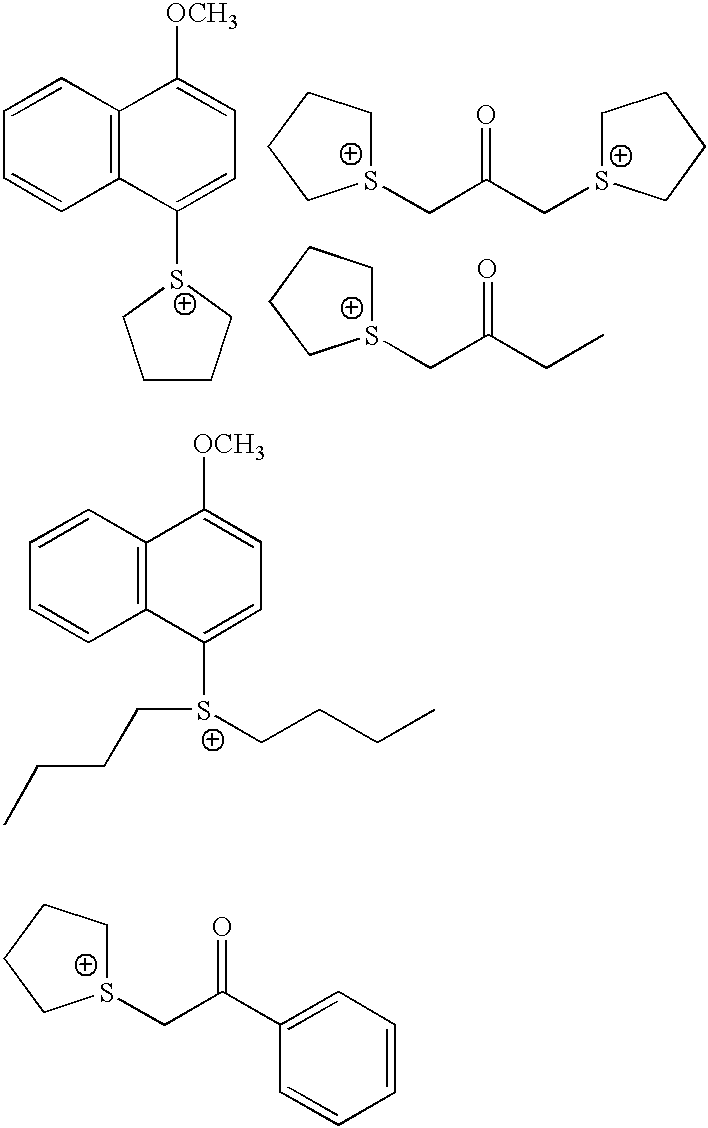
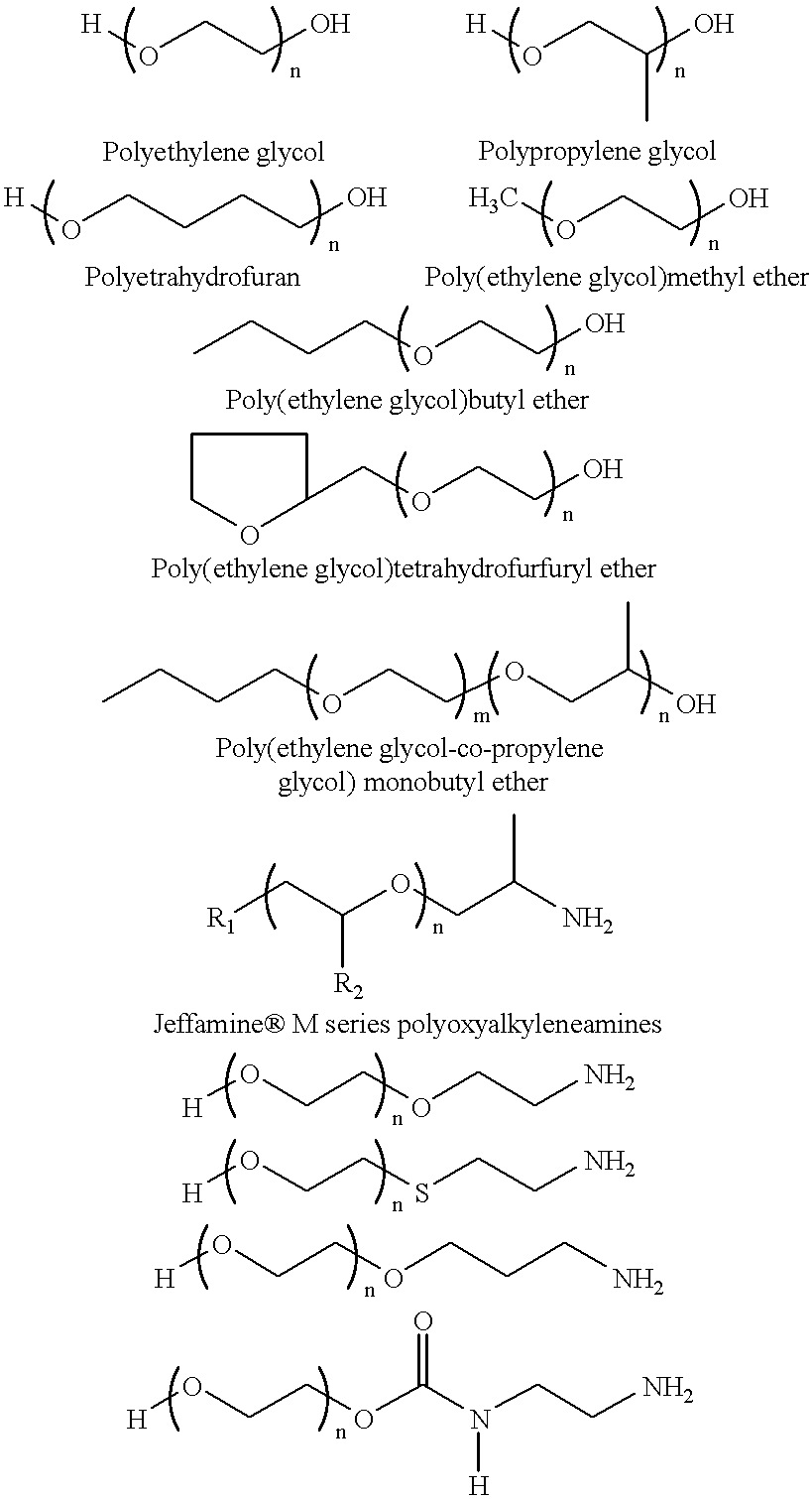
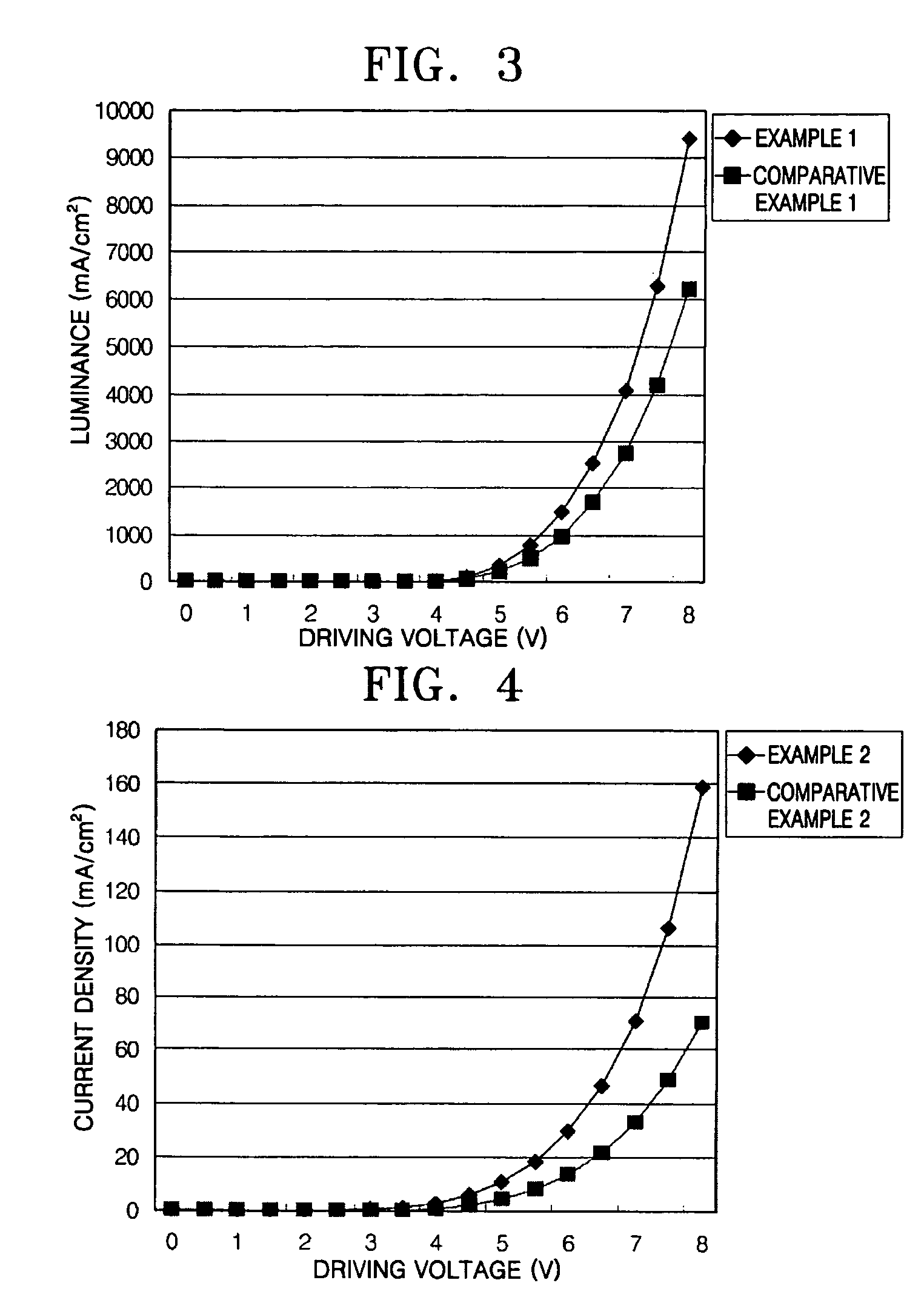
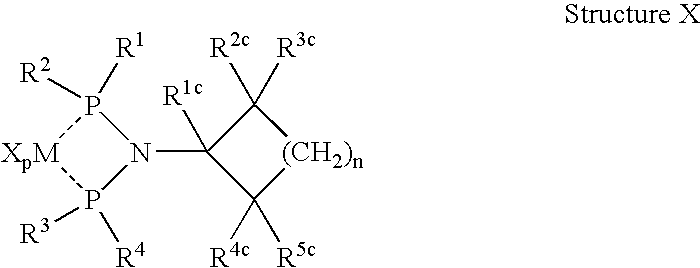
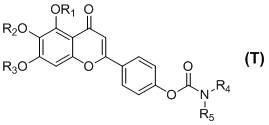
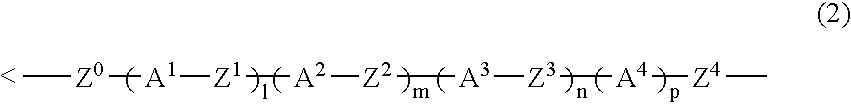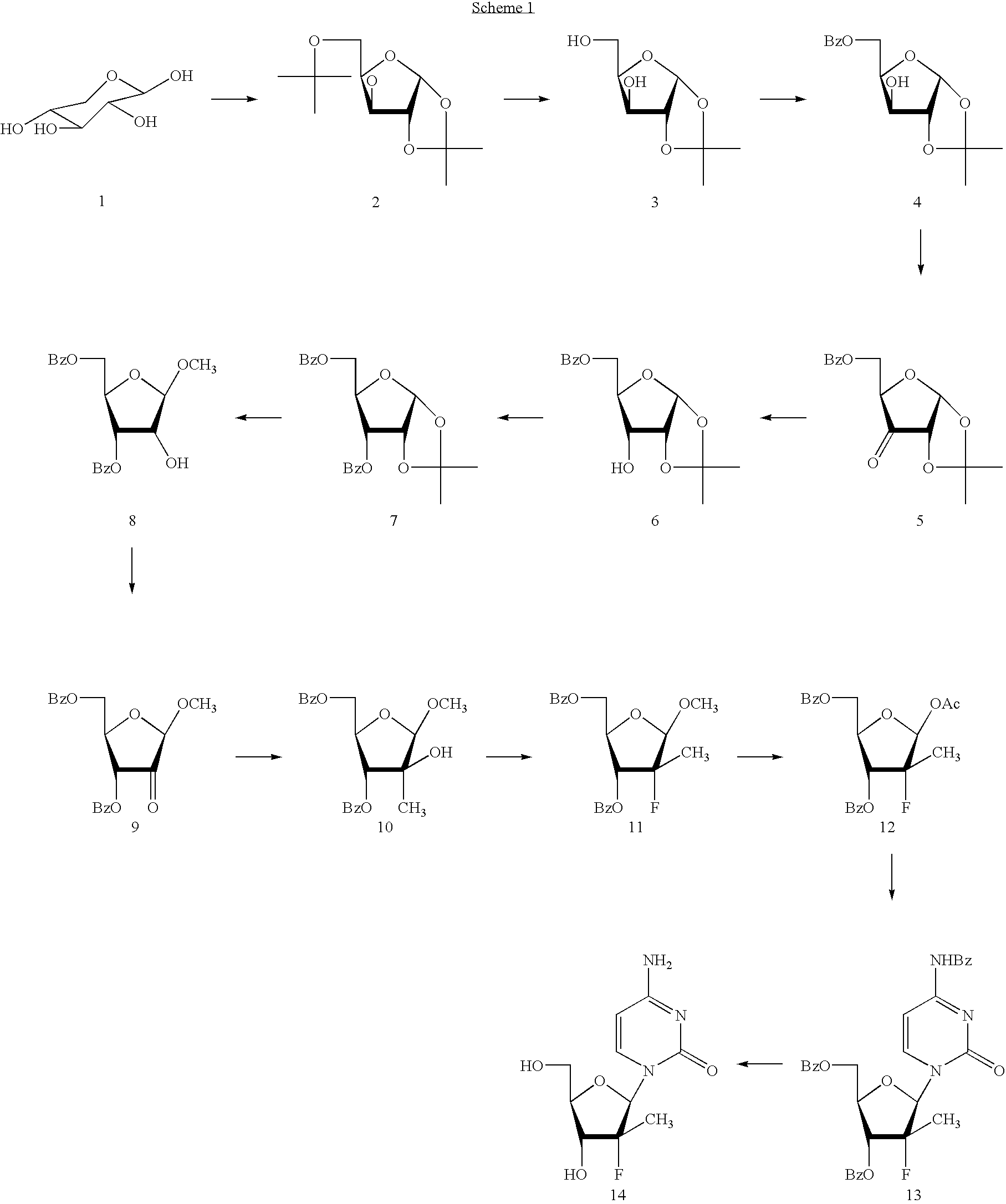Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2431 results about "Alkyl substitution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br, under basic conditions, where the attacking nucleophile is the OH− and the leaving group is Br−.
