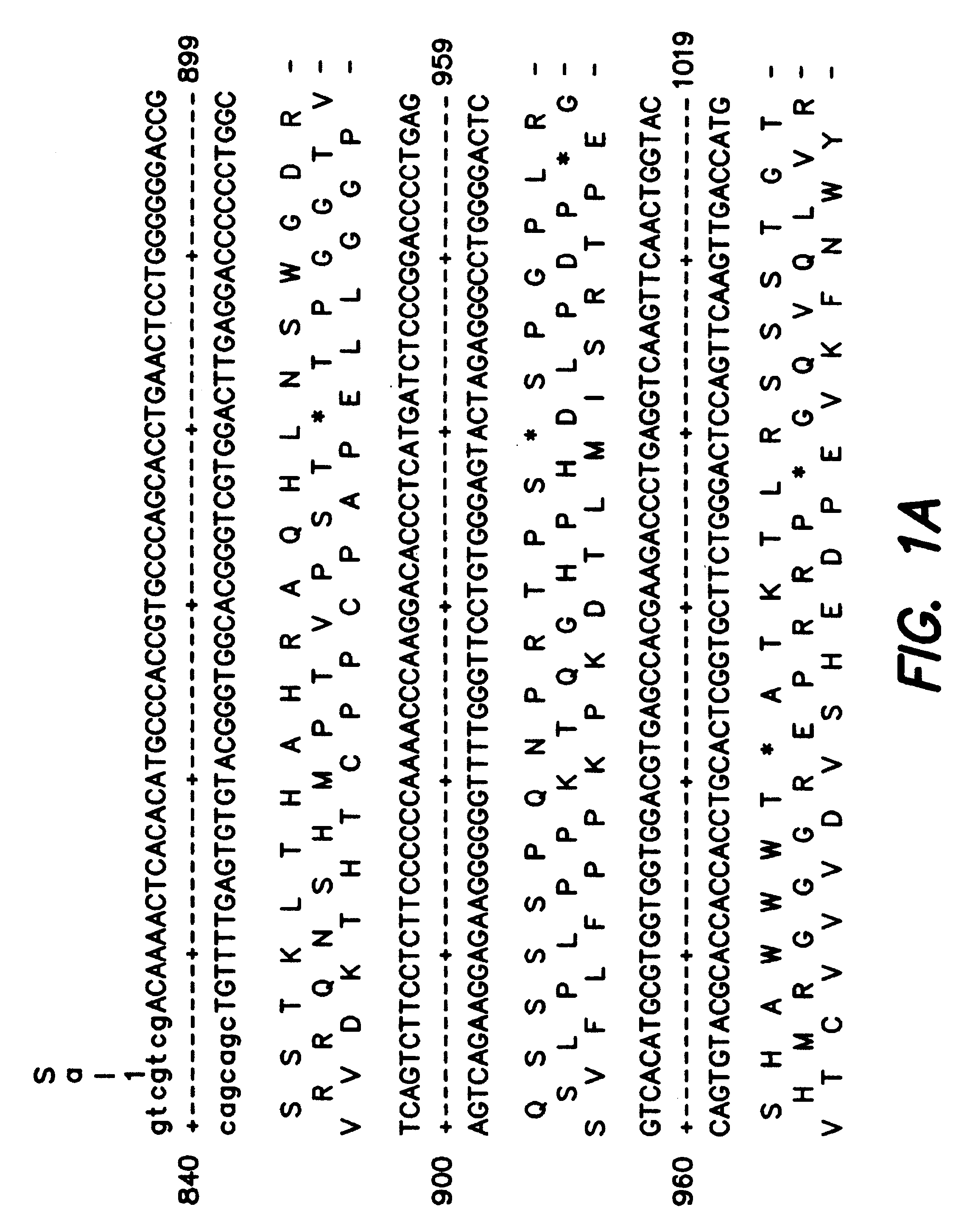
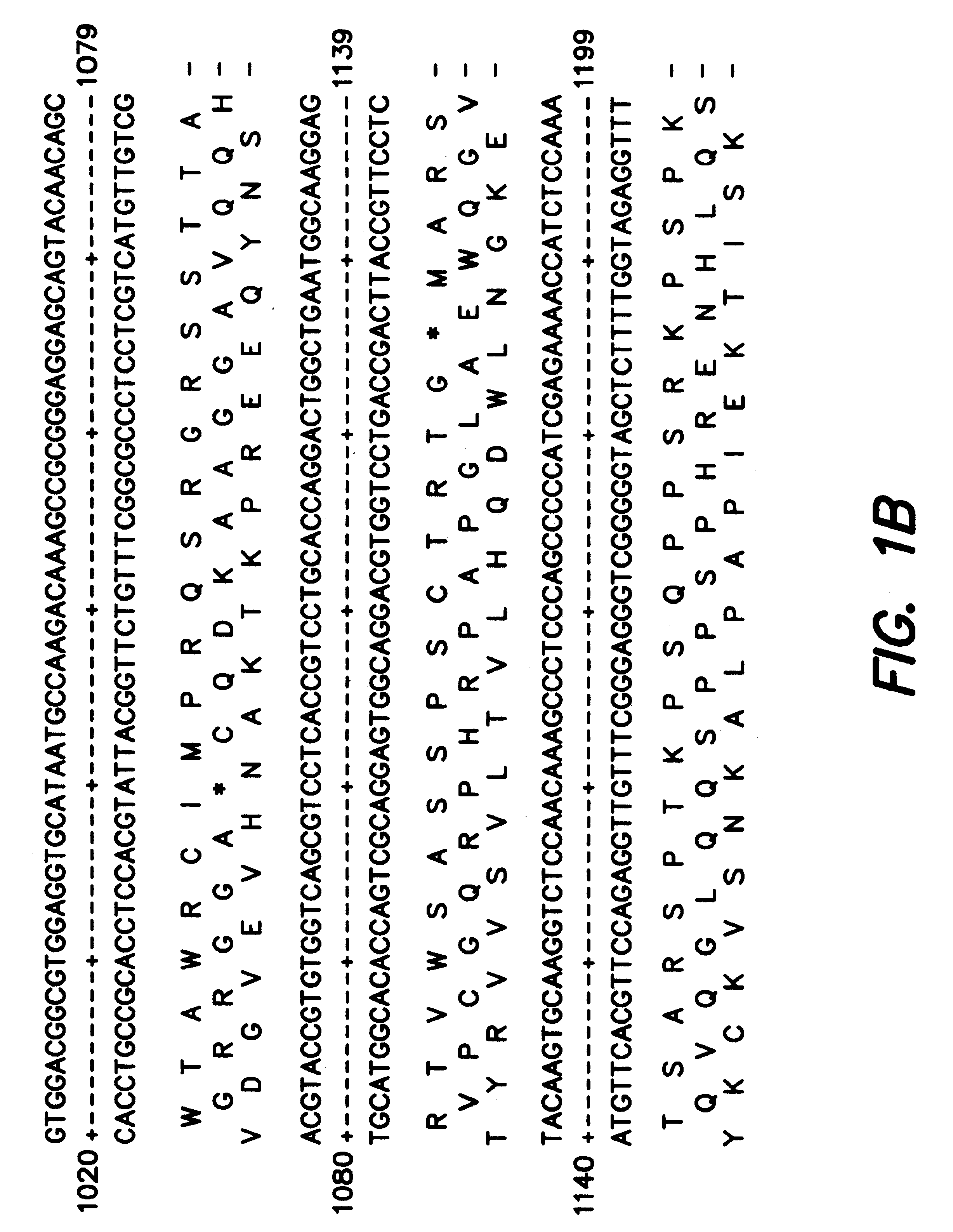
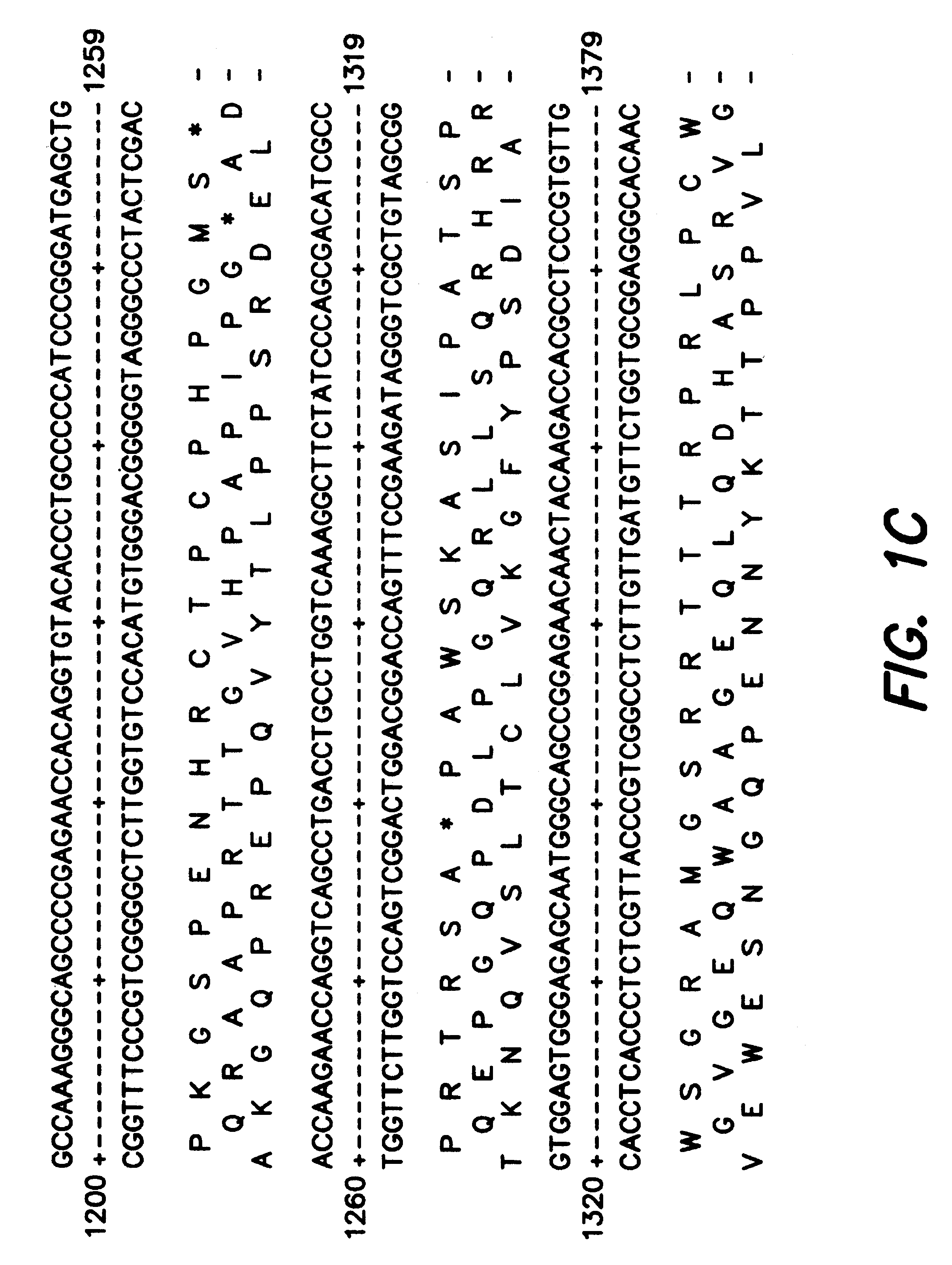
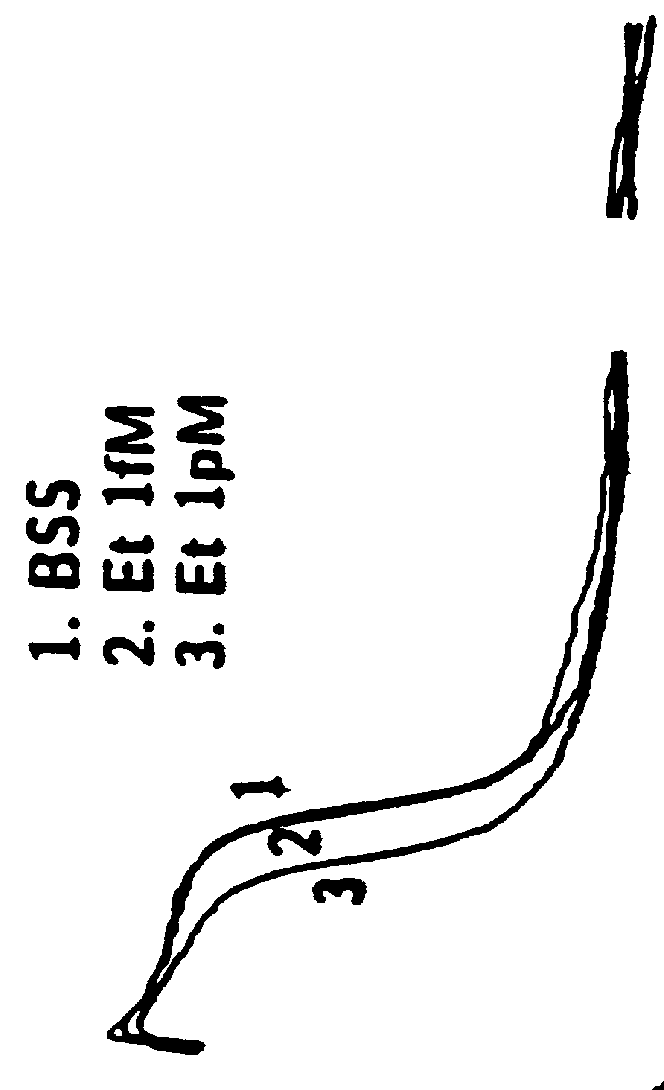
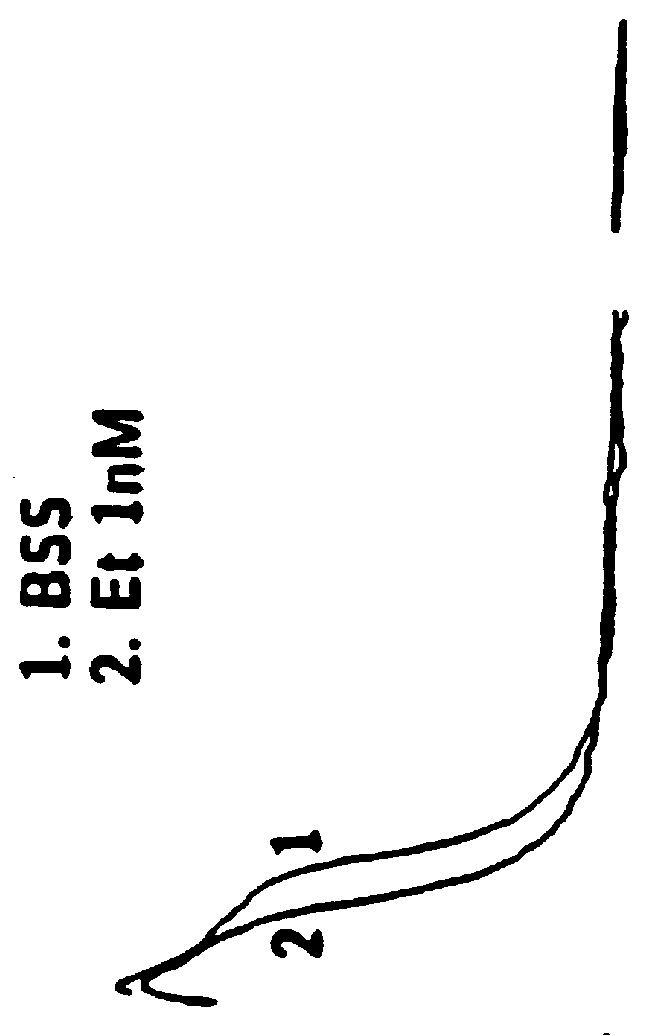
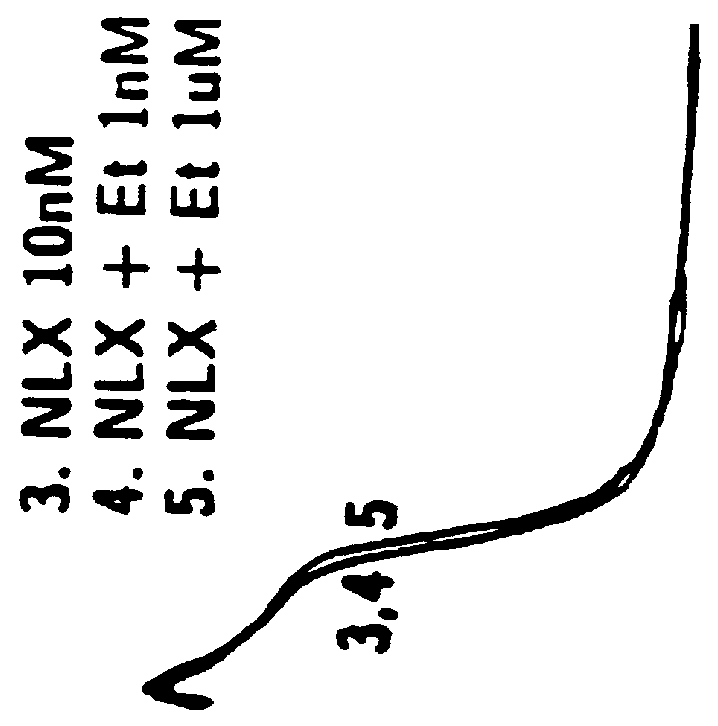
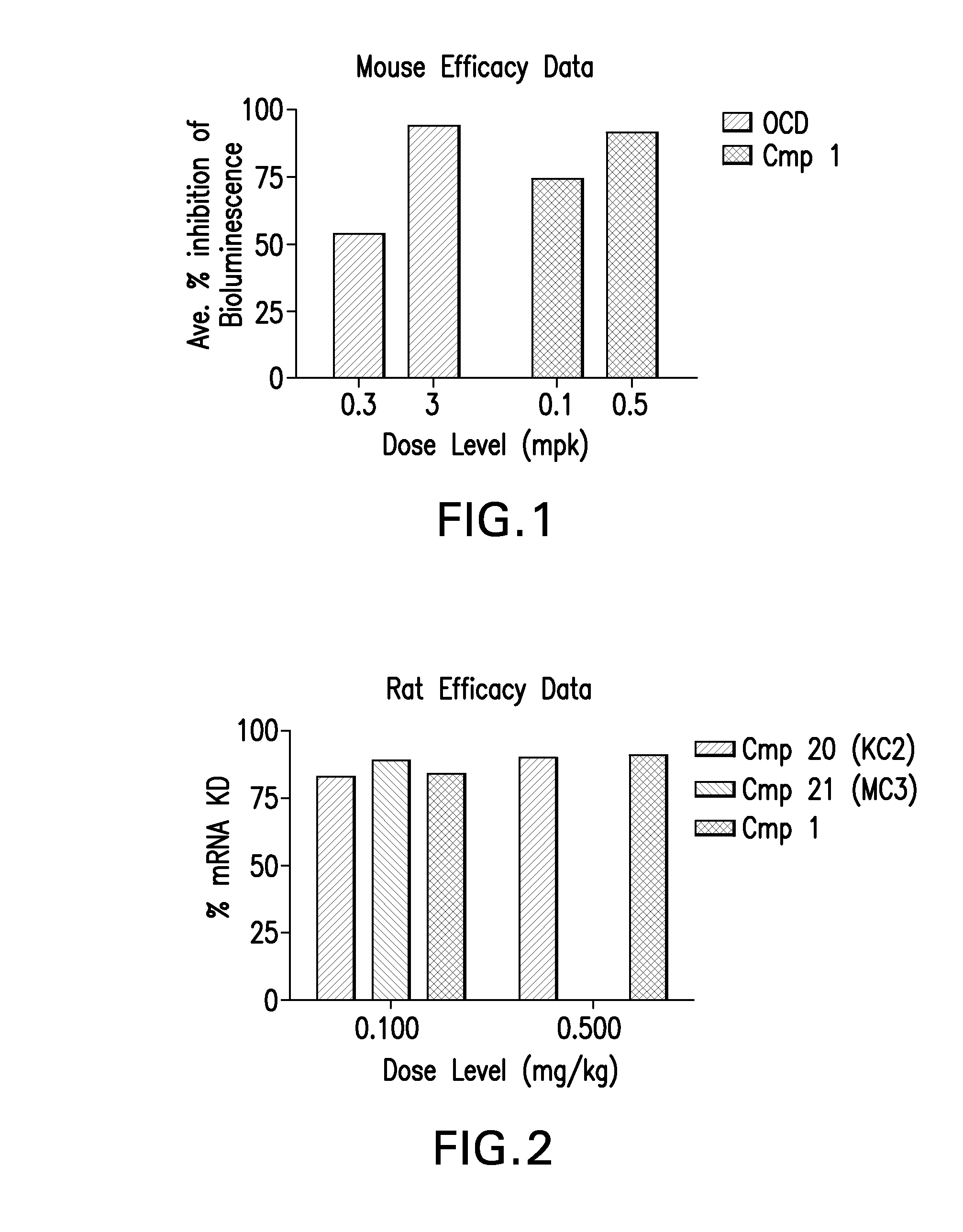
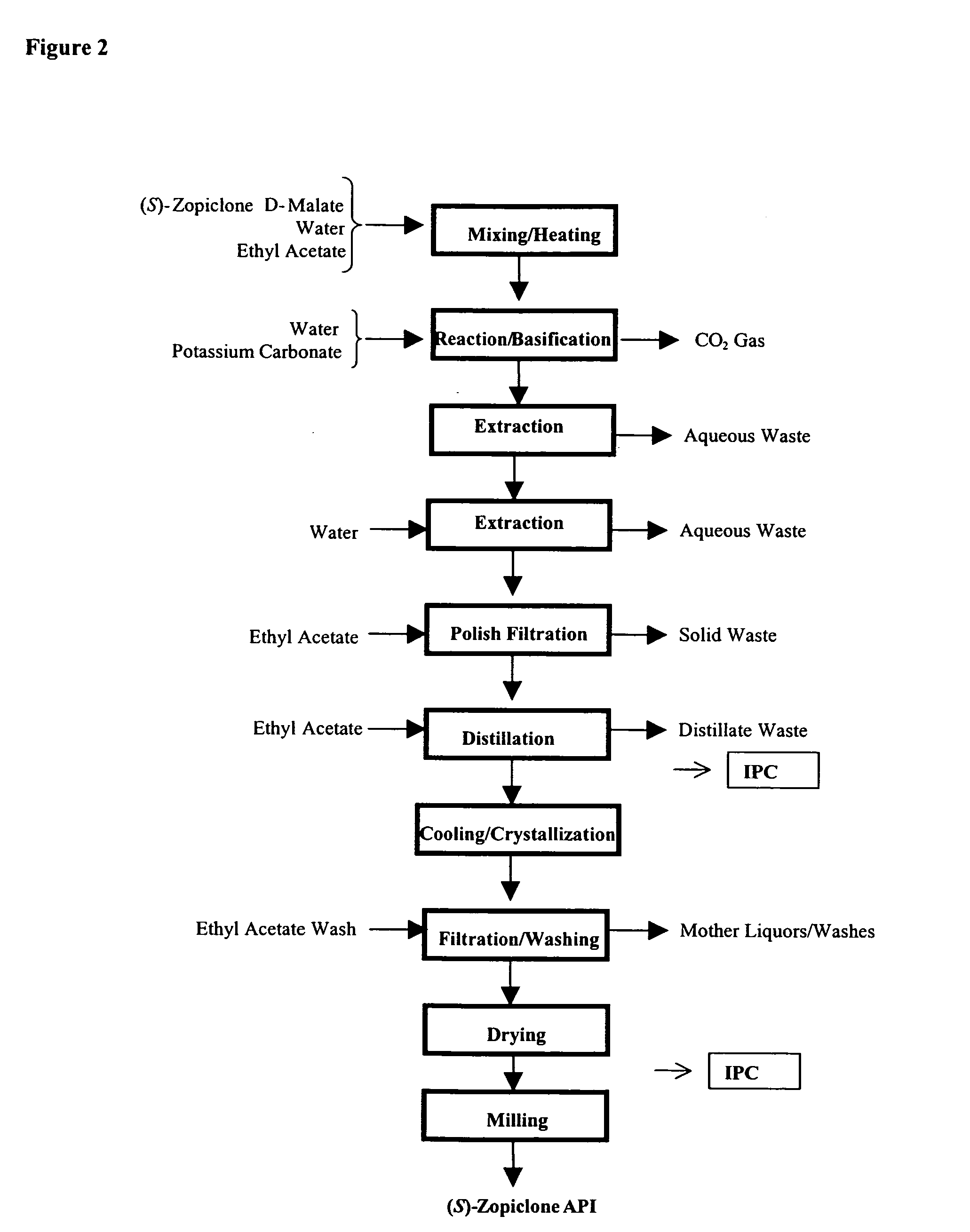
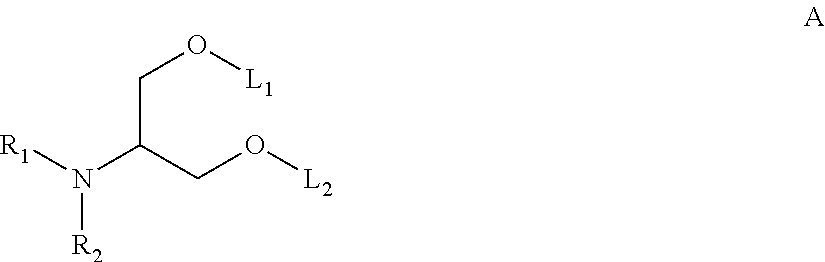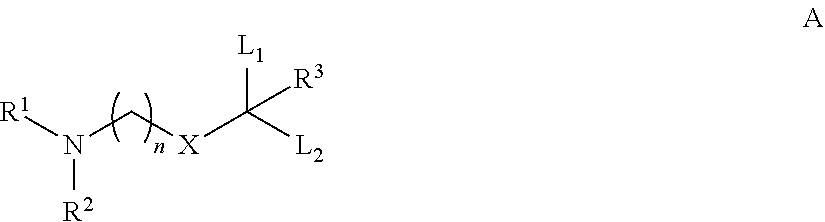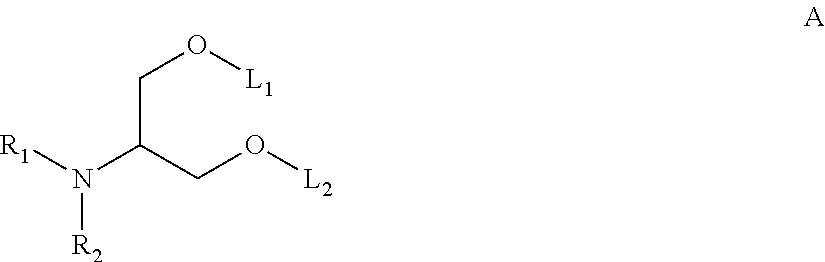Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
718 results about "Tolerability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tolerability refers to the degree to which overt adverse effects of a drug can be tolerated by a patient. Tolerability of a particular drug can be discussed in a general sense, or it can be a quantifiable measurement as part of a clinical study. Usually, it is measured by the rate of "dropouts", or patients that forfeit participation in a study due to extreme adverse effects. Tolerability, however, is often relative to the severity of the medical condition a drug is designed to treat. For instance, cancer patients will generally tolerate an immense amount of pain or discomfort during a chemotherapeutic study with the hope of prolonging survival or finding a cure, whereas patients experiencing a benign condition, such as a headache, will not.
