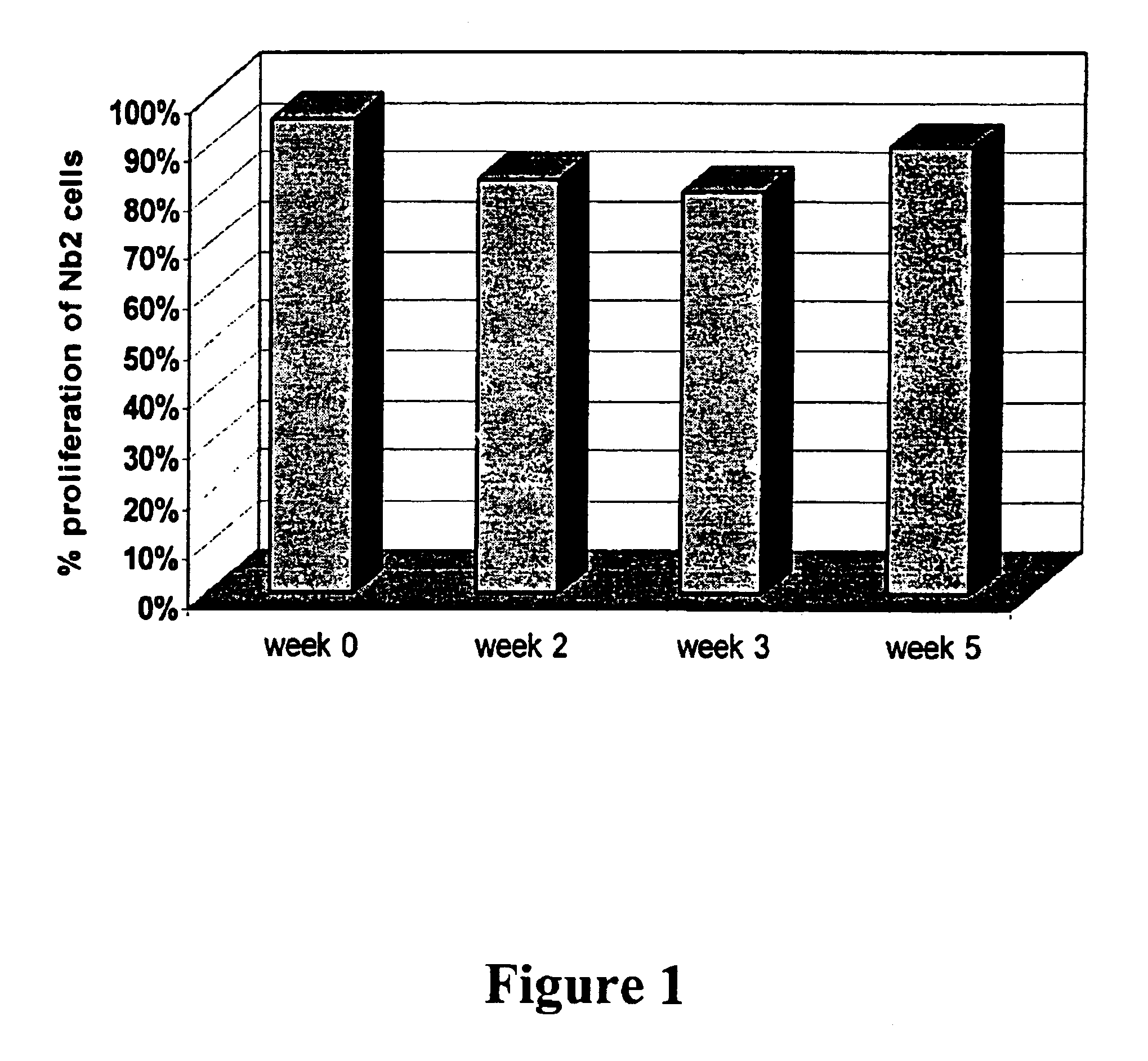
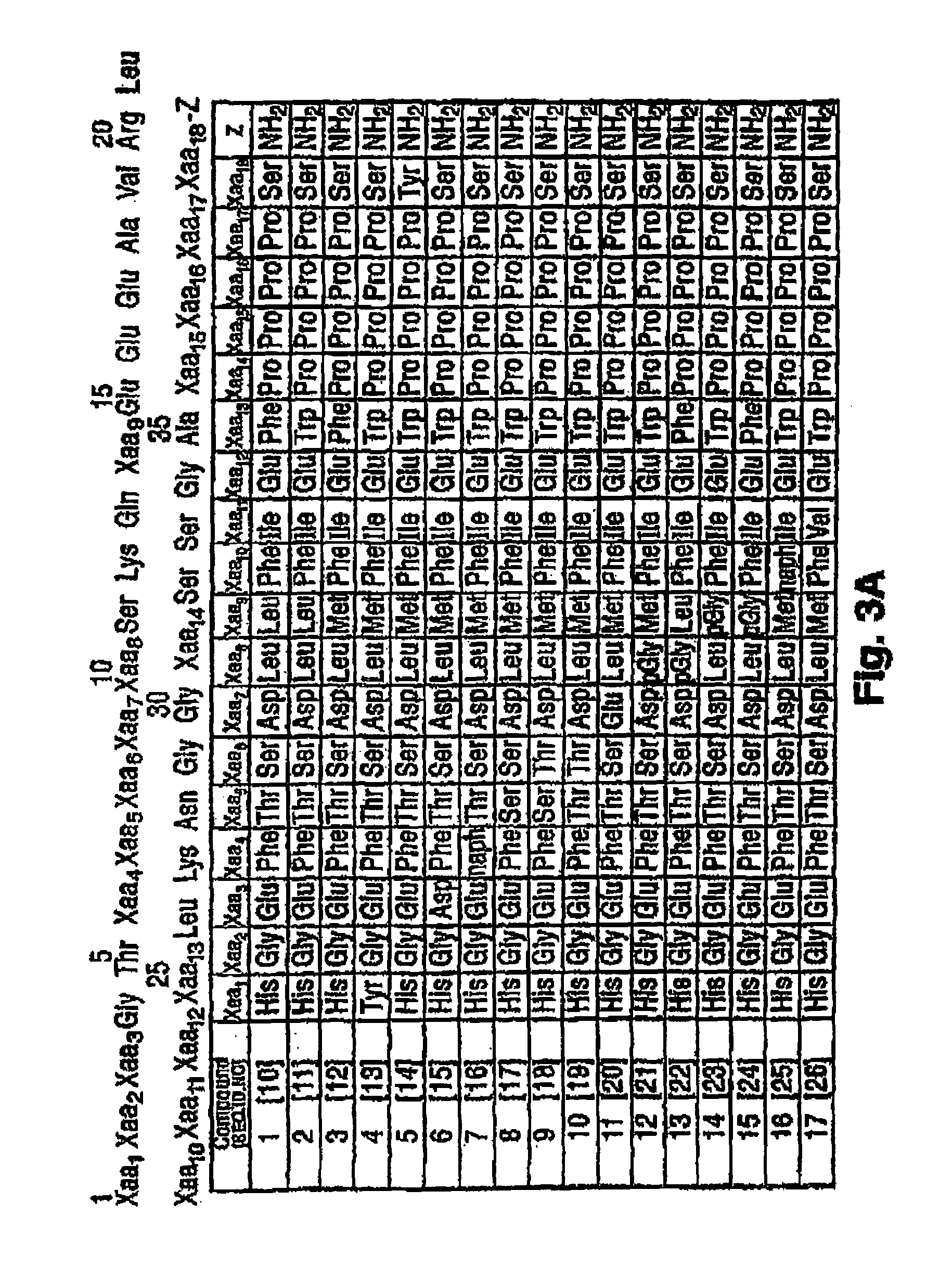
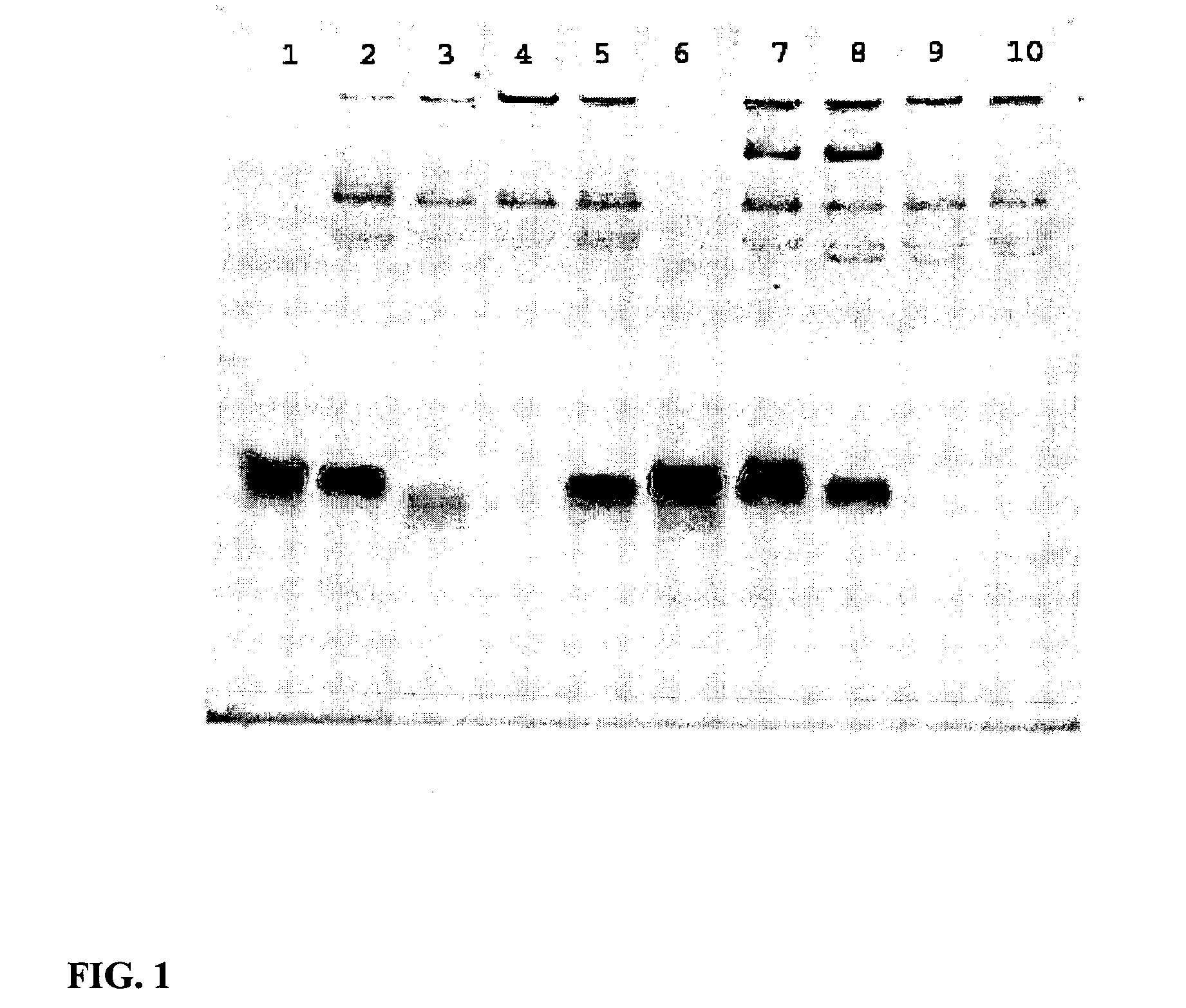
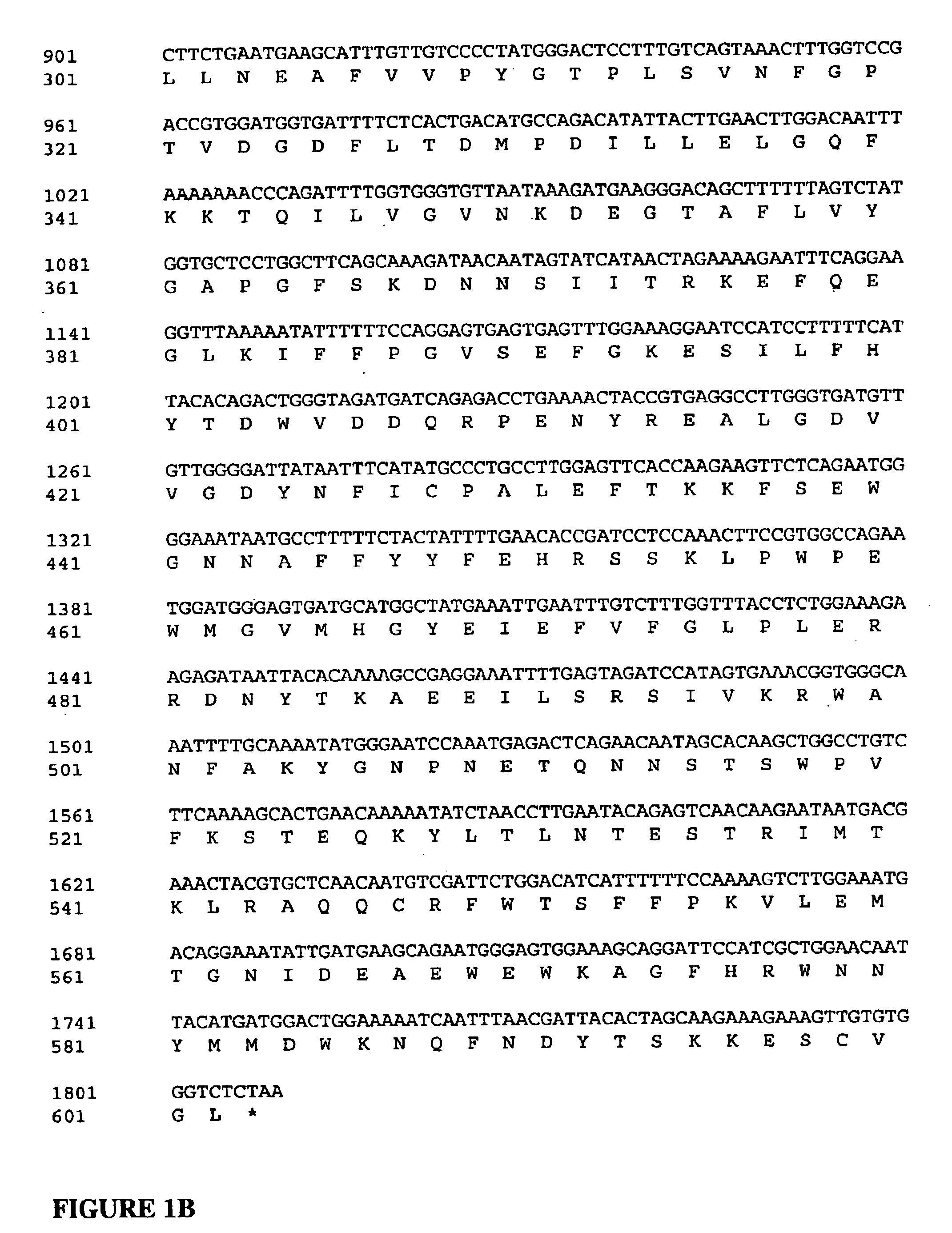
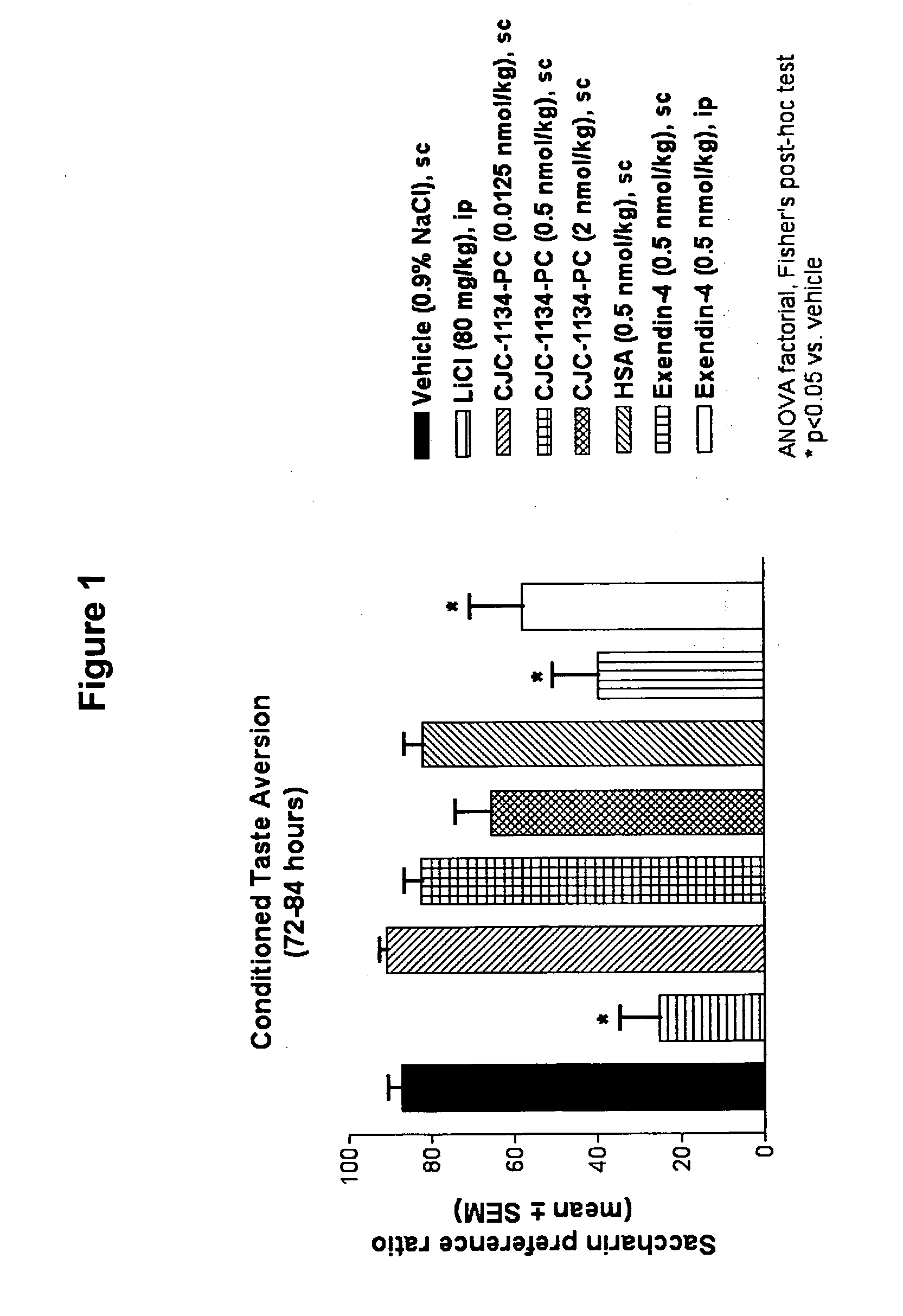
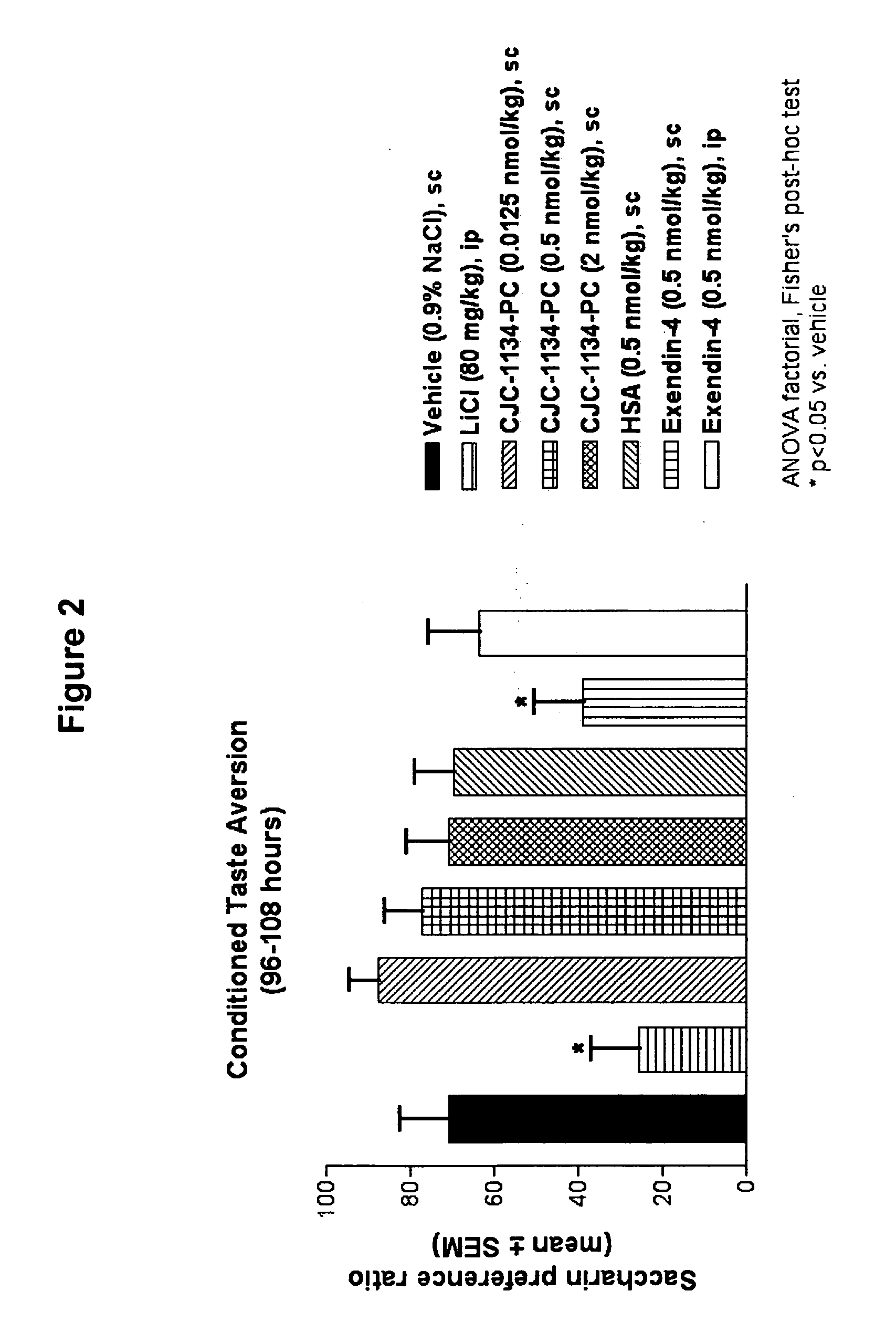
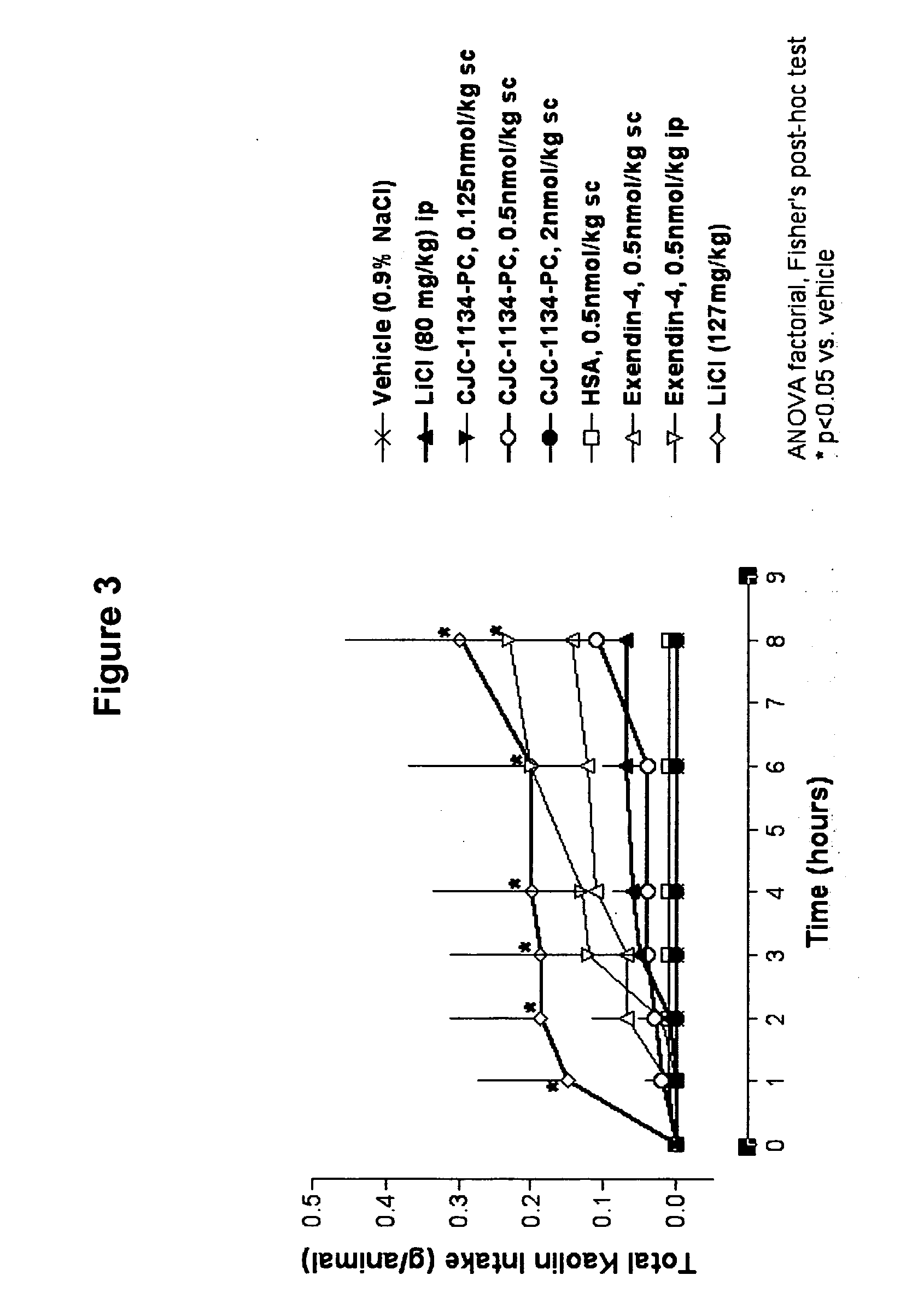
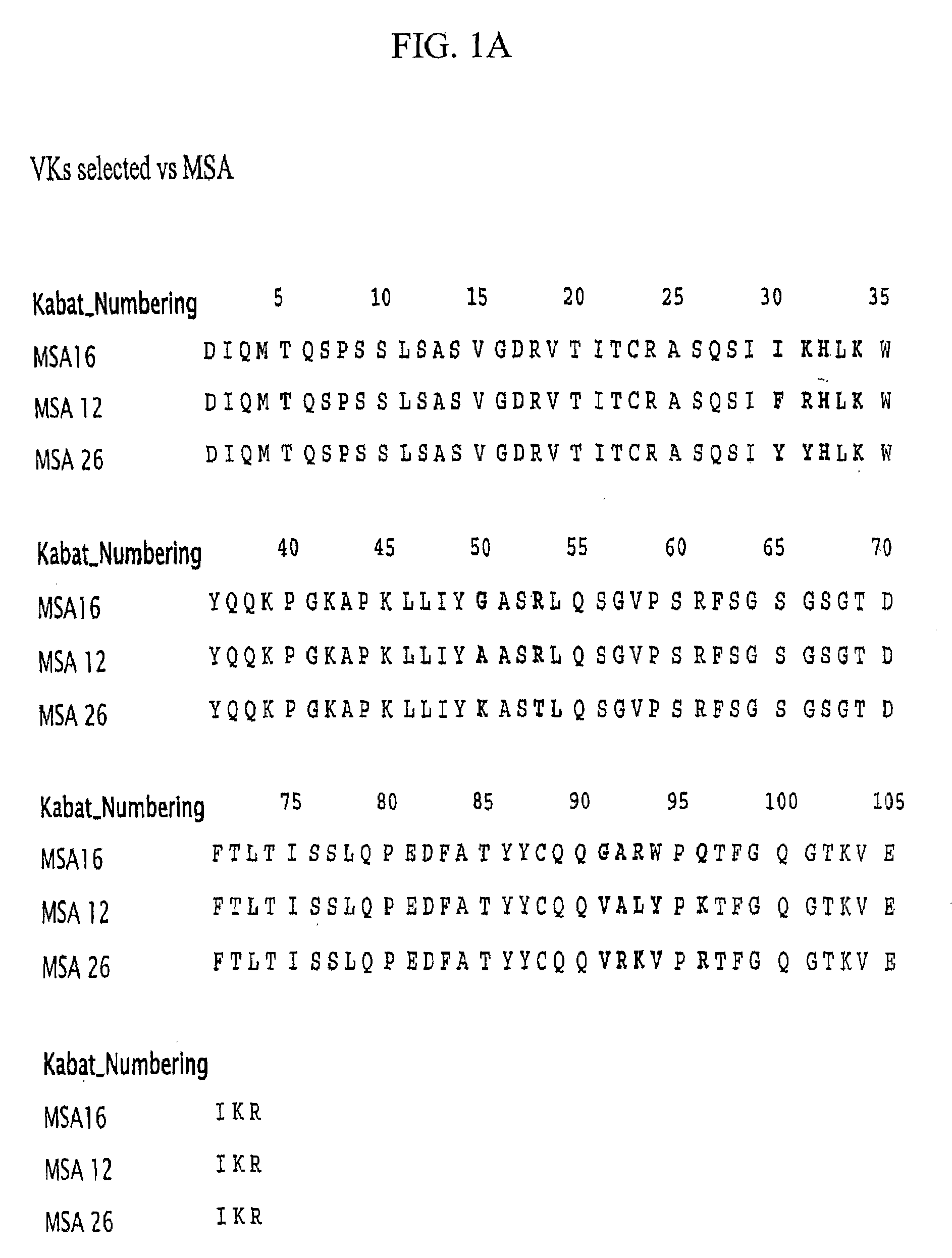
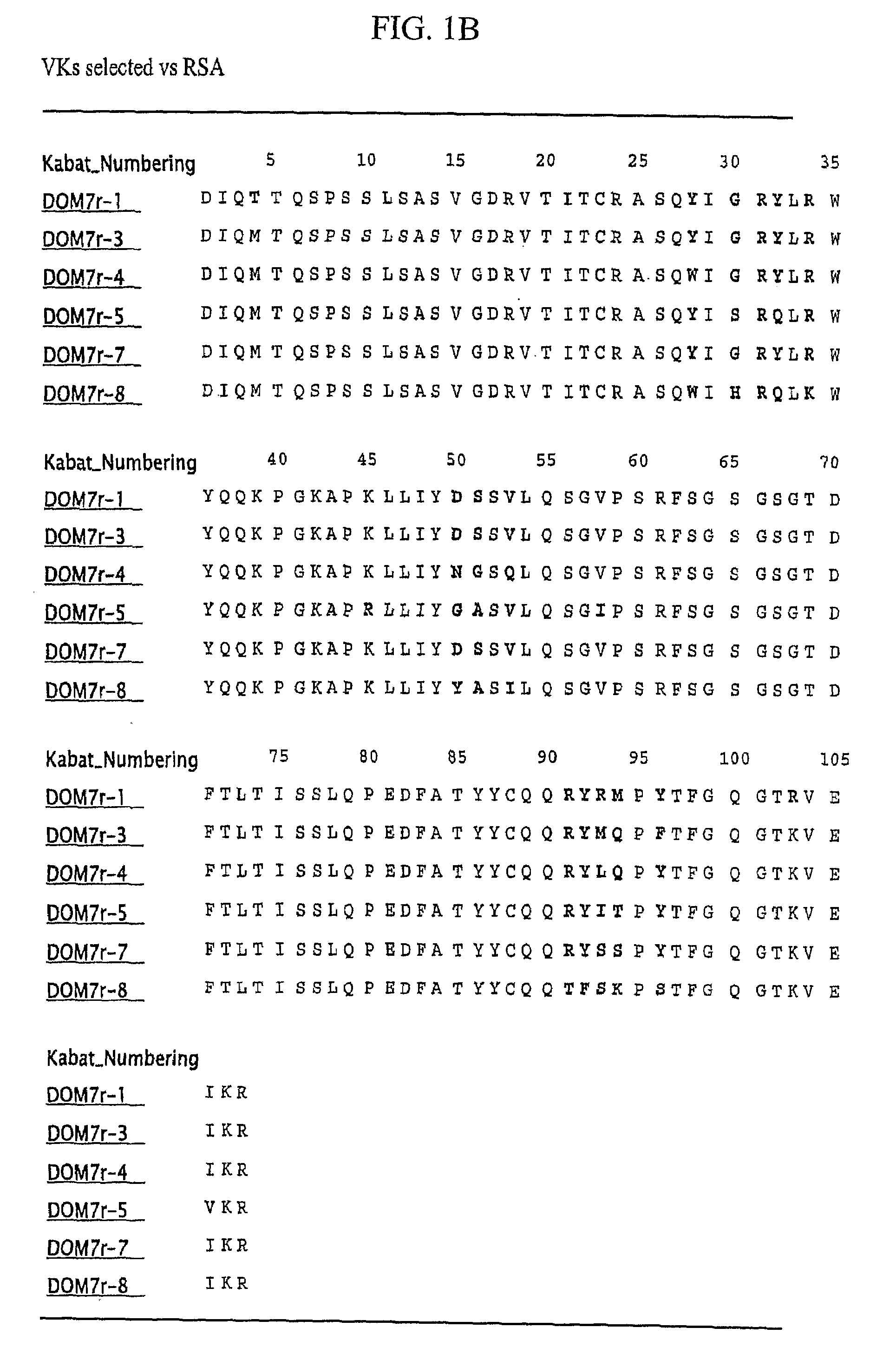
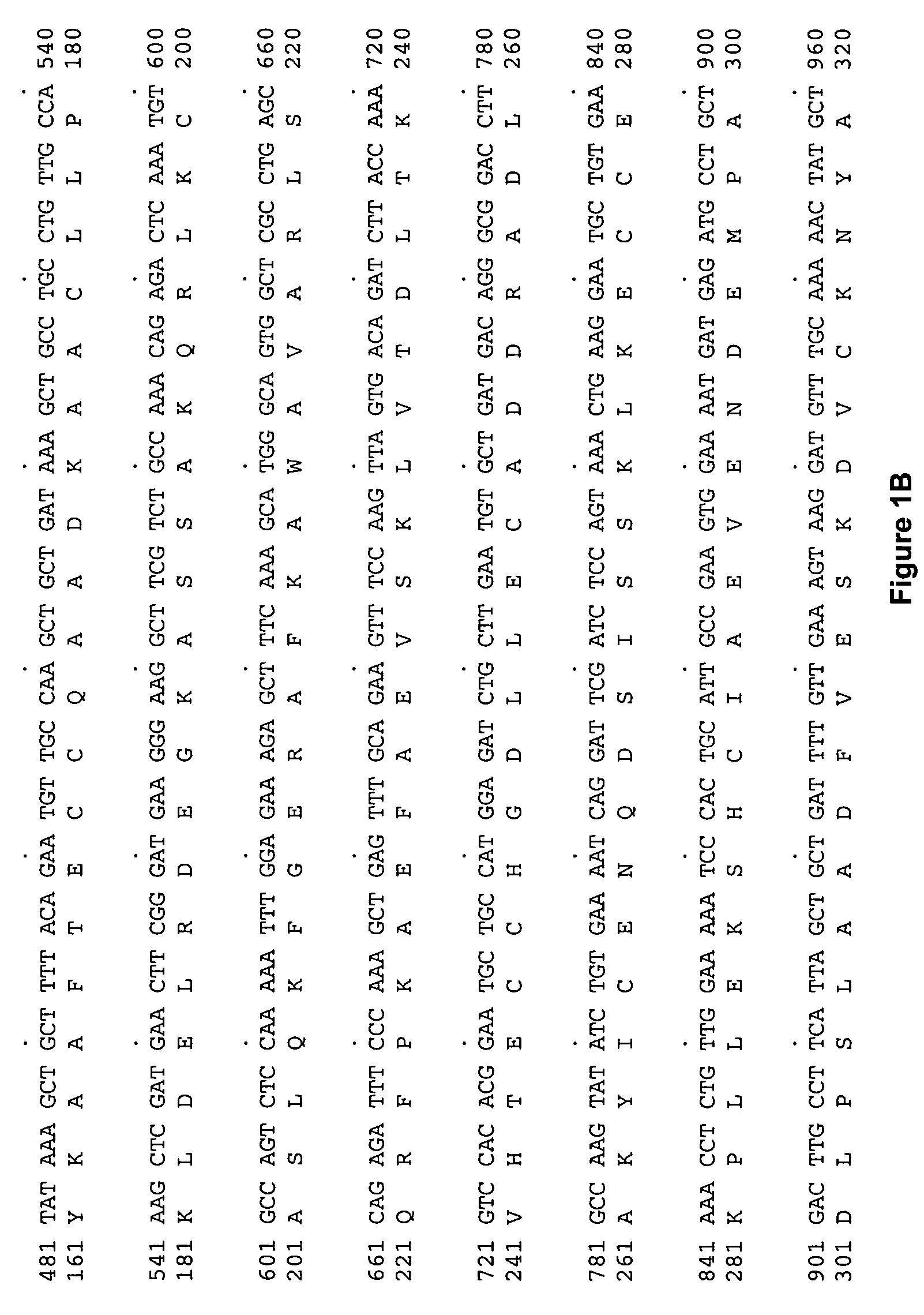
