Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
310 results about "Thianaphthene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thianaphthene may be used in the preparation of 2-thianapthenylphenyllith ium, via metalation by n-butyllithium. General description Reaction of ethyl diazoacetate with thianaphthene has been reported.
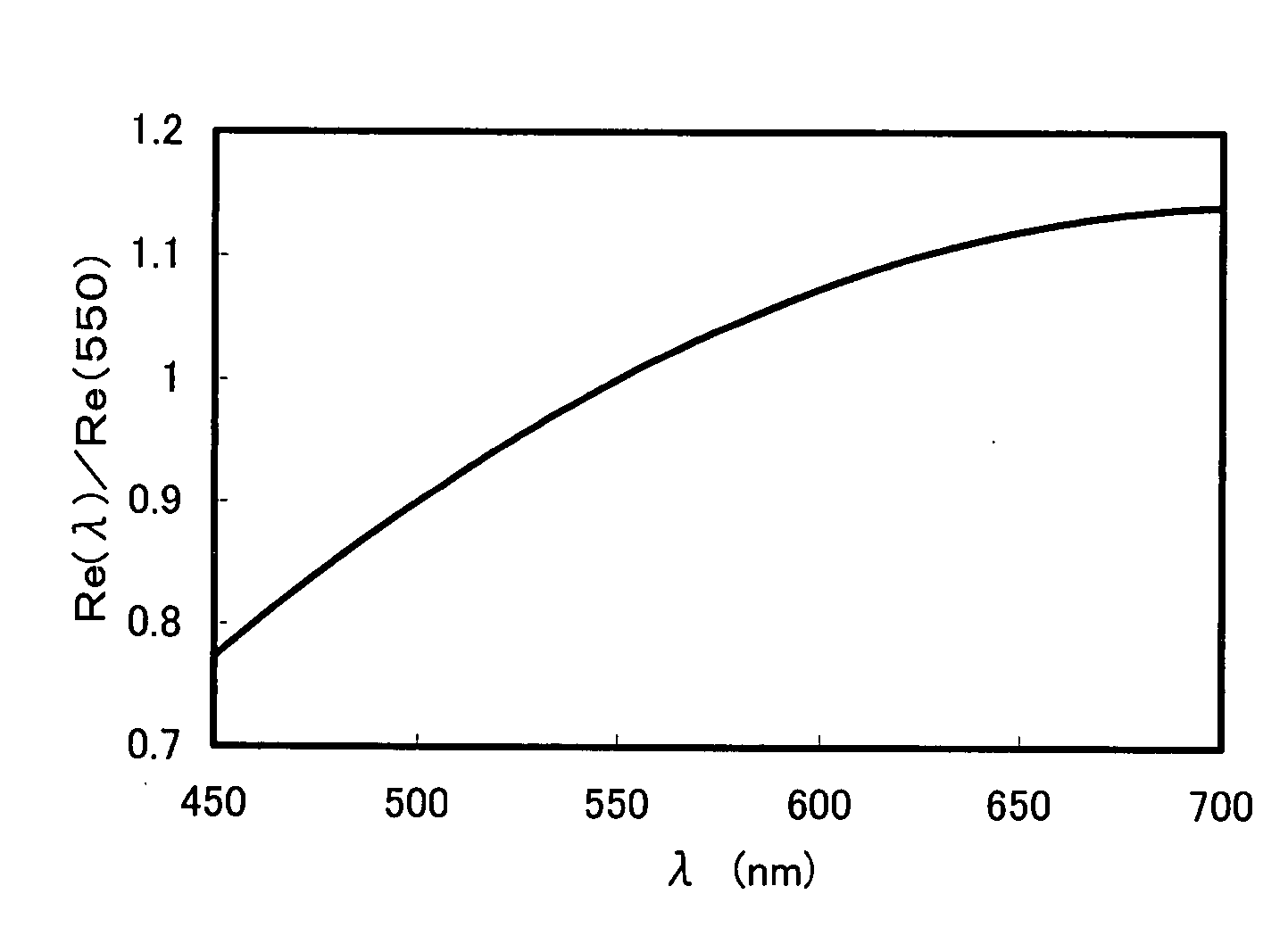
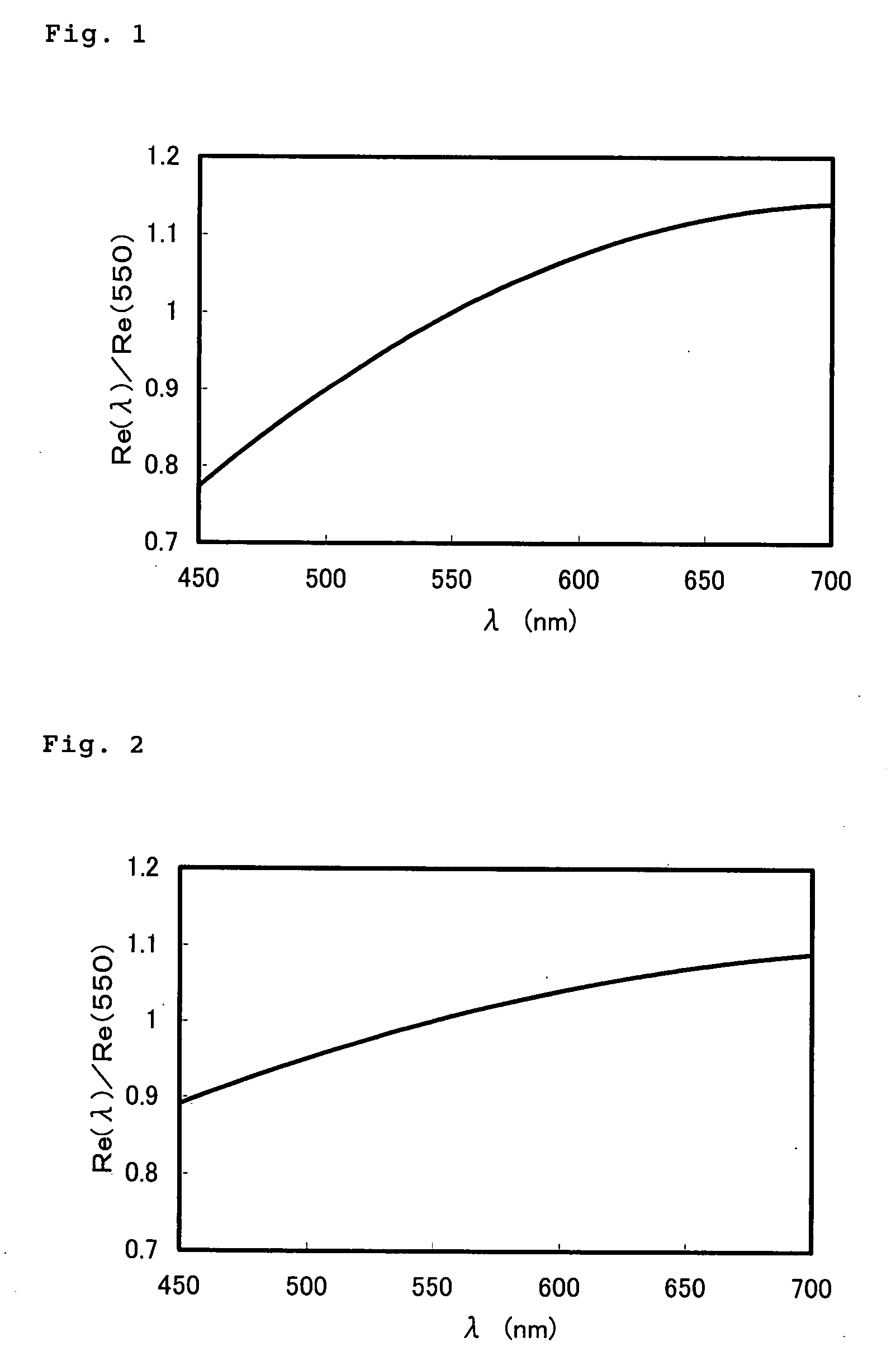
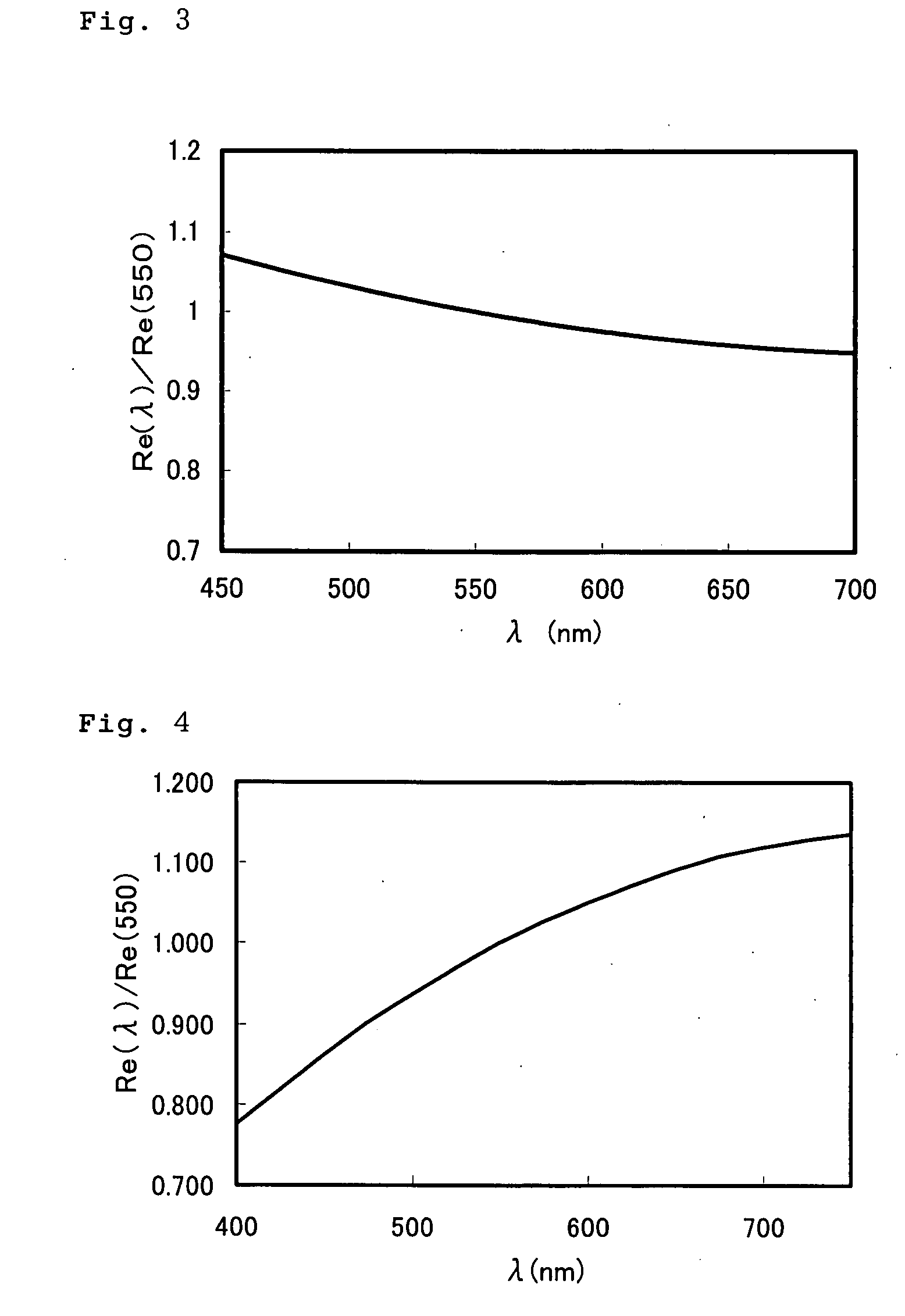
Optical Film
A composition comprising a polymerizable compound of the formula (1) and a rod-shaped polymerizable liquid crystal compound: P2-E2-X2-B2-A2-(G2)t-Y-(G1)s-A1-B1-X1-E1-P1 (1) (in the formula (1), Y represents a di-valent group, s and t represent each independently an integer of 0 or 1, G1 and G2 when s and t are 1 represent each independently —CR1R2—, R1 and R2 represent each independently an alkyl group having 1 to 4 carbon atoms, halogen atom or hydrogen atom, A1 and A2 represent each independently a di-valent cyclic hydrocarbon group, di-valent heterocyclic group, methylenephenylene group, oxyphenylene group or thiophenylene group, B1 and B2 represent each independently a di-valent group, X1 and X2 represent each independently a di-valent group, E1 and E2 represent each independently an alkylene group having 2 to 25 carbon atoms, and P1 and P2 represent a hydrogen atom or polymerizable group, at least one of P1 and P2 being a polymerizable group.).
