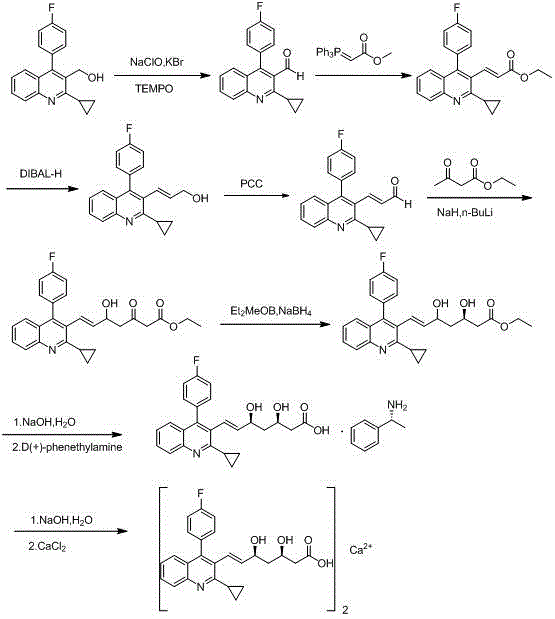
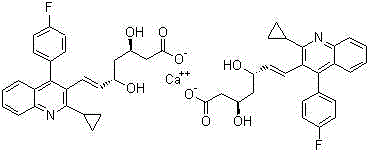
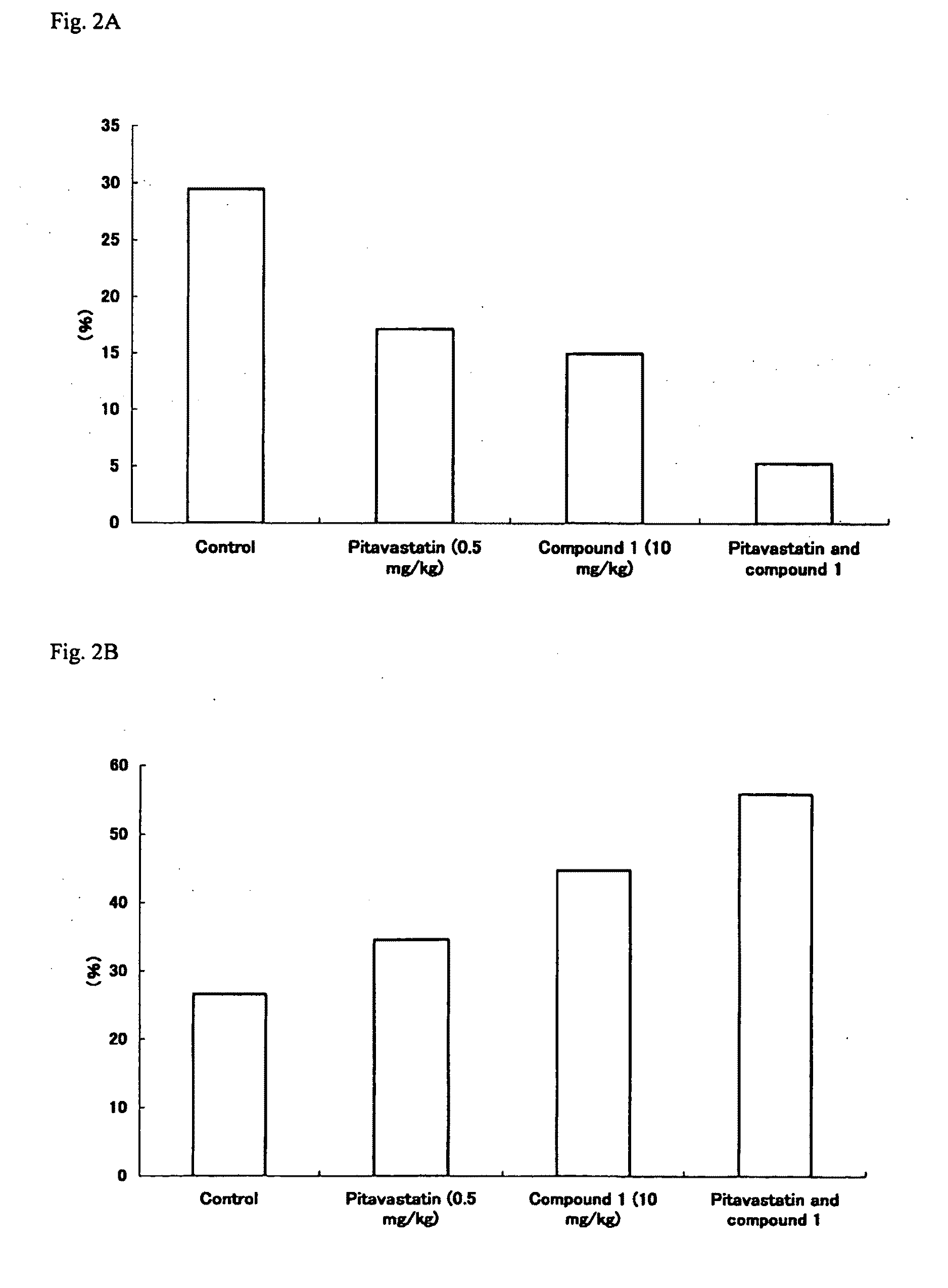
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
80 results about "Pitavastatin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pitavastatin (usually as a calcium salt) is a member of the blood cholesterol lowering medication class of statins. Like other statins, it is an inhibitor of HMG-CoA reductase, the enzyme that catalyses the first step of cholesterol synthesis.
Hyperlipemia therapeutic agent
The present invention relates to a hyperlipemia therapeutic agent comprising pitavastatins and eicosapentaenoic acid or an ester derivative thereof as effective ingredients.According to the present invention, a type IIb and type IV hyperlipemia therapeutic agent having an excellent effect of lowering the cholesterol and triglyceride in blood is provided.
Owner:KOWA CO LTD +1
Omega-3 fatty acid self-emulsifying composition
InactiveUS20180015038A1Reduce the amount requiredImprove compatibilityNervous disorderAntipyreticPolyoxyethylene castor oilEmulsion
A pharmaceutical composition comprising, in relation to 100% by weight of a total amount of a self-emulsifying composition, 70 to 90% by weight of eicosapentaenoic acid ethyl ester as a first medicinal component, 0.5 to 6% by weight of water, 1 to 29% by weight of polyoxyethylene sorbitan fatty acid ester (optionally further comprising polyoxyethylene castor oil) as an emulsifier, 1 to 25 parts by weight of lecithin in relation to 100 parts by weight of the eicosapentaenoic acid ethyl ester, and pitavastatin, rosuvastatin, or a salt thereof as a second medicinal component. The composition is excellent in any one of self-emulsifying property, dispersibility of the composition, emulsion stability, absorbability, and storage stability of the medicinal components and a preparation.
Owner:MOCHIDA PHARM CO LTD
Quinolines compounds and their intermediates, preparation method and application
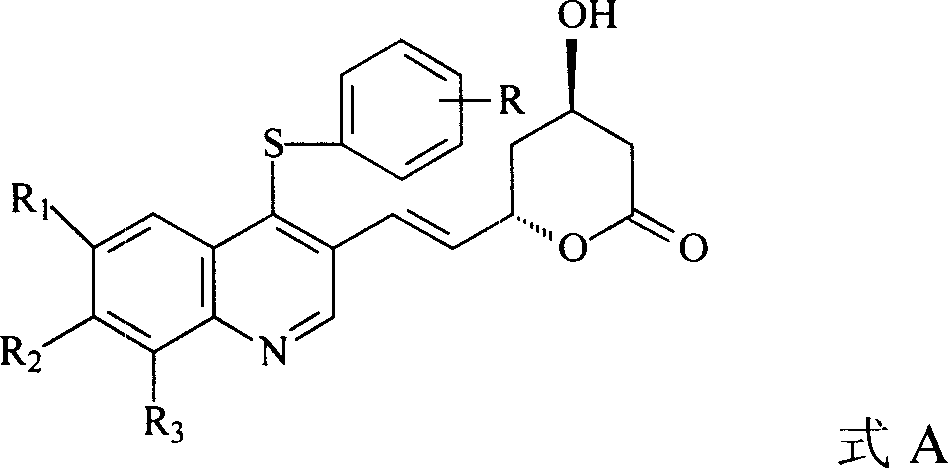
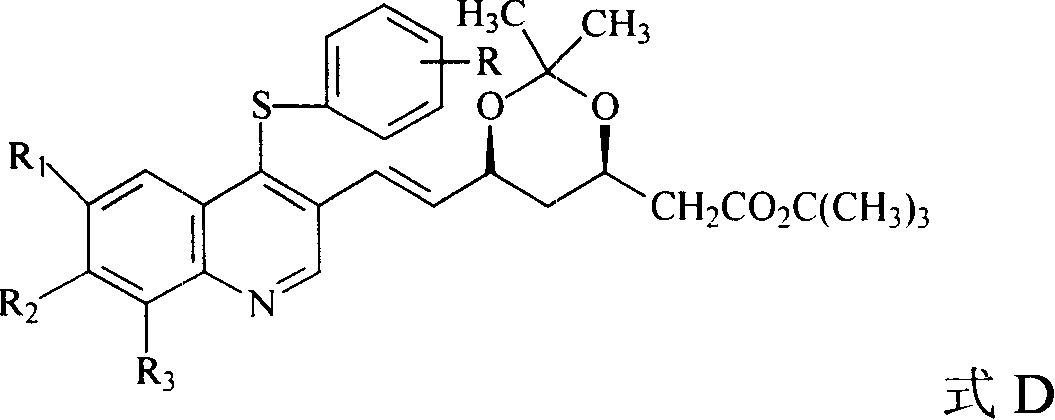
The invention discloses a quinolinic compound showed in the formula A and pharmacy acceptable solvate, optical isomers or polymorphic substance and a reaction intermediate compound showed in the formula D; wherein, R1, R2 and R3 are respective and independent H, halogen or group showed in the formula H; wherein, the R is H, the halogen, alkyl of C1-C4 or alkoxide of C1-C4. The invention further discloses a preparation method thereof and application for preparing medicines for inhibiting HMG CoA reductase and treating hyperlipemia related diseases. Compared with the existing fuvastatin, rosuvastatin and pitavastatin in the prior art, the quinolinic compound of the invention can better inhibiting the activity of the HMG CoA reductase and can be used for treating hyperlipemia related diseases.
Owner:SHANGHAI INST OF PHARMA IND
Novel boronate esters
The present invention relates to optically active boronate derivatives which are useful as intermediates for the synthesis of HMG-CoA enzyme inhibitors such as atorvastatin, cerivastatin, rosuvastatin, pitavastatin, and fluvastatin.
Owner:BIOCON LTD
Pitavastatin soluble tablet composition and preparation method thereof
InactiveCN1698609AQuality improvementHigh dissolution rateMetabolism disorderPill deliveryPitavastatinDissolution
The invention relates to a pitavastatin soluble tablet composition and preparation process wherein the composition is prepared from soluble medicinal auxiliary materials by a predetermined proportion, the composition becomes solution state in oral liquid, thus facilitating the dissolution, absorption and application of pitavastatin calcium.
Owner:南京馗珂生物医药技术有限公司
Medicinal composition stabilized by base reagent containing pitavastatin calcium and preparation technology thereof
The invention provides a drug compound containing Pitavastatin, pH regulator and other excipient and the preparation process. The invention is characterized in that the pH regulator is chosen from calcium hydrogenphosphate and sodium bicarbonate; the pH of the water solution or suspension of the compound is more than 9 but less than 10, ensuring the stability of the preparation.
