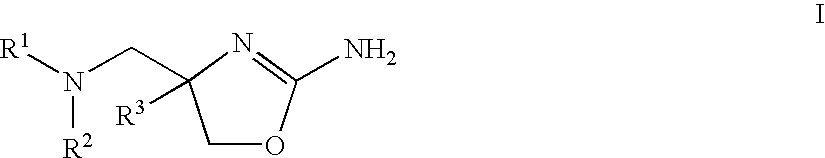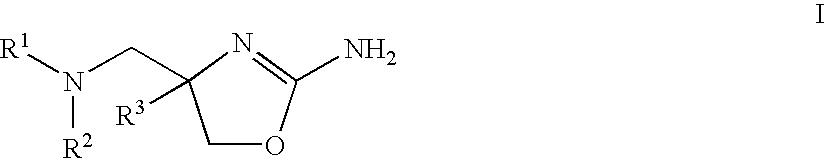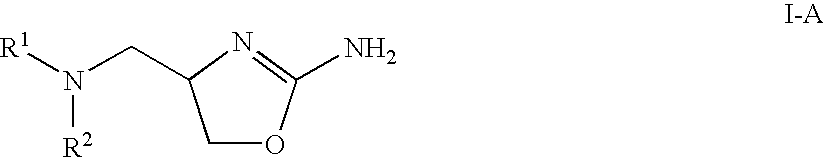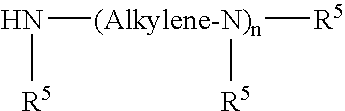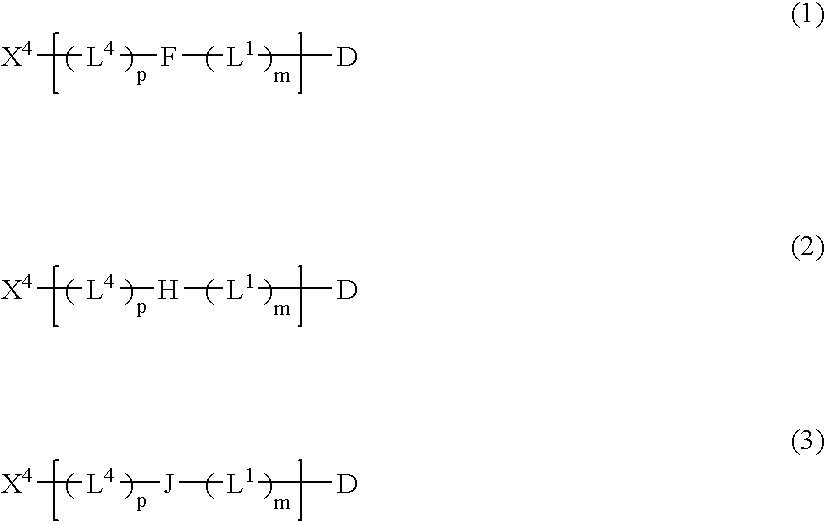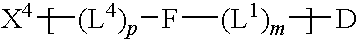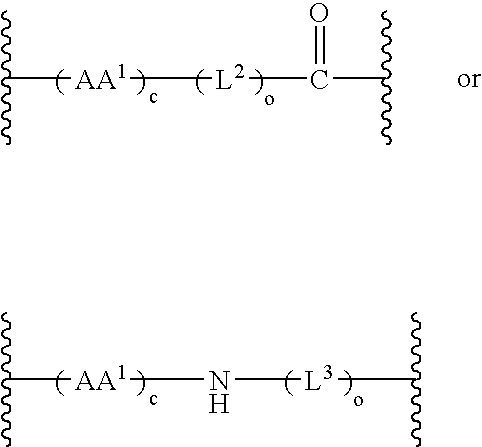Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
69456 results about "Medicinal chemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicinal chemistry and pharmaceutical chemistry are disciplines at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where they are involved with design, chemical synthesis and development for market of pharmaceutical agents, or bio-active molecules (drugs).
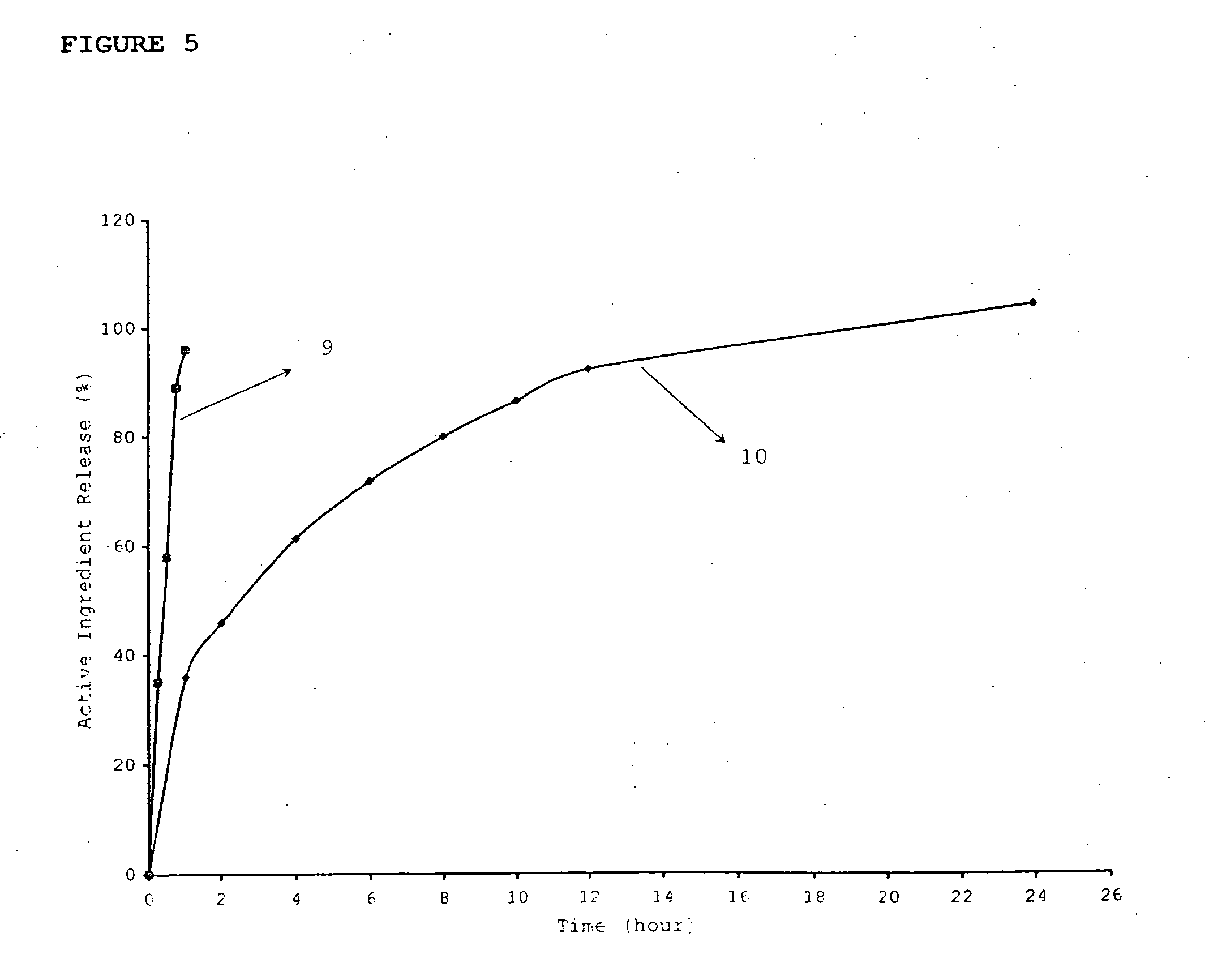
Means to achieve sustained release of synergistic drugs by conjugation
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
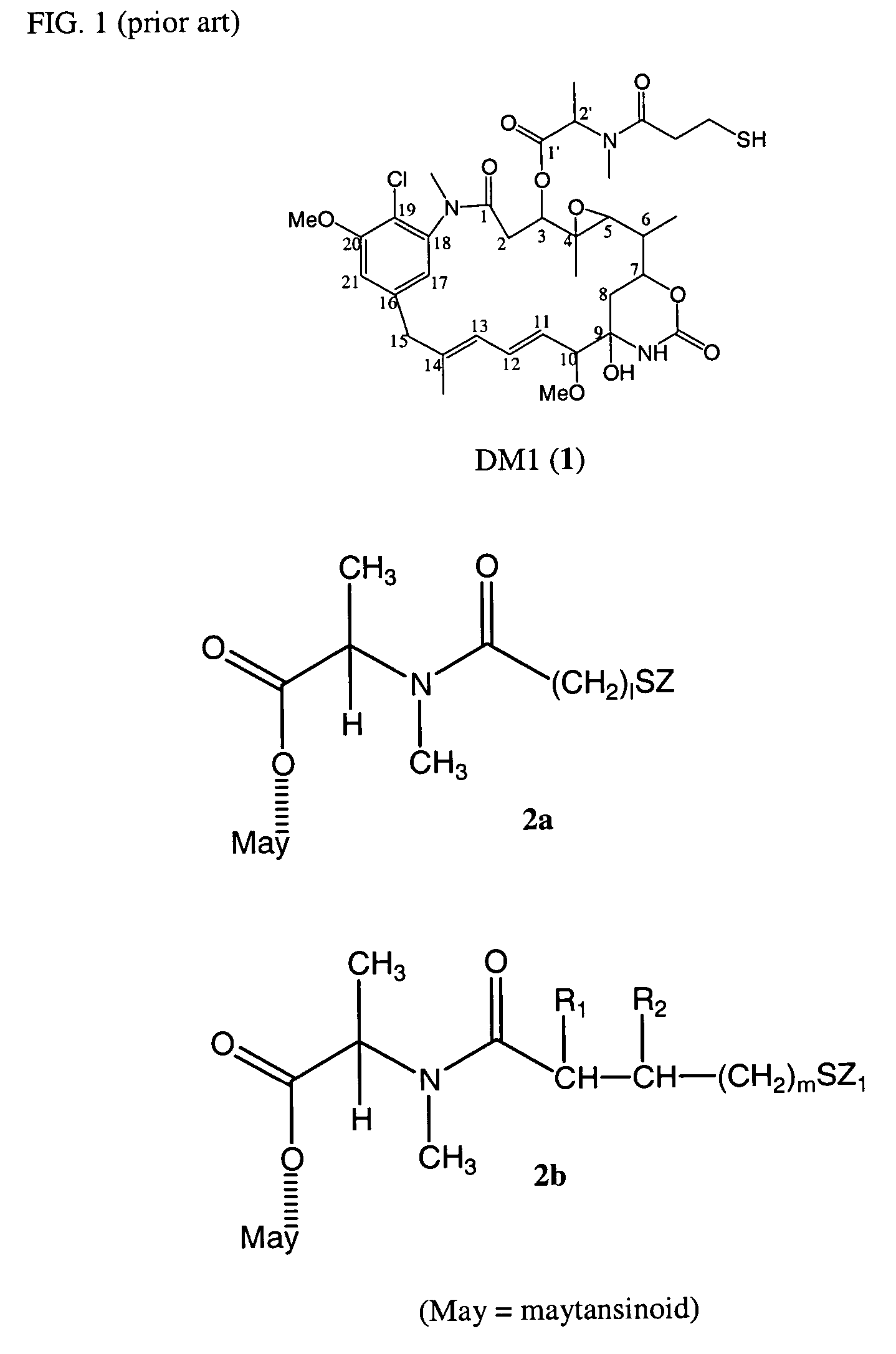
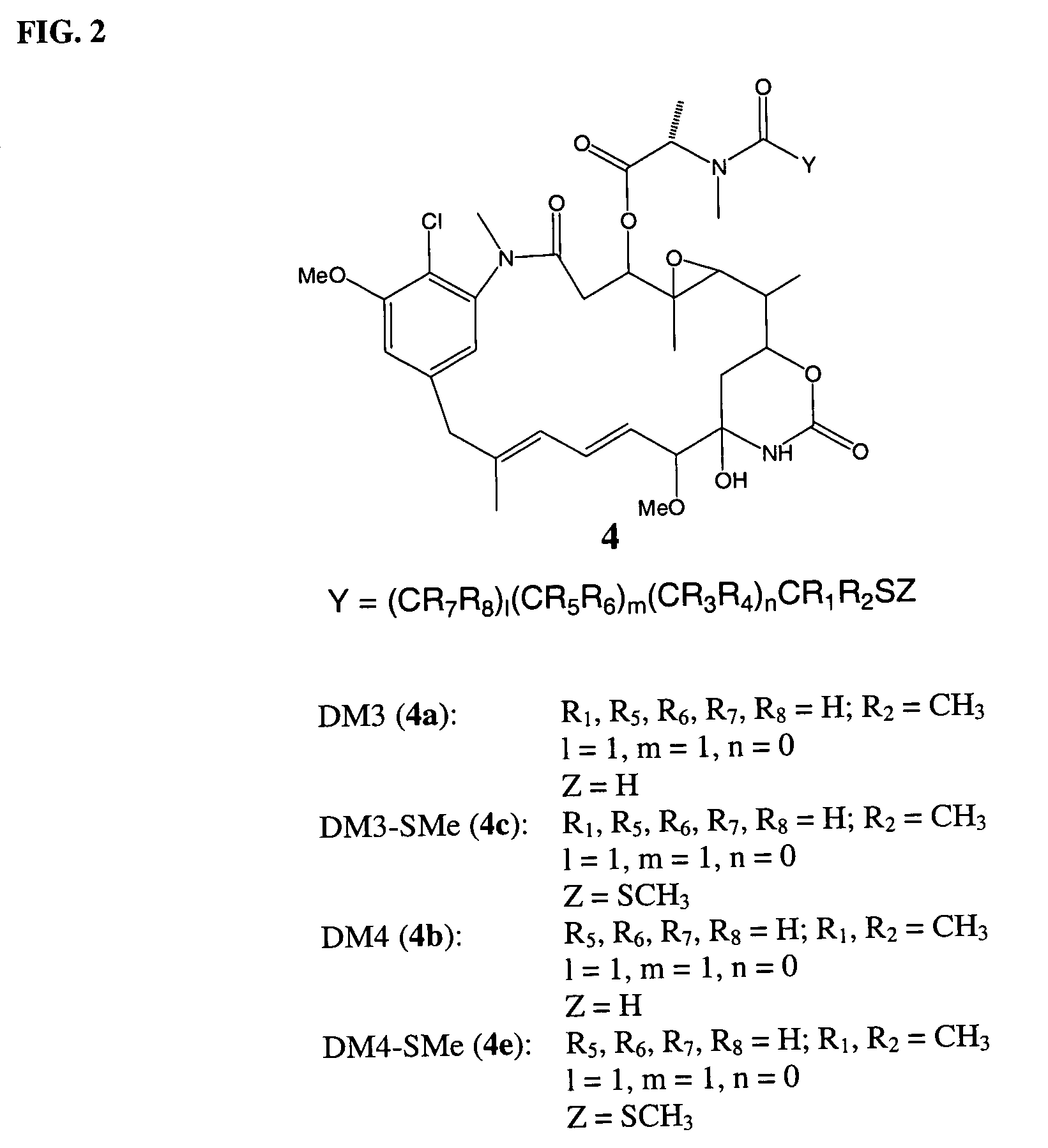
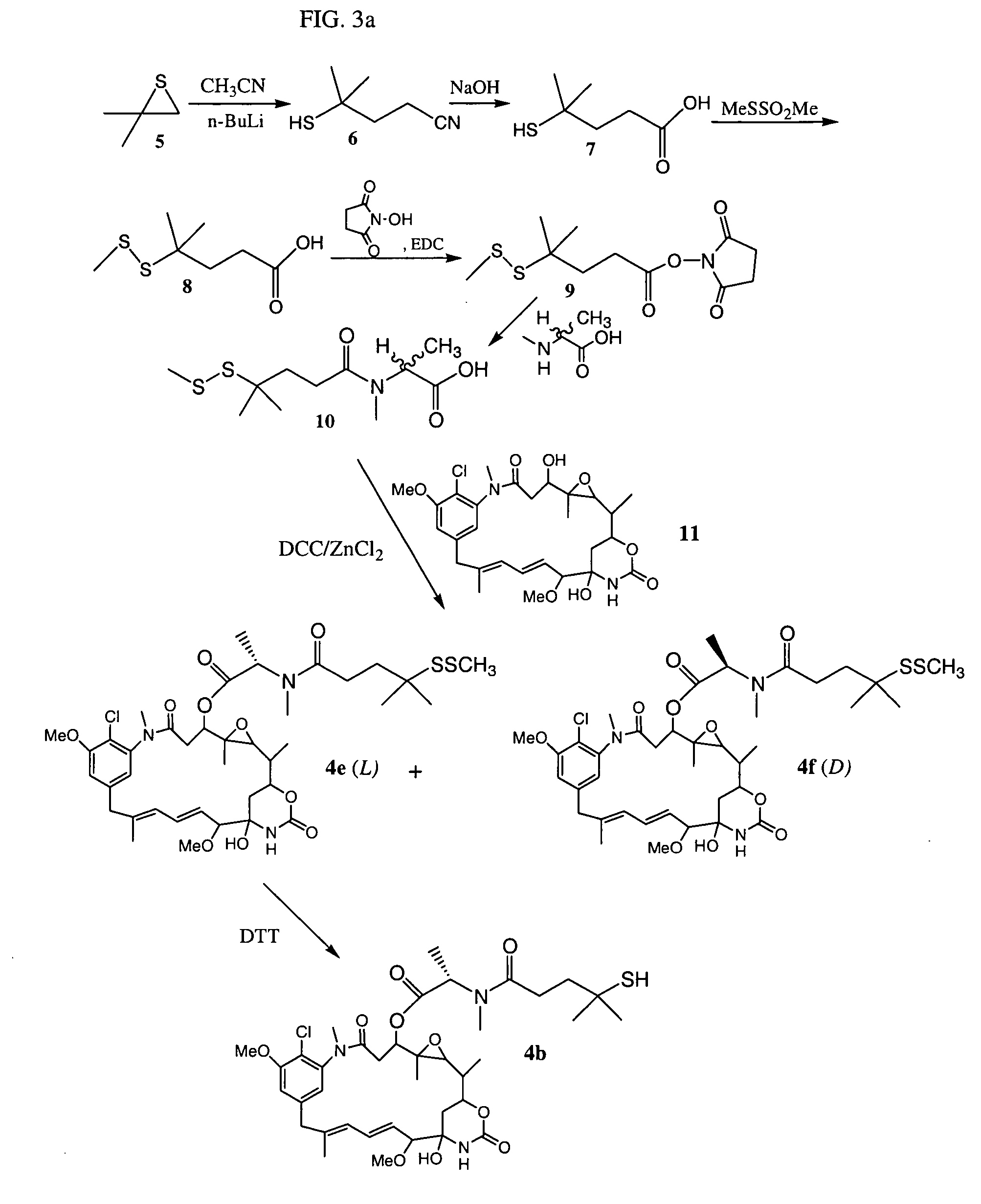
Cytotoxic agents comprising new maytansinoids
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
Chemical linkers and conjugates thereof
ActiveUS20060024317A1Increase solubilityDecrease aggregationMaterial nanotechnologyHybrid immunoglobulinsDrugToxin
The present disclosure provides drug-ligand conjugates that are potent cytotoxins, wherein the drug is linked to the ligand through either a peptidyl, hydrazine, or disulfide linker. The disclosure is also directed to compositions containing the drug-ligand conjugates, and to methods of treatment using them.
Owner:MEDAREX LLC
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Disulfide prodrugs and linkers and stabilizers useful therefor
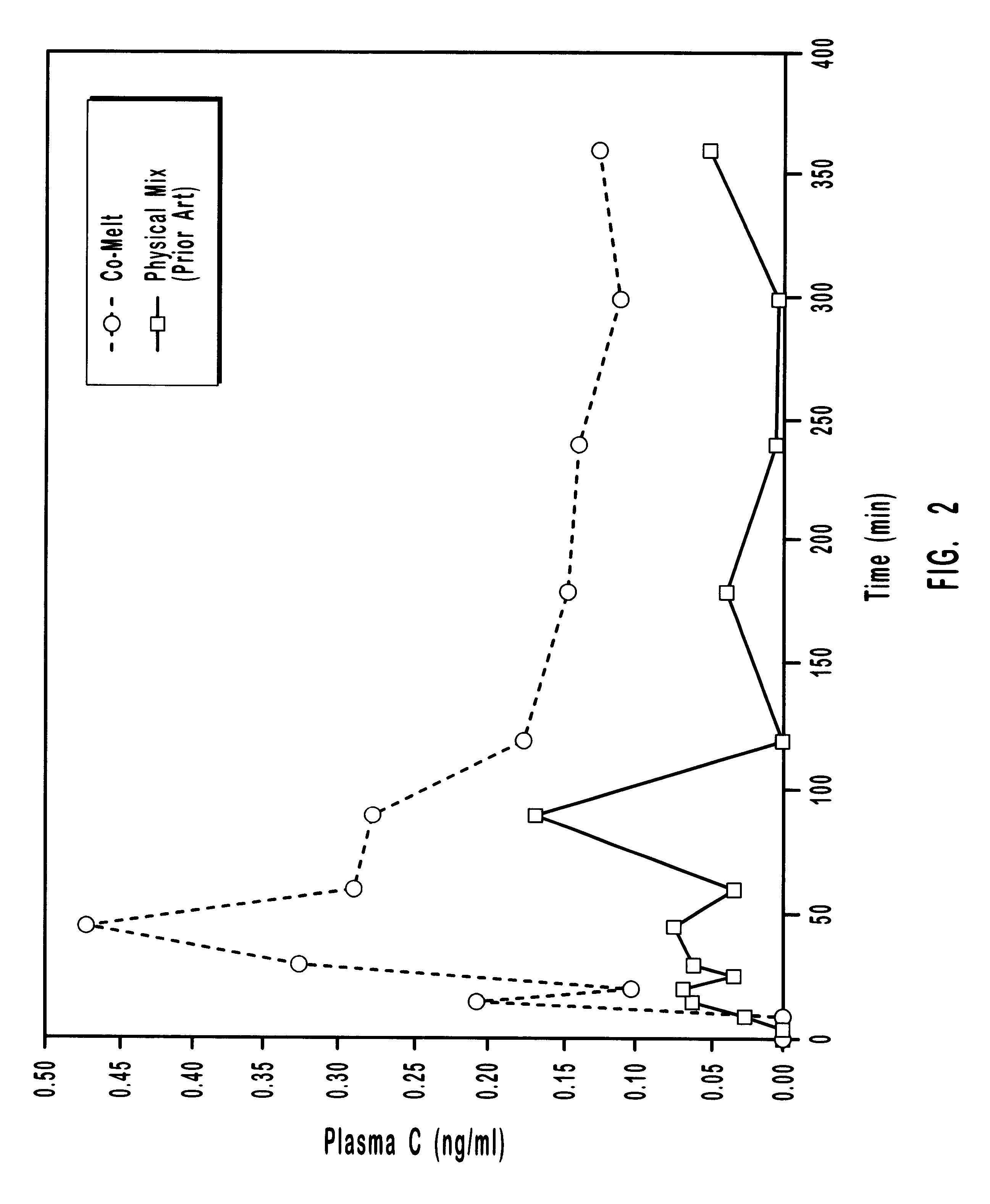
The present invention provides analogues of duocarmycins that are potent cytotoxins. Also provided are peptidyl and disulfide linkers that are cleaved in vivo. The linkers are of use in forming prodrugs and conjugates of the cytotoxins of the invention as well as other diagnostic and therapeutic moieties. The invention provides prodrugs and conjugates of the duocarmycin analogues with the linker arms of the invention.
Owner:ER SQUIBB & SONS INC
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
