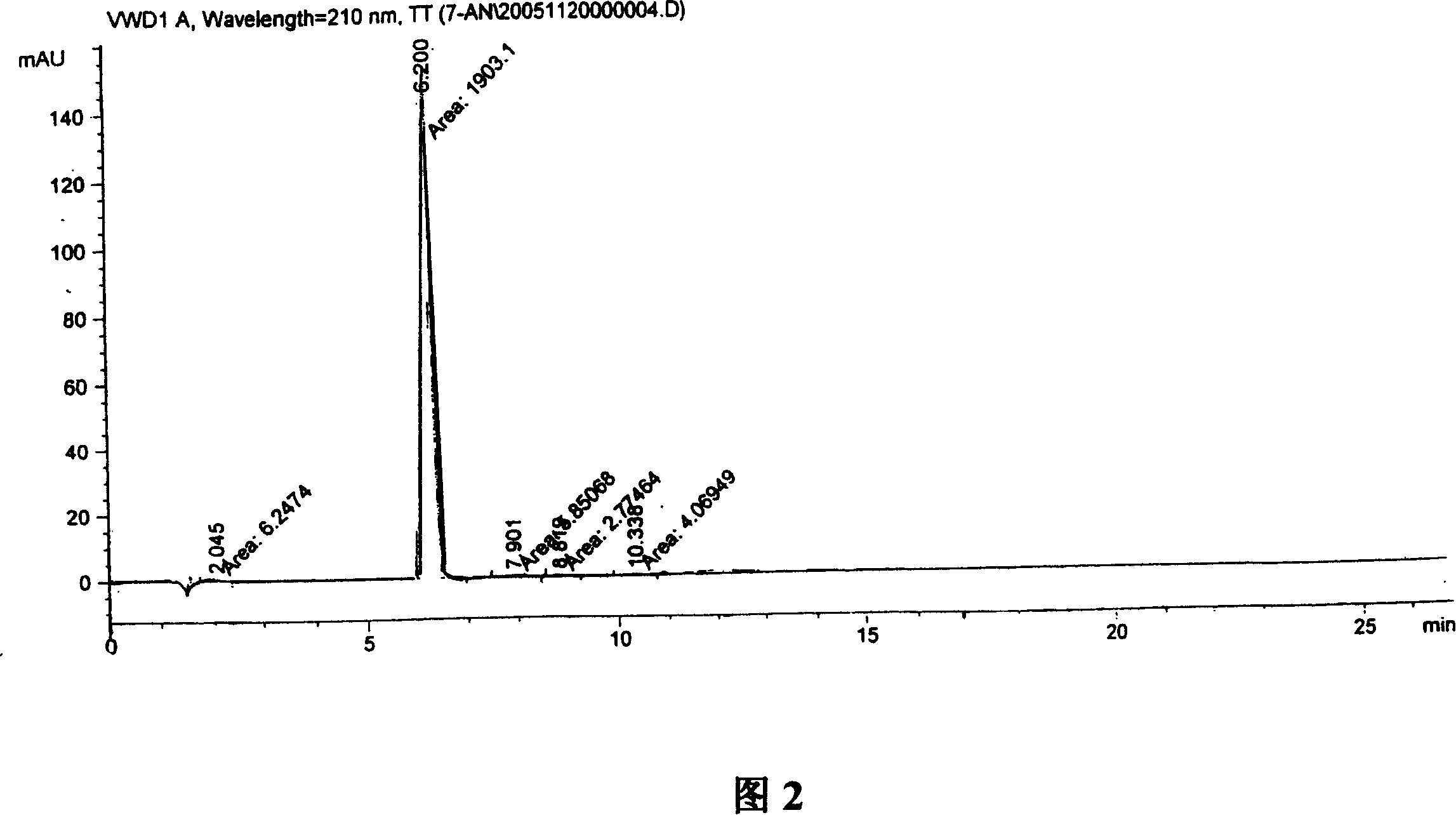
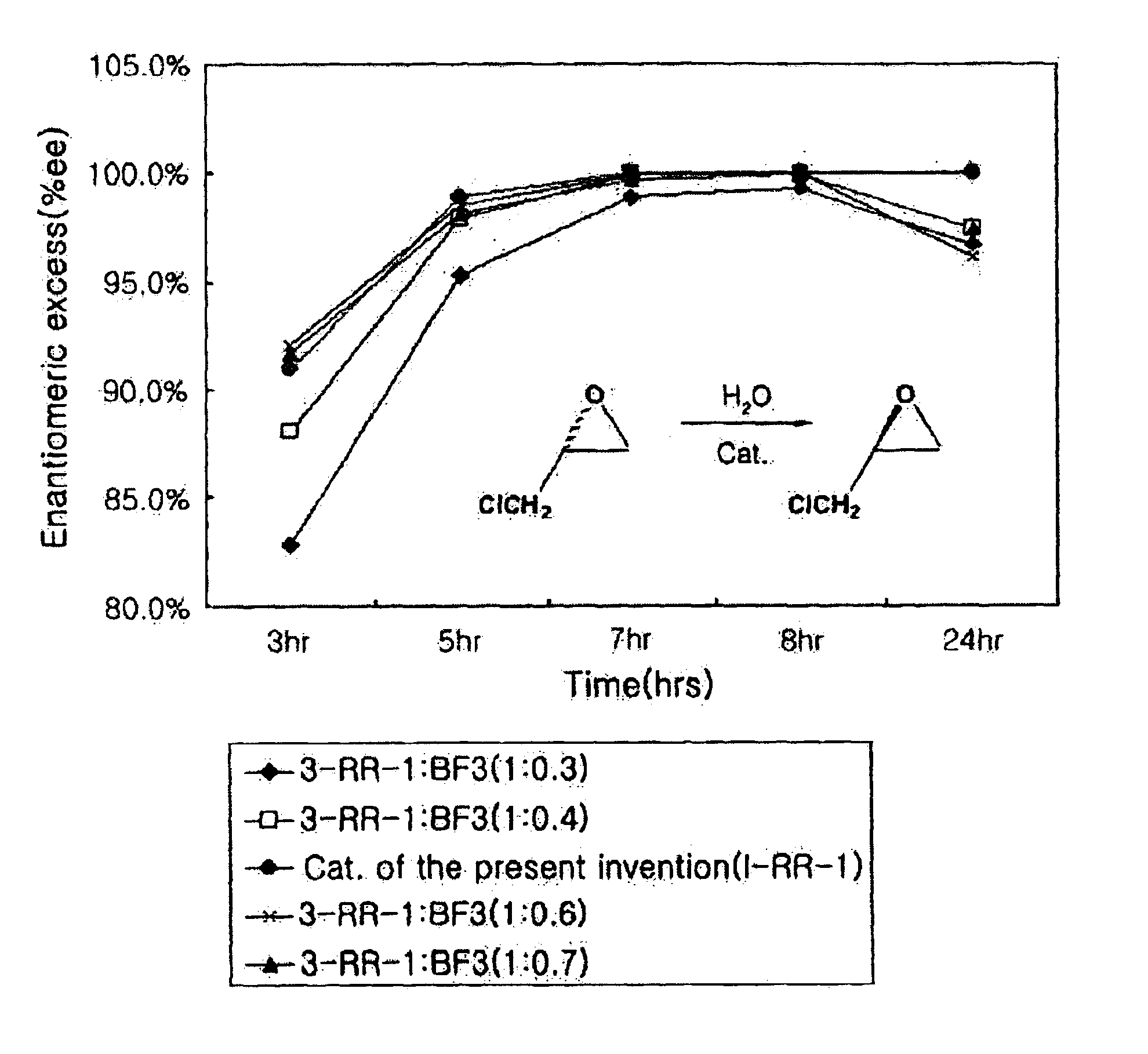
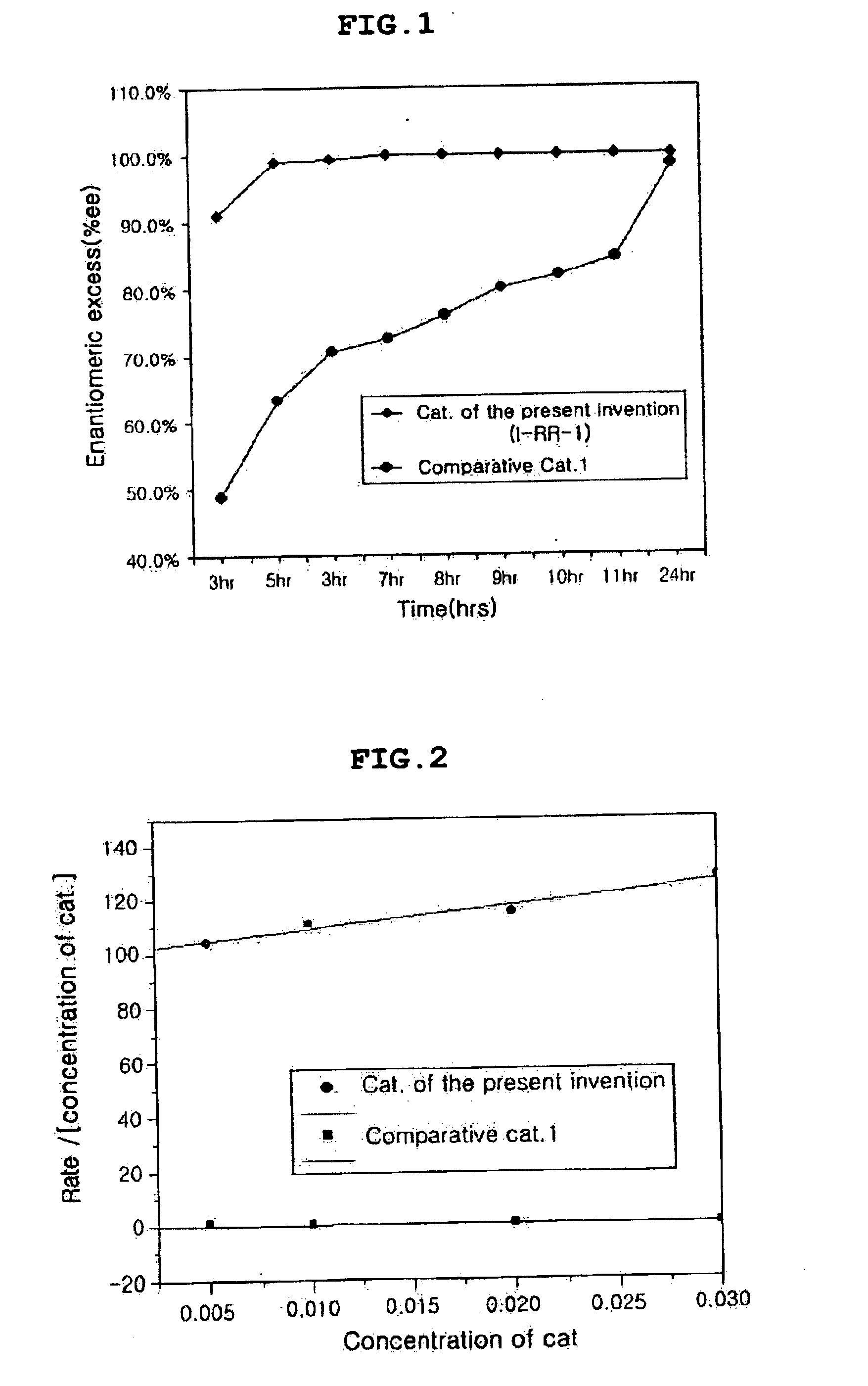
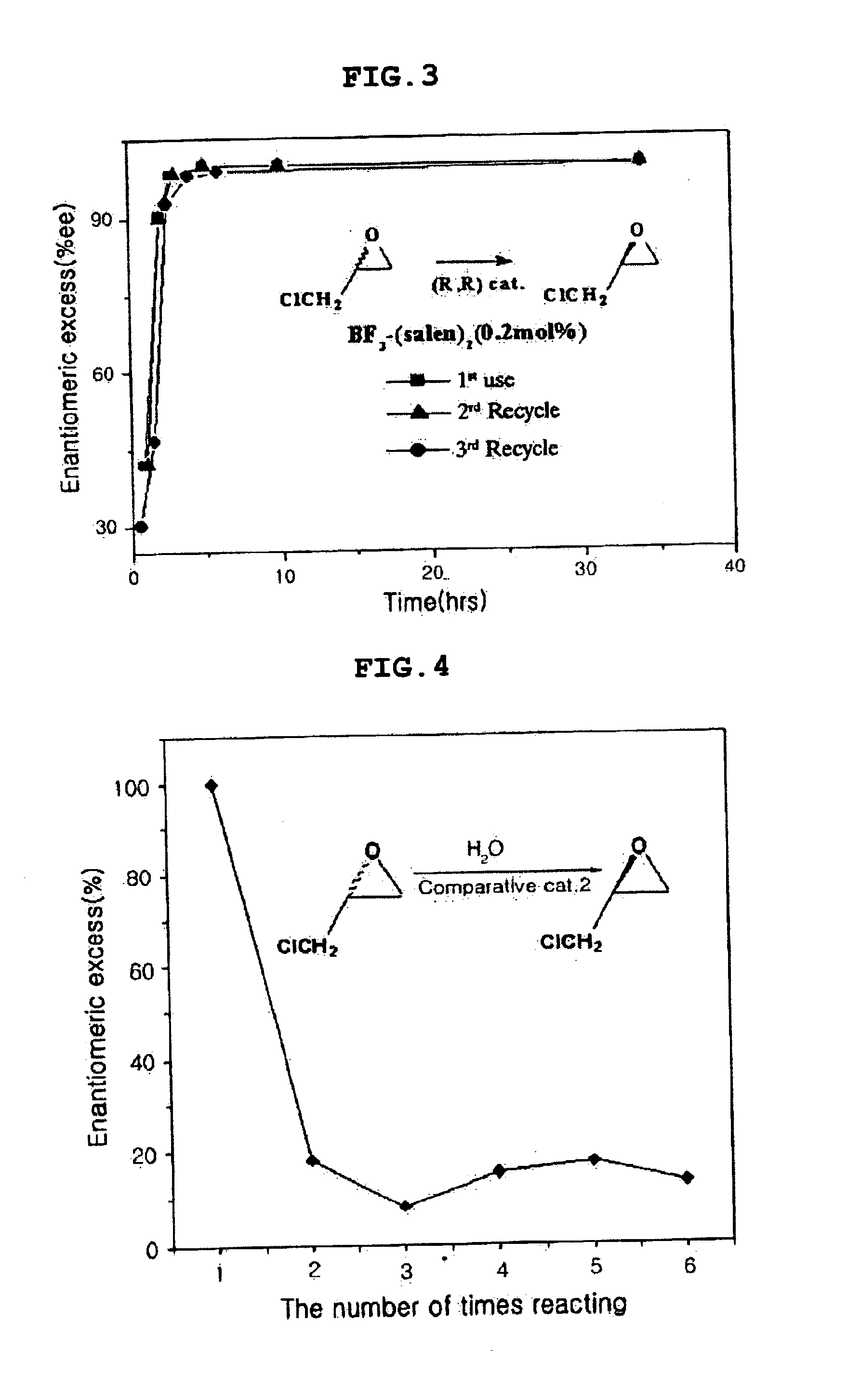
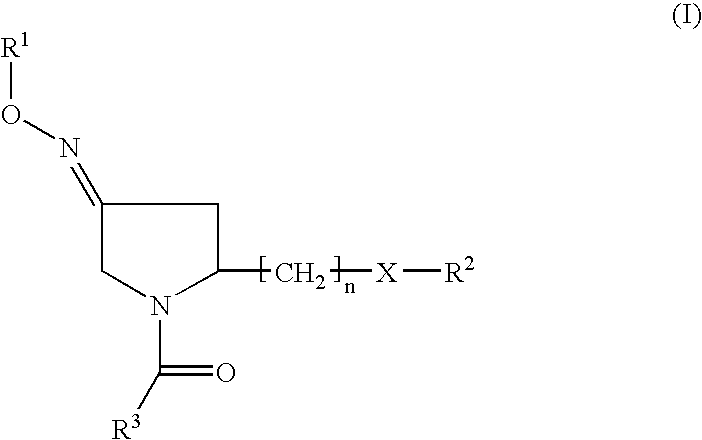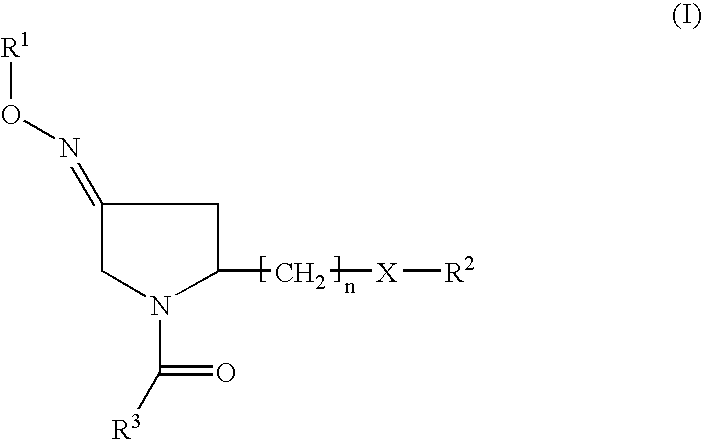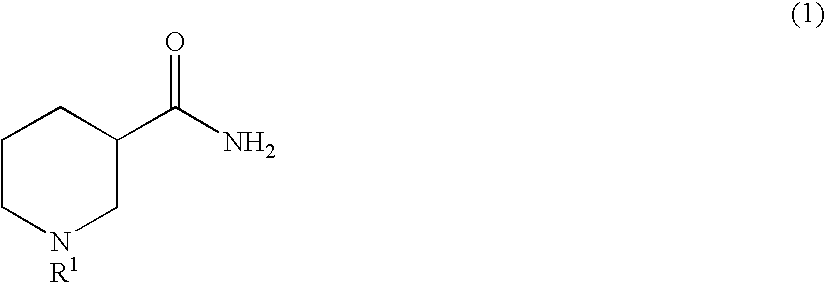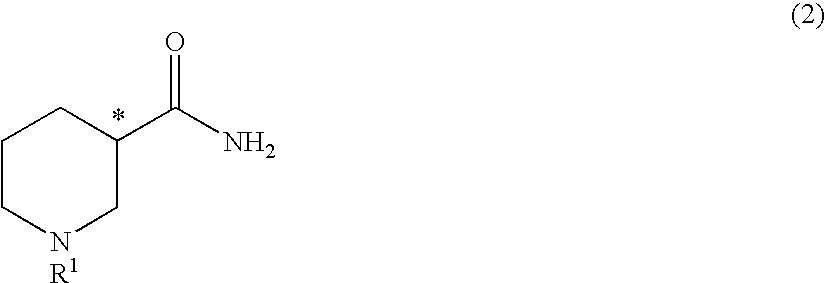Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1545 results about "Racemization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, Racemization is a conversion, by heat or by chemical reaction, of an optically active compound into an optically inactive form which half of the optically active substance becomes its mirror image (enantiomer) referred as racemic mixtures(i.e. contain equal amount of '+' and '-' forms) .If the racemization results in a mixture where the D and L enantiomers are present in equal quantities, the resulting sample is described as a racemic mixture or a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects.
