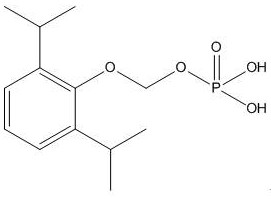
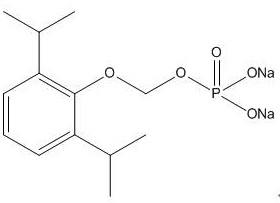
A kind of synthetic method and application of fospropofol sodium and intermediate thereof
A technology of fospropofol sodium and a synthesis method, applied in the field of drug synthesis and application, can solve the problems of large side effects, low biosafety and the like, and achieve the effects of simple operation, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
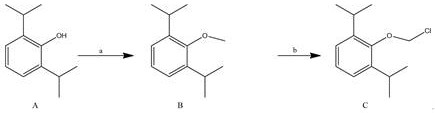
[0044] A preparation method of water-soluble propofol prodrug fospropofol sodium, the preparation method comprising the following steps:
Embodiment 1-a
[0046] Compound A (propofol) was dissolved in DMF (the volume-to-mass ratio of 5V:DMF to compound A was 5 mL / g), and potassium carbonate (2.0eq: the molar ratio of potassium carbonate to compound A was 2.0) was added, The reaction solution was cooled to 0-5°C, and CH was added dropwise with stirring. 3 I (2.0eq:CH 3 The molar ratio of I to Compound A is 2.0) in DMF (1V:DMF to CH 3 The volume-to-mass ratio of I is 1mL / g) solution, after the addition is completed, the reaction is raised to room temperature for 2-3h, TLC plate layer monitoring, after the reaction is completed, take the reaction solution and dilute it into ice water, separate out a large amount of solid, filter, dry to obtain the compound The crude product B, the mass yield of 106.8%, was used directly for the next reaction without further purification.
Embodiment 1-b
[0048] Compound B was dissolved in DMF (5V: The volume-to-mass ratio of DMF to Compound B was 5 mL / g), phosphorus pentachloride was added (1.0eq: the molar ratio of phosphorus pentachloride to Compound B was 1.0), and heated The reaction was carried out at 70°C for 3-4 hours, monitored by TLC, filtered after the reaction was completed, the filtrate was concentrated, water and ethyl acetate were added to the remaining liquid, and the liquid was separated. 3 The solution was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain a crude compound C with a mass yield of 115.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com