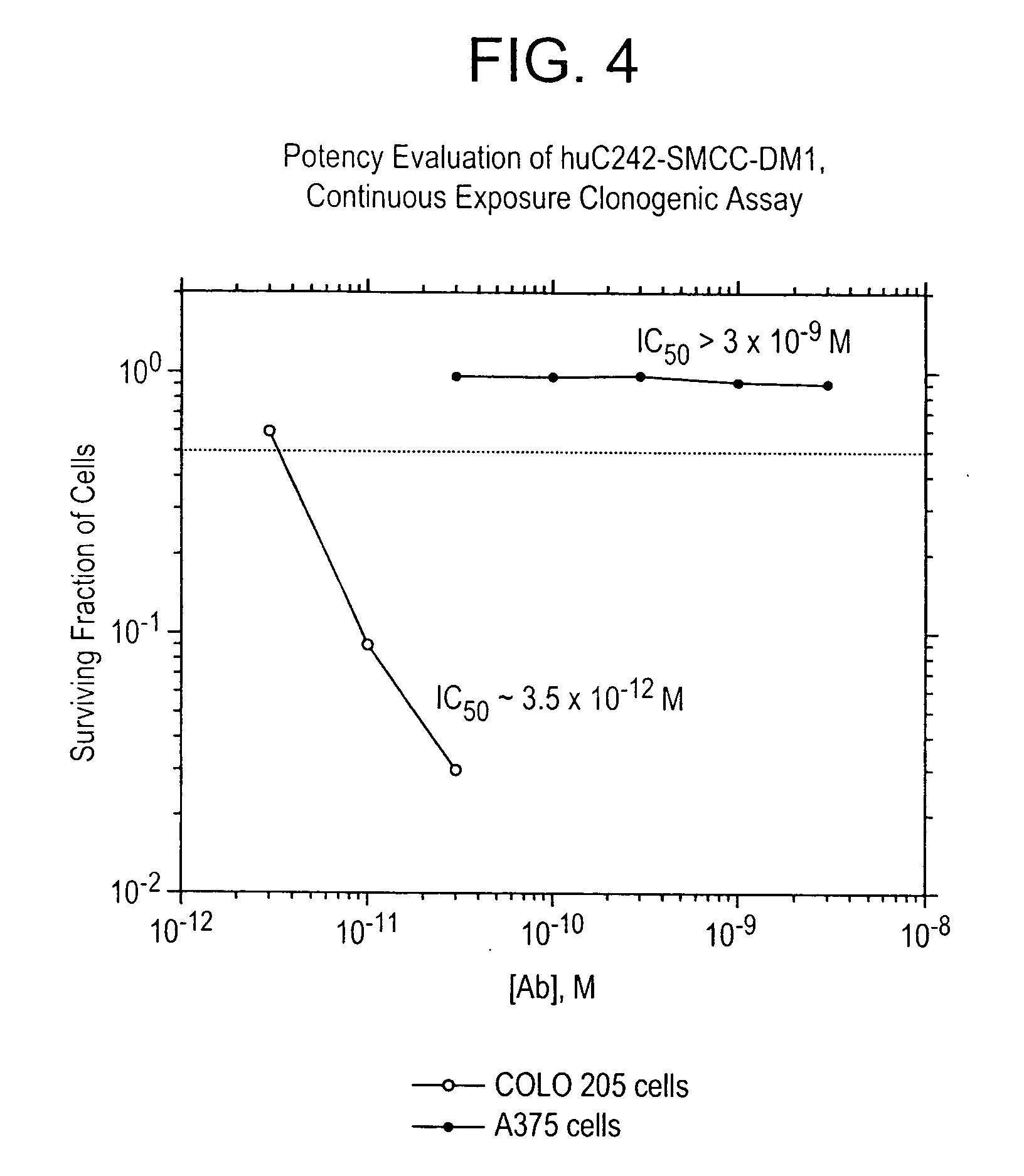
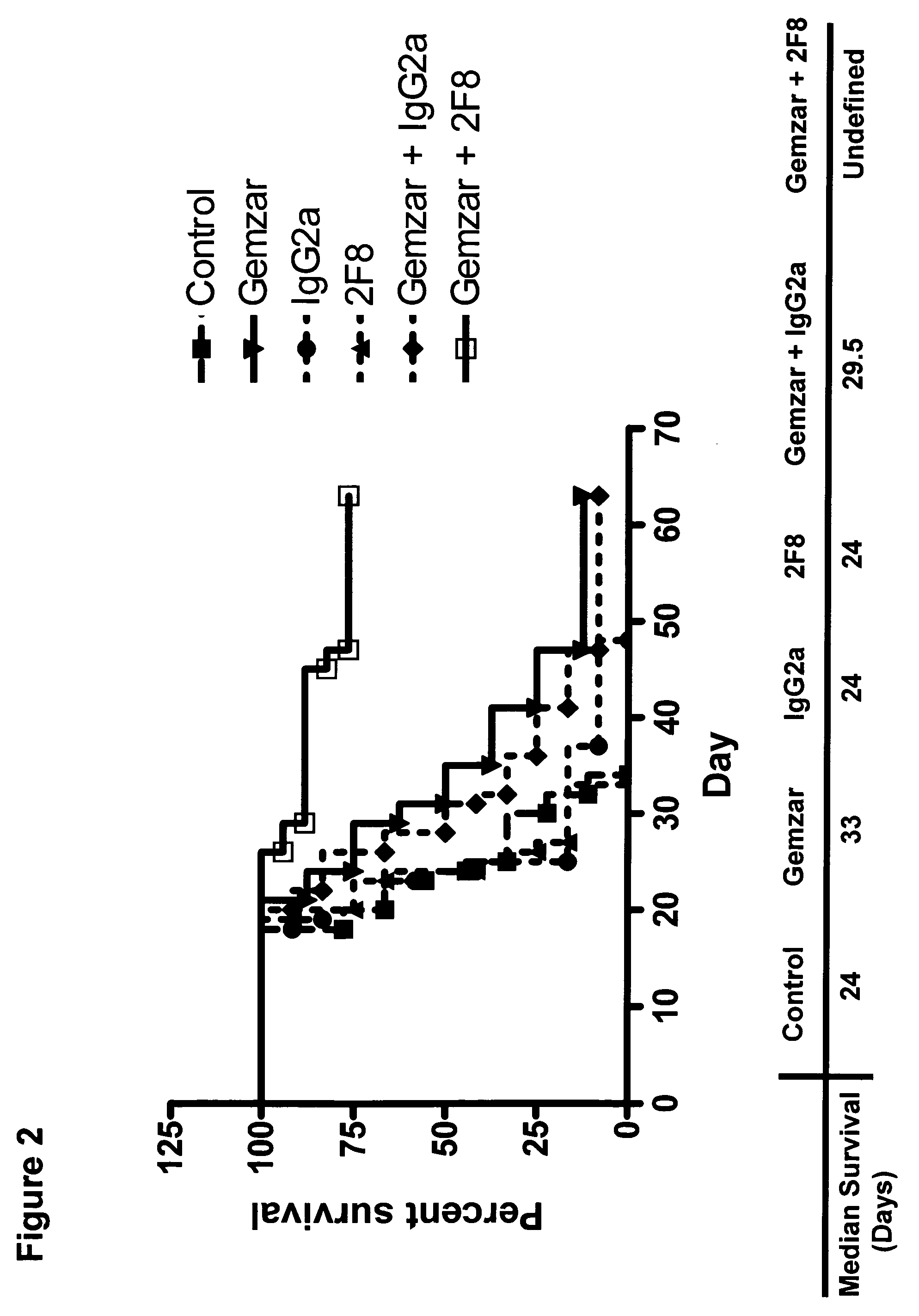
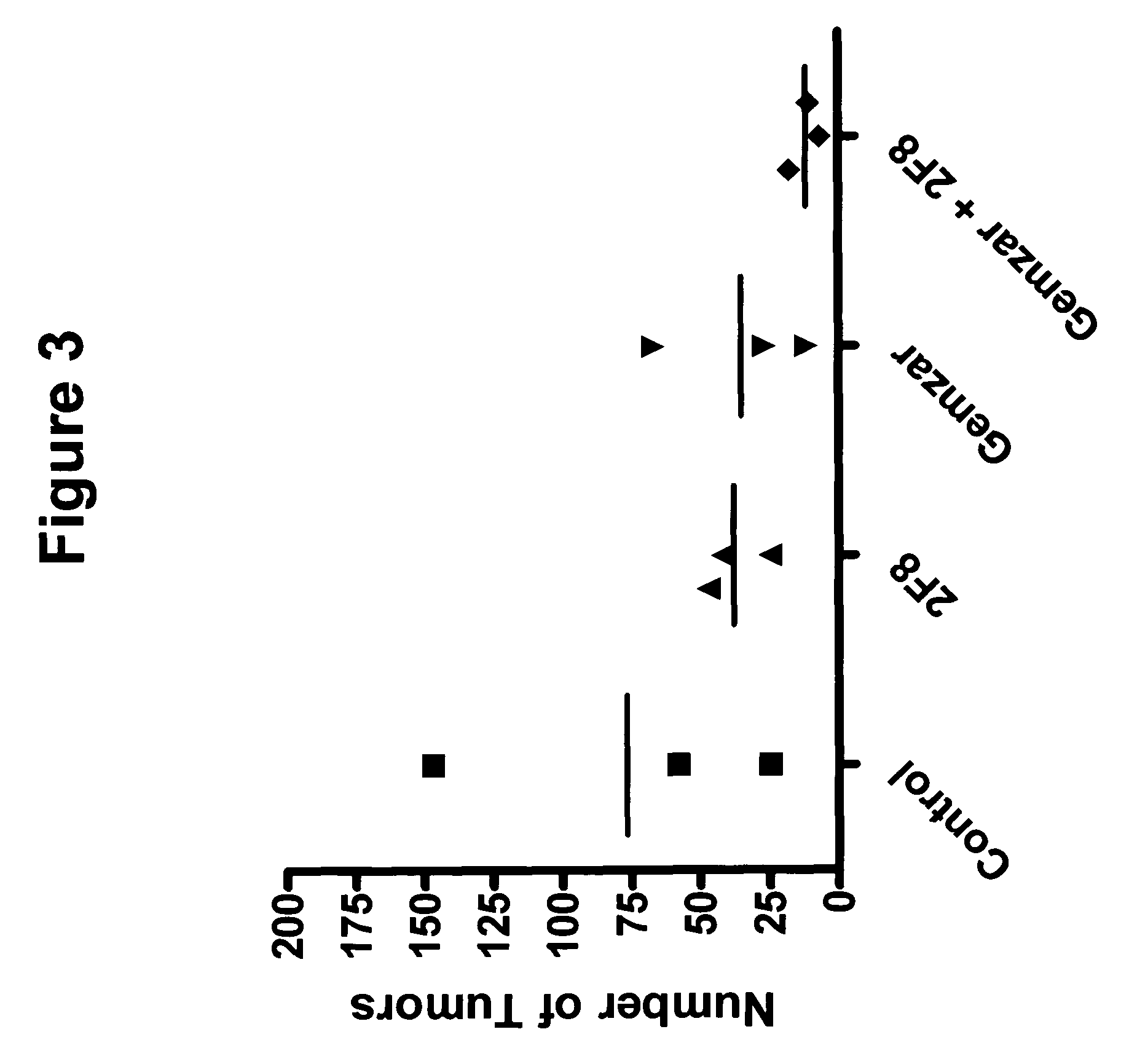
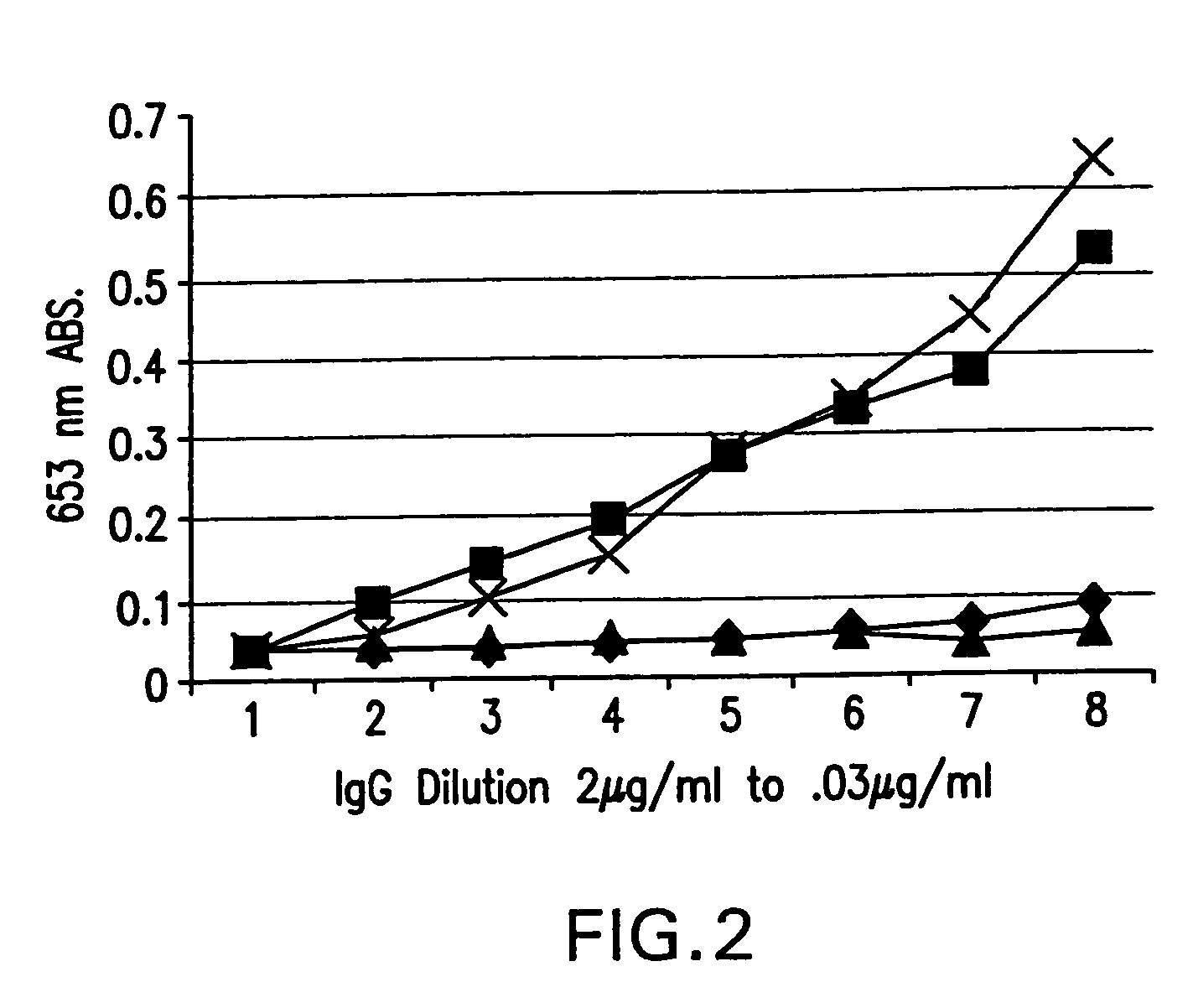
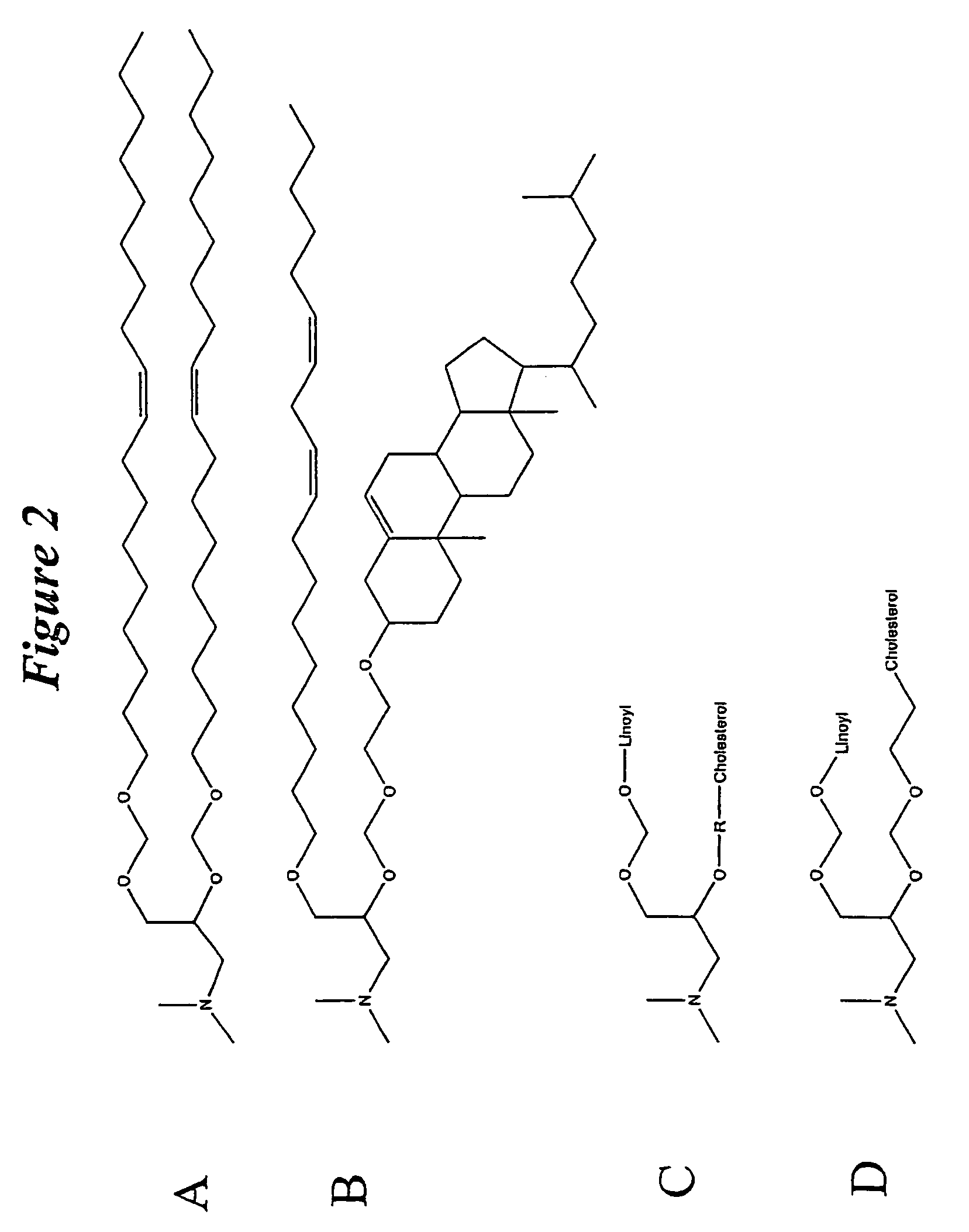
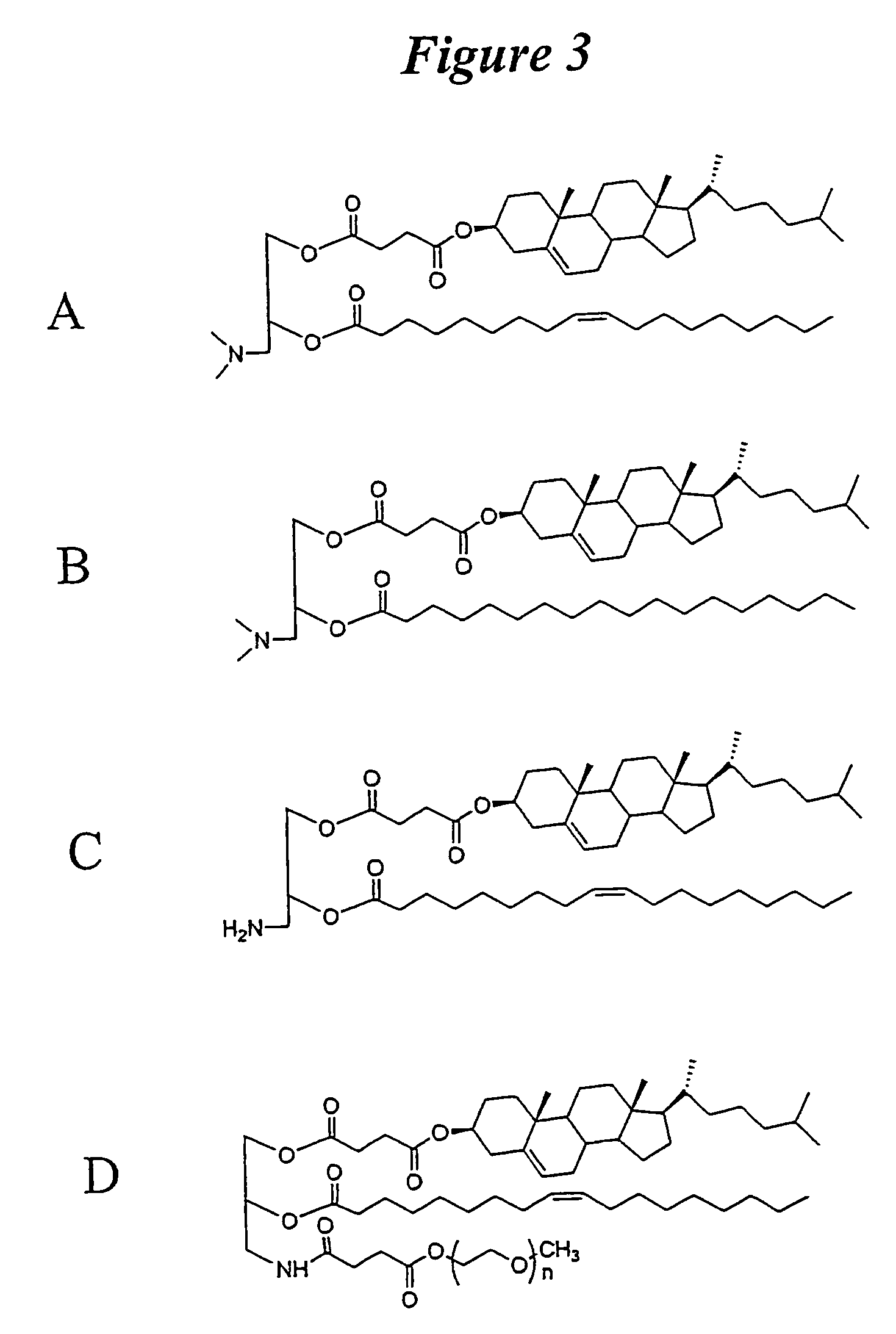
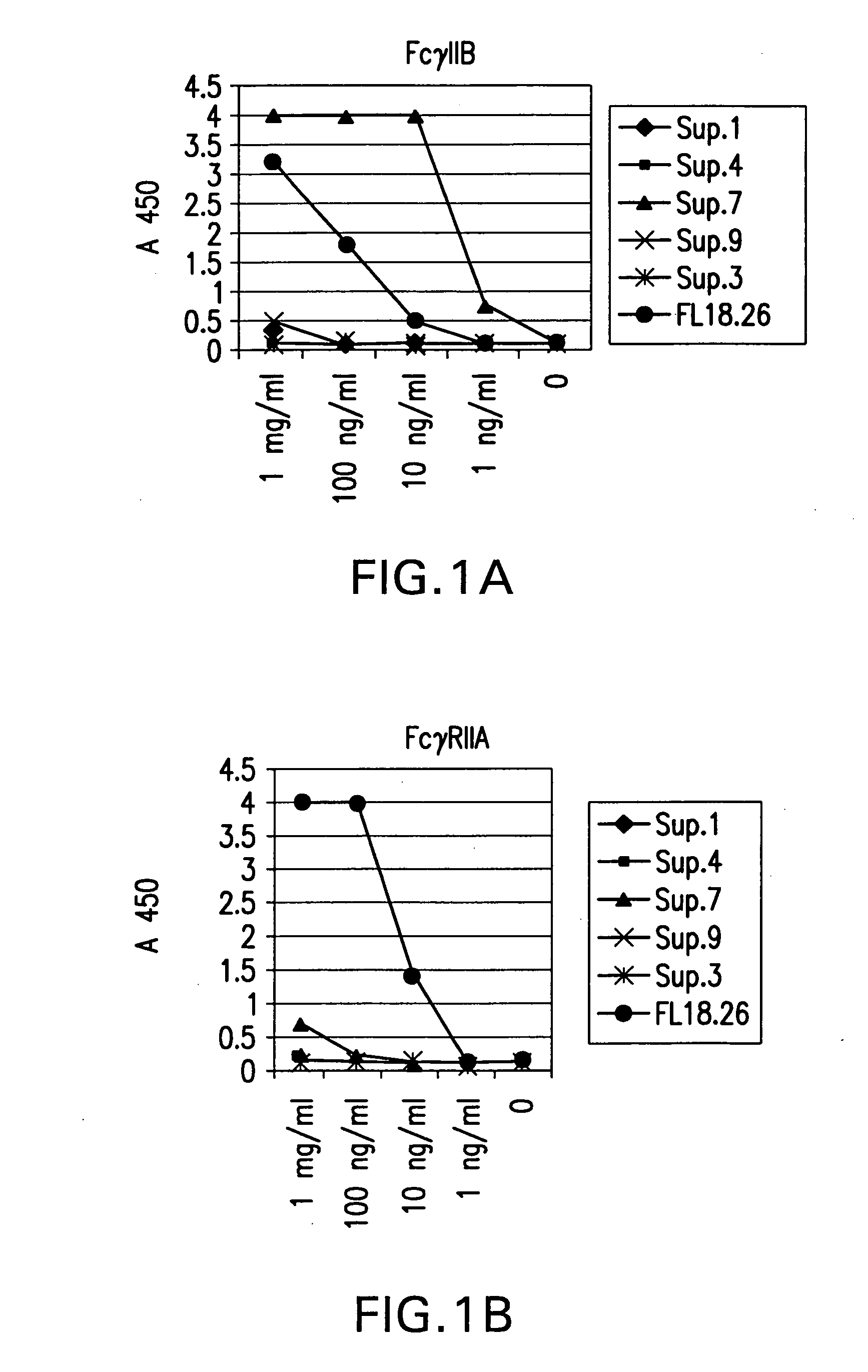
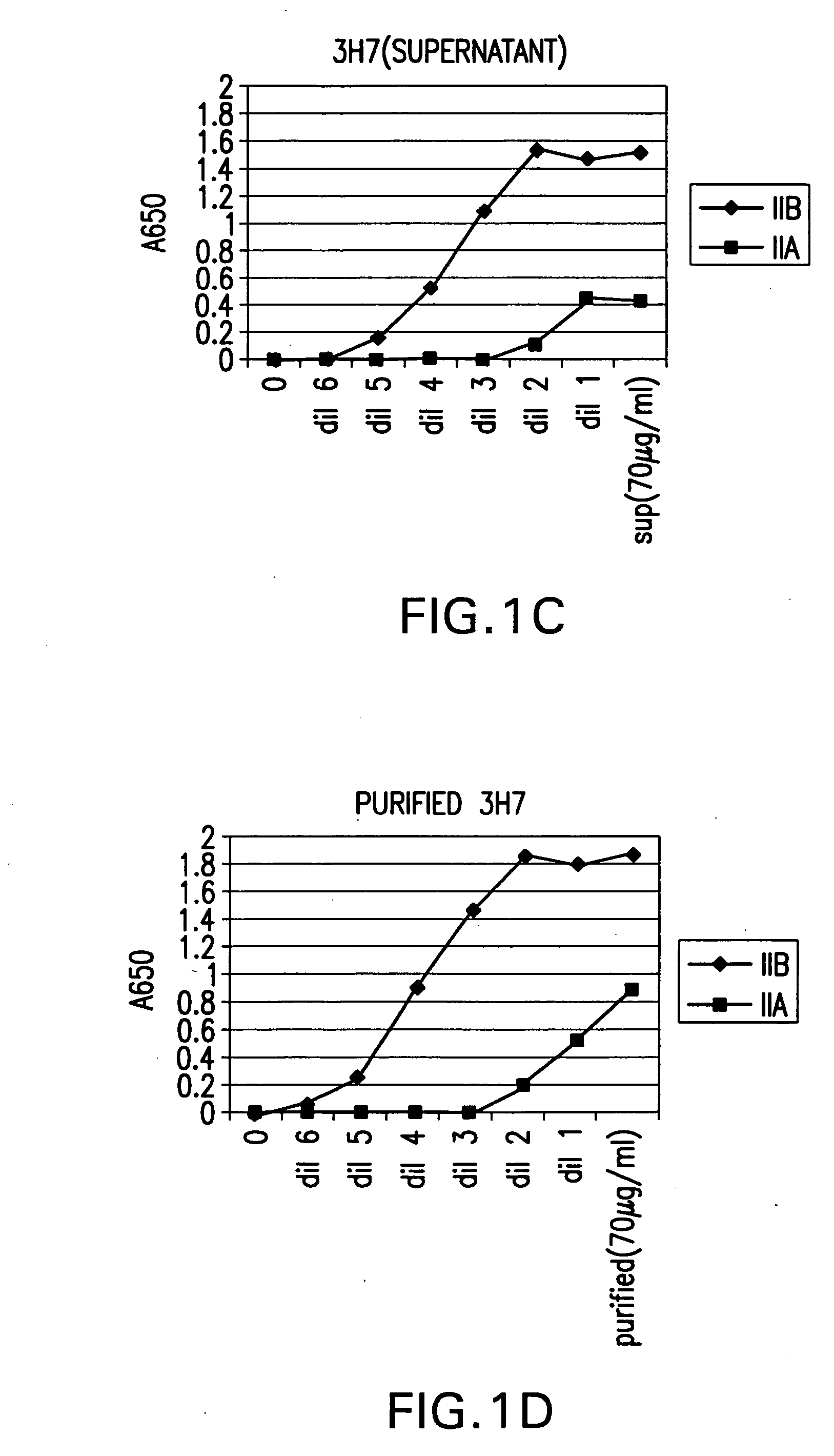
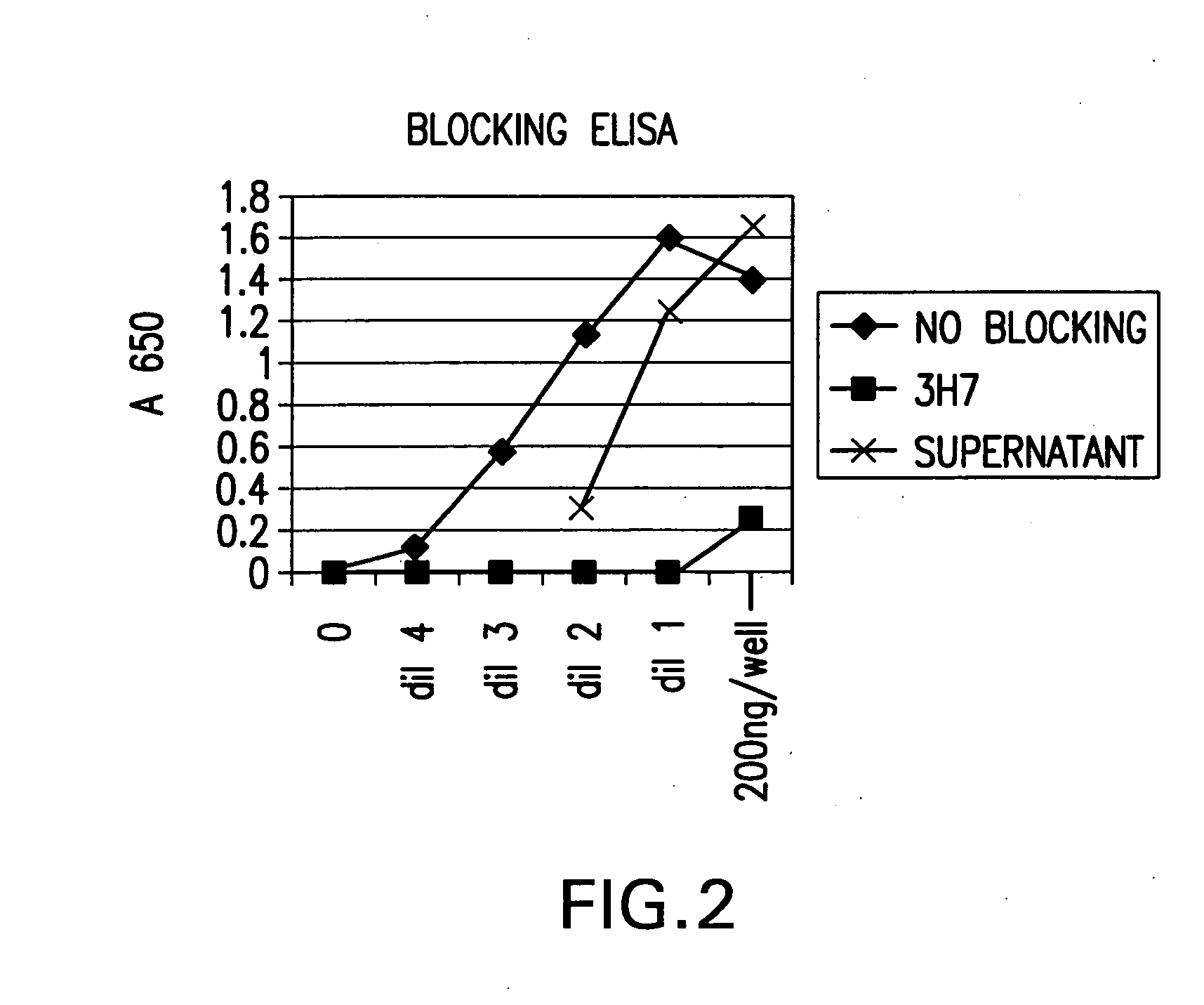
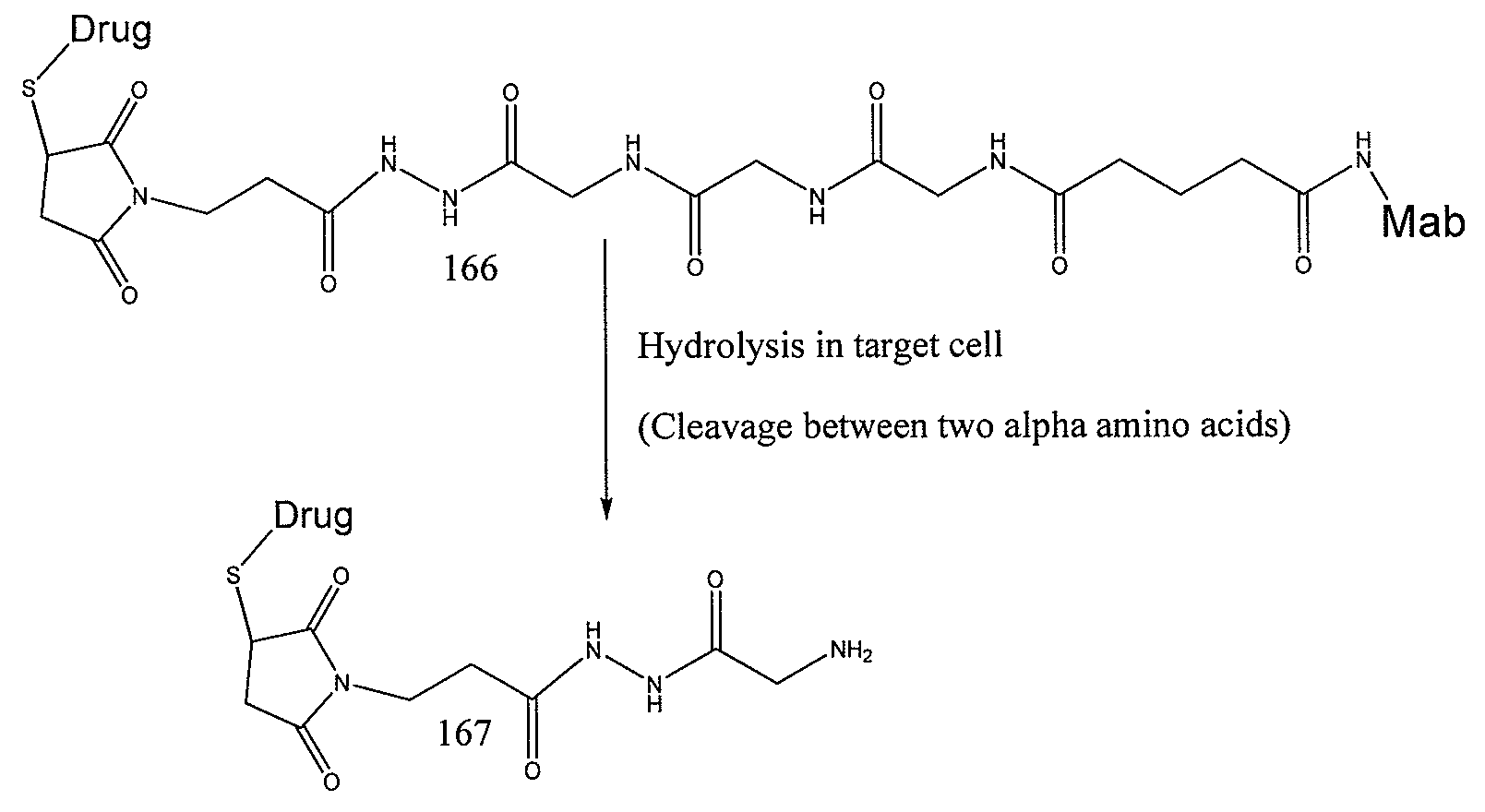
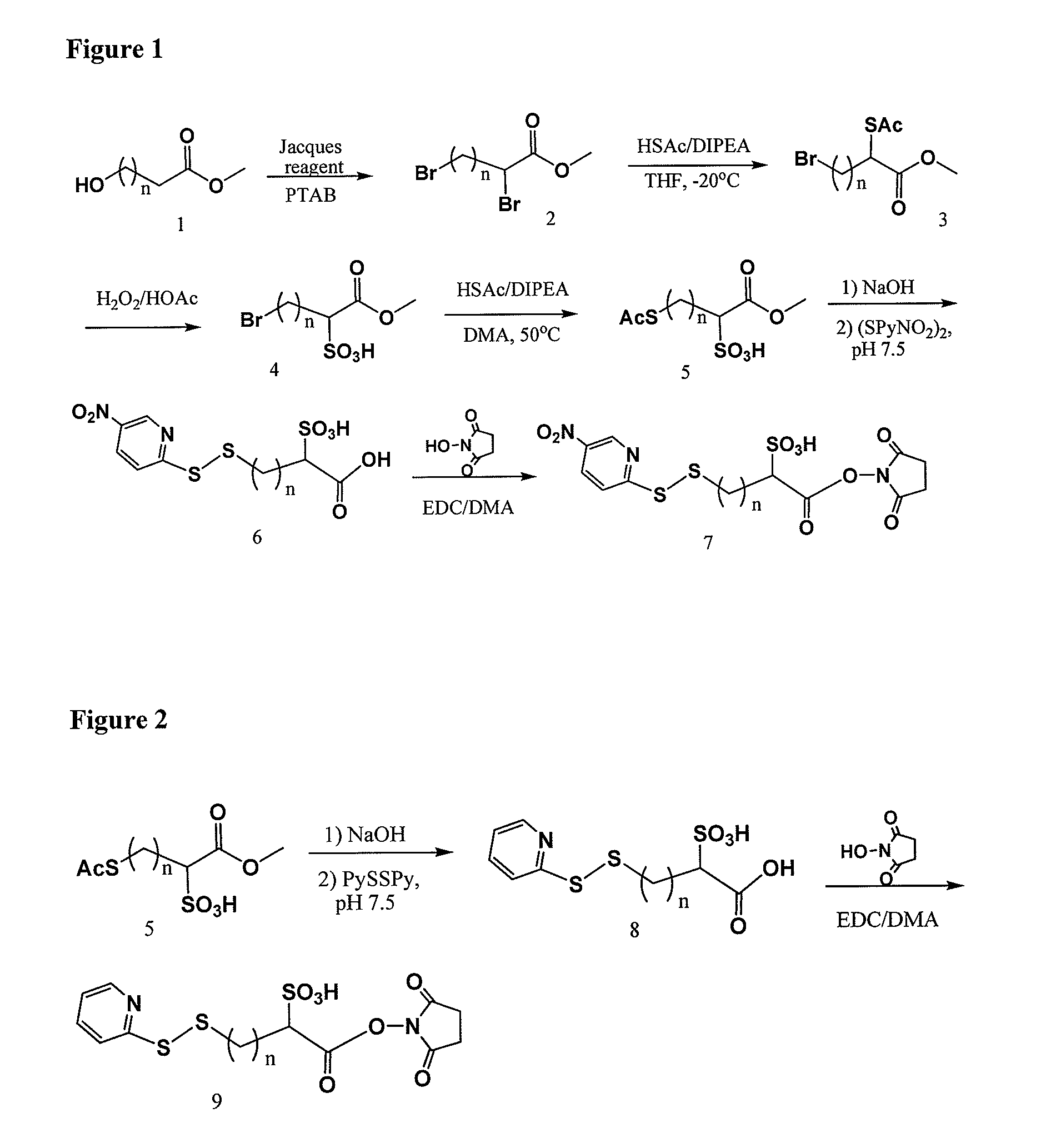
