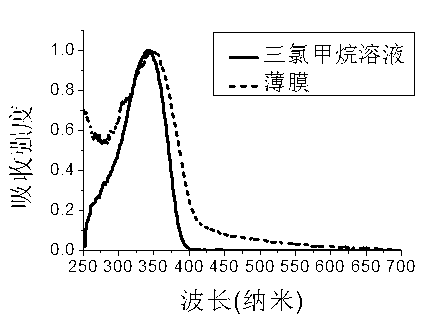
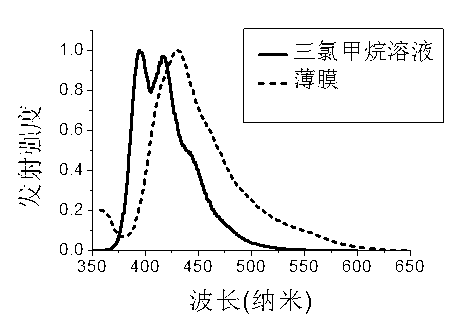
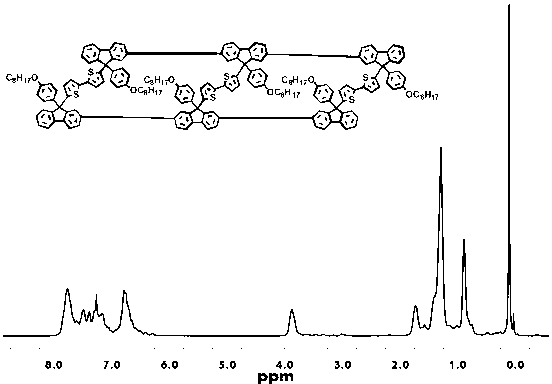
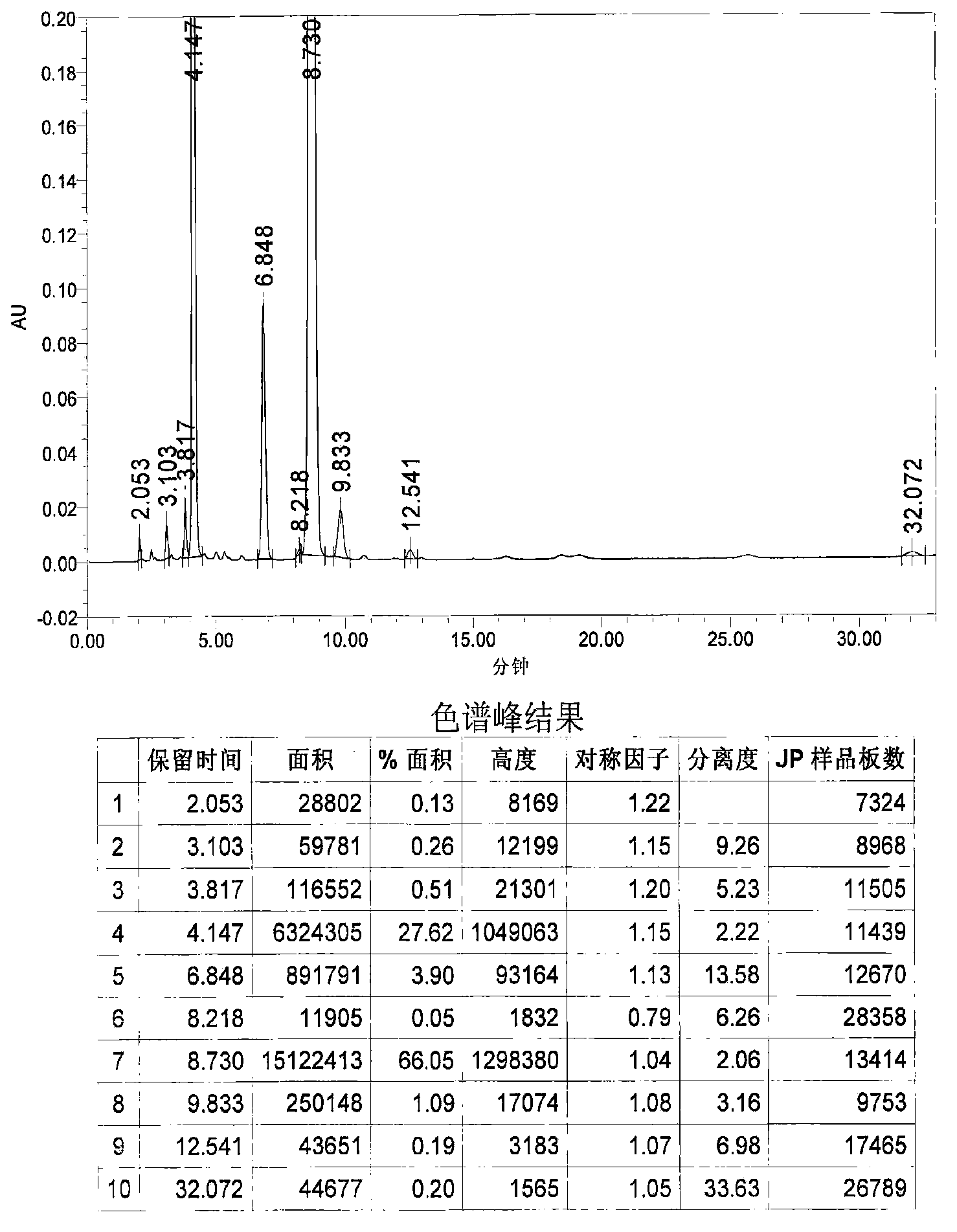
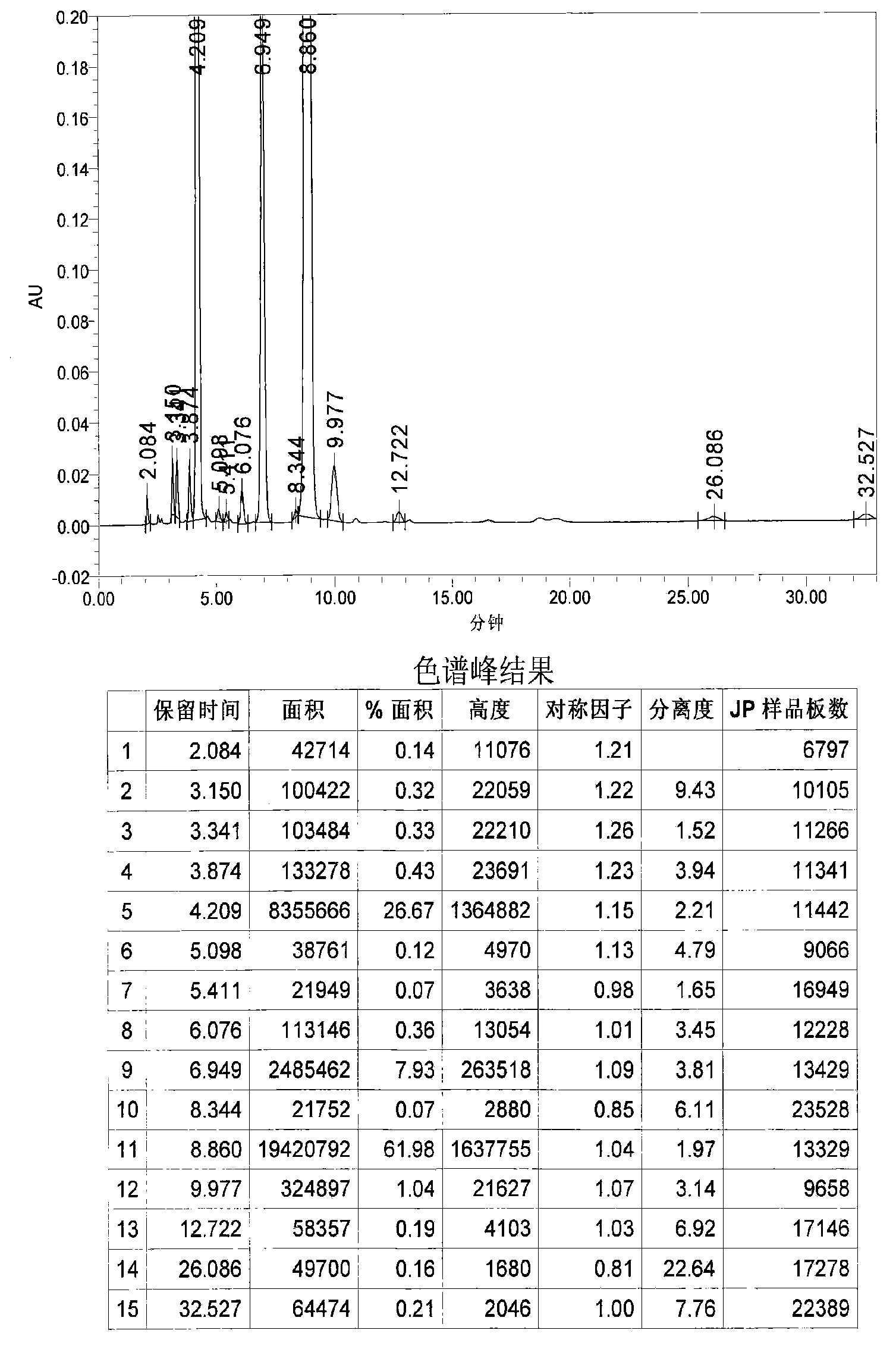
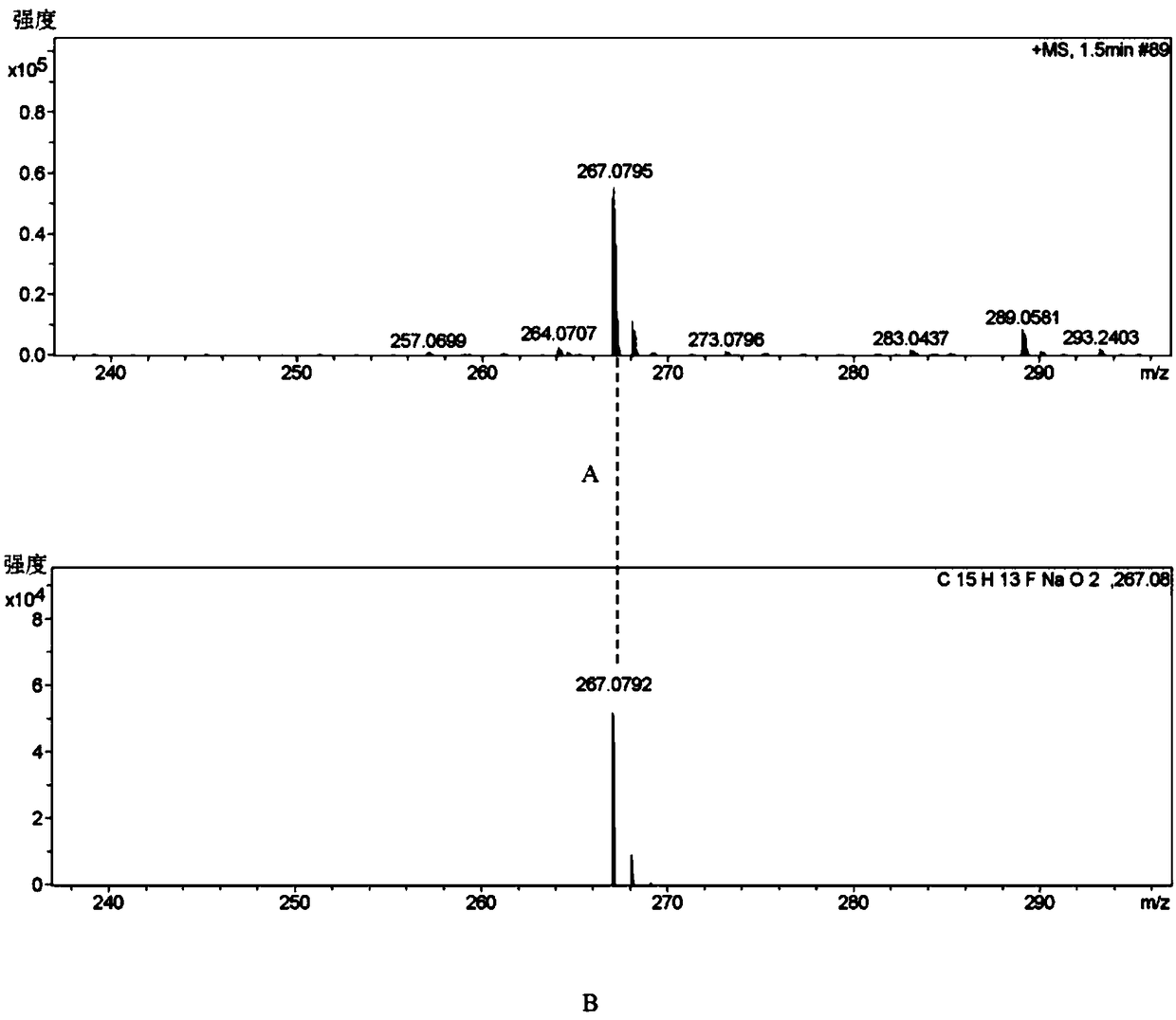
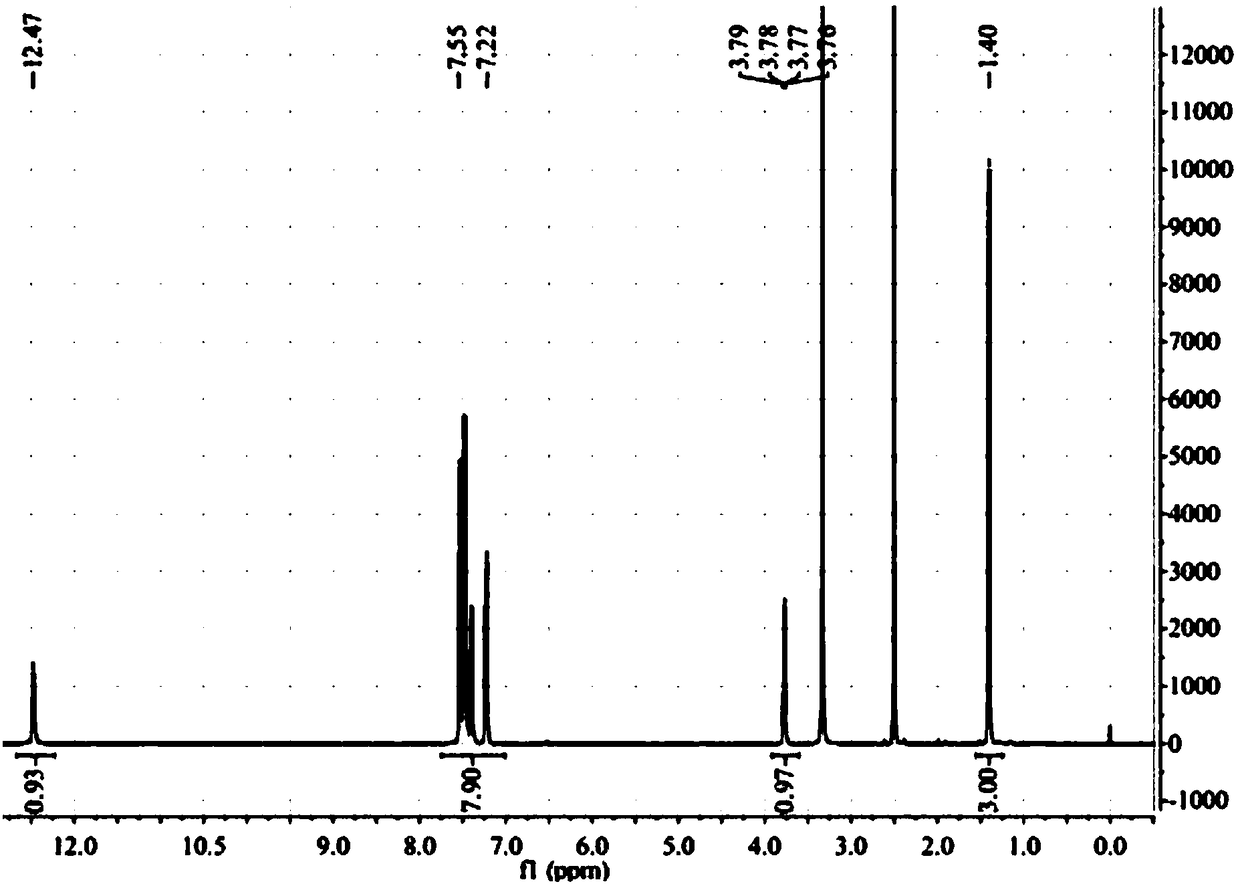
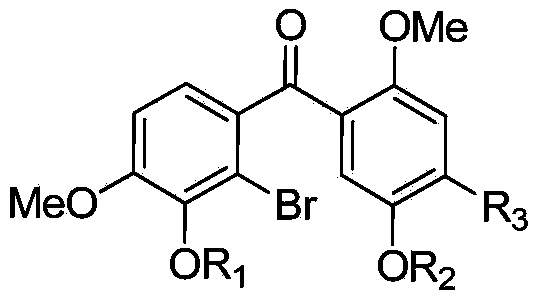
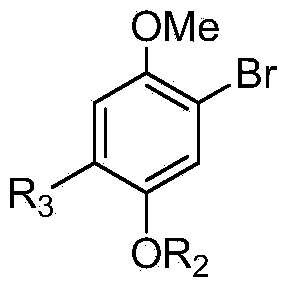
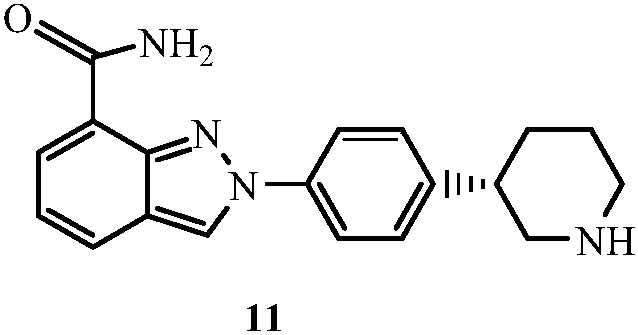
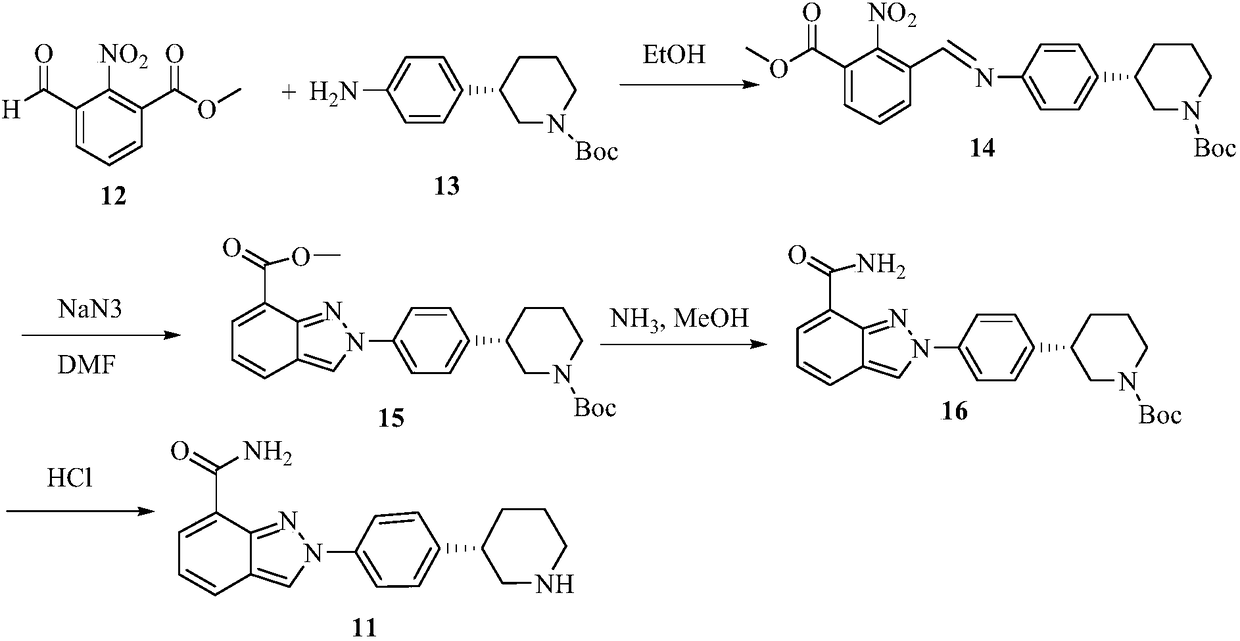
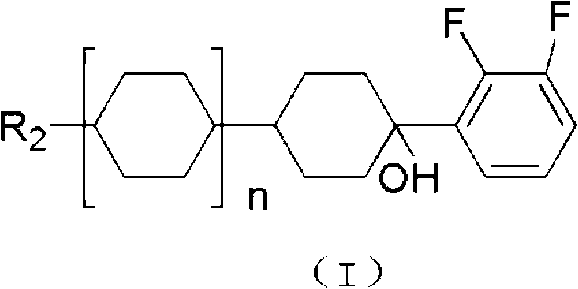
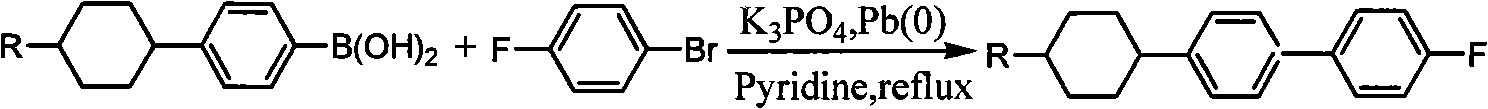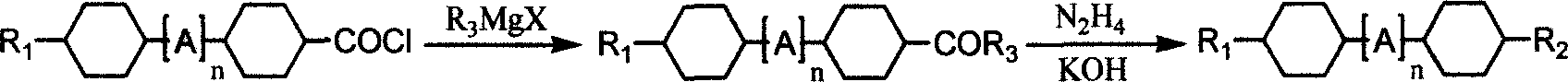Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
723 results about "Grignard reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
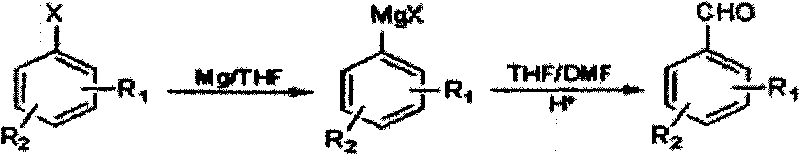
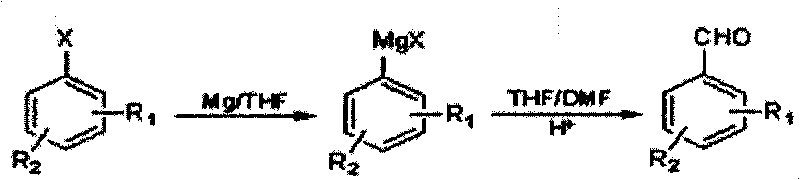
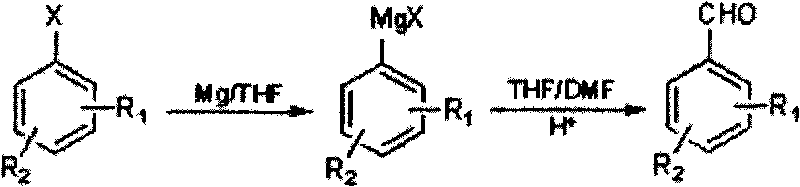
The Grignard reaction (pronounced /ɡriɲar/) is an organometallic chemical reaction in which alkyl, vinyl, or aryl-magnesium halides (Grignard reagent) add to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is not a Grignard reaction, but provides a Grignard reagent.
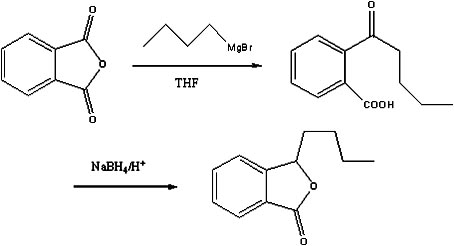
