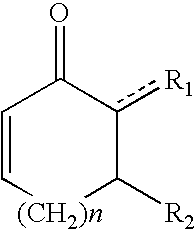
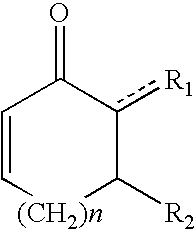
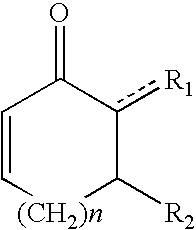
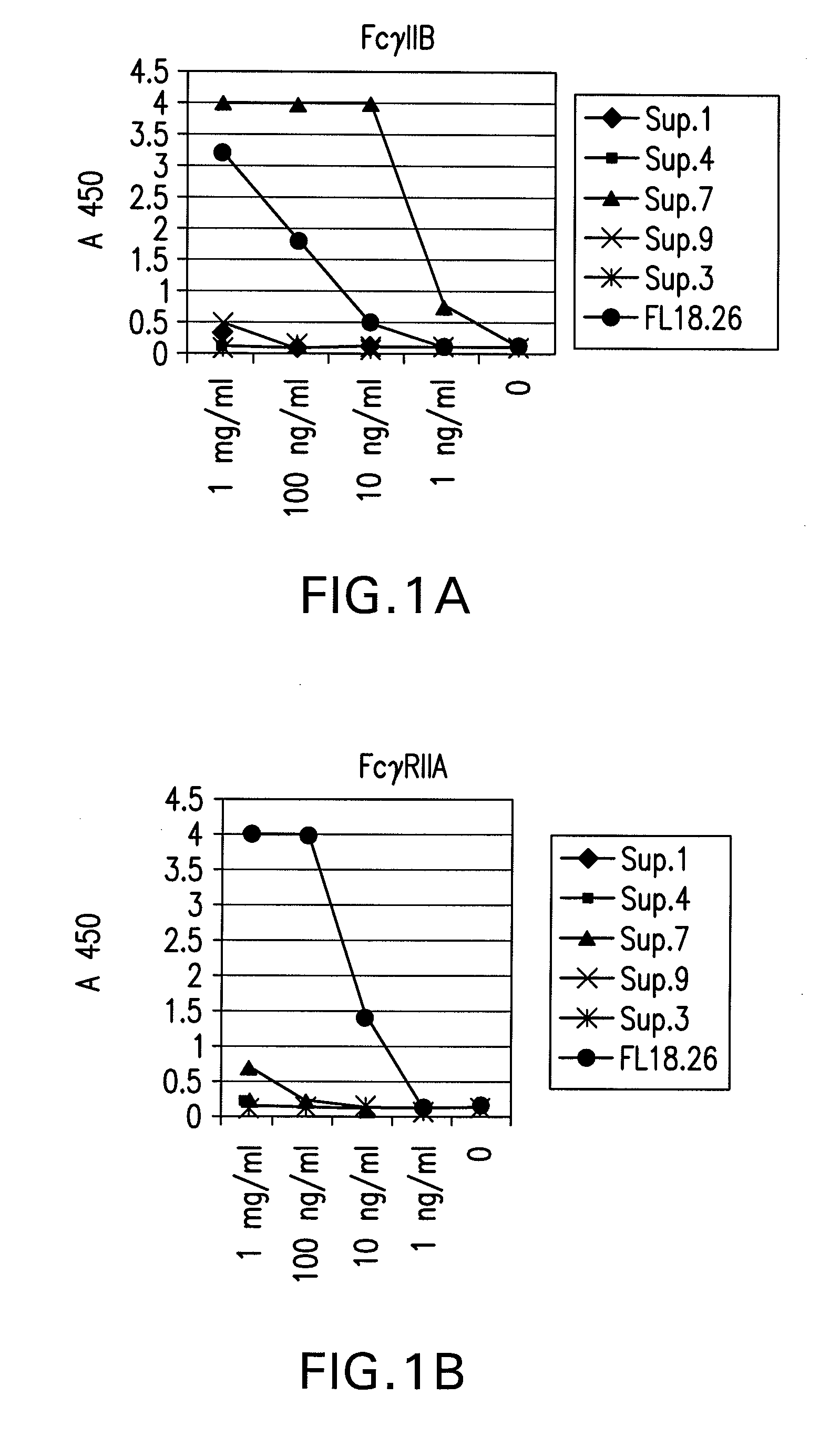
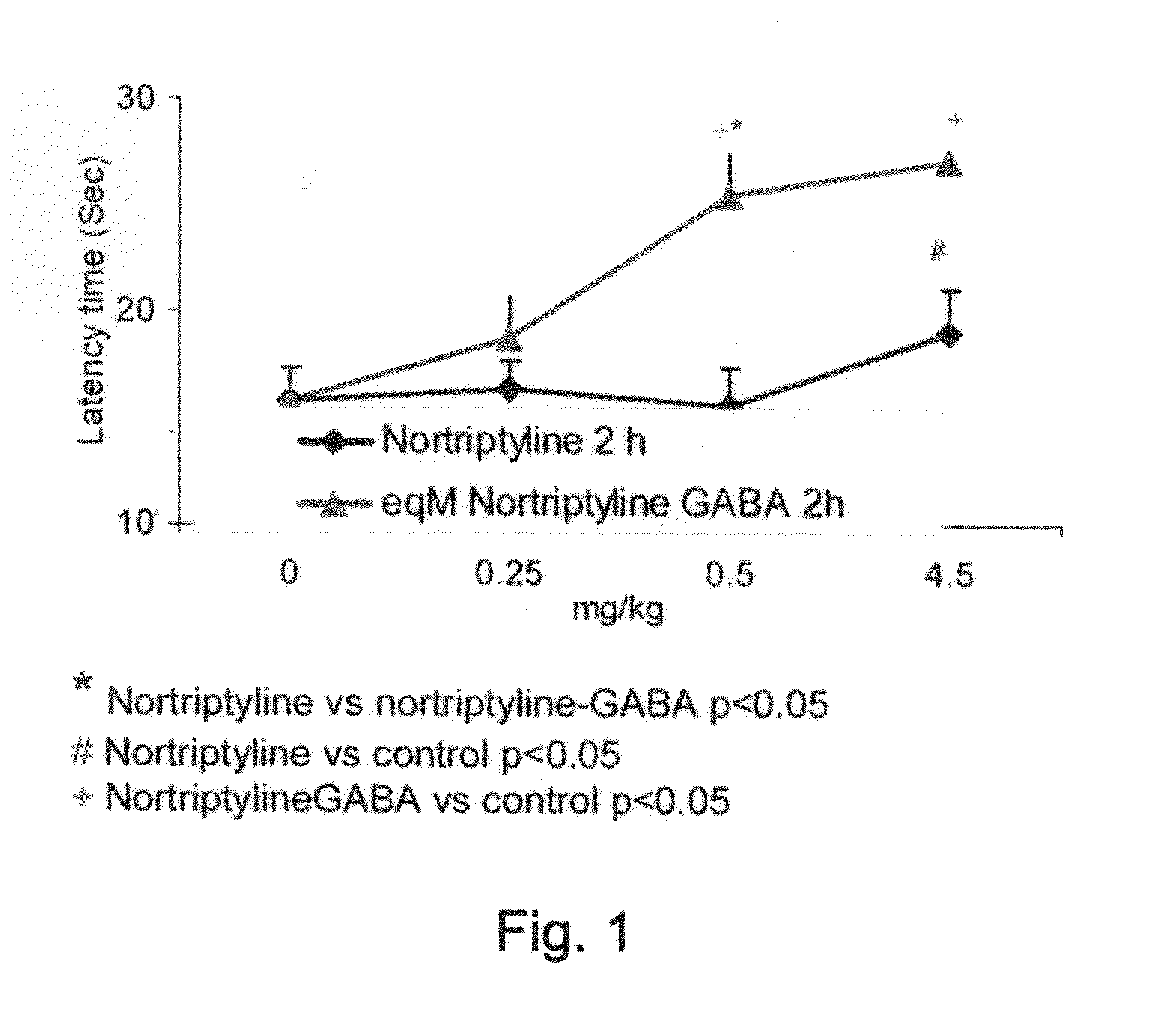
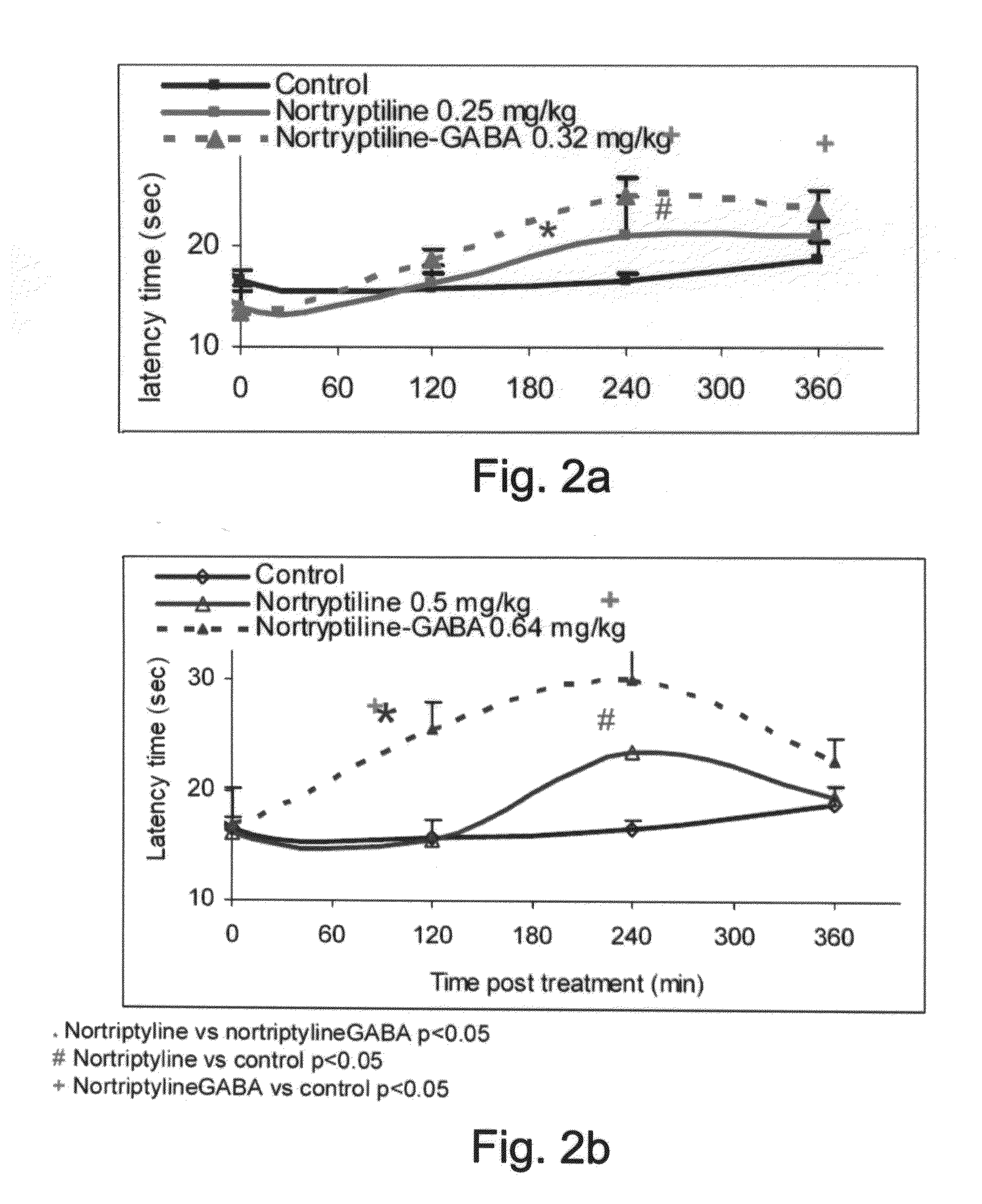
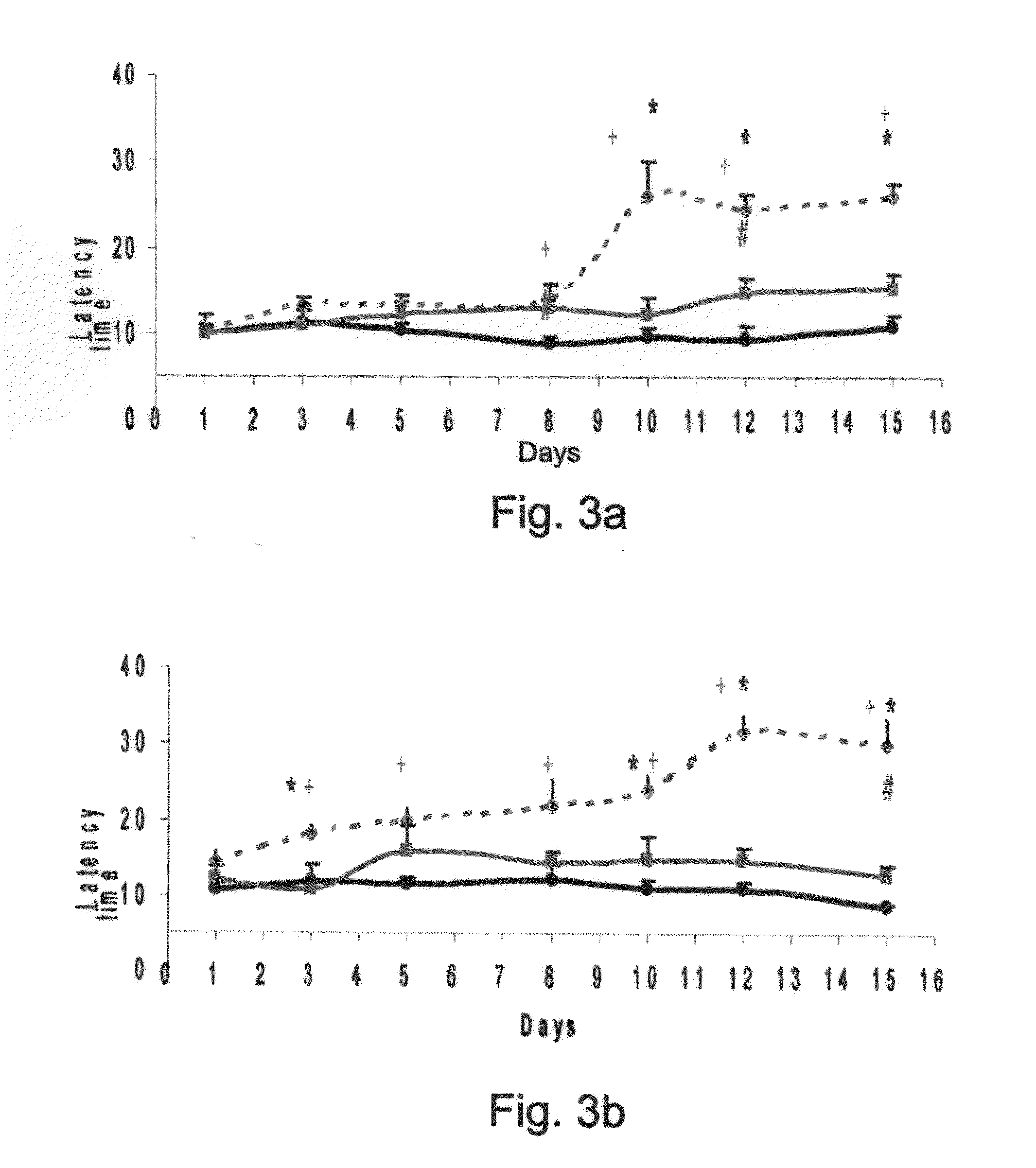
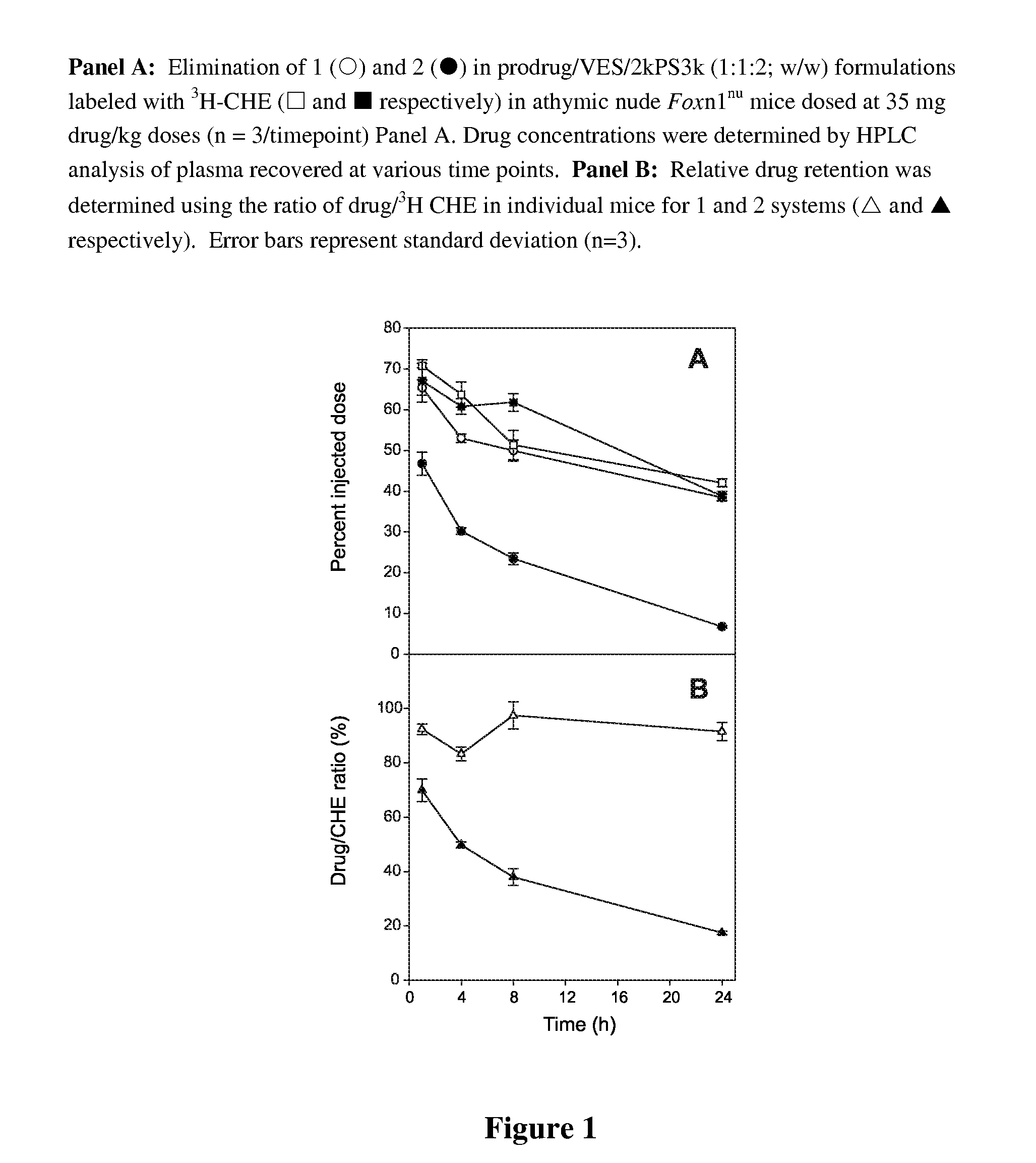
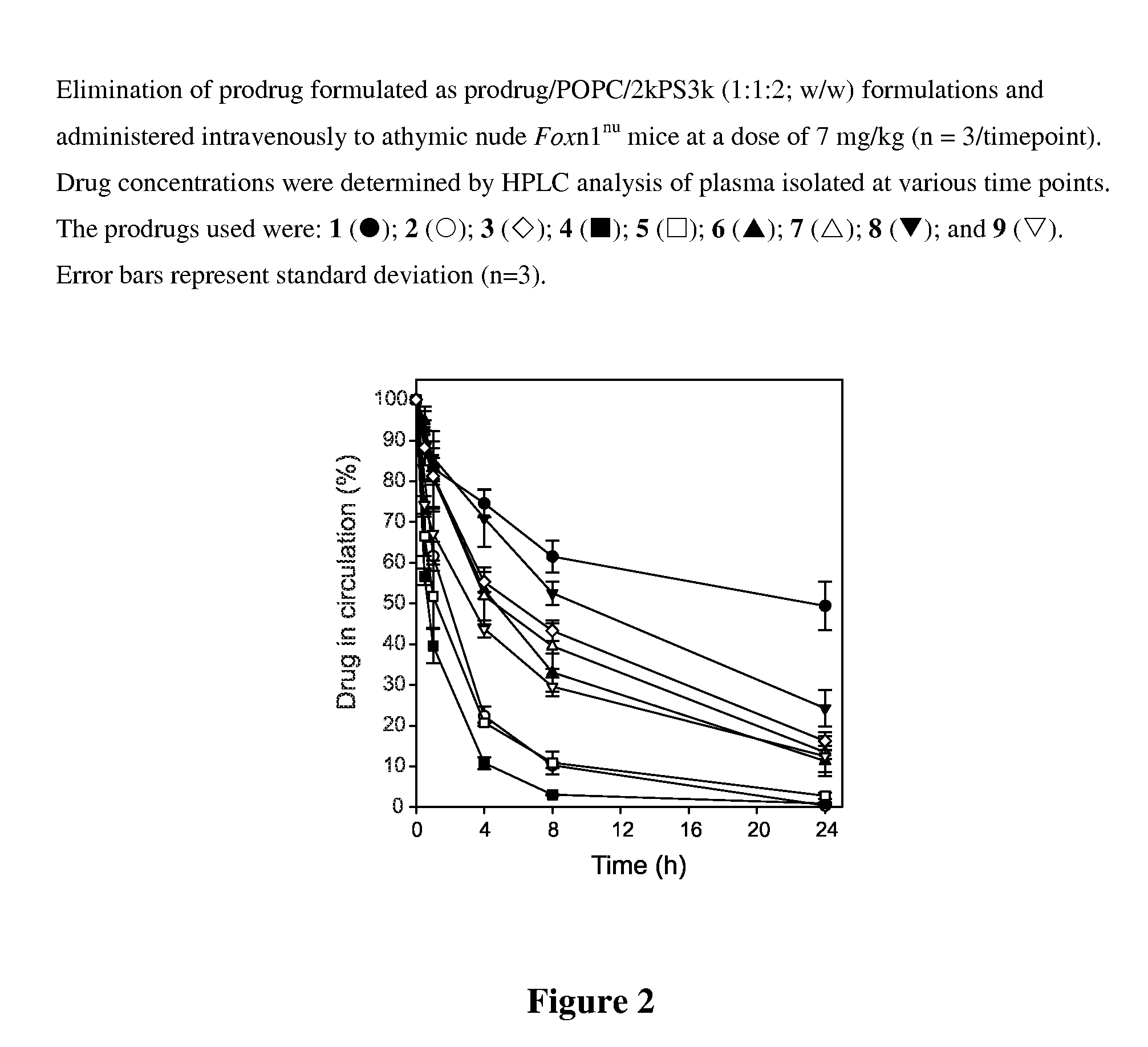
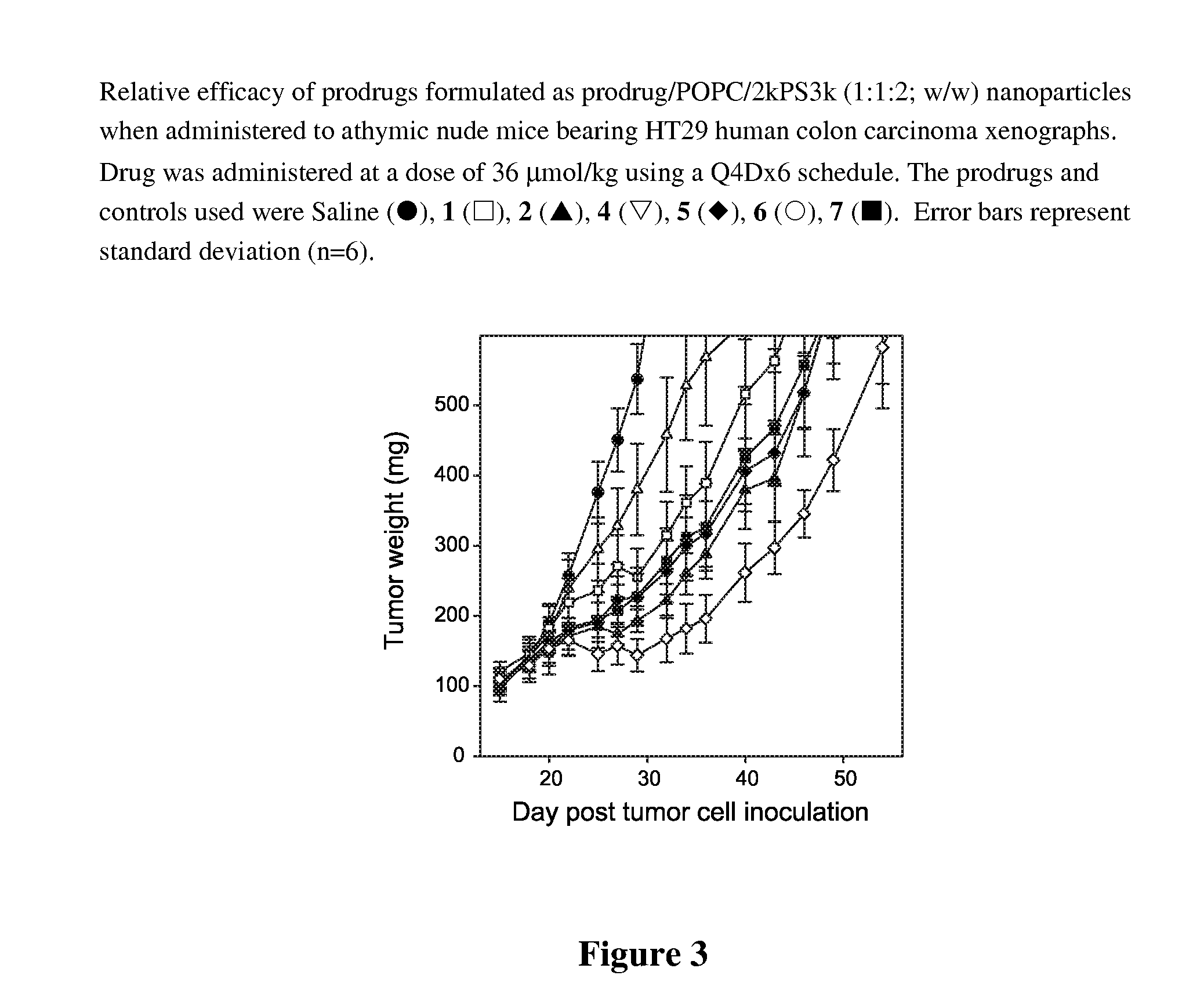
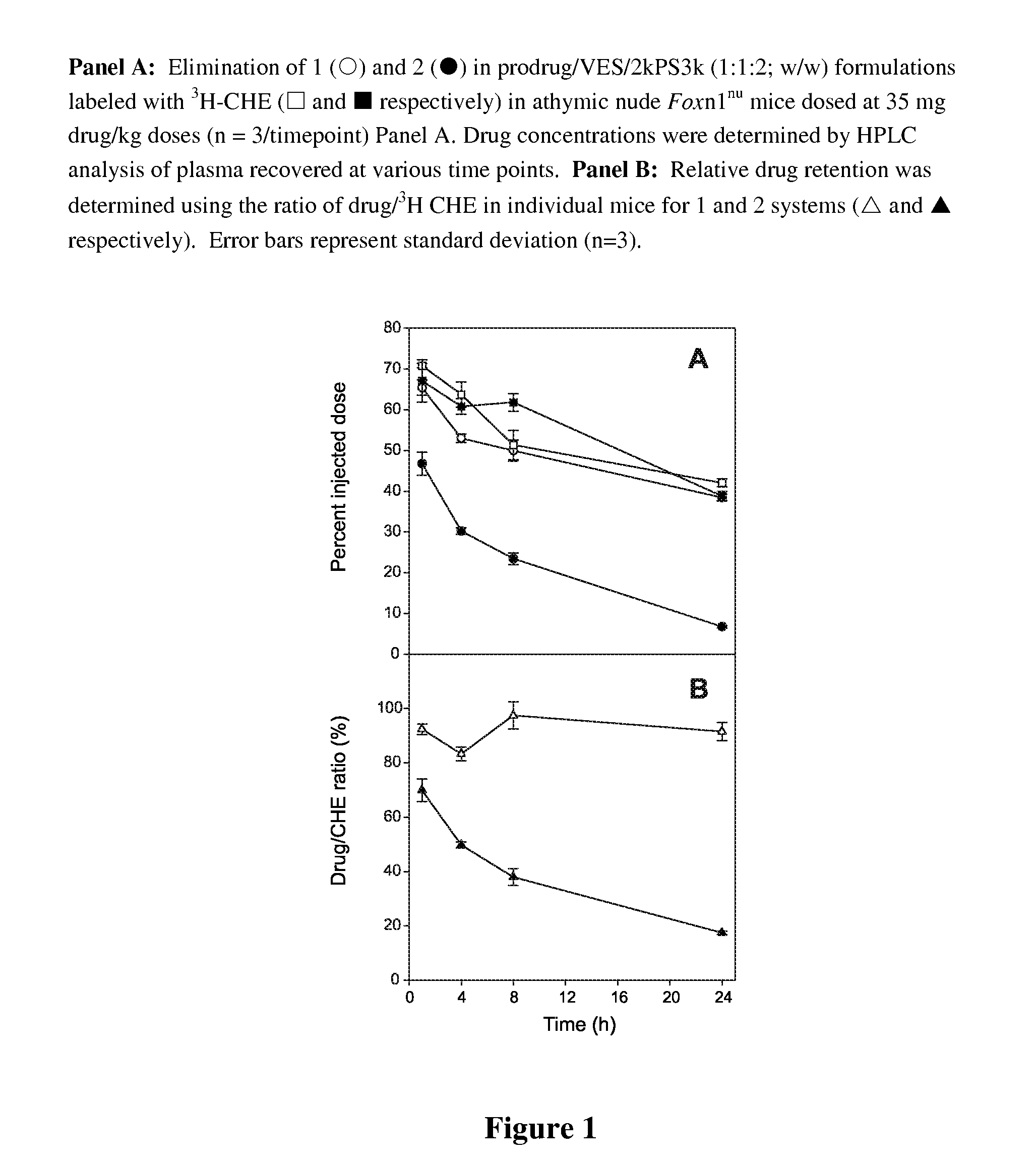
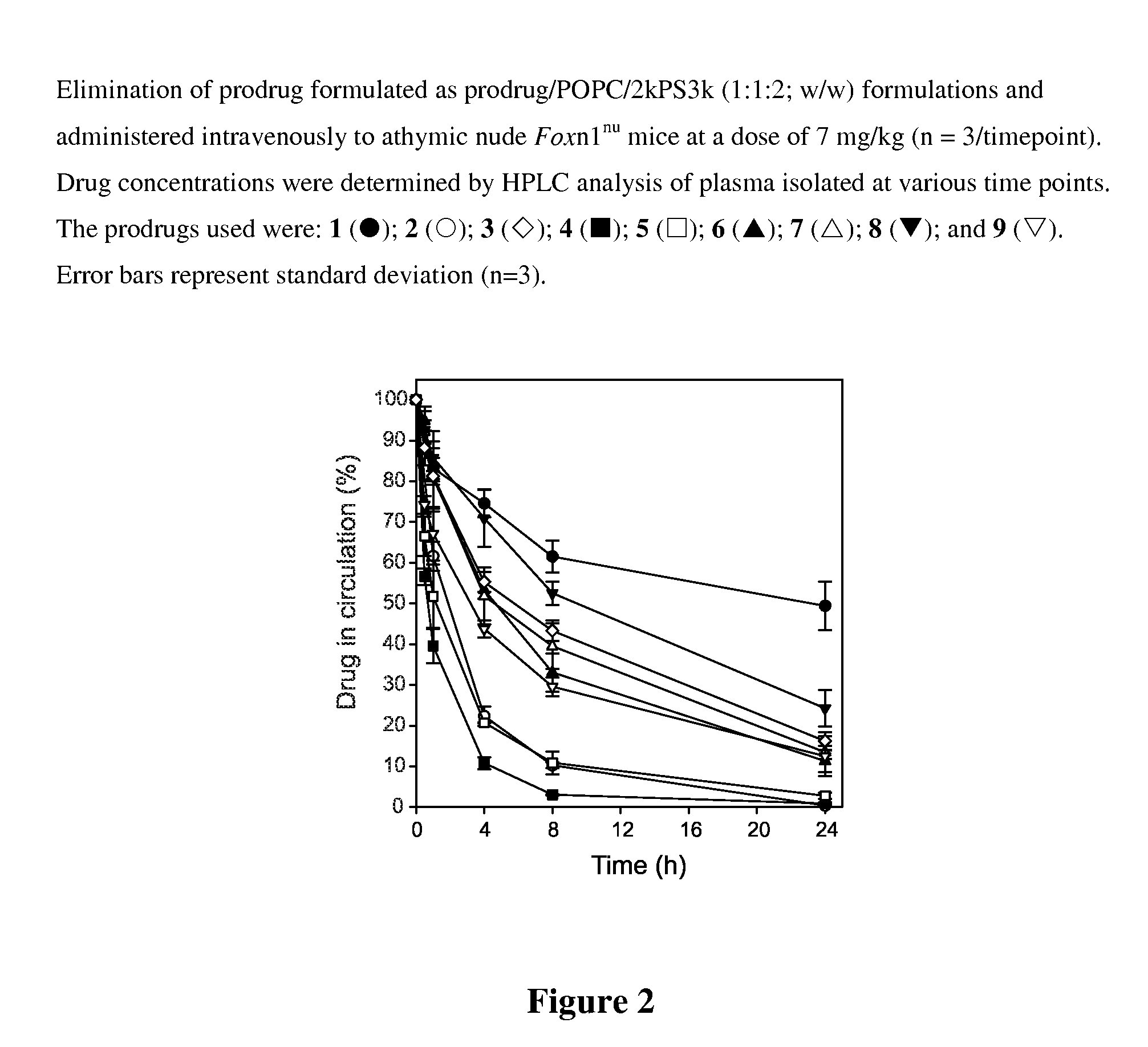
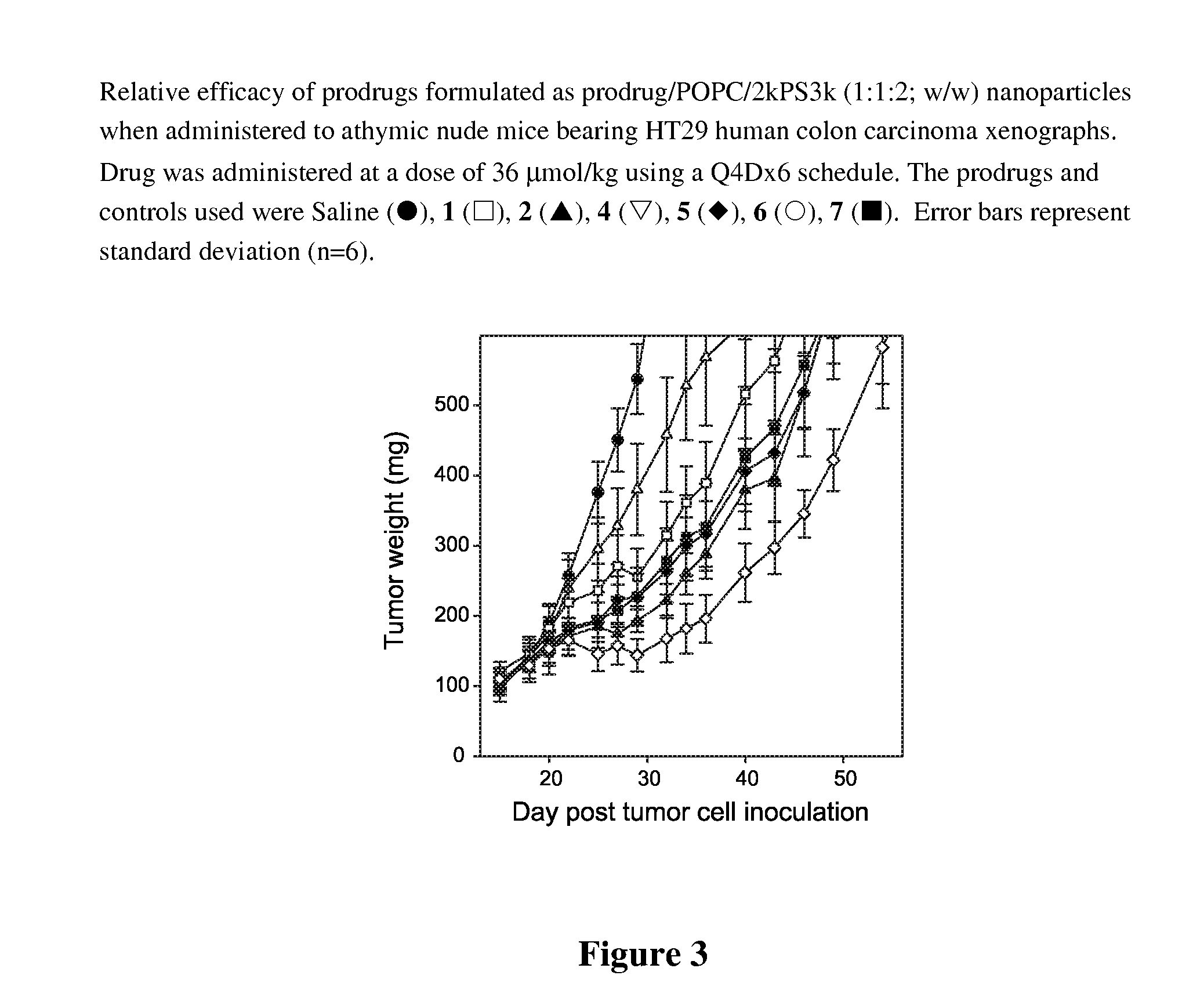
