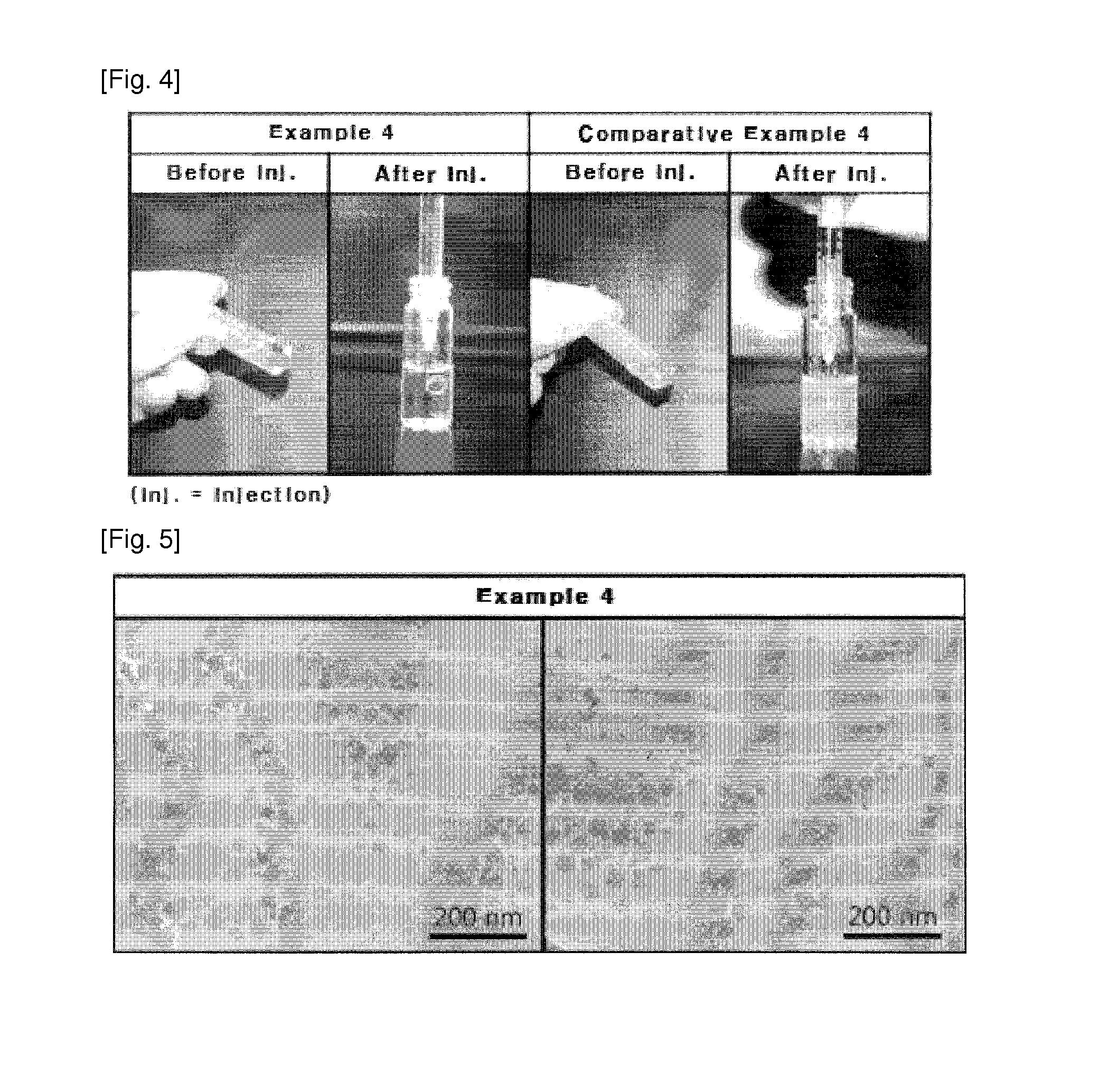
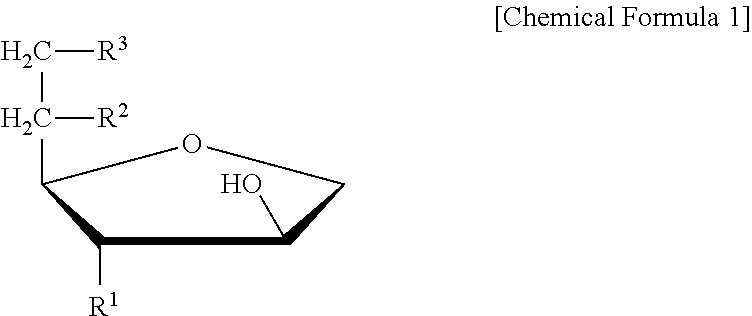
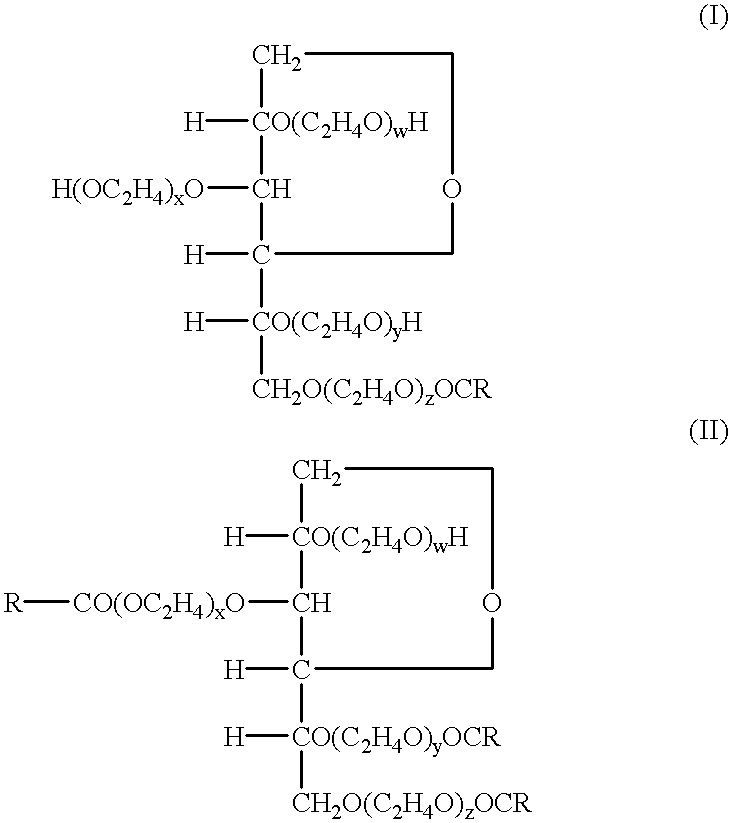
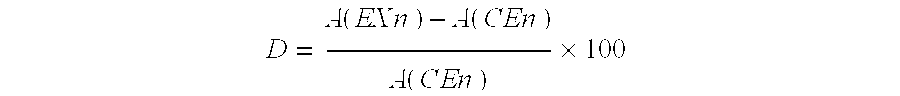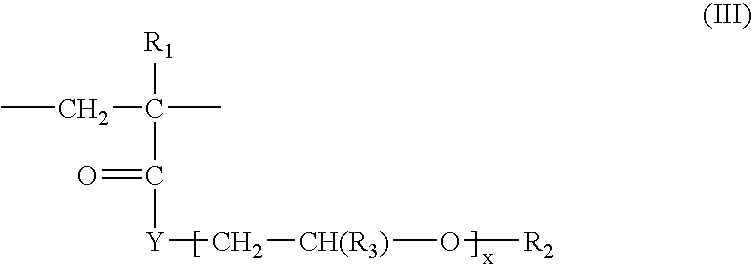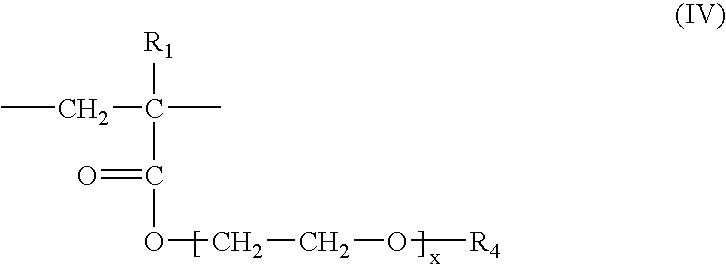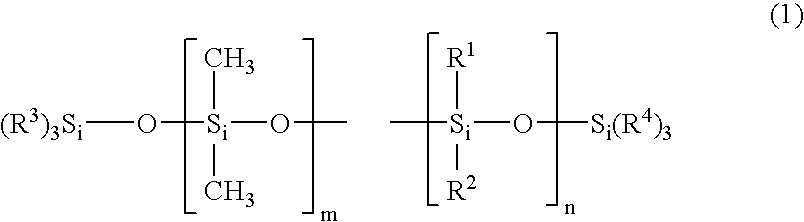Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1670 results about "Sorbitan" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sorbitan is a mixture of isomeric organic compounds derived from the dehydration of sorbitol and is an intermediate in the conversion of sorbitol to isosorbide. Sorbitan is primarily used in the production of surfactants such as polysorbates; which are important emulsifying agents, with a total annual demand of more than 10000 tons in 2012.