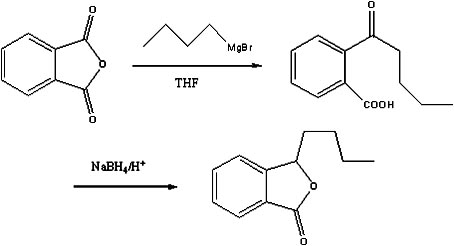
Preparation method of butylphthalide
InactiveCN101962374AReaction raw materials are readily availableFew reaction stepsOrganic chemistryBenzoic acidGrignard reagent
The invention discloses a preparation method of butylphthalide, which comprises the following steps: taking phthalic anhydride as a raw material; enabling the phthalic anhydride to carry out addition reaction with the Grignard reagent of butyl halide to obtain an intermediate of o-valeryl benzoic acid; and then, reducing by sodium borohydride, and carrying out acidic cyclization to obtain the butylphthalide. The phthalic anhydride and the butyl halide which are used as raw materials in the preparation method of the butylphthalide of the invention are commercial products, and the reaction raw materials can be obtained easily. Because the Grignard reaction, the sodium borohydride reduction and the acidic cyclization are classical reactions, the operation is simple, the industrialized production can be realized easily, the yield of the butylphthalide reaches 50-60%, and the purity of the butylphthalide reaches 97-98%.
