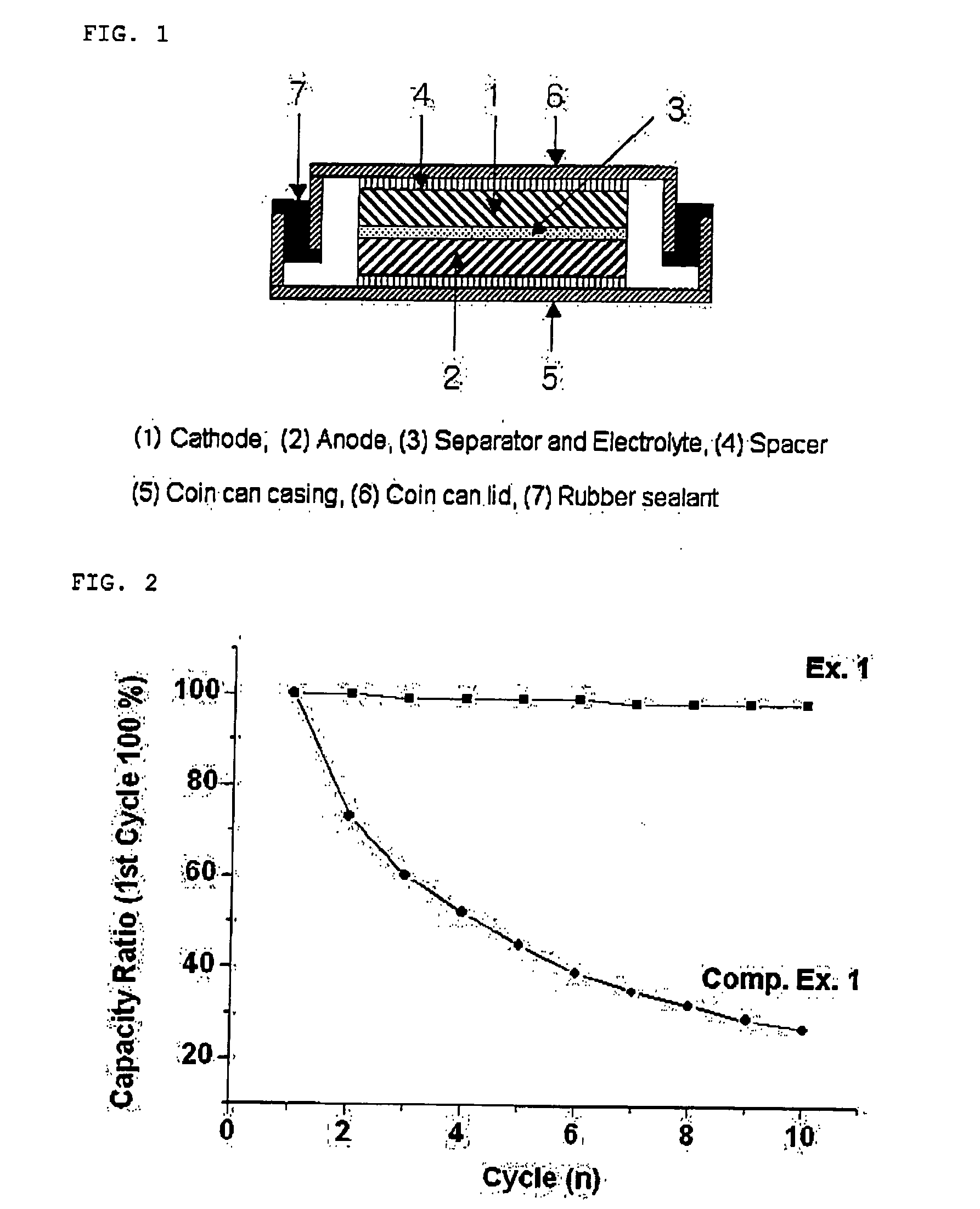
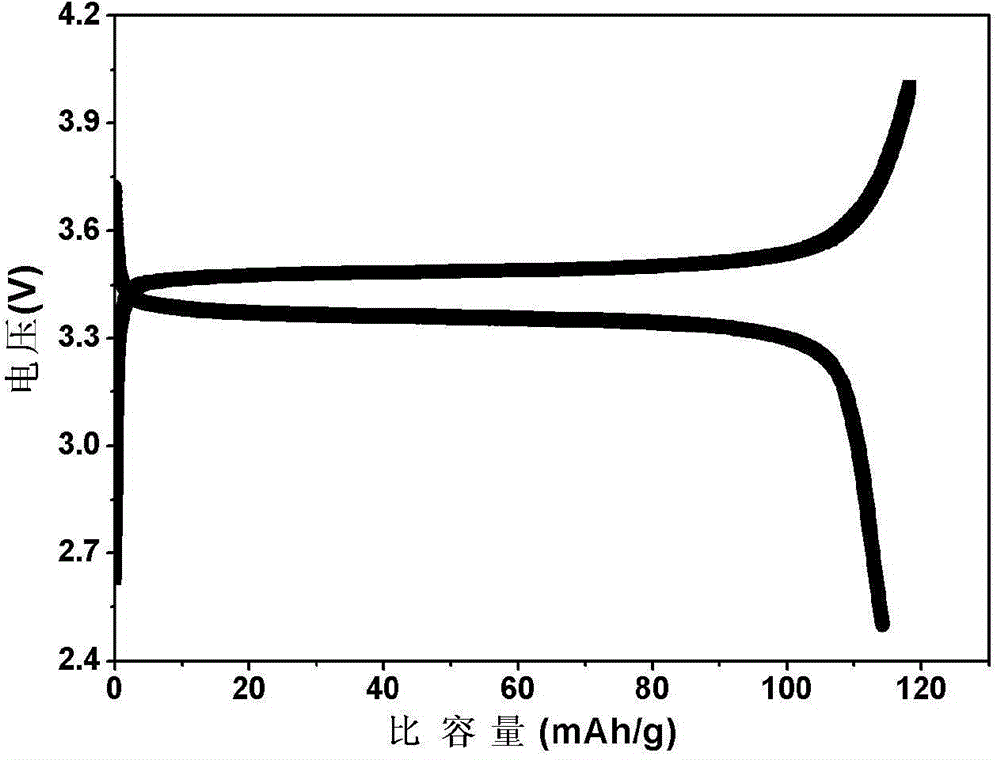
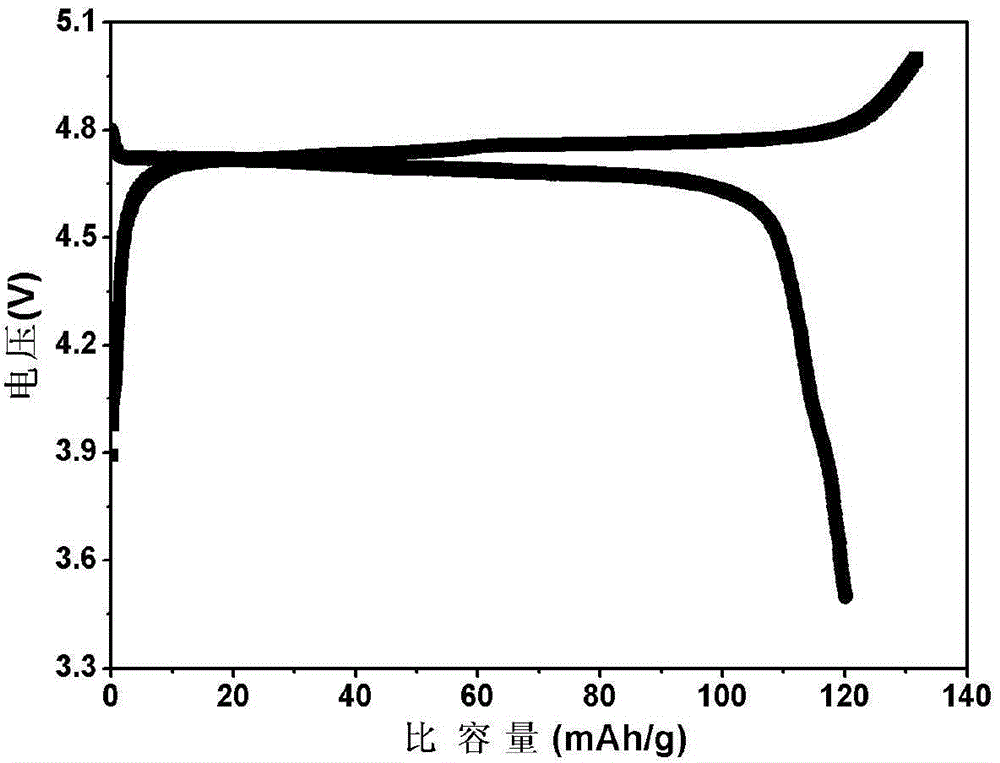
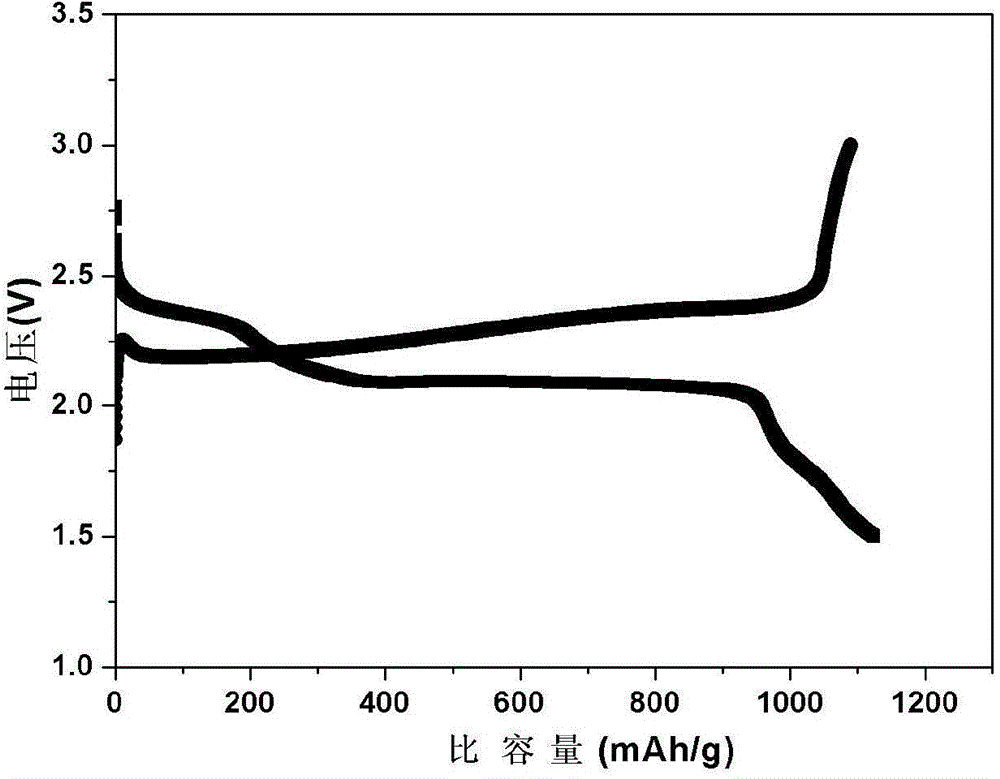
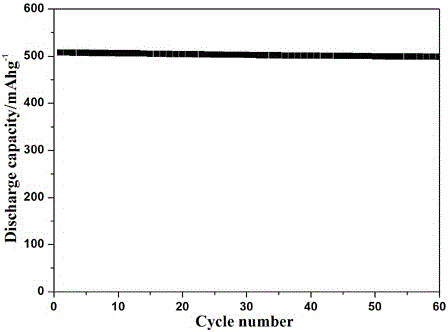
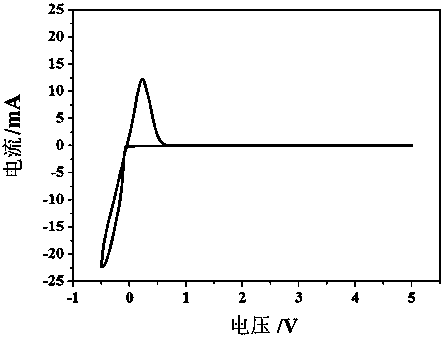
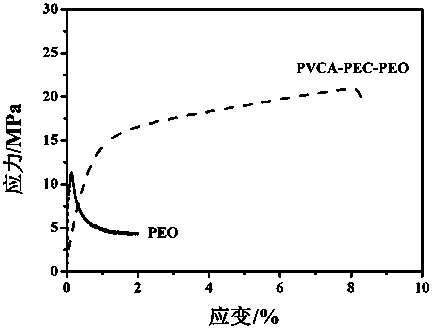
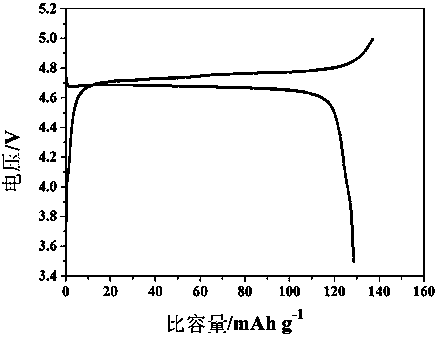
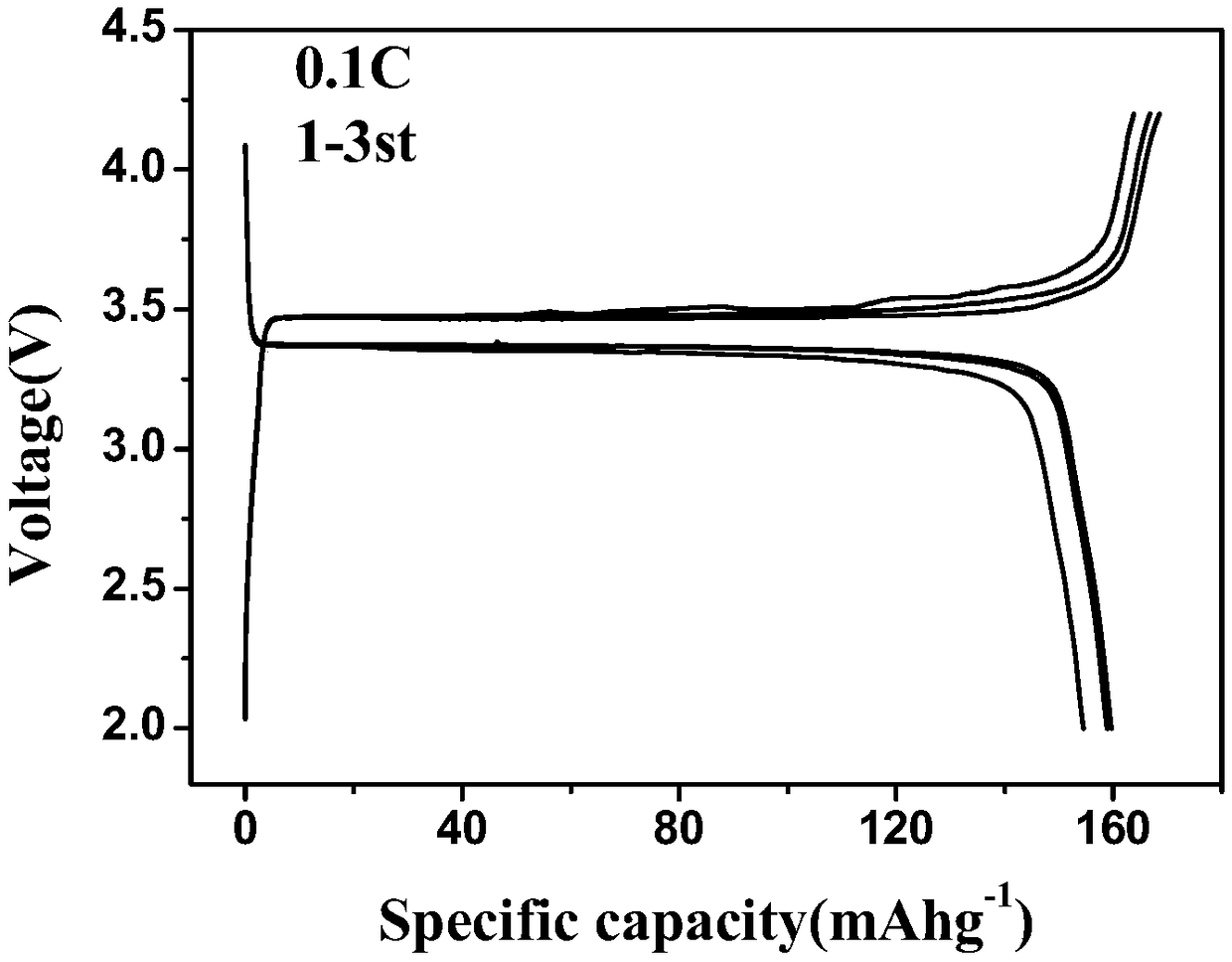
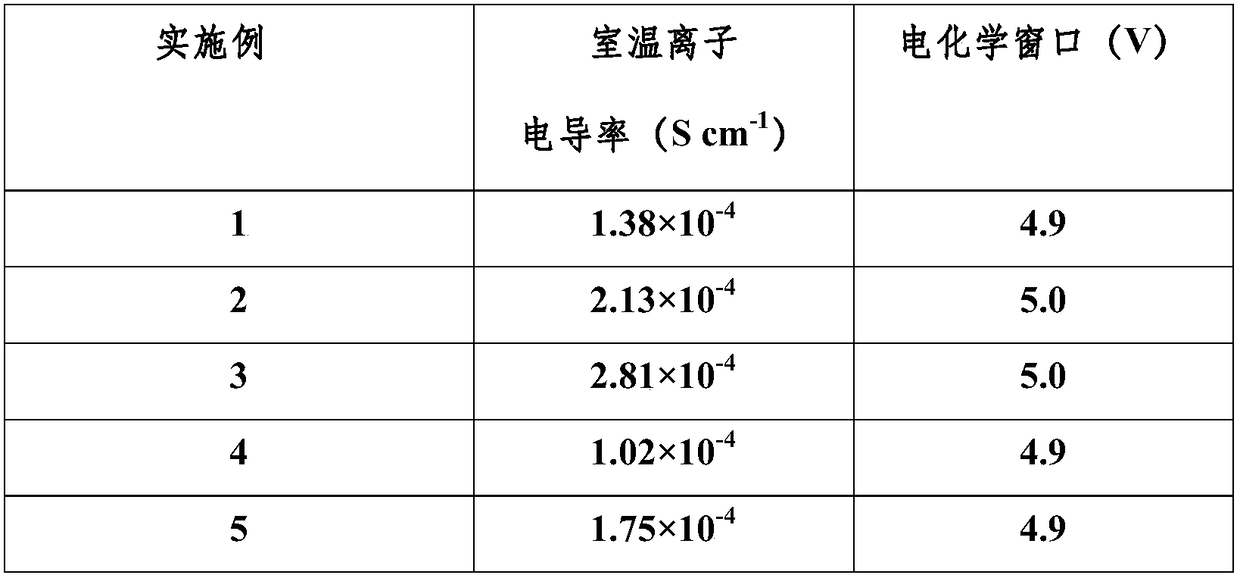
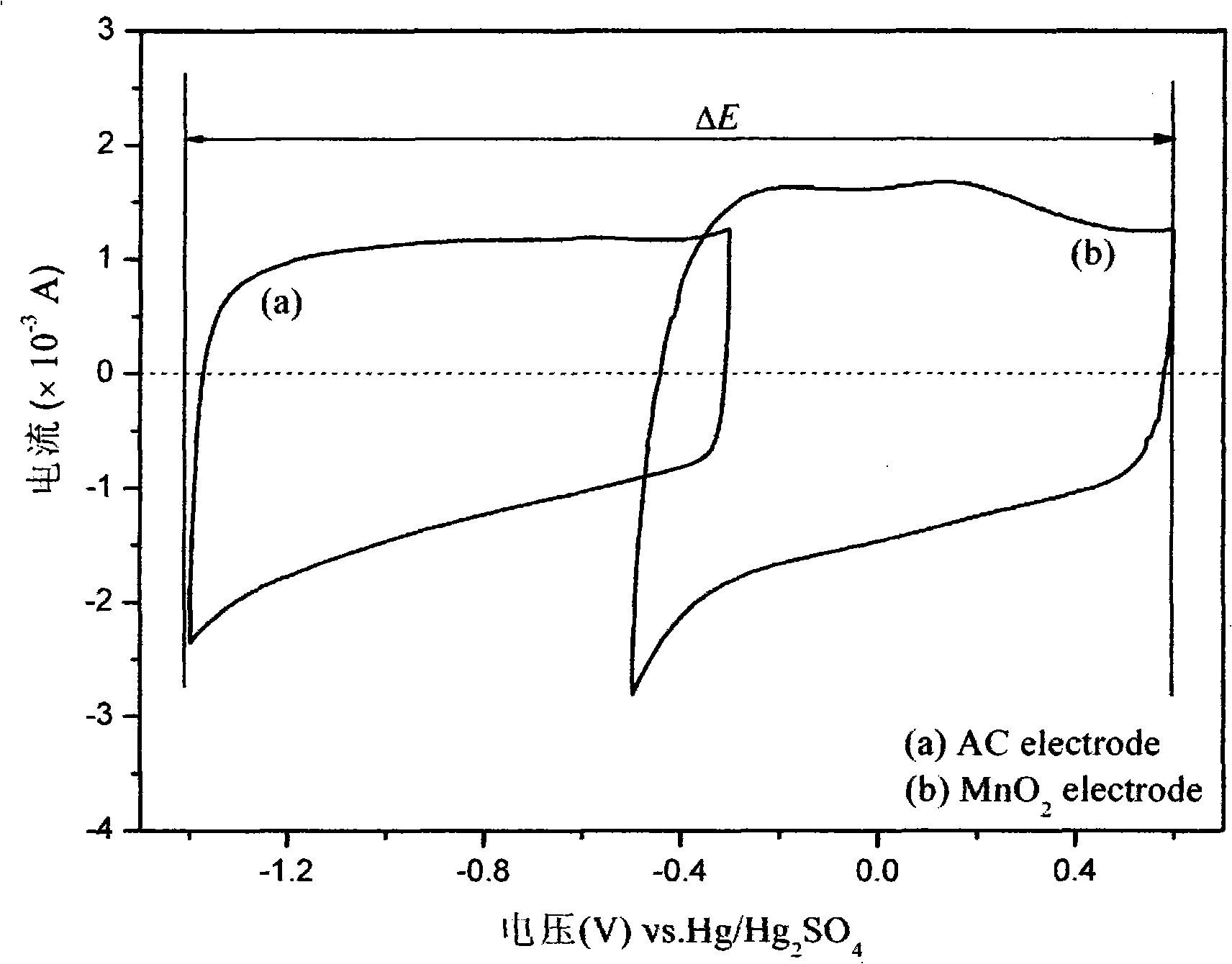
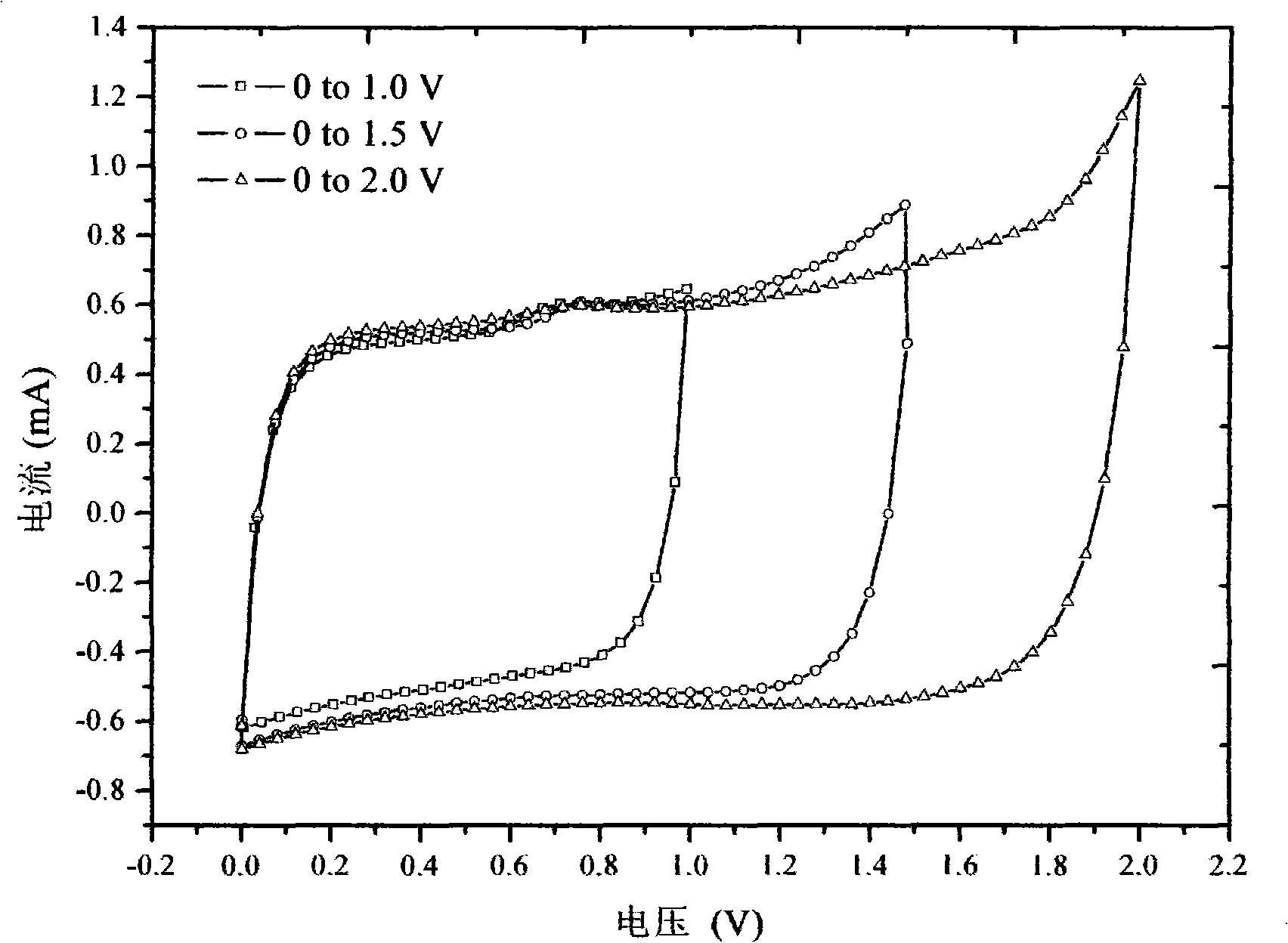
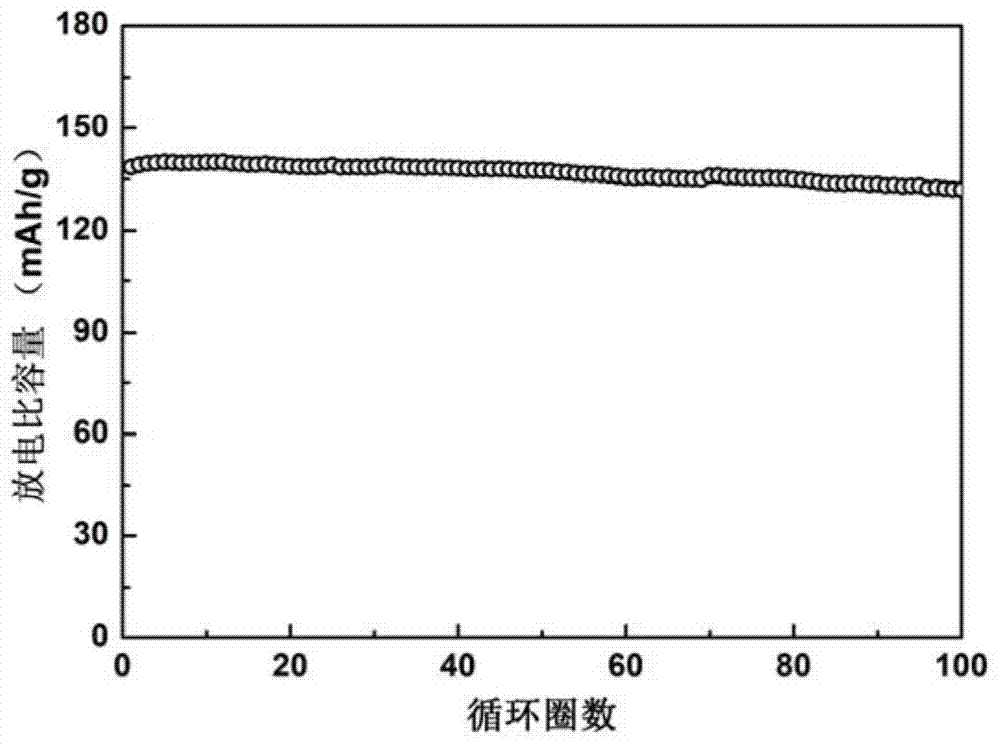
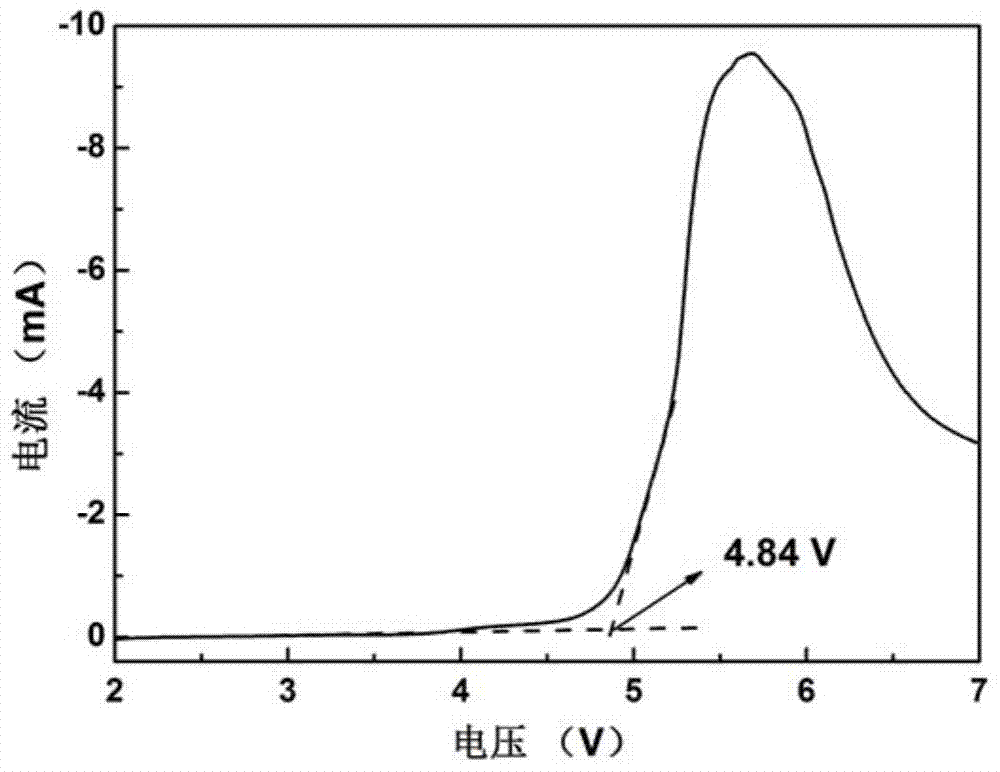
