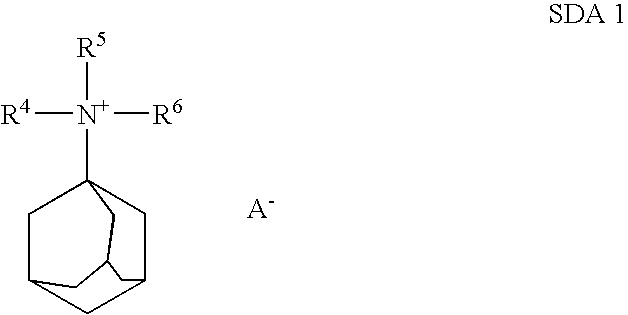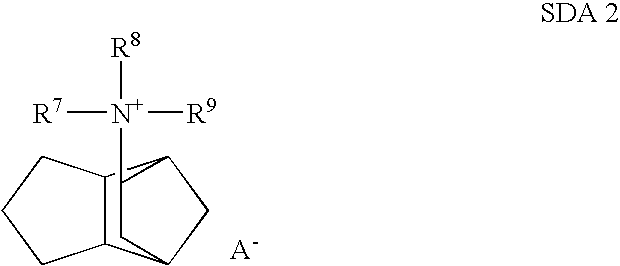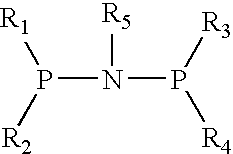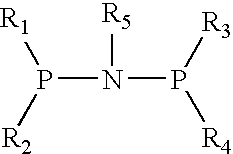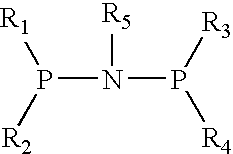Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5476 results about "Benzyl group" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C₆H₅CH₂–. Benzyl features a benzene ring attached to a CH₂ group.
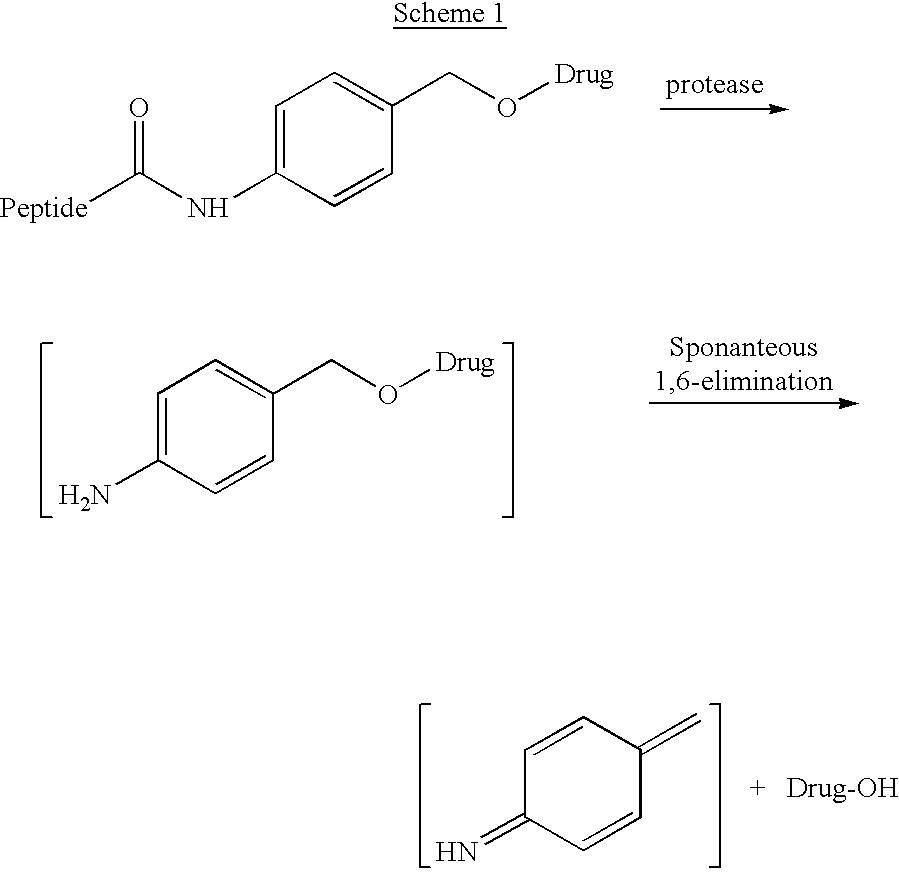
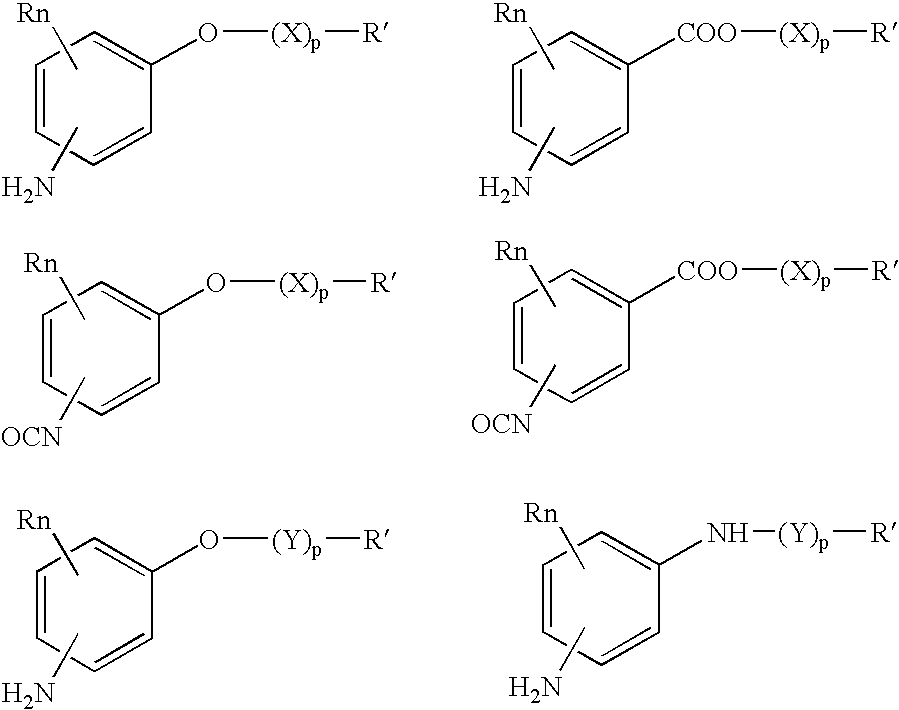
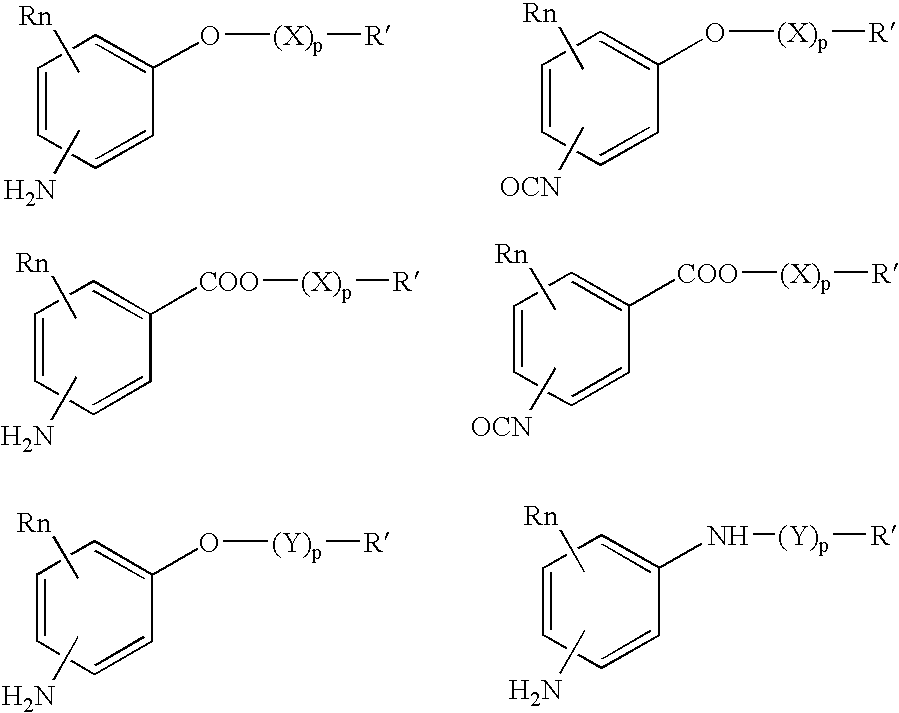
p-Amidobenzylethers in drug delivery agents
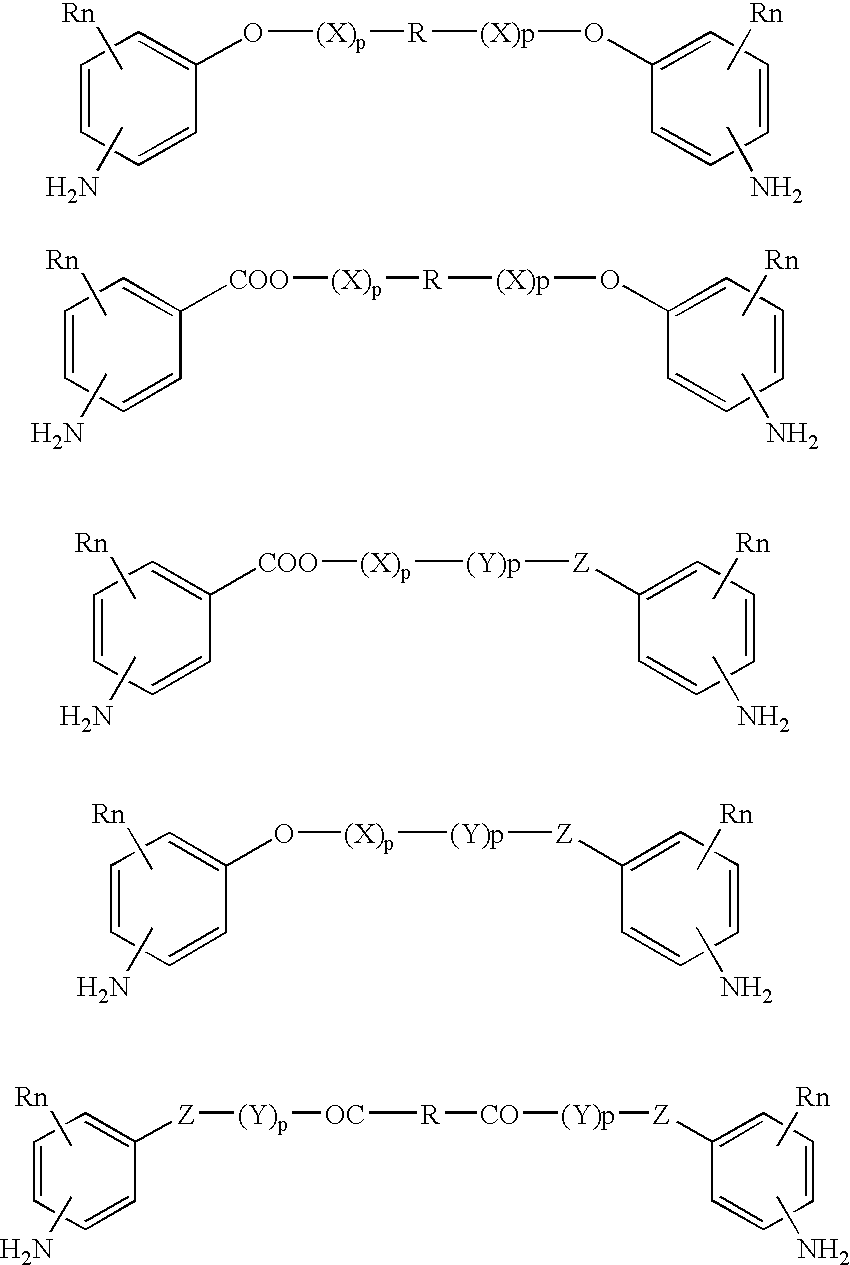
Compounds of the formulaeLAn-Z-X—WwD and BZ-X—WwDwherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide residue; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
Process for applying a streamable epoxy adhesive
InactiveUS20050070634A1Low viscosityHigh strength bondAdhesive processes with adhesive heatingEpoxy resin adhesivesBENZYL ALCOHOL/WATERViscosity
The invention is a composition comprising applying to a substrate a stream of an adhesive comprising: one or more epoxy resins; one or more rubber modified epoxy resins; one or more toughening compositions comprising the reaction product of one or more isocyanate terminated prepolymers and one or more capping compounds having one or more phenolic, benzyl alcohol, aminophenyl, or, benzylamino groups wherein the reaction product is terminated with the capping compounds; one or more curing agents for epoxy resins and one or more catalysts which initiate cure at a temperature of about 100° C. or greater; and optionally; fillers adhesion promoters, wetting agents or rheological additives useful in epoxy adhesive compositions; wherein the adhesive composition has a viscosity at 45° C. of about 20 Pa.s to about 400 Pa.s. The composition can be used as an adhesive and applied as a stream using a high speed streaming process.
