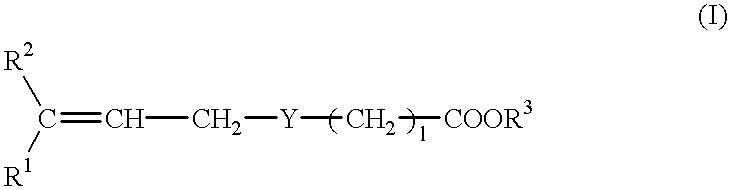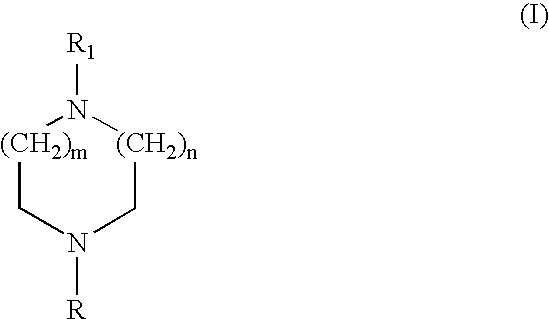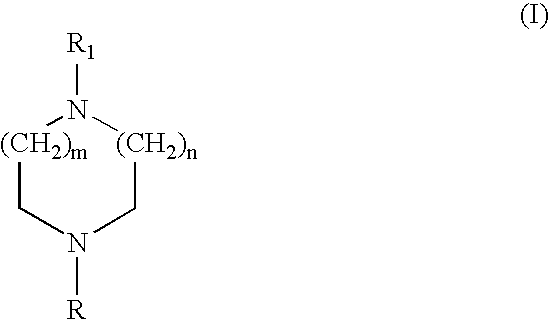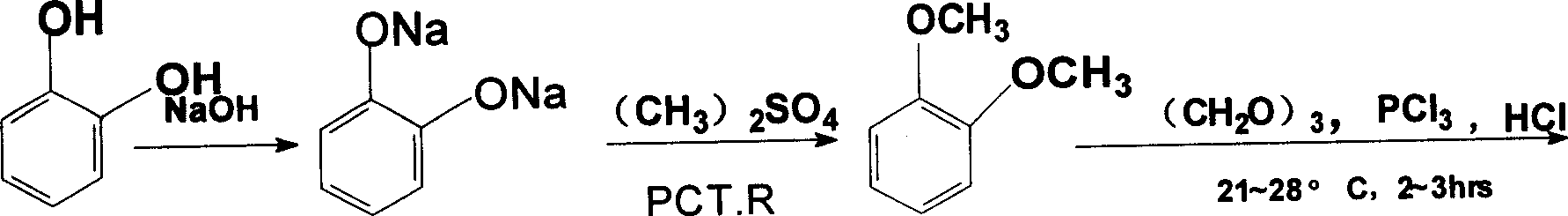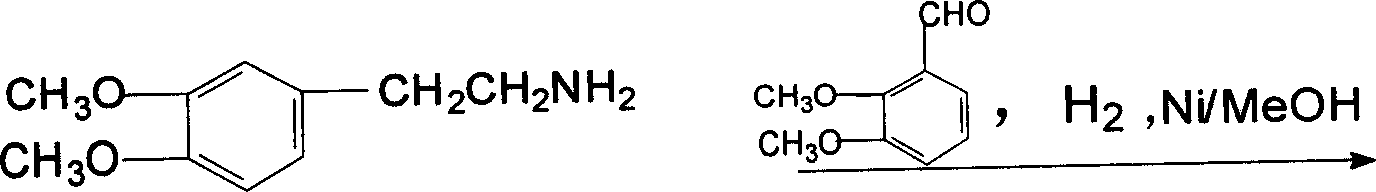Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
368 results about "Methylenedioxy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
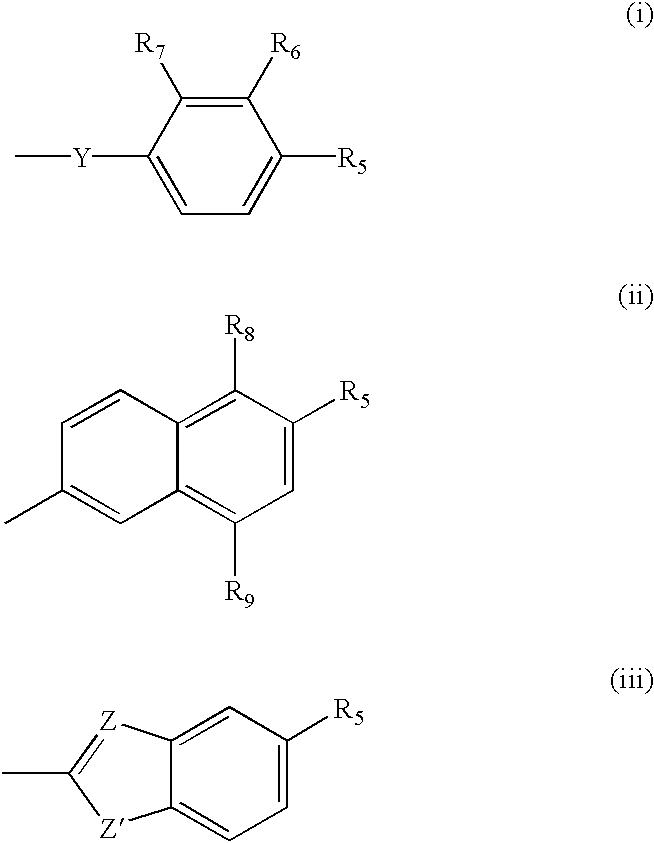
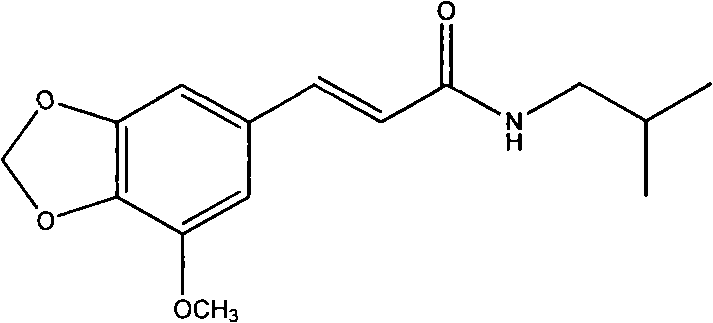
Methylenedioxy is the term used in the field of chemistry, particularly in organic chemistry, for a functional group with the structural formula R-O-CH₂-O-R' which is connected to the rest of a molecule by two chemical bonds. The methylenedioxy group consists of two oxygen atoms connected to a methylene bridge (-CH₂- unit). The methylenedioxy group is generally found attached to an aromatic structure such as phenyl where it forms the methylenedioxyphenyl or benzodioxole functional group which is widely found in natural products, including safrole, and drugs and chemicals such as tadalafil, MDMA, paroxetine and piperonyl butoxide.
N-benzyldioxothiazolidylbenzamide derivatives and process for producing the same
PCT No. PCT / JP96 / 01459 Sec. 371 Date Dec. 2, 1997 Sec. 102(e) Date Dec. 2, 1997 PCT Filed May 30, 1996 PCT Pub. No. WO96 / 38428 PCT Pub. Date Dec. 5, 1996The present invention provides novel N-benzyldioxothiazolidylbenzamide derivatives that improve the insulin resistance and have potent hypoglycemic and lipid-lowering effects and processes for preparing the same, and relates to N-benzyldioxothiazolidylbenzamide derivatives characterized by being represented by a general formula (1) wherein R1 and R2 denote identically or differently hydrogen atoms, lower alkyl groups with carbon atoms of 1 to 4, lower alkoxy groups with carbon atoms of 1 to 3, lower haloalkyl groups with carbon atoms of 1 to 3, lower haloalkoxy groups with carbon atoms of 1 to 3, halogen atoms, hydroxyl groups, nitro groups, amino groups which may be substituted with lower alkyl group(s) with carbon atoms of 1 to 3 or hetero rings, or R1 and R2 link to form a methylenedioxy group, R3 denotes a lower alkoxy group with carbon atoms of 1 to 3, hydroxyl group or halogen atom, and dotted line indicates double bond or single bond in combination with solid line, and processes for preparing the same.
Owner:KYORIN PHARMA CO LTD
Phenylethanolamine compounds useful as beta 3 agonists, process for producing the same, and intermediates in the production of the same