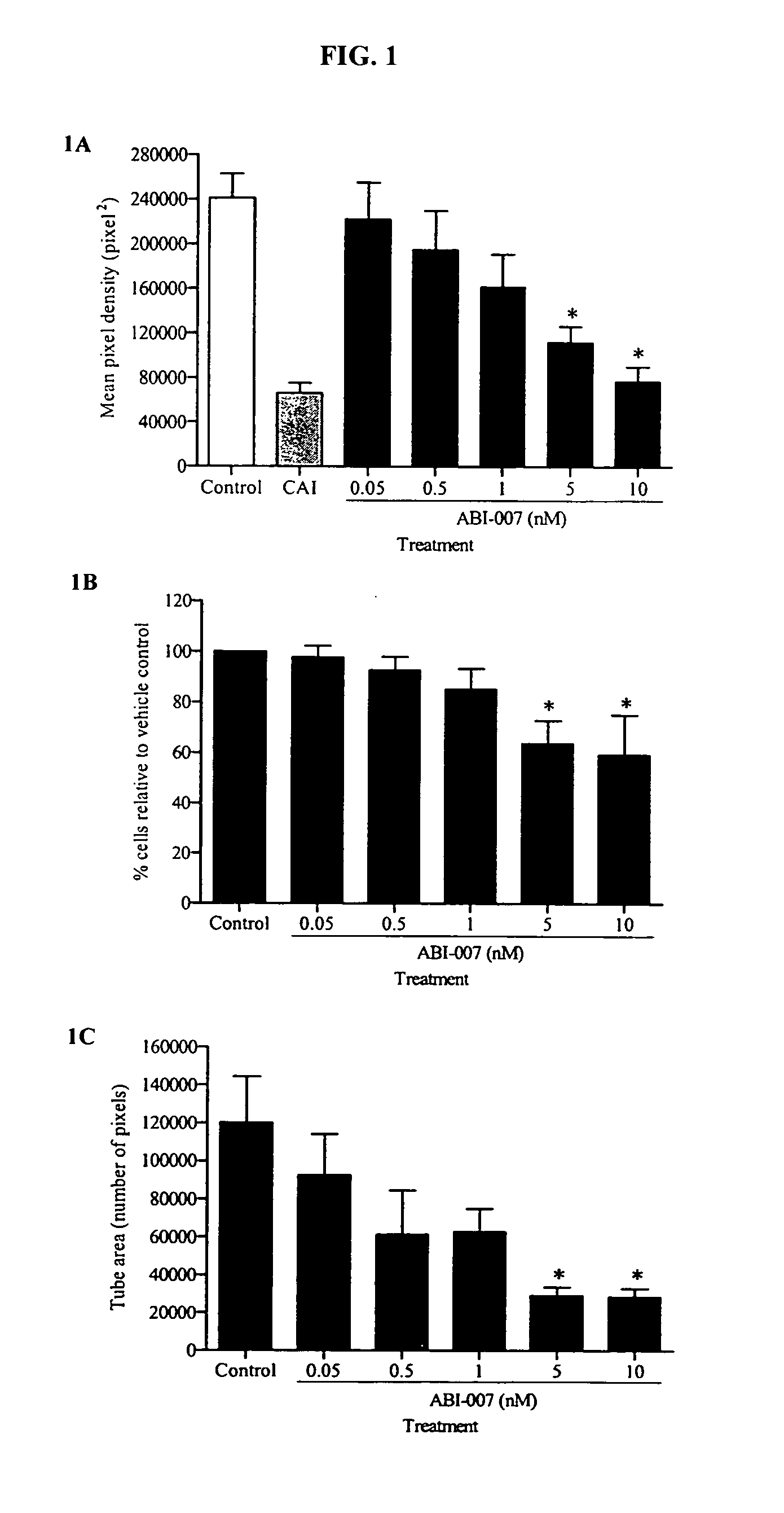
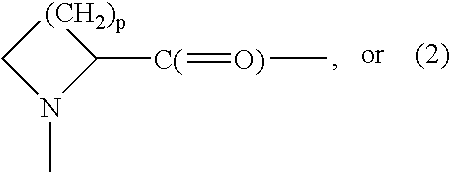Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3897 results about "Albumin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Albumin is a family of globular proteins, the most common of which are the serum albumins. All the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins, such as egg white, are called albuminoids.