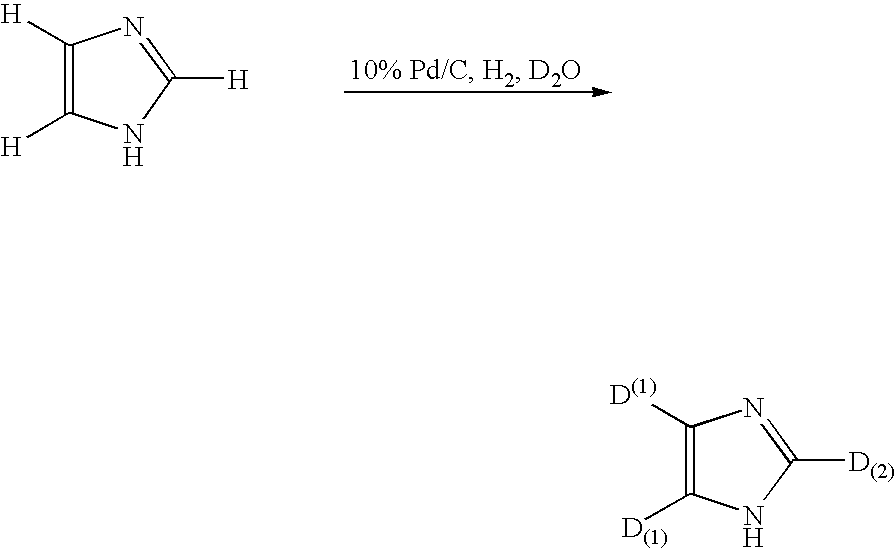
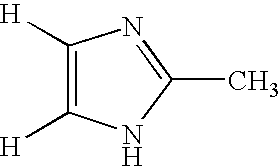
Method for deuteration of a heterocyclic ring
ActiveUS7517990B2Isotope introduction to heterocyclic compoundsSugar derivativesNickel catalystHydrogen atom
The present invention relates to a method for deuteration of a heterocyclic ring, which comprises subjecting a compound having a heterocyclic ring to sealed refluxing state in a deuterated solvent in the presence of an activated catalyst selected form a palladium catalyst, a platinum catalyst, a rhodium catalyst, a ruthenium catalyst, a nickel catalyst and a cobalt catalyst. In accordance with a method of the present invention, a hydrogen atom belonging to a heterocyclic ring of a compound having a heterocyclic ring can be very efficiently deuterated because temperature of deuteration reaction can be maintained at higher than boiling point of the solvent.Further, a method for deuteration of the present invention can be applied widely to deuteration of various compounds having a heterocyclic ring which are liable to decomposition under supercritical conditions or acidic conditions, leading to industrial and efficient deuteration of a compound having a heterocyclic ring.
Owner:FUJIFILM WAKO PURE CHEM CORP
2-aminooxazolines as taar1 ligands
Owner:F HOFFMANN LA ROCHE & CO AG
Lubricant and fuel compositions containing hydroxy carboxylic acid and hydroxy polycarboxylic acid esters
InactiveUS20050198894A1Reduce the amount requiredWithout diminishing anti-wear performanceOrganic chemistryLiquid carbonaceous fuelsCarboxylic acidMedicinal chemistry
Disclosed herein is a composition comprising: (A) a lubricant or a hydrocarbon fuel; (B) at least one hydroxy carboxylic acid ester or hydroxy polycarboxylic acid ester having the generic formula defined herein; and (C) at least one phosphorus-containing additive.
Owner:CHEMTURA CORP
Melt-extruded orally administrable opioid formulations
InactiveUS6261599B1Sustained effectSlow and control releaseBiocideOrganic active ingredientsMelt extrusionDosage form
Bioavailable sustained release oral opioid analgesic dosage forms, comprising a plurality of multiparticulates produced via melt extrusion techniques disclosed.