Owner:HAINAN SHENGKE LIFE SCI RES INST
Medicinal composition containing rosuvastatin or pitavastatin and B clan vitamin
ActiveCN1891219AGood curative effectImprove compliancePill deliveryGranular deliveryDiseasePitavastatin
The present invention relates to a medicine composition conlaining resulvatatine or pivatatine compound and vitamins B, in which the resulvatatine or pivatatine content is 1-40 mg and vitamins B content is 0.1-50 mg. Said invention also provides the application of said medicine composition in preparation of medicine for preventing and curing the diseases of atherosclerosis, angiocardiopathy and cerebrovascular disease.
Owner:SHENZHEN AUSA PHARM CO LTD
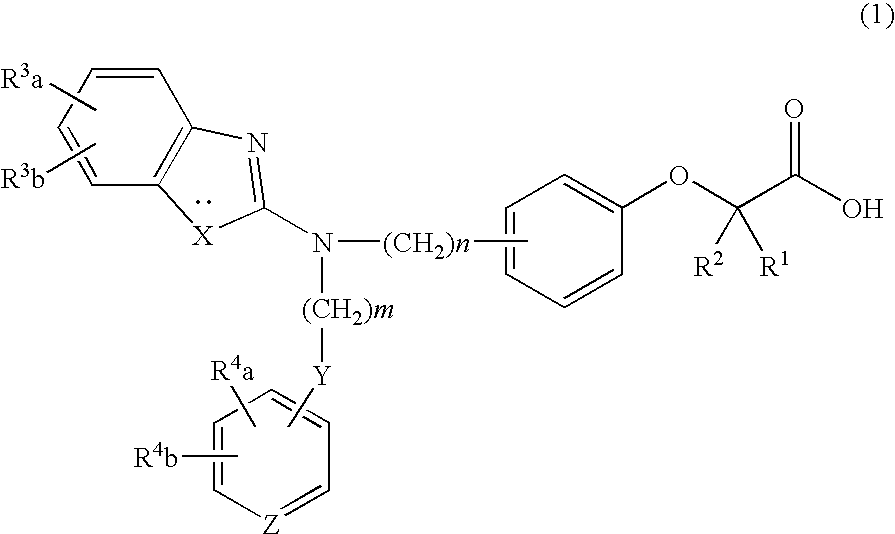
Prophylactic and/or therapeutic agent for hyperlipidemia
ActiveUS20100069433A1Improve concentrationGood effectBiocideOrganic chemistryEthyl groupBlood plasma
The present invention provides a therapeutic agent for hyperlipidemia having an excellent effect of lowering the cholesterol and triglyceride level in blood plasma.The present invention relates to a prophylactic and / or therapeutic agent for hyperlipidemia, a prophylactic and / or therapeutic agent for obesity or diabetes mellitus, and a prophylactic and / or therapeutic agent for metabolic syndrome, each agent including a compound represented by the following formula (1):, wherein:R1 and R2, which may be identical or different, each represent a hydrogen atom, a methyl group or an ethyl group; R3a, R3b, R4a and R4b, which may be identical or different, each represent a hydrogen atom, a halogen atom, a nitro group, a hydroxyl group, a C1-4 alkyl group, a trifluoromethyl group, a C1-4 alkoxy group, a C1-4 alkylcarbonyloxy group, a di-C1-4 alkylamino group, a C1-4 alkylsulfonyloxy group, a C1-4 alkylsulfonyl group, a C1-4 alkylsulfinyl group, or a C1-4 alkylthio group, or R3a and R3b, or R4a and R4b are joined to represent an alkylenedioxy group; X represents an oxygen atom, a sulfur atom or N—R5 (wherein R5 represents a hydrogen atom, a C1-4 alkyl group, a C1-4 alkylsulfonyl group, or a C1-4 alkyloxycarbonyl group); Y represents an oxygen atom, a S(O)l group (l represents a number from 0 to 2), a carbonyl group, a carbonylamino group, an aminocarbonyl group, a sulfonylamino group, an aminosulfonyl group, or an NH group; Z represents CH or N; n represents a number from 1 to 6; and m represents a number from 2 to 6,or a salt thereof, and a statin, particularly pitavastatin, in combination.
Owner:KOWA CO LTD
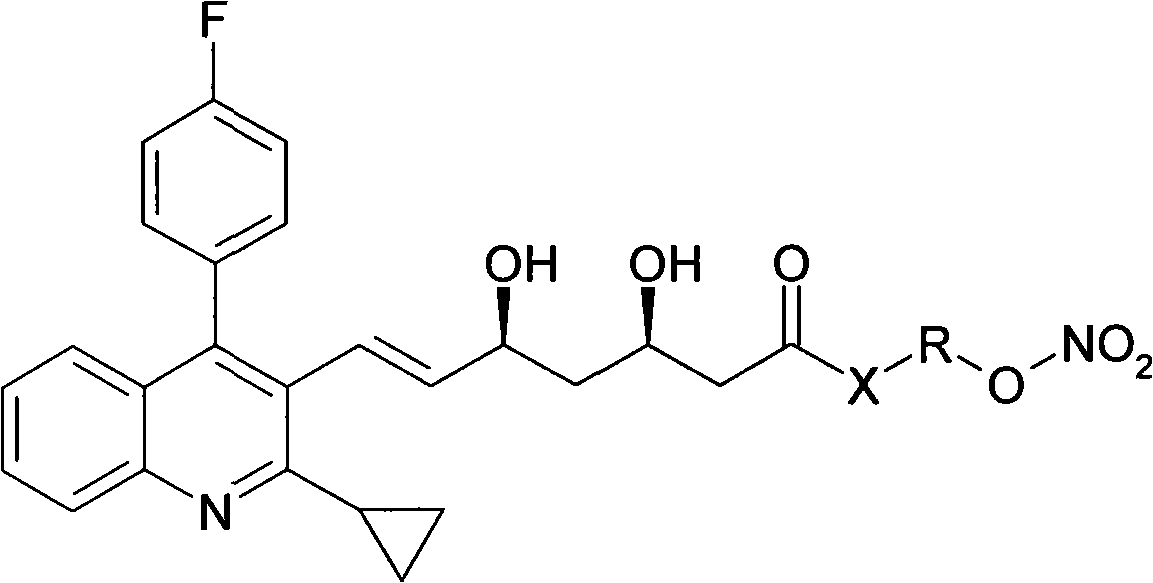
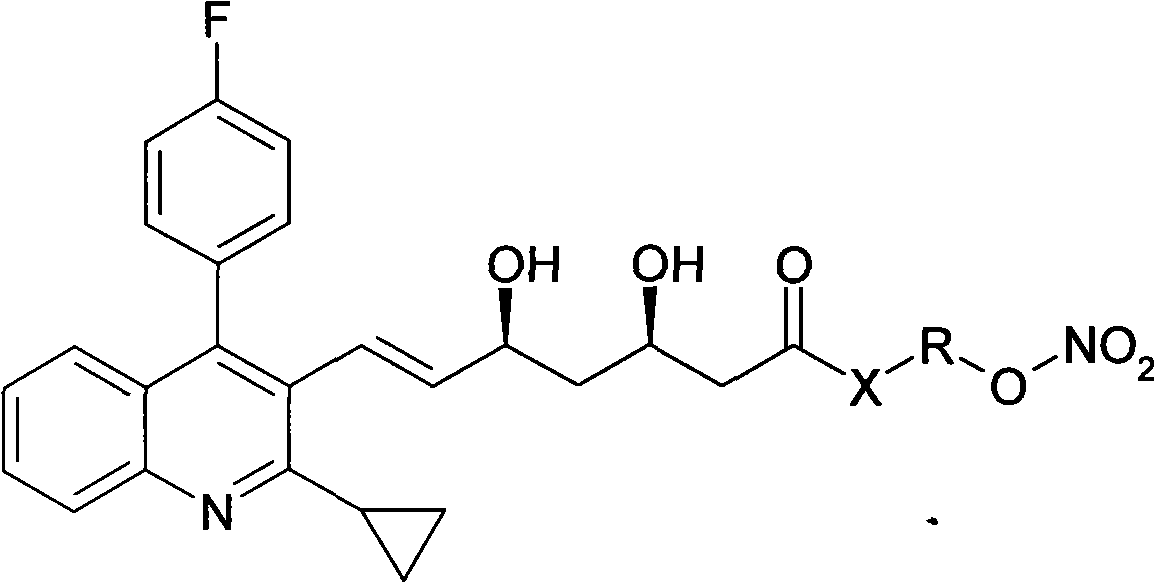
Novel pitavastatin nitryl ester derivant
InactiveCN101270084AReduce dosageSignificant effectOrganic chemistryMetabolism disorderPitavastatinThreonine
The present invention provides novel pitavastatin nitro ester derivative that is shown in the formula (I). The derivative comprises acceptable salt, hydrate or solvolyte in the pharmacy. In the formula (I), X stands for O, S and NH; R has a bivalent group defined as follows: (a) alkyl or substitutional alkyl with 1 to 20 carbon atoms that can be freely substituted by one or more than one substitutional group; or amino group with 3 to 7 carbon atoms that can be substituted by one or more than one substitutional group; (b) amino acid residue containing hydroxyl, such as serine and threonine residue; (c) n standing for an integral from 0 to 20 and m standing for an integral from 0 to 20.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Method for preparing intermediate of pitavastatin calcium
InactiveCN104016916AReduced purification stepsThe reaction steps are simpleOrganic chemistryMethyl aldehydePitavastatin
The invention discloses a method for preparing an intermediate of pitavastatin calcium. The method comprises the steps of reacting 2-cyclopropyl-4-(4-fluorophenyl) quinoline-3-methyl aldehyde with (triphenylphosphoranyl) methyl acetate to obtain an intermediate 1; reacting the intermediate 1 in presence of a strong reducing agent to obtain an intermediate 2; reacting the intermediate 2 in the presence of an oxidant to obtain an intermediate 3; reacting the intermediate 3 with ethyl acetoacetate under the action of a strong base reagent to obtain an intermediate 4; carrying out chiral reduction on the intermediate 4 and a carbonyl group under the action of a reducing agent to obtain an intermediate 5; and hydrolyzing the intermediate 5 under the action of an alkali to obtain pitavastatin heptenoic acid, and then reacting pitavastatin heptenoic acid with a chiral reagent, carrying out chiral resolution and hydrolyzing to obtain the pitavastatin heptenoic acid product. The method disclosed by the invention has the beneficial effects that the disadvantage of the basic patent route is overcome, no risk reagent is used, the purification step is reduced and the reaction steps are simplified and the method is applicable in large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Method for reduction, stabilization and prevention of rupture of lipid rich plaque
InactiveUS20090275595A1Good effectReduction and stabilization and prevention of ruptureBlood disorderCardiovascular disorderMedicinePitavastatin
There is to provide is an agent for reduction of a lipid rich plaque, stabilization of a lipid rich plaque and / or prevention of rupture of a lipid rich plaque in an atherosclerotic lesion comprising an effective amount of 2-[4-[2-(benzimidazole-2-ylthio)ethyl]piperazin-1-yl]-N-[2,4-bis(methylthio)-6-methyl-3-pyridyl]acetamide (hereinafter, referred to as compound 1), its pharmaceutically acceptable salt or a hydrate thereof and Pitavastatin, and a pharmaceutically acceptable carrier, wherein the agent is intended to be simultaneously administered, or separately administered with interval of time to a patient in need thereof. There is also to provide a method for reduction of a lipid rich plaque, stabilization of a lipid rich plaque and / or prevention of rupture of a lipid rich plaque in an atherosclerotic lesion, comprising simultaneously administering, or separately administering with interval of time an effective amount of the compound 1, its pharmaceutically acceptable salt or a hydrate thereof and an effective amount of Pitavastatin to a patient in need thereof.
Owner:KOWA CO LTD
Process for the preparation of HMG-COA reductase inhibitors and intermediates thereof
ActiveUS8476432B2Improve isolationReduce stepsOrganic active ingredientsOrganic chemistryHMG-CoA reductasePitavastatin
The present invention provides an improved process for preparing HMG-CoA reductase inhibitors such as rosuvastatin calcium, fluvastatin sodium, and pitavastatin calcium under a mild condition, using a novel amide-bond-containing compound having R2—N—O—R1 moiety as a key intermediate. And also, the present invention provides the novel compound, an intermediate useful for the preparation thereof, and a process for the preparation thereof.
