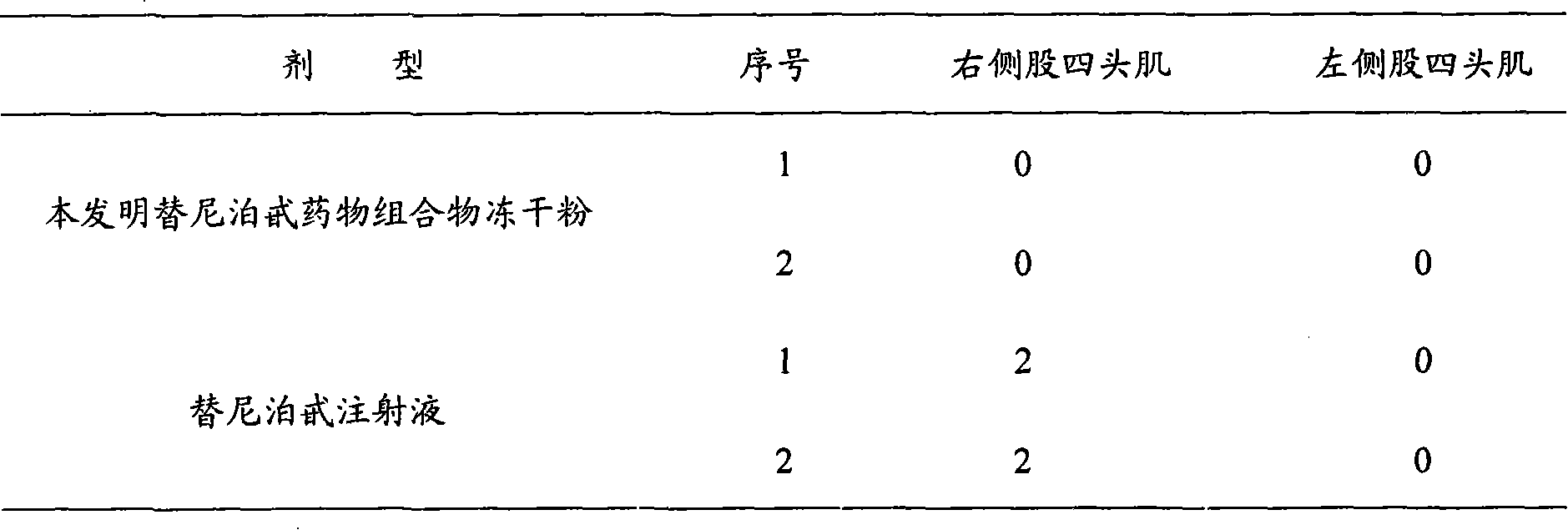
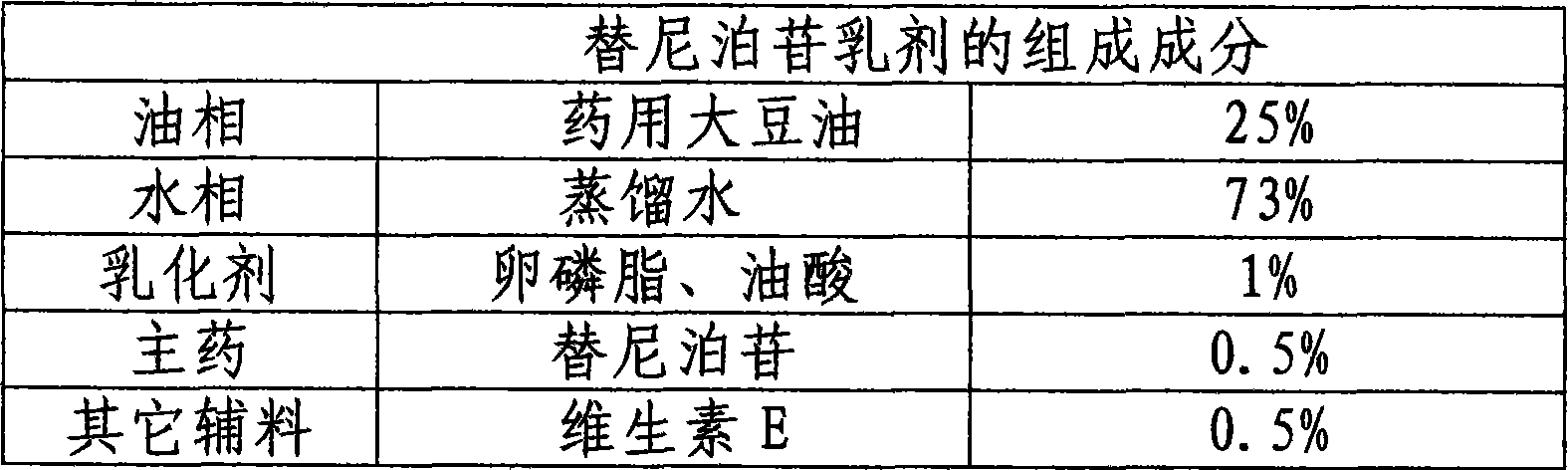
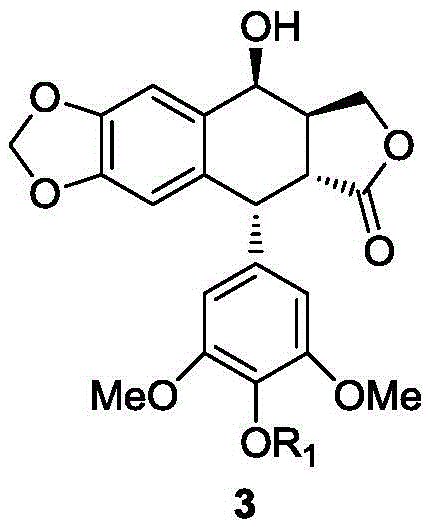
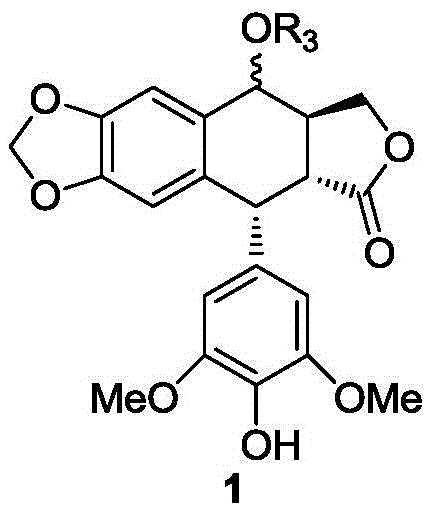
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Teniposide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Regorafenib is used to treat cancer of the colon and rectum which has spread to other parts of the body. It is also used to treat liver cancer and a certain cancer of the digestive system (gastrointestinal stromal tumor).
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
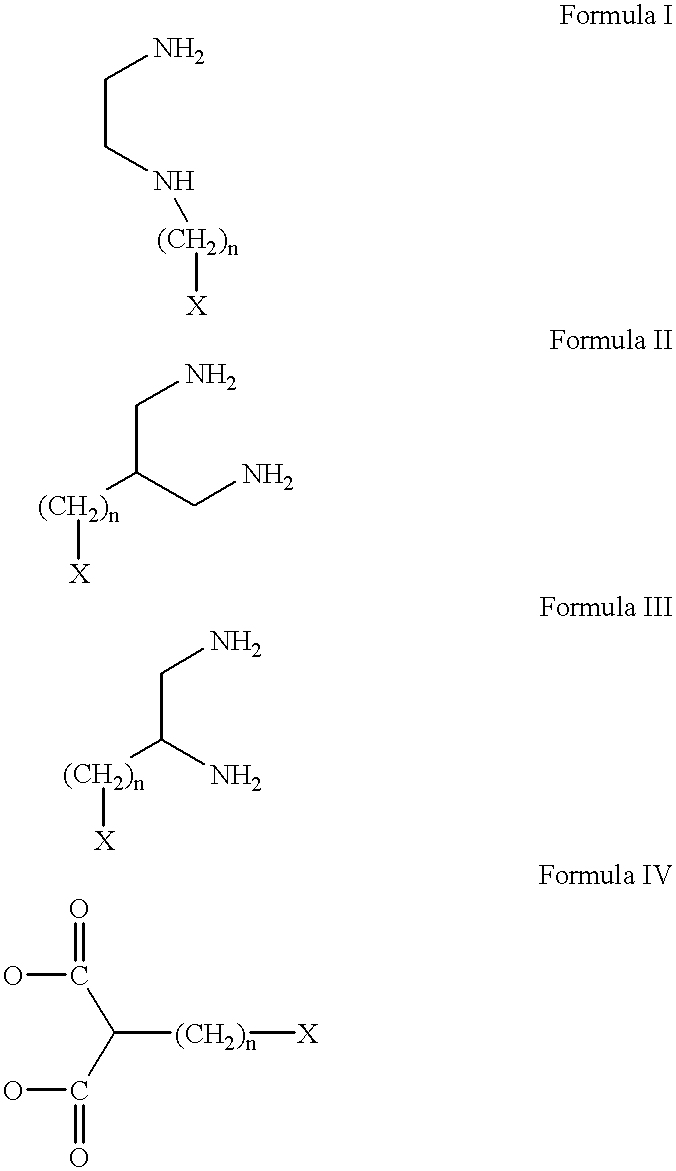
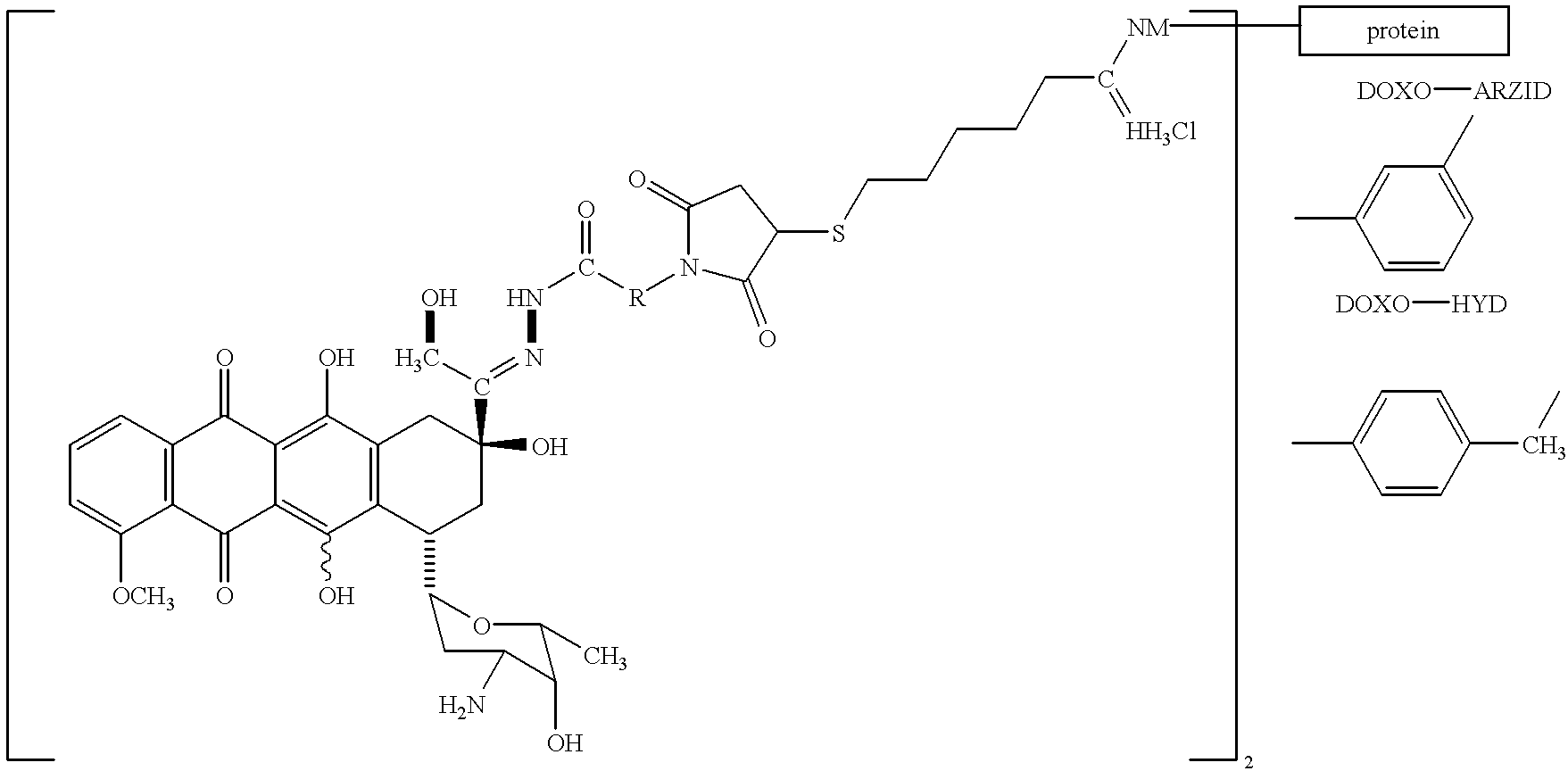
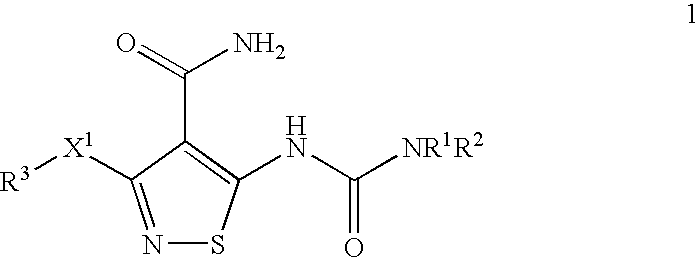
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Anticancer composition
InactiveCN1961864AOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantTreatment effect
Disclosed is an anti-cancer composition slow release injection which comprises slow release microspheres and dissolvent, the slow release microspheres comprise slow release auxiliary materials, Epothilone derivatives, glyoxaline piperazidine, Topo enzyme inhibitor combination selected from SN-38, CPT-11, HCPT, Topotecan, Irinotecan, Etoposide, Teniposide, Amrubicin, Valtaxin, XK469, AD312, ICRF-187 or ICRF-193, the dissolvent being specific dissolvent containing suspension adjuvant, the slow release auxiliary materials are selected from polylactic acid and its copolymer, polyethylene / polylactic acid, glycollic acid copolymer, aliphatic acid and sebacylic acid copolymer, the viscosity of the suspension adjuvant is 80-3000cp (at 20-30 deg C), and is selected from carboxymethylcellulose, The slow release microspheres can also be prepared into slow release implanting agent, for injection or placement in or around tumor with a release period of about 40 days. The slow release injection and slow release implanting agent can be used independently for effectively suppressing tumor accretion, or used in combination with non-operative methods such as chemotherapy and / or radiotheraphy with the function of improving their treatment effects.
