Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
573 results about "Chiral resolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chiral resolution in stereochemistry is a process for the separation of racemic compounds into their enantiomers. It is an important tool in the production of optically active drugs. Other terms with the same meaning are optical resolution and mechanical resolution.
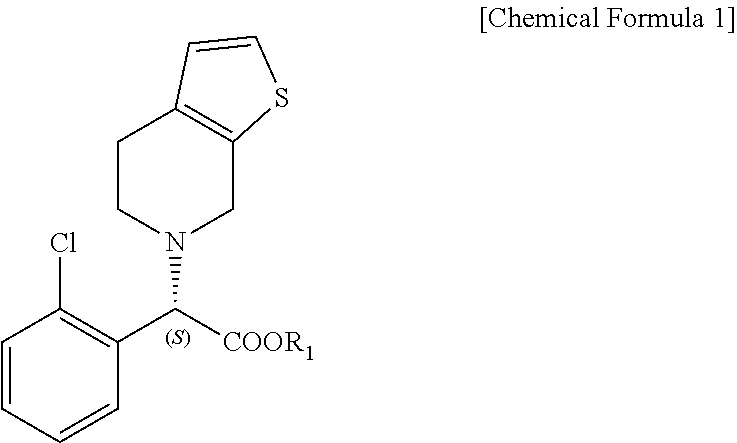
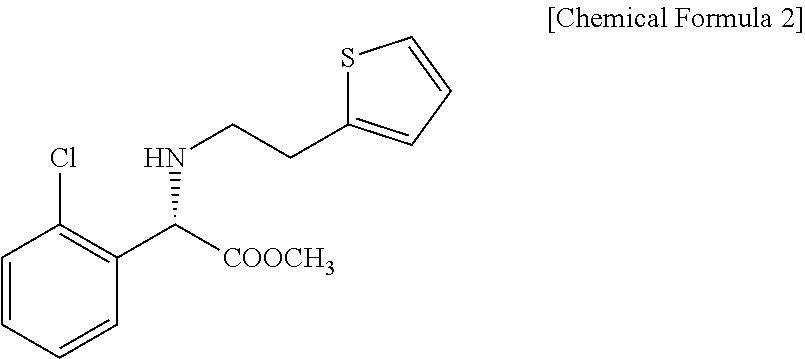
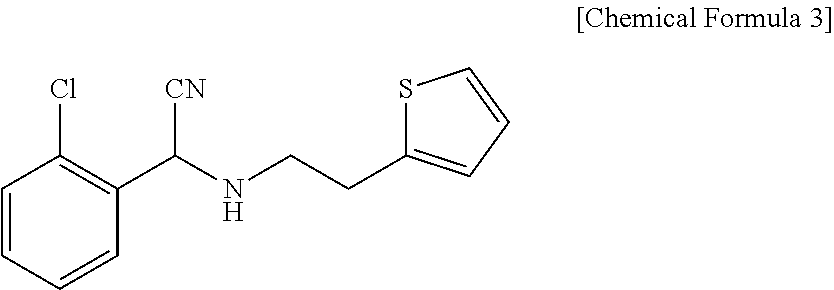
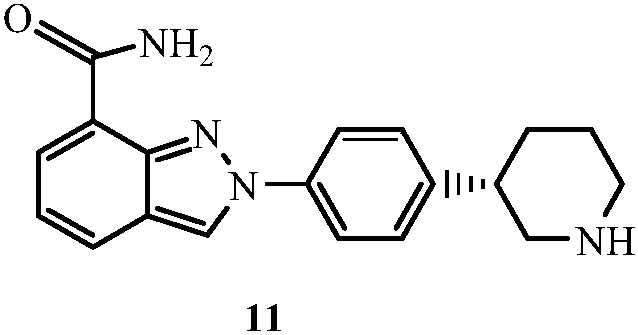
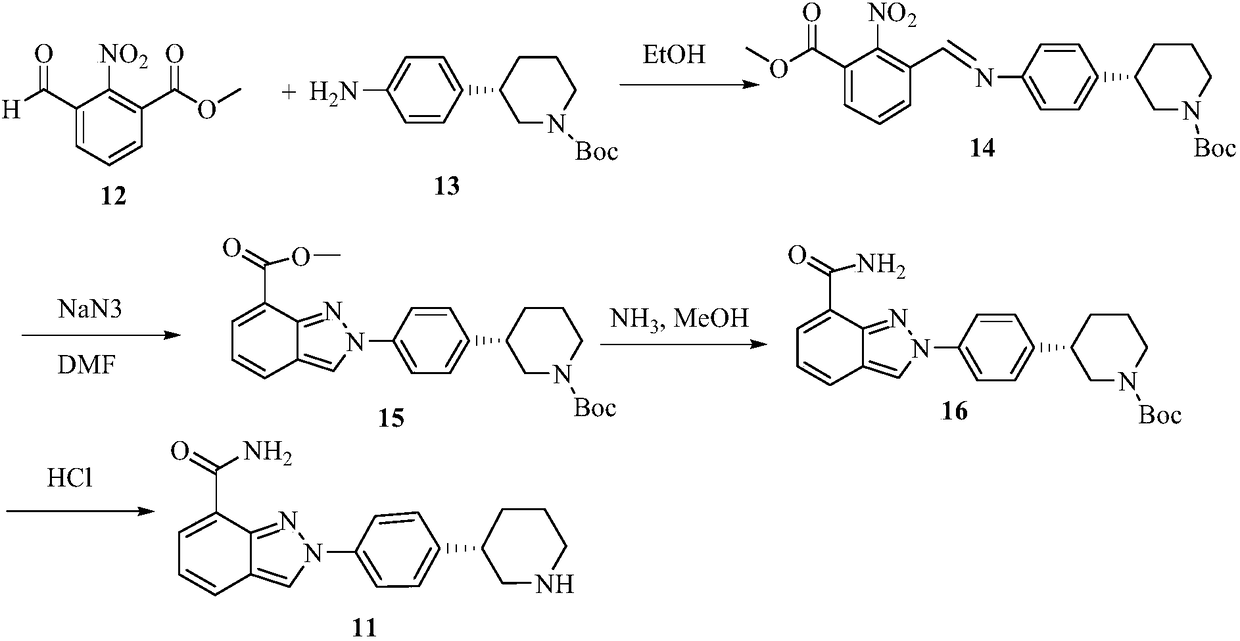
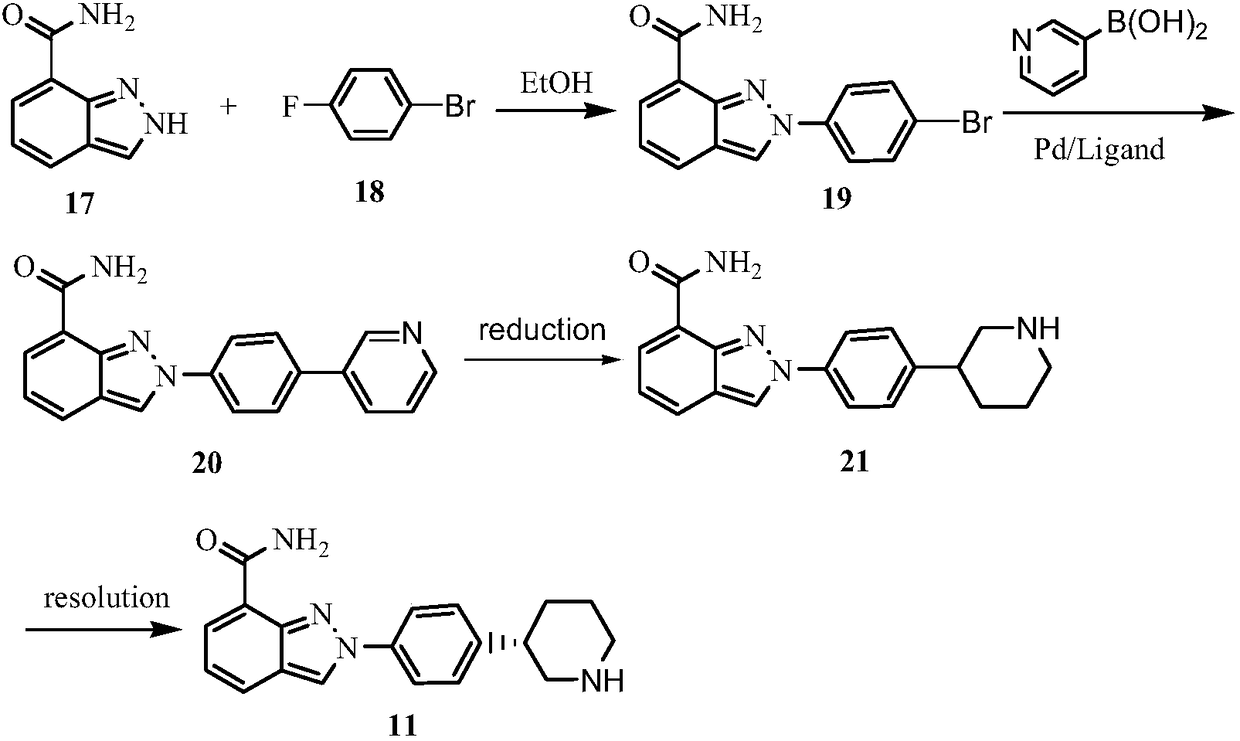
Method for preparing clopidogrel and its derivatives
The present invention relates to a method for preparing Clopidogrel and its derivatives. More particularly, the present invention is a method for preparation of (S)-2-Clopidogrel and its derivatives, which are active inhibitors of platelet aggregation, from an optically active (S)-2-chlorophenyl glycine alkyl ester through hydrolysis of racemic 2-chlorophenylglycine alkyl esters using an enzyme. The present invention employs a simple procedure to prepare Clopidogrel and its derivatives. Because no chiral resolving agents are used except for a small amount of enzyme, the cost of preparation can be reduced. In addition, the present invention is suitable for synthesizing highly optical-active Clopidogrel and its derivatives on a large scale by using optically active (S)-2-chlorophenylglycine alkyl ester obtained in high yield as an intermediate, and is also environmentally friendly since no highly toxic reagents are employed.
Owner:ENZYTECH LTD
Preparation method and application of nano material monolithic column immobilized enzyme biological micro-reactor
InactiveCN104195042AFast catalytic efficiencyHigh catalytic efficiencyEnzyme production/based bioreactorsMicroorganism fixing/supporting apparatusMicroreactorFunctional monomer
The invention discloses a preparation method and an application of a nano material monolithic column immobilized enzyme biological micro-reactor. The preparation method comprises the following steps of firstly, preparing a porous organic polymer monolithic column by using a mixed solution of a functional monomer, a crosslinking agent, a pore forming agent and an initiating agent through in-situ thermal-initiated or light-initiated polymerization in the column, and then bonding a nano material after functional modification to obtain a nano material monolithic column; and secondly, realizing immobilization of the enzyme on the monolithic column by using the nano material as an intermediate ligand to obtain the nano material monolithic column immobilized enzyme biological micro-reactor. The biological micro-reactor is successfully applied to proteomic analysis, medicament chiral resolution and catalyzed ester exchange reactions. The biological micro-reactor disclosed by the invention has the following advantages that the preparation method is simple, the immobilization amount of the enzyme is large, the catalytic activity is high, the enzymolysis speed is high, the efficiency is high, the service life is long, and the biological micro-reactor can be reused.