Owner:YUHAN
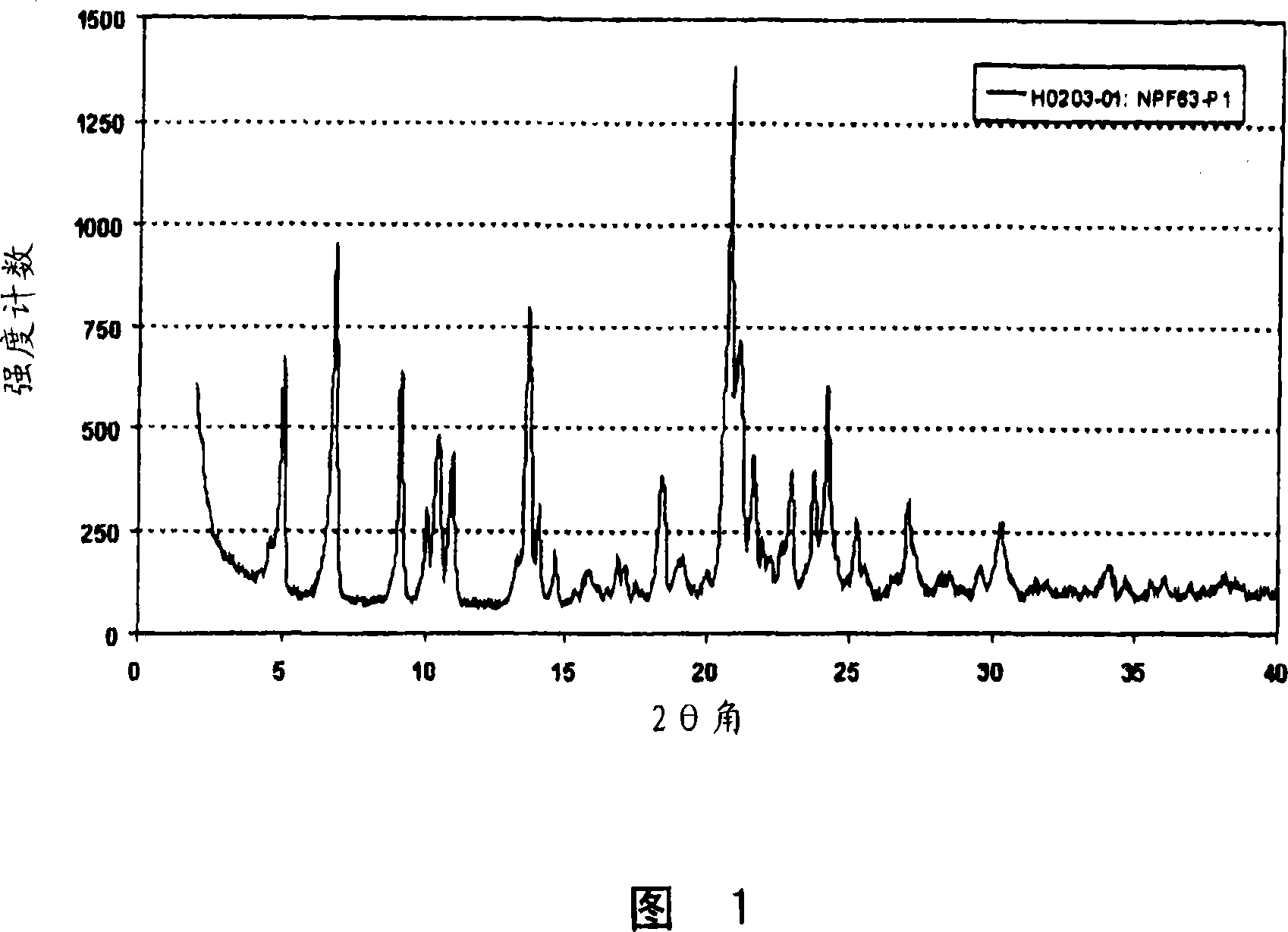
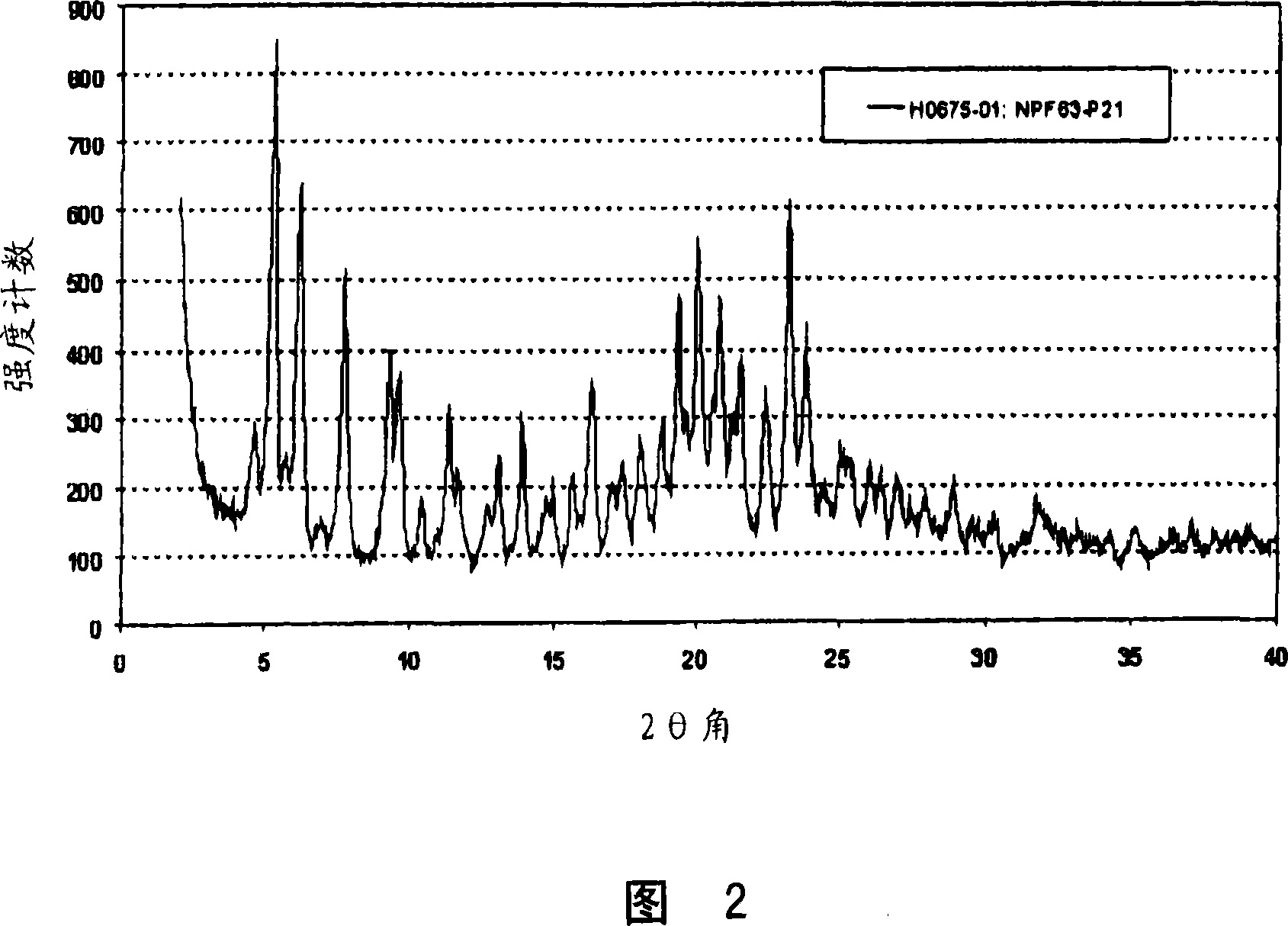
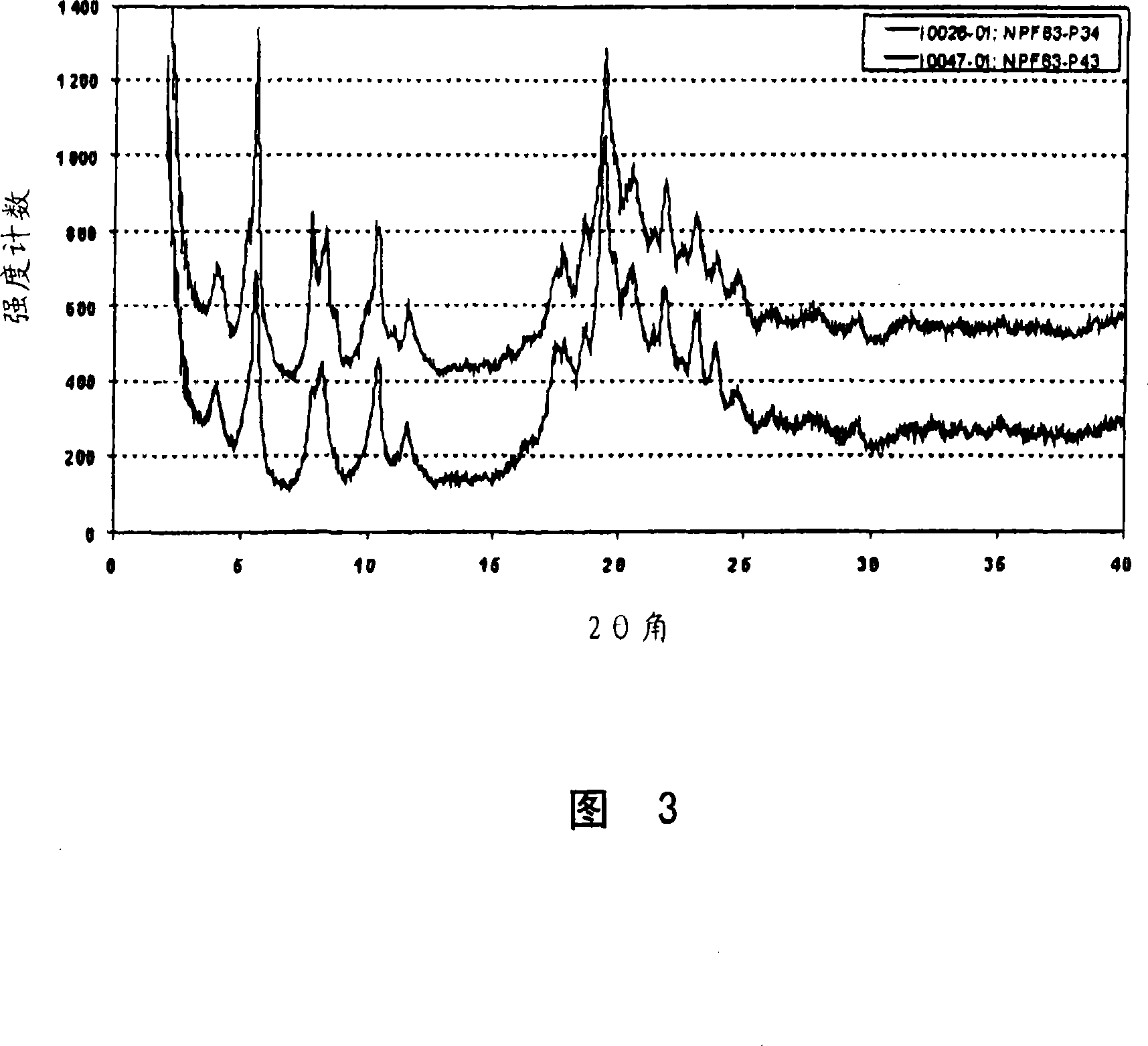
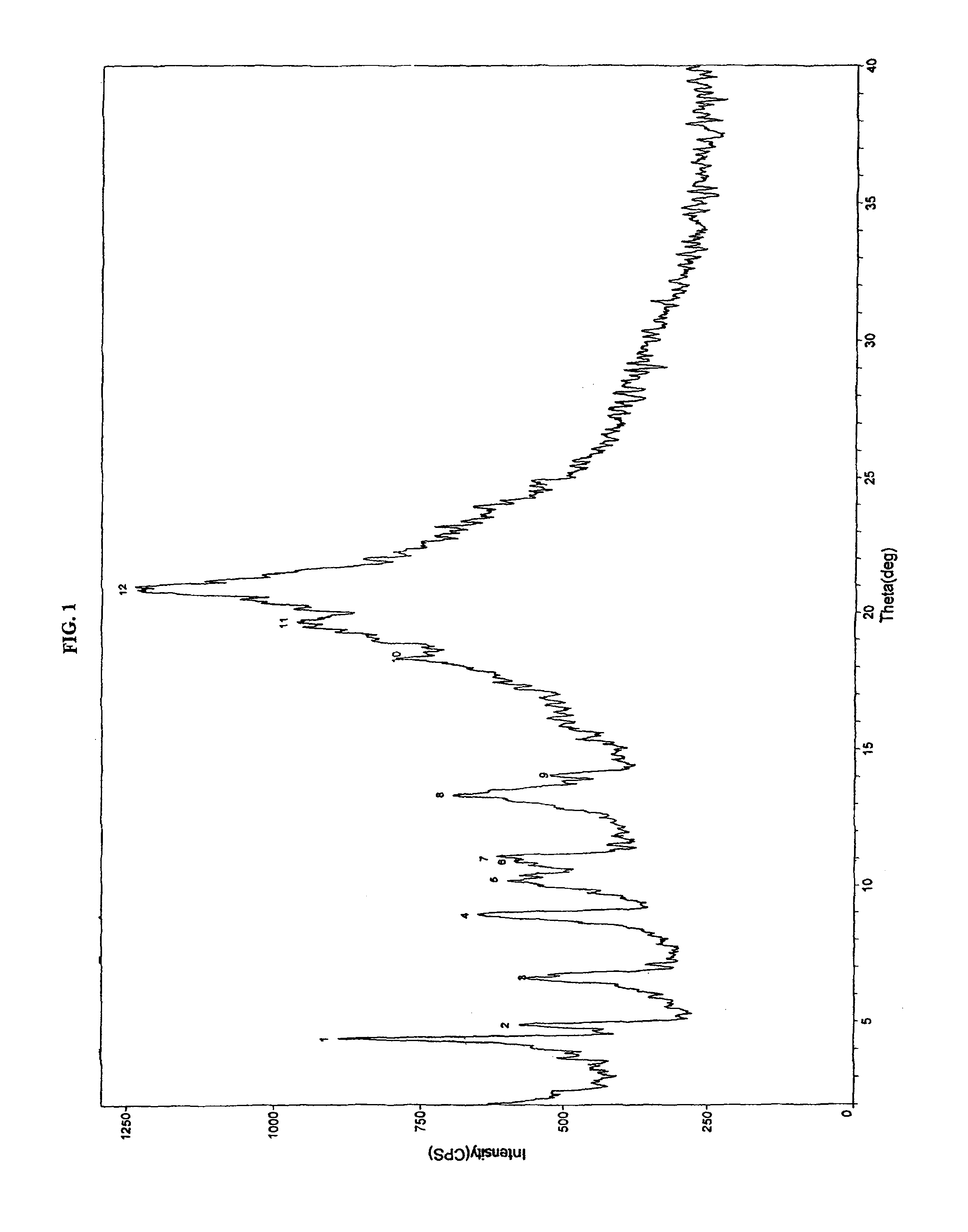
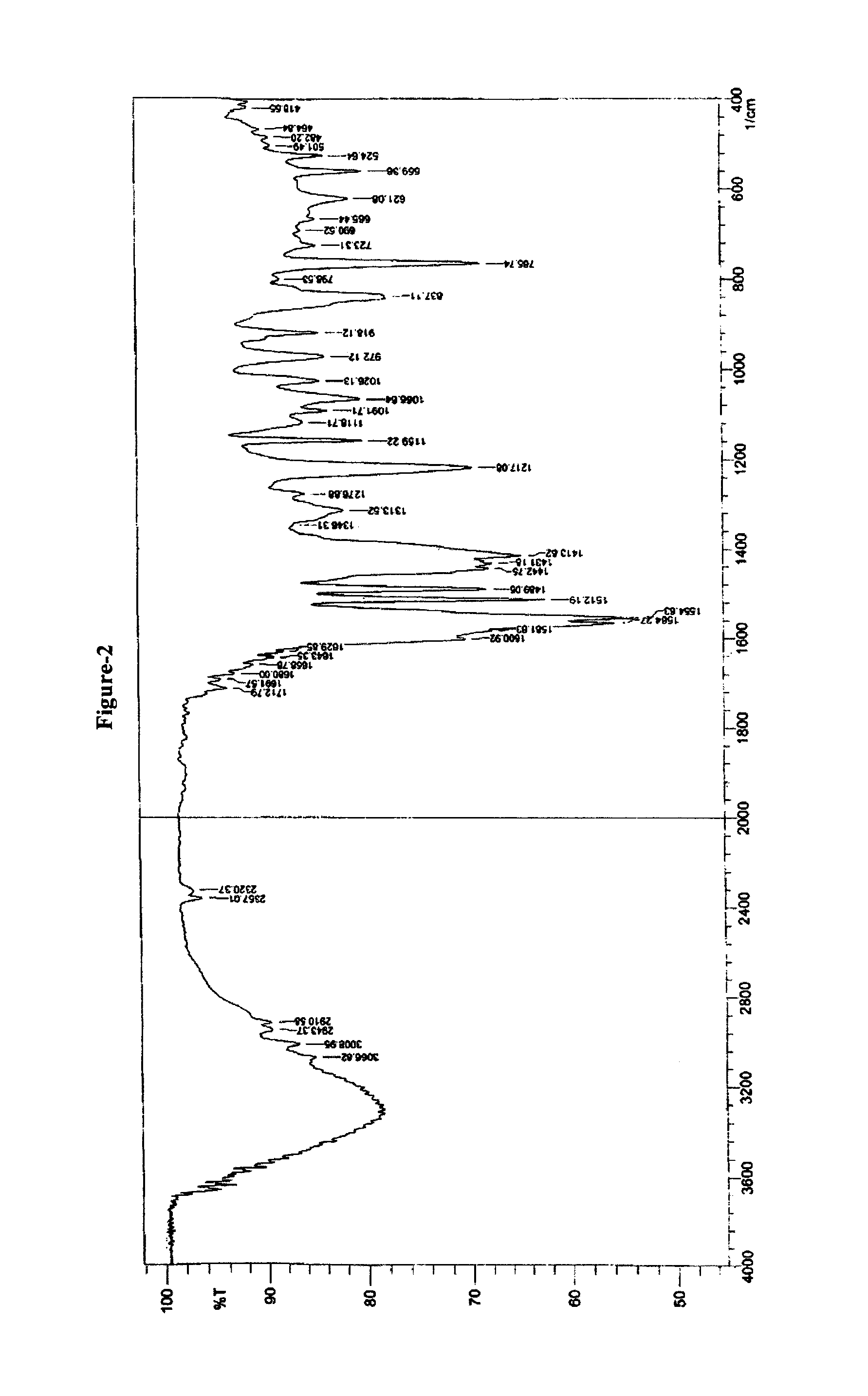
