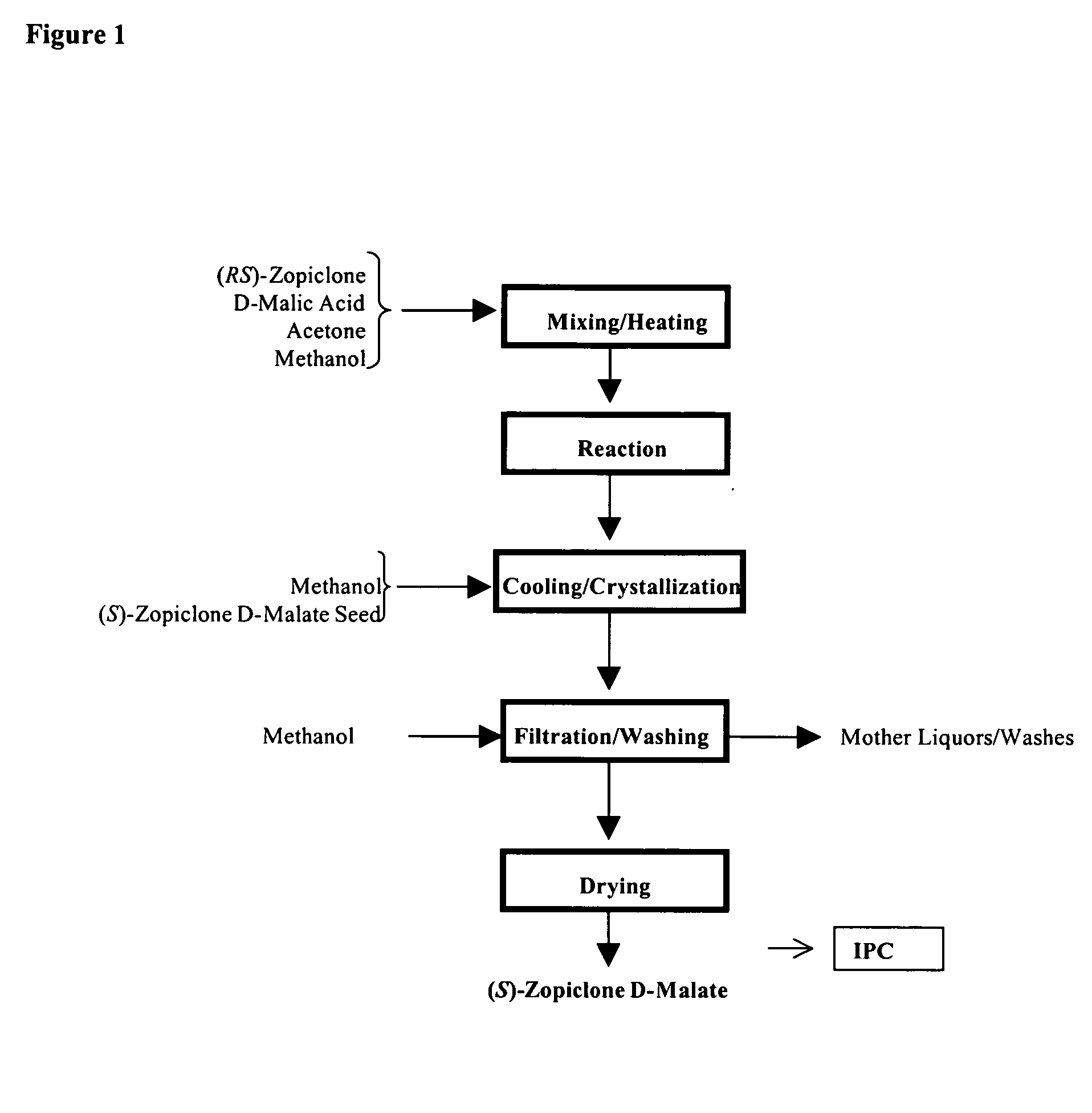
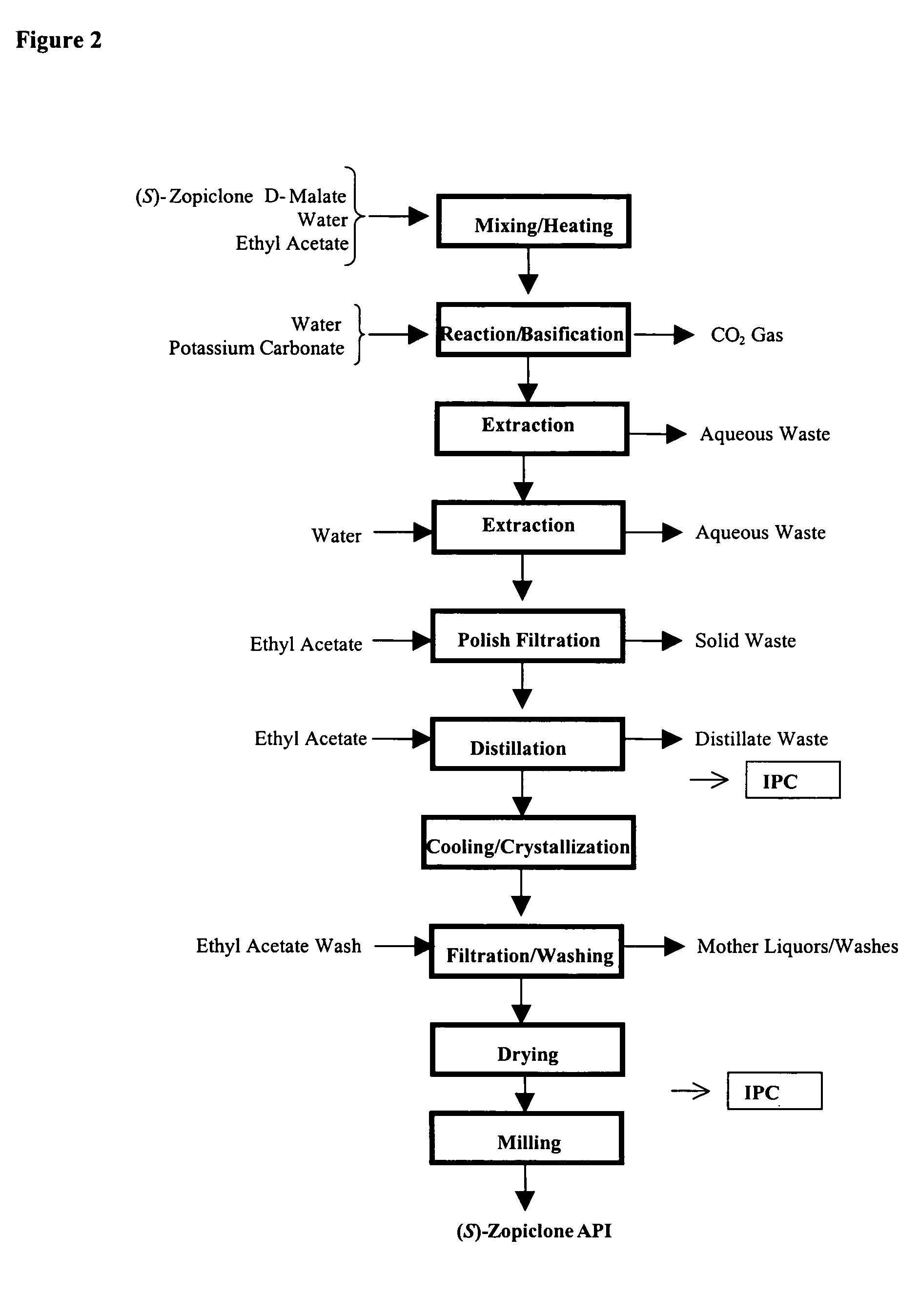
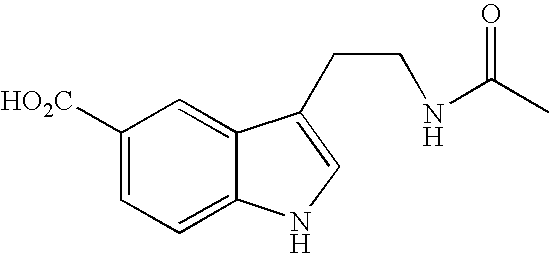
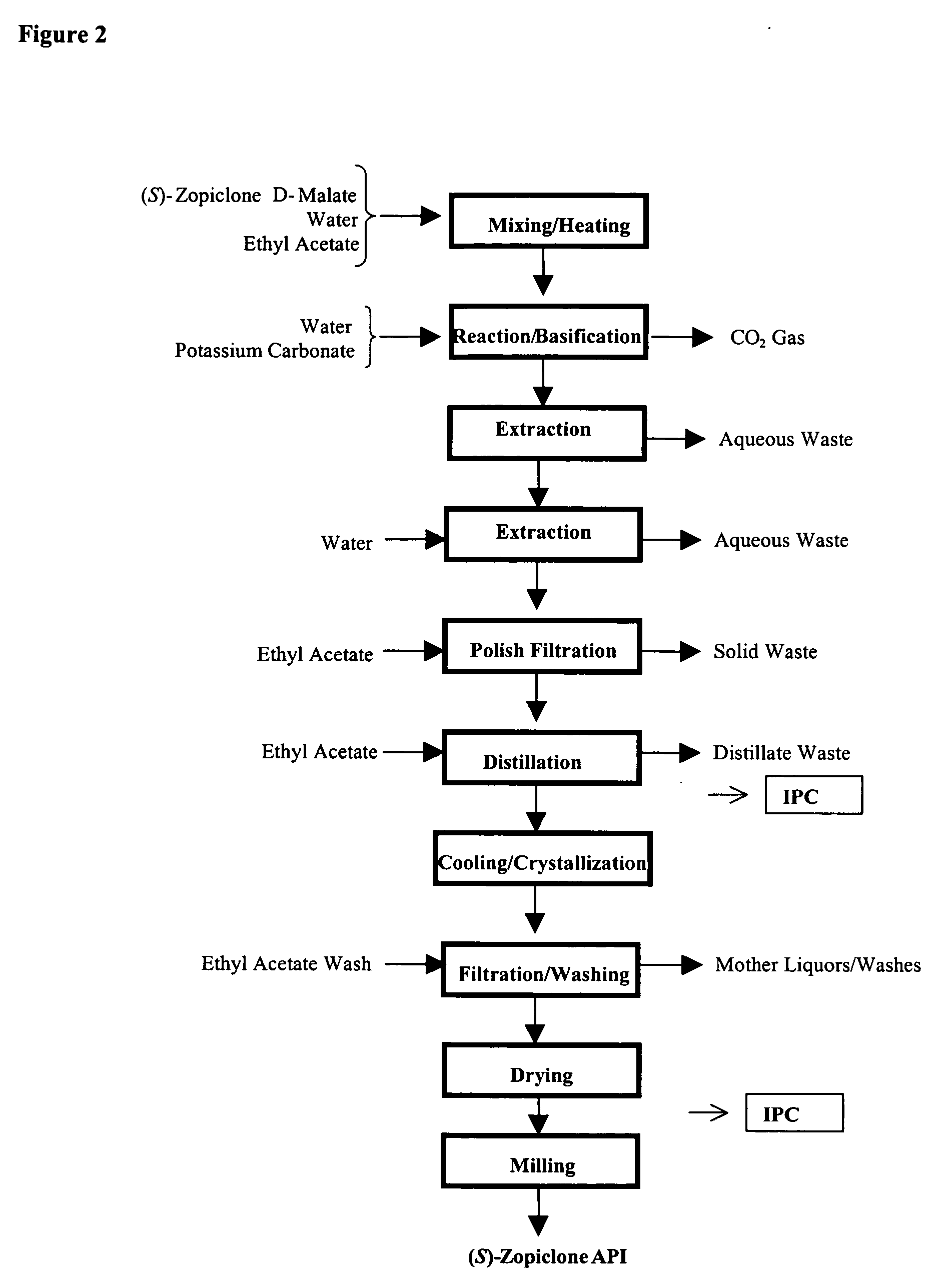
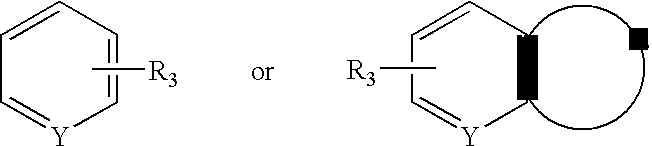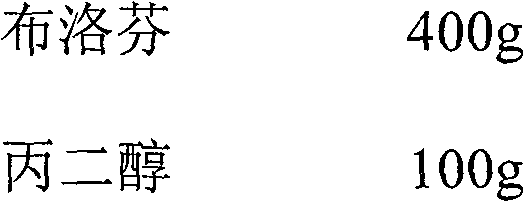Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
30 results about "Sedative agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Procedural sedation and analgesia, previously referred to as conscious sedation, is defined as "a technique of administering sedatives or dissociative agents with or without analgesics to induce a state that allows the patient to tolerate unpleasant procedures while maintaining cardiorespiratory function.".
Melatonin combination therapy for improving sleep quality
One aspect of the present invention relates to pharmaceutical compositions comprising a sedative agent; and melatonin or a melatonin analog, collectively referred to as “melatonin agents.” In a preferred embodiment, the sedative agent is eszopiclone. The pharmaceutical compositions of the invention are useful in the treatment of various sleep disorders. In addition, the present invention also relates to a method of treating a patient suffering from a sleep abnormality or insomnia comprising administering a therapeutically effective amount of a pharmaceutical composition of the invention.
Owner:SEPACOR INC
Dopamine-agonist combination therapy for improving sleep quality
InactiveUS20050267176A1Useful in treatmentOrganic active ingredientsBiocideLimb dyskinesiaSedative agent
The present invention generally relates to pharmaceutical compositions comprising a dopamine agonist and sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone. The pharmaceutical compositions of the invention are useful in the treatment of restless-leg syndrome and periodic-limb-movement disorder, as well as various sleep disorders. In addition, the present invention relates to a method of treating a patient suffering from restless-leg syndrome, periodic-limb-movement disorder, a sleep abnormality, or insomnia, comprising coadministering a therapeutically effective amount of a dopamine agonist and a therapeutically effective amount of a sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)-N-desmethylzopiclone.
Owner:SEPACOR INC
Dopamine-Agonist Combination Therapy For Improving Sleep Quality
The present invention generally relates to pharmaceutical compositions comprising a dopamine agonist and sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. The pharmaceutical compositions of the invention are useful in the treatment of restless-leg syndrome and periodic-limb-movement disorder, as well as various sleep disorders. In addition, the present invention relates to a method of treating a patient suffering from restless-leg syndrome, periodic-limb-movement disorder, a sleep abnormality, or insomnia, comprising coadministering a therapeutically effective amount of a dopamine agonist and a therapeutically effective amount of a sedative agent. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine. In a preferred embodiment, the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone. In a preferred embodiment, the dopamine agonist is optically pure (S)-didesmethylsibutramine; and the sedative agent is optically pure (S)-zopiclone or optically pure (S)—N-desmethylzopiclone.
Owner:WOODWARD SPECIALTY LLC
Opioid agonist in a fast dispersing dosage form
InactiveUS7090866B2Act quicklyMinimizing side-effectsBiocidePowder deliveryPremedicationTranquilizing Agents
This invention relates to a pharmaceutical composition for oral administration comprising a carrier and, as active ingredient, an opioid (μ receptor) agonist, such as fentanyl, or a salt thereof, characterized in that the composition is in the form of a fast-dispersing dosage form designed to release the active ingredient rapidly in the oral cavity. A process for preparing such a composition and the use of such a composition as an analgesic, for the treatment of chronic pain and / or breakthrough pain, as an anesthetic premedication, for the induction of anesthesia, as a sedative and / or for the treatment of anxiety are also provided.