Owner:JINAN SHUAIHUA PHARMA TECH
Combination therapy for hyperproliferative disease
InactiveUS20070197517A1Ease of detectabilityEasy to prepareBiocideHeavy metal active ingredientsCarboplatinDisease
This invention relates a method of treating hyperproliferative diseases. More particularly, the present invention relates to a method of treating hyperproliferative diseases, such as cancer, comprising the step of administering to a mammal in need of such treatment, either simultaneously or sequentially, (i) a therapeutically effective amount of a taxane derivative, a platinium coordination complex selected from the group consisting of carboplatin, tetraplatin, and topotecan, a nucleoside analog selected from the group consisting of gemcitabine hydrochloride and 5-FU, an anthracycline, a topoisomerase selected from the group consisting of etoposide, teniposide, amsacrine, topotecan, and Camptosar®, an aromatase inhibitor; and (ii) a therapeutically effective amount of an isothiazole derivative. The combinations of the present invention may optionally include an anti-hypertensive agent. This invention also relates to pharmaceutical compositions useful in the treatment of hyperproliferative diseases in mammals, containing such combinations. The present invention also relates to kits having a first compartment with a compound of formula 1 and a second compartment containing a taxane derivative, a platinum coordination complex, a nucleoside analog, an anthracycline, a topoisomerase inhibitor, or an aromatase inhibitor and a third compartment containing an anti-hypertensive agent.
Owner:PFIZER INC
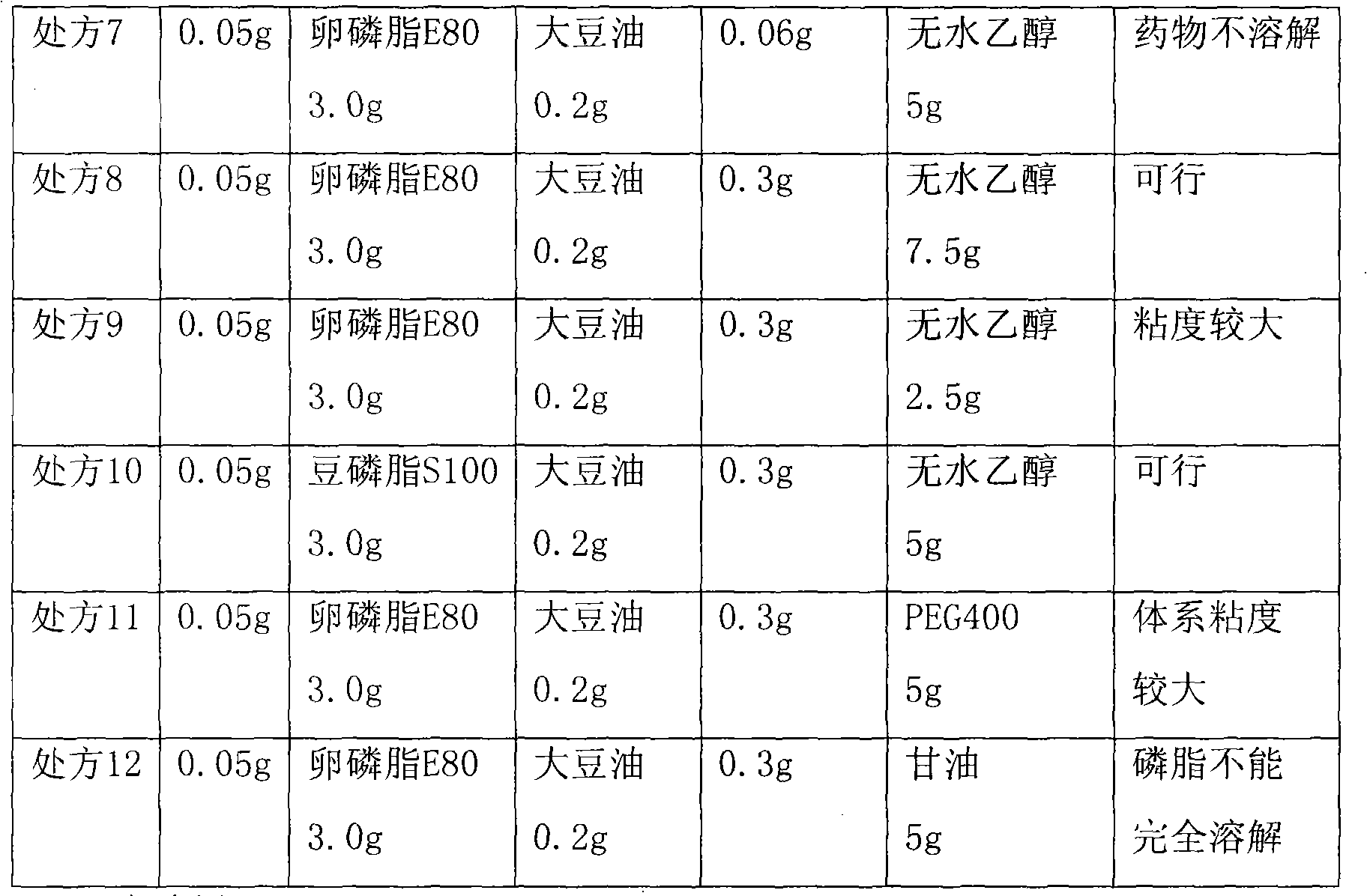
Teniposide liposome and preparation method thereof
InactiveCN101579312ATargetedGood curative effectOrganic active ingredientsPharmaceutical non-active ingredientsSolubilityTreatment effect
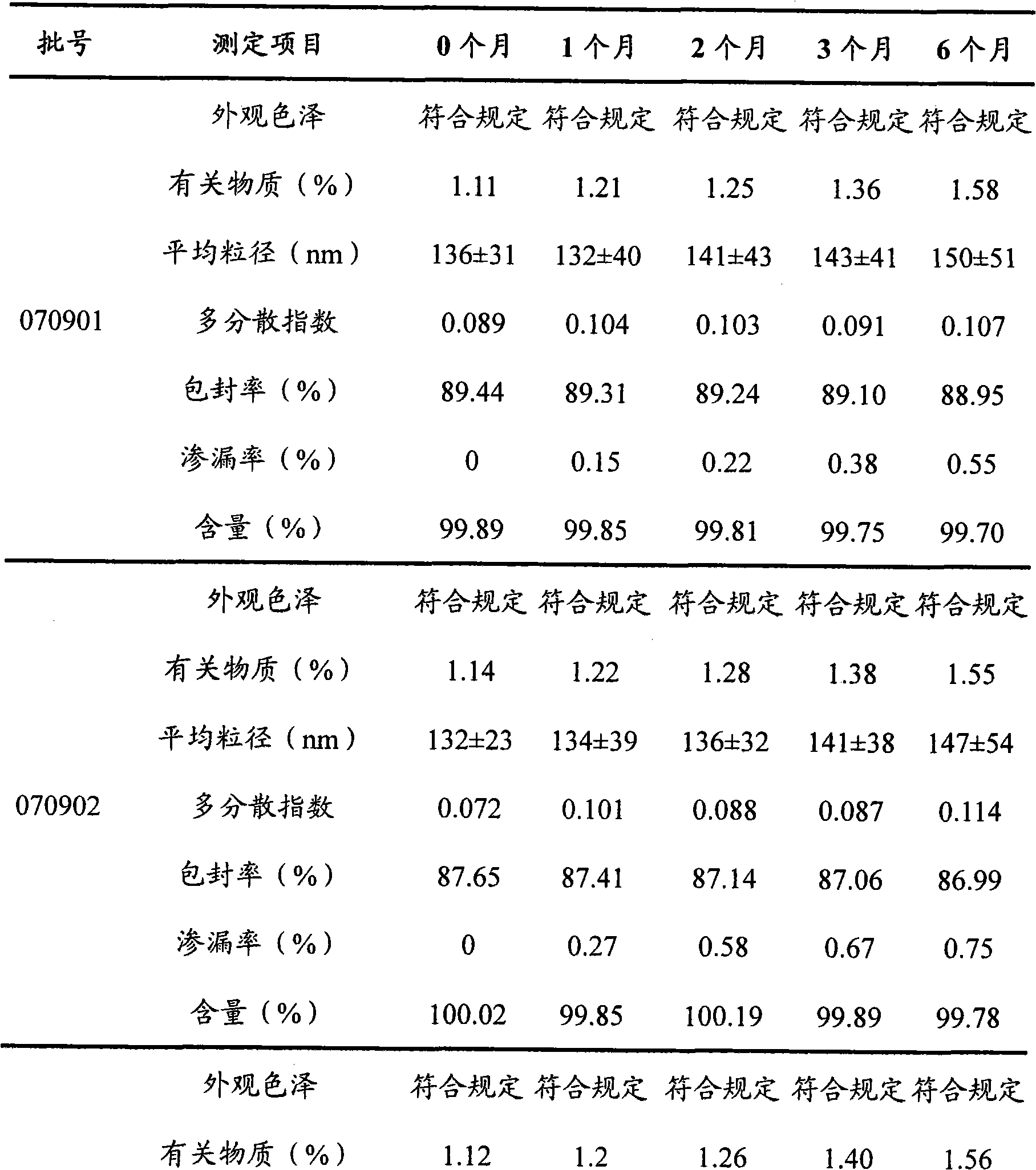
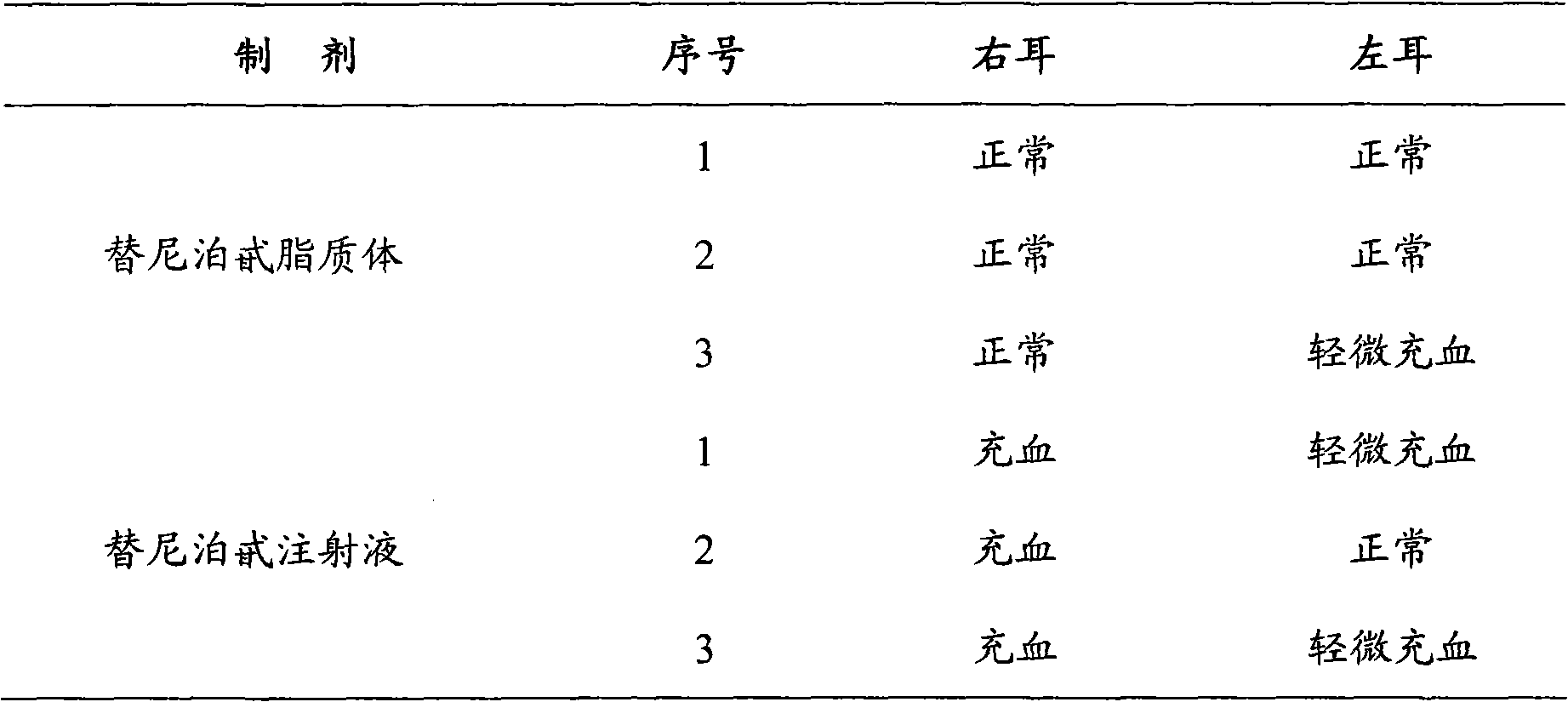
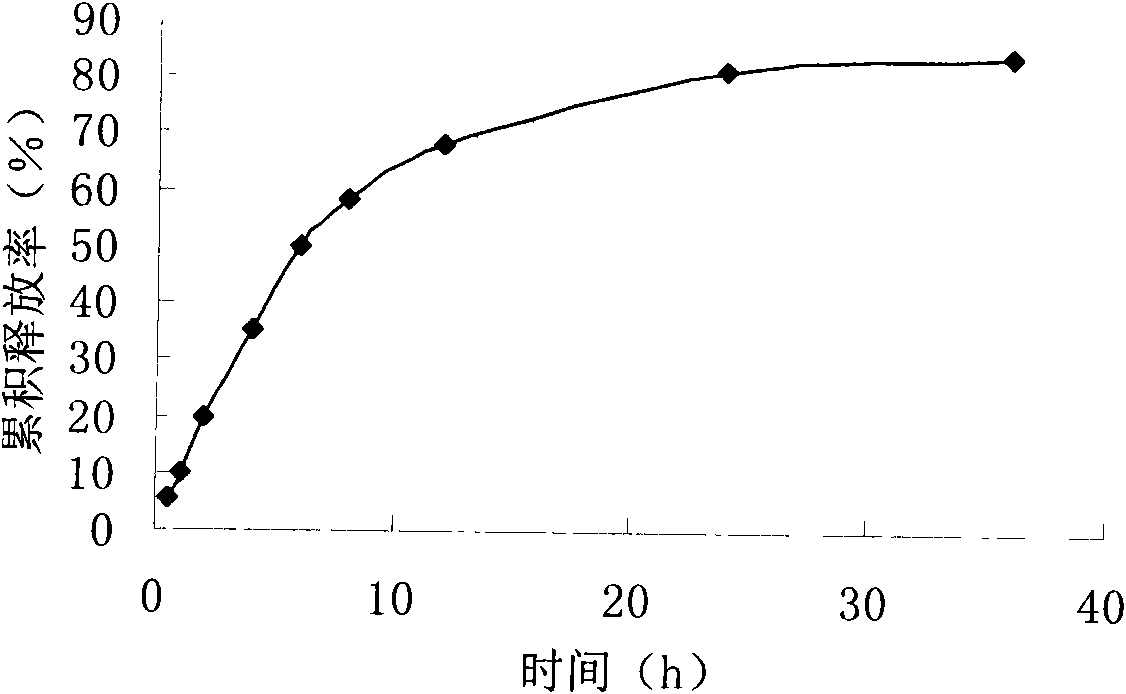
The invention provides teniposide liposome capable of being used for injection or oral administration, which is characterized in that teniposide is encapsulated by phospholipids, and the teniposide liposome with small grain diameter, high encapsulation rate, good stability and low toxic and side effect is prepared. The teniposide liposome prepared by the method improves the solubility and the stability of the teniposide, lowers the toxicity and prolongs the circulation time of drug in blood, thereby improving the treatment effect of the drug, and leading the preparation prepared by the liposome to have the characteristics of low toxicity, low hypersensitivity and high efficiency. The invention also relates to a preparation method of the teniposide liposome, which has simple process and lowcost and is suitable to industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method of determining acute myeloid leukemia response to treatment with farnesyltransferase inhibitors
ActiveUS20130130999A1Faster assayQuick forecastBiocideMicrobiological testing/measurementMyeloid leukemiaAptX
The disclosed method rapidly identifies with desired accuracy AML patients, including elderly AML patients, likely to respond to treatment with a combination of a farnesyltransferase inhibitor and one or more of etoposide, teniposide, tamoxifen, sorafenib, paclitaxel, temozolomide, topotecan, trastuzumab and cisplatinum. In an embodiment, the improvements include the use of whole blood rather than the customary bone marrow sample, thus making the assay more accurate, rapid, less intrusive, less expensive as well as less painful. The method includes evaluation of a two-gene expression ratio (RASGRP1:APTX), which with a corresponding threshold, provides sufficient accuracy for predicting the response to the combination treatment. In the preferred embodiment the combination treatment combines tipifarnib (R115777, ZARNESTRA®) with etoposide. Further, the elderly AML patients identified as being likely responsive to the combination treatment with tipinifarb and etoposide have a complete recovery rate comparable to the best therapy available for younger patients.
