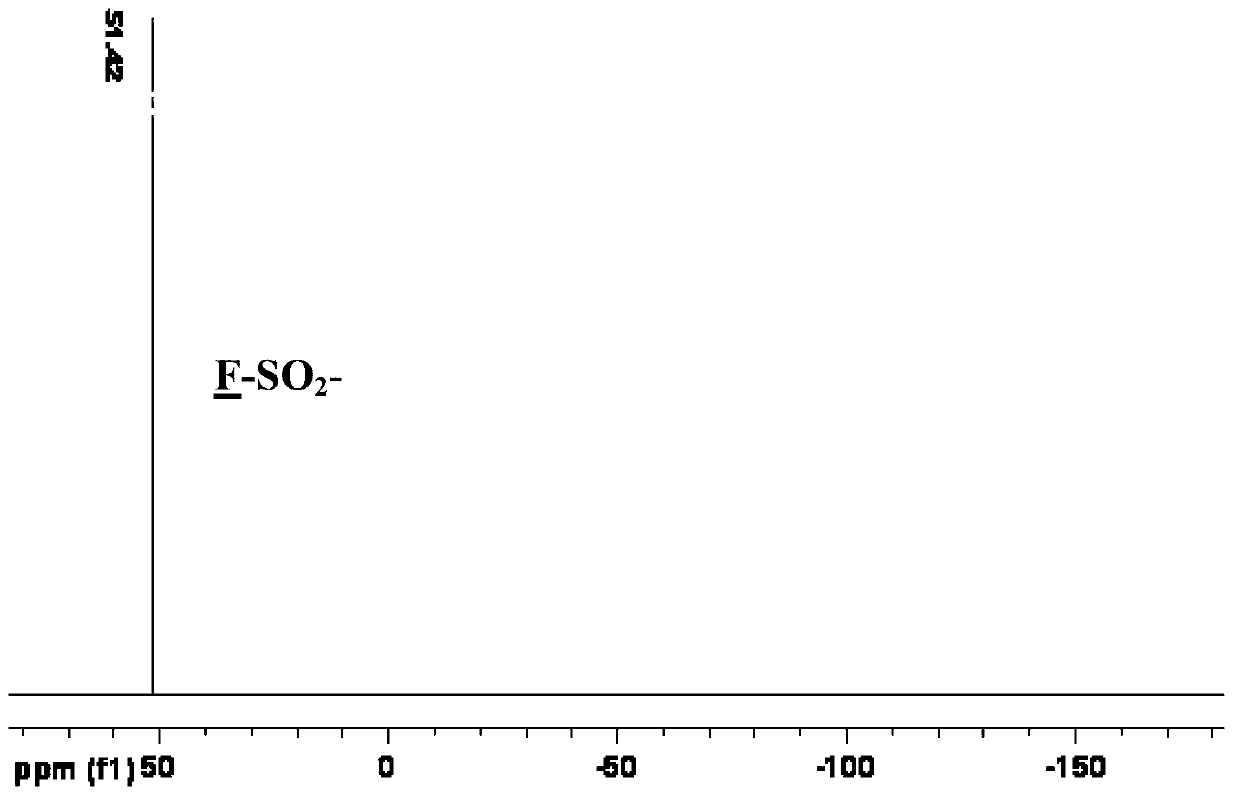
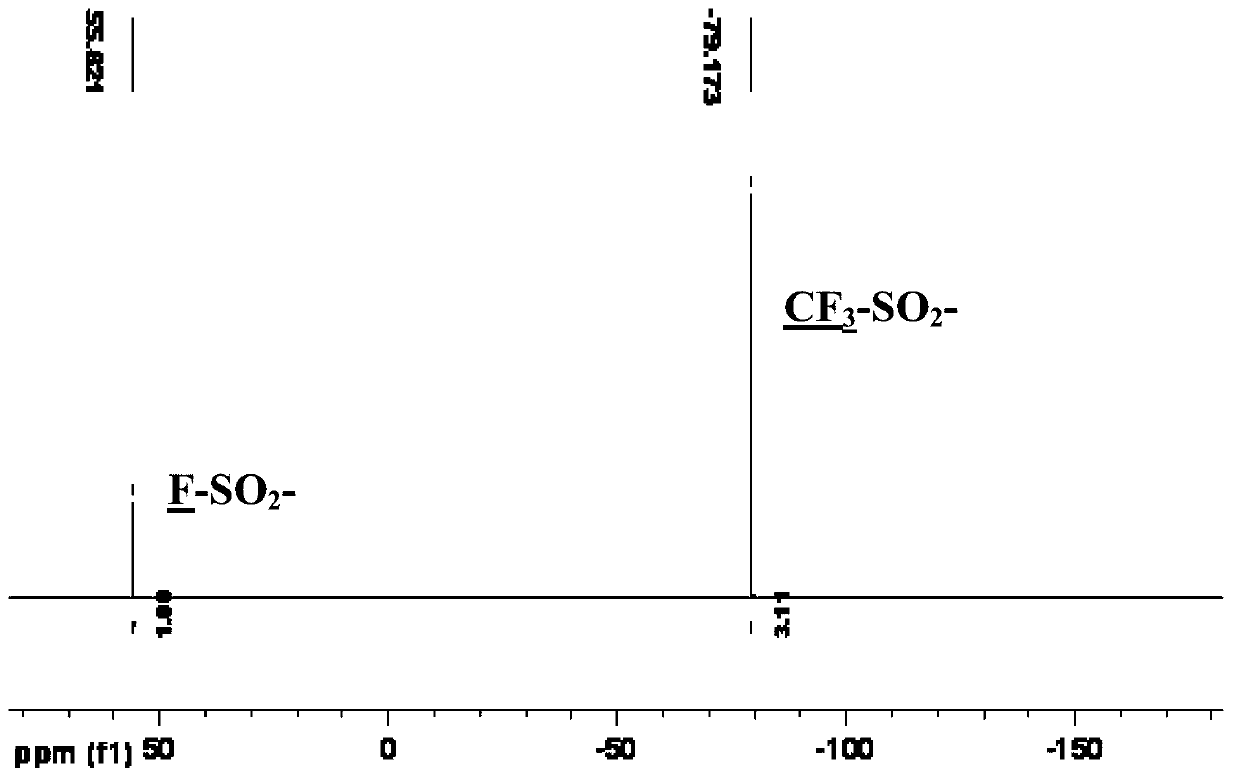
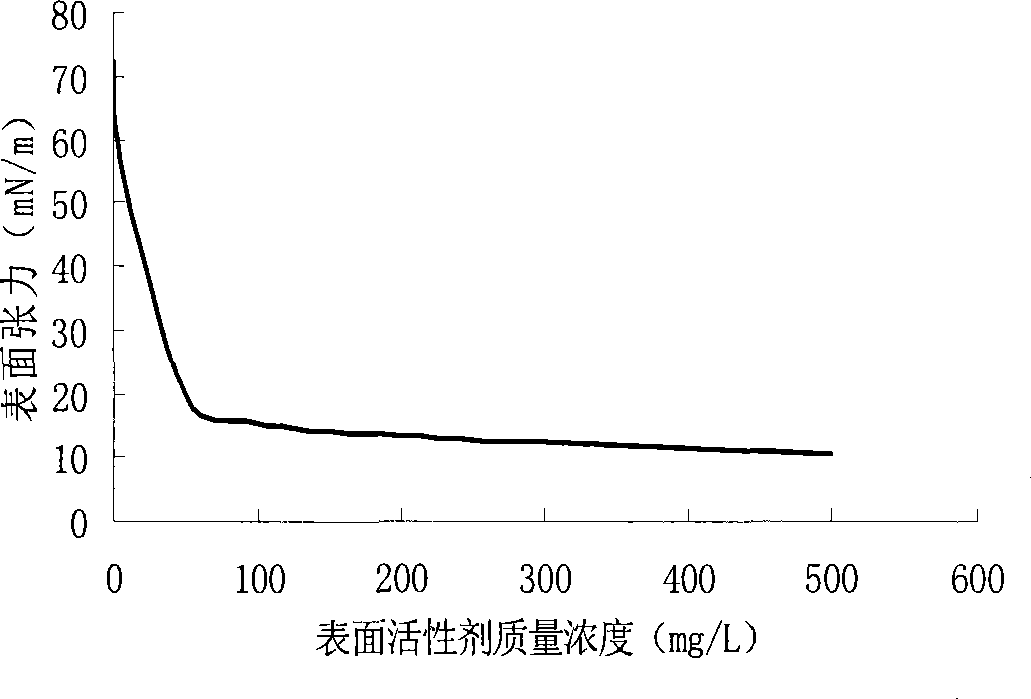
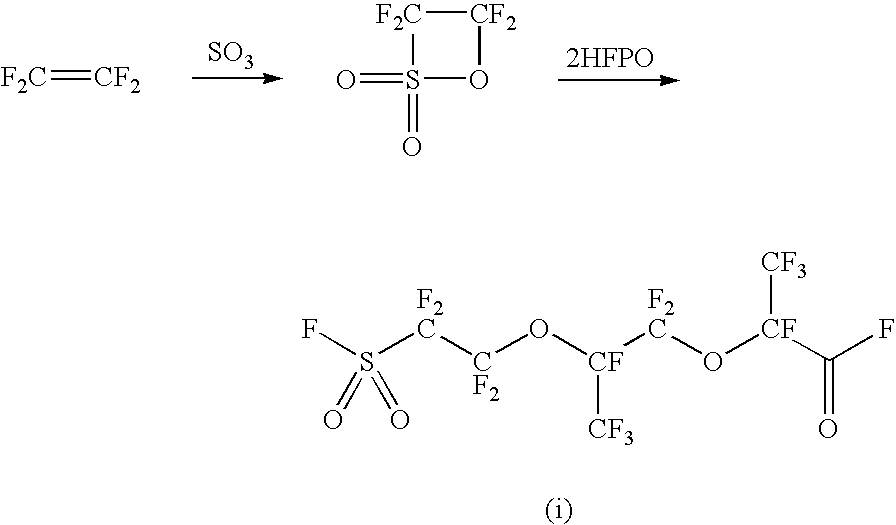
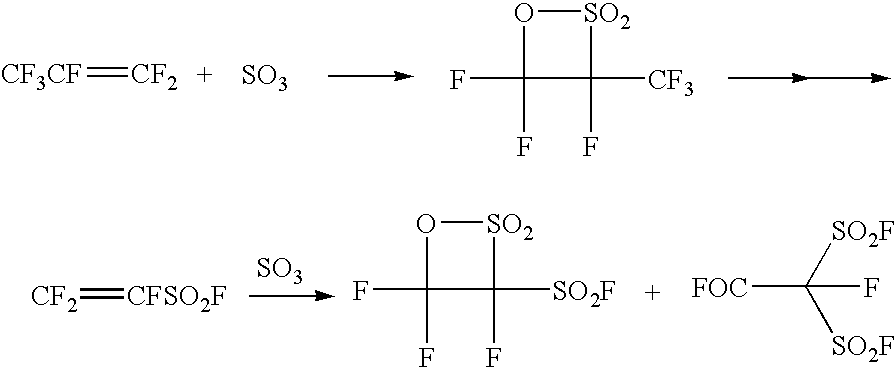
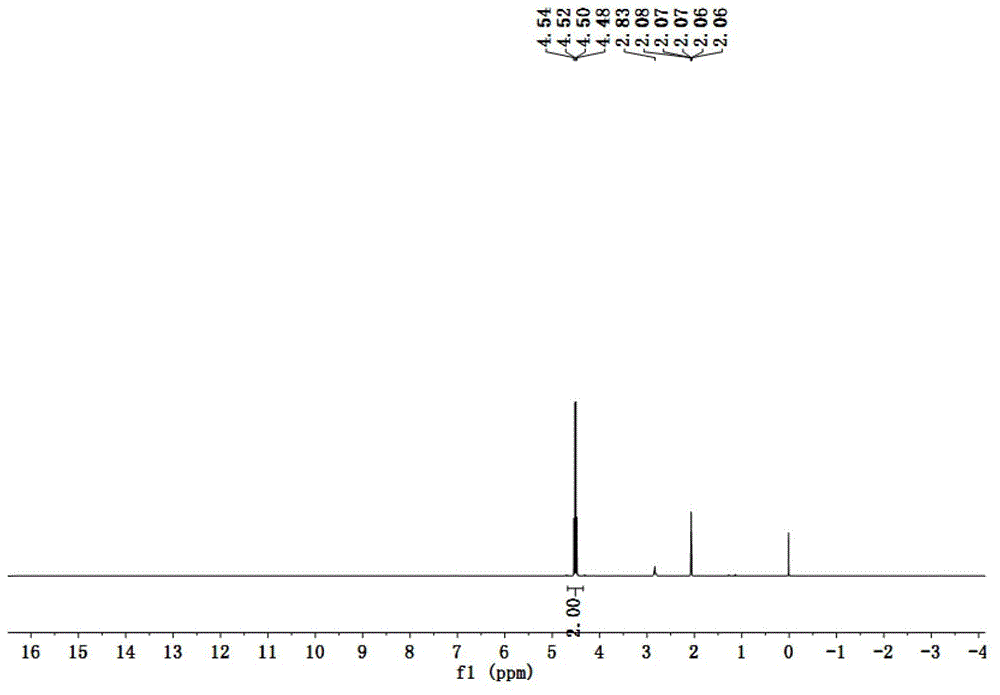
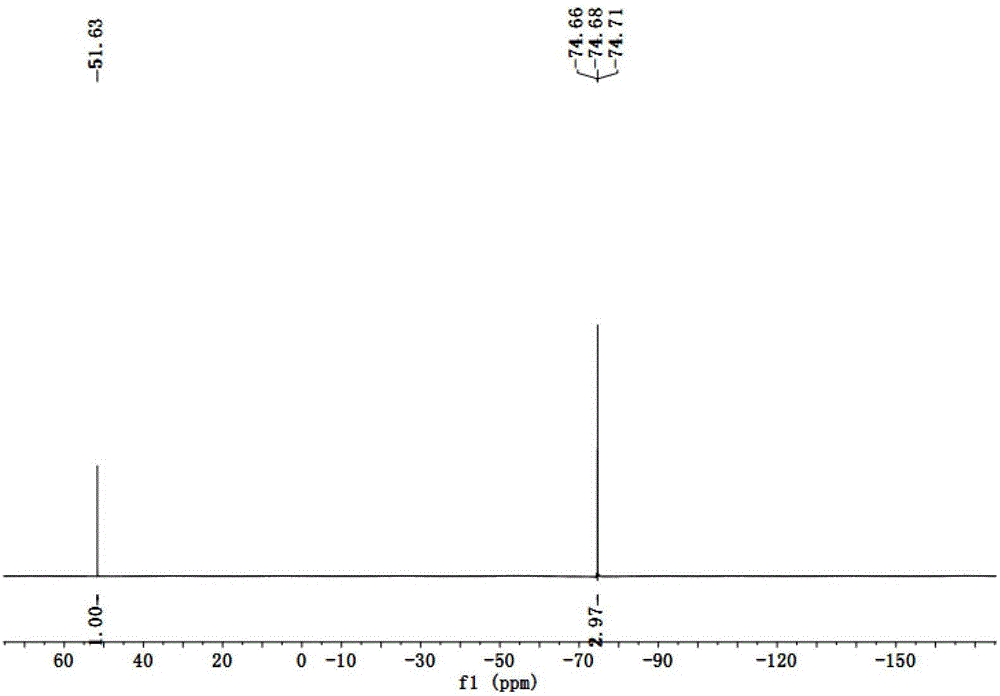
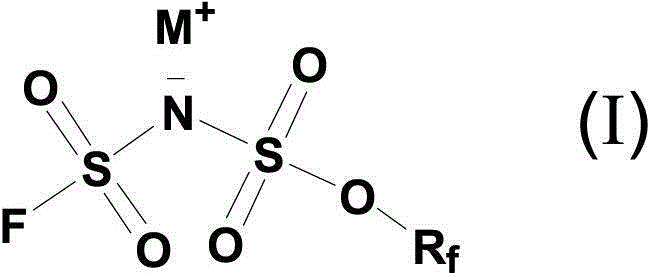
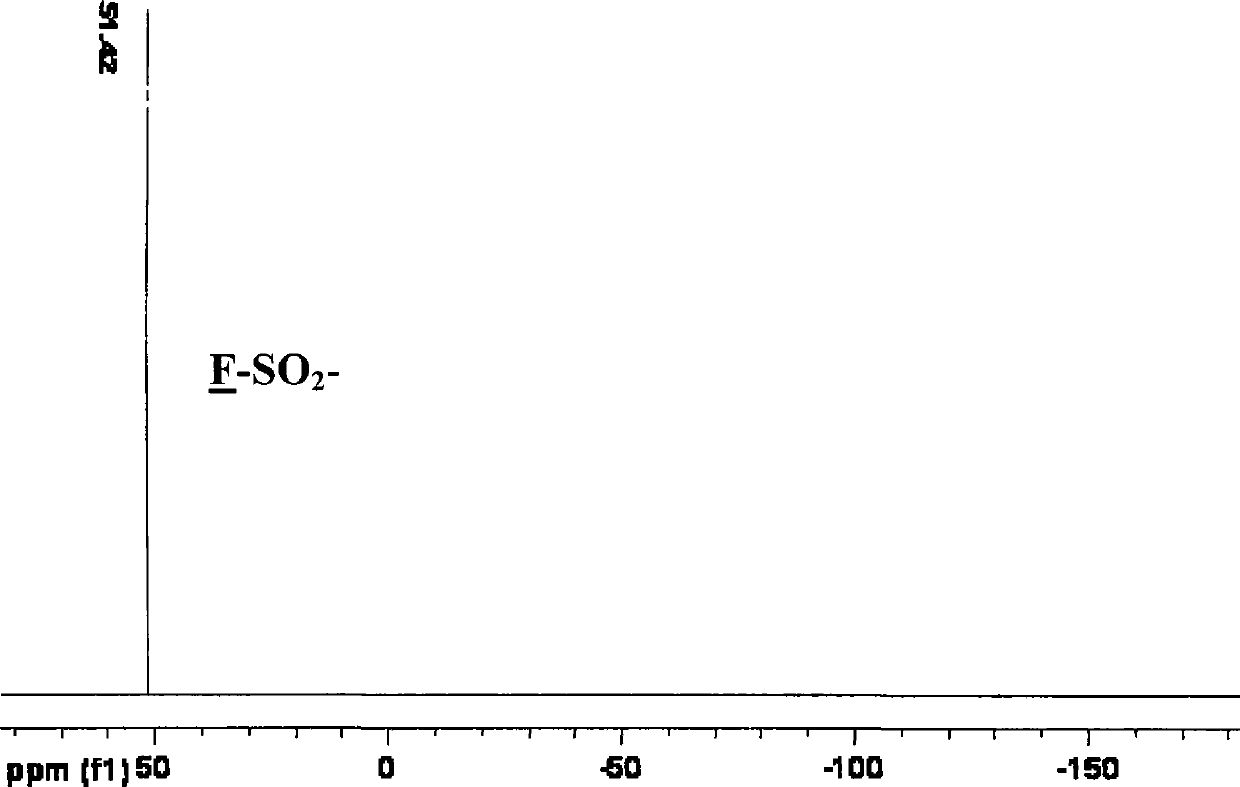
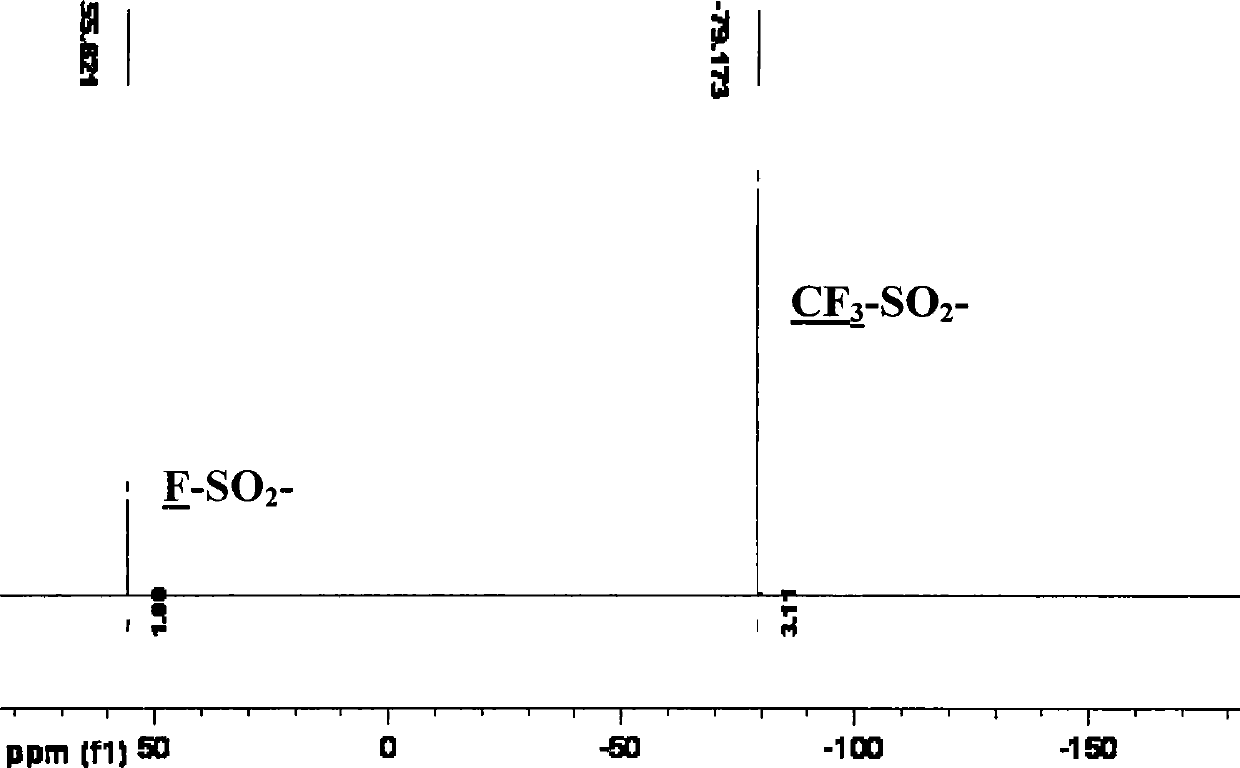
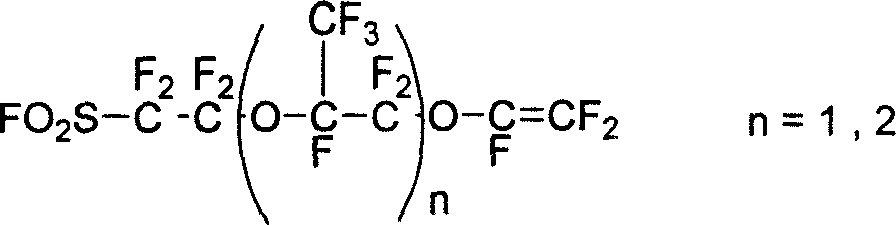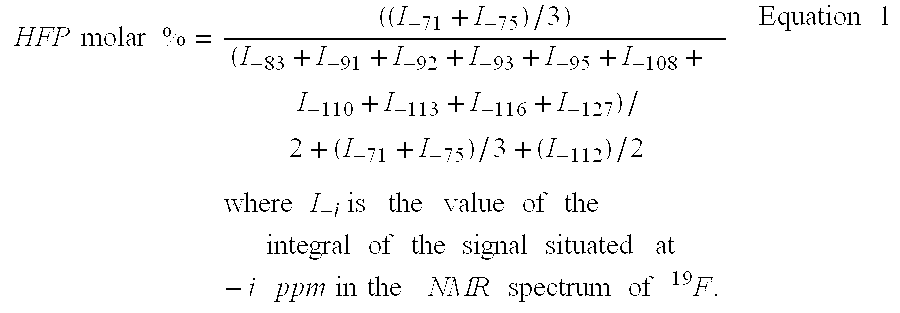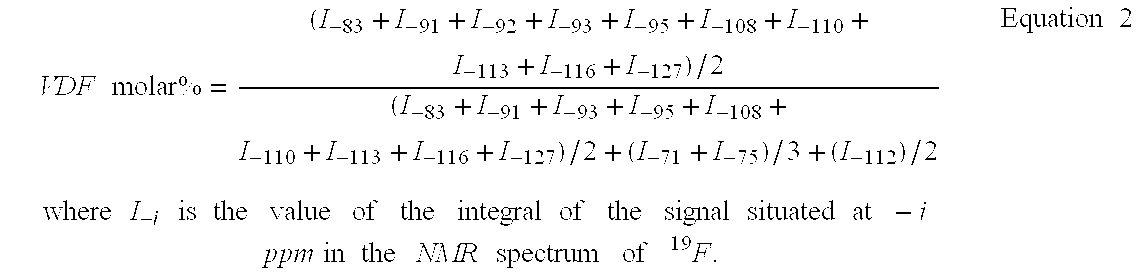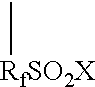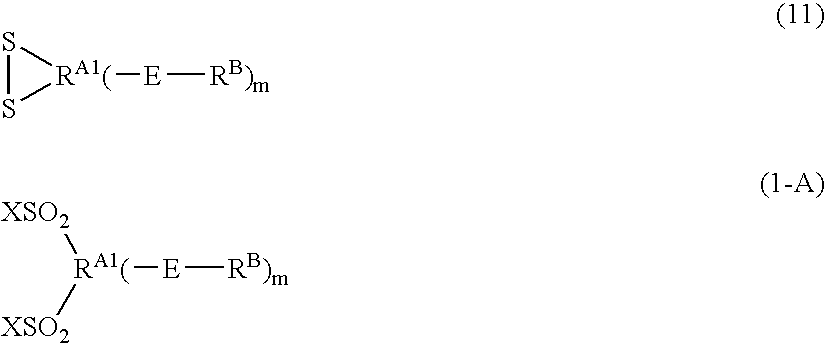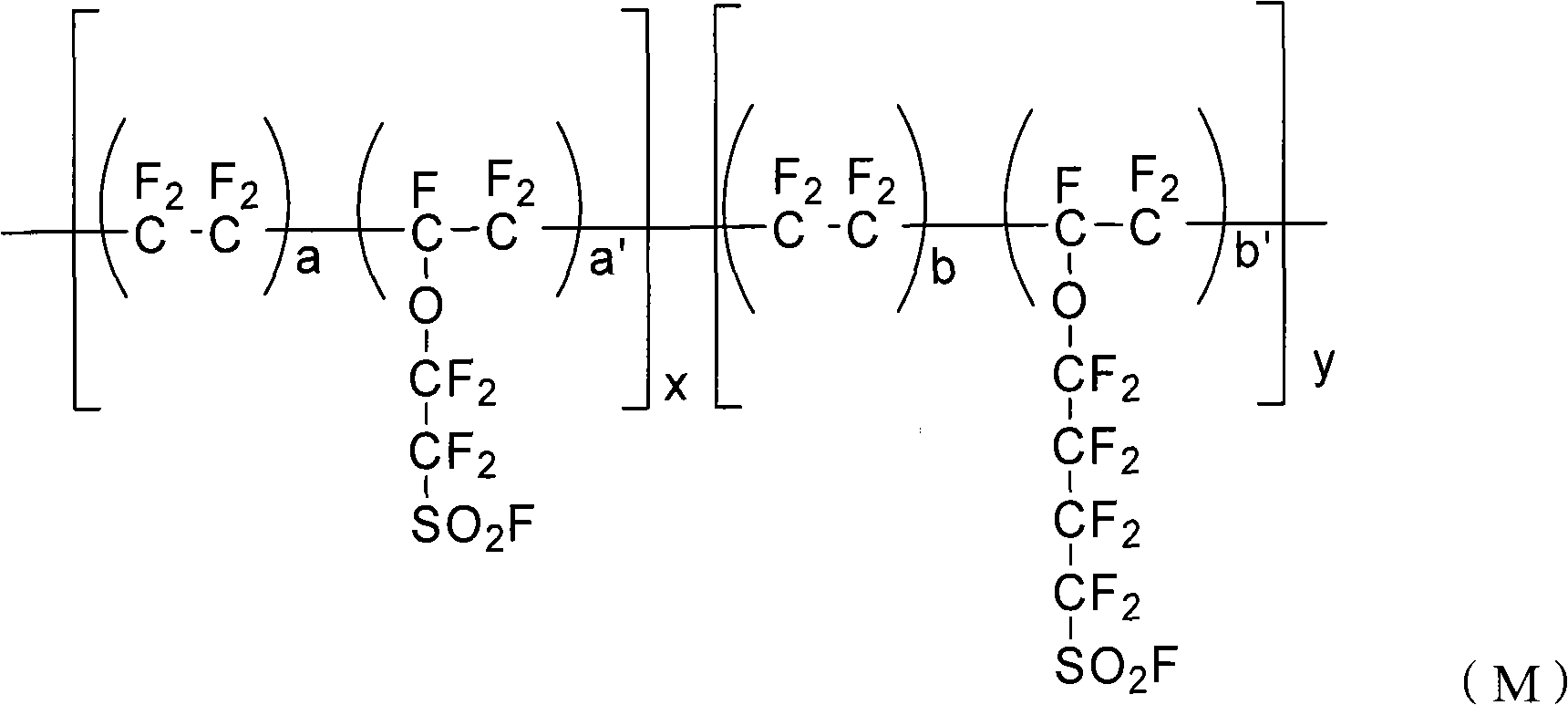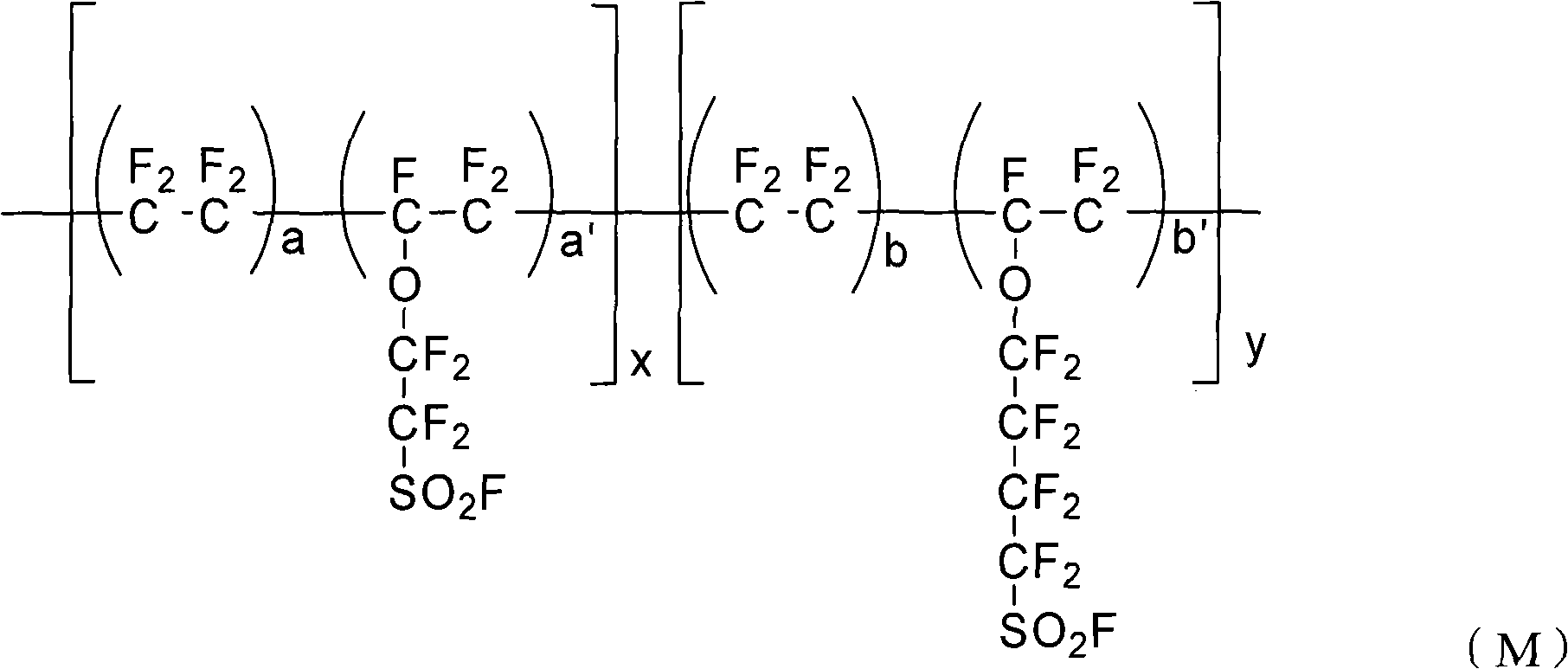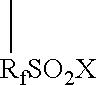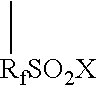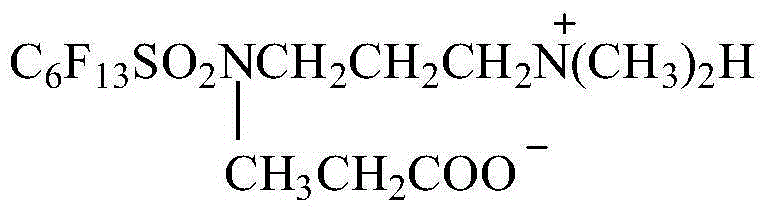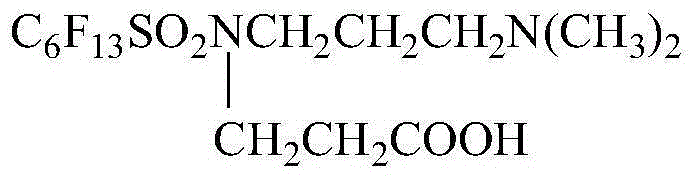Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
262 results about "Sulfonyl fluoride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
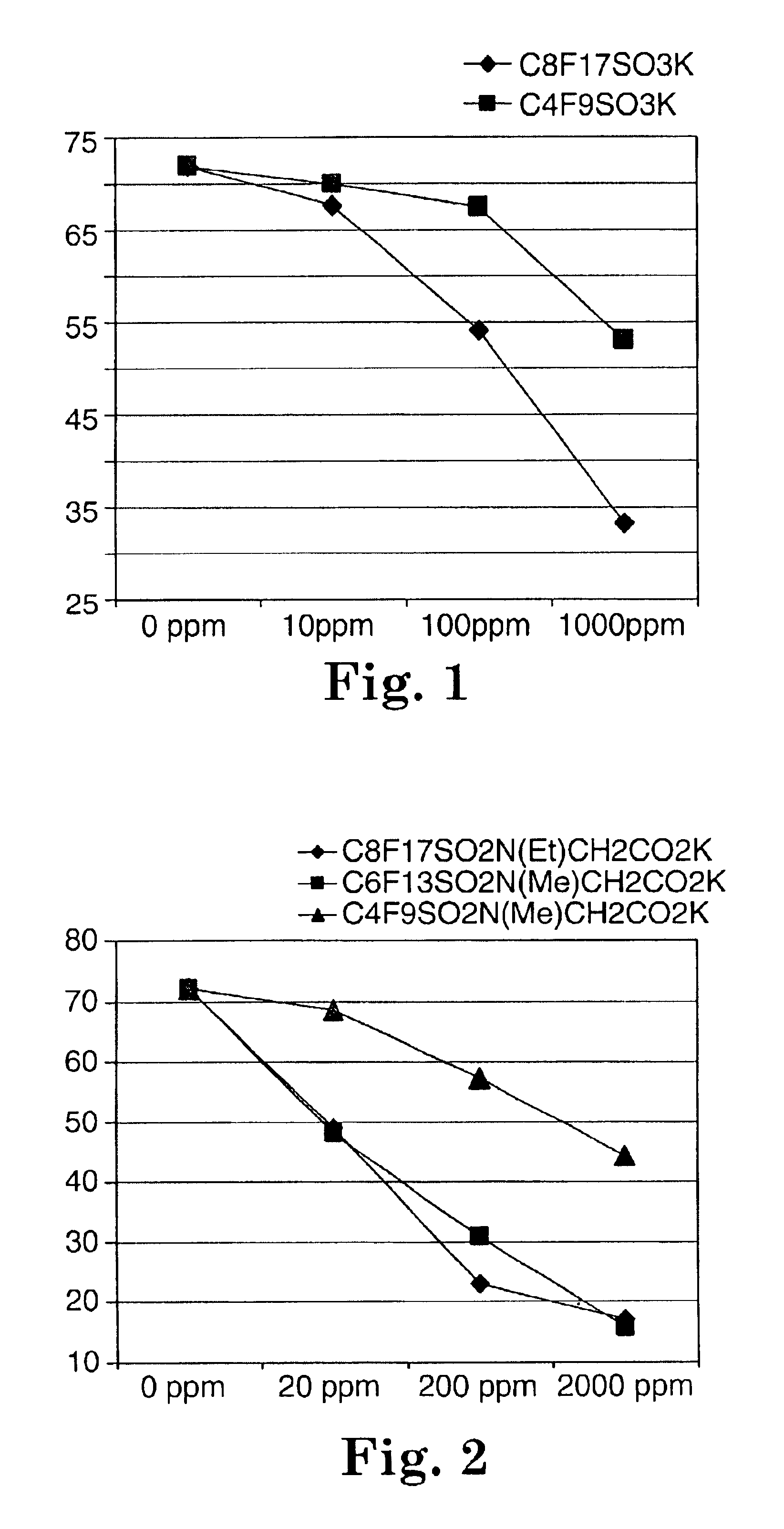
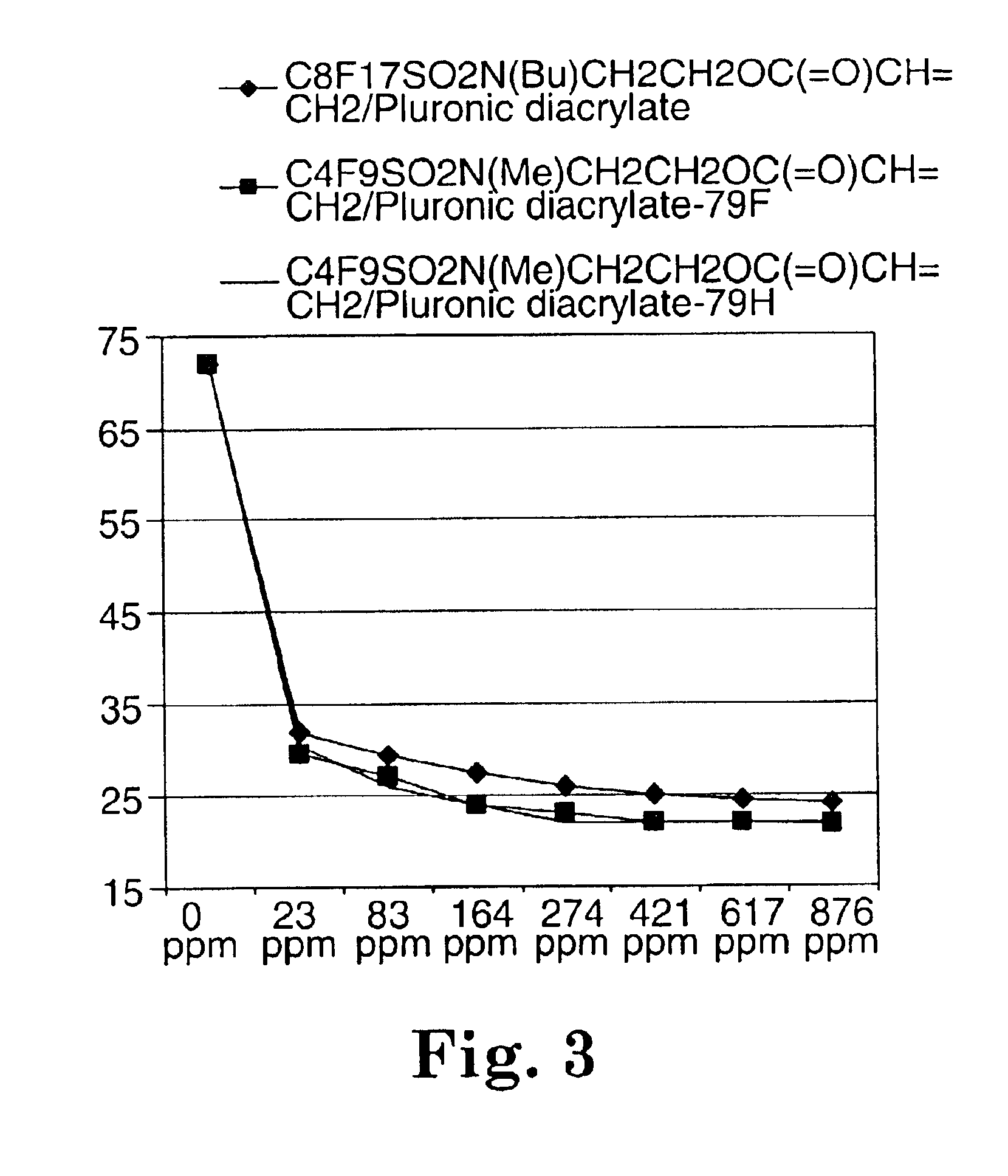
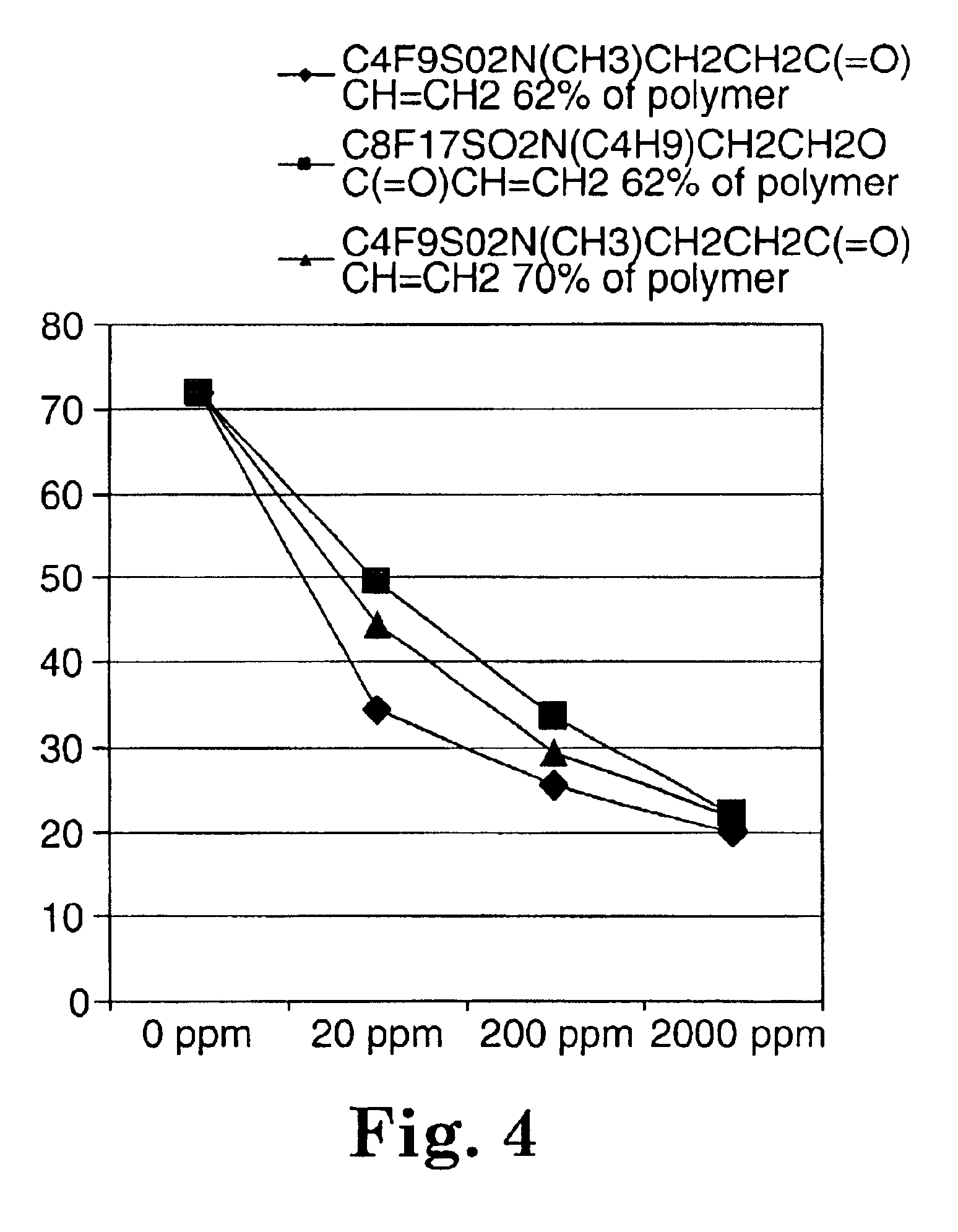
Fluorochemical sulfonamide surfactants
InactiveUS6852781B2High yieldLow costNon-macromolecular adhesive additivesPhotosensitive materialsSide chainSulfonyl fluoride
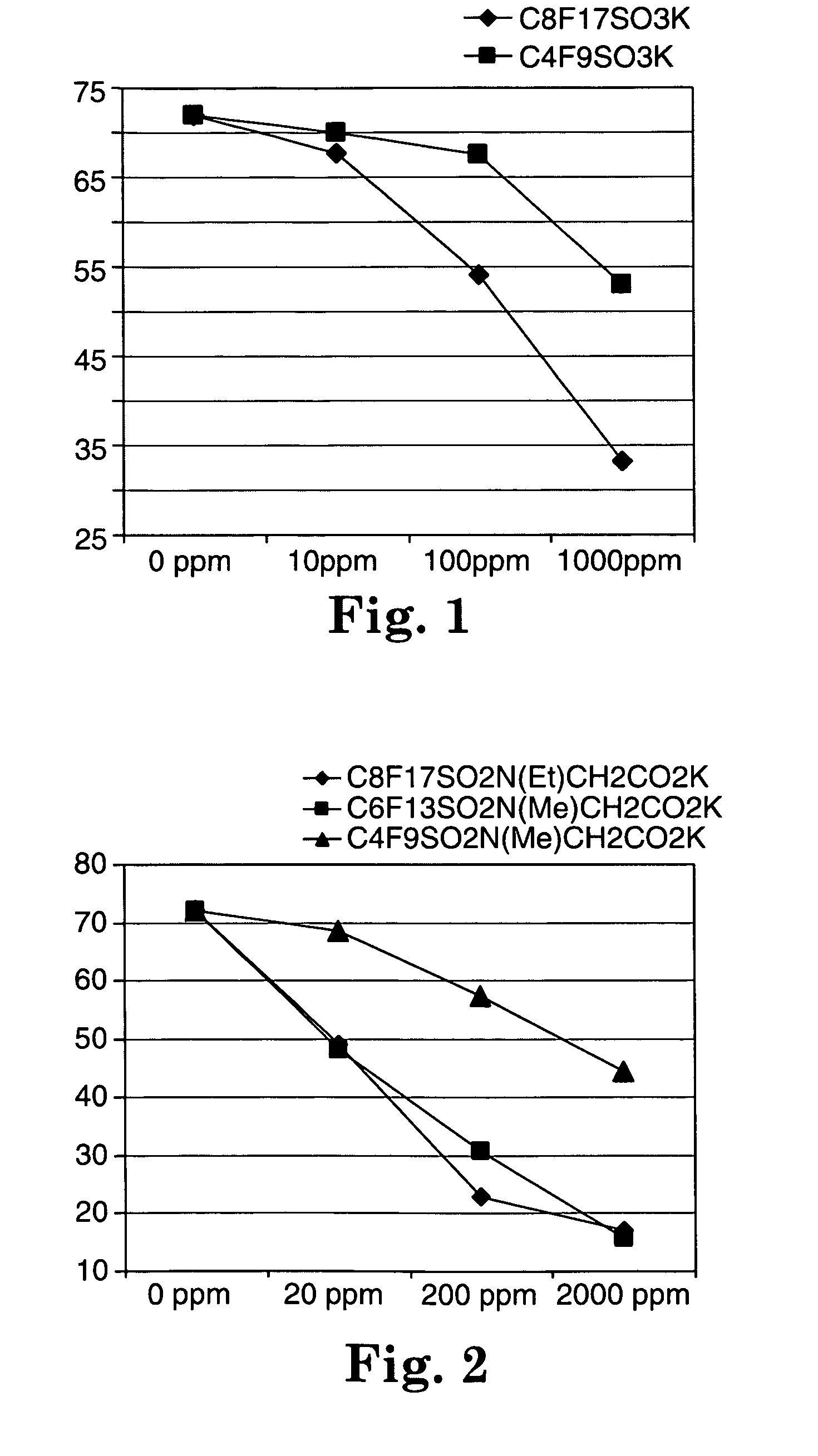
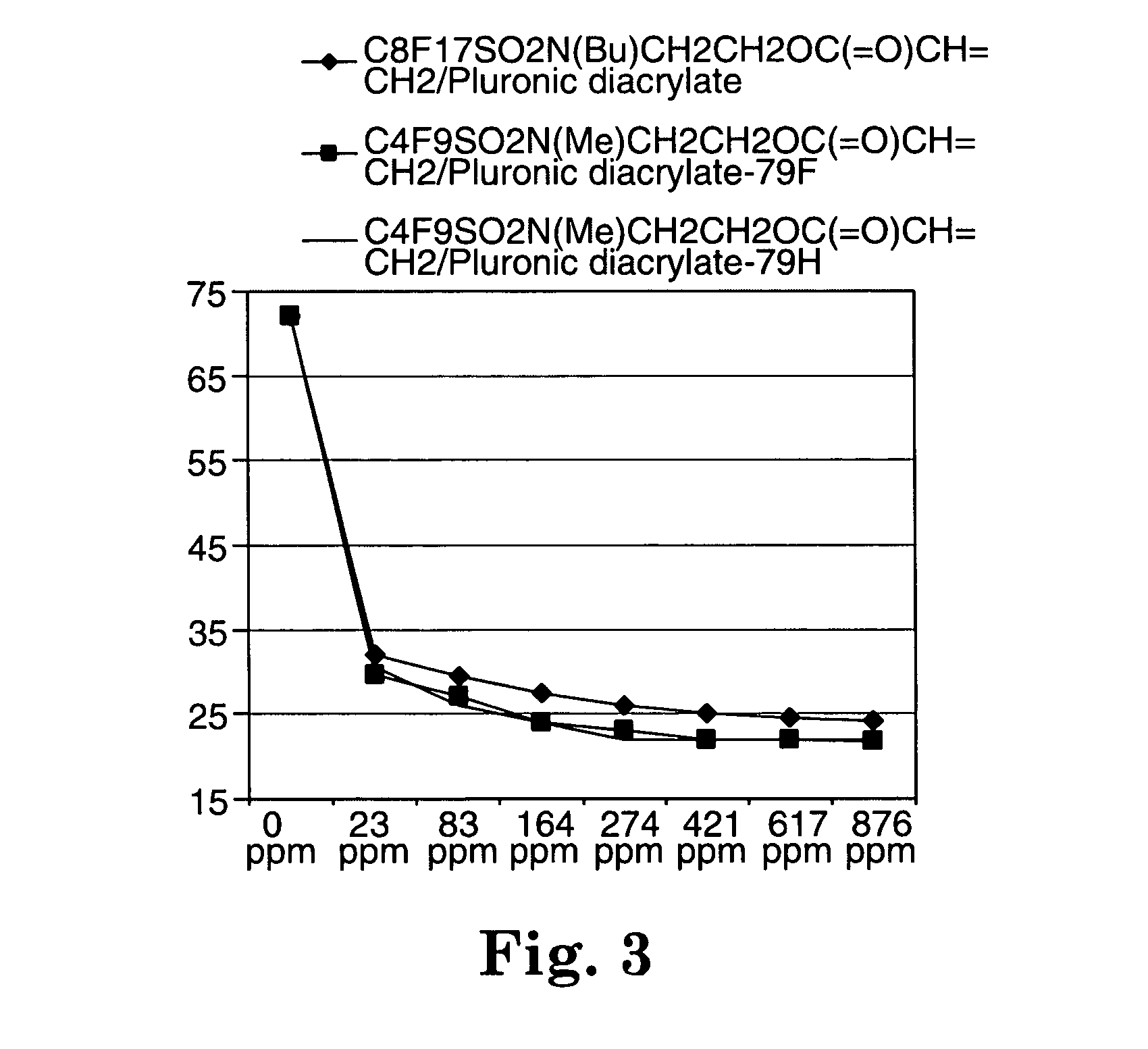
Described are fluorochemical surfactants derived from nonafluorobutanesulfonyl fluoride that contain polyalkyleneoxy side chains and may be copolymerized with acrylic acid or methacrylic acid to form polyacrylates or polymethacrylates. The surfactants surprisingly lower the surface tension of water and other liquids in the same or similar low values achieved by premier surfactants such as those derived from perfluorooctane sulfonyl fluoride.
Owner:3M INNOVATIVE PROPERTIES CO
Polymer of containing fluorin, and application as material of ion exchange fiber
InactiveCN101003588AIncrease the effective areaLower resistanceMelt spinning methodsVinyl etherAlkali ions
