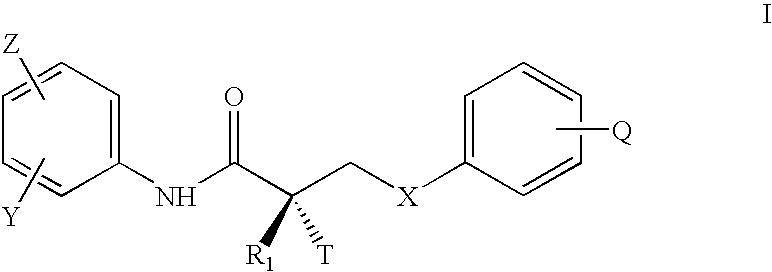
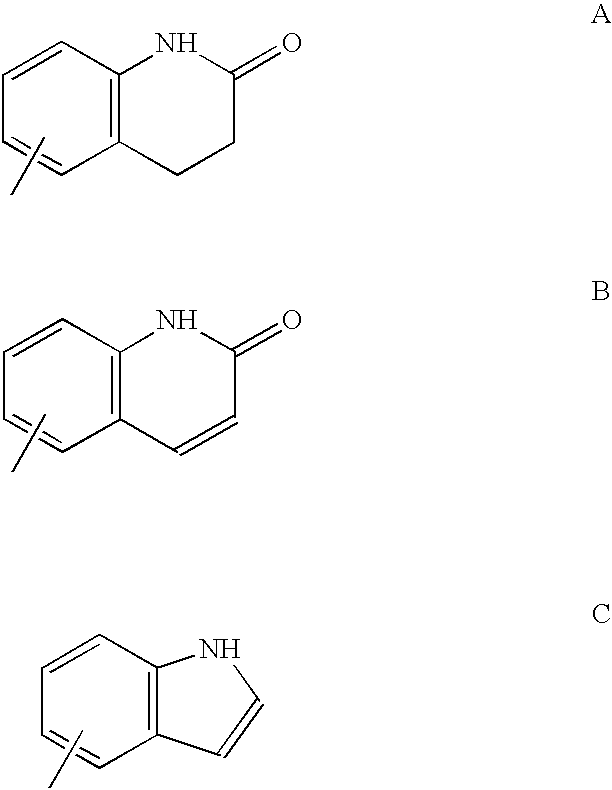
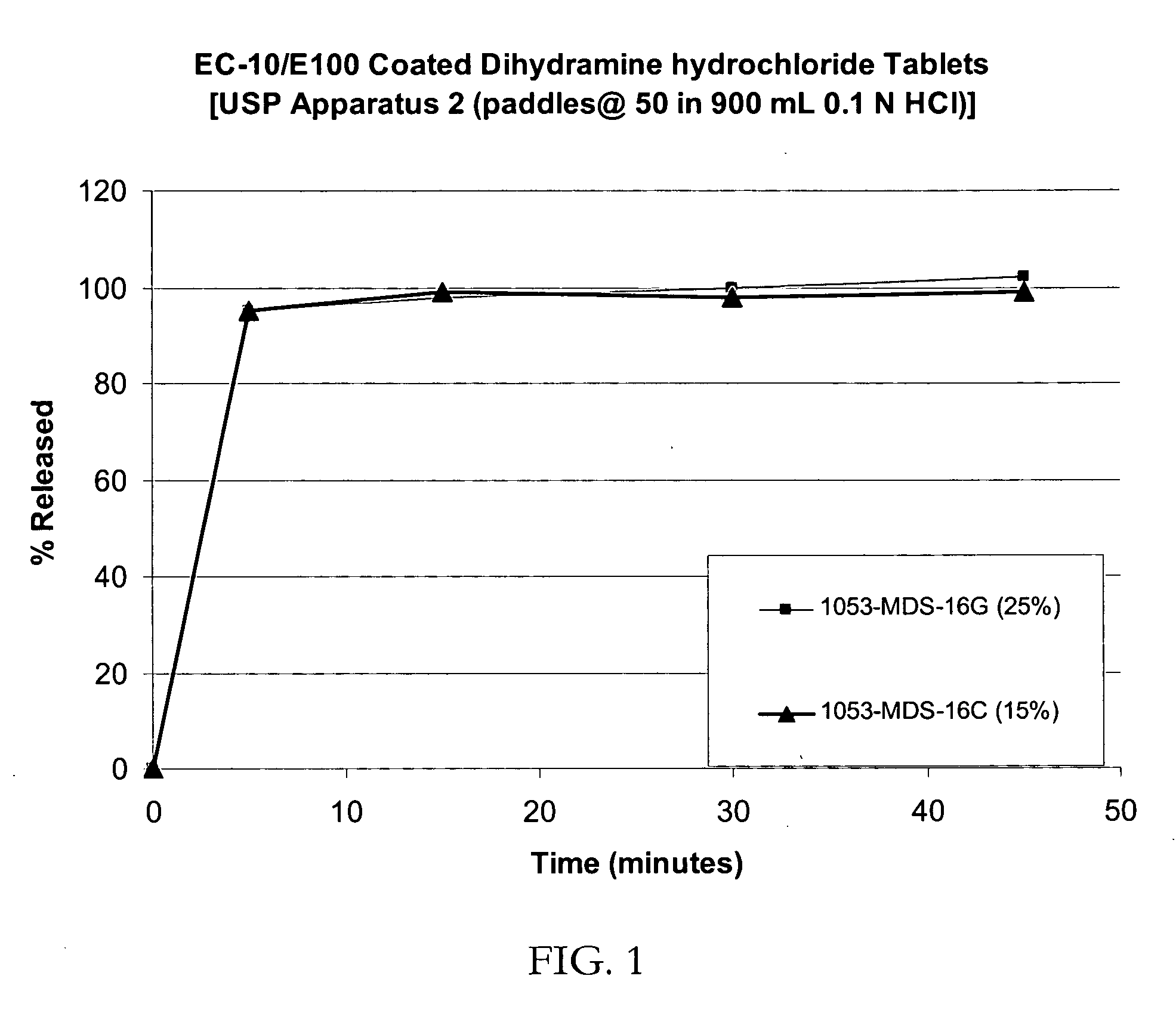
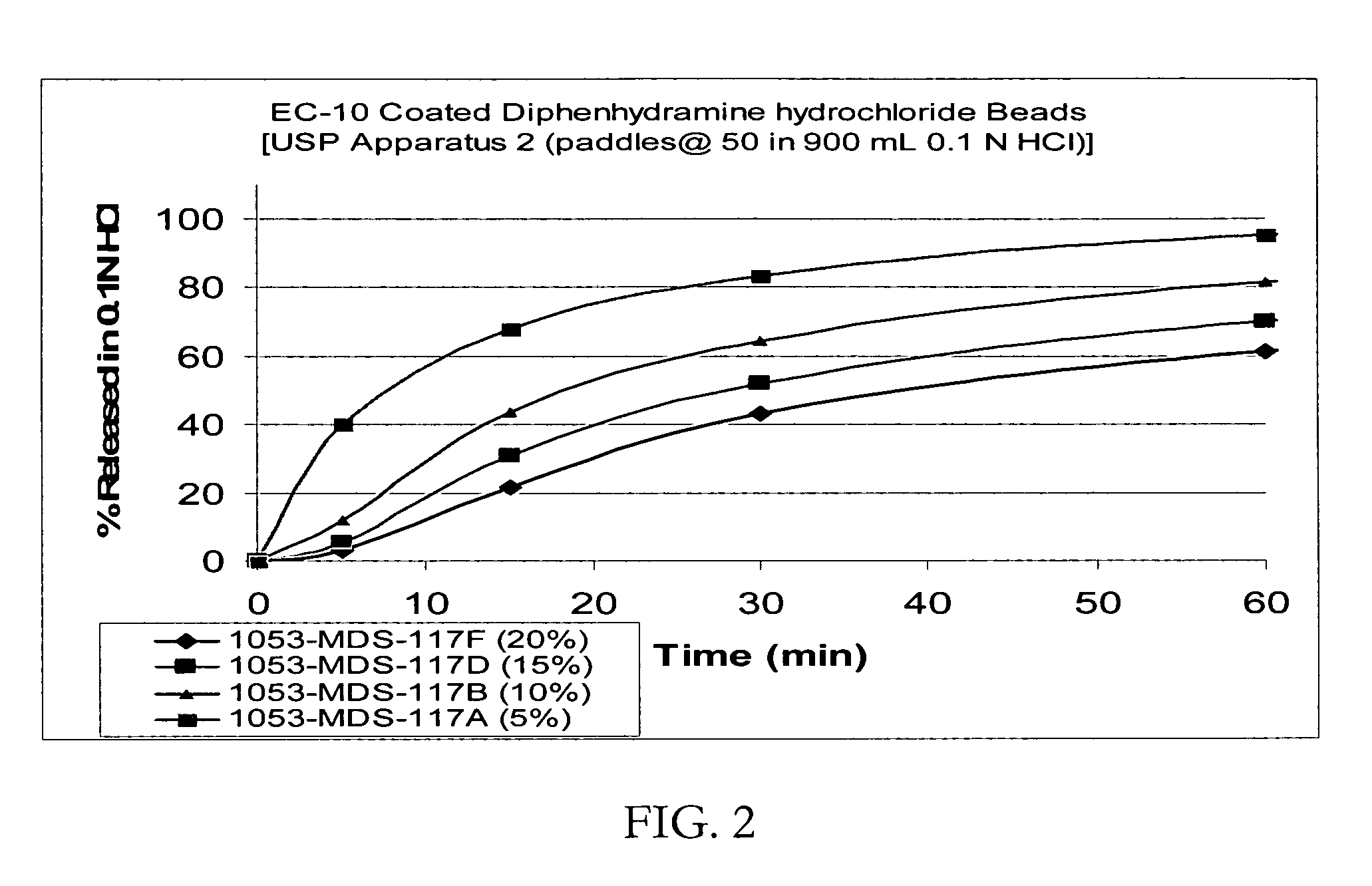
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
5529 results about "Pharmaceutical preservatives" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Some typical preservatives used in pharmaceutical formulations are Antioxidants like vitamin A, vitamin E, vitamin C, retinyl palmitate, and selenium The amino acids cysteine and methionine Citric acid and sodium citrate Synthetic preservatives like the parabens: methyl paraben and propyl paraben.
