Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
61 results about "Eszopiclone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used to treat a certain sleep problem (insomnia).
Melatonin combination therapy for improving sleep quality
One aspect of the present invention relates to pharmaceutical compositions comprising a sedative agent; and melatonin or a melatonin analog, collectively referred to as “melatonin agents.” In a preferred embodiment, the sedative agent is eszopiclone. The pharmaceutical compositions of the invention are useful in the treatment of various sleep disorders. In addition, the present invention also relates to a method of treating a patient suffering from a sleep abnormality or insomnia comprising administering a therapeutically effective amount of a pharmaceutical composition of the invention.
Owner:SEPACOR INC
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:SEPACOR INC
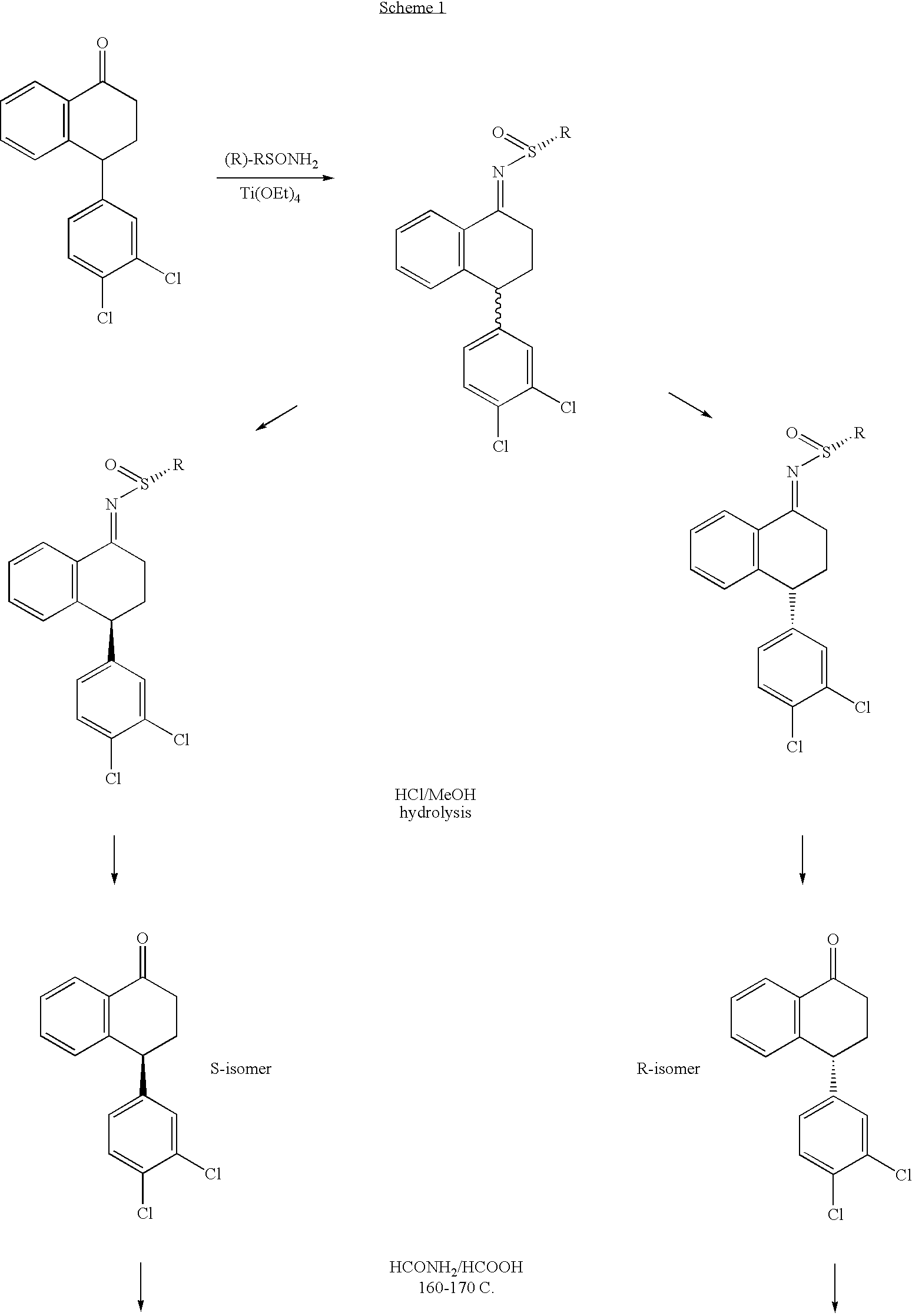
Combinations of Eszopiclone and Trans 4-(3,4-Dichlorophenyl)-1,2,3,4-Tetrahydro-N-Methyl-1-Napthalenamine or Trans 4-(3,4-Dichlorophenyl)-1,2,3,4-Tetrahydro-1-Napthalenamine, and Methods of Treatment of Menopause and Mood, Anxiety, and Cognitive Disorders
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is trans 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1-napthalenamine or trans 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-napthalenamine. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Combinations of Eszopiclone and an Antidepressant
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is an antidepressant. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Treatment of anxiety with eszopiclone
InactiveUS20080175903A1Improved liabilityImprove securityBiocideNervous disorderANXIETY COMPLEXAnxiety
The present disclosure provides a unit dosage form with an anxiolytic dosage of zopiclone particularly eszopiclone. Also provided is a method for treatment or prophylaxis of anxiety using a subsedative dosage of zopiclone particularly eszopiclone.
Owner:SEPACOR INC
Combination of sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., insomnia and / or depression. The first component of the pharmaceutical composition is a GABA receptor modulating compound. The second component of the pharmaceutical composition is a serotonin reuptake inhibitor, a norepinephrine reuptake inhibitor, a 5-HT2A modulator, or dopamine reuptake inhibitor. In certain embodiments, the pharmaceutical composition comprises eszopiclone. In a preferred embodiment, the pharmaceutical composition comprises eszopiclone and fluoxetine. The present invention also relates to a method of treating a sleep abnormality, treating insomnia, treating depression, augmenting antidepressant therapy, eliciting a dose-sparing effect, reducing depression relapse, improving the efficacy of antidepressant therapy or improving the tolerability of antidepressant therapy, comprising co-administering to a patient in need thereof a GABA-receptor-modulating compound; and a SRI, NRI, 5-HT2A modulator or DRI.
Owner:WOODWARD SPECIALTY LLC
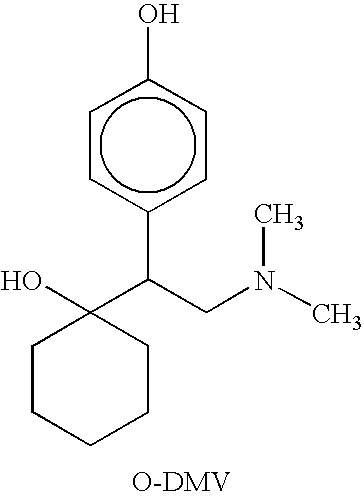
Combinations of Eszopiclone and O-Desmethylvenlafaxine, and Methods of Treatment of Menopause and Mood, Anxiety, and Cognitive Disorders
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is O-desmethylvenlafaxine. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Process for the preparation of eszopiclone
InactiveUS20080146800A1Minimizes problemEfficient and cost-effective processOrganic chemistryMetaclazepamPyrazine
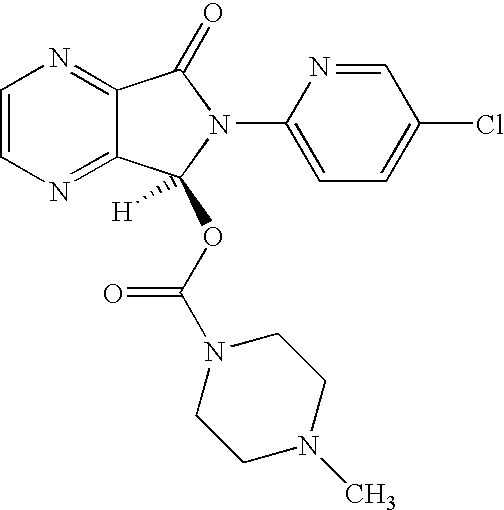
The invention relates to a process for making of 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo-[3,4-b] pyrazin-5-yl-4-methyl piperazine-1-carboxylate, also known as zopiclone. The invention further describes an effective method for resolving of zopiclone into its enantiomers (eszopiclone and (R)-zopiclone) and also provides a method of recycling of (R)-zopiclone.
Owner:CENTAUR CHEM PVT +1
Eszopiclone preparation method
The invention relates to an eszopiclone preparation method comprising main steps that: zopiclone is subjected to a reaction with D-dibenzoyltartaric acid or a hydrate thereof, such that dextral zopiclone-D-dibenzoyltartrate is produced; and the salt is dissociated, such that eszopiclone is obtained. According to the method, D-dibenzoyltartaric acid with a substance amount of a quarter to a half of that of zopiclone is adopted. The reaction conditions are mild, operation is convenient, product yield is high, and product purity is high. The method is suitable for large-scale industrialized productions.