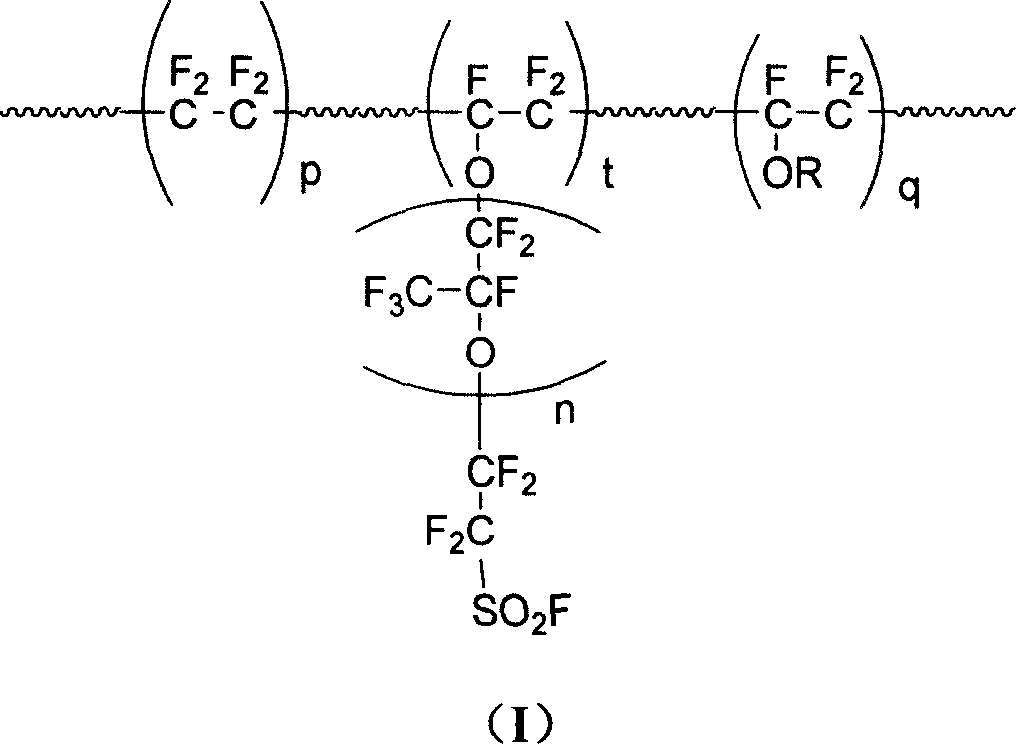
This invention discloses a fluorine-containing polymer and its application as ion exchange fiber material. The fluorine-containing polymer is a perfluoro resin containing sulfonylfluoride groups, and is shown in formula 1. The fluorine-containing polymer has ion exchange function, and is prepared by free radical copolymerization of perfluorosulfonyl vinyl ether, tetrafluoroethylene and hexafluoropropylene in the presence of dispersant, solvent and initiator. The dispersant / solvent is mixed solution of melamine derivative containing linear perfluoro hydrocarbon and water. The fluorine-containing polymer can be melt-spun into polymer fibers, which can be woven into fiber network that can be used as the reinforcing network material for ion exchange membranes and chlor-alkali ion membrane to reinforce and improve the ion exchange ability.
Owner:SHANDONG HUAXIA SHENZHOU NEW MATERIAL
Fluorochemical sulfonamide surfactants
InactiveUS7417099B2High yieldLow costNon-macromolecular adhesive additivesPhotosensitive materialsSide chainSulfonyl fluoride
Described are fluorochemical surfactants derived from nonafluorobutanesulfonyl fluoride that contain polyalkyleneoxy side chains and may be copolymerized with acrylic acid or methacrylic acid to form polyacrylates or polymethacrylates. The surfactants surprisingly lower the surface tension of water and other liquids in the same or similar low values achieved by premier surfactants such as those derived from perfluorooctane sulfonyl fluoride.
Owner:3M INNOVATIVE PROPERTIES CO
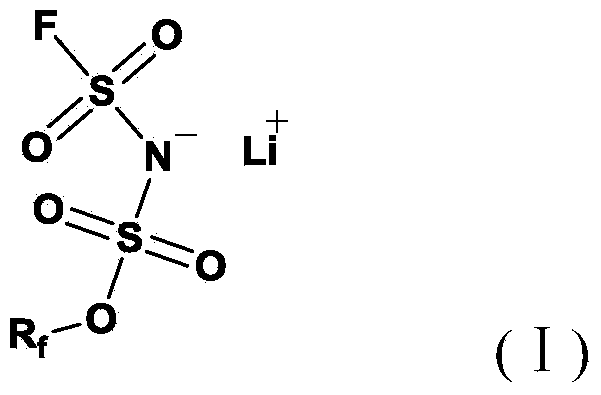
Preparation method of difluoro-sulfimide and lithium difluoro-sulfimide
InactiveCN106365132AFew reaction stepsSimple processNitrosyl chlorideAmidosulfonic acidChemical synthesisDistillation