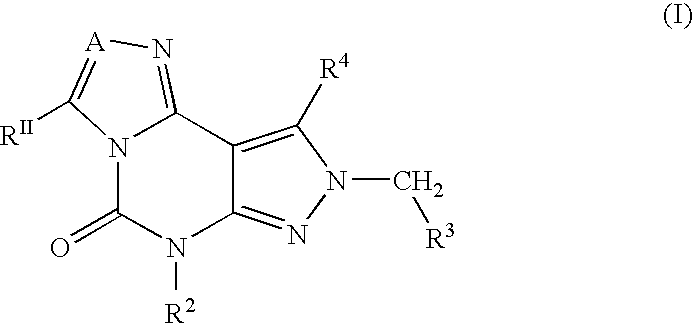
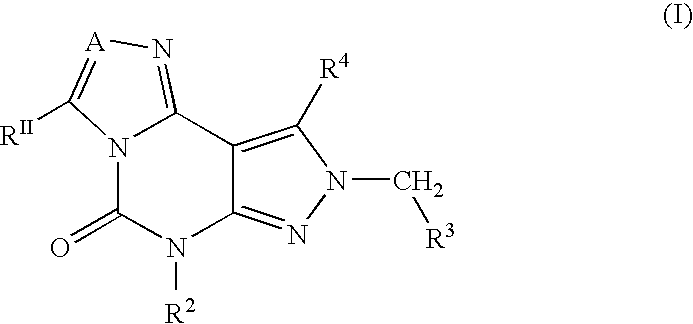
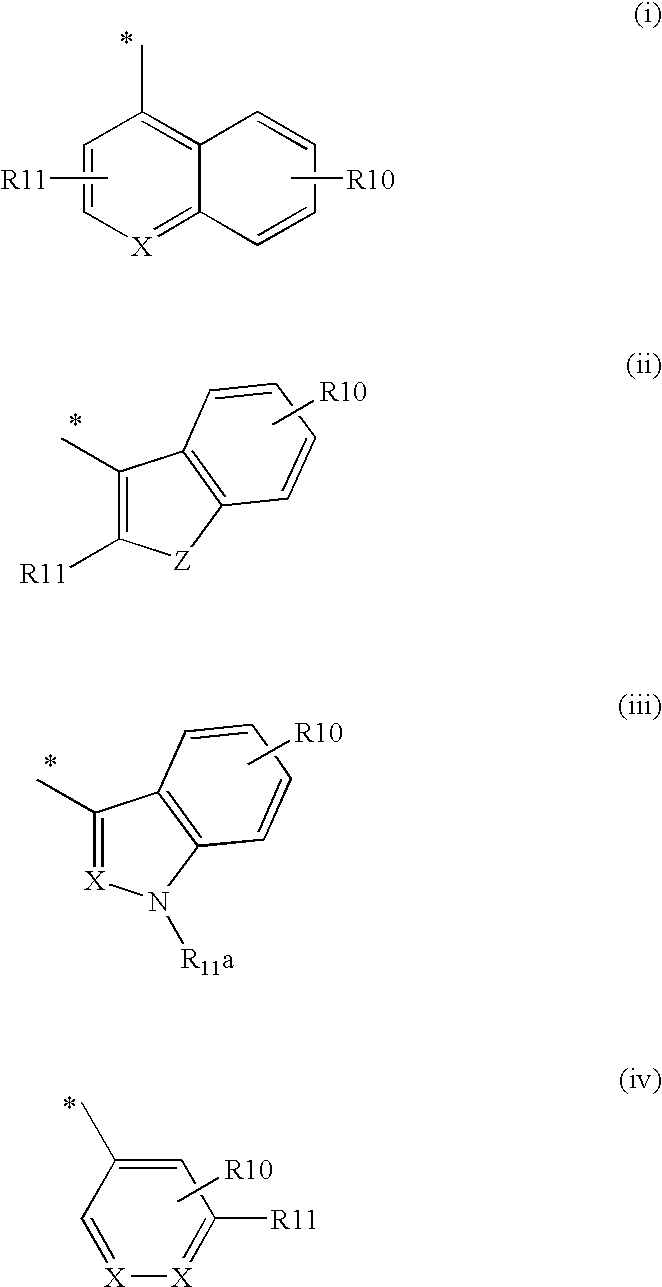
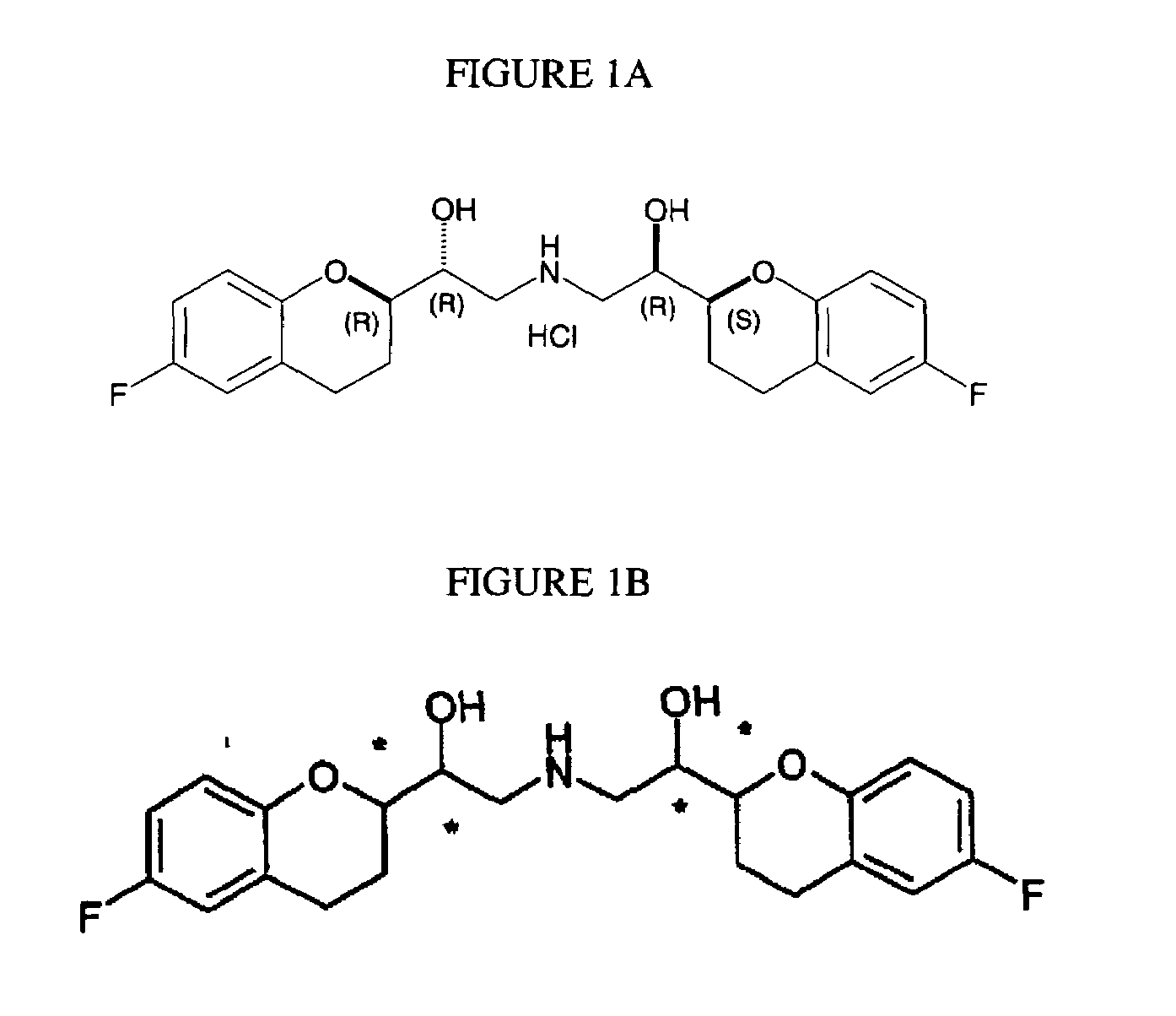
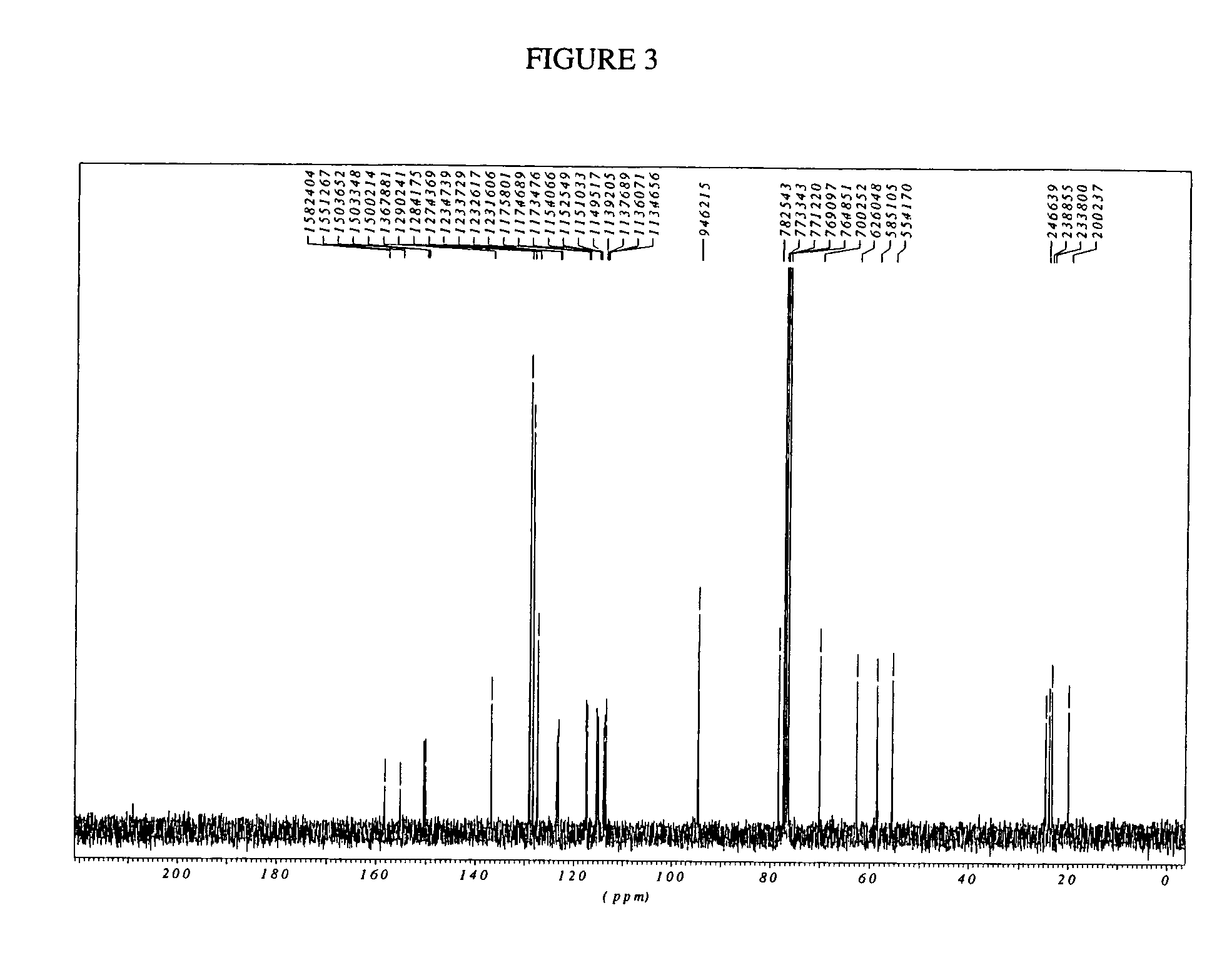
2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof
Disclosed are 2-arylimidazo[1,2-a]pyridine-3-acetamide derivatives represented by formula I, their tautomer, racemate or optical isomer, their pharmaceutically acceptable salt, or their solvates, wherein R1, R2, R3 and R4 are defined as in the specification. Preparation methods of said compounds and use of said compounds in treating and / or preventing central nervous system disease associated with TSPO functional disorder
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
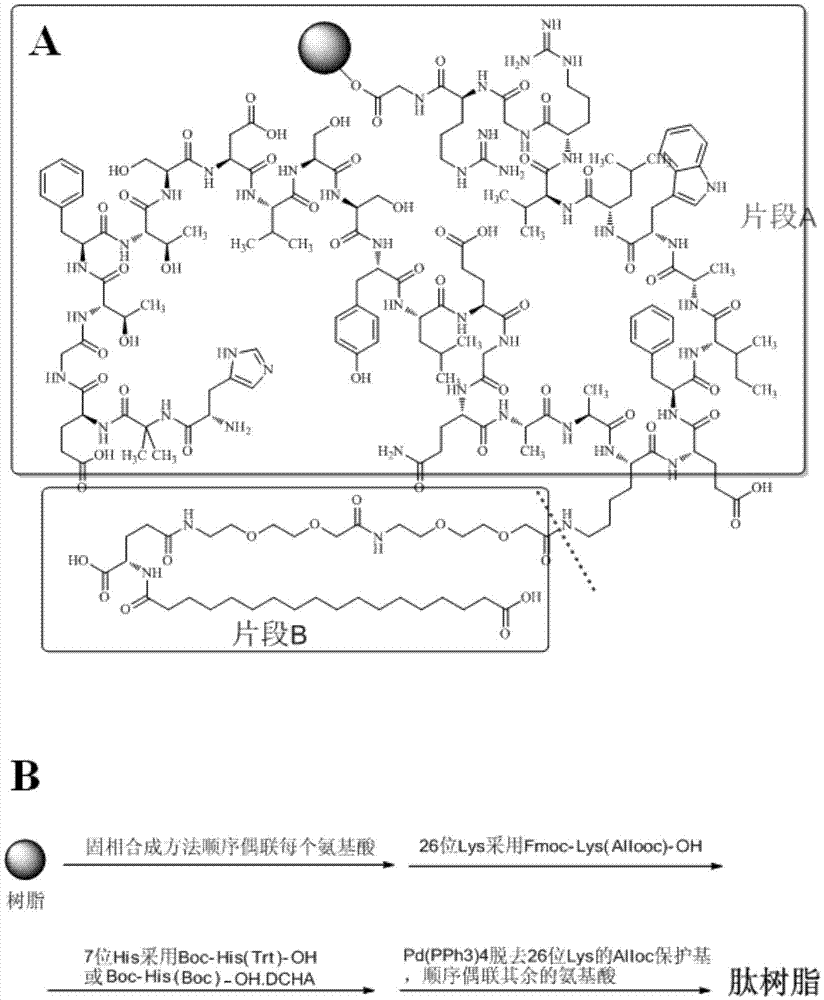
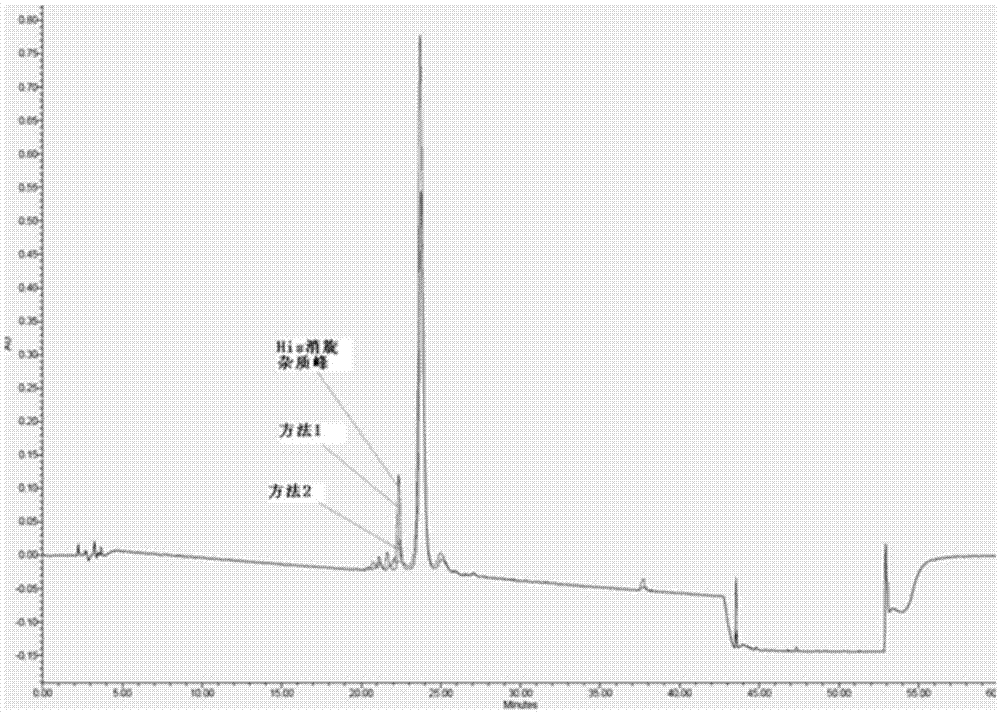
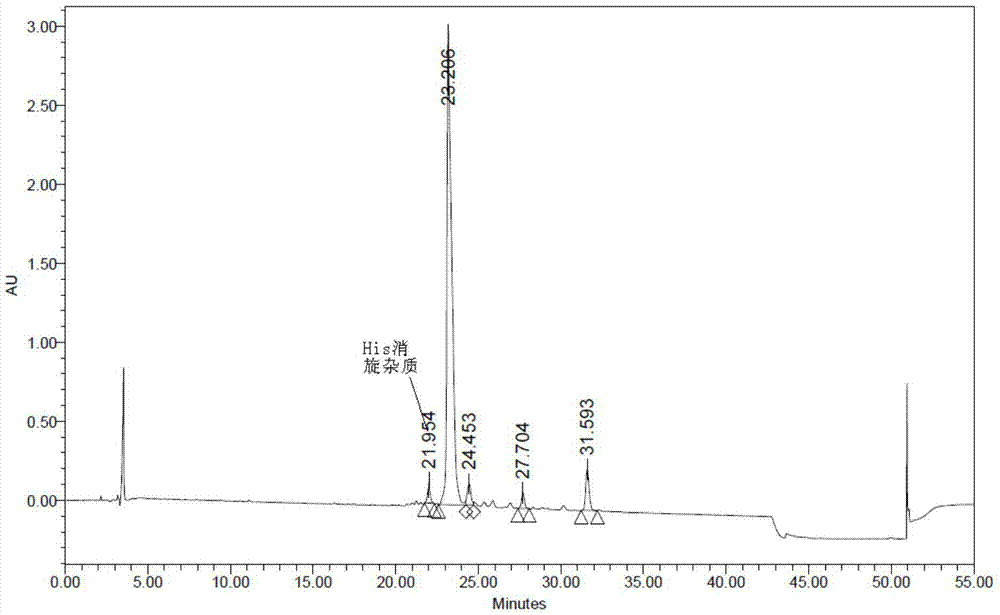
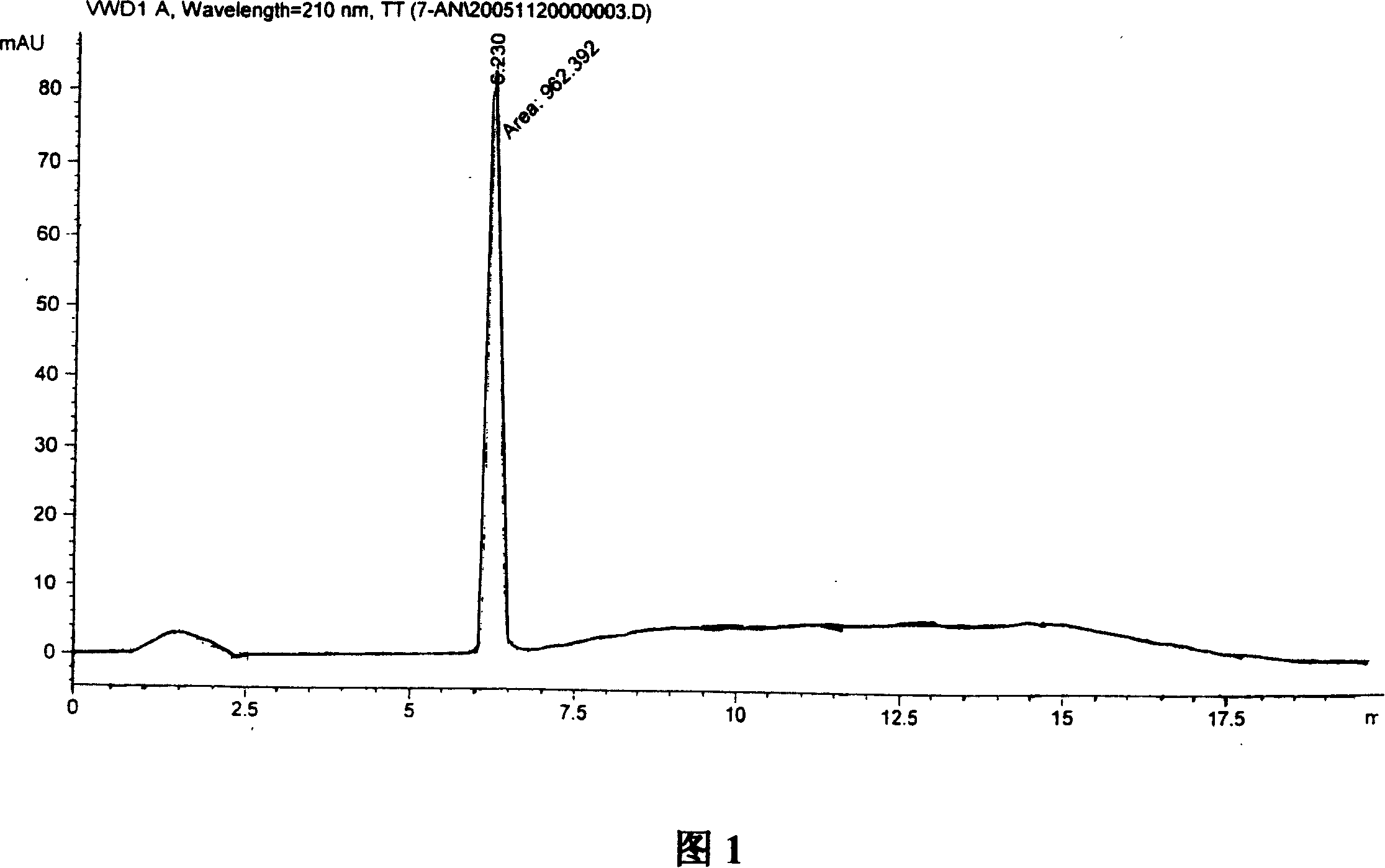
Method for preparing semaglutide
InactiveCN106928343AEasy to operateNo side effectsPeptide-nucleic acidsPeptide preparation methodsSide reactionSemaglutide
The invention relates to the field of polypeptides, in particular to a method for preparing semaglutide. The method has the advantages that Fmoc-Lys (Alloc)-OH protection amino acid is used as a raw material, de-protection is carried out by the aid of selected Pd (PPh3) 4, accordingly, operation procedures are simple, only 1-2 times of simple elimination reaction operation are required, each elimination reaction operation is carried out for 10-30 min, side reaction is prevented, the operation procedures