Owner:四川弘远药业有限公司
Methods for preparing eszopiclone crystalline form a, substantially pure eszopiclone and optically enriched eszopiclone
The present invention provides methods for preparing eszopiclone Form A, substantially chemically pure eszopiclone, or eszopiclone with low level(s) of residual solvent(s). The present invention also provides eszopiclone with low level(s) of residual solvent(s). The present invention also provides a process for optical enrichment of eszopiclone free base. For instance, one of the embodiments of the invention is directed to a method of preparing eszopiclone Form A, wherein the method comprises crystallizing eszopiclone free base from a solvent selected from the group consisting of isopropanol (IPA), methyl isobutyl ketone (MIBK), acetone, n-butanol, i-butanolisobutanol, 2-butanol, tetrahydrofuran (THF), dimethyl carbonate, methanol, ethanol, ethyl lactate, dimethylformamide (DMF), carbon tetrachloride, toluene, iso-butyl acetate and mixtures thereof.
Owner:TEVA PHARM USA INC
Treatment of obstructive sleep apnea syndrome with a combination of a carbonic anhydrase inhibitor and an additional active agent
InactiveUS20110224196A1Amelioration of the extent of each apneaAlleviate excessive daytime sleepinessBiocideNervous disorderZaleplonActive agent
This invention relates generally to methods and pharmaceutical formulations useful in treating patients suffering from obstructive sleep apnea syndrome (OSAS). Treatment of OSAS is effected by administering a carbonic anhydrase inhibitor to the patient in combination with at least one additional active agent. Examples of additional active agents include modafinil, eszopiclone, zolpidem, zaleplon, and phentermine.
Owner:VIVUS
Eszopiclone process
InactiveUS7476737B2Simple, efficient, inexpensive, ecofriendlyImprove scalabilityBiocideOrganic chemistryPyrazinePyridine
Owner:DR REDDYS LAB LTD +1
Dexzopiclone oral fast-dissolving film and preparation method thereof
InactiveCN105343887AMask bitternessGood water solubilityOrganic active ingredientsNervous disorderOlder peopleEszopiclone
The invention belongs to the field of a pharmaceutical preparation, and in particular relates to a dexzopiclone oral fast-dissolving film and a preparation method thereof. The film mainly consists of such ingredients as a dexzopiclone inclusion compound, a film-forming material, a filling agent, a plasticizer, a flavoring agent and an antioxidant. The preparation method comprises the following processes: (1) preparing the dexzopiclone inclusion compound; (2) preparing a blank glue solution; (3) preparing a dexzopiclone drug-containing glue solution; and (4) coating and drying the drug-containing glue solution. The oral fast-dissolving film is capable of masking the bitterness of the dexzopiclone, and the film is unnecessary to be taken with water and is suitable for children, old people and insomnia patients having difficulty in swallowing; and moreover, the oral fast-dissolving film is good in flexibility and not easy to become broken, and the film is capable of fast dissolving in an oral cavity and is high in bioavailability.
Owner:JINAN KANGHE MEDICAL TECH
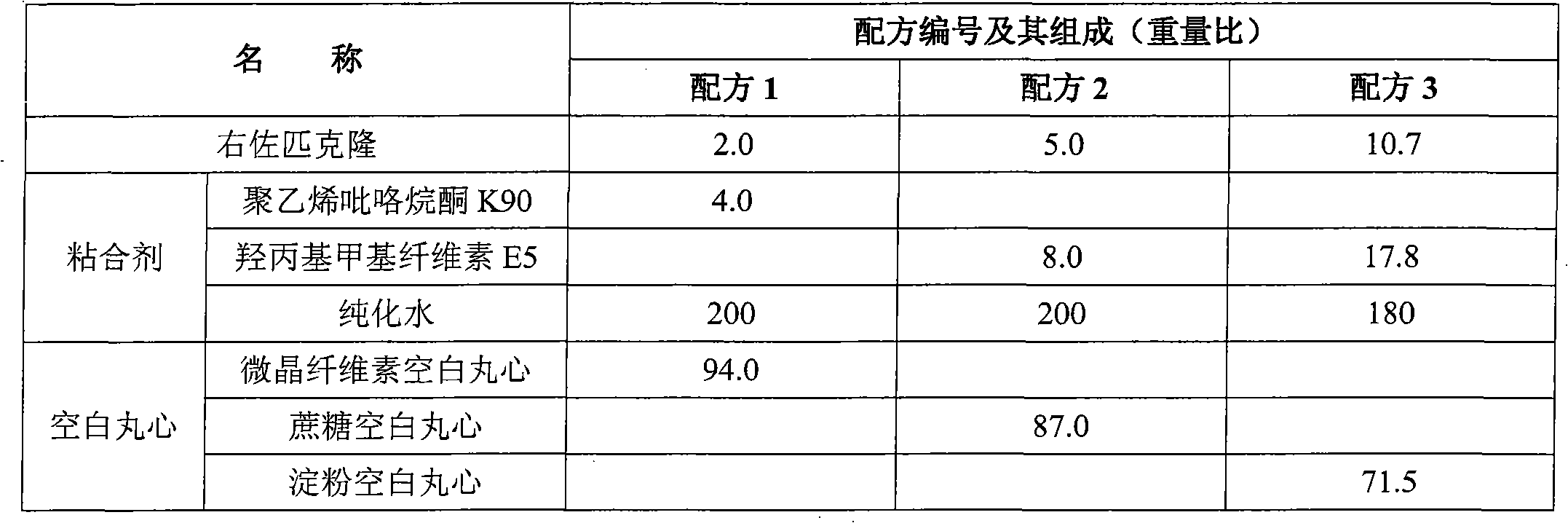
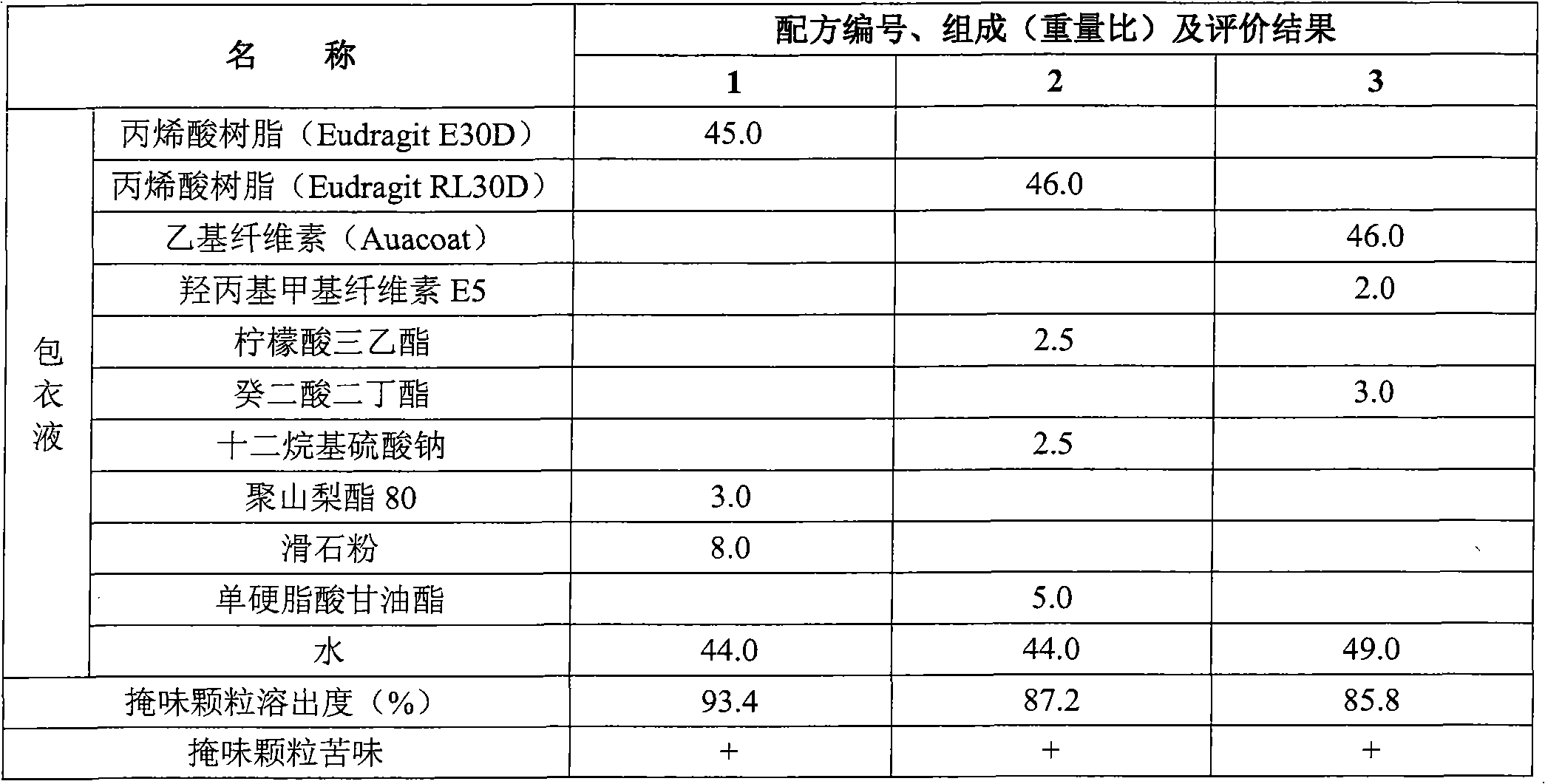
Eszopiclone-containing particle and its preparation method
ActiveCN102727452AAdvantages of preparation processImprove use complianceOrganic active ingredientsNervous disorderOrally disintegrating tabletDissolution
The invention relates to an eszopiclone-containing particle and its preparation method. The particle can cover the bitter taste of eszopiclone, has no obvious sandy feeling, has uniform contents and high dissolution rate, and can be used for preparing solid dosage forms such as orally disintegrating tablets, dispersing tablets, suspension granules and the like.