Owner:BEIJING UNIV OF CHEM TECH
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
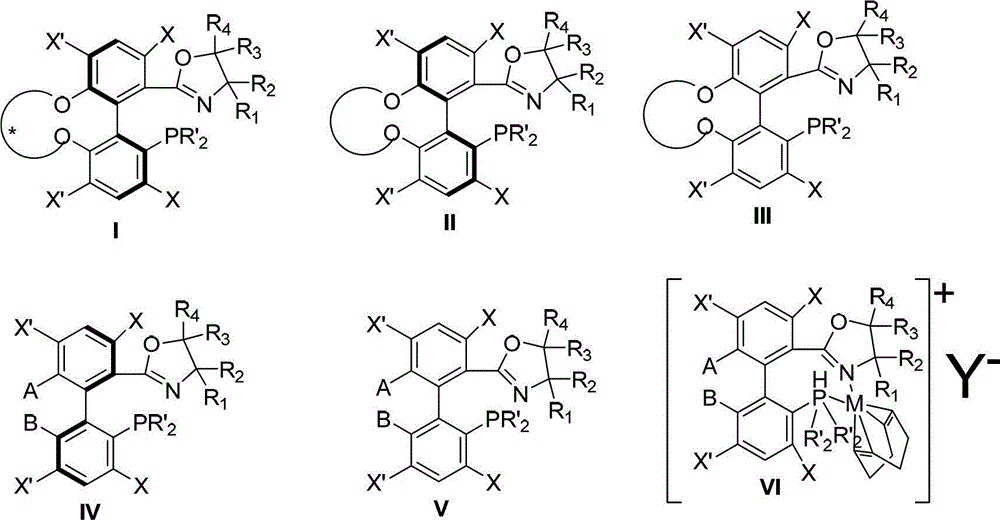
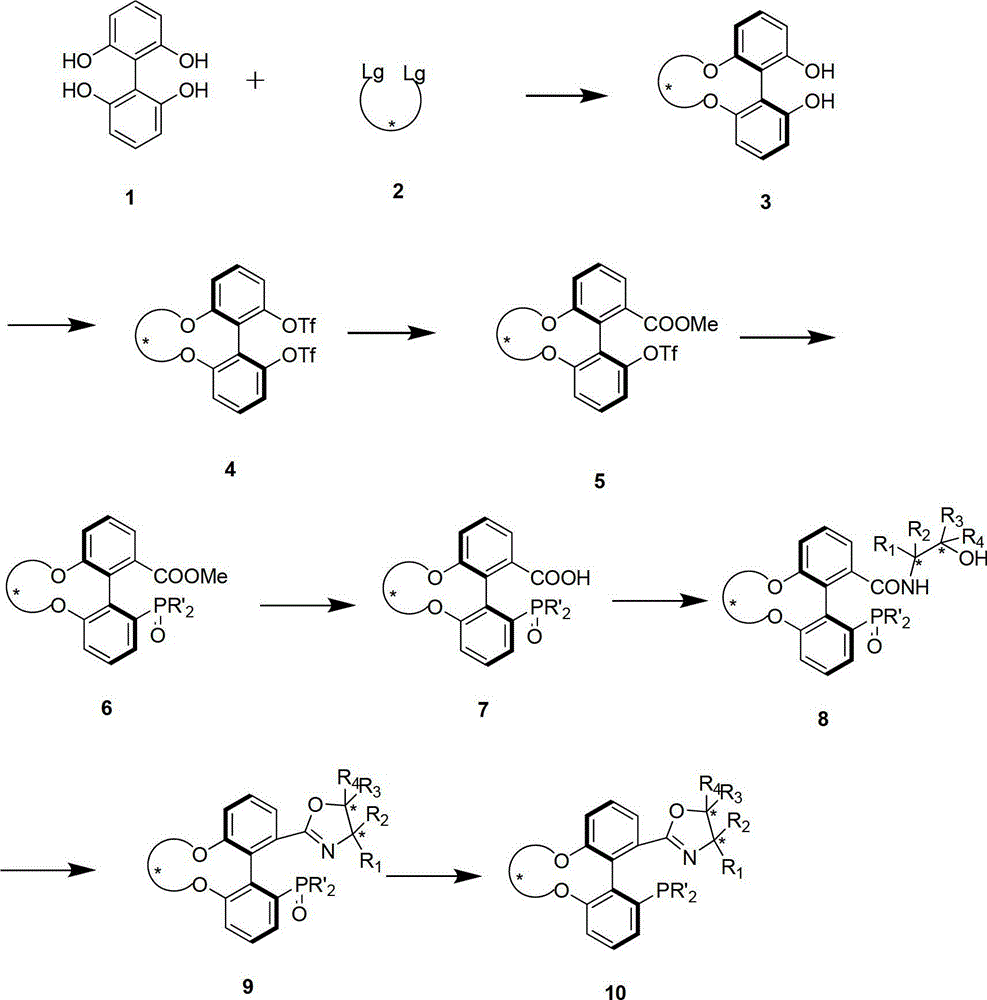
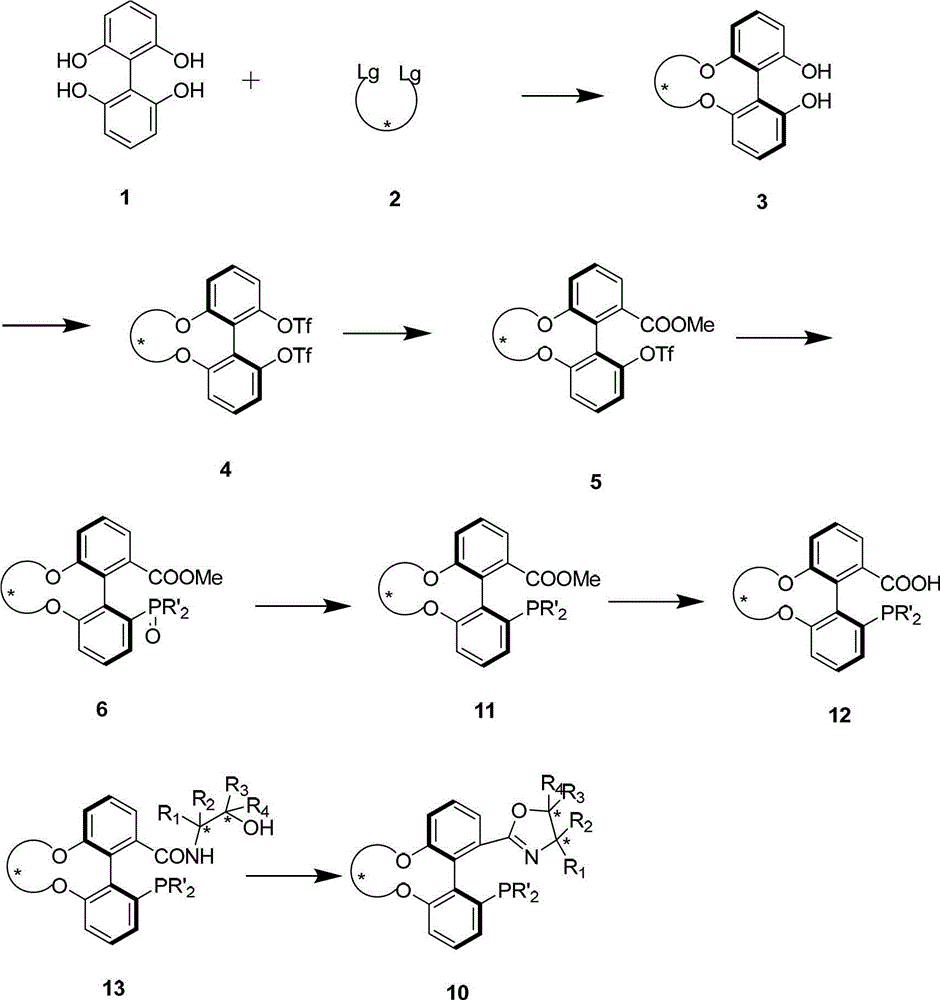
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
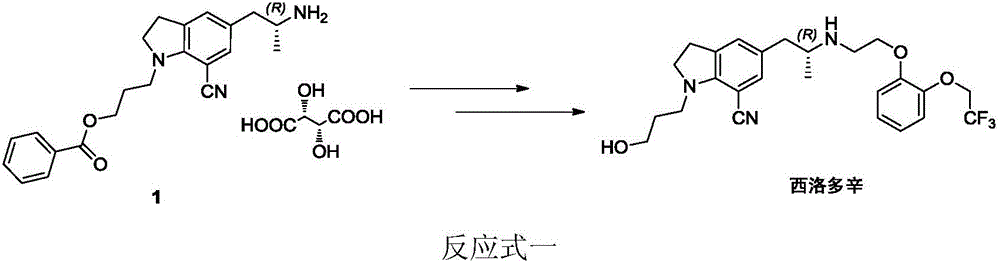
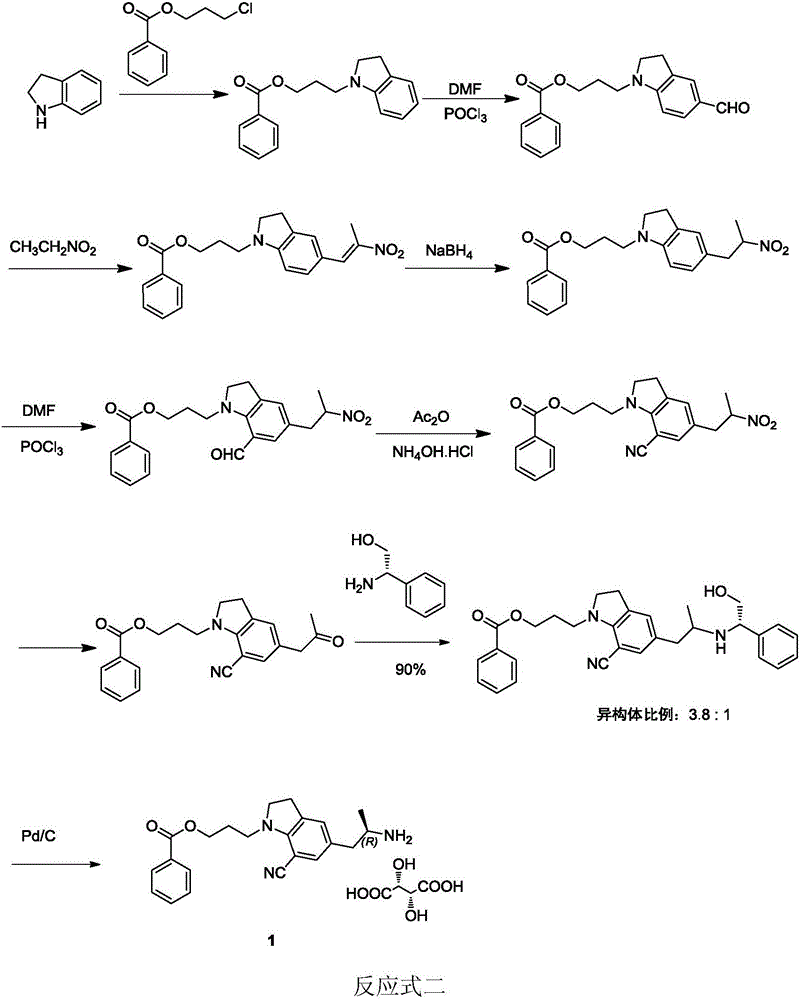
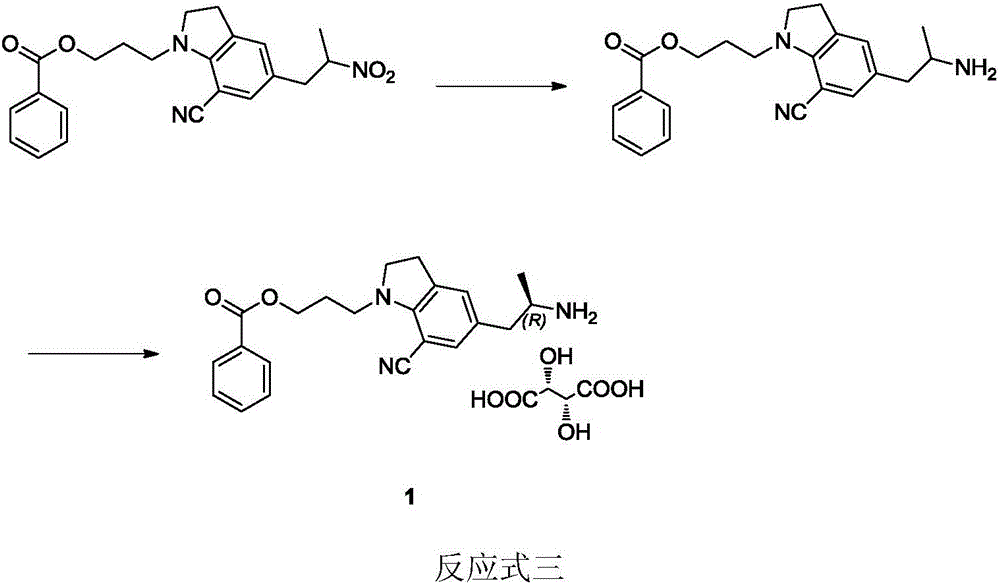
Preparation method of silodosin intermediate
ActiveCN104974072ALower purchase costReduce manufacturing costCarboxylic acid salt preparationIndolineCarbonyl reduction
The invention discloses a preparation method of a silodosin intermediate, wherein the structure of the silodosin intermediate is represented as the formula A. The preparation method, wherein indoline is employed as a start raw material, includes the reactions of Friedel-Crafts acylation, carbonyl reduction, Gabriel reaction and chiral resolution and the like. The preparation method is simple in operation, is low in cost, is high in yield, allows the product to purify easily and is stable in processes, and is suitable for industrial production. The invention also discloses a new intermediate compound which is related in the method.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
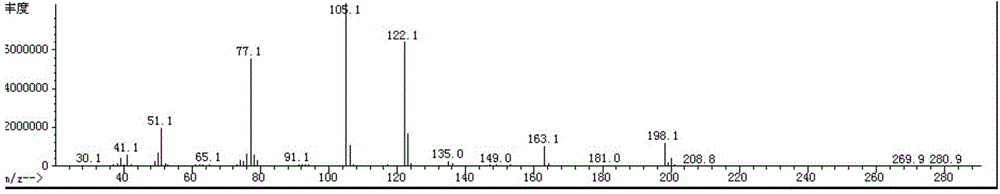
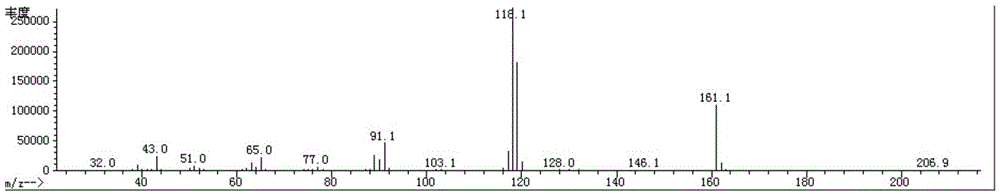
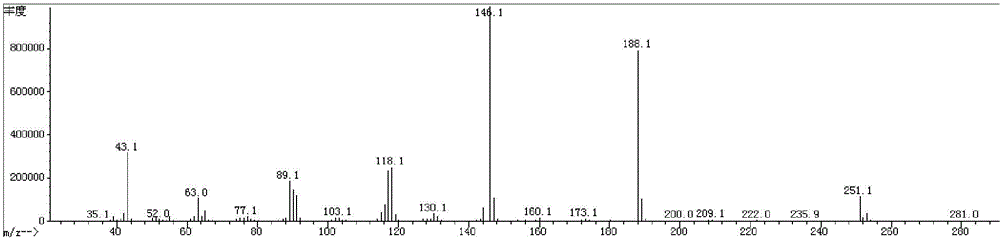
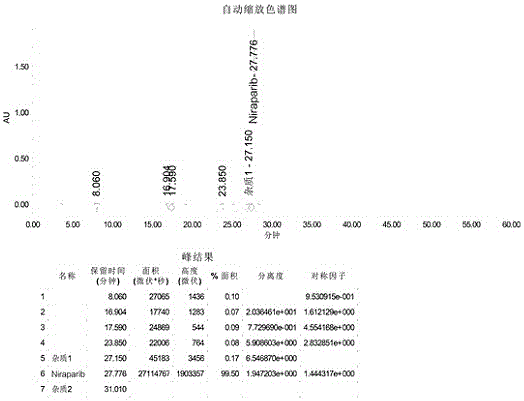
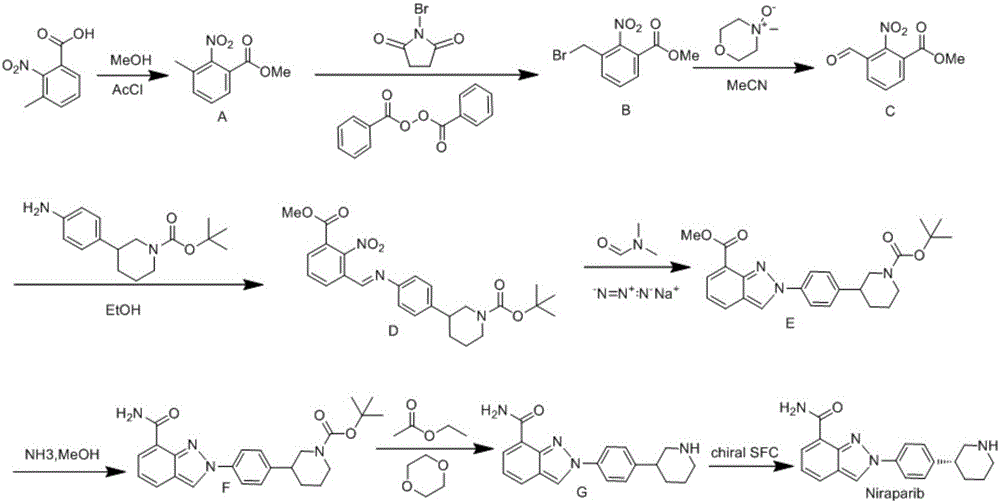
Synthesis method for preparing PARP inhibitor Niraparib
InactiveCN106496187ALow priceEasy to getOrganic chemistryBulk chemical productionSynthesis methodsMethyl anthranilate
The invention provides a novel synthesis method for preparing a PARP inhibitor Niraparib. The method comprises the steps that a starting material methyl anthranilate is subjected to diazo coupling, cyclization, amidation, BOC removal and chiral resolution, and then the Niraparib with the purity reaching 99.5% is obtained. The method is simple, convenient and easy to operate suitable for industrial production.
