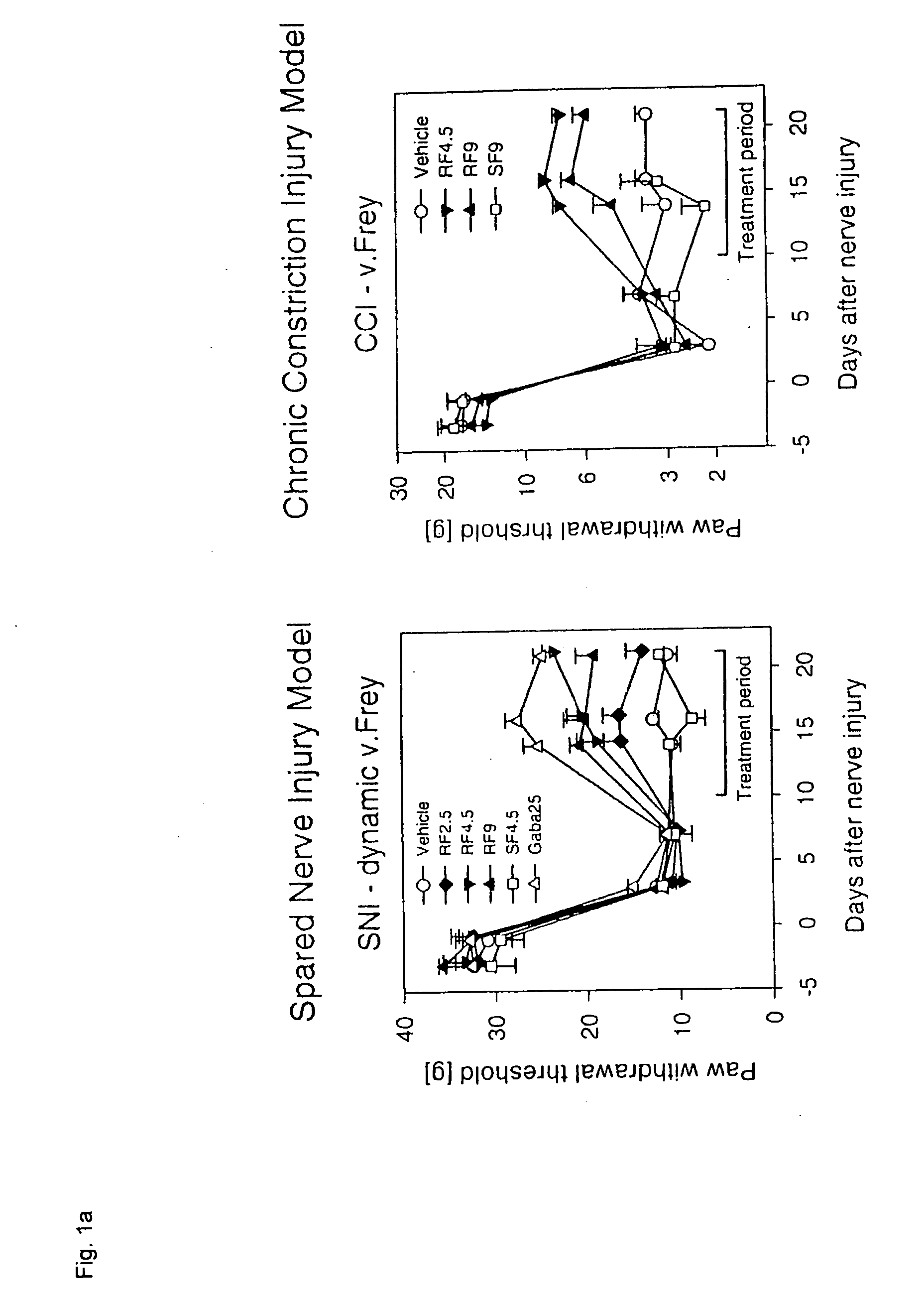
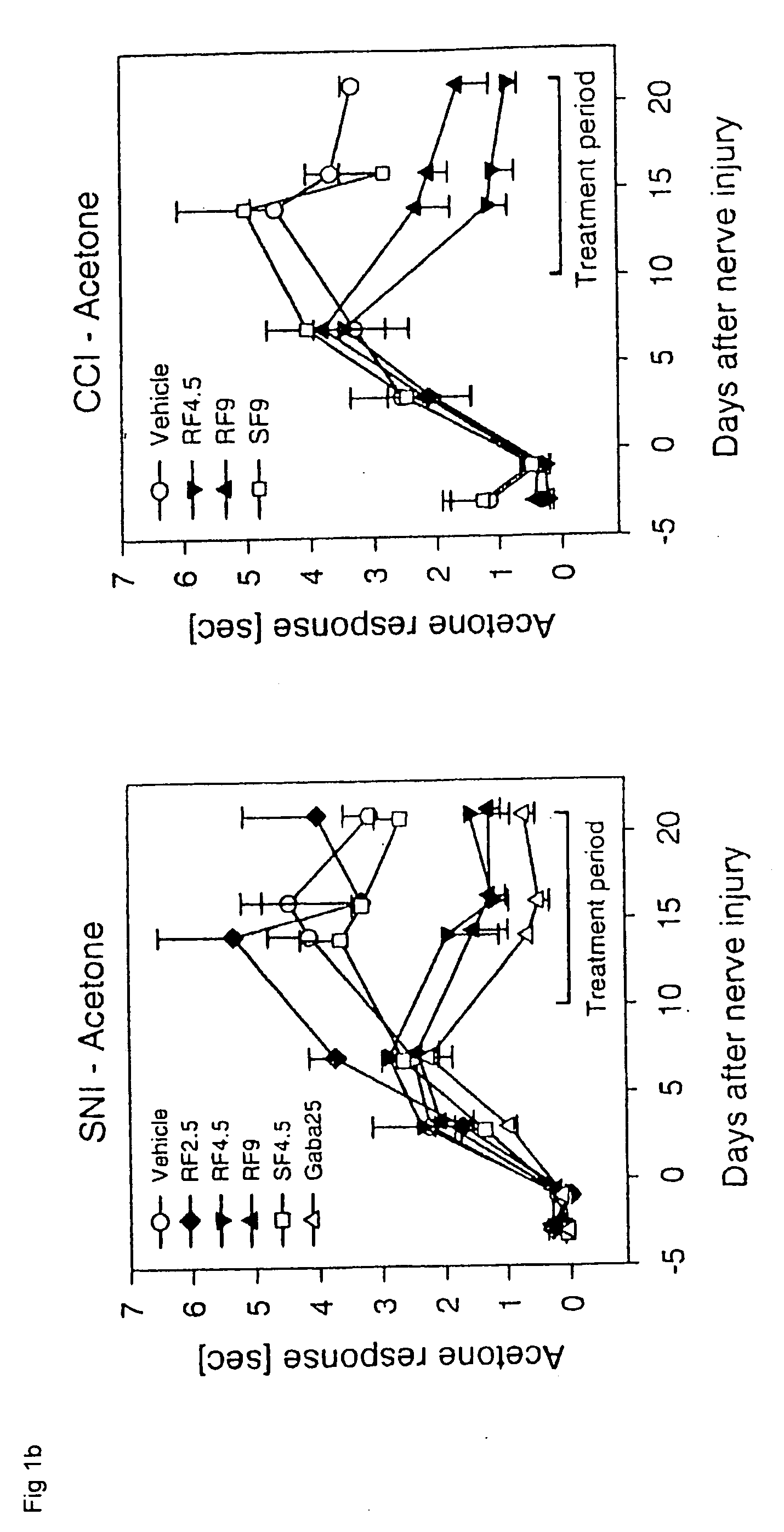
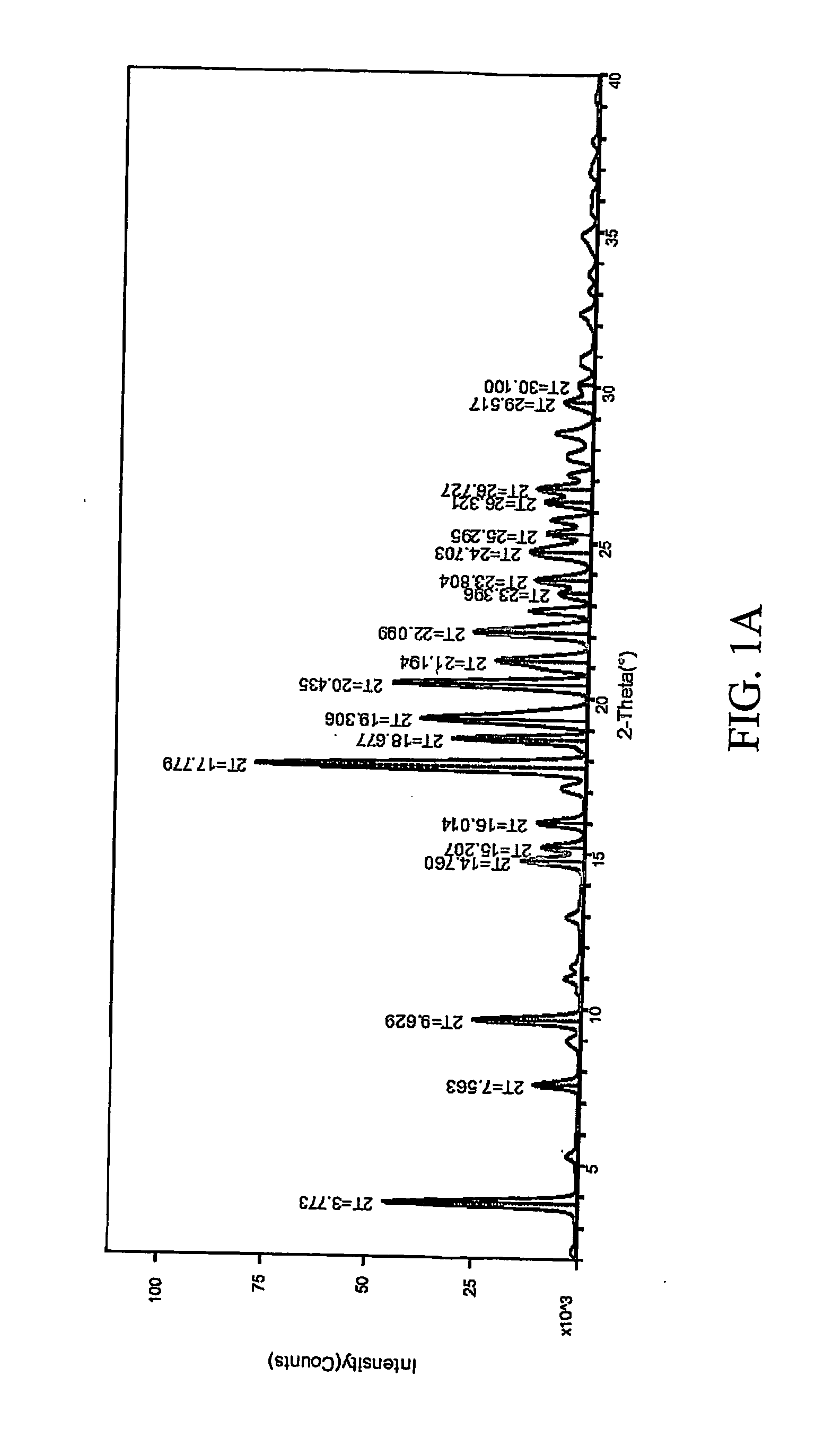
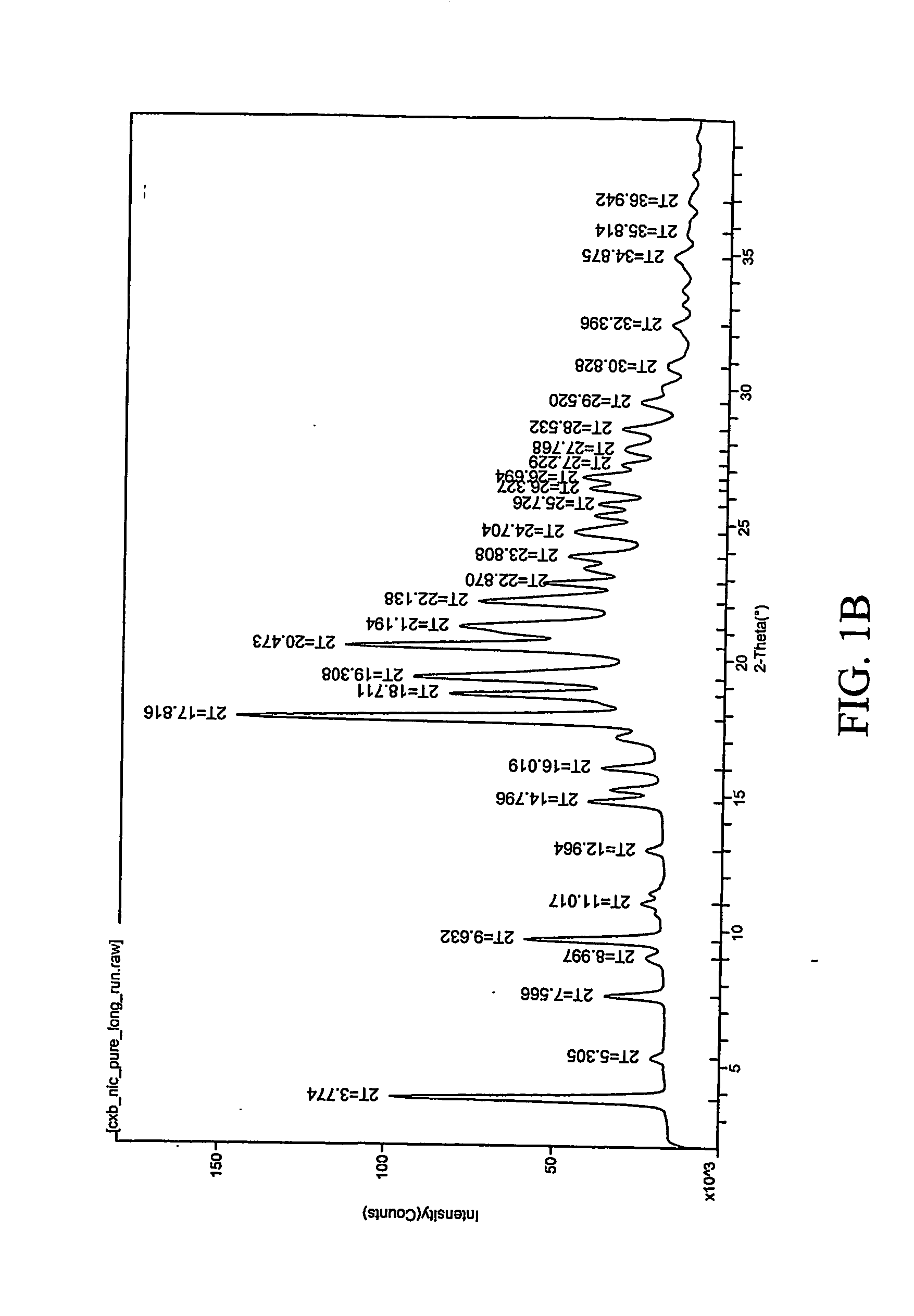
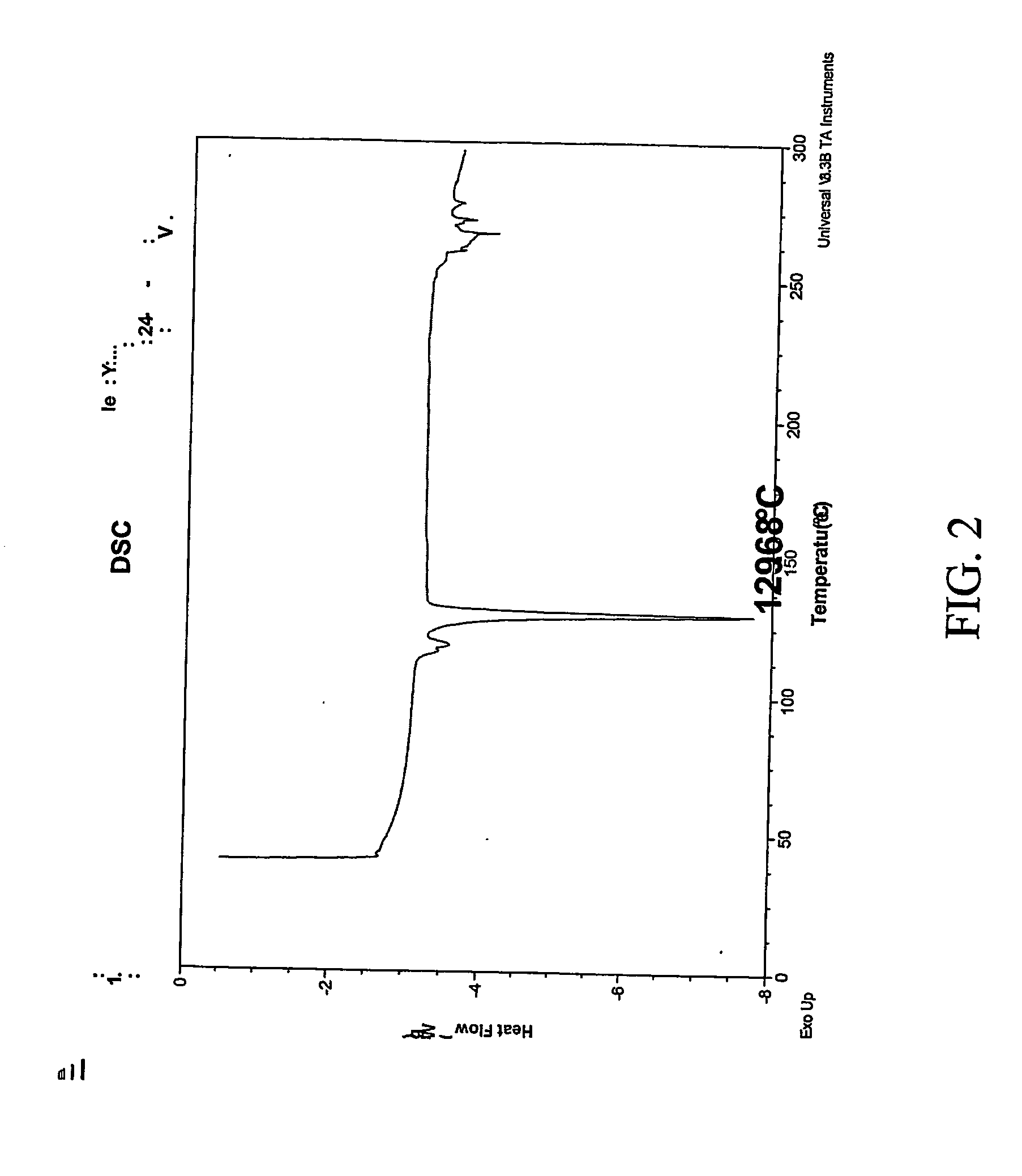
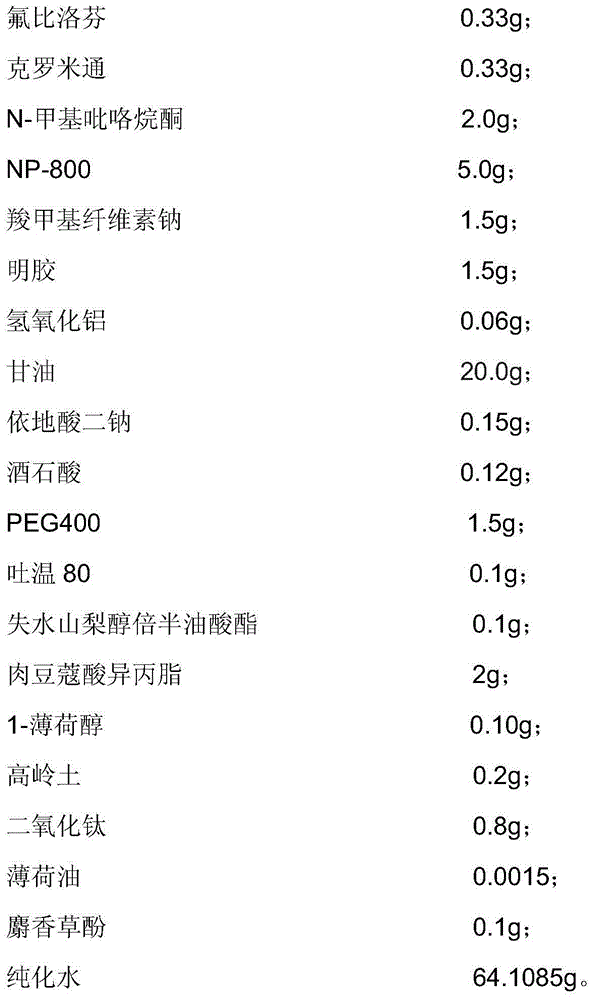
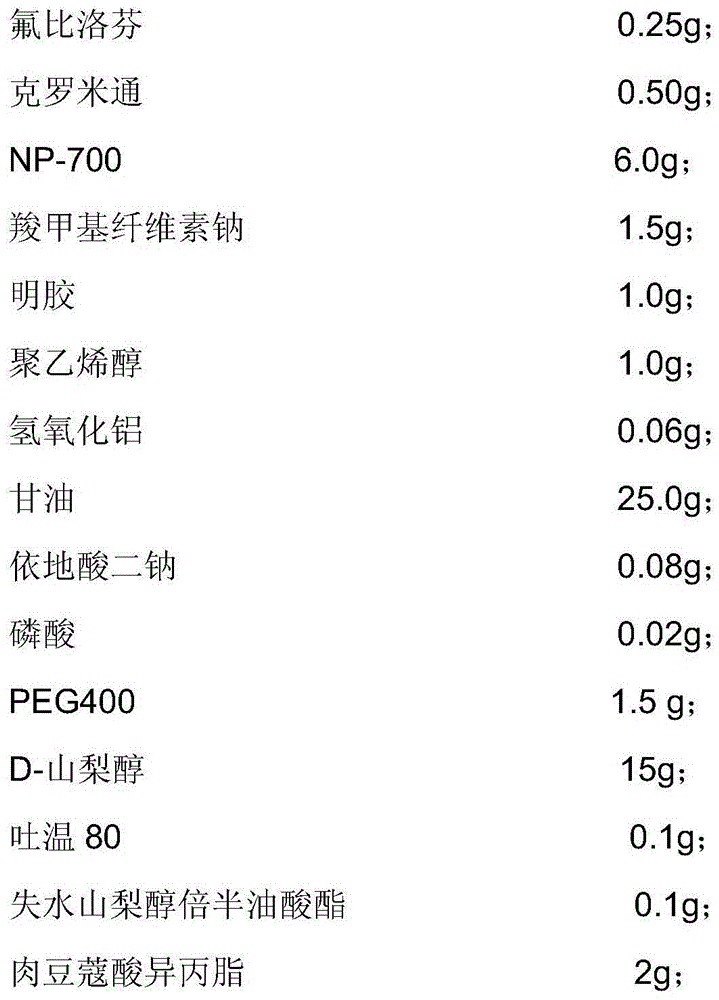
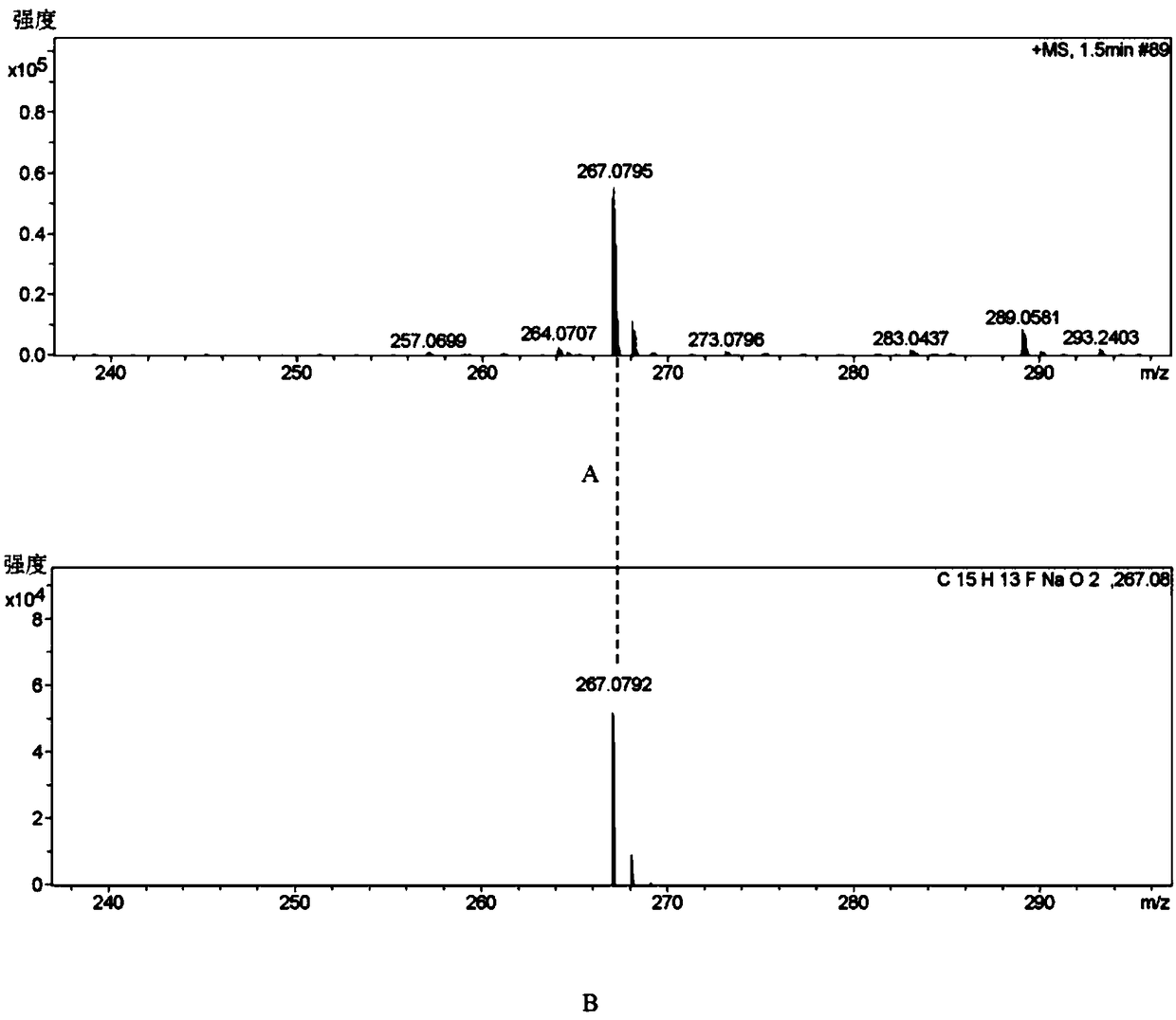
