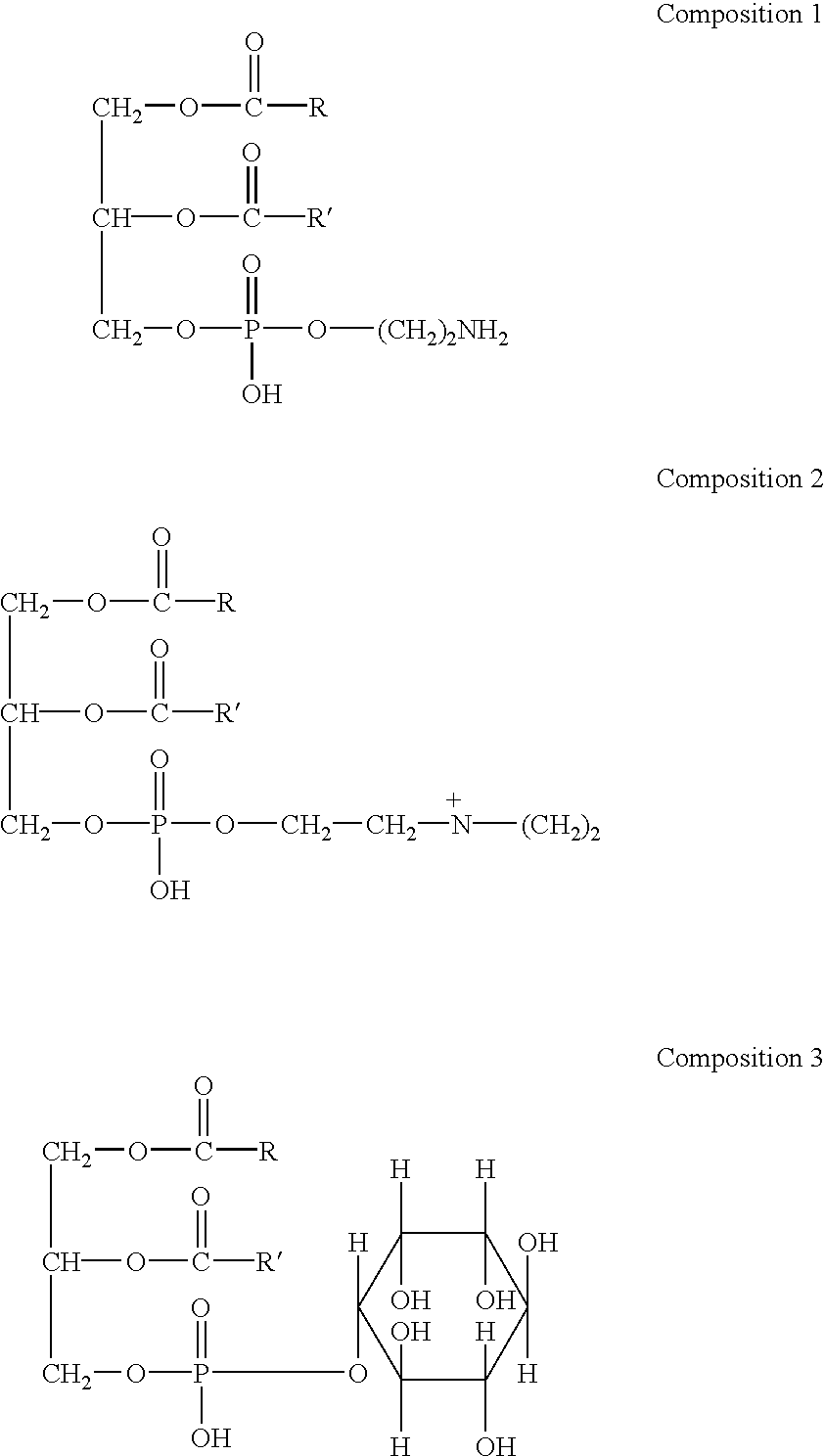Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
6699 results about "Lecithin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lecithin (UK: /ˈlɛsɪθɪn/, US: /ˈlɛsəθɪn/, from the Greek lekithos "yolk") is a generic term to designate any group of yellow-brownish fatty substances occurring in animal and plant tissues which are amphiphilic – they attract both water and fatty substances (and so are both hydrophilic and lipophilic), and are used for smoothing food textures, emulsifying, homogenizing liquid mixtures, and repelling sticking materials.
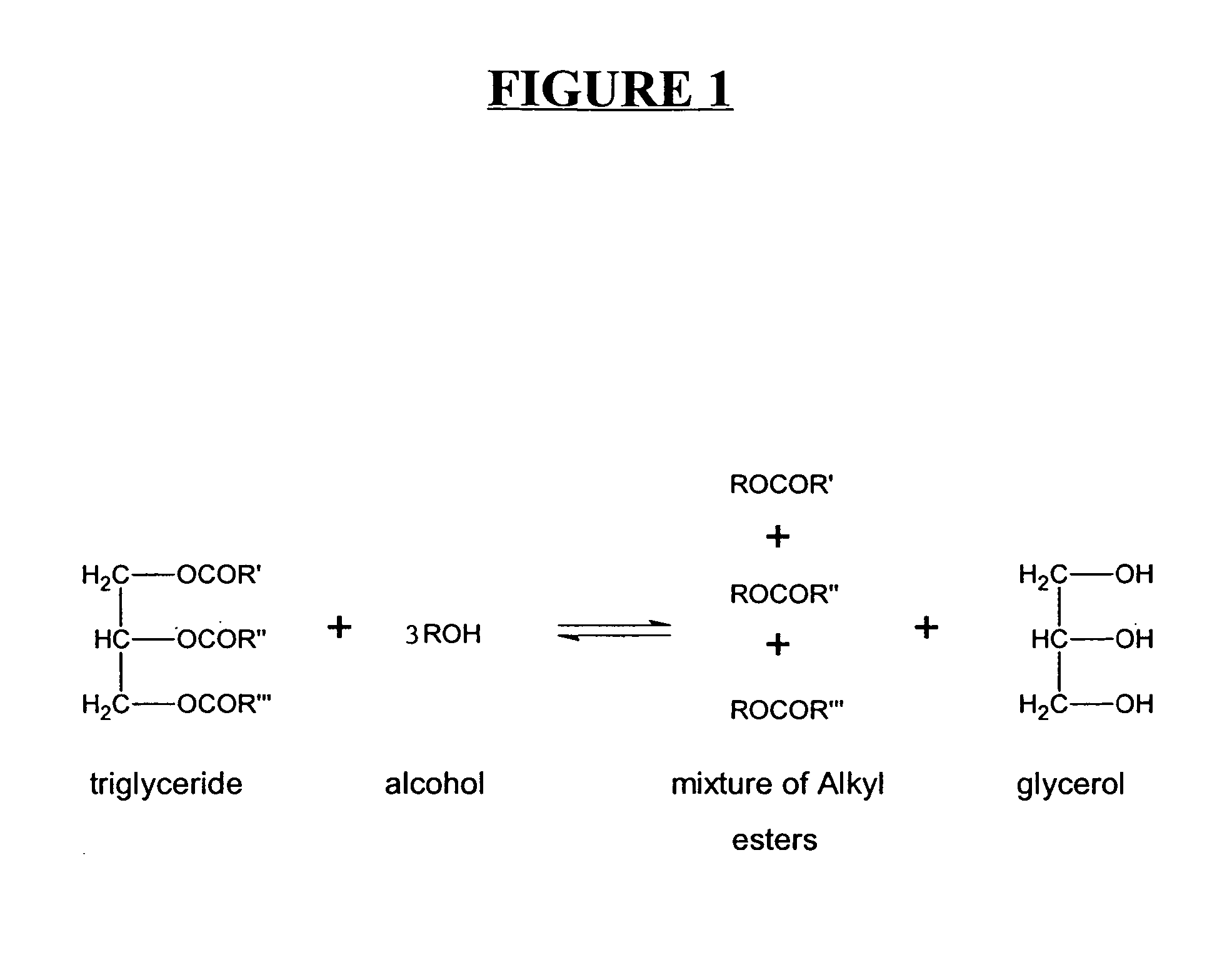
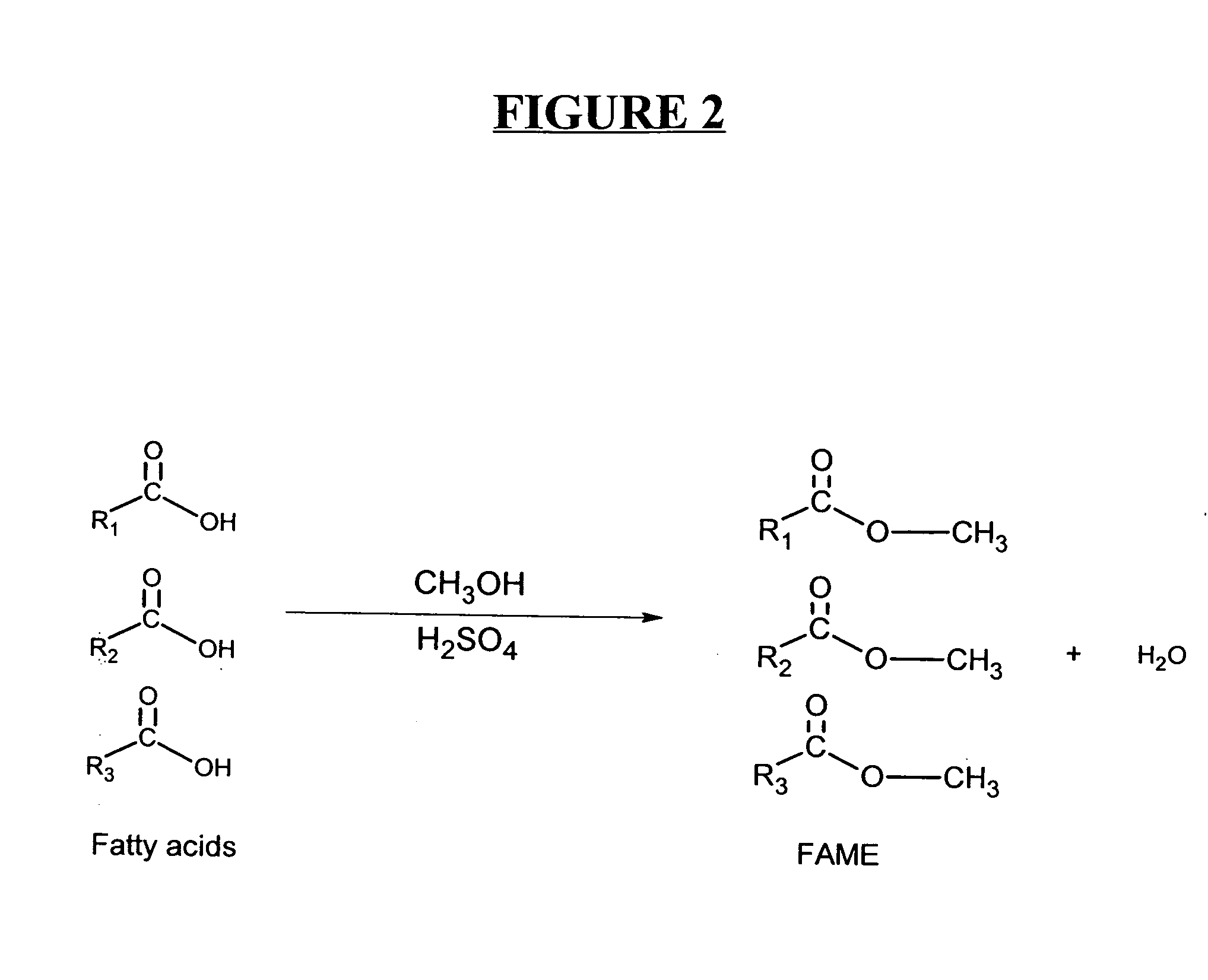
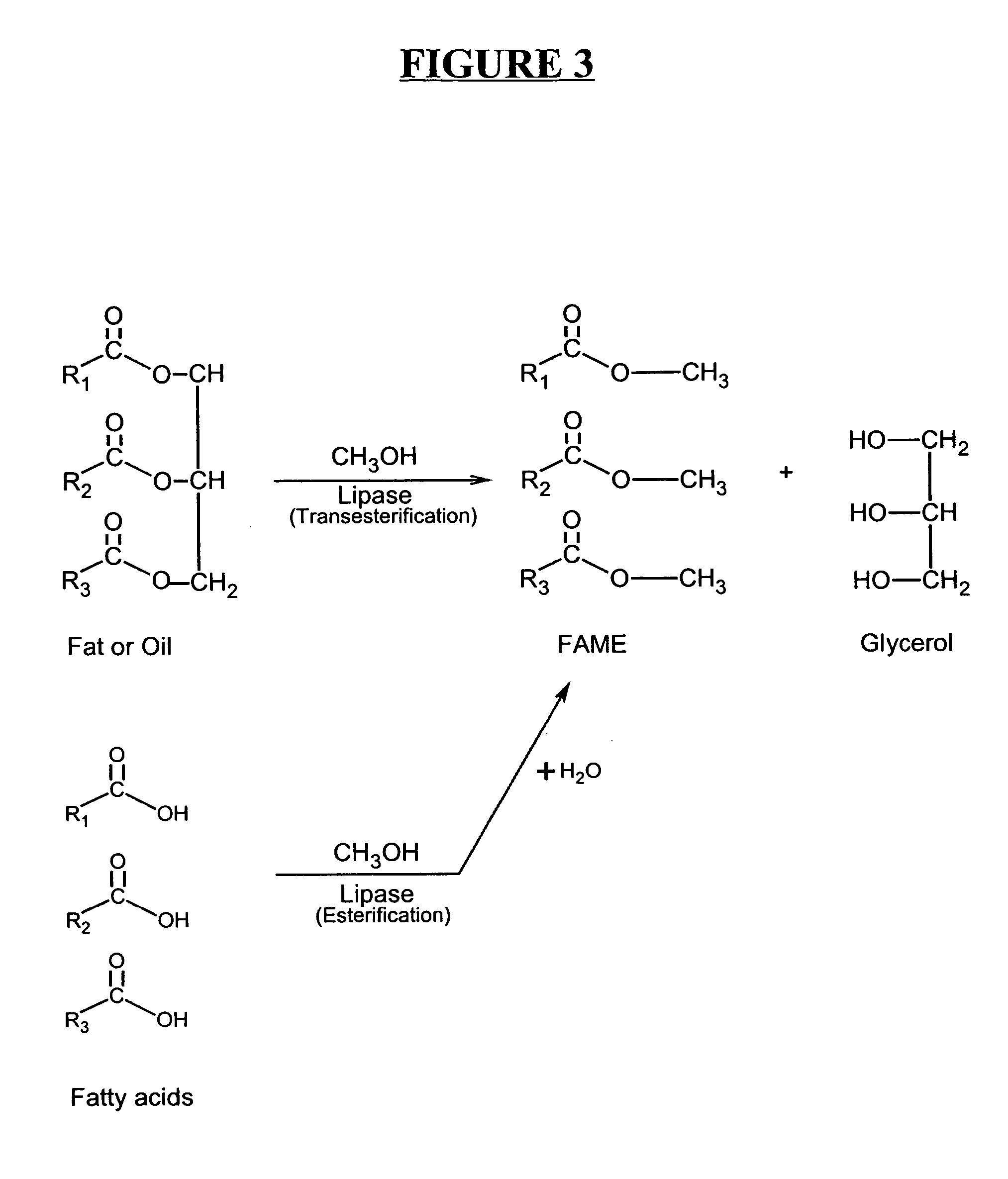
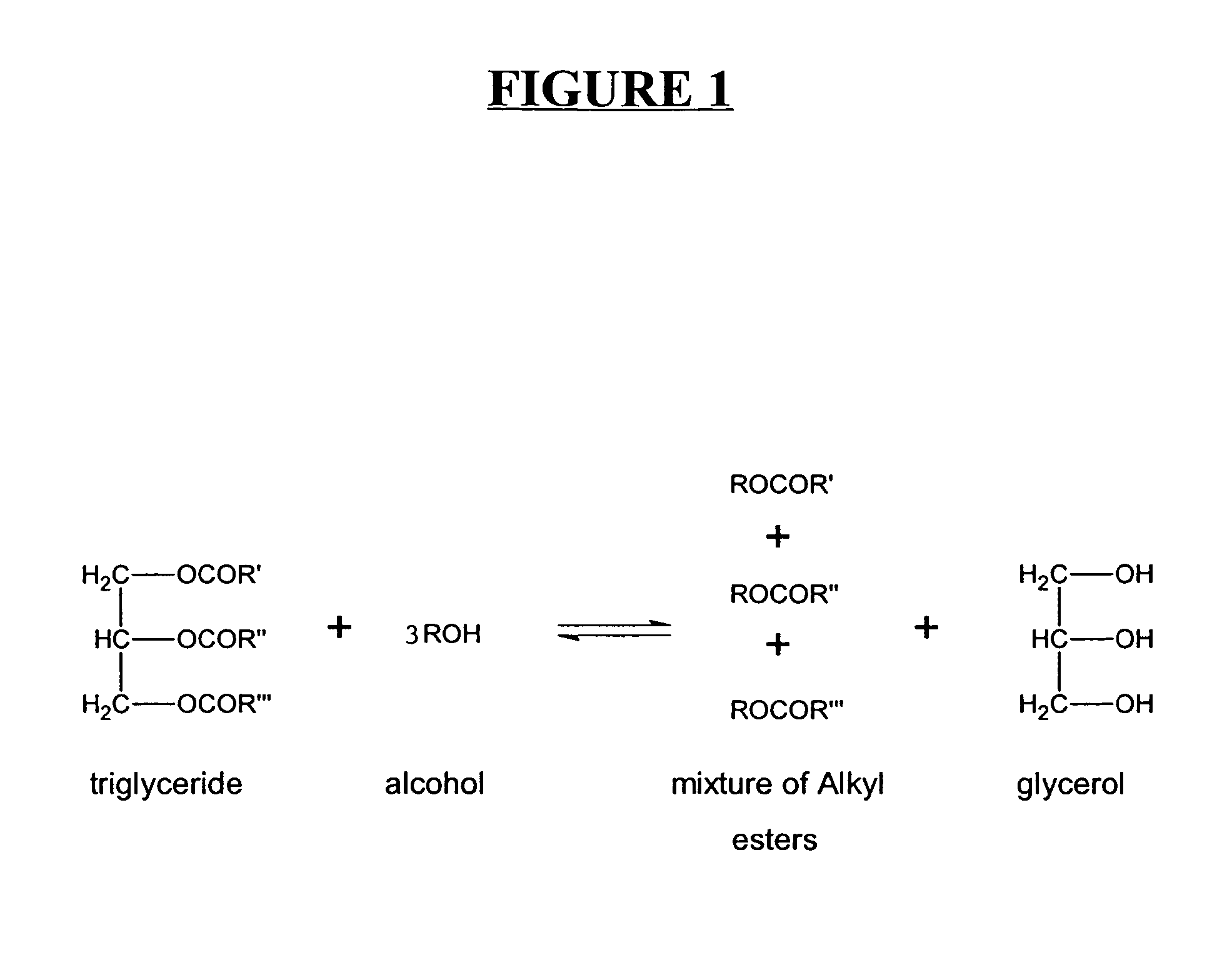
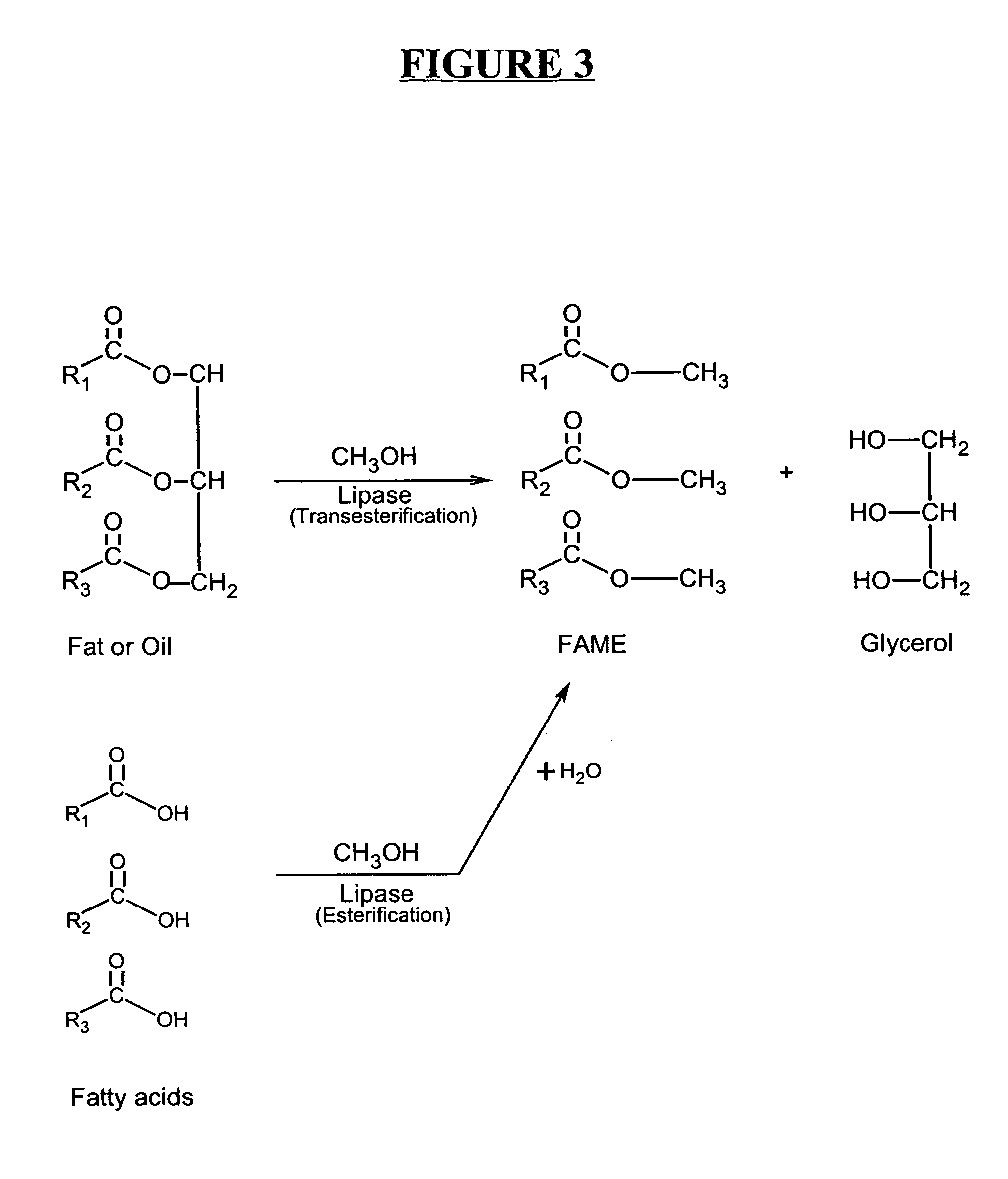
Production of biodiesel and other valuable chemicals from wastewater treatment plant sludges
ActiveUS20050112735A1Reduce the environmentPromote digestionBio-organic fraction processingByproduct vaporizationLipid formationSludge
A process for producing biodiesel has been invented by first extracting lipids from the sludges generated during primary and / or biological treatment of municipal, agricultural, and industrial wastewaters using primary, secondary, and tertiary treatments followed by the transesterification of the extracted lipids using transesterification conversion into alcohol-based esters. The resulting products from this process include biodiesel, glycerol, lipid-free proteins, various other useful chemicals and an aqueous-based substrate well suited for optimized digestion within subsequent biological digestion (either aerobic or anaerobic). The lipids extracted from the sludges containing high levels of microorganisms are phospholipids which can also be directly used as lecithin. The extraction of the lipids from the sludges will be performed using chemical extraction techniques with the transesterification of the extracted lipids accomplished using basic, acidic, and / or a combination of the two transesterification techniques.