Owner:SHANGHAI INST OF TECH
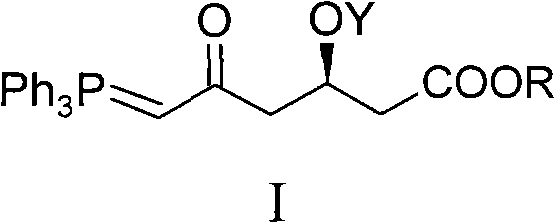
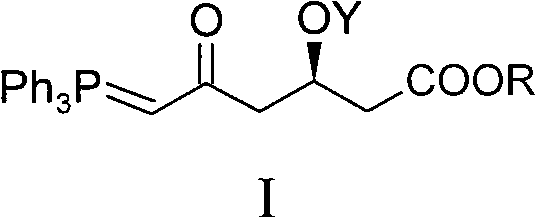
Method for preparing rosuvastatin calcium midbody
ActiveCN101735272AHigh yieldReduce pollutionGroup 5/15 element organic compoundsBulk chemical productionWittig reactionGrignard reaction
The invention discloses a method for preparing a rosuvastatin calcium midbody, namely a compound (R is C1-C10 alkyl, and Y is a hydroxyl protecting group) shown as the general formula I. Chloroethylene and R-epoxy chloropropane as initial raw materials are carried out seven steps of reaction, such as Grignard reaction, sodium cyanide nucleophilic substitution reaction, alcoholysis reaction, hydroxyl protection, oxidizing reaction, methylchloroformate acylation reaction and Wittig reaction to prepare the compound shown as the general formula I. The method has mild condition, simple and convenient operation, stable process, low cost and easy acquisition of raw materials, high product yield, easy disposal of the three wastes, less environmental pollution, low preparation cost and suitability for industrialized large-scale production.
Owner:JIANGXI DONGBANG PHARMA
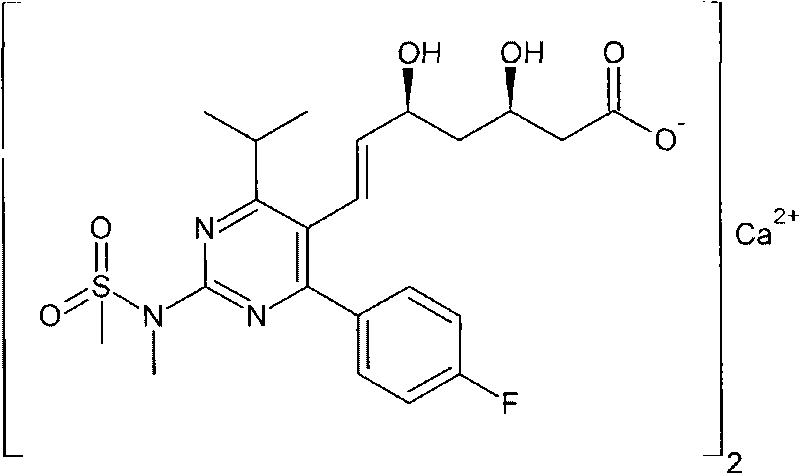
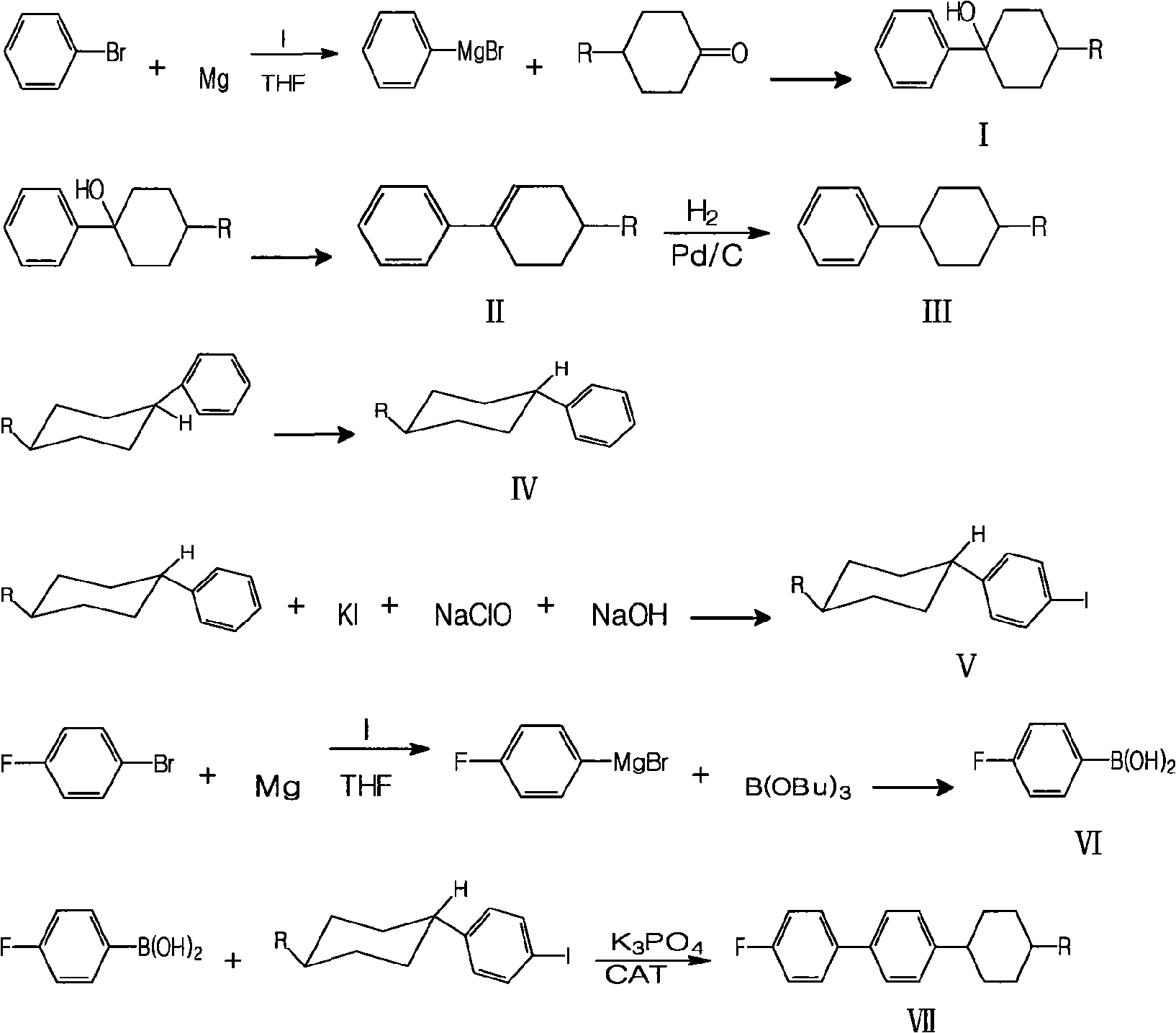
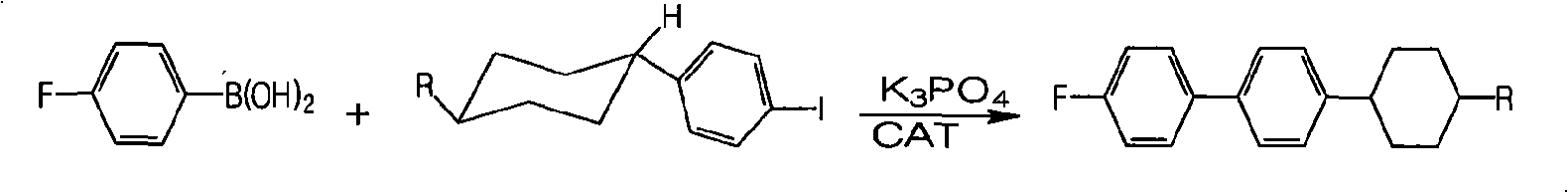
Method for synthesizing fluorine-containing antiform alkyl cyclohexyl biphenyl single liquid crystal
ActiveCN101560396AReduce usageHigh yieldLiquid crystal compositionsHalogenated hydrocarbon preparationCyclohexanoneIodide
The invention discloses a method for synthesizing fluorine-containing antiform alkyl cyclohexyl biphenyl single liquid crystal; in the method, bromobenzene and magnesium are selected to carry out Grignard reaction; then the reactants are coupled and hydrolyzed with alkyl cyclohexanone; dehydration, hydrogenation, transformation and iodization are carried out to synthesize alkyl cyclohexyl iodide; the Grignard reaction is carried out on fluorobromobenzene and magnesium, then low temperature coupling is carried out to synthesize p-fluoropheyl boric acid; finally, coupling reaction is carried out to synthesize the target compound; the method of the invention has the characteristics of use of highly active load catalyst, high yield, low production cost, little discharge of three wastes, reduction of generation of isomers and improvement of selectivity and yield of the target compound.
Owner:山东盛华新材料科技股份有限公司
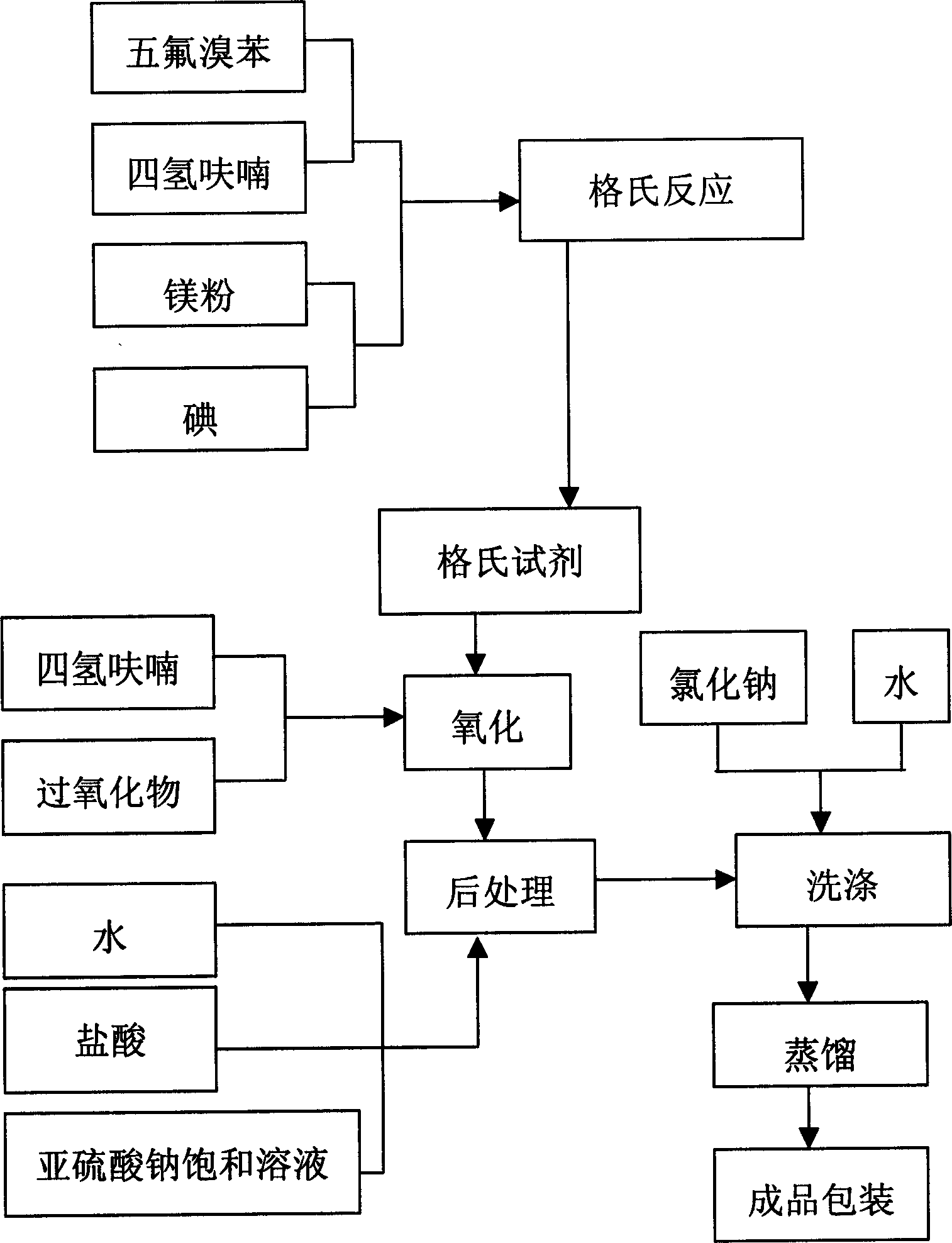
Process of producing pentafluorophenol
ActiveCN1847210ASimple production processHigh purityOrganic chemistryOrganic compound preparationGrignard reagentGrignard reaction
The present invention provides process of preparing pentafluoropheol. Pentafluoro bromobenzene as initial material is first made to produce Grignard reaction to result in Grignard reagent, and the Grignard reagent is then oxidized with peroxide to prepare pentafluoropheol. The process of the present invention has simple technological process, no need of special apparatus, high product yield, high product purity and low production cost.
