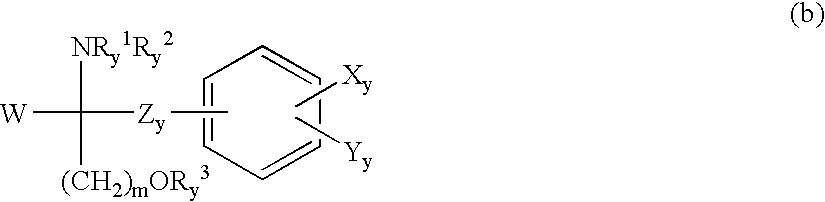Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
341 results about "HMG-CoA reductase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
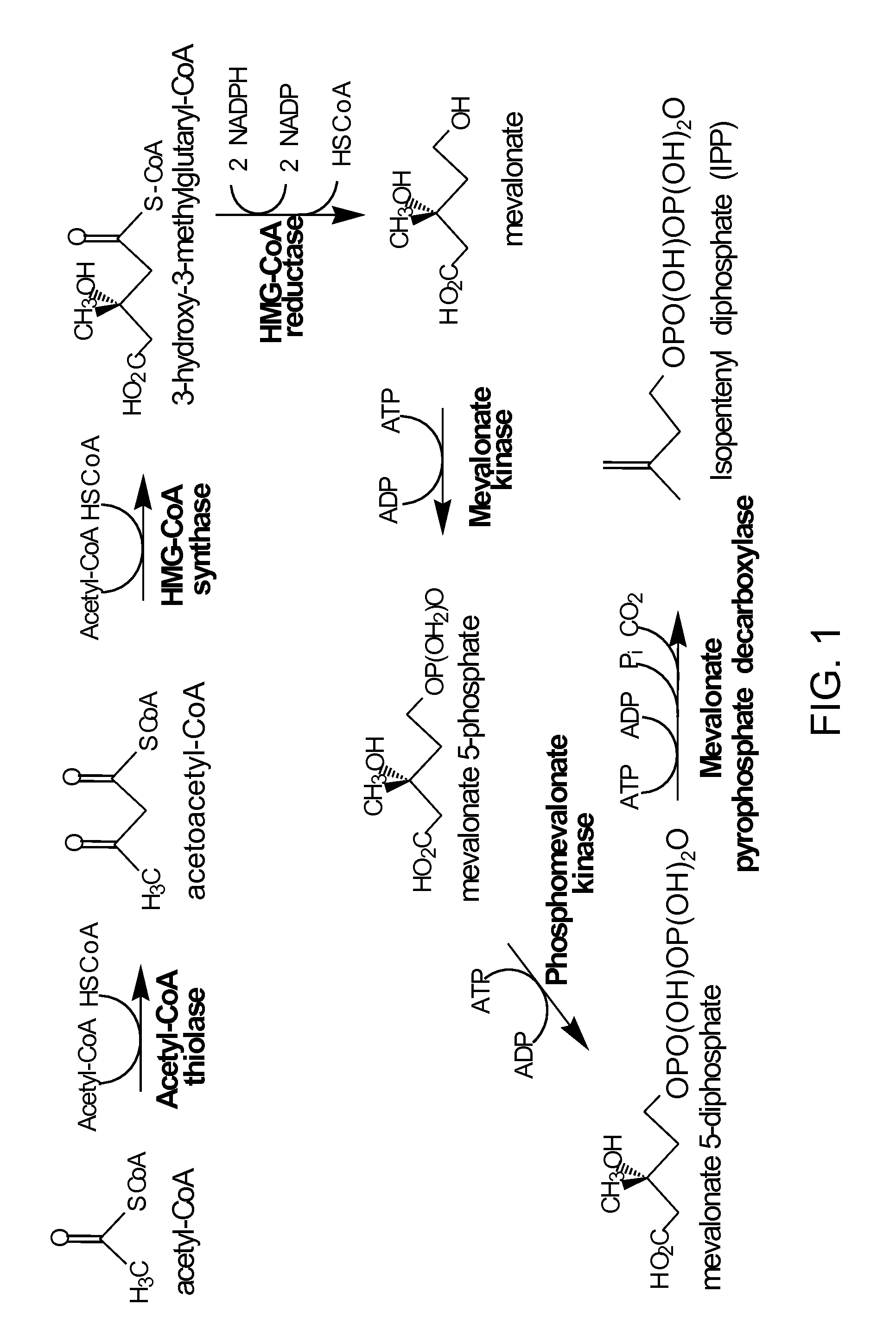
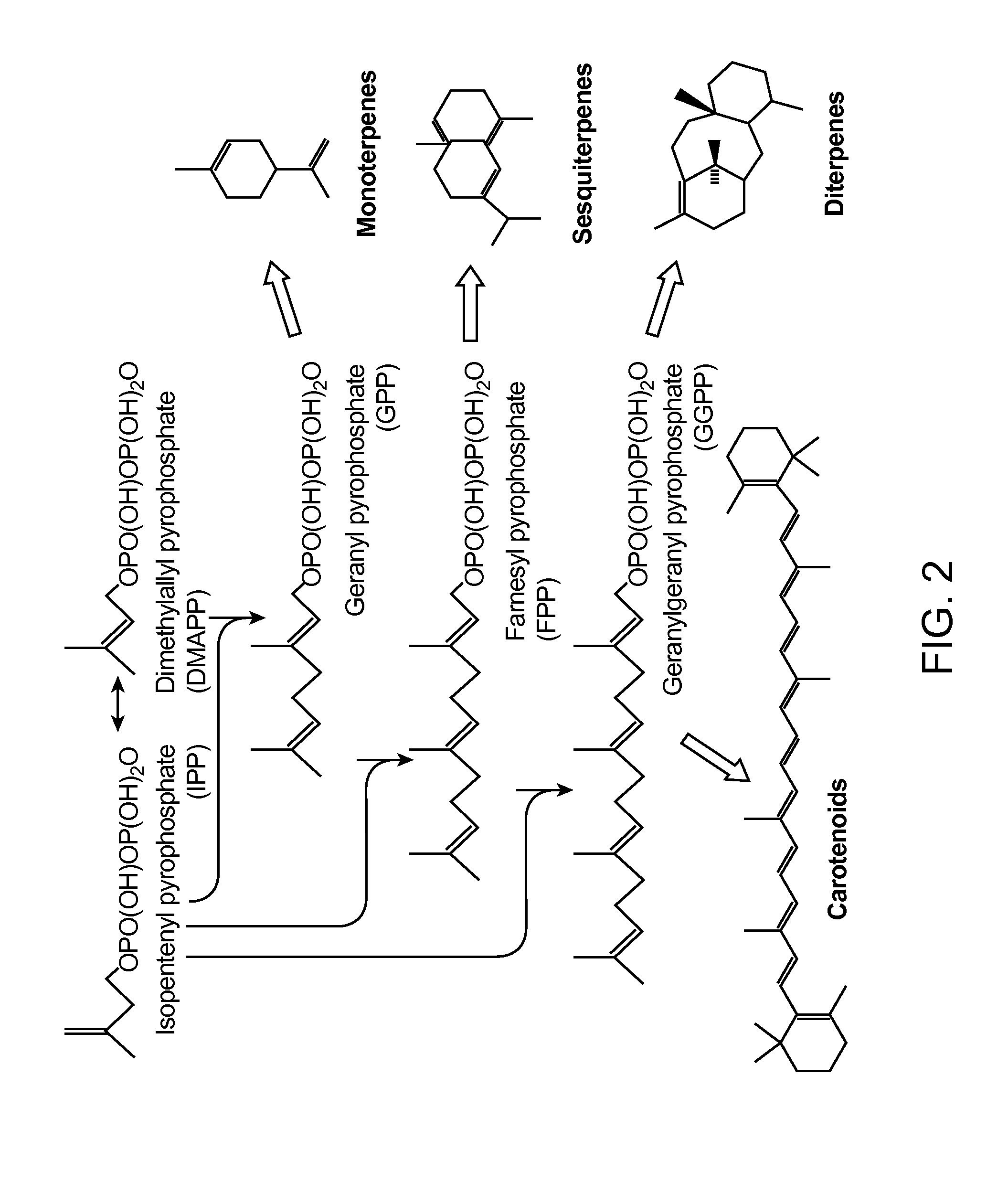
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, officially abbreviated HMGCR) is the rate-controlling enzyme (NADH-dependent, EC 1.1.1.88; NADPH-dependent, EC 1.1.1.34) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. Normally in mammalian cells this enzyme is suppressed by cholesterol derived from the internalization and degradation of low density lipoprotein (LDL) via the LDL receptor as well as oxidized species of cholesterol. Competitive inhibitors of the reductase induce the expression of LDL receptors in the liver, which in turn increases the catabolism of plasma LDL and lowers the plasma concentration of cholesterol, which is considered, by those who accept the standard lipid hypothesis, an important determinant of atherosclerosis. This enzyme is thus the target of the widely available cholesterol-lowering drugs known collectively as the statins.
Stent coatings containing HMG-CoA reductase inhibitors
InactiveUS20030077310A1Hydrolysis can be preventedAvoid accessStentsSurgeryHMG-CoA reductaseDepressant
Stents with coatings comprising a combination of a restenosis inhibitor comprising an HMG-CoA reductase inhibitor and a carrier. Also provided are methods of coating stents with a combination of an HMG-CoA reductase inhibitor and a carrier. A preferred example of a restenosis inhibitor is cerivastatin. The stent coatings have been shown to release restenosis inhibitors in their active forms.
Owner:ZIMMER ORTHOBIOLIGICS
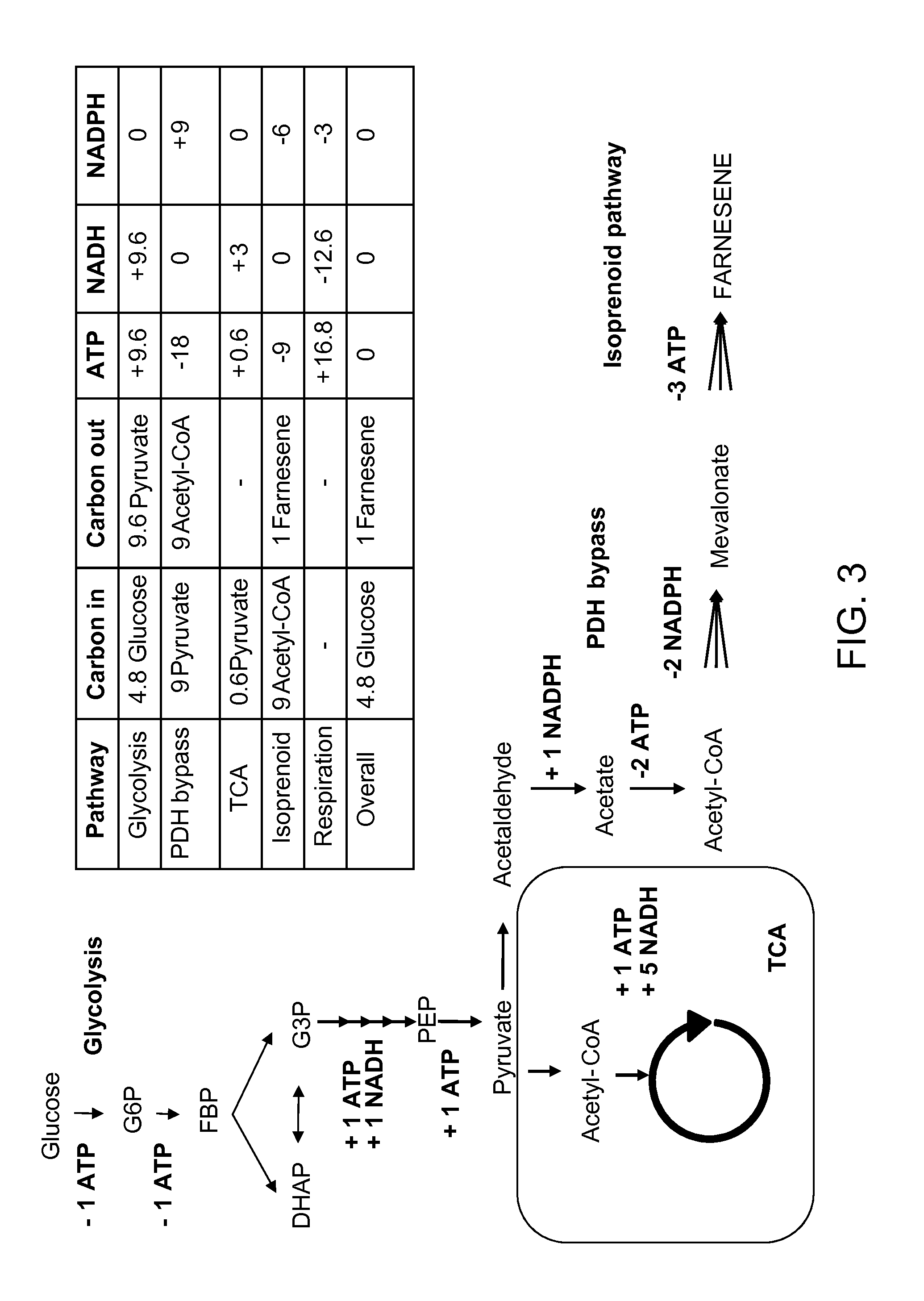
Production of acetyl-coenzyme a derived isoprenoids
ActiveUS8415136B1Avoid high forceThermodynamically favorableFungiTransferasesHeterologousHMG-CoA reductase
Provided herein are compositions and methods for the heterologous production of acetyl-CoA-derived isoprenoids in a host cell. In some embodiments, the host cell is genetically modified to comprise a heterologous nucleotide sequence encoding an acetaldehyde dehydrogenase, acetylating (ADA, E.C. 1.2.1.10) and an MEV pathway comprising an NADH-using HMG-CoA reductase. In some embodiments, the host cell is genetically modified to comprise a heterologous nucleotide sequence encoding an ADA and an MEV pathway comprising an acetoacetyl-CoA synthase. In some embodiments, the genetically modified host cell further comprises one or more heterologous nucleotide sequences encoding a phosphoketolase and a phosphotransacetylase. In some embodiments, the genetically modified host cell further comprises a functional disruption of the native PDH-bypass. The compositions and methods described herein provide an energy-efficient yet redox balanced route for the heterologous production of acetyl-CoA-derived isoprenoids.
Owner:AMYRIS INC
Cyclic amine bace-1 inhibitors having a benzamide substituent
ActiveUS20050119227A1Inhibition formationBiocideNervous disorderHMG-CoA reductaseNeuro-degenerative disease
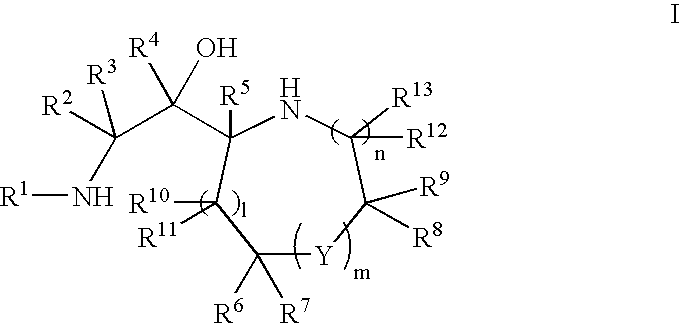
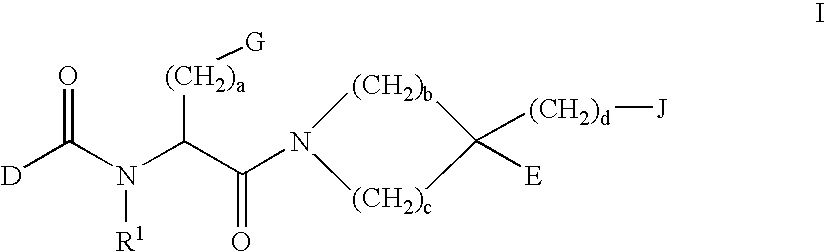
Disclosed are compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein [0001]R1 is [0002]R is —C(O)—N(R27)(R28) or and the remaining variables are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are pharmaceutical compositions and methods of treating cognitive or neurodegenerative diseases comprising the compounds of formula I in combination with a β-secretase inhibitor other than those of formula I, an HMG-CoA reductase inhibitor, a gamma-secretase inhibitor, a non-steroidal anti-inflammatory agent, an N-methyl-D-aspartate receptor antagonist, a cholinesterase inhibitor or an anti-amyloid antibody.