Owner:PURDUE PHARMA LP
Heterocyclic compounds
InactiveUS6329381B1Excellent interferon biosynthesis inducing activityInhibition thicknessAntibacterial agentsBiocideBULK ACTIVE INGREDIENTInterferon inducer
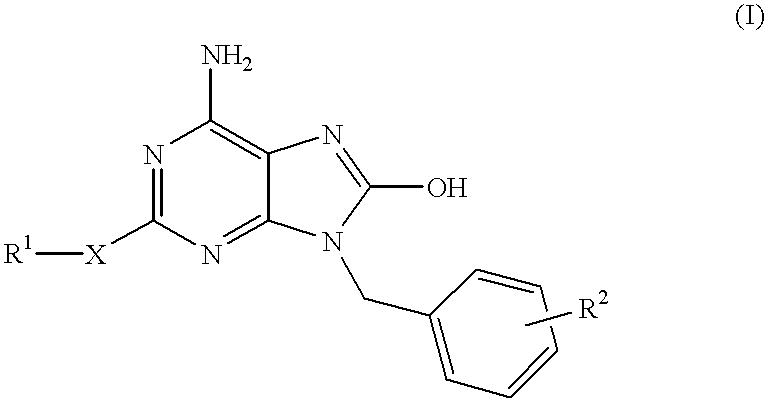
The present invention relates to a heterocyclic compound of the following general formula (I):wherein X is sulfur atom, oxygen atom or -NR3- (R3 may form a heterocyclic ring or a substituted heterocyclic ring with R1 via the nitrogen atom),R1 is alkyl group, substituted alkyl group, aryl group, substituted aryl group, heterocyclic group or substituted heterocyclic group, andR2 is hydrogen atom, halogen atom etc.;or its pharmaceutically acceptable salt and interferon inducers, antiviral agents, anticancer agents and therapeutic agents for immunologic diseases comprising the compound (I) or its pharmaceutically acceptable salt as active ingredients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Pharmaceutical formulation containing opioid agonist, opioid antagonist and irritant
ActiveUS20030068392A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid AgonistOpioid antagonist
Disclosed in certain embodiments is an oral dosage form comprising: a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and an irritant in an effective amount to impart an irritating sensation to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
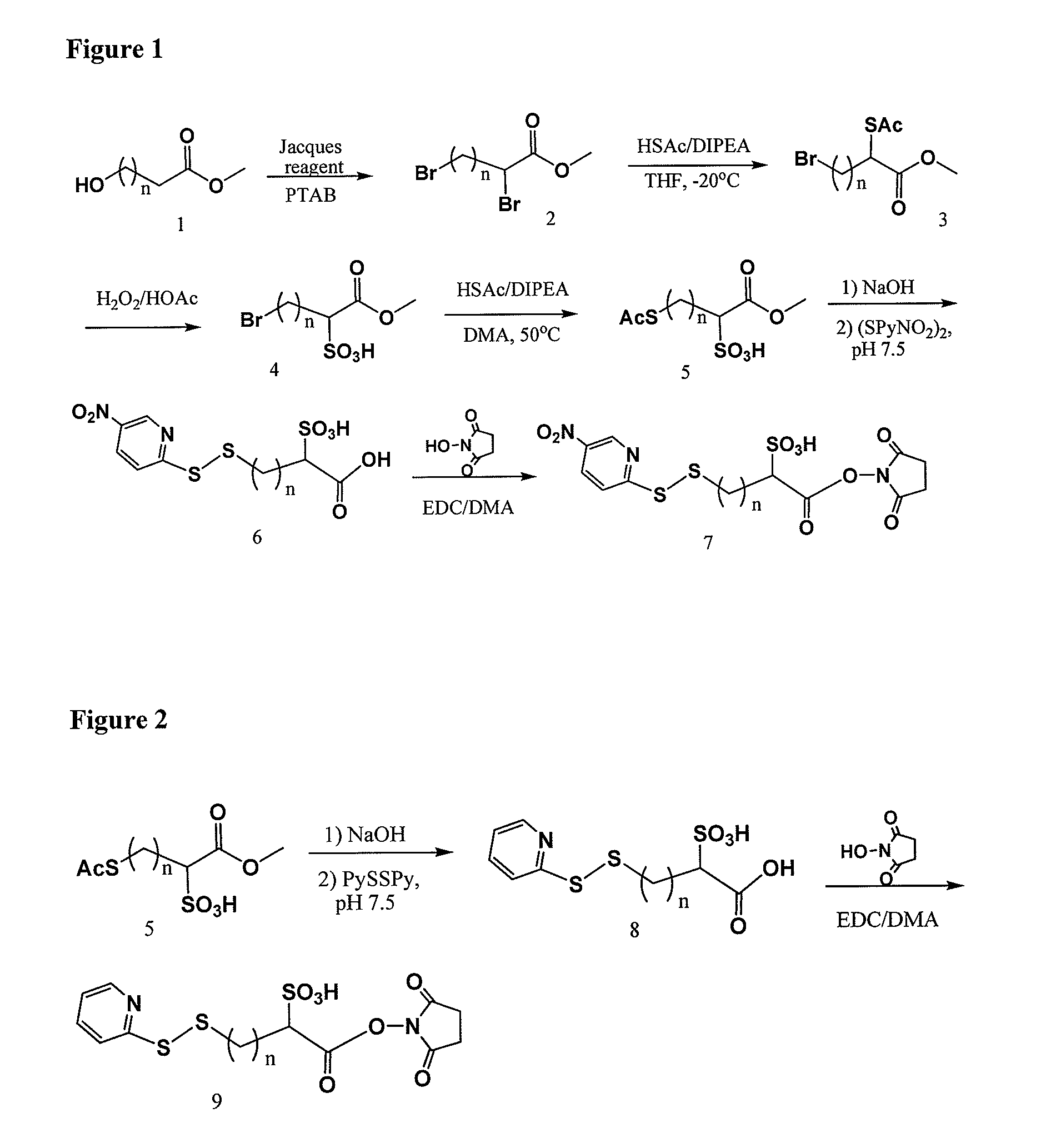
Cross-linkers and their uses
ActiveUS20090274713A1Good water solubilityHigh sensitivityOrganic active ingredientsTripeptide ingredientsCell bindingCross-link
Charged or pro-charged cross-linking moieties and conjugates of cell binding agents and drugs comprising the charged or pro-charged cross-linking moieties and method of making the same.
Owner:IMMUNOGEN INC
Pharmaceutical formulation containing opioid agonist, opioid antagonist and bittering agent
InactiveUS20030124185A1Analgesic and euphoric effect be reduce and eliminateCompromise integrityPowder deliveryPill deliveryOpioid antagonistDrug
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic; an opioid antagonist; and a bittering agent in an effective amount to impart a bitter taste to an abuser upon administration of the dosage form after tampering.
Owner:PURDUE PHARMA LP
