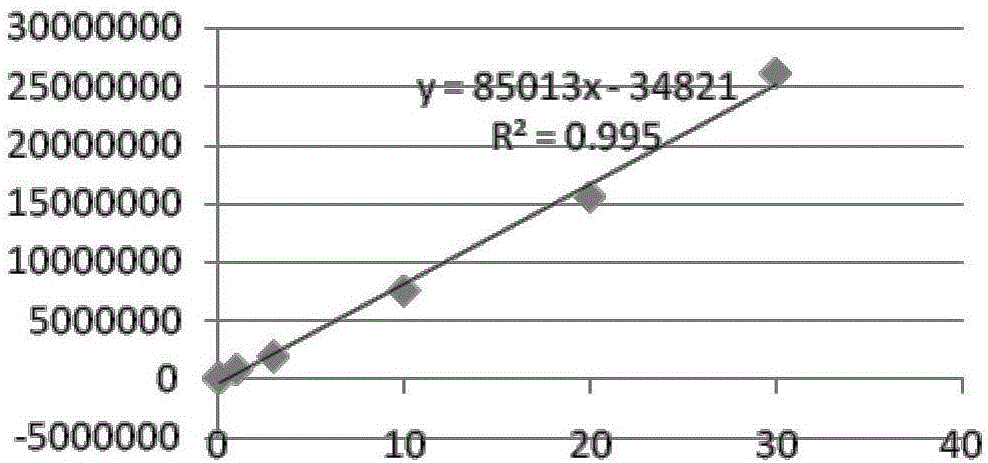
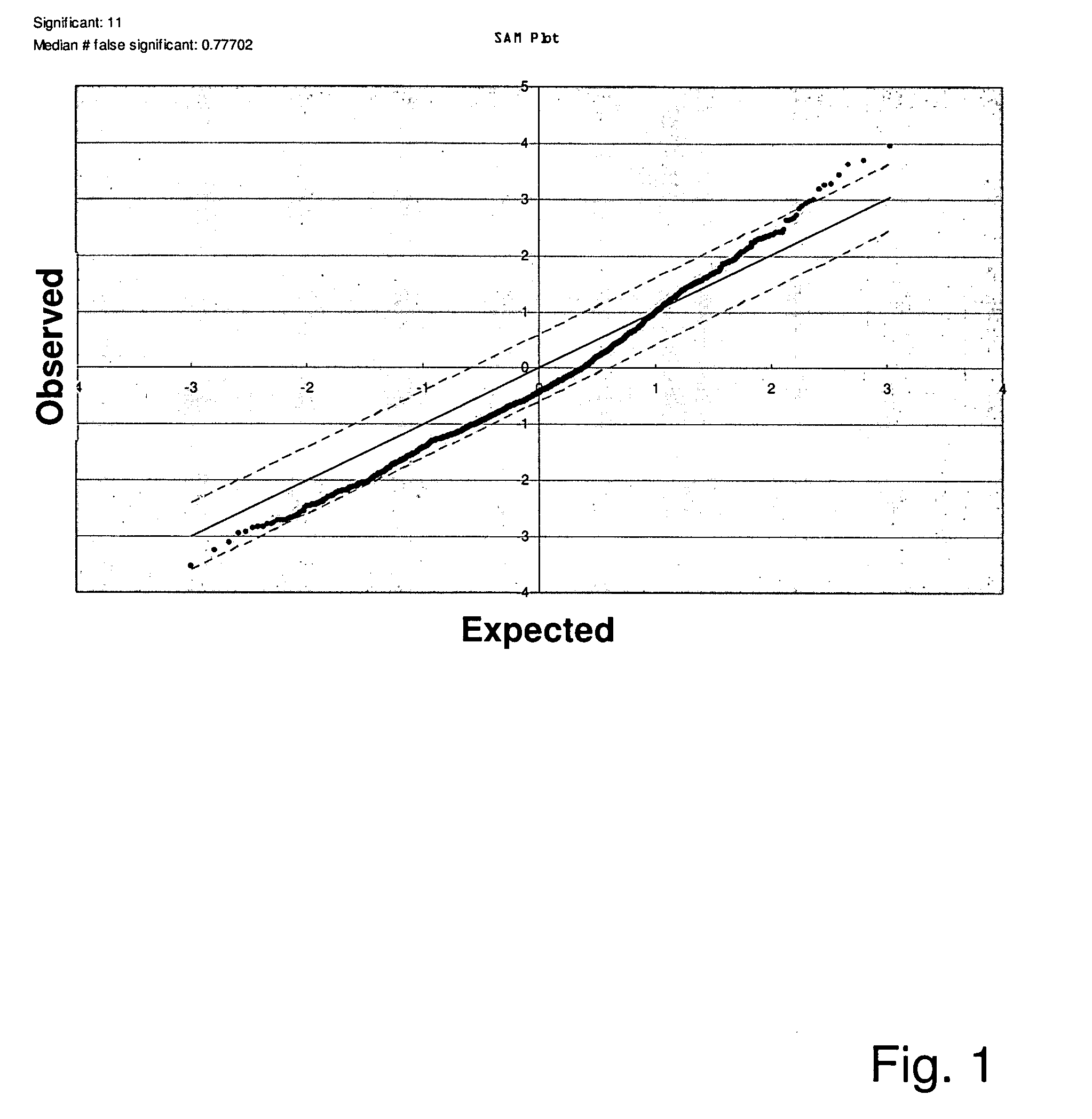
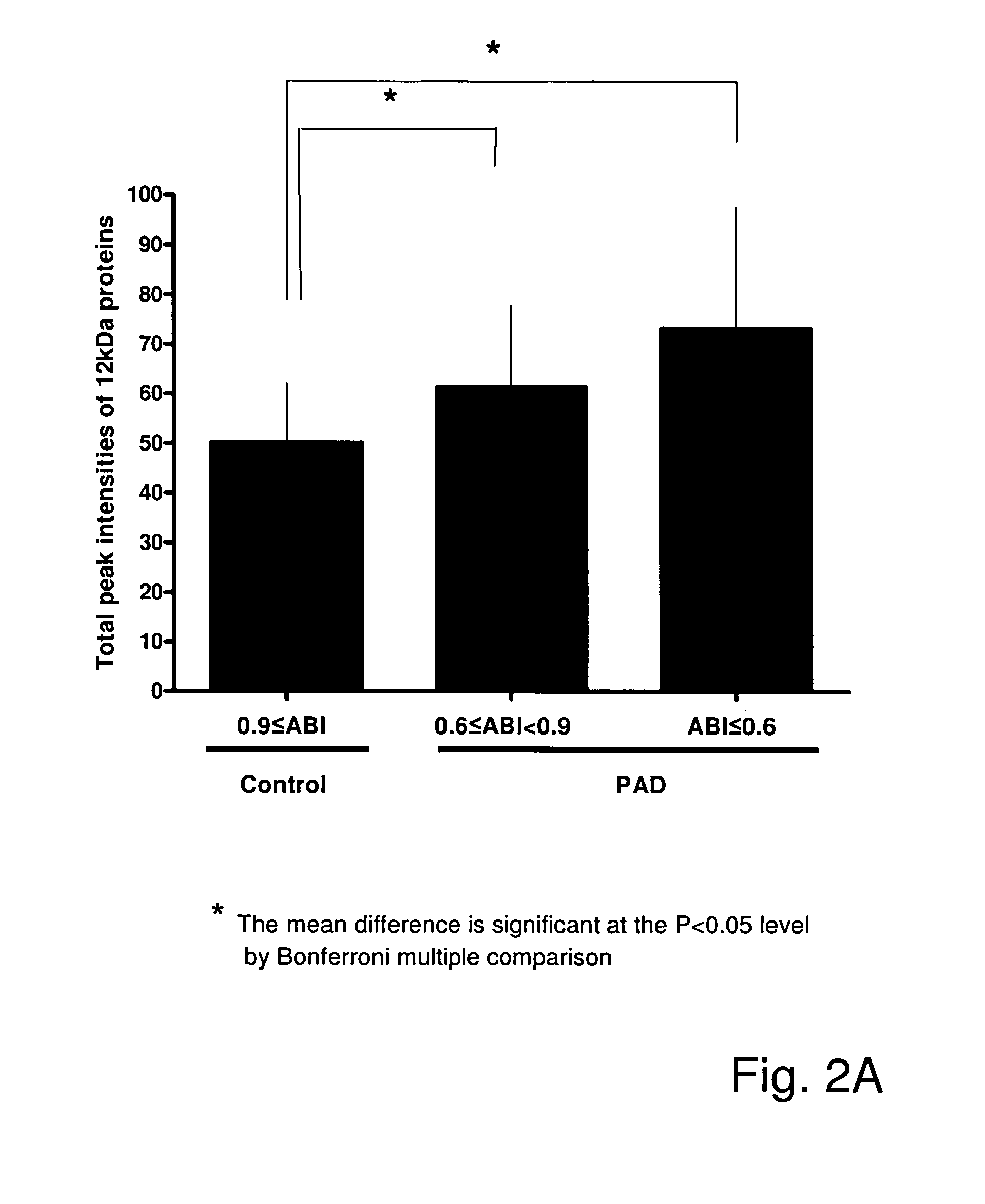
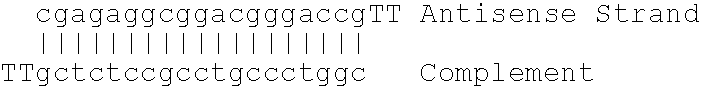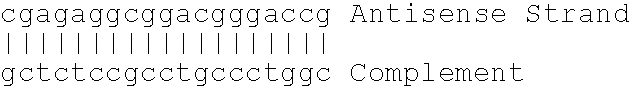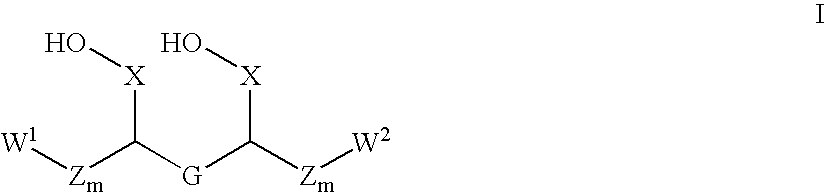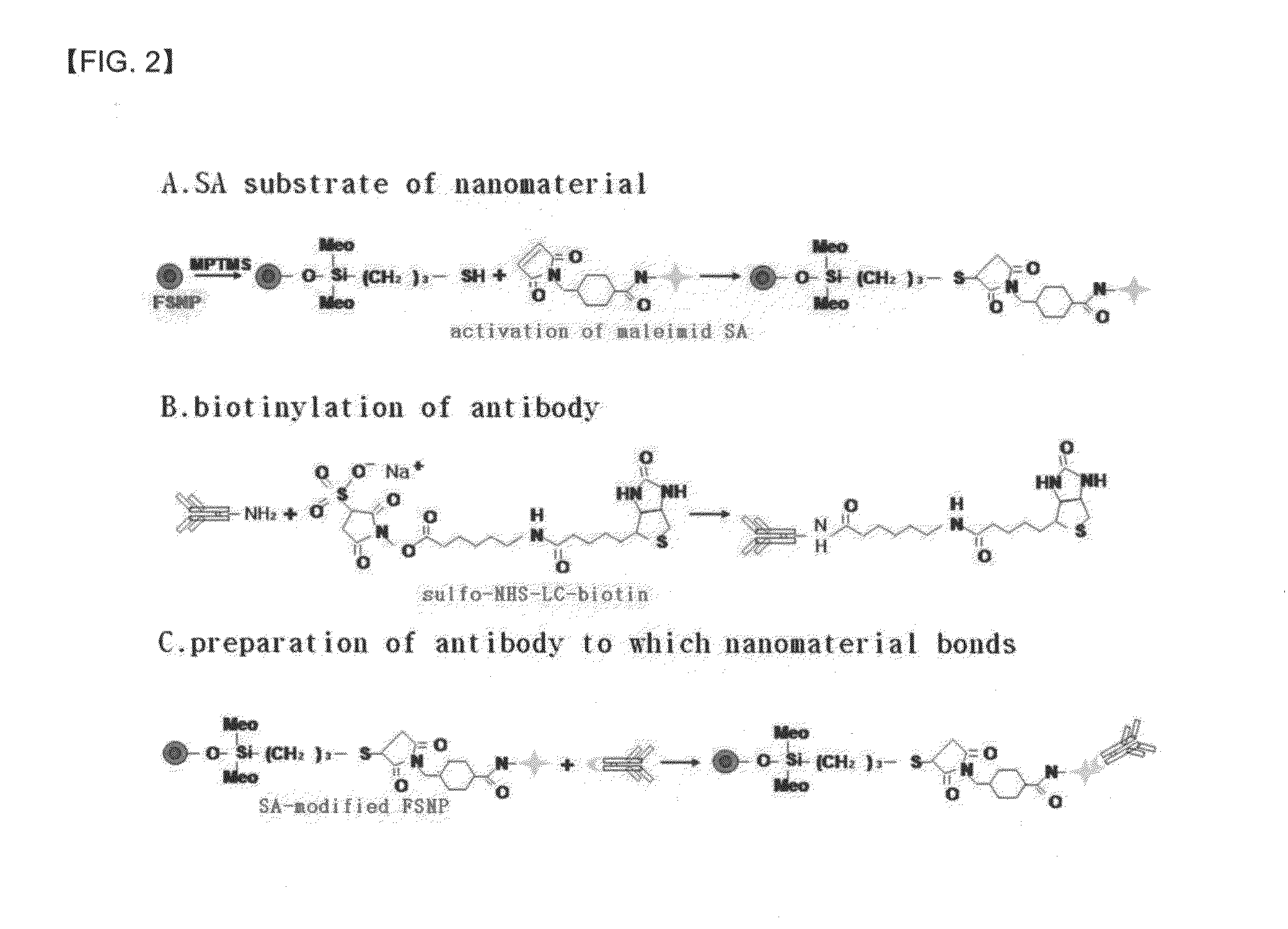Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
326 results about "C-reactive protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
<ul><li>A normal reading is < 10 mg/L.</li><li>A higher reading indicates inflammation which could be a result of infection, chronic disease or trauma.</li><li>The levels of CRP are variable, so it is recommended to perform the test twice in a gap of around two weeks and consider the average value from the two reports.</li><li>The test does not give information about the cause of inflammation and further tests are required to ascertain the cause.</li></ul>
Anti-inflammatory supplement compositions and regimens to reduce cardiovascular disease risks
InactiveUS20060172012A1Relieve symptomsPromotes fast digestionOrganic active ingredientsBiocideBlueberry extractApple extract
Disclosed are improvements in human nutrition involving a unique combination of natural products constituting anti-inflammatory compositions which can reduce cardiovascular disease risks as well as play a positive role in other conditions and diseases for which key indicators, especially selected from the group consisting of C-reactive protein (CRP) levels, cyclooxygenase-2 (COX-2), 5-lypoxygenase (5-LOX) expression and prostaglandin E2 (PGE-2) biosynthesis or any combination of these, are indicators. Therapeutic compositions preferably comprise curcumin, bilberry extract, grape seed extract, green tea extract and apple extract, in effective amounts individually and combined to provide a therapeutically significant reduction in one or more key indicators. Another exemplified therapeutic composition comprises: omega-3 rich refined fish oil, resveratrol, blueberry extract, grape seed extract, green tea extract and gamma and / or delta tocopherol, in effective amounts individually for the above benefits.
Owner:A M TODD
Modulation of C-reactive protein expression
InactiveUS7425545B2Reduce expressionUseful applicationAntibacterial agentsSenses disorderDiseaseOligonucleotide
Compounds, compositions and methods are provided for modulating the expression of C-reactive protein. The compositions comprise oligonucleotides, targeted to nucleic acid encoding C-reactive protein. Methods of using these compounds for modulation of C-reactive protein expression and for diagnosis and treatment of disease associated with expression of C-reactive protein are provided.
Owner:IONIS PHARMA INC
High-Potency Sweetener Composition With C-Reactive Protein Reducing Substance and Compositions Sweetened Therewith
InactiveUS20070116839A1Improve flavor profileImproving temporal profile profileSugar food ingredientsMetabolism disorderAdditive ingredientSweetness
The present invention relates generally to functional sweetener compositions comprising non-caloric or low-caloric natural and / or synthetic, high-potency sweeteners and methods for making and using them. In particular, the present invention relates to different functional sweetener compositions comprising at least one non-caloric or low-caloric natural and / or synthetic, high-potency sweetener, at least one sweet taste improving composition, and at least one functional ingredient, such as C-reactive protein reducing substances. The present invention also relates to functional sweetener compositions and methods that can improve the tastes of non-caloric or low-caloric high-potency sweeteners by imparting a more sugar-like taste or characteristic. In particular, the functional sweetener compositions and methods provide a more sugar-like temporal profile, including sweetness onset and sweetness linger, and / or a more sugar-like flavor profile.
Owner:THE COCA-COLA CO
High throughput multi-antigen microfluidic fluorescence immunoassays
InactiveUS20060263818A1Reduce total integrated backgroundIncreased signal noiseBioreactor/fermenter combinationsBiological substance pretreatmentsAntigenPoint of care
The development of a high-throughput multi-antigen microfluidic fluorescence immunoassay system is illustrated in a 100-chamber PDMS (polydimethylsiloxane) chip which performs up to 5 tests for each of 10 samples. Specificity of detection is demonstrated and calibration curves produced for C-Reactive Protein (CRP), Prostate Specific Antigen (PSA), ferritin, and Vascular Endothelial Growth Factor (VEGF). The measurements show sensitivity at and below levels that are significant in current clinical laboratory practice (with SIN>8 at as low as 10 pM antigen concentration). The chip uses 100 nL per sample for all four tests and provides an improved instrument for use in scientific research and “point-of-care” testing in medicine.