Owner:PHARMACOPEIA DRUG DISCOVERY +1
Composition and method for treating cancer
InactiveUS7074825B2Promote growthReduce the total massBiocidePeptide/protein ingredientsHMG-CoA reductaseCancer cell
A composition and an associated method of treating cancer cells by impeding cancer cell growth with the composition are disclosed. The composition includes at least a first and a second HMG-CoA reductase inhibitor, wherein the total amount of the first and second HMG-CoA reductase inhibitors is effective in synergistically impeding cancer cell growth and wherein the cancer cell growth synergistic impedance from the total amount of the first and second HMG-CoA reductase inhibitors is greater than a theoretical additive effect from the combined first and second HMG-CoA reductase inhibitors. The present composition does not simultaneously contain both a tocotrienol and an ionone when the composition contains only a first and a second HMG-CoA reductase inhibitor. The method includes treating cancer cells with the claimed composition to impede cancer cell growth.
Owner:MO HUANBIAO +3
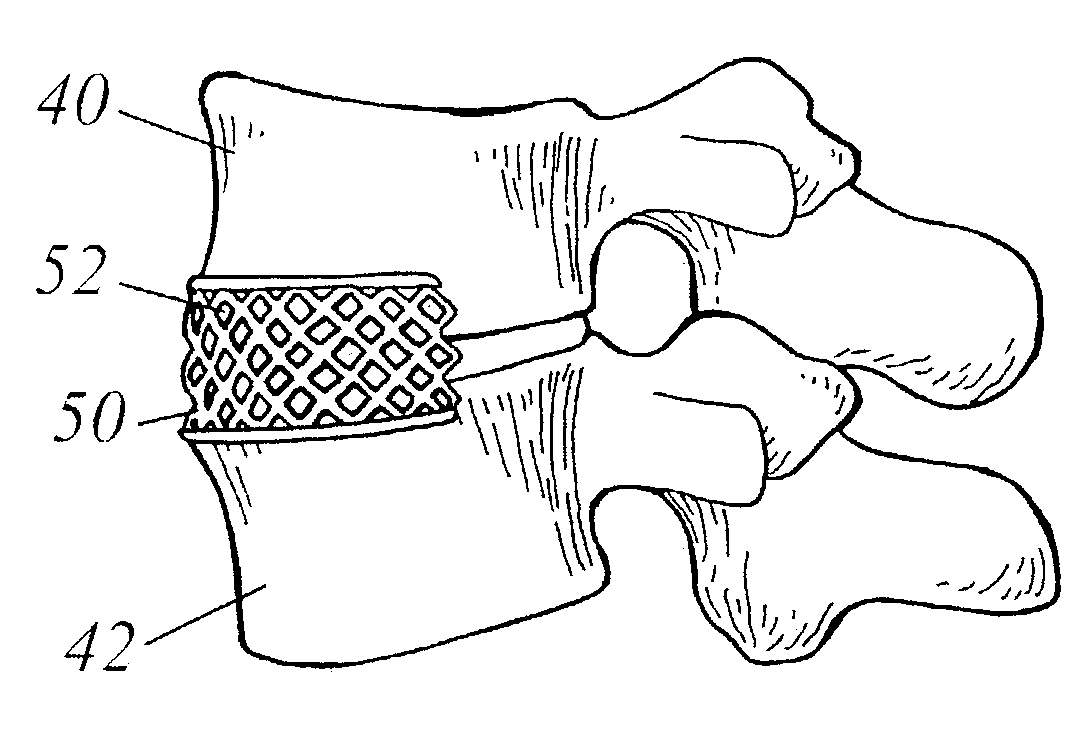
Spinal fusion using an HMG-CoA reductase inhibitor
ActiveUS7041309B2Promote bone growthImprove responseBiocideImpression capsHMG-CoA reductaseHeterotopic bone
An improved technique for spinal fusion including administration of an HMG-CoA reductase inhibitor to the fusion site. The HMG-CoA reductase inhibitor is preferably delivered to the site by a carrier. More preferably, the HMG-CoA reductase inhibitor is delivered to the site by a noncompressible delivery vehicle. The invention is suitable for promoting non-anatomic or heterotopic bone growth between any bony surfaces where bone growth is desired but does not naturally occur.
Owner:NEUROPRO TECH
Drug eluting patch for the treatment of localized tissue disease or defect
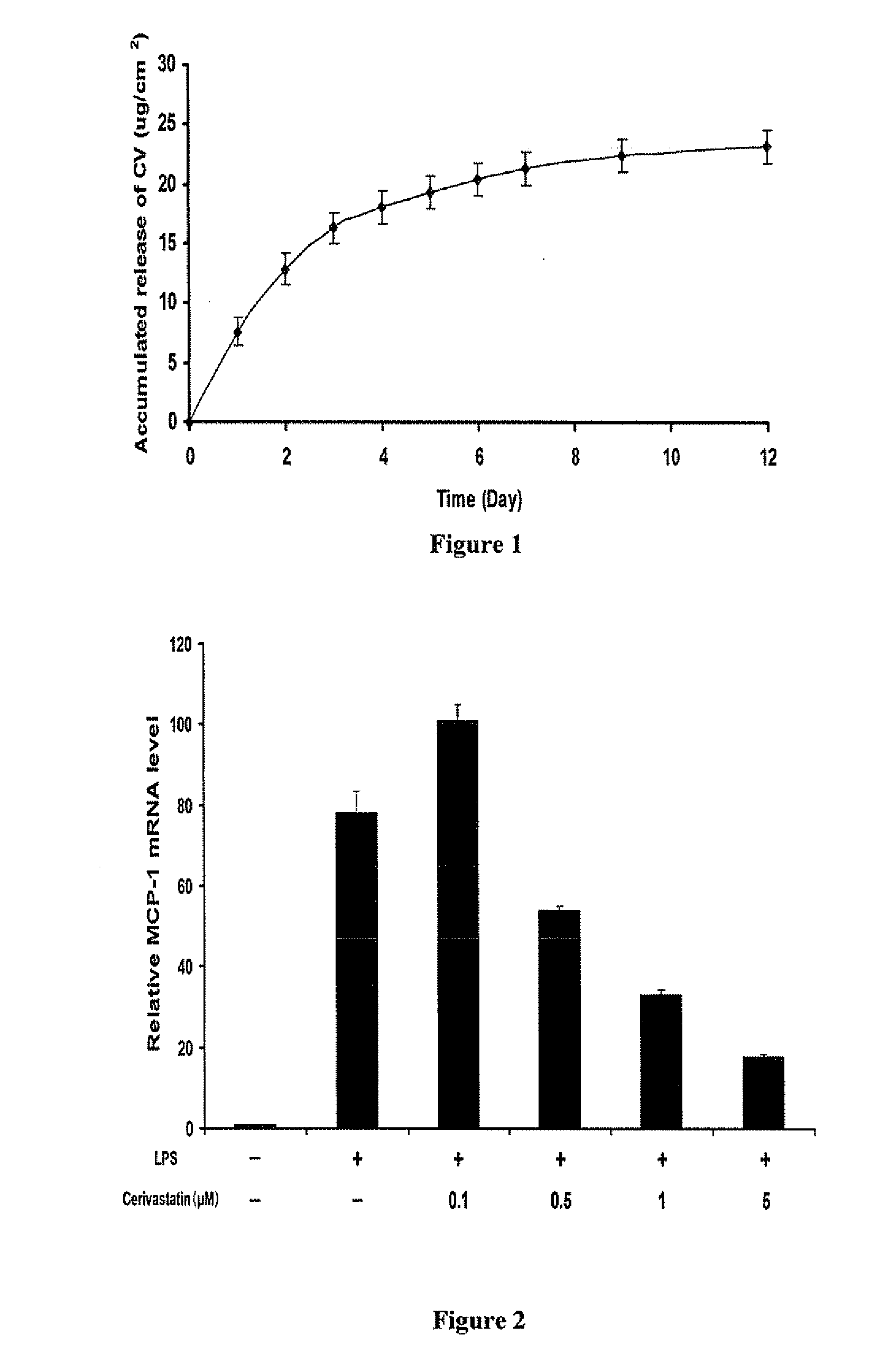
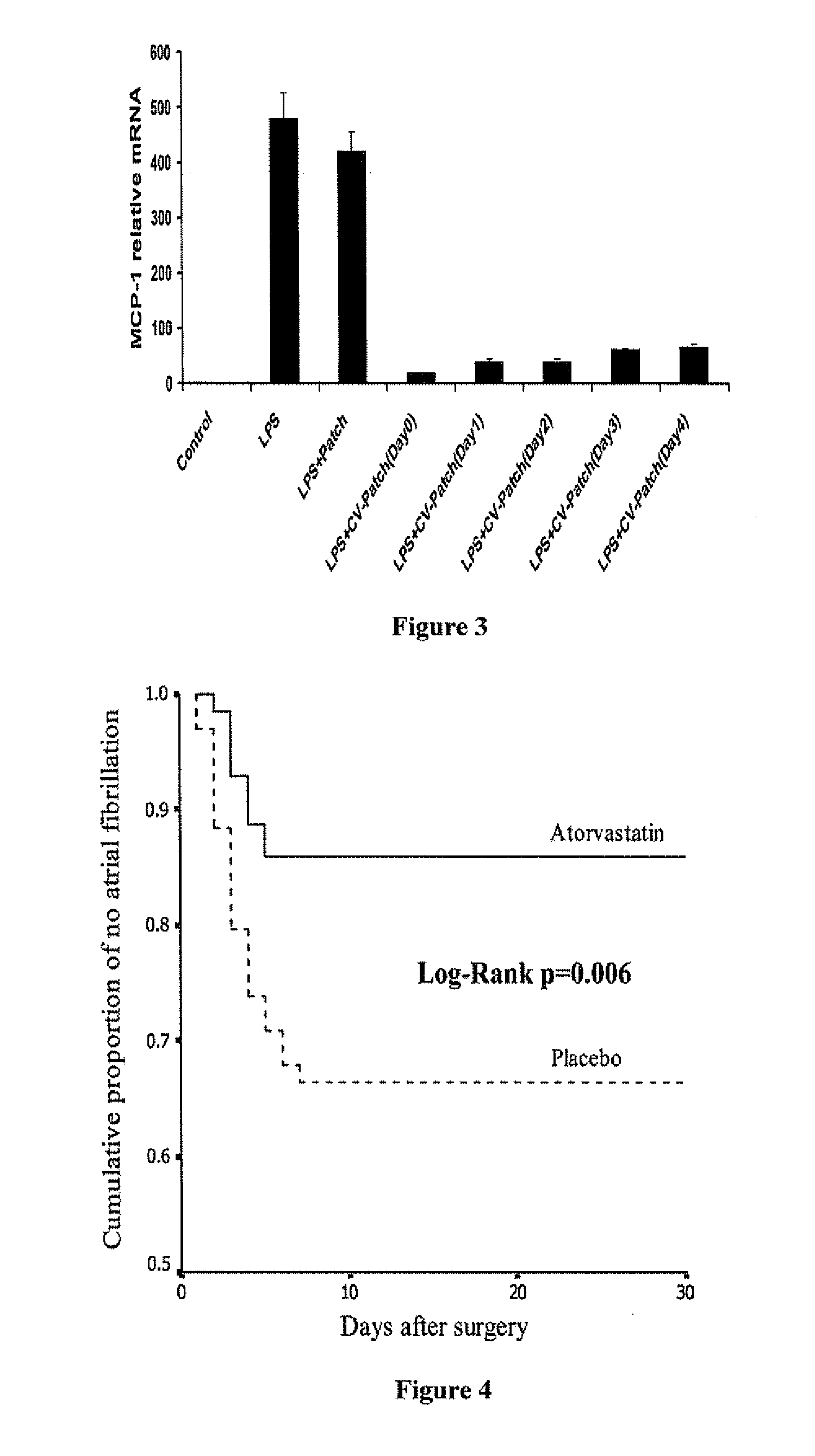
A polymeric matrix for delivery of an HMG CoA reductase inhibitor such as a statin to tissue such as cardiac tissue in need thereof for the treatment or prevention of a disease or defect such as atrial fibrillation has been developed. In the preferred embodiment, a statin is delivered by means of a patch sutured to cardiac tissue at the time of cardiothoracic surgery. In the most preferred embodiment, the patch is a biodegradable material providing controlled or sustained release over a prolonged period of time, such as a week. Suitable materials include extracellular matrix, or other biodegradable hydrogels or polymeric materials providing sustained or controlled release of statin at the site of application.
Owner:MIDCAP FINANCIAL TRUST AS AGENT
Composition for improving blood cholesterol levels
A nutritional composition for improving blood cholesterol by jointly and simultaneously inhibiting cholesterol absorption, decreasing blood LDL levels, increasing blood HDL levels and interfering with HMG-CoA reductase synthesis or degradation in an individual comprising, therapeutically effective amounts of plant sterols or plant stanols or derivatives thereof, procyanidins, policosanol and niacin or derivatives of niacin is provided. Both a composition and a method are provided by the present disclosure.
