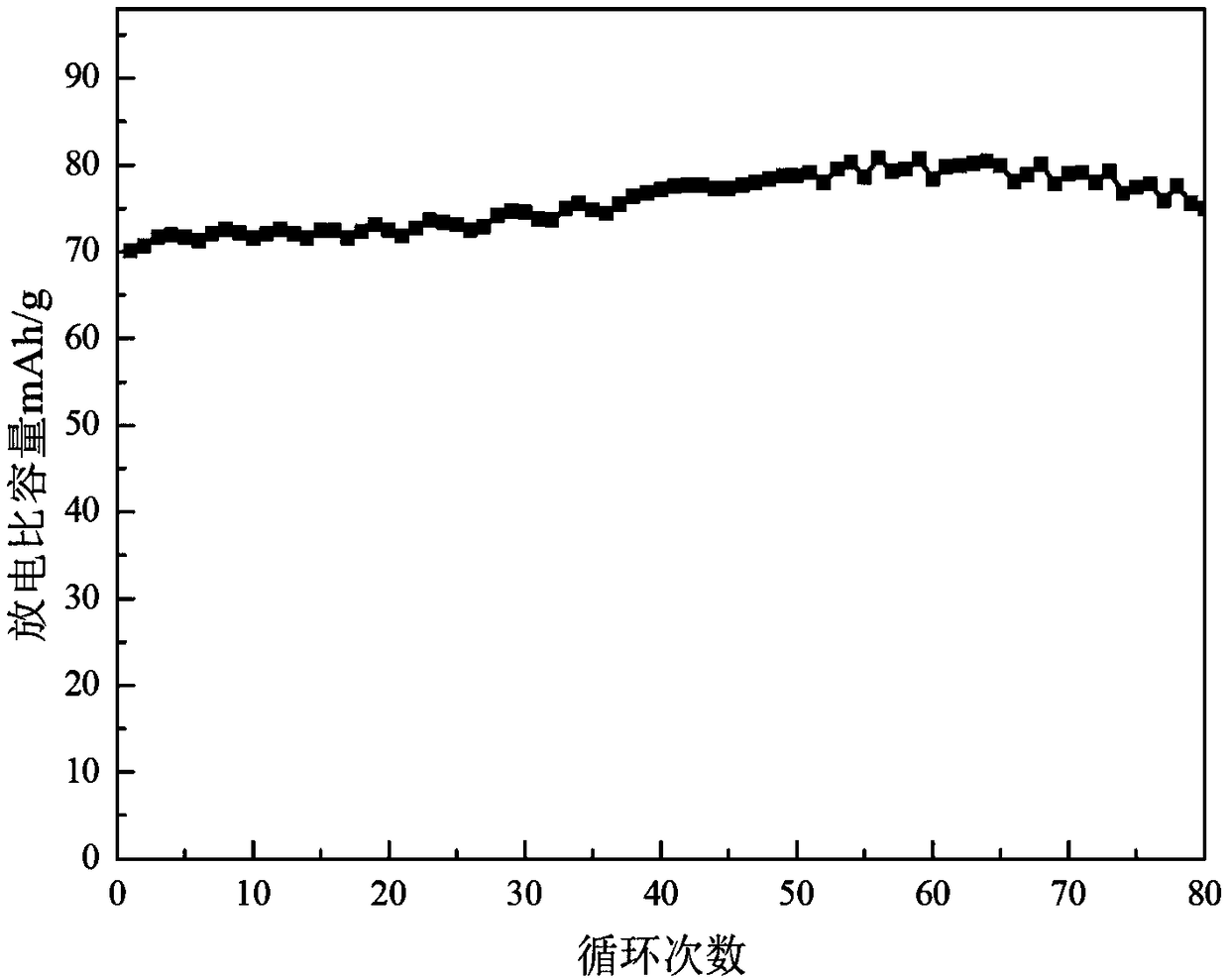
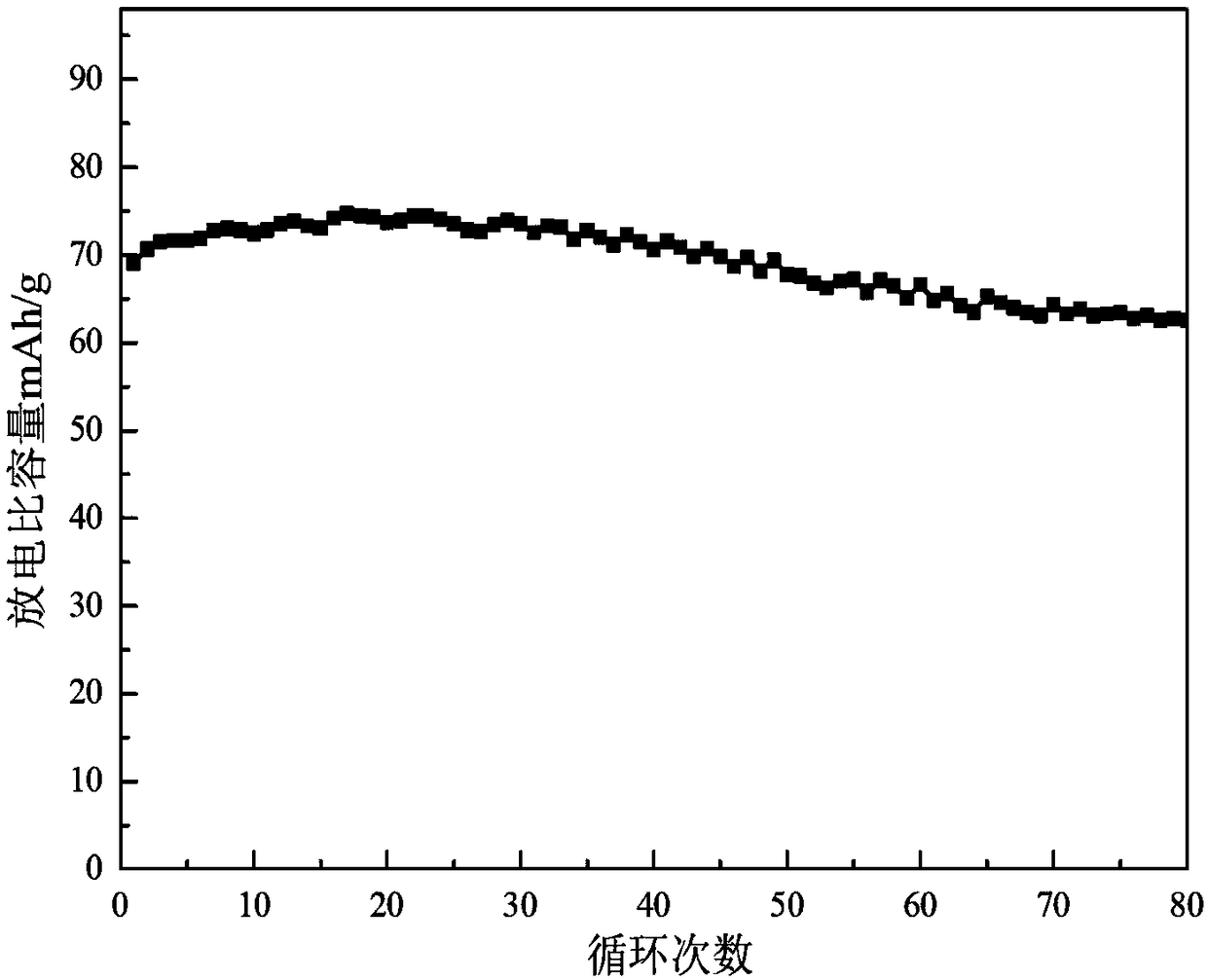
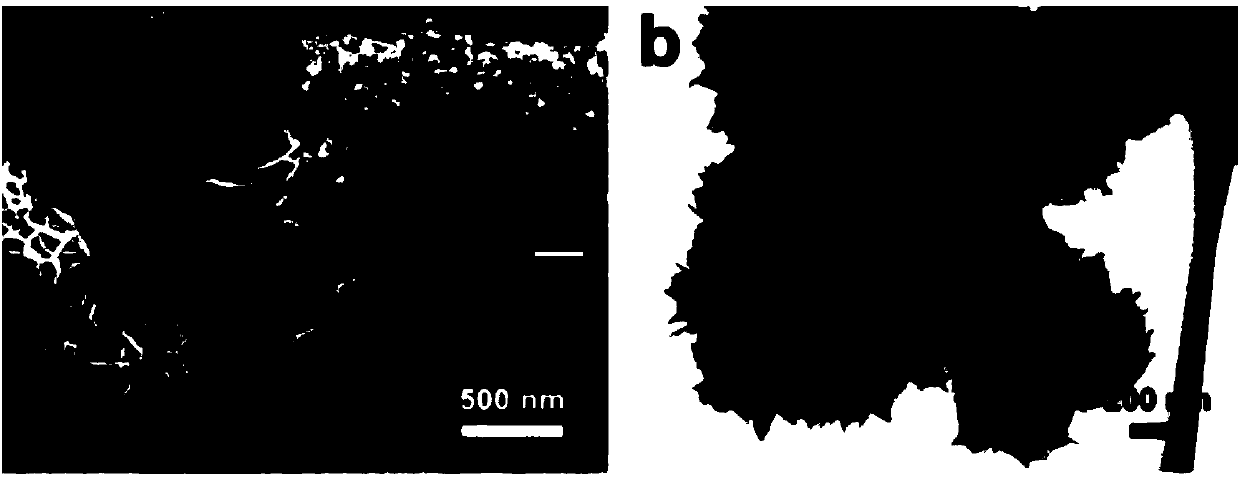
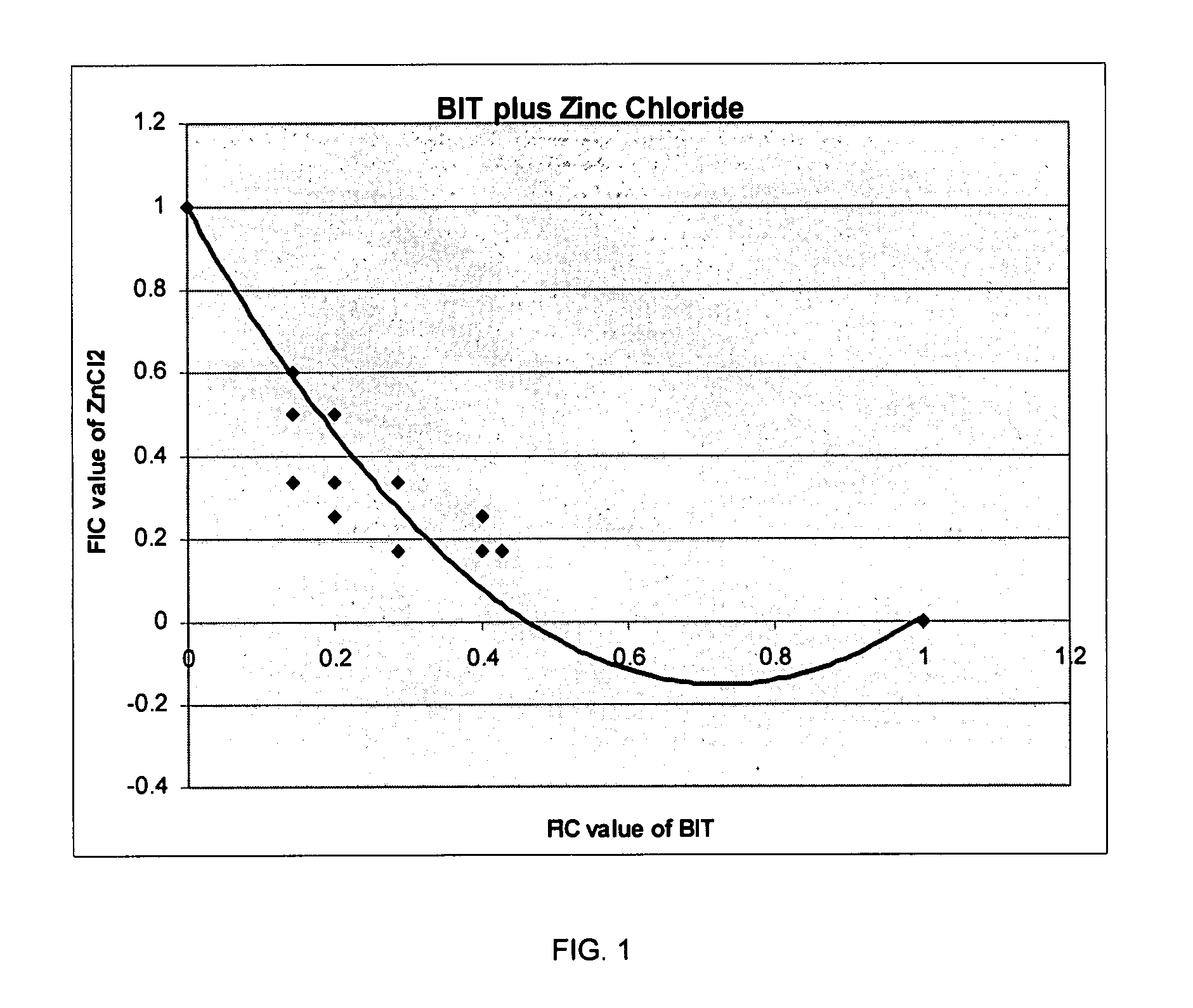
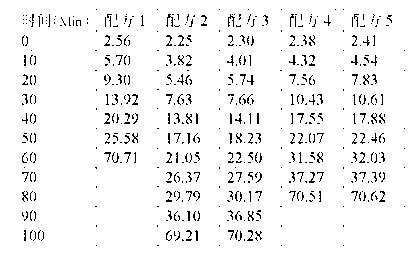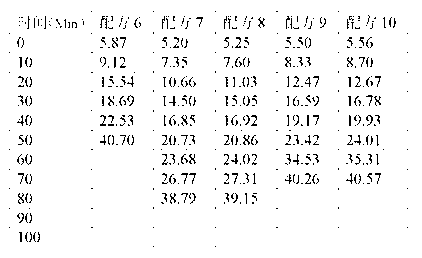Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1669 results about "Zinc ion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
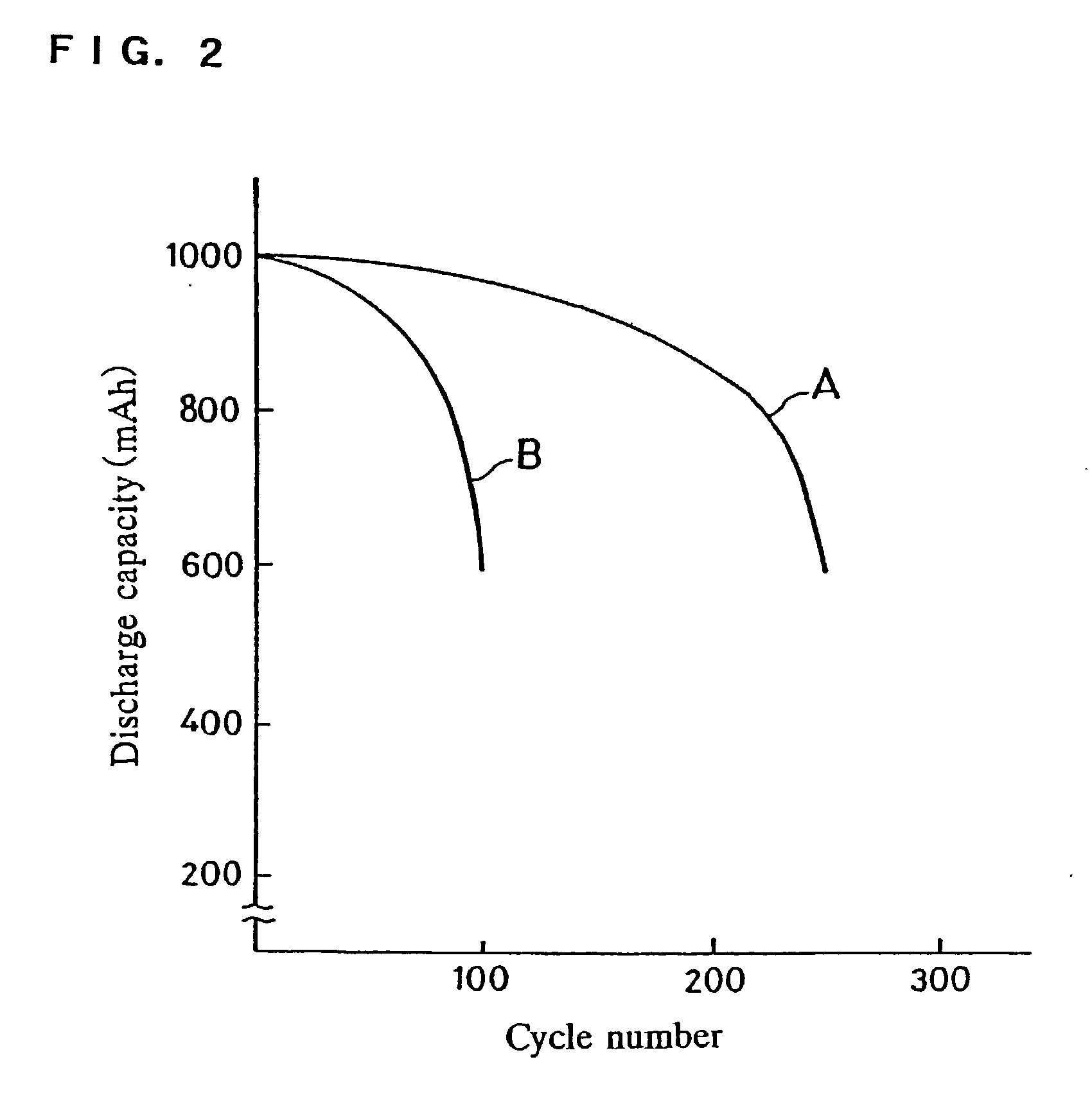
Zinc ion is a cation, and its formula is Zn^2+, This is because zinc atom requires 2 outer shell electrons to be removed in order to become stable.
