Preparation method of flurbiprofen and preparation method of flurbiprofen axetil
A technology of flurbiprofen axetil and flurbiprofen, which is applied in the field of preparation of flurbiprofen and flurbiprofen axetil, can solve the problems of low industrial reproducibility and low yield, and achieve human environmental hazards low cost, low raw material cost and short preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
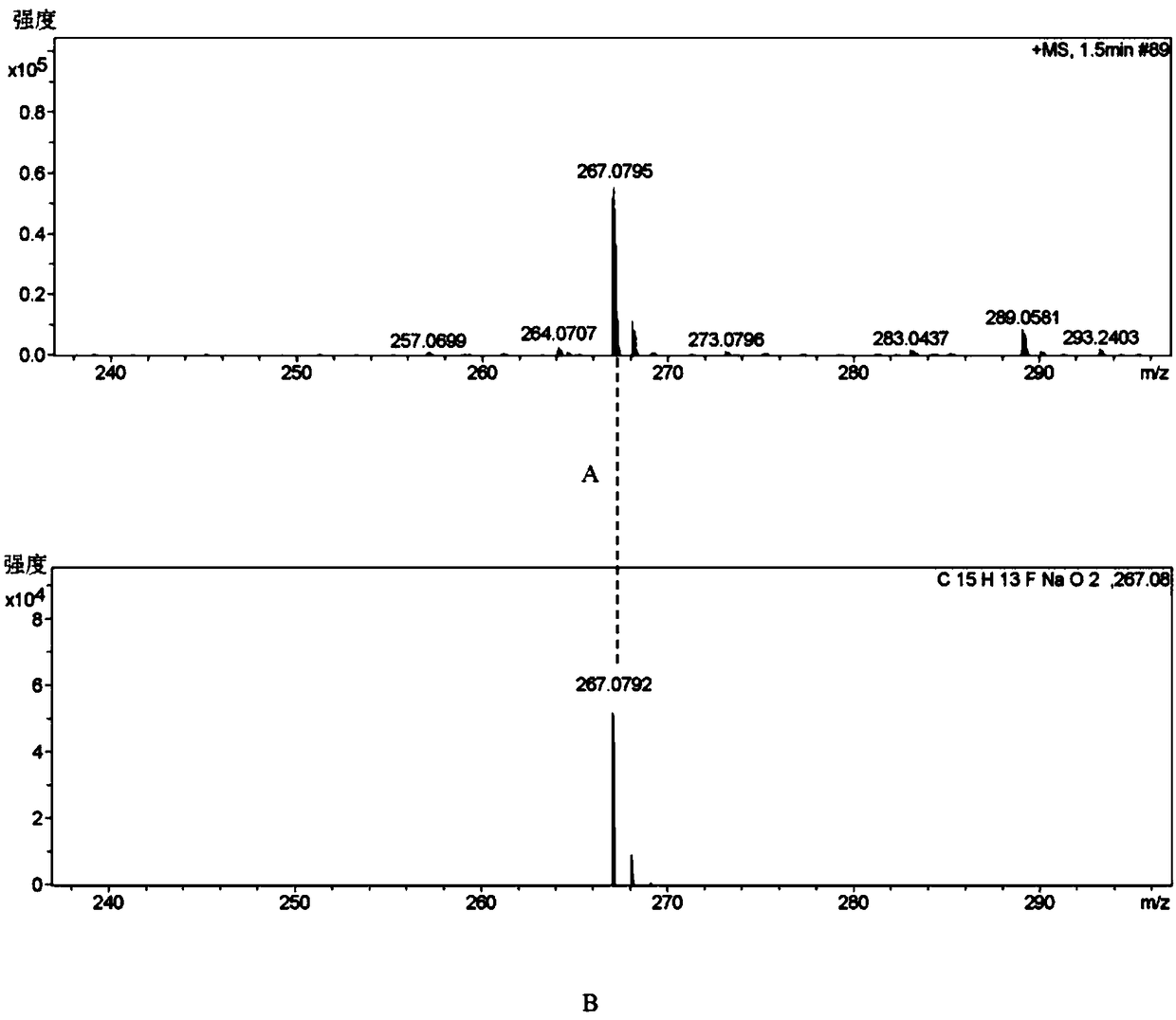
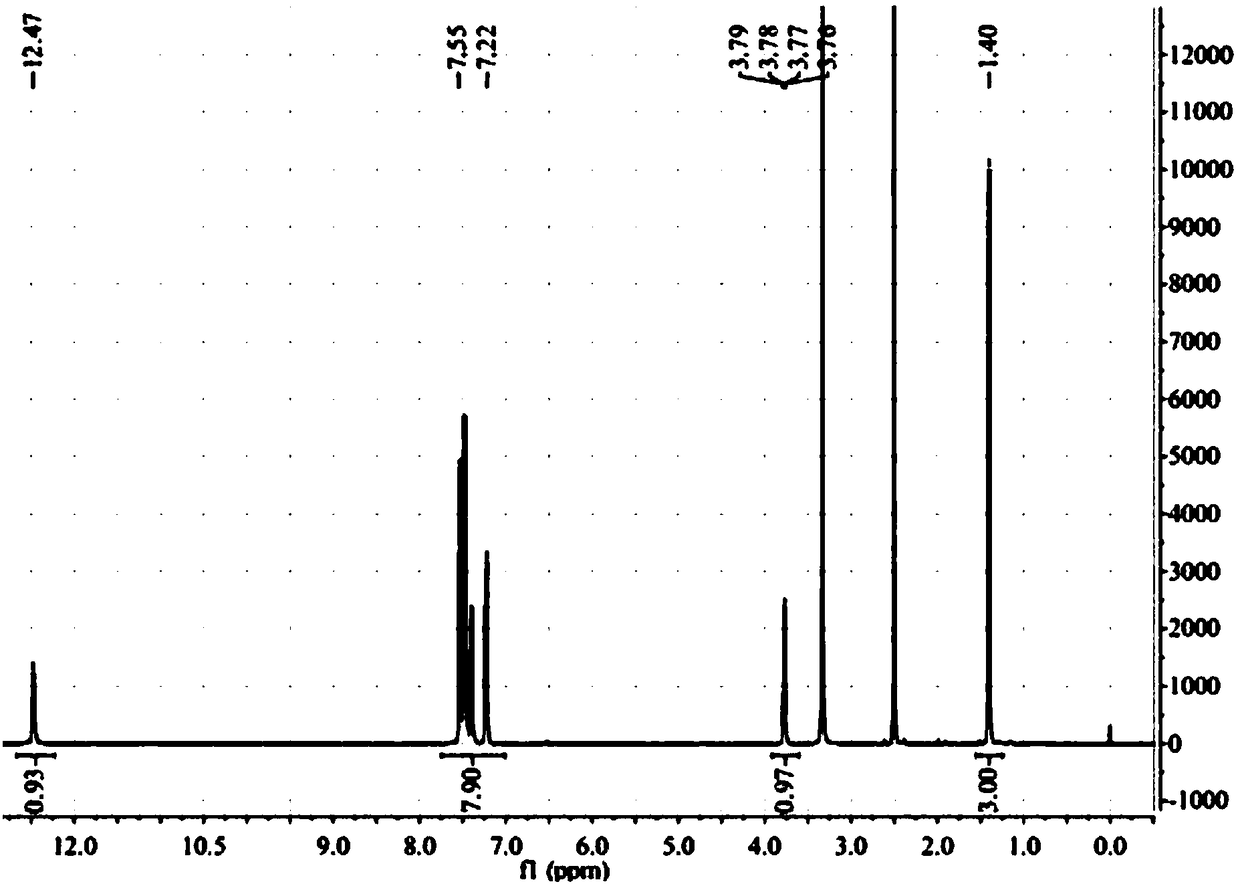
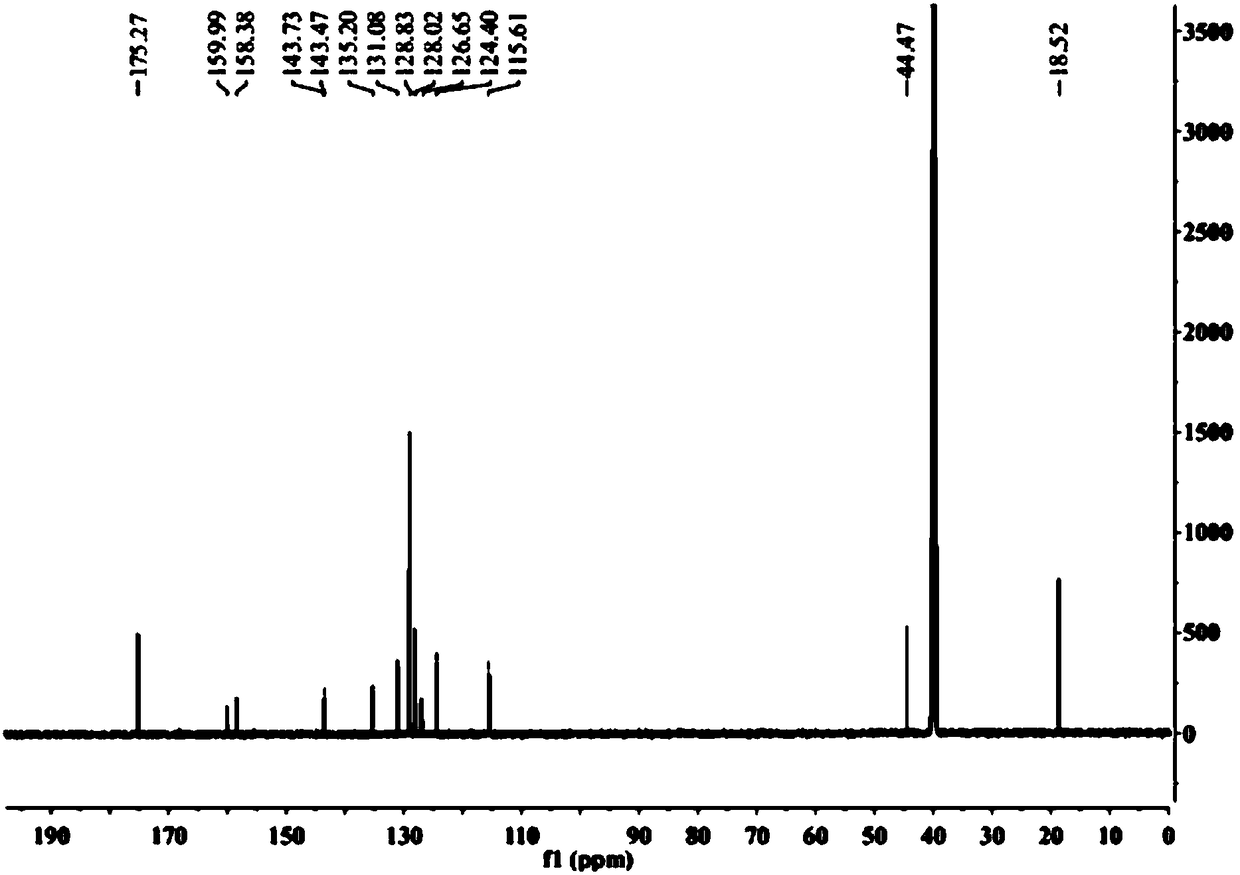
[0064] 1, the preparation method of flurbiprofen
[0065] 1) Put a 500mL three-necked flask equipped with a condenser (with a calcium chloride drying tube), a constant pressure dropping funnel and a thermometer on a magnetic stirrer, and add 1.9g (80.0mmol) of magnesium chips and tetrahydrofuran (THF) 100mL, 2 iodine capsules, nitrogen gas;