Owner:DOW GLOBAL TECH LLC
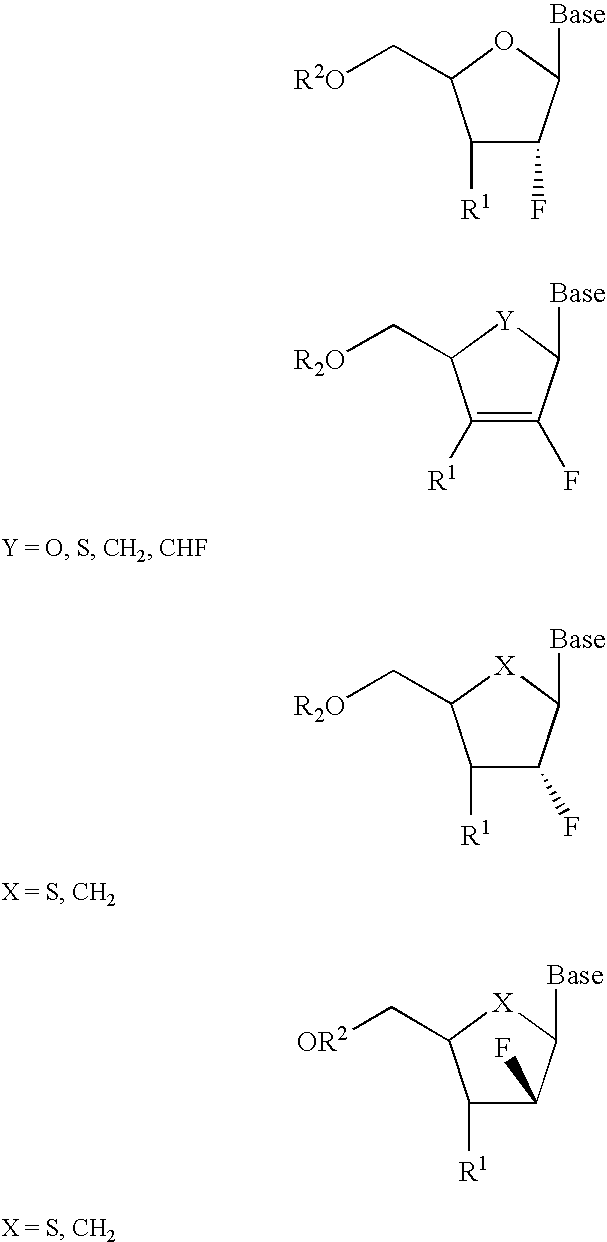
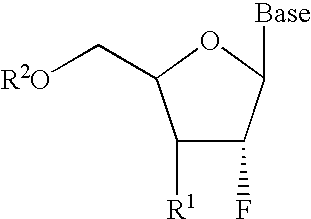
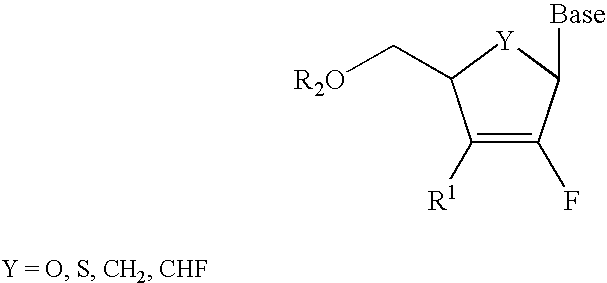
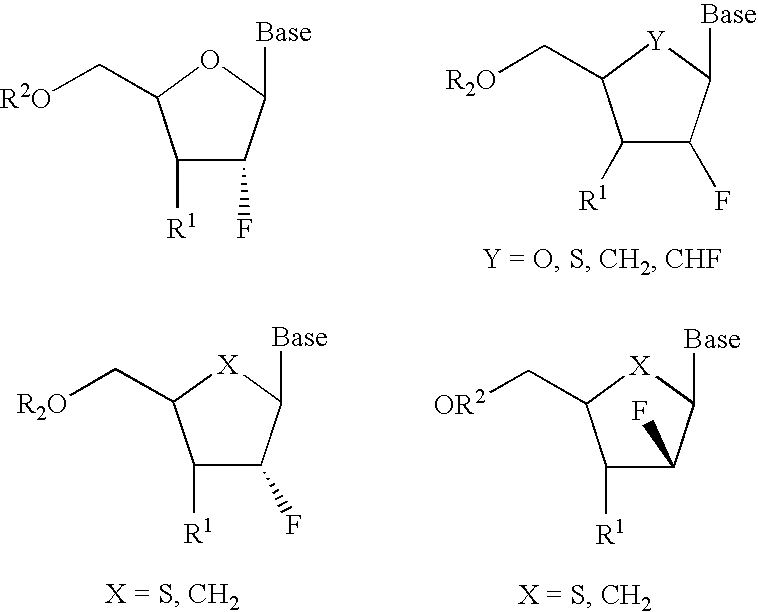
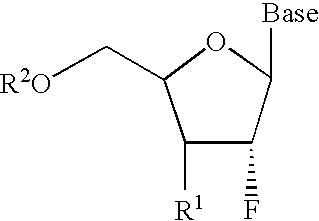
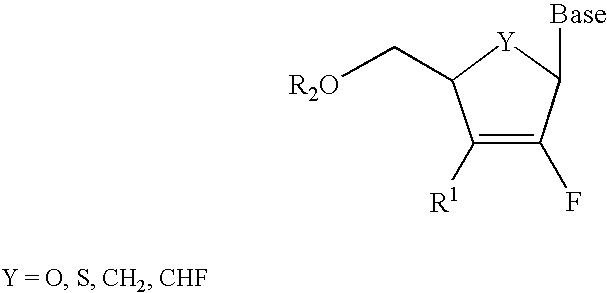
2′-fluoronucleosides
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
Inhibitors of P2X3
Compounds of formula 1 are modulators of P2X3 useful for the treatment of pain and genito-urinary, gastrointestinal, and respiratory disorders: wherein R1 is —C(═S)CH3, pyridyl, pyrimidinyl, pyrazinyl, thiazolyl, furyl, furylcarbonyl, acetyl, or carbamoyl; R2a and R2b are independently H, methyl, or ethyl; R3 is H or methyl; Y is a bond, —(CR4R5)n— or —CR4═CR5—; wherein R4 and R5 are each independently H or methyl and n is 1 or 2; X is N or CH; A is phenyl, 5-membered heterocyclyl, or 6-membered heterocyclyl; R6, R7 and R8 are each independently H, halo, lower alkyl, cycloalkyl, alkylthio, alkylthio-lower alkyl, alkylsulfonyl-lower alkyl, di(lower alkyl)amino-lower alkyl, morpholinyl-lower alkyl, 4-methyl-piperazinyl-methyl, trifluoromethyl, pyridyl, tetrazolyl, thiophenyl, phenyl, biphenyl, or benzyl (where thiophenyl, phenyl and benzyl are substituted with 0-3 lower alkyl, halo, sulfonamido, trifluoromethyl, lower alkoxy or lower alkylthio) or R6 and R7 together form a 5-membered or 6-membered carbocyclic or heterocyclic ring substituted with 0-3 substituents selected from the group consisting of lower alkyl, lower alkoxy, oxo, halo, thiophenyl-lower alkyl, phenyl, benzyl (where phenyl and benzyl are substituted with 0-3 lower alkyl, halo, sulfonamido, trifluoro-methyl, lower alkoxy, lower alkylthio, amino-lower alkyl, lower alkylamino-lower alkyl, or di(lower alkyl)amino-lower alkyl); and pharmaceutically acceptable salts thereof; wherein when R1 is pyrimidin-2-yl, X is N, Y is a bond and A is oxazol-5-yl the carbon atom at position 4 in said oxazol-5-yl is not substituted by propyl when the carbon atom at position 2 in said oxazol-5-yl is substituted by substituted phenyl and the carbon atom at position 4 in said oxazol-5-yl is not substituted by phenyl when the carbon atom at position 2 is substituted by unsubstituted or substituted phenyl.
Owner:ROCHE PALO ALTO LLC
Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
ActiveUS20070249544A1Prevent degradationImproving and restoring functionalityBiocideAntibiotics chemistryCrystallographyBenzene
The invention relates to a crystalline form of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
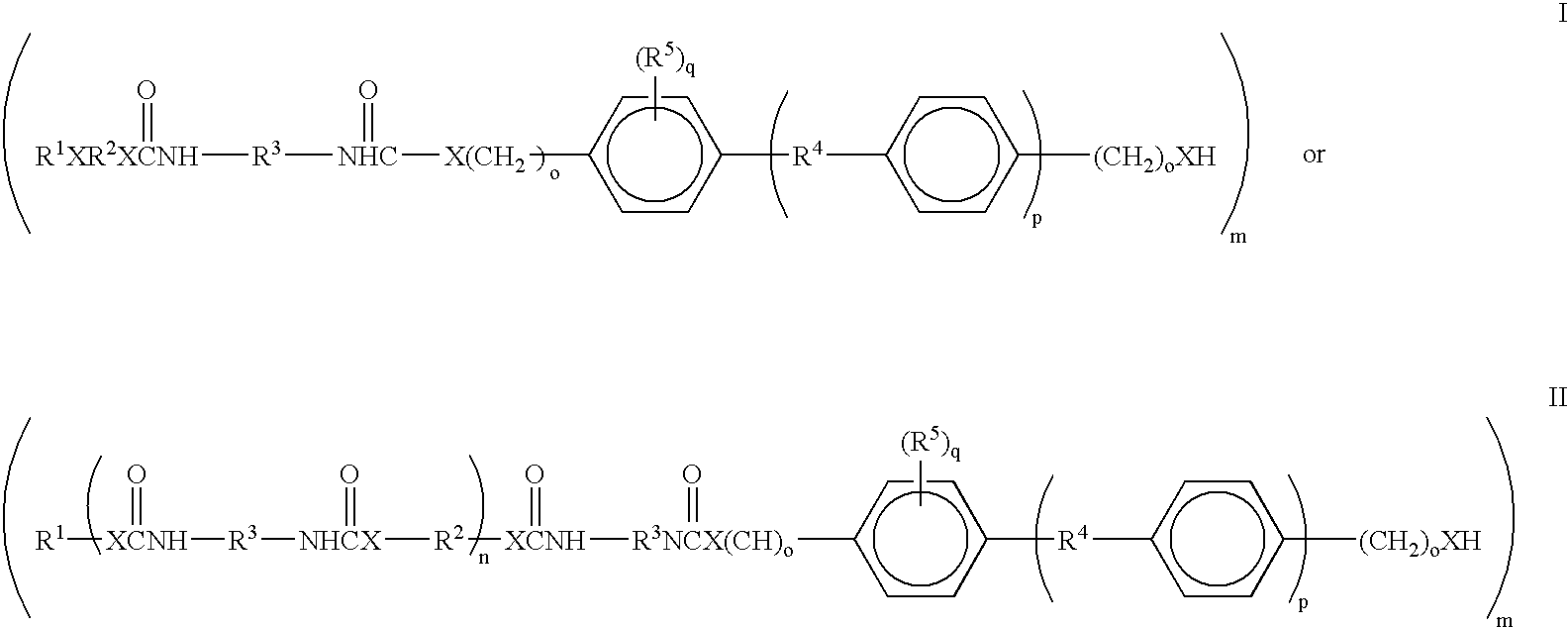
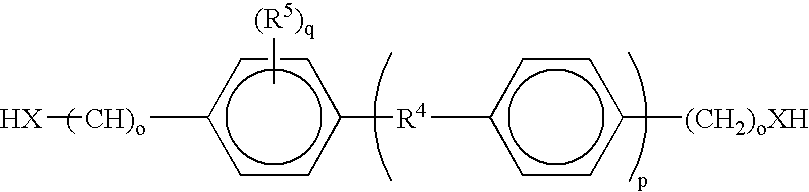
Bioabsorbable and biocompatible polyurethanes and polyamides for medical devices
ActiveUS20060188547A1Low toxicitySuture equipmentsOrganic active ingredientsAbsorbable polymersPolyester
Absorbable polyurethanes, polyamides and polyester urethanes prepared from at least one compound selected from: or the diamines and diisocyanates thereof, wherein each X represents a member independently selected from —CH2COO— (glycolic acid moiety), —CH(CH3)COO— (lactic acid moiety), —CH2CH2OCH2COO— (dioxanone), —CH2CH2CH2CH2CH2COO— (caprolactone moiety), —(CH2)yCOO— where y is one of the numbers 2, 3, 4 or 6-24 inclusive, and —(CH2CH2O)z′CH2COO— where z′ is an integer between 2 and 24, inclusive; each Y represents a member independently selected from —COCH2O— (glycolic ester moiety), —COCH(CH3)O— (lactic ester moiety), —COCH2OCH2CH2O— (dioxanone ester), —COCH2CH2CH2CH2CH2O— (caprolactone ester), —CO(CH2)mO— where m is an integer between 2, 3, 4 or 6-24 inclusive, —COCH2O(CH2CH2O)n— where n is an integer between 2 and 24, inclusive; R′ is hydrogen, benzyl or an alkyl group, the alkyl group being either straight-chained or branched; p is an integer between 1 and 4, inclusive; and Rn represents one or more members selected from H, alkoxy, benzyloxy, aldehyde, halogen, carboxylic acid and —NO2, which is attached directly to an aromatic ring or attached through an aliphatic chain. Absorbable polymers prepared from these compounds are useful for drug delivery, tissue engineering, tissue adhesives, adhesion prevention and other implantable medical devices.
Owner:BEZWADA BIOMEDICAL LLC
Prodrugs of piperazine and substituted piperidine antiviral agents
This invention provides for prodrug Compounds I, pharmaceutical compositions thereof, and their use in treating HIV infection.wherein:X is C or N with the proviso that when X is N, R1 does not exist;W is C or N with the proviso that when W is N, R2 does not exist;V is C;E is hydrogen or a pharmaceutically acceptable salt thereof; andY is selected from the group consisting ofAlso, this invention provides for intermediate Compounds II useful in making prodrug Compounds I.wherein:L and M are independently selected from the group consisting of C1-C6 alkyl, phenyl, benzyl, trialkylsilyl, -2,2,2-trichloroethoxy and 2-trimethylsilylethoxy.
Owner:VIIV HEALTHCARE UK (NO 4) LTD
2′-Fluoronucleosides
InactiveUS7307065B2Sure easyUseful in treatmentBiocidePeptide/protein ingredientsPhosphoric Acid EstersPhosphate
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae:whereinBase is a purine or pyrimidine base;R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; andR3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY +1
Polymers with anti-microbial properties
The invention relates to polymers with antimicrobial properties, consisting of: a) 99-40 wt % non functional vinylically polymerizable monomers and b) 1-60 wt % functional vinylically polymerizable monomers of general formula (I) wherein V=vinyl, (meth)acroyl, allyl or styryl, A=a possibly available linking unit, which can be alkyl, aryl, arylalkyl or hydroxy alkyl, which can also be interrupted by hetero atoms, e.g. by hetero atoms in urethane, carbonate, ester, amide or ether groups, wherein y=0 or is 1, Hsp=a hydrophilic spacer of general formula (i) -(O-CH2-CH2)r- and / or (ii) -(O-CH2-CH(CH3))s with r=0-40, s=0-40 and r+s=2-40, also m=1.2 or 3 and R1=CH3, ethyl or benzyl, R2=an alkyl radical with 8-20 C-atoms, wherein t=1, 2 or 3 and X-=Cl-, Br-, I- or alkyl sulphate.