Owner:JANSSEN PHARMA NV
Teniposide medicinal composition and preparation method thereof
InactiveCN101632683ASolve solubilityFix stability issuesOrganic active ingredientsMacromolecular non-active ingredientsSolubilitySide effect
The invention relates to a teniposide medicinal composition and a preparation method thereof. The teniposide medicinal composition comprises active component teniposide, cyclodextrin, and derivatives or mixture thereof, and unnecessary excipient, wherein the teniposide is included by the cyclodextrin and / or the derivatives of the cyclodextrin. The medicinal composition improves the water solubility and stability of the teniposide, reduces toxic and side effects of the teniposide, and can be used as an initial material or a component for preparing injection or an oral preparation of the teniposide.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Medical combination of teniposide, the preparing method and the function thereof
ActiveCN101062049AClinically convenientLess irritatingOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention discloses a medicinal component of substitute nipa glycosides, which is characterized by the following: incorporating substitute nipa glycosides, Tuwen surface activator, assisting solvent and inorganic salt; choosing the assisting solvent as mixture of dimethyl ethanolamine and carbowax; choosing the inorganic salt from one or multiple common salt, potassium chloride, sulphate and sulphate; setting this component as injection or freeze dry powder needle; adding into Tuwen and the assisting dissolvent; adding into the inorganic salt at the same time; increasing solubility of substitute nipa glycosides. This invention also relates to the preparing method and usage of this medicinal component.
Owner:河北道恩药业有限公司
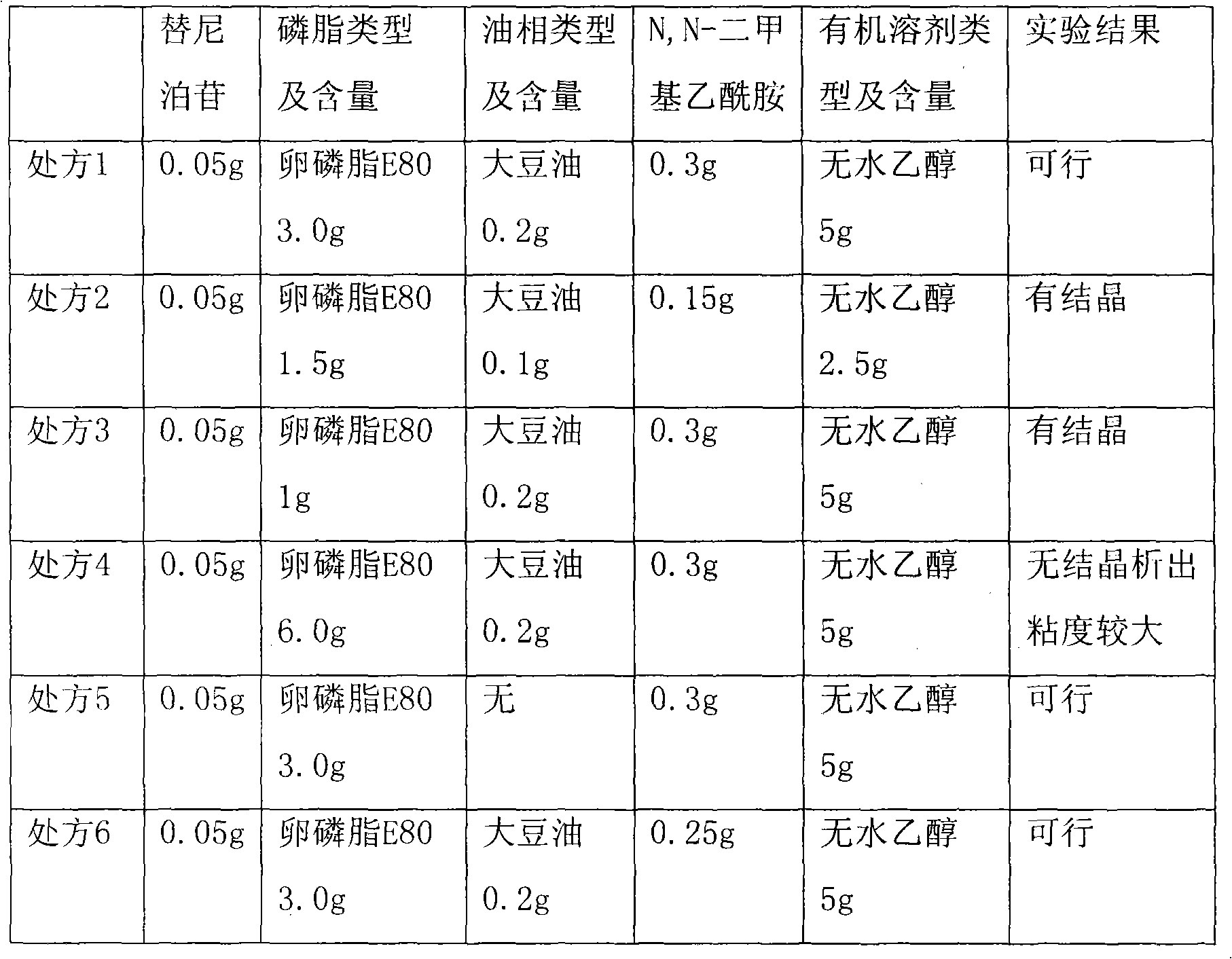
Teniposide emulsion and preparation method thereof
InactiveCN101502488AGood physical and chemical stabilityImprove bioavailabilityOrganic active ingredientsEmulsion deliveryOral medicationCurative effect
The invention relates to the technical field of medicine and discloses a teniposide emulsion and a preparation method thereof. The formula of the teniposide emulsion comprises the following components by the preparation mass: 5% to 30% of oil phase, 0.001% to 1.0% of teniposide, 0.5% to 5% of surfactant and 64% to 94.499% of water phase. The teniposide emulsion can be prepared through the three methods as follows: adding teniposide powder to blank micro-emulsion and mixing to obtain the teniposide emulsion; or, adding teniposide to a water-soluble medium containing an emulsifying agent, mixing with the oil phase and stirring on a magnetic basis to obtain the teniposide emulsion; or, adding teniposide to the oil phase containing an emulsifying agent, then, mixing with the water phase and stirring on a magnetic basis to obtain the teniposide emulsion. The invention improves the stability, compliance and curative effect of teniposide, the insoluble drug, reduces the toxicity and vascular irritation thereof. The invention can be clinically prepared into injections, lyophilized powder and emulsions for oral administration.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of etoposide, teniposide and analogs of etoposide and teniposide
InactiveCN105566412AEasy to operatePromote development and utilizationSugar derivativesSugar derivatives preparationProtecting groupGlycal
The invention discloses a preparation method of etoposide, teniposide and analogs of etoposide and teniposide. The preparation method includes the following steps of 1, selective protection of 4'domethylpodophyllotoxin4'hydroxy; 2, introduction of 4 hydroxy hydroxyl; 3, removal of a protecting group. The method is mild in reaction condition and environmentally friendly, and the yield and purity of the products are high.