are safe, and enlarged production can be facilitated; Boc-His (Boc)-OH. DCHA and Boc-His (Trt)-OH are used as raw materials in the procedures, and accordingly His racemization risks can be reduced to the greatest extent; special fragments are coupled, and accordingly the synthesis efficiency can be improved.
Owner:HYBIO PHARMA
Synthesis, split and racemization of chirality medicament levetiracetam midbody (S)-(+)-2-amido butyramide hydrochlorate
ActiveCN101130504AReduce dosageLow costOrganic compound preparationCarboxylic acid amides optical isomer preparationButyramidePyrrolidine
The invention discloses a new synthesizing technique of chiral drug (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetamide ( left Piracetam) intermediate (S)-(+)-2-aminobutanamide hydrochlorate, which comprises the following steps: adopting 2-brobutyrate as initial raw material; aminating; esterifying; ammonolyzing; detaching; looping; obtaining the object compound; making mixed rotary free alkaline (+-)-2-aminobutanamide; adopting half-quantum resolution method to connect chemical detaching salt to evolve salt; removing the detaching agent through alkalization; obtaining the (S)-(+)-2-aminobutanamide hydrochlorate with optical activity; using the mother liquor to make the product. The invention improves the receiving rate and saves the cost of raw material with simply technical operation and low cost, which resolves the resolved mother liquor after racemic action again to reduce the pollution of environment, therefore fitting for industrialized manufacturing.
