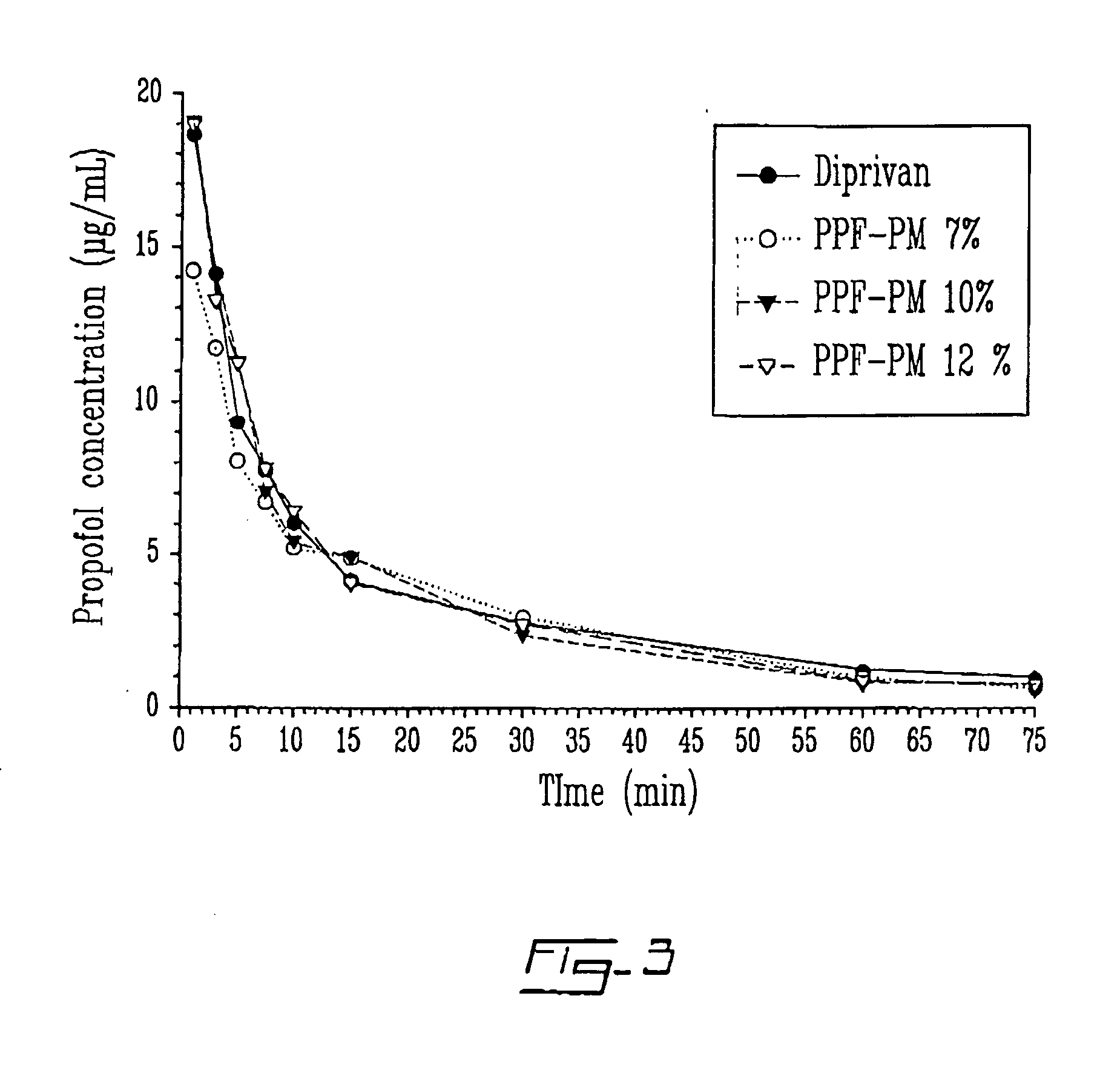
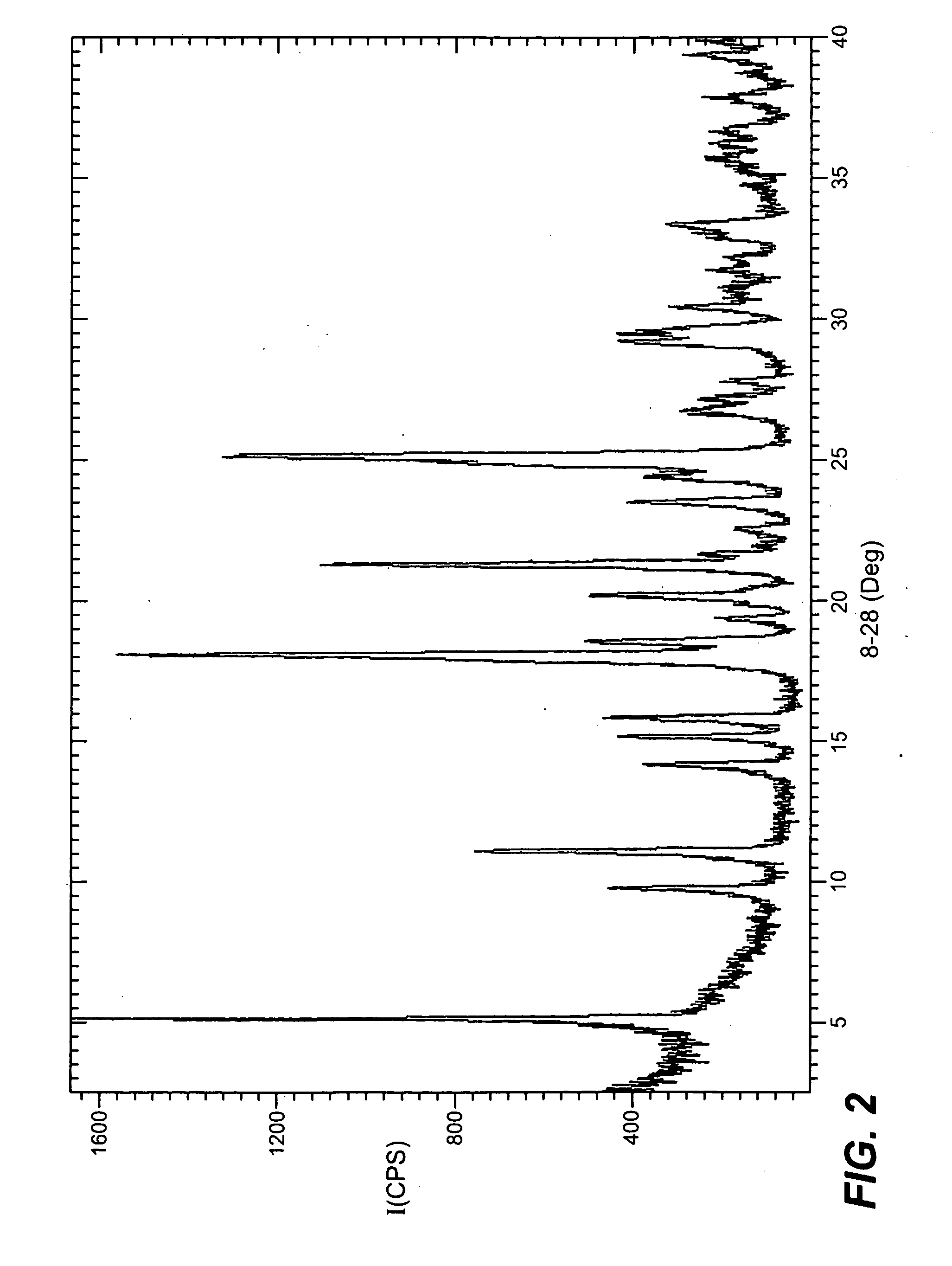
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
315 results about "Propofol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
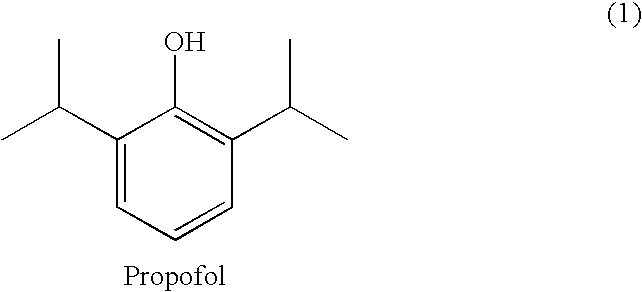
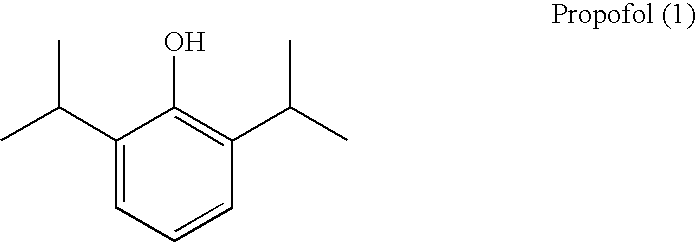
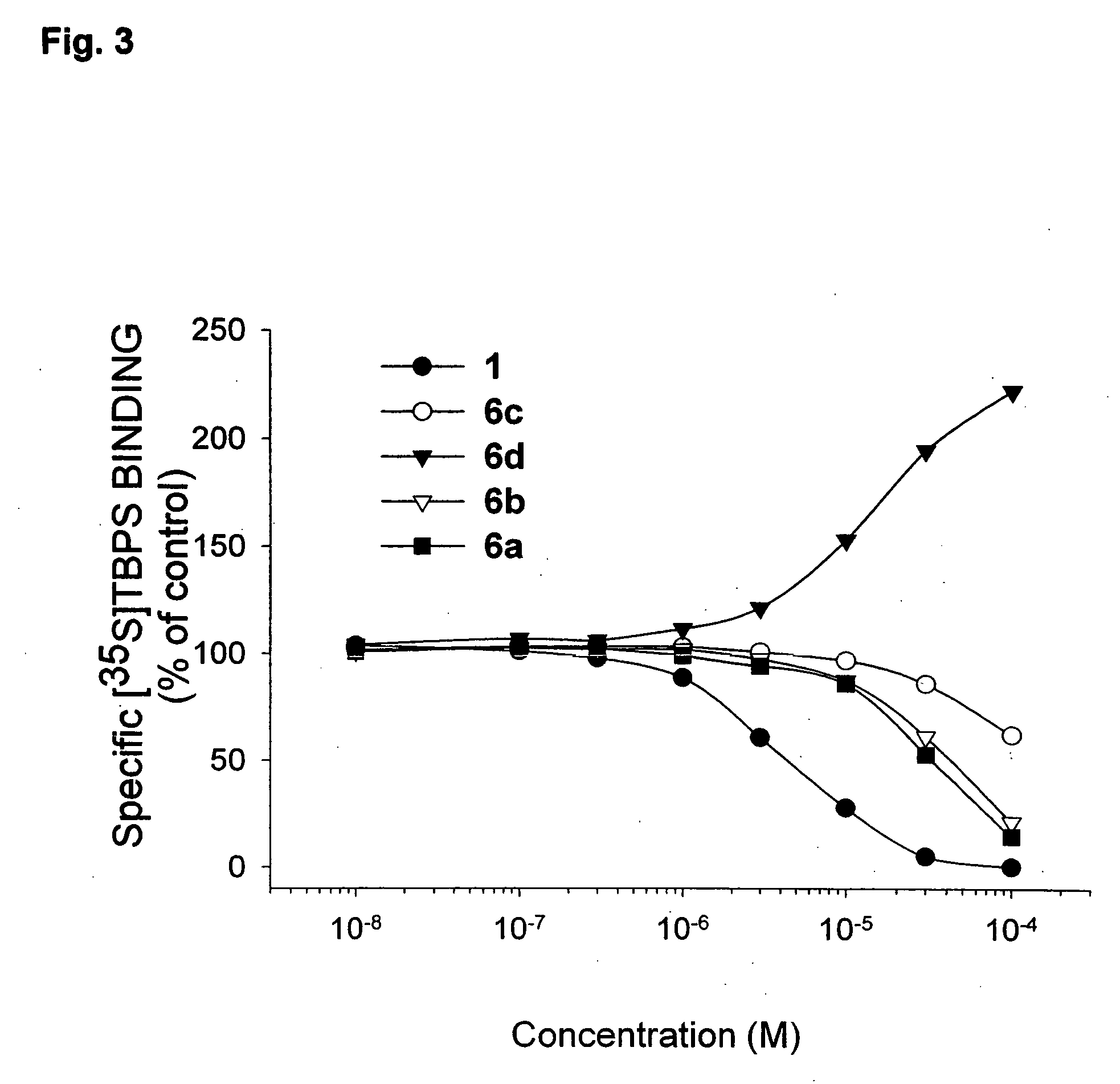
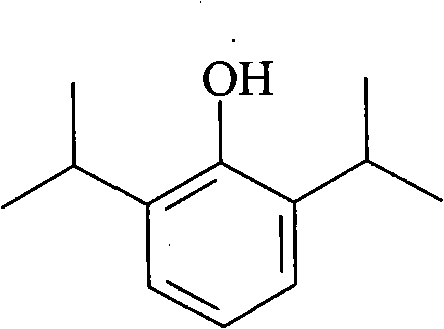
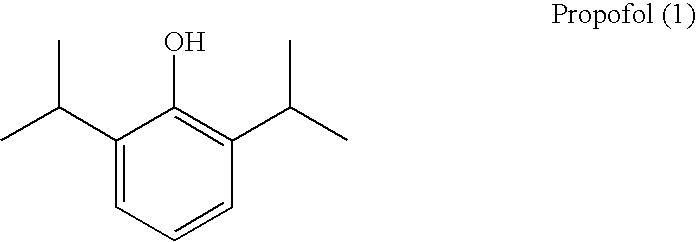
Propofol, marketed as Diprivan among other names, is a short-acting medication that results in a decreased level of consciousness and lack of memory for events. Its uses include the starting and maintenance of general anesthesia, sedation for mechanically ventilated adults, and procedural sedation. It is also used for status epilepticus if other medications have not worked. It is given by injection into a vein. Maximum effect takes about two minutes to occur and it typically lasts five to ten minutes.