Owner:SUMITOMO CHEM CO LTD
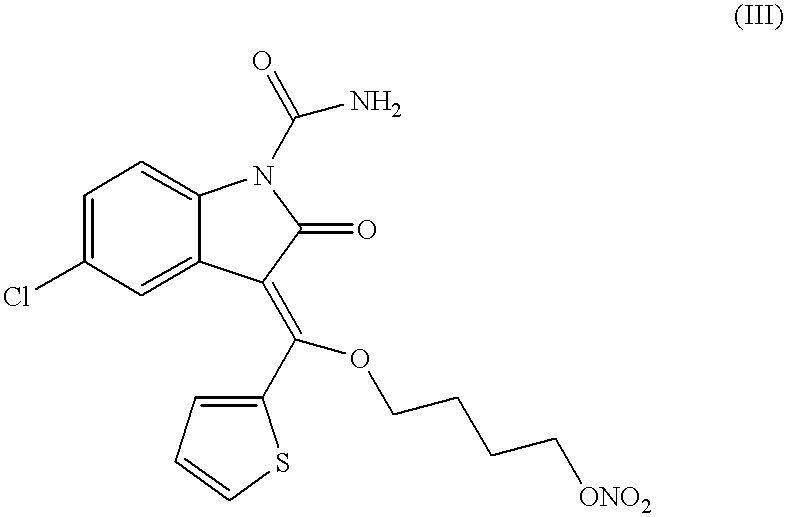
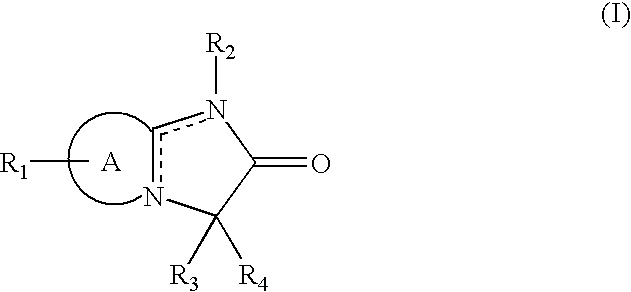
Nitric oxide releasing oxindole prodrugs for anagesic, Anti-inflammatory and disease-modifying use
Nitric oxide releasing oxindole prodrugs are described which are useful in methods of treating or preventing pain, inflammation, fever, or gastrointestinal lesions in a patient in need of such treatment, or of modifying an inflammatory disease or condition by favorably affecting the outcome thereof in said patient, wherein there is administered to said patient a therapeutically effective amount of a compound of Formula (I): and pharmaceutically acceptable salts thereof. In preferred embodiments, X is a covalent bond; RA and RB are both hydrogen; n is the integer 4; Y is -O-; Z is -NO2; RC is a member selected from the group consisting essentially of 5-Cl and 5-F; RD is a member selected from the group consisting essentially of 6-Cl and 6-F; and RE is a member selected from the group consisting essentially of benzyl, 2-furyl, 2-thienyl, 5-chloro-2-thienyl and 5-trifluoromethyl-2-thienyl.
Owner:PFIZER INC +1
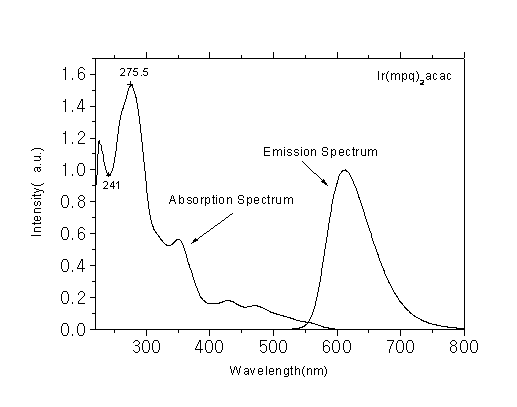
Phosphorescent luminescent materials and preparation method and application thereof
InactiveCN102703059AImprove luminous efficiencyThe synthesis method is simpleGroup 8/9/10/18 element organic compoundsSolid-state devicesIridiumQuinoline
The invention discloses phosphorescent luminescent materials and a preparation method and application thereof. The invention is characterized in that the phosphorescent luminescent materials are red light materials containing a metal iridium complex; a structural general formula of the phosphorescent luminescent materials is shown in the specification; and in the structural general formula, R1 and R2 are independently one of alkyl, phenyl, halogen-substituted phenyl, alkyl-substituted phenyl, naphthyl, anthryl, a halogen substituent, methoxy, phenoxy, cyano-substituted carbazolyl, substituted N-phenylcarbazolyl, quinolyl, thiazolyl, thienyl, an aromatic amino group, a group with an azole structure, an aromatic heterocyclic radical, a substituted aromatic heterocyclic radical and a silicon alkyl substituent respectively. The phosphorescent materials have high luminous efficiency; and the high luminous efficiency shows that the compounds can be taken as luminescent materials or main luminescent materials and applied to electroluminescent devices. Through data test and comparison, the materials are organic electroluminescent materials which have excellent performance and a good prospect, and particularly are phosphorescent red light materials which have good performance; and moreover, a method for synthesizing the luminescent materials is simple and low in cost.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
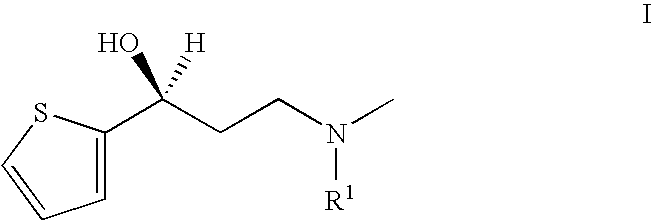
Process for the preparation of N-alkyl-N-methyl-3-hydroxy-3-(2-thienyl)-propylamines
InactiveUS20050197503A1High chemical purityHigh optical purityOrganic chemistryPropylamineAsymmetric hydrogenation
The present invention relates to an improved process for preparing chiral N-substituted N-methyl-3-hydroxy-3-(2-thienyl)-propylamine on an industrial scale using an asymmetric hydrogenation as a key step and optionally a special sequence of subsequent steps, using a catalyst system consisting of rhodium and (2R, 4R)-4-(dicyclohexylphosphino)-2-(diphenyl-phosphino-methyl)-N-methyl-aminocarbonyl-pyrrolidine.