Owner:CATALENT USA WOODSTOCK INC +3
Systems and methods for providing gastrointestinal pain management
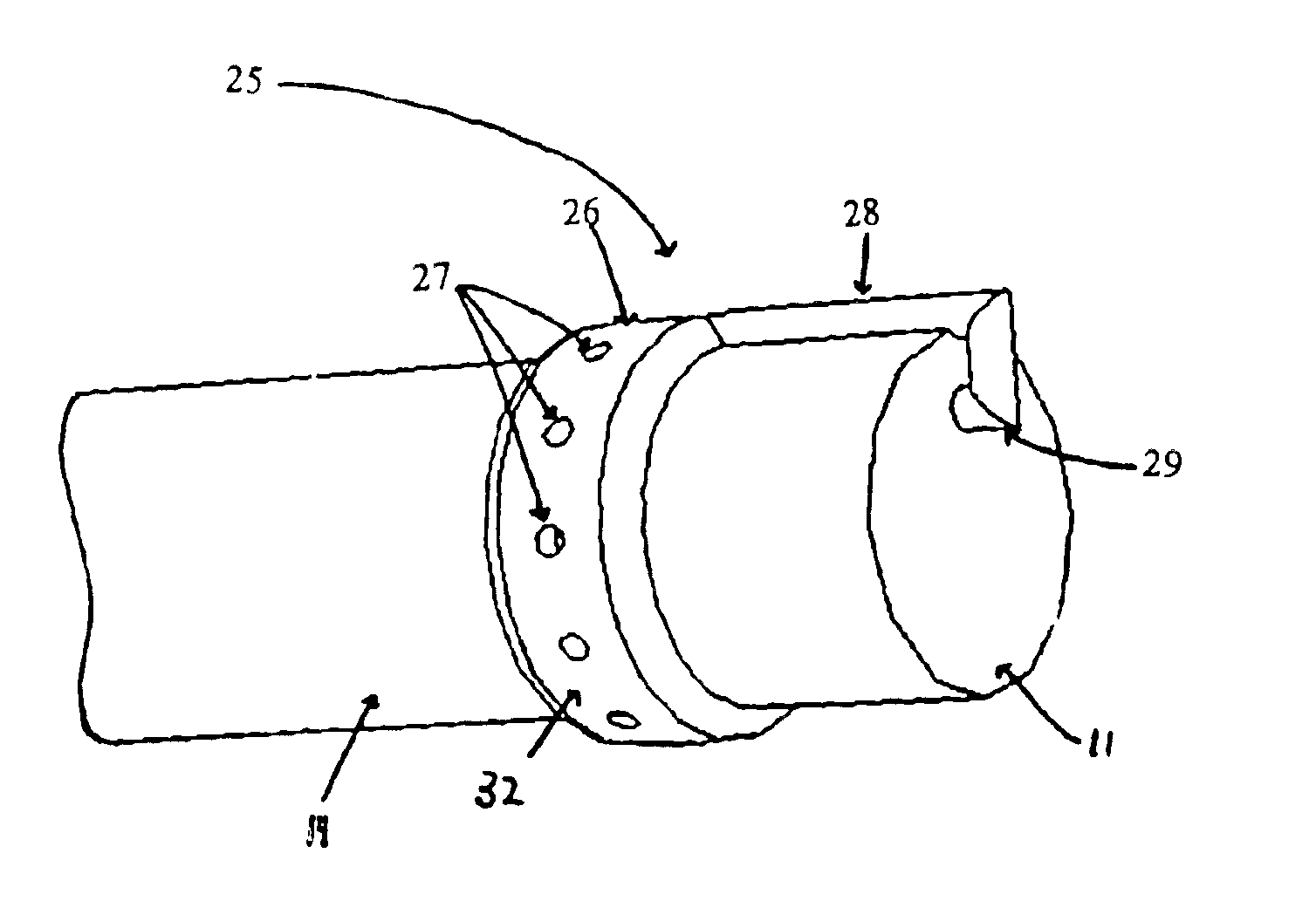
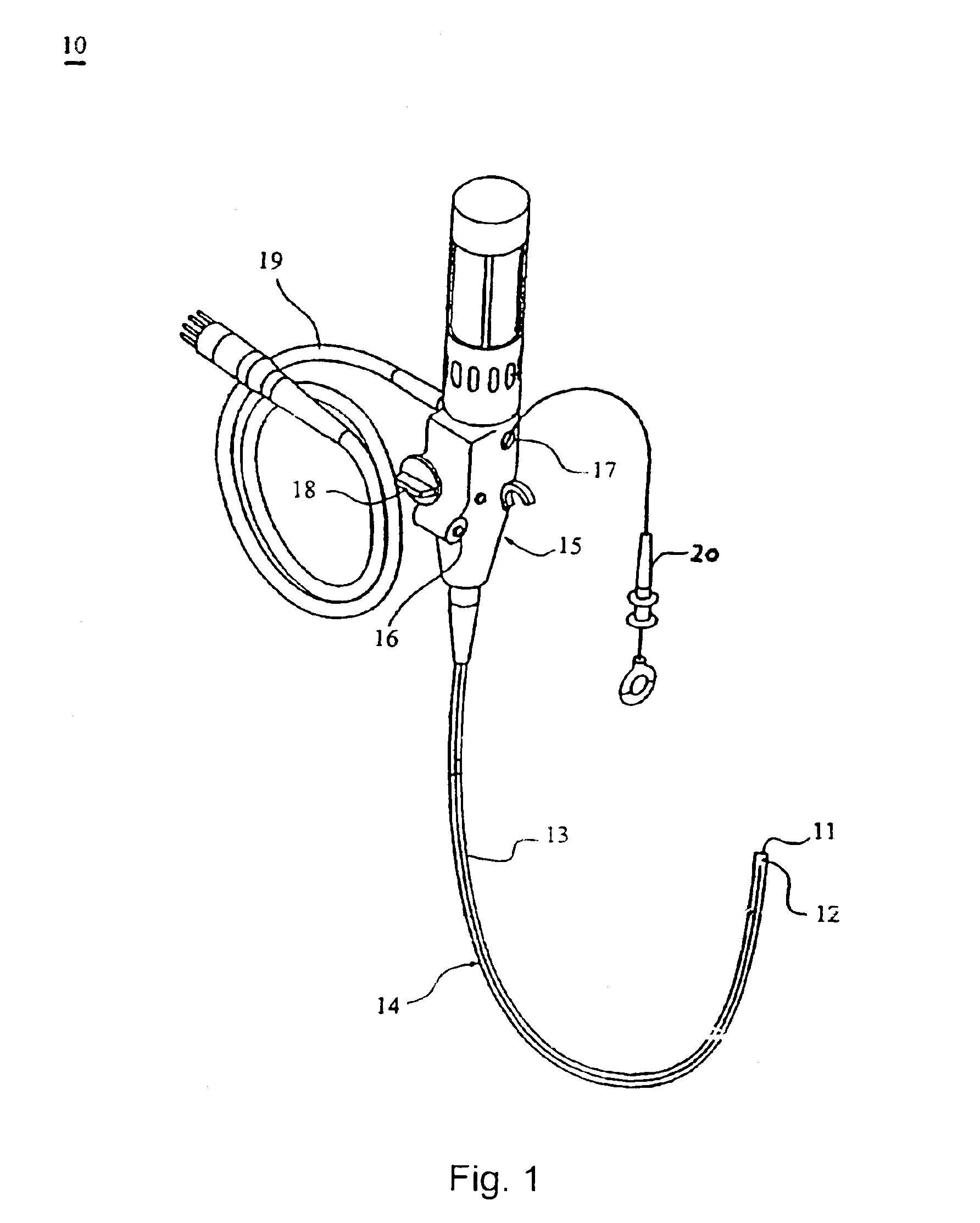
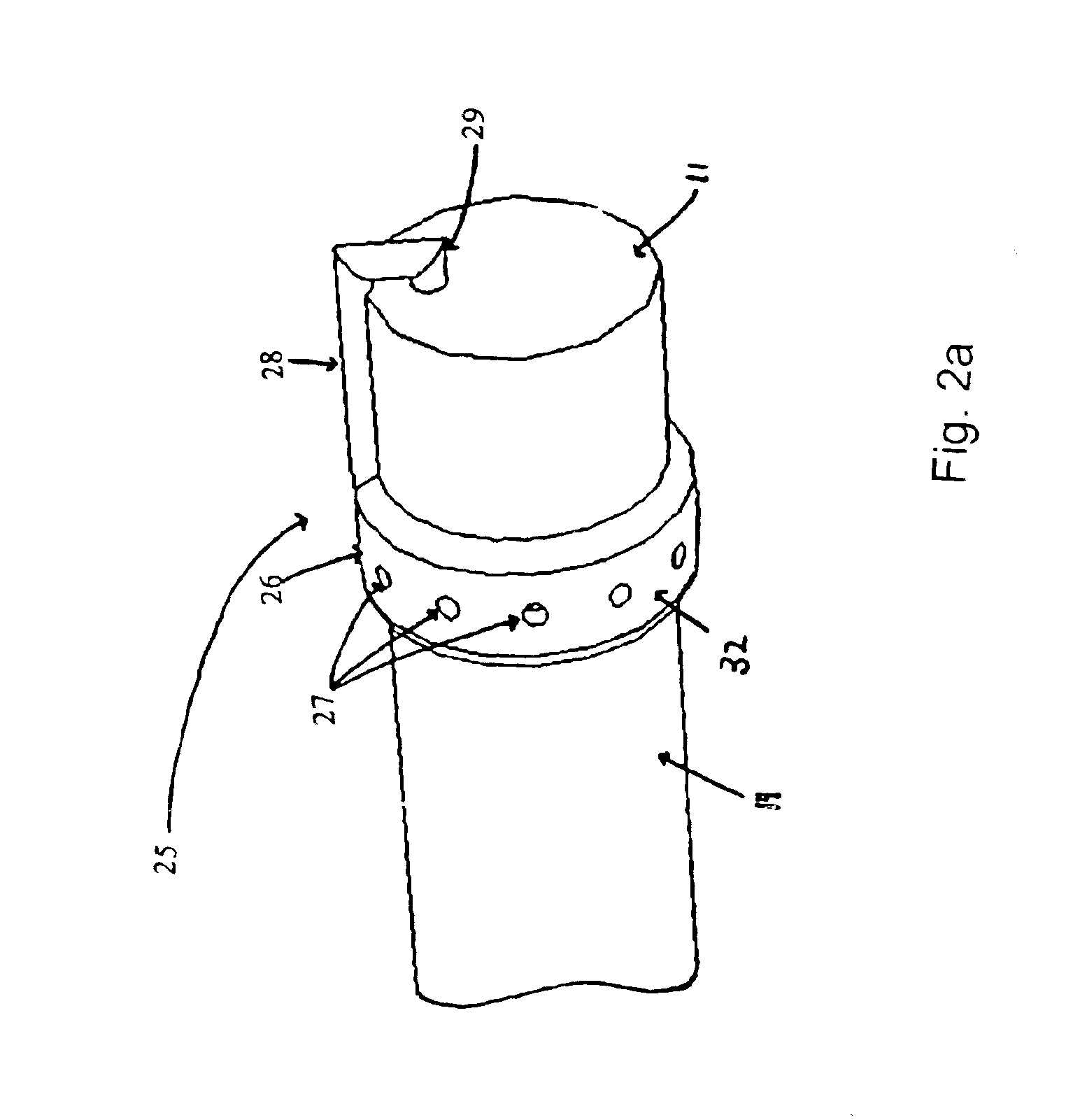
The present invention includes systems and methods for decreasing the pain and discomfort commonly associated with endoscopic procedures, where such procedures may be performed with lower dosage levels of sedative and analgesic drugs. The invention includes use of an anesthetic collar coupled to an endoscope with a flexible shaft. The anesthetic collar allows lubricants, local anesthetics, dyes, and / or other desirable fluids to be passed through the existing lumen of the flexible shaft into an annulus, where the fluid may be distributed through expulsion pores into the gastrointestinal tract. Utilizing the existing lumens found in endoscopes, the present invention allows those fluids that may reduce the pain and discomfort associated with endoscopies such as, for example, local anesthetics and lubricants, to be distributed in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, where such fluids may reduce the drug level requirements for sedative and analgesic agents. Alternatively, the endoscope may be redesigned for streamlined integration with the anesthetic collar or to accomplish the same function of distributing local anesthetics and lubricants, in an even fashion throughout the gastrointestinal tract or throughout the length and circumference of the endoscope, The invention can also be used with endoscopes without existing lumens.
Owner:SCOTT LAB
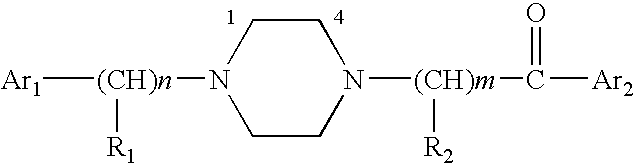
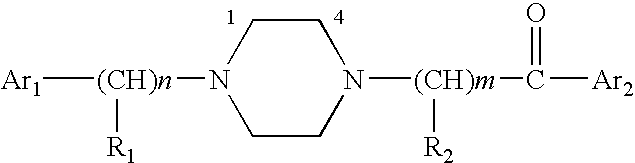
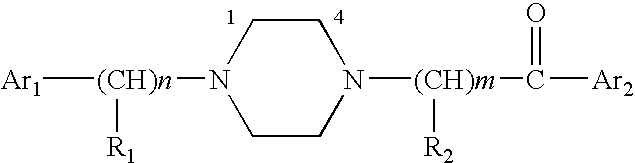
Aralkyl-ketone piperazine derivatives and their uses as new antalgic or ataractic agent
ActiveUS7332495B2High yield and purityQuality improvementOrganic active ingredientsNervous disorderSide effectMedicine
Owner:NHWA PHARMA CORPORATION
Dosing regimen of sedative
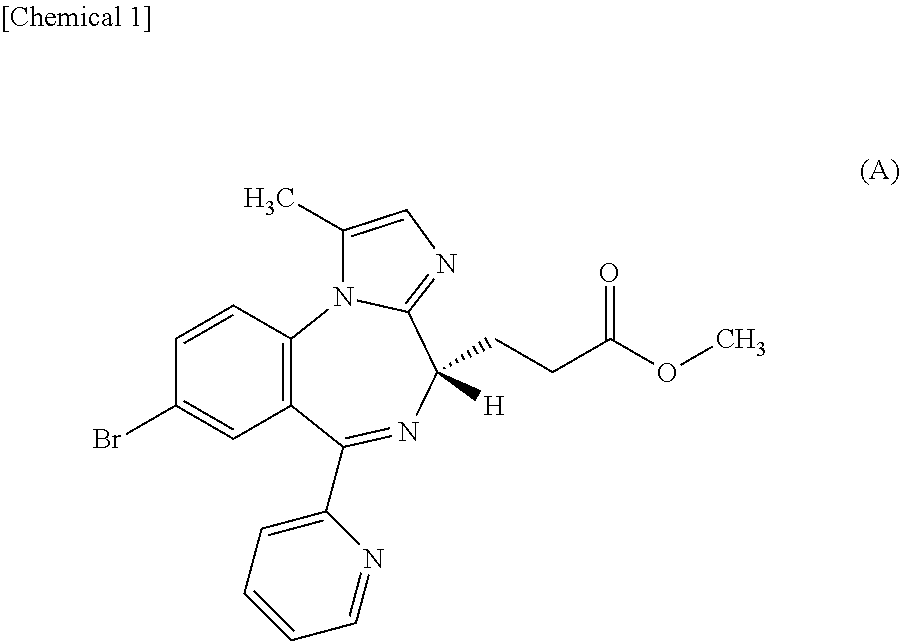
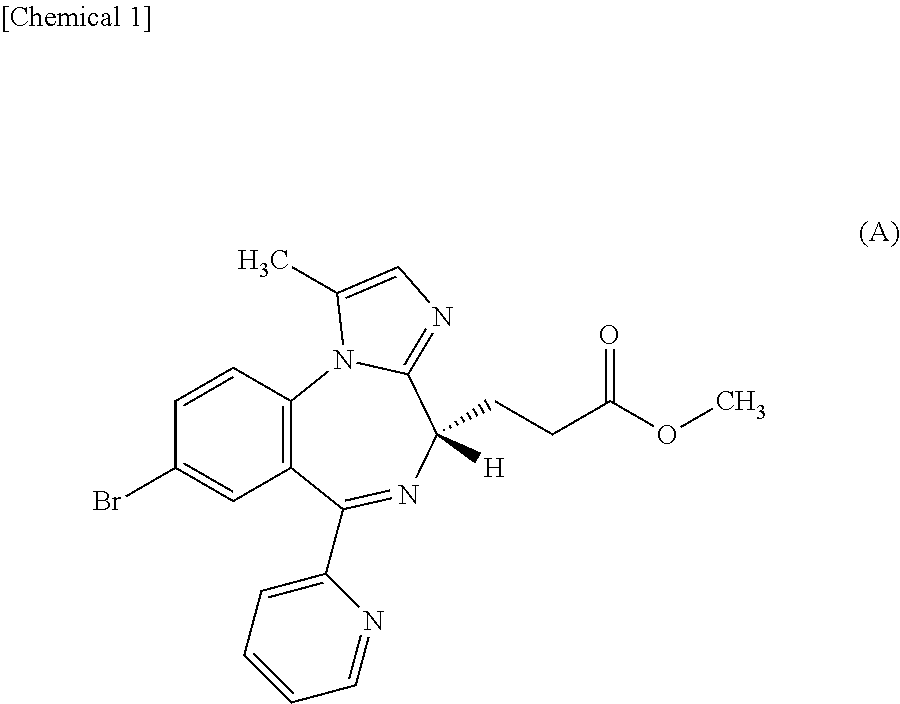
InactiveUS20150224114A1Rapid of consciousnessPromote recoveryBiocideNervous disorderDosing regimenPropanoic acid
By intravenously administering a sedative comprising methyl 3-[(4S)-8-bromo-1-methyl-6-(2-pyridinyl)-4H-imidazo[1,2-a][1,4]benzodiazepin-4-yl]propanoate or a salt thereof to a patient by a dosing regimen including a two-step process disclosed in the present invention, general anesthesia can be safely and promptly induced and maintained.