Owner:SHAANXI UNIV OF SCI & TECH
Phosphine ligand and enantiomer or racemic body thereof and preparation methods thereof
ActiveCN102532196AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsIridiumSynthesis methods
The invention discloses a phosphine ligand and an enantiomer or a racemic body thereof and preparation methods and applications thereof. The structural formulae of the phosphine ligand and the enantiomer or the racemic body are shown in the specifications; a phosphine ligand compound has a novel framework; complete transfer of planar chirality to axial chirality in a synthesis process is realized through a desymmetrization reaction; a synthesis method is simple and economical; during preparation of a chiral ligand, common complex chiral splitting processes are avoided; and an obtained chiral ligand has the advantages of high reaction activity, high enantioselectivity and the like in a model reaction, can be applied to catalytic reactions of a plurality of metals such as palladium, rhodium, nickel, copper, iridium, ruthenium, iron, cobalt, gold, platinum and the like, and can have a very good catalytic effect.
Owner:SUN YAT SEN UNIV
Method for preparing S-(-)-amlodipine and R-(+)-amlodipine by chirally resolving racemic amlodipine
ActiveCN101798280AWide variety of sourcesCheap sourceOptically-active compound separationOrganic racemisationSolventTartrate
The invention discloses a method for preparing S-(-)-amlodipine and R-(+)-amlodipine by chirally resolving racemic amlodipine. The resolving solvent is ethanol or a mixed solvent containing ethanol, chiral dibenzoyl-D-tartaric acid or dibenzoyl-L-tartaric acid is used as the resolving agent, the chiral resolving agent and the racemic amlodipine selectively form chiral amlodipine dibenzoyl tartrate, and the chiral amlodipine dibenzoyl tartrate is alkalified to obtain the chiral amlodipine. The invention adopts the ethanol as the resolving solvent; and when using industrial ethanol as the resolving solvent, the invention can also acquire good resolving effect and obtain the qualified medicinal amlodipine raw material, thereby obviously reducing the cost. The invention has the characteristics of simple reaction operation, easy control, low toxicity, environmental protection, high efficiency and the like, and is suitable for green large-scale production.
Owner:石家庄润柏医药科技有限公司
Magnetic nano particle enzyme immobilization as well as preparation method and uses thereof
InactiveCN101177678AEasy to manufactureEasy to operateFermentationOn/in inorganic carrierChiral selectivityMagnetite Nanoparticles
The invention relates to a functionalized magnetic nano particle immobilized enzyme and a preparation method thereof. Bisphenols with active functional groups are used to coordinate with defect sites on the surface of magnetic nanoparticles, thereby introducing active functional groups on the surface of magnetic nanoparticles, and forming covalent bonds with enzymes to achieve the purpose of immobilizing enzymes. This type of immobilized enzyme is easy to prepare, easy to operate, and exhibits high reactivity and chiral selectivity in chiral resolution and chiral synthesis. This type of immobilized enzyme is also very stable, and can still maintain high reactivity and chiral selectivity after repeated use. The method is universal and applicable to the immobilization of other biomacromolecules.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
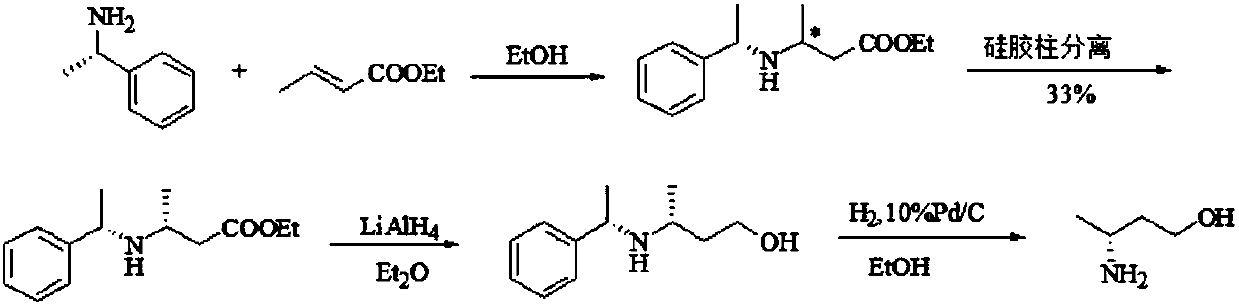
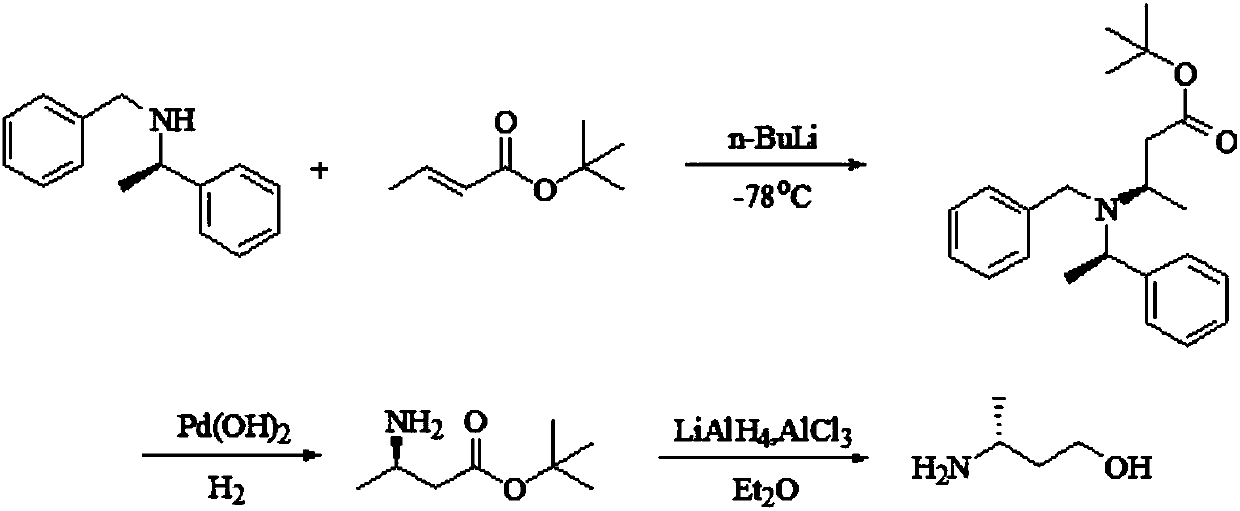
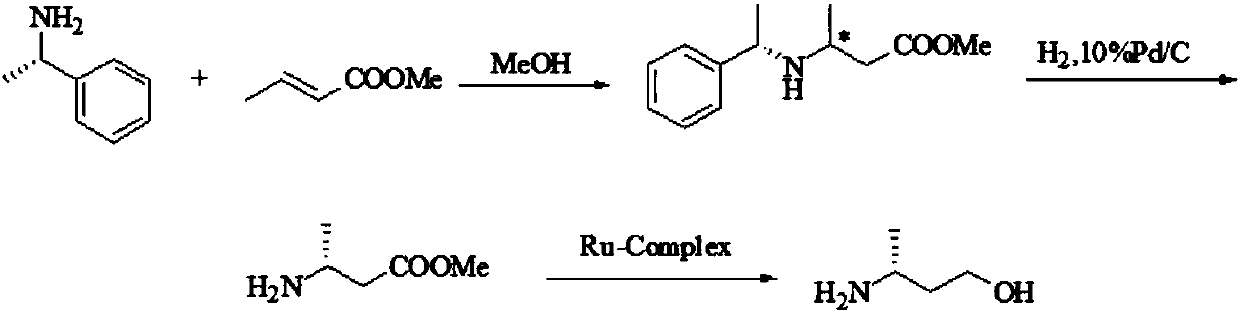
Preparation method of (R)-3-amino butanol
ActiveCN107805205ASimple process routeWide range of optionsOrganic compound preparationOrganic chemistry methodsAlcoholButanone
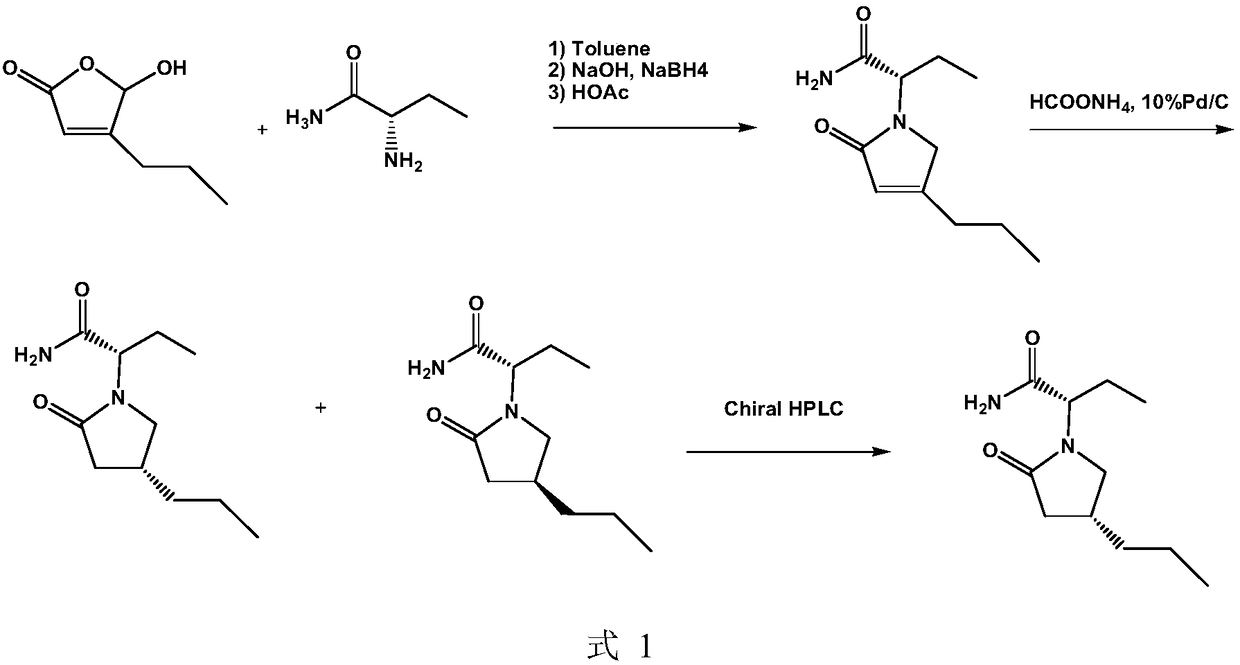
The invention discloses a preparation method of (R)-3-amino butanol. The preparation method includes following steps: (1), allowing (R)-1-methylbenzylamine and butanone alcohol to be in ammoniation reduction reaction to obtain a mixture of (R,R)-3-(1'-methylbenzylamine)-butanol and (R,S)-3-(1'methylbenzylamine)-butanol; (2), adopting an acidic chiral resolution reagent to split the mixture to obtain (R,R)-3-(1'-methylbenzylamine)-butanol; (3), allowing (R,R)-3-(1'-methylbenzylamine)-butanol to be in debenzylation reduction reaction to obtain (R)-3-amino butanol. According to the preparation method, a pair of epimers can be obtained by enabling butanone alcohol to react with a chiral reagent (R)-1-methylbenzylamine, the chiral reagent is added for further resolution, then subsequent reduction is performed, and obtained (R)-3-amino butanol has high purity and ee value.
Owner:ZHEJIANG NHU CO LTD
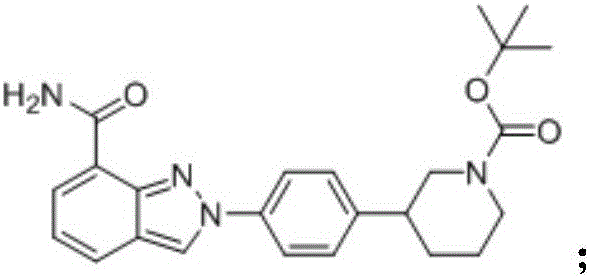
Synthesis method for (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method for chiral intermediate of niraparib
InactiveCN108203404AHigh reaction yieldMild reaction conditionsOrganic chemistry methodsSynthesis methodsOrganic synthesis
The invention belongs to the technical field of organic synthesis. The synthesis method firstly provided by the invention takes benzyl-4-oxopiperidine as a starting material, and the starting materialis subjected to Grignard reaction, elimination reaction, hydrogenation reduction reaction and chiral resolution in sequence to successfully obtain a target product (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine. The synthesis method sencondly provided by the invention takes the same starting raw material for Grignard reaction, organic silicon reagent is used for removing a hydroxide radical, and benzyl is removed by catalytic hydrogenation reaction; finally, the chiral resolution is carried out to obtain a target product. The (S)-3-phenylpiperidine can be synthesized according to the synthesis method. (S)-3-p-aminosalicylic phenylpiperidine can be synthesized according to the third aspect; or according to the fourth aspect, (S)-3-p-bromophenyl piperidine is synthesized to serve asthe key intermediate for preparing the niraparib. According to the synthesis method for (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine and the synthesis method for chiral intermediate of niraparib, production cost is obviously lowered, and the synthesis methods are favorable for the large-scale industrial production of a niraparib medicine.