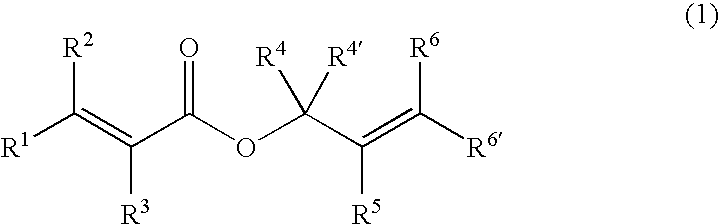
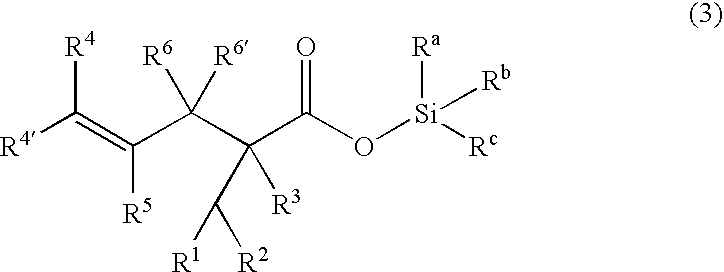
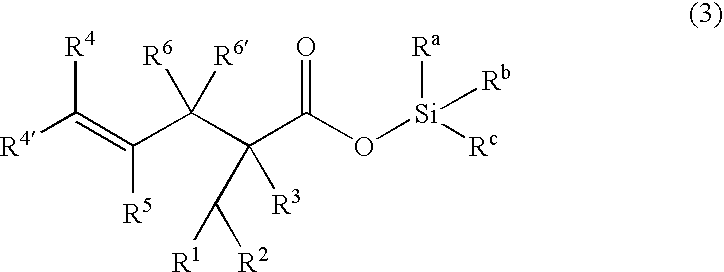
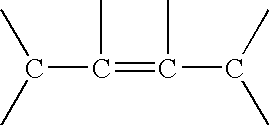
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS7307178B2Few stepsHigh yieldSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM CO LTD
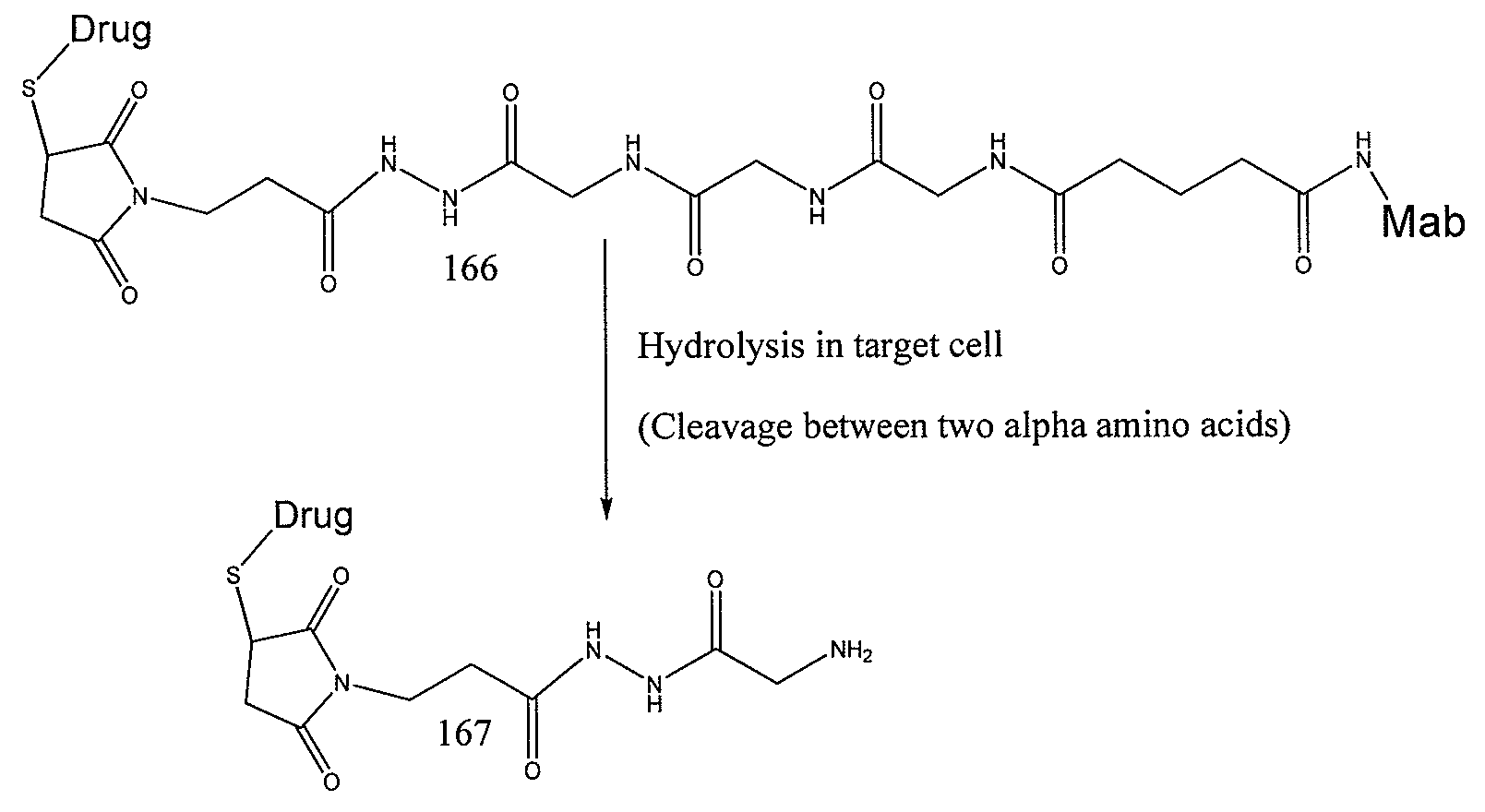
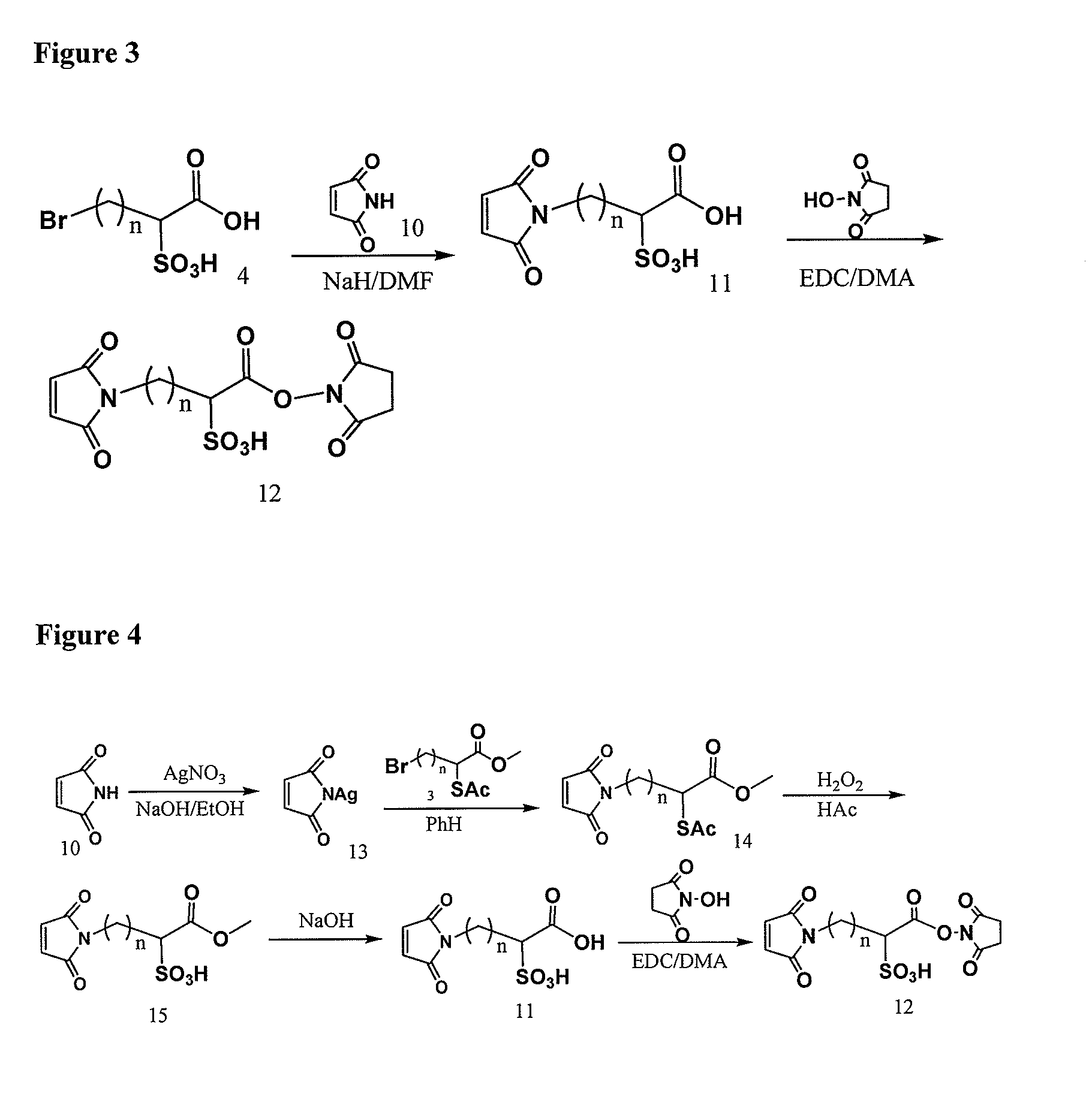
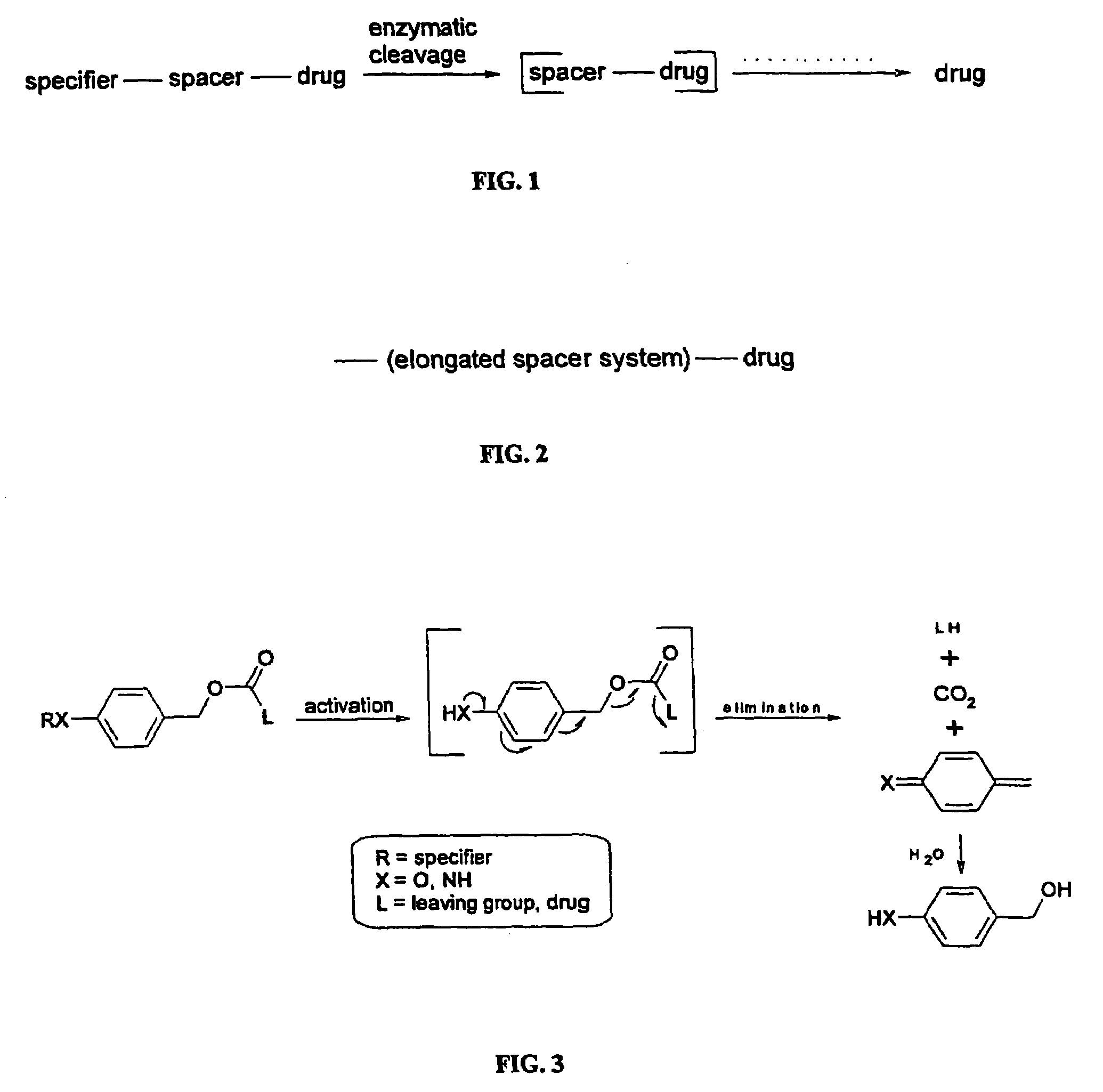
Elongated and multiple spacers in activatible prodrugs
InactiveUS7223837B2Improved kineticsFacilitate enzymatic cleavageAntibacterial agentsOrganic active ingredientsTumor cellsChemistry
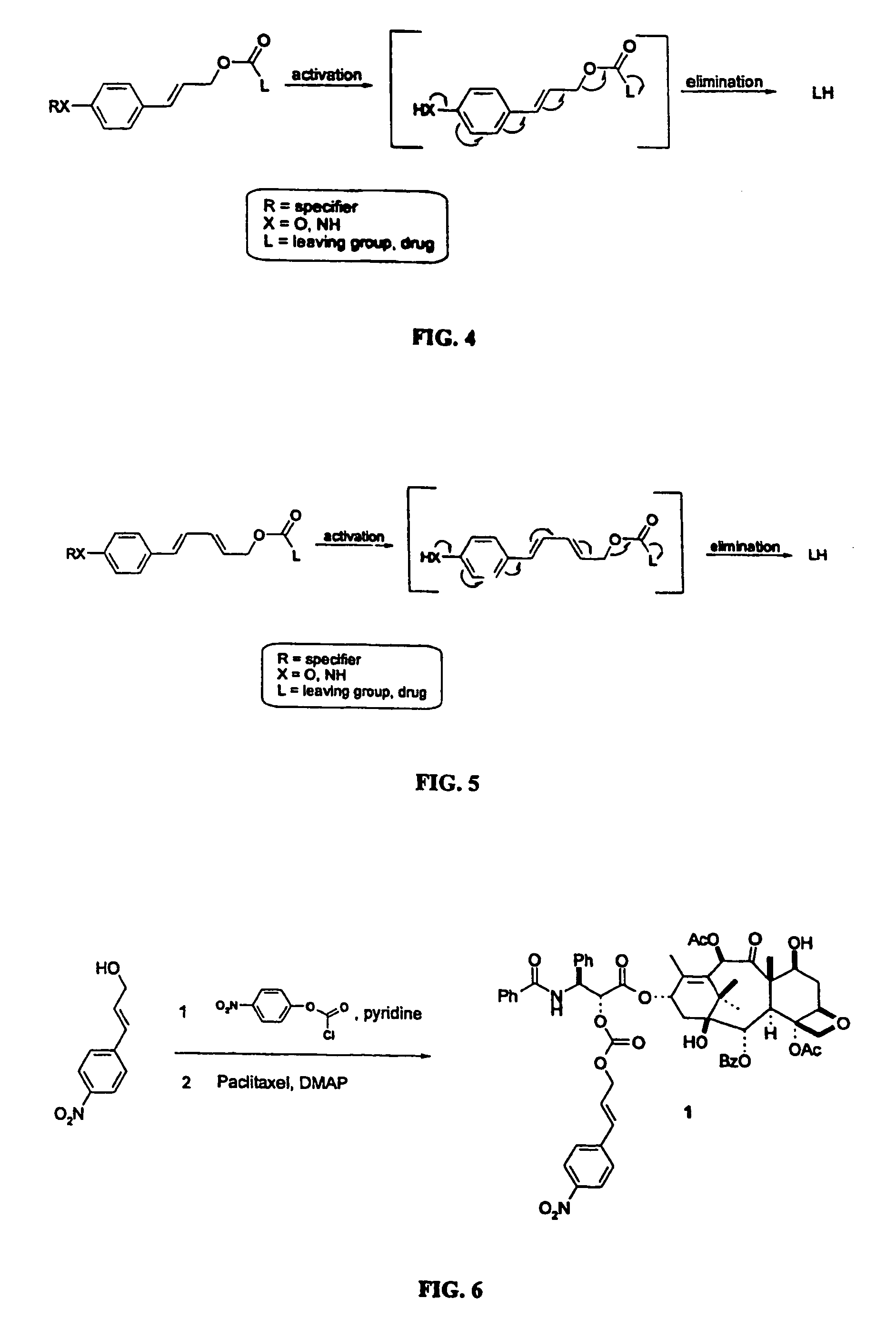
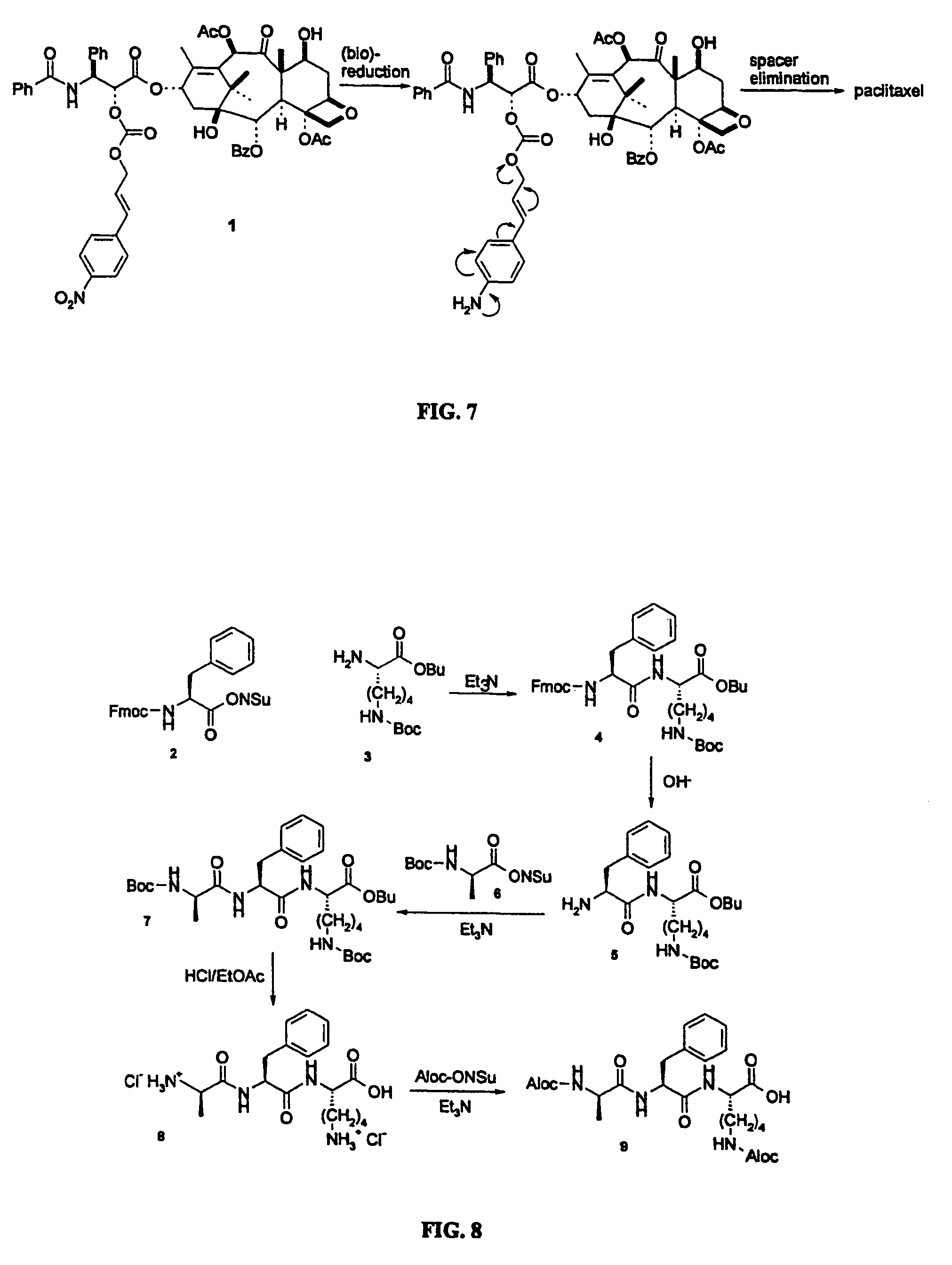
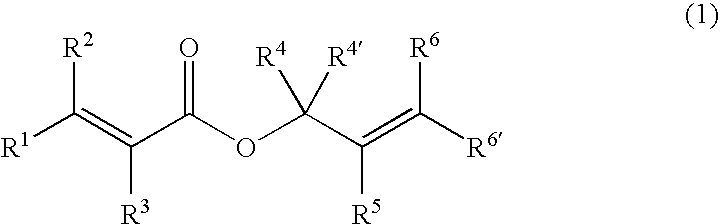
This invention is directed to prodrugs that can be activated at the preferred site of action in order to selectively deliver the corresponding therapeutic parent drugs to target cells or to the target site. This invention will therefore primarily but not exclusively relate to tumor cells as target cells. More specifically the prodrugs are compounds of the formula V—(W)k—(X)l—A—Z, wherein: V is a specifier; (W)k—(X)l—A is an elongated self-elimination spacer system; W and X are each a 1,(4+2n) electronic cascade spacer, being the same or different; A is either a spacer group of formula (Y)m wherein: Y is a 1,(4+2n) electronic cascade spacer, or a group of formula U being a cyclization elimination spacer; Z is a therapeutic drug; k, l and m are integers from 0 (included) to 5 (included); n is an integer of 0 (included) to 10 (included), with the provisos that: —when A is (Y)m: k+l+m≧1, and if k+l+m=1; —when A is U: k+l≧1.
Owner:BYONDIS BV
Processes of making gamma,delta-unsaturated carboxylic acid and silyl ester thereof, carboxyl group-containing organosilicon compound and process of making
ActiveUS20050070729A1High yieldFew stepsSilicon organic compoundsPreparation from carboxylic acid esters/lactonesCarboxyl radicalPerylene derivatives
A γ,δ-unsaturated carboxylic acid silyl ester is prepared by reacting an α,β-unsaturated carboxylic acid ester with a hydrosilane or hydrosiloxane in the presence of tris(pentafluorophenyl)borane. γ,δ-Unsaturated carboxylic acid derivatives are readily prepared through fewer steps and in high yields.