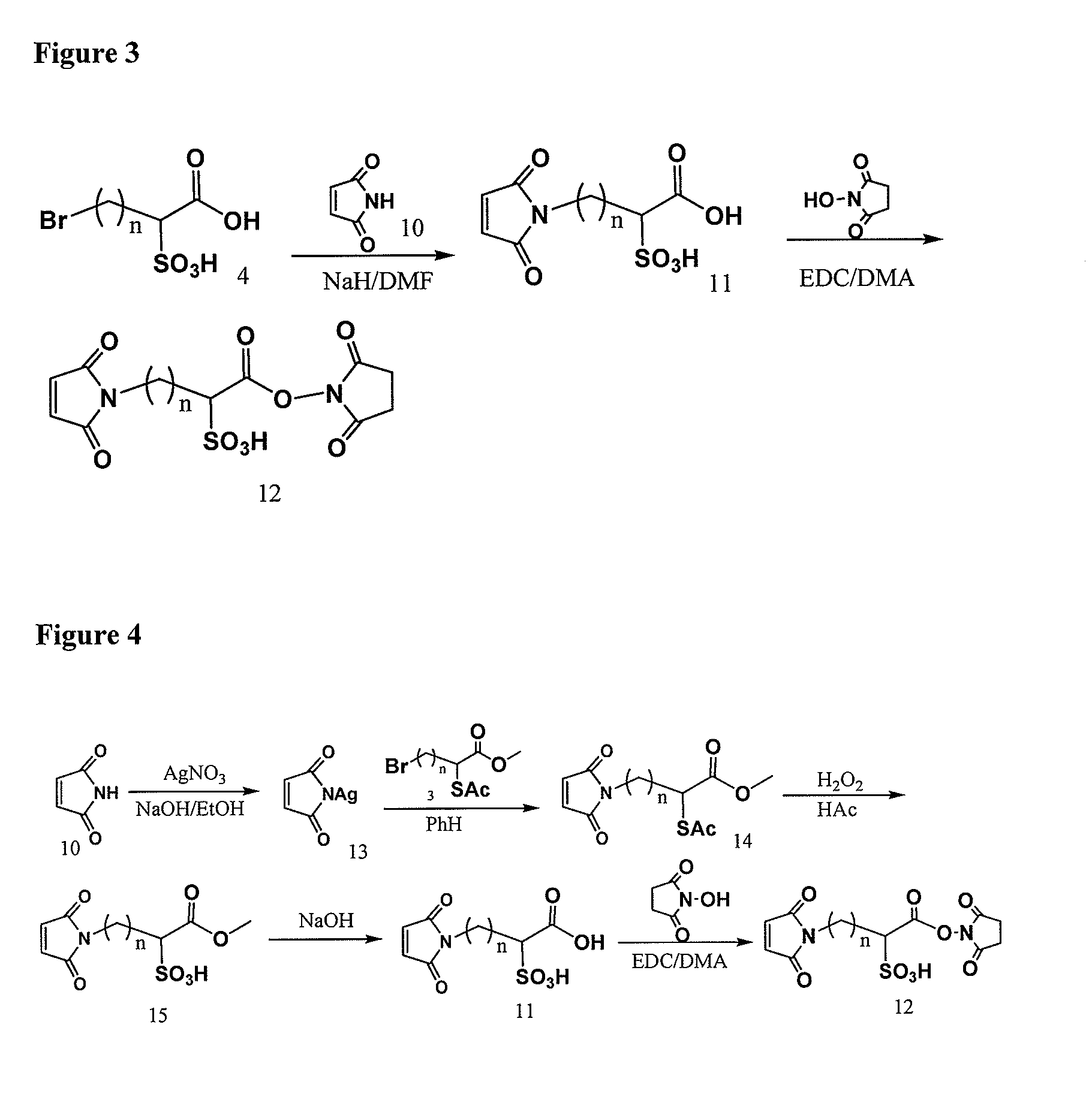
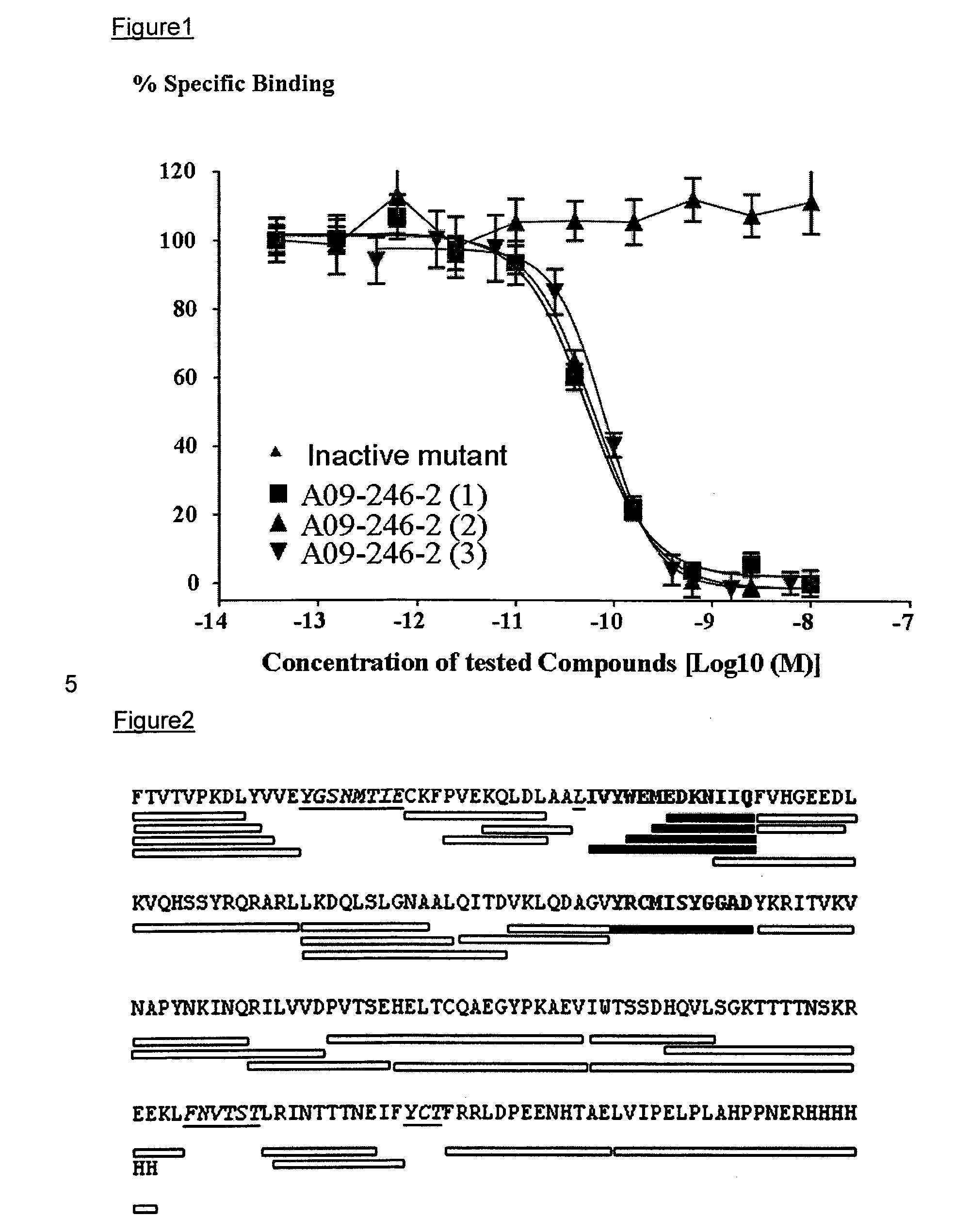
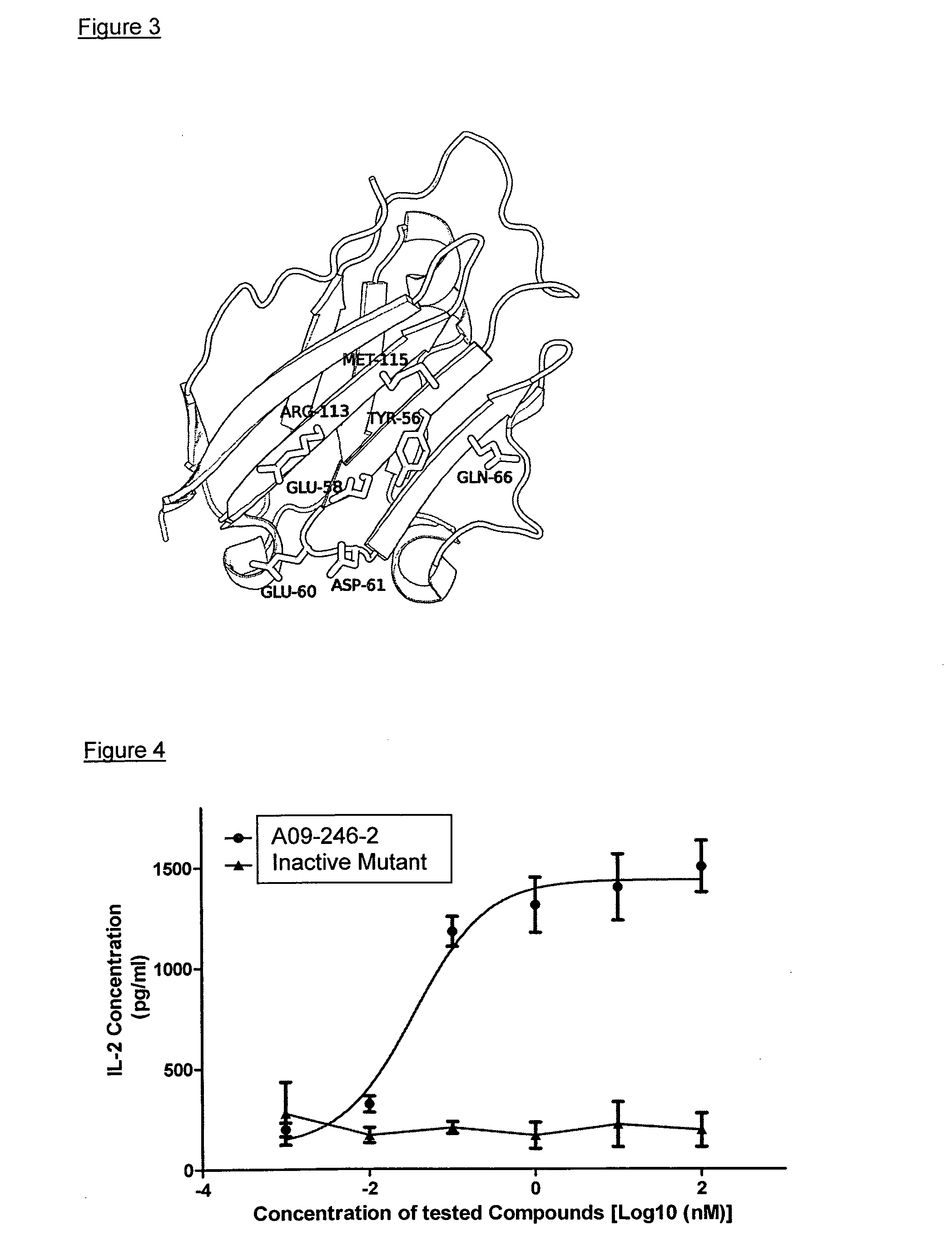
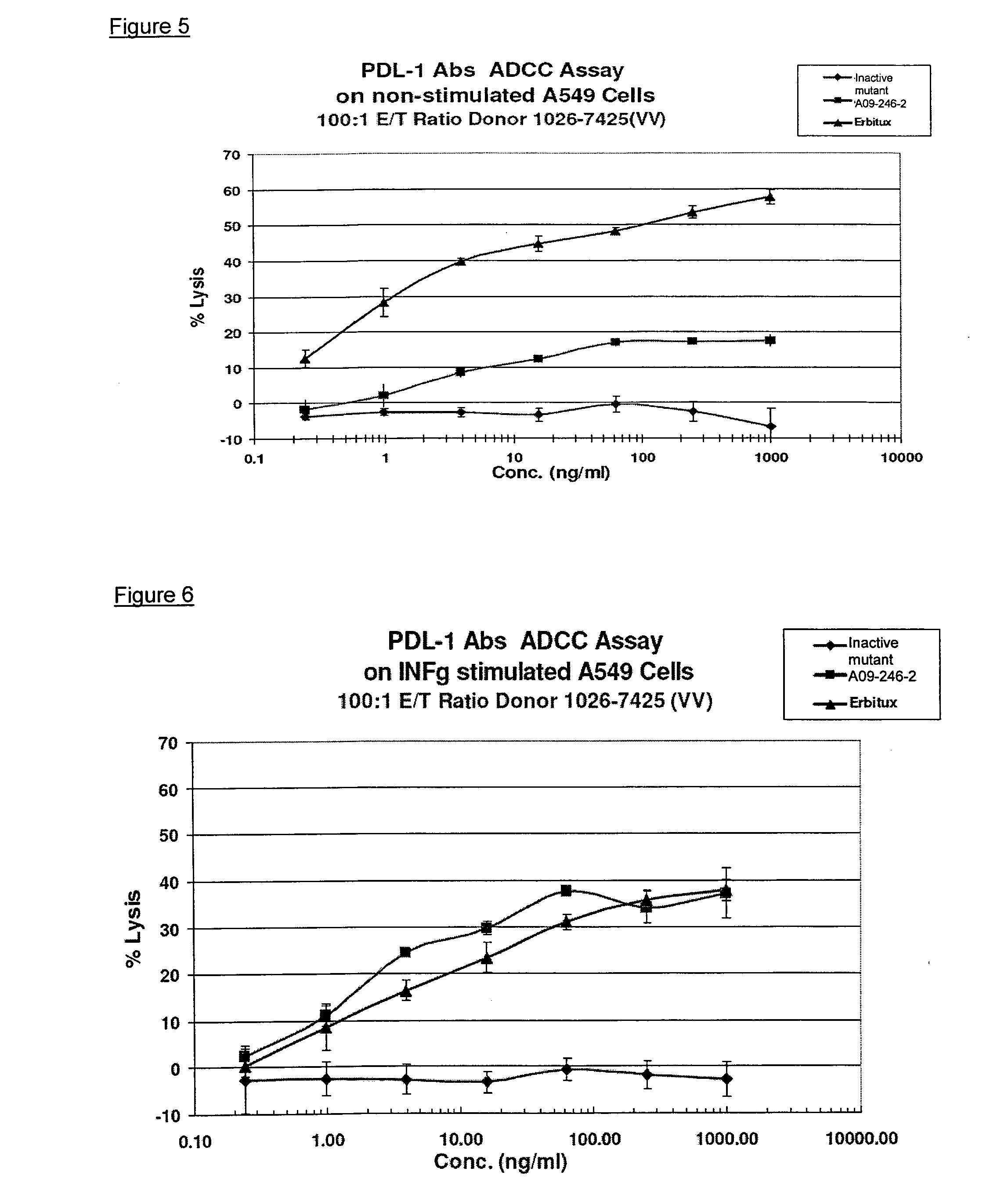
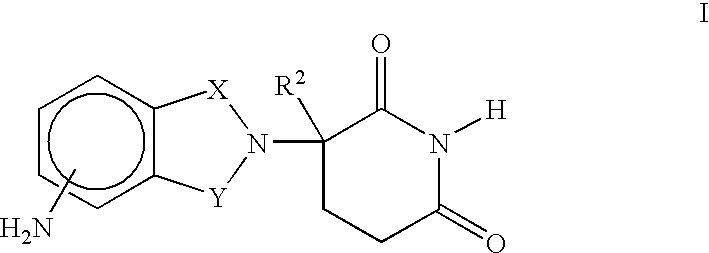
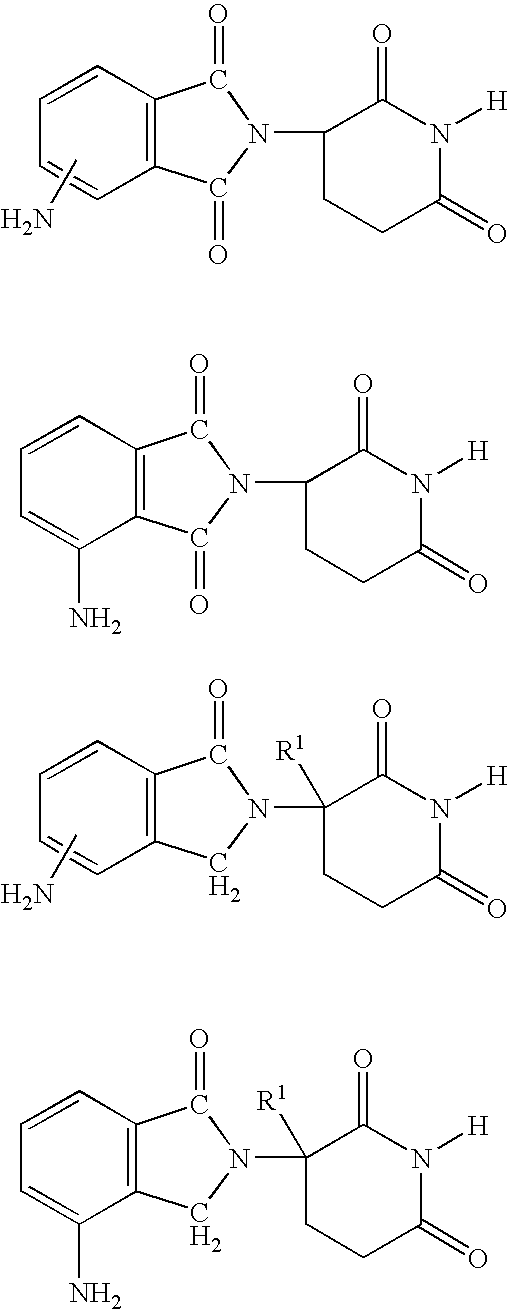
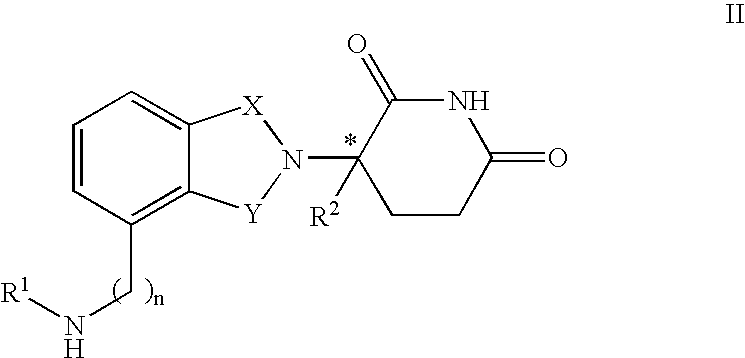
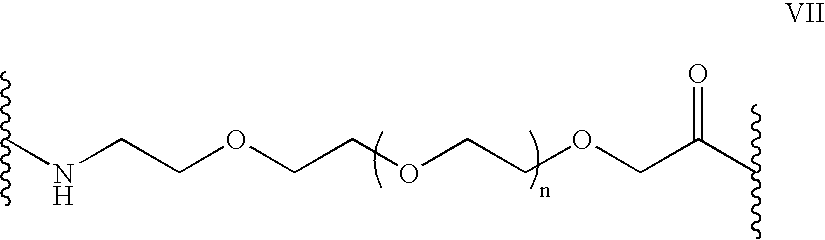
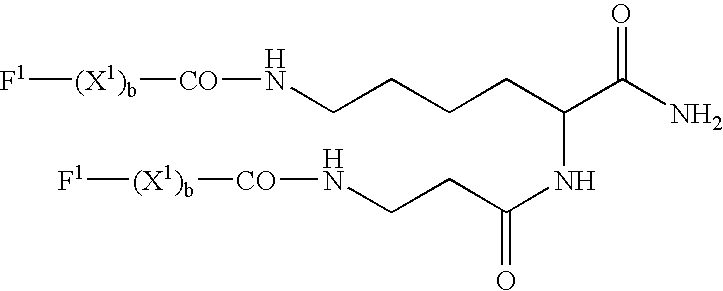
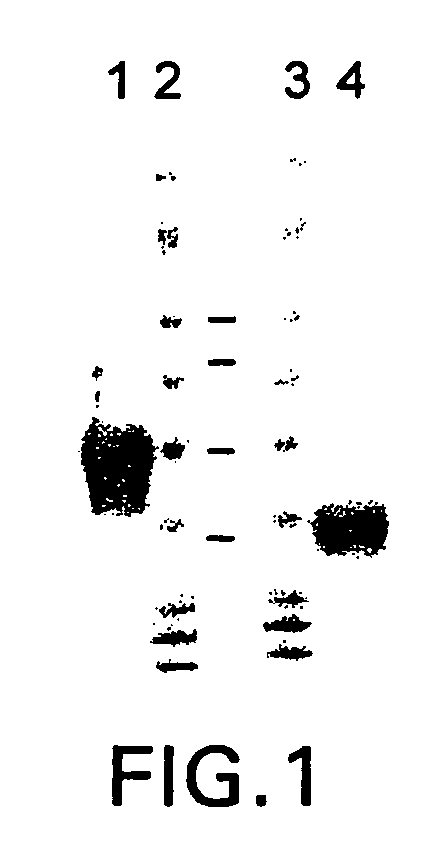Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