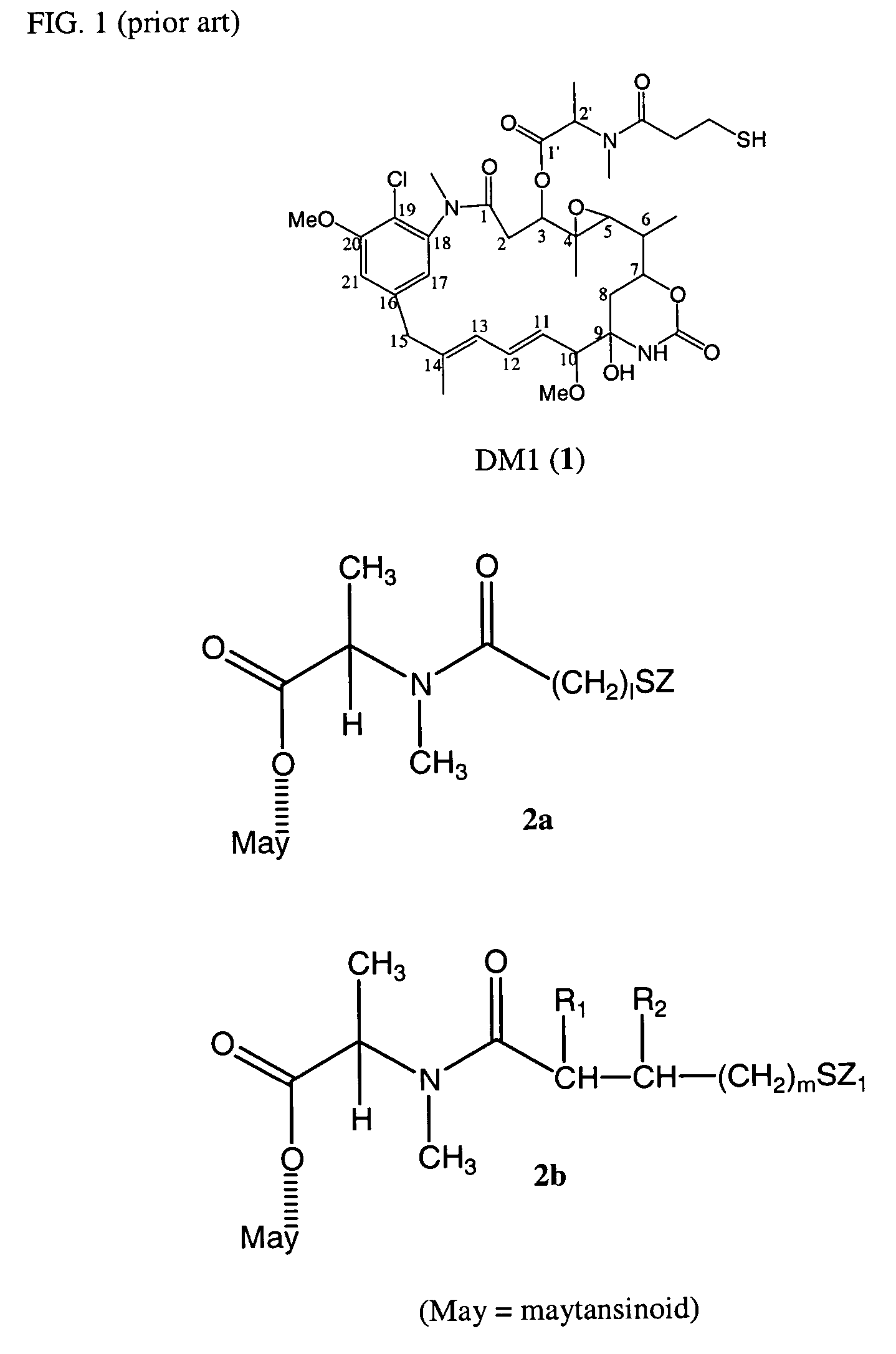
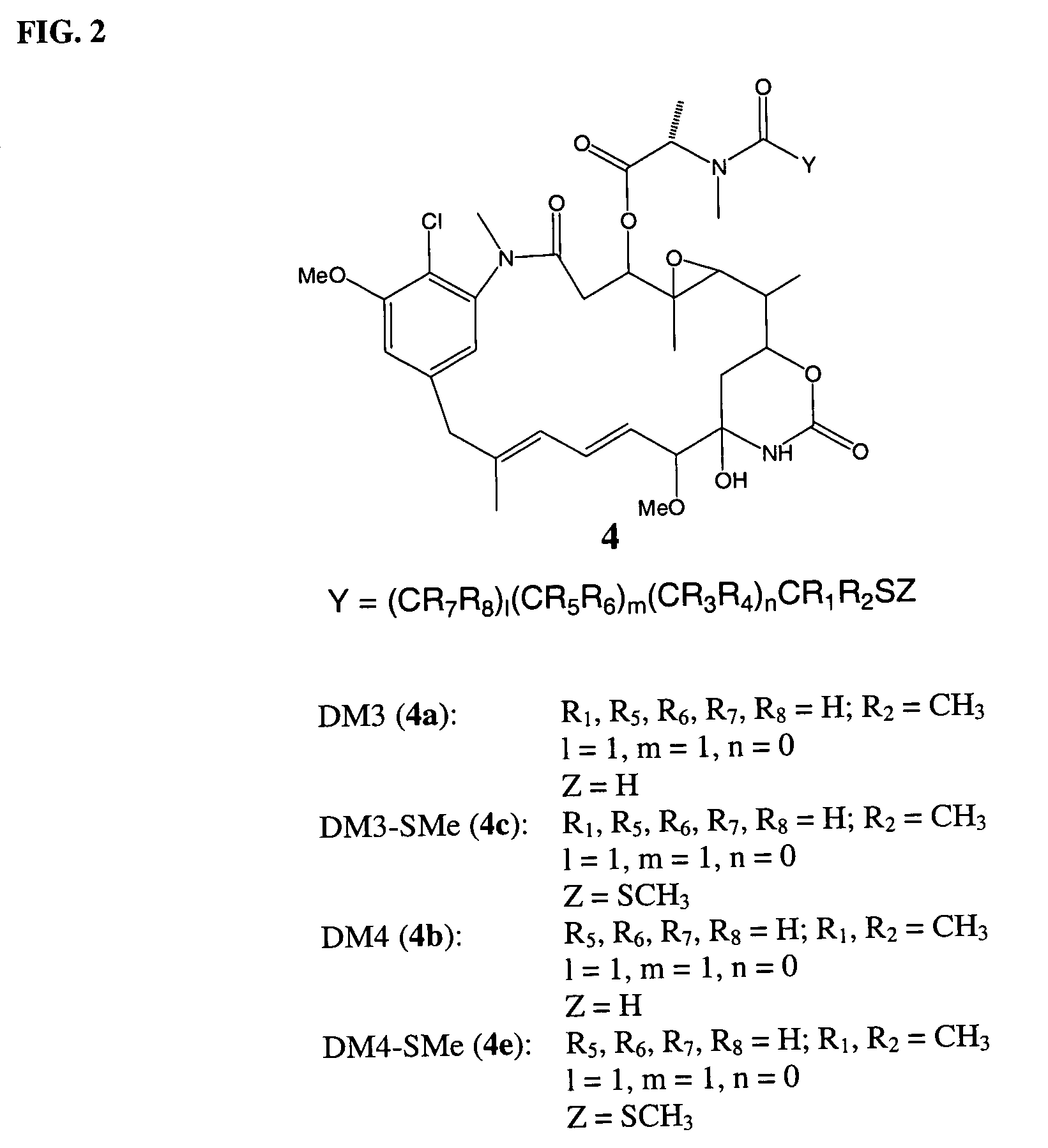
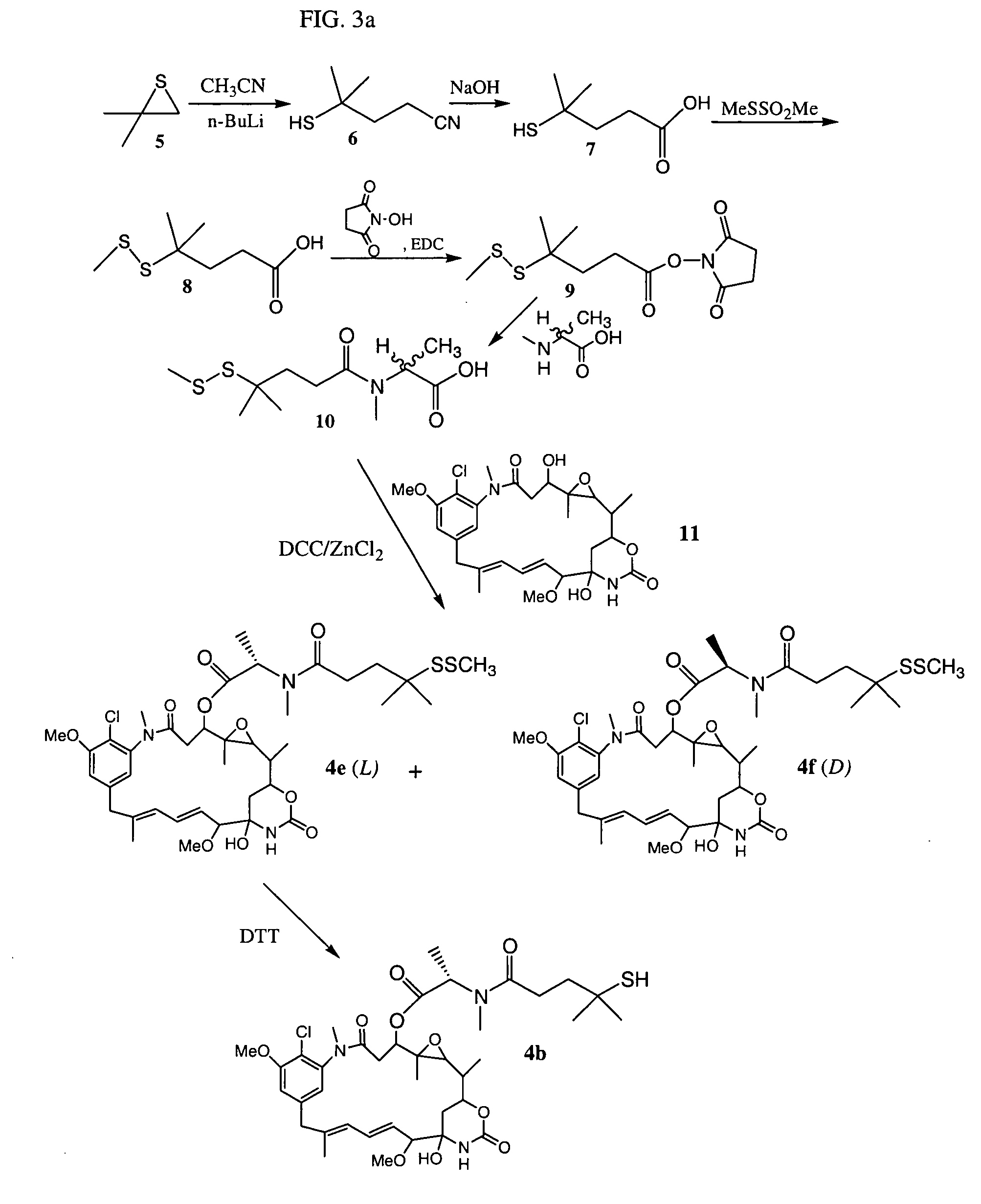
Cytotoxic agents comprising new maytansinoids
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
Heterocyclic compounds
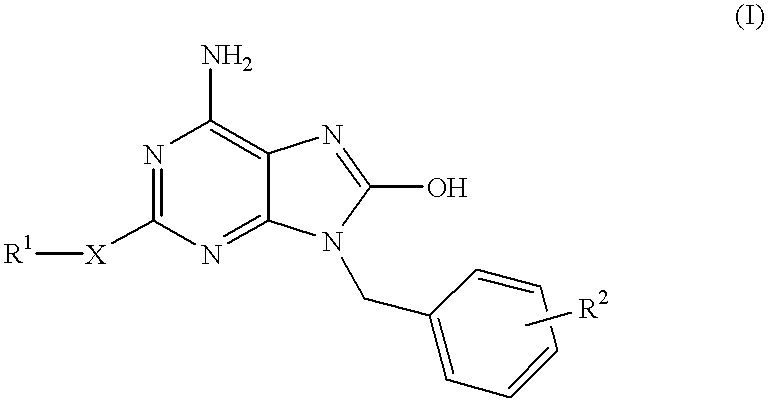
InactiveUS6329381B1Excellent interferon biosynthesis inducing activityInhibition thicknessAntibacterial agentsBiocideBULK ACTIVE INGREDIENTInterferon inducer
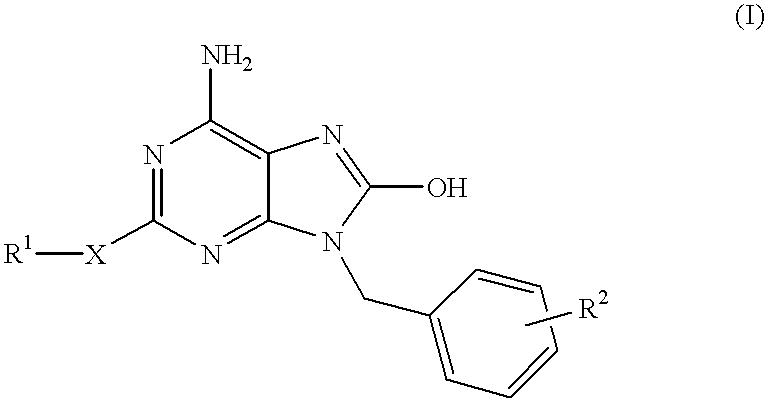
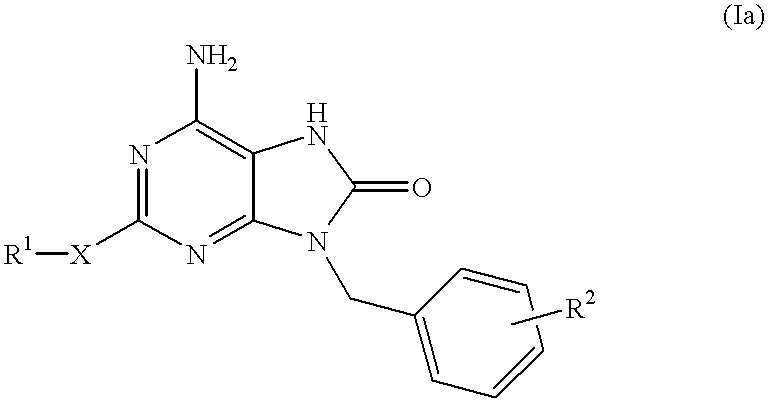
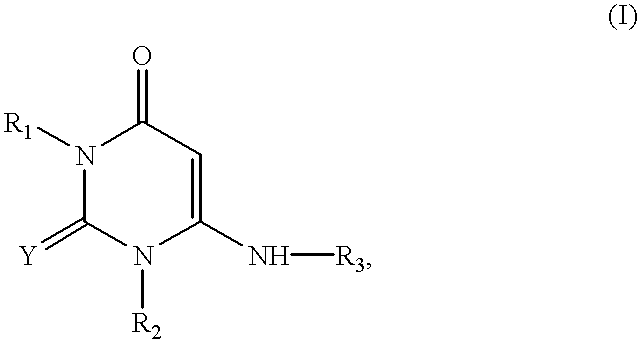
The present invention relates to a heterocyclic compound of the following general formula (I):wherein X is sulfur atom, oxygen atom or -NR3- (R3 may form a heterocyclic ring or a substituted heterocyclic ring with R1 via the nitrogen atom),R1 is alkyl group, substituted alkyl group, aryl group, substituted aryl group, heterocyclic group or substituted heterocyclic group, andR2 is hydrogen atom, halogen atom etc.;or its pharmaceutically acceptable salt and interferon inducers, antiviral agents, anticancer agents and therapeutic agents for immunologic diseases comprising the compound (I) or its pharmaceutically acceptable salt as active ingredients.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Photoacid generators for use in photoresist compositions
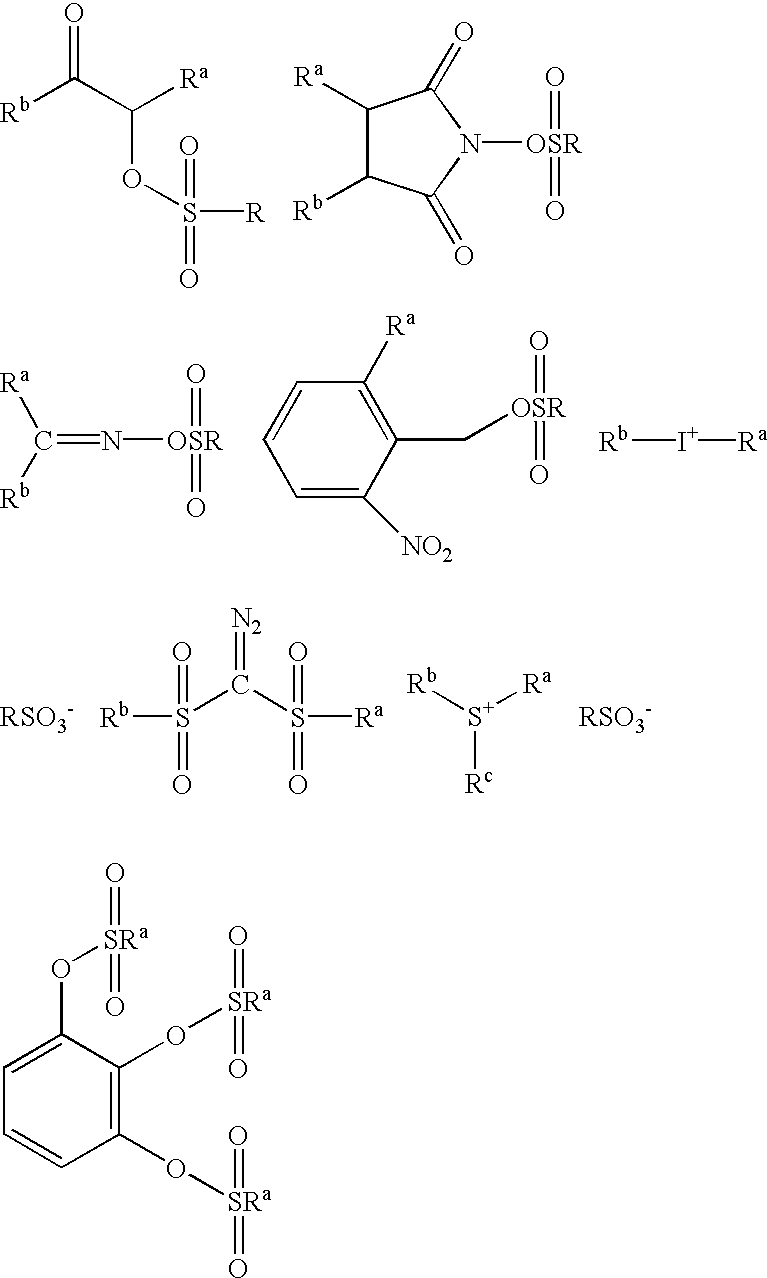
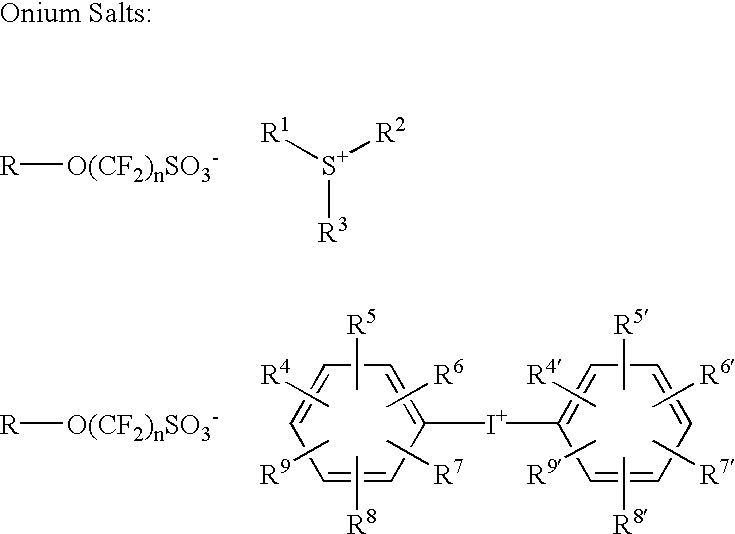
A photoacid compound having the following general structure:<paragraph lvl="0"><in-line-formula>R-O(CF2)nSO3X< / in-line-formula>wherein n is an integer between about 1 to 4; R is selected from the group consisting of: substituted or unsubstituted C1-C12 linear or branched alkyl or alkenyl, substituted or unsubstituted