Owner:CHENGDU KANGHONG PHARMA GRP
Medicinal composition containing Esopiclone and preparation method thereof
ActiveCN102232953AImprove bioavailabilityImprove securityOrganic active ingredientsNervous disorderMedicineDissolution
The invention discloses a medicinal composition containing Esopiclone and a preparation method thereof. The Esopiclone-containing medicinal composition consists of a main medicine and auxiliary materials. In the invention, by controlling the particle size of the main medicine, a pre-mixing method and the auxiliary materials, the medicinal composition which has high dissolution rate and high content uniformity can be obtained. The preparation method adopts a modern preparation technique, the quality of the product is stable, and an Esopiclone-containing solid oral preparation which is safe, effective, quick in absorption, high in bioavailability and accurate in dosage is provided for patients.
Owner:CHENGDU KANGHONG PHARMA GRP
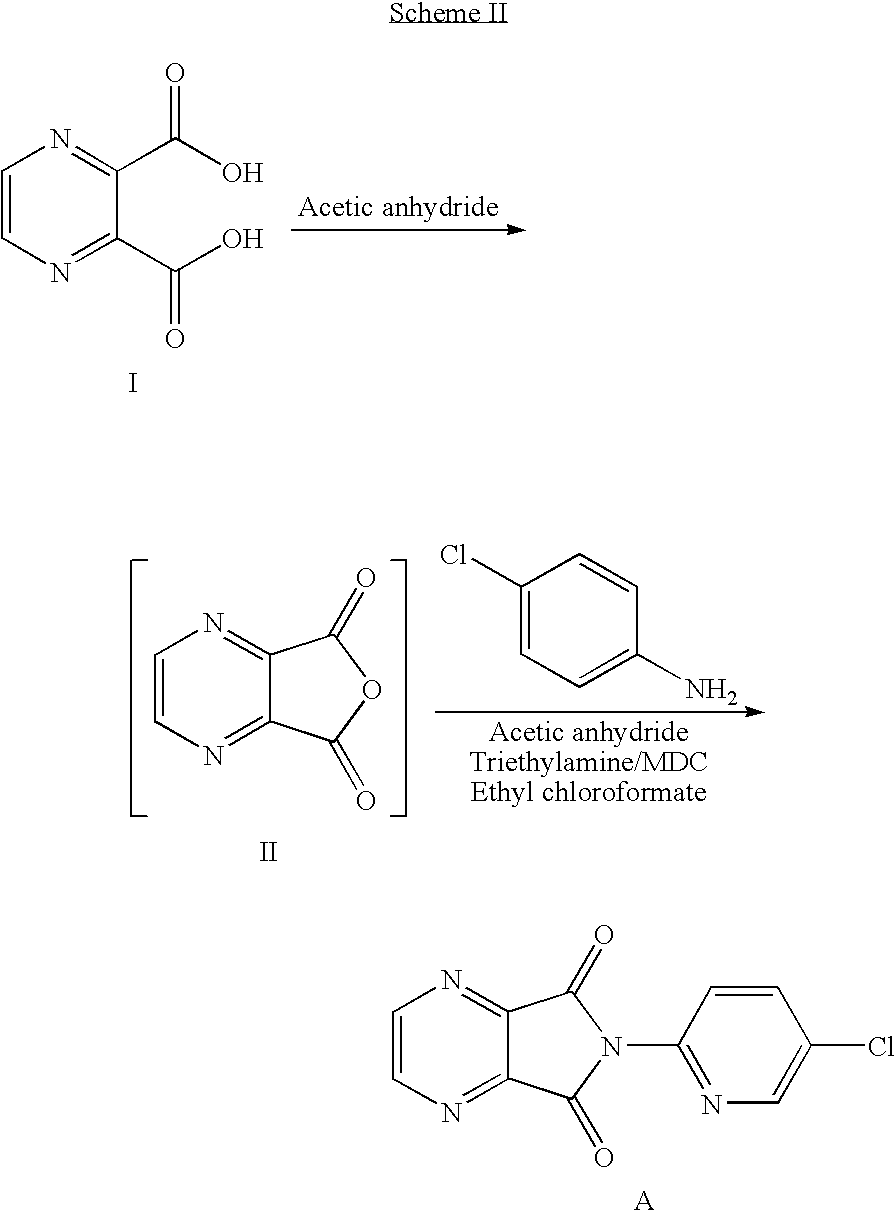
Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine
ActiveCN101058581AEasy post-processingPromote safe productionOrganic chemistryOrganic solventMorpholine
The invention discloses a making method of 6-(5-chlorine-2-pyridine)-5,7-dioxy-6,7-dihydrogen-5H-pyrrole [3,4-b] pyrazine (I) as intermediate of right-adjuvant clone, which comprises the following steps: dissolving 3-(5-chloropyridine-2-amino)formyl morpholine pyrazine-2-carboxyl acid (II) into the composite liquid of organic solvent / amine; adding chloroformate in the reacting liquid; obtaining the product I.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD +1
Sedative-hypnotic preparation, compound preparation, preparation method and pharmaceutical composition thereof
ActiveCN103919780AImprove stabilityQuality assuranceOrganic active ingredientsNervous disorderOrganic acidSedative/hypnotic
The invention relates to a sedative-hypnotic preparation, a compound preparation, a preparation method and a pharmaceutical composition thereof. The compound preparation of the sedative-hypnotic preparation includes zopiclone or eszopiclone and a pharmaceutically acceptable auxiliary material which includes a stabilizing agent with a dosage being, by mass, 0.1%-10% of the zopiclone or the eszopiclone. The stabilizing agent is one or more of a pharmaceutically acceptable organic acid salt, a pharmaceutically acceptable organic acid buffer pair and a pharmaceutical acceptable anti-oxidant. The pharmaceutical composition of the sedative-hypnotic preparation forms a compound preparation pharmaceutical composition with other pharmaceutical active substances. The preparation method of the sedative-hypnotic preparation or the compound preparation includes carrying out a wet granulation process to the pharmaceutical composition of the sedative-hypnotic preparation or the compound preparation pharmaceutical composition. The sedative-hypnotic preparation or the compound preparation is good in stability and is excellent in dissolution rate and content uniformity. Quality of the preparations can be ensured and the preparations are suitable for industrial production.
Owner:SHANGHAI ZHONGXI PHARMA
Novel Process
The present invention relates to a process for optically resolving eszopiclone, comprising chiral chromatography. Preferably the process comprises a multi-column continuous process or a simulated moving bed process. Preferably the stationary phase used in the chiral chromatography process comprises an amylose or cellulose derivative of tris(3,5-dimethylphenyl carbamate), or an amylose derivative of tris-α-methylbenzylcarbamate. The process of the present invention has the advantage that it is high yielding and can be carried out on an industrial scale.The present invention also provides eszopiclone, or a pharmaceutically acceptable salt thereof, obtained by the chiral chromatography process. The eszopiclone or salt thereof is suitable for use as a medicament, for example, for the treatment of anxiety or insomnia.
Owner:GENERICS UK LTD
Chiral synthesis of Eszopiclone
InactiveCN102675318AAtom utilization is highReasonable designOrganic chemistrySynthesis methodsPyrazine
The invention relates to a chiral synthesis method for Eszopiclone and belongs to the technical field of medicine. The chiral synthesis method for Eszopiclone comprises the following two steps of reaction: (1), reducing under the action of a 6-(5-chlorine-2-pyridyl)-5,6-dioxo-6,7-dihydro-5H-pyrrol(3,4-b)pyrazine chiral reagent to obtain 6-(5-chlorine-2-pyridyl)-5(S)-hydroxyl-7-oxo-6,7-dihydro-5H-pyrrol(3,4-b)pyrazine; and (2), reacting the 6-(5-chlorine-2-pyridyl)-5(S)-hydroxyl-7-oxo-6,7- dihydro-5H-pyrrol(3,4-b)pyrazine with 1-chloroformyl-4-methylpiperazine hydrochloride under the action of organic base to obtain the Eszopiclone. The chiral synthesis method for the Eszopiclone has the advantages of high atom utilization ratio, short steps and advanced technology.