Owner:JIANGXI NORMAL UNIVERSITY
Fluorouracil containing anti-cancer sustained-release injection
InactiveCN101234084AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Teniposide solid lipid nanoparticle and preparation method thereof
InactiveCN101596155ALow toxicityEliminate allergic reactionsOrganic active ingredientsAntineoplastic agentsSolubilityMedicine
The invention discloses a teniposide solid lipid nanoparticle and a preparation method thereof in industrial application. The teniposide solid lipid nanoparticle comprises an effective treatment dose of teniposide, a lipid material and an emulsifier, has smaller grain diameter, high entrapment rate and good stability, improves the solubility and the stability of the teniposide, reduces the toxicity of the teniposide, prolongs the cycle time of medicaments in blood and improves the therapeutic index of the medicaments, thereby having the characteristics of low toxicity and irritability and high efficiency in clinical application.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
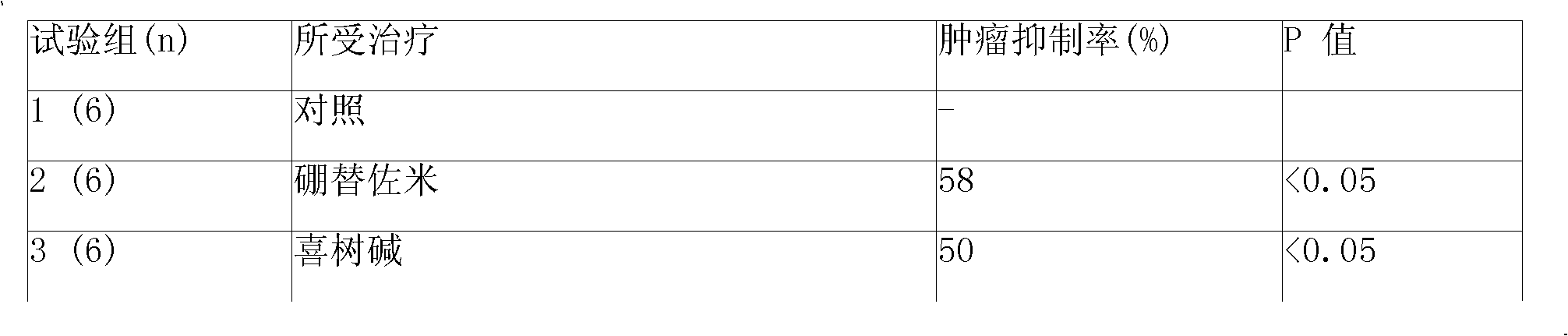
Sustained-released injection containing bortezomib and topology enzyme inhibitor
InactiveCN101336893ADipeptide ingredientsBoron compound active ingredientsLocal radiotherapyPhosphate
The invention relates to a slow-release injection containing bortezomib and topoismerase inhibitors. The slow-release injection is composed of slow-release microspheres and a solvent, wherein the slow-release microsphere contains an anticancer effective component selected from bortezomib and topoismerase inhibitor and a slow-release adjuvant, and the solvent is a common solvent or a special solvent containing suspending agent. The viscosity of the suspending agent is in the range from 100cp to 3000cp at a temperature ranging from 20 DEG C to 30 DEG C. The suspending agent is preferably sodium carboxymethylcellulose. The slow-release adjuvant is selected from a copolymer of poly(phosphate ester) (such as p(LAEG-EOP) and p(DAPG-EOP)) or a copolymer or a blend of poly(phosphate ester) and PLA or polifeprosan or PLGA or poly(erucic acid dipolymer-sebacic acid). The topoismerase inhibitor is selected from camptothecin, hydroxycamptothecine, topoteean, lurtotecan, irinotecan, etoposide and teniposide. The anticancer composition is also formulated as slow-release implant. After the intratumoral or peritumoral injection or implantation, the effective blood concentration lasts more than 60 days. Additionally, the slow-release injection can significantly reduce the general drug reaction and selectively enhance the chemotherapeutic effect, particularly the effect of non-operative treatment such as local radiotherapy. The slow-release injection is used for the treatment of various solid tumors.
Owner:济南基福医药科技有限公司
Intravenous injection microemulsion preparation of teniposide
This invention relates to a formulation and preparation method of micro emulsion of Teniposide for intravenous injection. The micro emulsion comprises active components and auxiliary materials, wherein the active components are Teniposide or mixture of Teniposide, paclitaxel, and etoposide; and auxiliary materials is composed of vegetable oil, emulsifying agent or assistant emulsifier, and organic solvent. The micro emulsion for intravenous injection is obtained by preparing active components and auxiliary materials to pre-concentration liquid and adding to 5% glucose solution for clinical use. The invention has the advatages of convenient adminstration, high bioavailability, and reduced toxic anaphylaxis.
Owner:PEKING UNIV
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
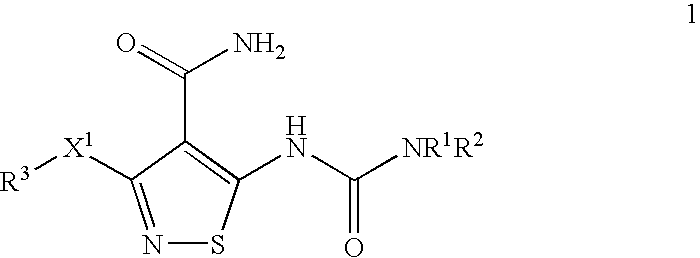
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Liposome preparation of teniposide phospholipid complexes and prepraring method thereof
InactiveCN101292957AImprove solubilityImprove lipophilicityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolEntrapment
The invention relates to a liposome preparation of teniposide phospholipid composite and the preparation method thereof. Clinical experiments prove that teniposide has a more broad-spectrum of anti-tumor activity, but the existing preparations in the process of use cause severe allergic reactions due to a large number of surfactants CremophorEL contained in the prescriptions. The invention provides a liposome preparation of teniposide phospholipid composite, which comprises the following components by weight percentages: 0.1-10 percent of the teniposide phospholipid composite, 0-80 percent of the phospholipid and 0-50 percent of cholesterol, wherein, the teniposide phospholipid composite is compounded by the teniposide and the phospholipid. The invention also provides the preparation method of the liposome preparation of teniposide phospholipid composite, as well as a lyophilized preparation, and not only overcomes the shortcomings of existing teniposide preparations in toxicity and allergy, but also improves the hydrophilicity and / or the lipotropy of the teniposide and enhance the entrapment rate and the stability of the liposome preparation thereof at the same time. The method is a novel drug sustained-release targeted preparation.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Fat emulsion pre-emulsifying concentrated solution for teniposide intravenous injection and preparation method thereof
InactiveCN101912362AImprove severe allergic deficienciesPrevent oxidationOrganic active ingredientsEmulsion deliveryOrganic solventFat emulsion
The invention belongs to the field of medicinal preparations and discloses fat emulsion pre-emulsifying concentrated solution for teniposide intravenous injection and a preparation method thereof. The fat emulsion pre-emulsifying concentrated solution for teniposide intravenous injection comprises the following components in percentage by weight: 0.01 to 10 percent of teniposide, 0 to 20 percent of oil phase, 10 to 80 percent of phospholipids and 10 to 99 percent of organic solvent for injection. The preparation method comprises the following steps of: dissolving the active ingredient, namely the teniposide, in the organic solvent or the oil phase or the mixture of the organic solvent and the oil phase, adding the other components in the formula into the mixture, and after stirring to uniformly mix the components, obtaining the transparent and clear pre-emulsifying concentrated solution.