Owner:MISSISSPPI STATE UNIV RES & TECH
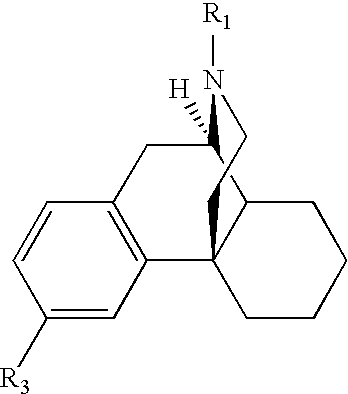
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Weight losing and meal replacement protein type solid beverage
ActiveCN102687750AEnhance nutritional propertiesAdd flavorMilk preparationBiotechnologyPROTEIN S HEERLEN
The invention provides a weight losing and meal replacement protein type solid beverage, which comprises ingredients including concentrated whey protein powder, soy isolate protein powder, dried skim milk, soluble dietary fiber, maltitol or erythritol, maltodextrin, fructo-oligose or fructose, soya bean lecithin, nutrose, low-fat pectin and the like. The protein in the body is effectively supplemented, the satiety is effectively prolonged, the appetite and the caloric intake are more strictly controlled, and further, the goal of losing the weight is reached. The product has the advantages that the nutrition is balanced, the mouth feeling is smooth, savoury and mellow, the carrying is convenient, the process is simple and feasible, and the solid beverage is suitable for mass production.
Owner:浙江诺特健康科技股份有限公司
Chewing gum base and chewing gum compositions
ActiveUS6986907B2High affinityEasy to processContainers for annular articlesChewing gumPolymer scienceSpray dried
Owner:WM WRIGLEY JR CO
Mixed micellar delivery system and method of preparation
InactiveUS6221378B1Antibacterial agentsOrganic active ingredientsOctylphenoxy PolyethoxyethanolChamomile extract
A mixed micellar pharmaceutical formulation includes a micellar proteinic pharmaceutical agent, an lkali metal C8 to C22 alkyl sulphate, alkali metal salicylate, a pharmaceutically acceptable edetate and at least one absorption enhancing compounds. The absorption enhancing compounds are selected from the group consisting of lecithin, hyaluronic acid, pharmaceutically acceptable salts of hyaluronic acid, octylphenoxypolyethoxyethanol, glycolic acid, lactic acid, chamomile extract, cucumber extract, oleic acid, linolenic acid, borage oil, evening of primrose oil, trihydroxy oxo cholanylglycine, glycerin, polyglycerin, lysine, polylysine, triolein and mixtures thereof. The amount of each absorption enhancing compound is present in a concentration of from 1 to 10 wt. / wt. % of the total formulation, and the total concentration of absorption enhancing compounds are less than 50 wt. / wt. % of the formulation. Preferably, the formulation is administered, in combination with a propellant, to the buccal cavity, using a metered dose dispenser.
Owner:GENEREX PHARMA INC
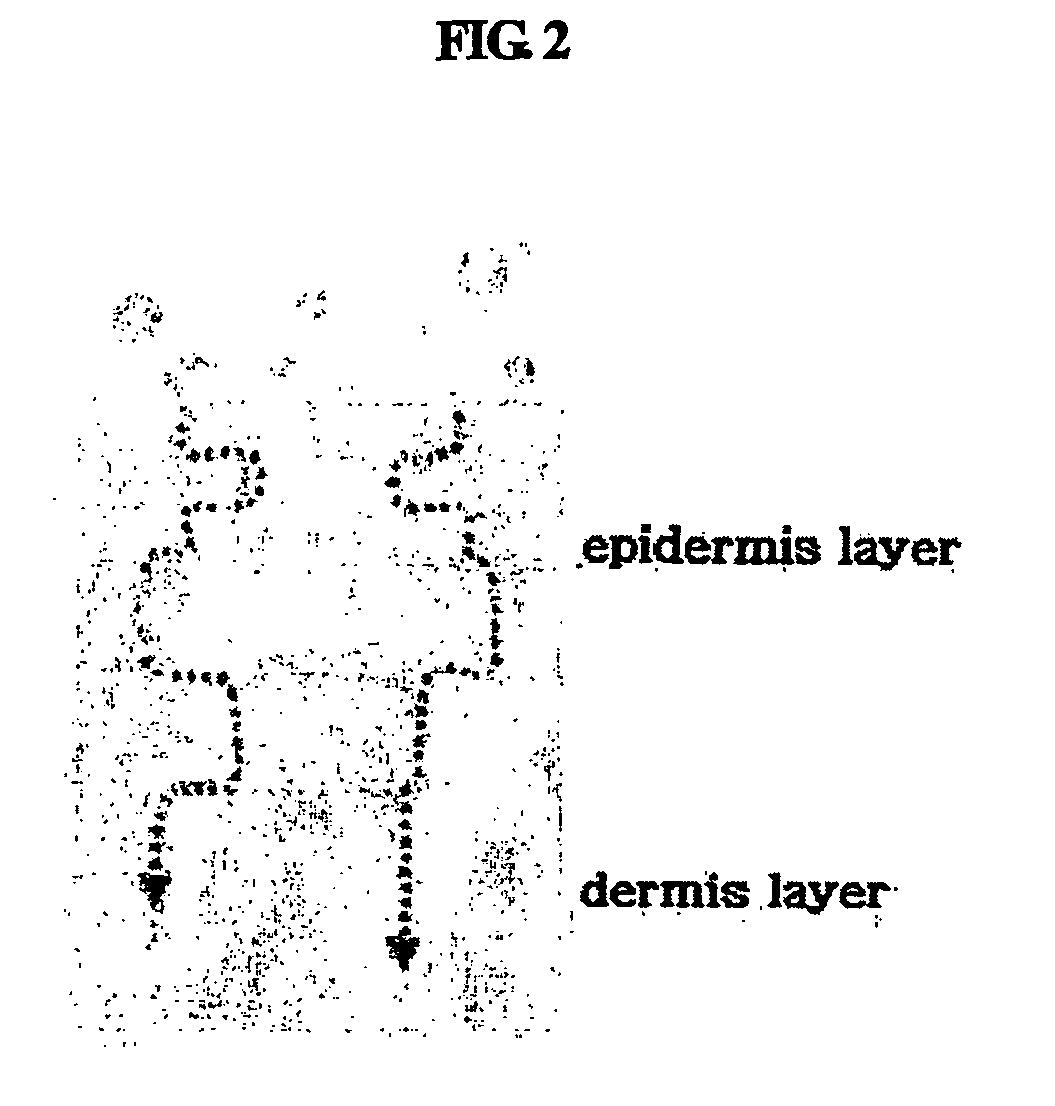
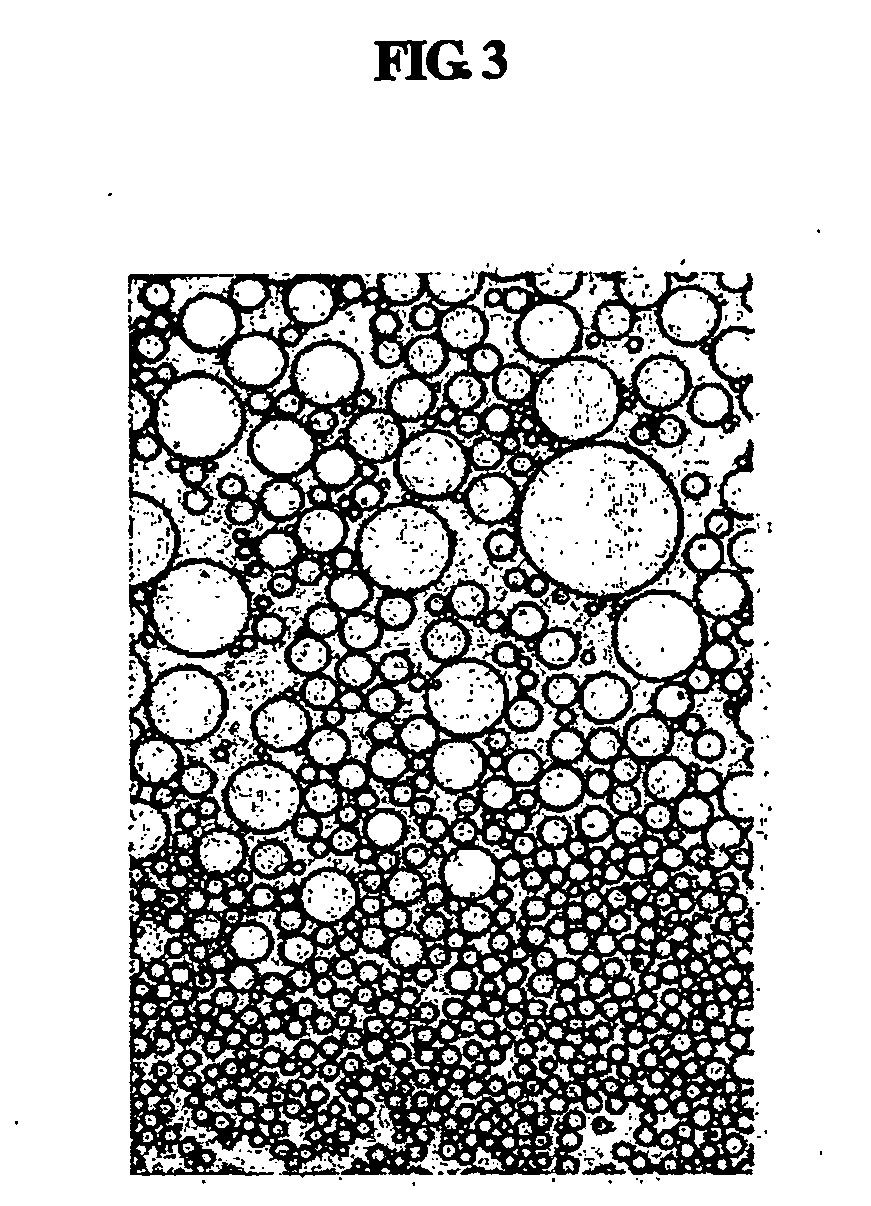
Intra-serum and intra-gel for modeling human skin tissue
InactiveUS6475800B1Diagnostics using spectroscopyScattering properties measurementsConfocalCrosslinking reagent
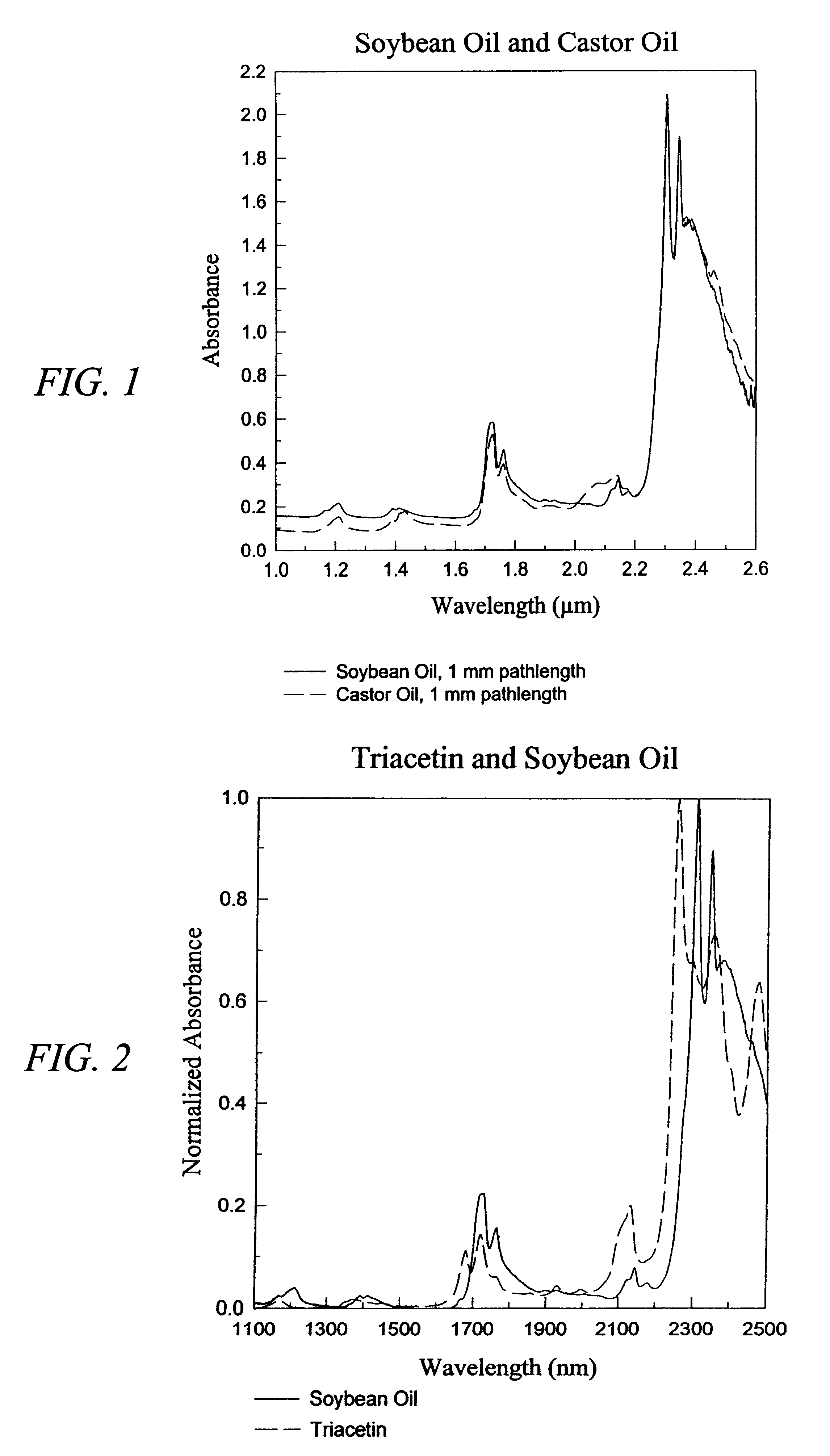
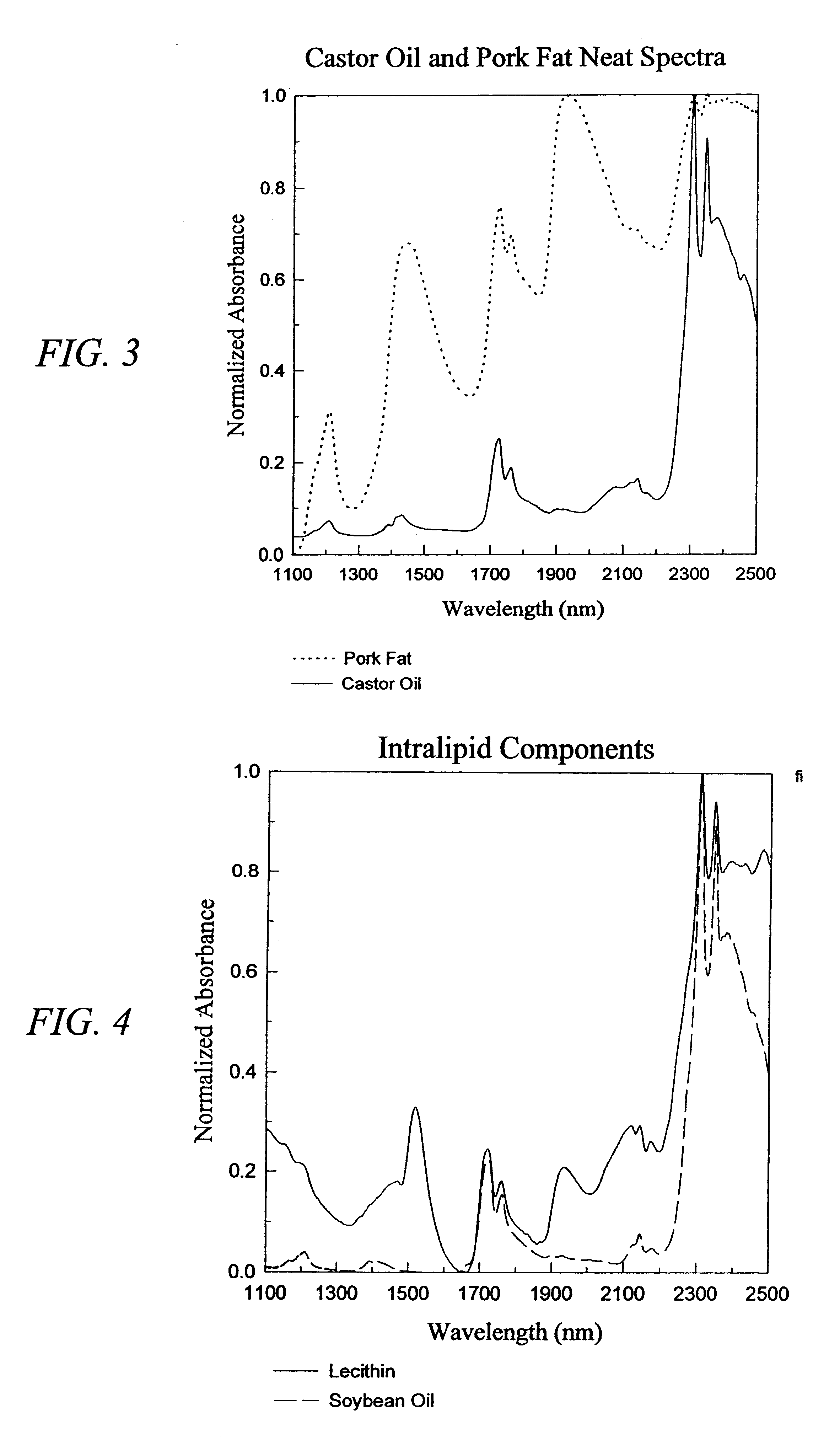
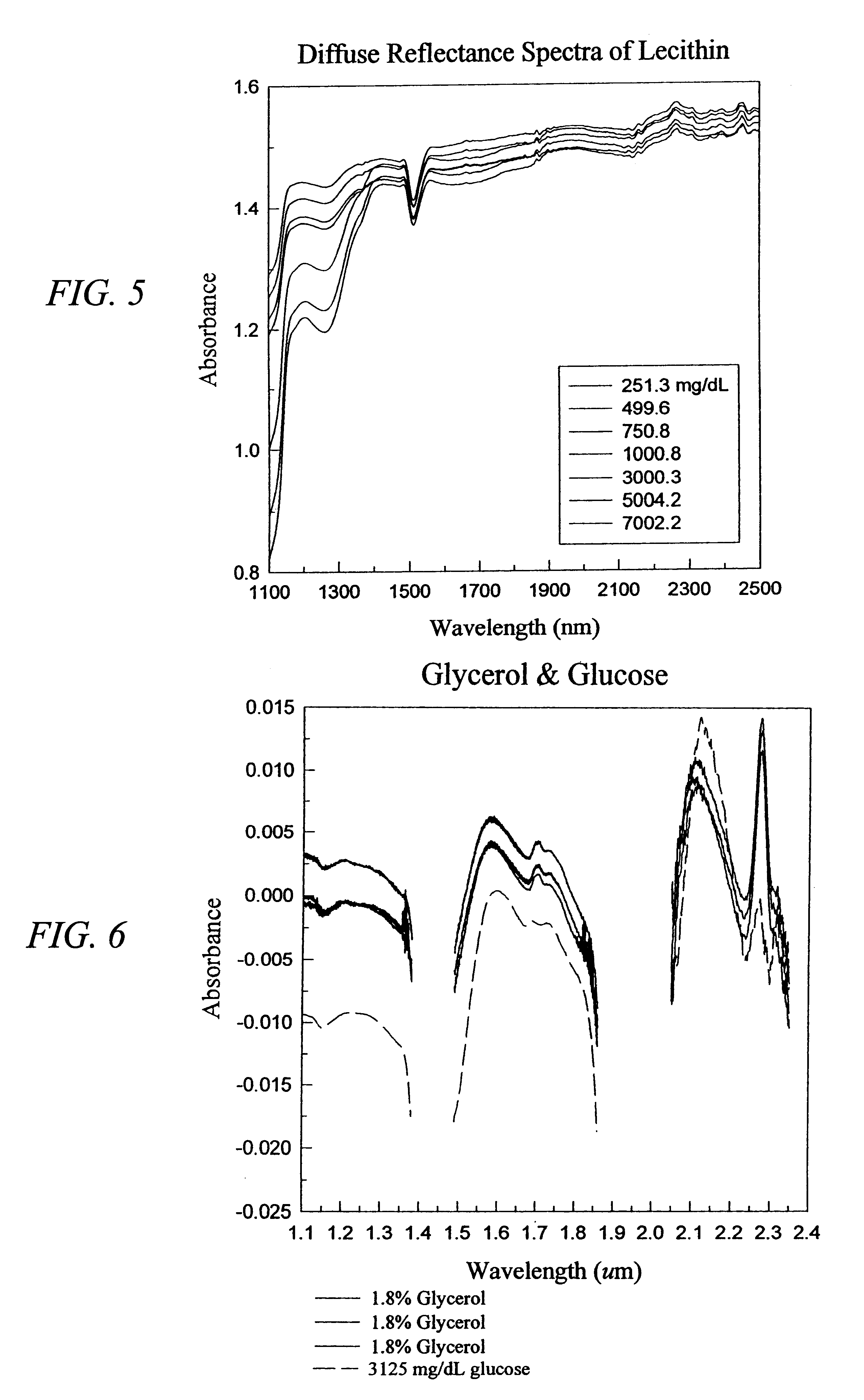