Owner:SHIN ETSU CHEM IND CO LTD
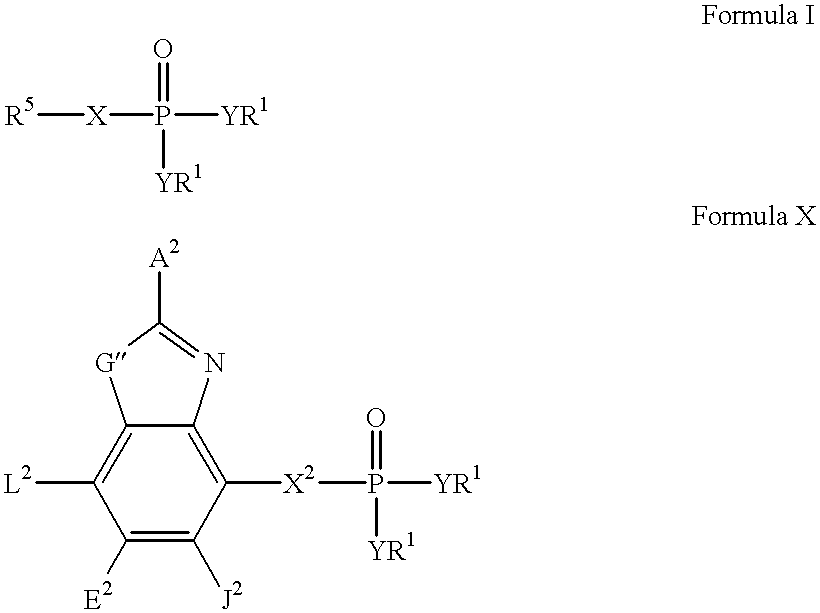
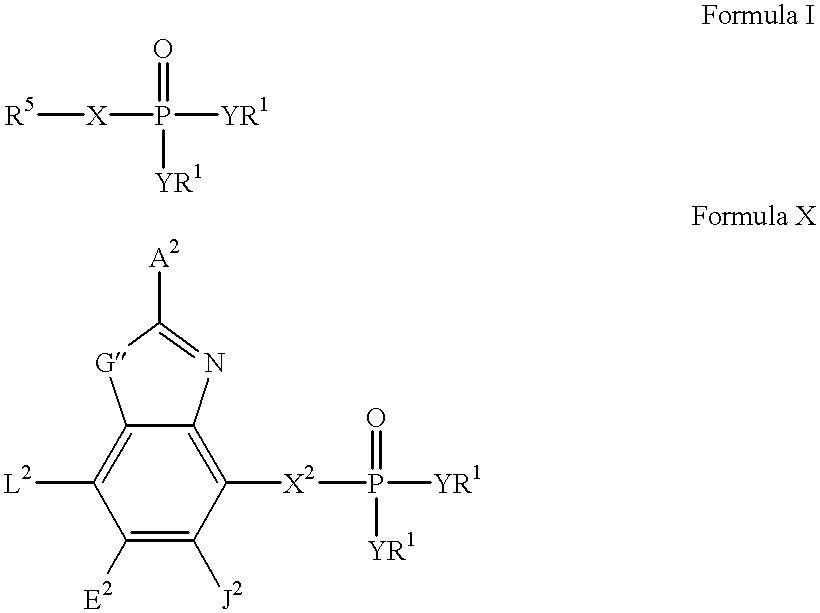
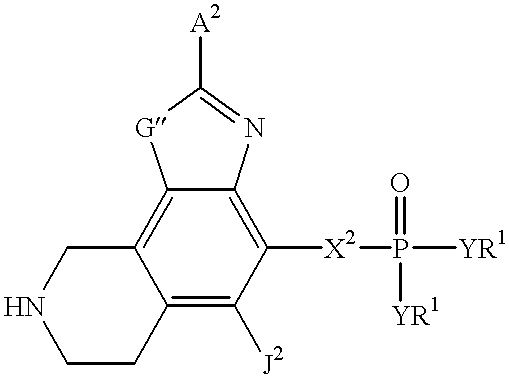
Heteroaromatic compounds containing a phosphonate group that are inhibitors of fructose-1,6-bisphosphatase
FBPase inhibitors of the formula I and Xare useful in the treatment of diabetes and other conditions associated with elevated blood glucose or excess glycogen storage.
Owner:METABASIS THERAPEUTICS INC
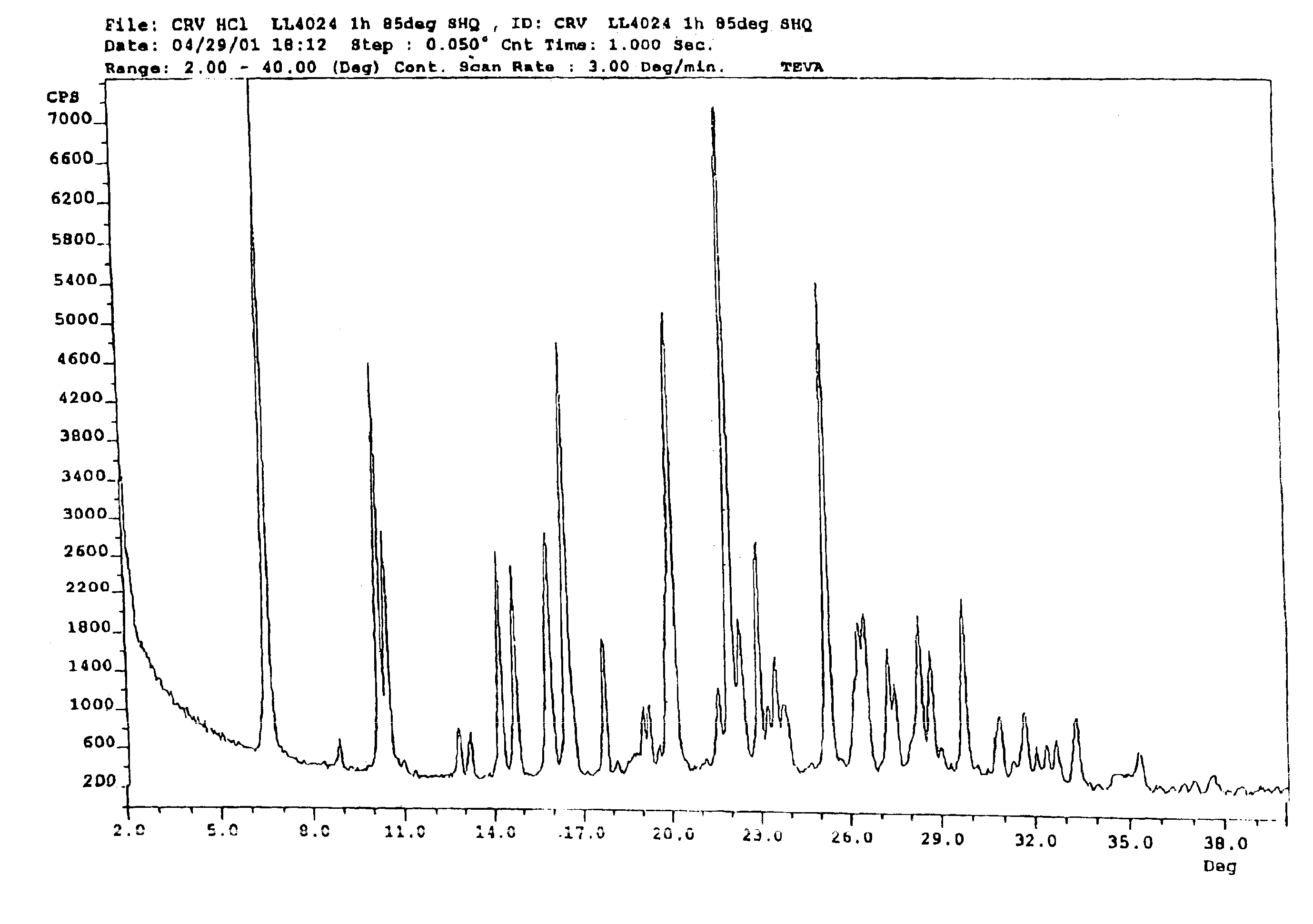
Carvedilol
InactiveUS7056942B2Lower the temperature of the solutionBiocideOrganic chemistryCarvedilolMedicinal chemistry
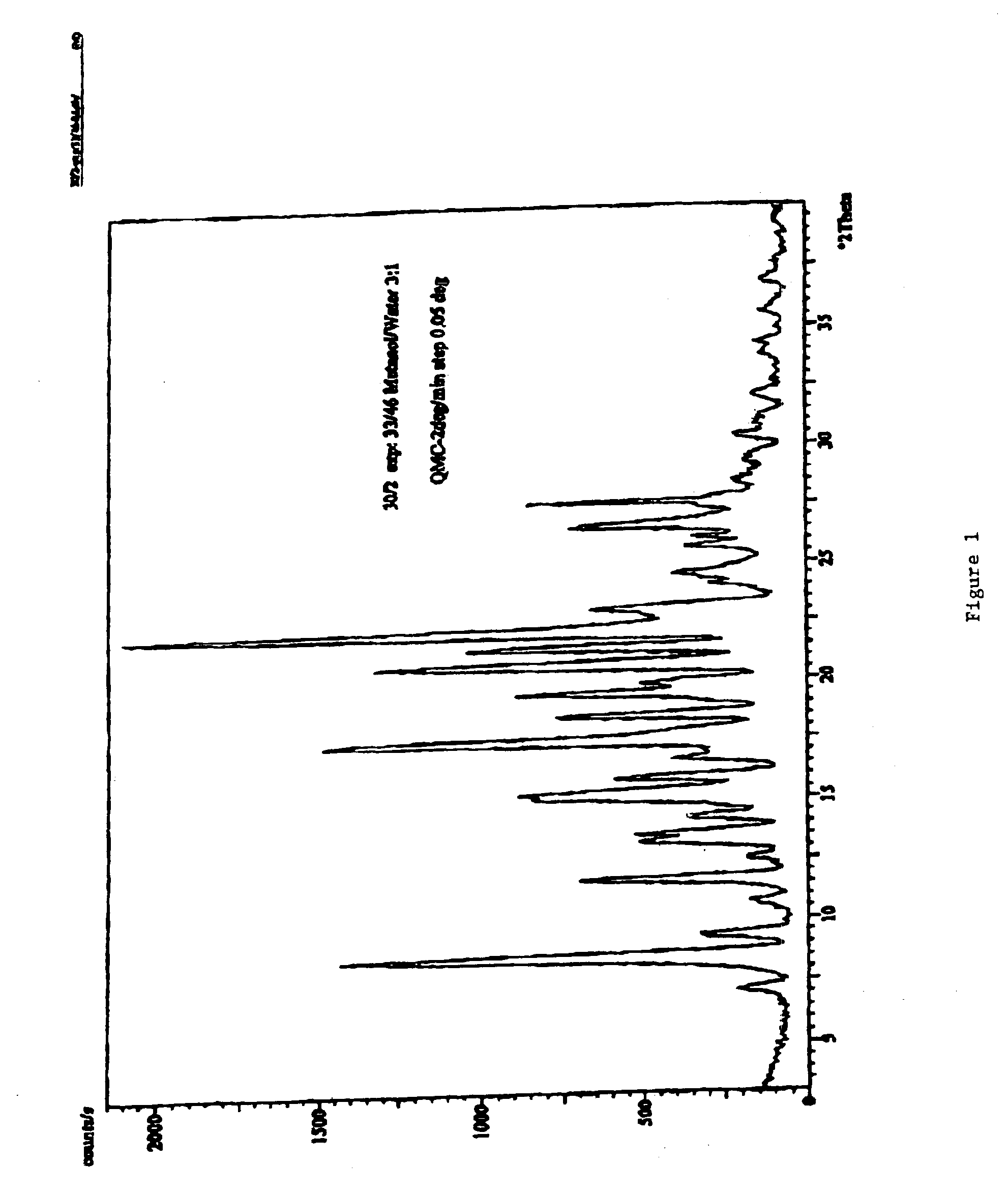
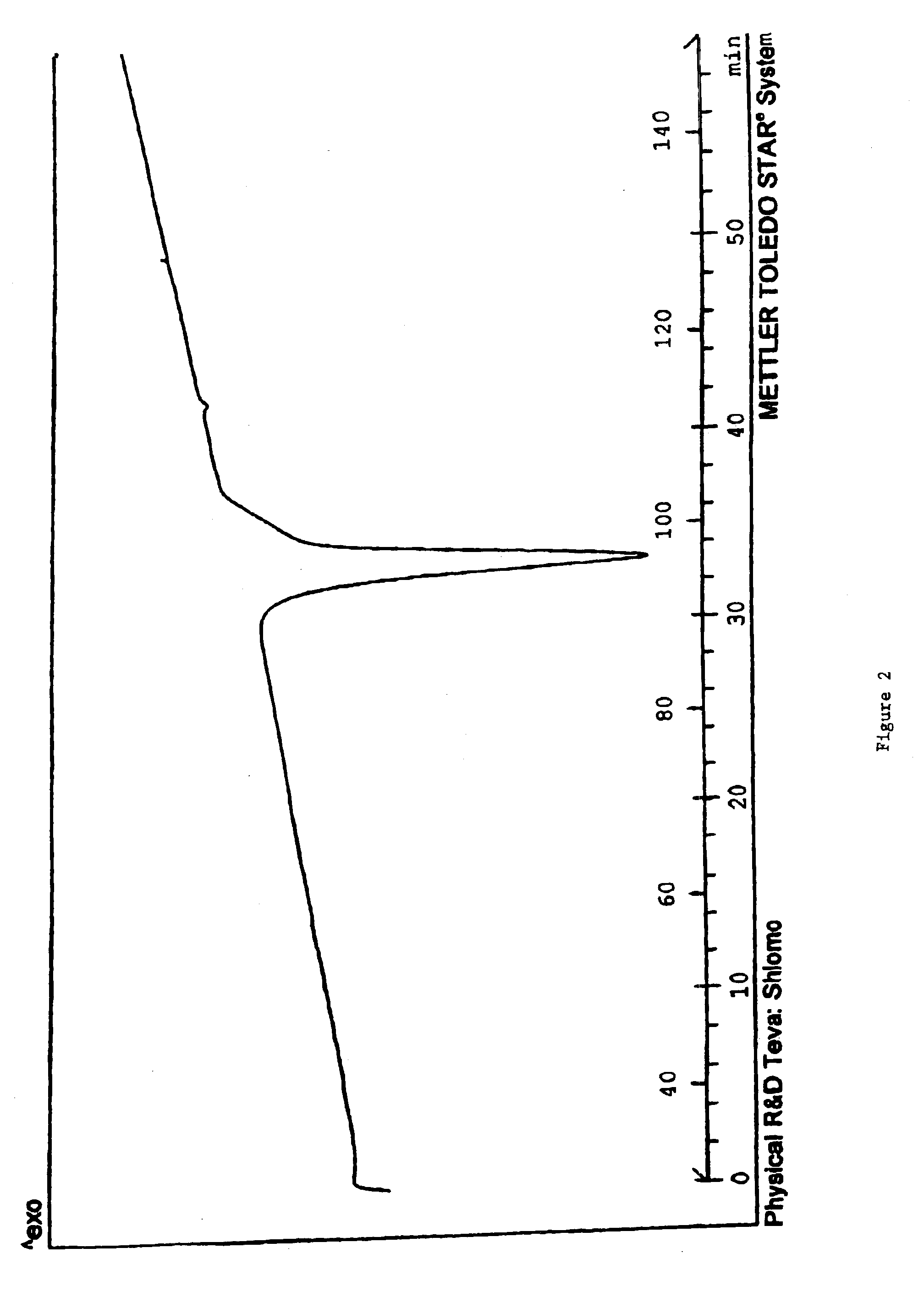
This invention relates to an improved process of preparing carvedilol, as well as a new crystalline hydrate and solvate and forms of carvedilol, processes for the manufacture thereof, and pharmaceutical compositions thereof.