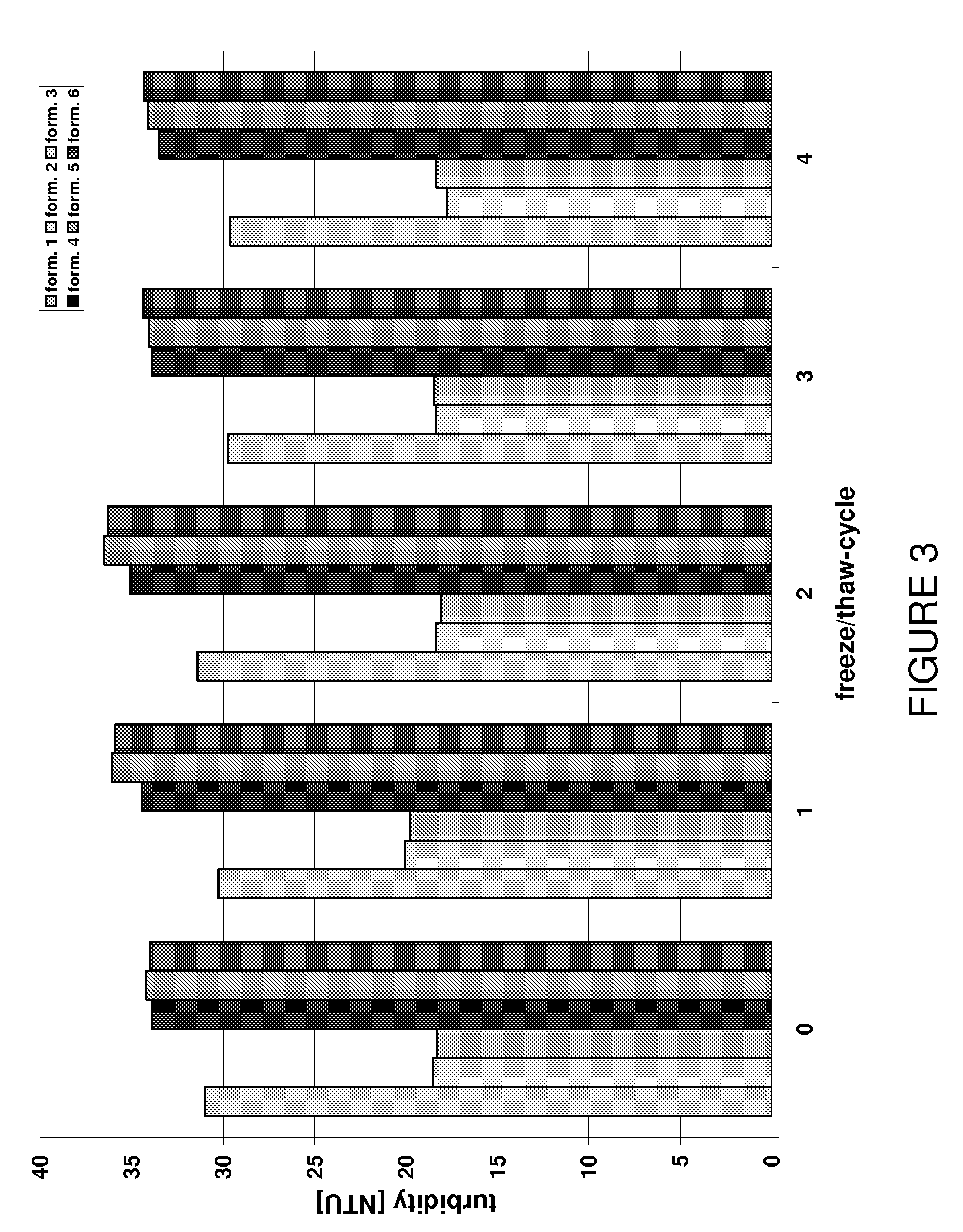
34234results about "Urinary disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
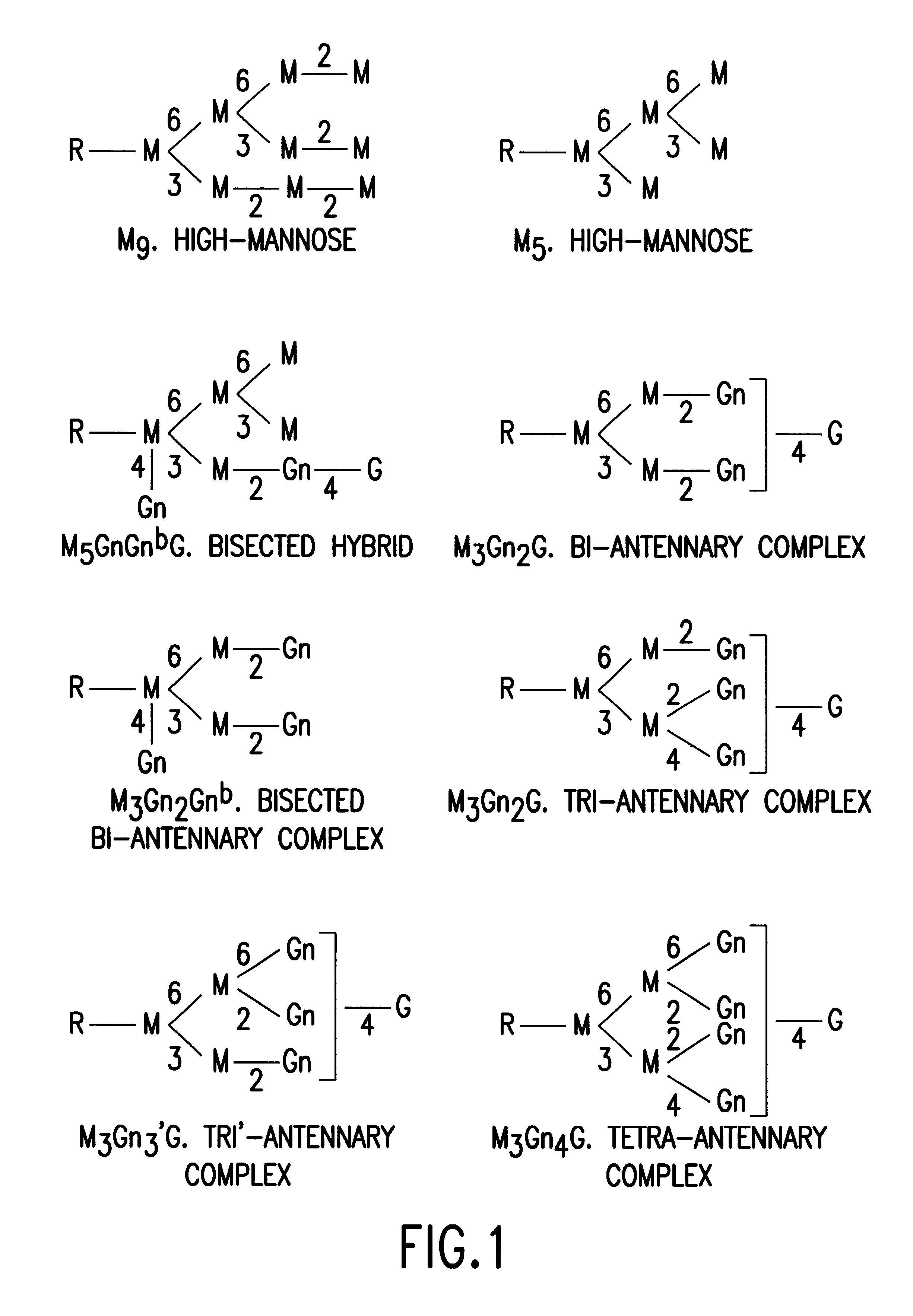
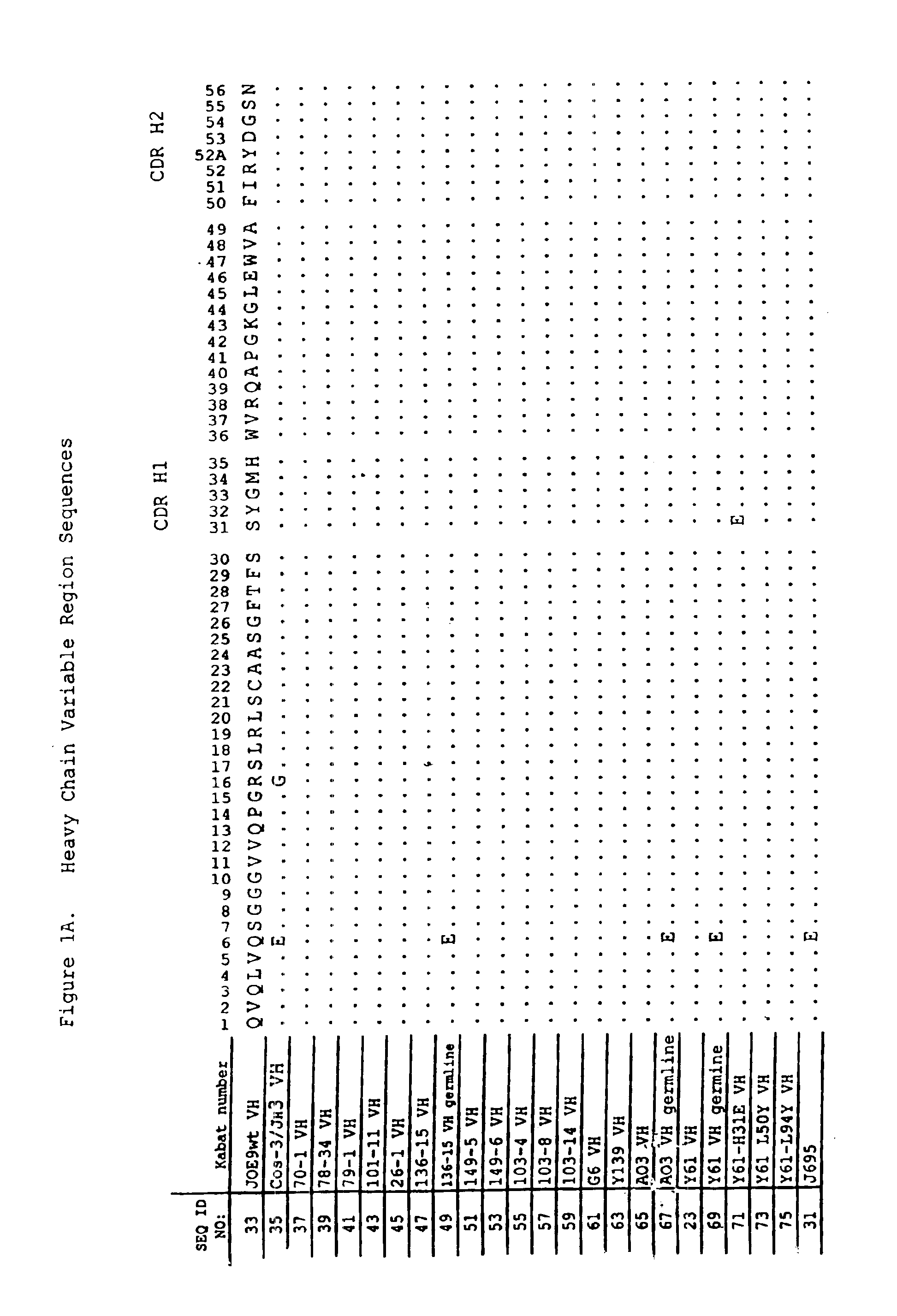
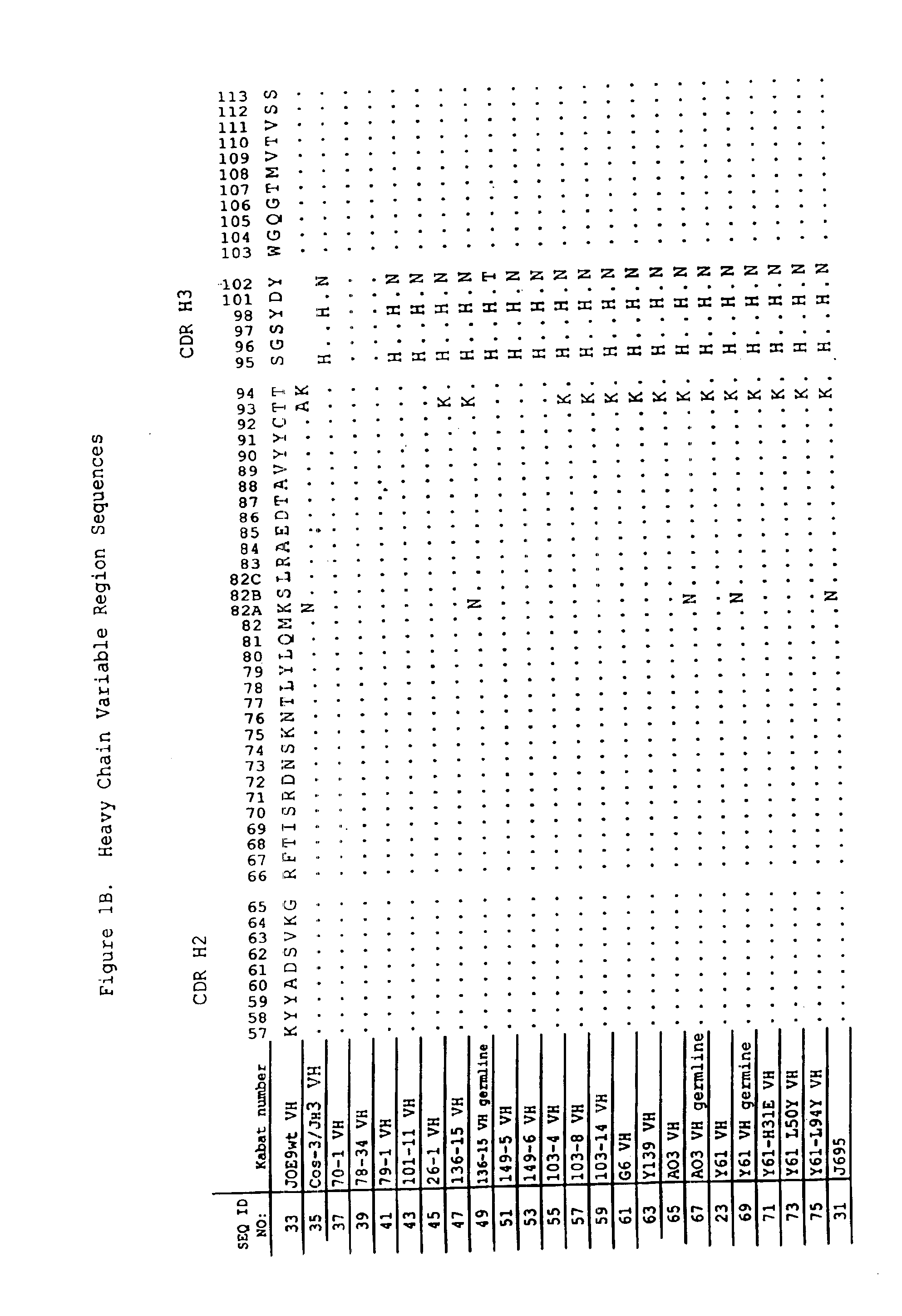
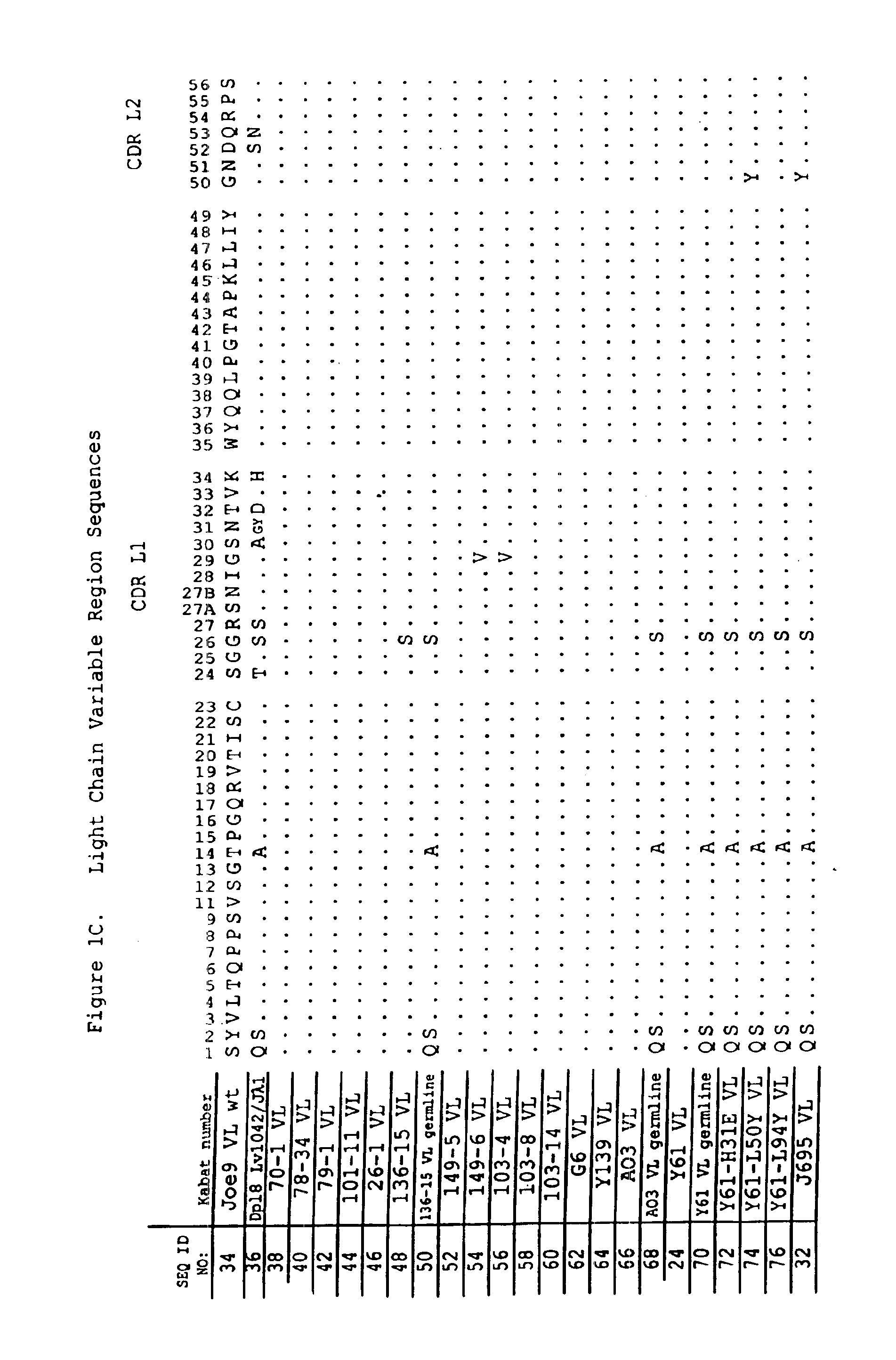
Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity
InactiveUS6602684B1Increase healing valueEnhanced Fc-mediated cellular cytotoxicityNanotechFungiAntibody fragmentsADAMTS Proteins
The present invention relates to the field glycosylation engineering of proteins. More particular, the present invention is directed to the glycosylation engineering of proteins to provide proteins with improved therapeutic properties, e.g., antibodies, antibody fragments, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin, with enhanced Fc-mediated cellular cytotoxicity.