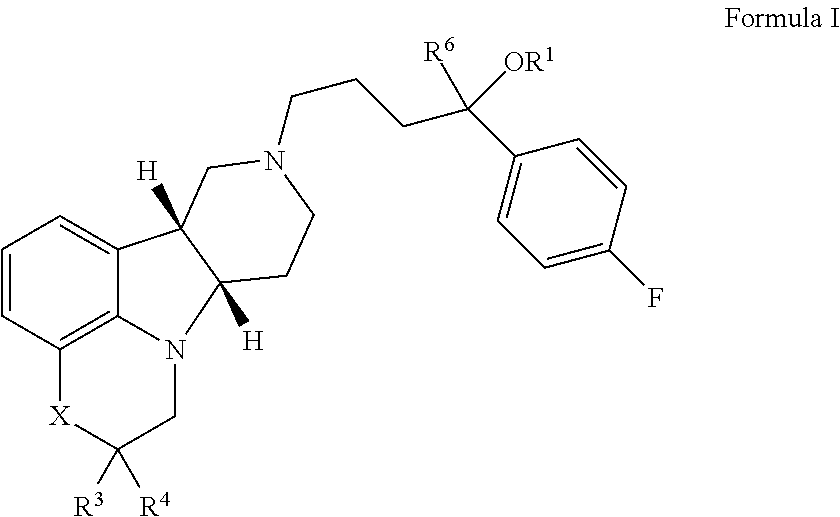
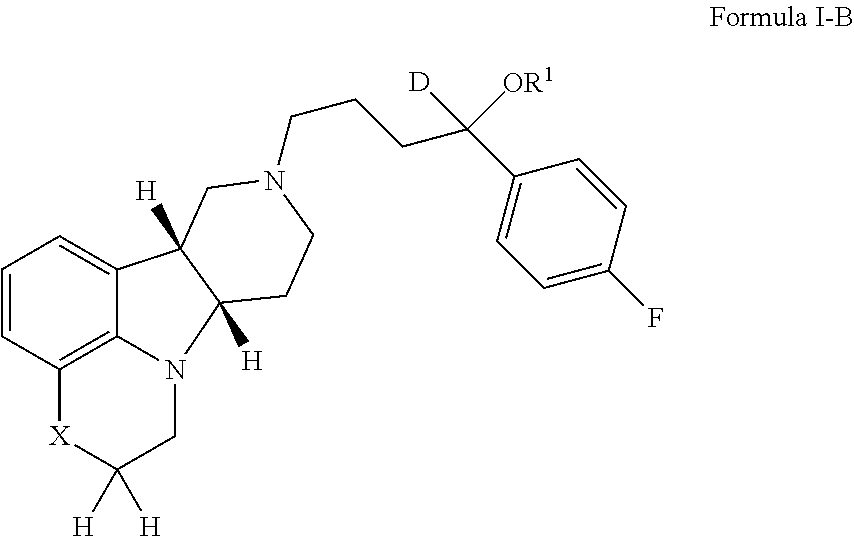
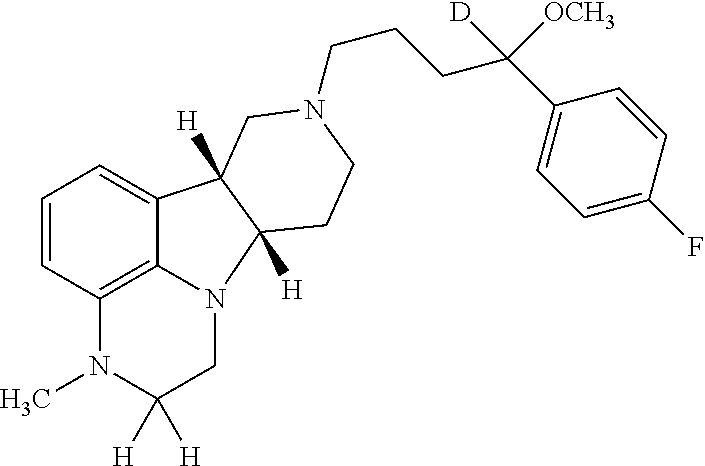
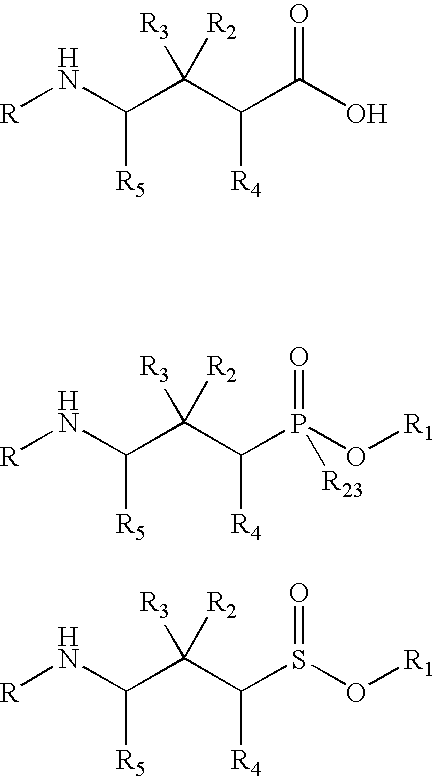
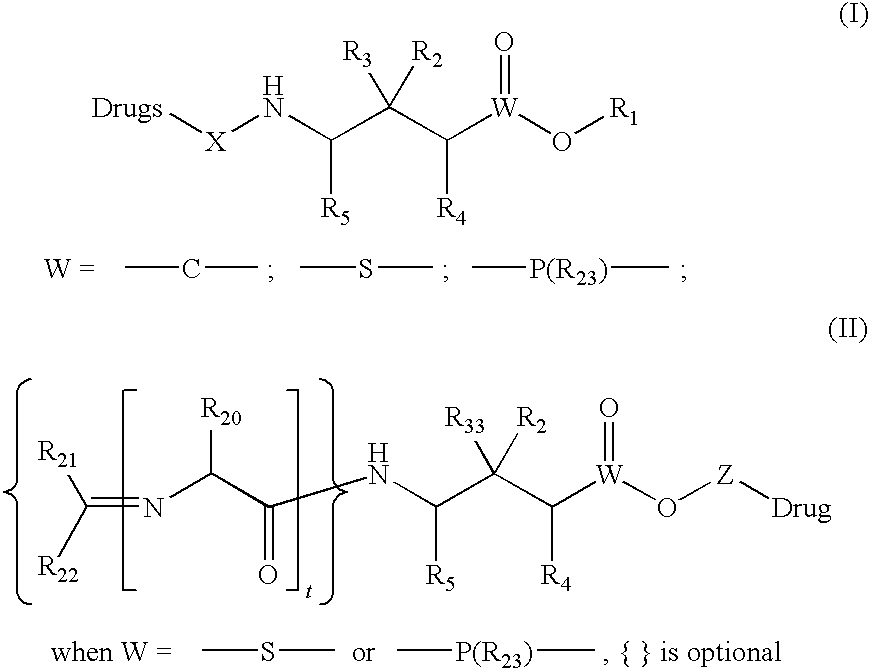
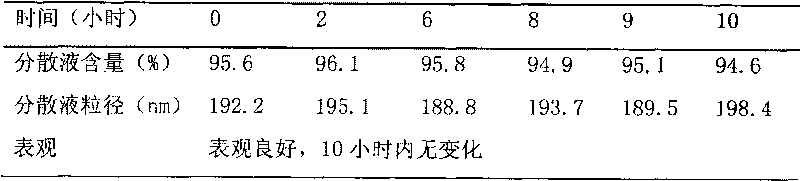
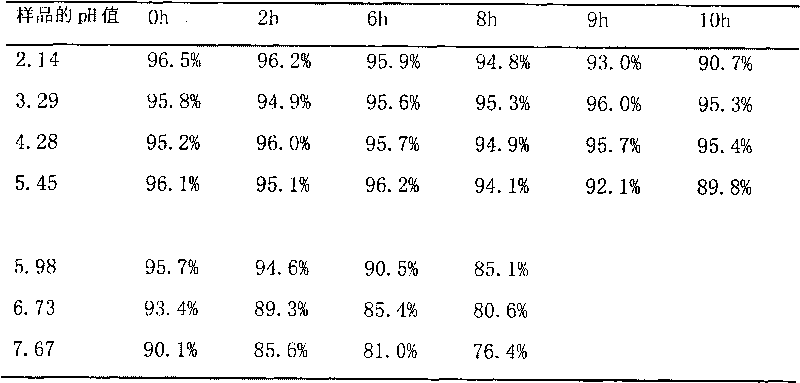
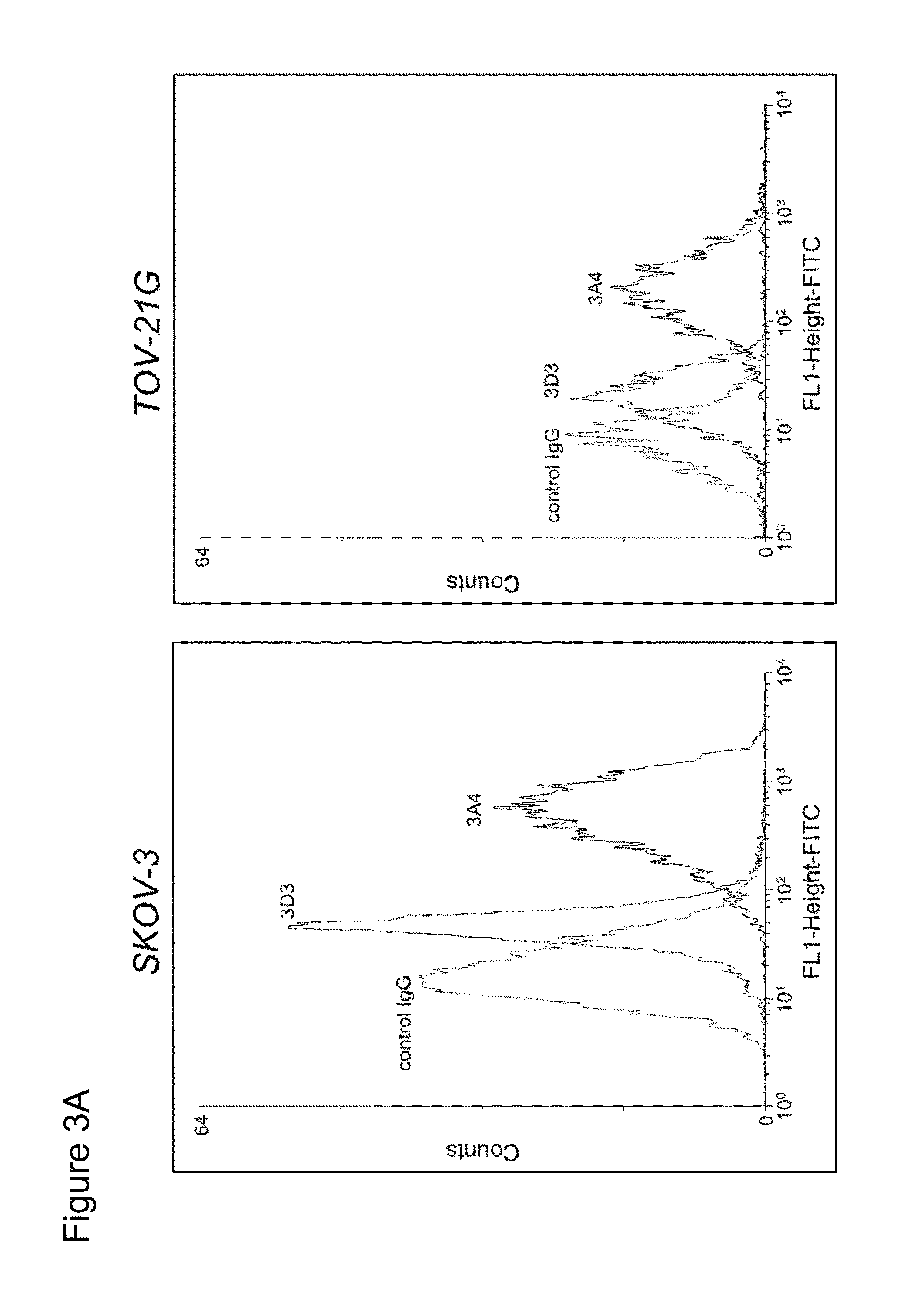
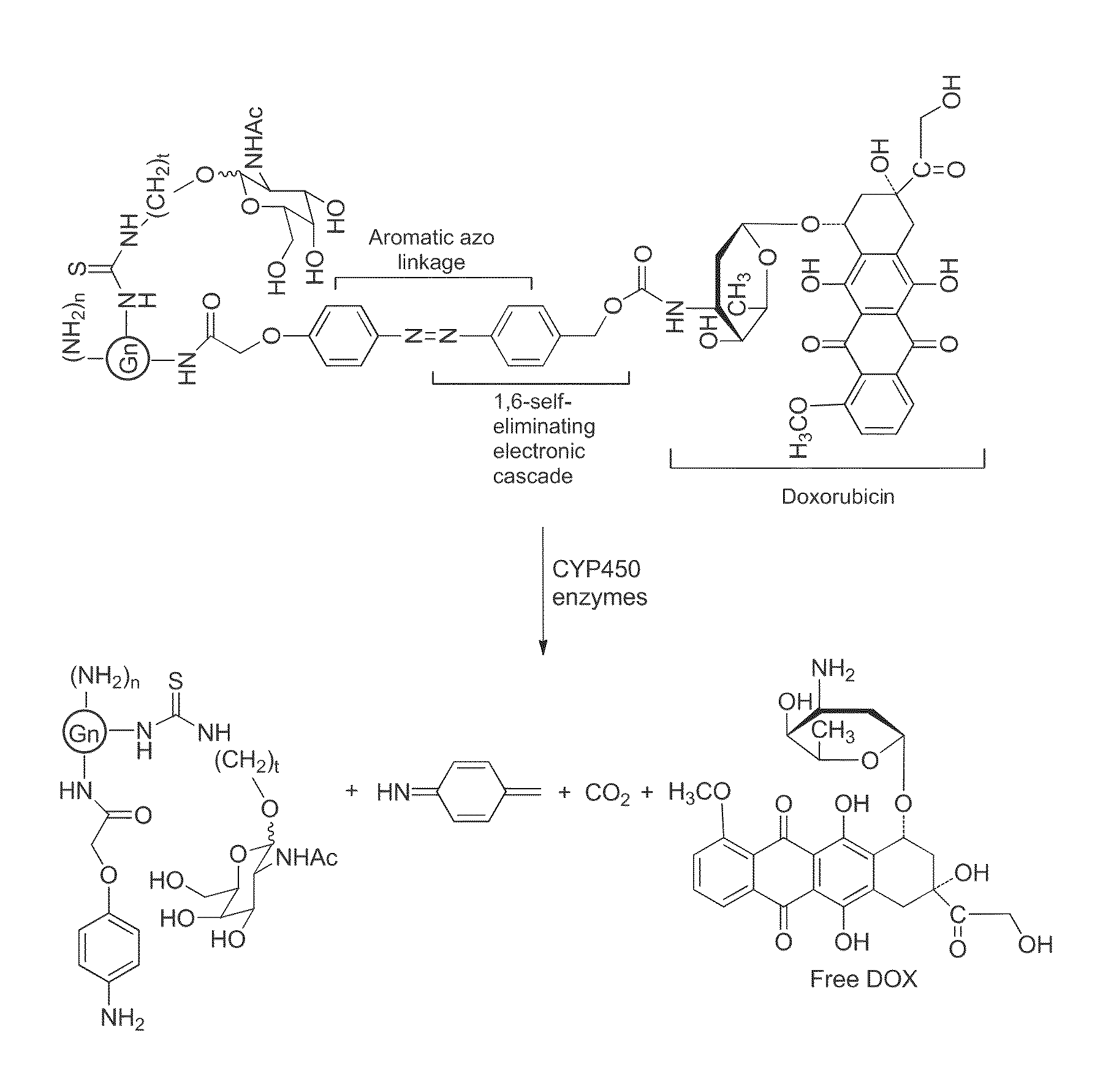
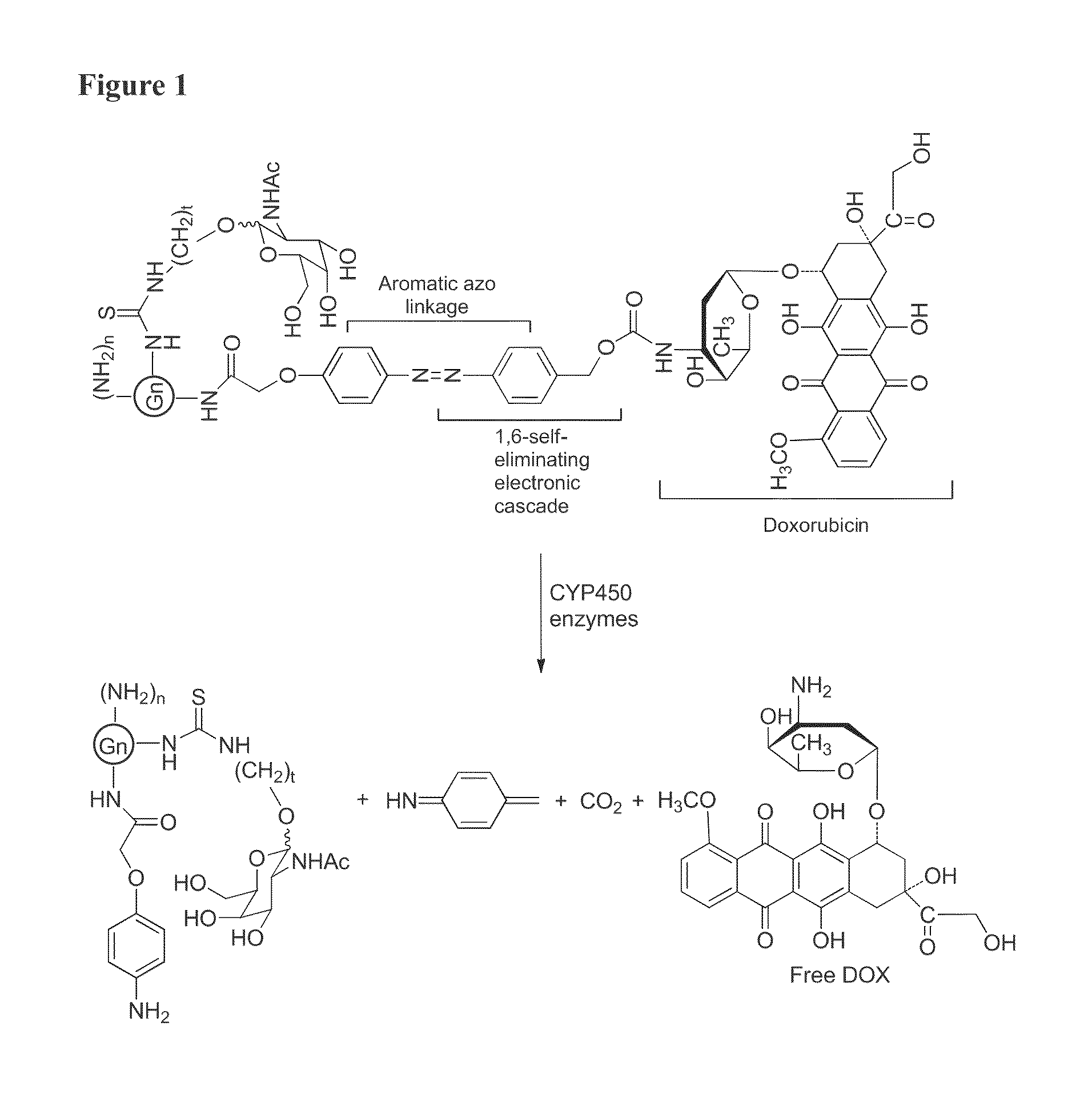
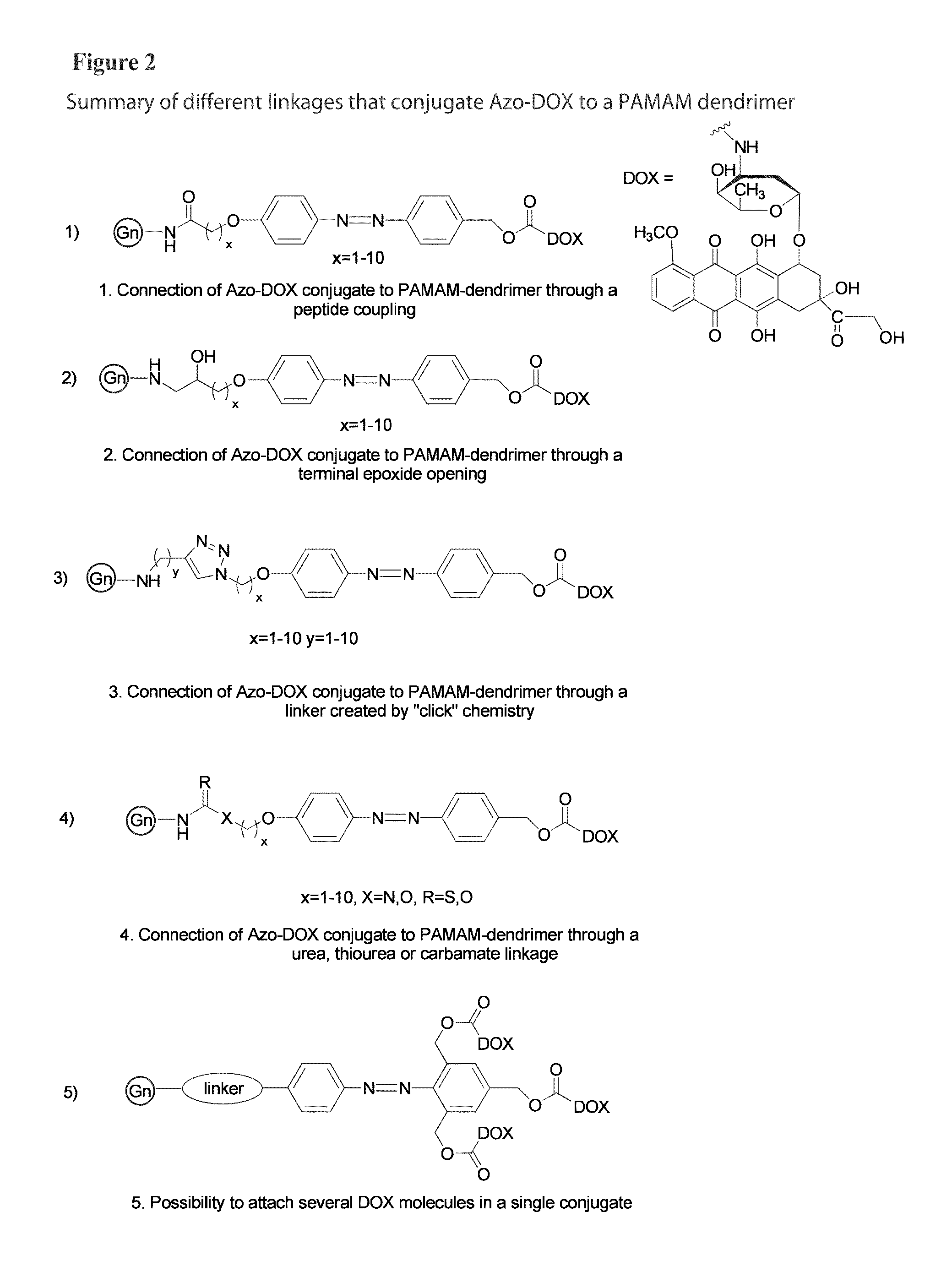
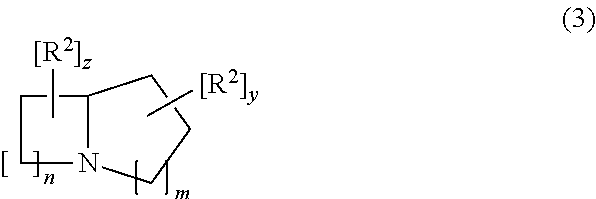Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
