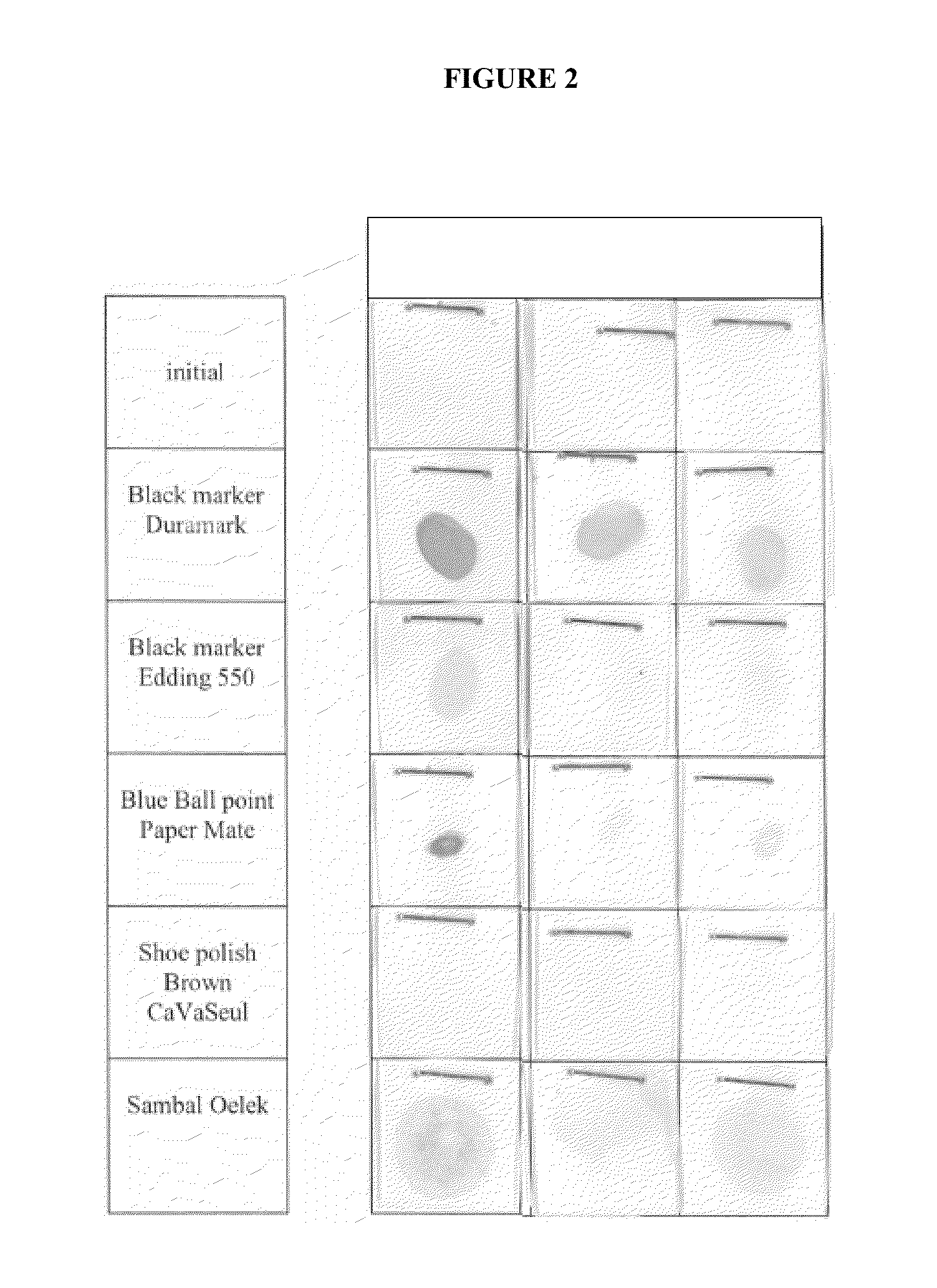
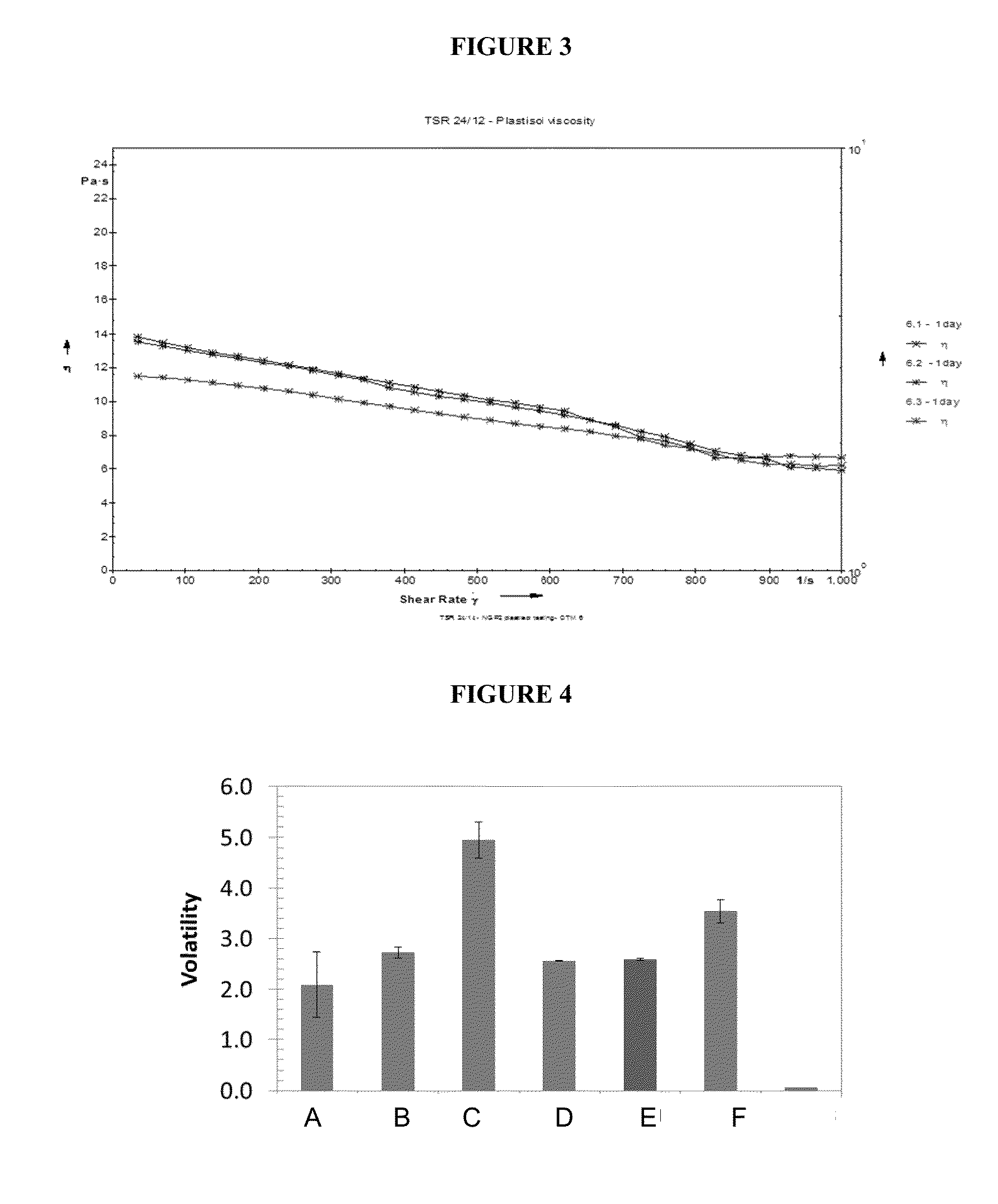
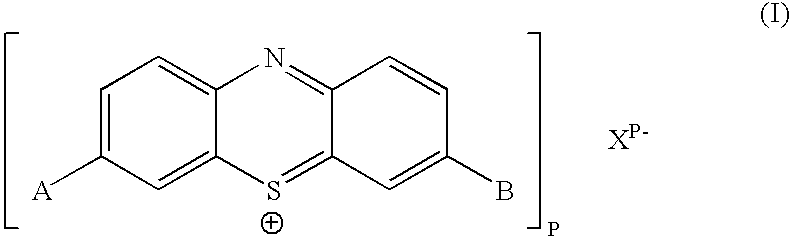
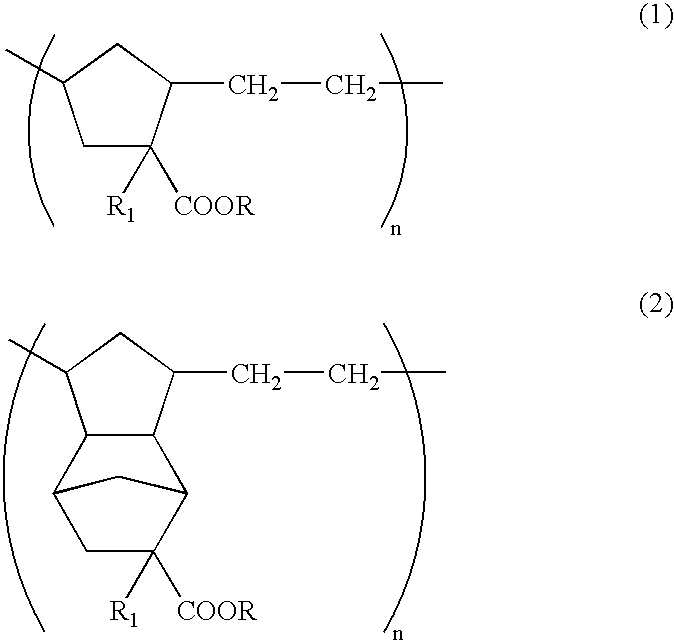
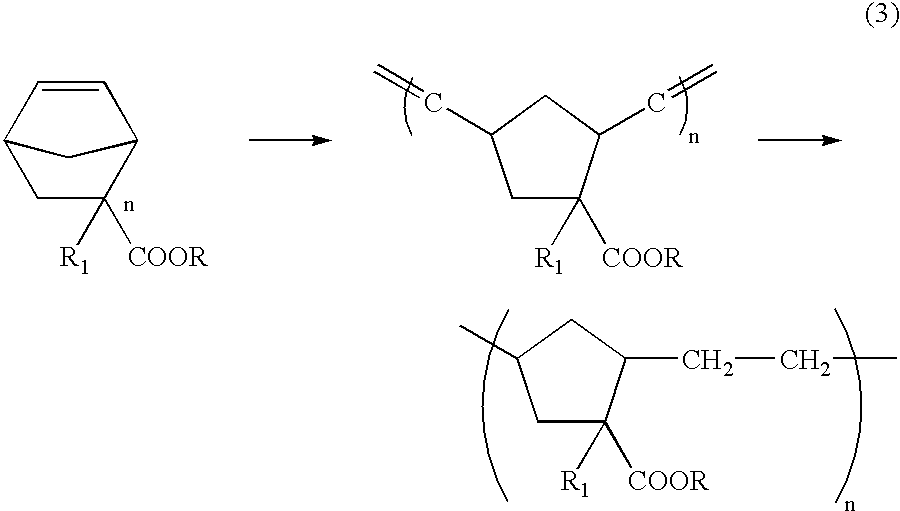
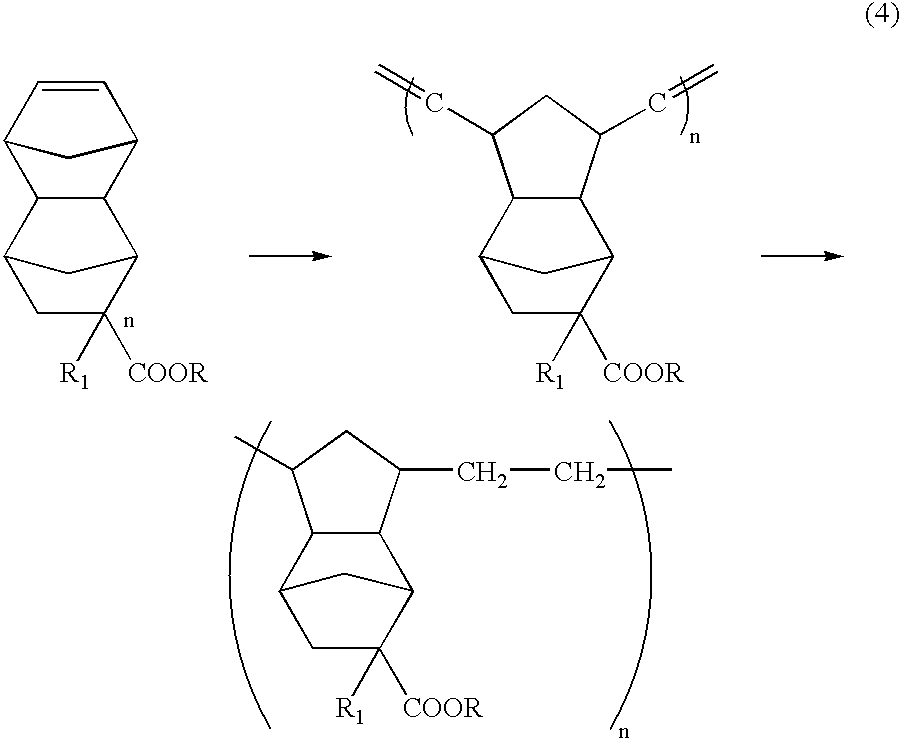
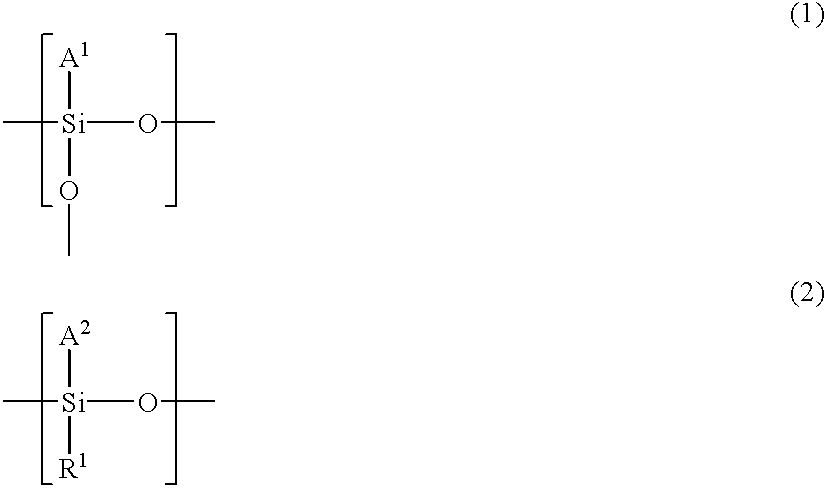
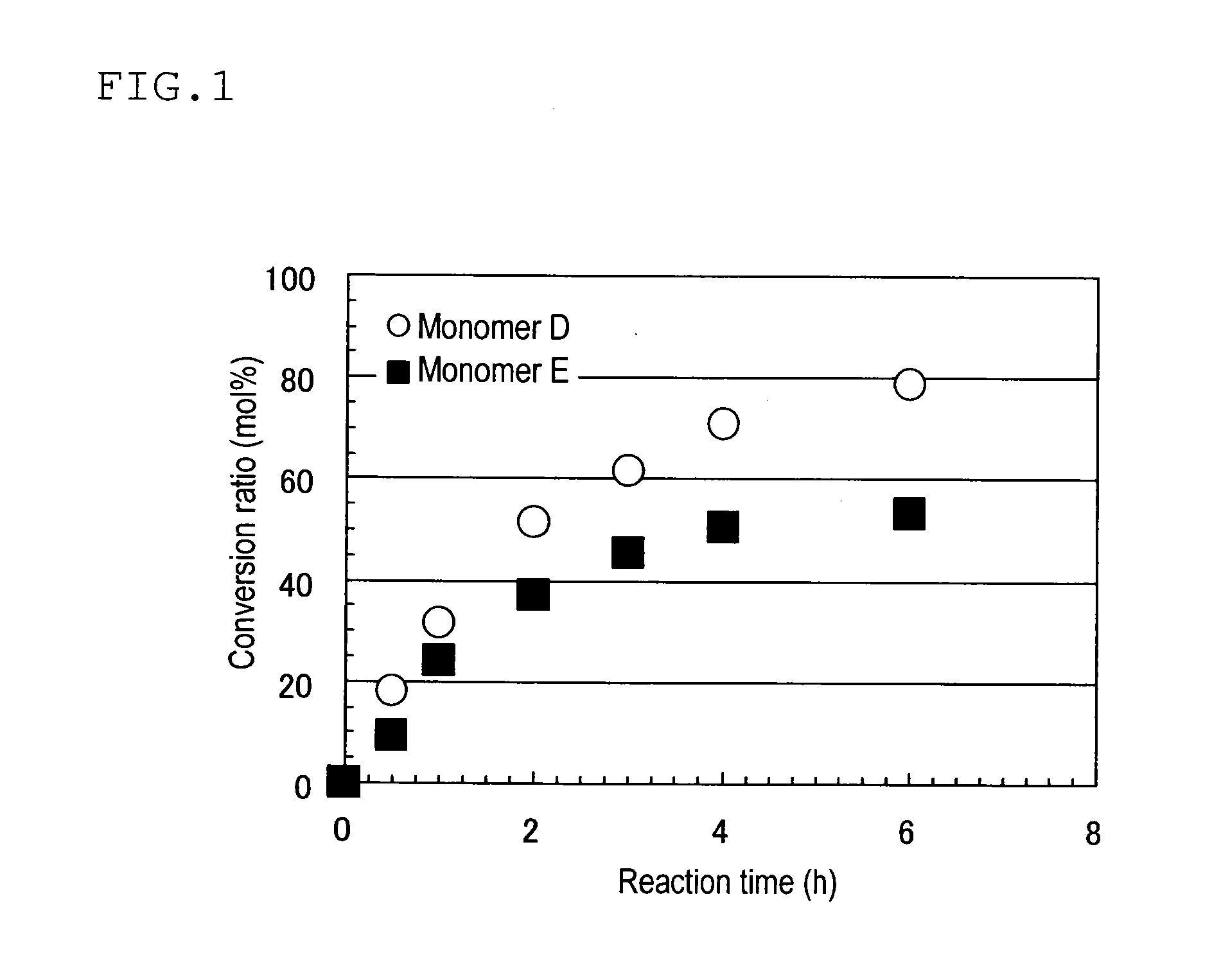
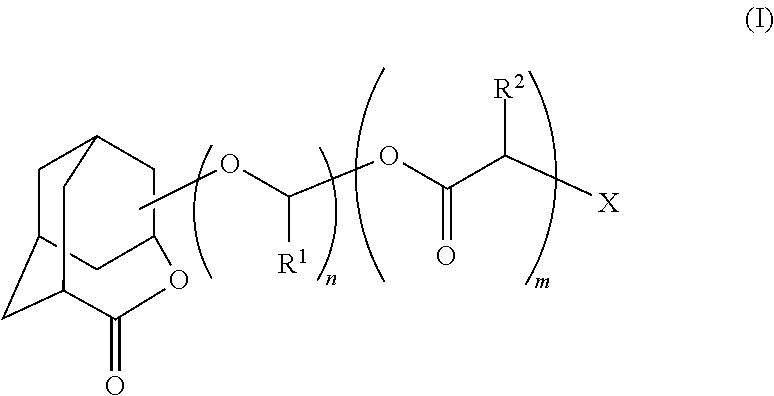
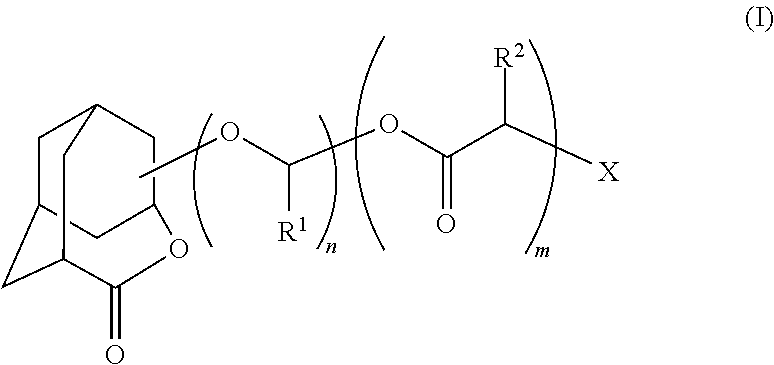
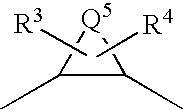
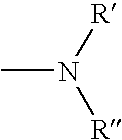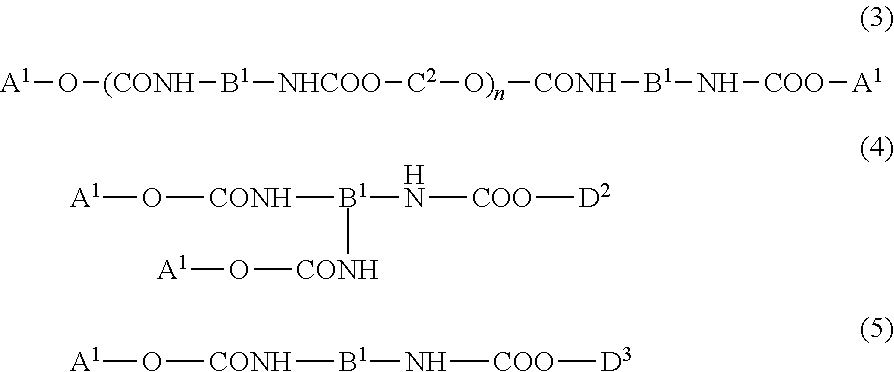Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
266 results about "Cyclic hydrocarbon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon bonds are single bonds. Like other alkanes, cycloalkanes are saturated compounds.
Optical Film
A composition comprising a polymerizable compound of the formula (1) and a rod-shaped polymerizable liquid crystal compound: P2-E2-X2-B2-A2-(G2)t-Y-(G1)s-A1-B1-X1-E1-P1 (1) (in the formula (1), Y represents a di-valent group, s and t represent each independently an integer of 0 or 1, G1 and G2 when s and t are 1 represent each independently —CR1R2—, R1 and R2 represent each independently an alkyl group having 1 to 4 carbon atoms, halogen atom or hydrogen atom, A1 and A2 represent each independently a di-valent cyclic hydrocarbon group, di-valent heterocyclic group, methylenephenylene group, oxyphenylene group or thiophenylene group, B1 and B2 represent each independently a di-valent group, X1 and X2 represent each independently a di-valent group, E1 and E2 represent each independently an alkylene group having 2 to 25 carbon atoms, and P1 and P2 represent a hydrogen atom or polymerizable group, at least one of P1 and P2 being a polymerizable group.).
Owner:SUMITOMO CHEM CO LTD
Stabilization of resorcinol derivatives in cosmetic compositions
InactiveUS6863897B2Improved storage/color stabilityImprove stabilityCosmetic preparationsToilet preparationsHydrogenEthyl group
Cosmetic compositions containing a micronized metal oxide along with a 4-substituted resorcinol derivatives of the general formula I exhibit improved storage stability: where each R1 and R2, independently, represents a hydrogen atom, —CO—R (acyl group), —COO—R, CONHR; where R represents saturated or unsaturated, linear, branched or cyclic C1-C18 hydrocarbon groups; andR3 represents (1) an alkyl group, having from 1 to 18 carbon atoms, preferably having from 2 to 12 carbon atoms, with or without substitution of one or more hydrogen atoms of a linear alkyl group with a methyl or ethyl group; e.g., R3 constitutes linear or branched chain alkyls, or (2) a group of the general formula formula (II) Where X is preferably H, n is 0 to 3 and the dashed lines represents an optional double bond.