araalkyl, substituted or unsubstituted aryl, substituted or unsubstituted bicycloalkyl, substituted or unsubstituted tricycloalkyl, hydrogen, alkyl sulfonic acid, substituted or unsubstituted perfluoroalkyl, the general structure F((CF2)pO)m(CF2)q- wherein p is between about 1 to 4, m is between about 0 to 3 and q is between about 1 to 4, and substituted or unsubstituted partially fluorinated alkyl, halofluoroalkyl, perfluoroalkylsulfonic, or glycidyl; and X is selected from the group consisting of: organic cations and covalently bonded organic radicals.
Owner:FUJIFILM ELECTRONICS MATERIALS US
Substituted nucleosides, preparation thereof and use as inhibitors of RNA viral polymerases
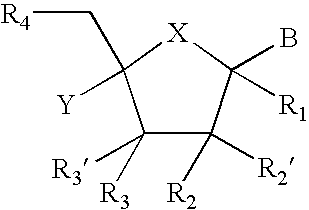
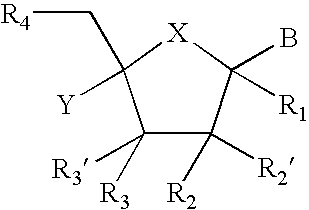
Provided are compounds represented by: X is O, S or NR6, R1 is H or (CH2)mR5, R2, R2', R3 and R3' are independently NO2, N3 or (CH2)mR5, OH R4 is H, OR6, SR6, NR6R6a, CN, C(O)OR6, C(O)NR6R6a, R6, OR7 or (CH2)nR7, R5 is H, halo, OR6, SR6, NR6R6a, CN, C(O)OR6, C(O)NR6R6a, R6, OR7 or (CH2)mR7, R6 and R6a are individually H, alkyl, substituted alkyl, alkenyl, substituted alkenyl, alkynyl, substituted alkynyl, aryl or substituted aryl, R7 is: R8 is H, F, SR9 or OR9, R9 is H, alkyl, alkenyl, alkynyl, aryl or hydroxyprotecting group, Y is H, CH3 or (CH2)mR5, Z is O or S W is CH2, CF2, CHF or O, m is 0-4, B is adenine, guanine, cytosine, uracil, thymine, modified purines and pyrimidines substituted pyridines, five membered heterocycles substituted by at least one of amines, substituted amines, amides, substituted amides, esters, halogens, alkyls, ethers; and pharmaceutically acceptable salts thereof and prodrugs thereof. These ring systems may be substituted.