Owner:ROCHE GLYCART AG
Antigen binding molecules with increased Fc receptor binding affinity and effector function
The present invention relates to antigen binding molecules (ABMs). In particular embodiments, the present invention relates to recombinant monoclonal antibodies, including chimeric, primatized or humanized antibodies specific for human CD20. In addition, the present invention relates to nucleic acid molecules encoding such ABMs, and vectors and host cells comprising such nucleic acid molecules. The invention further relates to methods for producing the ABMs of the invention, and to methods of using these ABMs in treatment of disease. In addition, the present invention relates to ABMs with modified glycosylation having improved therapeutic properties, including antibodies with increased Fc receptor binding and increased effector function.
Owner:ROCHE GLYCART AG
Human CTLA-4 antibodies
The present invention provides human sequence antibodies against CTLA-4 and methods of treating human diseases, infections and other conditions using these antibodies.
Owner:ER SQUIBB & SONS INC
Human antibodies that bind human IL-12 and methods for producing
InactiveUS6914128B1Avoid interferencePreservationNervous disorderPeptide/protein ingredientsAntigen bindingIn vivo
Human antibodies, preferably recombinant human antibodies, that specifically bind to human interleukin-12 (hIL-12) are disclosed. Preferred antibodies have high affinity for hIL-12 and neutralize hIL-12 activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. The antibodies, or antibody portions, of the invention are useful for detecting hIL-12 and for inhibiting hIL-12 activity, e.g., in a human subject suffering from a disorder in which hIL-12 activity is detrimental. Nucleic acids, vectors and host cells for expressing the recombinant human antibodies of the invention, and methods of synthesizing the recombinant human antibodies, are also encompassed by the invention.
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
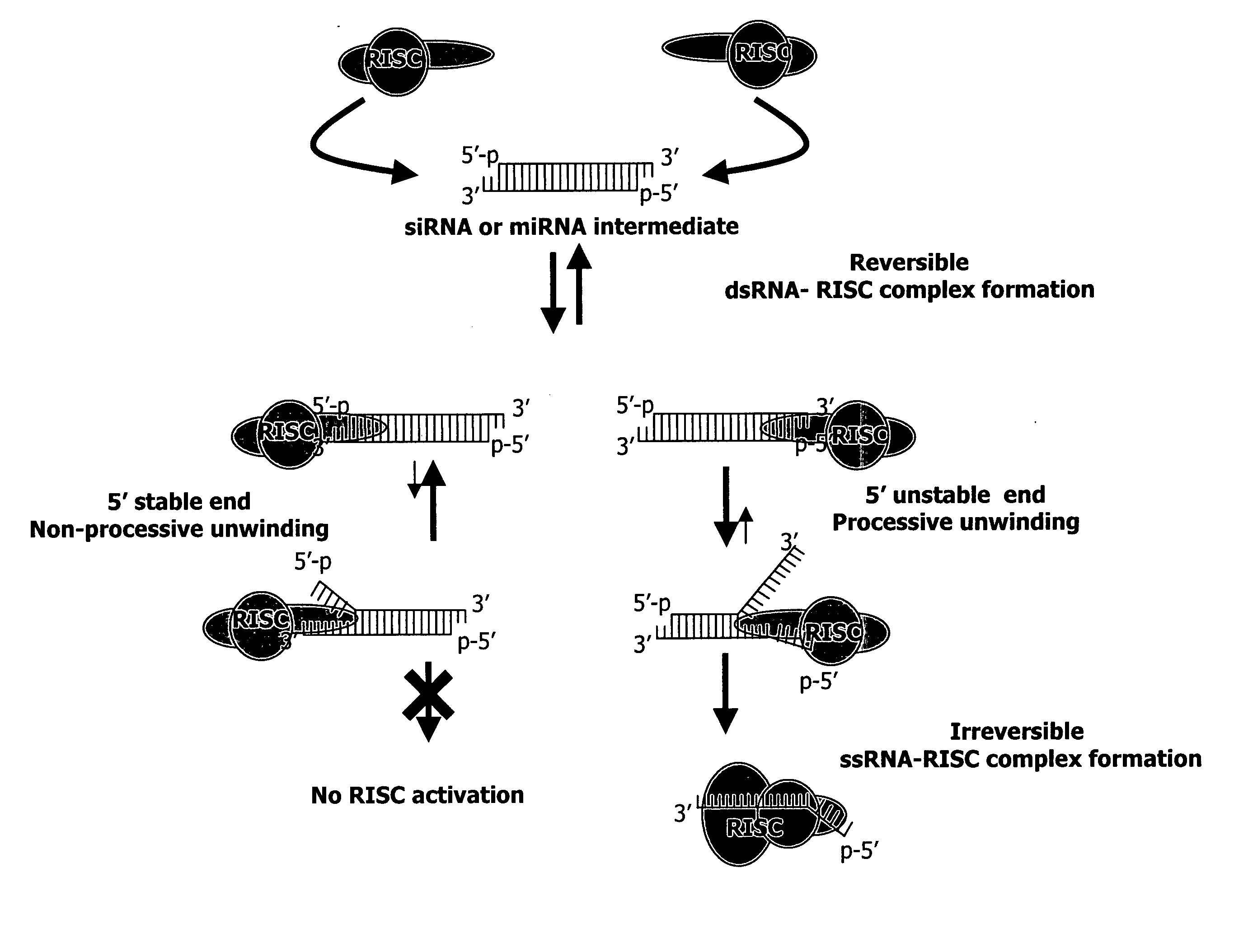
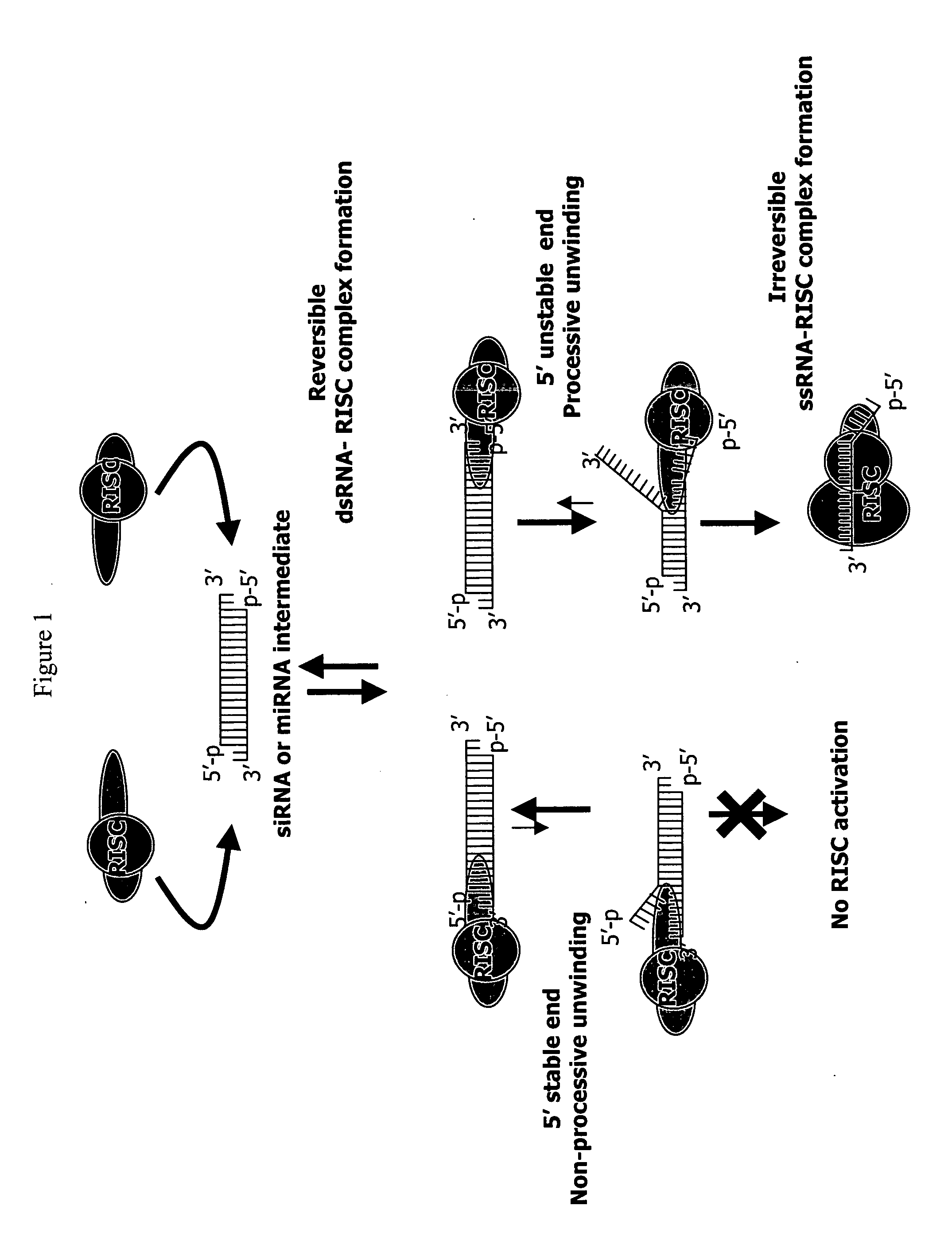
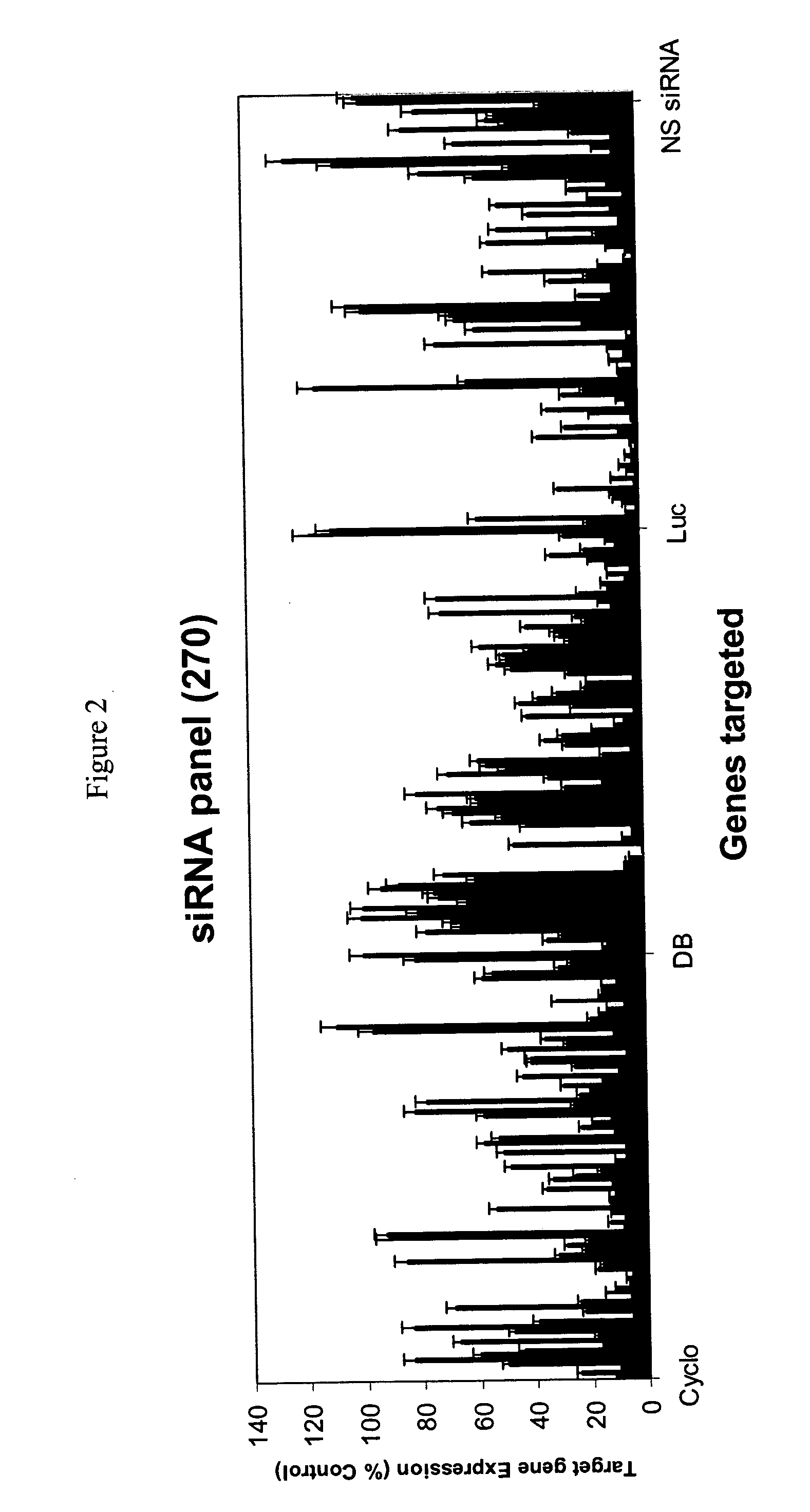
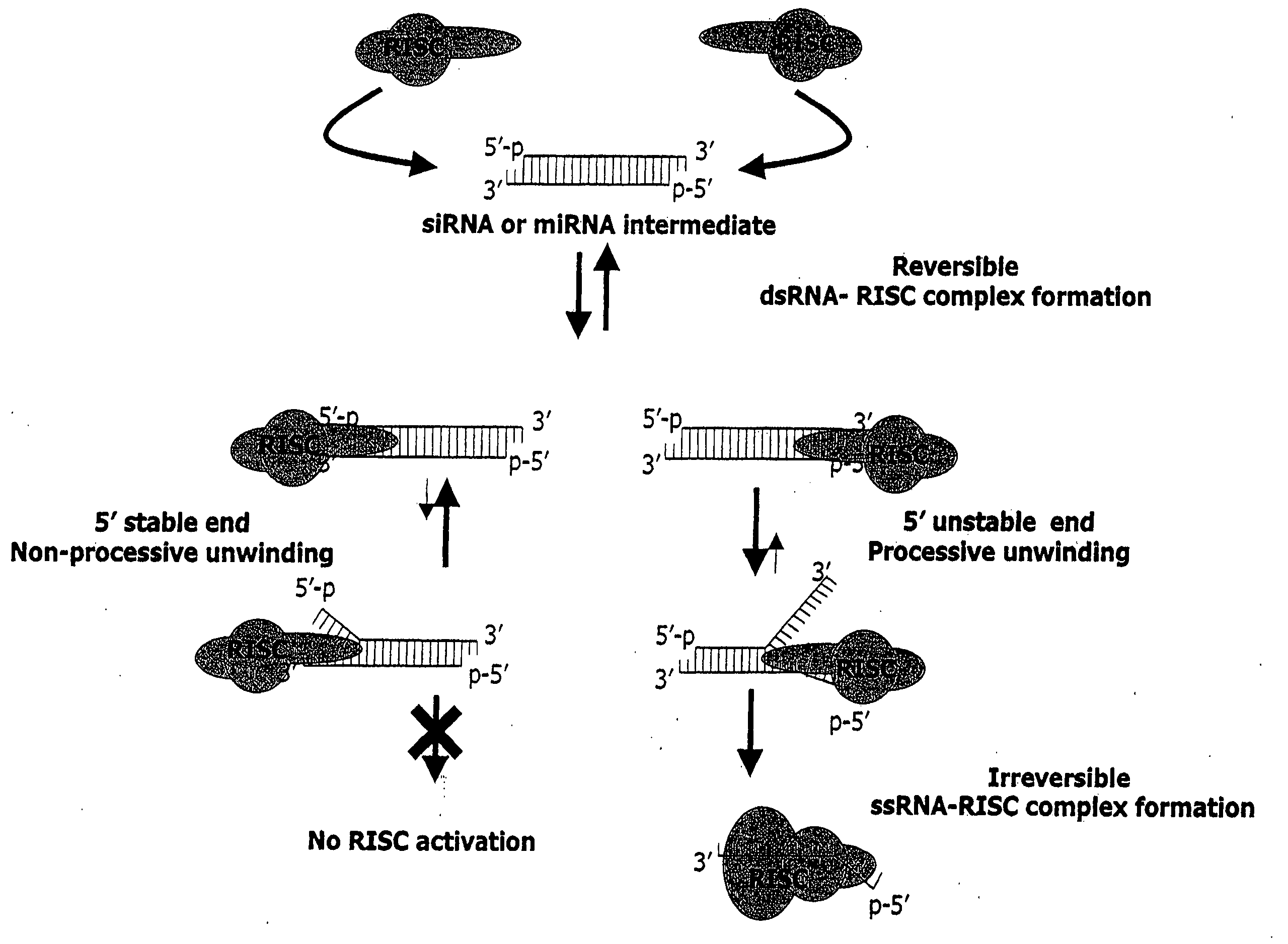
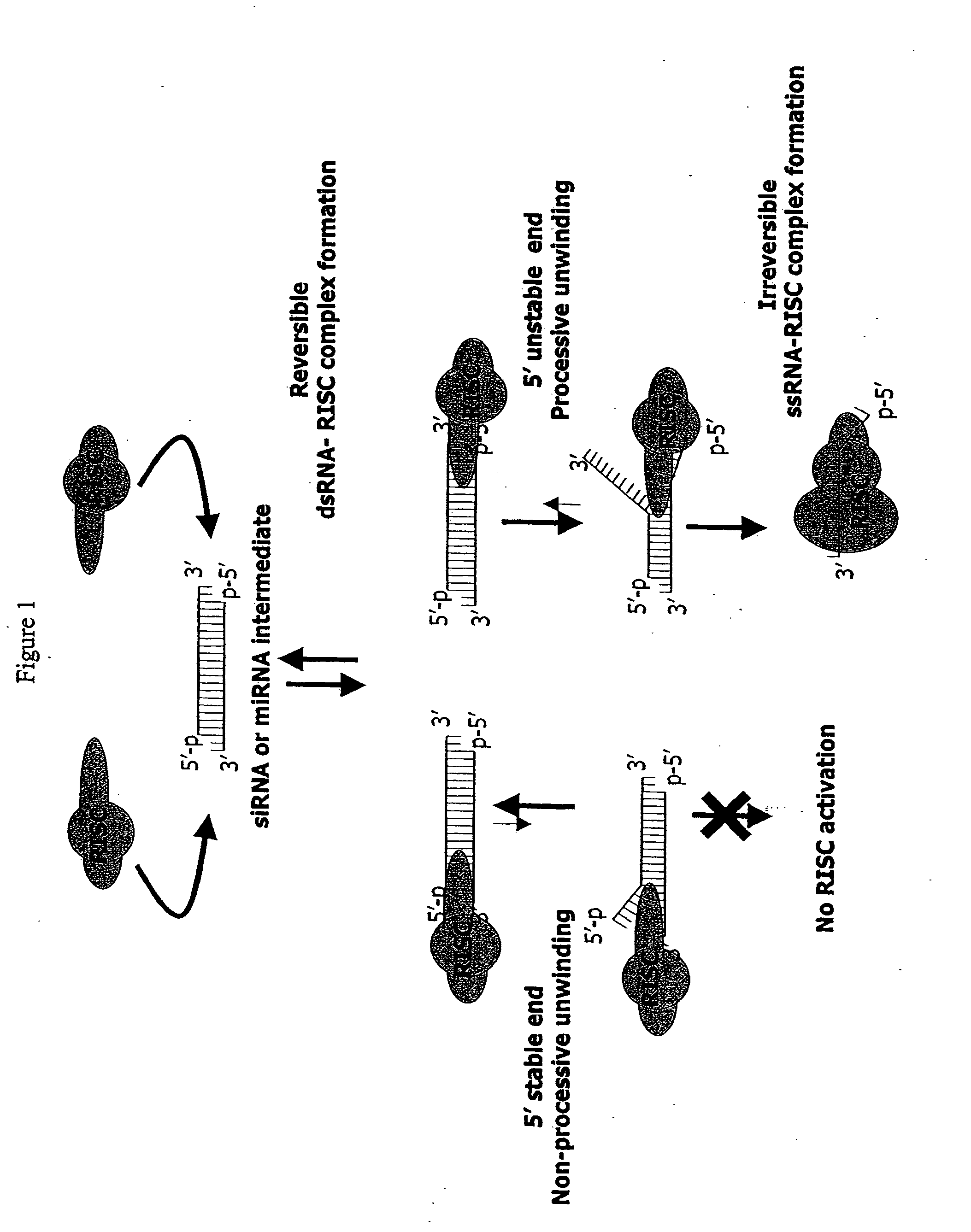
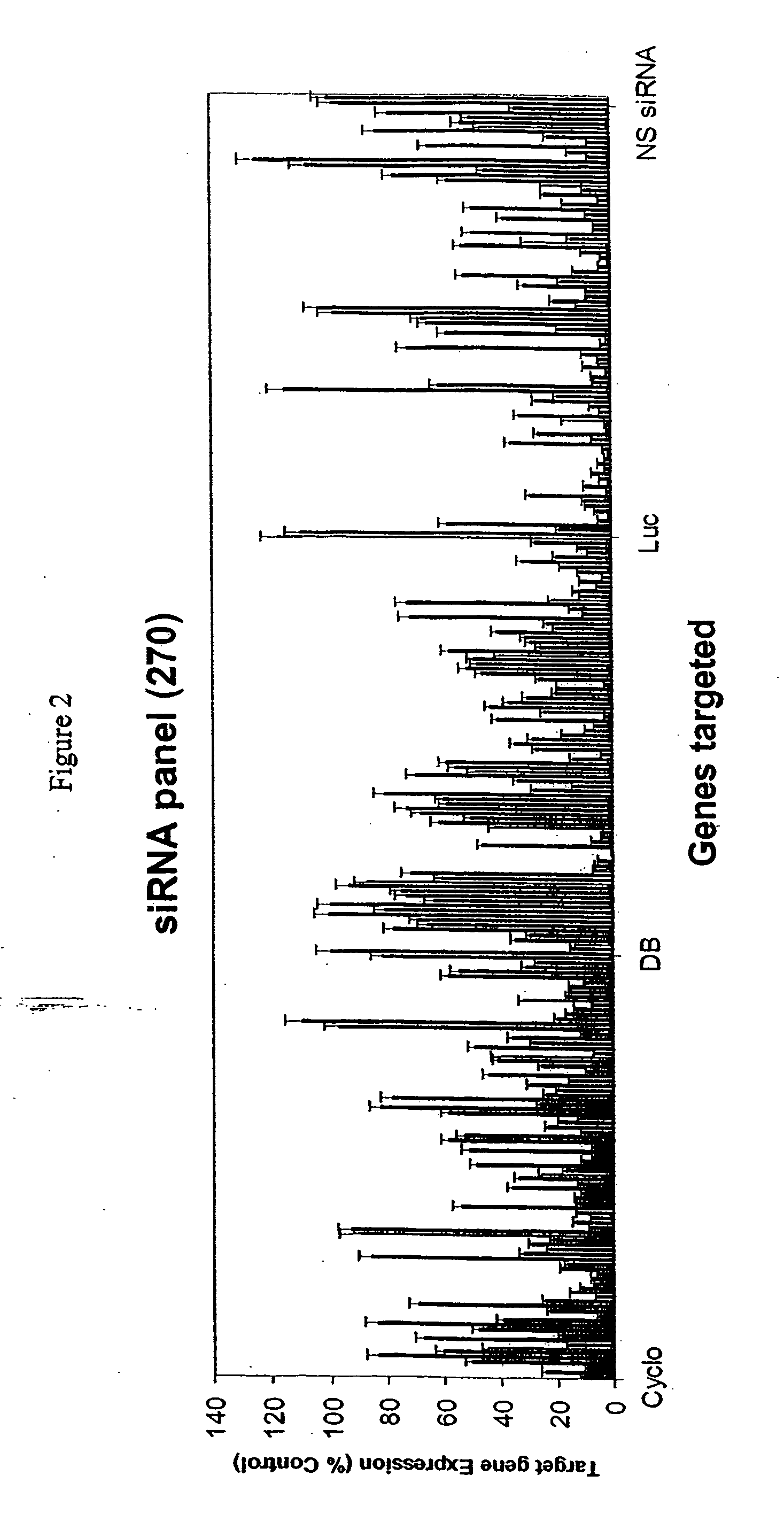
Methods and compositions for selecting siRNA of improved functionality
InactiveUS20050255487A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsGene silencingSilencing gene
Efficient sequence specific gene silencing is possible through the use of siRNA technology. By selecting particular siRNAs by rational design, one can maximize the generation of an effective gene silencing reagent, as well as methods for silencing genes. Methods, compositions, and kits generated through rational design of siRNAs are disclosed.
Owner:THERMO FISHER SCIENTIFIC INC