The invention relates to a preparation method of difluoro-sulfimide and lithium difluoro-sulfimide, and belongs to the field of fluorine chemical synthesis. The preparation method comprises the steps that difluoro-sulfimide is obtained by adding fluoro-sulfoxide into a mixture of sulfamic acid or amino sulfonyl fluoride and fluorosulfuric acid for reacting and then subjected to recrystallization and filtration which are conducted at the temperature of minus 100 DEG C to 16 DEG C and / or reduced / normal-pressure distillation and purification which are conducted at the temperature of 60 DEG C to 169 DEG C; difluoro-sulfimide and a lithium-containing substance react in a solvent to obtain lithium difluoro-sulfimide, and then recrystallization, filtration and purification are conducted. According to the preparation method, difluoro-sulfimide can be prepared through one step, lithium difluoro-sulfimide is obtained through lithiation, and the prepared products can be purified; few steps are needed, the technology is simple, the process is easy to control, the requirement on production devices is low, the production efficiency, the product yield and the purity are high, and large-scale production and application can be achieved.
Owner:718TH RES INST OF CHINA SHIPBUILDING INDAL CORP
Process for converting an alcohol to the corresponding fluoride
InactiveUS6248889B1Good choiceLow costOrganic compound preparationOrganic halogenationAlcoholOrganic base
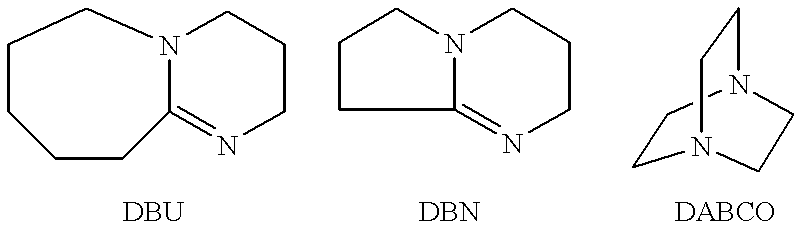
A process for preparing a fluoride from its corresponding alcohol comprises the steps of (a) forming a mixture comprising (i) at least one fluorinated, saturated aliphatic or alicyclic sulfonyl fluoride (for example, perfluorobutanesulfonyl fluoride) and (ii) at least one primary or secondary alcohol; and (b) adding a molar excess of at least one strong, aprotic, non-nucleophilic, hindered, double bond-containing, organic base (for example, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)) to the mixture.
Owner:3M INNOVATIVE PROPERTIES CO
Fluorochemical sulfonamide surfactants