Colon targeted delivery system
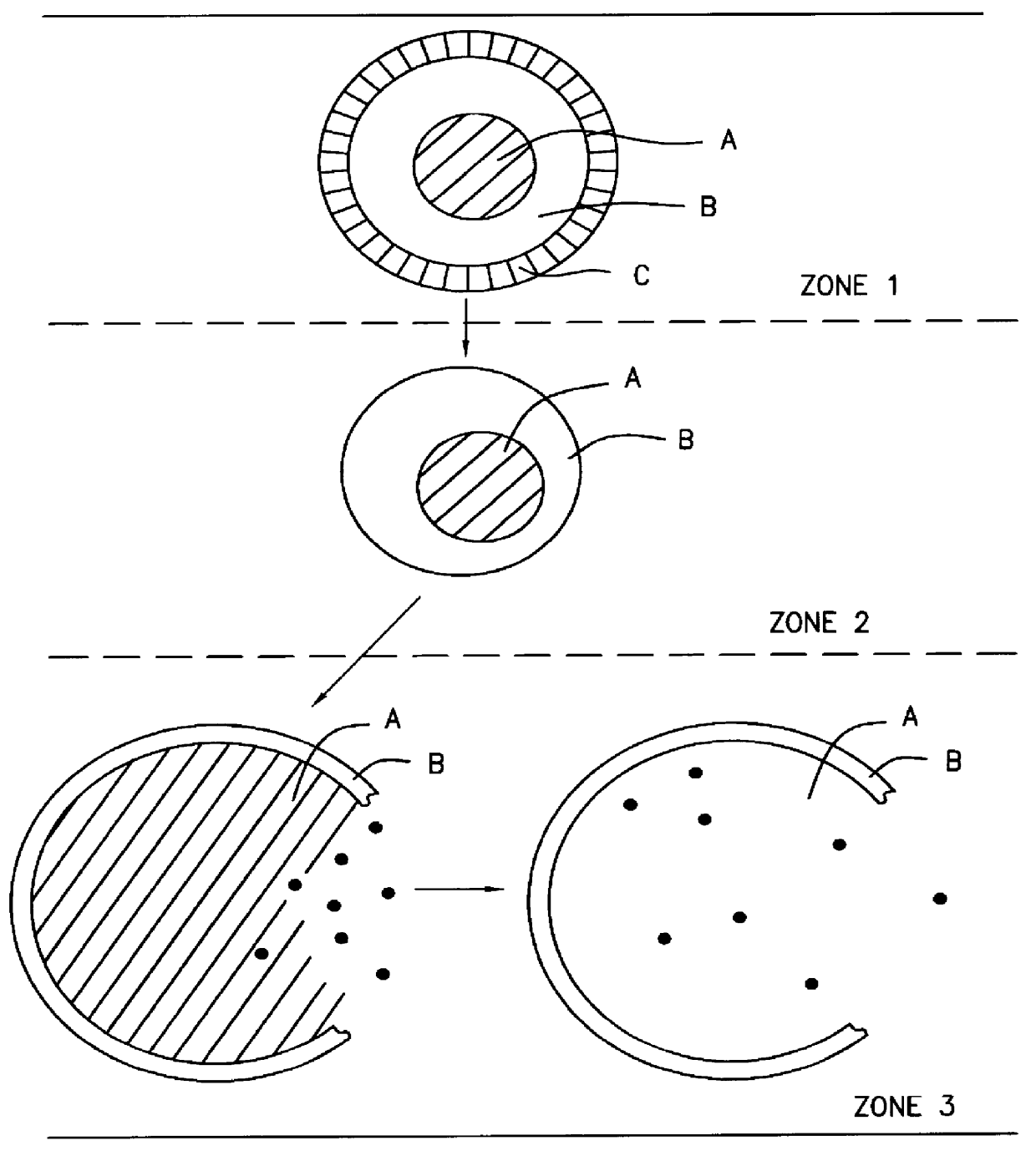
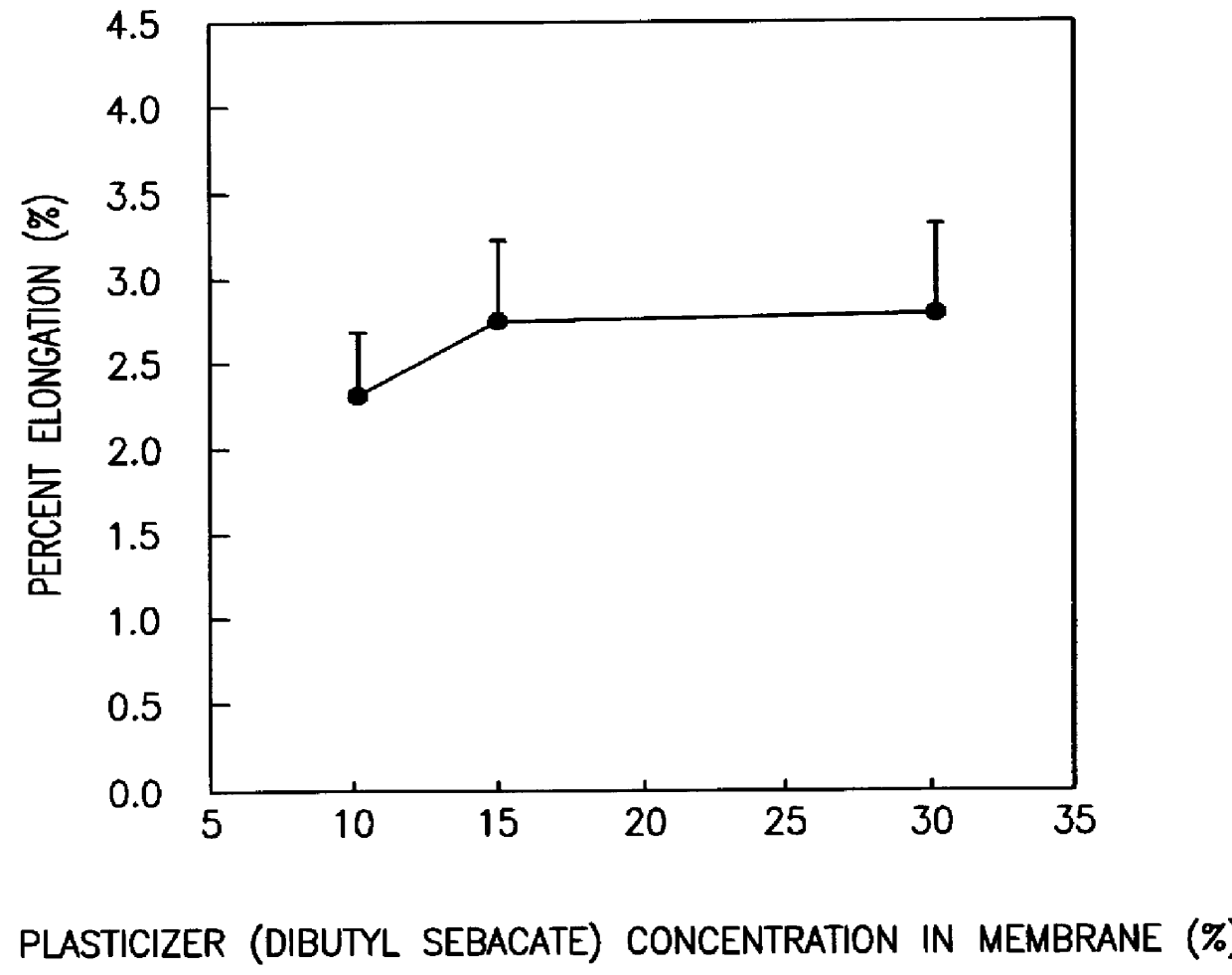
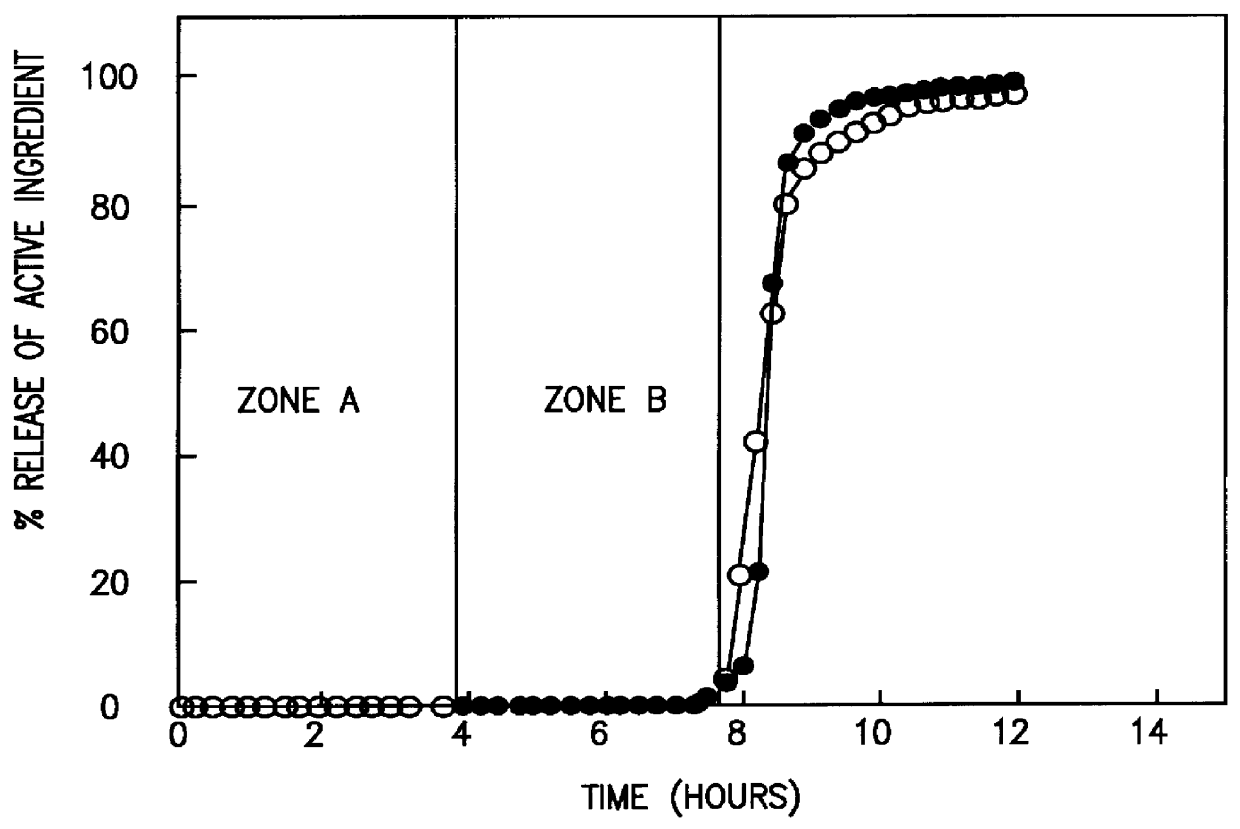
A novel delivery system for targeting drugs to the colon is herein described. The system is a tablet comprised of three parts: 1) an outer enteric coating, 2) an inner semi-permeable polymer membrane containing a plasticizer and 3) a central core comprising swelling excipients and an active ingredient. The novel dosage form described herein will release the drug consistently in the colon by a time-dependent explosion mechanism. This delivery system is particularly suitable for delivering viral protease inhibitors to the colon.
Owner:F HOFFMANN LA ROCHE & CO AG
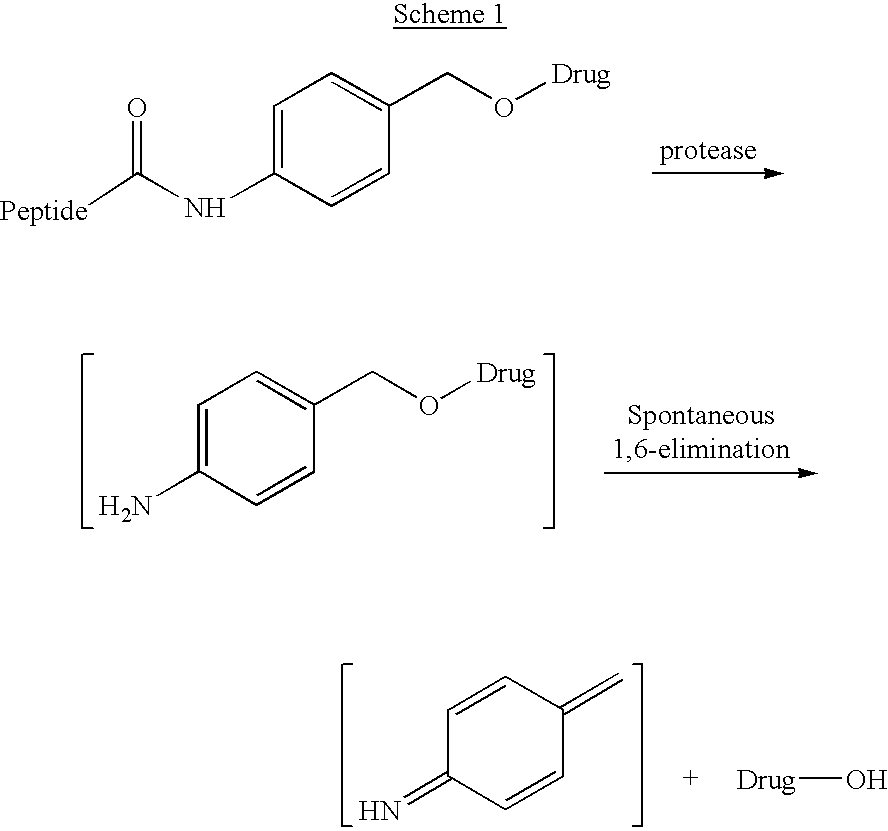
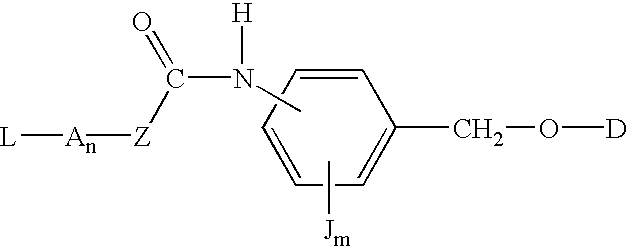
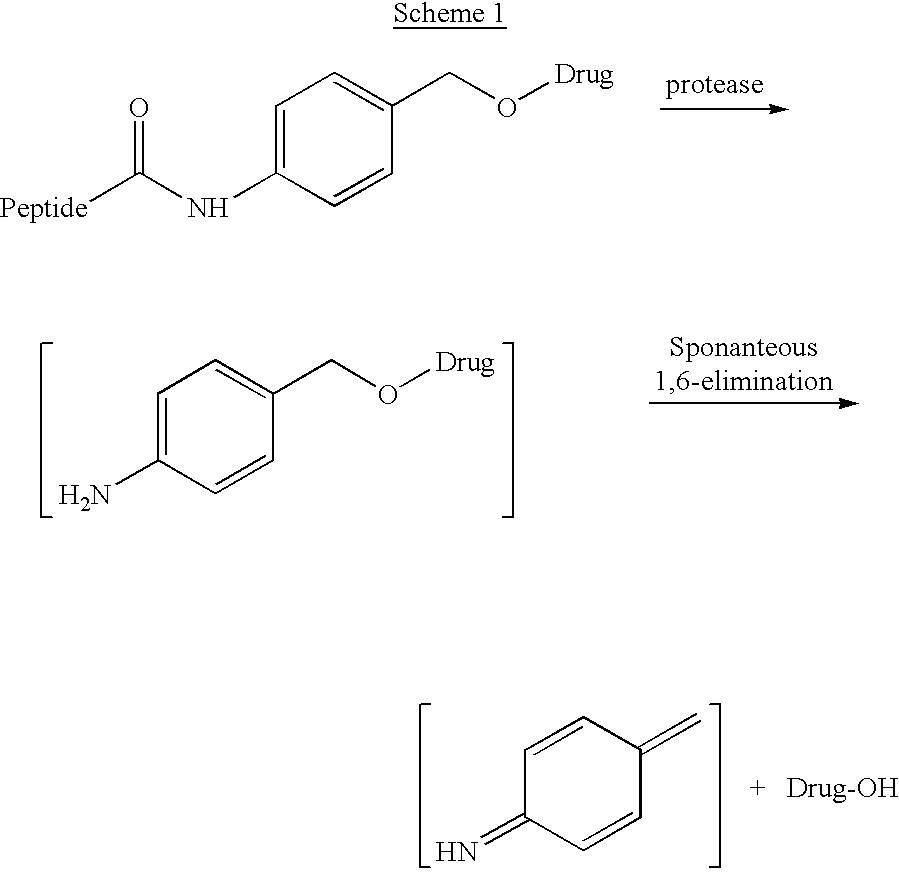
P-amidobenzylethers in drug delivery agents
InactiveUS20030130189A1Decrease in drug activityImprove stabilityTetrapeptide ingredientsTripeptide ingredientsEtherDiluent
Compounds of the formulas LAn-Z-X-WwD and BZ-X-WwD wherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
Pharmaceutical formulation containing opioid agonist,opioid antagonist and gelling agent
InactiveUS20030068371A1Reduce and eliminate effectInhibition effectBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
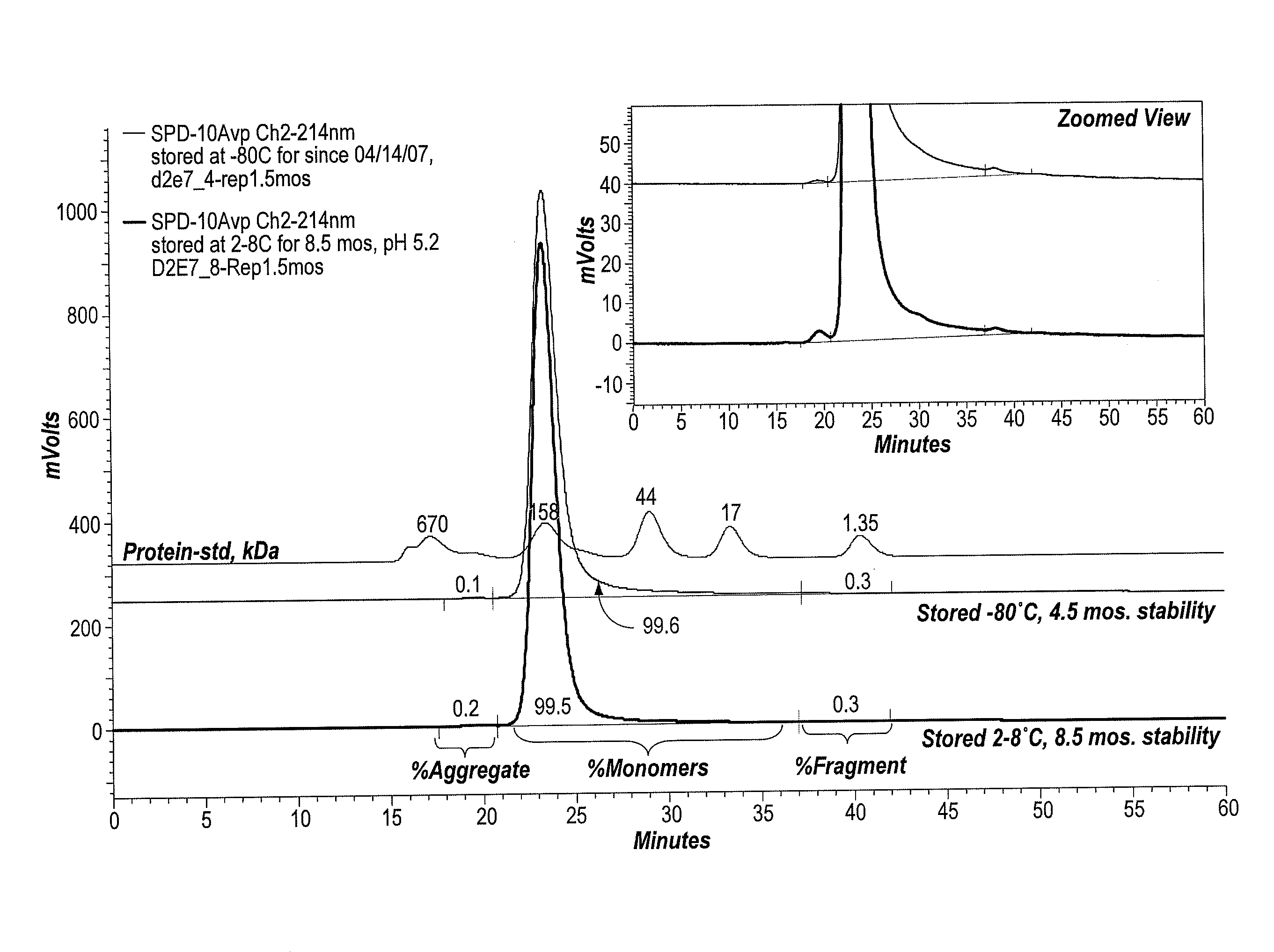
Protein formulations and methods of making same
ActiveUS20090291062A1Improve consistencyAccurate concentrationPeptide/protein ingredientsAntipyreticOsmolar ConcentrationExcipient
The invention provides an aqueous formulation comprising water and a protein, and methods of making the same. The aqueous formulation of the invention may be a high protein formulation and / or may have low levels of conductivity resulting from the low levels of ionic excipients. Also included in the invention are formulations comprising water and proteins having low osmolality.
Owner:ABBVIE BIOTECHNOLOGY LTD
Poly(beta-amino alcohols), their preparation, and uses thereof
ActiveUS20130302401A1Organic active ingredientsPeptide/protein ingredientsChemical structureFibrosis