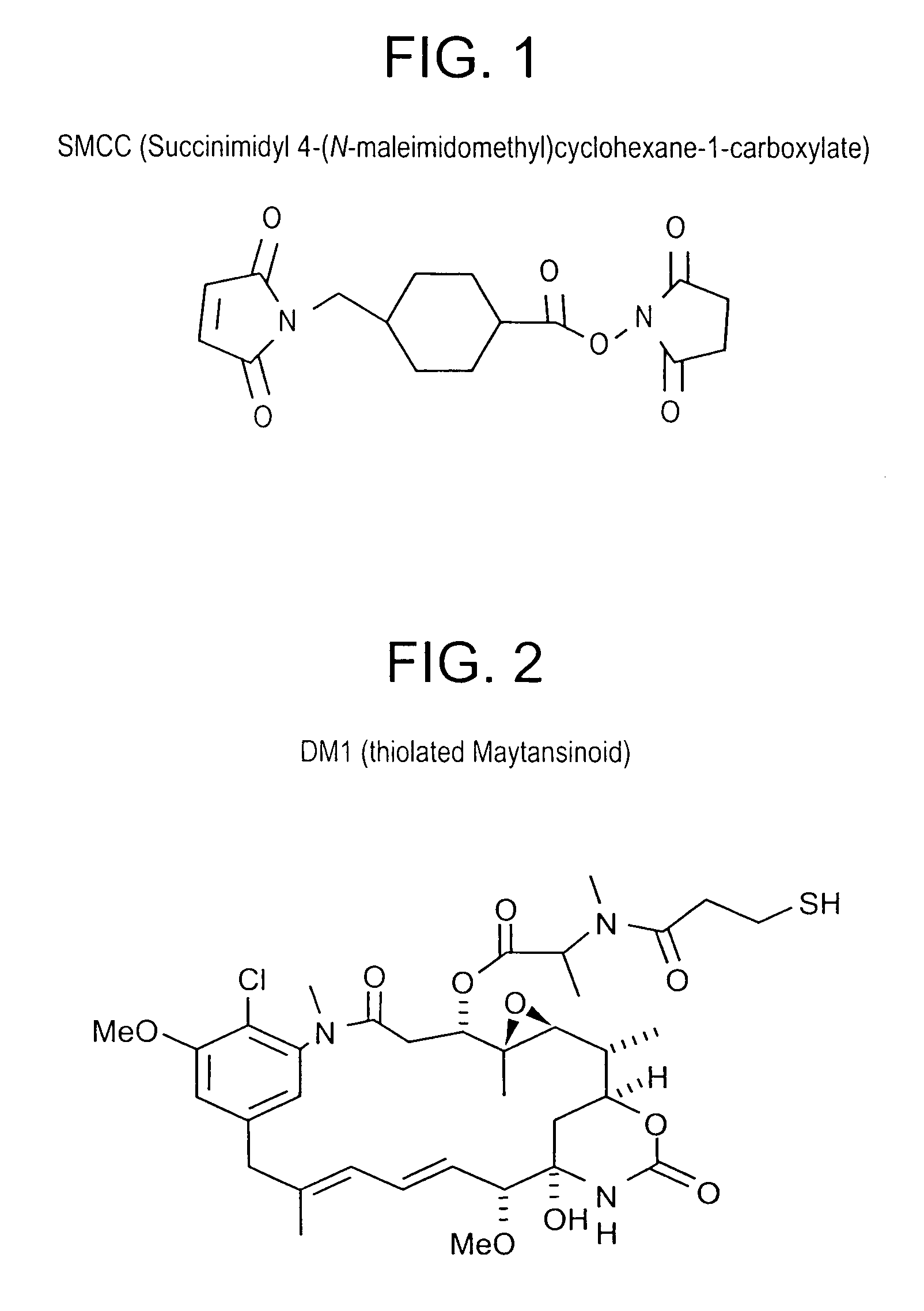
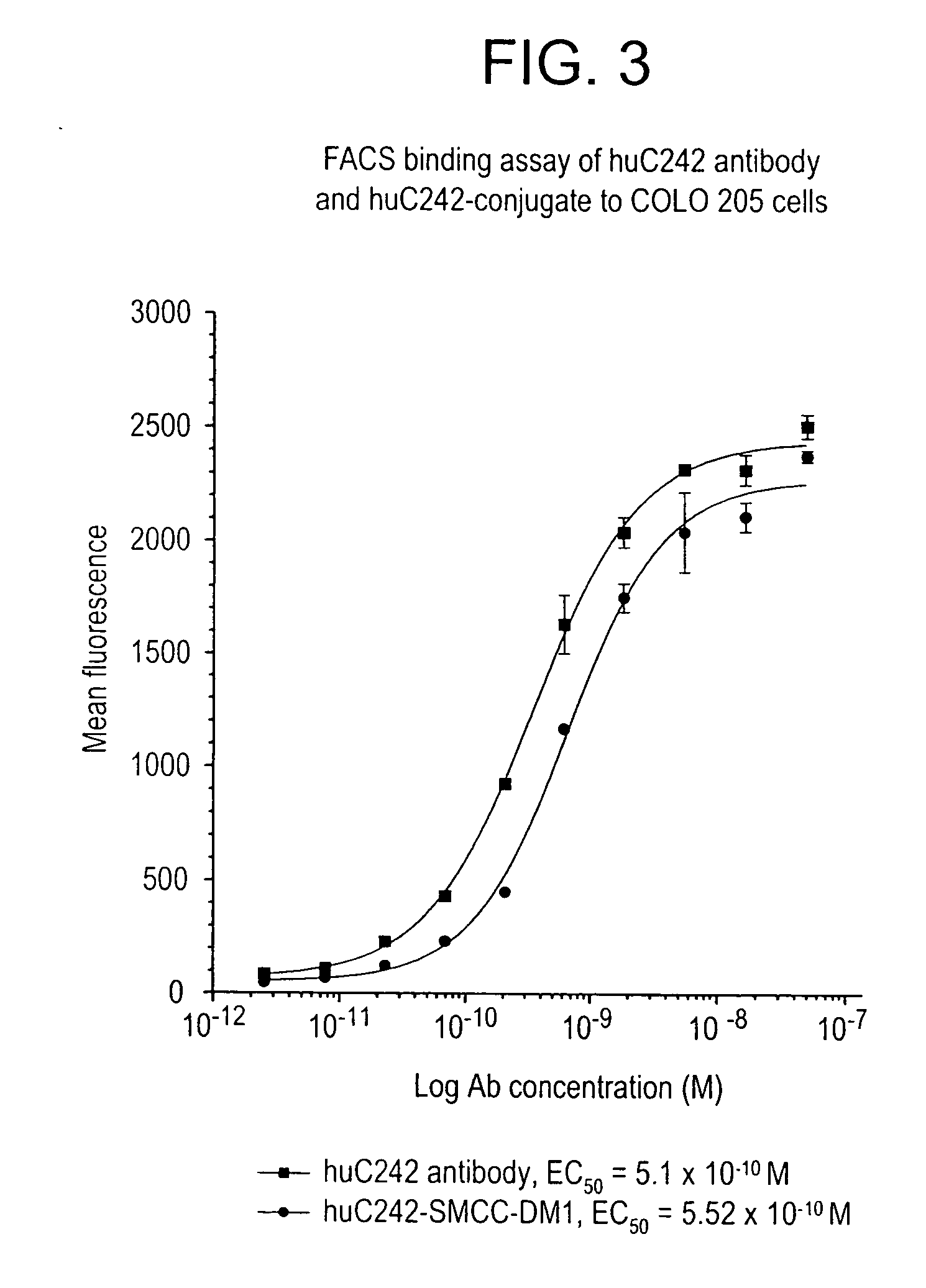
Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates
The present invention discloses a method for targeting maytansinoids to a selected cell population, the method comprising contacting a cell population or tissue suspected of containing the selected cell population with a cell-binding agent maytansinoid conjugate, wherein one or more maytansinoids is covalently linked to the cell-binding agent via a non-cleavable linker and the cell-binding agent binds to cells of the selected cell population.
Owner:IMMUNOGEN INC
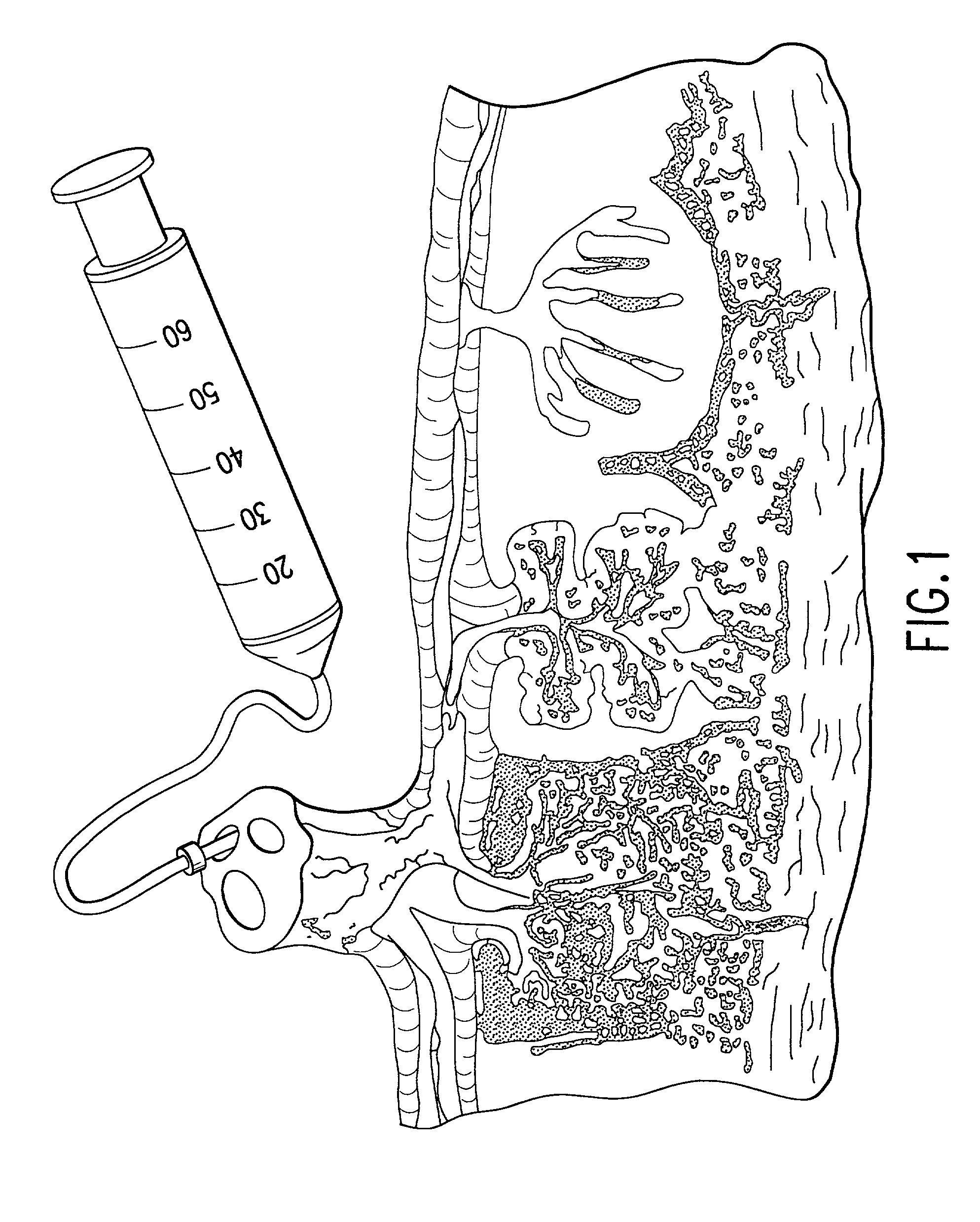
Collagen biofabric and methods of preparation and use therefor
InactiveUS20040048796A1Improved biophysical propertyImprove featuresSenses disorderPeptide/protein ingredientsSurgical GraftWound dressing
The present invention relates to collagenous membranes produced from amnion, herein referred to as a collagen biofabric. The collagen biofabric of the invention has the structural integrity of the native non-treated amniotic membrane, i.e., the native tertiary and quaternary structure. The present invention provides a method for preparing a collagen biofabric from a placental membrane, preferably a human placental membrane having a chorionic and amniotic membrane, by decellularizing the amniotic membrane. In a preferred embodiment, the amniotic membrane is completely decellularized. The collagen biofabric of the invention has numerous utilities in the medical and surgical field including for example, blood vessel repair, construction and replacement of a blood vessel, tendon and ligament replacement, wound-dressing, surgical grafts, ophthalmic uses, sutures, and others. The benefits of the biofabric are, in part, due to its physical properties such as biomechanical strength, flexibility, suturability, and low immunogenicity, particularly when derived from human placenta.
Owner:CELLULAR THERAPEUTICS DIV OF CELGENE +1
Combination therapies employing GITR binding molecules
ActiveUS8591886B2Small sizeImprove securityOrganic active ingredientsNervous disorderInternal medicine
Owner:GITR
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS7355008B2Function increaseGood curative effectAntibacterial agentsSenses disorderTherapeutic antibodyEffector cell
Owner:MARCOGENICS INC +1
Delivery and formulation of engineered nucleic acids
ActiveUS20120251618A1Improve the level ofIncrease in level of polypeptideNervous disorderAntipyreticNucleic acidProtein expression
Provided are formulations, compositions and methods for delivering biological moieties such as modified nucleic acids into cells to modulate protein expression. Such compositions and methods include the delivery of biological moieties, and are useful for production of proteins.