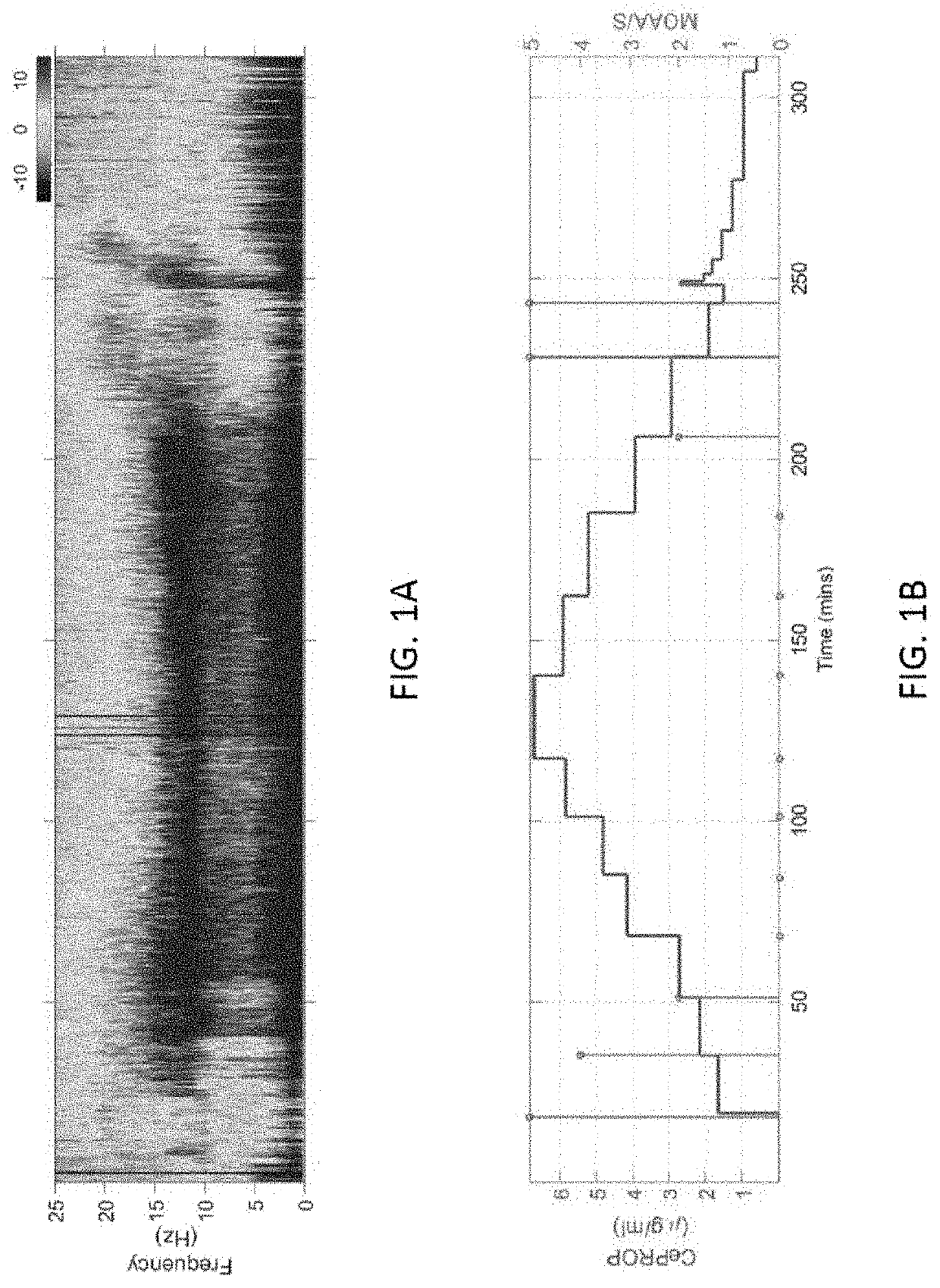
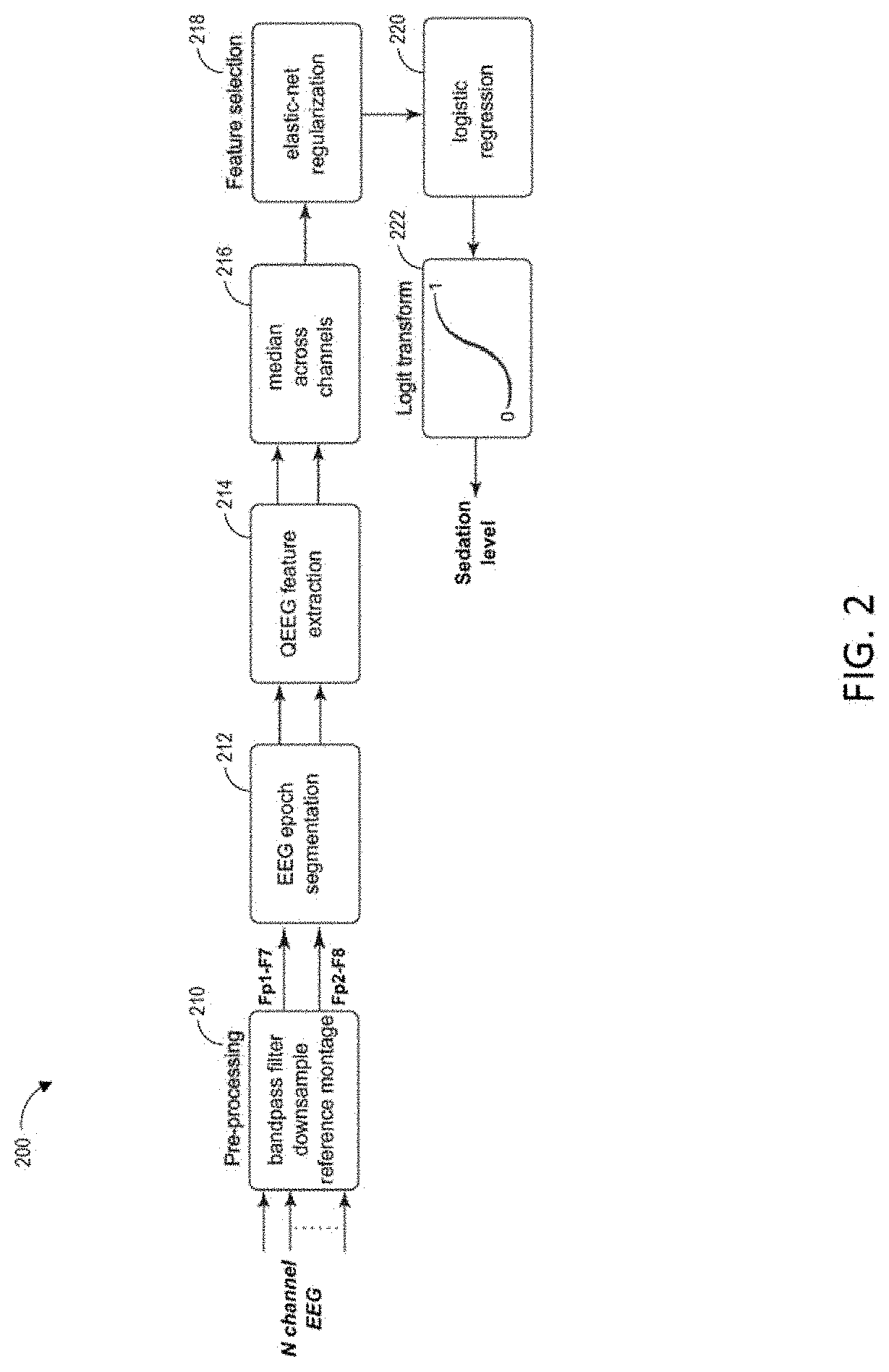
Combining multiple qeeg features to estimate drug-independent sedation level using machine learning
PendingUS20200253544A1Improve predictabilityImprove performanceElectroencephalographyMechanical/radiation/invasive therapiesSedative drugPhysical medicine and rehabilitation
The present disclosure describes systems and methods of estimating sedation level of a patient using machine learning. For example, the integration of multiple QEEG features into a single sedation level estimation system using machine learning could result in a significant improvement in the predictability of the levels of sedation, independent of the sedative drug used. The present disclosure advantageously allows for the incorporation of large numbers of QEEG features and machine learning into the next-generation monitors of sedation level. Different QEEG features may be selected for different sedation drugs, such as propofol, sevoflurane and dexmedetomidine groups. The sedation level estimation system can maintain a high performance for detecting MOAA / S, independent of the drug used.
Owner:MASIMO CORP
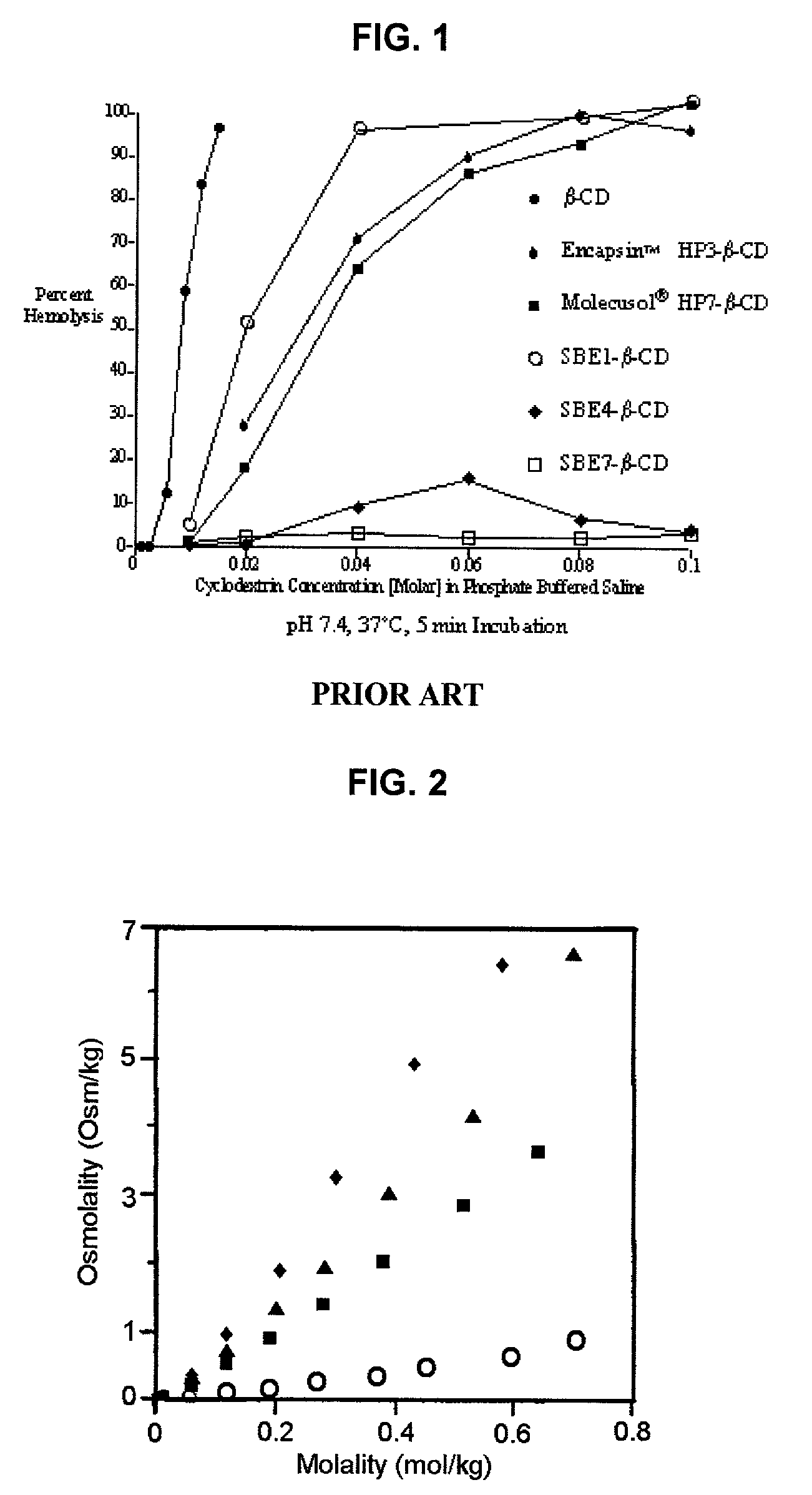
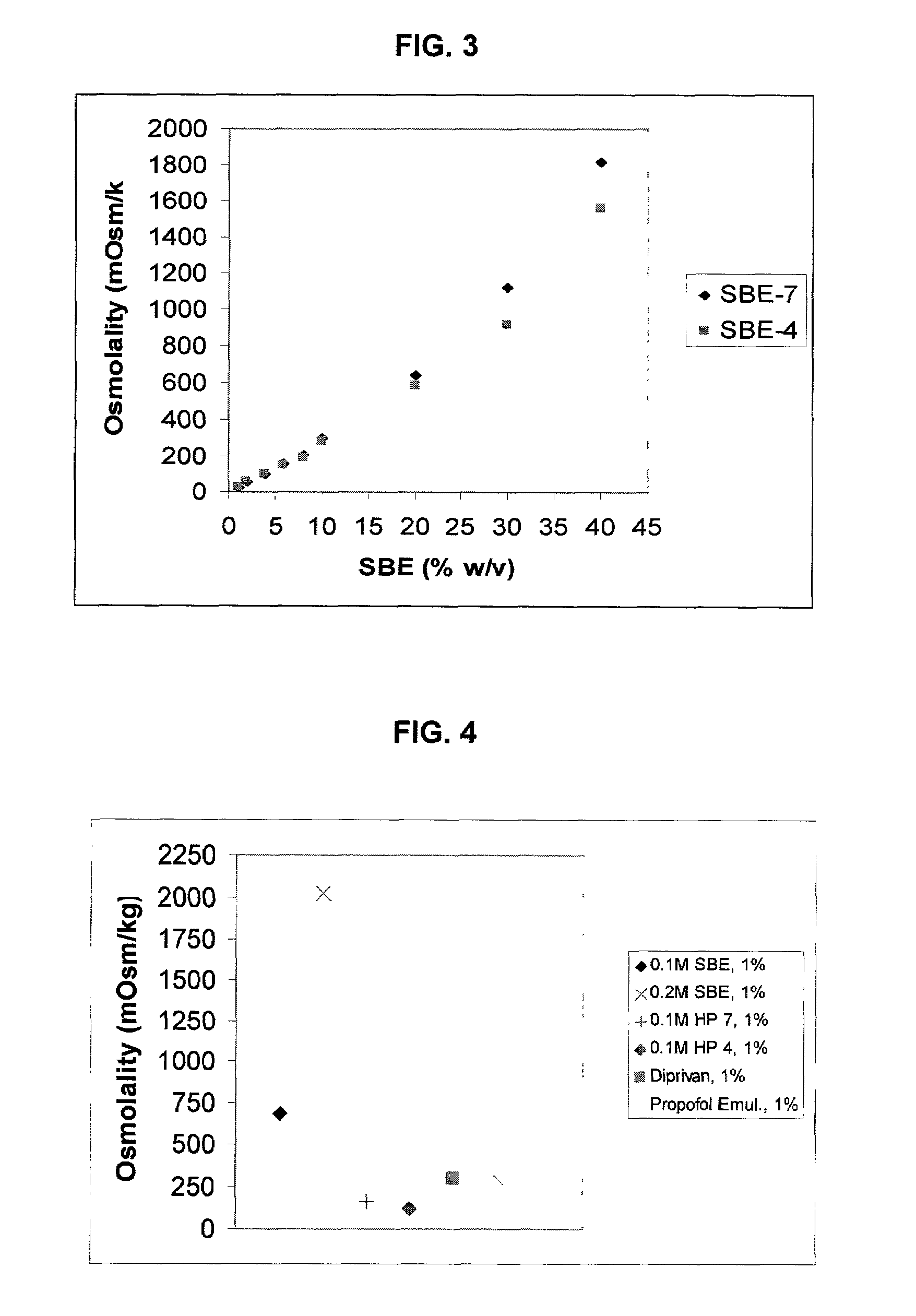
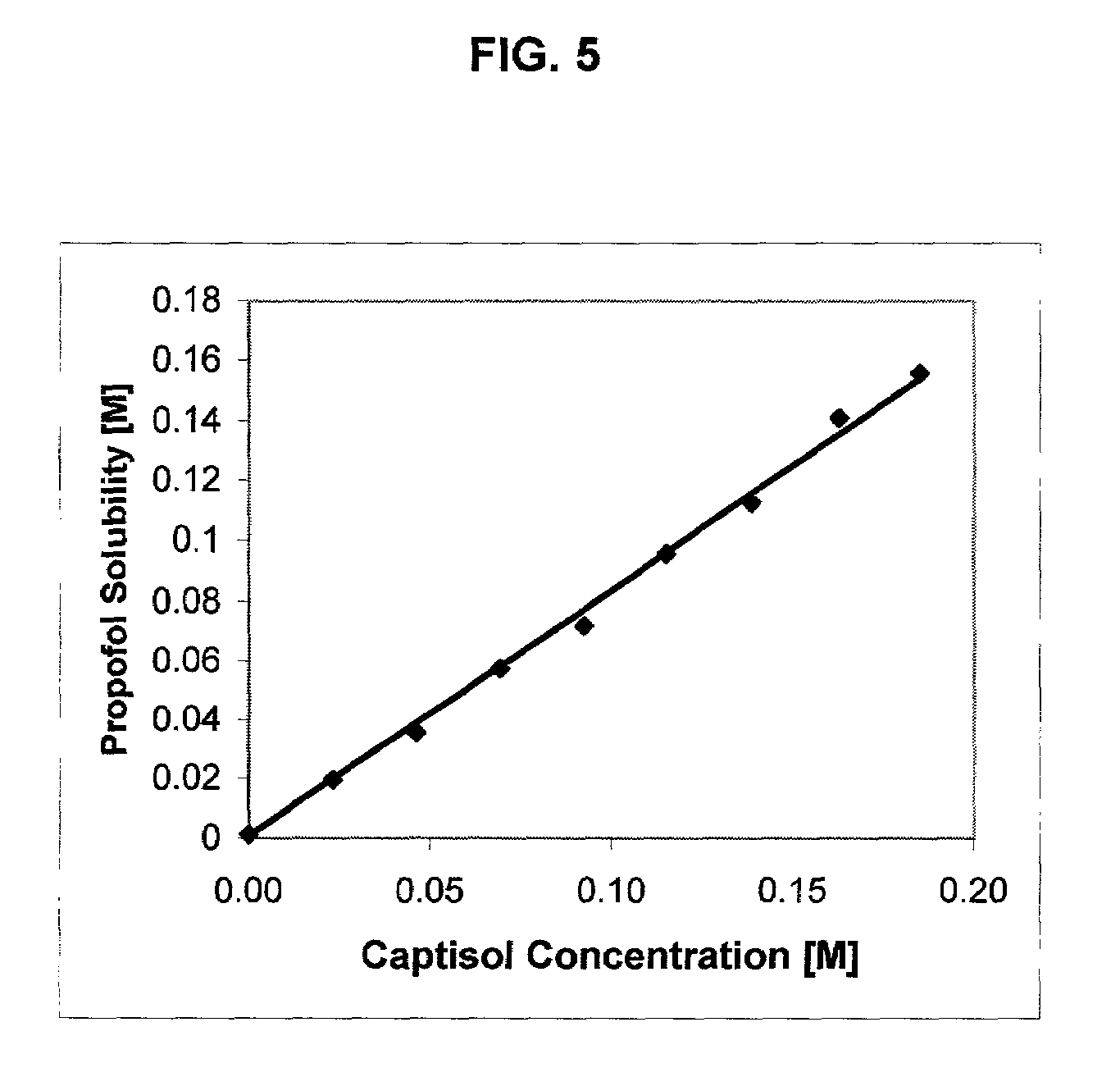
Formulations containing propofol and a sulfoalkyl ether cyclodextrin
InactiveUS7034013B2Reduce bacterial contaminationHigh photochemical stabilityBiocideHydroxy compound active ingredientsEmulsionAllergic response
An injectable formulation of a sedative hypnotic drug, such as the anesthetic drug propofol, that is pharmaceutically stable and demonstrates a reduced incidence of pain upon injection. The formulation of the present invention employs a sulfoalkyl ether cyclodextrin solubilizing and complexing excipient, such as CAPTISOL® cyclodextrin (sulfobutyl ether β-cyclodextrin) to form a true aqueous solution and not a suspension. This formulation minimizes the allergic response and microbial contamination issues typically associated with propofol parenteral formulations. The present formulation may also reduce pain on injection as compared to the known emulsion type propofol formulations. The liquid formulation can be sterile filtered unlike emulsion-type formulations of sedative hypnotics. The liquid formulation can be lyophilized or otherwise dried to yield a solid formulation.