The invention provides a class of samples that model the human body. This family of samples is based upon emulsions of oil in water with lecithin acting as the emulsifier. These solutions that have varying particle sizes may be spiked with basis set components (albumin, urea and glucose) to simulate skin tissues further. The family of samples is such that other organic compounds such as collagen, elastin, globulin and bilirubin may be added, as can salts such as Na+, K+ and Cl-. Layers of varying thickness with known index of refraction and particle size distributions may be generated using simple crosslinking reagents, such as collagen (gelatin). The resulting samples are flexible in each analyte's concentration and match the skin layers of the body in terms of the samples reduced scattering and absorption coefficients, mums and muma. This family of samples is provided for use in the medical field where lasers and spectroscopy based analyzers are used in treatment of the body. In particular, knowledge may be gained on net analyte signal, photon depth of penetration, photon radial diffusion, photon interaction between tissue layers, photon density (all as a function of frequency) and on instrument parameter specifications such as resolution and required dynamic range (A / D bits required). In particular, applications to delineate such parameters have been developed for the application of noninvasive glucose determination in the near-IR region from 700 to 2500 nm with an emphasis on the region 1000 to 2500 nm (10,000 to 4,000 cm-1).
Owner:GLT ACQUISITION
Rumen by-pass delivery system
InactiveUS20020127259A1Prevent oxidationEffective absorptionBiocideAnimal feeding stuffBiotechnologyNutrition
The present invention relates to a composition for the delivery of active agents that are protected from environmental oxidation and are resistant to ruminal fermentation degradation but not intestinal digestion and adsorption. The composition may contain biologically active ingredients such as for example, vitamins, minerals, proteins, amino acids, fatty acids, nutritional supplements, and pharmaceuticals together with natural and / or synthetic lecithin phospholipids.
Owner:ORTHOEFER FRAND T
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
Composition and method for reducing the risk or progression of cardiovascular, glaucoma, tardive dyskinesia and other diseases
InactiveUS20040087479A1Reduce riskShorten the progressBiocideOrganic active ingredientsBeta-CaroteneAdditive ingredient
Elevated levels of homocysteine have been implicated as an important risk factor for cardiovascular and other diseases. A composition for decreasing levels of plasma homocysteine and a method for administering the composition are provided, the composition containing dextromethorphan (DM), folic acid and vitamins B6 and B12. The composition provides a synergistic therapeutic effect so that lower amounts of the above ingredients may be employed to minimize any undesirable side effects caused by the use of high levels of a component such as DM. Preferred compositions for cardiovascular diseases further include lecithin, vitamin E, betacarotene, procyanidins / flavonoids, trimethylglycine, garlic oil and minerals. Other compositions for treating glaucoma include bilberry, bioflavonoids and beta-carotene and for treating tardive dyskinesia include an antioxidant such a grape seed extract and pine bark extract, lecithin and oligomeric proanthocyanidins. The compositions may be administered using any suitable means such as orally or intravenous.
