Preparation method of difluoro-sulfimide and lithium difluoro-sulfimide
A technology of lithium bisfluorosulfonimide and bisfluorosulfonimide, which is applied in the field of fluorine chemical synthesis, can solve the problems of long production process, difficulty in ensuring purity, and difficulty in controlling the reaction process, so as to meet the requirements of yield and quality. The needs of the production equipment, the residual impurity content is easy to control, and the effect of reducing the requirements of the production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
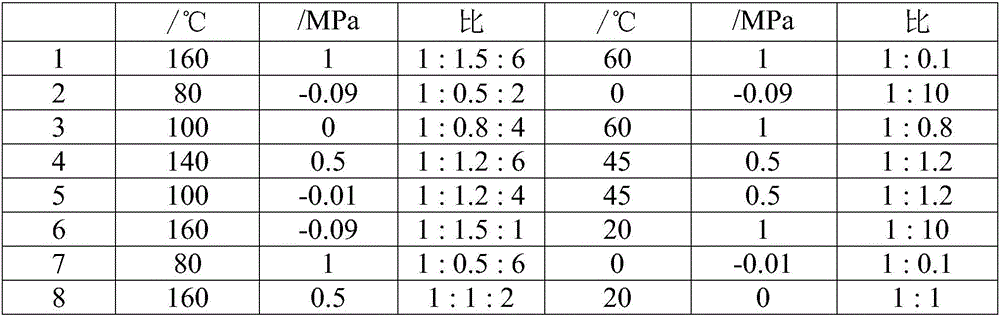
[0052] (1) Under stirring, add 97.1g of sulfamic acid (1mol) and fluorosulfonic acid into the dry 1L reactor, and flow 1.5m into the reactor 3 h -1 Slow introduction of sulfoxide fluoride SOF 2 Reaction, wherein, sulfamic acid: fluorosulfonic acid: SOF 2 The molar ratio is 1:1.5:6, the reaction temperature is 160°C, and the reaction pressure is 1MPa. After the introduction is completed, the reaction is continued for 18 hours to obtain the product, which is detected as bisfluorosulfonimide;
[0053] (2) Recrystallize and filter the bisfluorosulfonimide prepared in step (1) in the solvent diethyl carbonate at -100°C to obtain purified bisfluorosulfonimide with a yield of 90% , with a purity of 95%;
[0054] (3) Add the solvent nitromethane, and slowly add 31.2g of lithium-containing substance lithium fluoride (1.2mol) into the 1L reactor containing the bisfluorosulfonimide prepared in step (2) under stirring. The molar ratio of sulfonylimide and lithium-containing substance ...
Embodiment 2
[0057] (1) The raw materials are sulfamoyl fluoride, fluorosulfonic acid and SOClF, the molar ratio of sulfamoyl fluoride: fluorosulfonic acid: SOClF is 1:0.5:2, the reaction temperature is 80°C, the reaction pressure is -0.09MPa, and the rest With embodiment 1 step (1);
[0058] (2) Distilling the bisfluorosulfonimide prepared in step (1) under reduced pressure at 169° C. to obtain purified bisfluorosulfonimide with a yield of 90% and a purity of 92%;
[0059] (3) Add solvent dimethyl carbonate, the molar ratio of bisfluorosulfonimide and lithium-containing substance lithium oxide is 1:10, the reaction temperature is 0°C, and the reaction pressure is -0.09MPa, and the rest are the same as in Example 1 ( 3);
[0060] (4) adding solvent to the reaction product prepared in step (3) is tetrahydrofuran, adding 200 g of solvent tetrahydrofuran and recrystallizing at normal temperature for 2 hours, the yield is 93%, and the purity is more than or equal to 99.5%. Ion content<0.4ppm...
Embodiment 3
[0062] (1) Raw material sulfamic acid: fluorosulfonic acid: SOF 2 The mol ratio is 1:0.8:4, and the temperature of reaction is 100 DEG C, and the reaction pressure is 0MPa, and all the other are with embodiment 1 step (1);
[0063] (2) The bisfluorosulfonimide prepared in step (1) was distilled under reduced pressure at 60°C to obtain purified bisfluorosulfonimide with a yield of 91% and a purity of 93%;
[0064] (3) adding solvent isopentane, the ratio of bisfluorosulfonimide and lithium-containing substance lithium sulfide is 1:0.8, and the rest are the same as step (3) of Example 1;
[0065] (4) add solvent to the reaction product that step (3) makes is acetonitrile, add solvent acetonitrile 200g recrystallization 2h at normal temperature, productive rate is 94%, purity >=99.5%, wherein, chloride ion content<0.1ppm, fluorine Ion content<0.4ppm, water content<10ppm, sodium ion content<1ppm, potassium ion content<1ppm, all the other are the same as step (4) of embodiment 1. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com