Owner:EVONIK ROEHM GMBH
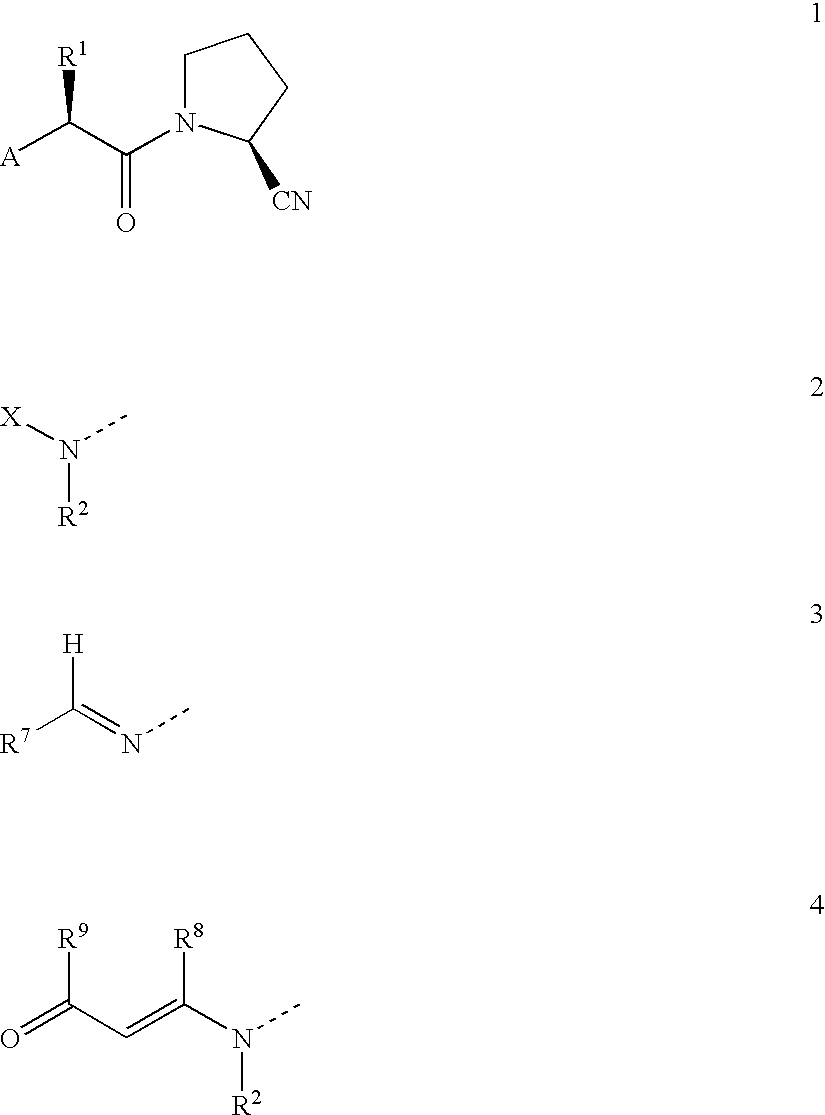
Novel antidiabetic agents
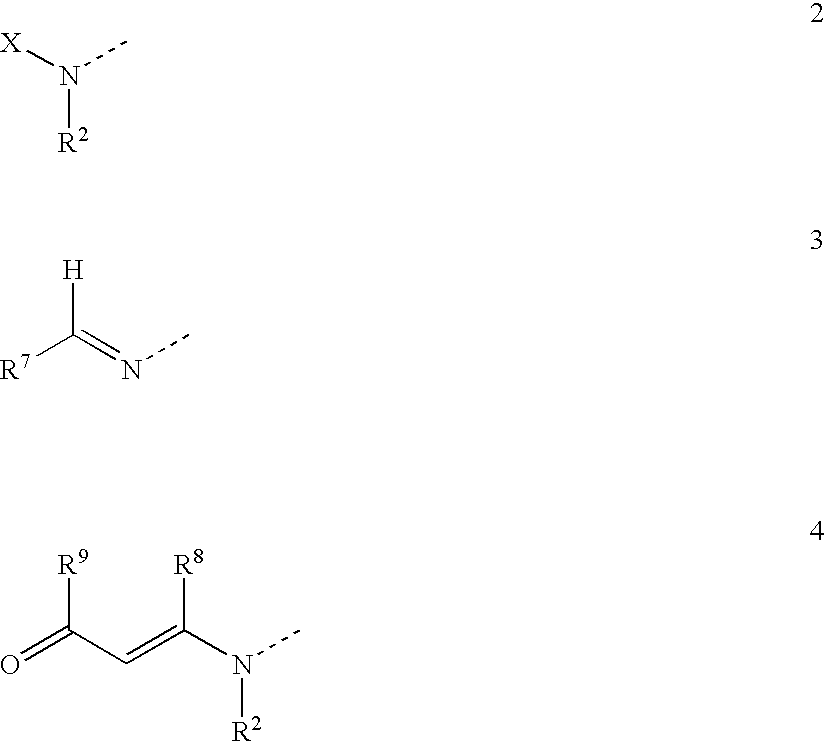
Compounds which are 1-(2'-aminoacyl)-2-cyanopyrrolidine derivatives according to general formula (1) are DP-IV inhibitors for treatment of impaired glucose tolerance or type 2 diabetes; wherein A is selected from groups (2,3 and 4); X is selected from aminoacyl groups corresponding to the natural amino acids, acyl groups R3CO, groups R4COOC(R5)(R6)OCO, methoxycarbonyl, ethoxycarbonyl and benzyloxycarbonyl; R1 is selected from H, C1-C6 alkyl residues, (CH2)aNHW1, (CH2)bCOW2, (CH2)cOW3, CH(Me)OW4, (CH2)d-C6H4-W5 and (CH2)eSW6, where a is 2-5, b is 1-4, c is 1-2, d is 1-2, e is 1-3, W1 is COW6, CO2W6 or SO2W6, W2 is OH, NH2, OW6 or NHW6, W3 is H or W6, W4 is H or W6, W5 is H, OH or OMe, and W6 is C1-C6 alkyl, optionally substituted phenyl, optionally substituted heteroaryl or benzyl and R2 is selected from H and (CH2)n-C5H3N-Y, where n is 2-4 and Y is H, F, Cl, NO2 or CN, or R1 and R2 together are -(CH2)p-where p is 3 or 4; R3 is selected from H, C1-C6 alkyl and phenyl; R4 is selected from H, C1-C6 alkyl, benzyl and optionally substitued phenyl; R5 and R6 are each independently selected from H and C<highlight>
Owner:FERRING BV
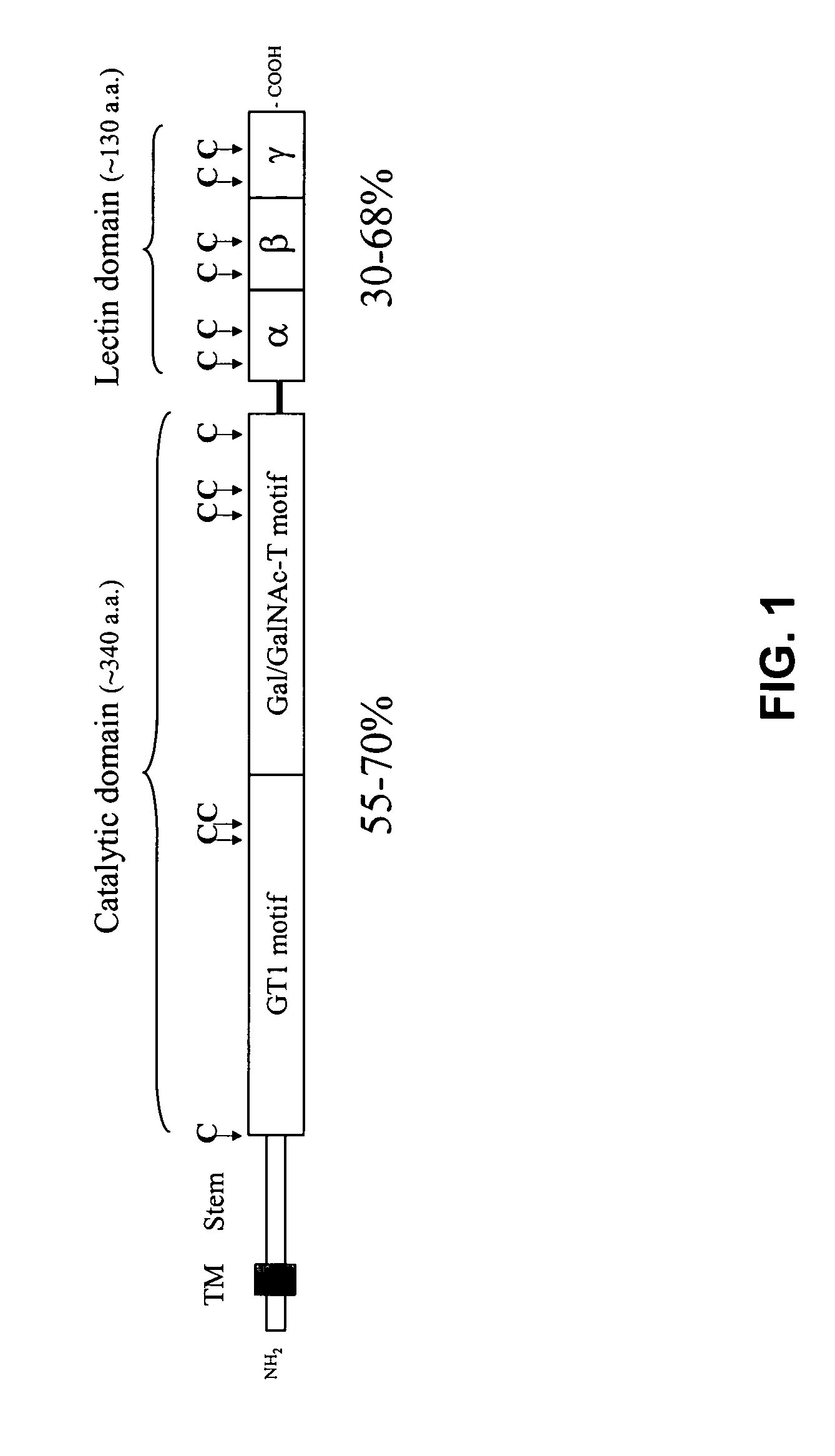
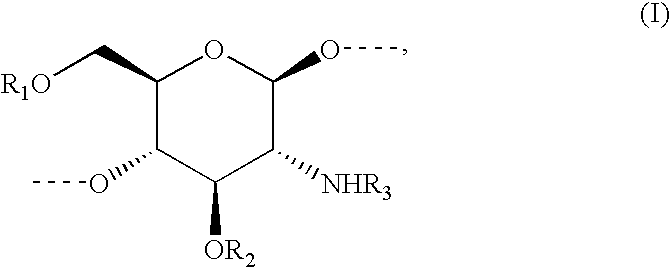
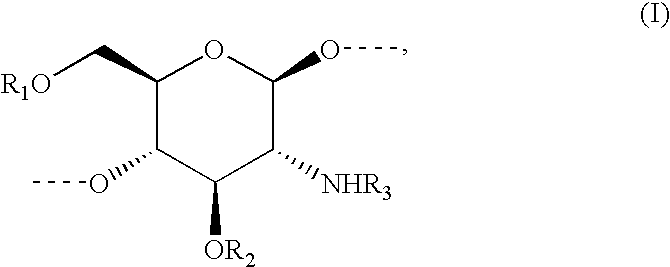
Methods to identify agents modulating functions of polypeptide galnac-transferases, pharmaceutical compositions comprising such agents and the use of such agents for preparing medicaments
Novel methods for identification of inhibitors or modulators of binding activities mediated by lectin domains of polypeptide GalNAc-transferases are disclosed. Direct binding activity of GalNAc-transferase lectins has been demonstrated for the first time and methods to measure lectin mediated binding of isolated lectins or enzymes with lectin domains are disclosed. The present invention specifically discloses a novel selective inhibitor of polypeptide GalNAc-transferase lectin domains, which provides a major advancement in that this inhibitor and related inhibitors sharing common characteristics of activity bind lectin domains without serving as acceptor substrate for glycosyltransferases involved in synthesis of O-glycans. This inhibitor is represented by the β-anomeric configuration of GalNAc-benzyl, GalNAcβ-benzyl. Methods for inhibiting intracellular transport, cell surface expression, and secretion of mucins and O-glycosylated glycoproteins without affecting O-glycosylation processing are disclosed using the novel selective inhibitor identified.
Owner:GLYCOZYM
Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
The invention relates to crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
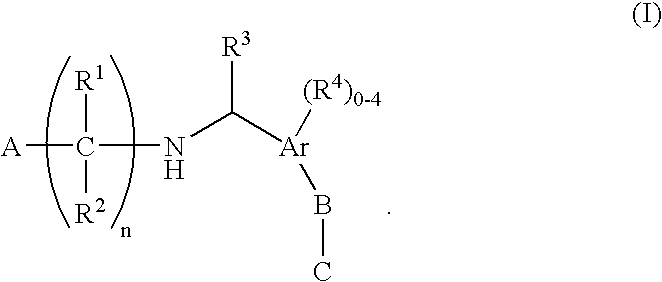
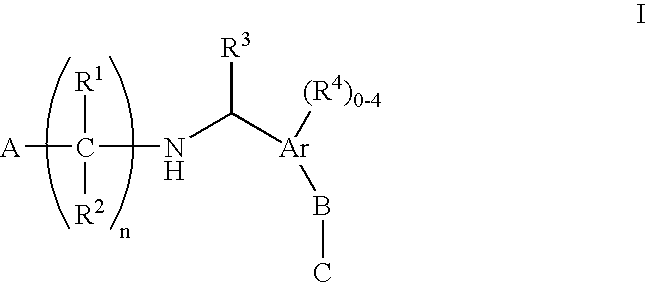
N-(benzyl)aminoalkylcarboxylates, phosphinates, phosphonates and tetrazoles as Edg receptor agonists
The present invention encompasses compounds of Formula (I) as well as the pharmaceutically acceptable salts and hydrates thereof. The compounds are useful for treating immune mediated diseases and conditions, such as bone marrow, organ and tissue transplant rejection. Pharmaceutical compositions and methods of use are included.
