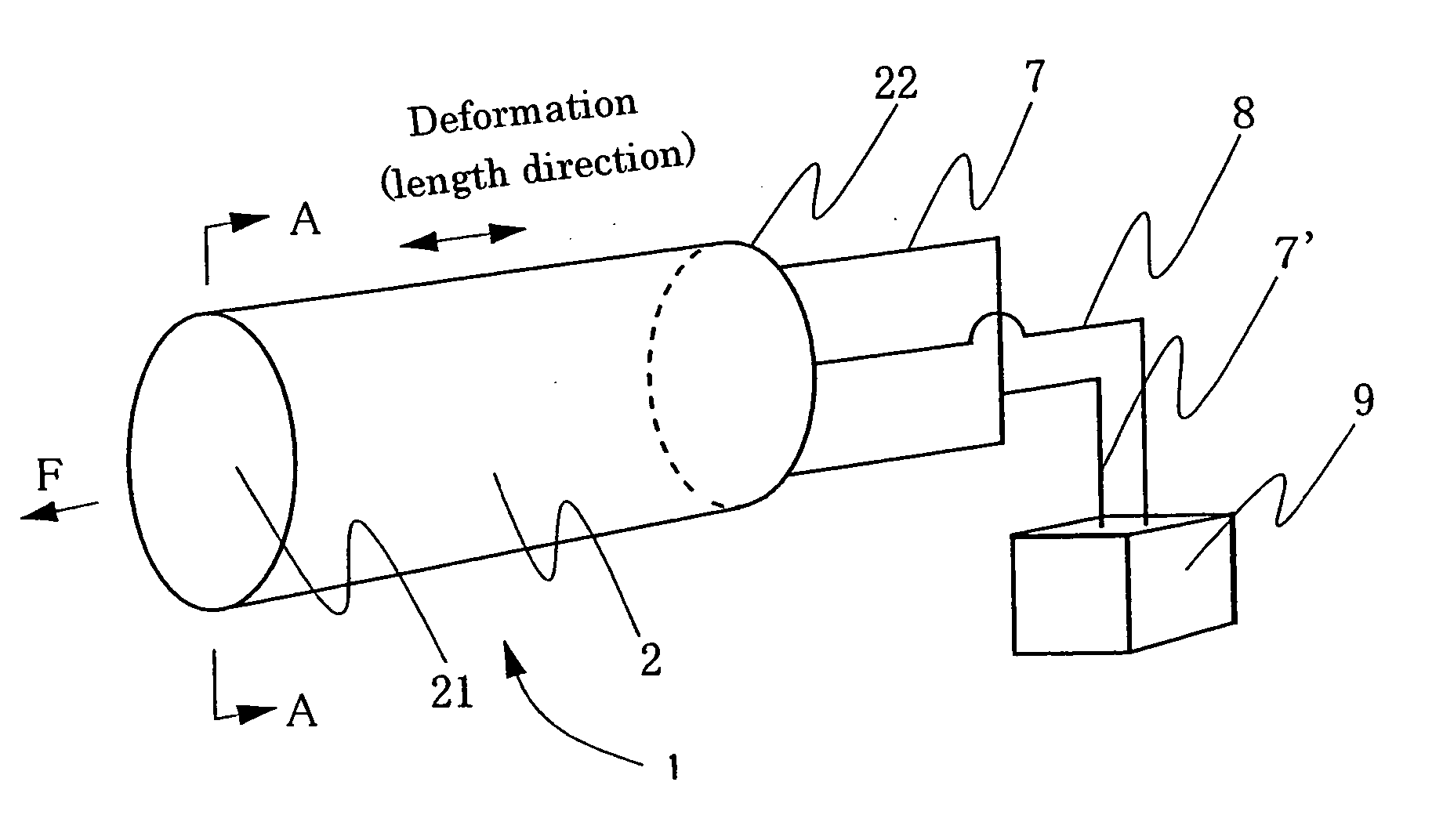
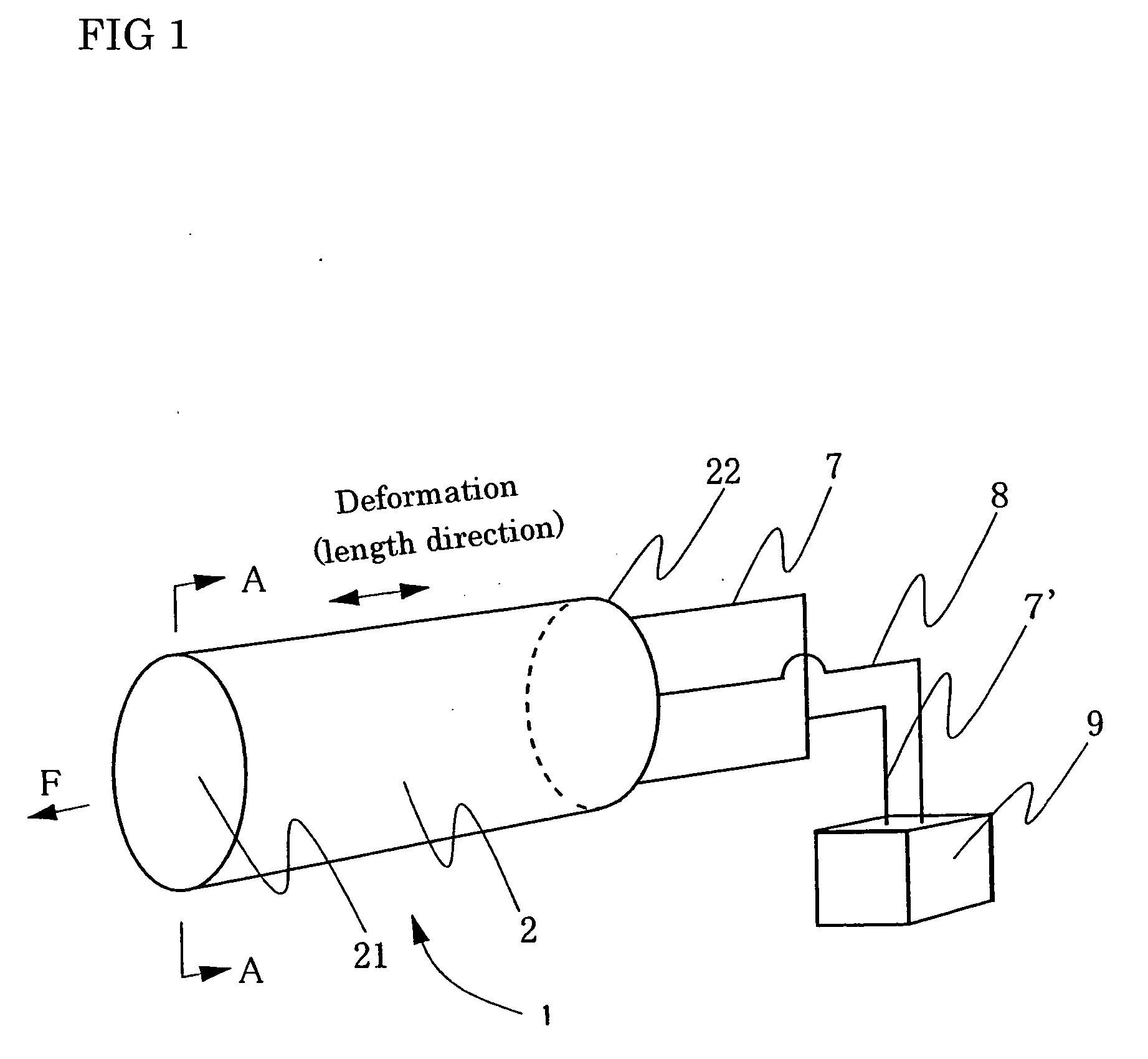
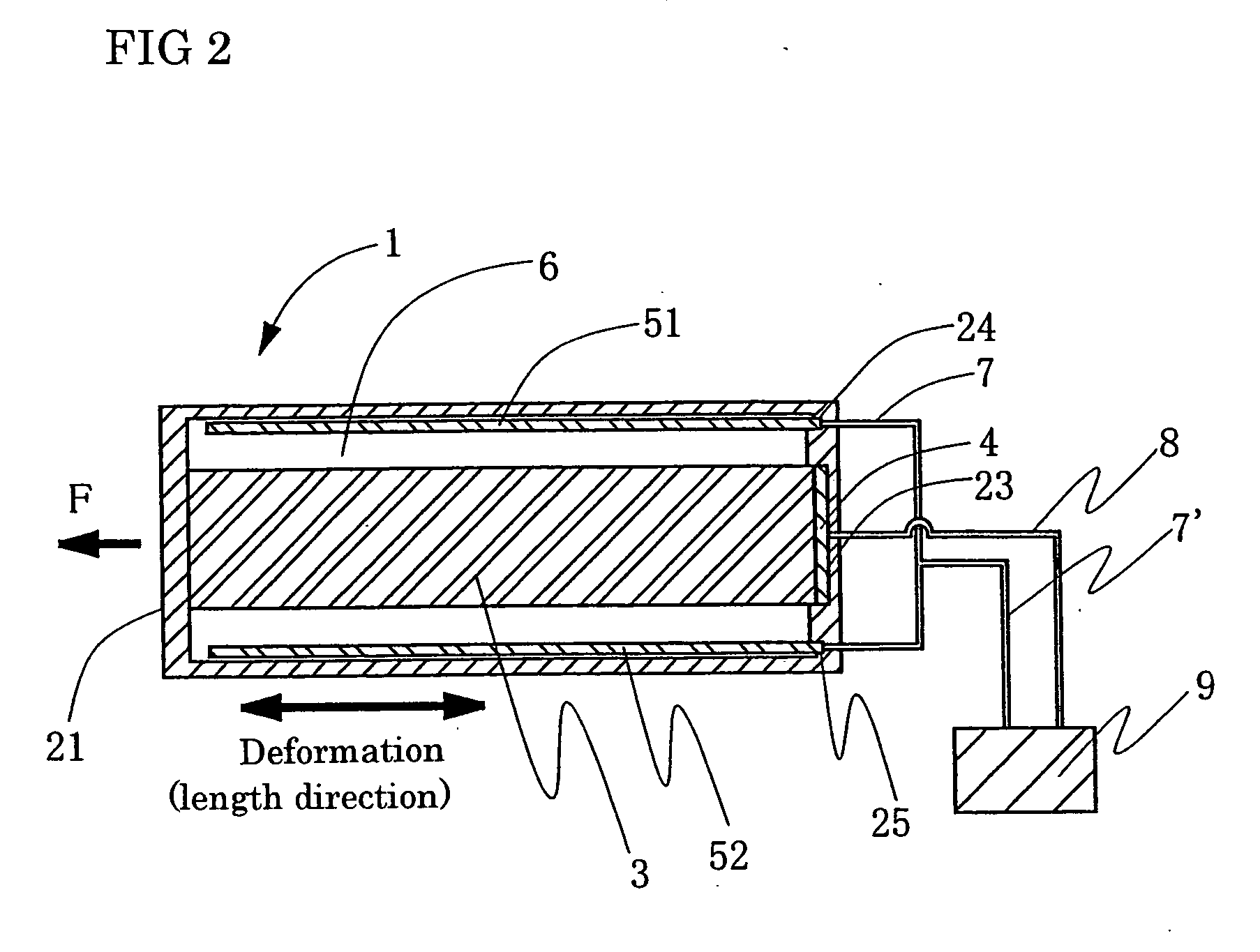
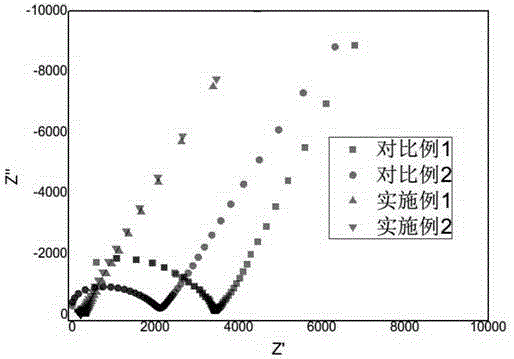
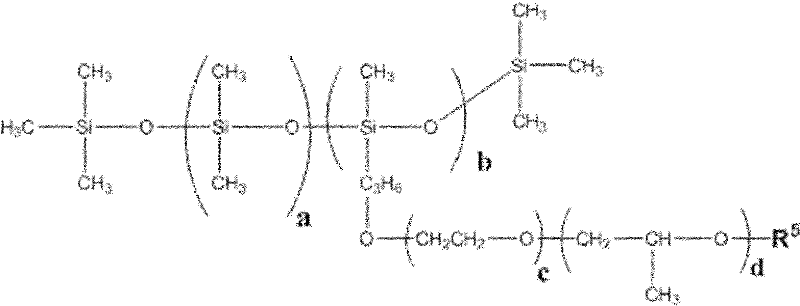
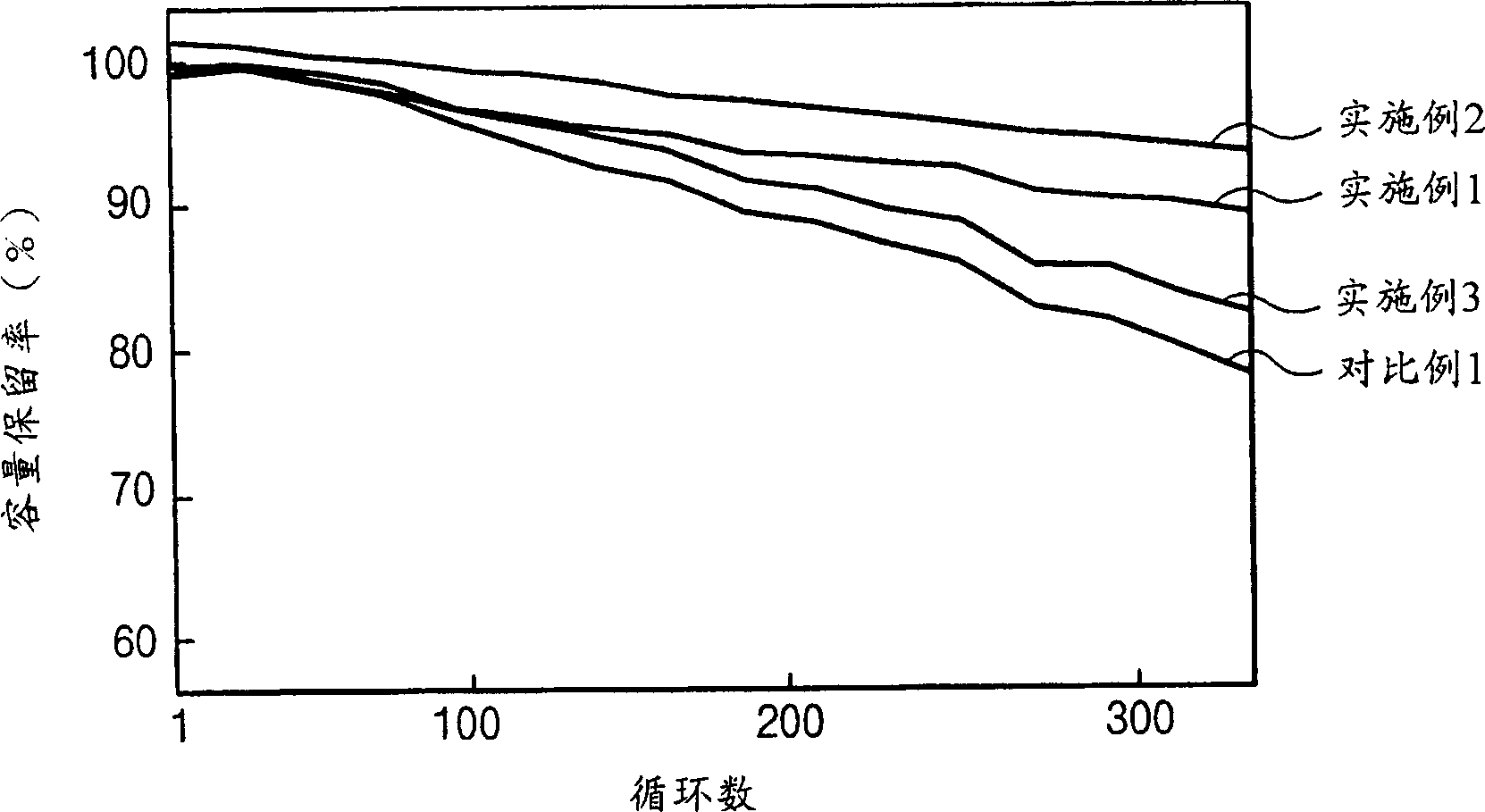
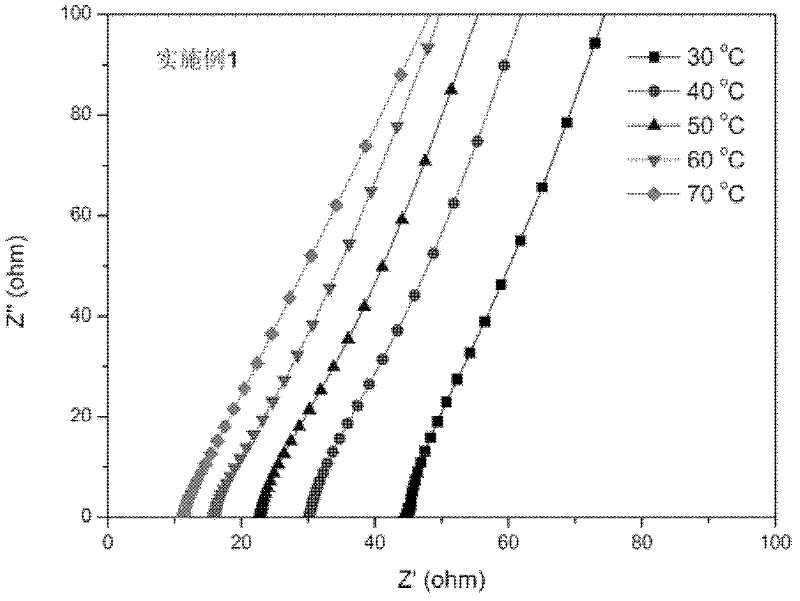
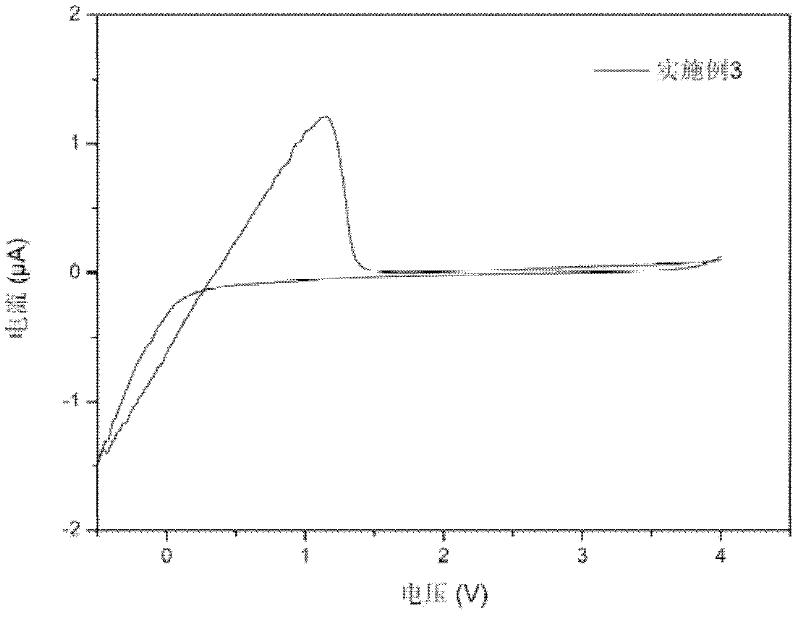
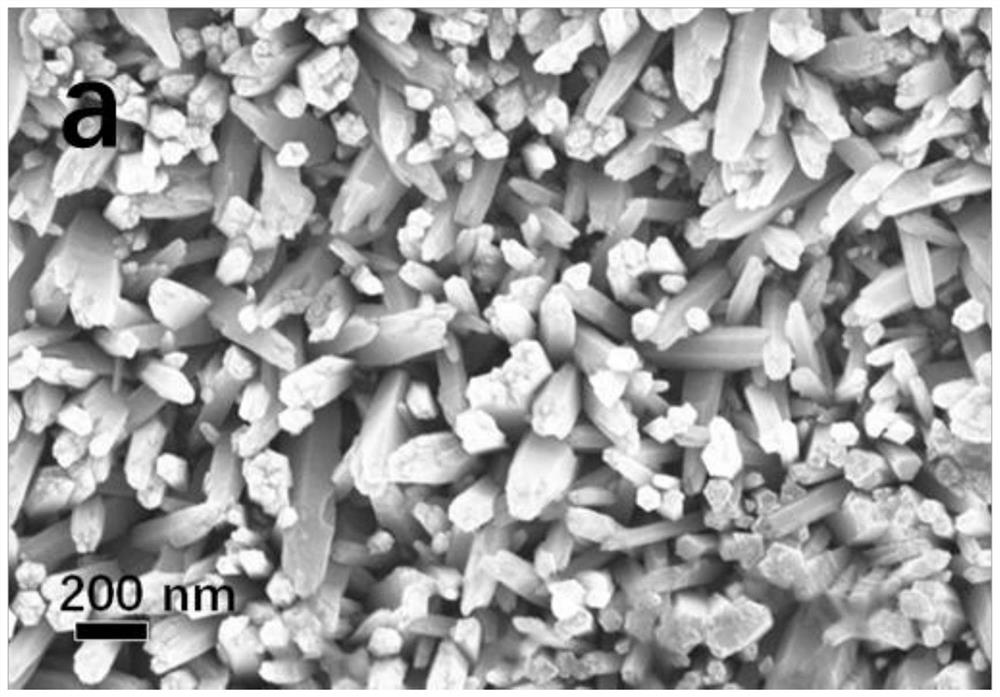
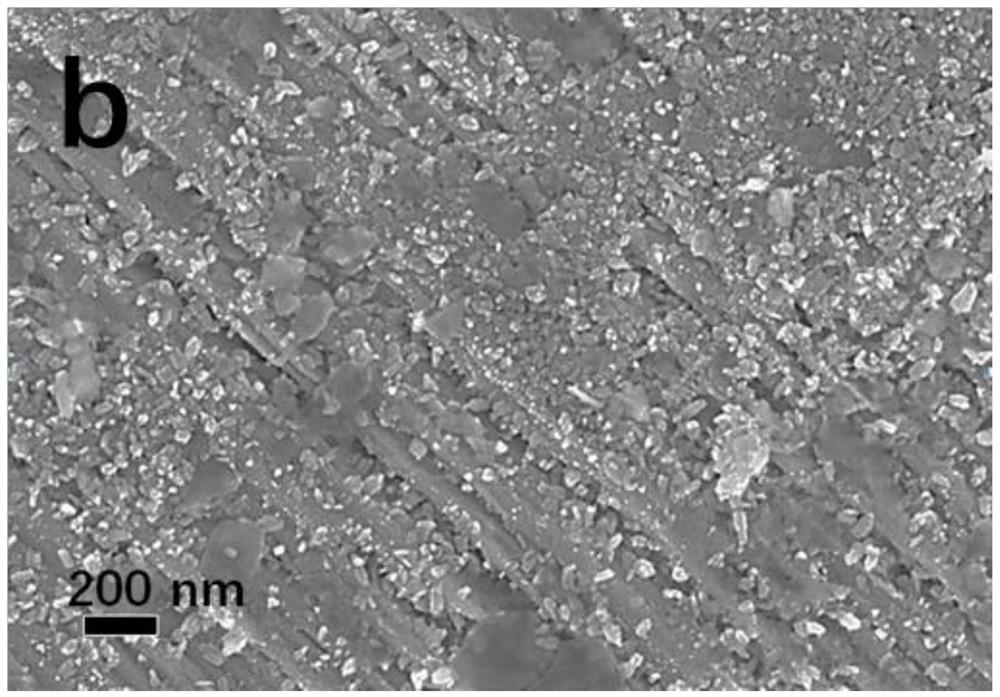
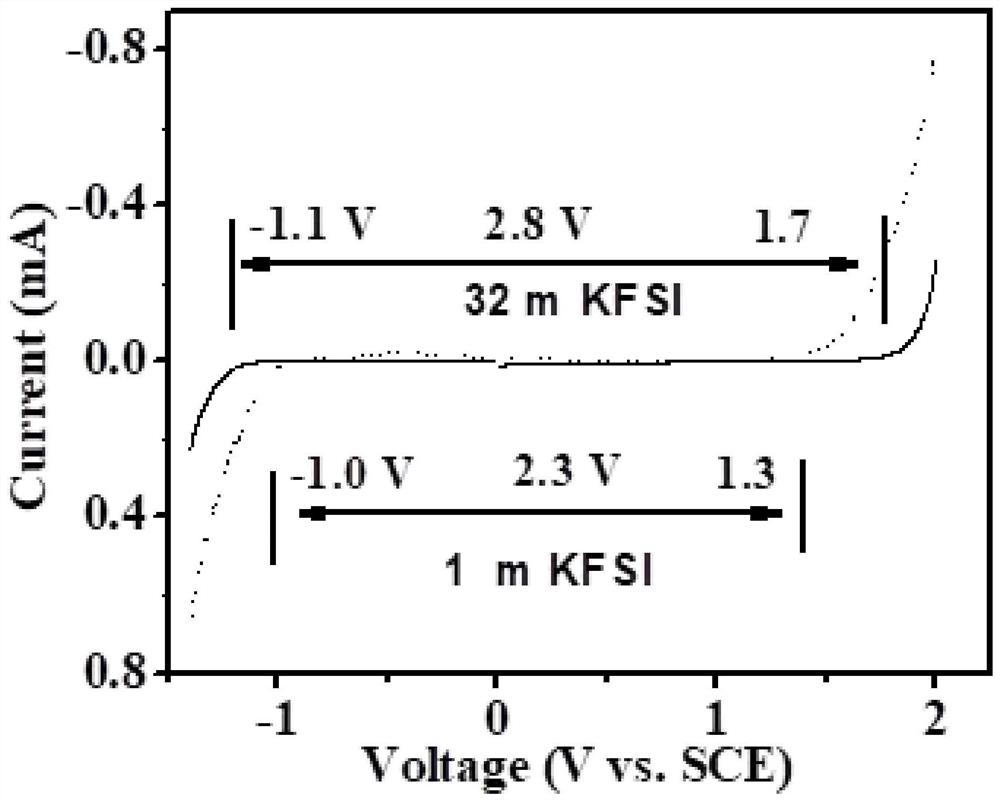
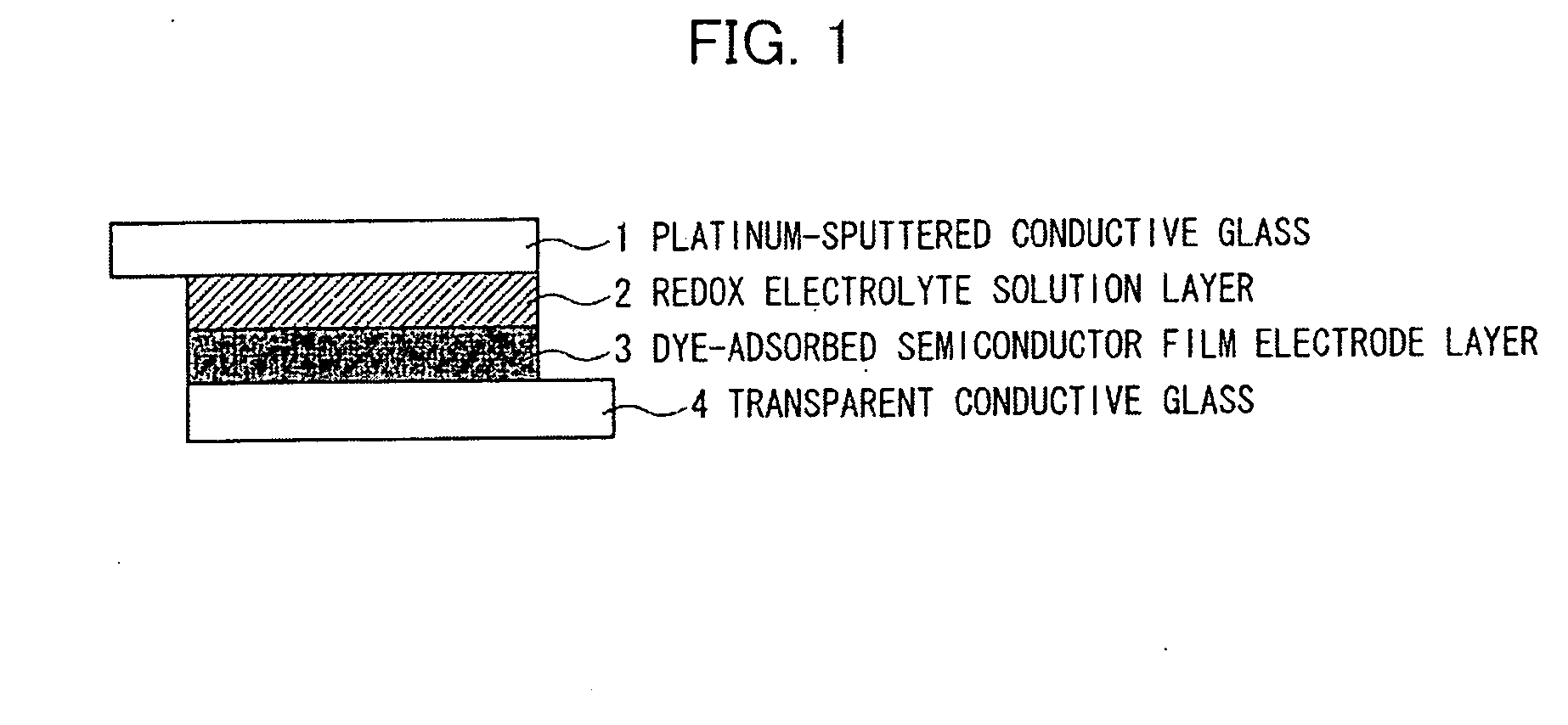
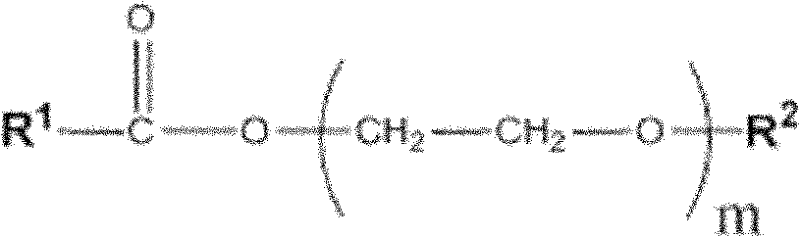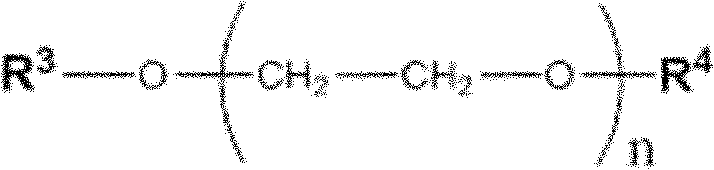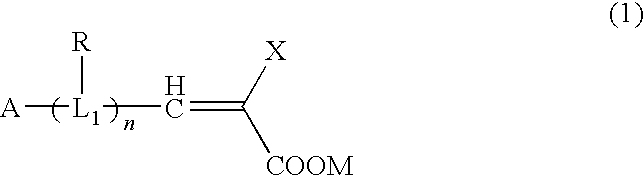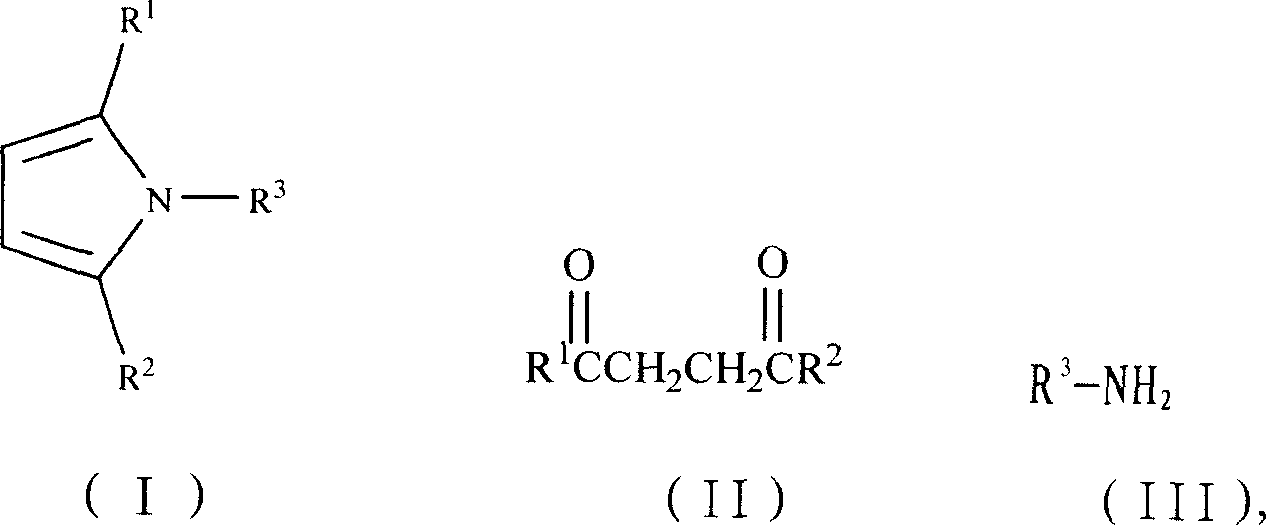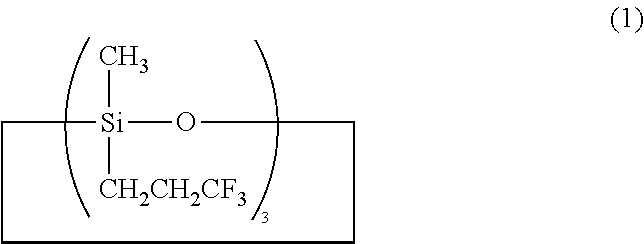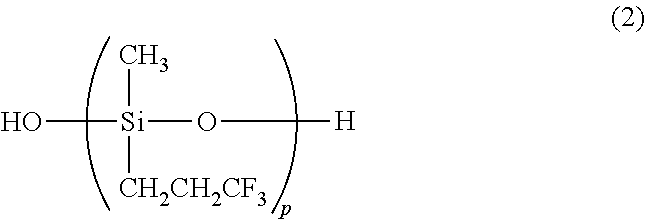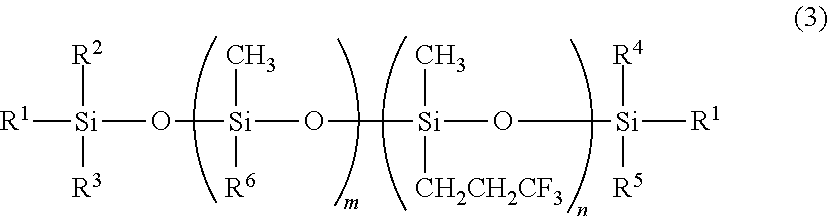Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
671 results about "Trifluoromethanesulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Triflate, also known by the systematic name trifluoromethanesulfonate, is a functional group with the formula CF₃SO₃−. The triflate group is often represented by −OTf, as opposed to −Tf (triflyl). For example, n-butyl triflate can be written as CH₃CH₂CH₂CH₂OTf.
Process for producing conductive polymer
A process for producing conductive polymers with excellent electrochemical strain per redox cycle is provided. A process for producing conductive polymers by an electrochemical polymerization method, wherein said conductive polymers have deformation property by electrochemical redox, said electrochemical polymerization method is a polymerization method using electrolyte including organic compounds as solvents, and wherein said organic compounds include (1) chemical bond species selected at least one from a group composed of the chemical bond consisting of ether bond, ester bond, carbon-halogen bond, and carbonate bond and / or (2) functional groups selected at least one from a group composed of functional groups consisting of hydroxyl group, nitro group, sulfone group, and nitryl group in a molecule, and said electrolyte includes anions which include trifluoromethanesulfonate ion and / or plural of fluorine atoms which bond to central atom is used.