Owner:TEVA PHARMA IND LTD
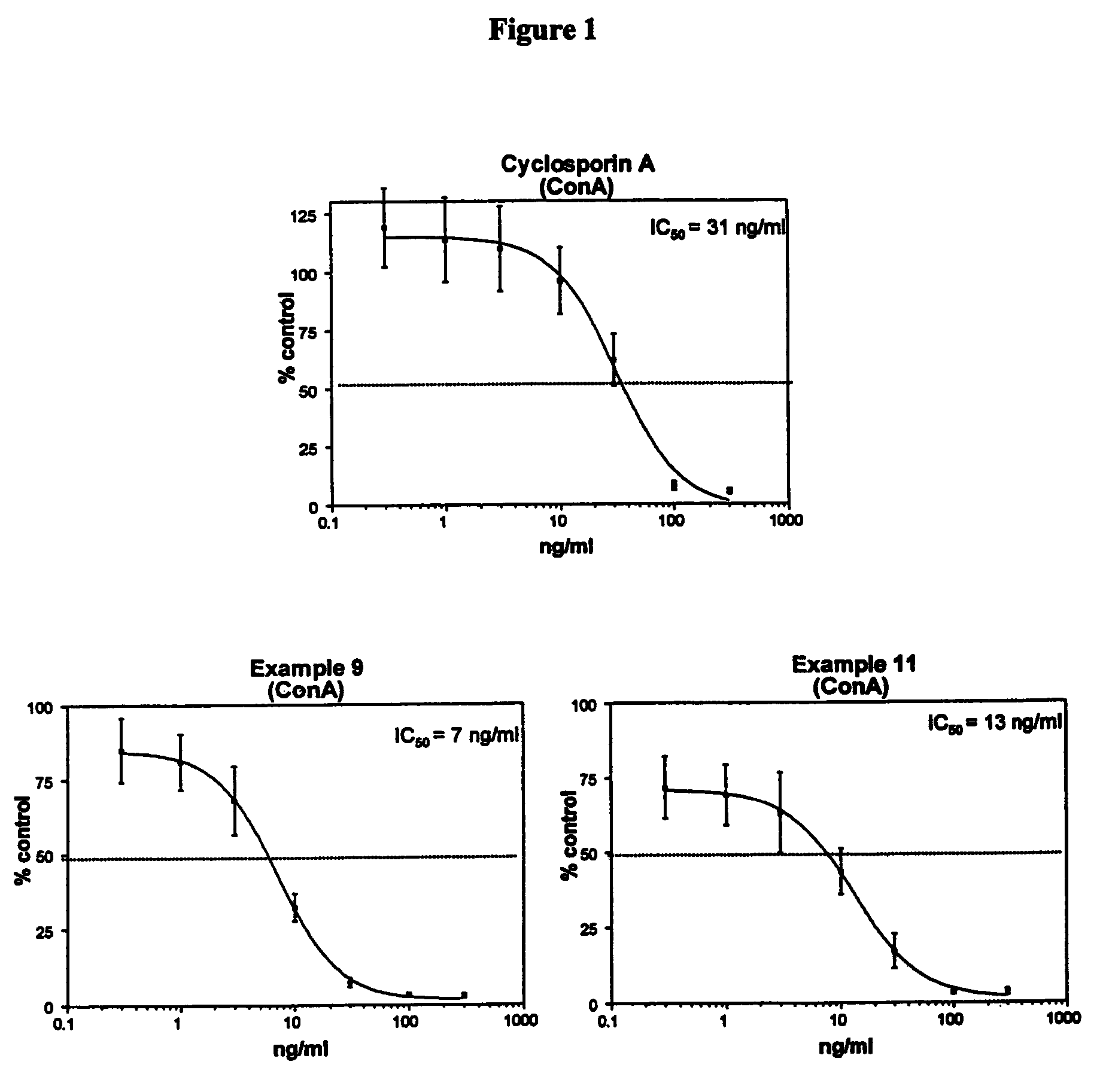
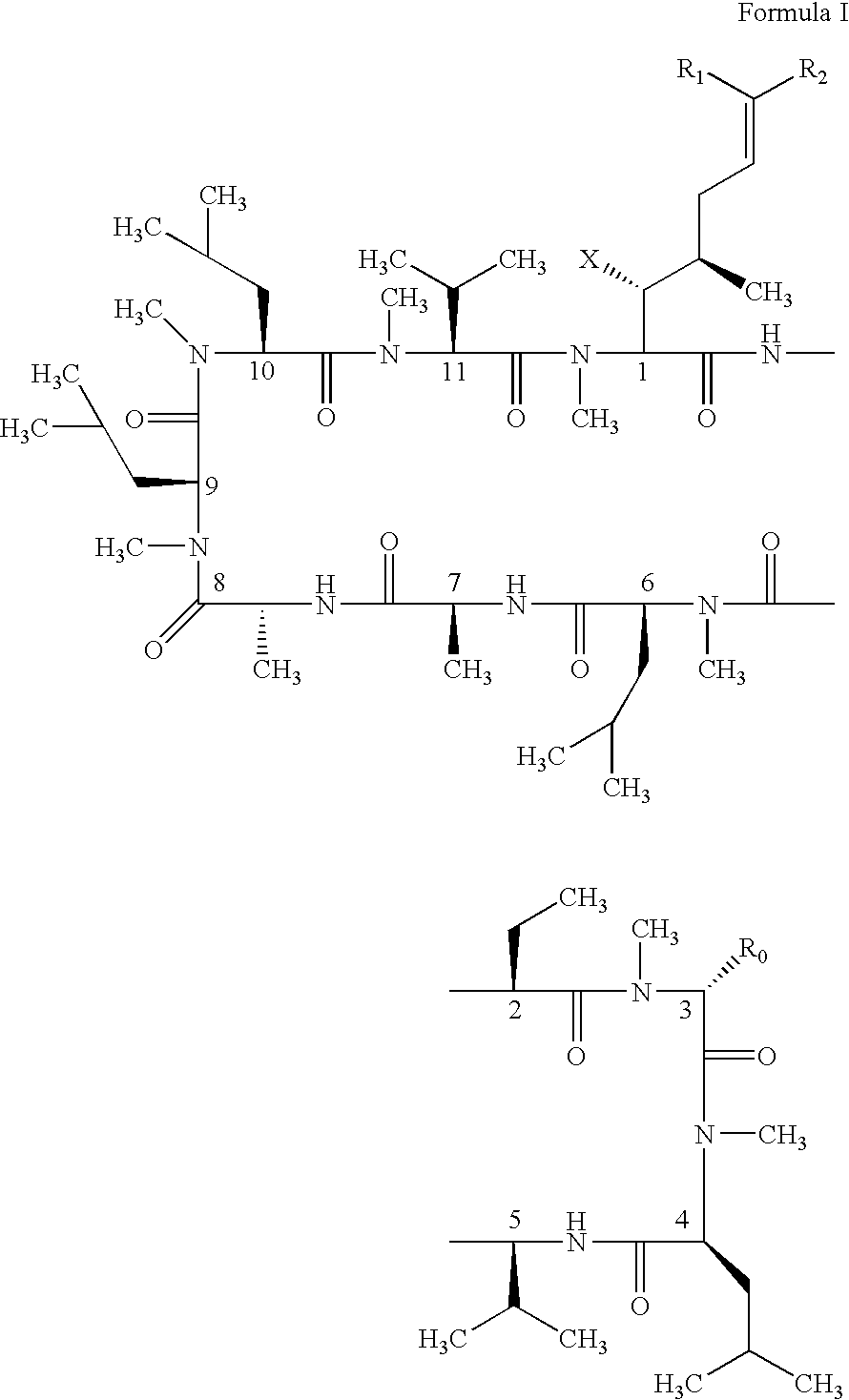
Cyclosporin analogues and their pharmaceutical uses
Owner:ALBANY MOLECULAR RESEARCH INC
Pyrrolobenzodiazepines as key intermediates in the synthesis of dimeric cytotoxic pyrrolobenzodiazepines
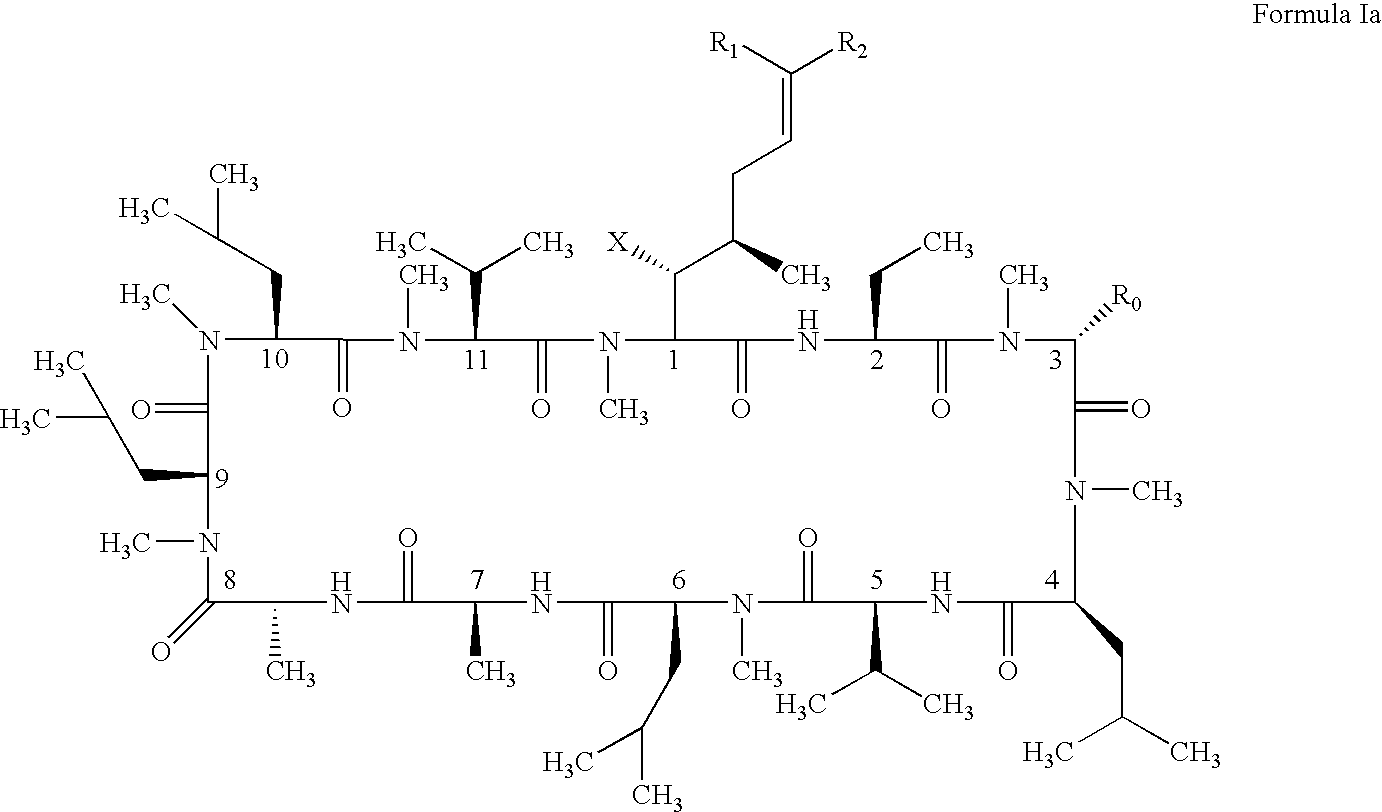
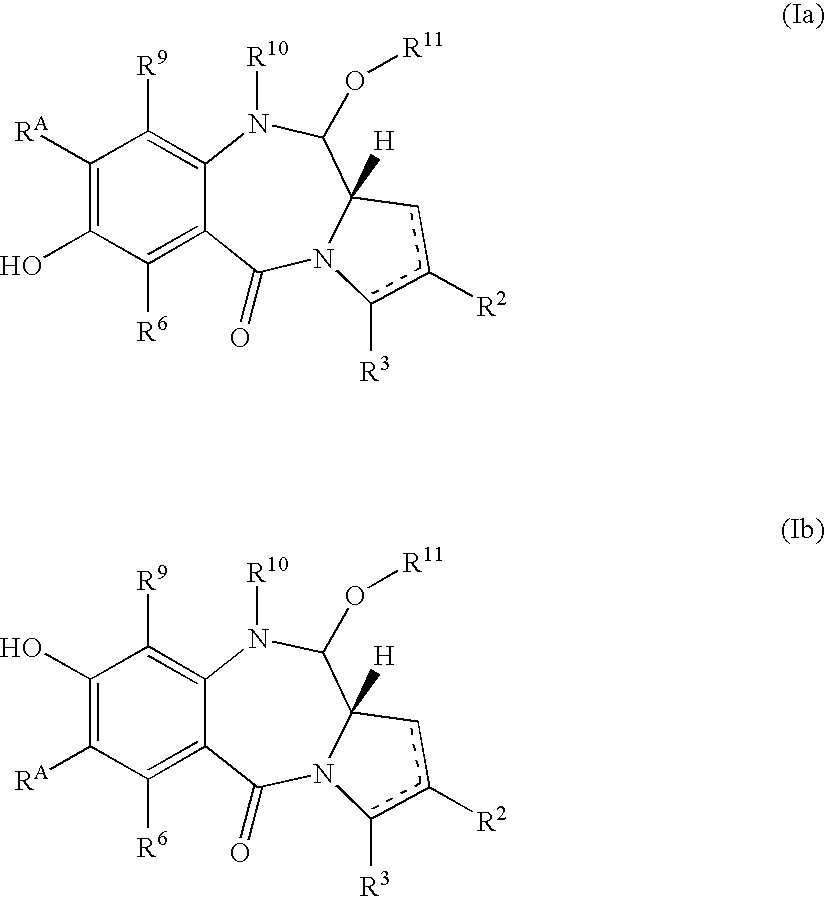
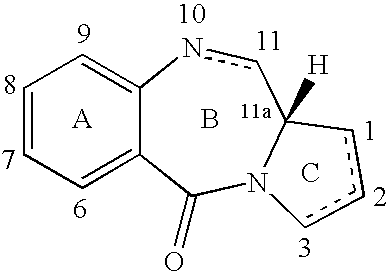
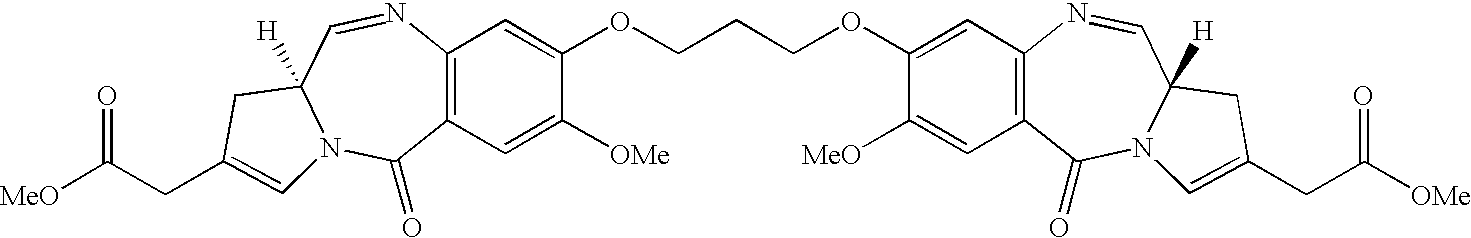
Compounds and a method of synthesis of compounds of formula (Ia) or (Ib): and salts, solvates, and chemically protected forms thereof, wherein the dotted lines indicate the optional presence of a double bond between C1 and C2 or C2 and C3; R2 and R3 are independently selected from —H, ═O, ═CH2, —CN, —R, OR, halo, ═CH—R, O—SO2—R, CO2R and COR; R10 is a carbamate-based nitrogen protecting group; and R11 is an oxygen protecting group.