Owner:UNILVER HOME & PERSONAL CARE USA DIV OF CONOPCO
Dimeric pharmaceutical compounds and their use
InactiveUS20050085413A1Prevent microbial infectionArresting its developmentAntibacterial agentsBiocidePharmaceutical formulationStereochemistry
The invention relates to a compound of general formula (I): X-L-Y (I) in which X and Y are pharmaceutically active moieties which may be the same or different; and L is a linker which is an optionally substituted saturated or unsaturated straight chain, branched and / or cyclic hydrocarbon radical having a backbone of at least 11 atoms, or a pharmaceutically acceptable derivative or salt thereof, methods for their preparation, pharmaceutical formulations containing them or their use in the prevention or treatment of a microbial infection.
Owner:BIOTA SCI MANAGEMENT PTI LTD
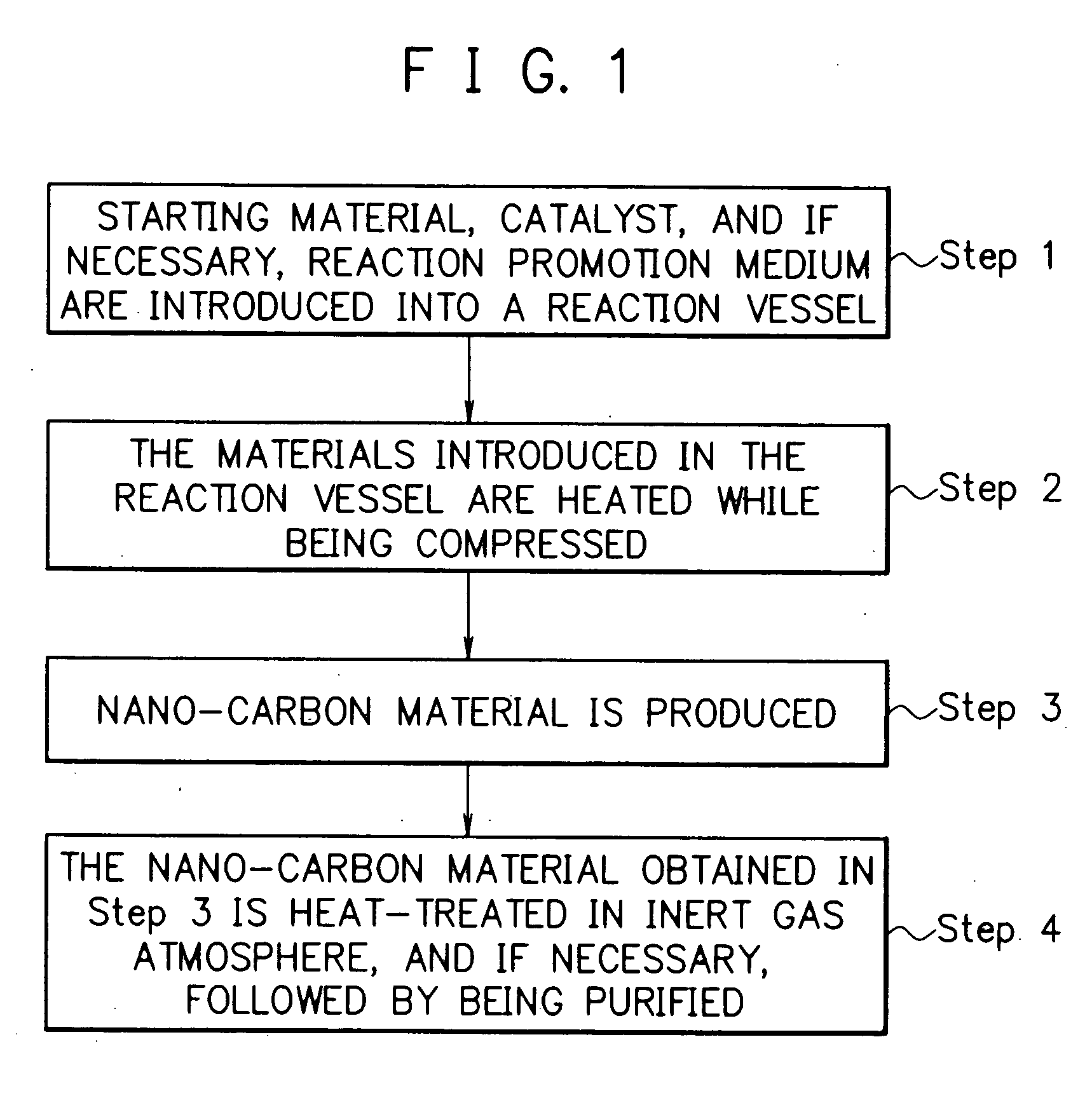
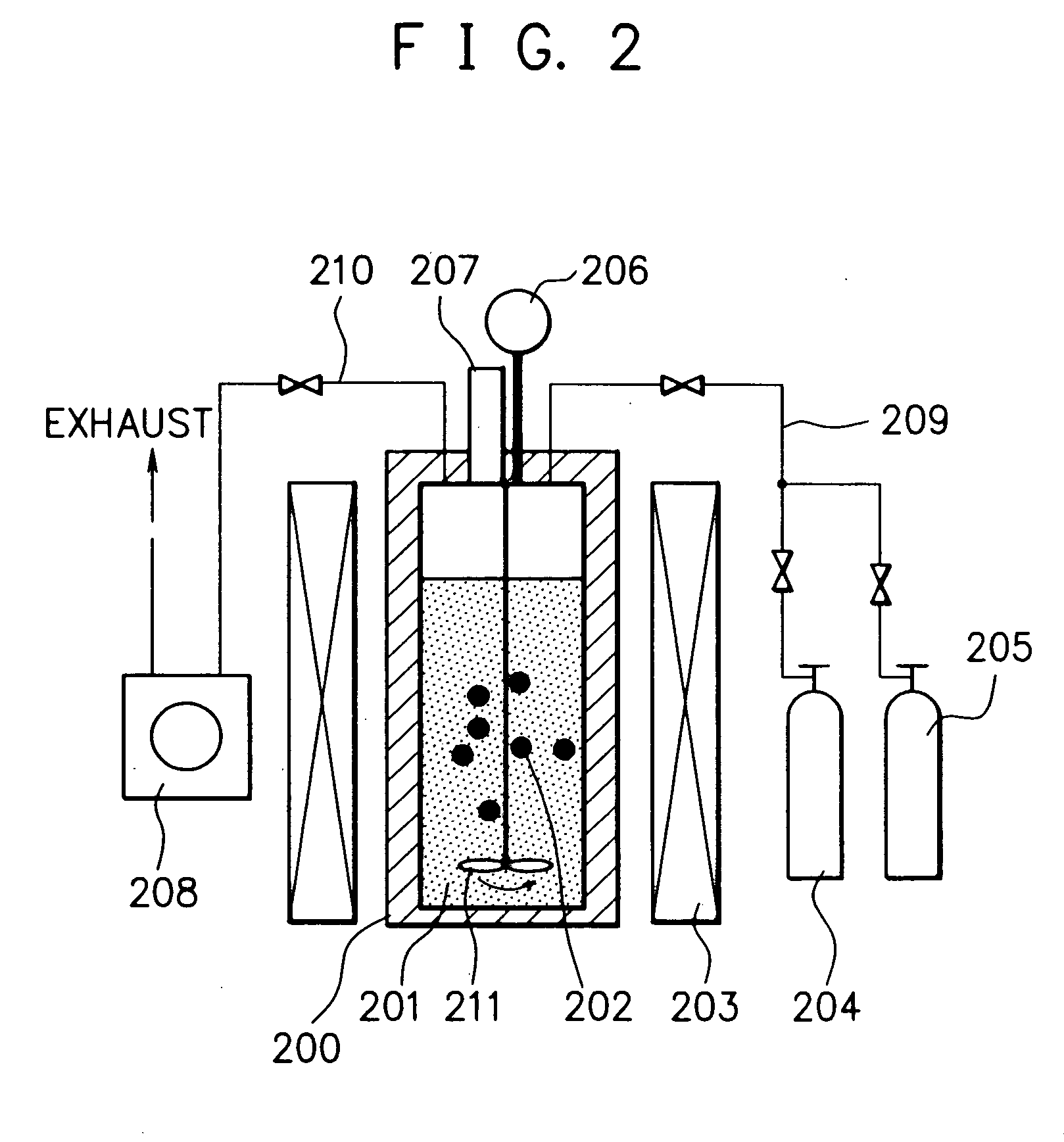
Method for producing nano-carbon materials
InactiveUS20050079119A1Efficiently and quantitatively produceLow production costMaterial nanotechnologyNanostructure manufacturePtru catalystUnsaturated hydrocarbon
A method for producing nano-carbon materials, having a step wherein a starting material comprising one or more kinds of compounds selected from the group consisting saturated hydrocarbons, unsaturated hydrocarbons, saturated cyclic hydrocarbons, and alcohols whose atomic ratio of the component carbon to the component oxygen is more than 2.0 and a catalyst are together treated at a temperature in a range of from 100 to 800° C. while being compressed at a pressure in a range of from 0.2 to 60 MPa, where said starting material is converted into a supercritical fluid or a subcritical fluid while said supercritical fluid or said subcritical fluid being contacted with said catalyst, or a step wherein said starting material, said catalyst and a supplementary material capable of functioning as a reaction promotion medium are together treated at a temperature in a range of from 100 to 800° C. while being compressed at a pressure in a range of from 0.2 to 60 MPa, where at least said supplementary material is converted into a supercritical fluid or a subcritical fluid and said starting material is contacted with said supercritical fluid or said subcritical fluid formed from said supplementary material while being contacted with said catalyst.