Owner:SOSNOWSKI ROBERT E +1
Cyclosporin compositions
A composition is disclosed herein comprising from about 0.001% to about 0.4% cyclosporin A, castor oil, and a surfactant selected from the group consisting of alcohol ethoxylates, alcohols, alkyl glycosides, alkyl polyglycosides, alkylphenol ethoxylates, amine oxides, block polymers, carboxylated alcohol or alkylphenol ethoxylates, carboxylic acids / fatty acids, cellulose derivatives, ethoxylated alcohols, ethoxylated alkylphenols, ethoxylated aryl phenols, ethoxylated fatty acids, ethoxylated fatty acids, ethoxylated fatty esters and oils, fatty alcohols, fatty esters, glycol esters, lanolin-based derivatives, lecithin and lecithin derivatives, lignin and lignin derivatives, methyl esters, monoglycerides and derivatives , phosphalipids, polyacrylic acids, polyethylene glycols, polyethylene oxide-polypropylene oxide copolymers, polyethylene oxides, polymeric surfactants, polypropylene oxides, propoxylated alcohols, propoxylated alkyl phenols, propoxylated fatty acids, protein-based surfactants, sarcosine derivatives, silicone-based surfactants, sorbitan derivatives, stearates, sucrose and glucose esters and derivatives, and combinations thereof.
Owner:SAINT REGIS MOHAWK TRIBE
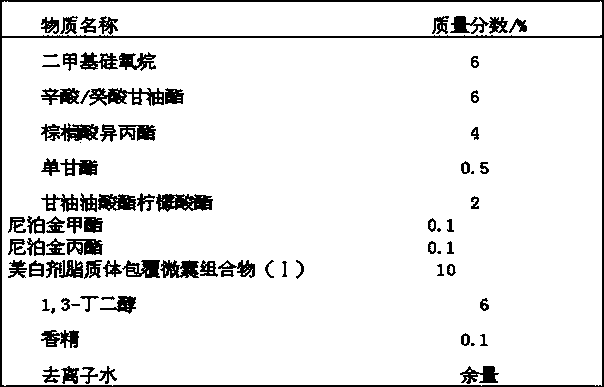
Whitening and freckle-removing cream and preparation method thereof
ActiveCN106580797AImprove antioxidant capacityEfficient hydrationCosmetic preparationsToilet preparationsGlycyrrhiza glabra RootGlycerol
The invention discloses whitening and freckle-removing cream and a preparation method thereof. The whitening and freckle-removing cream comprises water, butanediol, caprylic / capric triglyceride, isohexadecane, glycerol, cyclopentasiloxane, butyrspermum parkii butter, trehalose, cetearyl olivate, sorbitan olivate, cetearyl alcohol, cetearyl glucoside, magnolia sieboldii extract, citrus peel extract, hydrogenated lecithin, phytosterol, bisabolol, tocopheryl acetate, glycyrrhiza glabra root extract, thymus serpyllum extract, panthenol, sodium hyaluronate and the like. The whitening and freckle-removing cream disclosed by the invention can be used for inhibiting tautomerase activity of tyrosinase and dopachrome and hindering polymerization of DHI; the formation of melanin in a body is inhibited, the regeneration capability of pigment cells is improved, the pigment deposition is reduced and pigment cell detachment is promoted; the self-immunity of the cells is enhanced, and the skin elasticity and a metabolism function are improved; and the self-protection capability of skins is improved and the safe whitening and freckle-removing effects are realized.
Owner:GUANGZHOU KEYING COSMETICS CO LTD
Linker-Based Lecithin Microemulsion Delivery Vehicles
ActiveUS20080139392A1Improve solubilityPromote absorptionBiocideTransportation and packagingHigh concentrationSide effect
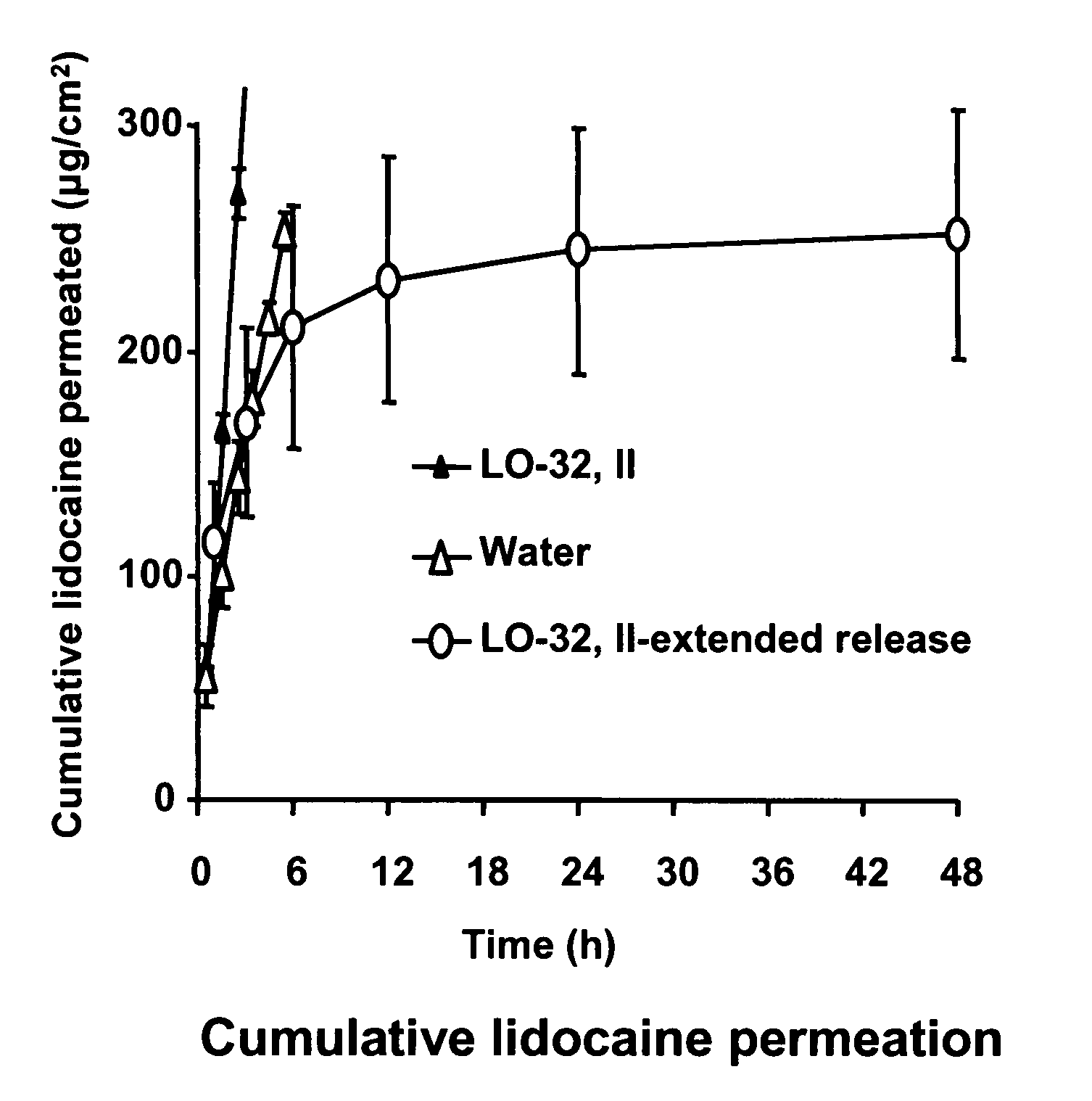
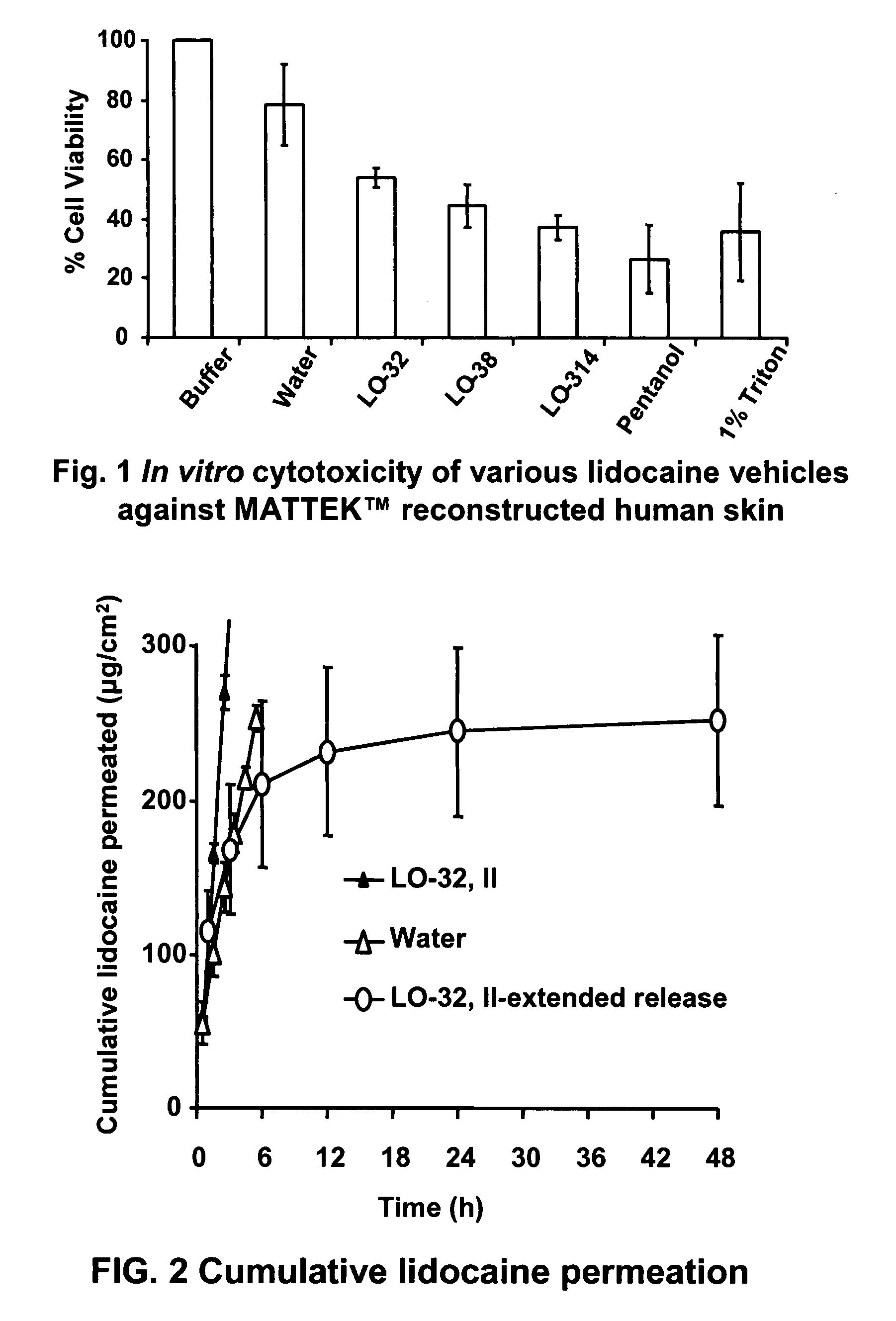
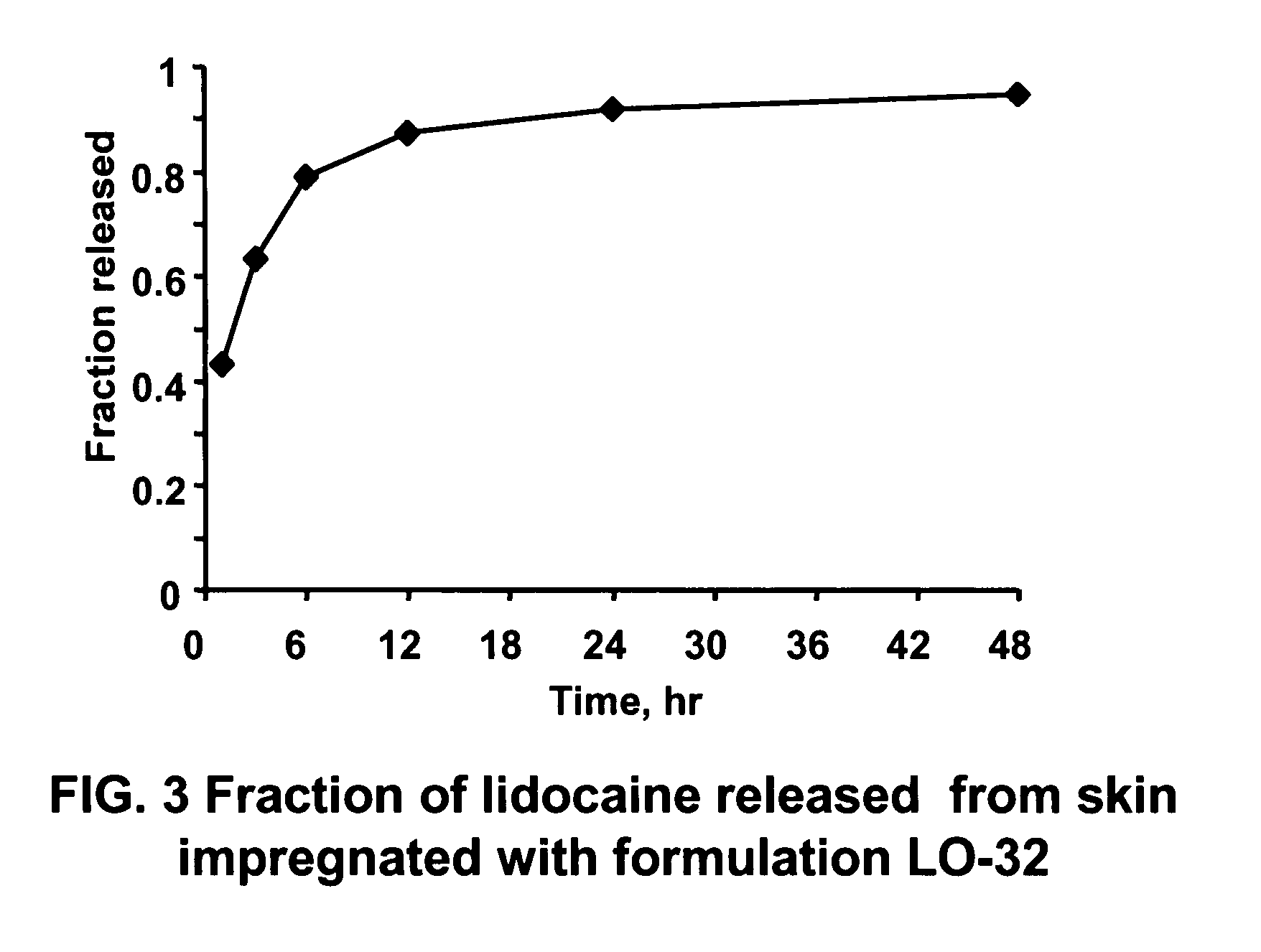
The present invention relates to biocompatible microemulsion systems designed for controlled release drug delivery applications formulated with phospholipids such as lecithin (surfactant), a lipophilic additive (linker) containing 9 or more carbons in their alkyl group and hydrophilic-lipophilic balance (HLB) of 5 or less, and a surfactant-like hydrophilic additive (linker) containing between 6 to 9 carbon atoms in their alkyl tail. The combination of linkers and phospholipids produce formulations capable of delivering high concentrations of poorly soluble drugs into epithelial tissue using low surfactant concentrations, with minimum cytotoxic side effects.
Owner:ACOSTA ZARA EDGAR JOEL +1
Transnasal anticonvulsive compositions and modulated process
InactiveUS6627211B1Promote absorptionIncrease permeationBiocideNervous disorderCo administrationHigh plasma
A method of vehicle modulated administration of an anticonvulsive agent to the nasal mucous membranes of humans and animals is disclosed. The vehicle system is an aqueous pharmaceutical carrier comprising an aliphatic alcohol, a glycol and a biological surfactant such as a bile salt or a lecithin. The pharmaceutical composition provides a means to control and promote the rate and extent of transmucosal permeation and absorption of the medicaments via a single and multiple administration. Nasal administration of the pharmaceutical preparation produces a high plasma concentration of the anticonvulsant nearly as fast as intravenous administration. Such compositions are particularly suitable for a prompt and timely medication of patients in the acute and / or emergency treatment of status epilepticus and other fever-induced seizures.
Owner:BIOPHARM
Omega-3 fatty acid self-emulsifying composition
InactiveUS20180015038A1Reduce the amount requiredImprove compatibilityNervous disorderAntipyreticPolyoxyethylene castor oilEmulsion
A pharmaceutical composition comprising, in relation to 100% by weight of a total amount of a self-emulsifying composition, 70 to 90% by weight of eicosapentaenoic acid ethyl ester as a first medicinal component, 0.5 to 6% by weight of water, 1 to 29% by weight of polyoxyethylene sorbitan fatty acid ester (optionally further comprising polyoxyethylene castor oil) as an emulsifier, 1 to 25 parts by weight of lecithin in relation to 100 parts by weight of the eicosapentaenoic acid ethyl ester, and pitavastatin, rosuvastatin, or a salt thereof as a second medicinal component. The composition is excellent in any one of self-emulsifying property, dispersibility of the composition, emulsion stability, absorbability, and storage stability of the medicinal components and a preparation.