Owner:BIOCRYST PHARM INC
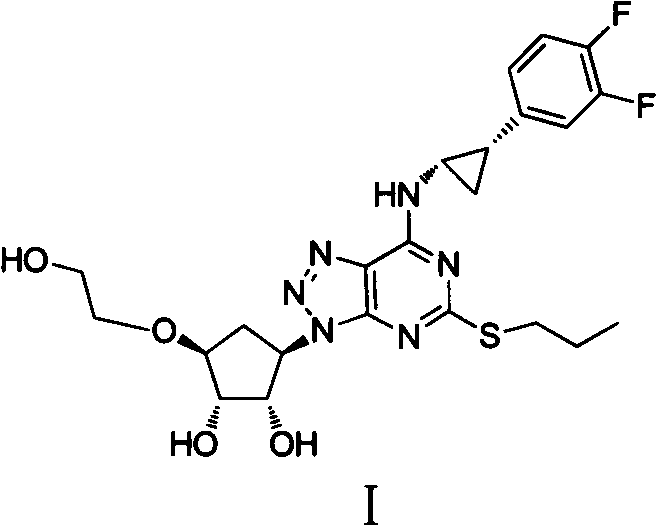
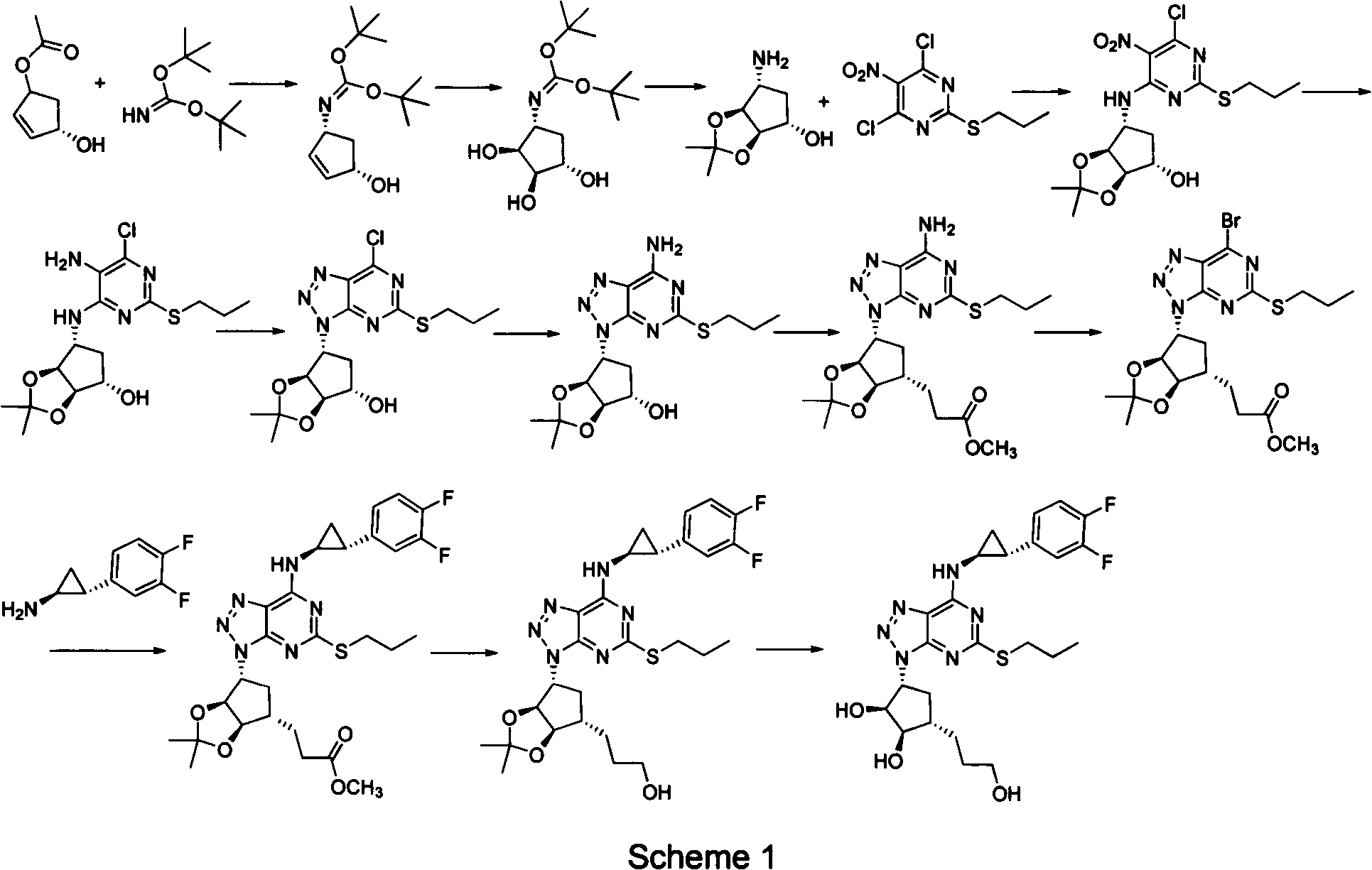
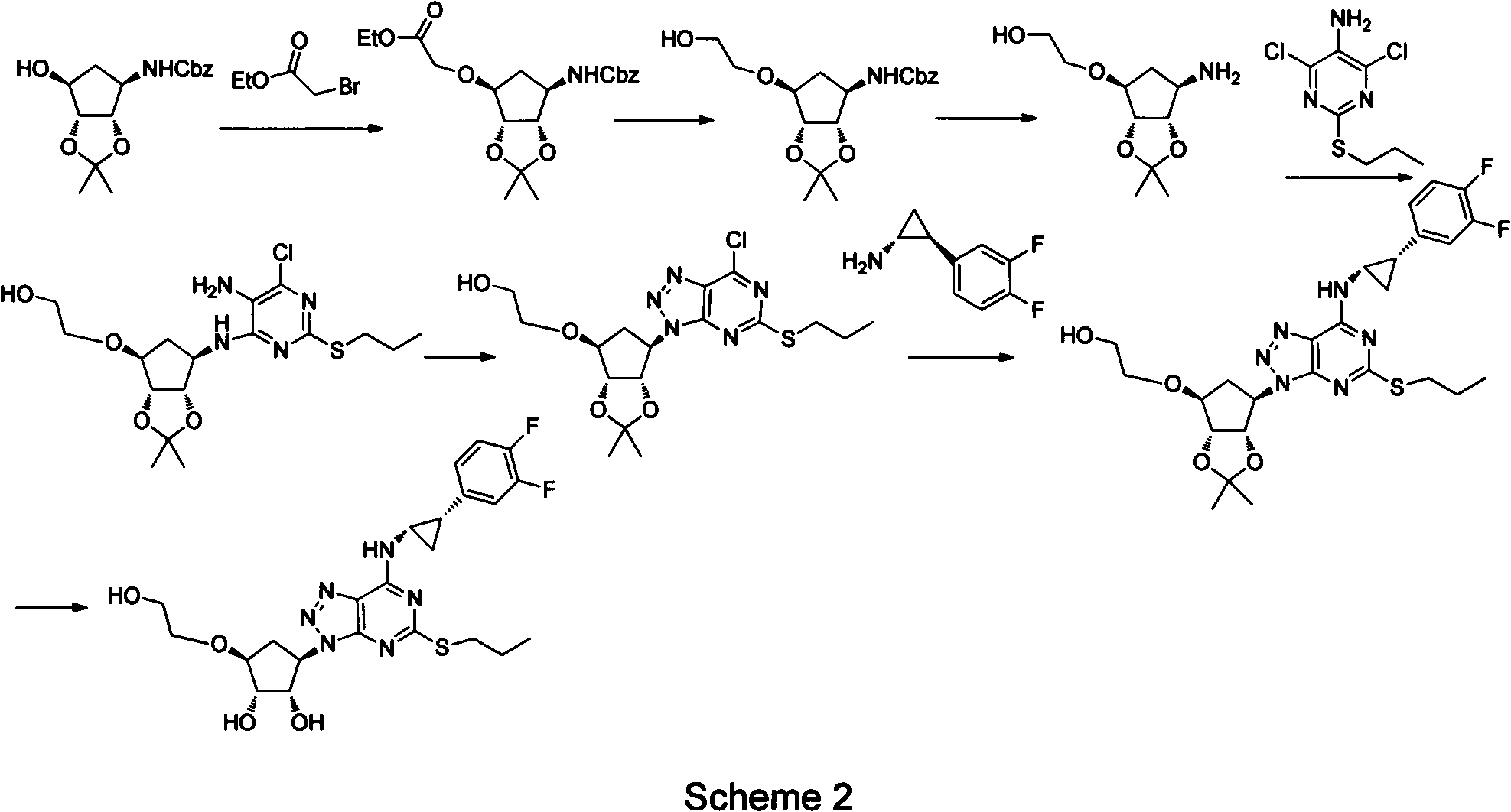
Preparation method of ticagrelor
ActiveCN102675321AHigh yieldReduce manufacturing costOrganic chemistryBulk chemical productionNitriteTicagrelor
The invention provides a preparation method of ticagrelor, belonging to the technical field of medicine manufacturing. According to the method, a compound VII is taken as a raw material, and the method comprises the steps of: carrying out a nucleophilic substitution reaction on the raw material to obtain a compound VI; hydrogenating the VI, removing carbamazepine (Cbz) protection to obtain a compound V; carrying out a reaction on the V and 4, 6-dichloro-2-(allyl sulfide)-5-amio-pyrimidine to obtain a compound IV; carrying out a reaction on the IV and nitrite of alkali metal to obtain a compound III; carrying out a reaction on the III and (1R, 2S)-2-(3, 4-difluoro phenyl) cyclopropylamine to obtain a compound II; and finally, removing protecting group of the II to obtain a compound I.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
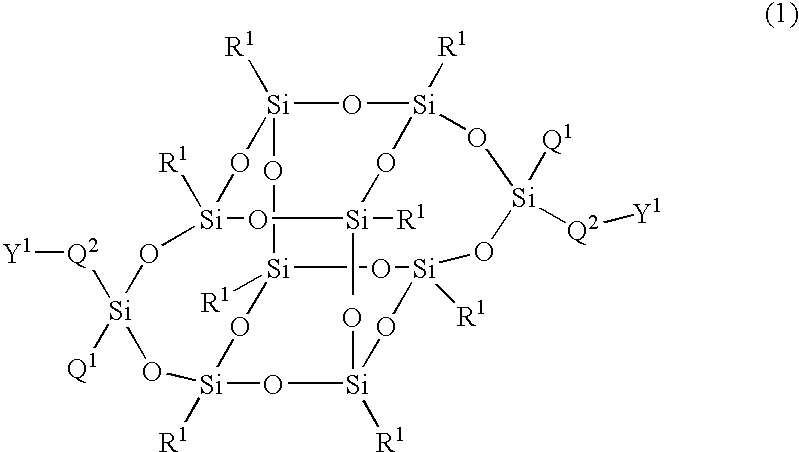
Compound having silsesquioxane skeleton and its polymer
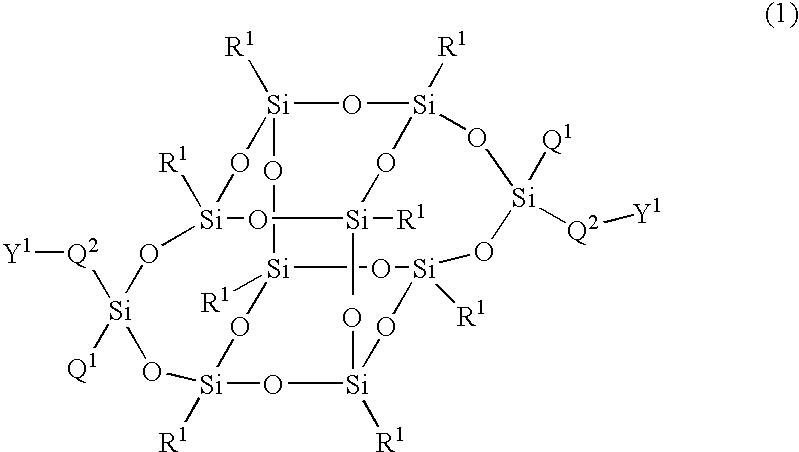
The present invention relates to a compound represented by Formula (1) and a polymer obtained using the compound: wherein R1 is phenyl which may have substituents, Q1 is hydrogen, halogen, alkyl having 1 to 10 carbon atoms, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl or phenyl in which optional hydrogen may be replaced by halogen or alkyl having 1 to 5 carbon atoms, and Q2 is a group represented by Formula (2) wherein the code < represents a bonding point with silicon, l, m, n and p are independently 0, 1, 2 or 3, A1 to A4 are independently a single bond, 1,4-cyclohexylene, 1,4-cyclohexenylene, a condensed ring group having 6 to 10 carbon atoms which is a divalent group, or 1,4-phenylene, Z0 to Z3 are independently a single bond, —CH═CR—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms, and Z4 is a single bond, —CH═CH—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms. And Y1 in Formula (1) is the group defined in Claim 1.
Owner:JNC PETROCHEM CORP +1
Covalent attachment of polymers onto macromolecular chromophores by nucleophilic substitution reaction for inkjet printing
InactiveUS6221932B1Easy to controlExceptional propertyDuplicating/marking methodsInksPolymer scienceOrganic solvent
The present invention relates to ink-jet ink compositions that comprise macromolecular chromophores having functional groups covalently attached for water solubility. Moreover, the MMCs have polymer chains covalently attached to the pigments by nucleophilic substitution to provide enhanced smearfastness, enhanced print quality, improved bleed control, and improved resistance to water when applied to the media. These inks have good viscosity and surface tension, are more soluble in organic solvents and are, therefore, useful in ink-jet printing, including thermal ink jet printing, piezoelectric ink jet printing, and continuous ink jet printing.
Owner:HEWLETT PACKARD DEV CO LP
Phenylcarbazole compounds and organic electroluminescence devices using the same
ActiveUS20060020136A1Improve efficiencyExtend your lifeOrganic chemistryDischarge tube luminescnet screensOrganic electroluminescencePolycyclic group
A phenylcarbazole compound of formula (1) below is provided, where each of R1 and R2 is independently a monosubstituted or polysubstituted functional group selected from the group consisting of hydrogen atom, a substituted or unsubstituted C1-C30 alkyl group, a substituted or unsubstituted C6-C30 aryl group, a substituted or unsubstituted C4-C30 heterocyclic group, a substituted or unsubstituted C6-C30 condensed polycyclic group, wherein groups adjacent to R1 and R2 bind and form a saturated or unsaturated cyclic hydrocarbon group, and Ar is a substituted or unsubstituted C6-C30 aryl group or a C6-C30 heteroaryl group, wherein the substituent R4 is defined herein. Also included is an organic electroluminescence device comprising the above phenylcarbazole compounds.
Owner:SAMSUNG DISPLAY CO LTD
Process for making an overbased, sulfurized salt of an alkylated hydroxyaromatic compound
ActiveUS20110124539A1Improve compatibilityReduced and had no endocrine disruption effectOrganic chemistryAdditivesIsomerizationAlpha-olefin
An overbased, sulfurized salt of at least one alkylated hydroxyaromatic compound, wherein the alkyl substituent of the hydroxyaromatic compound is a residue of at least one isomerized olefin having from about 15 to about 99 wt. % branching is disclosed. The overbased, sulfurized salt of at least one alkylated hydroxyaromatic compound is produced by the process comprising: (a) alkylating at least one hydroxyaromatic compound with at least one isomerized olefin having from about 15 to about 99 wt. % branching obtained by isomerizing at least one normal alpha olefin having from about 10 to about 40 carbon atoms, to provide at least one alkylated hydroxyaromatic compound; (b) neutralizing and sulfurizing the alkylated hydroxyaromatic compound in any order to provide at least one neutralized, sulfurized alkylated hydroxyaromatic compound; and (c) overbasing the at least one neutralized, sulfurized alkylated hydroxyaromatic compound.