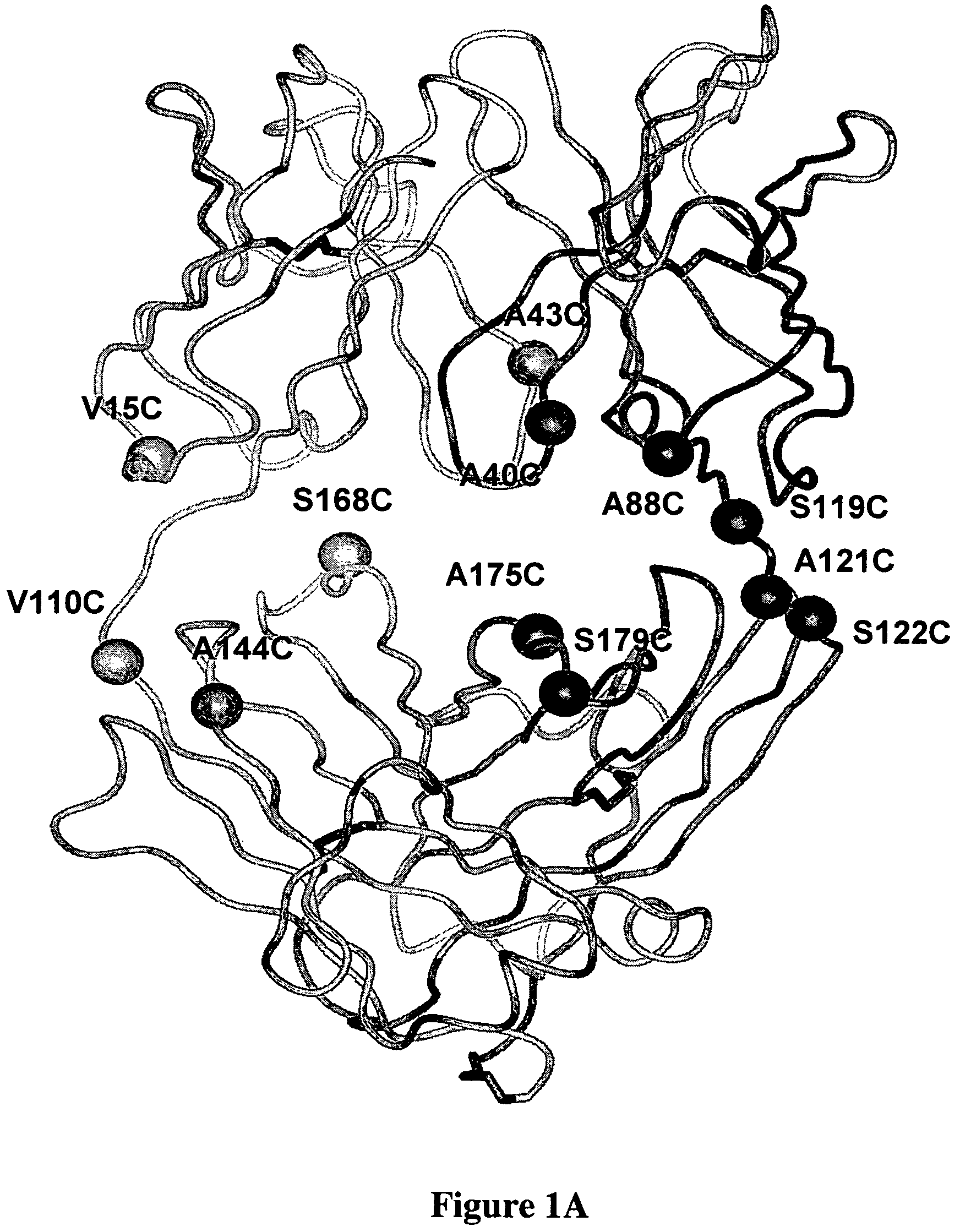
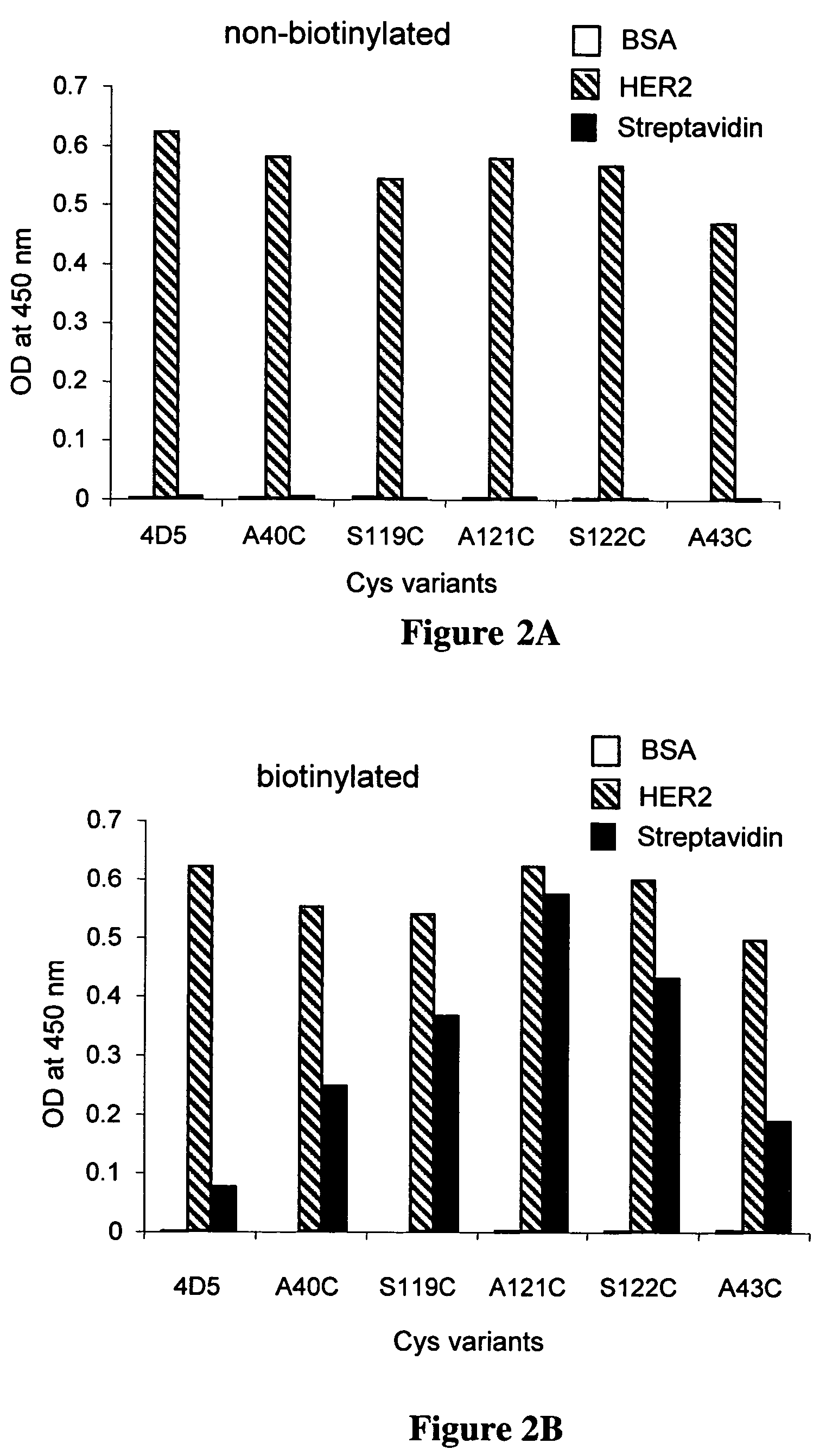
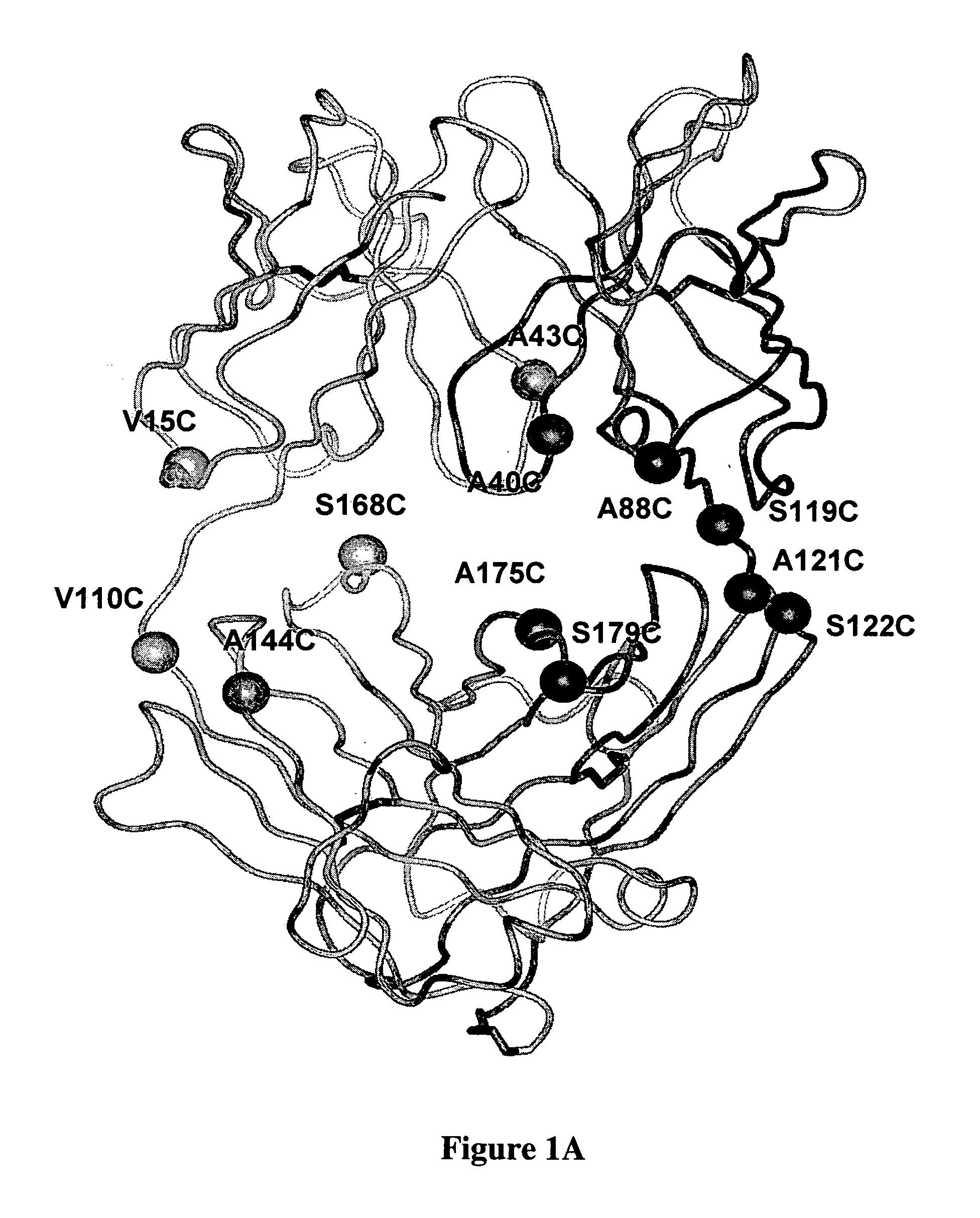
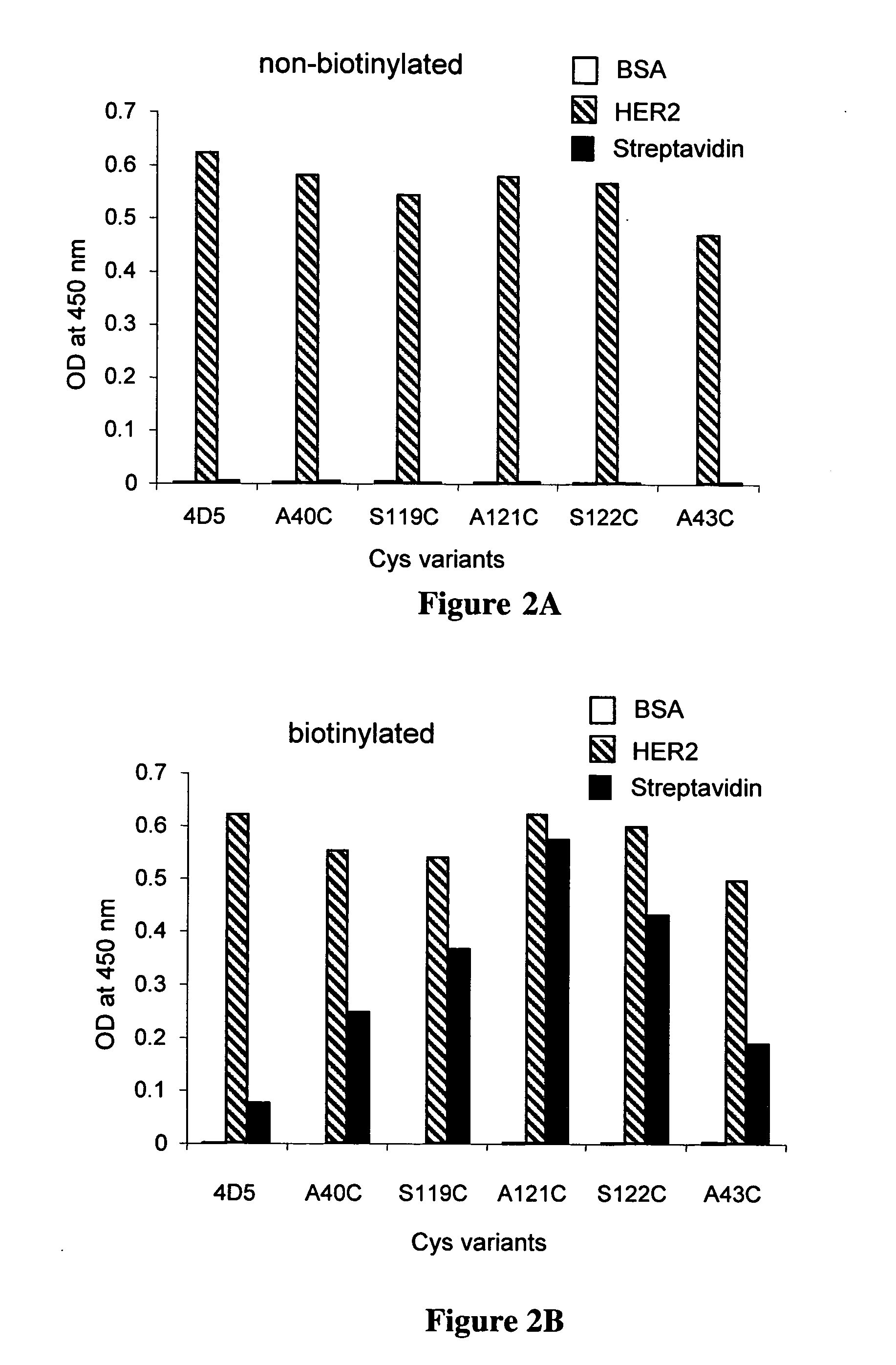
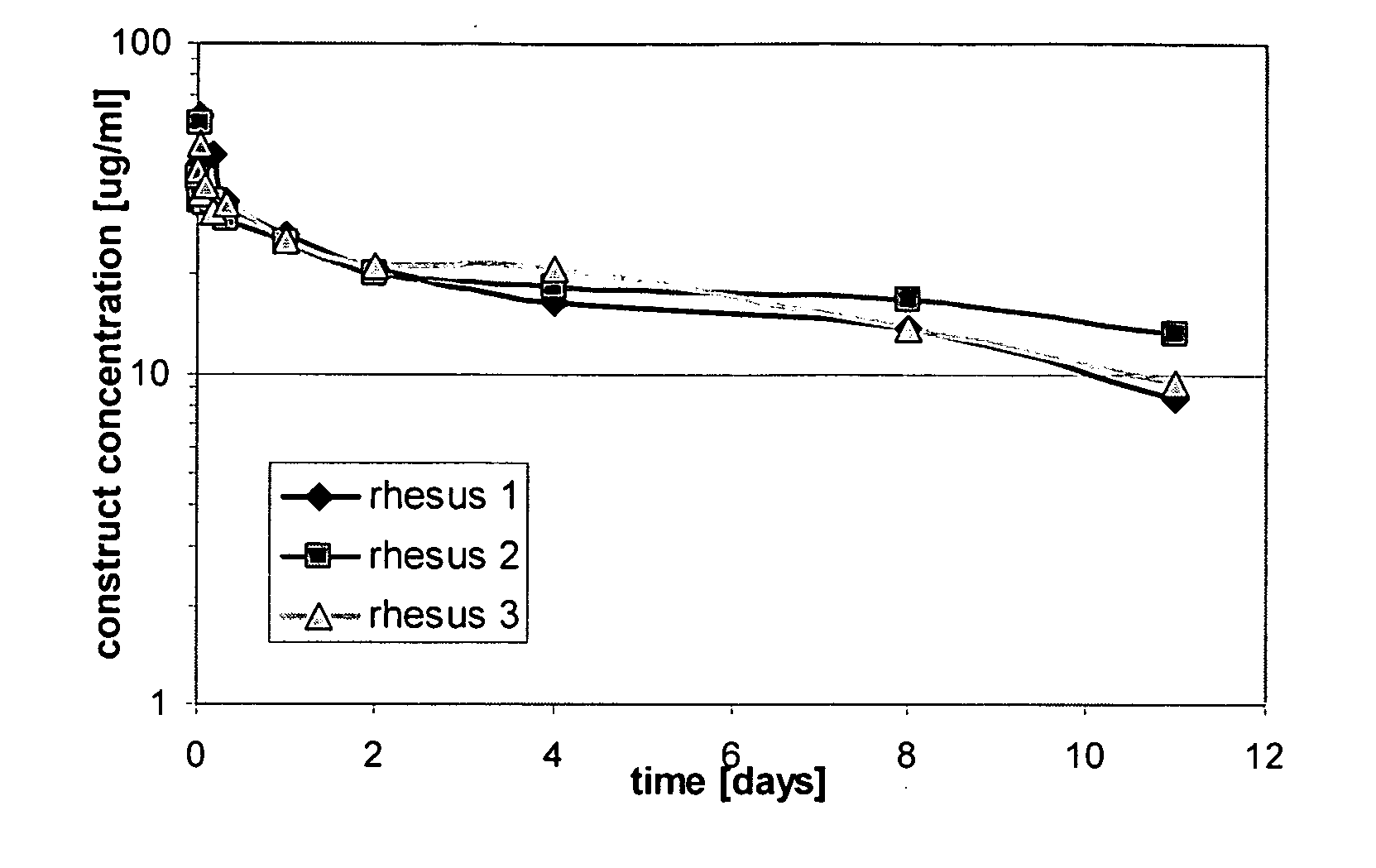
Cysteine engineered antibodies and conjugates
Owner:GENENTECH INC
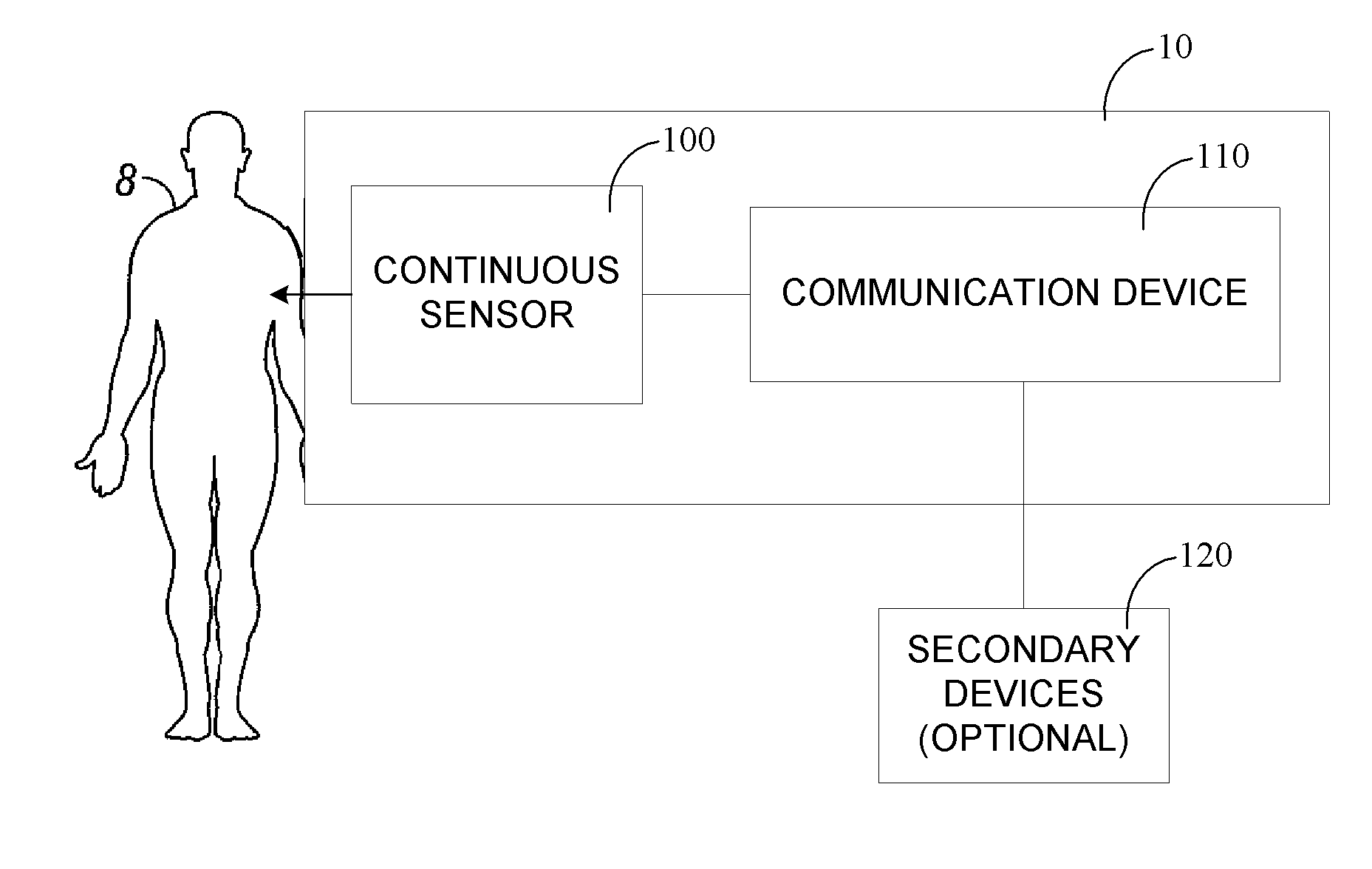
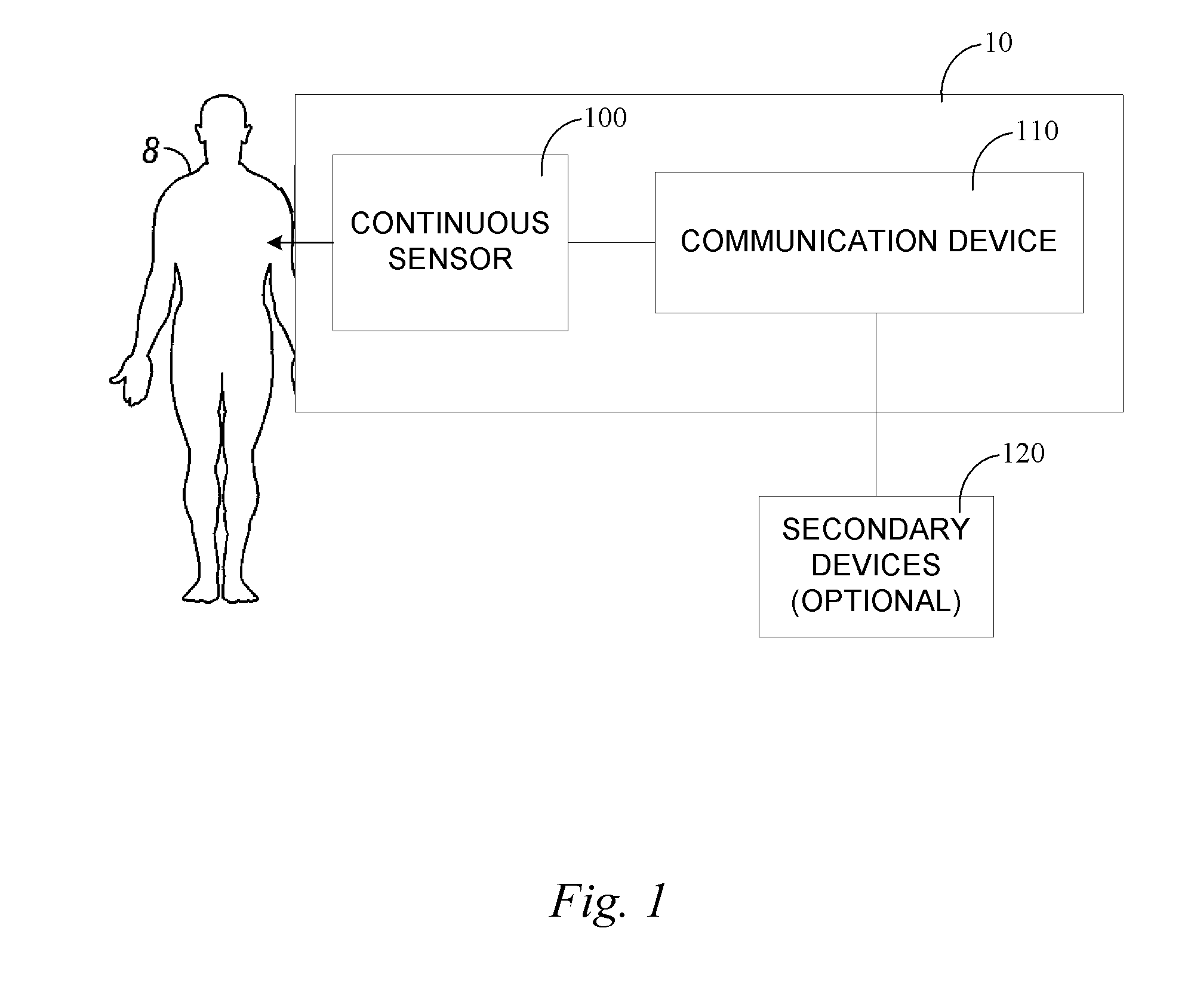
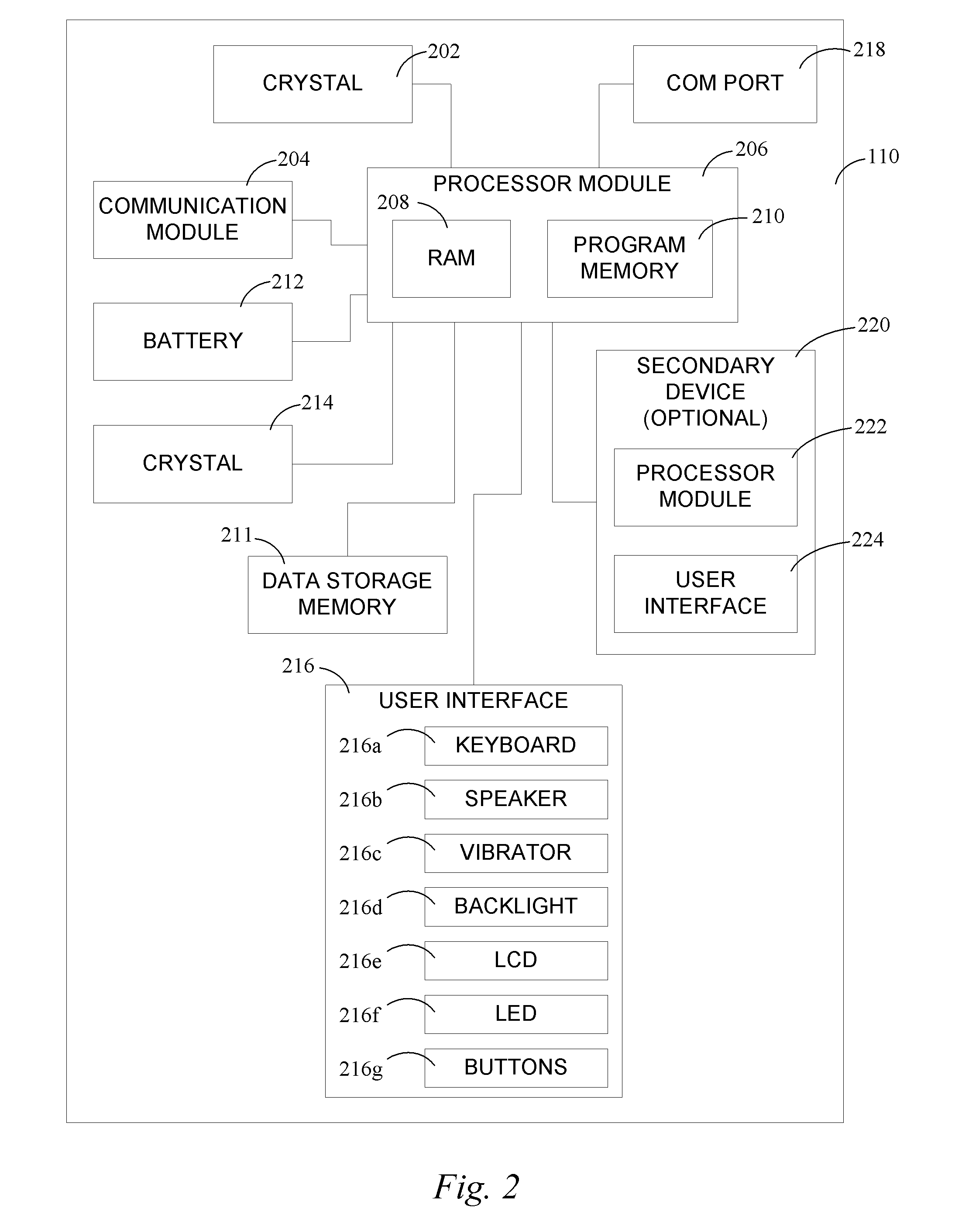
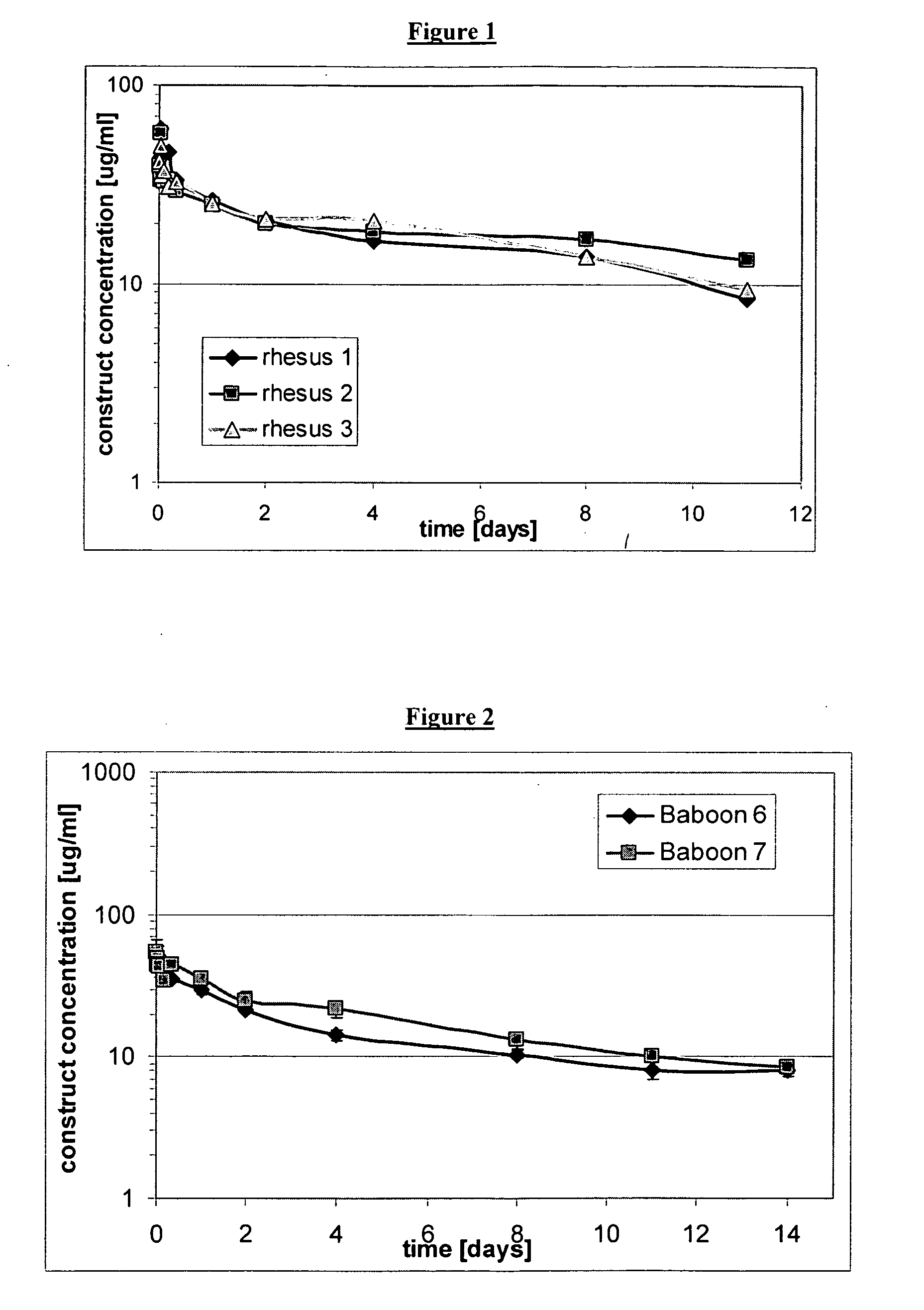
Continuous medicament sensor system for in vivo use
Systems and methods for continuous measurement of a medicament in vivo are provided. In some embodiments, the system is configured to provide information associated with medicament titration and includes a continuous analyte sensor and a communication device. In some embodiments, the system is configured for continuous ambulatory drug testing, including an ambulatory host monitor having a continuous sensor, a location module, a processor module and a transmitter. In some embodiments, the system is configured for continuously monitoring a hormone level and includes a continuous hormone sensor and a communication device configured to output hormone information in real time. Yet another embodiment provides an analyte sensor for continuous monitoring of a host's nutritional status, and is configured for both continuous glucose detection and continuous albumin detection.
Owner:DEXCOM
Cysteine engineered antibodies and conjugates
ActiveUS20070092940A1Sugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsCross-linkCysteine thiolate
Antibodies are engineered by replacing one or more amino acids of a parent antibody with non cross-linked, highly reactive cysteine amino acids. Antibody fragments may also be engineered with one or more cysteine amino acids to form cysteine engineered antibody fragments (ThioFab). Methods of design, preparation, screening, and selection of the cysteine engineered antibodies are provided. Cysteine engineered antibodies (Ab), optionally with an albumin-binding peptide (ABP) sequence, are conjugated with one or more drug moieties (D) through a linker (L) to form cysteine engineered antibody-drug conjugates having Formula I: Ab-(L-D)p I where p is 1 to 4. Diagnostic and therapeutic uses for cysteine engineered antibody drug compounds and compositions are disclosed.