Owner:MERCK SHARP & DOHME CORP
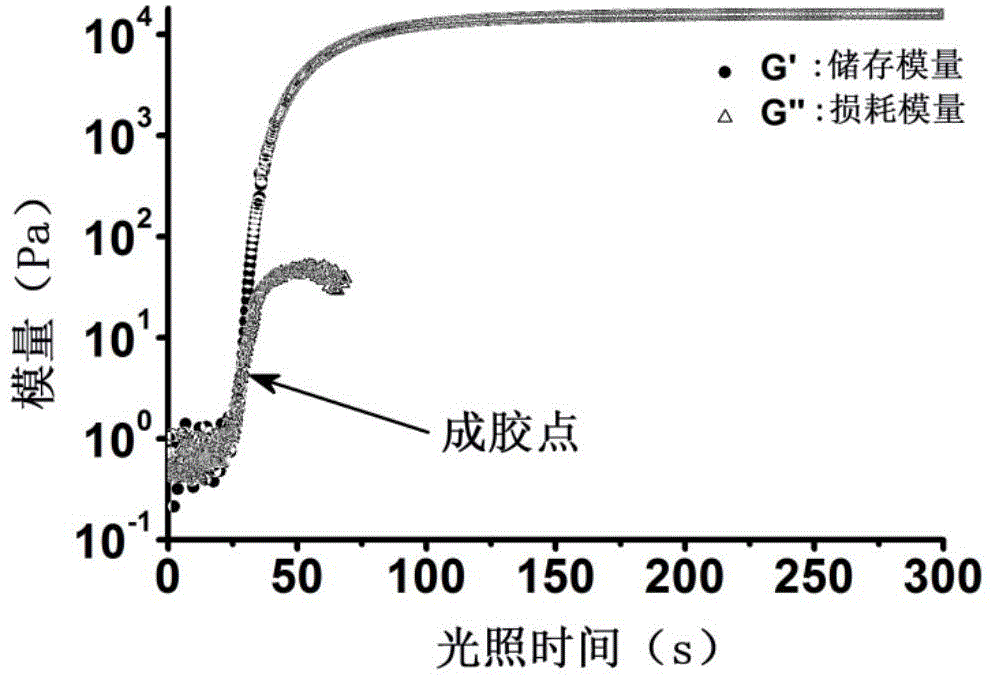
Non-radical photochemical crosslinked hydrogel material preparation method, product and application
ActiveCN105131315APrecise and controllable time and spaceEasy to operateOrganic active ingredientsCosmetic preparationsPolymer scienceHydroxylamine
The present invention provides a non-radical photo-crosslinked hydrogel preparation method, comprising the following steps: a component A is dissolved in a biocompatible medium to obtain a solution A, component B-hydrazide, hydroxylamine or primary amine high molecular derivative is dissolved in a biocompatible medium to obtain a solution B; the solution A and the solution B are evenly mixed to obtain a hydrogel precursor solution; under illumination, aldehyde group produced by light excitation of o-nitrobenzyl in the component A of the hydrogel precursor solution is crosslinked with hydrazone, hydroxylamine or primary amine group in the component B in the form of respective formation of oxime and Schiff base to produce the hydrogel. The present invention also provides a kit for the hydrogel preparation, and application of the hydrogel in tissue repair, beauty and as a cell, protein or drug carrier. The tissue surface light-situ gel can be achieved by the hydrogel, in particular, wound surface in-situ thin glue formation can be achieved, and the hydrogel is especially suitable for clinical wound surface tissue repair and isolation.
Owner:上海戴云化工科技有限公司 +2
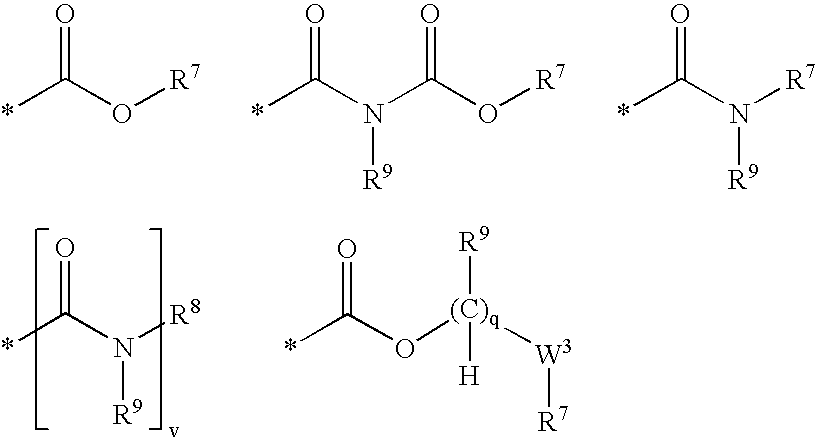
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Hair treatment compositions containing N-hydroxy-alkyl-O-benzyl chitosans and methods of using same
InactiveUS20050226838A1EffectiveMaintain good propertiesCosmetic preparationsHair cosmeticsMedicineAdditive ingredient
The hair treatment composition contains preferably from 0.01 to 20 percent by weight of at least one N-hydroxyalkyl-O-benzyl chitosan and from 0.01 to 20 percent by weight of at least one other hair treatment effective ingredient. The at least one N-hydroxyalkyl-O-benzyl chitosan has at least one hydroxylalkyl group, preferably a hydroxyethyl, hydroxypropyl or hydroxybutyl group, and has from 2 to 20 carbon atoms. Various methods of treating hair with hair treatment compositions containing one or more of the N-hydroxyalkyl-O-benzyl chitosans are described.
Owner:WELLA AG
Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments
The invention relates to crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, to a method for the preparation thereof, as well as to the use thereof for preparing medicaments.