Owner:IOMEDIX DEV INT
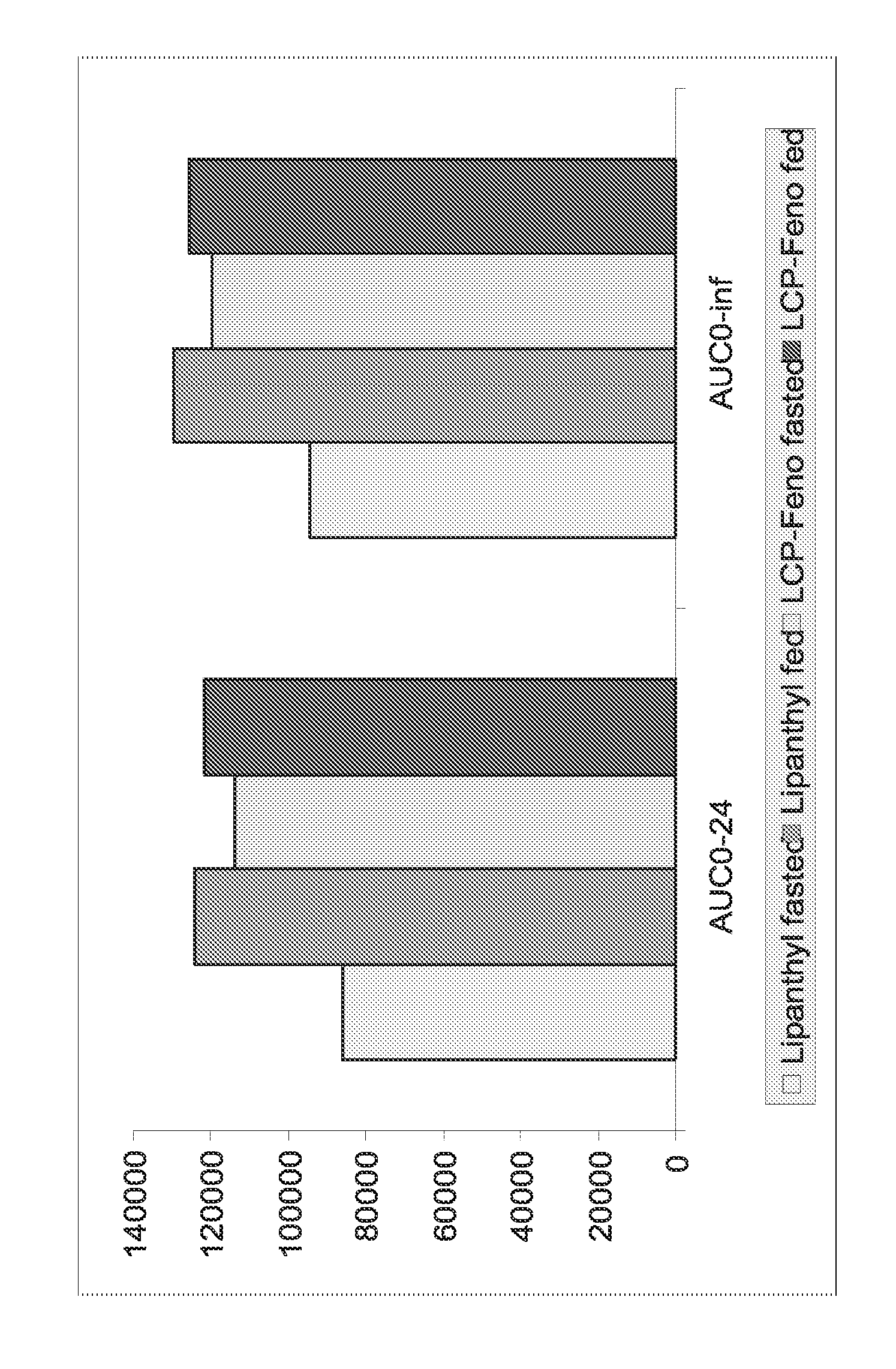
Compositions comprising fenofibrate and rosuvastatin
InactiveUS20050096391A1Improve bioavailability in vivoSubstance may accumulateBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor rosuvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCrosuvastatin) of between about 150 and about 12,000. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and rosuvastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
Nanoparticles for drug delivery
InactiveUS20070237827A1Sustained releaseReduce resistanceBiocidePowder deliveryHMG-CoA reductaseMedicine
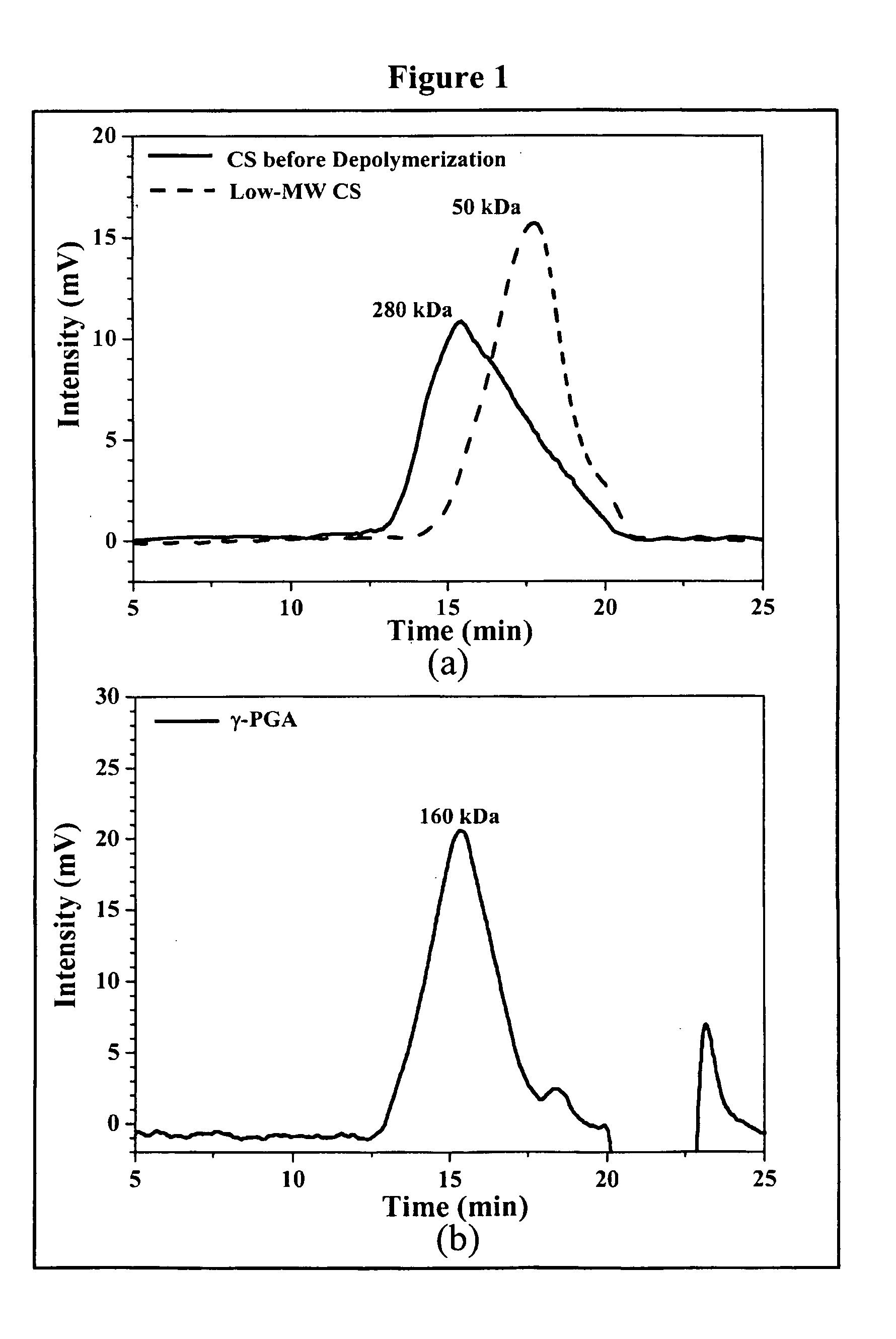
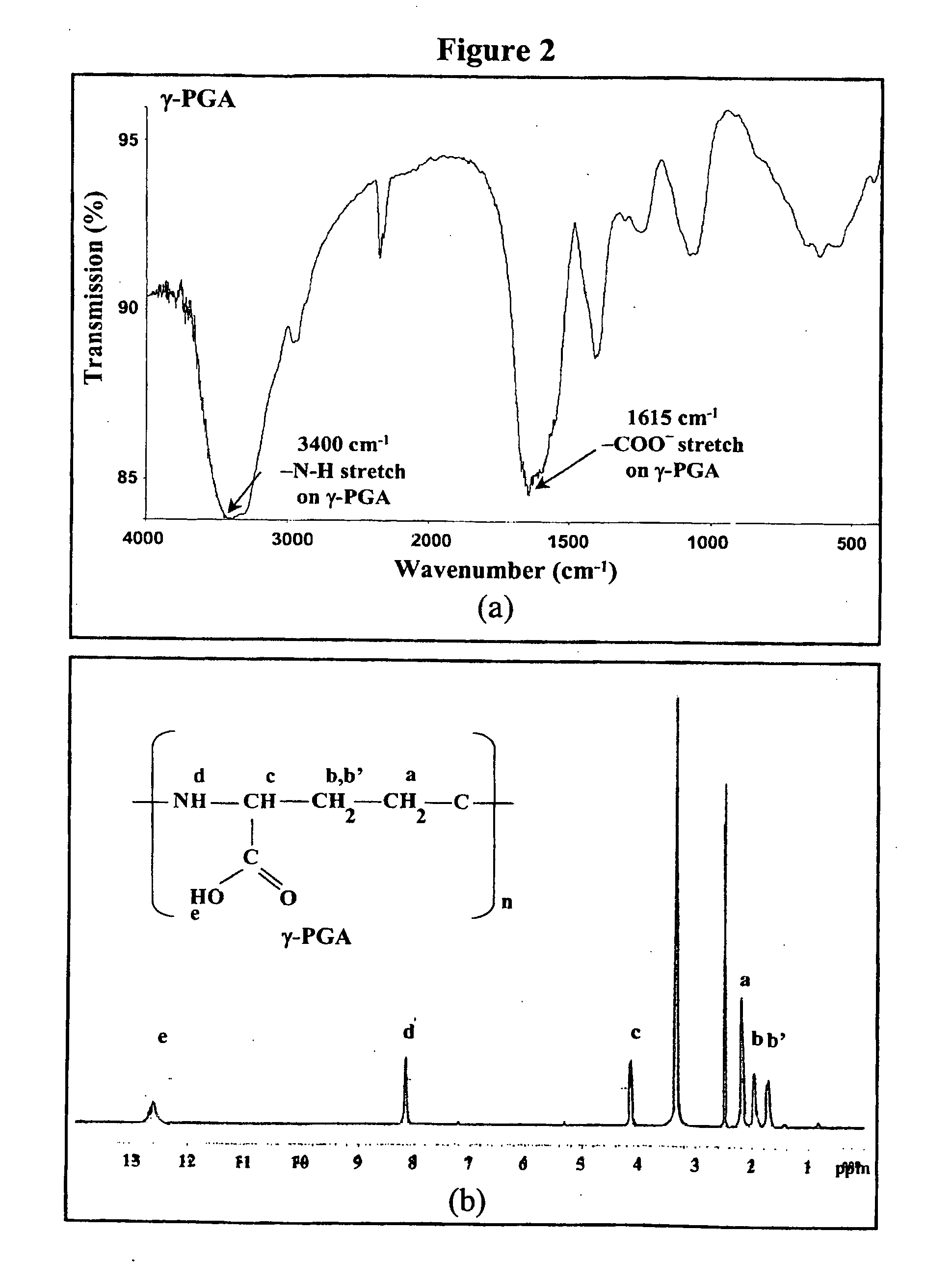
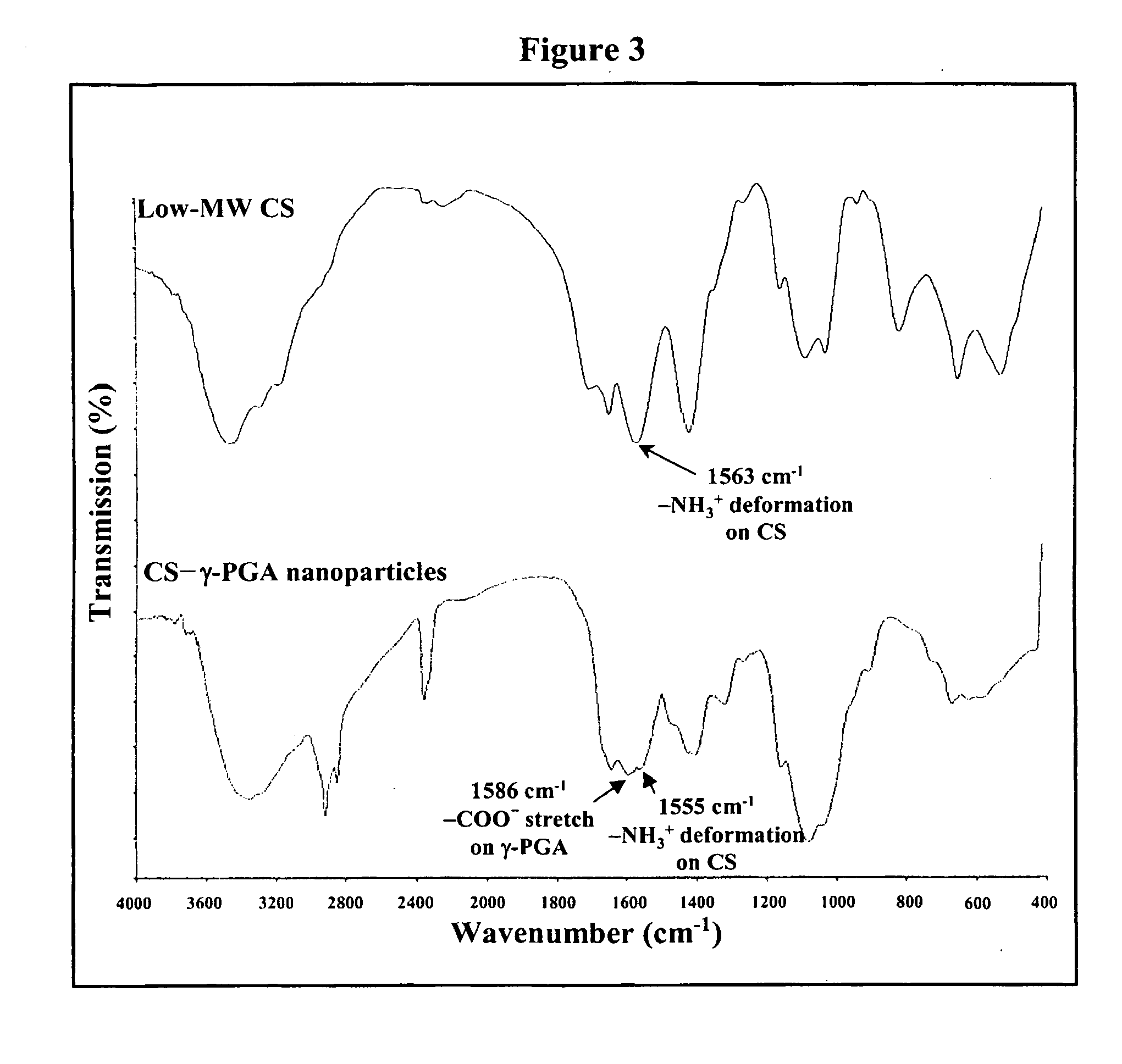
The invention discloses the nanoparticles composed of chitosan, poly-glutamic acid, and at least one bioactive agent of HMG-CoA reductase inhibitors or erythropoietin. The nanoparticles are characterized with a positive surface charge and their enhanced permeability for paracellular drug delivery.
Owner:NANOMEGA MEDICAL CORP
HMG CoA reductase inhibiting composition, method of preparation thereof and method for competitively inhibiting HMG CoA reductase using such composition
InactiveUS20030162827A1Rapid dissolvableMore solubilizedPowder deliveryBiocideHMG-CoA reductaseSemi solid
The present invention relates to a HMG CoA Reductase inhibiting composition with improved bioavailability. The present invention also relates to a method for preparation of such composition and to the competitive inhibition of HMG CoA Reductase using such composition. The active ingredients are present in a rapidly dissolvable and more solubilized state, thereby enabling sustained and complete solubilization, more rapid dissolution upon administration to a patient, improved absorption and / or bioavailability and also increased flexibility in terms of providing the active ingredient and being capable of administration in the form of a solution, suspension, solid dispersion, solid solution or semi-solid formulation.