Owner:CANON KK
Plasticizer Blends and Use Thereof
Provided are mixtures of 1) compounds of the formula:wherein R1 is a saturated or unsaturated cyclic hydrocarbon optionally substituted with an alkyl and / or an OXO-ester, and R2 is a C4 to C14 hydrocarbyl; and 2) one or more second plasticizers selected from the group consisting of alkyl terephthalates, alkyl phthalates, alkyl benzoate esters, di-benzoate esters, esters of cyclohexane polycarboxylic acids, and dialkyl adipates. Also provided are plasticized polymer compositions containing said mixtures.
Owner:EXXONMOBIL CHEM PAT INC
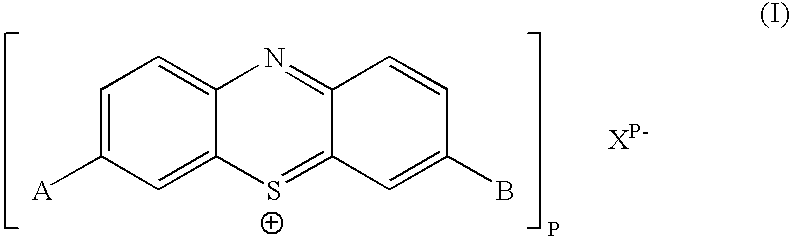
Biologically active methylene blue derivatives
InactiveUS20040147508A1Maintain long-termImprove stabilityAntibacterial agentsOrganic active ingredientsPhotodynamic therapyChemical compound
The present invention relates to a phenothiazinium compound of Formula (I): wherein: A and B each independently is in which R' and R'' each independently is a linear, branched or cyclic hydrocarbon group, or R' and R'' together with the N atom to which they are attached form an optionally substituted 5-, 6- or 7-membered ring; and where X<p-> is a counteranion and P is 1, 2 or 3; except for the compounds in which A and B are both either -N(CH3)2 or -N(CH2CH3)2 for use in a treatment that requires removal, deactivation or killing of unwanted tissues or cells. The invention also relates to compositions comprising the compounds of Formula I, to selected compounds of Formula I, use of the compounds of Formula I as medicaments and as a PDT agent or a photodiagnostic agent, a conjugate or composite formed between a compound of Formula I and a polymer; and to a method for sterilising fluids in which the fluid is passed over the conjugate or composite whilst it is illuminated. The compounds are biologically active photosensitisers which are strongly photocytotoxic and have application in the areas of photodynamic therapy (PDT), as well as for the diagnosis and detection of medical conditions and related uses in photochemical internalisation, in the production of cancer vaccines, in the treatment and prevention of microbial infections and in photodisinfection or photosterilisation.
Owner:PHOTOPHARMICA LTD
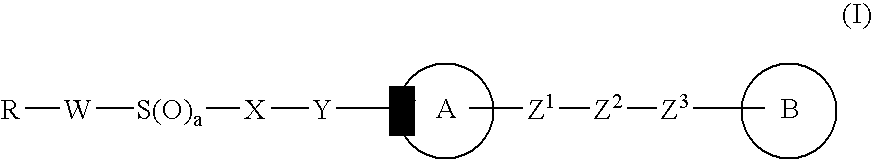
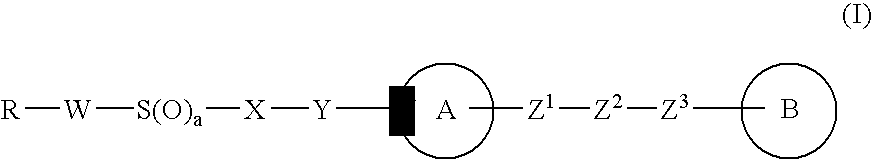
Imidazole derivative, process for producing the same, and use
InactiveUS20070004736A1Improve drug efficacyImproved oral absorbabilityBiocideOrganic chemistryCyclic hydrocarbonDivalent
There is provided an imidazole derivative useful as a thrombosis treating agent, which is represented by the formula (I): wherein R represents an optionally substituted cyclic hydrocarbon group or an optionally substituted heterocyclic group, W represents a bond or an optionally substituted divalent linear hydrocarbon group, X represents an optionally substituted divalent hydrocarbon group, Y represents —CO—, —S(O)—, —S(O)2— or a bond, ring A represents an optionally substituted pyrrolidine ring, an optionally substituted piperidine ring or an optionally substituted perhydroazepine ring, Z1 and Z3 independently represent a bond or an optionally substituted divalent linear hydrocarbon group, Z2 represents —N(R1)—, —O—, —S(O)—, —S(O)2—, —CO—, —CH(R1)— or a bond, ring B represents an optionally substituted imidazole ring, wherein a substituent which the optionally substituted imidazole ring represented by ring B may have may be taken together with R1 to form an optionally substituted ring, and a represents 0, 1 or 2.
Owner:TAKEDA PHARMA CO LTD
Metallocene compounds, catalysts comprising them, process for producing an olefin polymer by use of the catalysts, and olefin homo- and copolymers
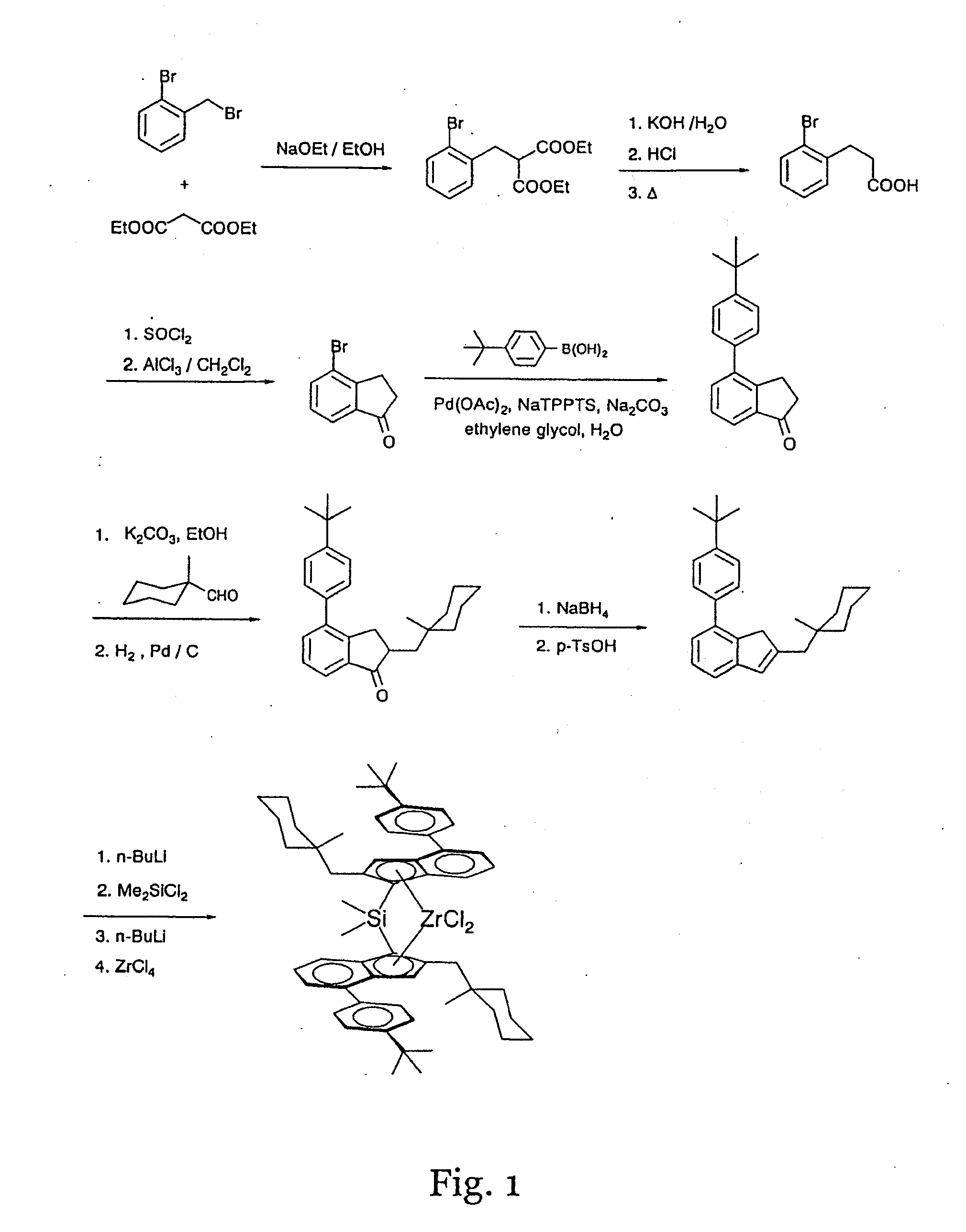
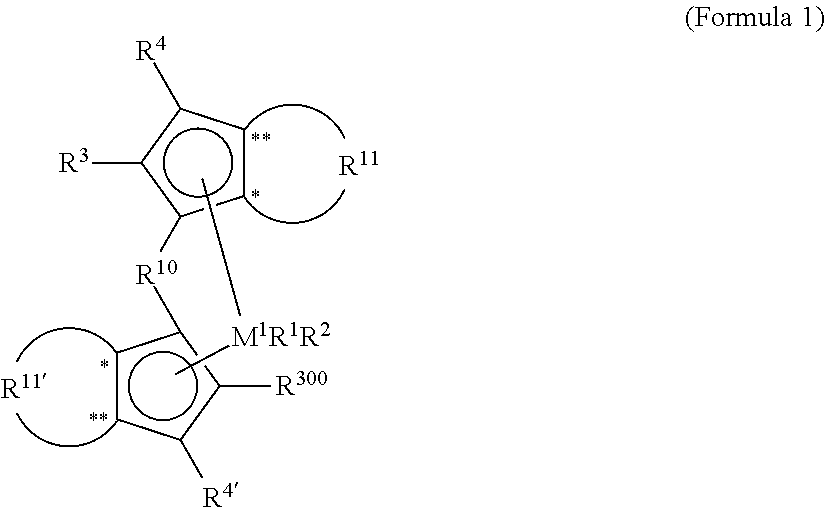
InactiveUS20110230630A1High melting pointQuality improvementOrganic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationPolypropyleneCopolymer
Owner:LUMMUS NOVOLEN TECH
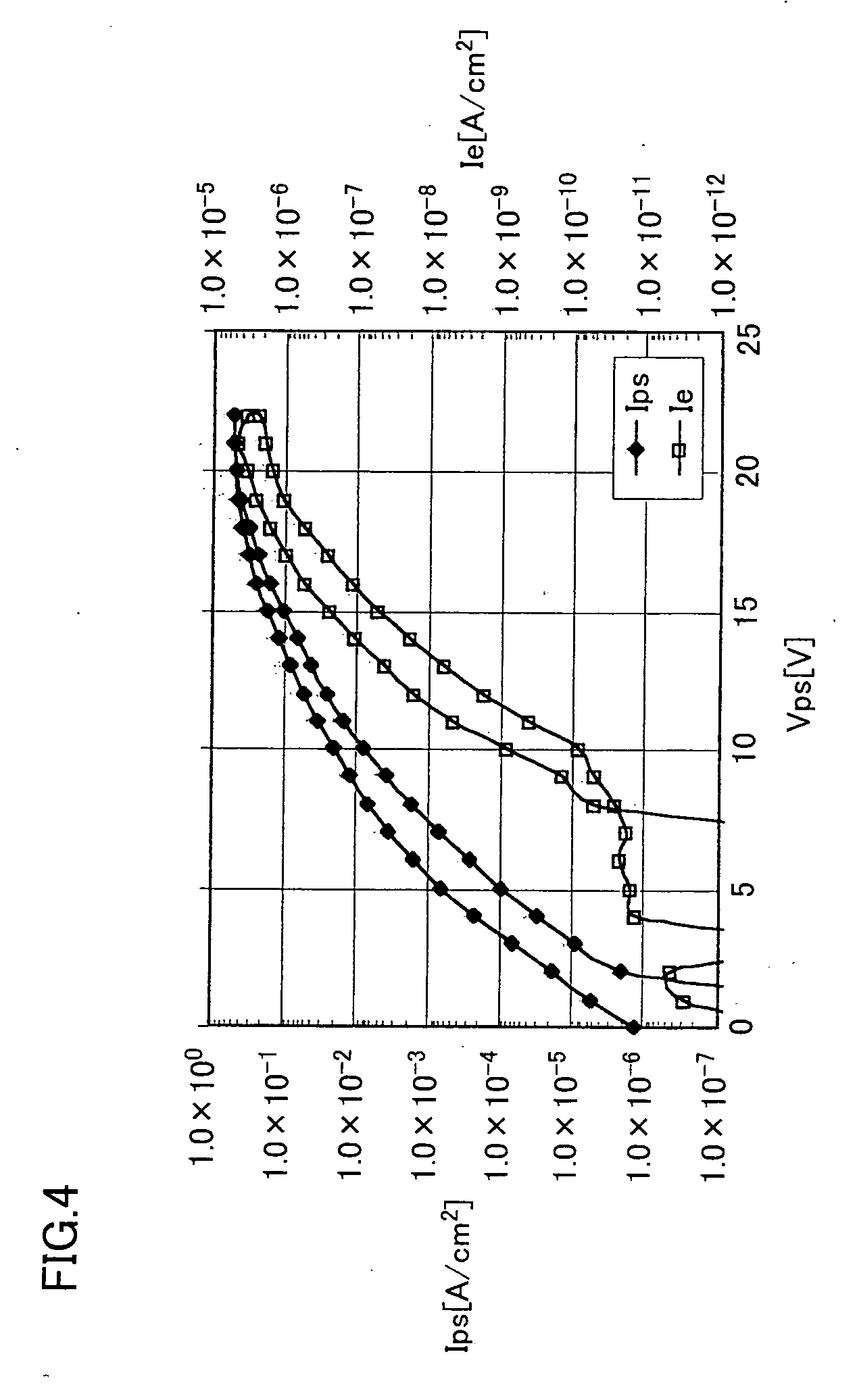
Electron emitting element and image forming apparatus employing it
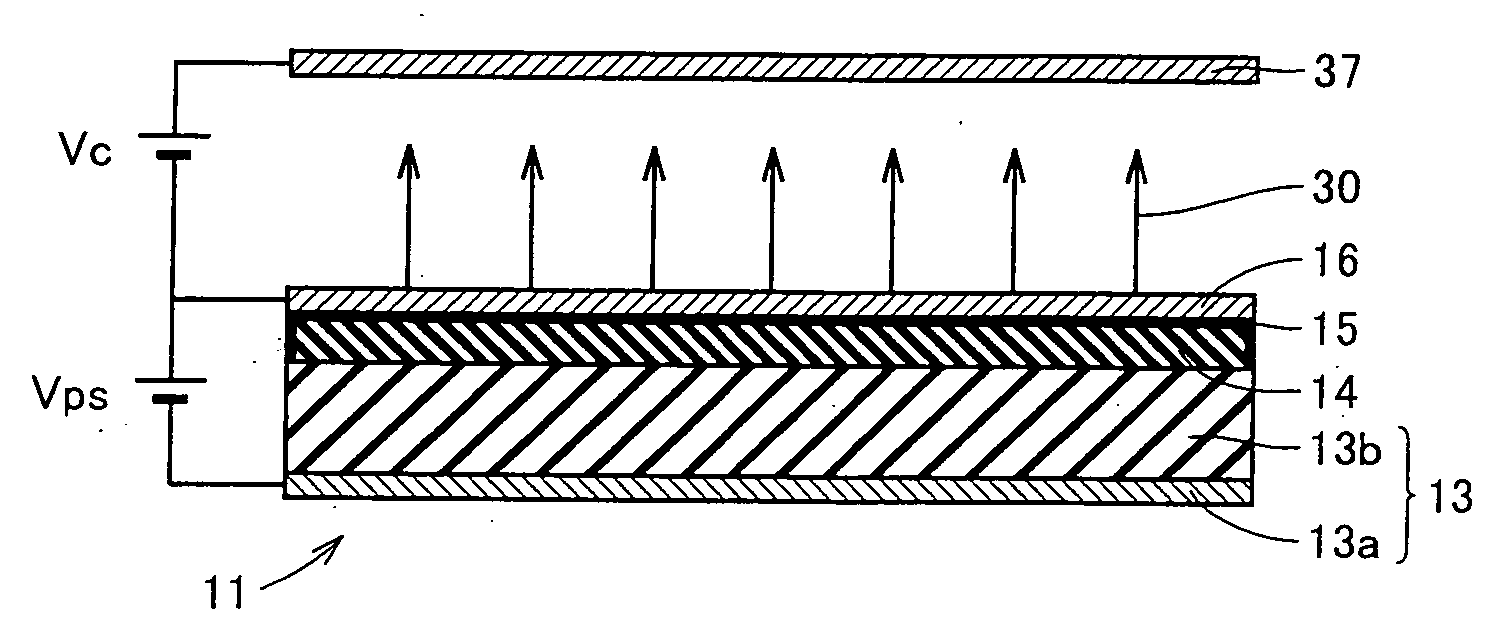
ActiveUS20060186786A1Discharge tube luminescnet screensLamp detailsSimple Organic CompoundsLow vacuum
An electron emitting element is of a structure in which a semiconductor layer is formed between an upper electrode and a lower electrode, wherein an organic compound adsorption layer is formed on a semiconductor surface of the semiconductor layer by causing the organic compound to be adsorbed on the semiconductor surface. Herein, the semiconductor layer can be made of silicon or polysilicon and partly or as a whole porous. The absorbed organic compound can be a non-cyclic hydrocarbon, a compound obtained by coupling at least an aldehyde group to a non-cyclic hydrocarbon, or a non-cyclic hydrocarbon having an unsaturated bond. As a result, there can be provided an electron emitting element capable of stably operating in the atmosphere or in a low vacuum even when being operated in the atmosphere or in the low vacuum and an imaging device using the electron emitting element.