Owner:PEKING UNIV
Sustained-release injection containing nitrosourea drugs
The invention provides a sustained-release injection containing nitrosourea drug (galamustine), which contains sustained-release microspheres and solvents. The sustained-release microspheres each comprise an anticancer-active component selected from nitrosourea drugs (such as nimustine and carmustine) and / or topoisomerase inhibitors, and a sustained-release agent. The solvents are common solvents or special solvents containing suspending agent. The viscosity of the suspending agent ranges from 100cp to 3000cp (at a temperature ranging from 20 DEG C to 30 DEG C). The suspending agent is selected from sodium carboxymethylcellulose and the like. The sustained-release agent is selected from p(LAEG-EOP) or p(DAPG-EOP) or other polyphosphate ester copolymers, or copolymer or blend of polyphosphate ester and PLA, polifeprosan, PLGA or poly(erucidic acid dipolymer-sebacic acid). The topoisomerase inhibitor is selected from camptothecin, hydroxycamptothecine, topotecan, lartotecan, irinotecan, etoposide or teniposide. The anticancer composition is also available in the dosage form of sustained-release implant, can retain the effective drug concentration for more than 60 days after intratumoral or local injection or implantation, can obviously reduce the systemic reaction to the drug, and can selectively enhance the curative effect of non-operative treatments such as radiotherapy and chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
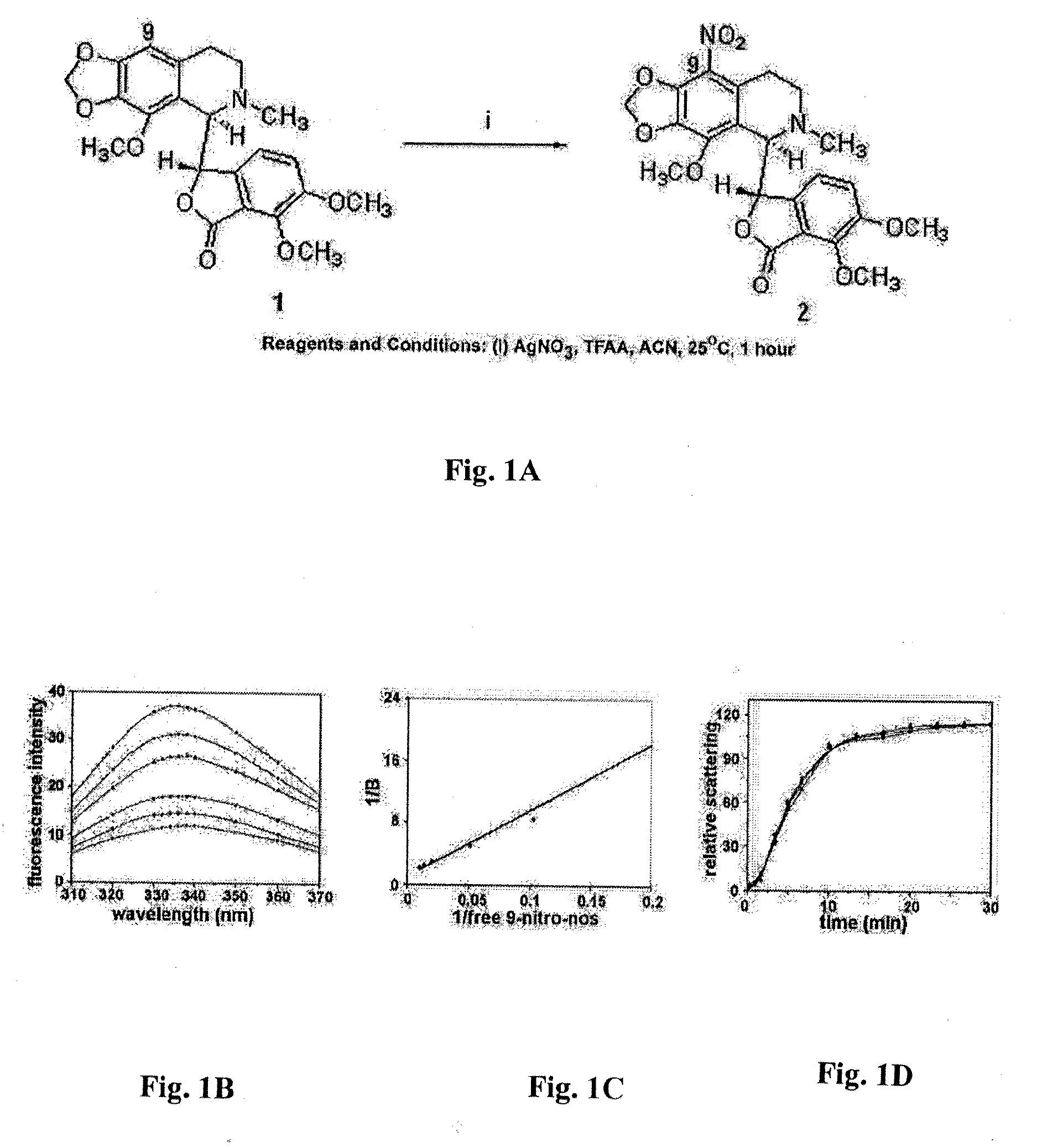
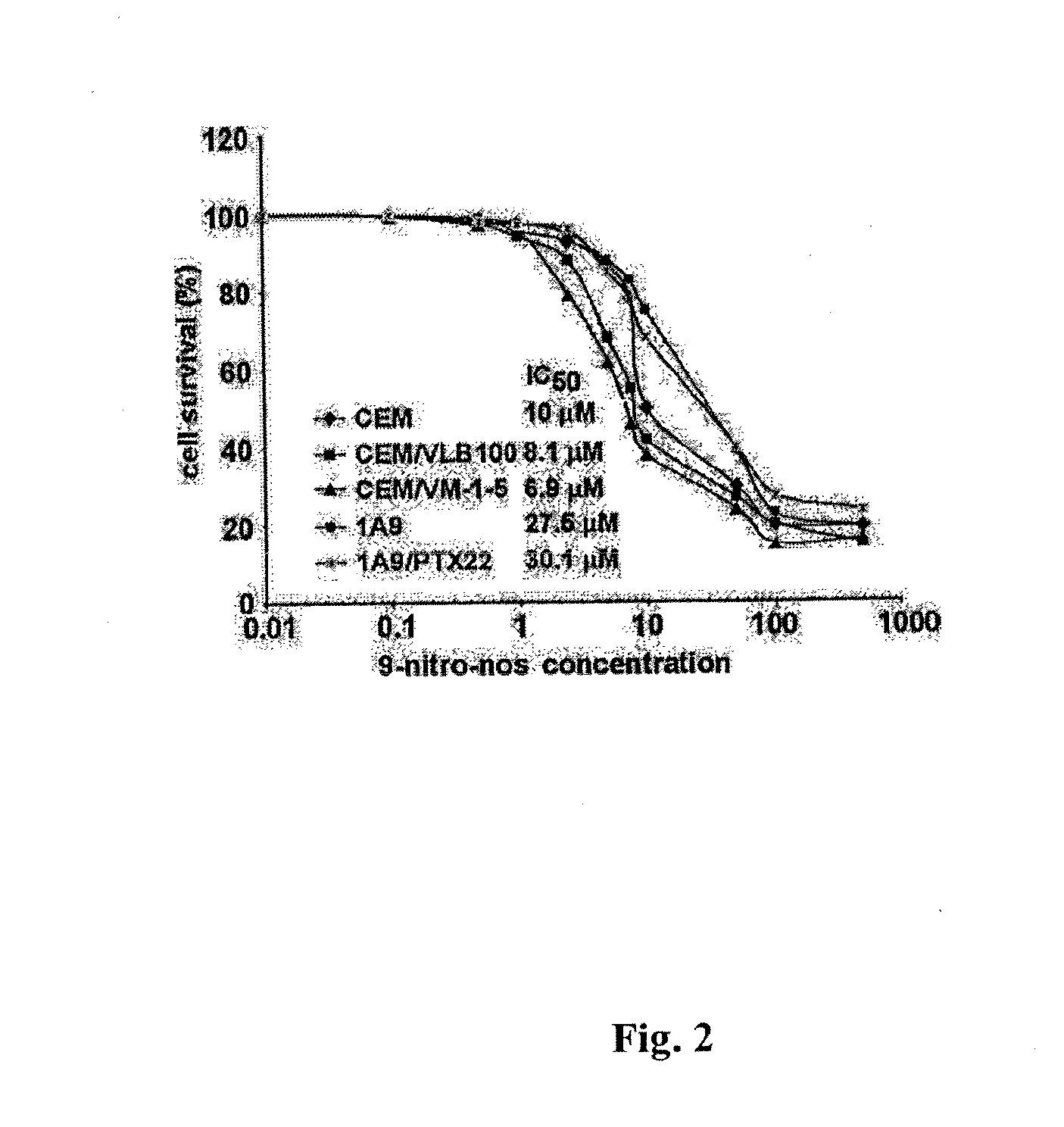
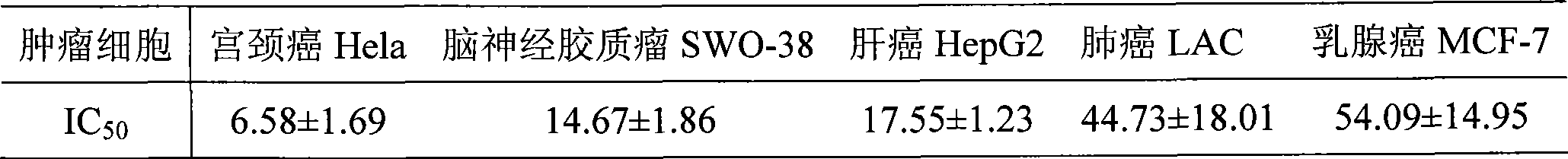
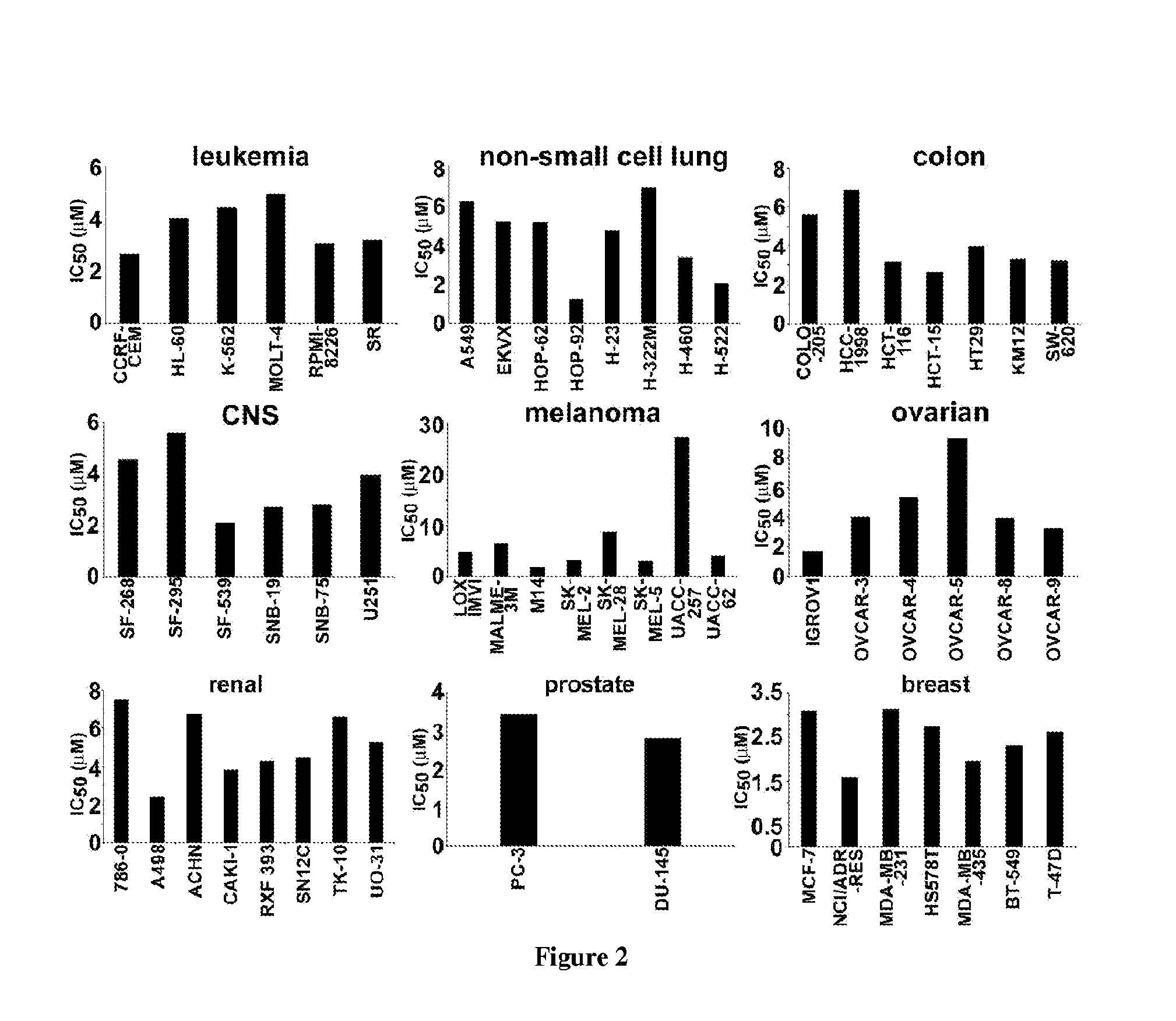
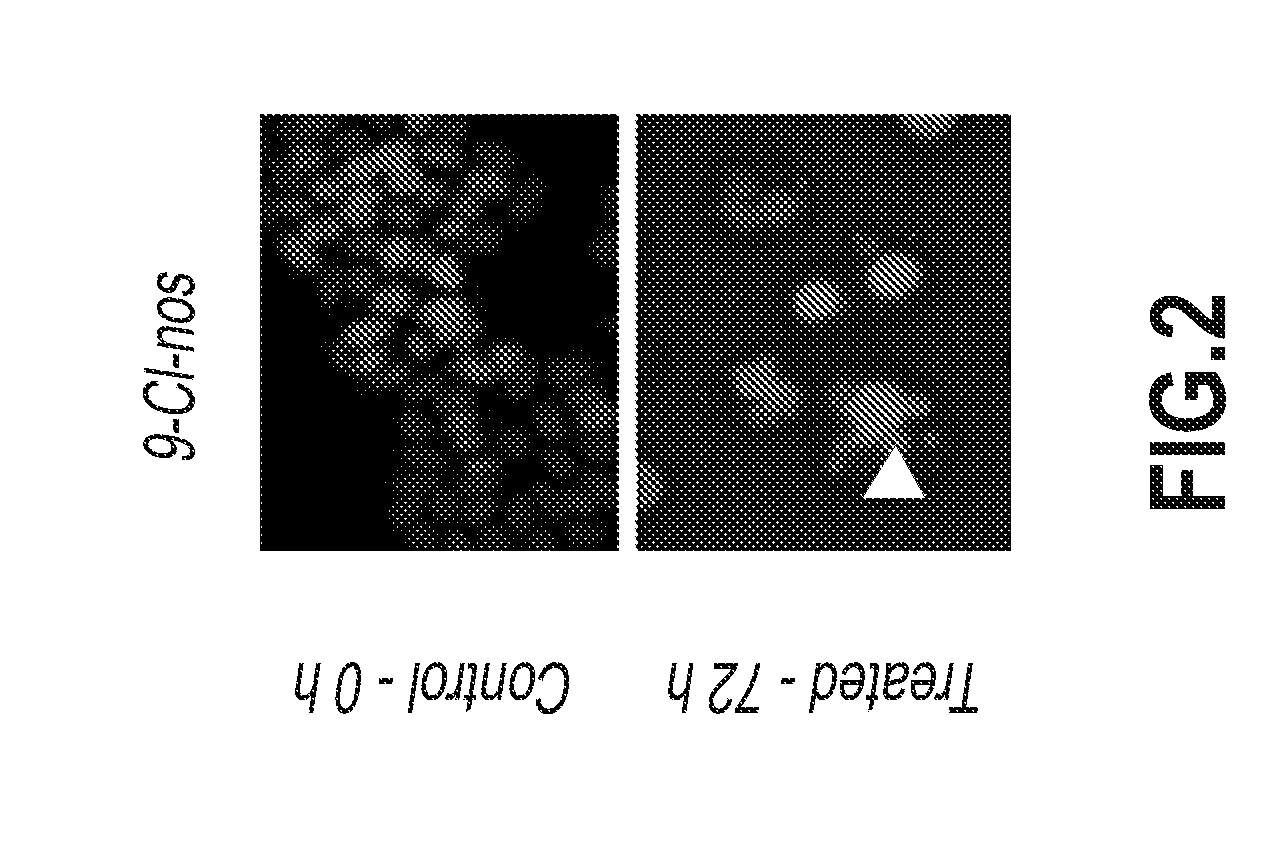
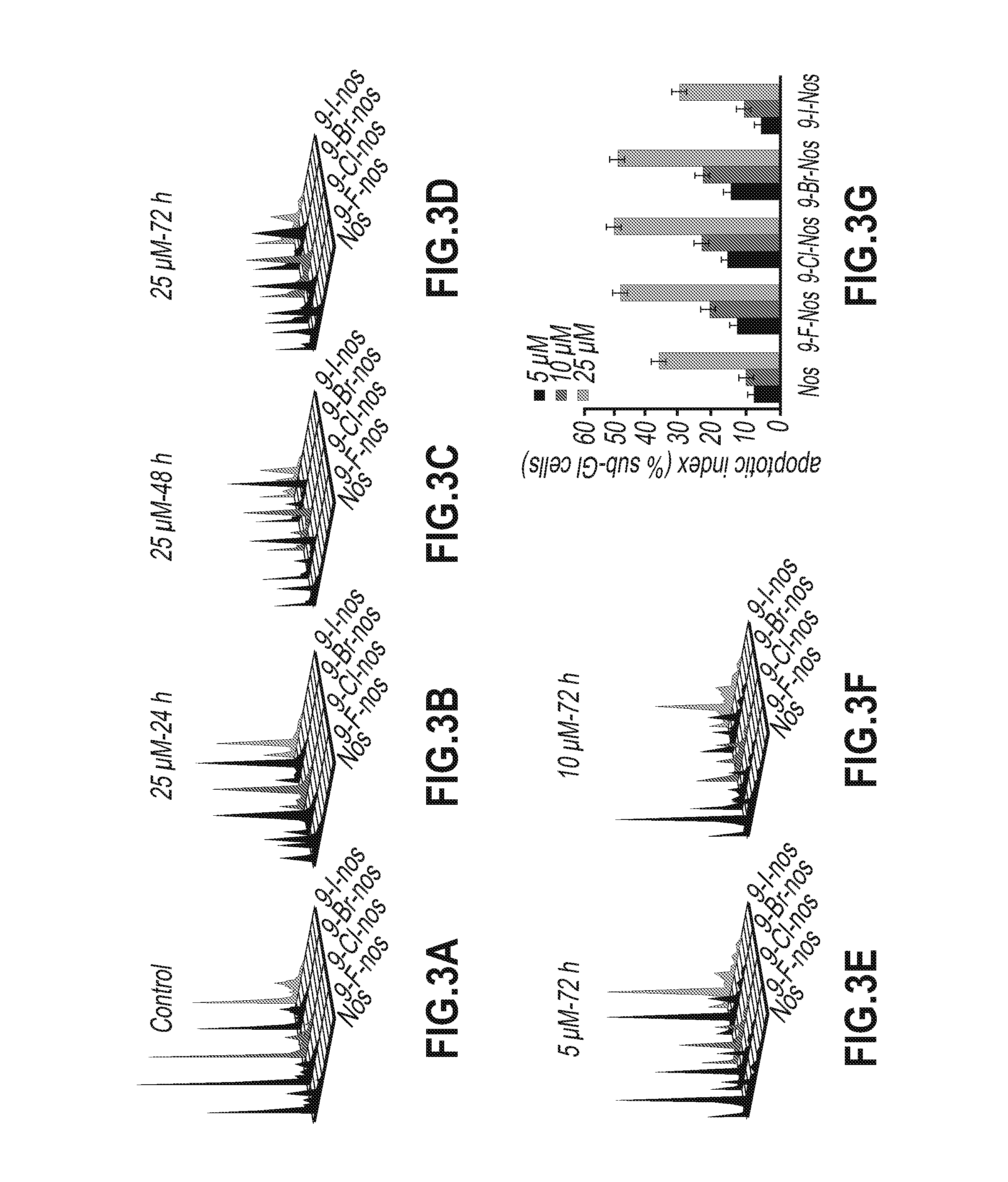
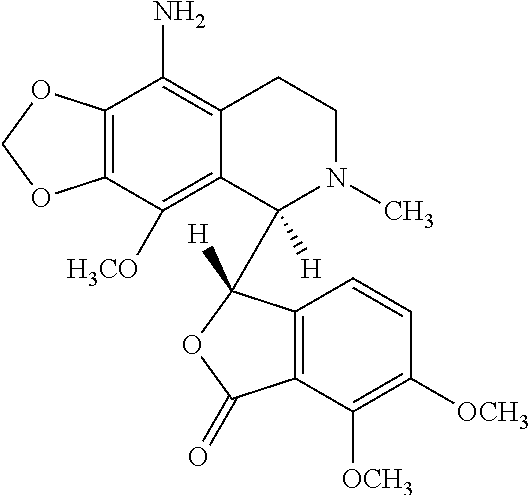