Owner:ABA CHEM CORP
Chiral salen catalyst and methods for the preparation of chiral compounds from racemic epoxides by using new catalyst
InactiveUS6884750B2High optical purityHigh activityOrganic-compounds/hydrides/coordination-complexes catalystsCobalt organic compoundsFood additiveEpoxide
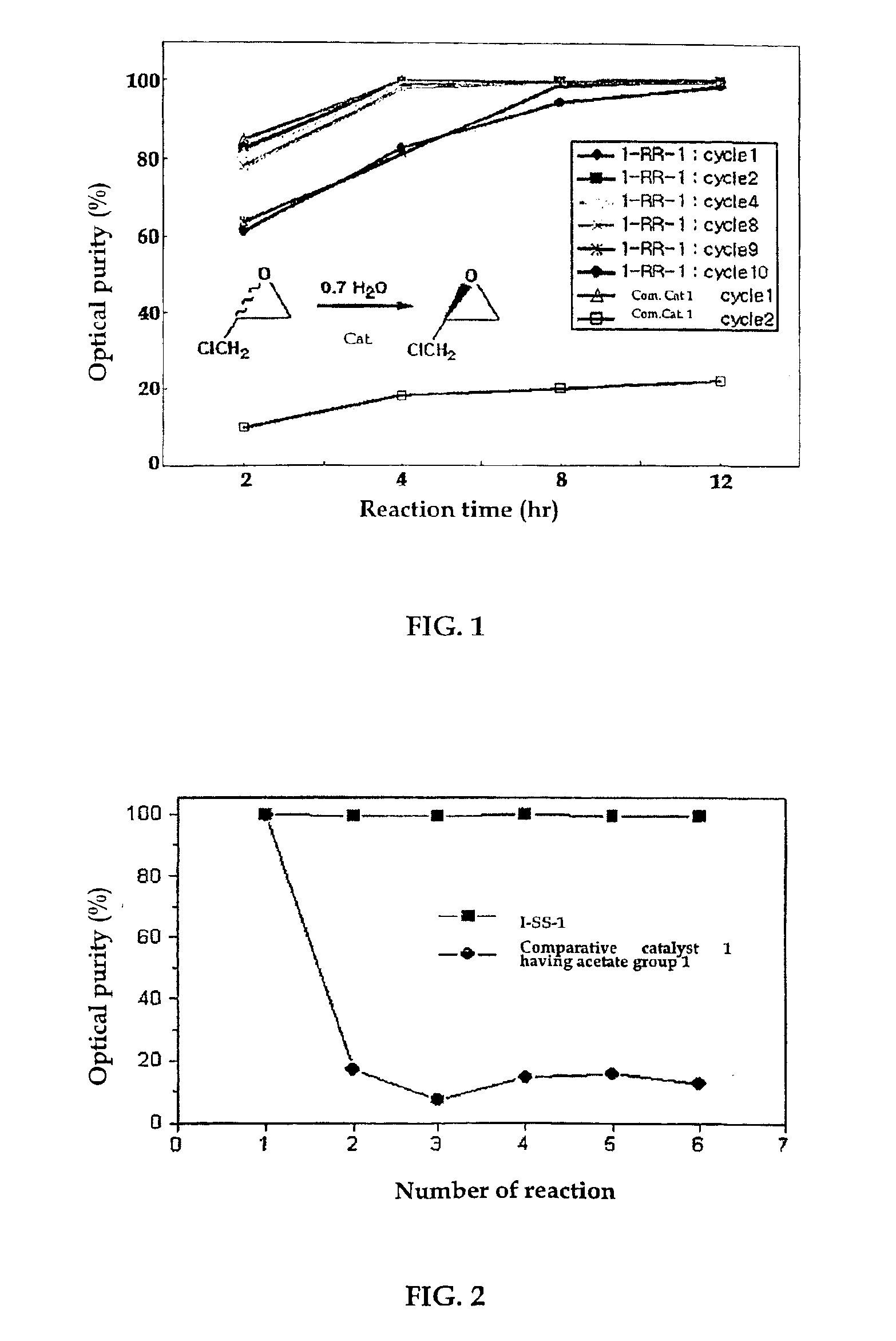
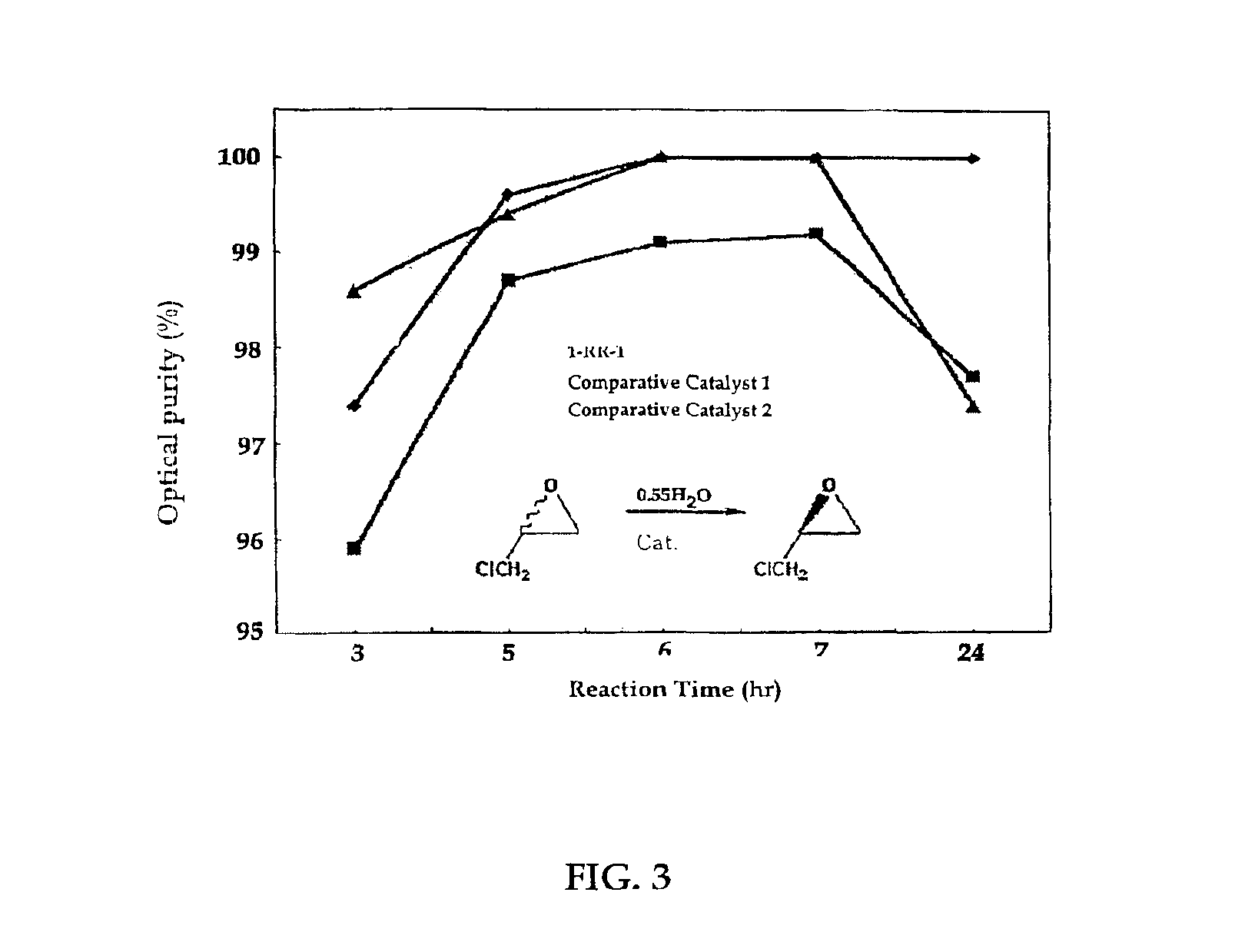
The present invention relates to new chiral salen catalysts and methods for the preparation of chiral compounds from racemic epoxides by using new catalyst. More particularly, the present invention is to provide novel chiral salen catalysts and their uses for producing chiral compounds having high optical purity to be used as raw materials for preparing chiral medicines or food additives in a large scale economically, wherein the chirl salen catalyst having a particular molecules structure can be reused continuously without any activating process of used catalysts and cause no or little racemization after the reaction is completed because it maintains its catalytic activity after the reaction process.
Owner:RS TECH CORP
Triazolo compounds as MMP inhibitors
InactiveUS20020151558A1Organic active ingredientsBiocideMedicinal chemistryMatrix metalloproteinase inhibitor
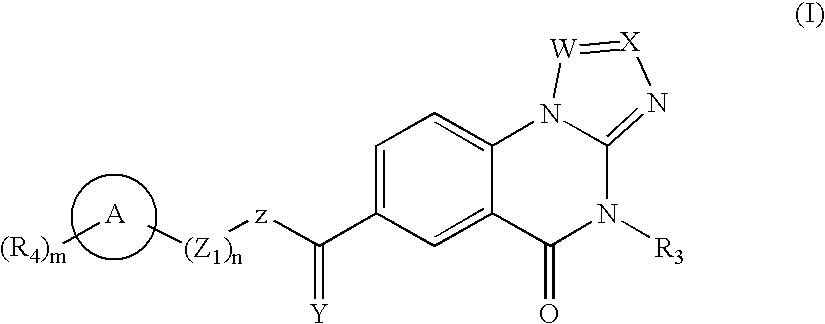
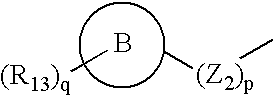
A compound selected from those of formula (I): in which: W represents N or C-R1; in which R1 is as defined in the description, X represents N or C-R2 in which R2 is as defined in the description, Y represents a group selected from oxygen, sulfur, -NH, and -Nalkyl, Z represents a group selected from oxygen, sulphur, -NR8 in which R8 is as defined in the description, and optionally carbon depending the definition of Y, n is an integer from 0 to 8 inclusive, Z1 represents a group -CR9R10 wherein R9 and R10, are as defined in the description, which group contains optionally multiple bonds or heteroatomes, A represents a cyclic group, m is an integer from 0 to 7 inclusive, the group(s) R4 is (are) as defined in the description, R3 represents a group selected from hydrogen, alkyl, alkenyl, alkynyl, and the group of formula: in which p, Z2, B, q, and R13 are as defined in the description, optionally, its racemic forms, isomers thereof, N-oxydes thereof, and its the pharmaceutically acceptable salts thereof, and medicinal products containing the same are useful as specific inhibitors of type-13 matrix mettaloprotease.
Owner:WARNER-LAMBERT CO
Ketone bodies and ketone body esters as blood lipid lowering agents
ActiveUS9211275B2Reduce serum cholesterol and/or triglyceride levelLowering of total serum cholesterol levelHydroxy compound active ingredientsMetabolism disorderChemistryNutritional composition
The subject disclosure provides compositions for reducing serum cholesterol and / or triglyceride levels in subjects. These compositions can comprise racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol or R-1,3 butandiol alone and can be, optionally, administered in conjunction with a low fat diet to a subject. Alternatively, compositions comprising racemic β-hydroxybutyrate or D-β-hydroxybutyrate, optionally in the acid form, physiologically compatible salts of racemic β-hydroxybutyrate or D-β-hydroxybutyrate, esters of D-β-hydroxybutyrate, oligomers of D-β-hydroxybutyrate containing from 2 to 20 or more monomeric units in either linear or cyclic form, racemic 1,3 butandiol, R-1,3 butandiol or combinations thereof can be formulated as nutritional supplements (also referred to as nutritional compositions) or incorporated into therapeutic compositions containing a) anti-hypertensive agents; b) anti-inflammatory agents; c) glucose lowering agents; or d) anti-lipemic agents) which are administered to a subject, optionally in combination with a low fat diet, in order to cause a reduction or lowering of: serum cholesterol levels; triglyceride levels; serum glucose levels, serum homocysteine levels, inflammatory proteins (e.g., C reactive protein) and / or hypertension in treated subjects. Alternatively, compositions disclosed herein can be administered alone, or in combination with other therapeutic agents to prevent or reverse vascular disease.
Owner:OXFORD UNIV INNOVATION LTD +1
Chiral polymeric salen catalyst, and a process for preparing chiral compounds from racemic epoxides by using them
InactiveUS6903043B2Avoid deactivationEasy to produceRuthenium organic compoundsOrganic compound preparationDiolHydrolysis
The present invention relates to chiral salen catalysts and a process for preparing chiral compounds from racemic epoxides by using them. More particularly, the present invention is to provide a chiral polymeric salen catalyst and its use for producing chiral compounds such as chiral epoxides and chiral 1,2-diols economically in high yield and high optical purity by performing stereoselective hydrolysis or racemic epoxides.
Owner:RSTECH CO LTD
Modafinil compositions
InactiveUS20070021510A1Improve solubilityHigh dissolution rateBiocideOrganic active ingredientsSolubilityMedicine
Co-crystals and solvates of racemic, enantiomerically pure, and enantiomerically mixed modafinil are formed and several important physical properties are modulated. The solubility, dissolution, bioavailability, dose response, and stability of modafinil can be modulated to improve efficacy in pharmaceutical compositions.
Owner:CEPHALON INC
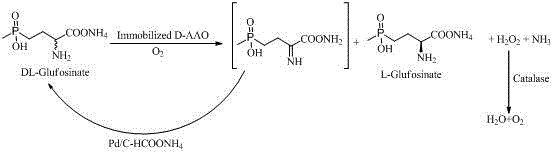
Enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium
The invention discloses an enzyme-chemocatalysis racemization removing preparation method for L-glufosinate-ammonium. According to the method, a one-pot reaction manner is adopted, under the molecular oxygen, immobilization D-amino acid oxidase catalyzes D-enantiomer in an enantioselectivity mode into 2-imino-4-(hydroxy methyl phosphonyl) butyric acid in a dehydrogenation mode, and palladium-ammonium formate catalyzes 2-imino -4-(hydroxy methyl phosphonyl) butyric acid into DL-glufosinate-ammonium in an in-situ reduction mode. Hydrogen peroxide produced in the process is efficiently decomposed into water and oxygen through catalase. Complete reacemization removing of DL-glufosinate-ammonium and efficient preparing of L-glufosinate-ammonium are achieved through biological oxidation-chemical reduction circulation. The method has the advantages that the process is simple, cost is low, environmental friendliness is achieved, and energy is saved. High-concentration DL-glufosinate-ammonium can be converted into L-glufosinate-ammonium. The yield is 90%, the optical purity of the product is 99%, and the method is suitable for industrial production of L-glufosinate-ammonium.
Owner:重庆惠健生物科技有限公司
Microorganism of producing D-pantothenic acid enternal ester hydrolase and process for preparing D-pantothenic acid thereof
The invention relates to a method used microorganism enzyme resolution DL-pantoic acid lactone to produce D-pantoic acid. It uses the D-pantoic acid lactone hydrolase strain of the fusarium, gibberella, aspergillus, penicillium, rhizopus, gliocladium, aureobasidium to ferment and culture, uses wet thallus as coarse enzyme, DL-pantoic acid lactone as substrate to produce D-pantoic acid. L- pantoic acid lactone can be reclaimed. The DL-pantoic acid lactone gained by racemization reaction can newly be used to do resolution.