Owner:BOEHRINGER INGELHEIM INT GMBH
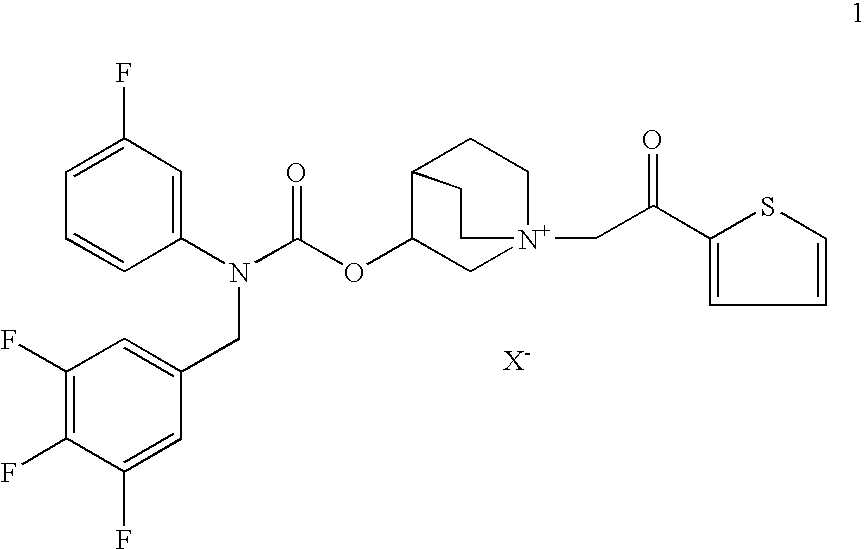
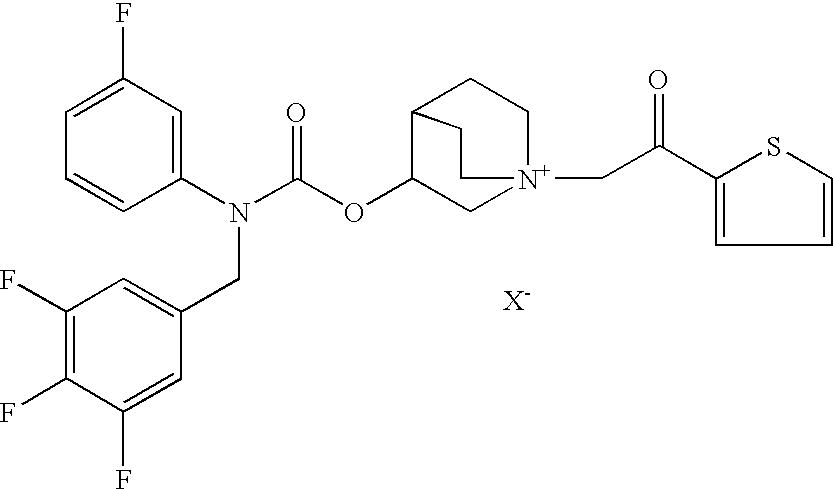
Compositions comprising an antimuscarinic and a long-acting beta-agonist
InactiveUS20090181935A1Substantial therapeutic benefitQuick effectBiocideAnimal repellantsDermatologyAirways disease
Compositions which comprise a combination of a salt of 3-[[[(3-fluorophenyl)[(3,4,5-trifluoro phenyl)methyl]amino]carbonyl]oxy]-1-[2-oxo-2-(2-thienyl)ethyl]-1-azoniabicyclo [2.2.2]octane, and a long-acting phenylalkylamino beta2-agonist are effective for the prevention and treatment of inflammatory or obstructive airways diseases.
Owner:CHIESI FARM SPA
Piperazine-containing compounds useful in the treatment of pain
The present invention is directed to a compound of general formula (I),wherein R<1 >is selected from phenyl, pyridinyl, thiophenyl, furanyl, imidazolyl; each phenyl ring and heteroaromatic ring optionally and independently being further substituted by 1, 2 or 3 substituents selected from straight and branched C1-C6 alkyl, NO2, CF3, C1-C6 alkoxy, chloro, fluoro, bromo, and iodo, as well as their pharmaceutically acceptable salts. The invention includes pharmaceutical compositions comprising these compounds and the use of the compounds in therapy, in particular in the management of pain.
Owner:ASTRAZENECA AB
Conjugated copolymer, production method thereof and capacitor using the copolymer
InactiveUS20070129534A1Improve conductivityAccelerating polymerizationHybrid capacitor electrodesCell electrodesElectrolysisCompound (substance)
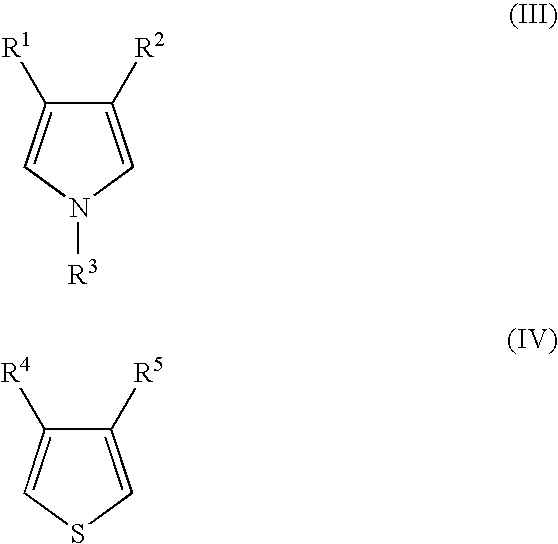
The present invention relates to a novel π-conjugated copolymer having a high conductivity, obtained through chamically oxidative polymerization of a thiophene-based unit represented by formula (IV) and a pyrrole-based unit represented by formula (III) at a low temperature, preferably in the presence of a compound containing a counter anion, and production method therefor, and further relates to an article coated with the copolymer, a solid electrolytic capacitor using the copolymer as solid electrolyte and production method for the capacitor.
Owner:MURATA MFG CO LTD
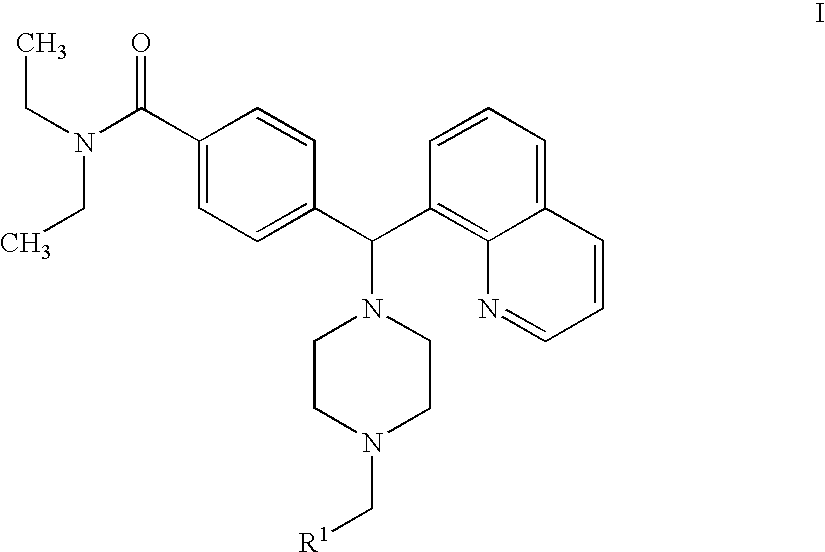
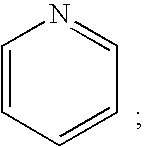
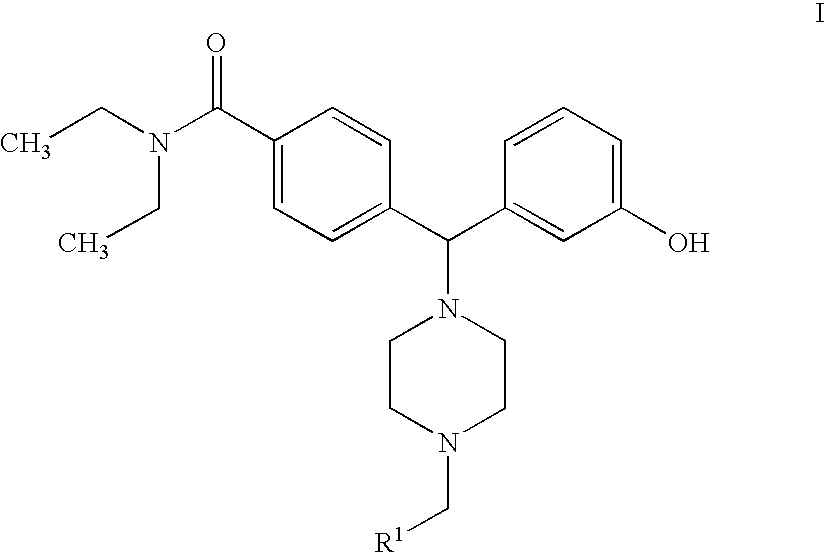
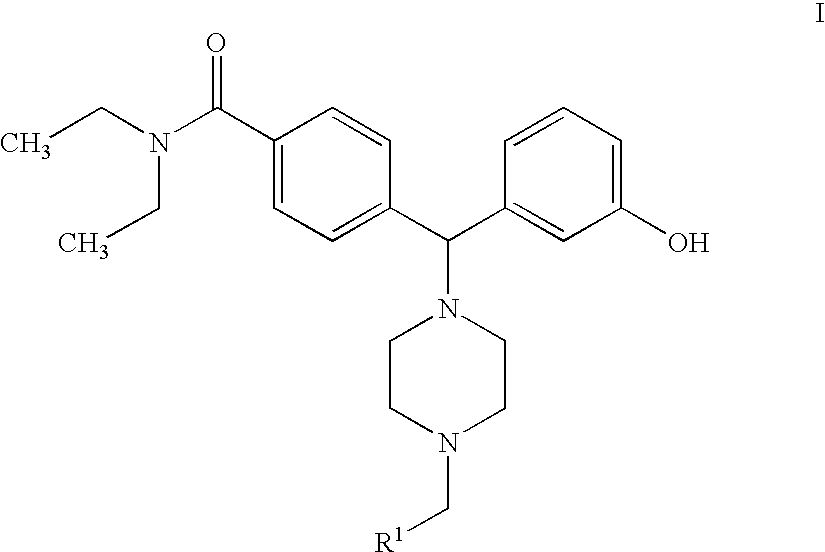
Hydroxyphenyl-piperazinyl-methyl-benzamide derivatives for the treatment of pain
The present application describes compounds of general formula Iwhere R<1 >is selected from any one of pyridinyl, thienyl, furanyl, imidazolyl, and triazolyl;and where each R<1 >heteroaromatic ring may optionally and independently be further substituted by 1, 2 or 3 substituents selected from straight and branched C1-C6 alkyl, NO2, CF3, C1-C6 alkoxy, chloro, fluoro, bromo, and iodo. The substitutions on the heteroaromatic ring may take place in any position on the ring system. The invention also includes enantiomers, salts and pharmaceutical compositions containing the compounds. The compounds may be used in treating patients for pain.