Noscapine analogs and their use in treating cancers, including drug-resistant cancers
InactiveUS20100227878A1Inhibit cell proliferationBiocideOrganic chemistryStructure functionMitotic arrest
Compounds, pharmaceutical compositions including the compounds, and methods of preparation and use thereof are disclosed. The compounds are noscapine analogs. The compounds and compositions can be used to treat and / or prevent a wide variety of cancers, including drug resistant cancers. While the antitussive plant alkaloid, noscapine, binds tubulin, displays anticancer activity, and has a safe pharmacological profile in humans, structure-function analyses pointed to a proton at position 9 of the isoquinoline ring that can be modified without compromising tubulin binding activity. Noscapine analogs with various functional moieties at position 9 on the isoquinoline ring kill human cancer cells resistant to other anti-microtubule agents, such as vincas and taxanes. Representative analogs include the 9-nitro, 9-bromo-, 9-iodo-, and 9-fluoro-noscapines, which bind tubulin and induce apoptosis selectively in tumor cells (ovarian and T-cell lymphoma) resistant to paclitaxel, vinblastine and teniposide. Surprisingly, treatment with one of the analogs, 9-nitro-nos, at doses as high as 100 μM, did not affect the cell cycle profile of normal human fibroblasts. This selectivity for cancer cells represents a unique edge over the other available antimitotics. The compounds can perturb the progression of cell cycle by mitotic arrest, followed by apoptotic cell death associated with increased caspase-3 activation and appearance of TUNEL-positive cells. Thus, the compounds are novel therapeutic agents for a variety of cancers, including ovarian and T-cell lymphoma cancers, even those that have become drug-resistant to currently available chemotherapeutic drugs.