Receptor specific transepithelial transport of therapeutics
InactiveUS6030613AEffective strategyImprove abilitiesPeptide/protein ingredientsAntibody mimetics/scaffoldsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
Receptor specific transepithelial transport of therapeutics
InactiveUS6485726B1Effective strategyImprove abilitiesBacterial antigen ingredientsPeptide/protein ingredientsAntigenTolerability
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:BRANDEIS UNIV +1
Herbicide resistance genes
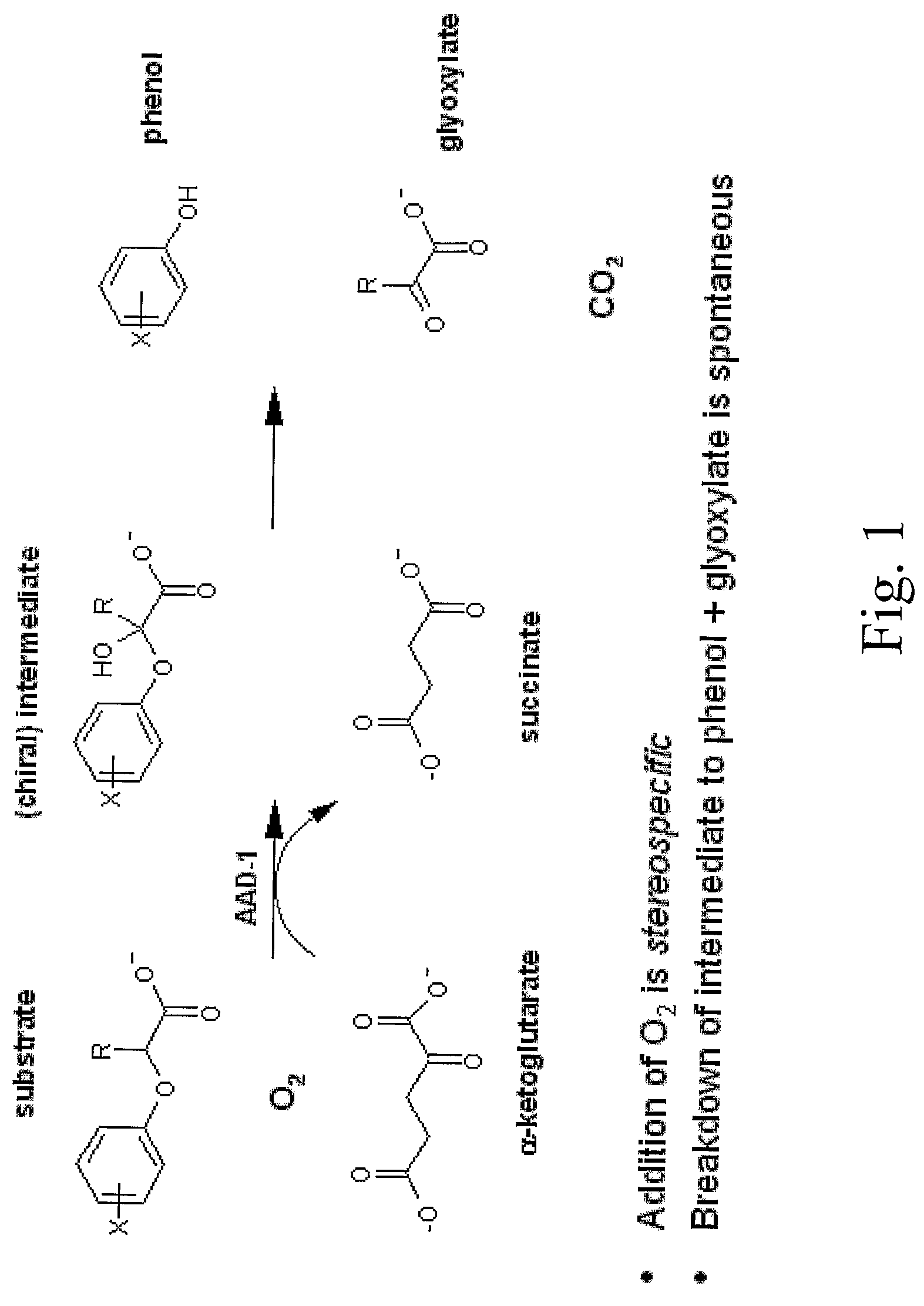
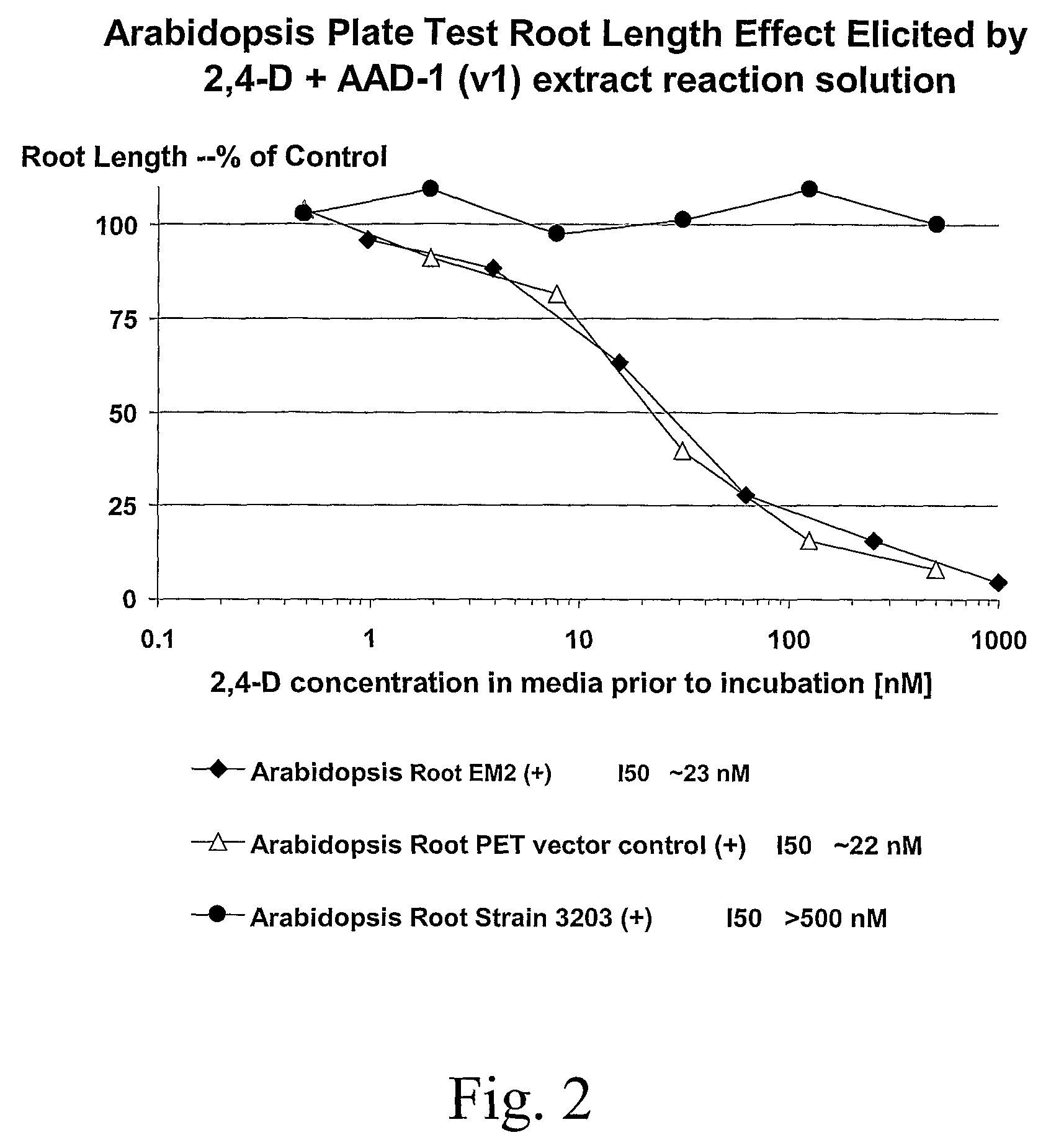
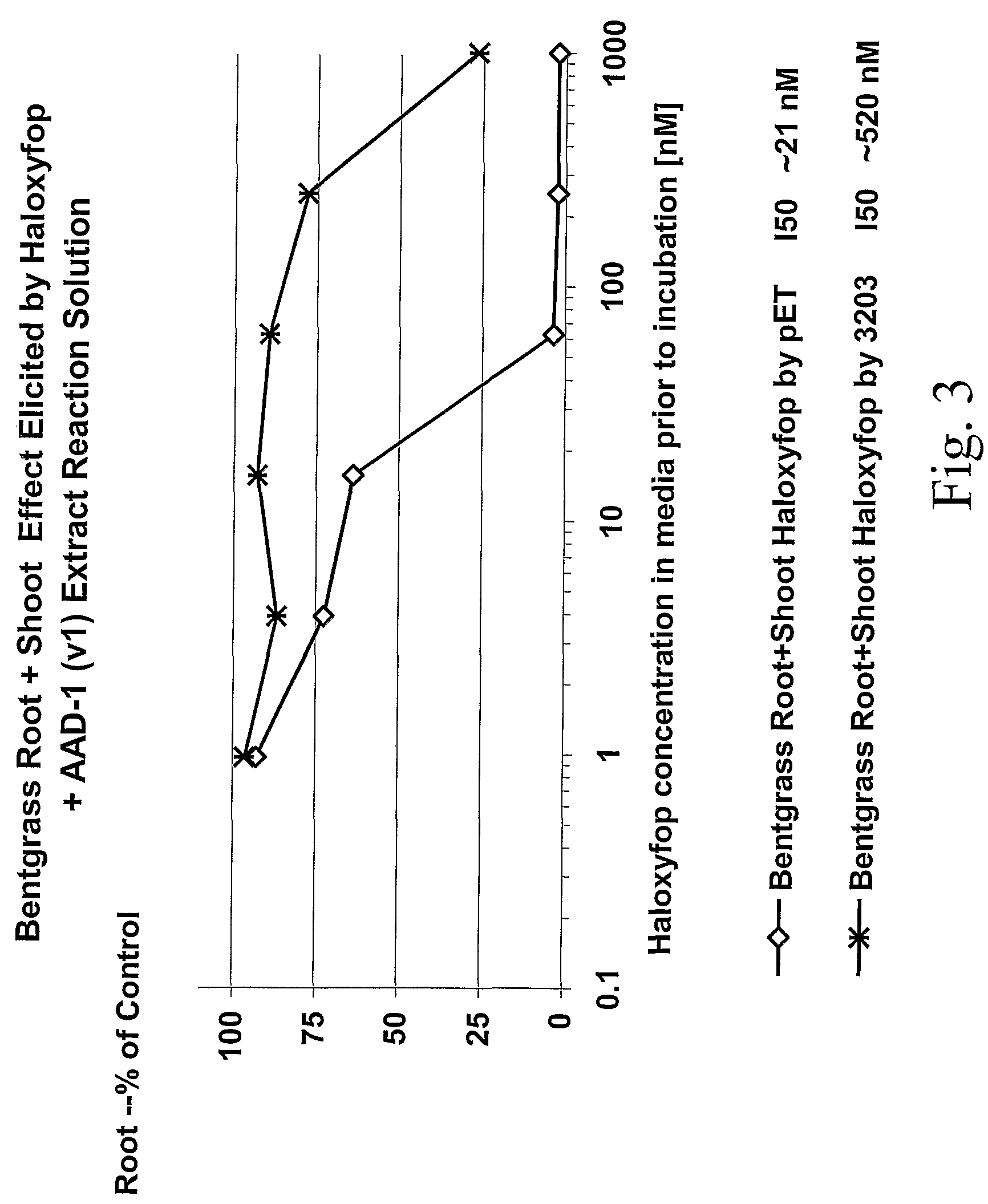
The subject invention provides novel plants that are not only resistant to 2,4-D and other phenoxy auxin herbicides, but also to aryloxyphenoxypropionate herbicides. Heretofore, there was no expectation or suggestion that a plant with both of these advantageous properties could be produced by the introduction of a single gene. The subject invention also includes plants that produce one or more enzymes of the subject invention alone or “stacked” together with another herbicide resistance gene, preferably a glyphosate resistance gene, so as to provide broader and more robust weed control, increased treatment flexibility, and improved herbicide resistance management options. More specifically, preferred enzymes and genes for use according to the subject invention are referred to herein as AAD (aryloxyalkanoate dioxygenase) genes and proteins. No α-ketoglutarate-dependent dioxygenase enzyme has previously been reported to have the ability to degrade herbicides of different chemical classes and modes of action. This highly novel discovery is the basis of significant herbicide tolerant crop trait opportunities as well as development of selectable marker technology. The subject invention also includes related methods of controlling weeds. The subject invention enables novel combinations of herbicides to be used in new ways. Furthermore, the subject invention provides novel methods of preventing the formation of, and controlling, weeds that are resistant (or naturally more tolerant) to one or more herbicides such as glyphosate.
Owner:CORTEVA AGRISCIENCE LLC
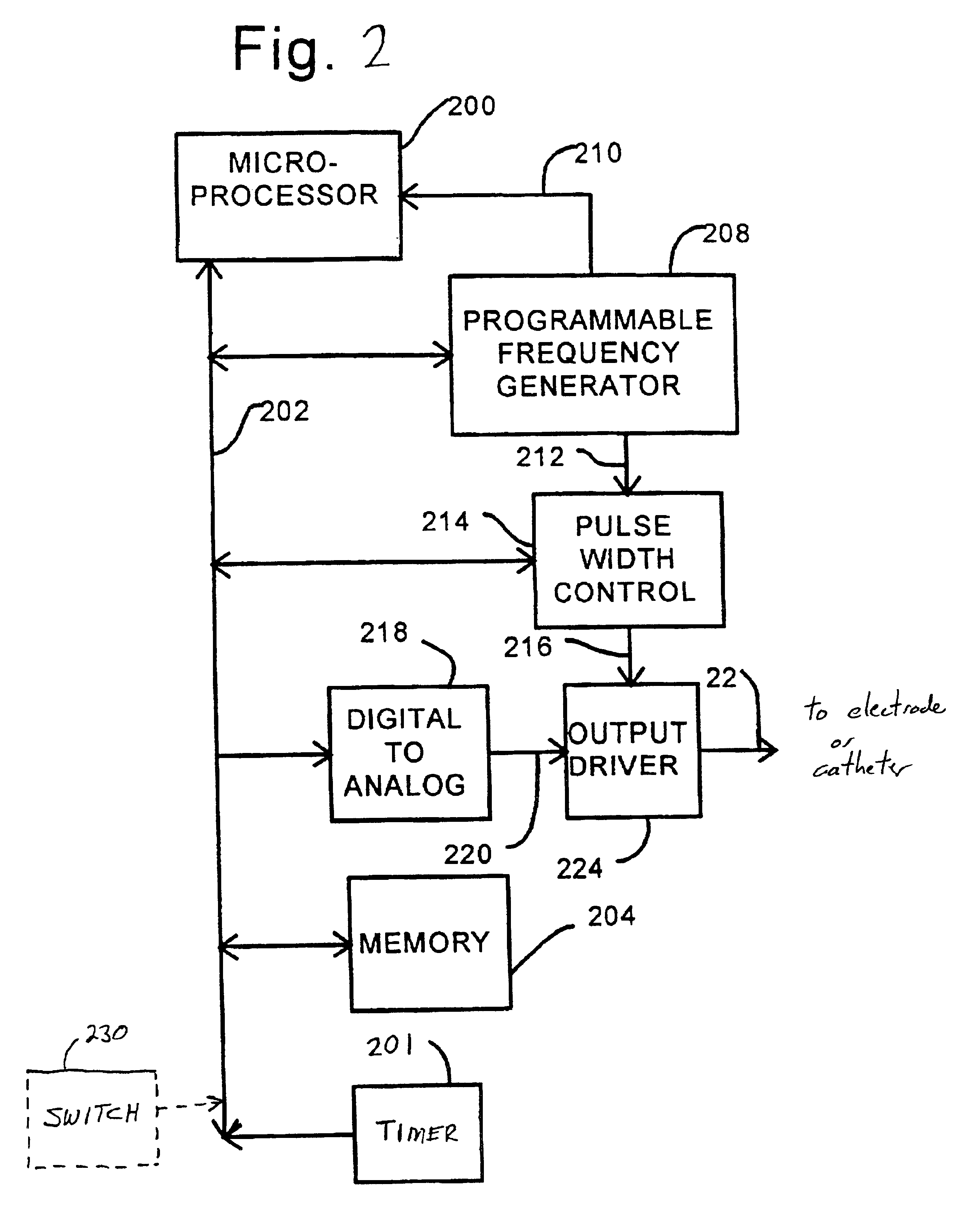
Therapeutic treatment of disorders based on timing information
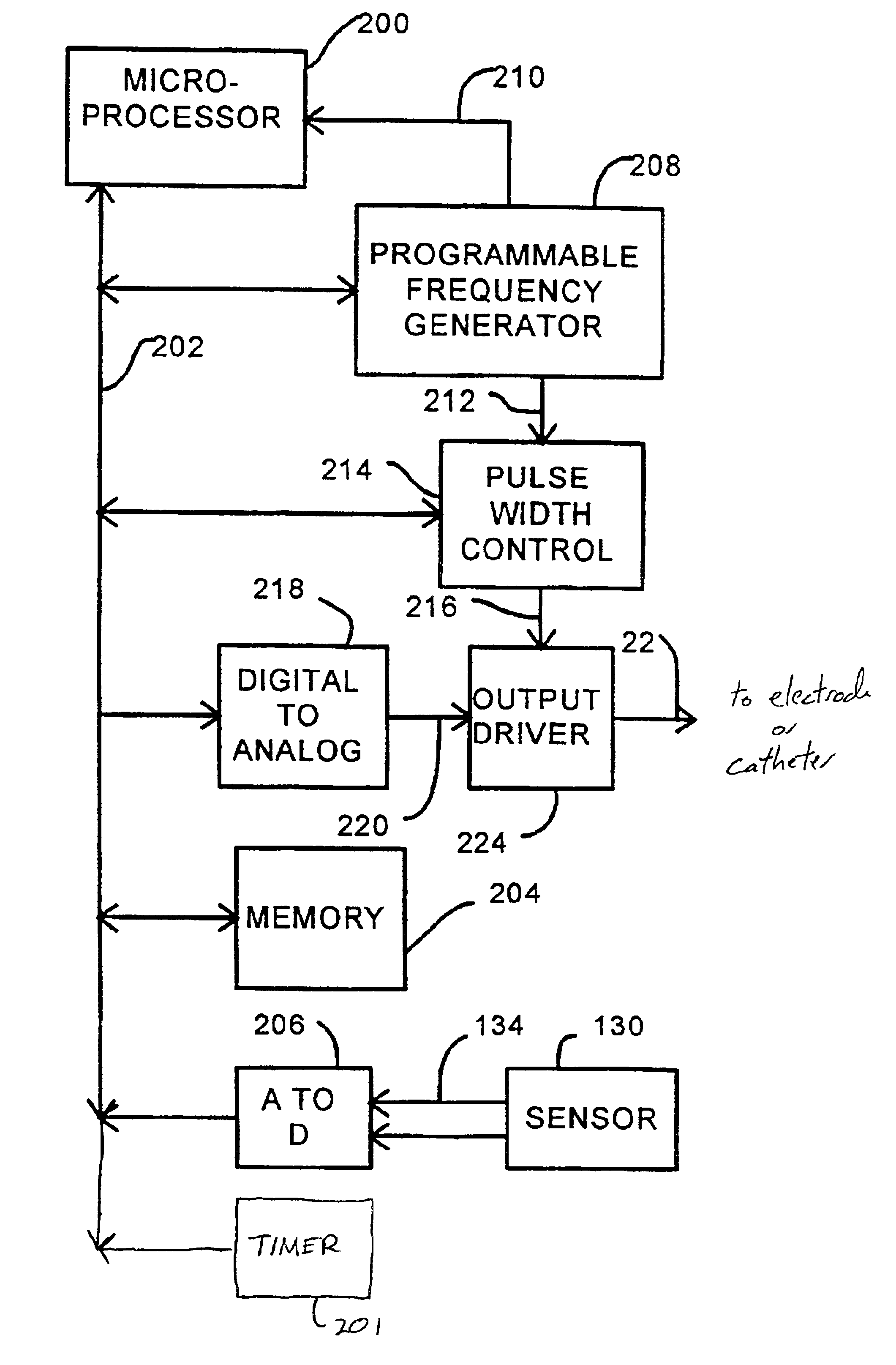
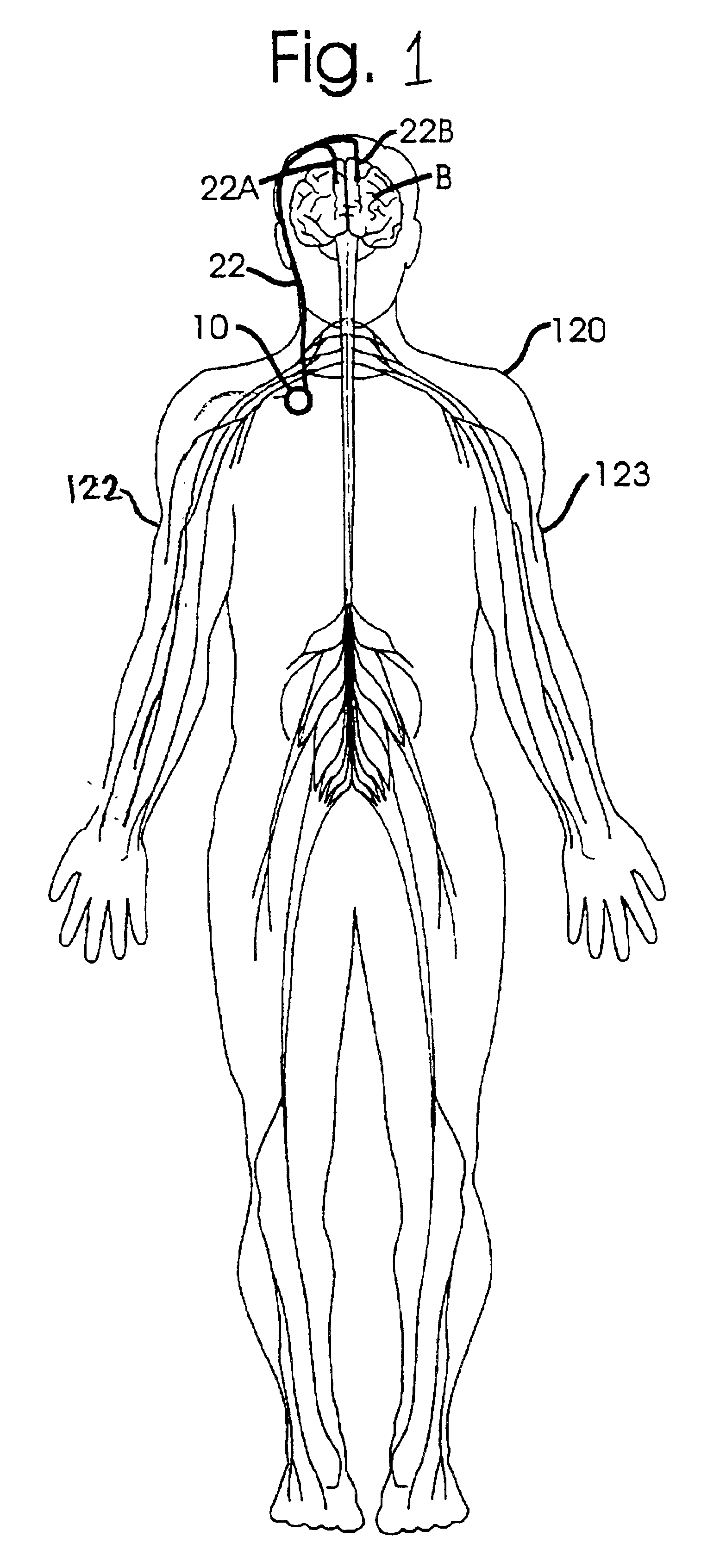
Disclosed are techniques for operation of neurostimulation or drug delivery devices to stop treatment therapy during times when the patient does not need to be treated. Advantageously, the present invention reduces battery usage and / or drug dosage during periods when treatment therapy need not be provided. Further, the present invention slows or reduces the tolerance the patient may develop from the electrical stimulation or treatment therapy. In one embodiment, the present invention includes a timer or a real time clock for shutting off the device during periods when the patient is sleeping in accordance with a preset schedule. The present invention preferably turns off after the patient has fallen asleep and right before the patient has awakened. Alternatively, the invention may include a sensor for sensing conditions indicative of whether the patient is awake or asleep. This sensed information may also be used to determine whether the treatment therapy should be delivered or stopped.