Owner:ASTRAZENECA AB
Fungicides for the control of take-all disease of plants
A method of controlling Take-All disease of plants by applying a fungicide of the formula wherein Z1 and Z2 are C and are part of an aromatic ring which is [benzothiophene]thiophene; and A is [selected from] -C(X)-amine [wherein the amine is an unsubstituted, monosubstituted or disubstituted amino radical]wherein the amine is a monosubstituted or a disubstituted amine, wherein one of the substituents has a cyclic moiety, said cyclic moiety which is chosen from the group consisting of thienyl, furanyl, and a non-heterocyclic substituent, wherein when the amine is disubstituted, the second substituent is a non-cyclic substituent, -C(O)-SR3, -NH-C(X)R4, [and] or -C(=NR3)-XR7; B is -Wm-Q(R2)3 or selected from [O-tolyl]o-tolyl, 1-naphthyl, 2-naphthyl, and 9-phenanthryl, each optionally substituted with halogen or R4; Q is C, Si, Ge, or Sn; W is -C(R3)pH(2-p)-; or when Q is C, W is selected from [-C(R3)pH(2-p),[-C(R3)pH(2-p)-, -N(R3)mH(1-m)-, [-S(O)p-,] -S(O)p-, and -O-, X is [0] O or S; n is [0, 1, 2, or 3]2; m is 0 or 1; p is 0, 1, or 2; wherein the two R groups are combined with the thiophene ring to form a fused ring which is benzothiophene; each R and R2 is independently defined herein; R3 is C1-C4 alkyl; R4 is C1-C4 alkyl, haloalkyl, alkoxy, alkylthio, alkylamino, or dialkylamino; and R7 is C1-C4 alkyl, haloalkyl, or phenyl, optionally substituted with halo, nitro, or R4; or an agronomic salt thereof.
Owner:MONSANTO TECH LLC
Heterocyclic compounds and cerebral function improvers containing the same as the active ingredient
InactiveUS7141579B2Remarkable effectAdvantageous in separating actionBiocideNervous disorderCombinatorial chemistryBULK ACTIVE INGREDIENT
Heterocyclic compound represented by the formula Iwhereinrepresentsor the like; R1 represents hydrogen atom, C1–C6 alkyl or benzyloxy; R2 represents methyl or nil; R3 represents hydrogen atom, C1–C6 alkyl, C2–C6 alkenyl, C3–C8 cycloalkyl or —CH2R5 [wherein R5 represents phenyl (which may be substituted with C1–C6 alkyl, halogen atom or cyano)] or thienyl; R4 represents C1–C6 alkyl, C2–C6 alkenyl, C3–C8 cycloalkyl or —CH2R6 [wherein R6 represents phenyl (which may be substituted with C1–C6 alkyl, halogen atom or cyano), naphtyl or thienyl]; or R3 is coupled with R4.
Owner:ZENYAKU KOGYO KK
Process for preparing a 4,7-bis(5-halothien-2-yl)-2,1,3-benzothiadiazole and a precursor therefor