Owner:SHANGHAI BIOBOND PHARMA
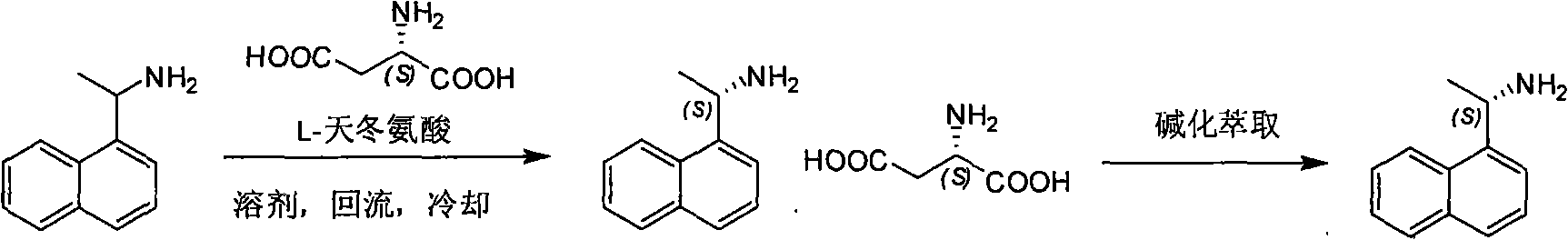
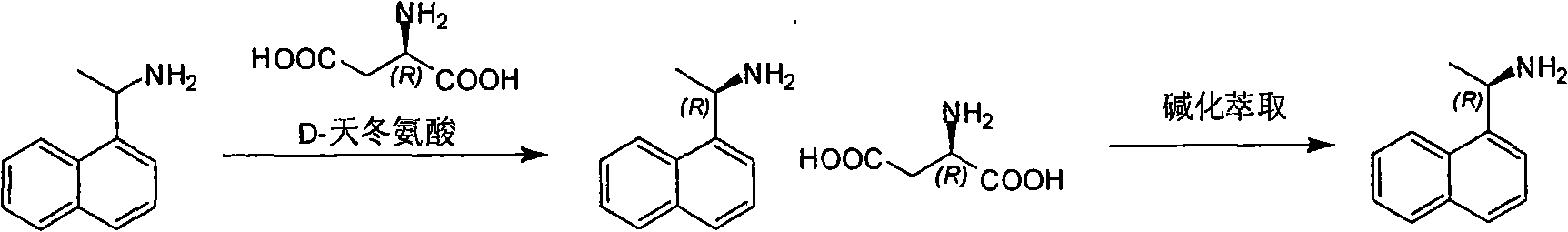
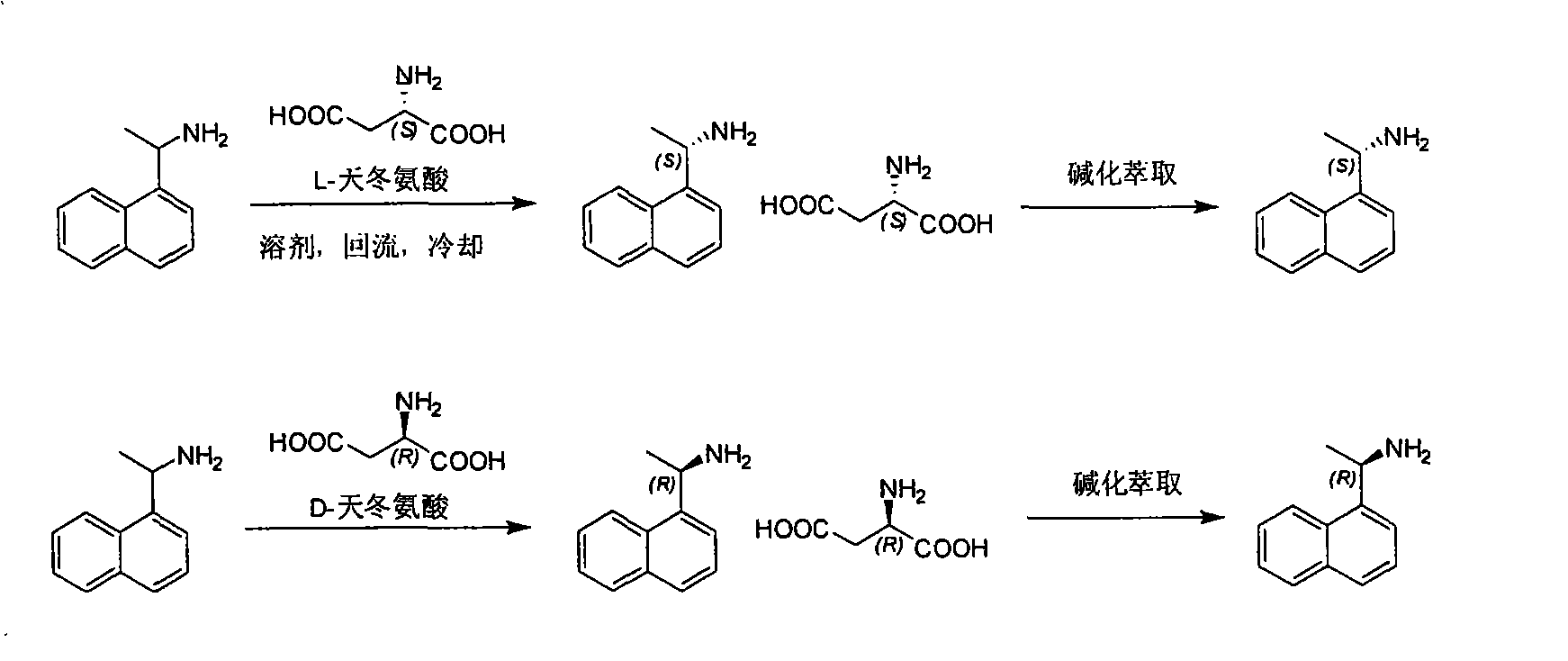
Method for preparing optical pure 1-(1-naphthyl)ethylamine by separation
ActiveCN101407465AReasonable choice of reaction processThe split method is simpleAmino compound purification/separationSolubilitySolvent
The invention relates to a method for preparing 1-(1-naphtaline) ethylamine possessing optical activity, which mainly solves problems of current process of expensive original raw material, long synthesizing process, low yield coefficient, and over-high cost and the like; the invention has chiral aspartic acid as chiral resolving agent, water or solution of water and dioxane as solvent; under a heating condition, racemate 1-(1-naphtaline) ethylamine is reacted with chiral aspartic acid to form enantiomeric salt. Then, according to different solubility of 1-(1-naphtaline) ethylamine to enantiomeric salt, splitting is carried out, and two configurations (S configuration and R configuration) of 1-(1-naphtaline) ethylamine are separated and prepared; chiral e.e. value for one time of splittingcan reach over 98 percent, with yield coefficient over 30 percent. The method is mainly used for preparing S configuration 1-(1-naphtaline) ethylamine and R configuration 1-(1-naphtaline) ethylamine drug intermediate possessing optical activity and template compound researched and developed by innovative small molecule drugs.
Owner:WUXI APPTEC (TIANJIN) CO LTD +1
Preparation method of silodosin intermediate
The invention discloses a preparation method of a silodosin intermediate. The preparation method comprises the following steps that in an organic solvent, under the action of lewis acid, friedel-crafts acylation reaction occurs between a compound 2 and a compound 3, so as to obtain a compound 4, wherein the lewis acid is one of or more of zinc trifluoromethanesulfonate, bismuth trifluoromethanesulfonate, scandium trifluoromethanesulfonate and aluminum trichloride; under the action of organic acid or boron trifluoride ether complex, the compound 4 reacts with triethyl silicane, so as to obtain a compound 5; the compound 5 reacts with sodium azide, so as to obtain a compound 6; under the action of catalysts, the compound 6 reacts with di-tert-butyl dicarbonate ester and hydrogen, so as to obtain a compound 7; under an acidic condition, the deamination protective reaction of the compound 7 occurs, so as to obtain a compound 8; the compound 8 reacts with L-tartaric acid, so as to obtain a compound 1, namely the silodosin intermediate. The preparation method of the silodosin intermediate has the advantages of simplicity, economy and mild reaction conditions, and chiral resolution is not needed.
Owner:ZHEJIANG TIANYU PHARMA
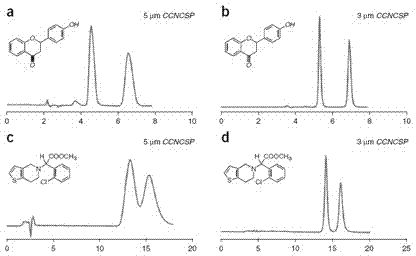
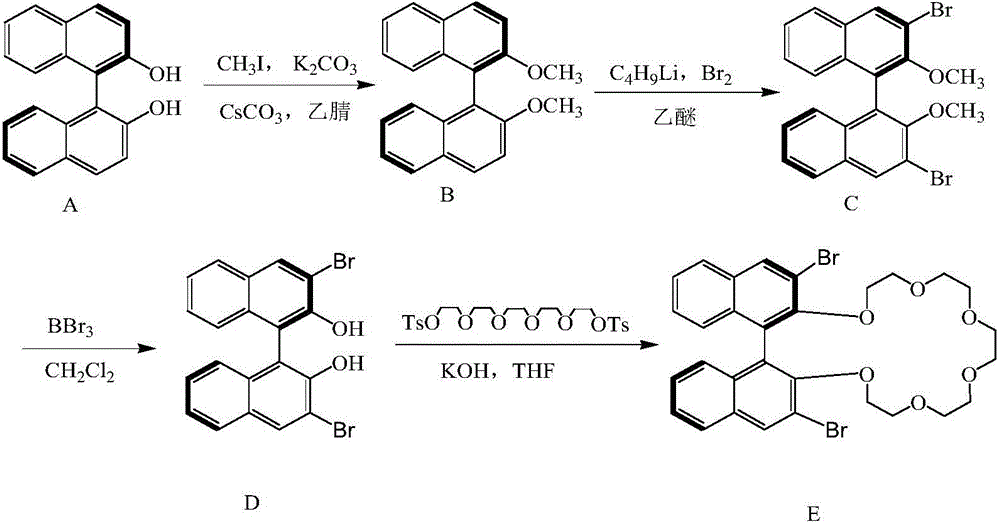
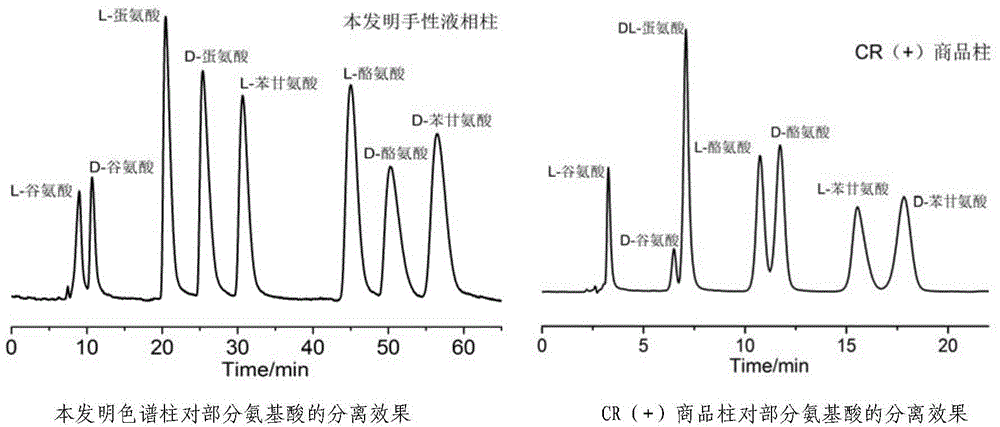
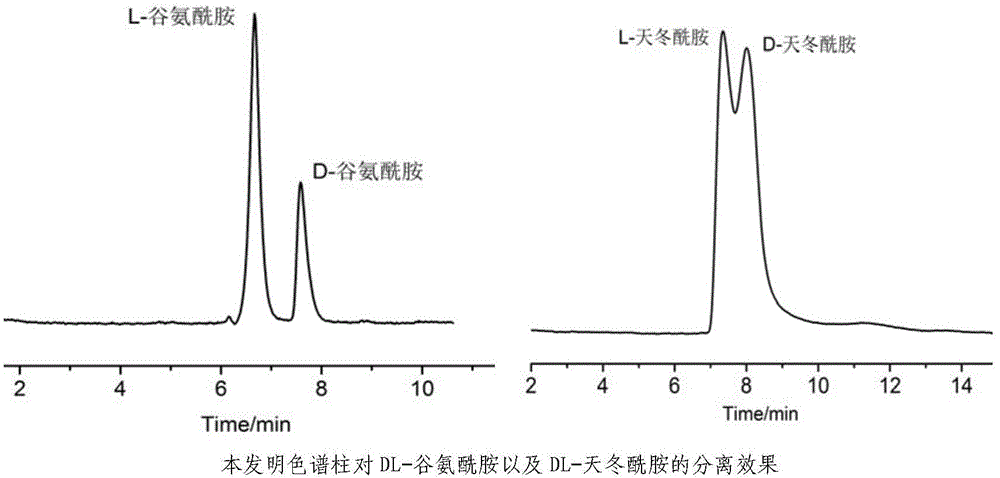
High performance liquid chromatography separating column suitable for amino acid chiral resolution
InactiveCN104475066AEfficient separationHigh resolutionOther chemical processesOrganic compound preparationEvaporationSlurry
The invention discloses a high performance liquid chromatography separating column suitable for amino acid chiral resolution. The separating column is prepared by the following steps: dissolving R(or S)-(3,3'-bromo-1,1'-dinaphthyl)-20-crown-6 in dichloromethane, uniformly dispersing the crown ether solution to C18 silica gel, carrying out rotary evaporation to remove the solvent to prepare a chiral stationary phase; and mixing the chiral stationary phase in a methanol water solution, stirring to obtain a homogenate solution, and filling the chromatographic column by a wet process by using the methanol water solution as a displacement fluid. The separating column can effectively separate all the 19 protein amino acids with chiral site at normal temperature, and can effectively separate chiral drugs with primary amine on the chiral site. The separating column has the obvious characteristics of high resolving power, high separating rate, high reproducibility, lower preparation cost and the like, and can be used repeatedly. The separating column has obviously better separating effect for amino acids than the like products at home and abroad.
Owner:YUNNAN NORMAL UNIV
Preparation method of magnetic microspheres-based levofloxacin surface imprinted material
InactiveCN104788612AImprove adsorption capacityGood magnetic responseOther chemical processesAlkali metal oxides/hydroxidesSynthesis methodsMicrosphere
The invention relates to a preparation method of a magnetic microspheres-based levofloxacin surface imprinted material. The preparation process mainly comprises three steps: preparation of magnetic microspheres; coating of the magnetic microspheres with a molecularly imprinted material; and elution of template molecules. A synthetic method provided by the invention is simple. The prepared magnetic microspheres-based surface molecularly imprinted material has specific recognition function, high adsorption capacity and rapid adsorption ability of levofloxacin and has chiral resolution function of ofloxacin, has good magnetic response and high mechanical strength, can be used as an absorption filler or a coating material, also can be used in preparation of a molecular imprinting sensor and a chip, and is of great significance for researches on specific recognition, chiral resolution and highly sensitive detection of floxacin drugs.