Owner:CHEVRON ORONITE CO LLC +1
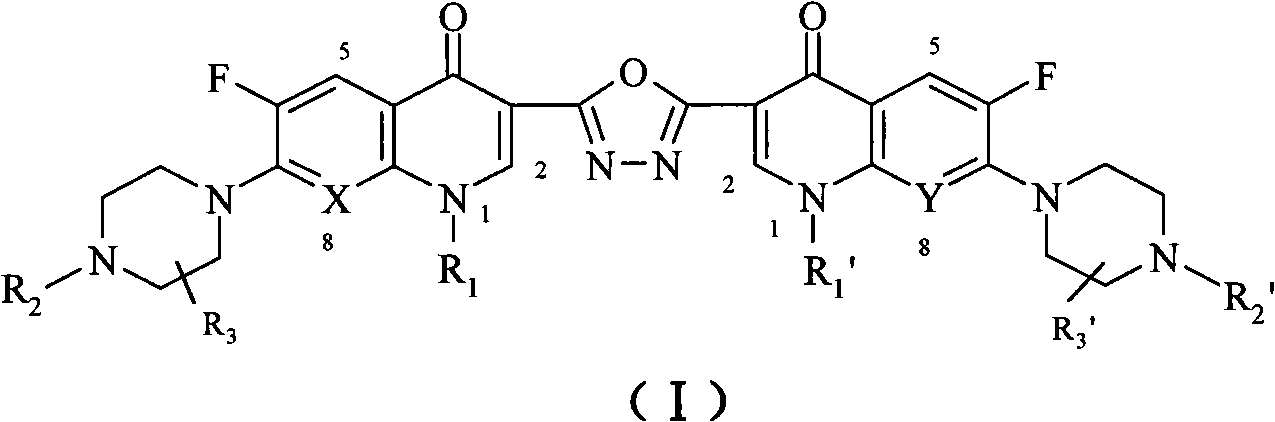
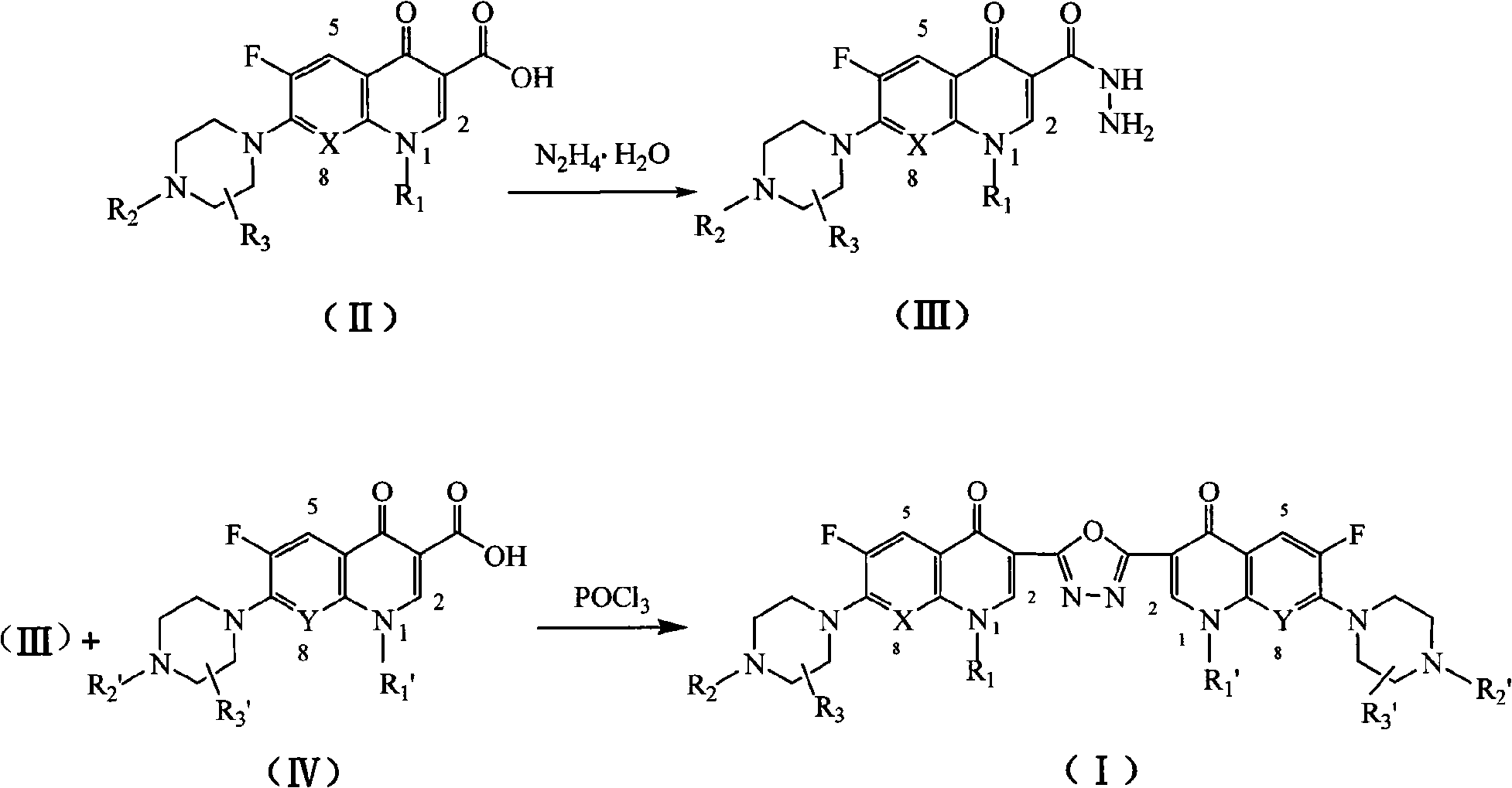
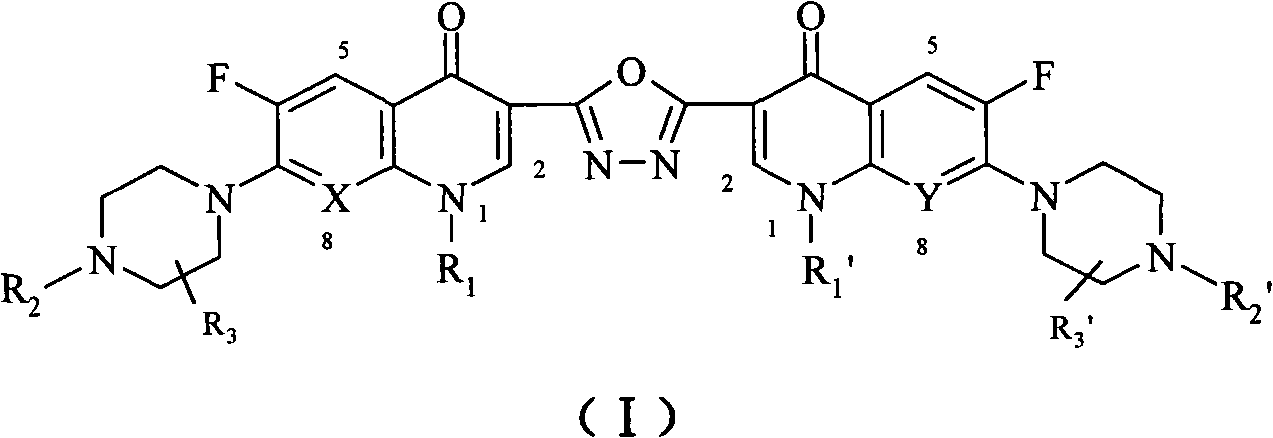
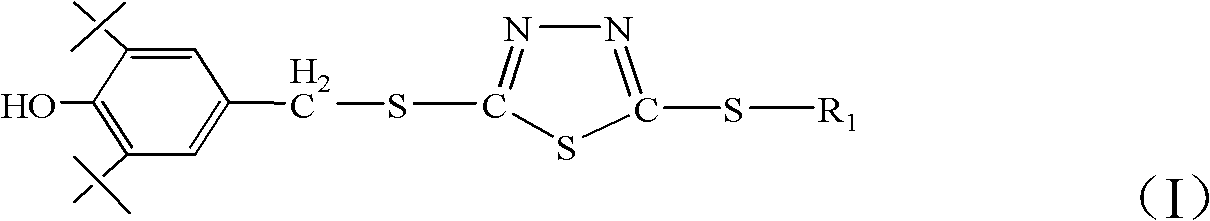
C3/C3 fluoroquinolone dimmer derivative using oxadiazole as connection chain as well as preparation method and application thereof
InactiveCN101643471AStrong cytotoxicityStrong growth inhibitory activityOrganic active ingredientsOrganic chemistryDimmerAryl radical
The invention discloses a C3 / C3 fluoroquinolone dimmer derivative using oxadiazole as a connection chain as well as a preparation method and an application thereof. The C3 / C3 fluoroquinolone dimmer derivative using oxadiazole as a connection chain is a compound which has the following structural general formula (I), wherein R1 and R1' are independently selected from H, (C1-C10) alkyl, (C3)-(C10) cycloalkyl, (C1)-(C10) haloalkyl and substituted aryl group or substituted heterocyclic aryl group; R2 and R2' are independently selected from H, (C1-C7)alkyl, (C3)-(C7) cycloalkyl, substituted aryl group, substituted heterocyclic aryl group and hydrocarbon acyl or sulfonyl; R3 and R3' are independently selected from H, (C1-C5) alkyl, (C3)-(C5) cycloalkyl, substituted aryl radical or substitutionalheterocyclic aryl radical; and X and Y are independently selected from CH, N or carbon atoms connected with halogen, alkyl, oxyl, sulfenyl, amino, substituted amino, substituted aryl radical or substituted heterocyclic aryl radical. The C3 / C3 fluoroquinolone dimmer derivative using oxadiazole as a connection chain can be used for preparing medicaments for treating tumors and preventing microbialinfection diseases.
Owner:河南省健康伟业生物医药研究股份有限公司
Phenylcarbazole compounds and organic electroluminescence devices using the same
ActiveUS7431997B2Improve efficiencyExtend your lifeOrganic chemistryDischarge tube luminescnet screensArylHydrogen atom
A phenylcarbazole compound of formula (1) below is provided,where each of R1 and R2 is independently a monosubstituted or polysubstituted functional group selected from the group consisting of hydrogen atom, a substituted or unsubstituted C1-C30 alkyl group, a substituted or unsubstituted C6-C30 aryl group, a substituted or unsubstituted C4-C30 heterocyclic group, a substituted or unsubstituted C6-C30 condensed polycyclic group, wherein groups adjacent to R1 and R2 bind and form a saturated or unsaturated cyclic hydrocarbon group, and Ar is a substituted or unsubstituted C6-C30 aryl group or a C6-C30 heteroaryl group, wherein the substituent R4 is defined herein. Also included is an organic electroluminescence device comprising the above phenylcarbazole compounds.
Owner:SAMSUNG DISPLAY CO LTD
Olefin oligomerization catalysts and methods of using same
InactiveUS7378537B2Organic-compounds/hydrides/coordination-complexes catalystsHydrocarbons from unsaturated hydrocarbon additionPtru catalystHeteroatom
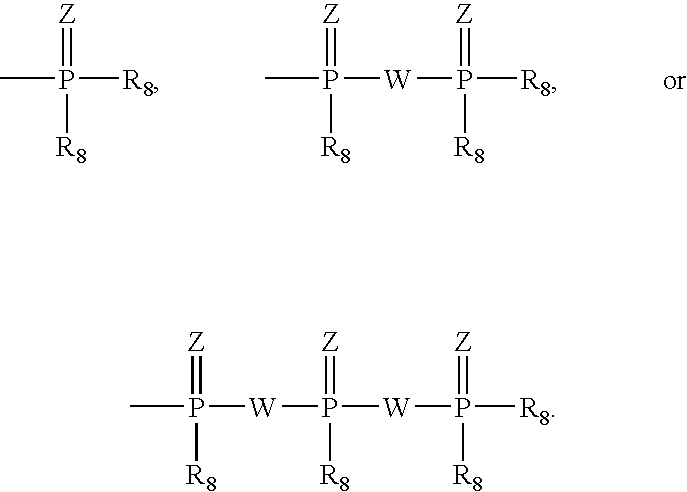
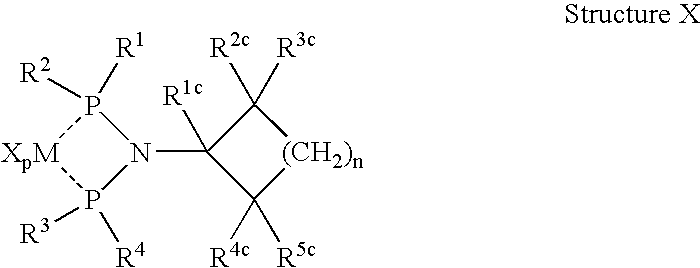
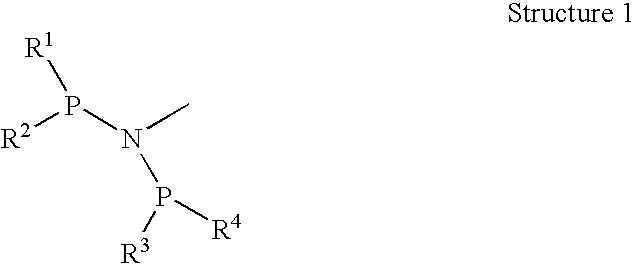
A metal complex comprising a metal compound complexed to a heteroatomic ligand, the metal complex having Structure X:wherein R1, R2, R3, and R4 are each independently an alkyl group, a cycloalkyl group, a substituted cycloalkyl group, an aromatic group, or a substituted aromatic group, R1c, R2c, R3c, R4c, and R5c are each independently hydrogen or an alkyl group, and MXp comprises a group IVB, VB, or VIB metal. A metal complex comprising a metal compound complexed to a diphosphino aminyl ligand comprising at least two diphosphino aminyl moieties and a linking group linking each aminyl nitrogen atom of the diphosphino aminyl moieties.