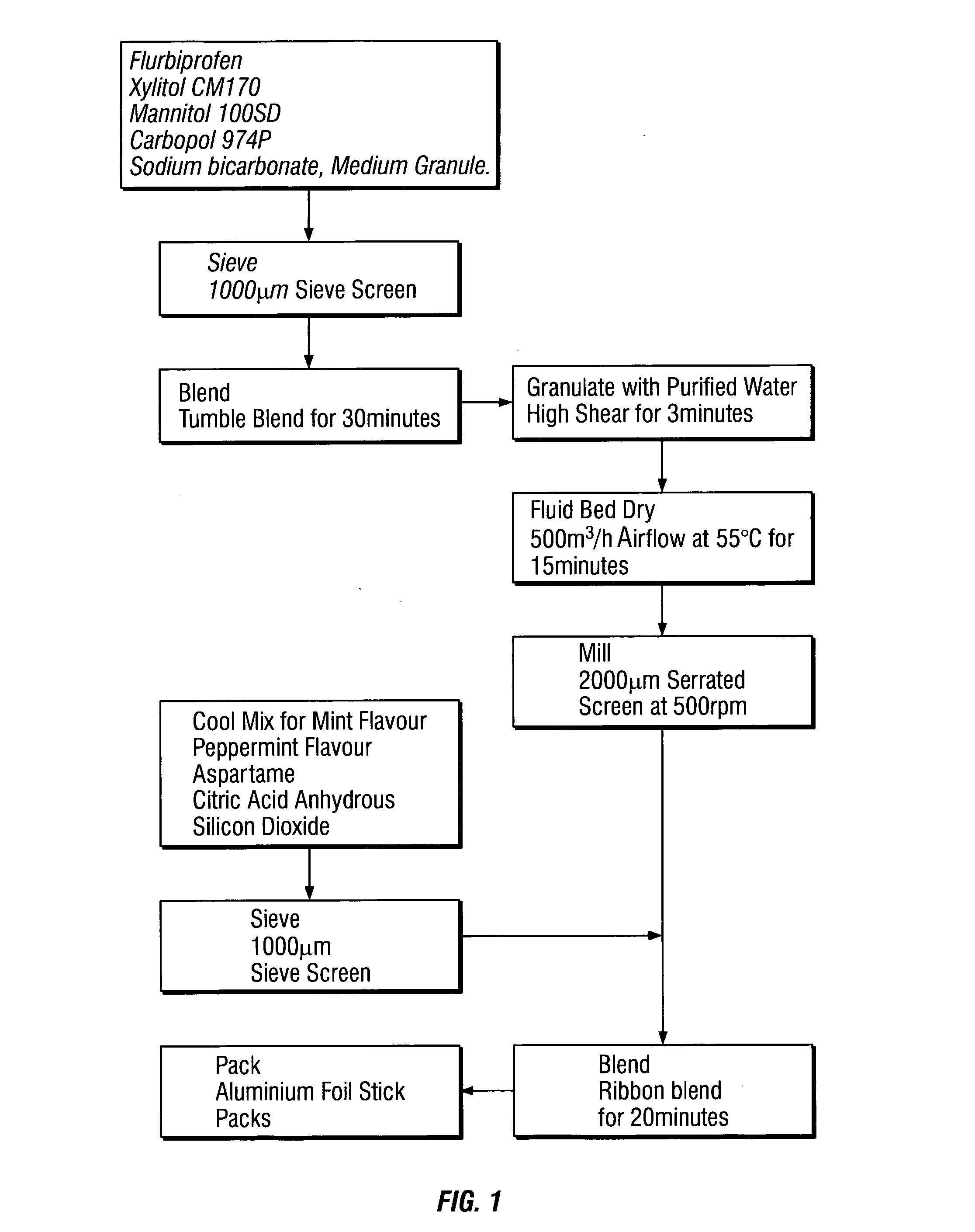
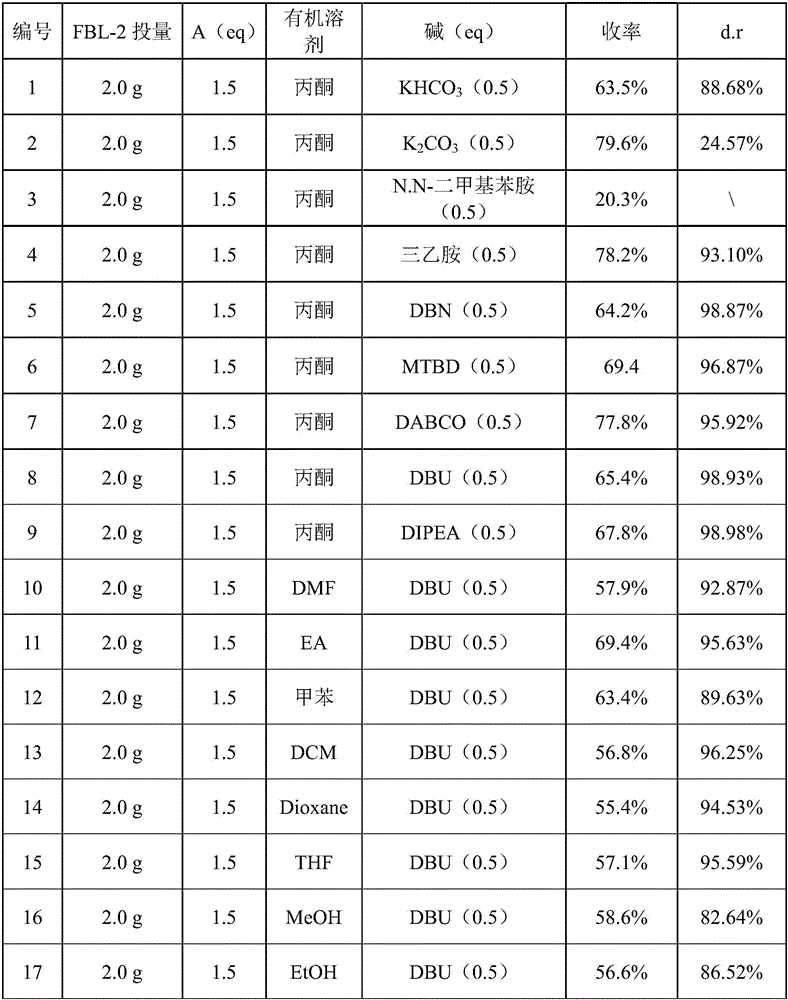
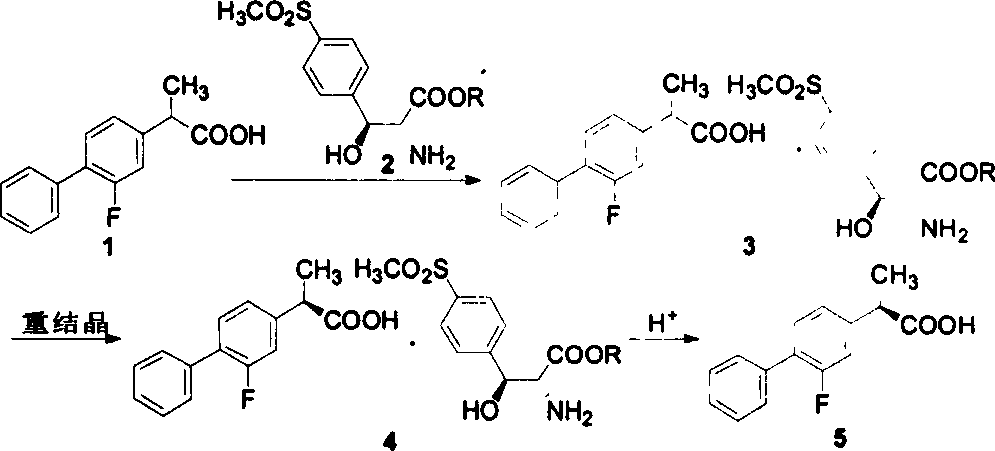
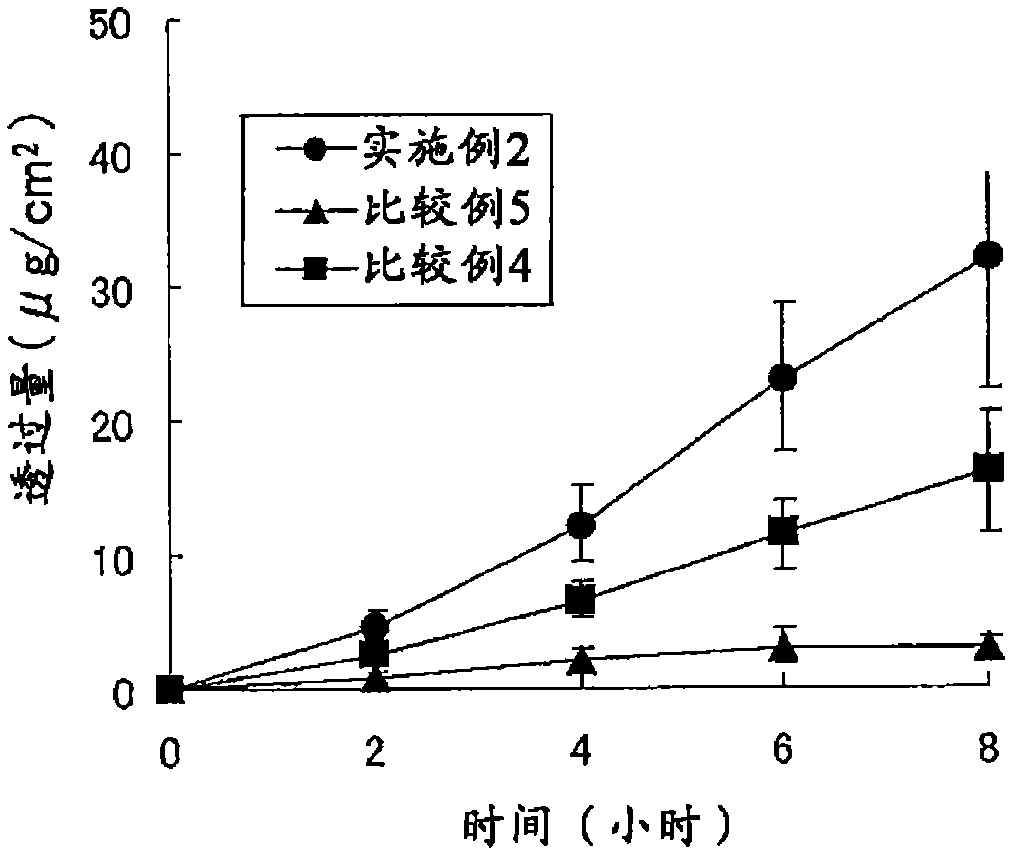
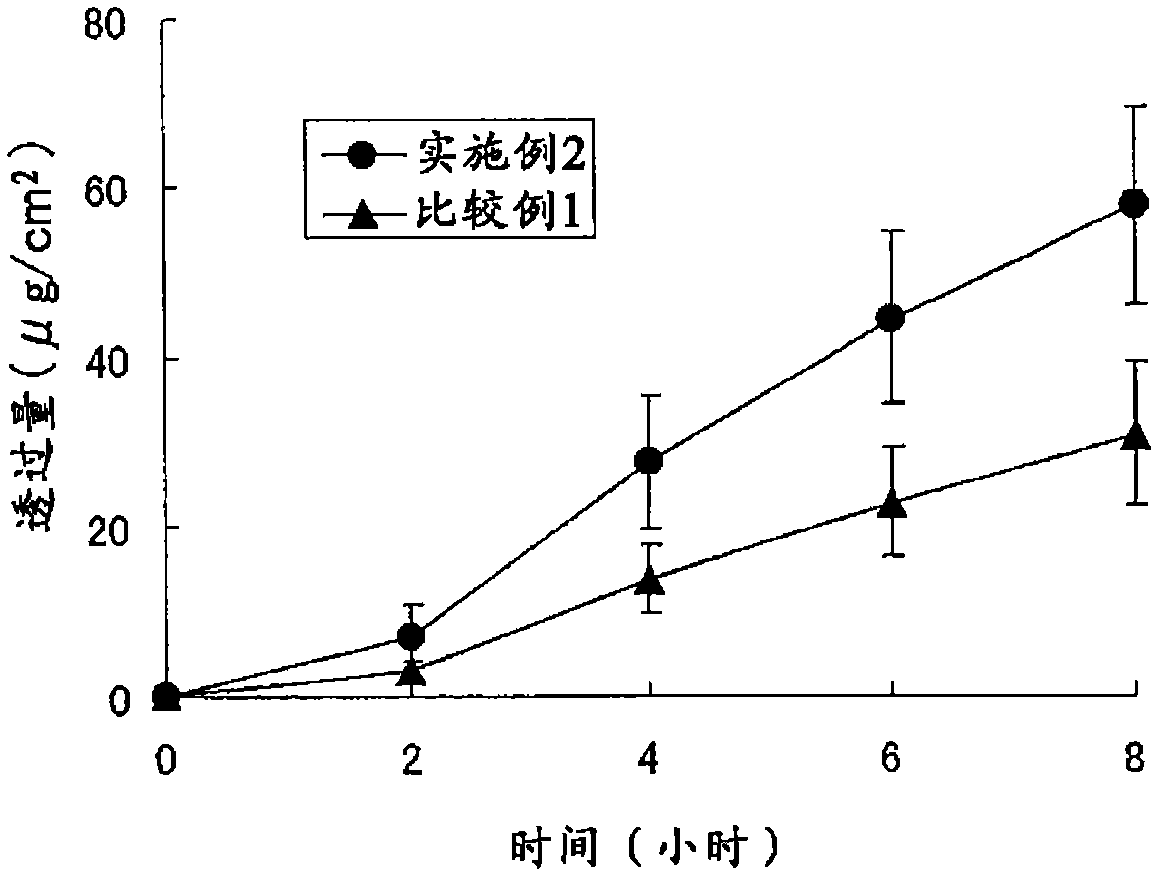
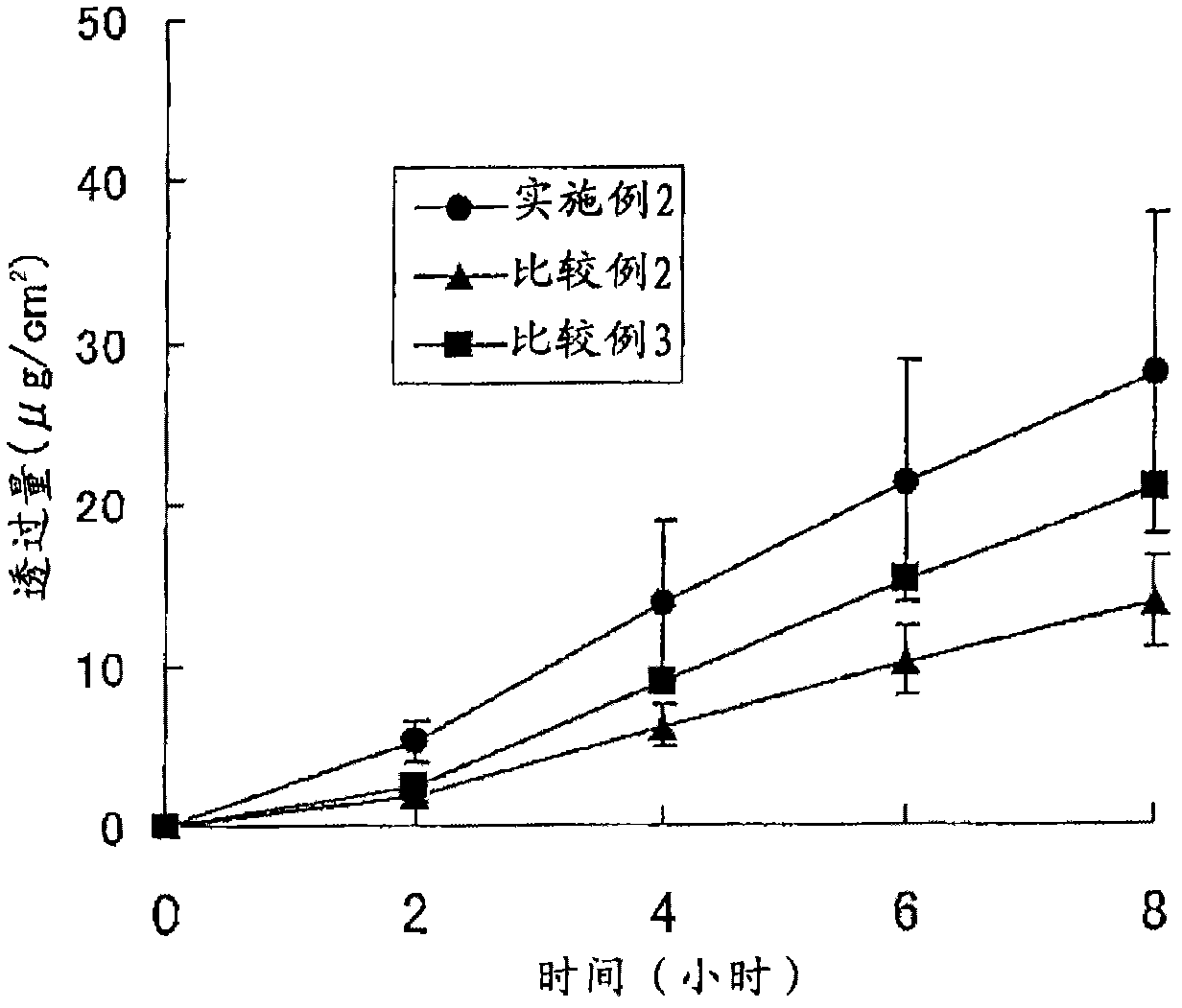
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
164 results about "Flurbiprofen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Flurbiprofen is used to reduce pain, swelling, and joint stiffness from arthritis.
Drugs as well as their production and use in the treatment of pain-associated neuropathies
InactiveUS20090162421A1Reduction of evoked neuropathic painReduce painBiocideAntipyreticPeripheral neuropathic painTreatment pain
The present invention relates to the use of tarenflurbil and / or a pharmaceutically tolerable salt or derivative thereof in enantiomerically-pure and / or essentially enantiomerically-pure form or a form that is enriched with respect to flurbiprofen racemate and / or a racemate of said salt or derivative, for the production of a drug for the treatment of pain-associated neuropathy, pain-associated neuropathy that is simultaneously accompanied by states of nociceptive pain, peripheral and / or predominantly peripheral neuropathic pain or central and / or predominantly central neuropathic pain.