Owner:CADILA HEALTHCARE LTD
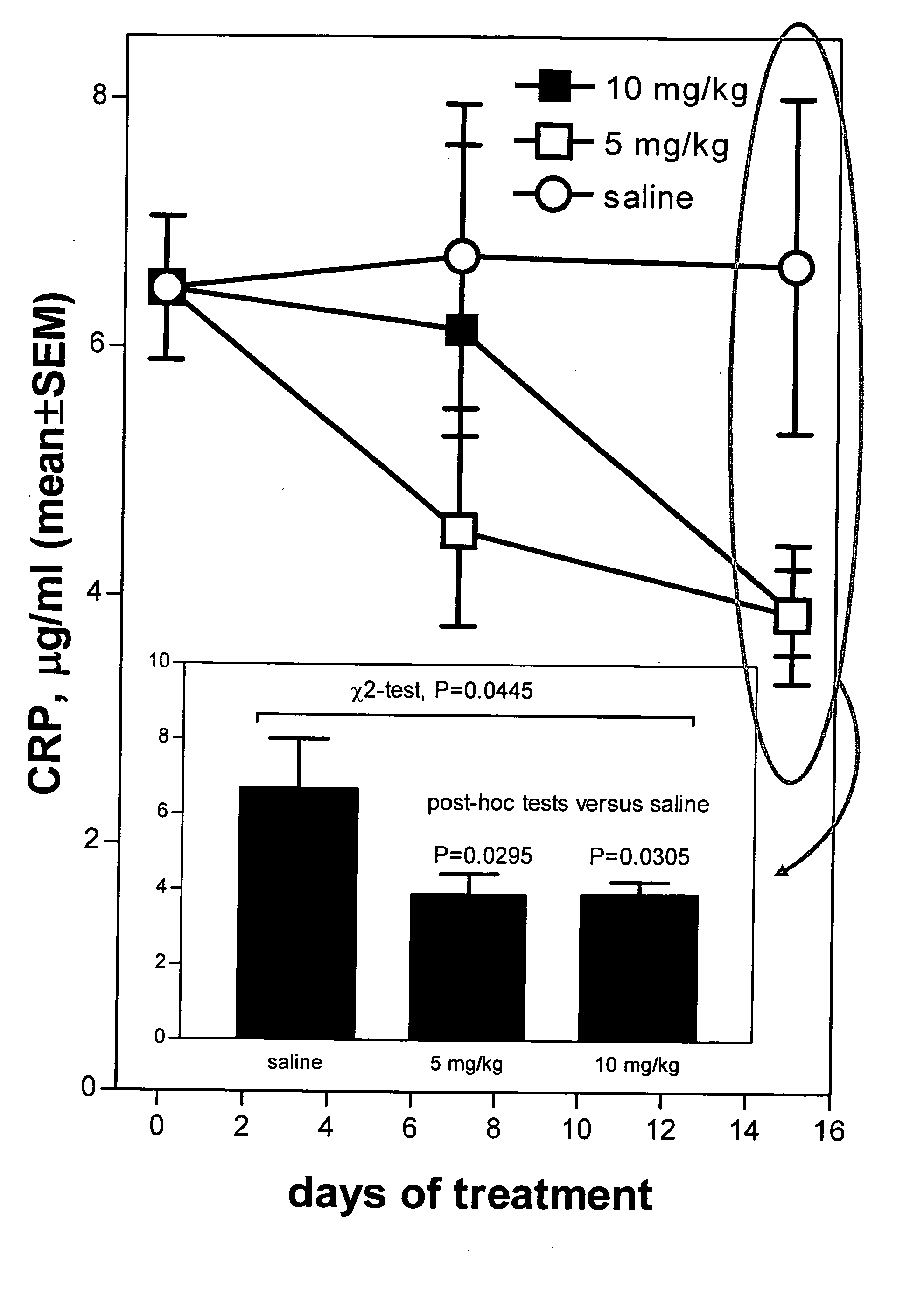
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
Macrocyclic beta-secretase inhibitors
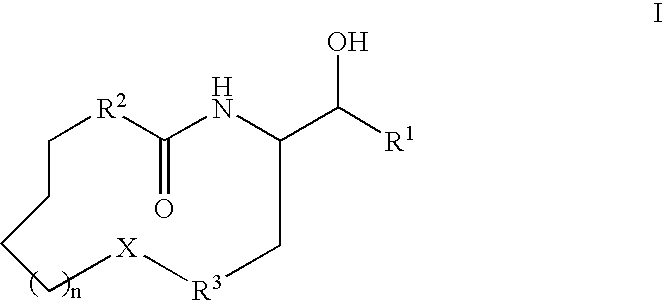
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, n and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
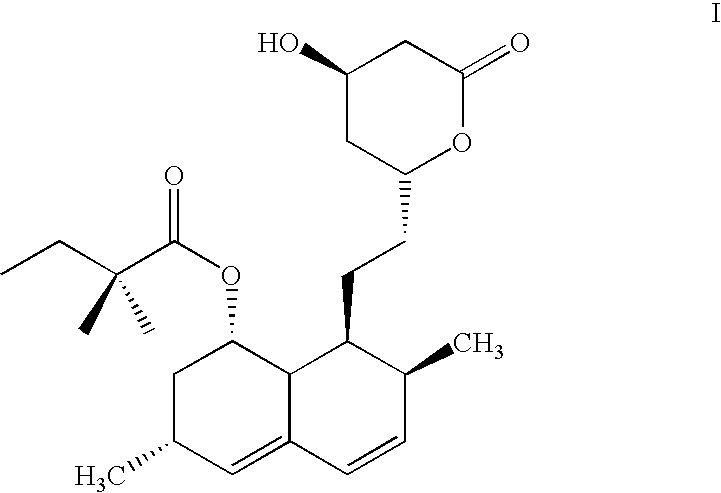
Process for producing simvastatin
InactiveUS6331641B1Improve efficiencyMild conditionsOrganic chemistryBulk chemical productionHMG-CoA reductaseAlcohol
This invention provides an easy and efficient process for producing a simvastatin of great use as an HMG-CoA reductase inhibitor, which comprises deacylation of lovastatin with an inorganic base and a secondary or tertiary alcohol and subjecting the resulting diol lactone to selective protection with a ketal or acetal protective group, acylation and deprotection-lactonization to give simvastatin.
Owner:KANEKA CORP
Substituted amide beta secretase inhibitors
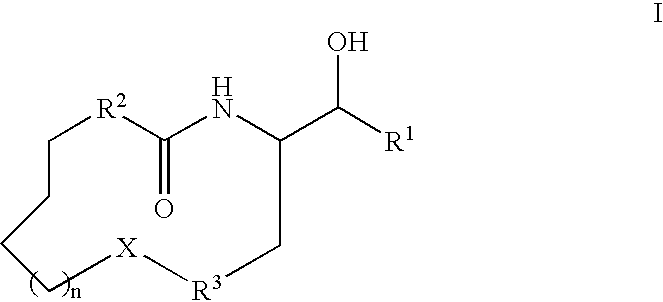
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, R4 and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
Medicinal composition as immunosuppressant
InactiveUS20070149597A1Easy to optimizeSimple compositionBiocideOrganic chemistryHMG-CoA reductaseMethyl group
A pharmaceutical composition useful as a preventive or therapeutic agent for immune-related diseases. The pharmaceutical composition includes at least HMG-COA reductase inhibitor and at least one amino alcohol compound having the following formula (I), a pharmacologically acceptable salt thereof, or a pharmacologically acceptable ester thereof wherein R1 and R2 each represents hydrogen; R3 represents lower alkyl or hydroxymethyl; R4 represents hydrogen, alkyl, alkoxy or halogen; R5 represents hydrogen, halogeno, cyclohexyl or phenyl; X represents vinylene (CH═CH), oxygen, sulfur or methylated nitrogen; Y represents a single bond, oxygen, sulfur or carbonyl; Z represents a single bond or C1-C8 alkylene; n is 2 or 3.
Owner:DAIICHI SANKYO CO LTD
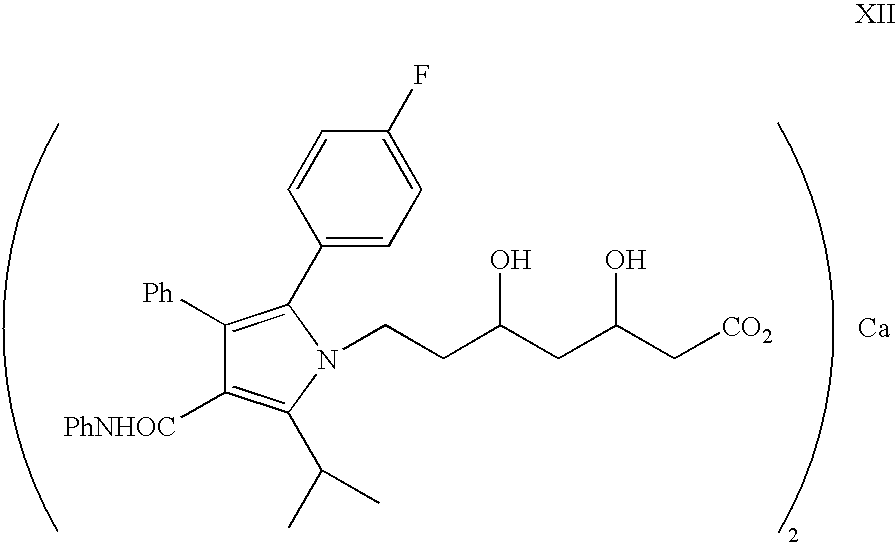
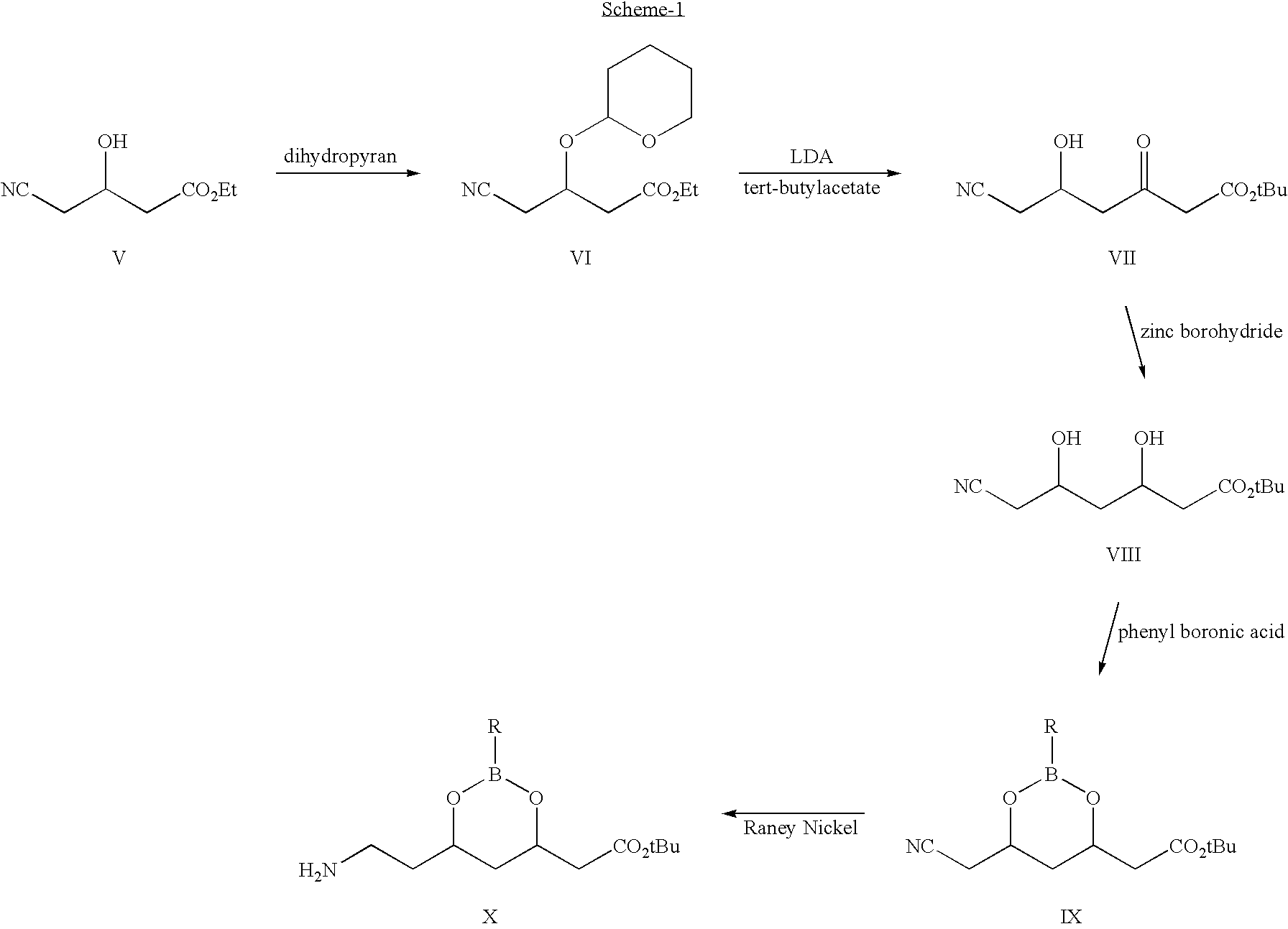
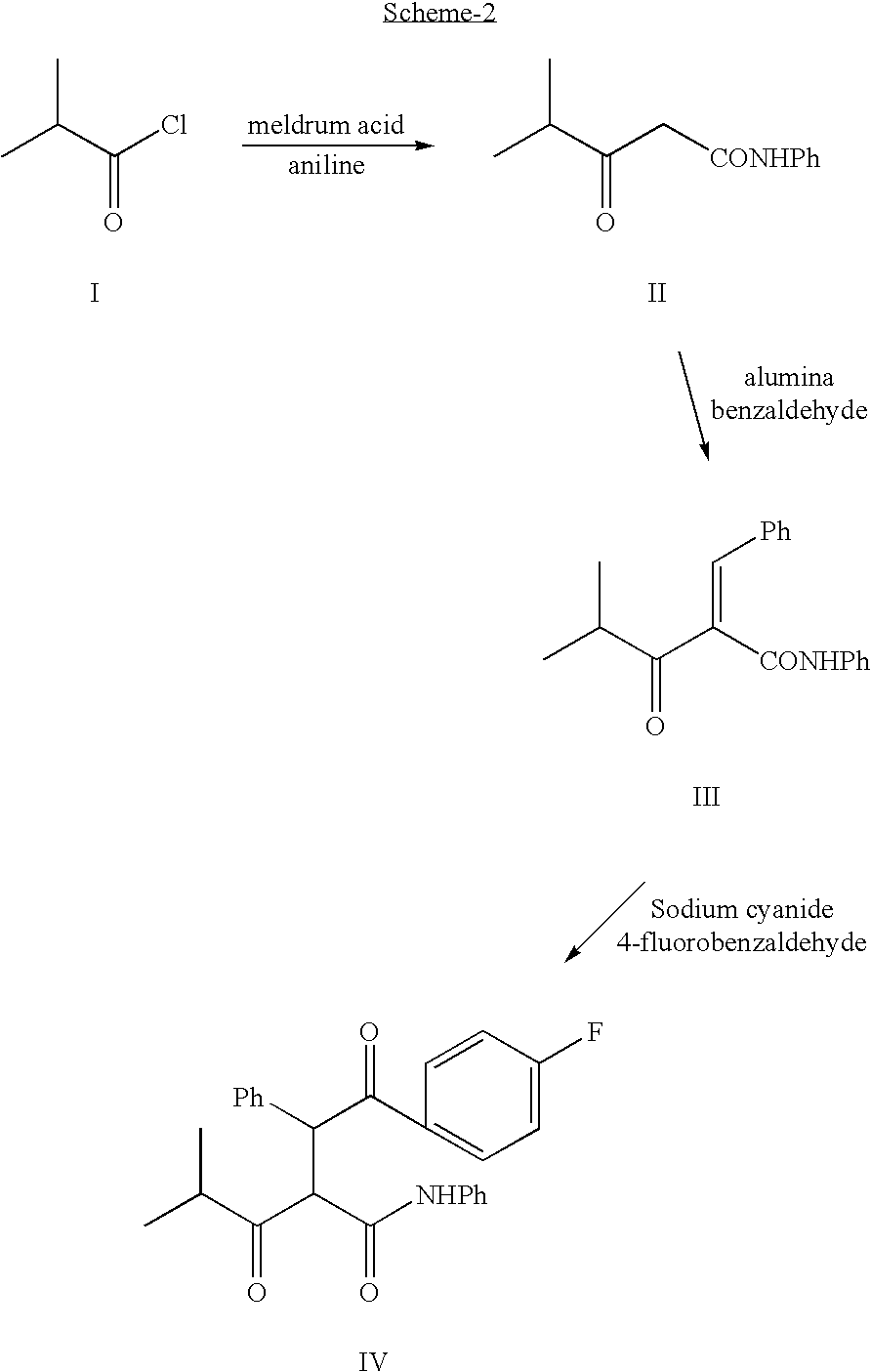
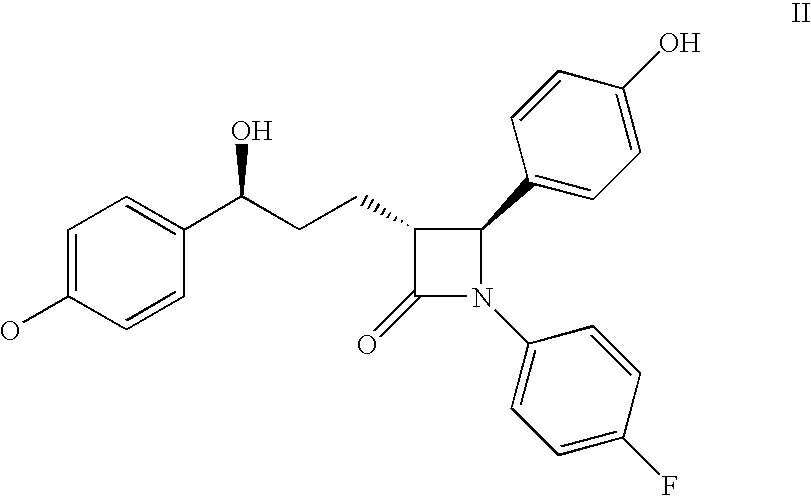
Process for the synthesis of atorvastatin and phenylboronates as intermediate compounds
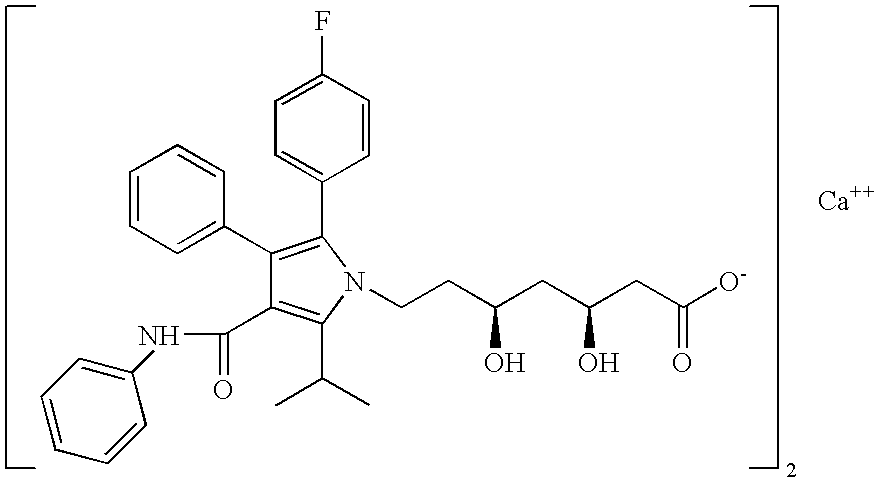
The present invention discusses a novel process for the synthesis of [R-(R*,R*)]-2-(4-fluorophenyl)-B,D-dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-heptanoic acid hemi calcium, atorvastatin. The compound so prepared is useful as inhibitor of the HMG-CoA reductase and may thus be used as hypolipidemic and hypocholesterolemic agent.