Owner:MEDTRONIC INC
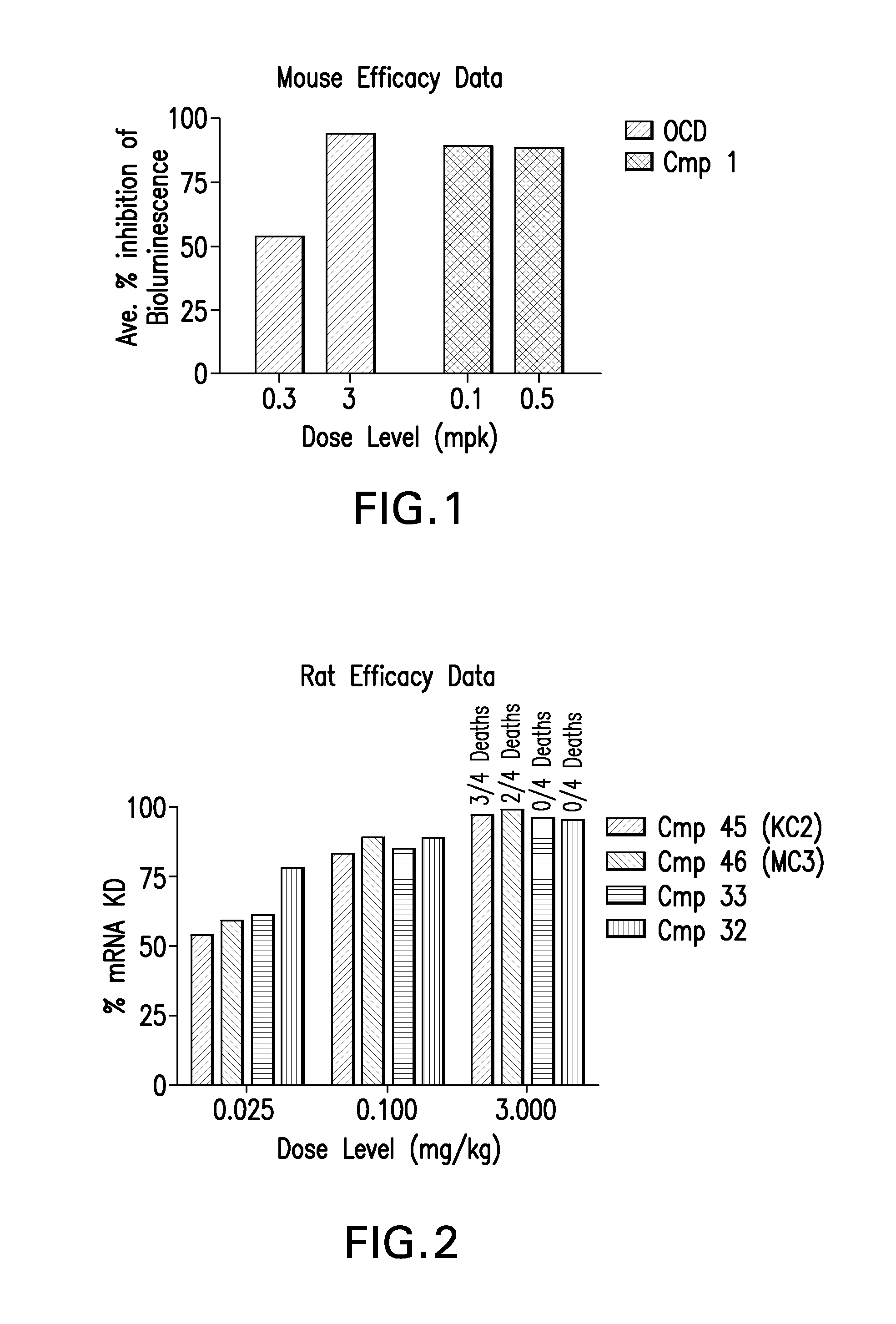
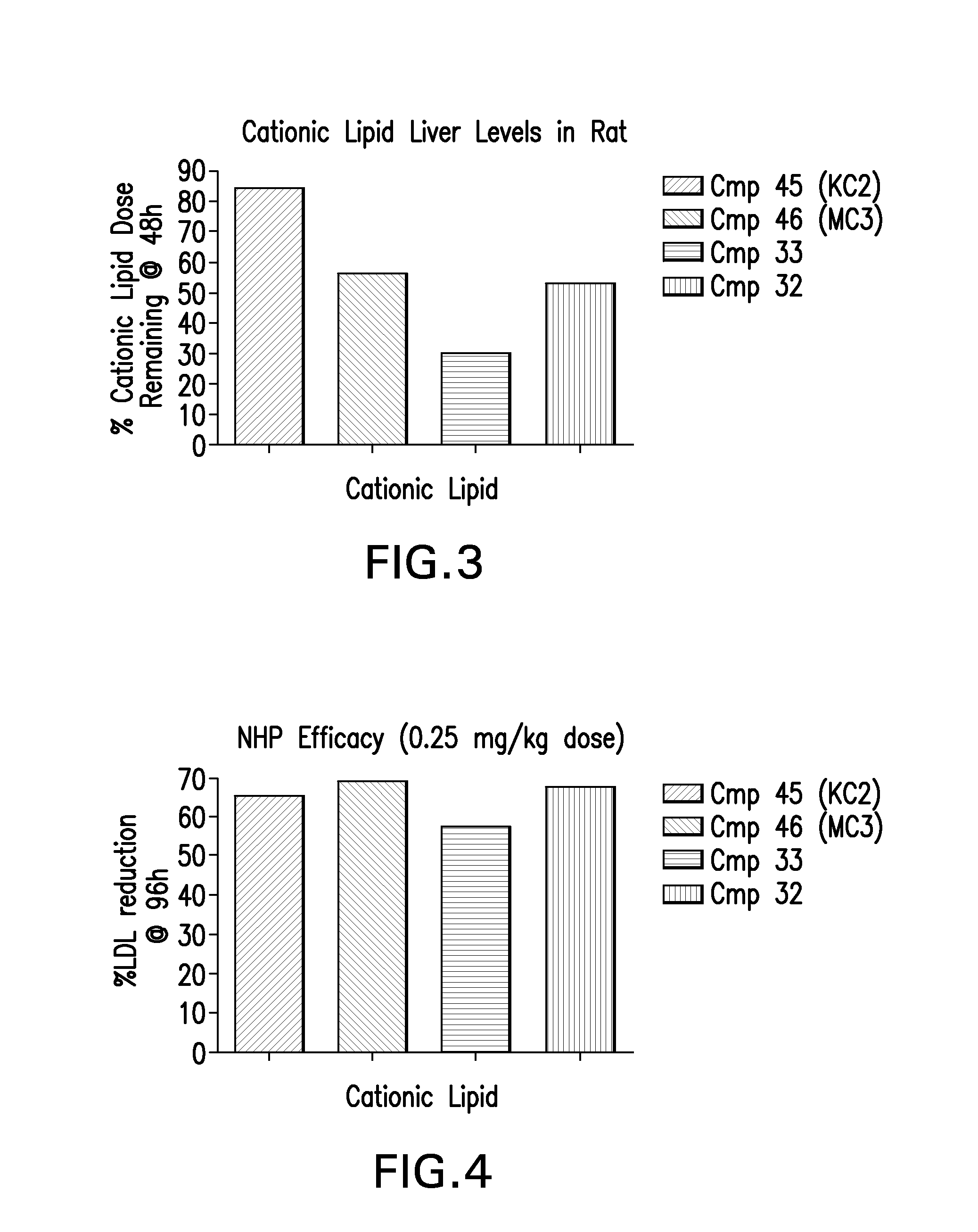
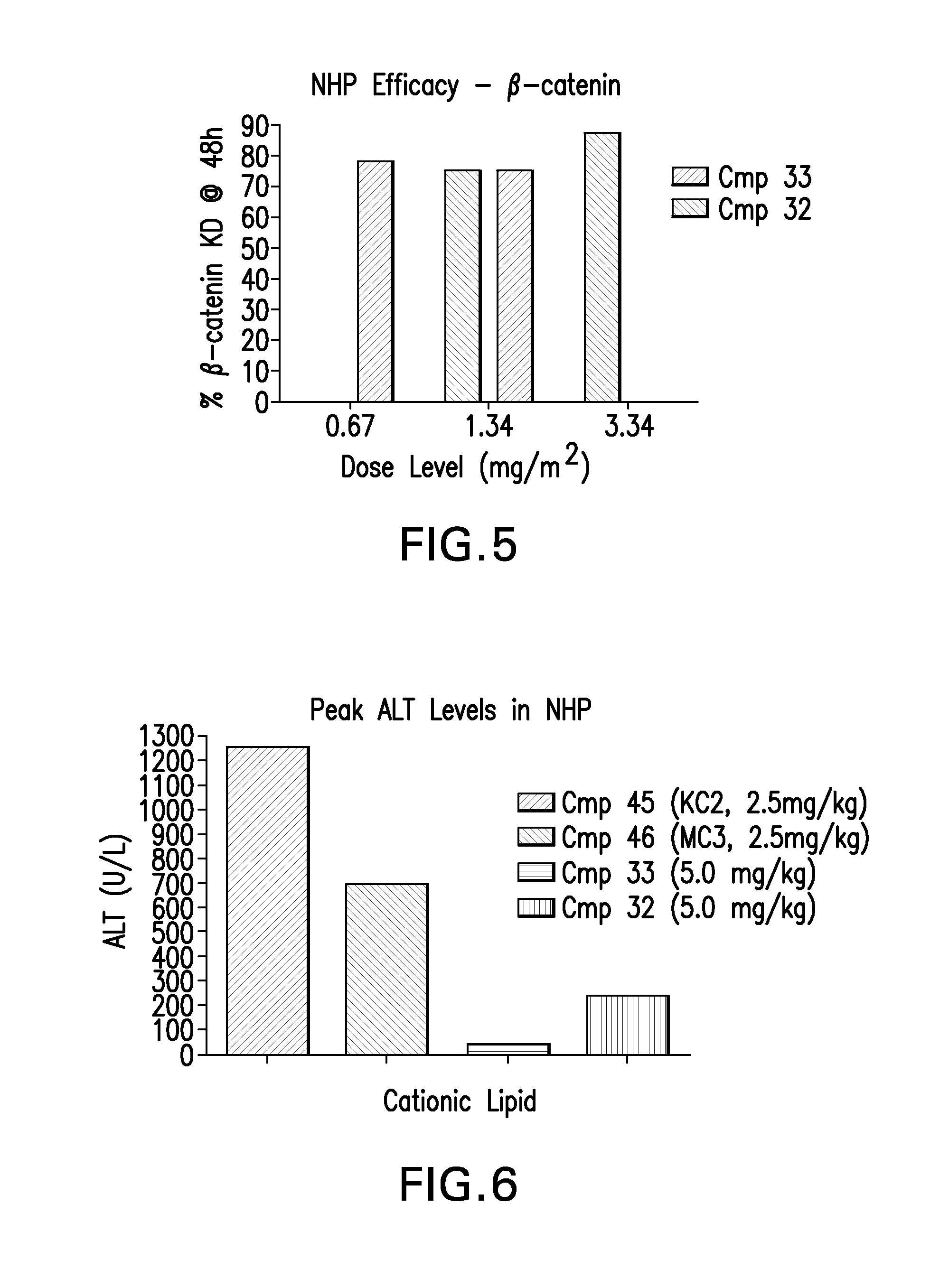
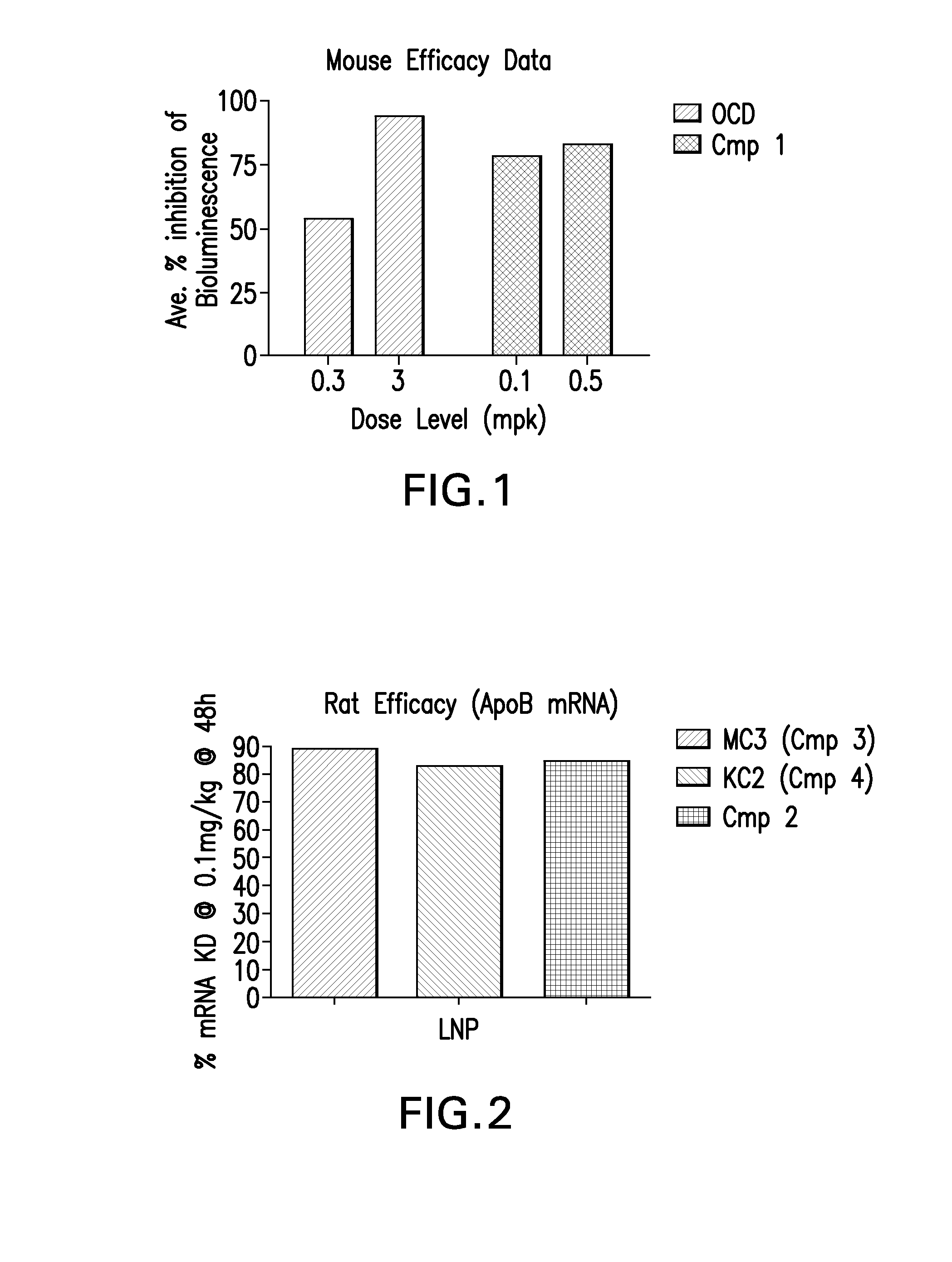
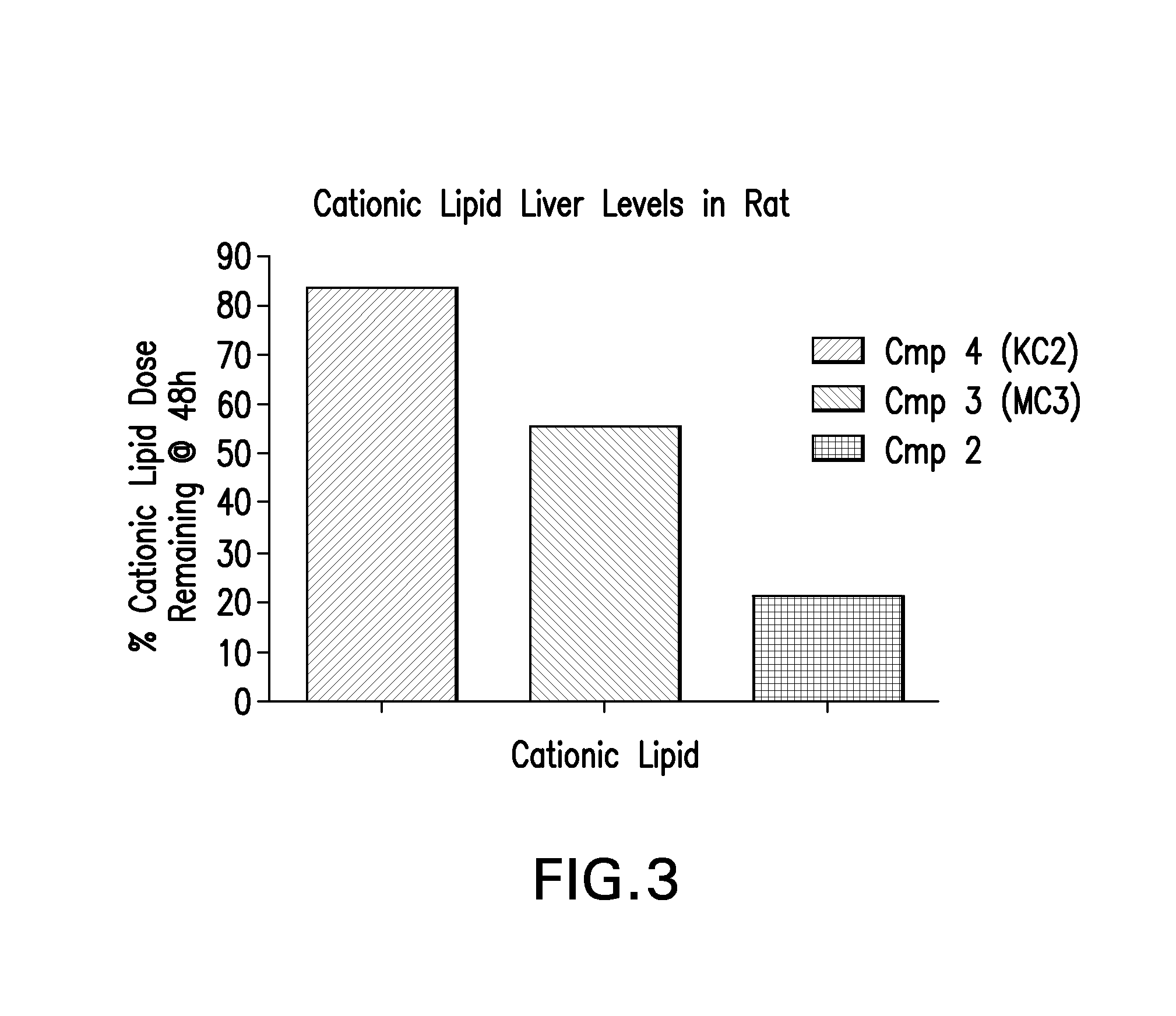
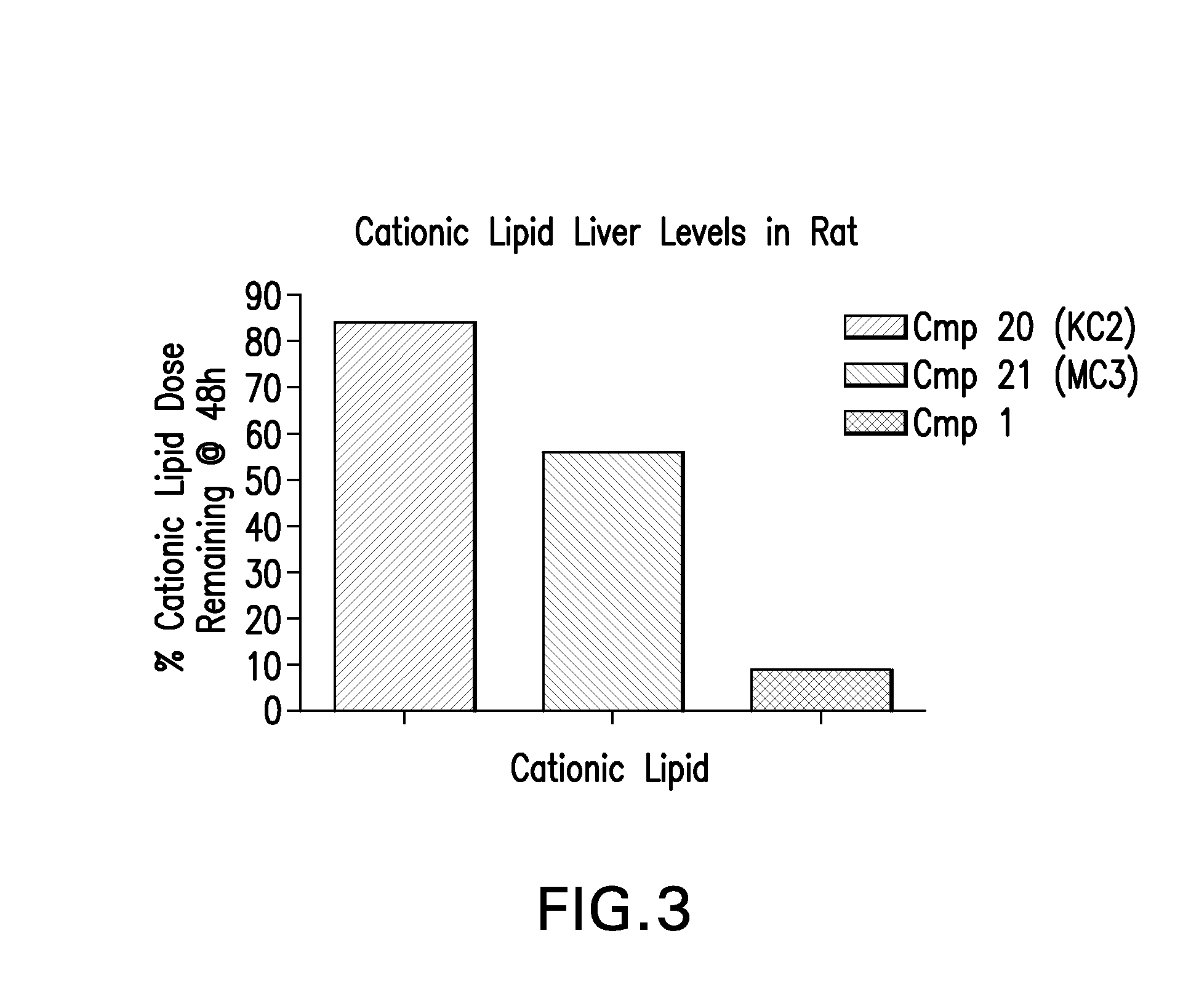
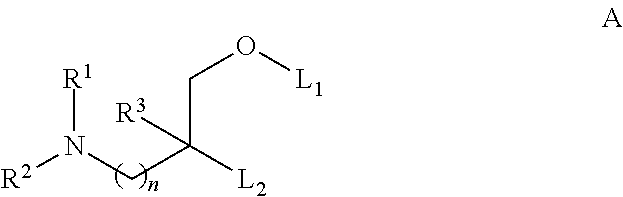
Novel low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS20130178541A1Good curative effectReduce liver toxicityBiocideMicroencapsulation basedTolerabilityNanoparticle
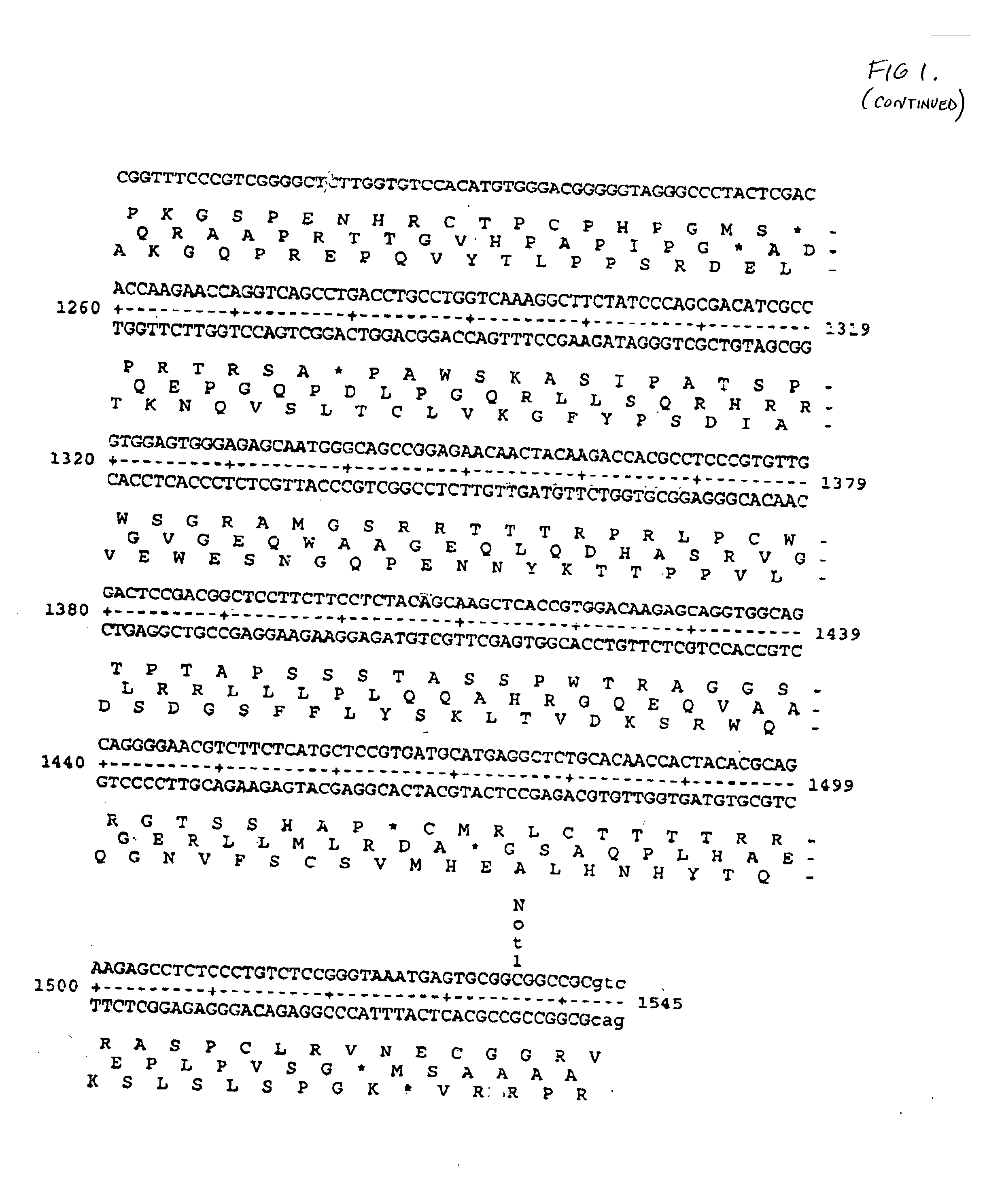
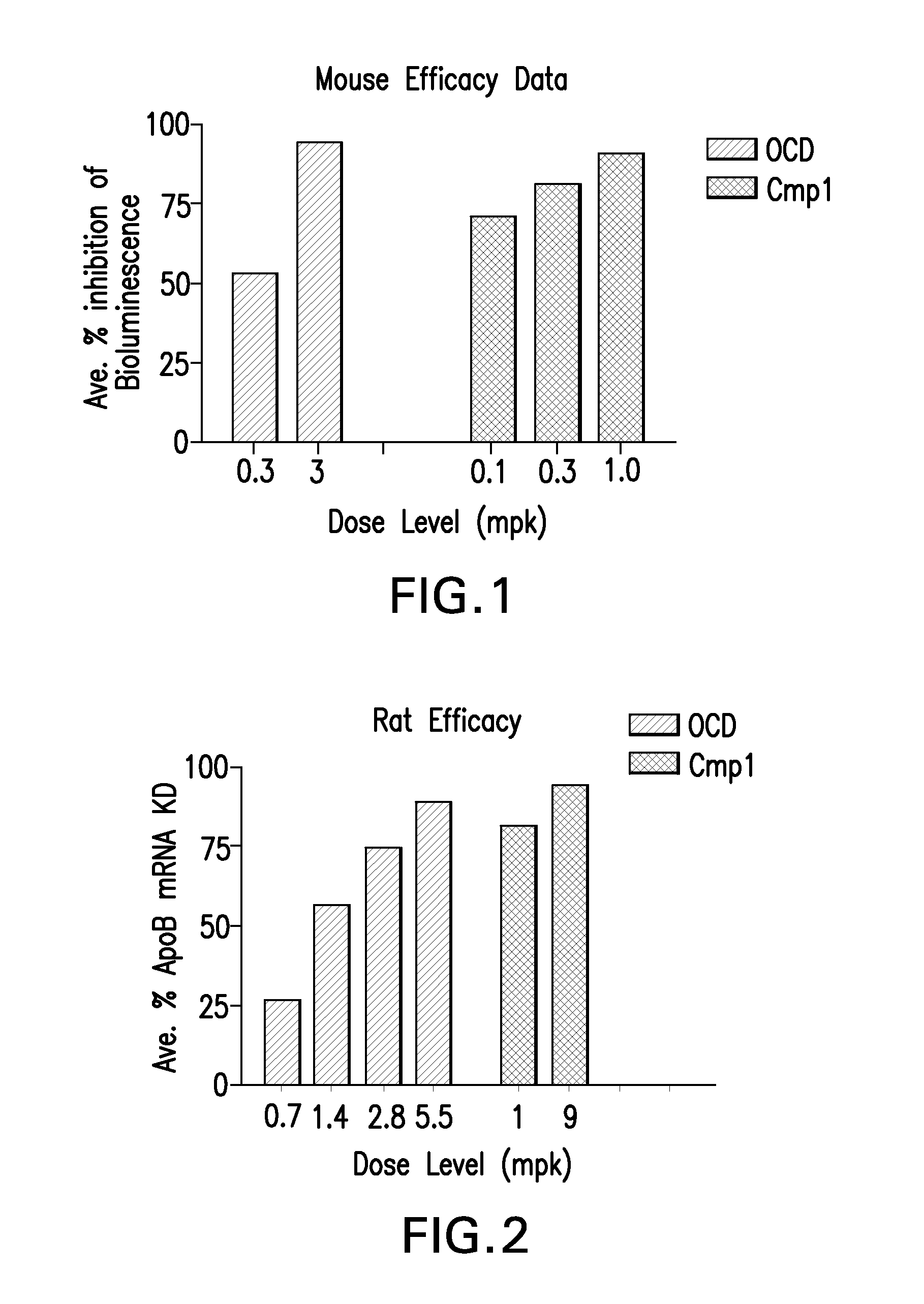
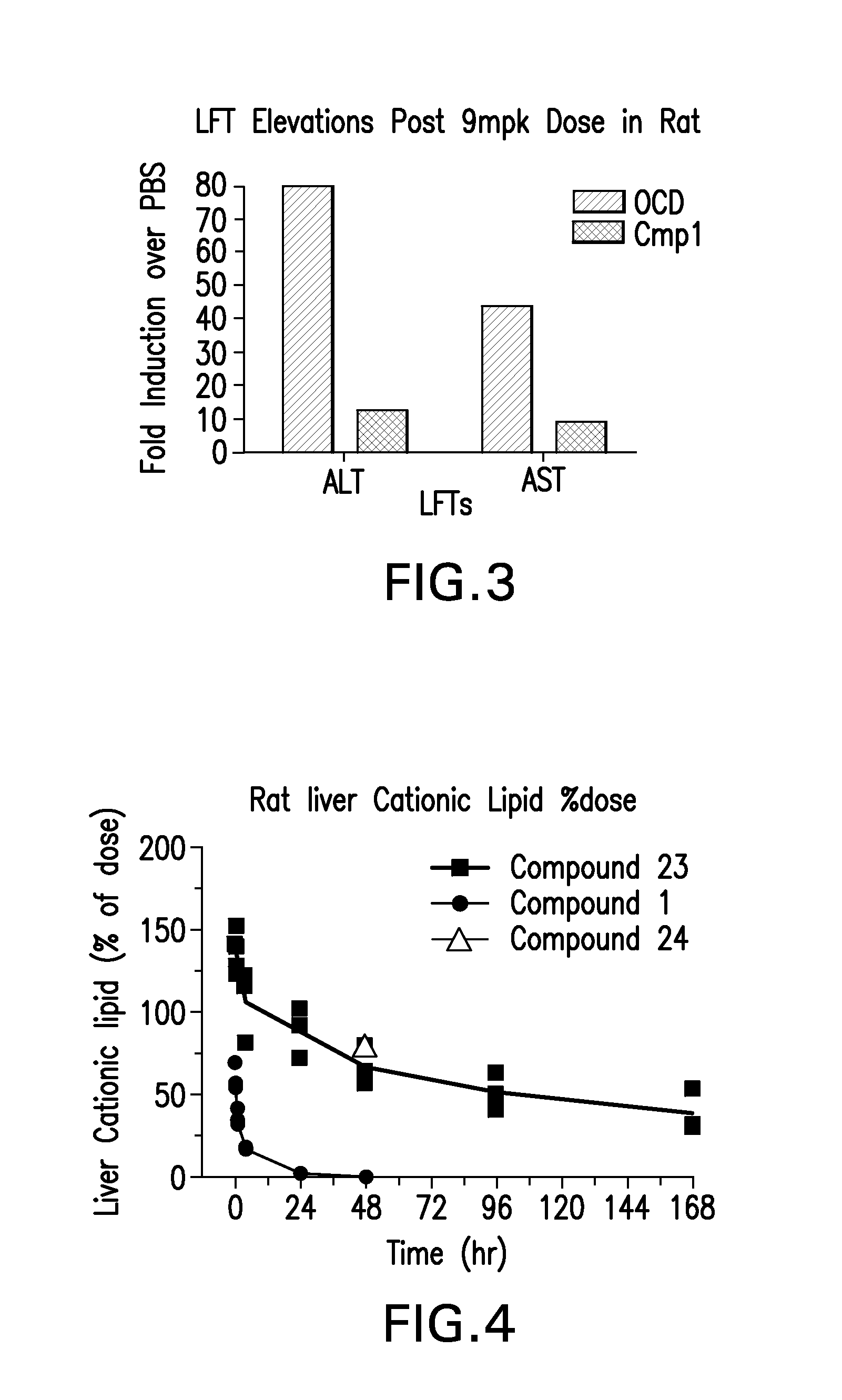
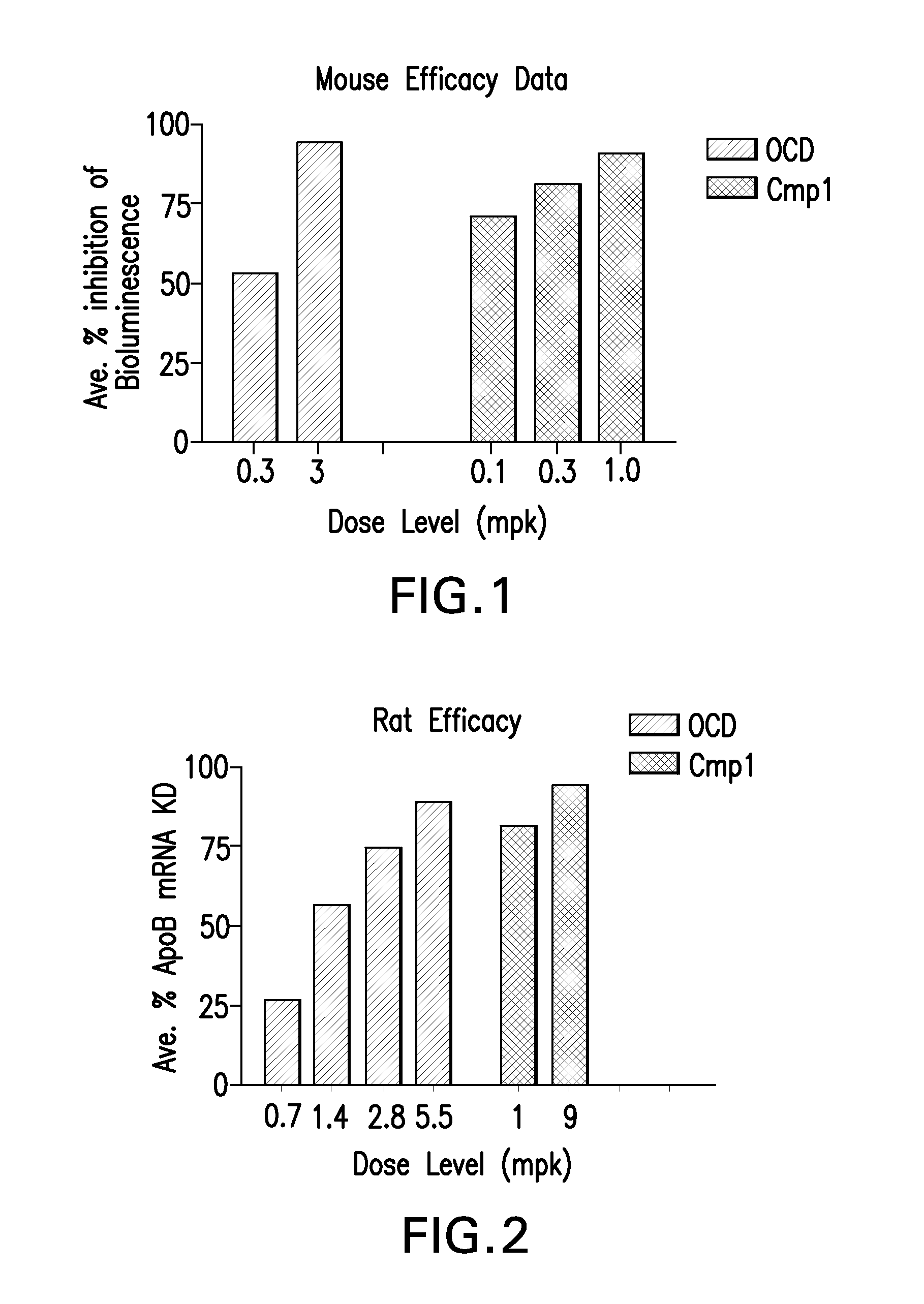
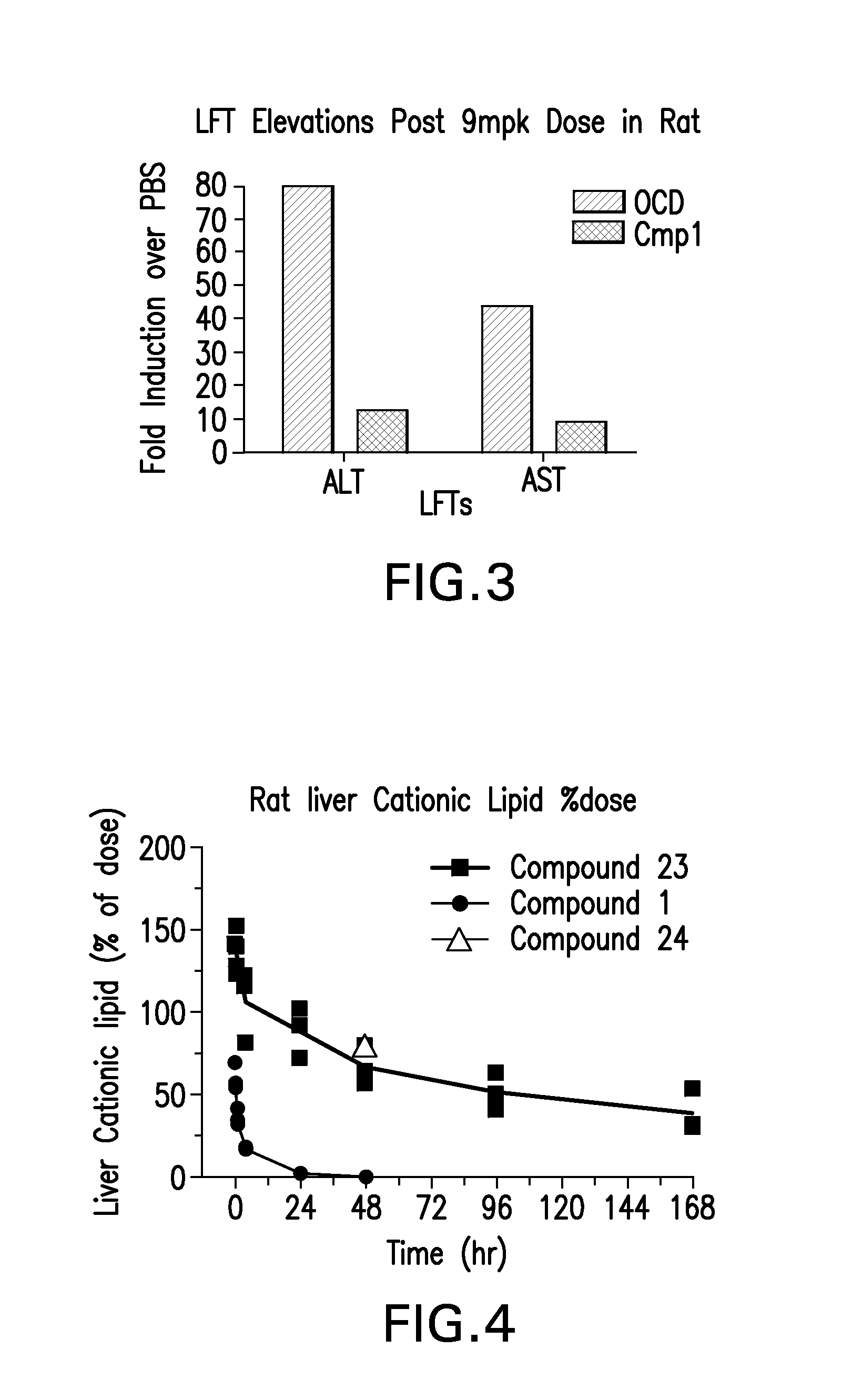
The instant invention provides for novel catiomc lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
Receptor specific transepithelial transport of therapeutics
The present invention relates in general to methods and products for initiating an immune response against an antigen, and in particular relates to transepithelial delivery of antigens to provoke tolerance and immunity. The present invention further relates to methods and products for the transepithelial delivery of therapeutics. In particular, the invention relates to methods and compositions for the delivery of therapeutics conjugated to a FcRn binding partner to intestinal epithelium, mucosal epithelium and epithelium of the lung. The present invention further relates to the synthesis, preparation and use of the FcRn binding partner conjugates as, or in, pharmaceutical compositions for oral systemic delivery of drugs and vaccines.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC +1
Combination therapy for depression, prevention of suicide, and various medical and psychiatric conditions
InactiveUS7973043B2Preventing disease progression/modifyingDelaying/preventing relapseBiocideNervous disorderInitial treatmentTherapy resistant
The present invention relates to a new method of treatment for persons meeting diagnoses for major depressive disorder, or other unipolar (non-bipolar, non-psychotic and non-treatment resistant) depression. The method comprises administering a combination of two categories of drugs, antipsychotics or dopamine system stabilizers, in combination with a newer antidepressant such as a selective serotonin reuptake inhibitor, as initial treatment or as soon as possible. The method targets the prevention of suicide, and provides other benefits including preventing disease progression development of tolerance toward the antidepressants. Another aspect of the invention relates to using the method for alleviating cognitive distortion and related functional impairment or health risks, and / or using the method for smoking cessation or nicotine withdrawal.