Owner:SHANGHAI ZNBIOCHEM
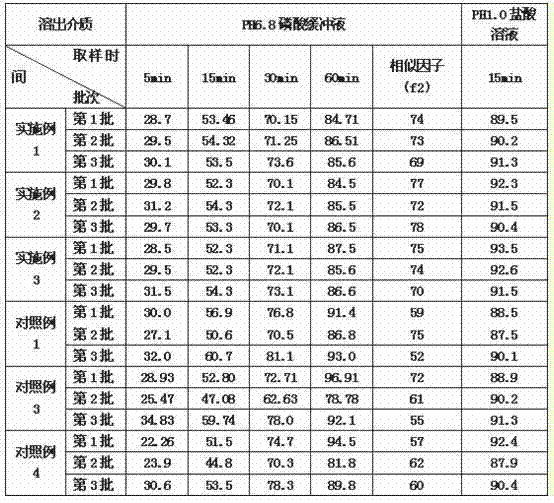
Eszopiclone solid preparation and preparation method thereof
ActiveCN102106824AEasy to operateImprove securityOrganic active ingredientsNervous disorderMechanical crushingZopiclone
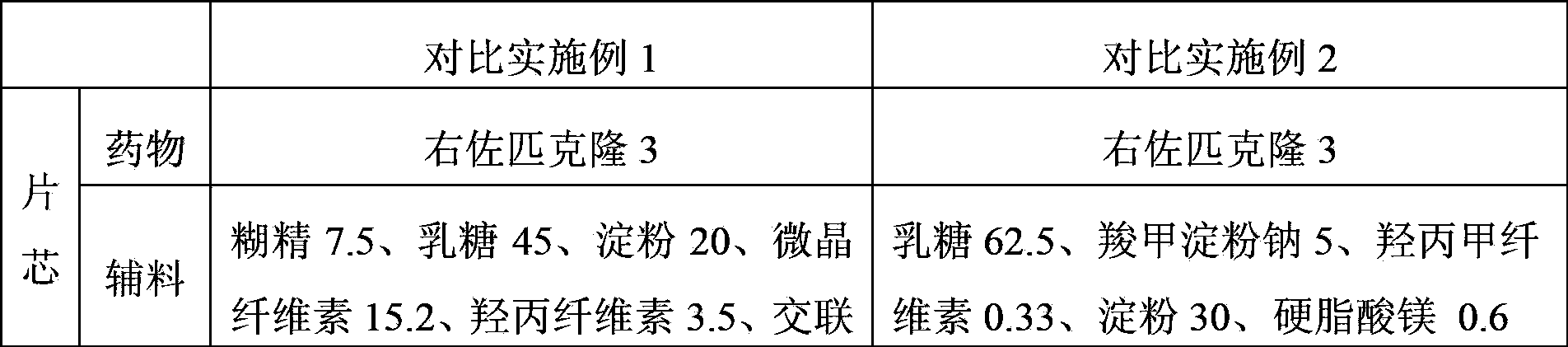
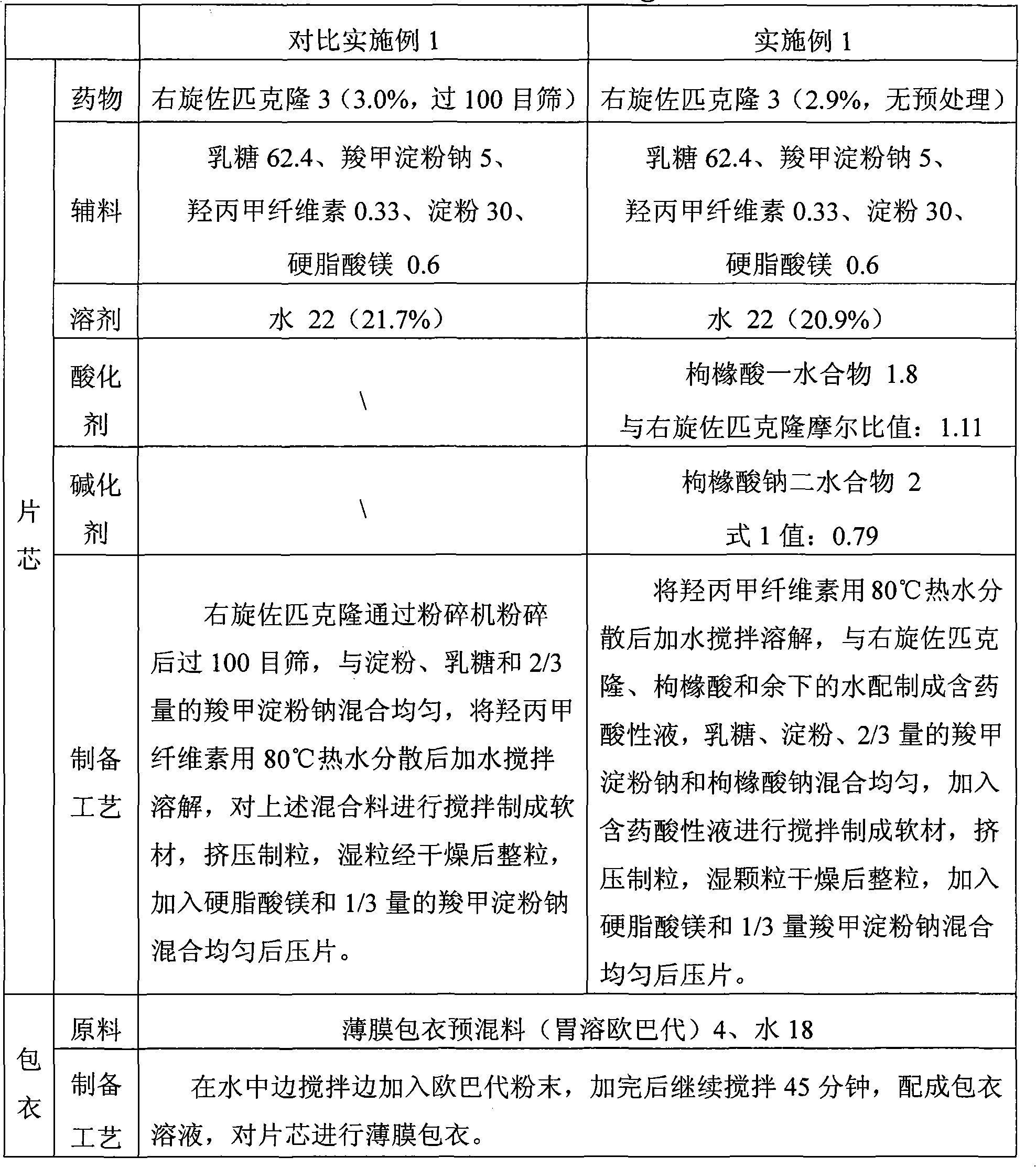
The invention discloses the preparation method of eszopiclone solid preparation, which comprises the following steps of dissolving eszopiclone into acidic solution containing an acidifying agent to obtain medicine-containing acidic solution; and uniformly mixing alkalinizing agent, accessories and the medicine-containing acidic solution to granulate by a wet method, wherein the alkalinizing agentis agent by which the acidity of mixed solution of the alkalinizing agent and the medicine-containing acidic solution is reduced relative to the acidity of the medicine-containing acidic solution. The invention also discloses eszopiclone solid preparation prepared by the method. According to the method disclosed by the invention, the defects of the serious pollution, high loss and serous potential safety hazards brought by mechanical crushing treatment are avoided; and the method is simple, convenient and feasible for operation and easy for industrial production and has high safety coefficient. The eszopiclone solid preparation prepared by the method has the advantages of excellent dissolution property, stability, and content uniformity.
Owner:SHANGHAI ZHONGXI PHARMA +1
Process for the preparation of eszopiclone
InactiveUS7786304B2Minimizes problemEfficient and cost-effective processOrganic chemistryPyrazineAcyl group
Owner:CENTAUR CHEM PVT +1
Racemization method of eszopiclone
The invention relates to a racemization method of eszopiclone. According to the invention, tetramethylguanidine which is cheap and easily available is used as a racemization agent. The method provided by the invention is simple to operate, is safe and stable, has advantages of high yield and good product quality, and is suitable for industrial production.
Owner:四川弘远药业有限公司
A kind of preparation method of eszopiclone
The invention relates to an eszopiclone preparation method comprising main steps that: zopiclone is subjected to a reaction with D-dibenzoyltartaric acid or a hydrate thereof, such that dextral zopiclone-D-dibenzoyltartrate is produced; and the salt is dissociated, such that eszopiclone is obtained. According to the method, D-dibenzoyltartaric acid with a substance amount of a quarter to a half of that of zopiclone is adopted. The reaction conditions are mild, operation is convenient, product yield is high, and product purity is high. The method is suitable for large-scale industrialized productions.
