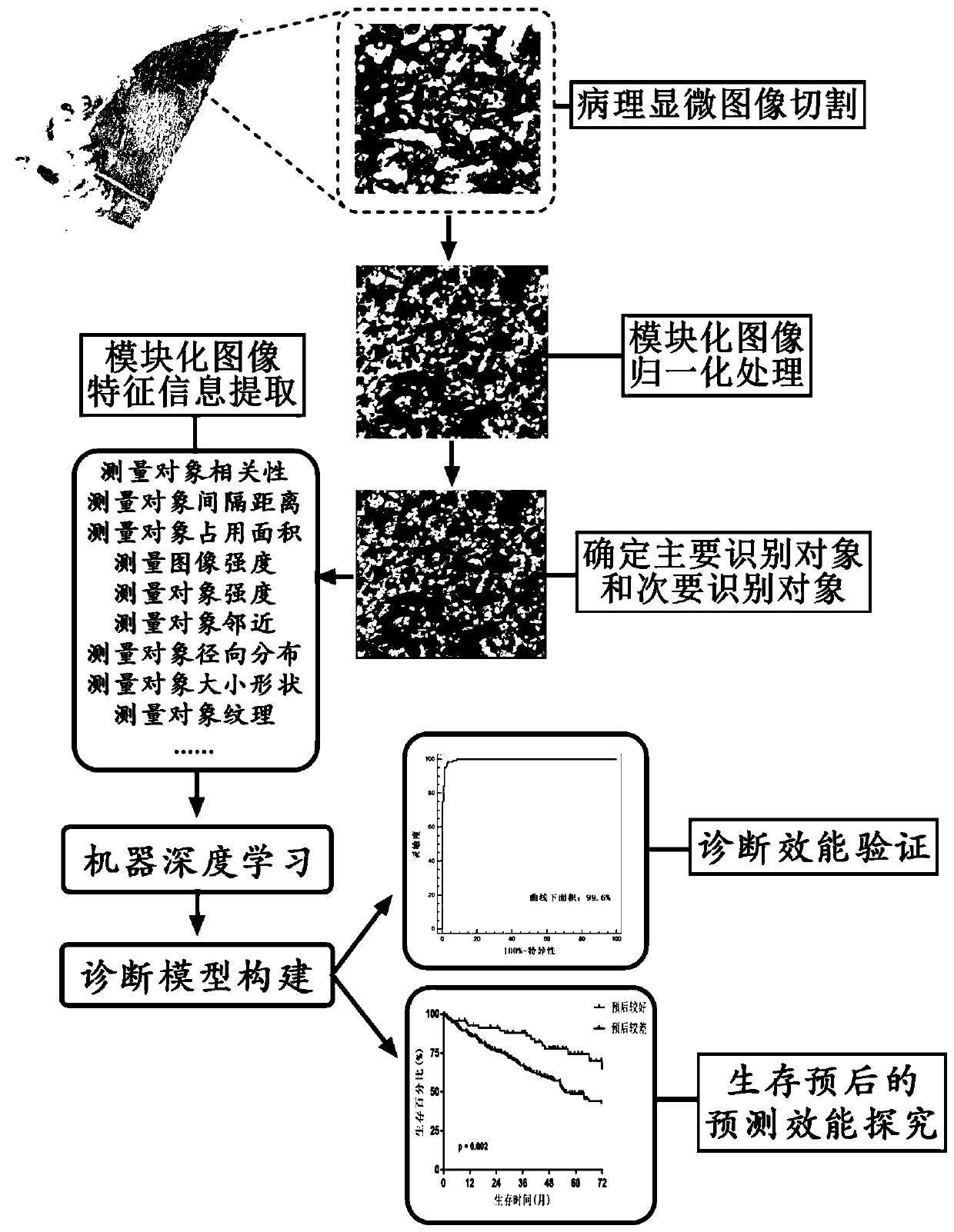
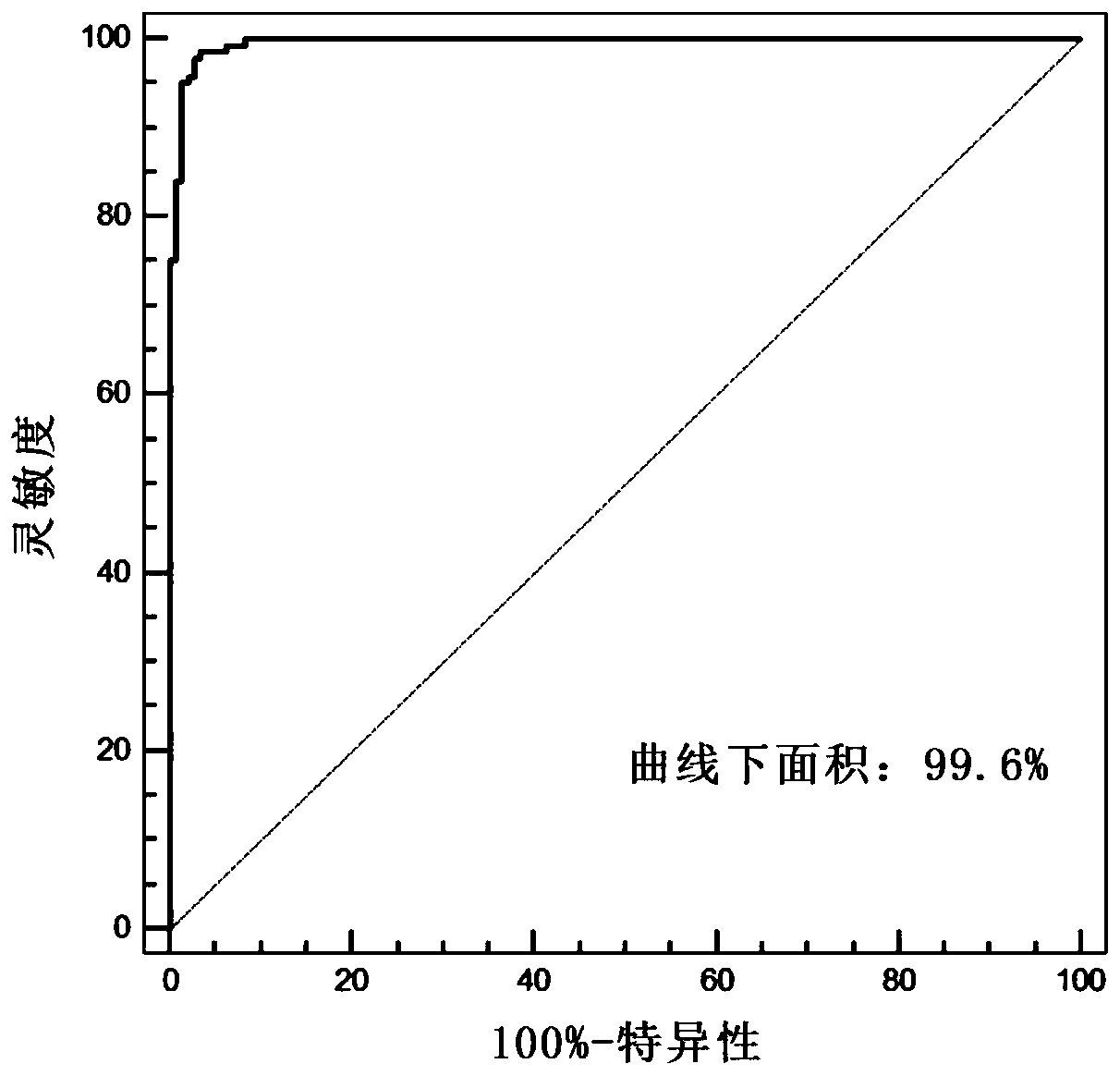
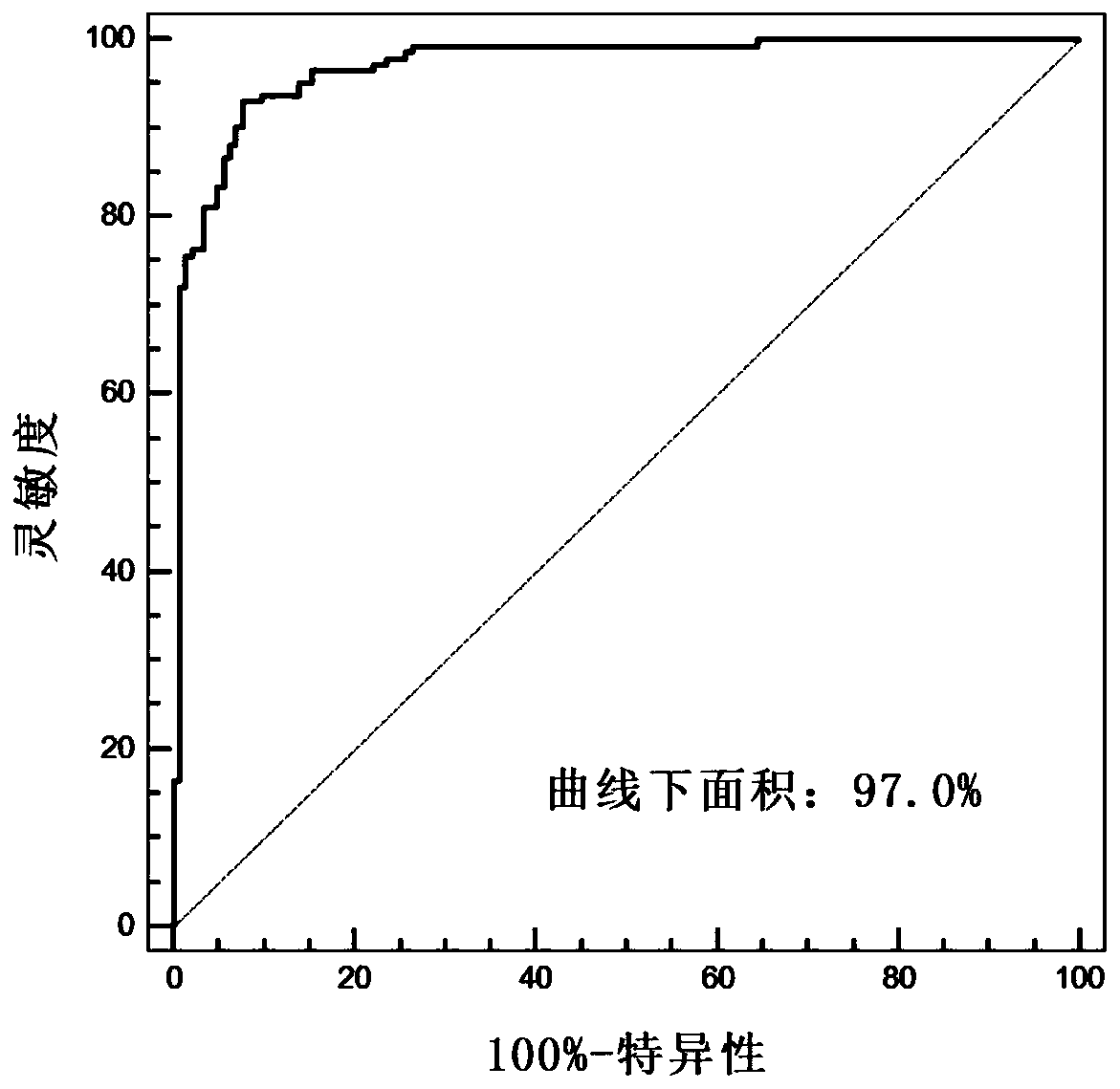
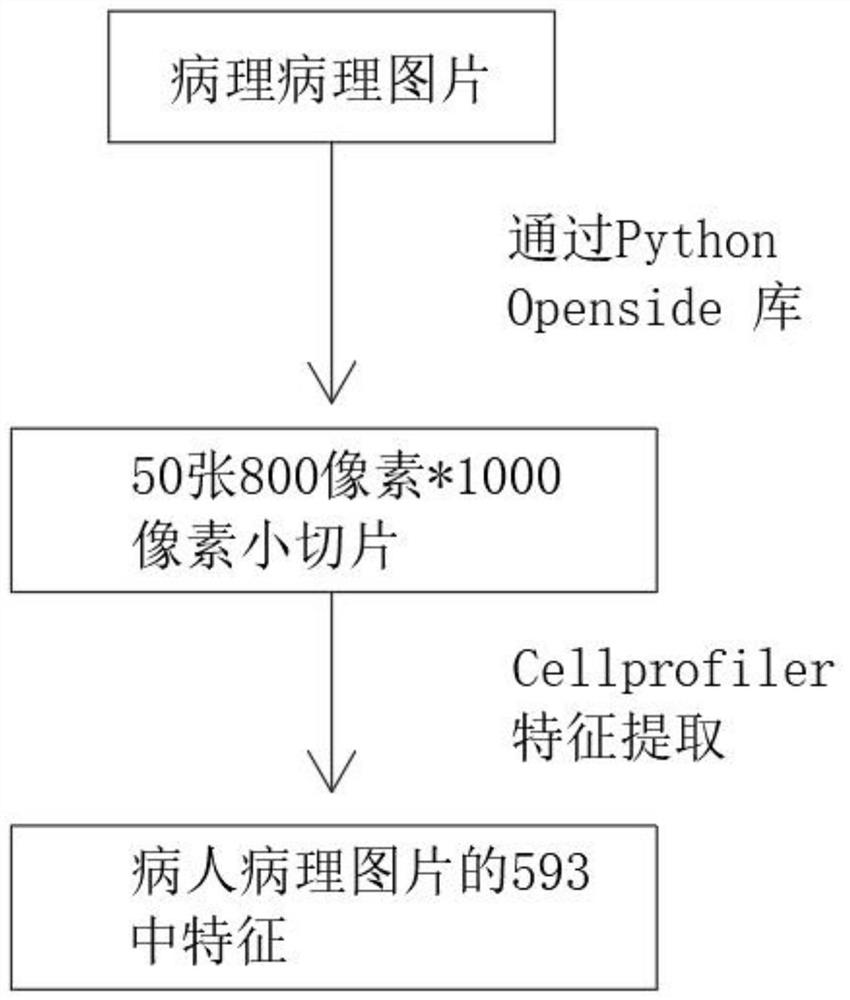
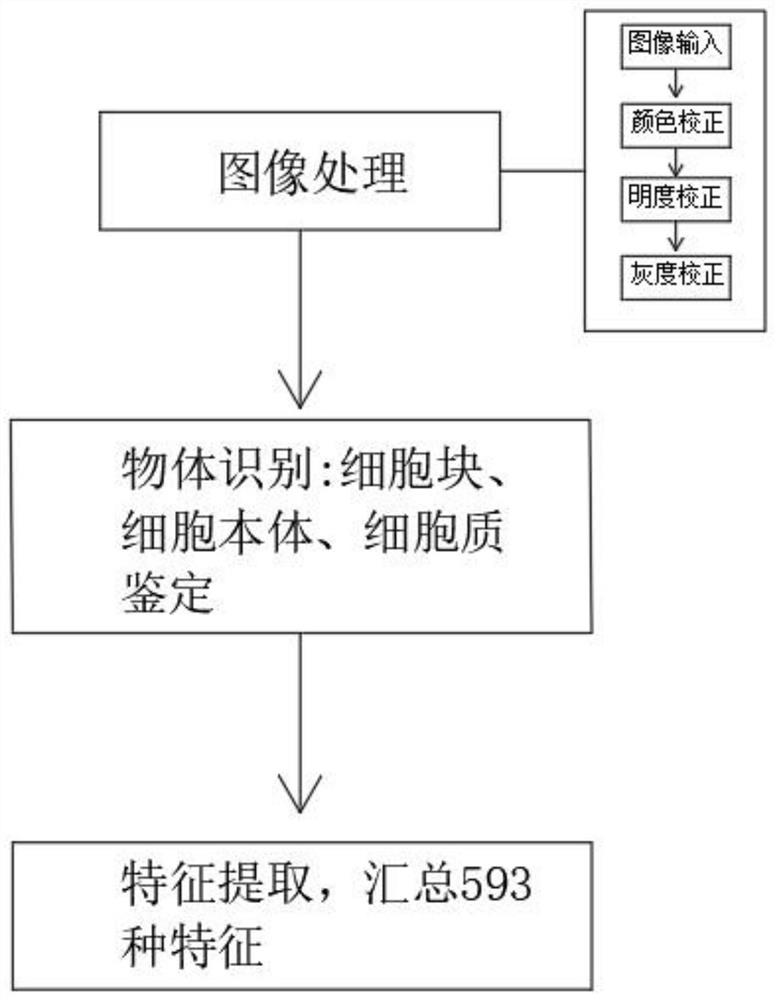
Artificial intelligence pathological diagnosis method for renal clear cell carcinoma based on deep learning
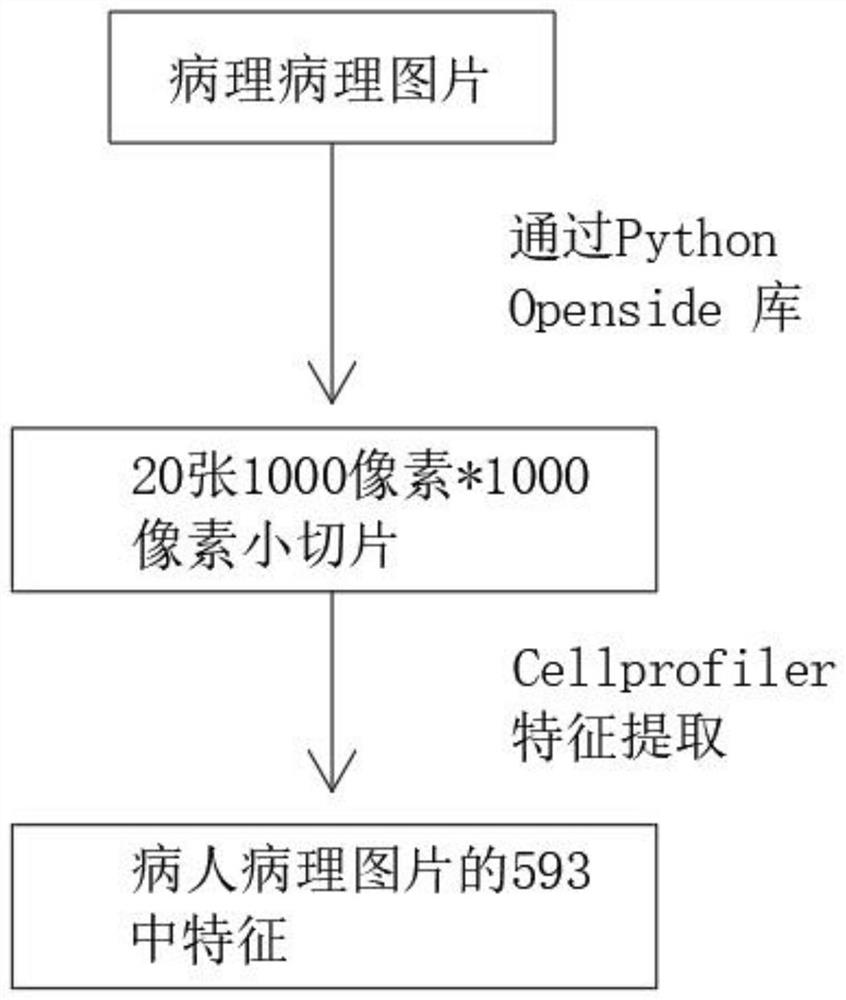
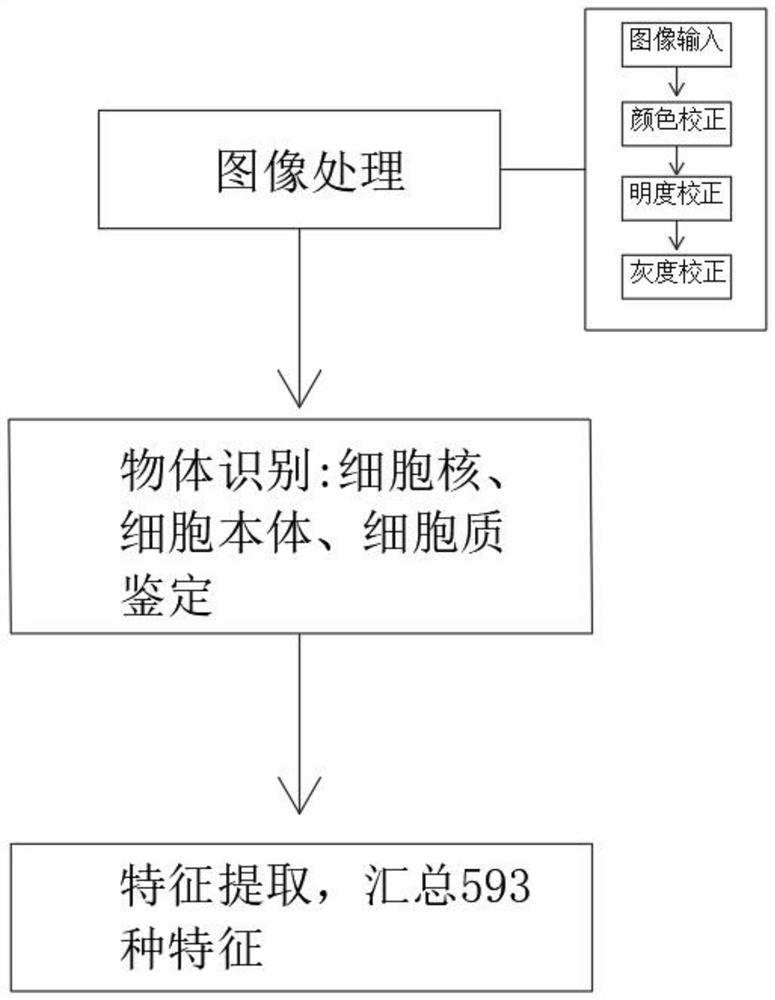
PendingCN111554381ASolve the problem of uneven pathological diagnosisEffectively predict survival prognosisImage enhancementImage analysisMicroscopic imageRenal clear cell carcinoma
The invention relates to an artificial intelligence pathological diagnosis method for renal clear cell carcinoma based on deep learning. The method comprises the following steps: S1, data acquisition;S2, pathological microscopic image processing; S3, modular image feature information extraction; S4, machine deep learning and diagnosis model construction; S5, diagnosis efficiency verification of the artificial intelligence diagnosis model: taking the artificial intelligence diagnosis model constructed by the image data in the training set as a diagnosis classifier, inputting the feature information data extracted by the test set for prediction, and evaluating the diagnosis efficiency of the artificial intelligence