Owner:PAION UK
Method for treating early severe diffuse acute respiratory distress syndrome
InactiveUS20120325209A1Vasopressor/inotrope requirementLower respiratory rateBiocideTracheal tubesAgonistSedative agent
A method for treatment of severe diffuse acute respiratory distress syndrome in an intubated-ventilated patient which includes sedating said patient with at least one alpha-2 agonist, maintaining spontaneous ventilation and applying pressure support ventilation of at least 5-10 cmH2O combined to a high positive end expiratory pressure (PEEP) of 10-24 cmH2O. A pharmaceutical composition containing at least one alpha-2 agonist suitable for treatment of ARDS in combination with, if appropriate, at least one sedative agent which does not depress ventilatory drive is also disclosed.
Owner:QUINTIN LUC
Pre-operative beverages
The invention provides a formulated beverage composition that can be administered to a patient, prior to anesthesia, sedation and / or surgical or dental operation, to reduce the risk of aspiration pneumonia while simultaneously improving the well-being of the patient. The beverage composition is designed to be taken orally, by a patient, within a specified short period of time prior to administration of an anesthesia and / or sedative / analgesic. Further described herein is the method of using the beverage composition. The method may include providing a written label of instructions affixed to the beverage container which instructions positively direct the patient's ingestion of the beverage composition.
Owner:SANTE
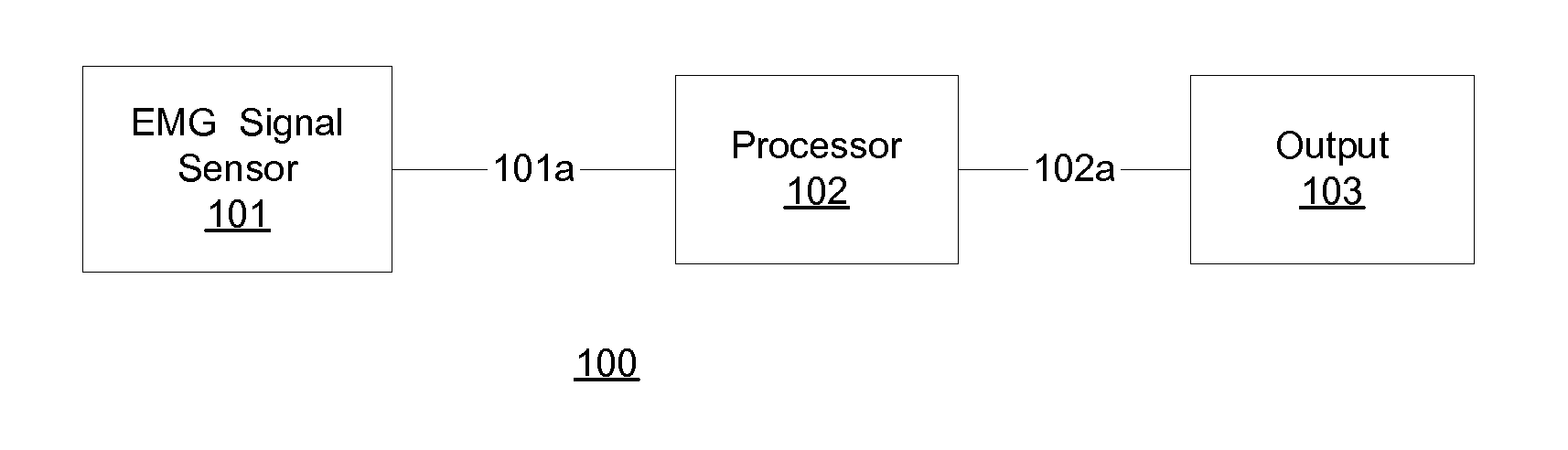
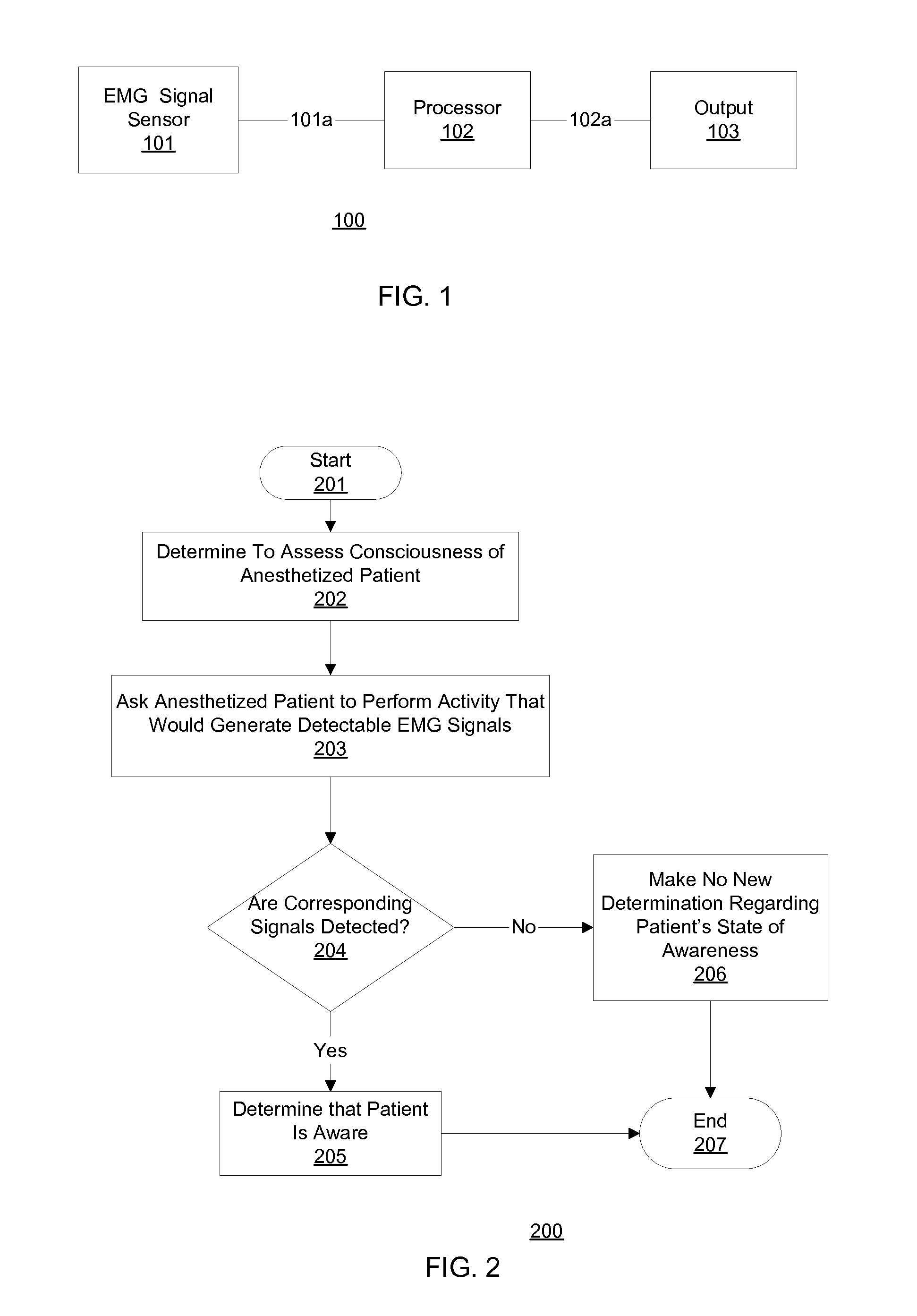
Communication with and consciousness-assessment of anesthetized surgery patients
In one embodiment, prior to undergoing surgery, a patient is trained in the use of an anesthesia-awareness detection (AAD) system to produce an observable output by attempting to move a muscle. The AAD system includes an electromyographic (EMG) signal monitor. While undergoing surgery, the patient is anesthetized with a sedative and a paralytic. During surgery, if the surgical team wishes to asses the consciousness state or communicative ability of the patient, then the surgical team asks the patient to move the muscle. If the patient is unable to speak due to the paralytic but is aware and attempts to comply, then the attempt is detected by the AAD system, which provides a corresponding output to inform the surgical team, which may take appropriate action to ask additional questions of the patient and / or address the patient's anesthesia awareness.
Owner:EGETH MARC J
Aralkyl-ketone piperazine derivatives and their uses as new antalgic or ataractic agent
ActiveUS20060148811A1High yield and purityQuality improvementOrganic active ingredientsNervous disorderSide effectMedicine
Owner:NHWA PHARMA CORPORATION
Method for treating early severe diffuse acute respiratory distress syndrome
InactiveUS8703697B2Lower respiratory rateAvoid RV dilatation or septal bulgingRespiratorsBiocideAgonistSedative agent
A method for treatment of severe diffuse acute respiratory distress syndrome in an intubated-ventilated patient which includes sedating said patient with at least one alpha-2 agonist, maintaining spontaneous ventilation and applying pressure support ventilation of at least 5-10 cmH2O combined to a high positive end expiratory pressure (PEEP) of 10-24 cmH2O. A pharmaceutical composition containing at least one alpha-2 agonist suitable for treatment of ARDS in combination with, if appropriate, at least one sedative agent which does not depress ventilatory drive is also disclosed.
Owner:QUINTIN LUC
Intelligent pharmaceutical delivery system with non-concentric pumping mechanism to reduce flow anomaly and method of using
ActiveUS10842932B1Reduces flow pulsationReduce riskElectrotherapyBioelectric signal measurementPharmaceutical drugSedative agent
The present invention relates to the titration and delivery of anesthetic and sedative medications to a subject. Further, the present invention relates to a device and methods for titrating and delivering such medications in a semi-automated or fully automated manner and which can be monitoring and controlled remotely. Even still further, the present invention relates to such a device that can perform the titration and delivery of medication in a manner that minimalizes occlusion and prevents back flow of the medication. More particularly, the present invention relates to a device for titration and delivery of medication using a non-concentric pumping mechanism that gradual or progressively increases and decreases occlusion in the medication delivery line within the pump to minimize and / or prevent sudden formation and release of occlusion in order to provide more steady and continuous flow of the medication through the device to the subject.
Owner:NEUROWAVE SYST
Sleep-improving agent
InactiveCN101711240AExcellent sleep improvement effectGood sedative effectOrganic active ingredientsNervous disorderSedative agentBULK ACTIVE INGREDIENT
An object of the present invention is to provide a novel sleep-improving agent and sedative agent. The above object is achieved by providing a sleep-improving agent and a sedative agent containing, as an active ingredient, oxypinnatanine or its derivative represented by the following chemical formula (I).
Owner:财团法人大坂生物科学研究所 +2
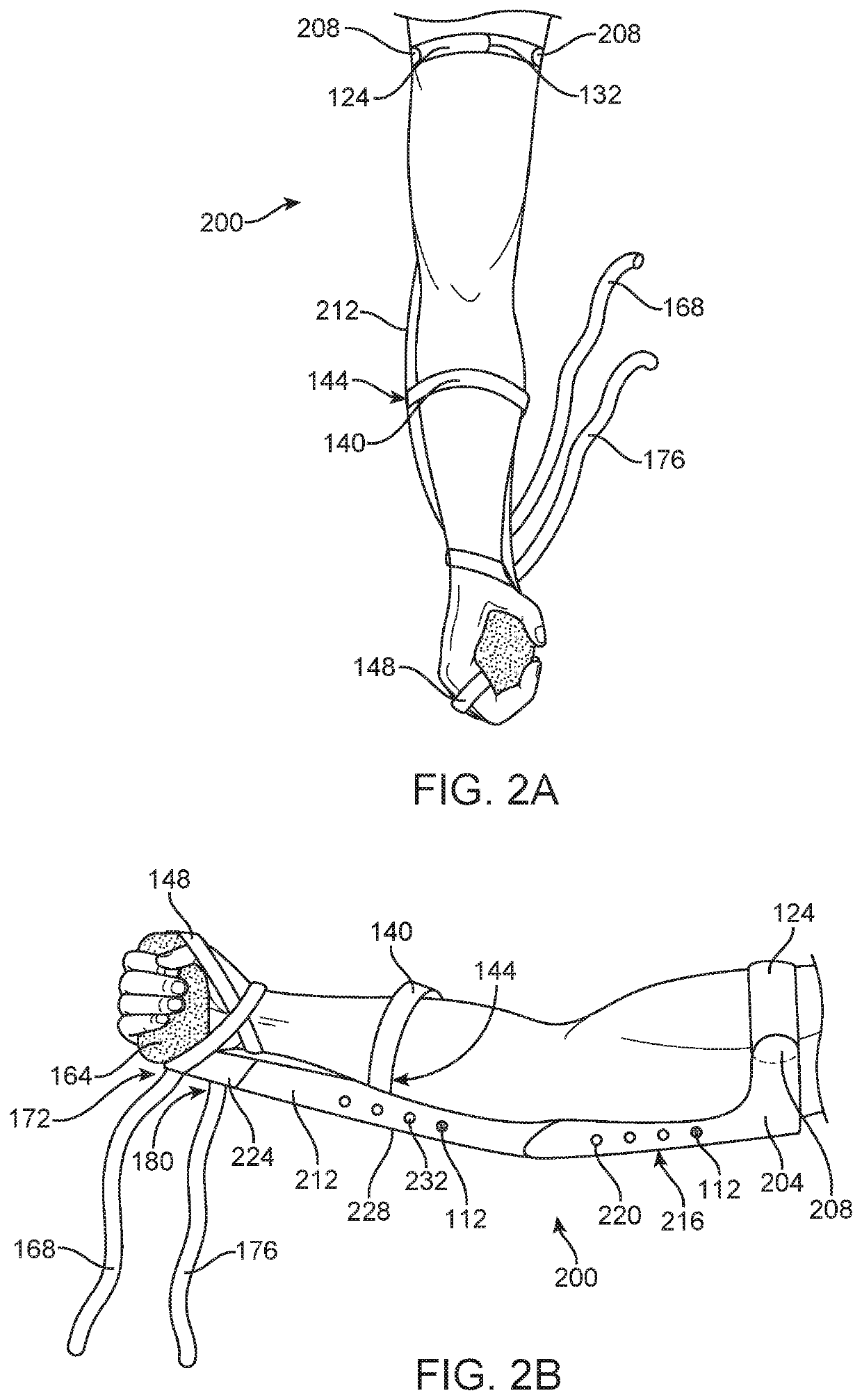
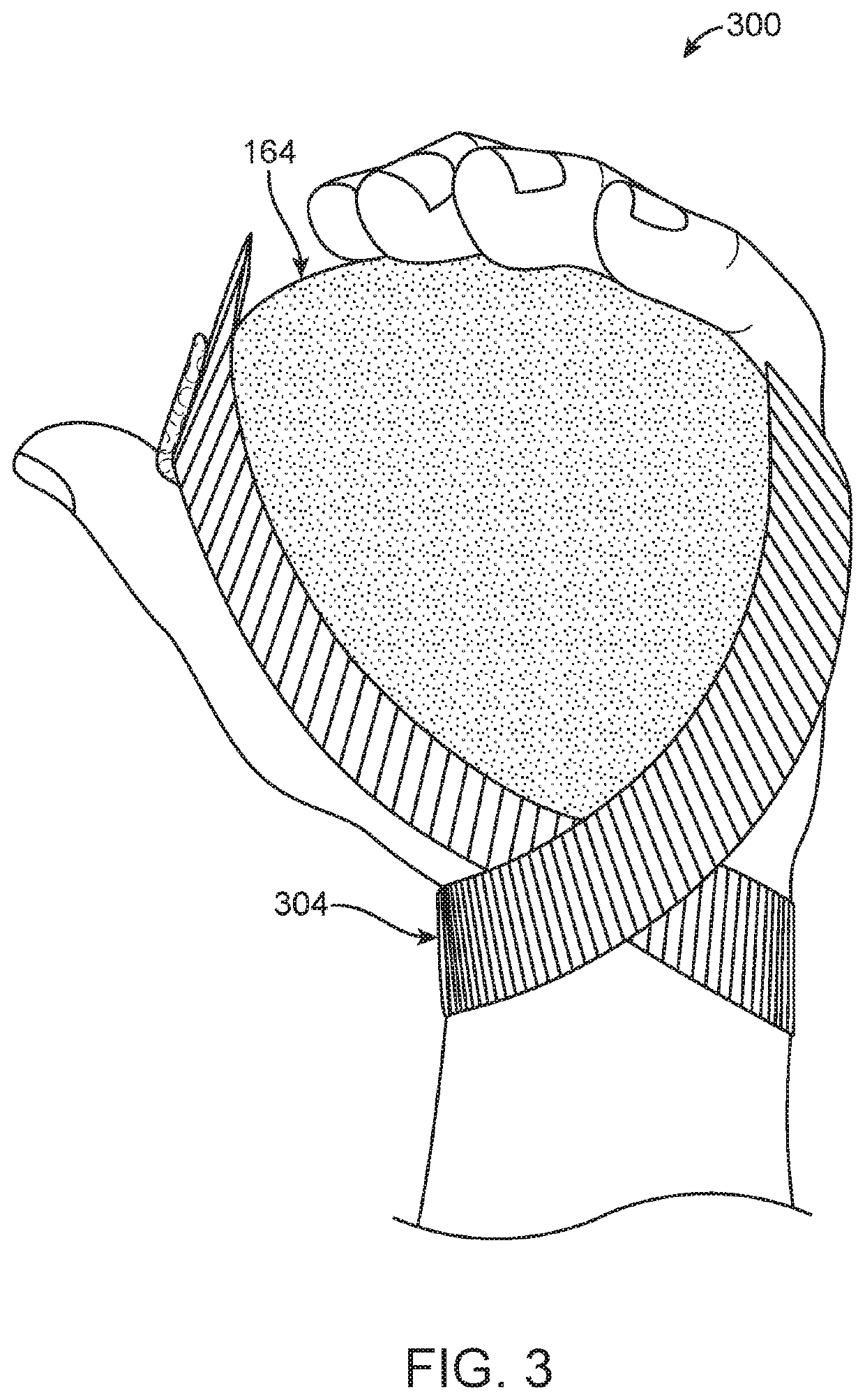
Medical Protective and Exercise Restraint Methods
ActiveUS20200253775A1Reduce riskMuch muscle strengthResilient force resistorsRestraining devicesDiaphragmatic movementMuscle strength
Intended to prevent self-extubation of nasal or oral tubes of an awake patient who may reflexively attempt removal of such and allow the early mobilization and exercise necessary for preservation of as much muscle strength as possible, promotion of diaphragmatic motion necessary for liberation from a ventilator, reduction of patient stress allowing for sedation minimization and improved cognition for reduction of delirium and post-intensive care syndrome, and relief of medical staff and healthcare dollars to focus more on healthcare rather than on protection from self-harm.