Crystalline forms of pitavastatin calcium
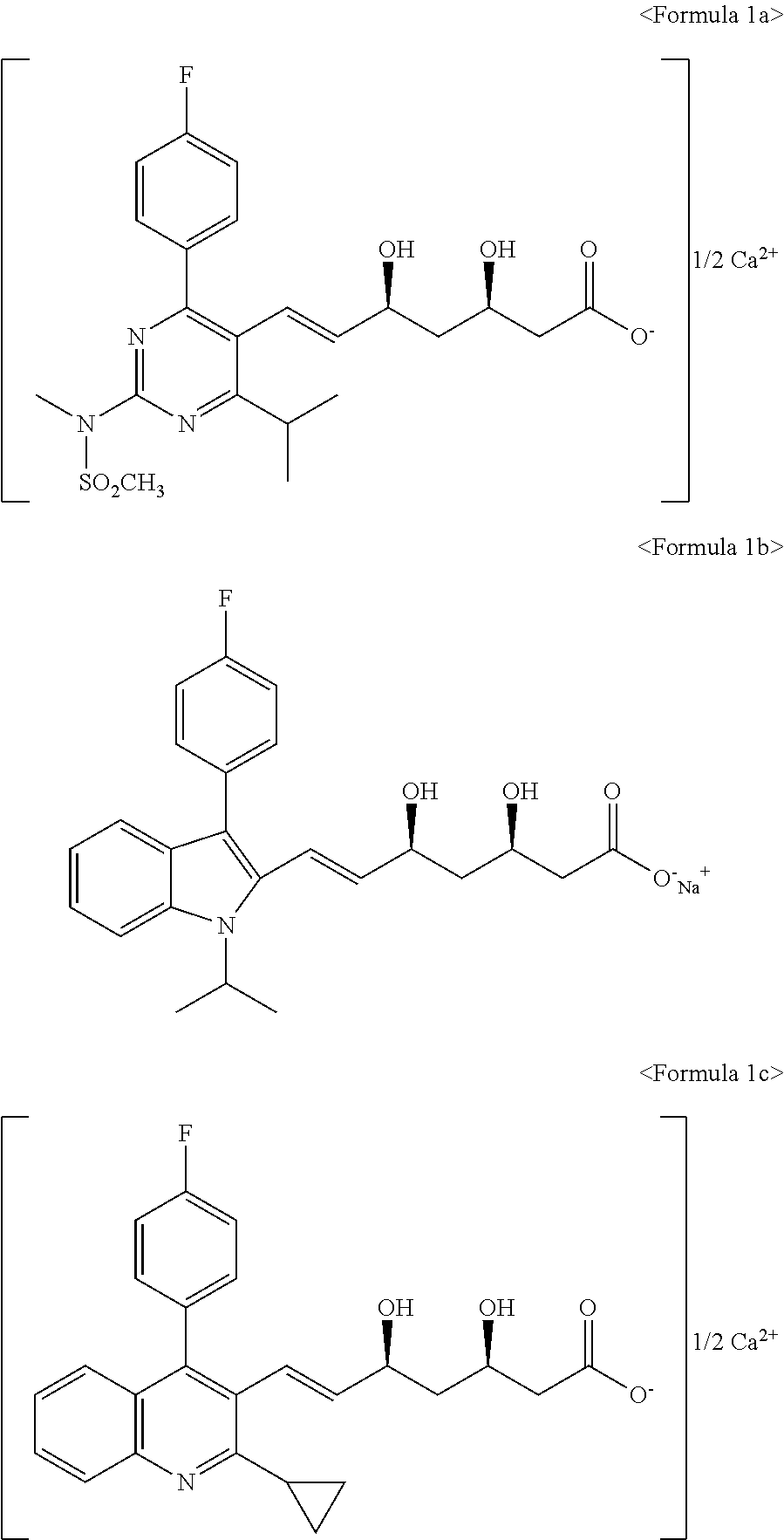
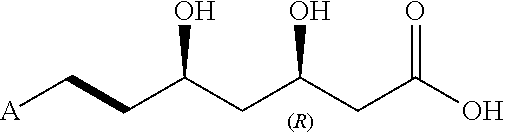
The amorphous form of (3R, 5S)-7-[2-cyclopropyl-4-(4-fluorophenyl) quinolin-3-yl]-3,5-dihydroxy-6 (E)-heptanoic acid hemicalcium salt.