Owner:GENENTECH INC
Controlled delivery of therapeutic agents by insertable medical devices
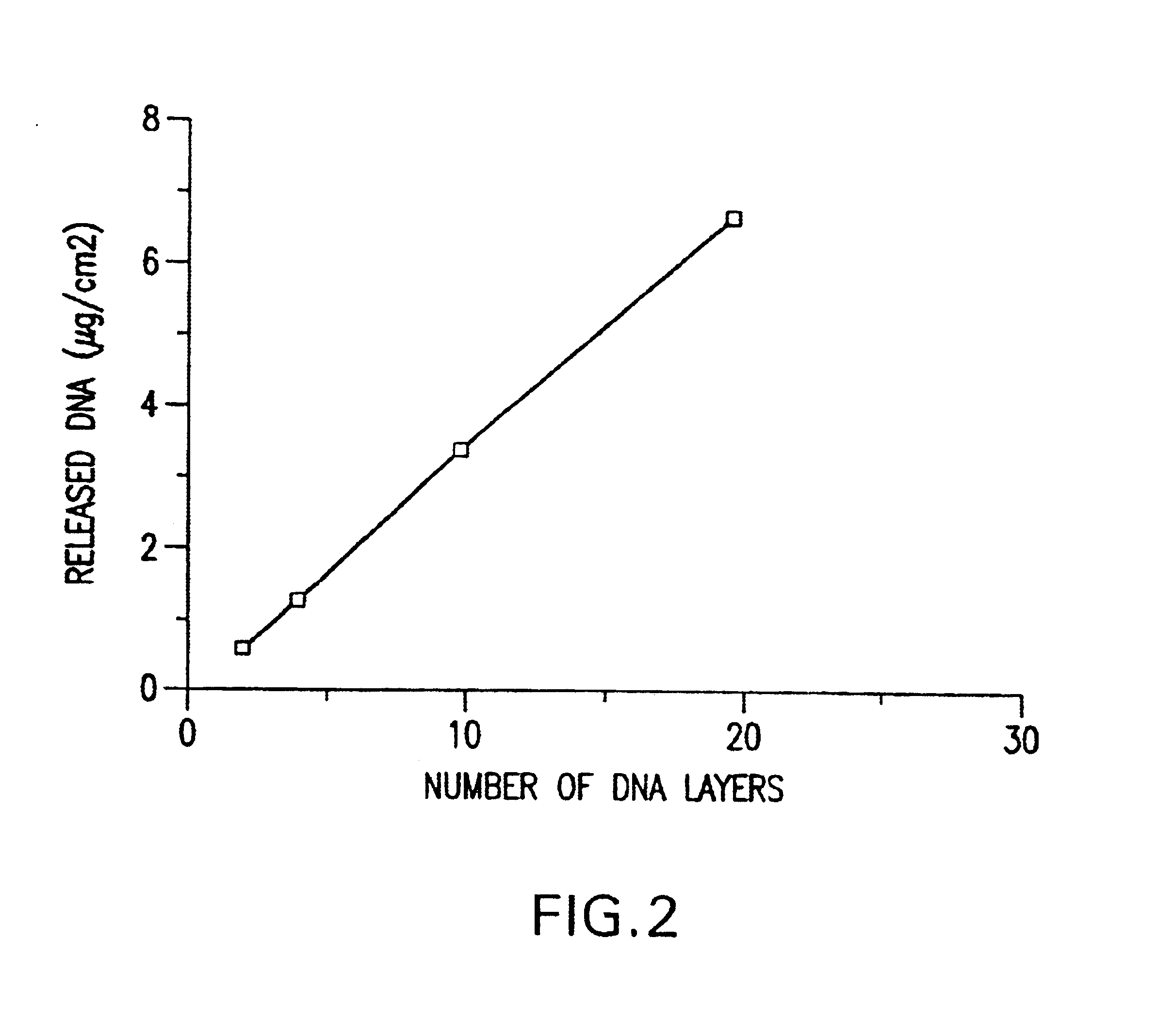
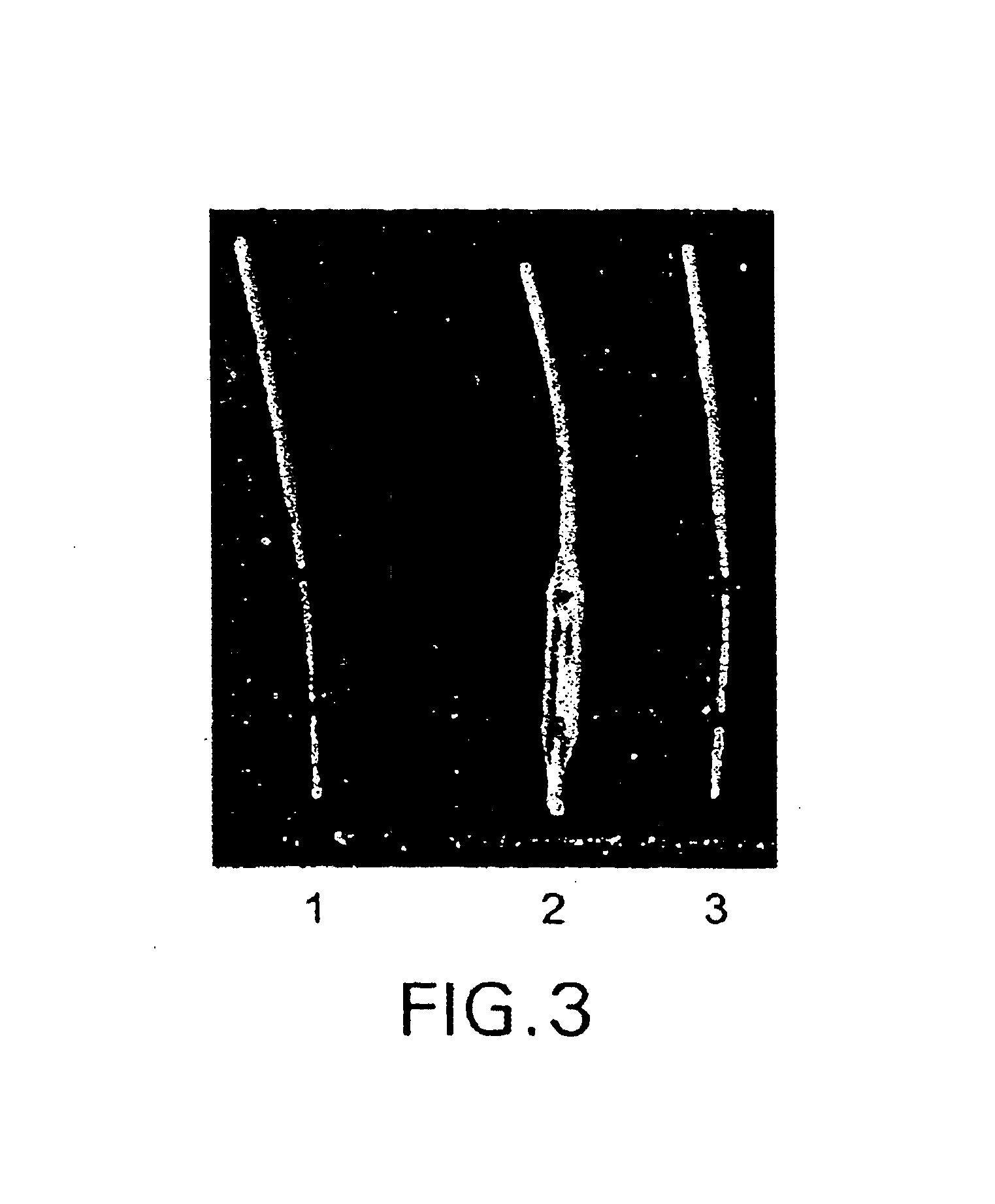
A medical device and method for transportation and release of a therapeutic agent into a mammalian body are disclosed. The medical device is coated with alternating layers of a negatively charged therapeutic agent and a cationic polyelectrolyte, following a controlled adsorption technique. The method is simple, with minimal perturbation to the therapeutic agent and uses clinically acceptable biopolymers such as human serum albumin. The amount of the therapeutic agent that can be delivered by this technique is optimized by the number of the layers of the therapeutic agent adsorbed on the surface of medical device. There is a washing step between alternate layers of the therapeutic agent and cationic polyelectrolyte carrier, so that the amount of the therapeutic agent on the insertable medical device represents the portion that is stably entrapped and adsorbed on to the medical device. The insertable medical device and method according to this invention are capable of reproducibly delivering therapeutic agent to a site in a mammalian body, and allow for a highly reproducible and controllable release kinetics of the therapeutic agent.
Owner:SCI MED LIFE SYST
Serum albumin binding peptides for tumor targeting
InactiveUS20050287153A1Altered pharmacodynamicsFacilitated DiffusionImmunoglobulins against blood coagulation factorsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPeptide ligand
Peptide ligands having affinity for serum albumin are useful for tumor targeting. Conjugate molecules comprising a serum albumin binding peptide fused to a biologically active molecule demonstrate modified pharmacokinetic properties as compared with the biologically active molecule alone, including tissue (e.g., tumor) uptake, infiltration, and diffusion.
Owner:GENENTECH INC
Albumin fusion proteins
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disordrs or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Cultivation of primate embryonic stem cells
InactiveUS20050148070A1Improve cloning efficiencyAvoid variabilityOrganic active ingredientsCulture processMammalFeeder Layer
The invention relates to methods for culturing human embryonic stem cells by culturing the stem cells in an environment essentially free of mammalian fetal serum and in a stem cell culture medium including amino acids, vitamins, salts, minerals, transferring, insulin, albumin, and a fibroblast growth factor that is supplied from a source other than just a feeder layer the medium. Also disclosed are compositions capable of supporting the culture and proliferation of human embryonic stem cells without the need for feeder cells or for exposure of the medium to feeder cells.
Owner:WISCONSIN ALUMNI RES FOUND
Albumin fusion proteins
InactiveUS20070048282A1Extended shelf lifeImprove stabilityAntibacterial agentsPeptide/protein ingredientsDiseaseAlbumin
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disorders or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Serum albumin binding proteins with long half-lives
InactiveUS20070269422A1Increased serum half-lifeReduce dosing frequencyOrganic active ingredientsPeptide/protein ingredientsSerum igePrimate
The present invention relates to amino acid sequences that are capable of binding to serum albumin; to compounds, proteins and polypeptides comprising or essentially consisting of such amino acid sequences; to nucleic acids that encode such amino acid sequences, proteins or polypeptides; to compositions, and in particular pharmaceutical compositions, that comprise such amino acid sequences, proteins and polypeptides; and to uses of such amino acid sequences, proteins and polypeptides. Particularly, the amino acid sequences and compounds of the present invention bind to or otherwise associate with serum albumin in such a way that, when the amino acid sequence or compound is bound to or otherwise associated with a serum albumin molecule in a primate, it exhibits a serum half-life of at least 50% of the natural half-life of serum albumin in said primate.
Owner:ABLYNX NV
Cultivation of primate embryonic stem cells
InactiveUS20050244962A1Maintain normalMaintaining the karyotype of the stem cellsCulture processArtificial cell constructsFeeder LayerStem cell culture
The invention relates to methods for culturing human embryonic stem cells by culturing the stem cells in an environment essentially free of mammalian fetal serum and in a stem cell culture medium including amino acids, vitamins, salts, minerals, transferring, insulin, albumin, and a fibroblast growth factor that is supplied from a source other than just a feeder layer the medium. Also disclosed are compositions capable of supporting the culture and proliferation of human embryonic stem cells without the need for feeder cells or for exposure of the medium to feeder cells.
Owner:WICELL RES INST
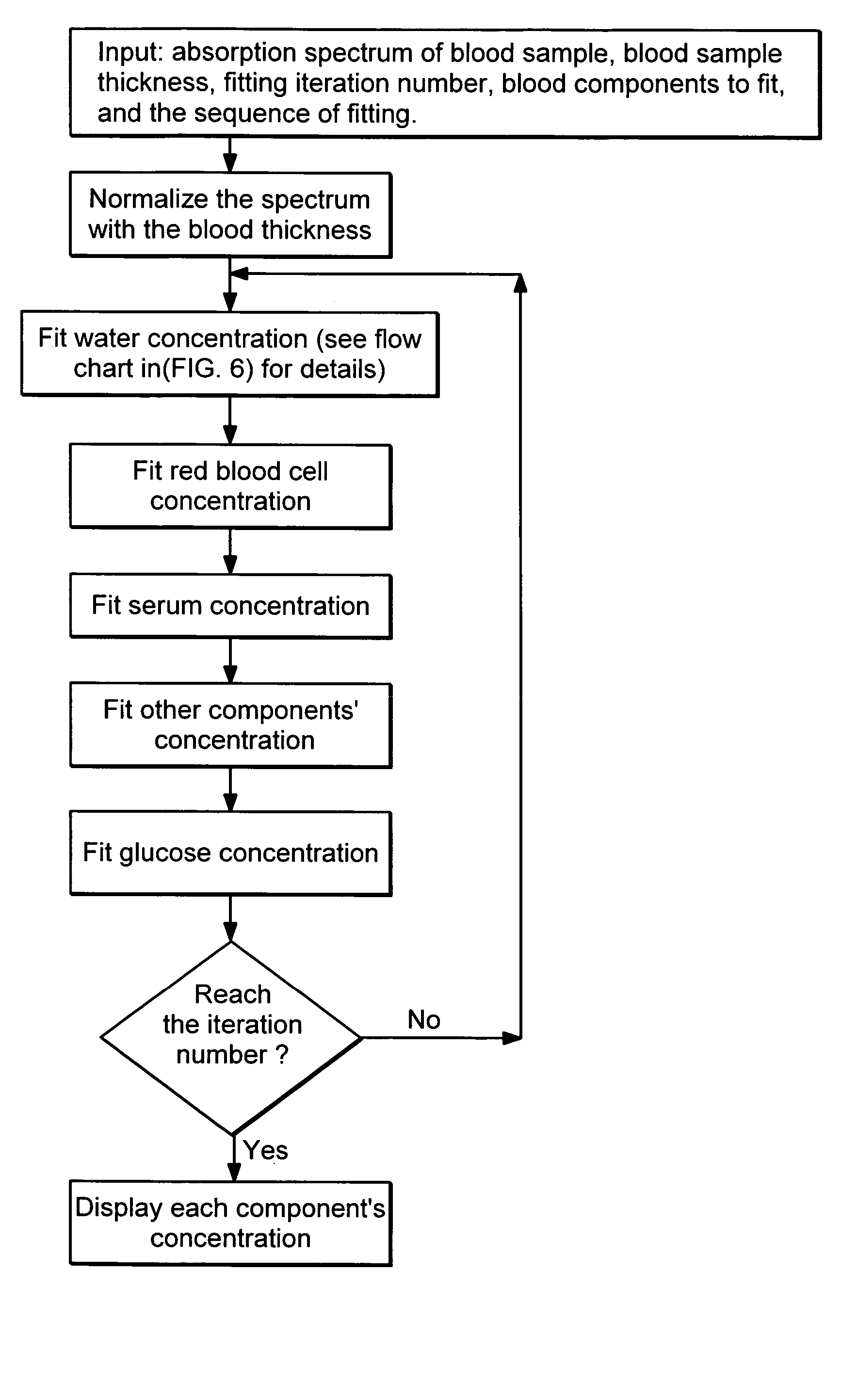
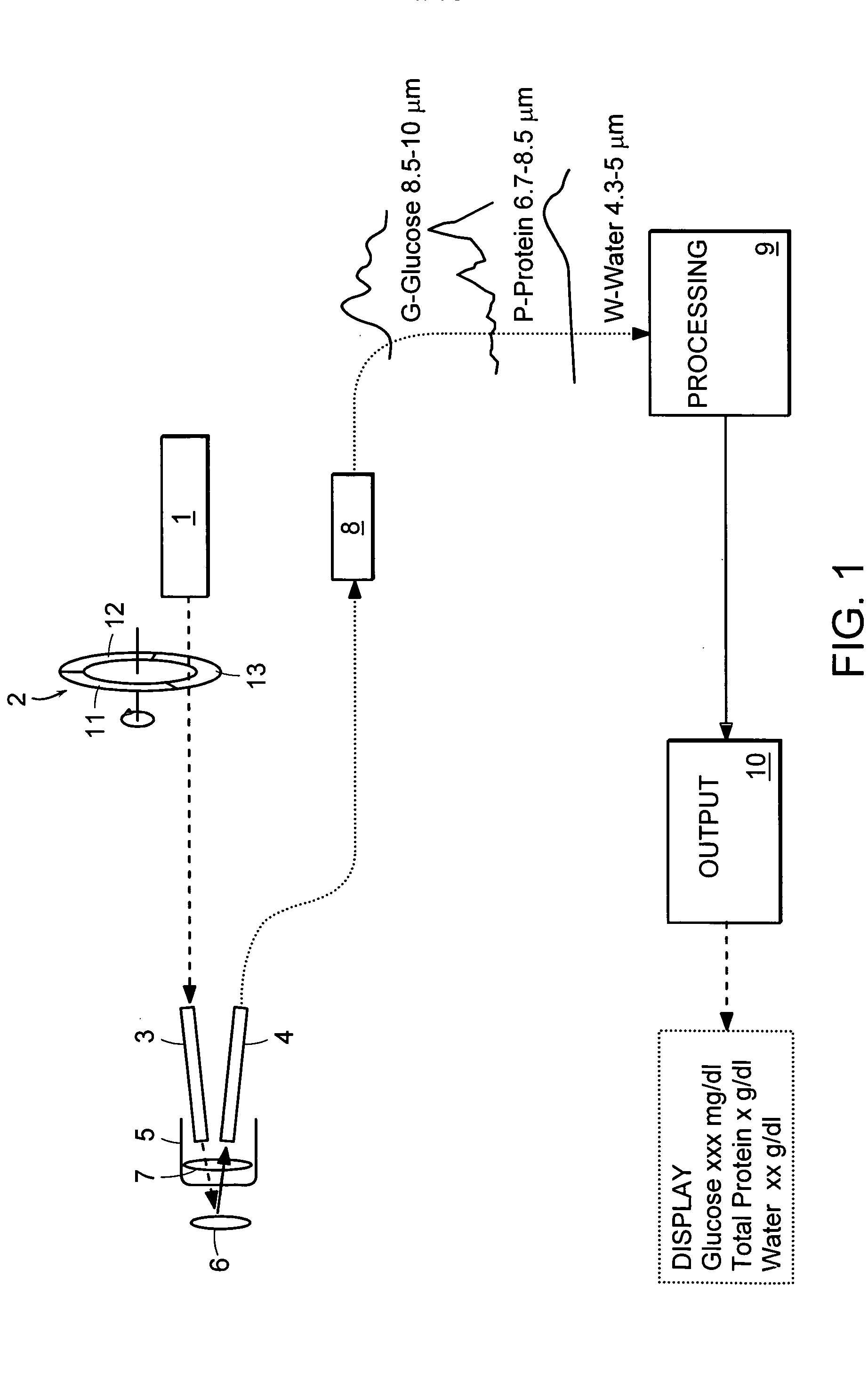
Non-invasive blood component measurement system
Non-invasive, optical apparatus and methods for the direct measurement of hemoglobin derivatives and other analyte concentration levels in blood using diffuse reflection and transmission spectroscopy in the wavelength region 400-1350 nm which includes the transparent tissue window from approximately 610 to 1311 nanometers and, using diffuse reflection spectroscopy, the mid-infrared region from 4.3-12 microns in wavelength. Large area light collection techniques are utilized to provide a much larger pulsate signal than can be obtain with current sensor technology. Sensors used in separate or simultaneous precision measurements of both diffuse reflection and transmission, either separately or simultaneously, from pulsate, blood-perfused tissue for the subsequent determination of the blood analytes concentrations such as arterial blood oxygen saturation (SaO2), carboxyhemoglobin (COHb), oxyhemoglobin (OHb), deoxyhemoglobin (dOHb), methemoglobin (metHb), water (H2O), hematocrit (HCT), glucose, cholesterol and proteins such as albumin and other analytes components.
Owner:3WAVE OPTICS
Albumin fusion proteins
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disordrs or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Preparation of recombinant factor VIII in a protein free medium
InactiveUS6171825B1Eliminate and at least greatly reduce riskImprove productivityFactor VIICulture processFactor iiManganese
Recombinant Factor VIII can be produced in relatively large quantities on a continuous basis from mammalian cells in the absence of any animal-derived proteins such as albumin by culturing the cells in a protein free medium supplemented with polyol copolymers, preferably in the presence of trace metals such as copper. In very preferred embodiments, the medium includes a polyglycol known as Pluronic F-68, copper sulfate, ferrous sulfate / EDTA complex, and salts of trace metals such as manganese, molybdenum, silicon, lithium and chromium. With an alternative medium which included trace copper ions alone (without polyol copolymers) we were also able to enhance the productivity of Factor VIII in recombinant cells such as BHK cells that are genetically engineered to express Factor VIII.
Owner:BAYER HEALTHCARE LLC +1
Albumin fusion proteins
InactiveUS6946134B1Extended shelf lifeRetain activityPeptide/protein ingredientsSerum albuminDiseaseAlbumin
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disordrs or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Methods for glucagon suppression using modified exendins
InactiveUS7153825B2Saccharide peptide ingredientsVasoactive intestinal peptideDiseaseBiological half-life
We claim a method of lowering plasma glucagon in a subject in need thereof comprising administering to the subject a composition comprising a modified exendin or modified exendin analog, wherein said modification comprises one or more molecule linked to an exendin or the exendin analog wherein said molecule is selected from the group consisiting of polyethylene glycol, gelatin and / or albumin. The modified exendin or the modified exendin analog has activity of suppressing glucagon secretion and / or lowering glucagon levels in the subject and possesses increased biological half-life compared to unmodified exendin or unmodified exendin analog. The method is useful in treating hyperglucagonemia and other disorders that would be benefited by lowering plasma glucagon or suppressing glucagon secretion.
Owner:AMYLIN PHARMA INC
Inhibitor nucleic acids
InactiveUS20050256071A1Low melting pointQuality improvementOrganic active ingredientsNervous disorderAptamerHalf-life
The present invention provides methods and compositions for attenuating expression of a target gene in vivo. In general, the method includes administering RNAi constructs (such as small-interfering RNAs (i.e., siRNAs) that are targeted to particular mRNA sequences, or nucleic acid material that can produce siRNAs in a cell), in an amount sufficient to attenuate expression of a target gene by an RNA interference mechanism. In particular, the RNAi constructs may include one or more modifications to improve serum stability, cellular uptake and / or to avoid non-specific effect. In certain embodiments, the RNAi constructs contain an aptamer portion. The aptamer may bind to human serum albumin to improve serum half life. The aptamer may also bind to a cell surface protein that improves uptake of the construct.