Owner:KOSHIDA NOBUYOSHI +1
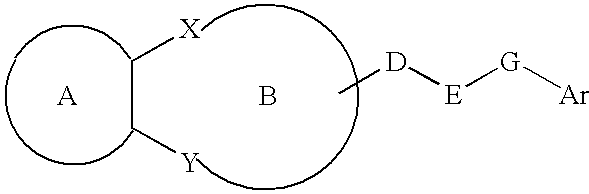
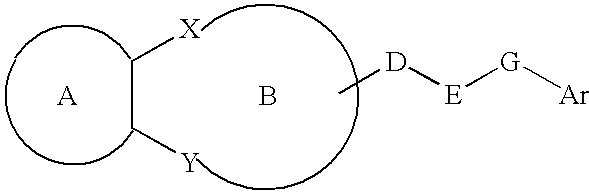
Lipid-rich plaque inhibitors
InactiveUS6974806B2Reduce usageUseful in treatmentBiocideOrganic chemistryArylHydrocotyle bowlesioides
The present invention provides a lipid-rich plaque regressing agent comprising a compound represented by Formula: in which ring A is a cyclic hydrocarbon or the like; ring B is a heterocyclic ring or the like; each of X and Y is —NR1— (in which R1 is a hydrocarbon or the like); D is a C1-3 alkylene group or the like; E is —NH— or the like; G is a bond or the like; and Ar is an aryl or the like; D may be taken together with a constituent atom of the ring B to form a ring, and R4 may be taken together with a constituent atom of the ring B to form a ring.
Owner:TAKEDA PHARMA CO LTD
Resist material and method for forming a resist pattern with the resist material
InactiveUS20030143483A1Precise patternRadiation applicationsSemiconductor/solid-state device manufacturingResistSide chain
A resist material is made of a polymer or copolymer having a cyclic hydrocarbon as a skeletal structure and an alkali-soluble group to which a protective group is attached as a side chain. Because of the protective group, the resist material is insoluble in alkali solution. In addition, an acid generating agent is added to the resist material. When the acid generating agent is irradiated with a radiation ray, an acid is generated from the acid generating agent, and the protective group is detached from the alkali-soluble group by the function of the acid. Therefore, a resist film made of the resist material can be formed in a desired pattern by irradiating the resist film with the radiation ray. Also, because the cyclic hydrocarbon is used as the skeletal structure of the polymer or copolymer, a superior dry-etching resistance is obtained as compared with a conventional resist material in which acrylate resin is used as a skeletal structure, so that there is no probability that a patterned resist film is over-etched or deformed even though the patterned resist film is used as a mask in an etching process.
Owner:FUJITSU SEMICON LTD
Radiation-sensitive resin composition
A radiation-sensitive resin composition comprising (A) an acid-dissociable group-containing polysiloxane and (B) a photoacid generator containing trifluoromethane sulfonic acid or a compound which generates an acid of the following formula (I),wherein Rf individually represents a fluorine atom or a trifluoromethyl group, and Ra represents a hydrogen atom, a fluorine atom, a linear or branched alkyl group having 1-20 carbon atoms, or a linear or branched fluoroalkyl group having 1-20 carbon atoms, a substituted or unsubstituted monovalent cyclic hydrocarbon group having 3-20 carbon atoms, or a substituted or unsubstituted monovalent cyclic fluoro-hydrocarbon group having 3-20 carbon atoms. The radiation-sensitive resin composition of the present invention exhibits superior resolution, while maintaining high transparency to radiations and high dry etching resistance. The resin composition thus can greatly contribute to the lithography process that will become more and more minute in the future.
Owner:JSR CORPORATIOON
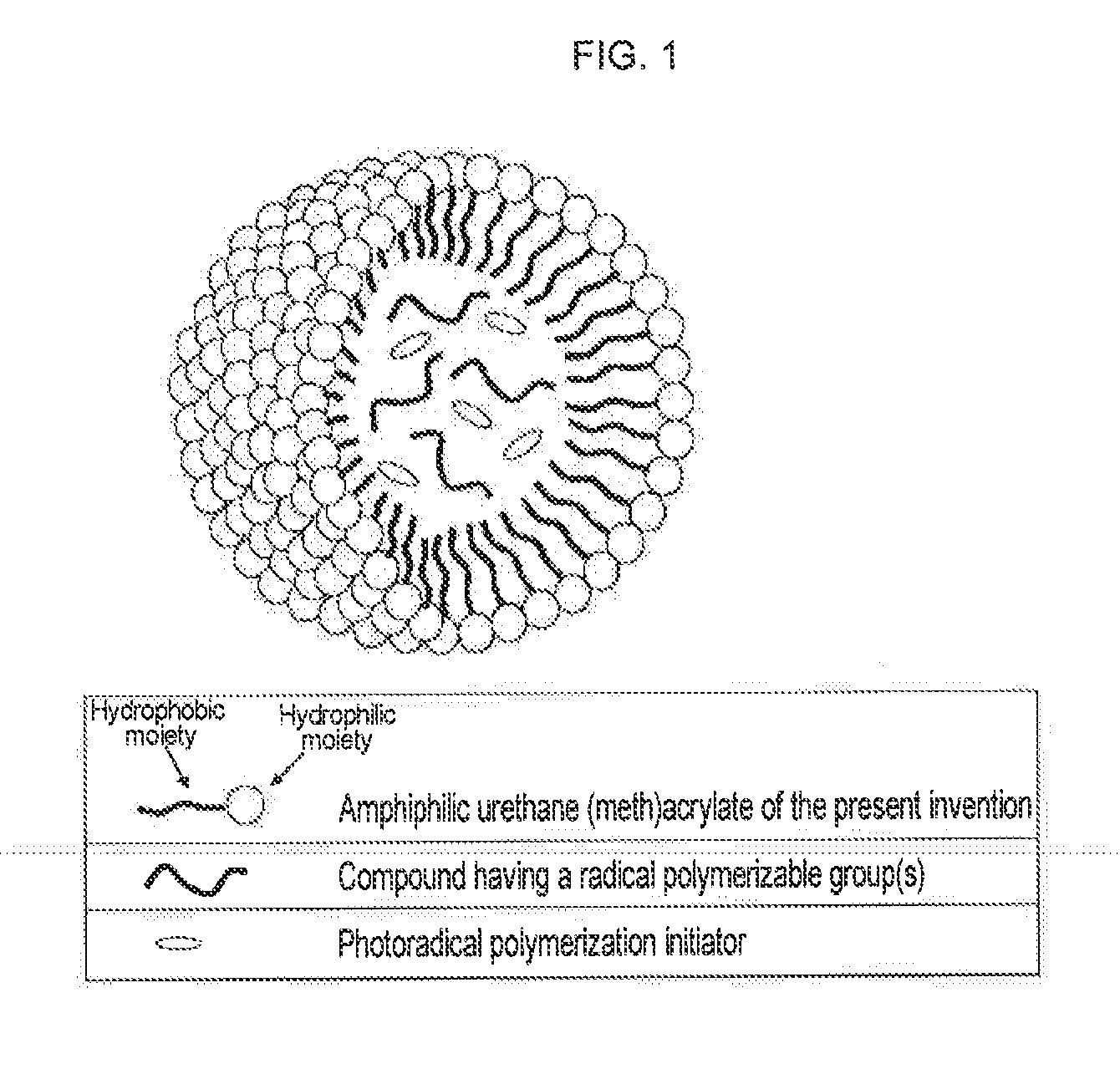
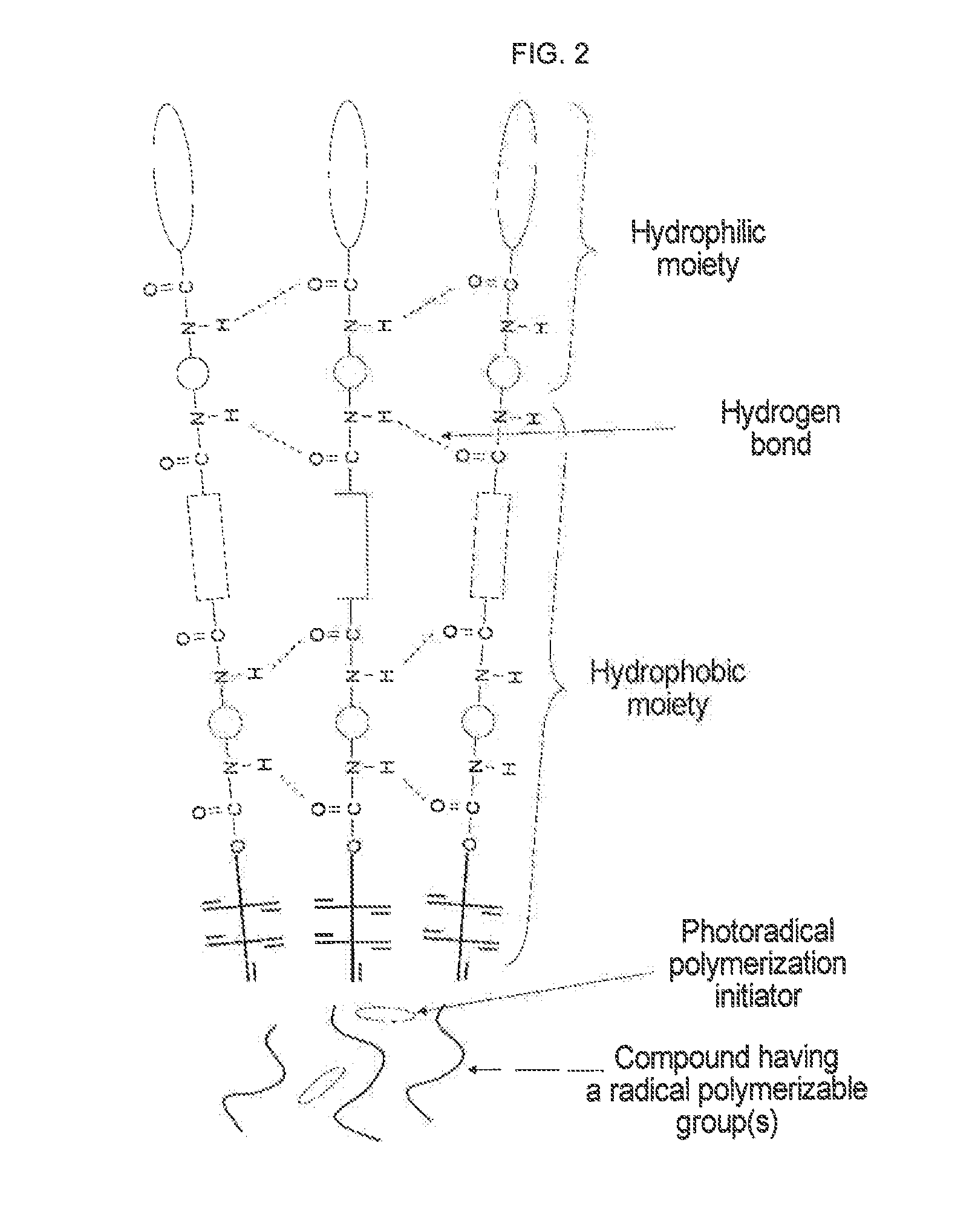
Urethane (METH) acrylate and production method thereof, cross-linked urethane (METH) acrylate and production method thereof, and light curable aqueous emulsion
InactiveUS20120225969A1High strengthGood dispersibilityPolyurea/polyurethane coatingsThermographyCross-linkEmulsion
For the purpose of providing a urethane(meth)acrylate excellent in emulsifiability in water and a production method thereof, and a light curable aqueous emulsion using the urethane(meth)acrylate having a low viscosity and excellent in the curability, provided is a urethane(meth)acrylate being represented by the following general formula (1) and having a weight average molecular weight of 1,000 to 10,000:A1-O—(CONH—B1-NHCOO—C1-O)n-CONH—B1-NH—COO-D1 (1)wherein in formula (1), n represents a natural number of 1 to 30, A1 represents a residue of a hydroxyl group-containing (meth)acrylate, B1 represents a residue of diisocyanate, C1 represents a residue of a diol of an acyclic hydrocarbon or a cyclic hydrocarbon, and D1 represents a residue of a polyoxyalkylene glycol monoalkyl ether.