Owner:NISSAN CHEM IND LTD
Pitavastatin calcium intermediate preparation method
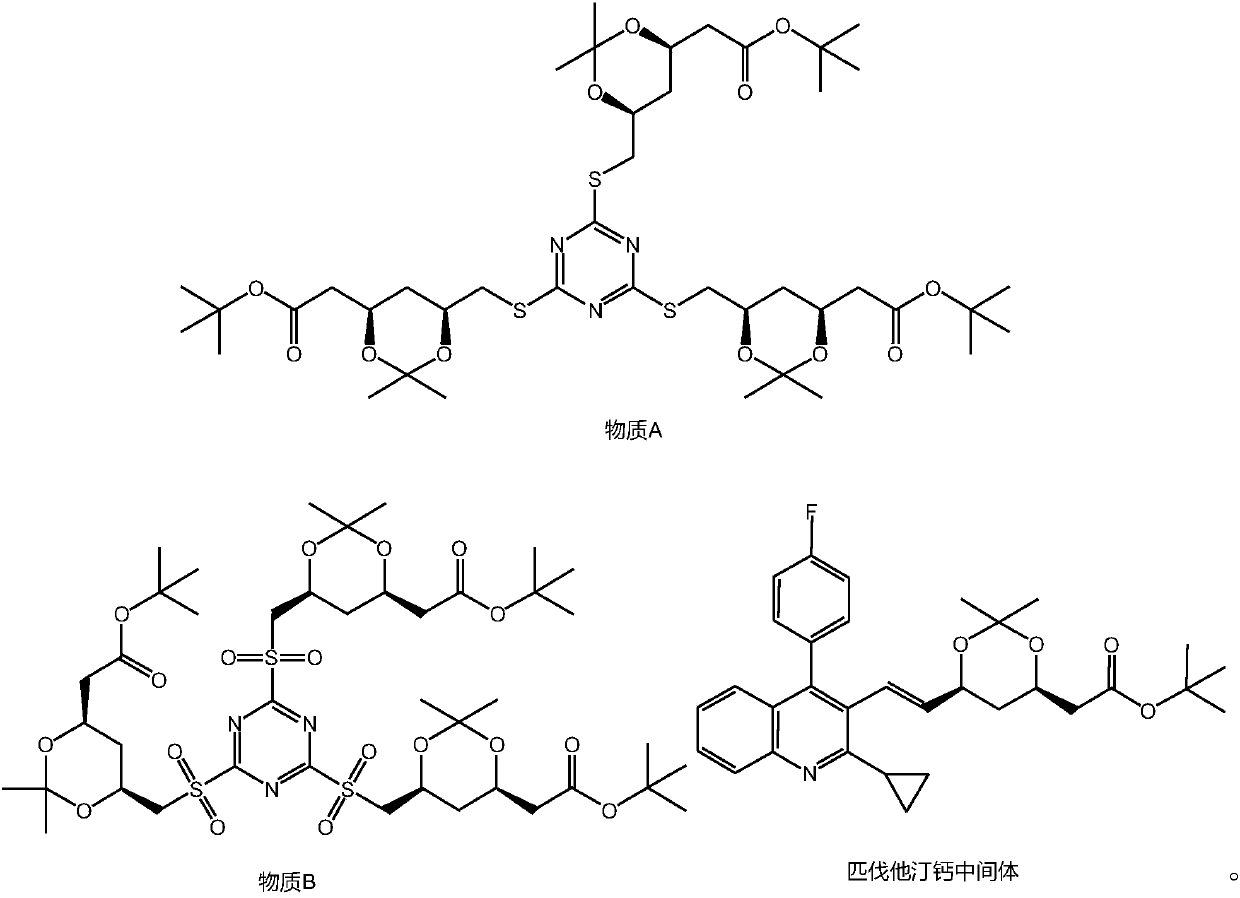
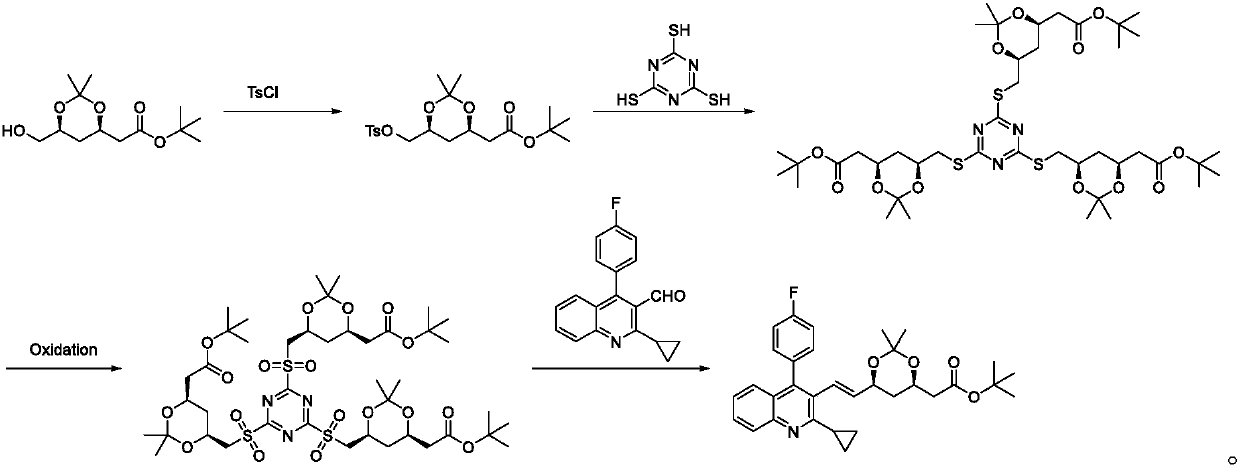
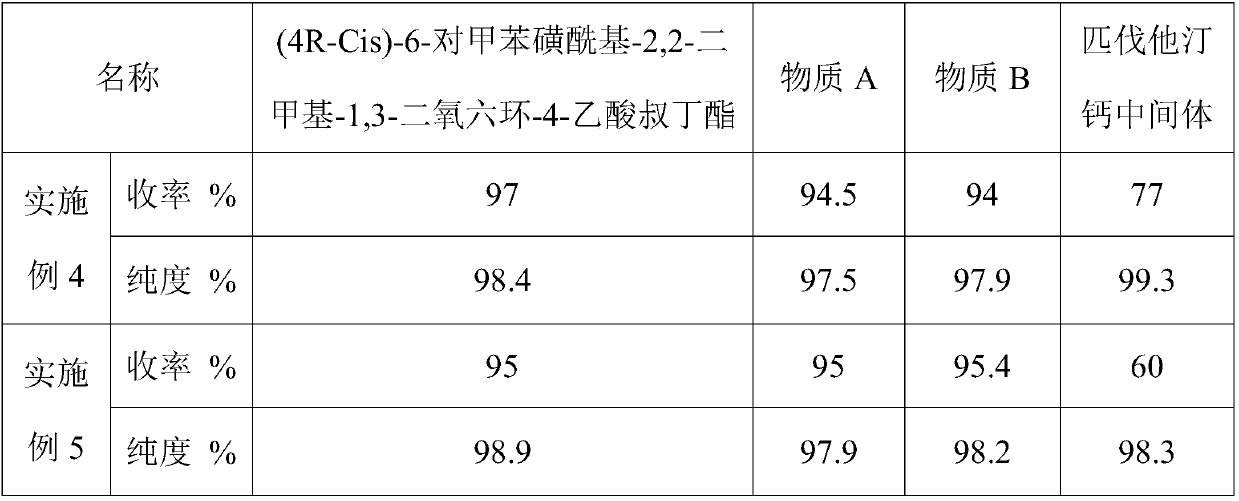
The invention discloses a pitavastatin calcium intermediate preparation method. The method includes steps: subjecting (4R-Cis)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethylester and paratoluensulfonyl chloride to sulfonylation reaction under the catalyst action to obtain (4R-Cis)-6-para-toluenesulfonyl-2,2-dimethyl-1,3-dioxane-4-acetic acid 1,1-dimethylethyl ester; subjecting to reaction with trimercapto-s-triazine under the action of a basic catalyst to obtain a substance A; subjecting to oxidization under the action of an oxidant to obtain a substance B; finally subjecting to reaction with 2-cyclopropyl-4-(4-fluorophenyl)quinoline-3-formaldehyde under the catalytic action of sodium hydride to obtain a pitavastatin calcium intermediate. The pitavastatin calciumintermediate preparation method has advantages of cheapness and easiness in acquisition of raw materials, high atom economy, environmental friendliness, mildness and controllability of reaction conditions, simplicity in operation and purification treatment, suitableness for industrial production, high stereoselectivity and high yield, and the prepared pitavastatin calcium intermediate is high inpurity.
Owner:ANHUI QINGYUN PHARMA & CHEM
Process for preparing statins
Process for the preparation of β-ketoester synthetic intermediates useful in the preparation of statins, in particular Pitavastatin.
Owner:DIPHARMA FRANCIS
Novel boronate esters
InactiveUS20050154213A1Inorganic boron active ingredientsOrganic chemistry methodsPitavastatinFluvastatin
The present invention relates to optically active boronate derivatives which are useful as intermediates for the synthesis of HMG-CoA enzyme inhibitors such as atorvastatin, cerivastatin rosuvastatin, pitavastatin, and fluvastatin.
Owner:BIOCON LTD
Medicine
InactiveUS20150164809A1Good disintegrationMaintain stabilityBiocideMetabolism disorderCrospovidonesPitavastatin
The present invention provides a pharmaceutical product which includes a solid preparation comprising pitavastatin or a salt thereof, in which production of a lactone form thereof is suppressed.The pharmaceutical product is characterized by including a solid preparation comprising the following ingredients (A) and (B): (A) pitavastatin or a salt thereof; and (B) at least one member selected from the group consisting of carmellose and a salt thereof, crospovidone, and microcrystalline cellulose, and the solid preparation having a water content of 2.9 mass % or less, wherein the solid preparation is stored in a tight package.