Owner:SCHERER AXEL +3
Immunoassay for C-reactive protein
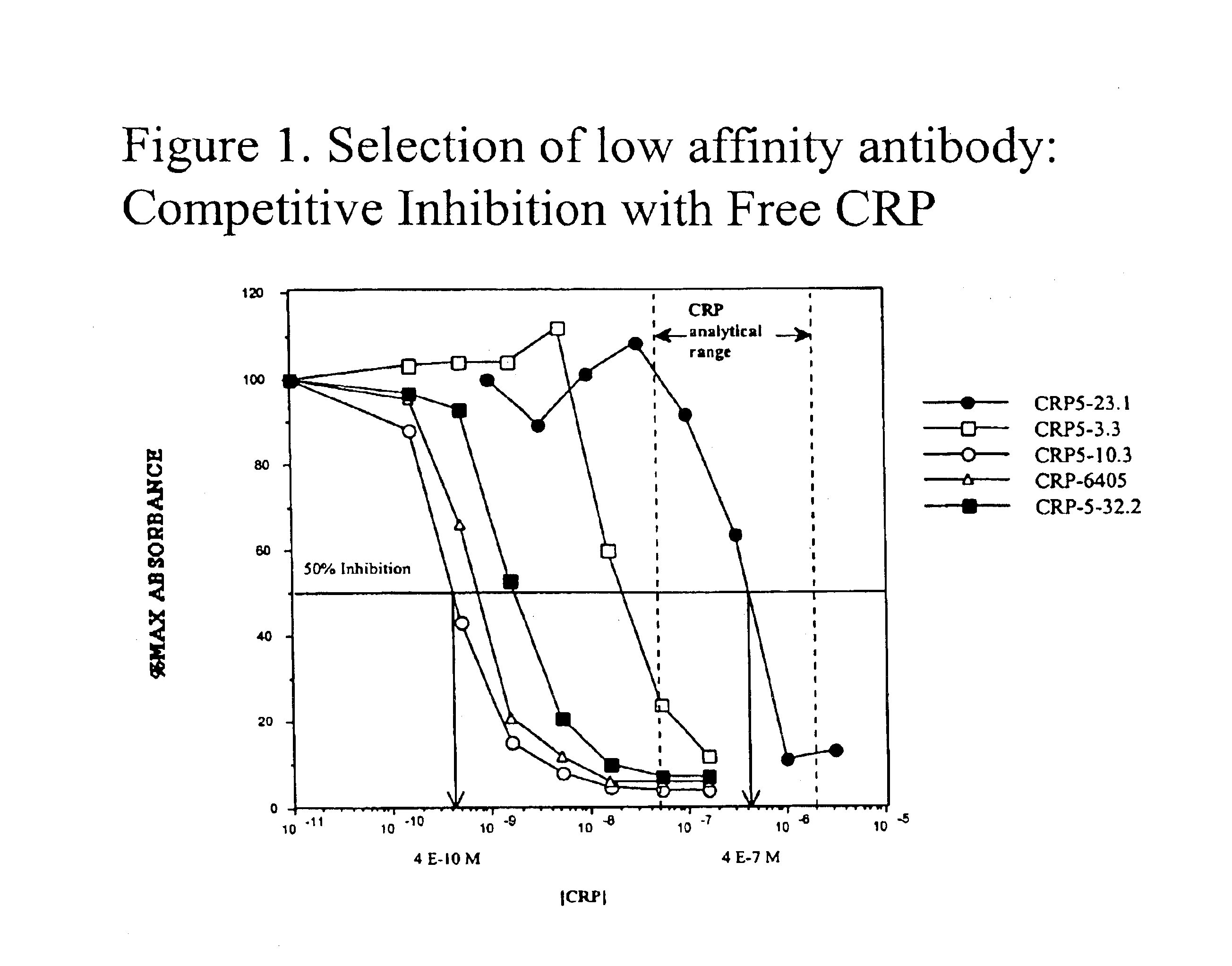
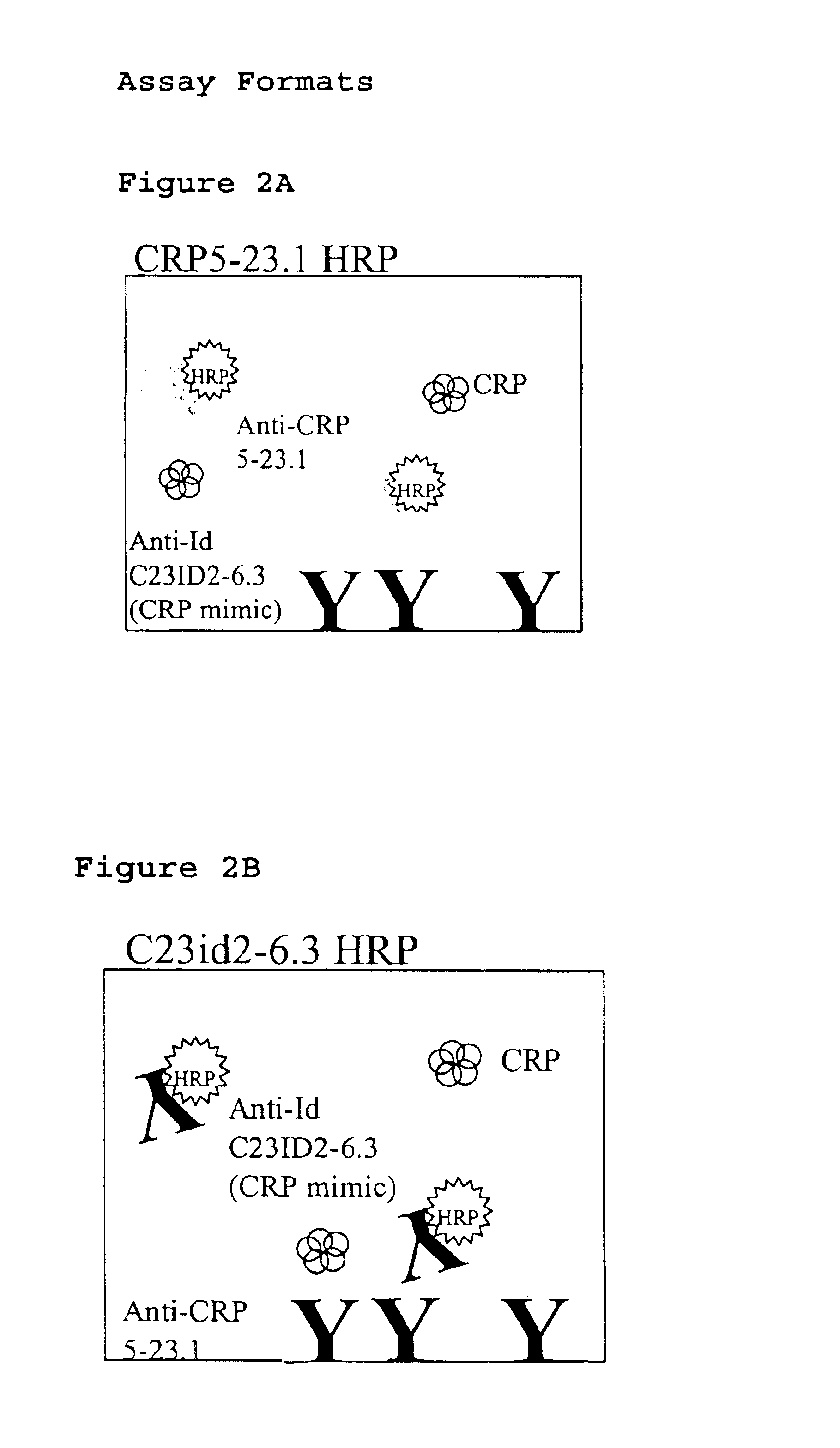
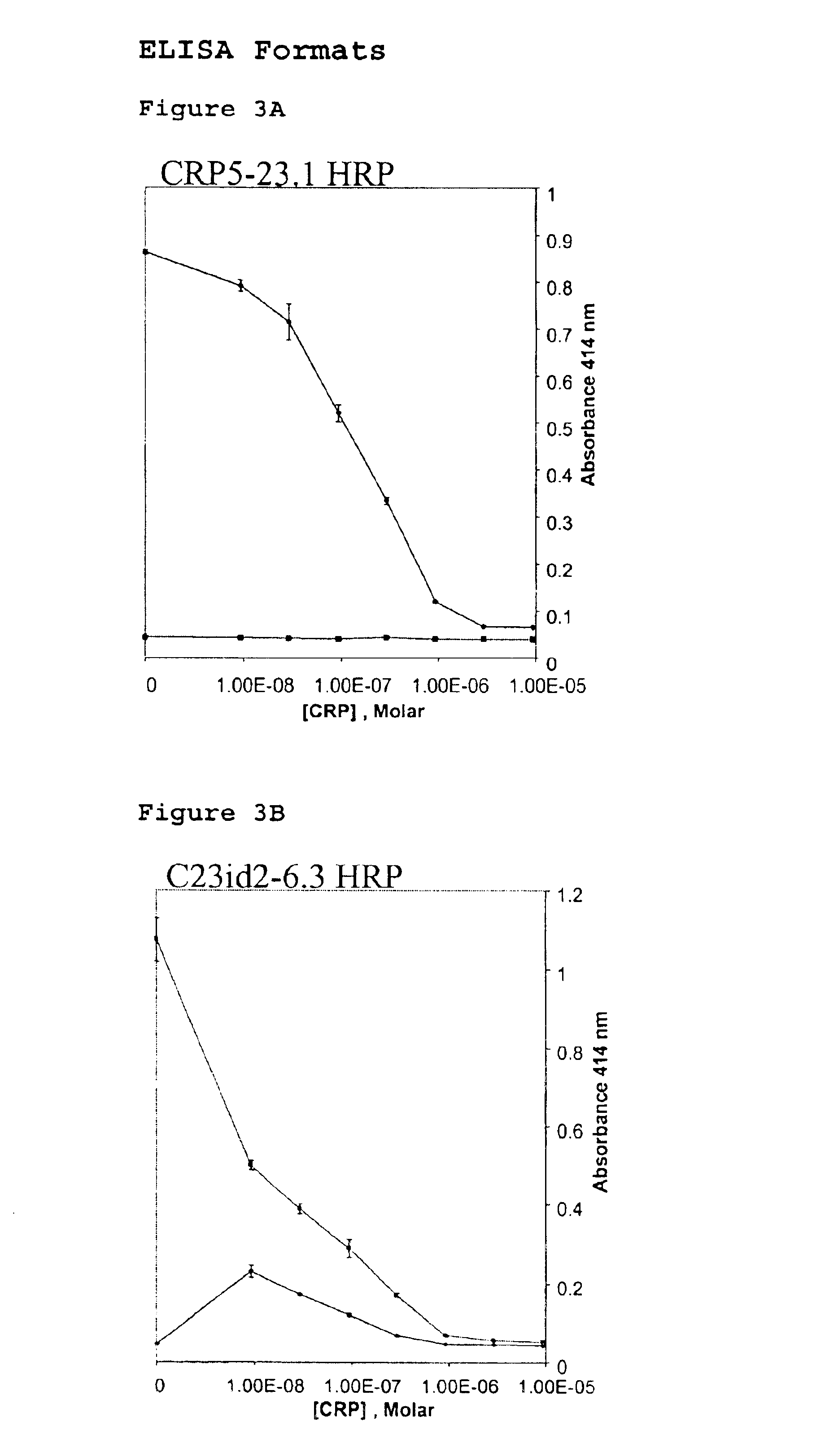
InactiveUS6838250B2High affinityWeak affinityAnimal cellsImmunoglobulins against animals/humansLow affinityAntigen
The present invention relates to new CRP immunoassay compositions. The compositions include a low affinity anti-human CRP monoclonal antibody, and an antiidiotypic antibody raised against it. The invention further provides a method for obtaining antiidiotypic monoclonal antibody populations directed to an antibody that is specific for a high concentration, high molecular weight target antigen.
Owner:ORTHO-CLINICAL DIAGNOSTICS
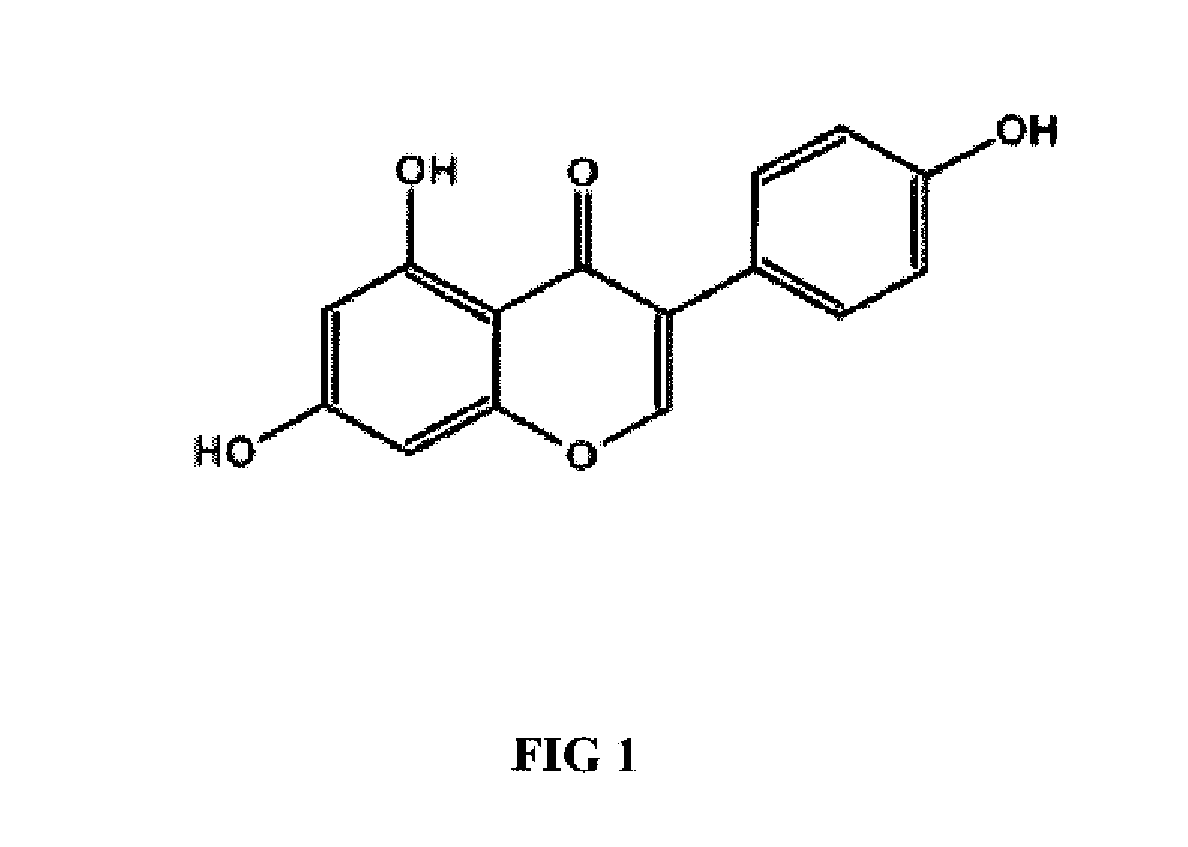
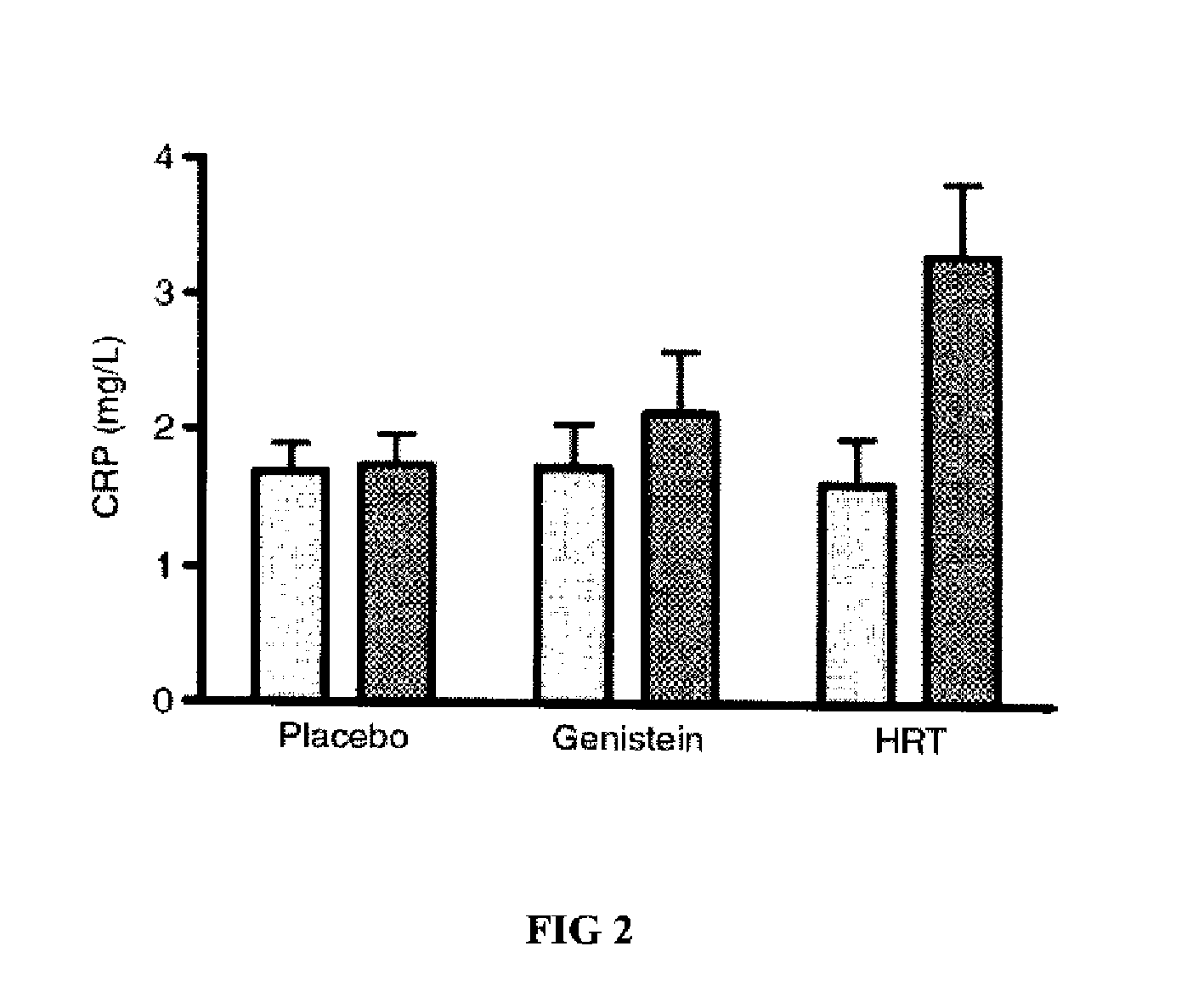
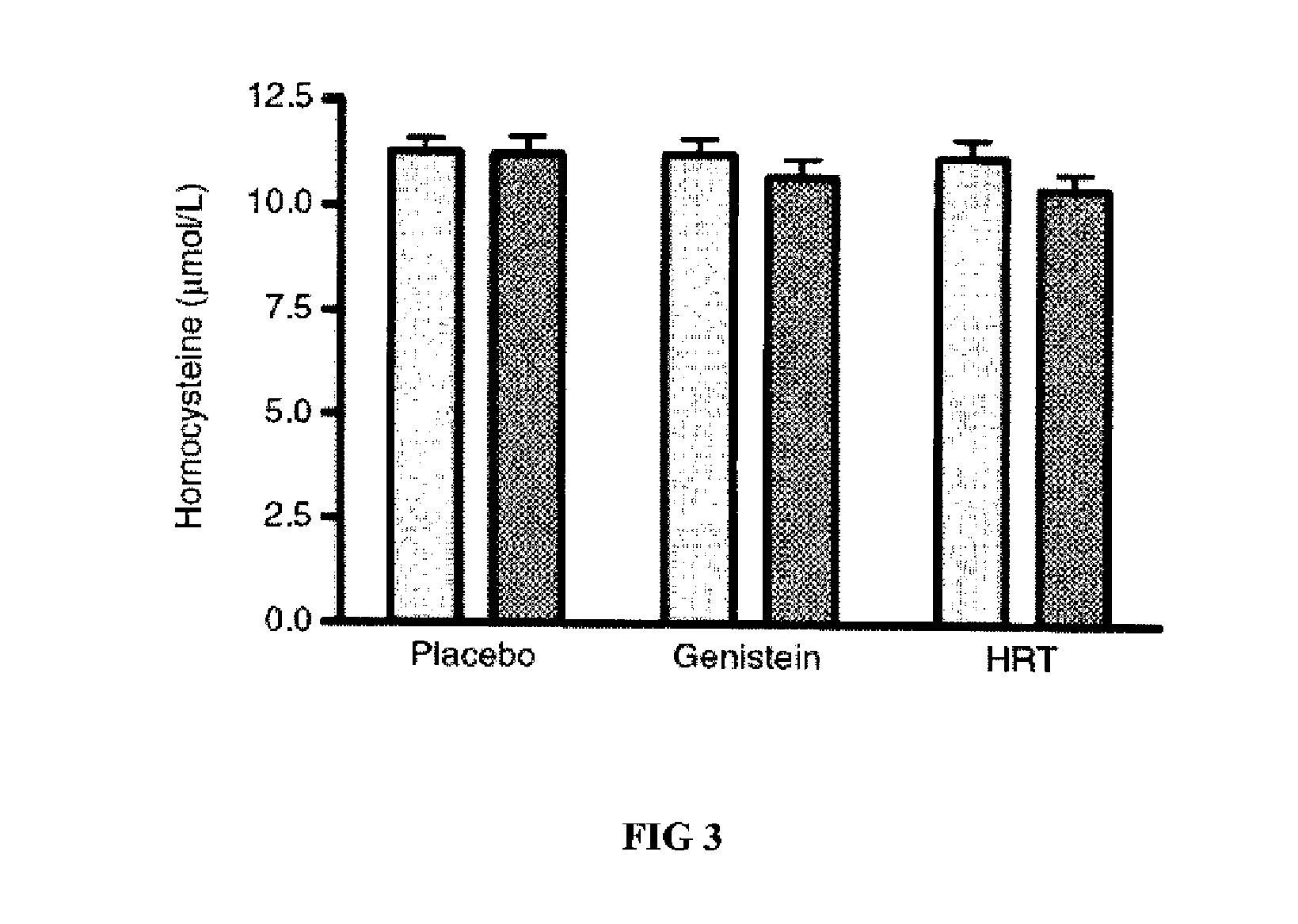
Genistein modulated reduction of cardiovascular risk factors
InactiveUS20070207225A1Reducing cardiovascular risk factorReduce risk factorBiocideAnimal repellantsFasting glucoseFibrinogen
The disclosed methods and compositions for reducing cardiovascular risk factors in mammals generally includes using genistein to modulate various inflammatory and cardiovascular risk markers including: homocysteine, C-reactive protein, fibrinogen, sex hormone-binding globulin, fasting glucose, insulin, insulin resistance, and osteoprotegerin.