PCT No. PCT / JP96 / 03097 Sec. 371 Date Apr. 24, 1998 Sec. 102(e) Date Apr. 24, 1998 PCT Filed Oct. 24, 1996 PCT Pub. No. WO97 / 15549 PCT Pub. Date May 1, 1997The present invention relates to phenylethanolamine compounds represented by general formula [I]: (where R1 represents hydrogen or halogen; R2 represents hydrogen, hydroxy, lower alkoxy, lower alkoxy substituted with one or two lower alkoxycarbonyl or carboxy groups, lower alkoxy substituted with lower alkylaminocarbonyl which may be substituted with lower alkoxy, lower alkoxy substituted with cyclic aminocarbonyl of 4 to 6 carbon atoms, lower alkoxycarbonyl or carboxy; R3 represents hydrogen, hydroxy, lower alkoxy or lower alkoxy substituted with one or two lower alkoxycarbonyl or carboxy groups; R2 and R3 may be bonded to each other to form methylenedioxy substituted with carboxy or lower alkoxycarbonyl; and m and n are 0 or 1), and their pharmacologically acceptable salts, which have a potent beta 3 adrenergic stimulating effect and high beta 3 adrenergic receptor selectivity, as well as to processes for their production and intermediates in their production.
Owner:TT PHARMA
Process for the manufacture of quinoline derivatives
Owner:ACTIVE BIOTECH AB
Process for the manufacture of quinoline derivatives
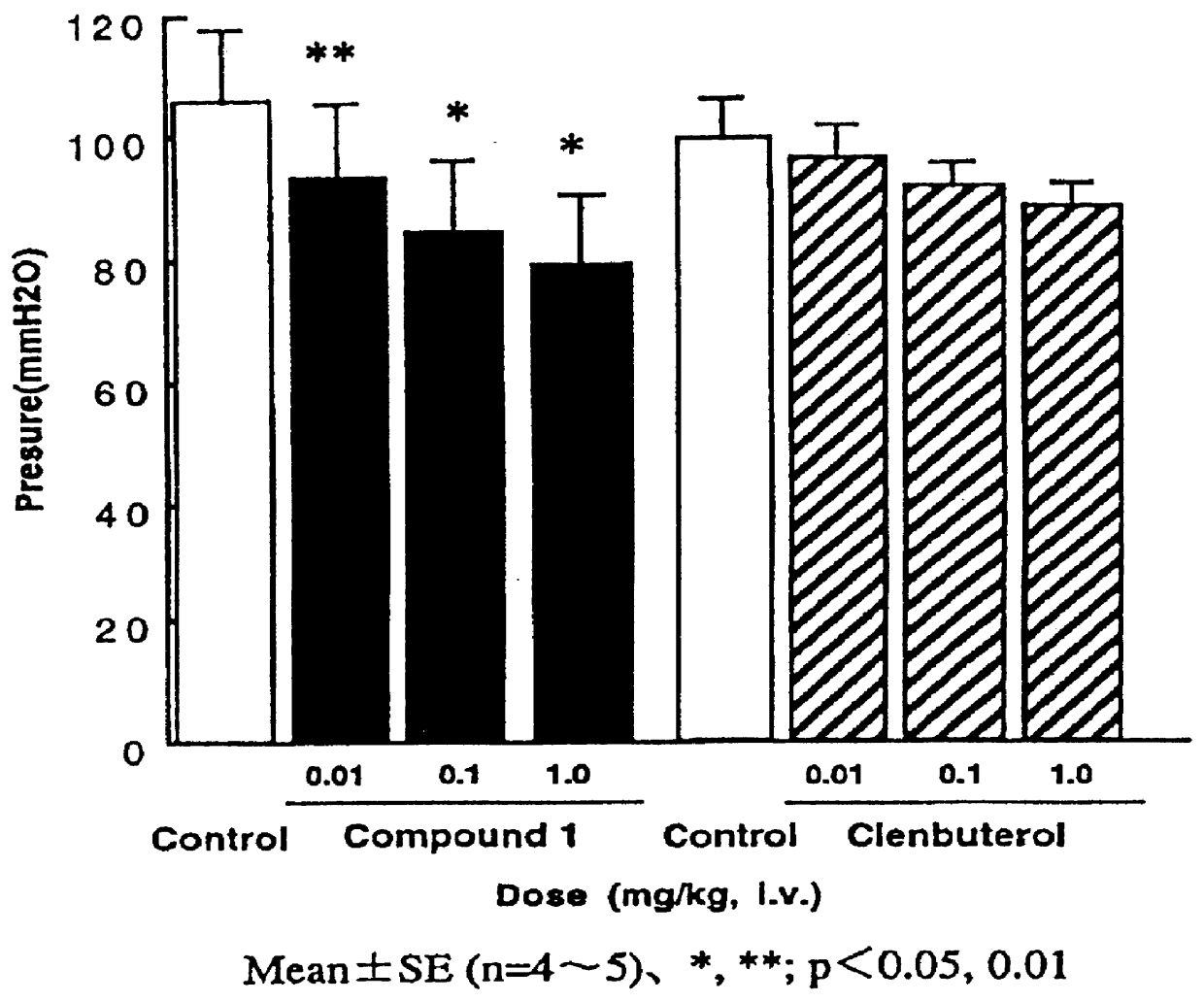
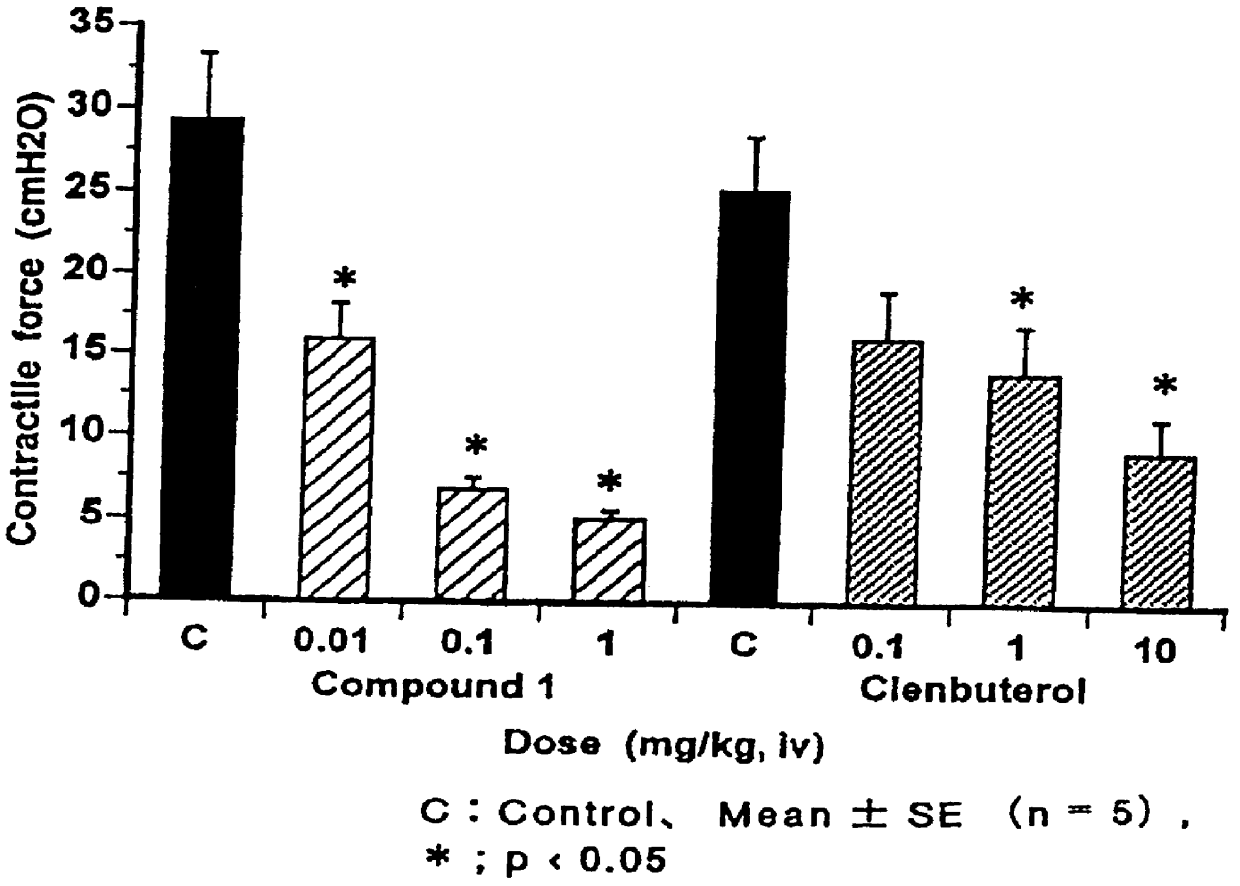
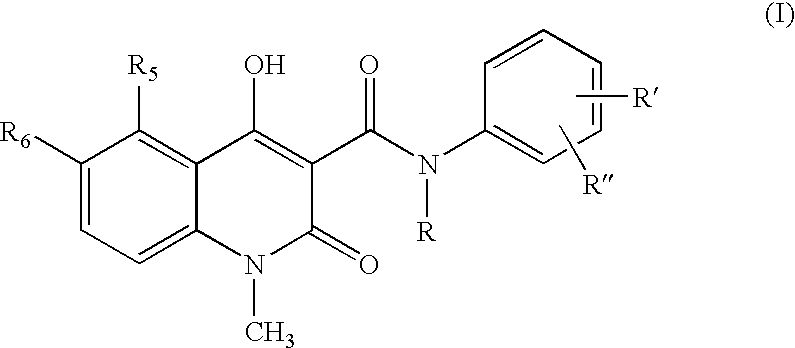
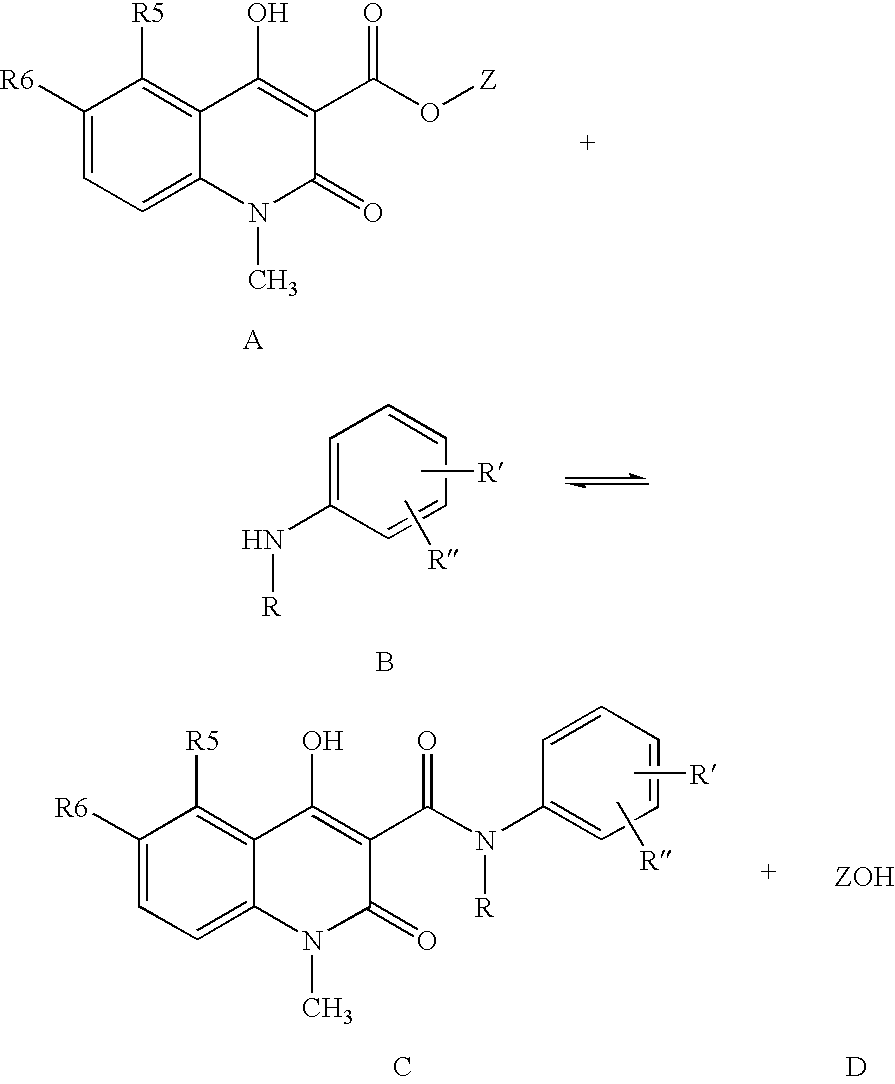
A process for the preparation of the compounds of general formula (I) wherein R is selected from methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec.-butyl and allyl; R5 is selected from methyl, ethyl, n-propyl, iso-propyl, methoxy, ethoxy, methylthio, ethylthio, n-propylthio, methylsulphinyl, ethylsulphinyl, fluoro, chloro, bromo, trifluoromethyl, and OCHxFy; wherein x=0−2, y=1−3 with the proviso that x+y=3; R6 is hydrogen; or R5 and R6 taken together are methylenedioxy; R′ is selected from hydrogen, methyl, methoxy, fluoro, chloro, bromo, trifluoromethyl, and OCHxFy, wherein x=0−2, y=1−3 with the proviso that x+y=3; R″ is selected from hydrogen, fluoro and chloro, with the proviso that R″ is selected from fluoro and chloro only when R′ is selected from fluoro and chloro; by reacting a quinoline-3-carboxylic acid ester derivative of formula A, where Z is methyl, with an aniline derivative of formula B according to the following reaction diagram, to give the compound of general formula (I), designated “C”, and an alcohol, designated “D”. in a solvent selected from straight or branched alkanes and cycloalkanes or mixtures thereof with a boiling point between 80 and 200° C.
Owner:ACTIVE BIOTECH AB
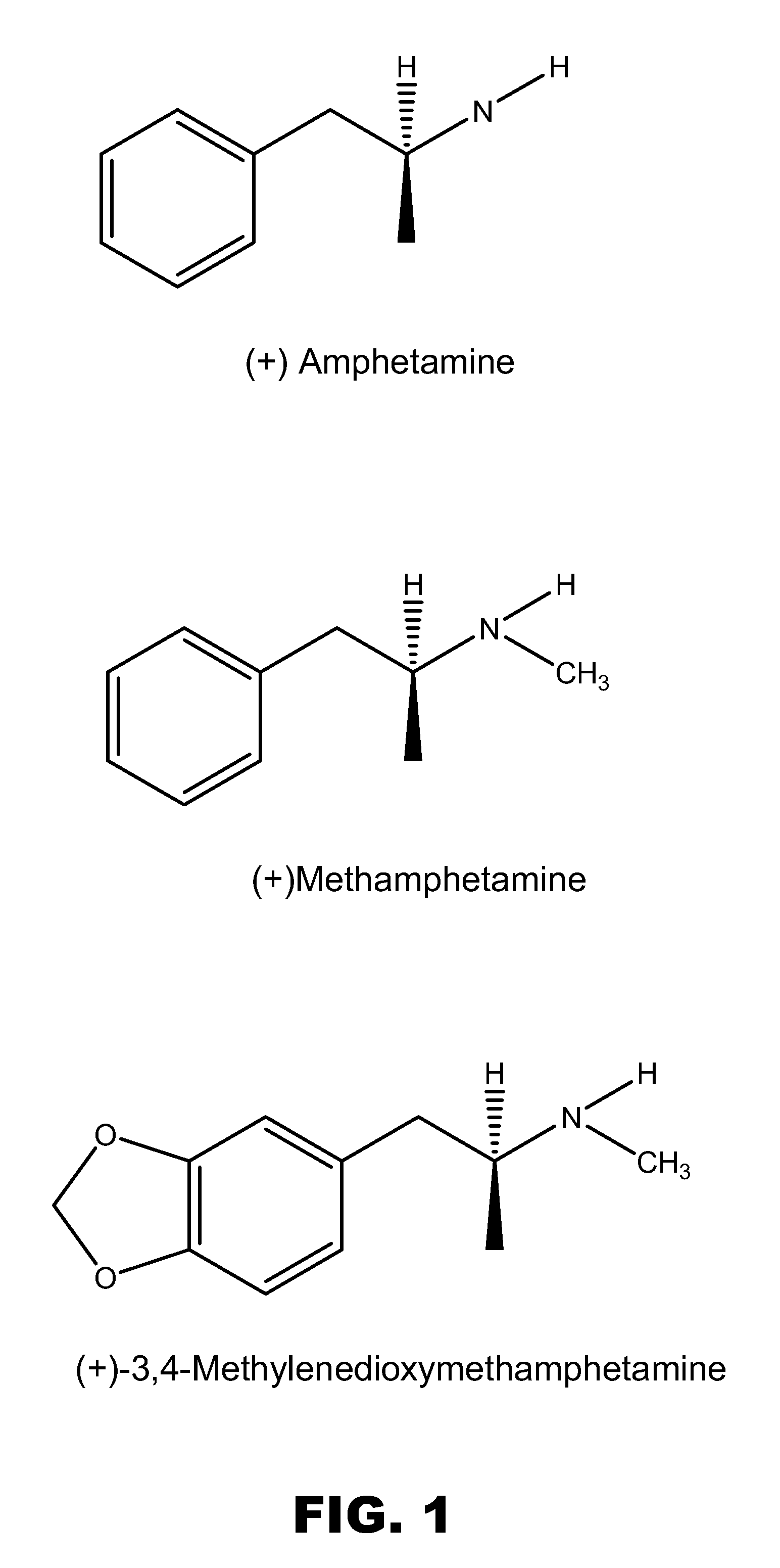
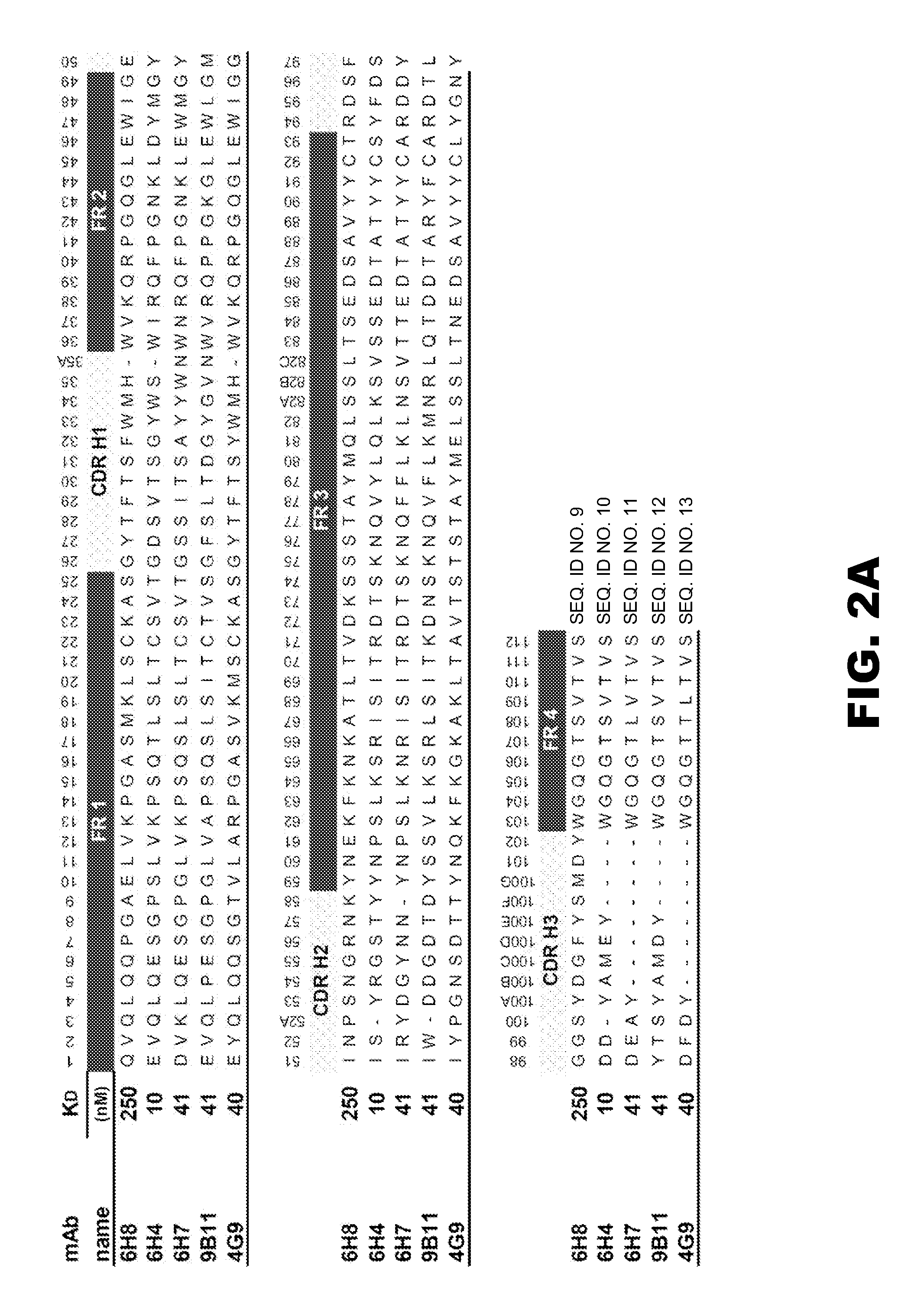
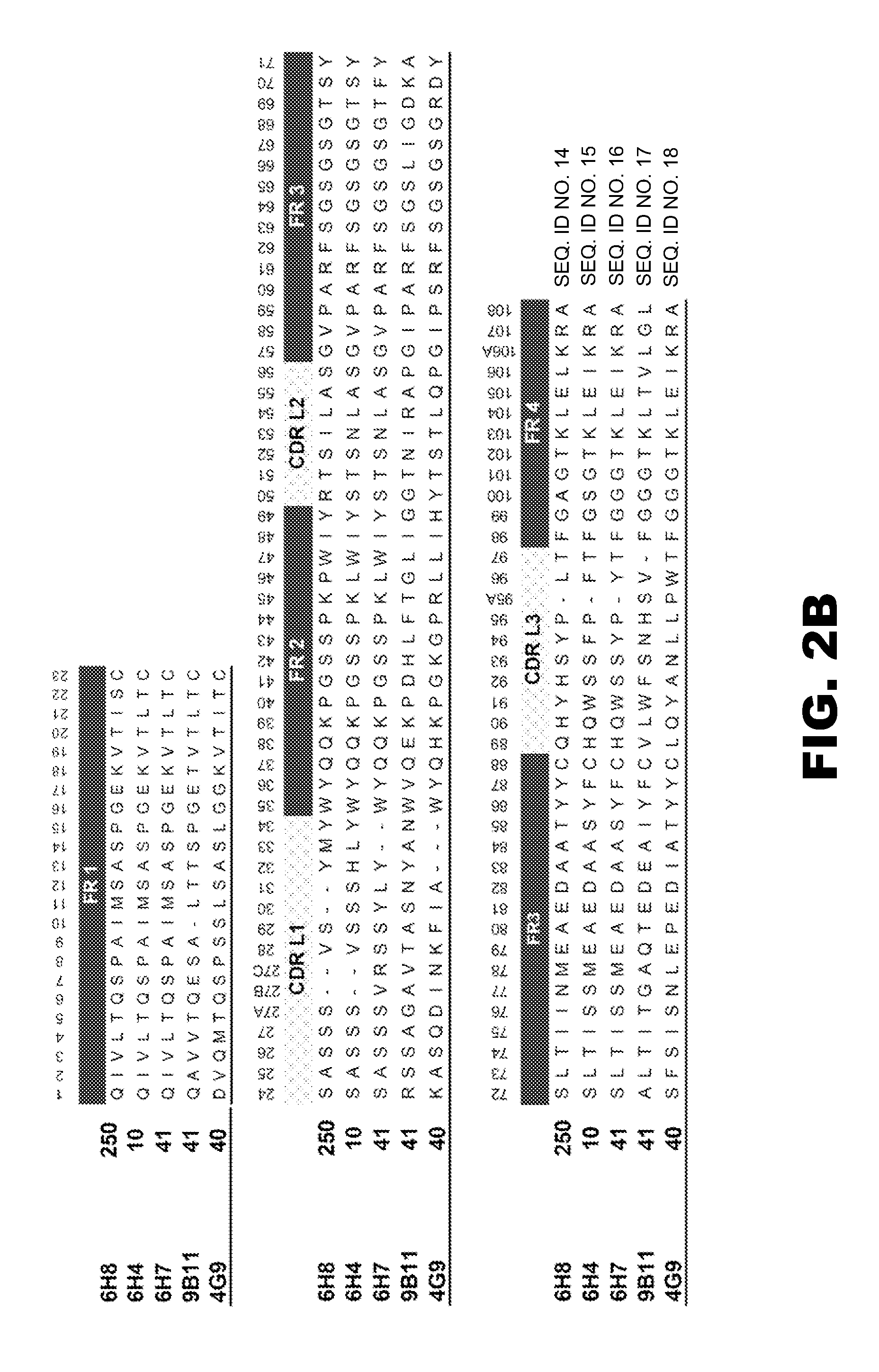
Monoclonal antibodies that selectively recognize methamphetamine and methamphetamine like compounds
ActiveUS20080125579A1Reduce concentrationImmunoglobulins against animals/humansAntibody ingredientsMonoclonal antibodyMDMA