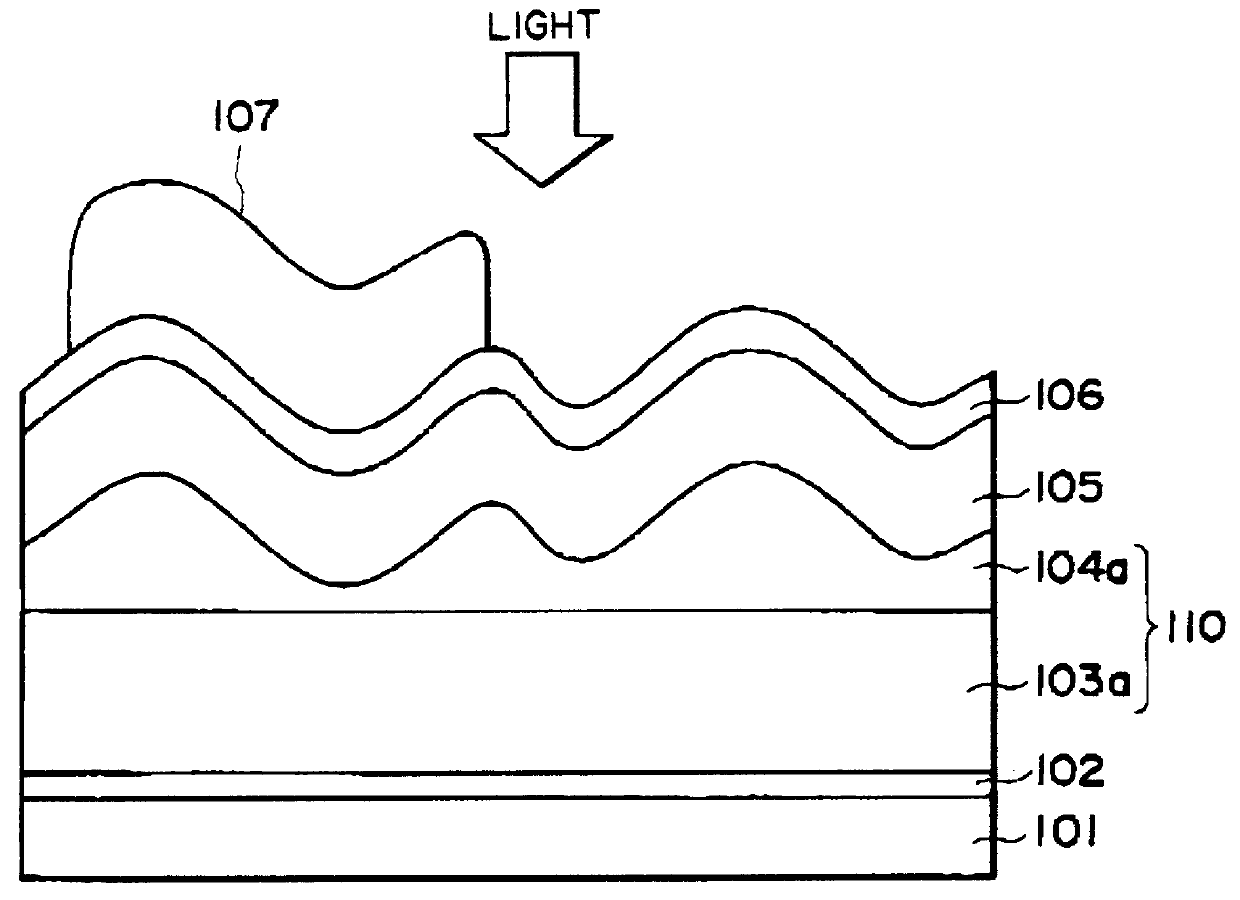
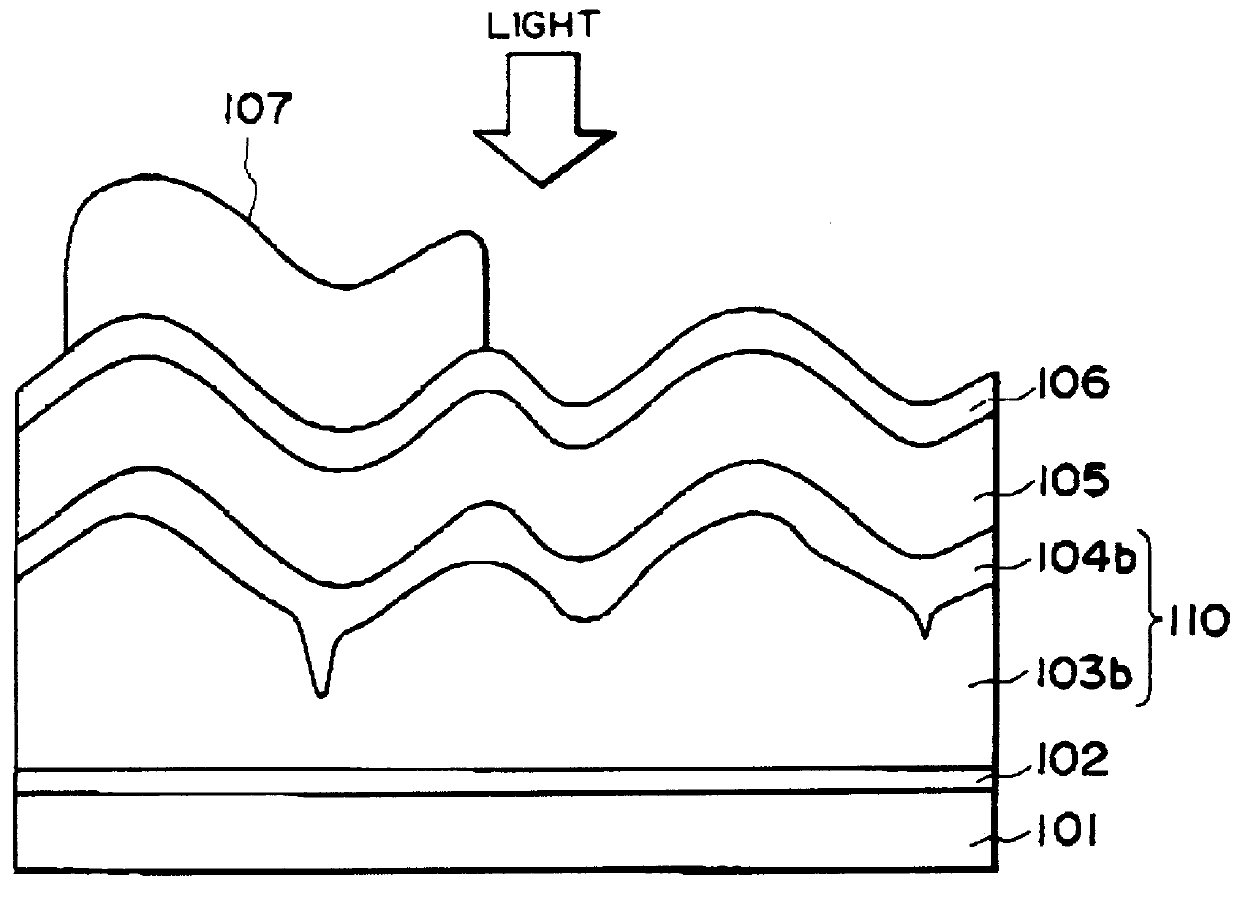
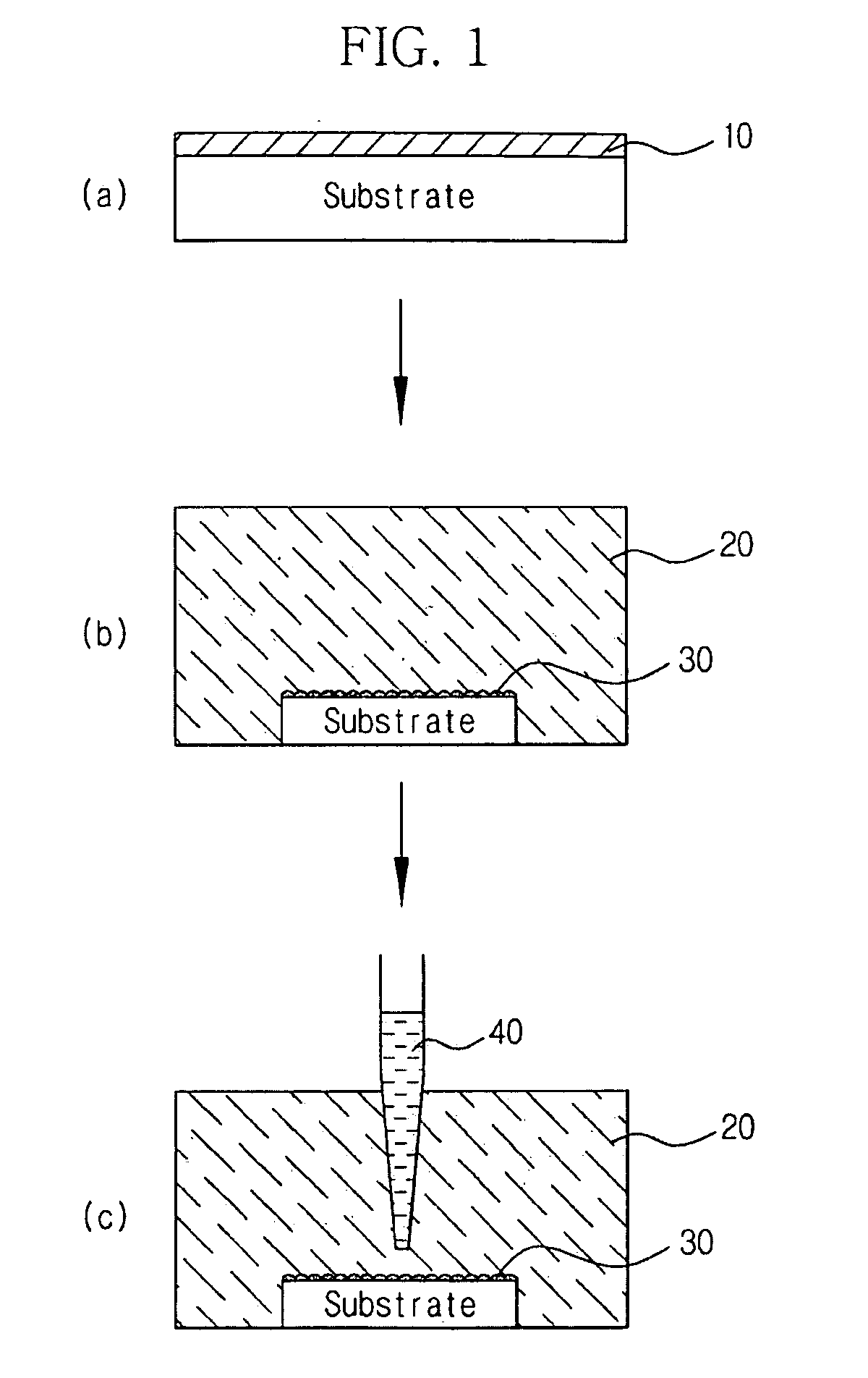
Process for producing photo-electricity generating device
InactiveUS6123824AInhibition of abnormal growthReduce leakage currentElectrolytic inorganic material coatingSurface reaction electrolytic coatingElectricityPower flow
A photo-electricity generating device is produced through the steps of: immersing an electrode and an electroconductive substrate in an aqueous solution comprising nitrate ions and zinc ions, supplying a current passing through a gap between the electrode and the electroconductive substrate to form a first zinc oxide layer on the electroconductive substrate, etching the first zinc oxide layer, and forming a semiconductor layer on the zinc oxide layer. The zinc oxide layer may preferably be formed in two zinc oxide layers under different electrudeposition conditions. In this case, the etching step may preferably be performed between steps for forming these zinc oxide layers. The zinc oxide layer is provided with an unevenness at its surface suitable for constituting a light-confining layer of a resultant photo-electricity generating device.