Owner:SEIKO EPSON CORP +1
Arylalkylamine Compound and Process for Preparing the Same
The present invention relates to an arylalkylamine compound represented by the following formula [I] or a pharmaceutically acceptable salt thereof, a process for preparing the same, and use of the above-mentioned compound as an activating compound (CaSR agonist) of a Ca sensing receptor, a pharmaceutical composition containing the above-mentioned compound as an effective ingredient, etc. The symbols in the formula represent the following meanings: Ar: optionally substituted aryl or optionally substituted heteroaryl here, the cyclic portion of the heteroaryl is bicyclic heterocyclic ring in which 5- to 6-membered monocyclic heterocyclic ring containing 1 or 2 hetero atom(s) and benzene ring are fused; R1: a group selected from the group consisting of optionally substituted cyclic hydrocarbon group, and optionally substituted heterocyclic group; n: an integer of 1 to 3; X: single bonding arm, —CH2—, —CO—, —(CH2)m—CO—, —CH(R2)—CO—, —(CH2)p—Y—(C(R3)(R4))q—CO—, —NH—CO— or —N(R5)—CO—; in the above-mentioned respective definitions of the X, the bonding arm described at the left end represents a bond with R1; m is an integer of 1 to 3; p is an integer of 0 to 2; q is an integer of 0 to 2; Y: —O— or —SO2—; R2: phenyl or lower alkyl; R3, R4: each independently represents hydrogen atom or lower alkyl; R5: lower alkyl; provided that the ring portion of the group represented by R1 is neither naphthylidine nor partially saturated group thereof, and, when X is —CH2— or —CO—, R1 is not naphthyl.
Owner:MITSUBISHI TANABE PHARMA CORP
Celastrol derivative and preparation method thereof and application of celastrol derivative to preparation of antitumor medicine
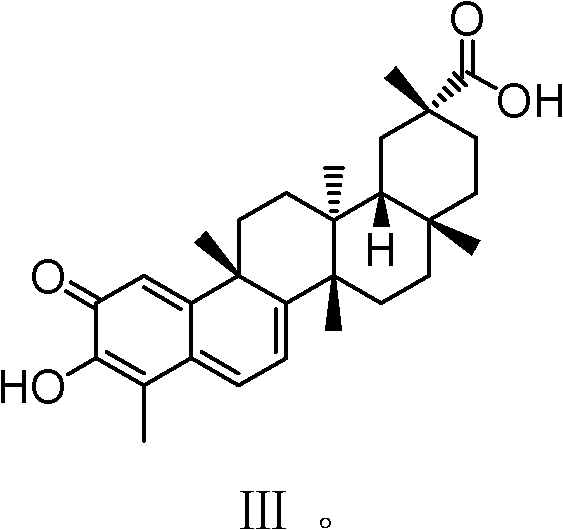
ActiveCN102432663AImprove water solubilityImprove bioavailabilityDigestive systemSteroidsChemistryOrganic acid
The invention relates to celastrol, a celastrol derivative, a method for preparing the celastrol derivative, biogenetic salt prepared from the celastrol derivative serving as a raw material, and application of the celastrol derivative to the preparation of an antitumor medicine. The celastrol derivative has a structure shown as a formula I, wherein R1 is H, C1-C6 straight-chain or branched-chain alkyl, benzyl and benzyl with a substituent group on a benzene ring; and R2 or R3 are independent C5-C6 cyclic hydroxyl, C1-C6 chain alkyl, phenyl and substituted phenyl respectively, or R2 and R3 are cyclized with N, and the cycle is hexahydric cyclic heterocycle containing N or O. The celastrol derivative has high antitumor activity, stability and water solubility. The celastrol derivative can be salified with one of medically acceptable inorganic acids (such as hydrochloric acid, sulfuric acid and phosphoric acid) or organic acids (citric acid, cinnamic acid, succinic acid and the like); and the salt has high water solubility.
Owner:ZHEJIANG UNIV OF TECH
Thermal transfer ink sheet, ink cartridge, coating composition for dye layer of thermal transfer ink sheet, and thermal transfer recording method
ActiveUS8114813B2Inhibited DiffusionAvoid stickingDecorative surface effectsAblative recordingHydrogen atomPhotochemistry
A thermal transfer ink sheet comprising, as formed on a support, a dye layer containing a thermal transferable dye in a resin binder, wherein the dye layer contains a polyvinyl acetal modified with at least one compound of the following formula [1]:wherein one of R1 and R2 is a branched hydrocarbon group and the other is a hydrogen atom or a hydrocarbon group, or R1 and R2 are groups that bond to each other to form a cyclic hydrocarbon.
Owner:FUJIFILM CORP
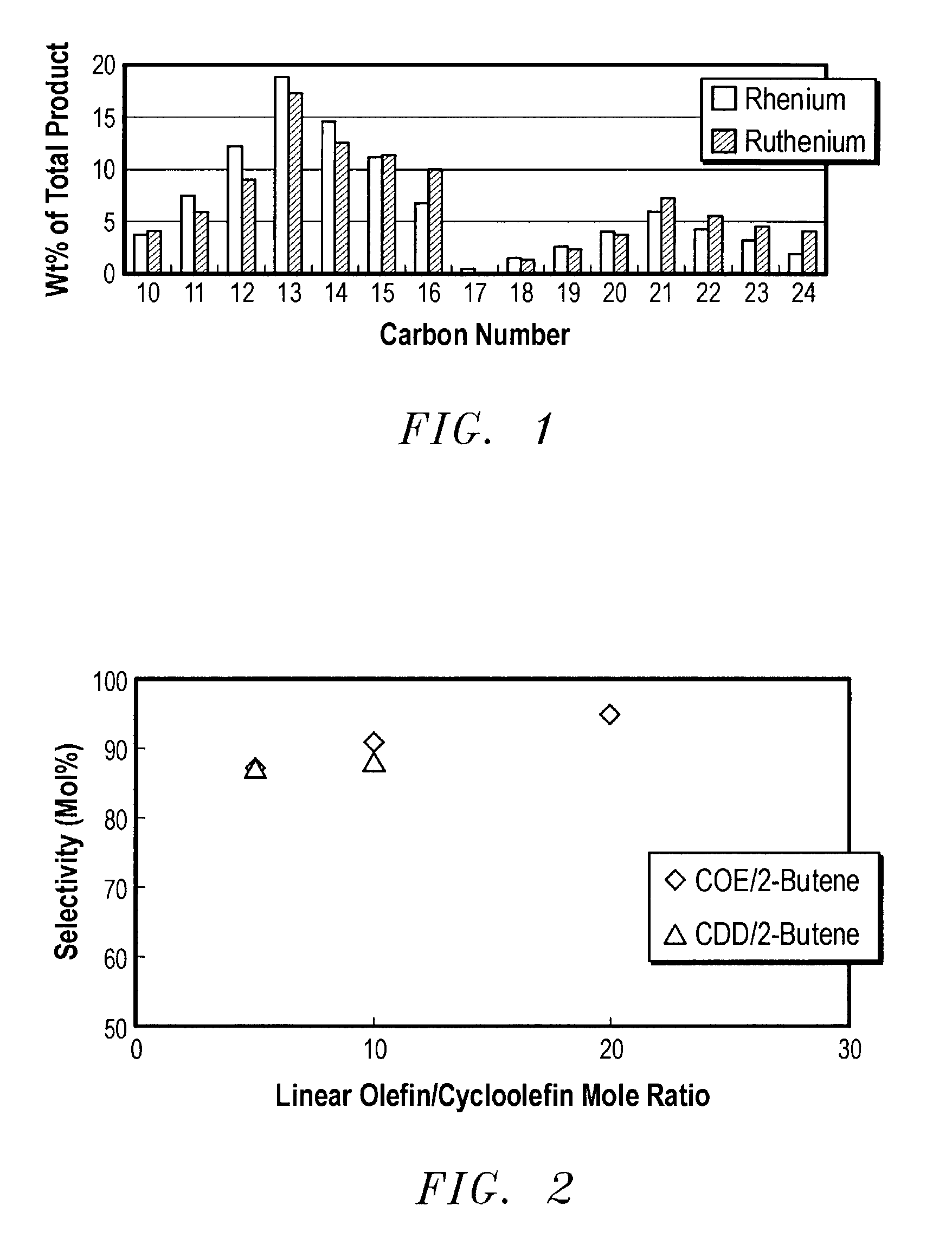
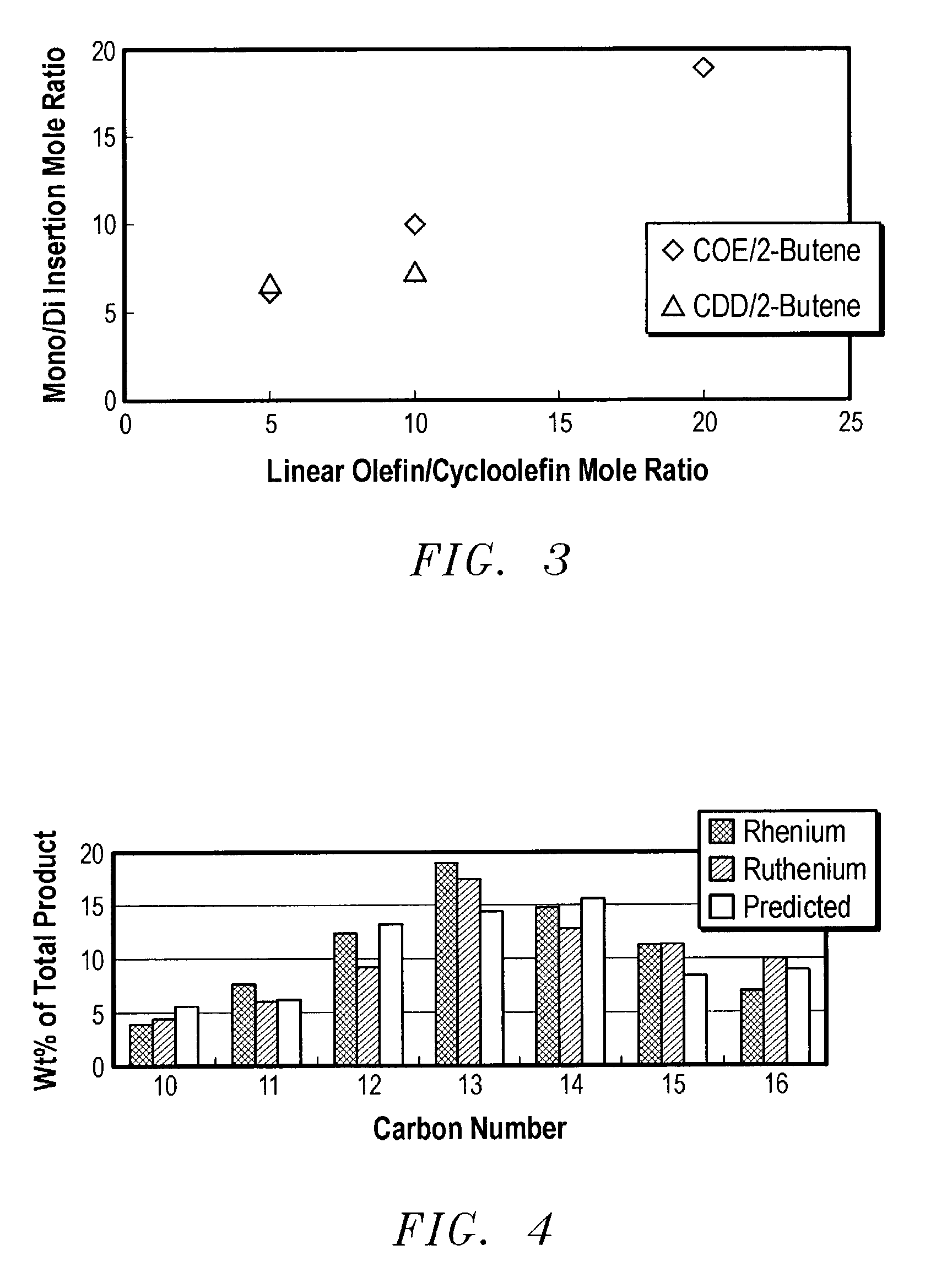
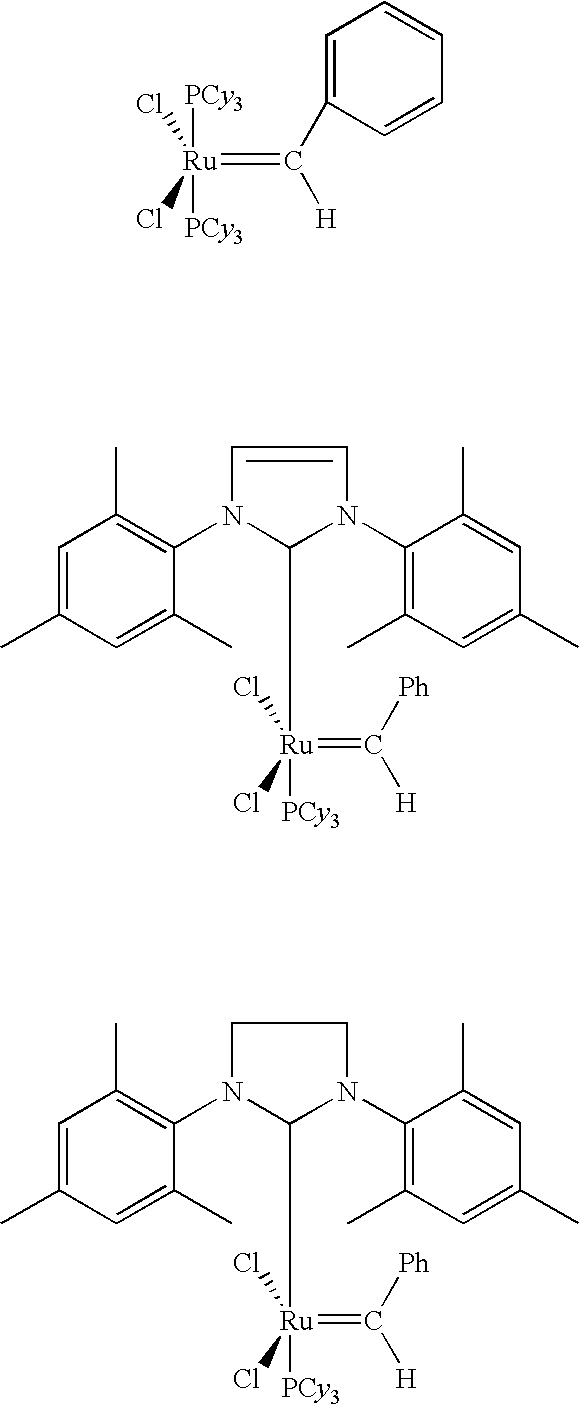
Linear and branched olefin production from cyclic olefin feedstocks
InactiveUS7041864B2Chemical industryHydrocarbon by metathesis reactionUnsaturated hydrocarbonCyclic Olefins
Ring opening cross metathesis of secondary non-cyclic hydrocarbons with cyclic unsaturated hydrocarbons having 8 carbon atoms or more to produce corresponding unsaturated product hydrocarbons having more than 8 carbon atoms.
Owner:SHELL OIL CO
Homoadamantane derivative, method for producing the same and photosensitive materials for photoresist
InactiveUS20130022914A1Reduced compatibilityReduce roughnessOrganic chemistryPhotosensitive materialsHalogenHydrogen atom
A homoadamantane derivative represented by the following formula (I): wherein R1 and R2 are independently a hydrogen atom or a linear, branched or cyclic hydrocarbon group having 1 to 6 carbon atoms, x is a hydroxyl group or a halogen atom, and n and m are independently an integer of 0 to 3, provided that n and m are not simultaneously 0.