Owner:CHINA PHARM UNIV
Method of synthesizing bepotastine or benzenesulfonic acid salt thereof and intermediates used therein
InactiveUS20140046068A1High optical puritySafe and effective and low-costOrganic chemistryAlpha hydroxy acidBenzenesulfonic acid
The present invention relates to a novel method of synthesizing bepotastine or its benzenesulfonic acid salt and novel intermediates used therein. The present invention uses L-α-hydroxy acid for chiral resolution to form an L-α-hydroxy acid salt of a compound represented by the following formula (VII-1), so as to synthesize bepotastine or its benzenesulfonic acid salt in high optical purity.
Owner:EVERLIGHT USA INC
Synthesis method of brivaracetam intermediate and brivaracetam
InactiveCN109134406AAvoid chiral resolutionRaw materials are easy to getOrganic compound preparationOrganic chemistry methodsSynthesis methodsBrivaracetam
The invention discloses a synthesis method of a brivaracetam intermediate and brivaracetam. The synthesis method of the brivaracetam intermediate includes the steps of stripping carboxyl, triggering ahydrogenation reaction, and then conducting chiral resolution to obtain the brivaracetam intermediate which can be used for further synthesis of brivaracetam, wherein specific information is shown inthe description. According to the synthesis method of the brivaracetam intermediate, applied raw materials are easy to obtain, reaction operation is simple, and reaction conditions are easy to control. The synthesis method of brivaracetam is high in yield, the purity of synthesized brivaracetam can reach 99% or above, and the overall synthesis cost is lower.
Owner:LIVZON NEW NORTH RIVER PHARMA
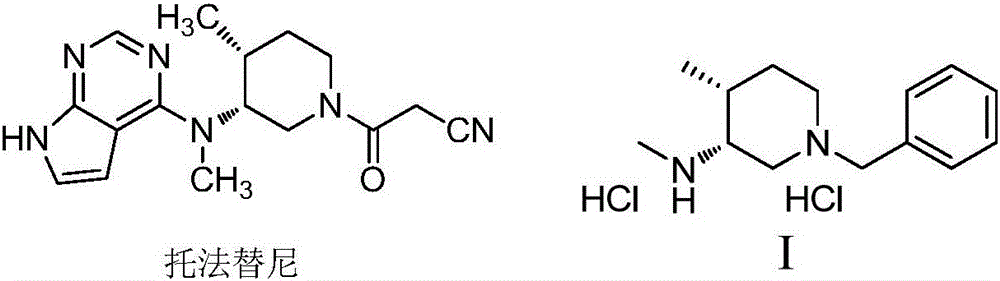
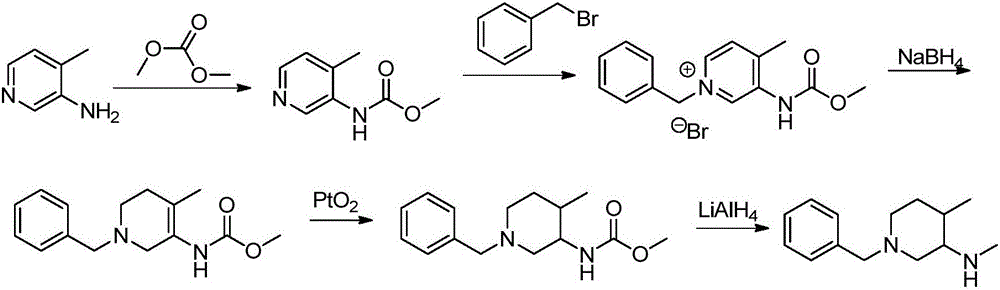
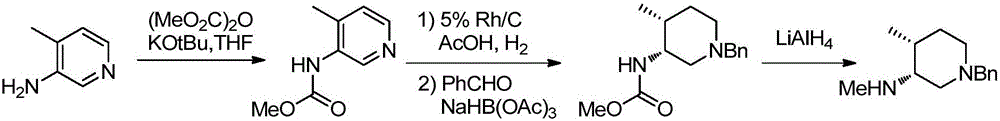
Preparation method of tofacitinib intermediate
The invention relates to a novel preparation method of a tofacitinib intermediate and in particular to a preparation method of the tofacitinib intermediate (3R,4R)-1-benzyl-N-4-dimethyl piperidine-3-amine dihydrochloride. The preparation method comprises the following steps of: by taking 1-benzyl-4-methyl-1,2,3,6-tetrahydropyridine as an initial raw material, oxidizing olefin to form a ketone II by means of a one-step process; forming imine III with amine; applying asymmetric reduction imine to form amine; removing a trans isomer by recrystallization to obtain a cis-form structure IV; and finally, applying chiral resolution to obtain a final product (3R,4R)-1-benzyl-N-4-dimethyl piperidine-3-amine dihydrochloride I. The preparation method is creative in process, the process steps are shortened, and the synthetic yield of an asymmetric compound is greatly increased, thereby laying a solid foundation for industrial production.
Owner:苏州楚凯药业有限公司
Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds
A process and diastereomeric salts useful for the optical resolution of racemic α-[4-(1,1-dimethylethyl)phenyl]-4-(hydroxydiphenylmethyl)-1 -piperidinebutanol, 4-[4-[4-(hydroxydiphenylmethyl)-1 -piperidinyl]-1-hydroxybutyl]-α,α-dimethylbenzeneacetic acid and lower alkyl 4-[4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-1-hydroxybutyl]-α,α-dimethylbenzeneacetates. The process comprises placing into solution a chiral resolving agent, either (+) / (−)-di-paratoluoyltartaric acid or (−) / (+)-mandelic acid, in an amount equimolar to a compound corresponding to the desired enantiomer of the above compound, precipitating the resulting diastereomeric salt between the chiral resolving agent and the target enantiomer and separating the enantiomer.
Owner:MERRELL DOW PHARMA INC
Crystal forms and methods of synthesis of (2r, 6r)-hydroxynorketamine and (2s, 6s)-hydroxynorketamine
ActiveCN109311801AOrganic active ingredientsCarbamic acid derivatives preparationL-Pyroglutamic AcidNorketamine
The disclosure provides a method for synthesizing free base forms of (2R,6R)-hydroxynorketamine (HNK) and (2S,6S)-hydroxynorketamine. In an embodiment synthesis of (2R,6R)-hydroxynorketamine (HNK) includes preparation of (R)-norketamine via chiral resolution from racemic norketamine via a chiral resolution with L-pyroglutamic acid. The disclosure also provided crystal forms of the corresponding (2R,6R)-hydroxynorketamine (HNK) and (2S,6S)-hydroxynorketamine hydrochloride salts.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
A preparation method for solid film dip-coated with a novel chiral recognition agent
InactiveCN103203187ASimple stepsLow requirementSemi-permeable membranesSeparation processHigh selectivity
A preparation method for solid film dip-coated with a novel chiral recognition agent belongs to the technical field of film separation. The method comprises: synthesis of a cyclodextrin-type chiral ionic liquid; preparation of a crosslinking solution; treatment of base film; soaking of film sheets in a chiral ionic liquid / chitosan crosslinking solution or a polyanionic film-making solution and a chiral ionic liquid film-making solution; washing of composite film sheets; and drying of film sheets. The method for preparing chiral solid film is easy, few in steps and low in requirements on equipment. Chiral separation experiments show that the use of the chiral separation solid film prepared by the method can realize high selectivity and low energy consumption in chiral separation processes.
Owner:BEIJING UNIV OF CHEM TECH
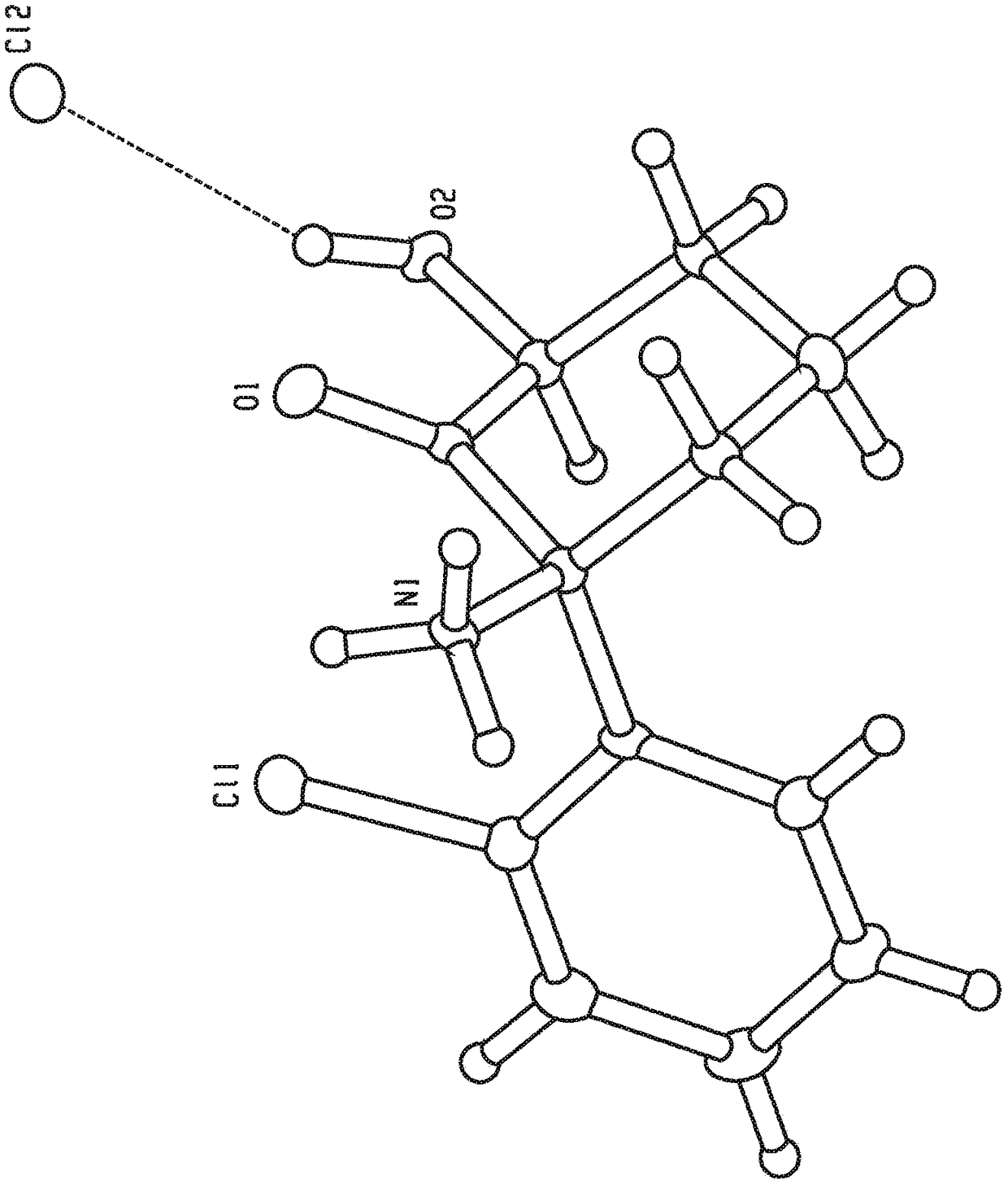
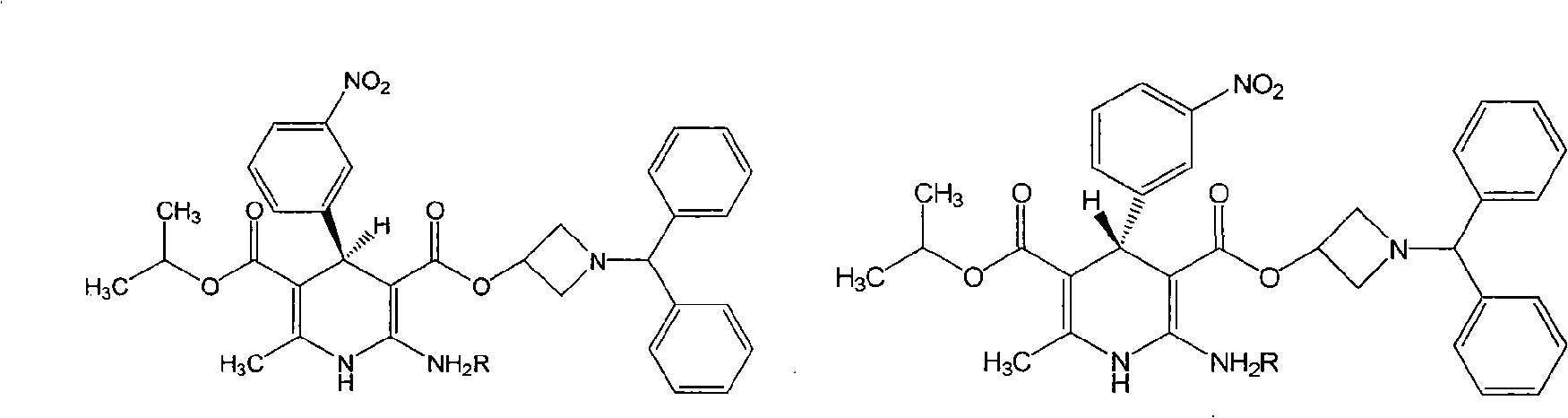
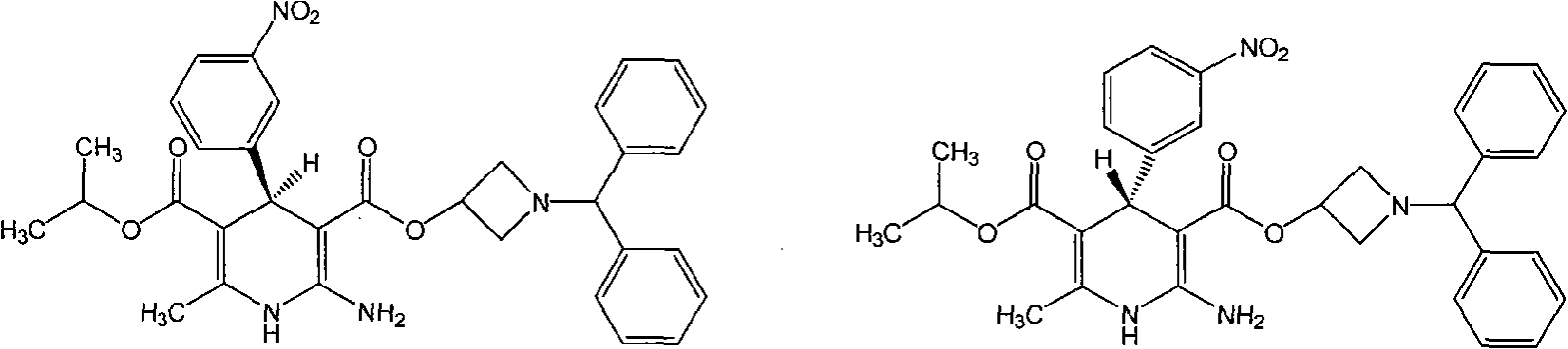
Method for preparing chiral azelnidipine and acceptable salt thereof
The invention relates to a method for preparing (S)-(+)-azelnidipine and (R)-(-)-azelnidipine as well as benzene sulfonic acid, paratoluenesulfonic acid, D-(+)-camphorsulfonic acid, L-(-)-camphorsulfonic acid, taurine and high taurine salt thereof by economically and effectively resoluting racemic azelnidipine. Compared with the prior method for preparing optically active azelnidipine and salt thereof by a high performance liquid chromatography which needs expensive instruments and equipment and has less treatment amount, the method adopts a recrystallization resolution method of the prior chiral resolution reagent and a cheap and easily-obtained solvent, has large sample treatment amount and simple operation, does not need special instruments, and is suitable for industrial production.