Owner:PRIMUS PHARM INC
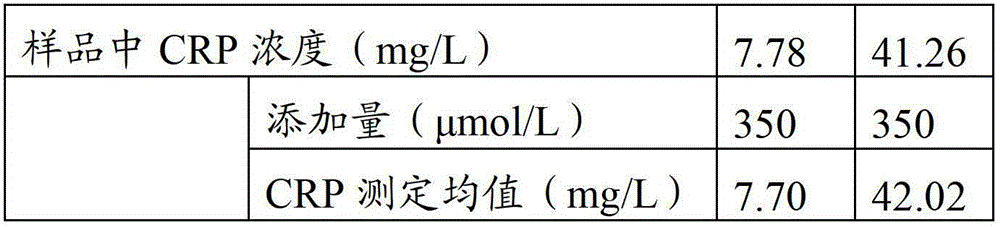
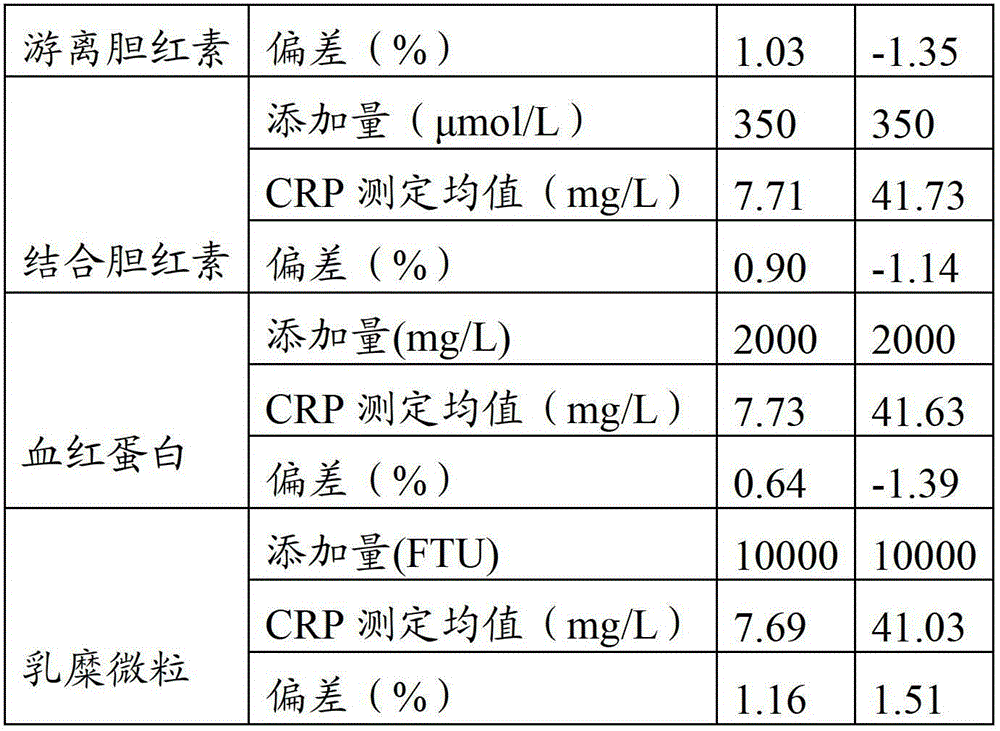
Full-scale C-reactive protein (CRP) colloidal gold immunoturbidimetric assay kit
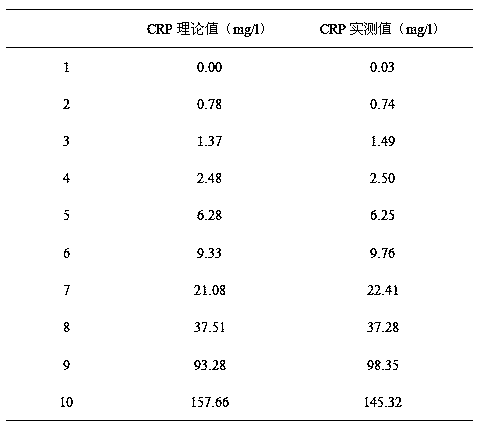
The invention provides a full-scale CRP colloidal gold immunoturbidimetric assay kit. A quantitative assay purpose is reached through enhancing the turbidity by the colloidal gold. The method used by the kit provided by the invention solves a problem that the generation of a precipitate after a latex reinforced immunoturbidimetric reaction goes against biochemical instrument cleaning. The kit provided in the invention comprises a reagent R1 and a reagent R2, wherein the reagent R2 is a proper buffer solution; and the reagent R2 is a buffer solution of the colloidal gold combined with an antihuman CRP antibody. The kit has the characteristics of high sensitivity, strong specificity and good stability, can be used for assaying the content of the CRP in serum or blood plasma, and is suitable for clinical fully-automatic biochemical analyzers. The CRP assay sensitivity of the kit can reach 0.01mg / L, and the upper CRP assay limitation of the kit is 500mg / L.
Owner:重庆沃康生物科技有限公司
Method of judging viral infection
InactiveUS20050227223A1Microbiological testing/measurementBiological material analysisViral infectionReagent
A method for diagnosing a viral infection without complicating bacterial infection, which comprises determining a C-reactive protein and an MxA protein in a biological sample; a kit for judging viral infection without complicating bacterial infection, which comprises a reagent for determining a C-reactive protein and a reagent for determining an MxA protein in a biological sample.
Owner:KYOWA MEDEX CO LTD
Immunochromatographic test strip for full quantitative detection of C-reactive protein and preparation method thereof
The invention discloses an immunochromatographic test strip for full quantitative detection of C-reactive protein and a preparation method thereof. The test strip is composed of a sample pad, a mark pad, a coating film and absorbent paper which are successively overlapped and adhered on a baseplate, wherein the mark pad is coated with C-reactive protein (CRP) monoclonal antibody labelled by fluorescent latex particles and rabbit IgG labelled by fluorescent latex particles; and the coating film is composed of a detection region and a quality control region, and the detection region is coated with another CRP monoclonal antibody which is at different epitope with the CRP monoclonal antibody labelled by fluorescent latex particles. The C-reactive protein immunochromatographic test strip can perform sensitive quantitative detection on the full CRP in 10 seconds, can diagnose diseases and identify infection more rapidly and accurately, and can detect infectious illness state and determine the curative effect of antibiotics; and the full CRP has two detection results, namely hs-CRP and conventional CRP, comprehensive linear range and good detection sensitivity and less required sample amount, and is very convenient to operate.
Owner:GUANGZHOU WONDFO BIOTECH
Treatment and diagnostic methods for fibrosis related disorders