Owner:BOEHRINGER INGELHEIM INT GMBH
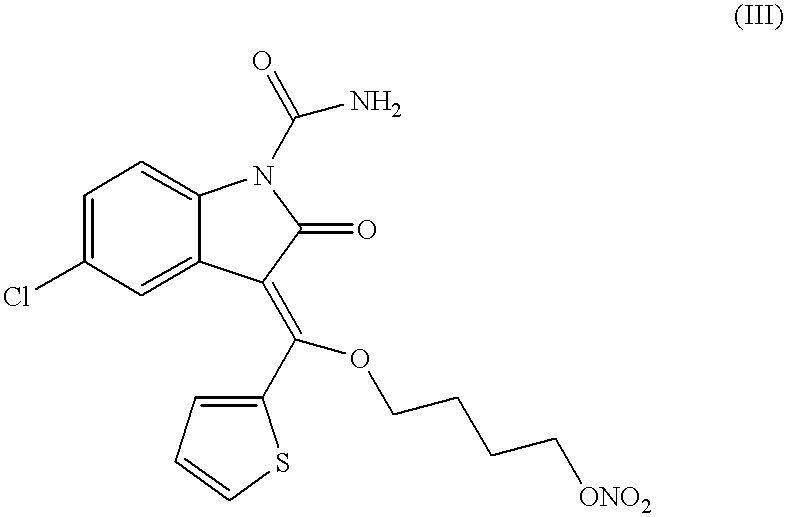
Nitric oxide releasing oxindole prodrugs for anagesic, Anti-inflammatory and disease-modifying use
Nitric oxide releasing oxindole prodrugs are described which are useful in methods of treating or preventing pain, inflammation, fever, or gastrointestinal lesions in a patient in need of such treatment, or of modifying an inflammatory disease or condition by favorably affecting the outcome thereof in said patient, wherein there is administered to said patient a therapeutically effective amount of a compound of Formula (I): and pharmaceutically acceptable salts thereof. In preferred embodiments, X is a covalent bond; RA and RB are both hydrogen; n is the integer 4; Y is -O-; Z is -NO2; RC is a member selected from the group consisting essentially of 5-Cl and 5-F; RD is a member selected from the group consisting essentially of 6-Cl and 6-F; and RE is a member selected from the group consisting essentially of benzyl, 2-furyl, 2-thienyl, 5-chloro-2-thienyl and 5-trifluoromethyl-2-thienyl.
Owner:PFIZER INC +1
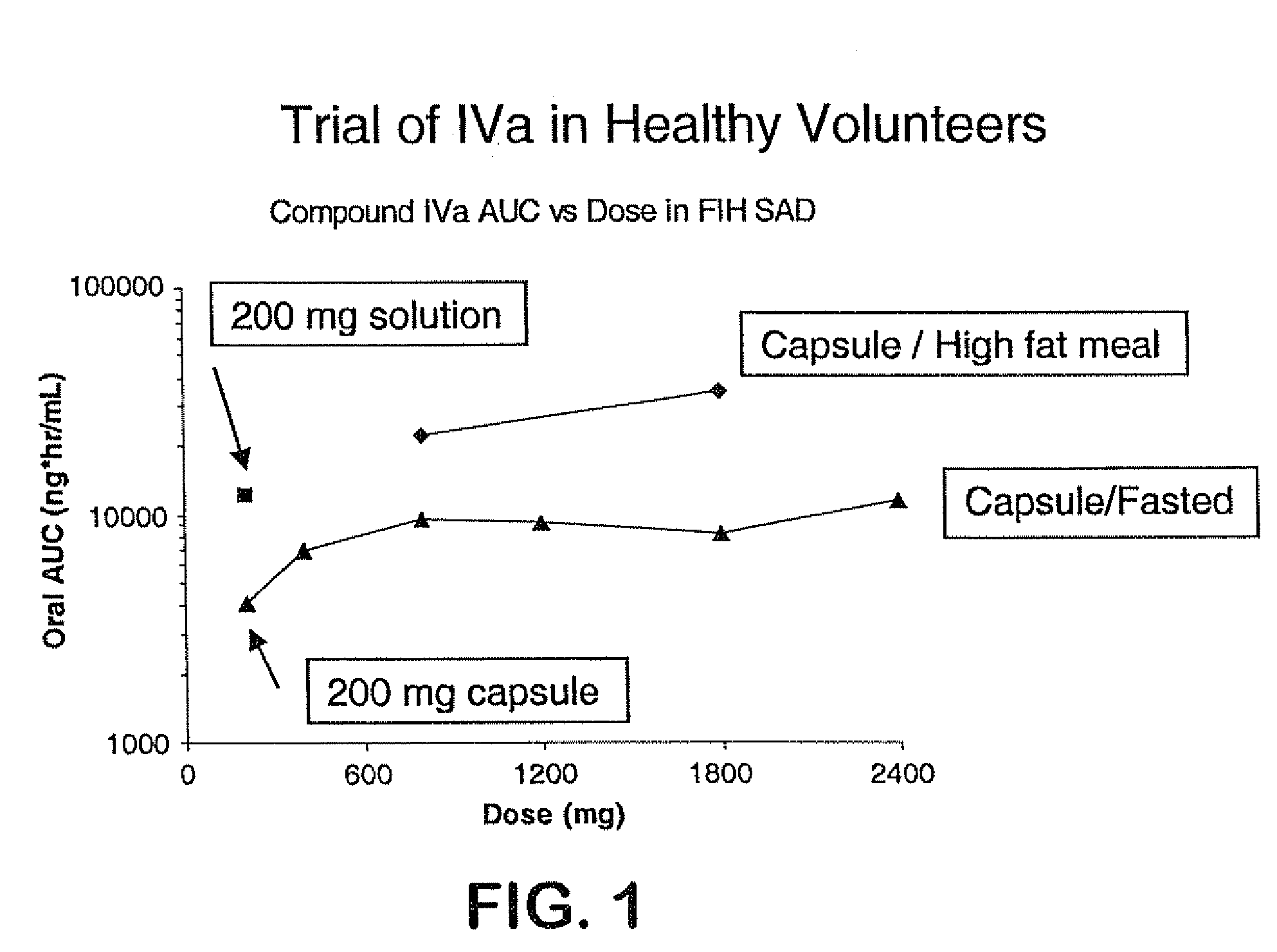
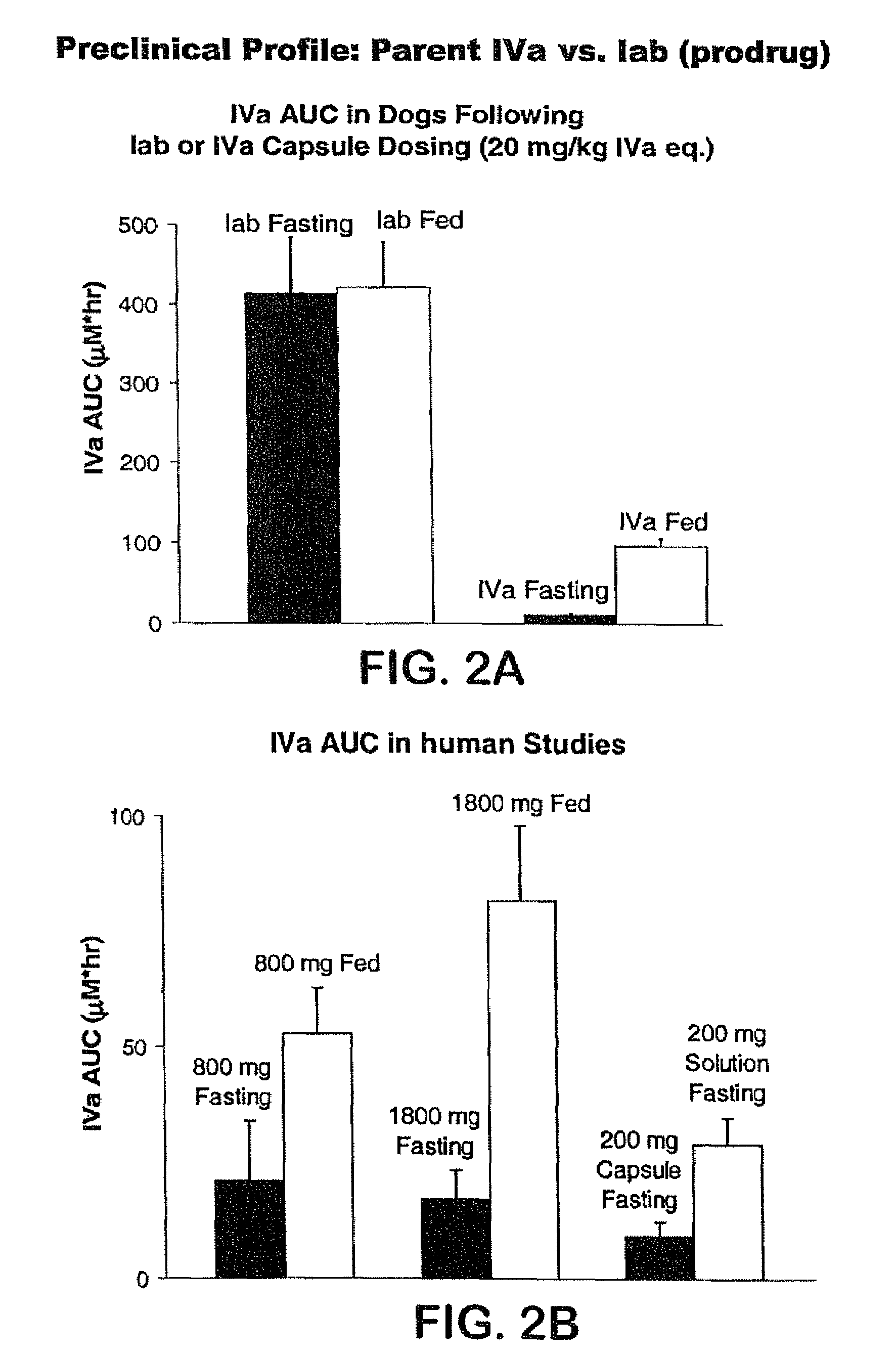
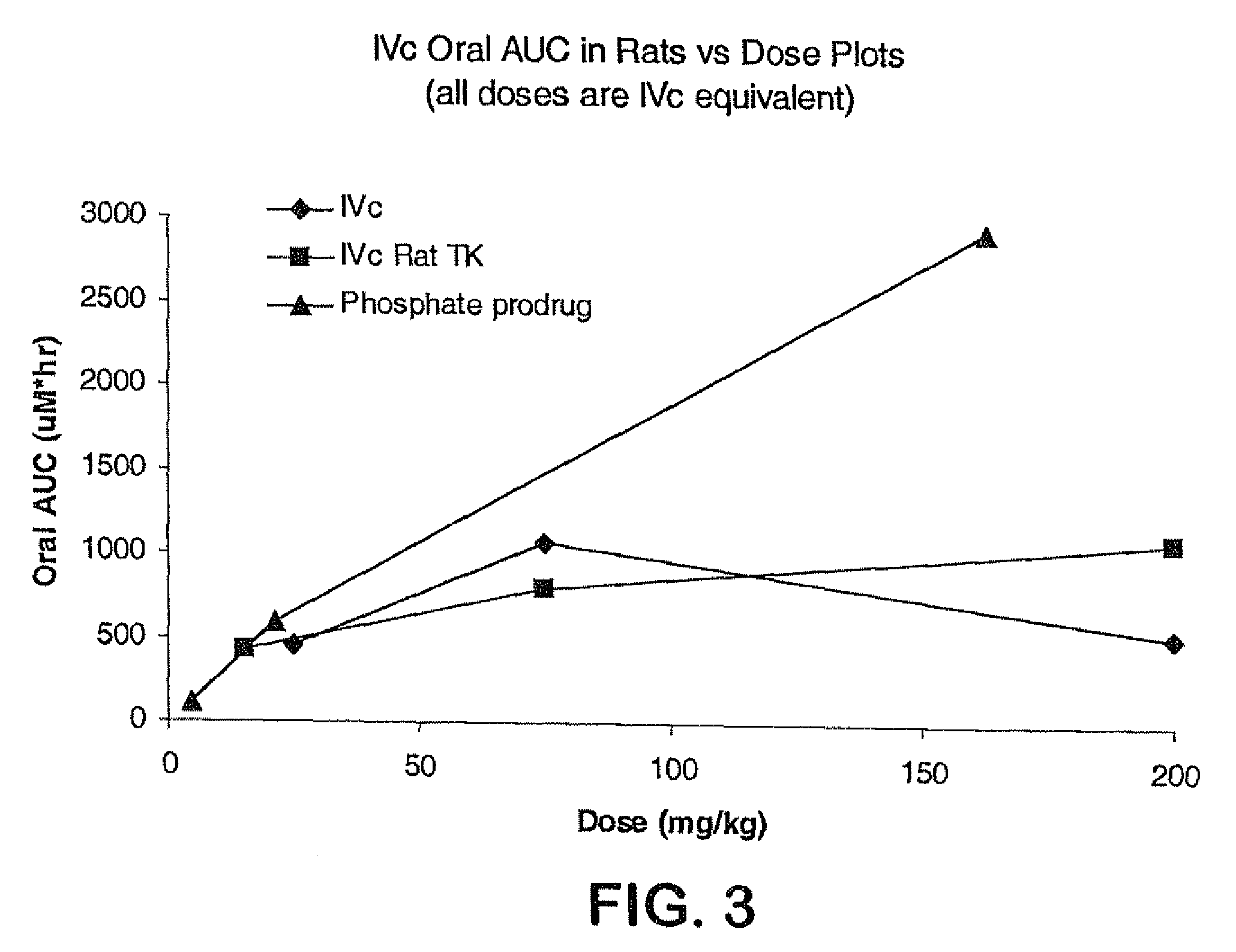
7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy
Owner:ONCOCEUTICS INC
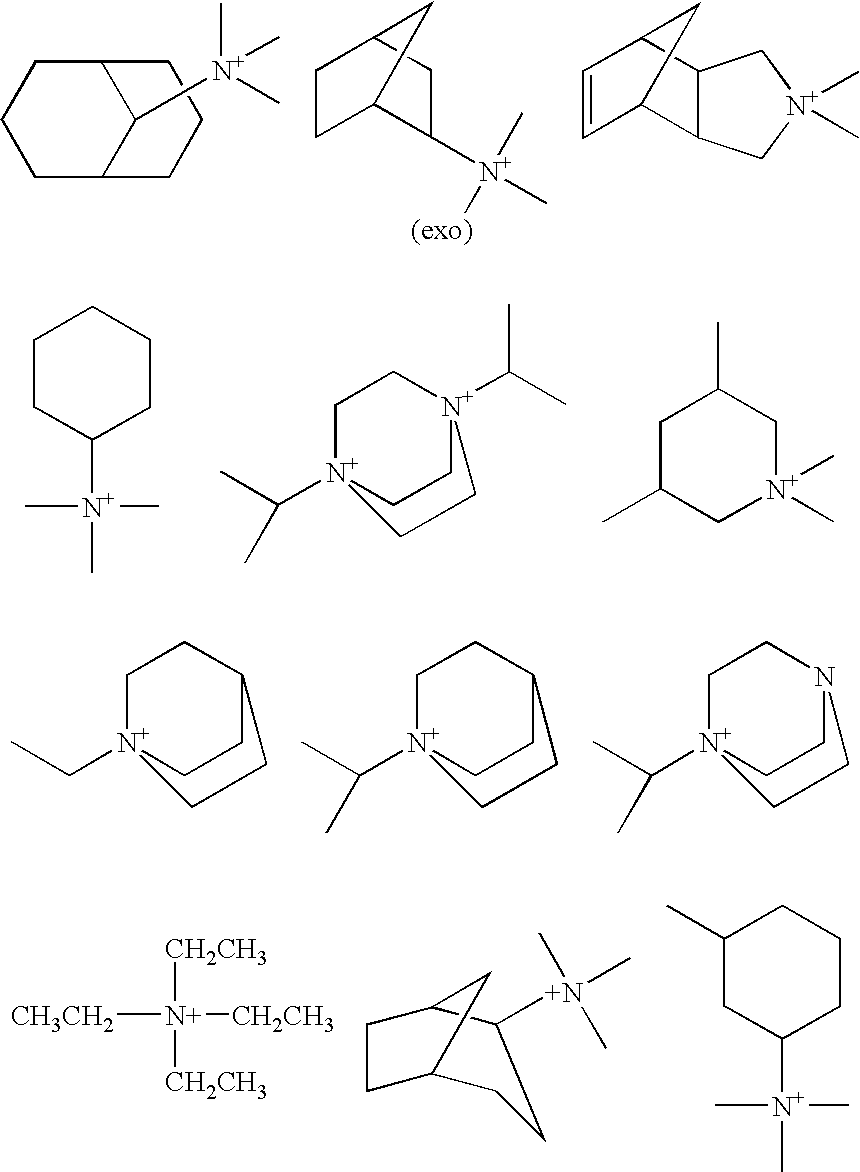
Preparation of Molecular Sieves Using a Structure Directing Agent and An N, N, N-Triakyl Benzyl Quaternary Ammonium Cation
Crystalline molecular sieves are prepared using a mixture comprising an organic structure directing agent capable of forming the molecular-sieve and an N,N,N-trialkyl benzyl quaternary ammonium cation.
Owner:CHEVROU USA INC
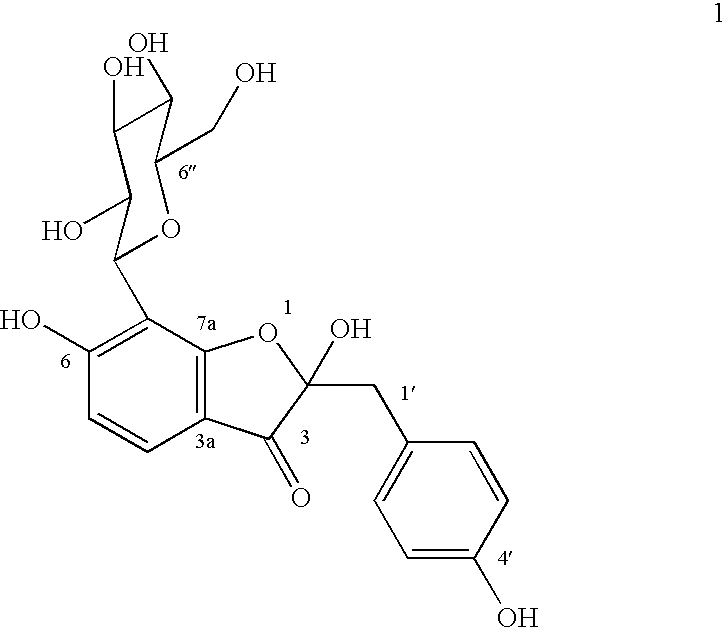
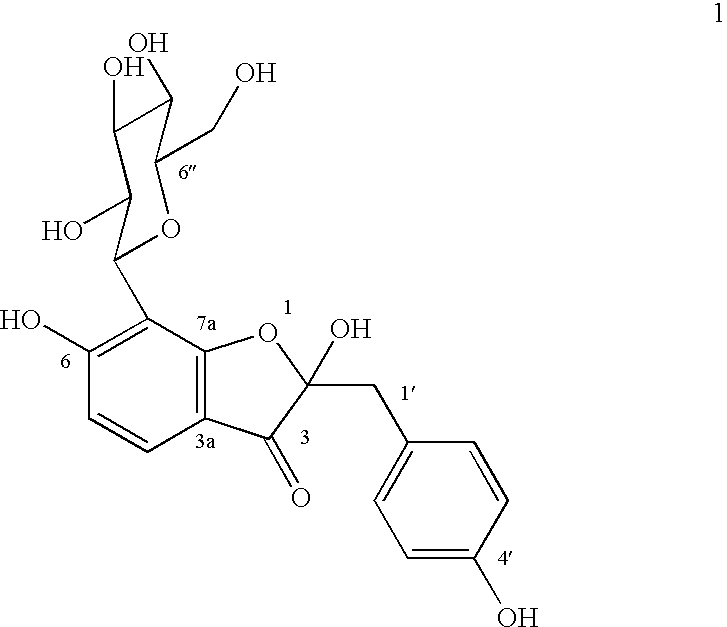
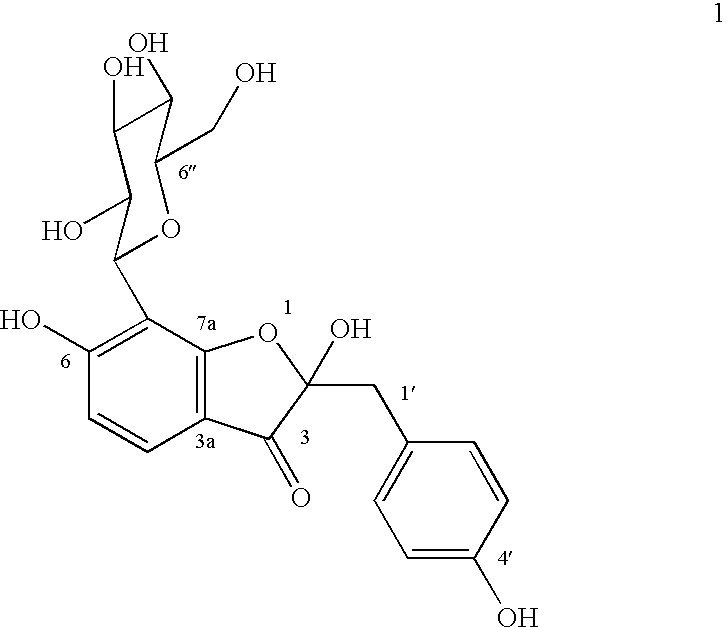
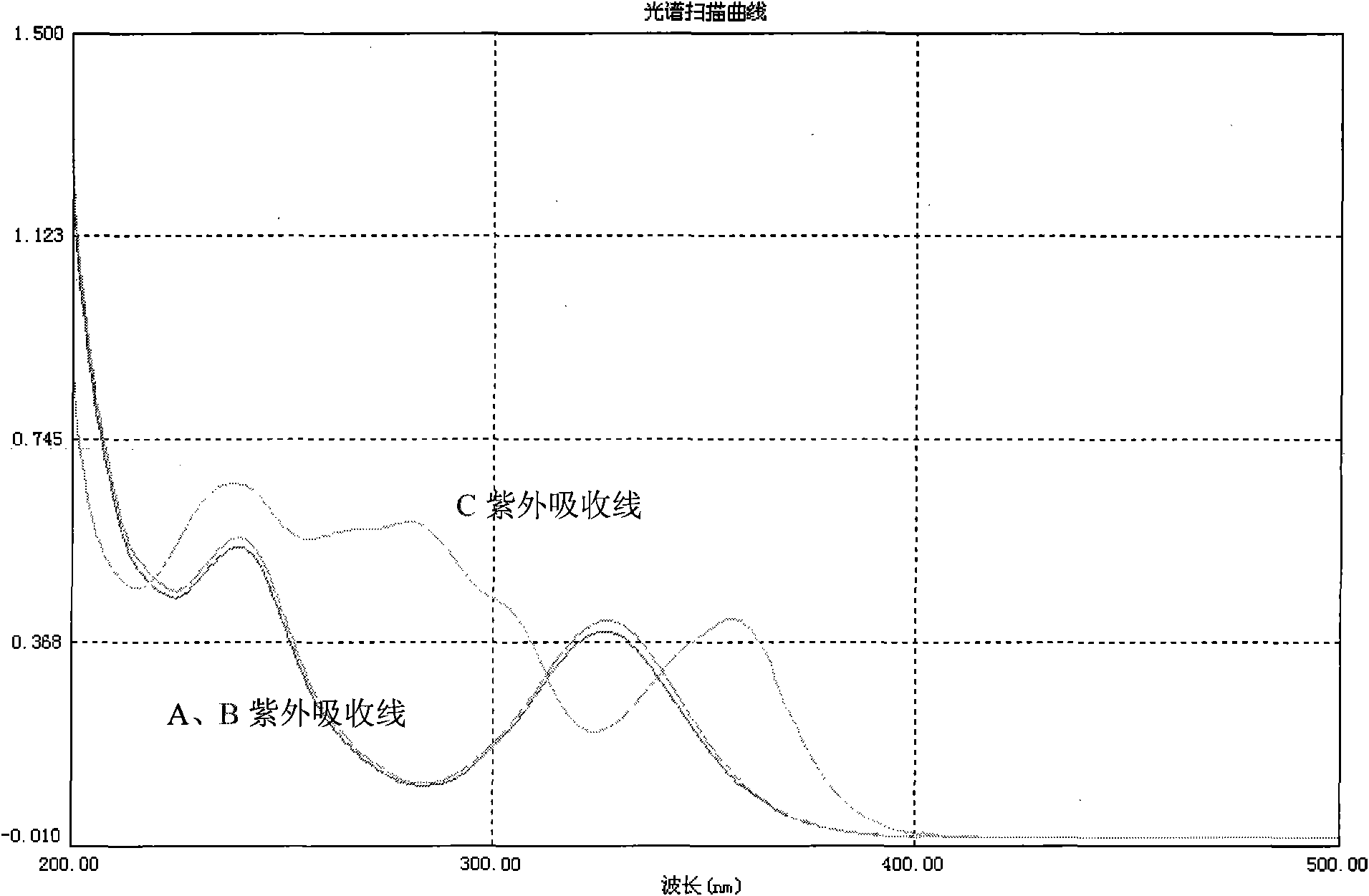
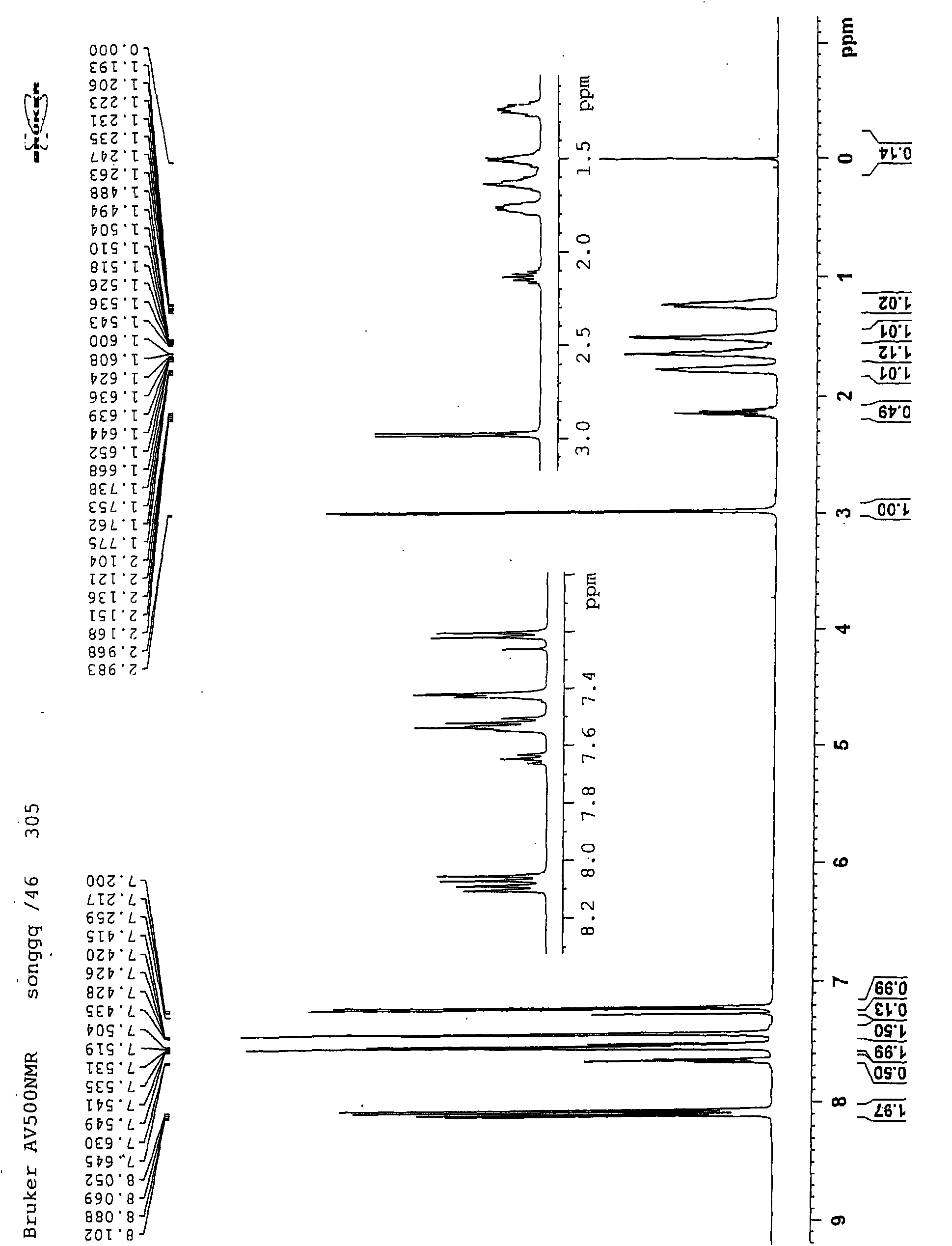
Glucopyranoside and process of isolation thereof from pterocarpus marsupium pharmaceutical composition containing the same and use thereof
A novel glucopyranoside, 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H) benzofuran-7-C-beta-D-glucopyranoside of the formula 1isolated from Pterocarpus marsupium and to a process for the isolation thereof is disclosed. The invention also relates to a pharmaceutical composition containing 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H)benzofuran-7-C-beta-D-glucopyranoside and to method for the treatment of diabetes using said compound.