Owner:内蒙古永太化学有限公司
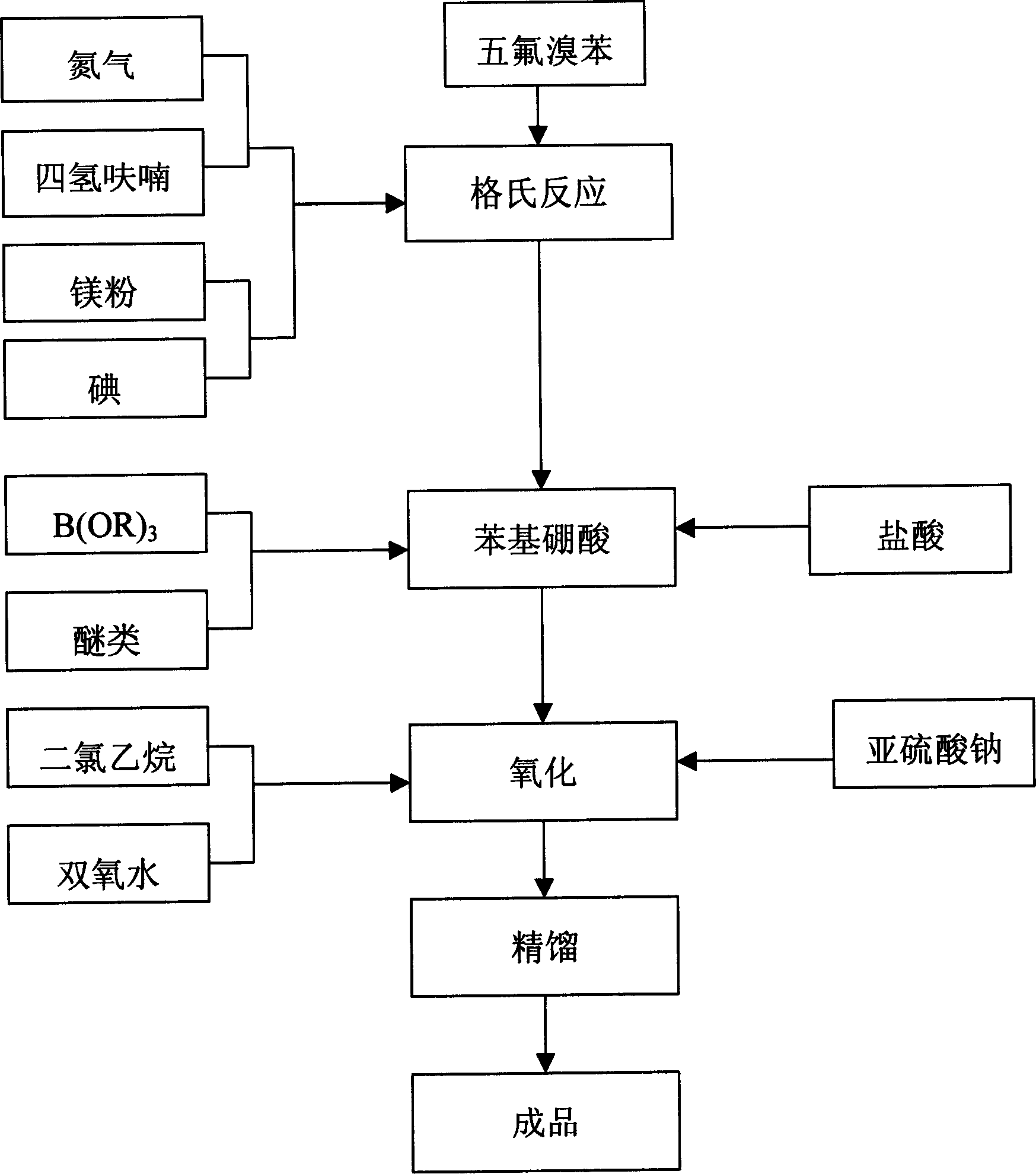
Preparation method of nicotinamide fungicide namely boscalid
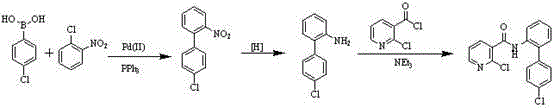
The invention discloses a preparation method of a nicotinamide fungicide namely boscalid. The preparation method comprises the following steps: starting from a raw material namely para chlorobromobenzene, performing Grignard reaction, and synthesizing p-chlorobenzeneboronic acid; starting from ortho-nitroaniline, synthesizing o-nitro halogenated benzene, or directly synthesizing o-amino halogenated benzene; and under the action of specific catalysts, enabling p-chlorophenylboronic acid to perform a series of reactions such as coupling, reduction, amidation and the like with o-nitro halogenated benzene or o-amino halogenated benzene to prepare boscalid. The preparation method disclosed by the invention is simple to operate, high in yield, small in pollution, low in cost and high in purity, and is very suitable for industrial production.
Owner:SUZHOU TOKIND CHEM CO LTD
Method for synthesizing 2-thiophene ethylamine
ActiveCN101885720AReduce manufacturing costMild reaction conditionsOrganic chemistryTerthiopheneEthylene oxide
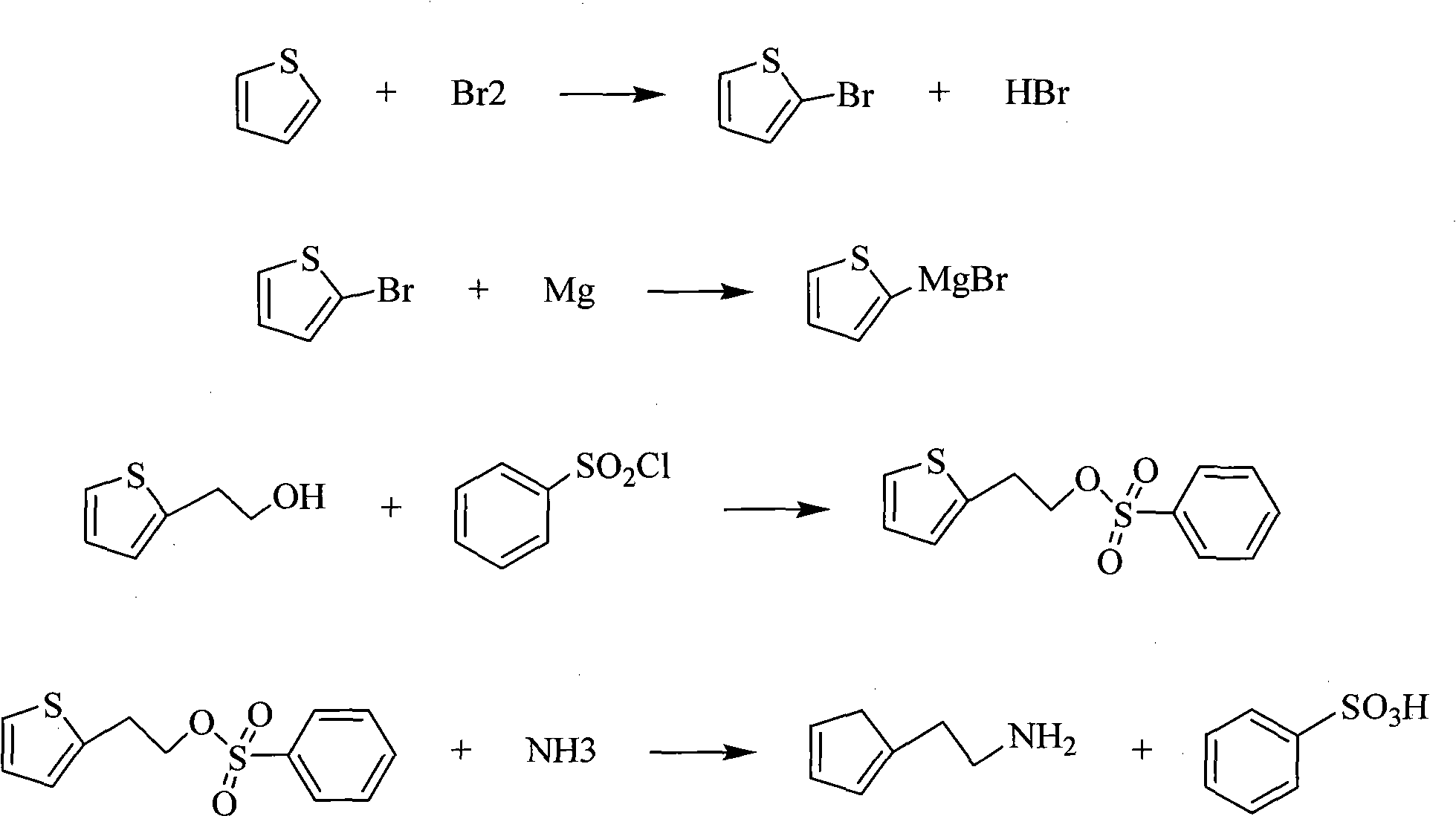
The invention relates to a method for synthesizing 2-thiophene ethylamine, which is characterized in that thiophene is taken as a raw material, intermediate 2-thiophene ethanol is firstly prepared, and then the 2-thiophene ethylamine is obtained through pressurization and ammonolysis. The method mainly comprises the following steps: bromizing thiofuran at low temperature to obtain 2-bromo thiophene; carrying out Grignard reaction on the 2-bromo thiophene and magnesiumchips and reacting with ethylene oxide to generate the 2-thiophene ethanol; and preparing 2-thiophene ethylamine from the 2-thiophene ethanol through the two steps of esterification and ammonolysis. The invention takes cheap and easily obtained thiophene as the raw material which is subjected to the fives reactions of bromination, Grignard, addition, esterification and ammonolysis, greatly reduces the production cost, has mild reaction condition, simple process and small pollution, facilities the realization of the industrialized production, does not use the reducing agent and avoids the utilization of expensive or toxic materials.
Owner:LIANYUNGANG DIPU CHEM
Synthesis method of 17alpha-hydroxyl progesterone
The invention discloses a synthesis method of 17alpha-hydroxyl progesterone, which comprises the steps of by taking 4-androstene-diketone as a starting raw material, carrying out vyanation via acetone cyanohydrin, protecting 3carbonyl by using triethyl orthoformate and ethyl alcohol, protecting 17hydroxy by using butyl vinyl ether, and carrying out hydrolysis after Grignard reaction to generate the 17alpha-hydroxyl progesterone. According to the synthesis method, the cost is reduced, the environment pollution is decreased, the reaction time is shortened, the aftertreatment process of the industrial production is simplified, the production time and cost are greatly saved, the productivity is improved and convenience is brought to the industrial implementation. Compared with the traditional process, the synthesis method has the characteristics of low raw material cost, simple and convenient method, high yield, good selectivity, mild reaction condition, small pollution and applicability to industrial production; and the method is stable and easy to realize.