The present invention is directed to a method for preparing a 4,7-bis(5-halothien-2-yl)-2,1,3-benzothiadiazole, more particularly, 4,7-bis(5-bromothien-2-yl)-2,1,3-benzothiadiazole, and a precursor therefor, namely, a 4,7-bis(thien-2-yl)-2,1,3-benzothiadiazole. The precursor is prepared by contacting in the presence of a palladium catalyst and a solvent a 4,7-dihalo-2,1,3-benzothiadiazole with a thienyl group adding reagent, which can either be a 2-thienylzinc halide, a 2-thienylmagnesium halide, or a 2-thiopheneboronic acid, under such conditions as to form 4,7-bis(thien-2-yl)-2,1,3-benzothiadiazole. The precursor can then be halogenated, preferably brominated, to form the desired dibrominated product, which is a particularly suitable monomer for the preparation of a copolymer of a 9,9-disubstituted fluorene. This copolymer is useful, for example, in polymeric light emitting diode (pLED) applications.
Owner:SUMITOMO CHEM CO LTD
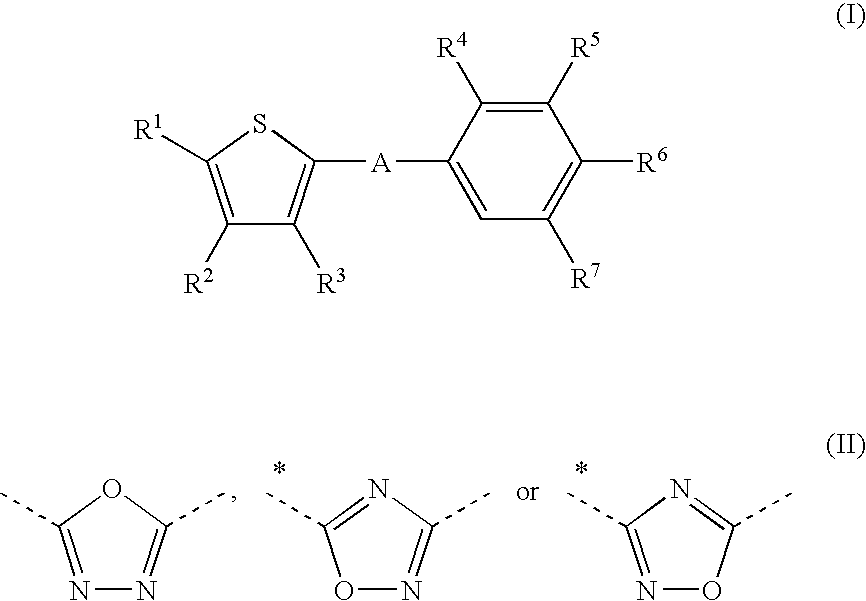
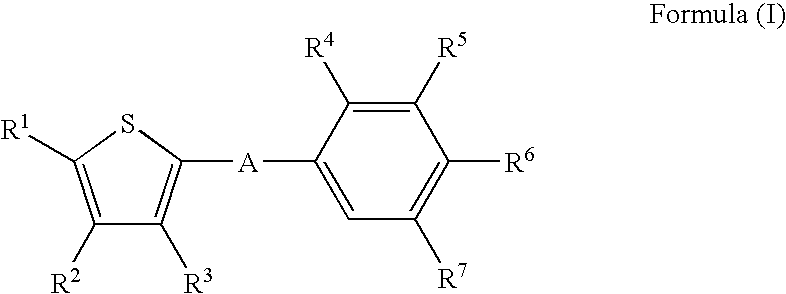
Thiophene derivatives as S1P1/EDG1 receptor agonists
The invention relates to thiophene derivatives of formula (I) / their preparation and their use as pharmaceutically active compounds. Said compounds particularly act as immunosuppressive agents wherein: A represents *—CO—CH═CH—, *—CO—CH2CH2—, *—CO—CH2—NH—, wherein the asterisks indicate the bond that is linked to the thiophene group of Formula (I), and R1-R3 are as defined in the claims.
Owner:ACTELION PHARM LTD
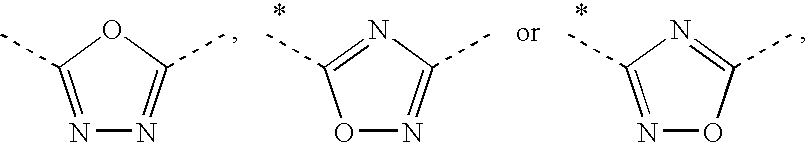
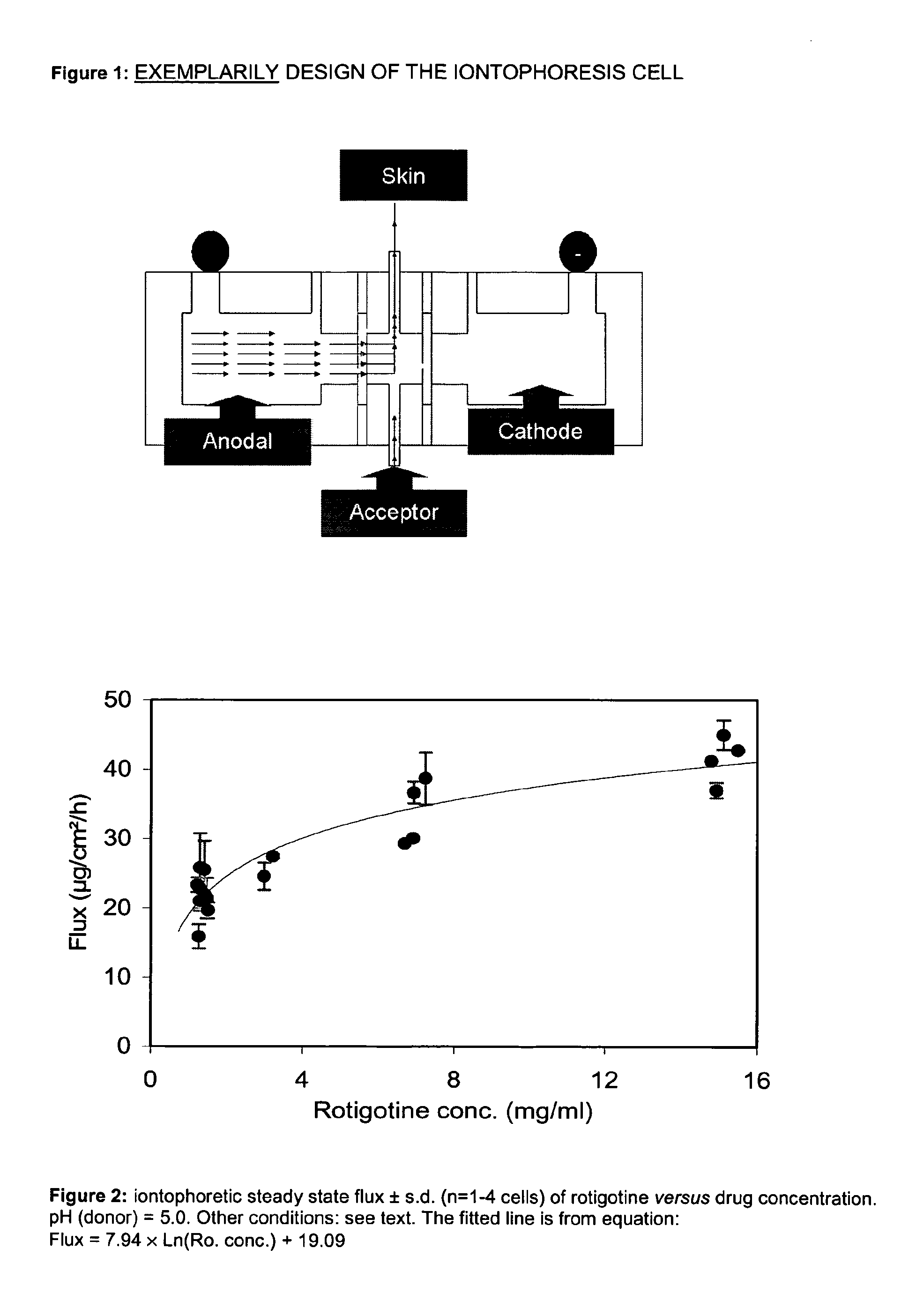
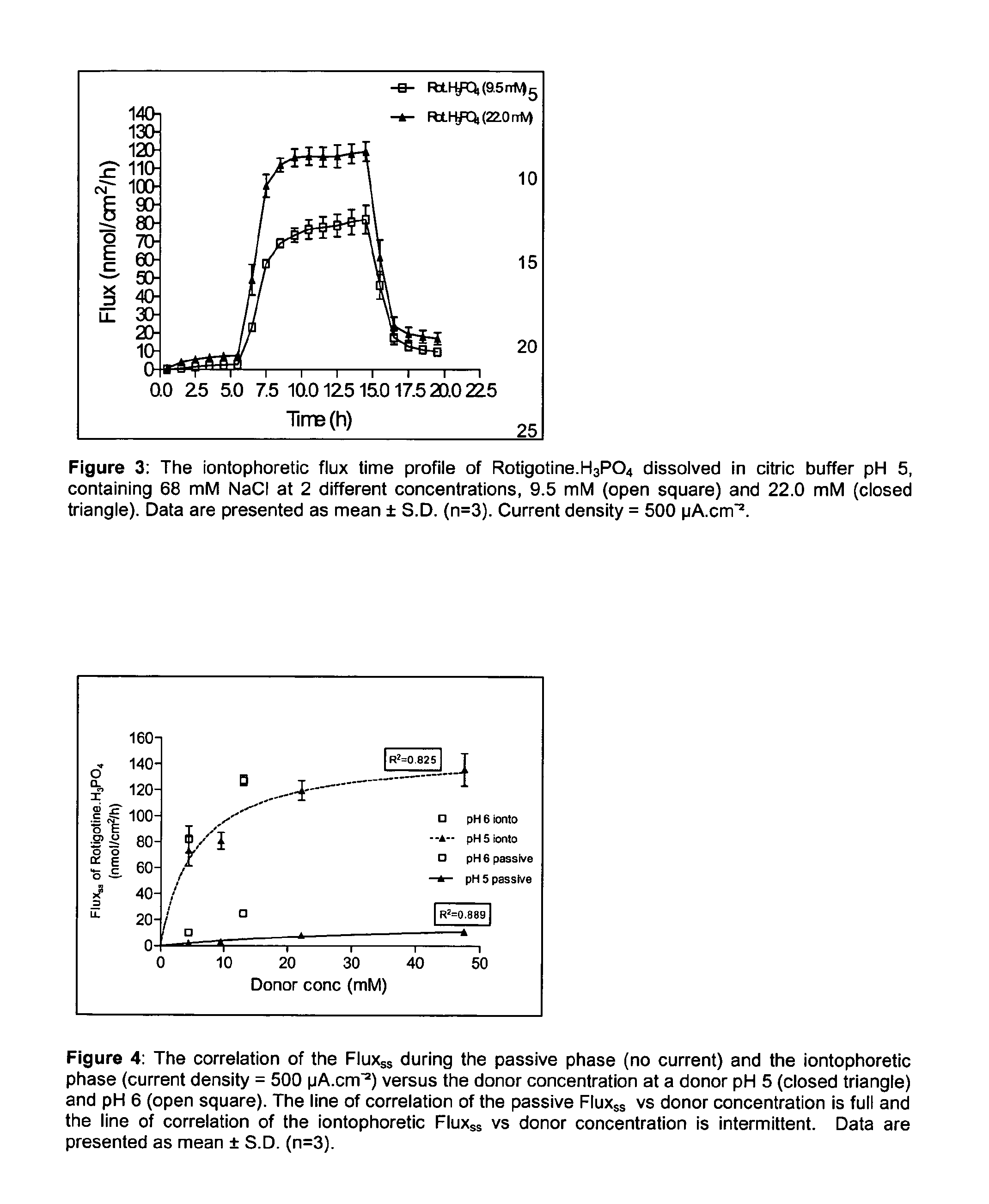
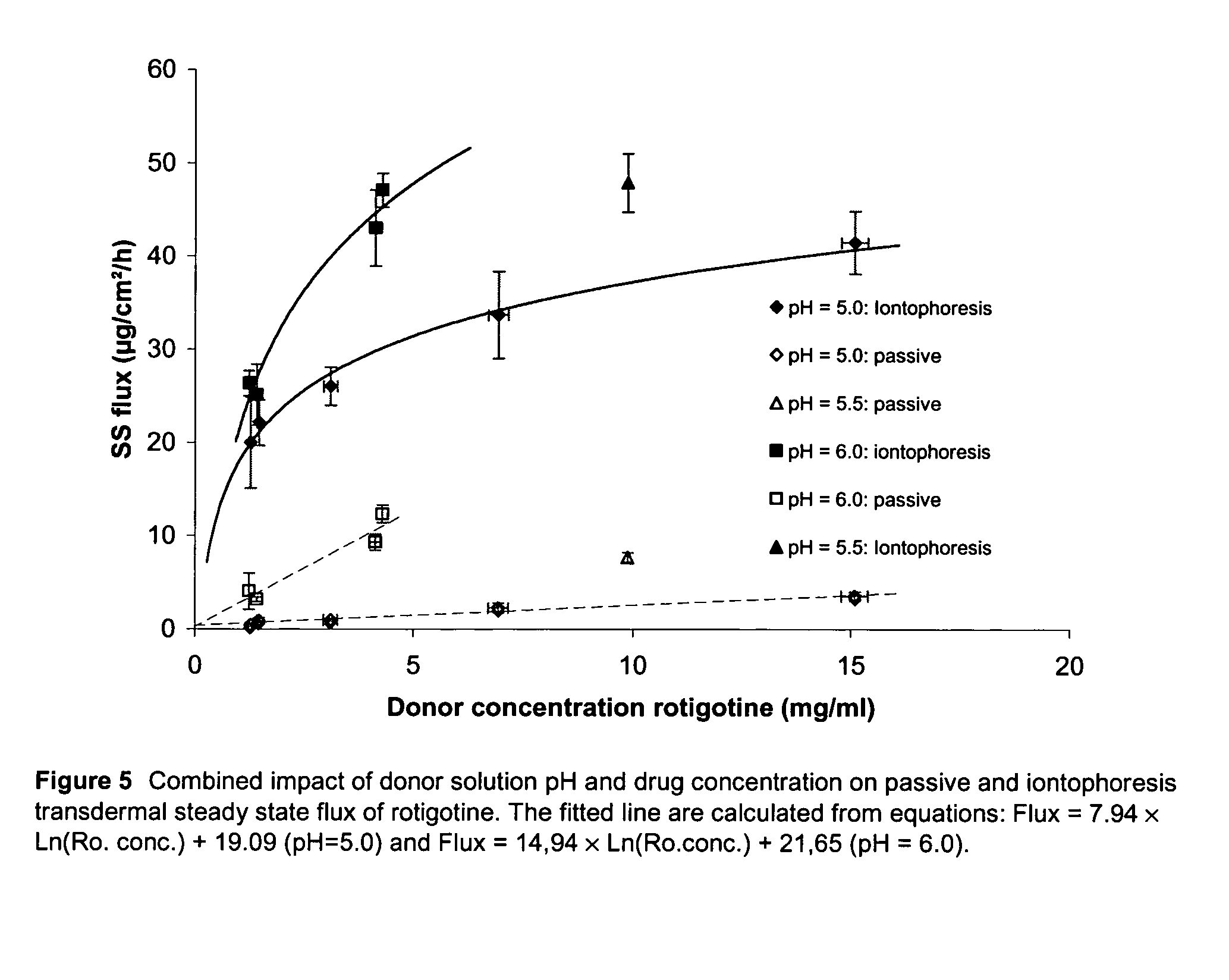
Pharmaceutical composition comprising rotigotine salts (acid or na), especially for iontophoresis
InactiveUS20120101146A1Enhanced iontophoretic deliveryGood to excellent solubilityBiocideElectrotherapyRotigotineDrug
The present invention relates to new salts of 6-(propyl-(2-thiophen-2-ylethyl)amino)tetralin-1-ol (rotigotine), their use as a medicament, for example for the treatment of CNS disorders like Parkinson Disease, RLS, fybromyalgia and / or depression, in particular through electromotive administration. The present invention relates to pharmaceutical formulations suitable for iontophoresis that provide enhanced iontophoretic delivery of rotigotine to at least one target tissue. The formulations are further characterized by good to excellent solubility of the salts in aqueous solutions.