Owner:MOCHIDA PHARM CO LTD
Self-emulsifying composition of omega-3 fatty acid
ActiveUS20170368184A1Reduce the amount requiredImprove compatibilityOrganic active ingredientsNervous disorderΩ 3 pufaSorbitan
A self-emulsifying composition contains: 70 to 90% by weight of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; 0.5 to 6% by weight of water; 1 to 29% by weight of a polyoxyethylene sorbitan fatty acid ester as an emulsifier (optionally including a polyoxyl castor oil, and not including lecithin); and lecithin in an amount of 3 to 40 parts by weight in relation to 100 parts by weight of ω3 polyunsaturated fatty acids and the like. The self-emulsifying composition is excellent in self-emulsifying property, composition dispersibility, emulsion stability, and absorbability, is free from ethanol and polyhydric alcohols or only has such an alcohol added thereto at a reduced concentration, and is useful for foods and pharmaceuticals.
Owner:MOCHIDA PHARM CO LTD
Self-emulsifying composition of omega3 fatty acid
InactiveUS20170348273A1Reduce the amount requiredImprove compatibilityOrganic active ingredientsNervous disorderAlcoholEmulsion
A self-emulsifying composition contains: 70 to 90% by weight of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; 0.5 to 6% by weight of water; 1 to 29% by weight of a polyoxyethylene sorbitan fatty acid ester as an emulsifier (optionally including a polyoxyl castor oil, and not including lecithin); and lecithin in an amount of 3 to 40 parts by weight in relation to 100 parts by weight of ω3 polyunsaturated fatty acids and the like. The self-emulsifying composition is excellent in self-emulsifying property, composition dispersibility, emulsion stability, and absorbability, is free from ethanol and polyhydric alcohols or only has such an alcohol added thereto at a reduced concentration, and is useful for foods and pharmaceuticals.
Owner:MOCHIDA PHARM CO LTD
Moisture barrier film coating composition, method and coated form
A dry moisture barrier film coating composition for forming a moisture barrier film coating for pharmaceutical tablets and the like comprises polyvinyl alcohol, soya lecithin, and optionally a flow aid, a colorant, and / or a suspending agent. A liquid coating solution or dispersion for forming a moisture barrier film coating for pharmaceutical tablets and the like comprises polyvinyl alcohol, soya lecithin, water, and optionally a flow aid, a colorant, and / or a suspending agent. A method of coating pharmaceutical tablets and the like with a moisture barrier film coating comprises forming a liquid coating solution or dispersion for forming a moisture barrier film coating for pharmaceutical tablets and the like comprising polyvinyl alcohol, soya lecithin, water, and optionally a flow aid, a colorant, and / or a suspending agent, applying the coating solution or dispersion onto the tablets to form a film coating on the tablets, and drying the film coating on the tablets.
Owner:BPSI HLDG LLC
Multiple-layered liposome and preparation method thereof
InactiveUS20070082042A1Good skin permeabilityImprove stabilityDermatological disorderLiposomal deliverySterolIntercellular space
Disclosed are multilayered liposomes for transdermal absorption and a method of preparing the liposomes. The multilayered liposomes are prepared using a mixture of oil-phase components comprising squalane, sterols, ceramides, neutral lipids or oils, fatty acids and lecithins, is 200 to 5000 nm in particle size, and is capable of entrapping a physiologically active substance. The multilayered liposomes entrap a larger amount of a physiologically active substance and are structurally stable when encapsulating the physiologically active substance, compared to unilamellar liposomes. Also, they are prepared by a simple and cost-effective process not using a high-pressure homogenizer but using a general homo mixer. Further, since the multilayered liposomes are prepared in a larger size than the intercellular spaces in the stratum corneum, they overcome the tension of surrounding cells when passing through the intercellular spaces and are thus able to penetrate into the dermal layer, compared to nano-sized unilamellar liposomes. Thus, the multilayered liposomes are useful for enhancing the transdermal absorption of physiologically active substances.
Owner:BIOSPECTRUM
Rapid disintegrating tablets (RDTs) for pharmaceutical use and method for preparing the same
InactiveUS20050053655A1Disintegrates quicklyBiocideSalicyclic acid active ingredientsAdditive ingredientActive agent
The present invention provides a fast-disintegrating tablet (RDT) and the method of preparing the RDT. The RDT contains a plurality of microcapsules which contains an active pharmaceutical ingredient surrounded by a polymeric matrix formed by a hydrogel. The microcapsules are separated from each other by a surfactant, particularly lecithin. The RDT is particularly suitable for use as a drug delivery system for antiacid or antiulcer drugs, such as famotidine. The RDT is further characterized by their its fast disintegration time of about 3 second to 3 minutes.