Owner:EAMEX
All-solid polymer electrolyte, and preparation method and application of all-solid polymer electrolyte
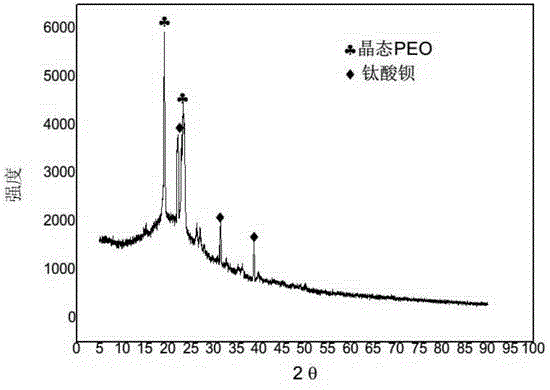
InactiveCN104538670AImprove conductivityHigh mechanical strengthSolid electrolytesFinal product manufacturePolyethylene oxideBarium titanate
The invention discloses an all-solid polymer electrolyte, and a preparation method and an application of the all-solid polymer electrolyte, and belongs to the field of lithium ion batteries. The all-solid polymer electrolyte comprises polyethylene oxide, lithium salt, inorganic nano particles and ion liquid, wherein a mass ratio of the lithium salt to polyethylene oxide is 0.1-0.5; the mass sum of the inorganic nano particles and the ion liquid is 10-30% of the mass of the all-solid polymer electrolyte; the lithium salt comprises one or several of bistrifluoromethane sulfonimide lithium salt, lithium tetrafluoroborate, lithium perchlorate, lithium hexafluorophosphate, lithium hexafluoroarsenate, lithium trifluoromethanesulfonate and lithium bis borate; and the inorganic nano particles comprise one or several of nano aluminum oxide, nano silicon oxide, nano zirconium oxide and nano barium titanate. The all-solid polymer electrolyte has better mechanical strength and higher ion conductivity. The method is simple in technology and low in cost; and raw materials are easy to obtain.
Owner:HUAZHONG UNIV OF SCI & TECH RES INST SHENZHEN
Sodium ion battery negative electrode sheet and sodium ion battery
InactiveCN104966813AEnhanced insertion siteIncrease profitCell electrodesSecondary cellsElectrolytic agentSodium tetrafluoroborate
The present invention discloses a sodium ion battery negative electrode sheet, the negative electrode sheet is a porous graphite film structure, the diameter of the pores is from 2 to 30 microns, the distance between the centers of the circles of the pores is 5 to 50 microns, mass ratio of carbon atoms in the porous graphite film is greater than 99%, the negative electrode