diagnosis model through a subject working feature curve; and S6, predictionefficiency research of survival prognosis of patients with renal clear cell carcinoma. The invention further provides a renal clear cell carcinoma artificial intelligence pathological diagnosis modelbased on deep learning. The method can effectively predict the survival prognosis of patients with renal clear cell carcinoma, can achieve the effect that cannot be achieved by traditional film reading diagnosis of pathologists, and can provide effective guidance opinions for judging whether the patients with renal clear cell carcinoma continue to be treated or not after operations.
Owner:SHANGHAI FIRST PEOPLES HOSPITAL
Application of arsenic trioxide in preparation of medicines for treating renal carcinoma
InactiveCN103211837AInorganic active ingredientsUrinary disorderCancer cellRenal clear cell carcinoma
The invention discloses application of arsenic trioxide in preparation of medicines for treating renal carcinoma. The key point of the technical scheme is that arsenic trioxide inhibits proliferation of human renal carcinoma cells 786-O by inhibiting the PI3K-Akt transduction path, so that arsenic trioxide can be used for preparing medicines for treating renal carcinoma, and in particular for preparing medicines for treating renal clear cell carcinoma.
Owner:XINXIANG MEDICAL UNIV
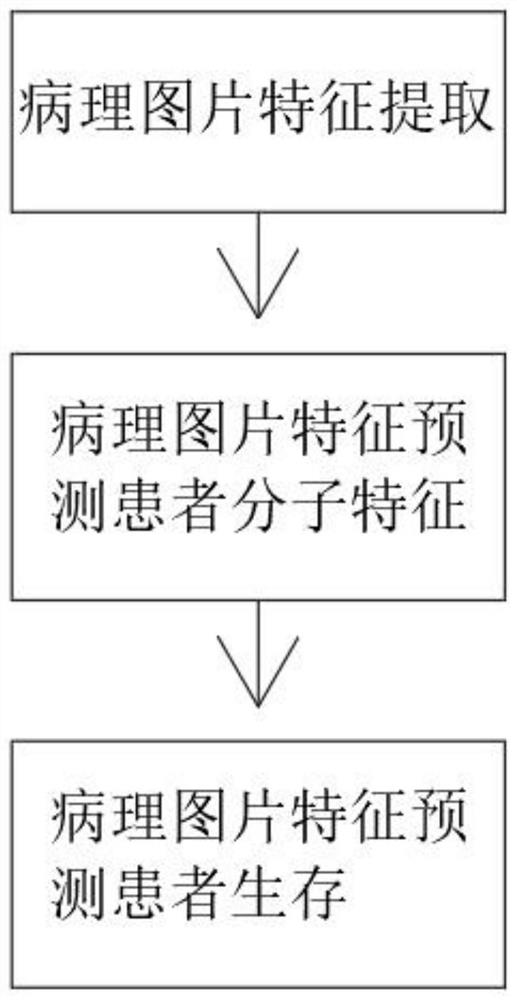
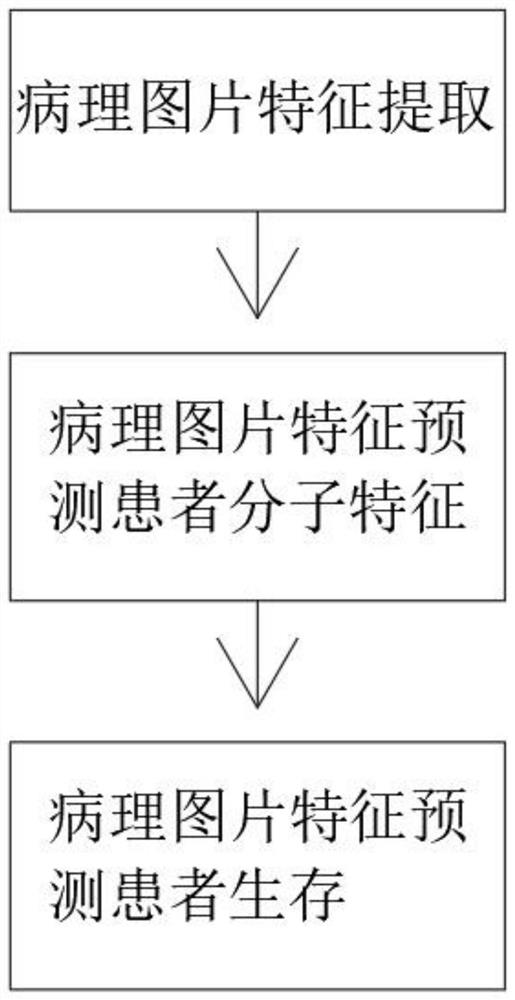
Pathological picture-based renal clear cell carcinoma molecular feature prediction and prognosis technology
PendingCN113436722ARapid economic quantificationHealth-index calculationEpidemiological alert systemsPatient survivalRenal clear cell carcinoma
The invention discloses a pathological picture-based renal clear cell carcinoma molecular feature prediction and prognosis technology. The renal clear cell carcinoma molecular feature prediction and prognosis technology comprises the following parts: 1, feature extraction of the pathological picture; 2, predicting the molecular characteristics of the patient through the pathological picture; 3, predicting the lifetime of the patient by integrating a single pathological picture and a pathological picture with multiple omics; according to the method, the pathological picture of the patient can be quickly and economically quantified, and the survival time of the patient with existing gene, transcription or proteomics data can be quickly, economically and more accurately judged when the important mutation state, molecular subtype attribution and survival of the patient are predicted.