Owner:CUDA PHARMA LLC
Buccal, polar and non-polar sprays containing propofol
InactiveUS20050002867A1Fast absorptionRapid onsetBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
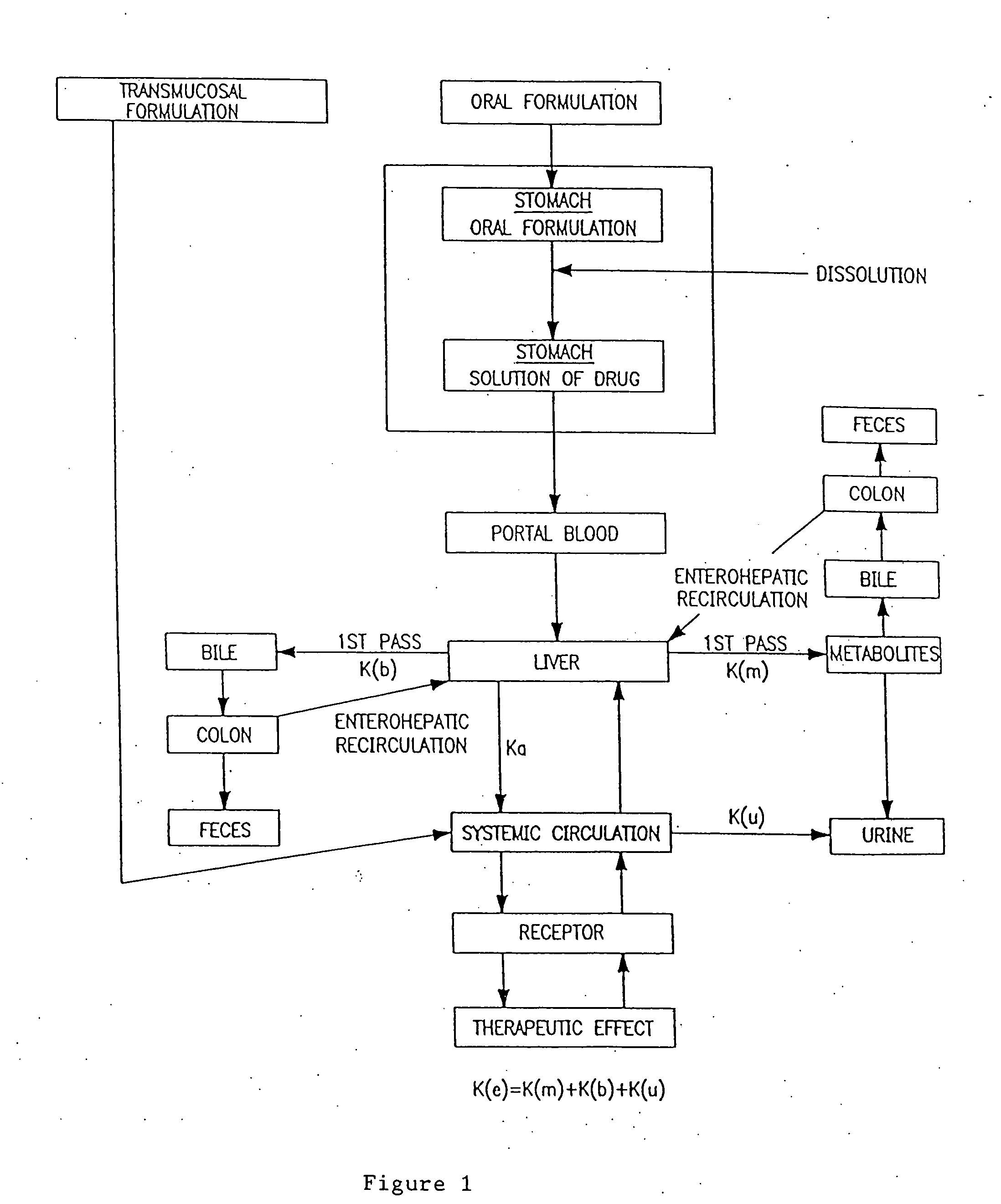
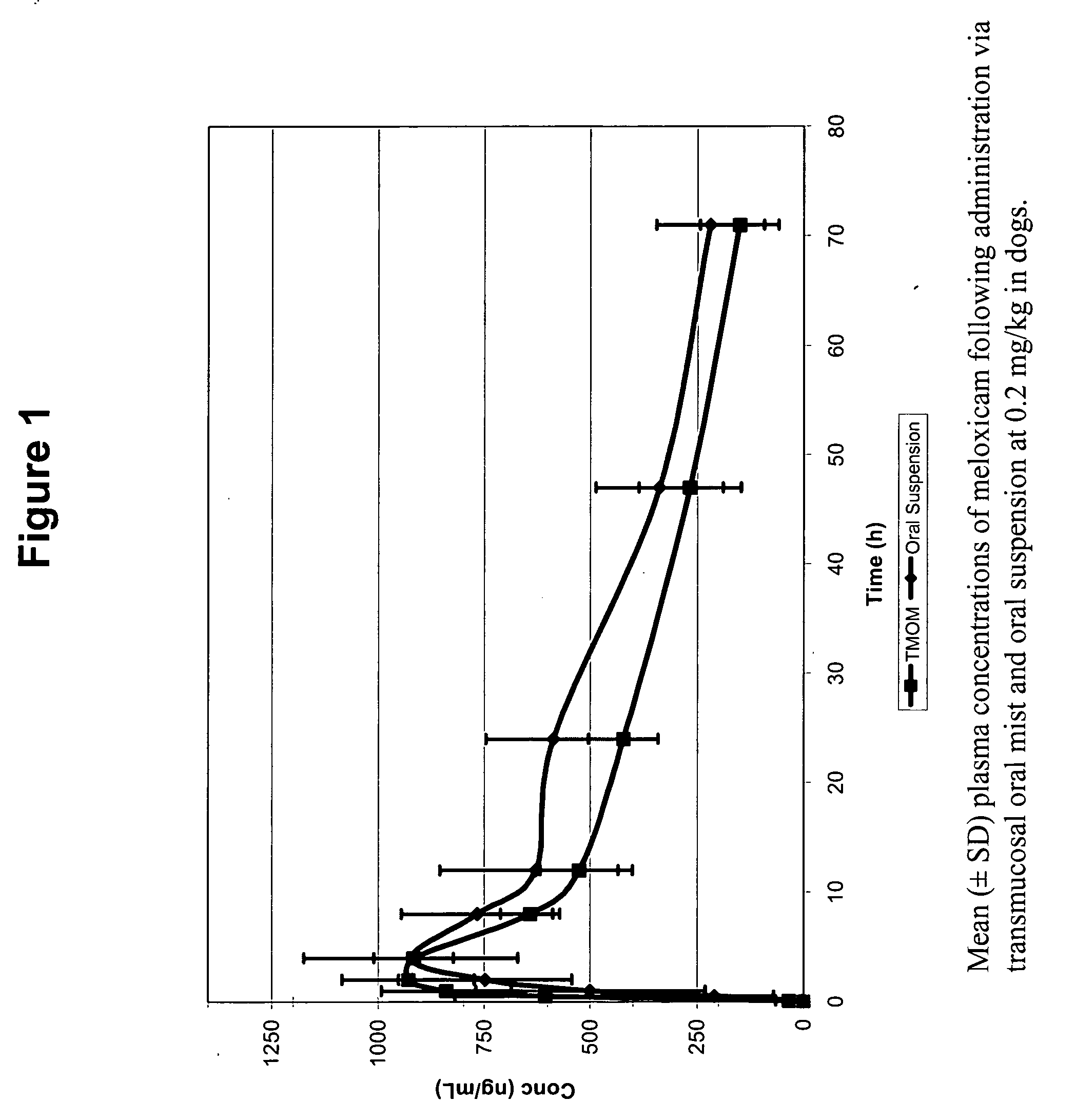
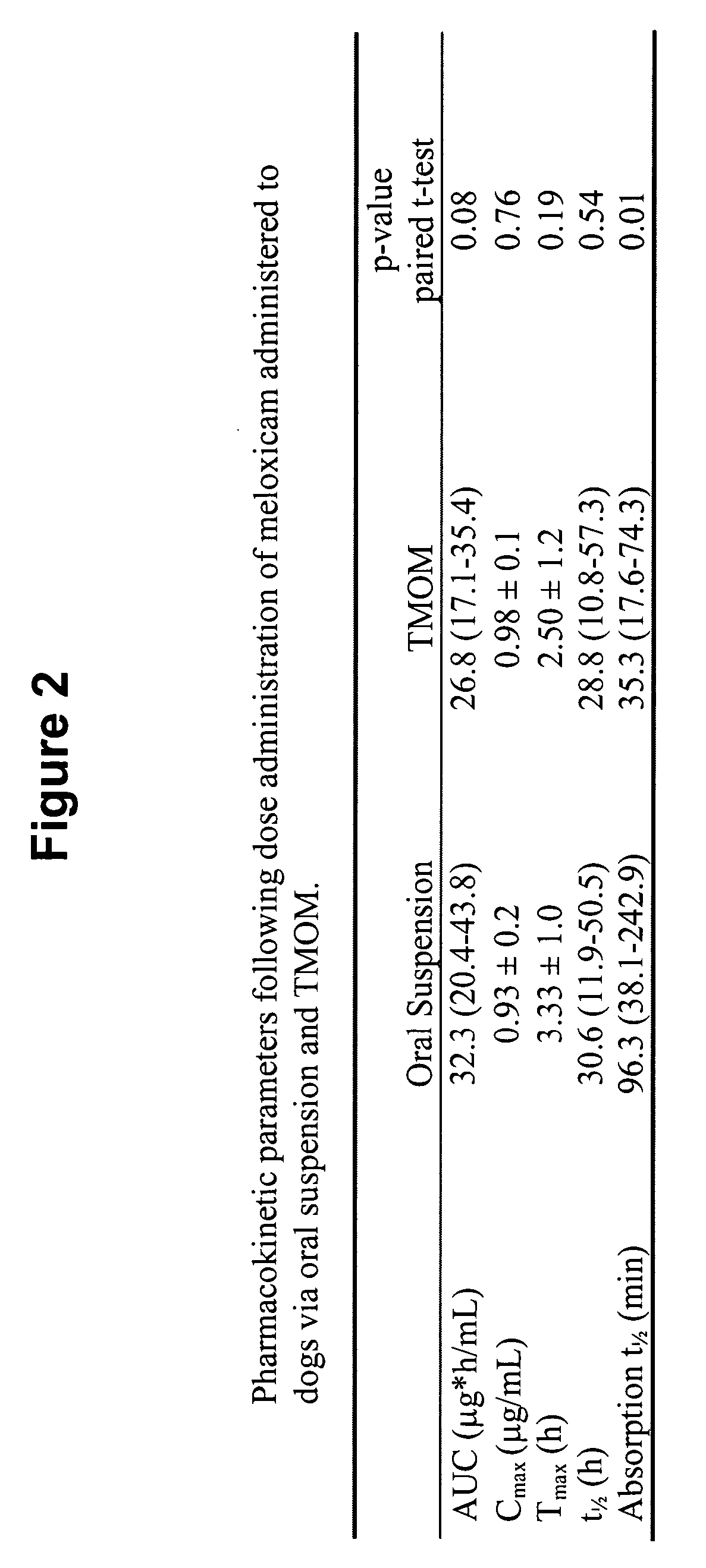
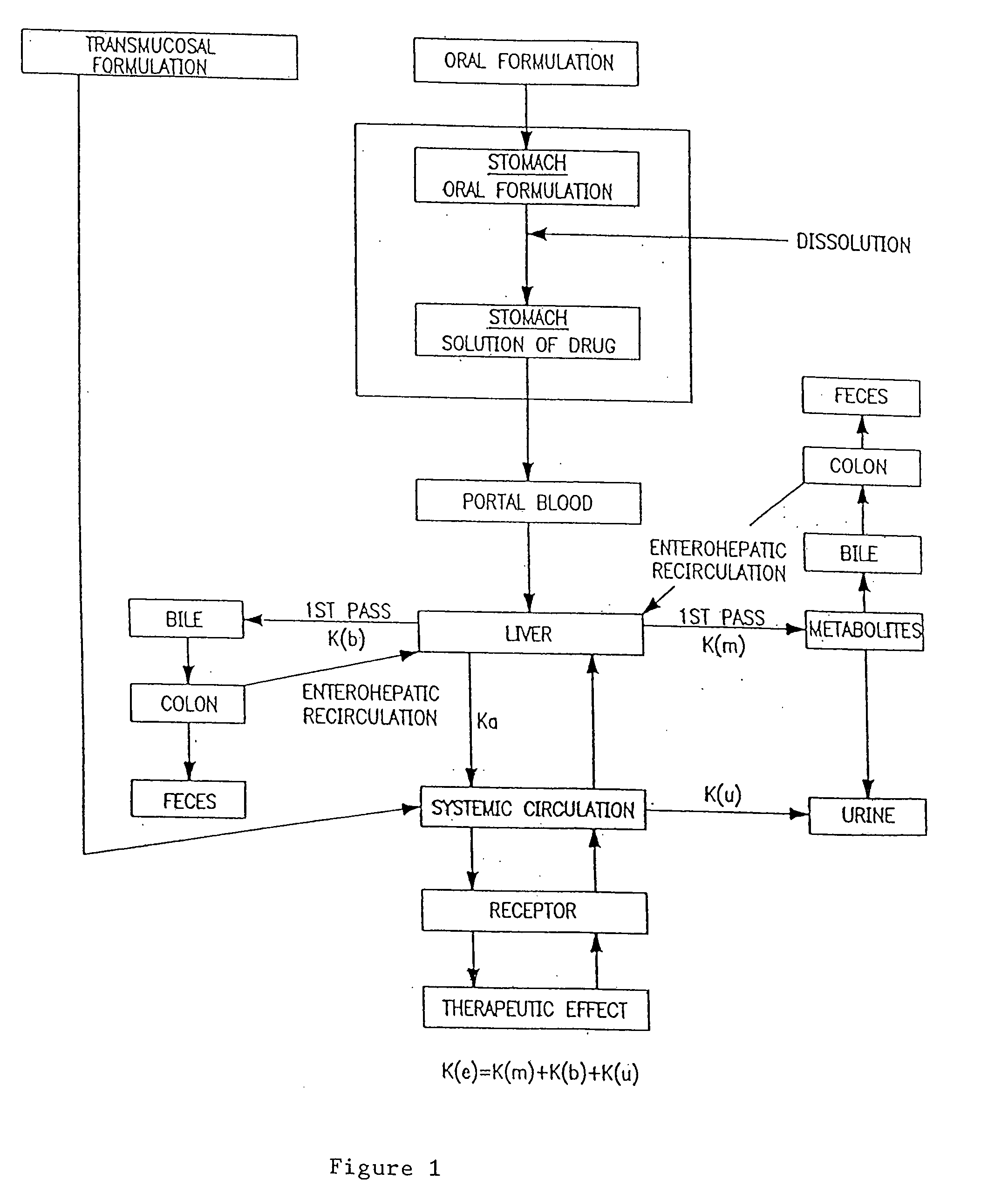
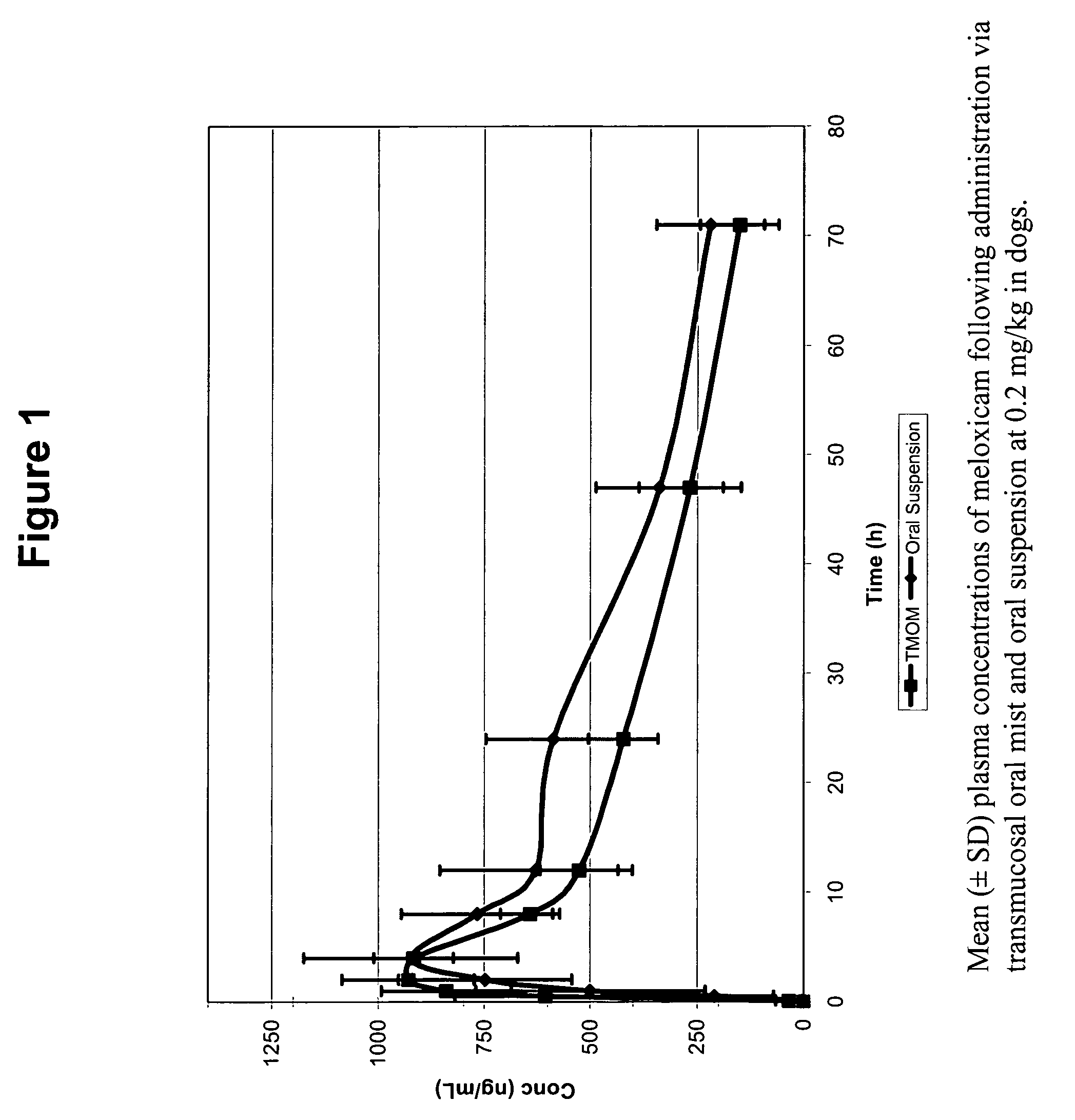
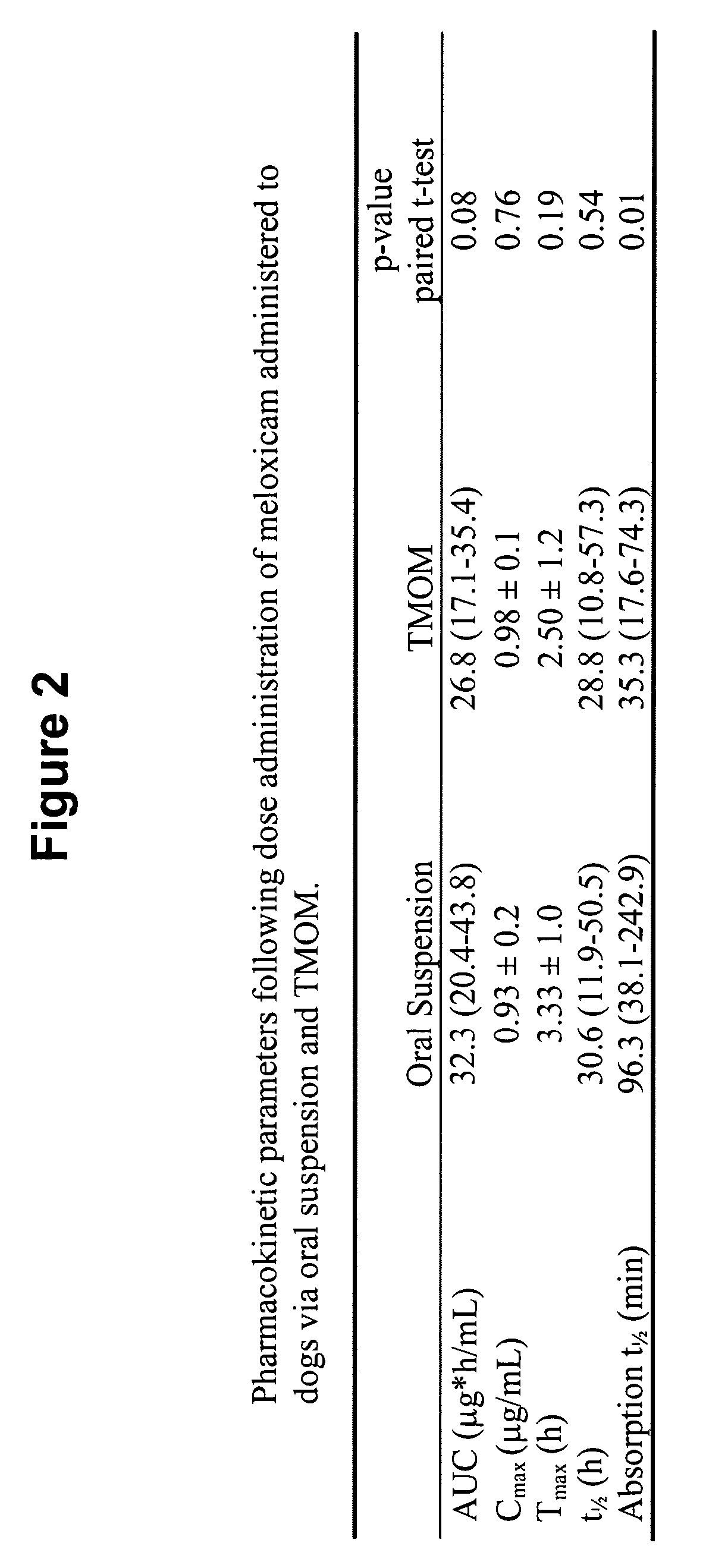
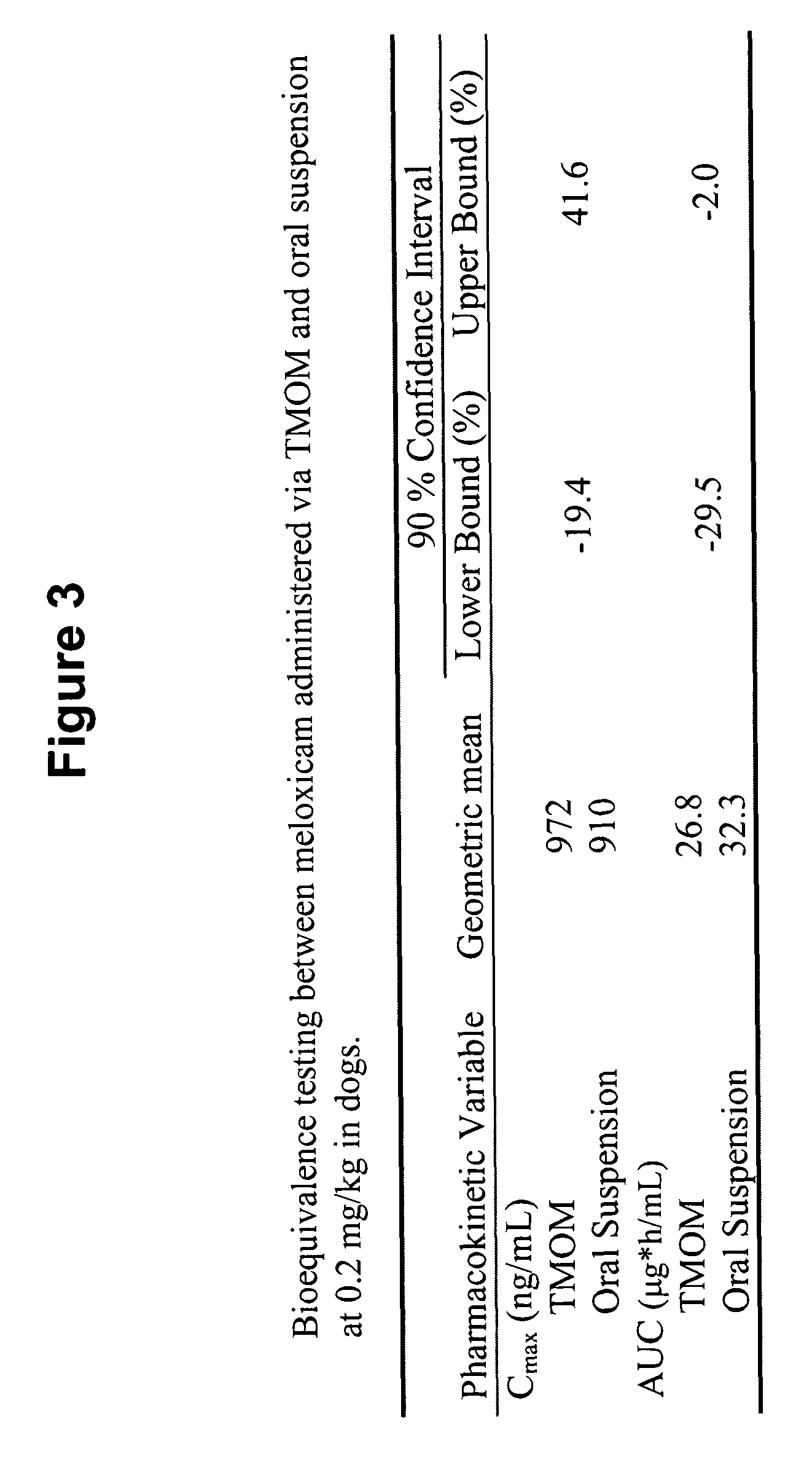
Transmucosal administration of drug compositions for treating and preventing disorders in animals
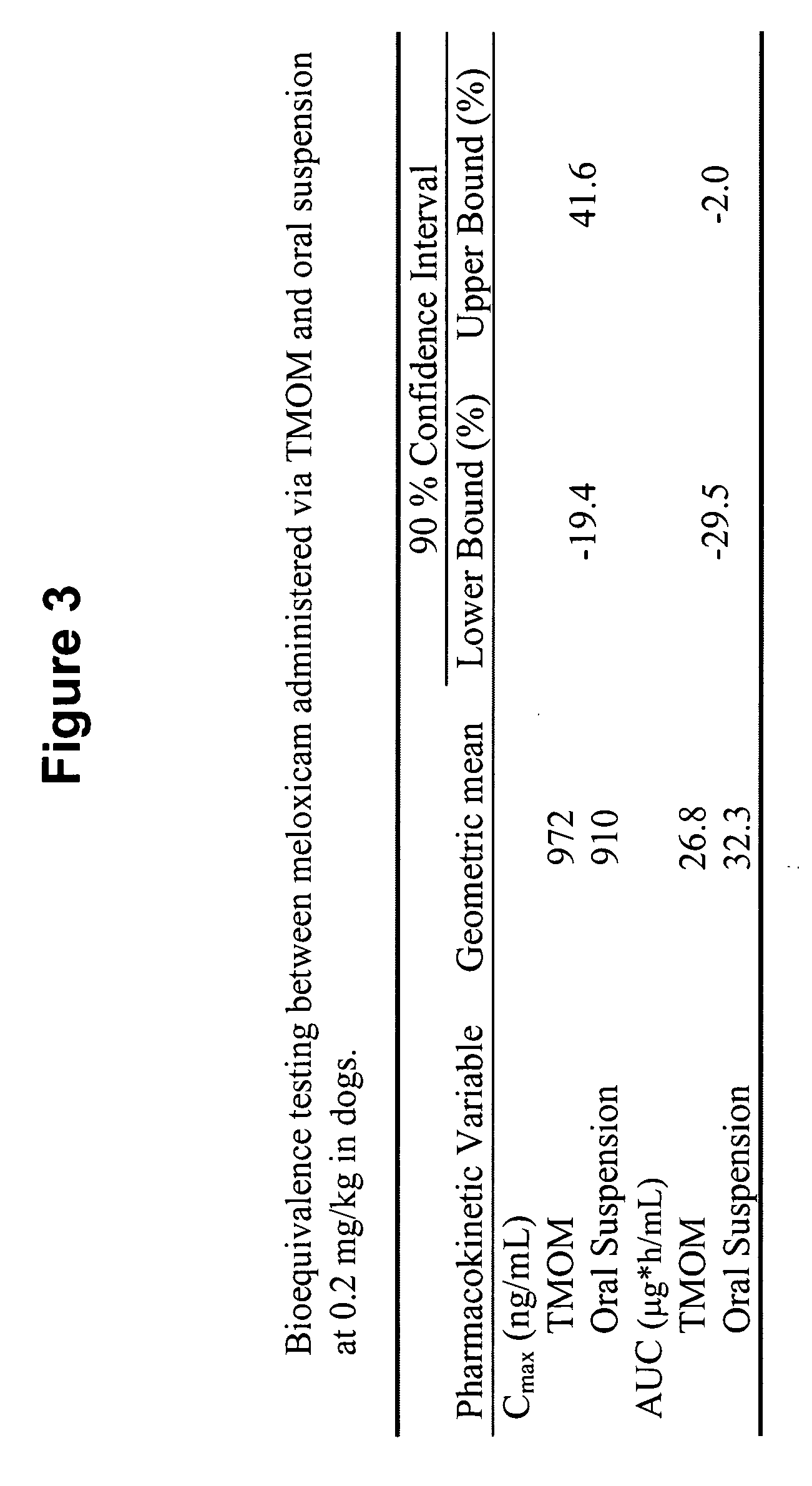
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Buccal, polar and non-polar sprays containing propofol
InactiveUS20060222597A1Rapid onsetFast absorptionBiocideHydroxy compound active ingredientsAerosol spraySolvent
Buccal aerosol sprays using polar and / or non-polar solvents have now been developed which provide propofol for rapid absorption through the oral mucosa, resulting in fast onset of effect. The buccal polar compositions of the invention comprise formulation I: propofol, a polar solvent and an optional flavoring agent; formulation II: propofol, a polar solvent, a propellant, and an optionally flavoring agent; formulation III: propofol, a non-polar solvent, and an optional flavoring agent; formulation IV: propofol, a non-polar solvent, a propellant, and an optional flavoring agent; formulation V: propofol, a mixture of a polar solvent and a non-polar solvent, and an optional flavoring agent; and formulation VI: propofol, a mixture of a polar solvent and a non-polar solvent, a propellant, and an optional flavoring agent.