111results about How to "Strong therapeutic activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
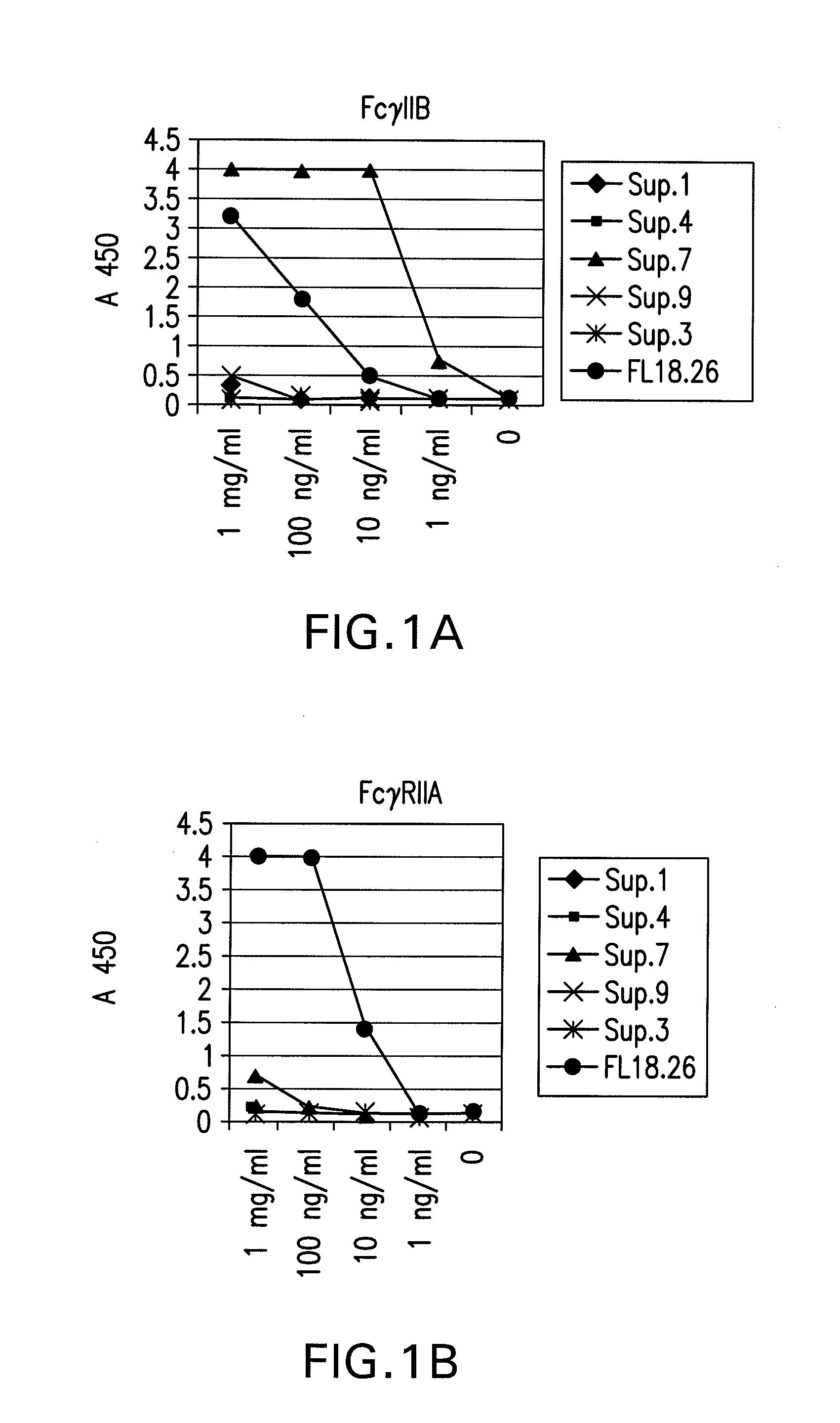
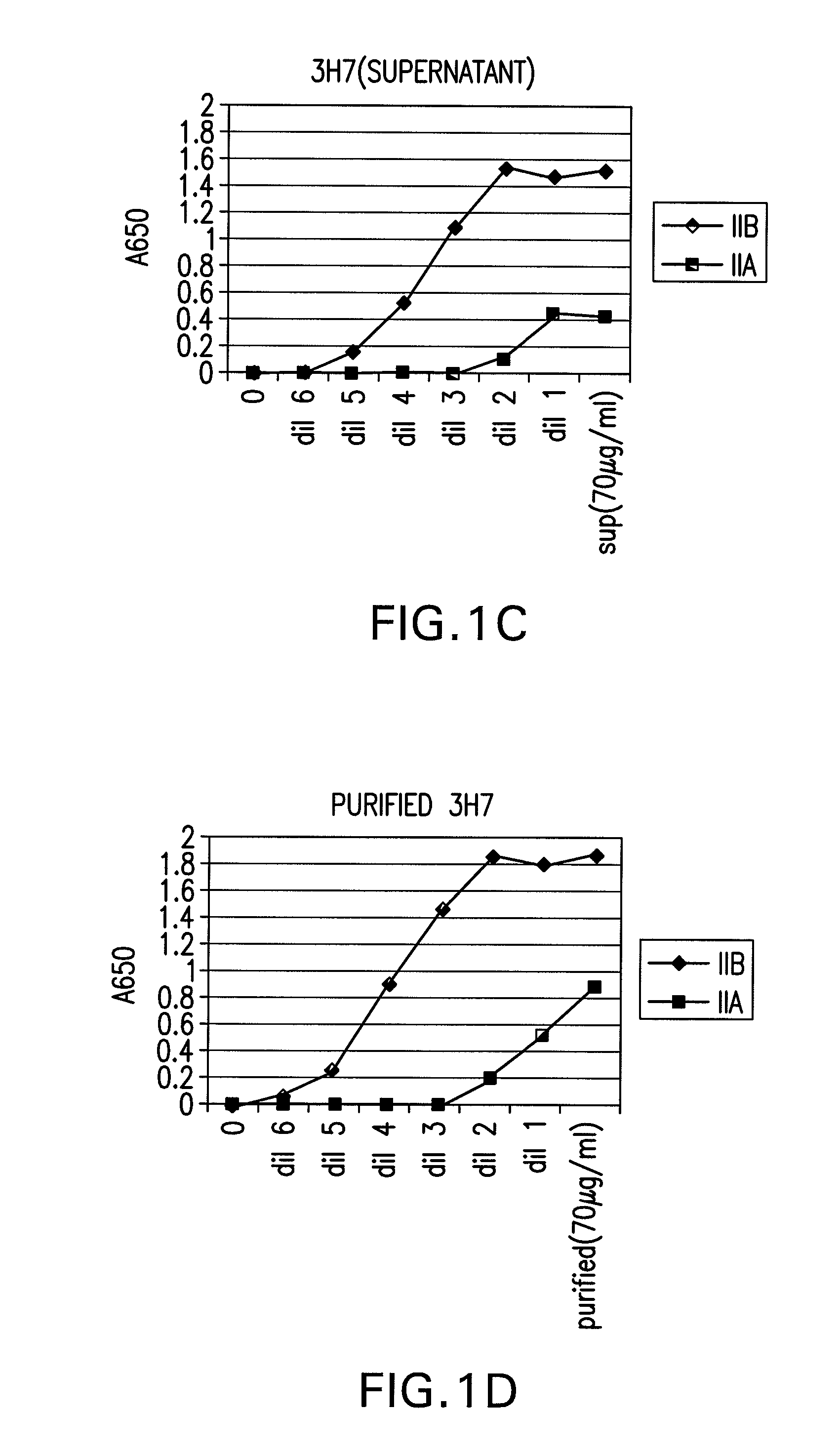
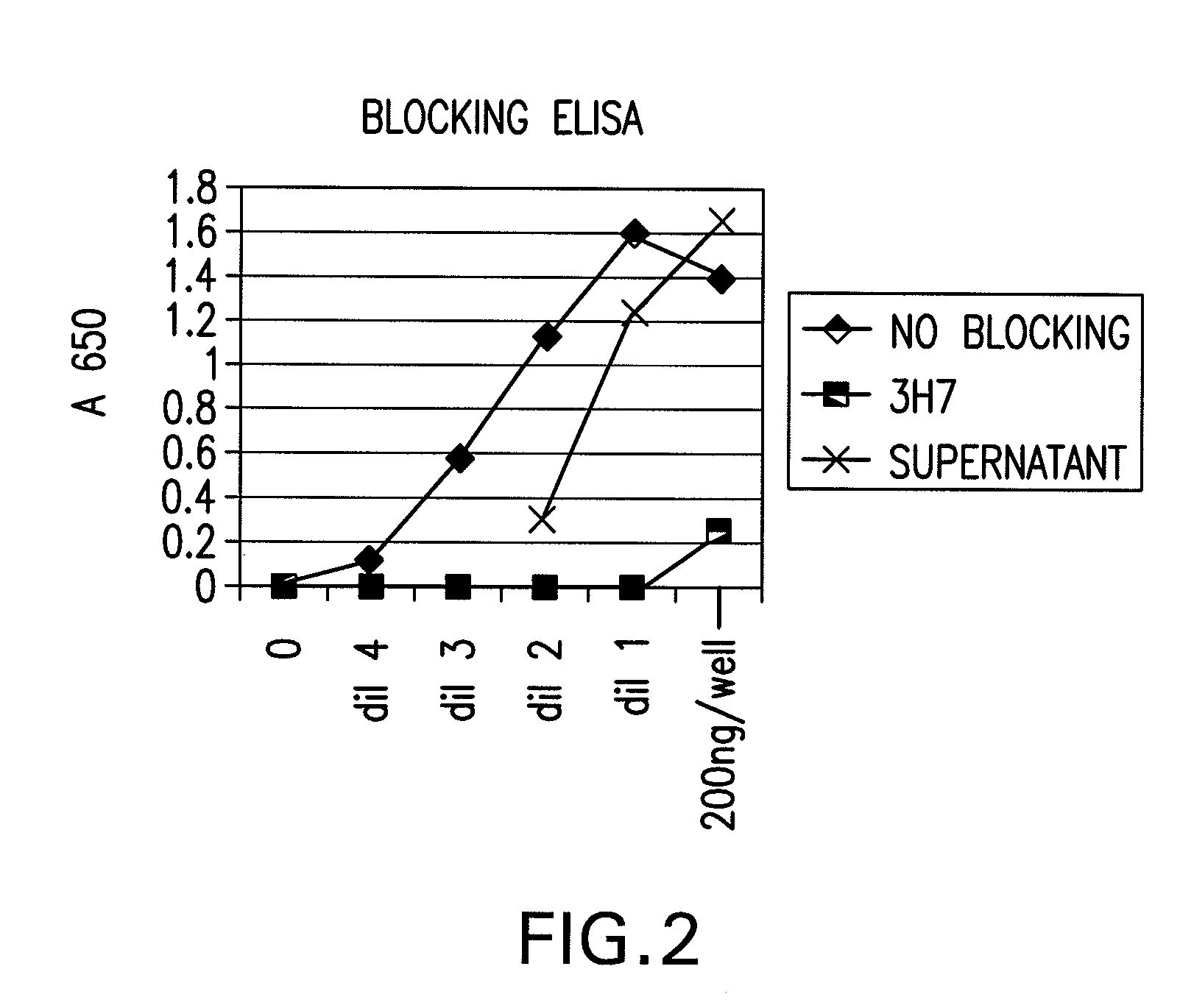
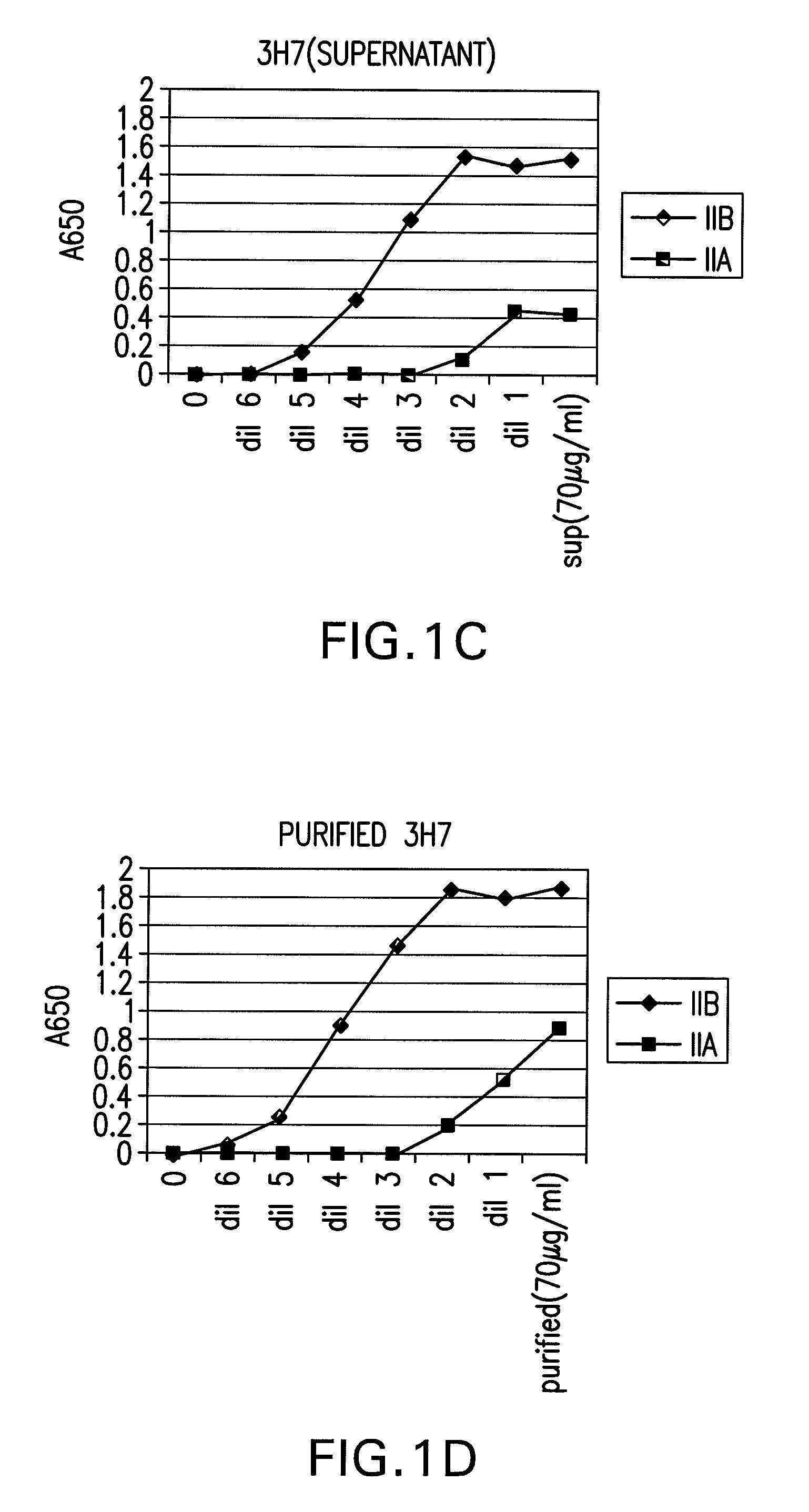
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
Organic compounds
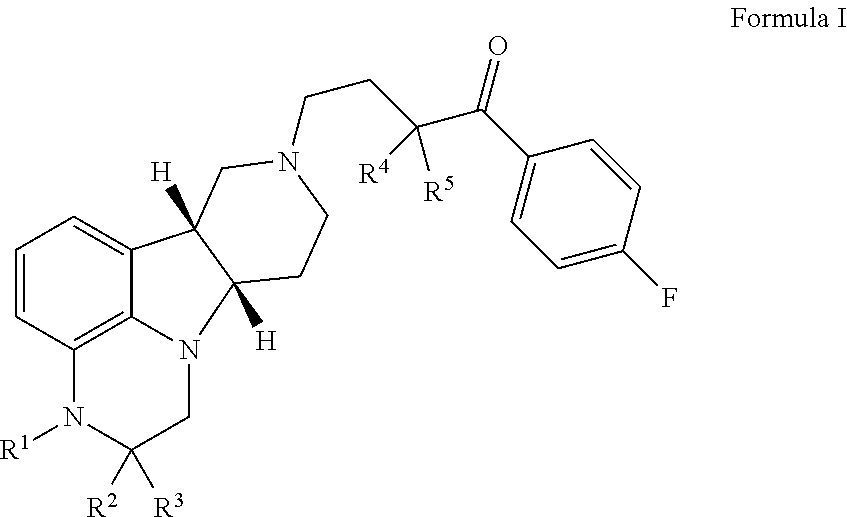
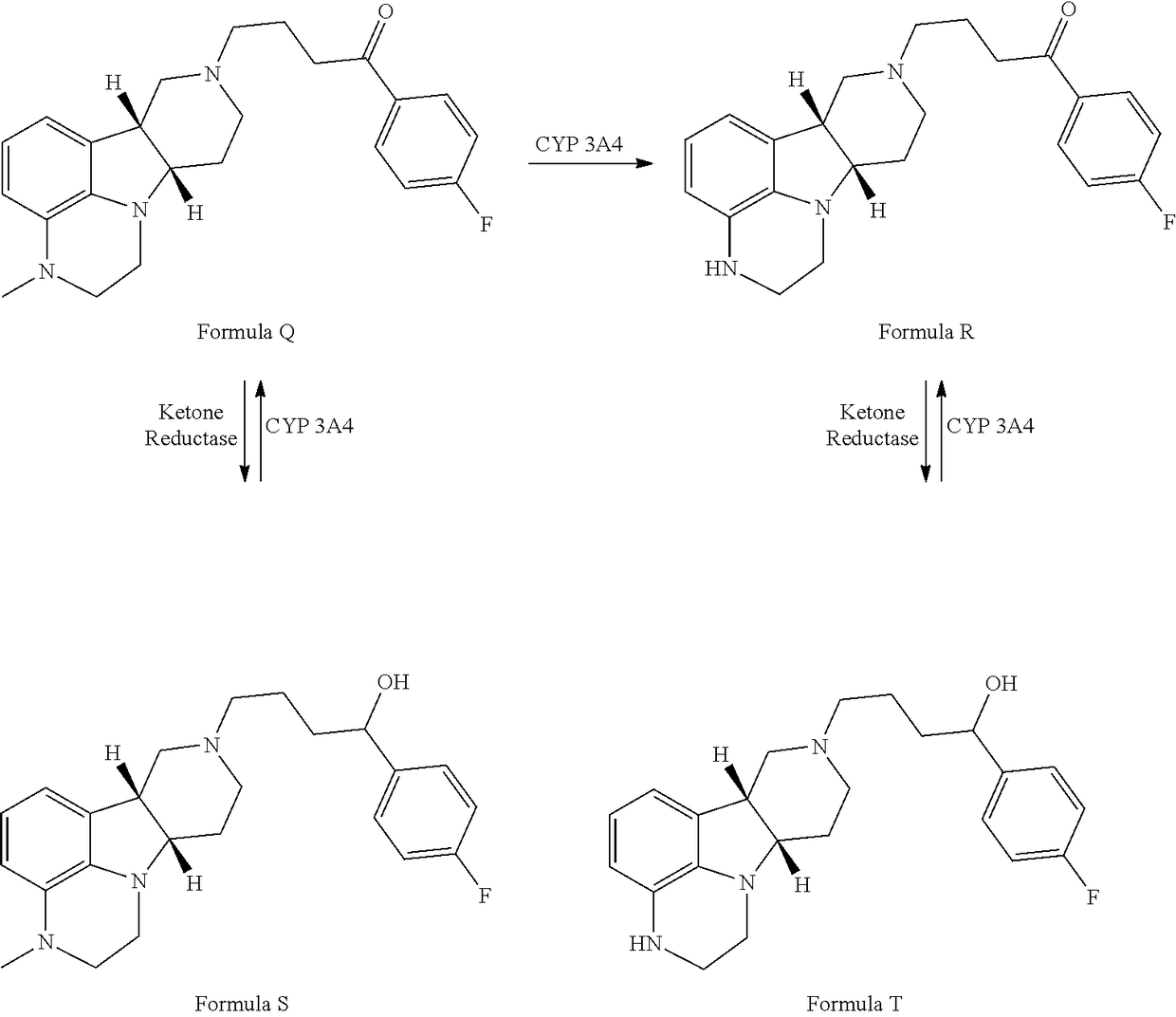
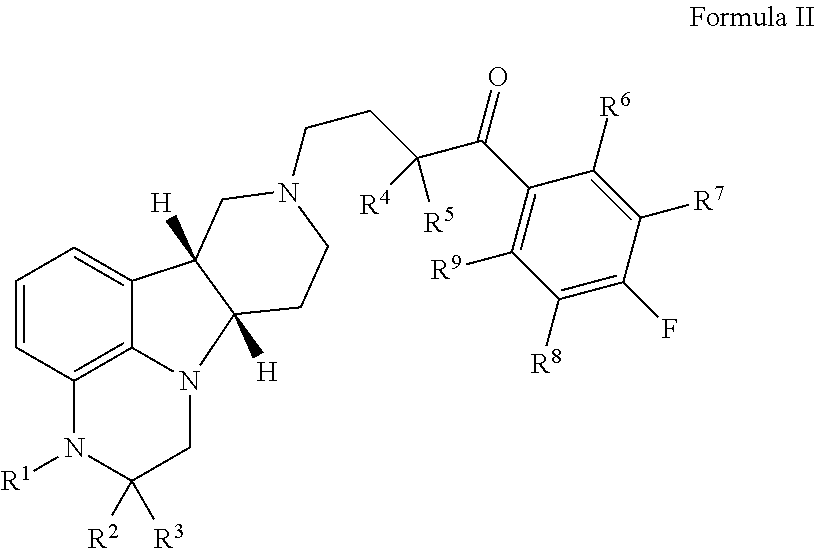
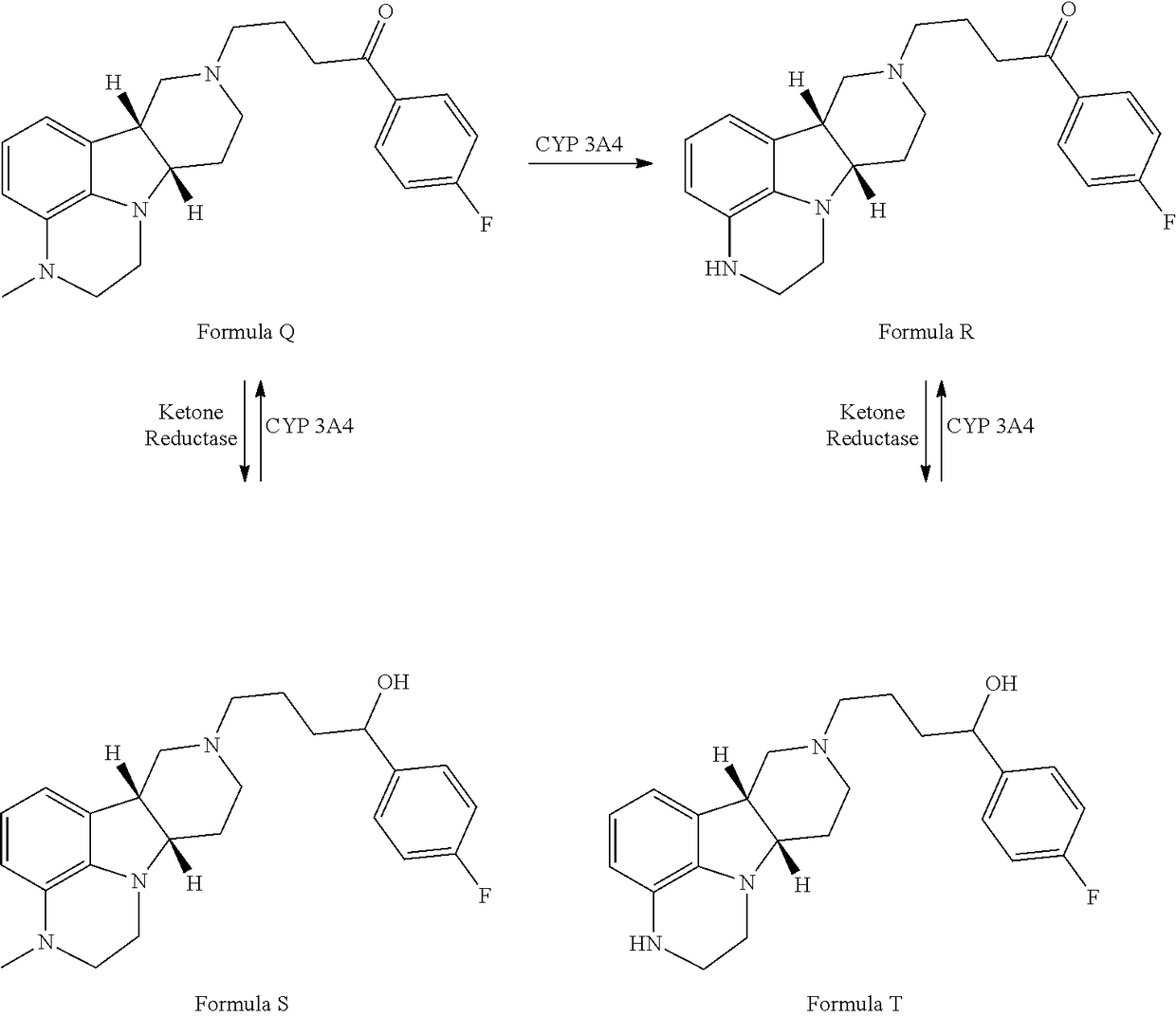