Owner:曾皓
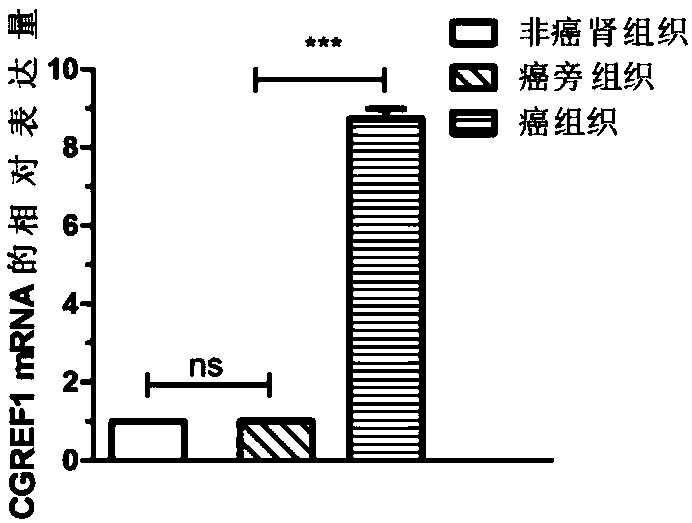
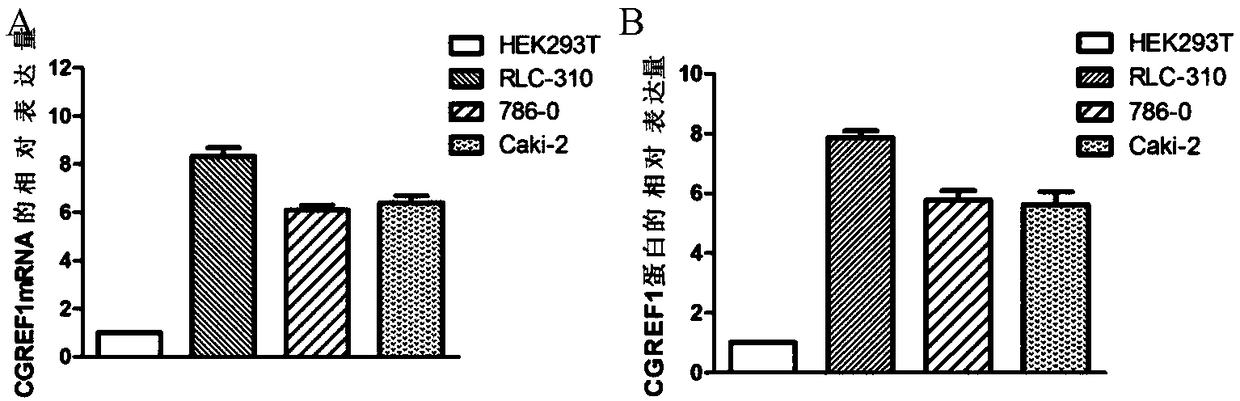
Application of CGREF1 as marker in diagnosis and treatment of renal clear cell carcinoma
PendingCN108220443AImprove accuracyPrevent invasionMicrobiological testing/measurementMaterial analysisRenal clear cell carcinomaOncology
The invention discloses application of CGREF1 as a marker in diagnosis and treatment of renal clear cell carcinoma. The invention discloses a product including a reagent for detecting the expression level of the CGREF1 and application of relevant reagents in preparing the product for diagnosing the renal clear cell carcinoma; meanwhile, the invention discloses a pharmaceutical composition including an inhibitor for inhibiting the expression level of the CGREF1 and application of relevant inhibitors in preparing the pharmaceutical composition for treating the renal clear cell carcinoma; the invention also discloses application of the CGREF1 in screening candidate compounds for treating the renal clear cell carcinoma.
Owner:QINGDAO MEDINTELL BIOMEDICAL CO LTD
Kidney clear cell carcinoma molecular feature prediction and prognosis technology based on pathological pictures
InactiveCN112489813AHigh-precision output prognostic risk scoreRapid economic quantificationProteomicsGenomicsPatient survivalRenal clear cell carcinoma
The invention discloses a renal clear cell carcinoma molecular feature prediction and prognosis technology based on a pathological picture. The renal clear cell carcinoma molecular feature predictionand prognosis technology comprises the following steps: 1, carrying out feature extraction of the pathological picture; 2, predicting the molecular characteristics of the patient through the pathological picture; 3, integrating a single pathological picture and a pathological picture into multiple omics to predict the lifetime of the patient. According to the method, pathological pictures of patients can be quantified quickly and economically, important mutation states, molecular subtype affiliation and survival time of the patients can be predicted, and the survival time of the patients withexisting gene, transcription or proteomics data can be judged quickly, economically and more accurately.
Owner:曾皓
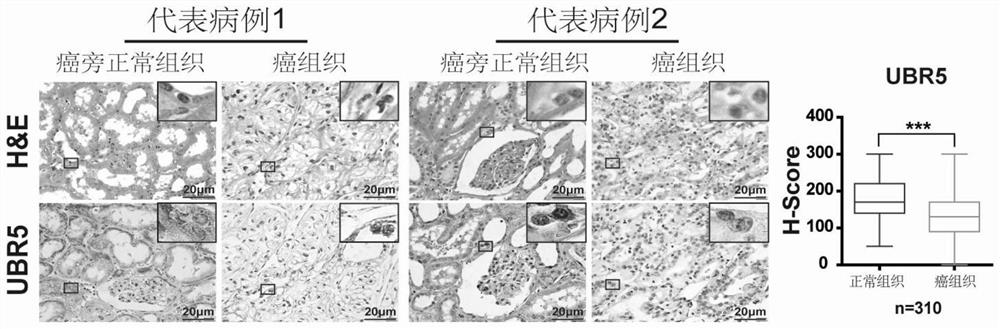
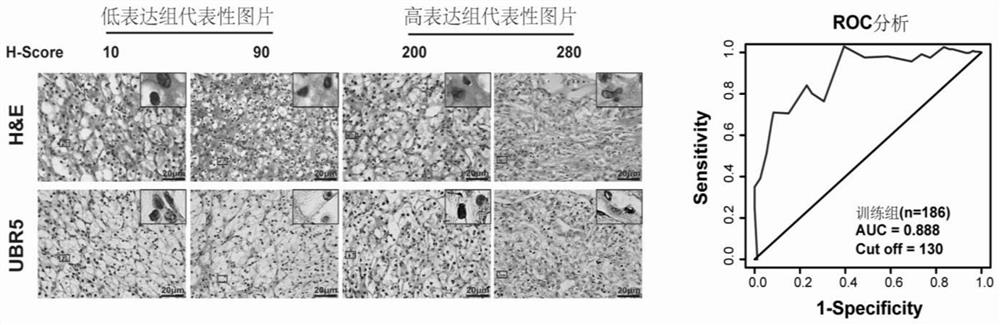
Application of E3 ubiquitination protein ligase UBR5 in preparation of tumor diagnosis or prognosis evaluation kit
PendingCN111912985APrecise riskAccurate Prognosis PredictionMaterial analysisProgression-free survivalUbiquitinated Proteins
The invention relates to the field of biotechnology and medical diagnosis, in particular to application of E3 ubiquitination protein ligase UBR5 in preparation of a renal clear cell carcinoma diagnosis or prognosis evaluation reagent or kit and a corresponding detection kit. Researches find that the expression of E3 ubiquitination protein ligase UBR5 molecules in patients with renal clear cell carcinoma is generally reduced, the expression level of the E3 ubiquitination protein ligase UBR5 molecules is remarkably related to prognosis of the patients, and the total lifetime (OS) and progression-free lifetime (PFS) of the patients with renal clear cell carcinoma can be better predicted by combining the expression of UBR5.
Owner:SHANGHAI CITY PUDONG NEW AREA GONGLI HOSPITAL
Separation and culture method of mouse kidney clear cell carcinoma circulating tumor cell line and human kidney clear cell carcinoma circulating tumor cells
ActiveCN111321120AThe method of isolation and culture is simpleEasy to operateCell culture active agentsTumor/cancer cellsRenal clear cell carcinomaOncology
The invention discloses a separation and culture method of a mouse kidney clear cell carcinoma circulating tumor cell line and kidney clear cell carcinoma circulating tumor cells, and relates to the technical field of separation and culture of tumor cells. The mouse kidney clear cell carcinoma circulating tumor cell line is circulating tumor cells of an in-situ kidney cancer NOD / SCID mouse. The separation and culture method of the circulating tumor cells mainly comprises the following steps of: firstly, infecting a human kidney clear cell carcinoma cell line 786-O with viruses, so that the human kidney clear cell carcinoma cell line is stably converted into green fluorescent protein and has Puromycin resistance, and culturing the cell line; and then establishing a mouse model, collecting peripheral blood of the mouse, and culturing and separating the peripheral blood to obtain the kidney cancer circulating tumor cells. The separation and culture method disclosed by the invention is simple and easy to operate, and the circulating tumor cells obtained by separation can be subjected to normal leaflet culture and can be widely applied to clinical research.