Owner:NOVADEL PHARMA
Solid formulations of liquid biologically active agents
InactiveUS20060198891A1Reduces and eliminates any sensation of painEasy loadingPowder deliveryDispersion deliveryActive agentSolvent
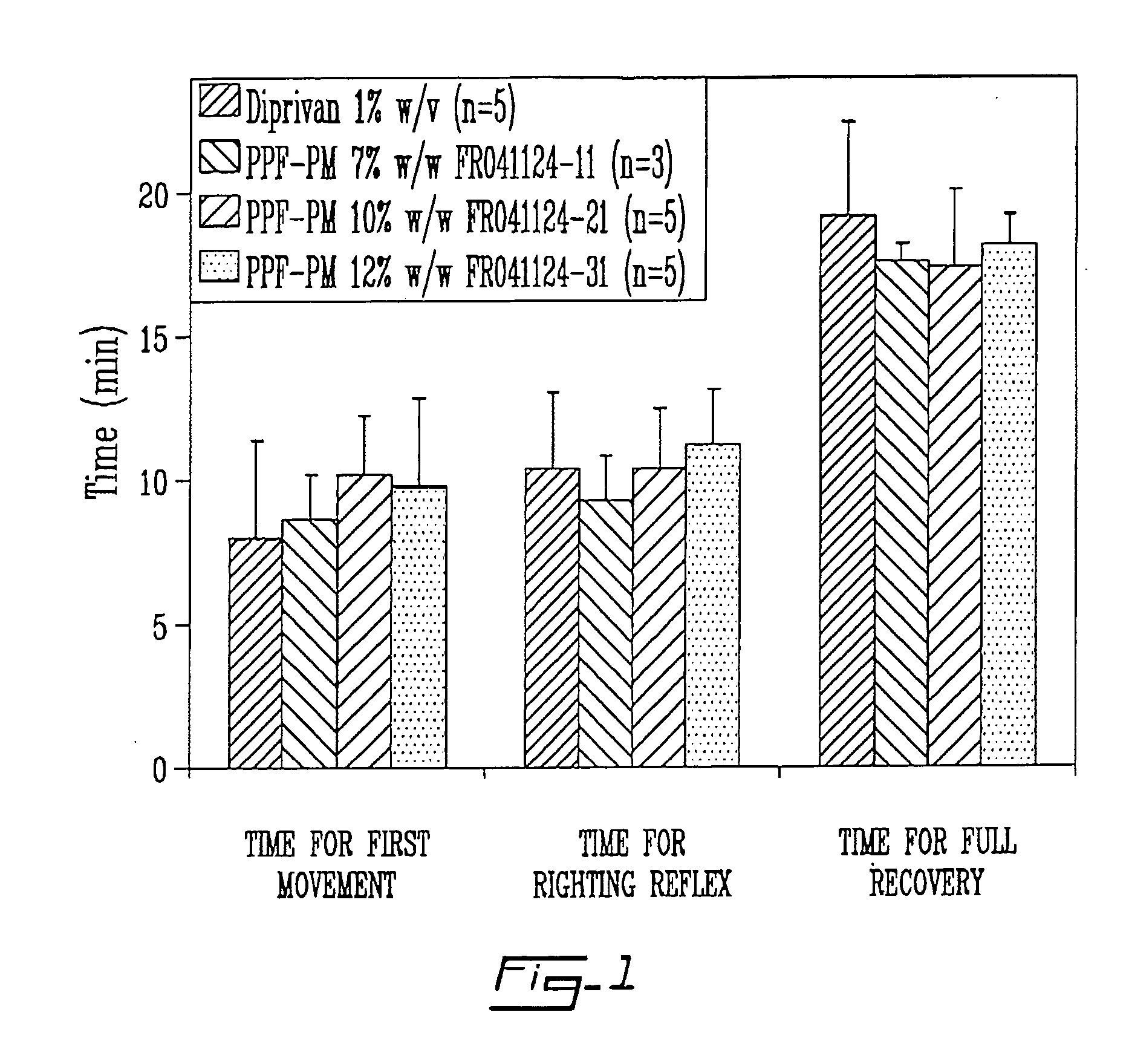
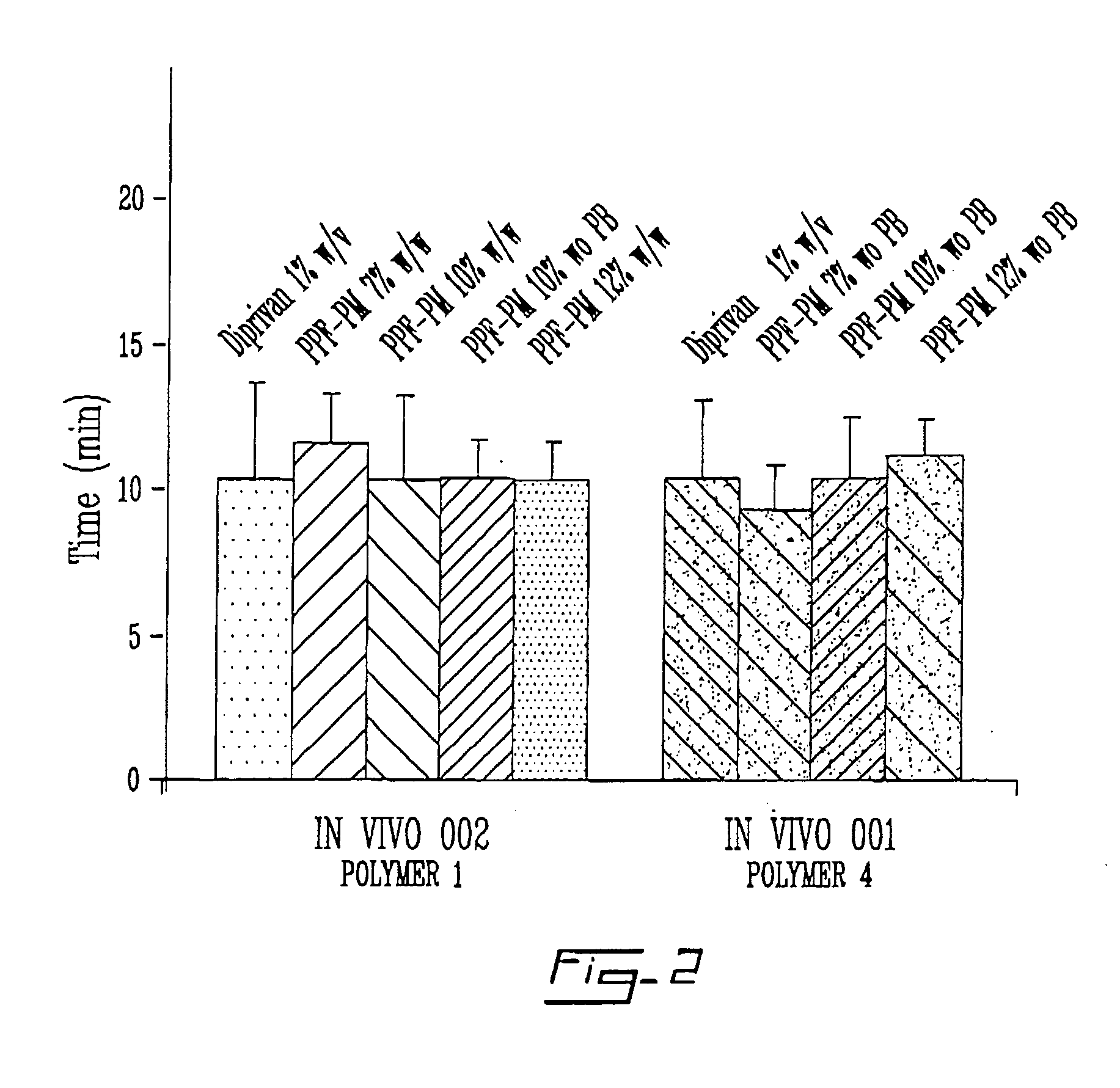
The instant invention relates to a solid product comprising a liquid biologically active agent which is intimately associated to a stabilizing agent; particularly a solid product that can be reconstituted to a clear, stable, stabilized nanodispersion or loaded micelles comprising a polymer as a stabilizing agent and a liquid, preferably water immiscible, biologically active agent. The instant invention is further directed toward a process for the production of the above solid product; particularly to micelles or nanodispersions produced by hydration of a cake or powder of the solid product, produced via an effective treatment of a stabilized solution comprising for example a polymer as a stabilizing agent, such as an amphiphilic block copolymer or a small molecular weight surfactant, loaded with a liquid biologically active agent, such as propofol, an optional additive, and a suitable solvent.
Owner:PALADIN LABS EURO LTD +2
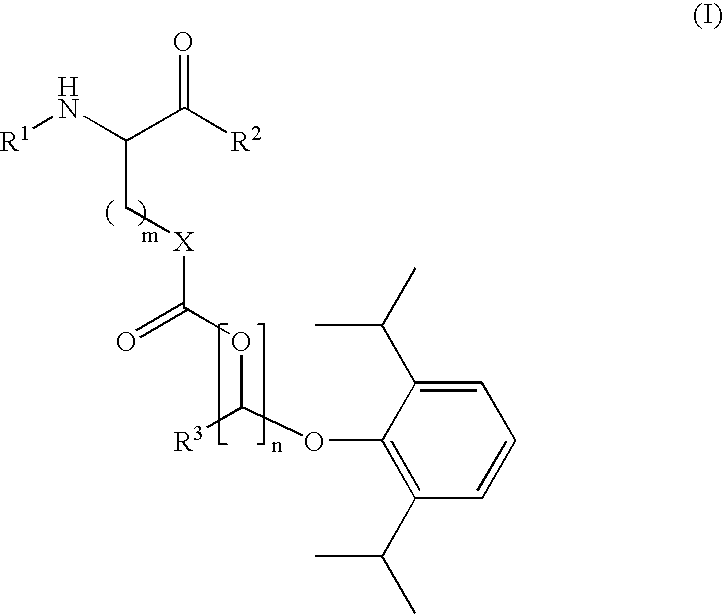
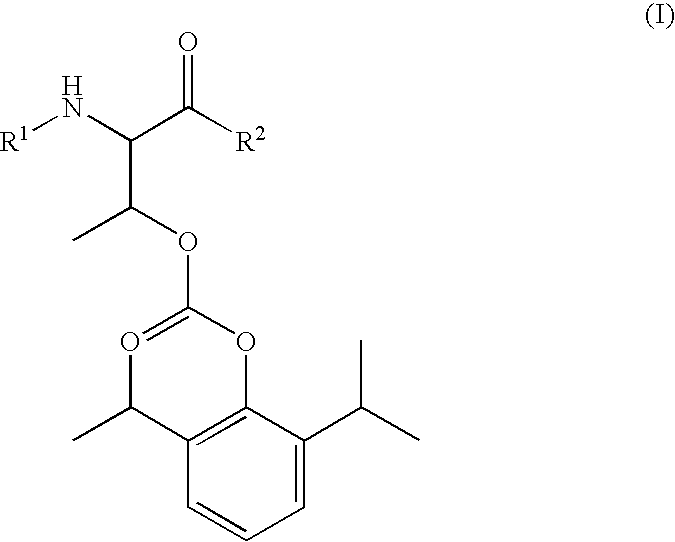
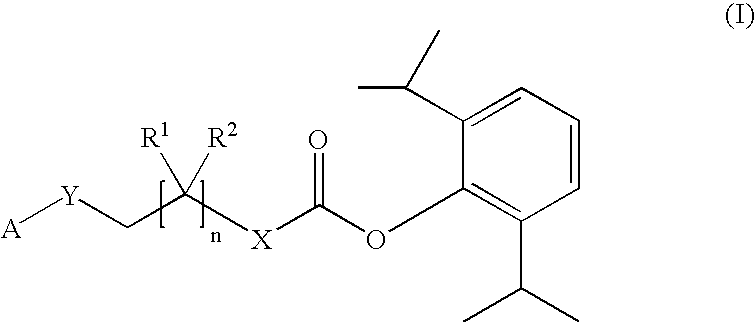
Amino acid derived prodrugs of propofol, compositions, uses and crystalline forms thereof
The present invention provides a prodrug of propofol and crystalline forms thereof, methods of making the propofol prodrug and crystalline forms thereof, pharmaceutical compositions of the propofol prodrug and crystalline forms thereof, methods of using the propofol prodrug and crystalline forms thereof and pharmaceutical compositions thereof to treat diseases or disorders such as headache pain, post-chemotherapy or post-operative surgery nausea and vomiting, neurodegenerative disorders, and mood disorders.
Owner:XENOPORT
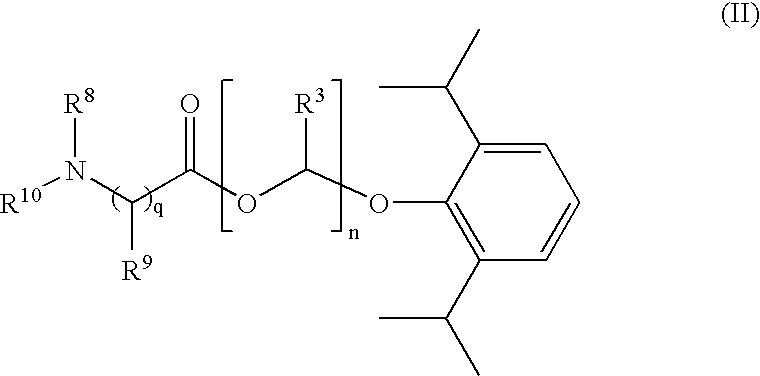
Amino acid derived prodrugs of propofol, compositions and uses thereof
The present invention provides propofol prodrugs, methods of making propofol prodrugs, pharmaceutical compositions of propofol prodrugs and methods of using propofol prodrugs and pharmaceutical compositions thereof to treat or prevent diseases or disorders such as migraine headache pain and post-chemotherapy or post-operative surgery nausea and vomiting.