Owner:OSAKA ORGANIC CHEM INDS
Diamine derivatives
ActiveUS7576135B2Enhanced inhibitory effectPreventing and treating thrombosisBiocideOrganic chemistryExtracorporeal circulationDisease
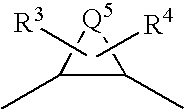
A compound represented by formula (1):Q1-Q2-T0-N(R1)-Q3-N(R2)-T1-Q4 (1)[wherein R1 and R2 are hydrogen atoms or the like; Q1 is a saturated or unsaturated, 5- or 6-membered cyclic hydrocarbon group which may be substituted, or the like; Q2 is a single bond or the like; Q3 represents the following group:(wherein Q5 is an alkylene group having 1 to 8 carbon atoms, or the like); and T0 and T1 are carbonyl groups or the like], a salt thereof, a solvate thereof, or an N-oxide thereof.The compound is useful as an agent for preventing and / or treating cerebral infarction, cerebral embolism, myocardial infarction, angina pectoris, pulmonary infarction, pulmonary embolism, Buerger's disease, deep venous thrombosis, disseminated intravascular coagulation syndrome, thrombus formation after artificial valve or joint replacement, thrombus formation and reocclusion after angioplasty, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), thrombus formation during extracorporeal circulation, or blood clotting upon blood drawing.
Owner:DAIICHI SANKYO CO LTD
Liquid for immersion exposure and immersion exposure method
InactiveUS20070164261A1Improve hydrophobicityHigh refractive indexDiffusing elementsSemiconductor/solid-state device manufacturingRefractive indexElution
An immersion exposure liquid which exhibits a high refractive index, prevents elution and dissolution of a photoresist film or its upper layer film component, and reduces defects during formation of a resist pattern when used in an immersion exposure method, and an immersion exposure method using the immersion exposure liquid. The immersion exposure liquid is used for an immersion exposure device or an immersion exposure method in which a substrate is exposed through a liquid provided between a lens of a projection optical system and the substrate, the immersion exposure liquid being liquid in an operating temperature range of the immersion exposure device and including an alicyclic hydrocarbon compound or a cyclic hydrocarbon compound containing a silicon atom in the ring structure.
Owner:JSR CORPORATIOON
Aziridinosilicones and the use thereof
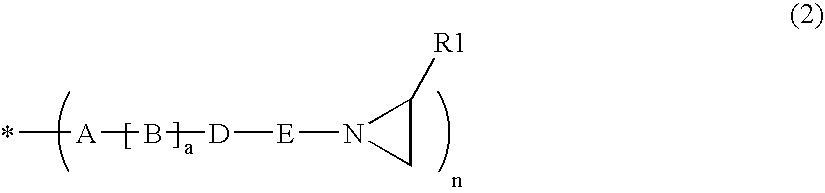
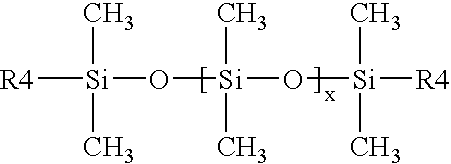
InactiveUS6906117B2Promote reproductionImprove accuracyImpression capsDental impression compositionsHeteroatomMedicinal chemistry
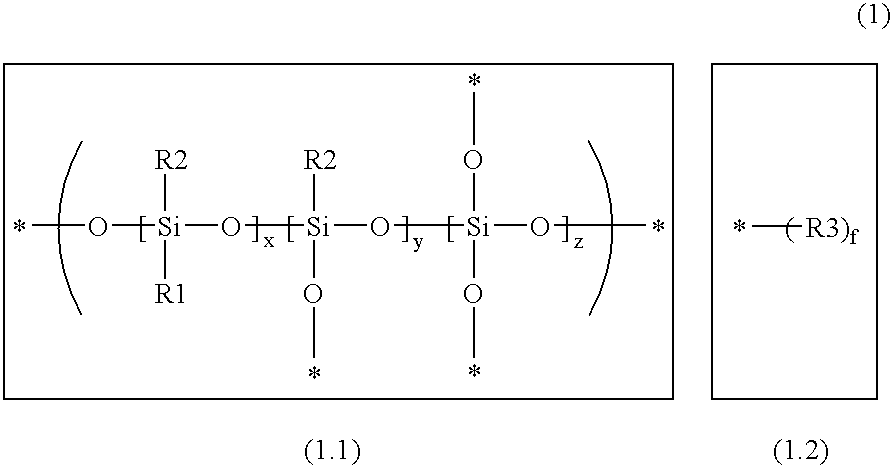
Arizidinosilicones of the general formula (1), wherein R1 represents H, C1-C12 alkyl, C2-C12 alkenyl, C2-C12 alkinyl, C7-C15 alkaryl, C7-C15 aralkyl, C3-C12 cycloalkyl and these groups can be substituted with Cl or F partially, completely or in a mixed manner and / or may contain 0 to 5 heteroatoms selected from O, N, S, R2 represents a group of the selection of R1 and / or R4, and R3 represents SiRl3 or SiR12R4, and wherein R4 represents formula (2), and A represents an (n+1) radical saturated, unsaturated or aromatic, linear, branched or cyclic hydrocarbon group that may contain 0 to 5 heteroatoms selected from O, N, S and that includes 1 to 18 carbon atoms, B is selected from O, S, NR1, D is selected from C(O)O, C(O)NR1, C(O), C(O)C(O), C(O)(CH2)m(C(O), C(S)NR1, CH2, E represents a diradical saturated or unsaturated, linear, branched or cyclic hydrocarbon group that may contain 0 to 5 heteroatoms selected from O, N, S and that includes 0 to 18 carbon atoms, a is 0 or 1, f is an integer from 2 to 1000, n, m is an integer from 1 to 10, and x, y, z each represents 0 or an integer, the sum of which should range between 1 and 10000, with the proviso that, if x is larger 0, y or z is smaller or equal x, preferably smaller or equal 0.05 times x, and especially preferred 0.02 times x. The invention also relates to the use of arizidinosilicones.
Owner:3M INNOVATIVE PROPERTIES CO
Anti-smudge body, display device, input device, and electronic device
InactiveUS20150239023A1Fouling preventionAntifouling/underwater paintsComputer graphics (images)Display device
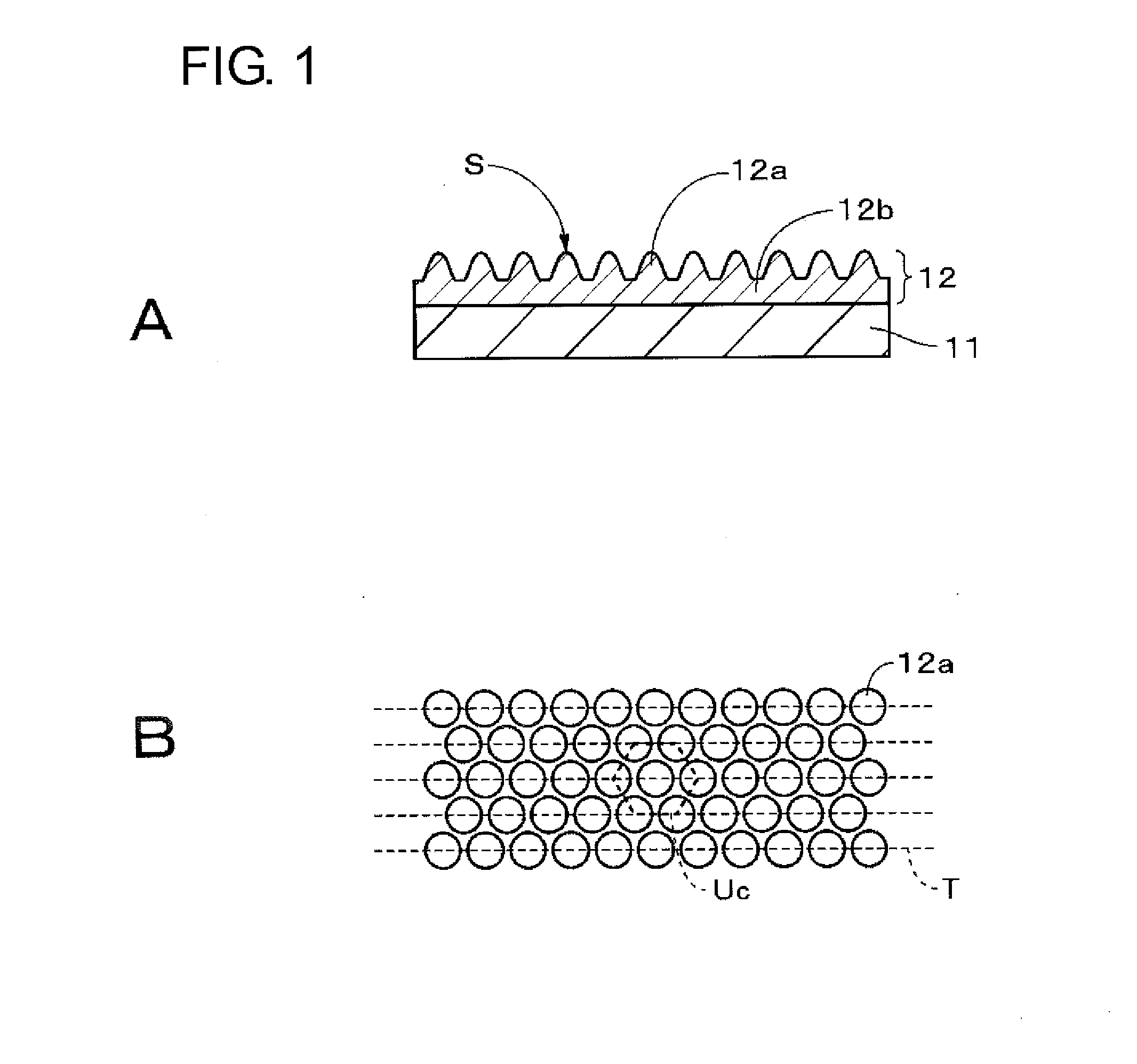
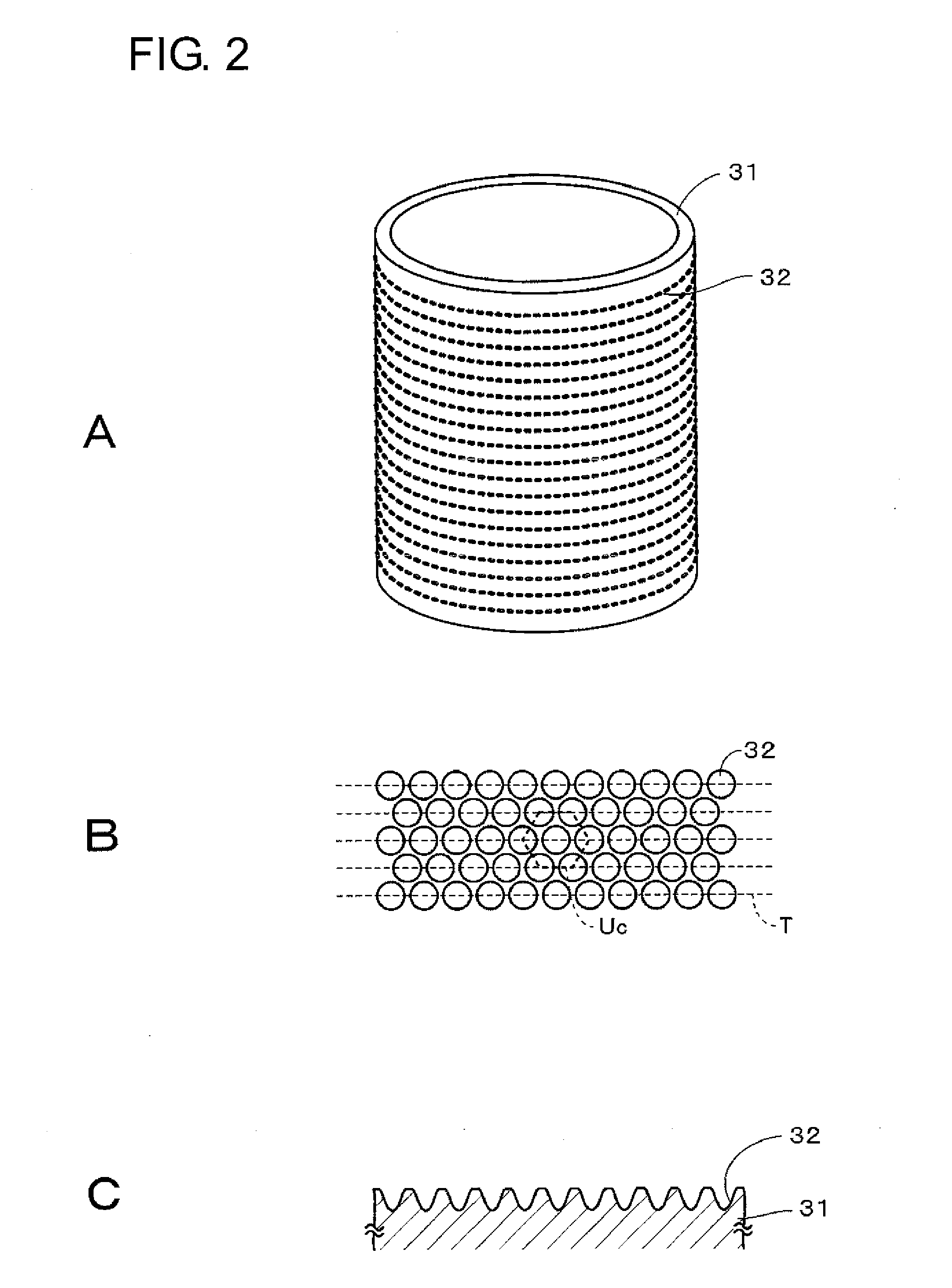
An anti-smudge body having a surface that, when fingerprints adhere to the surface, allows the fingerprint patterns to spread spontaneously to thereby cause the adhering fingerprints to become less noticeable has a surface and a fine irregular structure provided to the surface, wherein the irregular structure contains at least one of a first compound having an ester linkage in a portion other than terminal ends and a second compound having a cyclic hydrocarbon group.
Owner:DEXERIALS CORP
Nonaqueous electrolytes and nonaqueous-electrolyte secondary batteries employing the same
A nonaqueous electrolyte containing a monofluorophosphate and / or a difluorophosphate and a compound having a specific chemical structure or specific properties. The nonaqueous electrolyte can contain at least one of a saturated chain hydrocarbon, a saturated cyclic hydrocarbon, an aromatic compound having a halogen atom and an ether having a fluorine atom.