Owner:PAVINI MARIE
Premix formulation for parenteral use and packaging thereof
ActiveUS10632043B2Easy to storeEasy to transportOrganic active ingredientsPackage sterilisationPerioperative careSedation
The present invention relates to stable premix formulations for parenteral use and packaging for such formulations. The present invention relates to use of a single layer or multilayer film bags comprising polypropylene for packing premixed injectable formulations which remain stable during the storage period.The present invention relates to use of a single layer or multilayer film bags comprising polypropylene for packing dexmedetomidine premixed formulations. Such stable premixed formulations packed into a single layer or multilayer film bags comprising polypropylene are highly advantageous since they are ready-to-use, for example, in perioperative care of a subject in need thereof for sedation.
Owner:AUROBINDO PHARMA LTD
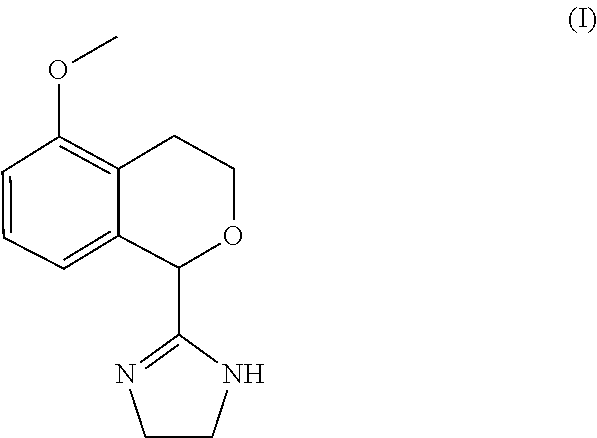
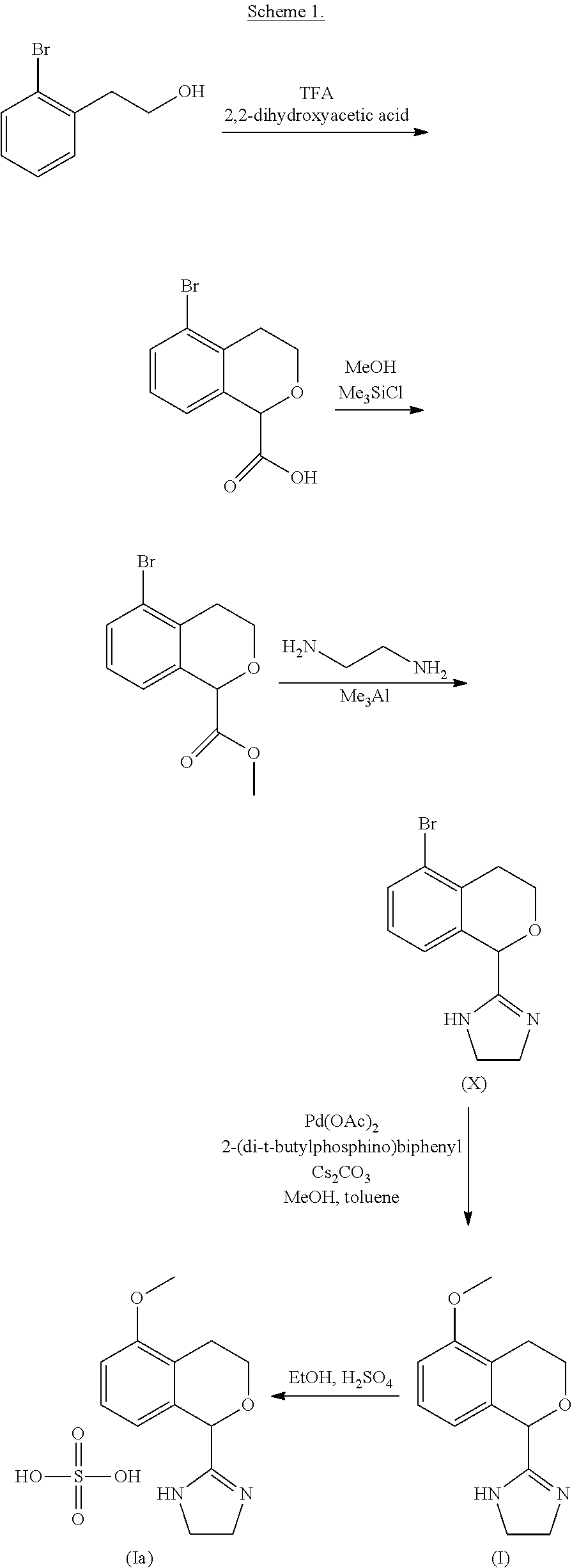
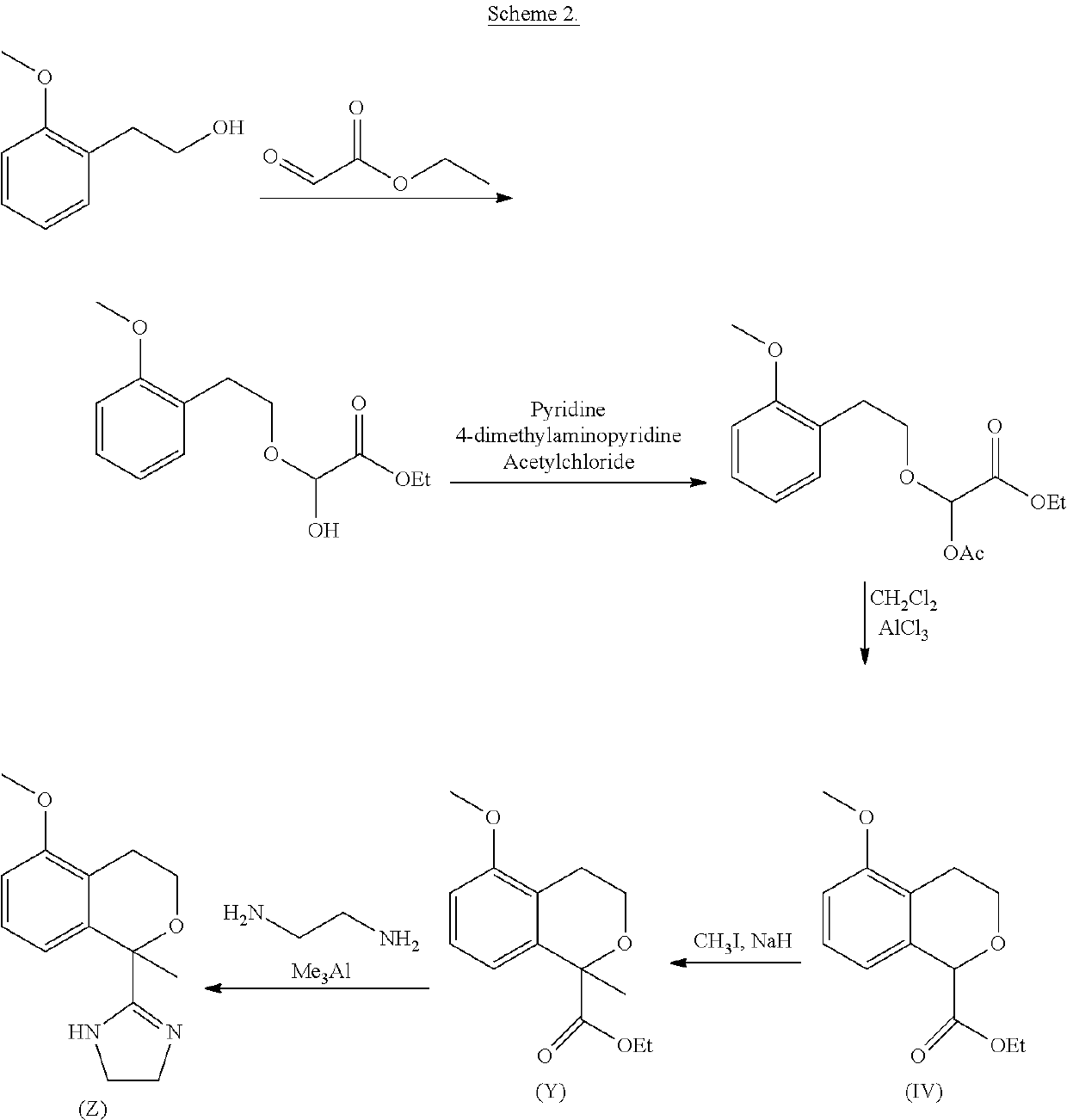
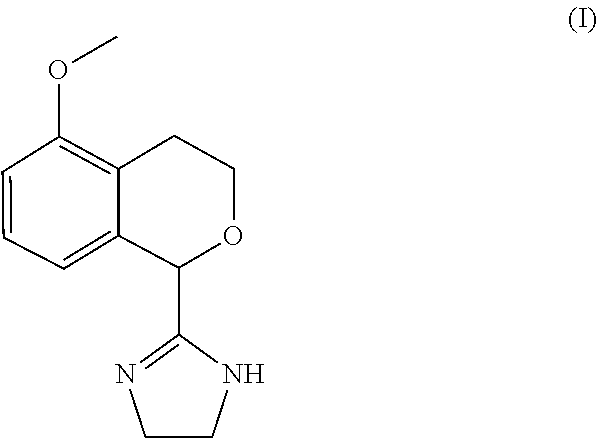
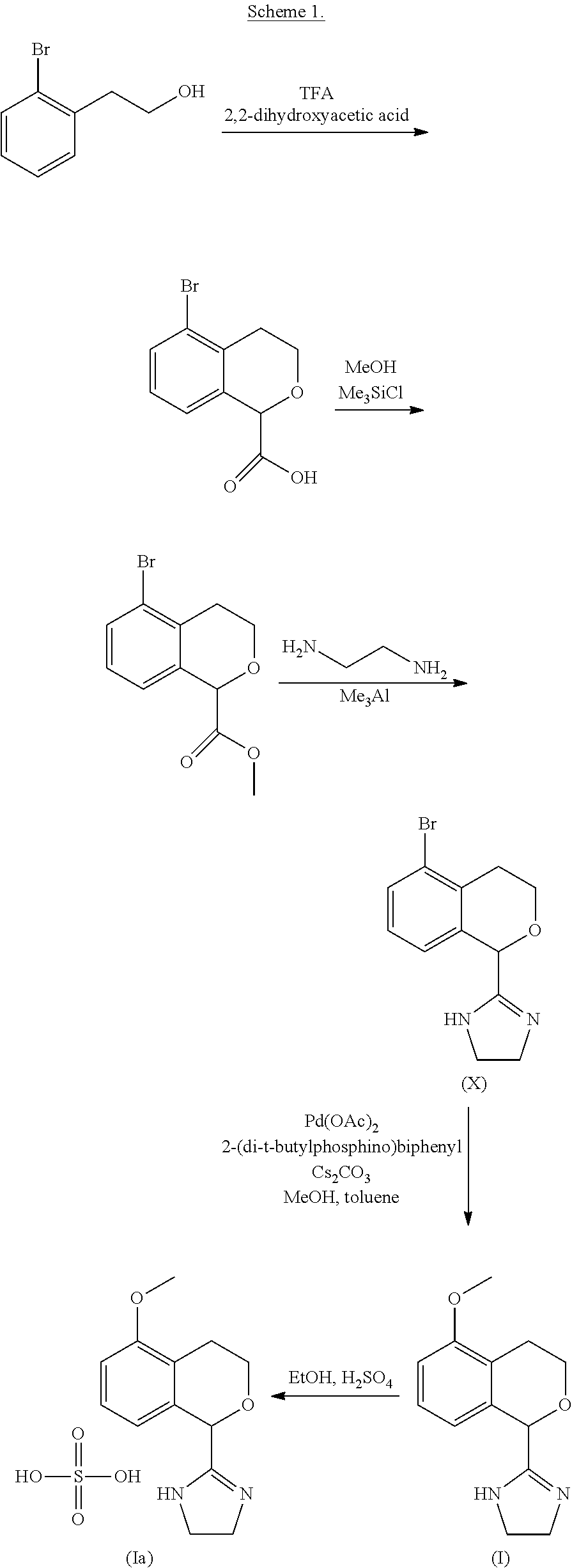
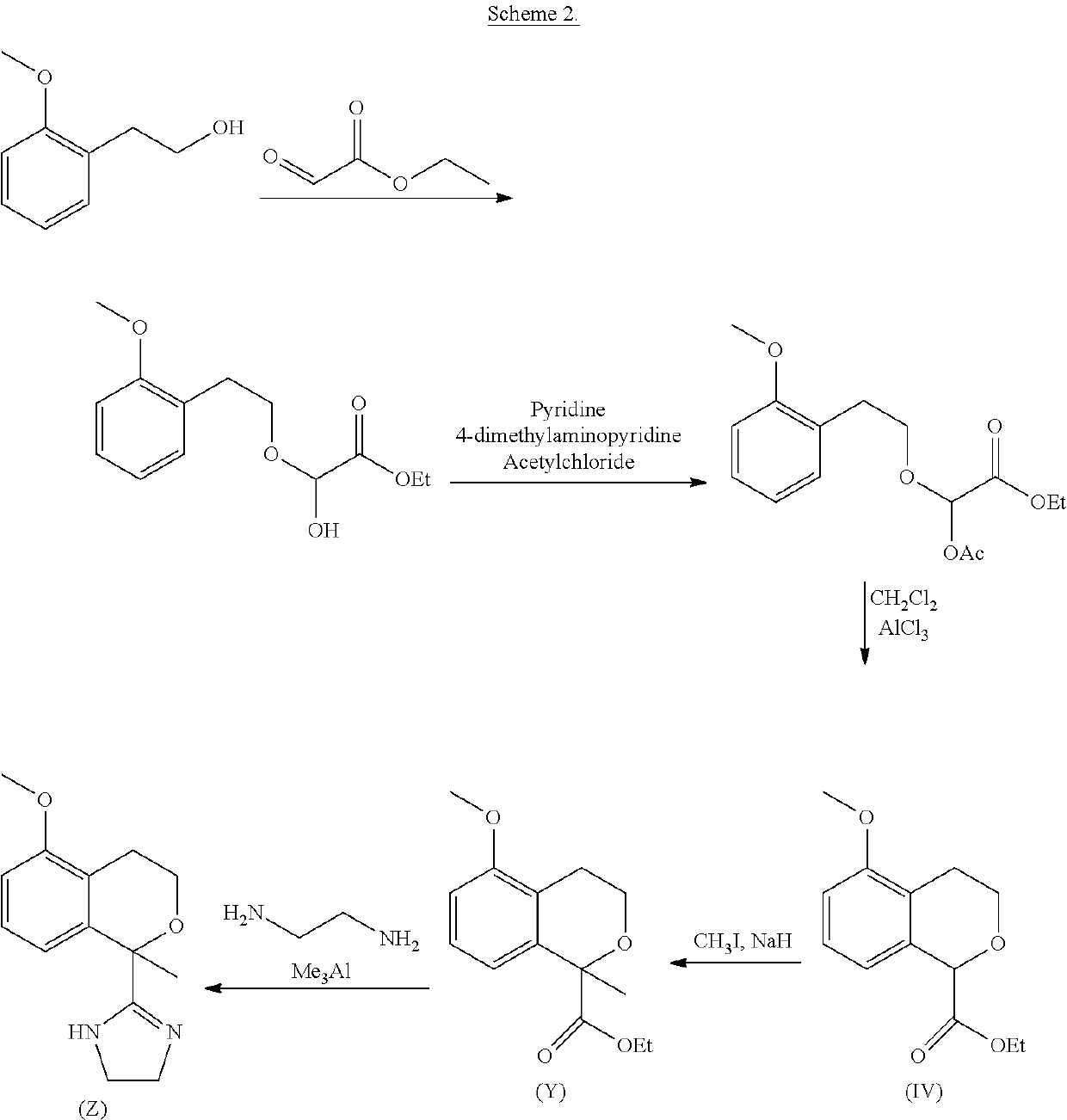
Process for the preparation of 2-(5-methoxyisochroman-1 -yl)-4,5-dihydro-1 h-imidazole and the hydrogensulfate salt thereof
ActiveUS20200308152A1Suitable for use in large industrial scaleHigh yieldOrganic active ingredientsGroup 4/14 element organic compoundsAdrenergicEthyl group
The present disclosure relates to an improved process for the preparation of isochroman structured alpha2A adrenoceptor agonist, namely 2-(5-methoxyisochroman-1-yl)-4,5-dihydro-1H-imidazole of formula (I) and a pharmaceutically acceptable salts thereof, such as 2-(5-methoxyisochroman-1-yl)-4,5-dihydro-1H-imidazole hydrogensulfate of formula (Ia), and to a novel intermediate compound used in the process, namely N-(2-aminoethyl)-5-methoxyisochroman-1-carboxamide monohydrate of formula (V). Alpha2A agonists are useful in the treatment of anxiety, and for use as a sedative or analgesic agent, and other diseases where alpha2A agonism is desired.