Owner:北京华禧联合科技发展有限公司
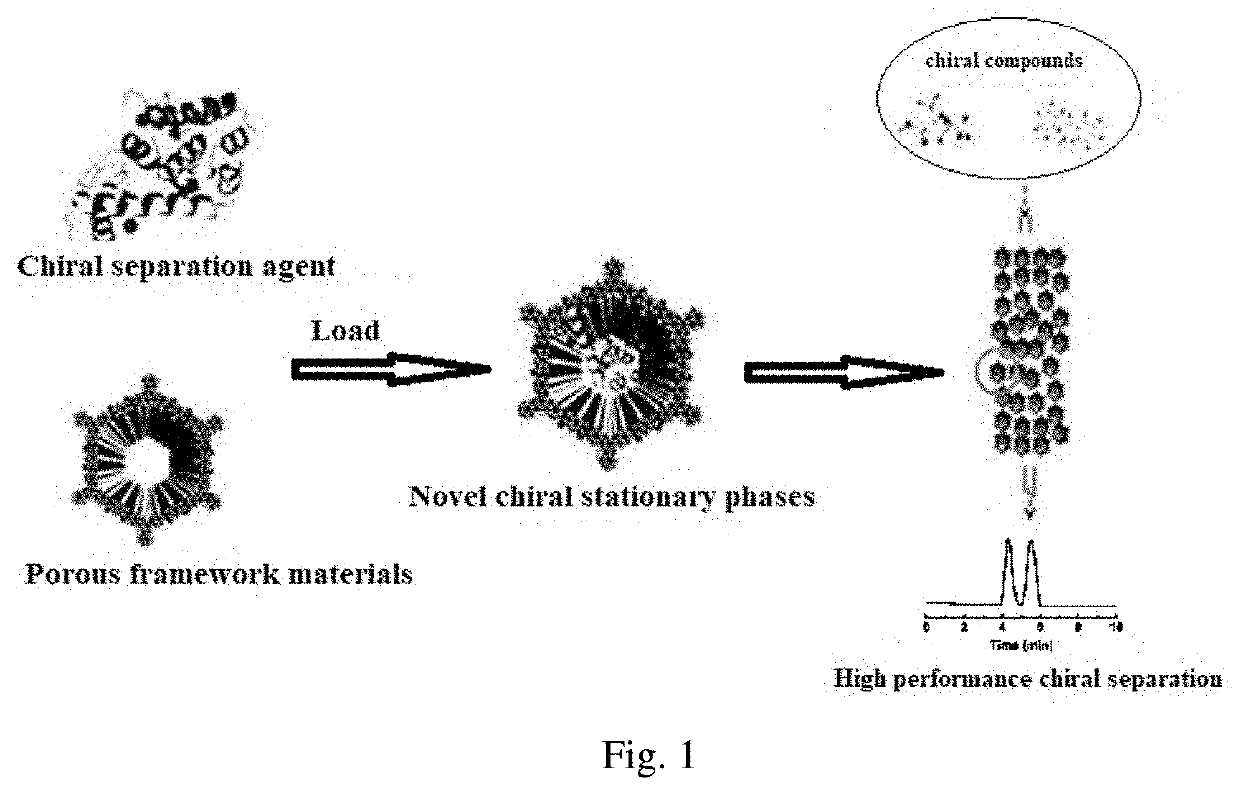
Preparation of chromatographic stationary phase having porous framework material as matrix for chiral separation
ActiveUS20210023528A1Improve stabilityImprove comprehensive applicabilityOther chemical processesSolid sorbent liquid separationChromatographic separationMetal-organic framework
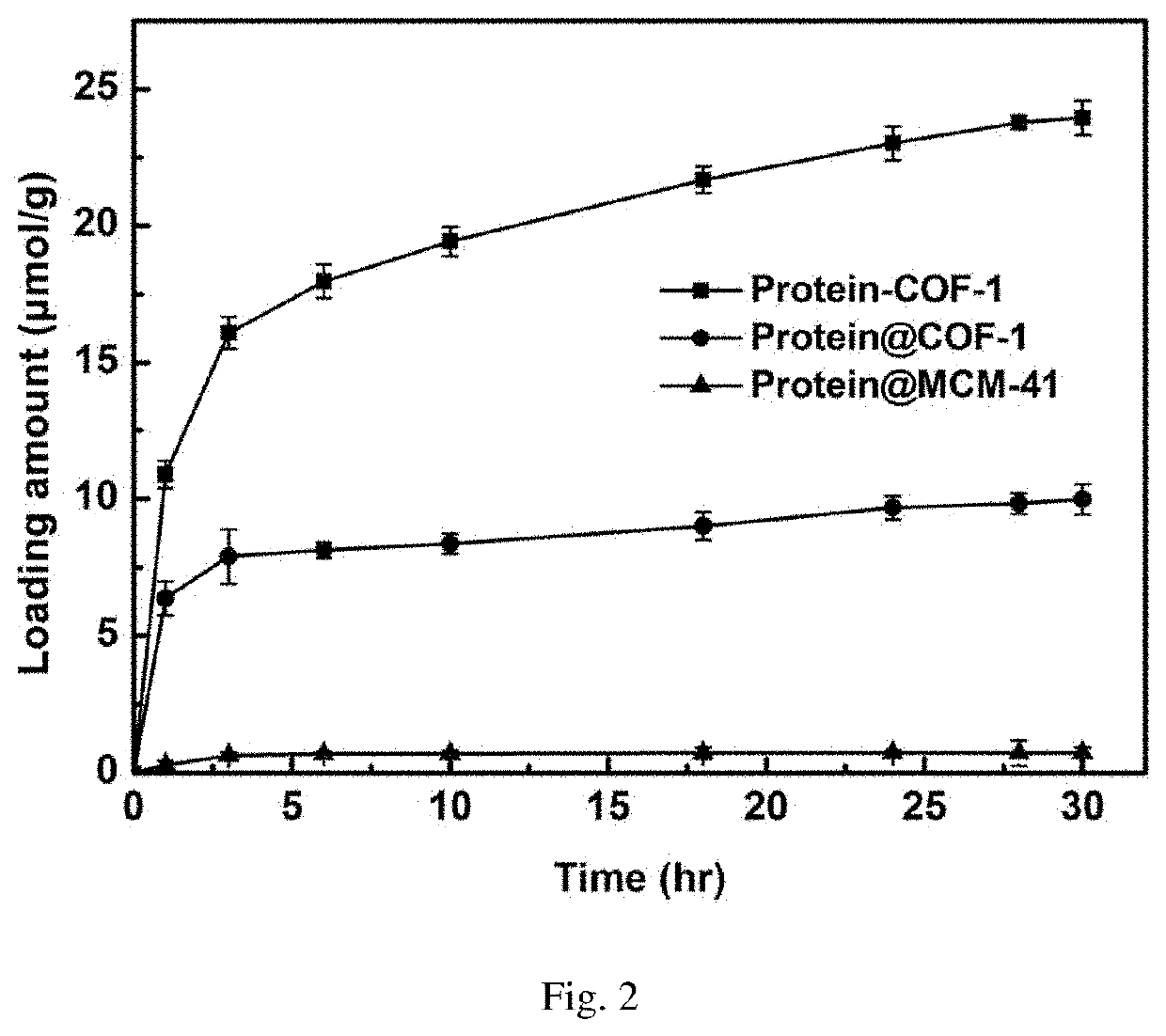
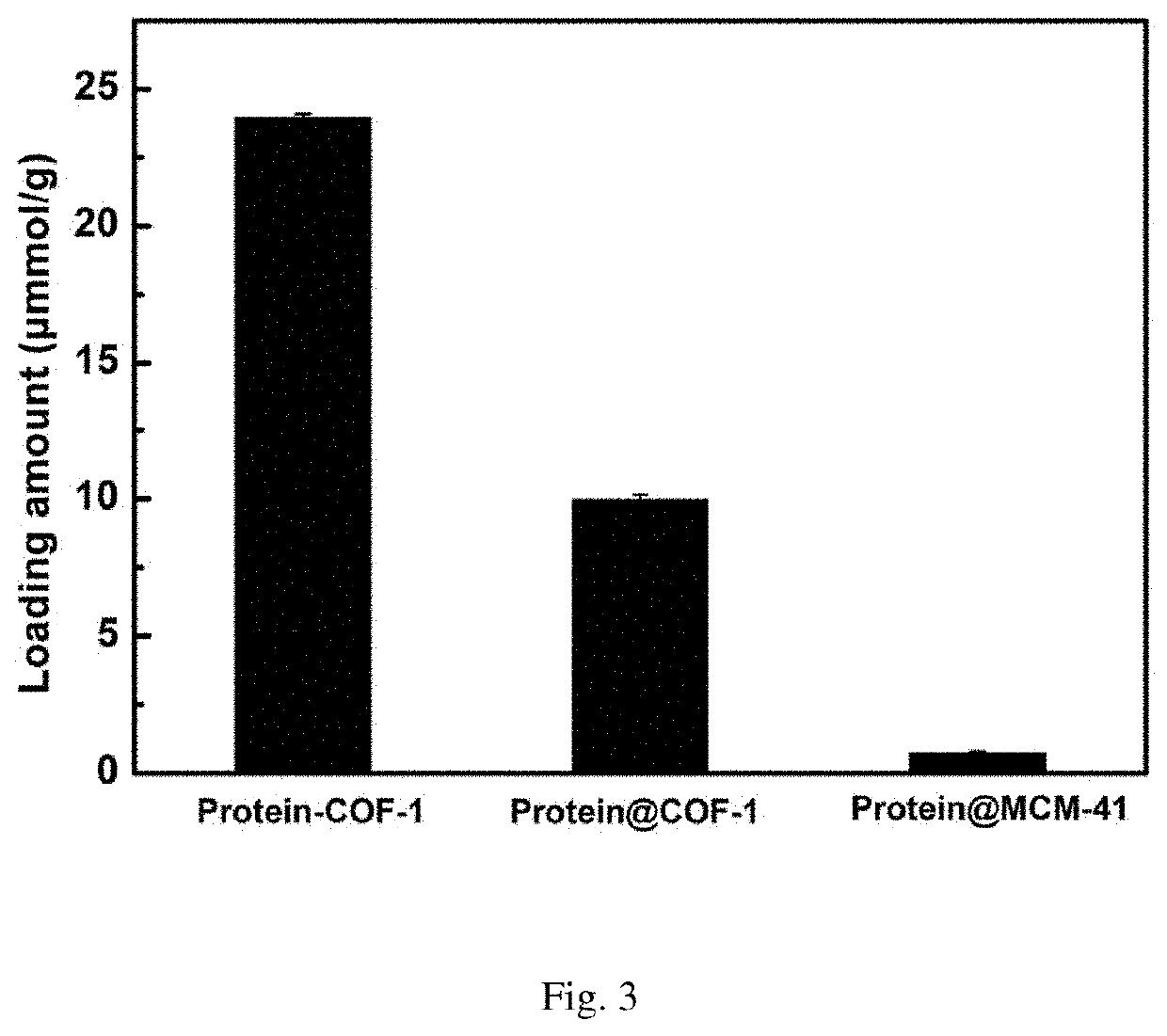
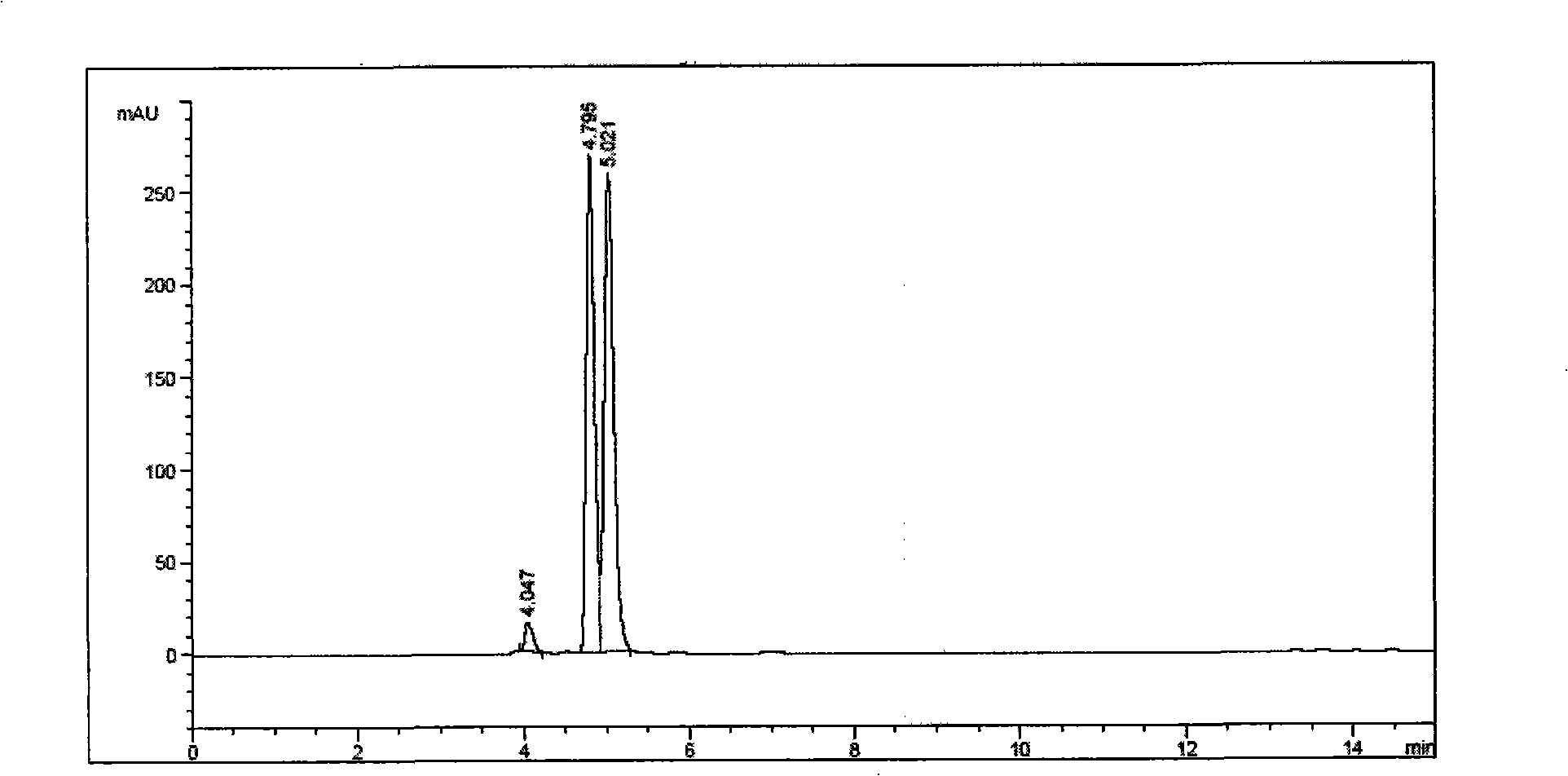
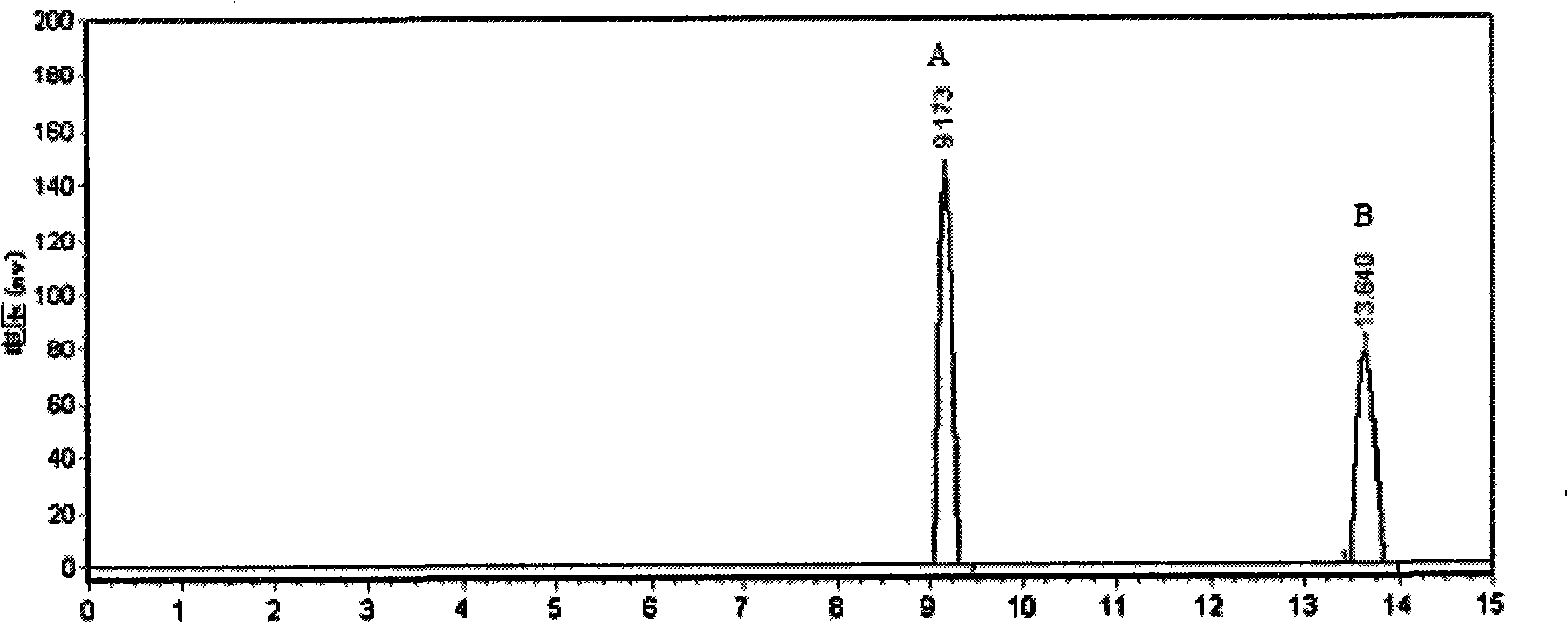
The novel porous framework materials (such as metal organic frameworks or covalent organic frameworks) having a wide range of applications, which was designed and developed in an inventive manner to resolve issues with respect to a carrier material in a stationary phase of a conventional chiral chromatographic column in which the carrier material has poor stability, a chiral resolving agent has a low loading rate, and the chiral resolving agent is prone to loss or is applied in a restricted manner. The porous framework material efficiently loads a chiral resolving agent (such as proteins, enzymes, or macrocyclic antibiotics) by means of covalent bonding, adsorption, embedding, and crosslinking, such that a variety of efficient and durable chiral stationary phases are prepared to serve as a novel high-performance chromatographic column filler used for chromatographic chiral separation (such as high-performance liquid chromatography or capillary chromatography). The various chiral stationary phases prepared by applying the above technique have high separation efficiency, high stability, and durability, and have been successfully applied to perform efficient separation of different kinds of chiral materials such as chiral amino acids and a chiral drug. The technique greatly widens the application range and extends the service life of a chiral chromatographic separation column.
Owner:NANKAI UNIV
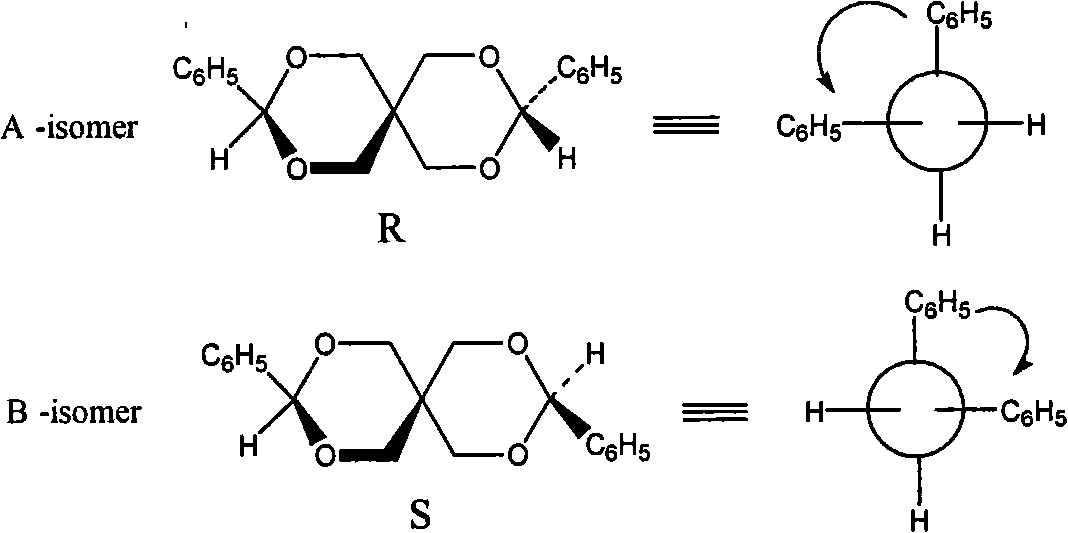
Pentaerythritols chiral spiro compound and synthesis and resolution method thereof
InactiveCN101357925AImprove protectionImprove securityOptically-active compound separationOrganic racemisationPentaerythritolBenzaldehyde