Owner:MIGALY PETER
Novel Low Molecular Weight Cationic Lipids for Oligonucleotide Delivery
ActiveUS20130090372A1Good curative effectReduce liver toxicityOrganic compound preparationOther foreign material introduction processesTolerabilityNanoparticle
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
Novel Low Molecular Weight Cationic Lipids For Oligonucleotide Delivery
ActiveUS20130274504A1Good curative effectReduce liver toxicityOrganic active ingredientsOrganic chemistryTolerabilityNanoparticle
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids comprising at least one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
Method of simultaneously enhancing analgesic potency and attenuating dependence liability caused by exogenous and endogenous opioid agonists
InactiveUSRE36547E1Enhance analgesic potencyDecrease dependence liabilityCompound screeningBiocideEndogenous OpiatesNervous system
This invention relates to a method of selectively enhancing the analgesic potency of morphine and other clinically used bimodally-acting opioid agonists and simultaneously attenuating development of physical dependence, tolerance and other undesirable side effects caused by the chronic administration of said bimodally-acting opioid agonists comprising the co-administration of a bimodally-acting opioid agonist which activates both inhibitory and excitatory opioid receptor-mediated functions of neurons in the nociceptive (pain) pathways of the nervous system and an opioid receptor antagonist which selectively inactivates excitatory opioid receptor-mediated side effects. This invention also relates to a method of using excitatory opioid receptor antagonists alone to block the undesirable excitatory side effects of endogenous bimodally-acting opioid agonists which may be markedly elevated during chronic pain. This invention further relates to a method of long-term treatment of previously detoxified opiate, cocaine and alcohol addicts utilizing said excitatory opioid receptor antagonists, either alone or in combination with low-dose methadone, to prevent protracted physical dependence, and to compositions comprising an excitatory opioid receptor antagonist of the invention and a bimodally-acting opioid agonist.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS20130274523A1Good curative effectReduce liver toxicityPowder deliveryNanotechTolerabilityCholesterol
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
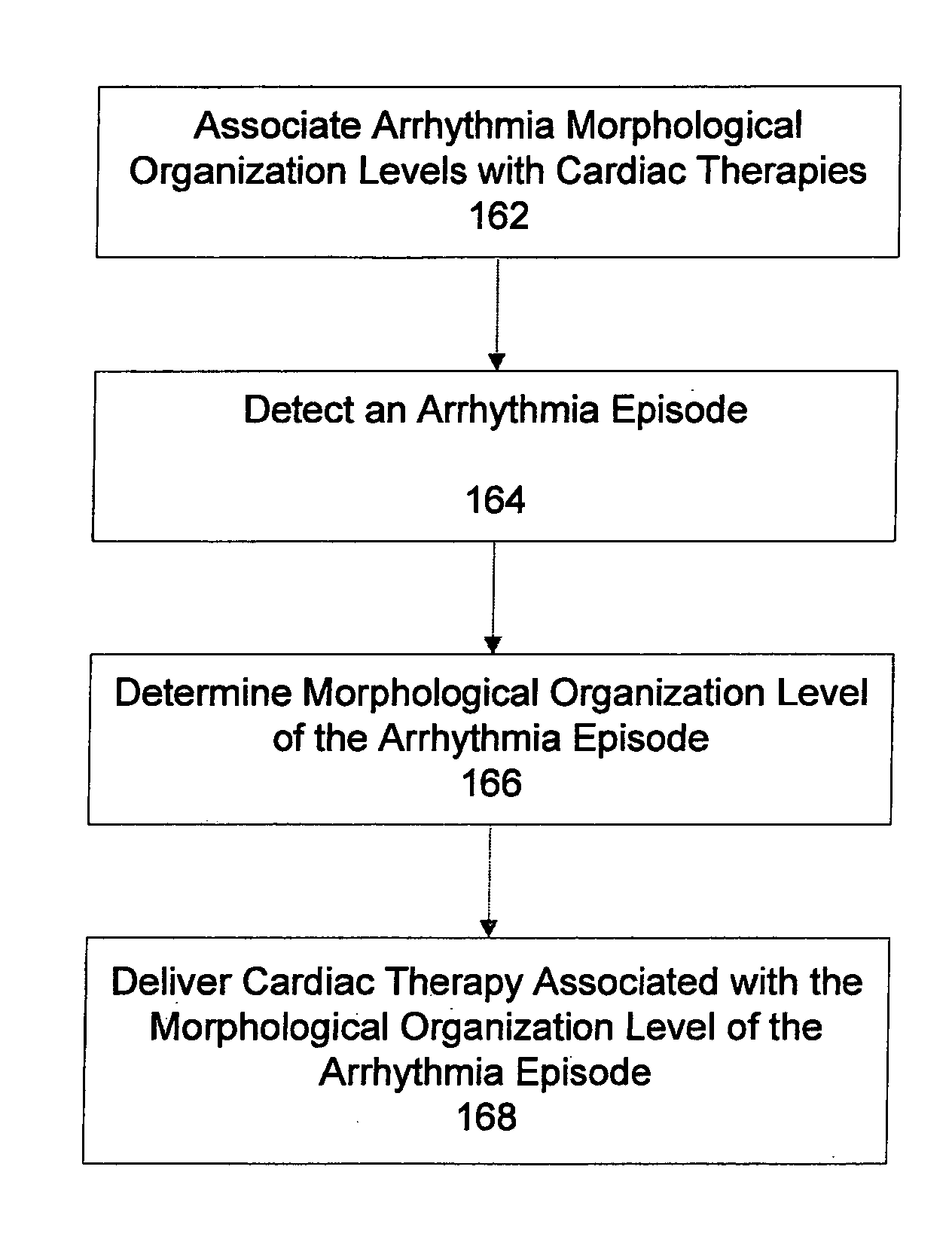
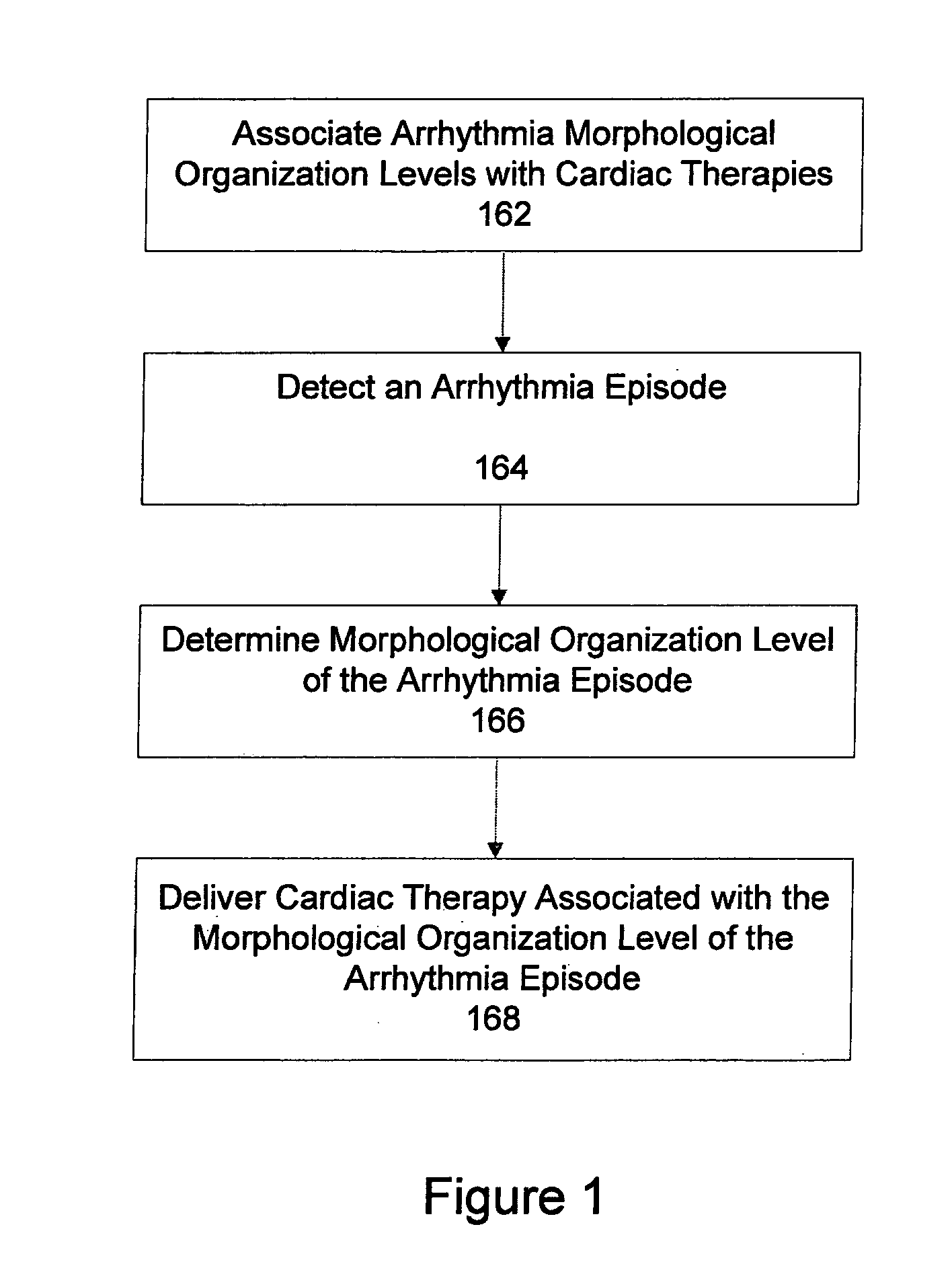
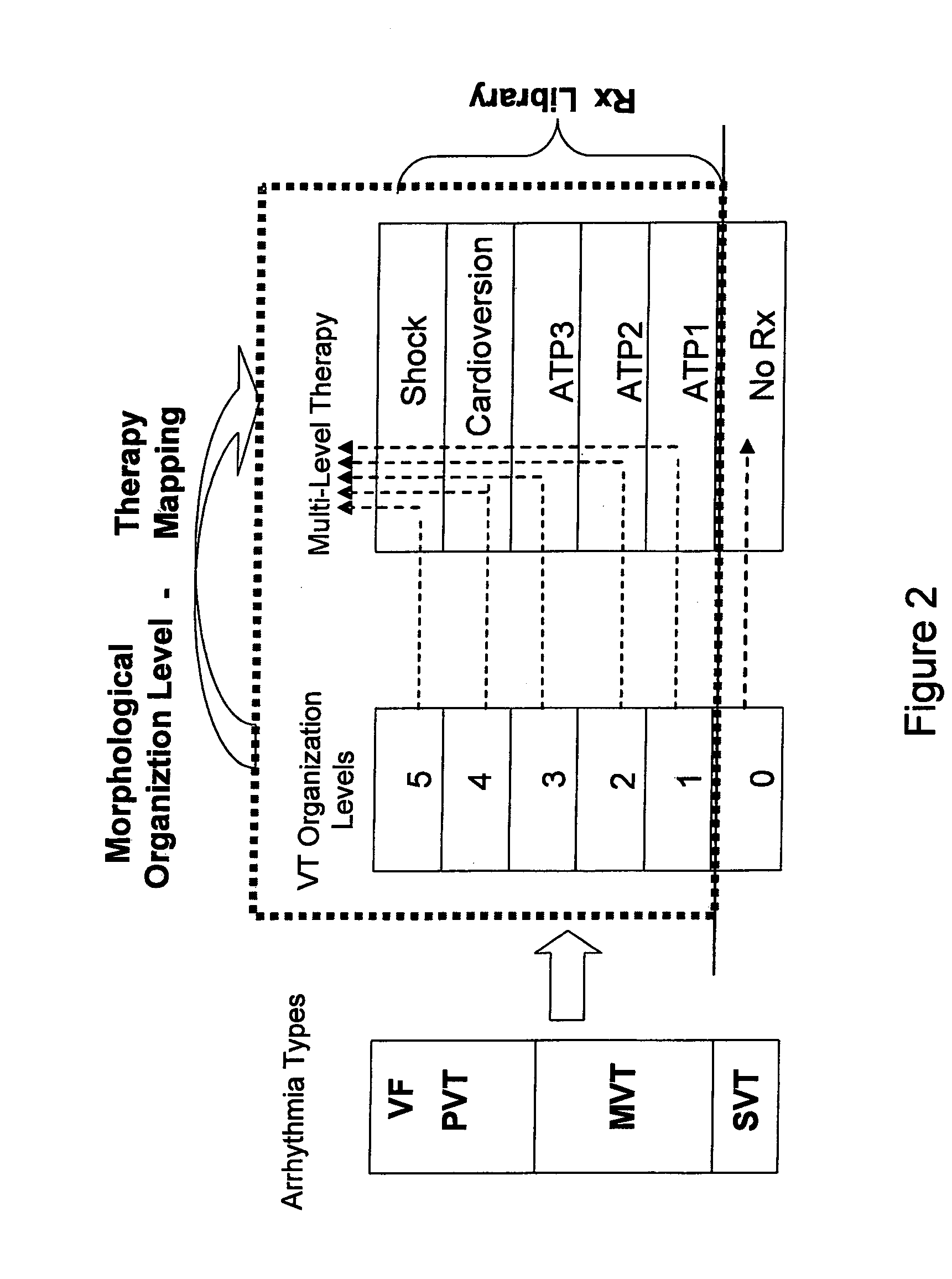
Automatic multi-level therapy based on morphologic organization of an arrhythmia
Methods and systems for selecting tachyarrhythmia therapy based on the morphological organization level of the arrhythmia are described. Morphological organization levels of arrhythmias are associated with cardiac therapies. The morphological organization levels are related to cardiac signal morphologies of the arrhythmias. An arrhythmia episode is detected and the morphological organization level of the arrhythmia episode is determined. A cardiac therapy associated with the morphological organization level of the arrhythmia episode is delivered to treat the arrhythmia. For example, the morphological organization levels may be associated with the cardiac therapies based on one or more of retrospective database analysis patient therapy tolerance, and physician input. The associations may be static or may be dynamically adjusted based on therapy efficacy.
Owner:CARDIAC PACEMAKERS INC
Methods and compositions for the prevention of tolerance to medications
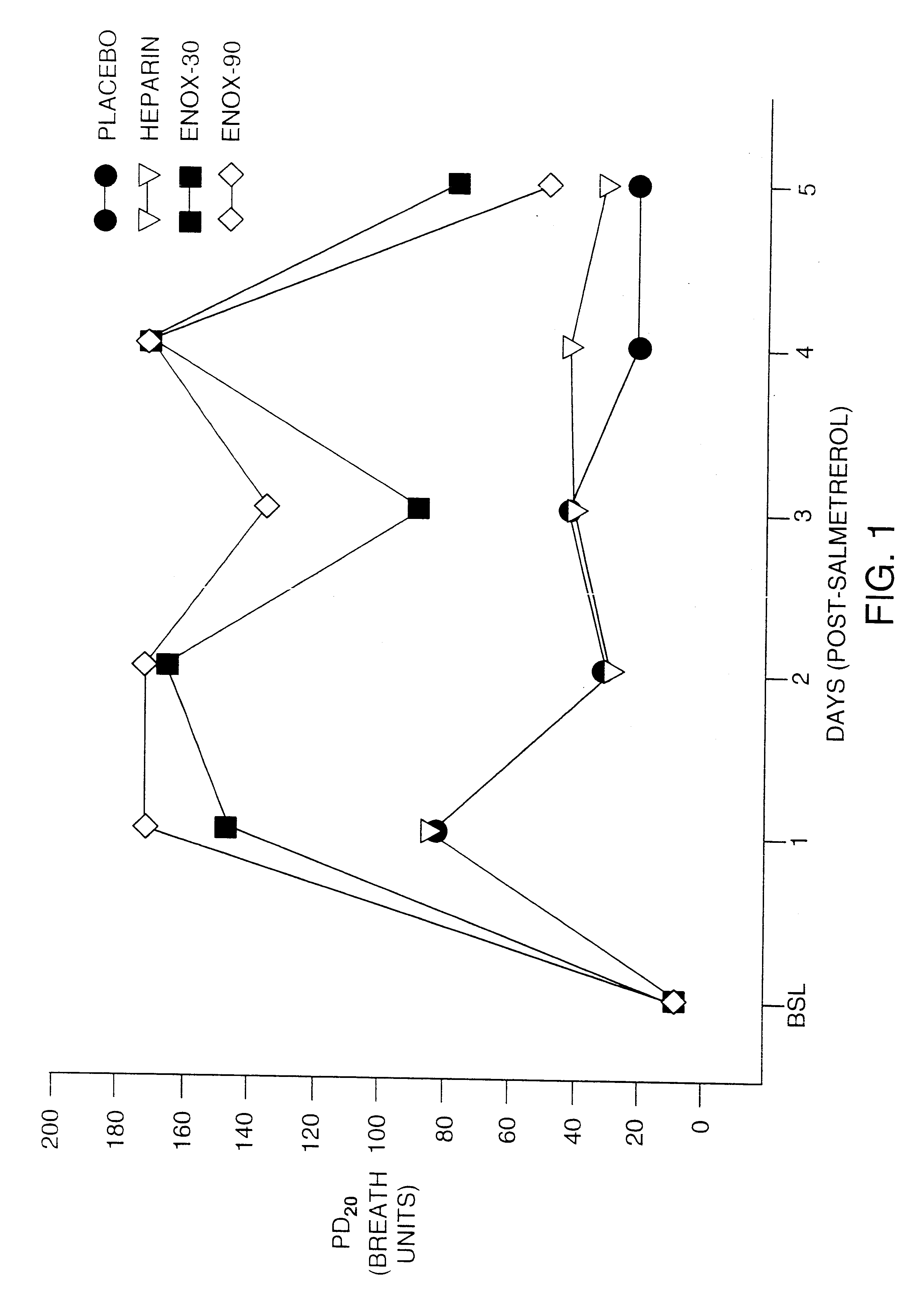
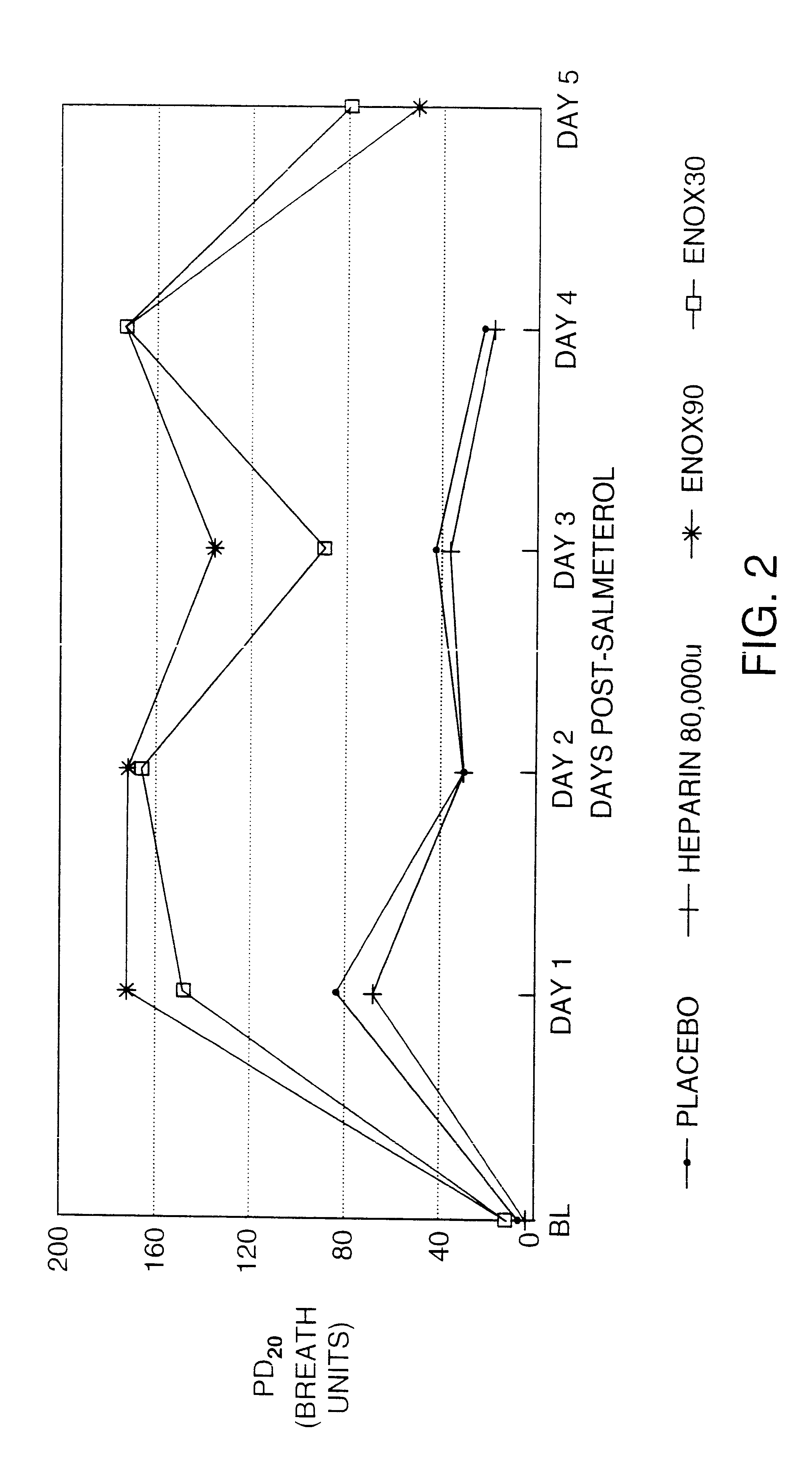
InactiveUS6235725B1Avoid toleranceEasy to managePowder deliveryBiocideTolerabilityAdrenergic receptor agonists
The present invention pertains to the identification of moieties and methods of using the same for preventing tolerance to bronchodilators. More specifically, the present invention pertains to the identification of compositions and methods which are capable of preventing tolerance to beta2-adrenergic agonists. The methods and compositions according to the invention are also useful as analytical tools for functional studies and as combination therapeutic tools.