Owner:ZHEJIANG PURUI PHARMA
Preparation method of flurbiprofen axetil
ActiveCN103012144AConvenient sourceMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationPropanoic acidDistillation
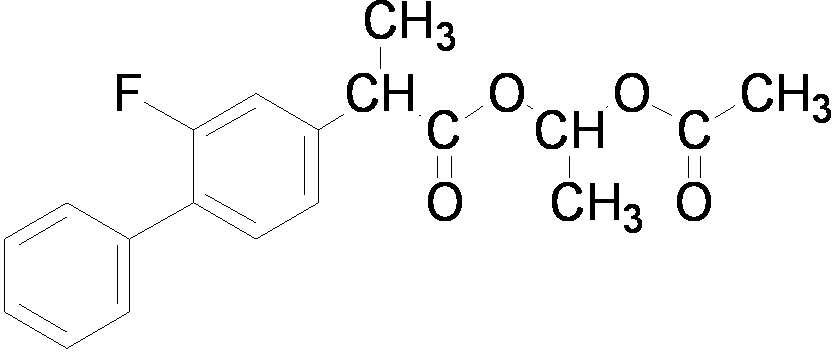
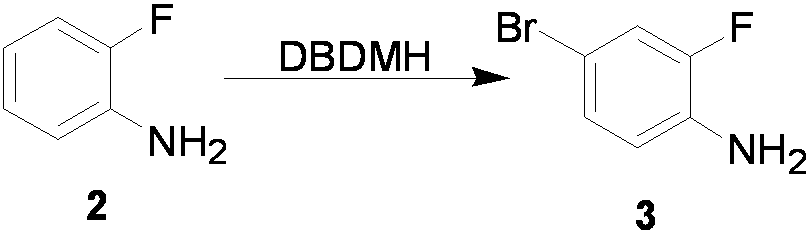
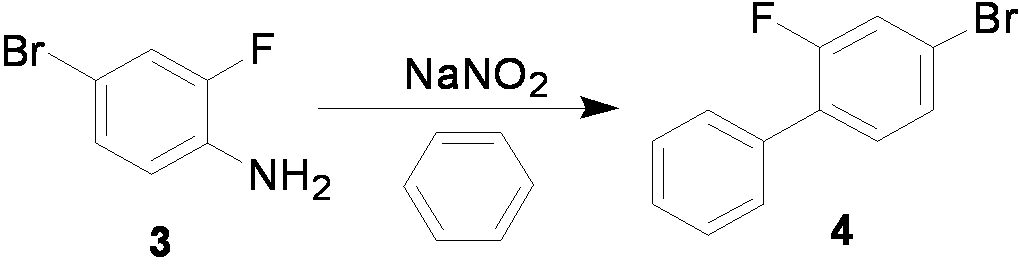
The invention relates to a preparation method of flurbiprofen axetil. The method comprises the following steps of: preparing 4-bromine-2-fluoroanili from fluoroaniline under the action of 1,3- dibromo-5,5-dimethyl hydantoin; condensing 4-bromine-2-fluoroanili with benzene under the action of a catalyst and sodium nitrite to synthesize 4-bromine-2-fluorobiphenyl; carrying out grignard reaction and acidity reaction on the 4-bromine-2-fluorobiphenyl and 2-bromine sodium propionate under the action of a catalyst to generate 2-(2- fluorine-4-biphenylyl) propionic acid; condensing the 2-(2- fluorine-4-biphenylyl) propionic acid with 1-chloroacetic ethyl acetate to generate a target compound flurbiprofen axetil; and carrying out molecular distillation on the flurbiprofen axetil crude product to obtain a flurbiprofen axetil final product.
Owner:哈药集团股份有限公司 +1
Method for preparing intermediate of Entecavir antiviral agent
InactiveCN101906113ASimple reaction conditionsRaw materials are cheap and easy to getGroup 4/14 element organic compoundsEntecavirSolvent
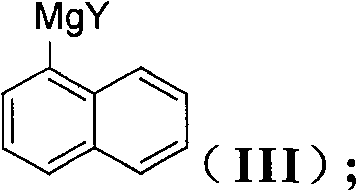
The invention discloses a method for preparing an intermediate (I) of an Entecavir antiviral agent, which comprises the following steps: (1) reacting a compound of a formula (II) with magnesium under a condition of a proper solvent to generate a compound of a formula (III); and performing the Grignard reaction of the compound of the formula (III) and a compound of a formula (IV). In the formulae, X and Y are identical or different and may be F, Cl and Br atoms. The method has the advantages of simple reaction conditions, cheap and readily available raw materials, simple and safe process, low cost and easy industrial production.
Owner:HANDE PHARMA
Fluorenyl organic framework material, preparation and application method thereof
ActiveCN103232473ATest thermal stabilitySave raw materialsOrganic chemistrySolid-state devicesSolubilityOrganic field-effect transistor
The invention relates to a fluorenyl organic framework material, a preparation and application method thereof. A material which is provided with a framework structure shaped as a Chinese character ri and constructed by the method disclosed by the invention is taken as a new generation organic semiconductor to be applied to an organic electronic device. The structure of the material is shown in the specification. The material has the characteristics that (1) monomers are synthesized via a Grignard reaction and a Friedel-Crafts reaction, the raw material is inexpensive and the synthesis method is simple; (2) the material has high heat stability and glass transition temperature; (3) the material is good in flexibility and high in solubility; and (4) due to the accumulation effect of diaryl fluorine, the material is good in electrooptic activity. The fluorenyl organic framework material can be applied to the organic electronic field of film devices such as organic light emitting display, organic light storage, organic photovoltaic cells, organic field effect transistors and organic laser, and the like. Electroluminescent devices prepared by using the material disclosed by the invention show satisfactory results in luminance, luminous efficiency and voltage resistance stability.
Owner:NANJING UNIV OF POSTS & TELECOMM
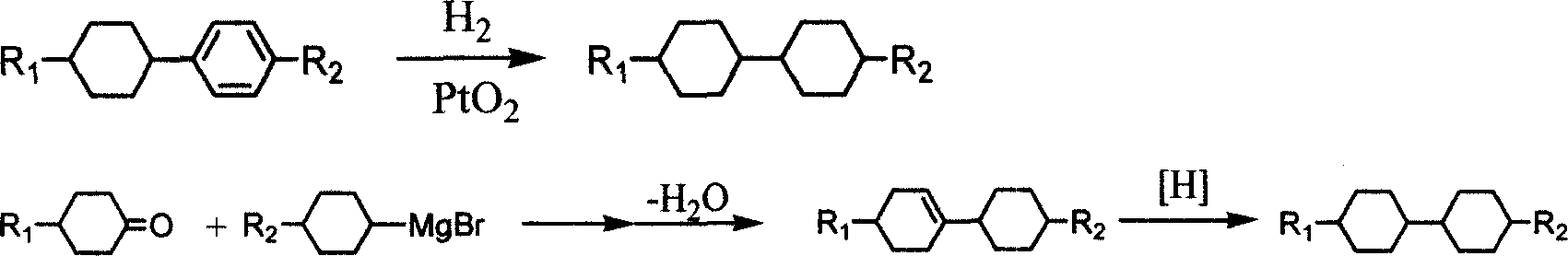
Method for preparing bis cyclohexane monomer liquid crystal using grignard reaction
InactiveCN1962580AAvoid drop in yieldLow costLiquid crystal compositionsHydrocarbon from oxygen organic compoundsGrignard reactionStructural formula
The invention discloses a preparing method of bicyclohexane monomer LCD in the monomer LCD preparing technical domain, which utilizes Grignard reaction to prepare the product with structural formula as graph I and synthetic feature route as graph II, wherein R1 and R2 is C2-C10; R3 is C1-C9 straight-chain alkyl; X is Cl or Br or I; A is C2H4 or cyclohexane; n is 0 or 1.
Owner:VALIANT CO LTD
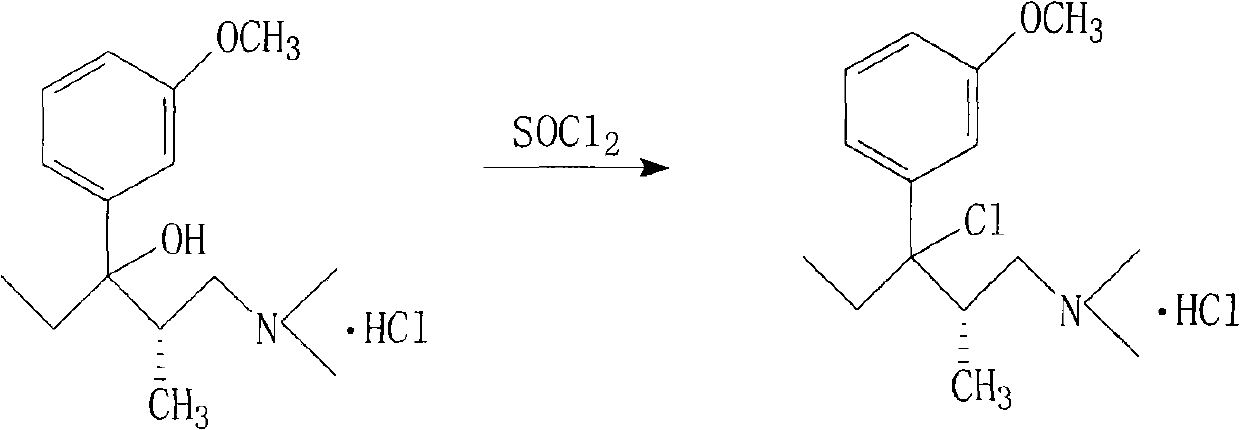
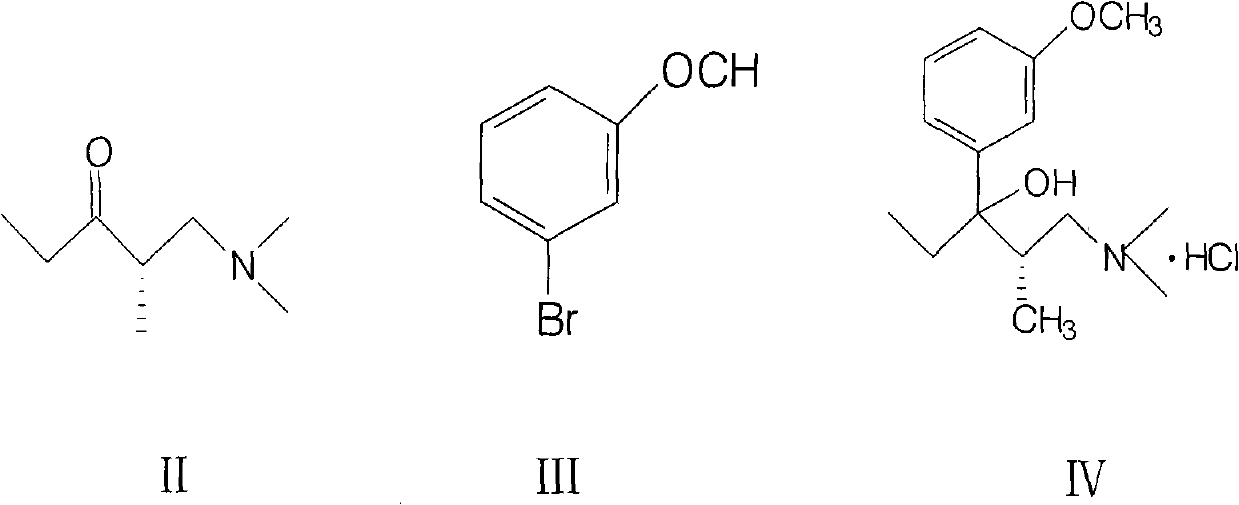