A new class of poly(beta-amino alcohols) (PBAAs) has been prepared using combinatorial polymerization. The inventive PBAAs may be used in biotechnology and biomedical applications as coatings (such as coatings of films or multilayer films for medical devices or implants), additives, materials, excipients, non-biofouling agents, micropatterning agents, and cellular encapsulation agents. When used as surface coatings, these PBAAs elicited different levels of inflammation, both in vitro and in vivo, depending on their chemical structures. The large chemical diversity of this class of materials allowed us to identify polymer coatings that inhibit macrophage activation in vitro. Furthermore, these coatings reduce the recruitment of inflammatory cells, and reduce fibrosis, following the subcutaneous implantation of carboxylated polystyrene microparticles. These polymers may be used to form polyelectrolyte complex capsules for cell encapsulation. The invention may also have many other biological applications such as antimicrobial coatings, DNA or siRNA delivery, and stem cell tissue engineering.
Owner:MASSACHUSETTS INST OF TECH
Use of matrix metalloproteinase inhibitors in skin care
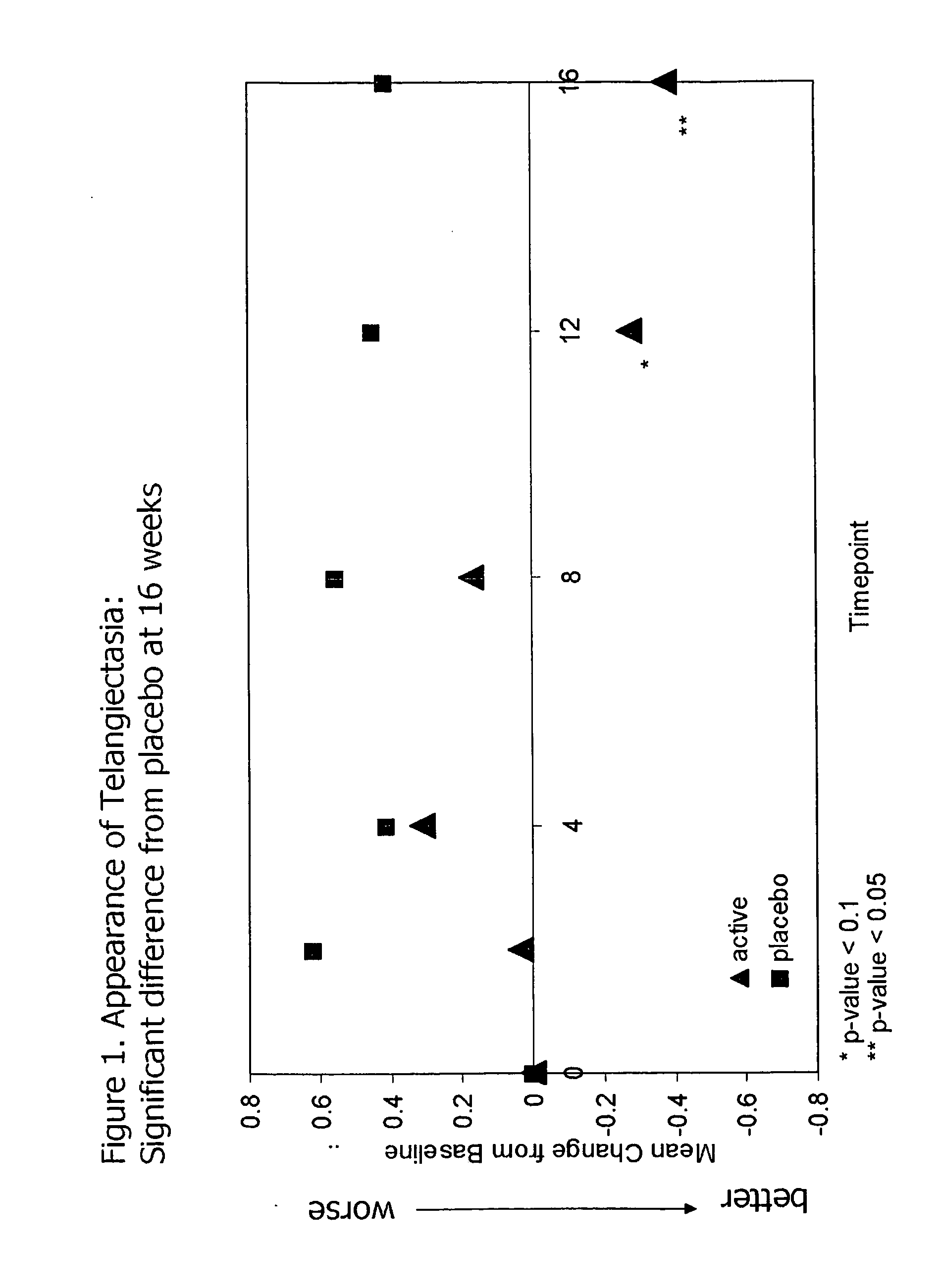
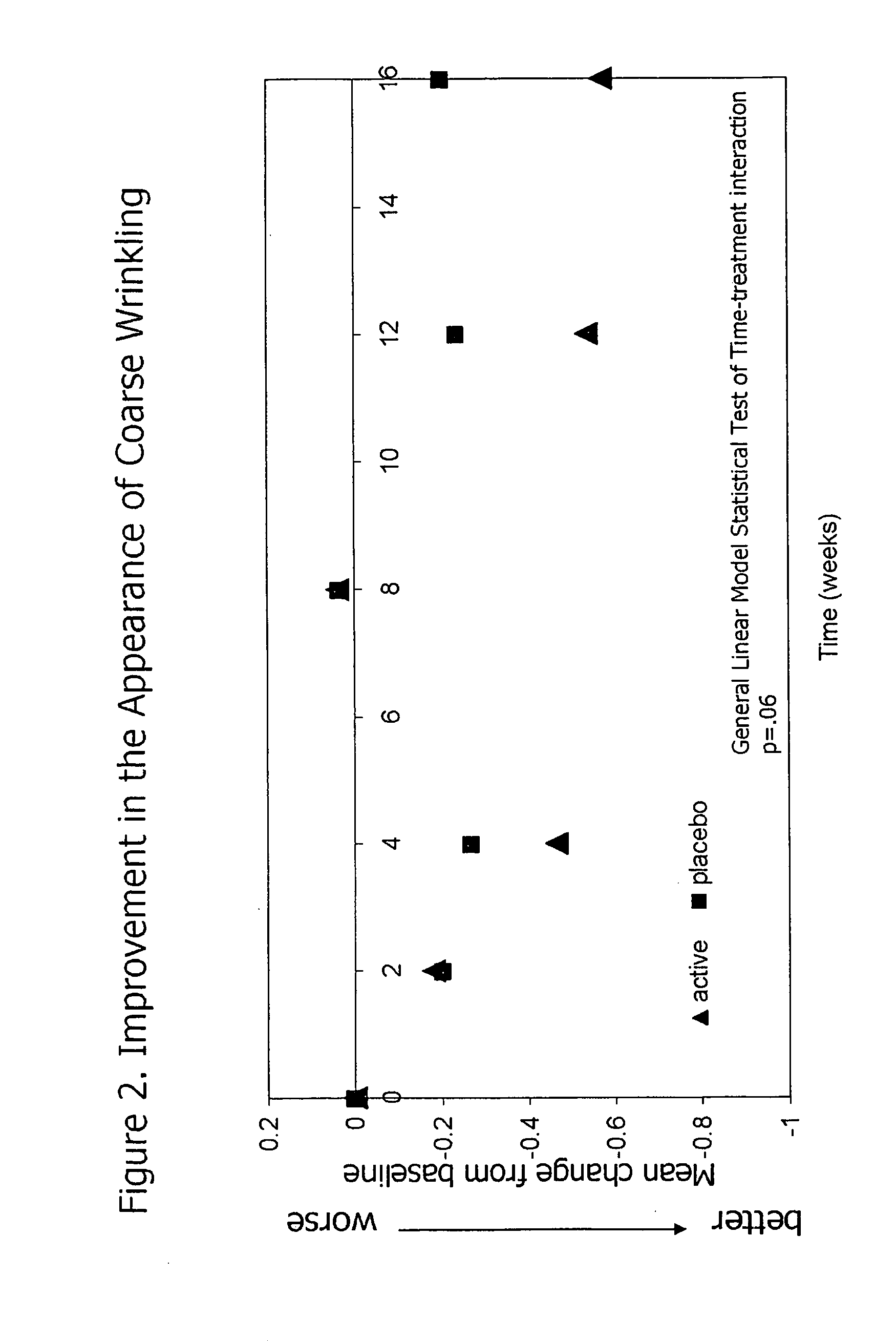
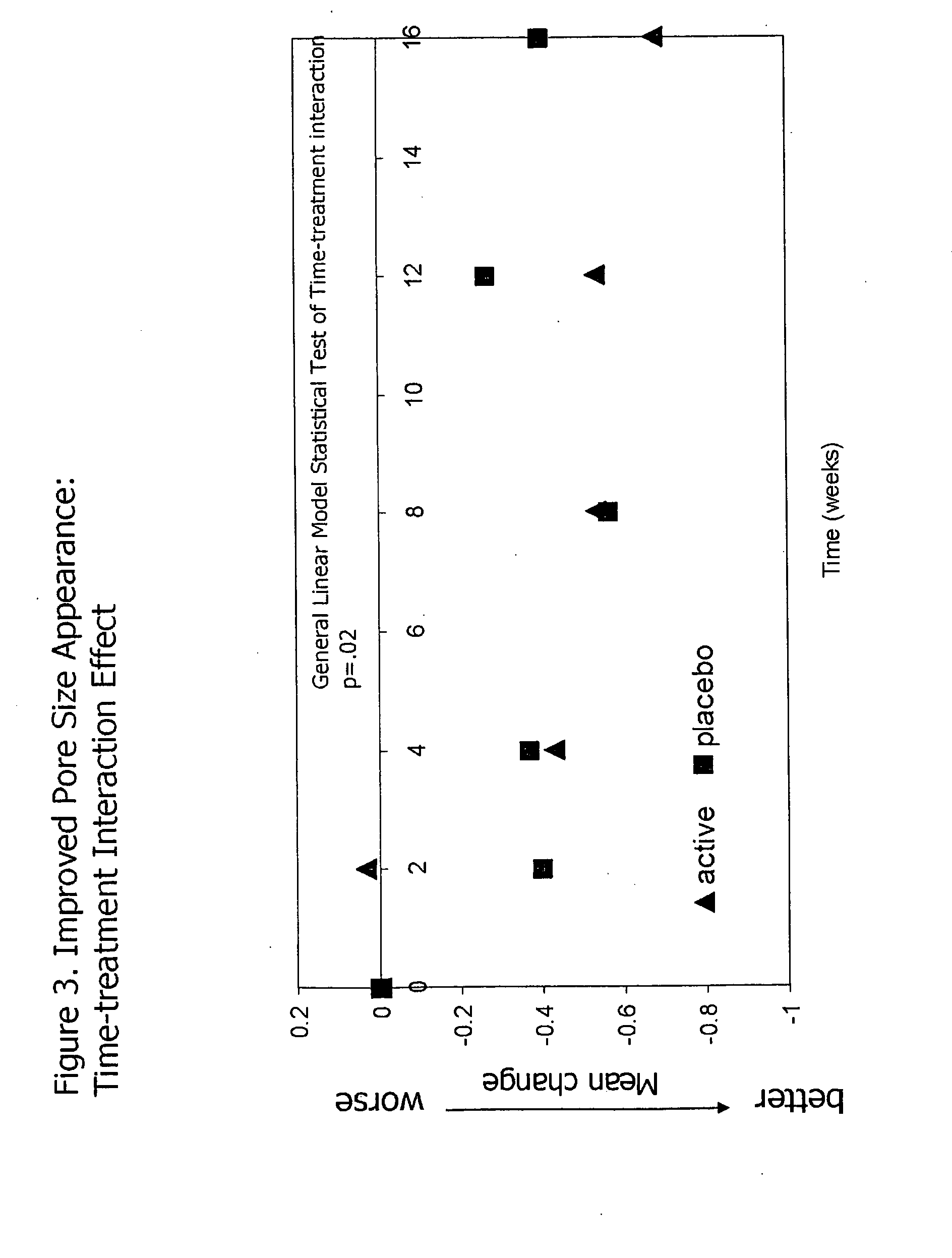
InactiveUS20090068255A1Preventing and reducing of and sun damageImprove skin appearanceBiocideCosmetic preparationsWrinkle skinDisease
The application of matrix metalloproteinase (MMP) inhibitors to the skin inhibits the degradation of proteins found in the skin including collagen, elastin, and other basement membrane and extracellular matrix protein. MMP inhibitors may be used in both cosmetic compositions and pharmaceutical compositions for application to skin. MMP inhibitors are formulated with a cosmetically suitable vehicle or pharmaceutically acceptable excipient for application to the skin as creams, lotions, ointments, solutions, face masks, etc. As cosmetics, the inventive MMP inhibitor compositions are applied to the skin to prevent or reduce the appearance of wrinkles, pigmentation changes, loss of elasticity, or other effects associated with aging or sun damage. As pharmaceuticals, the inventive MMP inhibitor compositions may also be applied to the skin to treat or prevent a skin disease (e.g., proliferative disease, inflammatory disease).