The invention generally relates to monoclonal antibodies that recognize at least one compound from the group consisting of (+) methamphetamine, (+) amphetamine, and (+) 3,4-methylenedioxymethamphetamine ((+) MDMA). Generally speaking, the monoclonal antibodies do not recognize (−) methamphetamine, (−) amphetamine, or (−) MDMA.
Owner:BIOVENTURES LLC
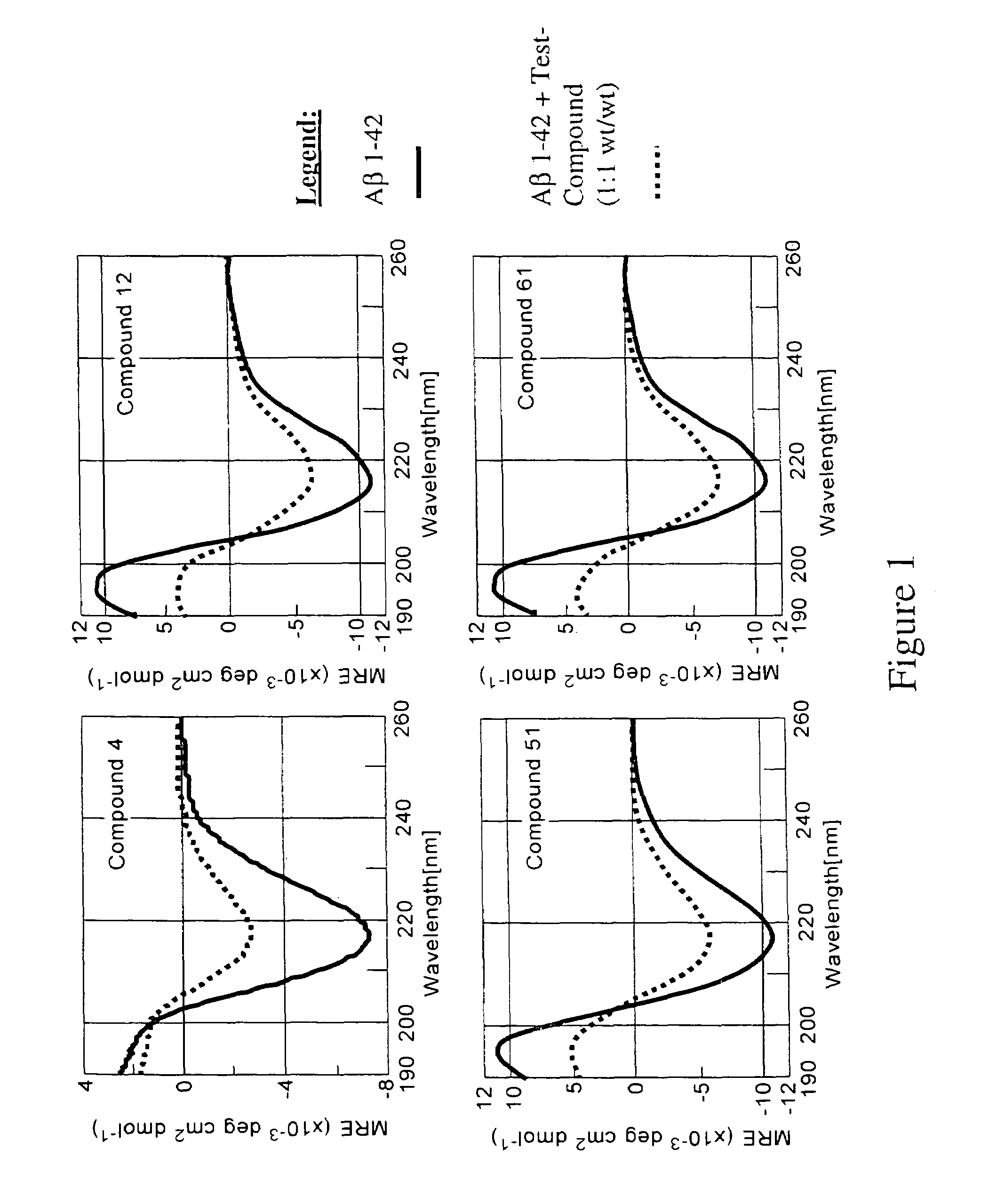
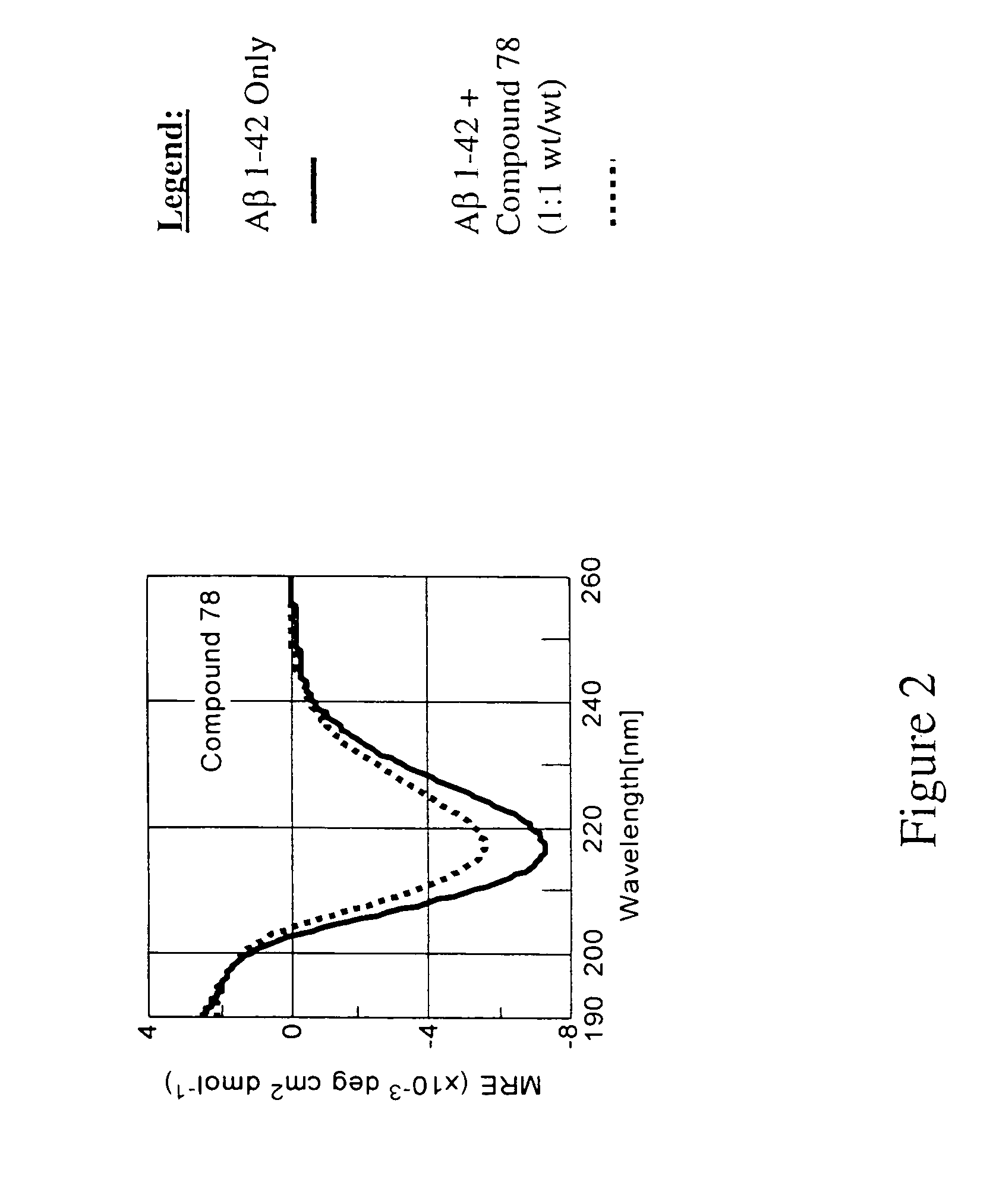
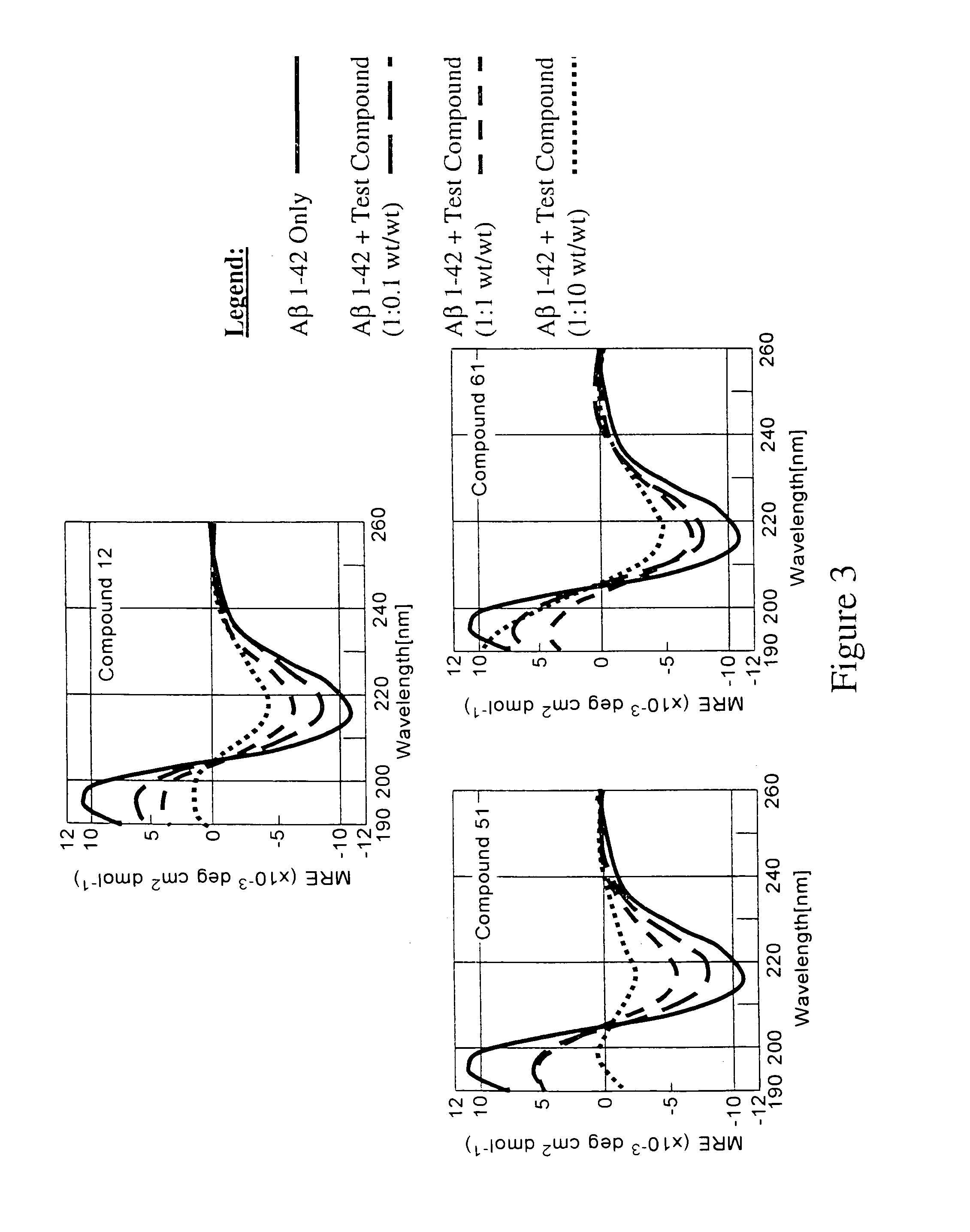
Compounds, compositions and methods for the treatment of synucleinopathies
Bis- and tris-dihydroxyaryl compounds and their methylenedioxy analogs and pharmaceutically acceptable esters, their synthesis, pharmaceutical compositions containing them, and their use in the treatment of synucleinopathies, such as Parkinson's disease, and the manufacture of medicaments for such treatment.
Owner:PROTEOTECH
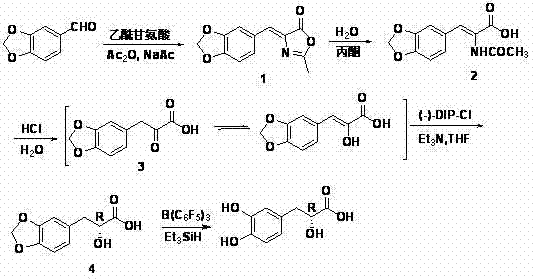
Asymmetric synthesis method of (+)-tanshinol
InactiveCN102924265ARaw materials are easy to getSimple and fast operationOrganic compound preparationCarboxylic compound preparationPropanoic acidCombinatorial chemistry
The invention relates to an asymmetric synthesis method of (+)-tanshinol, which comprises the following steps: carrying out Knoevenagel condensation on the raw material heliotropin, hydrolyzing for ring opening to obtain beta-(3,4-3,4-methylenedioxyphenyl)pyruvic acid, carrying out key asymmetric reduction reaction to obtain R-beta-(3,4-dibenzyloxyphenyl)-alpha-hydracrylic acid, and finally, deprotecting to obtain the (+)-tanshinol. The method has the advantages of accessible raw material and high optical purity of the product, is simple to operate, and can implement large-scale preparation.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Synthetic method of hydroxytyrosol
InactiveCN103664536AMild reaction conditionsLower reaction costOrganic chemistryOrganic compound preparationChemical synthesisHydroxytyrosol
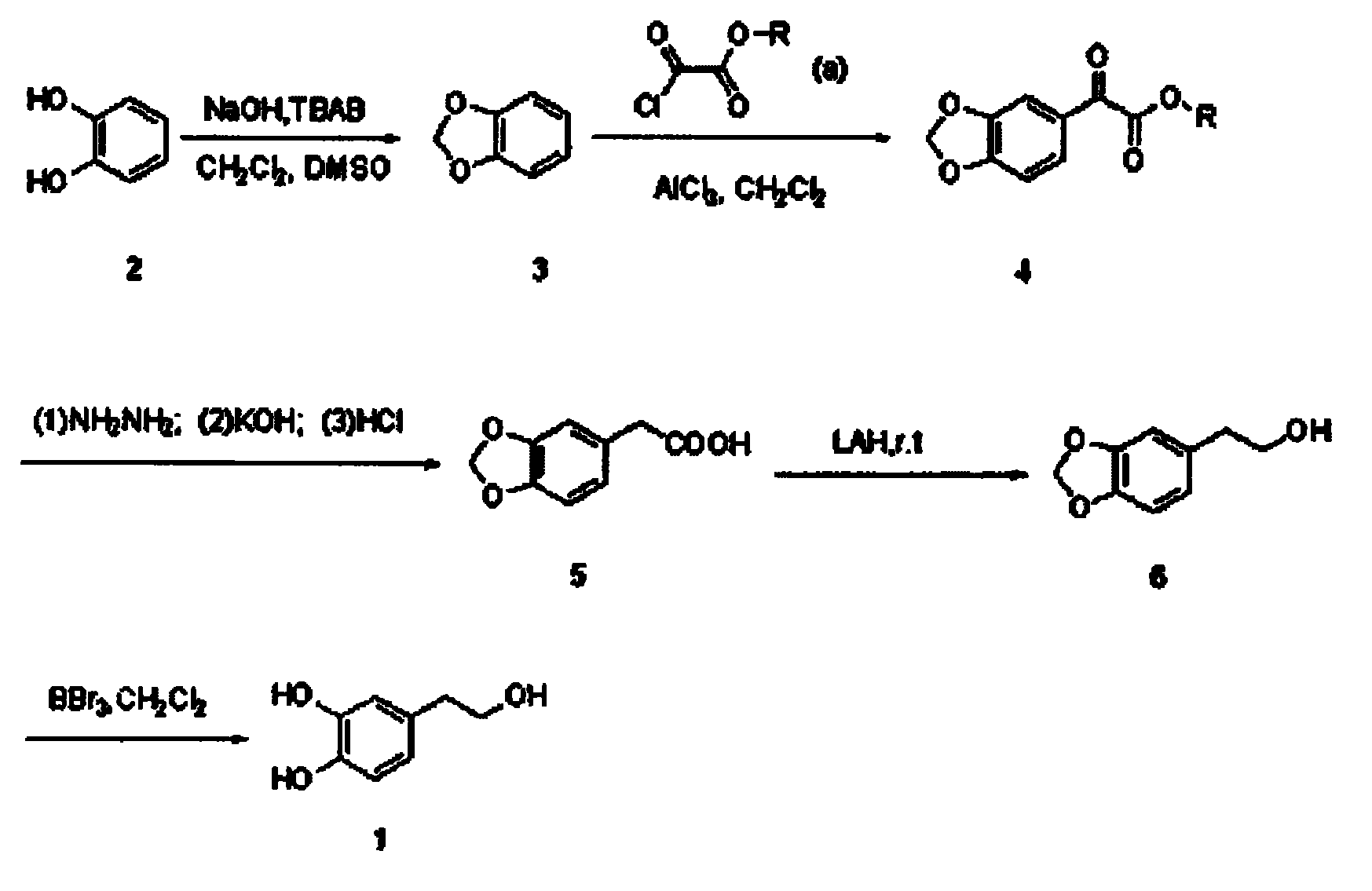
The invention belongs to the technical field of medicament synthesis, and in particular relates to a chemical synthetic method of hydroxytyrosol. The chemical synthetic method comprises the steps of (1) protecting two phenolic hydroxyl groups of catechol by using dichloromethane, and enabling catechol to react with dichloromethane to prepare 1,2-methylenedioxybenzene; (2) enabling 1,2-methylenedioxybenzene to react with various monoesters of oxalyl chloride to prepare 3,4-methylenedioxy phenylglyoxylic acid ester; (3) preparing 3,4-methylenedioxy phenylacetic acid by using 3,4-methylenedioxy phenylglyoxylic acid ester through a Wollff-kishner-Huang Minglong reduction reaction; and (4) reducing the 3,4-methylenedioxy phenylacetic acid by using lithium aluminum hydride, lithium borohydride or sodium borohydride to prepare 3,4-methylenedioxy phenethyl alcohol, and then removing methylene protection of the 3,4-methylenedioxy phenethyl alcohol by using boron tribromide or palladium on activated carbon to prepare hydroxytyrosol. A reactive reagent used in the synthetic method disclosed by the invention is easy to obtain and low in price, the reaction condition is mild, and the final yield of the whole reaction is 23%.