Method for preparing important intermediate of tapentadol hydrochloride analgesic
InactiveCN101948397AReduce manufacturing costSuitable for mass industrial productionOrganic compound preparationAmino-hyroxy compound preparationEnantiomerTapentadol Hydrochloride
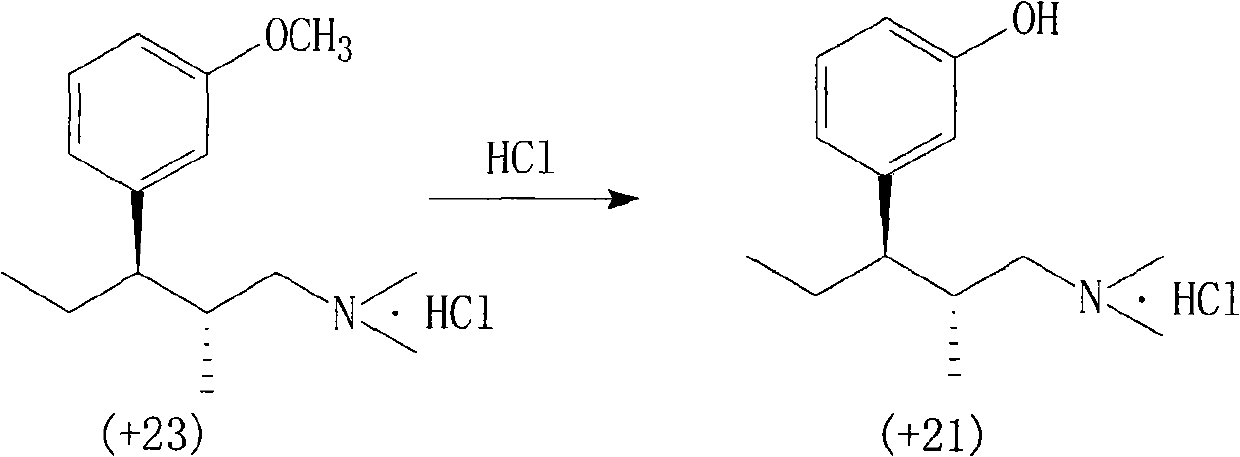
The invention relates to a method for preparing an import intermediate of a tapentadol hydrochloride analgesic, which is (2S)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentyl-3-ol hydrochloride. The (2S)-1-dimethylamino-3-(3-methoxyphenyl)-2-methylpentyl-3-ol hydrochloride with optical activity is obtained by the Grignard reaction of a compound II with optical activity and a compound III with optical activity. The Grignard reaction is performed by mixing magneson solution of the compound III and the tetrahydrofuran solution of the compound II at 5 to 10 DEG C and standing the mixed solution at room temperature overnight. The molar ratio of the compound II to the compound III is 0.8-1:1.0-1.5. The preparation method of the invention is not limited by a chiral column split instrument, solves the problem of processing enantiomers as waste chemicals, reduces production cost and is more suitable for large-batch industrial production.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV
Novel fluorine phenyl-containing silicone oil and method of producing the same
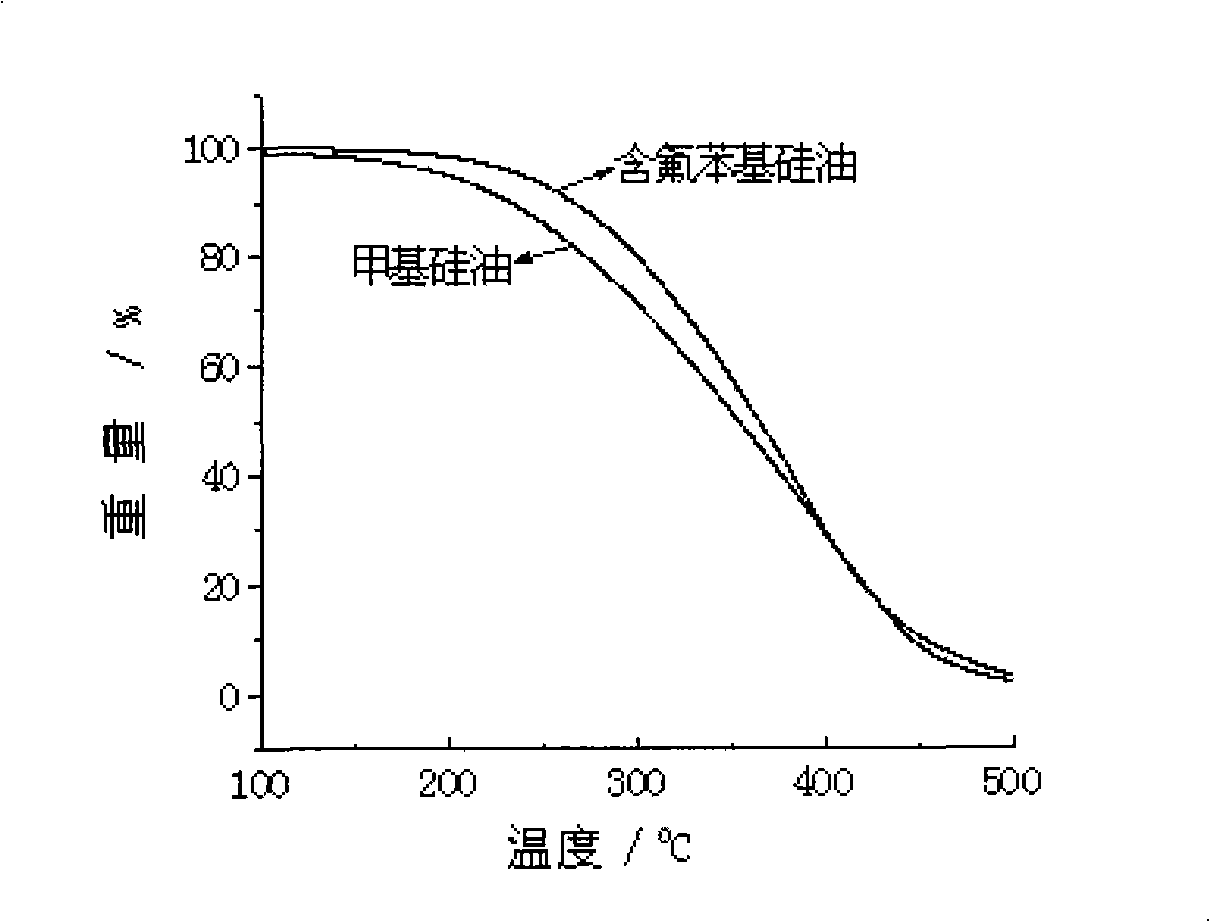
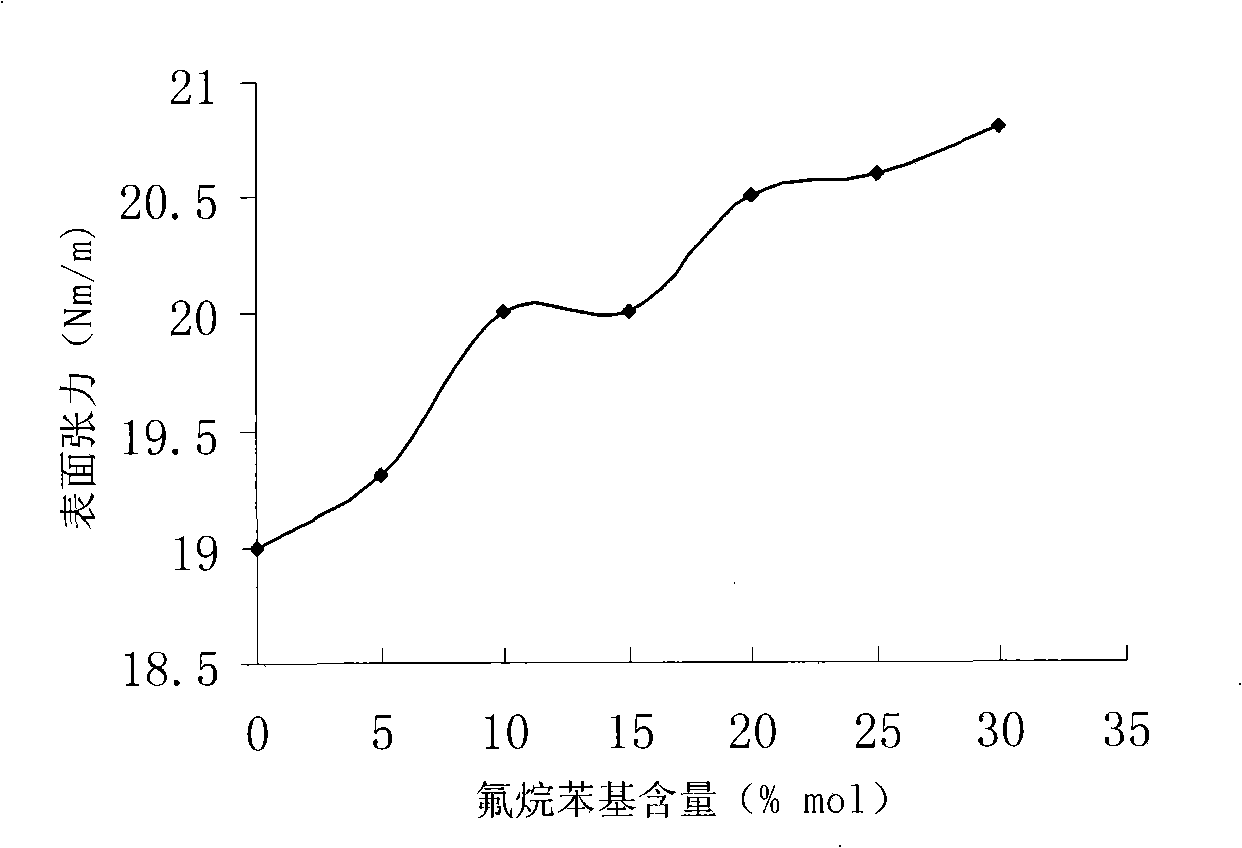
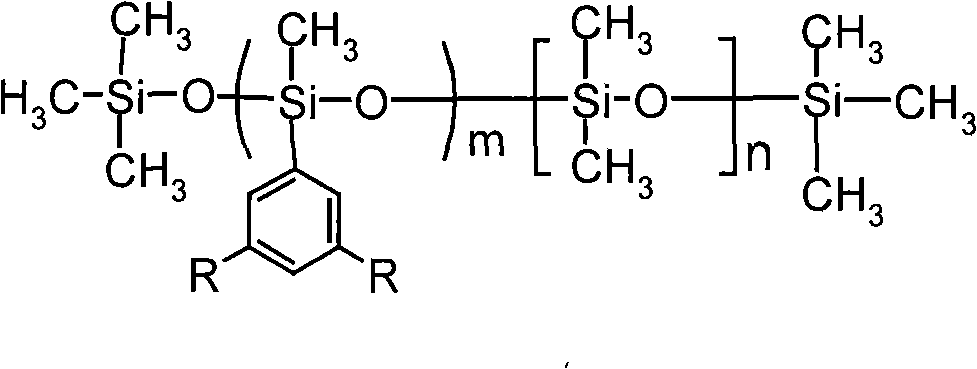
InactiveCN101402733AImprove heat resistanceRaw materials are easy to getHeat-exchange elementsBase-materialsChemical synthesisHeat conducting
The invention discloses novel fluoride phenyl silicone oil and a preparation method thereof, pertaining to the technical field of chemical synthesis and special-typed high molecular material. The silicone oil takes 3, 5-difluoride substituent-1-halogeno benzene and methyl-trimethoxy silicane as raw materials and prepares fluoride organoxilicon monomer 3, 5-difluoride substituent phenyl-methyl-dimethoxy-silicane monomer through grignard reaction and substitution reaction. Hydrolysis and equilibrium polymerization are imposed on the fluoride organoxilicon monomer synthesized and dimethyl-dimethoxy silicane together to prepare 3, 5-di (trifluoromethyl) phenyl silicone oil. The novel fluoride phenyl silicone oil product has good heat resistance, is supposed to be applied as heat conducting oil, hydraulic oil, surface treatment materials and the like, and has advantages of easy access to raw materials, easy realization of preparation technology and facilitation for promotion and application.
Owner:SUZHOU UNIV
Method for preparing halogenated methyl-benzaldehyde by Grignard reaction
ActiveCN101712603ARaw materials are easy to getSimple processOrganic compound preparationCarbonyl compound preparationGrignard reagentBenzaldehyde
The invention relates to a method for preparing halogenated methyl-benzaldehyde by Grignard reaction. The reaction is carried out according to the following reaction formula and the following steps: (1) adding magnesium chips, an organic solvent and an initiating agent into a dry reaction flask with nitrogen protection for stirring to obtain a mixed solution A, then dropwise adding 1 / 10 volume ofmixed solution B of halogenated benzene and the organic solvent into the mixed solution A for initiating the reaction, and dropwise adding residual 9 / 10 volume of the mixed solution B of the halogenated benzene and the organic solvent for reacting for 1h to prepare a Grignard reagent, wherein the reaction temperature is 10-80 DEG C, and iodine particles or 1,2-dibromoethane can be used as the initiating agent; and (2) cooling the Grignard reagent to -40-10 DEG C, dropwise adding a mixed solution of N,N-dimethyl formamide and the organic solvent into the Grignard reagent, then slowly heating the mixed solution to 10-45 DEG C for reacting for 0.5-5h, lowering the temperature below 20 DEG C, regulating the pH value to be less than or equal to 2, extracting with CH2Cl2, washing, removing the solvent, and distilling with water vapor to obtain the product. The invention has the advantages of easy acquisition of raw materials and simple processes, the product purity is greater than or equal to 99%, and the product yield is greater than 50%.