Owner:四川弘远药业有限公司
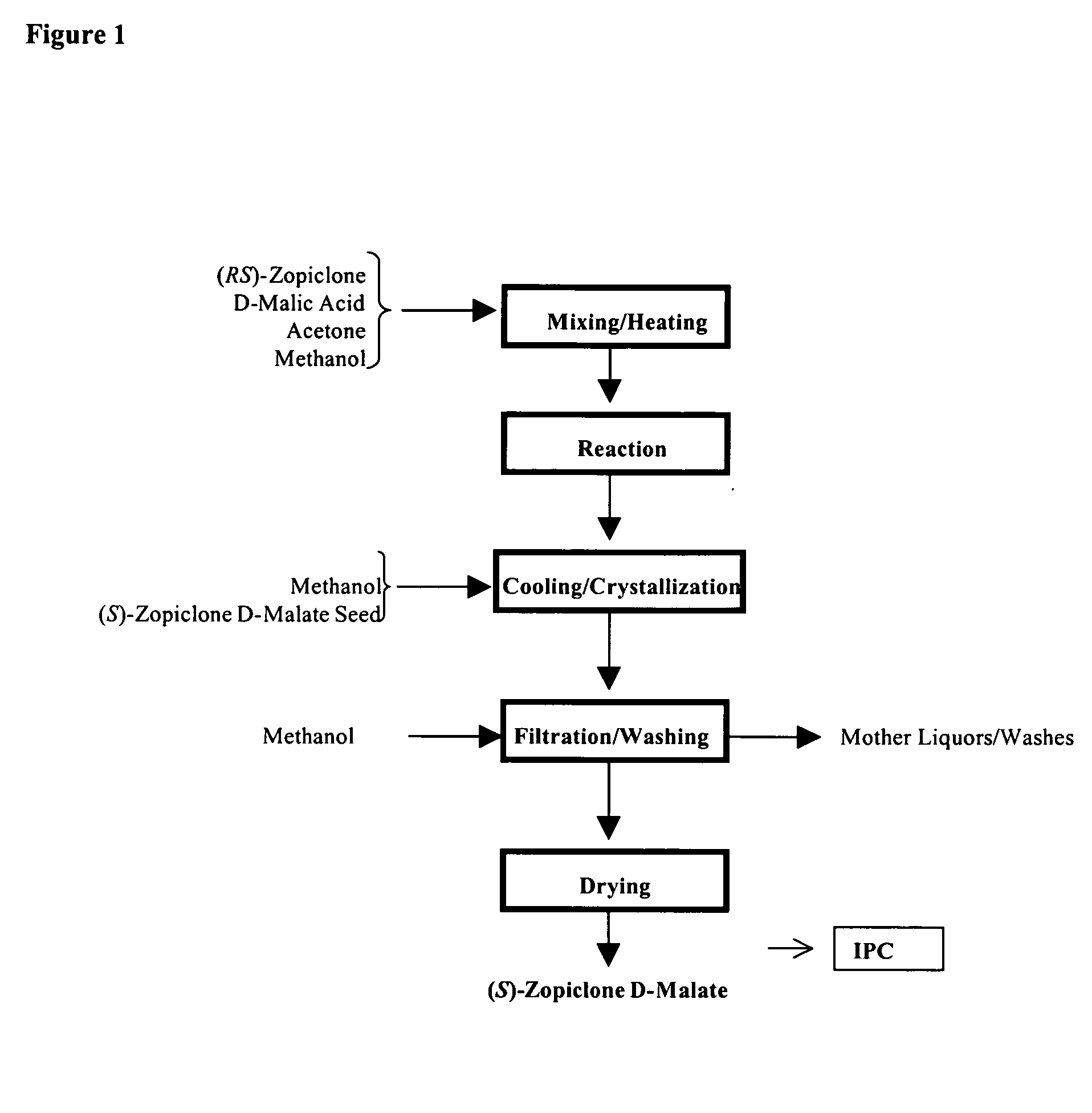
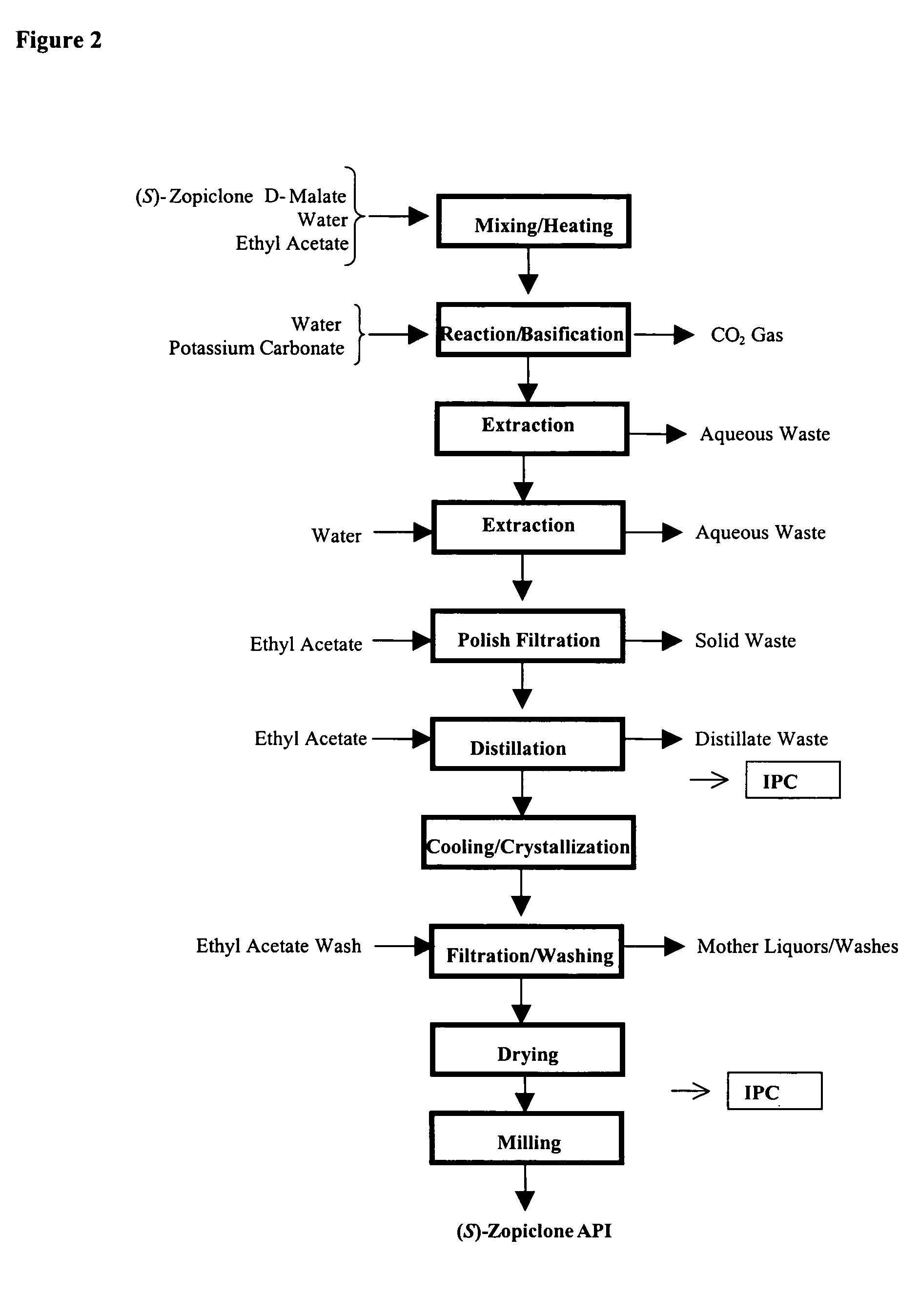
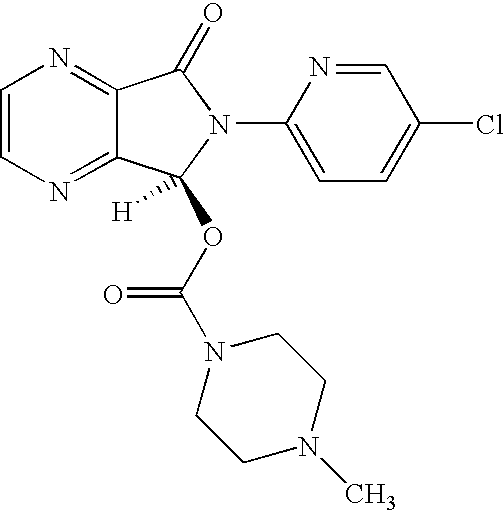
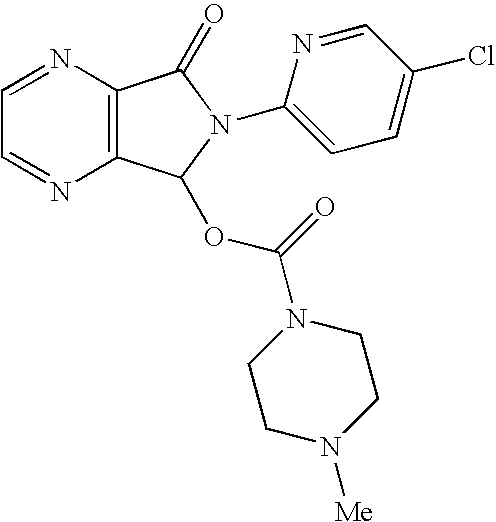
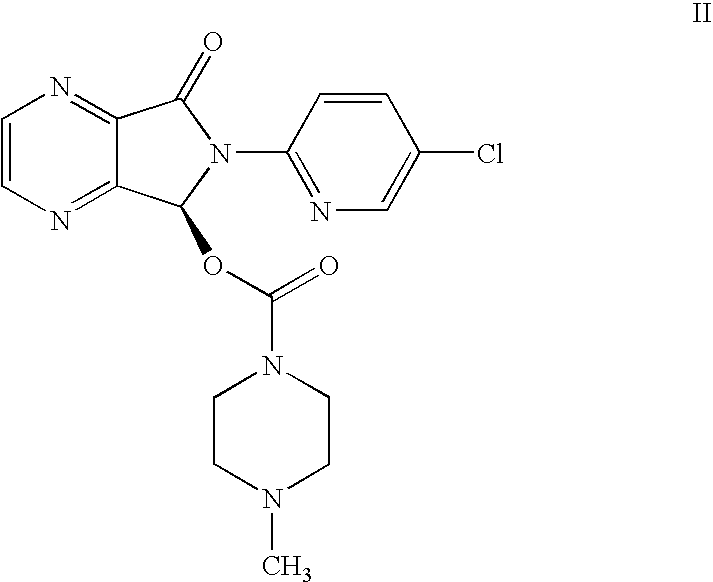
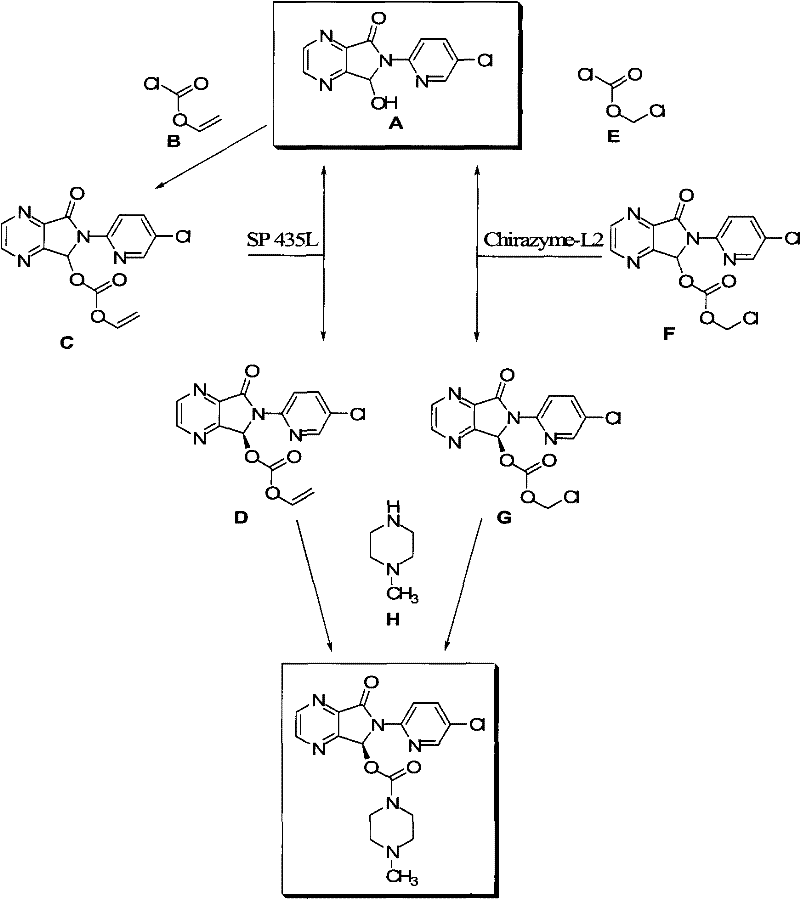
Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone)
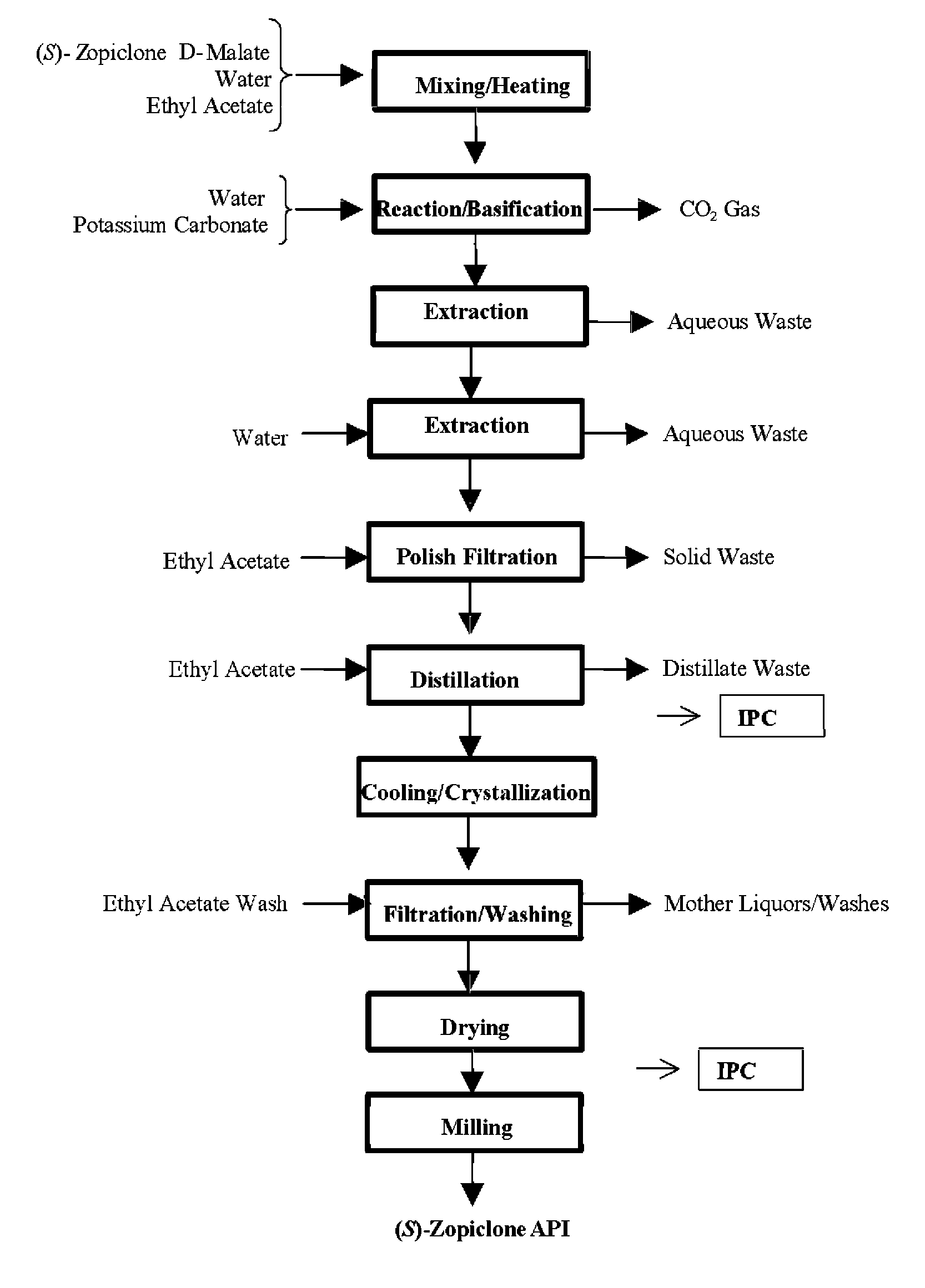