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
640 results about "Electrochemical window" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
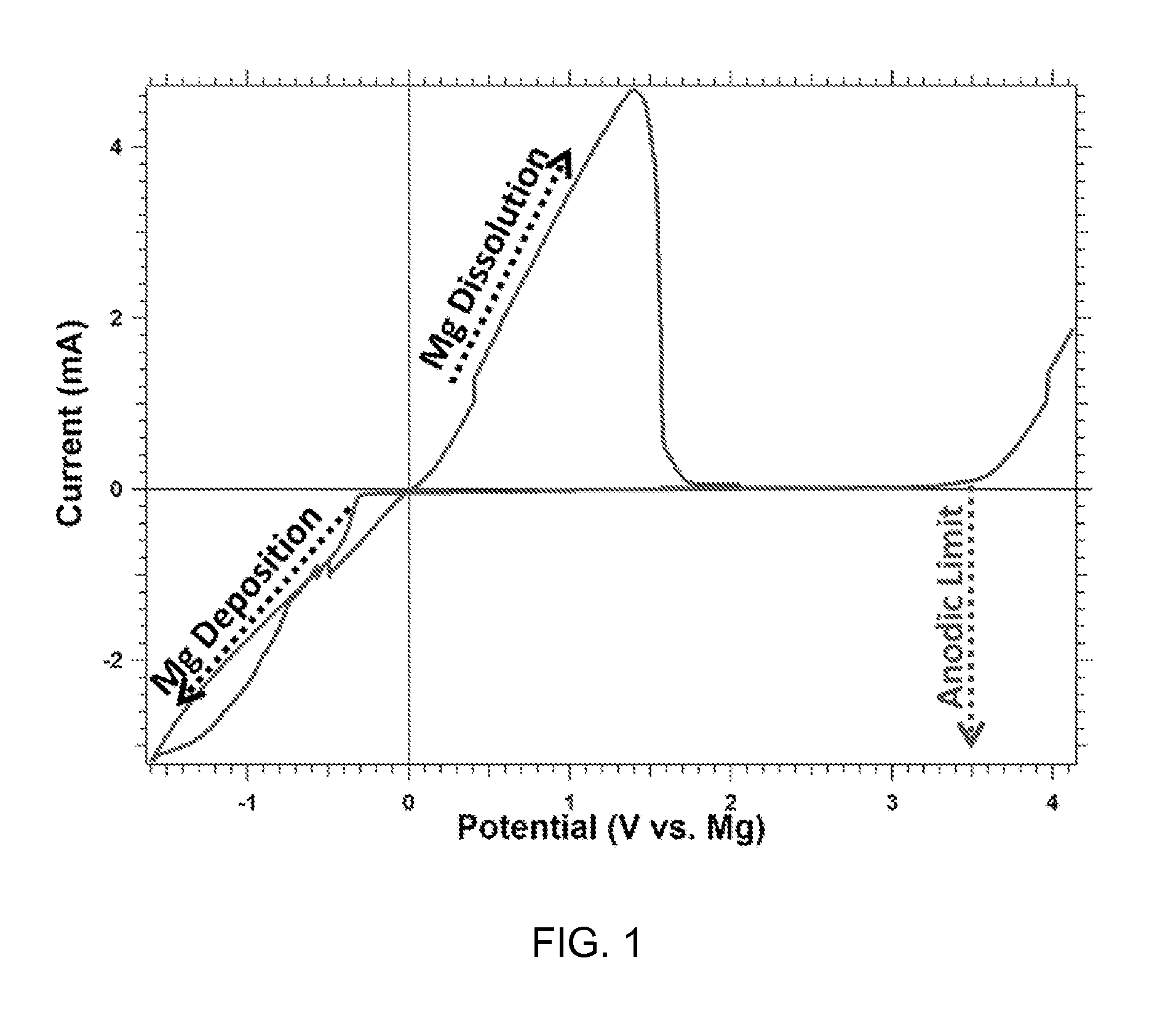
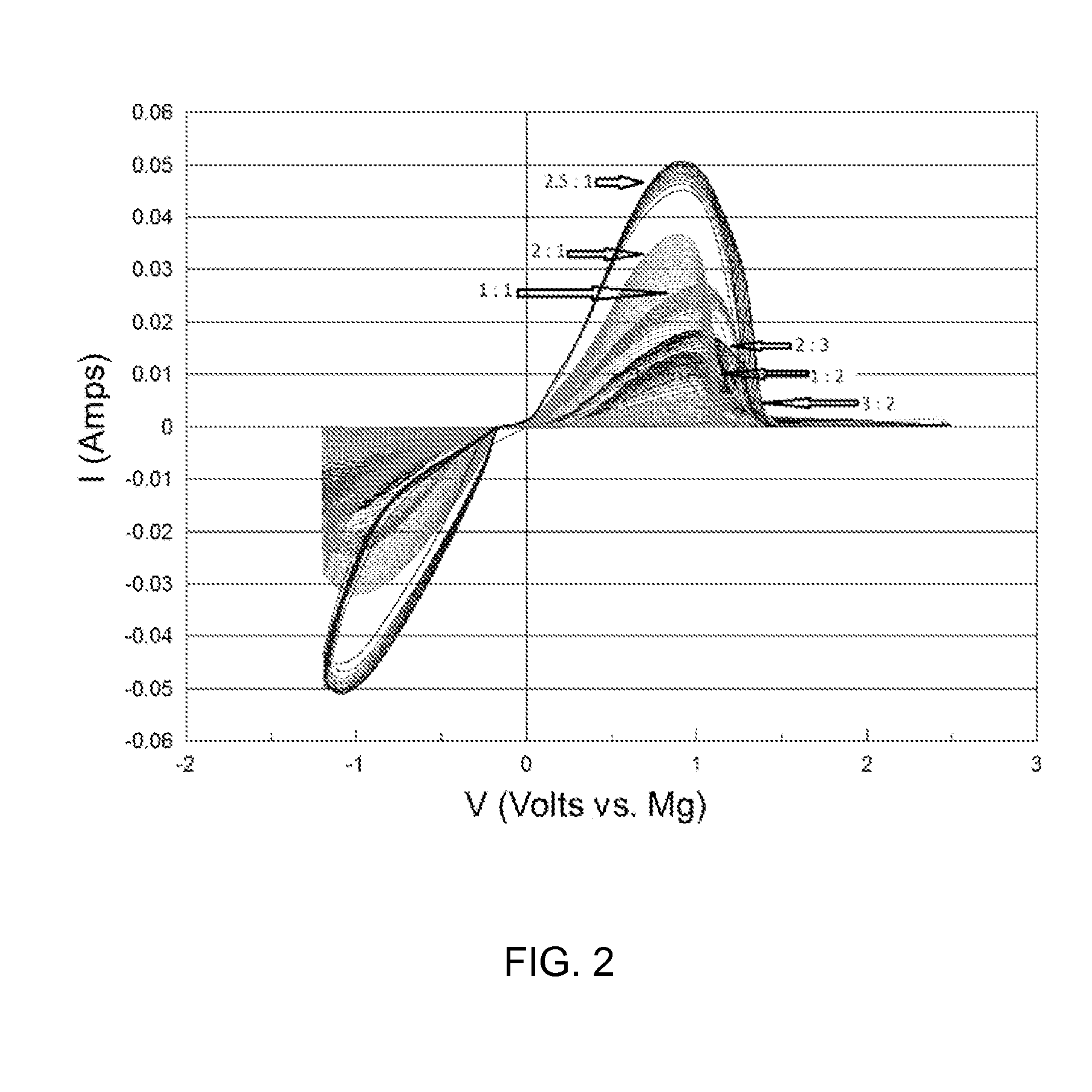
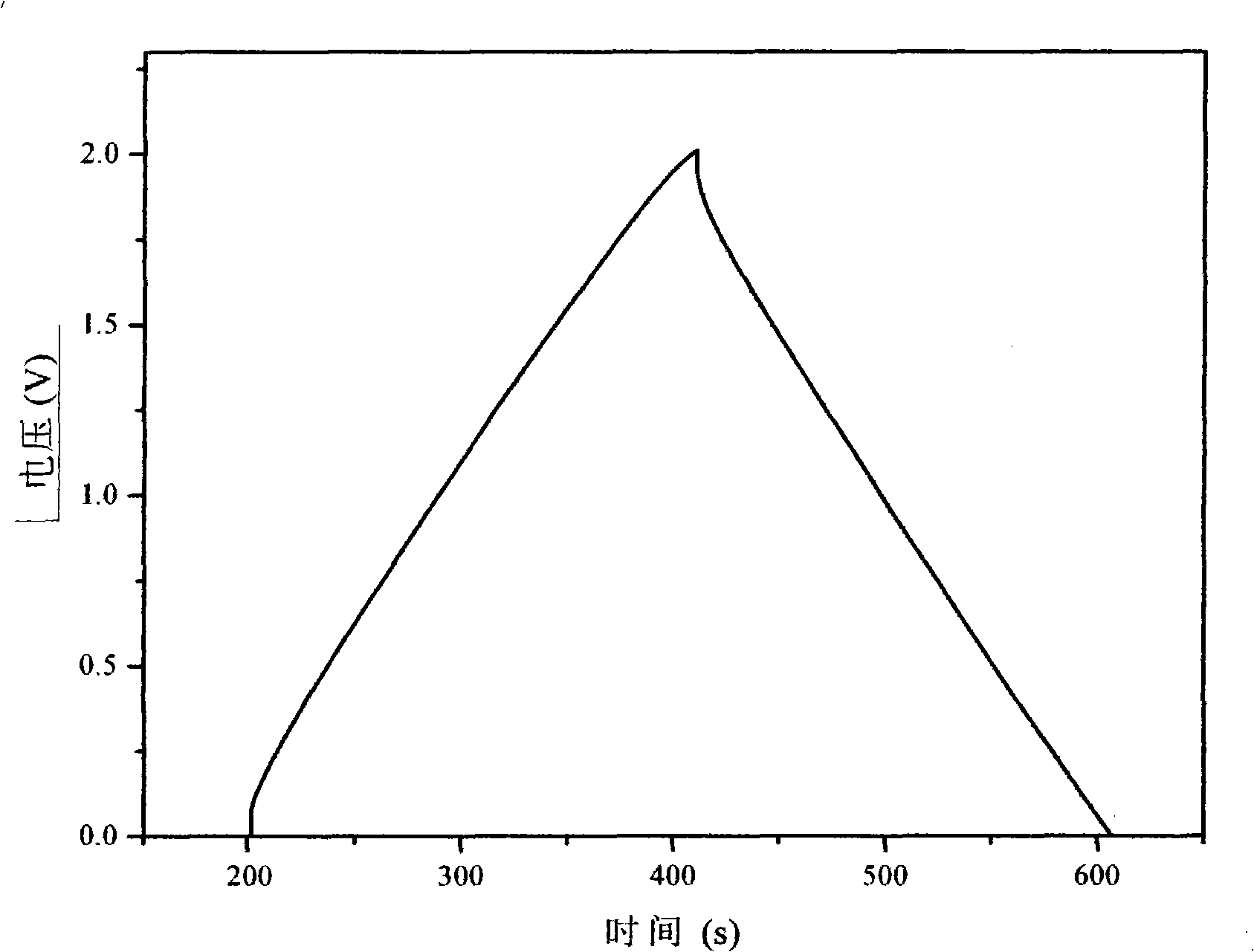
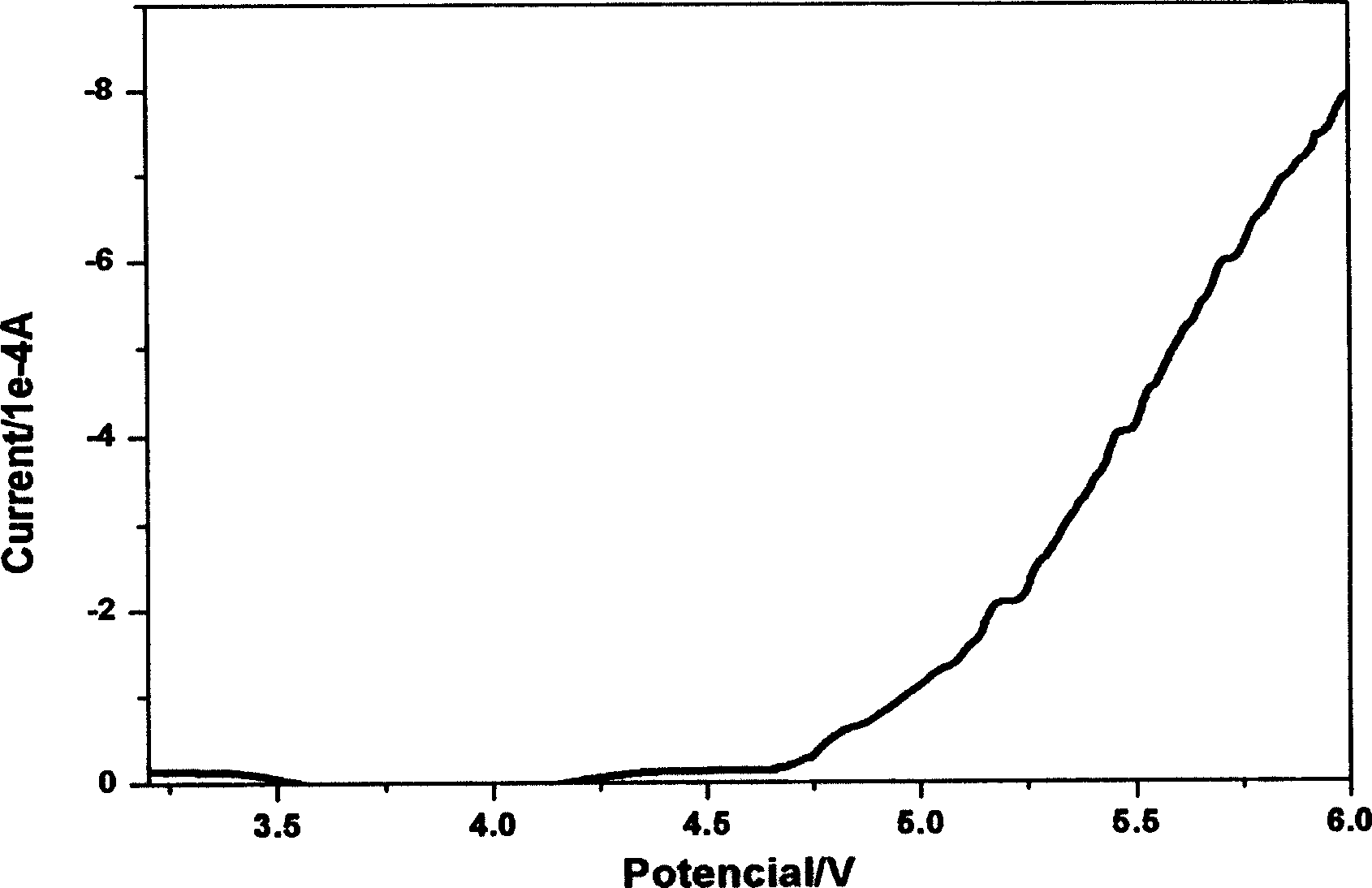
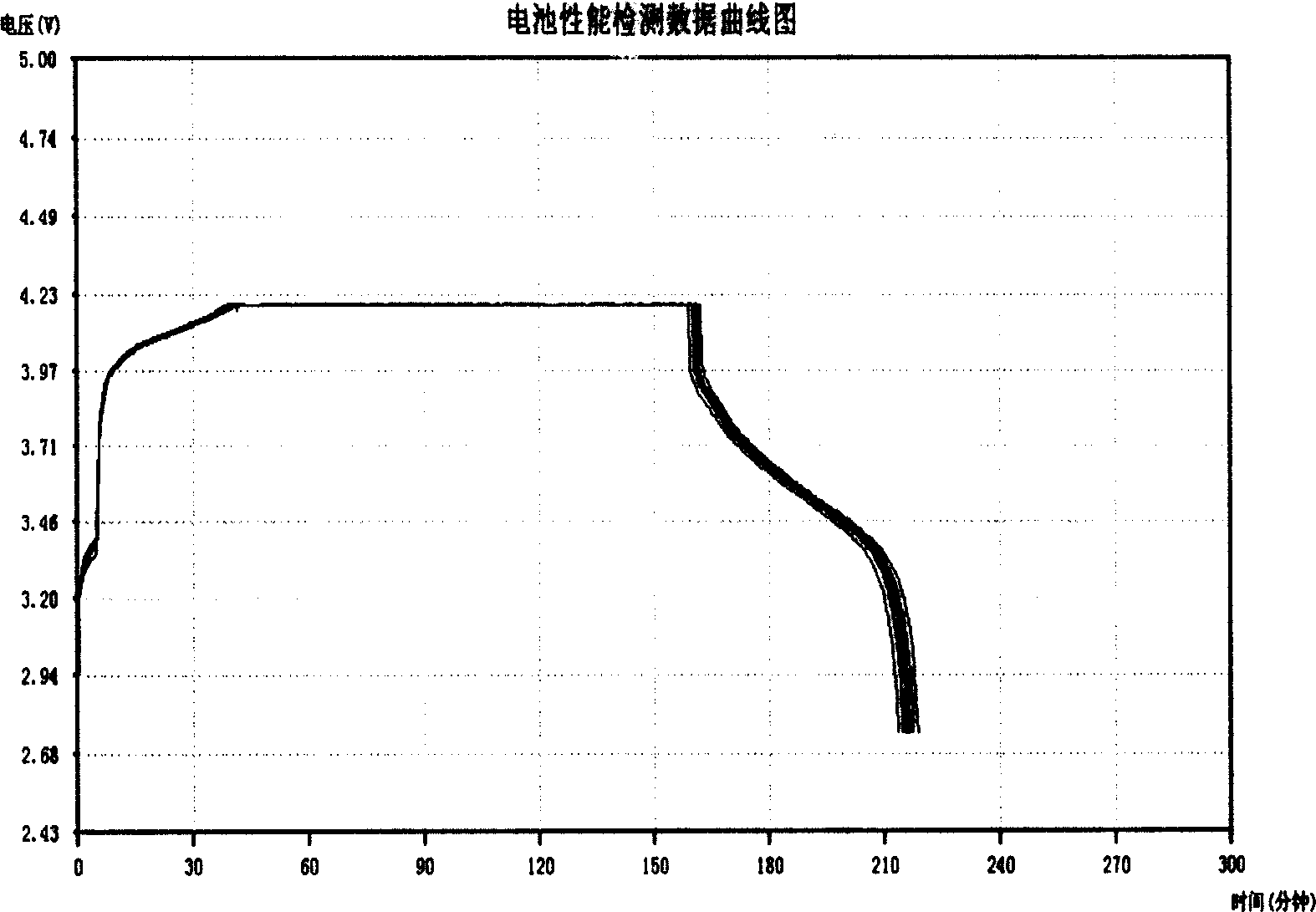
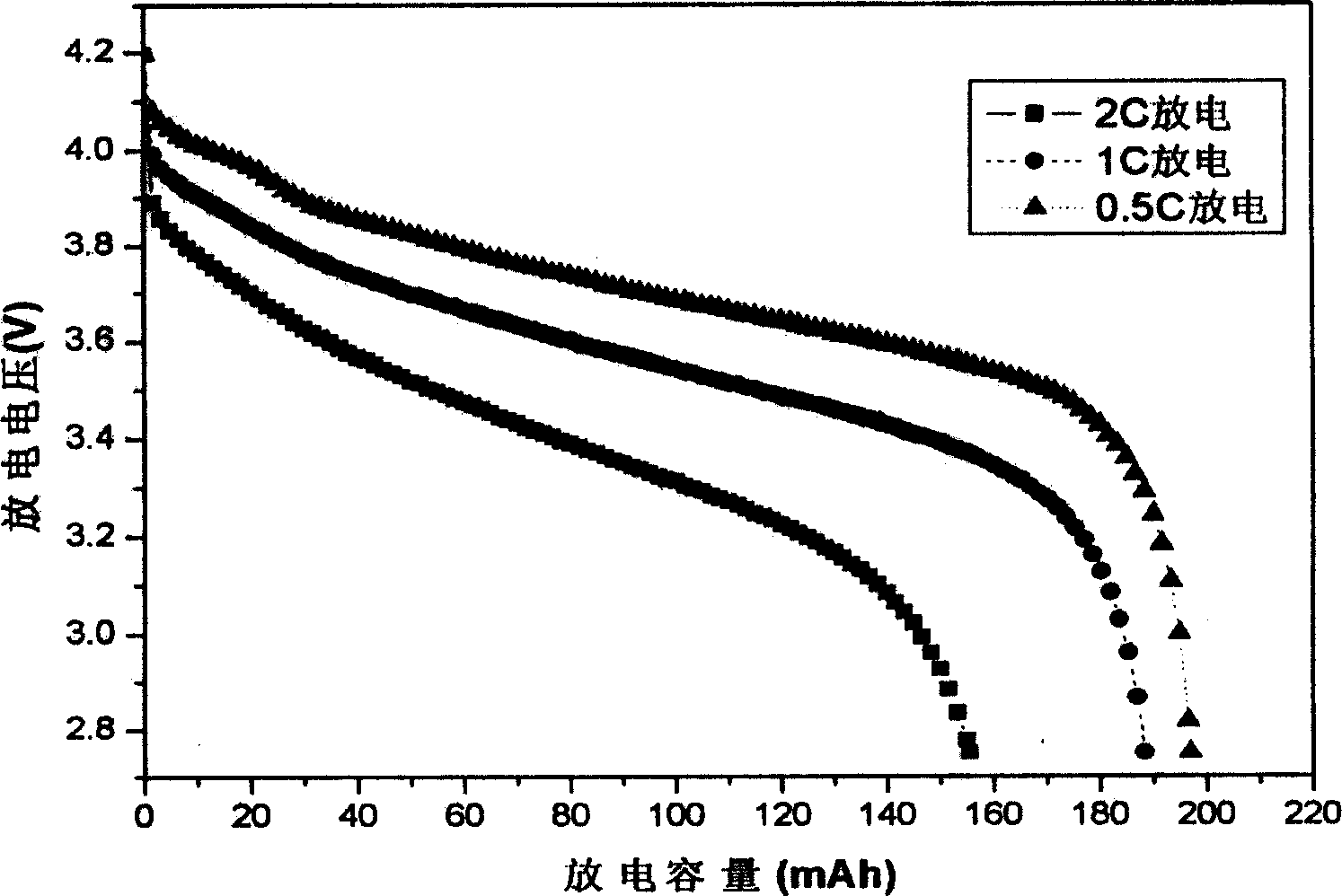
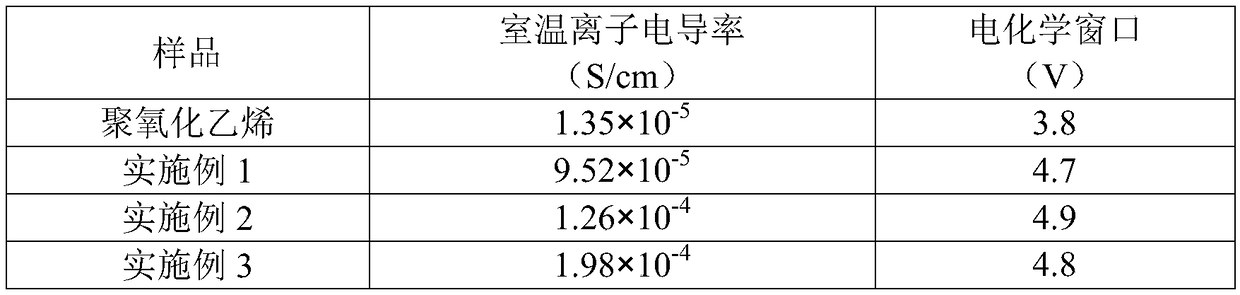
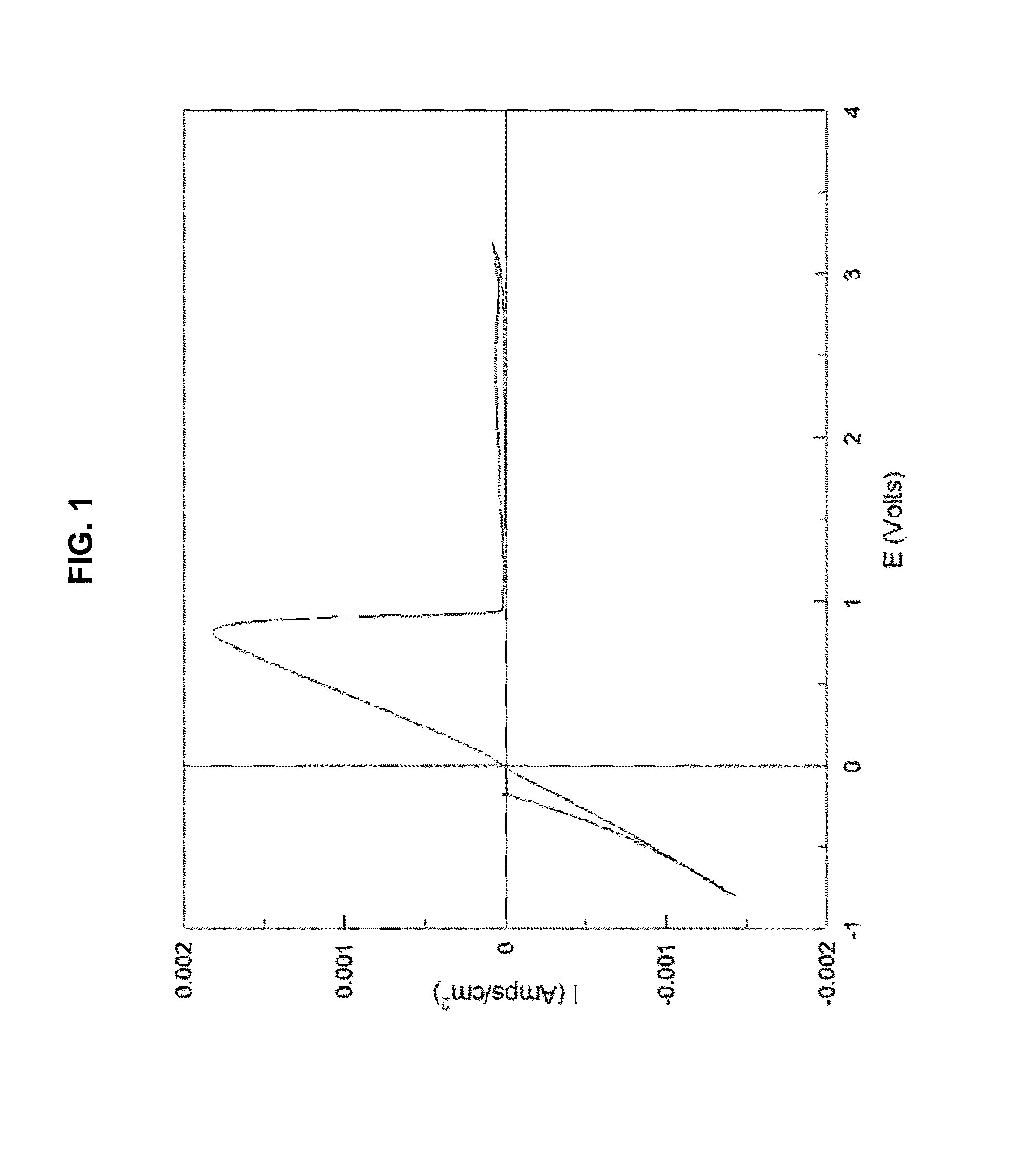
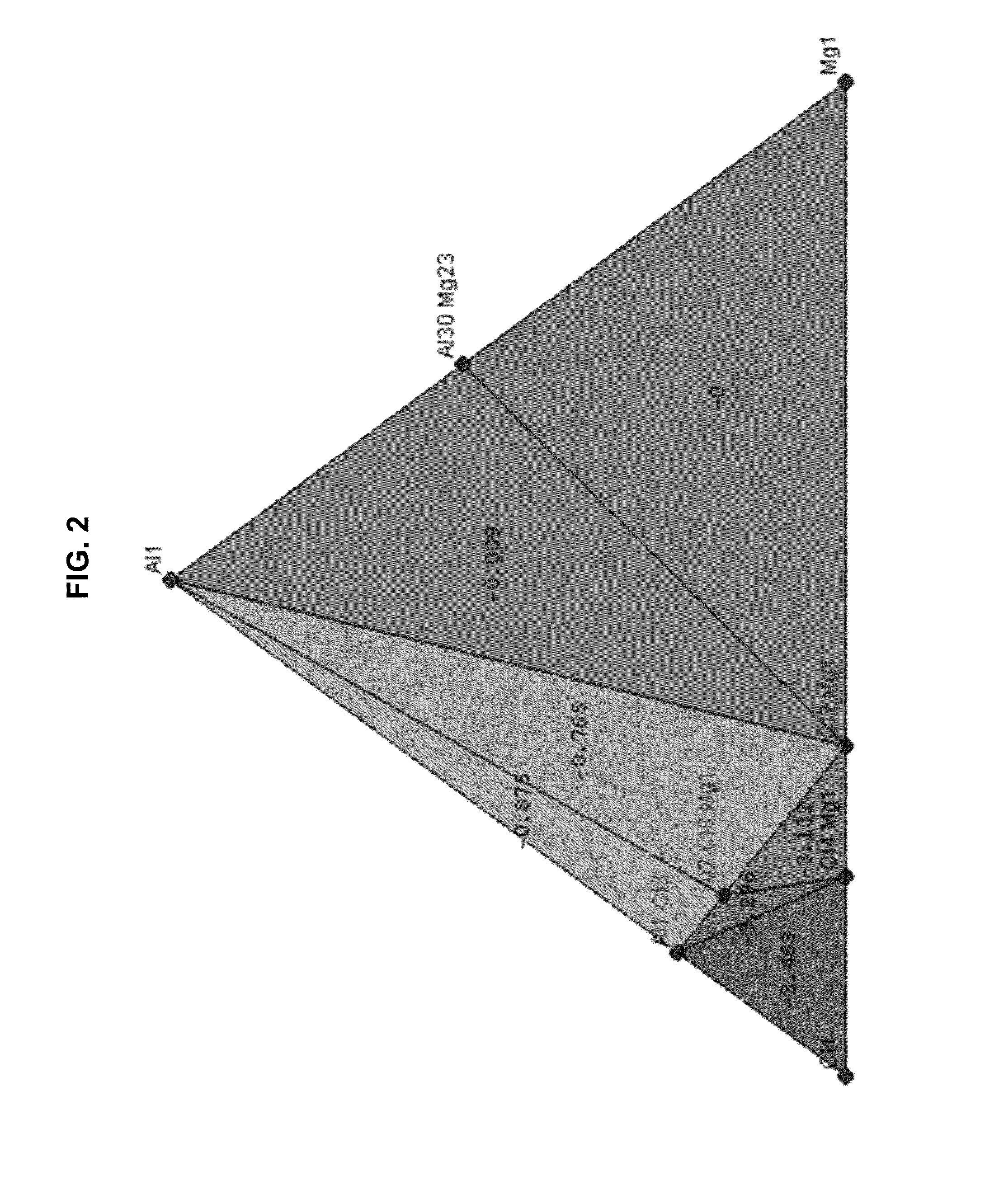
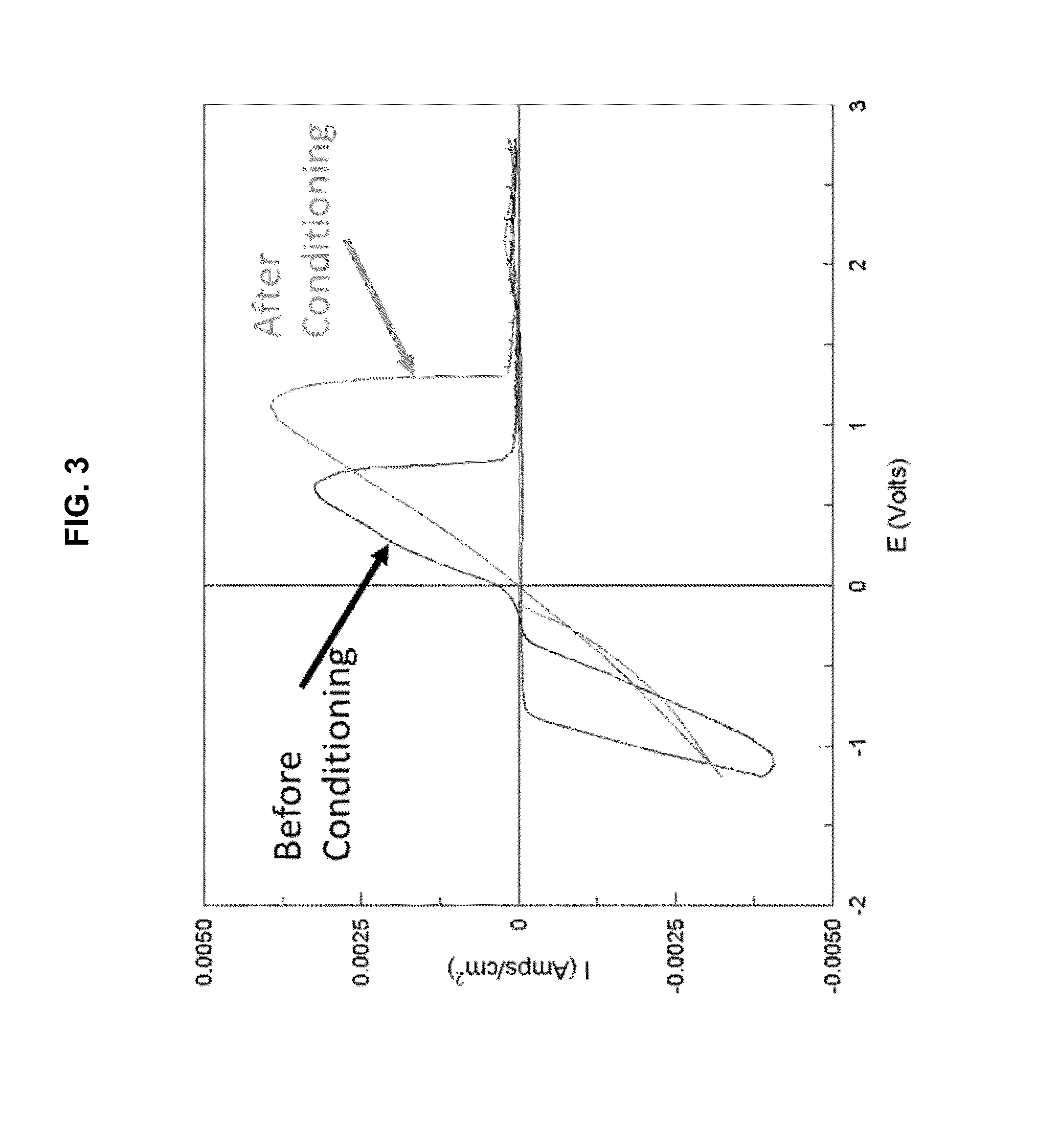
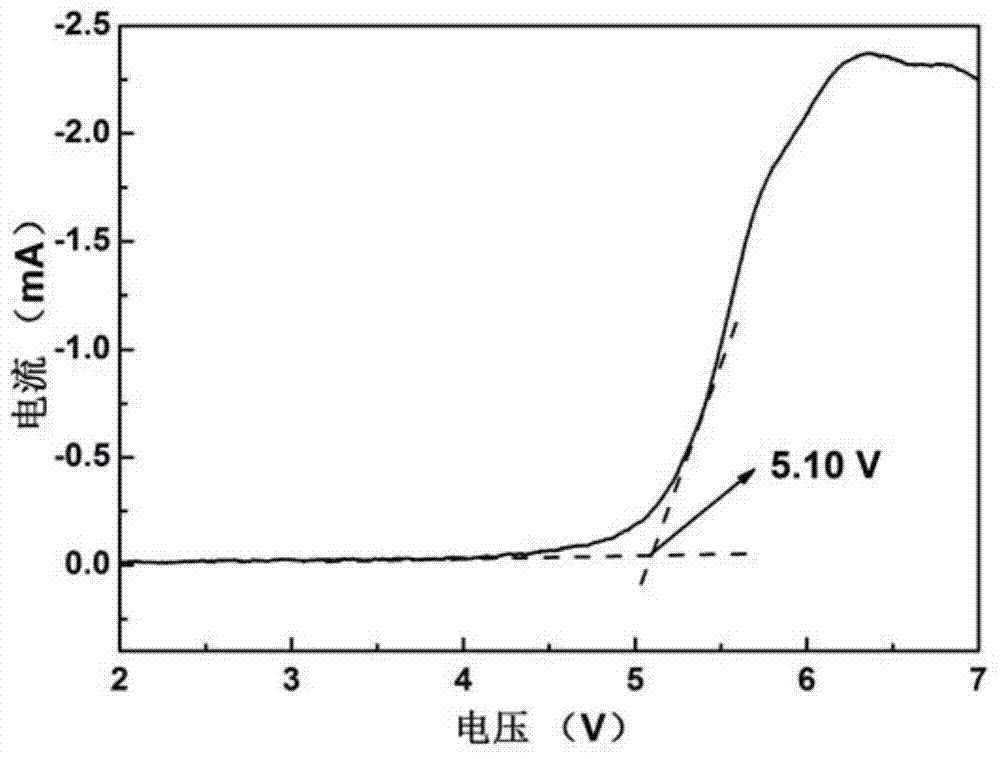