Owner:CANON KK
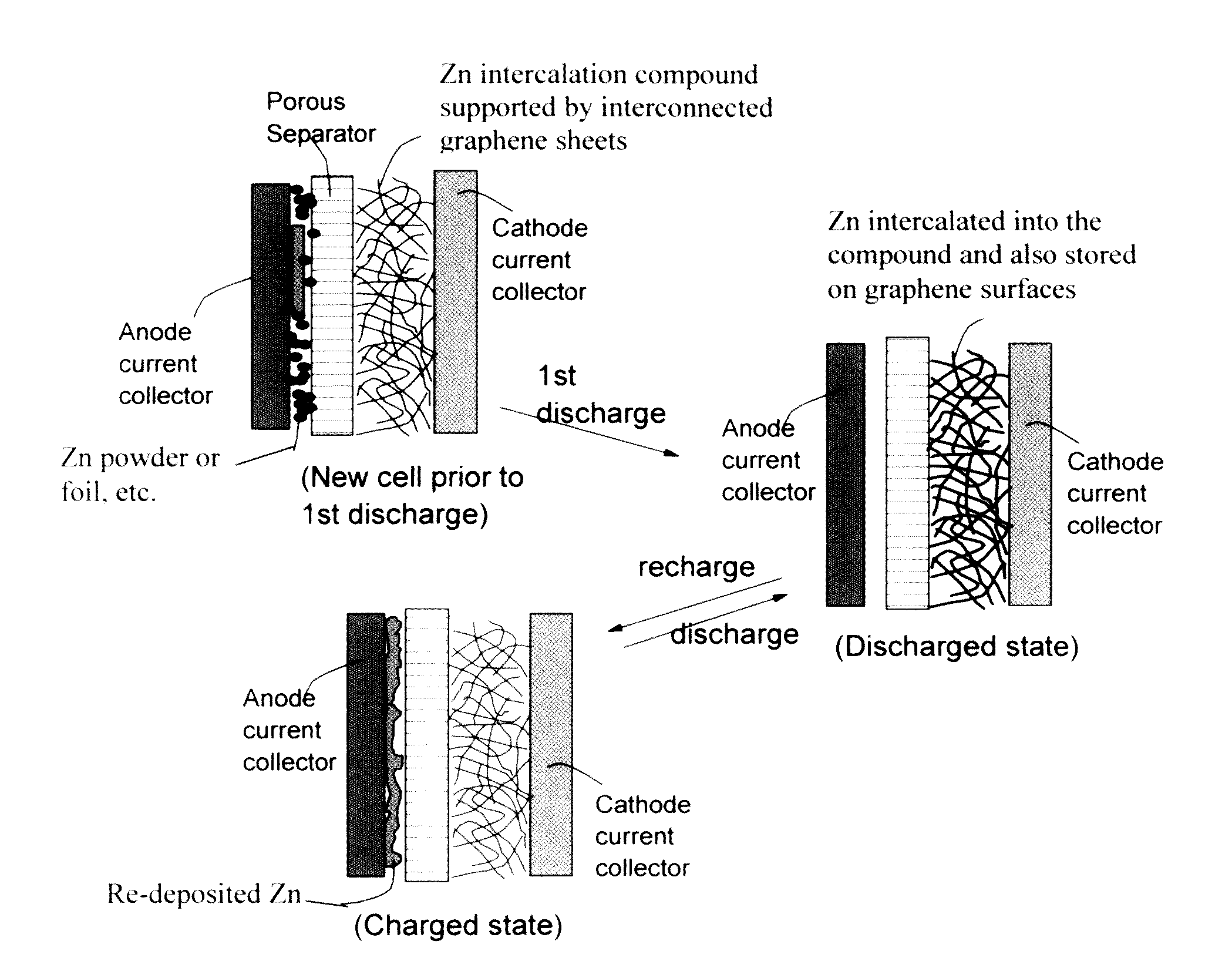
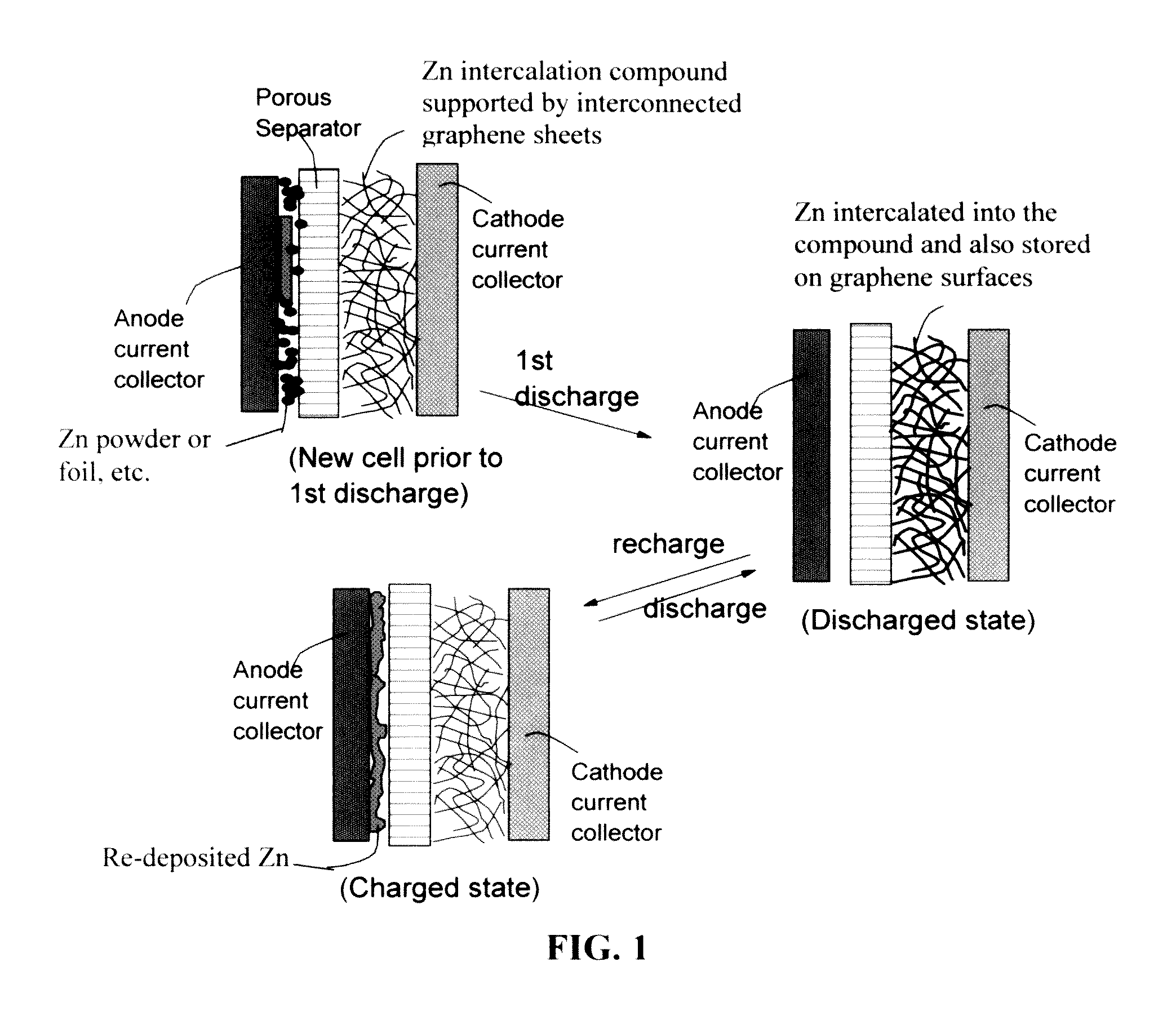
Zinc Ion-Exchanging Energy Storage Device
ActiveUS20160301096A1Quick releaseRapid depositionHybrid capacitor electrolytesAlkaline accumulatorsChemical treatmentZinc metal
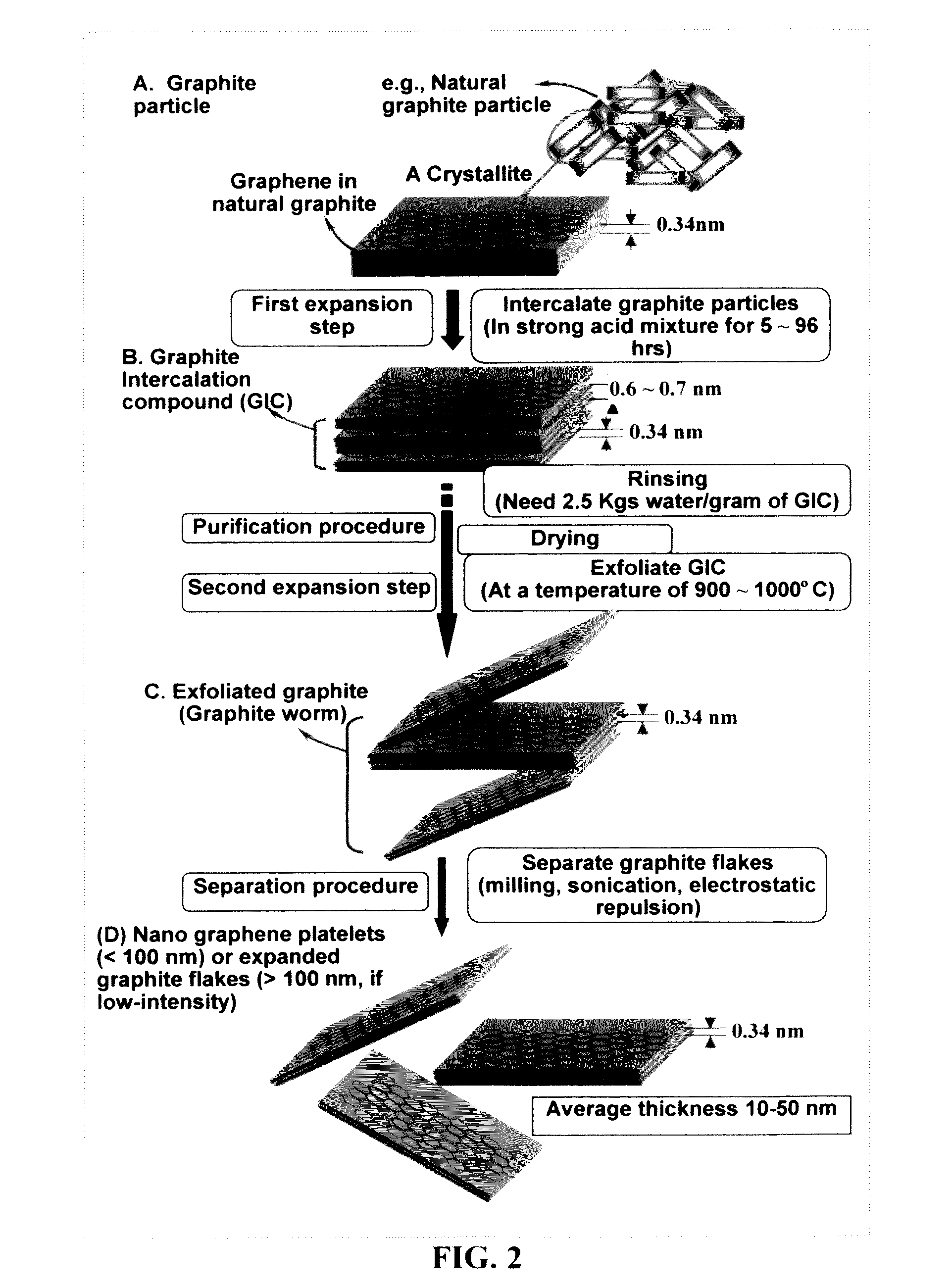
A zinc ion-exchanging battery device comprising: (A) a cathode comprising two cathode active materials (a zinc ion intercalation compound and a surface-mediating material); (B) an anode containing zinc metal or zinc alloy; (C) a porous separator disposed between the cathode and the anode; and (D) an electrolyte containing zinc ions that are exchanged between the cathode and the anode during battery charge / discharge. The zinc ion intercalation compound is selected from chemically treated carbon or graphite material having an expanded inter-graphene spacing d002 of at least 0.5 nm, or an oxide, carbide, dichalcogenide, trichalcogenide, sulfide, selenide, or telluride of niobium, zirconium, molybdenum, hafnium, tantalum, tungsten, titanium, vanadium, chromium, cobalt, manganese, iron, nickel, or a combination thereof. The surface-mediating material contains exfoliated graphite or multiple single-layer sheets or multi-layer platelets of a graphene material.
Owner:GLOBAL GRAPHENE GRP INC
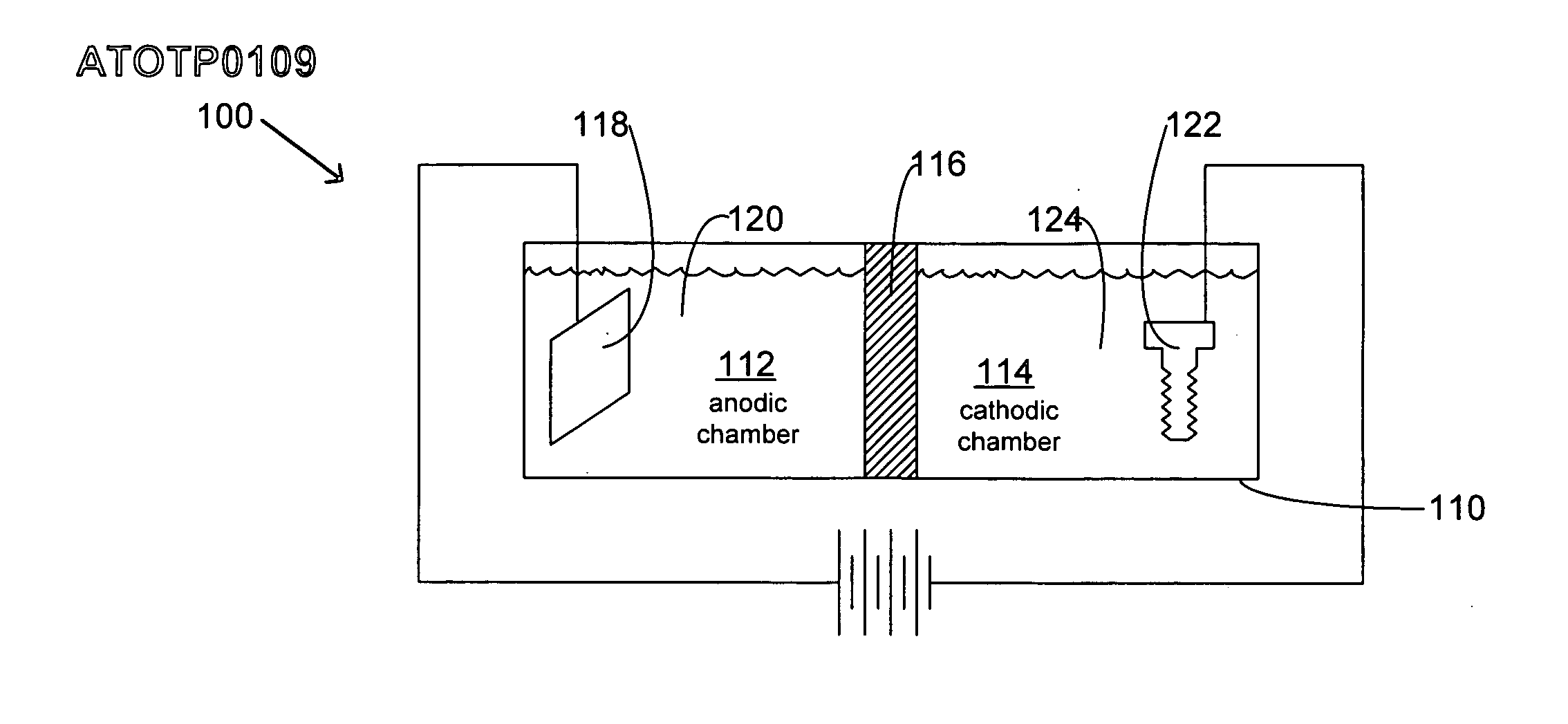
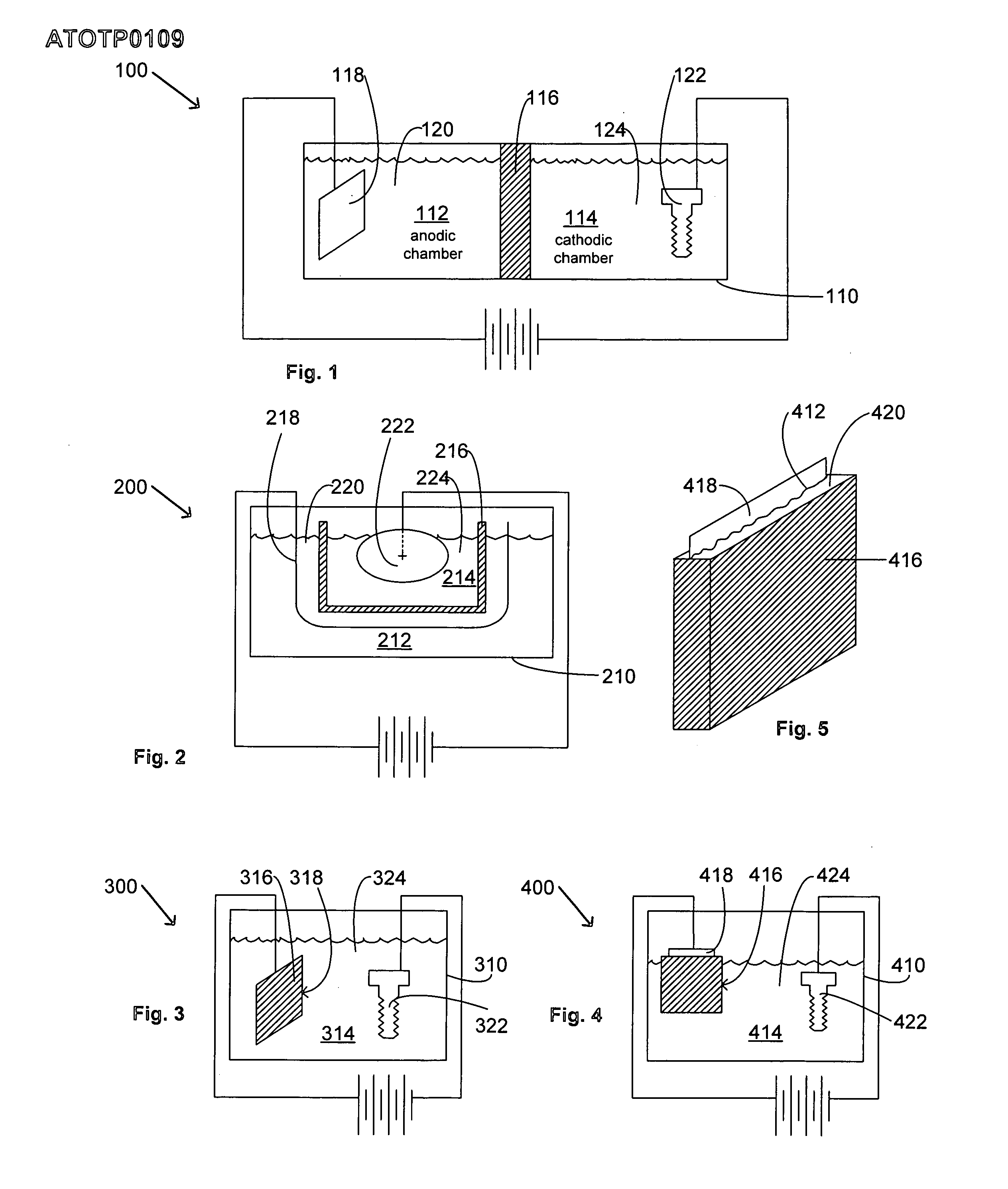
Articles with electroplated zinc-nickel ternary and higher alloys, electroplating baths, processes and systems for electroplating such alloys
An electroplating bath, a system, a process for, and the article obtained from, depositing a zinc-nickel ternary or higher alloy, a) zinc ions; b) nickel ions; and c) one or more ionic species selected from ions of Te+4, Bi+3 and Sb+3, and in some embodiments, further including one or more additional ionic species selected from ions of Bi+3, Sb+3, Ag+1, Cd+2, Co+2, Cr+3, Cu+2, Fe+2, In+3, Mn+2, Mo+6, P+3, Sn+2 and W+6. In some embodiments, the system includes a divider forming a cathodic chamber and an anodic chamber, with the electroplating bath in the cathodic chamber only. In various embodiments, the zinc-nickel ternary and higher alloys may provide improved properties to the conductive substrates upon which the alloys are deposited.