Owner:MODERNATX INC
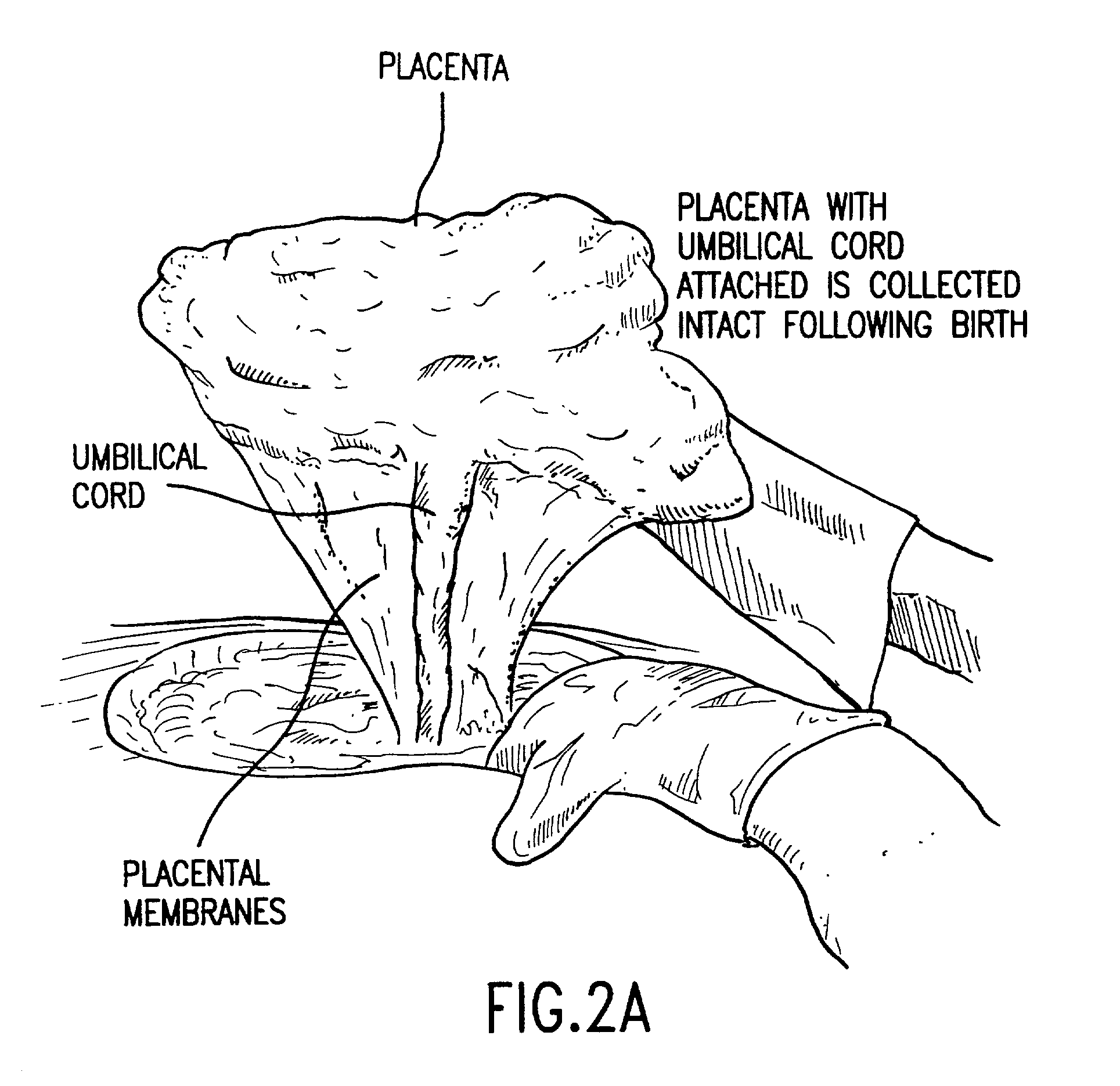
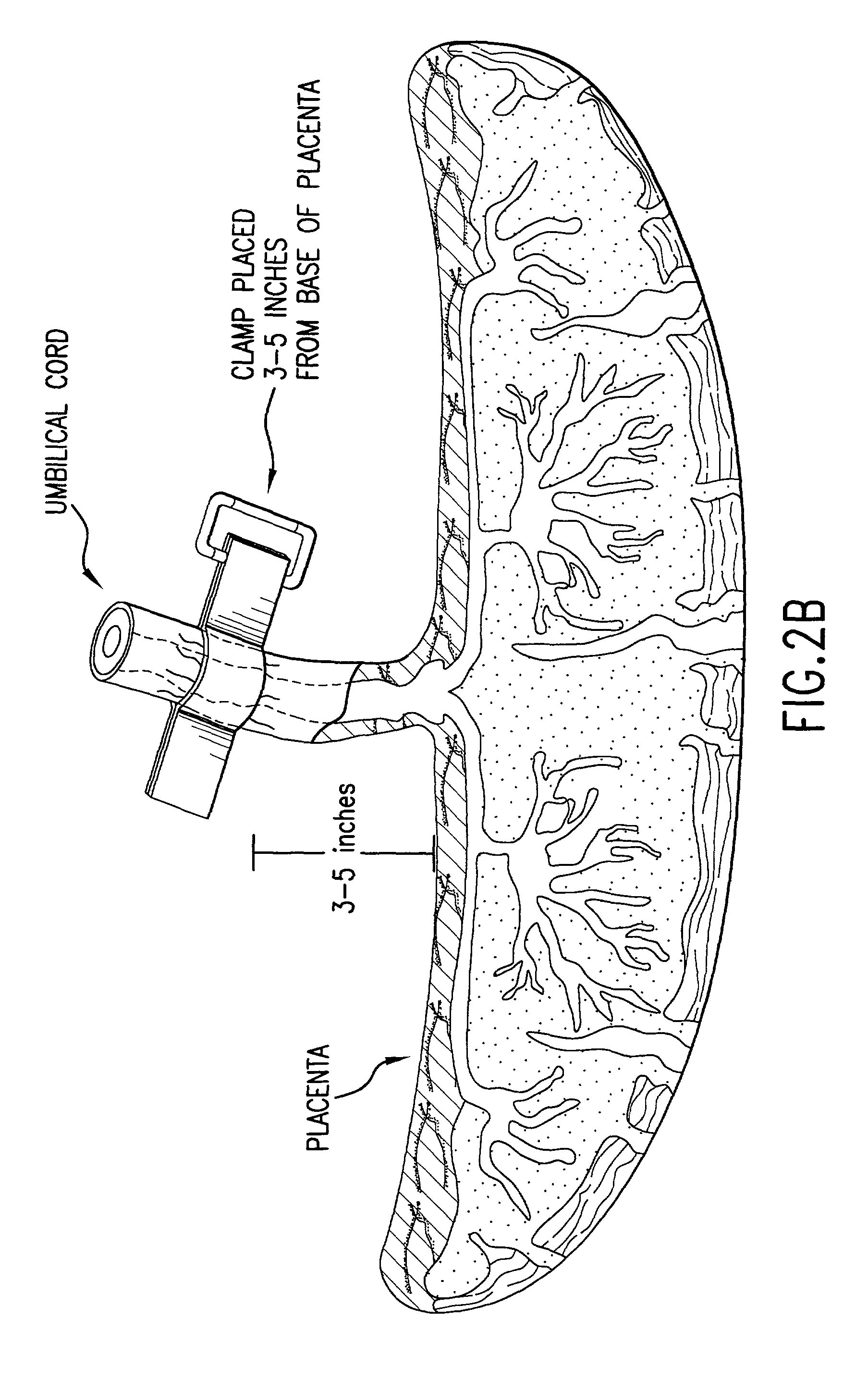
Post-partum mammalian placenta, its use and placental stem cells therefrom
InactiveUS20030032179A1Enhance exsanguinationEnhance sterile conditionSenses disorderAntipyreticAnticoagulant AgentEmbryo
The present invention provides a method of extracting and recovering embryonic-like stem cells, including, but not limited to pluripotent or multipotent stem cells, from an exsanguinated human placenta. A placenta is treated to remove residual umbilical cord blood by perfusing an exsanguinated placenta, preferably with an anticoagulant solution, to flush out residual cells. The residual cells and perfusion liquid from the exsanguinated placenta are collected, and the embryonic-like stem cells are separated from the residual cells and perfusion liquid. The invention also provides a method of utilizing the isolated and perfused placenta as a bioreactor in which to propagate endogenous cells, including, but not limited to, embryonic-like stem cells. The invention also provides methods for propagation of exogenous cells in a placental bioreactor and collecting the propagated exogenous cells and bioactive molecules therefrom.
Owner:CELULARITY INC
Methods of making conditioned cell culture medium compositions
InactiveUS6372494B1Eliminate wrinklesEliminate frown lineCosmetic preparationsPeptide/protein ingredientsReserve CellCell culture media
Novel products comprising conditioned cell culture medium compositions and methods of use are described. The conditioned cell medium compositions of the invention may be comprised of any known defined or undefined medium and may be conditioned using any eukaryotic cell type. The medium may be conditioned by stromal cells, parenchymal cells, mesenchymal stem cells, liver reserve cells, neural stem cells, pancreatic stem cells and / or embryonic stem cells. Additionally, the cells may be genetically modified. A three-dimensional tissue construct is preferred. Once the cell medium of the invention is conditioned, it may be used in any state. Physical embodiments of the conditioned medium include, but are not limited to, liquid or solid, frozen, lyophilized or dried into a powder. Additionally, the medium is formulated with a pharmaceutically acceptable carrier as a vehicle for internal administration, applied directly to a food item or product, formulated with a salve or ointment for topical applications, or, for example, made into or added to surgical glue to accelerate healing of sutures following invasive procedures. Also, the medium may be further processed to concentrate or reduce one or more factors or components contained within the medium.
Owner:ALLERGAN INC
Functional and hyperfunctional siRNA
ActiveUS20050246794A1Improve efficiencyGood curative effectOrganic active ingredientsGenetic material ingredientsSilent geneGene silencing
Owner:THERMO FISHER SCIENTIFIC INC
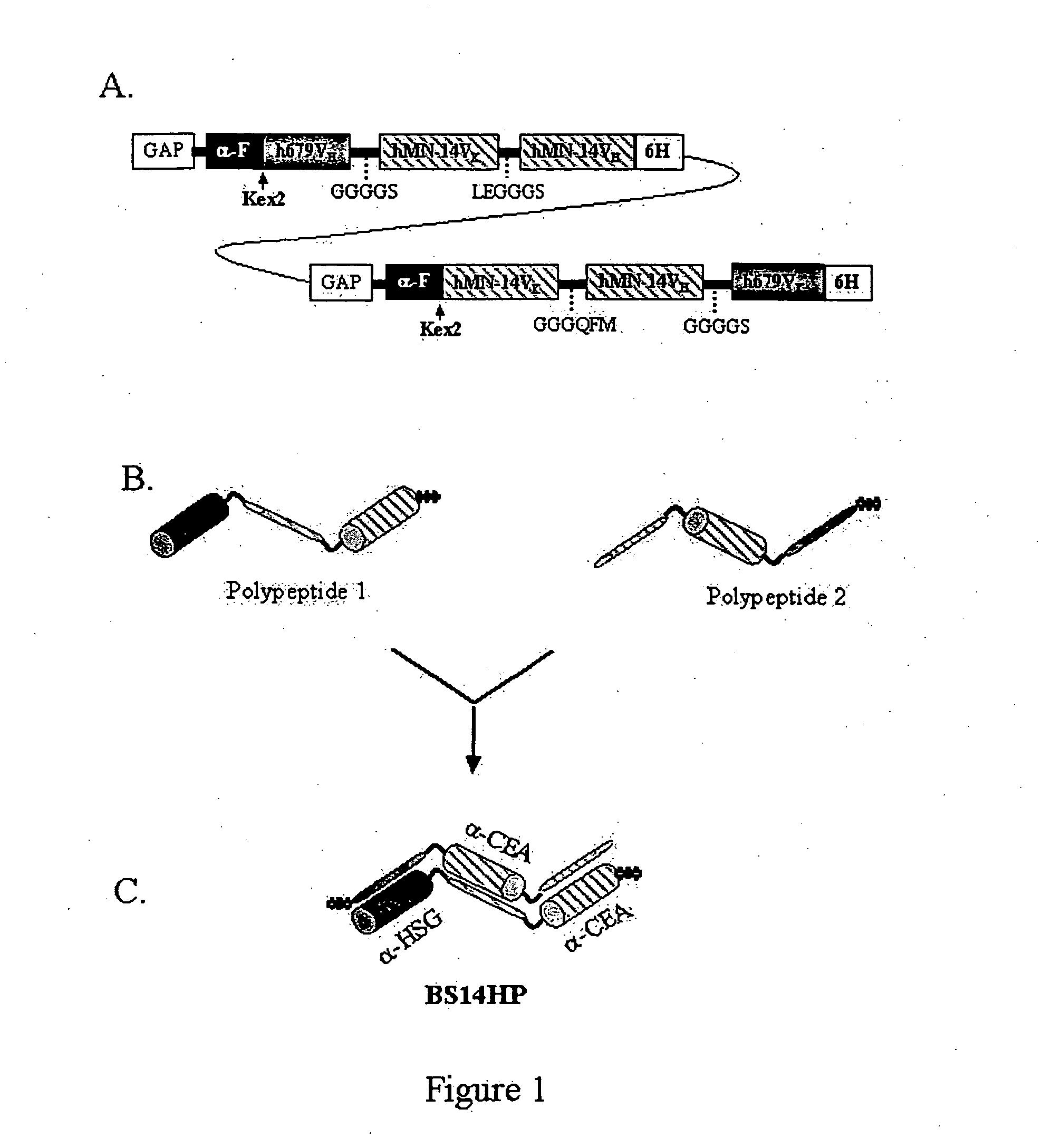
Polyvalent protein complex
The invention provides for a polyvalent protein complex (PPC) comprising two polypeptide chains generally arranged laterally to one another. Each polypeptide chain typically comprises 3 or 4 “v-regions”, which comprise amino acid sequences capable of forming an antigen binding site when matched with a corresponding v-region on the opposite polypeptide chain. Up to about 6 “v-regions” can be used on each polypeptide chain. The v-regions of each polypeptide chain are connected linearly to one another and may be connected by interspersed linking regions. When arranged in the form of the PPC, the v-regions on each polypeptide chain form individual antigen binding sites.
Owner:IBC PHARMACEUTICALS INC
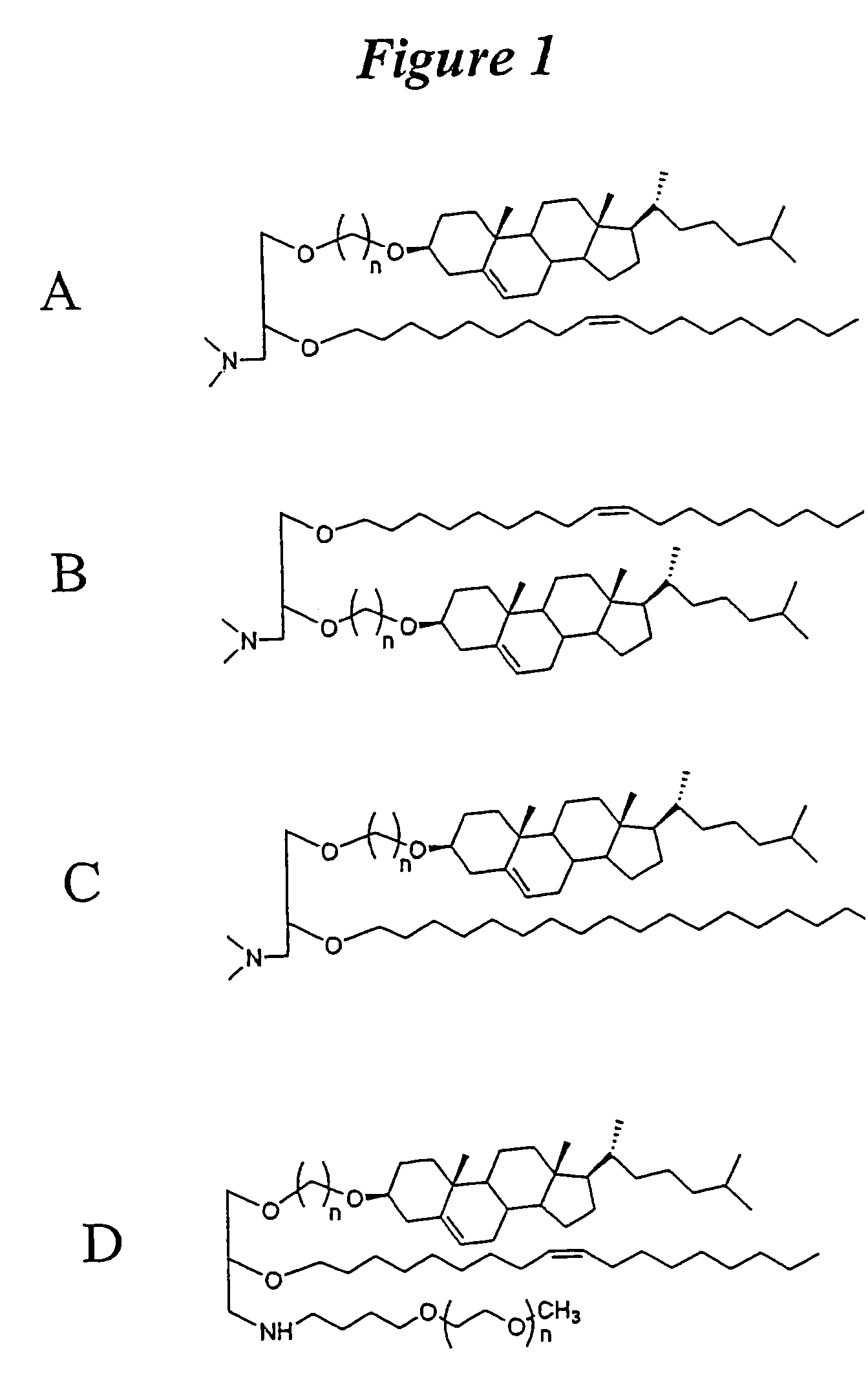
Lipid nanoparticle based compositions and methods for the delivery of biologically active molecules
ActiveUS7404969B2Reduce deliveryAntibacterial agentsOrganic active ingredientsLipid formationMolecular composition
The present invention relates to novel cationic lipids, transfection agents, microparticles, nanoparticles, and short interfering nucleic acid (siNA) molecules. Specifically, the invention relates to novel cationic lipids, microparticles, nanoparticles and transfection agents that effectively transfect or deliver short interfering nucleic acid (siNA). The compositions described herein are generally referred to as formulated molecular compositions (FMC) or lipid nanoparticles (LNP).
Owner:SIRNA THERAPEUTICS INC
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Cross-linkers and their uses
ActiveUS20090274713A1Good water solubilityHigh sensitivityOrganic active ingredientsTripeptide ingredientsCell bindingCross-link
Charged or pro-charged cross-linking moieties and conjugates of cell binding agents and drugs comprising the charged or pro-charged cross-linking moieties and method of making the same.
Owner:IMMUNOGEN INC
Modified binding molecules comprising connecting peptides
InactiveUS20050163782A1Well formedIncreased formationAntibacterial agentsSenses disorderDisulfide LinkagePeptide
The instant invention describes methods of separating or preferentially synthesizing dimers which are linked via at least one interchain disulfide linkage from dimers which are not linked via at least one interchain disulfide linkage from a mixture comprising the two types of polypeptide dimers. These forms can be separated from each other using hydrophobic interaction chromatography. In addition, the invention pertains to connecting peptides that result in the preferential biosynthesis of dimers that are linked via at least one interchain disulfide linkage or that are not linked via at least one interchain disulfide linkage. The invention also pertains to compositions in which a majority of the dimers are linked via at least one interchain disulfide linkage or are not linked via at least one interchain disulfide linkage. The invention still further pertains to novel binding molecules, e.g., comprising connecting peptides of the invention.