The electrochemical window (EW) of a substance is the voltage range between which the substance is neither oxidized nor reduced. The EW is one of the most important characteristics to be identified for solvents and electrolytes used in electrochemical applications. The EW is a term that is commonly used to indicate the potential range and the potential difference. It is calculated by subtracting the reduction potential (cathodic limit) from the oxidation potential (anodic limit) . When the substance of interest is water, it is often referred to as the water window.
Organic-inorganic all-solid-state composite electrolyte as well as preparation and application methods thereof
PendingCN106785009AIncrease transport activityImprove wettabilitySolid electrolytesSecondary cellsElectrical conductorComposite electrolyte
The invention relates to organic-inorganic all-solid-state composite electrolyte as well as preparation and application methods thereof, belonging to the field of lithium ion batteries. According to the organic-inorganic all-solid-state composite electrolyte, a highly-ordered three-dimensional connection network skeleton is formed by an inorganic fast lithium ion conductor, and a three-dimensional connection network is filled with a polymer and lithium salt. The organic-inorganic all-solid-state composite electrolyte which is flexible and has a controllable three-dimensional connection network structure is prepared. The electrolyte is high in lithium ion conductivity, wide in electrochemical window, good in mechanical property and stable to lithium metal. A lithium ion secondary battery assembled by a composite electrolyte membrane prepared by the method is high in capacity, stable in cycle performance, low in interface impedance and good in interface stability.