Owner:BIOCON LTD
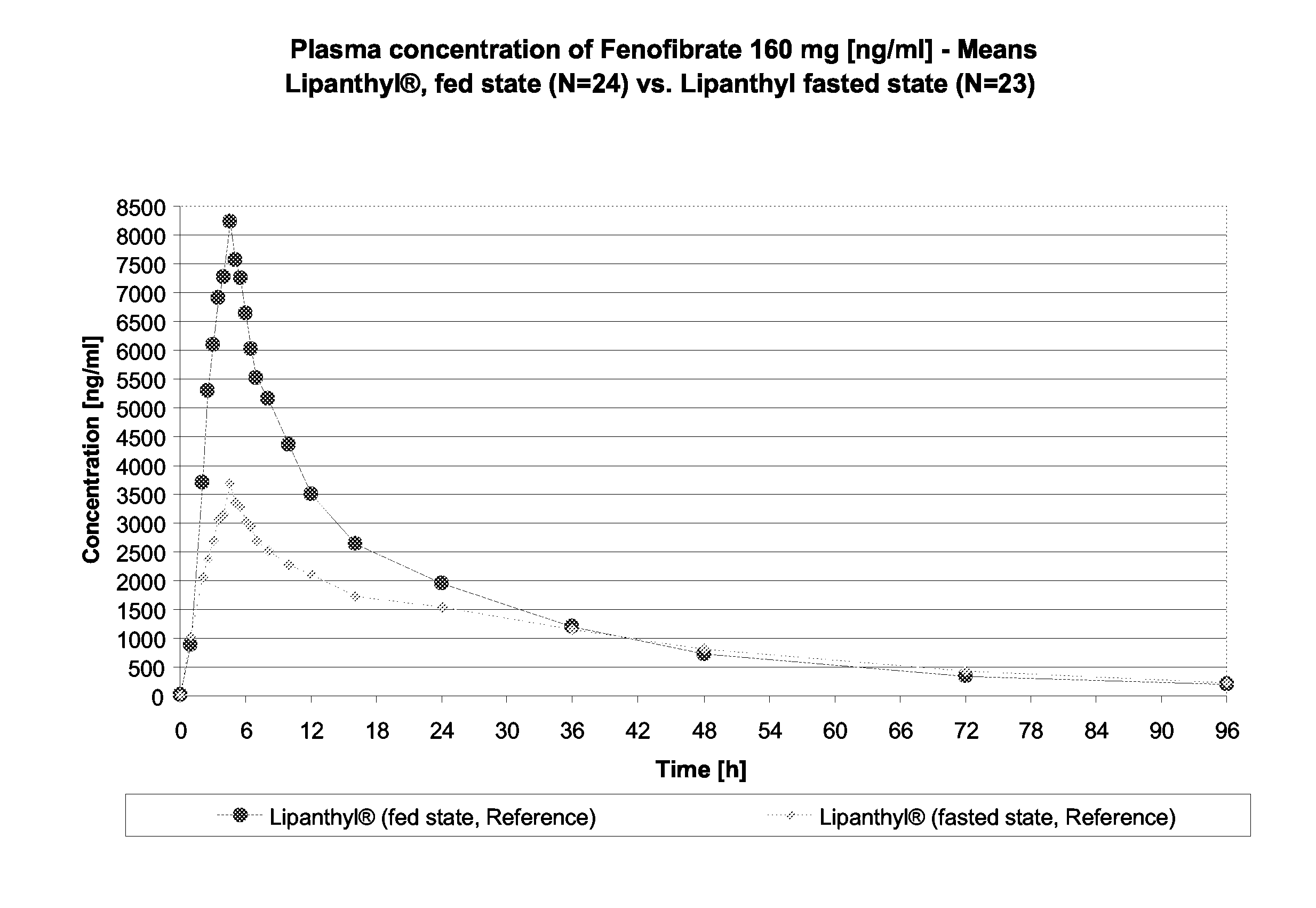
Pharmaceutical compositions comprising fenofibrate and atorvastatin
InactiveUS20070014846A1Improve bioavailabilityReducing inter-individual variationBiocidePill deliveryParticulatesHMG-CoA reductase
Pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor atorvastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCatorvastatin) of between about 250 and about 10,000. The solid compositions are manufactured without any need of addition of water or aqueous medium. Atorvastatin is optionally provided as a controlled release or a delayed release formulation resulting in a maintained LDL-lowering effect at a reduced dosage, and fenofibrate is provided in a formulation having increasing bioavailability and reduced food effect.
Owner:VELOXIS PHARMA
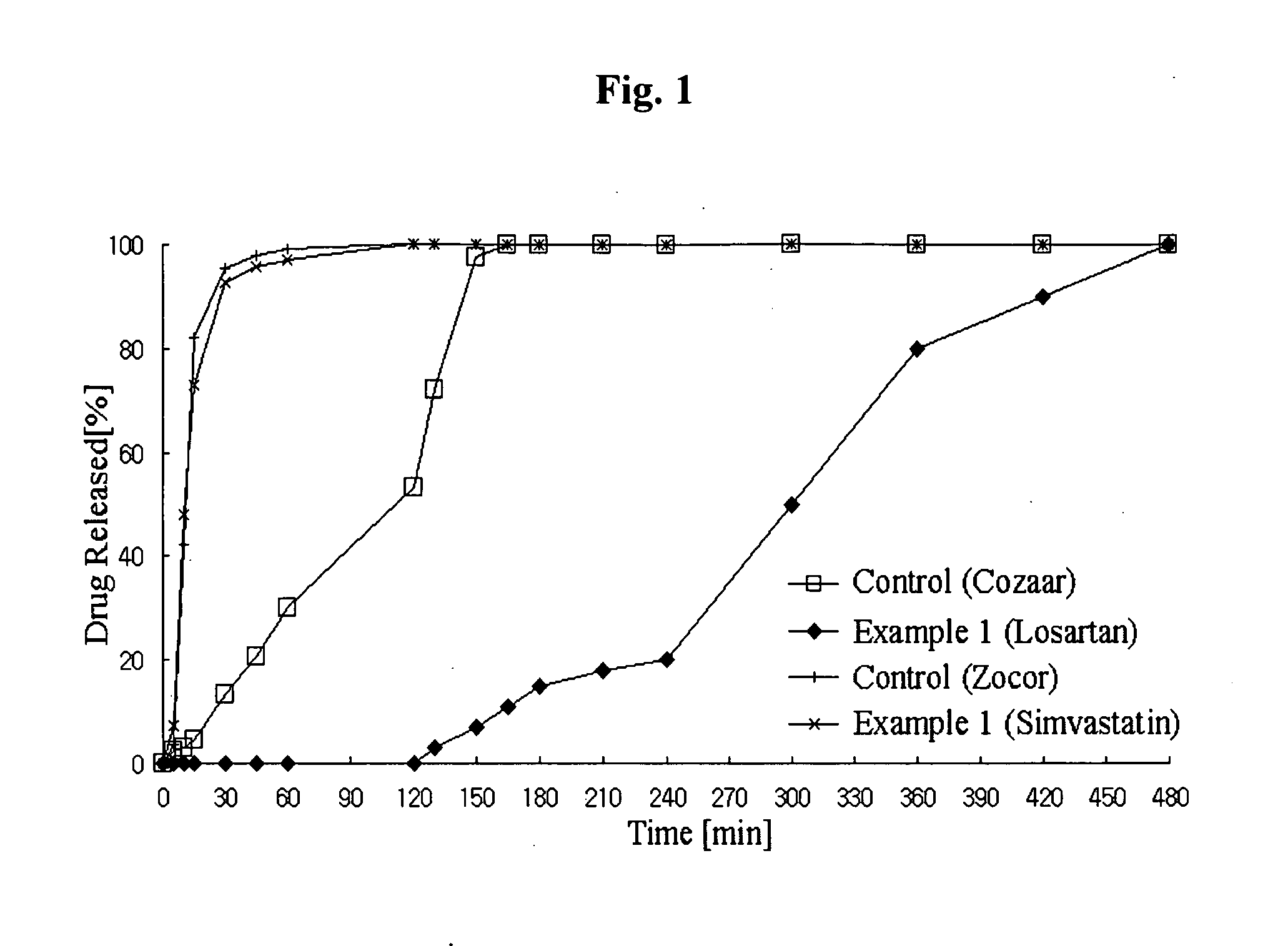
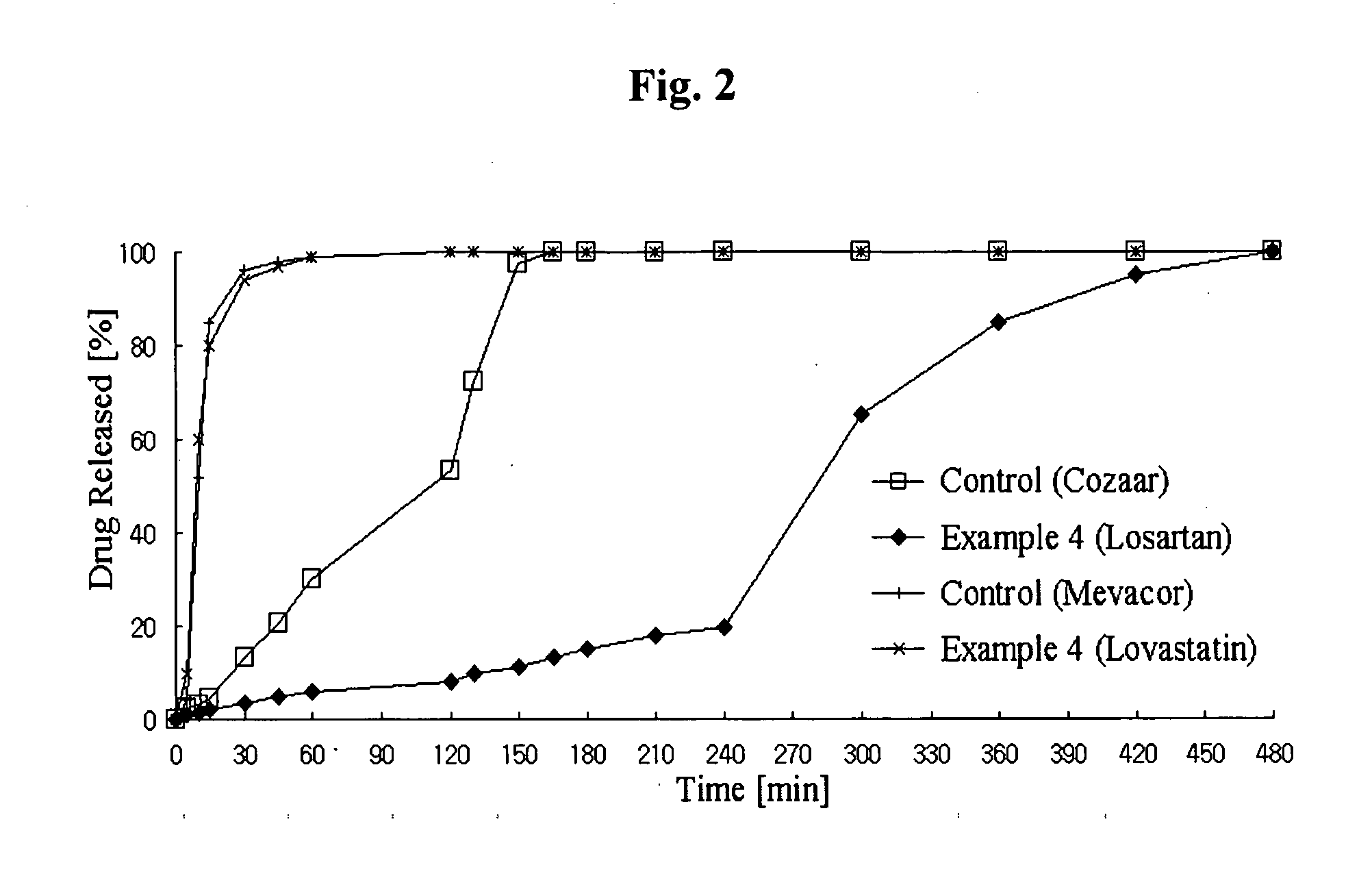
Controlled release complex composition comprising angiotensin-ii-receptor blockers and hmg-coa reductase inhibitors
ActiveUS20100074951A1Maximize the effect of treatmentPreventing and reducing side effectBiocideMetabolism disorderHMG-CoA reductaseCoronary artery disease
Disclosed herein is a lag time delayed-release combination pharmaceutical composition comprising of an angiotensin-II-receptor blocker and an HMG-CoA reductase inhibitor, as well as a preparation method thereof. The composition is designed based on chronotherapy in which active ingredients are administered to have different onset times, such that the release of each active ingredient of the composition in body can be lag time delayed to a specific rate. Also, the composition is very effective for the treatment of hypertension and the prevention of complications in patients having metabolic syndromes which show diabetes, obesity, hyperlipidemia, coronary artery diseases and the like. More specifically, the composition is a drug delivery system designed such that the release of each drug is controlled to a specific rate, and it can show the most ideal effect, when it is absorbed in body.
Owner:HANALL PHARMA CO LTD
Pharmaceutical formulation
The instant invention provides a pharmaceutical composition comprised of a cholesterol absorption inhibitor and an HMG-CoA reductase inhibitor, one or more anti-oxidants, microcrystalline cellulose, hydroxypropyl methylcellulose, magnesium stearate and lactose. The composition need not contain ascorbic acid in order to obtain desirable stability.