Vaccine formulations
ActiveUS20050079185A1Improve stabilityStable and safe and easily administrableAntibacterial agentsSsRNA viruses negative-senseEukaryotic plasmidsNon ionic
The present invention provides for a novel oil-in-water (O / W) emulsion, with increased stability in the presence of bacterial or viral suspensions, especially those concentrated and non-purified or weakly purified. The emulsion of the present invention can act as vehicle for the delivery of a pharmaceutical composition comprising at least one immunogen and, in particular, an immunogen selected from the group comprising an inactivated pathogen, an attenuated pathogen, a subunit, a recombinant expression vector, and a plasmid or combinations thereof. In one embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen, said immunogen selected from the group comprising an inactivated Mycoplasma hyopneumoniae bacterium, an inactivated porcine circovirus type 2 (PCV-2) virus or combinations thereof; (2) a mineral oil; (3) a non-ionic lipophilic surfactant; and (4) a non-ionic hydrophilic surfactant having a low HLB value which comprises ethoxylated fatty acid diesters of sorbitan (generally having HLB value between 11 and 13). In another preferred embodiment, the present invention provides for an injectable oil-in-water (O / W) emulsion comprising: (1) an aqueous solution containing an immunogen; (2) a non-ionic hydrophilic surfactant having a high hydrophilic-lipophilic balance (HLB) value greater than 13 and less than 40, in particular HLB≧13.5, and preferably HLB≧14; (3) a mineral oil; (4) a non-ionic lipophilic surfactant; and (5) a non-ionic hydrophilic surfactant having a low HLB value (HLB value of about 9 to about 13).
Owner:MERIAL INC
Liquid dispersion comprising dibenzylidene sorbital acetals and ethoxylated nonionic surfactants
InactiveUS6102999ALow viscosityInexpensive fluid dispersionOrganic chemistryTransportation and packagingPeristaltic pumpPolyolefin
This invention relates to a fluid dispersion of at least one dibenzylidene sorbitol acetal derivative. The sorbitol acetal derivative is useful as a clarifying agent for polyolefins and the inventive fluid dispersion permits improvements in the handling and processing of and mixing within the polyolefin composition. The inventive dispersion must be shelf stable, retain its nucleating effects, be compatible with polypropylene (and other polyolefins), and possess both short-term and long-term viscosities which permit acceptable transport through a standard polyolefin-manufacturing peristaltic pump. The preferred inventive dispersion thus comprises 3,4-DMDBS and at least one ethoxylated nonionic surfactant having an HLB of greater than about 8.5. Preferred surfactants include those selected from the group consisting essentially of ethoxylated sorbitan (C8-C22) monoesters and ethoxylated nonyl-phenol ethers. The inventive dispersion may be introduced within any polyolefin composition, preferably polypropylene, which may then be molded into any shape or form. A method of producing a polyolefin plastic utilizing the inventive dispersion is also provided.
Owner:MILLIKEN & CO
Omega-3 fatty acid self-emulsifying composition
InactiveUS20180015038A1Reduce the amount requiredImprove compatibilityNervous disorderAntipyreticPolyoxyethylene castor oilEmulsion
A pharmaceutical composition comprising, in relation to 100% by weight of a total amount of a self-emulsifying composition, 70 to 90% by weight of eicosapentaenoic acid ethyl ester as a first medicinal component, 0.5 to 6% by weight of water, 1 to 29% by weight of polyoxyethylene sorbitan fatty acid ester (optionally further comprising polyoxyethylene castor oil) as an emulsifier, 1 to 25 parts by weight of lecithin in relation to 100 parts by weight of the eicosapentaenoic acid ethyl ester, and pitavastatin, rosuvastatin, or a salt thereof as a second medicinal component. The composition is excellent in any one of self-emulsifying property, dispersibility of the composition, emulsion stability, absorbability, and storage stability of the medicinal components and a preparation.
Owner:MOCHIDA PHARM CO LTD
Self-emulsifying composition of omega3 fatty acid
InactiveUS20170348268A1Reduce the amount requiredImprove compatibilityOrganic active ingredientsNervous disorderEmulsionAlcohol
A self-emulsifying composition contains: 70 to 90% by weight in total of one or more compounds selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; 1 to 29% by weight of an emulsifying agent selected from among a polyoxyethylene sorbitan fatty acid ester, a sorbitan fatty acid ester, a glycerin fatty acid ester and a polyoxyl castor oil; and 0.5 to 6% by weight of water when the composition is defined to be 100% by weight as a whole. The self-emulsifying composition is excellent in self-emulsifying property, composition dispersibility, emulsion stability, and absorbability, is free from ethanol and polyhydric alcohols or only has such an alcohol added thereto at a reduced concentration, and is useful for foods and pharmaceuticals.
Owner:MOCHIDA PHARM CO LTD
Self-emulsifying composition of omega-3 fatty acid
ActiveUS20170368184A1Reduce the amount requiredImprove compatibilityOrganic active ingredientsNervous disorderΩ 3 pufaSorbitan
A self-emulsifying composition contains: 70 to 90% by weight of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; 0.5 to 6% by weight of water; 1 to 29% by weight of a polyoxyethylene sorbitan fatty acid ester as an emulsifier (optionally including a polyoxyl castor oil, and not including lecithin); and lecithin in an amount of 3 to 40 parts by weight in relation to 100 parts by weight of ω3 polyunsaturated fatty acids and the like. The self-emulsifying composition is excellent in self-emulsifying property, composition dispersibility, emulsion stability, and absorbability, is free from ethanol and polyhydric alcohols or only has such an alcohol added thereto at a reduced concentration, and is useful for foods and pharmaceuticals.
Owner:MOCHIDA PHARM CO LTD
Non-wood fiber plastic composites
This invention is directed to an extrudable compound, an extrusion method using a polymer, a cellulosic fiber, and at least one lubricant selected from the group consisting of ethoxylated esters of hydantoins, ethoxylated esters of sorbitol and sorbitan, and ethylene bisamides made from fatty acids containing 6-10 carbons and composites manufactured through such processes which simulate conventional wood products. Another embodiment of the invention is directed to a composition containing polymer, a cellulosic fiber from an agricultural waste product and a lubricant containing a mixture of an alkylene bisamide derived from a C10-C18 is fatty acid and an alkaline earth salt of a fatty acid.
Owner:ARXADA LLC
Self-emulsifying composition of omega3 fatty acid
InactiveUS20170348273A1Reduce the amount requiredImprove compatibilityOrganic active ingredientsNervous disorderAlcoholEmulsion
A self-emulsifying composition contains: 70 to 90% by weight of at least one compound selected from the group consisting of ω3 polyunsaturated fatty acids and their pharmaceutically acceptable salts and esters; 0.5 to 6% by weight of water; 1 to 29% by weight of a polyoxyethylene sorbitan fatty acid ester as an emulsifier (optionally including a polyoxyl castor oil, and not including lecithin); and lecithin in an amount of 3 to 40 parts by weight in relation to 100 parts by weight of ω3 polyunsaturated fatty acids and the like. The self-emulsifying composition is excellent in self-emulsifying property, composition dispersibility, emulsion stability, and absorbability, is free from ethanol and polyhydric alcohols or only has such an alcohol added thereto at a reduced concentration, and is useful for foods and pharmaceuticals.