Compositions and methods are provided for the treatment of fibrosis related disorders utilizing the ratio of the concentration of serum amyloid P(SAP) to C-reactive protein (CRP) in a patient. The methods may further comprise determining the R131 / H131 polymorphism of FcγRIIA. Diagnostic methods are also provided.
Owner:PROMEDIOR
Biomarkers for predicting major adverse events
InactiveUS20140187519A1Improve risk modelingIdentifying riskSalicyclic acid active ingredientsBiocideCvd riskBeta-2 microglobulin
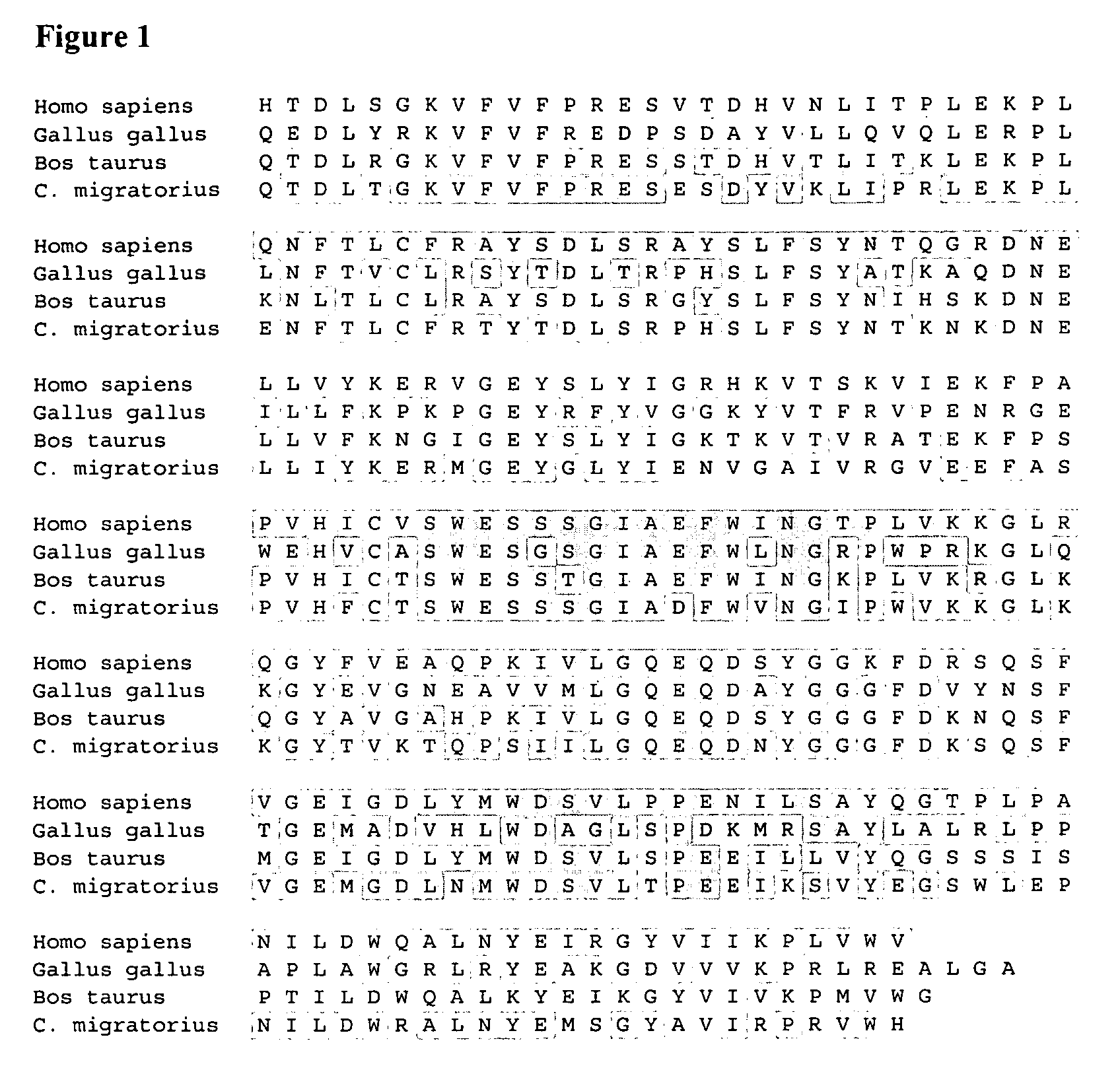
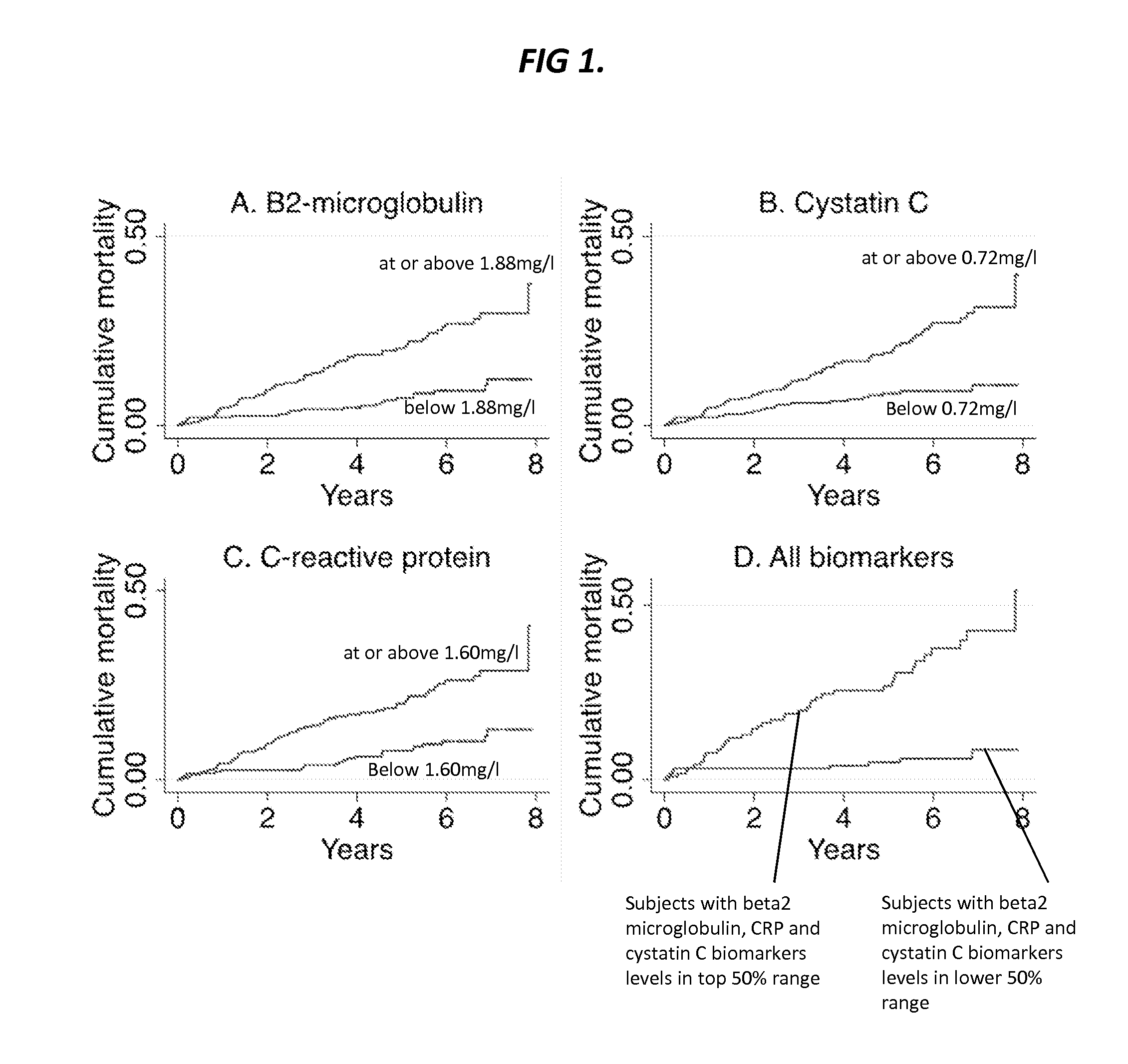
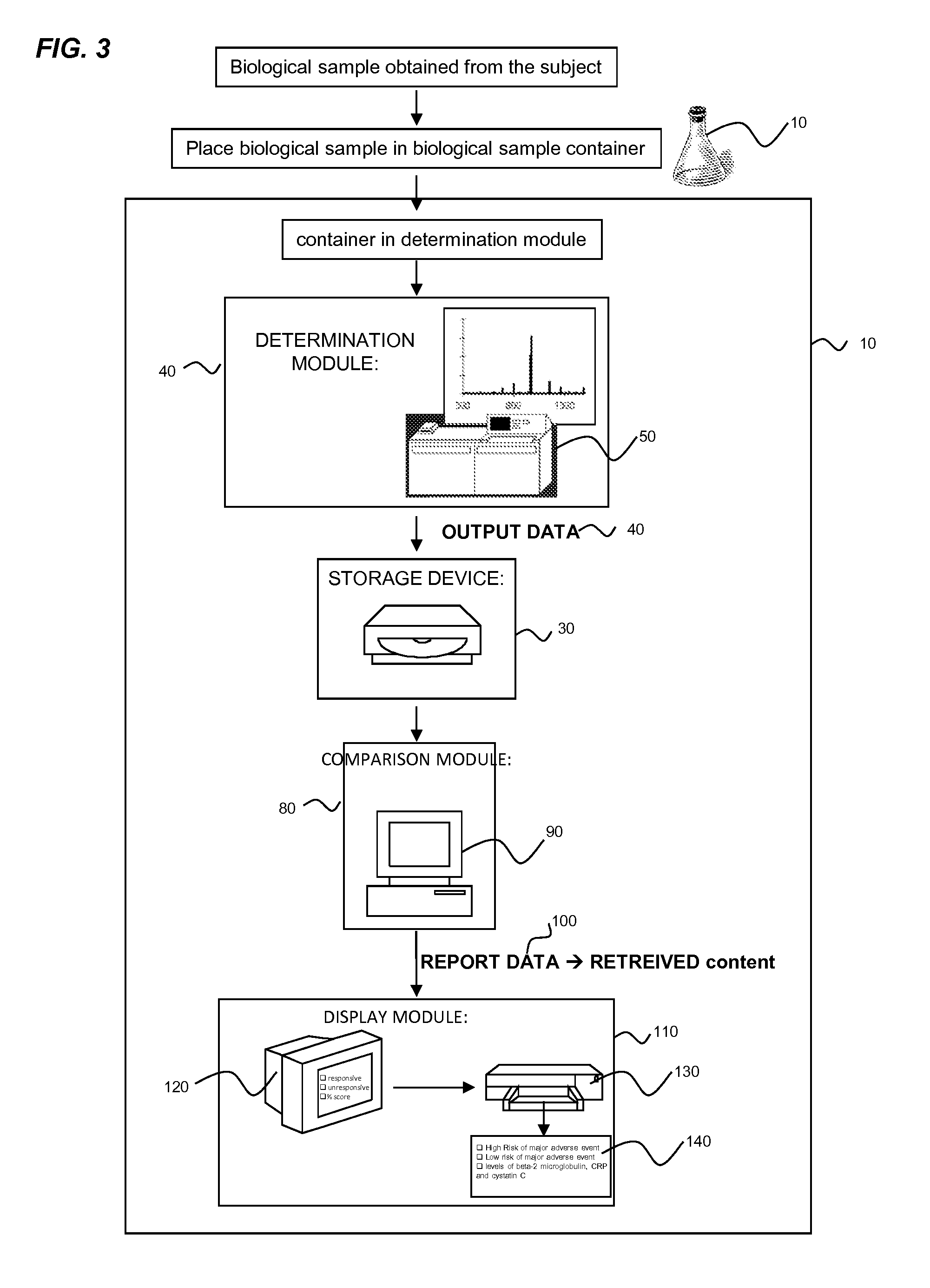
Provided herein are diagnostic markers and uses thereof for predicting if a subject is at risk of a major adverse event. In particular, one aspect provided herein relates to methods to determine if a subject is at risk of having a major adverse effect by measuring at least 2, or at least 3 of the biomarkers beta 2 microglobulin, c-reactive protein and cystatin C.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Reduction in complement activation and inflammation during tissue injury by carotenoids, carotenoid analogs, or derivatives thereof
InactiveUS20060270590A1Reducing and preventing and inhibiting complicationPrevent and inhibit accumulation/depositionBiocidePeptide/protein ingredientsComplement systemAstaxanthin
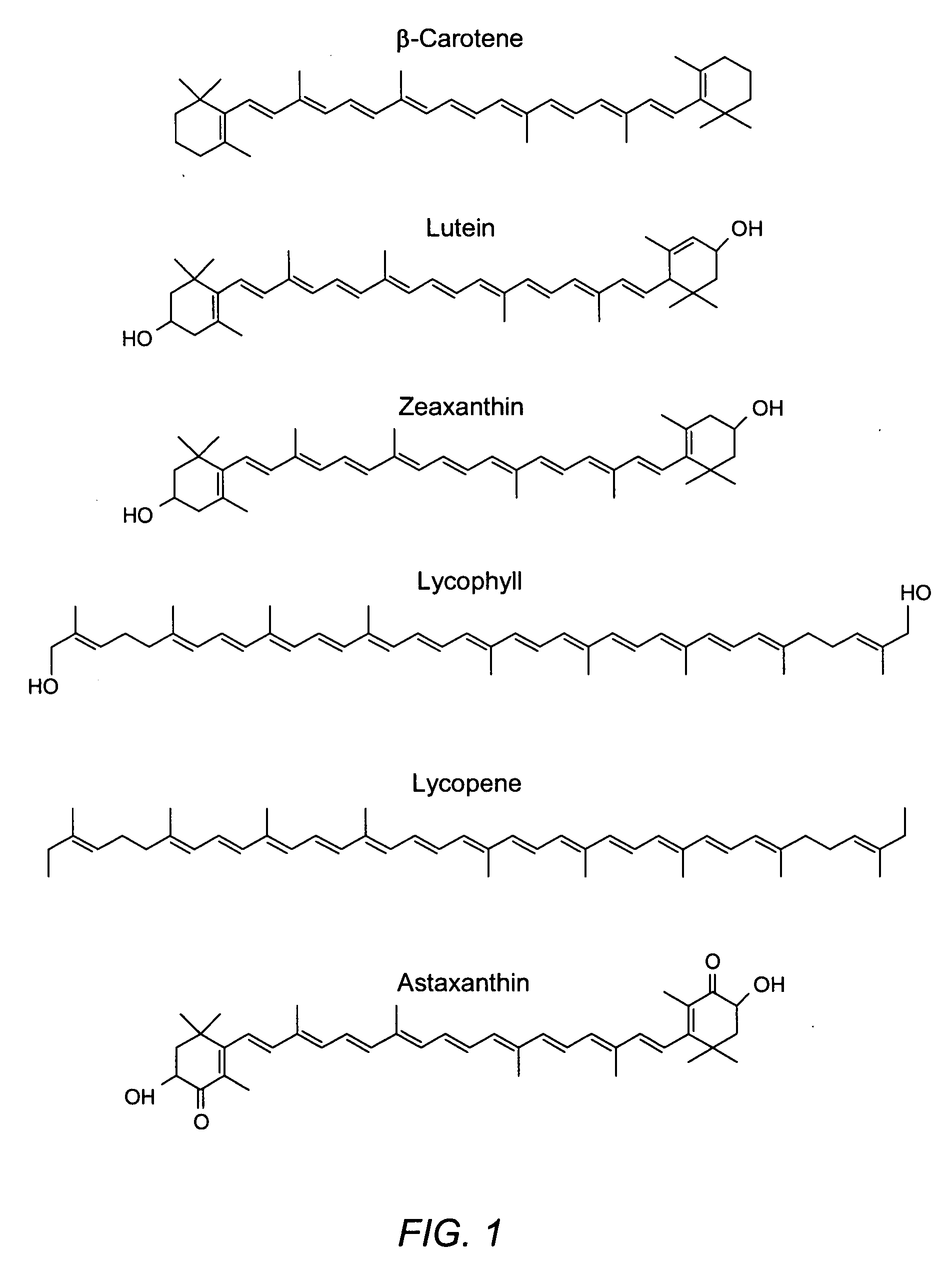
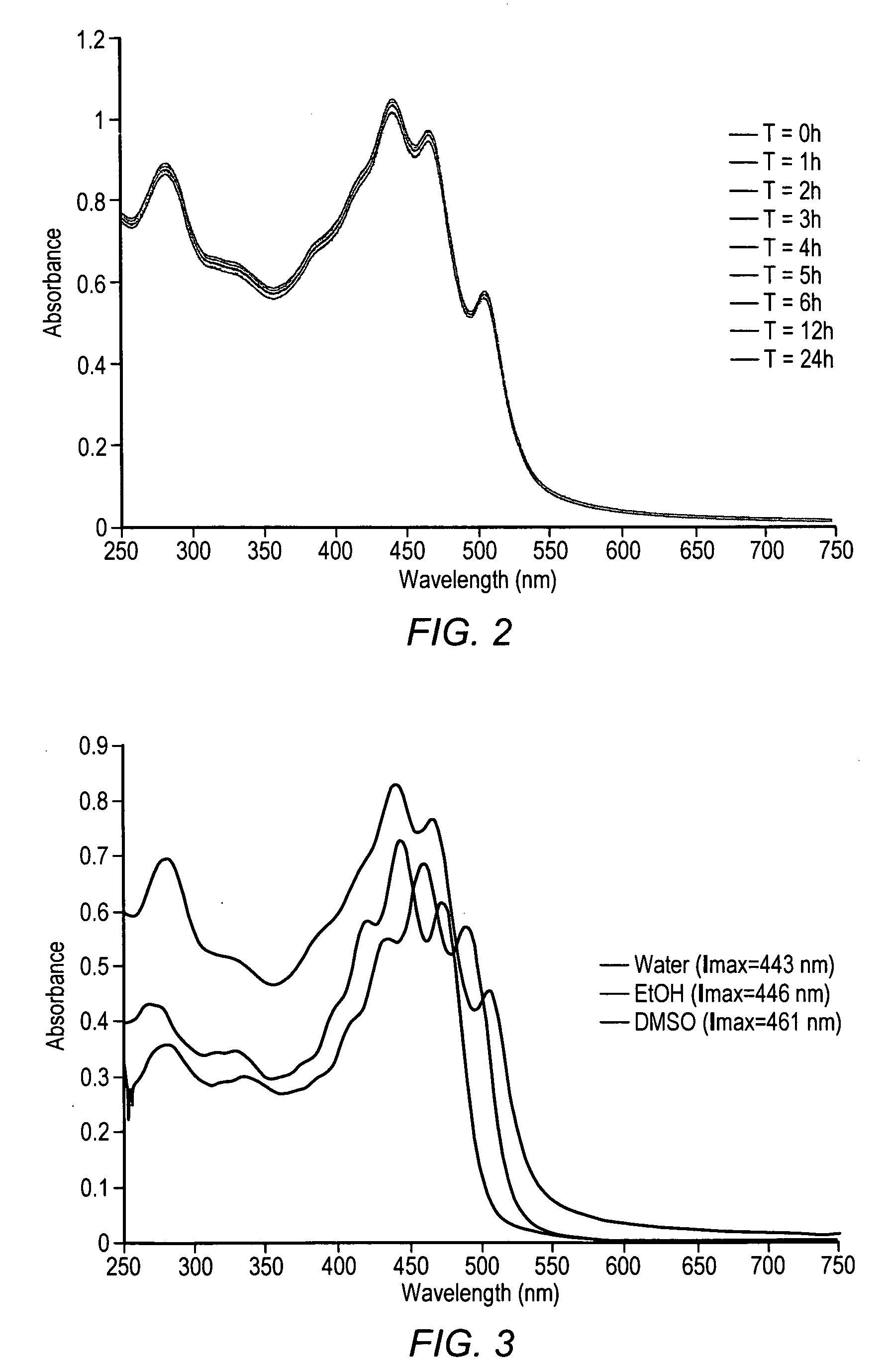
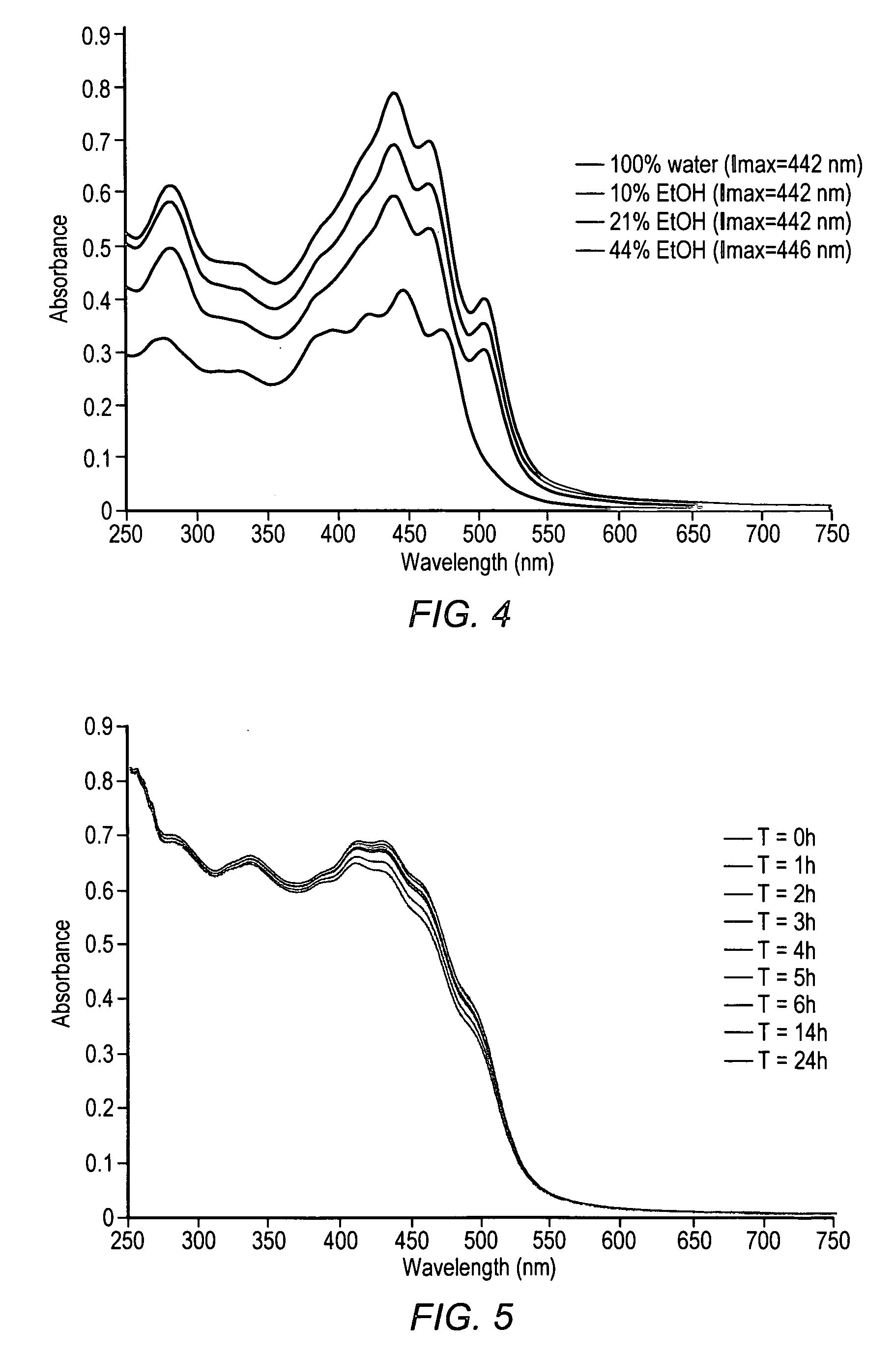
Administering water-soluble or dispersible synthetic analogs or derivatives of astaxanthin, lutein, zeaxanthin, or lycophyll and / or other carotenoids to a subject may reduce some of the adverse effects of inflammation in a body organ or tissue. The analogs or derivatives may be incorporated into pharmaceutical, over-the-counter, or nutraceutical preparations. Administration of the analogs or derivatives described herein may reduce deposition of inflammatory mediators such as C-reactive protein, complement system proteins or the membrane attack complex (MAC) in tissues. Reduced deposition of these molecules in tissues may reduce cell damage and / or lysis in the tissues.
Owner:CARDAX PHARMA
Dihydroxyl compounds and compositions for cholesterol management and related uses
The present invention relates to novel dihydroxyl compounds, compositions comprising hydroxyl compounds, and methods useful for treating and preventing a variety of diseases and conditions such as, but not limited to aging, Alzheimer's Disease, cancer, cardiovascular disease, diabetic nephropathy, diabetic retinopathy, a disorder of glucose metabolism, dyslipidemia, dyslipoproteinemia, hypertension, impotence, inflammation, insulin resistance, lipid elimination in bile, obesity, oxysterol elimination in bile, pancreatitis, Parkinson's disease, a peroxisome proliferator activated receptor-associated disorder, phospholipid elimination in bile, renal disease, septicemia, metabolic syndrome disorders (e.g., Syndrome X), thrombotic disorder. Compounds and methods of the invention can also be used to modulate C reactive protein or enhance bile production in a patient. In certain embodiments, the compounds, compositions, and methods of the invention are useful in combination therapy with other therapeutics, such as hypocholesterolemic and hypoglycemic agents.