Owner:TEVA WOMENS HEALTH RES INC
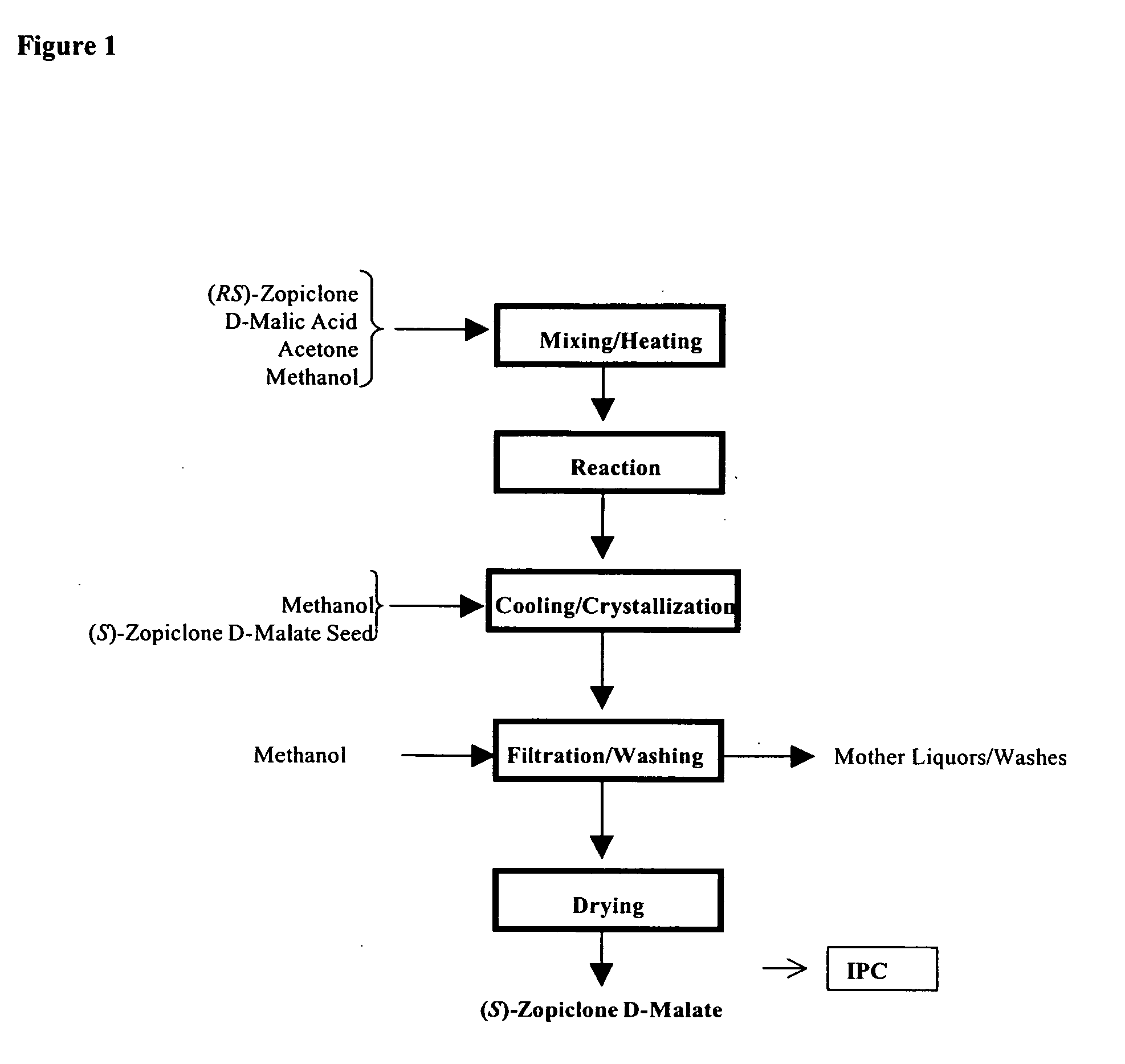
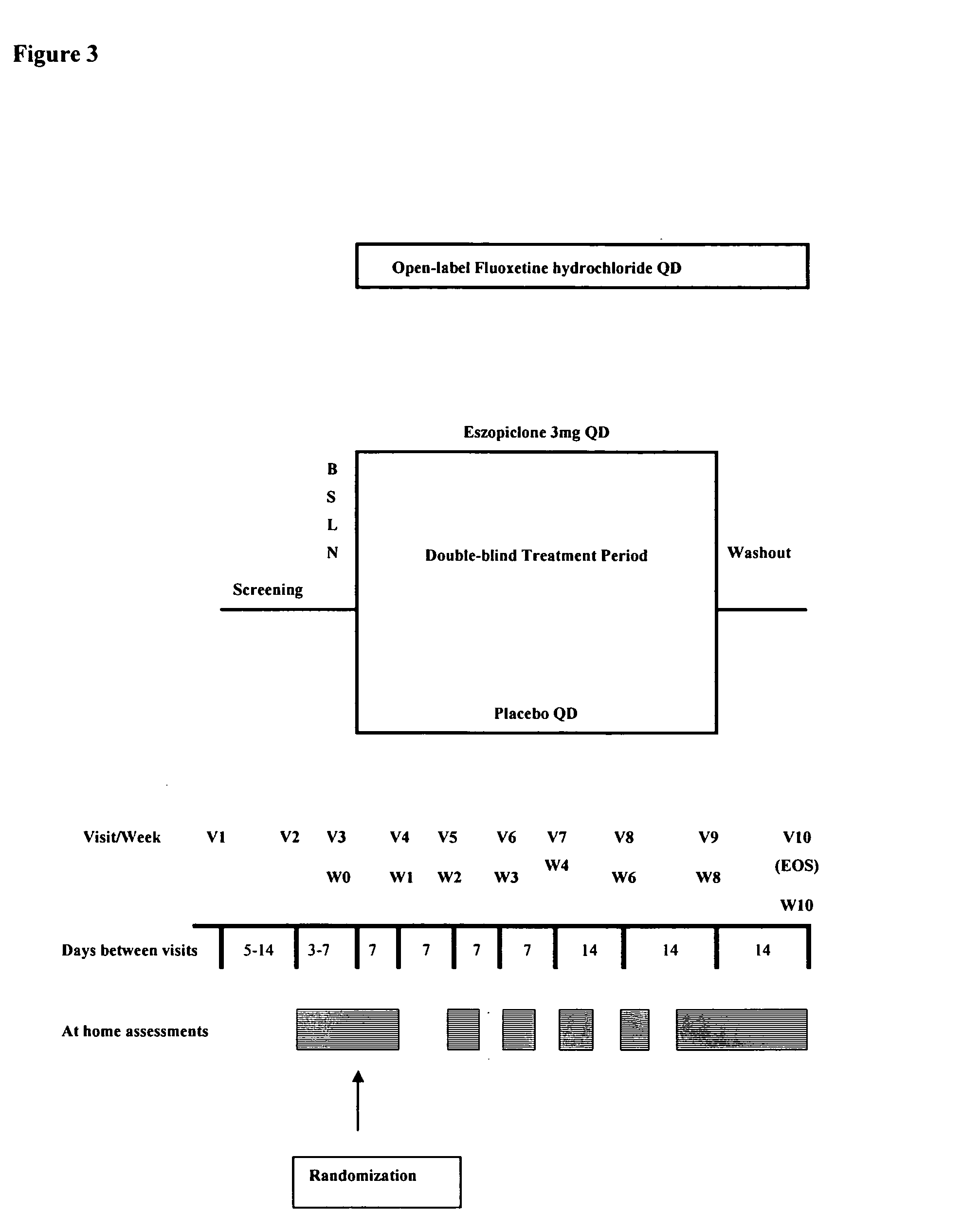
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:SEPACOR INC
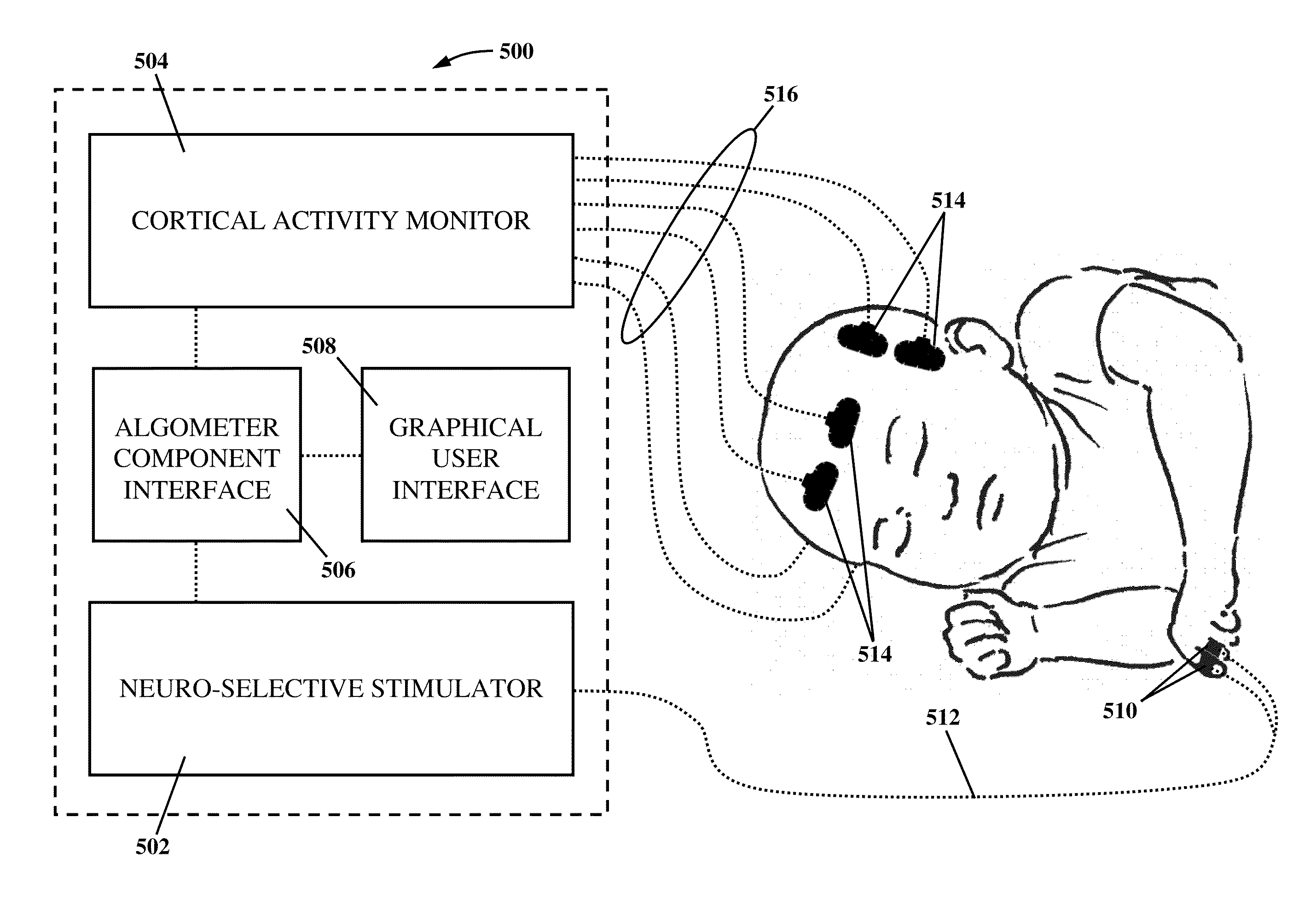
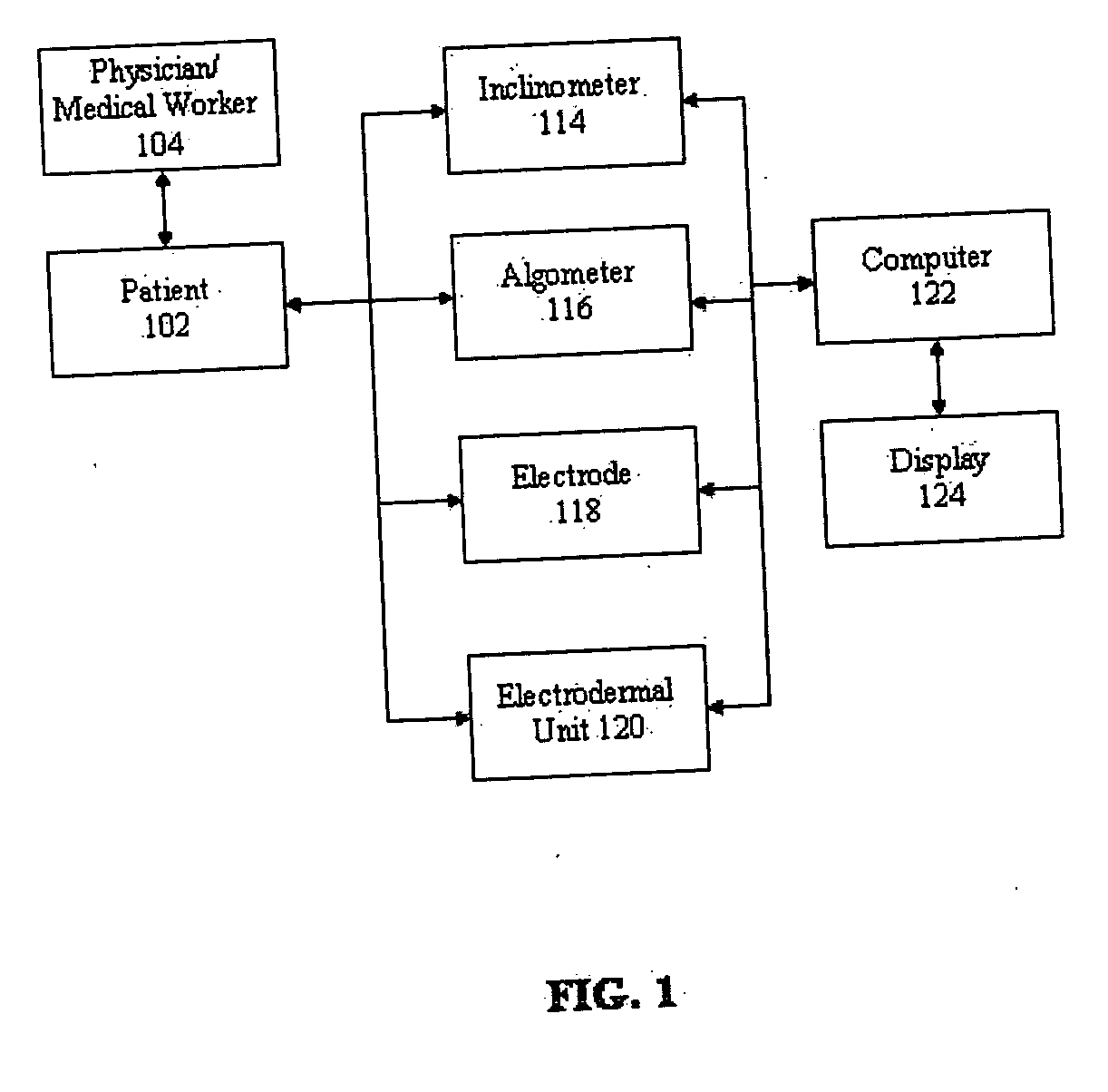
Apparatus and method for human algometry
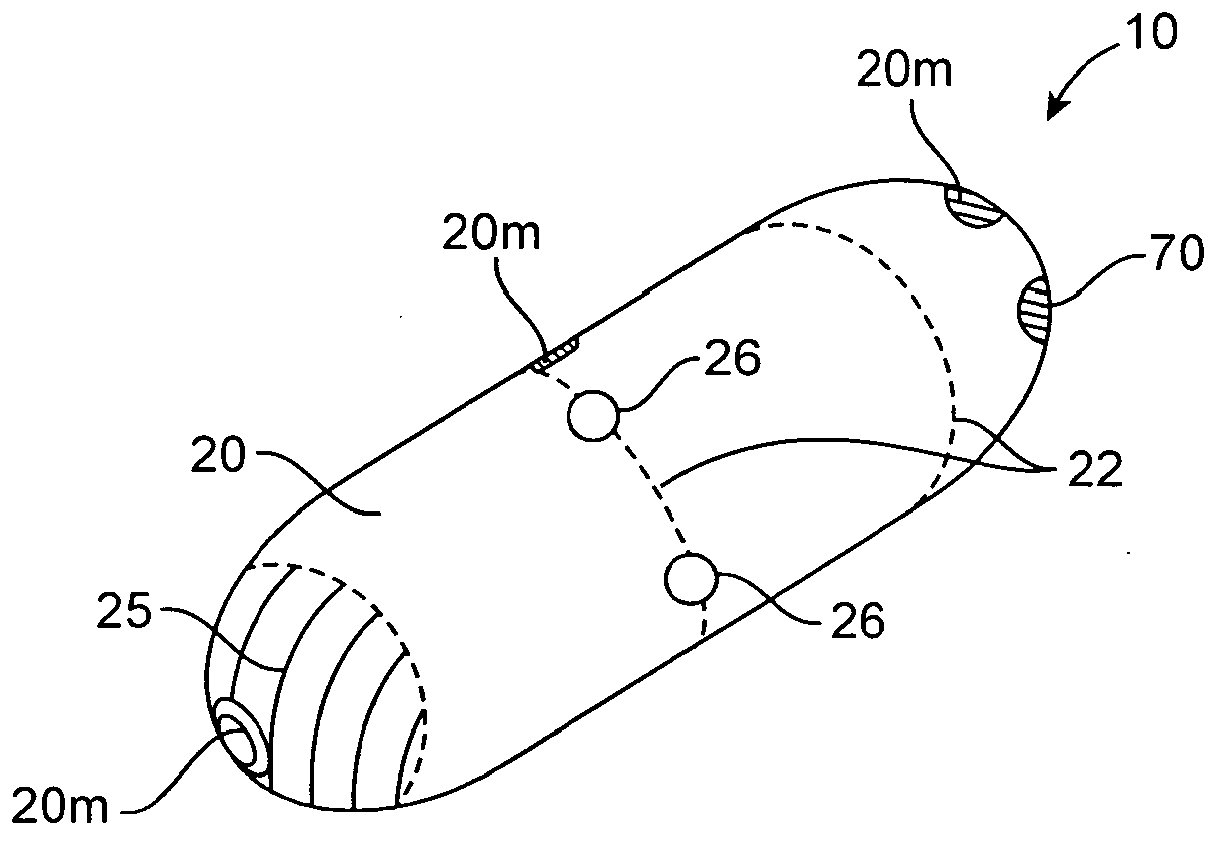
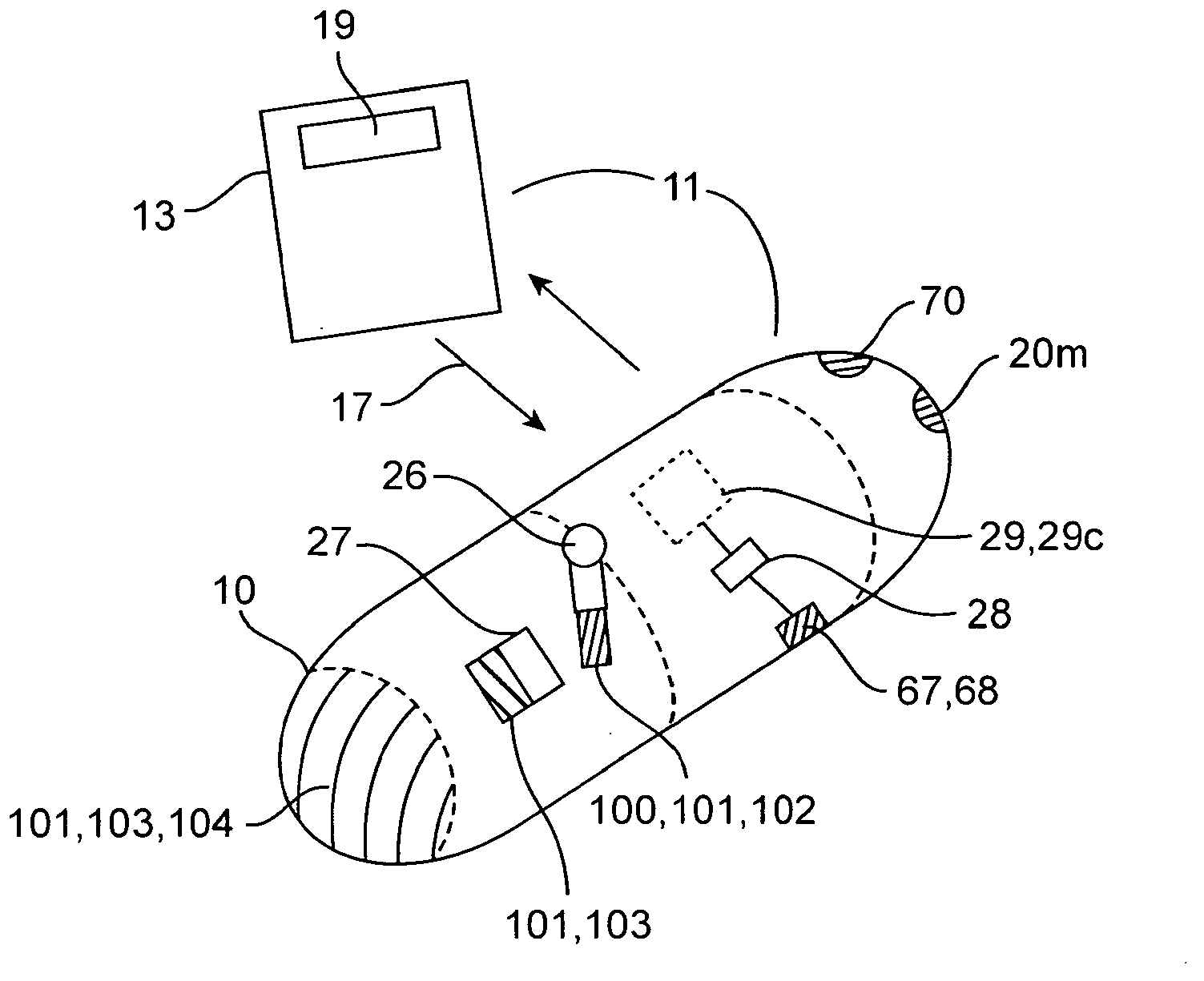
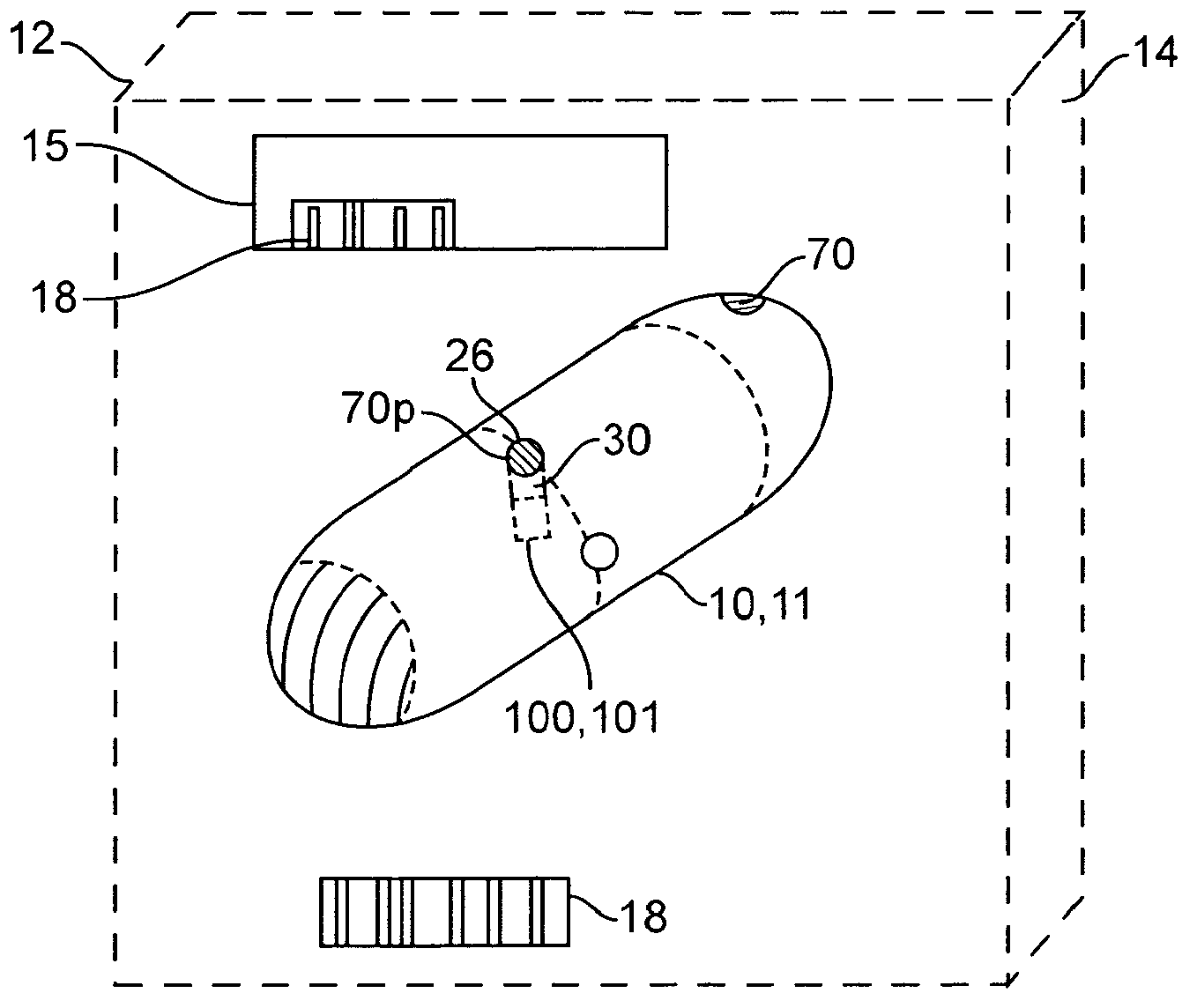
An apparatus and method for performing human algometry are disclosed. They include a stimulator configured to apply electrical stimulation of variable intensity to an area of a patient's body, a monitoring device configured to measure a. level of cortical activity in one or more regions of the patient's brain, and a microprocessor connected to the stimulator and the monitoring device that is configured to correlate the intensity of the electrical stimulation with the level of activity in the one or more regions of the patient's brain and to determine at least one of a measurement of pain intensity, a measurement of a sensory detection threshold (SDT), a measurement of a drug's analgesic impact, an indication of an onset of tolerance to a drug, an indication of an onset of analgesic-induced hyperalgesia, an indication of conditions of allodynia, a measurement of dose-response characteristics of pain management drugs, and a characterization of a pain condition.