Owner:MOCHIDA PHARM CO LTD
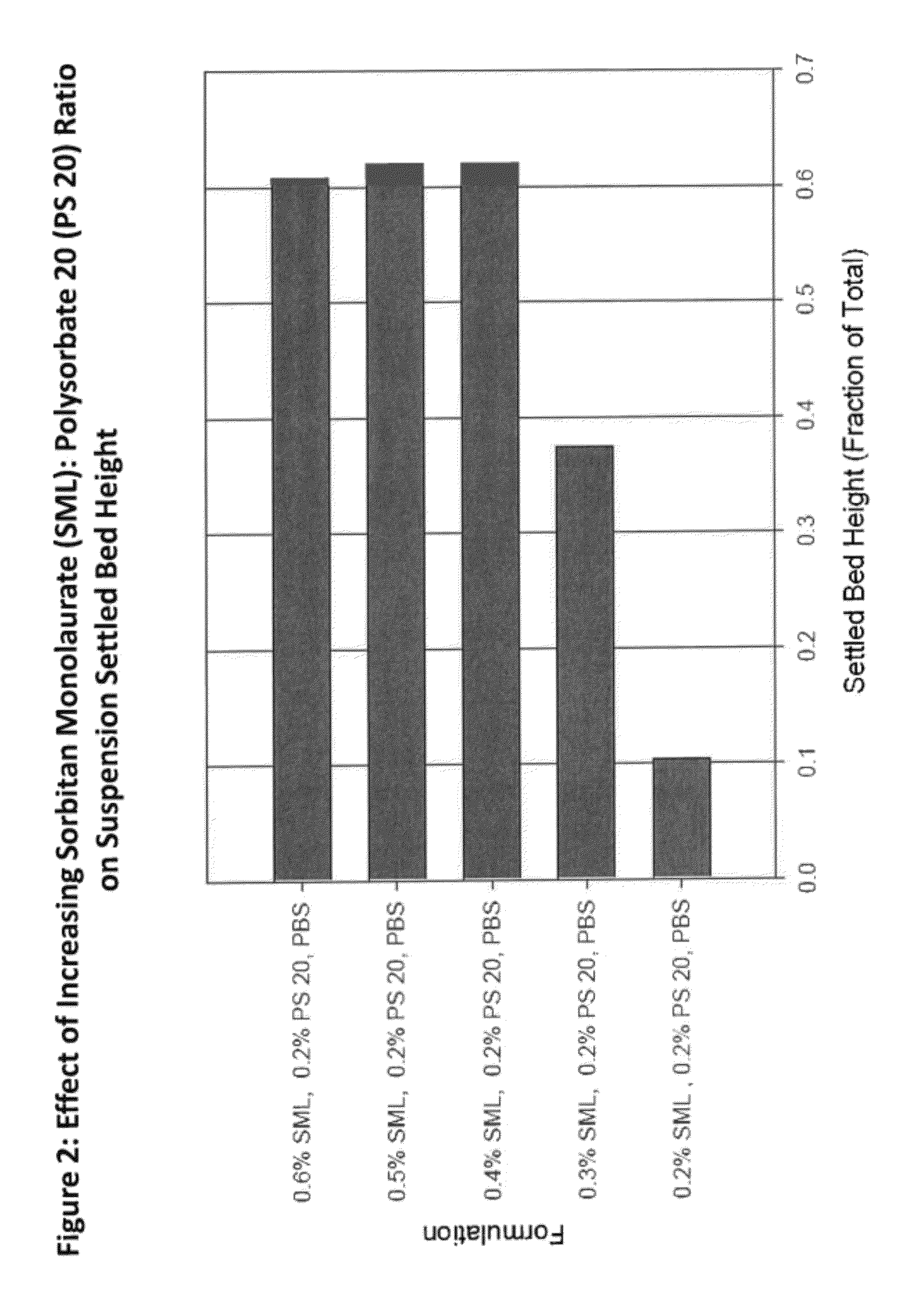
Pharmaceutical compositions comprising sorbitan esters
ActiveUS20120238552A1Patient compliance is goodOptimizing pharmacological profileBiocideNervous disorderCarboxylic acidSorbitan
The present invention relates to a pharmaceutical composition comprising sorbitan esters of carboxylic acids that are useful for the delivery of anti-psychotic drugs.
Owner:ALKERMES PHARMA IRELAND LTD
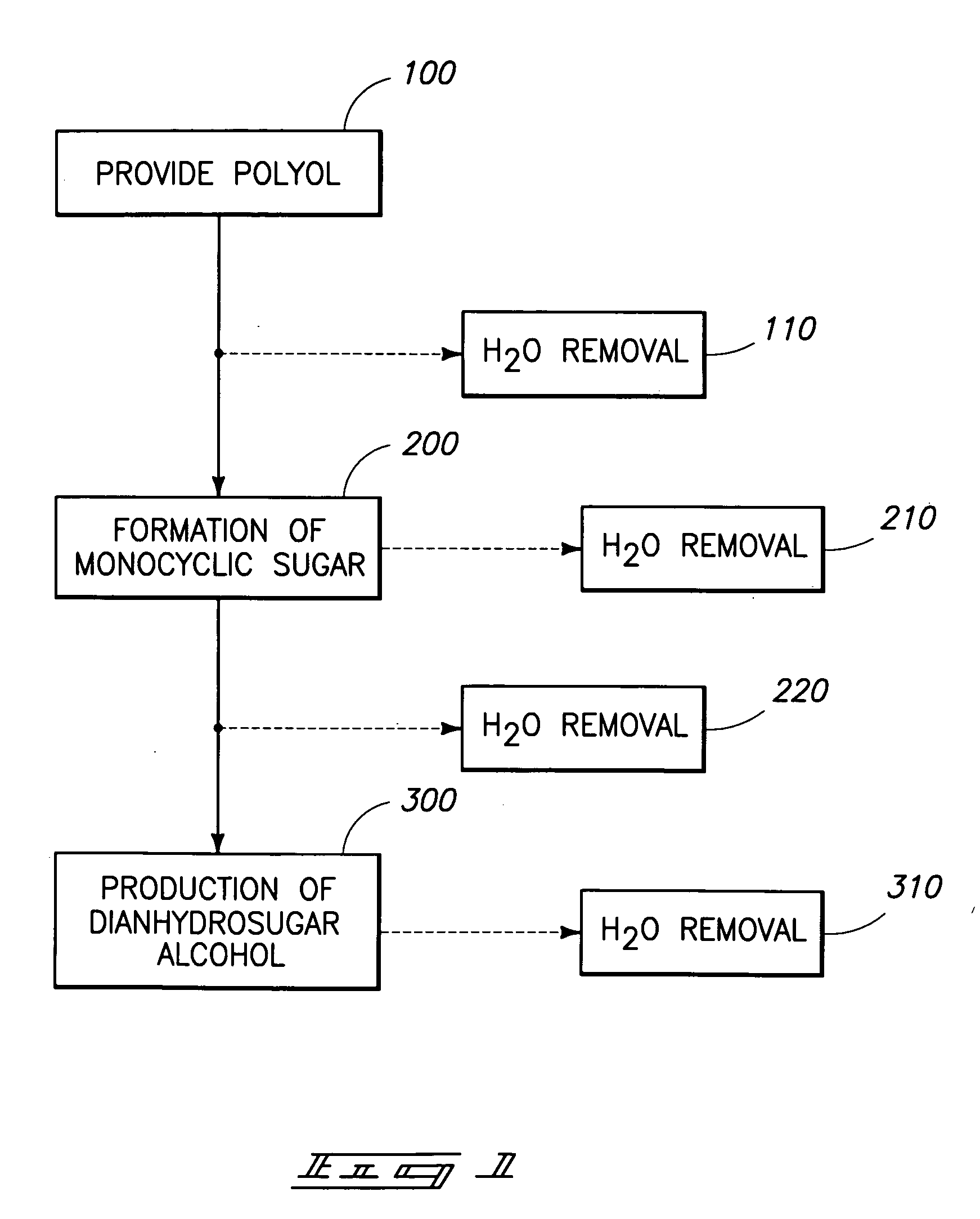
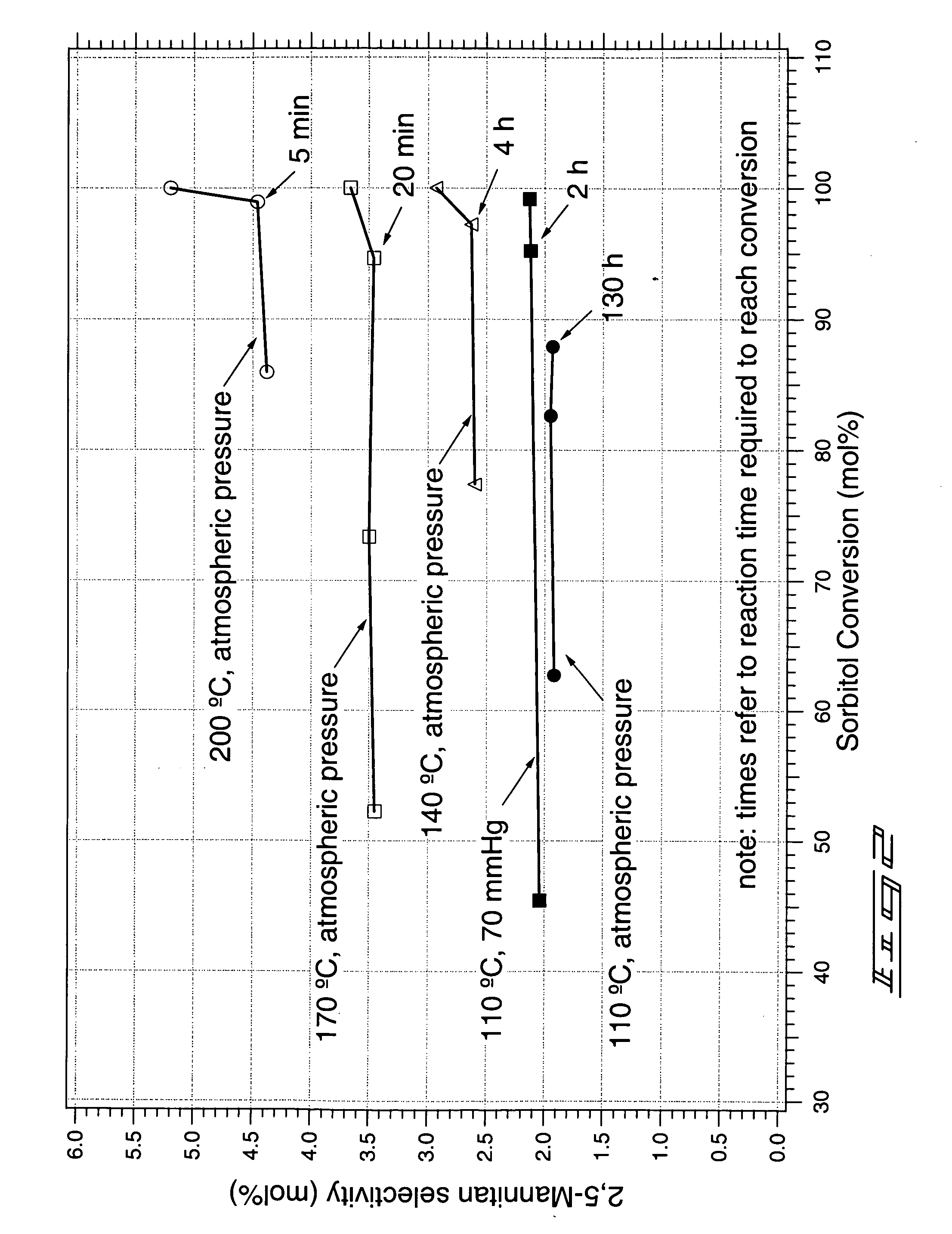
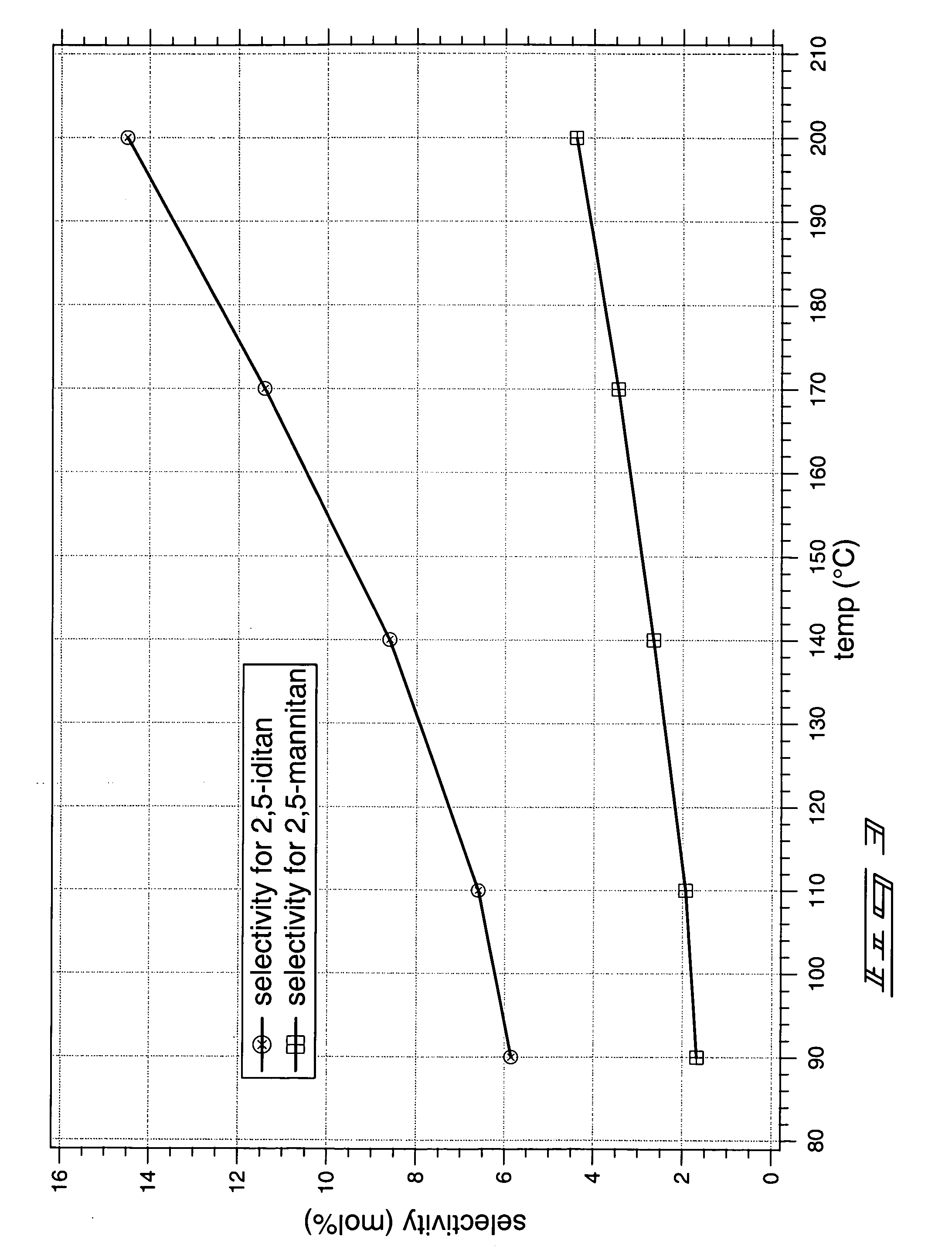
Method of forming a dianhydrosugar alcohol
The invention includes methods of producing dianhydrosugars. A polyol is reacted in the presence of a first catalyst to form a monocyclic sugar. The monocyclic sugar is transferred to a second reactor where it is converted to a dianhydrosugar alcohol in the presence of a second catalyst. The invention includes a process of forming isosorbide. An initial reaction is conducted at a first temperature in the presence of a solid acid catalyst. The initial reaction involves reacting sorbitol to produce 1,4-sorbitan, 3,6-sorbitan, 2,5-mannitan and 2,5-iditan. Utilizing a second temperature, the 1,4-sorbitan and 3,6-sorbitan are converted to isosorbide. The invention includes a method of purifying isosorbide from a mixture containing isosorbide and at least one additional component. A first distillation removes a first portion of the isosorbide from the mixture. A second distillation is then conducted at a higher temperature to remove a second portion of isosorbide from the mixture.