Owner:SECOND AFFILIATED HOSPITAL OF COLLEGE OF MEDICINEOF XIAN JIAOTONG UNIV
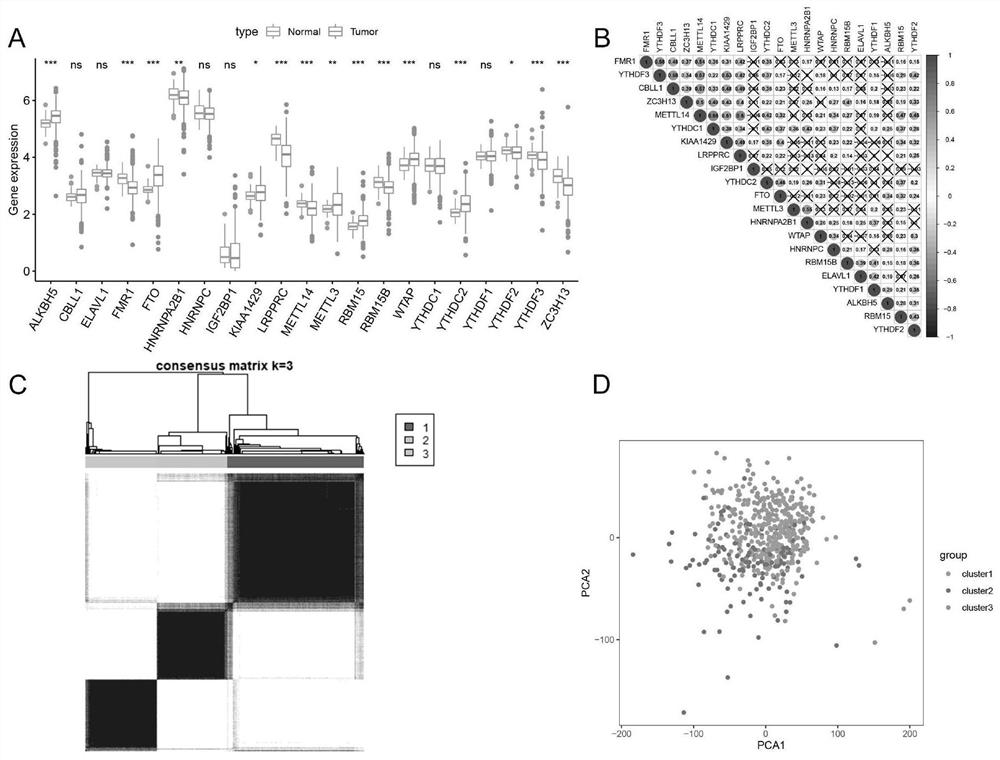
application of m6A modification related combined genome in predicting curative effect of immunotherapy for patients with renal clear cell carcinoma
ActiveCN113462776APredicted responseHigh predictive value of immunotherapy efficacyMicrobiological testing/measurementBioinformaticsTreatment effectRenal clear cell carcinoma
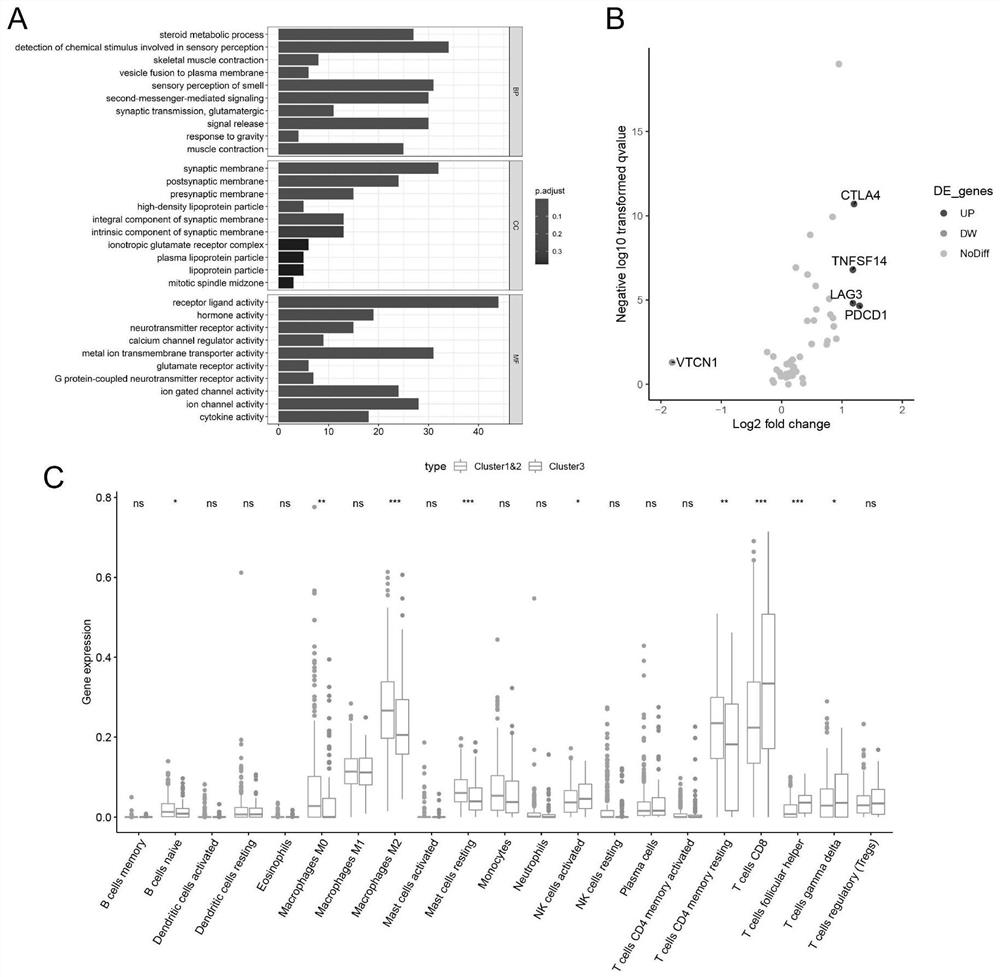
The invention relates to the technical field of medical biological detection, provides a new application of a combined genome of HNRNPA2B1 and ALKBH5, and particularly relates to an application in preparation of a reagent or a kit for predicting the curative effect of renal clear cell carcinoma immunotherapy. The invention also provides a renal clear cell carcinoma immunotherapy curative effect prediction kit and a renal clear cell carcinoma immunotherapy curative effect prediction system. The gene combination is derived from a renal clear cell carcinoma m6A modification mode differential expression mode, discovery of the m6A modification related gene combination model provides a brand-new strategy for predicting the immune treatment effect of a renal clear cell carcinoma patient, clinical doctors can be guided to implement an individualized precise treatment strategy, the survival rate of the patient is increased, and the method has important guiding significance for immunotherapy application of patients with renal clear cell carcinoma.