Owner:CHILDRENS NAT MEDICAL CENT
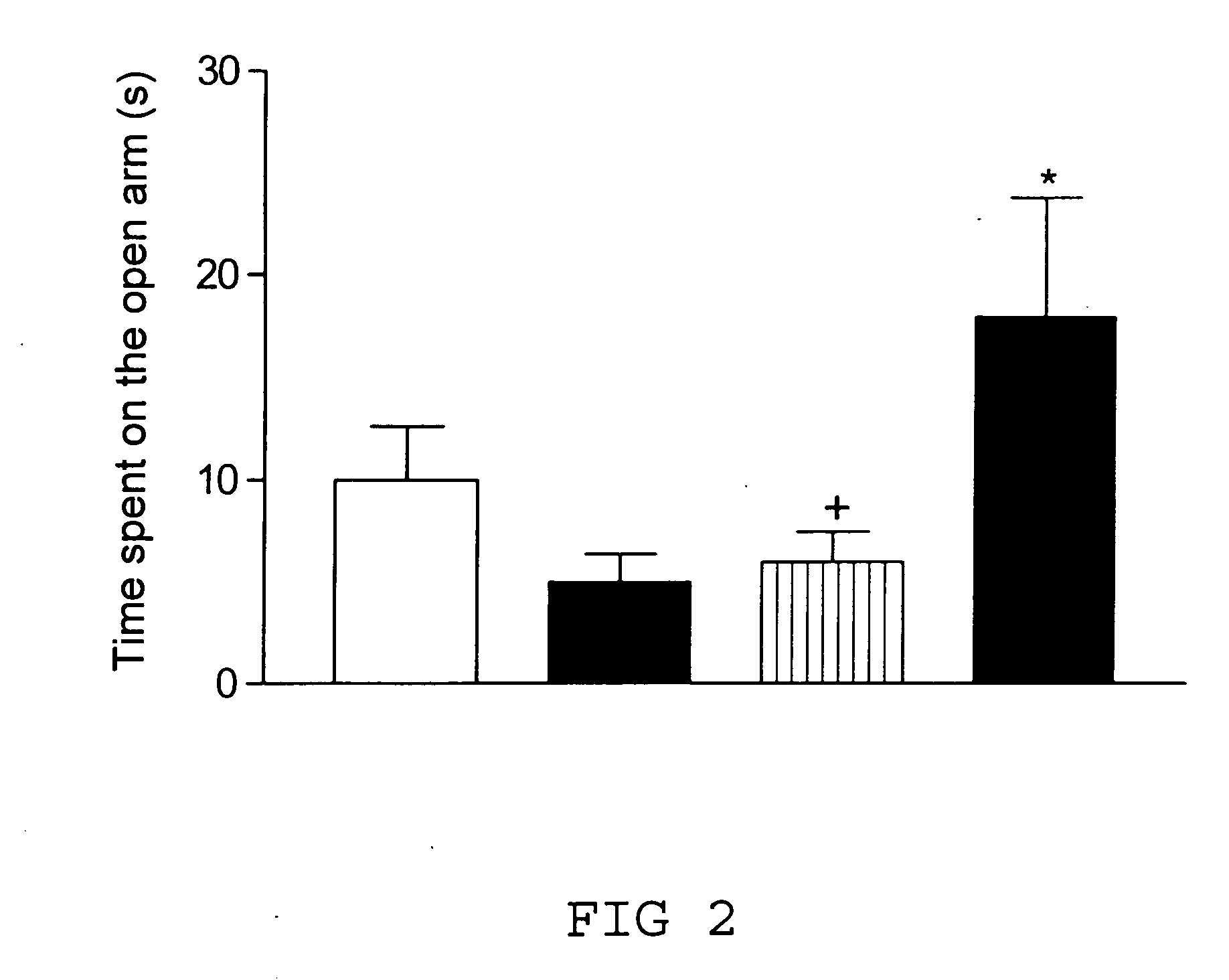
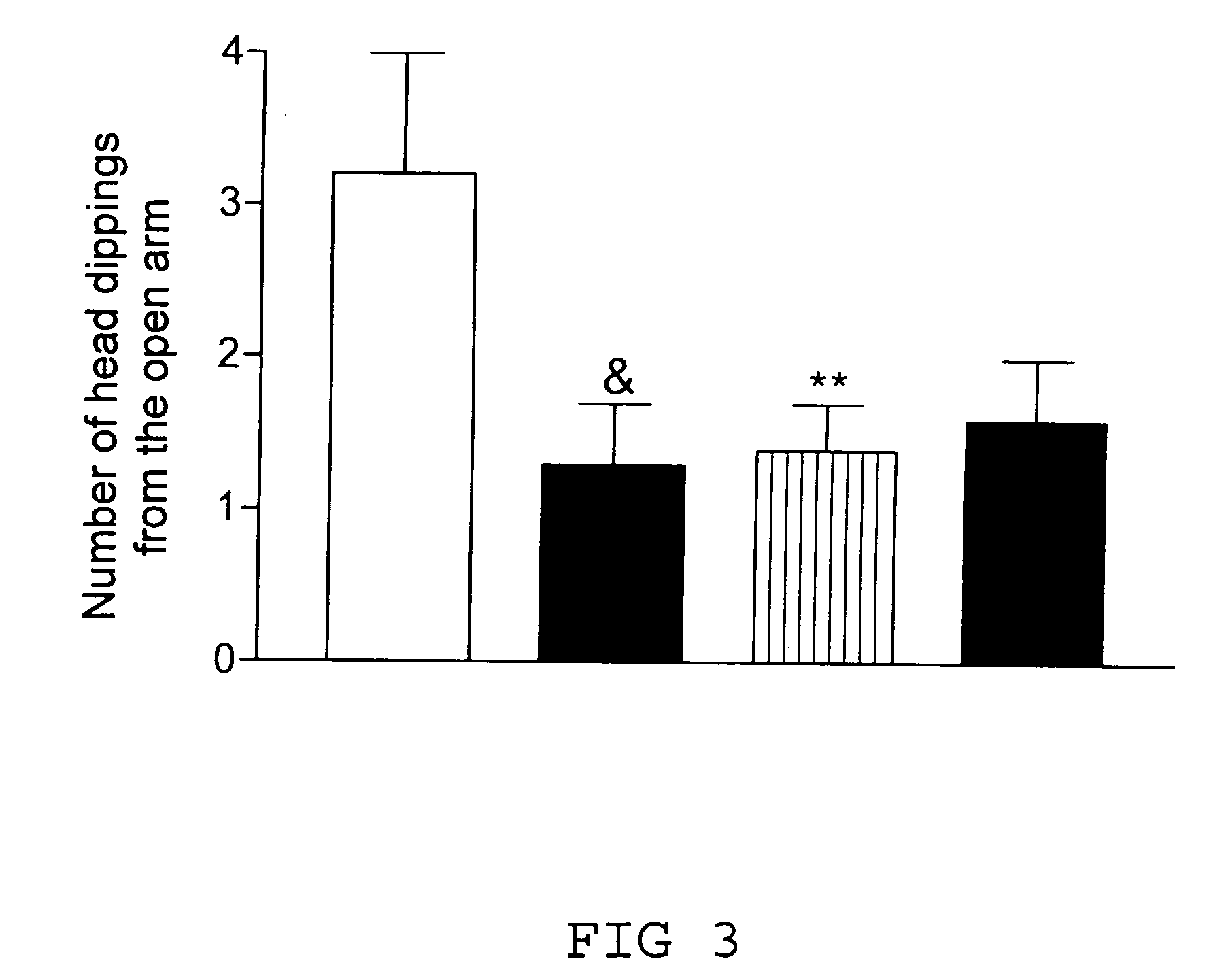
Transgenic animal model for modelling pathological anxiety, a method for identifying compounds for treatment of diseases or disorders caused by pathological anxiety and a method for using wfs1 protein as a target for identifying effective compounds against pathological anxiety
InactiveUS20100146645A1Low environmental changeImprove anxietyCell receptors/surface-antigens/surface-determinantsBiological testingTolerabilityClinical psychology
The invention discloses the transgenic animal model for pathological anxiety, the method to generate this model, the method to test drugs and drug candidates for the treatment of pathological anxiety and the method to use Wfs1 as target for screening of new anxiolytic drugs to treat pathological anxiety. This animal model is useful to test potential drug candidates for the treatment of diseases caused by pathological anxiety and to screen therapeutic compounds for the psychiatric disorders caused by reduces stress-tolerance and deficiency in adaptation to environmental challenges.
Owner:TARTU ULIKOOL THE UNIV OF TARTU
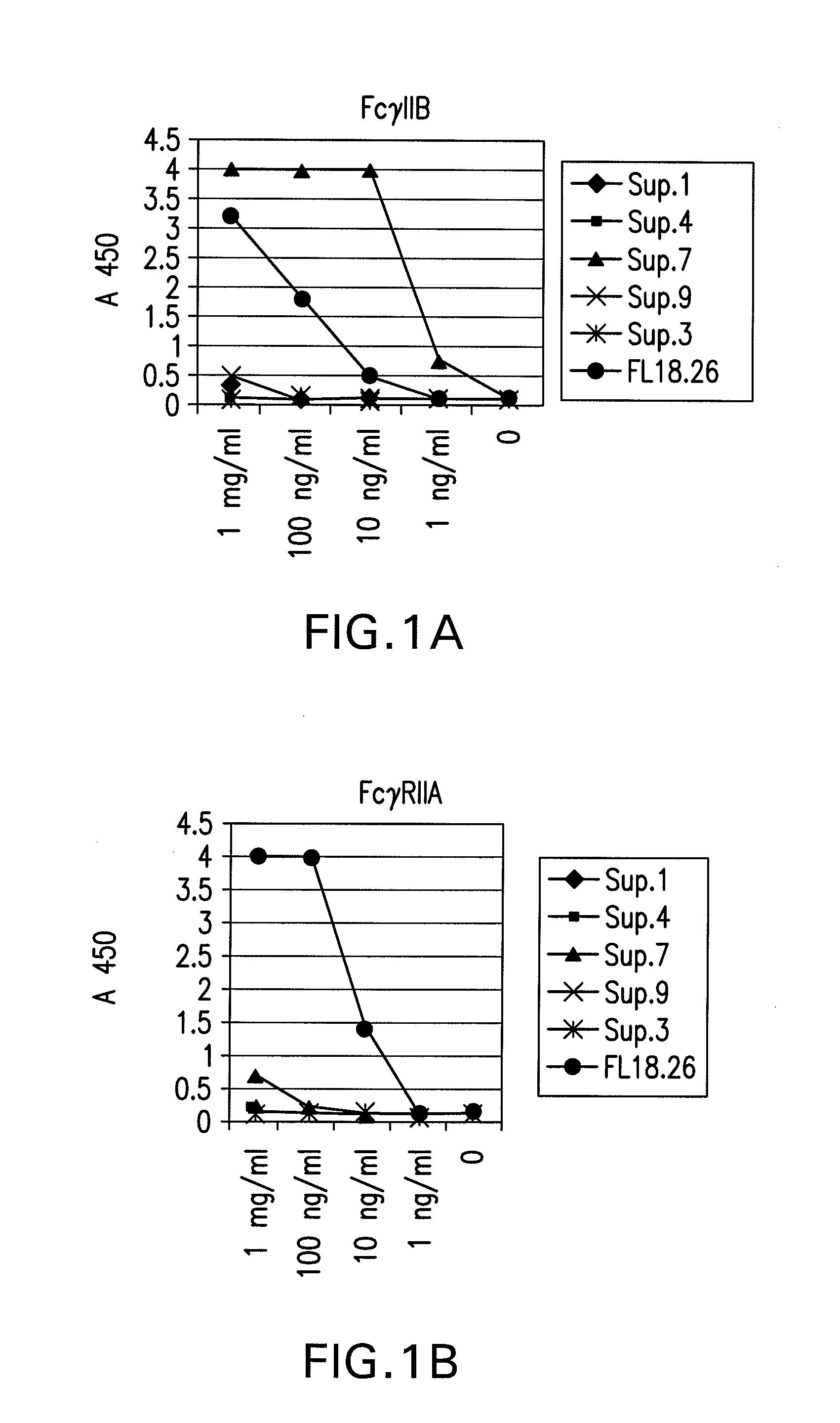
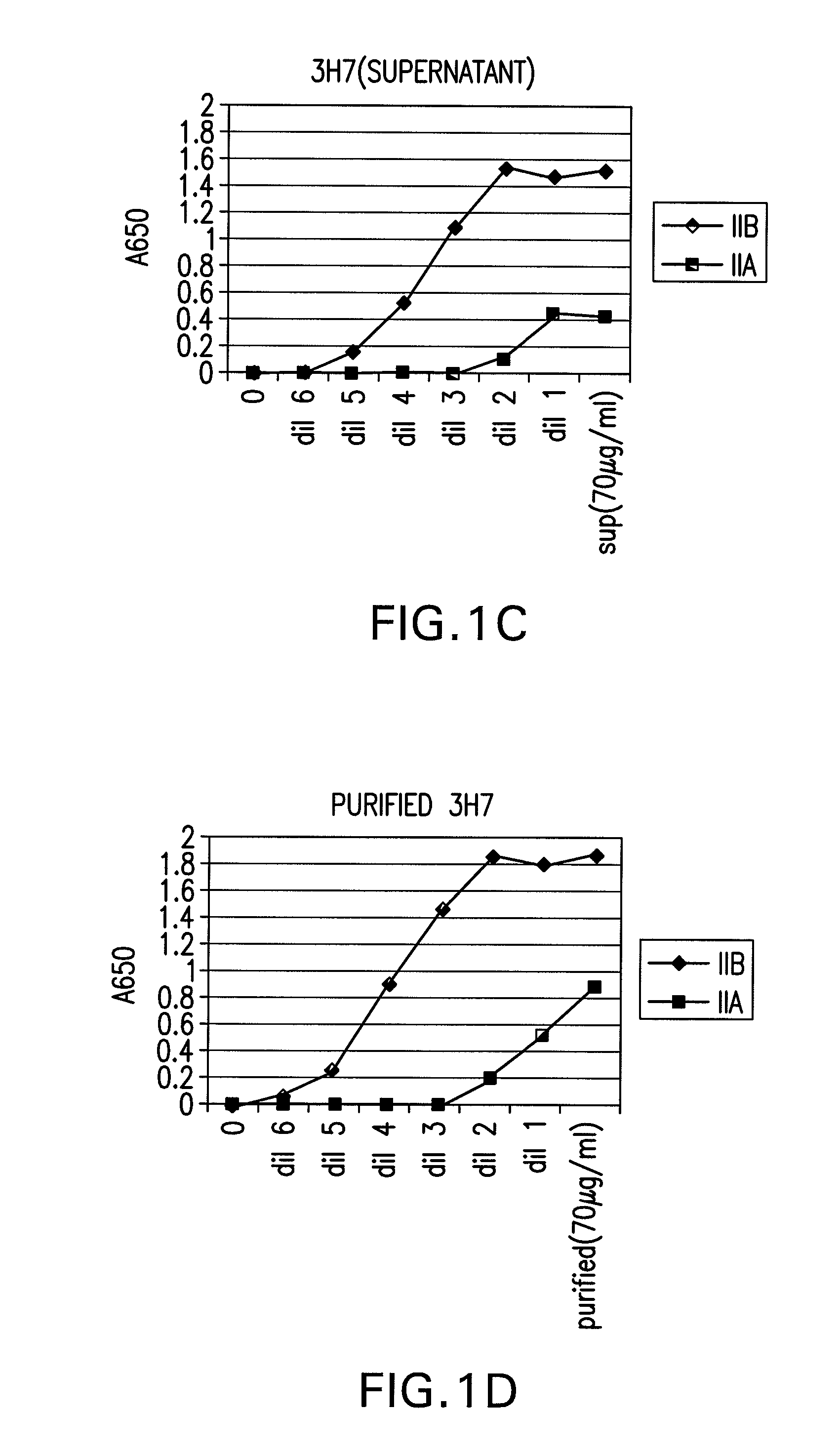
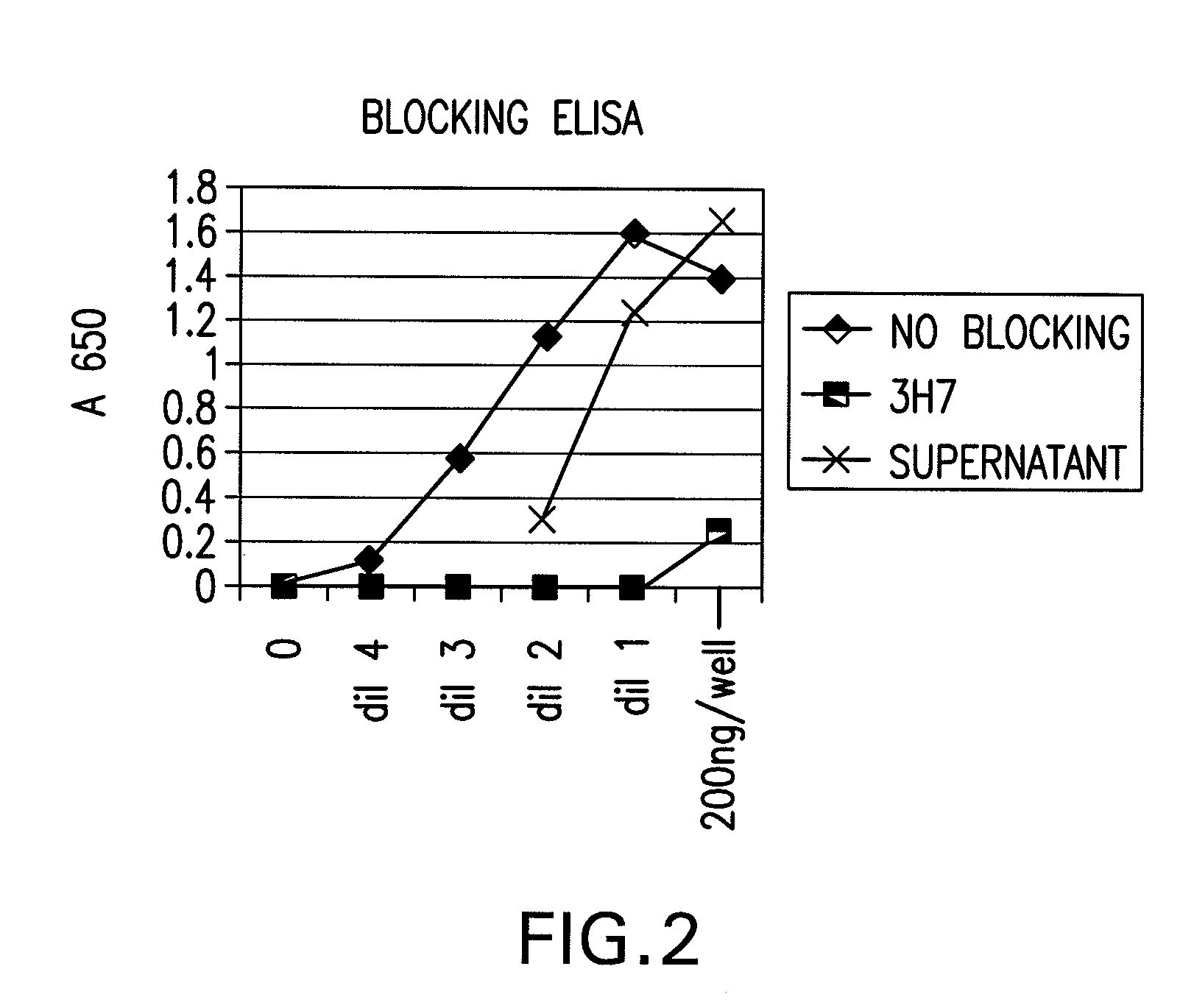
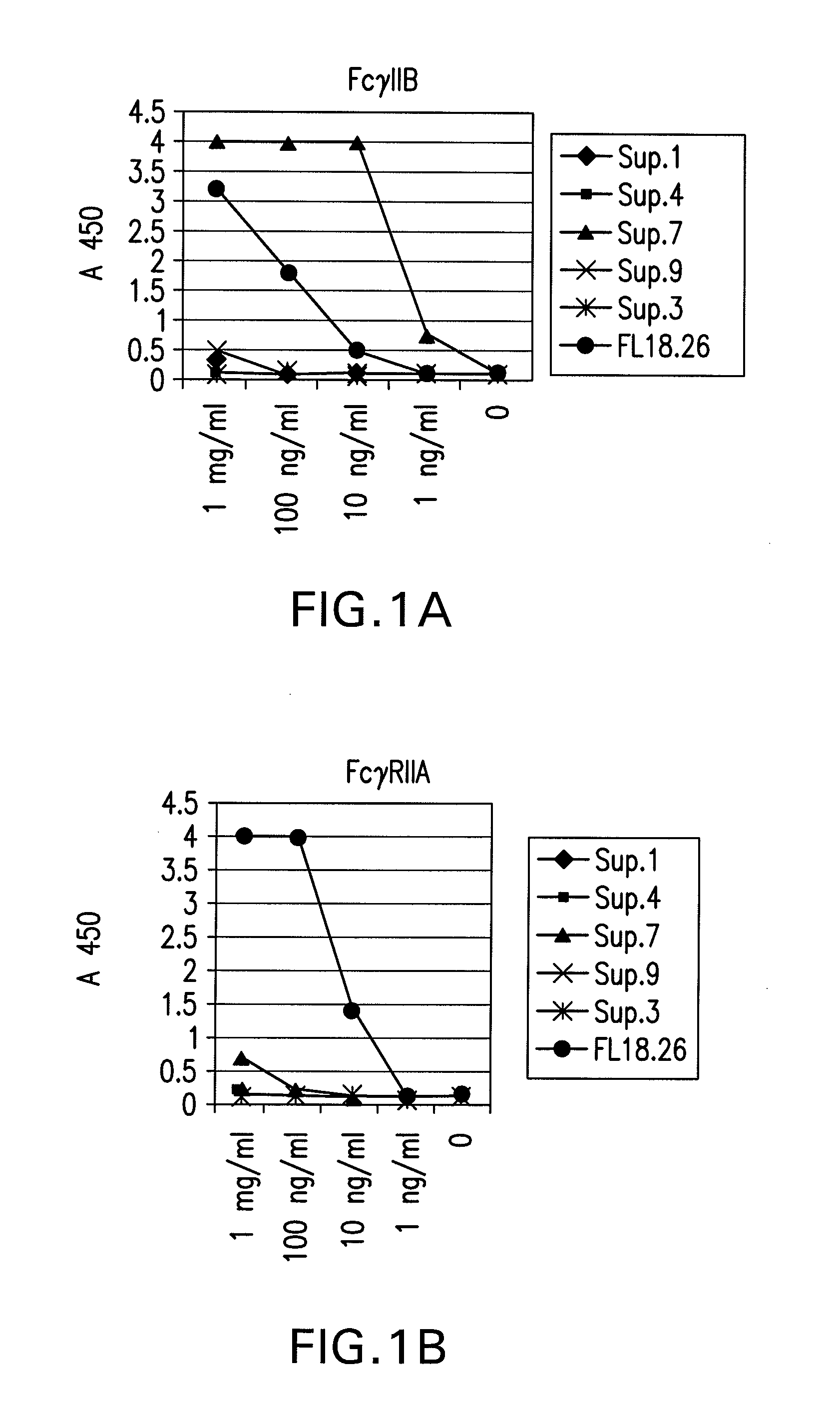
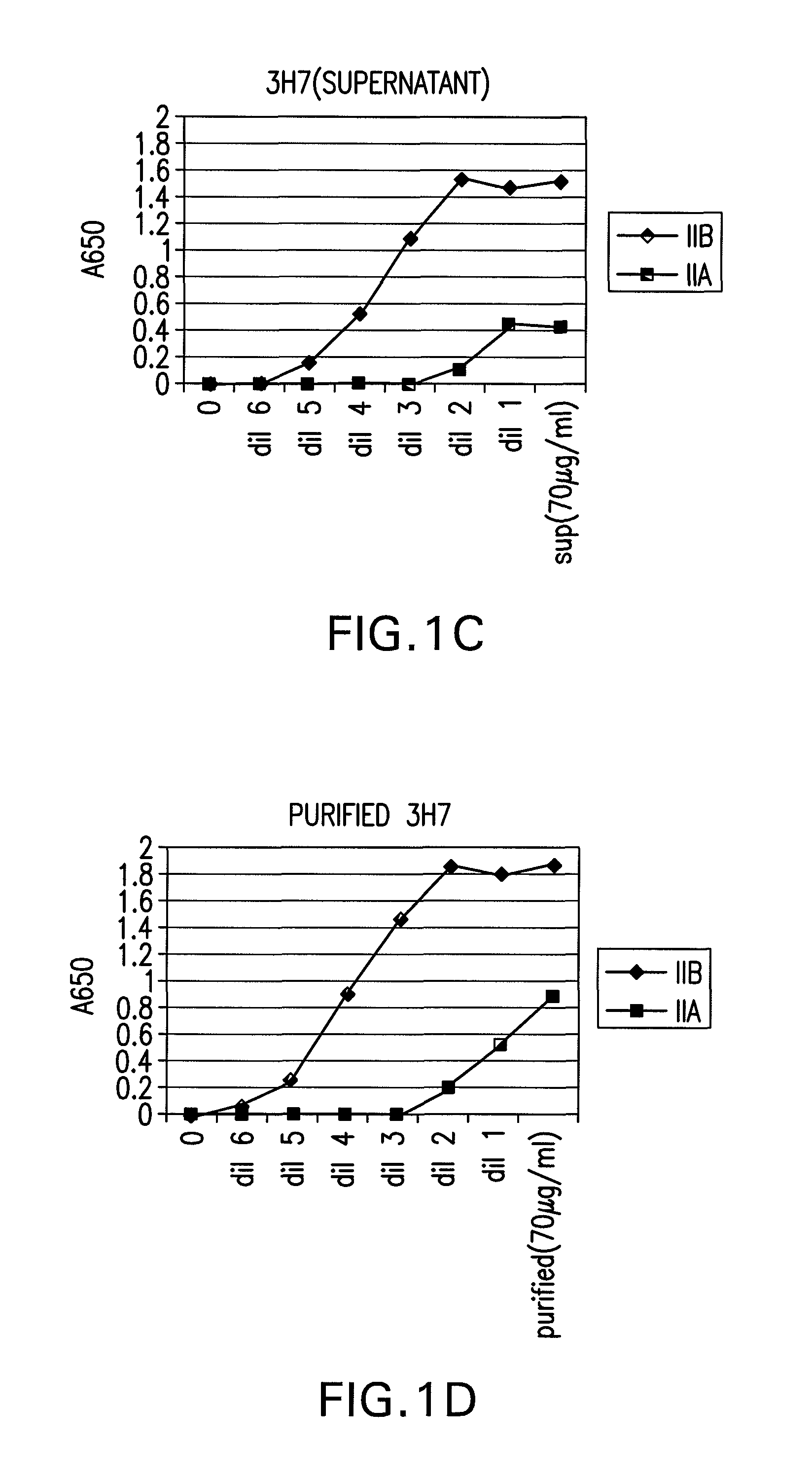
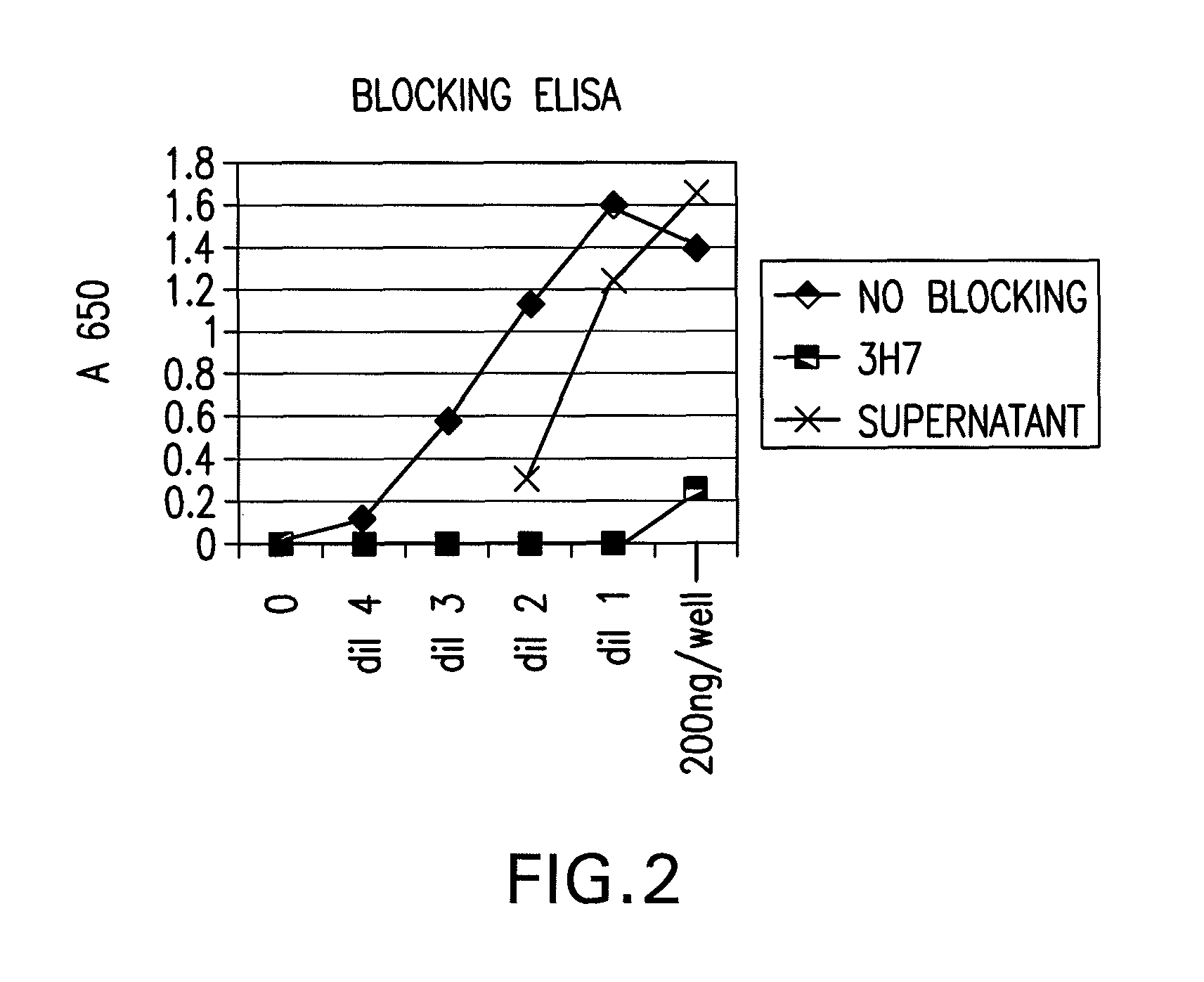
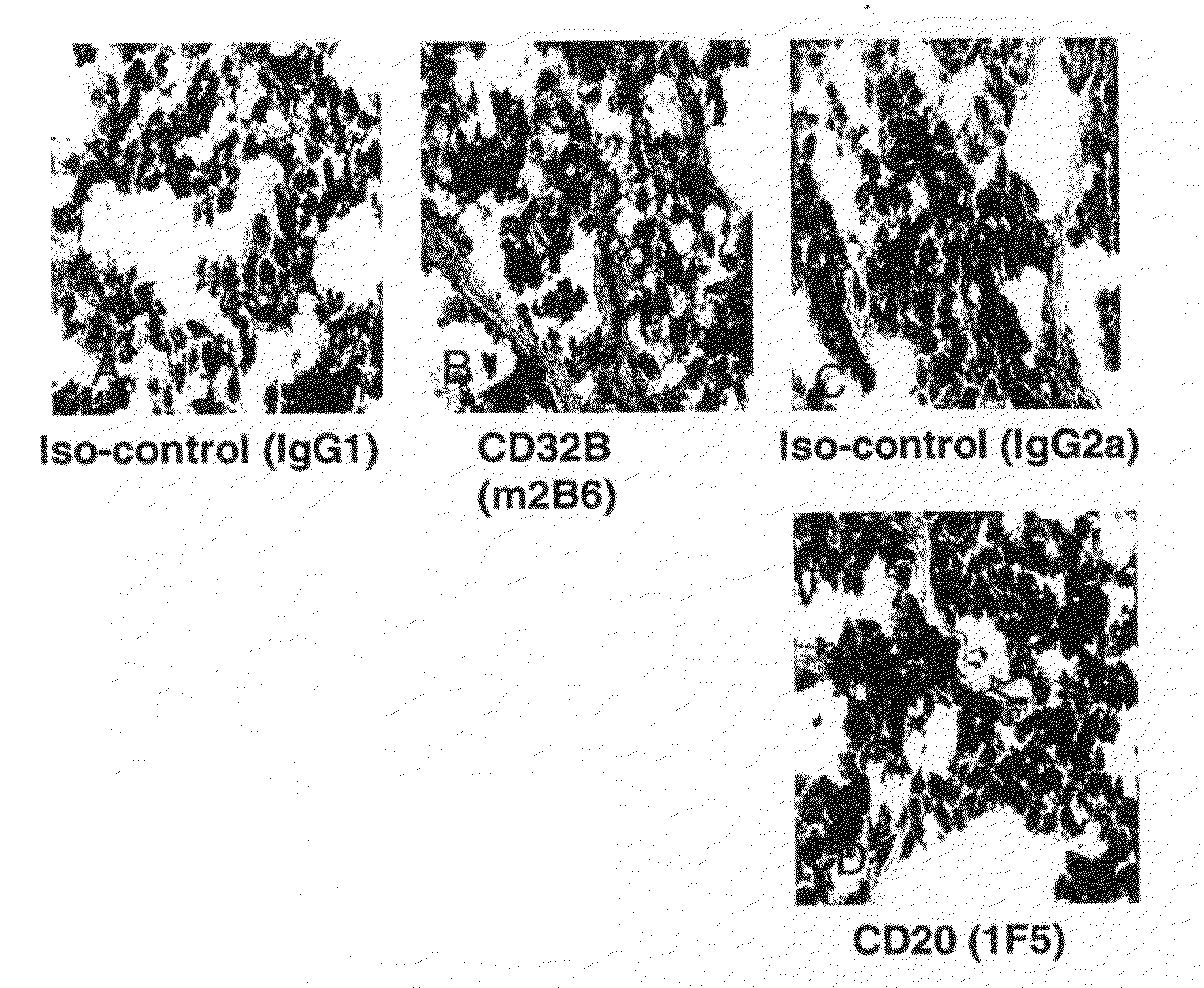
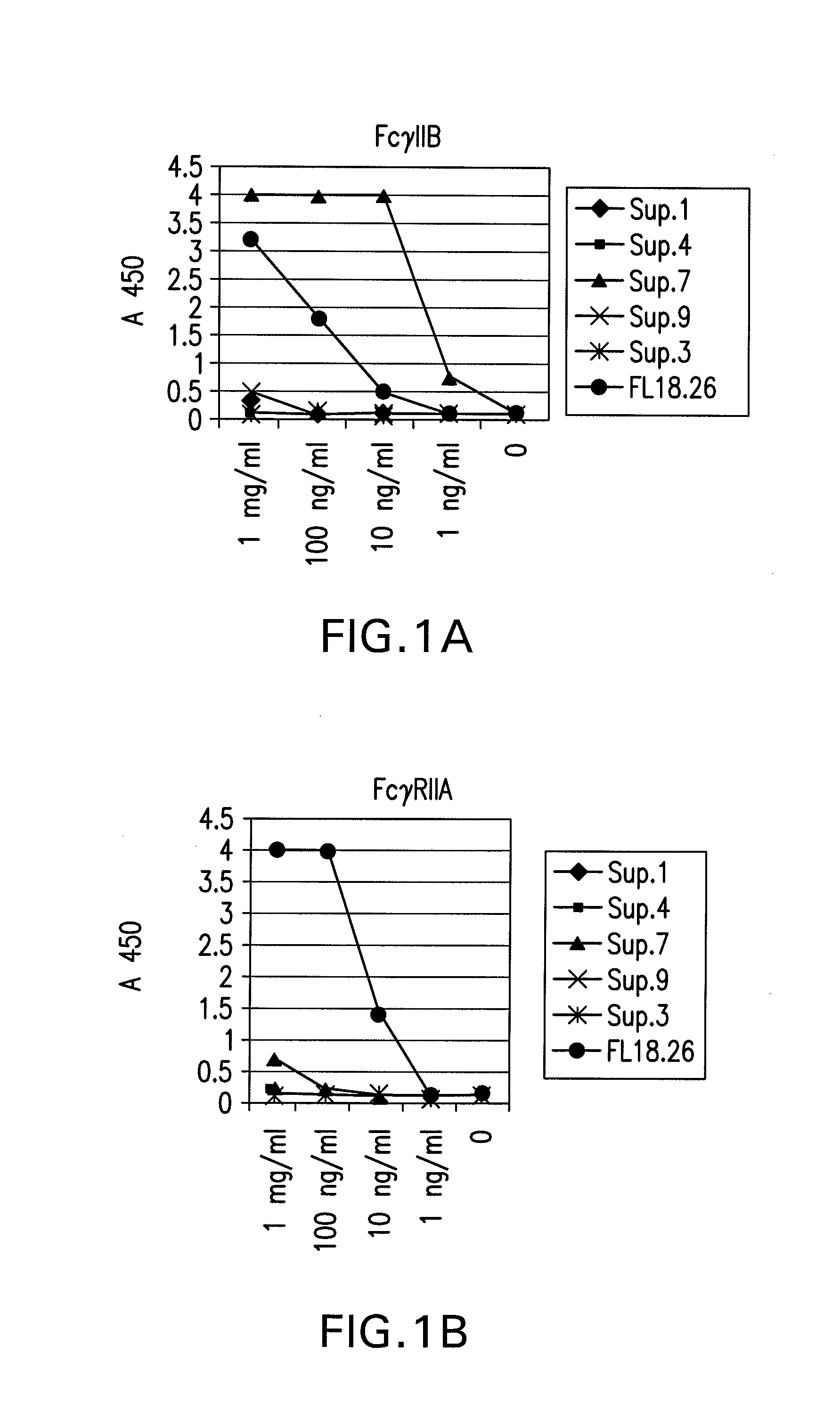
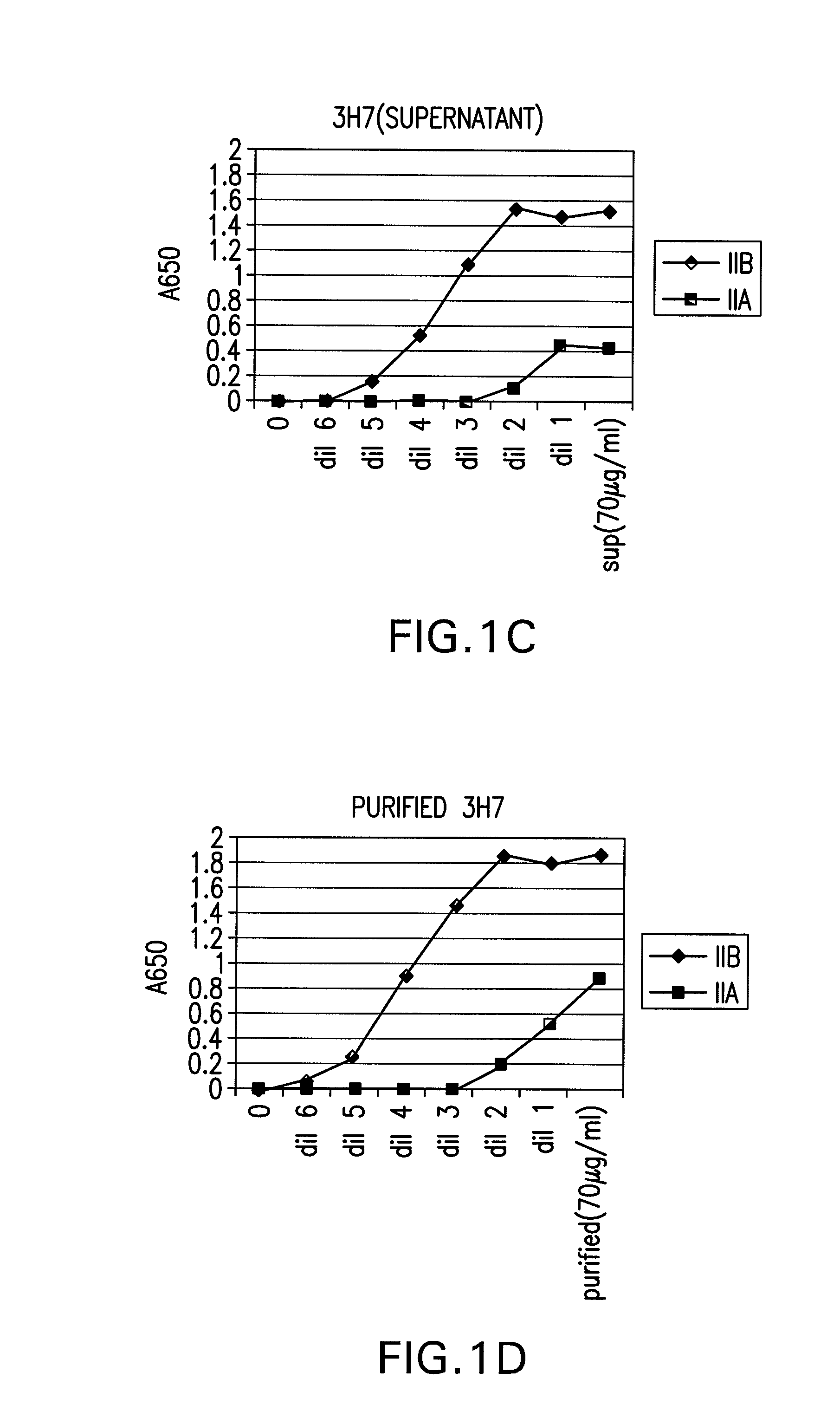
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090074771A1Strong therapeutic activityEnhancing antibody-mediated effector functionAntibody ingredientsImmunoglobulinsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
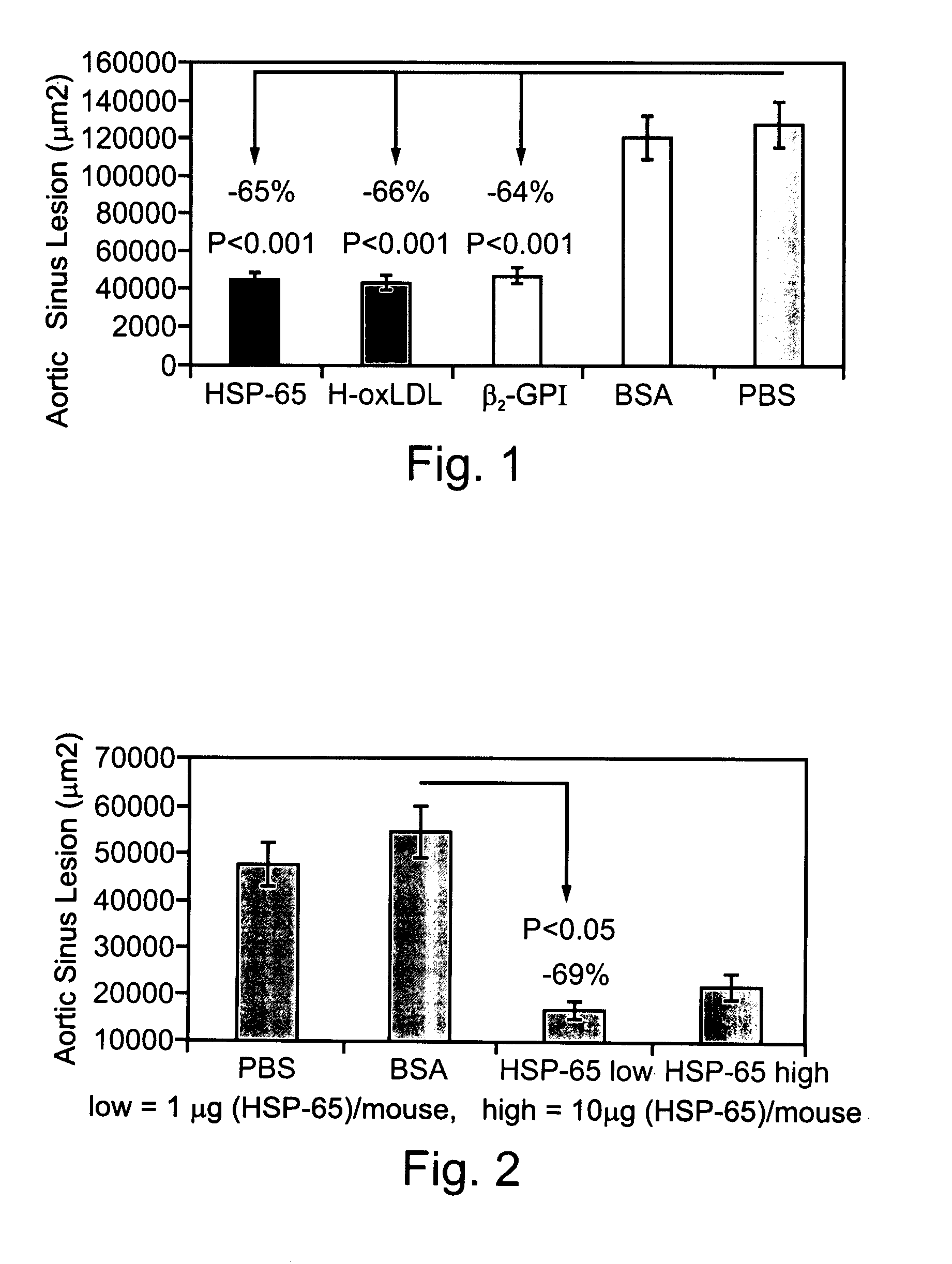
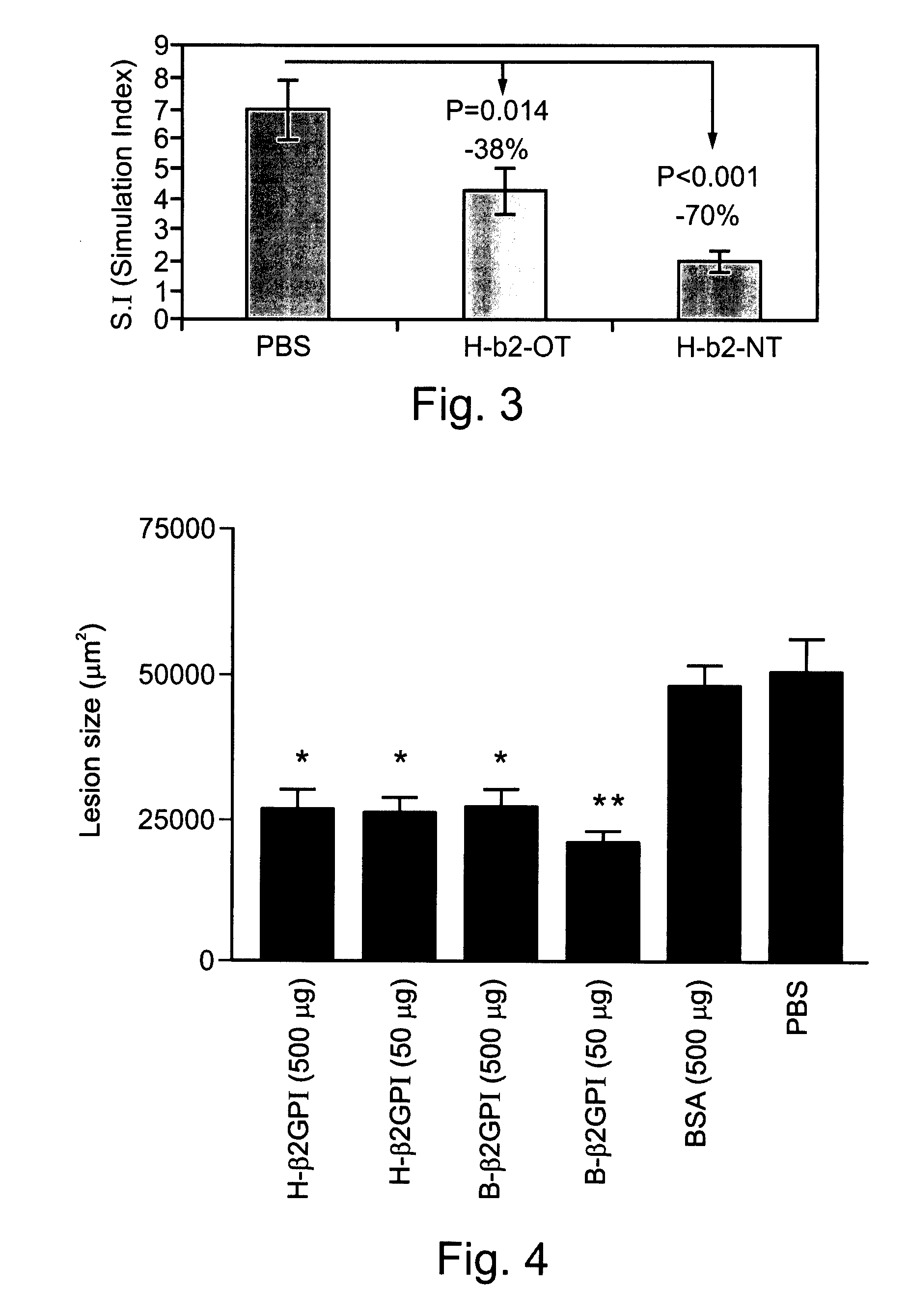
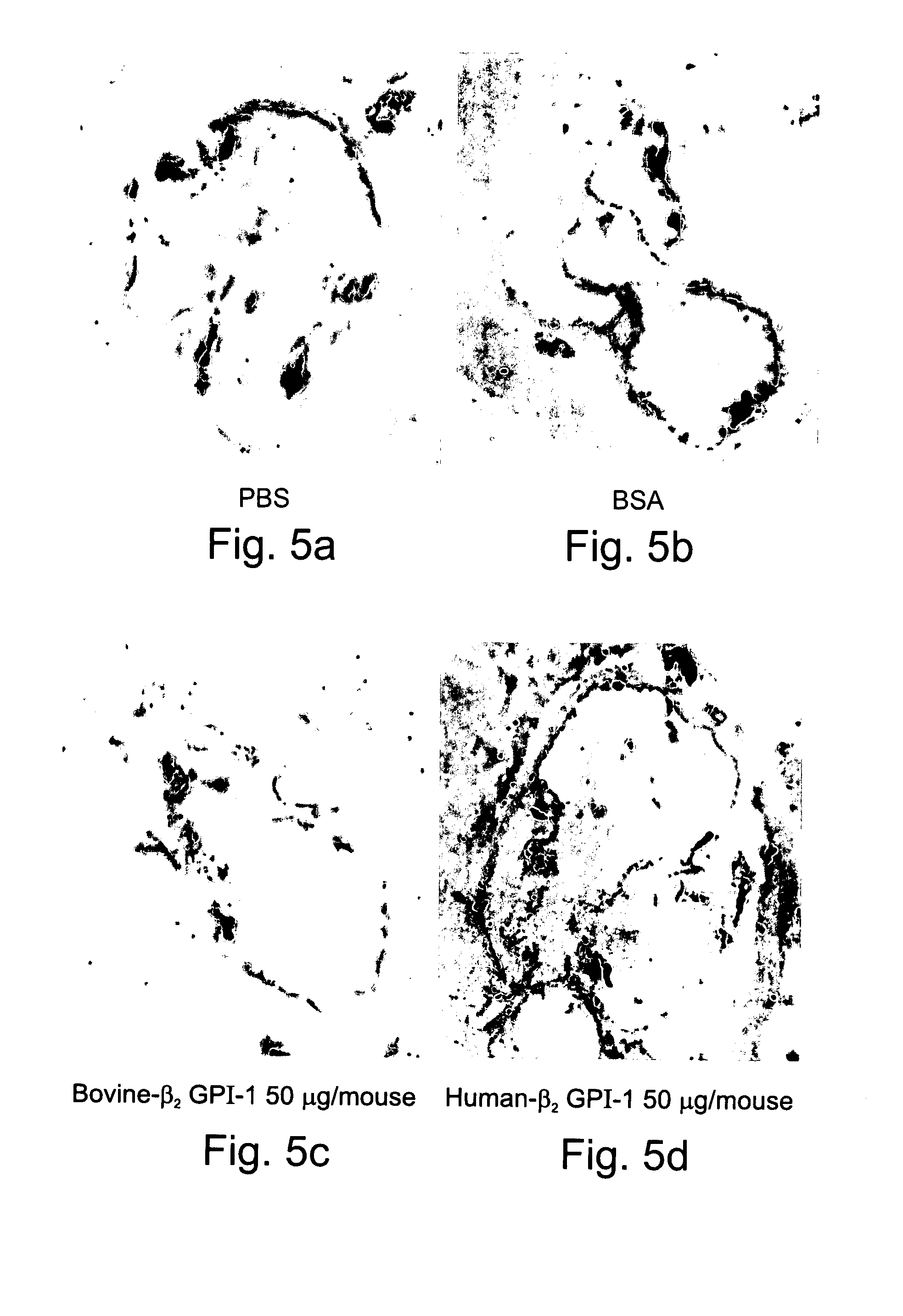
Compositions Containing Beta 2-Glycoprotein I-Derived Peptides for the Prevention and/or Treatment of Vascular Disease
Methods and compositions employing beta2-glycoprotein-1 (β2GPI)-derived peptides and combinations thereof effective in inducing mucosal tolerance to atheroma related antigens and effective in inhibiting inflammatory processes contributing to atheromatous vascular disease and sequalae are provided.
Owner:VASCULAR BIOGENICS
Low molecular weight cationic lipids for oligonucleotide delivery
ActiveUS8748667B2Good curative effectReduce liver toxicityOrganic compound preparationOther foreign material introduction processesTolerabilityNanoparticle
The instant invention provides for novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. It is an object of the instant invention to provide a cationic lipid scaffold that demonstrates enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