Owner:KOWA CO LTD
Use of statin and aspirin in preparation of medicine for treating blood high viscosity syndrome
InactiveCN1965843AImprove treatment efficiencyLower specific viscosityOrganic active ingredientsMetabolism disorderAspirinLovastatin
Owner:BEIJING HUAANFO BIOMEDICAL RES CENT
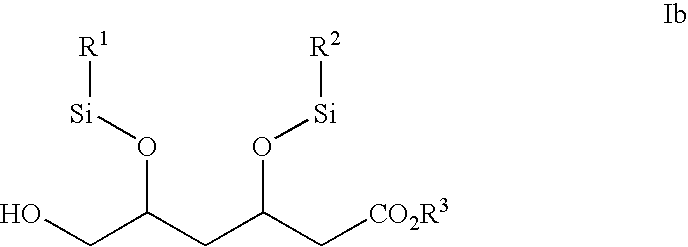
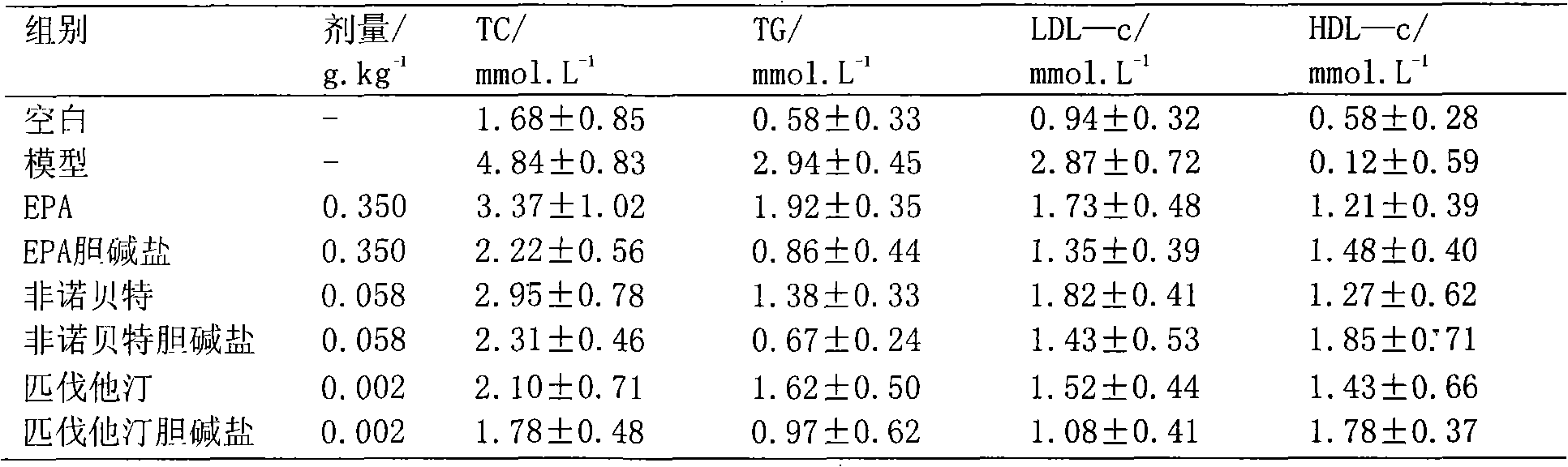
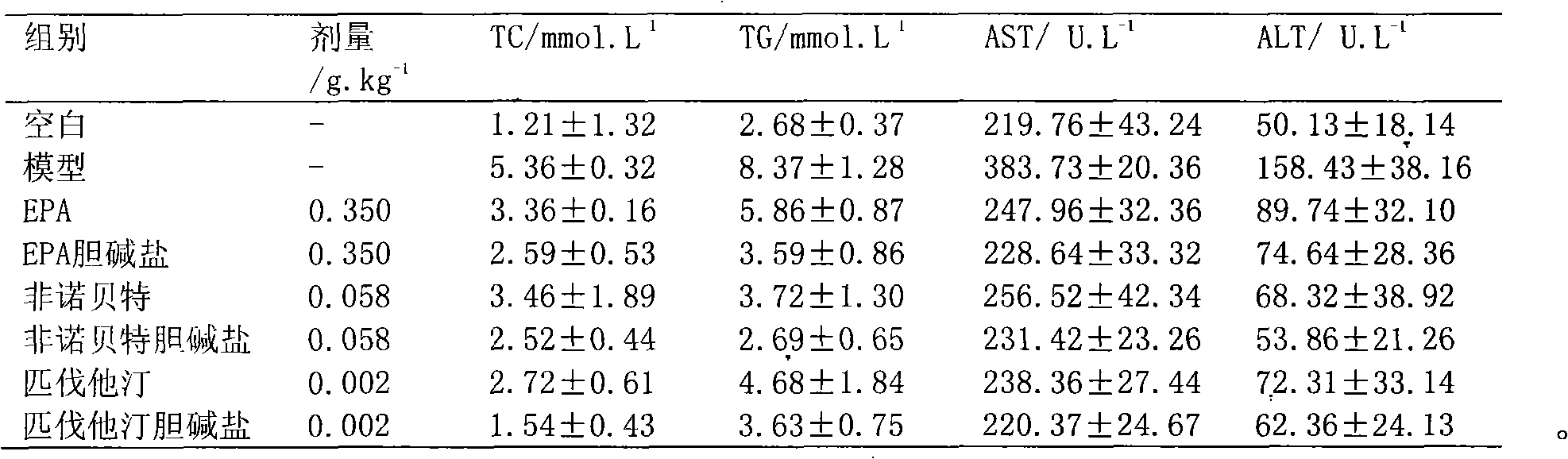
Choline salt of hypolipidemic drug and preparation method and pharmaceutical use thereof
InactiveCN101863780APromote absorptionEfficient use ofOrganic chemistryElcosanoid active ingredientsDocosahexaenoic acidPitavastatin
The invention relates to a choline salt of a hypolipidemic drug and a preparation method and a pharmaceutical use thereof. The invention provides a choline salt of a class of hypolipidemic drugs, and the hypolipidemic drugs include but not limited to clofibrate, libet, fenofibrate, ciprofibrate, gemfibrozil, acipimox, niacin, lovastatin, simvastatin, pravastatin, fluvastatin, atorvastatin, rosuvastatin, pitavastatin, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and other unsaturated fatty acids. The choline salt of the hypolipidemic drug can be used for treating hyperlipidemia and other cardiovascular diseases. The invention also provides a preparation method of the choline salt of the hypolipidemic drug.
Owner:北京利乐生制药科技有限公司
Pitavastatin-containing preparation and method for producing same
ActiveUS20140031390A1Suppresses generation of lactoneNot easy to change colorBiocideMetabolism disorderPitavastatinAqueous dispersion
The present invention provides a pitavastatin-containing preparation containing pitavastatin or a pharmacologically acceptable salt thereof and at least one kind of a basic additive selected from the group consisting of basic magnesium compounds and basic calcium compounds, and an aqueous solution or an aqueous dispersion of the pitavastatin-containing preparation having a pH of more than 8 and 10 or less; and a method for producing a pitavastatin-containing preparation, including blending at least one kind of a basic additive selected from the group consisting of basic magnesium compounds and basic calcium compounds with pitavastatin or a pharmacologically acceptable salt thereof, to make an aqueous solution or an aqueous dispersion of the pitavastatin-containing preparation have a pH of more than 8 and 10 or less.
Owner:SAWAI PHARMA
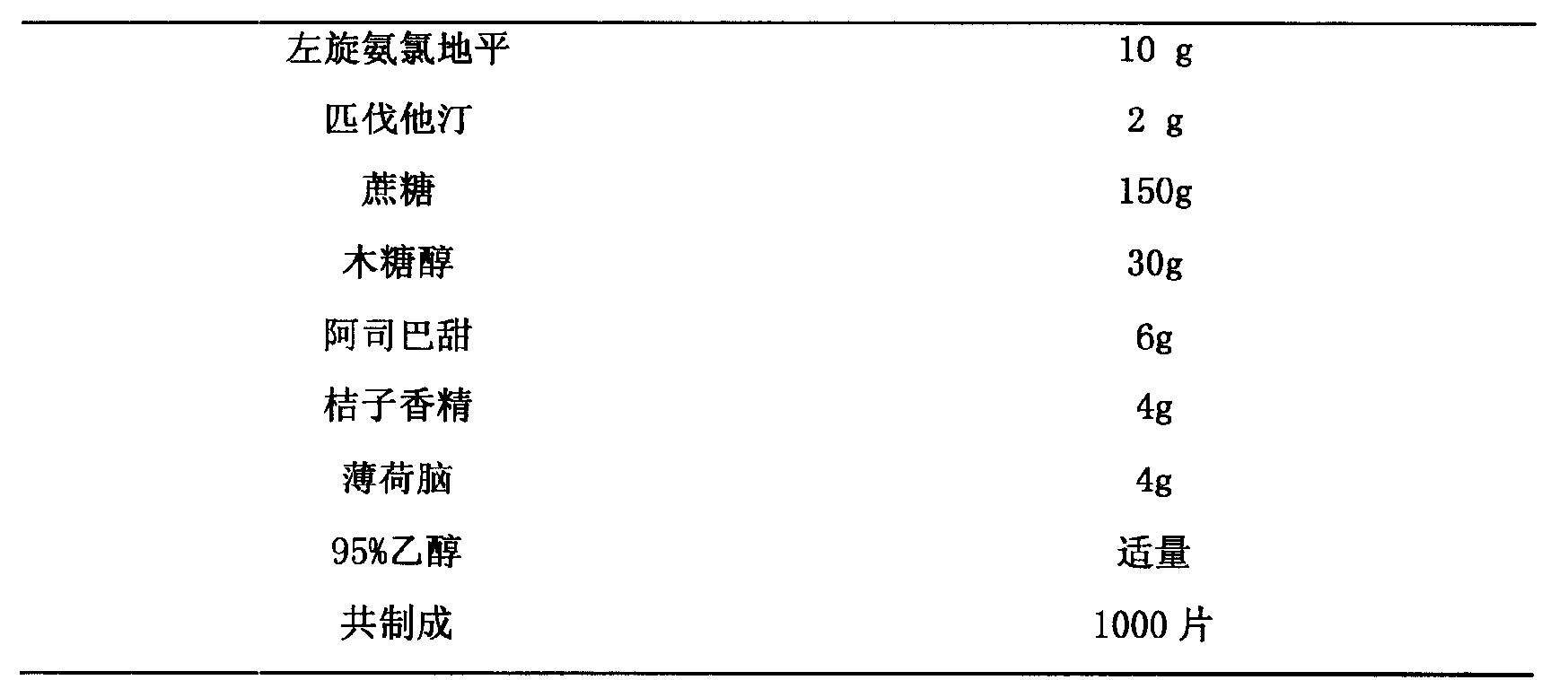
Medicine compounds containing levamlodipine and pitavastatin
The invention relates to a medical composition containing L-amlodipine and Pitavastatin and a preparation method thereof. The L-amlodipine and the Pitavastatin or the pharmaceutically acceptable salt of the two medicines are taken as medical active components and mixed with the pharmaceutically acceptable supplements to form a medical combination. The L-amlodipine and Pitavastatin or the pharmaceutically acceptable salt of the two medicines are taken as raw materials, a plurality of supplements with special type and proportion are added, and the oral preparations such as tablet, capsule, soft capsule, chewable tablet, orally disintegrating tablet, buccal tablet and dropping pill can be prepared and developed according to the technical method described by the invention. The medical composition of the invention can be applied to treat all kind of hypertensions.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Compound immobilized enzyme used for preparing statins and preparation method of compound immobilized enzyme
ActiveCN105925558AImprove utilization efficiencyEasy to separateCarbon-halide lyasesOxidoreductasesHydroxybutyric acidActive enzyme
The invention belongs to the technical field of medical industry, and mainly relates to a compound immobilized enzyme used for preparing statins, as well as a preparation method and applications of the compound immobilized enzyme. The compound immobilized enzyme provided by the invention is prepared through the manner of fixing active enzyme in an immobilized enzyme carrier through the fixing manner of adsorption, covalent binding, embedding, microencapsulation or crosslinking, wherein the active enzyme is composed of ketoreductase and / or halohydrin dehalogenase, and the mass percent of the ketoreductase in the active enzyme is 0%-100%. The immobilized enzyme product is applied to the process for producing the midbody, namely, ethyl(S)-4-chloro-3-hydroxybutanoate and / or ethyl(R)-4-cyano-3-hydroxybutyate of the products including atorvastatin, rosuvastatin, pitavastatin and the like. Compared with the liquid enzyme, the compound immobilized enzyme provided by the invention has the advantages that the production cost is reduced, the labor intensity is alleviated, the utilization efficiency of enzyme is improved, the discharge of waste water, waste gas and industrial residue is reduced, and the environment is protected.
Owner:河北周酶生物科技有限公司
Composition with lipase inhibitor and hydroxymethyl-glutaryl coenzyme A reductase inhibitor
The invention provides a composition with a lipase inhibitor and a hydroxymethyl-glutaryl coenzyme A reductase inhibitor, and a pharmaceutical preparation with the composition. Preferentially, the lipase inhibitor is one of orlistat and cetilistat, and the hydroxymethyl-glutaryl coenzyme A reductase inhibitor is one of atorvastatin, rosuvastatin, simvastatin, fluvastatin, pravastatin, lovastatin and pitavastatin or pharmaceutically-acceptable salt thereof. The composition can have the synergistic antibacterial effect and the synergistic histamine releasing inhibition effect, and therefore thetreatment effect and economic characteristics of bacterial infection-caused allergic disease patients can be improved.