InactiveUS20050148491A1High yieldLow costOrganic chemistryNon-macromolecular adhesive additivesSide chainSulfonyl fluoride
Described are fluorochemical surfactants derived from nonafluorobutanesulfonyl fluoride that contain polyalkyleneoxy side chains and may be copolymerized with acrylic acid or methacrylic acid to form polyacrylates or polymethacrylates. The surfactants surprisingly lower the surface tension of water and other liquids in the same or similar low values achieved by premier surfactants such as those derived from perfluorooctane sulfonyl fluoride.
Owner:3M INNOVATIVE PROPERTIES CO
Imine alkali metal salt and ion liquid and application of same as non water electrolyte
InactiveCN102816096AEasy to separate and purifyHigh yieldAmino preparation from aminesCarboxylic acid nitrile preparationCation compositionAlkali metal
The invention provides ion liquid composed of fluoroalkyl sulfimide salt alkali metal salt containing 'S-fluoroalkyl sulfimide group', fluoroalkyl sulfimide negative ions containing 'S-fluoroalkyl sulfimide group' and positive ions of sulfonium salt, ammonium salt and microcosmic salt. The ion liquid utilizes (fluoroalkyl sulfonyl) (fluoroalkyl sulfinyl) imine with sulfur valence state as +4 and hydroxylamine oxygen sulfonic acid to react to prepare intermediate fluoro alkyl (S- fluoroalkyl sulfimide group) sulfamide of fluoroalkyl sulfimide, effectively shortens the line that the (fluoroalkyl sulfonyl) (fluoroalkyl sulfinyl) imine is utilized to prepare fluoro alkyl (S- fluoroalkyl sulfimide group) sulfamide of fluoroalkyl sulfimide through the three steps of chlorination, fluorination and amination, and is convenient to operate and high in yield and purity. The alkali metal salt has good heat stability and hydrolysis resistance, has high conductivity and oxidation potential in traditional carbonic ester solution, and is good in compatibility with electrode materials widely used. The ion liquid can be applied to lithium ion batteries and carbon-based super capacitors.