Owner:BIOGEN MA INC
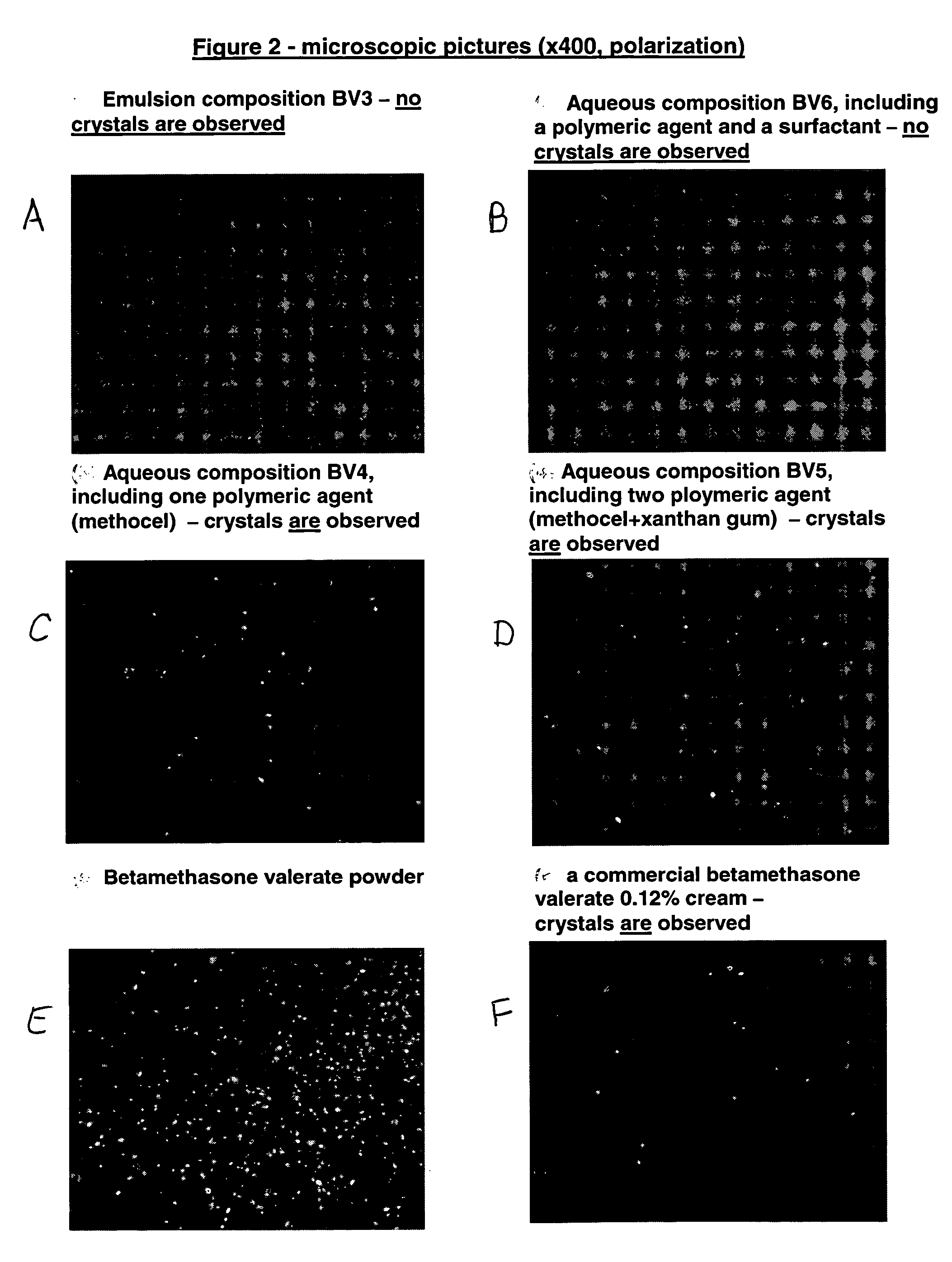
Pharmaceutical and cosmetic carrier or composition for topical application
A pharmaceutical or cosmetic carrier or composition for topical application characterized by rheological properties which render the carrier or composition semi-solid at rest and a liquid upon application of shear forces thereto. The composition or carrier are prepared by mixing 1-25 percent of a solidifying agent and 75-99 percent of a hydrophobic solvent, by weight, wherein at least one of them has therapeutic or cosmetic benefits, in the presence or absence of a biologically active substance.
Owner:VYNE PHARMA LTD
Anti-pd-l1 antibodies and uses thereof
ActiveUS20140341917A1Function increaseUpregulate cell-mediated immune responsesOrganic active ingredientsPeptide/protein ingredientsAntigen Binding FragmentAntigen binding
The present application relates to anti-PD-L1 antibodies or antigen binding fragments thereof, nucleic acid encoding the same, therapeutic compositions thereof, and their use to enhance T-cell function to upregulate cell-mediated immune responses and for the treatment of T cell dysfunctional disorders, such as tumor immunity, for the treatment of and cancer.
Owner:MERCK PATENT GMBH
Methods of using and compositions comprising immunomodulatory compounds for the treatment and management of myeloproliferative diseases
InactiveUS20040087546A1Reduce adverse effectsImprove toleranceBiocideNervous disorderActive agentMyeloproliferative disease
Methods of treating, preventing and / or managing a myeloproliferative disease are disclosed. Specific methods encompass the administration of an immunomodulatory compound, or a pharmaceutically acceptable salt, solvate, hydrate, stereoisomer, clathrate, or prodrug thereof, alone or in combination with a second active agent, and / or the transplantation of blood or cells. Particular second active agents are capable of suppressing the overproduction of hematopoietic stem cells or ameliorating one or more of the symptoms of a myeloproliferative disease. Pharmaceutical compositions, single unit dosage forms, and kits suitable for use in methods of the invention are also disclosed.
Owner:CELGENE CORP
Cartilage and bone repair and regeneration using postpartum-derived cells
Cells derived from postpartum tissue and methods for their isolation and induction to differentiate to cells of a chondrogenic or osteogenic phenotype are provided by the invention. The invention further provides cultures and compositions of the postpartum-derived cells and products related thereto. The postpartum-derived cells of the invention and products related thereto have a plethora of uses, including but not limited to research, diagnostic, and therapeutic applications, for example, in the treatment of bone and cartilage conditions.
Owner:DEPUY SYNTHES PROD INC
Polypeptide variants with altered effector function
InactiveUS20060067930A1High affinity bindingWeaker binding affinityCompound screeningNervous disorderEffector functionsMolecular biology
The invention provides polypeptides having IgG Fc regions with amino acid modifications that result in the polypeptides exhibiting altered Fc effector functions.
Owner:GENENTECH INC
Erythropoietin: remodeling and glycoconjugation of erythropoietin
InactiveUS20060088906A1Increase hematocrit levelImprove the level ofPeptide/protein ingredientsPeptide preparation methodsErythropoiesisErythropoietin
Owner:NEOSE TECH
Stable high protein concentration formulations of human Anti-tnf-alpha-antibodies
InactiveUS20100278822A1Suitable viscosityIncrease concentrationAntibacterial agentsSenses disorderHigh concentrationPolyol
The invention provides a liquid pharmaceutical formulation which does not include NaCl and comprises more than 20 mg of a polyol and at least about 100 mg / mL of a human anti-TNF-alpha antibody, or antigen-binding portion thereof. The invention provides a high concentration antibody formulation having long-term stability and advantageous characteristics for subcutaneous administration.
Owner:ABBVIE BIOTECHNOLOGY LTD
Method of collecting placental stem cells
InactiveUS20020123141A1Increase concentrationImprove the environmentSenses disorderAntipyreticCord blood stem cellEmbryo
A method of collecting embryonic-like stem cells from a placenta which has been treated to remove residual cord blood by perfusing the drained placenta with an anticoagulant solution to flush out residual cells, collecting the residual cells and perfusion liquid from the drained placenta, and separating the embryonic-like cells from the residual cells and perfusion liquid. Exogenous cells can be propagated in the placental bioreactor and bioactive molecules collected therefrom.
Owner:CELULARITY INC
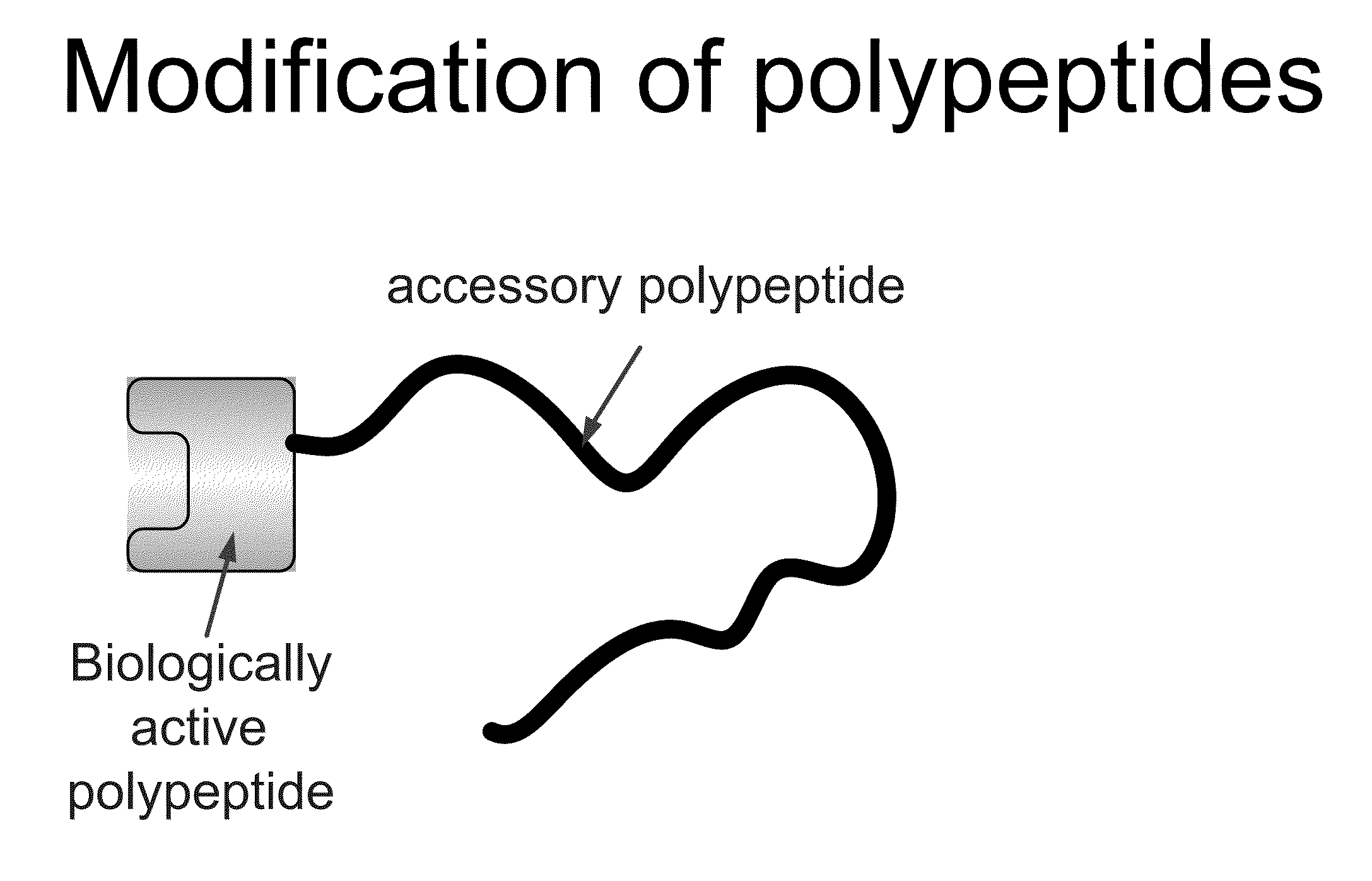
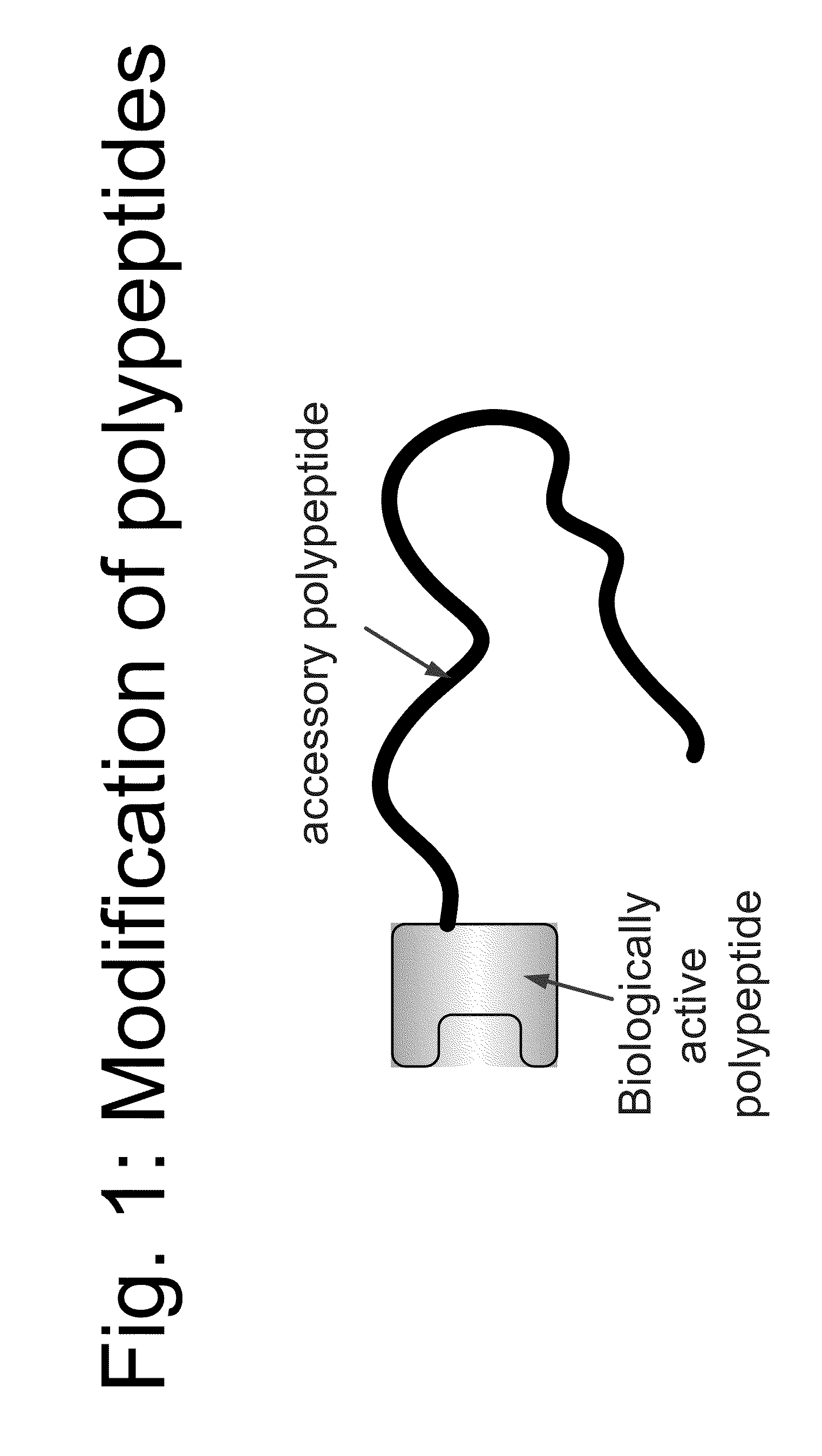
Compositions and methods for modifying properties of biologically active polypeptides
ActiveUS20090092582A1Improve protein solubilityReduce aggregationAntibacterial agentsSenses disorderDrug biological activityBiological activity
Owner:AMUNIX PHARMA INC
Steroid kit and foamable composition and uses thereof
InactiveUS20060018937A1Preventing and alleviatingCosmetic preparationsSenses disorderActive agentFilm-forming agent
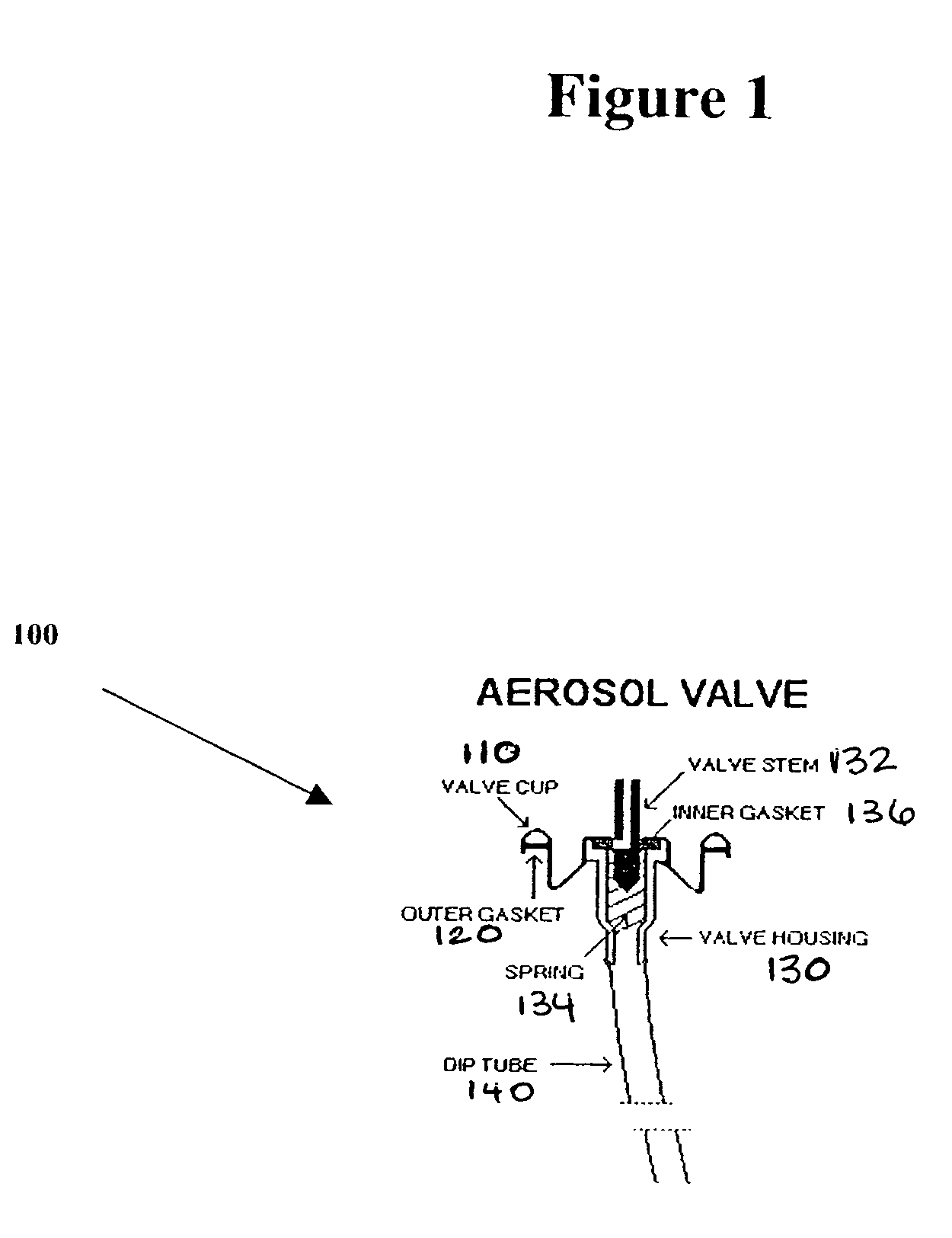
A composition and therapeutic kit including an aerosol packaging assembly including a container accommodating a pressurized product and an outlet capable of releasing a foamable composition, including a steroid as a foam. The pressurized product includes a foamable composition including: a container accommodating a pressurized product; and an outlet capable of releasing the pressurized product as a foam; wherein the pressurized product comprises a foamable composition including: i. a steroid; ii. at least one organic carrier selected from the group consisting of a hydrophobic organic carrier, a polar solvent, an emollient and mixtures thereof, at a concentration of about 2% to about 50% by weight; iii. a surface-active agent; iv. about 0.01% to about 5% by weight of at least one polymeric additive selected from the group consisting of a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent; v. water; and vi. liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. The composition further may include a therapeutically active foam adjuvant, selected from the group consisting of a fatty alcohol, a fatty acid, a hydroxyl fatty acid; and mixtures thereof.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com