Owner:XENOPORT
O/w-emulsions comprising semifluorinated alkanes
InactiveUS20140004197A1Improve antibacterial stabilityHigh drug loadingPowder deliveryBiocideAlkaneEmulsion
The invention provides liquid compositions in the form of physically stable emulsions comprising a semifluorinated alkane. The semifluorinated alkane is comprised in the dispersed phase, which may also include an active pharmaceutical ingredient. One of the preferred active ingredients is propofol. The compositions are optionally heat sterilisable and can be used for pharmaceutical or cosmetic product applications, and administered topically, intravenously, or via other routes.
Owner:NOVALIQ GMBH
Aqueous 2,6-diisopropylphenol pharmaceutical compositions
InactiveUS20050027019A1Good chemical stabilityImprove physical stabilityBiocideHydroxy compound active ingredientsAqueous solutionExcipient
The present invention relates to aqueous pharmaceutical compositions comprising 2,6-diisopropylphenol (propofol). A composition of the present invention can comprise propofol and two or more excipients as an aqueous mixture. The propofol containing compositions are preferably sterile and are parenterally administered to any animal, including humans. The compositions are also chemically and physically stable over a wide range of environmental conditions.
Owner:JANSSEN BIOTECH INC
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
Aqueous pharmaceutical compositions of 2,6-diisopropylphenol (propofol) and their uses
InactiveUS20040265388A1Simple preparation processEasy to storePowder deliveryHydroxy compound active ingredientsPolyethylene glycolPropofol
The present invention provides aqueous pharmaceutical compositions containing a lipophilic therapeutic agent. In particular, the invention provides aqueous pharmaceutical compositions containing the compound 2,6-diisopropylphenol (propofol). Preferred compositions of the invention contain propofol in the presence of at least one block copolymer (for example, P188 or another poloxamer) and a polyethylene glycol (PEG). Compositions of the invention are preferably sterile or are readily sterilized (e.g., by autoclaving) and are suitable for parenteral administration to any animal, including humans. The compositions are also chemically and physically stable over a wide range of environmental conditions and for extended periods of time.
Owner:JANSSEN BIOTECH INC
Measuring system for the determination of the concentration of propofol (2,6-diisopropylphenol) in the respiratory flow
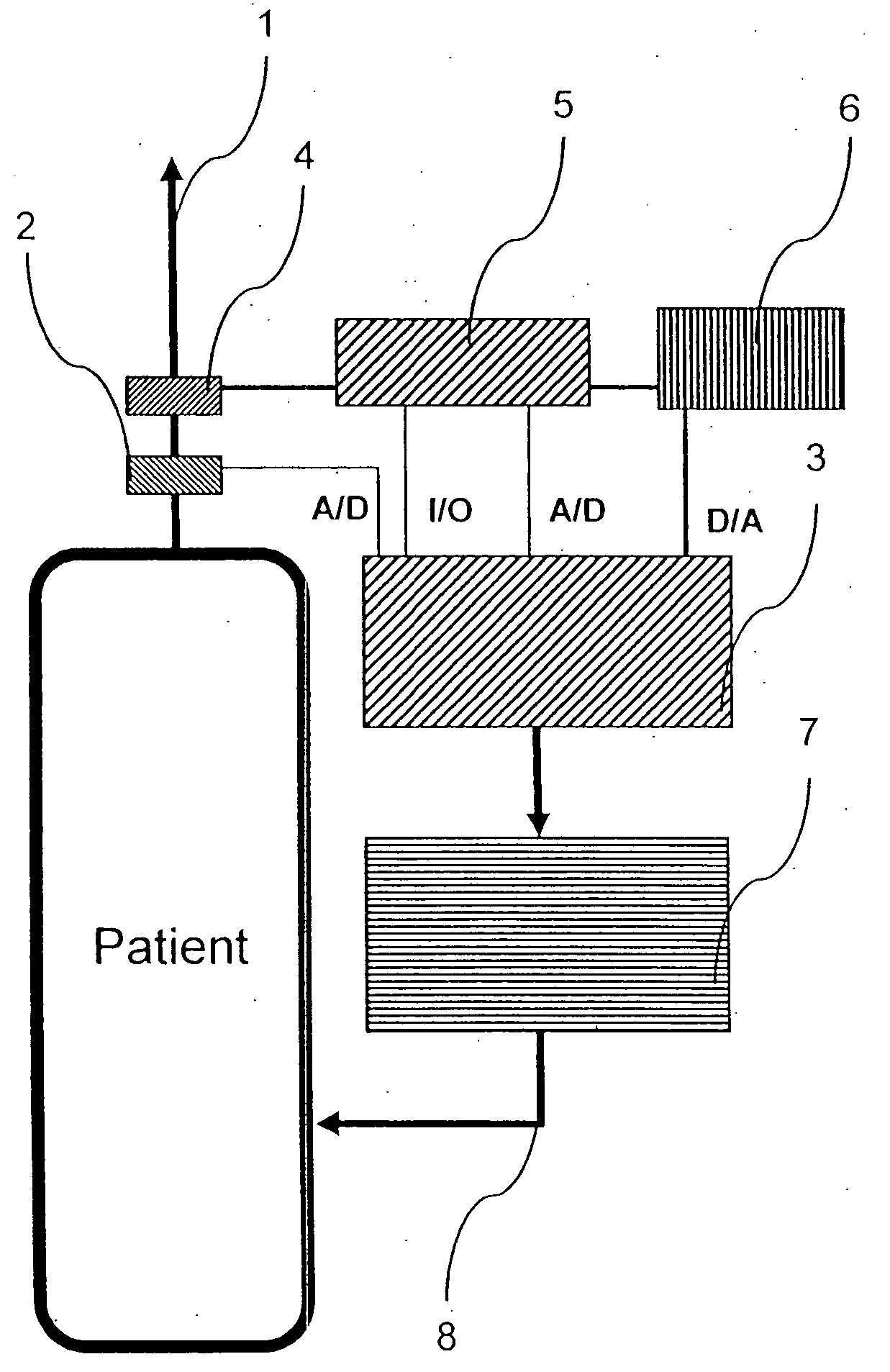
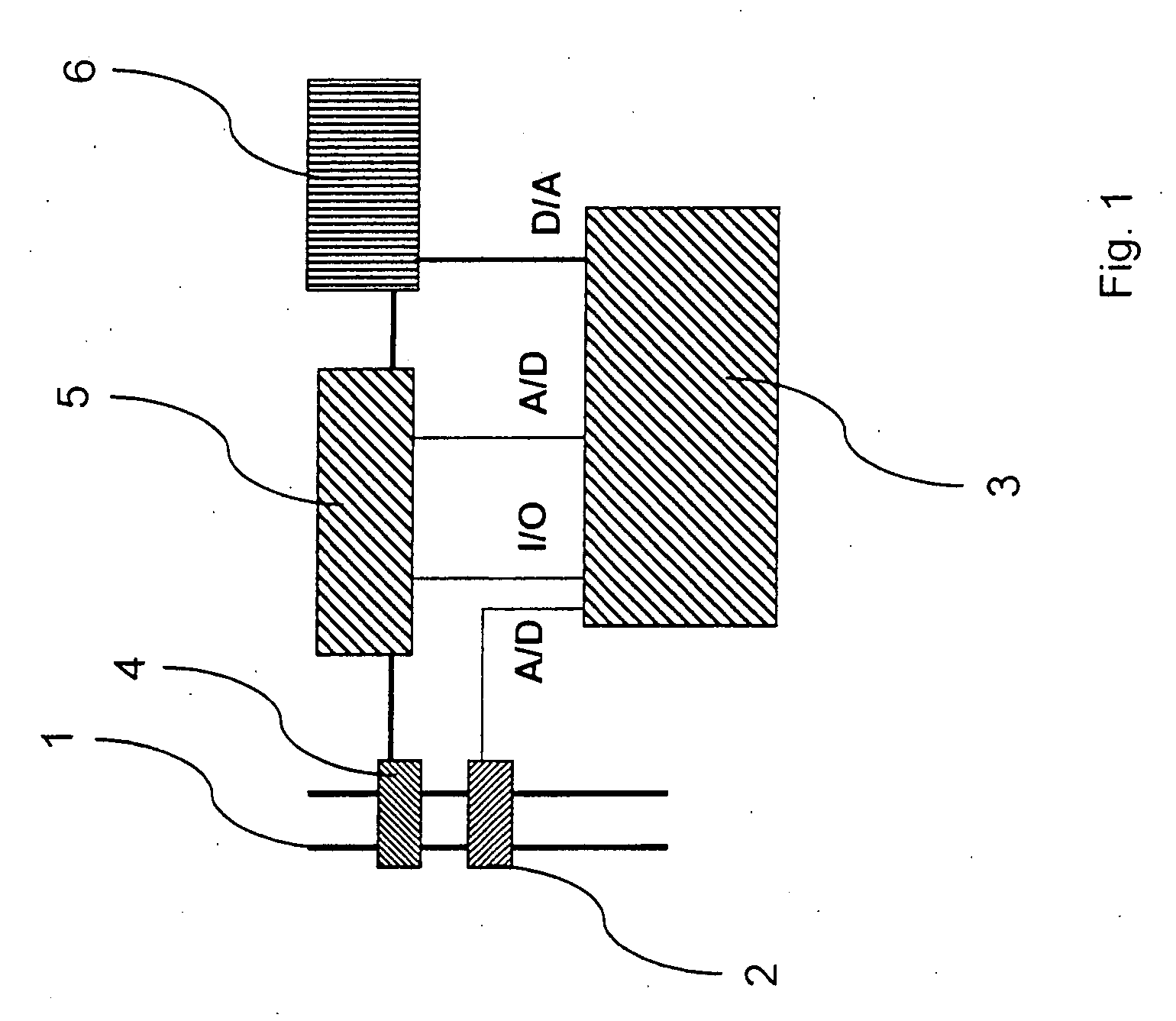
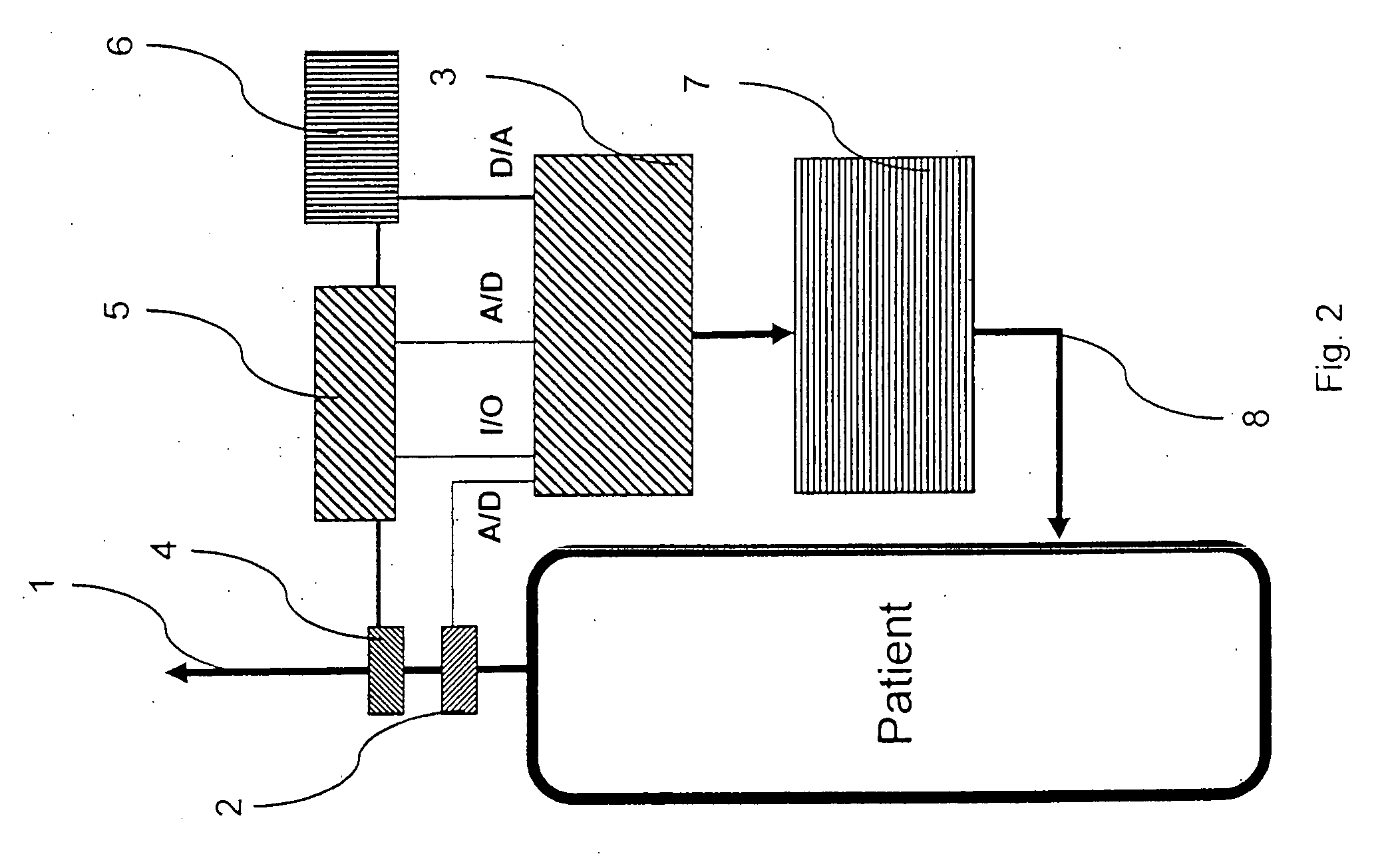
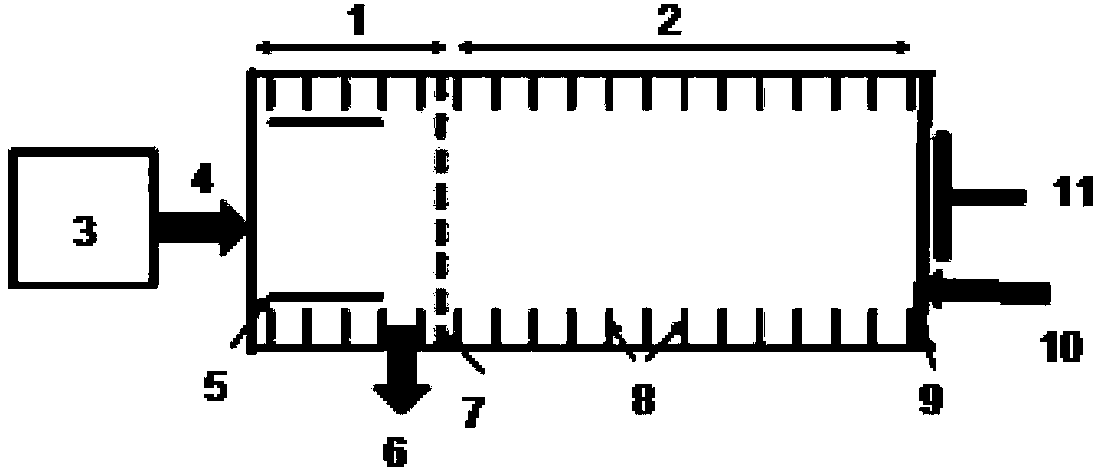
InactiveUS20050022811A1Easy to handleEasy to transportRespiratorsOperating means/releasing devices for valvesRespiratory flowMedicine
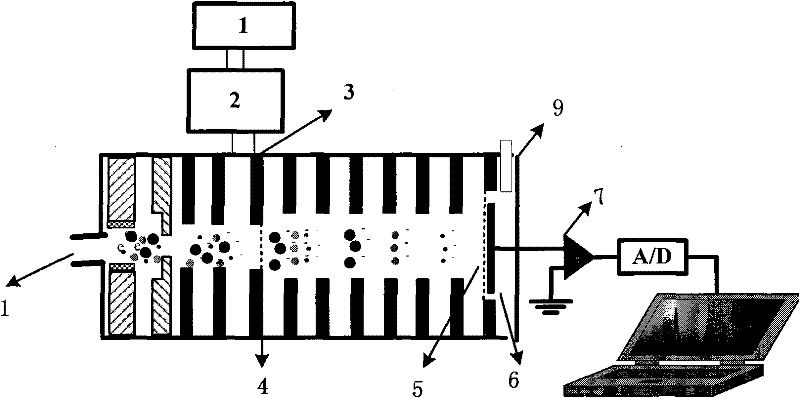
A rapid measuring system for the determination of the concentration of propofol in the respiratory flow, which can be designed in a compact form, has the features of a breathing gas line (1) including a breathing gas sensor (2) detecting the respiration, wherein the breathing gas sensor (2) is connected to an evaluating unit (3), a propofol sensor (5) with a downstream pump (6) is in gas flow connection with the breathing gas line (1), wherein the evaluating unit (3) is connected with the propofol sensor (5) and the pump (6), so that the evaluating unit (3) actuates the pump (6) for breathing gas sampling depending on the signal of the breathing gas sensor (2), and the propofol sensor (5) sends a measured signal for the concentration of propofol in the breathing gas to the evaluating unit (3).