Owner:重庆鑫富化工有限公司 +1
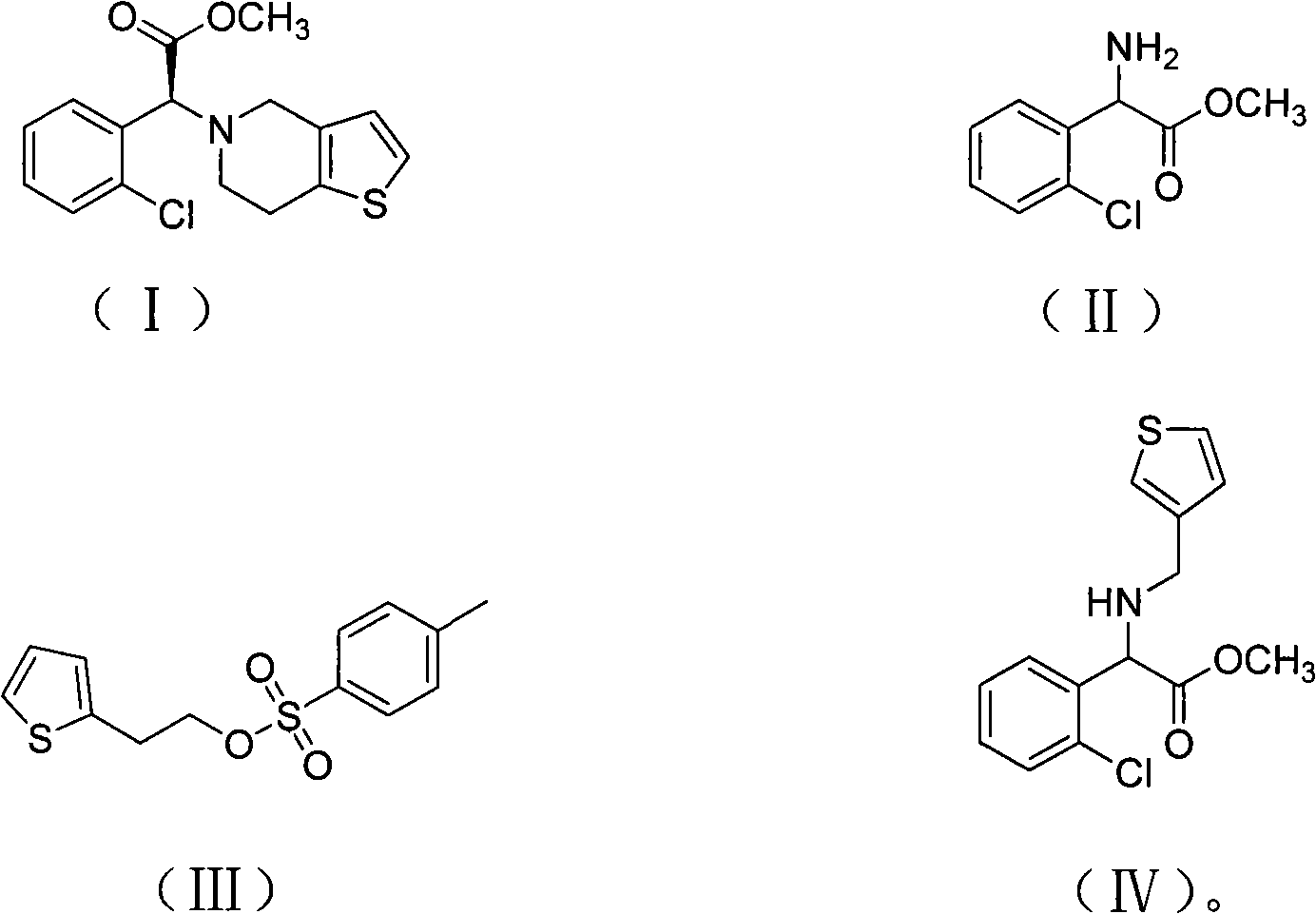
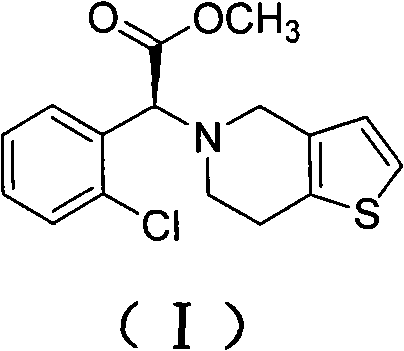
Method for preparing clopidogrel and salts thereof
The invention provides a preparation method for clopidogrel and the salts of clopidogrel, belonging to the field of medical and chemical technology. The invention solves the problems of more reaction steps, long technical route, high cost and low purity of the existing preparation method for clopidogrel. The preparation method for clopidogrel comprises the following steps: a. synchronous resolution and racemization; b. preparation of (plus) alpha-(2 - thiophene triethylamine yl) -2 - (2 - chlorophenyl) methyl acetate; c. preparation of target product clopidogrel through cyclization reaction. The clopidogrel can generate medicinal salt with acids in a solvent. The preparation method for clopidogrel and the salts of clopidogrel has simple technology, easy operation, lower costs and higher product purity.
Owner:江苏八巨药业有限公司
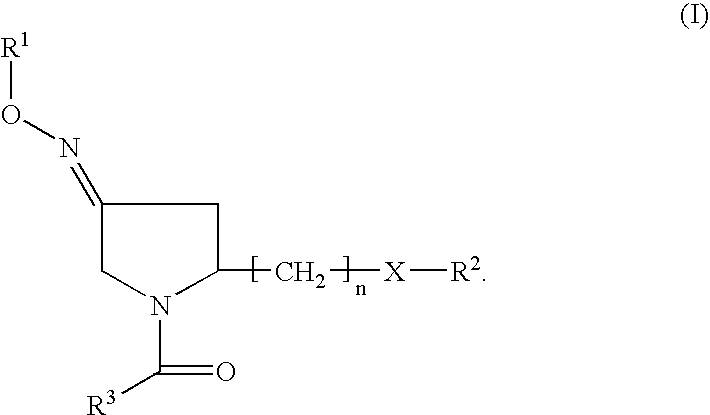
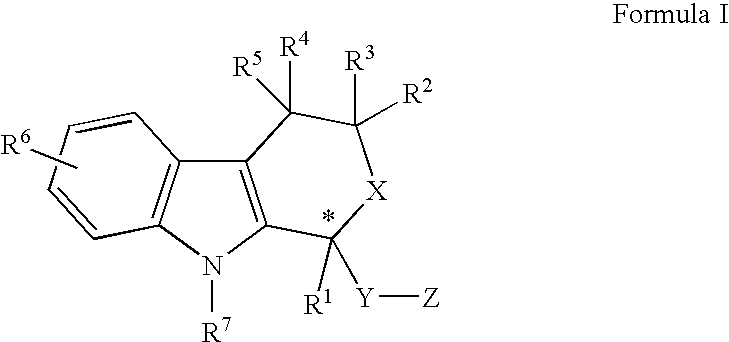
Pyrrolidine derivatives as oxytocin antagonists
The present invention relates to novel pyrrolidine derivative of formula (I), its geometrical isomers, its optically active forms as enantiomers, diastereomers, mixtures of these and its racemate forms, as well as salts thereof, wherein R1 is selected from the group comprising or consisting of H and C1–C6-alkyl, for the prevention and / or treatment of preterm labor, premature birth or dysmenorrhea
Owner:MERCK SERONO SA
Fused heterocycles as inhibitors of glutamate racemase(MURI)
Owner:ASTRAZENECA AB
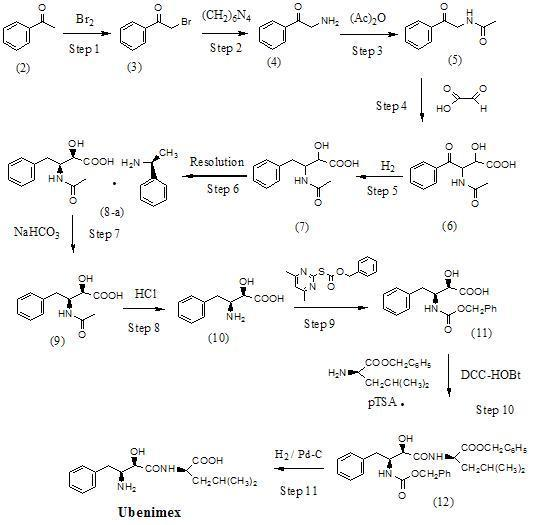
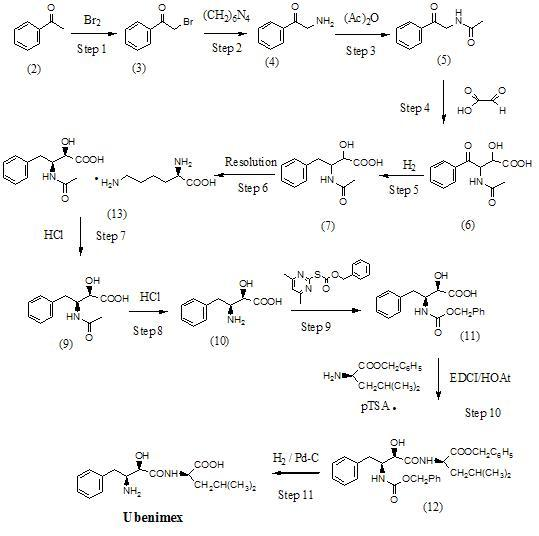
Preparation method for ubenimex
ActiveCN101891647AOrganic compound preparationCarboxylic acid amides optical isomer preparationArginine3-amino-2-hydroxy-4-phenylbutyric acid
The invention belongs to the field of antineoplastic agent preparation and provides a preparation method for ubenimex. The preparation method comprises the following steps of: preparing high-purity key intermediate (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid by taking L-lysine, L-arginine or L-histidine as a resolving reagent; and preserving the chirality of C-5 in forming a peptide chain by taking EDCI / HOAt as a condensing agent. Due to the adoption of the method, the problem that the (2S,3R)-3-amino-2-hydroxy-4-phenyl butyric acid and the (2S,3R)-3-acetamino-2-hydroxy-4-phenyl butyric acid cannot be completely separated by the conventional resolving agent and the racemization problem in the condensation of amide are effectively solved; and the purity of the ubenimex prepared by the method can reach over 99.5 percent.
Owner:ZHEJIANG APELOA KANGYU PHARMA +1
Stereoisomers of p-hydroxy-milnacipran, and methods of use thereof
The present invention relates generally to the enantiomers of para-hydroxy-milnacipran or congeners thereof. Biological assays revealed that racemic para-hydroxy-milnacipran is approximately equipotent in inhibiting serotonin and norepinephrine uptake (IC50=28.6 nM for norepinephrine, IC50=21.7 nM for serotonin). Interestingly, (+)-para-hydroxy-milnacipran is a more potent inhibitor of norepinephrine uptake than serotonin uptake (IC50=10.3 nM for norepinephrine, IC50=22 nM for serotonin). In contrast, (−)-para-hydroxy-milnacipran is a more potent inhibitor of serotonin uptake compared to norepinephrin uptake (IC50=88.5 nM for norepinephrine, IC50=40.3 nM for serotonin). The invention also relates to salts and prodrug forms of the aforementioned compounds. In certain embodiments, the compounds of the present invention and a pharmaceutically acceptable excipient are combined to prepare a formulation for administration to a patient. Finally, the present invention relates to methods of treating mammals suffering from various afflictions, e.g., depression, chronic pain, or fibromyalgia, comprising administering to a mammal in need thereof a therapeutically effective amount of a compound of the present invention.
Owner:COLLEGIUM PHARMA INC
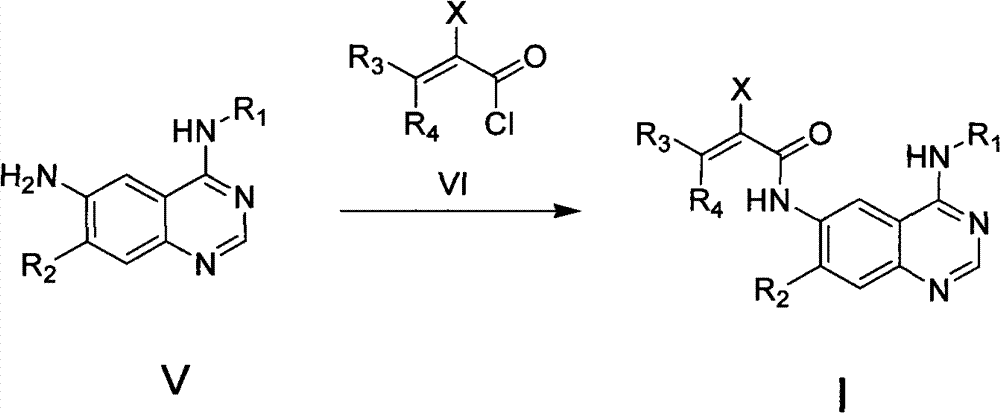
Quinazoline derivative, preparation method, intermediate, composition and application
ActiveCN102898386ASignificant inhibition of proliferationHas inhibitory effectOrganic active ingredientsGroup 5/15 element organic compoundsEnantiomerDiastereomer
The invention discloses a quinazoline derivative and a pharmaceutically acceptable salt thereof showed in a formula I, or their enantiomer, diastereoisomer, tautomer, racemate, solvate, metabolic precursor or prodrug. The invention also discloses a preparation method, an intermediate, a composition and an application. The quinazoline derivative has good antineoplastic activity.
Owner:SHANGAI PHARMA GRP CO LTD +1
Method for catalyzing dynamic kinetic resolution of arylamine via racemization catalyst
InactiveCN102533922AGood stability for repeated useMild reaction conditionsOrganic chemistry methodsChemical recyclingChlorobenzenePtru catalyst
The invention discloses a method for catalyzing dynamic kinetic resolution of arylamine via a racemization catalyst, comprising the following steps of: 1) adding p-chlorophenol, n-pentanoic acid, dicyclohexylcarbodiimide and 4-dimethylamino-pyridine, and carrying out mixing, filtration, drying, concentration and column chromatography to obtain a pentanoic acid p-chlorophenyl ester acyl donor; 2) carrying out coprecipitation on magnesium chloride solution and aluminum chloride solution and carrying out water-heat treatment to obtain chloridion intercalated hydrotalcite, adding the chloridion intercalated hydrotalcite in lauryl sodium sulfate aqueous solution, and carrying out backflow, cooling, centrifugation, water washing, acetone washing and drying to obtain a carrier; 3) adding palladium salt and the carrier, and carrying out heating, ascorbic acid addition, centrifugation, water washing, acetone washing and freeze-drying to obtain the racemization catalyst; and 4) adding arylamine, the acyl donor, lipase and the racemization catalyst in toluene and placing in a stainless steel reactor to add hydrogen so as to obtain amide. The method provided by the invention is used for catalyzing the dynamic kinetic resolution of arylamine, has rapid reaction rate, low temperature, high conversion rate and high product optical purity, and has great application value.