Owner:BATTELLE MEMORIAL INST
Two-stage dehydration of sugars
InactiveUS20070173651A1Oxygen-containing compound preparationSugar derivativesContinuous reactorAlcohol
The invention includes methods for producing dianhydrosugar alcohol by providing an acid catalyst within a reactor and passing a starting material through the reactor at a first temperature. At least a portion of the staring material is converted to a monoanhydrosugar isomer during the passing through the column. The monoanhydrosugar is subjected to a second temperature which is greater than the first to produce a dianhydrosugar. The invention includes a method of producing isosorbide. An initial feed stream containing sorbitol is fed into a continuous reactor containing an acid catalyst at a temperature of less than 120° C. The residence time for the reactor is less than or equal to about 30 minutes. Sorbitol converted to 1,4-sorbitan in the continuous reactor is subsequently provided to a second reactor and is dehydrated at a temperature of at least 120° C. to produce isosorbide.
Owner:BATTELLE MEMORIAL INST
Sustained-Release Lipid Pre-Concentrate of Pharmacologically Active Substance And Pharmaceutical Composition Comprising The Same
ActiveUS20140206616A1Patient compliance is goodGood sustained releaseBiocidePeptide/protein ingredientsLipid formationSaturated fatty acid ester
Disclosed is a sustained release lipid pre-concentrate, comprising: a) a sorbitan unsaturated fatty acid ester having a polar head with at least two or more —OH (hydroxyl) groups; b) a phospholipid; and c) a liquid crystal hardener, free of an ionizable group, having a hydrophobic moiety of 15 to 40 carbon atoms with a triacyl group or a carbon ring structure. The lipid pre-concentrate exists as a liquid phase in the absence of aqueous fluid and forms into a liquid crystal in the presence of aqueous fluid. Also, a pharmaceutical composition further comprising a pharmacologically active ingredient plus the pre-concentrate is provided.
Owner:CHONG KUN DANG PHARMA CORP
Coal slime flotation collector and preparation method thereof
The invention discloses a coal slime flotation collector and a preparation method thereof. The coal slime flotation collector comprises the following matters in percentage by weight: 20-50 percent of kerosene and / or light diesel oil, 1-5 percent of primary emulsion, 0.006-0.015 percent of auxiliary emulsion and the balance of water; wherein the primary emulsion is a mixture of polyoxyethylene sorbitan fatty acid ester and dehydrated sorbitol fatty acid ester, and the hydrophile-lipophile balance (HLB) value of the primary emulsion is within 12.8-14.3; the auxiliary emulsion is selected from the following (1) or (2), wherein the (1) is sodium dodecyl benzene sulfonate, and the (2) is a mixture obtained by mixing fatty alcohol polyoxyethylene ether sulfate and the sodium dodecyl benzene sulfonate according to the mass ratio of 1: 0.5-2. The coal slime flotation collector has good stability, simple preparation process and 40-60 percent of the oil-saving ratio on the premise of improving the float yield and the tail coal ash proportion. The collector is beneficial to saving the energy, reducing the emission and improving the economical benefit when being used for floating the coal slime.
Owner:SHANXI MEDICAL UNIV
Hydrocolloid adhesive mass useful for medical purposes
The present invention relates to a novel hydrocolloid adhesive mass useful for medical purposes, characterized in that said hydrocolloid adhesive mass comprises:a) 0.2 to 5 parts by weight of an ethoxylated sorbitan fatty acid ester;b) 20 to 50 parts by weight of a hydrocolloid;c) 32 to 120 to parts by weight of an adhesive matrix made up of one or more polymers selected from poly(styrene / olefin / styrene) block copolymers, low-molecular polyisobutylenes and high-molecular polyisobutylenes, and one or more compounds selected from sticky resins, or tackifying resins, plasticizers, polybutenes, antioxidants, ethylene / vinyl acetate copolymers, butyl rubbers and ethylene / propylene block copolymers; andd) 0 to 15 parts by weight of an acrylate copolymer with a glass transition temperature below -20° C. It further relates to the use of this hydrocolloid adhesive mass for the production of dressings, especially for the treatment of superficial, deep, chronic or acute dermo-epidermal lesions, exudative wounds and bums.
Owner:LABORATOIRE URGO
Materials composites of a moulded article of transparent or translucent dyeable plastics moulding compounds
InactiveUS20070128442A1Large flow lengthPerfect purityLayered productsThin material handlingPolymer scienceStearic acid
The present invention relates to materials composites of a moulded article of at least one transparent or translucent dyeable plastics moulding compound which moulded article is bonded to at least one transparent or translucent surface layer and / or to decorative films, functional films or coats or rubbers or other plastics, wherein the plastics moulding compound used for the manufacture of the moulded article, the surface layer or the other plastics contains in an amount of 0.01 to 5.0% by weight, preferably 0.01 to 2.0% by weight, each related to the total weight of the moulding compound, at least one lubricant selected from the group consisting of sorbitan esters, sebacic acid esters, dodecanedioic acid esters, docosanoic acid esters, glycerine, glycol, diethylene glycol, stearoyl amide, stearyl stearate, ethylene bissteroyl amide, octane pyrrolidone, and from the group consisting of non-polar paraffin oils and of tetracosanes, and wherein a permanent adhesion to the other plastics layers and / or sheets or coats or rubbers or other plastics is achieved.
Owner:EMS CHEM AG
Liquid dispersion comprising dibenzylidene sorbitol acetals ethoxylated nonionic surfactants
InactiveUS6127470ALow viscosityInexpensive fluid dispersionTransportation and packagingMixingPeristaltic pumpPolyolefin
This invention relates to a fluid dispersion of at least one dibenzylidene sorbitol acetal derivative. The sorbitol acetal derivative is useful as a clarifying agent for polyolefins and the inventive fluid dispersion permits improvements in the handling and processing of and mixing within the polyolefin composition. The inventive dispersion must be shelf stable, retain its nucleating effects, be compatible with polypropylene (and other polyolefins), and possess both short-term and long-term viscosities which permit acceptable transport through a standard polyolefin-manufacturing peristaltic pump. The preferred inventive dispersion thus comprises 3,4-DMDBS and at least one ethoxylated nonionic surfactant having an HLB of greater than about 8.5. Preferred surfactants include those selected from the group consisting essentially of ethoxylated sorbitan (C8-C22) monoesters and ethoxylated nonyl-phenol ethers. The inventive dispersion may be introduced within any polyolefin composition, preferably polypropylene, which may then be molded into any shape or form. A method of producing a polyolefin plastic utilizing the inventive dispersion is also provided.
Owner:MILLIKEN & CO
Bacteriostatic antistatic multifunctional non-woven fabric
ActiveCN101638846AFine foamImprove stabilityFibre typesUltrasonic/sonic fibre treatmentUltravioletNonwoven fabric
The invention relates to a bacteriostatic antistatic multifunctional non-woven fabric which is fabricated by the steps of unwinding non-woven gray fabric; applying finishing agent by using an ultrasonic foam applicator; extruding out redundant finishing agent by using a rolling mill; and then drying by using a baking oven and winding. The finishing agent includes the following components: sodium dodecyl sulfate, hydroxyethyl cellulose, nanometer silver powder, penetrating agent JFC, nano-silica, gamma-aminopropyltriethoxysilane, sorbitan ester, cetyltrimethyl ammonium chloride, methacrylic acid, beta-cyclodextrin and spices. The non-woven fabric has multiple functions of being antibacterial, anti-static, anti-ultraviolet, washing-resisting, fragrance lasting and the like. Especially the ultrasonic foam applicator is utilized to conduct foam finishing, and the finishing agent can be evenly penetrated in the fiber structure by means of the effect of ultrasonic waves, so as to lead nanometer material in the finishing agent and particles of the spices to disperse in the fabric and to be wrapped by organic matters to form a microcapsulation state, so that all functions and effects are lasting.
Owner:仙桃新发塑料制品有限公司
Guerbet based sorbitan esters
InactiveUS6013813AImprove propertiesCosmetic preparationsCationic surface-active compoundsSorbitanSorbitol
Owner:HANSOTECH
Composition containing ascorbic acid
The invention relates to a composition in oil-in-water emulsion form containing ascorbic acid or a derivative thereof, at least one polysaccharide hydrophilic gelling agent, at least one C16-C22 fatty acid sorbitan ester, at least one ethoxylated fatty acid ester and at least one polymer containing a sulpho-functional monomer.
