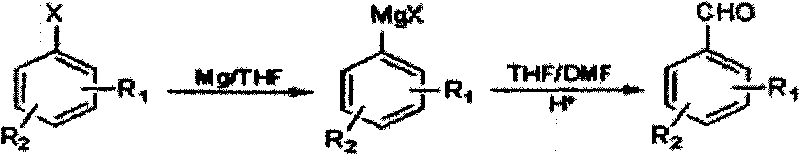
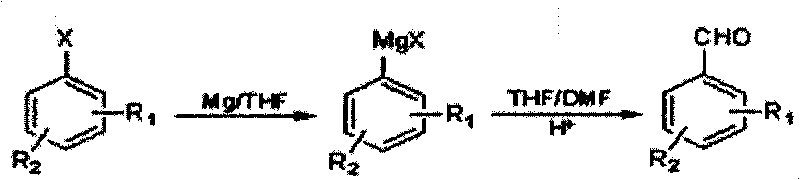
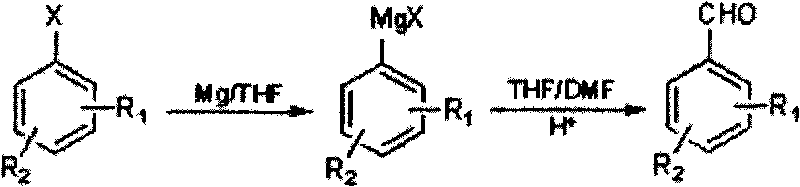
Method for preparing halogenated methyl-benzaldehyde by Grignard reaction
A technology of halogenated methylbenzaldehyde and Grignard reaction, which is applied in the field of preparation of halogenated benzaldehyde, can solve the problems of slow reaction and difficult industrial application, and achieve the effect of easy-to-obtain raw materials and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 6.5g (0.27mol) magnesium wire, 50mL tetrahydrofuran, 1g iodine pellets into a 500mL dry and clean device with a drying tube, stir, and add 1 / 10 volume of 51.4g (0.25mol) 3-chloro-4-bromotoluene dropwise Dissolve the mixed solution of 178g (200mL) anhydrous tetrahydrofuran to initiate the reaction, cool down to about 25°C, add dropwise the remaining 9 / 10 volume of 3-chloro-4-bromotoluene in anhydrous tetrahydrofuran solution, dropwise, and react at about 20°C 1h to get the Grignard reagent, lower the temperature to -5°C, add dropwise a mixture of 50mL tetrahydrofuran and 20g (0.27mol) N,N-dimethylformamide to the Grignard reagent, let it rise slowly to 20 ℃, react for 1h, below 20℃, adjust the pH ≤ 2, let it stand, separate the organic phase, use C for the water phase 2 h 4 Cl 2 Extract, combine the organic phases, remove the solvent, and steam distill to obtain 35.62g of 2-chloro-4-methylbenzaldehyde, with a yield of 69.3%.
Embodiment 2
[0022] With the technological operation step of embodiment 1, different conditions are:
[0023] 6.0g (0.25mol) of magnesium wire, 3g of iodine particles, 51.4g (0.25mol) of 3-chloro-4-bromotoluene in 154.2g (173mL) of anhydrous tetrahydrofuran solution, the reaction temperature after initiation is 10°C, -10°C Add N,N-dimethylformamide dropwise, yield 55.2%.
Embodiment 3
[0025] With the technological operation step of embodiment 1, different conditions are:
[0026] 5g iodine pellets, 514g (578mL) anhydrous tetrahydrofuran solution of 51.4g (0.25mol) 3-chloro-4-bromotoluene, the reaction temperature after initiation is 30°C, 18g (0.25mol) N, N-dimethylformaldehyde Amide, yield 64.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com