Owner:LIVING PROOF INC
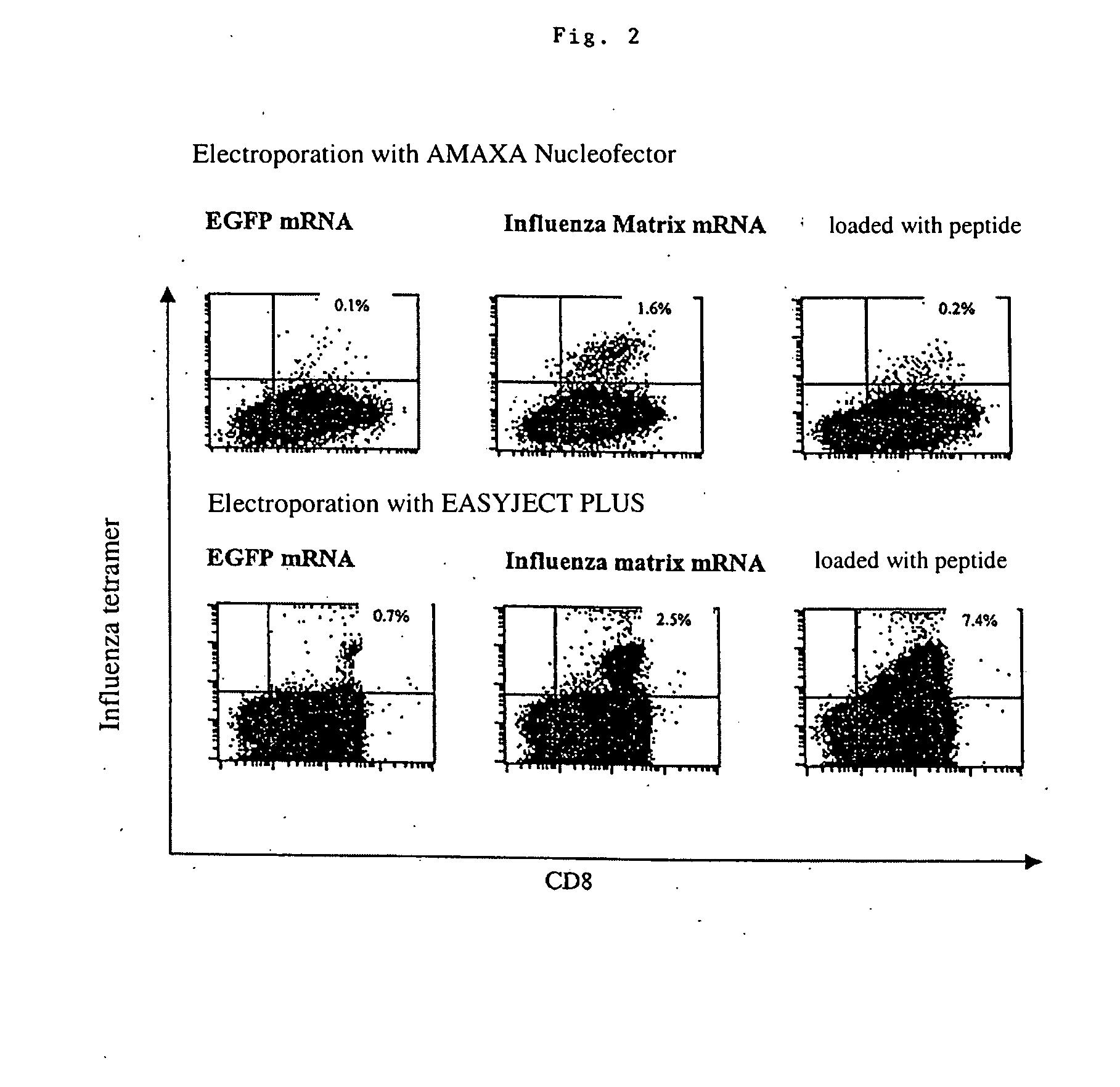
Transfection of blood cells with mRNA for immune stimulation and gene therapy
InactiveUS20060188490A1Improve stabilityIncrease transfectionSsRNA viruses negative-senseBiocideAntigenCancer prevention
The present invention relates to a pharmaceutical composition containing blood cells or haemopoietic cells, e.g. red blood cells (erythrocytes), granulocytes, mononuclear cells (PBMCs) and / or blood platelets, in combination with a pharmaceutically acceptable excipient and / or vehicle, wherein the cells are transfected with at least one mRNA comprising at least one region coding for at least one antigen. The invention further discloses a method of preparing the aforesaid pharmaceutical composition and the use of blood cells transfected in this way for the preparation of drugs or pharmaceutical compositions for immune stimulation against the antigens encoded by the mRNA. The subjects according to the invention are used especially for the therapy and / or prophylaxis of carcinoses or infectious diseases and can also be employed in gene therapy.
Owner:CUREVAC AG
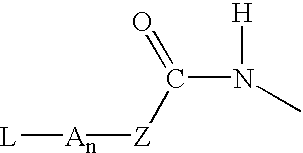
p-Amidobenzylethers in drug delivery agents
Compounds of the formulaeLAn-Z-X—WwD and BZ-X—WwDwherein: D is a drug moiety; L is a ligand; B is a blocking group; A is an optional acyl unit; Z is an amino acid or a peptide residue; X is an aminobenzyl ether self-immolative spacer group; W is an optional second self-immolative group; n is an integer of 0 or 1; and w is an integer of 0 or 1, and compositions of said compounds with pharmaceutically acceptable carrier, diluent and / or excipient, and methods of delivery the drug D via the compounds.
Owner:SEAGEN INC
Powdered protein compositions and methods of making same
InactiveUS20090226530A1Easy to moveWide concentration rangeAntibacterial agentsPowder deliveryBiotechnologyProtein composition
Owner:ABBVIE DEUTSHLAND GMBH & CO KG
Pharmaceutical formulation containing opioid agonist, opioid antagonist and gelling agent
InactiveUS7842307B2Reducing abuse potential of dosage formLower potentialBiocideNervous disorderOpioid antagonistOpioid Agonist
Disclosed in certain embodiments is an oral dosage form comprising a therapeutically effective amount of an opioid analgesic, an opioid antagonist and one or more pharmaceutically acceptable excipients; the dosage form further including a gelling agent in an effective amount to impart a viscosity unsuitable for administration selected from the group consisting of parenteral and nasal administration to a solubilized mixture formed when the dosage form is crushed and mixed with from about 0.5 to about 10 ml of an aqueous liquid.