Owner:DRAGERWERK AG
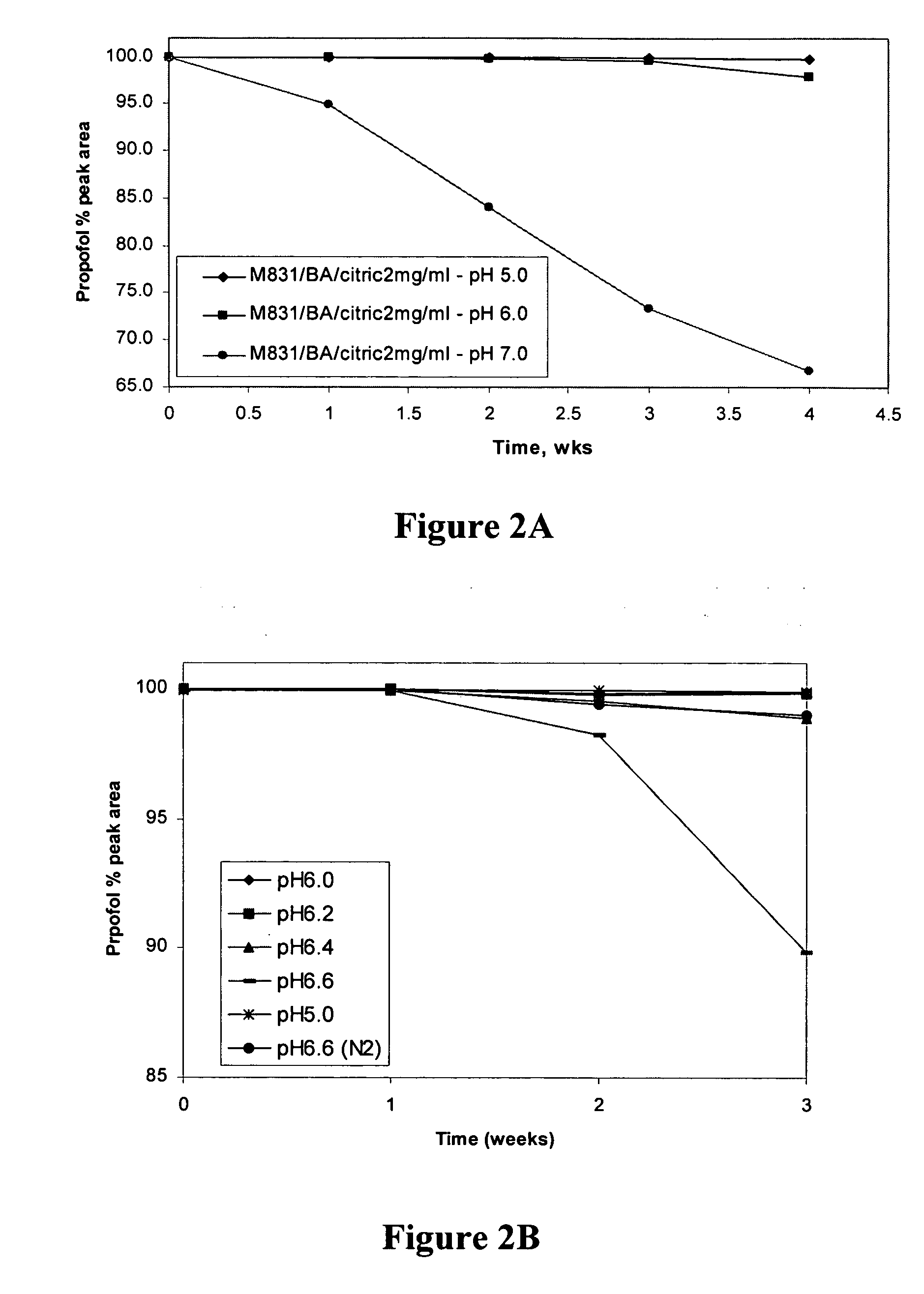
Propofol formulations with non-reactive container closures
ActiveUS20050009731A1Excellent exhibition of antimicrobial activityReduce riskBiocidePowder deliverySolventAlbumin
A sterile pharmaceutical composition for parenteral administration of propofol, said composition comprising propofol, optionally albumin, and less than about 10% by weight solvent for propofol, wherein said composition is stored in a container having a closure wherein said closure is inert to propofol.
Owner:FRESENIUS KABI USA LLC
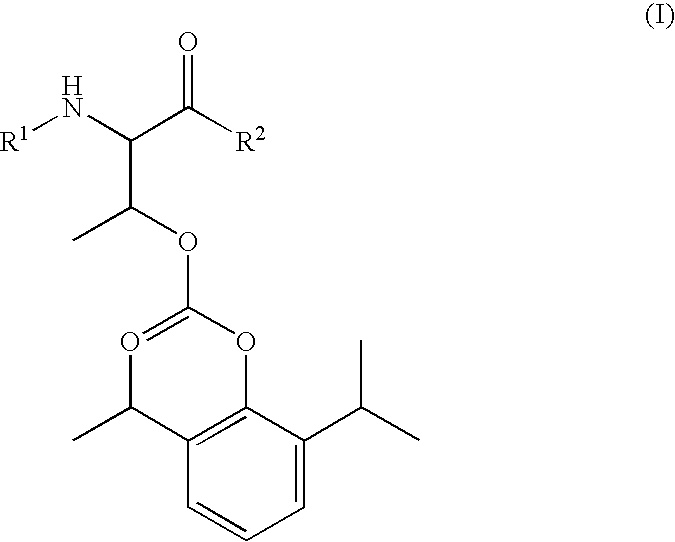
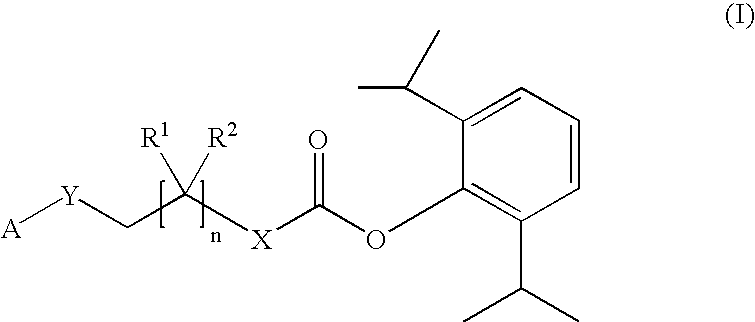
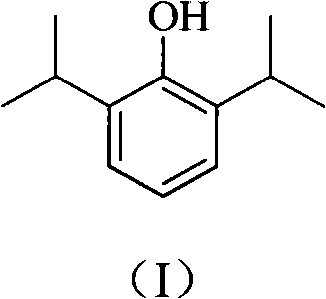
Phosphoryl carboxylic acid propofol ester derivative and preparation method thereof
ActiveCN101633671AImprove bioavailabilityGood water solubilityOrganic active ingredientsAnaestheticsO-Phosphoric AcidPhosphate
The invention relates to a phosphoryl carboxylic acid propofol ester derivative which has the general formula (I). The method comprises the following steps: propofol reacts with 2-halogenated carboxylic acid and a derivative thereof by alkali to obtain corresponding ester and then the product reacts with phosphoric acid or thiophosphoric acid and the derivative thereof by dissolvent to obtain a water-soluble product or the propofol reacts with a 2-halogenated carboxylic acid phosphate ester derivative by the alkali to obtain the corresponding ester and then the ester is catalyzed, hydrogenated and salified to obtain the water-soluble product (I). The preparation method has mild reaction condition, high yield, simple operation and industrialized prospect, and a prepared oral preparation has the characteristics of high bioavailability, rapid absorption, high stability, and the like; and auxiliary materials with safety defects, such as a surface active agent, and the like can not be added into the prepared injection, thereby improving the stability of the preparation, reducing or removing injection pain, increasing the compliance of patients, overcoming the defects of propofol emulsion and having the advantage of obvious effect. The invention has the structural general formula (I).
Owner:HANGZHOU ADAMERCK PHARMLABS INC
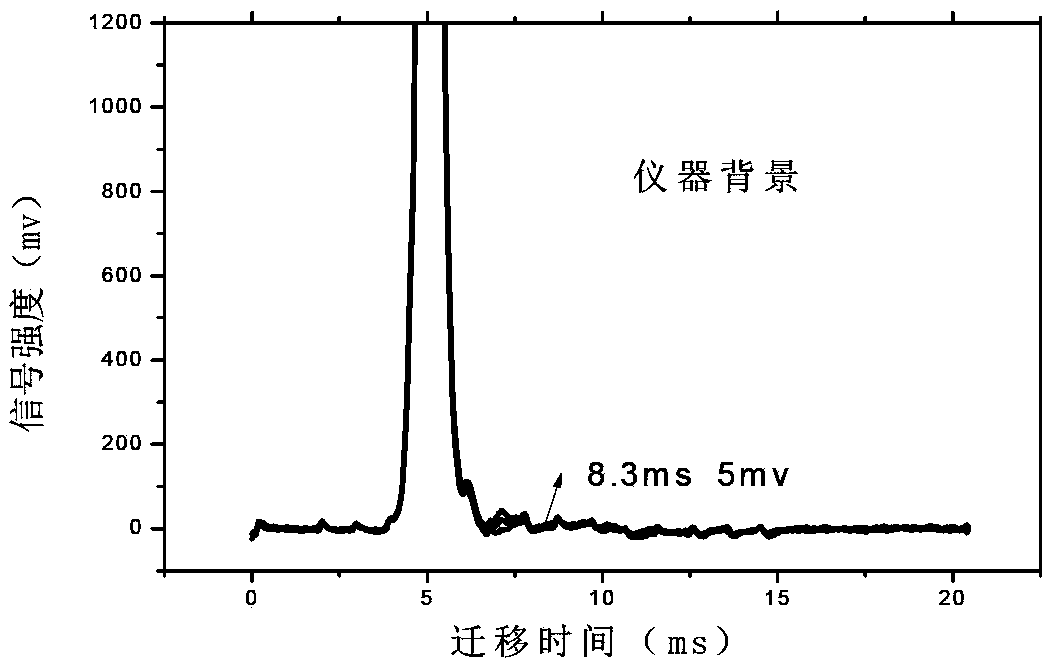
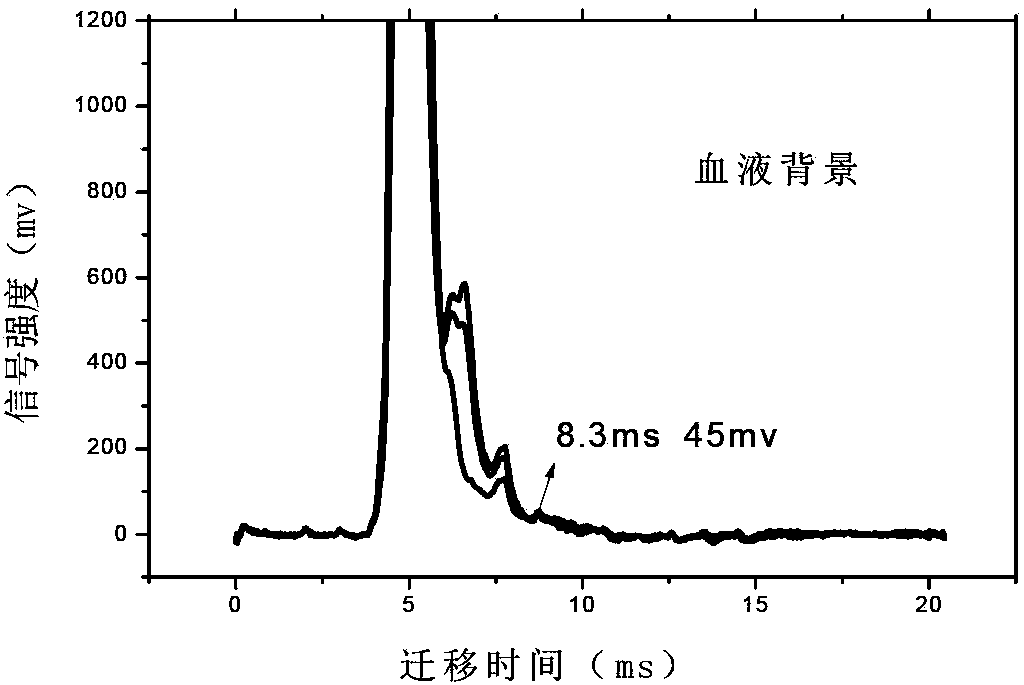
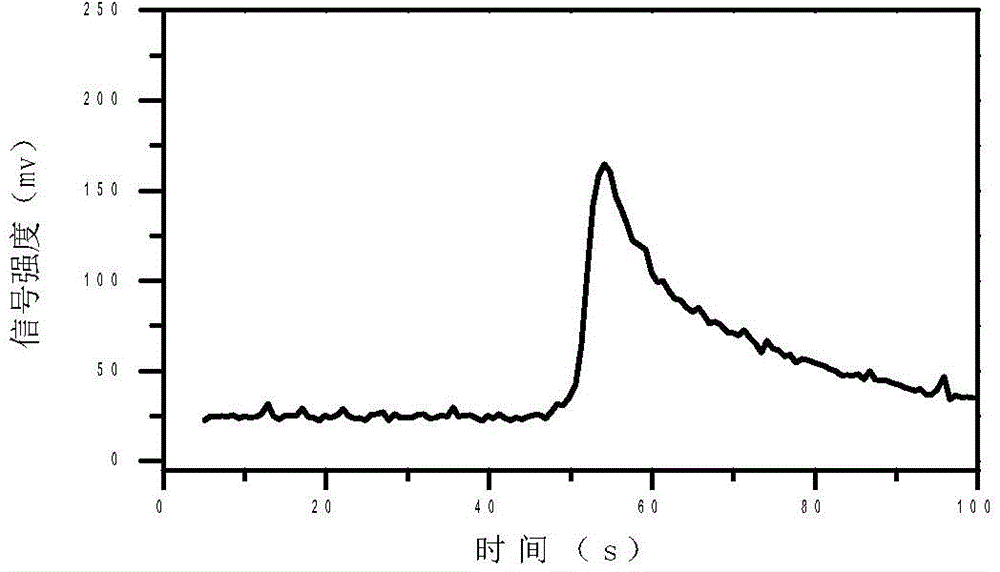
Accurate method for detecting propofol anesthetic in blood
InactiveCN103884771AShorten test timeEasy to carryMaterial analysis by electric/magnetic meansMedicineIon-mobility spectrometry
The invention discloses a rapid sensitive method for detecting a propofol anesthetic in blood. By adopting the ion-mobility spectrometry technology as a basic detection technology and adopting an anion mode, a rapid qualitative and quantitative analysis method for detecting the propofol anesthetic in the blood by ion-mobility spectrometry is established. The limit of detection can reach smaller than 1 ng (a concentration smaller than 0.1 ppm). The range of quantitative analysis concentrations is 0.5-20 ppm, thus meeting the human blood dose concentration analysis range. The analysis time of each sample is shorter than 30 s. No pretreatment technology is needed. The method is simple, rapid, efficient and high in reliability, and can be widely used for clinical-administration deep analysis and used for guiding the medication by a doctor.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Pharmaceutical composition comprising propofol
The invention provides novel pharmaceutical compositions comprising the active ingredient propofol. Preferably, propofol is dissolved in at least one semifluorinated alkane. The compositions, which are preferably liquid or gel-like, may optionally comprise further excipients. They may be used as fill material in capsules, as buccal or nasal sprays, or as aerosols for pulmonary administration. They are particularly useful for the transmucosal administration of propofol.