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Apoptosis inducing adamantyl derivatives and their usage as anti-cancer agents, especially for cervical cancers and dysplasias
InactiveUS6462064B1Easy to convertCombating the greasy appearance of the skin orBiocideHydroxy compound active ingredientsCancer preventionRetinoid
The invention relates to the discovery that specific adamantyl or adamantyl group derivatives containing retinoid-related compounds induce apoptosis of cancer cells and therefore may be used for the treatment of cancer, including advanced cancer. Also, the present invention relates to novel adamantyl or adamantyl group derivatives containing retinoid compounds and their usage for treatment and / or prevention of cancer, keratinization disorders, dermatological conditions, and other therapies More specifically, it has been shown that such adamantyl compounds, e.g., 6-[3-(1-adamantyl)-4-methoxyphenyl]-2-naphthoic acid, 2-[3-(1-adamantyl)-4-methoxyphenyl]-5-benzimidazole carboxylic acid, and 6-[3-(1-adamantyl)-4,5-methylenedioxyphenyl]-2-naphthoic acid, can be used to treat or prevent cervical cancers and precancers such as cervical dysplasias, including high grade and low grade dysplasias.
Owner:GALDERMA RES & DEV SNC +1
Application of 5'-methoxy-3',4'-methylenedioxy cinnamic acid isobutyl amide in preparing antidepressant medicaments
ActiveCN102240281ASignificantly antagonizes body temperature dropSignificantly antagonizes drooping eyelidsNervous disorderPill deliveryMethylenedioxyCinnamic acid
The invention belongs to the field of traditional Chinese medicine preparation, and relates to application of an amide compound 5'-methoxy-3',4'-methylenedioxy cinnamic acid isobutyl amide extracted from plants in preparing antidepressant medicaments.
Owner:TIANJIN TASLY PHARMA CO LTD
Compounds, compositions and methods for the treatment of amyloid diseases and synucleinopathies such as alzheimer's disease, type 2 diabetes, and parkinson's disease
Bis- and tris-dihydroxyaryl compounds and their methylenedioxy analogs and pharmaceutically acceptable esters, their synthesis, pharmaceutical compositions containing them, and their use in the treatment of amyloid diseases, especially Aβ amyloidosis, such as observed in Alzheimer's disease, IAPP amyloidosis, such as observed in type 2 diabetes, and synucleinopathies, such as observed in Parkinson's disease, and the manufacture of medicaments for such treatment.
