Novel pitavastatin nitryl ester derivant
A technology of pitavastatin and derivatives, which is applied in the field of medicine and achieves the effects of being suitable for long-term application, reducing blood lipids and having a small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
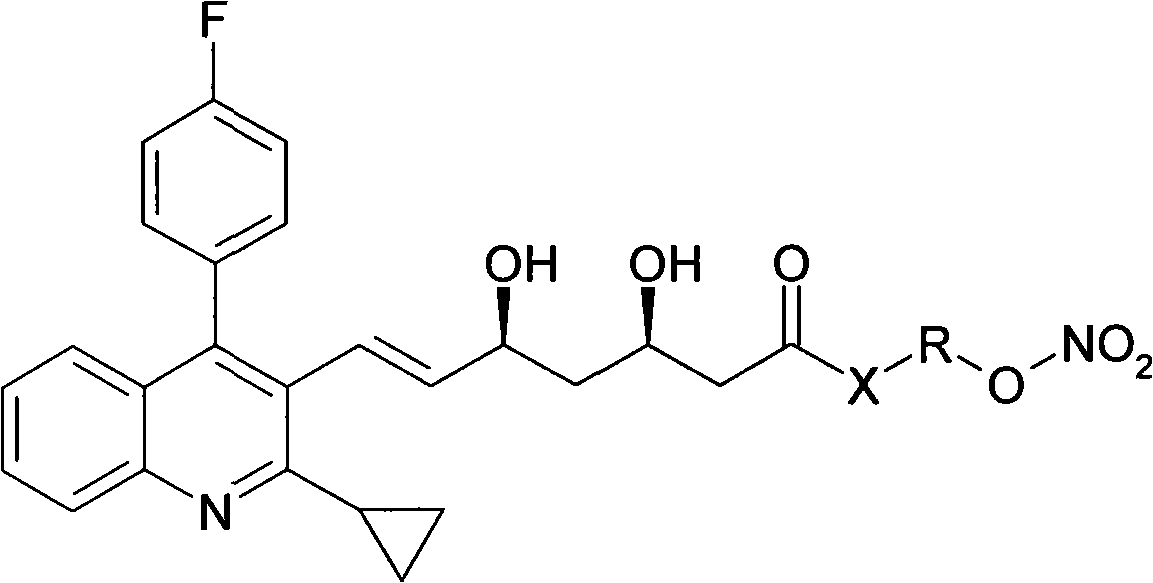
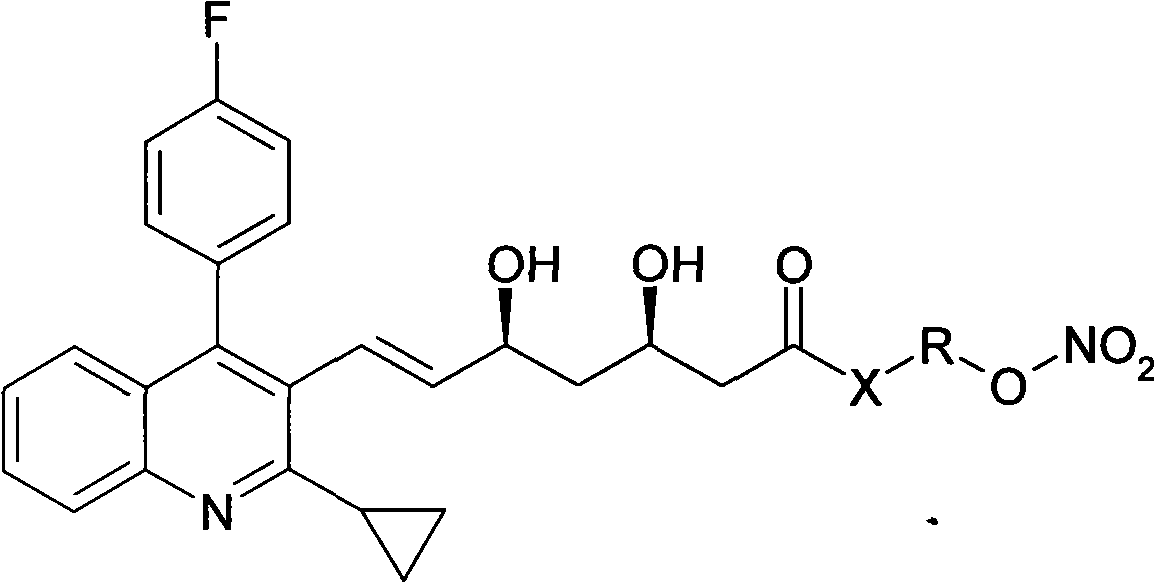
[0017] Example 1 Preparation of pitavastatin 4-(nitrooxy)butyl ester (I1)
[0018] Dissolve 8.9g of pitavastatin sodium in 60ml of DMF, cool in an ice-water bath, and add dropwise 6.5g-30ml of 1,4-dibromobutane in DMF. After dropping, the temperature was raised to room temperature for 22 hours. Concentrate to dryness under reduced pressure, add water and ether, dry the organic phase over anhydrous sodium sulfate and concentrate to dryness. After passing through the column, eluting with petroleum ether: ethyl acetate = 3:1, the desired components were collected and evaporated to dryness under reduced pressure to obtain pitavastatin 4-bromobutyl ester.