Owner:EMORY UNIVERSITY
Antineoplastic combined medicament with enhancing and poison-reducing character
InactiveCN101428034AGrowth inhibitionPossess synergistic and detoxifying effectOrganic active ingredientsAntineoplastic agentsUltrasound attenuationMedicine
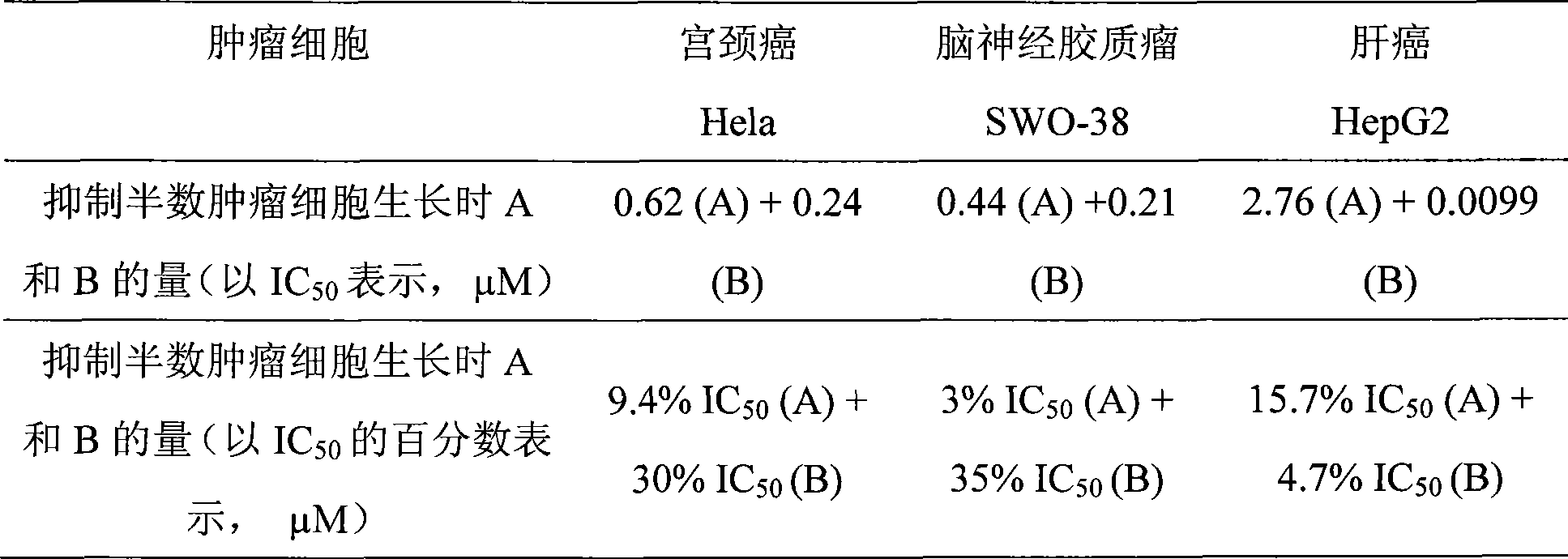
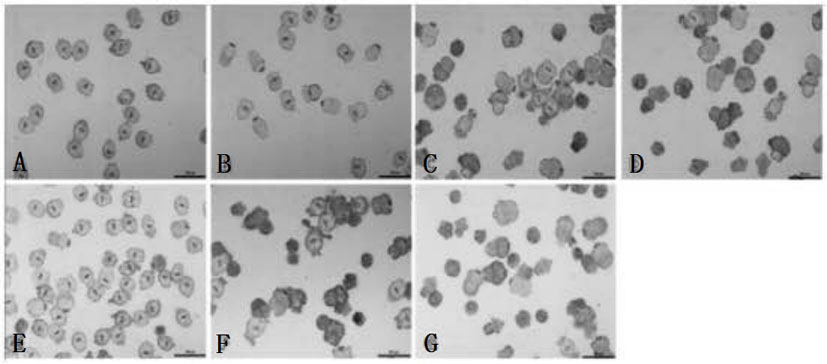
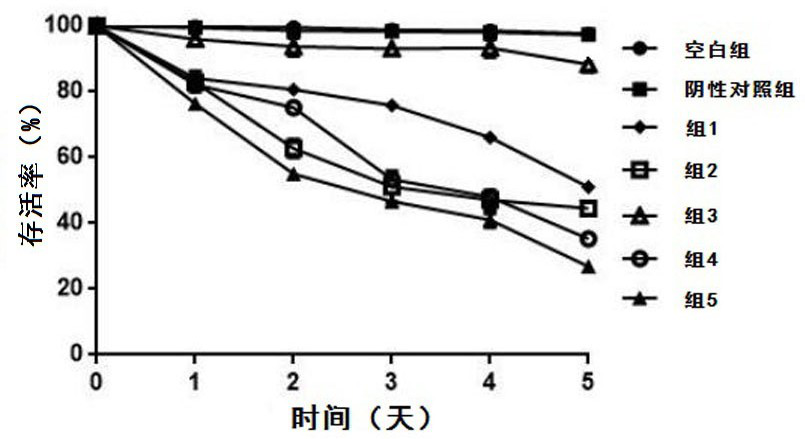
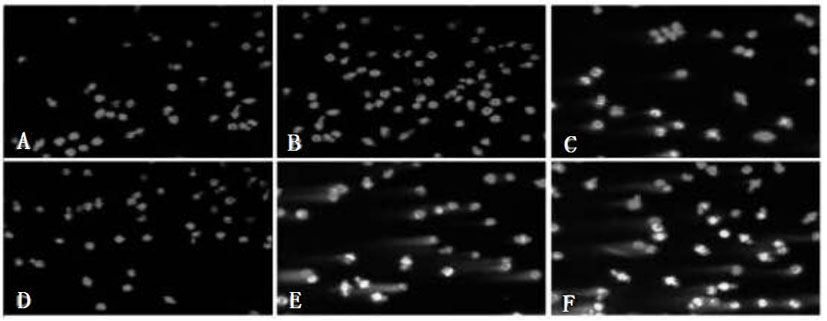
The invention relates to an antineoplastic compound medicine having synergism and attenuation effects. The compound medicine mainly comprises teniposide and derivant of arteannuin. Owing to the synergism and attenuation effects in inhibiting the growth of specific tumor cells, the compound medicine is suitable for preparing antineoplastic medicine, particularly for preparing medicines for treating cerebral glioma, liver cancer and cervical carcinoma.
Owner:GUANGZHOU YUNZHONG BIOTECH
Liposome preparation of teniposide phospholipid complexes and preparing method thereof
InactiveCN101292957BImprove solubilityImprove lipophilicityOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolPhospholipid complex
The invention relates to a liposome preparation of teniposide phospholipid composite and the preparation method thereof. Clinical experiments prove that teniposide has a more broad-spectrum of anti-tumor activity, but the existing preparations in the process of use cause severe allergic reactions due to a large number of surfactants CremophorEL contained in the prescriptions. The invention provides a liposome preparation of teniposide phospholipid composite, which comprises the following components by weight percentages: 0.1-10 percent of the teniposide phospholipid composite, 0-80 percent ofthe phospholipid and 0-50 percent of cholesterol, wherein, the teniposide phospholipid composite is compounded by the teniposide and the phospholipid. The invention also provides the preparation method of the liposome preparation of teniposide phospholipid composite, as well as a lyophilized preparation, and not only overcomes the shortcomings of existing teniposide preparations in toxicity and allergy, but also improves the hydrophilicity and / or the lipotropy of the teniposide and enhance the entrapment rate and the stability of the liposome preparation thereof at the same time. The method is a novel drug sustained-release targeted preparation.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Docetaxel-containing anti-cancer sustained-release injection
InactiveCN101234085AEasy to operateGood repeatabilityOrganic active ingredientsPharmaceutical delivery mechanismPolyethylene glycolSuspending Agents
The invention relates to anticancer sustained release injection which comprises sustained release microspheres and menstruum, wherein, the sustained release microspheres comprise anticancer active components and sustained release auxiliary material; the menstruum is special menstruum that contains suspending agent. The anticancer active components are fotemustine, nimustine, carmustine or combination of bendamustine and mitozolomide, docetaxel, etoposide, teniposide, vinblastine, anastrozole, tamoxifen, fluorouracil or mitomycin C; the sustained release auxiliary material is polylactic acid and polylactic acid copolymer, polyethylene glycol and polylactic acid copolymer of polyethylene glycol, terminal carboxyl group polylactic acid copolymer, EVAc, fatty acid and decanedioic acid copolymer, etc.; viscosity of the suspending agent is 100cp-3,000cp (at 25 DEG C-30 DEG C), and the suspending agent is selected from sodium carboxymethylcellulose, etc. The sustained release microspheres can also be made into sustained release implant; the injection or implant is injected or placed in or around tumor so as to reduce general reaction of the drug and selectively improve and keep local concentration for about 30-50 days. The anticancer sustained release injection can be used solely and can also promote anti-tumor effects of non-operative treatments, such as chemotherapy and / or radiotherapy, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Teniposide derivative, preparation method therefor and application of teniposide derivative
PendingCN111454308AImprove anti-tumor activitySmall toxicitySugar derivativesSugar derivatives preparationSide effectThiazole
The invention discloses a teniposide derivative, a synthesis method therefor and application of the teniposide derivative. The teniposide derivative represented by a formula (V) shown in the description. The anti-tumor activity of the teniposide derivative is improved remarkably and the toxic or side effects are lowered, is obtained through introducing a heteroaromatic compound with low toxicity such as 5-fluoro-benzothiazol-2-thiol or 5-fluoro-benzoxazol-2-thiol into 2' and 3' positions of a saccharide ring of teniposide by means of an ester bond or amide bond. Shown by in-vitro tumor cell activity inhibiting experiments, the toxic or side effects of the compound represented by the formula (V) disclosed by the invention are remarkably lowered compared with those of the teniposide on the basis that the anti-tumor activity of the compound is equivalent to that of the teniposide.
Owner:汤亚杰
Composition combining DNA damage causing compounds with DNA damage repair inhibitors, and preparation method and application of composition
InactiveCN111714638AGood treatment effectOrganic active ingredientsCyclic peptide ingredientsRAD51Chemical compound
The invention relates to the technical field of preparation of medicine for treating echinococcosis, in particular to a composition combining DNA damage causing compounds with DNA damage repair inhibitors, and a preparation method and application of the composition. The composition comprises the DNA damage causing compounds and the DNA damage repair inhibitors. The DNA damage causing compounds areone or more kind of materials of harmine, harmine derivatives, adriamycin, dactinomycin, daunorubicin, etoposide and teniposide; and the DNA damage repair inhibitors are one or more kind of materialsof an RAD51 inhibitor and a BRCA1 inhibitor. Through combined use of the DNA damage causing compounds and the DNA damage repair inhibitors, the composition provided by the invention is used for treating the echinococcosis; the effect of realizing the damage to worm body DNA by the DNA damage causing compounds and realizing the inhibition on the DNA damage repair by the DNA damage repair inhibitors at the same time can be achieved; the worm body DNA damage repair process is completely blocked; finally, the worm body apoptosis is caused; the anti-echinococcosis effect is achieved; and the anti-echinococcosis treatment effect is improved.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY +1
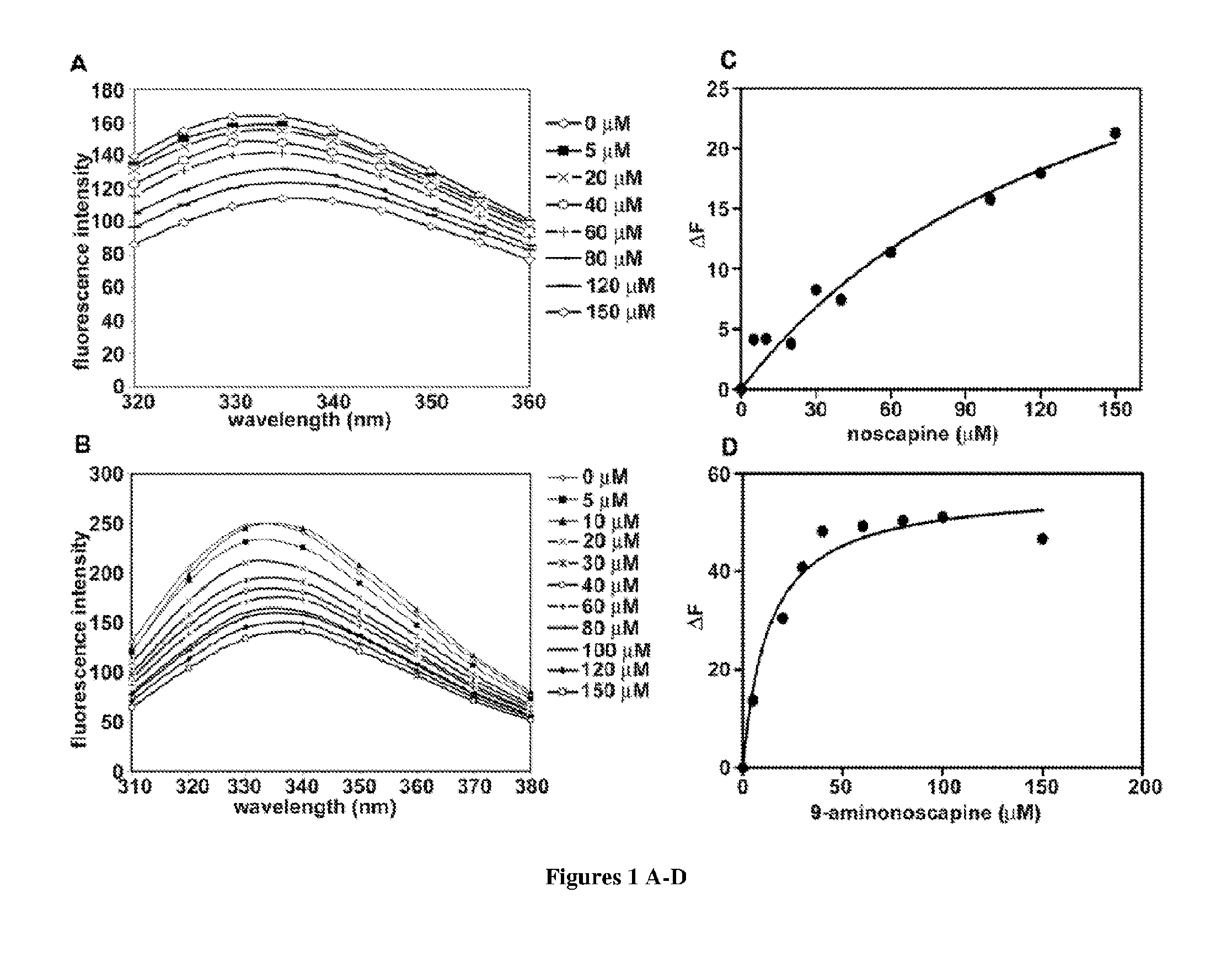
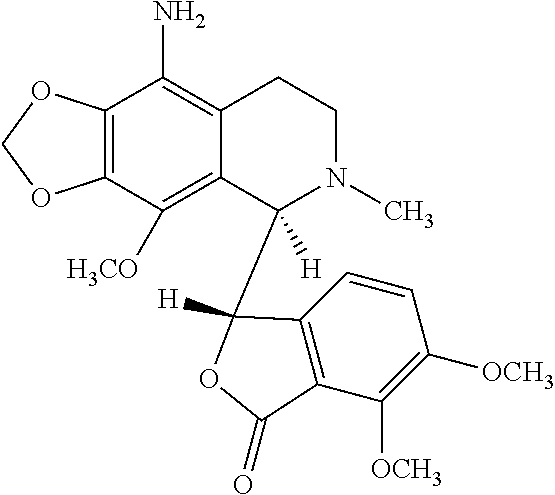
9-aminonoscapine and its use in treating cancers, including drug-resistant cancers
ActiveUS20110294844A1Inhibit cell proliferationHalt cell cycle progressionBiocideOrganic chemistryCaspaseBiological activation
9-aminonoscapine, prodrugs thereof, and pharmaceutically acceptable salts thereof, are disclosed. Pharmaceutical compositions including 9-aminonoscapine, and methods of preparation and use thereof are disclosed. 9-aminonoscapine is a noscapine analog that can be used to treat and / or prevent a wide variety of cancers, including drug resistant cancers, by binding tubulin and inducing apoptosis selectively in tumor cells (ovarian and T-cell lymphoma) resistant to paclitaxel, vinblastine and teniposide. 9-aminonoscapine can perturb the progression of cell cycle by mitotic arrest, followed by apoptotic cell death associated with increased caspase-3 activation and appearance of TUNEL-positive cells. Thus, 9-aminonoscapine is a novel therapeutic agents for a variety of cancers, including ovarian and T-cell lymphoma cancers, even those that have become drug-resistant to currently available chemotherapeutic drugs.