Owner:COUNCIL OF SCI & IND RES
Ketoxime ester photoinitiator
ActiveCN101565472AImprove applicabilityApplication performance (good sensitivityOrganic compound preparationPhotomechanical apparatusCarbazoleMethyl benzene
The invention relates to a ketoxime ester photoinitiator, in particular to a ketoxime ester photoinitiator for a photo-curing material. The ketoxime ester photoinitiator has a chemical structural formula as the right, wherein in a R1 structure, n is an integer of between 0 and 5; m is an integer of between 1 and 6; R2 is methyl, phenyl, substituted phenyl, benzyl or substituted benzyl; and R3 is diphenyl sulfide group, substituted diphenyl sulfide group, carbazole group or substituted carbazole group. The ketoxime ester photoinitiator solves the problems of poor application performance and poor thermal stability of the prior OXE-1 ketoxime ester photoinitiator.
Owner:CHANGZHOU TRONLY NEW ELECTRONICS MATERIALS
High-energy, rechargeable, electrochemical cells with non-aqueous electrolytes
InactiveUS6316141B1Non-aqueous electrolyte accumulatorsGroup 5/15 element organic compoundsElectrolysisHigh energy
A non-aqueous electrolyte for use in an electrochemical cell comprising: (a) at least one organic solvent; (b) at least one electrolytically active salt represented by the formula:in which: M' is selected from a group consisting of magnesium, calcium, aluminum, lithium and sodium; Z is selected from a group consisting of aluminum, boron, phosphorus, antimony and arsenic; R represents radical selected from the following groups: alkyl, alkenyl, aryl, phenyl, benzyl, and amido; X is a halogen (I, Br, Cl, F); m=1-3; and n=0-5 and q=6 in the case of Z=phosphorus, antimony and arsenic, and n=0-3 and q=4 in the case of Z=aluminum and boron. Rechargeable, high energy density electrochemical cells containing an intercalation cathode, a metal anode, and an electrolyte of the above-described type are also disclosed.
Owner:BAR ILAN UNIV
Aqueous laundry detergent compositions having improved softening properties and improved aesthetics
InactiveUS20050164905A1Cationic surface-active compoundsDetergent compounding agentsLiquid laundry detergentSurface-active agents
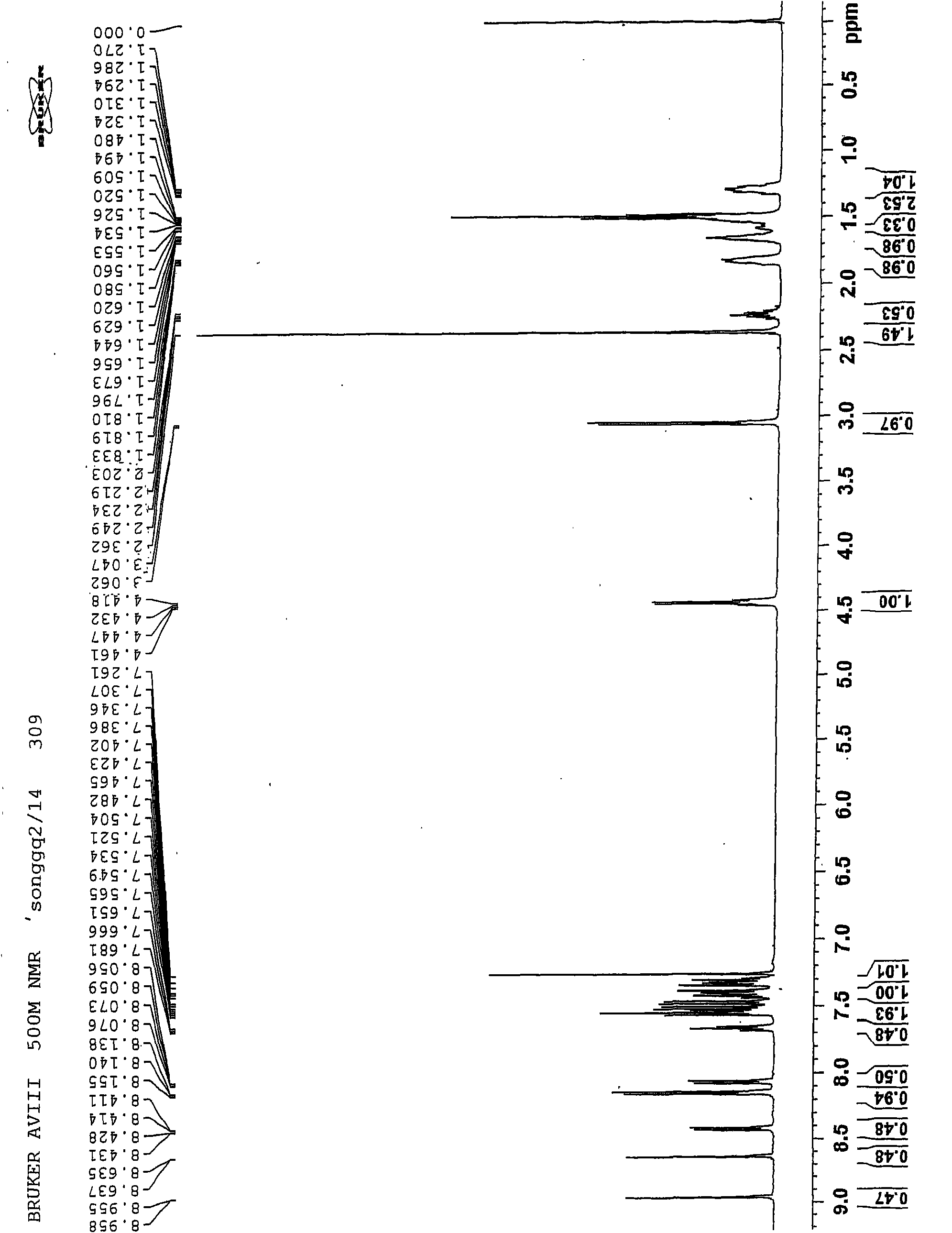
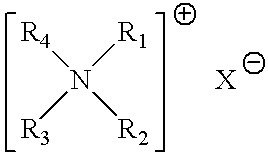
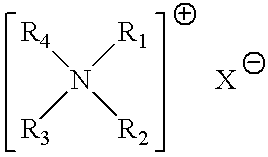
Aqueous heavy duty liquid laundry detergent compositions useful for laundering fabrics and providing cleaning and softening benefits. Such detergent compositions contain at least 5%, by weight of the composition, of a surfactant component; from about 0.1% to about 10% of a quaternary ammonium fabric-softening agent having the formula wherein R1 and R2 are individually selected from the group consisting of C1-C4 alkyl, C1-C4 hydroxy alkyl, benzyl, and —(C2H4O)xH where x has a value from about 2 to about 5; X is an anion; and (1) R3 and R4 are each a C8-C14 alkyl or (2) R3 is a C8-C22 alkyl and R4 is selected from the group consisting of C1-C10 alkyl, C1-C10 hydroxy alkyl, benzyl, and —(C2H4O)xH where x has a value from about 2 to about 5; and such compositions contain less than 10 ppm of trimethylamine and dimethylamine impurities.
Owner:THE PROCTER & GAMBLE COMPANY
Catalyst composition for ethylene oligomerization and the use thereof
ActiveUS20070232481A1High activityGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbons from unsaturated hydrocarbon additionChromium Compounds1-Octene
The present invention relates to a catalyst composition for ethylene oligomerization and the use thereof. Such catalyst composition includes chromium compound, ligand containing P and N, activator and accelerator; wherein the chromium compound is selected from the group consisting of acetyl acetone chromium, THF-chromium chloride and / or Cr(2-ethylhecanoate)3; general formula of the ligand containing P and N is shown as: in which R1, R2, R3 and R4 are phenyl, benzyl, or naphthyl. R5 is isopropyl, butyl, cyclopropyl, cyclopentyl, cyclohexyl or fluorenyl; the activatior is methyl aluminoxane, ethyl aluminoxane, propyl aluminoxane and / or butyl aluminoxane; general formula of the accelerator is X1R6X2, in which X1 and X2 are F, Cl, Br, I or alkoxyl, R6 is alkyl or aryl; the molar ratio of a, b, c and d is 1:0.5˜10:50˜3000:0.5˜10. After mixing the four components mentioned previously under nitrogen atmosphere for 10 minutes, they are incorporated to the reactor, or these four components are incorporated directly into the reactor. Then ethylene is introduced for oligomerization. Such catalyst can be used in producing 1-octene through ethylene oligomerization. It is advantageous in high catalysing activity, high 1-octene selectivity, etc. The catalytic activity is more than 1.0×106 g product·mol−1 Cr ·h−1, the fraction of C8 linear α-olefin is more than 70% by mass.