Owner:ZHEJIANG UNIV
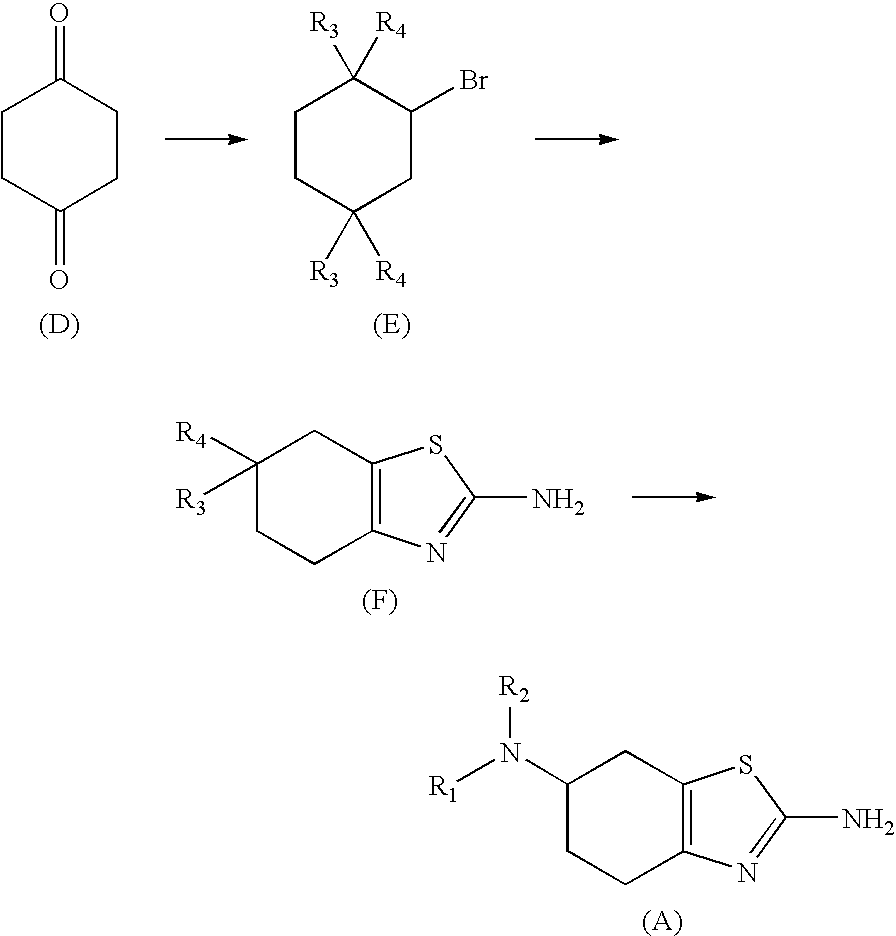
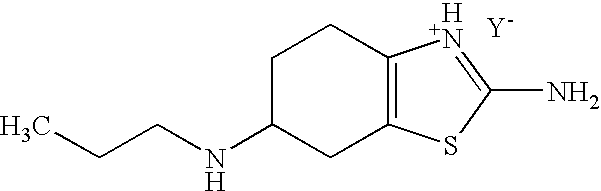
Method for the Resolution of 2-Amino-6-Propylamino-4,5,6,7-Tetrahydrobenzothiazol and Intermediate Compounds
InactiveUS20080194832A1Little reproducibilityHigh optical purityOrganic active ingredientsOrganic chemistryRacemic mixtureStereochemistry
The invention relates to a novel method for the resolution of the racemic mixture of compound (R,S)-2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, or the enrichment of same with in one of its enantiomers, and to intermediate compounds which can be used to perform said method.
Owner:CRYSTAL PHARMA SA
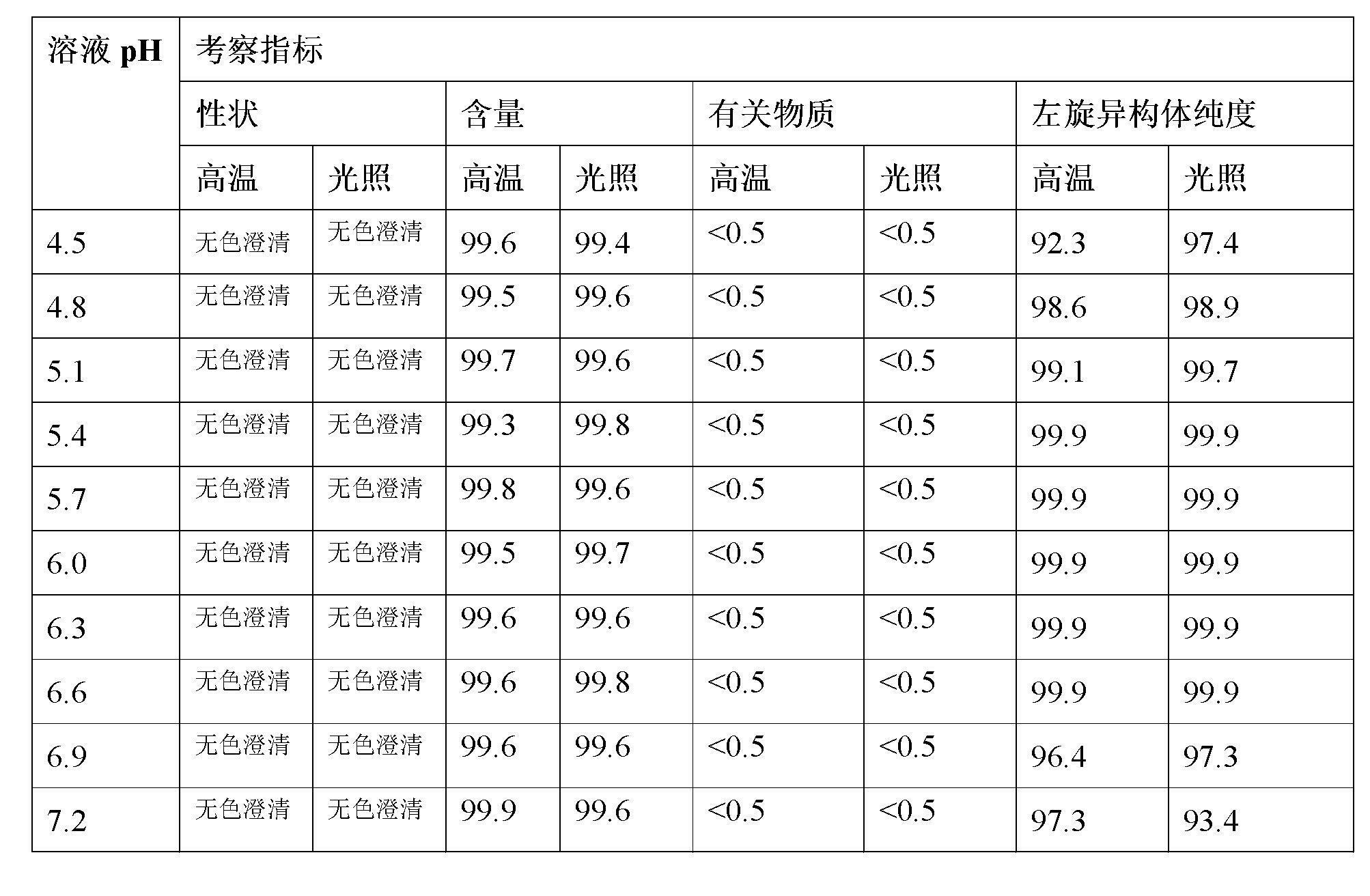
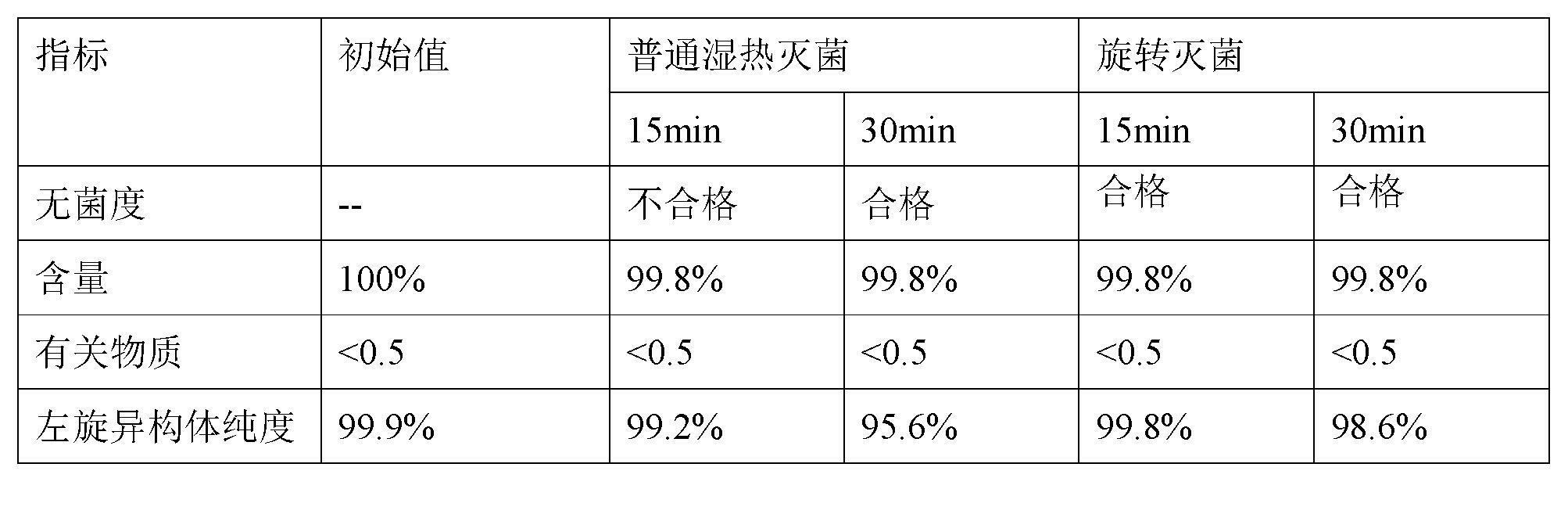
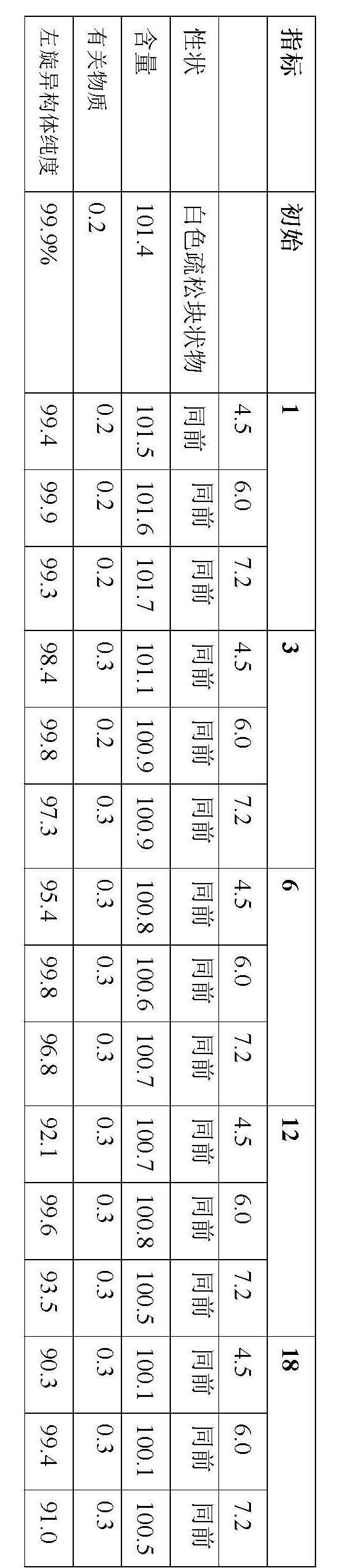
Stable S-oxiracetam preparation for injection and preparation method of same
The invention relates to a stable preparation for injection by taking S-oxiracetam as active ingredient. The preparation is a composition for injection, and is formed by the S-oxiracetam or salts thereof serving as active ingredient and pharmaceutically acceptable auxiliary material. To restrain the racemization of the S-oxiracetam, when the powder injection with the active ingredient is prepared, and only the pH value of the S-oxiracetam medicament solution ranges from 4.5 to 7.0, the pH value is between 5.4 to 6.6 preferably, so that the S-oxiracetam medicament solution can be produced, and the final freeze-dried product has acceptable long stability; and moreover, when injection is produced, a rotary steam sterilization method needs to be adopted, terminal rotating sterilization is performed for 15 to 45 minutes under the temperature of 121 DEG C, and the terminal rotating sterilization is performed for 15 to 20 minutes preferably, so that the S-oxiracetam injection with high purity can be obtained and has acceptable long stability.