A pentaerythritol chiral spirocyclic compound is characterized by being a chiral spirocyclic compound which can be compounded by substituted benzaldehyde and pentaerythritol, also can be split and contains the construction unit of pentaerythritol. The synthetic method comprises the procedures of charging materials, adding with a catalytic agent, stirring, filtering, recrystallizing, etc. The splitting method is as follows: the compound is dissolved in a moving phase, and chiral separation is carried out on the compound in a high-effective liquid chromatograph by a chiral column. The invention has the advantages that the chiral spirocyclic compound can be used as the intermediate compound of a novel chiral axial medicine and also can be used as a medicine monomer to be induced in different active functional groups; pure optical isomer is obtained by splitting an external racemic body, thereby improving the safety of medince and having significance in pharmacodynamics and medicine dosage. In the synthetic process, an environement friendly synthetic method is applied, thereby having low cost and convenient operation, and being beneficial to protecting environment and belonging to green chemistry.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Resolution method of R-(+)-1-(1-naphthyl) ethylamine
InactiveCN101735070AA simple and cyclic separation method for racemate 1-(1-naphthalene)ethylamineThe method of effective circular resolution racemate 1-(1-naphthalene) ethylamineAmino compound purification/separationSolubilityAlcohol
Owner:EAST CHINA UNIV OF SCI & TECH
Method for selective synthesis of alpha-narcotine with participation of blockage group
The invention belongs to the technical field of drug synthesis, and relates to position selective synthesis of alpha-narcotine with participation of a blockage group, preparation of serial phthalide tetrahydroisoquinoline compounds and preparation of various phthalide-3-carboxylic acid compounds and various 3-oxo isochroman-1-carboxylic acid compounds through a universal mode. In the method, 2, 3-dimethoxy benzoic acid is used as a raw material and condensed with glyoxylate through the universal mode to obtain 6, 7-dimethoxy phthalide-3-carboxylic acid, the carboxylic acid and 2-(2-bromo-3, 4-methylenedioxy-5-methoxy-phenyl)-ethylamine hydrochloride are condensed to prepare amide, the amide reacts with Bischler-Napieralski to prepare bromo-alpha-narcotine through reaction, methylation and salt formation, and (-)-alpha-narcotine is then prepared through dehalogenation and resolution. Due to the positioning function of the blockage group, the invention effectively reduces the position selectivity of Bischler-Napieralski cyclization, and realizes the simple and efficient synthesis of alpha-narcotine through removal of the blockage group and the chiral resolution.