Owner:ORION CORPORATION
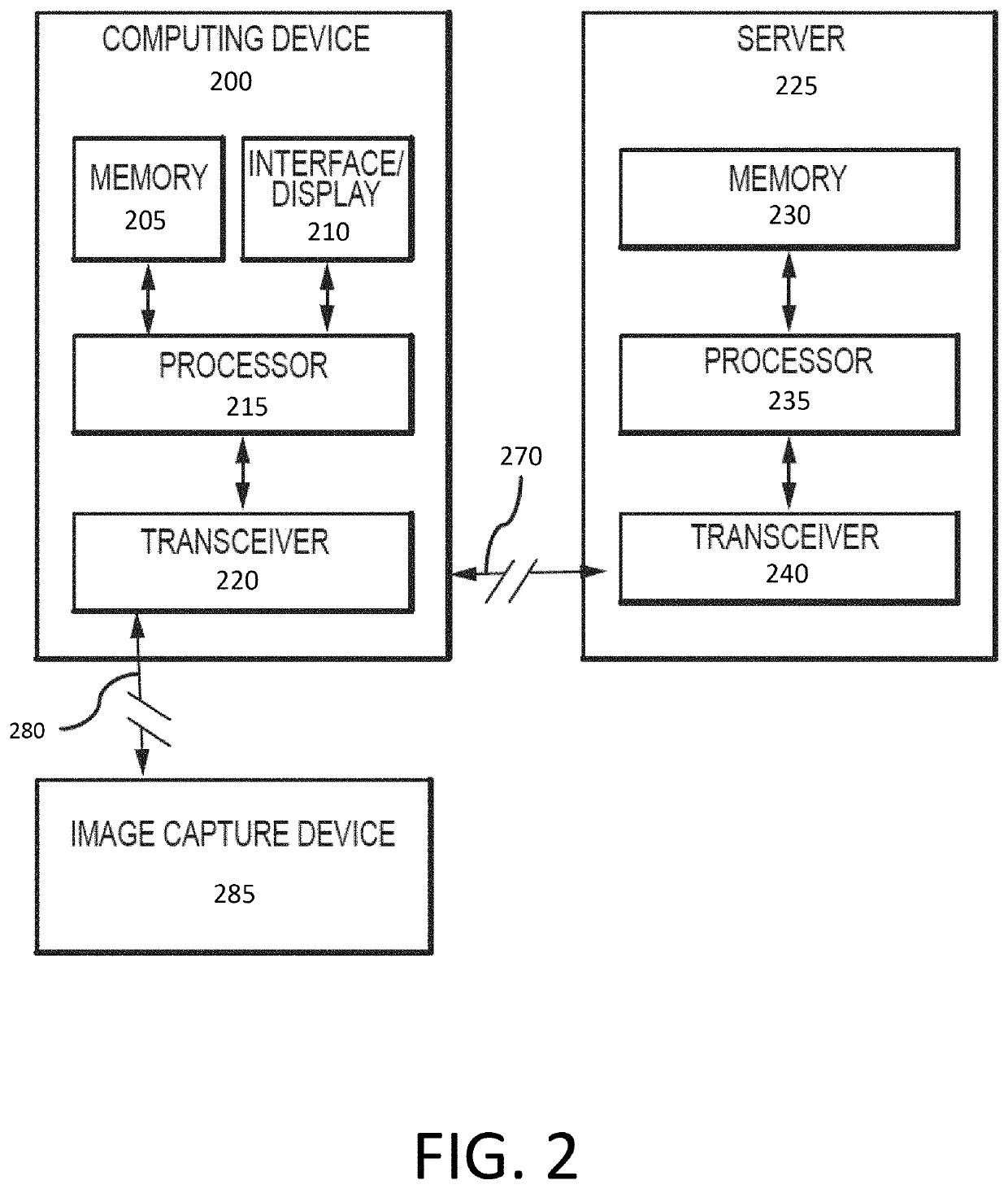
Systems and methods for sedation-level monitoring
PendingUS20210298635A1Well formedImprove toleranceTracheal tubesMedical devicesSedationMonitoring system
Systems and methods are provided for monitoring the sedation level of patients. Images may be captured of a patient and regions of interest identified. Changes to image properties or to one or more regions of interest may be analyzed to generate physiological parameters for the patient. Physiological parameters may include information related to the patient's breathing behavior and / or activity. Physiological parameters may be used to determine a sedation level of the patient. If it is determined that the patient is over- or under-sedated, appropriate action may be taken, such as displaying an indication, activating an alarm, and / or causing an amount of sedative to be administered to the patient.
Owner:TYCO HEALTHCARE GRP LP
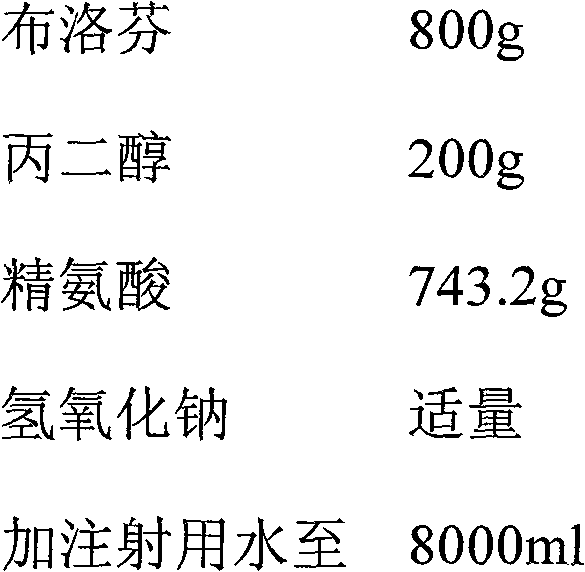
Ibuprofen-containing pharmaceutical composition and preparation method and application thereof
ActiveCN102258507BGood effectEffective treatmentOrganic active ingredientsAntipyreticDiseaseArginine
Owner:北京爱力佳医药科技有限公司
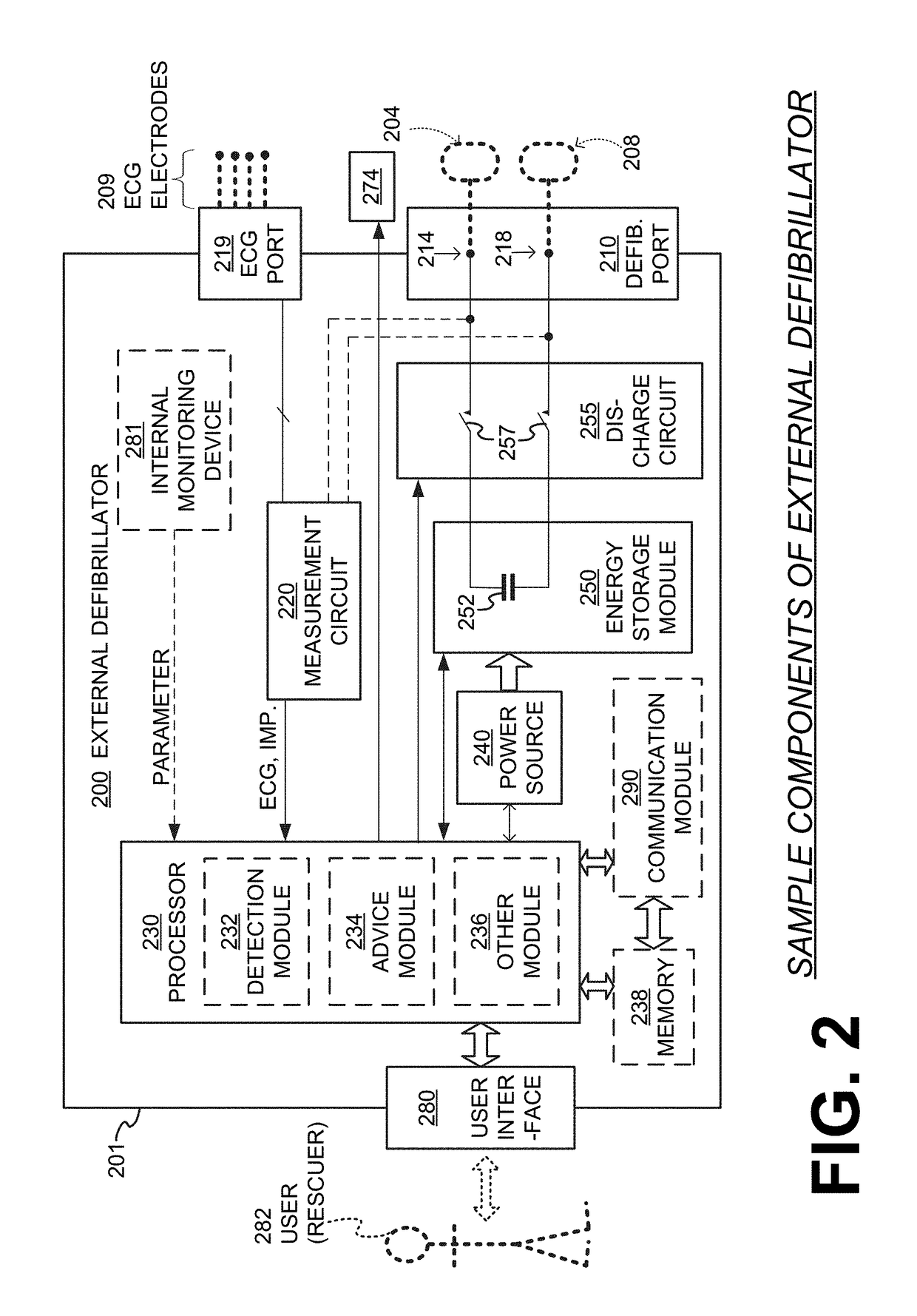
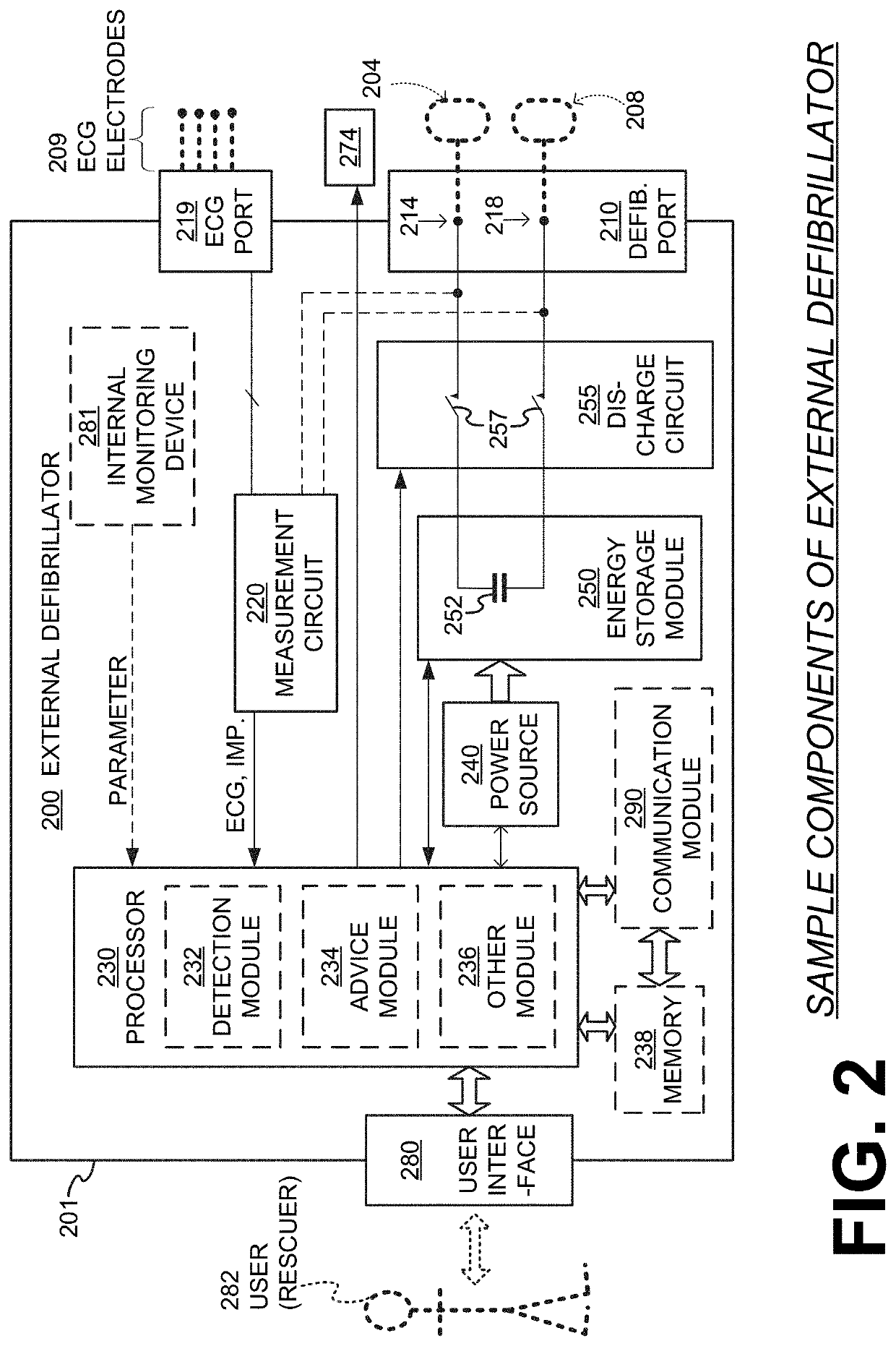
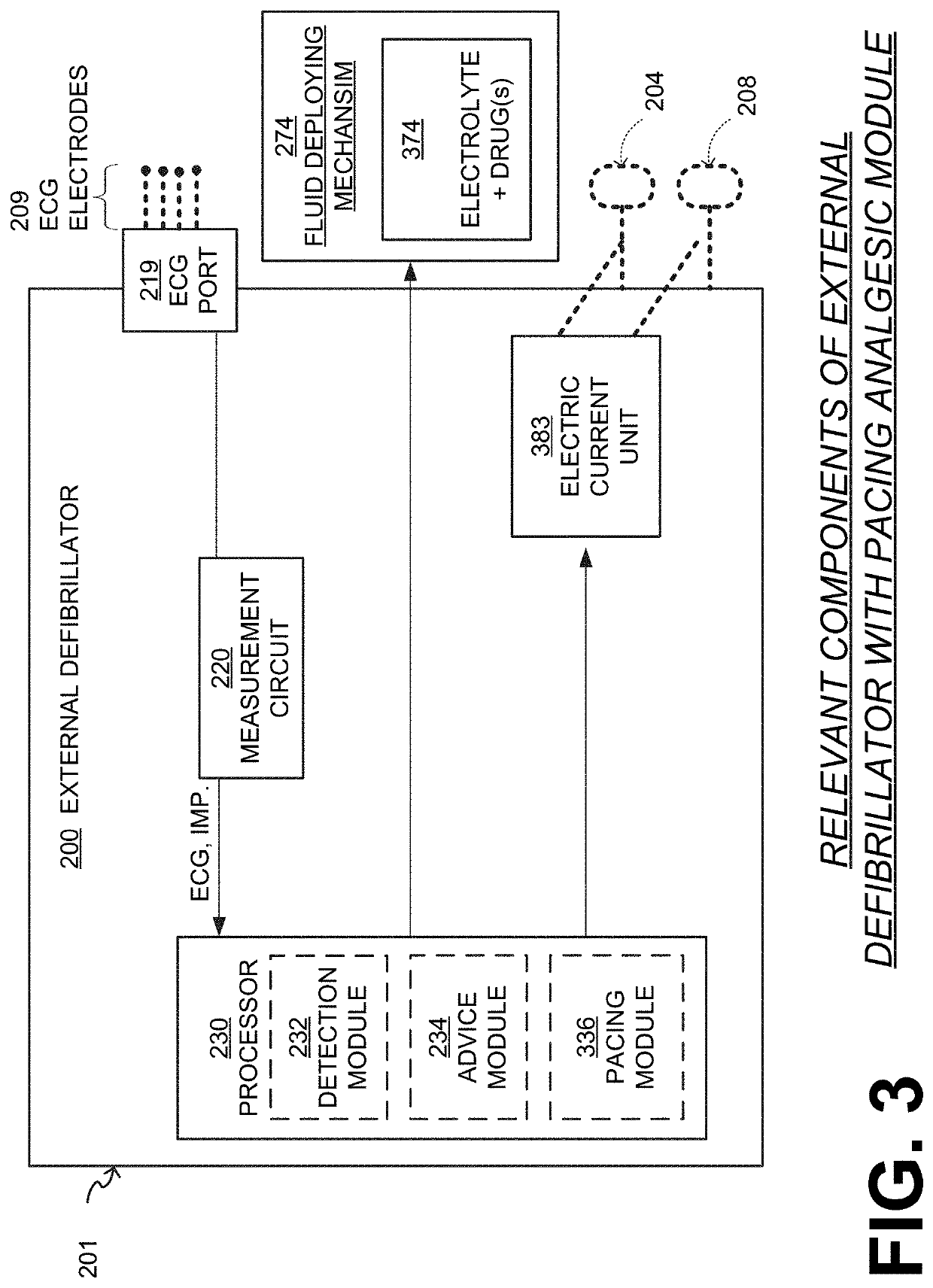
Wcd with pacing analgesia
An external defibrillator system such as a WCD is also capable of providing transthoracic pacing and drug delivery (e.g., pain-reducing drugs and / or a sedatives) to a patient. The drug(s) may be included in the therapy electrode electrolyte and dispensed for defibrillation, cardioversion and / or pacing therapy. Alternatively, the drug(s) may be stored in a separate reservoir and dispensed during pacing therapy. The drug(s) may be dispensed to a patient after a successful defibrillation therapy. The pacing therapy may be delivered a set time-period after the drug(s) were dispensed. A relatively small electric current may be delivered to the area of the patient on which the drug(s) were dispensed to facilitate drug absorption.
Owner:STRYKER CORP +1
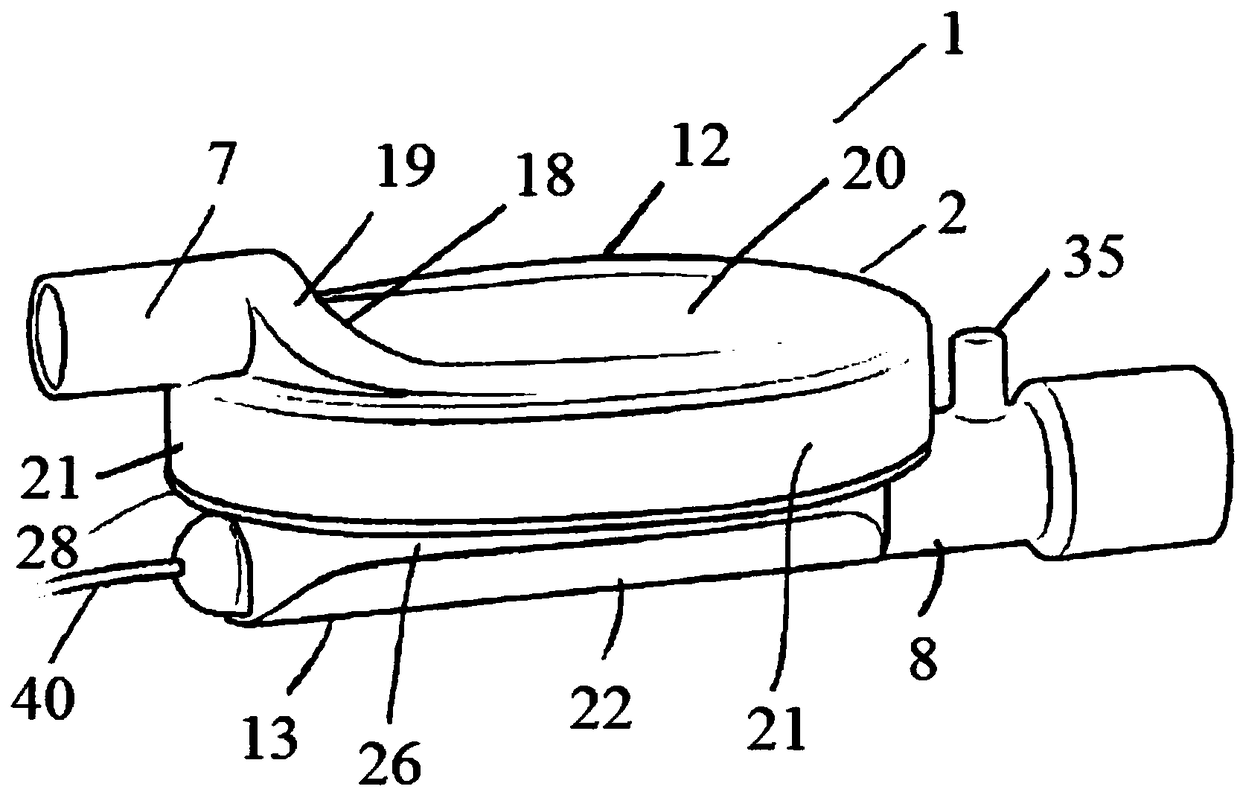
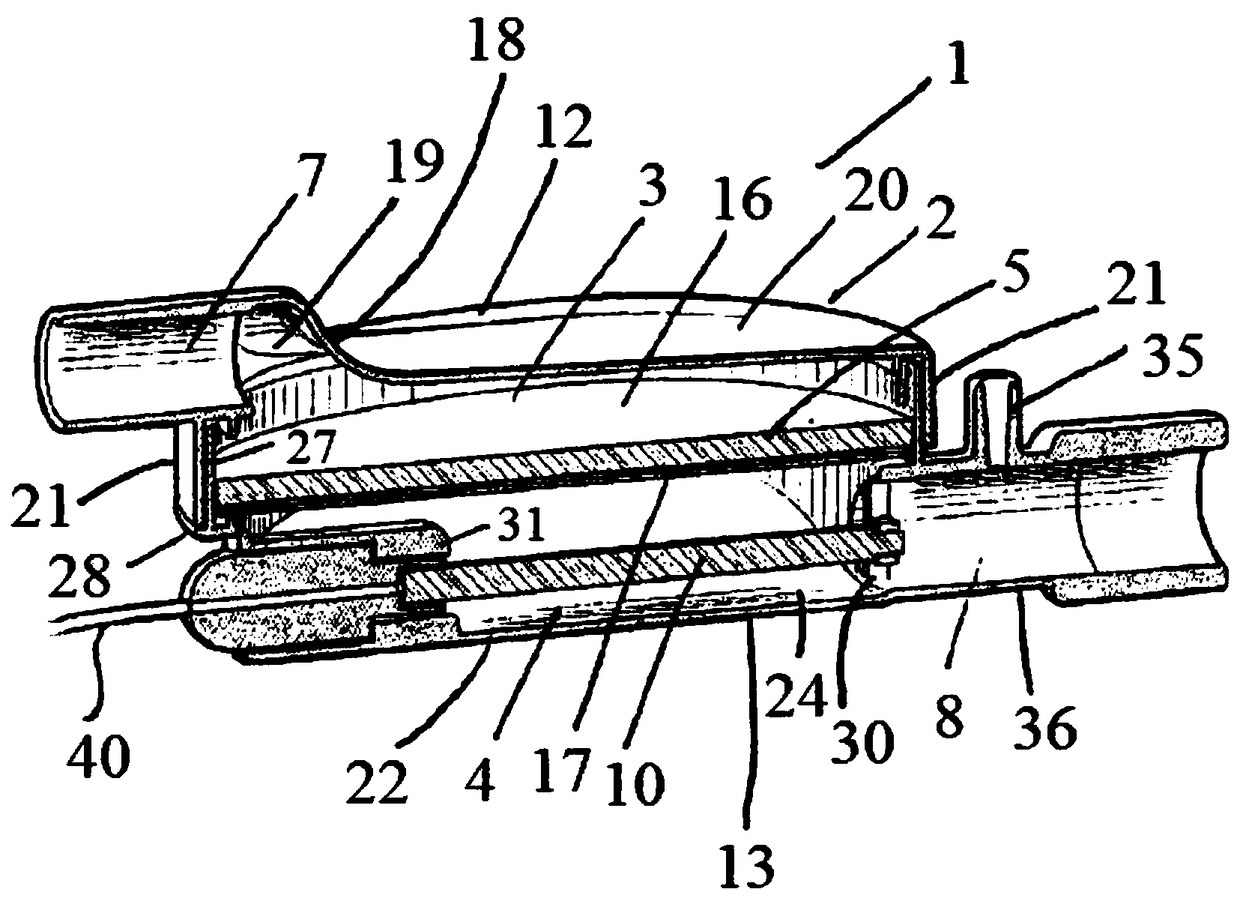
A sedation device
A sedation device (1) has a housing (2) divided internally into a ventilator chamber (3) and an associated evaporator chamber (4) which overlap and are separated by a filter (5) mounted between the chambers (3, 4) and forming a common gas-permeable dividing wail between the chambers (3, 4), An inlet port (7) is provided at one end of the ventilator chamber (3) at a top of the housing (2) for connection to a patient ventilator in use. An outlet port (8) on the evaporator chamber (4) can be connected via a breathing tube to a patient. An evaporator (10) is mounted within the evaporator chamber (4) for delivery of a volatile sedative into the evaporator chamber (4) during use.