Owner:UCB SA
Fungicidal Mixtures of Thiophene Derivative
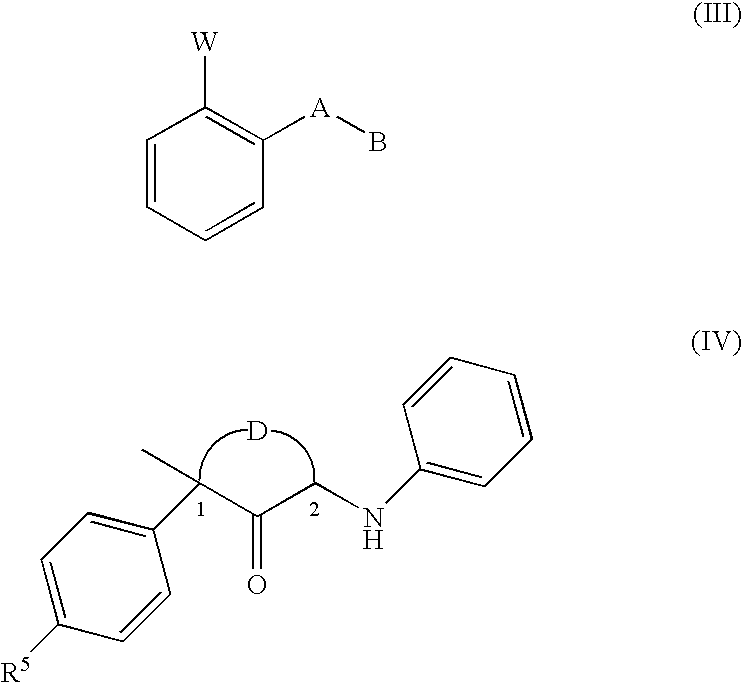
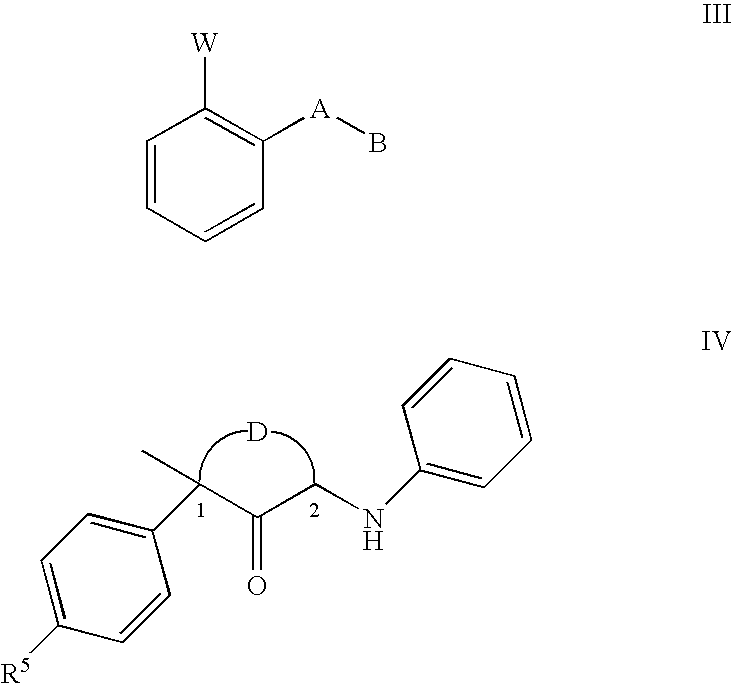
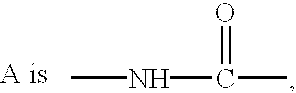
Disclosed are fungicidal mixtures, compositions and methods for controlling plant diseases relating to combinations comprising (a) N-[2-(1,3-dimethyl-butyl)-3-thienyl]-1-methyl-3-(tri-fluoromethyl)-1H-pyrazole-4 carboxamide (including all stereoisomers) or an agriculturally suitable salt thereof; and (b) at least one compound selected from the group consisting of compounds of Formula III or Formula IV which act at the bcl complex of the fungal mitochondrial respiratory electron transfer site; (INSERT FORMULA III HERE) (INSERT FORMULA IV HERE) wherein W, A, B, D and R5? are disclosed in this specification, and agriculturally suitable salts thereof; and optionally (c) at least one compound selected from the group of compounds acting at the demethylase enzyme of the sterol biosynthesis pathway and agriculturally suitable salts thereof.
Owner:EI DU PONT DE NEMOURS & CO
Pyrrolidine sulfonamides
A compound of Formula (I): wherein:R1 is C1-6 alkyl, benzyl, or (CH2)n—C(O)NH2; wherein the benzyl may be unsubstituted or substituted by one or two C1-6 alkyl, halogen, C1-6 alkoxy, or methylenedioxy groups;R2 is benzimidazolyl, quinolinyl, benzofuranyl, napthyl, indolyl, benzothiophenyl, phenyl, furanyl, thienyl, or pyridyl substituted or unsubstituted by one, two or three halogen, C1-3 alkyl, C1-3 alkoxy, or methylenedioxy groups;X1 and X2 are independently hydrogen, halogen, C1-3 alkyl, C1-3 alkoxy, nitro, CF3, or CN;n is 1, 2, or 3;m is 1, 2 or 3;or a pharmaceutically acceptable salt thereof.
Owner:SMITHKLINE BECKMAN CORP
Ether and amide compounds preparation thereof composition containing same and use thereof as antidiadetics
InactiveUS6414001B2Enhance insulin actionLow toxicityBiocideOrganic chemistryPharmaceutical medicineTert butyl
Ether and amide derivatives are disclosed, which are represented by the following formula (I) and its pharmaceutical acceptable salt, and which are useful for the treatment of diabetes.wherein(with the provisos that (i) when A is -O-, then n is 2 or 3 (ii) whenthenn is 1 or 2. R3 is OH-, CH3SO2NH-, CF3SO2NH-, CH3SO2NHCH2-, CF3SO2NHCH2-, HOOC-, CH3OOC-,HOOC-CH2SO2NH-, CF3-CH2SO2NH-, R8-NHSO2-,R8-NHSO2-CH2-, HOOC-CH2-O-, HSO3N=CH-, or R9-SO2NHCO-;R4 is H, OH, O-alkyl or O-CH2OCH3;R5 is H, halogen atom, -CH2COOH or OH;R6 and R7 are hydrogen, t-butyl or pyrolidyl;R8 is hydrogen or lower alkyl;R9 is alkyl or thienyl;R10 is lower alkyl)or a pharmaceutically acceptable salt.
Owner:KOTOBUKI PHARMA CO LTD
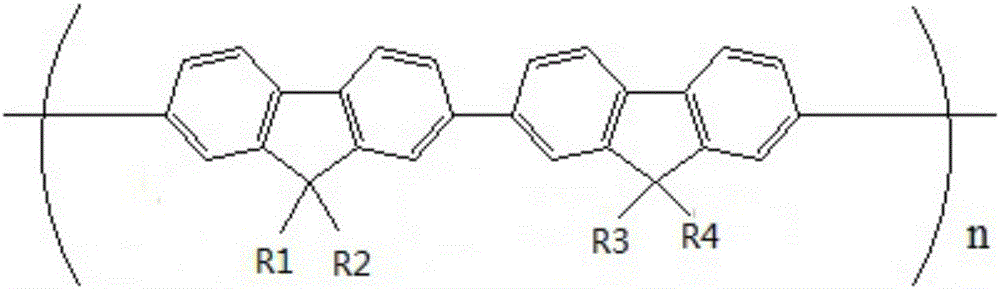
Carbazole-terminated heterofluorene main body material and preparation and application method
InactiveCN101775010AHigh triplet energy levelImprove thermal stabilityGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsCarbazoleSulfur
The invention discloses a carbazole-terminated heterofluorene main body material and a preparation and application method which is a general method for the carbazole-terminated heterofluorene main body material formed by introducing carbazole groups at the second and seventh sites or the third and sixth sites of heterofluorene. The carbazole-terminated heterofluorene main body material has the following basic structure, wherein X is one of heteroatoms such as nitrogen, oxygen, sulfur, phosphorus and the like; R1 and R2 are phenyl, alkyl (such as methyl, octyl and the like) with chain length of C1 to C18, thienyl and the like, or oxygen substituted group or are not existent; and R3 and R4 are one of ortho-para substituted groups such as methyl, alkoxyl, tertiary butyl and the like or are not existent.
Owner:NANJING UNIV OF POSTS & TELECOMM
Perovskite solar cell and preparation method for same
InactiveCN106784339AEasy to prepareImprove performanceSolid-state devicesSemiconductor/solid-state device manufacturingSide chainHole transport layer
The invention discloses a perovskite solar cell and a preparation method for the same. The perovskite solar cell sequentially comprises a conductive substrate, a hole transport layer, a perovskite layer, an electron transport layer, a cathode modification layer and a metal cathode from bottom to top, wherein the cathode modification layer comprises a conjugate polymer of which side chains contain at least two hydroxyl groups; the conjugate polymer comprises a conjugate main chain, the conjugate main chain comprises conjugate units A and B which are connected with each other, each of the conjugate units A and B comprises fluorene, dibenzothiophene, thianaphthene, BT, DBT or a phenyl group, the conjugate units A and B are connected with different side chains in which one side chain contains 2 to 6 hydroxyl groups. According to the perovskite solar cell and the preparation method for the same, an interface dipole modification effect is achieved by virtue of polar groups, so that the potential barrier between the metal cathode and the electron transport layer is reduced, the interface recombination effect is alleviated, and the performance of the perovskite solar cell is improved.
Owner:KUSN INNOVATION INST OF NANJING UNIV
Canagliflozin new preparation method
InactiveCN104151306AHigh reactivitySimple and fast operationOrganic chemistryBenzeneBiochemical engineering
The invention relates to a new synthetic method of 1-(beta-D-pyran glucosyl)-4-methyl-3-[5-(4-fluorinated phenyl)-2-thienyl methyl] benzene (canagliflozin), and the method is characterized in that: coupling and etherification steps are separately operated, at the same time in the etherification process, usage amount of methanesulfonic acid is greatly reduced compared with the prior art, and the method can reduce the cost, is convenient in operation and improves the quality of products.
Owner:XIANGBEI WELMAN PHARMA CO LTD
Novel chalcogen-containing organic compound and use thereof
ActiveUS20150014673A1Easy to synthesizeGood chemical stabilityTransistorOrganic chemistrySolventChemistry
[Problem] To provide an organic compound that is easy to synthesize, and has excellent chemical stability, semiconductor characteristics (high carrier mobility) and high solubility in a solvent.[Solution] A compound represented by formula (1) or formula (2):wherein, in formula (1), X is oxygen, sulfur or selenium; n is 0 or 1; R1 to R3 are hydrogen, fluorine, alkyl having 1 to 20 carbons, aryl, pyridyl, furyl, thienyl, thiazolyl or the like. However, except for a case where X is selenium, a case where all of R1 to R3 are simultaneously hydrogen is excluded, and a case where X is sulfur and all of R1 are simultaneously butyl is also excluded. In formula (2), X is oxygen, sulfur or selenium; n is 0 or 1; R1 to R2 are hydrogen, alkyl having 1 to 20 carbons, aryl, pyridyl, furyl, thienyl, thiazolyl or the like; however, a case where all of R1 to R2 are simultaneously hydrogen is excluded.