Owner:溧阳常大技术转移中心有限公司
Continuous production method of Grignard reagent
InactiveCN106674257AResolve triggerSolve the cooling problemMagnesium organic compoundsGrignard reagentGrignard reaction
The invention provides a continuous production method of a Grignard reagent, a mode of connecting n kettles in series is adopted, and the Grignard reagent is obtained through continuous Grignard reaction of halide and magnesium, wherein n is greater than or equal to 2; the production method comprises a continuous Grignard starting step and a continuous Grignard reaction operation step. The method effectively solves the problems of triggering and heat dissipating of the Grignard reaction, the potential safety hazards like blanking or explosion caused by the dramatic temperature rise of the Grignard reaction are avoided, meanwhile, the stability of control in the process of the Grignard reaction is improved, and the influences of human factors on the synthesis of the Grignard reagent are reduced.
Owner:江苏创拓新材料有限公司
Preparation method of butoconazole nitrate intermediate suitable for industrial production
ActiveCN103880596AHigh yieldReduce bumpsPreparation by OH and halogen introductionMagnesium organic compoundsGrignard reactionEthyl Chloride
The invention provides a method for industrial production of a butoconazole nitrate intermediate, that is 1-chloro-4-p-chlorophenyl-2-butanol (a compound in formula II). The method of the invention comprises: 1. a Grignard reaction, that is, adopting p-chlorobenzyl chloride as a raw material, and performing a Grignard reaction with magnesium powder in a mixed solvent of methyl tertiary butyl ether and tetrahydrofuran; 2. a condensation reaction, that is, continuing reaction by adding epichlorohydrin to obtain the compound II which is an important intermediate for preparing butoconazole nitrate. According to the method, the raw materials are cheap and easily available, the reaction solvent is safer, and the method is suitable for industrial production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Grignard reaction method in production of maltol
The invention discloses a Graber's reacting method in the maltol manufacturing course, which comprises the following steps: a. extracting the autoclave into vacuum under -0. 04Mpa; b. aerating chloromethane to do Graber's reaction; controlling the pressure below 0. 25Mpa and temperature below 80 deg. c; c. cooling the temperature below 35 deg. c; decompressing to aerate nitrogen to protect; adding the material into another autoclave to drip furfural to do additional reaction; adopting the retained 2%-3% Graber's agent in the Graber's autoclave as the catalyst to lead Graber's reaction. The invention improves the equipment utility and receiving rate under low pressure without heating, which shortens the manufacturing time with high equipment utility.
Owner:ANHUI JINGHE IND
Preparation method of flurbiprofen and preparation method of flurbiprofen axetil
InactiveCN108558651ALow costHigh purityPreparation from carboxylic acid saltsOxygen-containing compound preparationChemical synthesisBromine
The invention relates to the field of pharmaceutical chemical synthesis, in particular to a preparation method of flurbiprofen and a preparation method of flurbiprofen axetil. The preparation method of the flurbiprofen comprises the steps of carrying out a Grignard reaction by using 4-bromine-2-fluorine biphenyl as a raw material, carrying out a coupling reaction, and acidizing to obtain the flurbiprofen; the yield is 90%, and the purity is 99.5%; then, the flurbiprofen axetil is prepared by using the flurbiprofen, obtained by the method, as a raw material, the yield reaches up to 90%, and thepurity reaches up to 99.5%. The preparation methods are high in quality controllability and industrial reproducibility.
Owner:上海峰林生物科技有限公司
Functional diamine monomers having high planarity and containing naphthaline structure and synthesis method and application thereof
ActiveCN104744268AGood planaritySimple processAmino preparation from aminesOrganic compound preparationPolyesterPolymer science
The invention discloses functional diamine monomers having high planarity and containing a naphthaline structure and a synthesis method and application thereof. The novel functional diamine monomers are prepared from raw materials monomers such as dihalogenated naphthaline, naphthalic acid, naphthalenediol or naphthylenediamine through a series of chemical reactions such as substitution reaction, Suzuki reaction, amidation reaction, esterification reaction, Grignard reaction, Kumada coupling reaction. The diamine monomers containing a naphthaline structure, which have a lowest energy state 3D molecular structure and have high planarity, can be obtained. Due to planar space structure, the diamine monomers disclosed by the invention can serve as monomers used for preparing polymers with strong molecular chain interaction force, tight molecular chain packing and small free volume and the polymers can be endowed with an excellent barrier property. The synthesis method of the diamine monomers is simple in process and purification operation is easy; therefore, the synthesis method is suitable for industrial production. The diamine monomers disclosed by the invention can be used for synthesizing functional polymers such as polyamide, polyimide, polyamide-imide and polyester-imide.
Owner:江西有泽新材料科技有限公司
Method for recovering tetrahydrofuran from Grignard reaction waste residue of magnesium chloride
The invention relates to a method for recovering tetrahydrofuran from Grignard reaction waste residue of magnesium chloride. The method comprises the following steps: (1) dissolving and distilling: respectively adding the waste residue of magnesium chloride and water to a reaction kettle; stirring to be fully dissolved; heating so that tetrahydrofuran solvent is dissociated and continuously vaporized; then, condensing into liquid, receiving to obtain a crude product A; (2) drying and dewatering: drying by alkali and a molecular sieve until water content is smaller than 0.1 percent; standing still and precipitating, transferring clear liquid at an upper layer to a filter; filtering to obtain a crude product B; (3) rectifying: transferring the crude product B to a tower kettle of a rectifying tower; heating to vaporize and regurgitate the tetrahydrofuran in the rectifying tower; slowly distilling a front cut fraction after regurgitation is lasted for 1-2 hours until the tetrahydrofuran content is larger than 99.8 percent, and starting to collect a boiling reagent to obtain the rectified tetrahydrofuran. The technical scheme of the invention greatly lowers the unit consumption of thetetrahydrofuran in the Grignard reaction and has simple and convenient treatment method and low cost.
Owner:SHANGHAI YIMIN CHEM
Device for continuous preparation of Grignard reagent and method for continuous preparation of Grignard reagent through using device
ActiveCN102603775ANot prone to valve cloggingLess prone to plumbing problemsMagnesium organic compoundsEnvironmental resistanceVapor–liquid separator
The invention discloses a device for the continuous preparation of a Grignard reagent and a method for the continuous preparation of the Grignard reagent through using the device. The device comprises a solvent storage container, a solvent-raw material mixing container, a raw gas storage container, a preheater, a reactor, a magnesium adding bin, a gas-liquid separator, a Grignard reagent reception container and a tail gas recovery apparatus, wherein the reactor adopts a three-phase bubble slurry column reactor. A purpose of the continuous preparation of the Grignard reagent can be realized through using the device of the invention, and initiation and heat radiation problems of a Grignard reaction can be effectively solved through adopting the three-phase bubble slurry column reactor, so potential safety hazards of material rush-out, explosion and the like caused by the severe temperature rise of the Grignard reaction can be effectively avoided, the obstruction of valves and pipelines by magnesium shreds can be prevented, and the high purity Grignard reagent can be obtained; and the tail gas recovery apparatus is arranged in the invention to absorb and recycle tail gases, so the cost is saved, and the environmental pollution is reduced, thereby cleaning and environmental protection requirements of the preparation of the Grignard reagent are realized.
Owner:SHANGHAI HEGNO PHARMA HLDG +1
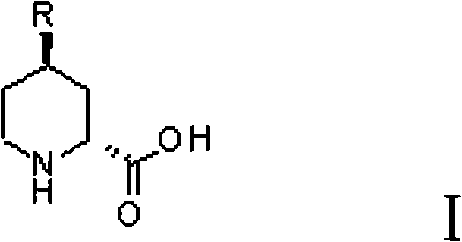
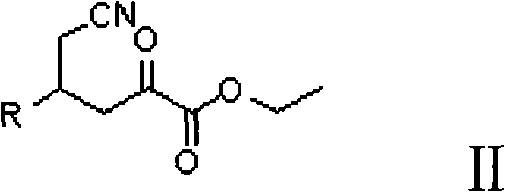
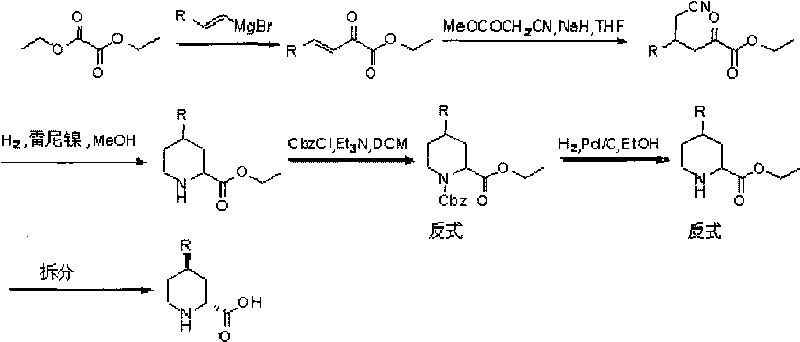
Preparation method for (2R, 4R)-4-substituted-2-piperidine carboxylic acid compound and intermediate thereof
InactiveCN101712645ARaw materials are easy to getSimple processAsymmetric synthesesSynthesis methodsOrganic synthesis
The invention relates to a synthesis method for preparing a (2R,4R)-4-methyl-2 -piperidine carboxylic acid compound taking diethyl oxalate as starting materials and an intermediate thereof, and belongs to the field of organic synthesis. The synthesis method comprises the following steps of: taking the diethyl oxalate and 1-bromo-substituted-propylene as the starting materials, performing a Grignard reaction and an addition reaction on the diethyl oxalate and 1-bromo-3-substituted-propylene to obtain intermediate 2-carbonyl-4-substituted-5 cyan ethyl valerate; and then performing a cyclization reaction, a benzyl ester protection reaction and a deprotection reaction on the intermediate 2-carbonyl-4-substituted-5 cyan ethyl valerate to obtain trans-4-substituted-2-piperidine ethyl formate; and finally, splitting the trans-4-substituted-2-piperidine carboxylic acid ethyl ester to obtain a chiral target product (2R,4R)-4-methyl-2-piperidine formic acid compound. The preparation method has the advantages of readily available raw materials, simple process, and mild reaction condition.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
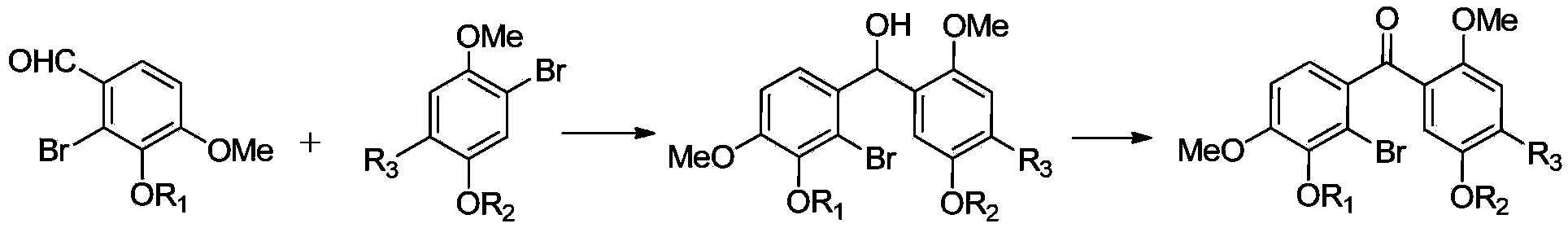
Substituted benzophenone and preparation method thereof
InactiveCN103524320AOvercome the shortcomings that cannot be widely usedEasy to operateGroup 4/14 element organic compoundsOrganic compound preparationDiphenylmethanolPyridinium
The invention discloses substituted benzophenone and a preparation method thereof. The substituted benzophenone has a benzophenone structure, can be used as a hydrogen abstraction type photoinitiator, can be widely applied to ultraviolet light curing and other fields, and can also be used as a key intermediate to synthesize a poly-substituted fluorenone type compound. The preparation method of the substituted benzophenone, provided by the invention, is simple to operate, toxic substances are not used, an intermediate compound B and an intermediate compound A are ingeniously utilized to construct a benzhydrol structure through Grignard reaction, and the compound with the benzophenone structure is further obtained by oxidation with pyridinium chlorodichromate; the defect that synthesis methods of benzophenone type compounds in the prior art can not be widely applied is overcome.