Owner:HUAZHONG UNIV OF SCI & TECH +1
Preparation method of bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
ActiveCN102786452AOvercome operabilityOvercome fatal shortcomings such as difficult product purificationSulfonic acid amide preparationTetrafluoroborateDecomposition
The invention discloses a method for preparing bis(sulfonyl fluoride) imine and (perfluoroalkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. According to the method, sulfamide is utilized to take reaction with thionyl chloride and chlorosulfonic acid for preparing bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine, then, the bis(sulfonyl fluoride) imine or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine takes reaction with antimony trifluoride and potassium (rubidium or caesium and the like) carbonate, and corresponding high-purity bis(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (perfluoroalkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt can be obtained; and the double decomposition exchange reaction of the potassium (rubidium or caesium) salt and lithium (or sodium) perchlorate or lithium (or sodium) tetrafluoroborate and the like in aprotic polar solvents is utilized to obtain corresponding high-purity lithium (or sodium) salt. The method provided by the invention has the characteristics that the operation step is simple, the products can be easily separated and purified, the purity and the yield are high, the environment pollution is avoided, the method is suitable for industrial mass production, and the like.
Owner:武汉市瑞华新能源科技有限公司
Hexafluoropropene-based fluorosulfonated elastomers with a low glass transition temperature, containing neither tetrafluoroethylene nor a siloxave group
InactiveUS20030153699A1Easy to operateImprove the immunityThin material handlingElastomerPolymer science
The present invention describes the synthesis of new fluorinated elastomers with very low glass transition temperatures (Tg), a good resistance to bases, gasoline and other carburants and good workability properties, these elastomers contain hexafluoropropene (HFP), perfluoro(4-methyl-3,6-dioxaoct-7-ene) sulfonyl fluoride (PFSO2F), vinylidene fluoride (VDF) and / or at least one fluorinated alkene and / or one vinyl perfluorinated ether. In a precise case, they are prepared by radical polymerisation of HFP and PFSO2F or by radical terpolymerisation HFP, PFSO2F and VDF in the presence of different organic initiator, such as peroxides, peresters or diazo compounds.
Owner:AMEDURI BRUNO MICHEL +3
Perfluorinated ion exchange resin as well as preparation method and application thereof
ActiveCN101768236ASolve the problem of insufficient molecular weightAct as a dispersantSemi-permeable membranesCell electrodesTetrafluoroethylenePolymer science
The invention provides a perfluorinated ion exchange resin as well as a preparation method and application thereof, wherein the perfluorinated ion exchange resin is provided with a phosphonate side group and sulfonyl fluoride short side groups with two different structures and has the function of high exchange capacity. The perfluorinated ion exchange resin is formed by tetrafluoroethylene, sulfonyl fluoride alkene ether monomers with the short side groups in two different structures, and a phosphonate side group alkene ether monomer through multi-copolymerization, and the repeated unit is represented by the following formula. An ion exchange membrane made of the resin can not only have various chemical media resistance, but also have high iron exchange capacity, high conductivity, high mechanical strength, high dimensional stability, low membrane resistance and long service life and is applicable for a fuel cell and a high-temperature fuel cell.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Bisphenol A type poly sulfuric acid (ammonia) ester compound and synthetic method thereof
ActiveCN104059228AImprove mechanical propertiesExcellent dielectric propertiesPolyesterPtru catalyst
The present invention discloses a bisphenol A type poly sulfuric acid (ammonia) ester compound, and belongs to the technical field of polymers. The bisphenol A type poly sulfuric acid (ammonia) ester compound is prepared as follows: using bisphenol A dioxygen sulfonyl fluoride as a raw material for reacting with trialkyl silicon group-protected bisphenol or diamine in the presence of an inert gas for protection and a catalyst at 30-120 DEG C for 3 to 36 hours; and pouring the reaction mixture into water to precipitate a polymerization product, namely the bisphenol A type poly sulfuric acid (ammonia) ester compound. The bisphenol A type poly sulfuric acid (ammonia) ester compound has excellent mechanical properties, dielectric properties, tolerance and wear resistance, and has broad application prospects in aerospace, electronic communications, electromechanical and microelectronic industries. In addition, the high reaction activity bisphenol A type dioxygen sulfonyl fluoride compound is used as a polyester synthetic raw material, and compared with a traditional polyester process, the reaction condition is mild and easy to control, the reaction process is simple and easy to operate; the after treatment process is simple, the environmental pollution is less, and the bisphenol A type poly sulfuric acid (ammonia) ester compound is favorable to industrial production.
Owner:内蒙古图微新材料科技有限公司
Graft oligomeric electrolytes
Disclosed are compositions prepared by free-radical-driven grafting onto hydrocarbons or hydrocarbon ethers of olefinically unsaturated fluorocarbons containing sulfonyl fluoride, fluorosulfonate, fluorosulfonimide, or fluorosulfonyl methide groups, wherein the grafting step is followed by a hydrolysis step in the case of sulfonyl fluoride.
Owner:EI DU PONT DE NEMOURS & CO
Fluorocarbon gemini surfactant as well as preparation method and application thereof
ActiveCN101502771AUse low concentrationReduce surface tensionTransportation and packagingMixingPotassium hydroxideEthyl acetate
The invention discloses a fluorine-carbon gemini surfactant for oil extraction and a preparation method thereof. The preparation method of the surfactant is as follows: 1) perfluorooctsulfunyl fluoride is dissolved in ethyl acetate, is firstly mixed with polyethylene polyamines and then reacts with potassium hydroxide after being heated to obtain diperfluoro octyl sulfamide; 2) diperfluoro octyl sulfamide is mixed with 2-chlorethanol in an organic solvent and reacts with potassium hydroxide to obtain N-N-alcohol diperfluoro octyl sulfamide; 3) N-N-alcohol diperfluoro octyl sulfamide is mixed with hydrogen peroxide and reacts with potassium hydroxide to obtain the target product. The fluorine-carbon gemini surfactant for oil extraction provided by the invention has comparatively low using concentration, can obviously reduce the surface tension of aqueous phase system, is excellent in temperature and salt resistance, can be used as a surfactant component in polymer-surfactant binary compound flooding and alkali-polymer-surfactant ternary compound flooding systems and can also be directly applied in surfactant flooding.
Owner:CHINA NAT OFFSHORE OIL CORP +1
Process for producing fluorinated sulfonyl fluoride compound
InactiveUS20060106252A1Organic compound preparationSulfonic acid preparationSulfonyl fluorideLiquid phase
It is an object of the present invention to solve difficulty in production and to provide a process to obtain fluorinated sulfonyl fluoride compound having various molecular structures efficiently at a low cost. That is, the present invention provides a process which comprises reacting (FSO2—)nRA(-E-RB)m (1F) with fluorine in a liquid phase to form (FSO2—)nRAF(-EF-RBF)m (2), and decomposing this compound to obtain (FSO2—)nRAF(-EF1)m (3), provided that RA is a (n+m)valent organic group having at least two carbon atoms, RAF is a group having RA fluorinated, or the like, each of RB and RBF is a fluorinated monovalent organic group, or the like, E is —COOCH2— or the like, EF is —COOCF2— or the like, EFl is —COF or the like, n is an integer of at least 2, and m is an integer of at least 1.
Owner:ASAHI GLASS CO LTD
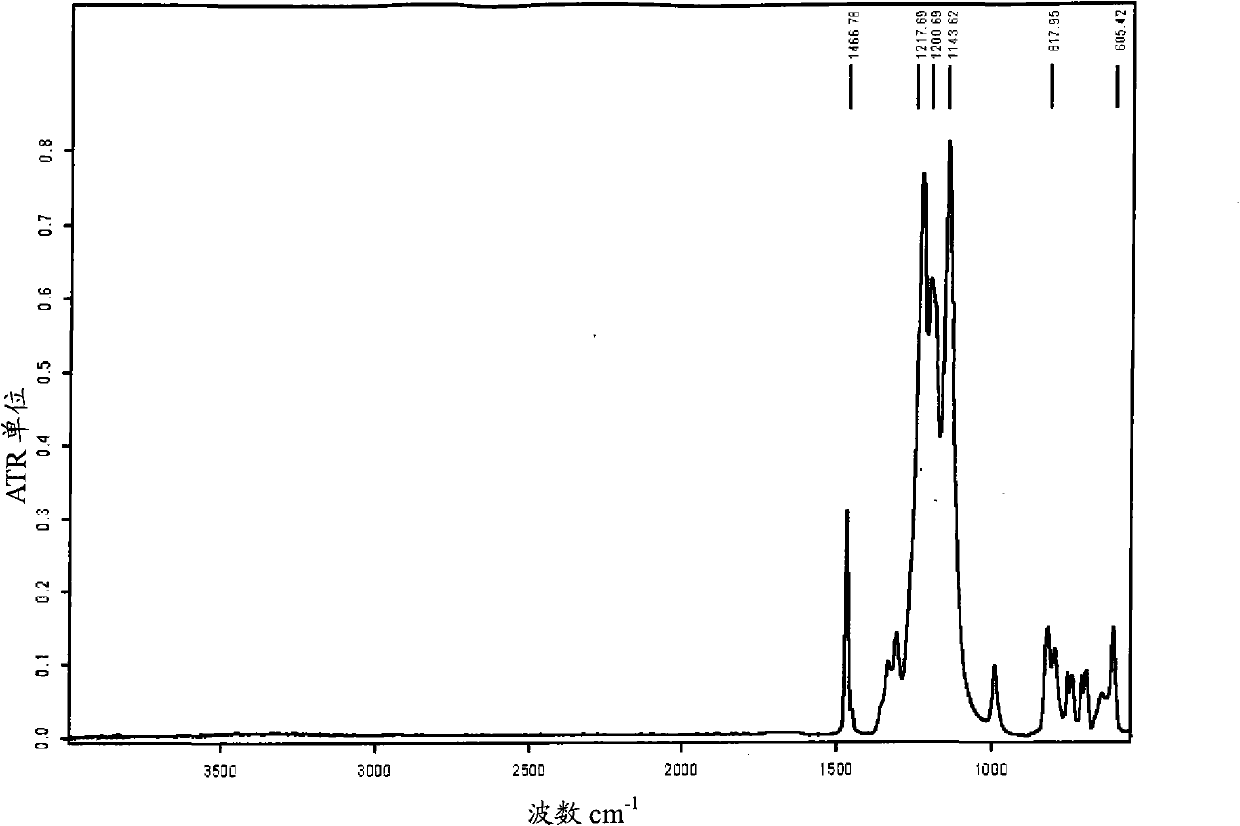
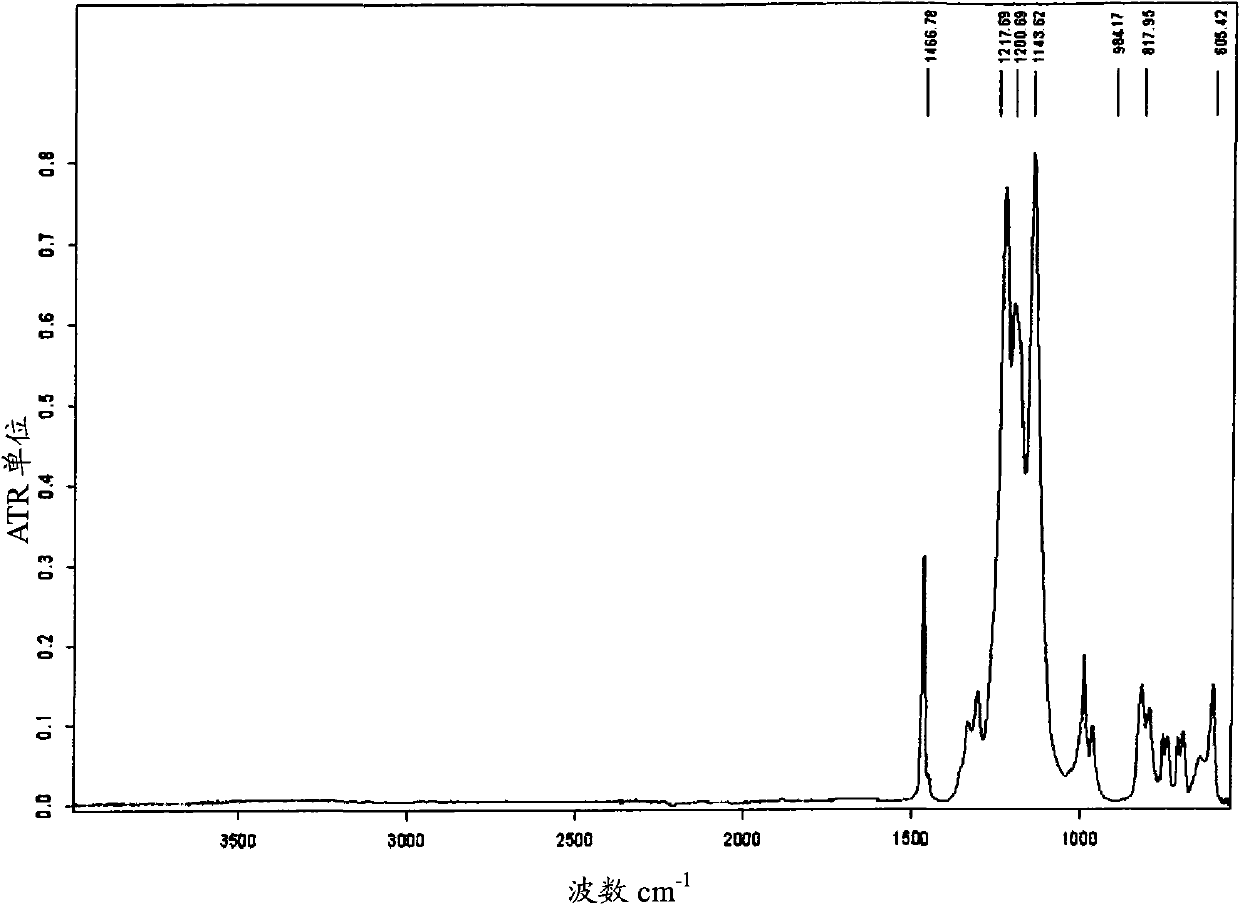
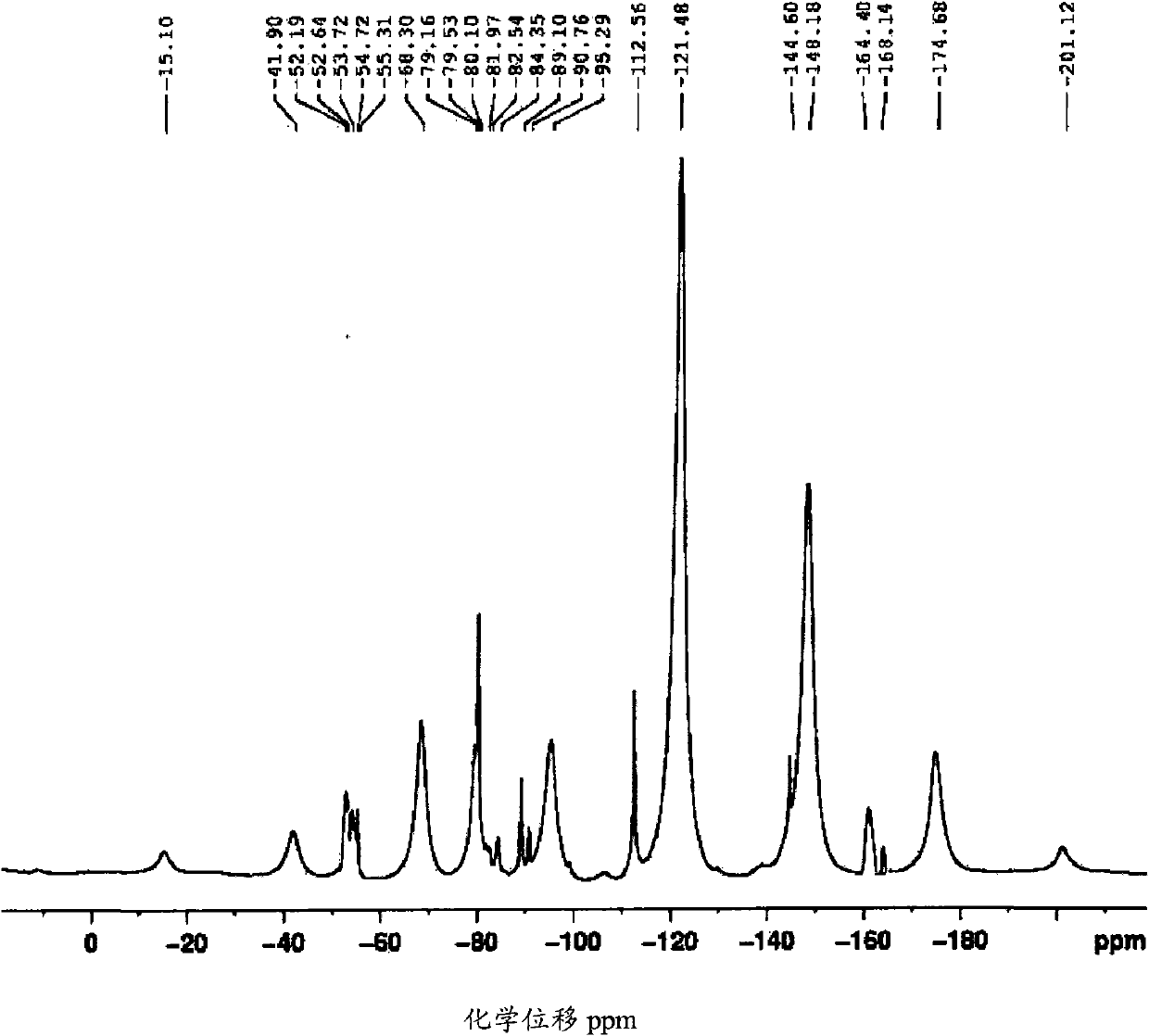
Fluorosulphonated elastomers with low glass transition based on vinylidene fluoride
The present invention describes the synthesis of new sulforated fluorinated elastomers having very low glass transition temperatures (Tg), a good resistance to bases, oils and fuels and good properties of workability. These elastomers contain, by way of example, from 80 to 60 mole % of vinylidene fluoride (VDF) and 20 to 40 mole % of perfluoro(4-methyl-3,6-dioxaoct-7-ene) sulfonyl fluoride (PFSO2F). In this case, they are prepared by radical copolymerisation of VDF and PFSO2F in the presence of different organic initiators, for example, peroxides, peresters or diazo compounds.