Owner:PAZ ARZNEIMITTEL ENTWICKLUNGSGMBH
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
Flurbiprofen hydrogel plaster and composition thereof
InactiveCN104546803AReduce dosageGood transdermal effectOrganic active ingredientsAntipyreticDiseaseAnkylosing spondylitis
The invention provides a flurbiprofen hydrogel plaster and a composition thereof. In the composition, the content of flurbiprofen is 0.1-3wt%, and a hydrogel substrate accounts for the rest. Based on the total weight of the hydrogel substrate, the hydrogel substrate comprises the following components: 0.1-40wt% of a hydrophilic base material, 0.01-2wt% of a crosslinking agent, 0.01-4wt% of a crosslinking regulator, 10-60wt% of a humectant, 0.05-45wt% of a penetration enhancer and 10-85wt% of a solvent. According to the flurbiprofen hydrogel plaster provided by the invention, a small number of medicines are used, and the permeability and stability are good; and the flurbiprofen hydrogel plaster can be applied to various arthralgia diseases such as rheumatic arthritis, gouty arthritis, ankylosing spondylitis, tenosynovitis, muscle pain and lumbago.
Owner:和心医药科技(上海)有限公司
Flurbiprofenbab preparation and its preparation method
The present invention relates to the field of medicine technology, in particular, it relates to a flurbiprofenbab preparation and its preparation method. Said preparation includes back lining layer, medicine storage layer and protecting film, said medicine storage layer is formed from flurbiprofen powder preparation or flurbiprofen emulsion, hydrophilic bab preparation matrix, osmotic acceleratorand additive. Its medicine-carrying quantity is large, moisture-holidng property and air permeability are good.
Owner:上海宝龙药业股份有限公司
Preparation method of flurbiprofen and preparation method of flurbiprofen axetil
InactiveCN108558651ALow costHigh purityPreparation from carboxylic acid saltsOxygen-containing compound preparationChemical synthesisBromine
The invention relates to the field of pharmaceutical chemical synthesis, in particular to a preparation method of flurbiprofen and a preparation method of flurbiprofen axetil. The preparation method of the flurbiprofen comprises the steps of carrying out a Grignard reaction by using 4-bromine-2-fluorine biphenyl as a raw material, carrying out a coupling reaction, and acidizing to obtain the flurbiprofen; the yield is 90%, and the purity is 99.5%; then, the flurbiprofen axetil is prepared by using the flurbiprofen, obtained by the method, as a raw material, the yield reaches up to 90%, and thepurity reaches up to 99.5%. The preparation methods are high in quality controllability and industrial reproducibility.
Owner:上海峰林生物科技有限公司
Preparation method of flurbiprofen axetil
The invention relates to a preparation method of flurbiprofen axetil. The method comprises the following steps: carrying out a reaction between flurbiprofen and bromoethyl acetate in the presence of a catalyst and a solvent; washing reaction products and separating grease; carrying out underpressure distillation; dissolving a fraction in an organic solvent, washing and drying; and removing the solvent. Purity of flurbiprofen axetil prepared by the method can reach more than 99.0%.
Owner:WUHAN DOCAN PHARMA
Flurbiprofen acetaminophen resin lipid microsphere injection, freeze-drying lipid microsphere injection and preparation methods
ActiveCN103054800AImprove solubilityImprove physical stabilityPowder deliveryOrganic active ingredientsSolubilityTreatment effect
The invention relates to a flurbiprofen acetaminophen resin lipid microsphere injection, a freeze-drying lipid microsphere injection and a preparation method. Through a reasonable composition proportion and a preparation process with controllable parameters, good stability and strong operability, the flurbiprofen acetaminophen resin which is an indissolvable drug is wrapped in an oil phase and an interfacial film of a lipid microsphere, so that not only is the solubility of the drug remarkably strengthened, but also precipitation and oxidation do not occur easily, the physical and chemical stabilities of the drug are greatly improved, the devitrification phenomenon is avoided in the use and storage processes, a slow release effect is exerted and the action time of the drug in the blood plasma is prolonged, meanwhile, as the lipid microsphere targets to an inflammation part, the toxicity and the vessel stimulation during injection are remarkably reduced, the clinical application of the injections is safer, the treatment effect is more remarkable, and the injections and the methods are suitable for industrialized production and clinical application.
Owner:WUXI ERYUN TECH CO LTD
External plaster containing flurbiprofen
ActiveCN101119716APrevent crystallizationHigh dose releaseOrganic active ingredientsAntipyreticAdditive ingredientStyrene-isoprene-styrene block copolymer
The present invention provides a transdermal external patch, which is an external patch in which an adhesive layer is laminated on a support, wherein the adhesive layer contains styrene-isoprene-benzene as an essential component. An ethylene block copolymer (SIS), a tackifying resin, and a softener, and flurbiprofen as an active ingredient are mixed therein. The external patch containing flurbiprofen makes possible the long-term stable release of the flurbiprofen contained, and the preparation has high stability and very high drug release.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Flurbiprofen liposome and preparation method thereof
InactiveCN101732255AReduce lossesGood biocompatibilityOrganic active ingredientsAntipyreticYolkWater baths
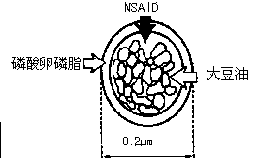
The invention relates to a flurbiprofen liposome and a preparation method thereof. The flurbiprofen liposome is prepared by weighting the following materials in part by weight: 1 to 10 parts of cholesterol, 5 to 40 parts of yolk lecithin, 2 to 50 parts of flurbiprofen and 1 to 10 parts of vitamin C; dissolving the materials with chloroform and methanol in a mass ratio of 4:1; uniformly mixing the materials to obtain mixed solution; putting the solution into a rotary evaporator; distilling the solution under reduced pressure for removing the chloroform and the methanol to obtain a lipid membrane; adding phosphate buffer with pH between 6 and 8 into the lipid membrane; rotating for 1 to 4 h in warm bath at the temperature of between 20 and 60 DEG C on the rotary evaporator; and carrying out water bath ultrasound for 5 to 30 min, and then filtering the mixture with a 0.22 to 0.45 mu m filter membrane to obtain the flurbiprofen liposome. The flurbiprofen liposome prepared by the invention has good stability, targeting property and slow-releasing property, can reduce administration dosage and reduce toxic and side effect of medicaments, is suitable for percutaneous administration and oral administration, and can be widely used for preparing oral liquid, aerosol, spray, and ophthalmic and external liposome administration formulations.
Owner:TONGJI UNIV
Flurbiprofen coating agent and preparation method thereof
InactiveCN105232496AAntisepticGood biocompatibilityOrganic active ingredientsAntipyreticAlcoholCurative effect
The invention discloses a flurbiprofen coating agent, comprising, by weight, 8-15 g of flurbiprofen, 5-20 g of chitosan, 8-15 g of carbomer, 83-125 g of glycerol, 8-15 g of laurocapram, 200-600 g of ethyl alcohol, and 500-900 g of purified water. A preparation method includes: 1, spreading 10 g of chitosan and 10 g of carbomer to the surface of 300 g of purified water so that the chitosan and the carbomer naturally swell, and performing heating so that the chitosan and the carbomer dissolve into glutinous slurry; 2, mixing 100 g of glycerol and 600 g of ethyl alcohol, adding 5 g of flurbiprofen, and performing uniform mixing to obtain mixed liquid; 3, adding the glutinous slurry of step 1 into the mixed liquid of step 2, and performing uniform mixing; 4, adding 10 g of laurocapram, adding 650 g of purified water, and performing full mixing to obtain the flurbiprofen coating agent. The flurbiprofen coating agent is convenient to administer and has exact curative effect; the preparation process is simple and reasonable and meets the requirement on coating agent quality.
Owner:CHENGDU AIBIKE BIOTECH
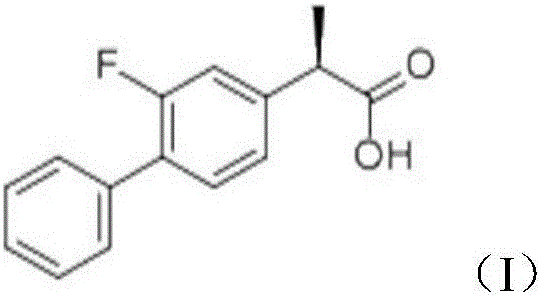
Novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition
ActiveCN103301101AReduce gastrointestinal adverse reactionsIndustrial applicabilityOrganic active ingredientsNervous disorderArgininePropionine
The invention provides a novel 2-(2-fluorine-4biphenyl)-propionic acid pharmaceutical composition. Flurbiprofen and basic amino acid arginine or lysine form a pharmaceutical composition solution. The composition can be administrated in an injection or oral administration manner and can also be further administrated in a freeze-drying manner. Compared with a flurbiprofen axetil injection, a flurbiprofen composition not only does not influence the antipyretic, anti-inflammation and analgestic effects of the flurbiprofen, but also has the advantages of simple technology, low cost, high quality, stable long shelf life and convenience in quality control, storage and transportation; moreover, compared with a conventional oral preparation, the flurbiprofen composition has the same low gastrointestinal tract adverse reaction as the flurbiprofen axetil injection.