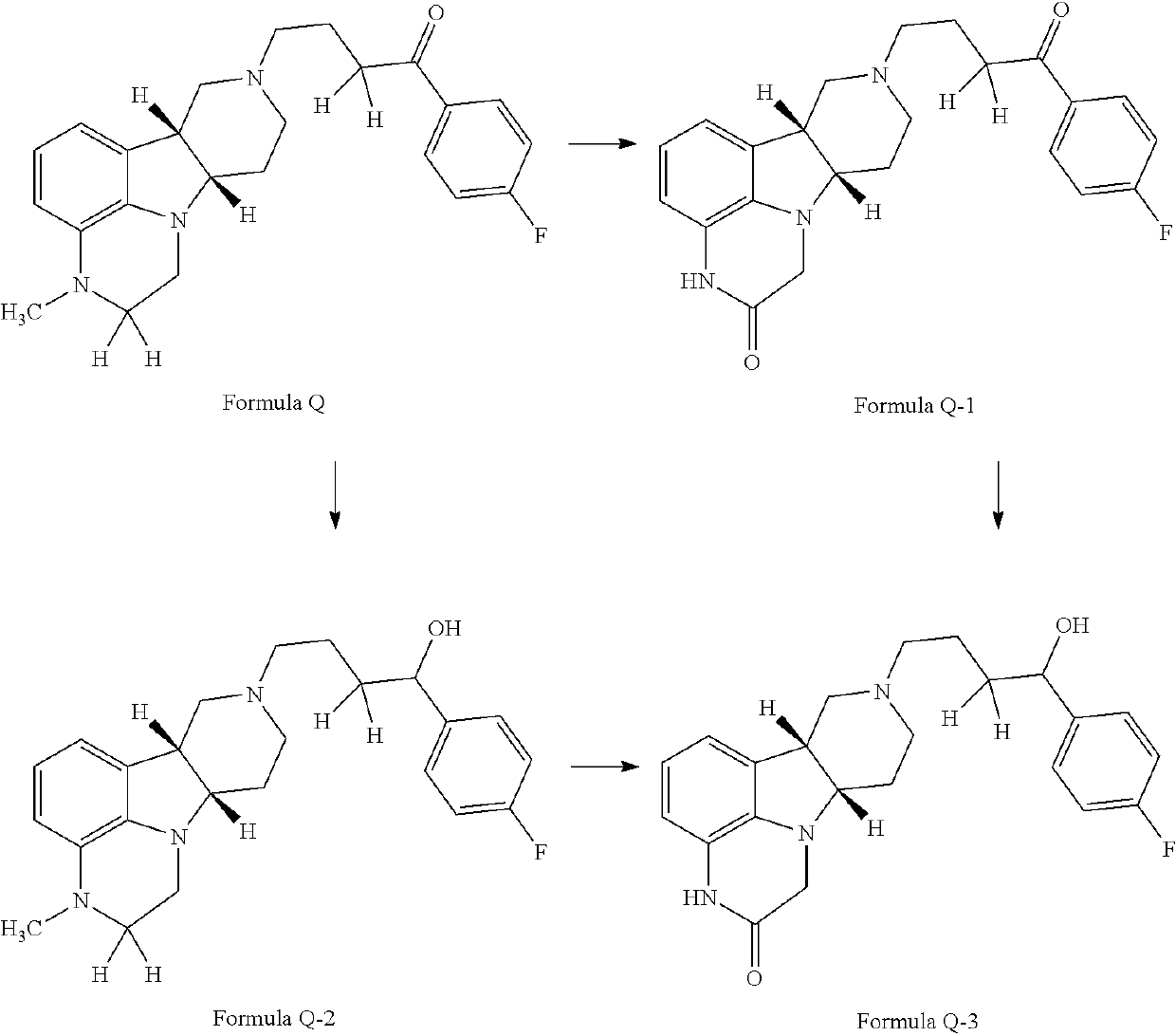
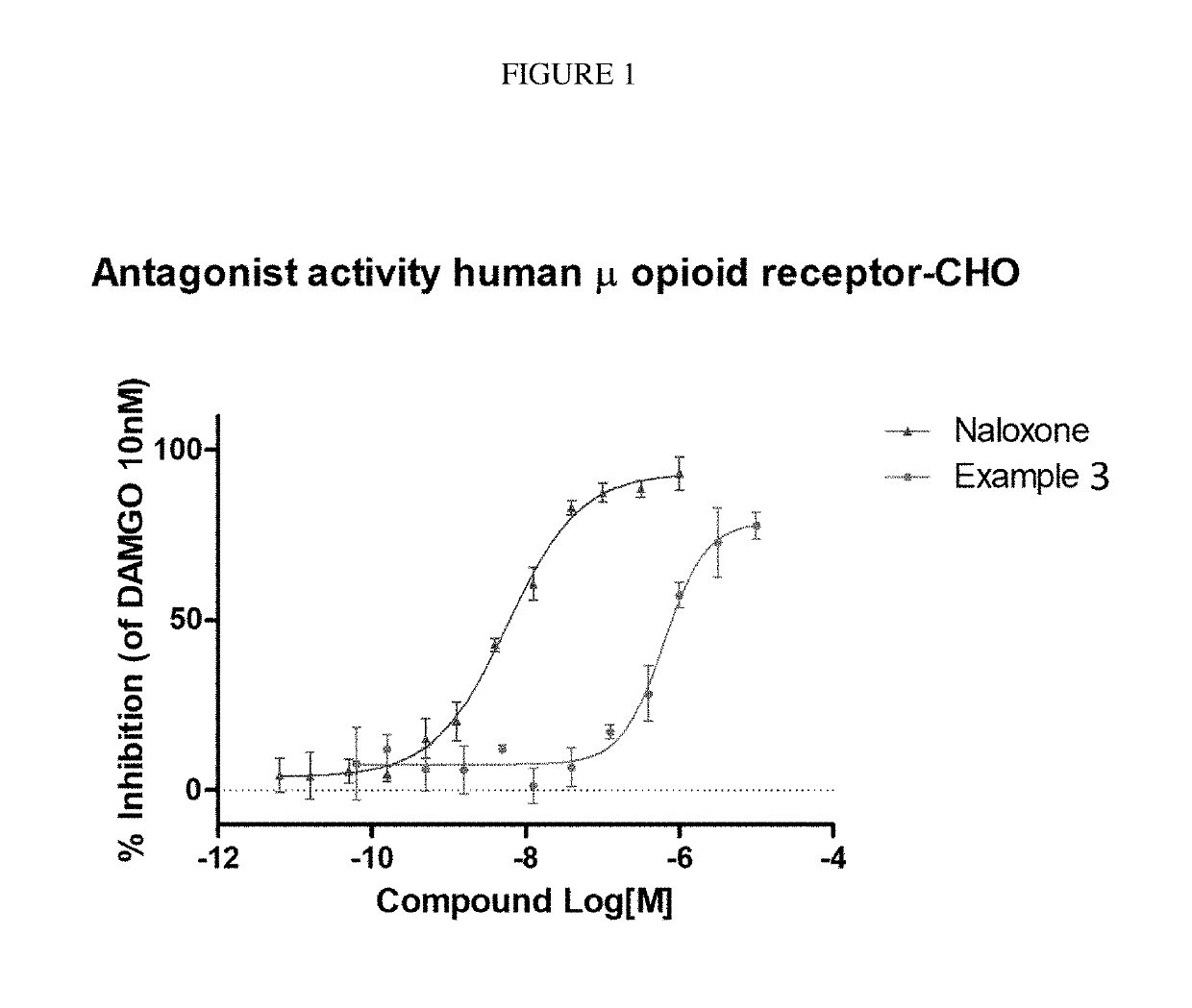
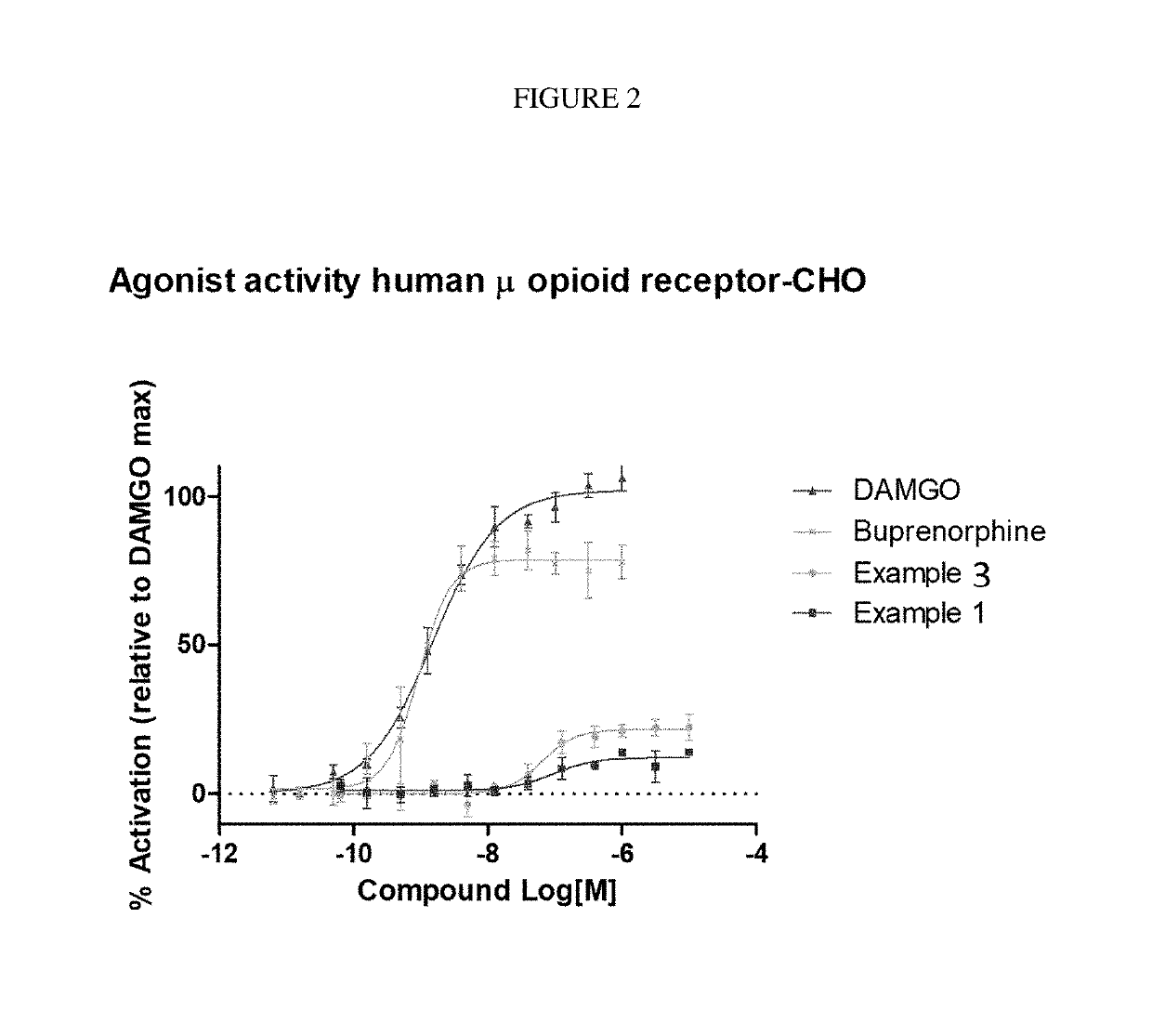
ActiveUS9745300B2Strong therapeutic activitySide effect can be reduced and minimizedOrganic active ingredientsIsotope introduction to heterocyclic compoundsDisease5-HT3 receptor
The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving 5-HT2A receptor, serotonin transporter (SERT) and / or pathways involving dopamine D1 / D2 receptor signaling systems, and / or the treatment of residual symptoms.