Owner:MEDICAL & PHARMA IND TECH & DEV CENT
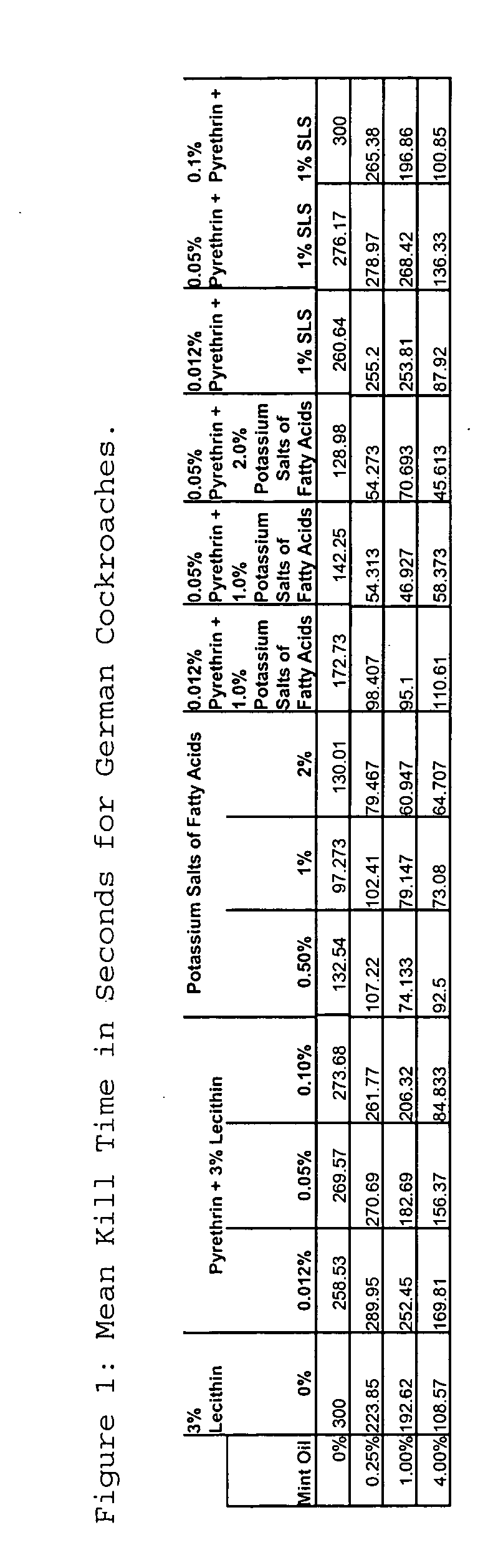
Insecticidal compositions and methods of using same
InactiveUS20050244445A1Efficacious productPoor efficacyBiocideDead animal preservationSulfateInsecticidal soap
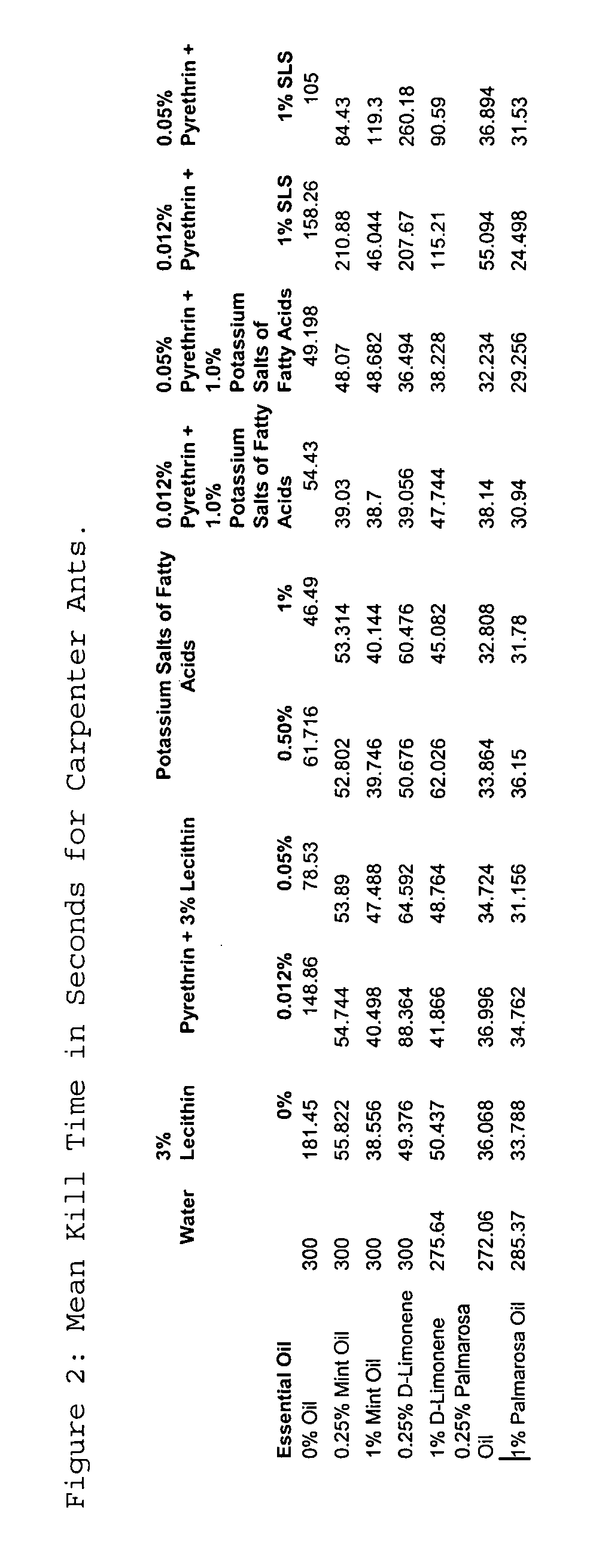
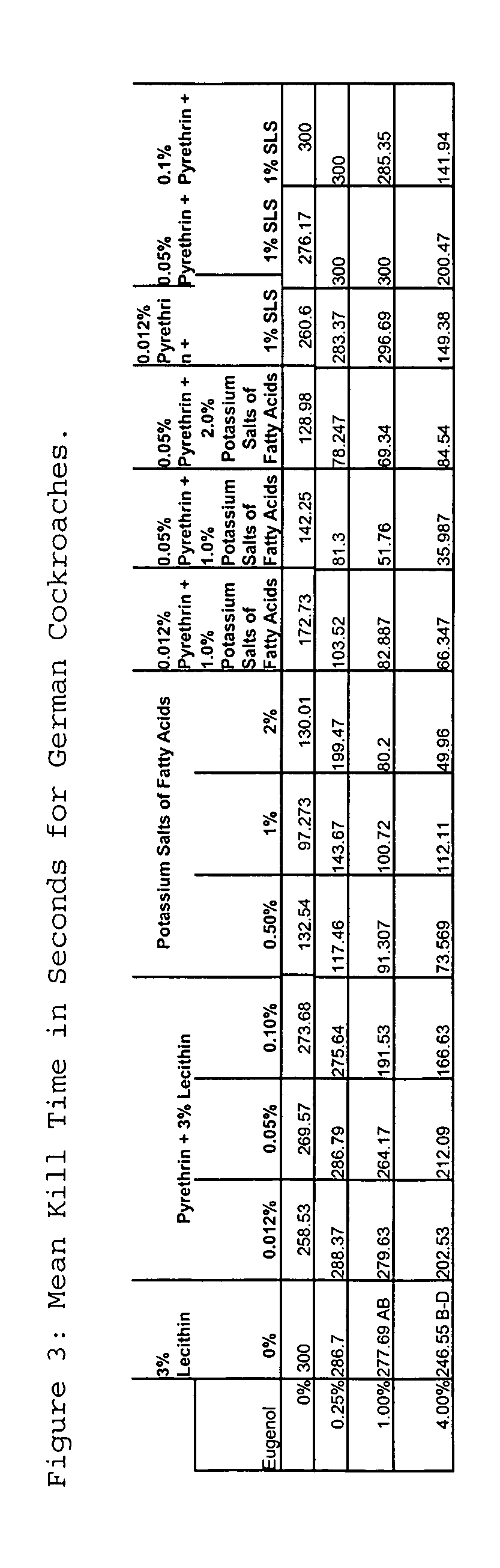
The present invention provides novel insecticidal formulations comprising an effective concentration of: 1) at least one or more essential oils and an insecticidal soap; 2) at least one or more essential oils, an insecticidal soap, and pyrethrins; 3) at least one or more essential oils and pyrethrins; 4) at least one or more essential oils, an insecticidal soap and a synergist, such as sodium lauryl sulfate, sodium dodecyl sulfate or lecithin; 5) at least one or more essential oils, an insecticidal soap, a synergist, and pyrethrins; and 6) at least one or more essential oils, a synergist, and pyrethrins. A carrier oil, such as mineral oil, may be added to any of the foregoing formulations.
Owner:WOODSTREAM CORP
Neuropathy cream
Owner:OZTURK BINNUR +1
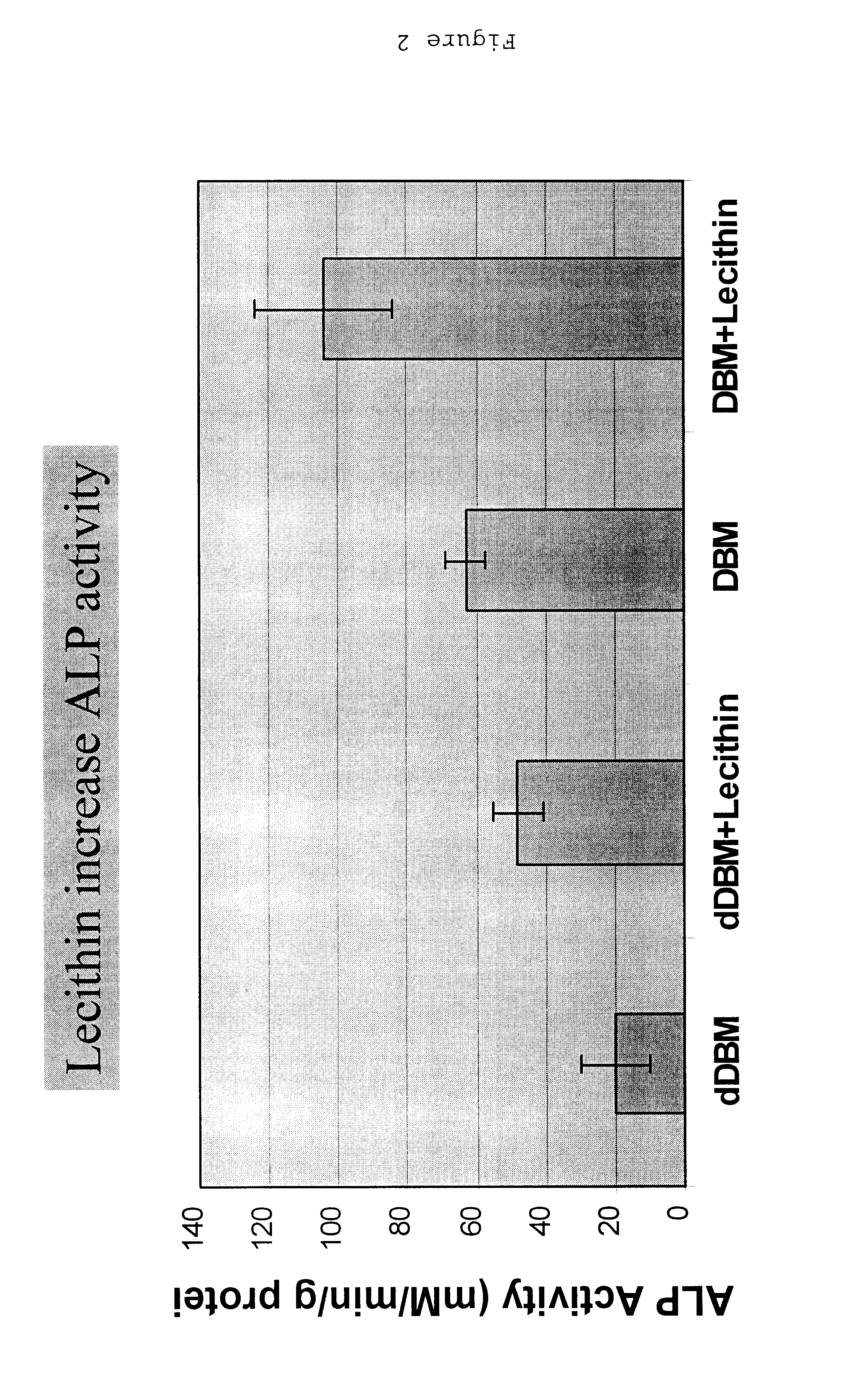
Bone graft material incorporating demineralized bone matrix and lipids
InactiveUS6565884B2Good osteoinductivityEasy to operateBiocidePowder deliveryHydrophilic polymersVitamin C
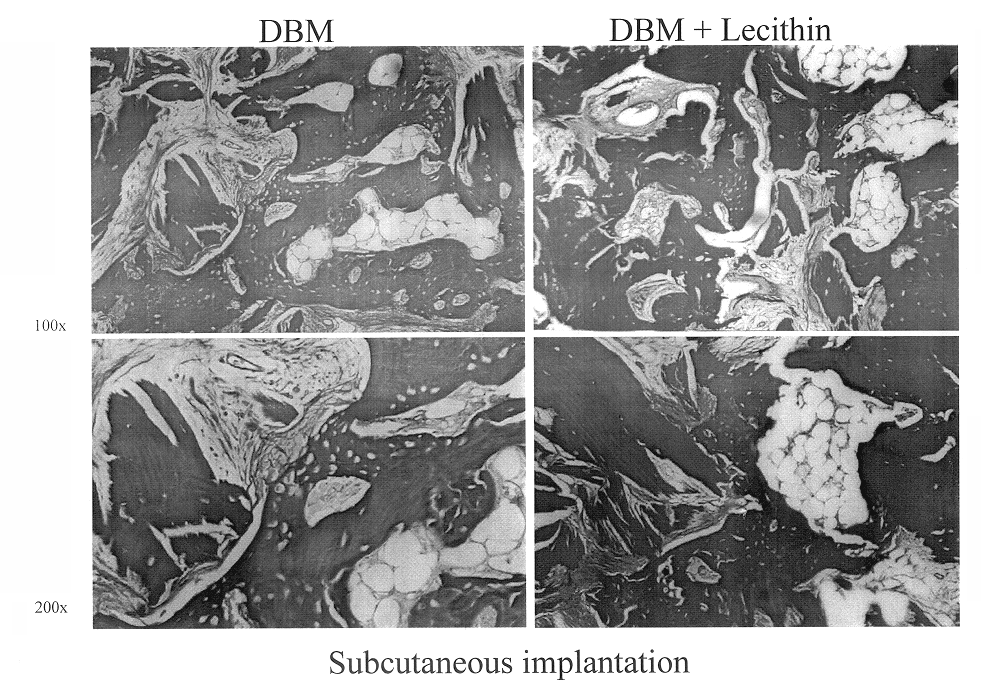
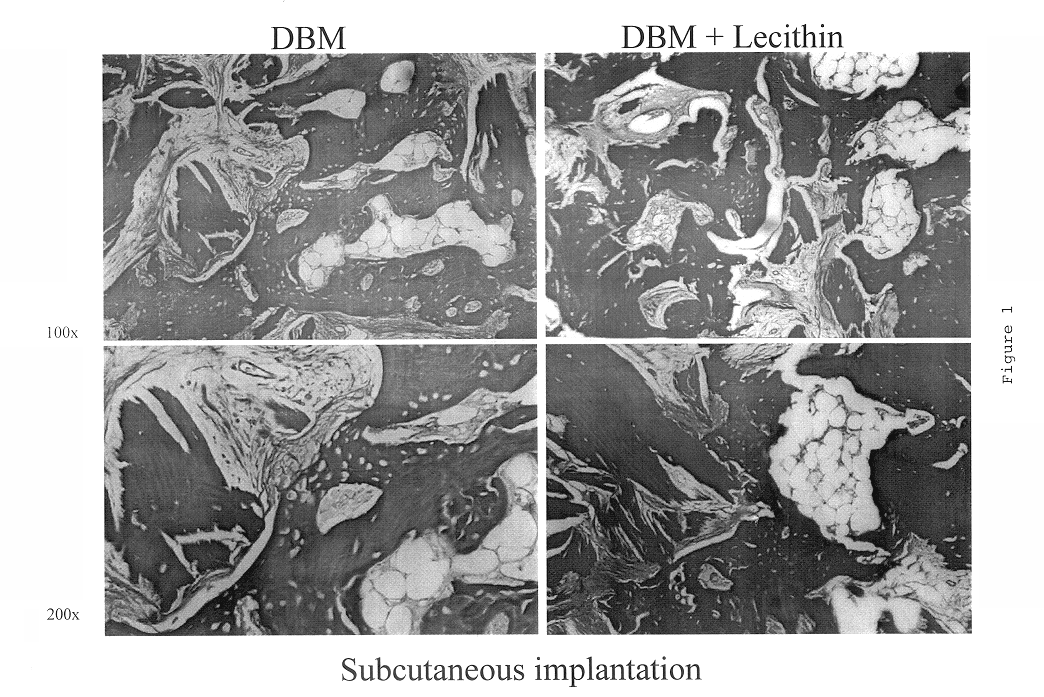
A demineralized bone putty composition comprises: (1) demineralized bone matrix (DBM); and (2) a lipid fraction selected from the group consisting of lecithin and a mixture of lecithin and triglycerides containing unsaturated fatty acids. The putty composition is moldable, biocompatible, slowly resorbable, and soluble in tissue fluids, and non-extrudable. The composition delivers a biologically active product to animals and humans that will enhance bone formation at sites where bone is lost, deficient, or present in suboptimal amounts. The composition can further comprise calcium, an antioxidant such as Vitamin E or Vitamin C, or a hydrophilic polymer such as methylcellulose or hydroxypropyl methylcellulose.
Owner:BIOMET MFG CORP
Freezing flour-dough improver and uses thereof
ActiveCN101411344AOvercome stabilityOvercome volumeDough treatmentPre-baking dough treatmentYeastVitamin C
The invention discloses a frozen dough modifying agent, which is prepared by evenly mixing an enzyme preparation (including one or a plurality of alpha-amylase, cellulose, hemicellulase, pentosanase, lipase, glucose oxidase, glutamine transaminage and so on), vitamin C, an emulsifying agent, a thickening agent, wheat gluten powder, lecithin and stuffing according to certain proportion. The frozen dough modifying agent has the advantages that the frozen dough modifying agent can effectively improve the stability of dough during fermentation and roasting processes, improve the freezing resistance property of yeasts, increase the volume of finished products, improve the tissue of the finished products, reduce the loss of the vitality of the yeasts during the freezing process, and effectively delay the aging of the finished products.
Owner:ANGELYEAST CO LTD
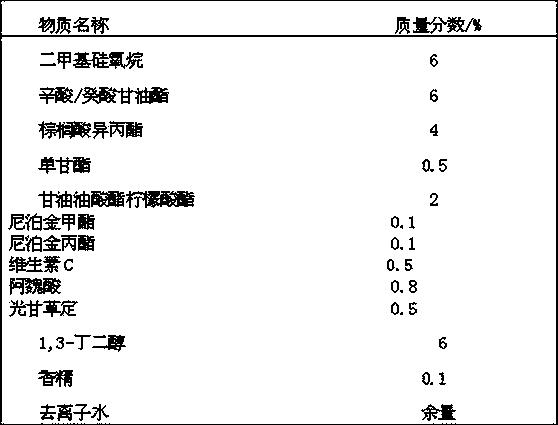
Whitening agent liposome coating micro-capsule composition as well as preparation method and application thereof
ActiveCN103432009AOvercome defects that are difficult to be absorbed by the skinOvercome the defect of being easily oxidized and discoloredCosmetic preparationsToilet preparationsPhospholipinPolyol
The invention discloses a whitening agent liposome coating micro-capsule composition as well as a preparation method and an application thereof. The whitening agent liposome coating micro-capsule composition comprises 5-30% of whitening agent, 2-50% of polyhydric alcohol, 1-30% of phospholipids, 1-30% of grease, 1-10% of emulsifying agent and 1-30% of deionized water, wherein the whitening agent is coated by lecithin which is very similar to a skin lipid structure and is prone to penetrating into the deep layer of skin, thus the defect that the whitening agent is unlikely to be absorbed by the skin is overcome; the whitening agent coated by lecithin is isolated from oxygen in air, thus the detect that the whitening agent is prone to being oxidized and discoloring is solved; when whitening products containing the whitening agent liposome coating micro-capsule composition are used, the whitening agent is slowly released and has a long-time whitening effect.
Owner:GUANGDONG IND TECHN COLLEGE +1
Tea jelly sweet
The invention discloses a tea jelly sweet which is prepared from raw material in the formula in parts by weight: 4-10 parts of functional composition, 30-60 parts of maltitol, 30-40 parts of starch syrup, 3.5-7 parts of plural gel, 0.1-10 parts of lecithin, 0.1-3 parts of hydrogenated vegetable oil, 1-3 parts of peppermint oil and 20-40 parts of water, wherein the functional composition comprises one or several of ultra-micropowder or extractions or original materials of pu-erh tea, black tea, green tea, Oolong tea, white tea, yellow tea and dark tea and other functional factors; and the plural gel comprises modified starch, gelatine, agar and carrageenan. The invention has the characteristics of defined functions, attractive appearance, natural aroma, good taste and the like, has no artificially-synthesized pigment and essence and sweetner causing decayed tooth and is suitable for groups to eat for a long term so as to improve the sub-health state.