Owner:AMEDURI BRUNO MICHEL +3
Preparation method of trifluoro methanesulfonic anhydride
ActiveCN102911086AHigh yieldReduce contentOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of trifluoro methanesulfonic anhydride. The method comprises the following steps of: firstly reacting trifluoro methanesulphonyl fluoride with alkali metal hydroxide to prepare trifluoro mesylate, purifying trifluoro mesylate by recrystallization by utilizing an organic solvent, reacting trifluoro methane sulfonyl chloride with trifluoro mesylate to generate a trifluoro methanesulfonic anhydride crude product, and finally purifying trifluoro methanesulfonic anhydride by atmospheric distillation. The preparation method of trifluoro methanesulfonic anhydride can be used for not only effectively simplifying reaction steps so that the operation process is simple and convenient and the operation is safe, but also avoiding byproducts generated in the process of the traditional method for producing trifluoro methanesulfonic anhydride, and effectively reducing the contents of F<-> and SO4<2-> in the product; by utilizing recrystallization, atmospheric distillation and other methods for purification, the product purity is up to 99.5%; and more importantly, the yield of anhydride is greatly increased and raised to 88% from original 60%.
Owner:JIANGXI GUOHUA IND CO LTD
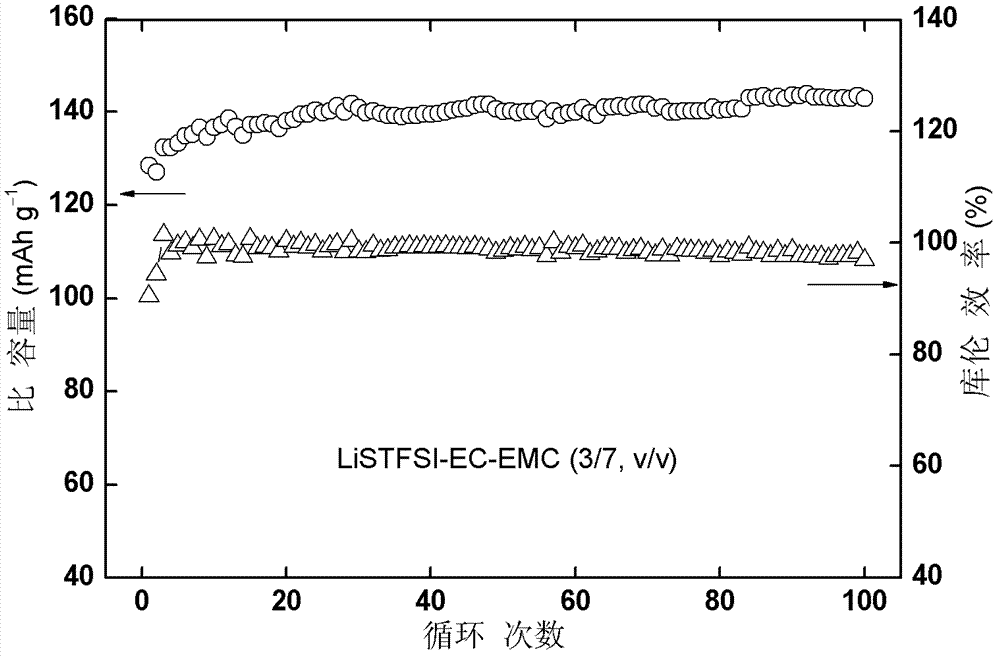
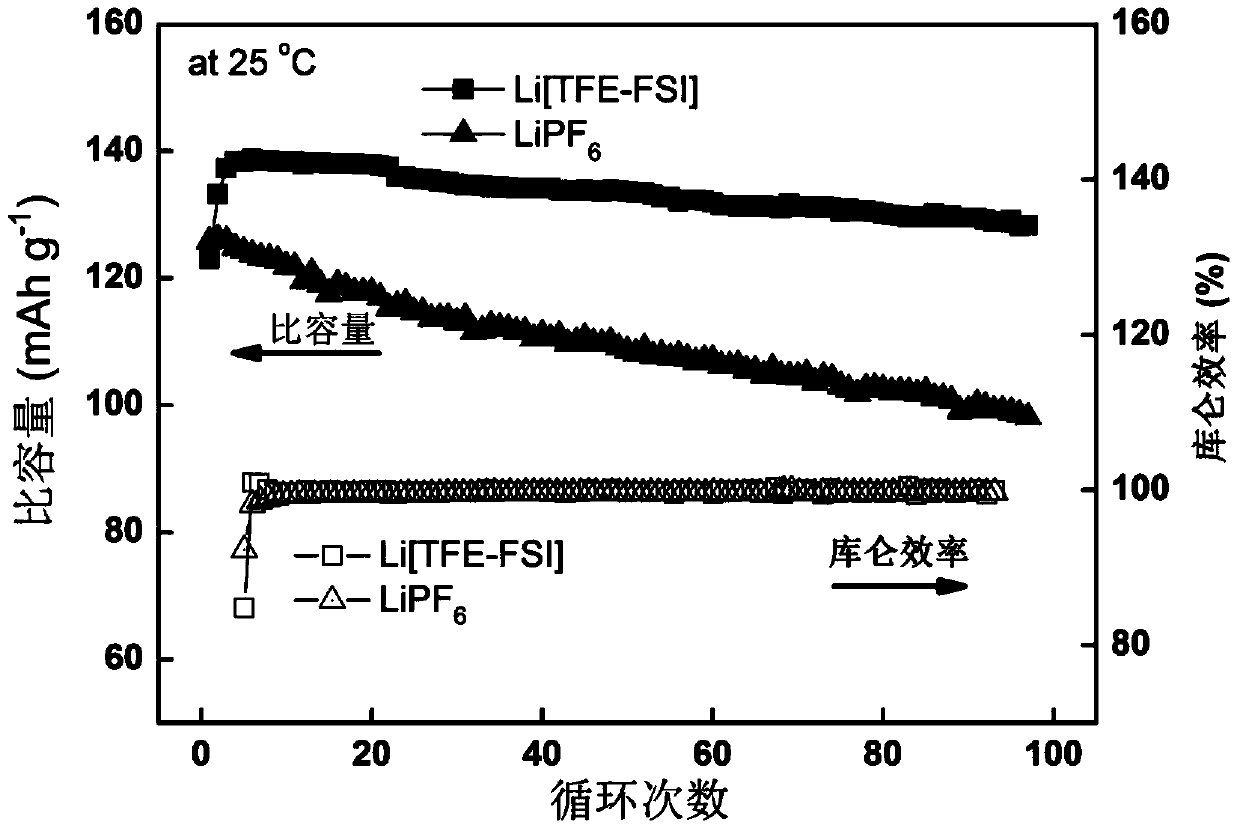
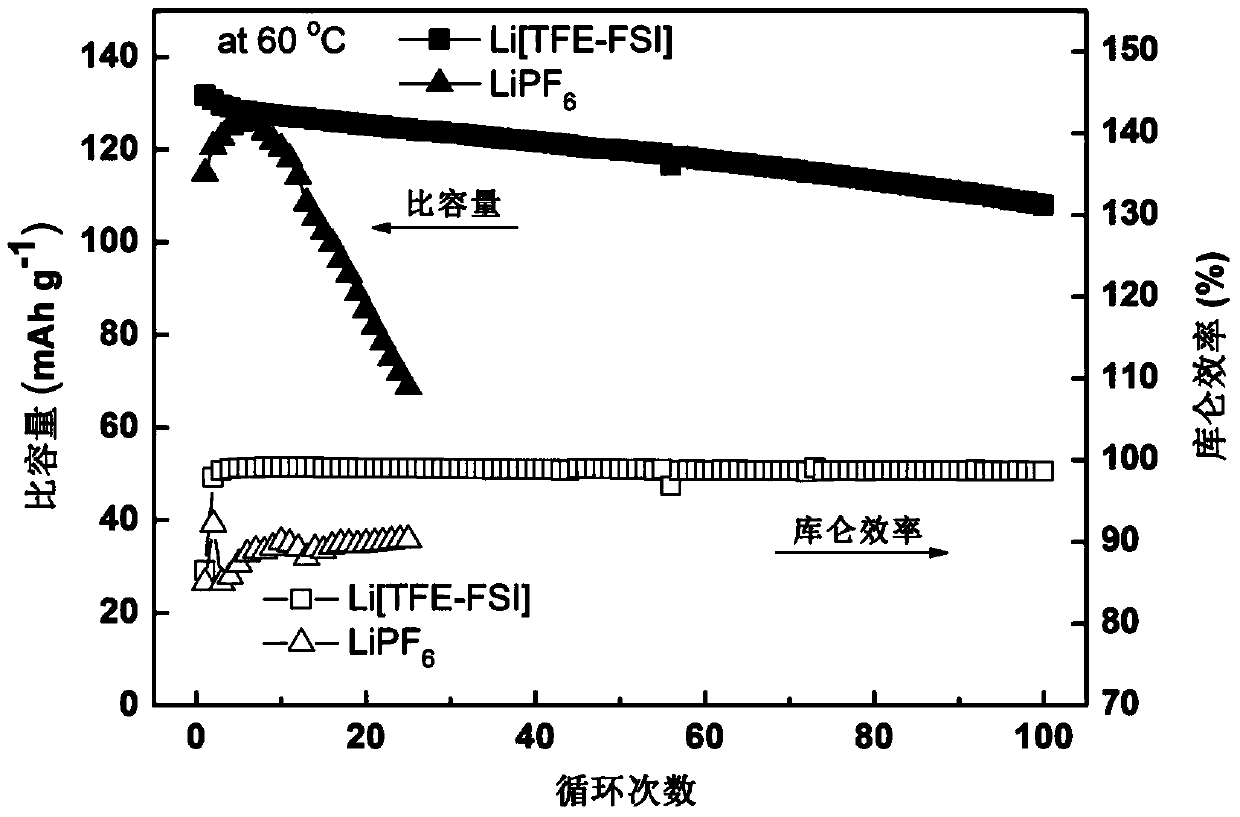
Lithium ion battery electrolyte and lithium ion battery
InactiveCN105449275AImprove securityImprove thermal stabilitySecondary cells servicing/maintenanceTriflic acidSulfonyl fluoride
The invention discloses a lithium ion battery electrolyte and a lithium ion battery. The lithium ion batter electrolyte comprises non-aqueous organic solvent, lithium salt, a functional additive, a flame-retardant additive and a negative electrode film-forming agent. According to the scheme of the lithium ion batter electrolyte, perfluoroalkyl diphenyl sulfide is used as the functional additive on the basis of reasonably optimizing the non-aqueous organic solvent, the lithium salt and the negative electrode film-forming agent; the problem that the novel lithium salt corrodes an aluminium current collector can be effectively solved, wherein the novel lithium salt comprises lithium trifluoromethanesulfonate (LiCF3SO3), perfluoroalkyl sulfonyl lithium methide (LiC (CF3SO2)3, bi(trifluoromethyl sulfonyl) lithium imide (LTFSI), bi(sulfonyl fluoride) lithium imide (LiFSI) and the like; the cycling performance of the lithium ion battery is improved; the LiPF6 can be well replaced by the lithium ion battery electrolyte, and the lithium ion battery electrolyte can be widely applied to the secondary lithium ion battery electrolyte, and particularly suitable for lithium ion power batteries for improving the thermal stability of the lithium ion power batteries.
Owner:OPTIMUM BATTERY CO LTD
Perfluorosulfonic composite proton exchange membrane for fuel cell
InactiveCN101777659AHigh mechanical strengthImprove proton conductivityCell component detailsFuel cell detailsManganeseCerium
The invention provides a perfluorosulfonic composite proton exchange membrane for a fuel cell and a preparation method thereof. Perfluorosulfonic resin formed by perfluorosulfonic resin, M-type perfluorosulfonic resin and porous polymer enhanced materials is H-type resin with sulfonic acid groups (-SO3-) or F-type resin with sulfuryl fluoride groups (-SO2F-). The M-type perfluorosulfonic resin is cerium or / and manganese metal ion type perfluorosulfonic resin formed in a way that cerium or / and manganese ions are fully exchanged with sulfonic acid groups or sulfuryl fluoride groups in perfluorosulfonic resin. Cerium or / and manganese ions can be evenly distributed on the membrane body, the thermal stability is high, the heat treatment temperature of the composite membrane is improved, the combination of the porous polymer enhanced materials with the perfluorosulfonic resin is facilitated, the prepared composite proton exchange membrane has good mechanical strength and proton conductivity capacity, and the performance of the fuel cell is improved.