Owner:SEDANA MEDICAL LTD
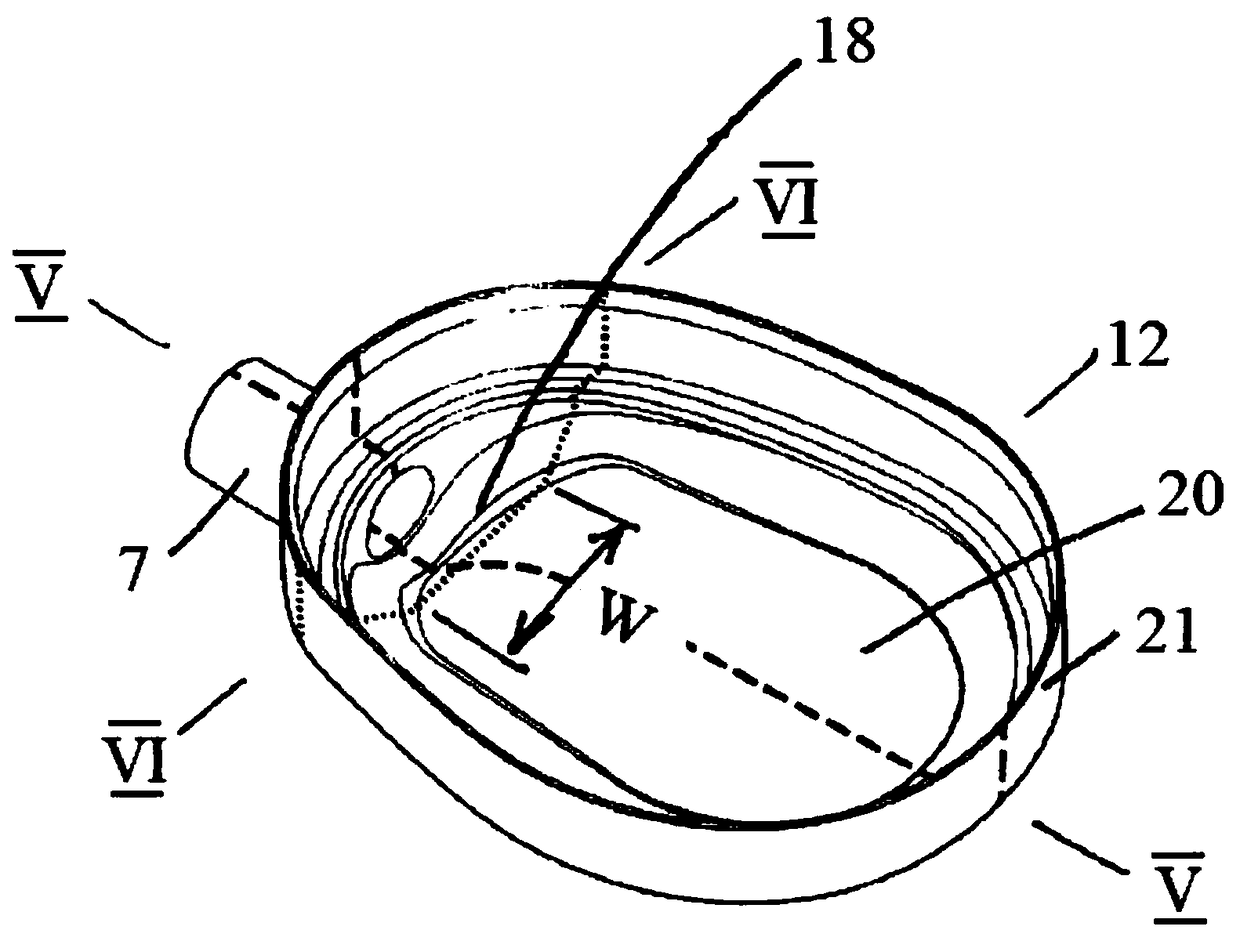
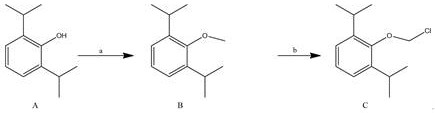
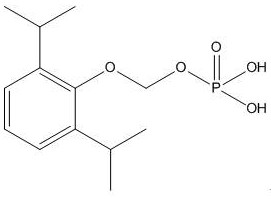
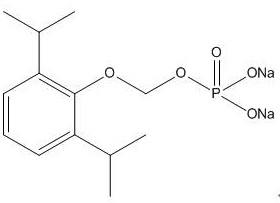
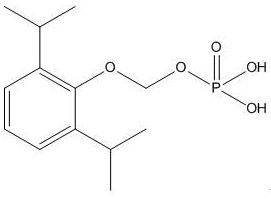
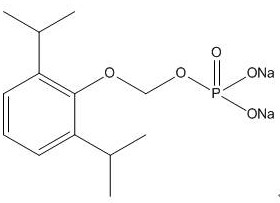
A kind of synthetic method and application of fospropofol sodium and intermediate thereof
ActiveCN113416121BThere is no security riskMild reaction conditionsOrganic active ingredientsNervous disorderSedative agentMedicinal chemistry
The invention provides a method for synthesizing fospropofol sodium and an intermediate thereof, including intermediate B and intermediate C, and the application of fospropofol sodium prepared by the synthetic method in an anesthetic sedative for animals . The method for synthesizing fospropofol sodium provided by the invention avoids using the highly toxic reagent chlorobromomethane, and has high safety; the prepared fospropofol intermediate has mild reaction conditions, simple and convenient operation, and the mass yield: >97%, which is easy to use. Industrialized production, the propofol prodrug prepared by the fospropofol sodium synthesized by the synthetic method provided by the present invention can produce the same clinical effect as the commercially available propofol emulsion injection, and no fospropofol anesthesia occurs induced myocardial depressive response.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Process for the preparation of 2-(5-methoxyisochroman-1-yl)-4,5-dihydro-1H-imidazole and the hydrogensulfate salt thereof
ActiveUS11352346B2Suitable for use in large industrial scaleMore economicalGroup 4/14 element organic compoundsOrganic active ingredientsAdrenergicEthyl group
The present disclosure relates to an improved process for the preparation of isochroman structured alpha2A adrenoceptor agonist, namely 2-(5-methoxyisochroman-1-yl)-4,5-dihydro-1H-imidazole of formula (I) and a pharmaceutically acceptable salts thereof, such as 2-(5-methoxyisochroman-1-yl)-4,5-dihydro-1H-imidazole hydrogensulfate of formula (Ia), and to a novel intermediate compound used in the process, namely N-(2-aminoethyl)-5-methoxyisochroman-1-carboxamide monohydrate of formula (V). Alpha2A agonists are useful in the treatment of anxiety, and for use as a sedative or analgesic agent, and other diseases where alpha2A agonism is desired.