Owner:JNC CORP
Flavone analog, preparation and application thereof as anti-diabetic medicament
The invention discloses a flavone analog and application of a pharmaceutically acceptable salt thereof to preparation of an anti-diabetic medicament. The flavone analog has a structure shown by a formula I-X or II-X, wherein R1 in the formula I-X or II-X is selected from a substituted phenyl group, a substituted or unsubstituted furan group and a substituted or unsubstituted thienyl group and the substituent group is selected from a C1-4 alkyl group, a C1-4 alkoxyl group, halogen, a hydroxyl group and a cyano group; R2 is selected from the substituted phenyl group, the substituted or unsubstituted furan group and the substituted or unsubstituted thienyl group and the substituent group is selected from the halogen, the hydroxyl group and the cyano group.
Owner:TIANJIN MEDICAL UNIV
Ethoxyphenyl thienyl compounds and methods for the treatment of bacterial infections
This invention relates generally to the discovery of a method of inhibiting, preventing or treating bacterial infections. The invention also relates to a method of inhibiting bacterial capsule biogenesis.
Owner:DUKE UNIV +2
Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester
The invention belongs to the field of medicinal chemistry, and relates to a preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester. Specifically, the preparation method comprises the following steps: enabling (R)-3-quinuclidinol (II) and oxalic acid or derivatives of the oxalic acid to be subjected to esterification reaction so as to obtain oxalic acid diquinine-3(R)-base ester (III): enabling the compound (III) and bimolecular 2-R base thiophene to be subjected to nucleophilic addition substitution, thus obtaining the target product (I). The preparation method has the advantages that the process route is short, reaction conditions are mild, raw materials are simple and easy to obtain, and the specific reaction routes are as shown in descriptions.
Owner:AVENTIS PHARMA HAINAN
Compound, display panel and display device
ActiveCN110078755AImprove stabilityExtend your lifeSilicon organic compoundsSolid-state devicesAcenaphthyleneBenzanthracenes
The invention provides a compound, a display panel and a display device. The compound has the structure shown in formula (I), L represents substituted or unsubstituted phenyl, naphthyl, pyridyl, pyrimidinyl and pyrazinyl; D is an electron-donating group and independently selected from any one of substituted or unsubstituted phenyl, biphenyl, naphthyl, anthryl, phenanthryl, acenaphthylene, pyrenyl,peryl, flurenyl, spiro bifluorenyl, benzophenanthryl, benzanthracene, fluoranthryl, picene, furyl, benzofuryl, dibenzofuryl, thienyl, benzothienyl, dibenzothienyl, phenoxazine, thianthryl, carbazolyl, acridinyl and diarylamino. The designed compound has a TADF characteristic and can emit light by triplet exciton of traditional fluorescence molecular transition inhibition so as to improve the device efficiency.
Owner:WUHAN TIANMA MICRO ELECTRONICS CO LTD
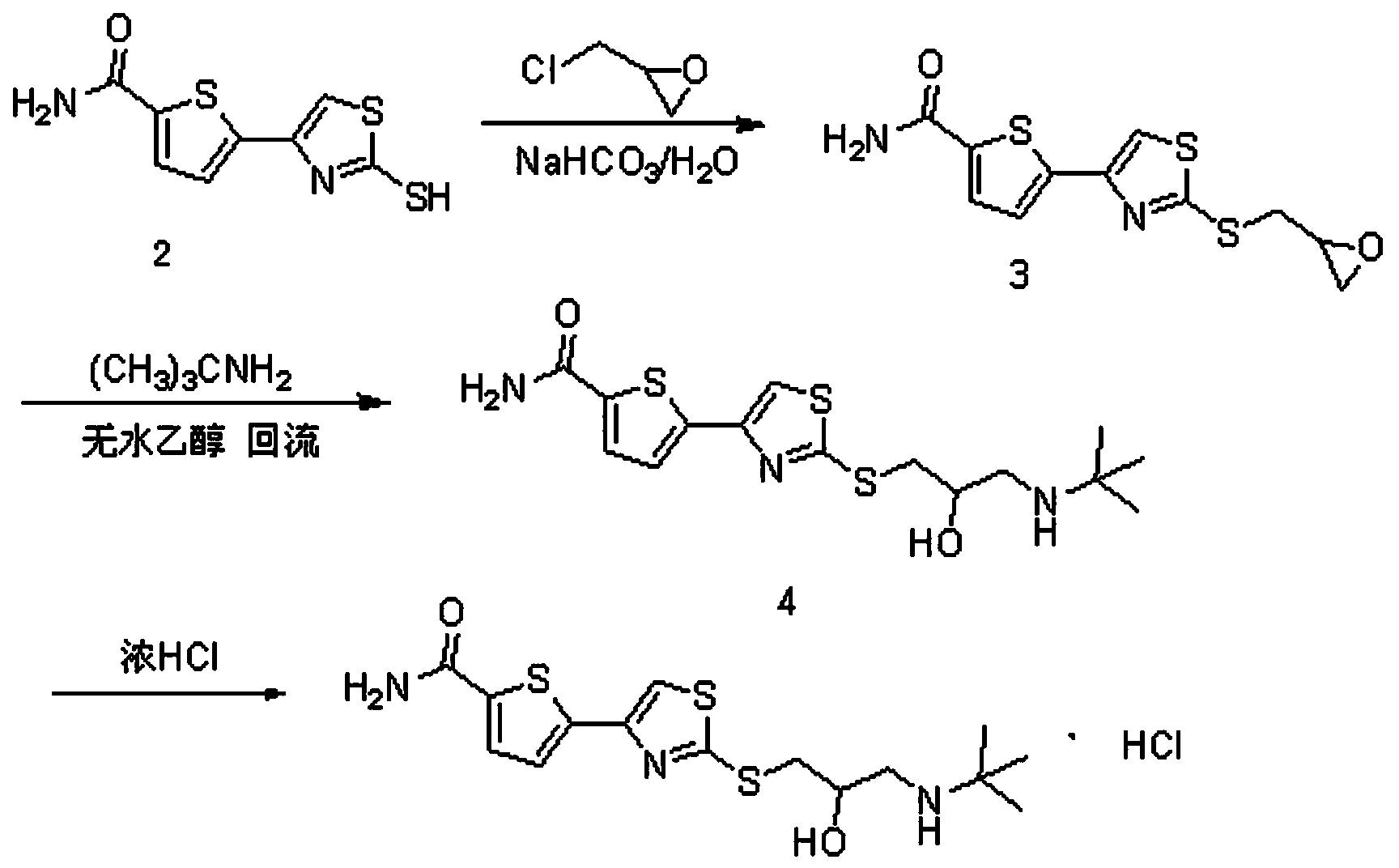
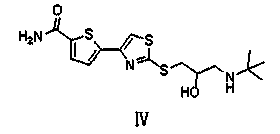
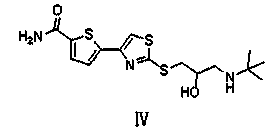
Preparation method of arotinolol hydrochloride
The invention relates to a preparation method of arotinolol hydrochloride. The preparation method comprises the following steps: (1) preparing 2-[2,3-glycidyl-4-(5-carbamyl-2-thienyl)] thiazole; (2) dissolving 2-[2,3-glycidyl-4-(5-carbamyl-2-thienyl)] thiazole in absolute ethyl alcohol, adding tert-butylamine and performing a reflux reaction to obtain arotinolol; and (3) dissolving arotinolol in dimethyl sulfoxide and then adding concentrated hydrochloric acid to generate the arotinolol hydrochloride. The preparation method disclosed by the invention is short in production period, simple in process, high in yield, safe and reliable, and the arotinolol hydrochloride prepared by the method disclosed by the invention is high in purity.
Owner:SHIJIAZHUANG GERUI PHARMA CO LTD
Bis-indolinedione D-A-D type polymer electrochromic material and preparation method thereof
The invention discloses a bis-indolinedione D-A-D type polymer electrochromic material and a preparation method thereof, and belongs to the field of polymer electrochromic materials, wherein the bis-indolinedione monomer structure is as a receptor (A), donors (D) comprise furan group, selenophene group, thiophene group and derivatives thereof, and pyrrole group and derivatives thereof; and the polymer electrochromic material is obtained by electrochemical polymerization method or a chemical method from an active precursor. Due to N atoms and dione functional groups in the structure of the polymer electrochromic material, bis-indolinedione as the receptor structure has strong electron-withdrawing ability, so that the bis-indolinedione D-A-D type polymer electrochromic material has lower bandwidth, a wide light absorption range, richer color, faster response time, higher coloration efficiency, better stability and other characteristics.