Owner:SHENYANG PHARMA UNIVERSITY
Pseudomonad esterase and application in preparing optical pure mandel and derivative thereof
InactiveCN101538542AHigh optical purityImprove stabilityBacteriaMicroorganism based processesPhenylacetic acidAcid derivative
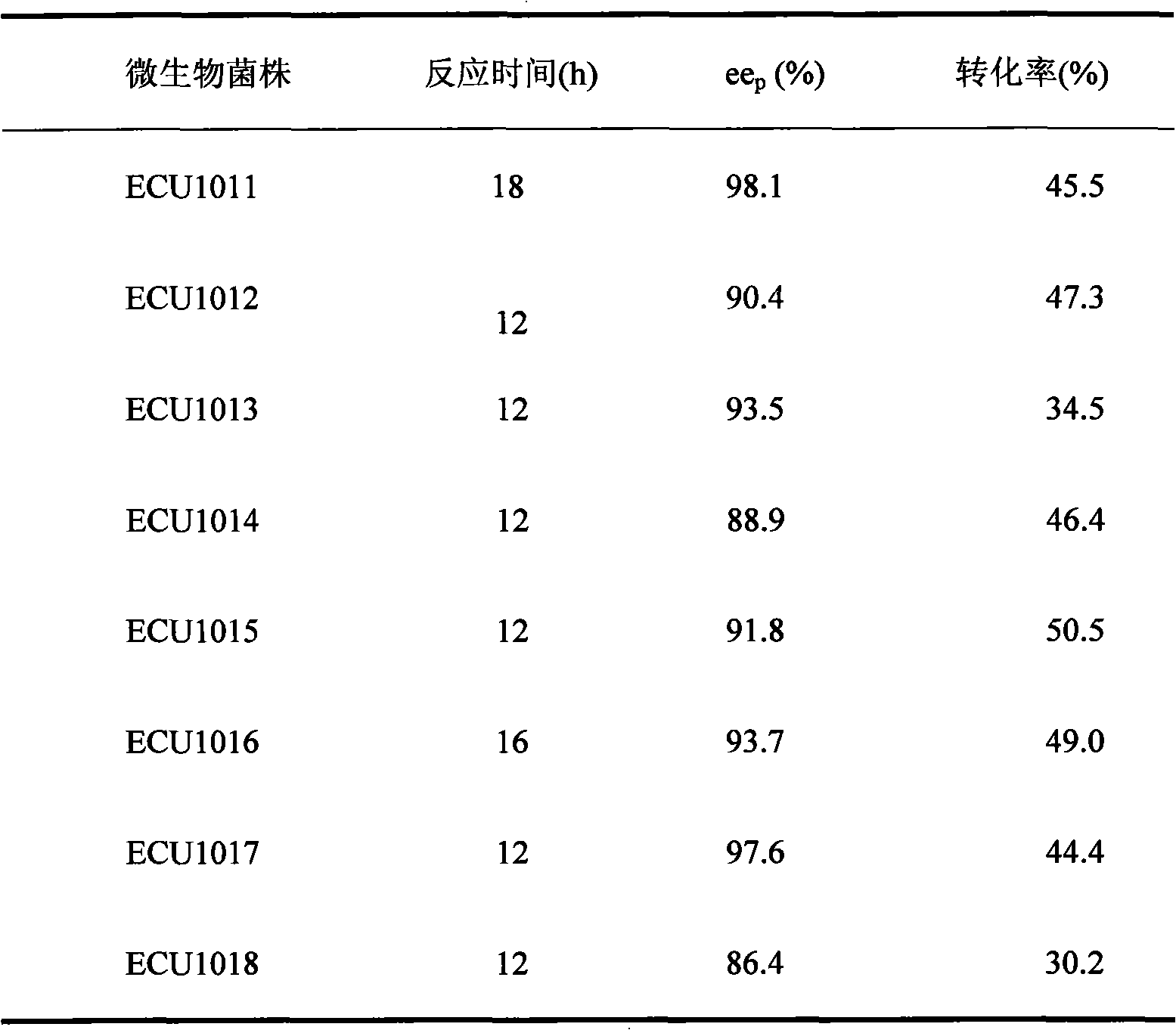
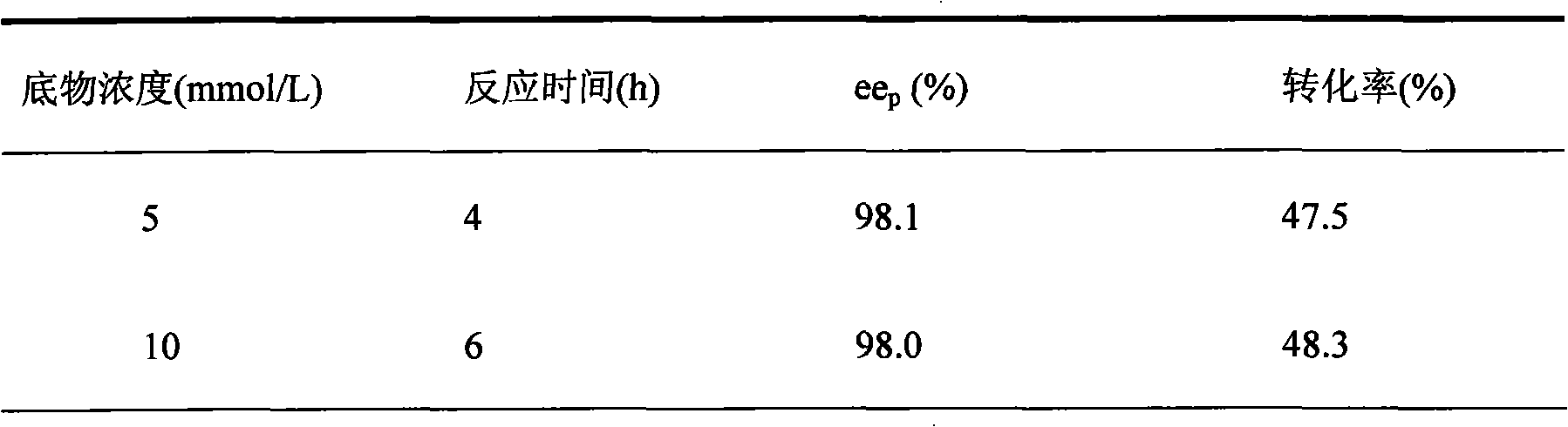
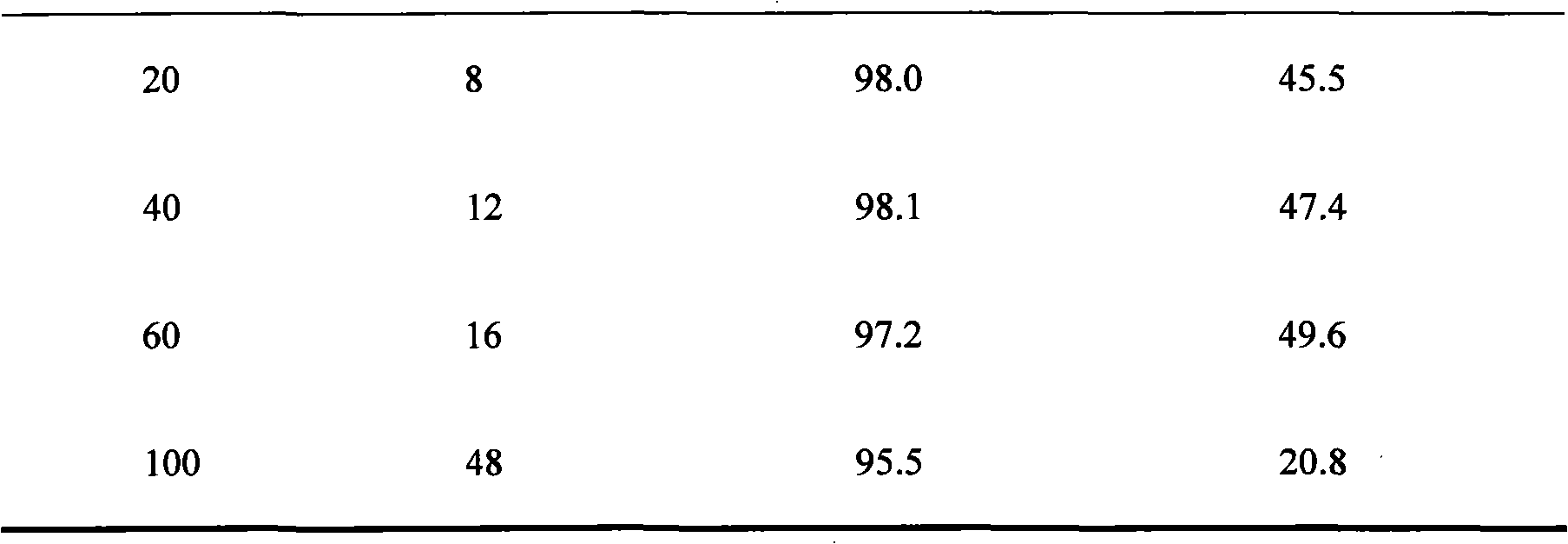
The invention discloses a pseudomonad which produces esterase and an application in preparing (S)-mandel, (R)-mandel or derivative thereof by using the enantiomorphous selective hydrolization of esterase catalysis 2-acetoxyl group-phenylacetic acid or the derivative thereof produced by the pseudomonad. The esterase is the bacteria body cultured by using soil isolated bacteria-pseudomonas sp.ECU1011 (preserving number is CGMCC No.2872). The technique of producing (S)-mandel and (R)-mandel by adopting the disclosed pseudomonad esterase to catalyzing enantiomorphous selective hydrolization of 2-acetoxyl group-phenylacetic acid is new, the reaction condition is mild, the enantiomorph of the product and the substrate have high purity, the enantiomeric excess values (ee) are 98.1% and more than 99% respectively. The biological catalyst has extensive substrate spectrum, can be used for chiral separation of various mandel derivative, and has excellent industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method and intermediate of ramelteon
InactiveCN102924410AReduce dosageRaw materials are cheap and easy to getOrganic chemistryBulk chemical productionPropionyl chlorideOrganic solvent
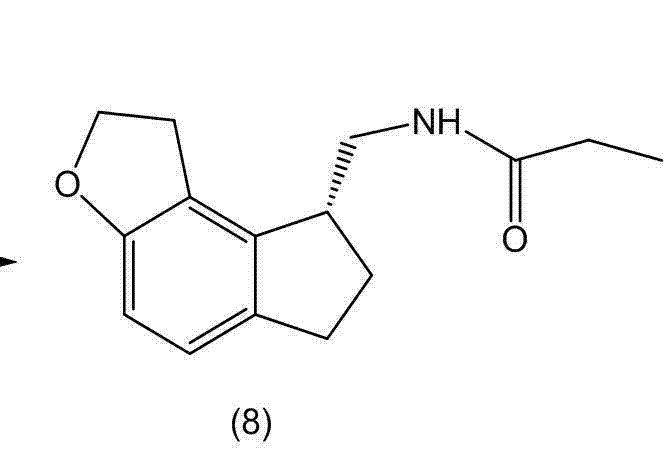
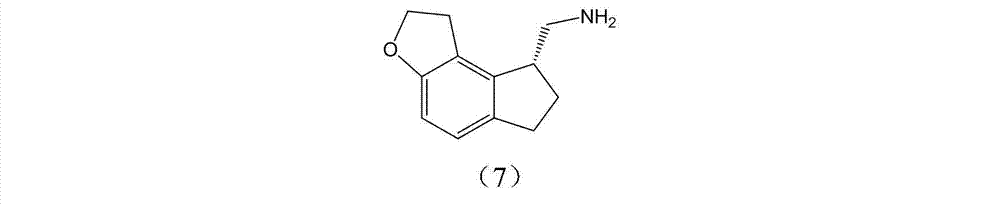
The invention discloses a preparation method and an intermediate of ramelteon. The preparation method comprises the following steps: 1. deprotecting a compound (5) in an organic solvent to obtain a compound (6); 2. after condensing the compound (6) and a chiral resolution reagent, crystallizing to obtain an S type condensate crystal, and hydrolyzing under alkaline conditions to obtain an S type optical isomer compound (7) of the compound (6); and 3. reacting the compound (7) with propionyl chloride under alkaline conditions to generate the ramelteon (8).
Owner:CHINA RESOURCES SAIKE PHARMA
Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof
InactiveCN101463001ASimple processEasy industrial operationOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupStructural formula
The invention relates to an octahydro cyclopentenopyridine (c) carboxylic acid derivative and a preparing method thereof, providing basis for designing and preparing compounds with different biological activities. A chemical structural formula is shown in the fight formula, wherein, R1 is one of alkoxy, bydroxy and amine. R2 and R3 are one of bydroxy, fluorine and hydrogen. N protecting group is tertbutyloxycarbonyl, benzyloxycarbonyl or benzyl. The compounds are optically pure. Starting from natural methionine ester or hydrochloride thereof, the compound of (S)-2-((benzyloxycarbonyl)(propargyl)-amine)butane-3-carboxylic ester (5) is obtained through substitution, oxidation and elimination. After reactions of cobalt carbonyl catalytic cyclization, and the like, (S)-2-benzyloxycarbonyl-5-octahydro cyclopentenopyridine (c)-1- carboxylic ester is obtained. Series of deritative compounds are obtained through reduction of carbonyl, fluorination, chiral separation, hydrolysis and condensation.
Owner:上海药明康德新药开发有限公司
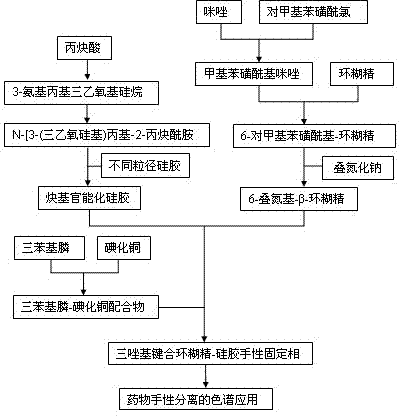
Triazolyl bonded cyclodextrin-silica gel chiral stationary phase and preparation method thereof
InactiveCN102343258AGood acid and alkali stabilityClick reaction implementationOther chemical processesStationary phasePtru catalyst
The invention discloses a method for preparing a triazolyl bonded cyclodextrin-silica gel chiral stationary phase through a click reaction and application of the method. In the invention, through selective azidation of the 6-hydroxyl of cyclodextrin and alkynylation of silica gel, and by making use of a click reaction with a newly developed catalyst, the triazolyl bonded cyclodextrin-silica gel chiral stationary phase with excellent chemical stability is then prepared, thus enriching the structural design of cyclodextrin. The triazolyl bonded cyclodextrin-silica gel chiral stationary phase ofthe invention shows an excellent chiral separation ability to amino acid, acid and neutral racemic drugs in a liquid chromatogram, thus being expected to be applied in the field of drug chiral separation of various chromatographic techniques as a chiral stationary phase.
Owner:NANJING XINLUOMEI NEW MATERIALS
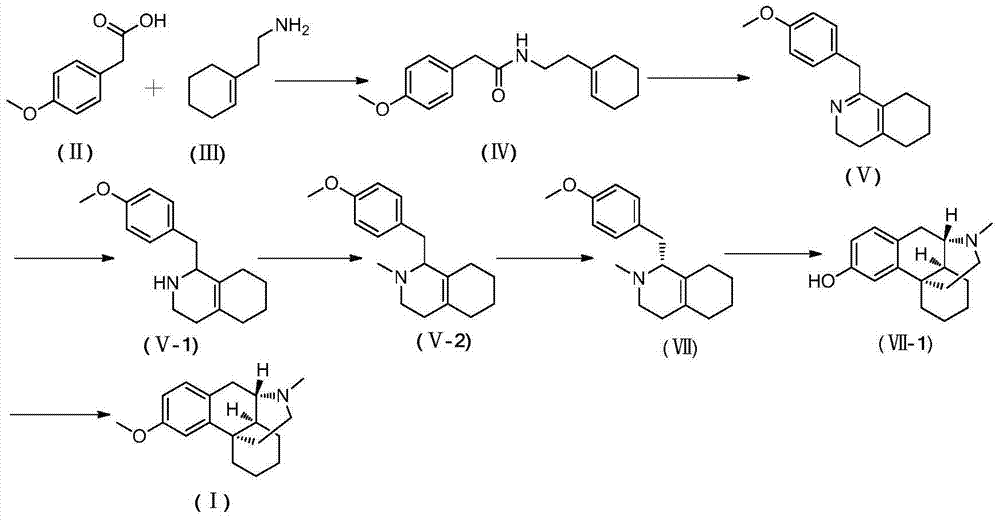
Novel method for preparing dextromethorphan
ActiveCN104119273AOrganic compound preparationCarboxylic acid amides preparationIsoquinolineQuinoline
The invention relates to a novel method for preparing dextromethorphan. When the method is used for preparing an intermediate (+)-1-(4-methoxy) benzyl-1,2,3,4,5,6,7,8-hexahydroisoquinoline (VI), a catalytic reducing method is adopted to carry out chiral reduction on 1-(4-methoxy) benzyl-3,4,5,6,7,8-hexahydroisoquinoline (VI), so that the intermediate is prepared with high selectivity. The novel method disclosed by the invention can cancel complex operations such as chiral resolution, is simple to operate, gentle in reaction condition, short in total time, wide in material source, and very suitable for industrially producing dextromethorphan.
Owner:SHANGHAI TIANCI INT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com











![Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b0c3f56-53f8-44bf-b54d-80c6f7053051/US20060014793A1-20060119-C00001.png)
![Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b0c3f56-53f8-44bf-b54d-80c6f7053051/US20060014793A1-20060119-C00002.png)
![Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds Process and diastereomeric salts useful for the optical resolution of racemic alpha-(4-(1, 1-dimethylethyl) phenyl] -4- (hydroxydiphenylmethyl) -1-piperidinebutanol and derivative compounds](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/0b0c3f56-53f8-44bf-b54d-80c6f7053051/US20060014793A1-20060119-C00003.png)






![Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28c5abf4-a2cd-42d3-bc98-d2d1badcb37c/A200710094607C00021.PNG)
![Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28c5abf4-a2cd-42d3-bc98-d2d1badcb37c/A200710094607C00022.PNG)
![Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof Octahydro cyclopenteno [c] pyrroletetrazole derivative and preparation thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28c5abf4-a2cd-42d3-bc98-d2d1badcb37c/A200710094607C00031.PNG)