Owner:云南龙润茶业集团有限公司
Production of biodiesel and other valuable chemicals from wastewater treatment plant sludges
ActiveUS7638314B2Reduce the environmentPromote digestionBio-organic fraction processingByproduct vaporizationSludgeFree protein
A process for producing biodiesel has been invented by first extracting lipids from the sludges generated during primary and / or biological treatment of municipal, agricultural, and industrial wastewaters using primary, secondary, and tertiary treatments followed by the transesterification of the extracted lipids using transesterification conversion into alcohol-based esters. The resulting products from this process include biodiesel, glycerol, lipid-free proteins, various other useful chemicals and an aqueous-based substrate well suited for optimized digestion within subsequent biological digestion (either aerobic or anaerobic). The lipids extracted from the sludges containing high levels of microorganisms are phospholipids which can also be directly used as lecithin. The extraction of the lipids from the sludges will be performed using chemical extraction techniques with the transesterification of the extracted lipids accomplished using basic, acidic, and / or a combination of the two transesterification techniques.
Owner:MISSISSPPI STATE UNIV RES & TECH
Transdermal cannabinoid formulations
The present invention includes a transdermal composition which contains a pharmaceutically effective amount of a cannabinoid for delivery of the cannabinoid to the bloodstream of a user. The composition may comprise the following components: a surfactant-lecithin organogel; and a cannabinoid. The composition may also comprise an exogenous terpene. The cannabinoid is capable of diffusing from the composition into the bloodstream of the user, and may be used in methods for treating a patient suffering from a condition such as pain, nausea and emesis, convulsions, muscle spasm, inflammation, depression, and cachexia.
Owner:MM TECH HLDG LLC
Nutritional balanced vegetarian diet and preparing method thereof
InactiveCN102763799AGood for healthEnhance anti-aging and life extensionFood preparationBiotechnologyYeast Proteins
The invention relates to a nutritional balanced vegetarian diet which is characterized by comprising components A, B and C. The component A comprises brown rice, fragrant rice, black rice, cooked brown rice, glutinous rice, oats, sorghum, millet, corn, soybeans, black beans, red beans, white beans, mung beans, peanuts, sesame seeds, barley, apple, banana, mango, orange, strawberry, tomato, carrot, pumpkin, celery, konjac, broccoli, cabbage, mushroom, undaria pinnatifida, seaweed, lily, green tea and pueraria. The component B comprises lecithin, oligosaccharide alcohol prebiotics, and rice bran. The component C comprises nutritional yeast and probiotics. Compared with the prior art, the nutritional balanced vegetarian diet provides all the essential nutrients to the human body.
Owner:NINGBO YUFANGTANG BIOTECH
Compositions comprising at least two nanoemulsions
InactiveUS20050048088A1Improve stabilityImprove bioavailabilityCosmetic preparationsPeptide/protein ingredientsRoom temperatureNutritional Supplementation
The stable compositions of the present inventions comprise at least two different nanoemulsions stabilized by lecithin each of which containing a liquid lipid, at least two of said lipids are incompatible to each other. Particularly said compositions comprise as incompatible lipids tocopherol and Coenzyme Q10. Said composition are useful in cosmetics, in cell cultures and in nutrient compliments. Processes are described for preparing such compositions form lipids which are solid at room temperature.
Owner:MIBELLE
Functional fatty powder and preparation method thereof
The invention discloses functional fatty powder and a preparation method thereof, and belongs to the technical field of feeds. The functional fatty powder is characterized in that raw materials comprise the following components in parts by weight: 30-50 parts of concentrated omega-fatty acid fat, 10-20 parts of lecithin, 8-15 parts of concentrated linolenic acid fat, 8-15 parts of MCT, 1-5 parts of choline, 1.5-5.5 parts of nicotinamide, 1-5 parts of a composite emulsifying agent, 3-8 parts of a composite antioxidant, 2-8 parts of vitamin C, and 40-70 parts of encrusting substances or 30-60 parts of carriers. The functional fatty powder provided by the invention is small in addition quantity, high in cost performance ratio, good in stability, high in nutrient value and high in fat utilization rate; the functional fatty powder not only can greatly improve the growing property and the reproductive property of animals but also can improve the immunity of the animals and promote the development of animal organisms, so that the functional fatty powder is an excellent feed additive.
Owner:GUANGZHOU YOUBAITE FEED SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com