[0019] The pitavastatin 4-bromobutyl ester obtained above was dissolved in 100 ml of acetonitrile, 3.4 g of silver nitrate was added, and the reaction was stirred at room temperature in the dark for 48 h. Filtrate, concentrate the filtrate to dryness under reduced pressure, pass through the column, elute with petroleum ether: ...
Embodiment 2
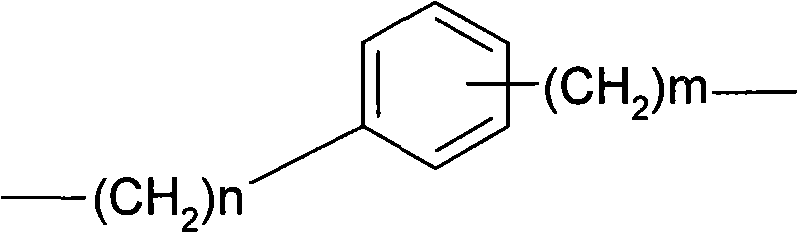
[0020] Example 2 Preparation of pitavastatin 4-(nitrooxymethyl)benzyl ester (I2)
[0021] Dissolve 8.9 g of pitavastatin sodium in 60 ml of DMF, cool in an ice-water bath, and add 7.0 g to 30 ml of p-dichloromethylbenzene in DMF solution dropwise. After dropping, the temperature was raised to room temperature for 24 hours. Concentrate to dryness under reduced pressure, add water and ethyl acetate, dry the organic phase over anhydrous sodium sulfate and concentrate to dryness. After passing through the column and eluting with petroleum ether: ethyl acetate = 1:1, the desired components were collected and evaporated to dryness under reduced pressure to obtain pitavastatin 4-chloromethylbenzyl ester.
[0022] The pitavastatin 4-chloromethylbenzyl ester obtained above was dissolved in 80 ml of acetonitrile, 4.0 g of silver nitrate was added, and the reaction was stirred at room temperature in the dark for 48 h. Filtrate, concentrate the filtrate to dryness under reduced pressure...
Embodiment 3
[0023] Example 3 Preparation of pitavastatin 2-(nitrooxymethyl)benzyl ester (I3)
[0024] Dissolve 8.9 g of pitavastatin sodium in 60 ml of DMF, cool in an ice-water bath, and add 7.0 g to 30 ml of o-dichloromethylbenzene in DMF solution dropwise. After dropping, the temperature was raised to room temperature for 24 hours. Concentrate to dryness under reduced pressure, add water and ethyl acetate, dry the organic phase over anhydrous sodium sulfate and concentrate to dryness. After passing through the column, eluting with petroleum ether: ethyl acetate = 1:1, the desired components were collected and evaporated to dryness under reduced pressure to obtain pitavastatin 2-chloromethylbenzyl ester.
[0025] The pitavastatin 2-chloromethylbenzyl ester obtained above was dissolved in 80 ml of acetonitrile, 3.6 g of silver nitrate was added, and the reaction was stirred at room temperature in the dark for 48 h. Filtrate, concentrate the filtrate to dryness under reduced pressure, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com