Owner:FUDAN UNIV SHANGHAI CANCER CENT
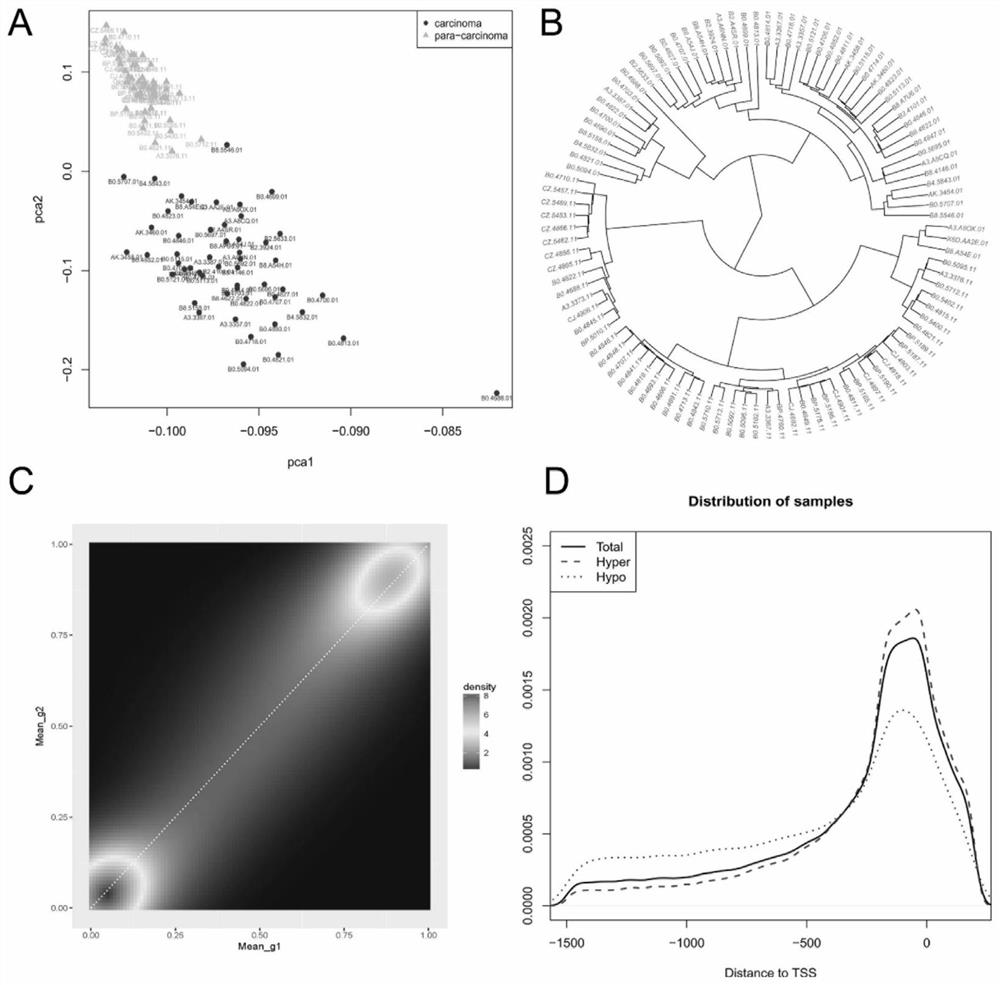
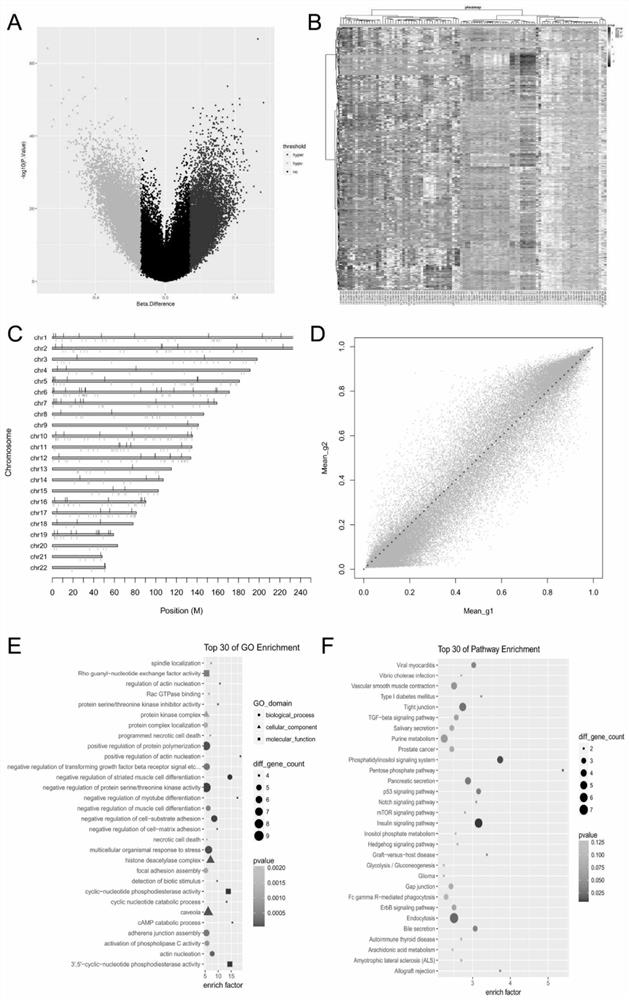
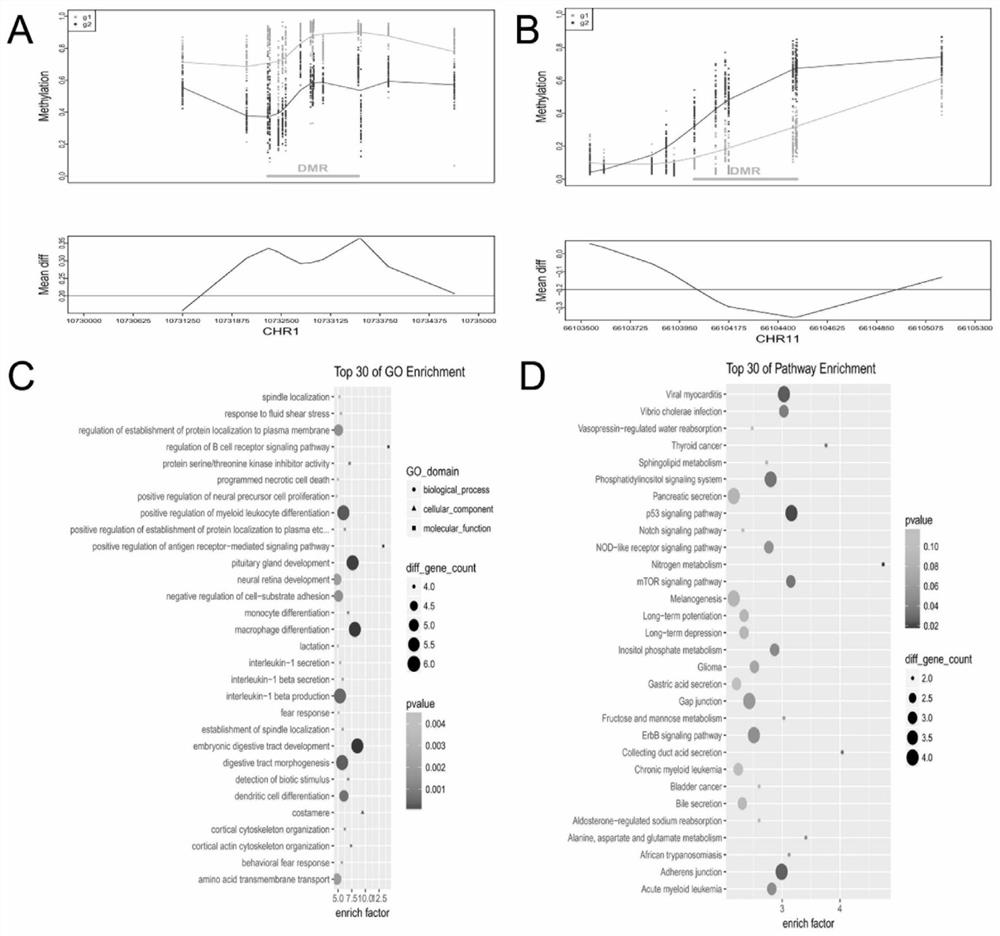
DNA methylated biomarker related to prognosis of renal clear cell carcinoma
The invention belongs to the technical field of biomedicine, and relates to a marker for prognosis of renal clear cell carcinoma and application of the marker. According to the invention, comparative analysis is carried out on the whole genome methylation level, differential methylation sites and differential methylation regions among tumor tissues and adjacent normal tissues by performing testing observation on the relationship between the change of DNA methylation related markers in renal clear cell carcinoma and the prognosis condition of diseases, and by utilizing an LASSO-Cox regression model, biological markers related to prognosis of renal clear cell carcinoma are determined to be TP73 (cg00295572, cg07382920, cg20611911 and cg01915516) and CTBP2 (cg05749728) respectively. The DNA methylation related markers disclosed by the invention can be used for preparing a product for evaluating the prognosis of the renal clear cell carcinoma.
Owner:FUDAN UNIV +1
Adjuvant Theraphy of G250-Expressing Tumors
ActiveUS20070207157A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAbnormal tissue growthRenal clear cell carcinoma
The invention relates to a method for the treatment of G250-antigen-expressing tumors, in particular renal clear cell carcinoma comprising the administration of G250-antigen-specific antibodies to high-risk patients diagnosed with non-metastasising disease.