Owner:PROTAMED
Pharmaceutical composition for treating transient ischemic attack
A pharmaceutical composition for treating a transient ischemic attack which comprises a compound of the formula: wherein R1 is a pyridyl group, R2 is a phenyl, thienyl, furyl, naphthyl, benzothienyl or pyridyl group, which may be substituted with a lower alkoxy group, a lower alkyl group, a halogen atom, trifluoromethyl group, a lower alkenyl group or / and methylenedioxy group, R3 is hydrogen atom or a lower alkyl group, and l is an integer of 0 to 6, Y is sulfur atom, methylene group or a group of the formula: wherein R4 is hydrogen atom or acetyl group, and m is 0 or 1, or a pharmaceutically acceptable salt.
Owner:TERASHITA ZEN ICHI +2
N-benzyldioxothiazolidylbenzamide derivatives and process for producing the same
The present invention provides novel N-benzyldioxothiazolidylbenzamide derivatives that improve the insulin resistance and have potent hypoglycemic and lipid-lowering effects and processes for preparing the same, and relates to N-benzyldioxothiazolidylbenzamide derivatives characterized by being represented by a general formula (1) [wherein R1 and R2 denote identically or differently hydrogen atoms, lower alkyl groups with carbon atoms of 1 to 4, lower alkoxy groups with carbon atoms of 1 to 3, lower haloalkyl groups with carbon atoms of 1 to 3, lower haloalkoxy groups with carbon atoms of 1 to 3, halogen atoms, hydroxyl groups, nitro groups, amino groups which may be substituted with lower alkyl group(s) with carbon atoms of 1 to 3 or hetero rings, or R1 and R2 link to form a methylenedioxy group, R3 denotes a lower alkoxy group with carbon atoms of 1 to 3, hydroxyl group or halogen atom, and dotted line indicates double bond or single bond in combination with solid line], and processes for preparing the same.
Owner:KYORIN PHARMA CO LTD
Fibriuretinin synthesis method
ActiveCN102040604AOptimizationSimple purification methodOrganic chemistryChemical synthesisBerberine
The invention relates to a fibriuretinin synthesis method. The method takes berberine hydrochloride as a starting material and comprises the following steps: selectively carrying out decomposition reaction on methylenedioxy of the berberine hydrochloride to obtain ortho-dyhydroxyl berberine hydrochloride; carrying out methylation reaction on hydroxyl of the ortho-dyhydroxyl berberine hydrochloride and dimethyl sulfate, recrystallizing, and adding hydrochloric acid to regulate pH value to obtain the target product, i.e. fibriuretinin. The fibriuretinin synthesis method has less synthesis steps, simple technology, and high total yield of 49.3% and the raw materials are easy to collect, so as to reduce cost, improve production efficiency and contribute a new synthesis method for fibriuretinin chemosynthesis industrialization.
Owner:YUNNAN PHYTOPHARML
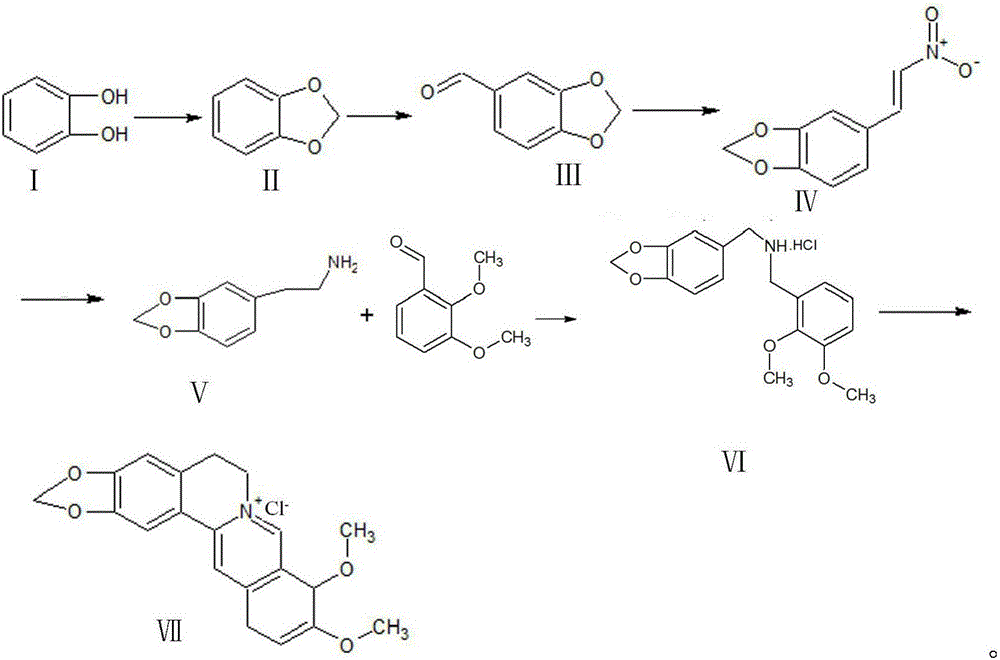
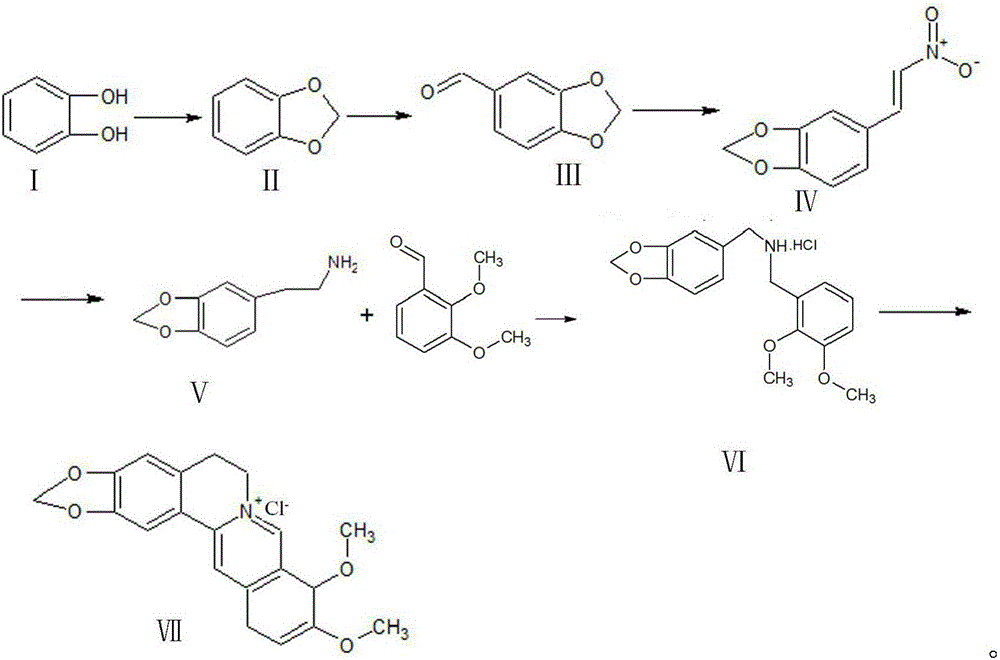
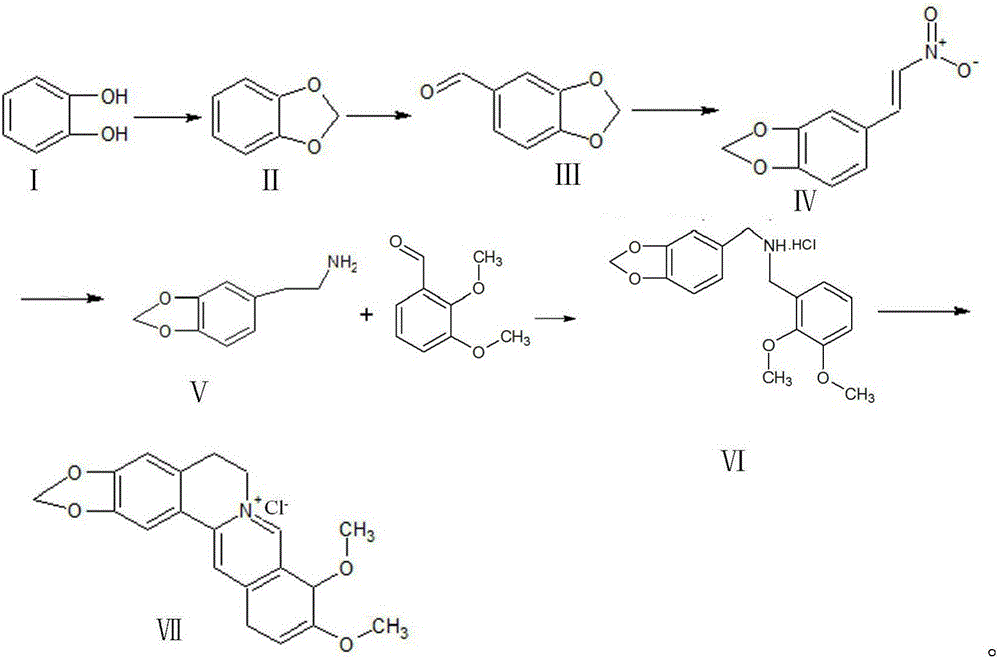
Synthetic process of berberine
The invention discloses a synthetic process of berberine. The synthetic process comprises the following synthetic route: pyrocatechol and dichloromethane are taken as raw materials and dimethyl sulphoxide is taken as a solvent to synthesize 1,3-benzodioxole; 1,3-benzodioxole is subjected to ViLsmeiar formylation to synthesize heliotropin; heliotropin undergoes nitration through a Henry reaction to generate beta-nitro-3,4-dioxomethenyl styrene; beta-nitro-3,4-dioxomethenyl styrene is subjected to Clemmensen reduction to generate 3,4-(methylenedioxyphenyl)ethylamine; and 3,4-(methylenedioxyphenyl)ethylamine and 2,3-dimethoxy benzaldehyde condense, and the obtained product is reduced to generate N-2,3-dimethoxybenzyl [3,4-(methylenedioxy)phenyl]ethylamine hydrochloride. N-2,3-dimethoxybenzyl [3,4-(methylenedioxy)phenyl]ethylamine hydrochloride is cyclized in a condition of glyoxal, formic acid and copper sulphate to generate berberine hydrochloride. According to the synthetic route, cyanation is avoided and toxicity is reduced. Zinc amalgam is adopted to replace H2Ni and LiAlH4, so that the cost is greatly reduced, the process difficulty is reduced, and the product yield is increased.
Owner:佑华制药(乐山)有限公司
Donor-Acceptor Compositions to Achieve High Contrast Broadly Absorbing Electrochromic Polymers
ActiveUS20160244553A1Easy to optimizeEasy to condenseCoatingsNon-linear opticsMethylenedioxyCopolymer
An improved contrast electrochromic polymers(ECP), is a copolymer having donor sequences with a normal distribution of at least one solubilizing donor repeating unit selected from substituted propylenedioxythiophene units (ProDOT) and / or substituted acyclic dioxythiophene units (AcDOT) and a plurality of monodispersed trimer sequences consisting of an acceptor unit bonded between two ethylenedioxythiophene units (EDOT), two methylenedioxythiophene units (MDOT), or a fused DAD trimer, where the monodispersed trimer sequence separates two of the donor sequences. One useful monodispersed trimer sequence is EDOT-BTD-EDOT (EBE). The donor sequences can have ProDOT units bound to the monodispersed trimer sequence. The donor sequence can have ProDOT units alternating with AcDOT units. Useful ECPs can have the structure: Pro-Pro / EBE; Ac-Ac / EBE; or Pro-Acx / EBE1−x where x is a fraction between 0.5 and 0.9.
Owner:GEORGIA TECH RES CORP
Process for the preparation of droxidopa
ActiveUS20170335357A1High yieldHigh purityHydrolasesOrganic compound preparationAspergillusMethylenedioxy
A novel process for the preparation of L-threo-dihydroxyphenylserine (Droxidopa) is described. It comprises of enantioselective hydrolysis of racemic (DL)-threo-N-acetyl-3-(3,4-methylenedioxyphenyl)-serine using commercially available L-amino acylase from Aspergillus sp. (EC 3.5.1.14) in the presence of cobalt ions, to obtain (L)-threo-3-(3,4-methylenedioxyphenyl)-serine followed by dealkylation to obtain Droxidopa. Protecting the amino group of (L)-threo-3-(3,4-methylenedioxyphenyl)-serine using either benzyloxycarbonyl or phthaloyl group before dealkylation followed by deprotection of the amino group results in obtaining Droxidopa in high yields and purity.