[0066] 2) Move to an oil bath, and when heated to 70°C (internal temperature 62-68°C), add 4-bromo-2-fluorobiphenyl (20.0g, 80.0mmol) dropwise in THF (100mL ) solution 3.0-5.0mL, about 35min, the color of reaction iodine disappears gradually;
[0067] 3) Lower the temperature to 62°C, add 4-bromo-2-fluorobiphenyl solution dropwise, drop the solution for about 2 hours, then raise the temperature to 72°C and stir the reaction until the magnesium disappears, and the magnesium chips basically react completely;
[0068] 4) Cool to room temperature 25°C, add 11.0mL (84.0mmol) ethyl 2-bromopropionate dissolved in 50mL THF solution (add 2ml tit...
Embodiment 2
[0116] 1, prepare flurbiprofen axetil with the flurbiprofen prepared in embodiment one as raw material, concrete method is as follows:
[0117] 1) Dissolve flurbiprofen in 600ml THF (three-neck flask, insert a thermometer in the left mouth, insert a dropping funnel in the middle mouth, and plug the bottle stopper in the right mouth), add 27.6g potassium carbonate, and stir at room temperature (25°C) for 30 min;
[0118] 2) Control the temperature at 15-25°C, and add bromoethyl acetate dropwise with a dropping funnel;
[0119] 3) After the dropwise addition, reflux reaction at 66-70°C for 4-5h, monitored by TLC;
[0120] 4) Cool to room temperature (25°C), evaporate THF under reduced pressure, and the yield of THF is above 90%, add 300mL ethyl acetate to dissolve, add 300mL water, separate the organic phase, extract the aqueous phase twice with 300mL ethyl acetate, and combine Organic phase 600ml, washed twice with sodium carbonate, washed twice with water, dried over anhydrou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com