Owner:INTRA CELLULAR THERAPIES INC
Organic compounds
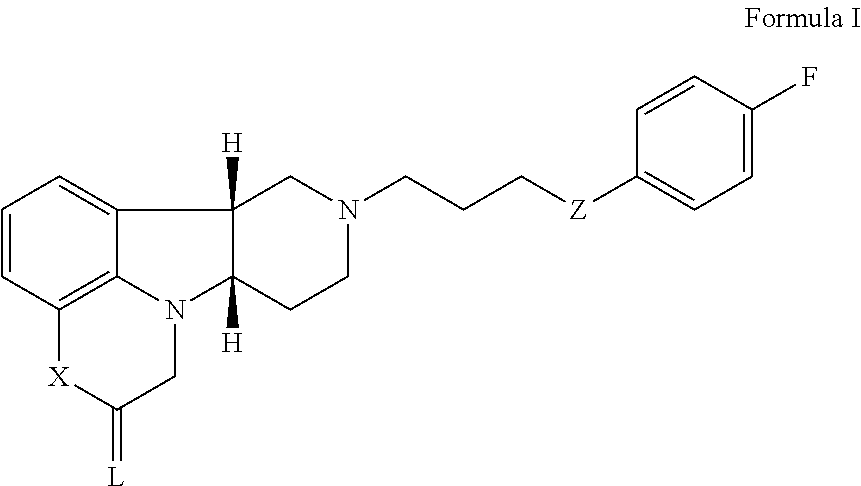
ActiveUS20170319580A1Avoid side effectsConvenient treatmentOrganic active ingredientsNervous disorder5-HT7 receptorOrganic compound
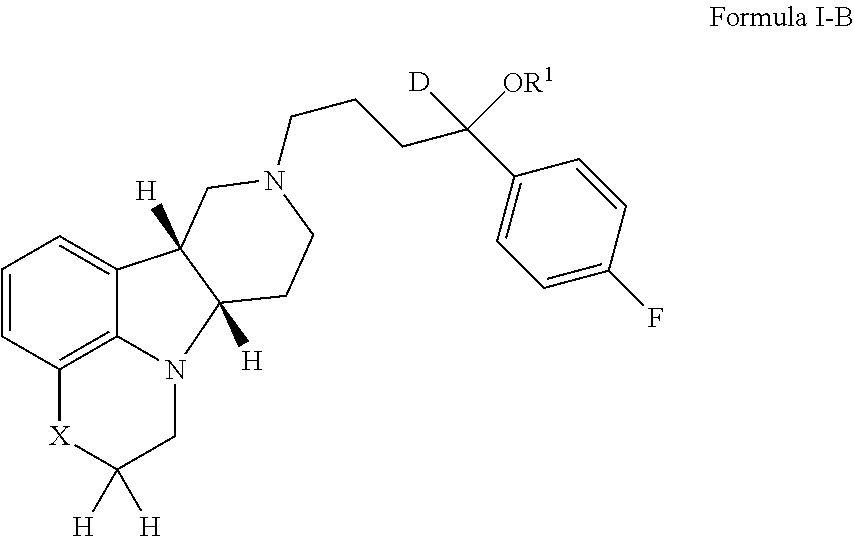
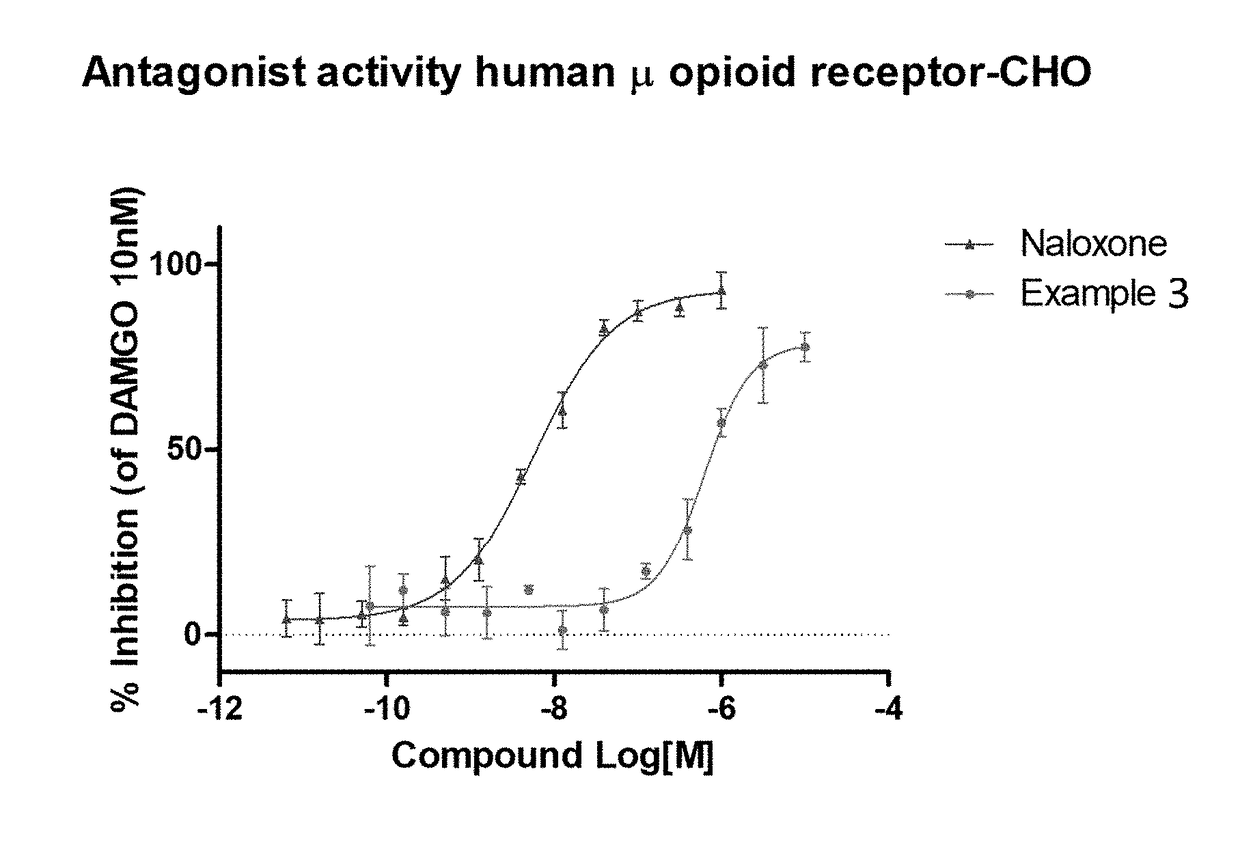
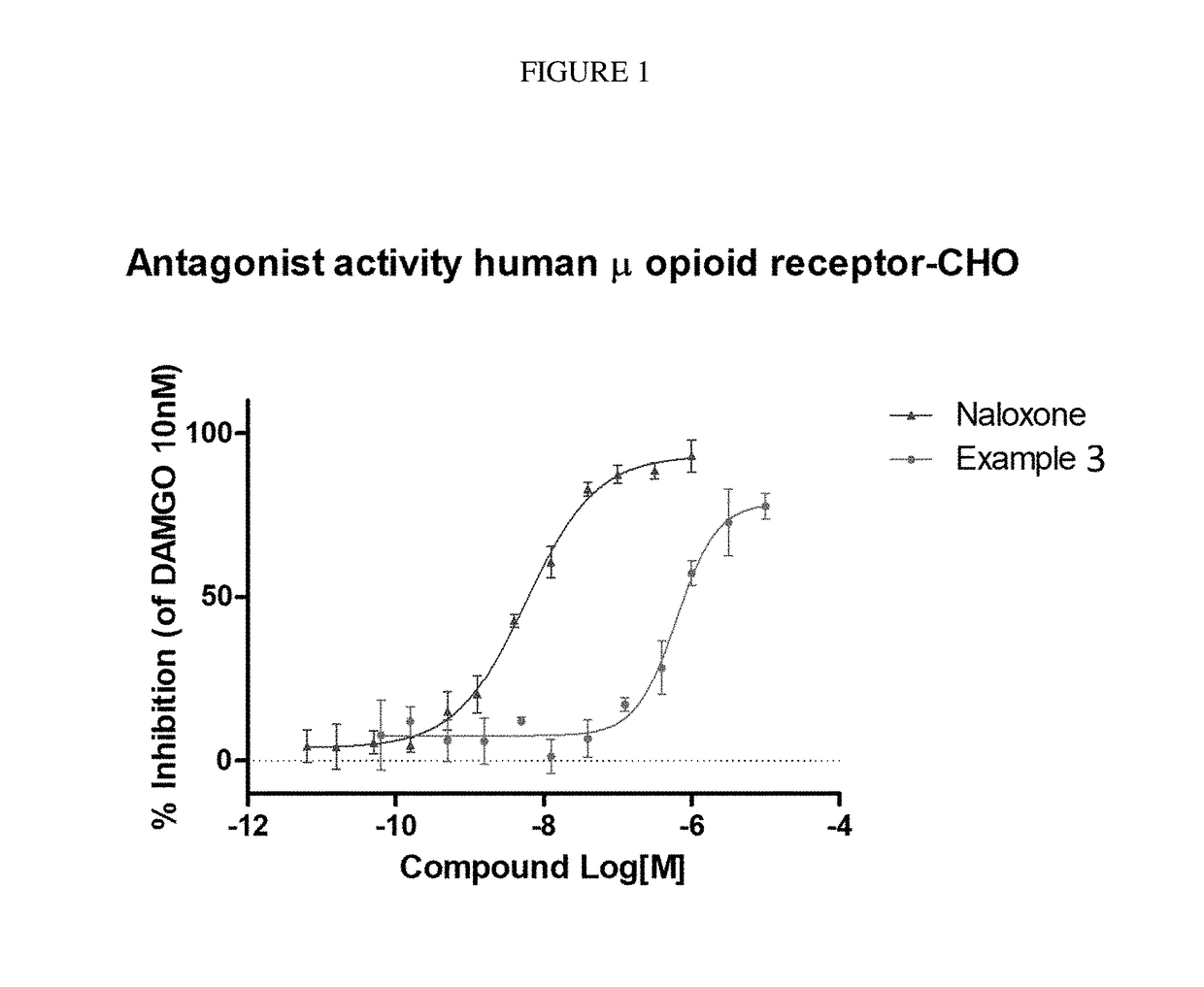
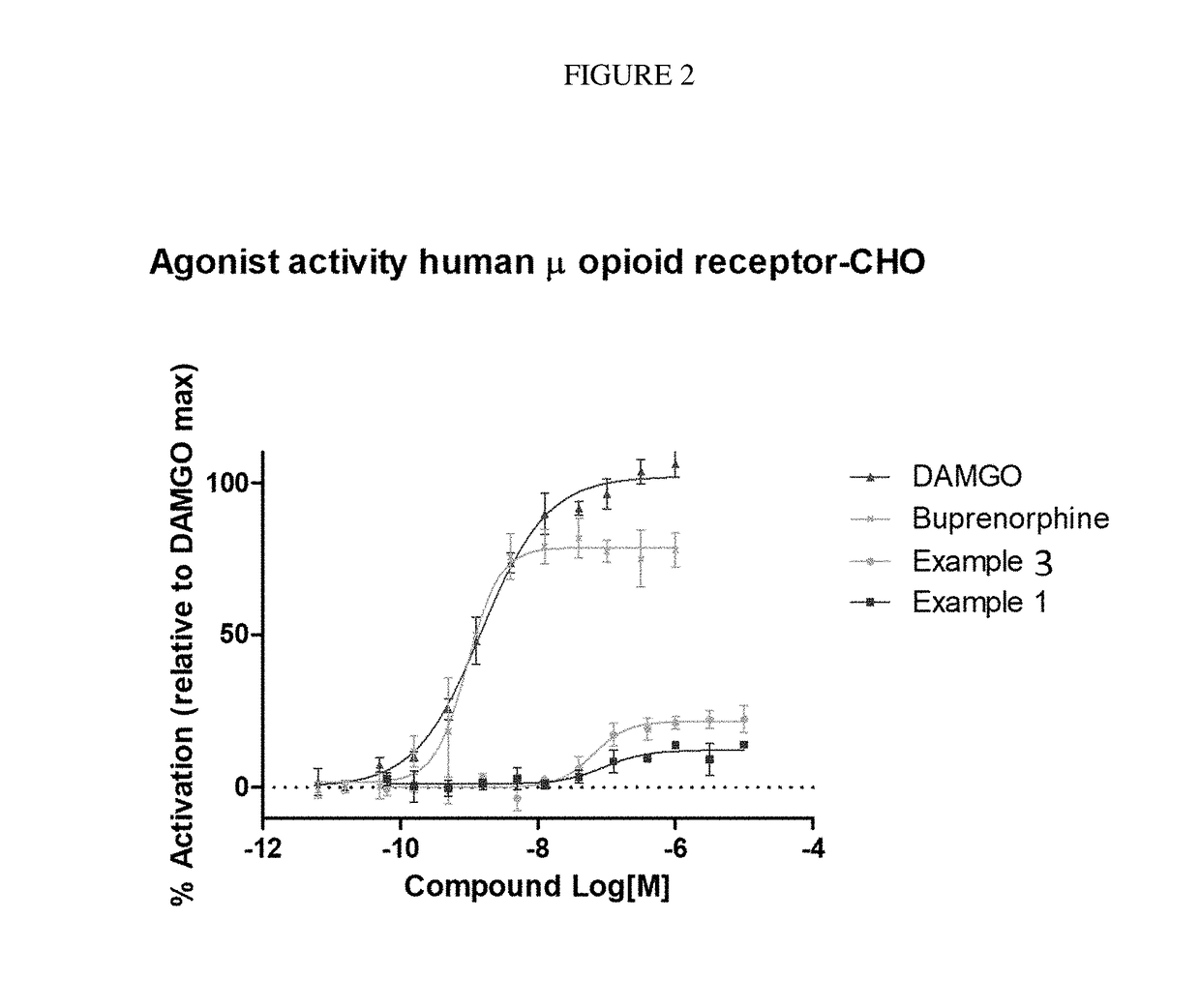
The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving the 5-HT2A receptor, the serotonin transporter (SERT), pathways involving the dopamine D1 and D2 receptor signaling system, and / or the μ-opioid receptor.
Owner:INTRA CELLULAR THERAPIES INC
Organic compounds
ActiveUS20170183350A1To promote metabolismImproves in vitro hepatic microsome stabilityOrganic active ingredientsNervous disorderDisease5-HT3 receptor
The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving 5-HT2A receptor, serotonin transporter (SERT) and / or pathways involving dopamine D1 / D2 receptor signaling systems, and / or the treatment of residual symptoms.
Owner:INTRA CELLULAR THERAPIES INC
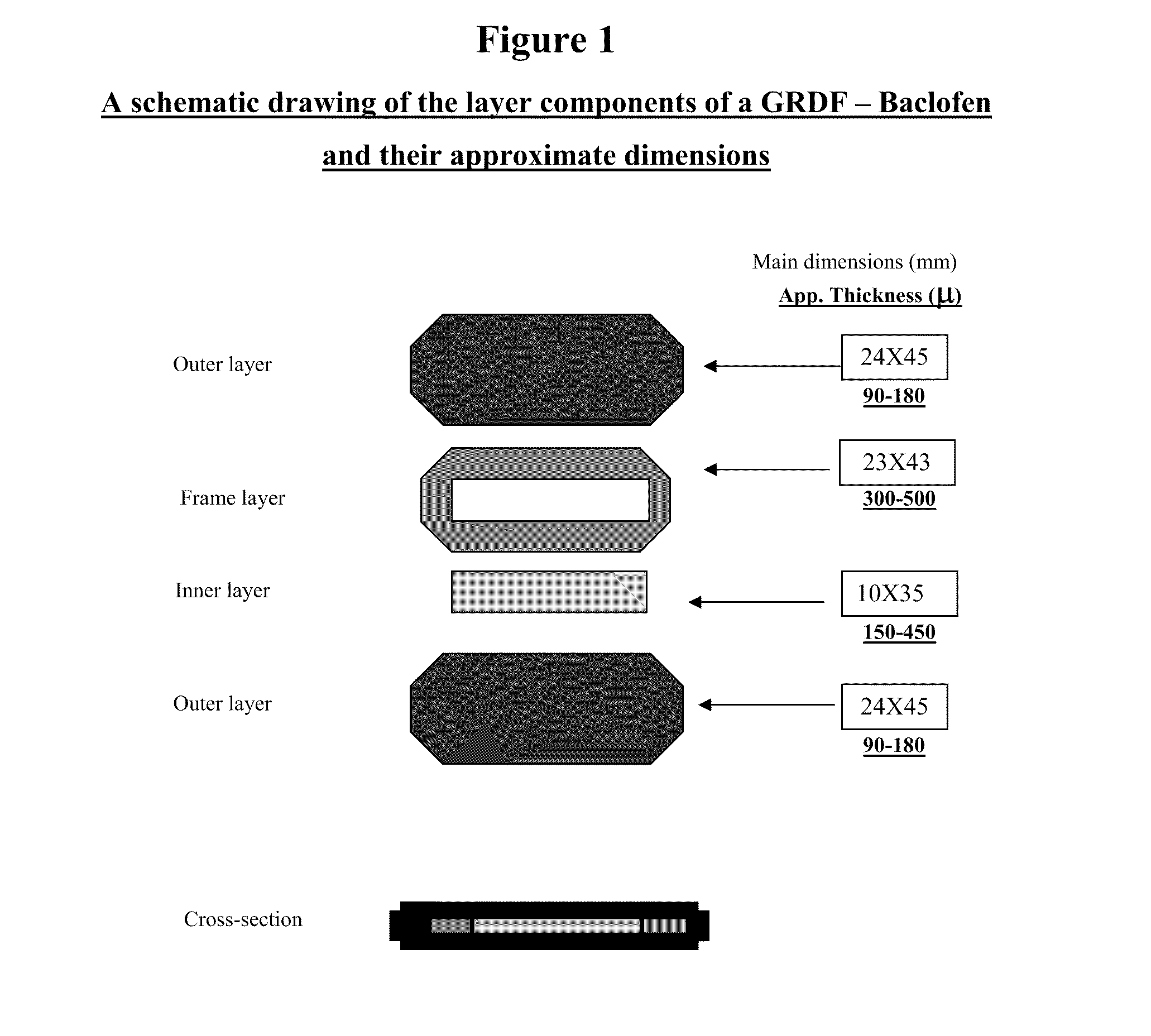
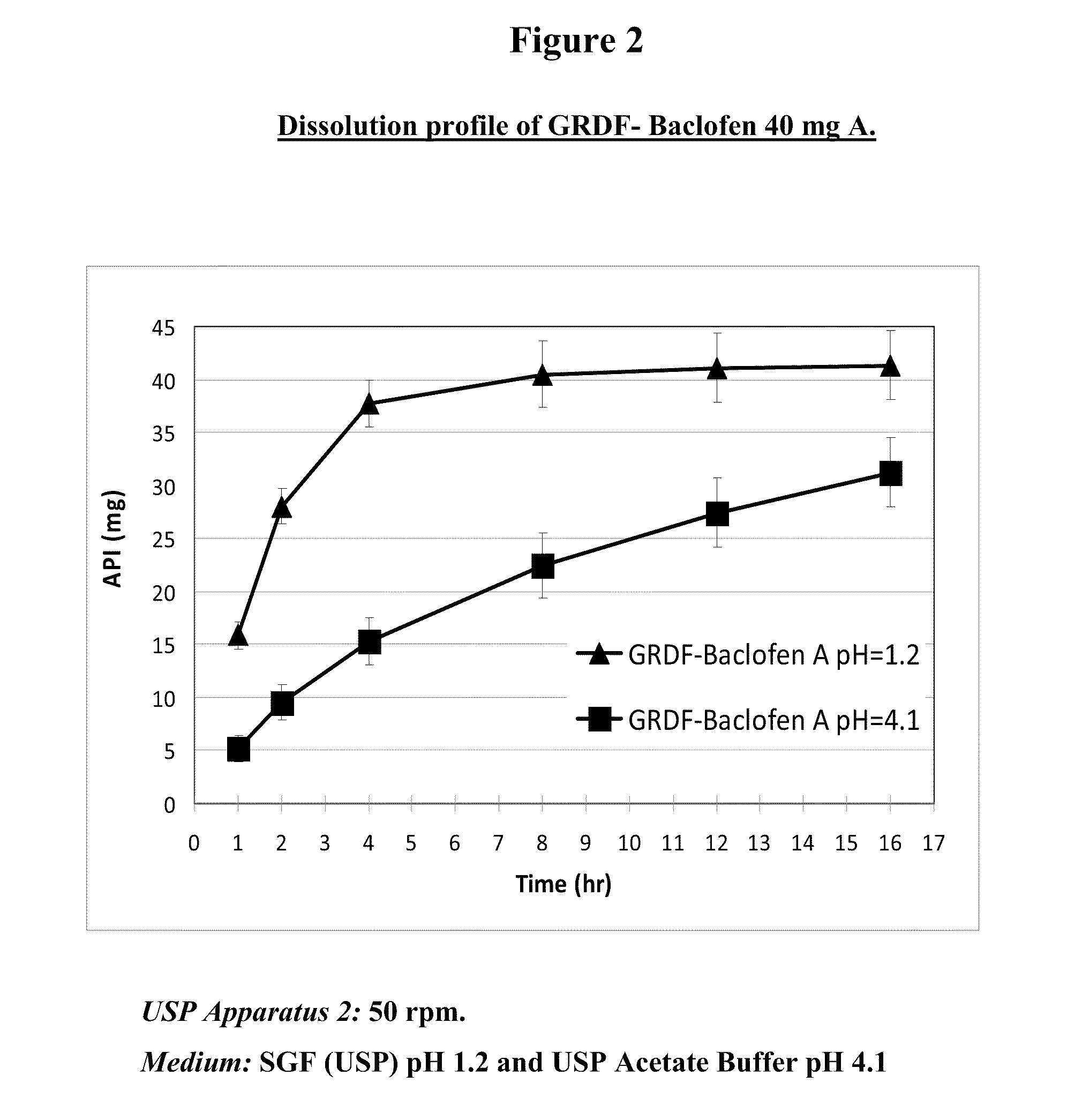
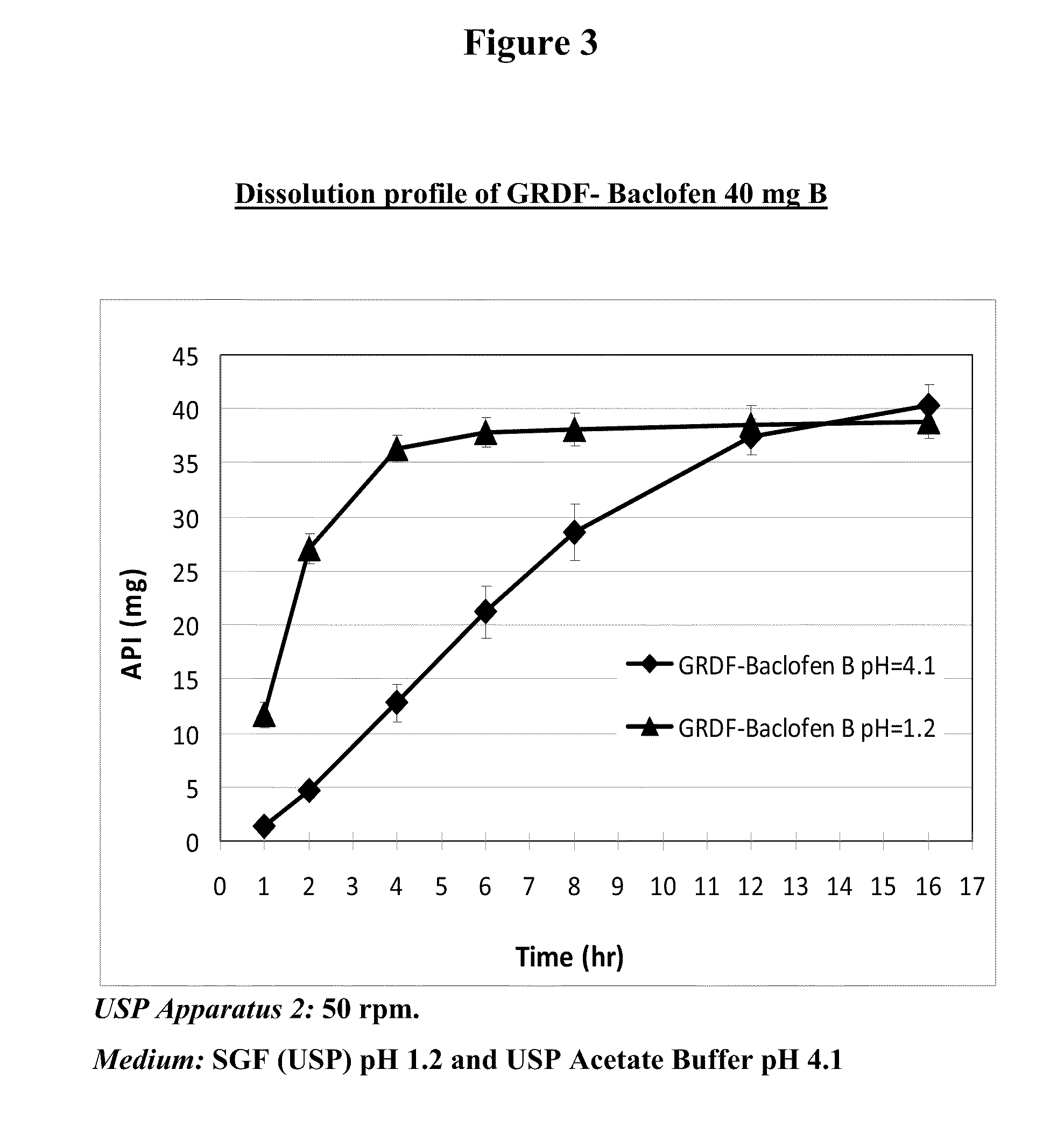
Baclofen and r-baclofen gastroretentive drug delivery systems
InactiveUS20110091542A1Reduce concentrationStrong therapeutic activityOrganic active ingredientsBiocideControlled releaseSide effect
A biodegradable, multi-layered controlled release gastroretentive baclofen or R-baclofen dosage form which is optionally divided into a first dosage of baclofen or R-baclofen for immediate release and a second dosage of baclofen or R-baclofen for controlled release in the stomach and gastrointestinal tract of a patient, folded into a capsule which disintegrates upon contact with gastric juice and the dosage form unfolds rapidly upon contact with gastric juice. The biodegradable, multi-layered gastroretentive dosage forms of the invention provide fast onset of baclofen or R-baclofen activity with prolonged absorption and minimal undesirable side effects.
Owner:INTEC PHARMA
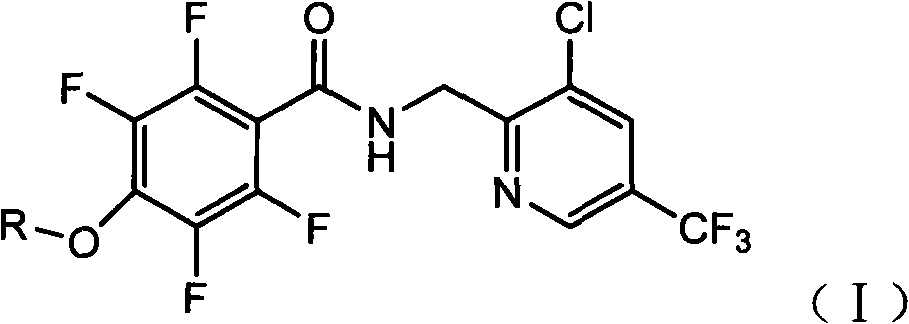
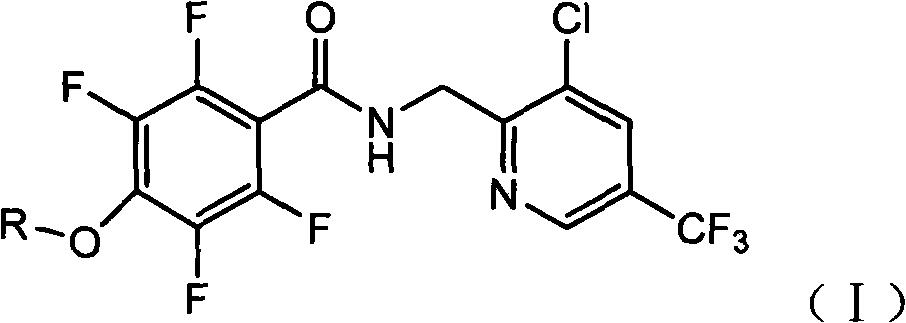
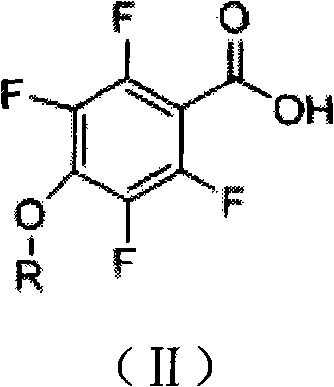
Tetrafluorophenoxy nicotinamide compound as well as preparation method and application thereof for sterilizing
ActiveCN102086173AGood sterilization effectHigh therapeutic activityBiocideOrganic chemistryDrugDisease
The invention discloses a tetrafluorophenoxy nicotinamide compound as well as a preparation method and application thereof for sterilizing and relates to the field of agricultural pesticide compounds and in particular to a crop bactericidal compound as well as a preparation method and application thereof. The tetrafluorophenoxy nicotinamide compound has the general formula (I) shown in the specification, in the formula, R is the groups of -CH3, -CH2CH3, -CH2CH2CH3, -CH(CH3)2, -CH2CH2CH2CH3, -C(CH3)3, -CH2CH(CH3)2, -CH2CF3 or -CH2CHF2. The compound can be used for controlling eumycetes diseases, such as epidemic diseases and downy mildews, and has bactericidal activity on drug-resistant mildews and drug-sensitive mildews.