Owner:ATOTECH DEUT GMBH
Stable solution of zinc ions and bicarbonate and/or carbonate ions
InactiveUS6015547APrevention and counteractingInorganic/elemental detergent compounding agentsBiocideZinc ionBicarbonate Ion
A storage stable aqueous solution or aqueous gel of zinc ions in the presence of bicarbonate ions is disclosed. The solution comprises: (a) a source of zinc ion, (b) a source of a stabilizing anion which can stabilize soluble zinc and bicarbonate and / or carbonate in solution; (c) a source of bicarbonate ion; and (d) a solvent therefor. The solvent comprises a major proportion of water. The zinc salt is present in an amount suitable for the intended purpose; the stabilizing anion in an amount B of at least 1.2 equivalents per equivalent of zinc ion; and the bicarbonate ion cannot exceed certain levels which are related to the level of the stabilizing anion.
Owner:CHURCH & DWIGHT CO INC
Chargeable zinc ion battery
ActiveCN101540417AImproved magnification performanceGood reversibilityAlkaline accumulatorsActive material electrodesZinc ionManganese
The invention discloses a chargeable zinc ion battery, which comprises an anode, a cathode, isolating membrane arranged between the anode and the cathode, and electrolyte which contains the anion and the cation and has ionicconductivity, wherein the cathode adopts active materials which are mainly zinc elements, the active materials of the anode are oxide materials of manganese which can occlude and release zinc ions, the electrolyte is liquid or gel materials with ionicconductivity which adopts the soluble salt of zinc as solute and water as solvent, and the pH value of the electrolyte lies between 3 and 7. The battery has the characteristics of high capacity, chargeable property, long cycle life and the like. Proven by experiments, the chargeable zinc ion battery has excellent rate performance, reversible performance and cycle performance.
Owner:SHENZHEN CITY THROUGH SCI & TECH OF NEW ENERGY CO LTD
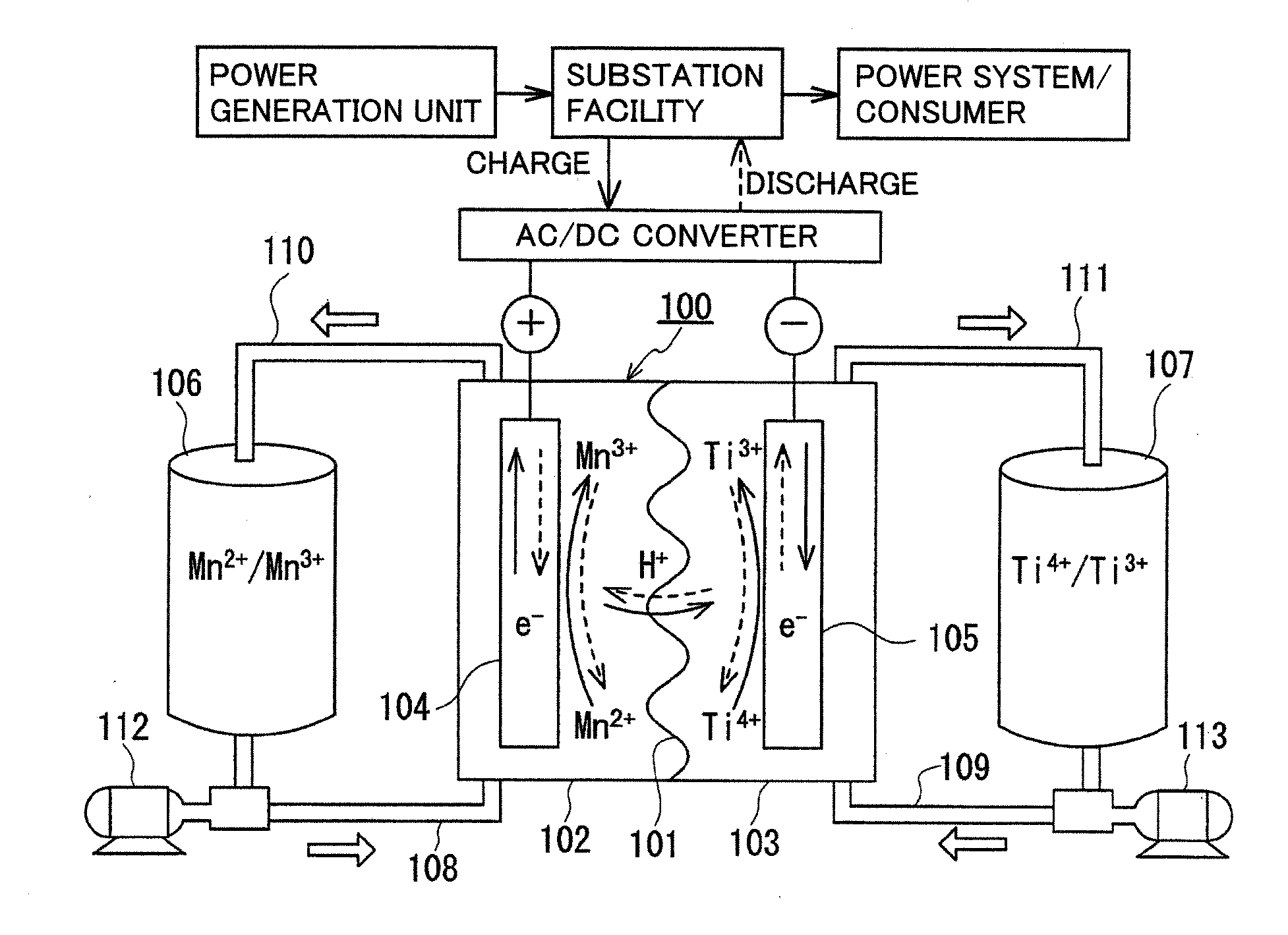
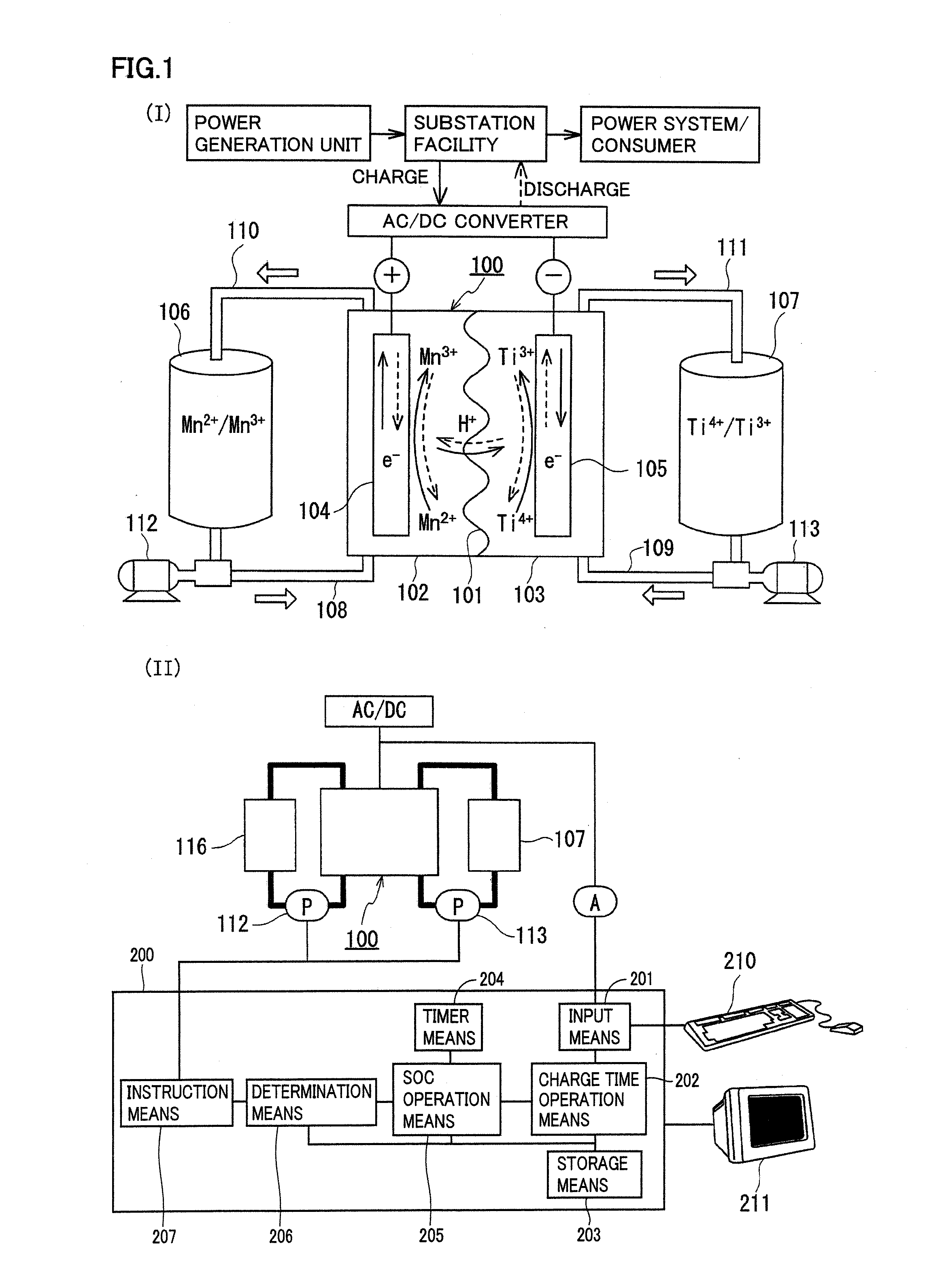
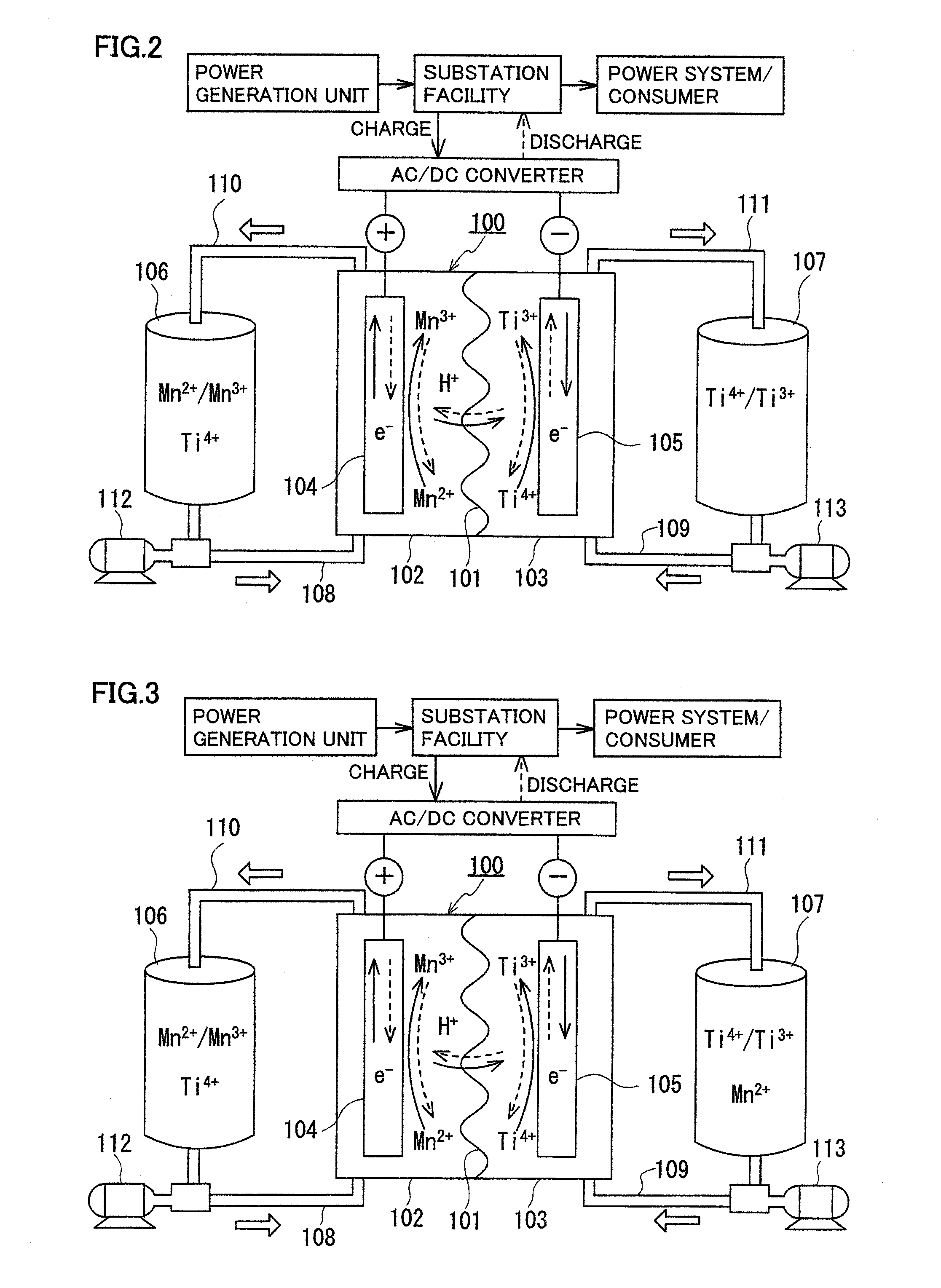
Redox flow battery
ActiveUS20120045680A1Suppress generationHigh electromotive forceElectrolyte moving arrangementsCell electrodesManganesePhysical chemistry
A redox flow battery having a high electromotive force and capable of suppressing generation of a precipitation is provided. In a redox flow battery 100, a positive electrode electrolyte and a negative electrode electrolyte are supplied to a battery cell including a positive electrode 104, a negative electrode 105, and a membrane 101 interposed between the electrodes 104 and 105, to charge and discharge the battery. The positive electrode electrolyte contains a manganese ion, or both of a manganese ion and a titanium ion. The negative electrode electrolyte contains at least one type of metal ion selected from a titanium ion, a vanadium ion, a chromium ion, a zinc ion, and a tin ion. The redox flow battery 100 can suppress generation of a precipitation of MnO2, and can be charged and discharged well by containing a titanium ion in the positive electrode electrolyte, or by being operated such that the positive electrode electrolyte has an SOC of not more than 90%. In addition, the redox flow battery 100 can have a high electromotive force equal to or higher than that of a conventional vanadium-based redox flow battery.
Owner:SUMITOMO ELECTRIC IND LTD
Warewashing composition for use in automatic dishwashing machines, and methods for manufacturing and using
InactiveUS20050020464A1Reduce corrosionInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsAluminum IonHard water
A warewashing detergent composition is provided according to the invention. The warewashing detergent composition includes a cleaning agent, an alkaline source, and a corrosion inhibitor. The cleaning agent comprises a detersive amount of a surfactant. The alkaline source is provided in an amount effective to provide a use composition having a pH of at least about 8. The corrosion inhibitor includes a source of aluminum ion and a source of zinc ion. The relative amounts of the source of zinc ion and the source of aluminum ion can be controlled to reduce visible filming when the warewashing detergent composition is used in the presence of hard water. Methods for using and manufacturing a warewashing detergent composition are provided.
Owner:ECOLAB USA INC
Warewashing composition for use in automatic dishwashing machines, comprising a mixture of aluminum and zinc ions
ActiveUS20050003979A1Reduce corrosionInorganic/elemental detergent compounding agentsOrganic detergent compounding agentsZincCorrosion inhibitor
A warewashing detergent composition is provided according to the invention. The warewashing detergent composition includes a cleaning agent, an alkaline source, and a corrosion inhibitor. The cleaning agent comprises a detersive amount of a surfactant. The alkaline source is provided in an amount effective to provide a use solution having a pH of at least about 8. The corrosion inhibitor includes a source of aluminum ion and a source of zinc ion. Methods for using and manufacturing a warewashing detergent composition are provided.