Owner:ESPERION THERAPEUTICS
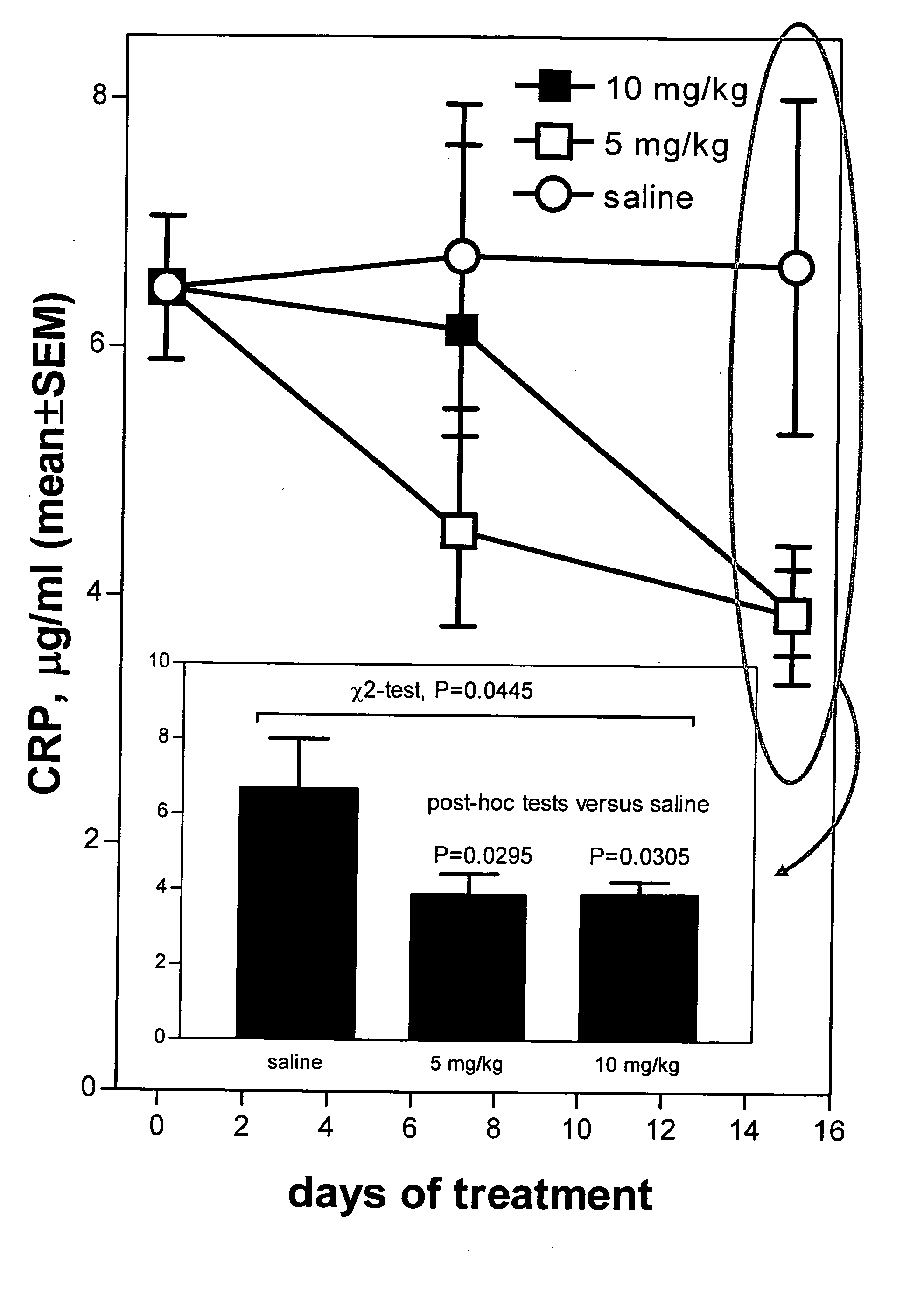
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
Full-range C-reactive protein detection kit
ActiveCN101769932AAccurate detectionAccurate measurementColor/spectral properties measurementsBiological testingBuffer solutionAntibody
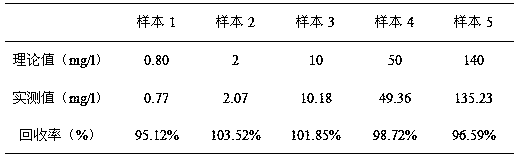
The invention discloses a full-range C-reactive protein detection kit, which comprises a reagent R1 and a reagent R2. The reagent R1 is a proper buffer solution; the reagent R2 is formed by the following steps: sensitizing two or more styrene latex with different average grain diameters by using an anti-human C-reactive protein antibody; placing the sensitized styrene latex with the different average grain diameters into the proper buffer solution respectively to form reagents; and finally mixing the reagents in different proportions, wherein the reagent R2 contains 0.8 to 3.5 mg / mL of the styrene latex combined with the anti-human C-reactive protein antibody. The full-range C-reactive protein detection kit not only can measure lower CRP content but also can measure high CRP content, and has the advantages of high sensitivity and stability and accurate measurement.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Methods of using IL-1 antagonists to reduce C-reactive protein
InactiveUS20060171948A1Reduce development riskEasy to controlBiocideAntibody mimetics/scaffoldsCancer researchAntagonist
Methods of reducing C-reactive protein (CRP) in a subject, comprising administering to the subject a therapeutic amount of an interleukin 1 (IL-1) antagonist, wherein CRP is reduced. The IL-1 antagonist is preferably an IL-1-binding fusion protein (IL-1 trap), preferably comprising SEQ ID NO:2.
Owner:REGENERON PHARM INC
Composition and application of composition as enzyme labeled compound preserving fluid
InactiveCN106771133AStable storageImprove stabilityChemiluminescene/bioluminescenceN-terminal pro-Brain Natriuretic PeptideBeta-2 microglobulin
The invention relates to the technical field of chemiluminescence immune assay, in particular to a composition and an application of the composition as enzyme labeled compound preserving fluid. The composition can improve the stability of an enzyme labeled compound, prolong a storage life of the enzyme labeled compound and stably preserve various enzyme labeled compounds for a long term. Experiments show that the stability difference between the un-aged enzyme labeled compounds of beta 2-microglobulin, C reactive protein or NT-proBNP (N-terminal pro-brain natriuretic peptide) and the aged enzyme labeled compounds for 6 days is within plus-minus 10%.
Owner:SINOCARE
Beta2-microglobulin and c reactive protein (CRP) as biomarkers for peripheral artery disease
InactiveUS20090042214A1Peptide/protein ingredientsMicrobiological testing/measurementArtery of PercheronAtheroma
The present invention relates to use of β-2-microglobulin (B2M or β2M) and C-reactive protein (CRP) levels as biomarkers of peripheral artery disease and / or atherosclerosis.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Methods and compositions for treating glucose-associated conditions, metabolic syndrome, dyslipidemias and other conditions
InactiveUS20060094693A1Lose weightAvoid weight gainBiocidePeptide/protein ingredientsDyslipidemiaFatty acid
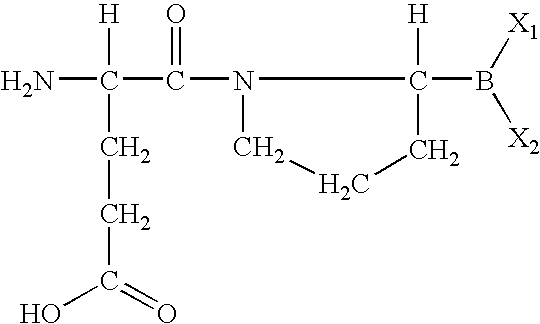
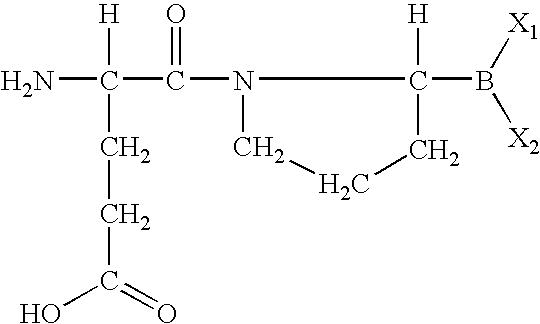
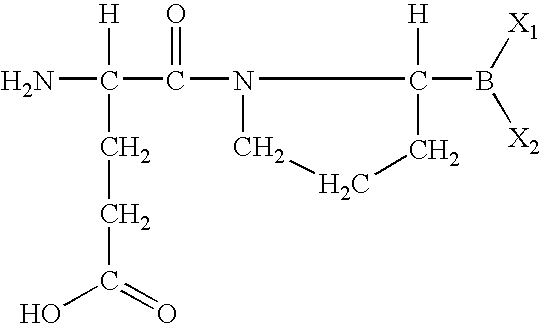
The invention relates, in part, to of Glu-boroPro containing compounds and methods of use thereof in the prevention or management of conditions that are associated with impaired glucose tolerance such as diabetes. The invention also relates to compositions of Glu-boroPro containing compounds and methods of use thereof in the prevention or management of conditions such as metabolic syndrome, dyslipidemias, inflammation, cardiovascular disorders such as hypertension and atherosclerosis, and to reduce body weight or prevent weight gain. The compounds of the invention are also useful in lowering levels of triglycerides, free fatty acids, C-reactive protein (CRP), HbA1C, total glycosylated hemoglobin (TGHb), in increasing insulin sensitivity index and in stimulating insulin release.
Owner:DARA BIOSCI
Composition for treating obesity and method of using the same
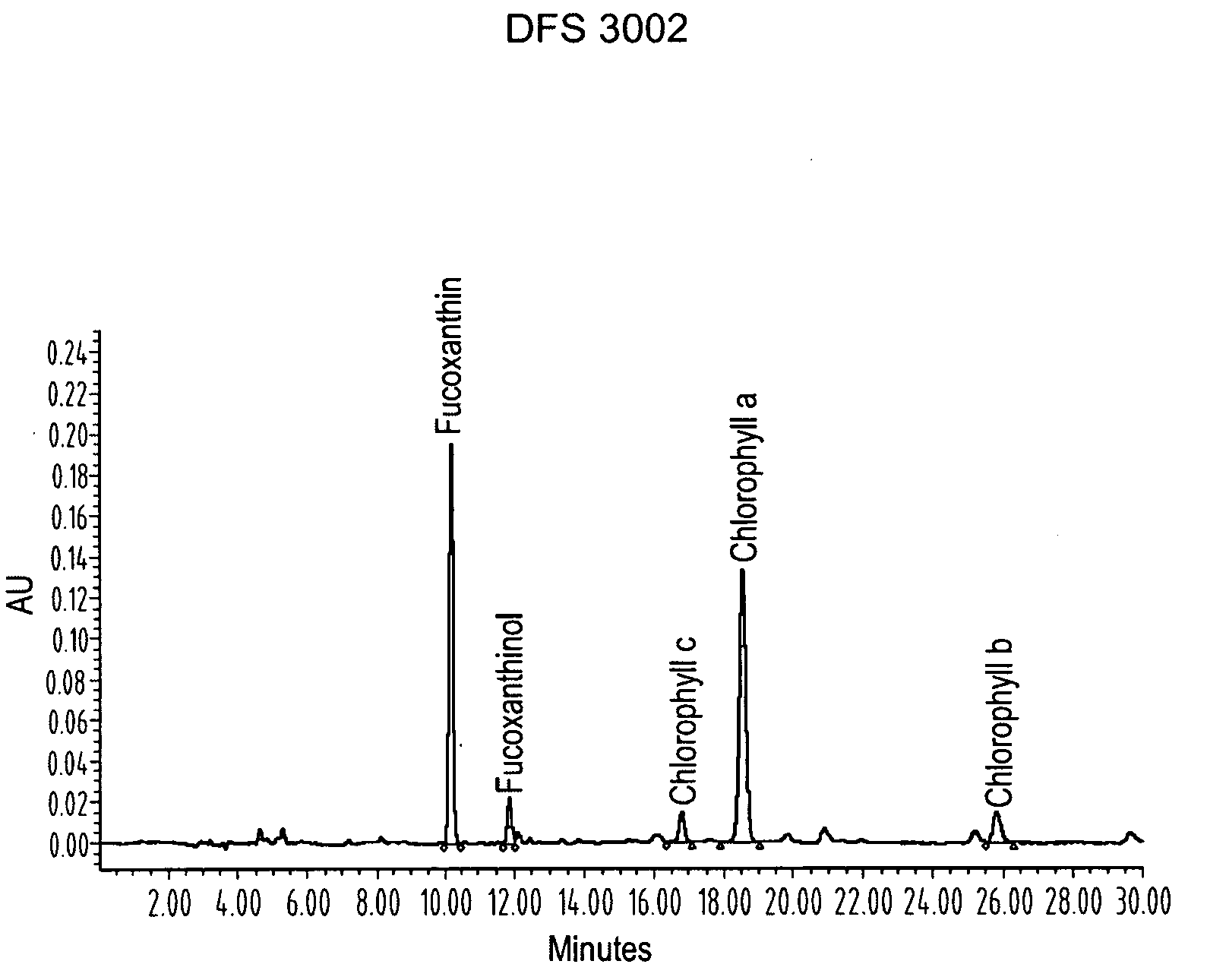
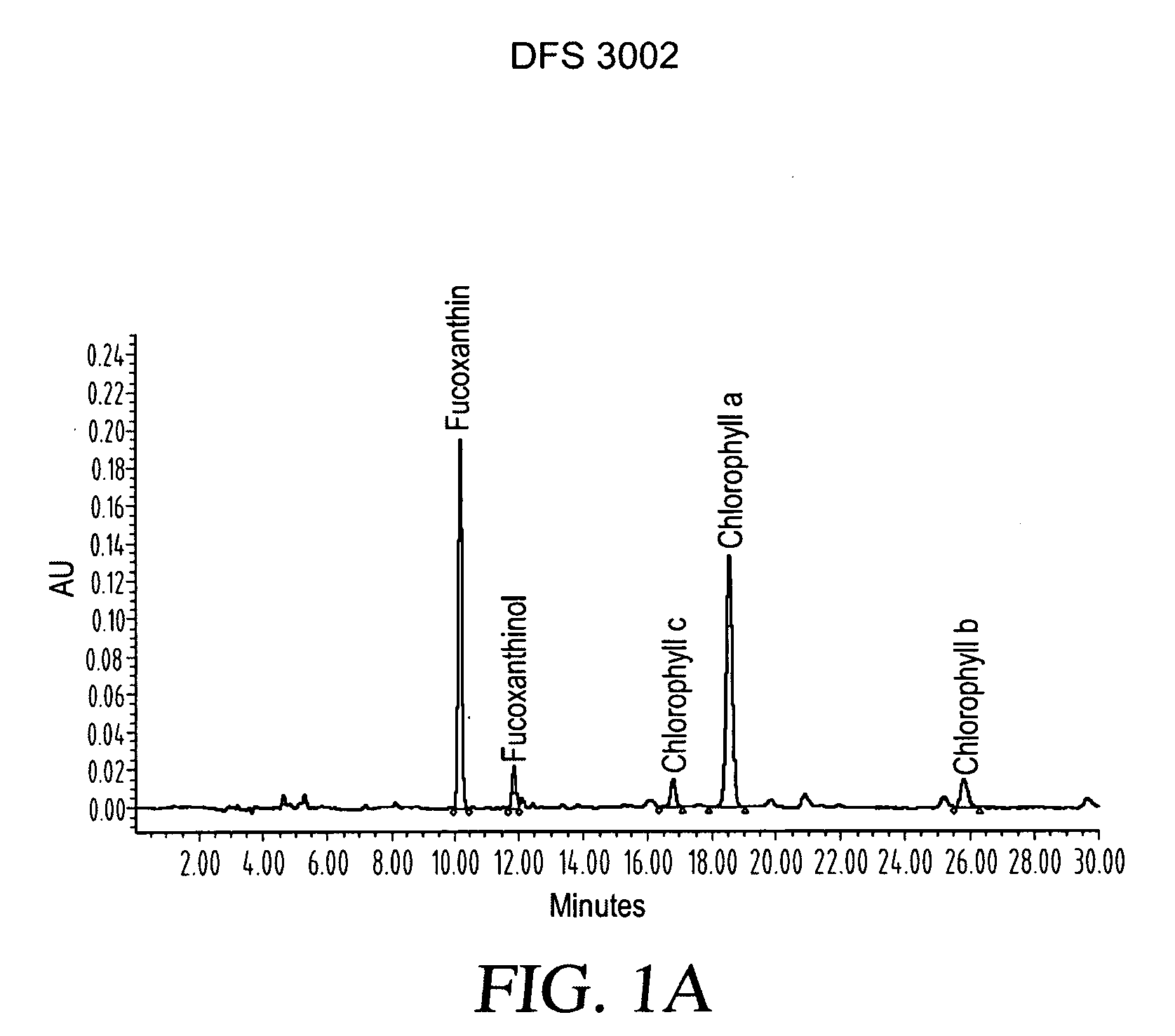
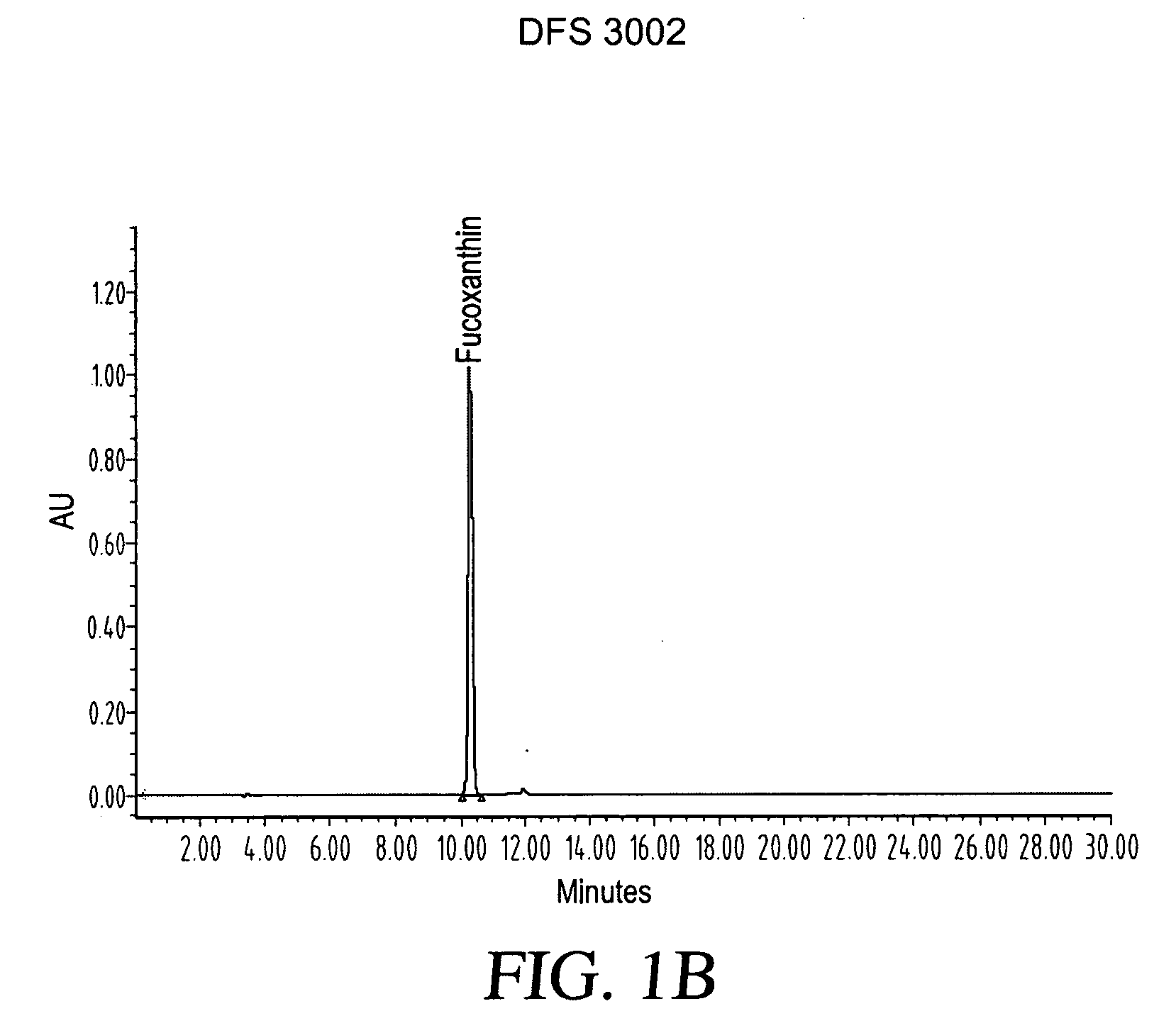
ActiveUS20080206275A1Reduce high blood pressureIncreasing energy expenditure rateBiocideMetabolism disorderFucoxanthinPOMEGRANATE SEED OIL
The present invention provides a composition for a medicinal or health effect of a treatment of liver fat and body fat, a reduction of blood pressure, an increase of the energy expenditure rate, a reduction of inflammatory C-reactive proteins and a reduction of plasma aminotransferase enzymes, comprising an effective amount of fucoxanthin alone or in combination with pomegranate seed oil, a pharmaceutically acceptable salt, a prodrug thereof, or a salt of the prodrug; and a method of using the same. The fucoxanthin may be used in pure form, or as a component of a brown marine vegetable extract.