Owner:南京星福星医药科技有限公司
A sustained-release microsphere preparation contain flurbiprofen and its preparation method
ActiveCN109223713AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsAntipyreticPolyesterMicrosphere
The invention relates to a sustained-release microsphere preparation containing flurbiprofen and a preparation method thereof, belonging to the pharmaceutical preparation field. In order to overcome the shortcomings of the prior art that flurbiprofen sustained-release microsphere preparation has poor stability and is prone to crystallization during use and storage, it is an object of the present invention to provide a sustained-release microsphere preparation containing flurbiprofen, wherein the sustained-release microsphere preparation is prepared from the following raw materials in parts byweight: 10-100 parts of polyester and 2-5 parts of flurbiprofen, and that sustained-release microsphere preparation further comprises an emulsifying agent and a stabilizing agent. A sustain release microsphere preparation prepared by that solvent evaporation method has high encapsulation efficiency and drug load, stable property and no obvious change in encapsulation efficiency and drug load during long-term storage, thus effectively prolonging the storage time of medicament and improving the quality stability of medicament.
Owner:GUANGDONG JIABO PHARM CO LTD
Preparation methods of flurbiprofen axetil compound
ActiveCN103664606AEasy to getLow costOrganic compound preparationCarboxylic acid esters preparationAcetic acidHigh volume manufacturing
The invention discloses preparation methods of a flurbiprofen axetil compound. The methods comprise the steps: firstly dropwise adding an ester compound into a mixture of flurbiprofen, an acid catalyst and an organic solvent to form a reaction system, or directly mixing flurbiprofen, an alcohol and the acid catalyst, after a reaction is finished, thus obtaining a reaction system, then carrying out spin steaming concentration treatment under reduced pressure, adding ethyl acetate and water to fully dissolve, standing and layering to obtain an organic phase, washing the organic phase, drying, purifying by passing through a silica gel column, and thus obtaining the flurbiprofen axetil compound. The preparation methods have the advantages of simple reaction operation, low requirements on production personnel and production equipment, small pollution degree and low production cost, are safe and effective, can avoid use of high-risk and high-toxic materials, and can be used for mass production.
Owner:GUANGDONG JIABO PHARM CO LTD
Antiprostaglandins for the treatment of ocular pathologies
Formulations and methods useful to treat ocular neovascularization (new blood vessel growth in the cornea, retina, conjunctiva, and / or choroid) are disclosed. According to the invention there is provided a formulation suitable for the treatment of ocular neovascularization that may comprise flurbiprofen in a concentration and dose suitable for treating ocular neovascularization, characterized in that said flurbiprofen may be at a substantially neutral pH in a pharmaceutically acceptable form suitable for delivery to the eye.
Owner:MINU
Flurbiprofen cataplasm
InactiveCN107157962AFast transdermal absorptionLow drug releaseOrganic active ingredientsAntipyreticCarboxymethyl celluloseGlycine
The invention provides flurbiprofen cataplasm. The cataplasm is composed of a backing layer, a drug reservoir and a protective layer. The cataplasm is characterized in that the drug reservoir is prepared from the following components in percentage by weight: 0.2%-0.5% of flurbiprofen as an active component, 5%-10% of an oil phase component, 5%-10% of partially neutralized sodium polyacrylate as an aqueous phase component, 15%-20% of glycerol, 0.2%-0.4% of aluminum glycinate, 0.1%-0.3% of sodium calcium edetate, 1%-1.5% of carbomer 934, 1.5%-3% of sodium carboxymethyl cellulose (CMC-Na), 0.05%- 0.1% of a pH adjusting agent and gellan gum, 1%-1.5% of L-glycine, 1%-3% of a filler and the balance of water, wherein the oil phase component is prepared from castor oil and benzyl alcohol, the ratio of castor oil to benzyl alcohol is 1 to (0.08 to 0.12), the aqueous phase component and water form hydrogel, the filler is arranged in the hydrogel dispersedly, and the oil phase component is emulsified and disperses in the hydrogel to form the drug reservoir.
Owner:北京茗泽中和药物研究有限公司
Method for fixing layered double hydroxide on surface of polypropylene and application of method
ActiveCN105148877AAchieve fixationCompact and stable structureOther chemical processesPreparing sample for investigationWater bathsDivalent metal ions
The invention discloses a method for fixing layered double hydroxide on the surface of polypropylene and application of the method. Firstly, a dopamine alkaline solution is prepared in a polypropylene centrifugal pipe, vortex preoxidation is carried out at normal temperature, a reaction is conducted for 6-8 hours at the water bath condition of 30-50 DEG C, and a poly-dopamine thin layer is formed; then, divalent metal ions, trivalent metal ions and a CO(NH2)2 solution are added into the centrifugal pipe modified by dopamine, layered double hydroxide is prepared and fixed after a heating reaction, and the inner surface of a device is provided with a layer-by-layer assembled structure of polydopamine and the layered double hydroxide. The preparation method is simple, no pollutant reagent needs to be added, and the formed layered double hydroxide structure is stable and compact. The device has a good effect on removing flurbiprofen, other acidic medicine active compounds, rhodamine B and other anionic dye in aqueous solutions. The device is small in size and convenient to carry, and can achieve the functions of adsorbing and removing anionic compounds in solutions through simple centrifugation.
Owner:WUHAN UNIV
Flurbiprofen acetaminophen ester solid dispersion and preparation method thereof
InactiveCN102871952APlay a synergistic anti-inflammatory and analgesic effectOrganic active ingredientsAntipyreticSolubilityAdjuvant
The invention relates to flurbiprofen acetaminophen ester solid dispersion and a preparation method thereof. Anti-inflammatory and analgesic prodrugs mainly comprise brand new compounds of flurbiprofen acetaminophen ester, and the flurbiprofen acetaminophen ester solid dispersion is prepared by adding hydrophilic carrier materials and by respectively adopting a solvent method, a solvent-melting method and a melting method and can be further prepared into oral tablets or capsules by being mixed with proper adjuvants. By the flurbiprofen acetaminophen ester solid dispersion and the preparation method thereof, solubility and dissolving-out speed of drugs can be obviously increased, the solubility of the drugs in water is increased by 10-100 times as compared with that of crude drugs, cumulative dissolving-out percentage of the drugs in dissolving-out media in 45 minutes is substantially increased accordingly, and finally oral absorbability of the drugs is improved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Flurbiprofen cataplasm
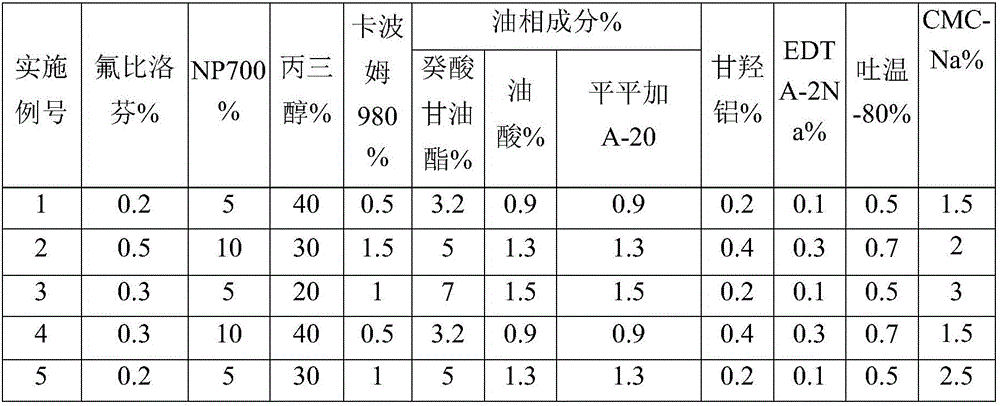
ActiveCN106667970AFast transdermal absorptionImprove the speed of transdermal absorptionOrganic active ingredientsAntipyreticCarboxymethyl celluloseEthylene diamine
The invention discloses a flurbiprofen cataplasm, which consists of a backing layer, a medicament storage space and a protective layer, wherein the medicament storage space consists of the following components in percentage by weight: 0.2 to 0.5 percent of flurbiprofen serving as an active ingredient, 5 to 10 percent of an oil-phase component, 5 to 10 percent of partial neutralization sodium polyacrylate, 20 to 40 percent of a humectant, 0.5 to 1.5 percent of carbomer 980, 1.5 to 3 percent of CMC-Na (sodium carboxymethyl cellulose), 0.2 to 0.4 percent of dihydroxyaluminium aminoacetate, 0.1 to 0.3 percent of EDTA-2Na (disodium ethylene diamine tetraacetic acid, 0.5 to 0.7 percent of tween 80, 1 to 3 percent of a filler and the balance of water, wherein the oil-phase component is prepared from caprin, oleic acid and peregal A-20 in a mass ratio of 1:(0.2 to 0.3):(0.2 to 0.3), and the partial neutralization sodium polyacrylate, the humectant, the carbomer 980, the CMC-Na, the dihydroxyaluminium aminoacetate, the EDTA-2Na and the tween 80 form a water-phase component.