Owner:SHANDONG HUAXIA SHENZHOU NEW MATERIAL
Perfluorinated ion exchange resin and preparation method and application thereof
ActiveCN101798365AHigh chemical stabilityHigh ion exchange capacityCation exchanger materialsOrganic diaphragmsIon exchangeIon
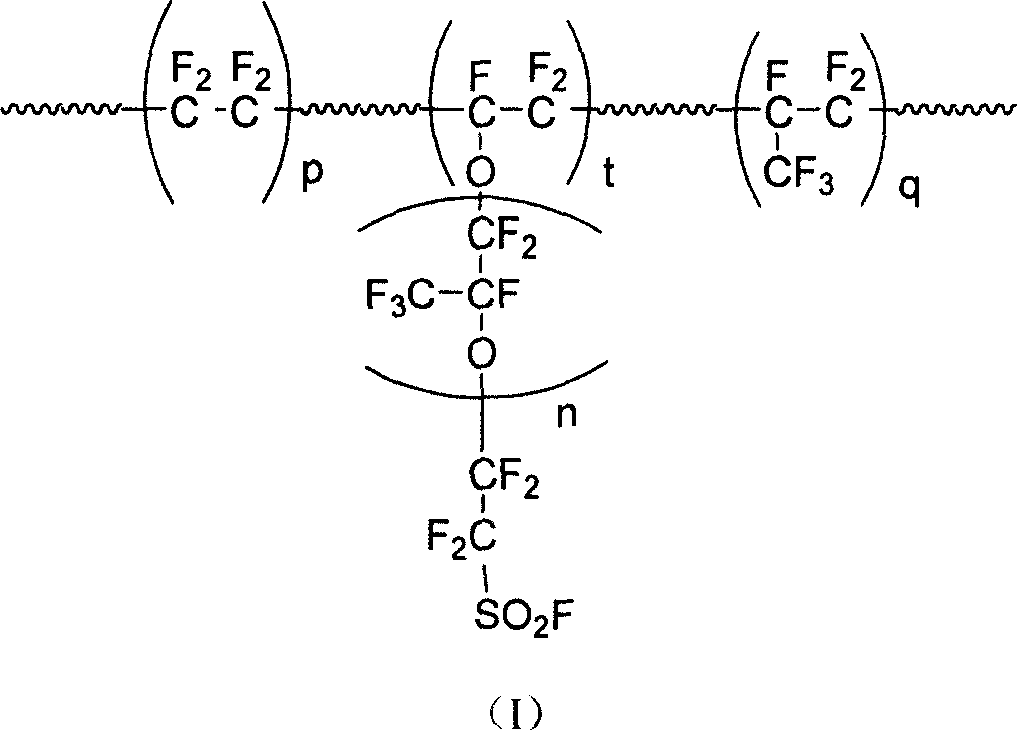
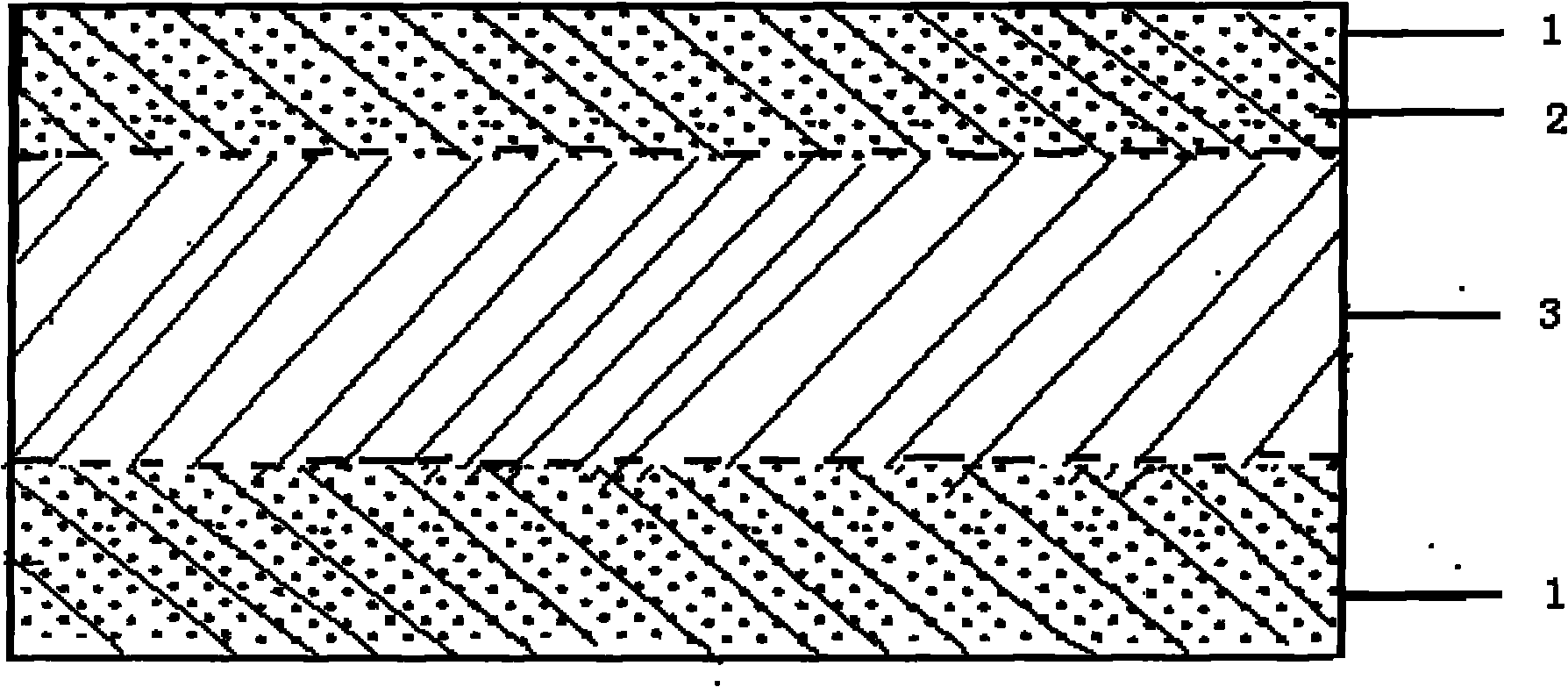
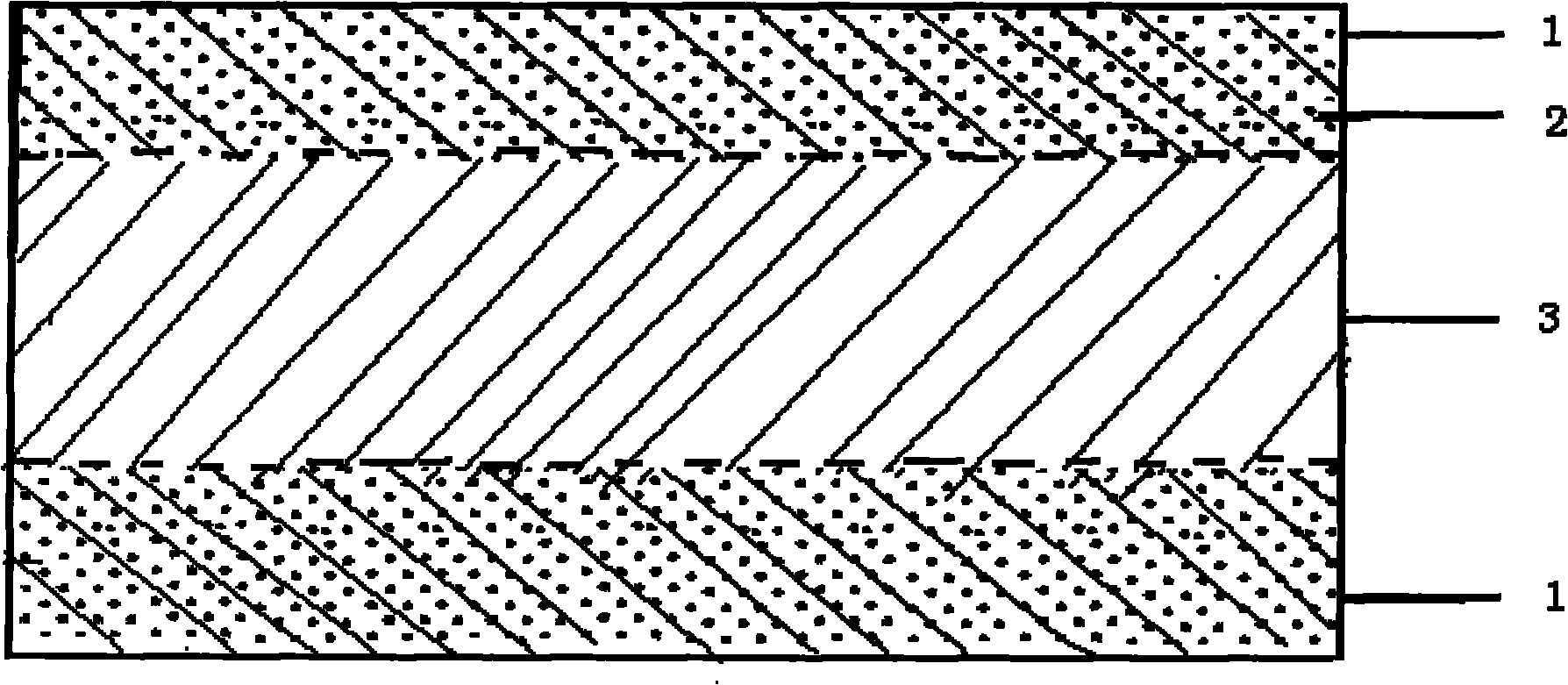
The invention provides a perfluorinated ion exchange resin and a preparation method and application thereof. The molecular formula of the perfluorinated ion exchange resin is shown as the formula M. The invention also provides a preparation method of the perfluorinated ion exchange resin; the method comprises: under the action of initiator, tetrafluoroethylene monomer and two sulfuryl fluoride vinyl ether monomers generate ternary copolymerization reaction. The perfluorinated ion exchange resin provided by the invention can simultaneously satisfy the requirements of mechanical strength and ion exchange volume and has favourable thermostability.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Graft oligomeric electrolytes
Disclosed are compositions prepared by free-radical-driven grafting onto hydrocarbons or hydrocarbon ethers of olefinically unsaturated fluorocarbons containing sulfonyl fluoride, fluorosulfonate, fluorosulfonimide, or fluorosulfonyl methide groups, wherein the grafting step is followed by a hydrolysis step in the case of sulfonyl fluoride.
Owner:EI DU PONT DE NEMOURS & CO
Nonaqueous electrolyte containing sulfonyl fluoride imidogen lithium salt as well as application of electrolyte
InactiveCN103794819AImprove thermal stabilityStrong redox resistanceCell electrodesSecondary cellsSulfonyl fluorideLithium-ion battery
The invention discloses nonaqueous electrolyte by taking asymmetrical (sulfonyl fluoride) (multi-fluorine alkoxy sulfonyl) lithium imide as conductive salt. The nonaqueous electrolyte has the characteristics of high thermal stability, high redox resistance, no aluminum foil corrosion and the like. Under the condition of no additive, the electrolyte material and the lithium ion battery electrode material have favorable compatibility; meanwhile, the electrolyte material has better room-temperature and high-temperature circulation stability than LiPF6, and can serve as a nonaqueous electrolyte additive for improving the high-temperature cycle and storage performances based on the LiPF6 electrolyte.
Owner:SUZHOU FLUOLYTE
Method for synthesizing perfluoroalkyl sulfimide alkali metal salt and ionic liquid synthesized by same
ActiveCN101747244AQuick responseEnhance nucleophilic attack capabilitySulfonic acid amide preparationRubidiumDecomposition
The invention discloses a method for synthesizing perfluoroalkyl sulfimide alkali metal salt (M[Rf1SO2NSO2Rf2]) which is abbreviated to M[PFSI], wherein Rf1 and Rf2=CmF2m+1, m=1-8, M=Li, Na, K, Rb, and Cs. The method utilizes potassium (rubidium and cesium) salt of perfluoroalkyl sulfamide and perfluoroalkyl sulfonyl fluoride for reaction in the existence of potassium carbonate (rubidium and cesium), so that the potassium (rubidium and cesium) salt of the perfluoroalkyl sulfimide can be conveniently prepared with high yield which is 70-90%; the high-purity corresponding lithium (or sodium) salt (M[PFSI], M=Li and Na) can be obtained through double decomposition exchange reaction of the potassium (rubidium and cesium) salt and lithium perchlorate (or sodium) in aprotic polar solvent (such as acetonitrile, dimethyl carbonate, nitromethane and the like); and the hydrophobic functionalized ionic liquid composed of cations of sulfonium, ammonium or phosphorus with the [PFSI] through reaction of the prepared alkali metal salt and the sulfonium salt, ammonium salt or phosphorus salt of which the side chain contains functionalized functional groups.
Owner:武汉市瑞华新能源科技有限公司
Perfluorinated ion exchange resin with high exchange capacity, preparation method and application thereof
ActiveCN101709101ASolve the ion exchange capacitySolve the strength problemSemi-permeable membranesOrganic diaphragmsVinyl etherSulfonyl fluoride
The invention provides a high exchange capacity perfluorinated resin of two types of short side-radical sulfonyl fluoride with different structures and bromine side-radical. The resin is a polyprotonic copolymer comprising the following components in total molar fraction: 50-85 percent of tetrafluoroethylene polymerization unit, 5-49 percent of the polymerization unit of the two types of short side-radical sulfonyl fluoride vinyl ether provided with different structures, and1-10 percent of bromine side-radical vinyl ether polymerization unit. A perfluorinated ion exchange membrane prepared by the resin not only has resistance to various chemical reagents, but also has high ion exchange capacity, electric conductivity, mechanical strength and dimensional stability, low membrane resistance and long service life, and is suitable for usage in fuel cells or high-temperature fuel cells. The invention also provides a preparation method and applications of the resin.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
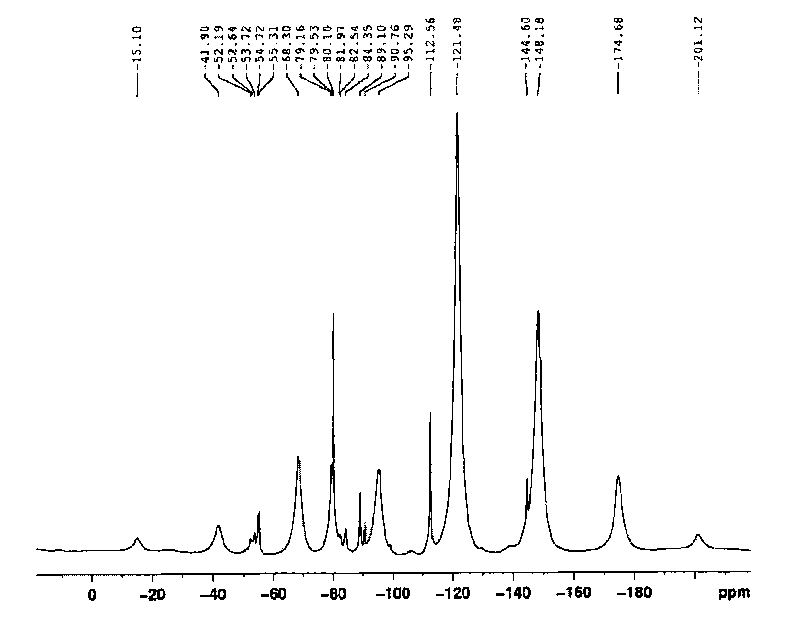
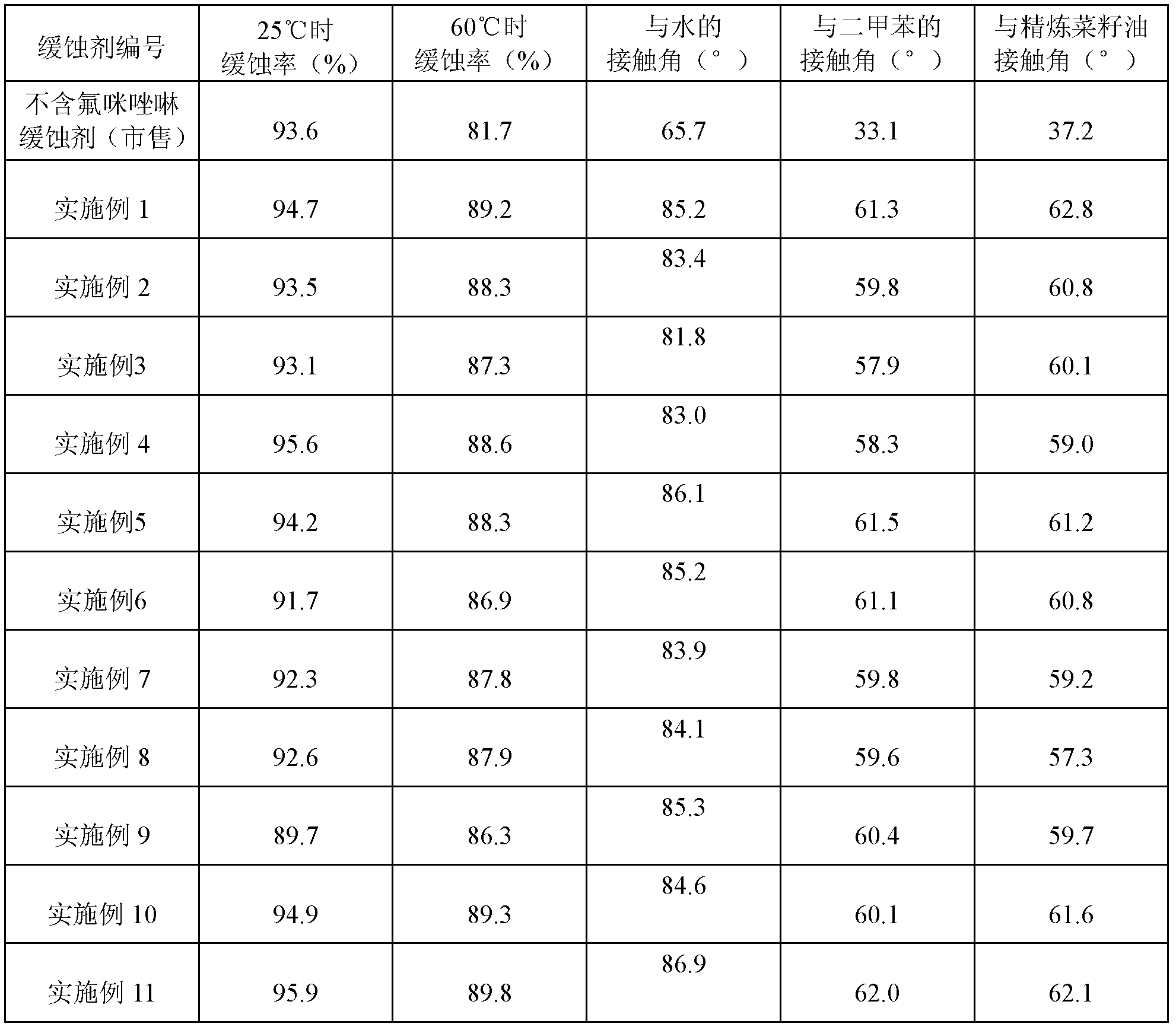
A kind of fluorine-containing cationic imidazoline corrosion inhibitor and preparation method thereof
ActiveCN102277577AReduce coefficient of frictionLow flow resistanceDiethylenetriamineCarboxylic acid
The invention provides a fluorine-containing cationic imidazoline corrosion inhibitor and a preparation method thereof. The method comprises the following steps: adding an organic carboxylic acid and diethylenetriamine into a reactor in a molar ratio of 1:(0.1-2); adding a benzene solvent, heating, and performing a reaction at the temperature of 130-165 DEG C; heating, and performing a cyclization reaction at the temperature of 190-210 DEG C; cooling to 80-110 DEG C after the cyclization reaction; dripping perfluoroalkglsulfonyl fluoride after cooling, and performing a thermostatic reaction; and dripping a quaternizing reagent after the thermostatic reaction, and performing a thermostatic reaction to prepare the fluorine-containing cationic imidazoline corrosion inhibitor. The corrosion inhibitor is a rufous sticky liquid and can be miscible with water in any proportion, wherein the flash point is more than 60 DEG C, the condensation point is less than minus 10 DEG C, and the density is between 0.86g / ml and 0.99g / ml. The fluorine-containing cationic imidazoline corrosion inhibitor has the advantages of good metal surface adsorption, easily formed oleophobic metal surface, less possibility of dropping and the like, and can be used for reducing the friction of organic media, such as crude oil, finished product oil and the like, to a vessel surface.