Owner:CALIFORNIA INST OF TECH
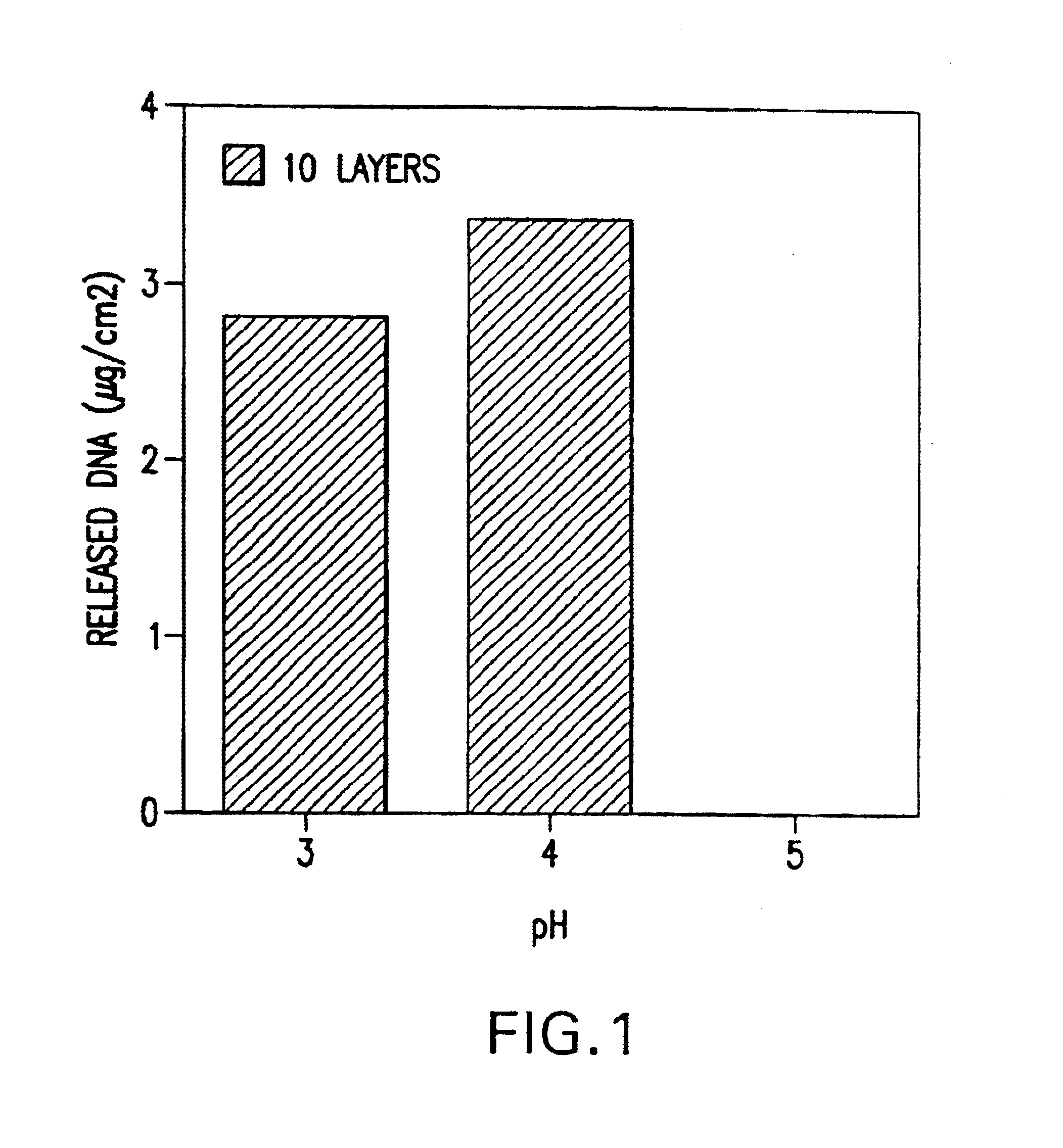
pH-triggered microparticles
InactiveUS20050123596A1Efficient deliveryGood biocompatibilityPowder deliveryMicroencapsulation basedPolyesterLipid formation
Microparticles that are designed to release their payload when exposed to acidic conditions are provided as a vehicle for drug delivery. Any therapeutic, diagnostic, or prophylatic agent may be encapsulated in a lipid-protein-sugar or polymeric matrix including a pH triggering agent to form pH triggerable microparticles. Preferably the diameter of the pH triggered microparticles ranges from 50 nm to 10 micrometers. The matrix of the particles may be prepared using any known lipid (e.g., DPPC), protein (e.g., albumin), or sugar (e.g., lactose). The matrix of the particles may also be prepared using any synthetic polymers such as polyesters. Methods of preparing and administering the particles are provided. Methods of immunization, transfection, and gene therapy are also provided by administering pH triggerable microparticles.
Owner:DANA FARBER CANCER INST INC +2
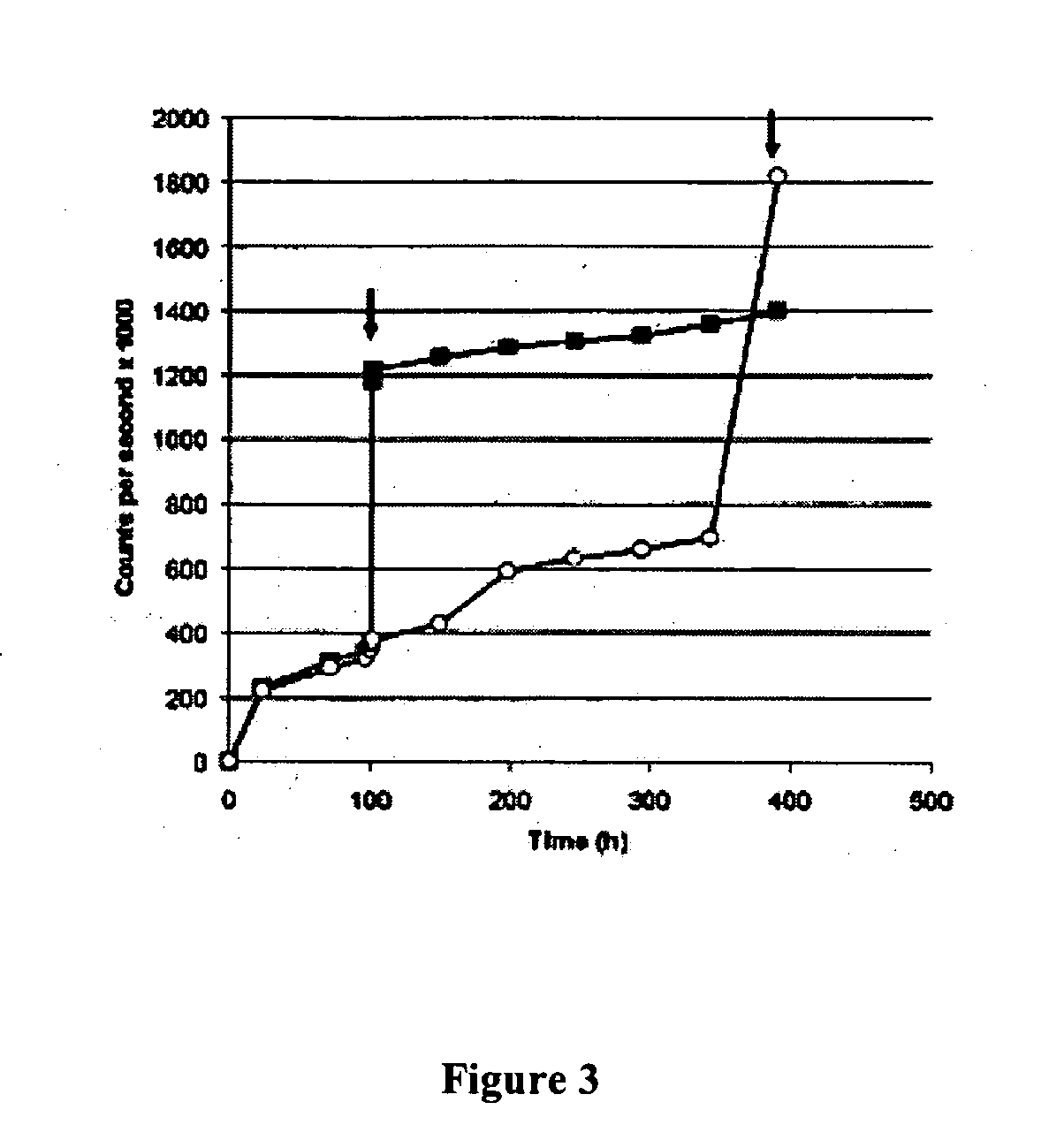
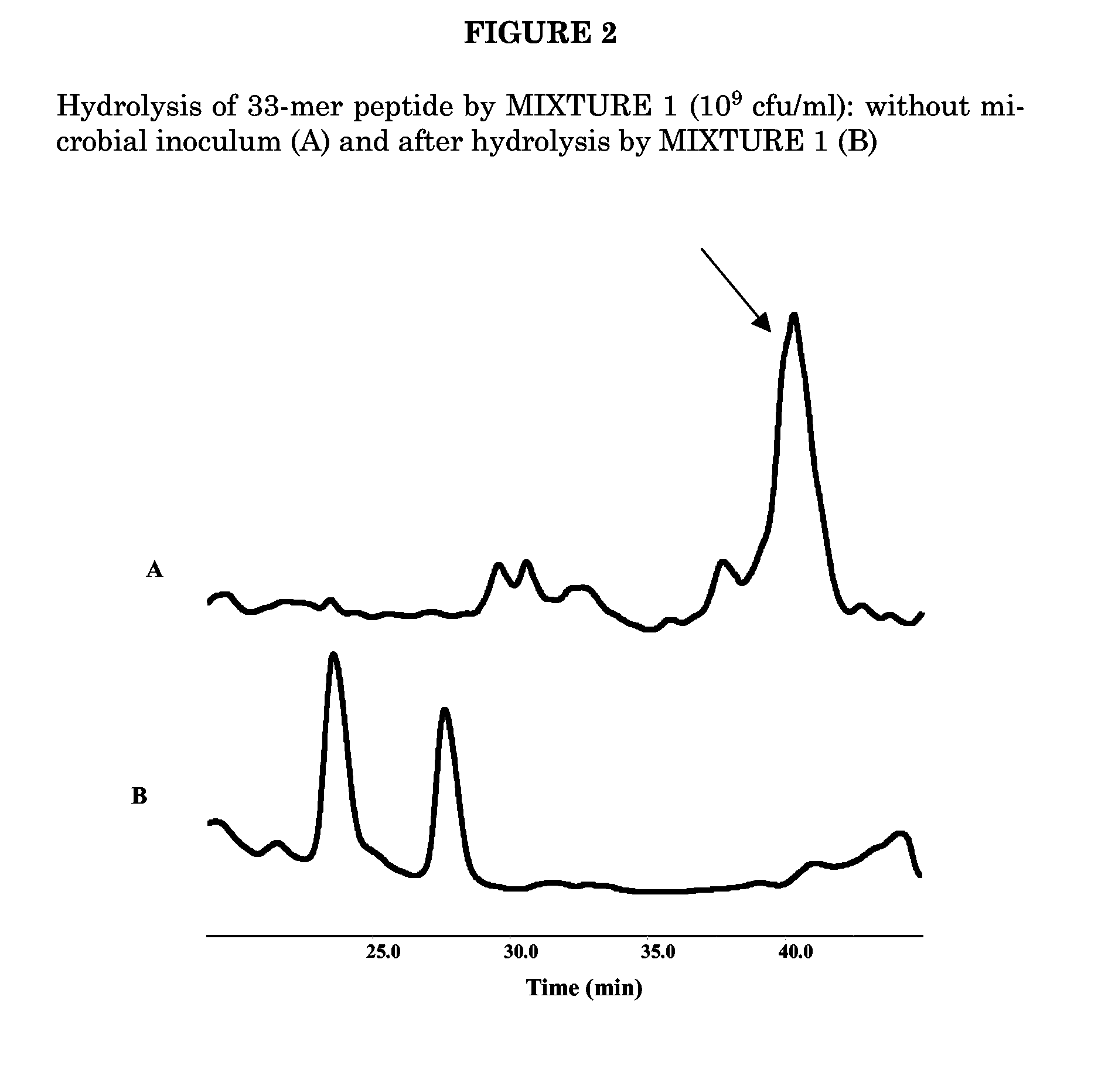
Mixture of at Least 6 Species of Lactic Acid Bacteria and/or Bifidobacteria in the Manufacture of Sourdough
A mixture of at least 6 species of lactic acid bacteria and / or Bifidobacteria is disclosed for use in bakery and medical field. The preferred mixture comprises Streptococcus thermophilus, Bifidobacterium infantis, Bifidobacterium longum, Bifidobacterium breve, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus. Said mixture is useful for a sourdough, a leavening composition. Baked goods and other food products obtained therefrom are disclosed. These goods have low or no gluten content and are suitable for the integration of the diet of a subject suffering from celiac disease, for decreasing the risk of allergies due to wheat flour albumins and globulins, for the treatment of schizophrenic symptoms, in the preparation of products for enteric diet.
Owner:VSL PHARMA INC
Production of hSA-linked butyrylcholinesterases in transgenic mammals
InactiveUS20060253913A1Promote formationImprove stabilityAnimal cellsVectorsMammalButyrylcholinesterase
The present invention provides methods for the large-scale production of recombinant butyrylcholinesterase fused to human serum albumin in cell culture, and in the milk and / or urine of transgenic mammals. The recombinant butyrylcholinesterase-albumin fusion protein of this invention can be used to treat and / or prevent organophosphate pesticide poisoning, nerve gas poisoning, cocaine intoxication, and succinylcholine-induced apnea.
Owner:PHARMATHENE
Method of treatment of diabetes and/or obesity with reduced nausea side effect
InactiveUS20070207958A1Prevent and reduce and eliminate nausea side effectReduces and eliminates side effectPeptide/protein ingredientsMetabolism disorderSide effectInsulinotropin
The present invention provides methods of administering an insulinotropic peptide in an amount effective to treat a disorder or condition while reducing nausea side effect by administering to a subject in need thereof an insulinotropic peptide conjugated to albumin. The present invention also provides methods of selecting a subject for administration of a conjugated insulinotropic peptide. Exemplary disorders or conditions treatable with an insulinotropic peptide include obesity and type II diabetes.