sheet may be used directly as the sodium ion battery negative electrode sheet, can avoid the use of a conductive agent, a binder and a metal collector, and has high capacity, corrosion resistance and good conductivity. The present invention also discloses a sodium ion battery using the negative electrode sheet, the sodium ion battery comprises a positive electrode sheet, a negative electrode sheet, a separator and an electrolyte, the sodium ion battery electrolyte solvent is one or more than one of diethanol dimethyl ether, dimethyl ether tetraethanol, and tetrahydrofuran, the electrolyte is one of sodium perchlorate, sodium hexafluorophosphate, sodium tetrafluoroborate and sodium trifluoromethanesulfonate, and the sodium ion battery is simple in production process and good in charging and discharging cycle stability, and has good prospects in the new energy field.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Liquid Chemical for Forming Protecting Film
ActiveUS20120017934A1Improve waterproof performanceReduce capillary forceNon-ionic surface-active compoundsOther chemical processesTrimethylsilyl trifluoromethanesulfonateCompound a
Disclosed is a liquid chemical for forming a water-repellent protecting film at least on a surface of a recessed portion of an uneven pattern at the time of cleaning a wafer having a finely uneven pattern at its surface and containing silicon at least a part of the uneven pattern. This liquid chemical contains a silicon compound A represented by the general formula: R1aSi(H)bX4-a-b and an acid A, the acid A being at least one selected from the group consisting of trimethylsilyl trifluoroactate, trimethylsilyl trifluoromethanesulfonate, dimethylsilyl trifluoroactate, dimethylsilyl trifluoromethanesulfonate, butyldimethylsilyl trifluoroactate, butyldimethylsilyl trifluoromethanesulfonate, hexyldimethylsilyl trifluoroacetate, hexyldimethylsilyl trifluoromethanesulfonate, octyldimethylsilyl trifluoroactate, octyldimethylsilyl trifluoromethanesulfonate, decyldimethylsilyl trifluoroacetate and decyldimethylsilyl trifluoromethanesulfonate.