Owner:SHANDONG UNITED PESTICIDE IND CO LTD
Methods for identifying and using IKK inhibitors
InactiveUS7053119B2Strong therapeutic activityInhibiting IKK activityBiocideCompound screeningDiseaseChemistry
Owner:BIOGEM S C A R L +2
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090017026A1Strong therapeutic activityEnhancing antibody-mediated effector functionImmunoglobulins against animals/humansAntibody ingredientsTherapeutic antibodyTherapeutic effect
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
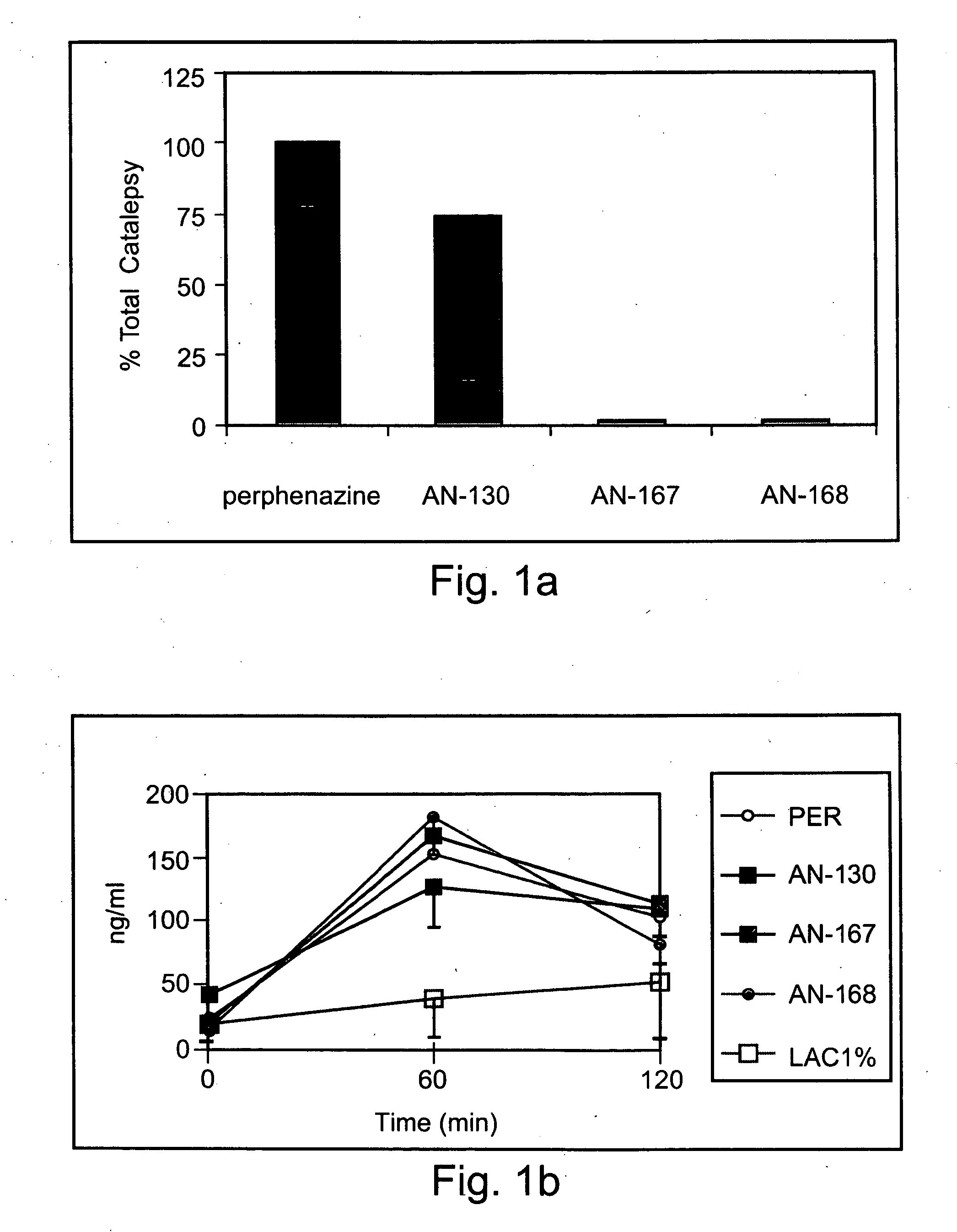
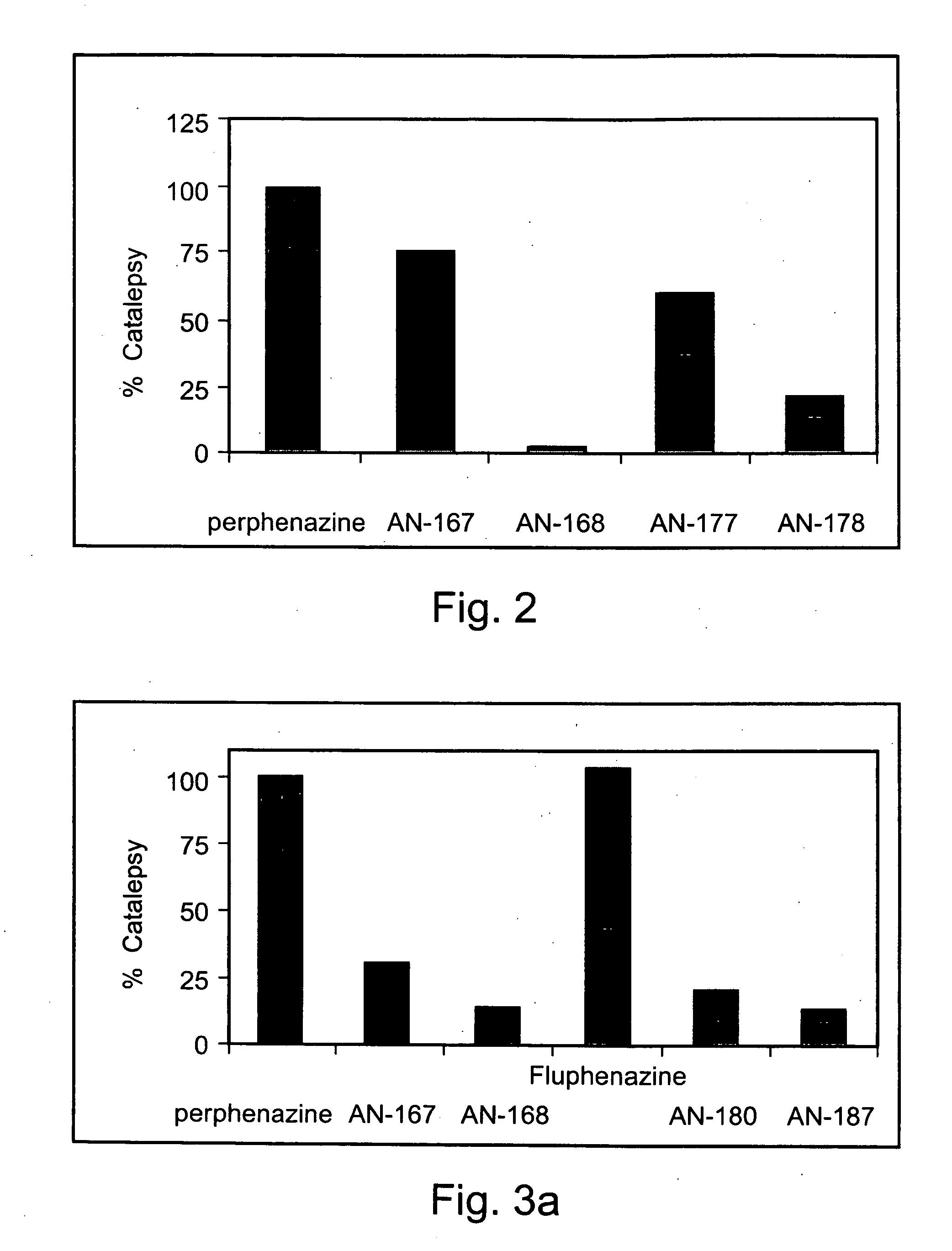
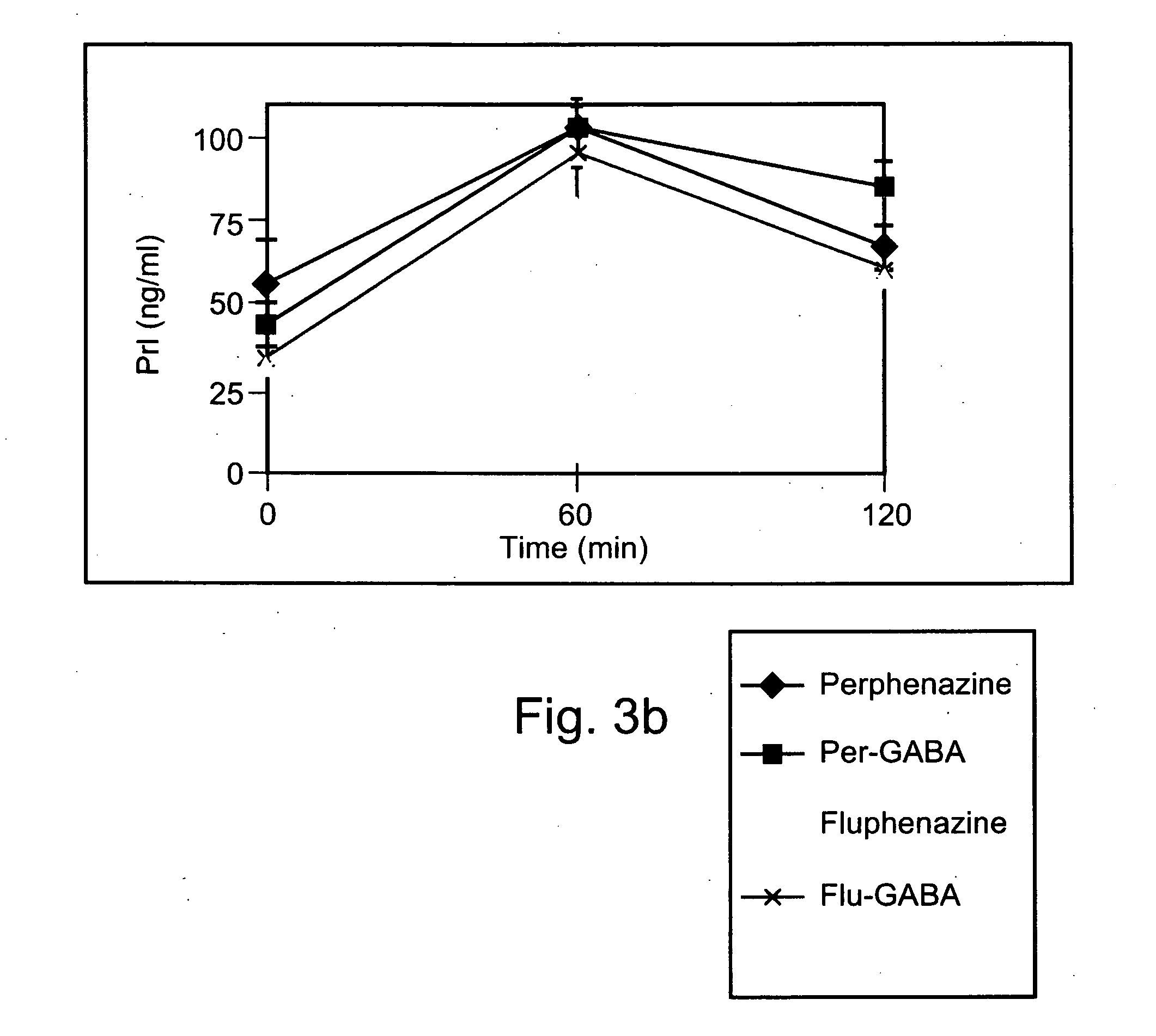
Conjugated psychotropic drugs and uses thereof
InactiveUS20070099977A1Minimize side effectsStrong therapeutic activityBiocideNervous disorderOrganic acidDisease
Owner:BAR ILAN UNIV +1
Novel salts of conjugated psychotropic drugs and processes of preparing same
InactiveUS20090298814A1Side-effect be reduceStrong therapeutic activityNervous disorderOrganic chemistryDrugChemistry
Novel chemical conjugates of a psychotropic drug residue and an amino-containing organic acid residue selected to reduce side effects induced by the psychotropic drug when administered per se, to enhance the therapeutic activity of the psychotropic drug and / or to exert anti-proliferative activity, in which the amino group is in the form of an acid addition salt thereof and which are characterized by high stability are disclosed. Further disclosed are processes for preparing the chemical conjugates and addition salts thereof, pharmaceutical compositions containing the chemical conjugates and methods utilizing the chemical conjugates for treating various medical conditions.
Owner:BAR ILAN UNIVERSITY +2
Organic compounds
ActiveUS10077267B2Similar biological activityTo promote metabolismOrganic active ingredientsIsotope introduction to heterocyclic compoundsDisease5-HT4 receptor
The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving 5-HT2A receptor, serotonin transporter (SERT) and / or pathways involving dopamine D1 / D2 receptor signaling systems, and / or the treatment of residual symptoms.
Owner:INTRA CELLULAR THERAPIES INC
Treatment of lysosomal storage disorders and other proteostatic diseases
InactiveUS20110237538A1Increase half-lifeInhibitory effectBiocideNervous disorderAcid CeramidaseAcid beta-galactosidase
Described are various compounds, in particular iminosugars, and methods for the treatment of proteostatic diseases, in particular lysosomal storage disorders. The compound may be a pharmacoperone of an enzyme selected from: (a) Acid alpha-glucosidase; (b) Acid beta-glucosidase; (c) glucocerebrosidase; (d) alpha-Galactosidase A; (e) Acid beta-galactosidase; (f) beta-Hexosaminidase A; (g) beta-Hexosaminidase B; (h) Acid sphingomyelinase; (i) Galactocerebrosidase; (j) Acid ceramidase; (k) Arylsulfatase A; (l) alpha-L-Iduronidase; (m) Iduronate-2-sulfatase; (n) Heparan N-sulfatase; (o) alpha-N-Acetylglucosaminidase; (p) Acetyl-CoA: alpha-glucosaminide N-acetyltransferase; (q) N-Acetylglucosamine-6-sulfate sulfatase; (r) N-Acetylgalactosamine-6-sulfate sulfatase; (s) Acid beta-galactosidase; (t) Arylsulfatase B; (u) beta-Glucuronidase; (v) Acid alpha-mannosidase; (w) Acid beta-mannosidase; (x) Acid alpha-L-fucosidase; (y) Sialidase; and (z) alpha-N-acetylgalactosaminidase.
Owner:SUMMIT
Organic compounds
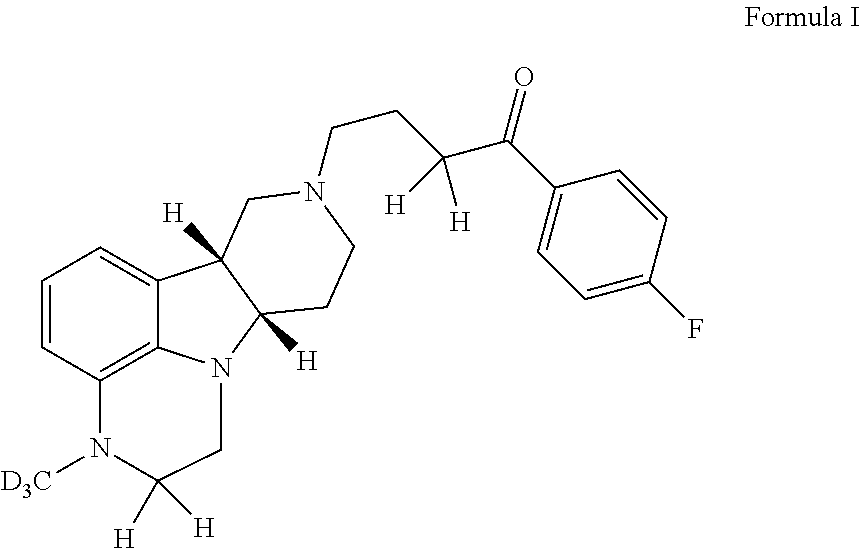
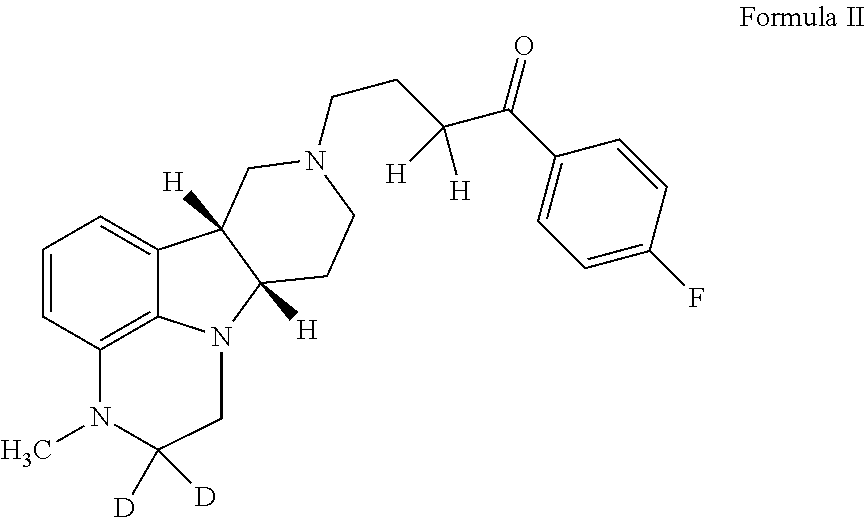
ActiveUS20190231780A1Avoid side effectsInhibit the serotonin re-uptake transporterOrganic active ingredientsNervous disorderDisease5-HT4 receptor
The invention relates to particular substituted deuterated heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving 5-HT2A receptor, serotonin transporter (SERT) and / or pathways involving dopamine D1 / D2 receptor signaling systems, and / or the treatment of residual symptoms.
Owner:INTRA CELLULAR THERAPIES INC
Conjugates Comprising a gaba-or glycine compound, pharmaceutical compositions and combinations thereof as well as their use in treating cns disorders
InactiveUS20100144869A1Strong therapeutic activityAccelerated onset of their protective effectBiocideNervous disorderDiseaseOrganic acid
Owner:RAMOT AT TEL AVIV UNIV LTD +1
Taxane delivery system
Nanoparticulate formulations for delivery of taxane conjugate prodrug formed from a taxane coupled to a hydrophobic moiety through a glycolate linker are described.
Owner:CELATOR PHARMA INC
Taxane delivery system
ActiveUS8486924B2Easy to controlStrong therapeutic activityBiocideOrganic chemistryGynecologyNanoparticle
Nanoparticulate formulations for delivery of taxane conjugate prodrug formed from a taxane coupled to a hydrophobic moiety through a glycolate linker are described.
Owner:CELATOR PHARMA INC
Organic compounds
ActiveUS20170037048A1Avoid side effectsStrong therapeutic activityOrganic active ingredientsIsotope introduction to heterocyclic compoundsDisease5-HT4 receptor
The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving 5-HT2A receptor, serotonin transporter (SERT) and / or pathways involving dopamine D1 / D2 receptor signaling systems, and / or the treatment of residual symptoms.
Owner:INTRA CELLULAR THERAPIES INC
Enhancement of drug therapy by mirna
InactiveUS20090280167A1Strong therapeutic activityHigh activityOrganic active ingredientsNervous disorderPharmaceutical drugHsp Inhibitor
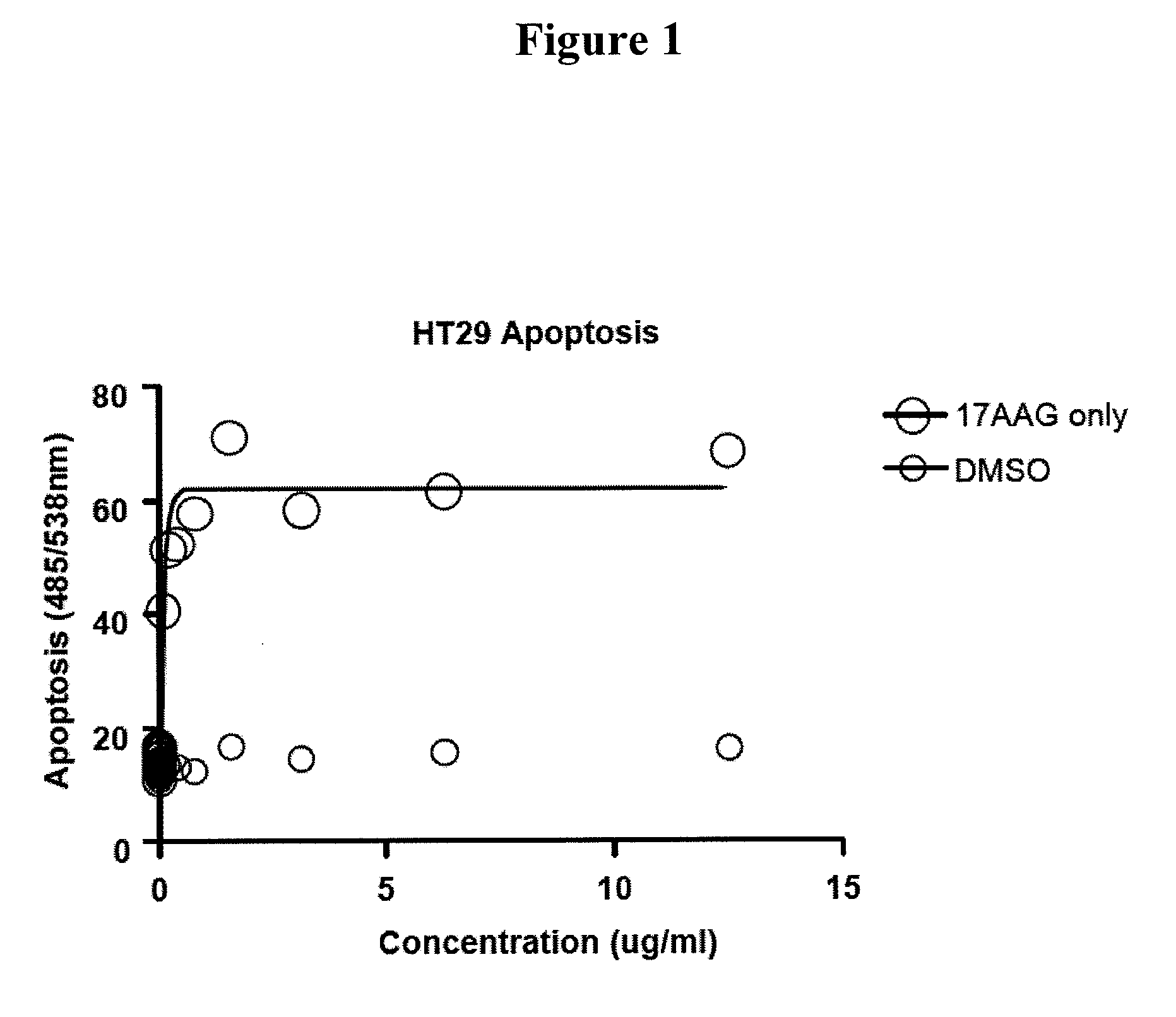
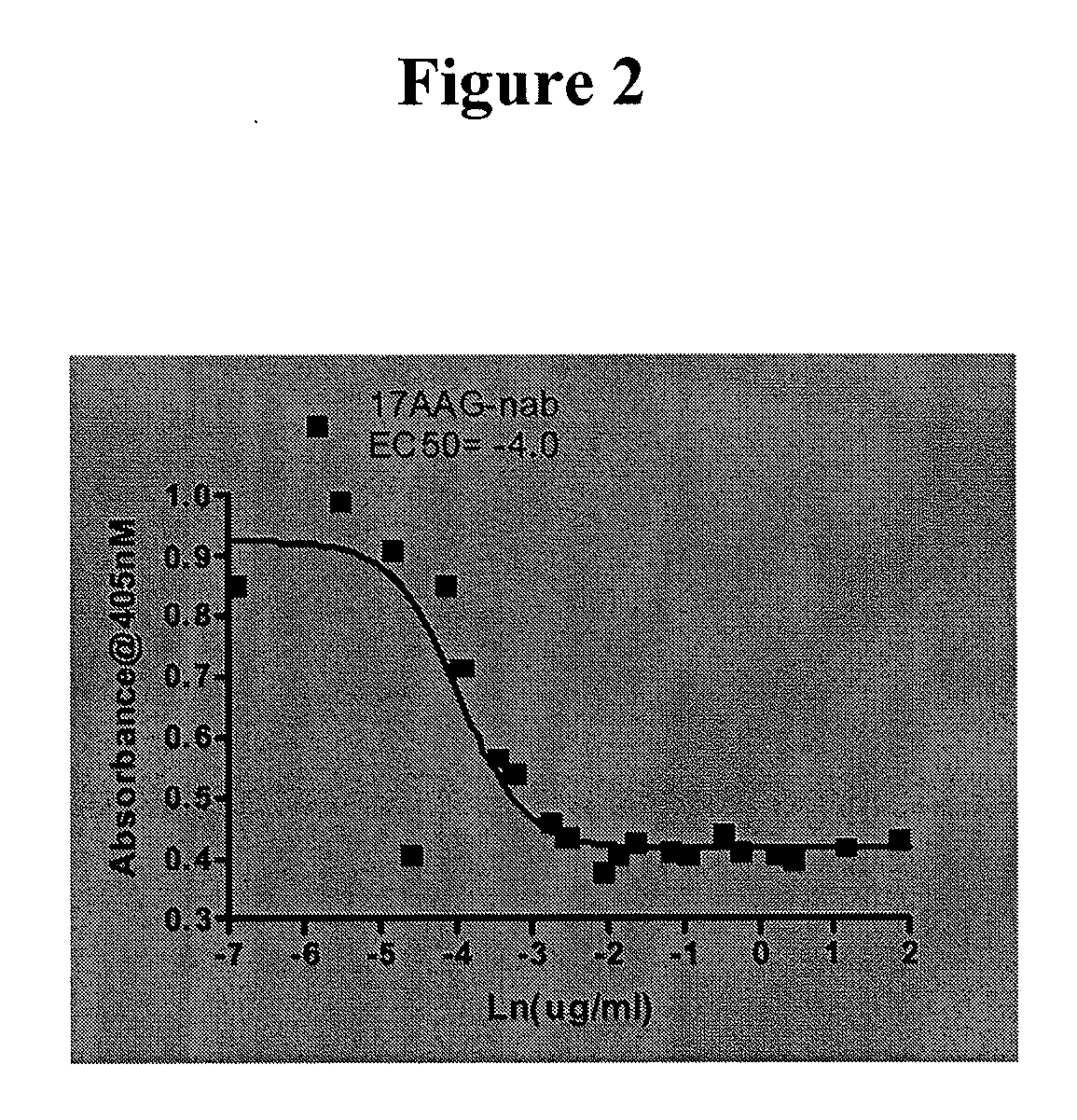
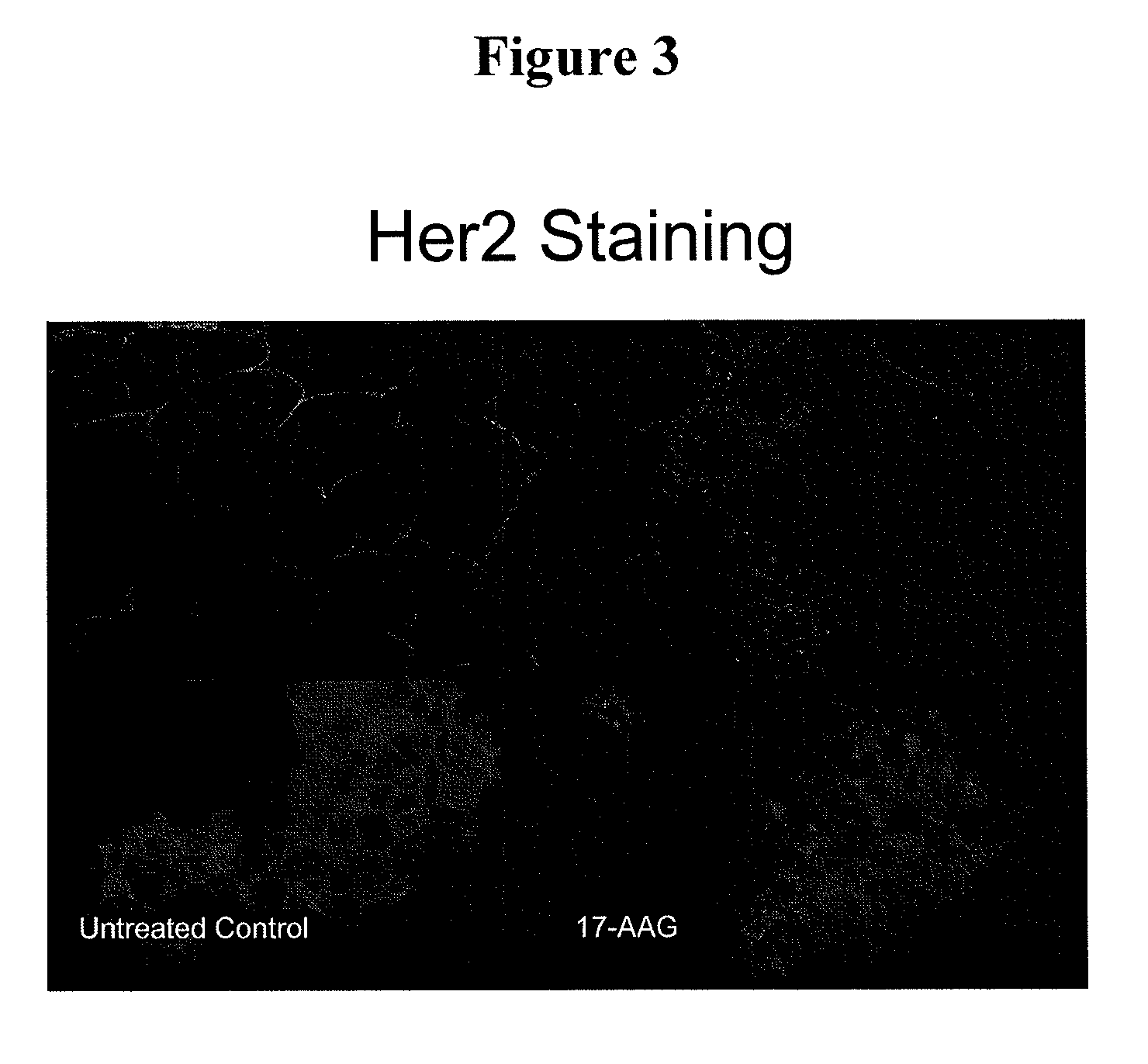
This invention provides methods and compositions for screening of microRNA capable of modulating gene expression in the apoptotic pathway in the presence of HSP90 inhibitor. The use of miRNA for enhancing the activity of therapeutic agents not limited to HSP90 inhibitor is also disclosed. The diagnostic use of miRNA for predicting response to therapy not limited to therapeutic agents is also disclosed. A method for the identification and therapeutic application of small molecules which are modulators of these nucleic acids are also included in this application
Owner:ABRAXIS BIOSCI LLC
Drug conjugates and methods of use thereof
ActiveUS8268887B2Eliminate side effectsGood curative effectBiocideNervous disorderDrug conjugationCombinatorial chemistry
In one aspect, the present invention provides a composition of a covalent conjugate of a GABA analog with a drug. In another aspect, the present invention provides methods for treating pain and neurological disorders using the conjugates of GABA analogs.