Owner:DIVI S LAB LTD
Phosphine compound, its intermediate, its complex with palladium and a manufacturing method of unsaturated compounds by using the palladium complex
ActiveUS7129367B2Carboxylic acid nitrile preparationOrganic compound preparationHydrogen atomMethylenedioxy
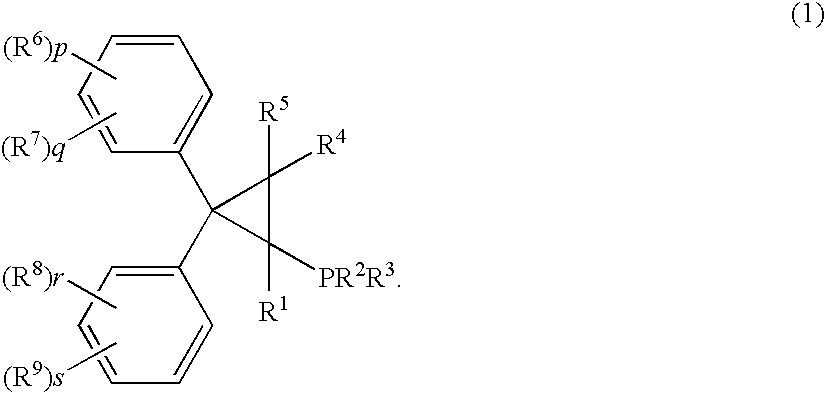
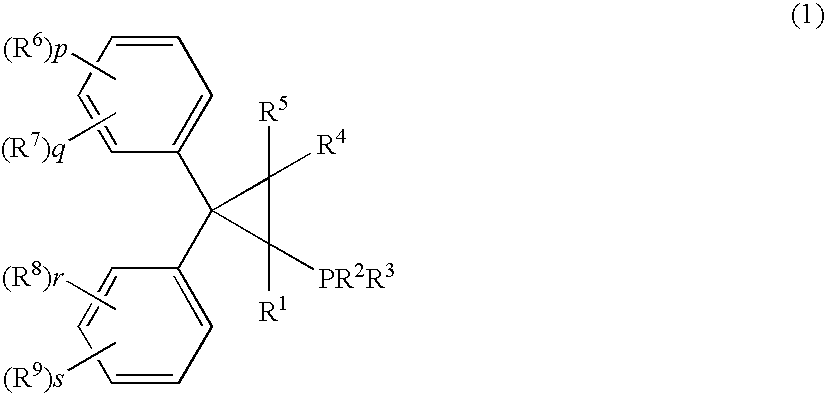
Palladum-phosphine complexes obtained by reacting a 5 compound of formula (1) below with a palladium compound: F(I) (wherein R1 is a hydrogen atom, an alkyl group, a cycloalkyl group or a phenyl group which may be substituted; R2 and R3 are each, the same or different, an alkyl group, a cycloalkyl group or a phenyl group which may be substituted; R4 and R5 are each, the same or different, a hydrogen atom, an alkyl group, a cycloalkyl group or a phenyl group which may be substituted; R6, R7, R8 and R9 are each, the same or different, an alkyl group, a cycloalkyl group, a phenyl group which may be substituted, an alkoxyl group, a dialkylamino group, a halogen atom, a phenyl group, a benzyl group, a naphthyl group or a halogenated alkyl group; R6 and R7, R8 and R9 may be combined to form, each, a fused ring, a trimethylene group, a tetramethylene group or a 20 methylenedioxy group; p, q, r and s are each an integer of 0 to 5; and p+q, and r+s are each in the range of 0 to 5.), which is a novel and efficient catalyst for manufacturing various useful compounds
Owner:TAKASAGO INTERNATIONAL CORPORATION
N-P flameresistant material and preparation method thereof and application in textiles
ActiveCN105348326ARapid responseFast processGroup 5/15 element organic compoundsHeat resistant fibresFire retardantHydroxymethyl
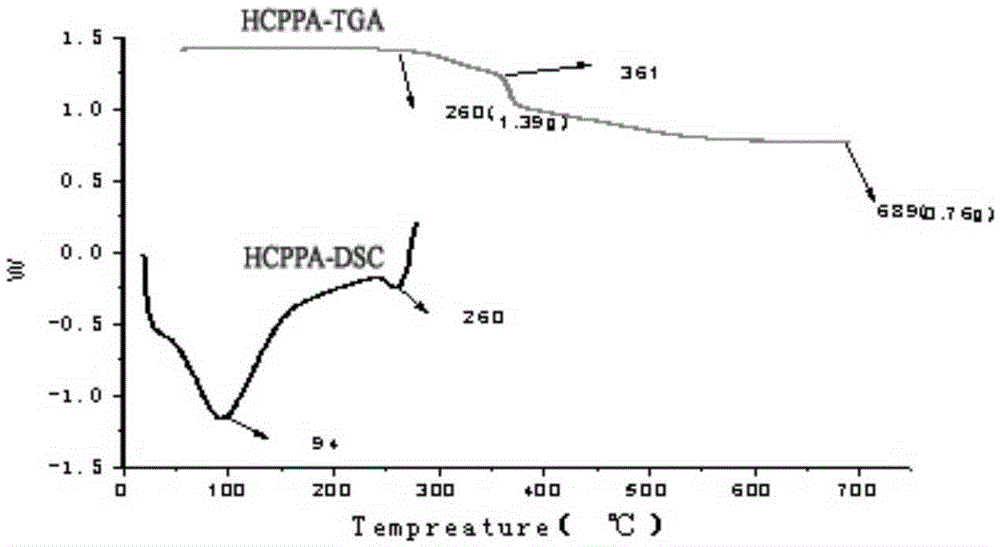
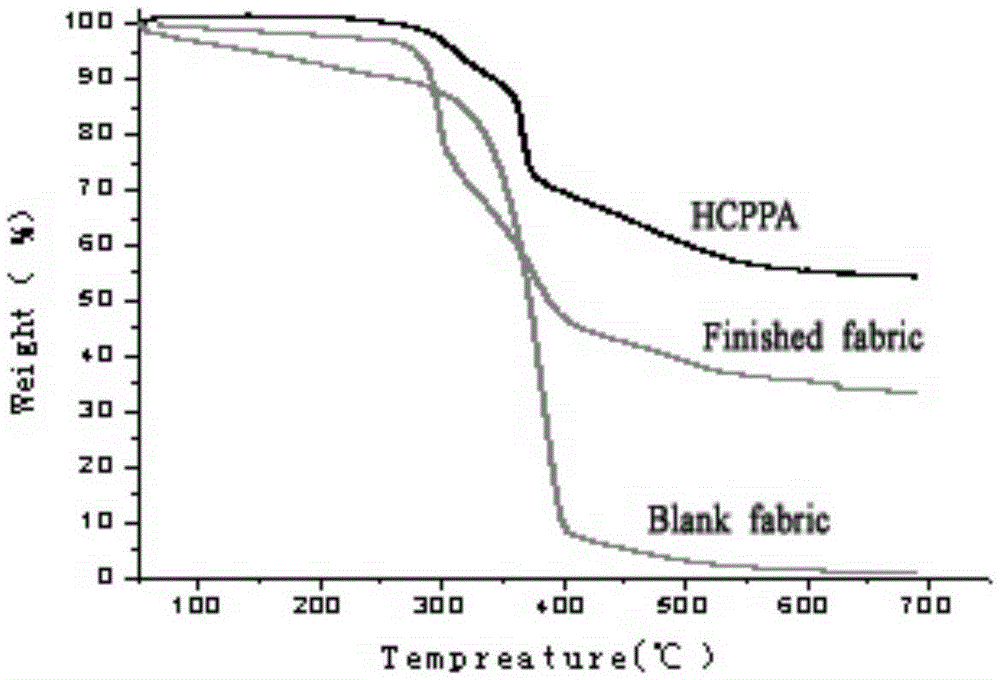
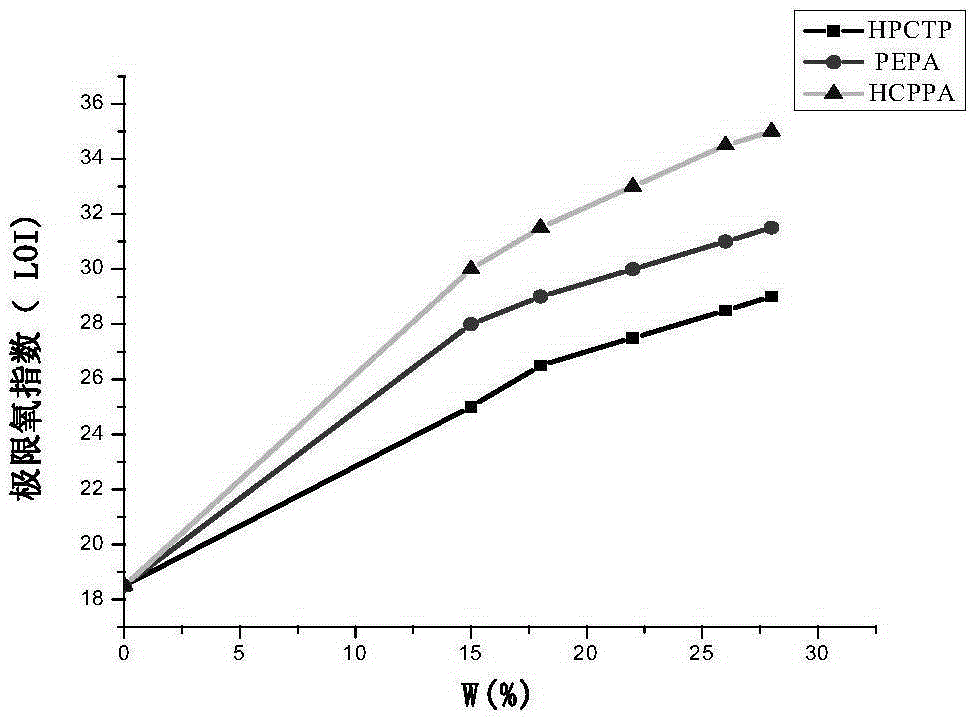
The invention discloses an N-P flameresistant material and a preparation method thereof and application in textiles. The chemical name of a flame retardant of the material is hexa(1-oxo-phospha-2,6,7-trioxabicyclo[2,2,2]octane-4-methylenedioxy)cyclotriphosphazene (HCPPA); the preparation method of the material comprises the steps of synthesizing hexachlorocyclotriphosphazene (HCPP) by reacting ammonium chloride with phosphorus pentachloride, wherein a catalyst is pyridine and ZnO; then synthesizing 1-oxo-phospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2,2,2]octane (PEPA) by reacting pentaerythritol with phosphorus oxychloride; finally synthesizing the HCPPA by reacting the HCPP with the PEPA. According to the preparation method, NaH is used as a catalyst, so that the synthesis reactions can be performed quickly, the reaction time is greatly shortened, and the product yield is improved. When the N-P flame retardant is used for retarding a flame of a cotton fabric, high limit oxygen index and char yield are achieved, and the wash durability is good.
Owner:HUNAN INSTITUTE OF ENGINEERING
Controlled odor mimic permeation system
The primary aspect of the Controlled Odor Mimic Permeation System (COMPS) is that it provides a field deployable instant and reproducible source of known amounts of target odors. This technology consists of a permeable polymer container (chosen to suit target odor and release rate required), stored inside a non-permeable package. The design allows for the pre-equilibration of the target odors such that the outer surface of the inner package can saturate with odor during storage. Removal of the inner item then provides an instant and reproducible source of known target vapor flux. We have successfully demonstrated this technology by placing the target odor chemicals within permeable membranes such as low density polyethylene which are then sealed within a non-permeable membranes such as metallized polyester. This design has multiple advantages including preventing cross contamination when storing multiple odor targets (5-10 targets are commonly employed) as well as being light-weight disposable, low unit cost potential, no external power / operating unit / machinery / hardware, simple to use and providing a known reproducible concentration of the target odors to the detector in the field. The applications of these COMPS include the whole range of biological (e.g. detector dog) and electronic (e.g. field sensors) detectors with examples such as explosives (e.g. 2-ethyl-1-hexanol simulating plasticized explosives), drugs (e.g. 3,4-methylenedioxybenzaldehyde simulating ecstasy), human remains (including dimethyl disulfide and pentanoic acid) and live human scent (including 5-heptene-2-one and nonanol).
Owner:FLORIDA INTERNATIONAL UNIVERSITY
Heteroaryl diazacycloalkanes, their preparation and use;
InactiveUS20040072823A1Decreased cholinergic functionUseful in treatmentBiocideNervous disorderArylHydrogen
The present invention discloses compounds of the formula any of its enantiomers or any mixture thereof, isotopes thereof or a pharmaceutically acceptable salt thereof; wherein n is 1, 2 or 3; m is 0, 1 or 2; R represents hydrogen, alkyl, cycloalkyl, cycloalkylalkyl, or aralkyl; and R<1 >represents aminophenyl; nitrophenyl; hydroxyphenyl, alkoxyphenyl; a monocyclic 5 to 6 membered heterocyclic group which may be substituted one or more times with substituents selected from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, alkenoxy, alkynoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino, nitro, -COOR<3>, -CONR<2>R<3>, -NH-CO2R<2>, NHCO-R<2>, -OCO-NR<2>R<3>; wherein R<2 >and R<3 >independently represents hydrogen or alkyl; aryl optionally substituted one or more times with alkyl, cycloalkyl, cycloalkylalkyl alkenyl, alkynyl, alkoxy, cycloalkoxy, alkenoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino and nitro; -X-alkyl-Y-alkyl wherein X and Y independently represents O, S, NH, N-alkyl or Se; and alkyl is optionally substituted with alkoxy or thioalkoxy; -X-(alkyl)o-aryl wherein o is 0 or 1 and X represents O, S, NH, N-alkyl or Se; optionally substituted one or more times with alkyl, cycloalkyl, cycloalkylalkyl alkenyl, alkynyl, alkoxy, cycloalkoxy, alkenoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino and nitro; -X-(alkyl)o-Z wherein o is 0 or 1 and X represents O, S, NH, N-alkyl or Se and Z represents a 5- or 6-membered monocyclic heterocyclic group; optionally substituted one or more times with alkyl, cycloalkyl, cycloalkylalkyl alkenyl, alkynyl, alkoxy, cycloalkoxy, alkenoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino and nitro; a monocyclic 5 to 6 membered heterocyclic group optionally substituted one or more times with alkyl, cycloalkyl, cycloalkylalkyl, alkenyl, alkynyl, alkoxy, cycloalkoxy, alkenoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino and nitro; or or R<1 >represents a bicyclic heterocyclic group, composed of a 5 to 6 membered monocyclic heterocyclic group fused to a benzene ring, and which may be substituted one or more times with substituents selected from the group consisting of alkyl, cycloalkyl, cycloalkylalkyl alkenyl, alkynyl, alkoxy, alkoxy-alkoxy, cycloalkoxy, alkenoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino, nitro, aryl optionally substituted one or more times with alkyl, cycloalkyl, cycloalkylalkyl alkenyl, alkynyl, alkoxy, cycloalkoxy, alkenoxy, alkynoxy, methylenedioxy, halogen, CF3, OCF3, CN, amino and nitro; and a monocyclic 5 to 6 membered heterocyclic group optionally substituted one or more times with
Owner:AIRTEX PROD +1
Pyrrolidine sulfonamides
A compound of Formula (I): wherein:R1 is C1-6 alkyl, benzyl, or (CH2)n—C(O)NH2; wherein the benzyl may be unsubstituted or substituted by one or two C1-6 alkyl, halogen, C1-6 alkoxy, or methylenedioxy groups;R2 is benzimidazolyl, quinolinyl, benzofuranyl, napthyl, indolyl, benzothiophenyl, phenyl, furanyl, thienyl, or pyridyl substituted or unsubstituted by one, two or three halogen, C1-3 alkyl, C1-3 alkoxy, or methylenedioxy groups;X1 and X2 are independently hydrogen, halogen, C1-3 alkyl, C1-3 alkoxy, nitro, CF3, or CN;n is 1, 2, or 3;m is 1, 2 or 3;or a pharmaceutically acceptable salt thereof.