Owner:CENT GLASS CO LTD
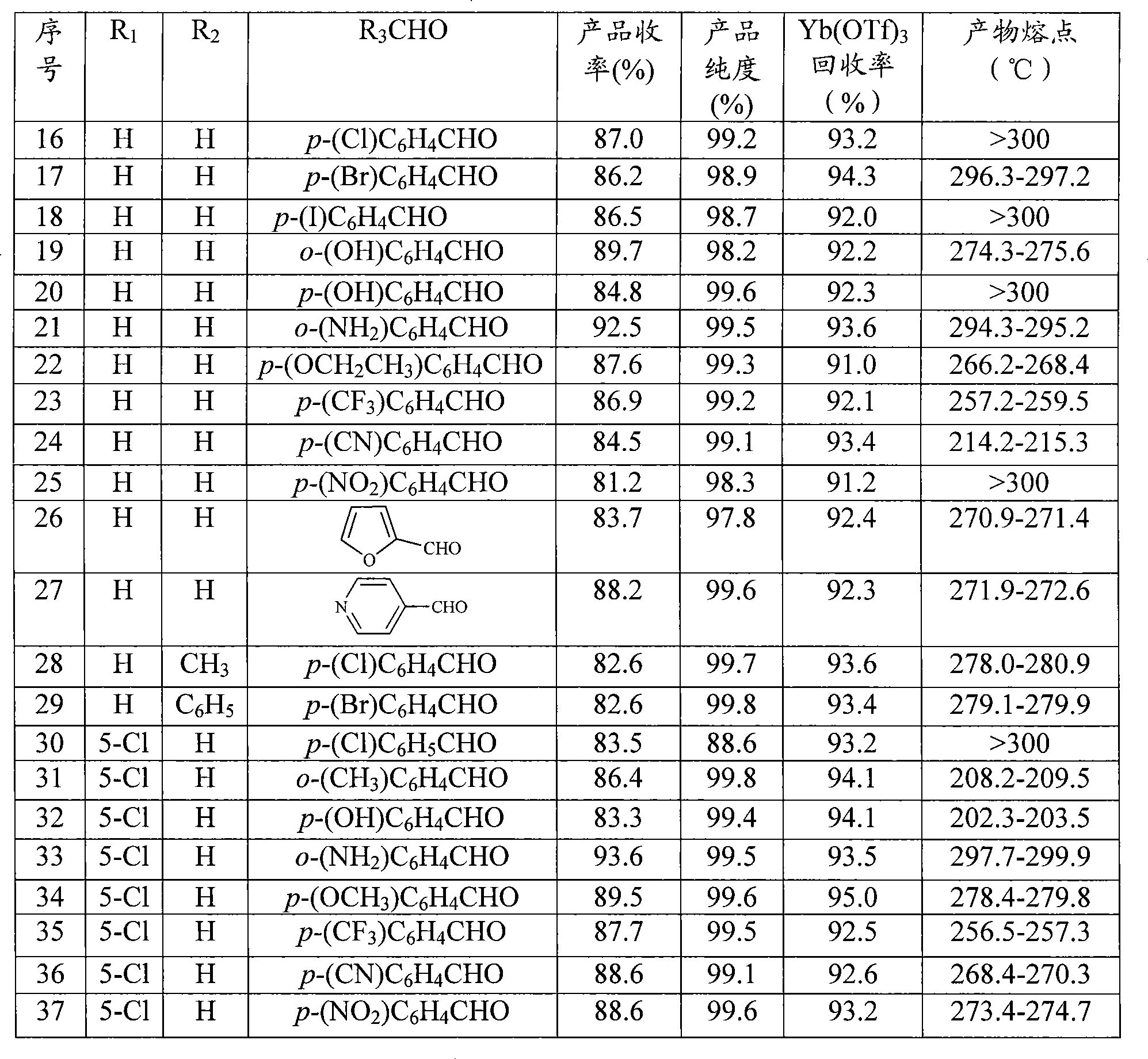
Synthesis of quinazoline ketone compounds
InactiveCN101429165AReduce dosageHigh reaction yieldOrganic chemistryChemical recyclingOrganic solventKetone
The invention provides a synthetic method for a quinazolinone compound. The structure of the quinazolinone compound is shown as formula (I). The method comprises the following steps: using o-amino benzamide compound shown in structural formula (II) and aldehyde shown in structural formula (III) as raw materials, using trifluoro mesylate as a catalyst, making the mixture reacted for 10 minutes to 24 hours in an inert organic solvent at a temperature of between 25 and 200 DEG C, and separating and purifying the reaction liquor after the reaction is finished to obtain the quinazolinone compound. The method has the advantages of high reaction yield up to more than 85 percent generally, advanced and reasonable process routes, mild reaction conditions, little dosage of the catalyst, reutilization, and substantially no three wastes.
Owner:WENZHOU UNIVERSITY
Electrolyte for power lithium ion battery
InactiveCN102544582AImprove wettabilityStable electrochemical propertiesSecondary cellsPolyolefinSolvent
The invention discloses high-wettability and high-pourability electrolyte for a power lithium ion battery. The electrolyte comprises a wetting additive, a lithium salt, a non-aqueous organic solvent and other additives, wherein the wetting additive is a nonionic surfactant, and the wetting additive accounts for 0.001 to 5 percent of the mass of the electrolyte; the non-aqueous solvent is one or a mixture of more of ethylene carbonate, propylene carbonate, methyl ethyl carbonate, dimethyl carbonate, diethyl carbonate and gamma-butyrolactone, and the non-aqueous solvent accounts for 50 to 90 percent of the mass of the electrolyte; the lithium salt at the concentration of 0.6 to 1.5 mol / L is at least one of lithium hexafluorophosphate, lithium tetrafluoroborate, lithium bis(oxalato)borate and lithium trifluoromethanesulfonate; and the other additives accounts for 0.5 to 8 percent of the mass of the electrolyte. The electrolyte has high wettability for a polyolefin diaphragm and electrode active materials, and has long cycle life.
Owner:DONGGUAN SHANSHAN BATTERY MATERIALS
Electrolyte for lithium secondary battery and lithium secondary battery comprising same
PURPOSE: Provided are a non-aqueous electrolyte capable of improving the electrochemical property, and a lithium secondary cell excellent in electrochemical property. CONSTITUTION: The electrolyte comprises a lithium salt; a non-aqueous, organic solvent; and at least one of organic compound selected from compounds of formula 1 to 4(wherein the formula 3 is R9-SO3-Si-(CmH2m+1)3 and formula 4 is CnX2n+1-SO3-Si(CmH2m+1)3). In the formulae, each of R1 to R9 independently represents primary, secondary, or tertiary alkyl group, alkenyl group or aryl group, X is hydrogen or halogen atom, and each of n and m is 0-3. The lithium salt is selected from the group consisting of lithium hexafluorophosphate(LiPF6), lithium tetrafluoroborate(LiBF4), lithium perchlorate(LiClO4), lithium trifluoromethanesulfonate(LiCF3SO3), lithium hexafluoroarsenate(LiAsF6), and mixture thereof.