Owner:PETROCHINA CO LTD
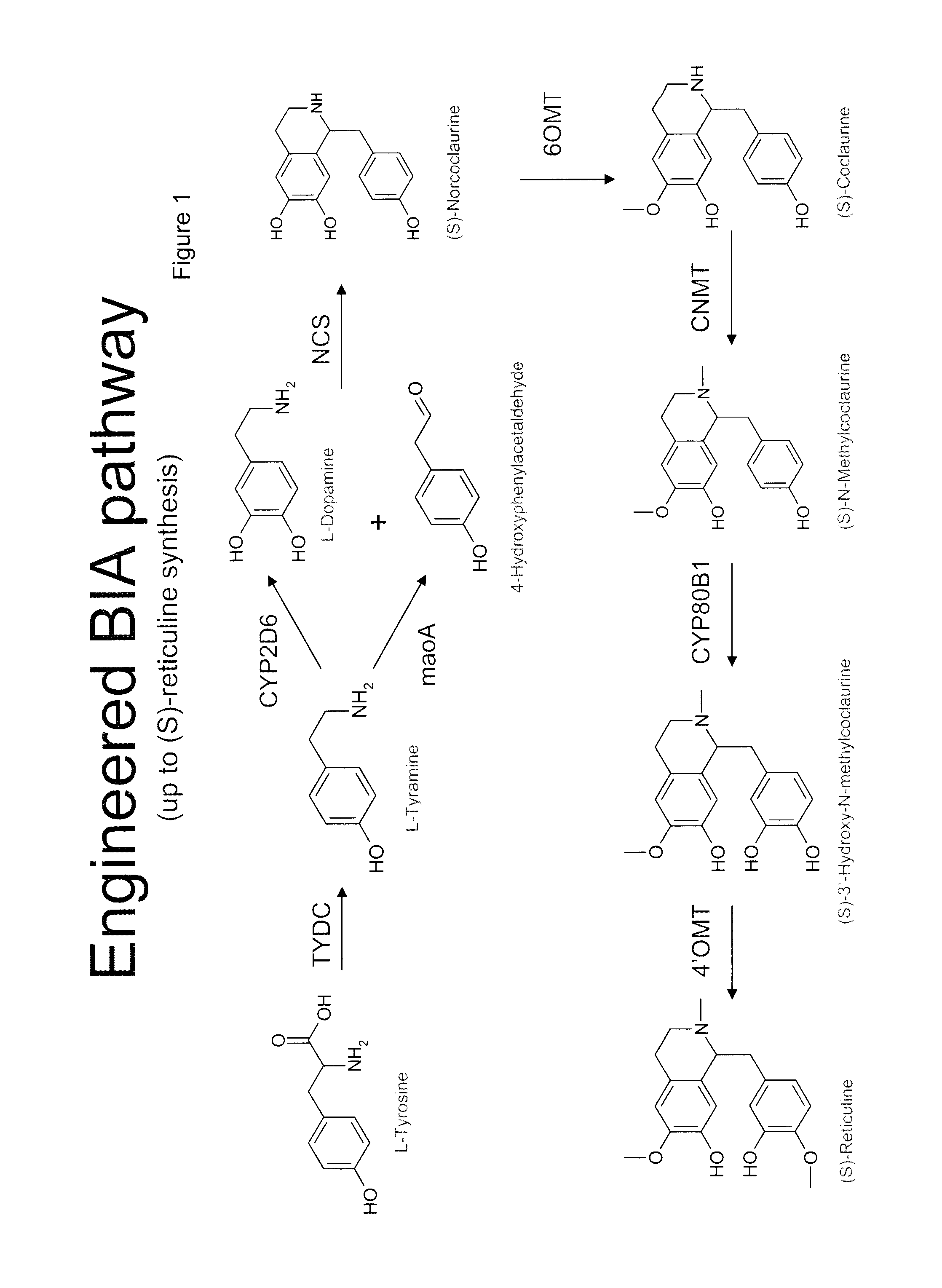
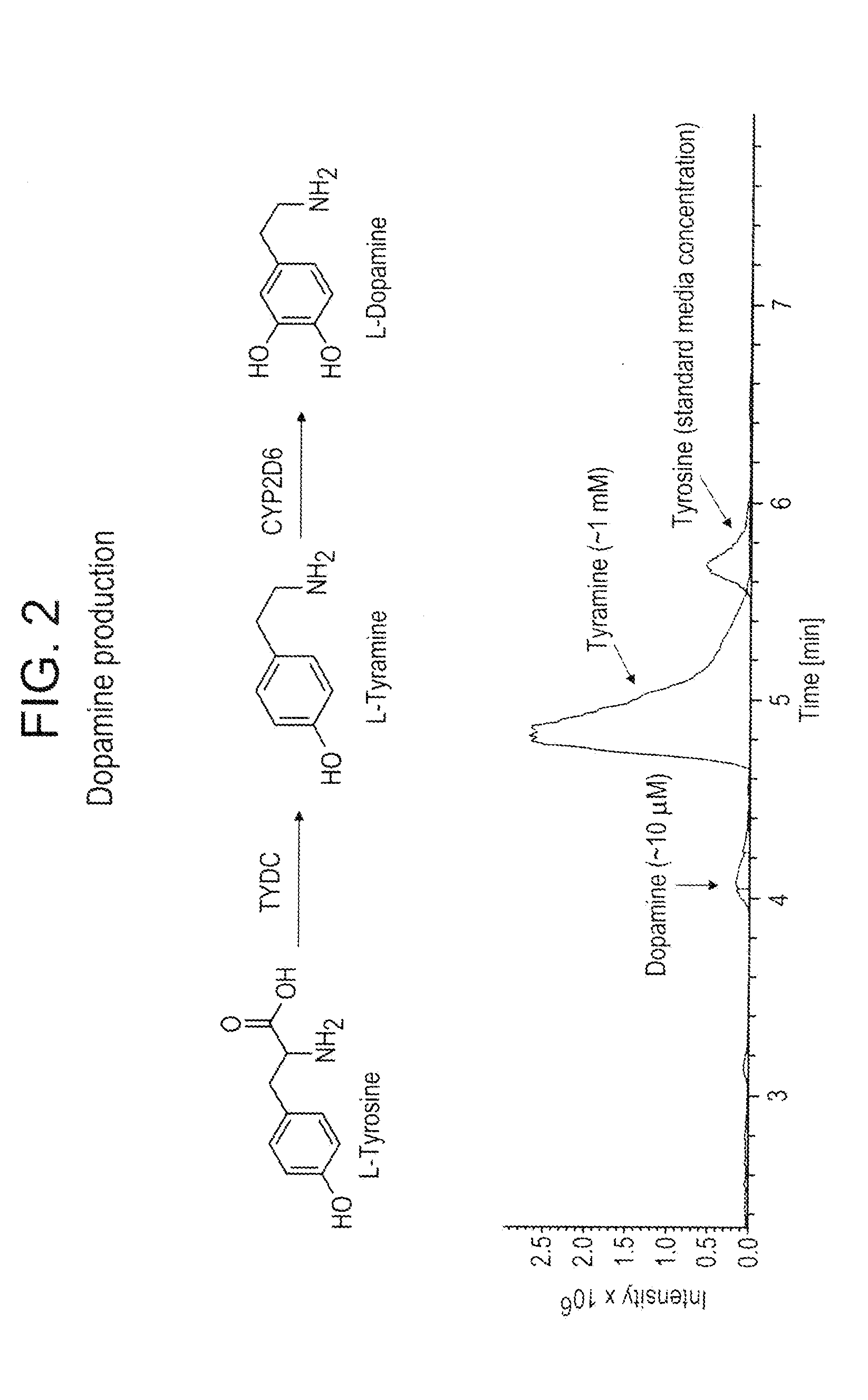
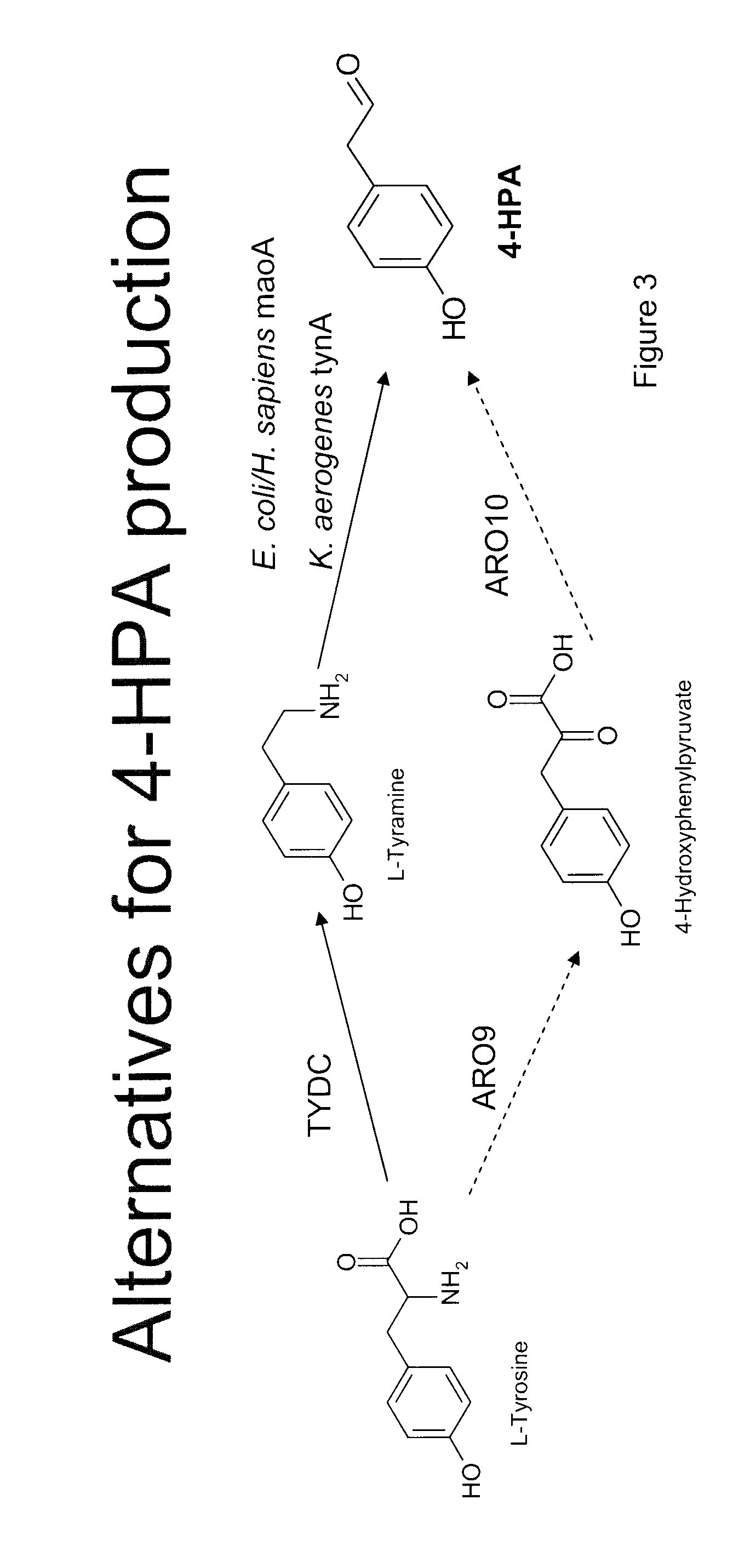
Compositions and methods for producing benzylisoquinoline alkaloids
ActiveUS20080176754A1Optimize growth ratePromote expression and activityTissue cultureLibrary member identificationMetabolic pathwayBenzylisoquinoline
The present invention relates to host cells that produce compounds that are characterized as benzylisoquinolines, as well as select precursors and intermediates thereof. The host cells comprise one, two or more heterologous coding sequences wherein each of the heterologous coding sequences encodes an enzyme involved in the metabolic pathway of a benzylisoquinoline, or its precursors or intermediates from a starting compound. The invention also relates to methods of producing the benzylisoquinoline, as well as select precursors and intermediates thereof by culturing the host cells under culture conditions that promote expression of the enzymes that produce the benzylisoquinoline or precursors or intermediates thereof.
Owner:CALIFORNIA INST OF TECH
Personal care compositions and methods for the beautification of mammalian skin and hair
Personal care composition comprising from about 0.05% to about 5% of at least one aquaporin-stimulating compound selected from the group consisting of xanthine, caffeine; 2-amino-6-methyl-mercaptopurine; 1-methyl xanthine; 2-aminopurine; theophylline; theobromine; adenine; adenosine; kinetin; p-chlorophenoxyacetic acid; 2,4-dichlorophenoxyacetic acid; indole-3-butyric acid; indole-3-acetic acid methyl ester; beta-naphthoxyacetic acid; 2,3,5-triiodobenzoic acid; adenine hemisulfate; n-benzyl-9-(2-tetrahydropyranyl)adenine; 1,3-diphenylurea; 1-phenyl-3-(1,2,3-thiadiazol-5-yl)urea; zeatin; indole-3-acetic acid; 6-benzylaminopurine; alpha-napthaleneacetic acid; 6-2-furoylaminopurine; green tea extract; white tea extract; menthol; tea tree oil; ginsenoside-RB1; ginsenoside-RB3; ginsenoside-RC; ginsenoside-RD; ginsenoside-RE; ginsenoside-RG1; ginseng root extract; ginseng flower extract; pomegranate extract, extracts from Ajuga turkestanica; extracts from viola tricolor and combinations thereof; an additional ingredient selected from the group consisting of niacinamide, glycerin and mixtures thereof, and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
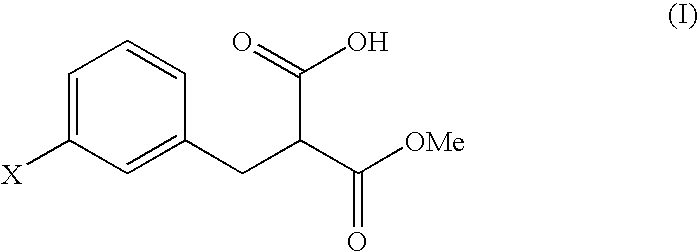
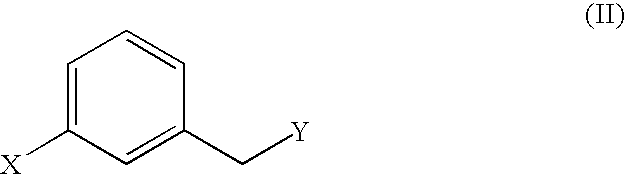
Malonic acid monomethyl derivatives and production process thereof
InactiveUS7109369B2Easy to makeProduct can be usedOrganic compound preparationCarboxylic acid esters preparationHydrogen atomMalonic acid
Owner:DAICEL CHEM IND LTD
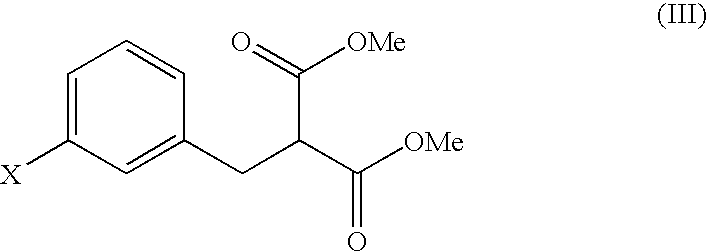
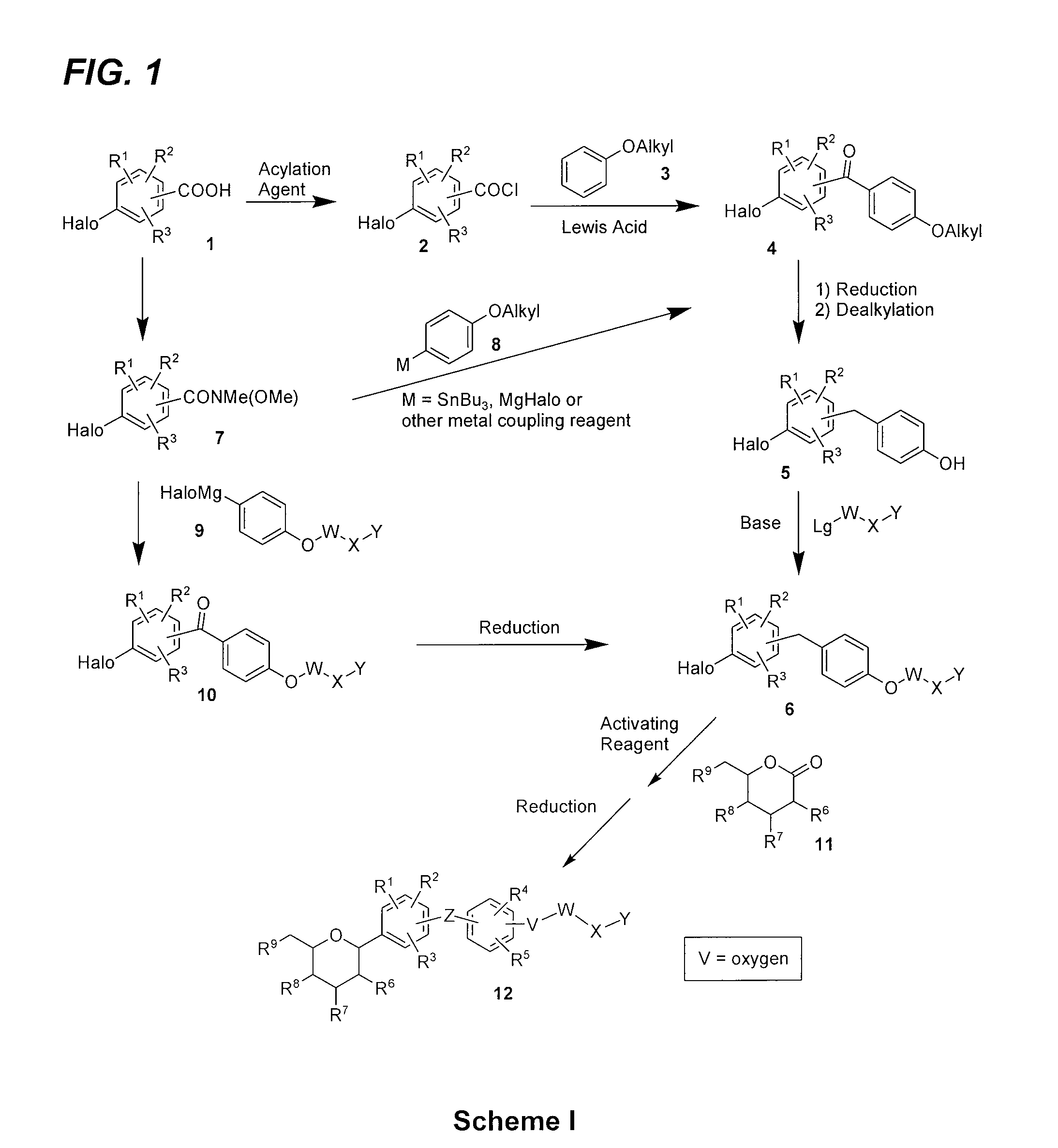
