Method for preparing S-(+)-flurbiprofen axetil high in optical purity
A flurbiprofen axetil and optical purity technology, applied in the field of chemical drug preparation, can solve the problems of easy decomposition of products, high temperature requirements, and inability to purify
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
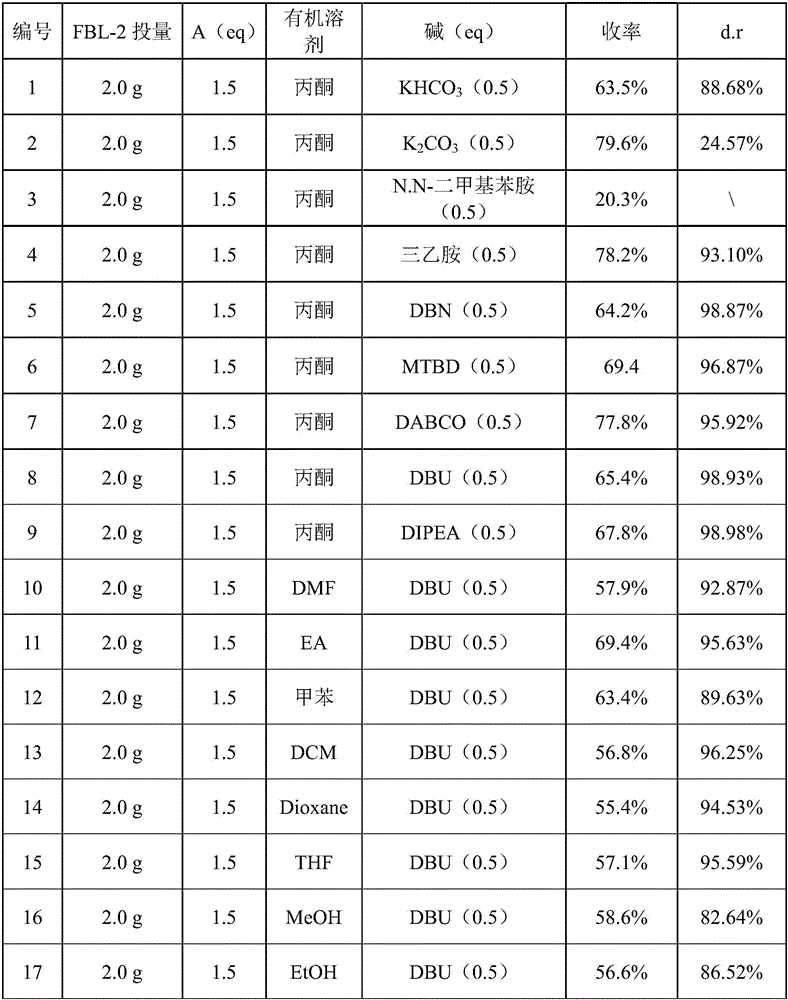
[0037] Embodiment 1: the impact of different bases and solvents on the Dr value and yield.
[0038] Accurately weigh S-(+)-flurbiprofen (2.0g, 8.19mmol) into a 100mL three-necked bottle, add 50mL of acetone and stir to dissolve it completely, the reaction temperature is lowered to 0°C, and slowly add KHCO 3 Solid (410mg, 4.10mmol), then (2.1g, 12.29mmol) 10mL of acetone solution of ethyl 1-bromoacetate was added dropwise to the above-mentioned S-(+)-flurbiprofen in acetone solution at 0°C, And stirred at 25°C for 4 hours, then added 100mL of water at 25°C to quench the reaction, then extracted 3 times with ethyl acetate, 50mL each time, the combined organic phase was washed 2 times with saturated sodium chloride solution, 30mL each time, and used Sufficiently dried over sodium sulfate water, evaporated in vacuo and purified by flash chromatography (filler is neutral aluminum oxide, PE:EA=10:1) to obtain 860 mg of the target compound as light yellow oil with a yield of 63.5%. ...
Embodiment 2
[0042] Embodiment 2: the influence of charging mode on Dr value and yield.
[0043]Accurately weigh S-(+)-flurbiprofen (2.0g, 8.19mmol) and ethyl 1-bromoacetate (2.1g, 12.29mmol) in a 100mL there-necked flask, add 50mL of acetone and stir to make it all dissolve, react The temperature was lowered to 0°C, and 10 mL of acetone solution of DBU (624 mg, 4.10 mmol) was slowly added dropwise to the above solution, and the reaction was stirred at 20°C for 4 hours. After the reaction was completed, 100 mL of water was added at 25°C to quench the reaction, followed by acetic acid Ethyl ester was extracted 3 times, 50mL each time, the combined organic phase was washed 2 times with saturated sodium chloride solution, 30mL each time, fully dried with anhydrous sodium sulfate, evaporated in vacuo and passed flash chromatography (the filler was neutral trioxide Dialuminium, PE:EA=10:1) was purified to obtain 880 mg of the target compound as a pale yellow oil, with a yield of 65.1% and Dr=98...
Embodiment 3
[0044] Embodiment 3: the influence of the molar equivalent of alkali on Dr value and yield.
[0045] Accurately weigh S-(+)-flurbiprofen (2.0g, 8.19mmol) and ethyl 1-bromoacetate (2.1g, 12.29mmol) in a 100mL there-necked flask, add 50mL of acetone and stir to make it all dissolve, react The temperature was lowered to 0°C, a solution of DBU (1.2g, 8.19mmol) in acetone (2mL) was slowly added dropwise to the above solution, and the reaction was stirred at room temperature at 25°C for 4 hours, and 100mL of water was added at 25°C to quench the reaction, followed by acetic acid Ethyl ester was extracted 3 times, 50mL each time, the combined organic phase was washed 2 times with saturated sodium chloride solution, 30mL each time, fully dried with anhydrous sodium sulfate, evaporated in vacuo and passed flash chromatography (the filler was neutral trioxide Dialuminium, PE:EA=10:1) was purified to obtain 2.0 g of the target compound as a pale yellow oil with a yield of 80.0% and Dr=88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com