Owner:NOVALIQ GMBH
Amino acid derived prodrugs of propofol, compositions and uses thereof
The present invention provides propofol prodrugs, methods of making propofol prodrugs, pharmaceutical compositions of propofol prodrugs and methods of using propofol prodrugs and pharmaceutical compositions thereof to treat or prevent diseases or disorders such as migraine headache pain and post-chemotherapy or post-operative surgery nausea and vomiting.
Owner:XENOPORT
Aqueous pharmaceutical compositions of 2,6-diisopropylphenol (propofol) and their uses
InactiveUS7550155B2Simple preparation processEasy to storePowder deliveryHydroxy compound active ingredientsPolyethylene glycolPropofol
The present invention provides aqueous pharmaceutical compositions containing a lipophilic therapeutic agent. In particular, the invention provides aqueous pharmaceutical compositions containing the compound 2,6-diisopropylphenol (propofol). Preferred compositions of the invention contain propofol in the presence of at least one block copolymer (for example, P188 or another poloxamer) and a polyethylene glycol (PEG). Compositions of the invention are preferably sterile or are readily sterilized (e.g., by autoclaving) and are suitable for parenteral administration to any animal, including humans. The compositions are also chemically and physically stable over a wide range of environmental conditions and for extended periods of time.
Owner:JANSSEN BIOTECH INC
Method for monitoring propofol narcotic in on-line manner
InactiveCN102455319AReduce volumeShort analysis timePreparing sample for investigationMaterial analysis by electric/magnetic meansIon-mobility spectrometryAnesthetic
The invention discloses a method for monitoring traced propofol narcotic rapidly and sensitively. In the method, an ion mobility spectrometry technology is taken as a basic detection technology, and an anion mode is adopted to establish a method for monitoring traced propofol narcotic. The method comprises the following steps: feeding a gas sample containing propofol into an ion mobility spectrometry tester for analysis to obtain a detecting signal, and then carrying out qualitation and quantitation. According to the invention, the measurement of a narcotic sample can be detected in real time, and the limit of detection of the propofol narcotic can reach 0.5ppb. The detecting method is simple, convenient, rapid and efficient and is good in reliability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Injectable aqueous dispersions of propofol
InactiveUS7041705B2Improve stabilityIncrease oil volumeBiocideHydroxy compound active ingredientsMean diameterWater insoluble
Irritation upon injection of a formulation containing propofol is reduced or substantially eliminated by administering a stable, sterile, and antimicrobial aqueous dispersion comprising a water-insoluble microdroplet matrix of mean diameter from about 50 nm to about 1000 nm consisting essentially of about 1% to about 15% of propofol, up to about 7% of a propofol-soluble diluent, and about 0.8% to about 4% of a surface stabilizing amphiphilic aent. The aqueous phase includes a pharmaceutically acceptable water-soluble polyhydroxy tonicity modifier. The propofol-containing dispersion is devoid of additional bactericidal or bacteriostatic preservative agents.
Owner:JAGOTEC AG
Methods of monitoring propofol through a supply chain
InactiveUS20120158610A1Improve productivityQuality improvementOther accessoriesContainer/bottle contructionEngineeringDistributor
Methods of monitoring Propofol through a supply chain are disclosed herein. Consequently, the methods perform authentication of propofol as it is transported through the supply chain to the end user whereby propofol manufacturers and distributors can achieve data and product integrity and ultimately minimize cost.
Owner:SMP LOGIC SYST
Watersoluble prodrugs of propofol
InactiveUS20050267169A1Good skin permeabilityEasy to refactorBiocideSugar derivativesWater soluble prodrugMammal
The present invention relates to propofol derivatives comprising a cyclic or linear amino acid, or a poly- or (oligo)saccharide moiety, a process for preparing said derivatives, a method for anesthetizing a mammal as well as a method for treating convulsions, migraine or related diseases, or for the inhibition of free radicals in a mammal to which said compounds are administered. Furthermore, the present invention relates to said compounds for use as a medicament and the use of said compounds for the preparation of a medicament for anesthetizing a mammal or for treating convulsions, migraine or related diseases, or for inhibition of free radicals in a mammal.
Owner:FRESENIUS KABI DEUT GMBH +1
Formula and preparation method of novel propofol fat emulsion preparation causing no pain and low injection stimulation
ActiveCN102085185ADissolve effectively and fullyHydroxy compound active ingredientsAnaestheticsGlycerolSugar
The invention discloses a formula and preparation method of a novel propofol fat emulsion preparation which causes no pain or can be used for obviously lowering injection stimulation and pain. The novel preparation comprises the main constituents of propofol, an emulsifier, refined soybean oil or other refined oil as an oil-soluble diluent, oleic acid or oleate with the effect of assisting emulsification, vitamin E or derivatives thereof with an antioxidation effect, an ionic complexing agent, and glycerol or micro molecular sugar as an isoosmotic adjusting agent.
Owner:XIAN LIBANG PHARMA
Formulations for anaesthetic use
InactiveUS20050004234A1Risk can be causedRisk of causing anaphylactoid reactions in susceptible individuals isBiocideHydroxy compound active ingredientsLipid formationSedation
A formulation for anaesthetic use is described. The formulation contains propofol, and may be used to induce and / or maintain anaesthesia or sedation in a vertebrae. The formulation additionally contains a solvent or a combination of solvents and is suitable for mixing with commonly used infusion fluids prior to injection in to a patient. The formulation may be terminally sterilised using moist heat in order to assure sterility, and contains no lipid, thereby avoiding complications associated with administration over prolonged periods of time, or to patients with disorders of fat metabolism.
Owner:PARNELL TECH
Prodrugs of propofol, compositions and uses thereof
The present invention provides propofol prodrugs, methods of making propofol prodrugs, pharmaceutical compositions of propofol prodrugs and methods of using propofol prodrugs and pharmaceutical compositions thereof to treat or prevent diseases or disorders such as migraine headache pain and post-chemotherapy or post-operative surgery nausea and vomiting.
Owner:XENOPORT
Transmucosal administration of meloxicam compositions for treating and preventing disorders in non-human domesticated animals
The invention includes compositions for transmucosal administration to an animal comprising at least one active agent and a pharmaceutically acceptable carrier. A preferred active agent is selected from the group consisting of meloxicam, carprofen, enrofloxacin, clemastine, diphenhydramine, digoxin, levothyroxine, cyclosporine, ondansetron, lysine, zolpidem, propofol, nitenpyram, ivermectin, milbemycin, and pharmaceutically acceptable salts, solvates and esters thereof. In another embodiment, the invention includes methods of treating or preventing a condition in an animal comprising transmucosally administering a composition comprising a therapeutically or prophylactically effective amount of an active agent and a pharmaceutically acceptable carrier.
Owner:ZOTTIS BELGIUM
Propofol medium-long-chain fatty emulsion
ActiveCN102366404AImprove stabilityImprove securityHydroxy compound active ingredientsAnaestheticsGlycerolMedium-chain triglyceride
The invention discloses a propofol medium-long-chain fatty emulsion, which is made from the following components of: by weight, 10-20 parts of propofol, 50-100 parts of soybean oil, 50-100 parts of medium-chain triglyceride, 20-25 parts of glycerin, 6-12 parts of phosphatidylcholine bilayer, 0.02-0.05 part of sodium hydroxide, 0.3-0.6 part of oleic acid and injection water.
Owner:四川国瑞药业有限责任公司
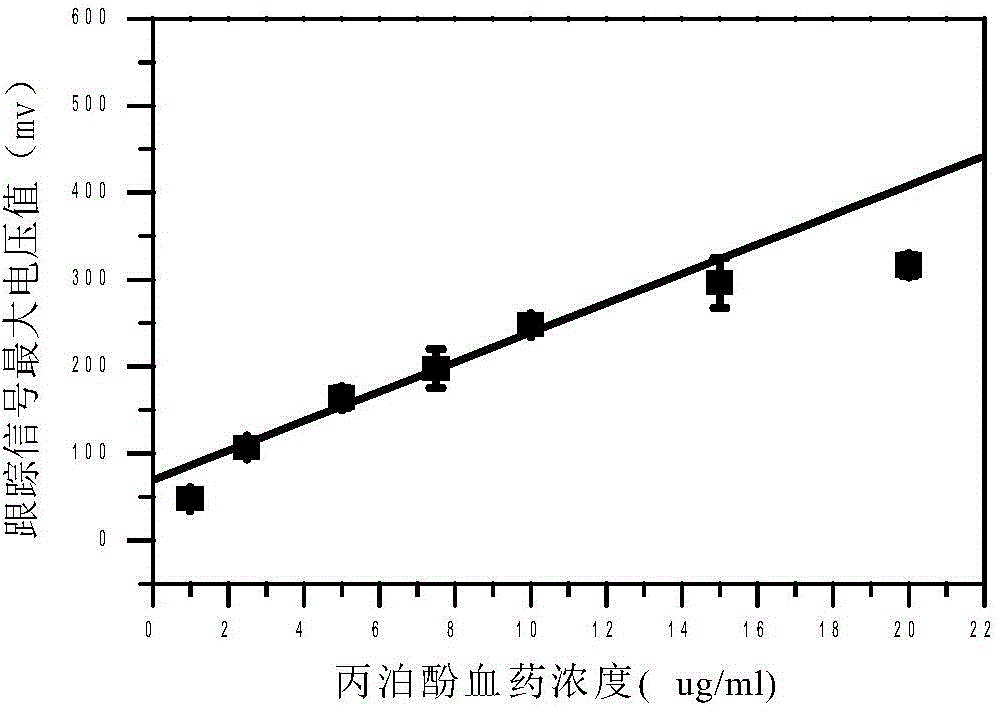
Quantitative analysis method for ion mobility spectrum
ActiveCN106198704AGood repeatabilityImprove accuracyMaterial analysis by electric/magnetic meansCorrelation coefficientQualitative analysis
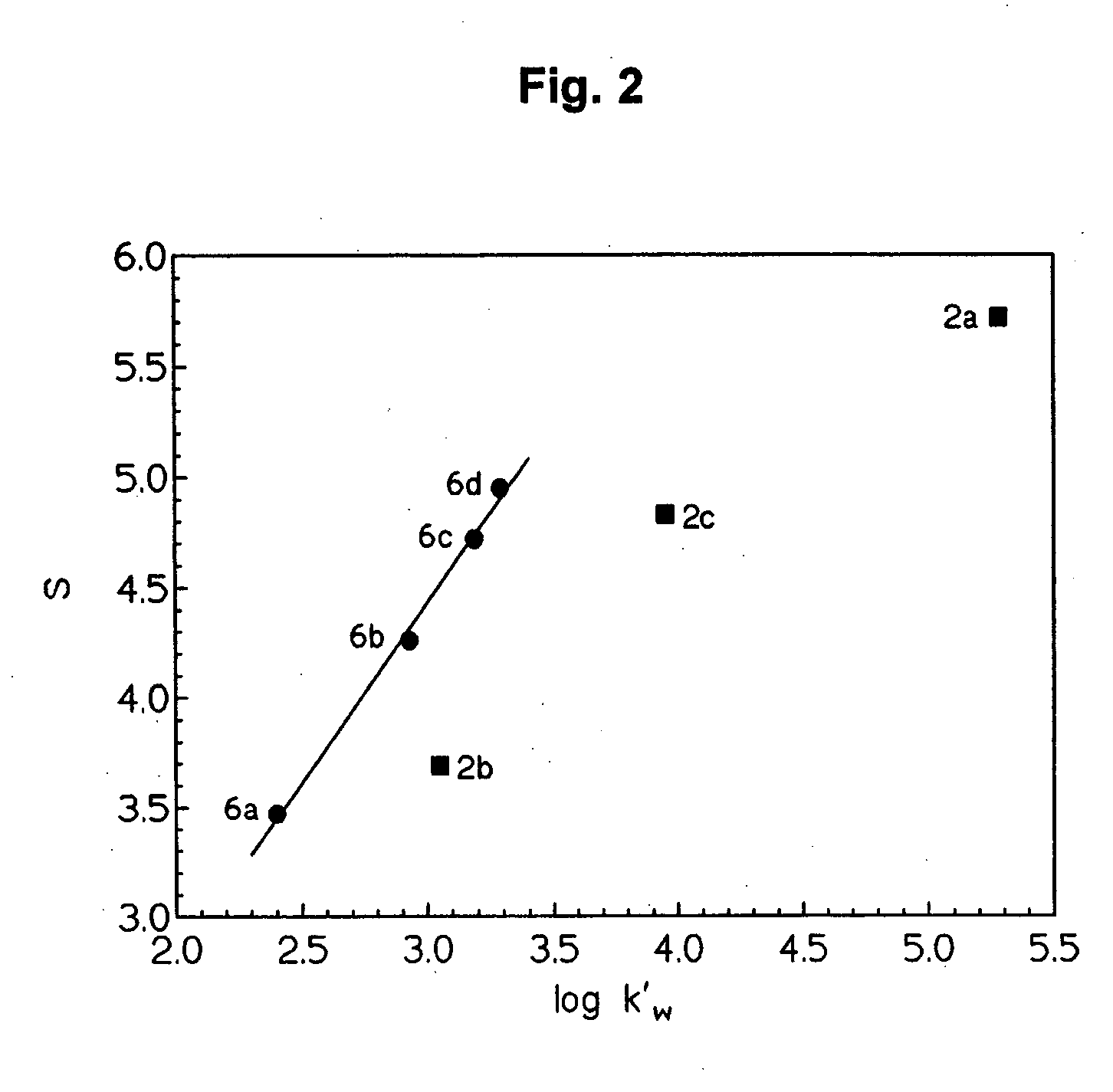
The present invention discloses an accurate quantitative analysis method for an ion mobility spectrum rapid detection sample. According to the method, based on the migration time obtained through qualitative analysis of an ion mobility spectrum, the thermal analysis process of the whole sample to be detected is completely recorded, and the recorded curve is defined as the tracking trend line of the thermal analysis of the sample; the samples with different concentrations are prepared by adopting the ion mobility spectrum technology as the base detection technology, and the tracking trend of the sample is subjected to data analysis; and by adopting the propofol in blood as an example, a standard curve equation y=671.48+934.42.x of the ion mobility spectrum detection is established between the plasma concentration of 1-20 ppm, wherein the correlation coefficient r is 0.9928. According to the present invention, the method is simple, rapid and efficient, and can be widely used for the quantitative analysis of the ion mobility spectrum rapid detection sample.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Novel method for preparing high-purity propofol
ActiveCN102603488AMild reaction conditionsLow costOrganic chemistryOrganic compound preparationPhenolImpurity
The invention provides a synthetic method for preparing a high-purity anesthetic, i.e., propofol (I). According to the method, the problem of difficulty in removing a large quantity of impurities with similar structures produced in the conventional preparation method is solved, and the emission of phenol pollutants is reduced.
Owner:云南龙海天然植物药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com