Owner:MEDIMMUNE LTD
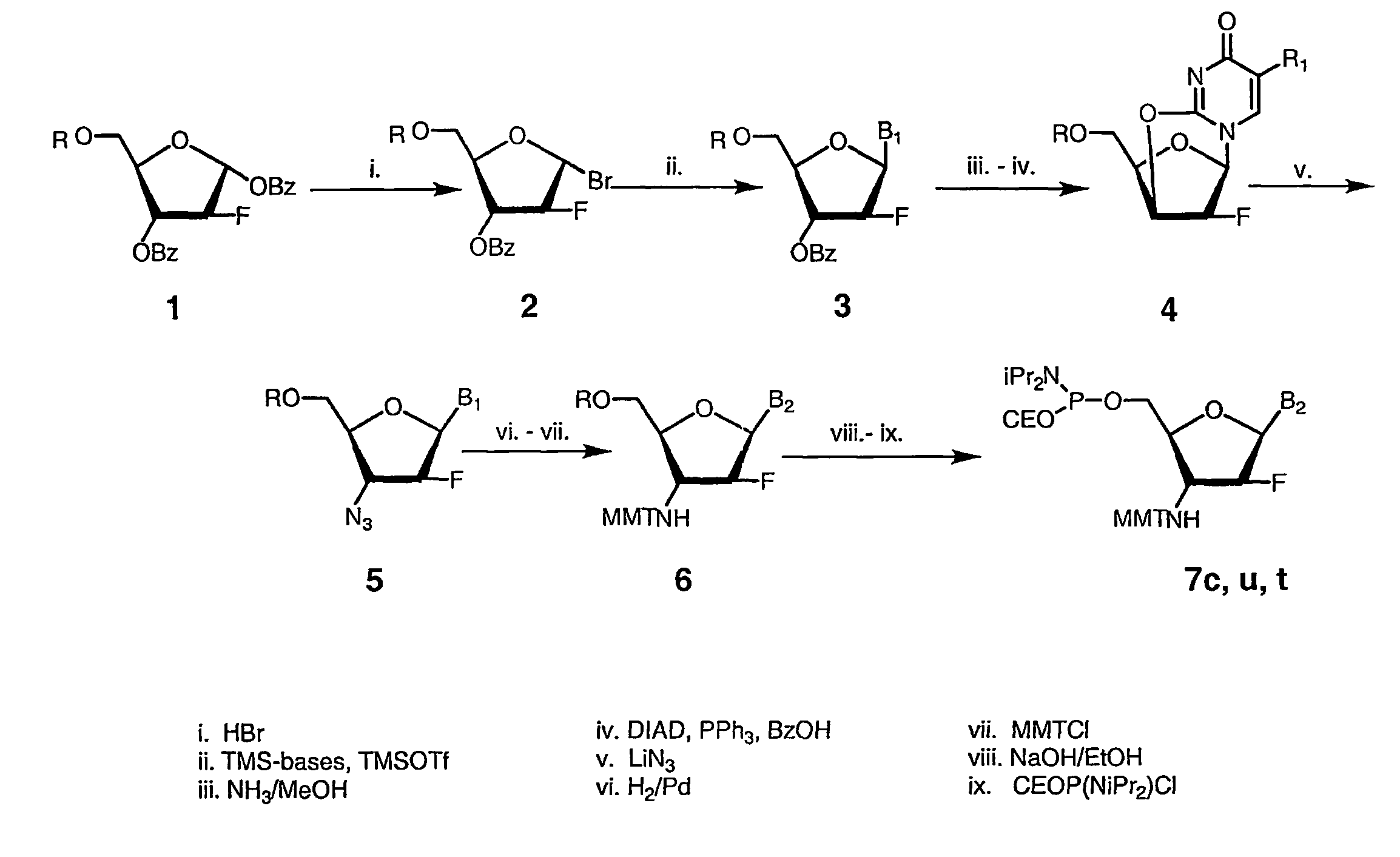
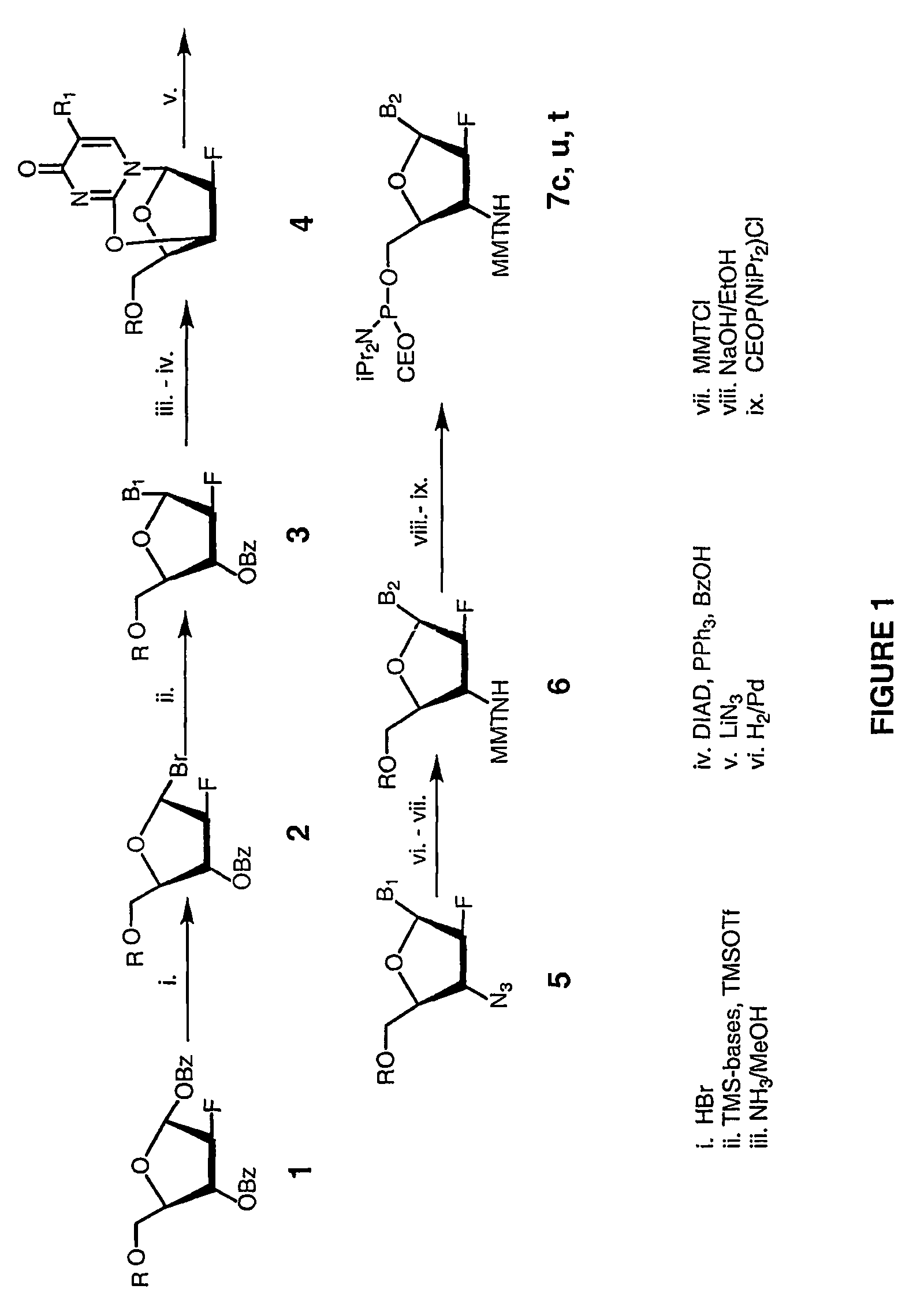
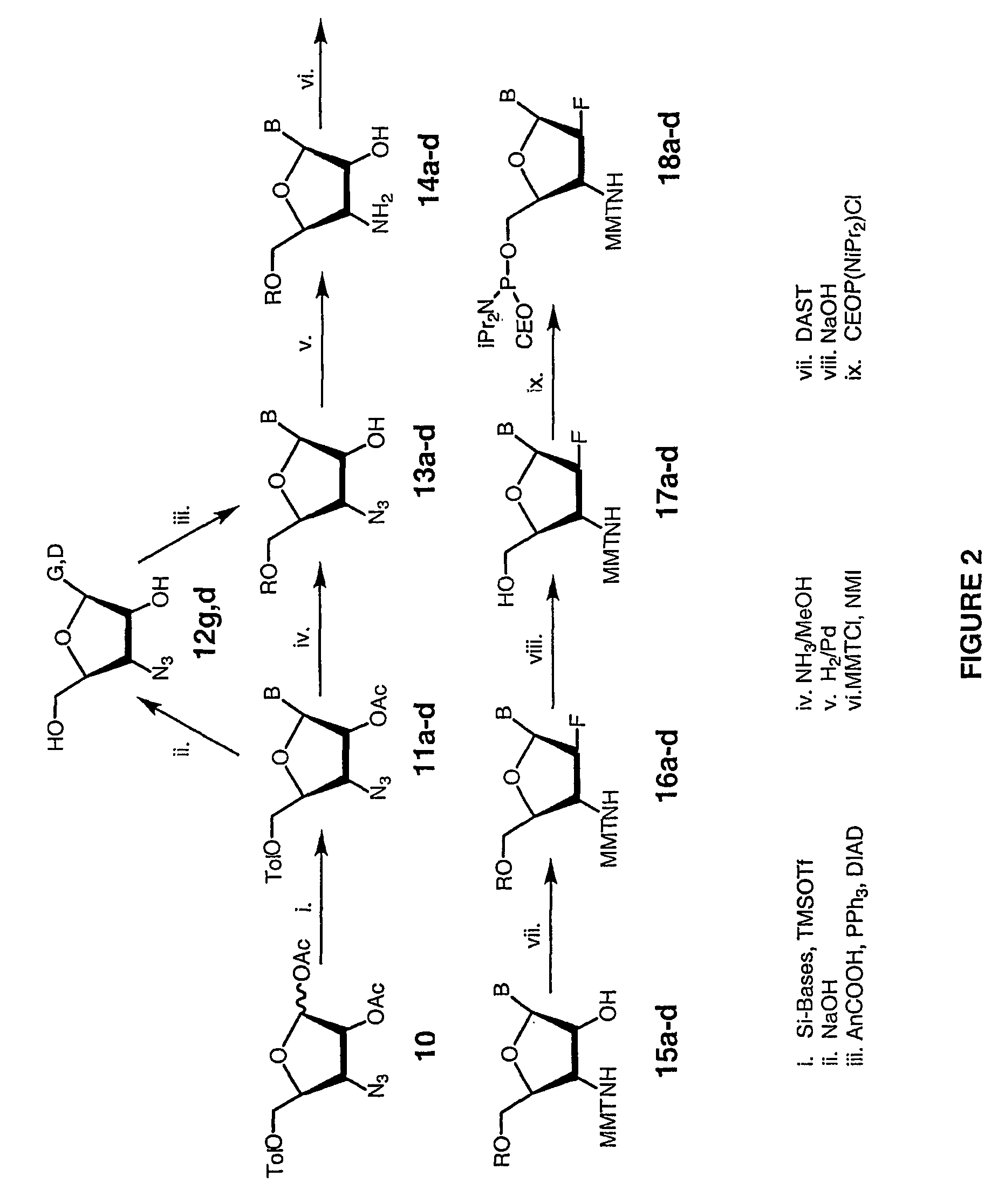
2'-arabino-fluorooligonucleotide N3'->P5' phosphoramidates: their synthesis and use
Oligonucleotides with a novel sugar-phosphate backbone containing at least one 2′-arabino-fluoronucleoside and an internucleoside 3′-NH—P(—O)(OR)—O-5′ linkage, where R is a positively charged counter ion or hydrogen, and methods of synthesizing and using the inventive oligonucleotides are provided. The inventive phosphoramidate 2′-arabino-fluorooligonucleotides have a high RNA binding affinity to complementary nucleic acids and are base and acid stable.