Owner:XGENE PHARMA LLC
Camptothecin medicament injection solution and injection and preparation method thereof
InactiveCN101708156AGood dispersionSolve problems that are difficult to make into injectionsOrganic active ingredientsEmulsion deliverySolubilityMedication injection
The invention relates to the technical field of medicaments, in particular to a camptothecin medicament injection solution and an injection and a preparation method thereof. The invention provides a camptothecin medicament injection which is good in stability, high in curative effect and low in toxicity and consists of the camptothecin medicament injection solution and a disperse medium, wherein the camptothecin medicament injection solution consists of a medicament active component, a stabilizing agent, a pH value regulator and an injection solvent. The medicament active component of the camptothecin medicament injection solution is a camptothecin medicament mainly existing in the form of a lactonic ring, which can improve the curative activity of the medicament and reduce toxic side effects; and the disperse medium is an injection emulsion, which can improve the dispersion degree of the camptothecin medicament and solve the problem that the camptothecin medicament is difficult to be prepared into the injection due to poor water solubility. The camptothecin medicament injection solution and the injection emulsion are packed and then stored respectively, and are mixed uniformly before use for intravenous administration, so the stability of the medicaments stored for a long time can be improved. The invention provides an intravenous injection which has low toxicity and is save and convenient to store for camptothecin medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
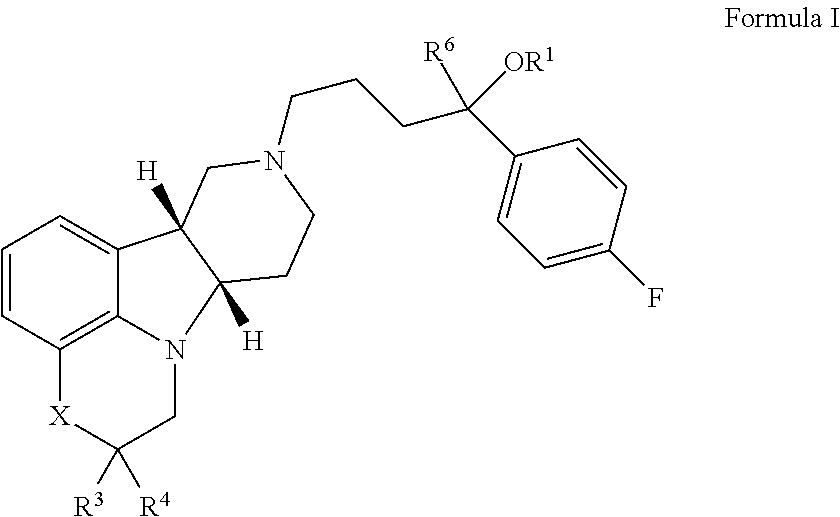
Tumor-treating compound and application thereof
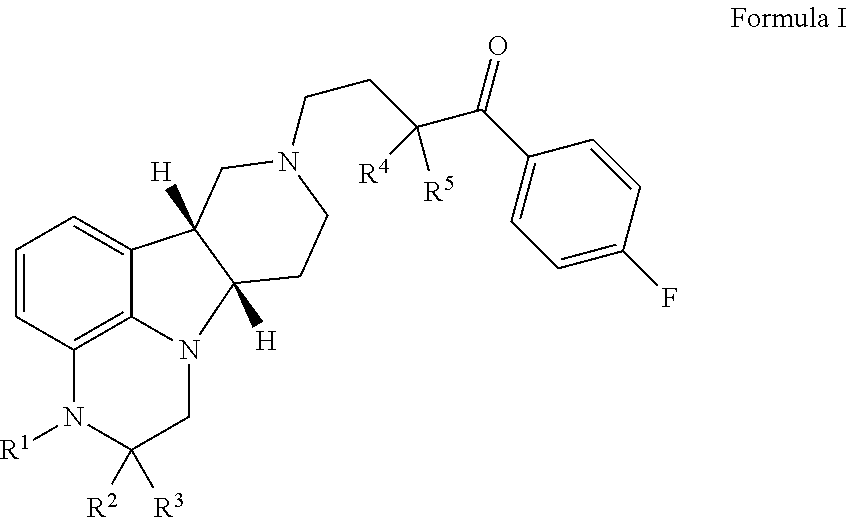
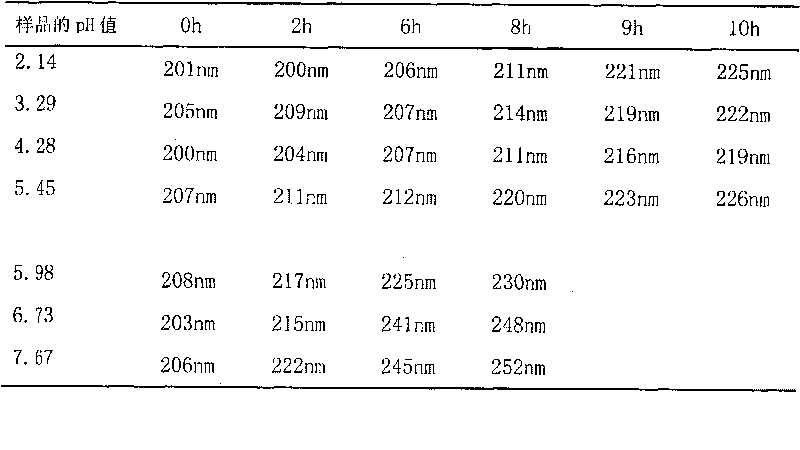
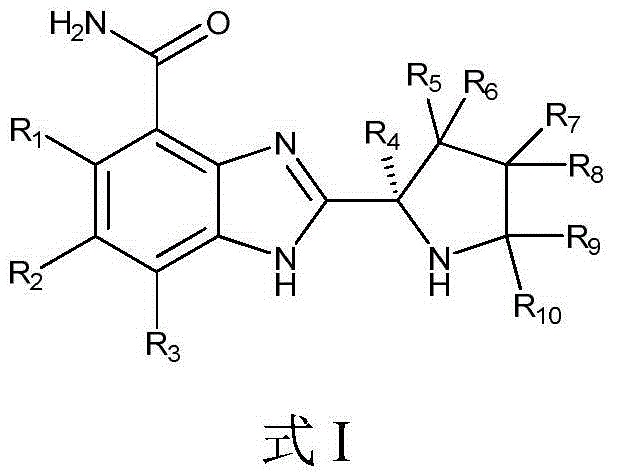
ActiveCN104926793AHas antitumor activityStrong therapeutic activityOrganic active ingredientsOrganic chemistry methodsMelanomaHalf-life
Provided in the present invention are a compound for treating tumours and a use thereof, and also provided are a compound as shown in formula I or a pharmaceutically acceptable salt thereof. The compound provided in the present invention has a high medicine peak concentration, a high medicine absorption and a long elimination half life, and can improve the efficacy of the medicine in clinical use and reduce the frequency of the dosages. The compound or the pharmaceutically acceptable salt thereof prepared by the present invention can act as a PARP inhibitor medicine, have a certain anti-tumour activity, and especially have a good therapeutic activity on triple negative, primary or metastatic breast cancer, colon cancer, uterine cancer, pancreatic cancer, lung cancer, stomach cancer, leukemia, melanoma, solid tumors or intracranial tumors, and provide a new choice for clinical medication.
Owner:HINOVA PHARM INC
Agents for treating tumors, use and method thereof
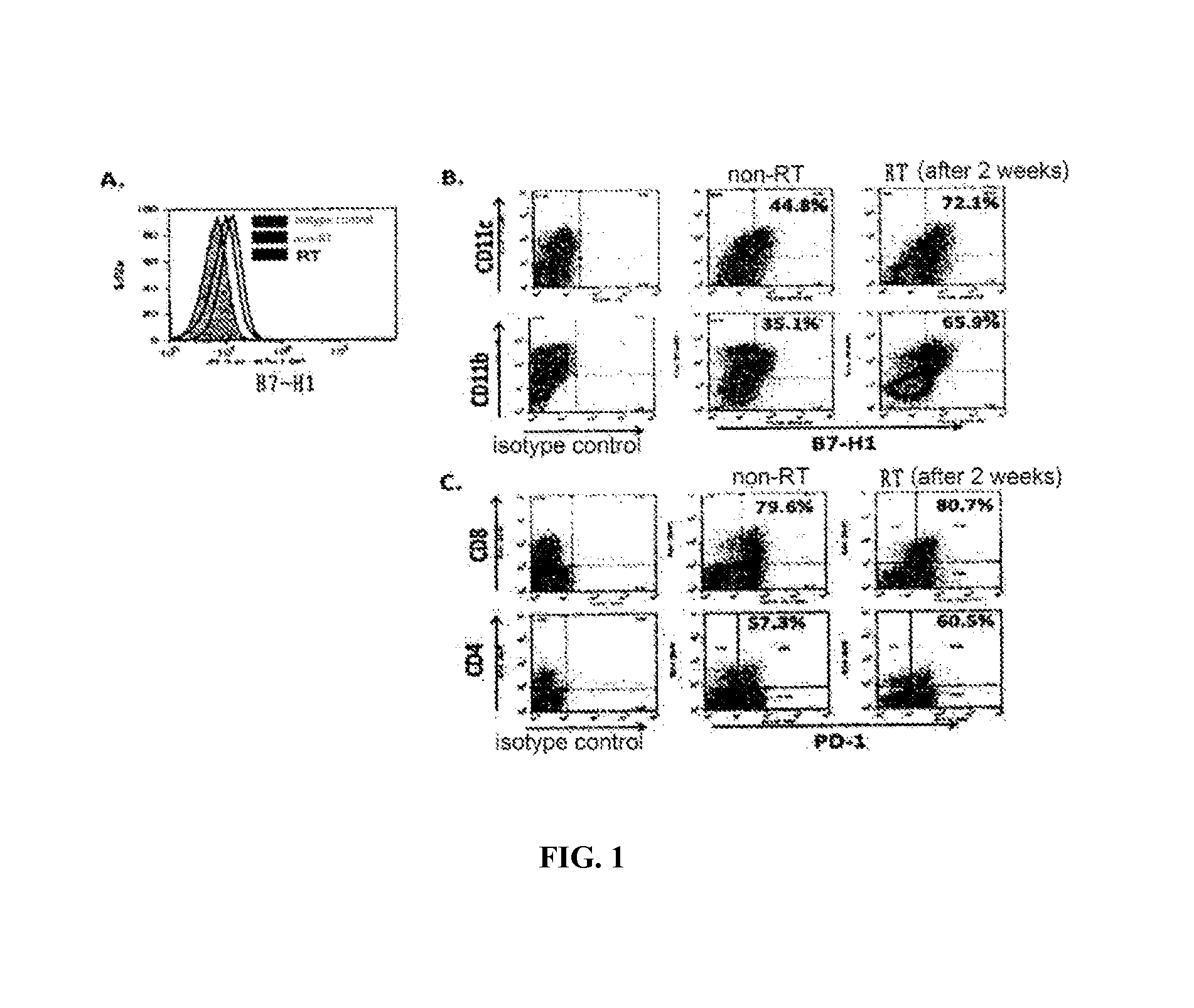
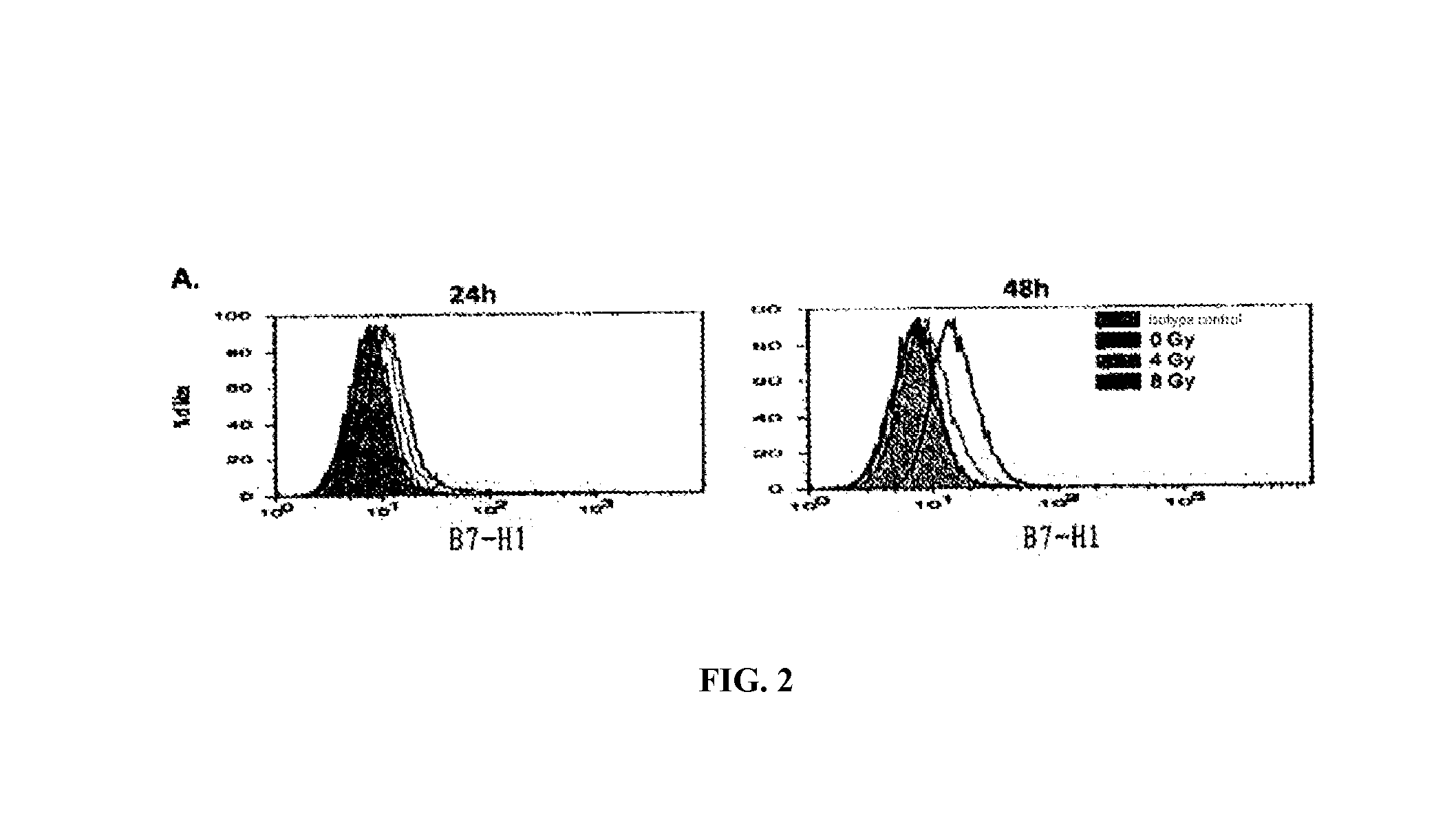
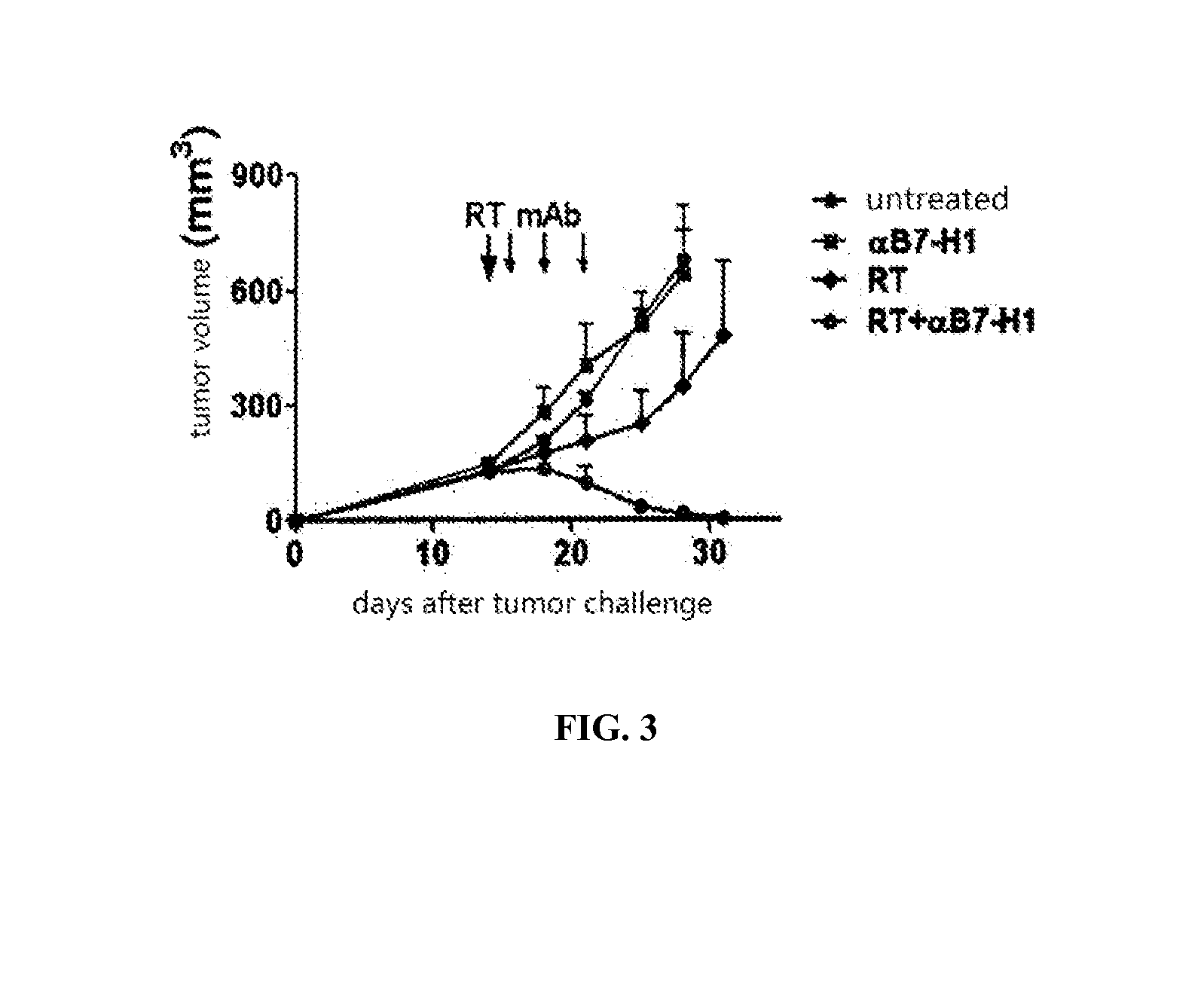
InactiveUS20150344577A1Reduce tumor burdenEnhance anti-tumor immunityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsRadiation therapyTumor cells
The present disclosure provides agents for treating and / or preventing resistance of tumor cells to radiation therapy (RT), the use and relevant method thereof.
Owner:SUZHOU DINGFU BIOTARGET CO LTD
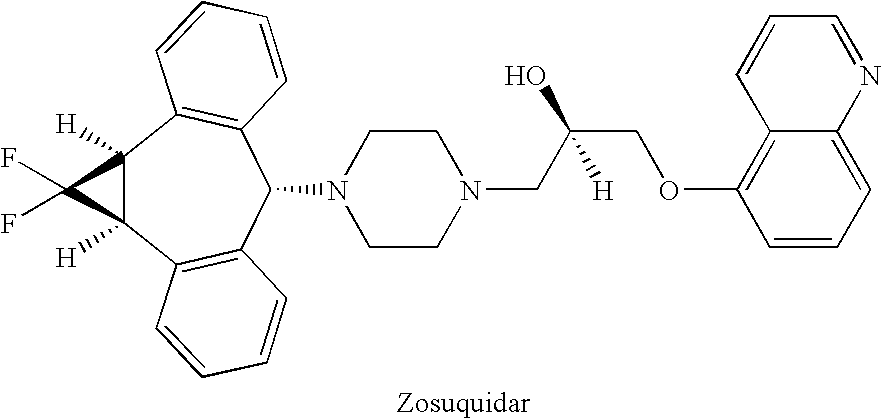
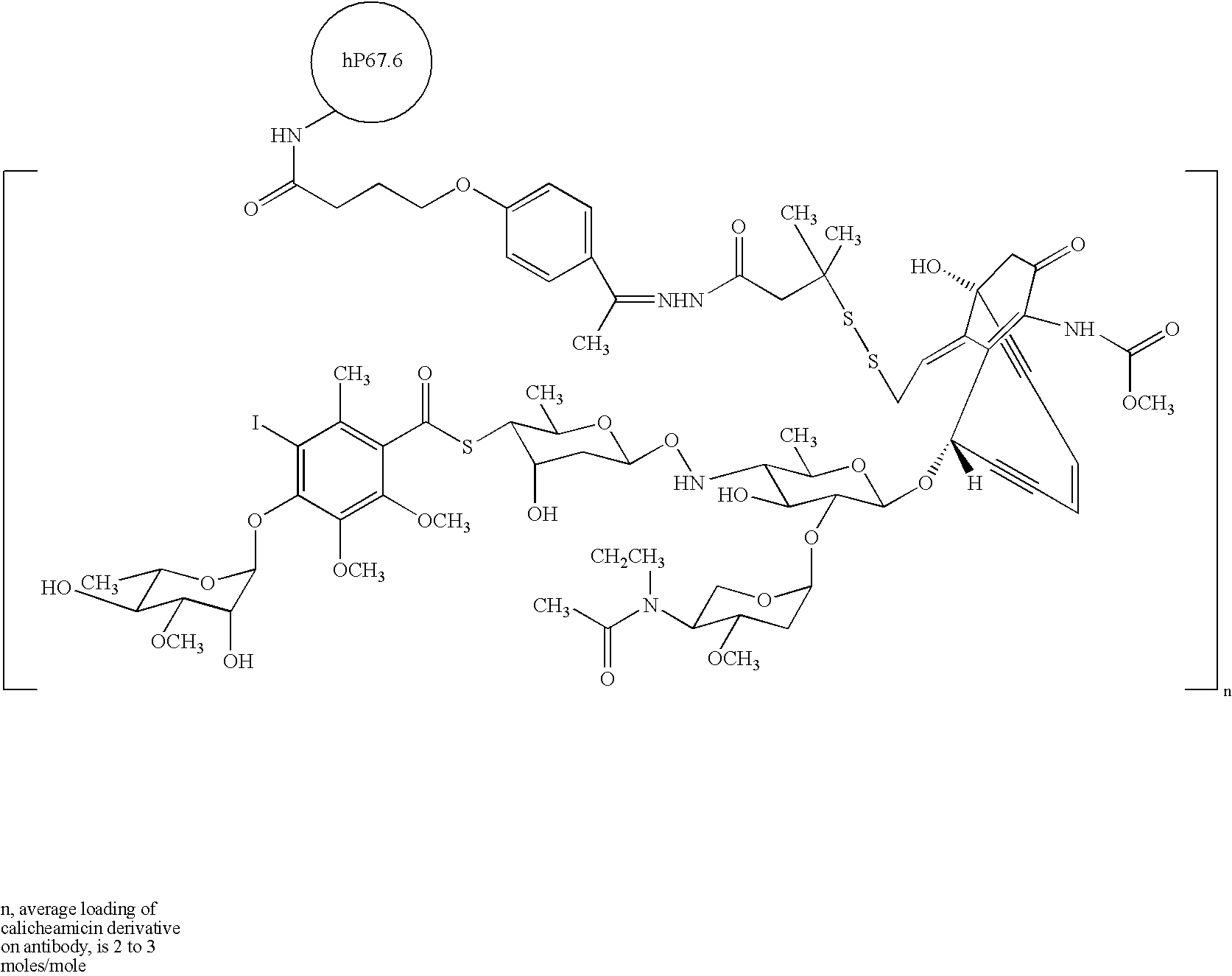
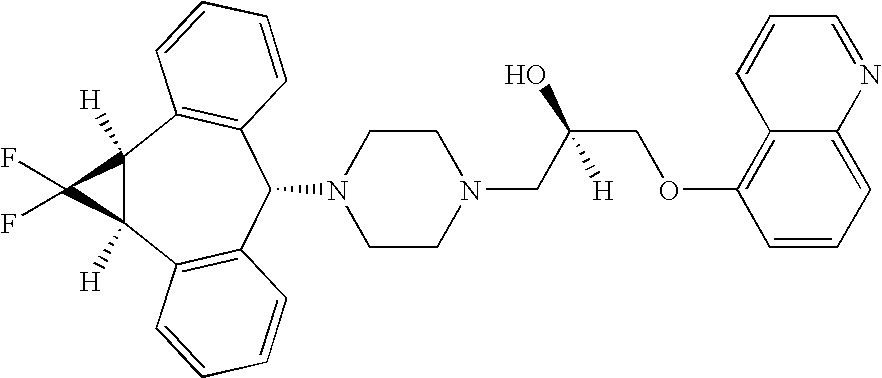
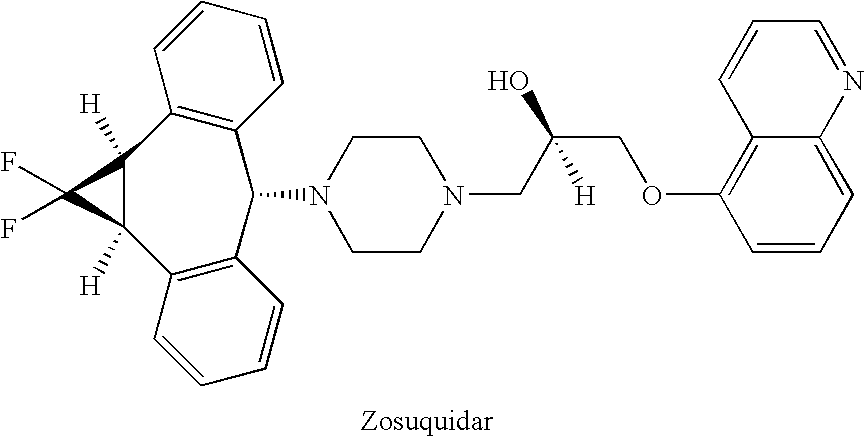
Treatment of patients with cancer using a calicheamicin-antibody conjugate in combination with zosuquidar
InactiveUS20070009532A1Strong therapeutic activityImprove completion rateBiocideAntibody ingredientsAntibody conjugateRecurrent acute
The present invention relates to a method of treating patients with solid tumors, leukemias, and other malignancies using a combination of zosuquidar and a calicheamicin-antibody conjugate, such as Mylotarg. The invention is also directed to pharmaceutical formulations comprising zosuquidar and calicheamicin-antibody conjugates. The formulations are particularly effective in treating relapsed Acute Myelogenous Leukemia (AML) and metastatic breast cancer.