Owner:黄泳华
Preparation method of pitavastatin calcium
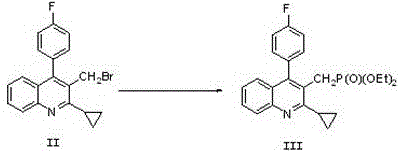
The invention relates to a preparation method of a cholesterol reduction drug, particularly relates to a preparation method of pitavastatin calcium as a crude drug of the cholesterol reduction drug, and aims at the problems that the pitavastatin calcium synthetic technology in the prior art is long in steps and complicated in operation, and uses strongly corrosive reagents which is environmentally unfriendly, causes serious corrosion to equipment, and may not facilitate industrial production. The invention provides the new preparation method of the pitavastatin calcium, the new preparation method is as follows: 3-bromomethyl-2-cyclopropyl-4-(4-fluorophenyl)quinoline is prepared from 2-cyclopropyl-4-(4-fluorophenyl)-3-quinolinecarboxaldehyde by a one step reaction, and then reacts with an organophosphorus reagent to obtain pitavastatin calcium intermediate phosphorus ylide, on the basis of improving of the yield to 80%, the reaction steps are reduced, and the reaction difficulty is reduced, and hydroxylamine hydrochloride is selected as a deprotection reagent, so that the new preparation method is mild in reaction conditions, environmentally friendly, high in yield, and beneficial to industrial production.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Therapeutic agent for thrombosis
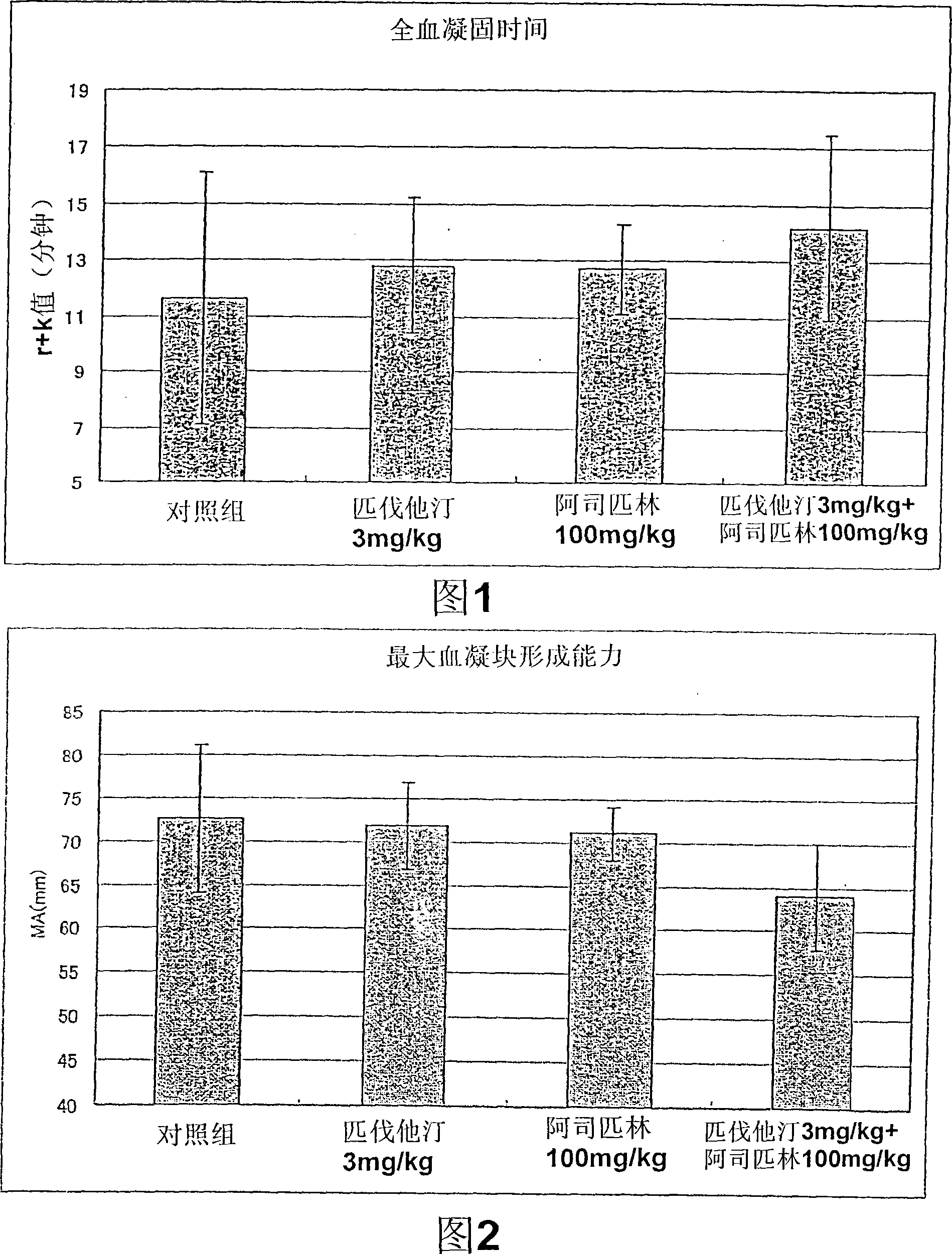
InactiveCN101146537AEffective treatmentAvoid side effectsBlood disorderHeterocyclic compound active ingredientsAspirinPitavastatin
The present invention relates to a therapeutic agent for thrombosis, in particular to a therapeutic agent for thrombosis effective both in inhibiting blood coagulation and in dissolving thrombus. The therapeutic agent for thrombosis is characterized by containing pitavastatins and aspirin.
Owner:KOWA CO LTD +1
Statin bioavailability enhancement delivery composition
Owner:PRUGEN IP HLDG
Local cochlear application of statins for stimulating neurite regrowth in the cochlea
ActiveUS20160022663A1Preventing and treating hearing lossBiocideOrganic chemistryPitavastatinStatine