FcγRIIB specific antibodies and methods of use thereof
InactiveUS8946387B2Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityImmune complex deposition
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
FcGammaRIIB Specific Antibodies and Methods of Use Thereof
InactiveUS20090076251A1Strong therapeutic activityEnhancing antibody-mediated effector functionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsTolerabilityAutoimmune responses
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, more particularly the extracellular domain of FcγRIIB with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA, and block the Fc binding site of FcγRIIB. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The present invention also encompasses the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in combination with other cancer therapies. The present invention provides pharmaceutical compositions comprising an anti-FcγRIIB antibody or an antigen-binding fragment thereof, in amounts effective to prevent, treat, manage, or ameliorate a cancer, such as a B-cell malignancy, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention further provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention with a vaccine composition. The invention further provides methods of treating cancer and / or regulating immune complex-mediated cell activation by administering the antibodies of the invention to enhance an immune response. The invention also provides methods of breaking tolerance to an antigen by administering an antigen-antibody complex and an antibody of the invention.
Owner:MACROGENICS INC
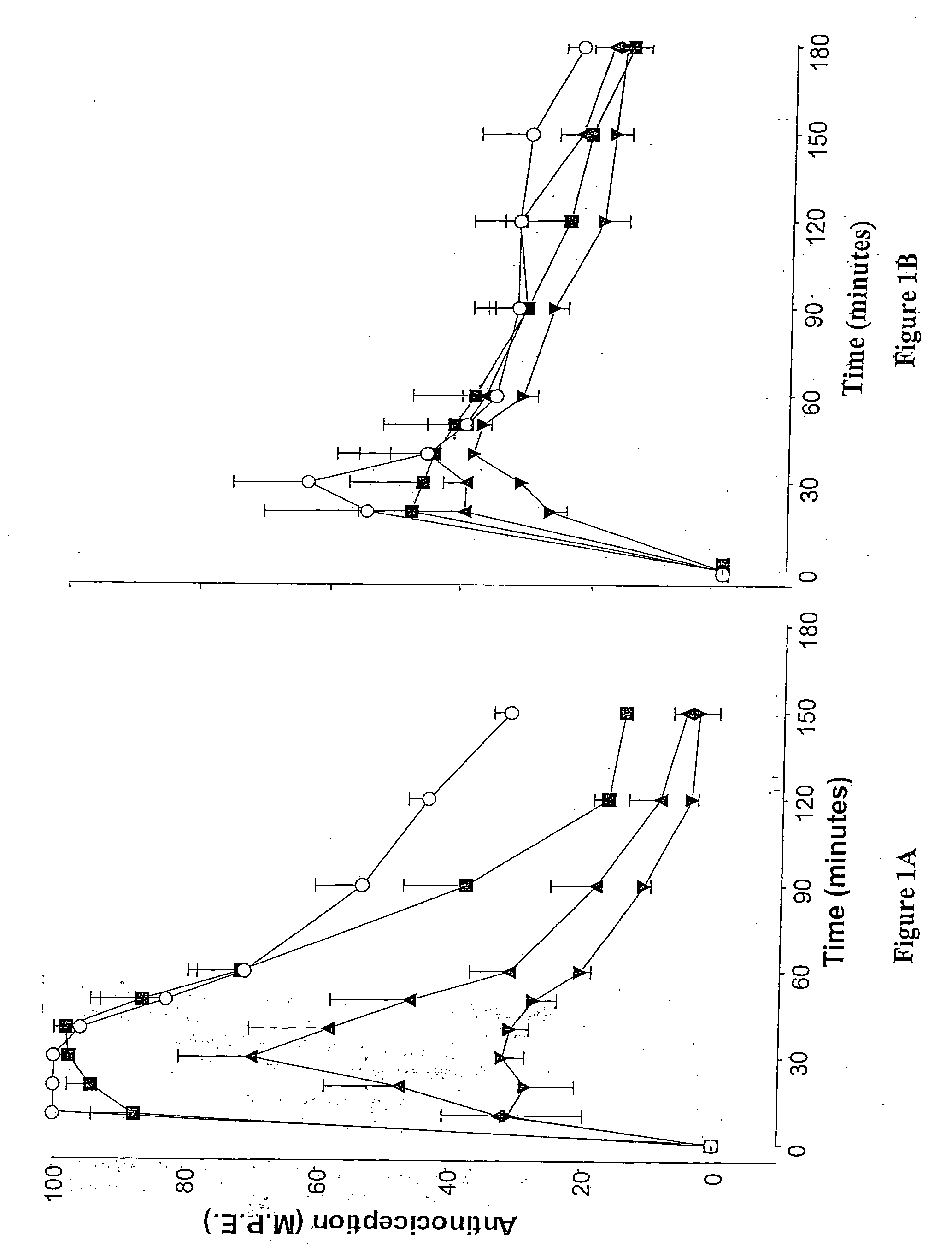
Methods and therapies for potentiating a therapeutic action of an opioid receptor agonist and inhibiting and/or reversing tolerance to opioid receptor agonists
Combination therapies of an opioid receptor agonist and an alpha-2 receptor antagonist in an amount effective to potentiate, but not antagonize, a therapeutic effect of the opioid receptor agonist are provided. Also provided are methods for use of these combination therapies in potentiating the therapeutic effects of opioid receptor agonists, inhibiting development of acute and / or chronic tolerance to opioid receptor agonists and treating conditions treatable by opioid receptor agonist therapy in a subject. In addition, a method for reversing opioid receptor agonist tolerance and / or restoring therapeutic effect of an opioid receptor agonist in a subject via administration of an alpha-2 receptor antagonist in an amount effective to potentiate, but not antagonize, the therapeutic effect of the opioid receptor agonist is provided.