Owner:MERCK SHARP & DOHME LTD +2
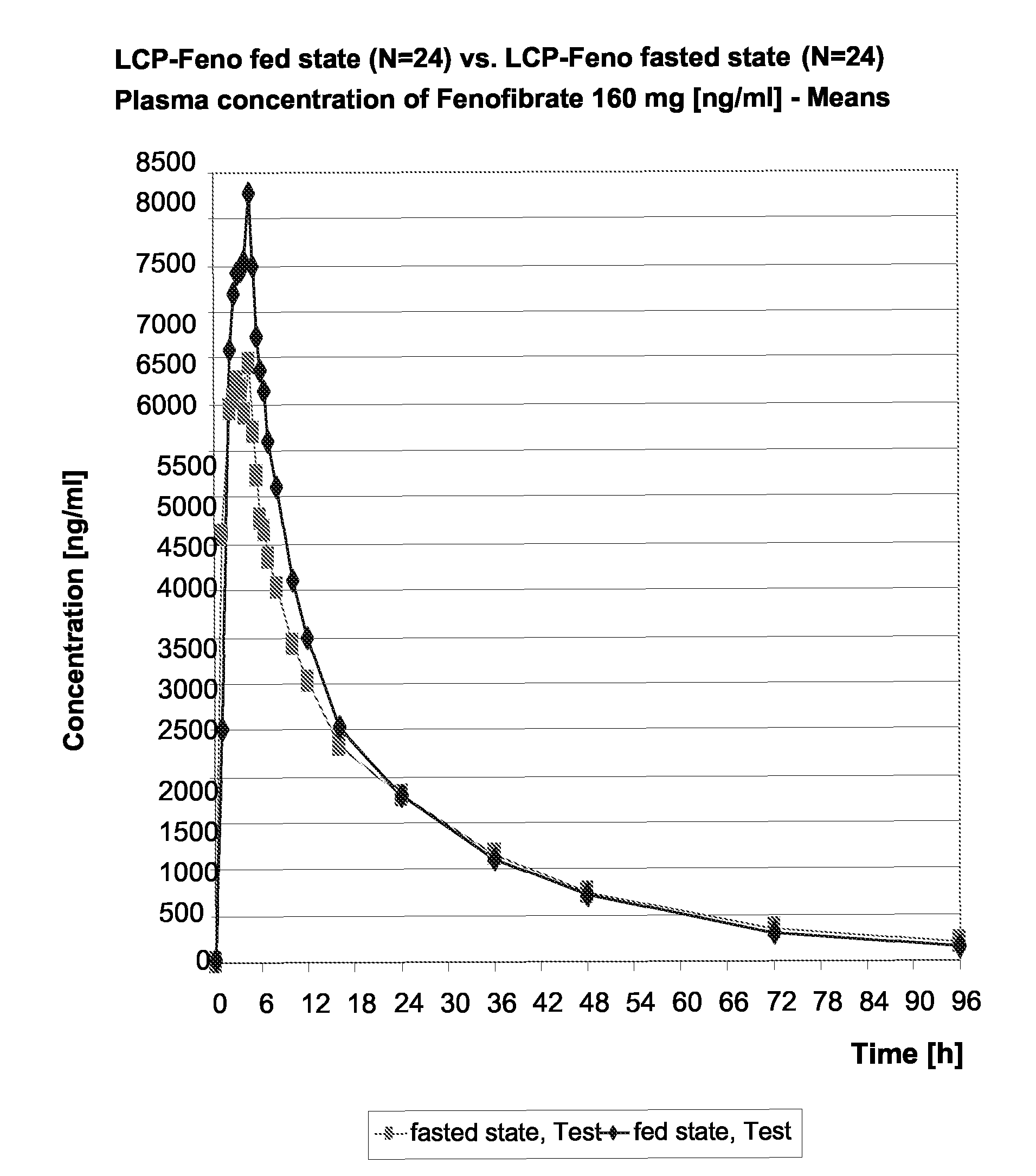
Stable Pharmaceutical Composition Comprising a Fixed Dose Combination of Fenofibrate and an Hmg-Coa Reductase Inhibitor
InactiveUS20080131503A1Avoid interactionImprove stabilityBiocideDrug compositionsHMG-CoA reductaseAdditive ingredient
Owner:VELOXIS PHARMA
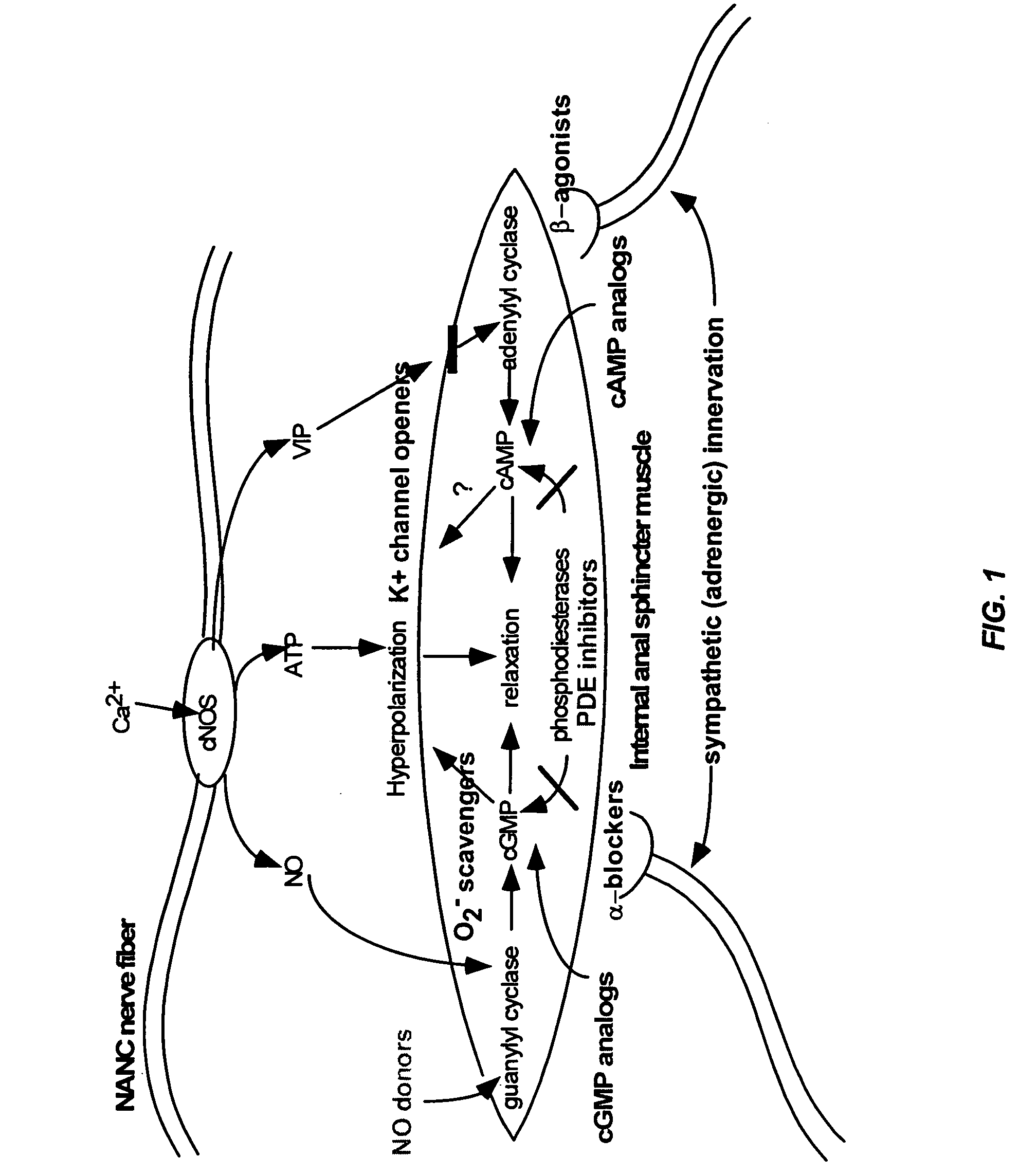
Compounds and methods for the treatment of urogenital disorders
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
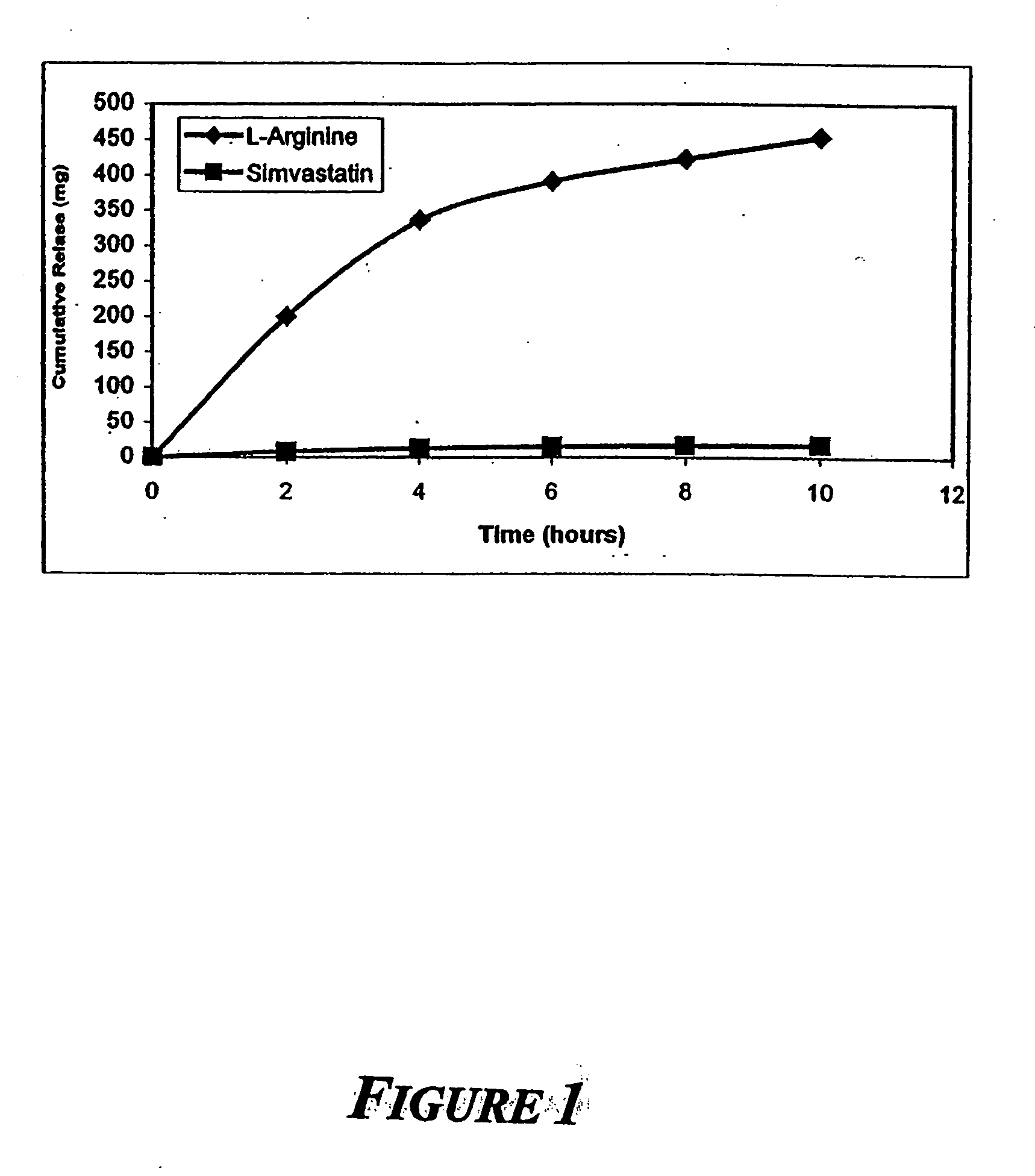
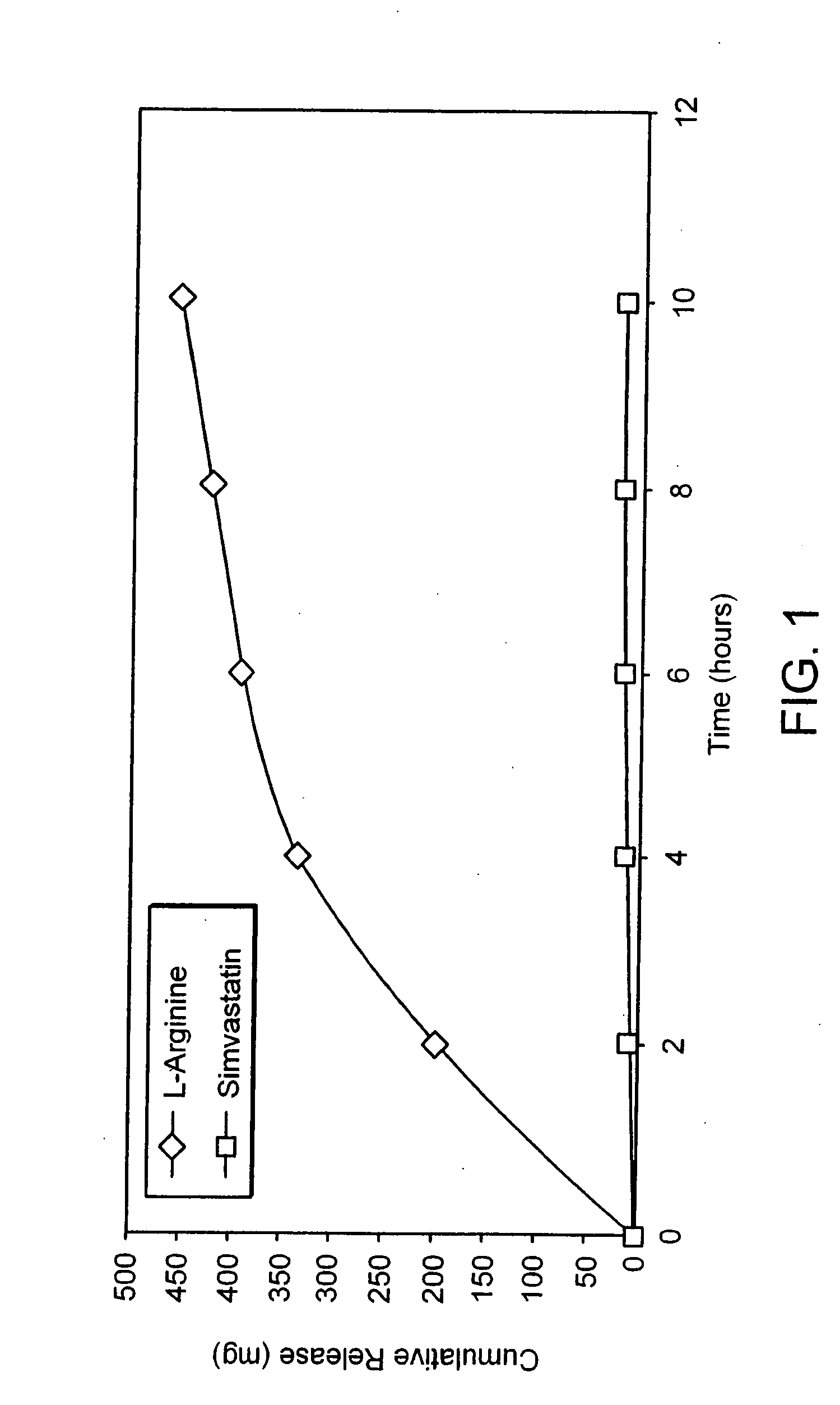
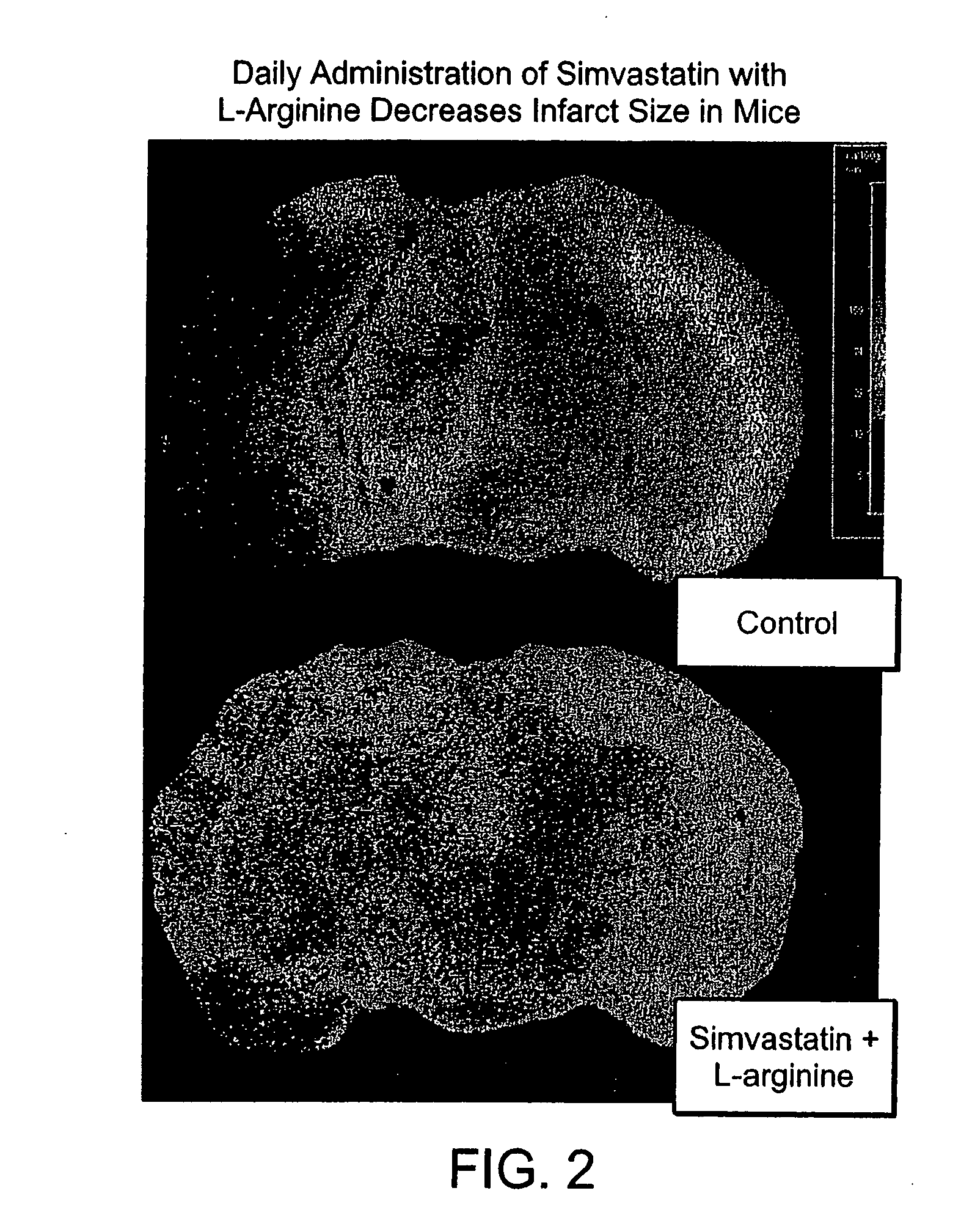
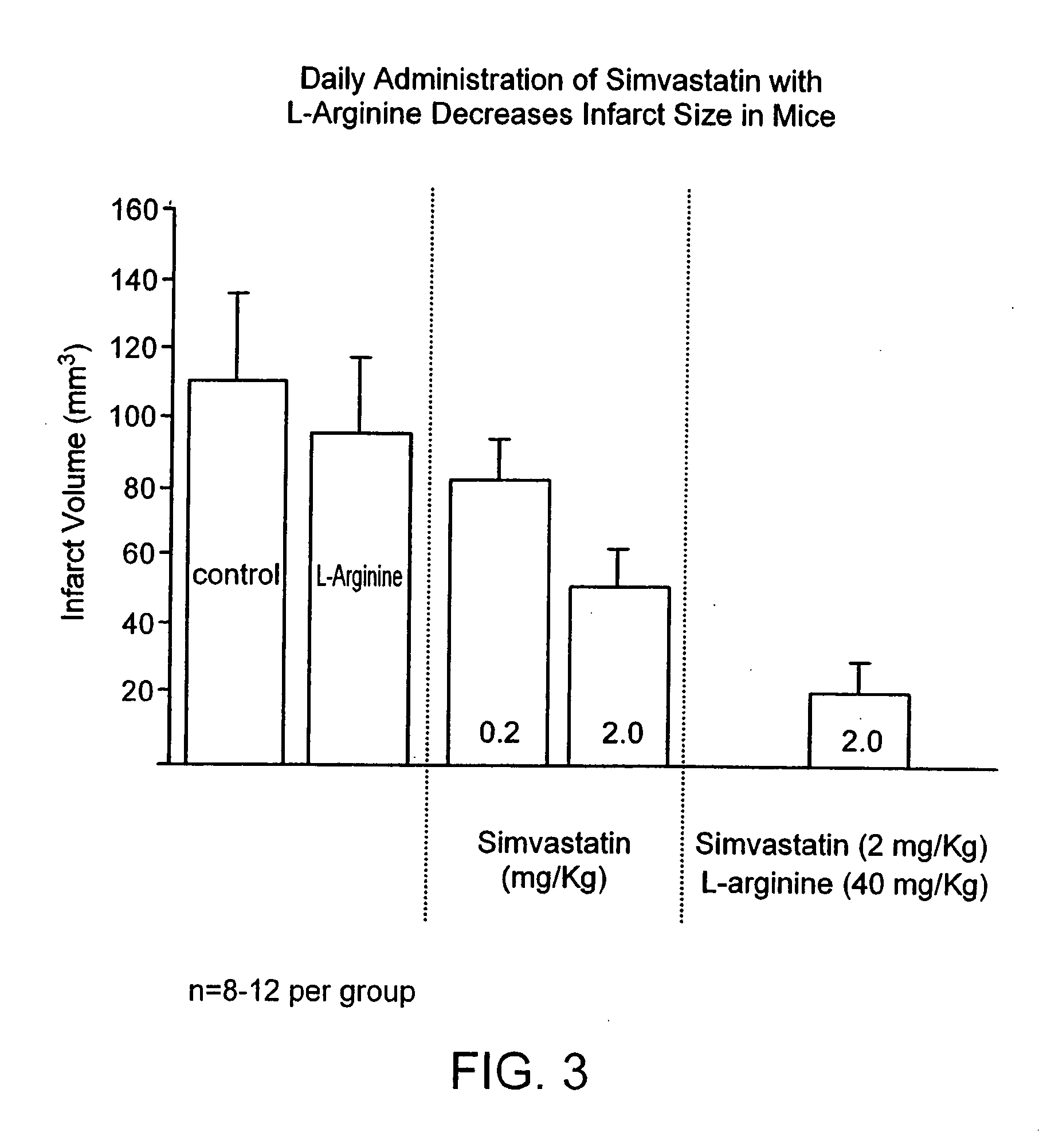
Sustained release L-arginine formulations and methods of manufacture and use
InactiveUS20050287210A1Easy to compressReduce cholesterolOrganic active ingredientsBiocideHMG-CoA reductaseArginine
The present invention provides methods and formulations for the treatment and prevention of cerebrovascular and cardiovascular diseases and disorders. The present invention is based, at least in part, on the discovery that administering to a subject a formulation comprising an agonist of endothelial nitric oxide synthase (eNOS), such as an HMG-CoA reductase inhibitor, and a formulation comprising a precursor of NO, such as L-arginine, may be used to treat or prevent cerebrovascular and / or cardiovascular diseases or disorders.
Owner:PALMETTO PHARMA
Oral pharmaceutical composition containing a combination pparalpha and a hmg-coa reductase inhibitor
InactiveUS20050032878A1Improve bioavailabilityBiocideMetabolism disorderHMG-CoA reductaseAdditive ingredient
Oral pharmaceutical composition containing, in the same pharmaceutical form, effective amounts of a HMG-CoA reductase inhibitor derivative and of a PPARa, especially fenofibrate. Also described is the use of some inactive ingredients which allow to improve the dissolution and / or bioavailability of the drugs from the said composition.