Owner:ORION CORPORATION
Synthesis method and application of fospropofol sodium and intermediates thereof
ActiveCN113416121AThere is no security riskMild reaction conditionsOrganic active ingredientsNervous disorderSedative agentOrganic chemistry
The invention provides a synthesis method of fospropofol sodium and intermediates thereof, the fospropofol sodium comprises an intermediate B and an intermediate C, the invention also provides application of the fospropofol sodium prepared by the synthesis method in an anesthetic sedative for animals. The synthesis method of fospropofol disodium avoids the use of a highly toxic reagent bromochloromethane, and has high safety; the prepared fosfopropofol intermediate is mild in reaction condition and simple and convenient to operate, the mass yield is greater than 97%, industrial production is easy, and a propofol prodrug prepared from fosfopropofol sodium synthesized by the synthesis method can generate the same clinical effect as that of a commercially available propofol emulsion injection, and the myocardial inhibition reaction caused by fospropofol anesthesia does not occur.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
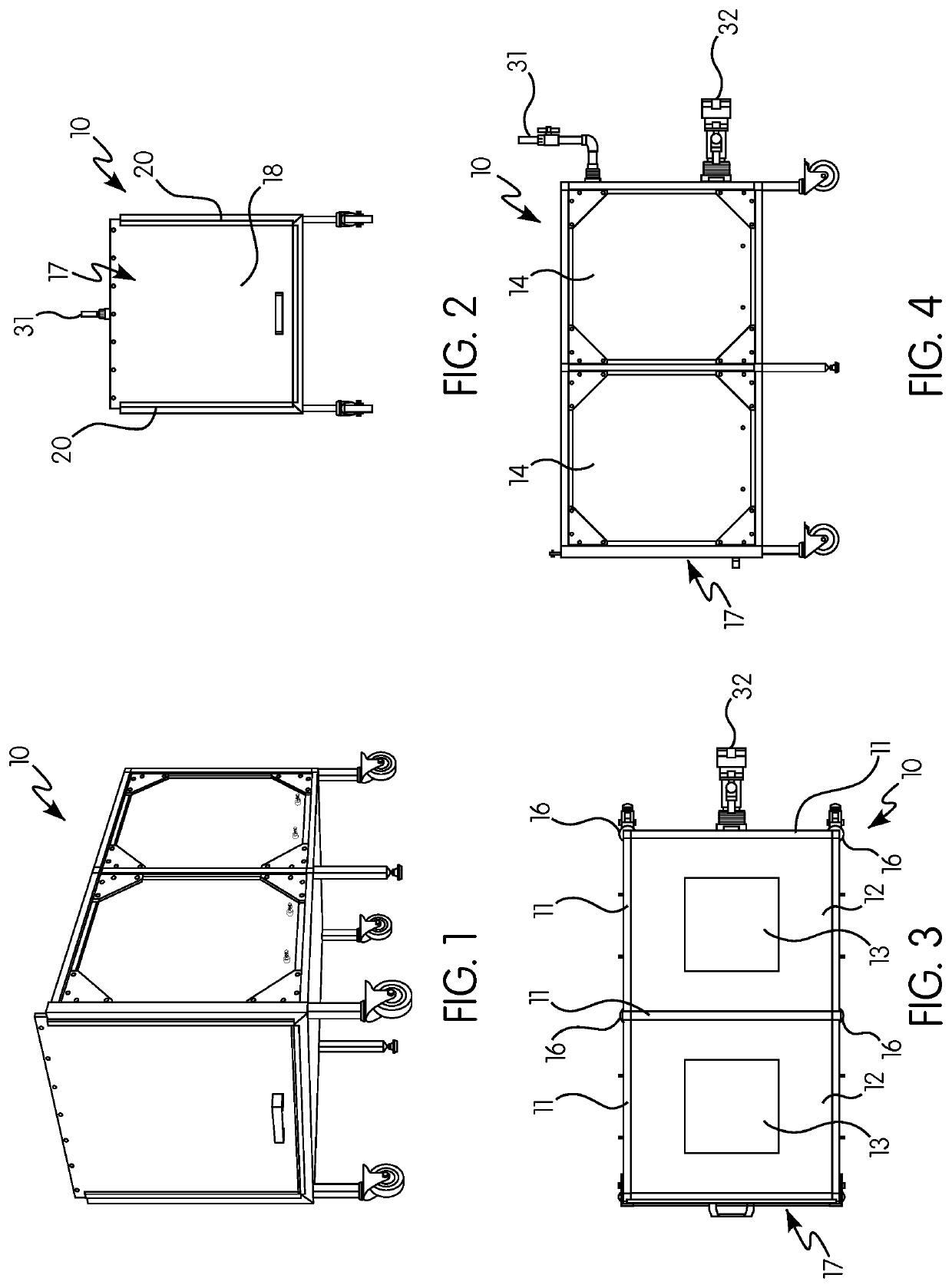
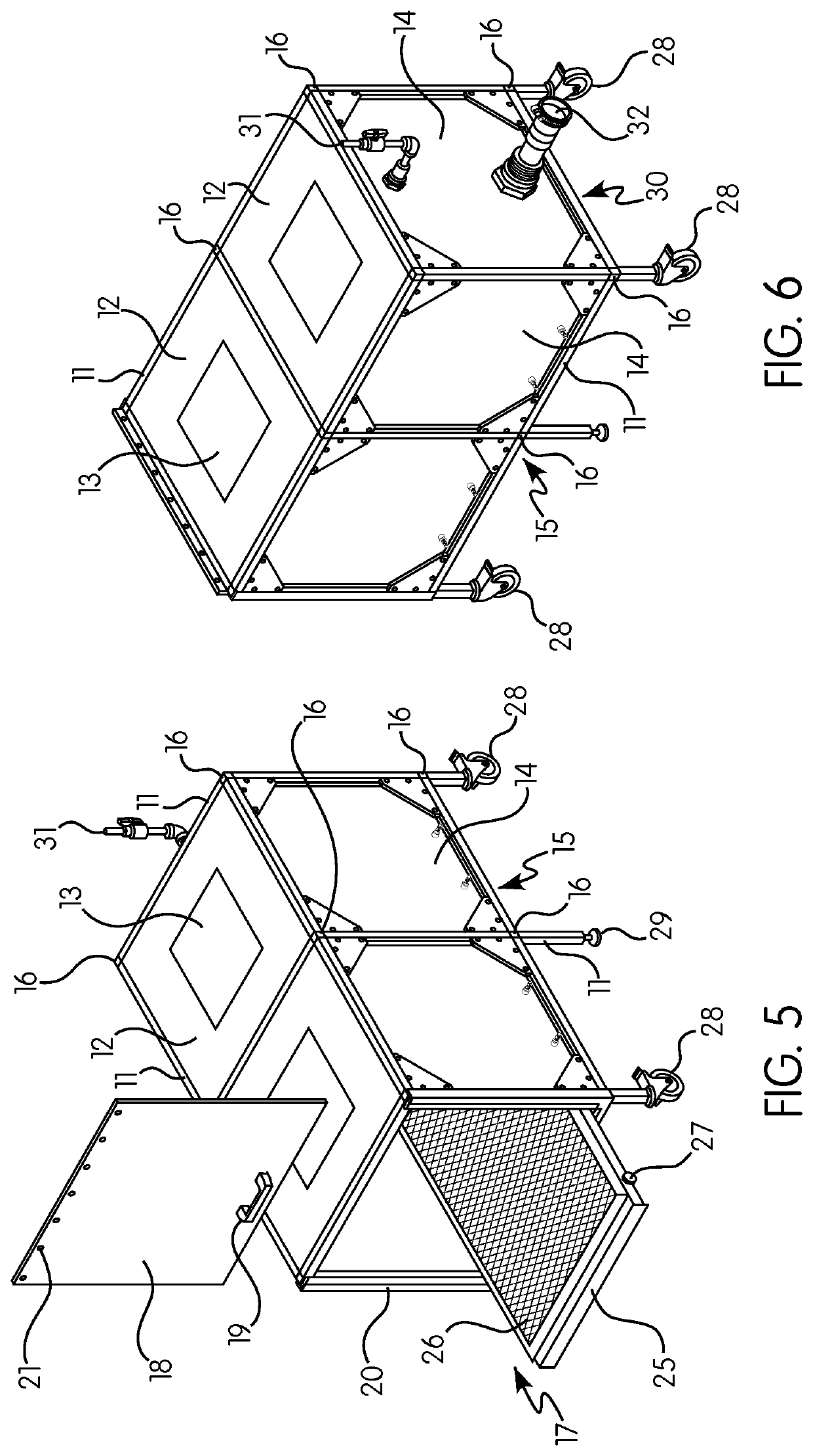
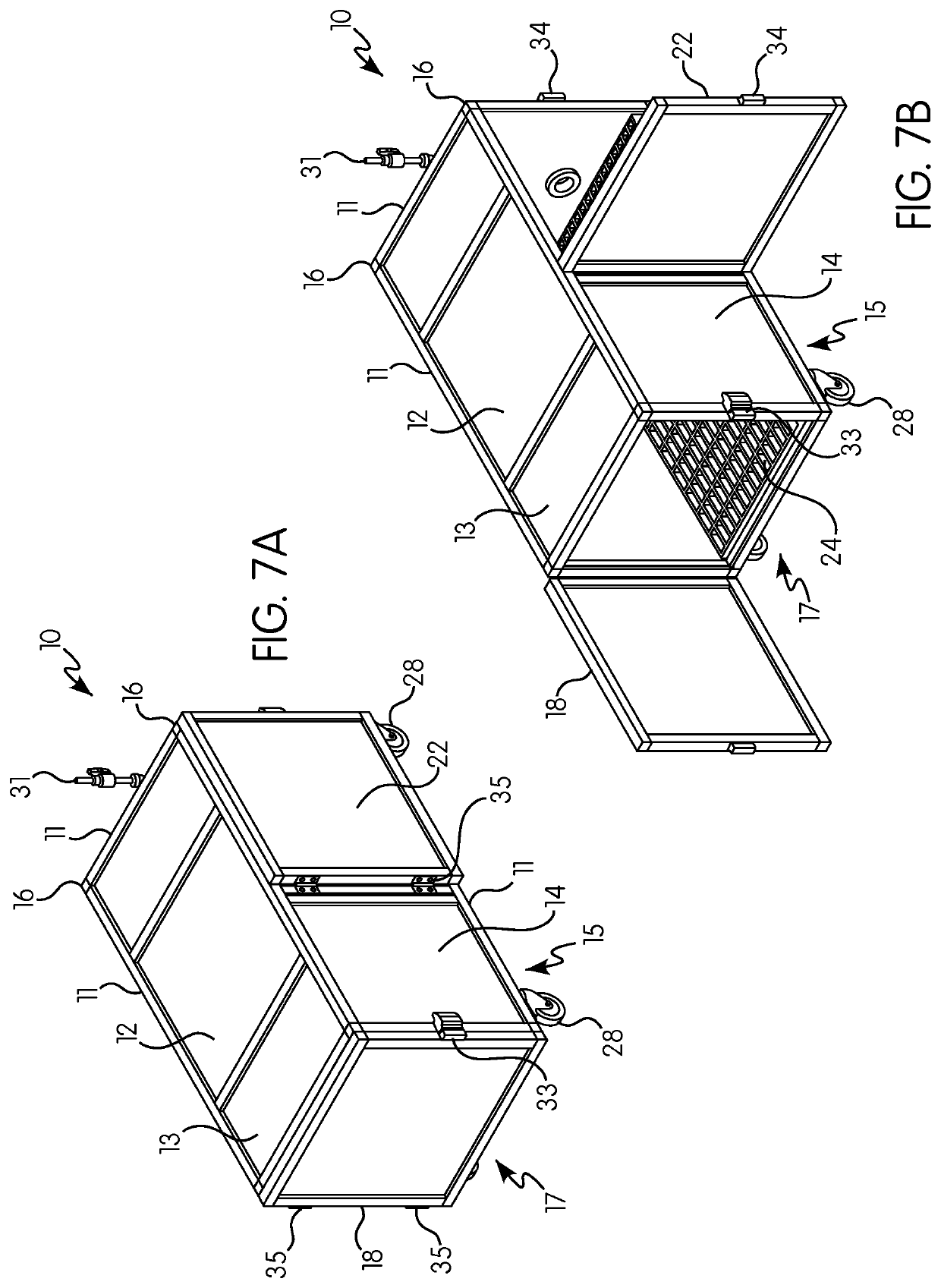
Gas induction chamber for large animals
The present invention is drawn to a gas induction chamber that is particularly suited to being scaled to larger animals, such as swine. The present invention solves the problems associated with researchers having to physically restrain animals to inject them with sedative and / or holding a cone over the animal's face to introduce sedation. That process can be dangerous for both the researcher and the animal. The present invention provides a gas induction chamber for use with larger animals comprised of a combination of interconnecting frame members for use in connecting side panels, roof panels, and floor panels into a leak-free chamber. A gas inlet pipe is located near the top of the back side panel and an extraction pipe located near the bottom of the back side panel.
Owner:DEZORT ENTERPRISES LLC
Dosing regimen of sedative
PendingUS20220152045A1Rapid of consciousnessOrganic active ingredientsNervous disorderDosing regimenPropanoic acid
By intravenously administering a sedative comprising methyl 3-[(4S)-8-bromo-1-methyl-6-(2-pyridinyl)-4H-imidazo[1,2-a][1,4]benzodiazepin-4-yl]propanoate or a salt thereof to a patient by a dosing regimen including a two-step process disclosed in the present invention, general anesthesia can be safely and promptly induced and maintained.