Owner:QUEENS UNIV OF KINGSTON
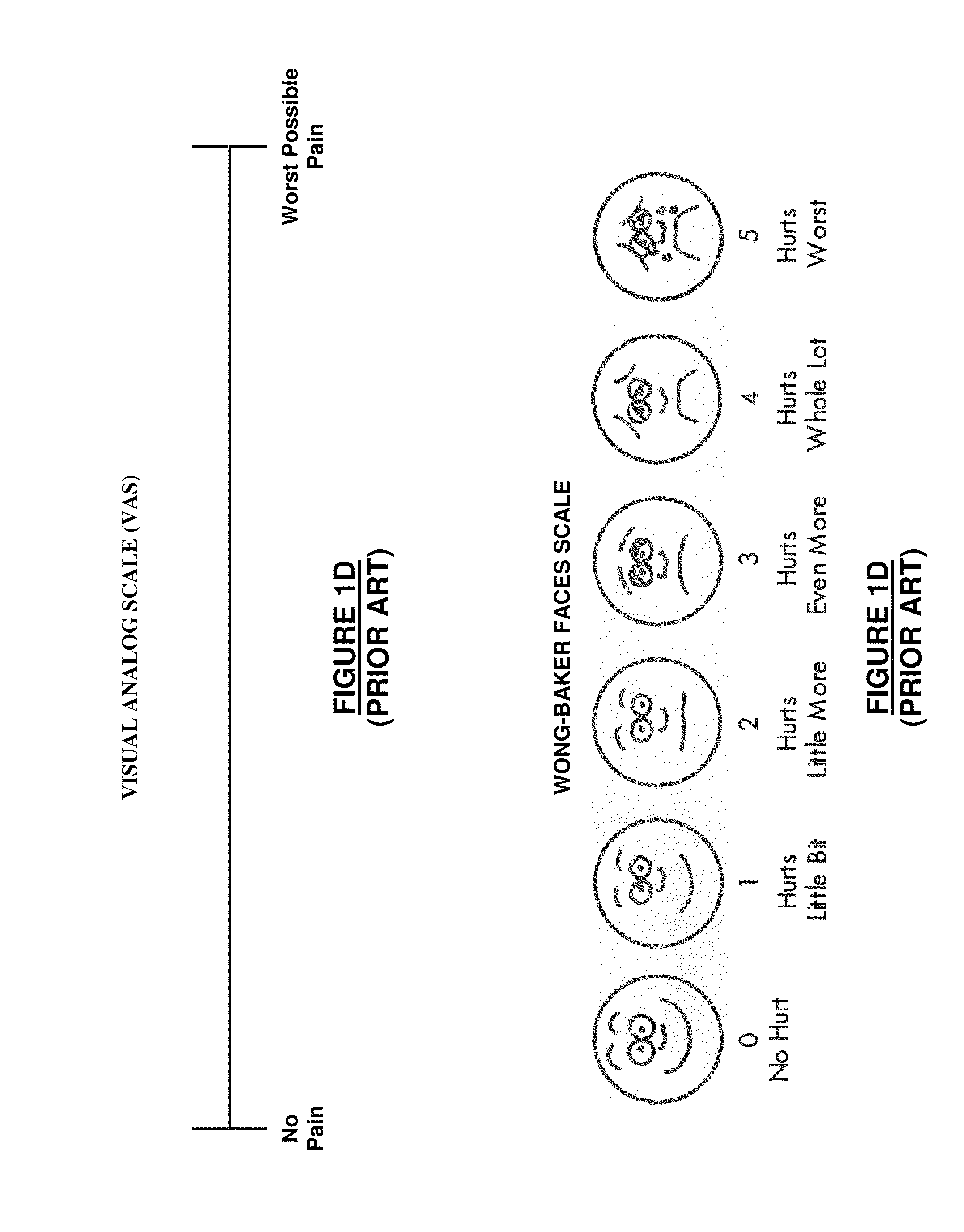
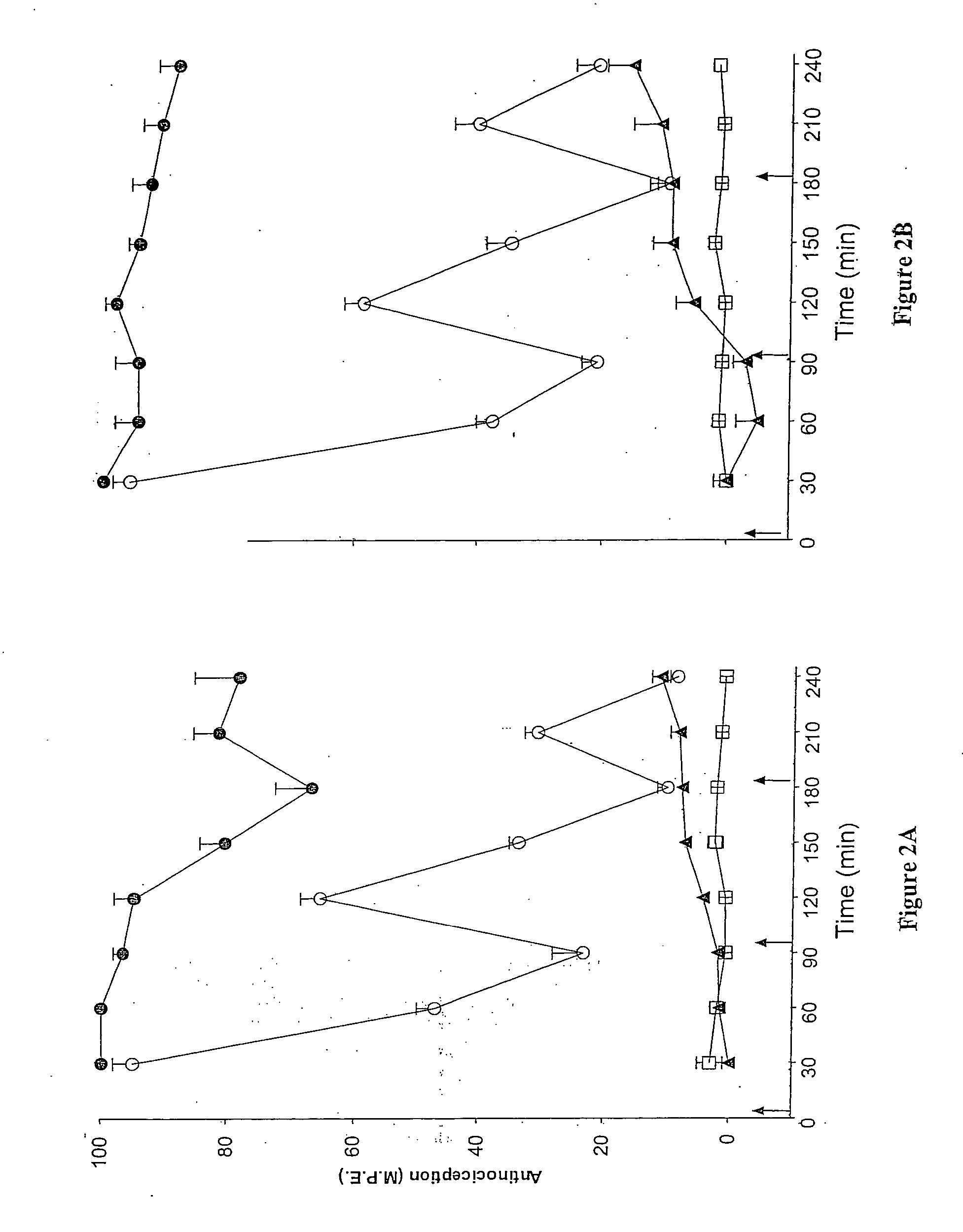
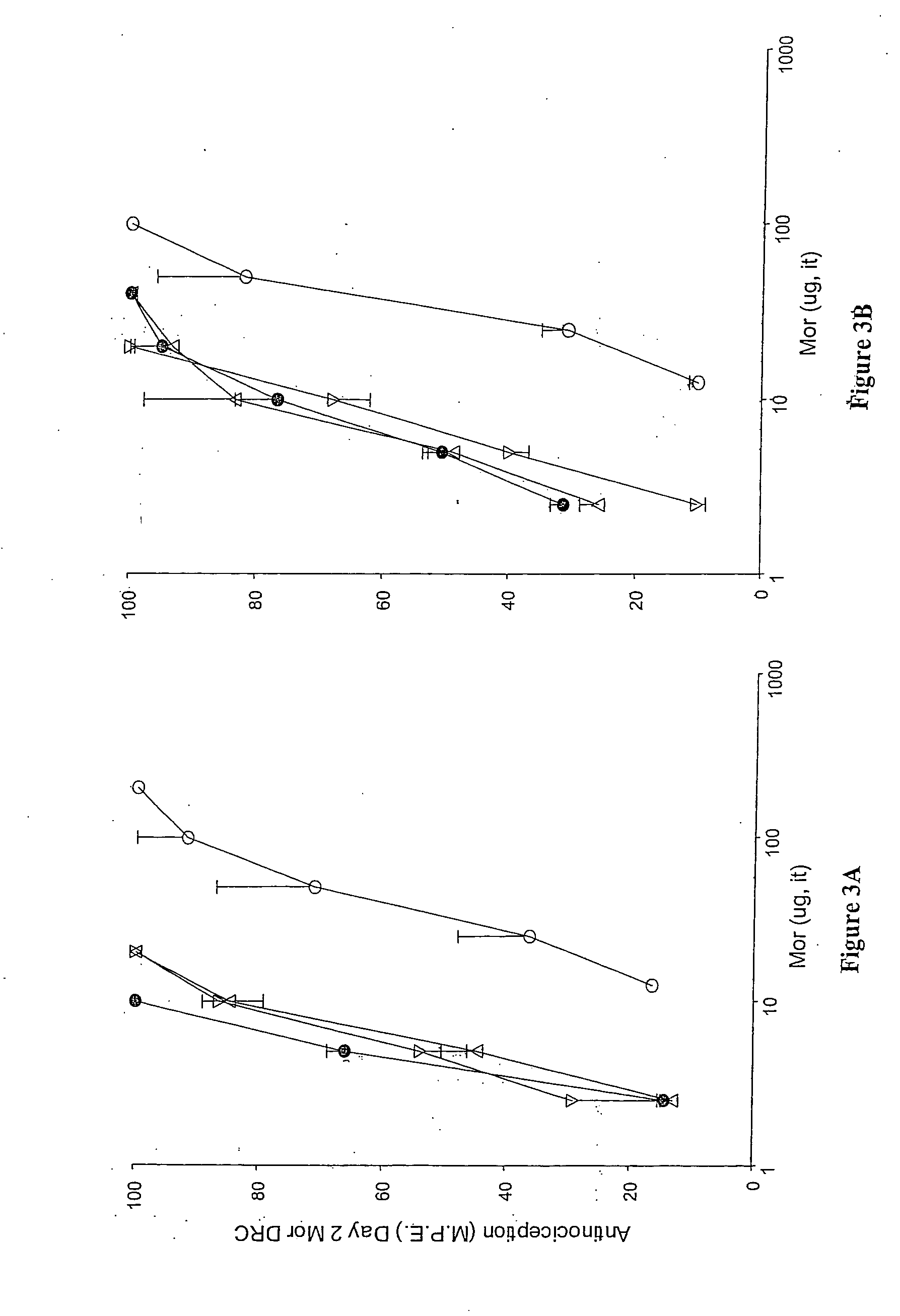
Evaluation of pain in humans
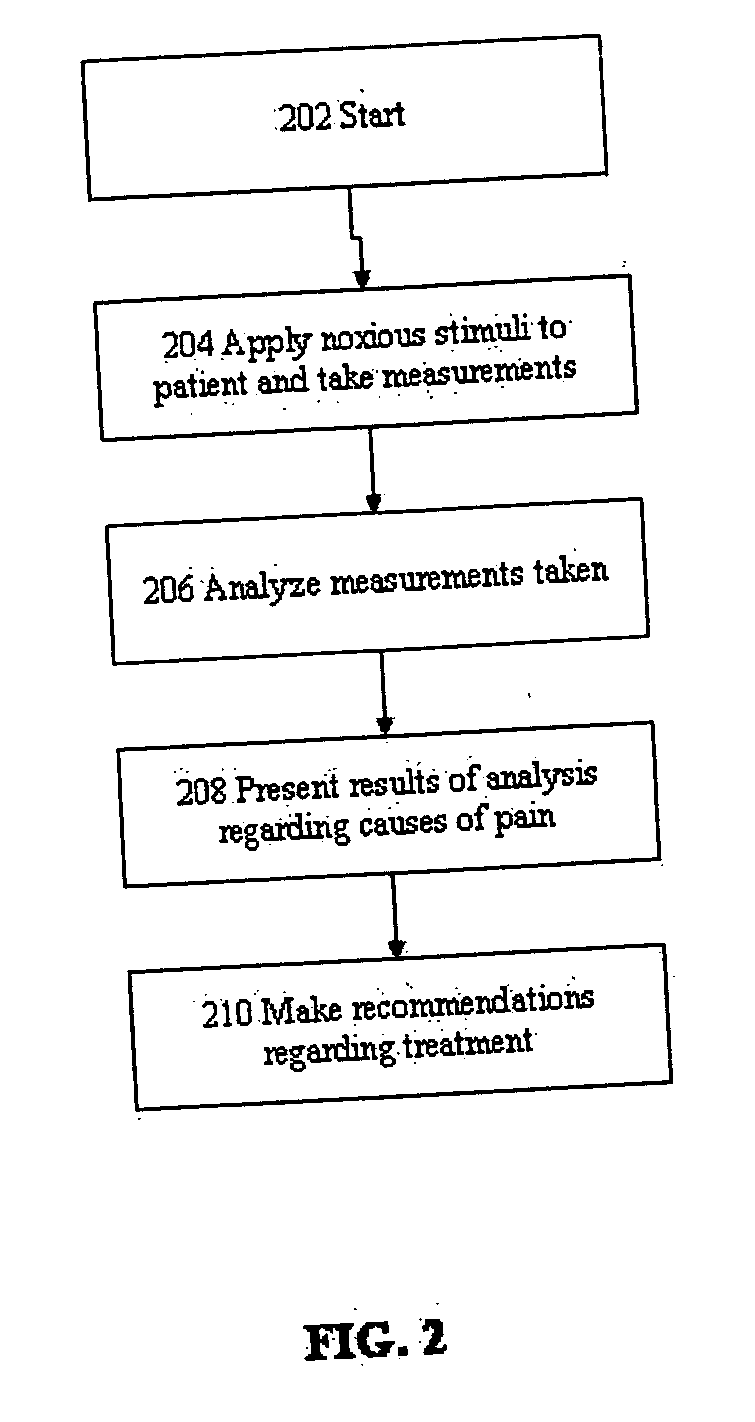
A method for evaluating pain experienced by a human is disclosed. The method includes applying a first noxious stimulus to a normative site on the human, wherein the first noxious stimulus is applied below a pain threshold of the human and logging a first information associated with the first noxious stimulus. The method further includes applying a second noxious stimulus to a source of the pain in the human, wherein the second noxious stimulus is applied until pain threshold is reached and logging a second information associated with the second noxious stimulus. The method further includes increasing the second noxious stimulus until pain tolerance is reached and logging a third information associated with the second noxious stimulus. The method further includes continuing to apply the second noxious stimulus until the human can no longer tolerate the second noxious stimulus and logging a fourth information associated with the second noxious stimulus.
Owner:ROSS DR DAVID BRUCE
Therapeutic agent preparations for delivery into a lumen of the intestinal tract using a swallowable drug delivery device
ActiveCN103025319AImprove pharmacokineticsGood curative effectAntibacterial agentsPeptide/protein ingredientsTolerabilityIntestinal walls
Embodiments of the invention provide swallowable devices, preparations and methods for delivering drugs and other therapeutic agents within the GI tract. Many embodiments provide a swallowable device for delivering the agents. Particular embodiments provide a swallowable device such as a capsule for delivering drugs into the intestinal wall or other GI lumen. Embodiments also provide various drug preparations that are configured to be contained within the capsule, advanced from the capsule into the intestinal wall and degrade within the wall to release the drug to produce a therapeutic effect. The preparation can be coupled to an actuator having a first configuration where the preparation is contained in the capsule and a second configuration where the preparation is advanced out of the capsule into the intestinal wall. Embodiments of the invention are particularly useful for the delivery of drugs which are poorly absorbed, tolerated and / or degraded within the GI tract.
Owner:RANI THERAPEUTICS
Colonic purgative composition with soluble binding agent
ActiveUS20050129781A1Improve dosage form characteristicSimple preparation processBiocideInorganic phosphorous active ingredientsBowel cleansingTolerability
This invention relates to novel colonic purgative compositions in a solid dosage form, comprising at least one purgative and at least one soluble, or soluble, nonfermentable binder, such as polyethylene glycol. Further, this invention relates to methods of using the colonic purgative compositions. The present compositions and methods are designed to improve patient tolerance and compliance, while at the same time improving the quality of bowel cleansing. The formulations and methods of this invention are particularly useful to cleanse the bowel prior to diagnostic and surgical procedures and can also be employed in lower dosages as a laxative to promote elimination and / or to relieve constipation.
Owner:SALIX PHARMA INC
Agents and methods for treatment of disease by oligosaccharide targeting agents
InactiveUS20050026866A1Reduce incidenceSpecific susceptibilityOrganic active ingredientsBiocideTolerabilityMammal
A method for targeting, treating, or diagnosing malignant mammalian tumor cells, comprising administering an effective amount of a β1,6-branched oligosaccharide specific binding agent to the mammal. As a treatment, the binding agent may be intrinsically cytotoxic, initiate an endogenous cytotoxic cascade, or play a role in a cytotoxic cascade involving exogenous factors. A preferred binding agent is Bordetella pertussis, which is both specific for the β1,6-branched oligosaccharide and well tolerated. Genetically engineered organisms may also be employed. Pharmaceutical compositions may also serve as binding agents.
Owner:YALE UNIV
DIETHER BASED BIODEGRADABLE CATIONIC LIPIDS FOR siRNA DELIVERY
ActiveUS20150057373A1Good curative effectReduce liver toxicityBiocideOrganic active ingredientsLipid formationTolerability
Disclosed herein are novel cationic lipids that can be used in combination with other lipid components such as cholesterol and PEG-lipids to form lipid nanoparticles with oligonucleotides. The cationic lipids can demonstrate enhanced efficacy along with lower liver toxicity as a result of lower lipid levels in the liver. The present invention employs low molecular weight cationic lipids with one short lipid chain coupled with inclusion of hydrolysable functionality in the lipid chains to enhance the efficiency and tolerability of in vivo delivery of siRNA.
Owner:SIRNA THERAPEUTICS INC
Surrogate tolerogenesis for the development of tolerance to xenografts
InactiveUS6060049AIncrease differentiationIncreased proliferationBiocideGenetic material ingredientsHematopoietic cellTolerability
PCT No. PCT / US94 / 05844 Sec. 371 Date Jun. 6, 1995 Sec. 102(e) Date Jun. 6, 1995 PCT Filed May 24, 1994 PCT Pub. No. WO94 / 27622 PCT Pub. Date Dec. 8, 1993This invention provides a method for developing immune tolerance in xenogeneic organ graft recipients, in which lympho-hematopoietic cells from an intended organ graft recipient are differentiated within a xenogeneic surrogate, such as a fetal animal. After birth of the surrogate, the matured lympho-hematopoietic cells containing antigen specific regulatory cells, including suppressor cells, veto cells, select B cells, anti-idiotype antibodies, and other related factors responsible for antigen specific tolerance in a surrogate animal are reintroduced into the intended organ graft recipient, in conjunction with an organ transplant or a tissue transplant from the xenograft surrogate. The invention also provides an organ graft repopulated with cells from the intended organ graft recipient produced in a surrogate animal.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Oligonucleotide chelate complexes
ActiveUS20120046348A1Reduce the impactSuppressing and reducingOrganic active ingredientsBiocideTolerabilityDivalent metal
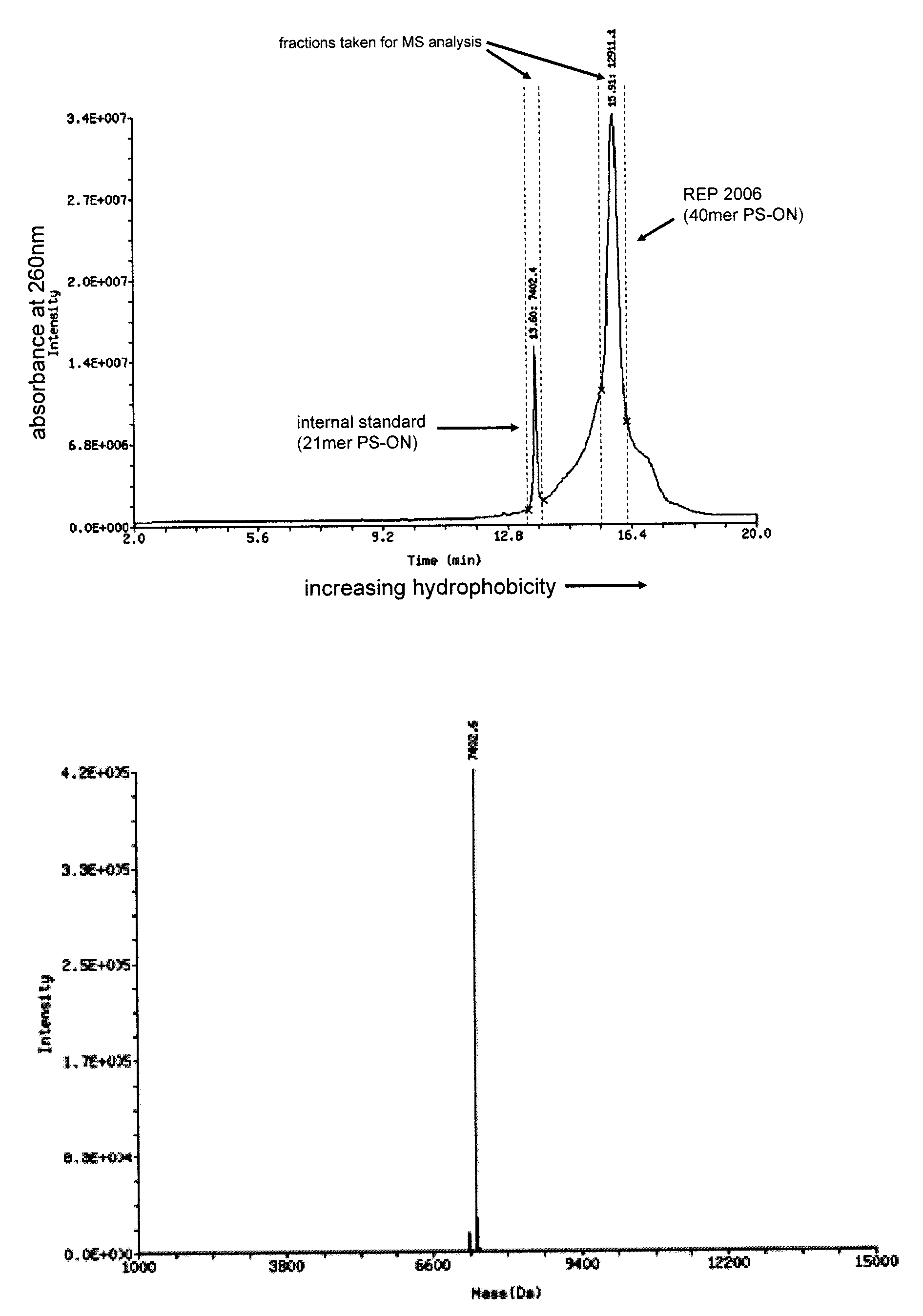
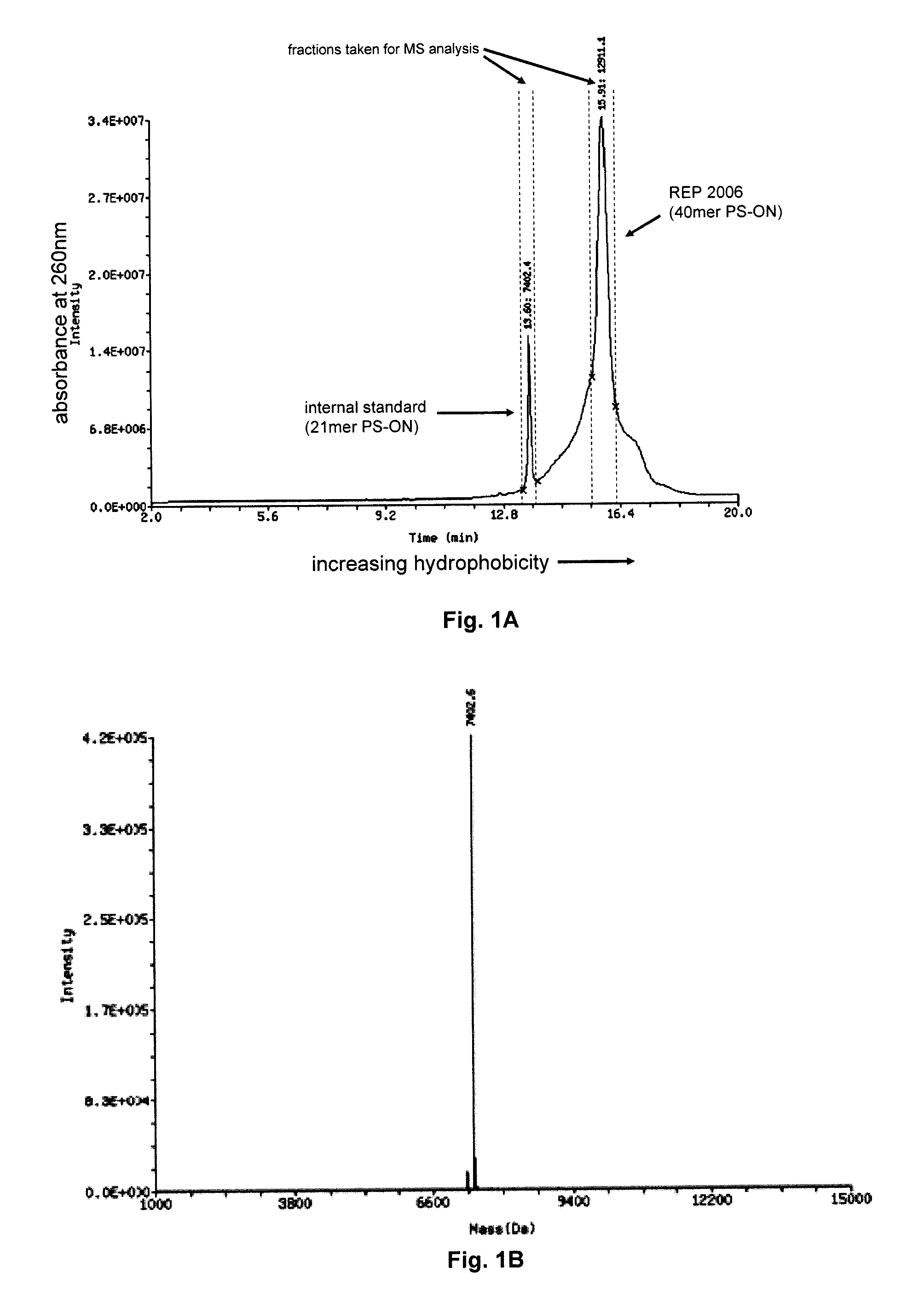
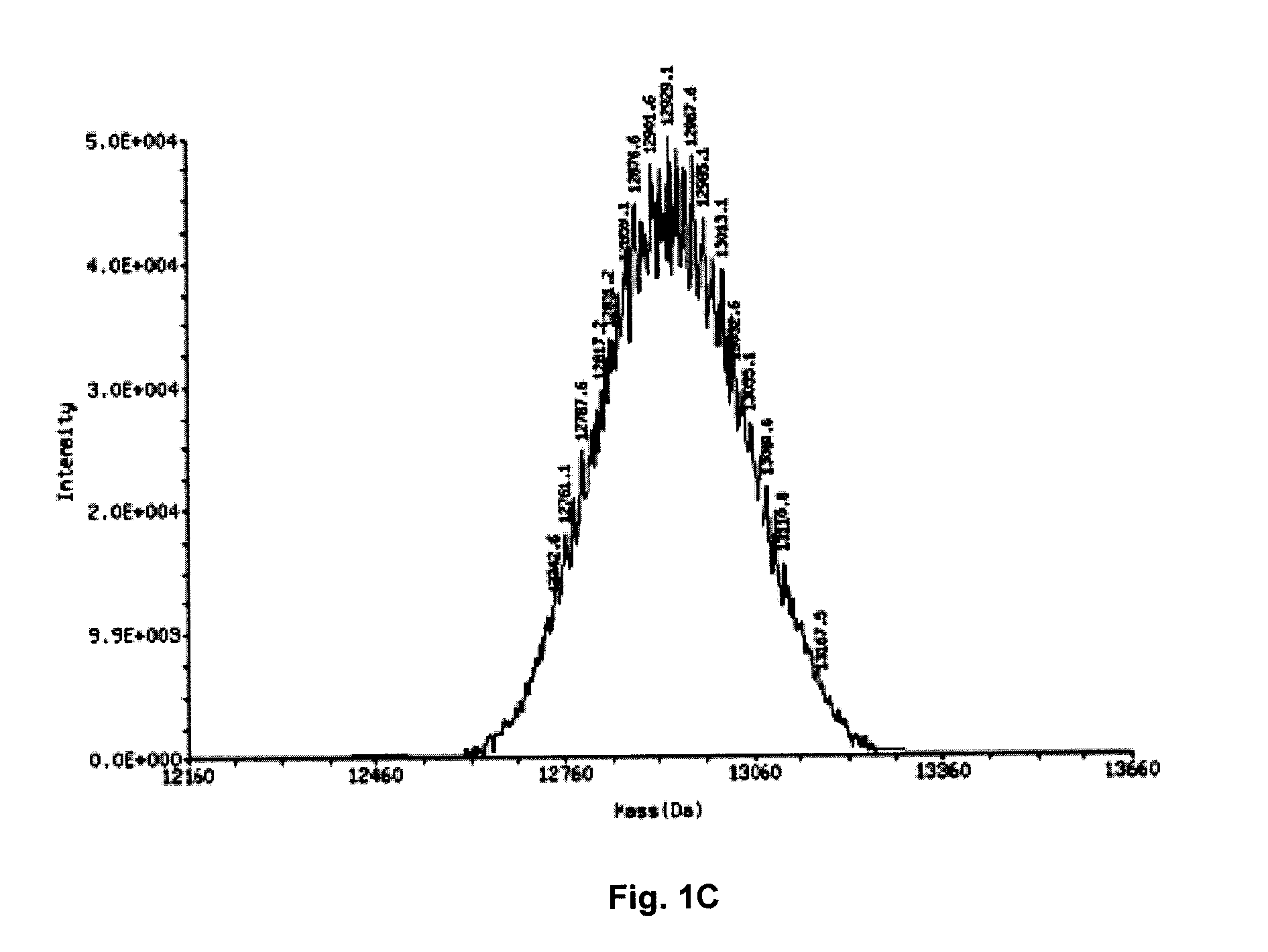
The present disclosure describes the broadly active chelation of diverse divalent 2+ metal cations by any oligonucleotide (ON), regardless of size or modification. This chelation effect is specific to cations which are divalent (or of higher valency) and results in the formation of oligonucleotide chelate complexes which do not behave like salts. It is described herein a novel composition of an ON chelate complex prepared using any ON and a divalent metal cation and methods for the suppression of anti-coagulation and or subcutaneous injection site reactions and or improved tolerability with oligonucleotides by the use of ON chelate complexes during oligonucleotide administration.
Owner:REPLICOR INC
Titration dosing regimen for controlled release tramadol
ActiveUS7413749B2Reduce adverse side effectsImprove toleranceOrganic active ingredientsNervous disorderDosing regimenControlled release
A titration dosing regimen for the administration of controlled release tramadol analgesic to patients. The titration dosing regimen provides a significant reduction in the occurrence of adverse effects from the introduction of controlled released tramadol dosing, thus increasing patient compliance and medication tolerability.
Owner:PURDUE PHARMA LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com