Owner:SAMSUNG SDI CO LTD +1
Synthesizing method of polyimide
ActiveCN102219900AEasy to recycleImprove protectionFiber1-Butyl-3-methylimidazolium hexafluorophosphate
The invention discloses a synthesizing method of polyimide. The method comprises the following step that: an aromatic diamine compound and an aromatic dianhydride compound are subject to a polymerization reaction in an ionic liquid, such that polyimide is obtained. The ionic liquid can be any one of 1-ethyl-3-methylimidazolium tetrafluoroborate, 1-ethyl-3-methylimidazolium acetate, 1-ethyl-3-methylimidazolium dicyanamide, 1-butyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium hexafluorophosphate, 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, and 1-butyl-3-methylimidazolium trifluoromethanesulfonate. The operation of the method is simple, the product made with the method is easy to purify and has high dissolvability (the product can be dissolved in regular solvents of N, N-dimethylacetamide, or tetrahydrofuran). The method has advantages of high yield, stable performance, good application prospect, and is suitable for industrialized production. Withthe method, polyimide films or fibers can be produced.
Owner:BEIJING RADIATION APPL RES CENT
Nonaqueous electrolytic solution containing magnesium ions, and electrochemical device using the same
ActiveUS20110111286A1Improve featuresManufacturing environmentAlkaline accumulatorsElectrode carriers/collectorsElectrolytic agentTriflic acid
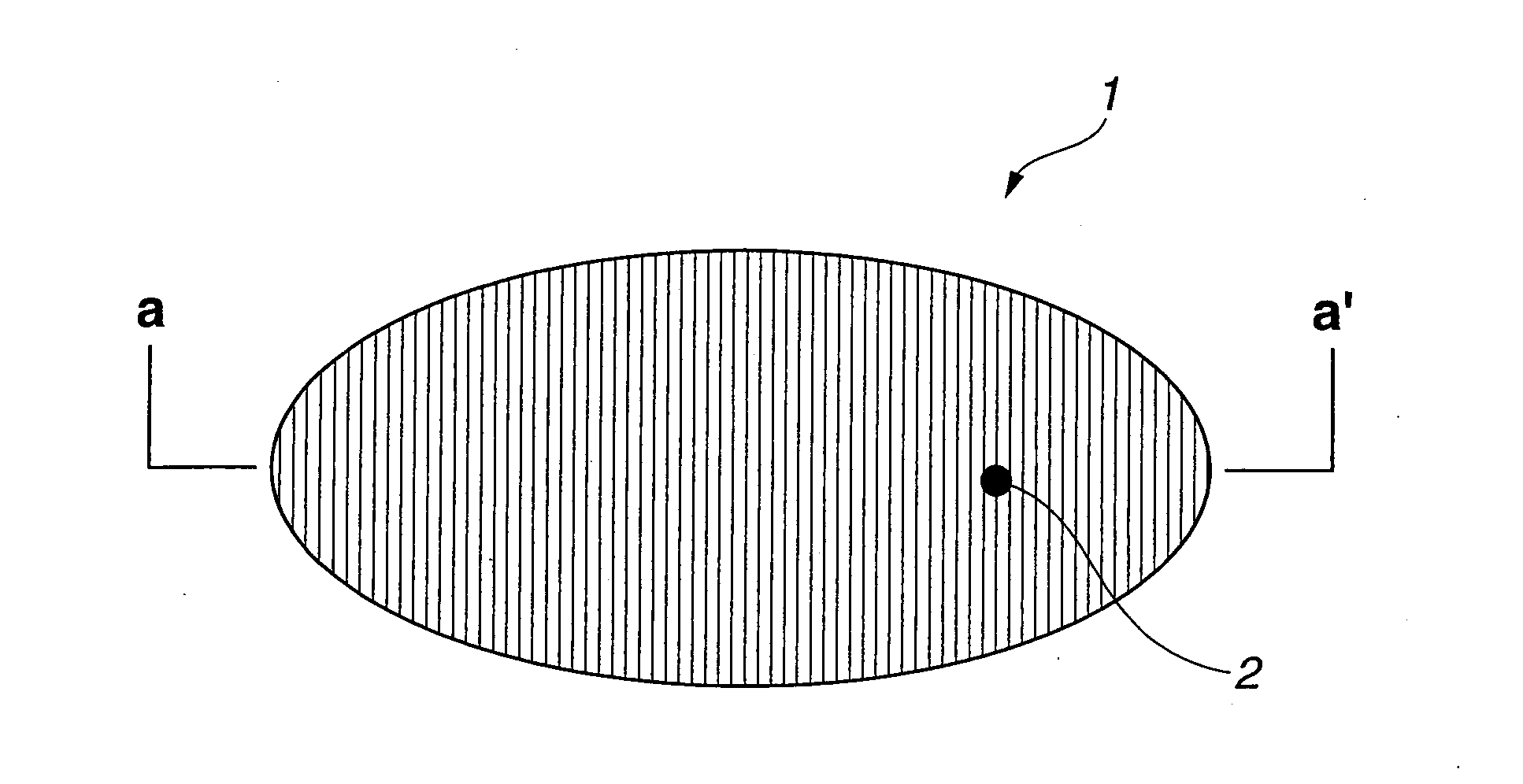
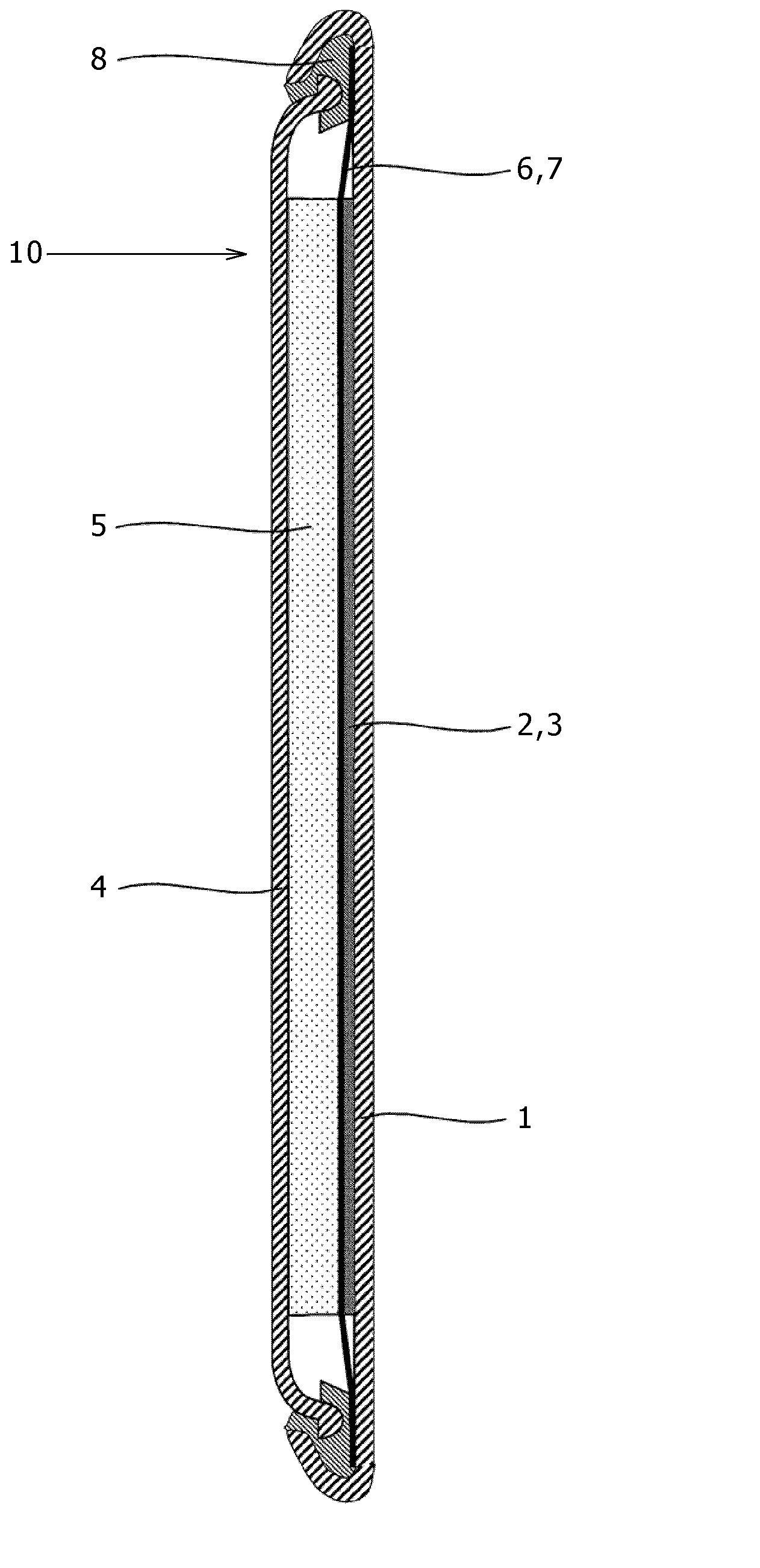
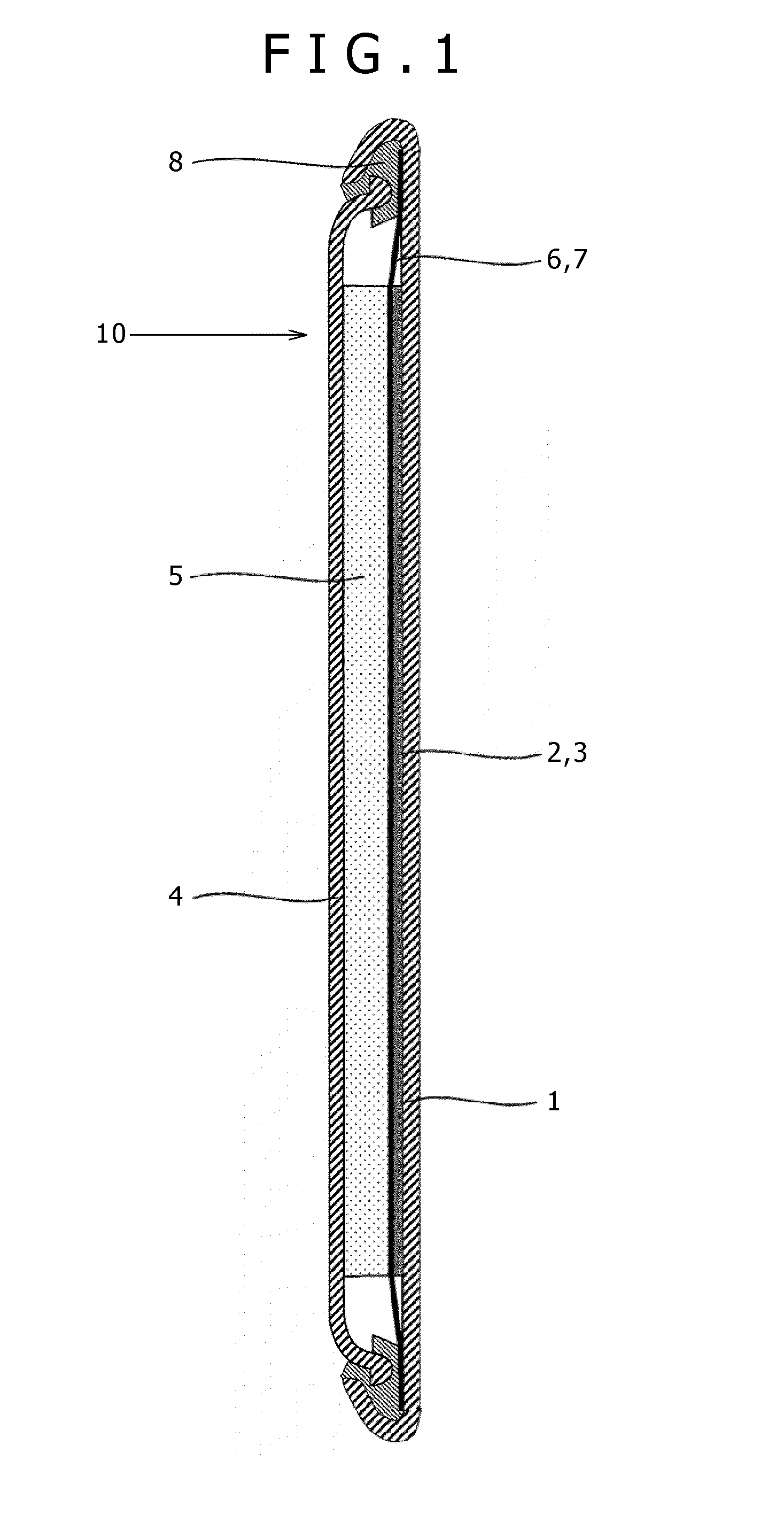
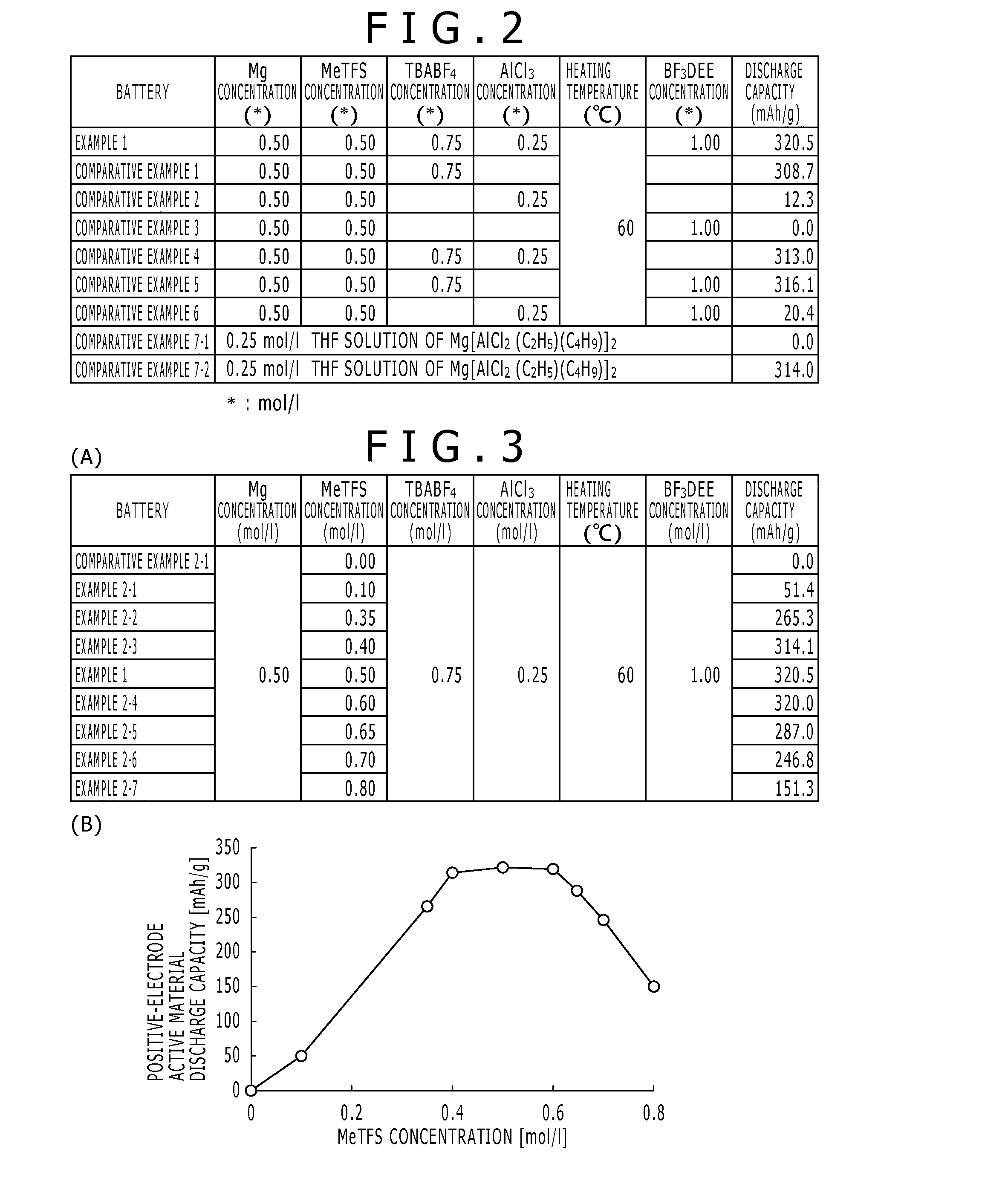
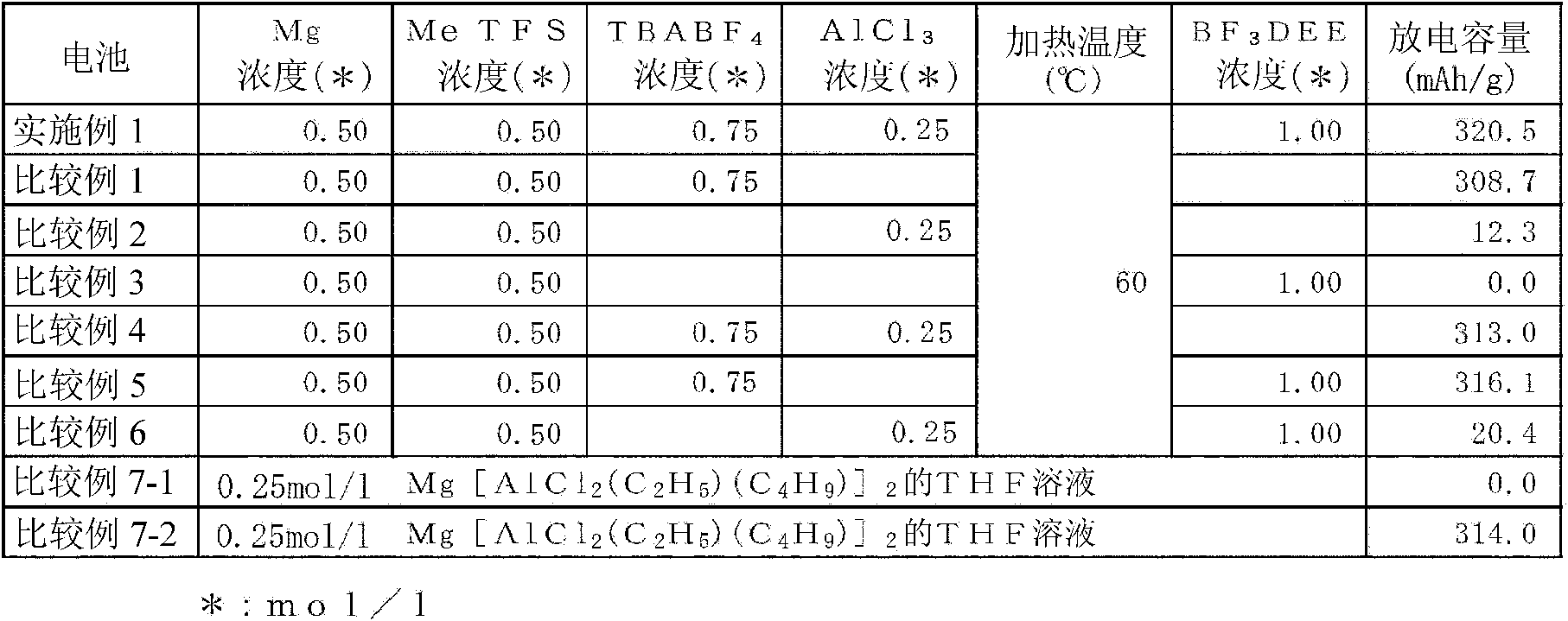
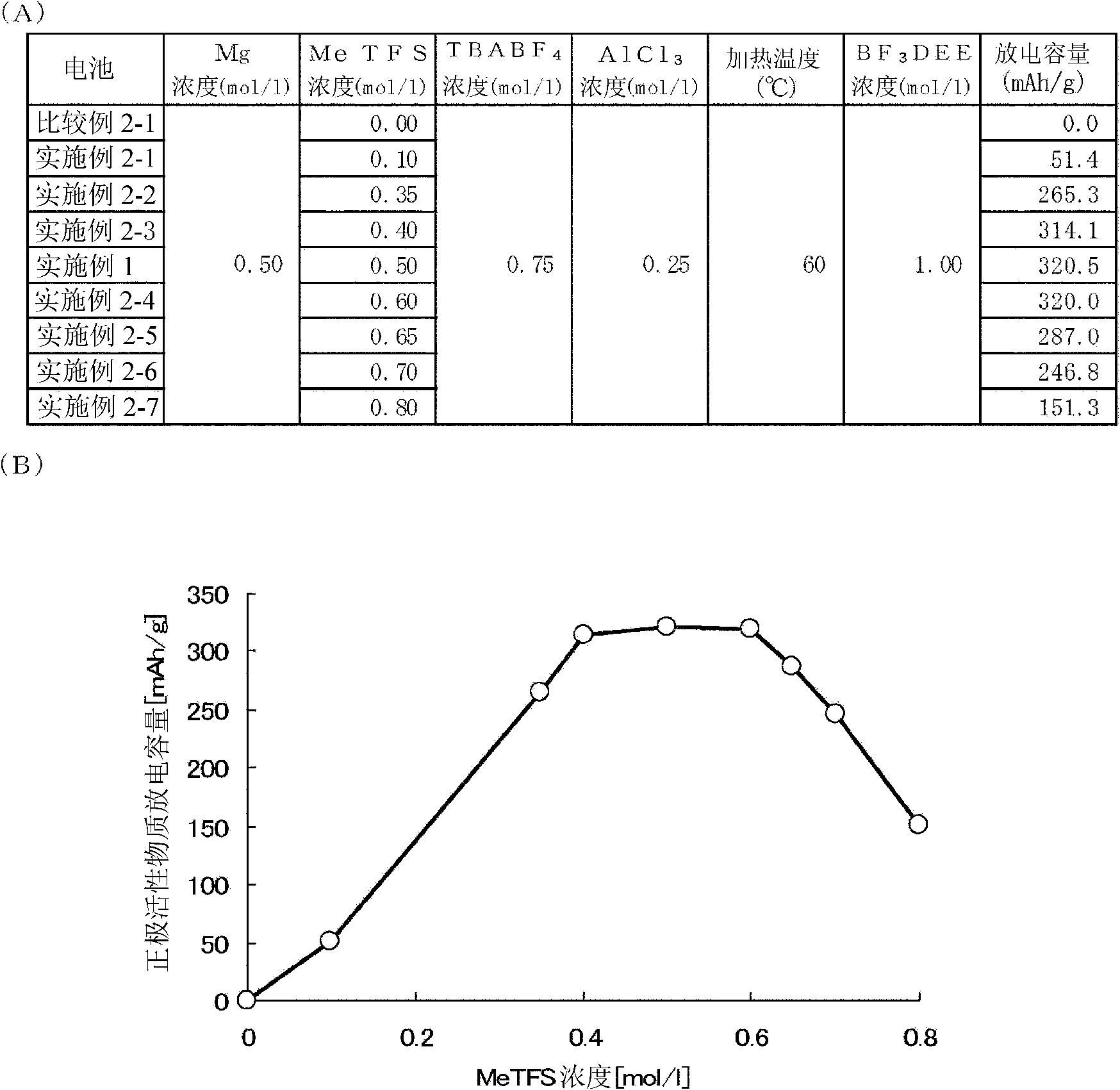
A nonaqueous electrolytic solution containing magnesium ions which shows excellent electrochemical characteristics and which can be manufactured in a general manufacturing environment such as a dry room, and an electrochemical device using the same are provided. A Mg battery has a positive-electrode can 1, a positive-electrode pellet 2 made of a positive-electrode active material or the like, a positive electrode 11 composed of a metallic net supporting body 3, a negative-electrode cup 4, a negative electrode 12 made of a negative-electrode active material 5, and a separator 6 impregnated with an electrolytic solution 7 and disposed between the positive-electrode pellet and the negative-electrode active material. Metal Mg, an alkyl trifluoromethanesulfonate, a quaternary ammonium salt or / and a 1,3-alkylmethylimidazolium salt, more preferably, an aluminum halide are added to an ether system organic solvent and are then heated, and thereafter, more preferably, a trifluoroborane-ether complex salt is added thereto, thereby preparing the electrolytic solution. By adopting a structure that copper contacts the positive-electrode active material, the electrochemical device can be given a large discharge capacity.