Owner:ECOLAB USA INC
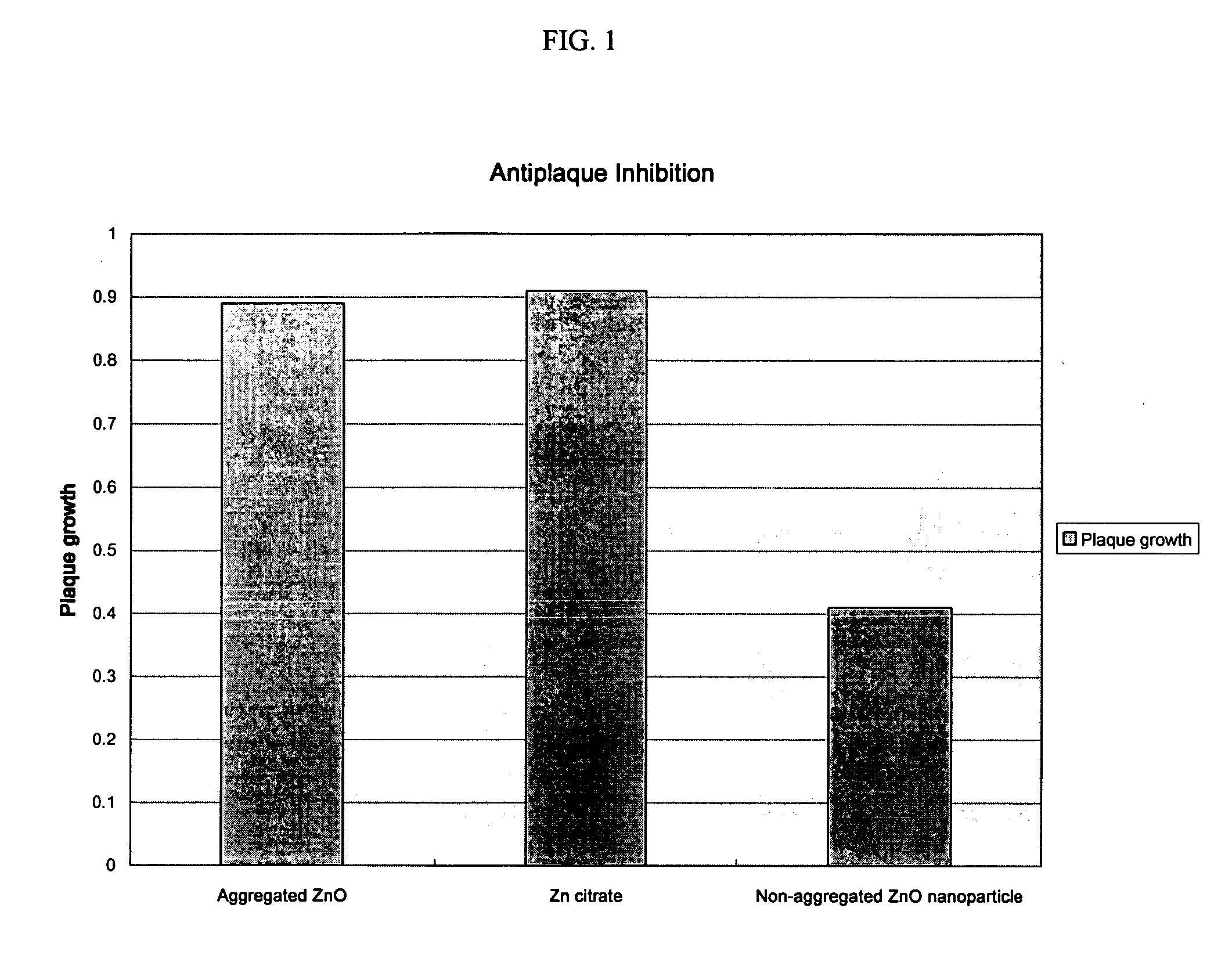
Oral composition containing non-aggregated zinc nanoparticles
An oral composition comprising a vehicle and a zinc ion source in the form of nanoparticles that are substantially non-aggregated and methods for use of such compositions are described. The composition provides antiplaque and anti malodor benefits to the user and the inclusion of nanoparticles permits a reduction in the amount of zinc ions present in the composition while maintaining efficacy.
Owner:COLGATE PALMOLIVE CO
Alkaline zinc-nickel alloy plating compositions, processes and articles therefrom
InactiveUS20050133376A1Easy to handleImprove ductilityAnti-corrosive paintsLiquid/solution decomposition chemical coatingZinc ionNickel alloy
The present invention relates to an aqueous zinc-nickel electroplating bath, including water; nickel ion; zinc ion; at least one complexing agent; and at least one non-ionogenic, surface active polyoxyalkylene compound, wherein the bath has an alkaline pH. In one embodiment, the zinc ion, the nickel ion and the non-ionogenic surface active polyoxyalkylene compound are present at concentrations sufficient to deposit a zinc-nickel alloy comprising a substantially gamma phase.
Owner:ATOTECH DEUT GMBH
Oral Stannous Compositions
ActiveUS20090136432A1High activityLow levelCosmetic preparationsToilet preparationsPhosphateDivalent metal ions
The present invention relates to an aqueous oral composition comprising:a) from 0.2% to 3% divalent metal ions comprising:i. from 0.1% to 1.5% of zinc ions;ii. from 0.1% to 2% of tin (II) ions; andb) a source of fluoride ions;c) a silica dental abrasive;d) one or more chelants having a MW of less than 1000 and capable of forming water-soluble complexes with the zinc ions, wherein the chelants comprise less than 0.2% linear polyphosphates having a chain length of four or more;e) an orally acceptable carrier comprising at least 20% total water;wherein the pH of the composition is from 5 to 6.5, the molar ratio of the chelants to the divalent metal ions is at least 0.70:1 and at least 80% by weight of the total zinc ions are solubilised within the composition.The composition of the invention has been found to give improved antimicrobial activity from the zinc / stannous combination without significant taste, staining or stability problems, compared to compositions having lower levels of chelants.
Owner:THE PROCTER & GAMBLE COMPANY
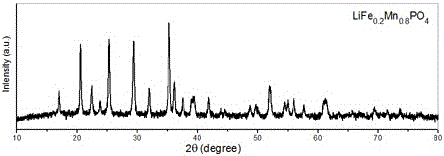
Water system high-voltage mixed ion secondary battery based on zinc-lithium ferric manganese phosphate
InactiveCN105826520AIncrease capacityExcellent rate performanceCell electrodesSecondary cellsElectrolytic agentElectrical battery
The invention relates to a water system high-voltage mixed ion secondary battery. A positive pole material of the battery is a high-voltage battery positive pole material, namely zinc-lithium ferric manganese phosphate (LiFe1-xMnxPO4), the element zinc serves as the majority of a negative pole material, and electrolyte is a liquid-state or gel-state material which is formed by lithium bis(trifluoromethane sulfonimide) (LiTFSI) and soluble zinc salt as solute and water as solvent and has ionic conductivity. The battery is based on the energy storage mechanisms of a dissolution-out / deposition reaction of zinc ions (Zn2+) on a negative pole and a reversible embedding / ejection reaction of the zinc ions (Zn2+) on a positive pole, meanwhile, through the water-in-salt electrolyte formed by high-concentration LiTFSI, the electrochemical water decomposition process is inhibited, a potential window of the water system electrolyte is remarkably broadened, the zinc-lithium mixed ion secondary battery has the advantages of being high in capacity, long in cycling life, safe, environmentally friendly, low in cost and the like, and the battery can be applied to the fields such as consumer electronic equipment, electromobiles and large-scale energy storage.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Aqueous zinc ion battery
InactiveCN108807910APromote circulationSimple preparation processFinal product manufactureSecondary cellsElectrical batteryZinc ion
The invention belongs to the technical field of batteries, and particularly relates to an aqueous zinc ion battery. The aqueous zinc ion battery comprises an anode, a cathode, an electrolyte and a diaphragm, wherein the diaphragm is arranged between the anode and the cathode; the cathode is a graphene-assisted zinc cathode; the electrolyte comprises a solvent and a solute, the solvent is water, and the solute comprises soluble zinc salt and manganese salt. Compared with the prior art, the aqueous zinc ion battery has the advantages that the graphene-assisted zinc cathode is applied to the aqueous zinc ion battery system; by adopting the graphene with excellent property and stable structure, the stability and conductivity of the zinc cathode are enhanced, so that the cycle property of the graphene-assisted zinc cathode is enhanced; especially, by adding the trace manganese salt and the corrosion inhibitor into the electrolyte, the problems of zinc corrosion, passivating and the like canbe effectively relieved; the cycle life of the graphene-assisted zinc cathode is further prolonged.
Owner:SHENZHEN CITY THROUGH SCI & TECH OF NEW ENERGY CO LTD
Inorganic complex antimicrobials containing zincium-rare earth as well as preparation method and application thereof
InactiveCN101176468AGrowth inhibitionBroad spectrum antibacterialBiocideAntifouling/underwater paintsFiberChemical synthesis
The utility model discloses an inorganic composite antimicrobial with zinc - rare earth and a preparation method and applications thereof. The antimicrobial uses natural or chemosynthetic ion exchange material as the carrier, and loads the double active centre of the zinc ions and the rare earth ions by an ion exchange method, wherein, the content of the zinc ions is 6.0 to 8.0wt%, the content of the rare earth ions is 2.0 to 4.0wt%. The antimicrobial exchanges the zinc ions and the rare earth ions into the carrier by means of a liquidoid or a solidoid ion exchange, and then the inorganic composite antimicrobial is prepared after a post treatment. Because having a double antimicrobial active centre of the zinc ions and the rare earth ions which can generate a synergistic effect, the antimicrobial has the advantages of broad antimicrobial spectrum, higher antimicrobial efficiency, good thermal stability and photostability, steady color and low cost; the antimicrobial is an ideal substitute for the silver-loaded inorganic antimicrobial, and can be added in plastic, rubber, fiber, paints, adhesive, paper or ceramic and other material for preparing antimicrobial functional material and products.
Owner:JINAN UNIVERSITY
Aqueous zinc-ion battery positive electrode material
ActiveCN107863485AImprove conductivityImprove electrochemical performanceMaterial nanotechnologyElectrode thermal treatmentHigh pressurePotassium permanganate
The invention discloses an aqueous zinc-ion battery positive electrode material. A preparation method of the material is a one-step hydrothermal method and comprises the steps of adding a certain amount of potassium permanganate into deionized water, performing stirring under a room temperature to obtain a deep violet solution, transferring the solution into a high-pressure kettle, and adding a three-dimensional substrate material into the solution for hydrothermal solution; washing the three-dimensional substrate material for many times after cooling of hydrothermal solution, and performing drying in a baking oven to obtain a uniform nanometer flower spherical Mn3O4 material grown on the three-dimensional substrate material. The prepared material is initially applied to prepare the aqueous zinc-ion battery positive electrode material, has high specific capacity and favorable cycle stability, is moderate in reaction condition and simple in process and is suitable for production on a large scale.
Owner:CENT SOUTH UNIV
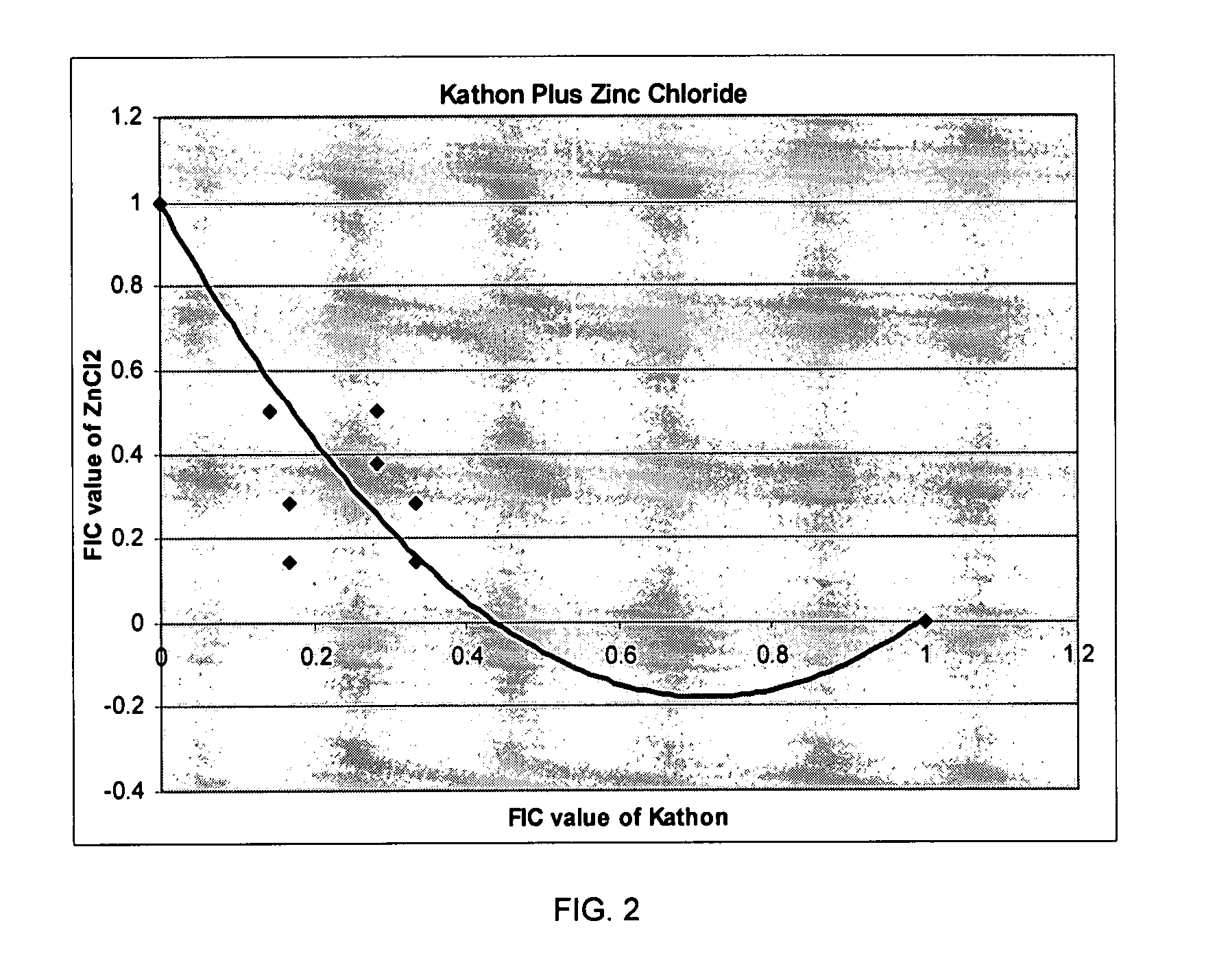
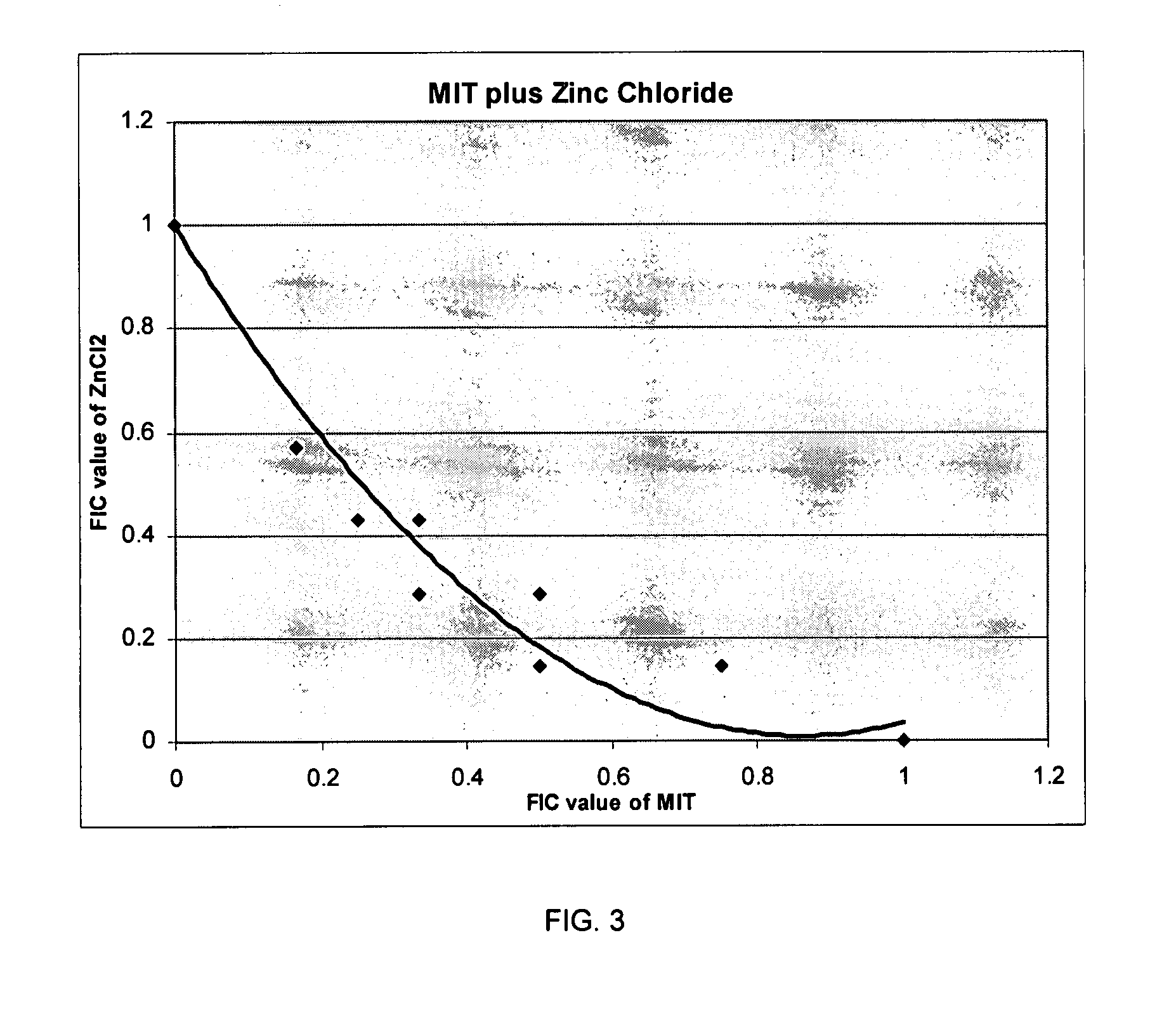
Isothiazolinone biocides enhanced by zinc ions
The present invention relates to an antimicrobial composition comprising an isothiazolinone, such as 1,2-benzisothiazolin-3-one, and a zinc compound selected from zinc salts, zinc oxides, zinc hydroxides or combinations thereof. Useful zinc salts include for example, oxides, sulfates, chlorides, and combinations thereof. In use, the zinc from the zinc compound enhances the antimicrobial activity to the isothiazolin-containing composition. This enhancement permits achieving the desired antimicrobial activity at a lower usage rate than is achieved using the isothiazolinone in the absence of the zinc compound. The antimicrobial composition can also contain co-biocides, such as pyrithiones, including zinc pyrithione or copper pyrithione.