Owner:FUKANGREN BIO PHARMA
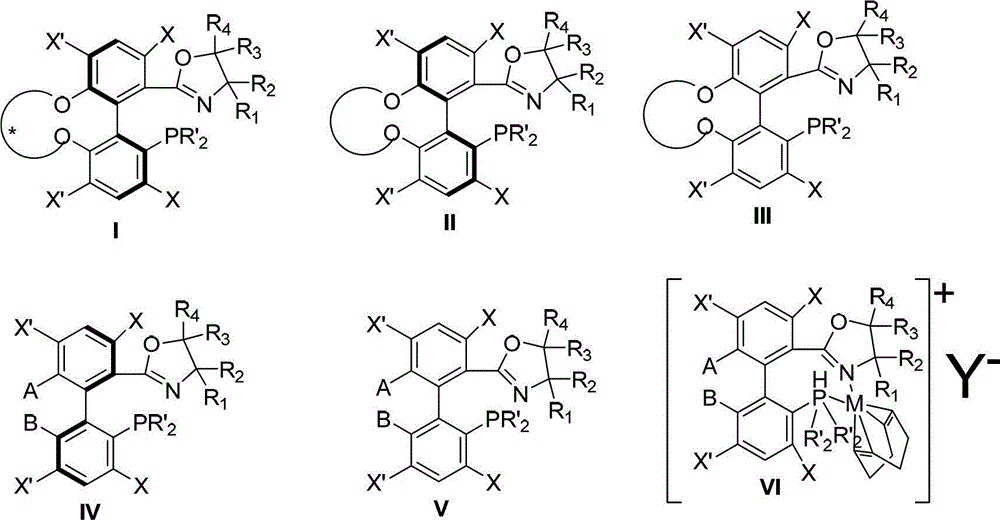
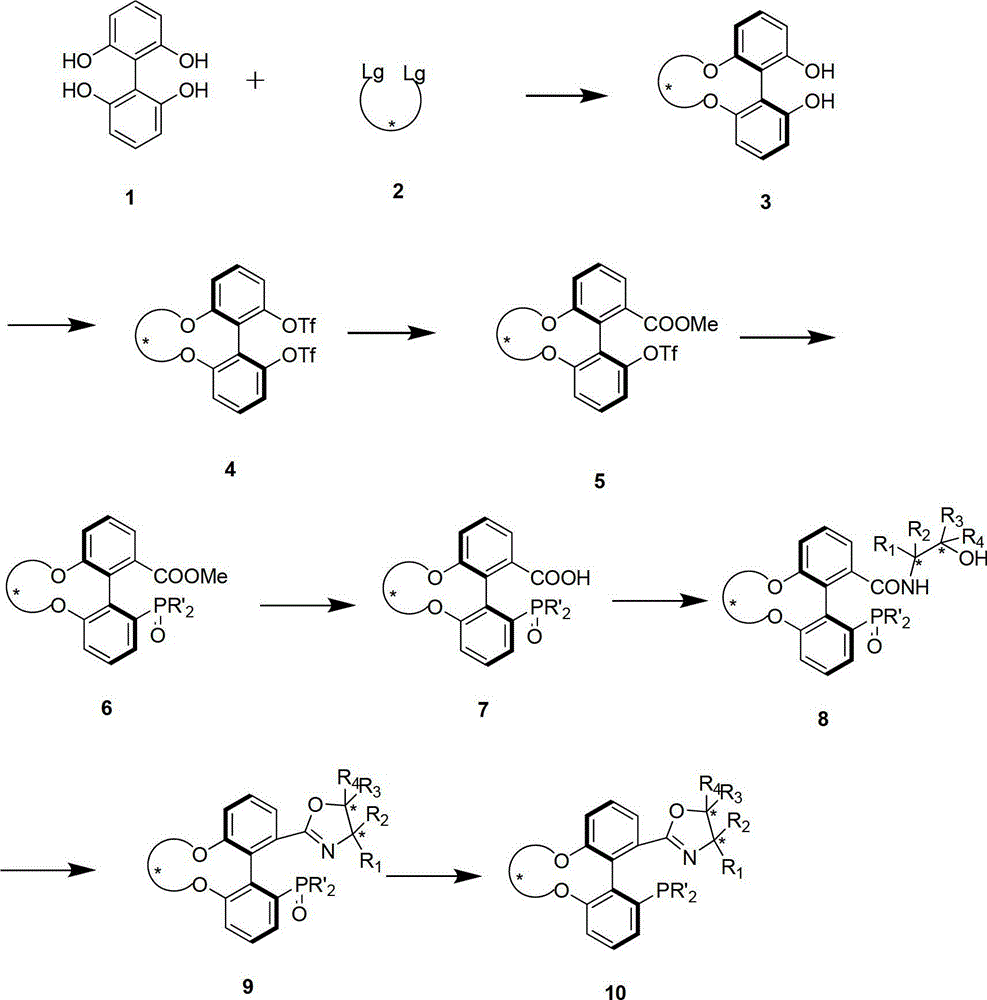
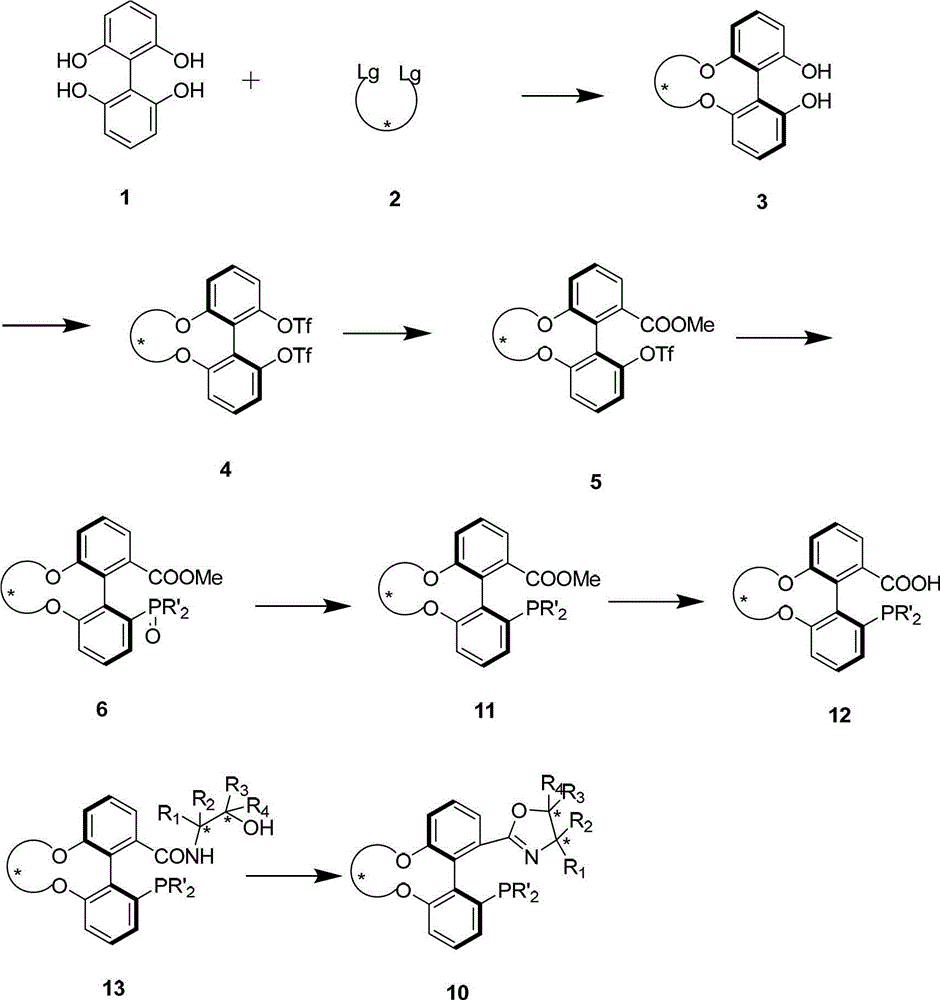
Preparation method and application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof
InactiveCN102875601AThe synthesis method is simpleSynthetic method is economicalOrganic compound preparationGroup 5/15 element organic compoundsPlanar chiralityStructural formula
The invention discloses a preparation method and an application for phosphine-oxazoline ligand, and ionic metal complex, enantiomer or racemate thereof. The ligand and the ionic metal complex thereof have the following structural formulas. The phosphine ligand related by the invention employs biphenyl as a skeleton, and realizes completely transmission from planar chirality to axial chirality through an asymmetric desymmerization. The synthetic method is simple and economic, omits a common and complex chiral separation process in the preparation of the chiral ligand. The obtained chiral ligand has the advantages of high reactive activity, good enantiomorphous selectivity and the like in a model reaction.
Owner:SUN YAT SEN UNIV
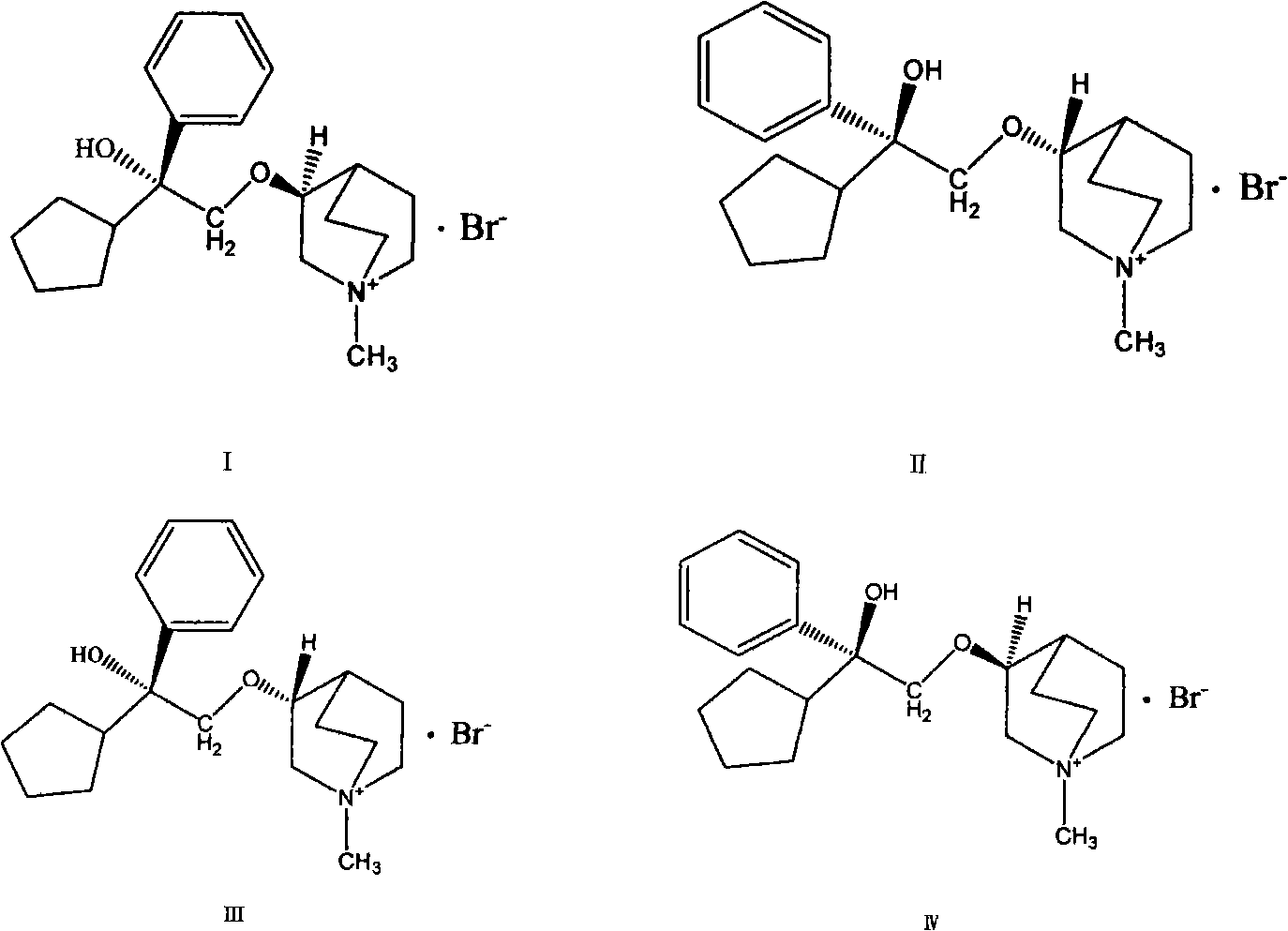
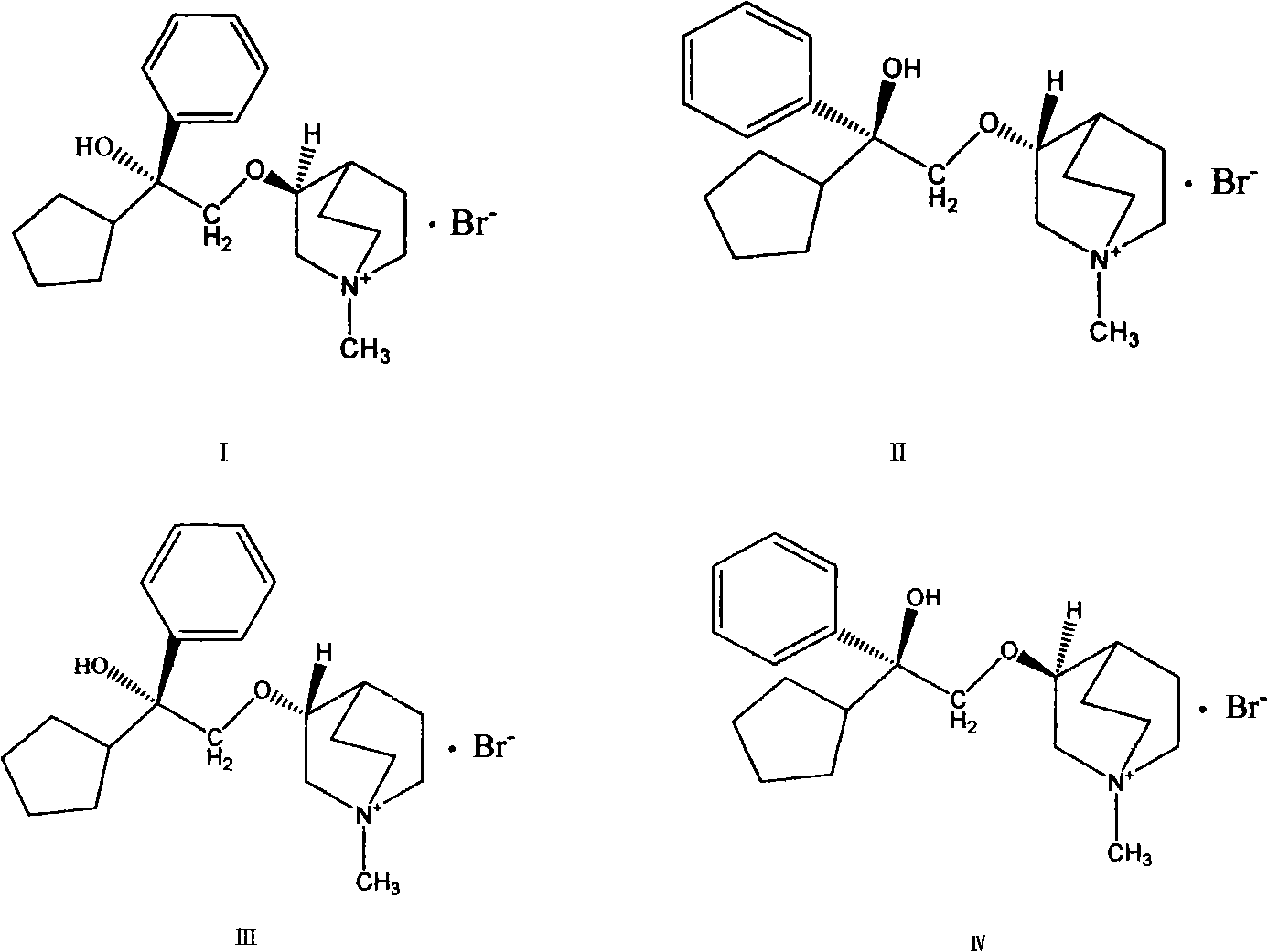
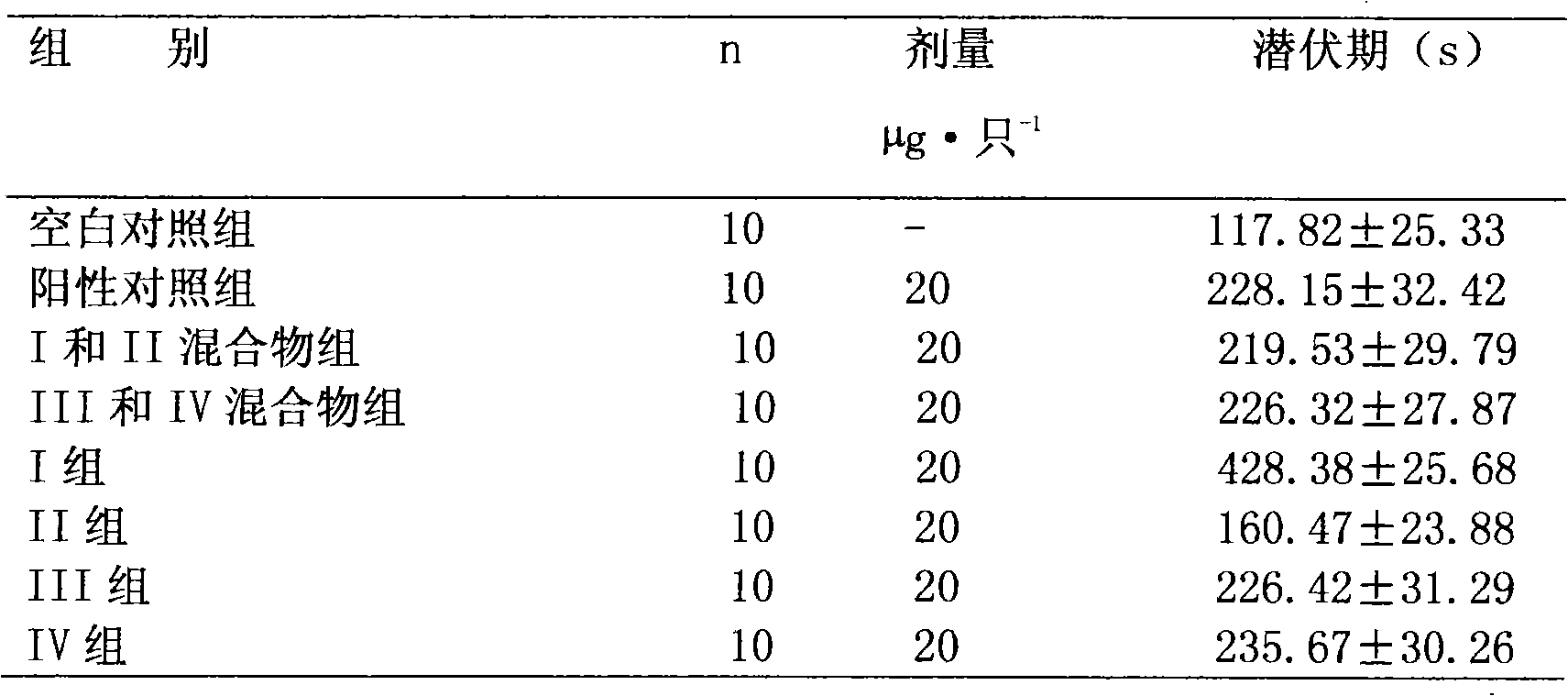
Preparation method and application of bencycloquidium bromide optical isomer and composition of bencycloquidium bromide optical isomer
The invention relates to the technical field of medicaments, in particular to a preparation method and application of a bencycloquidium bromide optical isomer. The isomer is obtained by a chemical resolution method, the activity of the isomer is far beyond that of other isomers and racemic forms; when the isomer is taken as a medicament to be taken, the taking dosage is reduced, and side effects caused by other isomers are simultaneously eliminated; moreover, the bencycloquidium bromide optical isomer has a romurtide cyclodextrin inclusion compound with higher pharmaceutical value and a preparation thereof. The romurtide is coated by the cyclodextrin, so that the problems of low water-solubility, unstable preparation prepared by the romurtide cyclodextrin inclusion compound and the like are solved. The romurtide cyclodextrin inclusion compound has the characteristics of high stability, good water-solubility, and low toxic and side effects, and is a quite good immunomodulator.