Owner:MURATA MFG CO LTD
Photo acid generator compounds, photo resists, and method for improving bias
InactiveUS6074800AEasy to controlImprove thermal stabilityOrganic chemistryOrganic compound preparationResistSulfonate
Several mid UV photo acid generators (PAGs), a chemically amplified photo resist (CAMP), and method for improving nested to isolated line bias are provided. Similarly, photo speed may also be improved. Unlike conventional mid UV PAGs, the present invention's PAG compounds, resist composition, and method do not require a mid UV sensitizer. Specifically, PAGs are provided that bear a chromophore capable of receiving mid UV radiation, particularly I-line, and that are suitable for use in a chemically amplified photo resist having a photo speed of 500 mJ / cm2 or less, but preferrably 200 mJ / cm2 or less. For example, the PAGs can be a sulfonium or iodonium salt, such as anthryl, butyl, methyl sulfonium triflate and bis(4-t-butylphenyl)iodonium 9,10-dimethoxyanthracene sulfonate. The chromophore forming a part of the PAGs can be selected from polyaromatic hydrocarbons, for example, chrysenes, pyrenes, fluoranthenes, anthrones, benzophenones, thioxanthones, anthracenes, and phenanthrenes, but preferably anthracenes.
Owner:GLOBALFOUNDRIES INC
Magnesium ion-containing nonaqueous electrolyte solution and electrochemical device using the same
InactiveCN102047491AImprove featuresImprove discharge capacityPrimary cellsElectrode carriers/collectorsOrganic solventTriflic acid
Disclosed is a magnesium ion-containing nonaqueous electrolyte solution having excellent electrochemical characteristics, which can be produced in a general production environment such as a dry room. An electrochemical device using the magnesium ion-containing nonaqueous electrolyte solution is also disclosed. An Mg battery comprises a positive electrode can (1), a positive electrode pellet (2) composed of a positive electrode active material and the like, a positive electrode (11) composed of a metal mesh support (3), a negative electrode cup (4), a negative electrode (12) composed of a negative electrode active material (5), and a separator (6) impregnated with an electrolyte solution (7) and arranged between the positive electrode pellet and the negative electrode active material. The electrolyte solution is prepared by adding and heating Mg metal, an alkyl trifluoromethanesulfonate, a quaternary ammonium salt or / and 1,3-alkylmethylimidazolium salt, preferably an aluminum halide in an ether organic solvent, and then adding a trifluoroborane-ether complex salt thereinto. Since the positive electrode active material is in contact with copper, a large discharge capacity can be obtained.
Owner:SONY CORP
Preparation method of trifluoro methanesulfonic anhydride
ActiveCN102911086AHigh yieldReduce contentOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of trifluoro methanesulfonic anhydride. The method comprises the following steps of: firstly reacting trifluoro methanesulphonyl fluoride with alkali metal hydroxide to prepare trifluoro mesylate, purifying trifluoro mesylate by recrystallization by utilizing an organic solvent, reacting trifluoro methane sulfonyl chloride with trifluoro mesylate to generate a trifluoro methanesulfonic anhydride crude product, and finally purifying trifluoro methanesulfonic anhydride by atmospheric distillation. The preparation method of trifluoro methanesulfonic anhydride can be used for not only effectively simplifying reaction steps so that the operation process is simple and convenient and the operation is safe, but also avoiding byproducts generated in the process of the traditional method for producing trifluoro methanesulfonic anhydride, and effectively reducing the contents of F<-> and SO4<2-> in the product; by utilizing recrystallization, atmospheric distillation and other methods for purification, the product purity is up to 99.5%; and more importantly, the yield of anhydride is greatly increased and raised to 88% from original 60%.
Owner:JIANGXI GUOHUA IND CO LTD
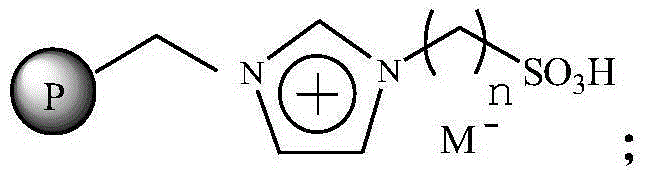
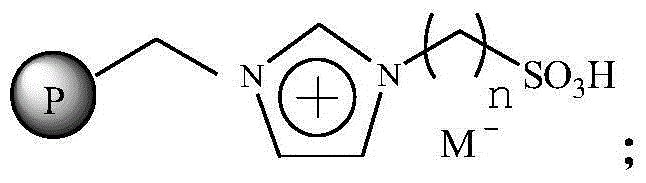
Immobilized ionic liquid catalyst and application thereof
InactiveCN106391112AHigh catalytic activityNot easy to inactivateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateTriflic acid
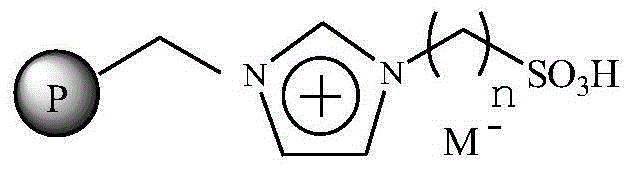
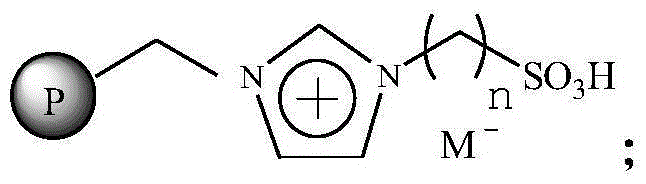
The invention relates to an immobilized ionic liquid catalyst and application thereof. The immobilized ionic liquid catalyst has a general structural formula as defined in the specification. In the general structural formula, P is a nanogel resin matrix; n is an integer in a range of 2 to 12; and M<-> is a negative ion selected from a group consisting of a trifluoromethanesulfonate group, a p-toluenesulfonate group, a benzenesulfonate group, a methanesulfonate group, a tetrafluoroborate group and a hexafluorophosphate group. The immobilized ionic liquid catalyst can be applied to industrial olefine acid addition for preparation of corresponding esters.
Owner:CHINA PETROLEUM & CHEM CORP +1
Lithium ion battery electrolyte and lithium ion battery
InactiveCN105449275AImprove securityImprove thermal stabilitySecondary cells servicing/maintenanceTriflic acidSulfonyl fluoride
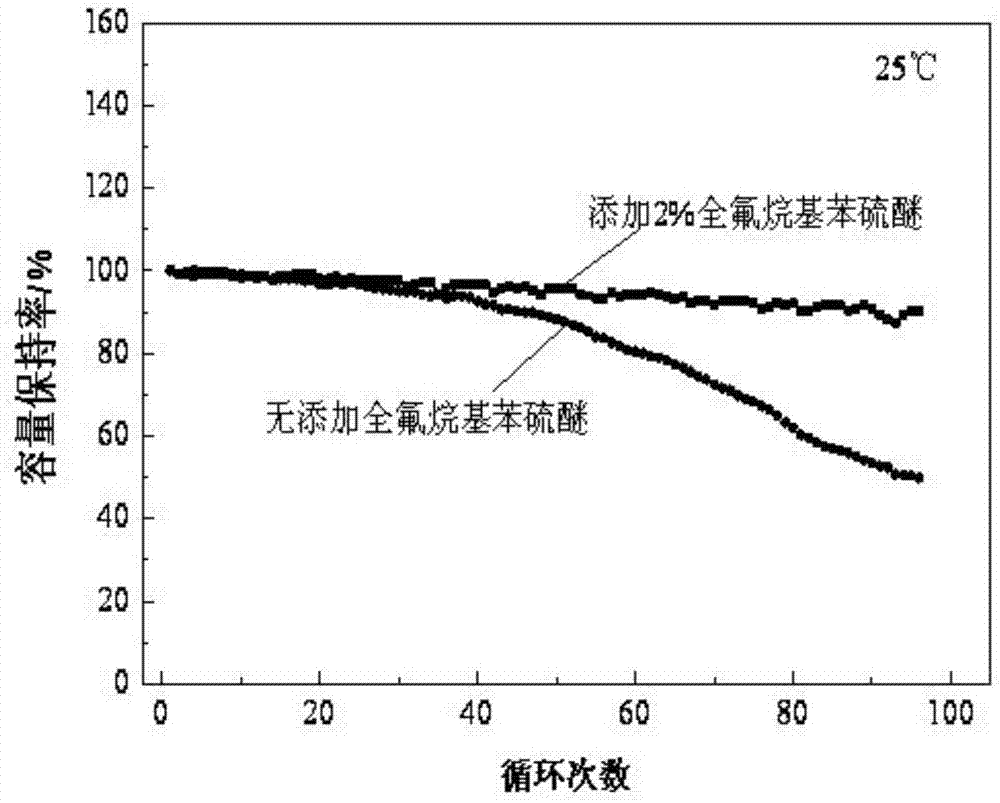
The invention discloses a lithium ion battery electrolyte and a lithium ion battery. The lithium ion batter electrolyte comprises non-aqueous organic solvent, lithium salt, a functional additive, a flame-retardant additive and a negative electrode film-forming agent. According to the scheme of the lithium ion batter electrolyte, perfluoroalkyl diphenyl sulfide is used as the functional additive on the basis of reasonably optimizing the non-aqueous organic solvent, the lithium salt and the negative electrode film-forming agent; the problem that the novel lithium salt corrodes an aluminium current collector can be effectively solved, wherein the novel lithium salt comprises lithium trifluoromethanesulfonate (LiCF3SO3), perfluoroalkyl sulfonyl lithium methide (LiC (CF3SO2)3, bi(trifluoromethyl sulfonyl) lithium imide (LTFSI), bi(sulfonyl fluoride) lithium imide (LiFSI) and the like; the cycling performance of the lithium ion battery is improved; the LiPF6 can be well replaced by the lithium ion battery electrolyte, and the lithium ion battery electrolyte can be widely applied to the secondary lithium ion battery electrolyte, and particularly suitable for lithium ion power batteries for improving the thermal stability of the lithium ion power batteries.
Owner:OPTIMUM BATTERY CO LTD
Mixtures of ionic liquids with lewis acids
ActiveUS20060264642A1Increase percentageLow applicabilityLiquid degasificationOrganic compound preparationImideAcid catalyzed
Ionic liquids comprising a mixture of one or more triflate or bis(trifluoromethylsulfonyl)imide salt(s) with one or more Lewis acids(s), wherein the total of the molar contents of the Lewis acid(s) in the mixture is from about 0.01-98%, are provided, that are useful as catalysts in Lewis acid catalyzed reactions.
Owner:NOVARTIS AG
Gel-type polymer electrolyte and preparation method thereof
The invention relates to a gel-type polymer electrolyte and a preparation method thereof. The gel-type polymer electrolyte membrane is prepared by using amine terminated nitrile rubber as a matrix of the solid electrolyte, dissolving the matrix, lithium salt, epoxidized polysilsesquioxane, and 1-butyl-3-methylimidazolium trifluoro methane sulfonate in an organic solvent, pouring the solvent on a teflon template, and carrying out heat treatment in a baking oven after volatilizing tetrahydrofuran. Compared with the prior art, the operation is simple; the membrane forming is easy; and the prepared membrane is smooth and uniform, has good mechanical properties, high room-temperature conductivity, and stable electrochemical window, and the prepared membrane used as solid electrolyte materials for the lithium ion battery has an application prospect.