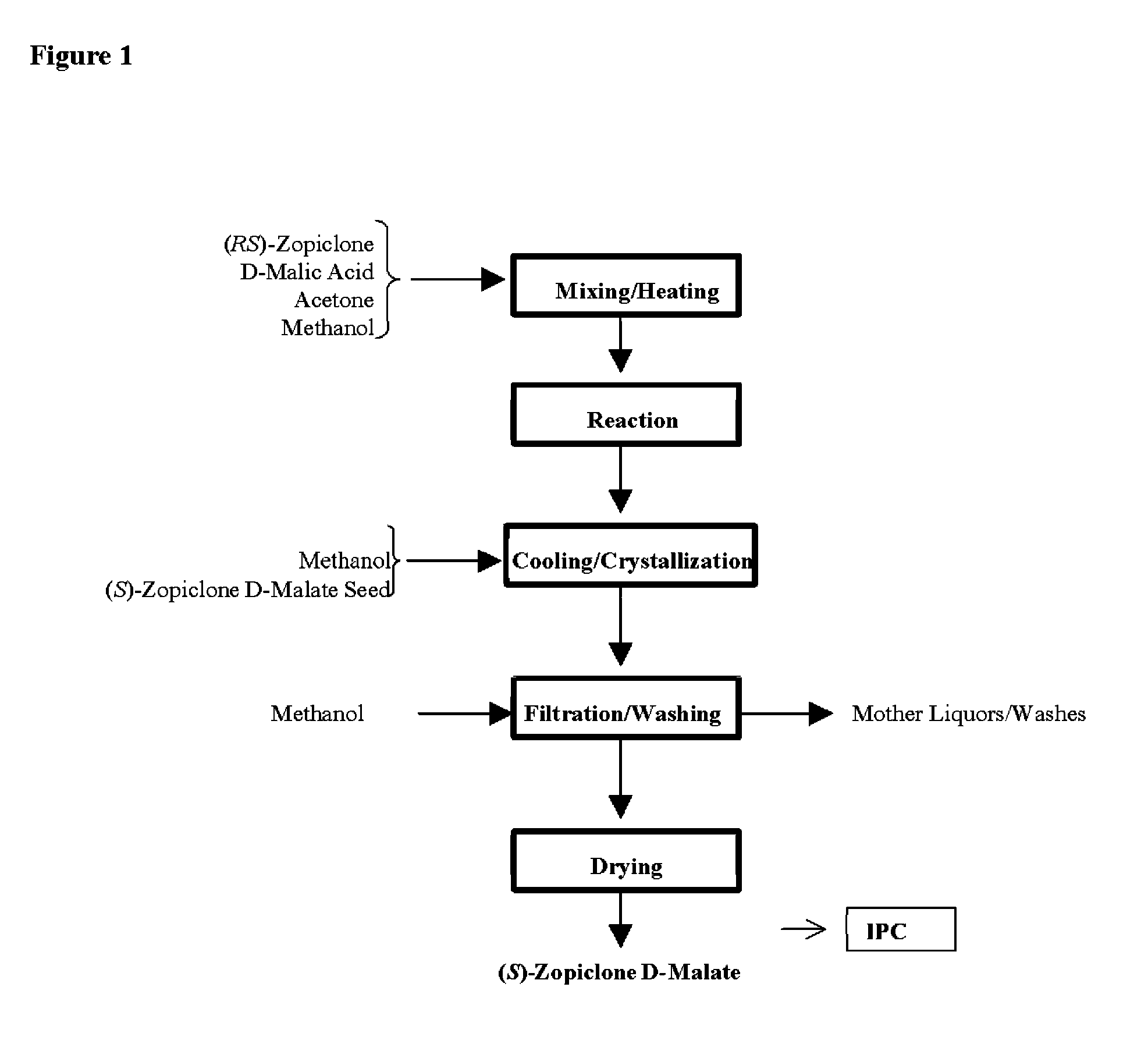
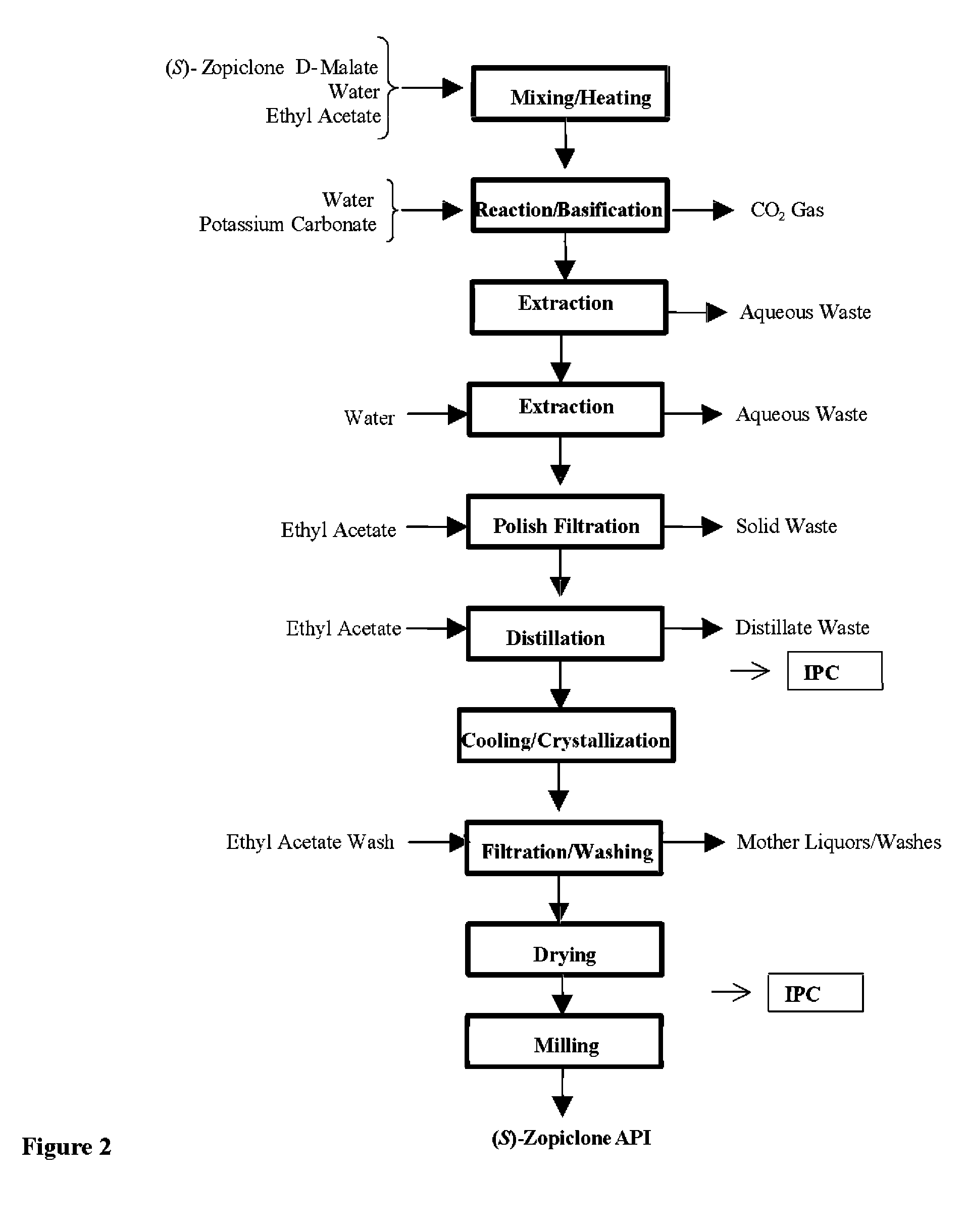
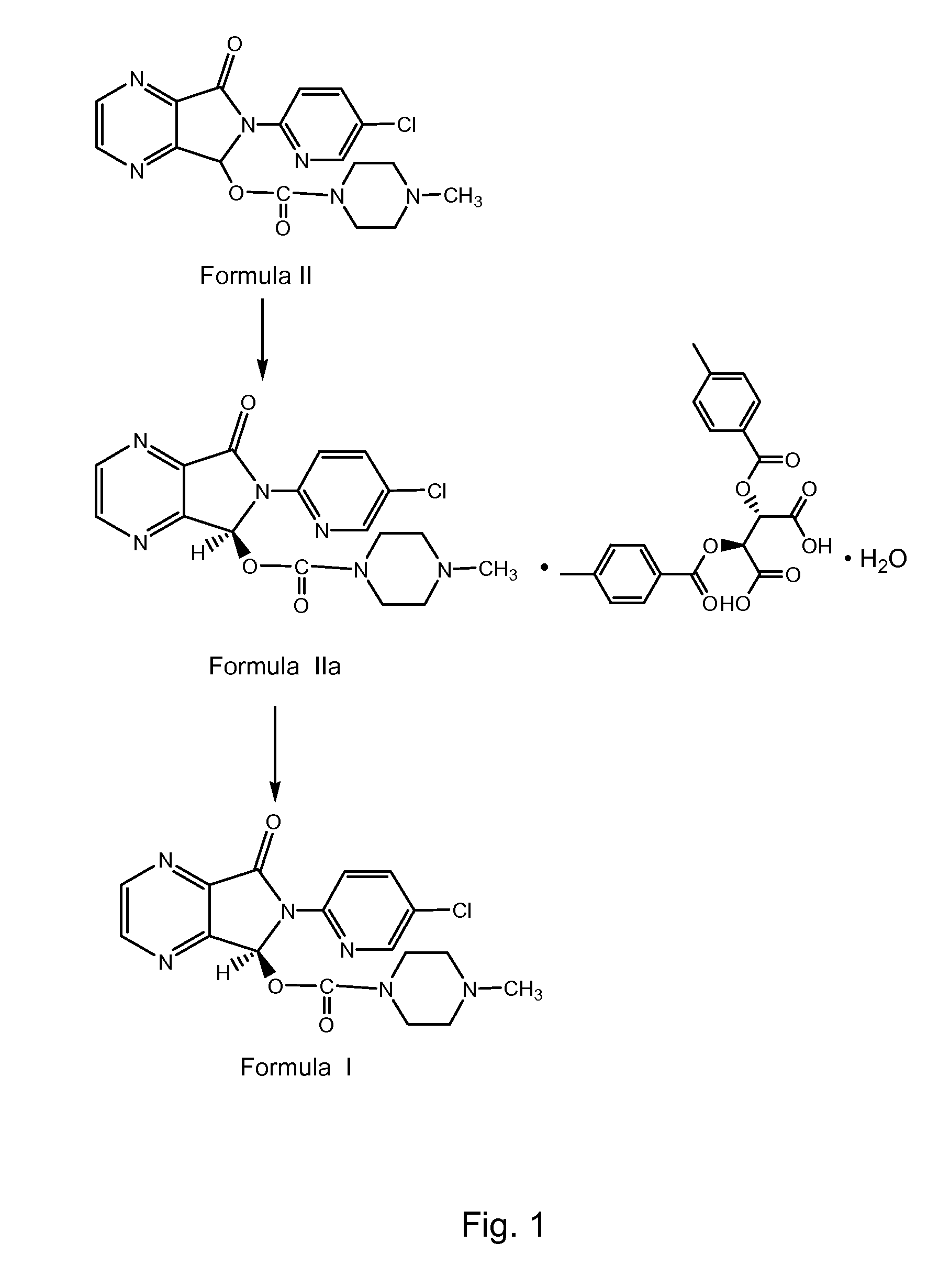
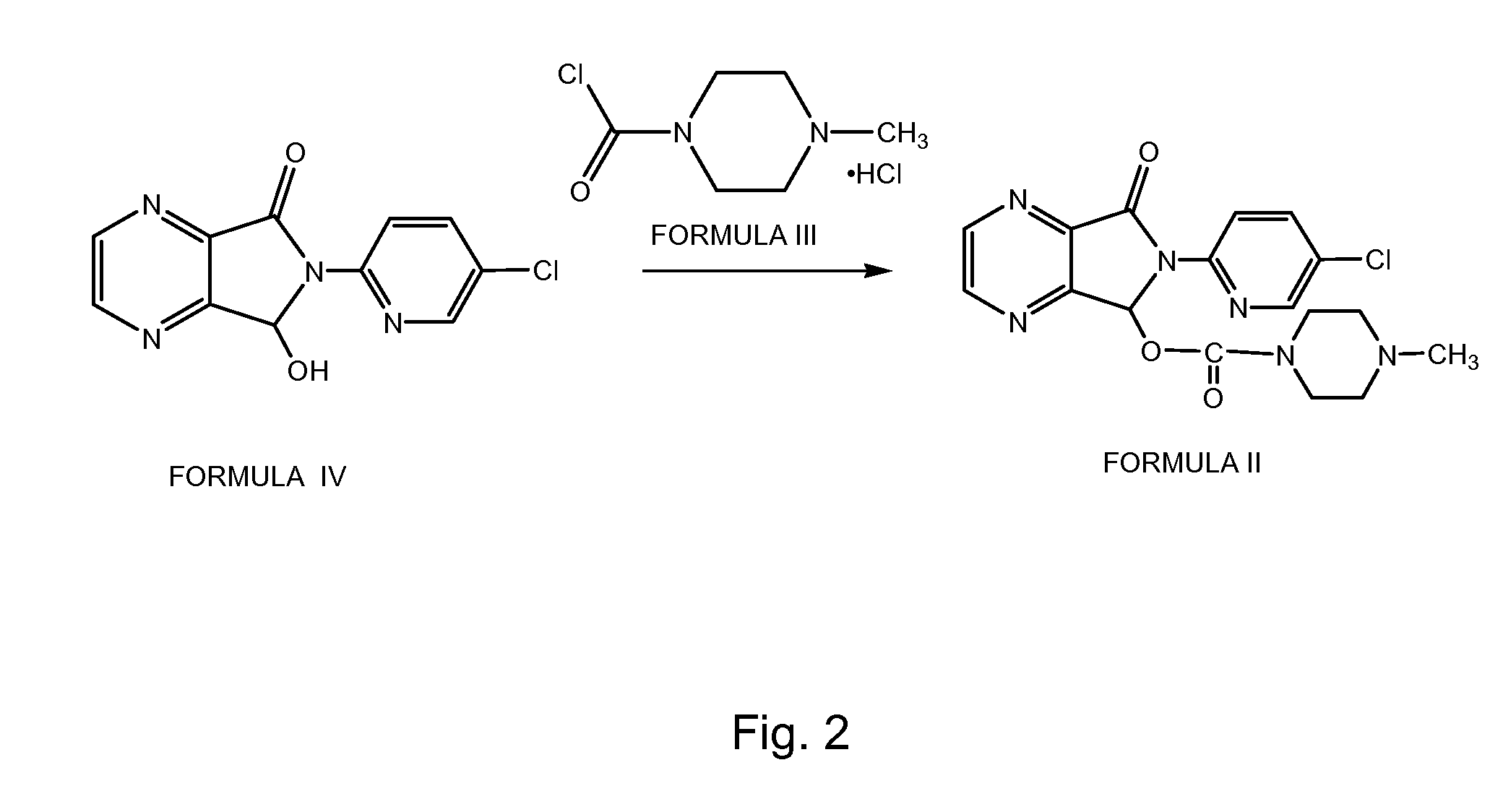
Disclosed herein is the process for preparation of 6-(5-chloro-pyrid-2-yl)-5-[(4-methyl-1-piperazinyl)carbonyloxy]-7-oxo-6,7-dihydro-5H-pyrrolo[3,4-b]pyrazine (Zopiclone), its resolution to get the dextrorotatory isomer of formula (I) substantially free of R(−) enantiomer and recovery of key raw material i.e. 6-(5-chloro pyrid-2-yl)-5-hydroxy-7-oxo-5,6-dihydropyrrolo[3,4-b]pyrazine from the R-isomer of Zopiclone followed by conversion of the recovered compound to get pure Eszopiclone (I) in high yield and high purity.