Owner:MU IONIC SOLUTIONS CORP
Ink composition, inkjet recording method, printed material, and process for producing lithographic printing plate
InactiveUS7553605B2Improve curing effectGood flexibilityPhotosensitive materialsPretreated surfacesPhotochemistryDivalent
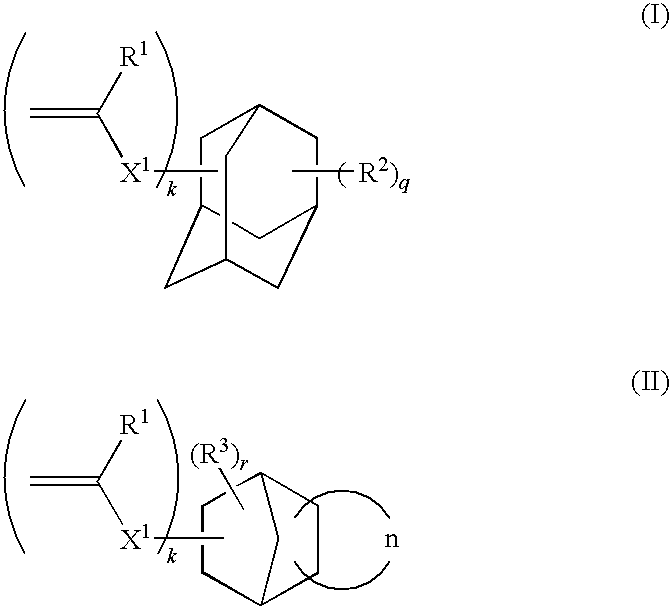
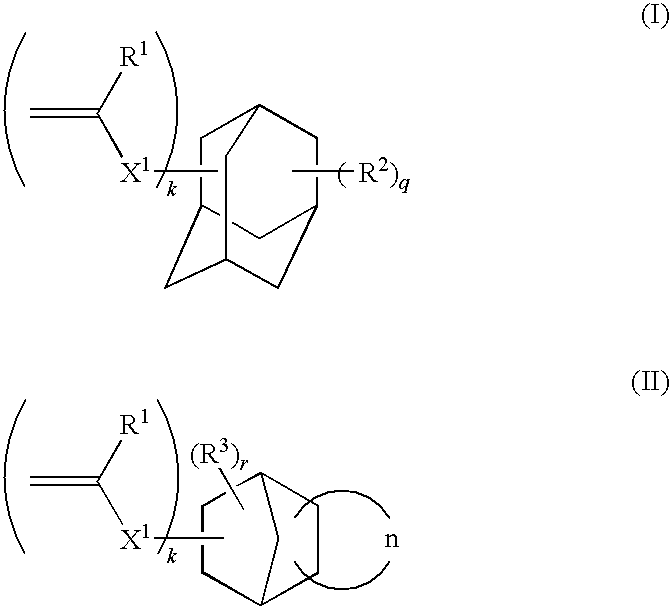
An ink composition is provided that includes (A) an N-vinyllactam, (B) a monomer represented by Formula (I), and (C) a radical polymerization initiator, or includes (A) an N-vinyllactam, (B) a monomer represented by Formula (II), (C) a radical polymerization initiator, and phenoxyethyl acrylate.(In Formula (I) and Formula (II), R1 denotes a hydrogen atom, a halogen atom, or an alkyl group having 1 to 4 carbons, X1 denotes a divalent linking group, R2 and R3 independently denote a substituent, k denotes an integer of 1 to 6, q and r independently denote an integer of 0 to 5, n denotes a cyclic hydrocarbon structure, the cyclic hydrocarbon structure may comprise in addition to hydrocarbon bonds a carbonyl bond (—C(O)—) and / or an ester bond (—C(O)O—), and the k R1s, the k X1s, the q R2s, and the r R3s may each be identical to or different from each other; furthermore, one carbon atom in the adamantane framework in Formula (I) may be replaced by a carbonyl bond (—C(O)—) and / or an ester bond (—C(O)O—), and one carbon atom in the norbornene framework in Formula (II) may be replaced by an ether bond (—O—) and / or an ester bond (—C(O)O—).) There are also provided an inkjet recording method, a printed material, and a process for producing a lithographic printing plate that employ the ink composition.
Owner:FUJIFILM CORP
Resist composition and method for forming resist pattern
ActiveUS20170369697A1High sensitivityReduce roughnessPhotomechanical exposure apparatusPhotosensitive material processingPolymer scienceUnsaturated hydrocarbon
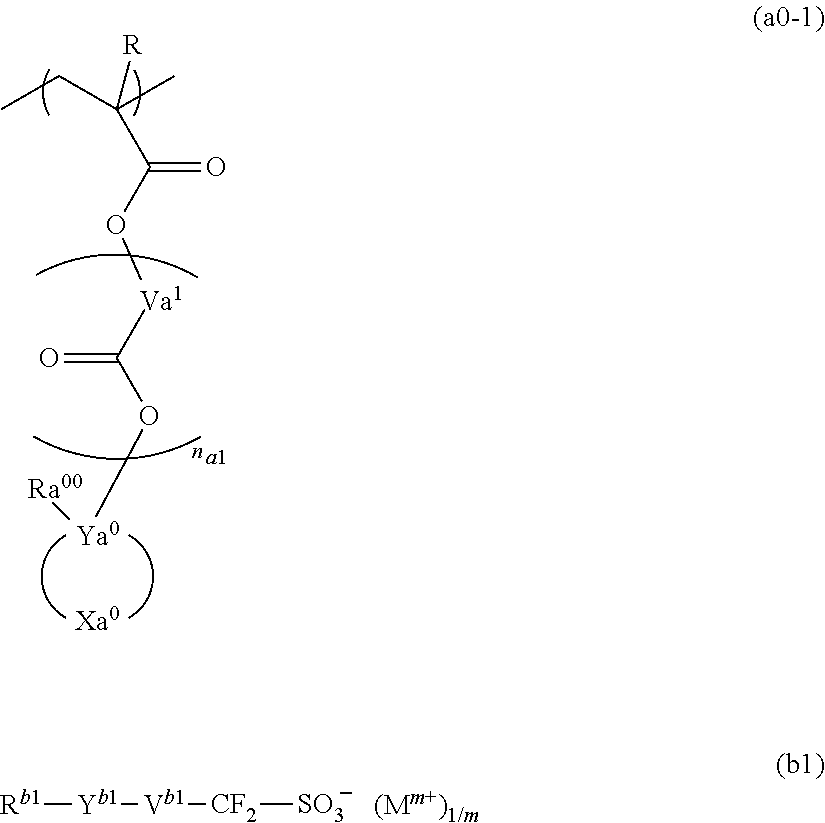
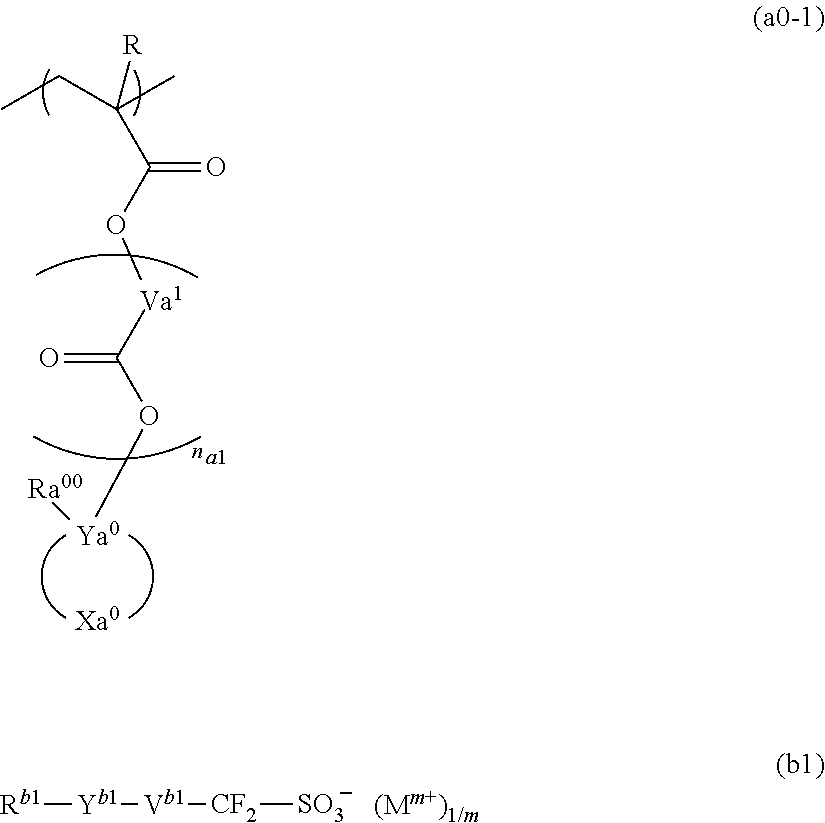
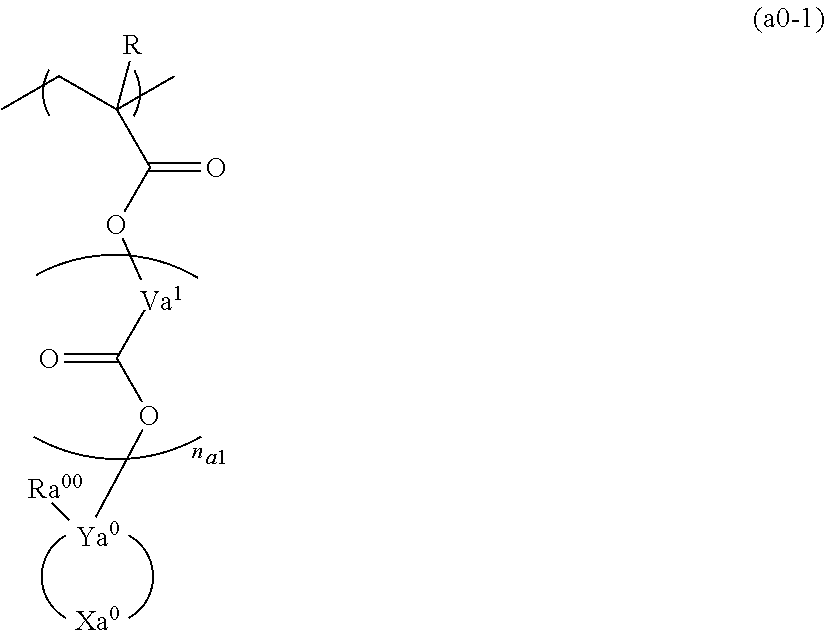
A resist composition containing a resin component having a structural unit represented by general formula (a0-1), and a compound represented by general formula (b1). In general formula (a0-1), R is a hydrogen atom, an alkyl group, or a halogenated alkyl group, Va1 is a divalent hydrocarbon group, na1 represents an integer of 0 to 2, Ya0 is a carbon atom, Xa0 is a group forming a monocyclic aliphatic hydrocarbon group together with Ya0, and Ra00 is an aromatic hydrocarbon group or a specific unsaturated hydrocarbon group. In general formula (b1), Rb1 represents a cyclic hydrocarbon group, Yb1 represents a divalent linking group containing an ester bond, Vb1 represents an alkylene group, a fluorinated alkylene group, or a single bond, and Mm+ is an m-valent organic cation.
Owner:TOKYO OHKA KOGYO CO LTD
Low-temperature aqueous-phase catalyst for lignin phenol derivative hydrodeoxygenation and preparation method thereof
InactiveCN104624225AHigh selectivityGood choiceMolecular sieve catalystsHydrocarbon from oxygen organic compoundsAlkanePhenol derivative
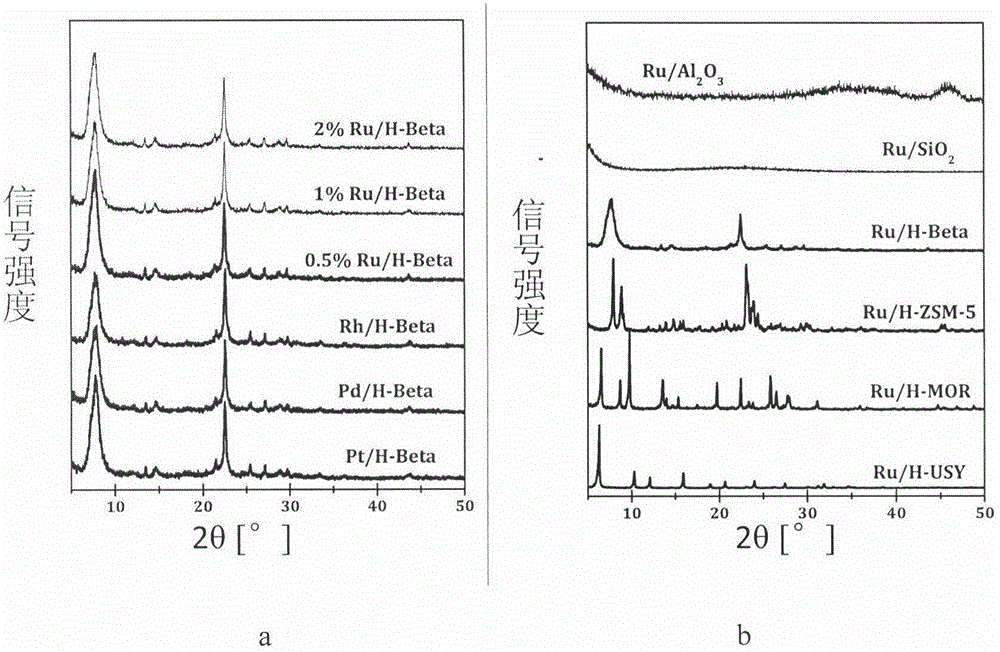
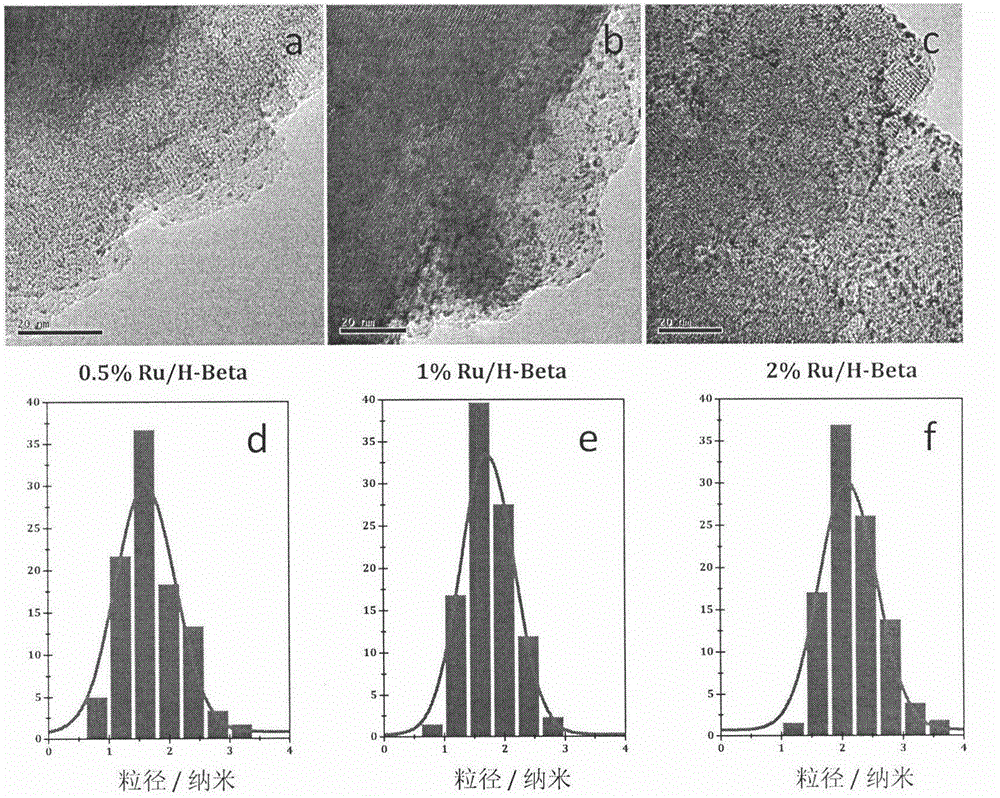
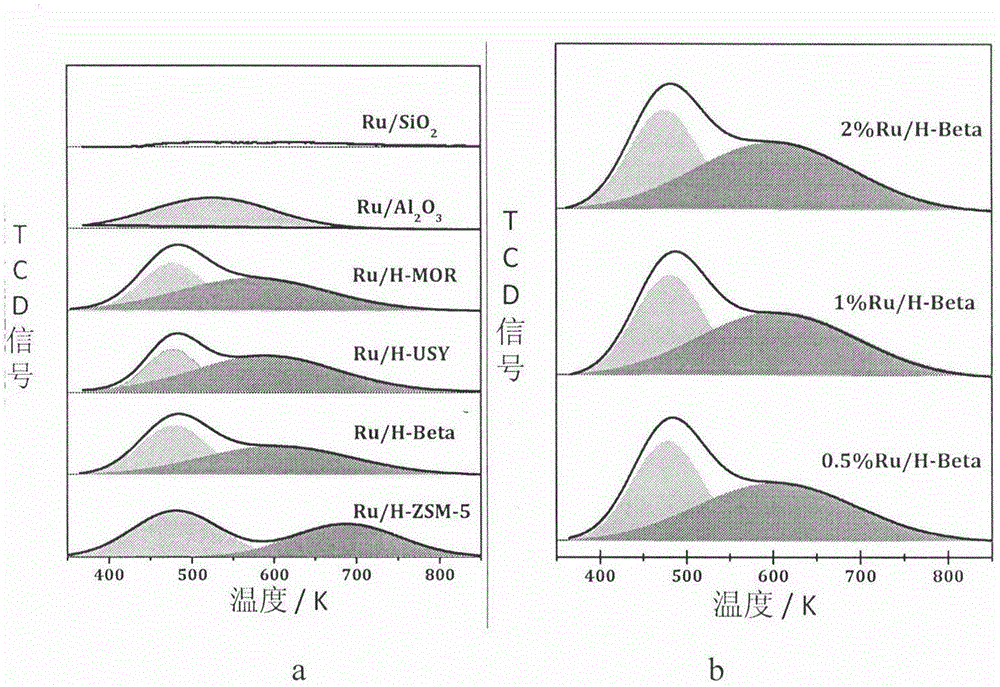
The invention relates to a low-temperature aqueous-phase catalyst for lignin phenol derivative hydrodeoxygenation and a preparation method thereof. The catalyst comprises a zeolite molecular sieve HBeta serving as a carrier and a load Ru, wherein the molar ratio of silicon to aluminum is 10-50, and the loading amount of Ru is 0.1 to 2%. The preparation method comprises the following steps: mixing measured aqueous solution of RuCl3 with a zeolite suspension, evaporating water and drying, and then reducing the obtained solid with hydrogen / argon mixed gas. A cyclic hydrocarbon (the conversion rate is greater than 98% and the selectivity is greater than 95%) is prepared at high selectivity under the relatively mild (100-150 DEG C and 1-4MPa) condition, so that the defects that the reaction conditions are harsh and the energy efficiency is low in the refining process of biomass pyrolysis oil can be overcome. The bifunctional catalyst provided by the invention has the advantages of simplicity and easiness in operation in the preparation process, stable physical and chemical properties, and direct application. At low temperatures, the catalyst shows excellent hydrodeoxygenation activity and extremely high selectivity of saturated hydrocarbons, has a simple and environment-friendly reaction process, and can be widely used in the refining of bio-gasoline.