Owner:LOREAL SA
Adjuvant Compositions, Agricultural Pesticide Compositions, and Methods for Making and Using Such Compositions
PendingUS20130123104A1Improved spray drift controlGood dispersionBiocideAnimal repellantsLiquid mediumAdjuvant
An agricultural adjuvant composition includes (a) one or more first nonionic surfactants selected from the group consisting of fatty acid glycol ester surfactants, polyalkoxylated triglyceride surfactants, alkoxylated fatty alcohol surfactants, and sorbitan fatty acid ester surfactants, (b) at least one of: (b)(i) one or more second nonionic surfactants selected from the group consisting of polyalkoxylated alkylphenol surfactants, polyalkoxylated alkarylphenol surfactants, amine oxide surfactants, alkanolamide surfactants, glycoside surfactants, and ethylene / propylene block copolymers, and (b)(ii) one or more anionic components selected from the group consisting of anionic surfactants and polyanionic polymers, (c) optionally, a liquid medium comprising one or more fatty acid (C1-C3)alkyl esters, optionally, one or more water soluble deposition aid polymers, and (e) optionally, one or more thickening agents.
Owner:RHODIA OPERATIONS SAS
Water-repellent/oil-repellent composition
InactiveCN101006149AExcellent water and oil repellencyImprove stabilityOther chemical processesFibre treatmentOrganic solventWater soluble
Disclosed is an aqueous water-repellent / oil-repellent composition containing the following components (A), (B) and (C). Fluorine-containing copolymer (A): a copolymer containing a polymerizable monomer (a1) having a perfluoroalkyl group with 1-6 carbon atoms or a perfluoroalkenyl group wherein the amount of (a1) is not less than 20% by weight relative to the copolymer. Surfactant (B): a surfactant essentially containing a sorbitan ester or an alkylene oxide addition product thereof. Aqueous medium (C): a medium substantially containing water only or water and a water-soluble organic solvent.
Owner:DAIKIN IND LTD
Neutral blockage removing agent composition used for oil recovery formation in oilfield and preparation method thereof
ActiveCN104194759AImprove cleanlinessNo pollutionDrilling compositionActivated attapulgitePolythylene glycol
The invention relates to a neutral blockage removing agent composition used for an oil recovery formation in an oilfield. The neutral blockage removing agent composition is prepared from the following raw materials in parts by weight: 30-36 parts of disodium polyepoxysuccinicate, 18-25 parts of sodium gluconate, 2-3 parts of polyethylene glycol, 2-4 parts of triethanolamine, 9-12 parts of ethyl lactate, 7-10 parts of fatty alcohol polyoxyethylene ether sodium sulfate, 8-12 parts of amino acid sodium, 10-15 parts of activated attapulgite, 0.1-0.2 part of vanadium pentoxide, 5-7 parts of lipase, 5-8 parts of sorbitan fatty acid ester, 15-18 parts of P-hydroxy sodium sulfonate, 3-7 parts of tert-butyl hydroperoxide and 0.1-0.3 part of diethylene glycol monolaurate. The neutral blockage removing agent composition has high blockage removing speed, is neutral, is free of corrosion and can quickly dissolve material scales, asphalt sediment scales, carbonate scales, silicate scales and the like generated by an ASP flooding system; waste liquid for blockage removal can be degraded and does not need to be discharged onto the ground to be subjected to sewage treatment, the formation in the oilfield is cleaned to remove the blockage, no corrosion, dead angle, precipitation or secondary well blockage is generated, and the blockage removal time does not exceed 24 hours.
Owner:GANSU HEIMA PETROCHEM ENG
Antiallergic microemulsion
InactiveCN105708757AImprove stabilityLong term storageCosmetic preparationsToilet preparationsVegetable oilStearate
The invention discloses an antiallergic microemulsion, which contains: sorbitan sesquioleate and PEG‑60 sorbitan stearate; one or more oily components, Selected from hydrocarbon oils, synthetic oils, animal and vegetable oils, silicone oils and mixtures thereof; polyhydric alcohols and water; one or more water-soluble antiallergic raw materials. The microemulsion is a water-in-oil system, has good stability, can be stored for a long time, has a good solubilizing effect on water-soluble anti-allergic raw materials, and can be made into products with higher functional components.
Owner:江西登云健康美业互联有限公司 +1
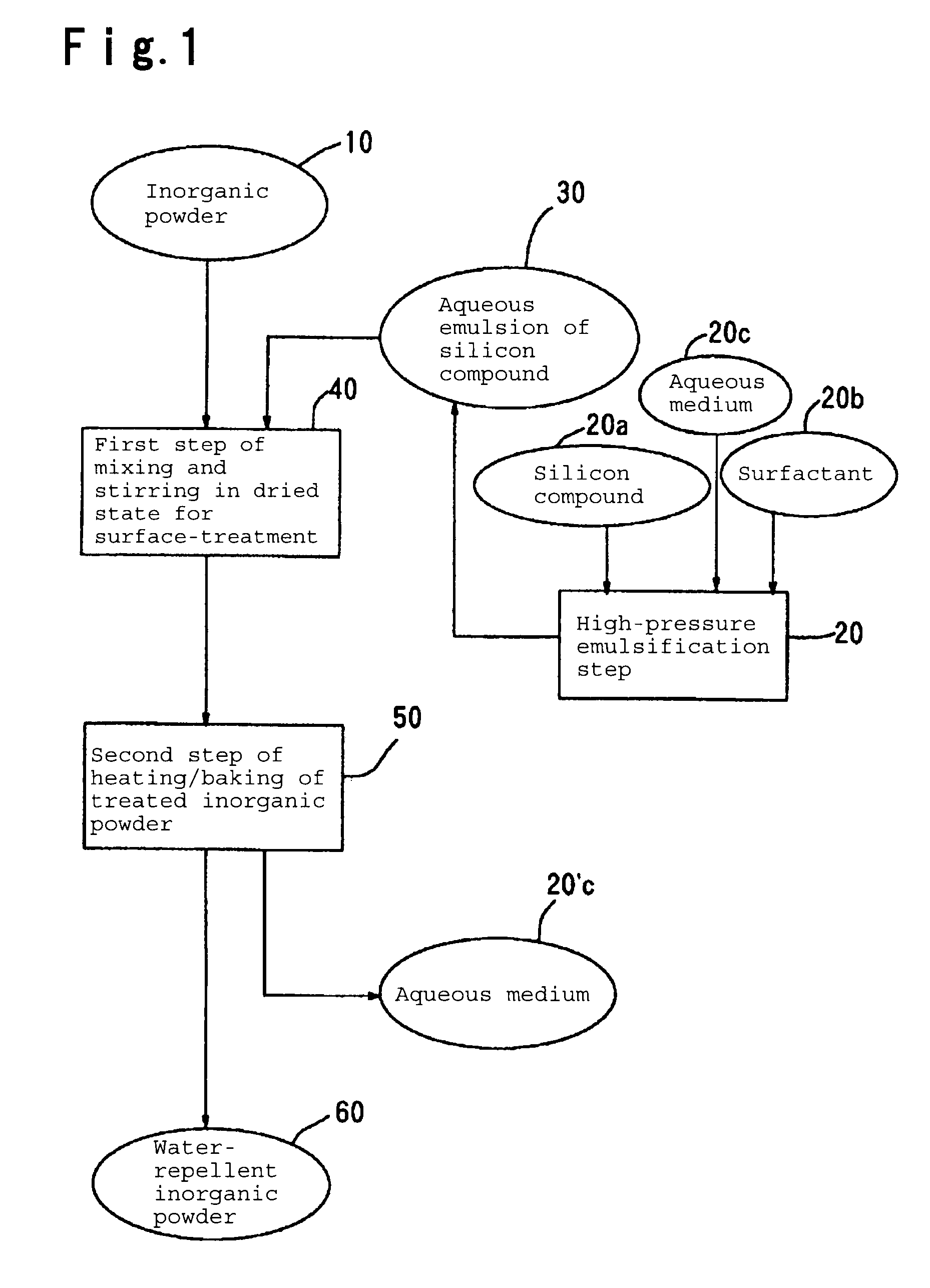
Water-repellent inorganic powder and process for its production
InactiveUS20080269358A1Stable water repellencyEffective treatmentMaterial nanotechnologySilicaEpoxyPolyethylene glycol
To provide a stable water-repellent inorganic powder which is substantially free from re-dissolution of a silicon compound used for surface treatment whether an organic solvent is polar or non-polar.A water-repellent inorganic powder which is surface-treated with a silicon compound, wherein the silicon compound is one having a group reactive with the inorganic powder, and when the inorganic powder is dispersed in any of polar organic solvents and non-polar organic solvents, the retention of the silicon compound is at least 90%. Preferably, the silicon compound is an aqueous emulsion of any of methylhydrogen silicone oil, alkoxy-modified silicone oil, amino-modified silicone oil, epoxy-modified silicone oil, polyether-modified silicone oil or carboxyl-modified silicone oil, and to the aqueous emulsion, at least one surfactant selected from the group consisting of a sorbitan fatty acid ester type, a polyoxyethylene sorbitan fatty acid ester type, a polyethylene glycol fatty acid ester type, a polyoxyethylene fatty acid ester type, an N-acylamino acid type, a polyoxyethylene alkyl ether type and a polyoxyethylene alkylphenyl ether type, is incorporated.