Owner:EMORY UNIVERSITY
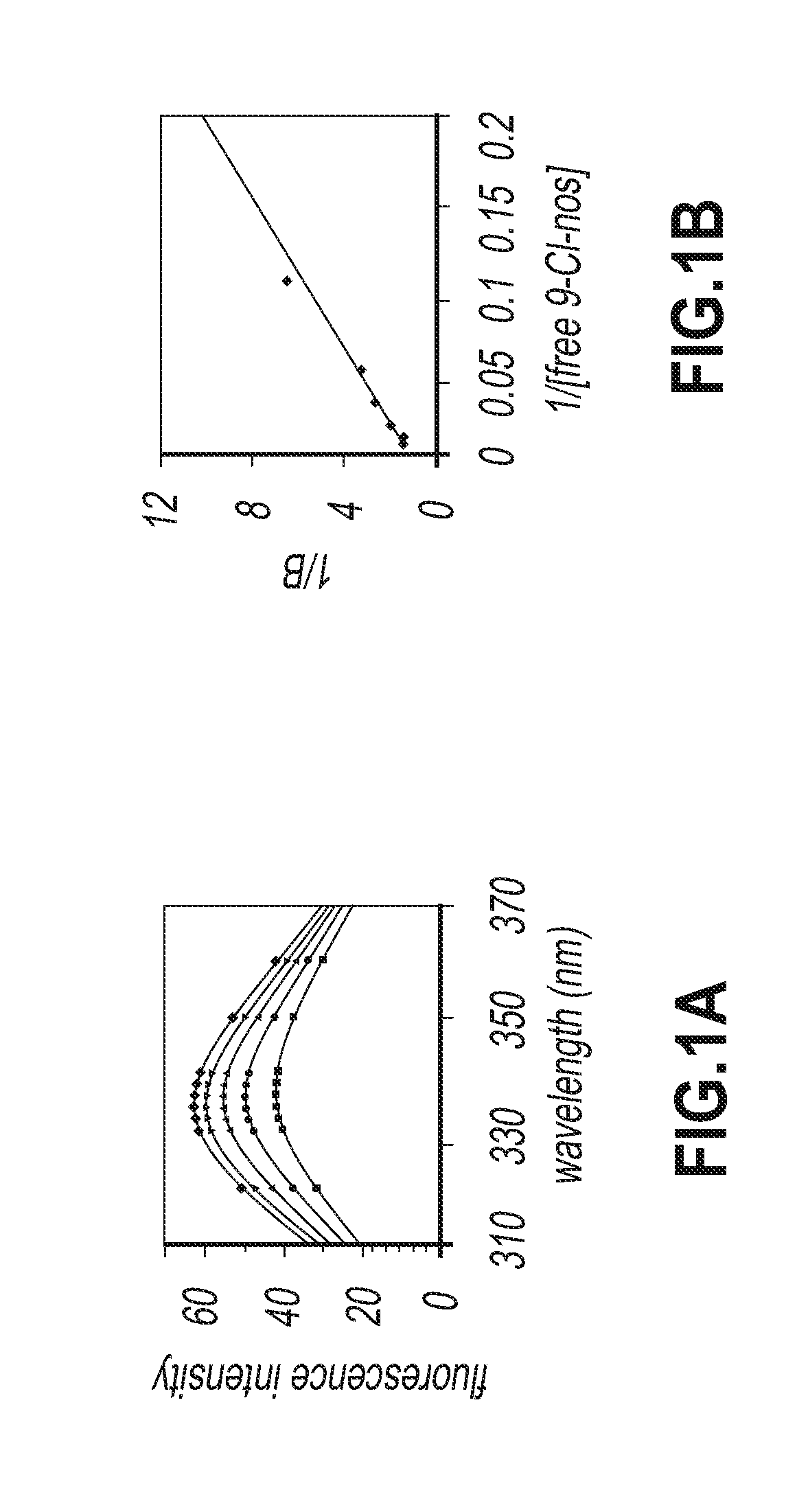
9-chloro noscapine and its use in treating cancers, including drug-resistant cancers
InactiveUS20100249172A1Inhibit cell proliferationBiocideOrganic chemistryCaspaseBiological activation
9-Chloro-nos, prodrugs thereof, and pharmaceutically acceptable salts thereof, are disclosed. Pharmaceutical compositions including 9-chloro-nos, and methods of preparation and use thereof are disclosed. 9-Chloro-nos is a noscapine analog that can be used to treat and / or prevent a wide variety of cancers, including drug resistant cancers, by binding tubulin and inducing apoptosis selectively in tumor cells (ovarian and T-cell lymphoma) resistant to paclitaxel, vinblastine and teniposide. 9-Chloro-nos can perturb the progression of cell cycle by mitotic arrest, followed by apoptotic cell death associated with increased caspase-3 activation and appearance of TUNEL-positive cells. Thus, 9-chloro-nos is a novel therapeutic agents for a variety of cancers, including ovarian and T-cell lymphoma cancers, even those that have become drug-resistant to currently available chemotherapeutic drugs.
Owner:EMORY UNIVERSITY
Application of teniposide in anti-mycobacterium tuberculosis drugs
ActiveCN110200983ASignificant anti-tuberculosis effectIncrease the value of reuseAntibacterial agentsOrganic active ingredientsMalignant lymphomaLymphocyte
The invention relates to an application of teniposide in anti-mycobacterium tuberculosis drugs. In clinic, teniposide is mainly used for treating malignant lymphoma and acute lymphoblastic leukemia; researches find out that teniposide has anti-tuberculosis effect and has good development value; the invention discloses an application of teniposide in anti-mycobacterium tuberculosis drugs and the obvious anti-mycobacterium tuberculosis effect of teniposide for the first time.
Owner:SHENZHEN UNIV
Gel injection of sustained-released topology enzyme inhibitor and preparation method thereof
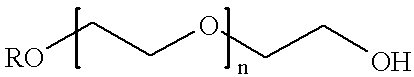
InactiveCN101336892APharmaceutical delivery mechanismPharmaceutical non-active ingredientsPolyesterPolyethylene glycol
The invention relates to a slow-release topoismerase inhibitor gel injection which contains an anticancer-effective amount of topoismerase inhibitor microspheres (or microsphere and topoismerase inhibitor micropowder), an amphiphilic block polymer composed of polyethylene glycol and polyester, a solvent and a certain amount of slow-release regulator. The amphiphilic block polymer aqueous solution is liquid at the room temperature and turns into a semisolid or solid biodegradable water-insoluble gel in vivo in warm-blooded animals, so that the topoismerase inhibitor encapsulated therein is sustainedly and locally released around the tumor and the microspheres can further control the slow release for a plurality of weeks to a plurality of months. After the intratumoral injection or peritumoral injection or post-operative intracavitary injection or arterial embolization, the slow-release topoismerase inhibitor gel injection can significantly reduce the general drug reaction and selectively enhance the curative effect of non-operative treatment such as chemotherapy and radiotherapy, and is used for the treatment of tumors of different stages. The topoismerase inhibitor is selected from camptothecin, topoteean, etoposide, teniposide, esorubicin, pirarubicin and valrubicin.
Owner:济南基福医药科技有限公司
An anticancer sustained release injection carrying tumor drug resistance reversal agent and reversal agent synergist
Disclosed is an anticancer slow release injection carrying tumor drug resistance reversal agents and reversal agent synergistic agent, which comprises slow release micro-balloons and dissolvent, wherein the slow release micro-balloons use slow release auxiliary materials as the carrying agent, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active ingredient in the micro-balloons is reversal agent synergistic agent selected from Tipifarnib, Ronafarnib, Valspodar, and / or selected from monohydric camptothecine, Mitozolomide, docetaxel, Oxaliplatin, Eptaplatin, Ifosfamide, Lomustine, Estramustine, Fotemustine, Samostine or Teniposide, the slow release auxiliary materials are selected from Polifeprosan, di-aliphatic acid and sebacylic acid copolymer, poly(erucic aciddipolymer-sebacylic acid), poly(fumaric acid-sebacylic acid) and EVAc.
Owner:JINAN KANGQUAN PHARMA TECH
Compound sustained-released injection containing marimastat as neovascularization inhibitor
InactiveCN101336911APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDepressantSuspending Agents
A compound sustained-released injection containing an angiogenesis inhibitor marimastat comprises sustained-released microspheres and a solvent. The sustained-released microspheres comprise a sustained-released adjuvant, an angiogenesis inhibitor selected from marimastat and fumagillin, and a cell toxicant selected from hydroxycamptothecin, mitozolomide, 4-carboxy temozolomide, docetaxel, oxaliplatin, sunplatinum, iphosphamide, lomustine, estramustine, fotemustine, semustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil and mitomycin c; and the solvent is a common solvent or a special solvent containing a suspending agent. The sustained-released adjuvant is selected from polifeprosan, poly(lactic acid), sebacic acid polymer such as poly(erucic acid dimmer-sebacic acid) and poly(fumaric acid-sebacic acid), EVAc, etc.; and the suspending agent has a viscosity of 100-3,000cp (20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc. The sustained-released microspheres can also be made into a sustained-released implant, which can enhance the curative effect of non-operative treatments such as chemotherapy and radiotherapy by intratumoral or peritumoral injection or placement.
Owner:JINAN KANGQUAN PHARMA TECH
9-aminonoscapine and its use in treating cancers, including drug-resistant cancers
InactiveUS20130150332A1Inhibit cell proliferationBiocideOrganic chemistryCaspaseBiological activation
9-aminonoscapine, prodrugs thereof, and pharmaceutically acceptable salts thereof, are disclosed. Pharmaceutical compositions including 9-aminonoscapine, and methods of preparation and use thereof are disclosed. 9-aminonoscapine is a noscapine analog that can be used to treat and / or prevent a wide variety of cancers, including drug resistant cancers, by binding tubulin and inducing apoptosis selectively in tumor cells (ovarian and T-cell lymphoma) resistant to paclitaxel, vinblastine and teniposide. 9-aminonoscapine can perturb the progression of cell cycle by mitotic arrest, followed by apoptotic cell death associated with increased caspase-3 activation and appearance of TUNEL-positive cells. Thus, 9-aminonoscapine is a novel therapeutic agents for a variety of cancers, including ovarian and T-cell lymphoma cancers, even those that have become drug-resistant to currently available chemotherapeutic drugs.
Owner:EMORY UNIVERSITY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com