Owner:CHEVRON PHILLIPS CHEMICAL CO LP
Multi-cyclic compounds
InactiveUS20090062529A1Proceed efficientlyValid conversionNervous disorderOrganic chemistryPolycyclic compoundPharmacometrics
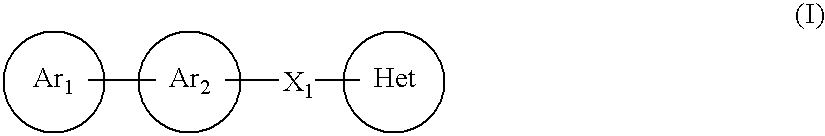
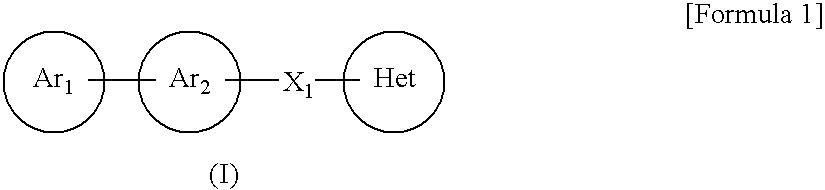
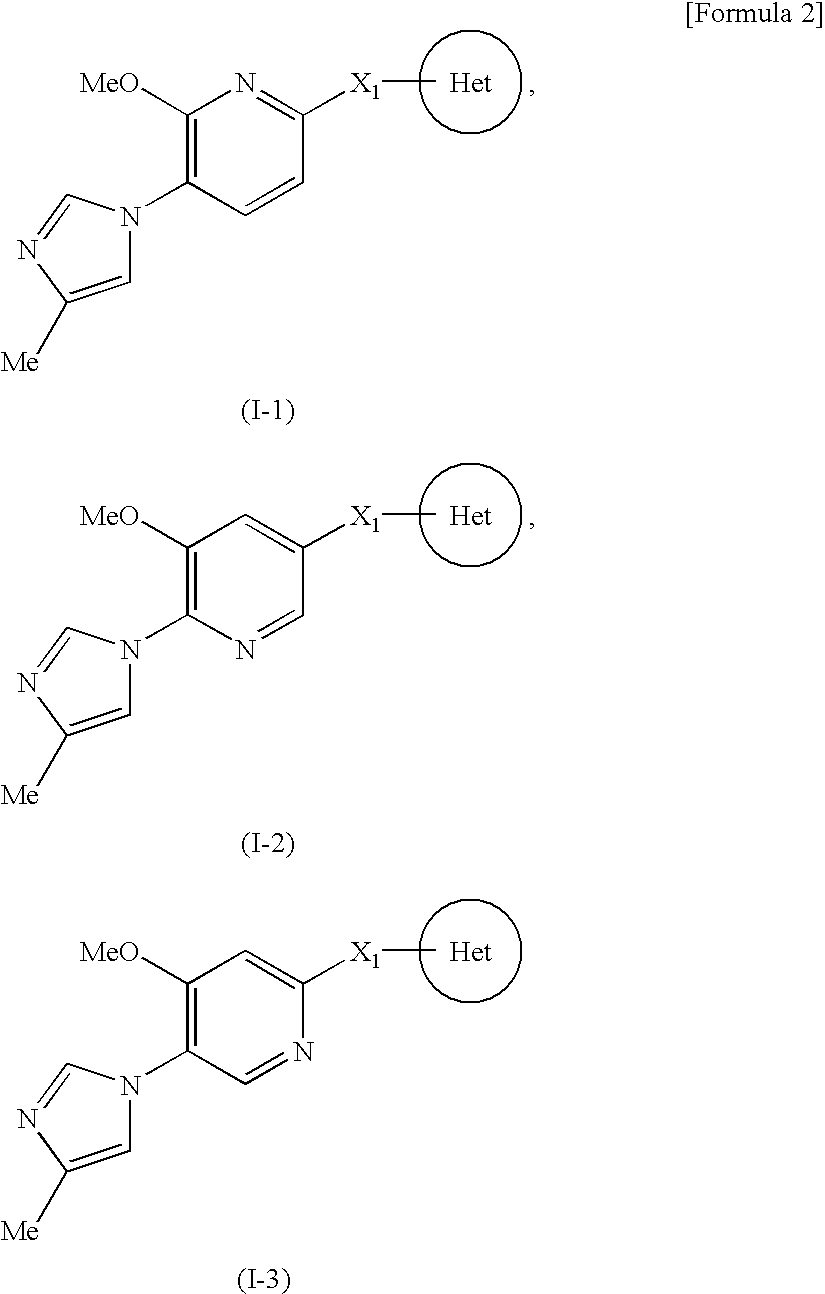
A compound represented by the formula (I):or a pharmacologically acceptable salt thereof, wherein Ar1 represents an imidazolyl group or the like which may be substituted with a C1-6 alkyl group, Ar2 represents a phenyl group or the like which may be substituted with a C1-6 alkoxy group, X1 represents a double bond or the like and Het represents a triazolyl group or the like which may be substituted with a C1-6 alkyl group or the like, is effective as a therapeutic or prophylactic agent for a disease caused by Aβ.
Owner:EISIA R&D MANAGEMENT CO LTD
Screen phenol-containing thiadiazole type antioxygen antiwear additive and preparation method thereof
InactiveCN103320199AAntioxidant is goodExcellent extreme pressureOrganic chemistryAdditivesAlkaneThio-
The invention discloses a screen phenol-containing thiadiazole type antioxygen antiwear additive and a preparation method thereof, wherein the chemical name of the additive is 2-(3,5-di-tert-butyl-4-hydroxy-benzyl)thio-5-alkylthio 1,3,4-thiadiazole. According to the preparation method, 2,5-dimercapto-1,3,4-thiadiazole is adopted as a raw material, the 2,5-dimercapto-1,3,4-thiadiazole and a halogenated alkane are subjected to a nucleophilic substitution reaction under a catalyst effect to generate monoalkyl thiadiazole, and the monoalkyl thiadiazole, formaldehyde and 2,6-di-tert-butylphenol are subjected to a condensation polymerization reaction under a catalyst effect to obtain 2-(3,5-di-tert-butyl-4-hydroxy-benzyl)thio-5-alkylthio 1,3,4-thiadiazole type lubricating oil multifunction additive, wherein the additive is characterized in that good oxidation resistance, extreme pressure property, wear resistance and corrosion resistance are provided for the lubricant with the additive, and the additive can be combined with other additives to provide good synergy effects, can be adopted to partly or completely replace ZDDP, and is applicable for internal combustion engine oils and industrial lubricating oils.
Owner:PETROCHINA CO LTD
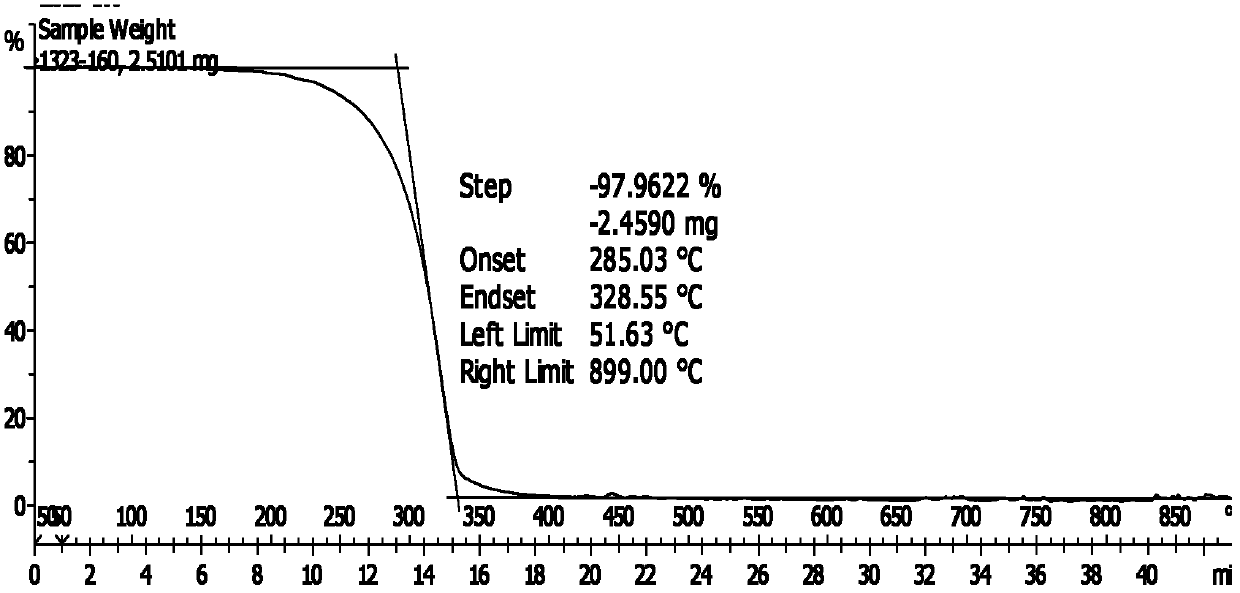
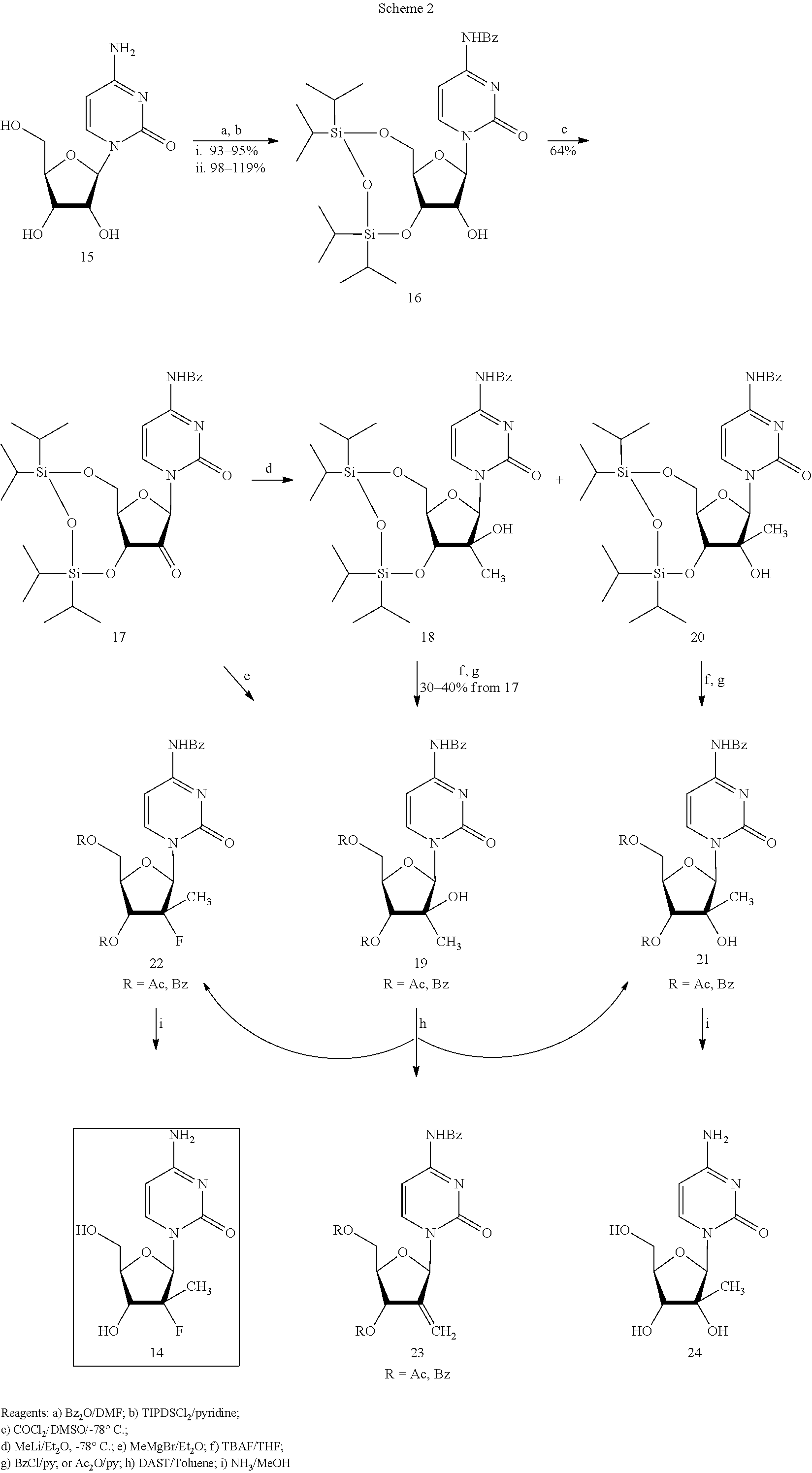
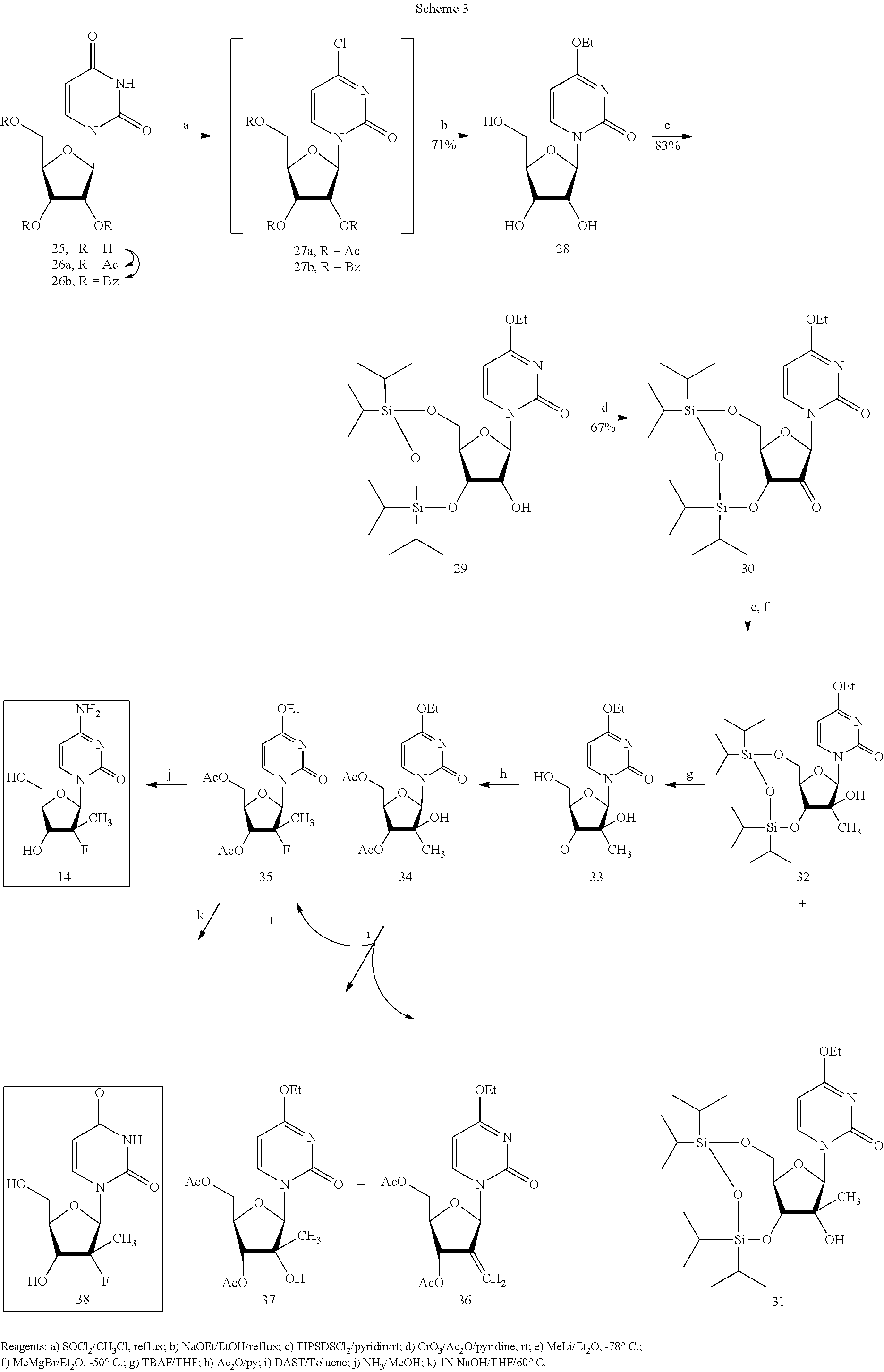
Preparation of 2'-fluoro-2'-alkyl-substituted or other optionally substituted ribofuranosyl pyrimidines and purines and their derivatives
The present invention provides (i) processes for preparing a 2′-deoxy-2′-fluoro-2′-methyl-D-ribonolactone derivatives, (ii) conversion of intermediate lactones to nucleosides with potent anti-HCV activity, and their analogues, and (iii) methods to prepare the anti-HCV nucleosides containing the 2′-deoxy-2′-fluoro-2′-C-methyl-β-D-ribofuranosyl nucleosides from a preformed, preferably naturally-occurring, nucleoside.
Owner:GILEAD SCI INC
Heterocyclic compound
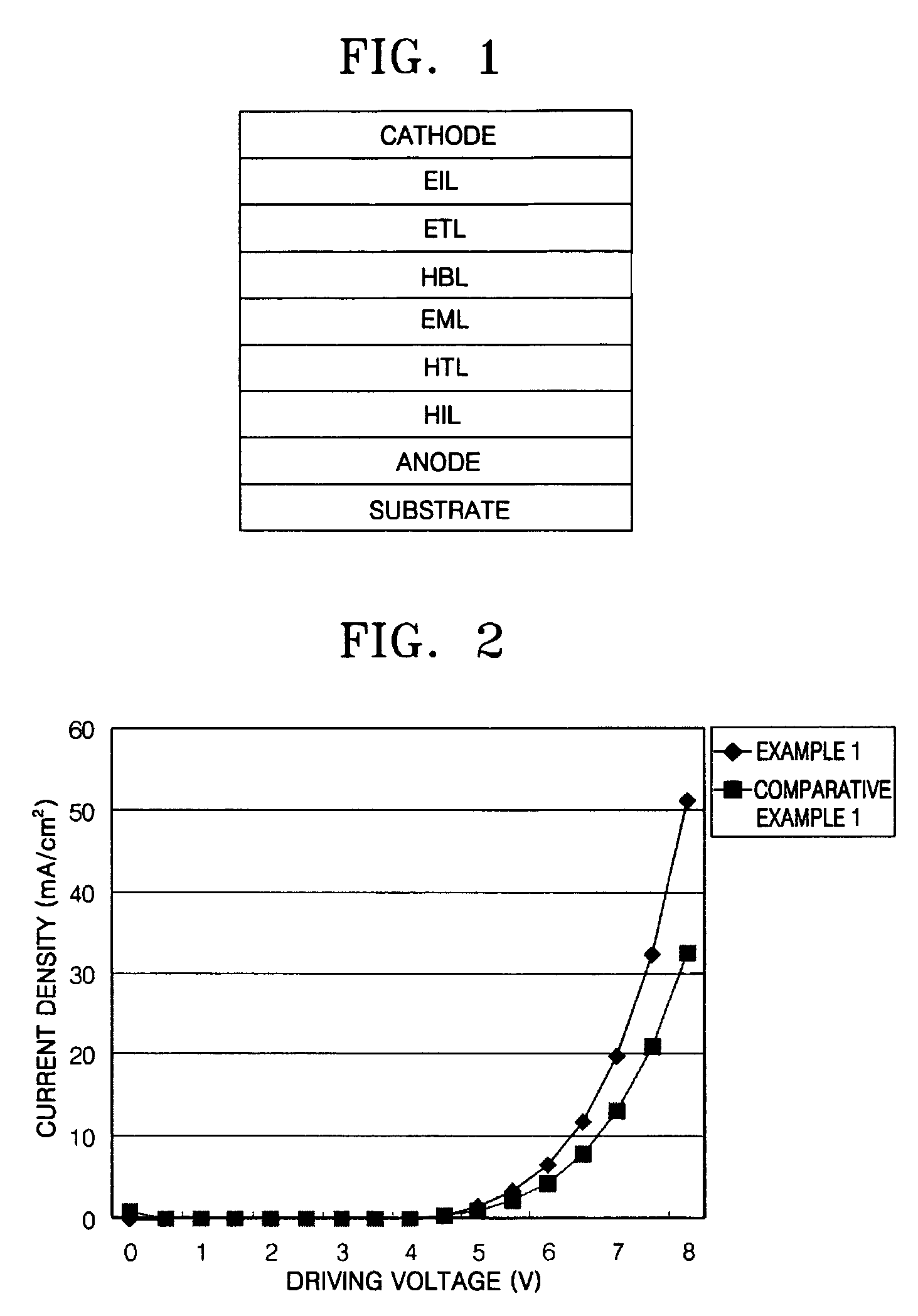
ActiveUS20120197020A1Reduce the driving voltageImprove current efficiencyOrganic chemistrySolid-state devicesCarbazoleDibenzofuran
Provided is a novel heterocyclic compound which can be used for a light-emitting element, as a host material of a light-emitting layer in which a light-emitting substance is dispersed. A heterocyclic compound represented by a general formula (G1) is provided. In the formula, A represents any of a substituted or unsubstituted dibenzothiophenyl group, a substituted or unsubstituted dibenzofuranyl group, and a substituted or unsubstituted carbazolyl group, R11 to R19 separately represent any of hydrogen, an alkyl group having 1 to 4 carbon atoms, and a substituted or unsubstituted aryl group having 6 to 14 carbon atoms, and Ar represents a substituted or unsubstituted arylene group having 6 to 13 carbon atoms.