Owner:WILEX AG
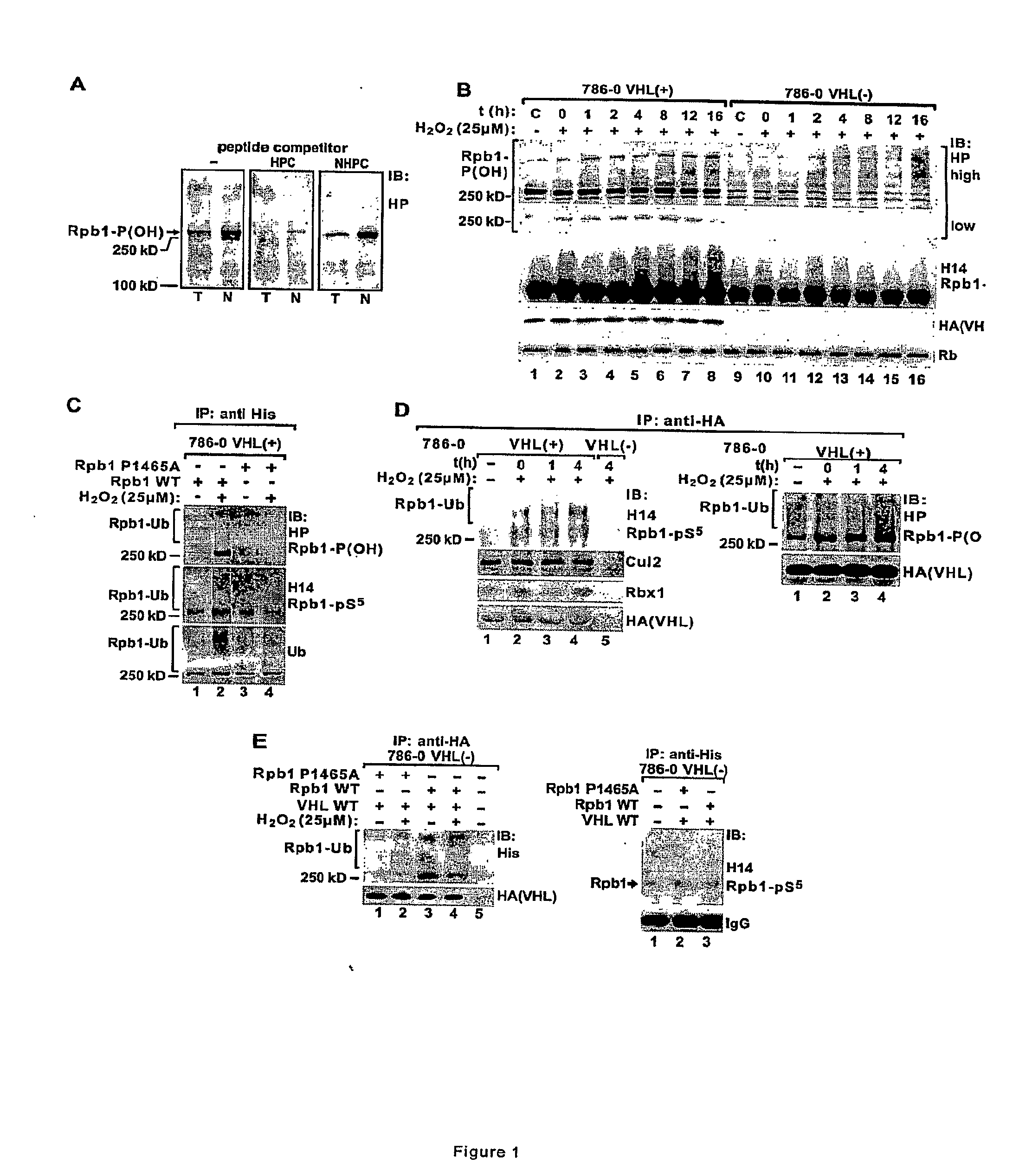
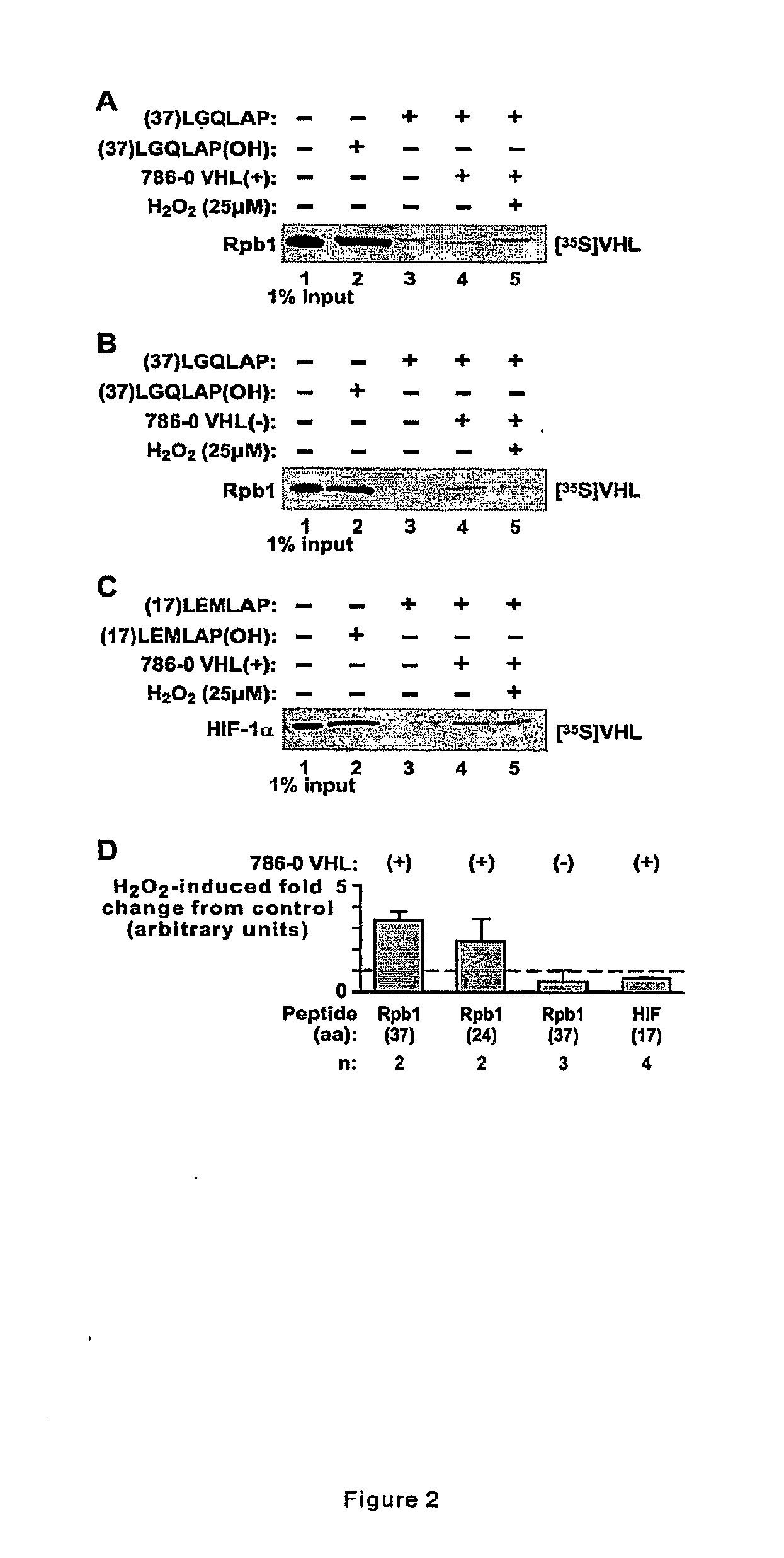
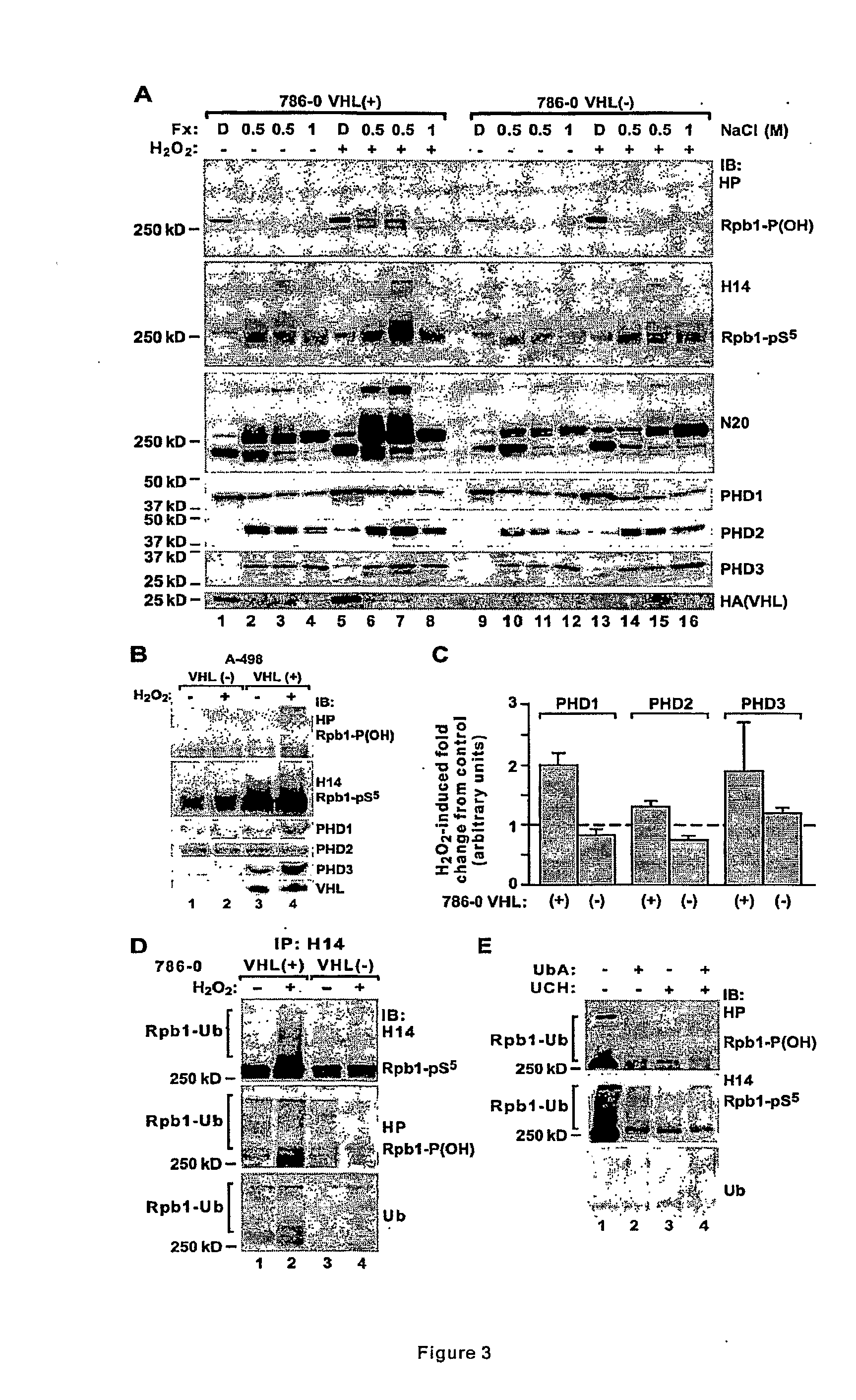
Methods for diagnosing and treating cancers via manipulations of a...pathway
InactiveUS20090220964A1Inhibit tumor growthIncreased riskMicrobiological testing/measurementTissue culturePhosphorylationRenal clear cell carcinoma
Methods for diagnosing, treating, and screening for cancer based on manipulations of a newly discovered pVHL dependent, non-degradative ubiquitylation pathway of Rpb1. In particular, methods comprising the use of biomarkers implicating the pathway, and promoters of either or both of P1465 hydroxylation and CTD Ser-5 phosphorylation of Rpb1. Specifically, the methods may be used to inhibit tumor growth, including, in particular, carcinomas such as renal clear cell carcinoma.