Owner:KANISA PHARMA INC
Organic compounds
ActiveUS10245260B2Convenient treatmentCompliance difficultNervous disorderOrganic chemistryDisease5-HT4 receptor
The invention relates to particular substituted heterocycle fused gamma-carbolines, their prodrugs, in free, solid, pharmaceutically acceptable salt and / or substantially pure form as described herein, pharmaceutical compositions thereof, and methods of use in the treatment of diseases involving the 5-HT2A receptor, the serotonin transporter (SERT), pathways involving the dopamine D1 and D2 receptor signaling system, and / or the μ-opioid receptor.
Owner:INTRA CELLULAR THERAPIES INC
Zosuquidar, daunorubicin, and cytarabine for the treatment of cancer
InactiveUS20070010465A1Strong therapeutic activityImprove completion rateBiocideCarbohydrate active ingredientsCytarabineRecurrent acute
The present invention relates to a method of treating patients with solid tumors, leukemias, and other malignancies using a combination of zosuquidar, daunorubicin, and cytarabine. The invention is also directed to pharmaceutical formulations comprising zosuquidar, daunorubicin, and cytarabine. The formulations are particularly effective in treating relapsed Acute Myelogenous Leukemia (AML).
Owner:KANISA PHARMA INC
Zosuquidar, daunorubicin, and cytarabine for the treatment of cancer
InactiveUS20070010478A1Improve completion rateStrong therapeutic activityBiocideCarbohydrate active ingredientsCytarabineRecurrent acute
The present invention relates to a method of treating patients with solid tumors, leukemias, and other malignancies using a combination of zosuquidar, daunorubicin, and cytarabine. The invention is also directed to pharmaceutical formulations comprising zosuquidar, daunorubicin, and cytarabine. The formulations are particularly effective in treating relapsed Acute Myelogenous Leukemia (AML).
Owner:KANISA PHARMA INC
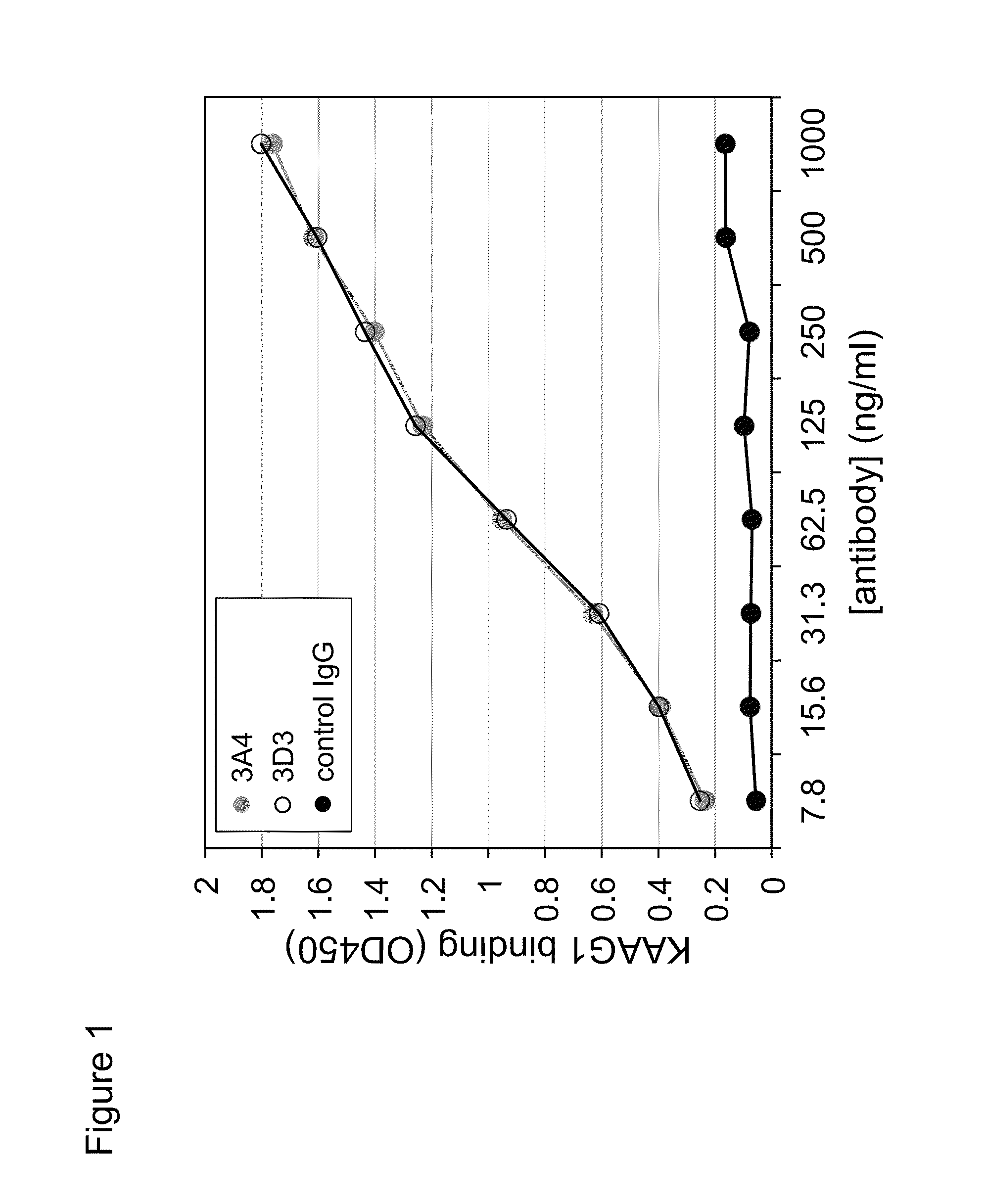
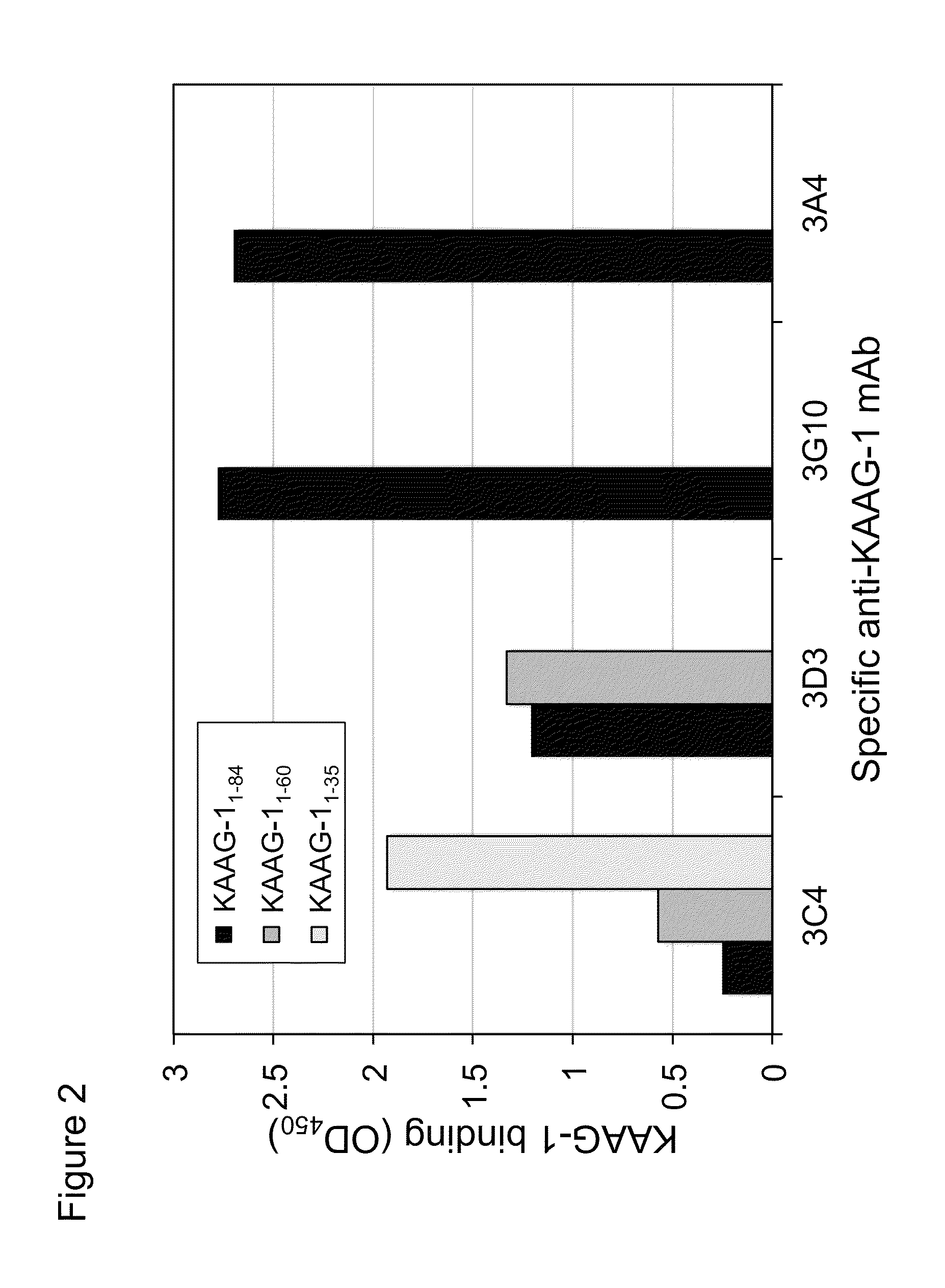
Antibodies against kidney associated antigen 1 and antigen binding fragments thereof
ActiveUS8937163B2Improve bindingImproved internalizationBiological material analysisTripeptide ingredientsAntigen Binding FragmentProstate cancer
Novel antibodies and antigen binding fragments that specifically bind to KAAG1 and which may be used in the treatment, detection and diagnosis of cancer comprising KAAG1-expressing cells are disclosed herein. Cells expressing the antibodies and antigen binding fragments as well as methods of detecting and treating cancer using the antibodies and fragments are also disclosed. Cancer indications which may benefit from such treatment or detection include ovarian cancer, renal cancer, lung cancer, colorectal cancer, breast cancer, brain cancer, and prostate cancer, as well as melanomas.
Owner:ADC THERAPEUTICS SA
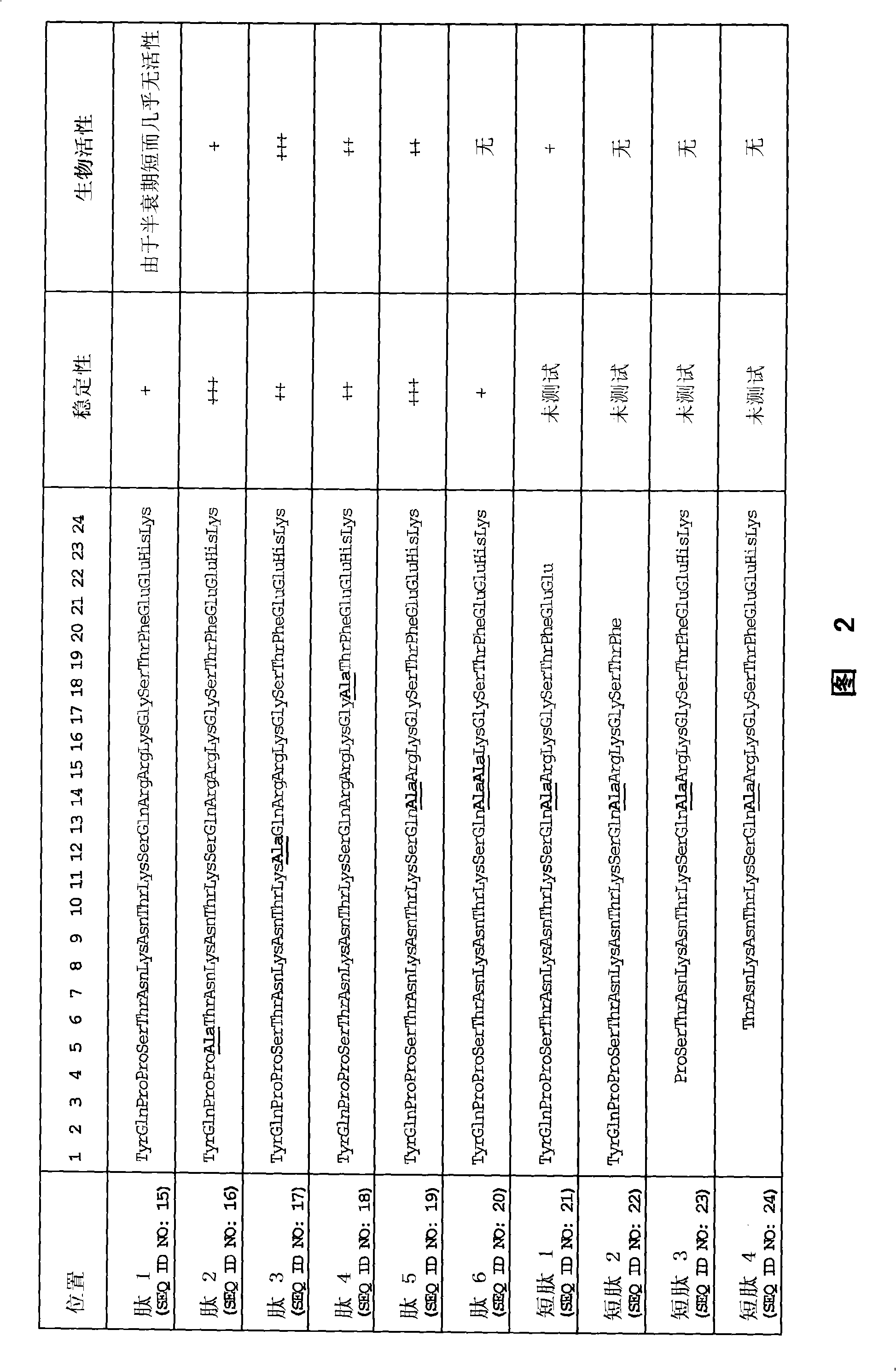
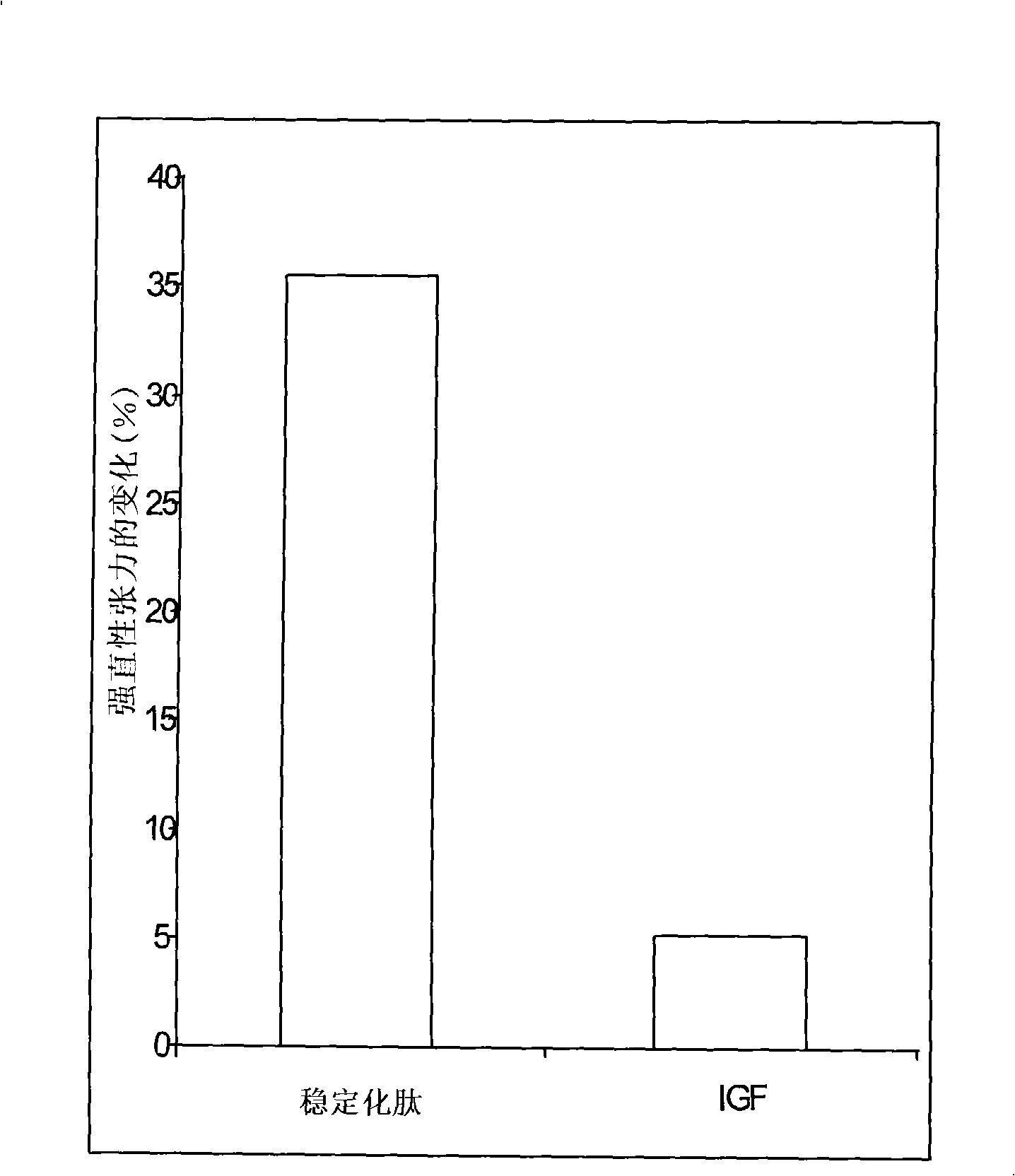
Mecano growth factor peptides and their use
InactiveCN101300269AImprove stabilityStrong therapeutic activityNervous disorderPeptide/protein ingredientsInsulin-like growth factorC-terminus
This invention relates to biologically active polypeptides derived from the E peptide that forms the C-terminus of the insulin-like growth factor I (IGF-I) splice variant known as mechano growth factor (MGF). These peptides are modified to improve their stability compared to the naturally occurring E peptide.
Owner:UCL BUSINESS PLC +1
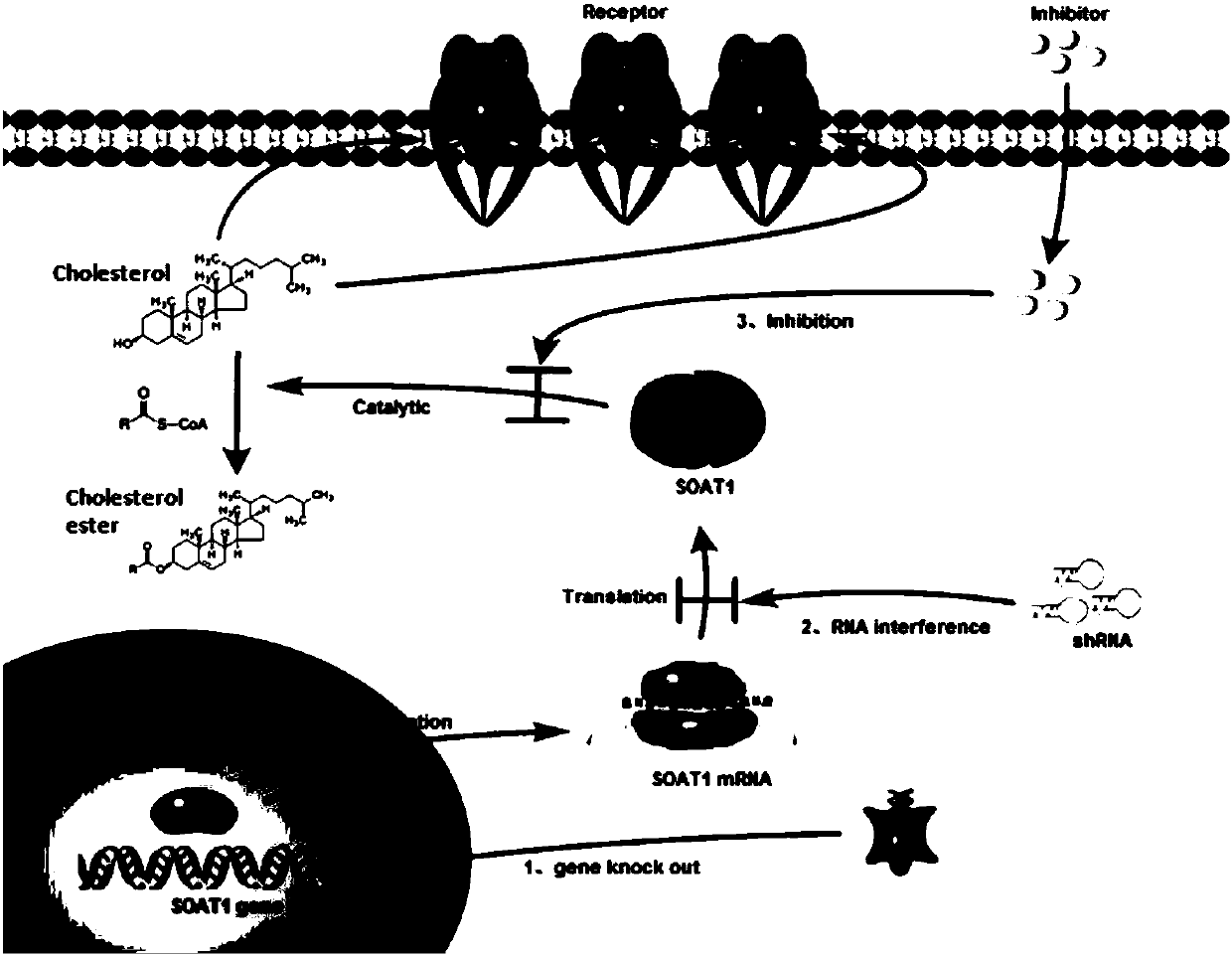
CAR-T cells with inhibited sterol o-acyltransferase 1 (SOAT1) as well as preparation method and application of CAR-T (Chimeric Antigen Receptor-T-cell Immunotherapy) cells
ActiveCN107058232ALimits the rate of conversion to cholesteryl estersIncrease contentImmunoglobulin superfamilyGenetically modified cellsCholesterolGene Knock-Down
The invention discloses CAR-T cells with inhibited sterol o-acyltransferase 1 (SOAT1). The CAR-T cells with inhibited SOAT1 comprise the following cells: a T cell subjected to SOAT1 gene knockout at DNA level and for expression of a hCAR19 receptor, a T cell subjected to SOAT1 gene knock-down at mRNA level and for expression of a hCAR19 receptor, and a T cell subjected to SOAT1 gene inhibiting effect by an inhibitor at protein level and for expression of a hCAR19 receptor. The invention also discloses a preparation method of the CAR-T cells with inhibited SOAT1 and application of the CAR-T cells in preparation of medicine for treating cells of tumors. A series of pre-clinical experiments prove that the CAR-T cells with inhibited SOAT1 have killing ability superior to that of CAR-T cells and have an extremely high application value in treatment of the cells of the tumors.
Owner:SHANGHAI UNICAR THERAPY BIOPHARM TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com