Owner:SHANGHAI JIAO TONG UNIV
Aqueous zinc ion battery electrolyte and application thereof
PendingCN111900497AReduce solubilityImprove cycle lifeFinal product manufactureSecondary cells servicing/maintenanceElectrolytic agentHigh concentration
The invention discloses an aqueous zinc ion battery electrolyte and application thereof. The aqueous zinc ion battery electrolyte disclosed by the invention contains solvent water, high-concentrationelectrolyte salt and zinc salt;the zinc salt is a water-soluble salt; the high-concentration electrolyte salt is potassium bis(fluorosulfonyl) imide and / or potassium trifluoromethanesulfonate, and themass molar concentration is not less than 10mol / kg. According to the electrolyte, due to the fact that high-concentration potassium bis(fluorosulfonyl) imide and / or potassium trifluoromethanesulfonate are / is added, a large number of water molecules in the electrolyte can be consumed due to the strong solvation effect, the hydration effect of zinc ions is reduced, and zinc dendrites formed by thezinc ions in dissolution and deposition are inhibited; in addition, the strong solvation effect of the high-concentration electrolyte salt and solvent water molecules can reduce the electrochemical activity of the water molecules, improve the electrochemical window of the electrolyte, reduce the hydrogen evolution reaction, reduce the dissolution effect of zinc, inhibit the decomposition of the water molecules on the surface of the electrode, reduce the corrosion dissolution of the zinc negative electrode and prolong the cycle life of the aqueous zinc ion battery.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
PBS (Polybuthylenesuccinate) /PLA (Polylactic Acid)/PHA (Polyhydroxyalkanoate) biodegradable composite material and preparation method thereof
The invention discloses a PBS (Polybuthylenesuccinate) / PLA (Polylactic Acid) / PHA (Polyhydroxyalkanoate) biodegradable composite material and a preparation method thereof. The PBS / PLA / PHA composite material is prepared from the following components in parts by weight: the PLA, the PBS, the PHA, citric acid, rubidium benzoate, bismuth trifluoromethanesulfonate, erucyl amide, filler, a compatilizer, a plasticizer, an antioxidant and an antibacterial agent. By reasonably selecting the materials and proportioning, the PBS / PLA / PHA composite material prepared by the preparation method disclosed by the invention plays the characteristics of respective properties of the PBS, the PLA and the PHA, and has higher impact-resisting strength, tensile strength and elongation at break, good comprehensive mechanical property, long service life and wide application range.
Owner:王泽陆
Organic compound, semiconductor film electrode employing the organic compound, photoelectric conversion element employing the organic compound, and photoelectrochemical solar cell employing the organic compound
InactiveUS20100174095A1Improve photoelectric conversion efficiencyIncrease heightMethine/polymethine dyesOrganic chemistryTriflic acidCarboxylic acid
The present invention provides: an organic compound increasing an open circuit voltage, and showing high photoelectric conversion efficiency; a semiconductor film electrode employing the organic compound as a dye; a photoelectric conversion element employing the semiconductor film electrode; and a photoelectrochemical solar cell employing the photoelectric conversion element. The organic compound is represented by the following general formula:wherein A is a carbazole ring; L1 is an electron transfer linking group having at least one heterocyclic ring selected from the group consisting of a thiophene ring, a furan ring, a pyrrole ring, and a condensed heterocyclic ring formed from any combinations of these rings; R is a substituent group bound to at least one electron transfer linking group selected from the group consisting of an alkyl group, an alkoxy group, and an aryl group; X is at least one electron withdrawing group selected from the group consisting of a cyano group, a carboxylic acid group, an ester group, an amide group, a trifluoromethyl group, a pentafluoroethyl group, a sulfonate group, and a trifluoromethanesulfonate group; M is a hydrogen atom or a salt-forming cation; and n is an integer of 1 to 12.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method for electrodepositing metal lanthanum in ionic liquid
The invention provides a method for electrodepositing metal lanthanum in an ionic liquid, relates to a method for electrodepositing metal lanthanum, and is used for solving the problem that a single metal lanthanum deposition layer can not be obtained in the ionic liquid 1-butyl-3-methylimidazolium trifluoro-methanesulfonate currently. The method comprises the following steps: adding anhydrous lanthanum chloride in the ionic liquid 1-methyl-3-ethyllimidazolium di(trifluoromethylsulfonyl)imine and evenly mixing, so as to obtain an electroplating liquid; and electroplating by using a constant current mode based on pretreated copper sheet as a cathode and platinum, graphite or titanium-based oxide as an anode, so as to obtain metal lanthanum deposited on the surface of a substrate. According to the invention, a La plated layer prepared by adopting an electrodeposition method is smooth and even, and the plating liquid components are simple and easy to control. In the invention, electrodeposited metal lanthanum can be applied to the field of functional material preparation.
Owner:HARBIN INST OF TECH
Hybrid polymer light-emitting devices
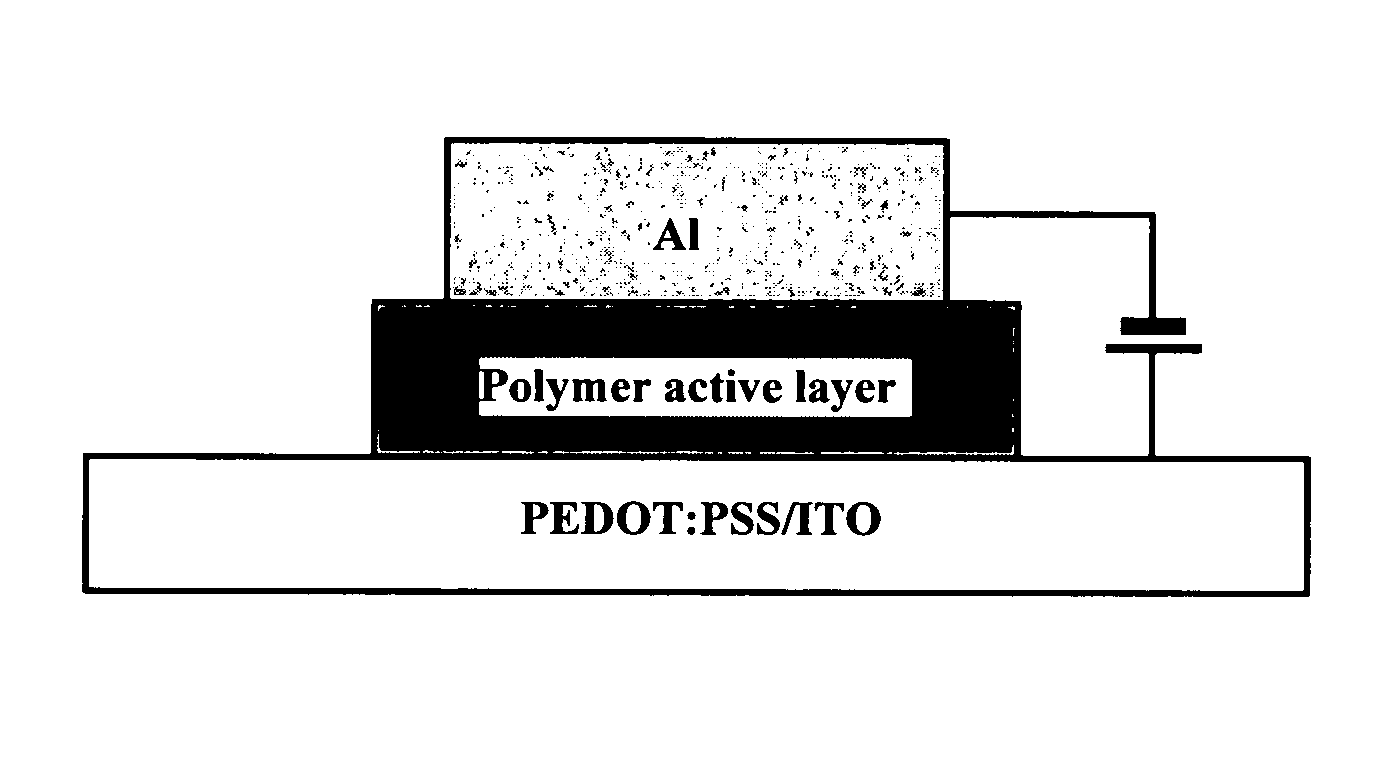
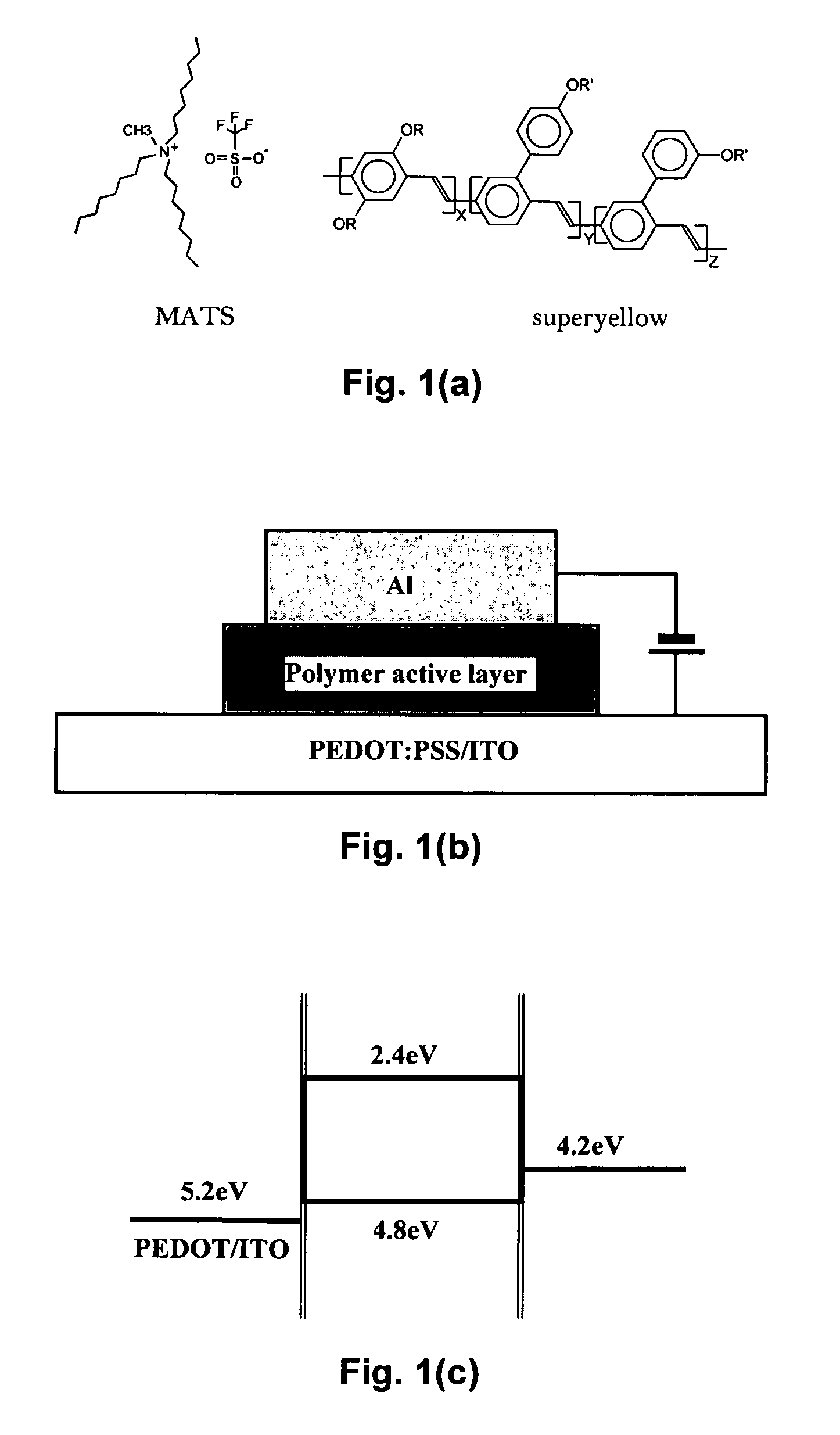
InactiveUS20080084158A1Maintain good propertiesShort response timeDischarge tube luminescnet screensLamp detailsPhotoelectrochemical cellLuminescent polymers
Mixtures and light-emitting devices that incorporate such mixtures are disclosed in which a soluble phenyl-substituted poly(para-phenylene vinylene) (PPV) copolymer (“superyellow”) is used as the host light-emitting polymer and methyltrioctylammonium trifluoromethanesulfonate, an ionic liquid, is used to introduce a dilute concentration of mobile ions into the emitting polymer layer. These mixtures and devices incorporating them are able to combine some of the characteristics of polymer light emitting diodes (PLEDs) and polymer light-emitting electrochemical cells (PLECs).
Owner:RGT UNIV OF CALIFORNIA
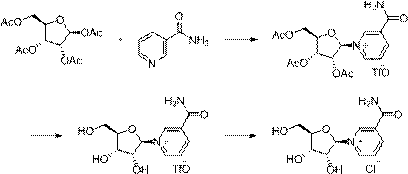
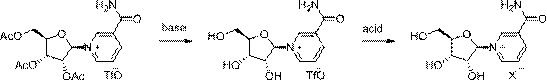
Method for preparing niacinamide nucleoside salt
InactiveCN108774278AAvoid degradationEasy to operateSugar derivativesSugar derivatives preparationSulfonateOrganic solvent
The invention relates to a method for preparing niacinamide nucleoside salt. The method comprises the following steps: replacing ions by niacinamide nucleoside trifluoromethane sulfonate prepared by deacetylation of triacetyl niacinamide nucleoside trifluoromethane in an organic solvent with acids, crystallizing to separate out a crude product, and pulping, thereby obtaining the corresponding niacinamide nucleoside salt. The ion replacement process in the invention overcomes the defects of chemical methods and extraction methods, and is suitable for industrial production.
Owner:张洪喜
Immobilized ionic liquid catalyst and application thereof
InactiveCN106391115AHigh catalytic activityNot easy to inactivateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTetrafluoroborateTriflic acid
The invention relates to an immobilized ionic liquid catalyst and application thereof. The immobilized ionic liquid catalyst has a general structural formula as defined in the specification. In the general structural formula, P is a nanometer macroporous resin matrix; n is an integer in a range of 2 to 12; M<-> is a negative ion selected from a group consisting of a trifluoromethanesulfonate group, a p-toluenesulfonate group, a benzenesulfonate group, a methanesulfonate group, a tetrafluoroborate group and a hexafluorophosphate group; and the nanometer macroporous resin matrix is a nanometer macroporous copolymer prepared from styrene monomer, copolymerization monomer, a graphene material and a pore-forming agent through in-situ copolymerization. The immobilized ionic liquid catalyst can be applied to industrial olefine acid addition for preparation of corresponding esters.