Owner:PURDUE PHARMA LP
Coated tablet formulation and method
ActiveUS20050266080A1Good chemical stabilityEasy to prepareBiocideMetabolism disorderCoated tabletsExcipient
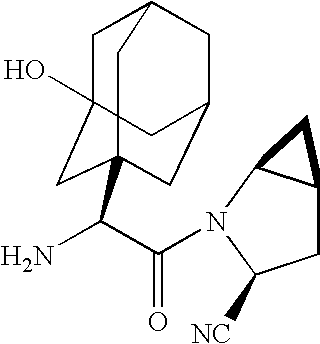
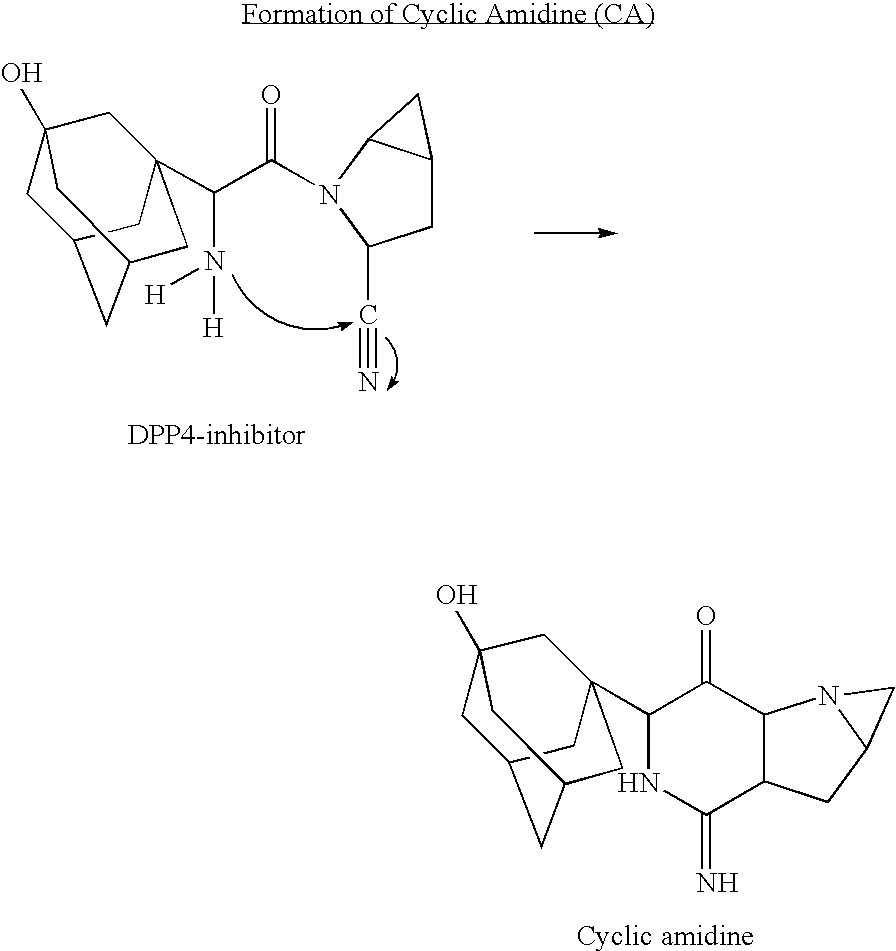
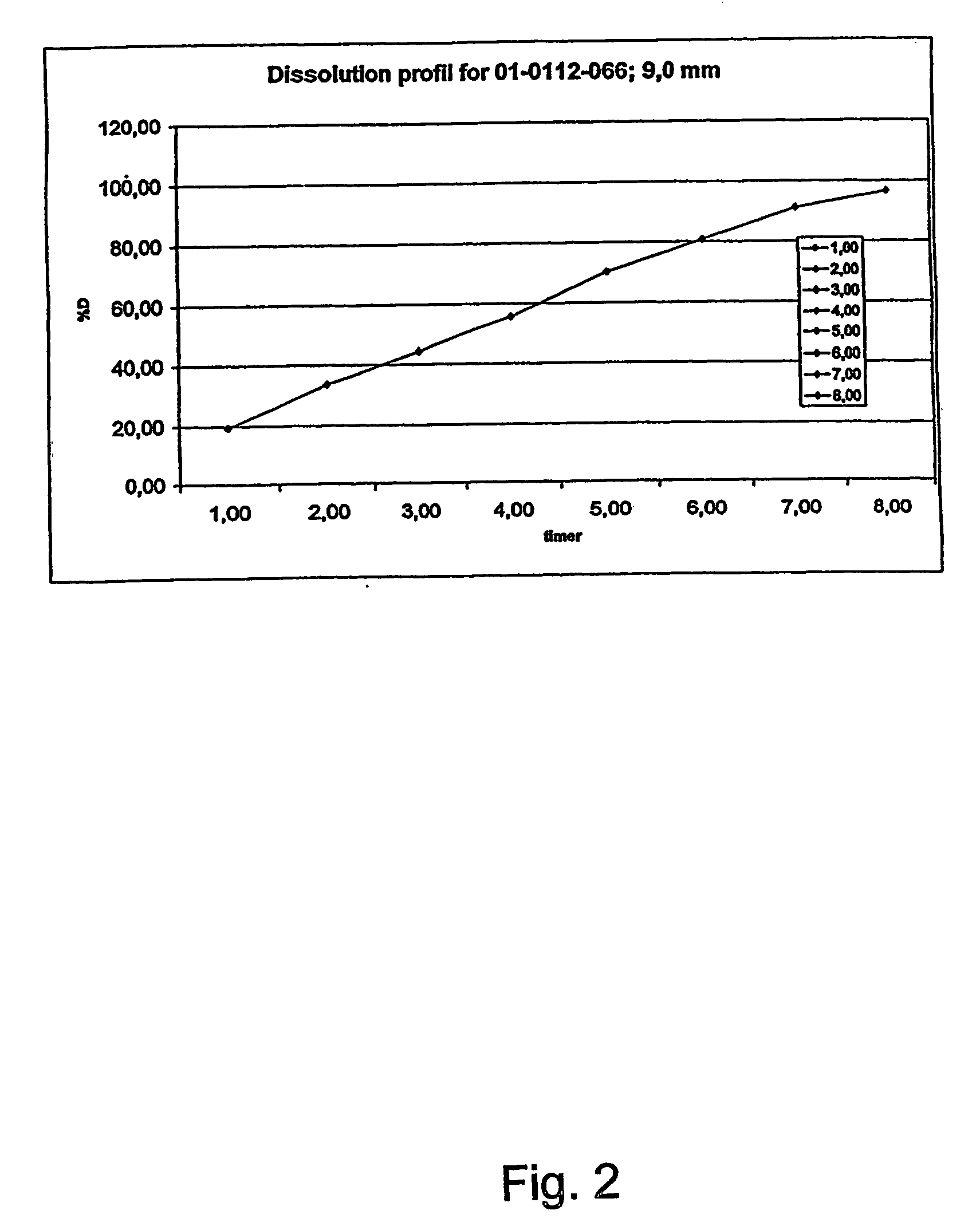
A coated tablet formulation is provided which includes a medicament such as the DPP4-inhibitor, saxaglipitin or its HCl salt, which is subject to intra-molecular cyclization, which formulation includes a tablet core containing one or more fillers, and other conventional excipients, which tablet core includes a coating thereon which may include two or more layers, at least one layer of which is an inner seal coat layer which is formed of one or more coating polymers, a second layer of which is formed of medicament which is the DPP4-inhibitor and one or more coating polymers, and an optional, but preferable third outer protective layer which is formed of one or more coating polymers. A method for forming the coated tablet is also provided.
Owner:ASTRAZENECA AB
Morphine controlled release system
InactiveUS20070003617A1Low administration frequencyAffecting extent of drug bioavailabilityBiocideNervous disorderMorphineDissolution
A composition for controlled release of an opioid from a pharmaceutical composition, the method comprises controlling the release of at least one opioid into an aqueous medium by erosion of at least one surface of a pharmaceutical composition comprising I) a matrix composition comprising a) polymer or a mixture of polymers, b) an opioid and, optionally, c) one or more pharmaceutically acceptable excipients, and (i) a coating. The matrix composition has a conus-like shape so the surface area exposed to the aqueous medium increases at least during initial erosion of the matrix composition, and the dissolution of the opioid-when tested in a Dissolution Test as described herein with or without application of sinkers-results in a zero order release of at least 80% of the opioid contained in the composition. Such compositions are especially suitable for controlled release of an opioid to obtain a delayed pead concentration and a prolonged therapeutically effective plasma concentration upon oral administration. Once or twice daily administration is possible. The matrix typically comprises PEO and the active substance is typically an opioid such as morphine or a glucuronide thereof.
Owner:EGALET LTD
Antibody formulations and methods of making same
ActiveUS8420081B2Minimal aggregationLow levelPeptide/protein ingredientsAntipyreticExcipientNuclear chemistry
Owner:ABBVIE BIOTECHNOLOGY LTD
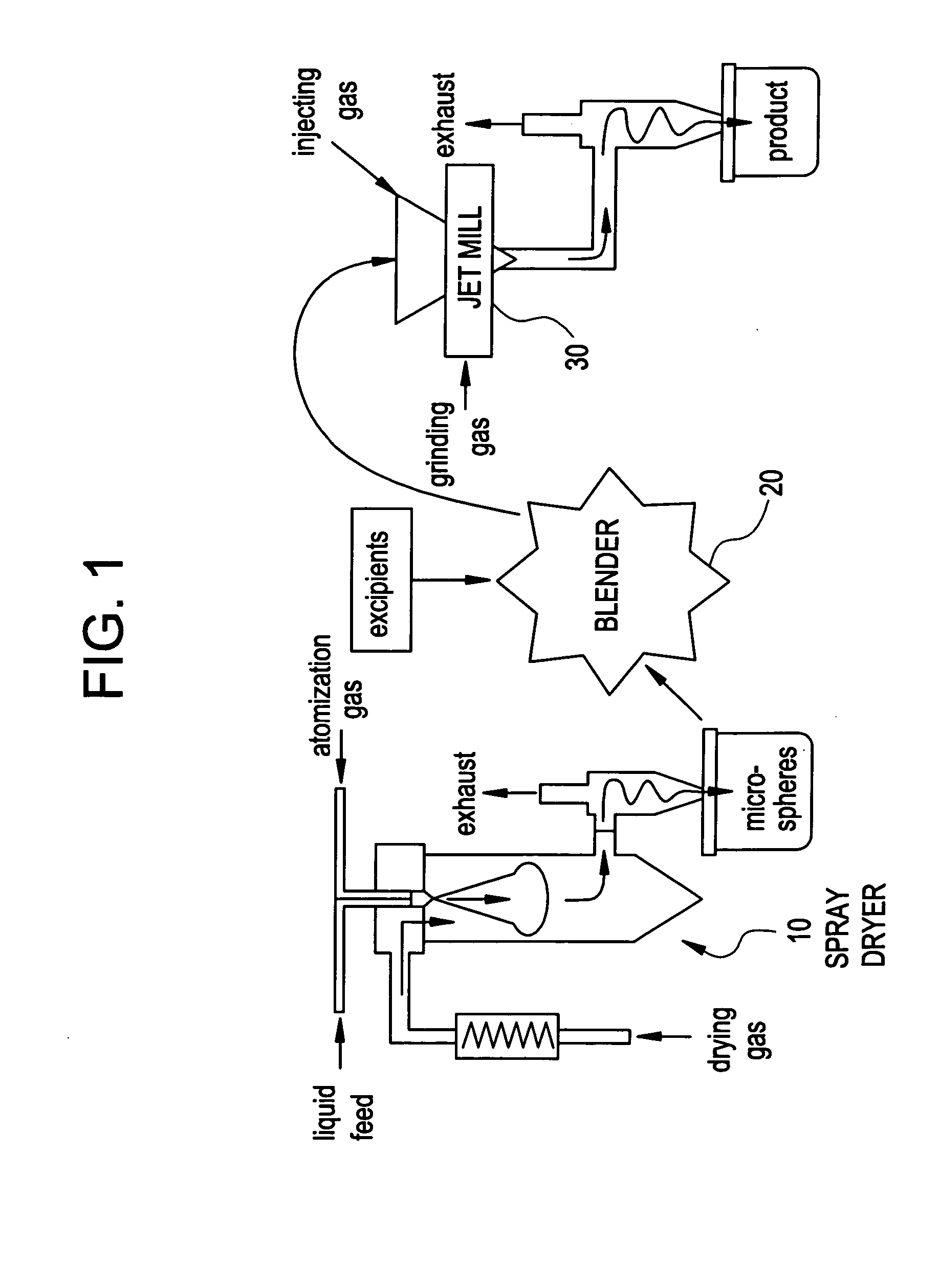
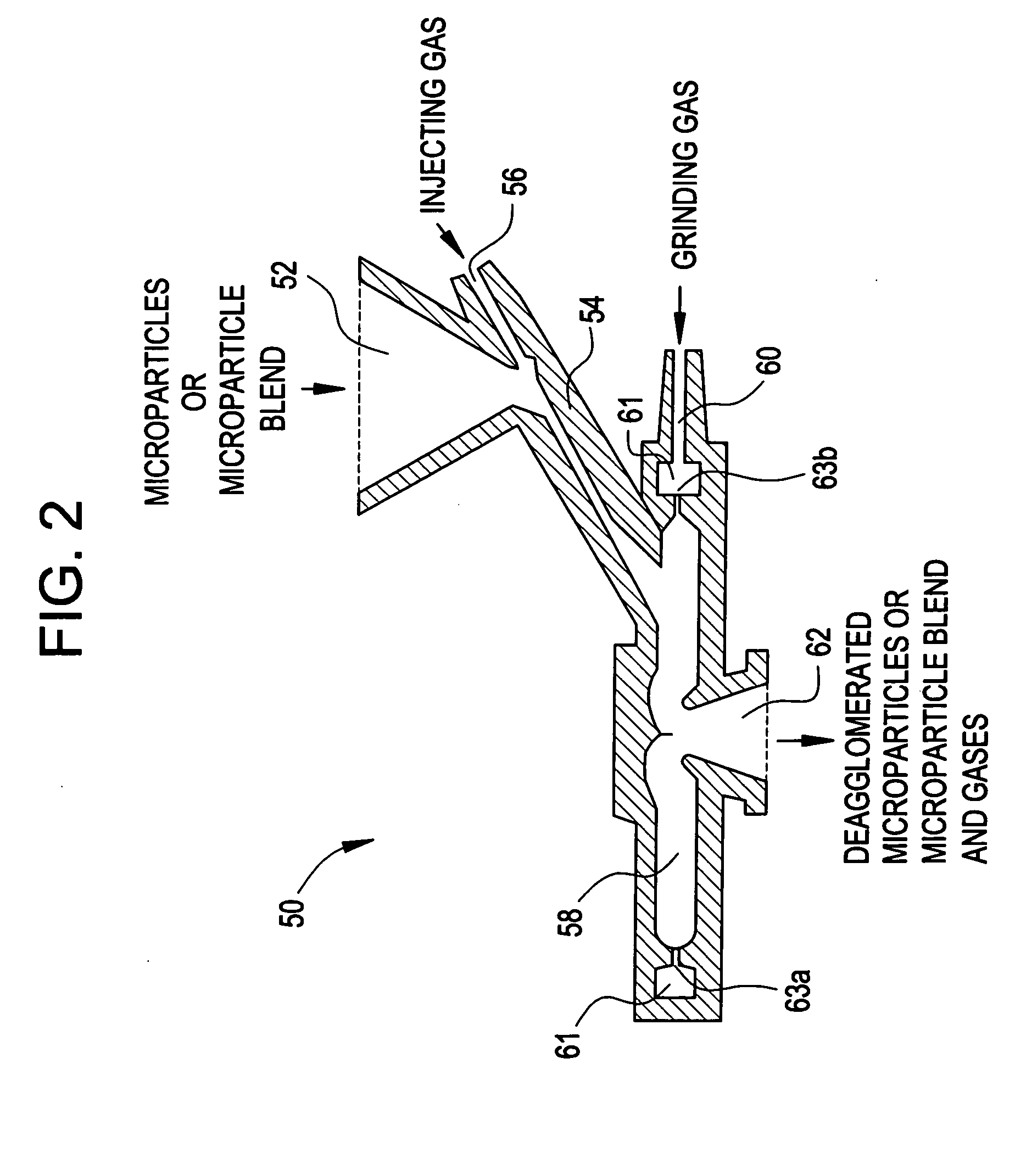
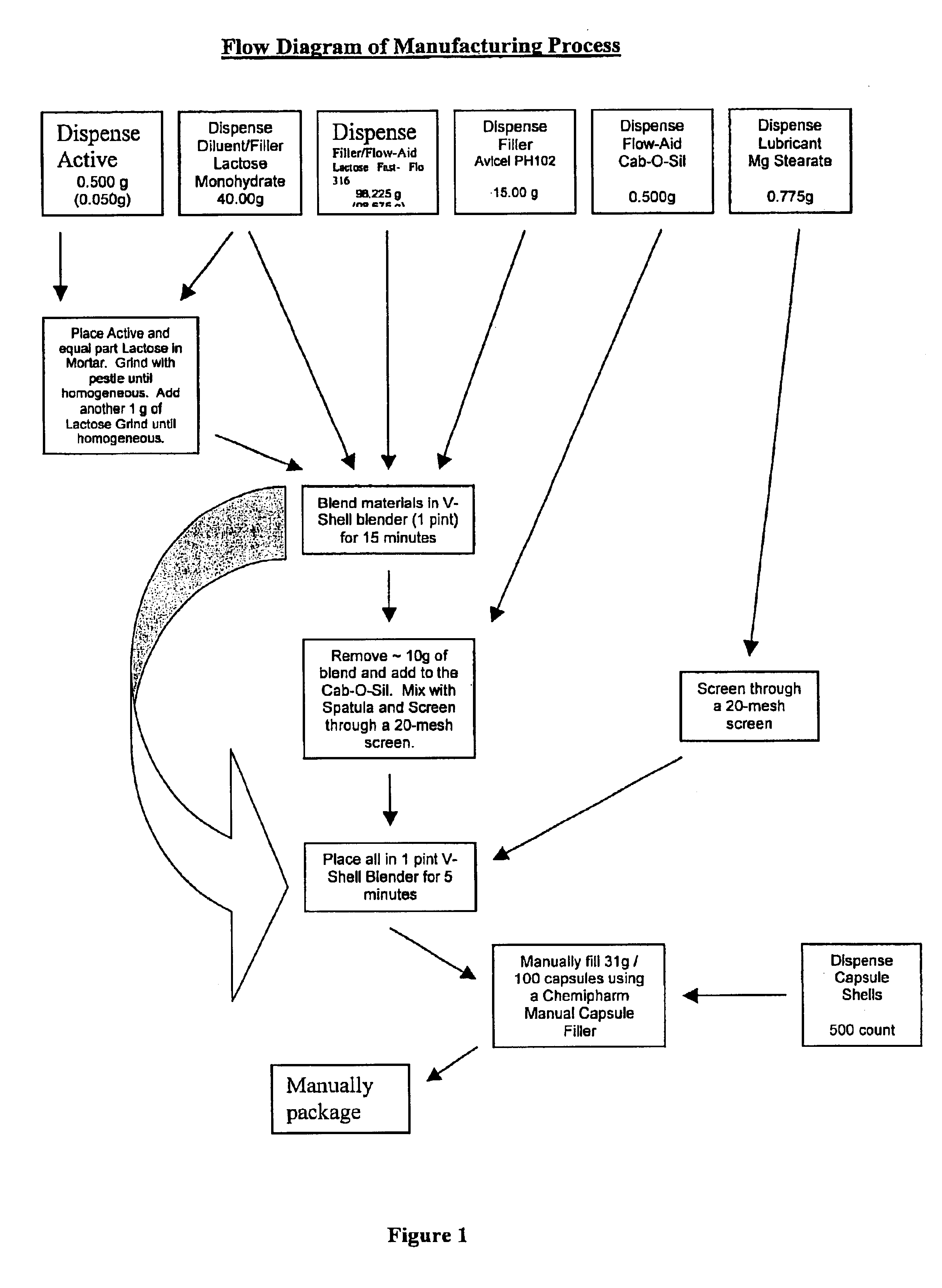
Methods for making pharmaceutical formulations comprising microparticles with improved dispersibility, suspendability or wettability
InactiveUS20050079138A1Good dispersibilityImproved suspendabilityPowder deliveryGranulation by liquid drop formationPowder mixtureMicroparticle
Methods are provided for making a dry powder blend pharmaceutical formulation, comprising the steps of: (a) providing microparticles which comprise a pharmaceutical agent; (b) blending the microparticles with at least one excipient in the form of particles to form a powder blend; and (c) jet milling the powder blend to form a dry powder blend pharmaceutical formulation having improved dispersibility, suspendability, or wettability as compared to the microparticles of step (a) or the powder blend of step (b). The method can further include dispersing the dry powder blend pharmaceutical formulation in a liquid pharmaceutically acceptable vehicle to make an formulation suitable for injection. Alternatively, the method can further include processing the dry powder blend pharmaceutical formulation into a solid oral dosage form. In one embodiment, the microparticles of step (a) are formed by a solvent precipitation or crystallization process.
Owner:ACUSPHERE INC