Owner:GALEPHAR M F
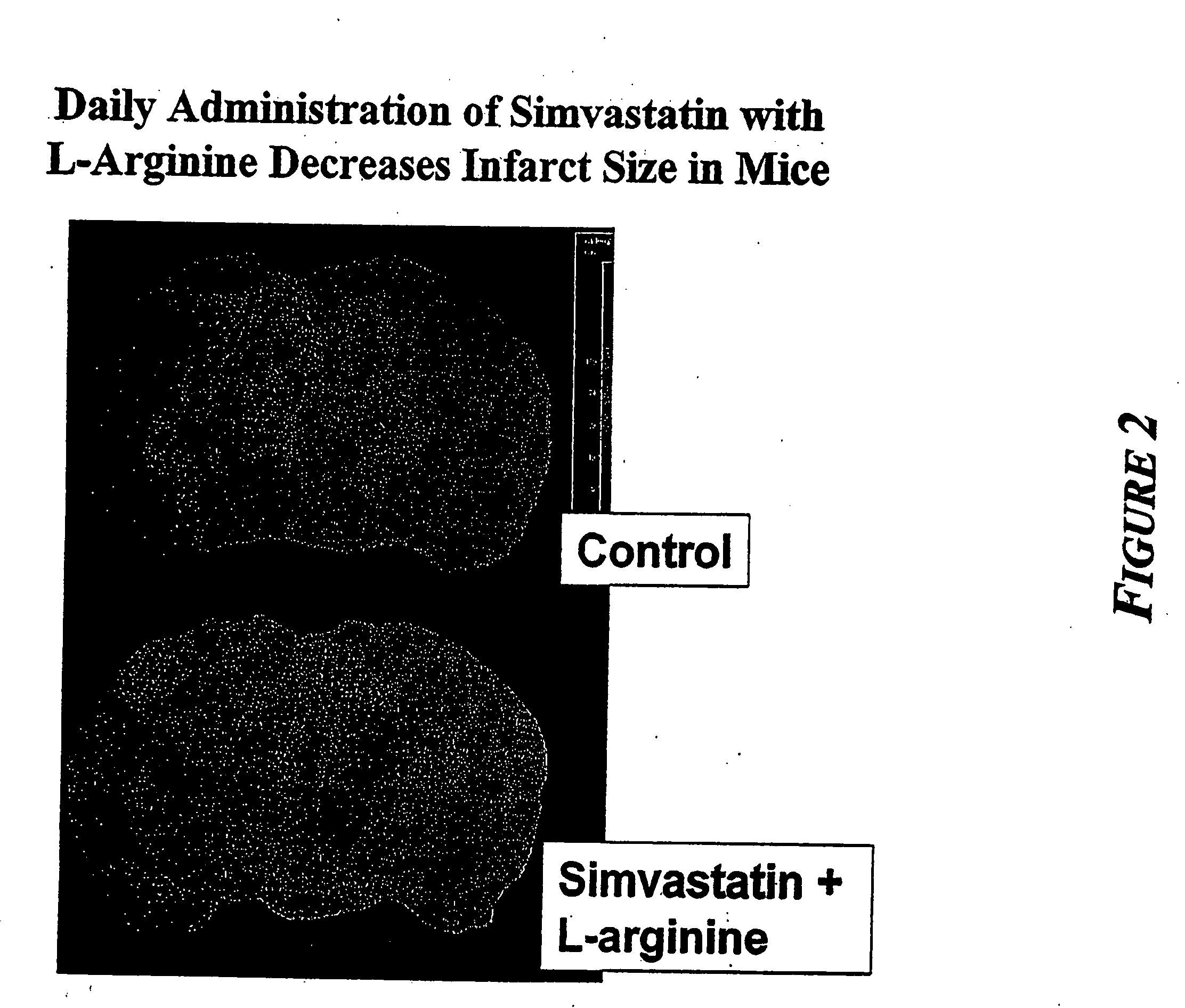
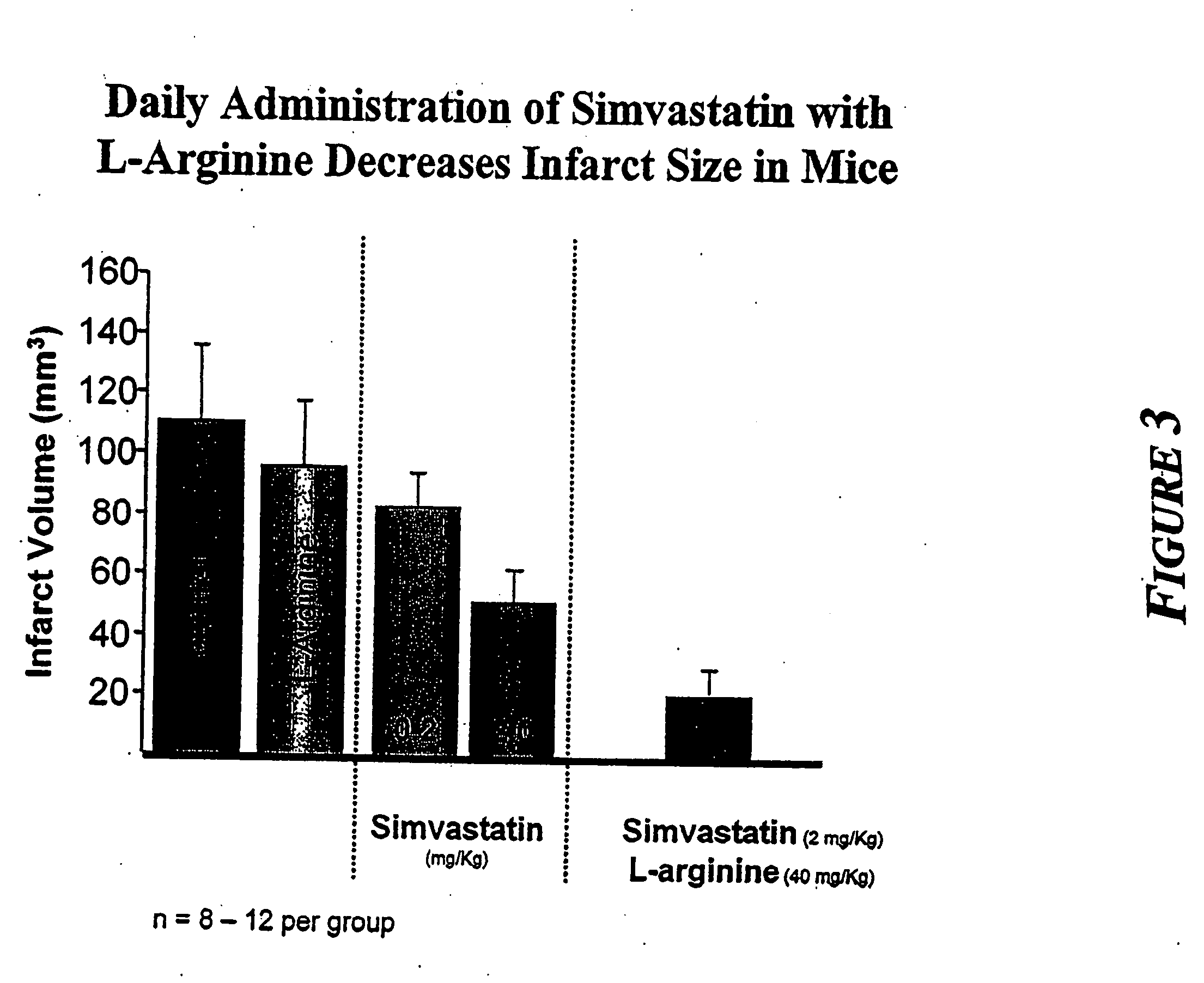
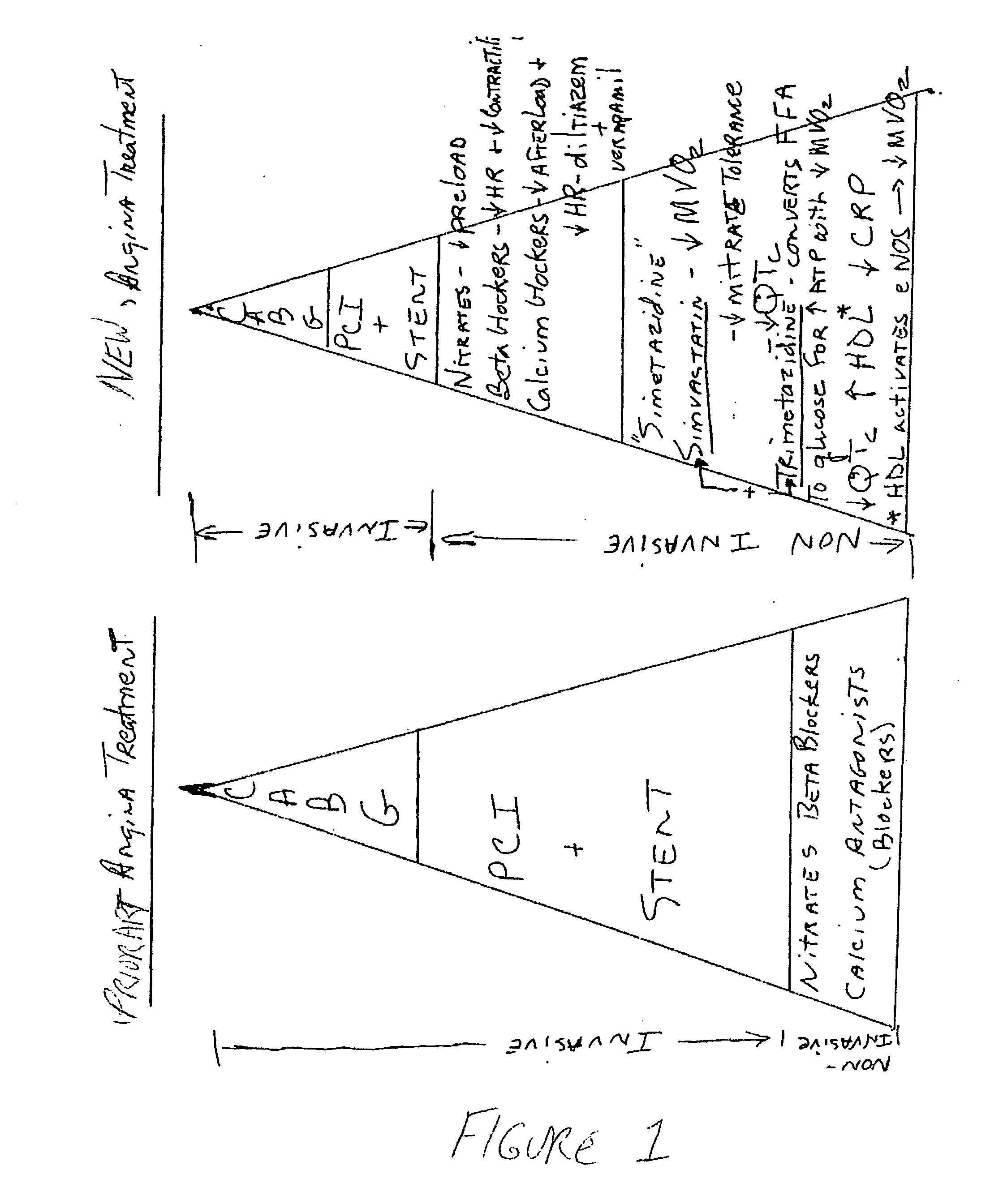
Combination therapy for endothelial dysfunction, angina and diabetes
InactiveUS20060205727A1Control blood sugar levelsIncrease productionBiocideMetabolism disorderHMG-CoA reductaseTrimetazidine
The combination of a HMG CoA reductase inhibitor like a statin, such as simvastatin, with a pFox inhibitor such as trimetazidine (“Simetazidine”) is particularly advantageous for treatment of end-stage complications, such as acute coronary syndrome (ACS) and chronic angina, especially in type II diabetics. The combination therapy is also useful in the treatment and / or prevention of chronic heart failure (CHF) and peripheral arterial disease (PAD). The combination of a nitric oxide (NO) mechanism with increased NO production with pFox inhibition simultaneously treats both the effect and the cause of angina. One or more oral hypoglycemic compounds (biguanides, insulin sensitizers, such as thiazolidinediones, α-glucosidase inhibitors, insulin secretagogues, and dipeptidyl peptidase IV inhibitors), protein kinase C (PKC) inhibitors, and acetyl-CoA carboxylase inhibitors can also be used in combination with the HMG CoA reductase inhibitors and / or pFox inhibitors, especially in type II diabetics, to control glucose levels and treat endothelial dysfunction. The drugs can be given in combination (e.g. a single tablet) or in separate dosage forms, administered simultaneously or sequentially. In the preferred form the statin is given in a dose of between 5 and 80 mg / day in two separate doses, and the pFox inhibitor is administered in a sustained or extended dosage formulation at a dose of 20 mg three times a day or 35 mg two times a day. The dose of the oral hypoglycemic, PKC inhibitor, or acetyl-CoA carboxylase inhibitor varies with the type of drug used.