Owner:NANKAI UNIV
Morpholine derivatives
InactiveUS20060241302A1Excellent inhibitory activityStrong inhibitory activityBiocideNervous disorderΒ amyloid proteinOxide
Provided is a compound capable of inhibiting production or secretion of β amyloid protein. A compound represented by the following formula (1): (wherein, R1 represents a heterocyclic group which may have a substituent, R2 represents a cyclic hydrocarbon group which may have a substituent or a heterocyclic group which may have a substituent, R3 represents a cyclic hydrocarbon group which may have a substituent or a heterocyclic group which may have a substituent, R4 represents a hydrogen atom or a C1-6 alkyl group, and X represents —S—, —SO— or —SO2—); an N-oxide or S-oxide thereof; a salt thereof; or a solvate thereof; and a medicament containing any of them.
Owner:DAIICHI PHARMA CO LTD
Positive resist composition
InactiveUS7179578B2High sensitivitySmall defocus latitude depended on line pitchPhotosensitive materialsRadiation applicationsEtchingSide chain
To provide a positive resist composition having high sensitivity, small defocus latitude depended on line pitch and less surface roughening at the etching, which can be suitably used for micro-photofabrication using far ultraviolet ray, particularly, ArF excimer laser ray.A positive resist composition comprising (A) a resin containing specific two kinds of repeating units, which has an aliphatic cyclic hydrocarbon group on the side chain and increases the dissolution rate in an alkali developer under the action of an acid, and (B) a specific compound capable of generating an acid upon irradiation with actinic rays or radiation, or a positive resist composition comprising (A) two kinds of resins as the resin having an aliphatic cyclic hydrocarbon group on the side chain and capable of increasing the dissolution rate in an alkali developer under the action of an acid, and (B) a compound capable of generating an acid upon irradiation with actinic rays or radiation.
Owner:FUJIFILM HLDG CORP +1
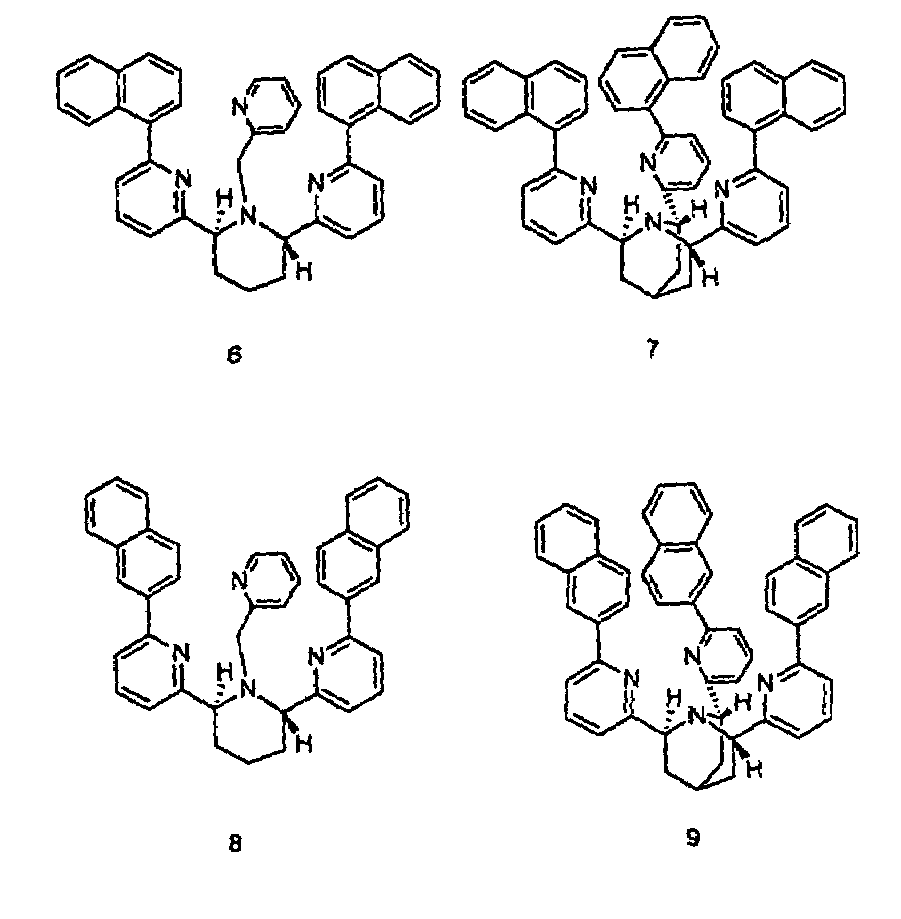
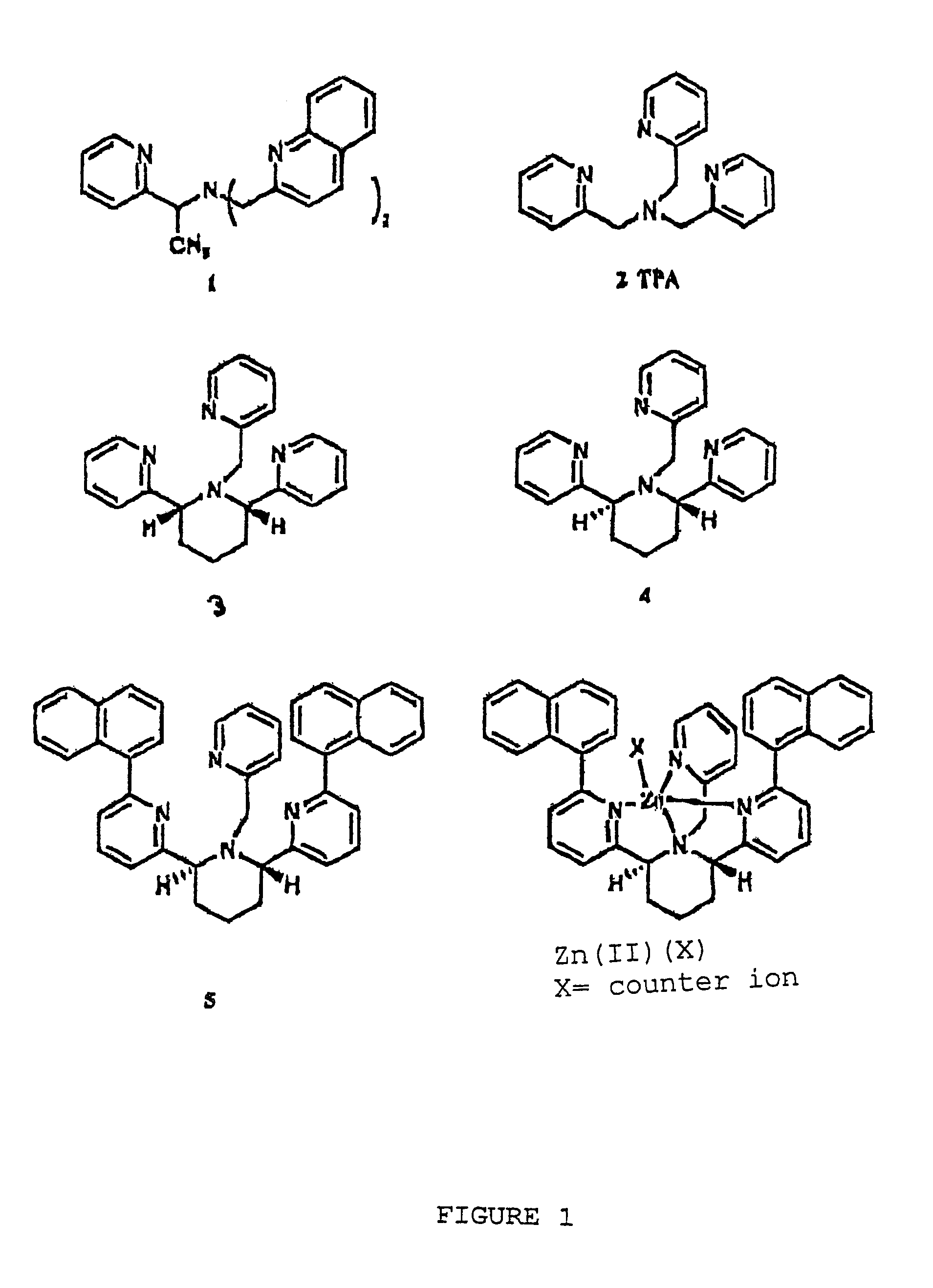
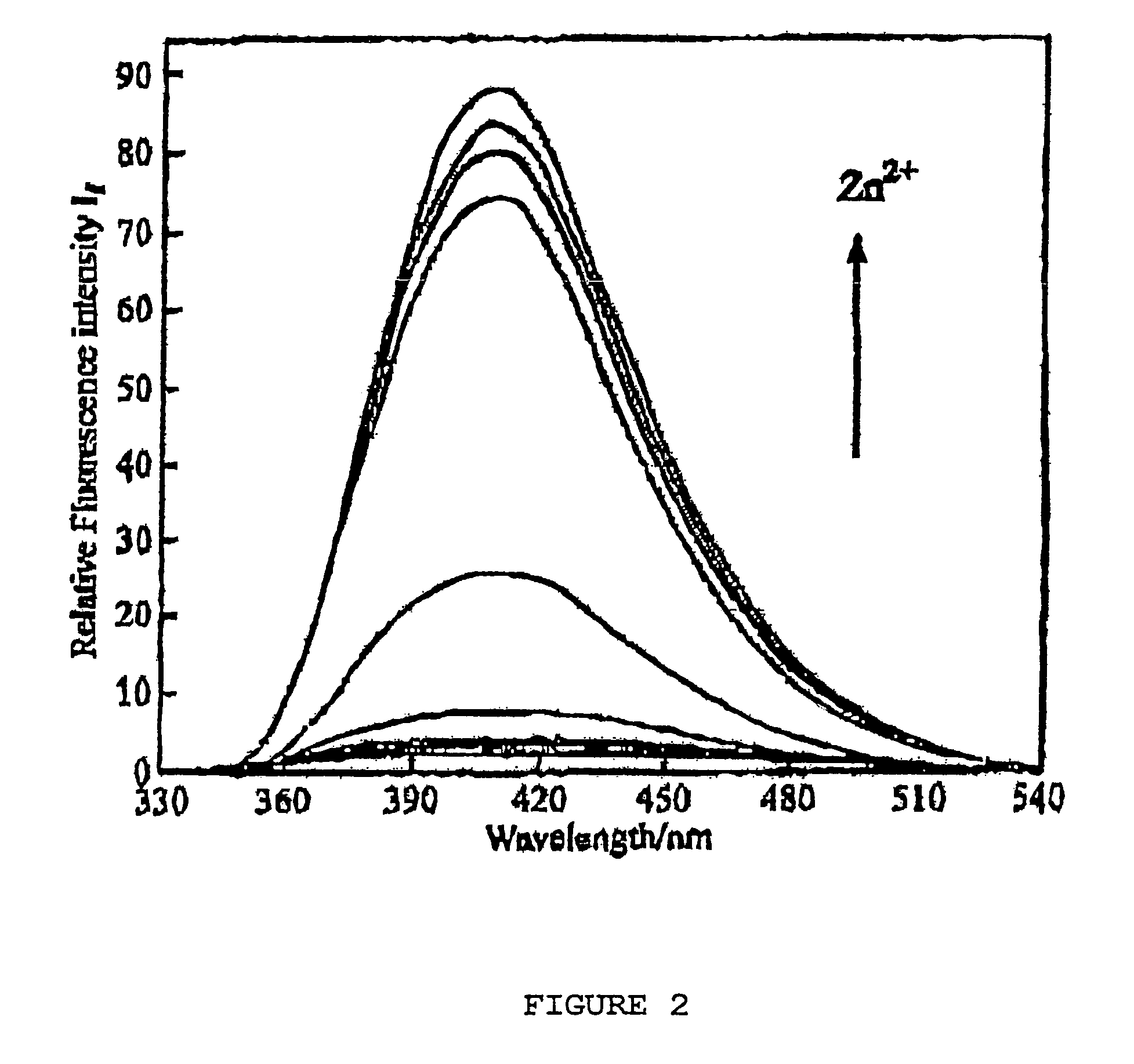
Chiral piperidine and quinucledine ligands
InactiveUS7491544B2High selectivityImprove rigidityAnalysis using chemical indicatorsChemiluminescene/bioluminescenceArylNaphthalene
Zn(II) is selectively detected in a sample by contacting the sample with a tripodal ligand with a piperidine or quinuclidine scaffold, one of which acts as a zinc sensor, in which the rigidity of the ligand scaffold is increased. The rigidity of the ligand scaffold can be increased by adding aromatic groups or cyclic hydrocarbon groups. Examples of aromatic groups include naphthalene and the like. Examples of cyclic groups include nitrogen-substituted cyclohexane and cyclohexene such as piperidine.
Owner:NEW YORK UNIV
Anti-smudge body, display device, input device, electronic device, and Anti-smudge article
InactiveUS20150240086A1Less noticeableAntifouling/underwater paintsDigital data processing detailsDisplay deviceEngineering
An anti-smudge body having a surface that, when fingerprints adhere to the surface, allows the fingerprint patterns to spread spontaneously to thereby cause them to become less noticeable has the surface and a plurality of protrusions provided thereto. The protrusions contain at least one of a first compound having an ester linkage in a portion other than terminal ends and a second compound having a cyclic hydrocarbon group.
Owner:DEXERIALS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com