Owner:PAION UK
Transcutaneous sedative release control in autonomous imaging
The invention relates to systems and methods for controlling the transdermal and automatic release of sedative during imaging of a subject. A sedation level of the patient is detected during the imaging procedure. An amount of a sedative to be applied to the patient to maintain a degree of sedation is calculated. Further, a time for releasing the amount of sedative is calculated. The calculated amount of sedative is percutaneously released to the patient at the calculated time.
Owner:KONINKLJIJKE PHILIPS NV
Compound with anesthetics activity, methods for its production and pharmaceutical compositions comprising the same
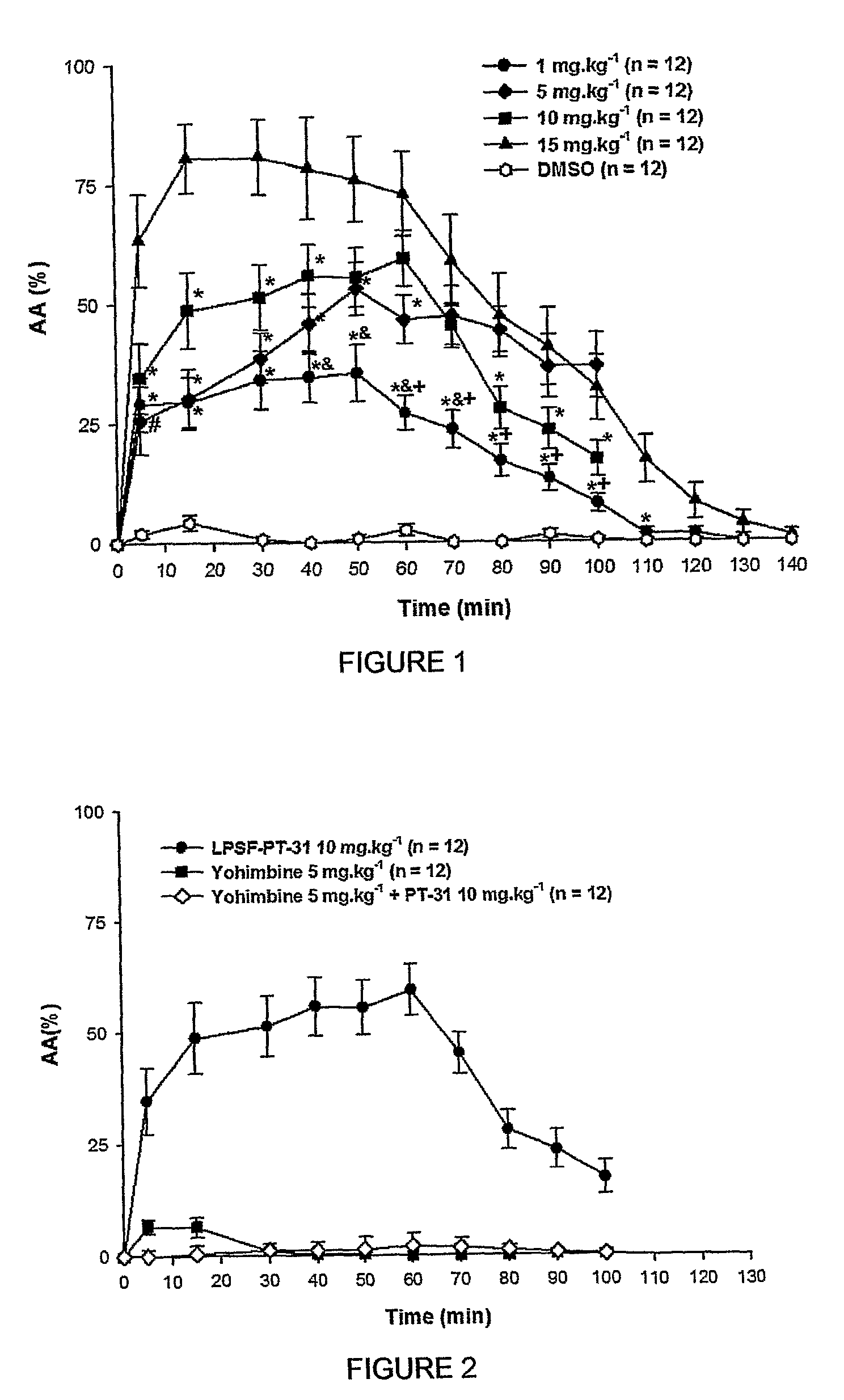
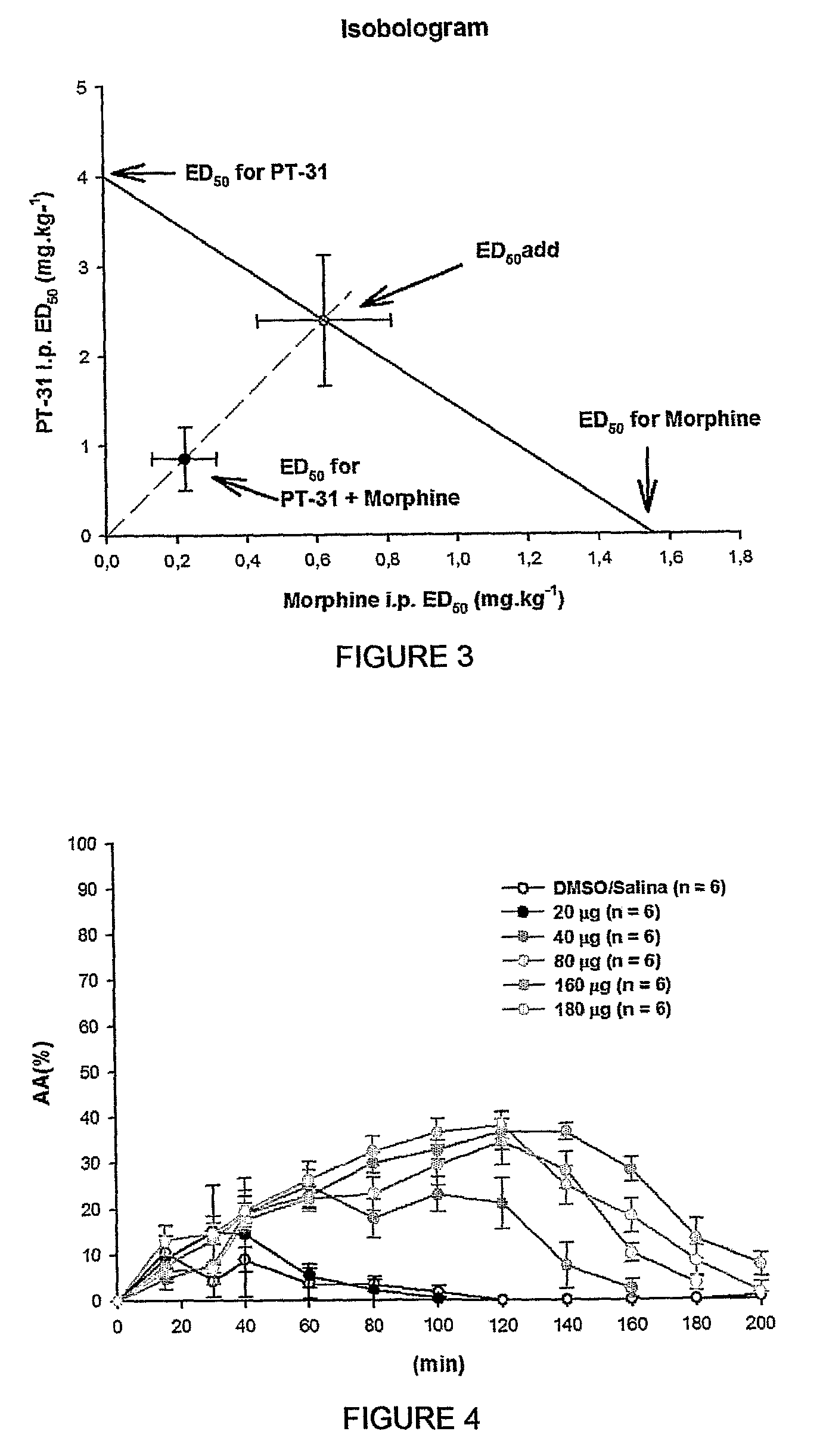
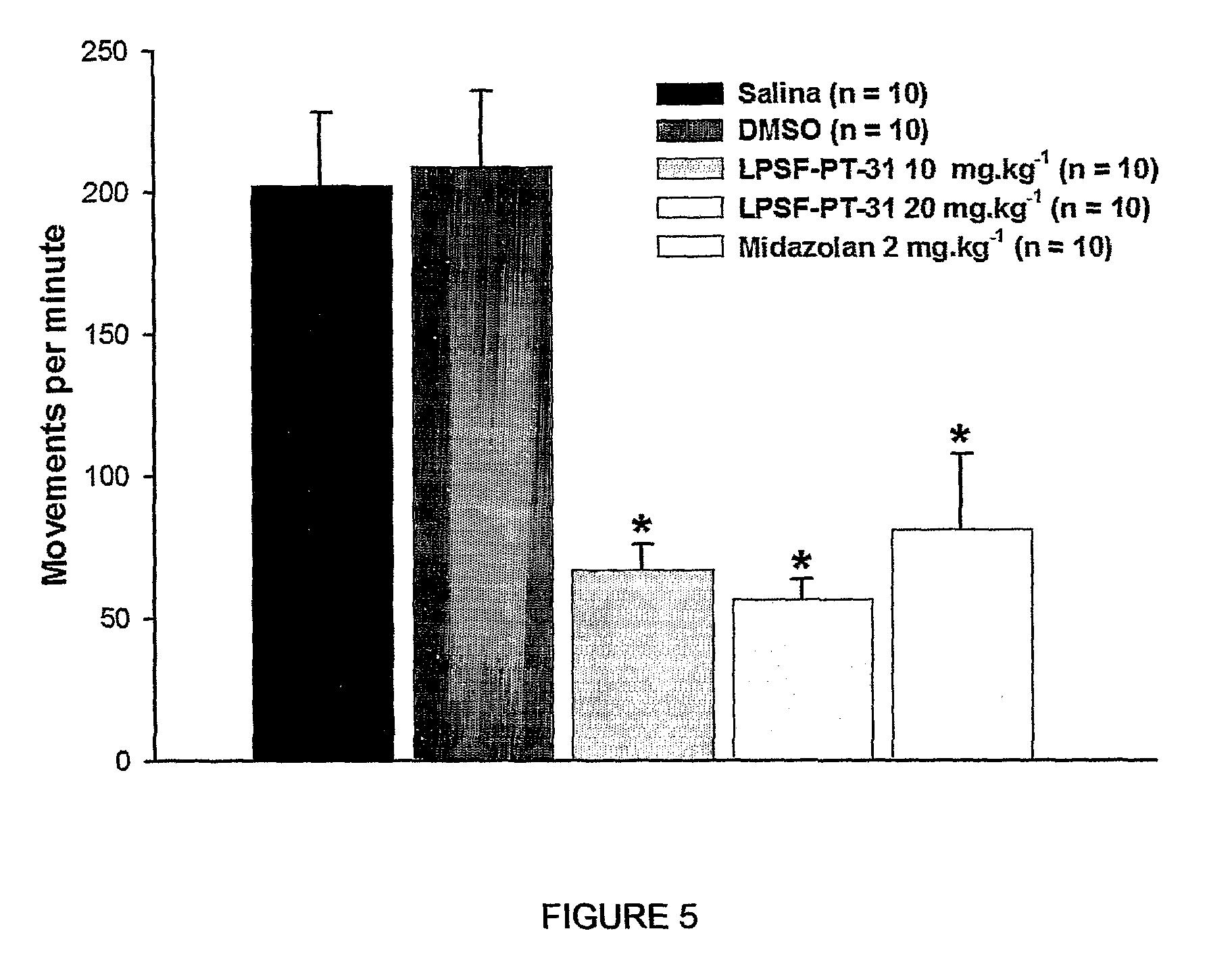
The present invention is related to a new series of chemical compounds, namely 3-benzyl-imidazolidine-2,4-dione substituted in the position 2 and / or 6 of benzyl ring by halogens as presented to the molecule named LPSF-PT-31, GIRSUPAN and its therapeutic use as drug with analgesic, sedative and adjuvant of anesthetics activities. The invention is also related to a process for production of said compounds as well as pharmaceutical compositions comprising them.
Owner:UFPE +1
WCD with pacing analgesia
An external defibrillator system such as a WCD is also capable of providing transthoracic pacing and drug delivery (e.g., pain-reducing drugs and / or a sedatives) to a patient. The drug(s) may be included in the therapy electrode electrolyte and dispensed for defibrillation, cardioversion and / or pacing therapy. Alternatively, the drug(s) may be stored in a separate reservoir and dispensed during pacing therapy. The drug(s) may be dispensed to a patient after a successful defibrillation therapy. The pacing therapy may be delivered a set time-period after the drug(s) were dispensed. A relatively small electric current may be delivered to the area of the patient on which the drug(s) were dispensed to facilitate drug absorption.
Owner:STRYKER CORP +1
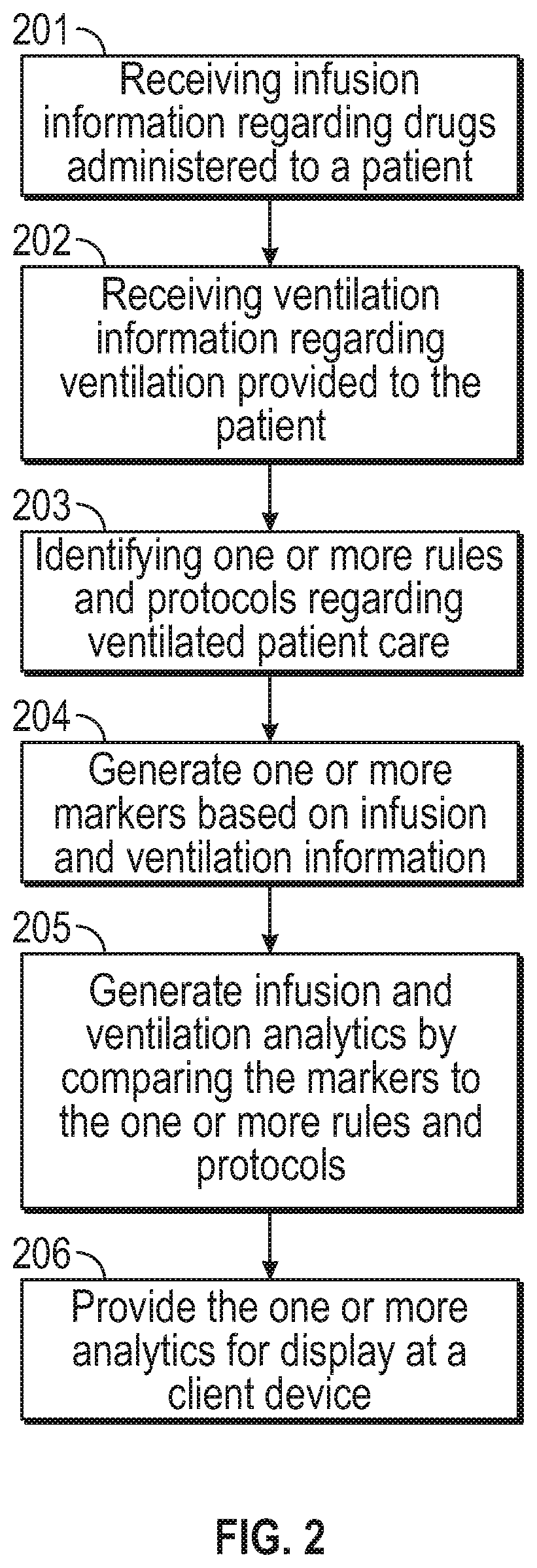
Analytics regarding patient care
ActiveUS11183287B2Facilitate managing rule and protocolRespiratorsDrug and medicationsPharmaceutical drugSedative agent
A method, system and computer-readable medium are provided for determining compliance with patient care protocols, comprising an infusion pump providing infusion information pertaining to one or more drugs administered to a patient, the one or more drugs including sedatives and analgesics, and a processor communicably coupled to the infusion pump and configured to determine information regarding the one or more drugs being administered to the patient based on the infusion information by determining a baseline threshold for the one or more drugs being administered to the patient according to the actual dosage of the one or more drugs administered to the patient prior to a current time, determining a dosage amount of the one or more drugs currently being administered to the patient and comparing the dosage amount of the one or more drugs currently being administered to the baseline threshold to determine a deviation from the baseline threshold.
Owner:CAREFUSION 303 INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com