Owner:CONJUCHEM
Intra-serum and intra-gel for modeling human skin tissue
InactiveUS6475800B1Diagnostics using spectroscopyScattering properties measurementsConfocalCrosslinking reagent
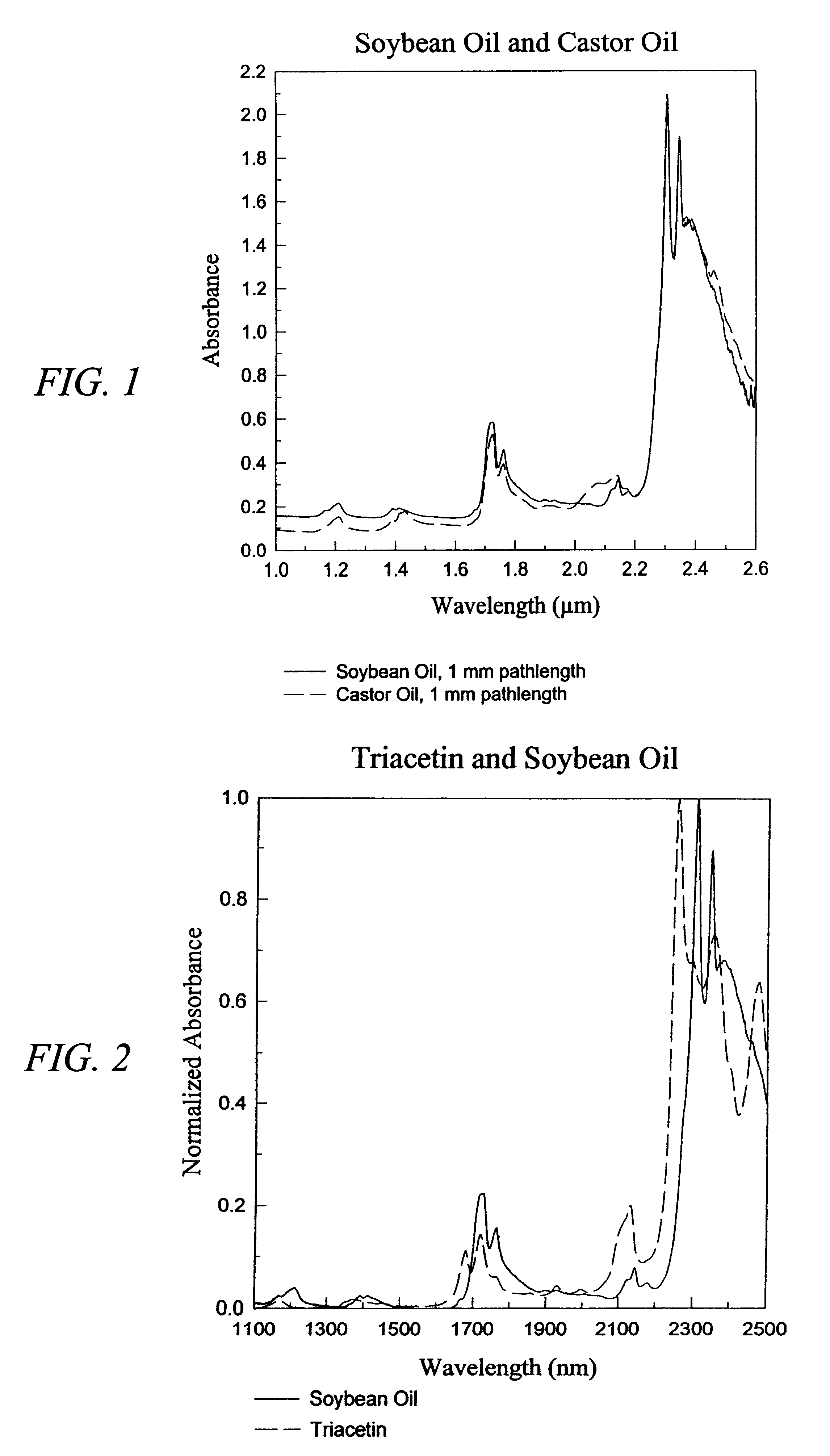
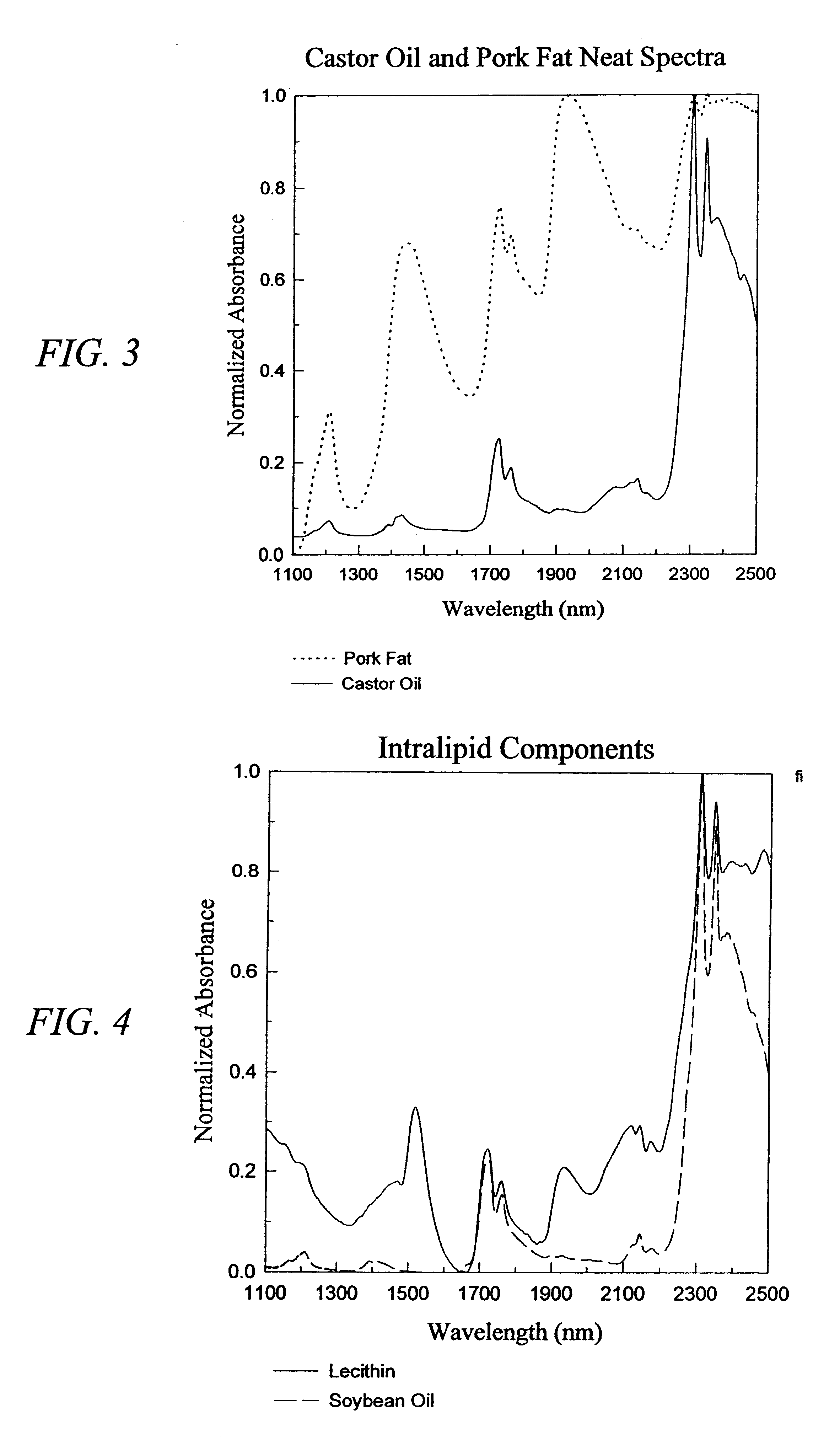
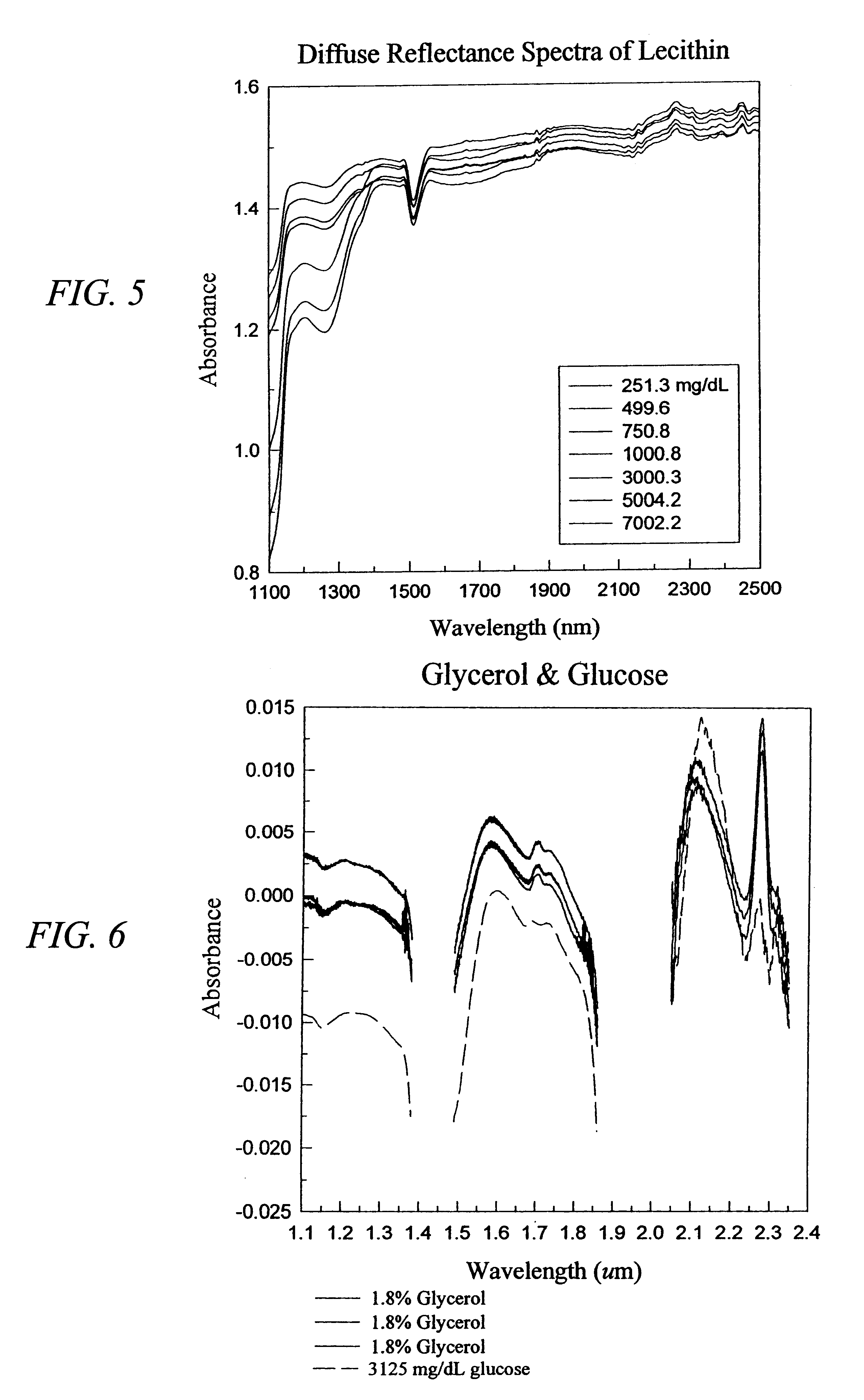
The invention provides a class of samples that model the human body. This family of samples is based upon emulsions of oil in water with lecithin acting as the emulsifier. These solutions that have varying particle sizes may be spiked with basis set components (albumin, urea and glucose) to simulate skin tissues further. The family of samples is such that other organic compounds such as collagen, elastin, globulin and bilirubin may be added, as can salts such as Na+, K+ and Cl-. Layers of varying thickness with known index of refraction and particle size distributions may be generated using simple crosslinking reagents, such as collagen (gelatin). The resulting samples are flexible in each analyte's concentration and match the skin layers of the body in terms of the samples reduced scattering and absorption coefficients, mums and muma. This family of samples is provided for use in the medical field where lasers and spectroscopy based analyzers are used in treatment of the body. In particular, knowledge may be gained on net analyte signal, photon depth of penetration, photon radial diffusion, photon interaction between tissue layers, photon density (all as a function of frequency) and on instrument parameter specifications such as resolution and required dynamic range (A / D bits required). In particular, applications to delineate such parameters have been developed for the application of noninvasive glucose determination in the near-IR region from 700 to 2500 nm with an emphasis on the region 1000 to 2500 nm (10,000 to 4,000 cm-1).
Owner:GLT ACQUISITION
Bispecific Fusion Antibodies With Enhanced Serum Half-Life
Drug compositions, fusions and conjugates are provided. The drug fusions and conjugates contain a therapeutic or diagnostic agent that is fused or conjugated to an antigen-binding fragment of an antibody that binds serum albumin. The drug compositions, fusions and conjugates have a longer in vivo half-life in comparison with the unconjugated or unfused therapeutic or diagnostic agent.
Owner:DORMANTIS LTD
Albumin fusion proteins
The present invention encompasses albumin fusion proteins. Nucleic acid molecules encoding the albumin fusion proteins of the invention are also encompassed by the invention, as are vectors containing these nucleic acids, host cells transformed with these nucleic acids vectors, and methods of making the albumin fusion proteins of the invention and using these nucleic acids, vectors, and / or host cells. Additionally the present invention encompasses pharmaceutical compositions comprising albumin fusion proteins and methods of treating, preventing, or ameliorating diseases, disorders or conditions using albumin fusion proteins of the invention.
Owner:HUMAN GENOME SCI INC
Compositions and methods of delivery of pharmacological agents
InactiveUS20070129448A1Eliminate side effectsInhibit microbial growthBiocideHydroxy compound active ingredientsSide effectPharmaceutical formulation
The present invention relates to a pharmaceutical composition comprising a pharmaceutical agent and a pharmaceutically acceptable carrier, which carrier comprises a protein, for example, human serun albumin and / or deferoxamine. The human serum albumin is present in an amount effective to reduce one or more side effects associated with administration of the pharmaceutical composition. The invention also provides methods for reducing one or more side effects of administration of the pharmaceutical composition, methods for inhibiting microbial growth and oxidation in the pharrmaceutical composition, and methods for enhancing transport and binding of a pharmaceutical agent to a cell.
Owner:ABRAXIS BIOSCI LLC
Administration of anti-cytokine f(ab')2 antibody fragments
InactiveUS20060210563A1Risk minimizationImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsAntibody fragmentsCytokine