Owner:SEMICON ENERGY LAB CO LTD
Process for production of delta-9-tetrahydrocannabinol
InactiveUS7674922B2High selectivityLower overall renovationOrganic compound preparationCarboxylic acid esters preparationArylPtru catalyst
The present invention relates to a process for preparation of a delta-9-tetrahydrocannabinol compound or derivative thereof involving treating a first intermediate compound with an organoaluminum-based Lewis acid catalyst, under conditions effective to produce the delta-9-tetrahydrocannabinol compound or derivative thereof. Another aspect of the present invention relates to a process for preparation of a cannabidiol or cannabidiolate compound involving reacting a first starting compound with a second starting compound in the presence of a metal triflate catalyst, under conditions effective to form the cannabidiol or cannabidiolate compound. The present invention also relates to a compound of the formula:where R8, R9, and R10 are the same or different and independently selected from the group consisting of H, substituted or unsubstituted alkyl, substituted or unsubstituted aryl, substituted or unsubstituted heteroaryl, or halo, with R1, R2, and R3 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Novel cyclic compound having 4-pyridylalkylthio group having substituted or unsubstituted amino group introduced therein
InactiveUS20070149574A1Useful in therapyExcellent cell proliferation inhibitory effectBiocideSenses disorderDiseaseAryl
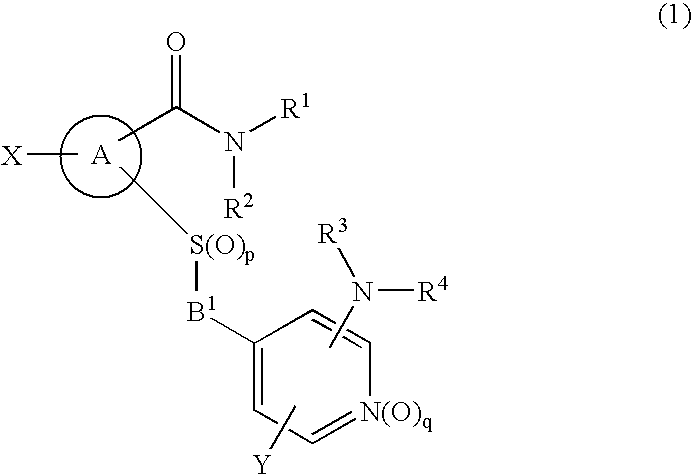
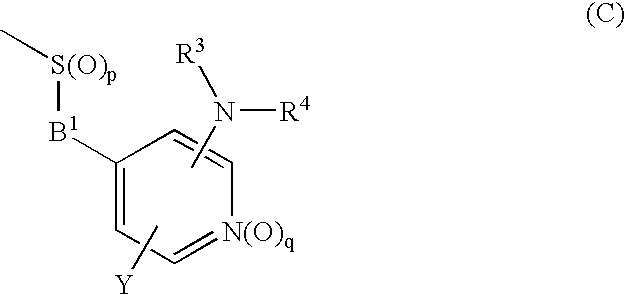
A novel cyclic compound having a 4-pyridylalkylthio group having an (un)substituted amino group introduced therein or a salt thereof. They are useful as a medicine. The cyclic compound is a compound represented by the following formula (1), which is useful for the treatment of diseases in which angiogenesis participates. In the following formula (1), ring A represents a benzene ring or a 5- or 6-membered aromatic heterocycle optionally fused with a cycloalkane ring; B represents alkylene; R1 and R2 each represents H, (substituted) aryl, (substituted) heterocyclic group, etc.; R3 and R4 each represents H, (substituted) alkyl, (substituted) cycloalkyl, -Z-R5, etc.; R5 represents (substituted) alkyl, (substituted) aryl, (substituted) heterocyclic group, etc.; X and Y each represents H, etc.; Z represents —CO—, —COO—, —CONR6—, —SO2—, etc.; R6 represents H, etc.; p is 0, 1, or 2; and q is 0 or 1.
Owner:SANTEN PHARMA CO LTD
Scutellarin carbamate derivative, preparation method and use thereof
The invention discloses a 4'-carbamate derivative of scutellarin shown in formula (I), a preparation method of the compound and application of the compound in preparing medicines for preventing and / or treating various symptoms and diseases caused by acetyl cholinesterase and / or mediated by free radicals, such as vascular dementia and Alzheimer's disease. In the formula, each of R1, R2 and R3 independently represents H, C1-C12 alkyl or R6CO, wherein R6 represents C1-C12 alkyl, but R1, R2 and R3 are not H at the same time; each of R4 and R5 independently represents H, C1-C12 alkyl, C1-C6 fatty alcohol, or an ester formed by the C1-C6 fatty alcohol and C1-C6 carboxylic acid, the C1-C6 carboxylic acid, or an ester formed by the C1-C6 carboxylic acid and the C1-C6 fatty alcohol; or R4NR5 represents a morpholine ring, a piperidine ring, a 4-benzyl piperidine ring, a piperazine ring, the piperazine ring with the 4-position substituted by C1-C12 alkyl, or a tetrahydropyrrole ring.