Owner:CHINA PETROLEUM & CHEM CORP +1
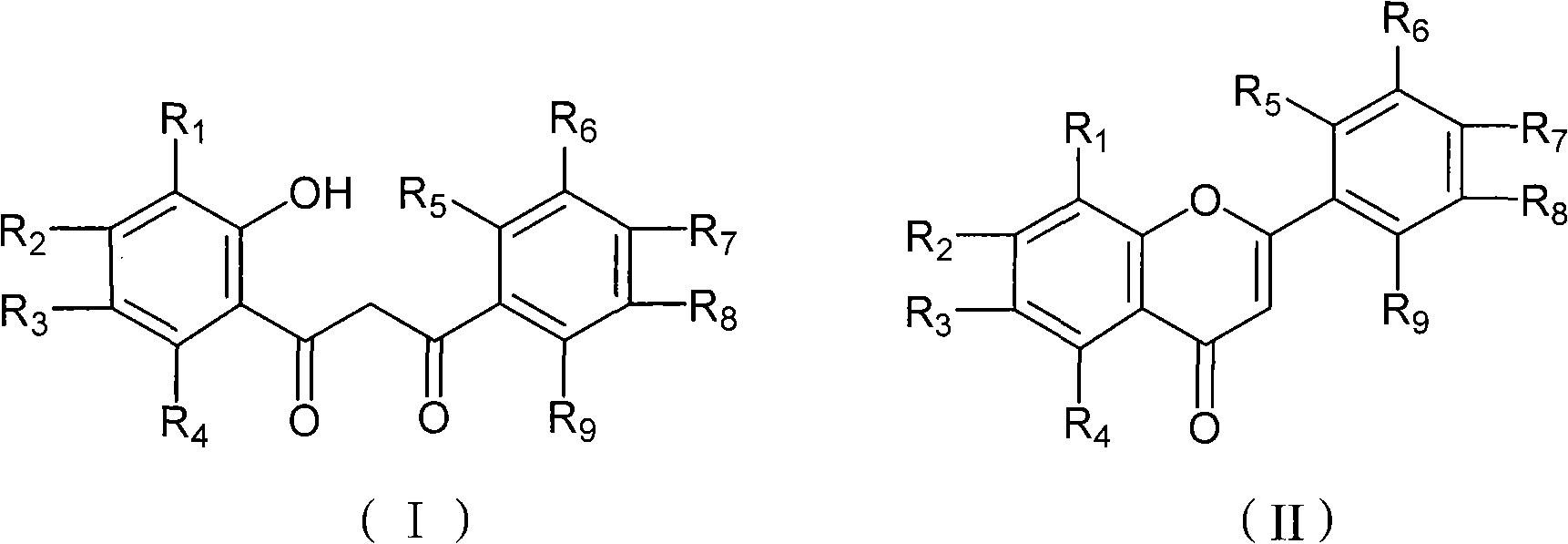
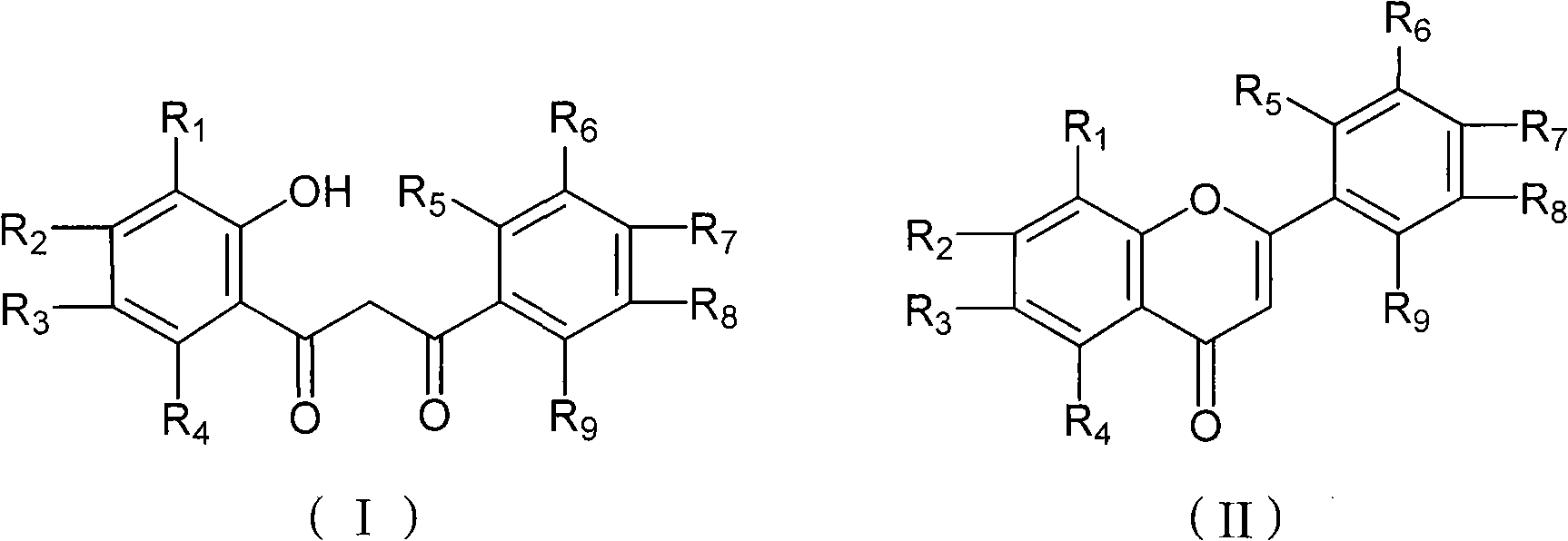
Chemical synthesis method of flavonoid compound
InactiveCN101555240AHigh reaction yieldReasonable process routeOrganic chemistryChemical recyclingChemical synthesisOrganic solvent
The invention discloses a chemical synthesis method of a flavonoid compound shown by a formula (II). The synthesis method comprises the following steps : under the catalysis of trifluoromethanesulfonic salt, a beta-propanedione derivant shown by a formula (I) fully reacts in an organic solvent under temperature of 0 DEG C to 100 DEG C, and the flavonoid compound shown by the formula (II) is obtained after reaction solution is separated and purified. The synthesis method has the advantages of reasonable technology, low production cost, simple operation, high reaction yield, little catalyst amount, environment protection, and has a favorable popularization and application prospect; in addition, the catalyst can be recycled and reused.
Owner:WENZHOU UNIVERSITY
Synthesis process of N-substituted pyrrole
InactiveCN1931839AAdvanced and reasonable process routeMild reaction conditionsOrganic chemistryRare-earth elementDiketone
The present invention is green synthesis process of N-substituted pyrrole as shown in expression I. The N-substituted pyrrole is prepared with diketone as shown in expression II and amine as shown in expression III as materials, the trifluoro mesylate of RE element or transition element as catalyst, and the reactant as solvent or added organic solvent, and through reaction at 0-150 deg.c for 10 min to 24 hr, filtering the reacted liquid to obtain filter cake, dissolving the filter cake in water and further filtering out the insoluble matter as the N-substituted pyrrole compound. The present invention has high reaction yield over 85 %, reasonable technological path, mild reaction condition, reusable catalyst consumption and no wastes produced basically.
Owner:WENZHOU UNIVERSITY
Lithium ion battery flame-retardant electrolyte
ActiveCN106025344AGuaranteed cycle performanceImprove conductivitySecondary cellsTetrafluoroborateTriflic acid
The invention discloses lithium ion battery flame-retardant electrolyte which comprises raw materials of an electrolyte lithium salt, an organic solvent and a flame-retardant additive, wherein the organic solvent is prepared from the following components in parts by volume: 15-40 parts of an organic basic solvent with a high dielectric constant, 5-45 parts of a low-vapor pressure solvent, 10-55 parts of a low-viscosity solvent and 2-35 parts of an indoor temperature ionic liquid; the anion of the indoor temperature ionic liquid is prepared from at least one ion of tetrafluoroborate, hexafluoroborate, trifluoromethanesulfonate, bi-trifluoro sulfonamide and trifluoroacetic acid; the cation of the indoor temperature ionic liquid is ammonium cation; the flame-retardant additive is at least one of fluoroproplylene carbonate, di-fluoroproplylene carbonate, trifluoroproplylene carbonate, fluorobenzene ether, fluoro ether and propyl-2,2,2-trifluoro-ethyl carbonate; the mass of the flame-retardant accounts for 1-20% of the total mass of the electrolyte. By adopting the lithium ion battery flame-retardant electrolyte, not only is the flame-retardant property of the electrolyte remarkably improved, but also the charge and discharge property of a battery is slightly affected.
Owner:DONGFENG COMML VEHICLE CO LTD
Preparation of triorganosiloxy end-capped organopolysiloxane
A triorganosiloxy end-capped organopolysiloxane, i.e., organopolysiloxane having a backbone consisting essentially of repeating (3,3,3-trifluoropropyl)methylsiloxane units and end-capped with triorganosiloxy groups is prepared by copolymerizing tris(3,3,3-trifluoropropyl)trimethylcyclo-trisiloxane with a silanol end-capped organopolysiloxane in the presence of an alkyllithium or lithium silanolate catalyst to form a silanol end-capped copolymer, and adding a trialkylsilyl triflate or strong acid in excess relative to the catalyst and a hexaorganodisilazane for effecting end-capping and neutralization.
Owner:SHIN ETSU CHEM IND CO LTD
Purrocoline derivative and synthetic method and application thereof
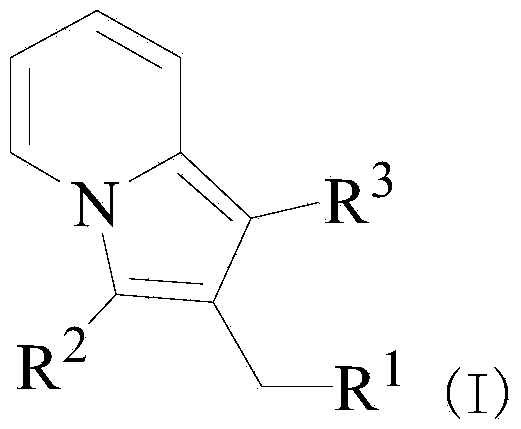
InactiveCN103641827ANovel structureReduced responseOrganic active ingredientsOrganic chemistrySynthesis methodsTriflic acid
The invention discloses a purrocoline derivative and a synthetic method and application thereof. The purrocoline derivative with a novel structure can be prepared by reacting propargyl alcohol and a 2-alkyl pyridine compound by taking samarium trifluoromethanesulfonate as a catalyst under the condition without a solvent; the reaction consumption and the chemical contamination are reduced, the cost is reduced and the operation is simplified; the obtained purrocoline derivative has better activity on a gastric cancer cell strain MGC80-3. The structural formula of the purrocoline derivative is shown in a formula (I) in the specification, wherein R<1> is phenyl, 4-methoxyphenyl, 4-fluorophenyl and 2-chlorphenyl or thienyl, R<2> is phenyl, and R<3> is H, phenyl, methyl, a nitrile group or an ethyl acetate group.
Owner:GUANGXI NORMAL UNIV
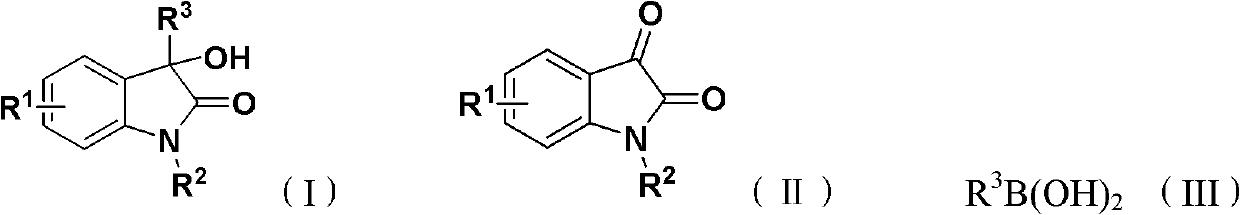
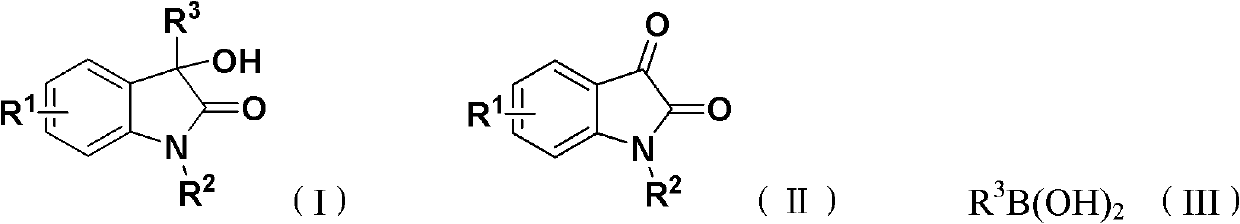
Method for synthesizing 3-hydroxy-3-arylindole-2-one derivative
The invention discloses a method for synthesizing a 3-hydroxy-3-arylindole-2-one derivative shown as a structural formula (I), which comprises the following steps of: fully reacting raw materials such as an isatin compound shown as a structural formula (II) and arylboric acid shown as a structural formula (III) in an inert organic solvent in the presence of a copper catalyst, a nitrogen-containing bidentate ligand and an alkaline compound, and separating and purifying reaction liquid after the reaction is finished to obtain the 3-hydroxy-3-arylindole-2-one derivative, wherein the copper catalyst is one or any combination of copper trifluoromethanesulfonate, copper acetylacetonate, copper acetate, cuprous iodide, copper bromide, copper fluoride and copper chloride. The method is high in implementation value and good in social benefit and economic benefit.
Owner:WENZHOU UNIVERSITY
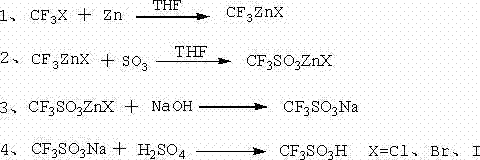
Trifluoromethanesulfonic acid preparation method
ActiveCN104725283AHigh yieldAvoid it happening againSulfonic acid preparationDistillationTriflic acid
Owner:JIANGXI GUOHUA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com