Teniposide emulsion and preparation method thereof
A technology of niposide emulsion and teniposide, which is applied in the field of medicine, can solve the problems of patients with blood vessel blockage, instability, and large blood vessel irritation, and achieve the effects of reducing irritation and toxicity, stable properties, and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A method for preparing a teniposide emulsion, the composition of which is as follows:
[0025]
[0026] Include the following steps:
[0027] Mix the prescribed amount of Tween 80, Span 80, medicinal soybean oil, and purified water evenly to prepare a medicinal liquid for later use. Add teniposide powder to the medicinal liquid, disperse it through an ultrasonic emulsifier, filter and fill it, and extinguish it. Bacteria, serial number, and the product is obtained.
Embodiment 2
[0029] A method for preparing a teniposide emulsion, the composition of which is as follows:
[0030]
[0031] Include the following steps:
[0032] Dissolve the prescribed amount of teniposide in the mixed solution of lecithin, Span 20, and MCT to obtain an oil phase. At the same time, mix glycerin and purified water with magnetic stirring, add the oil phase to the water phase and disperse through a ultrasonic emulsifier. Filtration filling, sterilization, numbering, the product is obtained.
Embodiment 3
[0034] A method for preparing a teniposide emulsion, the composition of which is as follows:
[0035]
[0036] Include the following steps:
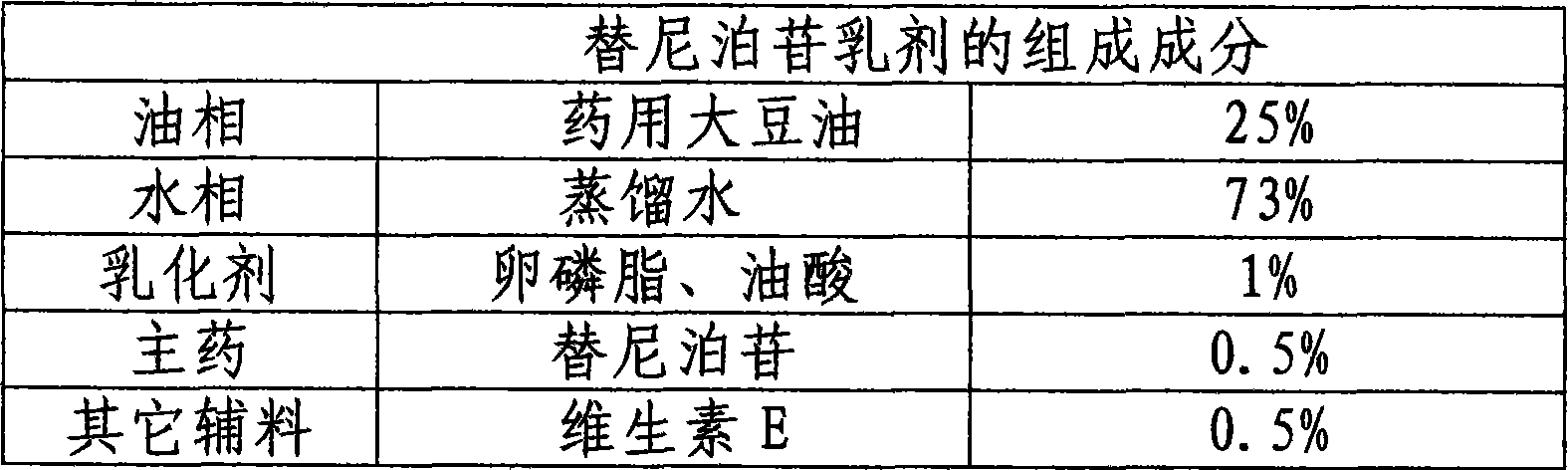
[0037] Dissolve the prescribed amount of teniposide in the purified water, lecithin, and oleic acid mixture solution, and stir for several minutes until each component dissolves to obtain an aqueous phase. At the same time, vitamin E is added to the medicinal soybean oil, heated and dissolved to obtain an oil phase. Add the oil phase to the water phase to disperse through an ultrasonic emulsifier, filter and fill, sterilize, and number to obtain the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com