Owner:UNIV OF SCI & TECH BEIJING
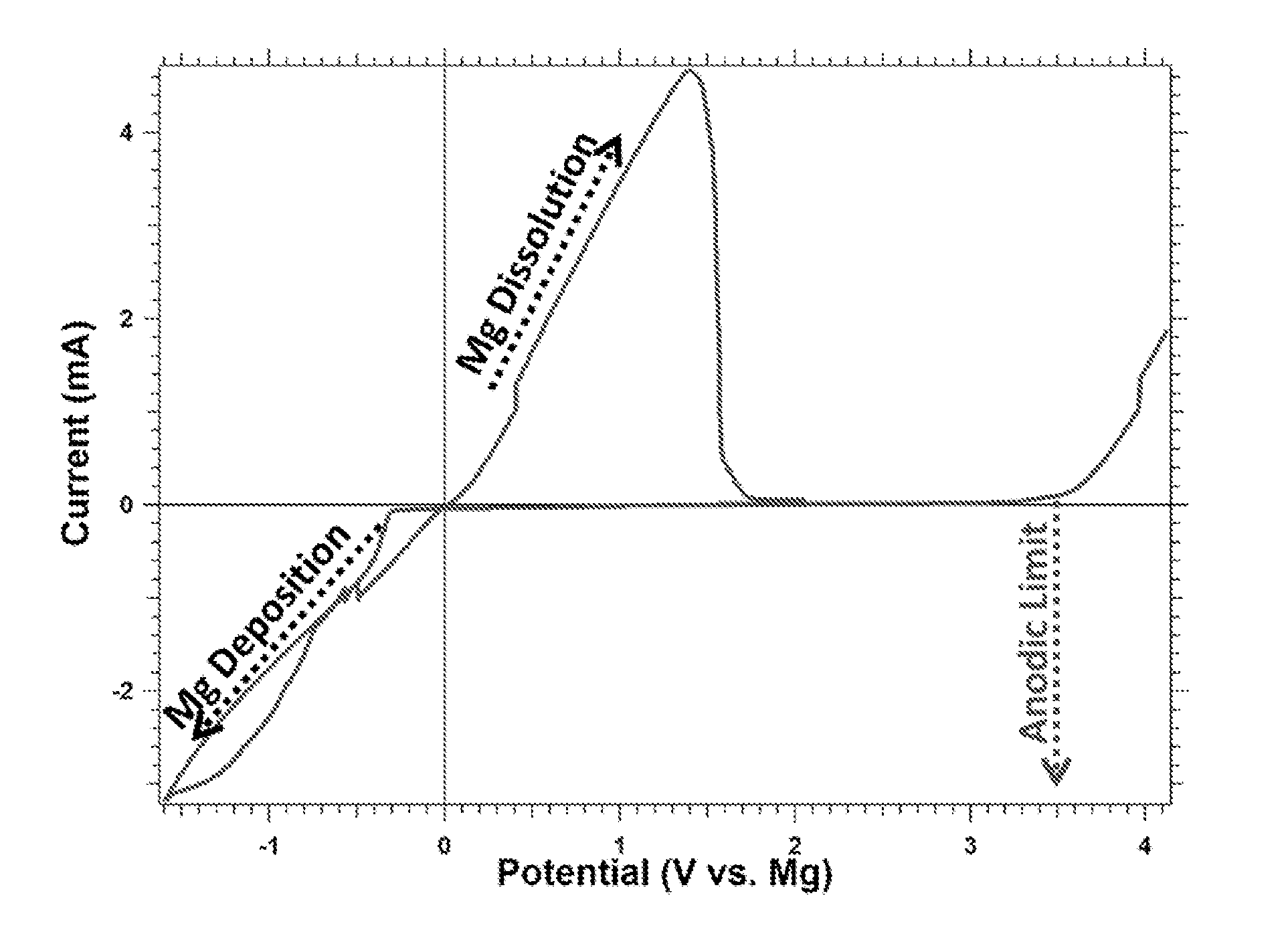
Rechargeable magnesium battery
ActiveUS20080182176A1Improved kineticsImprove conductivityMaterial nanotechnologyPhosphorus sulfur/selenium/tellurium compoundsElectrochemical windowCopper
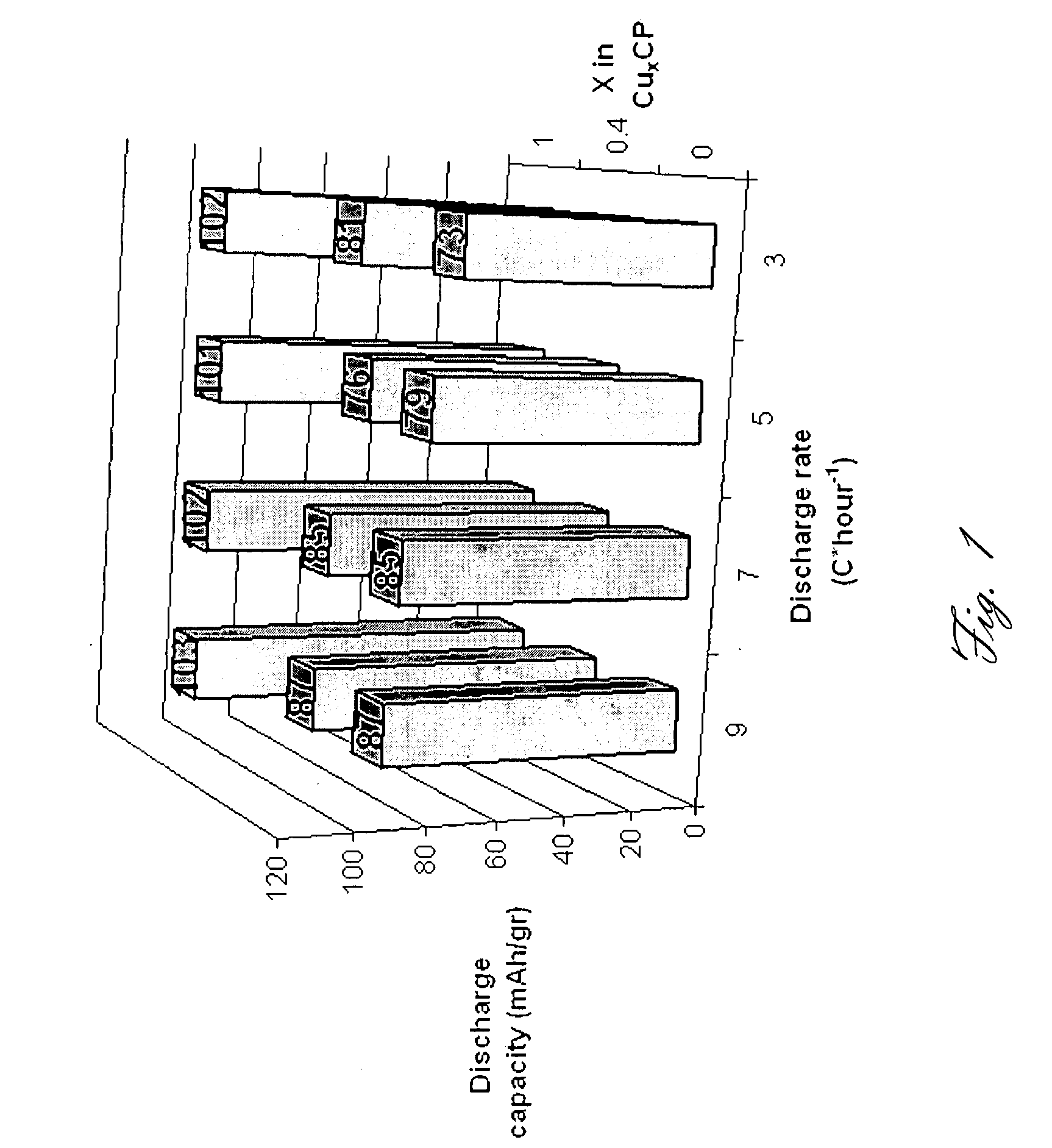
This invention generally relates to electrochemical cells utilizing magnesium anodes, new solutions and intercalation cathodes. The present invention is a new rechargeable magnesium battery based on magnesium metal as an anode material, a modified Chevrel phase as an intercalation cathode for magnesium ions and new electrolyte solution from which magnesium can be deposited reversibly, which have a very wide electrochemical window. The Chevrel phase compound is represented by the formula Mo6S8-YSeY in which y is higher than 0 and lower than 2 or by the formula MXMo6S8 in which M is selected from the group comprising of copper (Cu), nickel (Ni), silver (Ag) and / or any other transition metal; further wherein x is higher than 0 and lower than 2.
Owner:BAR ILAN UNIV
Secondary battery comprising eutectic mixture and preparation method thereof
ActiveUS20070099090A1Non-aqueous electrolyte accumulatorsCell electrodesDecompositionElectrochemical window
Disclosed is a secondary battery comprising a cathode, an anode, a separator and an electrolyte, wherein the electrolyte comprises a eutectic mixture formed of: (a) an amide group-containing compound, and (b) an ionizable lithium salt, and the anode comprises a metal or metal oxide having a potential vs. lithium potential (Li / Li+) within electrochemical window of the eutectic mixture. An electrolyte for a secondary battery comprising the eutectic mixture is also disclosed. Since the secondary battery uses a eutectic mixture as an electrolyte in combination with an anode having a potential vs. lithium potential (Li / Li+) within the electrochemical window of the eutectic mixture, it solves the problems occurring in a conventional battery using a eutectic mixture as an electrolyte, such problems including decomposition of an electrolyte and degradation of the quality of a battery. Also, due to the thermal and chemical stability, high conductivity and a broad electrochemical window of a eutectic mixture, it is possible to improve the quality as well as safety of a battery.
Owner:LG ENERGY SOLUTION LTD
Polycarbonate all-solid-state polymer electrolyte, all-solid-state secondary lithium battery made of same and preparation and application thereof
The invention relates to solid-state electrolytes, in particular to a polycarbonate all-solid-state polymer electrolyte, an all-solid-state secondary lithium battery made of the same and preparation and application thereof. The all-solid-state polymer electrolyte is prepared from polycarbonate macromolecules, lithium salt and a porous supporting material; the thickness is 20-800 micrometers, the mechanical strength is 10-80 MPa, the room temperature ion conductivity is 2*10<-5>S / cm-1*10<-3>S / cm, and the electrochemical window is higher than 4V. The all-solid-state polymer electrolyte is easy to prepare and form, good in mechanical property, high in ion conductivity and wide in electrochemical window; meanwhile, the solid-state electrolyte effectively inhibits growth of negative electrode lithium dendrites and improves interface stability and long circulation performance.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for preparing formic acid through electrochemical catalytic reduction of carbon dioxide
InactiveCN102190573AGood solubility and absorption propertiesImprove conductivityOrganic compound preparationCarboxylic compound preparationSupporting electrolyteOrganic solvent
The invention relates to a method for preparing formic acid through electrochemical catalytic reduction of carbon dioxide, and belongs to the technical field of carbon dioxide recycling. In the method, a proton exchange membrane separates an electrolytic tank into a cathode chamber and an anode chamber, organic solvent / ionic liquid / water mixed solution in which a large amount of carbon dioxide is dissolved is injected into the cathode chamber, and aqueous solution containing supporting electrolyte is injected into the anode chamber; and after an electrolysis power supply is connected, the carbon dioxide undergoes electroreduction reaction on the cathode to form the formic acid. By the method, the organic solvent / ionic liquid / water mixed solution with the advantages of good conductivity, low viscosity, high capacity of dissolving the carbon dioxide, wide electrochemical window, and low use cost can be obtained, and when the carbon dioxide is electrically reduced in the mixed solution, the current density in the electroreduction reaction of the carbon dioxide can be improved and the electrocatalytic activity and long-time stability of a cathode material are improved.
Owner:KUNMING UNIV OF SCI & TECH
Organic and inorganic composite all-solid-state electrolyte and all-solid-state battery formed from same
InactiveCN105811002AGood flexibilityGood chemical stabilitySolid electrolytesFinal product manufactureElectrical conductorInorganic compound
The invention relates to an organic and inorganic composite all-solid-state electrolyte, in particular to an organic polycarbonate macromolecule and inorganic fast-ion conductor composite all-solid-state electrode and preparation and application of an all-solid-state battery formed from the same. The organic and inorganic composite all-solid-state electrolyte comprises polycarbonate macromolecule, an inorganic fast-ion conductor, a lithium salt and a porous rigid support material, the thickness of the organic and inorganic composite all-solid-state electrolyte is 5-2,000 micrometers, the mechanical strength is 2-150MPa, the room-temperature ionic conductivity is 1*10<-4>-6*10<-3> S / cm, and an electrochemical window is greater than 4V. The organic and inorganic composite all-solid-state electrolyte provided by the invention is easy to prepare and simple to form, has favorable mechanical property, and is relatively high in room-temperature ionic conductivity and relatively wide in electrochemical window; and meanwhile, by the organic and inorganic composite all-solid-state electrolyte, the growth of lithium dendrites of a negative electrode can be effectively prevented, the interface stability is improved, and the long-circulation and safe application performance of the battery are further improved.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
High voltage-resistant multi-stage structure composite solid-state electrolyte for lithium battery
ActiveCN107732297AImprove ionic conductivityImprove mechanical propertiesSecondary cellsSolid state electrolyteHigh energy
The invention discloses a high voltage-resistant multi-stage structure composite solid-state electrolyte and its preparation method and use in a solid-state lithium battery. The lithium battery utilizes a multi-stage structure solid-state electrolyte containing different components. A polymer electrolyte with excellent electrode interface compatibility is used as an electrolyte in the negative electrode side. A high voltage-resistant polymer electrolyte is used as an electrolyte in the positive electrode side. A polymer electrolyte or an inorganic electrolyte with high ionic conductivity is used as a middle layer. The multi-stage structure solid-state electrolyte has advantages such as high mechanical properties, high ionic conductivity, a wide electrochemical window, excellent electrode interfacial compatibility and lithium dendrite growth inhibition of different components. Compared with the traditional liquid lithium ion battery, the battery with the multi-stage composite solid-state electrolyte has higher safety and higher energy density.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Method for synthesizing dioxalate group lithium borate
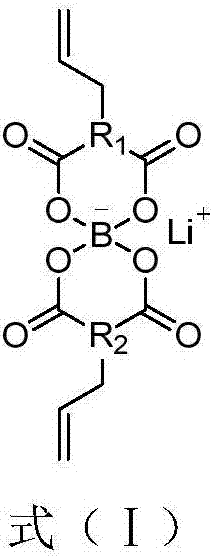
InactiveCN1687081AImprove high temperature cycle performanceInhibition of electrochemical co-intercalationGroup 3/13 element organic compoundsChemical reactionSynthesis methods
Owner:SHANDONG HIYI CHEM TECH
Electrolyte comprising eutectic mixture and electrochemical device using the same
ActiveUS20070042266A1Hybrid capacitor electrolytesElectrolytic capacitorsEvaporationElectrochemical window
Disclosed is an electrolyte comprising a eutectic mixture formed of: (a) an amide group-containing compound; and (b) a lithum-free ionizable salt. An electrochemical device comprising the electrolyte is also disclosed. The electrolyte improves the quality of and electrochemical device due to the excellent conductivity of the metal cation contained in the eutectic mixture, a broad electrochemical window and low viscosity. Additionally, since the eutectic mixture has excellent thermal and chemical stability, it is possible to solve the problems of evaporation, exhaustion and ignition of electrolytes, to minimize side reactions between constitutional elements of the device and the electrolyte, and to improve the safety of the electrochemical device.
Owner:LG ENERGY SOLUTION LTD
Aluminium ion battery and preparation method thereof
InactiveCN103825045AImprove securityImprove insulation performanceCell electrodesFinal product manufactureAluminum IonAluminium-ion battery
The invention relates to an aluminium ion battery and a preparation method thereof and belongs to the field of aluminium ion batteries and preparation thereof. The aluminium ion battery comprises a positive electrode, a negative electrode and an aluminium ion electrolyte, wherein the positive electrode is made of transition metal oxide; the negative electrode is made of high purity aluminium; the battery comprises a diaphragm material when the aluminium ion electrolyte is in a liquid state. Since abundant aluminium elements are stored, the cost for the ion battery is greatly reduced; the safety performance is improved; the transition metal oxide is applicable to hypervalent ion batteries due to relative stability under the variable valence states and different valence states. The ion liquid serves as the electrolyte for the hypervalent ion battery, so that aluminium ion is high in conductivity, good in heat stability, broad in electrochemical window and high in chemical stability and almost incapable of reacting with the positive electrode materials, the negative electrode materials, a current collector, a binder and a diaphragm in a battery system and capable of maintaining the liquid state in a board temperature range. The aluminium ion battery can be applied to various fields, such as electronic industries, communication industries and electric vehicles and the like.