Owner:SMITHKLINE BECKMAN CORP
Phenanthroindolizidine derivative and nfkb inhibitor containing same as active ingredient
InactiveCN102186850AExcellent NFκB inhibitionGood anticancer effectAntibacterial agentsOrganic active ingredientsHydrogen atomNf κb inhibition
A novel compound having an excellent NF?B inhibitory effect. Specifically disclosed is a compound represented by formula (1) or a salt thereof. (In the formula, R1 represents a hydrogen atom, a lower alkyl group or the like; R2 represents a hydrogen atom, a lower alkyl group, a halogen atom or the like; R3 represents a hydrogen atom, a lower alkyl group, a hydroxy group or a halogen atom; R4 represents a hydrogen atom or a lower alkyloxy group; R5 represents a hydrogen atom, a lower alkyloxy group, a halogen atom or a hydroxy group, or alternatively forms a methylenedioxy group or an isopropylidenedioxy group together with R6; R6 represents a hydrogen atom or a lower alkyloxy group, or alternatively forms a methylenedioxy group or an isopropylidenedioxy group together with R5; R7 represents a hydrogen atom or a lower alkyl group; and R8 represents a hydrogen atom, a hydroxy group, an amino group, a lower alkylcarbonyloxy group or a halogen atom.)
Owner:YAKULT HONSHA KK
Topical application for treating toenail fungus
InactiveUS6878365B2Powerful potencyNo skin irritationCosmetic preparationsBiocideNail InfectionFungal nail infection
A topical applications is used to treat and prevent the spread of nail infections or onychomycosis caused by bacteria, fungi and other pathogens. The topical application has a composition that comprises, as an active ingredient, at least one species selected from the group consisting of 2,2′-(alkyldioxy)bis-(alkyl-1,3,2-dioxaborinane) and 2,2′-oxybis(alkyl-1,3,2-dioxaborinane). More specifically, the composition comprises, as an active ingredient, at least one member selected from the group consisting of 2,2′-(1-methyltrimethylenedioxy)bis-(4-methyl-1,3,2-dioxaborinane) and 2,2′-oxybis(4,4,6-trimethyl-1,3,2-dioxaborinane).
Owner:BREHOVE JAMES EDWARD
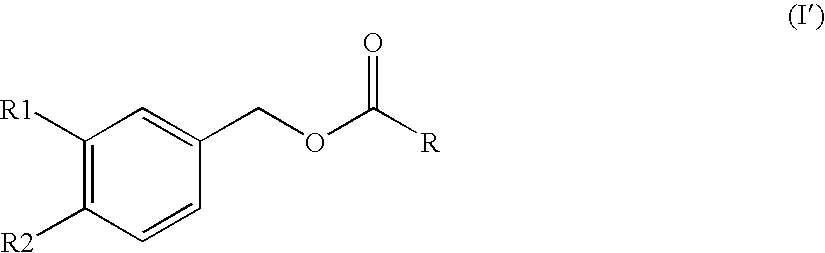
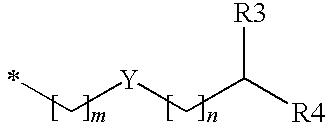
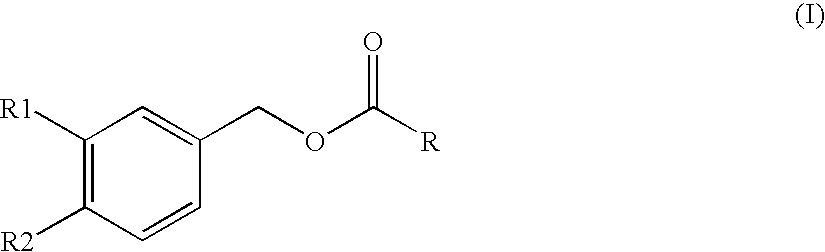
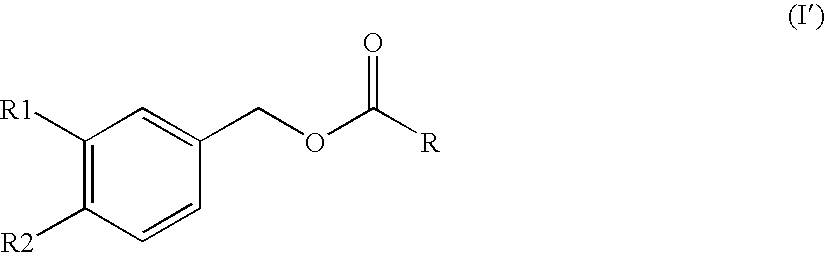
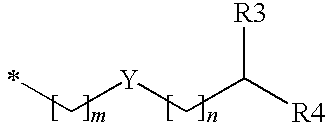
Substituted benzyl ester derivative and use thereof
The present invention relates to a pharmaceutical composition, a food composition or a cosmetic composition, containing one or more kinds of a compound represented by the following formula (I′)wherein R1 is a hydrogen atom, a hydroxyl group, a methoxy group or an ethoxy group, R2 is a hydroxyl group, a methoxy group or an acetoxy group, or R1 and R2 in combination optionally form a methylenedioxy group,R is represented by the following formulawherein Y is an ethylene group or a vinylene group, m and n are each an integer of 0 to 7, which satisfy m+n=2 to 8, and R3 and R4 are each independently a hydrogen atom, a methyl group or an ethyl group,provided that,(1) when R1 is a methoxy group, then R2 is not a hydroxyl group; and(2) when R1 is a hydroxyl group, then R2 is not a hydroxyl group and an acetoxy group.According to the present invention, a stable capsinoid derivative is provided, and a pharmaceutical composition, a food composition, a cosmetic composition and the like containing the derivative as an active ingredient can be provided.
Owner:AJINOMOTO CO INC
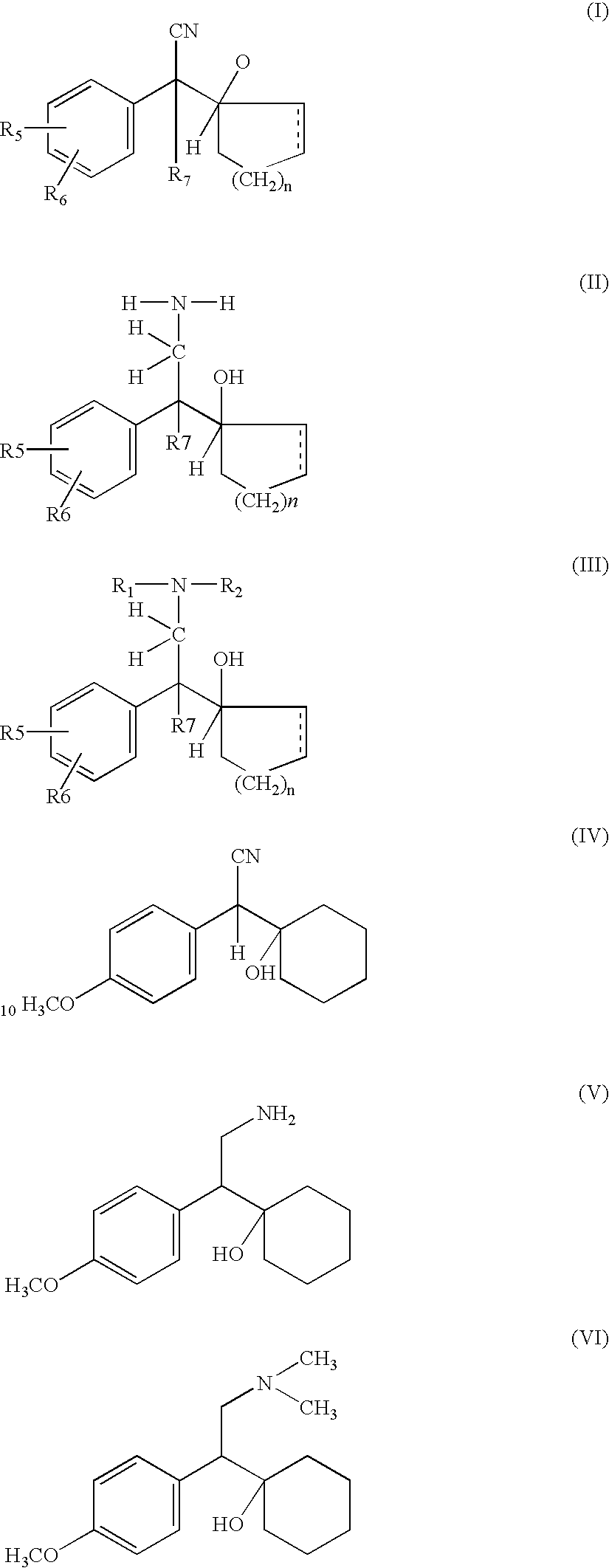
Manufacture of phenyl ethylamine compounds, in particular venlafaxine
InactiveUS7026513B2Reduce the cyanocarbinol most effectivelyPreparation by oxidation reactionsOrganic compound preparationPhenyl groupEthylamine
A process for the preparation of hydroxy(cycloalkane / cyclokene) phenylethyl amine of the general formula (III) comprising alkylation of its precursor amine of general formula (II) which is in turn produced by an effective reduction process from its precursor cyanide having the general formula (I) using Raney Nickel (CORMIII) as catalyst where, either of R5 and R6 independently could be in meta or para position and R5, R6 are independently hydrogen, hydroxyl, alkyl, alkanoyloxy, cyano, nitro, alkylmercapto, amino, alkylamino, allkanamido, halo, trifluoromethyl, or taken together methylenedioxy, n is 0, 1, 2, 3, 4, R7 is hydrogen of alkyl of 1–7 carbon atom, R1 IH or alkyl of 1–3 carbon atom and R2 is alkyl 1–3 carbon atom, the dotted line represents optional unsaturation. Compounds of formulae IV, V and VI are respectively derivatives of compounds I, II and III respectively.
Owner:NICHOLAS PIRAMAL INDIA LTD
1,8-naphthalimide derivative as well as synthesis method and application thereof
InactiveCN104557887ASignificant in vitro antitumor activityGood potential medicinal valueOrganic chemistryAntineoplastic agentsSynthesis methodsBromine
The invention discloses a 1,8-naphthalimide derivative as well as a synthesis method and an application thereof. The derivative is N-(3,4-methylene dioxophenethyl)-4-(3-N,N-dimethylamino)propylamino-1,8-naphthalimide; and the compound is prepared by taking 4-bromo-1,8-naphthalic anhydride as a raw material which sequentially reacts with 3,4-methylene dioxophenylethylamine and N,N-dimethyl-1,3-diaminopropane. The synthesis method is simple and easy to control. The applicant observes the proliferation inhibition activity of the 1,8-naphthalimide derivative on 9 human tumor cell strains and two normal cell strains, and the results indicate that the in-vitro antitumor activity is remarkable and the 1,8-naphthalimide derivative has relatively good potential medicinal value and is expected to be applied to the preparation of various anti-tumor drugs. The chemical structural formula of the compound disclosed by the invention is shown by the formula (I).
Owner:GUANGXI NORMAL UNIV
Synthesis process of palmatine and its salts
InactiveCN1733763ARaise quality standardsContent increased and stabilizedOrganic chemistryBenzeneAcetonitrile
The invention discloses a synthesis process of palmatine and its salts mainly comprising the following steps, (1) etherification, preparation of o-dimethoxybenzene, (2) acetonitrilizaiton, preparation of methylenedioxy benzene acetonitrile, (3) hydrogenization, preparation of o-dimethoxy-phenethylamine, (4) condensation, preparation of condensate hydrochlorates, (5) recondensation, preparation of palmatine, (6) preparation of corresponding salts from palmatine with related acids.