The present invention is directed to a method of treating a cytokine-mediated immune reaction in a patient in need of such treatment by administration of an effective amount of anti-cytokine F(ab′)2 antibody fragments. The antibody fragments are preferably free from albumin and of whole antibodies, as well as substantially free of pyrogens. The anti-cytokine F(ab′)2 antibody fragments may be administered with an effective amount of a pharmaceutically acceptable carrier.
Owner:LOPEZ DE SILANES JUAN +3
Breast cancer therapy based on hormone receptor status with nanoparticles comprising taxane
ActiveUS20100048499A1BiocidePhosphorous compound active ingredientsPR - Progesterone receptorNanoparticle
The present invention relates to methods and kits for the treatment of breast cancer based on hormone receptor status of progesterone receptor and estrogen receptor comprising the administration of a taxane alone, in combination with at least one other and other therapeutic agents, as well as other treatment modalities useful in the treatment of breast cancer. In particular, the invention relates to the use of nanoparticles comprising paclitaxel and albumin (such as Abraxane®) either alone or in combination with other chemotherapeutic agents or radiation, which may be used for the treatment of breast cancer which does not express estrogen receptor and / or progesterone receptor.
Owner:ABRAXIS BIOSCI LLC
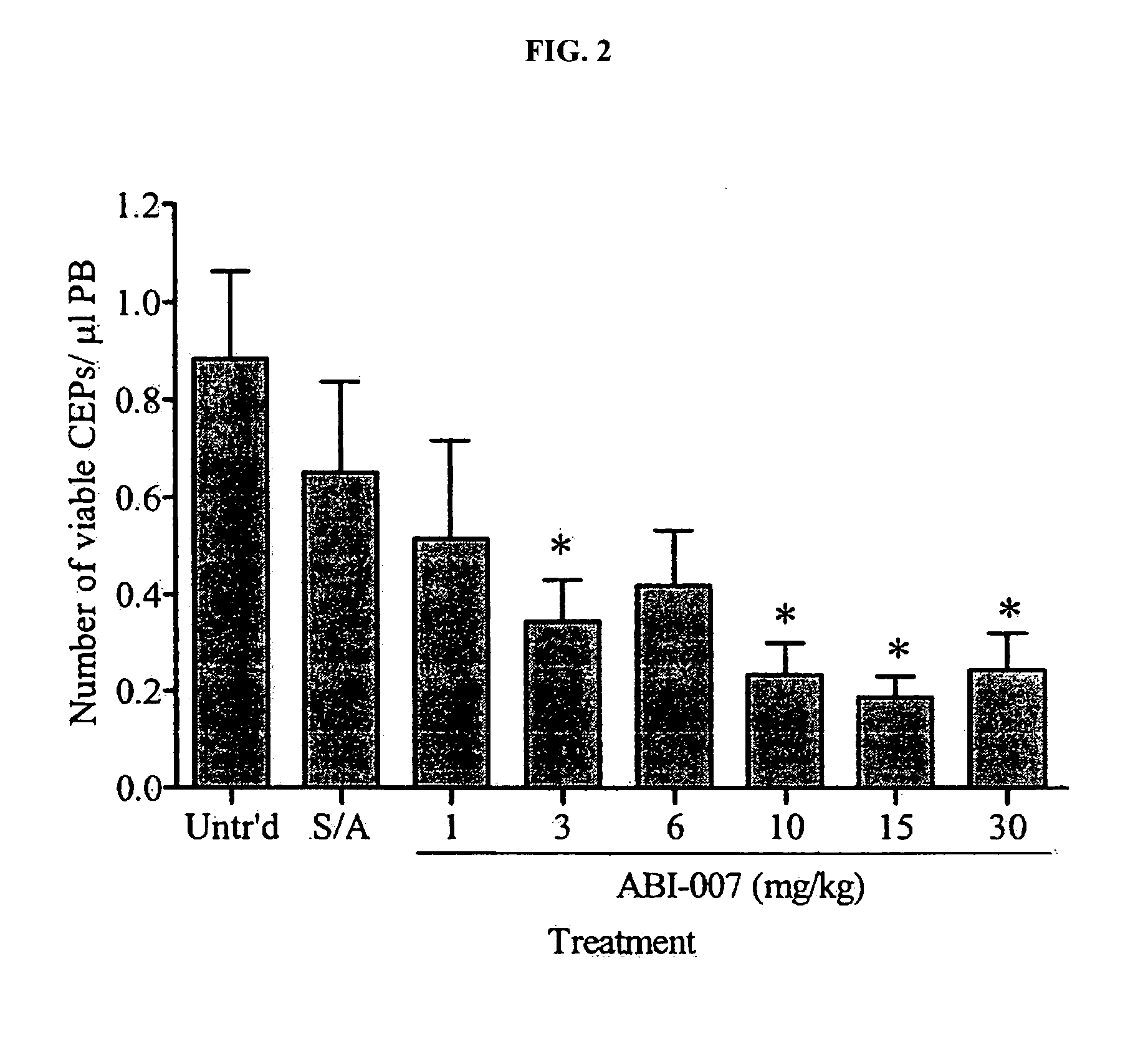
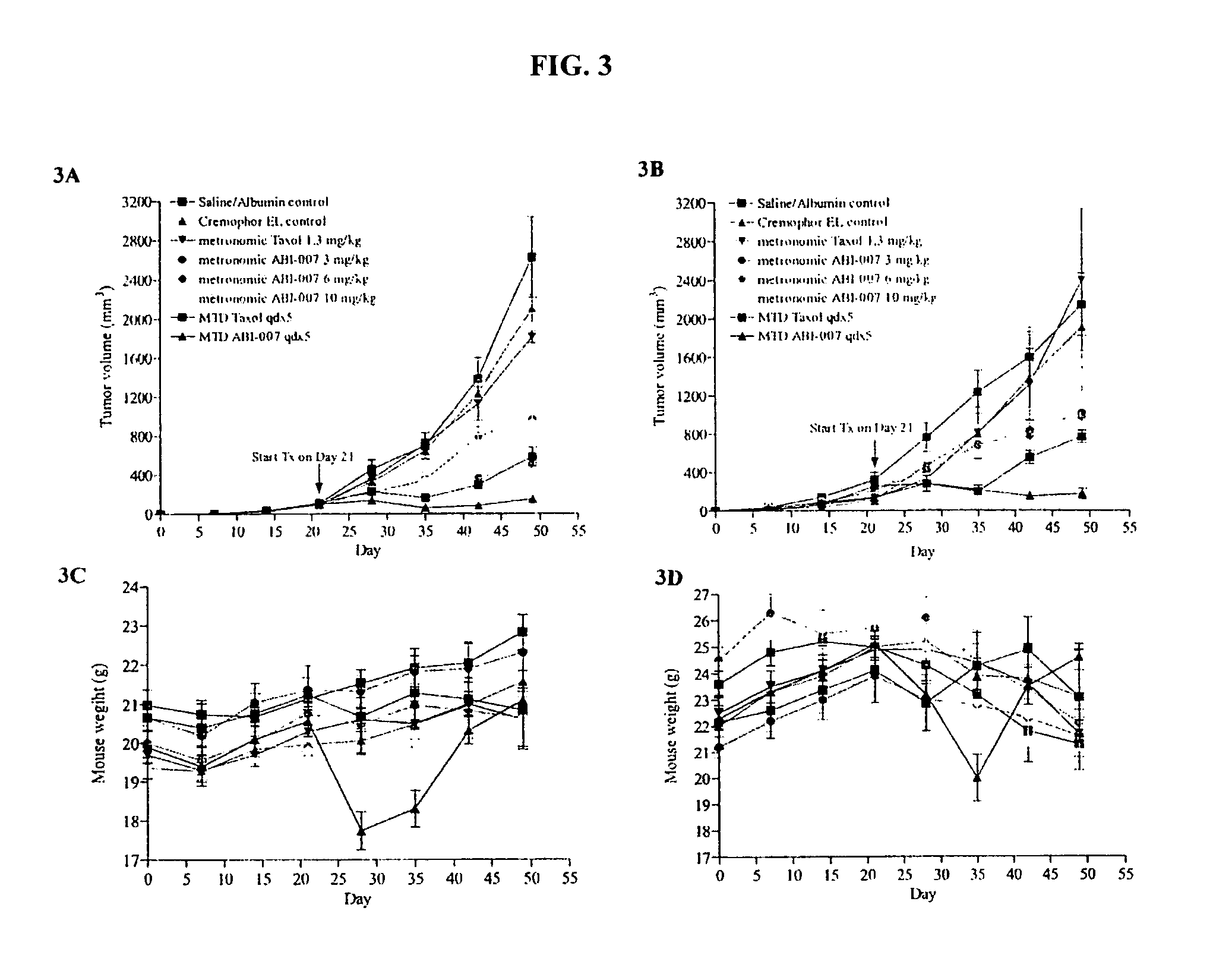
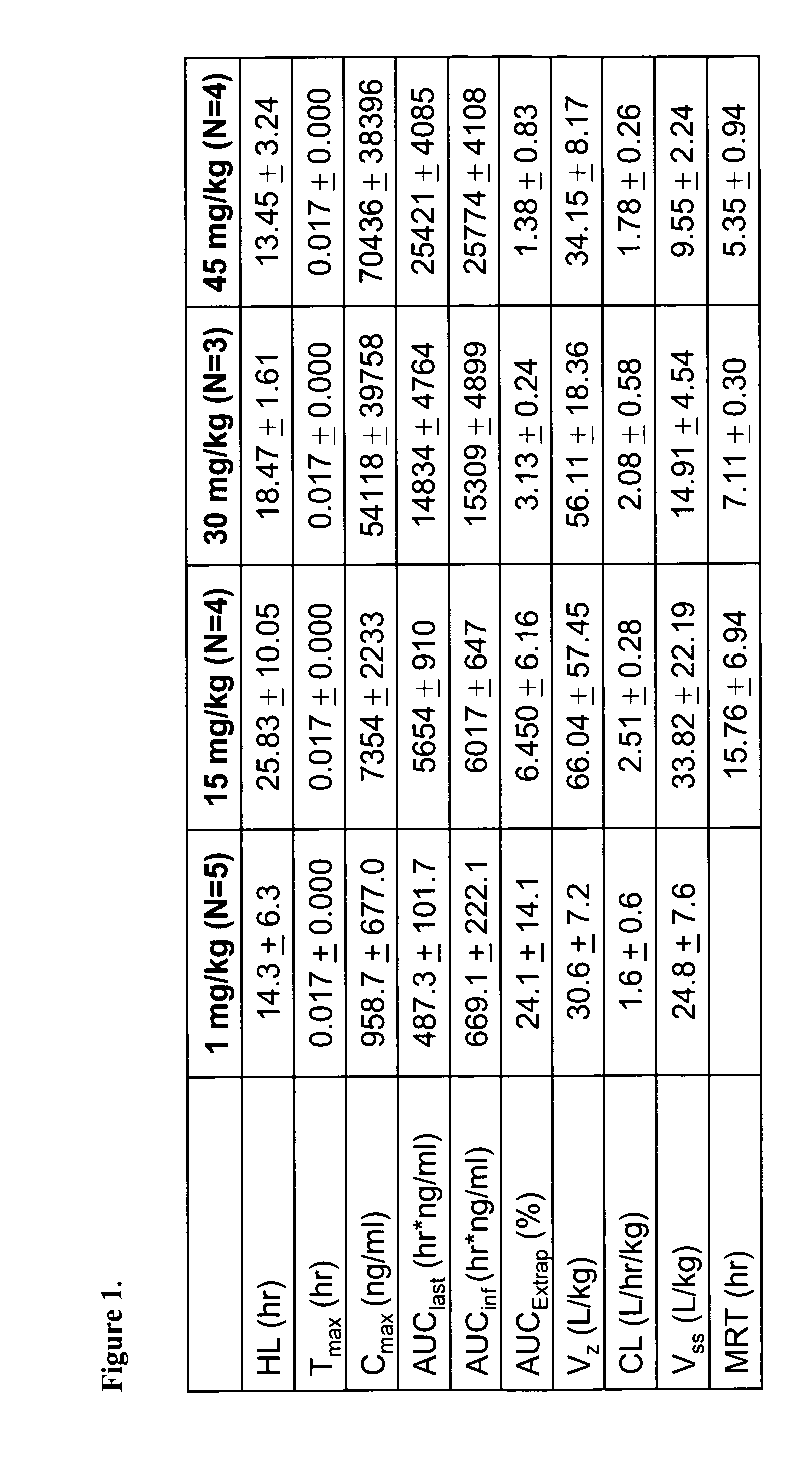
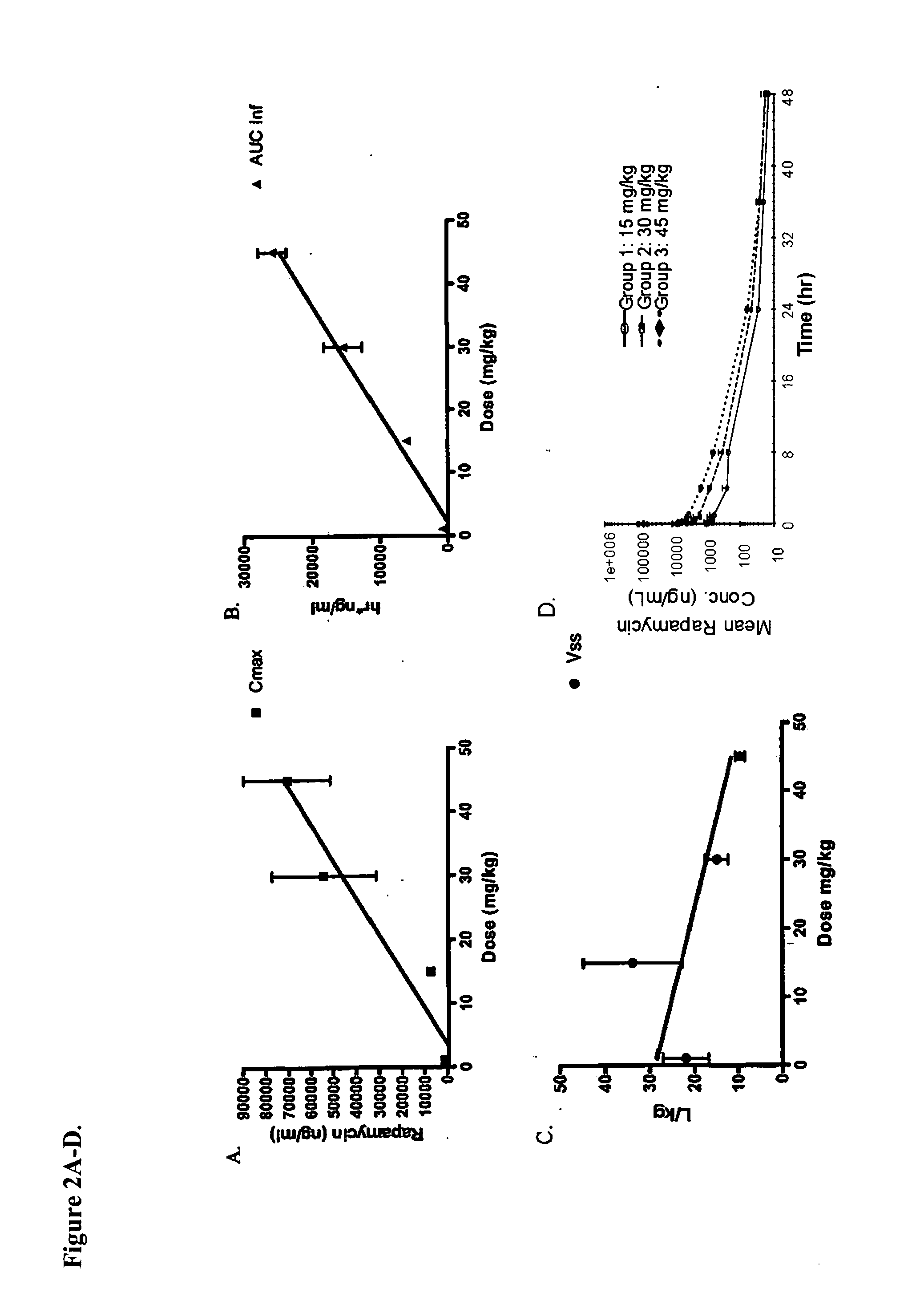
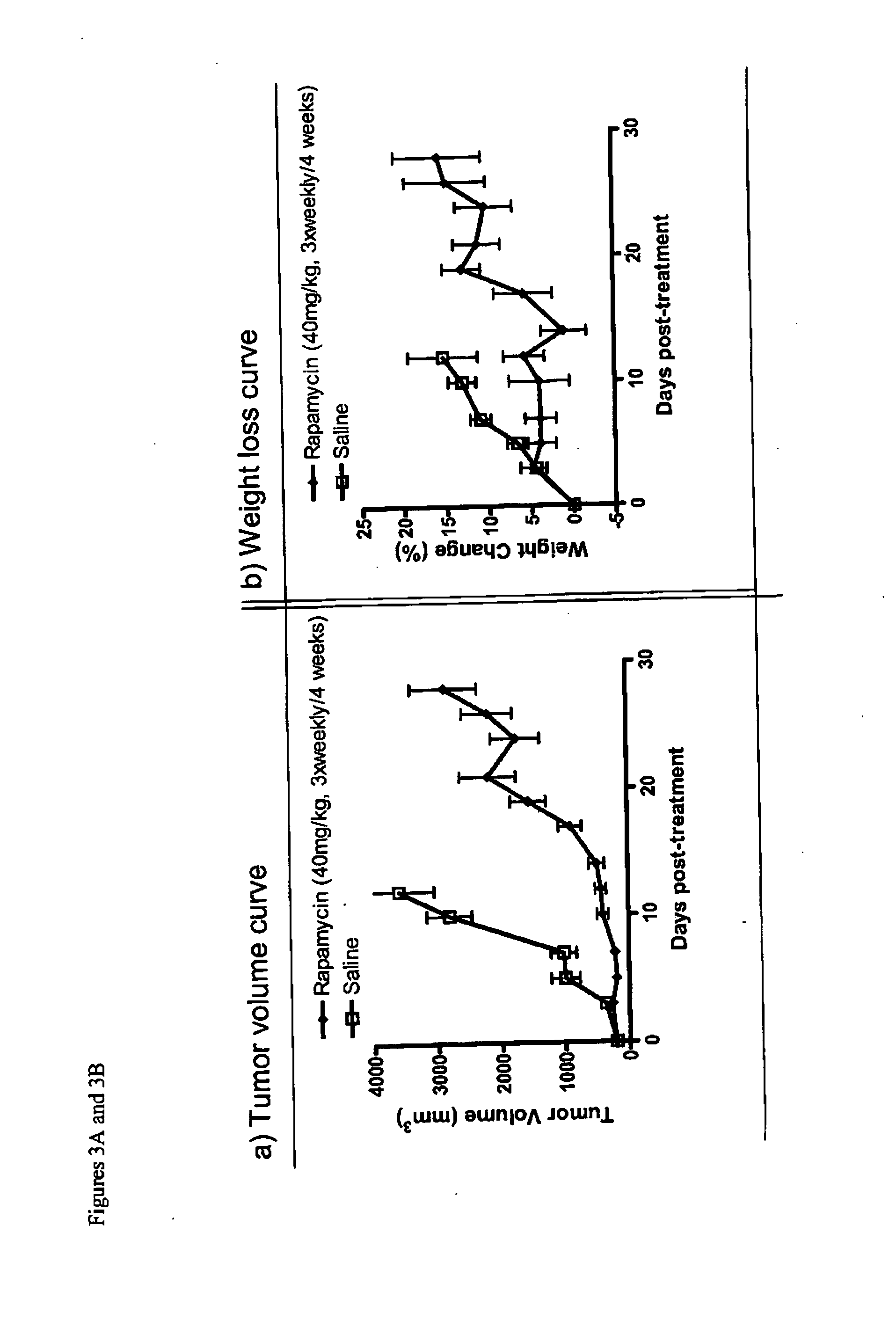
Nanoparticle comprising rapamycin and albumin as anticancer agent
The present invention features methods for treating, stabilizing, preventing, and / or delaying cancer by administering nanoparticles that comprise rapamycin or a derivative thereof. The invention also provides compositions (e.g., unit dosage forms) comprising nanoparticles that comprise a carrier protein and rapamycin or a derivative thereof. The invention further provides combination therapy methods of treating cancer comprising administering to an individual an effective amount of nanoparticles that comprise rapamycin or a derivative thereof and a second therapy.
Owner:ABRAXIS BIOSCI LLC
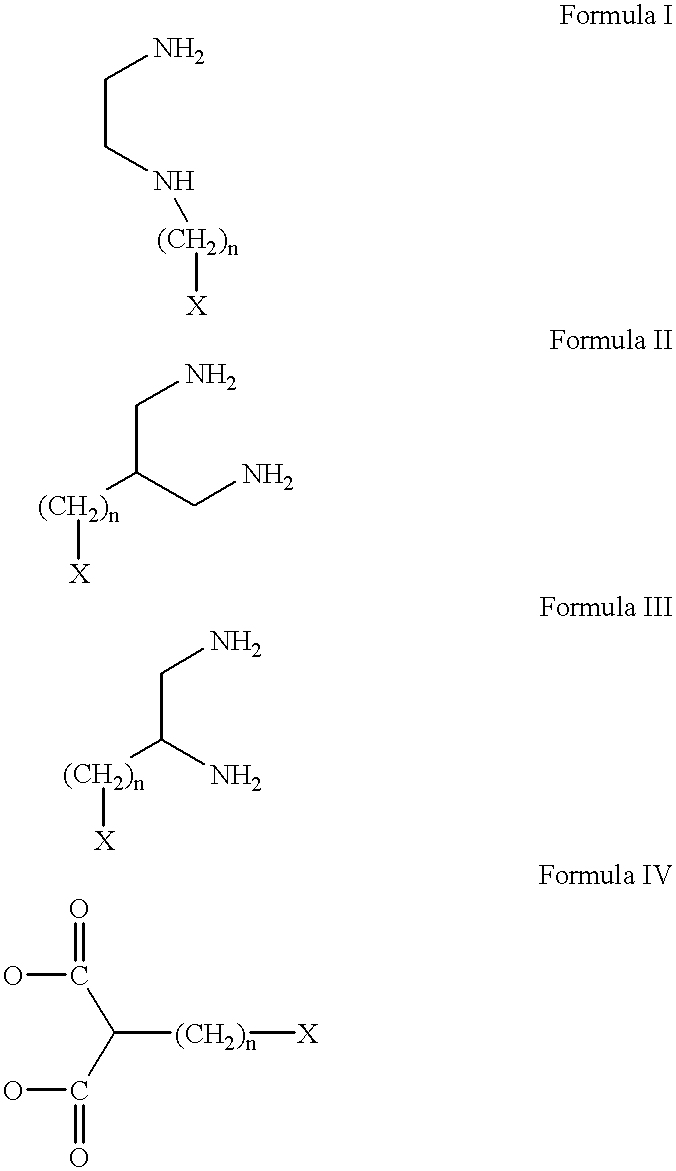
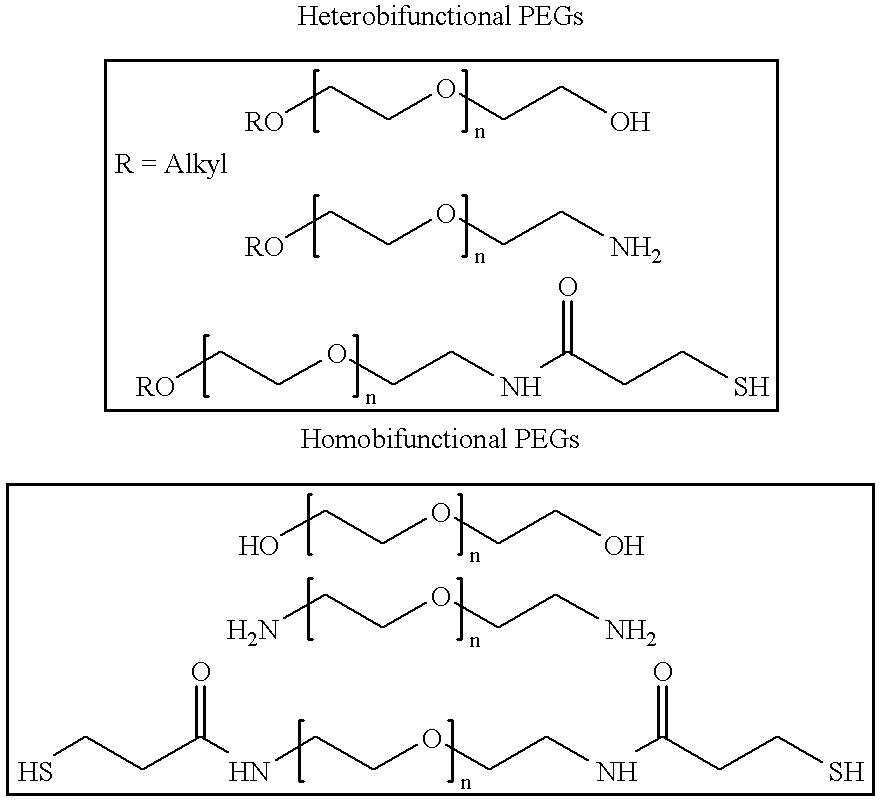
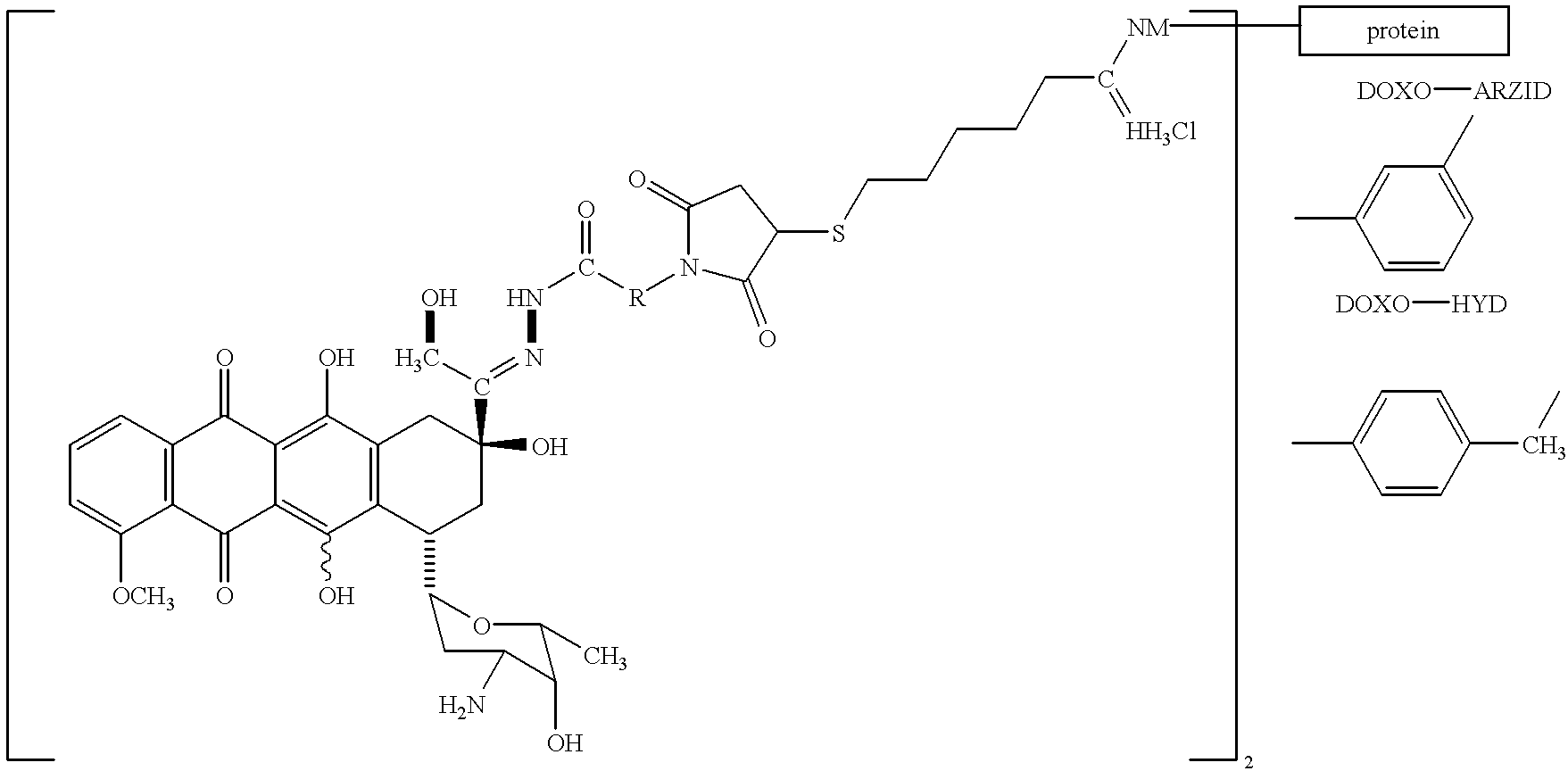
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Serum-free medium for mesenchymal stem cells
Serum-free media for growth and proliferation of chondrocytes and mesenchymal stem cells in culture are provided. A serum-free medium for growth of chondrocytes includes a serum-free composition comprising FGF-2, linoleic acid, ascorbic acid, B-mercaptoethanol, transferrin and dexamethasone. Further, the composition comprises EGF, PDGFbb, insulin and albumin. A method for growing chondrocytes in a serum free medium comprising the compostion is also provided. Also provided for mesenchymal stem cell growth, is a serum-free medium which includes a composition comprising FGF-2, LIF, SCF, pantotenate, biotin and selenium and method, therefore.
Owner:CONSORZIO PER LA GESTIONE DEL CENT DI BIOTECNOLOGIA AVANZATA +1
Methods of enhancing drug delivery and effectiveness of therapeutic agents
ActiveUS20120076862A1Promote absorptionConvenient treatmentPowder deliveryHeavy metal active ingredientsDiseaseCo administration
The present invention in one aspect provides methods of enhancing uptake of a therapeutic agent in a target tissue as well as methods of treating a disease (such as cancer) or enhancing effectiveness of treatment with a therapeutic agent in an individual by co-administering a composition comprising nanoparticles comprising albumin and a poorly water soluble drug such as a taxane with the therapeutic agent. The present invention in another aspect provides a method of treatment or a method of selecting patients for treatment of a disease (such as cancer) with the combination of a therapeutic agent and a composition comprising nanoparticles comprising albumin and a poorly water soluble drug such as a taxane based on one or more characteristics of the target tissue that correlates or indicates the capability of getting enhanced therapeutic agent uptake as a result of the co-administration of the taxane nanoparticle composition in the target tissue (referred to as “the drug uptake capability”). Also provided are pharmaceutical compositions, article of manufacture, and kits useful for methods described herein.
Owner:ABRAXIS BIOSCI LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com