Owner:UNIV OF SCI & TECH BEIJING
High voltage rechargeable magnesium batteries having a non-aqueous electrolyte
InactiveUS20130252112A1Improve conductivityImprove solubilityOrganic electrolyte cellsSecondary cellsGrignard reagentMetallic materials
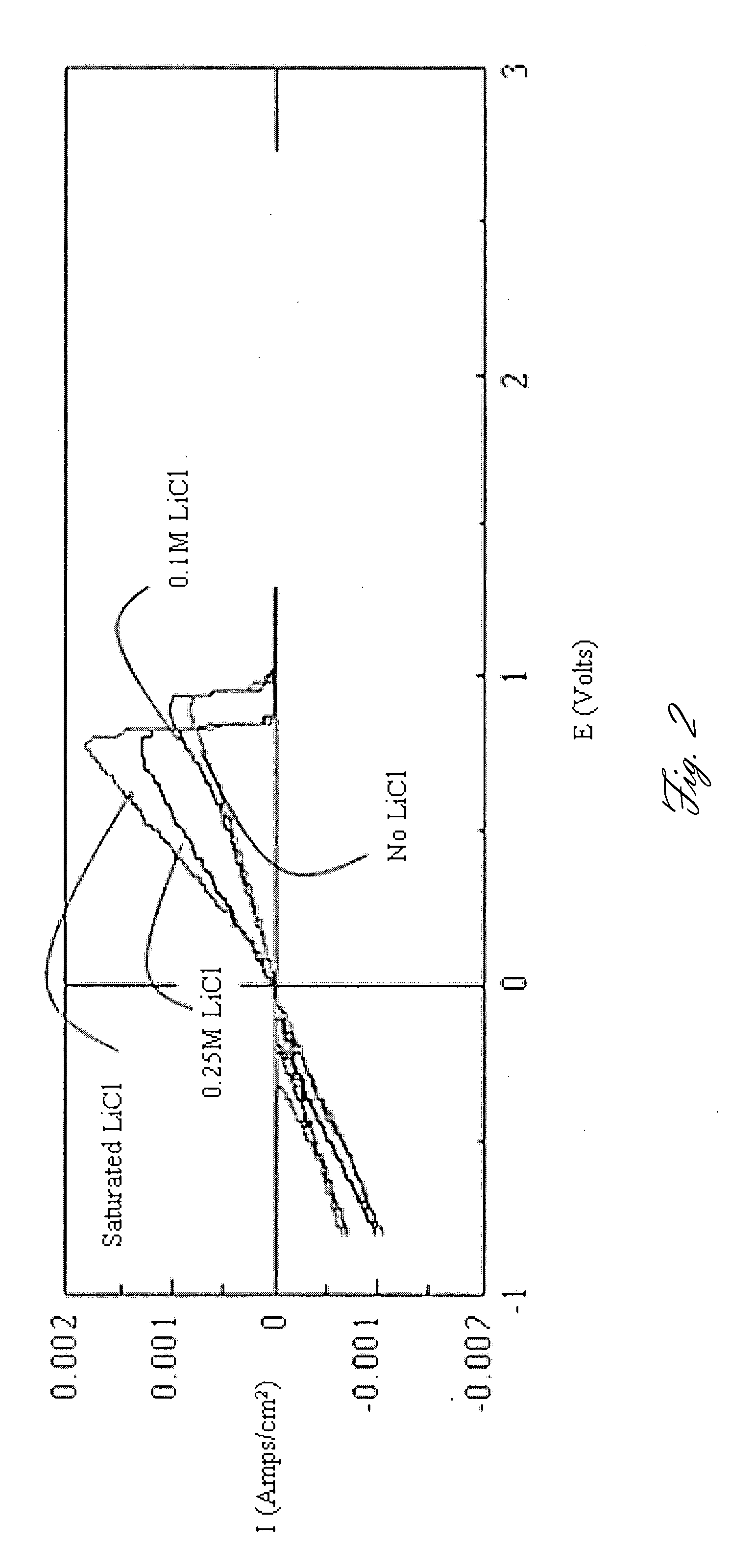
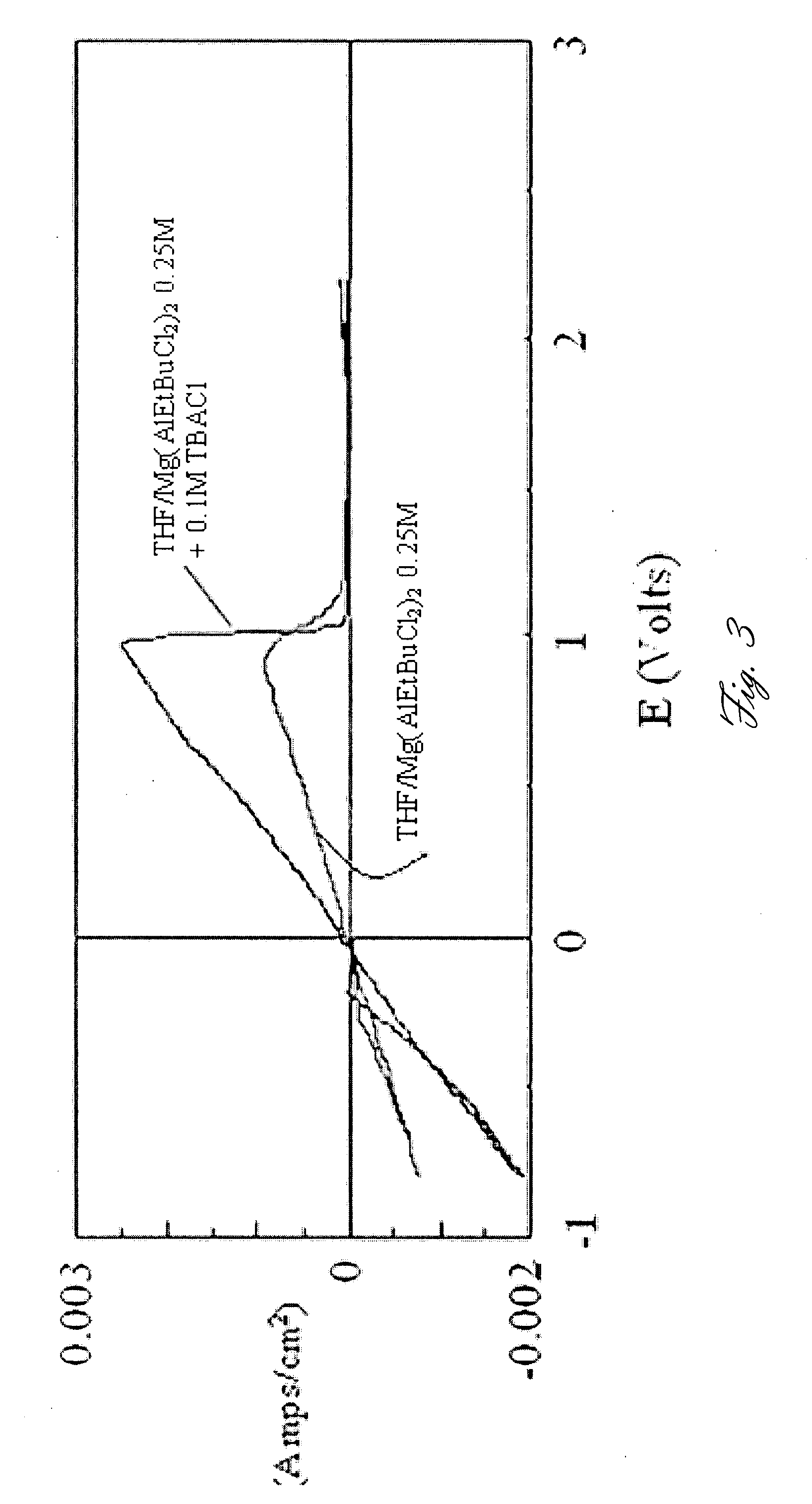
A rechargable magnesium battery having an non-aqueous electrolyte is provided. The properties of the electrolyte include high conductivity, high Coulombic efficiency, and an electrochemical window that can exceed 3.5 V vs. Mg / Mg+2. The use of the electrolyte promotes the electrochemical deposition and dissolution of Mg without the use of any Grignard reagents, other organometallic materials, tetraphenyl borate, or tetrachloroaluminate derived anions. Other Mg-containing electrolyte systems that are expected to be suitable for use in secondary batteries are also described.
Owner:PELLION TECH
Chargeable water-system zinc ion battery with long cycle life and high energy density
ActiveCN105958131AIncrease energy densitySolution to short lifeFinal product manufactureCell electrodesCarbon compositesHigh energy
Disclosed is a chargeable water-system zinc ion battery with long cycle life and high energy density. The chargeable water-system zinc ion battery comprises a positive electrode shell, an elastic sheet, a gasket, a positive electrode active substance, a diaphragm, a negative electrode active substance and a negative electrode shell, and all the components form a laminated layer structure in sequence, wherein the positive electrode active substance is positive ion defect type ZnMn<x>O<4> / C nanocomposite; the negative electrode is zinc foil or spherical zinc powder; the diaphragm is polyethylene nonwoven fabric or filter paper; and the electrolyte is a zinc trifluoromethanesulfonate water solution. The chargeable water-system zinc ion battery has the advantages that the ZnMn<x>O<4> / conductive carbon composite electrode material has simple and easily feasible preparation process; the synthesized ZnMn<x>O<4> nano crystals are uniformly embedded in the conductive carbon; due to the electrolyte, the Zn deposition / separation-out coulombic efficiency can reach about 100% and a wide electrochemical window of 0-2.5Vvs.Zn<2+> / Zn is realized; the positive electrode active substance and the novel electrolyte are applied to the water-system zinc ion battery, so that the battery shows a good electrochemical performance; and meanwhile, the battery has high reversible zinc storage capacity with high active substance content, and excellent cycling stability .
Owner:NANKAI UNIV
Composite solid-state polymer electrolyte and all-solid-state lithium battery
ActiveCN106654363AImprove electrochemical stabilityExcellent charge and discharge characteristicsSolid electrolytesSecondary cellsMicro nanoAll solid state
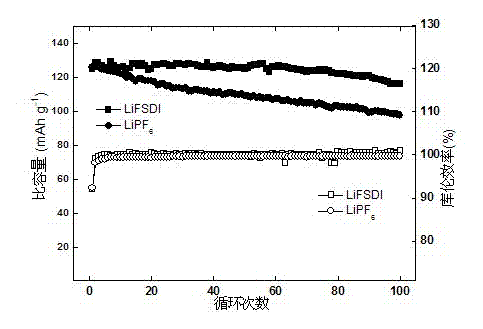
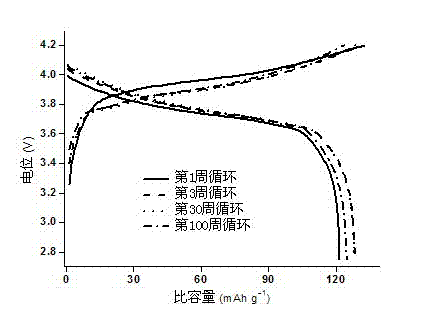
The invention discloses a composite solid-state polymer electrolyte and an all-solid-state lithium battery. The composite solid-state polymer electrolyte comprises organic micro-nano porous granules, a polymer having lithium ion conducting capability and a lithium salt; the composite solid-state polymer electrolyte takes the organic micro-nano porous granules as filler; and natural compatibility exists between the organic filler and the polymer matrix. The composite solid-state polymer electrolyte disclosed by the invention has high electrochemical window (4.2-5V), excellent interface stability with a lithium-based negative electrode material, and low interface impedance. The all-solid-state lithium battery, assembled by the composite solid-state polymer electrolyte disclosed by the invention, is high in cycling performance and rate capability.
Owner:UNIV OF SCI & TECH BEIJING
Preparation and application of organic-inorganic composite solid-state electrolyte
ActiveCN108878959AImprove mechanical propertiesImprove thermal stabilitySolid electrolytesSecondary cellsSolid state electrolytePtru catalyst
The invention relates to preparation and application of an organic-inorganic composite solid-state electrolyte, and relates to the technical field of a lithium ion battery electrolyte. The organic-inorganic composite solid-state electrolyte is prepared by selecting an isocyanate compound having rigid characteristic, a flexible chain segment compound capable of complexing and dissociating with lithium ions, inorganic nanoparticles, a conductive lithium salt and an organic solvent and adding a tin catalyst for crosslinking and curing. With the isocyanate compound, the mechanical property and thethermal stability of the composite solid-state electrolyte can be improved; by the flexible chain segment compound and the inorganic nanoparticles, the ion conductivity, the ion transfer number and the wide electrochemical window of the composite solid-state electrolyte can be improved, the charge-discharge performance of the lithium ion battery is improved, and the interface contact of the solid-state lithium ion battery is improved; and the organic-inorganic composite solid-state electrolyte has the advantages of excellent interface stability, wide electrochemical window, wide working temperature range, high ion conductivity and versatile shapes and is applicable to a lithium ion polymer battery.
Owner:BEIJING UNIV OF TECH
Method for producing aluminium and aluminium alloy by low temperature electrolysis
InactiveCN1664170ALight in massNon-volatilePhotography auxillary processesProcess efficiency improvementMetallic materialsIonic liquid
The invention relates to a method of making aluminum and aluminum alloy through electrolyzing in low temperature, which belongs to the metal material field, characterized in that it uses the alumina or silicate mineral containing aluminum as raw materials, and gets anhydrous aluminum chloride after chloridization, then prepares AlCl3 ionic liquid with anhydrous aluminum chloride, and uses the AlCl3 ionic liquid as ionogen, the aluminum will be produced in the cathode while the chlorine will be discharged from the anode. When producing the aluminum alloy, preparing the alloy elements chloridate-AlCl3 ionic liquid through adding alloy elements chloridate MeCln to the AlCl3 ionic liquid, Using the direct current to electrolyzing, wherein the voltage is above the higher decomposition voltage of the aluminium chloride and chloridate and below the electrochemistry window of the ionic liquid, and the aluminum will be produced in the cathode while the chlorine will be discharged from the anode.