Owner:AGC SI TECH
Cyclosporin compositions
A composition is disclosed herein comprising from about 0.001% to about 0.4% cyclosporin A, castor oil, and a surfactant selected from the group consisting of alcohol ethoxylates, alcohols, alkyl glycosides, alkyl polyglycosides, alkylphenol ethoxylates, amine oxides, block polymers, carboxylated alcohol or alkylphenol ethoxylates, carboxylic adds / fatty acids, cellulose derivatives, ethoxylated alcohols, ethoxylated alkylphenols, ethoxylated aryl phenols, ethoxylated fatty acids, ethoxylated fatty acids, ethoxylated fatty esters and oils, fatty alcohols, fatty esters, glycol esters, lanolin-based derivatives, lecithin and lecithin derivatives, lignin and lignin derivatives, methyl esters, monoglycerides and derivatives, phosphalipids, polyacrylic acids, polyethylene glycols, polyethylene oxide-polypropylene oxide copolymers, polyethylene oxides, polymeric surfactants, polypropylene oxides, propoxylated alcohols, propoxylated alkyl phenols, propoxylated fatty acids, protein-based surfactants, sarcosine derivatives, silicone-based surfactants, sorbitan derivatives, stearates, sucrose and glucose esters and derivatives, and combinations thereof.
Owner:SAINT REGIS MOHAWK TRIBE
Water-repellent/oil-repellent composition
ActiveUS7820745B2Improve water resistanceExcellent oil-repellencyOther chemical processesLiquid repellent fibresPolymer scienceActive agent
Disclosed is an aqueous water-repellent / oil-repellent composition containing the following components (A), (B) and (C). Fluorine-containing copolymer (A): a copolymer containing a polymerizable monomer (a1) having a perfluoroalkyl group with 1-6 carbon atoms or a perfluoroalkenyl group wherein the amount of (a1) is not less than 20% by weight relative to the copolymer. Surfactant (B): a surfactant essentially containing a sorbitan ester or an alkylene oxide addition product thereof. Aqueous medium (C): a medium substantially containing water only or water and a water-soluble organic solvent.
Owner:DAIKIN IND LTD
Resin matrix composite with aluminum for lubrication in drilling
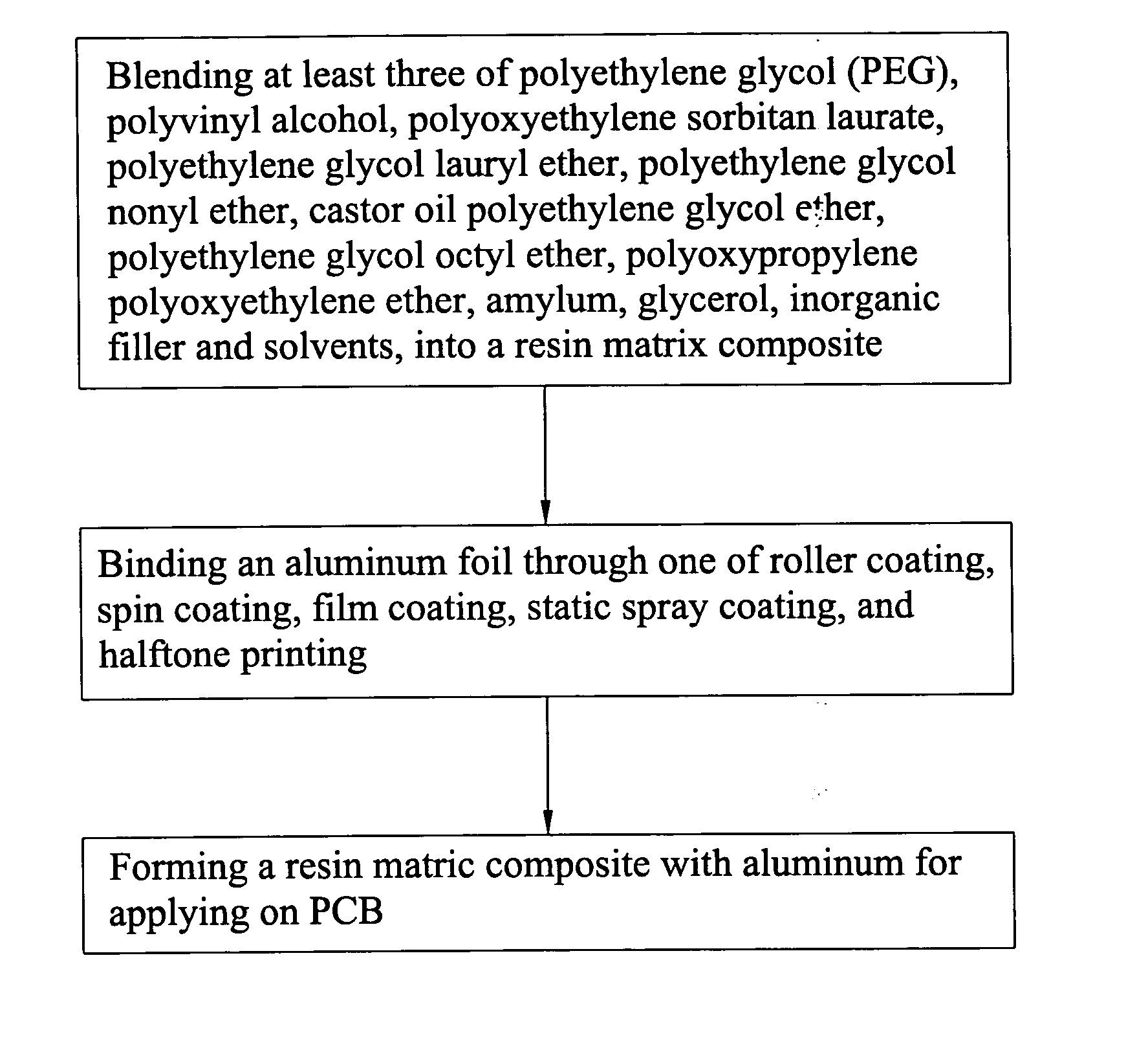
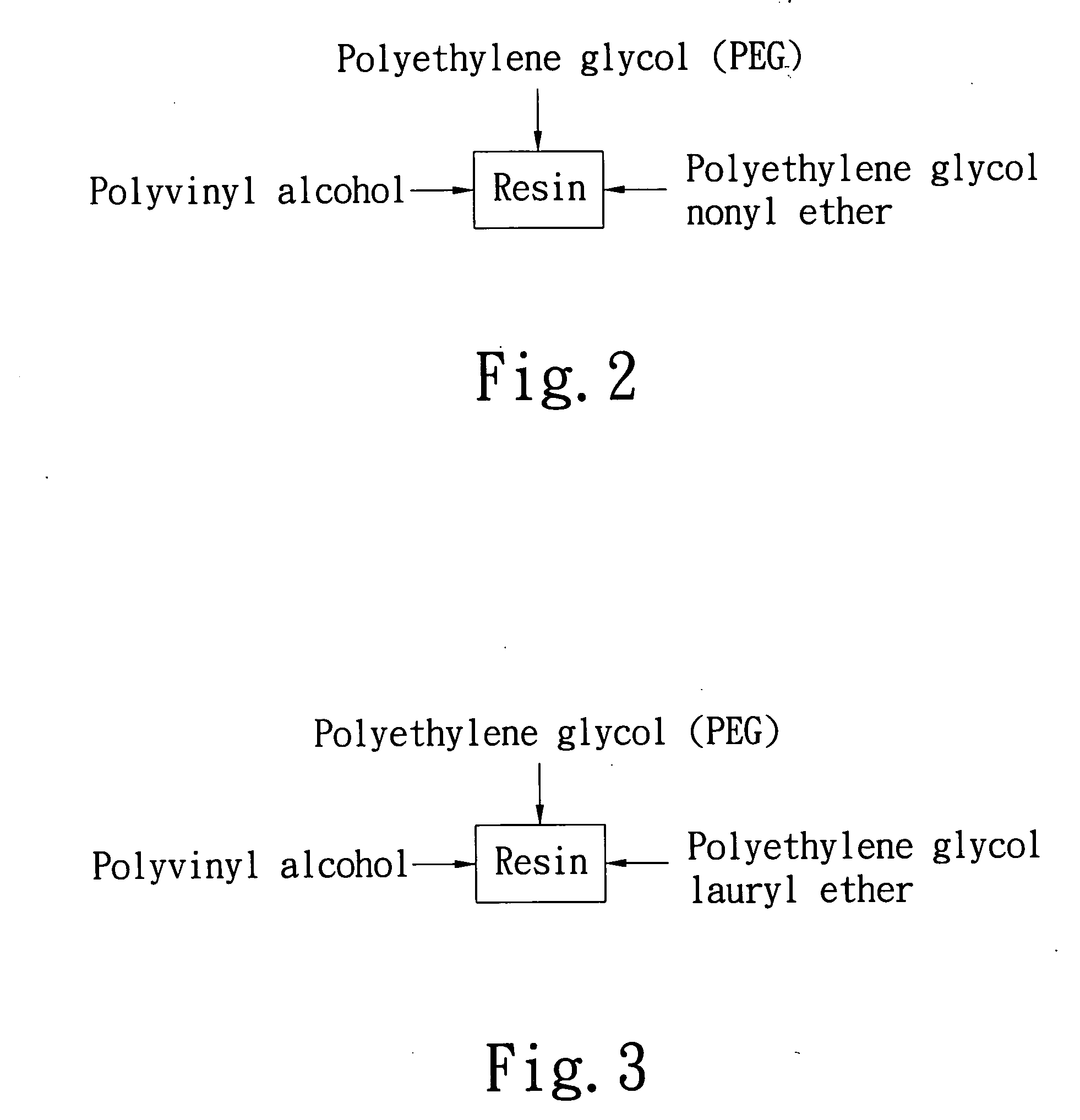
InactiveUS20060062996A1Simplified typesIncrease ratingsSynthetic resin layered productsPrinted circuit manufacturePolyvinyl alcoholPolyethylene glycol
A resin matrix composite with aluminum for lubrication in drilling comprises a resin matrix composite and an aluminum foil. The resin matrix composite is consisting of at least three of the following materials: PEG (polyethylene glycol), polyvinyl alcohol (PVA), polyoxyethylene sorbitan laurate, coconut alcohol polyethylene glycol ether, polyethylene glycol nonyl ether, castor oil polyethylene glycol ether, polyoxypropylene polyoxyethylene ether, amylum, glycerol, inorganic filler, and solvents. By one of roller coating, spin coating, film coating, static spray coating, or halftone printing, the resin matrix composite can bind the aluminum foil to form a resin matrix composite with aluminum foil for applying on PCB (printed circuit board) to function as lubricating in drilling.
Owner:YEH YUN CHAO
Preparation method for medical use hydrocolloid dressing
The invention discloses a preparation method for medical hydrocolloid dressing. The preparation method comprises the following steps:,1, dissolving styrene-butadiene-styrene block copolymer, then adding aliphatic series petroleum resins, carrying out the reaction, slowly adding cyclane oil, raising the temperature, then adding N.N-dibutyl dithio amido formic acid, and keeping the temperature to form a component A; 2, mixing anhydrosorbitol fatty acid ester and a polyoxyethylene sorbitan fatty ester condensation compound at normal temperature to form a liquid state component B; 3, taking sodium carboxymethyl cellulose, carboxymethyl chitosan, calcium alginate fibers and solid silver corpuscles, crushing the calcium alginate fibers into short fibers, mixing the substances at normal temperature to form a component C; and 4, adding the component B into the component A, raising the temperature, slowly stirring the mixture evenly and then adding the component C, stirring and cooling down the mixture, carrying out the debubbling in the vacuum, reducing the temperature, forming the dressing onto antiseize paper through injection moulding, and covering a PU film on the surface of the hydrocolloid to form a finished product.