Owner:NEKTIUM PHARMA SL
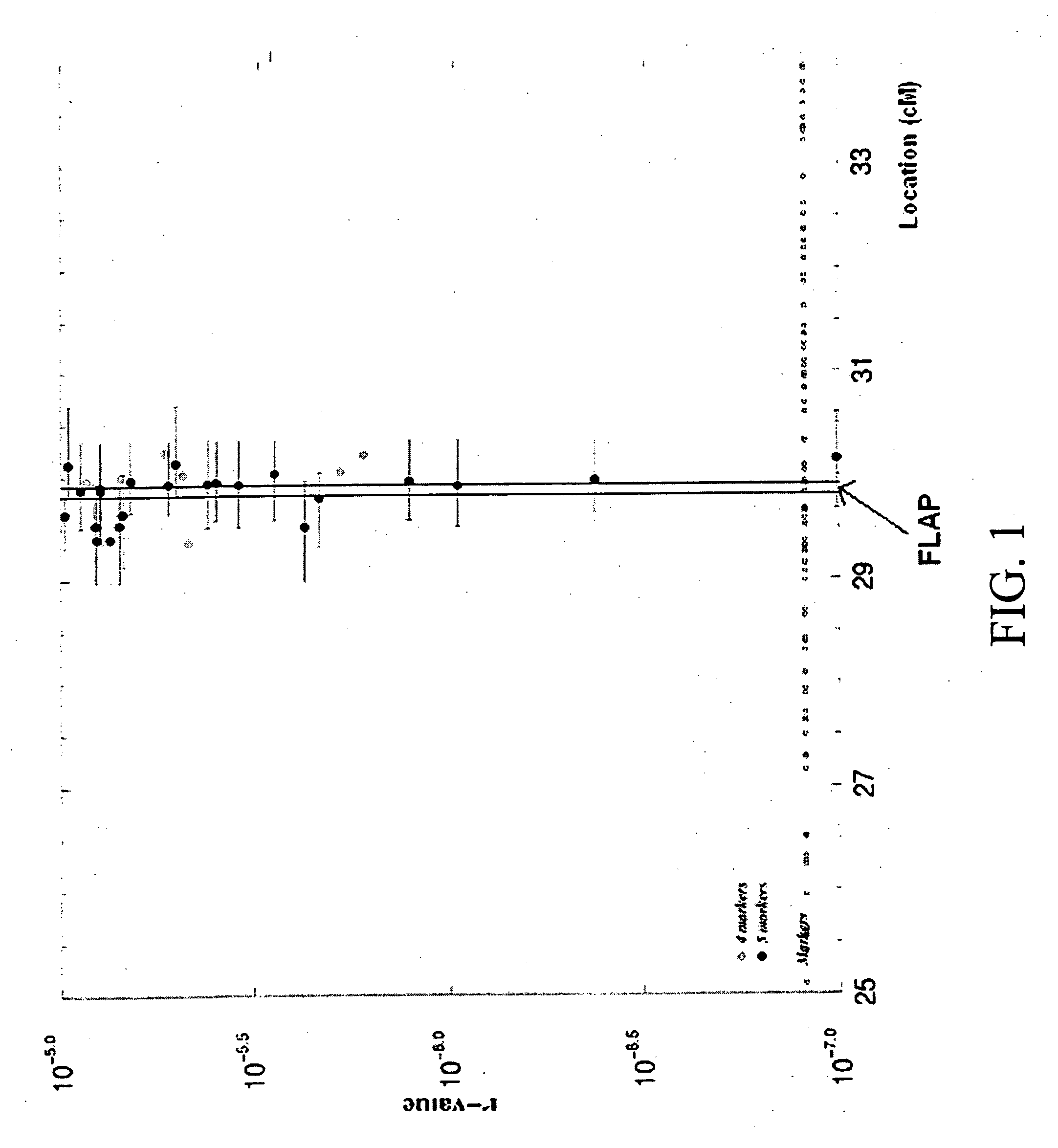
Susceptibility gene for myocardial infarction, stroke, and PAOD, methods of treatment
InactiveUS20060019269A1Reduce riskInhibit synthesisBiocidePeptide/protein ingredientsRisk strokeGenetic association analysis
Linkage of myocardial infarction (MI) and a locus on chromosome 13q12 is disclosed. In particular, the FLAP gene within this locus is shown by genetic association analysis to be a susceptibility gene for MI and ACS, as well as stroke and PAOD. Pathway targeting for treatment and diagnostic applications in identifying those who are at risk of developing MI, ACS, stroke or PAOD, in particular are described. The invention also provides for compositions comprising a leukotriene synthesis inhibitor and a stating and methods of using these compositions to reduce C-reactive protein in a human subject at risk of MI, ACS, stroke and / or PAOD.
Owner:DECODE GENETICS EHF
Preparation method of antigen-immobilized immuno- fluorescence slide and immuno-fluoroscence slide prepared thereby
InactiveUS20130029428A1Easily and highly-sensitively measuringBiological material analysisMammal material medical ingredientsAntigenBiotin-streptavidin complex
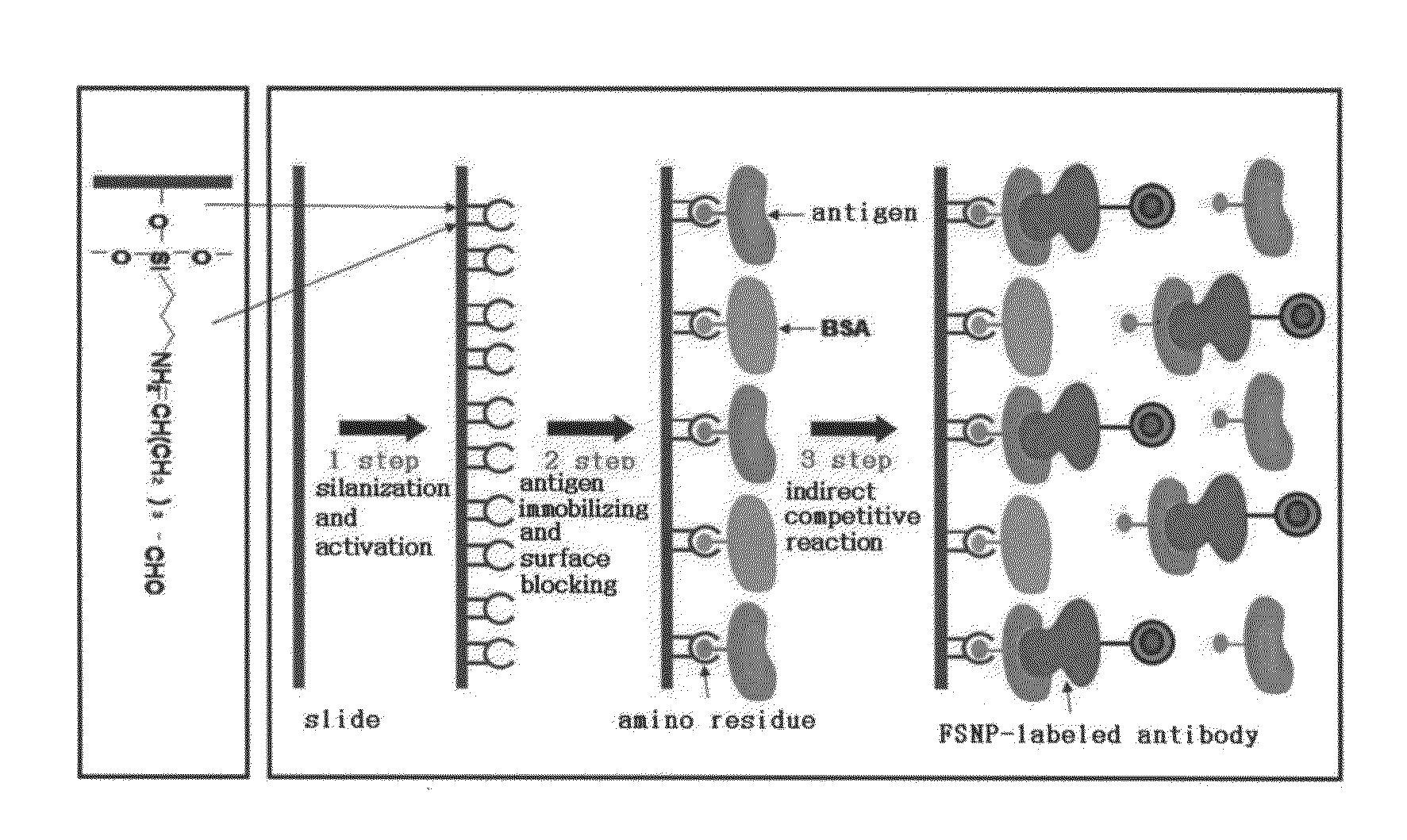
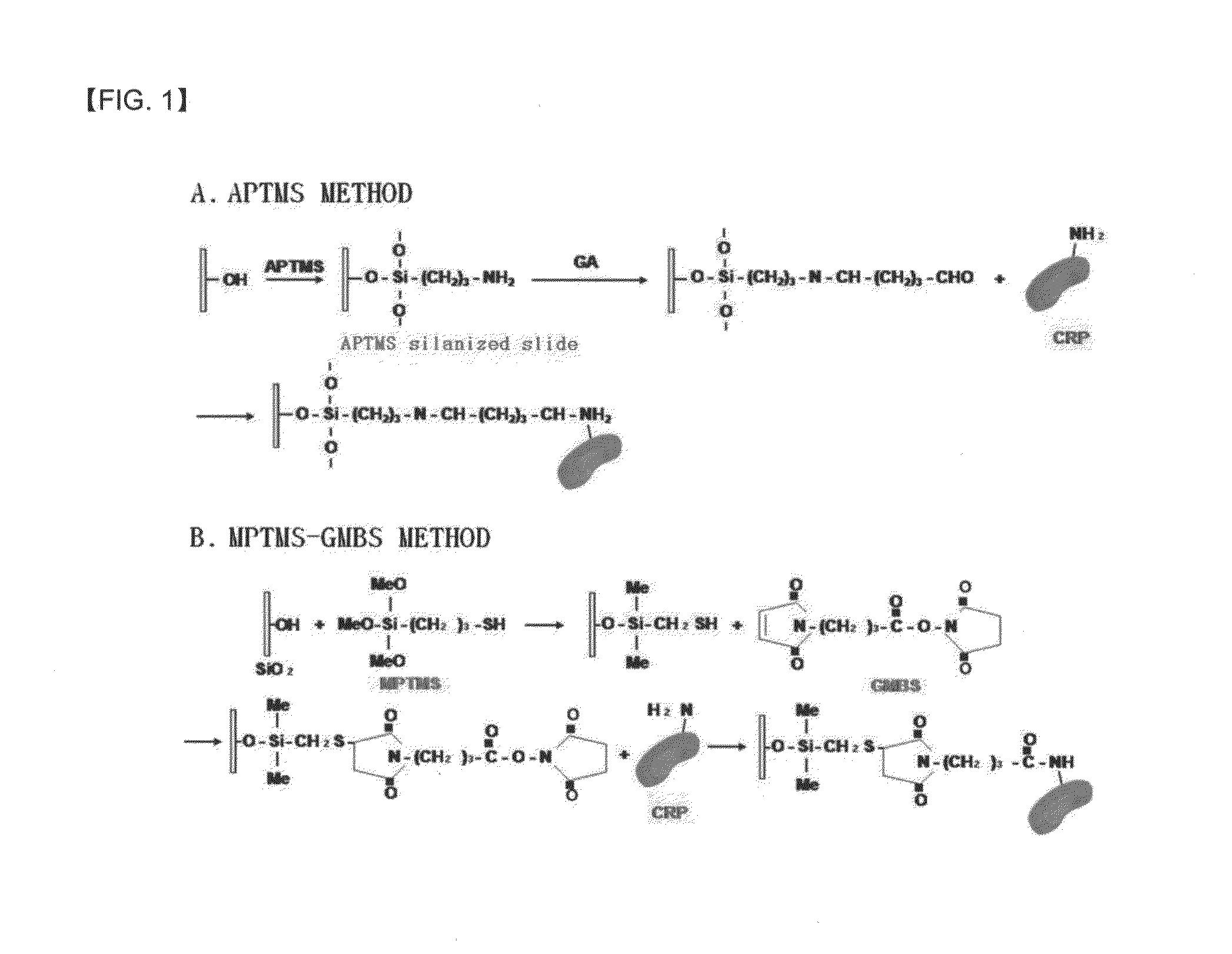
A method of preparing an antigen-immobilized immuno-fluorescence slide, the method comprising: immobilizing a C-reactive protein on a slide to prepare a protein chip; mixing an antibody that specifically binds to a target protein, with streptavidin to label the antibody with a fluorescent nanoparticle; immuno-reacting the antibody by competitive mixing, assaying with a fluorescence camera, wherein the immobilizing of the C-reactive protein on the slide comprises: modifying the slide with 3-aminopropyltrimethoxysilane to prepare a modified slide; hydrating the slide modified with 3-aminopropyltrimethoxysilane; activating the modified slide by using a glutaraldehyde solution; dissolving a C-reactive protein at a concentration of 0.01-0.5 mg / ml in a 30-70 mM phosphate buffer solution (pH 6.5-7.8) to prepare an antigen solution for immobilization; placing a petri dish comprising the slide on a spotting guide and spotting 1-100 μl of the antigen solution on spotting points; and performing a reaction on the slide prepared as described above for 1-6 hours to immobilize the antigen, and an immune-fluorescence slide prepared by using the method.