Multi-particulate, modified-release composition
A multi-particulate, modified-release pharmaceutical composition for the oral administration of an active ingredient to the colon, wherein said particles comprise: (a) a core comprising an active ingredient or a pharmaceutically acceptable salt or ester thereof, and optionally one or more excipients; (b) a first coating applied to the surface of the core, wherein said first coating is insoluble in gastric juice and in intestinal juice below pH 7, but soluble in colonic intestinal juice; and (c) a second coating applied to the surface of the first coating.
Owner:SANDOZ AG
Topical Pharmaceutical Foam Composition
InactiveUS20070154402A1Reduced intensity of colorReduce odor intensityAntibacterial agentsBiocideAlcohol freeActive agent
A stable topical alcohol-free aerosol foam containing one or more keratolytic agents is provided. The foam-forming formulation is an emulsion which contains an HFA propellant and one or more keratolytic agents. The emulsion has an oil phase and an aqueous, i.e. water-containing, phase. The active agent(s) may be present in either phase of the emulsion or dispersed in the emulsion. The oil phase may consist at least in part of the HFA propellant. Either or both of the oil phase and the aqueous phase may contain one or more surfactants, emulsifiers, emulsion stabilizers, buffers, and / or other excipients. The foam is stable on the skin, for example, for at least 5 minutes at body temperature, preferably at least 20 minutes at body temperature, and disappears into the skin upon rubbing or after prolonged standing. In one embodiment, the formulation contains an HFA propellant which does not contain additional co-solvents or co-propellants. The formulations demonstrate reduced intensity of the odor and / or color associated with the keratolytic agent(s) as compared to conventional formulations containing keratolytic agents.
Owner:PRECISION DERMATOLOGY
Porous drug matrices and methods of manufacture thereof
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Polyclonal antibody composition for treating allergy
InactiveUS6849259B2Efficient removalPotential clinical advantageImmunoglobulins against animals/humansImmunoglobulins against plantsMicrosphereBULK ACTIVE INGREDIENT
A pharmaceutical composition for treating allergy is described. The composition comprises as an active ingredient a recombinant polyclonal antibody or a mixture of different monoclonal antibodies capable of reacting with or binding to an allergen together with one or more pharmaceutically acceptable excipients. The composition may be used topically as a solution, dispersion, powder, or in the form of microspheres. The polyclonal antibody is preferably a recombinant polyclonal antibody produced by phage display technology. The pairing of specific immunoglobulin variable region light chain and heavy chain maintained from the original polyclonal immune response or selected by panning using the allergen in question is preferably maintained by bulk transfer of the pairs into an expression vector.
Owner:SYMPHOGEN AS
Sprayable formulations for the treatment of acute inflammatory skin conditions
A topical spray or foam, methods of making the formulation, and methods of use thereof, has been developed. In one preferred embodiment, the composition includes one or more active agents and exhibits both antibacterial activity and antifungal activity. Excipients such as chemical disinfectants, anti-pruritic agents to minimize itching, and skin protective compounds may be added. The composition may be formulated to be dispensed as a spray or foam and the spray or foam may be administered either by a hand pump or by an aerosolizing propellant. A second single phase formulation has also been developed. The formulation comprises a first drug which is water soluble or hydrophilic and a second drug which is lipid soluble or hydrophobic, wherein at least one of the drugs is bound to an ion-exchange resin. The use of binding resins, such as ion-exchange resins, allows drugs with incompatible solvent requirements to be prepared in a single-phase formulation.