Owner:北京茗泽中和药物研究有限公司
Flurbiprofen-containing external preparation for skin
PendingCN110787150AImprove adhesionOrganic active ingredientsAntipyreticDrug reservoirTopical preparation
The invention discloses a flurbiprofen-containing external preparation for skin. The preparation is composed of a backing layer, a drug reservoir matrix layer and a protective film, the flurbiprofen-containing external preparation contains 1-10 parts of flurbiprofen, 30 to 100 parts of a gel skeleton component; 0.2-3.5 parts of a cross-linking agent, 1-4 parts of a cross-linking regulator, 1-15 parts of an accelerator, 5.0-52.5 parts of a filling agent, 1-10 parts of a bacteriostatic agent, 5.5-50 parts of a solubilizer and 40-160 parts of a tackifiers. The invention provides the flurbiprofen-containing external preparation, which is safe, effective, non-irritant, good in adhesion and not easy to fall off.
Owner:BEIJING TIDE PHARMA
Process for producing optically active flurbiprofen
InactiveUS20060135617A1High optical purityEfficient productionPreparation from carboxylic acid saltsBiocideOrganic solventAbsolute configuration
The present invention provides a method for producing optically active flurbiprofen. The method of the present invention includes mixing racemic flurbiprofen and (S)- or (R)-3-methyl-2-phenylbutylamine in an organic solvent to produce a diastereomeric salt; and treating the diastereomeric salt with an acid in a second solvent. In the method of the present invention, flurbiprofen having a desired absolute configuration can be obtained very efficiently without repeating the procedure for optical resolution a plurality of times.
Owner:NAGASE & COMPANY
Flurbiprofen axetil emulsion for injection and preparation method thereof
ActiveCN109223712AImprove stabilityGood emulsifying effectOrganic active ingredientsAntipyreticMedicinePharmaceutical drug
The invention provides a flurbiprofen axetil emulsion for injection and a preparation method thereof. The preparation method comprises the following steps: under the protection of nitrogen, the oil phase is added into the aqueous phase step by step and shearing and mixing for many times to obtain colostrum; 40-60wt% of total oil phase is added into that water phase, shear and mixing are carried out for 10-30min to obtain crude emulsion A, wherein the crude emulsion A is obtained by adding 40-60 wt% of total oil phase into the water phase and mixing for 10-30min; 20-30wt% of total oil phase isadded into that crude emulsion A, and the crude emulsion B is obtained by shear and mixing for 10-30min; Shearing and mixing the crude emulsion B and the remaining oil phase for 10 to 30 minutes; Theoil phase includes flurbiprofen esters, oil phase solvents, emulsifiers and stabilizers, and the aqueous phase includes water for injection and / or osmotic pressure regulators. Under the precondition of the presence of the stabilizer in the oil phase, the invention adopts the oil phase step-by-step and multiple emulsification technology, which can effectively improve the emulsification effect, makethe particle size of the obtained emulsion more uniform, the encapsulation efficiency of the drug higher, and the targeting of the drug to the wound tissue better.
Owner:WUHAN DOCAN PHARMA
Flurbiprofen chalcone compound as well as preparation method and application thereof
ActiveCN108101780ASignificant anti-neuroinflammatory activityThe dose-effect relationship is obviousOrganic active ingredientsSenses disorderHuntingtons choreaSpinal cord
The invention discloses a novel flurbiprofen chalcone compound (I), pharmaceutically acceptable salt thereof, a preparation method thereof, a pharmaceutical composition and application to preparationof drugs for treating and / or preventing diseases related to neurodegeneration, including but not limited to neurodegeneration diseases of vascular dementia, an Alzheimer's disease, a Parkinson's disease, a Huntington's disease, HIV-related dementia, MS (multiple sclerosis), progressive lateral sclerosis spinal cord, neuropathic pain, glaucoma and the like.
Owner:SICHUAN UNIV
Controlled Release Flurbiprofen and Muscle Relaxant Combinations
This invention is a novel controlled release (CR) flurbiprofen and muscle relaxant combinations for oral administration with anti-inflammatory, analgesic, myorelaxant activity and methods of its manufacture. The pharmaceutical composition of the present invention is administered orally in tablet, multilayer tablet, multicoated tablet and capsule form.
Owner:SANOVEL ILAC SANAYI & TICARET ANONIM SIRKETI
Lipid microsphere preparation and preparation method thereof
The invention provides a lipid microsphere preparation comprising propofol, flurbiprofen ester, oil phase solvent and emulgator. The invention also provides a method for preparing the lipid microsphere preparation. The method comprises the following steps: (1) mixing an oil phase mixture containing the propofol, the flurbiprofen ester, the oil phase solvent and the emulgator to generate uniform oil phase; (2) adding the oil phase to water phase to form colostrum; (3) homogenizing the colostrums. The lipid microsphere preparation is used as a dope and has improved abirritation simultaneously.
Owner:WUHAN DOCAN PHARMA
Compositions
ActiveUS8569375B2Reduce painLess discomfortBiocideSmall article dispensingParticulatesParticle composition
An ingestible particulate composition comprises: a) at least one compound selected from the group consisting of 2,4-dichlorobenzyl alcohol, amylmetacresol, cetylpyridinium chloride, hexitidine, hexylresorcinol, flurbiprofen, lidocaine, benzocaine, ibuprofen, paracetamol, pectin, menthol, and benzydamine; and b) one or more bioadhesive materials. Resulting particulate compositions have excellent flow characteristics, dust suppression, organoleptic properties and stability. They are highly suitable for administration direction into a patient's mouth, and ingested to alleviate the symptoms of a sore throat.
Owner:RECKITT BENCKISER HEALTHCARE (UK) LTD
Method for the prevention and treatment of alzheimer's disease
Oral non-steroidal anti inflammatory drugs (NSAIDs) are not an effective treatment of Alzheimer's disease, because the brain dose is too low. Nasal delivery of NSAIDs such as ibuprofen, flurbiprofen, indomethacin, diclofenac, or naproxen, which inhibit the enzymes cyclooxygenase-1 (cox-1) and cox-2, are used to prevent and / or treat Alzheimer's disease, a low grade brain inflammation. The large amount of nasal NSAIDs (e.g., ibuprofen, flurbiprofen, indomethacin, diclofenac, or naproxen) that reaches the brain far exceeds that from an oral dose. Low-molecular-weight lipophilic drugs, such as ibuprofen, naproxen, indomethacin, diclofenac, and flurbiprofen, are readily absorbed into the brain by the intranasal route. Alzheimer's starts in the entorhinal cortex, which is closely connected to the olfactory nerves, and spreads anatomically in a defined pattern. Therefore, a nasal NSAID would readily reach the region of the brain where it acts therapeutically.
Owner:LEHRER STEVEN
Method for preparing S-(+)-flurbiprofen axetil high in optical purity
ActiveCN105777544AGuaranteed responseRacemization not foundOrganic active ingredientsAntipyreticOrganic baseReaction temperature
The invention discloses a method for preparing S-(+)-flurbiprofen axetil high in optical purity.The method comprises the steps of 1, making S-(+)-flurbiprofen axetil react with 1-substituted ethyl acetate in the presence of alkali and organic solvent for 3-15 h at the temperature of 0-25 DEG C; 2, extracting and washing a reaction product, and separating out grease; 3, conducting column chromatographic purification on the grease; 4, removing organic solution residues with the solvent coevaporation method, and then conducting vacuum drying to obtain the target product.Inorganic base is not used, organic base highly intersoluble with organic solvent is adopted, a proper solvent ratio is selected to guarantee the proceeding of reaction, and racemization and product breakdown which occur often are avoided during preparation.The total yield of the prepared S-(+)-flurbiprofen axetil can be 80% or more, and optical purity is higher than 99%.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Preparation of levo flurbi profen
InactiveCN1556089AReduce manufacturing costMild reaction conditionsCarboxylic compound separation/purificationAcetic acidOrganic solvent
A process for preparing leavo-flurbiprofen includes dissolving dl-flurbiprofen in solvent, adding (2R, 3S)-2-amino-3-hydroxy-3-p-methylsulfonyl phenylpropionate, raecting, cooling, crystallizing, filtering, recrystallizing, suspending in organic solvent, adding inorganic acid or its aqueous solution, separating liquid, and concentrating organic phase. Its advantages are high output rate and high optical purity (more than 99%).
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
External plaster containing flurbiprofen
ActiveUS20090022778A1Keep for a long timeGood release effectBiocideAntipyreticMedicineAdditive ingredient
In a plaster for external use for transdermal absorption in which an adhesive layer is laminated on a plastic backing, the adhesive layer contains a styrene-isoprene-styrene block copolymer (SIS), a tackifying resin and a softener which are essential ingredients, and further contains flurbiprofen blended as an active ingredient. The present plaster for external use is a flurbiprofen containing plaster for external use enabling long-term stable release of contained flurbiprofen, and having excellent stability and very high drug releasing property.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Flurbiprofen-containing external patch
ActiveCN102028673APrevent crystallizationHigh dose releaseOrganic active ingredientsAntipyreticPercutaneous absorptionBULK ACTIVE INGREDIENT
The invention provides a percutaneous absorption external patch formed by laminating an adhesive layer on a support body. The adhesive layer contains styrene-isoprene-styrene (SIS) block copolymer, tackifying resin and a softener which are taken as essential components, and flurbiprofen which is taken as an active ingredient is mixed with the essential components. In the flurbiprofen-containing external patch, flurbiprofen is possibly released for a long term stably, and the preparation has high stability and extremely high medicament release property.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com