Owner:KOREA FOOD RES INST
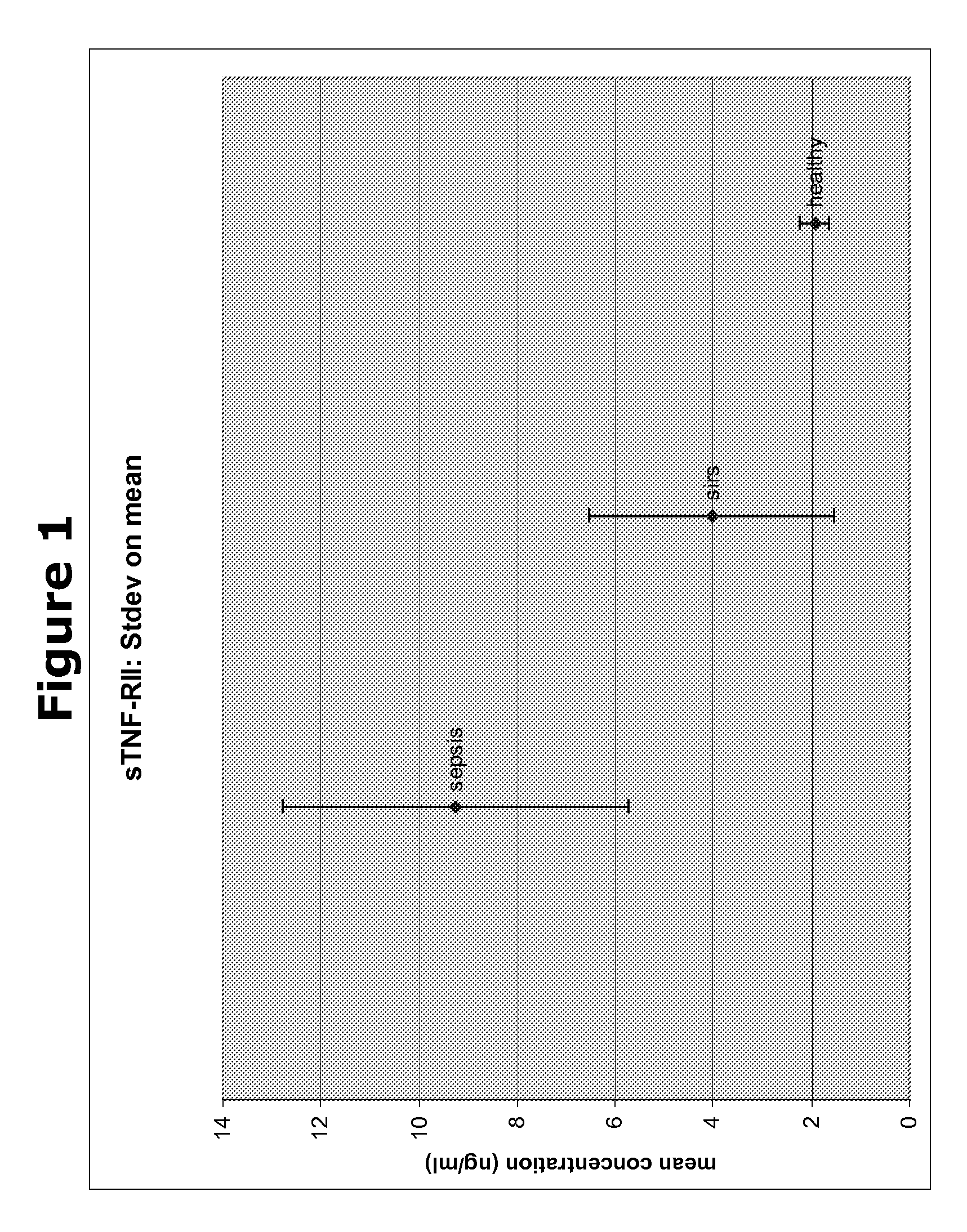
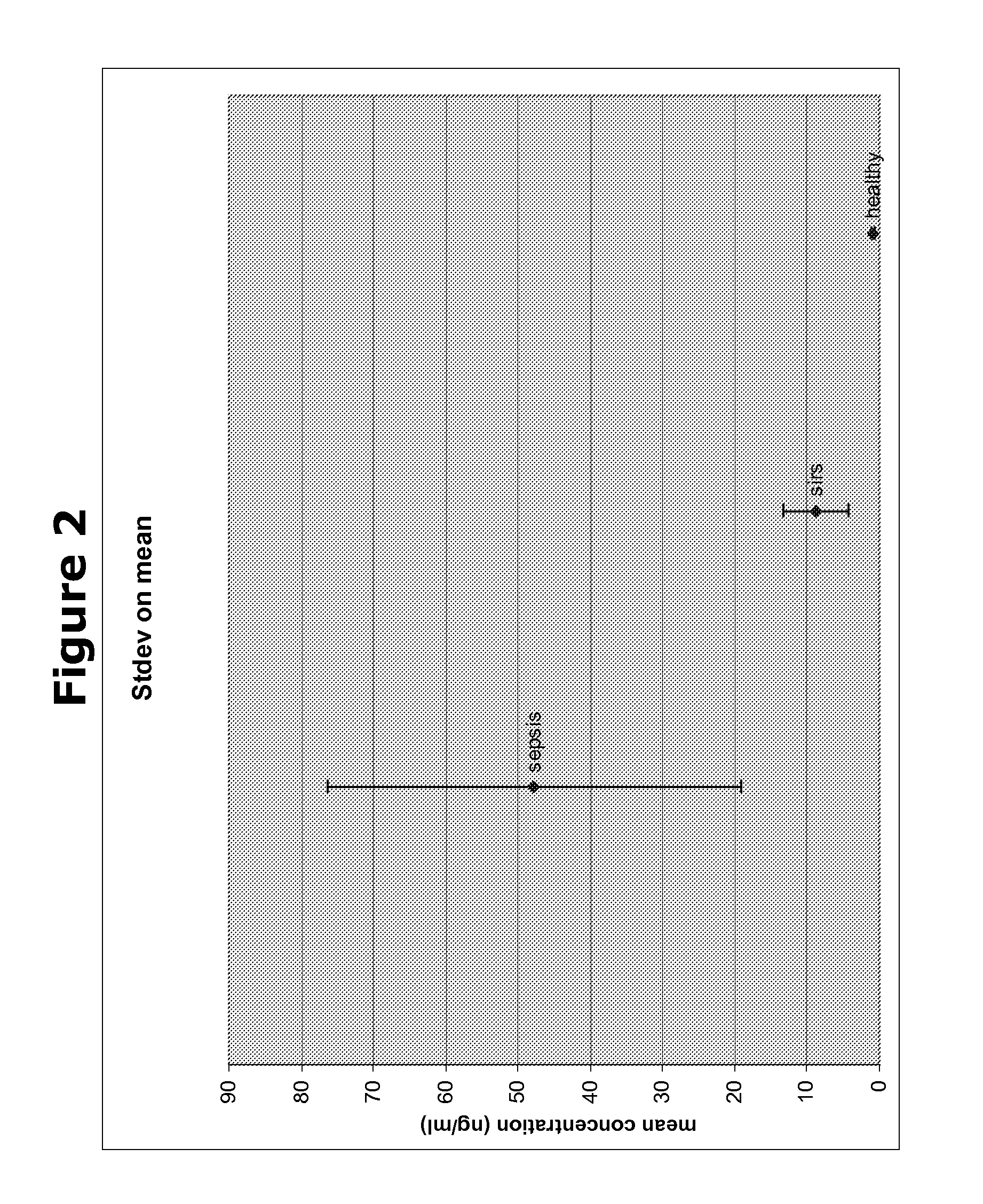
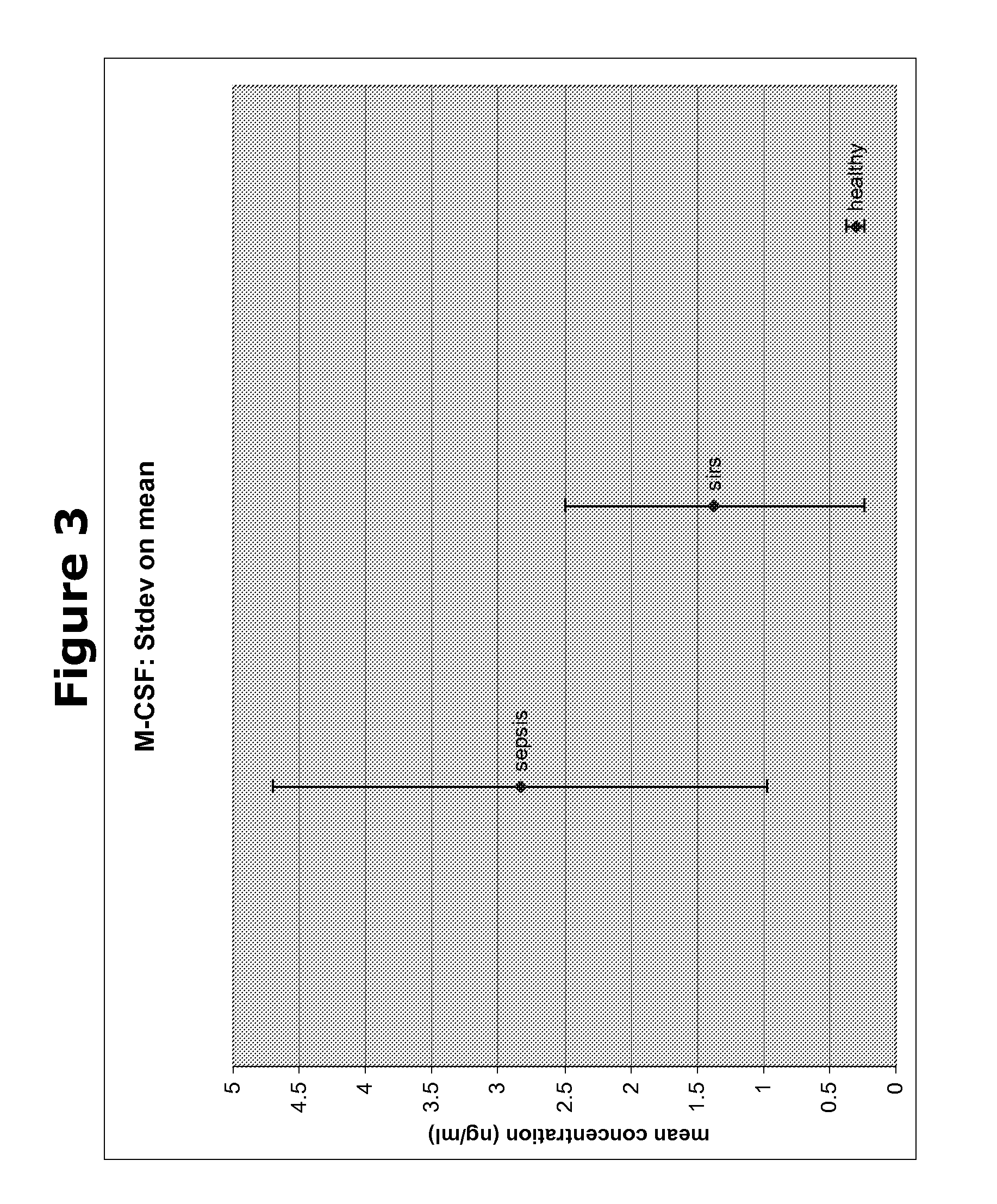
Biomarkers and methods for diagnosing, predicting and/or prognosing sepsis and uses thereof
InactiveUS20100292131A1Peptide librariesPeptide/protein ingredientsHealthy subjectsBiomarker (petroleum)
The present invention provides kits and methods for the diagnosis, prognosis and prediction of sepsis in a subject or for the differentiation between sepsis and SIRS in a subject, the method comprising(a) measuring the level of pro-hepcidin (pro-HEPC) in a biological sample taken from said subject, (b) measuring the level of at least one further biomarker selected from the group consisting of soluble TNF-receptor 2 (sTNFR2), Pentraxin-3 (PTX-3), Macrophage Colony-Stimulating Factor (MCSF), pro-Brain Natriuretic Protein (pro-BNP), one or more members of the Histone protein family, Procalcitonin (PCT) and c-Reactive Protein (CRP) in a biological sample from said subject, (c) using said measurements obtained in steps (a) and (b) to create a profile for said biomarkers and (d) comparing said profile with a reference biomarker profile obtained form a patient having SIRS or from a healthy subject.
Owner:BIOCARTIS NV
C reactive protein detection kit
ActiveCN103941017AImmunoglobulins against animals/humansBiological material analysisAntiendomysial antibodiesProtein.monoclonal
The invention relates to a C reactive protein detection kit, which contains a C reactive protein monoclonal antibody coated porous plate, an enzyme working solution, a sample diluent, an enzyme reaction substrate, a C reactive protein calibrator, and a C reactive protein quality control product. The kit provided by the invention can be used for quantitative detection of the CRP content in serum or plasma to realize early diagnosis of inflammation, and has the advantages of fast detection speed, high sensitivity, and good specificity.
Owner:BEIJING PERGRANDE BIOTECH DEV
Antisense modulation of C-reactive protein expression
Antisense compounds, compositions and methods are provided for modulating the expression of C-reactive protein. The compositions comprise antisense compounds, particularly antisense oligonucleotides, targeted to nucleic acids encoding C-reactive protein. Methods of using these compounds for modulation of C-reactive protein expression and for treatment of diseases associated with expression of C-reactive protein are provided.
Owner:IONIS PHARMA INC
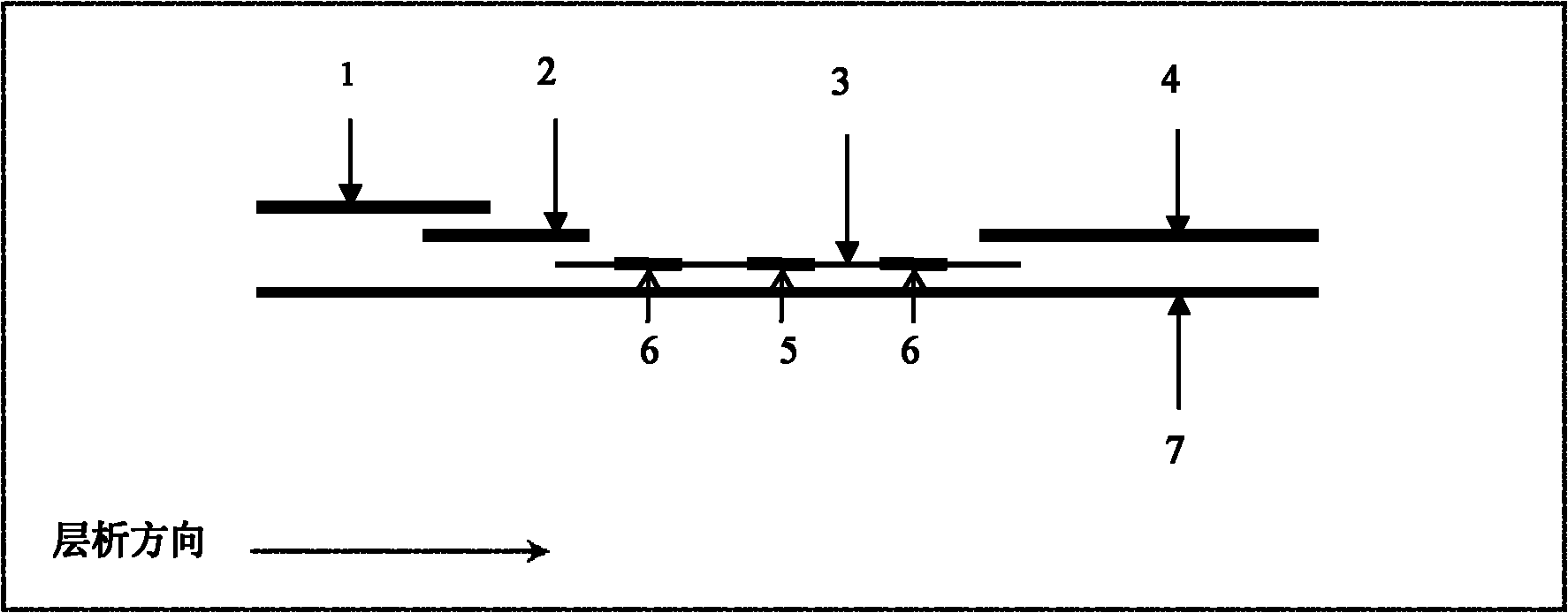
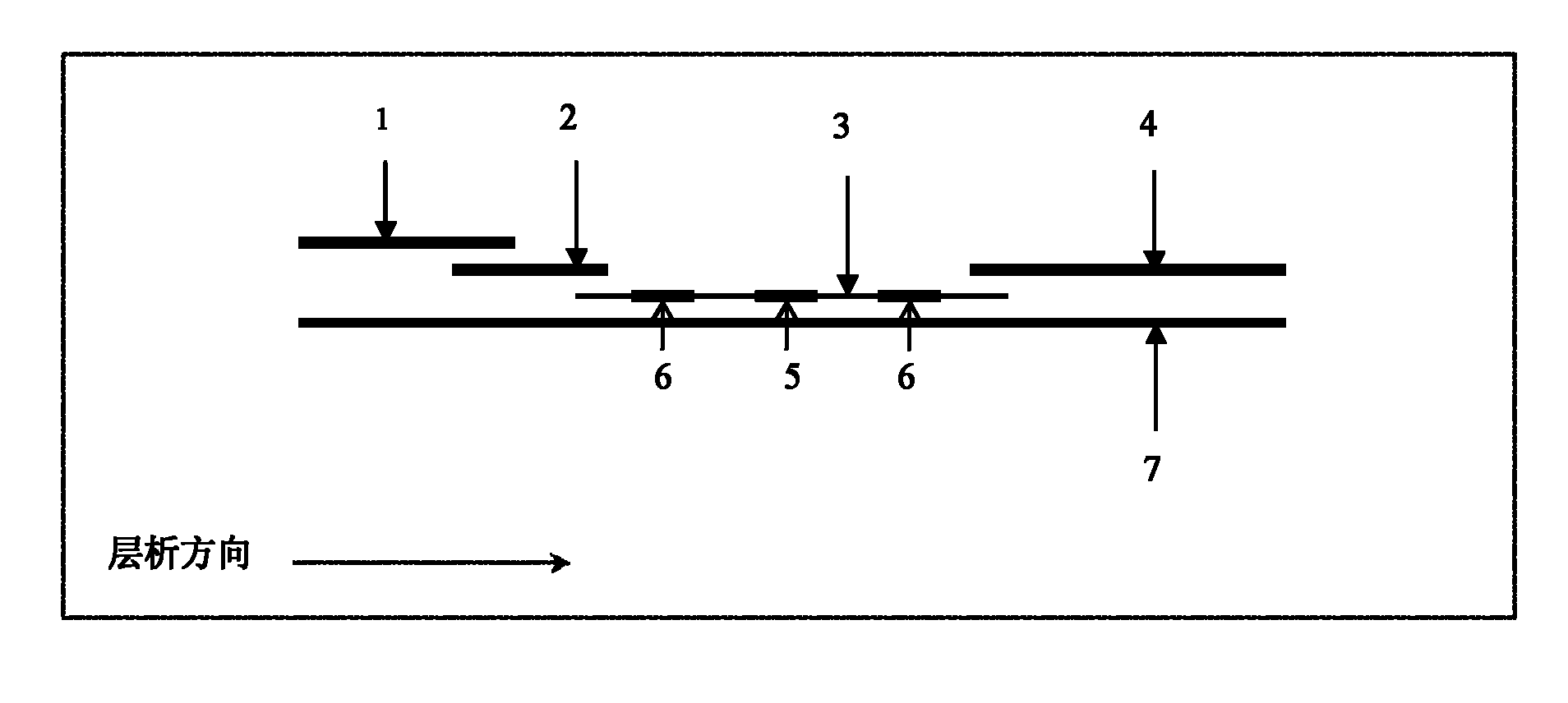
Human C-reactive protein colloidal gold immunochromatographic assay quantitative test paper
InactiveCN101887063AEasy to operateImprove detection efficiencyMaterial analysis by observing effect on chemical indicatorBiological testingAdhesiveColloid
The invention relates human C-reactive protein (CRP) colloidal gold immunochromatographic assay quantitative test paper, which comprises a sample pad, a bonding pad, a nitrocellulose membrane and absorbent paper mutually lapped on a bottom plate with an adhesive in turn. A colloidal gold-labeled CRP antibody complex is coated on the bonding pad; and a detection line and a quality control line are bonded on the nitrocellulose membrane, and are antibody-enveloped lines. During detection, the samples to be detected contact the lower end of the test paper and are subjected to chromatography along the test paper; if the quality control line and a quantitative line do not develop, the test is ineffective; if only the quality control line develops, the CRP concentration is lower than the lowest detection concentration; and if both the quality control line and the quantitative line become red, the content of the CRP is read through a quantitative detection device. The test paper is characterized in that the quality control line and the quantitative line are respectively arranged from the lower end to the upper end of the test paper along the sample chromatography direction.