Owner:COLLEGIUM PHARMA INC
Foam incorporating eutetic mixture
InactiveUS20050075407A1Broaden applicationEvenly distributedCosmetic preparationsBiocideAlcohol freeMedicine
The invention relates to an alcohol-free cosmetic or pharmaceutical foam carrier comprising water, a surface-active agent a gelling agent and a combination of active agents, which creates, upon admixing, a eutectic mixture. The foam carrier further comprises active agents and excipients with therapeutic properties.
Owner:FOAMIX PHARMACEUTICALS LIMITED
Methods and related compositions for reduction of fat and skin tightening
InactiveUS20060127468A1Efficient tighteningTighten regionBiocideCosmetic preparationsCelluliteExcipient
Compositions and methods useful in the reduction of localized fat deposits and tightening of loose skin in subjects in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion or anti-dispersion agents and pharmaceutically acceptable excipients. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including, for example, lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:RGT UNIV OF CALIFORNIA +1
Imiquimod cream formulation
InactiveUS20070264317A1Composition is stablePowder deliveryBiocideExcipientPharmaceutical preservatives
Owner:AGIS INDUSTRIES (1983) LTD
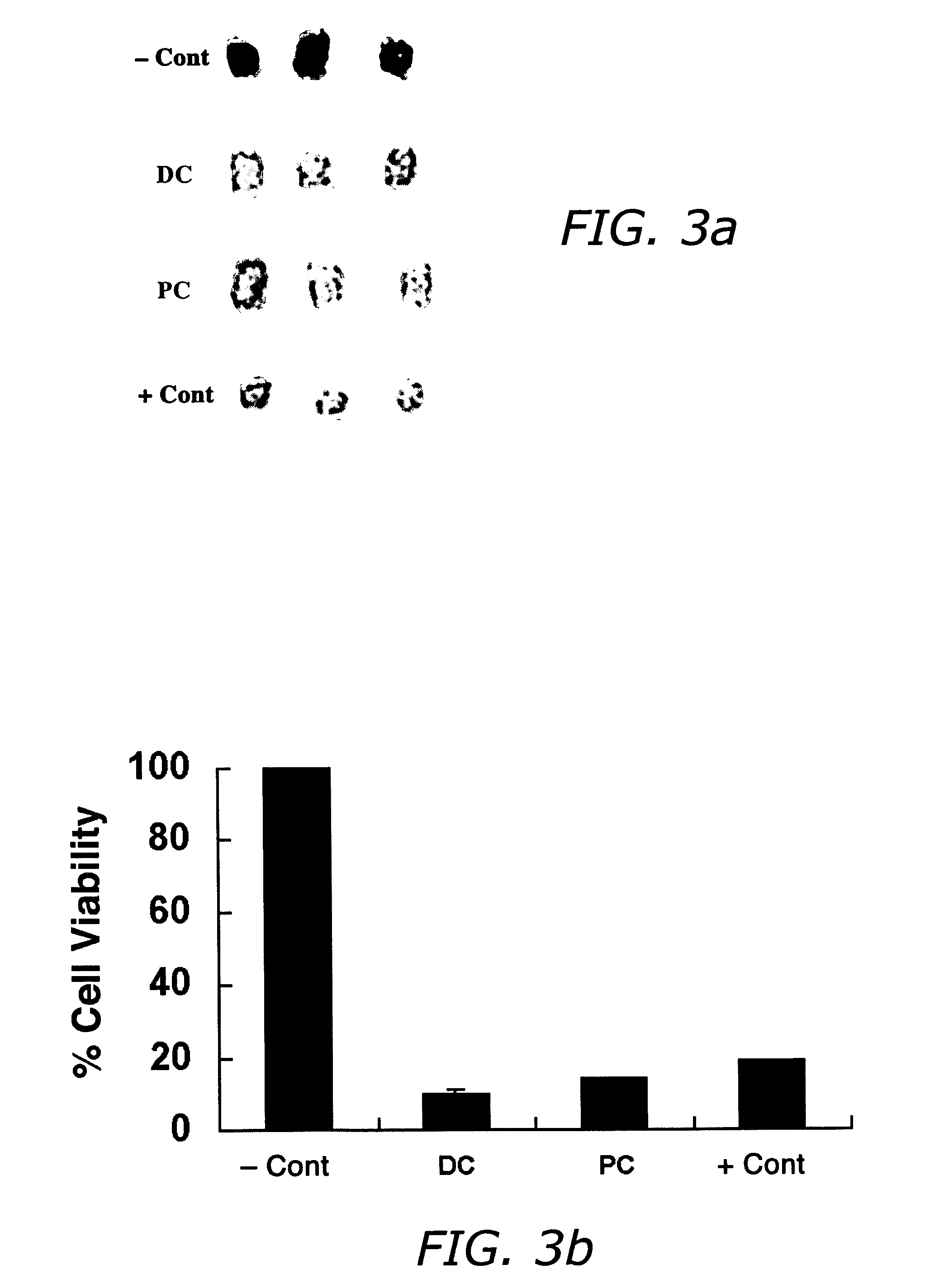
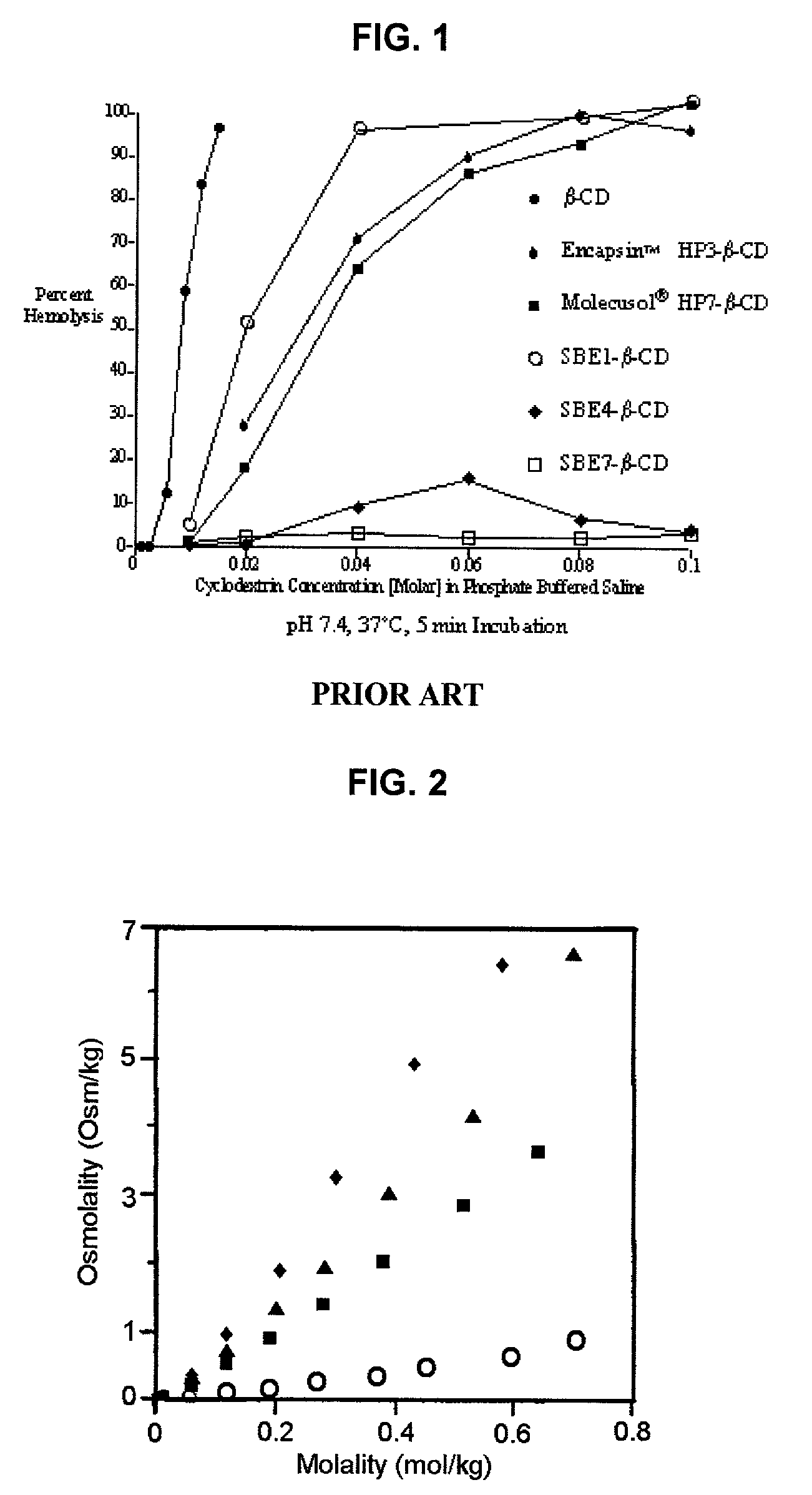
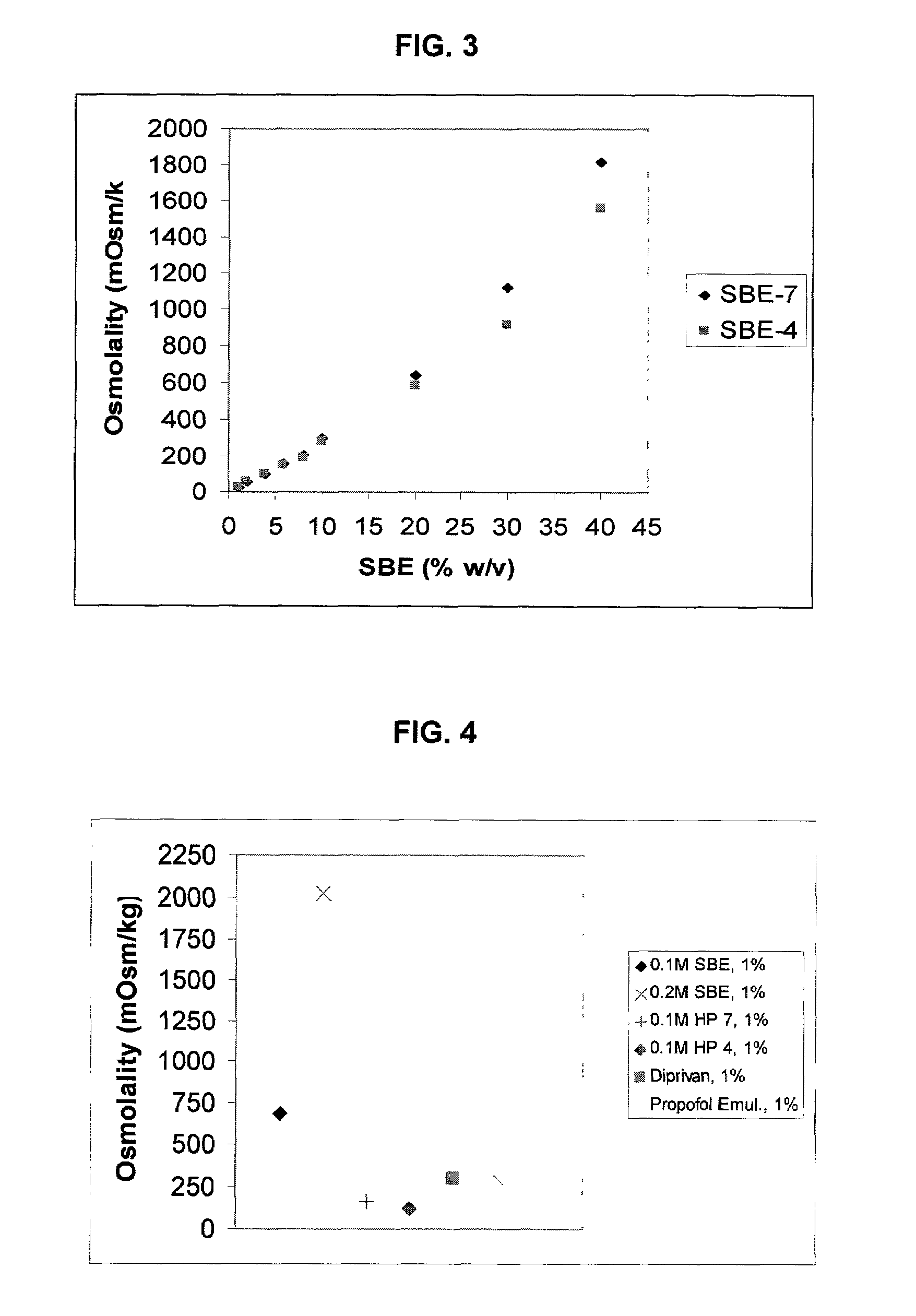
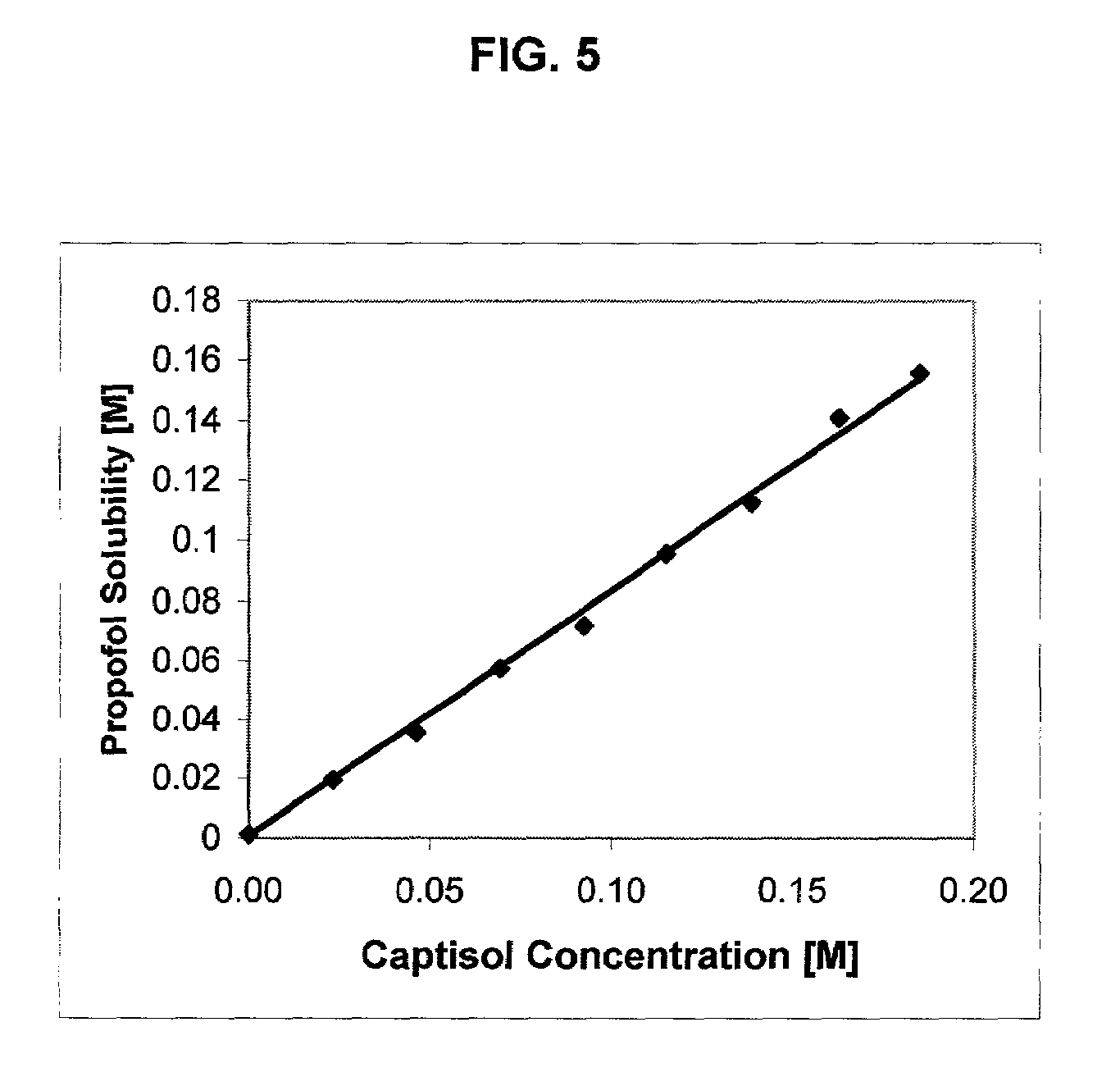
Formulations containing propofol and a sulfoalkyl ether cyclodextrin
InactiveUS7034013B2Reduce bacterial contaminationHigh photochemical stabilityBiocideHydroxy compound active ingredientsEmulsionAllergic response
An injectable formulation of a sedative hypnotic drug, such as the anesthetic drug propofol, that is pharmaceutically stable and demonstrates a reduced incidence of pain upon injection. The formulation of the present invention employs a sulfoalkyl ether cyclodextrin solubilizing and complexing excipient, such as CAPTISOL® cyclodextrin (sulfobutyl ether β-cyclodextrin) to form a true aqueous solution and not a suspension. This formulation minimizes the allergic response and microbial contamination issues typically associated with propofol parenteral formulations. The present formulation may also reduce pain on injection as compared to the known emulsion type propofol formulations. The liquid formulation can be sterile filtered unlike emulsion-type formulations of sedative hypnotics. The liquid formulation can be lyophilized or otherwise dried to yield a solid formulation.
Owner:CUDA PHARMA LLC
Pharmaceutical tablet suitable to deliver the active substance in subsequent and predeterminable times
Pharmaceutical tablet, capable of delivering the active substance or the active substances, according to a predeterminable release profile, comprising a core with an external partial coating in which said core consists of 3 layers, wherein the upper layer contains an amount of the active substance with suitable excipients, the intermediate layer consists of polymeric material with retarding barrier function and the lower layer contains the remaining amount of the active substance with suitable excipients and said external coating consists of controlled permeability polymeric materials, applied by compression to the lower surface and to the lateral surface of the core.
Owner:JAGOTEC AG
Penetrating pharmaceutical foam
ActiveUS20050074414A1Broaden applicationEvenly distributedAntibacterial agentsBiocideAlcohol freeActive agent
The invention relates to an alcohol-free cosmetic or pharmaceutical foam composition comprising water, a hydrophobic solvent, a surface-active agent, a gelling agent, an active component selected from the group of urea, hydroxy acid and a therapeutic enhancer and a propellant. The foam further comprises active agents and excipients with therapeutic properties having enhanced skin penetration.