Owner:UNIV OF SCI & TECH BEIJING
A manganese bioxide electrochemical super capacitor
InactiveCN101286418AThe average output voltage increasesIncrease capacityCapacitor electrolytes/absorbentsSecondary cellsCapacitanceHigh energy
The invention discloses a manganese dioxide electrochemical supercapacitor, comprising the following steps: the pseudocapacitance of the manganese dioxide and a double electrode layer capacitance mechanism of large specific surface area carbon material are combined into a maintaining power, the positive electrode adopts manganese dioxide material with high capacity, the negative electrode adopts high large specific surface area carbon material, an aqueous solution system or a non-aqueous solution system containing bivalent cations, thus forming the asymmetric electrochemical capacitor. As different materials are adopted in different electrochemical windows of the same electrolyte, individual voltage of the asymmetric electrochemical capacitor can be up to 2V or more, and as the bivalent cations are adopted as the cations of the electrolyte, the specific capacity of the positive electrode and the negative electrode are improved; therefore, the supercapacitor is characterized of high energy density and high power density, etc.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV +1
Method for preparing nanometer aluminum or nanometer aluminum coating in low-temperature electro-deposition form by using ion liquid/additive system
ActiveCN102888630AHigh purityWide variety of sourcesMaterial nanotechnologyElectrochemical windowIonic liquid
The invention designs a method for preparing nanometer aluminum or nanometer aluminum coating in an electro-deposition form by using an ion liquid / additive system. The method is characterized in that: ion liquid and anhydrous aluminum trichloride are mixed into a low-temperature electrolyte and suitable additives are added for preparing the ion liquid / additive system. A processed substrate is taken as a deposition cathode; the direct current electro-deposition is adopted for preparing the nanometer aluminum or nanometer aluminum coating; and the size of the aluminum grains is adjusted according to requirements. According to the method, the problems of high cost and small output of the present technology for producing the nanometer aluminum are solved; the adopted ion liquid is conventional ion liquid and is characterized by wide source, low cost, high conductivity, wide electrochemical window and being non-volatile and environment-friendly; the suitable additives are used, so that the high-quality nanometer aluminum or nanometer aluminum coating obtained in the conventional ion liquid is realized; the obtained aluminum deposited coating is compact, smooth and flat; a nanometer material can be obtained by using the ion liquid / additive system at lower temperature; the reaction is easy to control; the energy consumption is low; the obtained nanometer aluminum is high in quality and high in current efficiency; the size of the nanometer aluminum can be effectively controlled by adjusting the dosage and formula of the additives; the process is simple; the cost is low; and the application prospect is better.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Binary or ternary fluorine-containing sulfimide alkali metal salt and ionic liquid and applications thereof
InactiveCN102786443AEasy to separate and purifyHigh yieldElectrolytic capacitorsSecondary cellsElectrochemical windowHydrolysis
The invention discloses a method for preparing binary or ternary fluorine-containing sulfimide alkali metal salts, a method for preparing ionic liquid by the binary or ternary fluorine-containing sulfimide alkali metal salts, and applications of the alkali metal salts and ionic liquid as electrolytes in carbon-based super capacitors, secondary lithium (ion) batteries, and the like. The method for preparing the binary or ternary fluorine-containing sulfimide alkali metal salts provided by the invention is short in operation steps, easy for product separation and purification, and high in product yield and purity; the binary or ternary fluorine-containing sulfimide lithium provided by the invention has good thermal stability and hydrolysis resistance; a nonaqueous electrolytic solution of the binary or ternary fluorine-containing sulfimide lithium has high conductivity and lithium ion transference number, and also exhibits good oxidation resistance and good compatibility with widely-used electrode materials; meanwhile, the ionic liquid containing the binary or ternary fluorine-containing sulfimide anions exhibits the properties of low viscosity and high conductivity, and has a wide electrochemical window.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Production for gel polymer electrolyte
ActiveCN1645662AHigh mechanical strengthImprove electrochemical stabilityOrganic electrolyte cellsSecondary cellsPolymer electrolytesPolymer science
The method includes following step: two monomers are mixed according to the ratio of 1.0 to 1.0-9.0; then it mixes with thermal booster and liquid lithium ion battery solution to form the fore body of gel polymer dielectric; the fore body is injected into half finished battery. the half finished battery is vacuumized and sealed; the half finished battery is made thermal polymerization reaction, and the gel polymer dielectric is obtained.
Owner:SHENZHEN CAPCHEM TECH
Lithium ion battery electrolyte and secondary battery containing lithium ion battery electrolyte
InactiveCN103715454AIncrease capacityGood charge and discharge cycle performanceFinal product manufactureElectrolyte accumulators manufactureElectrochemical windowHigh pressure
The invention provides a lithium ion battery electrolyte and a secondary battery containing the lithium ion battery electrolyte, and relates to the technical field of electrochemistry. The lithium ion battery electrolyte contains a lithium salt, an organic solvent, 0.5-5% by weight of a film-forming additive, 1-15% by weight of a fluorobenzene compound, 0.01-0.5% of a wetting agent, and 0.01-2% of a stabilizer. The lithium ion secondary battery comprises a positive electrode material, a negative electrode material and an electrolyte, wherein the positive electrode material comprises, by mass, 85% of LiCoO2, 7.5% of polyvinylidene fluoride (PVDF) and 7.5% of conductive carbon black SP, and the negative electrode material comprises, by mass, 90% of high compactness graphite, 7% of polyvinylidene fluoride (PVDF) and 3% of conductive carbon black SP. According to the present invention, the battery capacity can be increased, the charge / discharge cycle performance can be improved, and the electrochemical window can be widened.
Owner:JIANGXI YOULI NEW MATERIALS
Preparation method of all-solid-state polymer electrolyte
ActiveCN109037770AImprove mechanical propertiesImprove electrochemical stabilitySecondary cellsElectrolyte immobilisation/gelificationHigh energyElectrochemical window
The preparation method of an all-solid-state polymer electrolyte comprises the following steps: adding a carbonic acid structure crosslinking agent, a polyether structure monomer, an olefine acid monomer and a functional polymer together with a solvent into a reactor for stirring to form a uniform precursor solution; Adding an initiator, a lithium salt, an inorganic filler and / or a fast ionic conductor to the precursor solution, stirring to obtain a mixed solution, uniformly coating the mixed solution on a mold, evaporating the solvent, and initiating a reaction under an inert gas; At that endof the initiation reaction, vacuum dry is carried out to obtain an all-solid polymer electrolyte. As that carbonate polyalkenyl structure monome is used as a crosslink agent, the polyether monomer and the olefine acid monomer are crosslinked and copolymerize, and the electrochemical window of the all-solid-state polymer electrolyte can be effectively improved by introducing the carbonate crosslinking structure, and the all-solid-state polymer battery with higher energy density and a better application range can be assembled.
Owner:ZHUHAI COSMX BATTERY CO LTD
High-salinity non-aqueous electrolyte and use thereof
InactiveCN103219542AImprove thermal stabilityReduce volatilitySecondary cellsElectricityOrganic solvent
The invention discloses a high-salinity non-aqueous electrolyte. The high-salinity non-aqueous electrolyte comprises a lithium salt and a non-aqueous organic solvent, and the concentration of the lithium salt is 2-10mol / L. The invention also discloses a use of the high-salinity non-aqueous electrolyte in a lithium or sodium cell system. The high-salinity non-aqueous electrolyte has the advantages of high thermal stability, low volatility, high safety, electrochemical widow and aluminum foil corrosivity, and lithium or sodium dendrite overcoming.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Secondary batteries comprising eutectic mixture and preparation method thereof
InactiveUS20100021815A1Good chemical and thermal stabilityQuality improvementAlkaline accumulatorsLead-acid accumulatorsLithiumLower limit
Disclosed is a secondary battery comprising a cathode, an anode, a separator and an electrolyte, wherein the electrolyte comprises: (a) a eutectic mixture; and (b) a first compound reduced at a higher potential vs. lithium potential (Li / Li+) than the lowest limit of the electrochemical window of the eutectic mixture. The electrolyte uses a eutectic mixture in combination with an additive reduced in advance of the eutectic mixture upon the initial charge to form a solid electrolyte interface (SEI) layer. Therefore, the electrolyte can solve the problem of electrolyte decomposition occurring when using a eutectic mixture alone as an electrolyte for a battery, and thus can prevent degradation of the quality of a battery.
Owner:LG CHEM LTD
Composite solid electrolyte, and preparation method and applications thereof
ActiveCN108598560AGrowth inhibitionAvoid chalkingFinal product manufactureSecondary cellsElectrochemical windowElectrical resistivity and conductivity
Owner:蓝谷智慧(北京)能源科技有限公司
Preparation for self-crosslinking compound solid electrolyte and all-solid lithium ion battery composed of self-crosslinking compound solid electrolyte
ActiveCN108899579AReduce crystallinityImprove ionic conductivitySolid electrolytesFinal product manufactureSolid state electrolyteIon transport number
The invention relates to preparation for a self-crosslinking compound solid electrolyte and an all-solid lithium ion battery composed of the self-crosslinking compound solid electrolyte and relates tothe field of the electrolyte of the lithium ion battery. Specifically, a compound solid electrolyte is prepared according to the following steps: adopting silane terminated polyether (MS) as a prepolymer and then stirring and uniformly mixing with inorganic nano-particles with acidity and alkalinity or organic polymer materials, conductive lithium salt and organic solvents, and preparing the compound solid electrolyte through the self-crosslinking curing of MS and inorganic nano-particles with acidity and alkalinity or organic polymer materials. The self-crosslinking compound curing of MS andinorganic nano-particles with acidity and alkalinity or organic polymer materials is capable of reducing the degree of crystallinity of the compound solid electrolyte, promoting the ionic conductivity, ion transference number, mechanical properties, electrochemical stability window and battery rate charge-discharge properties of the compound solid electrolyte and solving the problem of interfacecontact of the solid lithium ion battery. The ionic conductivity can reach up to 10<-4>Scm<-1>, the electrochemical window is above 5V, the shrinking rate of the product is low and the electrochemicalstability is high.
Owner:BEIJING UNIV OF TECH
Alkali metal salts of binary or ternary fluorine-containing sulfimide and ionic liquid and applications thereof
InactiveCN103641751AEasy to separate and purifyHigh yieldHybrid capacitor electrolytesCarboxylic acid nitrile preparationElectrochemical windowHydrolysis
The invention discloses alkali metal salts of binary or ternary fluorine-containing sulfimide, a method for preparing ionic liquid from the alkali metal salts of binary or ternary fluorine-containing sulfimide, and applications of the alkali metal salts and the ionic liquid as electrolytes in carbon-based supercapacitors, secondary lithium (ion) batteries, and the like. The method for preparing the alkali metal salts of binary or ternary fluorine-containing sulfimide provided by the invention is short in operation steps, and the product is easy to separate and purify, and very high in yield and purity; the binary or ternary fluorine-containing lithium sulfimide provided by the invention is good in thermal stability and hydrolysis resistance; the non-aqueous electrolyte has high conductivity and lithium ion transport number, exhibits good oxidation resistance, and has good compatibility with widely-used electrode materials; meanwhile, the ionic liquid containing binary or ternary fluorine-containing sulfimide anions is characterized by low viscosity and high conductivity, and has a wide electrochemical window.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Lithium single ion conduction polymer electrolyte based on functionalized lithium borate
ActiveCN106935904AEasy to synthesizeRaw materials are cheap and easy to getSolid electrolytesCell electrodesHigh rateElectrochemical window
The invention discloses a lithium single ion conduction polymer electrolyte based on functionalized lithium borate. A double-bond-containing functionalized lithium borate and a sulfydryl-containing compound are subjected to an alkene-sulfydryl click reaction, or the double-bond-containing functionalized lithium borate and the sulfydryl-containing compound and a double-bond-containing polyether are subjected to a poly-alkene-sulfydryl click reaction to prepare a linear or network-shaped all-solid-state or gel catalyst. The lithium single ion conduction polymer electrolyte prepared by the invention has the advantages of simple and easily implemented synthesis, low-cost and easily available raw materials, high room-temperature conductivity, high lithium ion mobility, wide electrochemical window, and the like; and a lithium battery assembled by the electrolyte provided by the invention has high safety, high rate capability, long cycle life and stability.
Owner:SUN YAT SEN UNIV +1
Polymer ion liquid electrolyte and preparation method thereof
InactiveCN104031193AImprove conductivityImprove solubilitySecondary cellsComposite electrolyteElectrical battery
The invention discloses an ether group-containing polymer ion liquid composite-electrolyte and a preparation method thereof. The polymer ion liquid composite-electrolyte has the advantages of high room-temperature conductivity, high lithium ion transport number, low vitrification point, good mechanical strength, good film forming performances, wide electrochemical window and good heat stability, and has wide application values in fields of lithium (ion) cells, carbon-based supercapacitors and solar cells.
Owner:HUAZHONG UNIV OF SCI & TECH
Non-aqueous electrolyte for rechargeable magnesium ion cell
InactiveUS20140220450A1Improve conductivityEasy to depositNon-aqueous electrolyte cellsSecondary cellsHigh energyMagnesium salt
An electrolyte for use in electrochemical cells is provided. One type of non-aqueous Magnesium electrolyte comprises: at least one organic solvent; at least one electrolytically active, soluble, inorganic Magnesium salt complex represented by the formula: MgnZX3+(2*n), in which Z is selected from a group consisting of aluminum, boron, phosphorus, titanium, iron, and antimony; X is a halogen and n=1-5. The properties of the electrolyte include high conductivity, high Coulombic efficiency, and an electrochemical window that can exceed 3.5 V vs. Mg / Mg+2 and total water content of <200 ppm. The use of this electrolyte promotes the electrochemical deposition and dissolution of Mg from the negative electrode without the use of any additive. Other Mg-containing electrolyte systems that are expected to be suitable for use in secondary batteries are also described. Rechargeable, high energy density Magnesium cells containing a cathode, an Mg metal anode, and an electrolyte are also disclosed.
Owner:PELLION TECH
Cross-linked polymer-based all-solid-state electrolyte material and application of cross-linked polyoxyethylene ether
ActiveCN103500845AImprove conductivityImprove conduction abilitySecondary cellsCross-linkAll solid state
The invention discloses a cross-linked polymer-based all-solid-state electrolyte material and an application of cross-linked polyoxyethylene ether. The cross-linked polymer-based all-solid-state electrolyte material comprises cross-linked polyoxyethylene ether, lithium salt and a modifying agent. An electrolyte membrane prepared from the electrolyte material containing the cross-linked polyoxyethylene ether has relatively high conductivity and a wide electrochemical window; and an assembled half-cell has good high-temperature cycle performance, and the retention rate of the charge-discharge specific capacity of the half-cell is high.
Owner:王海斌
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com