Owner:HONG KONG NITRIC OXIDE
Sustained release L-arginine formulations and methods of manufacture and use
InactiveUS20060029668A1Improved release profileEasy to compressBiocideOrganic active ingredientsHMG-CoA reductaseArginine
The present invention provides methods and formulations for the treatment and prevention of cerebrovascular and cardiovascular diseases and disorders. The present invention is based, at least in part, on the discovery that administering to a subject a formulation comprising an agonist of endothelial nitric oxide synthase (eNOS), such as an HMG-CoA reductase inhibitor, and a formulation comprising a precursor of NO, such as L-arginine, may be used to treat or prevent cerebrovascular and / or cardiovascular diseases or disorders.
Owner:PALMETTO PHARMA
Combination of an H3 antagonist/inverse agonist and an appetite suppressant
The present invention relates to pharmaceutical compositions comprising therapeutic combinations comprising: one or more H3 antagonists / inverse agonists; one or more appetite suppressants selected from the group consisting of CB1 antagonists / inverse agonists, sibutramine, phentermine and topiramate; and optionally one or more HMG-CoA reductase inhibitors. The invention also relates to medicaments and kits comprising the pharmaceutical compositions of the present invention, and methods of treating obesity, obesity related disorders and diabetes using the pharmaceutical compositions of the present invention.
Owner:SCHERING CORP
Co-formulations or kits of bioactive agents
Provided, among other things, is a formulation or kit comprising: (a) a pharmaceutically effective dosage of one or more a glucose-level-controlling bioactive agents selected from an α-glucodase inhibitor, sulfonylurea, meglitinide, thiazolidinediones, biguanide, insulin, dual PPARα / γ agonist, PPARγ agonist or insulin secretagogue; and (b) a pharmaceutically effective dosage of (i) one or more of an antihypertensive bioactive agent selected from an ACE inhibitor, calcium channel blocker, beta blocker, angiotension II receptor antagonist or diuretic, or (ii) one or more of an anti-dyslipidemia bioactive agent selected from a HMG-CoA reductase inhibitor, bile acid sequestrant, fibric acid derivative, sterol, cholesterol absorption inhibitor, MTP inhibitor or nicotinic acid derivative; wherein: in the case of (i) a combination of a first bioactive agent of group (a) that is metformin with a second bioactive agent of group (b), or (ii) a combination of a first bioactive agent of group (a) that is a thiazolidinedione or dual PPARα / γ agonist with an angiotension II receptor antagonist, one or more of the following applies: (I) one of the first bioactive agent or the second bioactive agent is formulated for sustained release, and the other is formulated for immediate release, each formulated for once-a-day dosing; or (II) the co-formulation or kit comprises (A) a biguanide and a thiazolidinedione and (B) one or more group (b) bioactive agents.
Owner:ABEILLE PHARMA
Compositions of choleseteryl ester transfer protein inhibitors and HMG-CoA reductase inhibitors
InactiveUS20040132771A1Overcomes drawbackImprove concentrationPowder deliveryBiocideHMG-CoA reductaseBioavailability
A composition comprises (1) a solid amorphous adsorbate comprising a cholesteryl ester transfer protein (CETP) inhibitor and a substrate; and (2) an HMG-CoA reductase inhibitor. The solid amorphous adsorbate provides concentration enhancement of the CETP inhibitor relative to a control composition consisting essentially of the unadsorbed CETP inhibitor alone, resulting in improved bioavailability.
Owner:BEND RES
Compositions comprising fenofibrate and pravastatin
InactiveUS20050096390A1Substance may accumulateImprove bioavailability in vivoBiocidePill deliveryHMG-CoA reductasePharmaceutical medicine
The present invention relates to pharmaceutical compositions in particulate form or in solid dosage forms comprising a combination of fenofibrate and the HMG CoA reductase inhibitor pravastatin or a pharmaceutically active salt thereof, which upon oral administration provides a relative AUC0-24 value (AUCfibric acid / AUCpravastatin) of between about 90 and about 6300. The solid compositions are manufactured without any need of addition of water or aqueous medium and comprise at least 80% of the active substances fenofibrate and pravastatin in dissolved form, or, optionally, atorvastatin in micronized form, in order to ensure suitable bioavailability.
Owner:LIFECYCLE PHARMA AS
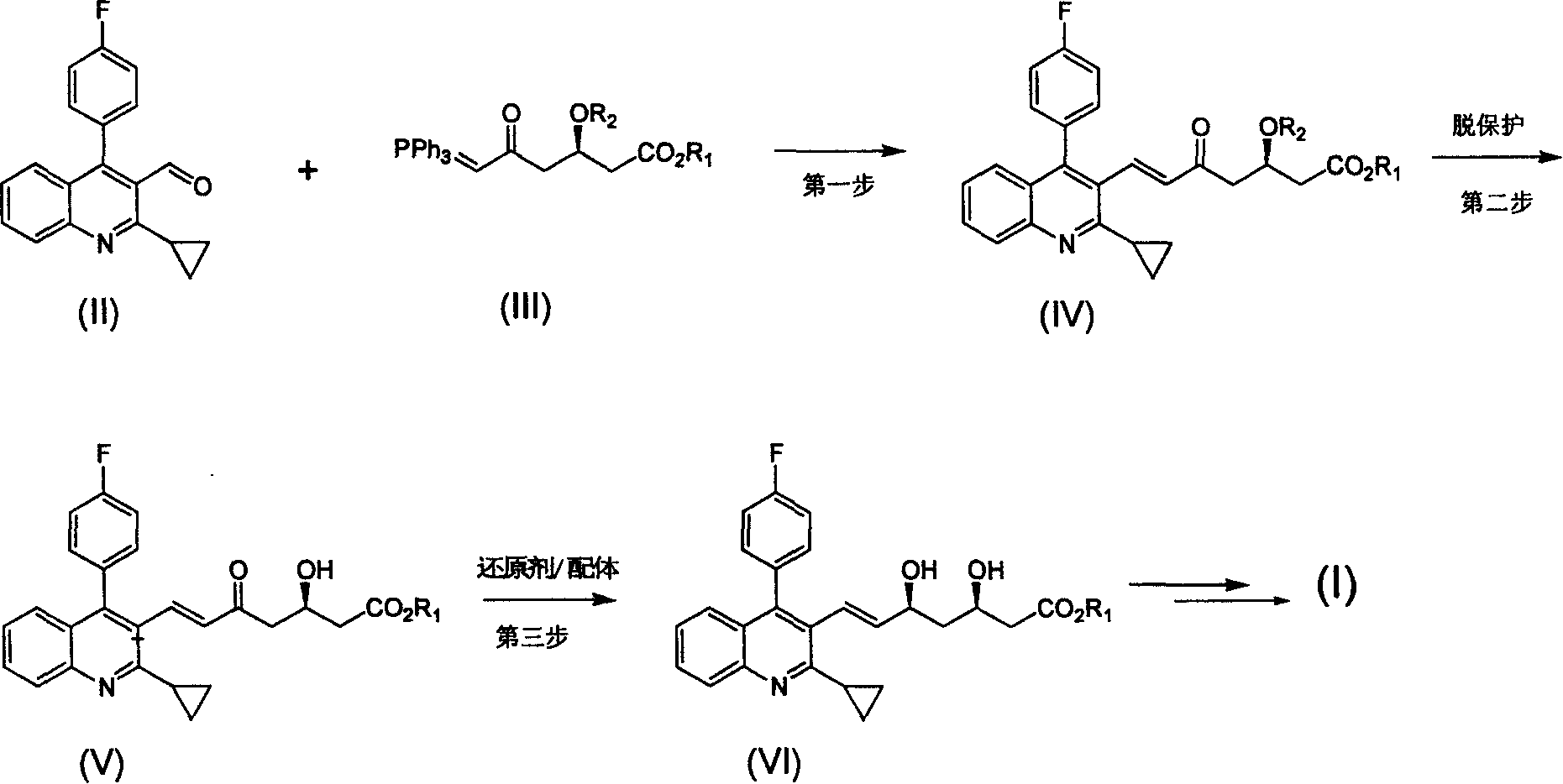
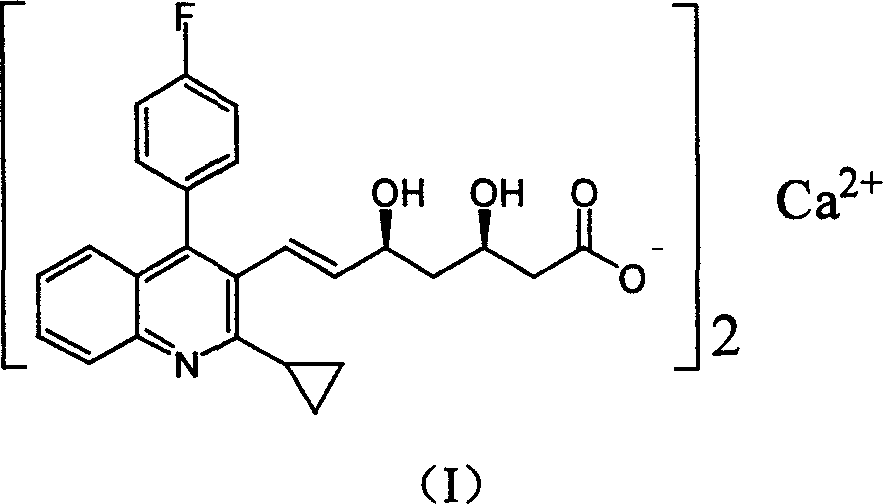
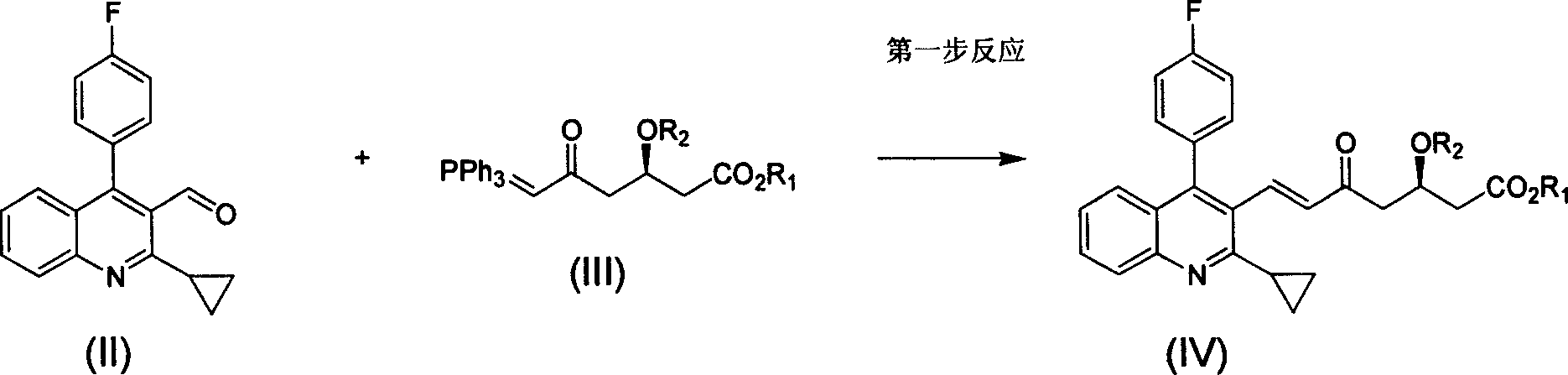
Method for preparing high optical purity pitavastatin calcium raw material drug
The invention relates the method of preparing the raw material of high optical purity pravastatin calcium. The method comprises the following steps: adding the 2- cyclopropyl-4-(4- fluorophenyl)-3-quinoline aldehyde II and (3R)-3- alkoxy silane-5- carbonyl-6- triphenyl phosphor heptene acid ester III in dissolvent, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)-3- alkoxy silane-6- triphenyl phosphor heptene acid ester IV, removing the protection of IV, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-5- carbonyl-(3R)- hydroxyl -6- triphenyl phosphor heptene acid ester V, deacidizing it in the mixture dissolvent of alcohol and ether with NaBH4 or KBH4 at -100-0Deg.C, getting (E)-7- [2-2- cyclopropyl-4-(4- fluorophenyl)-3- chinoline]-(3R, 5S)- dihydroxy -6- triphenyl phosphor heptene acid ester VI, hydrolyzing it with alkali, and getting pravastatin calcium. The material is used to prepare HMG-CoA reductase inhibiting agent.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com