Statin compositions are disclosed for stimulating neurite growth from spiral ganglion neurons in the inner ear, as well as methods and kits for preventing damage to or treating damage of auditory neurons and / or hair cells of the cochlea following acoustic or toxic insult. An exemplary statin for these methods and kits includes Pitavastatin having the compound formula (VIII):
Owner:NORTHWESTERN UNIV
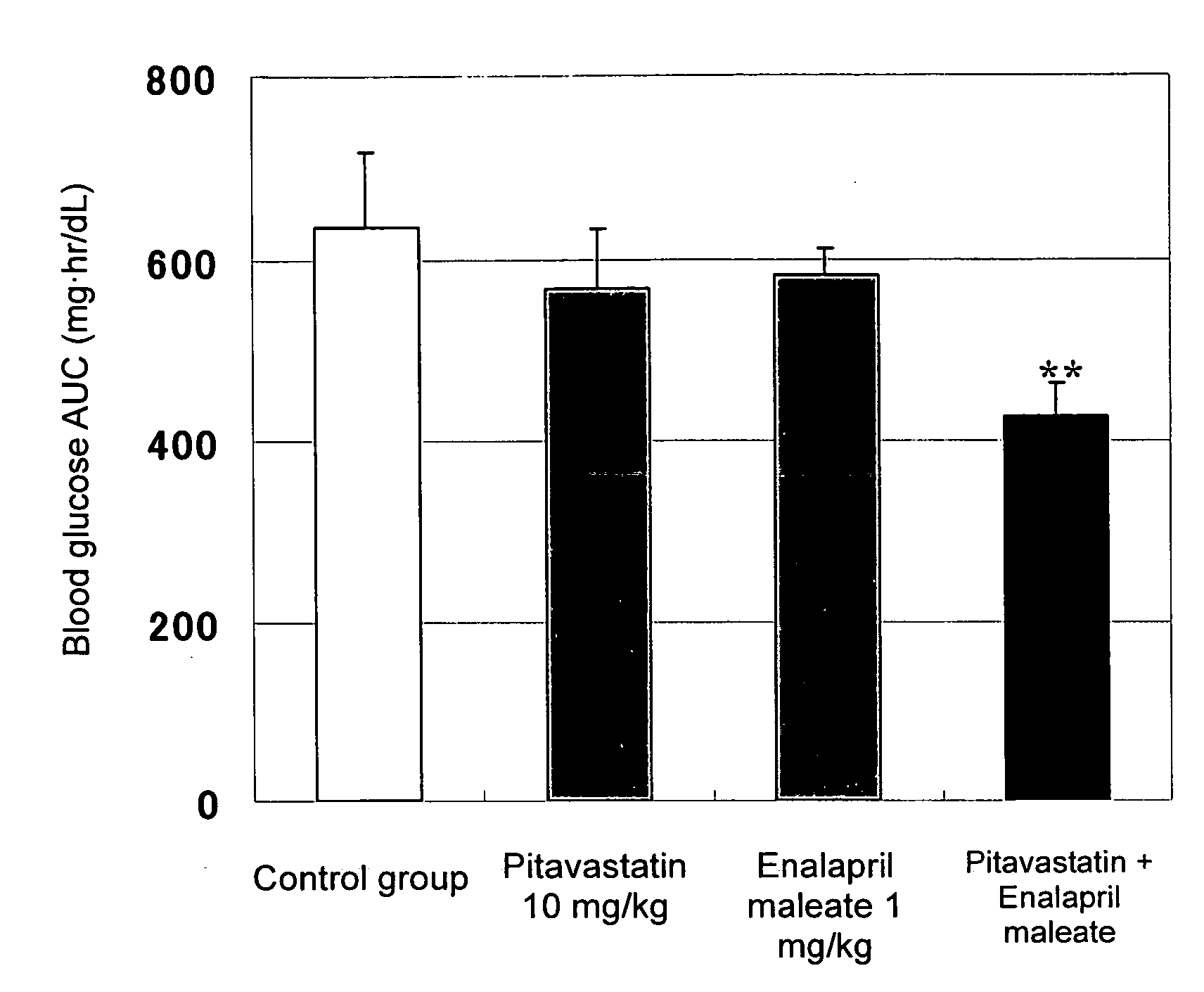
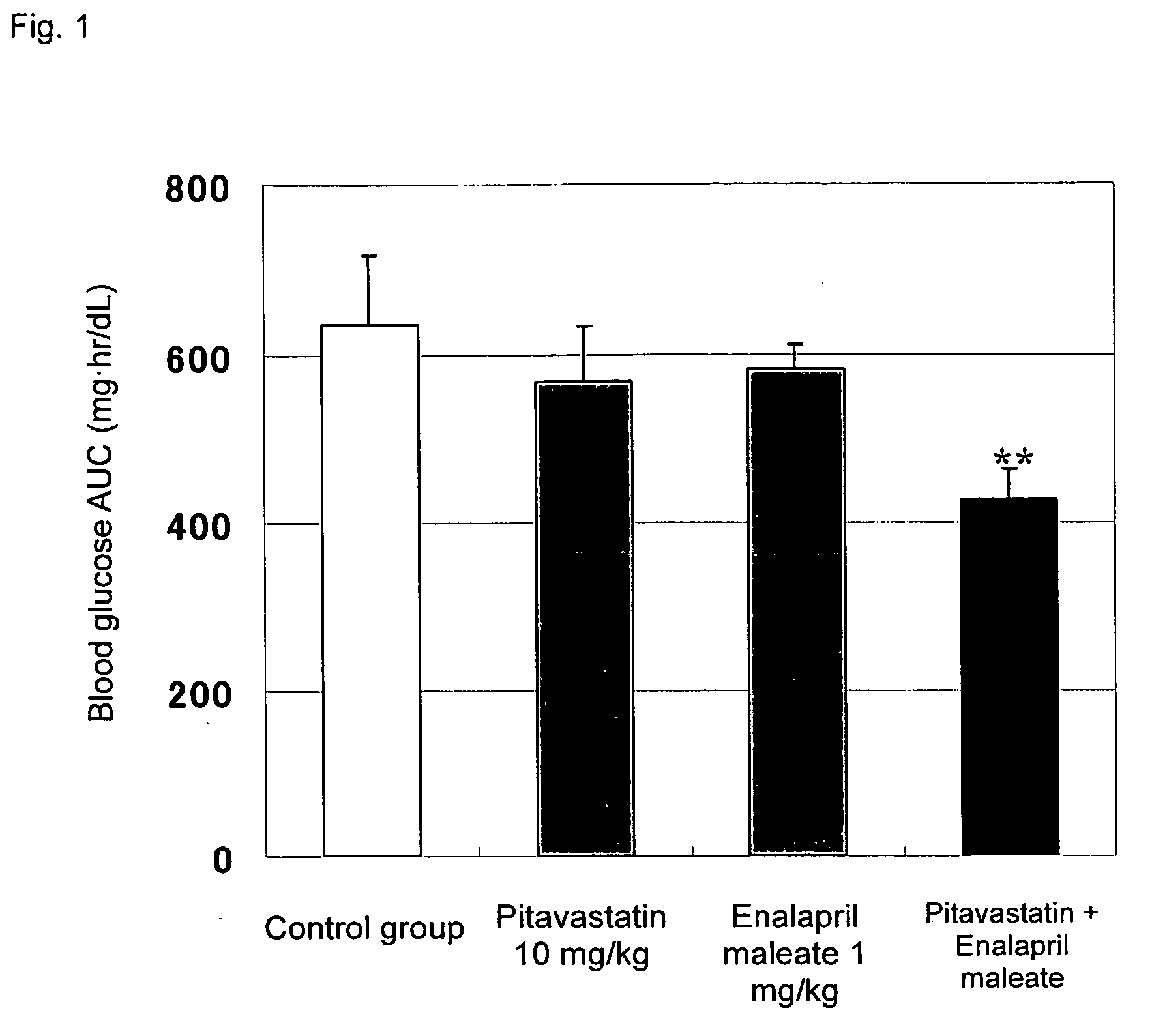
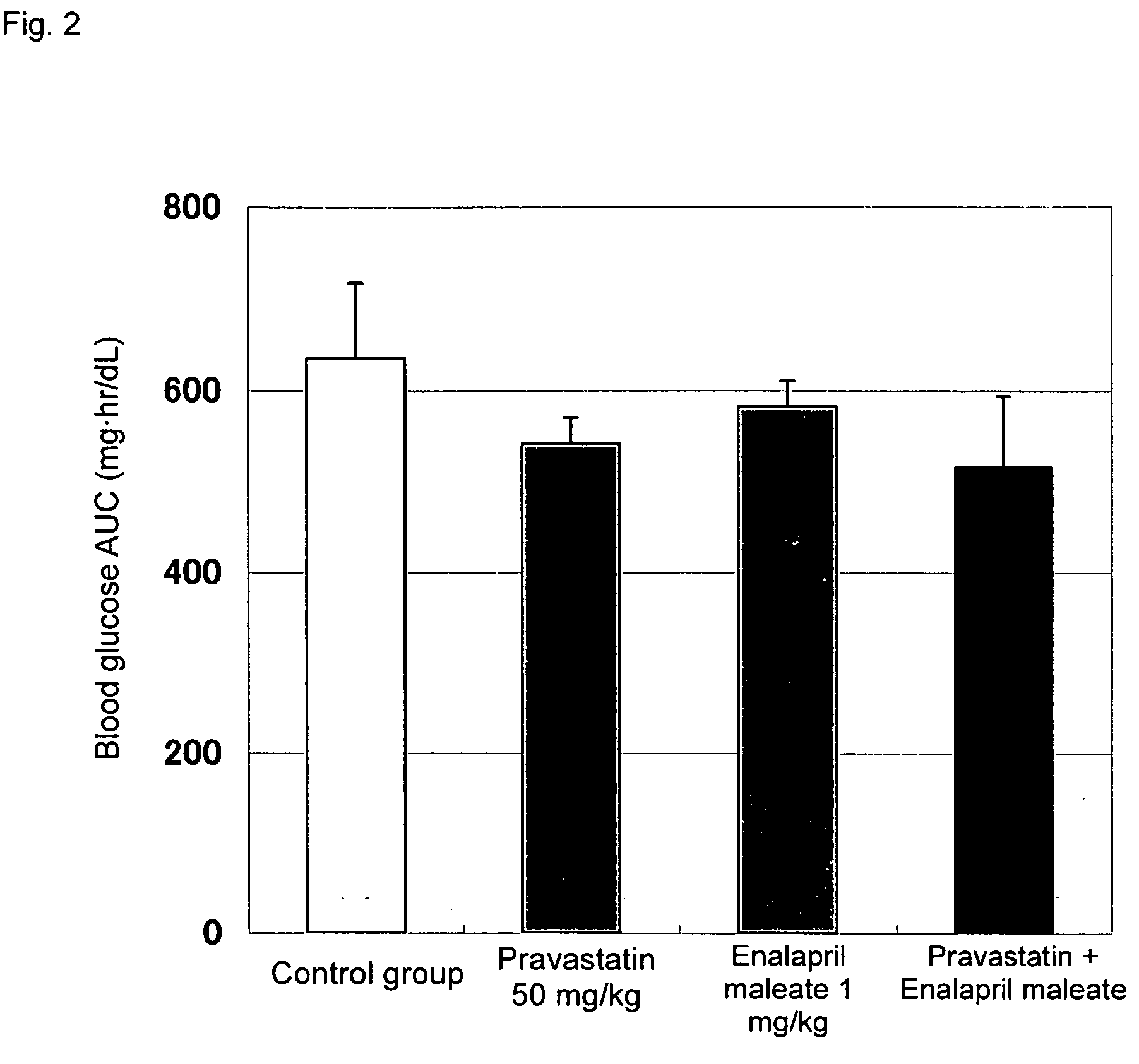
Method for the treatment of diabetes
InactiveUS20070179194A1Eliminate side effectsGood effectBiocideMetabolism disorderDiabetes mellitusPitavastatin
The present invention provides a method for treatment of diabetes, comprising administering a pitavastatin, and in combination therewith, enalapril or a salt thereof.
Owner:KOWA CO LTD +1
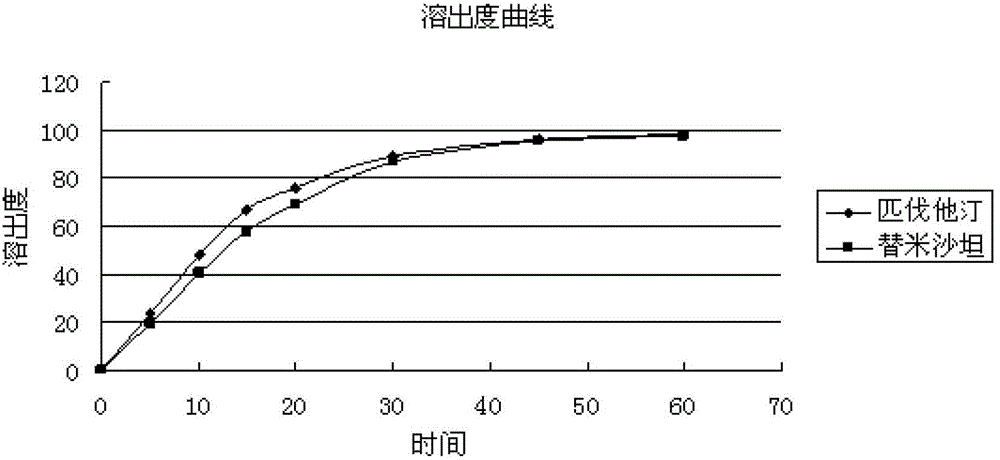
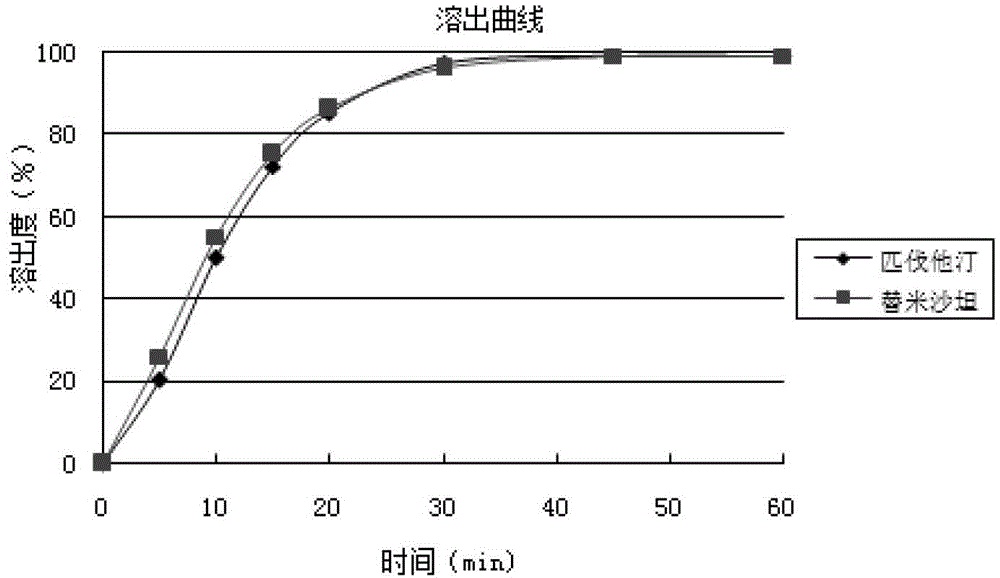
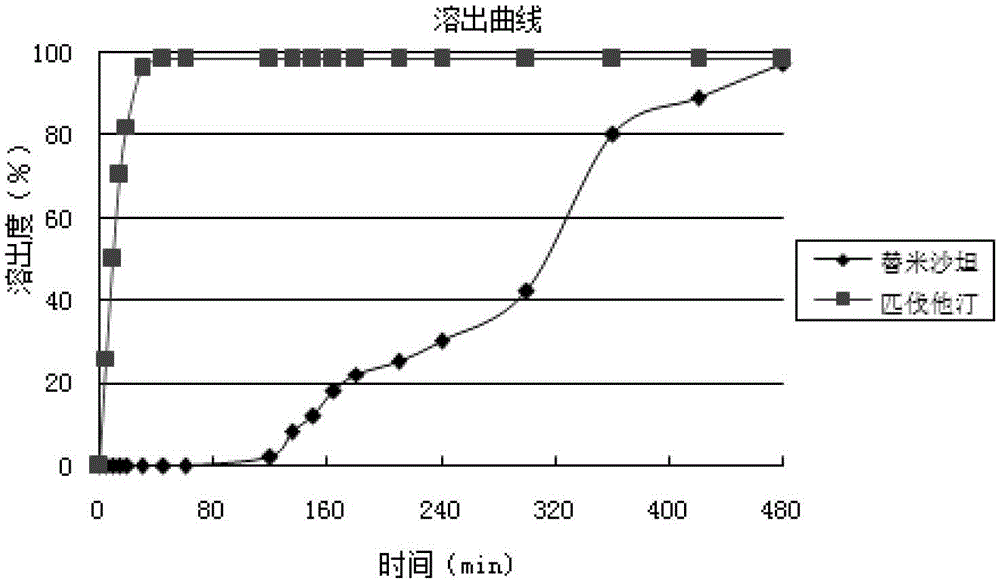
Medicinal composition containing telmisartan and pitavastatin
ActiveCN103142596ALow interaction potentialReduce interactionOrganic active ingredientsMetabolism disorderMedicineSecondary hyperlipidemia
The invention relates to a medicinal composition containing pitavastatin and telmisartan. The medicinal composition contains pitavastatin or a salt thereof, and telmisartan or a salt thereof. The medicinal composition has a synergistic effect in the field of the treatment of hyperlipidemia and hypertension, and is better than medicines in the prior art.
Owner:SICHUAN UNIV
Pitavastatin calcium and process for its preparation
The invention provides the process for the preparation of pitavastatin and its pharmaceutically acceptable salts thereof. In particular, the invention provides a process for the preparation of stable pitavastatin calcium in crystalline form having water content less than 5% wt / wt. The present invention also provides stable crystalline form of pitavastatin calcium substantially free from crystal Form-A and use thereof for pharmaceutical compositions.
Owner:CADILA HEALTHCARE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com