Owner:XI AN JIAOTONG UNIV
Synthesis method for (R)-3-phenylpiperidine or/and (S)-3-phenylpiperidine and synthesis method for chiral intermediate of niraparib
InactiveCN108203404AHigh reaction yieldMild reaction conditionsOrganic chemistry methodsSynthesis methodsOrganic synthesis
The invention belongs to the technical field of organic synthesis. The synthesis method firstly provided by the invention takes benzyl-4-oxopiperidine as a starting material, and the starting materialis subjected to Grignard reaction, elimination reaction, hydrogenation reduction reaction and chiral resolution in sequence to successfully obtain a target product (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine. The synthesis method sencondly provided by the invention takes the same starting raw material for Grignard reaction, organic silicon reagent is used for removing a hydroxide radical, and benzyl is removed by catalytic hydrogenation reaction; finally, the chiral resolution is carried out to obtain a target product. The (S)-3-phenylpiperidine can be synthesized according to the synthesis method. (S)-3-p-aminosalicylic phenylpiperidine can be synthesized according to the third aspect; or according to the fourth aspect, (S)-3-p-bromophenyl piperidine is synthesized to serve asthe key intermediate for preparing the niraparib. According to the synthesis method for (R)-3-phenylpiperidine or / and (S)-3-phenylpiperidine and the synthesis method for chiral intermediate of niraparib, production cost is obviously lowered, and the synthesis methods are favorable for the large-scale industrial production of a niraparib medicine.
Owner:SHANGHAI BIOBOND PHARMA
Preparation method of umeclidinium bromide
InactiveCN105461710AMild reaction conditionsReduce cooling costsOrganic chemistryBenzeneChlorobenzene
The invention discloses a preparation method of umeclidinium bromide. The method is suitable for industrial routine reactions, and industrial production schemes exist at present. A technical scheme adopted in the invention is characterized in that a Grignard reagent is adopted to substitute a lithium reagent, bromobenzene, chlorobenzene and other benzene halides are adopted as raw materials, and ether and tetrahydrofuran can be used as a solvent. Compared with the prior art, the method disclosed in the invention has the following advantages: 1, the lithium reagent with high price is substituted by magneson; 2, reaction conditions are mild, too low temperature is not needed, and the reaction temperature is about 0-5DEG C, so the refrigeration cost is reduced; and 3, a routine device can be used, and the Grignard reaction is a classic reaction and industrially exists for a hundred years or above, so people can be simply trained, and the device is mature and has high operating stability.
Owner:ANHUI DEXINJIA BIOPHARM
Epoxiconazole intermediate 1-chloro-3-(2-chlorophenyl)-2-(4-fluorophenyl)-2-propanol synthesis process
ActiveCN105130757AEasy to recycleProcess conditions are mild and controllableOrganic compound preparationHydroxy compound preparationPropanolChloride
The present invention discloses an epoxiconazole intermediate 1-chloro-3-(2-chlorophenyl)-2-(4-fluorophenyl)-2-propanol synthesis process, and belongs to the technical field of fine chemical industry. According to the process, 2-chlorobenzyl chloride and 2-chloro-4'-fluoroacetophenone are adopted as raw materials, diethoxymethane and toluene are adopted as solvents, and a two-step reaction comprising as a Grignard reaction and a nucleophilic addition reaction is performed to obtain the target product. According to the present invention, the high yield intermediate is obtained while the process operation is simplified, the raw material cost and the production cost are reduced, the environmental pollution is reduced, and the safety requirements of the industrial scale-up production are met.
Owner:内蒙古佳瑞米精细化工有限公司
Preparation method for m-trifluoromethyl acetophenone and intermediate thereof
InactiveCN105461538AHigh yieldShort reaction timeOrganic compound preparationCarbonyl compound preparation by condensationCyclohexyl chlorideGrignard reagent
The invention discloses a preparation method for m-trifluoromethyl acetophenone and an intermediate thereof. The preparation method comprises the steps of (a) Grignard reaction, wherein a compound A reacts with a Grignard reagent in a non-protonic solvent at the reaction temperature of 0-200 DEG C to obtain a compound B, the Grignard reagent is one or more of isopropylmagnesium chloride, isopropylmagnesium bromide, cyclohexylmagnesium chloride, cyclohexylmagnesium bromide, tert-butylmagnesium chloride and tert-butylmagnesium bromide, and the molar ratio of the Grignard reagent to the non-protonic solvent to the compound A is (0.9-2) to (2-20) to 1; (b) acetylization, wherein the compound B obtained in the step (a) reacts with an acetylization reagent in a non-protonic solvent at the reaction temperature between -40 DEG C and 200 DEG C to obtain the m-trifluoromethyl acetophenone. The reaction process is mild, and the preparation method is suitable for industrial mass production.
Owner:LIAONING TIANYU CHEM +4
Siliceous aromatic ether and aryne polymer and preparation method thereof
The invention relates to a siliceous aromatic ether and aryne polymer and a preparation method thereof. The polymer is structurally characterized by comprising silicon atoms, aryne groups and an aromatic ether structure on a main chain. The siliceous aromatic ether and aryne polymer is polymerized from diacetylene-benzene or a dyhydroxy aromatic compound and dichlorosilane by Grignard reaction under an inert atmosphere. The preparation method of the polymer comprises the following steps of: firstly reacting haloalkane with magnesium powder to produce an alkyl magnesium halide Grignard reagent; and then reacting with the dyhydroxy aromatic compound to obtain an alkyl magnesium halide Grignard reagent of the dyhydroxy aromatic compound or reacting with diacetylene-benzene to obtain an alkyl magnesium halide Grignard reagent of diacetylene-benzene; and finally reacting with dichlorosilane to obtain the siliceous aromatic ether and aryne polymer. The invention has the advantages of simple process, short reaction time, easily controlled process condition and simple subsequent processing and is convenient to operate; in addition. The product of the method is stable at room temperature and easy to store, the condensate of the polymer has excellent heat-resisting stability and mechanical property and is a high-performance thermosetting resin with good heat-resisting property.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method for liquid crystal monomer of o-difluoroalkoxybenzene derivative
ActiveCN102826966AOvercome the disadvantage that the cis structure cannot be isomerizedOvercome the disadvantage of not being able to isomerizeEther preparation by ester reactionsIsomerizationLiquid-crystal display
The invention relates to the field of liquid crystal displays and particularly relates to a preparation method for a liquid crystal monomer of an o-difluoroalkoxybenzene derivative used for liquid crystal displays. According to the preparation method, 1,2-difluorobenzene or 1,2-difluorobromobenzene is used as a raw material and undergoes metallization or a Grignard reaction with a cyclohexyl ketone compound, then dehydration and hydrogenation are carried out to prepare a compound containing cis-trans-isomers, and finally isomerization and oxyalkylation are successively carried out. The method overcomes the disadvantage of incapable isomerization of a cis structure of liquid crystal monomers containing alkoxy groups, increases product yield, reduces production cost, is beneficial for mass production and has a great application prospect.
Owner:VALIANT CO LTD
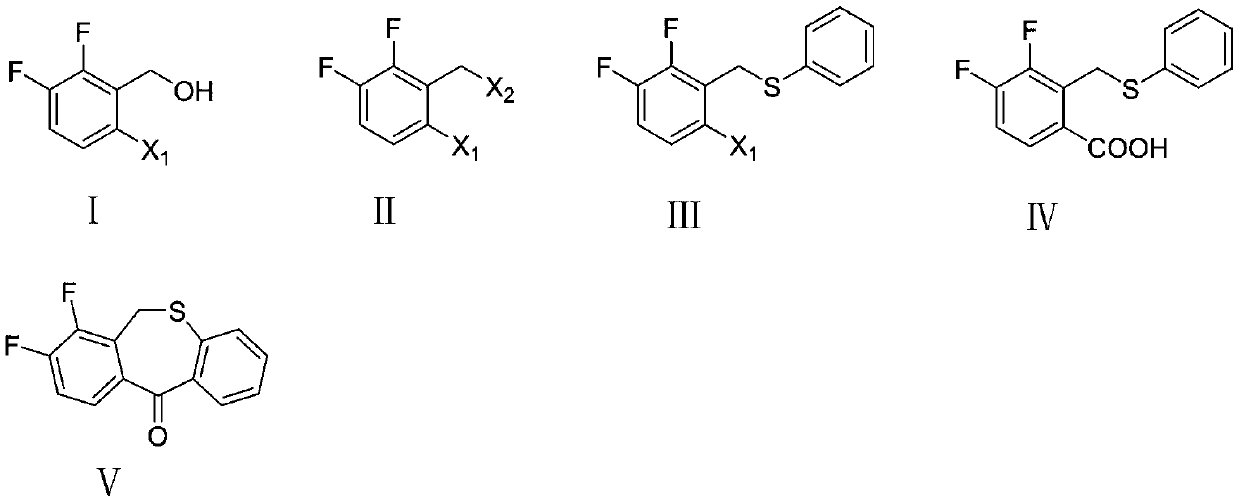
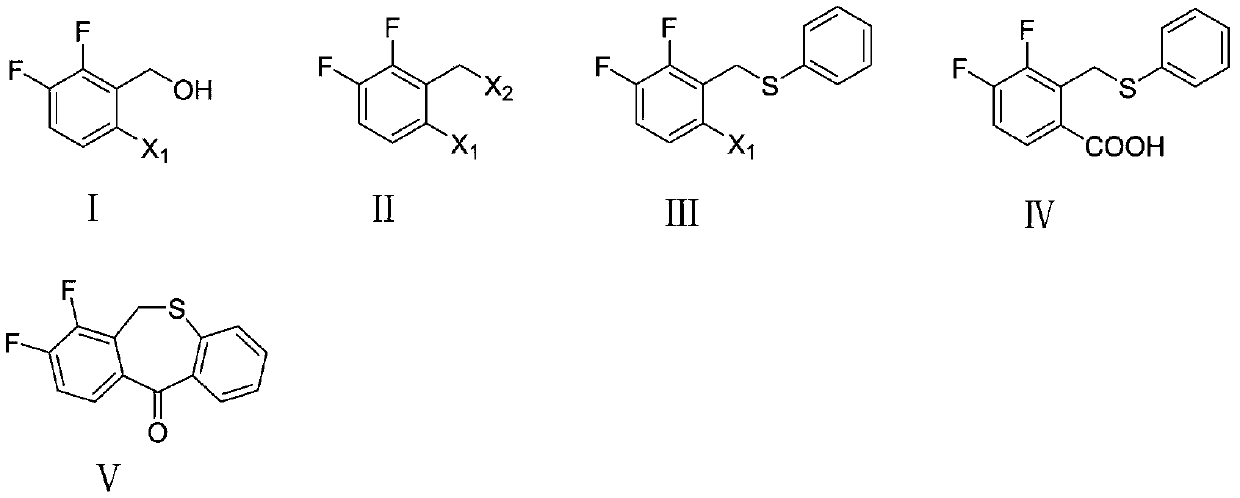
Preparation method of key intermediate of baloxavir marboxil
The invention discloses a preparation method of a key intermediate of baloxavir marboxil. The preparation method comprises the following steps: carrying out a nucleophilic substitution reaction on polysubstituted 2,3-difluoro-6-halogenated benzyl alcohol represented by a formula (I) to synthesize 2,3-difluoro-6-halogenated benzyl halide represented by a formula (II), carrying out a nucleophilic substitution reaction on the polysubstituted benzyl halide represented by the formula (II) to synthesize 2,3-difluoro-6-halogenated benzyl phenylsulfide represented by a formula (III), carrying out a Grignard reaction on the polysubstituted benzyl phenylsulfide represented by the formula (III) to synthesize 3,4-difluoro-2-(phenylthio)methyl)benzoic acid represented by a formula (IV), and carrying out a Friedel-Crafts acylation reaction on the polysubstituted benzoic acid to synthesize 7,8-difluorodibenzo[b,e]thiophene-11(6H)-one represented by a formula (V). In the formula (I), the formula (II)and the formula (III), X<1> and X<2> are separately and independently selected from chlorine, bromine or iodine. According to the preparation method, the polysubstituted benzyl alcohol is used as a starting raw material, and the steps of two-step nucleophilic substitution, Grignard exchange, Friedel-Crafts acylation and the like are carried out to prepare the key intermediate of the anti-influenzadrug baloxavir marboxil, so that original innovation is achieved.
Owner:ZHEJIANG UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com