Owner:VYNE THERAPEUTICS INC
Methods and related compositions for reduction of fat
ActiveUS20050267080A1Reduce fat depositionReduce decreaseAntibacterial agentsBiocideCelluliteExcipient
Compositions and methods useful in the reduction of localized fat deposits in patients in need thereof using pharmacologically active detergents are disclosed. The pharmacologically active detergent compositions can additionally include anti-inflammatory agents, analgesics, dispersion or anti-dispersion agents and pharmaceutically acceptable excipients. The pharmacologically active detergent compositions are useful for treating localized accumulations of fat including, for example, lower eyelid fat herniation, lipodystrophy and fat deposits associated with cellulite and do not require surgical procedures such as liposuction.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT +1
Taste-masked pharmaceutical compositions prepared by coacervation
InactiveUS20060105038A1Effective taste-maskingSmooth tasteOrganic active ingredientsPill deliveryAdditive ingredientWater insoluble
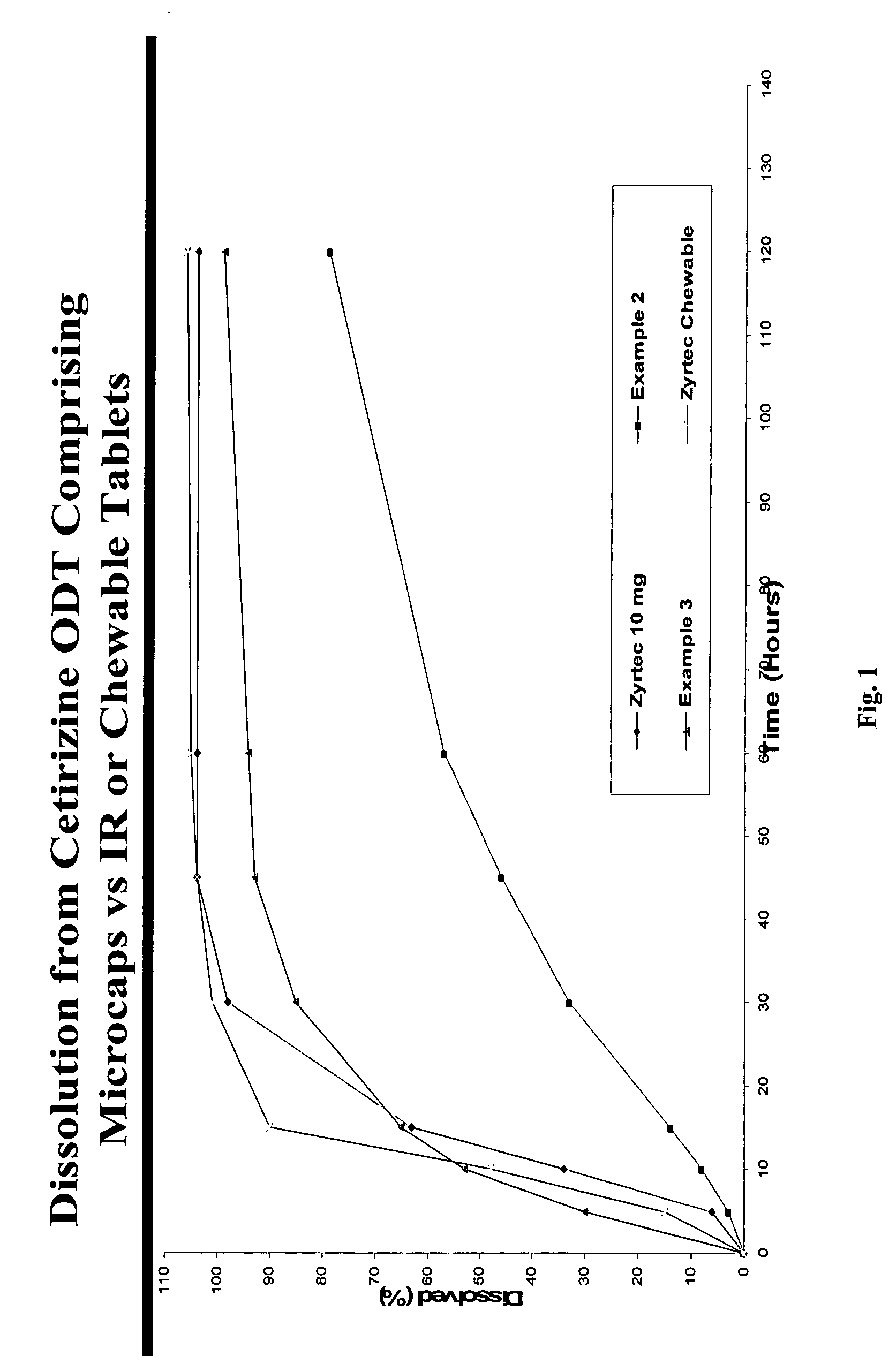
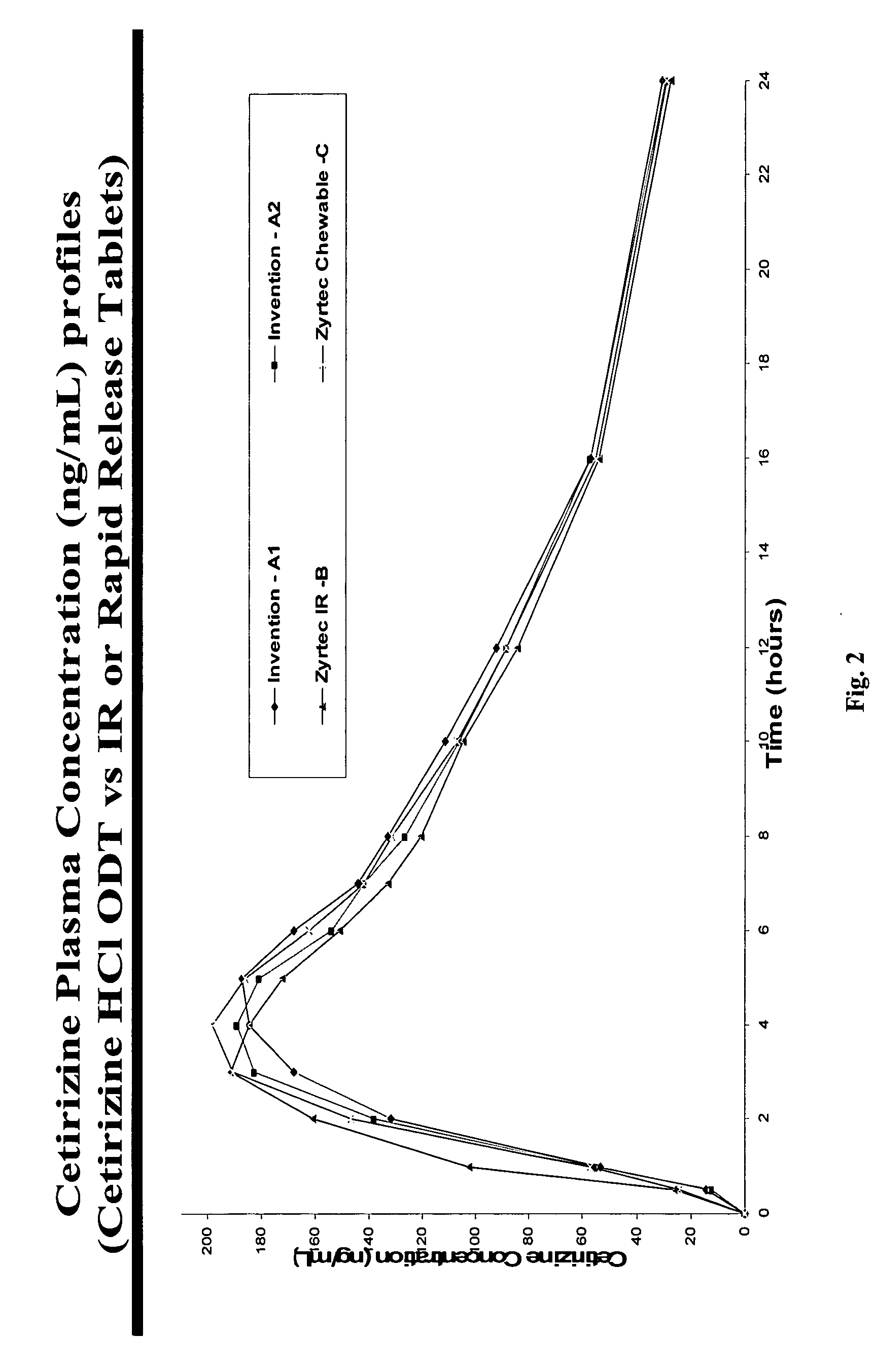
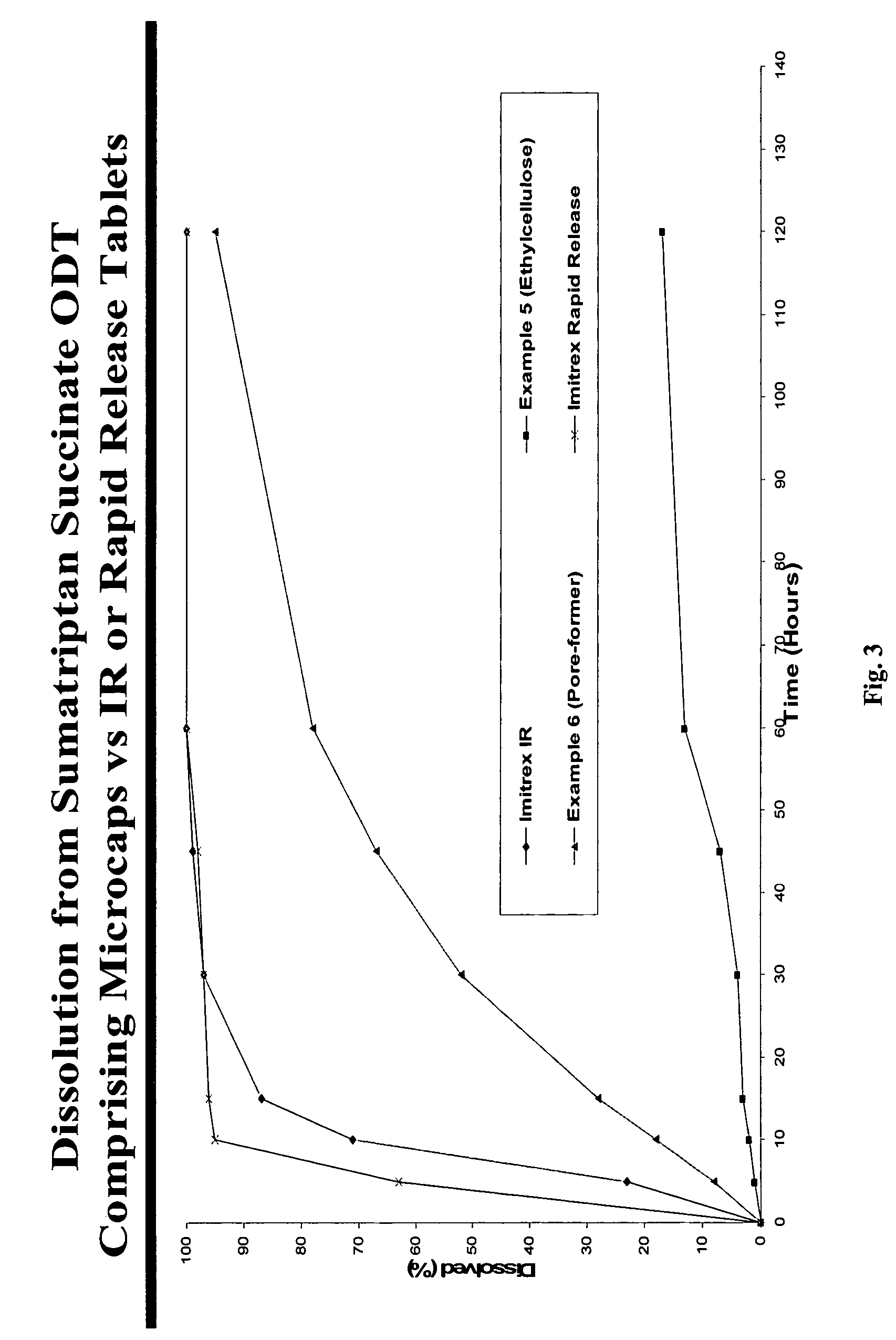
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredients, rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates rapidly with saliva in the buccal cavity forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing one or more actives) with a taste-masking membrane applied by a modified solvent coacervation process comprising a water-insoluble polymer and at least one gastrosoluble inorganic or organic pore-former, exhibit a pleasant taste when placed in the oral cavity and provide rapid, substantially-complete release of the dose on entry into the stomach.
Owner:EURAND PHAMACEUTICALS LTD
Formulations comprising selective androgen receptor modulators
InactiveUS6838484B2Decreased libidoAlteration in mood and cognitionBiocideOrganic chemistryDiseaseAging male
The present invention relates to pharmaceutical compositions and formulations comprising a novel class of androgen receptor targeting agents (ARTA) which demonstrate androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. The agents define a new subclass of compounds which are selective androgen receptor modulators (SARM) which are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia,osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of chronic muscular wasting; and / or e) decreasing the incidence of, halting or causing a regression of prostate cancer. The present invention provides pharmaceutical compositions comprising the selective androgen receptor modulator compounds, together with pharmaceutically acceptable excipients.
Owner:UNIV OF TENNESSEE RES FOUND
Taste-masked pharmaceutical compositions
ActiveUS20060078614A1Effective taste-maskingRapid/complete releasePill deliveryAdditive ingredientOrally disintegrating tablet
There is provided a method for preparing an orally disintegrating tablet (ODT) composition comprising microparticles of one or more taste-masked active pharmaceutical ingredient(s), rapidly-dispersing microgranules, and other optional, pharmaceutically acceptable excipients wherein the ODT disintegrates on contact with saliva in the buccal cavity in about 60 seconds forming a smooth, easy-to-swallow suspension. Furthermore, the microparticles (crystals, granules, beads or pellets containing the active) applied with a taste-masking membrane comprising a combination of water-insoluble and gastrosoluble polymers release not less than about 60% of the dose is in the stomach in about 30 minutes, thus maximizing the probability of achieving bioequivalence to the reference IR product having rapid onset of action (short Tmax). A process for preparing such compositions for oral administration using conventional fluid-bed equipment and rotary tablet press is also disclosed.
Owner:ADARE PHARM INC
Dosage Form with Impeded Abuse
InactiveUS20090004267A1Prevent crushingPrevent the subsequent abuseOrganic active ingredientsPowder deliveryWaxBreaking strength
A multiparticulate dosage form formulated to make misuse more difficult containing least one active substance with potential for misuse (A), at least one synthetic or natural polymer (C), optionally at least one natural, semi-synthetic or synthetic wax (D), at least one disintegrant (E) and optionally one or more additional physiologically compatible excipients (B), wherein the individual particles of the dosage form display a breaking strength of at least 500 N and a release of active substance of at least 75% after 45 minutes measured according to Ph.Eur. in the paddle mixer with sinker in 600 ml of aqueous buffer solution with a pH value of 1.2 at 37° C. and 75 rpm.
Owner:GRUNENTHAL GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com