Owner:GERON CORPORATION
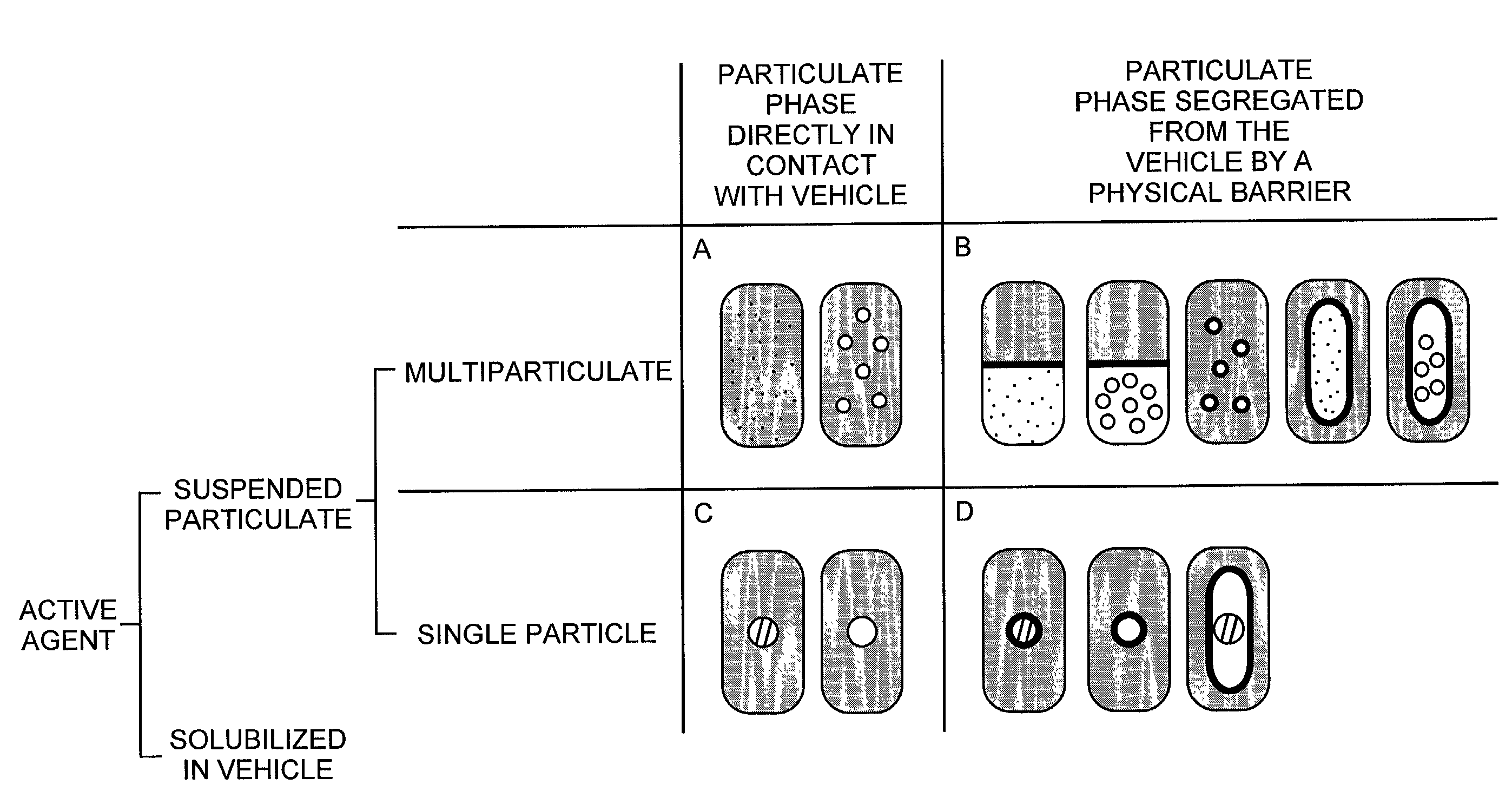
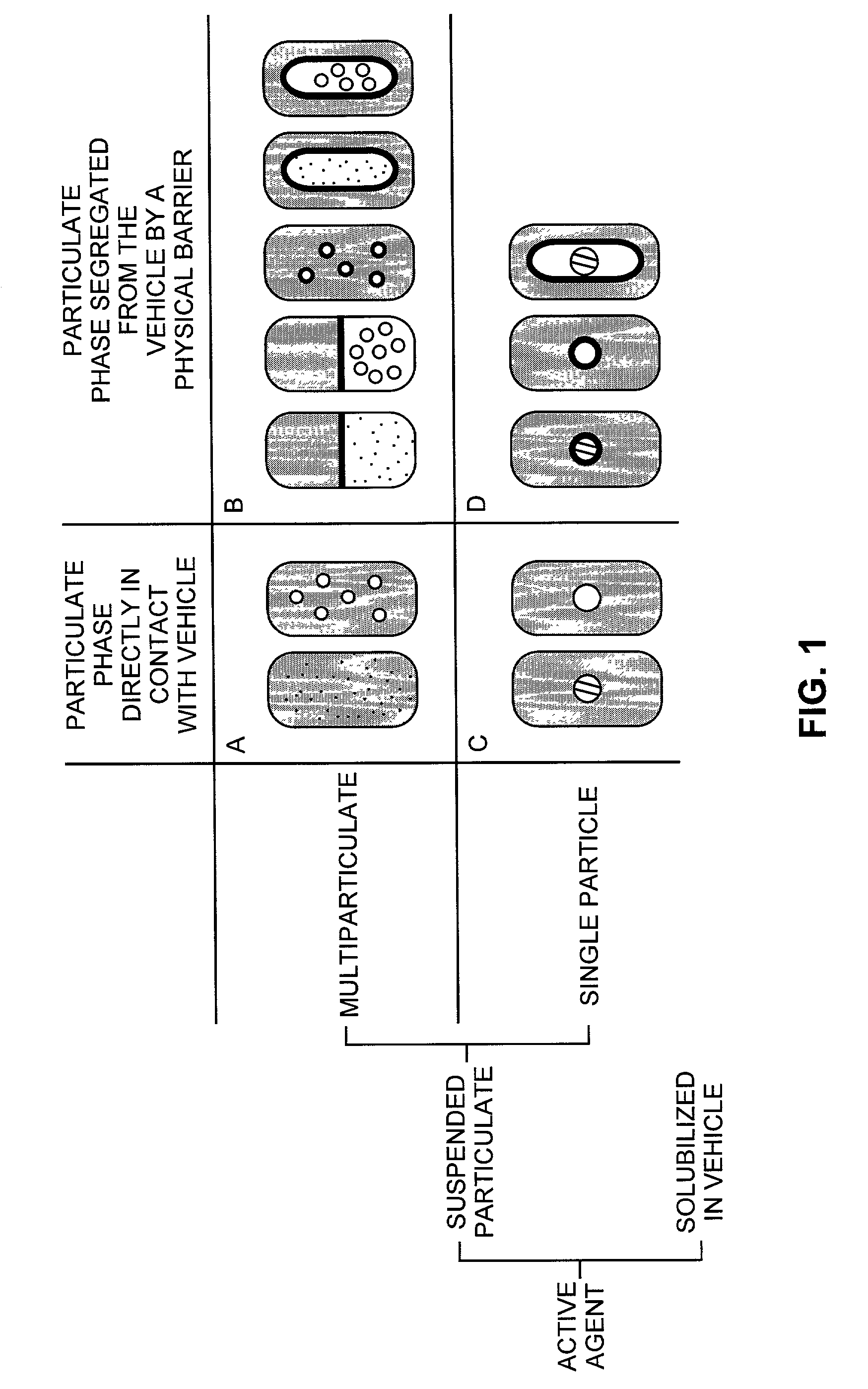
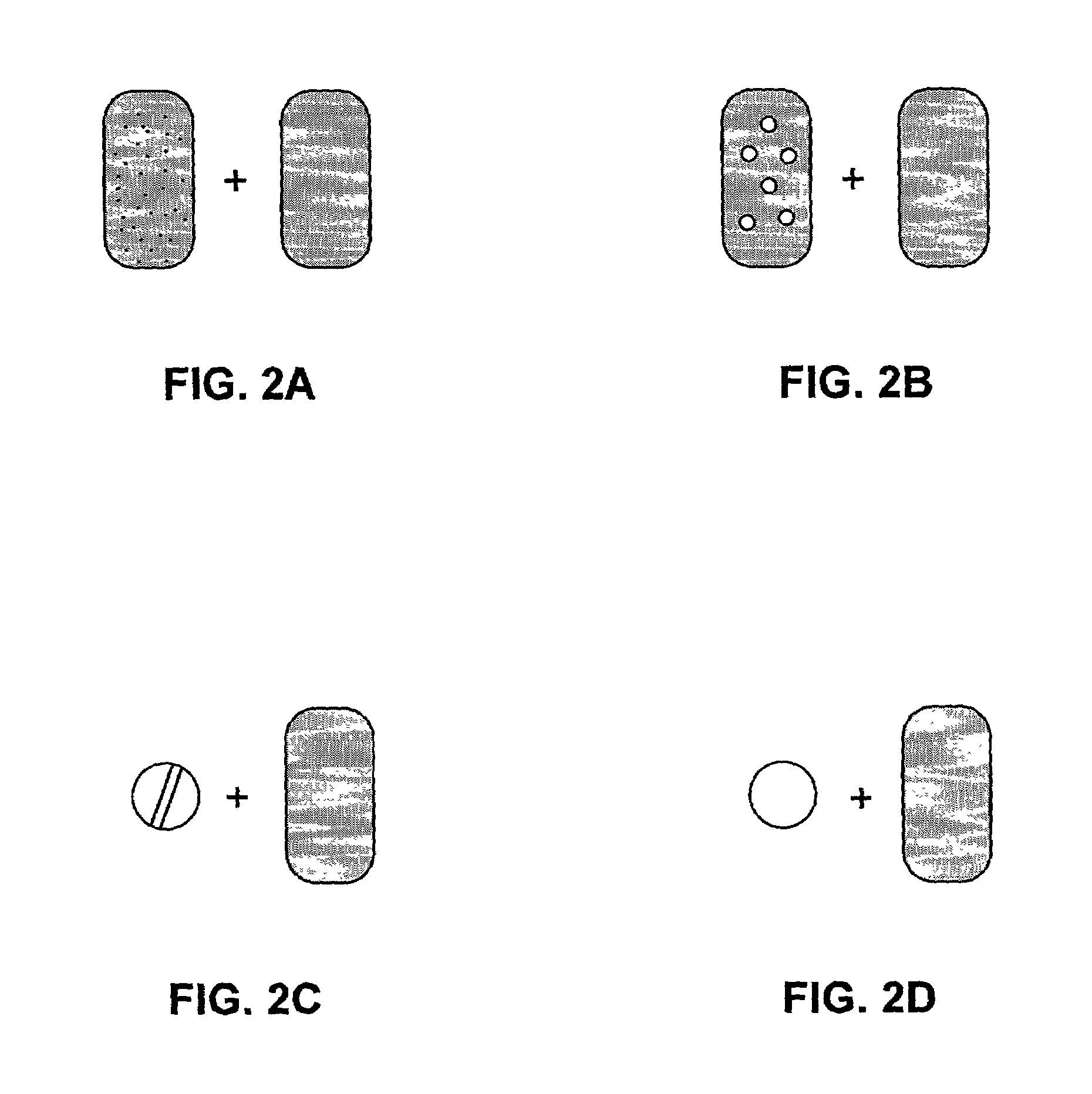
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
Anti-infection compound preparation and its preparation method
InactiveCN1380098AImprove immunityEffective excretionAntibody ingredientsUnknown materialsSide effectSuppository
The anti-infective medicine, including powder, mixture, aerosol, capsule, injection, suppository, ointment and microcapsule, is characterized by that on the theroretical basis of combining traditional Chinese medicine and modern immunology the immunoglobulin, effective components of Chinese medicinal materials which are extracted according to the compound prescription and auxiliary preparation are combined together organically, and undergone the fine preparation process to obtain a high-efficiency, safe, stable, environment-protecting type anti-infection medicine having no toxic side effect and having no drug resistance.
Owner:张勇飞 +2
Film forming foamable composition
InactiveUS20060193789A1Improve solubilityReduce deliveryCosmetic preparationsBiocideAlcohol freeFilm-forming agent
A foamable composition, includes (1) about 6% to about 70% by weight of at least one organic carrier; (2) about 0.1% to about 5% by weight of at least one surface-active agent; (3) about 0.01% to about 5% by weight of at least one film forming agent; (4) water; and (5) about 3% to about 25% by weight of the total composition of at least one liquefied or compressed gas propellant. The composition is substantially alcohol free and is used in treating, alleviating or preventing a disorder.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Novel dosage form
ActiveUS20060024365A1Effectively control release rateReduce sizePill deliveryEster active ingredientsHigh dosesSolubility
A dosage form comprising of a high dose, high solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of modified release active ingredient per unit is from 500 mg to 1500 mg; a process for preparing the dosage form.
Owner:TORRENT PHARMA LTD
Quaternary Ammonium Salt of a Polyalkene-Substituted Amine Compound
InactiveUS20080113890A1Organic compound preparationTransportation and packagingCompound aQuaternary ammonium cation
A quaternary ammonium salt detergent made from the reaction product of the reaction of: (a) polyalkene-substituted amine having at least one tertiary amino group; and (b) a quaternizing agent suitable for converting the tertiary amino group to a quaternary nitrogen and the use of such quaternary ammonium salt detergents in a fuel composition to reduce intake valve deposits.
Owner:THE LUBRIZOL CORP
Chemical linkers and conjugates thereof
The present disclosure provides drug-ligand conjugates that are potent cytotoxins, wherein the drug is linked to the ligand through either a peptidyl, hydrazine, or disulfide linker. The disclosure is also directed to compositions containing the drug-ligand conjugates, and to methods of treatment using them.
Owner:ER SQUIBB & SONS INC
Microbicidal composition
Owner:DDP SPECIALTY ELECTRONICS MATERIALS US 8 LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com