Owner:陕西日新石油化工有限公司
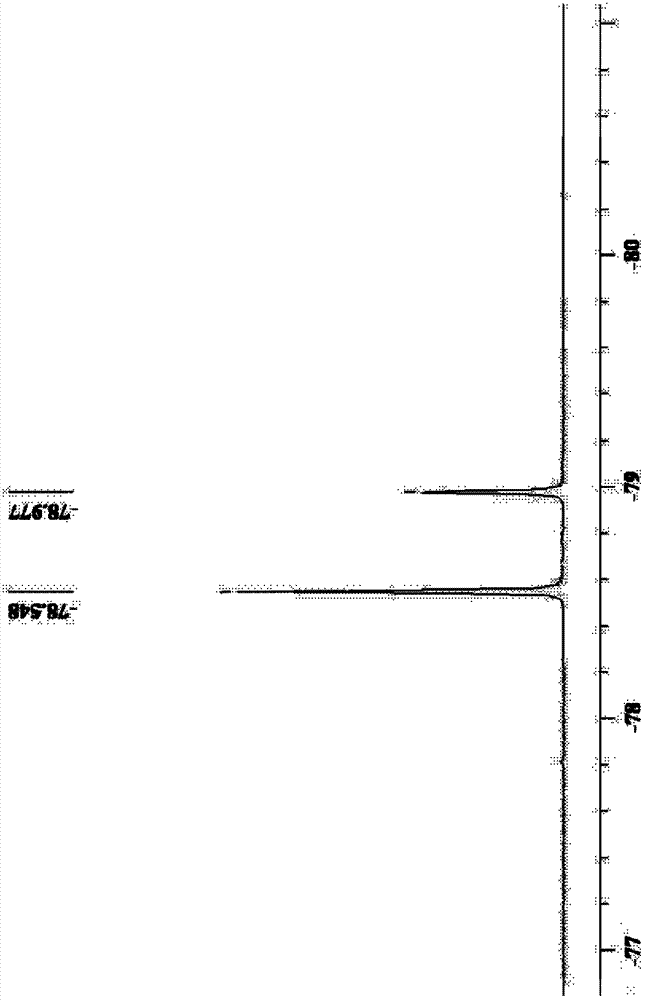
Preparation method of perfluorovinyl ether sulfonyl fluoride
ActiveCN108863854AMild conditions for fluorination reactionEasy post-processingSulfonic acid preparationEtherSulfonyl fluoride
The invention belongs to the technical field of perfluorinated resin, and particularly relates to a preparation method of Perfluorovinyl ether sulfonyl fluoride. A CF2=CF(OCF2CFX)mO(CF2)nSO2F productis directly generated from perfluorovinyl ether sulfonate CF2=CF(OCF2CFX)mO(CF2)nSO2OM by a one-step reaction under the action of a fluorinating reagent CHClFCF2N(C2H5)2,CF3CHFCF2N(C2H5)2. The preparation method has the advantages of simple process, easiness in operation, low reagent cost, avoidance of the production of waste acid gas and waste liquid as well as the increase of multi-step wastageand by-products; reaction steps are reduced, and the use of high-toxicity and high-risk reagents is avoided at the same time, and the yield can be up to 80 percent or more.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Preparation method for surfactant N-carboxyethyl, N-3-dimethylaminopropyl-perfluoro hexyl sulfonamide
ActiveCN105017097AReduce volatilityReduce self-polymerizationTransportation and packagingMixingPropylamineSolvent
The invention discloses a preparation method for a surfactant N-carboxyethyl, N-3-dimethylaminopropyl-perfluoro hexyl sulfonamide, wherein the surfactant N-carboxyethyl, N-3-dimethylaminopropyl-perfluoro hexyl sulfonamide is prepared by carrying out a two-step reaction on perfluoro hexyl sulfonyl fluoride and dimethylamino propylamine. According to the method, the reaction is carried out at a relatively low temperature, thereby avoiding byproduct increase caused by a high temperature reaction; a solvent is added into a second step of the reaction, thereby reducing polymerization reaction of acrylic acid. The fluorine-containing amphoteric surfactant product prepared by the invention is high in purity, and the surface tension (has extremely low surface tension) of an aqueous solution under extremely low addition concentration can be reduced; moreover, the product does not have poisonous and harmful effects on human body and the environment, and is expected to replace perfluorooctylsulfonyl (PFOS) surfactants.
Owner:应城市武化研化工新材料有限责任公司
Alkali metal salt of (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine and ionic liquids
ActiveCN104151206ANo pollution in the processReduce usageSecondary cellsSulfuric acid amide preparationSulfoniumSulfonyl fluoride
The invention provides a method for preparing an alkali metal salt of (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine, and preparing corresponding ionic liquids through a replacement reaction of the alkali metal salt and an ammonium salt, a phosphor salt, a sulfonium salt and the like. The invention provides an electrolyte material of a secondary lithium battery or carbon-based super capacitor based on an ionic liquid of an asymmetric (sulfonyl fluoride)( multi-fluorine alkoxy sulfonyl) imine negative ion. The electrolyte material has good compatibility with electrode materials of LiCoO2, LiFePO4, Li, graphite, Li4Ti5O12 and active carbon and the like.
Owner:武汉市瑞华新能源科技有限公司
Lithium ion battery electrolyte additive cyclic ethylene carbonate sulfate and preparation method thereof
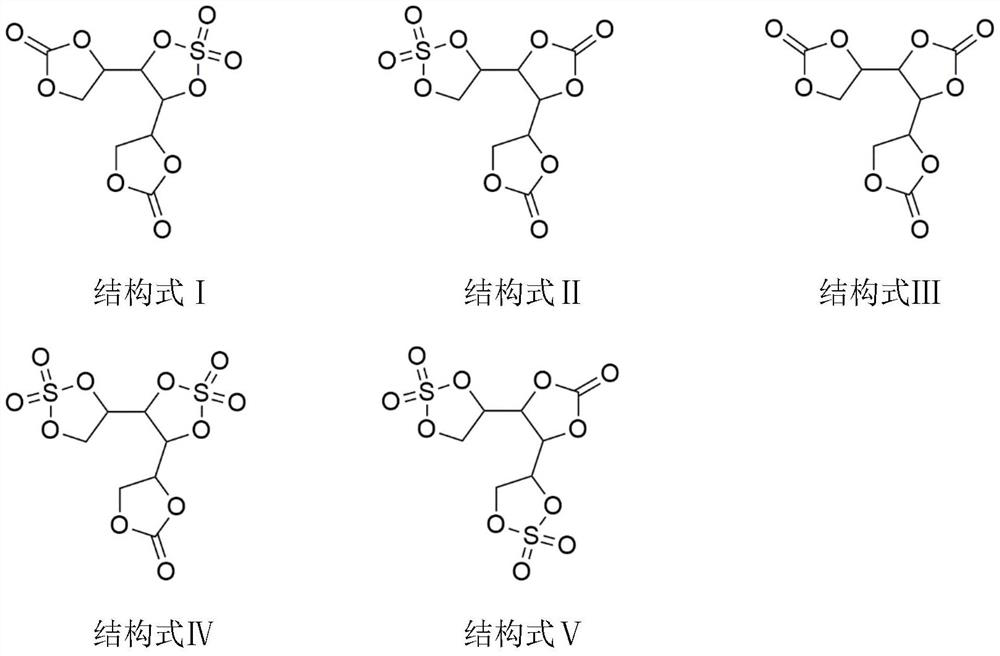
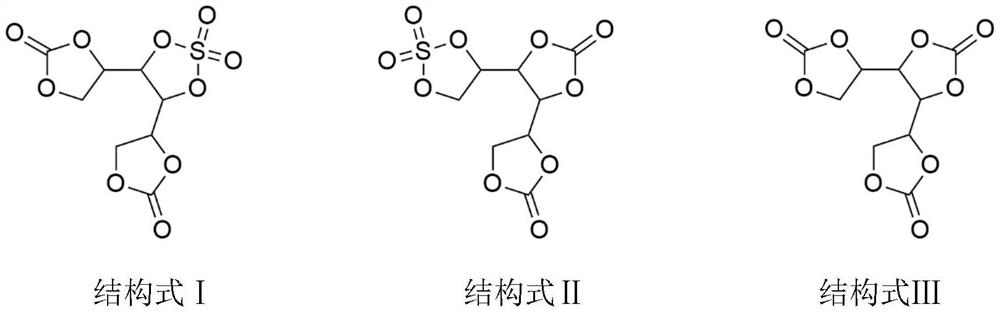
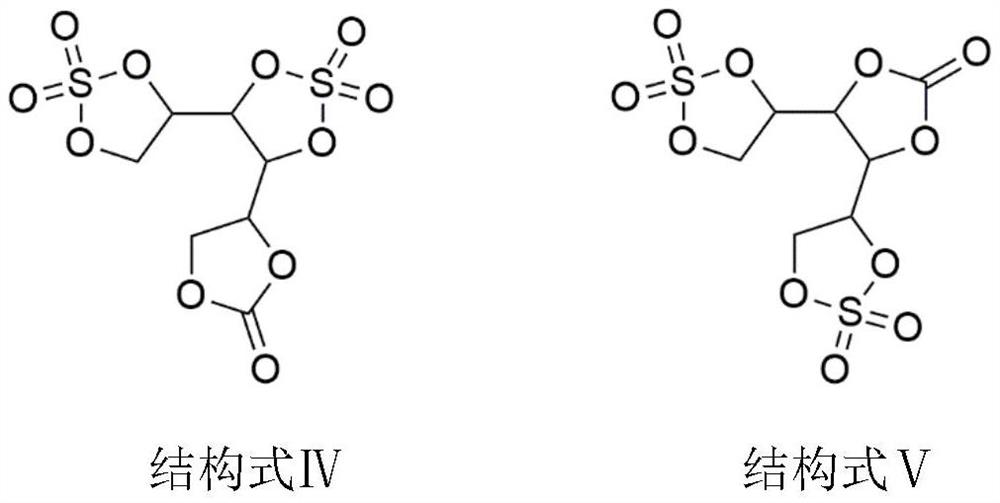
ActiveCN111755753ALow costRaw materials are easy to getOrganic chemistrySecondary cellsSulfonyl chlorideElectrolytic agent
The invention discloses a lithium ion battery electrolyte additive cyclic ethylene carbonate sulfate and a preparation method thereof. The lithium ion battery electrolyte additive cyclic ethylene carbonate sulfate comprises compounds as shown in structural formulas I-V. The preparation method comprises the steps of putting hexahydric alcohol and carbonic ester into a reaction container, then adding organic base or inorganic base as a catalyst, heating to carry out transesterification, and separating by-products under reduced pressure; and adding sulfonyl chloride or sulfonyl fluoride to reactto remove residual hydroxyl, and separating by-products and redundant raw materials under reduced pressure to prepare the cyclic ethylene carbonate sulfate. According to the method, polyol and carbonic ester are subjected to transesterification to prepare cyclic ethylene carbonate, then sulfonyl chloride and sulfonyl fluoride react to remove residual hydroxyl, cyclic ethylene carbonate sulfate isprepared, and the compound structure contains an ethylene sulfate structure and can replace part of DTD to reduce the electrolyte cost.
Owner:香河昆仑新能源材料股份有限公司
Method for preparing bi-(sulfonyl fluoride) imine and (fluorinated alkyl sulfonyl fluorine sulfonyl) imine alkali metal salt
The invention discloses a method for preparing bi-(sulfonyl fluoride) imine and (fluorinated alkyl sulfonyl fluorine sulfonyl) imine alkali metal salt. The method comprises the following steps of: reacting sulfamide and thionyl chloride and chlorosulfonic acid to prepare bi-(sulfonyl chlorine) imine and (fluorinated alkyl sulfonyl chlorine sulfonyl) imine, reacting with antimony trifluoride and potassium carbonate (rubidium or caesium) to obtain corresponding high-purity bi-(sulfonyl fluoride) imine potassium (rubidium or caesium) salt or (fluorinated alkyl sulfonyl fluorine sulfonyl) imine potassium (rubidium or caesium) salt, performing double decomposition exchange reaction using the potassium (rubidium or caesium) salt with the lithium perchlorate (or sodium) or lithium tetrafluoroborate (or sodium) in the aprotic polar solvent to obtain corresponding lithium (or sodium) salt with high purity. The method in the invention has the characteristics of simple operating steps, easy separation and extraction of output, high purity and yield, no environmental pollution, and the like, and is suitable for mass industrial production.
Owner:武汉市瑞华新能源科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com