Owner:ARCH CHEM INC
Active carbon fibre and preparation thereof
InactiveCN101348952ALarge specific surface areaPore structure highOther chemical processesAlkali metal oxides/hydroxidesActivated carbonPolyvinyl alcohol
The invention relates to an activated carbon fiber and a preparation method thereof. The compositions in weight percentage of the activated carbon fiber are: 80 to 95 percent of fiber-forming polymer, and 20 to 5 percent of functional additive component, wherein the fiber-forming polymer can be polyacrylonitrile, modified polyacrylonitrile, polyvinyl alcohol or regenerated cellulose fiber; and the functional additive component can be a superfine absorbent of supporting metal with the average diameter of between 0.01 and 20 mu meter; and the supporting metal can be one of a silver ion, a copper ion or a zinc ion. The preparation method comprises the following steps: a base material fiber-forming polymer fiber containing the functional additive component is prepared first, wherein the base material fiber can be a polyacrylonitrile fiber, a modified polyacrylonitrile, a polyvinyl alcohol fiber or a regenerated cellulose fiber base fiber; and different physical activation treatments or / and chemical activation treatments are carried out according to different base material fibers to produce the activated carbon fiber corresponding to the base material.
Owner:TIANJIN POLYTECHNIC UNIV
Zinc-nickel alloy electroplating system
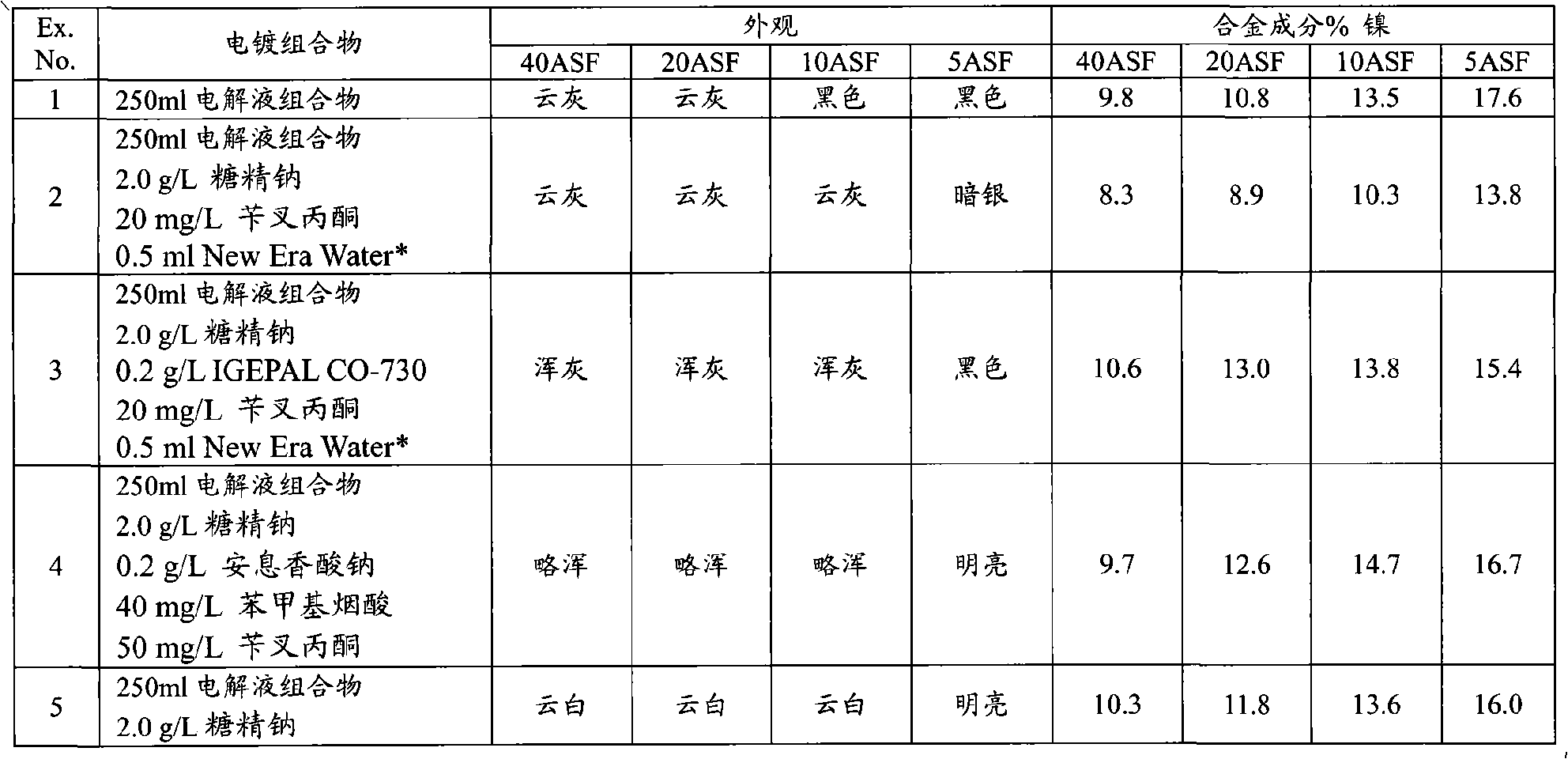
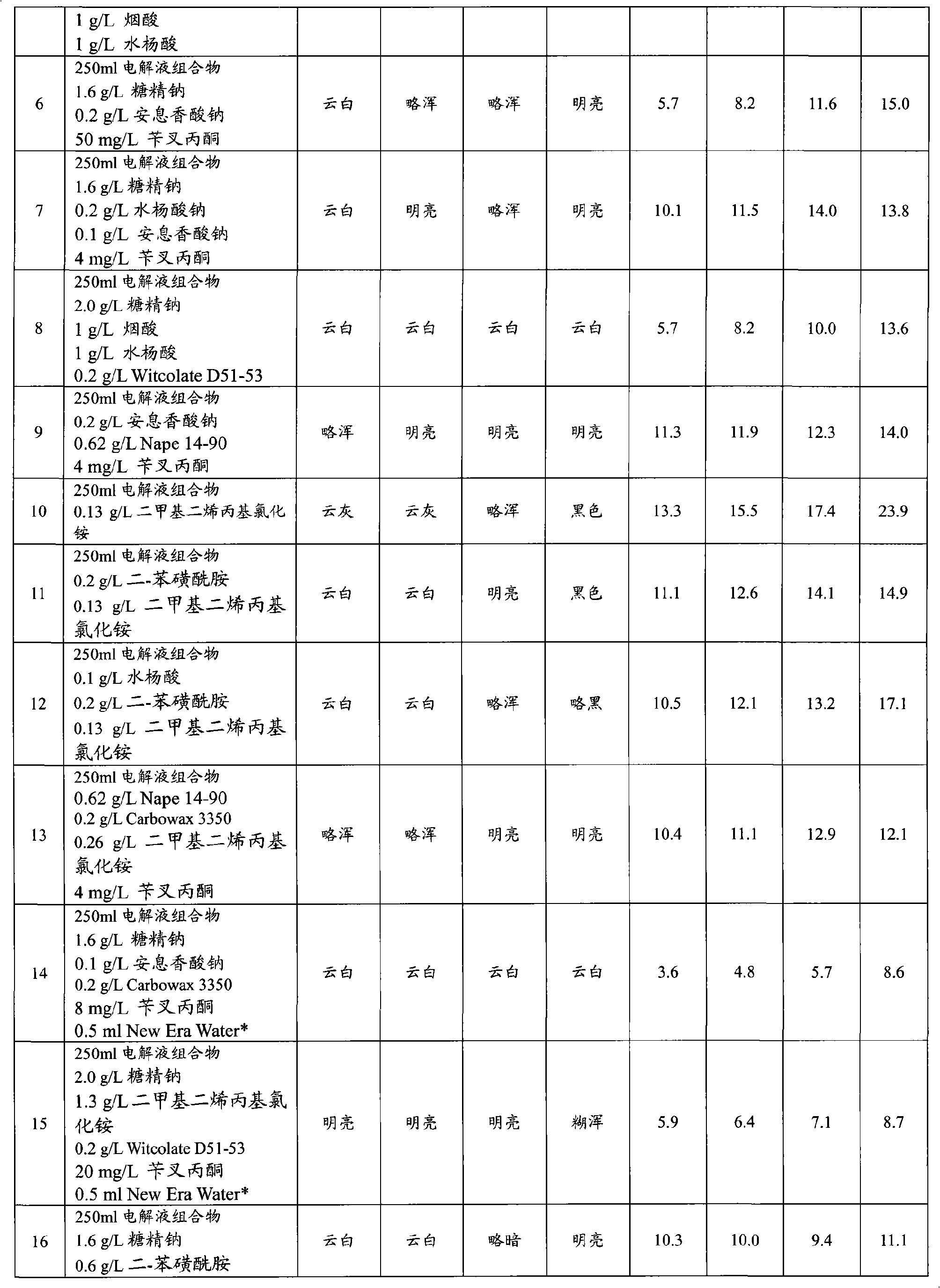
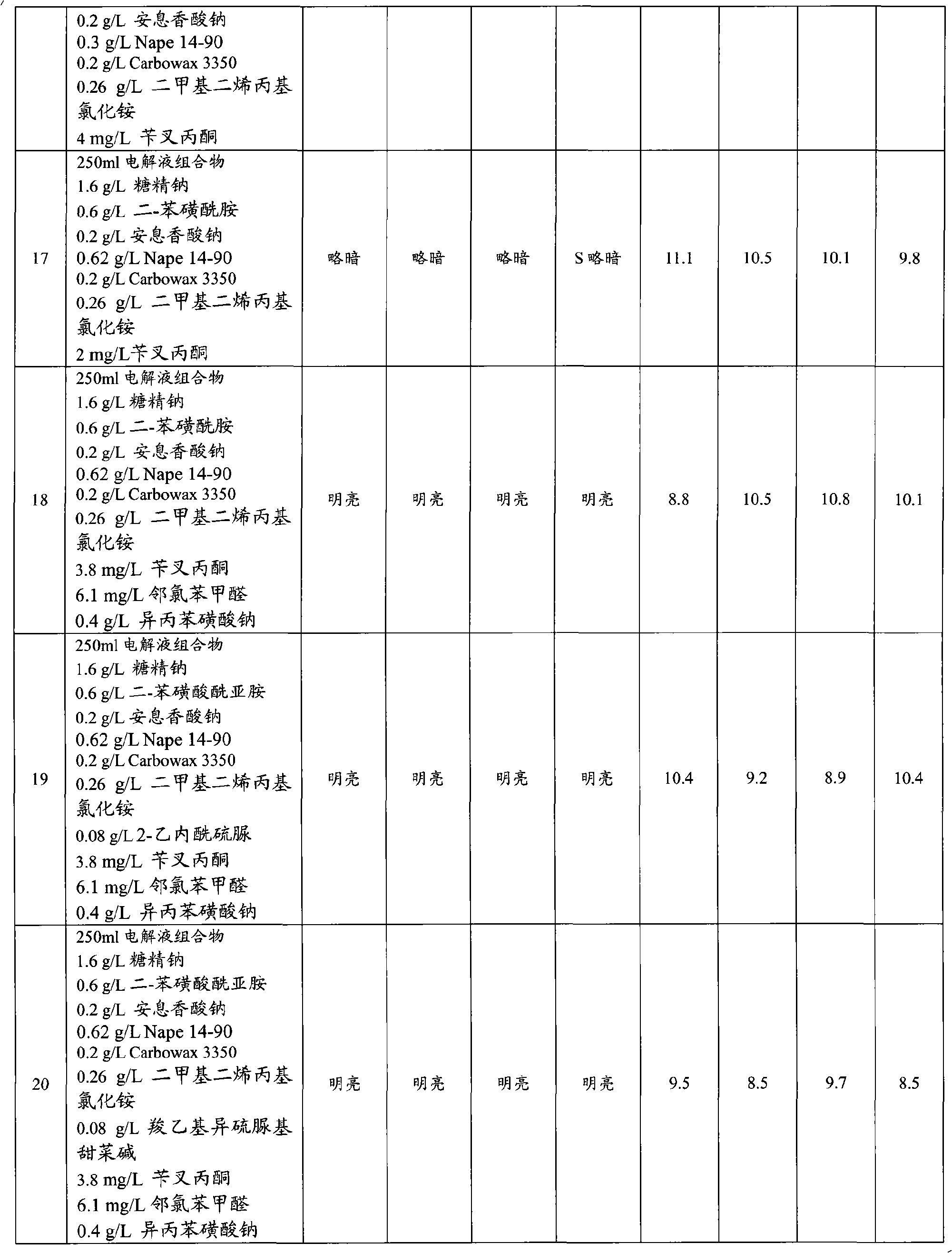
The invention provides an aqueous zinc-nickel alloy electroplating composition particularly useful in an electroplating method for depositing a zinc-nickel alloy layer on a substrate, wherein the deposited layer exhibits uniform nickel concentration and good aesthetics across a broad range of current densities. The electroplating composition comprises an electrolyte composition and an organic composition. In one embodiment, the electrolyte composition comprises a zinc ion source, a nickel ion source, a pH buffering agent and at least one additional salt, and the organic composition comprises a Class I nickel brightener, a Class II nickel brightener, an aromatic carboxylic acid, an aldehyde or ketone compound, and a non-ionic or anionic surfactant. The electroplating composition is particularly free of chelators and free ammonium-producing agents.