Owner:褚加冕
Acne treatment composition and application thereof and acne treatment cream containing acne treatment composition
ActiveCN105012479ASoothe rednessRelieve discomfort such as inflammationCosmetic preparationsToilet preparationsCentella asiatica extractEthylhexyl palmitate
The invention discloses an acne treatment composition and application thereof and an acne treatment cream containing the acne treatment composition. The acne treatment composition comprises shea butter, Chinese corktree bark extract, salix nigra bark extract, herba portulacae extract, purple perilla extract, asiatic pennywort herb extract and mung bean extract, effectively conditions acne skins and inhibits reproduction of acne, smoothens acne and removes acne marks, and can be applied to skin directly or added into acne treatment products. The acne treatment cream comprises the acne treatment composition, cetearyl alcohol, ethylhexyl palmitate, polydimethylsiloxane, glyceryl stearate, PEG-100 stearate, caprylic / capric triglyceride, propylene glycol, polyacrylamide, polysorbate-60, sorbitan stearate, laurocapram, PEG-20 methl glucse sesquistearate, menthol, borneol, edta disodium, methylparaben, propyl hydroxybenzoate and water. The acne treatment cream prepared by a hypo-allergenic formula is mild and non-irritating, easy to absorb, non-greasy, safe and effective.
Owner:广州科玛生物科技股份有限公司
Transparent anti-fog anti-splash coating compositions
The present invention is directed to an anti-fogging / anti-splash composition that can be used for anti-fog / anti-splash applications. When applied to the surface of a transparent or reflective substrate, the composition dries relatively clear and comprises a solution of a non-toxic, fast drying solvent or alcohol and a surface active agent containing sodium lauryl ether sulfate, and either a block ethylene oxide / polyethylene oxide copolymer, an ethoxylated amine, an ethoxylated acetylenic alcohol, sodium sulfosuccinate, ethoxylated sorbitan ester, random EO / PO polymers on butyl alcohol, or mixtures thereof. When applied to the surface of a substrate, the present invention provides a coating of high transparency which cures rapidly, is sufficiently surface active to be an anti-fog agent when "dry", will persist when "wet" under water spray conditions (such as on a dental mirror) for a period substantial enough to be practicable, and will provide anti-splash benefits throughout the use cycle.
Owner:MACHSON ROGER
Moisture absorption and sweat releasing antibacterial cotton polyester knitted fabric
The invention discloses moisture absorption and sweat releasing antibacterial cotton polyester knitted fabric. The moisture absorption and sweat releasing antibacterial cotton polyester knitted fabric is formed by blending and weaving, by weight, 55 parts of anionic polyester fibers and 45 parts of cotton fibers, and the fabric is manufactured according to the following composite arranging process: the fabric is placed in deionization aqueous solutions, the bath ratio is 3:10, the 4-5g / L nano-silver antimicrobials, 2-3g / L polyvinylpyrrolidone, 2-3g / L polyphenol sulfonic acid condensation products and 1-2g / L polyoxyethylene sorbitan fatty acid ester are firstly added, and are heated to 60 DEG C, and standing is carried out for 25 minutes; then, the 1-2g / L paraffin and 8-9g / L polyester linear block compounds are added, and are heated to 70 DEG C, and standing is carried out for 25 minutes; finally, isopropanolamine is added to adjust the pH value to be 4.0, the isopropanolamine is heated to 85 DEG C, and standing is carried out for 35 minutes, the fabric is taken out for being washed for 35 minutes by water, water removing is performed, and the fabric is dried for 2 hours at the temperature of 85 DEG C. The cotton polyester knitted fabric is comfortable in hand feeling, and has the excellent moisture absorption and sweat releasing antibacterial performance.
Owner:JIANGSU JINHONG KNITTING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com