Owner:USV LTD
Pharmaceutical composition
InactiveCN104706615AImprove the operating environmentSolve the problem of large differences in dissolution between different batchesOrganic active ingredientsNervous disorderStearic acidCroscarmellose sodium
The invention relates to a pharmaceutical composition containing a dexzopiclone tablet, belonging to the technical field of medicine. Commercially available dexzopiclone tablets are of three specifications, i.e., 1 mg, 2 mg and 3 mg. Due to small specifications of the dexzopiclone tablets, content uniformity of prepared tablets can hardly achieve standards; and since the dexzopiclone is hardly soluble in water, reproducibility of the dissolution data of tablets of different batches is poor. The technical scheme of the invention is as follows: the dexzopiclone tablet is characterized by containing, by mass, 1% of dexzopiclone with D90 of below 30 mu m, 0 to 77.5% of dicalcium phosphate dihydrate, 20 to 97.5% of microcrystalline cellulose, 1% of croscarmellose sodium and 0.5% of magnesium stearate. The technical scheme of the invention overcomes the above-mentioned problems in the prior art.
Owner:WEIHAI DISU PHARMA CO LTD +1
Eszopiclone composition
InactiveCN108498471AImprove stabilityDoes not diffuse odorOrganic active ingredientsNervous disorderPhosphateCroscarmellose sodium
The invention relates to an eszopiclone composition and a preparation method thereof and belongs to the technical field of pharmaceutical preparations. According to the eszopiclone composition provided by the invention, the unit dose of the composition is prepared from 1 to 3mg of eszopiclone, 40 to 87mg of microcrystalline cellulose, 8 to 12mg of dicalcium phosphate, 3 to 5mg of croscarmellose sodium, 10 to 30mg of colloidal silicon dioxide, 10 to 20mg of oily auxiliary material and 1.4 to 2mg of magnesium stearate. According to the technical scheme provided by the invention, the stable and safe eszopiclone tablet composition is obtained.
Owner:DISHA PHARMA GRP +1
Combinations of eszopiclone and O-desmethylvenlafaxine, and methods of treatment of menopause and mood, anxiety, and cognitive disorders
InactiveCN101257898AConvenient treatmentOrganic active ingredientsNervous disorderActive agentCognitive diseases
One aspect of the present invention relates to pharmaceutical compositions containing two or more active agents that when taken together can be used to treat, e.g., menopause, mood disorders, anxiety disorders, or cognitive disorders. The first component of the pharmaceutical composition is a sedative eszopiclone. The second component of the pharmaceutical composition is O-desmethylvenlafaxine. The present invention also relates to a method of treating menopause, perimenopause, mood disorders, anxiety disorders, and cognitive disorders.
Owner:SEPACOR INC
Eszopiclone oral disintegrating tablet and preparation method thereof
ActiveCN107669647AFast disintegrationShorten the timeOrganic active ingredientsNervous disorderEconomic benefitsDissolution
The invention relates to the technical filed of a medicine, and discloses an eszopiclone oral disintegrating tablet and a preparation method thereof. The eszopiclone oral disintegrating tablet is prepared by a bulk drug and excipients, wherein the weight percentage of the bulk drug to the excipients is 1-5%:95-99%, the eszopiclone oral disintegrating tablet comprises the following components by weight percentage: 1-5% of eszopiclone, 5-20% of a disintegrating agent, 70-90% of a filler, 1-5% of a flavouring, and 0.5-2.5% of a lubricant; wherein the sum of the weight percentage of the raw materials is 100%. By aiming at the disadvantages of difficult dissolution of eszopiclone in water, slow dissolution speed, and poor mouthfeel of eszopiclone, various excipients capable of promoting disintegration of the bulk drug eszopiclone and effectively covering the adverse smell of eszopiclone can be selected from many drugs, the obtained eszopiclone oral disintegrating tablet has the advantages of fast disintegration speed, short disintegration time, good palatability, smooth and clean surface, and no spot and the like. The oral disintegrating tablet is prepared by a direct tabletting method,the process is simple, and the economic benefit is good.
Owner:湖南中医药高等专科学校
Crystalline of eszopiclone, its composition, preparation and uses thereof
ActiveUS20110098307A1Improve securityGood linear relationshipBiocideNervous disorderIr absorptionZopiclone
The present invention discloses a crystalline form of S-zopiclone having a powder X-Ray diffraction spectrum excited by Cu-Ka radiation with characteristic peaks expressed in terms of 28 at about 11.08°, about 12.38°, about 15.86°, about 17.88°, about 19.98° and about 20.58°; a DSC thermogram with a peak at about 207.7° C. and an infrared absorption spectrum (IR) with characteristic peaks at about 3078 cm−1, about 2942-2838 cm−1, about 2790 cm−1, about 1716 cm−1, about 1463 cm−1, about 1372 cm−1 and about 757 cm−1. The present invention also discloses a method for preparing the crystalline form of eszopiclone, its pharmaceutical composition and its use in preparing a medicament for treating sleep disorders.
Owner:JIANGSU TASLY DIYI PHARMA CO LTD
A method for synthesizing eszopiclone
InactiveCN107445961BSimple and fast operationReduce manufacturing costOrganic chemistryHemiacetalThiazole
The invention provides a method for synthesizing eszopiclone. According to the method, chiral imidazo thiazole is taken as a catalyst to catalyze a racemic hemiacetal intermediate and chloro-formic ester to produce kinetic resolution reaction, and (S)-hemiacetal carbonic ester with good yield and enantioselectivity is obtained. The (S)-hemiacetal carbonic ester is reacted with N-methyl piperazine, so that eszopiclone can be obtained. The method has the advantages that the chiral imidazo thiazole which is low in cost and easy to obtain is used as the catalyst, operation procedures are simple, the production cost is low, and the method has very high application value for the industrial preparation of eszopiclone.
Owner:SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com















![Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/11bca340-5b61-4c10-8e8c-878ea939c844/A20061001349100071.PNG)
![Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/11bca340-5b61-4c10-8e8c-878ea939c844/A2006100134910002C1.PNG)
![Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine Method of preparing eszopiclone intermediate 6-(5-chloro-2-pyridyl)-5,7-dioxy-6,7-dihydro-5H-pyrrolo[3,4-b] pyrazine](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/11bca340-5b61-4c10-8e8c-878ea939c844/A20061001349100041.PNG)










![Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone) Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead77da4-24d6-4dd8-acbd-4c9164c74e4d/US20090198058A1-20090806-D00001.png)
![Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone) Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead77da4-24d6-4dd8-acbd-4c9164c74e4d/US20090198058A1-20090806-C00001.png)
![Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone) Process for Preparation of Dextrorotatory Isomer of 6-(5- chloro-pyrid-2-yl)-5-[(4-methyl -1-piperazinyl) carbonyloxy] -7-oxo-6,7-dihydro-5H-pyrrolo [3,4-b] pyrazine (Eszopiclone)](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead77da4-24d6-4dd8-acbd-4c9164c74e4d/US20090198058A1-20090806-C00002.png)