Owner:沈鹤柏
Detection of asymptomatic coronary artery disease using atherogenic proteins and acute phase reactants
InactiveUS20050181451A1Easy to detectDelay disease progressionDisease diagnosisBiological testingCoronary arteriesCoronary Artery Disease Symptoms
A method of assessing the likelihood that a person who is asymptomatic for coronary artery disease does in fact have the disease is disclosed. The levels of an atherogenic protein and acute phase reactant and optionally of anti-atherogenic protein for an individual are obtained and compared to one or more cut-points related to those substances and, based on the comparison(s), an assessment is made of the likelihood that the individual has coronary artery disease. The atherogenic protein may be OxLDL, the acute phase reactant may be C-reactive protein or fibrinogen, and the anti-atherogenic protein may be HDL.
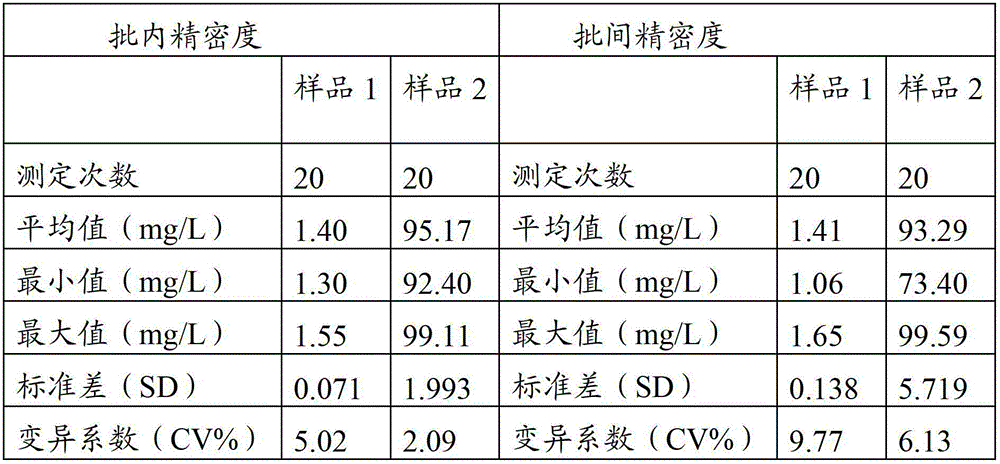
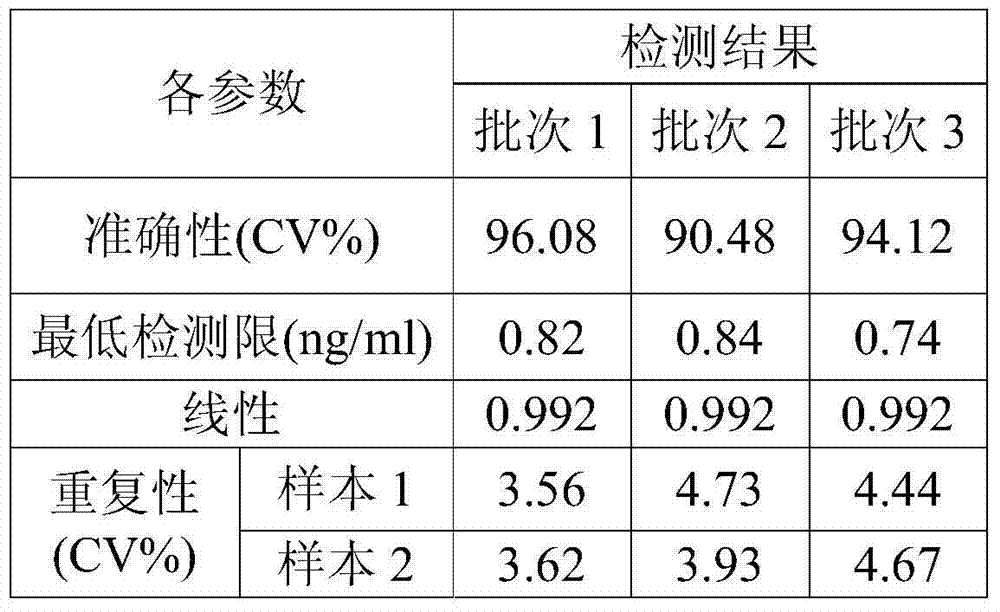
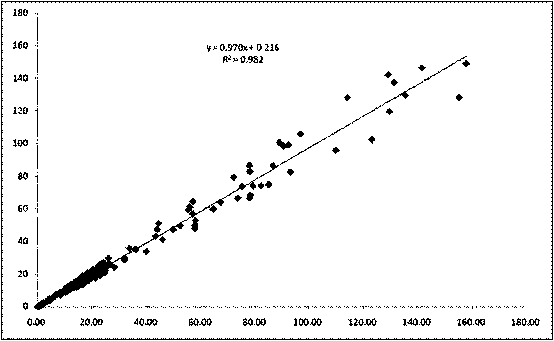
Method for improving sensitivity and linearity of latex reagent
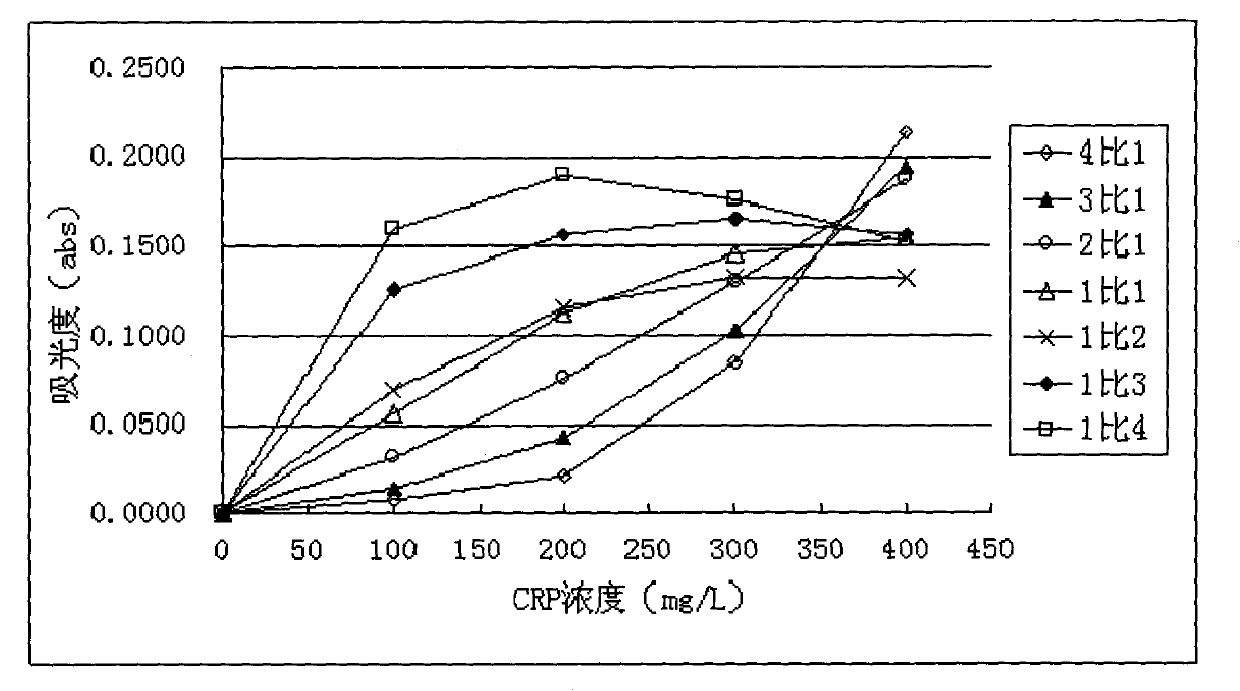
The invention provides a method for improving the sensitivity and linearity of a latex reagent. An adopted detection kit comprises a reagent R1 and a reagent R2. The method is characterized in that the latex reagent is a latex reagent mixed with latexes with large and small particle sizes, and is processed to prepare a C-creative protein latex turbidimetric detection kit, and the kit comprises the reagent R1 and the reagent R2. The method comprises the following steps: (1) directionally coating latex microspheres with large particle sizes with mouse to-be-tested antigen monoclonal antibodies; (2) directionally coating latex microspheres with small particle sizes with rabbit or sheep to-be-tested antigen monoclonal antibodies; (3) mixing the coated latex microspheres with the large particle sizes and the coated latex microspheres with the small particle sizes.
Owner:浙江夸克生物科技有限公司
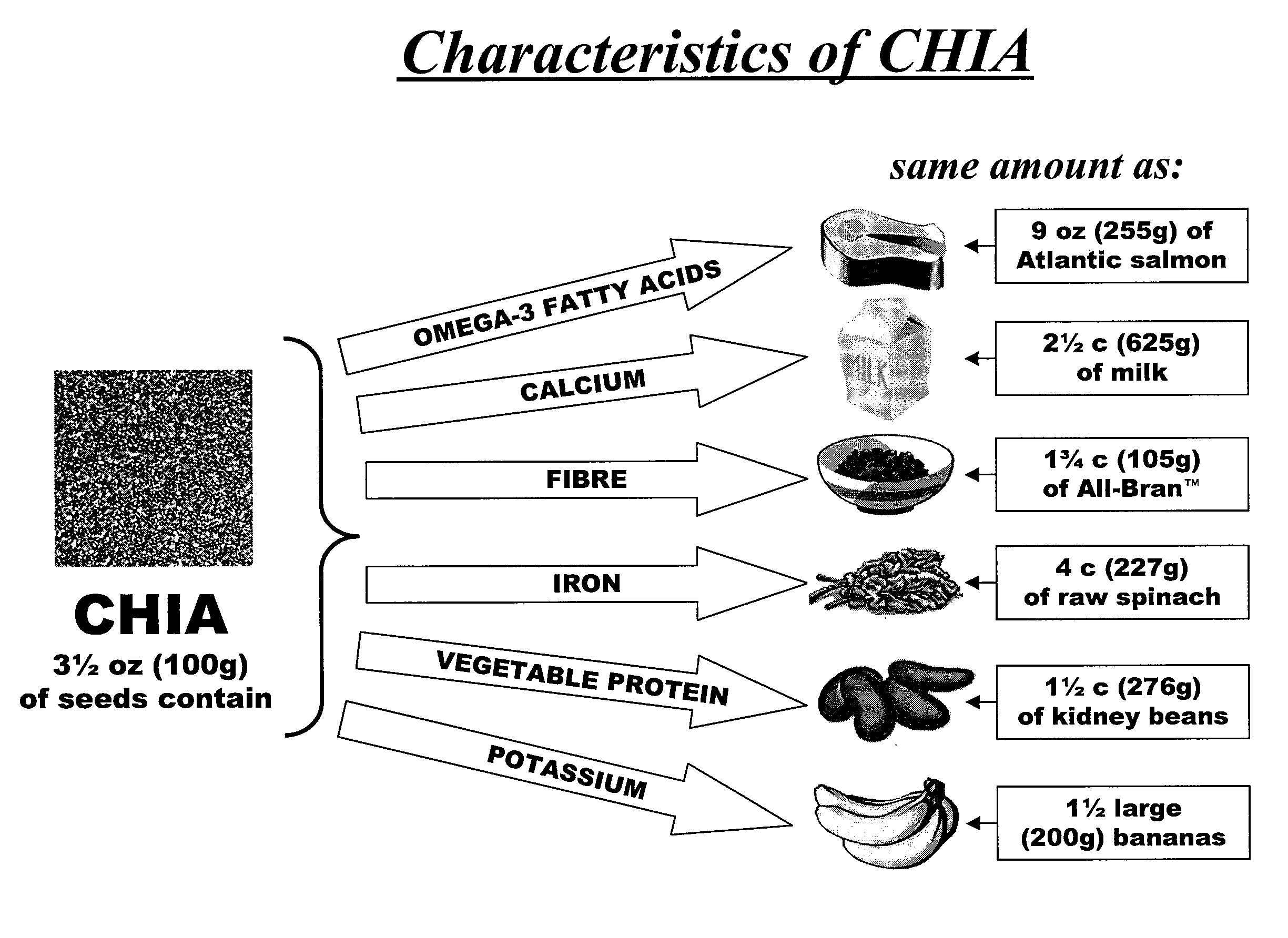
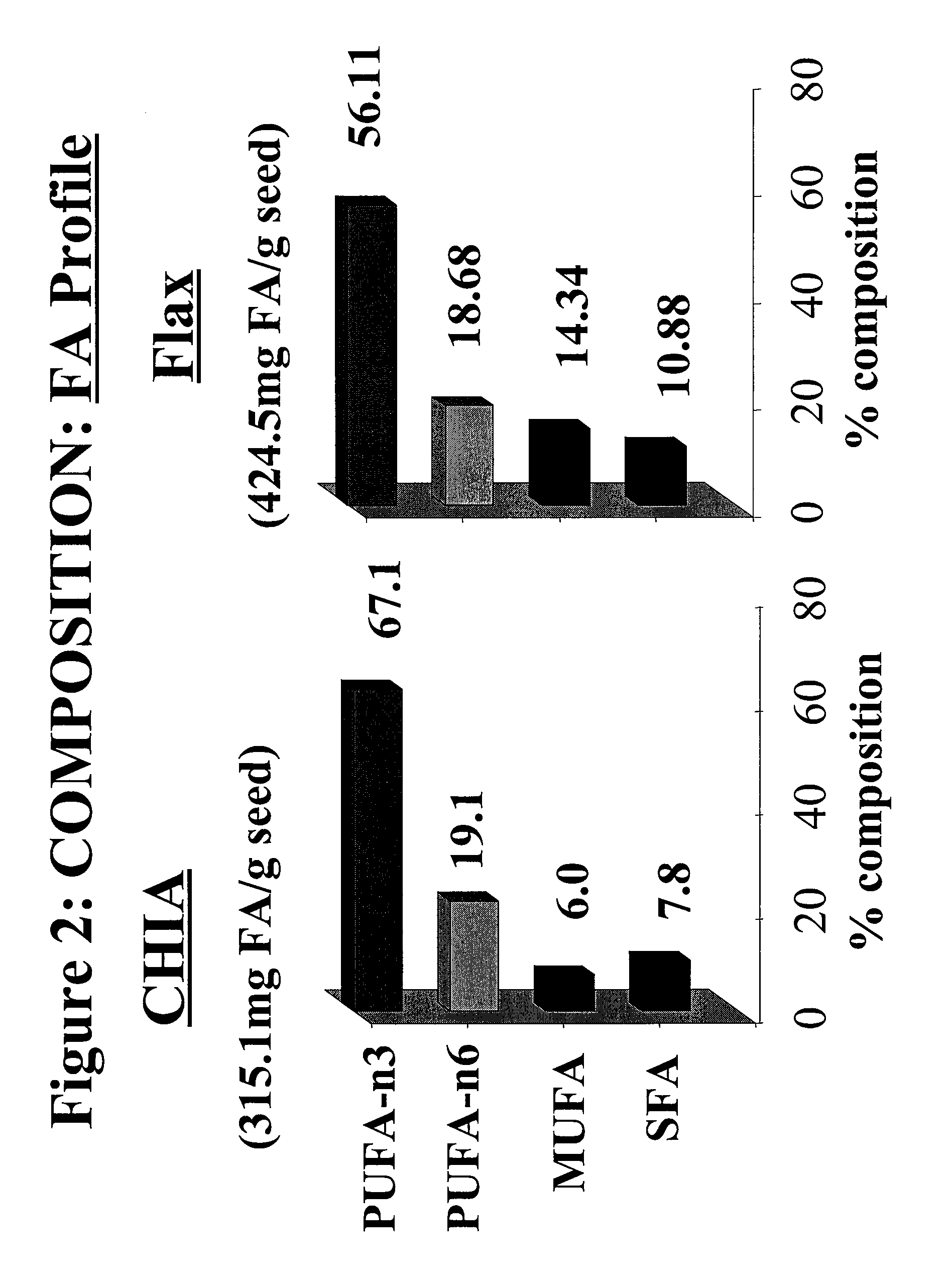
Salvia hispanica I (Chia) in the management and treatment of cardiovascular disease, diabetes and associated risk factors
InactiveUS20080305190A1Improve treatment outcomesReduces fasting and postprandial blood glucoseBiocideMetabolism disorderInflammatory factorsFactor ii
Described is use of Salvia hispanica L. (Chia) for controlling, in one embodiment reducing, blood glucose levels, preferably post-prandial blood glucose levels. This is useful in both non-diabetic and diabetic individuals, but especially in diabetic individuals. Also described is the use of chia in reducing postprandial blood glucose, insulin sensitivity, blood pressure, and oxidative stress in such individuals. The present invention further found that Chia can be used to improve endothelial function, coagulation, fibrinolysis and iron status. The present invention further encompasses the use of Chia in the treatment and / or management of diabetes and / or the treatment and management of diabetes associated conditions or risk factors, such as one or more of the following: blood pressure and blood glucose levels, post-prandial glycemia, inflammatory factors (C-reactive protein), coagulation (fibrinogen, factor VIII, von Willenbrand factor), and fibronolytic factors (such as t-PA), iron status and endothelial function, (such as increase in nitric oxide generation). In one embodiment the invention relates to dietary approaches to such treatment and management.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
Turbidimetric rapid detection kit for myocardial infarction nano-immunoenhancement and use method thereof
InactiveCN103185798ANo special training requiredNo need to waitMaterial analysis by observing effect on chemical indicatorBiological testingDisease courseBiomarker (petroleum)
The invention provides a turbidimetric rapid detection kit for myocardial infarction nano-immunoenhancement. The turbidimetric rapid detection kit comprises a reaction-detection integrated device, and a detection kit arranged in the reaction device, wherein a reagent R1, a reagent R2 and a calibrator are contained in the detection kit; simultaneously, quantitative detection for five important myocardial infarction-related biomarkers in one sample can be realized, and the five important biomarkers include content of troponin-I, content of D-dimer, content of myoglobin, content of hypersensitive C-reactive protein (hs-CRP) and content of heart-type fatty acid binding protein (FABP); and the turbidimetric rapid detection kit has an important clinical significance in the aspects of early diagnosis and disease course monitoring for myocardial infarction, treatment monitoring for medicines, and the like. The turbidimetric rapid detection kit provided by the invention realizes the integration of the reagents and the reaction device, and is simple, rapid and accurate to operate, high in sensitivity, strong in specificity, and low in detection cost; and the turbidimetric rapid detection kit is a detection / reagent measurement integrated kit suitable for outpatient and emergency treatment / clinical detection, and wide in instrument application range.
Owner:SUZHOU DIAGVITA BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com