Owner:UNIVERSITY OF CINCINNATI
Application of PBLD gene in preparation of medicine for diagnosing renal clear cell carcinoma and predicting prognosis of same
InactiveCN109022585ASimple methodReliable resultsMicrobiological testing/measurementKidney cancerRenal clear cell carcinoma
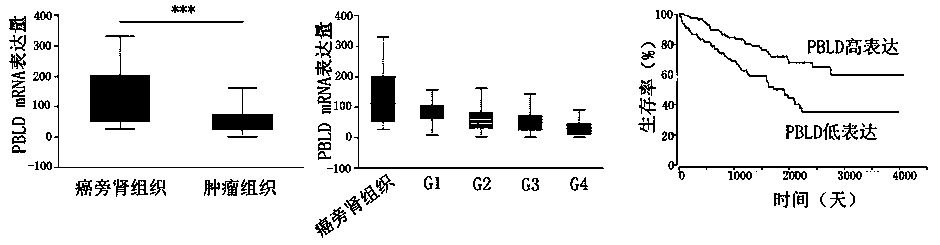
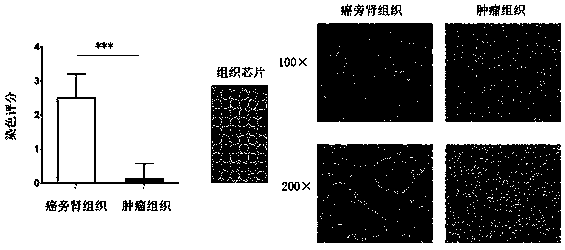
The invention provides an application of PBLD gene in preparation of a medicine for diagnosing renal clear cell carcinoma and predicting prognosis of same. By combining Q-PCR and immunohistochemical methods, mRNA and proteins in PBLD gene in renal clear cell carcinoma tissue and para-carcinoma tissue are detected; it is found that the PBLD is significantly under-expressed in the renal clear cell carcinoma tissue, which identifies that the PBLD, as a new specific renal cancer marker, can supply a new target point to therapy of the renal cancer.
Owner:路君
Characteristic miRNA expression profile combination and early prediction method for renal clear cell carcinoma
PendingCN111733251AFast predictionQuick forecastMicrobiological testing/measurementBiostatisticsEarly predictionNucleotide
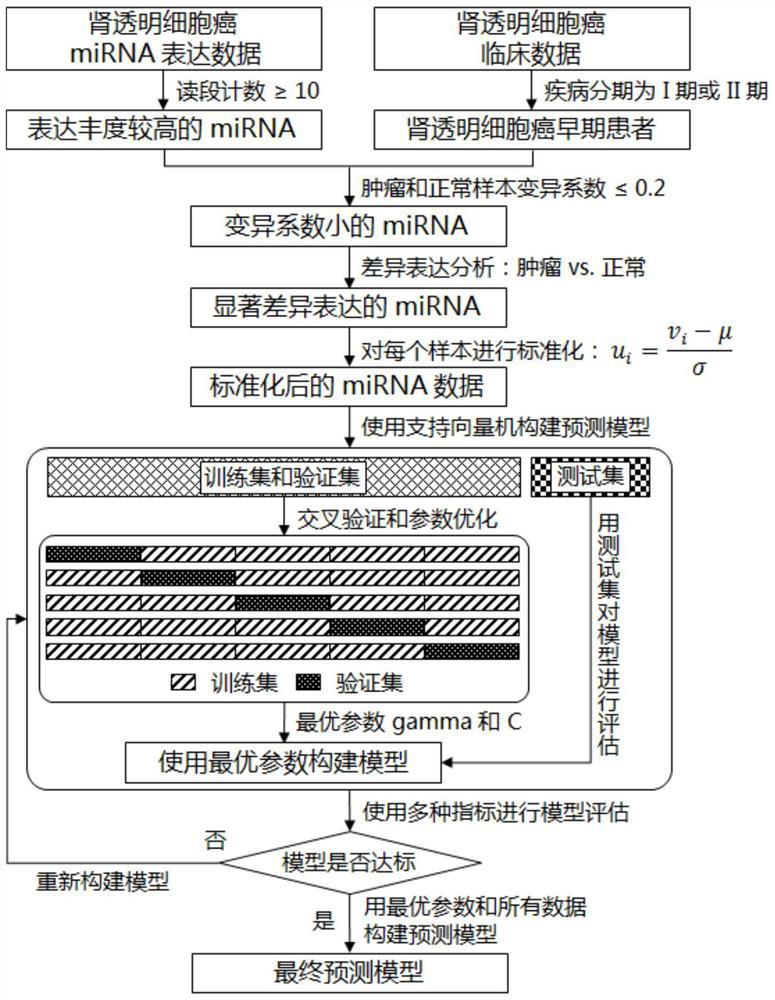
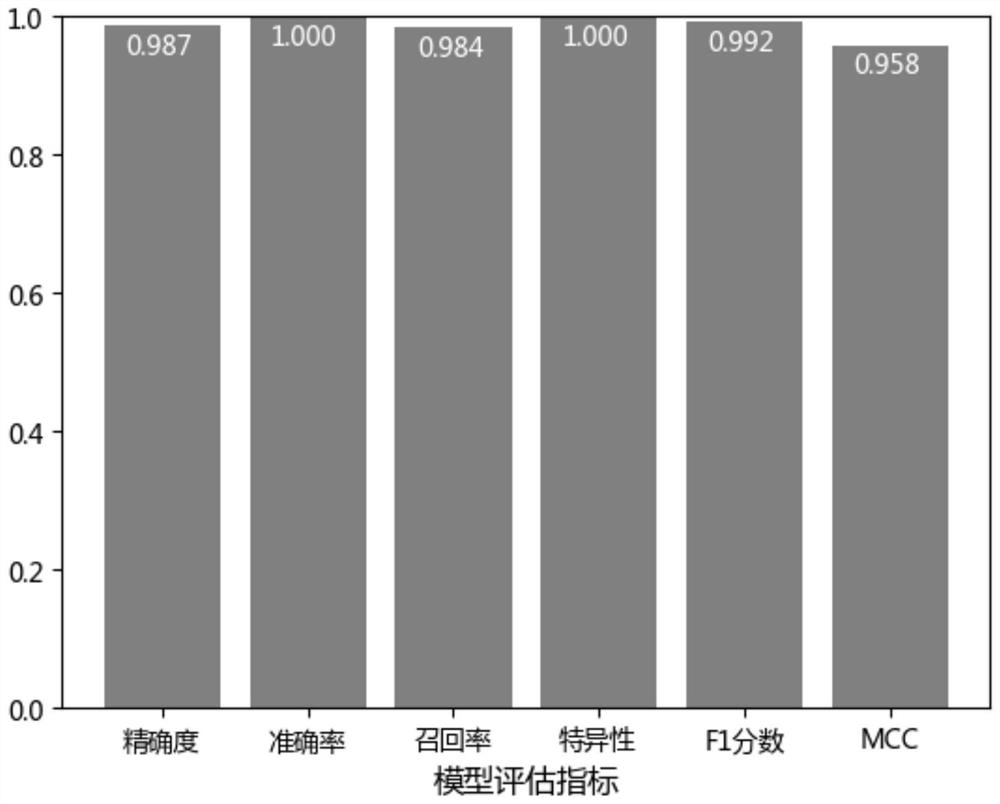
The invention discloses a characteristic miRNA expression profile combination and an early prediction method for renal clear cell carcinoma. The miRNA comprises hsa-let-7g, hsa-mir-10a, hsa-mir-10b, hsa-mir-125a, hsa-mir-21, hsa-mir-30b, hsa-mir-30c-1, hsa-mir-30c-2 and hsa-mir-532, and the nucleotide probe sequence is shown as SEQ ID NO. 1-9. The evaluation of the early risk of renal clear cell carcinoma based on the miRNA expression profile combination characteristics has high accuracy and accuracy (the area AUC under the ROC curve = 0.992). The early risk of renal clear cell carcinoma can be calculated by a support vector machine model only by acquiring the relative expression quantity of the nine miRNAs.
Owner:ANHUI MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com