Owner:YINGU PHARMA
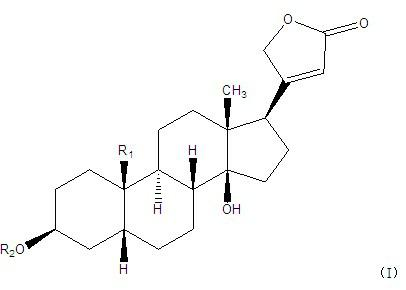
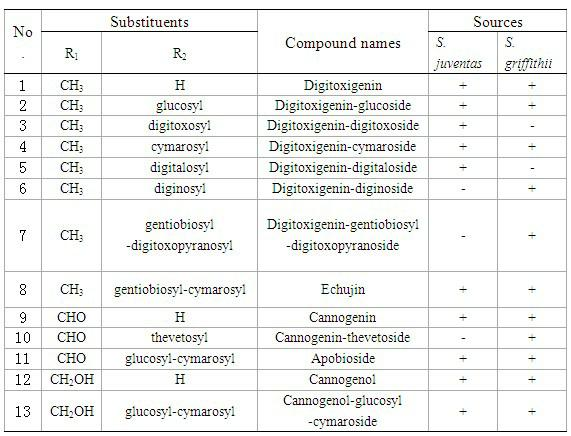
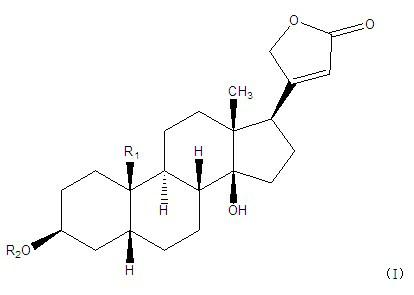
Cardiac glycoside compounds and antitumor application thereof
InactiveCN102219821AStrongly inhibits proliferative activityOrganic active ingredientsGlycoside steroidsBenzoic acidO-Phosphoric Acid
The invention belongs to the technical field of medicaments and in particular relates to cardiac glycoside compounds with a general formula (I), as well as derivatives, stereoisomers, a racemic mixture or a non-racemic mixture of the stereoisomers and pharmaceutically acceptable acid addition salts or solvates, wherein R1 is CH3, CHO or CH2OH; R2 is H or a linear saccharide chain or a branched saccharide chain, the linear saccharide chain or the branched saccharide chain is formed by saccharides; and these acids comprise inorganic acids like hydrochloric acid, sulfuric acid, hydrobromic acid, phosphoric acid, nitric acid, carbonic acid and the like as well as organic acids like methanoic acid, acetic acid, succinic acid, citric acid, lactic acid, fumaric acid, tartaric acid, benzoic acid, p-methyl-benzenesulfonic acid, methylsulphonic acid, naphthalenesulfonic acid, gluconic acid and the like. The cardiac glycoside compounds can be obtained by separation from plants, in particular Streptocaulon plant juventas or Streptocaulon griffithii, by using multiple conventional separating means or obtained through synthesis, semisynthesis or bioconversion means. These compounds have excellent inhibiting and treatment effects on multiple tumor cells.
Owner:SHENYANG PHARMA UNIVERSITY
Direct racemization of indole derivatives
The present invention discloses processes for the racemization of enantiomers of etodolac and other tetra-hydropyrano indole derivatives.
Owner:CEPHALON INC
Process for preparation of 1,3-dioxolane-4-methanol compounds
PCT No. PCT / JP97 / 03165 Sec. 371 Date Feb. 8, 1999 Sec. 102(e) Date Feb. 8, 1999 PCT Filed Sep. 9, 1997 PCT Pub. No. WO98 / 11087 PCT Pub. Date Mar. 19, 1998A process for preparing easily and economically a 1,3-dioxolane-4-methanol compound in a racemic form or an optically active form with high purity and in high yield. The process comprises reacting an alkali metal or alkaline earth metal salt of an alcohol or a carboxylic acid with a halogenomethyl-1,3-dioxolane which is prepared by acetalizing a halogeno-1,2-propanediol of a formula (1) wherein X is a halogen atom, in an acid catalyst to conduct esterification or etherification, and then hydrolyzing the ester group and hydrogenolyzing the ether group to prepare a 1,3-dioxolane-4-methanol compound of a formula (5) wherein R1 and R2 are hydrogen atom, alkyl having 1 to 4 carbon atoms or phenyl, and R1 and R2 may form a cycloalkyl ring having 3 to 6 carbon atoms with the adjacent carbon atoms.
Owner:DAISO CO LTD
Method for producing optically active 3-aminopiperidine or salt thereof
InactiveUS20100105917A1Inexpensive and readily availableEasy to produceFermentationOptically-active compound separationEnzymeStereoselectivity
The present invention relates to a method for producing an optically active 3-aminopiperidine or salt thereof. In the method, a racemic nipecotamide is stereoselectively hydrolyzed to obtain an optically active nipecotamide and an optically active nipecotic acid in the presence of an enzyme source derived from an organism, and then the optically active nipecotamide is derived into an optically active aminopiperidine or salt thereof by aroylation, Hofmann rearrangement, deprotection of the amino group and further deprotection; or the optically active nipecotamide is derived into an optically active aminopiperidine or salt thereof by selective protection with BOC, Hofmann rearrangement and further deprotection. It is possible by the present invention to produce an optically active 3-aminopiperidine or salt thereof useful as a pharmaceutical intermediate from an inexpensive and easily available starting material by easy method applicable to industrial manufacturing.
Owner:KANEKA CORP
Process for preparation of racemic Nebivolol
Owner:UNIV ZURICH +1
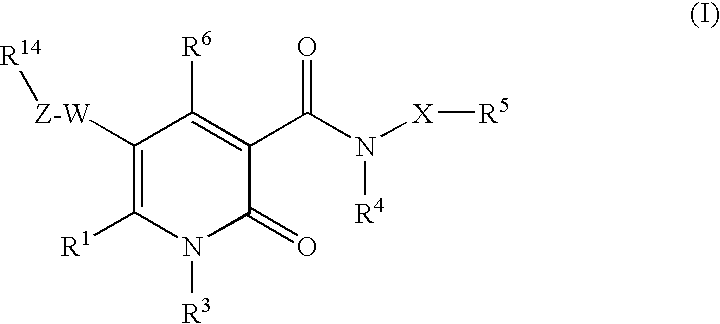
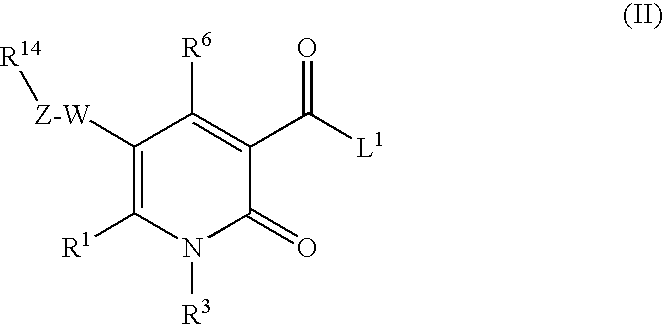
2-pyridine derivatives as inhibitors of neutrophile elastase
The invention provides compounds of formula wherein R1, R3, R4, R5, R6, R14, X, W and Z are as defined in the specification and optical isomers, racemates and tautomers thereof, and pharmaceutically acceptable salts thereof; together with processes for their preparation, pharmaceutical compositions containing them and their use in therapy. The compounds are inhibitors of human neutrophil elastase.
Owner:ASTRAZENECA AB
Method for Preparing 3-Hydroxy-4-Hydroxymethyl-Pyrrolidine Compounds
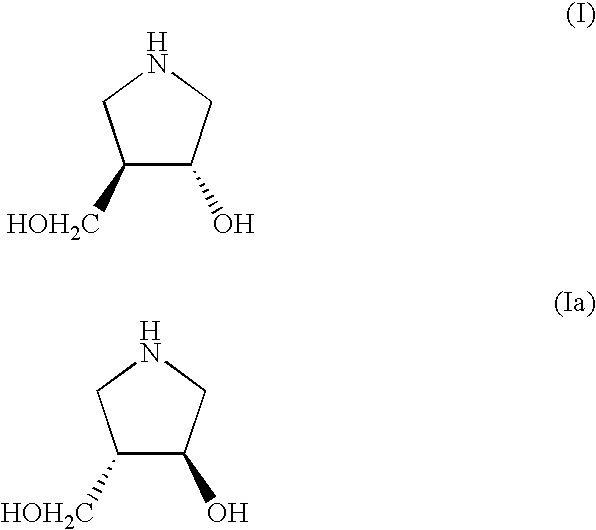
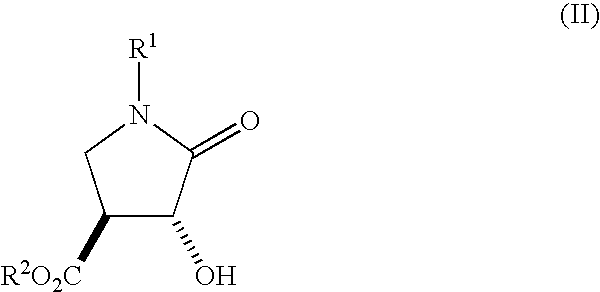
A process is disclosed for preparing (3R,4R)-3-hydroxy-4-hydroxymethylpyrrolidine, the compound of formula (I), or (3S,4S)-3-hydroxy-4-hydroxymethylpyrrolidine, the compound of formula (Ia) involving, as a key step, the enzyme-catalysed enantioselective hydrolysis of a racemic 3,4-trans-disubstituted pyrrolidinone compound of formula (II).
Owner:VICTORIA LINK LTD
Phosphine ligand and enantiomer or racemic body thereof and preparation methods thereof
ActiveCN102532196AHigh reactivityHigh enantioselectivityOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsIridiumSynthesis methods
The invention discloses a phosphine ligand and an enantiomer or a racemic body thereof and preparation methods and applications thereof. The structural formulae of the phosphine ligand and the enantiomer or the racemic body are shown in the specifications; a phosphine ligand compound has a novel framework; complete transfer of planar chirality to axial chirality in a synthesis process is realized through a desymmetrization reaction; a synthesis method is simple and economical; during preparation of a chiral ligand, common complex chiral splitting processes are avoided; and an obtained chiral ligand has the advantages of high reaction activity, high enantioselectivity and the like in a model reaction, can be applied to catalytic reactions of a plurality of metals such as palladium, rhodium, nickel, copper, iridium, ruthenium, iron, cobalt, gold, platinum and the like, and can have a very good catalytic effect.
Owner:SUN YAT SEN UNIV
Method for producing lactic acid
InactiveUS20070161098A1High optical purityGood physical propertiesOrganic compound preparationMicroorganismsGlycerolPhotochemistry
Lactic acid with high optical purity that has not previously been achieved is produced. It has been found that the optical purity of lactic acid is reduced as the racemization reaction of lactic acid proceeds when lactic acid coexists with glycerol. By reducing the amount of glycerol prior to concentrating lactic acid by heating, the optical purity of lactic acid after concentration by heating can be maintained at a high level.
Owner:TEIJIN LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof 2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/07f778de-8490-4c00-9e27-aeee582ed3d4/US20130203754A1-20130808-D00001.png)
![2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof 2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/07f778de-8490-4c00-9e27-aeee582ed3d4/US20130203754A1-20130808-D00002.png)
![2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof 2-Aryl Imidazo[1,2-a]Pyridine-3-Acetamide Derivatives, Preparation Methods and Uses Thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/07f778de-8490-4c00-9e27-aeee582ed3d4/US20130203754A1-20130808-D00003.png)