Owner:JIANGXI SCI & TECH NORMAL UNIV
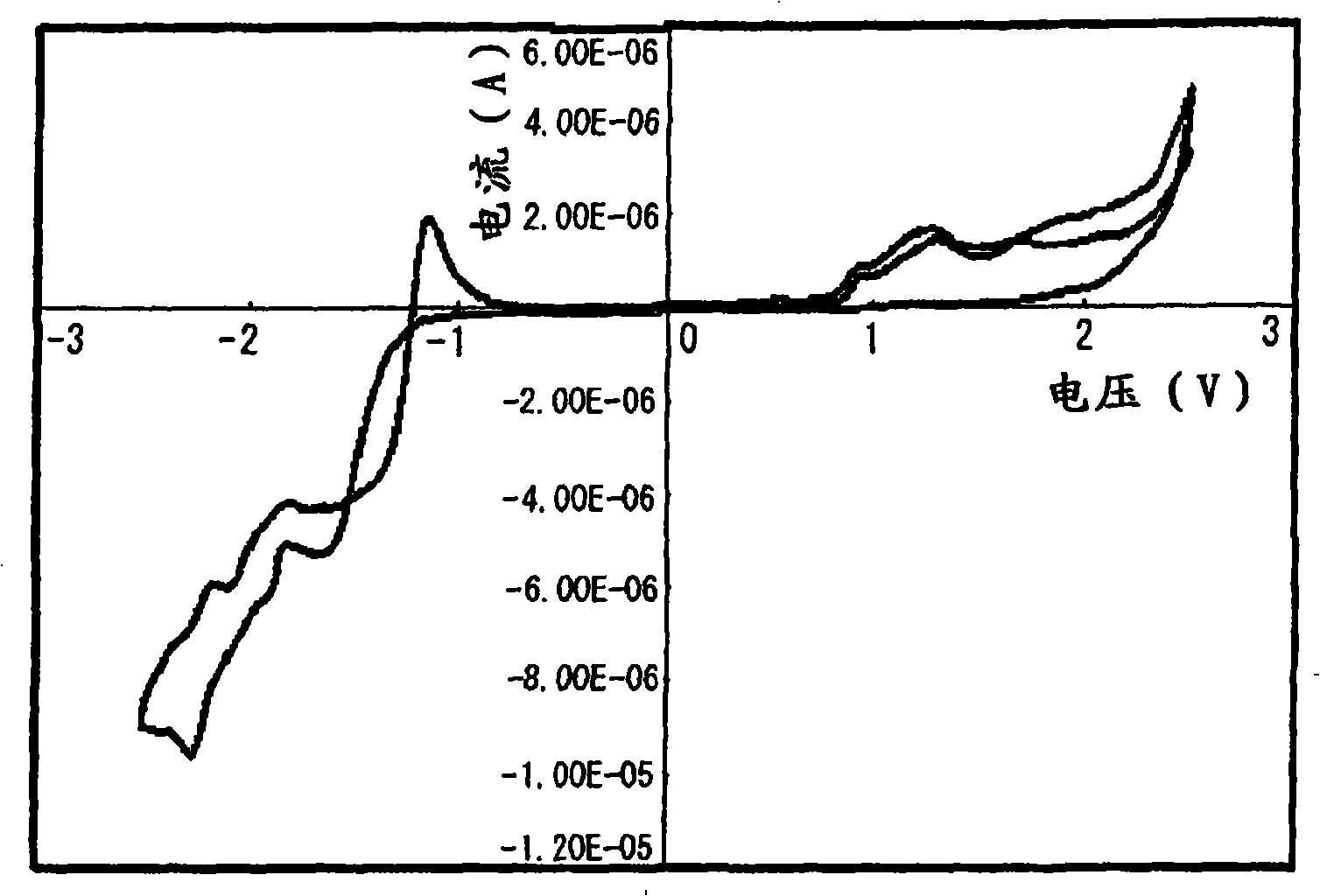
Thienyl polymer-containing polymer photocatalyst with high photocatalytic water splitting hydrogen production activity and preparation method thereof
ActiveCN113578382ALower bandgapGood coplanarityOrganic-compounds/hydrides/coordination-complexes catalystsHydrogen productionPhotocatalytic water splittingThiophene derivatives
The invention discloses a thienyl polymer-containing photocatalyst with high photocatalytic water splitting hydrogen production activity and a preparation method thereof. The photocatalyst is prepared by adopting a simple ternary copolymerization Suzuki coupling reaction, and the construction unit of the photocatalyst comprises pyrene, thiophene or thiophene derivative and dibenzothiophene sulfone. The pyrenyl monomer and the dibenzothiophene sulfuryl monomer for polymerization have the same polymerizable functional groups and can be subjected to Suzuki coupling reaction with thiophene or thiophene derivative monomers at the same time so as to ensure that pyrenyl units and dibenzothiophene sulfuryl units in the polymer structure are connected through thiophene or thiophene derivative units. The polymer photocatalyst has the characteristics of high photocatalytic hydrogen production activity, high apparent quantum efficiency, narrow optical band gap and continuous and adjustable structure and composition, is simple in preparation process, high in yield and stable in performance, can release hydrogen under sunlight, and can be used in the field of photocatalytic hydrogen production.
Owner:SHAANXI NORMAL UNIV
Antineoplastic medicament antifolate, its salt and midbody
InactiveCN101195625AStrong growth inhibitory activityOvercoming drug resistanceOrganic active ingredientsOrganic chemistryAnti cancer drugsAnti-Tumor Drugs
The invention discloses an antifolic for anti-tumor therapy, a relative salt and intermediate, wherein the antifolic and relative salt are represented as below, R1=NH2, R2=CH3, or R1=NHCH3 and R2=H, Ar is 1, 4-phenyl or 2, 5-thiofuran, R3 is H, metal cation, ammonium ion, or organic ammonium cation. The invention further discloses an application of the antifolic and relative salt for preparing anti-cancer drug. The inventive antifolic and relative salt have significant growth restraining activity on multiple tumor cells.
Owner:SHANGHAI JIAO TONG UNIV
Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto
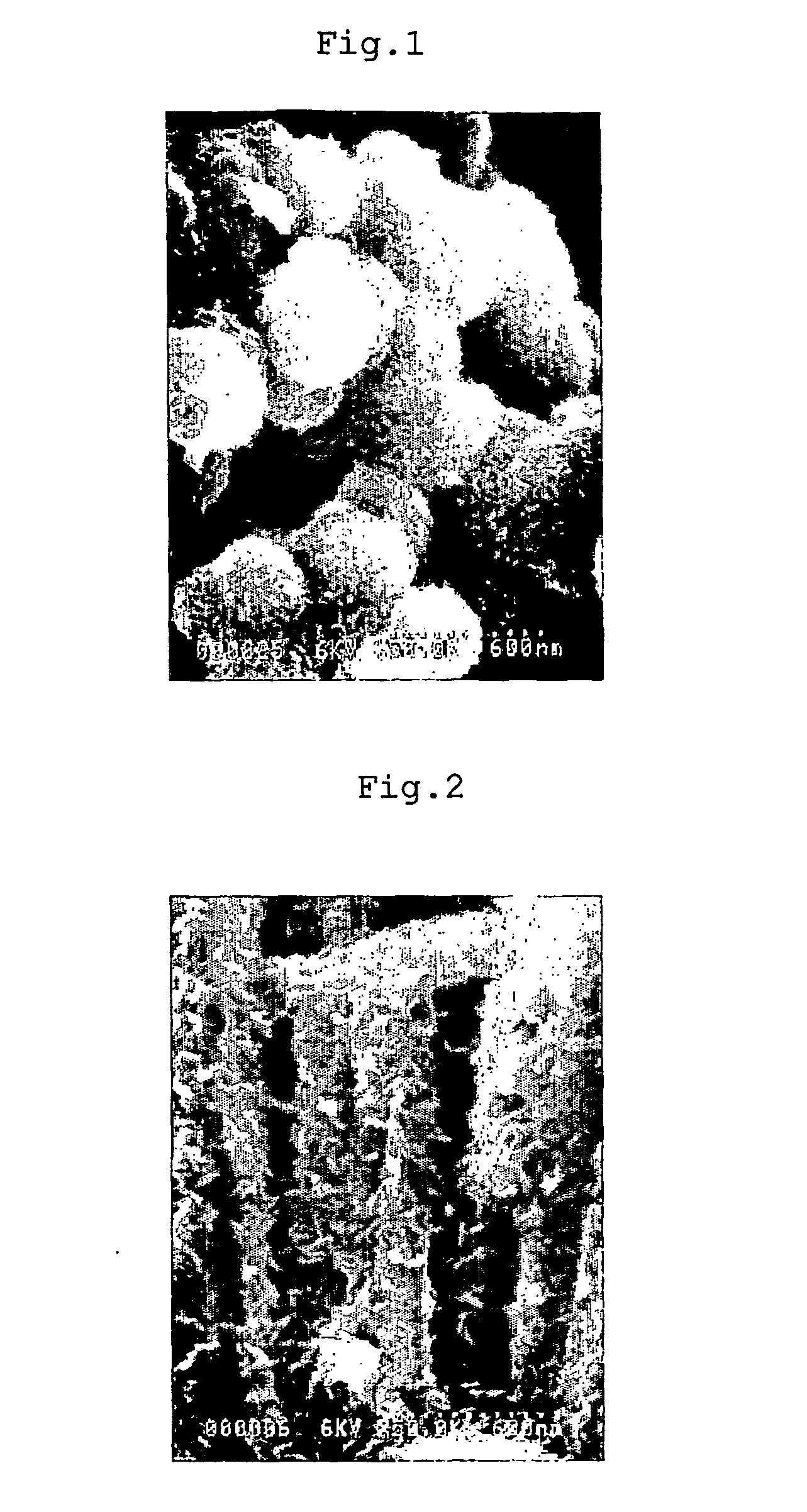
The invention discloses N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazole-[1,5-α]-pyrimidin-7-yl}phenyl)acetamide (compound ( 1)), and their use as sedative-hypnotics, anxiolytics, anticonvulsants and skeletal muscle relaxants. At the same time, methods for preparing the substance and related compositions are also disclosed, especially for the treatment of insomnia. And provides the exceptional physical and thermal stability possessed by the Form I polymorph. Type II polymorphs are also contemplated.
Owner:NEUROCRINE BIOSCI INC
Thiophene compound having sulfonyl group and process for producing the same
InactiveCN101282959AImprove heat resistanceImprove solubilityOrganic chemistryFinal product manufactureAcyl groupPhenyl group
A thiophene compound having sulfonyl groups which is represented by the formula [1]. It has high heat resistance and high unsusceptibility to oxidation and can improve solubility and dispersibility in various solvents. [In the formula, R1 and R2 each independently represents hydrogen, halogeno, cyano, etc.; and R3 and R3' each independently represents C1-20 alkyl, C1-20 haloalkyl, phenyl optionally substituted by W, thienyl optionally substituted by W, etc. (W represents chlorine, etc.)].
Owner:NISSAN CHEM CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






























![Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b0e4ea2b-a760-45a3-8844-17f82418db14/2014100045375100002DEST_PATH_IMAGE002.PNG)
![Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b0e4ea2b-a760-45a3-8844-17f82418db14/2014100045375100002DEST_PATH_IMAGE004.PNG)
![Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester Preparation method of 2-hydroyxl-2,2-dithienyl-2-polyglycolic acid-1-azabicyclo[2, 2, 2] octyl-3(R)-base ester](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b0e4ea2b-a760-45a3-8844-17f82418db14/2014100045375100002DEST_PATH_IMAGE010.PNG)






![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe5cdd4d-2af8-4f4e-8238-58deb8d51f2f/A0081315800441.PNG)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe5cdd4d-2af8-4f4e-8238-58deb8d51f2f/A0081315800442.PNG)
![Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto Polymorphs of N-methyl-N-(3-{3-[2-thienylcarbonyl]-pyrazol-[1,5-alpha]-pyrimidin-7-yl}phenyl)acetamide and compositions and methods related thereto](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/fe5cdd4d-2af8-4f4e-8238-58deb8d51f2f/A0081315800451.PNG)