Owner:PAVCO INC
Water-based zinc-manganese single flow battery
ActiveCN105336971AIncrease the speed of mass transferEliminate deformationRegenerative fuel cellsWater basedManganese oxide
The invention relates to a water-based zinc-manganese single flow battery; a positive electrode active material is an oxide of manganese, a metal composite oxide, a metal oxide or a carbon material, a negative electrode is a zinc electrode, an electrolyte solution is a nearly neutral aqueous solution containing a zinc salt and a manganese salt, positive electrode active ions and negative electrode active ions can coexist in one electrolyte solution, and an ion exchange membrane is not required for separating the positive electrode and the negative electrode; in processes of charging and discharging, the electrolyte solution constantly flows between an electrolyte solution storage tank and an electric pile through a pipeline under pushing of a liquid pump. During charging, zinc ions are deposited onto a negative electrode current collector from the electrolyte solution, the zinc ions and manganese ions are co-embedded into the positive electrode active substance at the same time, and the manganese ions are subjected to oxidation deposition; during discharging, the negative electrode deposited zinc is dissolved into the electrolyte solution, and the positive electrode manganese oxide is partially reduced and dissolved and is extricated to the electrolyte solution with the zinc ions simultaneously. The battery has the outstanding characteristics of simple manufacture, relatively high specific energy and specific power, low cost, long cycle life, environmental friendly and the like, and is widely applied in electric power, transportation, electronics and other fields.
Owner:NO 63971 TROOPS PLA
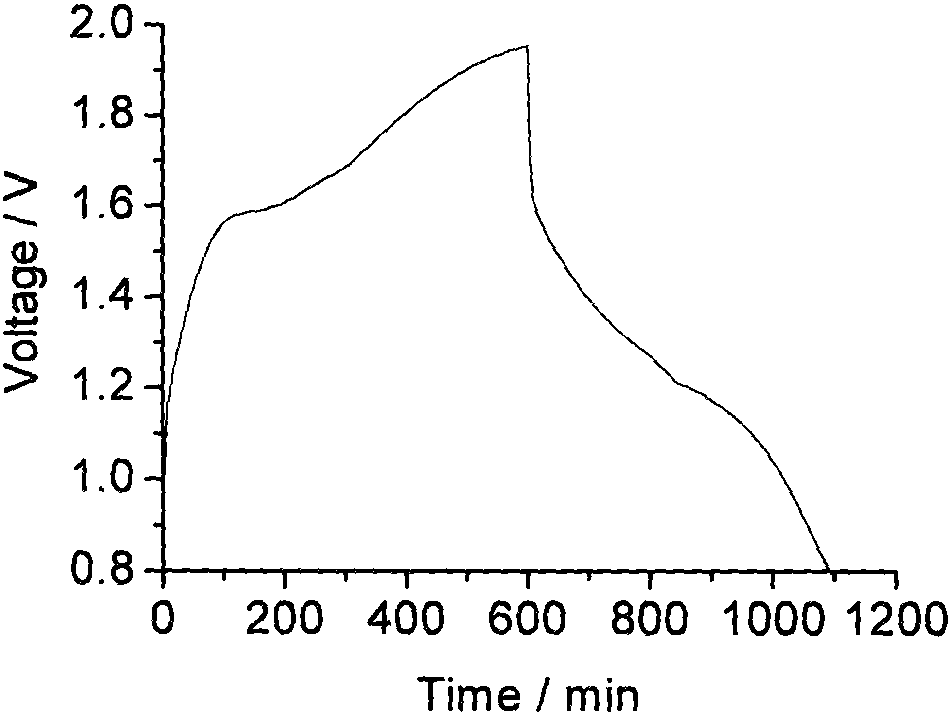
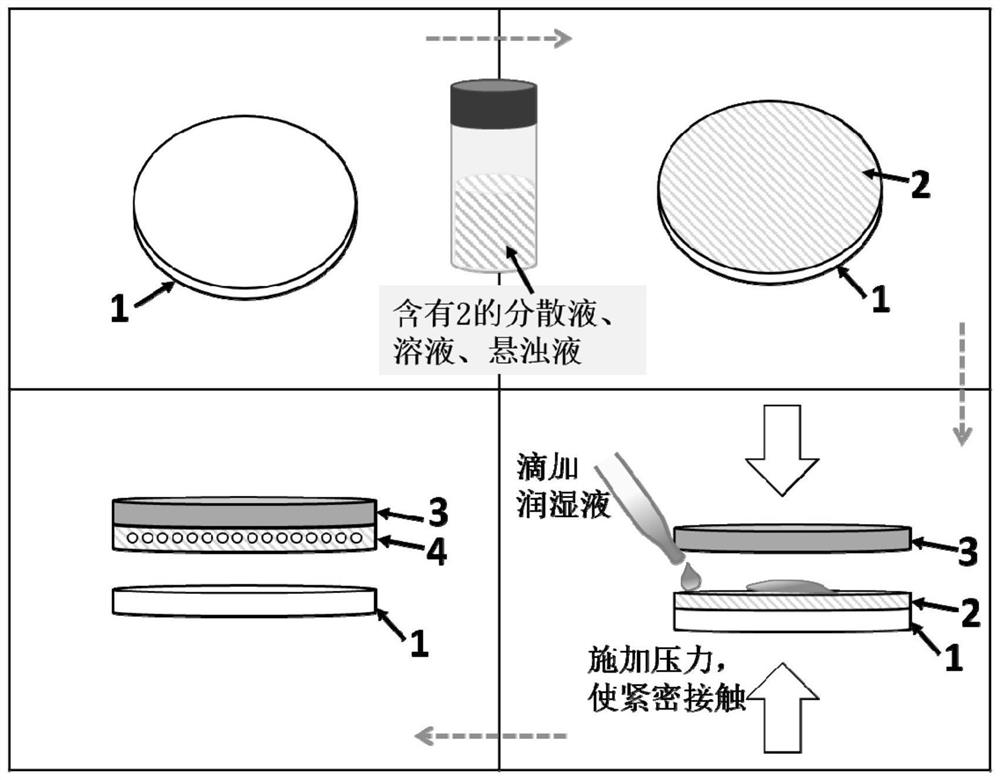
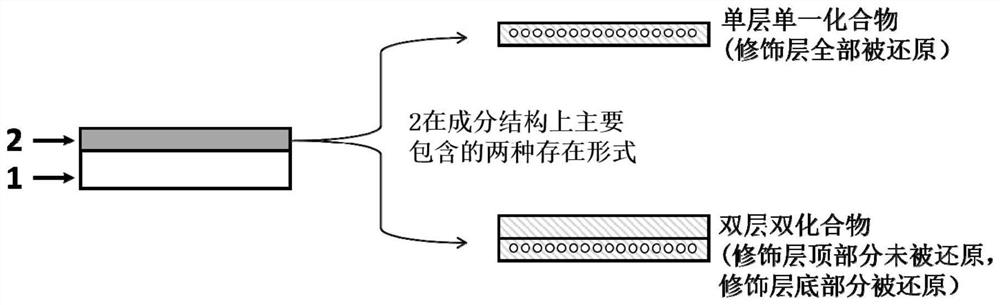
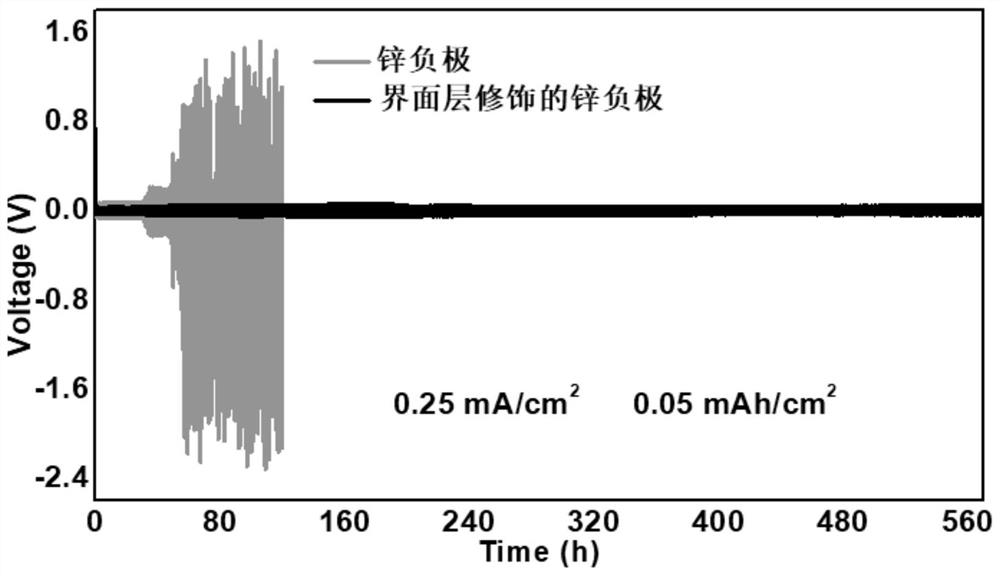
Zinc negative electrode with zinc ion conductivity interface modification layer, battery and preparation method
ActiveCN111933912AImprove Interface StabilitySolve the large interface impedanceSecondary cellsChemical electrode manufacturingElectrical batteryZinc ion
The invention provides a zinc negative electrode with a zinc ion conductivity interface modification layer, a battery and a preparation method, and belongs to the field of aqueous zinc battery metal zinc cathodes. The preparation method comprises: in air atmosphere, pre-constructing an interface modification material M on a base membrane; in a soaking environment by a wetting liquid, the interfacemodification material M layer on the base film being in close contact with the metal zinc to form the short-circuit primary battery, and the contacted interface modification material M and the metalzinc having spontaneous redox reaction, to make the interface modification material convert to ZnxM with zinc ion conductivity from M, meanwhile, transferring the interface modification material layerto the surface of the metal zinc negative electrode from the base film, and finally, obtaining the metal zinc negative electrode with the ZnxM interface modification layer with zinc ion conductivityon the surface. The ZnxM interface modification layer with the zinc ion conductivity can effectively inhibit dendritic crystal growth of a zinc negative electrode during charging and discharging of azinc battery, so that the interface stability of a metal zinc negative electrode is improved, and meanwhile, the cycling stability of a water-based zinc battery is improved. The method is simple and has a good actual effect.
Owner:HUAZHONG UNIV OF SCI & TECH
Zinc-nickel alloy electroplating system
The invention provides an aqueous zinc-nickel alloy electroplating composition particularly useful in an electroplating method for depositing a zinc- nickel alloy layer on a substrate, wherein the deposited layer exhibits uniform nickel concentration and good aesthetics across a broad range of current densities. The electroplating composition comprises an electrolyte composition and an organic composition. In one embodiment, the electrolyte composition comprises a zinc ion source, a nickel ion source, a pH buffering agent and at least one additional salt, and the organic composition comprises a Class I nickel brightener, a Class II nickel brightener, an aromatic carboxylic acid, an aldehyde or ketone compound, and a non- ionic or anionic surfactant. The electroplating composition is particularly free of chelators and free ammonium-producing agents.
Owner:百富可公司
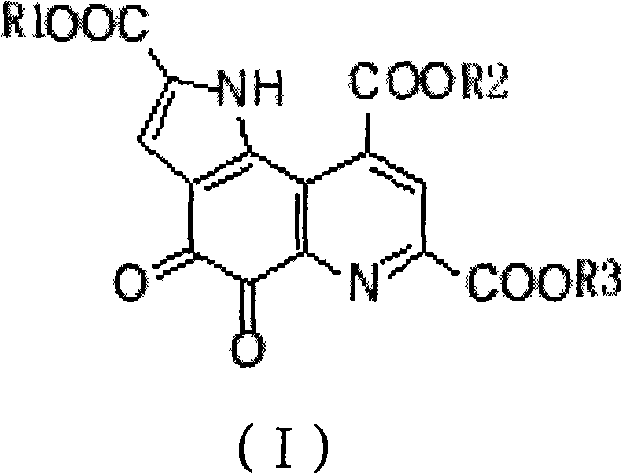
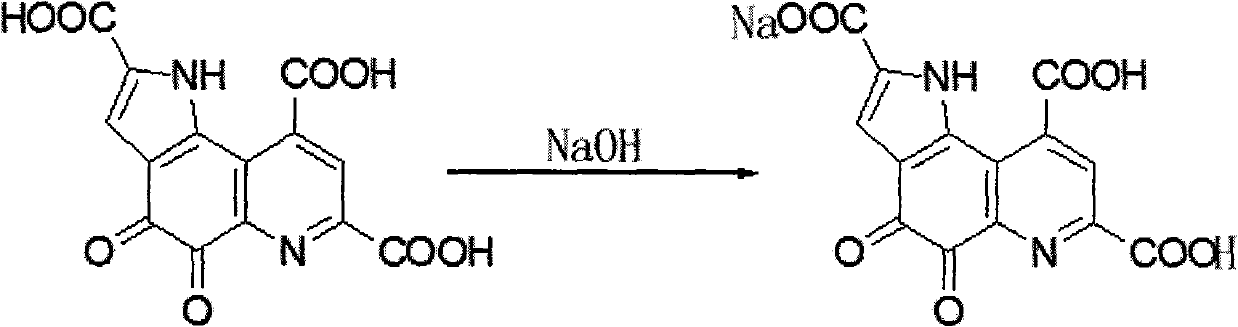
Pyrro-quinoline quinine sodium salt derivative and preparation method thereof
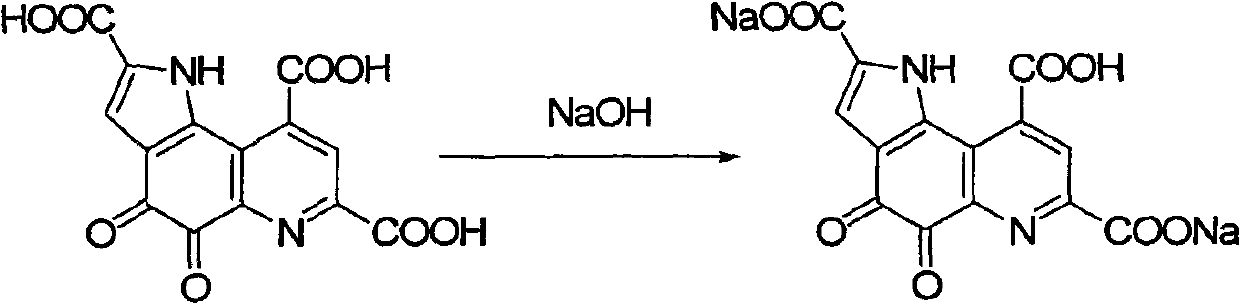
InactiveCN101885725AGood water solubilityMild reaction conditionsOrganic active ingredientsNervous disorderQuinolineStructural formula
The invention relates to a pyrro-quinoline quinine (PQQ) sodium salt derivative and a preparation method thereof, belonging to the field of pharmacy. In the preparation method, PQQ is used as a raw material, and an acid-base neutralization reaction with PQQ is carried out in a basic solvent of sodium hydroxide to prepare the PQQ sodium salt derivative represented in the structural formula (I), wherein R1, R2 and R3 can be identical or different and respectively represents H, ammonium ion (NH3), potassium ion, sodium ion, magnesium ion, calcium ion or zinc ion, and at least one of R1, R2 and R3 is sodium ion. The invention has mild reaction conditions, easy product refining and purification, simple preparation steps and high yield more than 80%. The PQQ sodium salt derivative has the functions of inhibiting GSK-3 activity, reducing senile pigment formation in a transgenic mouse brain, reducing tau protein phosphorylation and the like. Thus, the PQQ sodium salt derivative can be medically used for treating senile dementia and other diseases.
Owner:SHANGHAI RIXIN BIOTECHNOLOGY CO LTD
Cyanide-free zinc dipping solution and cyanide-free electroplating method of filter aluminium alloy by using the same
The invention discloses a cyanide-free zinc dipping solution and a cyanide-free electroplating method of filter aluminium alloy by using the cyanide-free zinc dipping solution. The cyanide-free zinc dipping solution, measured as per liter of cyanide-free zinc dipping solution, comprises 450-600 ml of plating bath solution and 400-550 ml of water, wherein the plating bath solution comprises 20-40 g / L zinc ions, 2-10 g / L nickel ions, 0.001-0.400 g / L copper ions, 0.05-1.00 g / L iron ions, 1-4 g / L cobalt ions, 80-200 g / L alkali metal hydroxide, 50-100 g / L main complexing agent, 13-40 g / L auxiliary complexing agent, and 0.07-2.00 g / L crystallization refiner. Zinc dipping and electroplating on materials with high silica content can be carried out by using the cyanide-free zinc dipping solution by just conducting zinc dipping once, the operation is simple, and the control is easy.
Owner:GUANGZHOU HONWAY TECH CORP +1
Akali zinc secondary cell and method for preparation thereof
InactiveUS20020164530A1Excellent in maintaining alkaline aqueous solutionIncrease internal resistanceCell seperators/membranes/diaphragms/spacersNon-aqueous electrolyte cellsAlkaline waterElectrical battery
An alkaline zinc secondary battery in accordance with the present invention comprises a separator layer comprising a gel electrolyte comprising a water absorbent polymer and an alkaline aqueous solution, disposed between a negative electrode comprising at least one selected from the group consisting of zinc and zinc oxide and a positive electrode. Because the separator layer is in a gel form, transfer of zinc ions is limited. Thereby, deformation of the negative electrode and generation of dendrite due to charge and discharge of the battery are substantially inhibited. Moreover, since impurity ions such as nitrate ions become less prone to move, self-discharge of the battery is also inhibited.