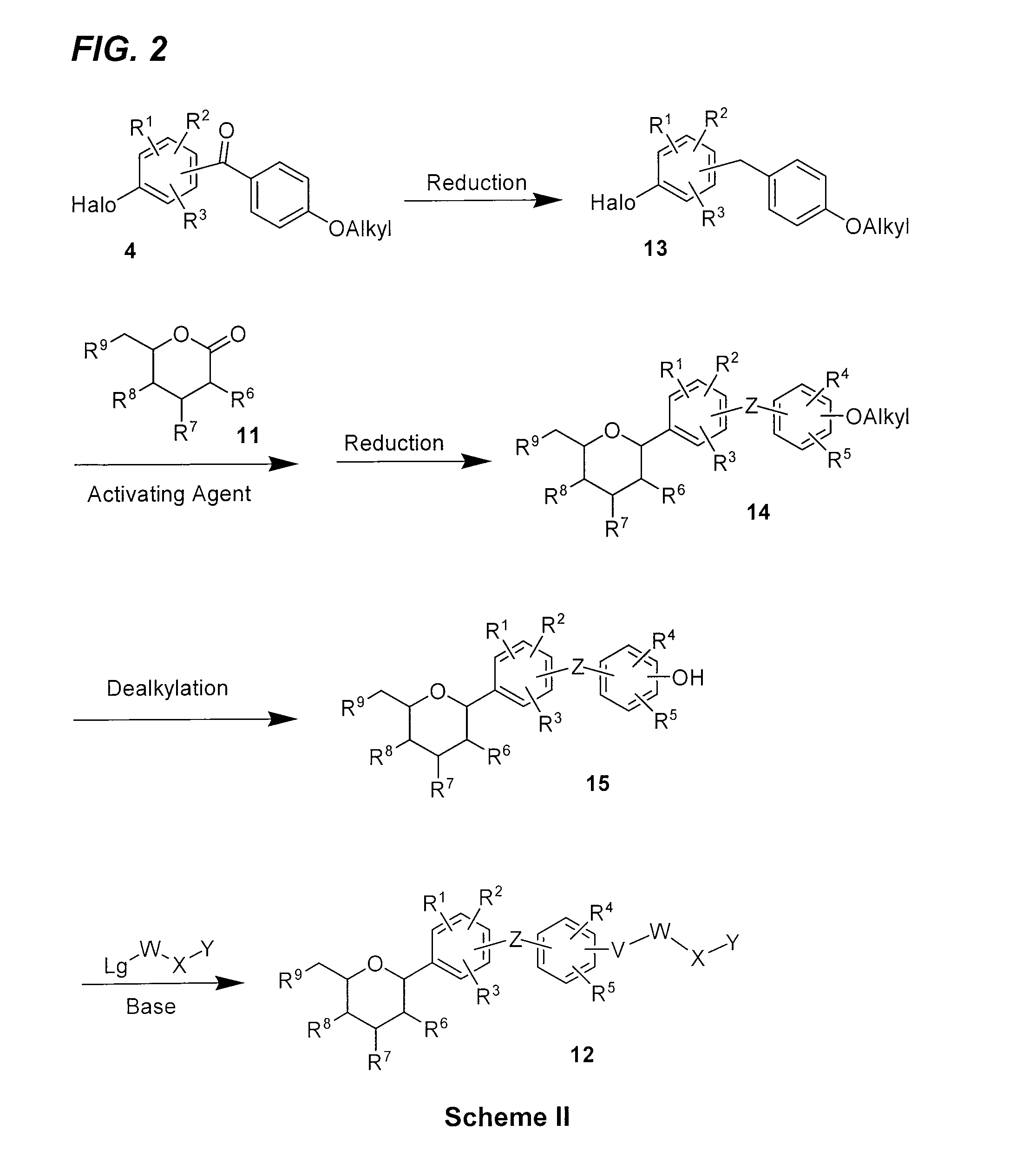
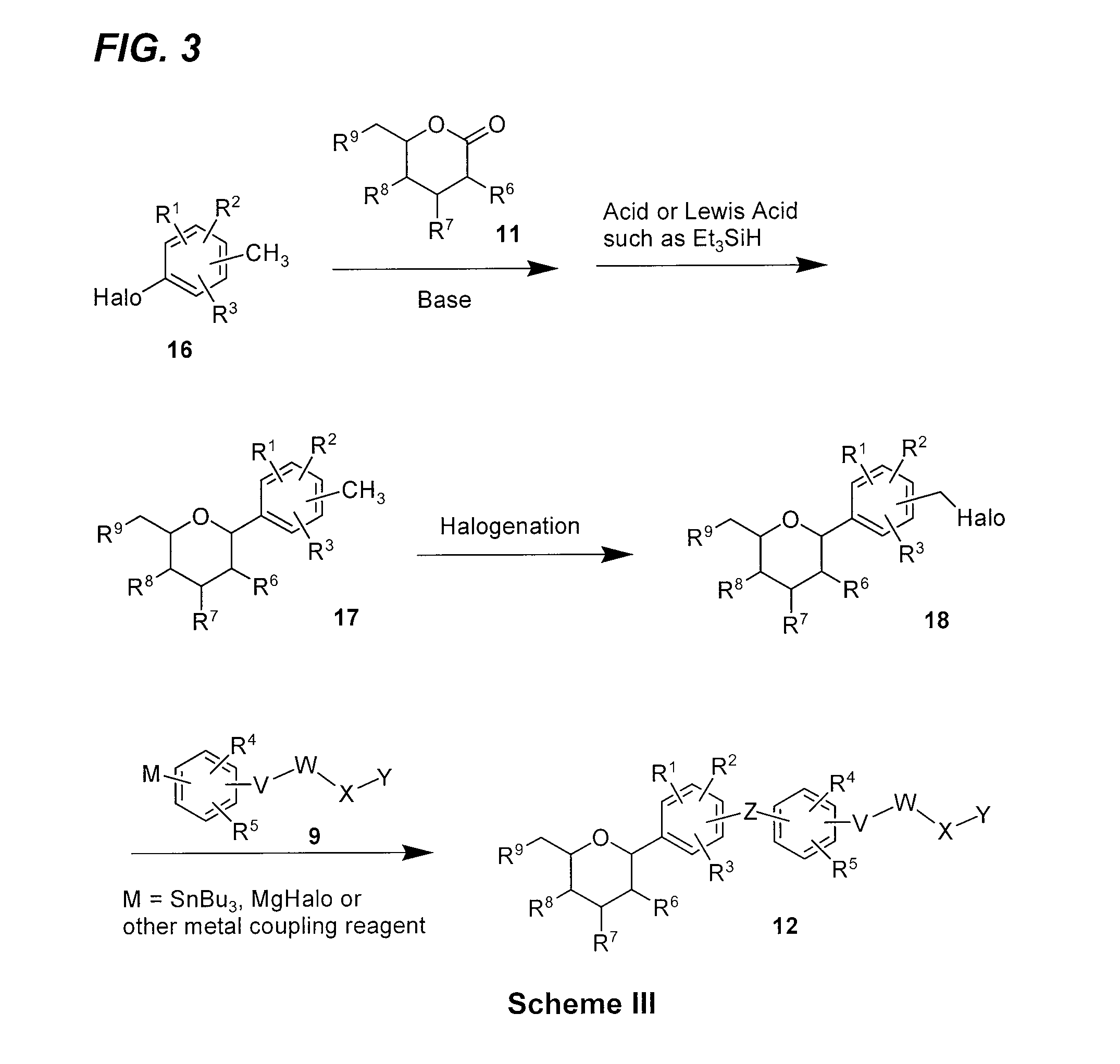
Benzylbenzene derivatives and methods of use
Provided are compounds having an inhibitory effect on sodium-dependent glucose cotransporter SGLT. The invention also provides pharmaceutical compositions, methods of preparing the compounds, synthetic intermediates, and methods of using the compounds, independently or in combination with other therapeutic agents, for treating diseases and conditions which are affected by SGLT inhibition.
Owner:THERACOSBIO LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
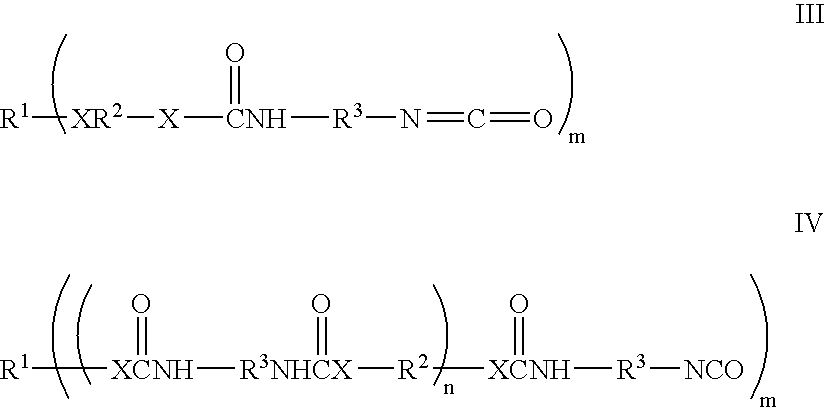


![Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e018e28e-a3d7-43a6-9df9-4da07d78e0e6/US20070249544A1-20071025-D00001.png)
![Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e018e28e-a3d7-43a6-9df9-4da07d78e0e6/US20070249544A1-20071025-C00001.png)
![Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline form of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((S)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e018e28e-a3d7-43a6-9df9-4da07d78e0e6/US20070249544A1-20071025-C00002.png)
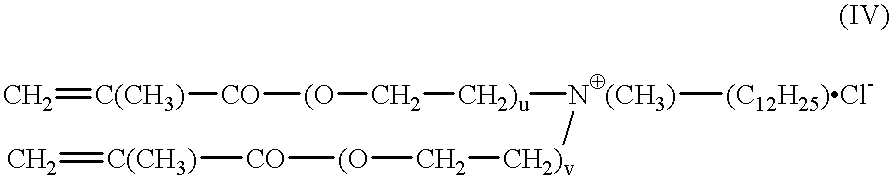
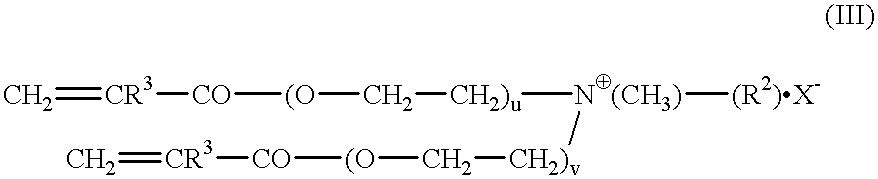
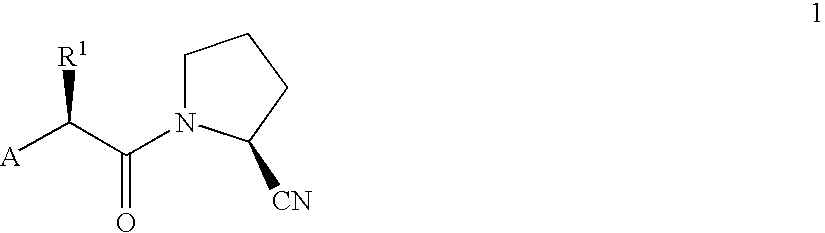
![Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1037191-23f6-4415-95ef-79a218e0cb8b/US20060251728A1-20061109-D00000.png)
![Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1037191-23f6-4415-95ef-79a218e0cb8b/US20060251728A1-20061109-D00001.png)
![Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(beta-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c1037191-23f6-4415-95ef-79a218e0cb8b/US20060251728A1-20061109-D00002.png)



![Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/713cb55c-505a-47ae-a0dd-2350f315dca1/US07723309-20100525-D00001.png)
![Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/713cb55c-505a-47ae-a0dd-2350f315dca1/US07723309-20100525-D00002.png)
![Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments Crystalline forms of 1-chloro-4-(β-D-glucopyranos-1-yl)-2-[4-((R)-tetrahydrofuran-3-yloxy)-benzyl]-benzene, a method for its preparation and the use thereof for preparing medicaments](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/713cb55c-505a-47ae-a0dd-2350f315dca1/US07723309-20100525-C00001.png)


![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-D00001.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-C00001.png)
![7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy 7-benzyl-4-(2-methylbenzyl)-2,4,6,7,8,9-hexahydroimidazo[1,2-a]pyrido[3,4-- e]pyrimidin-5(1H)-one, Salts Thereof and Methods of Using the Same in Combination Therapy](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b2faab5b-d907-46ea-8973-de72880bc4ce/US20140335048A1-20141113-C00002.png)