Owner:余娟
Substituted benzyl ester derivative and use thereof
The present invention relates to a pharmaceutical composition, a food composition or a cosmetic composition, containing one or more kinds of a compound represented by the following formula (I′)wherein R1 is a hydrogen atom, a hydroxyl group, a methoxy group or an ethoxy group, R2 is a hydroxyl group, a methoxy group or an acetoxy group, or R1 and R2 in combination optionally form a methylenedioxy group,R is represented by the following formulawherein Y is an ethylene group or a vinylene group, m and n are each an integer of 0 to 7, which satisfy m+n=2 to 8, and R3 and R4 are each independently a hydrogen atom, a methyl group or an ethyl group,provided that,(1) when R1 is a methoxy group, then R2 is not a hydroxyl group; and(2) when R1 is a hydroxyl group, then R2 is not a hydroxyl group and an acetoxy group.According to the present invention, a stable capsinoid derivative is provided, and a pharmaceutical composition, a food composition, a cosmetic composition and the like containing the derivative as an active ingredient can be provided.
Owner:AJINOMOTO CO INC
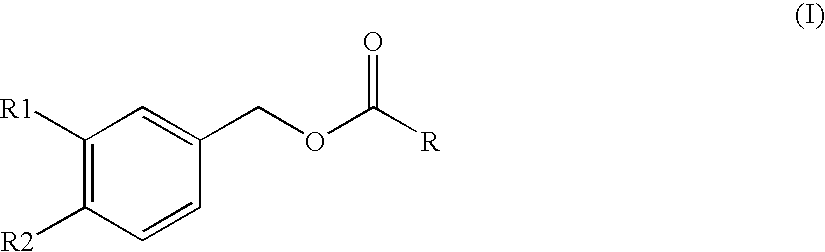
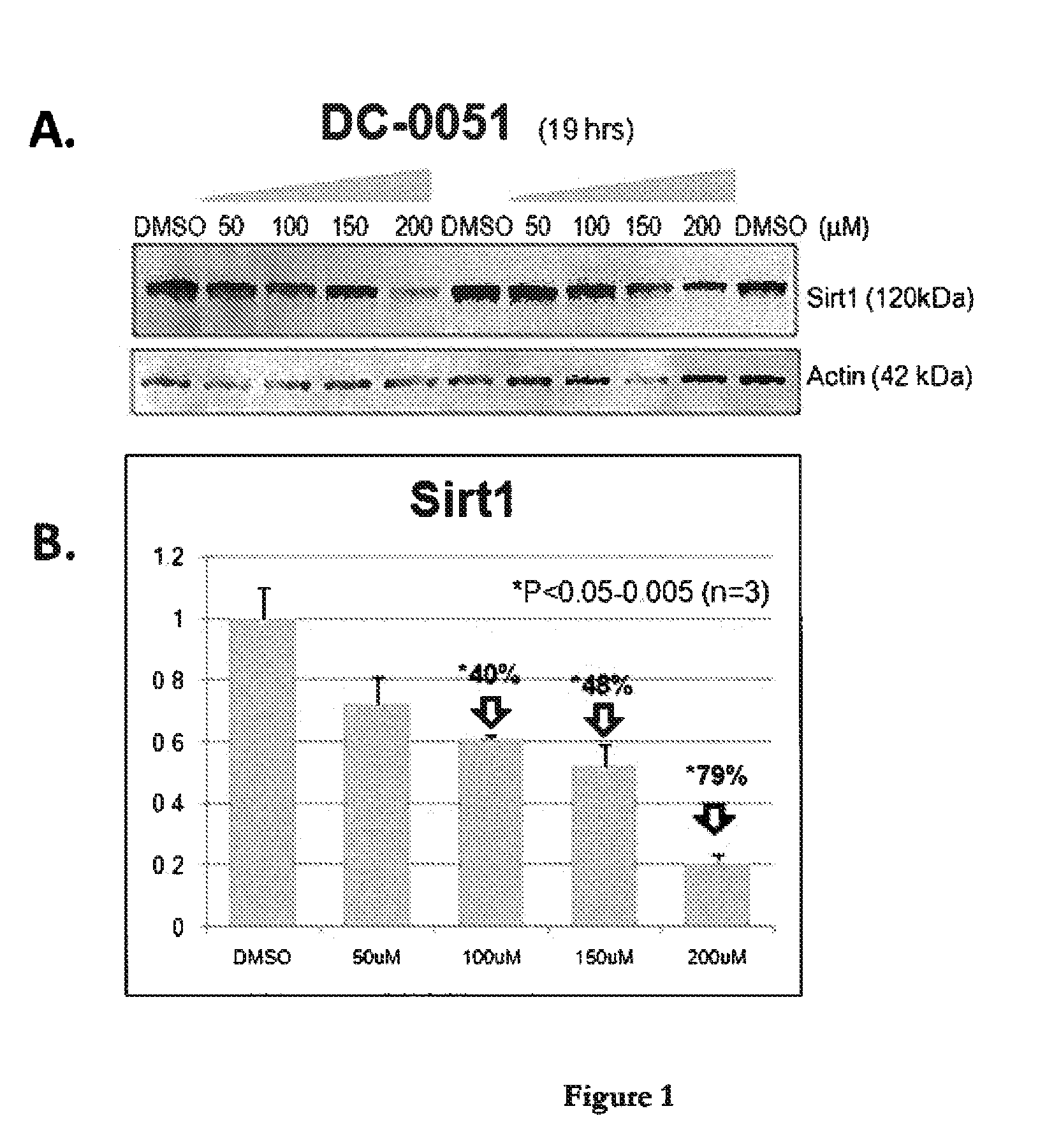
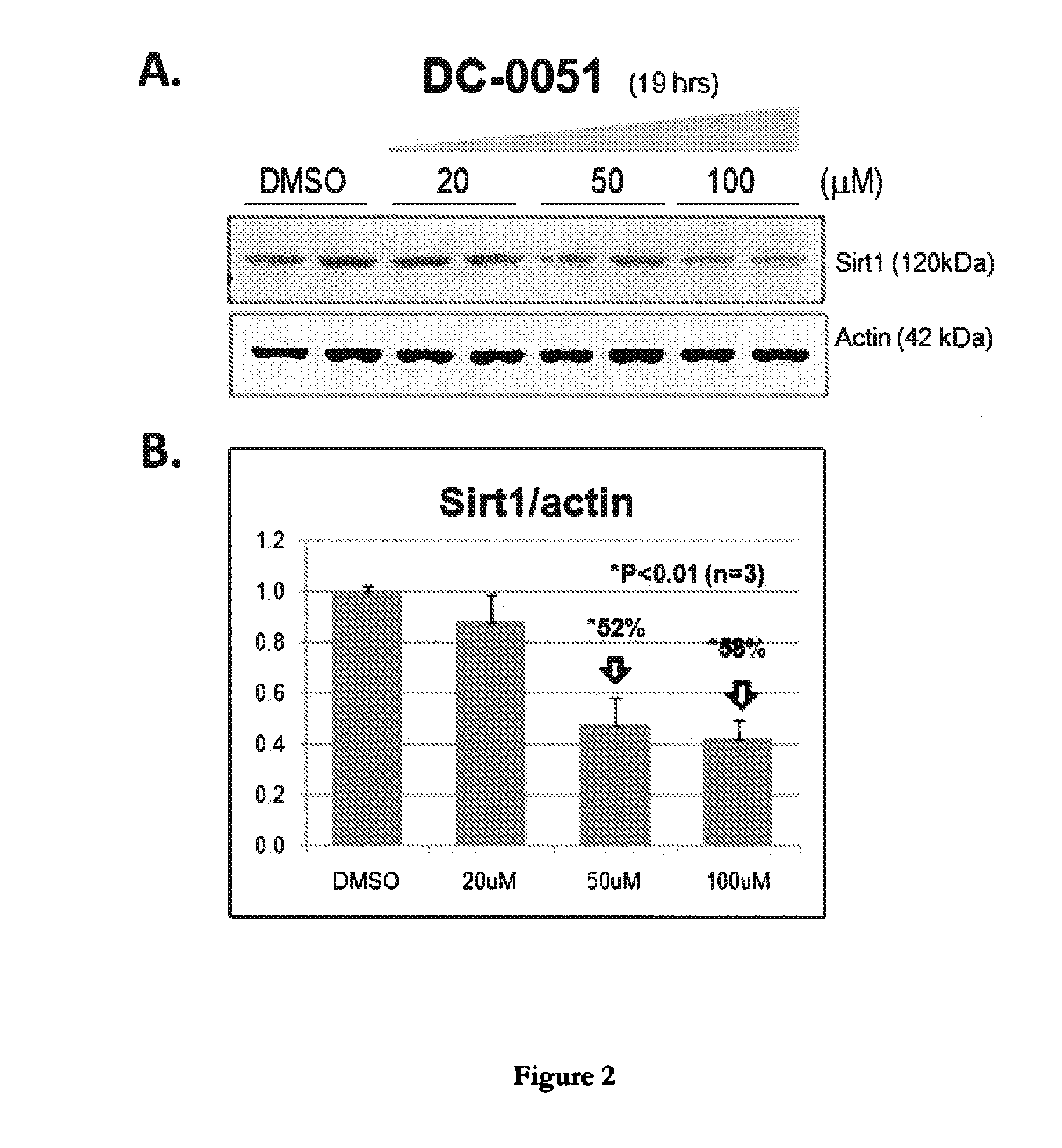
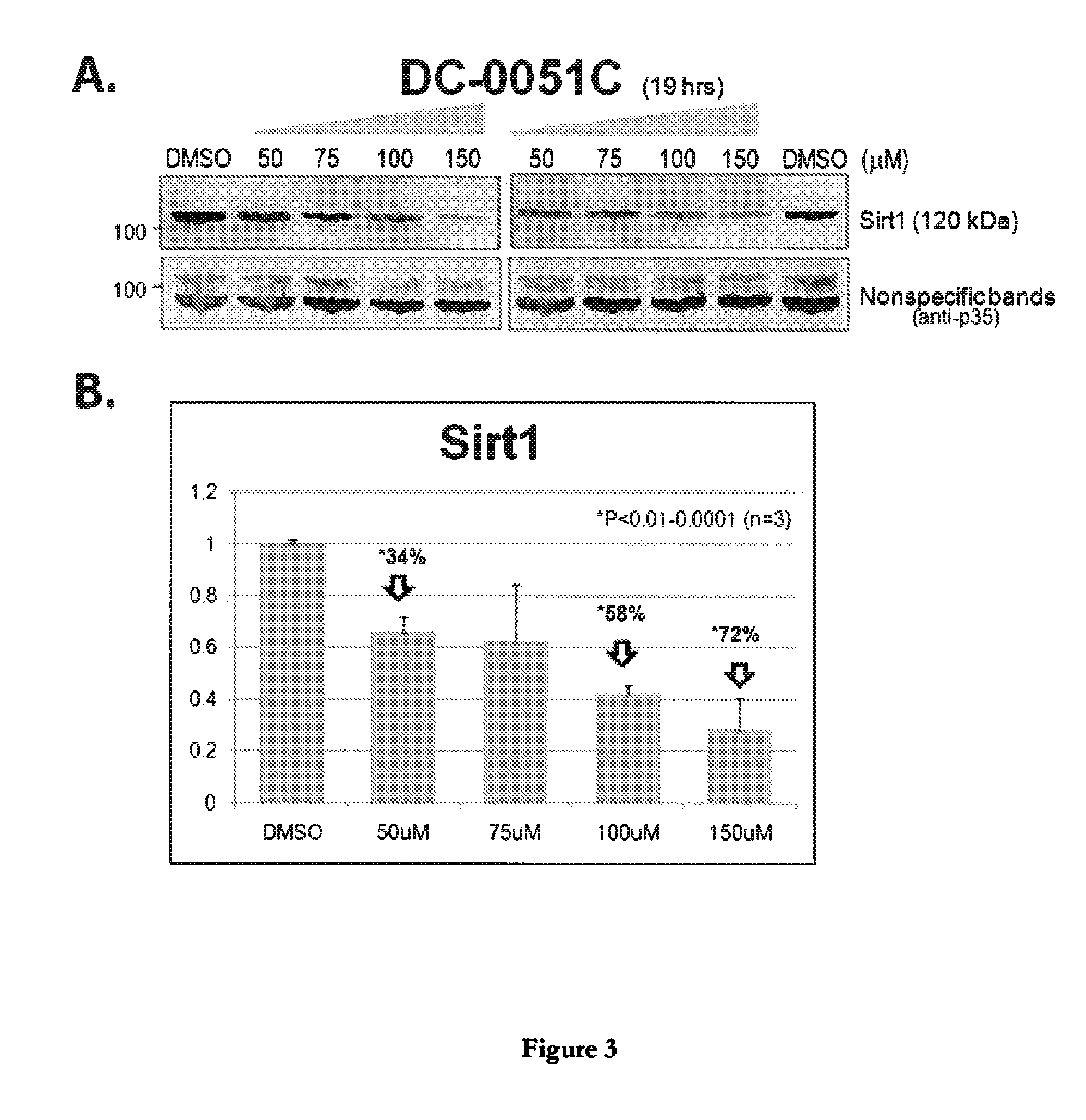
Sirtuin 1 and the Treatment of Neurodegenerative Diseases
InactiveUS20110015272A1Urea derivatives preparationBiocideHuntingtons choreaDementia with Lewy bodies
This invention relates to bis- and tris-dihydroxyaryl compounds and their methylenedioxy analogs and pharmaceutically acceptable salts and their use in the modulation of Sirtuin 1 (Sirt1) and there use in neuroprotection for subject suffering from neurodegenerative diseases such as Alzheimer's disease, Huntington's disease, Amyotrophic lateral sclerosis, frontotemporal dementia, Parkinson's disease, including Parkinson's plus diseases such as multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration and dementia with Lewy bodies, and in the manufacture of medicaments for such Sirt1 modulation and neuroprotection.
Owner:PROTEOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com