Owner:PANASONIC CORP
Sanitary product structure for human body with functions of sterilization and deodorization
InactiveUS20150164680A1Improve the bactericidal effectImprove securityNon-surgical orthopedic devicesBaby linensAluminum IonPlatinum
The invention relates to a sanitary product structure for human body with functions of sterilization and deodorization. It comprises a main body and at least one metal ion layer with the functions of sterilization and deodorization. The metal ion layer is at least disposed on the surface of the main body, wherein the metal ion layer is a cover layer composed of germanium ions, molybdenum ions, magnesium ions, manganese ions, chromium ions, vanadium ions, zinc ions, silicon ions, platinum ions, aluminum ions, selenium ions, calcium ions, titanium ions or any combinations thereof. Accordingly, the human body sanitary product has the effects of sterilization and deodorization.
Owner:CHEN TENG LAI
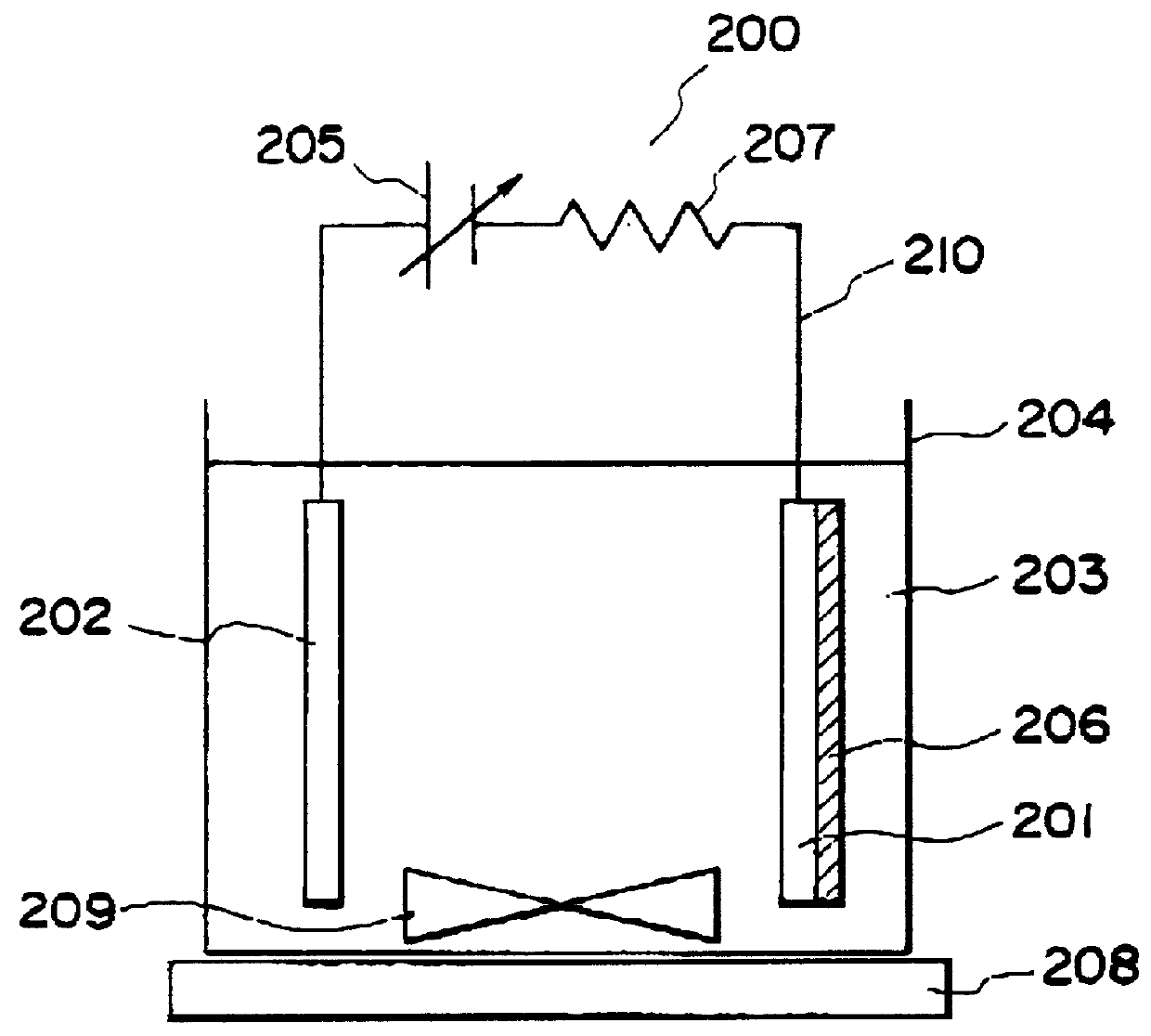
Method for preparing zinc oxide nanostructures and zinc oxide nanostructures prepared by the same
InactiveUS20090098043A1Increase probabilityIncrease ratingsMaterial nanotechnologyLiquid surface applicatorsZinc ionHexamethylenamine
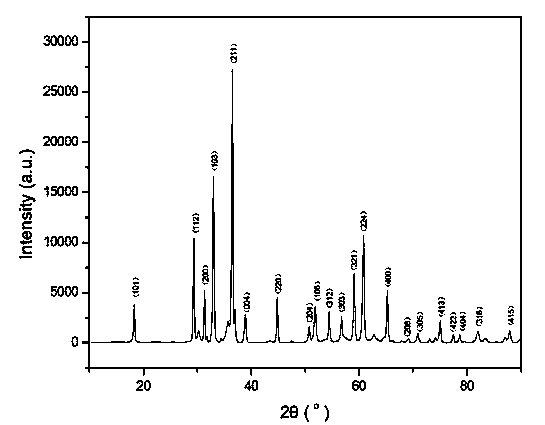
Example embodiments provide a method for preparing zinc oxide nanostructures. According to the method, zinc oxide nanostructures are prepared by dipping a substrate having a zinc (Zn) seed layer thereon in an aqueous solution of hexamethyleneamine and dropwise adding an aqueous solution of zinc nitrate to the aqueous solution of hexamethyleneamine. In addition, zinc ions can be continuously supplied in a constant amount as the reactions of the reactants proceed to prepare high-quality zinc oxide nanostructures at a high growth rate. Furthermore, zinc oxide nanostructures can be prepared on a large-area substrate at a low processing temperature regardless of the substrate material. Example embodiments also provide zinc oxide nanostructures prepared by the method.
Owner:SAMSUNG ELECTRONICS CO LTD
Hybrid energy storage device with zinc ion battery and supercapacitor
The invention relates to a hybrid energy storage device with a zinc ion battery and a supercapacitor. The hybrid energy storage device with the zinc ion battery and the supercapacitor comprises a core body, an electrolyte and a shell body. The core body is composed of a positive electrode, a negative electrode and a separator between the positive electrode and the negative electrode. The positive electrode and the negative electrode are respectively formed by active substances, a conductive agent and a current collector, wherein the active substances and the conductive agent adhere to the current collector. The two positive electrode active materials are provided. The first positive electrode active material is a composite metal oxide, and the second positive electrode active material is a carbon material capable of conducting reversible adsorption of ions. The two negative electrode active materials are provided. The first negative electrode active material is zinc and the second negative electrode active material is a carbon material capable of conducting reversible adsorption of ions. The electrolyte is composed of zinc salt and deionized water. The composite metal oxide is simple in preparation technology and environmentally friendly.
Owner:CHINA FIRST AUTOMOBILE
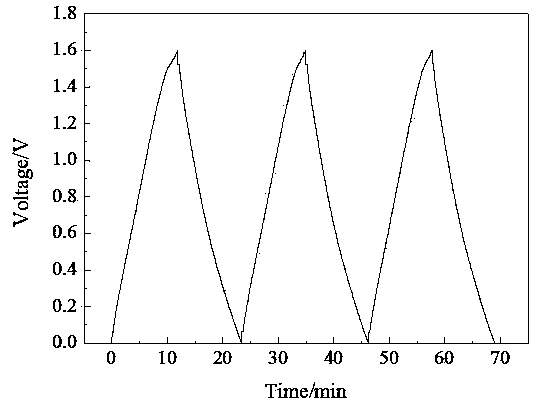
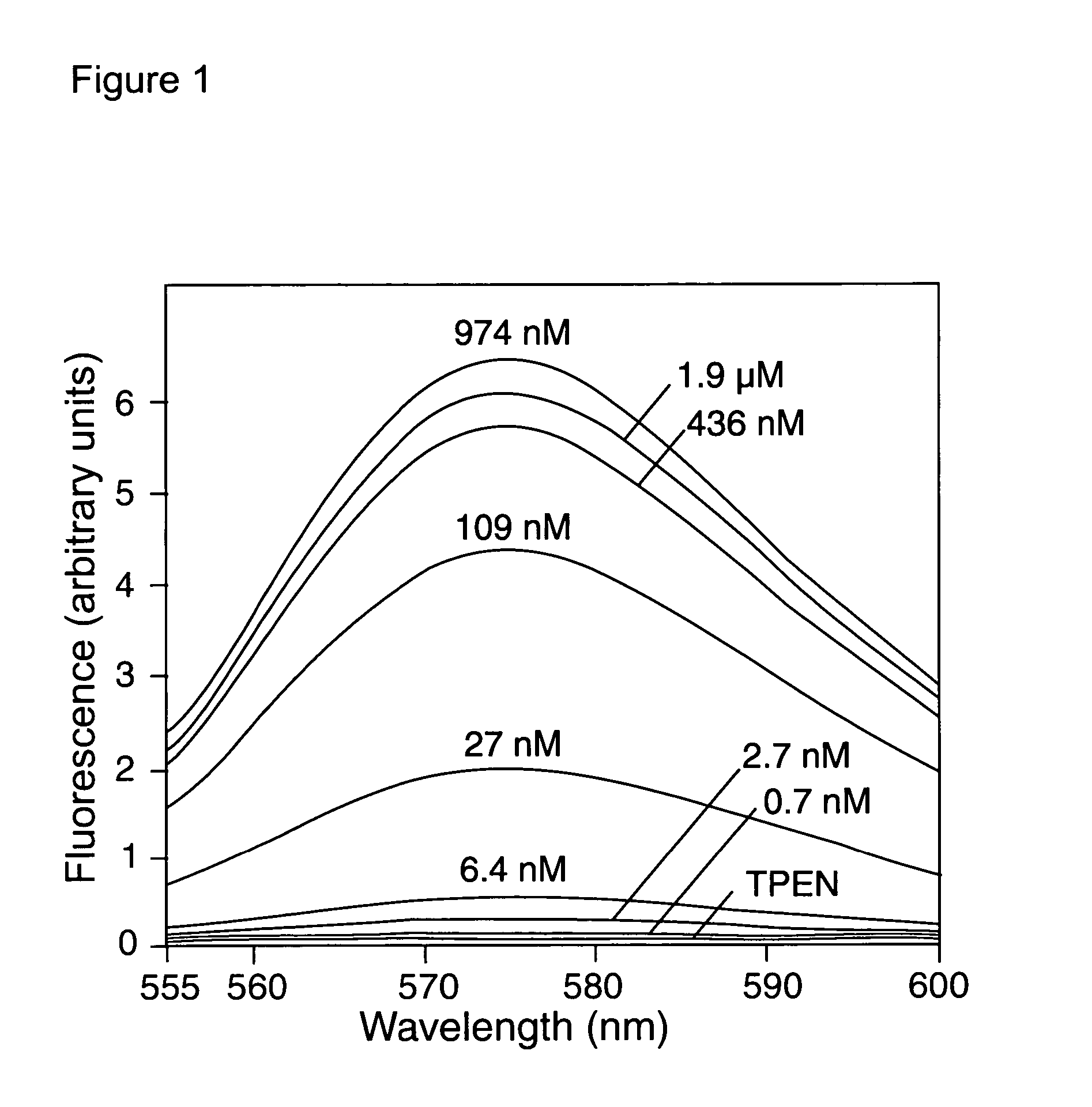
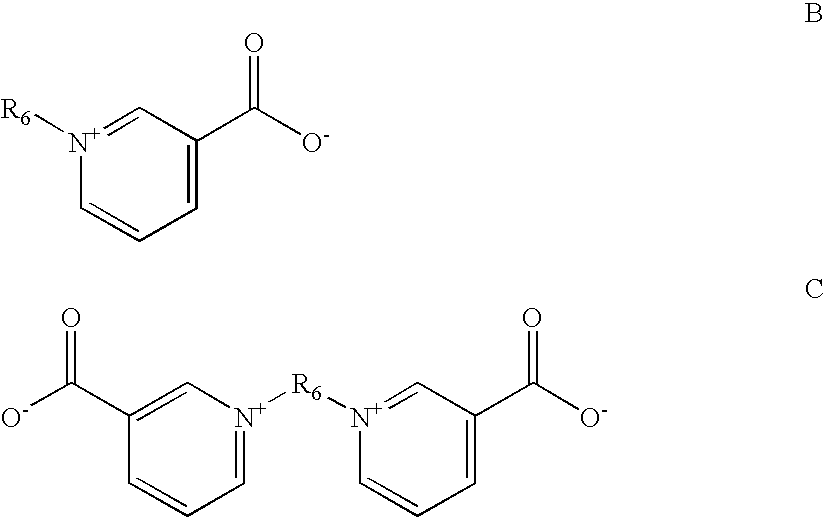
Zinc binding compounds and their method of use
InactiveUS20050250214A1Efficient ConcentrationAnalysis using chemical indicatorsOrganic chemistryMetal chelateZinc binding
The present invention provides a metal chelator and methods that facilitate binding, detecting, monitoring and quantitating of zinc ions in a sample. The metal chelating moiety of the zinc-binding compound is an analog of the well-known calcium chelator, BAPTA (1,2-bis(2-aminophenoxy)ethane-N,N,N′N′-tetraacetic acid), wherein the chelating moiety has been modified from a tetraacetic acid moiety to a tri- di- or monoacetic moiety. This change in acetic acid groups on the metal chelating moiety results in the selective bindings of zinc ions in the presence of calcium ions, both of which are present in biological fluids and intracellular cytosolic fluid and organelles.
Owner:MOLECULAR PROBES
Alkaline zinc-nickel alloy plating compositions, processes and articles therefrom
The present invention relates to a process for electroplating a zinc-nickel alloy on a substrate, including electroplating the substrate in an aqueous zinc-nickel electroplating bath, including water; nickel ion; zinc ion; at least one complexing agent; and at least one non-ionogenic, surface active polyoxyalkylene compound, wherein the bath has an alkaline pH. In one embodiment, the zinc ion, the nickel ion and the non-ionogenic surface active polyoxyalkylene compound are present at concentrations sufficient to deposit a zinc-nickel alloy comprising a substantially gamma phase.
Owner:OPASKAR VINCENT C +1
Calcium zinc heat stabilizer used for PVC, zinc-containing compound and application
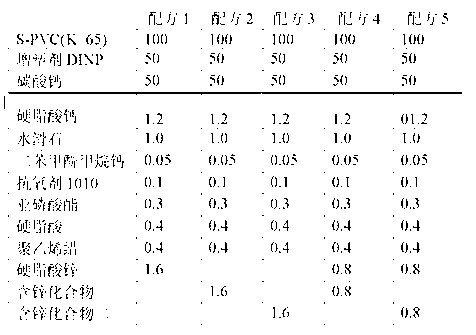
ActiveCN103183690AHigh activityStable unstable chlorineZinc organic compoundsCompounds of zincZinc ion
The invention relates to a calcium zinc heat stabilizer used for PVC. The calcium zinc heat stabilizer comprises a compound formed by a hydroxyl phenyl carboxylic acid, imidazole ligands and zinc ions, wherein the molecular formula of the compound is Zn(L1)2(L2)2, wherein L1 represents salicylic acid; L2 represents imidazole or L2 represents 1,2-dimethyl imidazole; a frequently-used fatty acid calcium, fatty acid zinc, an auxiliary stabilizer and a lubricating agent. Compared with a calcium zinc heat stabilizer prepared by using the fatty acid zinc and the like as raw materials, the calcium zinc heat stabilizer provided by the invention can effectively improve initial dyeing property and prolong zinc burnt blackening time of a PVC product, has easily available raw materials and is convenient for preparation.
Owner:SHENZHEN AIMSEA IND
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com