Owner:SICHUAN UNIV
Fused purine derivatives
A condensed purine derivative represented by Formula (I): wherein X—Y-Z represents R1N—C═O or N═C—W, R2 represents a hydrogen atom, a substituted or unsubstituted lower alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted aromatic heterocyclic group, a substituted or unsubstituted alicyclic heterocyclic group or the like, n represents an integer of from 0 to 3, V1 represents a hydrogen atom, a substituted or unsubstituted lower alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, or a substituted or unsubstituted aromatic heterocyclic group, V2 represents a substituted lower alkyl group or a substituted or unsubstituted aromatic heterocyclic group, and when V1 represents a hydrogen atom, a lower alkyl group, a substituted or unsubstituted aralkyl group, or a substituted or unsubstituted aryl group, and for example, X—Y-Z represents R1aN—C═O and R2 represents a substituted lower alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted alicyclic heterocyclic group, a halogen atom, a lower alkylthio group, —NR7R8, —CO2H, a lower alkoxycarbonyl group, —COHal, —CONR9R10 or —CHO, V2 may represent a lower alkyl group, a substituted or unsubstituted aralkyl group, or a substituted or unsubstituted aryl group; or a pharmacologically acceptable salt thereof.
Owner:KYOWA HAKKO KOGYO CO LTD
Electric Double-Layer Capacitor
ActiveUS20090103241A1Improve electrostatic capacityIncrease capacityHybrid capacitor electrolytesOrganic chemistryMethyl groupElectric double-layer capacitor
An activated carbon comprising a carbonized and activated compound represented by the formula (1):(wherein, R represents a hydrocarbon group having 1 to 12 carbon atoms, said hydrocarbon group may be optionally substituted with hydroxyl group, alkyl group, alkoxy group, aryl group, aryloxy group, sulfonyl group, halogen atoms, nitro group, thioalkyl group, cyano group, carboxyl group, amino group or amide group, R′ represents hydrogen atom or methyl group, and n represents an integer of 4, 6, or 8).
Owner:SUMITOMO CHEM CO LTD
Modified graphene and preparation method thereof
The invention discloses modified graphene and a preparation method thereof. The method comprises the following steps: performing oxidation treatment on natural flake graphite to obtain graphene oxide, and enabling graphene oxide to react with sulfoxide chloride to obtain graphene containing acyl chloride groups on the surface; then performing nucleophilic substitution reaction with 1,3-propane diamine, cyanuric chloride and a dihydric alcohol compound in sequence to obtain water-soluble graphene with hydroxyl groups on the surface, wherein the hydroxyl group content is 0.005-0.02%. Obtained modified graphene has relatively good water solubility, and is beneficial to stable dispersion of graphene; by adopting the characteristics of modified graphene, modified graphene can be mixed and uniformly dispersed with other concrete preparation materials very conveniently to prepare a reinforcing and toughening concrete product. In addition, operating conditions for preparing modified graphene can be easily met; moreover, modified graphene disclosed by the invention is easily-available in raw material, can be used for effectively reducing the cost and improving the economic benefits, and has relatively good application prospects.
Owner:FUJIAN JIANGXIA UNIV
Cyclic compounds
There is provided a CRF receptor antagonist comprising a compound of the formula (I):A-W—Ar (I)wherein, A is a group represented by the formula (A1) or (A2): (wherein, ring Aa is a 5- or 6-membered ring which may be further substituted; ring Ab is a 5- or 6-membered ring which may be further substituted; ring Ac is a 5- or 6-membered ring which may be substituted; R1 is optionally substituted alkyl, substituted amino, substituted hydroxy, etc.; X is carbonyl, —O—, —S—, etc.; Y1, Y2 and Q are independently optionally substituted carbon or nitrogen; is a single or double bond); W is a bond, optionally substituted methylene, optionally substituted imino, —O—, —S—, etc.; Ar is optionally substituted aryl or optionally substituted heteroaryl; or a salt thereof or a prodrug thereof.
Owner:TAKEDA PHARMA CO LTD
Compound for organic electroluminescent device and organic electroluminescent device having the same
ActiveUS20110309345A1Group 4/14 element organic compoundsGroup 5/15 element organic compoundsArylCarbazole
The present invention provides a compound of formula (I) for an organic electroluminescent device:wherein X and Y are each independently selected for the group consisting of an alkyl substituted, aryl substituted or unsubstituted carbazole, indolocarbazole, triphenylsilyl and diphenylphosphine oxide represented by formula (A), (B), (C), (D) or (E),in which R1, R2, and R3 are each independently selected from the group consisting of a hydrogen, an alkyl having 1 to 15 carbons atoms, an aryl group having 6 to 15 carbons atoms, an alkyl substituted, an aryl substituted or unsubstituted triphenylsilyl, and a diphenylphosphine oxide represented by the formula (D) or (E); m and n are each independently 0 or 1, provided that m+n is 1 or more; and Ar1 and Ar2 are each independently selected from the group consisting of an alkyl substituted, aryl substituted or unsubstituted phenyl, tolyl, naphthyl, fluorenyl, anthracenyl, and phenanthryl.
Owner:E RAY OPTOELECTRONICS TECH
Fluorene derivative, light-emitting element, light-emitting device, electronic device, and lighting device
ActiveUS20110095678A1High color purityImprove efficiencyOrganic chemistryDischarge tube luminescnet screensPhenyl groupLight emitting device
An object is to provide a new fluorene derivative as a good light-emitting material for organic EL elements. A fluorene derivative represented by General Formula (G1) is provided.In the formula, R1 to R8 separately represent a hydrogen atom, an alkyl group having 1 to 6 carbon atoms, a substituted or unsubstituted phenyl group, or a substituted or unsubstituted biphenyl group. Further, α1 to α4 separately represent a substituted or unsubstituted phenylene group. Ar1 represents a substituted or unsubstituted condensed aromatic hydrocarbon having 14 to 18 carbon atoms forming a ring. Ar2 represents a substituted or unsubstituted aryl group having 6 to 13 carbon atoms forming a ring. Ar3 represents an alkyl group having 1 to 6 carbon atoms or a substituted or unsubstituted aryl group having 6 to 12 carbon atoms. Further, j, m, and n separately represent 0 or 1, and p represents 1 or 2.
Owner:SEMICON ENERGY LAB CO LTD
Synthesis path of Timisatem
InactiveCN1344712AReduce generationHigh yieldOrganic chemistryCardiovascular disorderActive componentEthyl group
The present invention relates to the preparation of Timisatan as one active component for antihypertensive medicine. The carboxylic ester derivative is first obtained through the nucleophilic substitution reaction between some intermediate and 4'-bromomethyl biphenyly-2-carboxylic ester, and then hydrolyzed to eliminnate protection radical and to obtain the destination product. In the technological process of the present invention, methyl radical and ethyl radical are used as protecting radical, and this facilitates and stabilizes the preparation of 4'-bromomethyl biphenylyl-2-methyl carboxylic ester and 4'-bromomethyl biphenylyl-2-ethyl carboxylic ester, and results in easy to control reaction with the intermediate, less impurity, easy elimination of protecting radical and high product yield and quality.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
NH2-modified 6-aminouracils as stabilizers for halogenated polymers
A description is given of compounds of the general formula IwhereY is oxygen or sulfur, andR1 and R2 independently of one another are C1-C18-alkyl, C3-C6-alkenyl, unsubstituted or C1-C4-alkoxy-, C5-C8-cycloalkyl-, -OH- and / or Cl-substituted C1-C18-alkyl, C5-C8-cycloalkyl, phenyl or C7-C9-phenylalkyl which is unsubstituted or substituted on the phenyl ring by C1-C4-alkyl, C1-C4-alkoxy, C5-C8-cycloalkyl, -OH and / or Cl, andR3 is unsubstituted C9-C18-alkyl or is C1-C18-alkyl substituted by -OH, C1-C12-alkoxy, phenoxy, C6-C8-alkylphenoxy, C7-C9-alkoxyphenyl, -C=O(OR4) and / or -O-COR4, or is C3-C6-alkenyl, C5-C8-cycloalkyl, or mono- to tri-OH-, -C1-C4-alkyl-, -C1-C4-alkoxy-, -C=O(OR4)- and / or -O-COR4-substituted phenyl or naphthyl, and whereR4 is C1-C12-alkyl,which are suitable for stabilizing chlorine-containing polymers, especially PVC.
Owner:CHEMTURA VINYL ADDITIVES
Method for preparing rosuvastatin calcium midbody
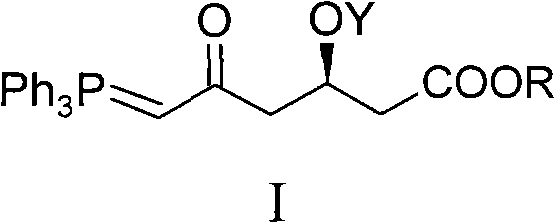
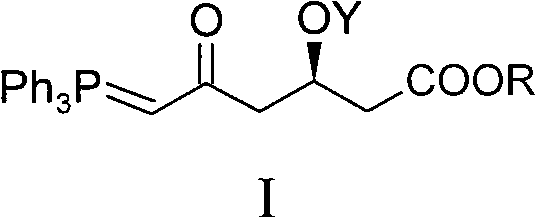
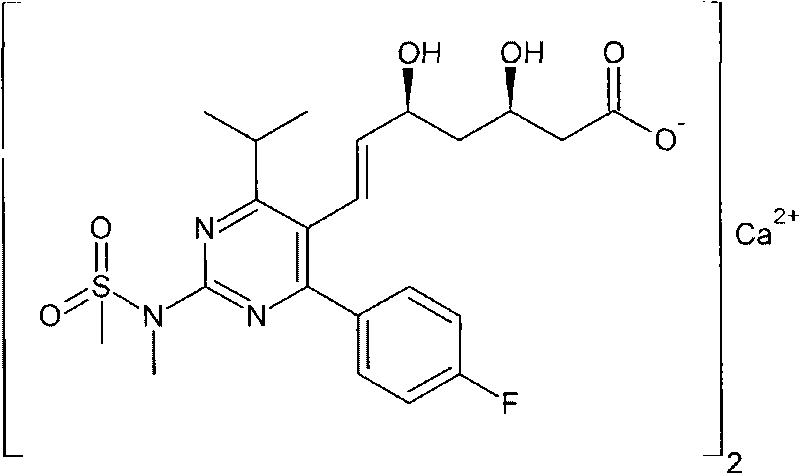
ActiveCN101735272AHigh yieldReduce pollutionGroup 5/15 element organic compoundsBulk chemical productionWittig reactionGrignard reaction
The invention discloses a method for preparing a rosuvastatin calcium midbody, namely a compound (R is C1-C10 alkyl, and Y is a hydroxyl protecting group) shown as the general formula I. Chloroethylene and R-epoxy chloropropane as initial raw materials are carried out seven steps of reaction, such as Grignard reaction, sodium cyanide nucleophilic substitution reaction, alcoholysis reaction, hydroxyl protection, oxidizing reaction, methylchloroformate acylation reaction and Wittig reaction to prepare the compound shown as the general formula I. The method has mild condition, simple and convenient operation, stable process, low cost and easy acquisition of raw materials, high product yield, easy disposal of the three wastes, less environmental pollution, low preparation cost and suitability for industrialized large-scale production.
Owner:JIANGXI DONGBANG PHARMA
Phosphocholine linked prodrug derivatives
InactiveUS7060290B1Increase bioactivity and bioavailabilityImprove bioavailabilityBiocideNervous disorderIndependent groupCarbonyl group
Disclosed are compounds of general formula (I) that function as prodrugs, thereby increasing bioavailabilities of the linked therapeutic agents, wherein the LINKER is (i) substituted or unsubstituted alkyl, (ii) substituted or unsubstituted alkenyl, (iii) substituted or unsubstituted alkanoyl, (iv) substituted or unsubstituted alkenoyl wherein the double bond is cis, and (v) (ortho or para) carbonyl-substituted aryl; and wherein the subtituent is each an independent group or linked together thereby forming a ring; and wherein X is one or more substituted or unsubstituted group containing one or more O, N, or S atom and wherein the substituent is each an independent group or linked together thereby forming a ring; and wherein the therapeutic agent is an alcohol-containing water-insoluble steroids or another alcohol containing compounds and methods to prepare such compounds.
Owner:SUPERGEN
Heterocyclic compound, light-emitting element, light-emitting device, electronic device, and lighting device
ActiveUS20130048971A1Reduce the driving voltageImprove emission efficiencyOrganic chemistrySolid-state devicesHydrogenDibenzofuran
Provided is a novel heterocyclic compound which can be used in a light-emitting layer of a light-emitting element as a host material in which a light-emitting substance is dispersed. A heterocyclic compound represented by a general formula (G1) is provided. Any one of R1 to R10 represents a substituent represented by a general formula (G1-1), another one of R1 to R10 represents a substituent represented by a general formula (G1-2), and the others separately represent hydrogen, an alkyl group having 1 to 6 carbon atoms, a substituted or unsubstituted phenyl group, or a substituted or unsubstituted biphenyl group. Further, α1 and α2 separately represent a substituted or unsubstituted phenylene group or a substituted or unsubstituted biphenyldiyl group, and A1 and A2 separately represent a substituted or unsubstituted carbazolyl group, a substituted or unsubstituted dibenzothiophenyl group, or a substituted or unsubstituted dibenzofuranyl group.
Owner:SEMICON ENERGY LAB CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com